Abstract
Background:
Resection of parotid carcinomas involving the parapharyngeal space is challenging. How this affects tumor margin control, recurrence, and survival is unclear.
Methods:
Patients who underwent resection of parotid carcinomas between 1985 and 2015 at Memorial Sloan Kettering Cancer Center were evaluated for the impact of parapharyngeal extension (PPE) on margin status, local recurrence-free probability (LRFP), and disease-specific survival (DSS).
Results:
Out of 214 patients in whom preoperative imaging was available for review, 22 (10.3%) had PPE. Matched by histotypes, carcinomas with PPE had comparable margin positivity (p = 0.479), T classification (p = 0.316), pathologic risk (p = 0.936), and adjuvant therapy (p = 0.617) to those without PPE. The 3-year LRFP was 88.9% versus 95.4% (hazard ratio [HR] 2.23 after adjusting for pT classification, p = 0.342) and the 5-year DSS was 74.2% versus 69.5% (adjusted HR 0.45, p = 0.232) in patients with and without PPE.
Conclusion:
PPE does not appear to worsen oncologic outcomes in the resection of parotid carcinomas.
Keywords: parapharyngeal space, parotid carcinoma, recurrence, surgical margin, survival
1 |. INTRODUCTION
The parapharyngeal space (PPS) is a deep neck space situated immediately lateral to the nasopharynx and oropharynx, extending from skull base to hyoid bone craniocaudally, pterygomandibular raphe to prevertebral space anteroposteriorly, superior constrictor to the medial pterygoid muscle and styloid apparatus transversely.1 Much has been written about the surgical resection of benign tumors in the PPS,2–9 but the oncologic outcomes of the resection of malignancies of the PPS have not been well described. The majority of the malignancies of the PPS are salivary gland carcinomas as evidenced by a systematic review of 1293 PPS tumors published between 1988 and 2014,10 in which 18% were malignant. Among them, 61% were salivary gland carcinomas, 11% sarcomas, 11% lymphomas, and the rest a mix of rare tumors such as malignant nerve sheath tumors or malignant paragangliomas. Salivary gland carcinomas of the PPS can arise de novo from a minor salivary gland or be a direct extension of a parotid carcinoma. Because salivary gland carcinomas are primarily treated by surgical resection but access to the PPS is challenging, we sought to determine how margin control and oncologic outcomes might be influenced by PPS involvement. Traditional surgical approaches to the PPS are transcervical, transparotid, transmandibular, or transoral, the most common being transcervical with or without the addition of a parotidectomy.10–13 Exposure of the tumor medial to the styloid process and the mandible is required to achieve margin control in the resection of malignancies in the PPS. However, medial and superior mobilization of the tumor in the PPS during the transcervical or transparotid approach may need to be performed without direct visualization, predisposing the tumor to rupture and spillage. Thus, a medial approach, such as mandibulotomy, may be needed to provide superior margin control in the resection of parapharyngeal carcinomas. With the development of transoral robotic surgery (TORS)2 and endoscopic surgery14 allowing alternative medial approaches to the PPS, how best to improve margin control becomes more pertinent. However, the paucity of data on the margin status, local control, and survival of patients with PPS involvement by salivary gland carcinomas complicates the decision process. In this paper, we describe a cohort of patients with parotid carcinomas involving the PPS to provide data on margin status, recurrence pattern, and disease-specific survival (DSS) and compare them to those without parapharyngeal extension (PPE) to determine if PPE is an adverse prognostic factor deserving greater attention in therapeutic decision making.
2 |. MATERIALS AND METHODS
After approval from the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSKCC) and obtaining a waiver of informed consent, we analyzed a database of 374 patients with parotid carcinomas resected at MSKCC, United States from 1985 to 2015. Preoperative axial imaging with either computed tomography or magnetic resonance imaging was available in 214 out of 374 patients. These images were reviewed by author HL who classified the tumors according to PPE and verified the classification with senior author IG when in doubt. We defined PPE as tumor extension medial to a plane passing from the styloid process to the posterior border of the ramus of the mandible, termed the stylomandibular plane (Figure 1). Tumors with more than 50% of the cross-sectional area medial to the stylomandibular plane on axial imaging were classified as major PPE, whereas those with 50% or less cross-sectional area medial to the stylomandibular plane were classified as minor PPE (Figure 1). Using Mann–Whitney U or Fisher’s exact tests, patients with and without PPE were compared for age, sex, pathologic risk group,15,16 clinical TNM and pathological TN classification per AJCC 8th Edition Staging Manual, adjuvant therapy, neck dissection, positive or close margin, perineural or vascular invasion and pattern of recurrence. Close and positive margins were determined on formalin-fixed paraffin-embedded specimens with close being defined as less than 5 mm. Postoperative radiotherapy was indicated in pathologically T3 or T4 tumors, nodal metastasis, positive margin, perineural invasion, vascular invasion, or high pathologic grade. Postoperative chemoradiotherapy was discussed on a case-by-case basis in a multidisciplinary tumor board for patients with high grade, high stage carcinomas (pT3/T4, pN+) and positive surgical margins. Pathologic risk groups were categorized by DSS and defined in our previous publications.15,16 High-risk tumors include salivary duct carcinoma, adenosquamous carcinoma, high-grade mucoepidermoid carcinoma (MEC), high-grade carcinoma ex-pleomorphic adenoma (CEPA), high-grade-adenocarcinoma, high-grade adenoid cystic carcinoma, high-grade acinic cell carcinoma, and high-grade epithelial-myoepithelial carcinoma. Intermediate-risk tumors include low to intermediate grade adenoid cystic carcinoma. Low-risk tumors include low-grade acinic cell carcinoma, low and intermediate-grade MEC, low-grade adenocarcinoma, low-grade CEPA, polymorphous low-grade adenocarcinoma, and low to intermediate-grade epithelial-myoepithelial carcinoma. Twenty-three patients without PPE had histotypes not present in those with PPE. These patients were excluded to reduce the confounding effect of pathologic risk on oncologic outcomes, leaving a total of 191 patients for analysis.
FIGURE 1.
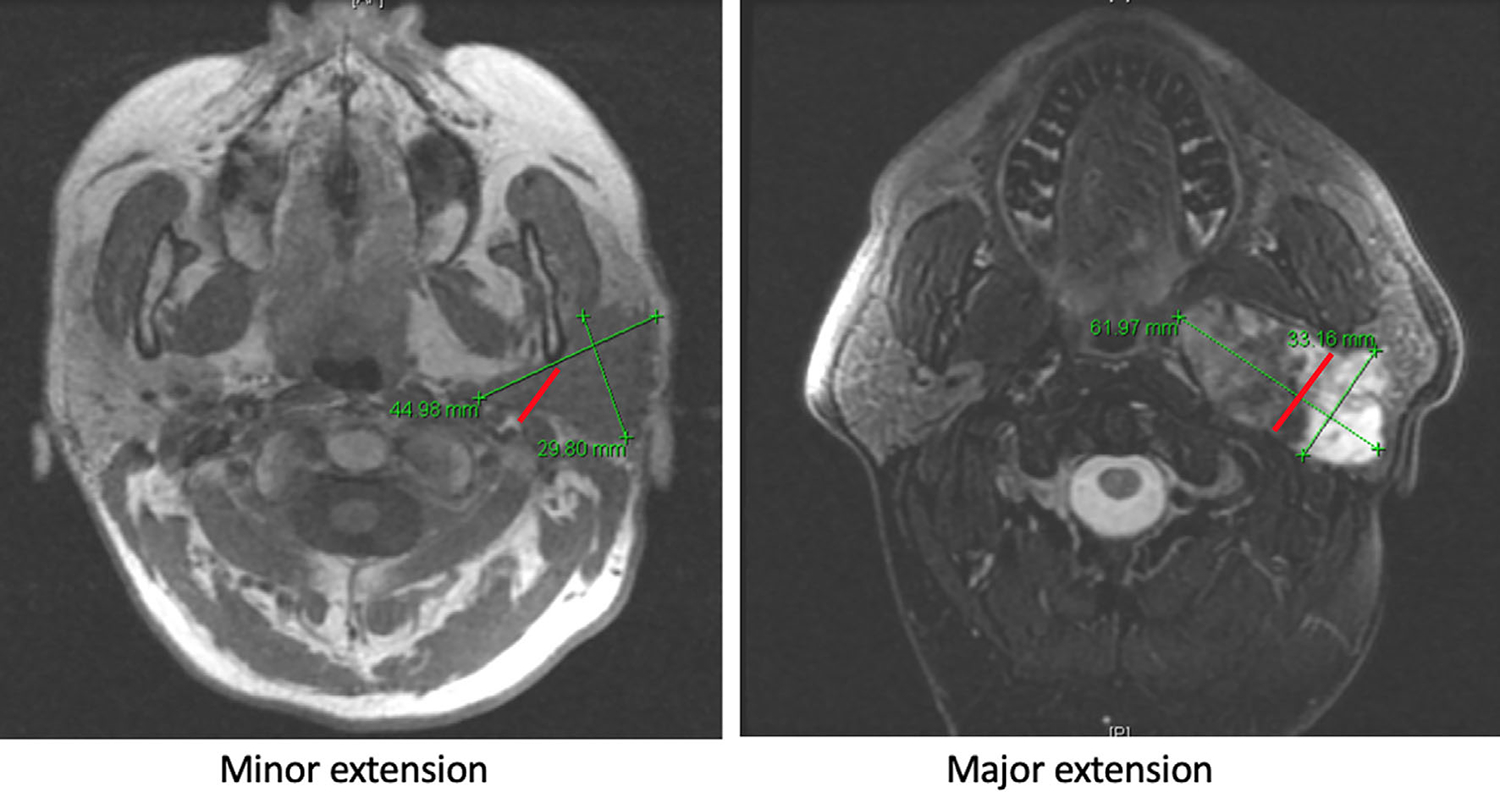
Classification of parapharyngeal extension (PPE). Red line denotes the entrance point to the parapharyngeal space during surgery. It passes from the styloid process to the posterior border of the ramus of the mandible. We term this the stylomandibular plane. Minor PPE is defined as 50% or less of the tumor extending medial to the stylomandibular plane, major PPE is defined as greater than 50% of the tumor extending medial to the stylomandibular plane, both assessed on the axial images of either computed tomography or magnetic resonance imaging
Parotid carcinomas were resected with the principle of achieving a grossly clear margin while preserving functionally important structures, such as a functionally intact facial nerve not encased or invaded by tumor, grossly normal mandible, skin, external auditory canal or styloid apparatus. Sites of recurrence were determined by chart review. The impact of PPE on surgical margin status was determined on univariable and multivariable logistic regression so that the effect of clinical T classification and pathologic risk on margin status could be adjusted. The impact of PPE on local recurrence-free probability (LRFP) and DSS was determined by univariable and multivariable Cox regression so that the effect of age, margin positivity, pathologic risk, T classification, stage and PORT/CRT on recurrence and survival could be adjusted. All statistical tests were 2-tailed and performed with STATA (version 16.0, StataCorp LLC, College Station, TX). A p < 0.05 was considered significant.
3 |. RESULTS
3.1 |. Clinical and pathologic characteristics of parotid carcinomas with and without PPE
A total of 22 out of 214 (10.3%) patients with parotid carcinomas had PPE. After excluding the histotypes not present in those with PPE, the clinical and pathologic characteristics of the remaining 191 patients are presented in Table 1. There were no statistically significant differences in the clinicopathologic characteristics between those with and without PPE. Fifty percent of patients whose carcinomas had PPE received postoperative radiotherapy and 13.6% received postoperative chemoradiotherapy, compared to 40.2% and 11.8% of patients whose carcinomas did not have PPE (p = 0.617). Carcinoma ex-pleomorphic adenoma was the commonest parotid carcinoma with PPE, whereas mucoepidermoid carcinoma was the commonest parotid carcinoma without PPE (see Table S1, Supporting Information for a complete list of histotypes in both groups). When grouped by pathologic risk,15,16 the distribution of low, intermediate, and high-risk carcinomas was similar between those with and without PPE.
TABLE 1.
Characteristics of patients with parotid carcinomas stratified by parapharyngeal extension
| Characteristics | No parapharyngeal extension (no. of patients = 169; 88.5%) | Parapharyngeal extension (no. of patients = 22; 11.5%) | p-value |
|---|---|---|---|
| Age | |||
| Median (range) | 60.0 (6–98) | 55.5 (31–82) | 0.422 |
| Sex | |||
| Female | 76 (45.0%) | 10 (45.5%) | 1.000 |
| Male | 93 (55.0%) | 12 (54.5%) | |
| Neck dissection | |||
| No | 87 (51.5%) | 12 (54.6%) | 0.824 |
| Yes | 82 (48.5%) | 10 (45.4%) | |
| cT classification (AJCC 8th ed.) | |||
| T1 | 53 (31.4%) | 4 (17%) | 0.316 |
| T2 | 62 (36.7%) | 13 (61%) | |
| T3 | 31 (18.3%) | 3 (13%) | |
| T4 | 22 (13.0%) | 2 (9%) | |
| Tx | 1 (0.6%) | 0 | |
| cN status (AJCC 8th ed.) | |||
| N0 | 141 (83.4%) | 19 (86.4%) | 0.505 |
| N+ | 28 (16.6%) | 3 (13.6%) | |
| cM classification (AJCC 8th ed.) | |||
| M0 | 165 (97.6%) | 22 (100%) | 0.610 |
| M1 | 4 (2.4%) | 0 | |
| pT classification (AJCC 8th ed.) | |||
| T1 | 61 (36.1%) | 3 (13.7%) | 0.143 |
| T2 | 44 (26.0%) | 8 (36.3%) | |
| T3 | 13 (7.7%) | 2 (9.1%) | |
| T4 | 50 (29.6%) | 9 (40.9%) | |
| Tx | 1 (0.6%) | 0 | |
| pN status (AJCC 8th ed.) | |||
| N0/Nx | 132 (78.1%) | 17 (77.3%) | 1.000 |
| N+ | 37 (21.9%) | 5 (22.7%) | |
| pStage (AJCC 8th ed.) | |||
| Stage I | 59 (34.9%) | 3 (13.6%) | 0.132 |
| Stage II | 39 (23.1%) | 7 (31.8%) | |
| Stage III | 8 (4.7%) | 2 (9.1%) | |
| Stage IV | 62 (36.7%) | 10 (45.5%) | |
| Unknown | 1 (0.6%) | 0 | |
| Perineural invasion | |||
| No | 92 (54.4%) | 11 (50.0%) | 0.812 |
| Yes | 65 (38.5%) | 9 (40.9%) | |
| Unknown | 12 (7.1%) | 2 (9.1%) | |
| Vascular invasion | |||
| No | 115 (68.0%) | 15 (68.2%) | 1.000 |
| Yes | 36 (21.3%) | 4 (18.2%) | |
| Unknown | 18 (10.7%) | 3 (13.6%) | |
| Adjuvant therapy | |||
| None | 80 (47.4%) | 8 (36.4%) | 0.617 |
| RT | 68 (40.2%) | 11 (50%) | |
| CRT | 20 (11.8%) | 3 (13.6%) | |
| Chemotherapy alone | 1 (0.6%) | 0 | |
| Histology/grade | |||
| Mucoepidermoid carcinoma | 57 (33.7%) | 4 (18.2%) | |
| Low | 31 | 4 | |
| Intermediate | 13 | ||
| High | 13 | ||
| Carcinoma ex-pleomorphic adenoma | 30 (17.8%) | 8 (36.4%) | |
| Low | 5 | 1 | |
| Intermediate | 1 | ||
| High | 24 | 7 | |
| Acinic cell carcinoma | 26 (15.4%) | 2 (9.1%) | |
| Low | 20 | 2 | |
| Intermediate | 1 | ||
| High | 5 | ||
| Salivary duct carcinoma | 23 (13.6%) | 2 (9.1%) | |
| High | 23 | 2 | |
| Adenoid cystic carcinoma | 13 (7.7%) | 1 (4.6%) | |
| Low | 3 | ||
| Intermediate | 9 | 1 | |
| High | 1 | ||
| Adenocarcinoma | 8 (4.7%) | 1 (4.6%) | |
| Low | 3 | ||
| Intermediate | 1 | ||
| High | 4 | 1 | |
| Epithelial-myoepithelial carcinoma | 8 (4.7%) | 1 (4.6%) | |
| Low | 5 | 1 | |
| Intermediate | 2 | ||
| High | 1 | ||
| Adenosquamous carcinoma | 3 (1.8%) | 1 (4.6%) | |
| High | 3 | 1 | |
| Polymorphous low-grade adenocarcinoma | 1 (0.6%) | 2 (9.1%) | |
| Low | 1 | 2 | |
| Pathologic risk group15,16 | |||
| Low | 83 (49.1%) | 10 (45.5%) | 0.936 |
| Intermediate | 12 (7.1%) | 1 (4.5%) | |
| High | 74 (43.8%) | 11 (50.0%) | |
| Surgical margin | |||
| Negative | 65 (38.4%) | 9 (40.9%) | 0.479 |
| Close | 42 (24.9%) | 3 (13.6%) | |
| Positive | 59 (34.9%) | 10 (45.5%) | |
| Unknown | 3 (1.8%) | 0 |
Abbreviations: AJCC, American Joint Committee on Cancer; c, clinical; CRT, chemoradiotherapy; p, pathological; RT: radiotherapy.
3.2 |. Surgical approaches and surgical margin status in the resection of parotid carcinomas with PPE
Among the 22 patients with PPE, 11 had minor and 11 had major PPE. The transparotid approach assisted by the transcervical exposure was employed in the resection of 73% of tumors with minor PPE (8 out of 11) and 64% of tumors with major PPE (7 out of 11) (Table 2). The transparotid approach combined with segmental mandibulectomy was employed in the resection of 18% of tumors with minor PPE (2 out of 11) and 27% of tumors with major PPE (3 out of 11). A mandibulotomy was employed in the resection of 9% of tumors with major PPE (1 out of 11) but not in any tumor with minor PPE. One tumor with minor PPE (9%) required a lateral temporal bone resection in addition to a transparotid-transcervical approach. None of them were resected transorally. Margin status did not differ significantly between carcinomas with minor and major PPE (18% vs. 9% for close margins, 55% vs. 36% for positive margins, respectively, p = 0.561). As a whole, the rates of positive, close, and negative surgical margins in tumors with PPE were 45.5%, 13.6%, and 40.9% versus 34.9%, 24.9%, and 38.4% in tumors without PPE (p = 0.479) (Table 1).
TABLE 2.
Surgical approaches and surgical margin status in the resection of parotid carcinomas with parapharyngeal extension
| Minor parapharyngeal extension (no. of patients = 11; 50%) | Major parapharyngeal extension (no. of patients = 11; 50%) | p-value (Fisher’s exact) | |
|---|---|---|---|
| Surgical approach | |||
| Transparotid +/− transcervical | 8 (72.7%) | 7 (63.6%) | |
| Transparotid + segmental mandibulectomy | 2 (18.2%) | 3 (27.3%) | |
| Mandibulotomy | 0 | 1 (9.1%) | |
| Transparotid + transcervical + lateral temporal bone resection | 1 (9.1%) | 0 | |
| Surgical margin status | |||
| Negative | 3 (27.3%) | 6 (54.5%) | 0.561 |
| Close | 2 (18.2%) | 1 (9.1%) | |
| Positive | 6 (54.5%) | 4 (36.4%) |
3.3 |. Impact of PPE on surgical margin positivity
Analyzing all 191 patients together, univariable logistic regression did not show a significant association between PPE and positive surgical margin (odds ratio [OR] 1.51, 95% CI 0.62–3.71, p = 0.367) (Table 3), but high pathologic risk (OR 2.99, 95% CI 1.57–5.69, p = 0.001) and clinical T4 classification (OR 7.11, 95% CI 2.44–20.71, p < 0.001) were significant. On multivariable analysis, the association between PPE and a positive surgical margin remained nonsignificant (OR 1.74, 95% CI 0.67–4.53, p = 0.258) after adjusting for pathologic risk and clinical T classification. Only clinical T4 classification (OR 5.15, 95% CI 1.55–17.13, p = 0.008) remained significantly associated with a positive surgical margin.
TABLE 3.
Impact of parapharyngeal extension on surgical margin positivity
| Positive surgical margin |
||||||
|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
|||||
| Variables | Unadjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value |
| Parapharyngeal extension | ||||||
| No | Ref | |||||
| Yes | 1.51 | 0.62–3.71 | 0.367 | 1.74 | 0.67–4.53 | 0.258 |
| Pathologic risk group15,16 | ||||||
| Low | Ref | |||||
| Intermediate | 2.69 | 0.82–8.85 | 0.104 | 2.39 | 0.70–8.15 | 0.163 |
| High | 2.99 | 1.57–5.69 | 0.001 | 1.68 | 0.78–3.60 | 0.183 |
| cT classification (AJCC 8th ed.) | ||||||
| T1 | Ref | |||||
| T2 | 1.24 | 0.57–2.72 | 0.593 | 1.12 | 0.50–2.50 | 0.791 |
| T3 | 2.31 | 0.93–5.74 | 0.071 | 1.85 | 0.69–4.98 | 0.221 |
| T4 | 7.11 | 2.44–20.71 | <0.001 | 5.15 | 1.55–17.13 | 0.008 |
Abbreviations: AJCC 8th ed., American Joint Committee on Cancer Staging Manual, 8th Edition; c, clinical; OR, odds ratio.
3.4 |. Impact of PPE on LRFP
Analyzing all 191 patients together, the 3-year LRFP was 88.9% versus 95.4% in patients with and without PPE (hazard ratio [HR] of PPE on LRFP: 3.15, 95% CI 0.59–15.70, p = 0.183) (Figure 2). Univariable Cox regression showed that high pathologic risk and pT3/T4 were associated with local recurrence. However, the small number of local recurrences (7 in total) limited the statistical power to adjust for all these variables in the multivariable analysis. By adjusting for pT3/T4 alone, the HR of PPE for local recurrence decreased to 2.23 (95% CI 0.43–11.58, p = 0.342) (Table 4).
FIGURE 2.
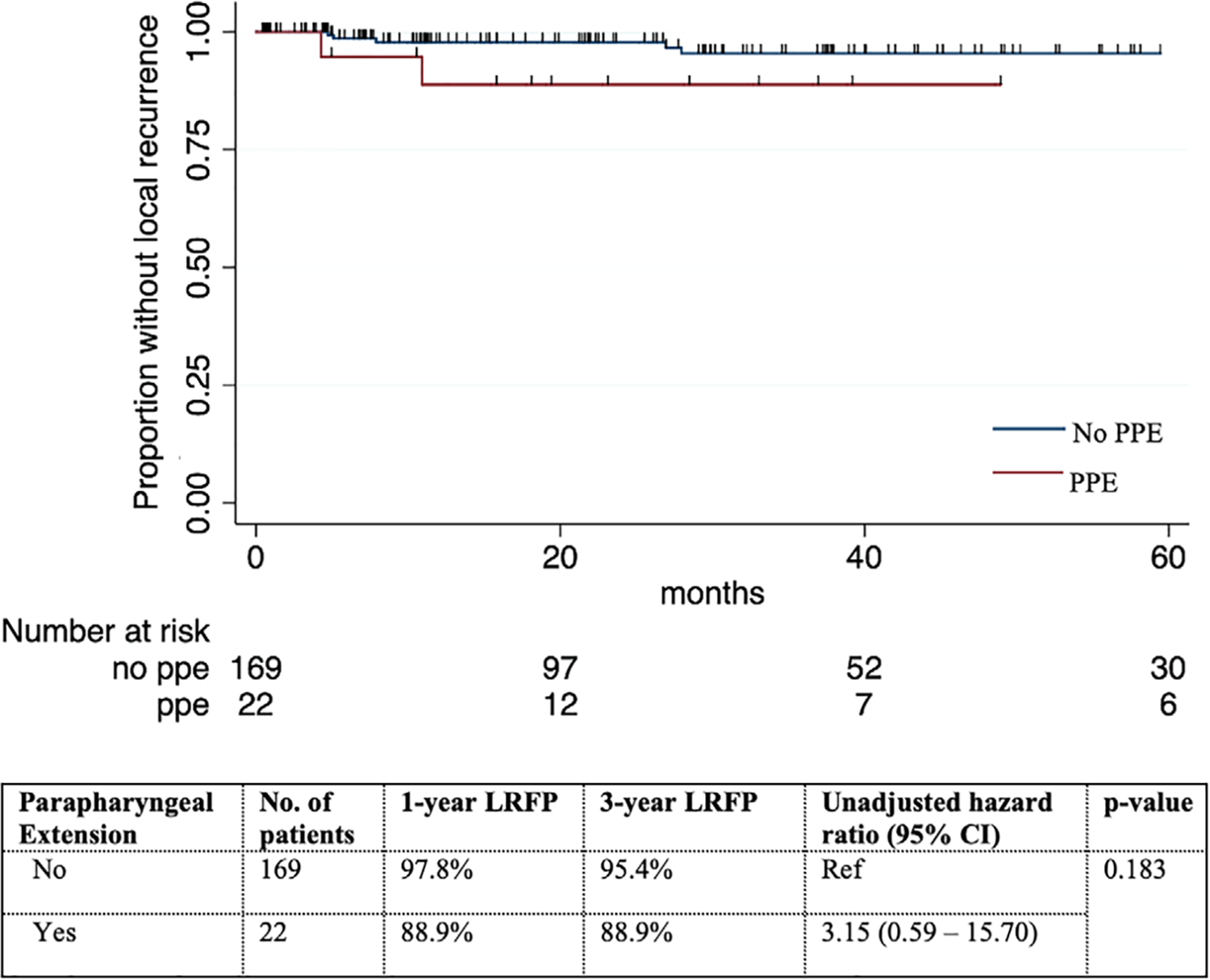
Local recurrence-free probability (LRFP) stratified by parapharyngeal extension (PPE) in patients with parotid carcinomas treated initially by surgery
TABLE 4.
Impact of parapharyngeal extension on local recurrence-free probability in patients with parotid carcinomas
| Local recurrence-free probability |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariablea |
Multivariableb (parsimonious) |
|||||||
| Variables | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Parapharyngeal extension | |||||||||
| No | Ref | ||||||||
| Yes | 3.15 | 0.59–15.70 | 0.183 | 2.10 | 0.43–10.97 | 0.377 | 2.23 | 0.43–11.58 | 0.342 |
| Age | |||||||||
| ≤60 | Ref | ||||||||
| >60 | 1.58 | 0.35–7.05 | 0.551 | ||||||
| Pathologic risk group15,16 | |||||||||
| Low/intermediatec | Ref | ||||||||
| High | 8.73 | 1.05–72.51 | 0.045 | 4.30 | 0.47–39.40 | 0.197 | |||
| Surgical margin | |||||||||
| Negative/closed | Ref | ||||||||
| Positive | 4.45 | 0.86–22.95 | 0.075 | 2.35 | 0.44–12.47 | 0.315 | |||
| pT classification (AJCC 8th ed.) | |||||||||
| T1-T2 | Ref | ||||||||
| T3-T4 | 9.31 | 1.12–77.39 | 0.039 | 3.66 | 0.39–34.05 | 0.258 | 8.57 | 1.02–72.01 | 0.048 |
| pStage (AJCC 8th ed.) | |||||||||
| Stage I-II | Ref | ||||||||
| Stage III-IV | 7.64 | 0.92–63.45 | 0.060 | ||||||
| PORT/CRT | |||||||||
| No | Ref | ||||||||
| Yes | 1.79 | 0.35–9.22 | 0.488 | ||||||
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; CRT, chemoradiotherapy; HR, hazard ratio; p, pathological; PORT, postoperative radiotherapy; Ref, reference variable.
pStage was not included in the multivariable analysis because of collinearity with pT classification.
The parsimonious model was built because the paucity of local recurrence (7 in total) limited the statistical power to the detection of only one significant association.
Low and intermediate pathologic risk groups were combined because there was no local recurrence in the low-risk group.
Patients with negative and close surgical margins were combined because there was no local recurrence in those with negative margin.
3.5 |. Impact of PPE on DSS
Analyzing all 191 patients together, the 5-year DSS was 74.2% versus 69.5% in patients with and without PPE (HR of PPE on DSS: 0.76, 95% CI 0.23–2.50, p = 0.652) (Figure 3). On univariable Cox regression, age, pathologic risk, surgical margin positivity, pT, pStage, and postoperative radiotherapy (including chemoradiotherapy) were significantly associated with DSS. On multivariable analysis, high pathologic risk (HR 9.82, 95% CI 2.03–47.50, p = 0.005), pStage III/IV (HR 26.25, 95% CI 2.05–335.27, p = 0.012) remained associated with poorer DSS, whereas postoperative radiotherapy (including chemoradiotherapy) was associated with a better DSS (HR 0.22, 95% CI 0.07–0.66, p = 0.007). PPE remained to be not predictive of DSS (Table 5).
FIGURE 3.
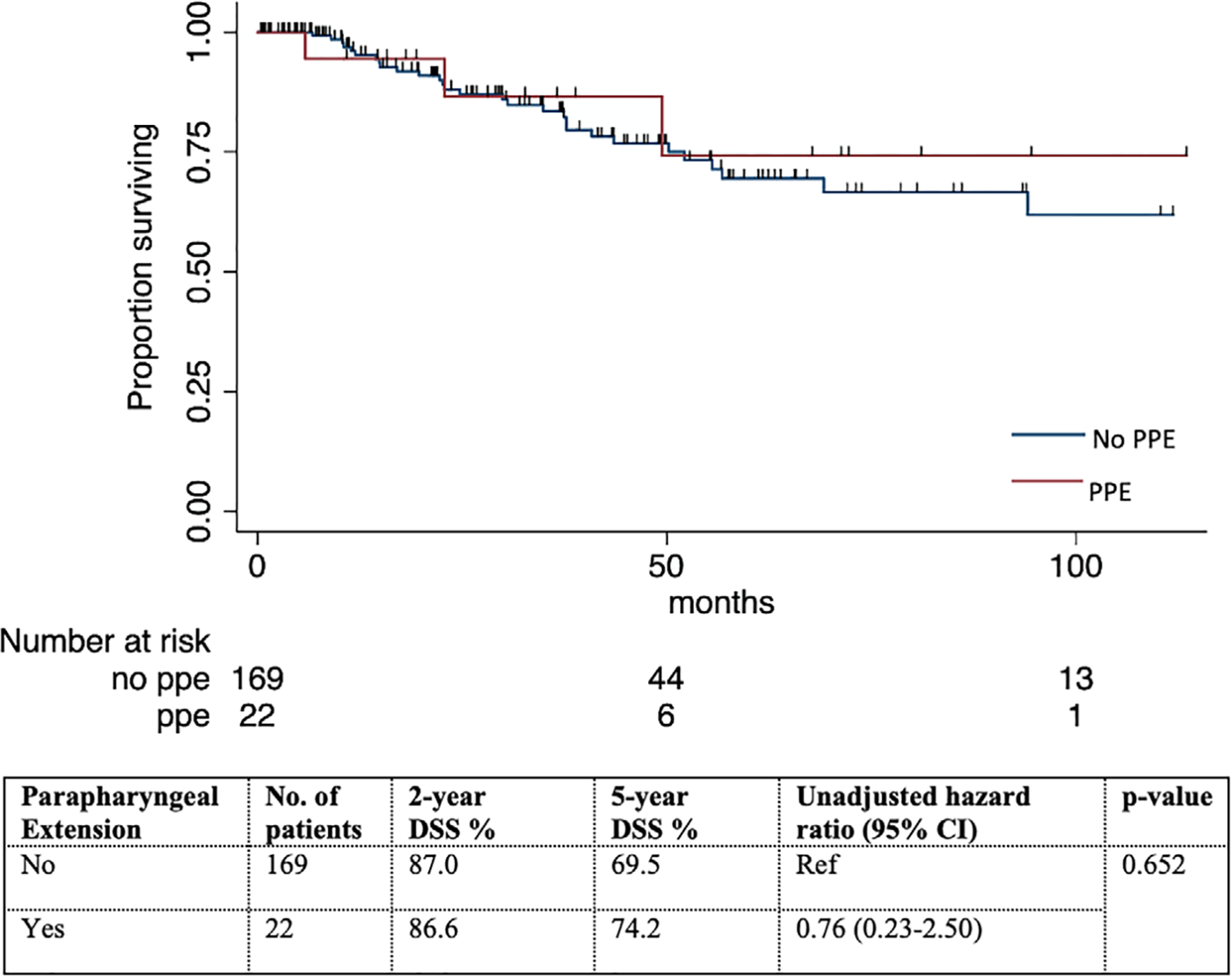
Disease-specific survival (DSS) stratified by parapharyngeal extension (PPE) in patients with parotid carcinomas treated initially by surgery
TABLE 5.
Impact of parapharyngeal extension on disease-specific survival in patients with parotid carcinomas
| Disease-specific survival |
||||||
|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
|||||
| Variables | Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value |
| Parapharyngeal extension | ||||||
| No | Ref | |||||
| Yes | 0.76 | 0.23–2.50 | 0.652 | 0.45 | 0.12–1.66 | 0.232 |
| Age | ||||||
| Continuous | 1.04 | 1.01–1.06 | 0.003 | 1.01 | 0.98–1.04 | 0.524 |
| Pathologic risk group15,16 | ||||||
| Low/intermediatea | Ref | |||||
| High | 20.24 | 4.84–84.70 | <0.001 | 9.82 | 2.03–47.50 | 0.005 |
| Surgical margin | ||||||
| Negative | Ref | |||||
| Close | 2.37 | 0.86–6.54 | 0.094 | 0.93 | 0.30–2.83 | 0.894 |
| Positive | 2.86 | 1.12–7.32 | 0.028 | 0.83 | 0.30–2.27 | 0.720 |
| pT classification (AJCC 8th ed.) | ||||||
| T1 | Ref | |||||
| T2 | 5.65 | 0.63–50.61 | 0.122 | 1.76 | 0.17–18.15 | 0.634 |
| T3 | 27.40 | 3.03–247.92 | 0.003 | 1.44 | 0.11–18.52 | 0.777 |
| T4 | 24.13 | 3.25–178.94 | 0.002 | 2.17 | 0.23–20.32 | 0.496 |
| pStage (AJCC 8th ed.) | ||||||
| I-II | Ref | |||||
| III-IV | 38.31 | 5.23–280.81 | <0.001 | 26.25 | 2.05–335.27 | 0.012 |
| PORT/CRT | ||||||
| No | Ref | |||||
| Yes | 2.81 | 1.16–6.84 | 0.023 | 0.22 | 0.07–0.66 | 0.007 |
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; CRT, chemoradiotherapy; HR, hazard ratio; p, pathological; PORT, postoperative radiotherapy; Ref, reference variable.
Low and intermediate risks are combined because there was no disease-specific death in the low-risk category.
3.6 |. Pattern of recurrence in parotid carcinomas with and without PPE
Over a median follow-up period of 26 months (range 0.1–163.4 months), 6 of the 22 (27.3%) patients whose carcinomas had PPE recurred after treatment. Two patients recurred in the parapharyngeal space and one of them also developed distant recurrence. Two patients recurred in the neck and one of them also recurred distantly. The other two patients had only distant recurrences. In patients whose carcinomas had no PPE, 33 out of 169 (19.5%) recurred. Five recurred locally, out of which one also recurred in the neck and three also recurred distantly. Four recurred in the neck but also distantly. The other 24 patients only recurred distantly. Comparing the pattern of recurrence between patients with and without PPE, there was no statistically significant difference in the probability of local (9.09% vs. 2.96%, p = 0.186), regional (9.09% vs. 2.96%, p = 0.186), or distant recurrence (18.18% vs. 18.34%, p = 1.000) (Figure 4).
FIGURE 4.
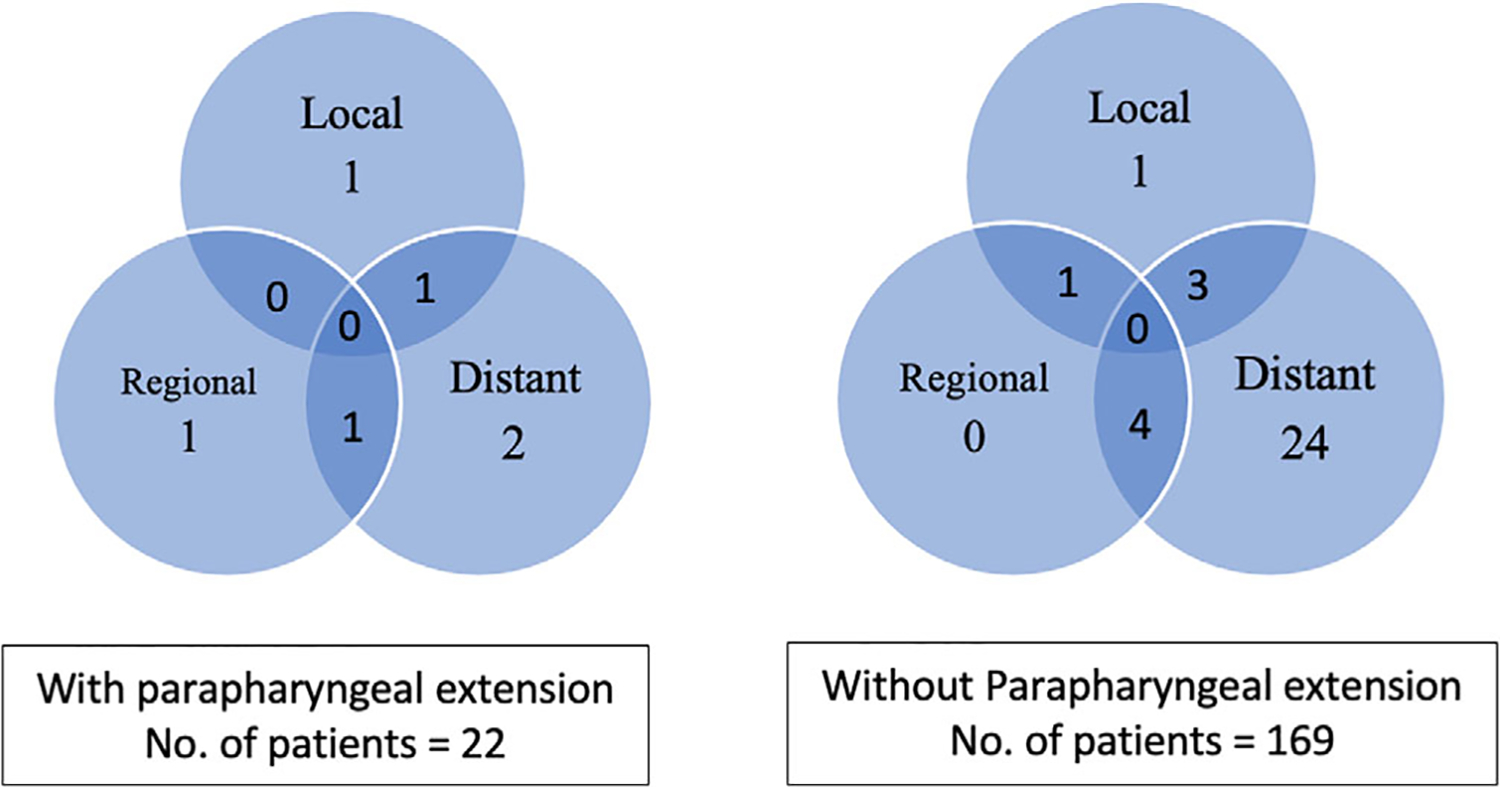
Pattern of recurrence in parotid carcinomas with and without parapharyngeal extension. Legend: Numbers in these Venn diagrams denote the number of patients who developed recurrence during a median follow-up period of 26 months. Overlapping areas indicate multiple sites of recurrence. All patients were treated by surgical resection and adjuvant therapy if there were high risk features (see section 2)
4 |. DISCUSSION
In the resection of parotid gland carcinomas, a pathologically clear surgical margin is associated with improved disease-specific,17 overall,17,18 and recurrence-free survival.19 However, the likelihood of a positive surgical margin and its impact on oncologic outcomes in patients with parotid carcinomas which involve the parapharyngeal space has been sparsely described. Lombardi et al. reported a margin positivity of 32.7% in 42 malignancies of the parapharyngeal space (PPS) and its association with worse locoregional control,20 but salivary gland carcinomas comprised a minority of these patients (16 out of 42). Stodulski et al. reported parapharyngeal space invasion to worsen disease-free survival by an odds ratio of 9.8 (95% CI 2.1–45.1) in 109 resected parotid carcinomas, but this association disappeared on multivariable analysis.21 In the literature, there is also a lack of consensus on how parapharyngeal space involvement should be defined. We defined parapharyngeal involvement, or PPE, as tumor extension medial to the radiologically identifiable stylomandibular plane because intraoperatively, the corridor bounded by the mandible, styloid process and the stylomandibular ligament is the narrowest point through which the deep lobe of the parotid gland passes into the PPS. Visualization of the tumor medial to the stylomandibular plane is difficult during the commonly performed transcervical approach, even though nasotracheal intubation, complete dental occlusion, division of the stylomandibular ligament, digastric muscle, stylohyoid muscle, and external carotid artery can improve visualization to some extent. To evaluate the impact of this unfavorable tumor location on surgical margin and oncologic outcomes, we identified tumors with and without PPE from a database of parotid carcinomas. Our data show that parotid carcinomas with PPE are commonly carcinoma ex-pleomorphic adenoma. This is probably because pleomorphic adenomas are the commonest salivary gland neoplasms of the PPS and parapharyngeal neoplasms tend to remain occult.10 In our experience, three quarters of carcinomas with PPE were resected via a transparotid, transcervical approach or their combination, 20% needed a segmental mandibulectomy and 5% needed a mandibulotomy. None were resected transorally. That a mandibulotomy and transoral approach was not commonly employed in our series may be explained by the parotid origin of these carcinomas, whereas carcinomas of the parapharyngeal minor salivary glands should be more medially located and require these approaches more often.
In our series of conventionally resected parotid carcinomas, we did not find PPE to be significantly associated with a positive surgical margin, although a small effect may not be detectable by the statistical power of our data. Interestingly, a positive surgical margin appears to be more common in tumors with minor PPE than tumors with major PPE. This suggests that the site of the positive margin may not be in the PPS. Perhaps, parotid carcinomas with minor PPE may be more likely to straddle the facial nerve by involving both the superficial and the deep lobe, whereas those with major PPE may be more likely to involve only the deep lobe. Therefore, the preservation of the facial nerve may contribute more strongly to a positive surgical margin than PPE. Certainly, the border, size and invasiveness of the tumors can also influence margin positivity. This is evident in our analysis of factors predictive of a positive margin, in which clinical T4 classification, a surrogate of tumor size and local extension,22 remains associated with margin positivity on multivariate analysis. A close or microscopically positive surgical margin may be unavoidable in the resection of a T4 tumor should the surgeon elect to preserve neighboring anatomical structures or a grossly uninvolved facial nerve, but a grossly positive surgical margin should be avoided by careful preoperative planning.
Despite a positive margin rate of 45.5% in our series of parotid carcinomas with PPE, the 3-year local control was 88.9%. This may be attributable to the use of postoperative radiotherapy when high-risk pathologic features are present. PPE was not associated with local recurrence but pathologic risk and T classification were, and both these factors reduced the hazard of local recurrence attributable to PPE. This implies tumor location is less important than its size, grade and local extension in predicting local recurrence. Distant recurrence appears to be more common than local or regional recurrence in parotid carcinomas with or without PPE. Distant recurrence occurred in 90% of those who recurred. This likely accounted for the poorer DSS in our patients as compared to their local control. PPE may have little impact on DSS based on the results of our multivariable analysis, but high pathologic risk and advanced pathologic stage significantly worsen DSS, whereas postoperative radiotherapy/chemoradiotherapy appears to improve it. In spite of that, distant recurrence remained common, affecting about one-fifth of our patients.
Our study has several limitations. First, parapharyngeal carcinomas of minor salivary gland origin that are radiologically separate from the deep lobe of the parotid gland are not included in this study. Those tumors may exhibit different clinicopathologic characteristics and oncologic outcomes. Second, by chart review, we could not ascertain the exact location of the positive margin in tumors with PPE. A prospective study can be done to determine the status of the parapharyngeal margin directly in order to further optimize the choice of surgical approach to the PPS.
In conclusion, despite the constraints of surgical access, parapharyngeal extension per se does not appear to adversely predict surgical margin positivity, local recurrence or DSS in parotid carcinomas. Advanced T classification, stage and high-risk pathology more likely determine these outcomes. However, a positive surgical margin can be common in the resection of parotid carcinomas with parapharyngeal extension. Surgical techniques or technologies that delineate the cancer margin more precisely may enhance the margin control during tumor resections in the parapharyngeal space. Effective distant control is also needed to further improve the survival of patients with parotid carcinomas.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NCI grant (No. P30 CA008748).
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Accepted as a podium presentation (abstract ID 105051) at the 2020 International Conference of American Head and Neck Society.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Stambuk HE, Patel SG. Imaging of the parapharyngeal space. Otolaryngol Clin North Am. 2008;41(1):77–101. [DOI] [PubMed] [Google Scholar]
- 2.Chan JY, Tsang RK, Eisele DW, Richmon JD. Transoral robotic surgery of the parapharyngeal space: a case series and systematic review. Head Neck. 2015;37(2):293–298. [DOI] [PubMed] [Google Scholar]
- 3.Chiang TY, Chen MK. Endoscope-assisted transoral excision of a huge parapharyngeal pleomorphic adenoma. B-ENT. 2011;7 (2):143–146. [PubMed] [Google Scholar]
- 4.Hakeem AH, Hazarika B, Pradhan SA, Kannan R. Primary pleomorphic adenoma of minor salivary gland in the parapharyngeal space. World J Surg Oncol. 2009;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendelsohn AH, Bhuta S, Calcaterra TC, Shih HB, Abemayor E, St John MA. Parapharyngeal space pleomorphic adenoma: a 30-year review. Laryngoscope. 2009;119(11):2170–2174. [DOI] [PubMed] [Google Scholar]
- 6.Panoussopoulos D, Yotakis J, Pararas B, Theodoropoulos G, Papadimitriou K. Giant pleomorphic adenoma of the parotid gland involving the parapharyngeal space treated by a totally extraoral transparotid approach. J Surg Oncol. 2002;81(3): 155–157. [DOI] [PubMed] [Google Scholar]
- 7.Schutt C, Cordero J. A transoral surgical approach to a parapharyngeal-space pleomorphic adenoma. Ear Nose Throat J. 2014;93(10–11):E29–E31. [PubMed] [Google Scholar]
- 8.Guo Y, Guo C, Zhang L, Yu G. Extracapsular dissection of the parapharyngeal space for a pleomorphic adenoma: a 10-year review. Br J Oral Maxillofac Surg. 2014;52(6):557–562. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz G, Ben-Ari O, Wasserzug O, Weizman N, Yehuda M, Fliss DM. The transcervical approach for parapharyngeal space pleomorphic adenomas: indications and technique. PLoS One. 2014;9(2):e90210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuet ML, Kasbekar AV, Masterson L, Jani P. Management of tumors arising from the parapharyngeal space: a systematic review of 1,293 cases reported over 25 years. Laryngoscope. 2015;125(6):1372–1381. [DOI] [PubMed] [Google Scholar]
- 11.Paderno A, Piazza C, Nicolai P. Recent advances in surgical management of parapharyngeal space tumors. Curr Opin Otolaryngol Head Neck Surg. 2015;23(2):83–90. [DOI] [PubMed] [Google Scholar]
- 12.Basaran B, Polat B, Unsaler S, Ulusan M, Aslan I, Hafiz G. Parapharyngeal space tumours: the efficiency of a transcervical approach without mandibulotomy through review of 44 cases. Acta Otorhinolaryngol Ital. 2014;34(5):310–316. [PMC free article] [PubMed] [Google Scholar]
- 13.Khafif A, Segev Y, Kaplan DM, Gil Z, Fliss DM. Surgical management of parapharyngeal space tumors: a 10-year review. Otolaryngol Head Neck Surg. 2005;132(3):401–406. [DOI] [PubMed] [Google Scholar]
- 14.Duek I, Sviri GE, Billan S, Gil Z. Minimally invasive surgery for resection of parapharyngeal space tumors. J Neurol Surg B Skull Base. 2018;79(3):250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay AJ, Migliacci J, Karassawa Zanoni D, McGill M, Patel S, Ganly I. Minor salivary gland tumors of the head and neck—Memorial Sloan Kettering experience: incidence and outcomes by site and histological type. Cancer. 2019;125(19):3354–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimica X, McGill M, Hay A, et al. Sex disparities in salivary malignancies: Does female sex impact oncological outcome? Oral Oncol. 2019;94:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amit M, Na’ara S, Trejo-Leider L, et al. Defining the surgical margins of adenoid cystic carcinoma and their impact on outcome: an international collaborative study. Head Neck 2017;39 (5):1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morse E, Fujiwara RJT, Judson B, Prasad ML, Mehra S. Positive surgical margins in parotid malignancies: institutional variation and survival association. Laryngoscope. 2019;129(1): 129–137. [DOI] [PubMed] [Google Scholar]
- 19.Ali S, Palmer FL, Yu C, et al. A predictive nomogram for recurrence of carcinoma of the major salivary glands. JAMA Otolaryngol Head Neck Surg. 2013;139(7):698–705. [DOI] [PubMed] [Google Scholar]
- 20.Lombardi D, Ferrari M, Paderno A, et al. Selection of the surgical approach for lesions with parapharyngeal space involvement: a single-center experience on 153 cases. Oral Oncol. 2020;109:104872. 10.1016/j.oraloncology.2020.104872. [DOI] [PubMed] [Google Scholar]
- 21.Stodulski D, Mikaszewski B, Stankiewicz C. Are all prognostic factors in parotid gland carcinoma well recognized? Eur Arch Otorhinolaryngol. 2012;269(3):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lydiatt WM, Mukherji SK, O’Sullivan B, Patel SG, Shah JP. Major salivary glands. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 8th ed. New York: Springer; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


