Abstract
A method for incorporating cyclopropane motifs into complex molecules has been developed. Herein we report a zinc dust-mediated cross-electrophile coupling reaction of 1,3-dimesylates to synthesize cyclopropanes. 1,3-Dimesylates can be readily accessed from 1,3-diols, a functionality prevalent in many natural products and medicinal agents. The reaction conditions are mild, such that functional groups, including amides, esters, heterocycles, and alkenes, are tolerated. Notably, we have demonstrated late-stage cyclopropanation of statin medicinal agents.
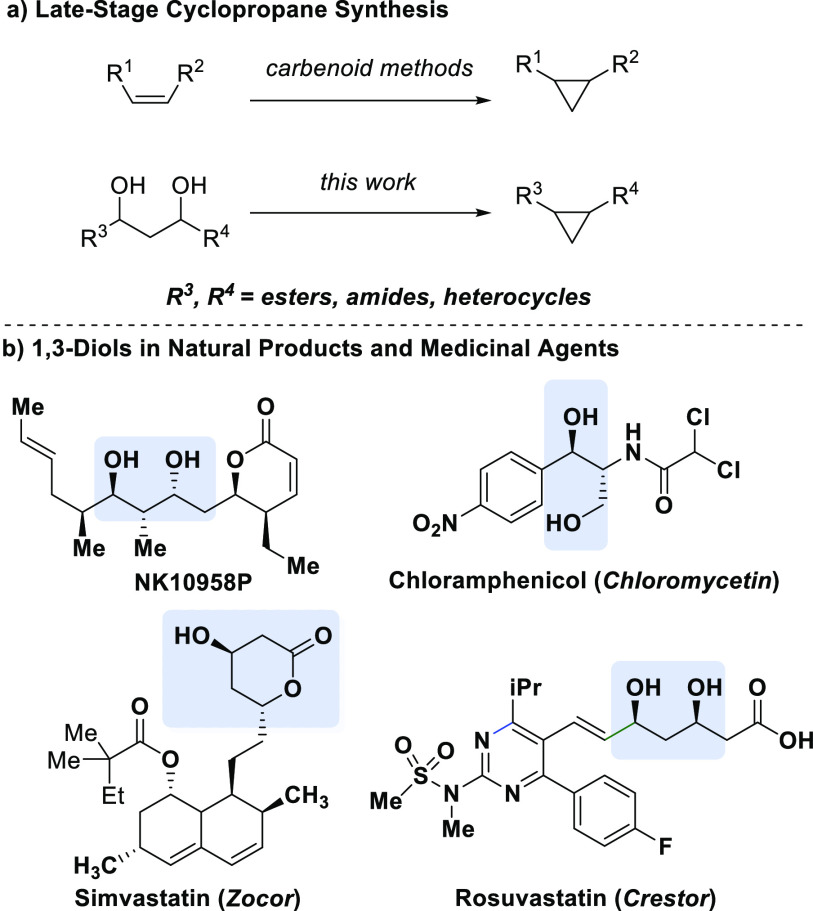
Natural products provide the structural frameworks and starting points for the discovery of many medicinal agents; >35% of drugs approved from 1981 to 2019 are natural products and synthetic analogues.1 Late-stage modification provides a strategy for remodeling the structures of complex scaffolds and altering activity.2 This field has evolved significantly in the past two decades, with exciting developments in chemoselective and site-selective reactions, including alcohol functionalization and C–H activation.3−6 Late-stage introduction of cyclopropane moieties would also be desirable, because the cyclopropane motif is important in medicinal chemistry.7 Alkene cyclopropanation has been employed to introduce cyclopropanes as epoxide isosteres, for example, in epothilone derivatives,8 and as a derivatization and tagging strategy for chemical biology studies.9 These strategies require alkenes in the natural product scaffold (Figure 1a).
Figure 1.
We envisioned conversion of 1,3-diols to cyclopropanes as an orthogonal approach. The appeal of this strategy is the prevalence of the alcohol functional group in natural products and medicinal agents (Figure 1b).10 The 1,3-diol motif is central to the backbone of polyketides, secondary metabolites with diverse biological activity ranging from anticancer to antibiotic to cholesterol-lowering activity.11 1,3-Diols are also found in medicinal agents such as rosuvastatin and lumigan. In addition to modifying the pharmacokinetic properties, transformation of a 1,3-diol moiety to a cyclopropane could be employed to alter the overall conformation and relative orientation of functional groups while retaining a C(sp3)-rich backbone.12 We set out to establish a method that would achieve late-stage synthesis of cyclopropanes from complex 1,3-diols employing mild reagents. On the basis of our prior work in the development of nickel-catalyzed intramolecular cross-electrophile coupling (XEC) reactions of 1,3-dimesylates,13 we hypothesized that 1,3-dimesylates would undergo a reducing metal-mediated XEC reaction to form cyclopropanes.14−17 In this work, we report a zinc-mediated conversion of 1,3-dimesylates to cyclopropanes and demonstrate this reaction on several natural product and medicinal agent cores, including a series of statins.
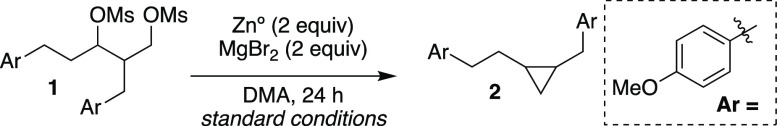
To initiate our investigation, we chose 1,3-dimesylate 1 as a suitable test substrate to identify the reaction conditions for an intramolecular XEC reaction to generate cyclopropane 2 (Table 1). Due to the functional group compatibility of reducing metal reagents, zinc dust was chosen as the reductant.18 We also included halide salts, including MgX2 and NaX, in the reaction based on a working hypothesis that these reactions would proceed through 1,3-dihalide intermediates.19,20 In the presence of zinc dust and magnesium bromide in DMA, 1,3-dimesylate 1 was converted to cyclopropane 2 in 69% yield (entry 1). We observed no product formation in the absence of MgBr2 (entry 2). Increasing the number of equivalents of MgBr2 did not significantly impact the yield (entry 3). Diminished yields were observed with the alternative halide salts MgI2, NaBr, and NaI (entries 4–6, respectively). Notably, NaI resulted in 49% recovered starting material. Increasing the amount of NaI from 2 to 8 equiv and changing the solvent from DMA to THF provided a moderate increase in conversion from entry 6 (entry 7). Finally, combining MgBr2 and NaI did not afford a higher yield (entry 8).
Table 1. Optimization of Zinc-Mediated XEC Reactions of 1,3-Dimesylates.
| entry | deviation from standard conditions | dimesylate | cyclopropane (%)a | dr (trans:cis) |
|---|---|---|---|---|
| 1 | none | 12 | 69 | 3.6:1 |
| 2 | no MgBr2 | 63 | <5 | NA |
| 3 | 3.0 equiv of MgBr2 | 21 | 68 | 3.9:1 |
| 4 | Mgl2 instead of MgBr2 | 40 | 19 | 3.8:1 |
| 5 | NaBr instead of MgBr2 | 21 | 52 | 4.2:1 |
| 6 | Nal instead of MgBr2 | 49 | 33 | 4.5:1 |
| 7b | 8.0 equiv of Nal instead of MgBr2 | 35 | 47 | 3.7:1 |
| 8 | add 2.0 equiv of Nal | 11 | 50 | 4.1:1 |
Yields determined by comparison to PhTMS as the internal standard.
THF instead of DMA.
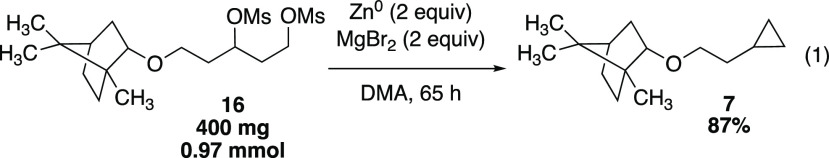
With suitable reaction conditions in hand, a variety of mono- and disubstituted cyclopropanes were synthesized to demonstrate the functional group compatibility of this transformation. Monosubstituted cyclopropanes 3–8 were synthesized in good to great yields (Figure 2a). The reaction was tolerant of trifluoromethyl groups (3), PMB-protected diols (4), β-branched substrates (3 and 5), and aryl ethers (6). Cyclopropane derivatives of a terpene, (−)-borneol, and a steroid, β-sitosterol, were synthesized (7 and 8, respectively). We next sought to evaluate the synthesis of aryl- and alkyl-1,2-disubstituted cyclopropanes (Figure 2b). Aryl ether and alkyl ether substituents were well tolerated (9, 11, and 12). Notably, cyclopropane 11 containing a pendant ether was synthesized in a 47% yield. Dibenzofuran-substituted cyclopropane 10 and furanyl cyclopropane 12 demonstrate this method’s compatibility with heterocyclic motifs. Additionally, a series of 1,2-disubstituted cyclopropanes from polyketide scaffolds were synthesized (Figure 2c). An aldol reaction followed by sodium borohydride reduction provides rapid access to the desired diol motifs from commercially available β-ketoesters and the corresponding aldehydes or primary alcohols.21 To facilitate the XEC reaction toward cyclopropanes 13–15, we found that Zn0 dust with 8 equiv of NaI in THF provided the best yield for this class of substrate. Cyclopropane 13 was formed in 46% yield. The natural product (S)-citronellal was derivatized into a 1,3-dimesylate to form cyclopropane 14 in 41% yield. Likewise, (R)-nopol was derivatized to form cyclopropane 15 in 43% yield. To determine whether the reaction would be amenable to larger scales, we performed the reaction of 1,3-dimesylate 16 on a 400 mg scale and were pleased to see that the yield improved to 87% (eq 1).
 |
1 |
Figure 2.
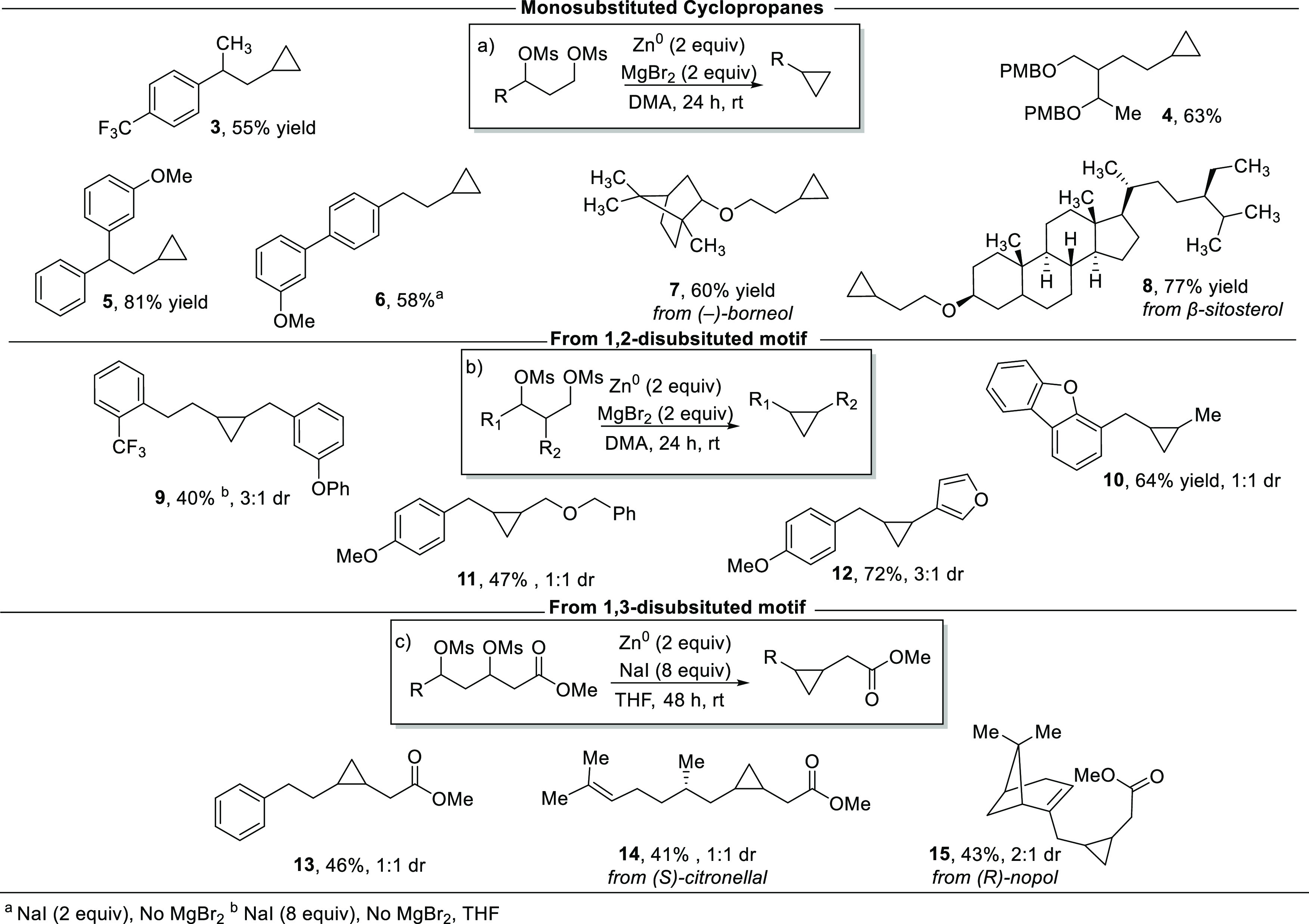
Scope of cyclopropane formation.
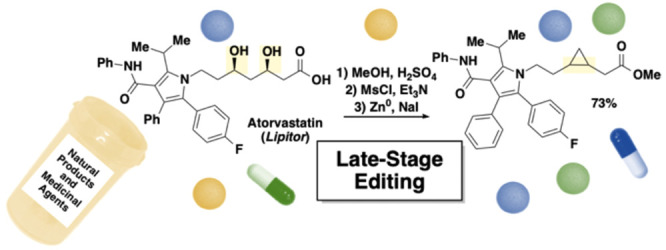
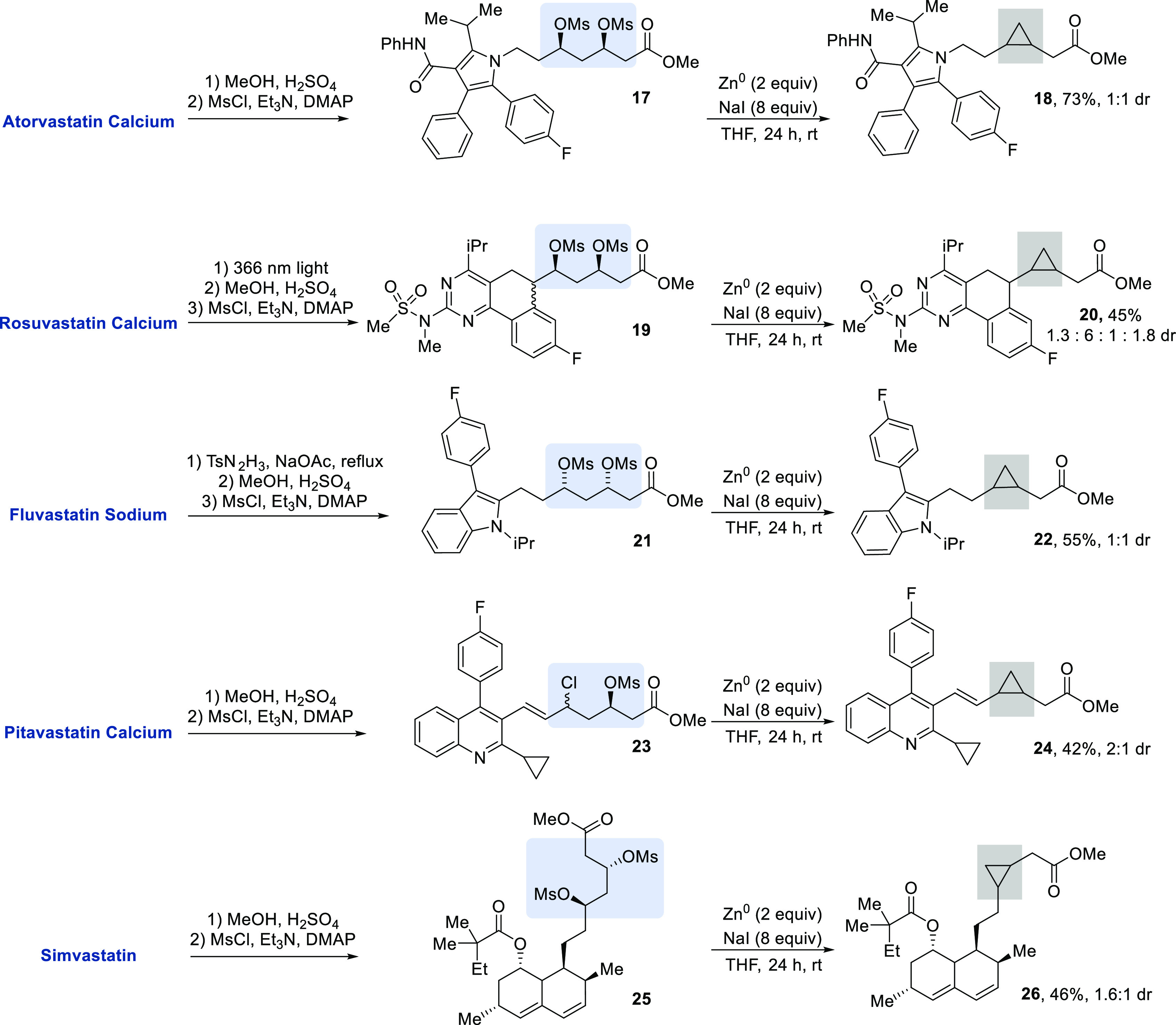
Statins contain or can be rapidly converted into 1,3-diol motifs, making them optimal substrates for our XEC reaction. Natural statins, such as mevastatin and lovastatin, are polyketides isolated from fungal sources.22 The potent ability of statins to regulate cholesterol metabolism led to the creation of many synthetic variants, such as atorvastatin and rosuvastatin.23,24 Many synthetic statins are available as 3,5-dihydroxy carboxylate salts. However, natural statins such as lovastatin and simvastatin are instead available as the β-hydroxy lactone. We aimed to perform a late-stage modification on these medicinal agents utilizing mild reaction conditions to form cyclopropane products (Scheme 1). To cyclize these complex diols, the carboxylic acids were protected as esters and 1,3-diols were converted to 1,3-dimesylates. In some cases, a styrene was reduced or arylated prior to the XEC reaction.25 Derivatives of atorvastatin (17), rosuvastatin (19), fluvastatin (21), pitavastatin (23), and simvastatin (25) provided cyclopropanes 18, 20, 22, 24, and 26, respectively. In addition to 1,3-dimesylates, 1-chloro-3-mesylates undergo the reaction. Treatment of pitavastatin methyl ester with mesyl chloride resulted in the formation of allylic chloride 23; this 1-chloro-3-mesylate underwent the zinc-mediated reaction to afford cyclopropane 24.26
Scheme 1. Formation of Cyclopropanes from Statin Derivatives.
In summary, we report a zinc-mediated cross-electrophile coupling reaction to afford alkyl and aryl cyclopropanes for late-stage modification of natural products and medicinal agents. This transformation allows for the synthesis of cyclopropanes from monosubstituted 1,3-dimesylates, 1,2-disubstituted 1,3-dimesylates, and polyketide scaffolds. As an application of this method, statin medicinal agents were converted into 1,2-disubstituted cyclopropanes.
Acknowledgments
This work was supported by the National Institute of Health (NIH) (R01GM135603) and the UC Irvine Brython-Davis Fellowship (TAT). Dr. Felix Grun and the UC Irvine Mass Spectrometry Facility are acknowledged for mass spectrometry data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c02362.
Experimental details and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For natural products in drug discovery, see:; a Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]; b Rodrigues T.; Reker D.; Schneider P.; Schneider G. Counting on Natural Products for Drug Design. Nat. Chem. 2016, 8, 531–541. 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]; c Truax N. J.; Romo D. Bridging the Gap Between Natural Product Synthesis and Drug Discovery. Nat. Prod. Rep. 2020, 37, 1436–1453. 10.1039/D0NP00048E. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Henkel T.; Brunne R. M.; Müller H.; Reichel F. Statistical Investigation into the Structural Complementarity of Natural Products and Synthetic Compounds. Angew. Chem., Int. Ed. 1999, 38, 643–647. . [DOI] [PubMed] [Google Scholar]
- For reviews of synthetic modification of natural products, see:; a Shugrue C. R.; Miller S. J. Applications of Nonenzymatic Catalysts to the Alteration of Natural Products. Chem. Rev. 2017, 117, 11894–11951. 10.1021/acs.chemrev.7b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Robles O.; Romo D. Chemo- and Site-Selective Derivatizations of Natural Products Enabling Biological Studies. Nat. Prod. Rep. 2014, 31, 318–334. 10.1039/C3NP70087A. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Majhi S.; Das D. Chemical Derivatization of Natural Products: Semisynthesis and Pharmacological Aspects- A Decade Update. Tetrahedron 2021, 78, 131801–131823. 10.1016/j.tet.2020.131801. [DOI] [Google Scholar]
- a Lewis C. A.; Miller S. J. Site-Selective Derivatization and Remodeling of Erythromycin A by Using Simple Peptide-Based Chiral Catalysts. Angew. Chem., Int. Ed. 2006, 45, 5616–5619. 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]; b Peddibhotla S.; Dang Y.; Liu J. O.; Romo D. Simultaneous Arming and Structure/Activity Studies of Natural Products Employing O–H Insertions: An Expedient and Versatile Strategy for Natural Products-Based Chemical Genetics. J. Am. Chem. Soc. 2007, 129, 12222–12231. 10.1021/ja0733686. [DOI] [PubMed] [Google Scholar]
- a Moir M.; Danon J. J.; Reekie T. A.; Kassiou M. An Overview of Late-Stage Functionalization in Today’s Drug Discovery. Expert Opinion on Drug Discovery 2019, 14, 1137–1149. 10.1080/17460441.2019.1653850. [DOI] [PubMed] [Google Scholar]; b Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachal P.; Krska S. W. The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-Like Molecules. Chem. Soc. Rev. 2016, 45, 546–576. 10.1039/C5CS00628G. [DOI] [PubMed] [Google Scholar]; c Zhang L.; Ritter T. A Perspective on Late-Stage Aromatic C–H Bond Functionalization. J. Am. Chem. Soc. 2022, 144, 2399–2414. 10.1021/jacs.1c10783. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Guillemard L.; Kaplaneris N.; Ackermann L.; Johansson M. Late-Stage C–H Functionalization Offers New Opportunities in Drug Discovery. Nature Reviews Chemistry 2021, 5, 522–545. 10.1038/s41570-021-00300-6. [DOI] [PubMed] [Google Scholar]; e White M. C.; Zhao J. Aliphatic C–H Oxidations for Late-Stage Functionalization. J. Am. Chem. Soc. 2018, 140, 13988–14009. 10.1021/jacs.8b05195. [DOI] [PMC free article] [PubMed] [Google Scholar]; For an example of a late-stage C–H activation of atorvastatin, see:; f Nagib D. A.; MacMillan D. W. C. Trifluoromethylation of Arenes and Heteroarenes by Means of Photoredox Catalysis. Nature 2011, 480, 224–228. 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of deamination reactions for natural product editing, see:; a Basch C. H.; Liao J.; Xu J.; Piane J. J.; Watson M. P. Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C–N Bond Activation. J. Am. Chem. Soc. 2017, 139, 5313–5316. 10.1021/jacs.7b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fier P. S.; Maloney K. M. NHC-Catalyzed Deamination of Primary Sulfonamides: A Platform for Late-Stage Functionalization. J. Am. Chem. Soc. 2019, 141, 1441–1445. 10.1021/jacs.8b11800. [DOI] [PubMed] [Google Scholar]; c Kennedy S. H.; Dherange B. D.; Berger K. J.; Levin M. D. Skeletal Editing Through Direct Nitrogen Deletion of Secondary Amines. Nature 2021, 593, 223–227. 10.1038/s41586-021-03448-9. [DOI] [PubMed] [Google Scholar]; d Jurczyk J.; Lux M. C.; Adpressa D.; Kim S. F.; Lam Y.; Yeung C. S.; Sarpong R. PhotoMediated Ring Contraction of Saturated Heterocycles. Science 2021, 373, 1004–1012. 10.1126/science.abi7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For representative late-stage cross-coupling and cross-electrophile coupling reactions, see:; a Leroux M.; Vorherr T.; Lewis I.; Schaefer M.; Koch G.; Karaghiosoff K.; Knochel P. Late-Stage Functionalization of Peptides and Cyclopeptides Using Organozinc Reagents. Angew. Chem., Int. Ed. 2019, 58, 8231–8234. 10.1002/anie.201902454. [DOI] [PubMed] [Google Scholar]; b Mennie K. M.; Vara B. A.; Levi S. M. Reductive sp3–sp2 Coupling Reactions Enable Late-Stage Modification of Pharmaceuticals. Org. Lett. 2020, 22, 556–559. 10.1021/acs.orglett.9b04320. [DOI] [PubMed] [Google Scholar]; c Dong Z.; MacMillan D. W. C. Metallaphotoredox-Enabled Deoxygenative Arylation of Alcohols. Nature 2021, 598, 451–456. 10.1038/s41586-021-03920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For cyclopropane as a privileged motif in medicinal chemistry, see:; a Talele T. T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]; b Salaün J.Cyclopropane Derivatives and their Diverse Biological Profile. In Small Ring Compounds in Organic Synthesis VI; De Meijere A., Ed.; Springer-Verlag: Berlin, 2000; pp 1–67. [Google Scholar]
- Johnson J.; Kim S.–H.; Bifano M.; Dimarco J.; Fairchild C.; Gougoutas J.; Lee F.; Long B.; Tokarski J.; Vite G. Synthesis, Structure Proof, and Biological Activity of Epothilone Cyclopropanes. Org. Lett. 2000, 2, 1537–1540. 10.1021/ol0058240. [DOI] [PubMed] [Google Scholar]
- Robles O.; Serna-Saldivar S. O.; Gutierrez-Uribe J. A.; Romo D. Cyclopropanations of Olefin-Containing Natural Products for Simultaneous Arming and Structure Activity Studies. Org. Lett. 2012, 14, 1394–1397. 10.1021/ol300105q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the prevalence of alcohols in natural products and medicinal agents, see:; a Ref (1d); b Cramer J.; Sager C. P.; Ernst B. Hydroxyl Groups in Synthetic and Natural-Product-Derived Therapeutics: A Perspective on a Common Functional Group. J. Med. Chem. 2019, 62, 8915–8930. 10.1021/acs.jmedchem.9b00179. [DOI] [PubMed] [Google Scholar]
- a Koskinen A. M. P.; Karisalmi K. Polyketide Stereotetrads in Natural Products. Chem. Soc. Rev. 2005, 34, 677–690. 10.1039/b417466f. [DOI] [PubMed] [Google Scholar]; b Walsh C. T.; Tang Y. In Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery; The Royal Society of Chemistry: Croyden, U.K., 2017. [Google Scholar]; c Mander L.; Liu H.-W. In Comprehensive Natural Products II Chemistry and Biology; Townsend C. A., Ebizuka Y., Eds.; Elsevier: Kidlington, U.K., 2010; Vol. 1. [Google Scholar]
- a Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; b Brown D. G.; Bostrom J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?. J. Med. Chem. 2016, 59, 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- Sanford A. B.; Thane T. A.; McGinnis T. M.; Chen P.–P.; Hong X.; Jarvo E. R. Nickel-Catalyzed Alkyl–Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes. J. Am. Chem. Soc. 2020, 142, 5017–5023. 10.1021/jacs.0c01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reducing metal-mediated conversion of 1,3-dihalides to cyclopropanes, see:; a With Na0:Freund A. Ueber Trimethylen. J. Prakt. Chem. 1882, 26, 367–377. 10.1002/prac.18820260125. [DOI] [Google Scholar]; b With Zn0:Gustavson G. Ueber Eine Neue Darstellungsmethode Des Trimethylens. J. Prakt. Chem. 1887, 36, 300–303. 10.1002/prac.18870360127. [DOI] [Google Scholar]; c With Zn0:Shortridge R. W.; Craig R. A.; Greenlee K. W.; Derfer J. M.; Boord C. E. The Synthesis of Some Cyclopropane and Spirane Hydrocarbons. J. Am. Chem. Soc. 1948, 70, 946. 10.1021/ja01183a017. [DOI] [Google Scholar]; d With CrII:Kochi J.; Singleton D. Reductive Cyclization of. alpha.,.omega.-Dihalides with Chromium-(II) Complexes. J. Org. Chem. 1968, 33, 1027. 10.1021/jo01267a019. [DOI] [Google Scholar]; e With CoII:Chock P. B.; Halpern J. Reactions of Pentacyanocobaltate(II) with Some Organic Halides. J. Am. Chem. Soc. 1969, 91, 582–588. 10.1021/ja01031a010. [DOI] [Google Scholar]; f With t-BuLi:Bailey W. F.; Gagnier R. P.; Patricia J. J. Reactions of tert-butyllithium with. alpha.,.omega.-dihaloalkanes. Evidence for Single-Electron-Transfer-Mediated Metal-Halogen Interchange Involving Alkyl Radical-Halide Ion Adducts. J. Org. Chem. 1984, 49, 2098–2107. 10.1021/jo00186a004. [DOI] [Google Scholar]; g With SmI2:Ohkita T.; Tsuchiya Y.; Togo H. Radical 3-exo-tet Cyclization of 1,3-Dihalopropanes with SmI2 to Form Cyclopropanes. Tetrahedron 2008, 64, 7247–7251. 10.1016/j.tet.2008.05.081. [DOI] [Google Scholar]; h With In:Tsuchiya Y.; Izumisawa Y.; Togo H. 3-exo-tet Cyclization of 2,2-Disubstituted 1,3-Dihalopropanes with Indium in Aqueous and Ionic Liquid Solvent System. Tetrahedron 2009, 65, 7533–7537. 10.1016/j.tet.2009.06.123. [DOI] [Google Scholar]
- Jana S. K.; Maiti M.; Dey P.; Maji B. Photoredox/Nickel Dual Catalysis Enables the Synthesis of Alkyl Cyclopropanes via C(sp3)–C(sp3) Cross Electrophile Coupling of Unactivated Alkyl Electrophiles. Org. Lett. 2022, 24, 1298–1302. 10.1021/acs.orglett.1c04268. [DOI] [PubMed] [Google Scholar]
- For general reviews of XEC reactions, see:; a Knappke C. E. I.; Grupe S.; Gärtner D.; Corpet M.; Gosmini C.; Jacobi von Wangelin A. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. - Eur. J. 2014, 20, 6828–6842. 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]; b Hewitt K. A.; Lin P. C.; Raffman E. T. A.; Jarvo E. R.. Comprehensive Organometallic Chemistry IV; 2021. [Google Scholar]
- For intramolecular XEC of 1,5-dihalides and 1,6-dihalides, see:; a With alkali naphthalenes:Garst J. F.; Barbas J. T. Reactions of 1,4- and 1,5-Dihaloalkanes with Alkali Naphthalenes. J. Am. Chem. Soc. 1974, 96, 3239–3246. 10.1021/ja00817a035. [DOI] [Google Scholar]; b With a Ni catalyst and Zn:Xue W.; Xu H.; Liang Z.; Qian Q.; Gong H. Nickel-Catalyzed Reductive Cyclization of Alkyl Dihalides. Org. Lett. 2014, 16, 4984–4987. 10.1021/ol502207z. [DOI] [PubMed] [Google Scholar]
- a Knochel P.; Singer R. D. Preparation and Reactions of Polyfunctional Organozinc Reagents in Organic Synthesis. Chem. Rev. 1993, 93, 2117–2188. 10.1021/cr00022a008. [DOI] [Google Scholar]; b Jana R.; Pathak T. P.; Sigman M. S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See refs (12) and (13)
- For related coupling reactions of alkyl sulfonates that likely proceed through alkyl halide intermediates, see:; a Do H. Q.; Chandrashekar E. R.; Fu G. C. Nickel/Bis(oxazoline)-Catalyzed Asymmetric Negishi Arylations of Racemic Secondary Benzylic Electrophiles to Generate Enantioenriched 1,1-Diarylalkanes. J. Am. Chem. Soc. 2013, 135, 16288–16291. 10.1021/ja408561b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liang Z.; Xue W.; Lin K.; Gong H. Nickel-Catalyzed Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate. Org. Lett. 2014, 16, 5620–5623. 10.1021/ol502682q. [DOI] [PubMed] [Google Scholar]; c Xu H.; Zhao C.; Qian Q.; Deng W.; Gong H. Nickel-Catalyzed Cross-Coupling of Unactivated Alkyl Halides Using Bis(pinacolato)diboron as Reductant. Chem. Sci. 2013, 4, 4022–4029. 10.1039/c3sc51098k. [DOI] [Google Scholar]; d Komeyama K.; Ohata R.; Kiguchi S.; Osaka I. Highly Nucleophilic Vitamin B12-Assisted Nickel-Catalysed Reductive Coupling of Aryl Halides and Non-Activated Alkyl Tosylates. Chem. Commun. 2017, 53, 6401–6404. 10.1039/C7CC01932G. [DOI] [PubMed] [Google Scholar]
- Procedure modified from:; Ciabatti R.; Maffioli S. I.; Panzone G.; Canavesi A.; Michelucci E.; Tiseni P. S.; Marzorati E.; Checchia A.; Giannone M.; Jabes D.; Romanò G.; Brunati C.; Candiani G.; Castiglione F. Synthesis and Preliminary Biological Characterization of New Semisynthetic Derivatives of Ramoplanin. J. Med. Chem. 2007, 50, 3077–3085. 10.1021/jm070042z. [DOI] [PubMed] [Google Scholar]
- Tobert J. A. Lovastatin and Beyond: The History of the HMG-CoA Reductase Inhibitors. Nat. Rev. Drug Discovery 2003, 2, 517–526. 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- Endo A. A Historical Perspective on the Discovery of Statins. Proc. Jpn. Acad. Ser. B 2010, 86, 484–493. 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S.; Goldstein J. L. A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science 1986, 232, 34–47. 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- a Fluvastatin was reduced with tosylhydrazide prior to esterification.; b Rosuvastatin was subjected to photochemical cyclization prior to esterification:Litvic M.; Smic K.; Vinkovic V.; Filipan-Litvic M. A Study of Photodegradation of Drug Rosuvastatin Calcium in Solid State and Solution Under UV and Visible Light Irradiation: The influence of Certain Dyes as Efficient Stabilizers. J. Photochem. Photobiol., A 2013, 252, 84–92. 10.1016/j.jphotochem.2012.11.008. [DOI] [Google Scholar]
- See the Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.