Abstract
Background
High efficacy in terms of protection from severe COVID‐19 has been demonstrated for several SARS‐CoV‐2 vaccines. However, patients with compromised immune status develop a weaker and less stable immune response to vaccination. Strong immune response may not always translate into clinical benefit, therefore it is important to synthesise evidence on modified schemes and types of vaccination in these population subgroups for guiding health decisions. As the literature on COVID‐19 vaccines continues to expand, we aimed to scope the literature on multiple subgroups to subsequently decide on the most relevant research questions to be answered by systematic reviews.
Objectives
To provide an overview of the availability of existing literature on immune response and long‐term clinical outcomes after COVID‐19 vaccination, and to map this evidence according to the examined populations, specific vaccines, immunity parameters, and their way of determining relevant long‐term outcomes and the availability of mapping between immune reactivity and relevant outcomes.
Search methods
We searched the Cochrane COVID‐19 Study Register, the Web of Science Core Collection, and the World Health Organization COVID‐19 Global literature on coronavirus disease on 6 December 2021.
Selection criteria
We included studies that published results on immunity outcomes after vaccination with BNT162b2, mRNA‐1273, AZD1222, Ad26.COV2.S, Sputnik V or Sputnik Light, BBIBP‐CorV, or CoronaVac on predefined vulnerable subgroups such as people with malignancies, transplant recipients, people undergoing renal replacement therapy, and people with immune disorders, as well as pregnant and breastfeeding women, and children. We included studies if they had at least 100 participants (not considering healthy control groups); we excluded case studies and case series.
Data collection and analysis
We extracted data independently and in duplicate onto an online data extraction form. Data were represented as tables and as online maps to show the frequency of studies for each item. We mapped the data according to study design, country of participant origin, patient comorbidity subgroup, intervention, outcome domains (clinical, safety, immunogenicity), and outcomes.
Main results
Out of 25,452 identified records, 318 studies with a total of more than 5 million participants met our eligibility criteria and were included in the review. Participants were recruited mainly from high‐income countries between January 2020 and 31 October 2021 (282/318); the majority of studies included adult participants (297/318).
Haematological malignancies were the most commonly examined comorbidity group (N = 54), followed by solid tumours (N = 47), dialysis (N = 48), kidney transplant (N = 43), and rheumatic diseases (N = 28, 17, and 15 for mixed diseases, multiple sclerosis, and inflammatory bowel disease, respectively). Thirty‐one studies included pregnant or breastfeeding women.
The most commonly administered vaccine was BNT162b2 (N = 283), followed by mRNA‐1273 (N = 153), AZD1222 (N = 66), Ad26.COV2.S (N = 42), BBIBP‐CorV (N = 15), CoronaVac (N = 14), and Sputnik V (N = 5; no studies were identified for Sputnik Light). Most studies reported outcomes after regular vaccination scheme.
The majority of studies focused on immunogenicity outcomes, especially seroconversion based on binding antibody measurements and immunoglobulin G (IgG) titres (N = 179 and 175, respectively). Adverse events and serious adverse events were reported in 126 and 54 studies, whilst SARS‐CoV‐2 infection irrespective of severity was reported in 80 studies. Mortality due to SARS‐CoV‐2 infection was reported in 36 studies.
Please refer to our evidence gap maps for more detailed information.
Authors' conclusions
Up to 6 December 2021, the majority of studies examined data on mRNA vaccines administered as standard vaccination schemes (two doses approximately four to eight weeks apart) that report on immunogenicity parameters or adverse events. Clinical outcomes were less commonly reported, and if so, were often reported as a secondary outcome observed in seroconversion or immunoglobulin titre studies. As informed by this scoping review, two effectiveness reviews (on haematological malignancies and kidney transplant recipients) are currently being conducted.
Plain language summary
Immunity in vulnerable groups after COVID‐19 vaccination
What did we want to find out?
We wanted to find out which studies on the most commonly used COVID‐19 vaccines in vulnerable subgroups have been published, and which outcomes were reported (e.g. effectiveness outcomes, safety, or immune response), to decide on the most relevant questions and answer these in further effectiveness systematic reviews (syntheses of the medical literature).
What did we do?
We searched medical databases and trial registries for studies on COVID‐19 vaccines that were authorised for use in the European Union (European Medicines Agency (EMA)‐approved) and those approved in at least 10 countries worldwide at the time of our search.
We included studies on additional conditions (comorbidities) that can reduce the immune reaction to vaccination, if they had more than 100 participants; they could include any age, sex, ethnicity, or country of recruitment.
We excluded studies looking at the general population and other than preselected COVID‐19 vaccines and subgroups.
Once we found the studies, we categorised the vaccines into the following groups: EMA‐approved COVID‐19 vaccines, other COVID‐19 vaccines, and schemes with different COVID‐19 vaccines. We summarised the results in an interactive online map. We mapped the study outcomes, the country in which the study was conducted, the study design, and the vulnerable population.
What did we find?
We included 318 studies. Most studies came from high‐income countries and included adults. We found that haematological malignancies (cancers that affect the blood, bone marrow, and lymph nodes) and solid tumours were examined in many studies, followed by people receiving dialysis and kidney transplants, rheumatic diseases, and others. Thirty‐one studies included pregnant or breastfeeding women. The majority of studies explored mRNA vaccines (N = 283 and N = 153 for BNT162b2 and mRNA‐1273) at two doses, and EMA‐approved vaccines were more commonly administered than other vaccines and schemes with different COVID‐19 vaccines.
Outcomes related to immunogenicity (how well a vaccine works, or the ability to stimulate the development of antibodies), especially the presence or absence of antibodies in the blood of patients or an estimate for the amount of these antibodies, were the most frequently reported outcome in more than 170 studies each. In addition, adverse events were assessed often (N = 126 studies), whilst SARS‐CoV‐2 infection was reported in only 80 studies.
What are the limitations of the evidence?
Due to the quick development of the pandemic, the research landscape may have changed. The newer Omicron variant has become the dominant variant, and a new vaccine has been approved by the EMA, which is not covered by our search.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to December 2021.
What are the next steps?
Based on the overview from this review, we have decided to conduct two detailed systematic reviews on haematological malignancies and kidney transplant recipients.
Summary of findings
Background
The coronavirus disease 2019 (COVID‐19) outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was declared a pandemic in March 2020 (WHO 2020). Despite intense international efforts to contain its spread, COVID‐19 has resulted in over 500 million confirmed cases and more than 6 million deaths worldwide as of May 2022 (WHO 2022a). Evolving SARS‐CoV‐2 variants with antigen escape and potentially altered transmission or disease characteristics have raised concerns, as these could impair established disease control measures as well as vaccines and therapeutic approaches (WHO 2022b).
Whilst effective treatments for COVID‐19 are limited, active immunisation against SARS‐CoV‐2 has a substantial impact on case incidences and hospitalisations, therefore vaccination is critical to controlling the pandemic (Pandey 2020). Vaccines have been shown to be highly effective at reducing transmission and preventing severe illness and death from COVID‐19 (Public Health Ontario 2021). As of 23 May 2022, 38 vaccines were approved by at least one country, and there are about 200 vaccine candidates under development in clinical trials (Basta 2022; Shrotri 2021). However, the logistical distribution process is time‐consuming, and global access to vaccines varies widely (Wouters 2021). The majority of vaccines have been administered in high‐income countries. Despite extensive international efforts for equitable access to vaccines such as COVAX (WHO 2022c), unequal access to vaccines continues to leave a substantial proportion of the world’s most vulnerable people at high risk of becoming seriously ill from COVID‐19.
Diverse technologies are used for COVID‐19 vaccine development based on the spike protein as the primary antigen (Krammer 2020), including the most recent and now most widely used nucleic acid vaccines, weakened and inactivated viruses, replicating and non‐replicating viral vectors, protein‐subunits, and virus‐like particles (Barajas‐Nava 2021). Most COVID‐19 vaccines require a two‐dose schedule for primary immunisation, administered weeks apart to allow the induction of long‐term immunity. Whilst this holds true for the general population, immunocompromised people frequently fail to achieve serological response, with the lowest probability of seroconversion observed in the solid organ transplant‐receiving population, with rates of only about 30% after the second vaccine dose (Lee 2022).
Waning immunity is indicated by evidence showing that vaccine efficacy against symptomatic infection decreases within 6 months by about 20 percentage points in people of all ages and by over 36% in people at least 50 years old (Feikin 2022). Tober‐Lau 2021 even indicated an absence of detectable serum neutralising activity against the Delta variant of concern at six‐month follow‐up in 39% of older participants (median age 82.5 years). At the same time, studies show that the viral load of breakthrough infections decreases after booster vaccination (Levine‐Tiefenbrun 2022). Hence, first booster vaccinations have been implemented in numerous countries for the general population. Second doses have recently been authorised for individuals with certain kinds of immunocompromise as well as those aged 50 years and older (CDC 2022).
Reduced immune response can occur due to the underlying cause of immunocompromise itself, such as primary immunodeficiency, malignancy, autoimmune disease, or due to immunosuppressive treatment itself. For solid malignancies, for example, a generally high immune response has been reported, whereas certain haematologic malignancies like chronic lymphocytic leukaemia show reduced seroconversion rates even if patients are treatment‐naive (Herishanu 2021). Cytotoxic chemotherapies, immune checkpoint inhibitors, and hormonal therapies seem to reduce immune response to only a relatively small extent, but specific immunosuppressive treatment, especially lymphocyte‐depleting therapies (CD‐20 antibodies, mycophenolate mofetil), chimeric antigen receptor (CAR) T‐cell therapy, and stem cell transplantation, are associated with significantly lower seroconversion rates (Thakkar 2021). In solid organ transplant recipients, the use of antimetabolites has been shown to reduce seroresponse, and the temporary hold of antimetabolite treatment substantially increased vaccine response in kidney transplant recipients (Osmanodja 2022; Schrezenmeier 2022).
Several immunoassays that measure humoural and cellular immune responses are commercially available. Qualitative seroresponse and quantification of antibody titres are now usually assessed by determination of SARS‐CoV‐2‐IgG‐antibodies against the full spike‐trimer, the spike‐1‐(S1) subunit, and the receptor‐binding domain (RBD). Neutralising antibodies primarily bind the RBD, a part of the S1 subunit, and block the interaction of the spike‐protein of the virus with the angiotensin‐converting enzyme type 2 receptor (ACE2) on the host cell surface, thereby preventing cell entry by SARS‐CoV‐2, and thus infection. This ability to block viral entry correlates with protection from symptomatic SARS‐CoV‐2 infection (Khoury 2021), thus neutralising antibody levels seem to be reliable predictors of protection against COVID‐19‐infection. The gold standard for assessing neutralisation capacity is the plaque reduction neutralisation test (PRNT), which requires biosafety level 3 conditions for isolation of live pathogenic SARS‐CoV‐2 virus, thereby limiting its large‐scale applicability for diagnostic routine (Rubio‐Acero 2021). Alternative platforms to evaluate virus neutralisation have been developed: pseudotyped virus neutralisation assays and surrogate enzyme‐linked immunosorbent assays measuring inhibition of the RBD binding to the ACE2‐receptor (Sholukh 2021). Furthermore, several studies have shown a high correlation between anti‐RBD‐IgG and anti‐S1‐IgG titres with virus neutralisation (Mahmoud 2021; Ramos 2022; Rubio‐Acero 2021), suggesting the use of binding antibody titres, which are easier to measure, as a correlate of protection (Earle 2021). This has, however, complicated comparison between cohorts, since multiple assays with different thresholds and arbitrary units are being used for immunity assessment worldwide. Though studies investigating different assays report a better correlation of the results within them, absolute titres from different assays are still not interchangeable (Perkmann 2021). The lack of an international consensus for the measurement of immunity after SARS‐CoV‐2 vaccination also holds true for the assessment of cellular immunity, which likely plays an important role in virologic control and disease severity (Sette 2021). An international standard consisting of pooled convalescent plasma for harmonisation of serological results across laboratories in order to define an antibody cut‐off predictable for vaccine efficacy has been established by the World Health Organization (WHO) for the quantification of both neutralising and binding antibodies, which could help reach the goal of defining a common antibody cut‐off predictable for vaccine efficacy (WHO 2020).
Another problem to face whilst discussing the feasibility of antibody titres as correlates of protection are the recent variants of concern, particularly the Omicron variant, against which neutralisation capacity after full vaccination is significantly lower than against the wild‐type (Cele 2022; Dejnirattisai 2022), resulting in a marked reduction in vaccine effectiveness (Abu‐Raddad 2022). The Omicron variant has evolved to escape neutralising activity by incorporating a high number of mutations in the RBD and has been able to spread rapidly in the population (Viana 2022), with several recent studies demonstrating that booster immunisations are required to fight the high immune escape of this variant (Garcia‐Beltran 2022; Gruell 2022; Lusvarghi 2021; Schmidt 2022).
In light of the rapid evolution of the virus, a better understanding of the immune response of the vulnerable population after COVID‐19 vaccination is critical for the optimisation of vaccination programmes, reducing the fatality rate, and blocking the infection chain.
Rationale for conducting a scoping review
As COVID‐19 is a new, globally spread disease, research on vaccine efficacy against severe COVID‐19 in various settings is just developing, with a rapidly increasing number of registered and published clinical trials. However, there is considerable heterogeneity of such studies with regard to study populations, vaccination settings, as well as definition, measurement, and reporting of outcomes. For example, the measurement tools for immunity parameters are in constant development. They may vary widely from study to study and provide differing results (Hillus 2021; Khoury 2021; Muecksch 2020; Patel 2021; Schmidt 2021; Schwarz 2021). Similarly, there is still no clearly defined minimum core outcome set for studies observing immunity. Immune responses to different vaccines, vaccine combinations, and possible booster vaccines after natural infection are hypothesised to vary, and the examined populations and their exposure to different SARS‐CoV‐2 variants may differ substantially. Although there is evidence of high long‐term efficacy of vaccination against hospitalisation and death in the general population (Krause 2021), the question of the duration of immunity in vulnerable individuals like children, immunocompromised individuals, and pregnant women involves numerous scenarios.
A scoping review, a systematic knowledge synthesis approach for identifying important concepts, sources, and knowledge gaps on a broadly defined topic, can provide an overview of the currently published literature (Tricco 2018). We will use the overview and classification of studies identified in this scoping review to flag evidence gaps and clusters of evidence that would allow the formulating of more specific, clearly defined questions for further systematic reviews.
Objectives
To provide an overview of the availability of existing literature on immune response and long‐term clinical outcomes after COVID‐19 vaccination, and to map this evidence according to the examined populations, specific vaccines, immunity parameters, and their way of determining relevant long‐term outcomes and the availability of mapping between immune reactivity and relevant outcomes.
Methods
The protocol of this scoping review was published in the Open Science Framework (Kreuzberger 2021). We adhered to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR) to ensure complete reporting (Tricco 2018). Please see Appendix 1 for the completed checklist for this scoping review.
Due to the unexpected amount of studies to screen and extract, we modified our inclusion and exclusion criteria during the process to keep the workload feasible. These changes are noted in Differences between protocol and review.
Criteria for considering studies for this review
Target population/intervention
We included studies that quantitatively assessed immunity outcomes after at least one vaccine dose. Hereby, we limited ourselves to those vaccines authorised for use in the European Union by the European Medicines Agency (EMA), and those approved, authorised, licensed, or granted an emergency use authorisation in at least 10 countries worldwide at the time of our search by 6 December 2021. At that time, the following vaccines were authorised by the EMA:
BNT162b2 (Comirnaty, Pfizer/BioNTech);
mRNA‐1273 (Spikevax, Moderna; and its equivalent TAK‐919, Moderna formulation, Takeda);
AZD1222 (Vaxzevria, Oxford/AstraZeneca; and its equivalent Covishield, Oxford/AstraZeneca formulation, Serum Institute of India);
Ad26.COV2.S (COVID‐19 Vaccine Janssen) (EMA 2021a).
Additional vaccines approved in at least 10 countries worldwide, according to Basta 2022, by 6 December 2021:
Sputnik V (Gamaleya);
Sputnik Light (Gamaleya);
BBIBP‐CorV (Vero Cells, Sinopharm (Beijing));
CoronaVac (Sinovac, COVID‐19 Vaccine (Vero Cell) Inactivated).
We excluded vaccines that were under EMA rolling review at the time of our literature search (e.g. NVX‐CoV2373 (Novovax CZ AS), CVnCoV, Vidprevtyn) (Shrotri 2021).
Due to the large number of studies identified that were to map, we ad hoc excluded studies focusing on the general population and kept only those on predefined subgroups and medical conditions that may affect vaccine response (e.g. pregnant and breastfeeding women, paediatric studies, haematological malignancies, solid tumours, and more comorbidities; for details, see Table 4).
1. Characteristics of identified studies: population.
| Characteristic | Number of studies | Number of participants (sum) | Number of participants (median, range) | |
| Enrolment period (minimum start, maximum end) | 30 January 2020 to 31 October 2021 | |||
| Missing dates | 79 | |||
| Overall | 318 | 5,061,795 | 317 (100 to 1,277,747) | |
| Age class | ||||
| Adult | 297 | |||
| Paediatric | 10 | |||
| Adult ‐ neonate | 13 | |||
| Include previously SARS‐CoV‐2‐positive participants | 84 | |||
| Report ethnicity in at least the baseline characteristics | 98 | |||
| Population: comorbidities | ||||
| Solid tumours | 47 | 183,974 | 200 (34 to 95,935) | |
| Haematological malignancies | 54 | 46,928 | 149 (14 to 32,156) | |
| Haematological malignancies, stem cell transplant | 22 | 2456 | 108.5 (12 to 397) | |
| Kidney disease | 6 | 41,903 | 50.5 (21 to 41,597) | |
| Dialysis | 48 | 189,142 | 172.5 (26 to 142,826) | |
| Chronic heart disease | 9 | 73,147 | 178 (21 to 70,716) | |
| Liver disease | 7 | 40,796 | 92 (12 to 40,074) | |
| Lung disease | 5 | 2377 | 104 (16 to 1893) | |
| Transplant: kidney | 43 | 12,991 | 148 (19 to 2350) | |
| Transplant: heart | 9 | 540 | 46 (16 to 134) | |
| Transplant: liver | 12 | 843 | 60 (11 to 161) | |
| Transplant: other or mixed | 19 | 56,514 | 187 (5 to 48,213) | |
| HIV/AIDS | 10 | 3508 | 105 (4 to 2103) | |
| Multiple sclerosis | 17 | 6331 | 239 (58 to 912) | |
| Inflammatory bowel disease | 15 | 40,842 | 436 (58 to 14,697) | |
| Rheumatoid arthritis | 5 | 6385 | 189 (129 to 5493) | |
| Systemic lupus erythematosus | 7 | 1761 | 126 (19 to 696) | |
| Psoriasis | 5 | 1452 | 101 (51 to 788) | |
| Rheumatic diseases, mixed | 28 | 55,175 | 393.5 (45 to 35,475) | |
| Other autoimmune diseases | 13 | 8344 | 108 (17 to 6380) | |
| Other | 24 | 161,134 | 287 (24 to 279,145) | |
| Population: healthy | ||||
| Pregnancy | 26 | 360,738 | 1071 (84 to 130,875) | |
| Breastfeeding | 7 | 12,433 | 180 (31 to 6815) | |
| Comparator group | Healthy control | 116 | 3,523,037 | 91.5 (7 to 963,962) |
| Country | ||||
| Multiple countries | 12 | |||
| Austria | 3 | |||
| Belgium | 3 | |||
| Brazil | 3 | |||
| Canada | 7 | |||
| China | 9 | |||
| Denmark | 2 | |||
| France | 28 | |||
| Germany | 10 | |||
| Greece | 7 | |||
| India | 2 | |||
| Iran, Islamic Rep. | 5 | |||
| Israel | 51 | |||
| Italy | 24 | |||
| Kuwait | 2 | |||
| Lithuania | 2 | |||
| Norway | 3 | |||
| Poland | 4 | |||
| Portugal | 4 | |||
| Qatar | 2 | |||
| Spain | 10 | |||
| Switzerland | 2 | |||
| United Kingdom | 31 | |||
| United States | 81 | |||
| Other* | 11 | |||
| Abbreviations: SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | ||||
*1 study each: Argentina, Australia, Japan, Mexico, the Netherlands, Peru, Russian Federation, Saudi Arabia, South Africa, Sweden, United Arab Emirates.
Setting
We did not restrict this scoping review to any specific setting. In contrast to our protocol, we did not capture the predominance of different SARS‐CoV‐2 variants during study conduct, as this was mostly not reported. The Omicron variant (B.1.1.529) was identified in November 2021 as a new variant of concern (WHO 2022a) and was therefore not covered by our search.
Study design and publication formats
For our scoping review, we searched for systematic reviews to gain an overview of the literature, redefine eligible outcome measures, and cross‐check included primary studies. We included systematic reviews that:
included only in vivo studies (in humans);
provided their search strategy; and
were clear in their inclusion and exclusion criteria.
We did not exclude systematic reviews based on format (i.e. rapid, living, or scoping reviews).
For our primary study search, we included the following study designs with more than 100 participants in our predefined subgroups:
retrospective cohort study;
prospective cohort study;
randomised controlled trial;
case‐control study.
We excluded the following study design: case reports.
We aimed to include:
peer‐reviewed full‐text publications;
articles uploaded to preprint servers (e.g. medRxiv, bioRxiv, ResearchSquare);
letters to the editor.
We excluded conference abstracts, reports, and other grey literature. In contrast to our protocol, we excluded records from clinical trial registries for ongoing studies; a list of potentially relevant studies can be found in our online database.
Outcomes
We were interested in exploring the outcomes that were available in the current literature, therefore we did not limit inclusion to specific outcomes, but required at least one of the following:
clinical outcomes (e.g. SARS‐CoV‐2 infection, admission to hospital, admission to intensive care unit (ICU), mortality, adverse events, etc.);
immunity parameters (immunoglobulin titres, neutralising antibody titres, B‐ or T‐cell response).
Timing
The minimum median follow‐up time was 14 days after complete vaccination. One vaccine dose usually represents incomplete vaccination (except for those with previous COVID‐19 infection and the Ad26.COV2.S vaccine); two doses are full vaccination independent of precise schedule; and the third dose onwards represents booster doses.
Unit of analysis
We included aggregated data from studies that investigated immunity after vaccination at the individual level. We therefore excluded studies that looked at the population level, such as at vaccination rates between countries with different vaccination strategies. We did not collect individual participant data.
Identification of relevant studies
Our Information Specialist (IM) searched the following electronic databases for systematic reviews from November 2019 to 25 August 2021:
Evidence Aid Coronavirus (COVID‐19) (evidenceaid.org/evidence/coronavirus-covid-19);
Usher Network for COVID‐19 Evidence Reviews (https://www.ed.ac.uk/usher/uncover/register‐of reviews);
US Department of Veterans Affairs Evidence Synthesis Program (www.covid19reviews.org/);
Epistemonikos, L*OVE List Coronavirus disease (COVID‐19) (app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?utm=aile);
MEDLINE (via Ovid).
We searched the following databases from November 2019 to 6 December 2021 to retrieve potentially relevant primary studies.
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org), including:
PubMed, weekly searches;
Embase.com, weekly searches;
ClinicalTrials.gov (www.clinicaltrials.gov), daily searches;
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch), weekly searches;
medRxiv (www.medrxiv.org), weekly searches;
Cochrane Central Register of Controlled Trials (CENTRAL), monthly searches.
-
Web of Science Core Collection:
Science Citation Index Expanded (1945 to present);
Emerging Sources Citation Index (2015 to present).
WHO COVID‐19 Global literature on coronavirus disease (https://search.bvsalud.org/global‐literature‐on novel‐coronavirus‐2019‐ncov/)
The search strategies for systematic reviews are shown in Appendix 2. The search strategies for primary studies were informed by included systematic reviews and were peer reviewed; they are provided in Appendix 3.
Study selection
After de‐duplication, the resulting records were screened independently by at least two review authors in a two‐stage process based on titles and abstracts, then as full‐texts, via Covidence (Covidence). Conflicts at each step were resolved by discussion or by involving a third review author when necessary. We documented the screening process in a PRISMA flow diagram (Moher 2009), where we described the reasons for exclusion of studies at the full‐text stage.
To ensure consistency between review authors, we developed a screening guidance sheet and discussed the screening and conflicts after the first 300 records, and regularly throughout the process. The guidance contained:
an overview on the screening steps (first‐level title and abstract screening versus full‐text screening);
an overview on the inclusion criteria (see table below) and a list of relevant subgroups;
predefined exclusion reasons at full‐text screening; and
additional notes, e.g. missing full‐text publications, how to tag, etc., gathered from questions arising during our weekly meetings.
| Population | Vaccines | Study design |
|
|
|
| Setting | Vaccination strategies | Publication format |
|
|
|
| 1We originally planned to list studies per age group, i.e. elderly participants separately; however, due to change in eligibility criteria (exclusion of the general population), the account may be incomplete, therefore adult participants are not further divided into age classes. | ||
Data collection and charting
Two review authors extracted the following data into the data extraction tool in Covidence (Covidence).
| General information |
|
|
Study characteristics/setting |
|
| Participant characteristics |
|
| Vaccine characteristics |
|
| Outcomes | Outcomes investigated
|
A first version of the data collection form was developed in MS Excel (MS Excel), and circulated to all review authors for feedback. After feedback was obtained, we transferred the items to Covidence (Covidence), and three review authors (CH, CSt, NK) piloted the form on a randomly selected sample of studies. Feedback from piloting was discussed at the next group meeting and accepted changes were implemented.
Pairs of review authors charted data independently in duplicate, and one of the two review authors or a third review author could subsequently compare the two extractions and decide on a final version. During data charting, we decided to skip multiple items due to feasibility. After having been exported to MS Excel, data were again checked for duplicate references and final eligibility of studies, and then summarised using the software R (R Core Team).
Data synthesis and presentation of results
As we anticipated high heterogeneity in study characteristics, populations, vaccines, and outcomes, we aimed to create an evidence map of studies investigating immunity after vaccination. We classified the data according to various factors, as follows:
population (i.e. general population, immunosuppressed, risk factors, etc.);
setting (country);
vaccine type (e.g. Oxford/AstraZeneca AZD1222, Pfizer/BioNTech BNT162b2, Moderna mRNA‐1273, Janssen (Johnson & Johnson) Ad26.COV2.S), combination scheme;
vaccination scheme (homologous, heterologous);
availability of outcomes (immune parameters, clinical outcomes).
We used multiple ways of presenting the results of our scoping search, as follows.
Table format: we charted data according to general characteristics, population characteristics, vaccine characteristics, and outcomes.
Evidence gap map: we created an interactive map using the software 3ie EGM (International Initiative for Impact Evaluation).
Narratively: we additionally summarised the characteristics narratively in the main body of the review.
Results
Results of the search
After de‐duplication of our identified 25,452 records, we screened 23,664 records based on title and abstract. We excluded 20,035 records as clearly irrelevant. After title and abstract screening, we decided that including studies of all listed study designs performed in the general population would not be feasible for us regarding data extraction and presentation, therefore we excluded studies on only the general population at the full‐text screening stage. After the exclusion of 3235 records including ongoing studies, studies with a sample size smaller than 100 participants, studies on the general population or healthcare workers only, studies that did not specify the vaccine received, and records published as ineligible format (i.e. conference abstracts, commentaries), 318 studies (in 394 articles) were included in our scoping review (Figure 1).
1.
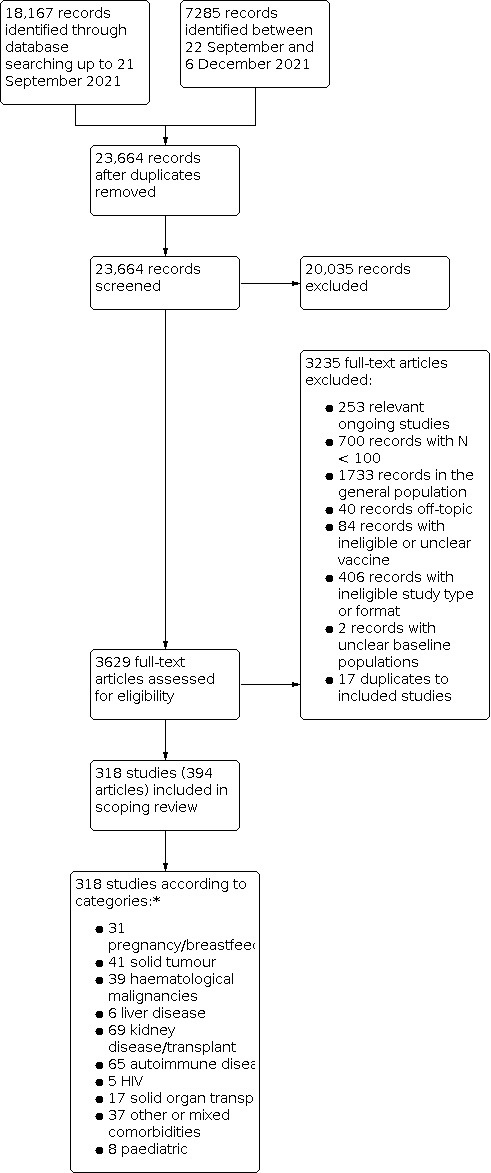
PRISMA flow diagram.
*Categories are mutually exclusive and sorted according to the online database. Numbers do not align with Table 4, as one study can include more than one comorbidity subgroup.
Description of studies
Included studies
We have presented our studies in an evidence gap map based on a template provided by the International Initiative for Impact Evaluation (International Initiative for Impact Evaluation). For reasons of clarity of representation, we created two versions of the map:
one version for the overall view of studies: egmopenaccess.3ieimpact.org/evidence-maps/scoping-review-covid-19-vaccines; and
one version to filter studies according to subgroups: egmopenaccess.3ieimpact.org/evidence-maps/scoping-review-covid-19-vaccines-duplicated-subgroups.
The program does not currently allow us to upload studies including multiple subgroups. The first map thus represents a general overview, but should not be used for filtering, as only studies with one subgroup will show. However, as the representation of subgroups is the main purpose of this scoping review, we duplicated studies so that it is possible to filter all studies belonging to one population subgroup. In the second map, the overall view is distorted by duplicating studies.
Additional current limitations include the representation of countries and study design: a) studies with multiple countries cannot be filtered according to country, and the People's Republic of China is missing in the drop‐down option; and b) randomised controlled trials can be filtered in the overall map (map 1), but not in the subgroup map.
Static versions of the map are included as summary of findings tables (Table 1; Table 2; Table 3) and in Figure 2 and Figure 3. The data and references to included and excluded studies can be accessed via the Open Science Framework project website. A list of references of included studies, sorted by subgroups, is provided in Appendix 4.
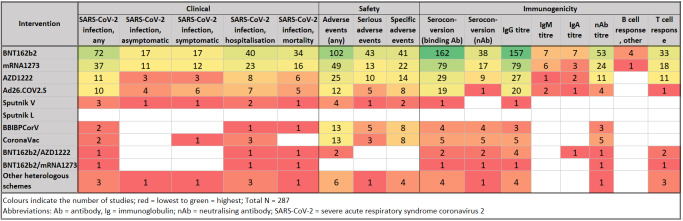
Summary of findings 1. Intervention ‐ outcome*.
| Clinical | Safety | Immunogenicity | ||||||||||||||
| Intervention | SARS‐CoV‐2 infection, any | SARS‐CoV‐2 infection, asymptomatic | SARS‐CoV‐2 infection, symptomatic | SARS‐CoV‐2 infection, hospitalisation | SARS‐CoV‐2 infection, mortality | Adverse events (any) | Serious adverse events | Specific adverse events | Seroconversion (binding Ab) | Seroconversion (neutralising Ab) | IgG titre | IgM titre | IgA titre | Neutralising Ab titre | B‐cell response, other | T‐cell response |
| BNT162b2 | 72 | 17 | 17 | 40 | 34 | 102 | 43 | 41 | 162 | 38 | 157 | 7 | 7 | 53 | 4 | 33 |
| mRNA‐1273 | 37 | 11 | 12 | 23 | 16 | 49 | 13 | 22 | 79 | 17 | 79 | 6 | 3 | 24 | 1 | 18 |
| AZD1222 | 11 | 3 | 3 | 8 | 6 | 25 | 10 | 14 | 29 | 9 | 27 | 1 | 2 | 11 | 0 | 11 |
| Ad26.COV2.S | 10 | 4 | 6 | 7 | 5 | 12 | 5 | 8 | 19 | 1 | 20 | 2 | 1 | 4 | 0 | 1 |
| Sputnik V | 3 | 1 | 1 | 2 | 1 | 4 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Sputnik L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BBIBPCorV | 2 | 0 | 0 | 1 | 1 | 13 | 5 | 8 | 4 | 4 | 3 | 0 | 0 | 3 | 0 | 0 |
| CoronaVac | 2 | 0 | 1 | 3 | 0 | 13 | 3 | 8 | 5 | 5 | 5 | 0 | 0 | 5 | 0 | 0 |
| BNT162b2/AZD1222 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 2 | 2 | 4 | 0 | 1 | 1 | 0 | 2 |
| BNT162b2/mRNA‐1273 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Other heterologous schemes | 3 | 1 | 1 | 3 | 1 | 6 | 1 | 4 | 4 | 1 | 4 | 0 | 0 | 1 | 0 | 3 |
| Abbreviations: Ab: antibody, Ig: immunoglobulin, SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | ||||||||||||||||
| An interactive evidence gap map is available at egmopenaccess.3ieimpact.org/evidence-maps/scoping-review-covid-19-vaccines (for filtering according to subgroups, please see Table 2; multicountry studies cannot be filtered). | ||||||||||||||||
*The overall number of studies was 286, excluding studies on pregnant and lactating women due to a different outcome set.
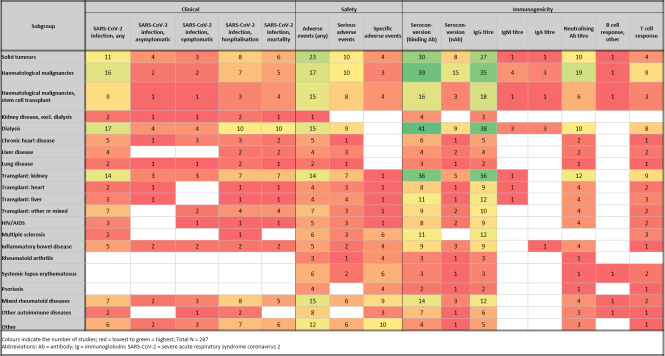
Summary of findings 2. Subgroup ‐ outcome*.
| Clinical (N studies, N population) | Safety | Immunogenicity | ||||||||||||||
| Subgroup | SARS‐CoV‐2 infection, any | SARS‐CoV‐2 infection, asymptomatic | SARS‐CoV‐2 infection, symptomatic | SARS‐CoV‐2 infection, hospitalisation | SARS‐CoV‐2 infection, mortality | Adverse events (any) | Serious adverse events | Specific adverse events | Seroconversion (binding Ab) | Seroconversion (neutralising Ab) | IgG titre | IgM titre | IgA titre | Neutralising Ab titre | B‐cell response, other | T‐cell response |
| Solid tumours | 11 (167,485) | 4 (1003) | 3 (96,584) | 8 (174,587) | 6 (166,084) | 23 (5645) | 10 (2227) | 4 (1187) | 30 (7851) | 8 (1466) | 27 (5778) | 1 (141) | 1 (141) | 10 (1409) | 1 (136) | 4 (807) |
| Haematological malignancies | 16 (37,390) | 2 (320) | 2 (32,294) | 7 (38,206) | 5 (35,278) | 17 (3154) | 10 (2125) | 3 (226) | 39 (8133) | 15 (2553) | 35 (6669) | 4 (736) | 3 (576) | 19 (3401) | 1 (123) | 9 (1459) |
| Haematological malignancies, stem cell transplant | 9 (1379) | 1 (113) | 1 (113) | 3 (437) | 4 (457) | 15 (1818) | 8 (1287) | 4 (461) | 16 (1863) | 3 (392) | 18 (2261) | 1 (23) | 1 (23) | 6 (699) | 1 (20) | 3 (253) |
| Kidney disease, excluding dialysis | 2 (41,759) | 1 (162) | 1 (162) | 2 (41,759) | 1 (41,597) | 1 (162) | 0 (0) | 0 (0) | 4 (144) | 0 (0) | 3 (122) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dialysis | 17 (179,469) | 4 (5321) | 4 (5622) | 10 (166,727) | 10 (167,721) | 15 (6403) | 9 (3473) | 0 (0) | 41 (181,208) | 9 (5106) | 38 (21,892) | 3 (452) | 3 (381) | 10 (9869) | 0 (0) | 8 (4653) |
| Chronic heart disease | 5 (72,841) | 1 (615) | 3 (71,361) | 3 (72,549) | 2 (71,934) | 5 (1106) | 1 (86) | 0 (0) | 6 (598) | 1 (262) | 5 (577) | 0 (0) | 0 (0) | 2 (283) | 0 (0) | 1 (262) |
| Liver disease | 4 (40,275) | 0 (0) | 0 (0) | 2 (40,174) | 2 (40,174) | 4 (574) | 3 (562) | 0 (0) | 4 (241) | 2 (393) | 4 (241) | 0 (0) | 0 (0) | 2 (393) | 0 (0) | 2 (60) |
| Lung disease | 2 (1939) | 1 (1893) | 1 (1893) | 2 (1997) | 1 (104) | 2 (1939) | 1 (16) | 0 (0) | 3 (380) | 1 (46) | 2 (62) | 0 (0) | 0 (0) | 1 (46) | 0 (0) | 1 (46) |
| Transplant: kidney | 14 (7922) | 3 (562) | 3 (945) | 7 (5579) | 7 (5703) | 14 (1919) | 7 (746) | 1 (609) | 36 (6815) | 5 (802) | 36 (7492) | 1 (148) | 0 (0) | 12 (2400) | 0 (0) | 9 (2786) |
| Transplant: heart | 2 (150) | 1 (23) | 0 (0) | 1 (23) | 1 (23) | 4 (253) | 3 (237) | 1 (46) | 8 (474) | 1 (16) | 9 (540) | 1 (46) | 0 (0) | 4 (207) | 0 (0) | 2 (62) |
| Transplant: liver | 3 (184) | 1 (56) | 0 (0) | 1 (56) | 1 (56) | 4 (165) | 4 (166) | 1 (58) | 11 (765) | 1 (11) | 12 (843) | 1 (58) | 0 (0) | 4 (158) | 0 (0) | 3 (207) |
| Transplant: other or mixed | 7 (51,842) | 0 (0) | 2 (2708) | 4 (4799) | 4 (51,596) | 7 (1296) | 3 (197) | 1 (89) | 9 (1652) | 2 (171) | 10 (2452) | 0 (0) | 0 (0) | 4 (257) | 0 (0) | 2 (75) |
| HIV/AIDS | 3 (2197) | 0 (0) | 1 (4) | 1 (2103) | 1 (2103) | 5 (497) | 3 (350) | 1 (90) | 8 (740) | 2 (243) | 9 (1405) | 0 (0) | 0 (0) | 4 (436) | 0 (0) | 2 (243) |
| Multiple sclerosis | 2 (1308) | 0 (0) | 0 (0) | 1 (753) | 0 (0) | 6 (2256) | 3 (1332) | 6 (2256) | 11 (3380) | 0 (0) | 12 (3510) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (603) |
| Inflammatory bowel disease | 5 (36,955) | 2 (4483) | 2 (4483) | 2 (26,910) | 2 (26,910) | 5 (6720) | 2 (424) | 4 (6474) | 9 (7397) | 3 (369) | 9 (7397) | 0 (0) | 1 (58) | 4 (805) | 0 (0) | 1 (4047) |
| Rheumatoid arthritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (752) | 1 (189) | 4 (6245) | 3 (458) | 1 (129) | 3 (458) | 0 (0) | 0 (0) | 1 (129) | 0 (0) | 0 (0) |
| Systemic lupus erythematosus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (1742) | 2 (149) | 6 (1742) | 3 (168) | 1 (126) | 3 (168) | 0 (0) | 0 (0) | 1 (126) | 1 (126) | 2 (145) |
| Psoriasis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (1401) | 0 (0) | 4 (1401) | 2 (152) | 1 (101) | 2 (152) | 0 (0) | 0 (0) | 1 (101) | 0 (0) | 1 (101) |
| Mixed rheumatoid diseases | 7 (37,940) | 2 (1034) | 3 (1944) | 8 (42,916) | 5 (36,757) | 15 (10,613) | 6 (2587) | 9 (7384) | 14 (5416) | 3 (1678) | 12 (5208) | 0 (0) | 0 (0) | 4 (1801) | 1 (82) | 2 (182) |
| Other autoimmune diseases | 2 (79) | 0 (0) | 1 (26) | 2 (6433) | 0 (0) | 8 (1628) | 0 (0) | 3 (1125) | 7 (519) | 1 (53) | 6 (493) | 0 (0) | 0 (0) | 3 (346) | 1 (133) | 1 (17) |
| Other | 6 (131,575) | 2 (10,756) | 3 (38,578) | 7 (144,720) | 6 (142,283) | 12 (17,855) | 6 (13,905) | 10 (3318) | 4 (441) | 1 (284) | 5 (649) | 0 (0) | 0 (0) | 3 (431) | 0 (0) | 1 (24) |
| Abbreviations: Ab: antibody; Ig: immunoglobulin, SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | ||||||||||||||||
| An interactive evidence gap map is available at egmopenaccess.3ieimpact.org/evidence-maps/scoping-review-covid-19-vaccines-duplicated-subgroups (studies are represented as intervention ‐ outcome table, but can be filtered according to subgroup due to duplication of subgroups with multiple patient groups; multicountry studies cannot be filtered; the labelling g + number are artefacts from duplicating a study for representing the subgroups and can be ignored). | ||||||||||||||||
*The overall number of studies was 287, excluding studies on pregnant and lactating women due to a different outcome set.
Summary of findings 3. Pregnancy studies only; intervention ‐ outcome*.
| Clinical | Safety | Birth outcomes | Immunogenicity | ||||||||||||
| Intervention | SARS‐CoV‐2 infection, any | Adverse events (any) | Serious adverse events | Abortion | Stillbirth | Preterm birth | Small size for gestational age | Congenital anomaly | Neonate mortality | IgG titre or seroconversion | IgA titre | IgM titre | Neutralising Ab seroconversion or titre | Cord blood Ab measurements | Other outcomes |
| BNT162b2 | 10 | 10 | 3 | 10 | 9 | 11 | 8 | 4 | 5 | 9 | 2 | 5 | 2 | 7 | 16 |
| mRNA‐1273 | 7 | 5 | 2 | 7 | 6 | 5 | 4 | 3 | 4 | 5 | 2 | 3 | 1 | 4 | 10 |
| AZD1222 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| Ad26.COV2.S | 2 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 1 | 3 | 1 | 1 | 0 | 2 | 6 |
| Sputnik V | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sputnik L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BBIBPCorV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CoronaVac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BNT162b2/AZD1222 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BNT162b2/mRNA‐1273 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other heterologous schemes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abbreviations: Ab: antibody; Ig: immunoglobulin, SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | |||||||||||||||
*Pregnancy studies only, N = 26.
2.
Heat map: intervention ‐ outcome.
3.
Heat map: subgroup ‐ outcome.
Study characteristics
Of the 318 included studies, 181 were published as full text, 110 as letters to the editor, research letters, or short communication, and 27 as preprints. The majority of the included studies were prospective cohort studies (N = 203). We also included 47 retrospective cohort studies, 32 cross‐sectional studies, 15 studies based on registry or surveillance system data, 8 case‐control studies, 7 randomised controlled trials, 5 mixed cohorts, and 1 diagnostic test accuracy study. The majority of studies were conducted in a single centre (N = 165); 146 were multicentre studies, and 7 studies did not report the number of centres (Table 5).
2. Characteristics of identified studies: general information.
| Characteristic | Number of studies | |
| Overall | 318 | |
| Publication format | ||
| Full text | 181 | |
| Letter to the editor/research letter | 110 | |
| Preprint | 27 | |
| Study design | ||
| Randomised controlled trial | 7 | |
| Prospective cohort study | 203 | |
| Retrospective cohort study | 47 | |
| Registry or surveillance system | 15 | |
| Cross‐sectional study | 32 | |
| Case‐control study | 8 | |
| Diagnostic test accuracy study | 1 | |
| Mixed | 5 | |
| Centres | ||
| Single centre | 165 | |
| Multiple centres | 146 | |
| Not reported | 7 | |
Population
Across studies, participants were enrolled between 30 January 2020 and 31 October 2021. The early enrolment start date can be explained by the fact that although vaccination was not yet available at the beginning of the pandemic, the identified studies published data on ongoing cohorts that started in early 2020. Participants were recruited mainly from high‐income countries (282/318), most commonly the United States (N = 81), Israel (N = 51), the United Kingdom (N = 31), France (N = 28), and Italy (N = 24); 12 studies were conducted in multiple countries. Eighty‐four studies reported that they included participants with previous SARS‐CoV‐2 infection, although this was usually a large minority of participants. Information on race or ethnicity as at least baseline characteristics was provided in 98 studies. The complete list of countries and population characteristics can be found in Table 4.
Most studies included adults only (N = 297), whilst 10 reports focused on adolescents and children. Overall, 26 studies included pregnant women, and 7 studies included breastfeeding women. Of the pregnancy and breastfeeding studies combined (N = 31), 13 studies included data on neonates in addition to parturient data (i.e. cord blood immunoglobulin values).
We identified 47 studies on participants with solid tumours and 54 studies on participants with haematological malignancies. Included in these or in addition to them, 22 studies explored vaccination in stem cell transplant recipients.
Six studies included participants with kidney disease; 48 studies included dialysis patients; and 43 studies included participants having received a kidney or combined kidney‐pancreas transplant. Nine, seven, and five studies investigated outcomes in participants with chronic heart disease, liver disease, and lung disease, respectively. Heart and liver transplant patients were investigated in 9 and 12 studies each, and 19 studies included other transplant patients or a group of mixed transplant patients without reporting subgroup data. Ten studies included participants with HIV/AIDS.
Several studies combined participants with multiple autoimmune and rheumatic diseases (N = 13 and N = 28, respectively); however, separate data were reported for multiple sclerosis in 17 studies, inflammatory bowel disease in 15 studies, rheumatoid arthritis in 5 studies, systemic lupus erythematosus in 7 studies, and psoriasis in 5 studies. Other patient populations were reported in 24 studies and included, amongst others, chronic neurological diseases in general, dementia, epilepsy, schizophrenia, mixed groups of immunosuppressed patients if not assigned to any of the above‐mentioned subgroups, myelodysplastic disorders, chronic hepatitis B infection, substance use disorders, mastocytosis, bleeding disorders, immune thrombocytopenia, spinal cord injuries, asthma reported separately, and allergies.
Studies do not add up to 318 included records, as studies commonly reported multiple participant subgroups. For a detailed description to filter the subgroups, please refer to the evidence gap map or the data extraction file at OSF.
Intervention
We identified 283 studies that included participants vaccinated with BNT162b2, of which 171 reported outcomes after one dose; 258 reported outcomes after the second dose; and 14 studies reported results after a third booster dose (Table 6). mRNA‐1273 was examined in 153 studies, of which 92 reported outcomes after the first dose; 140 studies reported outcomes after the second dose; 6 studies reported outcomes after a booster dose.
3. Characteristics of identified studies: interventions*.
| Characteristic | Intervention | Dose | Number of studies |
| Vaccine | |||
| Study included non‐vaccinated | 63 | ||
| BNT162b2 | 283 | ||
| 1 dose | 171 | ||
| 2 doses | 258 | ||
| 3 doses (booster) | 14 | ||
| mRNA‐1273 | 153 | ||
| 1 dose | 92 | ||
| 2 doses | 140 | ||
| 3 doses (booster) | 6 | ||
| AZD1222 | 66 | ||
| 1 dose | 48 | ||
| 2 doses | 52 | ||
| 3 doses (booster) | 1 | ||
| Ad26.COV2.S | 42 | ||
| 1 dose | 41 | ||
| 2 doses (booster) | 1 | ||
| 3 doses (booster) | 0 | ||
| Sputnik V | 5 | ||
| 1 dose | 3 | ||
| 2 doses | 4 | ||
| 3 doses (booster) | 0 | ||
| Sputnik Light | 0 | ||
| BBIBP‐CorV | 15 | ||
| 1 dose | 12 | ||
| 2 doses | 13 | ||
| 3 doses (booster) | 0 | ||
| CoronaVac | 14 | ||
| 1 dose | 13 | ||
| 2 doses | 13 | ||
| 3 doses (booster) | 0 | ||
| Heterologous schemes | 16 | ||
| Reporting of intervention: multiple vaccines analysed together only | 94 | ||
| 1 dose | 74 | ||
| 2 doses | 81 | ||
| 3 doses | 5 | ||
| Dose | |||
| 1 dose | 211 | ||
| 2 doses | 288 | ||
| 3 doses | 16 | ||
| 4 doses | 0 | ||
*For all vaccines except Ad26.COV2.S, one vaccine dose represents incomplete vaccination; two doses constitute full vaccination; and three or more doses are additional boosters. We extracted these data independent of the exact dosing schedule.
AZD1222 was vaccinated in 66 studies, of which 48, 52, and 1 study reported outcomes after one, two, and three doses, respectively. In 42 studies, AD26.COV2.S was given, and of these 41 studies reported outcomes after one dose (the full vaccination scheme), and one study reported outcomes after a second dose.
The remaining vaccines were examined much less frequently: BBIBP‐CorV was vaccinated in 15 studies, and CoronaVac in 14 studies. Sputnik V was applied in five studies, and no study adhering to our search frame was found for Sputnik Light. Heterologous vaccination schemes were reported in 16 studies.
Frequently, if multiple vaccines were administered in the same study, outcomes were reported only for groups who received different vaccines together (N = 94).
In total, 211 studies reported results after one dose, 288 studies after two doses, and 16 studies after three doses. No study identified up to our search date explored reported outcomes after a fourth vaccine dose in our defined subgroups.
Outcomes
Comorbidity subgroups
Out of 287 studies that included comorbidity or paediatric subgroups, 80 studies reported the occurrence of any SARS‐CoV‐2 infection (Table 7). Only 19 studies each divided these into asymptomatic and symptomatic infections. Forty‐four studies reported hospitalisation due to SARS‐CoV‐2 infection, and 36 studies reported mortality (including studies reporting that no deaths occurred).
4. Characteristics of identified studies: outcomes*.
| Outcome domain | Outcome | Number of studies* | Percentage of studies* |
| Overall | 287 | ||
| Clinical | |||
| SARS‐CoV‐2 infection, any | 80 | 27.9 | |
| SARS‐CoV‐2 infection, asymptomatic | 19 | 6.6 | |
| SARS‐CoV‐2 infection, symptomatic | 19 | 6.6 | |
| SARS‐CoV‐2 infection, hospitalisation | 44 | 15.3 | |
| SARS‐CoV‐2 infection, mortality | 36 | 12.5 | |
| Safety | |||
| Any adverse events | 126 | 43.9 | |
| Serious adverse events | 54 | 18.8 | |
| Disease‐specific adverse events | 50 | 17.4 | |
| Immunogenicity | |||
| Seroconversion, binding Ab | 179 | 62.2 | |
| Seroconversion, neutralising Ab | 49 | 17.1 | |
| IgG titres | 175 | 61.0 | |
| IgA titres | 7 | 2.4 | |
| IgM titres | 9 | 3.1 | |
| Neutralising Ab titres | 64 | 22.3 | |
| B‐cell response | 4 | 1.4 | |
| T‐cell response | 37 | 12.9 | |
| Abbreviations: Ab: antibody; Ig: immunoglobulin, SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | |||
*Excluding studies on pregnant and lactating women.
Adverse events in any form were reported in 126 studies, and serious adverse events were reported in 54 studies. Another 50 studies reported specific adverse events (e.g. disease flares for autoimmune diseases).
Immunogenicity was the most commonly reported outcome domain, with 179 studies reporting seroconversion based on binding antibody measurements and 49 studies based on neutralising antibodies.
Immunoglobulin G (IgG) titres were reported in 175 studies, whilst immunoglobulin A (IgA), immunoglobulin M (IgM), and neutralising antibodies (NAbs) were less frequent (7, 9, and 64 studies, respectively).
Other B‐cell response outcomes were reported in 4 studies, while T‐cell responses, such as interferon‐gamma measurements, were examined in 37 studies.
Studies on pregnant and breastfeeding women
Overall, 31 studies included pregnant and/or breastfeeding women (N = 26 and N = 7, respectively; see Table 8). Of the 26 studies on pregnant women, 10 reported the occurrence of SARS‐CoV‐2 infections; 10 reported adverse events; and 3 reported serious adverse events. Overall, 19 studies reported birth outcomes (abortion: 11 studies, stillbirth: 10 studies, preterm birth: 12 studies, small for gestational age: 8 studies, congenital anomalies: 4 studies, and neonate mortality: 6 studies). Immunogenicity was reported in nine studies, led by the outcome IgG titres or seroconversion in all nine studies, antibody titres in cord blood (7 studies), whilst IgM and IgA titres and NAb titres or seroconversion were reported less frequently (5, 2, and 2 studies, respectively).
5. Characteristics of identified studies on pregnancy and breastfeeding: outcomes.
| Outcome domain | Outcome | Number of studies | Percentage of studies |
| Pregnancy | 26 | ||
| Clinical | |||
| SARS‐CoV‐2 infection, any | 10 | 38.5 | |
| Safety | |||
| Any adverse events | 10 | 38.5 | |
| Serious adverse events | 3 | 11.5 | |
| Birth outcomes | |||
| Abortion | 11 | 42.3 | |
| Stillbirth | 10 | 38.5 | |
| Preterm birth | 12 | 46.2 | |
| Small for gestational age | 8 | 30.8 | |
| Congenital anomalies | 4 | 15.4 | |
| Neonate mortality | 6 | 23.1 | |
| Immunogenicity | |||
| IgG titres or seroconversion, mother | 9 | 34.6 | |
| NAb titres or seroconversion, mother | 2 | 7.7 | |
| IgM titres | 5 | 19.2 | |
| IgA titres | 2 | 7.7 | |
| Ab titres in cord blood | 7 | 26.9 | |
| Additional outcomes | 17 | 65.4 | |
| Breastfeeding | 7 | ||
| Clinical | |||
| SARS‐CoV‐2 infection, any | 1 | 14.3 | |
| Safety | |||
| Any adverse events, mother | 6 | 85.7 | |
| Any adverse events, neonate | 5 | 71.4 | |
| Immunogenicity | |||
| IgG titres or seroconversion, serum, mother | 3 | 42.9 | |
| NAb titres or seroconversion, serum, mother | 1 | 14.3 | |
| IgM titres, mother | 2 | 28.6 | |
| IgA titres, serum, mother | 3 | 42.9 | |
| B‐ or T‐cell response, mother | 0 | 0 | |
| IgG titres or seroconversion, milk | 3 | 42.9 | |
| IgA titres, milk | 3 | 42.9 | |
| Ab titres or seroconversion in serum, neonate | 0 | 0 | |
| Abbreviations: Ab: antibody; Ig: immunoglobulin, NAb: neutralising antibodies; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | |||
Excluded studies
We excluded 3235 records at full‐text stage for the following reasons:
253 records were ongoing studies that potentially include subgroups;
700 records were on studies with fewer than 100 participants;
1733 records included participants from the general population;
40 records were off‐topic, i.e. studies on immunity after infection, seroprevalence studies;
84 records assessed ineligible/unclear vaccines;
406 records were published in an ineligible format (i.e. conference abstract) or study design (e.g. cases only without baseline population);
2 records had an unclear baseline population;
17 were duplicates of included studies.
A complete list of excluded studies can be found at OSF.
Discussion
Summary of the main results
This review provides an overview of vaccination research activities on different vaccines approved in the European Union or in at least 10 countries worldwide at the time of our search 6 December 2021, and in relation to our prioritised endpoints of clinical efficacy, immunogenicity, and safety. We identified 318 eligible studies that explored at least 1 out of 8 predefined COVID‐19 vaccines in comorbidity subgroups, paediatric populations, or pregnant and breastfeeding women. The most commonly included patient populations were those with haematological malignancies, followed by patients with solid tumours, kidney disease including dialysis, and kidney transplant patients. Vaccine reaction to autoimmune diseases was commonly explored, although more often in populations with mixed diagnoses than separately. Of the autoimmune diseases, multiple sclerosis and inflammatory bowel disease were often reported separately. The majority of studies were conducted in high‐income countries (United States, Israel, Europe).
In our defined comorbidity subgroups, BNT162b2 was the most commonly used vaccine (N = 283 studies), followed by mRNA‐1273, AZD1222, and AD26.COV2.S. Results on vaccines that were not authorised by the EMA, but that were approved by at least 10 countries at the time of our search (Sputnik V and L, BBIBP‐CorV, CoronaVac), were rarely published. The majority of studies reported results after a standard vaccination scheme, which is usually after two vaccine doses (except for AD26.COV2.S).
The most commonly reported outcomes were seroconversion (62.2% of studies) and IgG titres (61.0% of studies) based on binding antibody titres in serum, followed by reporting of adverse events in 43.9% of studies. Any SARS‐CoV‐2 infection was reported in 27.9% of studies. Neutralising antibody seroconversion and titres were reported in 17.1% and 22.3% of studies, respectively.
To summarise, most of the identified evidence was for mRNA vaccines, whilst data for vector‐ and protein‐based vaccines, as well as vaccines based on inactivated virus, remain incomplete in relation to our endpoints and for the subgroups selected for this review. Evidence regarding heterologous vaccination schemes also remains very deficient.
We found that significantly more studies focused on the immunological effects and safety aspects of the vaccines than on their clinical efficacy, that is reduction in infections in general and severe infections with hospitalisation. With regard to immunogenicity, the humoural IgG response was examined most intensively, less frequently the capacity of the antibodies to neutralise the SARS‐CoV‐2 virus. Data on T‐cell immune responses were much more limited, whilst evidence on other B‐cell parameters is largely deficient.
Overall completeness and applicability of evidence
Due to the extreme output of COVID‐19‐related research, in particular vaccine research that is not comparable with any other topic in medical research before, it is extremely difficult to stay up‐to‐date with peer‐reviewed as well as pre‐printed manuscripts included in this scoping review, as evidence is growing exponentially.
A concern regarding this quick expansion of the literature, and the SARS‐CoV‐2 situation itself, is the change of research focus with any new variant of concern that shows different virological and clinical characteristics. Vaccines developed for one variant might have only limited efficacy for another variant, especially due to mutations in the targeted spike protein, which differs significantly between variants. Due to the time limit of the search in December 2021, this scoping review lacks studies that have been carried out on the currently predominant Omicron variant of concern. In addition, since our search, a new vaccine, Novavax, was approved by the EMA in December 2021, which is not included in this review (EMA 2021a; EMA 2021b). This limits overall completeness as well as applicability to a certain extent.
Due to the vast amount of studies presenting SARS‐CoV‐2 vaccination data, we had to limit ourselves in several ways. Foremost, we chose eight vaccines as feasible choices for efficient data collection. However, data on several vaccines administered to significant population sizes (e.g. Covaxin (BBV152) in India or Convidecia (Ad5‐nCoV) primarily in China) and possibly heterologous application schemes are thus missing. Likewise, whilst we listed children and adolescents amongst our vulnerability subgroups separately ‐ a status based foremost on general medical terms rather than COVID‐19 disease load itself ‐ we refrained from doing so for other subgroups of age because subgroups of elderly are often reported in publications on the general population, which we could have missed out on if not presented in the abstract, resulting in an incomplete account of this group. In particular the representation of studies including elderly, which have been threatened by high mortality rates from COVID‐19 and ‐ at least theoretically ‐ may be at risk for a weaker immune response to vaccination due to age (Brockmann 2022; Collier 2021) would have been highly important. Furthermore, we neglected the types of treatment (including immunosuppressive regimens); different treatments are associated with different seroconversion rates and antibody titres (Ligumsky 2022), and thus may have a significant impact on the immune response. A reduced immune response may therefore not only be a consequence of the underlying disease, but may also be related to the complex confounder of drug treatments. Finally, including studies of at least 100 participants of a particular subgroup was chosen as an arbitrary lower study‐size limit to ensure the practicability of data extraction as well as the robustness of findings. Likewise, we are aware that as a result studies on particularly rare diseases and thus smaller achievable subgroups (e.g. subgroups of rare inborn errors of immunity, subgroups on patients on specific biological therapeutics) may be underrepresented in this review. Similarly, this inclusion criterion also led to the exclusion of the first studies reporting on results of four vaccinations in subgroups as well as studies reporting on extensive or elaborate immunological diagnostics with small sample sizes.
Nevertheless, this scoping review might serve as an overview of the available evidence for the clinician who is as overwhelmed by the literature as the researcher trying to scope the evidence. By concentrating on patients with an expected reduced vaccination response due to the underlying disease or drug‐based immunosuppression, we aimed to help clinicians achieve an overview of which studies exist for guidance and for researchers identifying knowledge gaps. This scoping review might help to stimulate research closing these knowledge gaps, which is clinically extremely important to avoid severe disease courses.
Potential biases in the review process
One of the potential sources of bias in the review process is that although we published a protocol before commencing this scoping review, due to the large number of studies identified by our search, we had to change our eligibility criteria from a very broad scope to a somewhat more limited scope (see Differences between protocol and review). Abstracts that included the general population, but that reported on subgroups only within the main body of their publication, may have been excluded on the level of title and abstract screening as a result of our deviations.
Due to heterogeneity in data extraction, we also reduced the number of items to extract per study to reduce workload. All changes were discussed in weekly sessions with all present review authors and communicated via e‐mail to those who were absent.
This review was conducted by a large group of authors, which may have introduced heterogeneity in screening and data extraction. The completed data extraction sheet was therefore randomly checked for consistency between extractions by one review author.
We identified no other potential sources of bias.
Implications for a subsequent effectiveness review
This scoping review identified areas with insufficient evidence, and thereby crucially informs future systematic reviews. With the help of this scoping review, reasonable comparisons and outcome measures have been identified and have been transferred to two resulting systematic reviews, which accelerated the time‐critical process of conducting a systematic review in the rapidly evolving research landscape of SARS‐CoV‐2 vaccination. These systematic reviews focus on the overall response to vaccines in haematological malignancies, Piechotta 2022, and kidney transplant recipients (Hausinger 2022), the latter also including the most recent EMA‐approved vaccine, Novavax. An interesting question is whether those individuals who did not seroconvert after the standard scheme (usually two doses) will seroconvert after one or more booster doses; another important point to consider is that although a lot of focus lies on immune parameters, these do not always translate into clinical improvements. Other systematic reviews on immunocompromised groups are constantly emerging (e.g. Lee 2022), thus before commencing additional systematic reviews, the search used to identify systematic reviews could be updated.
Authors' conclusions
The majority of evidence on our predefined subgroups up to 6 December 2021 reported immunogenicity surrogate parameters or adverse events after mRNA vaccines administered as standard scheme. Clinical efficacy outcomes were less commonly reported, and if so, often as a secondary outcome observed in seroconversion or immunoglobulin titre studies, with a similar follow‐up time. There was considerably more data for European Medicines Agency‐approved vaccines than the additionally defined vaccines approved in more than 10 countries, or heterologous vaccination schemes.
Overall, based on insights from this scoping review, we defined two follow‐up review questions in response to data availability, one on the effectiveness and safety of COVID‐19 vaccines in haematological malignancies, and the other in kidney transplant recipients.
Acknowledgements
We would first like to thank Kathrin Grummich (Institute for Evidence in Medicine, Faculty of Medicine and Medical Center, University of Freiburg & Cochrane Germany, Cochrane Germany Foundation, Freiburg, Germany) for a quality check of our search strategy. In addition, we thank the International Initiative for Impact Evaluation (3ie) for providing access and support for their software EviAtlas.
Cochrane Haematology supported the authors in the development of this scoping review. Caroline Hirsch, Nina Kreuzberger, Ina Monsef, and Nicole Skoetz are members of Cochrane Haematology but were not involved in the editorial process or decision‐making for this review. The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Harald Herkner, Medical University of Vienna, Austria; Coordinating Editor of the Cochrane Emergency and Critical Care Group
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support
Peer reviewers (provided comments and recommended an editorial decision): Alexandre R Marra, Hospital Israelita Albert Einstein, Sao Paulo, SP, Brazil; the University of Iowa Hospitals and Clinics, Iowa City, IA, USA (clinical review); Abhijit Dutta, Maynaguri RH, Department of Health & Family Welfare (Govt. of West Bengal), India (consumer review); Robert Walton, Cochrane UK (summary versions review); Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review); Robin Featherstone, Cochrane Central Editorial Service (search review). Two additional peer reviewers provided clinical peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. PRISMA‐ScR checklist
| Recommended section | Item | PRISMA‐ScR checklist item | Reported | Section |
| TITLE | ||||
| Title | 1 | Identify the report as a scoping review. | yes | Title |
| ABSTRACT | ||||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | yes | Abstract |
| INTRODUCTION | ||||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | yes | Background ‐ Rationale for conducting a scoping review |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g. population or participants, concepts, and context) or other relevant key elements used to conceptualise the review questions and/or objectives. | yes | Objectives |
| METHODS | ||||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | yes | Methods |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g. years considered, language, and publication status), and provide a rationale. | yes | Methods |
| Information sources | 7 | Describe all information sources in the search (e.g. databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | yes | Methods and appendices |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | yes | Appendices |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e. screening and eligibility) included in the scoping review. | yes | Methods |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g. calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | yes | Methods |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | Yes | Methods |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | NA | ‐ |
| Synthesis of results | 13 | Describe the methods of handling and summarising the data that were charted. | yes | Methods |
| RESULTS | ||||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | yes | Methods, Figure 1 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | yes | Online data maps (egmopenaccess.3ieimpact.org/evidence-maps/scoping-review-covid-19-vaccines; egmopenaccess.3ieimpact.org/evidence-maps/scoping-review-covid-19-vaccines-duplicated-subgroups) and at OSF (osf.io/qmcgv/) |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | Not done | ‐ |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | partly | Online at OSF (osf.io/qmcgv/) |
| Synthesis of results | 18 | Summarise and/or present the charting results as they relate to the review questions and objectives. | yes | Results, table 1, table 2, table 3, table 4 |
| DISCUSSION | ||||
| Summary of evidence | 19 | Summarise the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | yes | Discussion ‐ Summary of the main results |
| Limitations | 20 | Discuss the limitations of the scoping review process. | yes | Discussion |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | yes | Discussion, Authors' conclusions |
| FUNDING | ||||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | no/yes | Sources of funding of included studies not described; source of funding for scoping review reported |
| See Tricco 2018 | ||||
Appendix 2. Search strategies for systematic reviews, up to 25 August 2021
1.Evidence Aid Coronavirus (Covid-19)
searched and screened: vaccin, mRNA, BNT162b2, Astrazeneca, AZD, JNJ, Chad, Ad26, moderna, Pfizer, BioNTech, Covishield, Janssen, Johnsons, rAd5, rAd26, Sputnik, BBiBP, sinopharm, CoronaVac, vero cell or TAK‐919 or boost and screened (24.08.2021)
2.Usher Network for COVID-19 Evidence Reviews
searched and screened: vaccin, mRNA, BNT162b2, Astrazeneca, AZD, JNJ, Chad, Ad26, moderna, Pfizer, BioNTech, Covishield, Janssen, Johnsons, rAd5, rAd26, Sputnik, BBiBP, sinopharm, CoronaVac, vero cell or TAK‐919 or boost and screened (24.08.2021)
3.U.S. Veterans’ Affairs (VA) Evidence Synthesis Program
searched and screened: vaccin, mRNA, BNT162b2, Astrazeneca, AZD, JNJ, Chad, Ad26, moderna, Pfizer, BioNTech, Covishield, Janssen, Johnsons, rAd5, rAd26, Sputnik, BBiBP, sinopharm, CoronaVac, vero cell or TAK‐919 or boost and screened (24.08.2021)
4.L*OVE
Searched by PICO (24.08.2021)
Public health SARS‐CoV‐2 vaccines RNA vaccines: BNT162b1; Pfizer‐BioNTech COVID‐19 vaccine or moderna COVID‐19 vaccine
Public health SARS‐CoV‐2 vaccines vector vaccines: Janssen AD26.COV.S, Oxford‐AstraZeneca COVID‐19 vaccine, Sputnik V COVID‐19 vaccine
Public health SARS‐CoV‐2 vaccines inactivated vaccines: CoronaVac, BBIBP‐CorV, SARS‐CoV‐2 vaccine (vero cells)
5. MEDLINE (via Ovid) (searched 25.08.2021)
# Searches
1 COVID‐19 Vaccines/
2 (vaccin* adj3 (COVID* or COVID‐19* or COVID19* or "SARS‐CoV‐2" or "SARS‐CoV2" or SARSCoV2 or "SARSCoV‐2" or "coronavirus disease 2019" or nCoV‐19)).tw,kf.
3 COVID‐19/ or SARS‐CoV‐2/
4 (COVID* or COVID‐19* or COVID19* or "SARS‐CoV‐2" or "SARS‐CoV2" or SARSCoV2 or "SARSCoV‐2" or "coronavirus disease 2019" or nCoV‐19).tw,kf.
5 or/3‐4
6 (vaccin* adj1 (respons* or candidate*)).tw. or boost*.tw. or (vaccin* adj1 therap*).ti.
7 (vaxzevria* or AZD1222* or AZD‐1222 or covishield* or ChAdOx1*).af. or AstraZeneca.tw,kf. or (oxford adj2 vaccin*).tw,kf.
8 (biontech* or pfizer*).tw,kf. or (comirnaty* or BNT162b2* or BNT‐162b2* or tozinameran*).af.
9 (moderna* or spikevax* or mRNA‐1273* or mRNA1273* or TAK‐919* or TAK919* or modernatx*).af.
10 (JNJ‐78436735* or JNJ78346735* or "Ad26.COV2.S*" or Ad26COVS1).af. or ("Johnsons&Johnson" or Janssen).tw,kf.
11 ("BBIBP‐CorV*" or BBIBPCorV* or sinopharm* or "sino‐pharm*").af.
12 (coronaVac* or "corona‐Vac*" or sinovac* or "sino‐vac*" or PiCoVacc* or "PiCo‐Vacc*" or "vero adj1 cell?").af.
13 (gamaleya* or "Gam‐COVID‐Vac*" or rAd26* or "recombinant adenovirus type 26 vector" or rAd5* or "recombinant adenovirus type 5 vector" or "adenoviral vector5").af. or sputnik*.tw,kf.
14 or/6‐13
15 1 or 2 or (5 and 14)
16 cochrane database of systematic reviews.jn. or search*.tw. or meta analysis.pt. or medline.tw. or systematic review.tw. or systematic review.pt. [17. Wong 2006 – systematic reviews filter –modified by adding systematic review.pt]
17 15 and 16
Appendix 3. Search strategies for primary literature, up to 6 December 2021
1. CCSR – Cochrane COVID‐19 Study Register
vaccin* or biontech* or pfizer* or corminaty* or comirnaty* or BNT162* or "BNT 162" or "bnt 162b2" or tozinameran* or moderna* or spikevax* or 1273* or mRNA1273* or "TAK‐919" or "CX‐024414" or CX024414* or astrazeneca* or vaxzevria* or AZD1222* or covishield* or ChAdOx* or Janssen* or "JNJ‐78436735" or JNJ78436735* or VAC31518* or "VAC‐31518" or "Johnson COVID‐19" or "Johnson COVID19" or Ad26* or Ad5* or Sputnik* or rAd26* or rAd5* or gamaleya* or "Gam‐COVID‐Vac" or "recombinant adenovirus type" or "adenovirus vector" or "combined vector" or BBIBP* or sinopharm* or sinovac* or PiCoVac* or Coronavac* or "heterologous boost" or "heterologous booster" or "homologous boost" or "homologous booster" or "post‐boost" or "post‐booster" or "boost schedule" or "boost schedules" or "bost dose" or "booster dose" or "after boost" or "after booster" or "variant boost" or "variant booster" or "nonvariant boost" or "nonvariant booster" or "non‐variant boost" or "non‐variant booster" or "delayed boost" or "delayed booster" or boosted or "prime boost" or "prime booster" or "boost regimen" or "three dosis" or "three doses" or "third dosis" or "third doses" or "third dose"
filter according to:
Study Characteristics: Study Design ‐ Case Series/Case Control/Cohort ‐ Cross‐sectional ‐ Parallel/Crossover ‐ Other ‐ Time series ‐ Unclear
Study Characteristics: Intervention Assignment ‐ Randomised ‐ Quasi‐Randomised ‐ Unclear
2. Web of Science
#1 search string Covid
(TI=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")) OR AB=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#2 search string vaccine ‐ vaccine names
(TI=(biontech* or pfizer* or corminaty* or comirnaty* or BNT162* or "BNT 162" or "bnt 162b2" or tozinameran* or moderna* or spikevax* or 1273* or mRNA1273* or "TAK‐919" or "CX‐024414" or CX024414* or astrazeneca* or vaxzevria* or AZD1222* or covishield* or ChAdOx* or Janssen or "JNJ‐78436735" or JNJ78436735* or "Johnson COVID‐19" or "Johnson COVID19" or Ad26* or Ad5* or VAC31518* or Sputnik* or rAd26* or rAd5* or gamaleya* or "Gam‐COVID‐Vac" or "recombinant adenovirus type" or "adenovirus vector" or "combined vector" or BBIBP* or sinopharm* or sinovac* or PiCoVac* or Coronavac*)) OR AB=(biontech* or pfizer* or corminaty* or comirnaty* or BNT162* or "BNT 162" or "bnt 162b2" or tozinameran* or moderna* or spikevax* or 1273* or mRNA1273* or "TAK‐919" or "CX‐024414" or CX024414* or astrazeneca* or vaxzevria* or AZD1222* or covishield* or ChAdOx* or Janssen or "JNJ‐78436735" or JNJ78436735* or "Johnson COVID‐19" or "Johnson COVID19" or Ad26* or Ad5* or VAC31518* or Sputnik* or rAd26* or rAd5* or gamaleya* or "Gam‐COVID‐Vac" or "recombinant adenovirus type" or "adenovirus vector" or "combined vector" or BBIBP* or sinopharm* or sinovac* or PiCoVac* or Coronavac*)
#3 search string booster
(TI=(boost*)) OR AB=(boost*)
#4 search string "third dose"
(TI=("three dosis" or "three doses" or "third dosis" or "third doses" or "third dose")) OR AB=("three dosis" or "three doses" or "third dosis" or "third doses" or "third dose")
#5 search string SARS‐CoV‐2 vaccine
(TI=((vaccin* NEAR/5 (COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")))) OR AB=((vaccin* NEAR/5 (COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")))
#6 search string combining COVID with vaccine names, booster and third dose
#1 AND (#2 OR #3 OR #5)
#7 search string SARS‐Cov‐2 vaccine (general) and COVID with vaccine names booster and third dose
#5 OR #6
3. WHO COVID‐19 Global literature
search 1
biontech* or pfizer* or corminaty* or comirnaty* or BNT162* or "BNT 162" or "bnt 162b2" or tozinameran* or moderna* or spikevax* or 1273* or mRNA1273* or "TAK‐919" or "CX‐024414" or CX024414* or astrazeneca* or vaxzevria* or AZD1222* or covishield* or ChAdOx* or Janssen* or "JNJ‐78436735" or JNJ78436735* or VAC31518* or "VAC‐31518" or "Johnson COVID‐19" or "Johnson COVID19" or Ad26* or Ad5* or Sputnik* or rAd26* or rAd5* or gamaleya* or "Gam‐COVID‐Vac" or "recombinant adenovirus type" or "adenovirus vector" or "combined vector" or BBIBP* or sinopharm* or sinovac* or PiCoVac* or Coronavac* or "heterologous boost" or "heterologous booster" or "homologous boost" or "homologous booster" or "post‐boost" or "post‐booster" or "boost schedule" or "boost schedules" or "bost dose" or "booster dose" or "after boost" or "after booster" or "variant boost" or "variant booster" or "nonvariant boost" or "nonvariant booster" or "non‐variant boost" or "non‐variant booster" or "delayed boost" or "delayed booster" or boosted or "prime boost" or "prime booster" or "boost regimen" or "three dosis" or "three doses" or "third dosis" or "third doses" or "third dose"
search 2
Vaccin* AND COVID or COVID19 or "SARS‐CoV‐2" or "SARS‐CoV2" or SARSCoV2 or "SARSCoV‐2" or "SARS coronavirus 2" or "2019 nCoV" or "2019nCoV" or "2019‐novel CoV" or "nCov 2019" or "nCov 19" or "severe acute respiratory syndrome coronavirus 2" or "novel coronavirus disease" or "novel corona virus disease" or "corona virus disease 2019" or "coronavirus disease 2019" or "novel coronavirus pneumonia" or "novel corona virus pneumonia" or "severe acute respiratory syndrome coronavirus 2"
Appendix 4. References of included studies
1. Studies on pregnant and breastfeeding women
1. Atyeo CG, Shook LL, Brigida S, De Guzman RM, Demidkin S, Muir C, et al. Maternal immune response and placental antibody transfer after COVID‐19 vaccination across trimester and platforms. medRxiv. 2021:2021.11.12.21266273. 2. Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa‐Cohen Y, et al. Efficient maternal to neonatal transfer of antibodies against SARS‐CoV‐2 and BNT162b2 mRNA COVID‐19 vaccine. Journal of Clinical Investigation. 2021;131(13). 3. Bertrand K, Honerkamp‐Smith G, Chambers CD. Maternal and Child Outcomes Reported by Breastfeeding Women Following Messenger RNA COVID‐19 Vaccination. Breastfeeding Medicine. 2021;16(9):697‐701. 4. Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID‐19 vaccination during pregnancy: coverage and safety. American Journal of Obstetrics and Gynecology. 2022;226(2):236.e1‐.e14. 5. Bleicher I, Kadour‐Peero E, Sagi‐Dain L, Sagi S. Early exploration of COVID‐19 vaccination safety and effectiveness during pregnancy: interim descriptive data from a prospective observational study. Vaccine. 2021;39(44):6535‐8. 6. Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben‐David A, et al. Short‐term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID‐19 vaccine. Ultrasound in Obstetrics & Gynecology. 2021;58(3):450‐6. 7. Butt AA, Chemaitelly H, Al Khal A, Coyle PV, Saleh H, Kaleeckal AH, et al. SARS‐CoV‐2 vaccine effectiveness in preventing confirmed infection in pregnant women. Journal of Clinical Investigation. 2021;131(23). 8. Dagan N, Barda N, Biron‐Shental T, Makov‐Assif M, Key C, Kohane IS, et al. Effectiveness of the BNT162b2 mRNA COVID‐19 vaccine in pregnancy. Nat Med. 2021;27(10):1693‐5. 9. Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS‐CoV‐2 infection in pregnant women. JAMA. 2021;326(8):728‐35. 10. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. American Journal of Obstetrics and Gynecology. 2021;225(3):303.e1‐.e17. 11. Hillson K, Clemens SC, Madhi SA, Voysey M, Pollard AJ, Minassian AM. Fertility rates and birth outcomes after ChAdOx1 nCoV‐19 (AZD1222) vaccination. Lancet. 2021;398(10312):1683‐4. 12. Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short‐term reactions among pregnant and lactating individuals in the first wave of the COVID‐19 vaccine rollout. JAMA Network Open. 2021;4(8). 13. Kharbanda EO, Haapala J, DeSilva M, Vazquez‐Benitez G, Vesco KK, Naleway AL, et al. Spontaneous abortion following COVID‐19 vaccination during pregnancy. JAMA. 2021. 14. Lechosa‐Muniz C, Paz‐Zulueta M, Mendez‐Legaza JM, Irure‐Ventura J, Gonzalez RC, Montes JC, et al. Induction of SARS‐CoV‐2‐Specific IgG and IgA in serum and milk with different SARS‐CoV‐2 vaccines in breastfeeding women: a cross‐sectional study in northern Spain. International Journal of Environmental Research and Public Health. 2021;18(16). 15. Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid‐19 vaccination during pregnancy and first‐trimester miscarriage. New England Journal of Medicine. 2021;385(21):2008‐10. 16. McLaurin‐Jiang S, Garner CD, Krutsch K, Hale TW. Maternal and Child Symptoms Following COVID‐19 Vaccination Among Breastfeeding Mothers. Breastfeeding Medicine. 2021;16(9):702‐9. 17. Morgan JA, Biggio JR, Jr., Martin JK, Mussarat N, Chawla HK, Puri P, et al. Maternal outcomes after Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) infection in vaccinated compared with unvaccinated pregnant patients. Obstetrics and gynecology. 2022;139(1):107‐9. 18. Morze K, Kotlińska A, Ura‐Polak S, Brojanowska‐Aleksandrowicz A. COVID‐19 vaccination outcomes among breastfeeding woman and their children – a pharmacovigilance survey research (preprint). Lancet Preprints. 2021. 19. Plitman Mayo R, Raz T, David BB, Meir G, Barr H, Solmesky LJ, et al. Waning of the humoral response to SARS‐CoV‐2 in pregnancy is variant‐dependent. medRxiv. 2021:2021.11.03.21265478. 20. Prabhu M, Murphy EA, Sukhu AC, Yee J, Singh S, Eng D, et al. Antibody response to Coronavirus Disease 2019 (COVID‐19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstetrics and gynecology. 2021;138(2):278‐80. 21. Romero Ramírez DS, Lara Pérez MM, Carretero Pérez M, Suárez Hernández MI, Martín Pulido S, Pera Villacampa L, et al. SARS‐CoV‐2 antibodies in breast milk after vaccination. Pediatrics. 2021;148(5). 22. Rottenstreich A, Zarbiv G, Oiknine‐Djian E, Vorontsov O, Zigron R, Kleinstern G, et al. Timing of SARS‐CoV‐2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clinical Microbiology and Infection. 2021. 23. Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener‐Well Y, Grisaru‐Granovsky S. Covid‐19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG: an international journal of obstetrics and gynaecology. 2022;129(2):248‐55. 24. Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) vaccination in pregnancy: Measures of immunity and placental histopathology. Obstetrics and Gynecology. 2021;138(2):281‐3. 25. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary Findings of mRNA Covid‐19 Vaccine Safety in Pregnant Persons. The New England journal of medicine. 2021;384(24):2273‐82. 26. Shook LL, Atyeo CG, Yonker LM, Fasano A, Gray KJ, Alter G, et al. Durability of anti‐Spike antibodies in the infant after maternal COVID‐19 vaccination. medRxiv. 2021:2021.11.17.21266415. 27. Stock SJ, Carruthers J, Calvert C, Denny C, Donaghy J, Goulding A, et al. SARS‐CoV‐2 infection and COVID‐19 vaccination rates in pregnant women in Scotland. Nature Medicine. 2022. 28. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS‐CoV‐2 vaccination in pregnancy. American Journal of Obstetrics & Gynecology MFM. 2021;3(6):100467. 29. Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID‐19 vaccination in pregnancy: early experience from a single institution. American Journal of Obstetrics & Gynecology MFM. 2021;3(6):100464. 30. Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID‐19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037‐40. 31. Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, et al. Receipt of mRNA Covid‐19 vaccines and risk of spontaneous abortion. New England Journal of Medicine. 2021.
2. Studies including patients with solid cancers or solid cancers and haematological malignancies
1. Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, et al. Immunogenicity of SARS‐CoV‐2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091‐8.e2. 2. Advani P, Chumsri S, Pai T, Li Z, Sharma A, Parent E. Temporal metabolic response to mRNA COVID‐19 vaccinations in oncology patients. Annals of Nuclear Medicine. 2021. 3. Agbarya A, Sarel I, Ziv‐Baran T, Agranat S, Schwartz O, Shai A, et al. Efficacy of the mRNA‐based BNT162b2 COVID‐19 vaccine in patients with solid malignancies treated with anti‐neoplastic drugs. Cancers (Basel). 2021;13(16):4191. 4. Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat‐Panah D, Dehghani M, Nabavifard M, et al. Immunogenicity and safety of the inactivated SARS‐CoV‐2 vaccine (BBIBP‐CorV) in patients with malignancy. Cancer Investigation. 2022;40(1):26‐34. 5. Baltas I, Boshier FAT, Williams CA, Bayzid N, Cotic M, Guerra‐Assunção JA, et al. Post‐vaccination COVID‐19: A case‐control study and genomic analysis of 119 breakthrough infections in partially vaccinated individuals. Clinical Infectious Diseases. 2021. 6. Barrière J, Chamorey E, Adjtoutah Z, Castelnau O, Mahamat A, Marco S, et al. Impaired immunogenicity of BNT162b2 anti‐SARS‐CoV‐2 vaccine in patients treated for solid tumors. Annals of Oncology. 2021;32(8):1053‐5. 7. Benda M, Mutschlechner B, Ulmer H, Grabher C, Severgnini L, Volgger A, et al. Serological SARS‐CoV‐2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID‐19 vaccine in haematological and oncological patients. British Journal of Haematology. 2021;195(4):523‐31. 8. Bernstine H, Priss M, Anati T, Turko O, Gorenberg M, Steinmetz AP, et al. Axillary lymph nodes hypermetabolism after BNT162b2 mRNA COVID‐19 vaccination in cancer patients undergoing 18F‐FDG PET/CT: a cohort study. Clinical Nuclear Medicine. 2021;46(5):396‐401. 9. Buttiron Webber T, Provinciali N, Musso M, Ugolini M, Boitano M, Clavarezza M, et al. Predictors of poor seroconversion and adverse events to SARS‐CoV‐2 mRNA BNT162b2 vaccine in cancer patients on active treatment. European Journal of Cancer. 2021;159:105‐12. 10. Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, et al. COVID‐19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. A prospective observational study in Italy. European Journal of Cancer. 2021;157:441‐9. 11. Cohen D, Hazut Krauthammer S, Wolf I, Even‐Sapir E. A sigh of relief: vaccine‐associated hypermetabolic lymphadenopathy following the third COVID‐19 vaccine dose is short in duration and uncommonly interferes with the interpretation of [(18)F]FDG PET‐CT studies performed in oncologic patients. European Journal of Nuclear Medicine and Molecular Imaging. 2021:1‐7. 12. Di Giacomo AM, Giacobini G, Gandolfo C, Lofiego MF, Cusi MG, Maio M. Severe acute respiratory syndrome coronavirus 2 vaccination and cancer therapy: A successful but mindful mix. European Journal of Cancer. 2021;156:119‐21. 13. Di Noia V, Pimpinelli F, Renna D, Barberi V, Maccallini MT, Gariazzo L, et al. Immunogenicity and safety of COVID‐19 vaccine BNT162b2 for patients with solid cancer: A large cohort prospective study from a single institution. Clinical Cancer Research. 2021;27(24):6815‐23. 14. Ehmsen S, Asmussen A, Jeppesen SS, Nilsson AC, Østerlev S, Vestergaard H, et al. Antibody and T cell immune responses following mRNA COVID‐19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034‐6. 15. Fendler A, Shepherd STC, Au L, Wilkinson KA, Wu M, Byrne F, et al. Adaptive immunity and neutralizing antibodies against SARS‐CoV‐2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021. 16. Figueiredo JC, Ihenacho U, Merin NM, Hamid O, Darrah J, Gong J, et al. SARS‐CoV‐2 vaccine uptake, perspectives, and adverse reactions following vaccination in patients with cancer undergoing treatment. Annals of Oncology. 2022;33(1):109‐11. 17. Goshen‐Lago T, Waldhorn I, Holland R, Szwarcwort‐Cohen M, Reiner‐Benaim A, Shachor‐Meyouhas Y, et al. Serologic status and toxic effects of the SARS‐CoV‐2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncology. 2021;7(10):1507‐13. 18. Gounant V, Ferré VM, Soussi G, Charpentier C, Flament H, Fidouh N, et al. Efficacy of Severe Acute Respiratory Syndrome Coronavirus‐2 Vaccine in Patients With Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses. Journal of Thoracic Oncology. 2022;17(2):239‐51. 19. Grinshpun A, Rottenberg Y, Ben‐Dov IZ, Djian E, Wolf DG, Kadouri L. Serologic response to COVID‐19 infection and/or vaccine in cancer patients on active treatment. ESMO Open. 2021;6(6):100283. 20. Heudel P, Favier B, Assaad S, Zrounba P, Blay JY. Reduced SARS‐CoV‐2 infection and death after two doses of COVID‐19 vaccines in a series of 1503 cancer patients. Annals of Oncology. 2021;32(11):1443‐4. 21. Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine among actively treated cancer patients. Journal of the National Cancer Institute. 2022;114(2):203‐9. 22. Linardou H, Spanakis N, Koliou G‐A, Christopoulou A, Karageorgopoulou S, Alevra N, et al. Responses to SARS‐CoV‐2 vaccination in patients with cancer (ReCOVer Study): a prospective cohort study of the Hellenic Cooperative Oncology Group. Cancers. 2021;13(18):4621. 23. Ma Y, Liu N, Wang Y, Zeng J, Hu YY, Hao W, et al. Immune checkpoint blocking impact and nomogram prediction of COVID‐19 inactivated vaccine seroconversion in patients with cancer: a propensity‐score matched analysis. Journal for Immunotherapy of Cancer. 2021;9(11). 24. Mair MJ, Berger JM, Berghoff AS, Starzer AM, Ortmayr G, Puhr HC, et al. Humoral immune response in hematooncological patients and health care workers who received SARS‐CoV‐2 Vaccinations. JAMA Oncology. 2022;8(1):106‐13. 25. Massarweh A, Eliakim‐Raz N, Stemmer A, Levy‐Barda A, Yust‐Katz S, Zer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS‐CoV‐2 in patients undergoing treatment for cancer. JAMA Oncology. 2021;7(8):1133‐40. 26. McKenzie DR, Muñoz‐Ruiz M, Monin L, Alaguthurai T, Lechmere T, Abdul‐Jawad S, et al. Humoral and cellular immunity to delayed second dose of SARS‐CoV‐2 BNT162b2 mRNA vaccination in patients with cancer. Cancer Cell. 2021;39(11):1445‐7. 27. Monin L, Laing AG, Muñoz‐Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID‐19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncology. 2021;22(6):765‐78. 28. Naranbhai V, St. Denis KJ, Lam EC, Ofoman O, Garcia‐Beltran WF, Mairena CB, et al. Neutralization breadth of SARS‐CoV‐2 viral variants following primary series and booster SARS‐CoV‐2 vaccines in patients with cancer. Cancer Cell. 2022;40(1):103‐8.e2. 29. Nelli F, Fabbri A, Onorato A, Giannarelli D, Silvestri MA, Giron Berrios JR, et al. Effects of active cancer treatment on safety and immunogenicity of COVID‐19 mRNA‐BNT162b2 vaccine: preliminary results from the prospective observational Vax‐On study. Annals of Oncology. 2022;33(1):107‐8. 30. Nishino M, Hatabu H, Ricciuti B, Vaz V, Michael K, Awad MM. Axillary lymphadenopathy after Coronavirus disease 2019 vaccinations in patients with thoracic malignancy: Incidence, predisposing factors, and imaging characteristics. Journal of Thoracic Oncology. 2022;17(1):154‐9. 31. Palich R, Veyri M, Marot S, Vozy A, Gligorov J, Maingon P, et al. Weak immunogenicity after a single dose of SARS‐CoV‐2 mRNA vaccine in treated cancer patients. Annals of Oncology. 2021;32(8):1051‐3. 32. Peeters M, Verbruggen L, Teuwen L, Vanhoutte G, Vande Kerckhove S, Peeters B, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5):100274. 33. Scoccianti S, Delli Paoli C, Grilli Leonulli B, Paoletti L, Alpi P, Caini S, et al. Acute tolerance of Moderna mRNA‐1273 vaccine against COVID‐19 in patients with cancer treated with radiotherapy. Lancet Oncology. 2021;22(9):1212‐4. 34. Shmueli ES, Itay A, Margalit O, Berger R, Halperin S, Jurkowicz M, et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy ‐ a single centre prospective study. European Journal of Cancer. 2021;157:124‐31. 35. Singer J, Le NS, Mattes D, Klamminger V, Hackner K, Kolinsky N, et al. Evaluation of antibody responses to COVID‐19 vaccines among solid tumor and hematologic patients. Cancers (Basel). 2021;13(17). 36. So ACP, McGrath H, Ting J, Srikandarajah K, Germanou S, Moss C, et al. COVID‐19 vaccine safety in cancer patients: a single centre experience. Cancers (Basel). 2021;13(14). 37. Thakkar A, Gonzalez‐Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID‐19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081‐90.e2. 38. Tsimafeyeu I, Volkova M, Alekseeva G, Berkut M, Nosov A, Myslevtsev I, et al. Safety and preliminary efficacy of the Gam‐COVID‐Vac vaccine and outcomes of SARS‐CoV‐2 infection in Russian patients with genitourinary malignancies. Journal of Hematology & Oncology. 2021;14(1):192. 39. Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short‐term safety of the BNT162b2 mRNA COVID‐19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncology. 2021;22(5):581‐3. 40. Waldhorn I, Holland R, Goshen‐Lago T, Shirman Y, Szwarcwort‐Cohen M, Reiner‐Benaim A, et al. Six‐Month Efficacy and Toxicity Profile of BNT162b2 Vaccine in Cancer Patients with Solid Tumors. Cancer Discovery. 2021;11(10):2430‐5. 41. Zeng C, Evans JP, Reisinger S, Woyach J, Liscynesky C, Boghdadly ZE, et al. Impaired neutralizing antibody response to COVID‐19 mRNA vaccines in cancer patients. medRxiv. 2021:2021.10.20.21265273.
3. Studies including patients with haematological malignancies
1. Ali H, Ngo D, Aribi A, Arslan S, Dadwal S, Marcucci G, et al. Safety and tolerability of SARS‐CoV2 Emergency‐Use Authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplantation and Cellular Therapy. 2021;27(11):938.e1‐.e6. 2. Attolico I, Tarantini F, Carluccio P, Schifone CP, Delia M, Gagliardi VP, et al. Serological response following BNT162b2 anti‐SARS‐CoV‐2 mRNA vaccination in haematopoietic stem cell transplantation patients. British Journal of Haematology. 2022;196(4):928‐31. 3. Benjamini O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidt N, et al. Safety and efficacy of the BNT162b mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2022;107(3):625‐34. 4. Chan WY, Howells L, Wilson W, Sanchez E, Ainley L, Chavda SJ, et al. Serological response to the BNT162b2 mRNA or ChAdOx1 nCoV‐19 COVID‐19 vaccine after first and second doses in patients with plasma cell disorders: influence of host and disease factors. British Journal of Haematology. 2022;196(3):e21‐e6. 5. Chevallier P C‐BMLBAPPGABMCIBMDTLGSMPM. Safety and immunogenicity of a first dose of SARS‐CoV‐2 mRNA vaccine in allogeneic hematopoietic stem‐cells recipients. Ejhaem. 2021. 6. Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MS, et al. Disease‐ and Therapy‐Specific Impact on Humoral Immune Responses to COVID‐19 Vaccination in Hematologic Malignancies. Blood Cancer Discovery. 2021;2(6):568‐76. 7. Dhakal B, Abedin S, Fenske T, Chhabra S, Ledeboer N, Hari P, et al. Response to SARS‐CoV‐2 vaccination in patients after hematopoietic cell transplantation and CAR T‐cell therapy. Blood. 2021;138(14):1278‐81. 8. Gavriatopoulou M, Terpos E, Ntanasis‐Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS‐CoV‐2: a prospective study. Blood Advances. 2021;5(21):4398‐405. 9. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS‐CoV‐2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031‐3. 10. Gurion R, Rozovski U, Itchaki G, Gafter‐Gvili A, Leibovitch C, Raanani P, et al. Humoral serological response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti‐CD2O antibodies. Haematologica. 2022;107(3):715‐20. 11. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165‐73. 12. Herzog Tzarfati K, Gutwein O, Apel A, Rahimi‐Levene N, Sadovnik M, Harel L, et al. BNT162b2 COVID‐19 vaccine is significantly less effective in patients with hematologic malignancies. American Journal of Hematology. 2021;96(10):1195‐203. 13. Kastritis E, Terpos E, Evangelakou Z, Theodorakakou F, Fotiou D, Manola MS, et al. Kinetics of anti‐SARS‐CoV‐2 neutralizing antibodies development after BNT162b2 vaccination in patients with amyloidosis and the impact of therapy. American Journal of Hematology. 2021. 14. Le Bourgeois A, Coste‐Burel M, Guillaume T, Peterlin P, Garnier A, Béné MC, et al. Safety and antibody response after 1 and 2 doses of BNT162b2 mRNA vaccine in recipients of allogeneic hematopoietic stem cell transplant. JAMA Network Open. 2021;4(9):e2126344‐e. 15. Le Bourgeois A, Coste‐Burel M, Guillaume T, Peterlin P, Garnier A, Imbert BM, et al. Interest of a third dose of BNT162b2 anti‐SARS‐CoV‐2 messenger RNA vaccine after allotransplant. British Journal of Haematology. 2021. 16. Lim SH, Campbell N, Johnson M, Joseph‐Pietras D, Collins GP, O'Callaghan A, et al. Antibody responses after SARS‐CoV‐2 vaccination in patients with lymphoma. Lancet Haematology. 2021;8(8):e542‐e4. 17. Lindemann M, Klisanin V, Thümmler L, Fisenkci N, Tsachakis‐Mück N, Ditschkowski M, et al. Humoral and cellular vaccination responses against SARS‐CoV‐2 in hematopoietic stem cell transplant recipients. Vaccines (Basel). 2021;9(10). 18. Malard F, Gaugler B, Gozlan J, Bouquet L, Fofana D, Siblany L, et al. Weak immunogenicity of SARS‐CoV‐2 vaccine in patients with hematologic malignancies. Blood Cancer Journal. 2021;11(8):142. 19. Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, et al. Immunogenicity of the BNT162b2 COVID‐19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematology. 2021;8(8):e583‐e92. 20. Marasco V, Carniti C, Guidetti A, Farina L, Magni M, Miceli R, et al. T‐cell immune response after mRNA SARS‐CoV‐2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. British Journal of Haematology. 2022;196(3):548‐58. 21. Marchesi F, Pimpinelli F, Sperandio E, Papa E, Falcucci P, Pontone M, et al. The 12‐week kinetics of anti‐SARS‐CoV‐2 antibodies in different haematological cancers after vaccination with BNT162b2. British Journal of Haematology. 2021. 22. Marlet J, Gatault P, Maakaroun Z, Longuet H, Stefic K, Handala L, et al. Antibody responses after a third dose of COVID‐19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines. 2021;9(10). 23. Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA Covid‐19 Vaccine in Patients with Hematological Neoplasms. Blood. 2021. 24. Ollila TA, Lu S, Masel R, Zayac A, Paiva K, Rogers RD, et al. Antibody response to COVID‐19 vaccination in adults with hematologic malignant disease. JAMA Oncology. 2021;7(11):1714‐6. 25. Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S, et al. Antibody responses after first and second Covid‐19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer Journal. 2021;11(7):136. 26. Piñana JL, López‐Corral L, Martino R, Montoro J, Vazquez L, Pérez A, et al. SARS‐CoV‐2‐reactive antibody detection after SARS‐CoV‐2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. American Journal of Hematology. 2022;97(1):30‐42. 27. Ramasamy K, Sadler R, Jeans S, Varghese S, Turner A, Larham J, et al. COVID symptoms, testing, shielding impact on patient‐reported outcomes and early vaccine responses in individuals with multiple myeloma. British Journal of Haematology. 2022;196(1):95‐8. 28. Ramasamy K, Sadler R, Jeans S, Weeden P, Varghese S, Turner A, et al. Immune response to COVID‐19 vaccination is attenuated by poor disease control and antimyeloma therapy with vaccine driven divergent T‐cell response. British Journal of Haematology. 2021;n/a(n/a). 29. Re D, Barrière J, Chamorey E, Delforge M, Gastaud L, Petit E, et al. Low rate of seroconversion after mRNA anti‐SARS‐CoV‐2 vaccination in patients with hematological malignancies. Leukemia & Lymphoma. 2021;62(13):3308‐10. 30. Salton NS, Szwarcwort M, Tzoran I, Horowitz NA, Zuckerman T, Horesh N, et al. Attenuated humoral immune response following anti‐SARSCoV‐2 vaccine in heavily pretreated patients with multiple myeloma and AL amyloidosis. American Journal of Hematology. 2021;96(12):E475‐E8. 31. Shapiro LC, Thakkar A, Gali R, Gonzalez‐Lugo JD, Bazarbachi A‐H, Rahman S, et al. High seroconversion rates amongst Black and Hispanics with hematologic malignancies after SARS‐CoV‐2 vaccination. medRxiv. 2021:2021.09.13.21263365. 32. Shem‐Tov N, Yerushalmi R, Danylesko I, Litachevsky V, Levy I, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in haematopoietic stem cell transplantation recipients. British Journal of Haematology. 2022;196(4):884‐91. 33. Shen Y, Freeman JA, Holland J, Solterbeck A, Naidu K, Soosapilla A, et al. COVID‐19 vaccine failure in chronic lymphocytic leukemia and monoclonal B‐lymphocytosis; humoral and cellular immunity. medRxiv. 2021:2021.10.28.21265549. 34. Stampfer SD, Goldwater MS, Jew S, Bujarski S, Regidor B, Daniely D, et al. Response to mRNA vaccination for COVID‐19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534‐41. 35. Tamari R, Politikos I, Knorr DA, Vardhana SA, Young JC, Marcello LT, et al. Predictors of Humoral Response to SARS‐CoV‐2 Vaccination after Hematopoietic Cell Transplantation and CAR T‐cell Therapy. Blood Cancer Discovery. 2021;2(6):577‐85. 36. Terpos E, Gavriatopoulou M, Fotiou D, Giatra C, Asimakopoulos I, Dimou M, et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS‐CoV‐2: a prospective study. Cancers. 2021;13(17):4480. 37. Terpos E, Gavriatopoulou M, Ntanasis‐Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. The neutralizing antibody response post COVID‐19 vaccination in patients with myeloma is highly dependent on the type of anti‐myeloma treatment. Blood Cancer Journal. 2021;11(8):138. 38. Van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly variable SARS‐CoV‐2 spike antibody responses to two doses of COVID‐19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028‐30. 39. Yeshurun M, Pasvolsky O, Shargian L, Yahav D, Ben‐Zvi H, Rubinstein M, et al. Humoral serological response to the BNT162b2 vaccine after allogeneic haematopoietic cell transplantation. Clinical Microbiology and Infection. 2022;28(2):303.e1‐.e4.
4. Studies on patients with liver disease and liver transplant
1. Calleri A, Saracco M, Pittaluga F, Cavallo R, Romagnoli R, Martini S. Seroconversion after Coronavirus Disease 2019 vaccination in patients awaiting liver transplantation: Fact or fancy? Liver Transplantation. 2022;28(2):180‐7. 2. John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, et al. Association of BNT162b2 mRNA and mRNA‐1273 vaccines with COVID‐19 infection and hospitalization among patients with cirrhosis. JAMA Internal Medicine. 2021;181(10):1306‐14. 3. Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS‐CoV2‐specific humoral and T‐cell immune response after second vaccination in liver cirrhosis and transplant patients. Clinical Gastroenterology and Hepatology. 2022;20(1):162‐72.e9. 4. Strauss AT, Hallett AM, Boyarsky BJ, Ou MCT, Werbel WA, Avery RK, et al. Antibody response to Severe Acute Respiratory Syndrome‐Coronavirus‐2 messenger RNA vaccines in liver transplant recipients. Liver Transplantation. 2021;27(12):1852‐6. 5. Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID‐19 vaccination in liver transplant recipients and those with chronic liver diseases. Journal of Hepatology. 2021;75(6):1434‐9. 6. Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, et al. Safety and immunogenicity of COVID‐19 vaccination in patients with non‐alcoholic fatty liver disease (CHESS2101): A multicenter study. Journal of Hepatology. 2021;75(2):439‐41.
5. Studies on patients with kidney disease, dialysis, or kidney transplant
1. Agur T, Ben‐Dor N, Goldman S, Lichtenberg S, Herman‐Edelstein M, Yahav D, et al. Antibody response to mRNA SARS‐CoV‐2 vaccine among dialysis patients ‐ a prospective cohort study. Nephrology Dialysis Transplantation. 2021. 2. Anand S, Montez‐Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. Antibody response to COVID‐19 vaccination in patients receiving dialysis. Journal of the American Society of Nephrology. 2021;32(10):2435‐8. 3. Anand S, Montez‐Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. SARS‐CoV‐2 vaccine antibody response and breakthrough infection in dialysis. medrxiv. 2021:2021.10.12.21264860. 4. Bassi J, Giannini O, Silacci‐Fregni C, Pertusini L, Hitz P, Terrot T, et al. Poor neutralization and rapid decay of antibodies to SARS‐CoV‐2 variants in vaccinated dialysis patients. medRxiv. 2021:2021.10.05.21264054. 5. Ben‐Dov IZ, Oster Y, Tzukert K, Alster T, Bader R, Israeli R, et al. Impact of tozinameran (BNT162b2) mRNA vaccine on kidney transplant and chronic dialysis patients: 3‐5 months follow‐up. Journal of nephrology. 2022;35(1):153‐64. 6. Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi‐Kremer S, et al. Antibody response after a third dose of the mRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063‐5. 7. Benotmane I, Gautier‐Vargas G, Cognard N, Olagne J, Heibel F, Braun‐Parvez L, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA‐1273 SARS‐CoV‐2 vaccine. Kidney International. 2021;99(6):1498‐500. 8. Benotmane I, Gautier‐Vargas G, Cognard N, Olagne J, Heibel F, Braun‐Parvez L, et al. Weak anti‐SARS‐CoV‐2 antibody response after the first injection of an mRNA COVID‐19 vaccine in kidney transplant recipients. Kidney International. 2021;99(6):1487‐9. 9. Bertrand D, Hanoy M, Edet S, Lemée V, Hamzaoui M, Laurent C, et al. Antibody response to SARS‐CoV‐2 mRNA BNT162b2 vaccine in kidney transplant recipients and in‐centre and satellite centre haemodialysis patients. Clinical Kidney Journal. 2021;14(9):2127‐8. 10. Broseta JJ, Rodríguez‐Espinosa D, Bedini JL, Rodríguez N, Maduell F. Antibody maintenance 3 months after complete messenger RNA COVID‐19 vaccination in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association ‐ European Renal Association. 2021;36(12):2340‐1. 11. Broseta JJ, Rodríguez‐Espinosa D, Rodríguez N, Mosquera MDM, Marcos M, Egri N, et al. Humoral and cellular responses to mRNA‐1273 and BNT162b2 SARS‐CoV‐2 vaccines administered to hemodialysis patients. American Journal of Kidney Diseases. 2021;78(4):571‐81. 12. Brunelli SM, Sibbel S, Karpinski S, Marlowe G, Walker AG, Giullian J, et al. Comparative effectiveness of Ad26.COV2.S vs. BNT162b2 for the prevention of SARS‐cov‐2 infection among dialysis patients. Journal of the American Society of Nephrology. 2021;32:95. 13. Cann A, Clarke C, Brown J, Thomson T, Prendecki M, Moshe M, et al. SARS‐CoV‐2 antibody lateral flow assay for antibody prevalence studies following vaccine roll out: A diagnostic accuracy study. medRxiv. 2021:2021.07.14.21260488. 14. Carr EJ, Wu M, Harvey R, Wall EC, Kelly G, Hussain S, et al. Neutralising antibodies after COVID‐19 vaccination in UK haemodialysis patients. Lancet. 2021;398(10305):1038‐41. 15. Charmetant X, Espi M, Benotmane I, Heibel F, Buron F, Gautier‐Vargas G, et al. Comparison of infected and vaccinated transplant recipients highlights the role of Tfh and neutralizing IgG in COVID‐19 protection. medRxiv. 2021:2021.07.22.21260852. 16. Chavarot N, Ouedrani A, Marion O, Leruez‐Ville M, Vilain E, Baaziz M, et al. Poor anti‐SARS‐CoV‐2 humoral and T‐cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105(9):e94‐e5. 17. Chemaitelly H, AlMukdad S, Joy JP, Ayoub HH, Yassine HM, Benslimane FM, et al. SARS‐CoV‐2 vaccine effectiveness in immunosuppressed kidney transplant recipients. medRxiv. 2021:2021.08.07.21261578. 18. Clarke CL, Martin P, Gleeson S, Thomson T, Edwards H, Mortimer P, et al. Comparison of immunogenicity between BNT162b2 and ChAdOx1 SARS‐CoV‐2 vaccines in a large haemodialysis population. medRxiv. 2021:2021.07.09.21260089. 19. Crespo M, Barrilado‐Jackson A, Padilla E, Eguía J, Echeverria‐Esnal D, Cao H, et al. Negative immune responses to two‐dose mRNA COVID‐19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. American Journal of Transplantation. 2022;22(3):786‐800. 20. Cserep G, Morrow D, Latchford K, Jesset R, Dosa A, Kirmizis D. The effect of a single dose of BNT162b2 vaccine on the incidence of severe COVID‐19 infection in patients on chronic hemodialysis: a single‐centre study. Clinical and Experimental Nephrology. 2022;26(1):54‐8. 21. Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco‐Zevallos J, Casals‐Urquiza J, et al. Cellular and humoral response after MRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients. American Journal of Transplantation. 2021;21(8):2727‐39. 22. Danthu C, Hantz S, Dahlem A, Duval M, Ba B, Guibbert M, et al. Humoral response after SARS‐CoV‐2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. Journal of the American Society of Nephrology. 2021;32(9):2153‐8. 23. Dębska‐Ślizień A, Ślizień Z, Muchlado M, Kubanek A, Piotrowska M, Dąbrowska M, et al. Predictors of humoral response to mRNA COVID19 vaccines in kidney transplant recipients: A longitudinal study‐the COViNEPH project. Vaccines (Basel). 2021;9(10). 24. Ducloux D, Colladant M, Chabannes M, Bamoulid J, Courivaud C. Factors associated with humoral response after BNT162b2 mRNA COVID‐19 vaccination in kidney transplant patients. Clinical Kidney Journal. 2021;14(10):2270‐2. 25. Espi M, Charmetant X, Barba T, Koppe L, Pelletier C, Kalbacher E, et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS‐CoV‐2 mRNA vaccine in virus‐unexposed patients receiving maintenance hemodialysis. Kidney International. 2021;100(4):928‐36. 26. Espi M, Charmetant X, Barba T, Mathieu C, Pelletier C, Koppe L, et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney International. 2022;101(2):390‐402. 27. Fazendeiro Matos J, Peralta R, Felix C, Pinto B, Ponce P. Vaccination against COVID‐19 in a network of hemodialysis units in Portugal: A promising experience. Acta Medica Port. 28. Frantzen L, Cavaillé G, Thibeaut S, El‐Haik Y. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in a haemodialysis cohort. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association ‐ European Renal Association. 2021;36(9):1756‐7. 29. Garcia P, Anand S, Han J, Montez‐Rath M, Sun S, Shang T, et al. COVID19 vaccine type and humoral immune response in patients receiving dialysis. medRxiv. 2021. 30. Goupil R, Benlarbi M, Beaubien‐Souligny W, Nadeau‐Fredette A‐C, Chatterjee D, Goyette G, et al. Short‐term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. Canadian Medical Association Journal. 2021;193(22):E793‐E800. 31. Grupper A, Katchman E, Ben‐Yehoyada M, Rabinowich L, Schwartz D, Schwartz IF, et al. Kidney transplant recipients vaccinated before transplantation maintain superior humoral response to SARS‐CoV‐2 vaccine. Clinical transplantation. 2021;35(12):e14478. 32. Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben‐Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. American Journal of Transplantation. 2021;21(8):2719‐26. 33. Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clinical Journal of the American Society of Nephrology. 2021;16(7):1037‐42. 34. Hod T, Ben‐David A, Olmer L, Levy I, Ghinea R, Mor E, et al. Humoral response of renal transplant recipients to the BNT162b2 SARS‐CoV‐2 mRNA vaccine using both RBD IgG and neutralizing antibodies. Transplantation. 2021;105(11):e234‐e43. 35. Holt SG, Mahmoud S, Ahmed W, Acuna JM, Al Madani AK, Eltantawy I, et al. An analysis of antibody responses and clinical sequalae of the Sinopharm HB02 COVID19 vaccine in dialysis patients in the United Arab Emirates. Nephrology (Carlton). 2022;27(3):260‐8. 36. Hsu CM, Weiner DE, Aweh GN, Manley HJ, Ladik V, Frament J, et al. Seroresponse to SARS‐CoV‐2 vaccines among maintenance dialysis patients. American Journal of Kidney Diseases. 2022;79(2):307‐10. 37. Ivanauskaite G, Rimsevicius L, Avizienyte E, Vinikovas A, Maciuleviciene A, Brauklyte J, et al. Successful COVID‐19 vaccination for patients on dialysis in Vilnius County. Hemodialysis International. 2021. 38. Kaiser RA, Haller MC, Apfalter P, Kerschner H, Cejka D. Comparison of BNT162b2 (Pfizer‐BioNtech) and mRNA‐1273 (Moderna) SARS‐CoV‐2 mRNA vaccine immunogenicity in dialysis patients. Kidney International. 2021;100(3):697‐8. 39. Kantauskaite M, Müller L, Kolb T, Fischer S, Hillebrandt J, Ivens K, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS‐CoV‐2 vaccination in kidney transplant recipients. American Journal of Transplantation. 2022;22(2):634‐9. 40. Kolb T, Fischer S, Müller L, Lübke N, Hillebrandt J, Andrée M, et al. Impaired immune response to SARS‐CoV‐2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360. 2021;2(9):1491‐8. 41. Lacson E, Jr., Argyropoulos CP, Manley HJ, Aweh G, Chin AI, Salman LH, et al. Immunogenicity of SARS‐CoV‐2 vaccine in dialysis. Journal of the American Society of Nephrology. 2021;32(11):2735‐42. 42. Longlune N, Nogier MB, Miedougé M, Gabilan C, Cartou C, Seigneuric B, et al. High immunogenicity of a messenger RNA‐based vaccine against SARS‐CoV‐2 in chronic dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association ‐ European Renal Association. 2021;36(9):1704‐9. 43. Manley HJ, Aweh GN, Hsu CM, Weiner DE, Miskulin D, Harford AM, et al. SARS‐CoV‐2 vaccine effectiveness and breakthrough infections in maintenance dialysis patients. medRxiv. 2021:2021.09.24.21264081. 44. Martin‐Garcia J CCGMBRRRCMGDBGDFFC. Suboptimal humoral immunological response to the 2nd dose of anti‐COVID19 mRNA‐1273 vaccine (Moderna) in kidney transplant patients. Nefrologia. 2021. 45. Masset C, Kerleau C, Garandeau C, Ville S, Cantarovich D, Hourmant M, et al. A third injection of the BNT162b2 mRNA COVID‐19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney International. 2021;100(5):1132‐5. 46. Matsunami M, Suzuki T, Terao T, Kuji H, Matsue K. Immune response to SARS‐CoV‐2 vaccination among renal replacement therapy patients with CKD: a single‐center study. Clinical and Experimental Nephrology. 2021. 47. McEvoy CM, Lee A, Misra PS, Lebovic G, Wald R, Yuen DA. Real‐world effectiveness of 2‐dose SARS‐CoV‐2 vaccination in kidney transplant recipients. medRxiv. 2021:2021.09.21.21263457. 48. Middleton RJ, Gorton J, O'Riordan E, Knight S, Kalra PA, Poulikakos D. Impact of Shielding and First Dose of COVID‐19 Vaccination in Kidney Transplant Recipients. NEPHRON. 2021. 49. Ou MT, Boyarsky BJ, Chiang TPY, Bae S, Werbel WA, Avery RK, et al. Immunogenicity and reactogenicity after SARS‐CoV‐2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105(9):2119‐23. 50. Paal M, Arend FM, Lau T, Hasmann S, Soreth‐Rieke D, Sorodoc‐Otto J, et al. Antibody response to mRNA SARS‐CoV‐2 vaccines in haemodialysis patients. Clinical Kidney Journal. 2021;14(10):2234‐8. 51. Polewska K, Tylicki P, Biedunkiewicz B, Rucińska A, Szydłowska A, Kubanek A, et al. Safety and Tolerability of the BNT162b2 mRNA COVID‐19 Vaccine in Dialyzed Patients. COViNEPH Project. Medicina (Kaunas). 2021;57(7). 52. Prendecki M, Thomson T, Clarke CL, Martin P, Gleeson S, De Aguiar RC, et al. Immunological responses to SARS‐CoV‐2 vaccines in kidney transplant recipients. Lancet. 2021;398(10310):1482‐4. 53. Rosa‐Diez G, Papaginovic Leiva MM, Lombi F, Crucelegui MS, Martínez RD, Trimarchi H, et al. Safety and effectiveness of COVID‐19 SPUTNIK V vaccine in dialysis patients. medRxiv. 2021:2021.10.21.21265349. 54. Santos‐Araújo C, Mota Veiga P, Santos MJ, Santos L, Romãozinho C, Silva M, et al. Time‐dependent evolution of IgG antibody levels after first and second dose of mRNA‐based SARS‐CoV‐2 vaccination in haemodialysis patients: a multicentre study. Nephrology Dialysis Transplantation. 2021;37(2):375‐81. 55. Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub‐Hohenbleicher H, et al. Impaired humoral and cellular immunity after SARS‐CoV‐2 BNT162b2 (tozinameran) prime‐boost vaccination in kidney transplant recipients. Journal of Clinical Investigation. 2021;131(14). 56. Sibbel S, McKeon KL, Luo J, Wendt K, Walker AG, Lazar R, et al. Real‐world effectiveness and immunogenicity of BNT162B2 in dialysis patients. Journal of the American Society of Nephrology. 2021;32:94‐. 57. Simon B, Rubey H, Treipl A, Gromann M, Hemedi B, Zehetmayer S, et al. Haemodialysis patients show a highly diminished antibody response after COVID‐19 mRNA vaccination compared with healthy controls. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association ‐ European Renal Association. 2021;36(9):1709‐16. 58. Song CC, Christensen J, Kumar D, Vissichelli N, Morales M, Gupta G. Early experience with SARs‐CoV‐2 mRNA vaccine breakthrough among kidney transplant recipients. Transplant Infectious Disease. 2021. 59. Speer C, Schaier M, Nusshag C, Töllner M, Buylaert M, Kälble F, et al. Longitudinal humoral responses after COVID‐19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines (Basel). 2021;9(10). 60. Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, et al. Humoral and cellular immunity to SARS‐CoV‐2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA‐1273 or BNT162b2 mRNA vaccine. Lancet Regional Health Europe. 2021;9:100178. 61. Tau N, Yahav D, Schneider S, Rozen‐Zvi B, Abu Sneineh M, Rahamimov R. Severe consequences of COVID‐19 infection among vaccinated kidney transplant recipients. American Journal of Transplantation. 2021;21(8):2910‐2. 62. Tillmann FP, Figiel L, Ricken J, Still H, Korte C, Plassmann G, et al. Evolution of SARS‐CoV‐2‐neutralizing antibodies after two standard dose vaccinations, risk factors for non‐response and effect of a third dose booster vaccination in non‐responders on hemodialysis: A prospective multi‐centre cohort Study. Journal of Clinical Medicine. 2021;10(21). 63. Torreggiani M, Blanchi S, Fois A, Fessi H, Piccoli GB. Neutralizing SARS‐CoV‐2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID‐19 vaccine: the war is far from being won. Kidney International. 2021;99(6):1494‐6. 64. Van Praet J, Reynders M, De Bacquer D, Viaene L, Schoutteten MK, Caluwé R, et al. Predictors and dynamics of the humoral and cellular immune response to SARS‐CoV‐2 mRNA vaccines in hemodialysis patients: a multicenter observational study. Journal of the American Society of Nephrology. 2021;32(12):3208‐20. 65. Veerle PWMW, Kevin KA, Steven A, Marie MC, Fabienne M, Joachim M, et al. mRNA‐1273 vaccine (Moderna): a better option than BNT162b2 (Pfizer) in kidney transplant recipients and dialysis patients? medRxiv. 2021. 66. Weigert A, Bergman M‐L, Gonçalves L, Godinho I, Duarte N, Abrantes R, et al. Longitudinal analysis of antibody responses to the Pfizer BNT162b2 vaccine in Patients Undergoing Maintenance Hemodialysis. medRxiv. 2021:2021.07.20.21260849. 67. Yanay NB, Freiman S, Shapira M, Wishahi S, Hamze M, Elhaj M, et al. Experience with SARS‐CoV‐2 BNT162b2 mRNA vaccine in dialysis patients. Kidney International. 2021;99(6):1496‐8. 68. Yau K, Abe KT, Naimark D, Oliver MJ, Perl J, Leis JA, et al. Evaluation of the SARS‐CoV‐2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Network Open. 2021;4(9):e2123622. 69. Yi SG, Knight RJ, Graviss EA, Moore LW, Nguyen DT, Ghobrial RM, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID‐19 vaccine administration. Transplantation. 2021;105(7):e72‐e3.
6. Studies on autoimmune diseases
1. Achiron A, Dolev M, Menascu S, Zohar DN, Dreyer‐Alster S, Miron S, et al. COVID‐19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Multiple Sclerosis Journal. 2021;27(6):864‐70. 2. Achiron A, Mandel M, Dreyer‐Alster S, Harari G, Magalashvili D, Sonis P, et al. Humoral immune response to COVID‐19 mRNA vaccine in patients with multiple sclerosis treated with high‐efficacy disease‐modifying therapies. Therapeutic Advances in Neurological Disorders. 2021;14:17562864211012835. 3. Al‐Janabi A, Littlewood Z, Griffiths CEM, Hunter HJA, Chinoy H, Moriarty C, et al. Antibody responses to single‐dose SARS‐CoV‐2 vaccination in patients receiving immunomodulators for immune‐mediated inflammatory disease. British Journal of Dermatology. 2021;185(3):646‐8. 4. Araujo CSR, Medeiros‐Ribeiro AC, Saad CGS, Bonfiglioli KR, Domiciano DS, Shimabuco AY, et al. Two‐week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS‐CoV‐2 vaccine: a randomised clinical trial. Annals of the Rheumatic Diseases. 2022:annrheumdis‐2021‐221916. 5. Barbhaiya M, Levine JM, Bykerk VP, Jannat‐Khah D, Mandl LA. Systemic rheumatic disease flares after SARS‐CoV‐2 vaccination among rheumatology outpatients in New York City. Annals of the Rheumatic Diseases. 2021;80(10):1352‐4. 6. Barbhaiya M, Levine JM, Siegel CH, Bykerk VP, Jannat‐Khah D, Mandl LA. Adverse events and disease flares after SARS‐CoV‐2 vaccination in patients with systemic lupus erythematosus. Clinical Rheumatology. 2021:1‐4. 7. Bardazzi F, Abbenante D, Filippi F, Sacchelli L, Loi C. The initial experience of COVID‐19 vaccination in autoimmune blistering diseases patients from a reference care center in Italy. Dermatologic Therapy. 2021;34(5):e15057. 8. Bartels LE, Ammitzboll C, Andersen JB, Vils SR, Mistegaard CE, Johannsen AD, et al. Local and systemic reactogenicity of COVID‐19 vaccine BNT162b2 in patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatology International. 2021. 9. Ben‐Tov A, Banon T, Chodick G, Kariv R, Assa A, Gazit S. BNT162b2 messenger RNA COVID‐19 vaccine effectiveness in patients with inflammatory bowel disease: Preliminary real‐world data during mass vaccination campaign. Gastroenterology. 2021;161(5):1715‐7.e1. 10. Botwin GJ, Li D, Figueiredo J, Cheng S, Braun J, McGovern DPB, et al. Adverse events after SARS‐CoV‐2 mRNA vaccination among patients with inflammatory bowel disease. The American journal of gastroenterology. 2021;116(8):1746‐51. 11. Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik‐Wang JM, et al. Antibody response to a single dose of SARS‐CoV‐2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases. 2021. 12. Braun‐Moscovici Y, Kaplan M, Braun M, Markovits D, Giryes S, Toledano K, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS‐CoV‐2. Annals of the Rheumatic Diseases. 2021;80(10):1317‐21. 13. Brill L, Rechtman A, Zveik O, Haham N, Oiknine‐Djian E, Wolf DG, et al. Humoral and T‐Cell response to SARS‐CoV‐2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurology. 2021;78(12):1510‐4. 14. Bugatti S, De Stefano L, Balduzzi S, Greco MI, Luvaro T, Cassaniti I, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic inflammatory arthritis. Annals of the Rheumatic Diseases. 2021;80(12):1635‐8. 15. Cannatelli R, Ferretti F, Carmagnola S, Bergna IMB, Monico MC, Maconi G, et al. Risk of adverse events and reported clinical relapse after COVID‐19 vaccination in patients with IBD. Gut. 2021. 16. Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, et al. Safety of the ChAdOx1 nCoV‐19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post‐vaccination cross‐sectional survey. Rheumatology International. 2021;41(8):1441‐5. 17. Chiang TP, Connolly CM, Ruddy JA, Boyarsky BJ, Alejo JL, Werbel WA, et al. Antibody response to the Janssen/Johnson & Johnson SARS‐CoV‐2 vaccine in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases. 2021;80(10):1365‐6. 18. Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, et al. Safety of the first dose of mRNA SARS‐CoV‐2 vaccines in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases. 2021. 19. Connolly CM, Ruddy JA, Boyarsky BJ, Barbur I, Werbel WA, Geetha D, et al. Disease Flare and Reactogenicity in Patients With Rheumatic and Musculoskeletal Diseases Following Two‐Dose SARS‐CoV‐2 Messenger RNA Vaccination. Arthritis & rheumatology (Hoboken, NJ). 2022;74(1):28‐32. 20. Cook C, Patel NJ, D’Silva KM, Hsu TY‐T, DiIorio M, Prisco L, et al. Clinical characteristics and outcomes of COVID‐19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Annals of the Rheumatic Diseases. 2022;81(2):289‐91. 21. Dailey J, Kozhaya L, Dogan M, Hopkins D, Lapin B, Herbst K, et al. Antibody responses to SARS‐CoV‐2 after infection or vaccination in children and young adults with inflammatory bowel disease. medRxiv. 2021:2021.06.12.21258810. 22. Disanto G, Sacco R, Bernasconi E, Martinetti G, Keller F, Gobbi C, et al. Association of disease‐modifying treatment and anti‐CD20 infusion timing with humoral response to 2 SARS‐CoV‐2 vaccines in patients with multiple sclerosis. JAMA Neurology. 2021;78(12):1529‐31. 23. Edelman‐Klapper H, Zittan E, Bar‐Gil Shitrit A, Rabinowitz KM, Goren I, Avni‐Biron I, et al. Lower serologic response to COVID‐19 mRNA vaccine in patients with inflammatory bowel diseases treated with anti‐TNFα. Gastroenterology. 2022;162(2):454‐67. 24. Esquivel‐Valerio J. A. S‐TCMM‐AIAC‐dlGJAG‐AGG‐GPLA. Adverse events of six COVID‐19 vaccines in patients with autoimmune rheumatic diseases: a cross‐sectional study. Rheumatology international. 2021. 25. Etemadifar M, Abhari AP, Nouri H, Sigari AA, Piran Daliyeh SM, Maracy MR, et al. Self‐reported safety of the BBIBP‐CorV (Sinopharm) COVID‐19 vaccine among Iranian people with multiple sclerosis. medRxiv. 2021:2021.10.17.21265114. 26. Etemadifar M, Sedaghat N, Nouri H, Lotfi N, Chitsaz A, Khorvash R, et al. SARS‐CoV‐2 serology among people with multiple sclerosis on disease‐modifying therapies after BBIBP‐CorV (Sinopharm) inactivated virus vaccination: Same story, different vaccine. Multiple Sclerosis and Related Disorders. 2022;57:103417. 27. Fan Y, Geng Y, Wang Y, Deng XR, Li GT, Zhao J, et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real‐world survey on inactivated COVID‐19 vaccines. Annals of the Rheumatic Diseases. 2021. 28. Fattizzo B, Giannotta JA, Cecchi N, Barcellini W. SARS‐CoV‐2 vaccination in patients with autoimmune cytopenias: The experience of a reference center. American Journal of Hematology. 2021;96(11):E413‐e6. 29. Felten R, Kawka L, Dubois M, Ugarte‐Gil MF, Fuentes‐Silva Y, Piga M, et al. Tolerance of COVID‐19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatology. 2021;3(9):e613‐e5. 30. Ferri C, Ursini F, Gragnani L, Raimondo V, Giuggioli D, Foti R, et al. Impaired immunogenicity to COVID‐19 vaccines in autoimmune systemic diseases. High prevalence of non‐response in different patients' subgroups. Journal of Autoimmunity. 2021;125. 31. Frey S, Connolly CM, Chiang TP, Teles M, Alejo JL, Boyarsky BJ, et al. Antibody kinetics in patients with rheumatic diseases after SARS‐CoV‐2 mRNA vaccination. Lancet Rheumatology. 2021;3(11):e753‐e4. 32. Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Annals of the Rheumatic Diseases. 2021;80(10):1330‐8. 33. Gadani SP, Reyes‐Mantilla M, Jank L, Harris S, Douglas M, Smith MD, et al. Discordant humoral and T cell immune responses to SARS‐CoV‐2 vaccination in people with multiple sclerosis on anti‐CD20 therapy. medRxiv. 2021:2021.08.23.21262472. 34. Garrido I, Lopes S, Macedo G. "Safety of COVID‐19 vaccination in inflammatory bowel disease patients on biologic therapy". Journal of Crohn's & Colitis. 2021. 35. Georgieva ZG, Dӧffinger R, Kumararatne D, Coles AJ, McCarthy C. Diminished seroconversion following a single SARS‐COV‐2 vaccine in ocrelizumab‐treated relapsing‐remitting multiple sclerosis patients. Multiple Sclerosis. 2021:13524585211046786. 36. Ghadiri F, Sahraian MA, Azimi A, Moghadasi AN. The study of COVID‐19 infection following vaccination in patients with multiple sclerosis. Multiple Sclerosis and Related Disorders. 2022;57:103363. 37. Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID‐19 vaccine in immune‐mediated inflammatory disease. Annals of the Rheumatic Diseases. 2021;80(10):1339‐44. 38. Hadi YB, Thakkar S, Shah‐Khan SM, Hutson W, Sarwari A, Singh S. COVID‐19 vaccination is safe and effective in patients with inflammatory bowel disease: Analysis of a large multi‐institutional research network in the United States. Gastroenterology. 2021;161(4):1336‐9.e3. 39. Kappelman MD, Weaver KN, Boccieri M, Firestine A, Zhang X, Long MLD. Humoral immune response to messenger RNA COVID‐19 vaccines among patients with inflammatory bowel disease. Gastroenterology. 2021;161(4):1340‐+. 40. Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV‐19 SARS‐CoV‐2 vaccines in patients with IBD. Gut. 2021;70(10):1884‐93. 41. Khan N MN. Effectiveness of SARS‐CoV‐2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology 2021;161(3):827‐36. 42. König M, Lorentzen Å R, Torgauten HM, Tran TT, Schikora‐Rustad S, Vaage EB, et al. Humoral immunity to SARS‐CoV‐2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. Journal of Neurology, Neurosurgery, and Psychiatry. 2021. 43. König M, Torgauten HM, Øverås MH, Chopra A, Rudjord Lorentzen Å, Tran TT, et al. Efficacy and safety of a third SARS‐CoV‐2 vaccination in multiple sclerosis vaccine non‐responders. medRxiv. 2021:2021.10.15.21264977. 44. Li X, Tong X, Yeung WWY, Kuan P, Yum SHH, Chui CSL, et al. Two‐dose COVID‐19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Annals of the Rheumatic Diseases. 2021. 45. Mahil SK, Bechman K, Raharja A, Domingo‐Vila C, Baudry D, Brown MA, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID‐19 vaccine BNT162b2: a cohort study. Lancet Rheumatology. 2021;3(9):e627‐e37. 46. Medeiros‐Ribeiro AC, Aikawa NE, Saad CGS, Yuki EFN, Pedrosa T, Fusco SRG, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27(10):1744‐51. 47. Melmed GY, Botwin GJ, Sobhani K, Li D, Prostko J, Figueiredo J, et al. Antibody responses after SARS‐CoV‐2 mRNA vaccination in adults with inflammatory bowel disease. Annals of Internal Medicine. 2021;174(12):1768‐70. 48. Moyon Q, Sterlin D, Miyara M, Anna F, Mathian A, Lhote R, et al. BNT162b2 vaccine‐induced humoral and cellular responses against SARS‐CoV‐2 variants in systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2021:annrheumdis‐2021‐221097. 49. Papagoras C, Fragoulis GE, Zioga N, Simopoulou T, Deftereou K, Kalavri E, et al. Better outcomes of COVID‐19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Annals of the Rheumatic Diseases. 2021. 50. Pavlotsky F, Segal Z, Barzilai A. Antibody response to BNT162b2 vaccine in immune modifiers–treated psoriatic patients. Journal of Psoriasis and Psoriatic Arthritis. 2022;7(1):24‐8. 51. Peet CJ, Papadopoulou C, Sombrito BRM, Wood MR, Lachmann HJ. COVID‐19 and autoinflammatory diseases: prevalence and outcomes of infection and early experience of vaccination in patients on biologics. Rheumatology Advances in Practice. 2021;5(2):rkab043. 52. Pitzalis M, Idda ML, Lodde V, Loizedda A, Lobina M, Zoledzwieska M, et al. Effect of different disease‐modifying therapies on humoral response to BNT162b2 vaccine in Sardinian multiple sclerosis patients. medRxiv. 2021:2021.09.26.21264067. 53. Pozdnyakova V, Botwin GJ, Sobhani K, Prostko J, Braun J, McGovern DPB, et al. Decreased antibody responses to Ad26.COV2.S relative to SARS‐CoV‐2 mRNA vaccines in patients with inflammatory bowel disease. Gastroenterology. 2021;161(6):2041‐3.e1. 54. Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher‐Stine L, Garonzik‐Wang J, et al. High antibody response to two‐dose SARS‐CoV‐2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases. 2021;80(10):1351‐2. 55. Sahraian AM, Ghadiri F, Azimi A, Naser Moghadasi A. Adverse events reported by Iranian patients with multiple sclerosis after the first dose of Sinopharm BBIBP‐CorV. Vaccine. 2021;39(43):6347‐50. 56. Sattui SE, Liew JW, Kennedy K, Sirotich E, Putman M, Moni TT, et al. Early experience of COVID‐19 vaccination in adults with systemic rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7(3). 57. Sciascia S, Costanzo P, Radin M, Schreiber K, Pini M, Vaccarino A, et al. Safety and tolerability of mRNA COVID‐19 vaccines in people with antiphospholipid antibodies. Lancet Rheumatology. 2021;3(12):e832. 58. Shehab M, Abu‐Farha M, Alrashed F, Alfadhli A, Alotaibi K, Alsahli A, et al. Immunogenicity of BNT162b2 vaccine in patients with inflammatory bowel disease on infliximab combination therapy: A multicenter prospective study. Journal of Clinical Medicine. 2021;10(22):5362. 59. Shehab M, Alrashed F, Alfadhli A, AlOtaibi K, AlSahli A, Mohammad H, et al. Serological response to BNT162b2 and ChAdOx1 nCoV‐19 vaccines in patients with inflammatory bowel disease on biologic therapies; a multi‐center prospective study. medRxiv. 2021:2021.10.31.21265718. 60. Shenoy P, Ahmed S, Cherian S, Paul A, Shenoy V, Vijayan A, et al. Immunogenicity of the ChAdOx1 nCoV‐19 and the BBV152 vaccines in patients with autoimmune rheumatic diseases. medRxiv. 2021:2021.06.06.21258417. 61. Shinjo SK, de Souza FHC, Borges IBP, Dos Santos AM, Miossi R, Misse RG, et al. Systemic autoimmune myopathies: A prospective phase 4 controlled trial of an inactivated virus vaccine against SARS‐CoV‐2. Rheumatology (Oxford). 2021. 62. Tallantyre EC, Vickaryous N, Anderson V, Asardag AN, Baker D, Bestwick J, et al. COVID‐19 vaccine response in people with multiple sclerosis. Annals of Neurology. 2022;91(1):89‐100. 63. Tzioufas AG, Bakasis AD, Goules AV, Bitzogli K, Cinoku, II, Chatzis LG, et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS‐CoV‐2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. Journal of Autoimmunity. 2021;125:102743. 64. Wang QL, Lv CZ, Han X, Shen MX, Kuang YH. A web‐based survey on factors for unvaccination and adverse reactions of SARS‐CoV‐2 vaccines in Chinese patients with psoriasis. Journal of Inflammation Research. 2021;14:6265‐73. 65. Zavala‐Flores E, Salcedo‐Matienzo J, Quiroz‐Alva A, Berrocal‐Kasay A. Side effects and flares risk after SARS‐CoV‐2 vaccination in patients with systemic lupus erythematosus. Clinical Rheumatology. 2021.
7. Studies on patients with HIV/AIDS
1. Brumme ZL, Mwimanzi F, Lapointe HR, Cheung P, Sang Y, Duncan MC, et al. Humoral immune responses to COVID‐19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. medRxiv. 2021:2021.10.03.21264320. 2. Levy I, Wieder‐Finesod A, Litchevsky V, Biber A, Indenbaum V, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in people living with HIV‐1. Clinical Microbiology and Infection. 2021;27(12):1851‐5. 3. Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 (AZD1222) vaccine against SARS‐CoV‐2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double‐blind, placebo‐controlled, phase 1B/2A trial. Lancet HIV. 2021;8(9):E568‐E80. 4. Nault L, Marchitto L, Goyette G, Tremblay‐Sher D, Fortin C, Martel‐Laferrière V, et al. Covid‐19 vaccine immunogenicity in people living with HIV‐1. bioRxiv. 2021:2021.08.13.456258. 5. Noe S, Ochana N, Wiese C, Schabaz F, Von Krosigk A, Heldwein S, et al. Humoral response to SARS‐CoV‐2 vaccines in people living with HIV. Infection. 2021:1‐7.
8. Studies on other solid organ transplants or mixed groups
1. Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID‐19 vaccination in solid organ transplant recipients. Transplant Infectious Disease. 2021;23(5):e13705. 2. Boyarsky BJ, Barbur I, Chiang TPY, Ou MT, Greenberg RS, Teles AT, et al. SARS‐CoV‐2 messenger RNA vaccine immunogenicity in solid organ transplant recipients with prior COVID‐19. Transplantation. 2021;105(11):E270‐E1. 3. Boyarsky BJ, Chiang TP, Ou MT, Werbel WA, Massie AB, Segev DL, et al. Antibody response to the Janssen COVID‐19 vaccine in solid organ transplant recipients. Transplantation. 2021;105(8):e82‐e3. 4. Boyarsky BJ, Chiang TP, Teles AT, Greenberg RS, Krach MR, Ou MT, et al. Antibody kinetics and durability in SARS‐CoV‐2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2021;105(10):e137‐e8. 5. Boyarsky BJ, Ou MT, Greenberg RS, Teles AT, Werbel WA, Avery RK, et al. Safety of the first dose of SARS‐CoV‐2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56‐e7. 6. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐6. 7. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784‐6. 8. Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, et al. Efficiency of a boost with a third dose of anti‐SARS‐CoV‐2 messenger RNA‐based vaccines in solid organ transplant recipients. American Journal of Transplantation. 2022;22(1):322‐3. 9. Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak‐Kita B, et al. Humoral and cellular immune response and safety of two‐dose SARS‐CoV‐2 mRNA‐1273 vaccine in solid organ transplant recipients. American Journal of Transplantation. 2021;21(12):3980‐9. 10. Hallett AM, Greenberg RS, Boyarsky BJ, Shah PD, Ou MT, Teles AT, et al. SARS‐CoV‐2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. The Journal of Heart and Lung Transplantation. 2021;40(12):1579‐88. 11. Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole‐González E, et al. Cellular and humoral immune response after mRNA‐1273 SARS‐CoV‐2 vaccine in liver and heart transplant recipients. American Journal of Transplantation. 2021;21(12):3971‐9. 12. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. New England Journal of Medicine. 2021;385(7):661‐2. 13. Kumar D, Ferreira VH, Hall VG, Hu Q, Samson R, Ku T, et al. Neutralization of SARS‐CoV‐2 variants in transplant recipients after two and three doses of mRNA‐1273 vaccine: secondary analysis of a randomized trial. Annals of Internal Medicine. 2022;175(2):226‐33. 14. Malinis M, Cohen E, Azar MM. Effectiveness of SARS‐CoV‐2 vaccination in fully vaccinated solid organ transplant recipients. American Journal of Transplantation. 2021;21(8):2916‐8. 15. Mazzola A, Todesco E, Drouin S, Hazan F, Marot S, Thabut D, et al. Poor antibody response after two doses of SARS‐CoV‐2 vaccine in transplant recipients. Clinical Infectious Diseases. 2021. 16. Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, et al. Safety and reactogenicity of 2 doses of SARS‐CoV‐2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(10):2170‐4. 17. Ravanan R, Mumford L, Ushiro‐Lumb I, Callaghan C, Pettigrew G, Thorburn D, et al. Two doses of SARS‐CoV‐2 vaccines reduce risk of death due to COVID‐19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021;105(11):e263‐e4.
9. Studies on other comorbidities or mixed cohorts, i.e. mixed immunosuppressed
1. Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post‐vaccination SARS‐CoV‐2 infection in UK users of the COVID Symptom Study app: a prospective, community‐based, nested, case‐control study. Lancet Infectious Diseases. 2022;22(1):43‐55. 2. Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS‐CoV‐2 in five groups of immunocompromised patients and healthy controls in a prospective open‐label clinical trial. eBioMedicine. 2021;74:103705. 3. Boekel L, Kummer LY, van Dam KPJ, Hooijberg F, van Kempen Z, Vogelzang EH, et al. Adverse events after first COVID‐19 vaccination in patients with autoimmune diseases. Lancet Rheumatology. 2021;3(8):E541‐E5. 4. Caminati M, Guarnieri G, Batani V, Scarpieri E, Finocchiaro A, Chieco‐Bianchi F, et al. COVID‐19 vaccination in patients with severe asthma on biologic treatment: Safety, tolerability, and impact on disease control. Vaccines. 2021;9(8). 5. Chodick G TLRRSPTGSB‐TAWCGITGCDMK. The effectiveness of the two‐dose BNT162b2 vaccine: analysis of real‐world data. Clinical Infectious Diseases. 2021. 6. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS‐CoV‐2: a prospective cohort study. Annals of Internal Medicine. 2021;174(11):1572‐85. 7. Di Fusco M, Moran MM, Cane A, Curcio D, Khan F, Malhotra D, et al. Evaluation of COVID‐19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. Journal of Medical Economics. 2021;24(1):1248‐60. 8. Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, Natarajan K, et al. Effectiveness of 2‐dose vaccination with mRNA COVID‐19 vaccines against COVID‐19‐associated hospitalizations among immunocompromised adults ‐ nine States, January‐September 2021. MMWR Morbidity and mortality weekly report. 2021;70(44):1553‐9. 9. Fang X, Qiao S, Zhang R, Yang T, Wang Z, Kong Q, et al. Effect of COVID‐19 vaccination on seizures in patients with epilepsy: A multicenter, retrospective study. Research Square. 2022. 10. Firinu D, Perra A, Campagna M, Littera R, Fenu G, Meloni F, et al. Evaluation of antibody response to BNT162b2 mRNA COVID‐19 vaccine in patients affected by immune‐mediated inflammatory diseases up to 5 months after vaccination. Clinical and Experimental Medicine. 2021. 11. Fusco F, Scognamiglio G, Merola A, Roma AS, Nicastro C, Spatarella M, et al. COVID‐19 vaccination in adults with congenital heart disease: Real‐world data from an Italian tertiary centre. International Journal of Cardiology Congenital Heart Disease. 2021;6:100266. 12. Haidar G, Agha M, Lukanski A, Linstrum K, Troyan R, Bilderback A, et al. Immunogenicity of COVID‐19 vaccination in immunocompromised patients: An observational, prospective cohort study interim analysis. medRxiv. 2021:2021.06.28.21259576. 13. Lee EJ, Beltrami Moreira M, Al‐Samkari H, Cuker A, DiRaimo J, Gernsheimer T, et al. SARS‐CoV‐2 Vaccination and Immune Thrombocytopenia in de novo and pre‐existing ITP patients. Blood. 2021. 14. Liao S‐Y, Gerber AN, Zelarney P, Make B, Wechsler ME. Impaired SARS‐CoV‐2 mRNA Vaccine Antibody Response in Chronic Medical Conditions: A Real‐World Analysis. Chest. 2022. 15. Lin S, Kennedy NA, Saifuddin A, Sandoval DM, Reynolds CJ, Seoane RC, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS‐CoV‐2 vaccine doses in infliximab‐ and vedolizumab‐treated patients. medRxiv. 2021:2021.11.10.21266168. 16. Lotan I, Romanow G, Levy M. Patient‐reported safety and tolerability of the COVID‐19 vaccines in persons with rare neuroimmunological diseases. Multiple Sclerosis and Related Disorders. 2021;55:103189. 17. Lotan I, Wilf‐Yarkoni A, Friedman Y, Stiebel‐Kalish H, Steiner I, Hellmann MA. Safety of the BNT162b2 COVID‐19 vaccine in multiple sclerosis (MS): Early experience from a tertiary MS center in Israel. European Journal of Neurology. 2021;28(11):3742‐8. 18. Lu L, Zhang Q, Xiao J, Zhang YY, Peng W, Han X, et al. COVID‐19 vaccine take‐up rate and safety in adults with epilepsy: Data from a multicenter study in China. Epilepsia. 2021. 19. Marfella R, D'Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, et al. Does poor glycaemic control affect the immunogenicity of the COVID‐19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes, Obesity and Metabolism. 2021. 20. Nadesalingam A, Cantoni D, Wells DA, Aguinam ET, Ferrari M, Smith P, et al. Paucity and discordance of neutralising antibody responses to SARS‐CoV‐2 VOCs in vaccinated immunodeficient patients and health‐care workers in the UK. Lancet Microbe. 2021;2(9):e416‐e8. 21. Nittner‐Marszalska M, Rosiek‐Biegus M, Kopeć A, Pawłowicz R, Kosińska M, Łata A, et al. Pfizer‐BioNTech COVID‐19 vaccine tolerance in allergic versus non‐allergic individuals. Vaccines (Basel). 2021;9(6). 22. Peck RC, Clark A, Shapiro S. Experience of Covid 19 vaccination in patients with bleeding disorders. Haemophilia. 2022;28(1):e9‐e11. 23. Prendecki M, Clarke C, Edwards H, McIntyre S, Mortimer P, Gleeson S, et al. Humoral and T‐cell responses to SARS‐CoV‐2 vaccination in patients receiving immunosuppression. Annals of the Rheumatic Diseases. 2021;80(10):1322‐9. 24. Rahav G, Lustig Y, Lavee J, Ohad B, Magen H, Hod T, et al. BNT162b2 mRNA COVID‐19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine. 2021;41:101158. 25. Ramanathan M, Murugesan K, Yang LM, Costales C, Bulterys PL, Schroers‐Martin J, et al. Cell‐Mediated and humoral immune response to 2‐Dose SARS‐CoV2 mRNA vaccination in Immunocompromised patient population. medRxiv. 2021:2021.07.21.21260921. 26. Rojas‐Pérez‐Ezquerra P, Crespo Quirós J, Tornero Molina P, Baeza Ochoa de Ocáriz ML, Zubeldia Ortuño JM. Safety of new mRNA vaccines against COVID‐19 in severely allergic patients. Journal of Investigational Allergology & Clinical Immunology. 2021;31(2):180‐1. 27. Rotondo C, Cantatore FP, Fornaro M, Colia R, Busto G, Rella V, et al. Preliminary data on post market safety profiles of COVID 19 vaccines in rheumatic diseases: assessments on various vaccines in use, different rheumatic disease subtypes, and immunosuppressive therapies: a two‐centers study. Vaccines (Basel). 2021;9(7). 28. Sankary KM, Sippel JL, Eberhart AC, Burns SP. Breakthrough cases of COVID‐19 in vaccinated United States Veterans with spinal cord injuries and disorders. Spinal Cord. 2021. 29. Shapiro Ben David S, Potasman I, Rahamim‐Cohen D. Rate of recurrent Guillain‐Barre syndrome after mRNA COVID‐19 vaccine BNT162b2. JAMA Neurology. 2021. 30. Shapiro Ben David S, Shamir‐Stein N, Baruch Gez S, Lerner U, Rahamim‐Cohen D, Ekka Zohar A. Reactogenicity of a third BNT162b2 mRNA COVID‐19 vaccine among immunocompromised individuals and seniors ‐ A nationwide survey. Clinical Immunology. 2021;232:108860. 31. Shavit R, Maoz‐Segal R, Iancovici‐Kidon M, Offengenden I, Yahia SH, Maayan DM, et al. Prevalence of allergic reactions after Pfizer‐BioNTech COVID‐19 vaccination among adults with high allergy risk. JAMA Network Open. 2021;4(8). 32. Skroza N, Bernardini N, Tolino E, Proietti I, Mambrin A, Marchesiello A, et al. Safety and Impact of Anti‐COVID‐19 Vaccines in Psoriatic Patients Treated with Biologics: A Real Life Experience. Journal of Clinical Medicine. 2021;10(15). 33. Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, et al. Effect of SARS‐CoV‐2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. 34. Sriskandarajah P, Hobart J, Radia DH, Whyte AF. A UK survey examining the experience of adults with mastocytosis receiving COVID‐19 vaccination. Hemasphere. 2021;5(11):e650. 35. Tzur Bitan D, Kridin K, Cohen AD, Weinstein O. COVID‐19 hospitalisation, mortality, vaccination, and postvaccination trends among people with schizophrenia in Israel: a longitudinal cohort study. Lancet Psychiatry. 2021;8(10):901‐8. 36. Wang L, Wang Q, Davis PB, Volkow ND, Xu R. Increased risk for COVID‐19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry. 2022;21(1):124‐32. 37. Xiang TD, Liang BY, Wang H, Quan XF, He SS, Zhou HL, et al. Safety and immunogenicity of a SARS‐CoV‐2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cellular & Molecular Immunology. 2021;18(12):2679‐81.
10. Studies on paediatric participants
1. Aiano F, Campbell C, Saliba V, Ramsay ME, Ladhani SN. COVID‐19 vaccine given to children with comorbidities in England, December 2020‐June 2021. Archives of Disease in Childhood. 2021. 2. Alamer E, Alhazmi A, Qasir NA, Alamer R, Areeshi H, Gohal G, et al. Side effects of COVID‐19 Pfizer‐BioNTech mRNA vaccine in children aged 12–18 years in Saudi Arabia. Vaccines. 2021;9(11):1297. 3. Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado‐Voges M, et al. Evaluation of mRNA‐1273 SARS‐CoV‐2 Vaccine in Adolescents. New England Journal of Medicine. 2021;385(24):2241‐51. 4. Frenck RW, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid‐19 vaccine in adolescents. New England Journal of Medicine. 2021;385(3):239‐50. 5. Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy children and adolescents: a double‐blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infectious Diseases. 2021;21(12):1645‐53. 6. Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID‐19 vaccine safety in adolescents aged 12‐17 years ‐ United States, December 14, 2020‐July 16, 2021. MMWR Morbidity and mortality weekly report. 2021;70(31):1053‐8. 7. Olson Sm NMMHNBPAMBJASLCIKWTCSS. Effectiveness of Pfizer‐BioNTech mRNA vaccination against COVID‐19 hospitalization among persons aged 12‐18 years ‐ United States, June‐September 2021. MMWR Morbidity and mortality weekly report. 2021;70(42):1483‐8. 8. Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, et al. Evaluation of the BNT162b2 Covid‐19 Vaccine in Children 5 to 11 Years of Age. The New England journal of medicine. 2022;386(1):35‐46.
X. Additional references to included studies (clinical trial records, preprints, secondary publications)
1. Achiron A, Mandel M, Dreyer‐Alster S, Harari G, Dolev M, Menascu S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross‐sectional study. Journal of Neuroimmunology. 2021;361:577746. 2. Ammitzbøll C, Bartels LE, Bøgh Andersen J, Risbøl Vils S, Elbaek Mistegård C, Dahl Johannsen A, et al. Impaired antibody response to the BNT162b2 messenger RNA Coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatology. 2021;3(9):622‐8. 3. Anand S, Montez‐Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. SARS‐CoV‐2 vaccine antibody response and breakthrough infections in patients receiving dialysis. MedRxiv. 2021. 4. Anand S M‐RMEHJGPCLHPKRBPDMBGABS. Antibody response to COVID‐19 vaccination in patients receiving dialysis (preprint). MedRxiv. 2021:2021.05.06.212567682021.05.06.21256768. 5. Araujo CSR, Medeiros‐Ribeiro AC, Saad CGS, Bonfiglioli KR, Domiciano DS, Shimabuco AY, et al. A randomized clinical trial of 2‐week methotrexate discontinuation in rheumatoid arthritis patients vaccinated with inactivated SARS‐COV‐2 vaccine. MedRxiv. 2021. 6. Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat‐Panah D, Dehghani M, Nabavifard M, et al. Immunogenicity and Safety of the inactivated SARS‐CoV‐2 vaccine (BBIBP‐CoV) in patients with malignancy. MedRxiv. 2021. 7. Atyeo C, DeRiso EA, Davis C, Bordt EA, De Guzman RM, Shook LL, et al. COVID‐19 mRNA vaccines drive differential antibody Fc‐functional profiles in pregnant, lactating, and nonpregnant women. Science Translational Medicine. 2021;13(617). 8. Atyeo C, Deriso EA, Davis C, Bordt EA, De Guzman RM, Shook LL, et al. COVID‐19 mRNA vaccines drive differential Fc‐functional profiles in pregnant, lactating, and non‐pregnant women (preprint). BioRxiv. 2021:2021.04.04.438404. 9. Avivi I, Balaban R, Shragai T, Sheffer G, Morales M, Aharon A, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. British Journal of Haematology. 2021;195(2):186‐93. 10. Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa‐Cohen Y, et al. Efficient maternal to neonatal transfer of SARS‐CoV‐2 and BNT162b2 antibodies (preprint). MedRxiv. 2021:2021.03.31.21254674. 11. Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa‐Cohen Y, et al. Efficient maternal to neonatal transfer of SARS‐CoV‐2 and BNT162b2 antibodies. MedRxiv. 2021. 12. Benotmane I, Gautier‐Vargas G, Cognard N, Olagne J, Heibel F, Braun‐Parvez L, et al. Weak anti‐SARS‐CoV‐2 antibody response after the first injection of an mRNA COVID‐19 vaccine in kidney transplant recipients (preprint). MedRxiv. 2021:2021.03.08.21252741. 13. Bernstine H, Priss M, Anati T, Turko O, Gorenberg M, Steinmetz AP, et al. Axillary lymph nodes hypermetabolism after BNT162b2 mRNA COVID‐19 vaccination in cancer patients undergoing F‐18‐FDG PET/CT ‐ a cohort study. Clinical Nuclear Medicine. 2021;46(5):396‐401. 14. Boekel L, Steenhuis M, Hooijberg F, Besten Y, van Kempen Z, Kummer L, et al. Antibody development after SARS‐CoV‐2 vaccinations in elderly patients with autoimmune diseases: data from a prospective controlled cohort study (preprint). SSRN. 2021. 15. Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, Kummer LY, et al. Antibody development after COVID‐19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatology. 2021;3(11):e778‐e88. 16. Botwin GJ, Li D, Figueiredo J, Cheng S, Braun J, McGovern DPB, et al. Adverse events following SARS‐CoV‐2 mRNA vaccination among patients with Inflammatory Bowel Disease (preprint). MedRxiv. 2021:2021.03.30.21254607. 17. Braun‐Moscovici Y, Kaplan M, Markovits D, Giryes S, Toledano K, Tavor Y, et al. Humoral response to Pfizer mRNA vaccine against SARS CoV2, in patients with autoimmune inflammatory rheumatic diseases and the impact on the rheumatic disease activity. MedRxiv. 2021. 18. Brumme ZL, Mwimanzi F, Lapointe HR, Cheung P, Sang Y, Duncan MC, et al. Humoral immune responses to COVID‐19 vaccination in people living with HIV on suppressive antiretroviral therapy. MedRxiv. 2021. 19. Brunelli SM, Sibbel S, Karpinski S, Marlowe G, Walker AG, Giullian J, et al. Comparative Effectiveness of BNT162b2 versus Ad26.COV2.S for the Prevention of COVID‐19 among Dialysis Patients. medRxiv. 2021:2021.10.21.21265339. 20. Buttiron Webber T, Provinciali N, Musso M, Ugolini M, Boitano M, Clavarezza M, et al. Predictors of poor seroconversion and adverse events to SARS‐CoV‐2 mRNA BNT162b2 vaccine in cancer patients on active treatment (preprint). Lancet Preprints. 2021. 21. Caroline A, Elizabeth AD, Christine D, Evan AB, Rose MDG, Lydia LS, et al. COVID‐19 mRNA vaccines drive differential Fc‐functional profiles in pregnant, lactating, and non‐pregnant women. BioRxiv. 2021. 22. Carr EJ, Wu M, Harvey R. Neutralising antibodies after COVID‐19 vaccination in UK haemodialysis patients (vol 398, pg 1038, 2021). Lancet. 2021;398(10305):1042‐. 23. Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, et al. COVID‐19 vaccines in adult cancer patients with solid tumors undergoing active treatment: seropositivity and safety. A prospective observational study in Italy. European Journal of Cancer. 2021. 24. Cohen D, Hazut Krauthammer S, Cohen YC, Perry C, Avivi I, Herishanu Y, et al. Correlation between BNT162b2 mRNA Covid‐19 vaccine‐associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. European Journal of Nuclear Medicine and Molecular Imaging. 2021;48(11):3540‐9. 25. Cohen D, Krauthammer SH, Wolf I, Even‐Sapir E. A sigh of relief: vaccine‐associated hypermetabolic lymphadenopathy following the third COVID‐19 vaccine dose is short in duration and uncommonly interferes with the interpretation of [F‐18]FDG PET‐CT studies performed in oncologic patients. European Journal of Nuclear Medicine and Molecular Imaging.49:1338‐44. 26. Dailey J, Kozhaya L, Dogan M, Hopkins D, Lapin B, Herbst K, et al. Antibody responses to SARS‐CoV‐2 after infection or vaccination in children and young adults with Inflammatory Bowel Disease (preprint). MedRxiv. 2021:2021.06.12.21258810. 27. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El‐Qunni AA, et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS‐CoV‐2. MedRxiv. 2021. 28. Edelman‐Klapper H, Zittan E, Bar‐Gil Shitrit A, Rabinowitz KM, Goren I, Avni‐Biron I, et al. Decreased Immune Response to COVID‐19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases Treated with Anti TNFα. MedRxiv. 2021. 29. Elhalel MD, Tzukert K, Levi IMY, Burstain I, Chen HP, Oster Y, et al. Five‐month impact of tozinameran (BNT162b2) vaccine on kidney transplant and dialysis patients: Serology and clinical outcomes. Journal of the American Society of Nephrology. 2021;32:101‐. 30. Eliakim‐Raz N, Massarweh A, Stemmer A, Stemmer SM. Durability of response to SARS‐CoV‐2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncology. 2021. 31. Ente Ospedaliero Ospedali G. BNT162b2 Messenger Ribonucleic Acid (mRNA) Covid‐19 Vaccine in Cancer Patients on Active Treatment. ClinicalTrialsgov. 2021. 32. Etemadifar M, Sedaghat N, Nouri H, Lotfi N, Chitsaz A, Khorvash R, et al. SARS‐CoV‐2 Serology Among People with Multiple Sclerosis on Disease‐Modifying Therapies after BBIBP‐CorV (Sinopharm) Inactivated Virus Vaccination: Same Story, Different Vaccine (preprint). Lancet Preprints. 2021. 33. Felten R, Kawka L, Dubois M, Ugarte‐Gil MF, Fuentes‐Silva Y, Piga M, et al. Tolerance of COVID‐19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study Comment. Lancet Rheumatology. 2021;3(9):E613‐E5. 34. Gounant V, Ferré VM, Soussi G, Charpentier C, Flament H, Fidouh N, et al. Efficacy of SARS‐CoV‐2 vaccine in thoracic cancer patients: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. MedRxiv. 2021. 35. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. COVID‐19 vaccine response in pregnant and lactating women: a cohort study. MedRxiv. 2021. 36. Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate Hampers Immunogenicity to BNT162b2 mRNA COVID‐19 Vaccine in Immune‐Mediated Inflammatory Disease (preprint). Medrxiv. 2021:2021.05.11.212569172021.05.11.21256917. 37. Healy K, Pin E, Chen P, Soderdahl G, Nowak P, Mielke S, et al. Appearance of IgG to SARS‐CoV‐2 in saliva effectively indicates seroconversion in mRNA vaccinated immunocompromised individuals. MedRxiv. 2021. 38. Hellenic Cooperative Oncology G. Responses to COVID‐19 Vaccination in Patients With Cancer. ClinicalTrialsgov. 2021. 39. Hepatopancreatobiliary Surgery Institute of Gansu P. Effectiveness and Safety of the COVID‐19 Vaccination for Patients With Liver Disease (CHESS2101). ClinicalTrialsgov. 2021. 40. Hospices Civils de L. Response of Haemodialysis Patients to BNT162b2 mRNA Cov‐19 Vaccine. ClinicalTrialsgov. 2021. 41. Hsu CM, Weiner DE, Manley HJ, Aweh GN, Ladik V, Frament J, et al. Seroresponse to SARS‐CoV‐2 vaccines among maintenance dialysis patients over six months. medRxiv. 2021:2021.09.13.21263535. 42. Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV‐19 SARS‐CoV‐2 vaccines. MedRxiv. 2021. 43. Lacson E, Jr., Argyropoulos CP, Manley HJ, Aweh G, Chin AI, Salman LH, et al. Immunogenicity of SARS‐CoV‐2 vaccine in dialysis (preprint). MedRxiv. 2021:2021.04.08.21254779. 44. Lim SH, Campbell N, Johnson M, Joseph‐Pietras D, Collins GP, O'Callaghan A, et al. Antibody responses after SARS‐CoV‐2 vaccination in lymphoma. MedRxiv. 2021. 45. Lim SH, Campbell N, Joseph‐Pietras D, Johnson M, Mundy C, Coleman H, et al. Serological responses after SARS‐CoV‐2 vaccination first dose in patients with lymphoid malignancy: first interim analysis of the UK proseco study. Haematological Oncology. 2021;39(S2). 46. Lymphoma, Leukemia S. The Lymphoma and Leukemia Society COVID‐19 Registry. ClinicalTrialsgov. 2021. 47. Marfella R, D'Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, et al. Does poor glycemic control affect the immunogenicity of COVID‐19 vaccination in patients with Type 2 Diabetes: the CAVEAT study. Diabetes, Obesity & Metabolism. 2021;24(1):160‐5. 48. Masset C, Kerleau C, Garandeau C, Ville S, Cantarovich D, Hourmant M, et al. A third injection of BNT162b2 mRNA Covid‐19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney International. 2021. 49. Medical University of G. Assessment of Immune Response After Vaccination Against COVID‐19 in Dialyzed Patients. ClinicalTrialsgov. 2021. 50. ModernaTx I. A Study to Evaluate the Safety, Reactogenicity, and Effectiveness of mRNA‐1273 Vaccine in Adolescents 12 to <18 Years Old to Prevent COVID‐19. ClinicalTrialsgov. 2020. 51. Monin‐Aldama L, Laing AG, Muñoz‐Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Interim results of the safety and immune‐efficacy of 1 versus 2 doses of COVID‐19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. MedRxiv. 2021. 52. Moyon Q, Sterlin D, Miyara M, Anna F, Mathian A, Lhote R, et al. BNT162b2 vaccine‐induced humoral and cellular responses against SARS‐CoV‐2 variants in Systemic Lupus Erythematosus (preprint). MedRxiv. 2021:2021.07.07.21260124. 53. Nadesalingam A, Cantoni D, Wells DA, Aguinam ET, Ferrari M, Smith P, et al. Breadth of neutralising antibody responses to SARS‐CoV‐2 variants of concern is augmented by vaccination following prior infection: studies in UK healthcare workers and immunodeficient patients. MedRxiv. 2021. 54. Peeters M, Verbruggen L, Teuwen L, Vanhoutte G, Kerckhove SVPB, Raats S, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021:100274‐. 55. Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with B‐cell non‐Hodgkin lymphoma. Blood Advances. 2021;5(16):3053‐61. 56. Prabhu M, Murphy EA, Sukhu AC, Yee J, Singh S, Eng D, et al. Antibody response to SARS‐CoV‐2 mRNA vaccines in pregnant women and their neonates. BioRxiv. 2021. 57. Prendecki M, Thomson T, Clarke CL, Martin P, Gleeson S, De aguiar RC, et al. Comparison of humoral and cellular responses in kidney transplant recipients receiving BNT162b2 and ChAdOx1 SARS‐CoV‐2 vaccines. MedRxiv. 2021. 58. Ramasamy K, Sadler R, Jeans S, Varghese S, Turner A, Larham J, et al. COVID symptoms, testing, shielding impact on patient reported outcomes and early vaccine responses in individuals with multiple myeloma (preprint). MedRxiv. 2021:2021.05.20.21257379. 59. Rottenstreich A, Zarbiv G, Oiknine‐Djian E, Vorontsov O, Zigron R, Kleinstern G, et al. Early versus late third trimester maternal SARS‐CoV‐2 BNT162b2 mRNA immunization maximizes transplacental antibody transfer and neonatal neutralizing antibody levels. MedRxiv. 2021. 60. Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener‐Well Y, Grisaru‐Granovsky S. Covid‐19 vaccination during the third trimester of pregnancy: rate of vaccination and the maternal and neonatal outcomes, a multicenter retrospective cohort study. BJOG: an international journal of obstetrics and gynaecology. 2021. 61. Rozen‐Zvi B, Yahav D, Agur T, Zingerman B, Ben‐Zvi H, Atamna A, et al. Antibody response to SARS‐CoV‐2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clinical Microbiology and Infection. 2021;27(8). 62. Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Budde K, et al. Impaired humoral and cellular immunity after SARS‐CoV2 BNT162b2 (Tozinameran) prime‐boost vaccination in kidney transplant recipients (preprint). MedRxiv. 2021:2021.04.06.21254963. 63. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA Covid‐19 vaccine safety in pregnant persons (vol 384, pg 2273, 2021). New England Journal of Medicine. 64. Shook LL, Atyeo CG, Yonker LM, Fasano A, Gray KJ, Alter G, et al. Durability of anti‐Spike antibodies in the infant after maternal COVID‐19 vaccination (preprint). MedRxiv. 2021. 65. Simon B, Rubey H, Treipl A, Gromann M, Hemedi B, Zehetmayer S, et al. Hemodialysis patients show a highly diminished antibody response after COVID‐19 mRNA vaccination compared to healthy controls. MedRxiv. 2021. 66. Strauss AT, Hallett AM, Boyarsky BJ, Ou MCT, Werbel WA, Avery RK, et al. Antibody response to SARS‐CoV‐2 messenger RNA vaccines in liver transplant recipients. Liver Transplantation. 2021;27(12):1852‐6. 67. Tel‐Aviv Sourasky Medical C. The Capability of Haemato‐oncology Patients to Generate Antibodies Against COVID‐19. ClinicalTrialsgov. 2021. 68. Thakkar A, Gonzalez‐Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID‐19 vaccination amongst patients with malignant disease‐ the impact of diagnosis and cancer‐directed therapies. MedRxiv. 2021. 69. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS‐CoV‐2 vaccination in pregnancy (preprint). MedRxiv. 2021:2021.05.17.21257337. 70. Tylicki L, Biedunkiewicz B, Dąbrowska M, Ślizień W, Tylicki P, Polewska K, et al. Humoral response to SARS‐CoV‐2 vaccination promises to improve the catastrophic prognosis of hemodialysis patients as a result of COVID‐19: the COViNEPH Project. Polish Archives of Internal Medicine. 2021;131(9):797‐801. 71. University Hospital Southampton NHSFT. Immune Responses to COVID‐19 Vaccination in Lymphoma Patients. ClinicalTrialsgov. 2021. 72. University of Witwatersrand, South Africa. COVID‐19 vaccine (ChAdOx1 nCoV‐19) trial in South African adults with and without HIV‐infection. ClinicalTrialsgov. 2020. 73. Vilnius University. Analysis of Immunogenicity, Safety and Efficacy of COVID‐19 Vaccines in Immunosuppressed Individuals. ClinicalTrialsgov. 2021. 74. Wang JT, Hou ZY, Liu JX, Gu Y, Wu YH, Chen ZH, et al. Safety and immunogenicity of Coronavirus disease 2019 vaccination in patients with chronic liver disease: a multicenter study. Journal of Hepatology. 2021;75:S359‐S. 75. Yau K, Abe KT, Naimark D, Oliver MJ, Perl J, Leis JA, et al. The humoral response to the BNT162b2 vaccine in hemodialysis patients. medRxiv. 2021:2021.05.24.21257425. 76. Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, et al. Receipt of mRNA COVID‐19 vaccines preconception and during pregnancy and risk of self‐reported spontaneous abortions, CDC v‐safe COVID‐19 Vaccine Pregnancy Registry 2020‐21. Research Square. 2021.
Differences between protocol and review
During the review process, we modified several decisions made at protocol stage during weekly group discussions, as follows.
| Protocol stage | Full review | Reasons | |
| Inclusion criteria | Complete vaccination | From one vaccine onwards | To obtain a more comprehensive account of outcome reporting (we also listed outcomes that were reported after one dose in publications on two or more doses). |
| No restriction on population; we planned to include any study on healthy general population as well as subgroups ≥ 100 participants | Limitation to certain subgroups ≥ 100 participants | Feasibility; inclusion and data extraction of ≥ 2000 publications not feasible | |
| ≥ 10 participants for studies with booster vaccination | ≥ 100 participants for studies with booster vaccination | Increase in available booster studies as compared to time point of protocol registration | |
| We originally planned to list studies per age group, i.e. children, young adults, adults, elderly. | The age group of children and adolescents (< 18) vs adults of any age | Due to change in inclusion criteria (limit to certain subgroups), the account may be incomplete, therefore adult participants are not further divided into age classes. | |
| Ongoing studies (e.g. trial registry entries, study protocols) without accompanying full‐text publication included | Ongoing studies listed along with excluded studies in an online appendix | The relevant ongoing studies are available via an online repository, but are not further sorted in the scoping review. As many of the included studies are based on retrospective data, a detailed charting on registered trials would provide an incomplete account on what to expect in the near future. | |
| Data extraction | Data extraction to be performed in a standardised MS Excel file | The Covidence data extraction form option | Easier implementation of duplicate extraction with numerous extractors. |
| Capturing the predominance of different SARS‐CoV‐2 variants during study conduct | SARS‐CoV‐2 variants during study conduct were not captured. | The majority of publications would not have reported this, which would have resulted in guesses based on date and place of enrolment. | |
| Outcomes | Correlation between immunity parameters and clinical outcomes, transmissibility, time to infection | Correlation between immunity parameters and clinical outcomes, transmissibility, and time to infection were not assessed. | This led to very heterogeneous data extraction and was not feasible. |
Contributions of authors
| Task | Contributors |
| Conception of the review | NK, CH, BL, CSc, MS, CSt, NS |
| Development of the search strategy | IM |
| Study selection | NK, CH, MA, LB, PJB, VDC, MG, RH, TL, SM, AM, VP, KV, SW, CSt, NS |
| Data extraction | NK, CH, MA, LB, VDC, MG, RH, VK, TL, AM, YSP, FW, SW, CSt, NS |
| Representation (map/tables/flow diagram) | NK, CH |
| Writing of the manuscript | NK, CH, RH, NS |
| Comments and approval of the final draft | NK, CH, MA, LB, PJB, VDC, MG, RH, VK, BL, TL, SM, AM, IM, YSP, VP, CSc, MS, KV, FW, SW, CSt, NS |
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Cochrane Haematology, Department I of Internal Medicine
-
Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt‐Universität zu Berlin, Berlin, Germany, Germany
Department of Infectious Diseases and Respiratory Medicine
External sources
-
Federal Ministry of Education and Research, Germany
This review was initiated as part of the project "COVIM", NaFoUniMedCovid19 (funding number: 01KX2021)
-
Federal Ministry of Education and Research, Germany
This review was contributed to by members of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
Declarations of interest
Nina Kreuzberger: no relevant interests; staff role at Cochrane Haematology, University Hospital Cologne, Germany; member of the prognosis methods group.
Caroline Hirsch: no relevant interests; Managing Editor at Cochrane Haematology, but was not involved in the editorial process for this review.
Marike Andreas: none known.
Lena Böhm: none known.
Paul Bröckelmann: BeiGene USA, Inc. (grant/contract), Bristol‐Myers Squibb Foundation (grant/contract), Celgene (travel), Takeda Oncology (consultant); consultant for internal medicine fellow in haematology/oncology, Department of Internal Medicine, University Hospital Cologne, Germany.
Veronica Di Cristanziano: no relevant interests; virologist at the Institute of Virology of the University Hospital Cologne, Germany; involved in the study 'Immune responses to SARS‐CoV‐2 infection and vaccination in dialysis patients and kidney transplant recipients'. The study at hand was supported by intramural funds.
Martin Golinski: no relevant interests; anesthesiologist consultant in intensive care medicine, Department of Anesthesiology, University Medicine Center Goettingen, Georg August University, Goettingen, Germany.
Renate Hausinger: no relevant interests; resident, Department of Nephrology at Klinikum rechts der Isar, University Hospital, Munich, Germany.
Verena Kappler: none known.
Berit Lange: Bundesministerium für Bildung und Forschung (grant/contract for MuSPAD seroprevalence study).
Tina Lischetzki: none known.
Sibylle Mellinghoff: Gilead Foundation (travel), Octapharma USA Inc (consultant).
Agata Mikolajewska: no relevant interests; co‐ordination of Section COVRIIN and Work in Office of STAKOB (Competence and Treatment Centres for high consequence infectious diseases) at Robert Koch Institute Centre for Biological Threats and Special Pathogens (ZBS), Section Clinical Management and Infection Control.
Ina Monsef: no relevant interests; Information Specialist, Cochrane Haematology.
Yun Soo Park: none known.
Vanessa Piechotta: none known.
Christoph Schmaderer: none known.
Miriam Stegemann: no relevant interests; medical doctor, Charité Universitätsmedizin Berlin, Germany.
Kanika Vanshylla: patent regarding SARS‐2 neutralising antibodies filed by the University of Cologne (pending); Tober‐Lau P, Gruell H, Vanshylla K, et al. doi:10.3201/eid2805.220271 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Federal Institute for Drugs and Medical Devices German Center for Infection Research (DZIF); Vanshylla K, Tober‐Lau P, Gruell H, et al. doi:10.1016/S1473‐3099(22)00135‐9 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Federal Institute for Drugs and Medical Devices German Center for Infection Research (DZIF) Deutsche Forschungsgemeinschaft (DFG); Gruell H, Vanshylla K, Tober‐Lau P, et al. doi:10.1038/s41591‐021‐01676‐0 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Federal Institute for Drugs and Medical Devices German Center for Infection Research (DZIF) Deutsche Forschungsgemeinschaft (DFG); Mellinghoff SC, Robrecht S, Mayer L, et al. doi:10.1038/s41375‐021‐01500‐1 German Center for Infection Research (DZIF) Mildred Scheel School of Oncology German Cancer Aid; Müller L, Andrée M, Ostermann PN, et al. doi:10.3389/fmed.2021.746644 VIRus ALliance NRW (VIRAL) from the Ministry of Culture and Science NRW Jürgen Manchot Foundation; Tober‐Lau P, Schwarz T, Vanshylla K, et al. doi:10.1016/S2213‐2600(21)00456‐2 COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung Berlin Institute of Health (BIH) and Berlin University Alliance Deutsche Forschungsgemeinschaft (DFG); Hillus D, Schwarz T, Tober‐Lau P, et al. doi:10.1016/S2213‐2600(21)00357‐X COVIM: “NaFoUniMedCovid19” Bundesministerium für Bildung und Forschung, Laboratory of Experimental Immunology, Institute of Virology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Florencia Weber: no relevant interests; resident of anaesthesiology, Universitätsklinikum Würzburg, Germany.
Stephanie Weibel: no relevant interests; editor with Cochrane Anaesthesia.
Caspar Stephani: no relevant interests; medical doctor on an intensive care unit in Göttingen, Germany.
Nicole Skoetz: no relevant interests; editor with Cochrane Haematology, but was not involved in the editorial process for this review.
contributed equally: last authors
contributed equally: last authors
New
References
Additional references
Abu‐Raddad 2022
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. New England Journal of Medicine 2022;386:1804-16. [DOI: 10.1056/NEJMoa2200797] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barajas‐Nava 2021
- Barajas-Nava L. Development of SARS-CoV-2 vaccines. Boletín Médico del Hospital Infantil de México 2021;78(1):66-74. [DOI: 10.24875/BMHIM.20000217] [DOI] [PubMed] [Google Scholar]
Basta 2022
- Basta NE, Moodie EMM on behalf of the VIPER (Vaccines, Infectious disease Prevention, and Epidemiology Research) Group COVID-19 Vaccine Development and Approvals Tracker Team. COVID-19 vaccine tracker. covid19.trackvaccines.org/vaccines/ (accessed 20 December 2021).
Brockmann 2022
- Brockmann MA, Mwimanzi F, Lapointe HR, Sang Y, Agafitei O, Cheung PK, et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. Journal of Infectious Diseases 2022;225(7):1129-40. [DOI: 10.1093/infdis/jiab592] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2022
- Centers for Disease Control and Prevention. Joint statement from HHS Public Health and medical experts on COVID-19 booster shots. www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed 2 February 2022).
Cele 2022
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654-6. [DOI: 10.1038/s41586-021-04387-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collier 2021
- Collier DA, Ferreira IATM, Kotagiri P, Datir RP, Lim EY, Touizer E, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021;596:417-22. [DOI: 10.1038/s41586-021-03739-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 21 December 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Dejnirattisai 2022
- Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022;399:234-6. [DOI: 10.1016/S0140-6736(21)02844-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Earle 2021
- Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021;39(32):4423-8. [DOI: 10.1016/j.vaccine.2021.05.063] [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2021a
- European Medicines Agency. Human regulatory - COVID-19 vaccines. www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed 14 March 2022).
EMA 2021b
- European Medicines Agency. EMA recommends Nuvaxovid for authorisation in the EU. www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu (accessed 31 May 2022).
Feikin 2022
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg V, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399(10328):924-44. [DOI: 10.1016/S0140-6736(22)00152-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Beltran 2022
- Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022;185:457-66. [DOI: 10.1016/j.cell.2021.12.033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruell 2022
- Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nature Medicine 2022;28:477-80. [DOI: 10.1038/s41591-021-01676-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hausinger 2022
- Hausinger R, Bachmann Q, Crone-Rawe T, Hannane N, Schmaderer C, Kreuzberger N, et al. Efficacy, immunogenicity and harms of SARS-CoV-2 booster vaccination for kidney transplant recipients: a systematic review. osf.io/nsyq4 (accessed prior to 8 July 2022).
Herishanu 2021
- Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021;137(23):3165-73. [DOI: 10.1182/blood.2021011568] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hillus 2021
- Hillus D, Schwarz T, Tober-Lau P, Hastor H, Thibeault C, Kasper S, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. Lancet Respiratory Medicine 2021;9(11):1255-65. [DOI: 10.1016/S2213-2600(21)00357-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
International Initiative for Impact Evaluation
- International Initiative for Impact Evaluation. Evidence gap maps. www.3ieimpact.org/evidence-hub/evidence-gap-maps (accessed 31 May 2022).
Khoury 2021
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nature Medicine 2021;27(7):1205-11. [DOI: 10.1038/s41591-021-01377-8] [DOI] [PubMed] [Google Scholar]
Krammer 2020
- Krammer F. SARS-CoV-2 vaccines in development. Nature 2020;586(7830):516-27. [DOI: 10.1038/s41586-020-2798-3] [DOI] [PubMed] [Google Scholar]
Krause 2021
- Krause PR, Fleming TR, Peto R, Longini IM, Figueroa JP, Sterne JAC, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021;398(10308):1377-80. [DOI: 10.1016/S0140-6736(21)02046-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Andreas M, Böhm L, Bröckelmann PJ, Di Cristanziano V, et al. Immunity after COVID-19 vaccination: protocol for a scoping review. doi.org/10.17605/OSF.IO/HTGB8 (accessed prior to 8 July 2022). [DOI: 10.17605/OSF.IO/HTGB8] [DOI] [PMC free article] [PubMed]
Lee 2022
- Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022;376:e068632. [DOI: 10.1136/bmj-2021-068632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine‐Tiefenbrun 2022
- Levine-Tiefenbrun M, Yelin I , Alapi H, Katz R, Herzel E, Kuint J, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nature Medicine 2021;27:2108-10. [DOI: 10.1038/s41591-021-01575-4] [DOI] [PubMed] [Google Scholar]
Ligumsky 2022
- Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. Journal of the National Cancer Institute 2022;114(2):203-9. [DOI: 10.1093/jnci/djab174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lusvarghi 2021
- Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster–elicited serum but evades most convalescent serum and therapeutic antibodies. Science Translational Medicine 2021;14(645):eabn8543. [DOI: 10.1126/scitranslmed.abn8543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmoud 2021
- Mahmoud SA, Ganesan S, Naik S, Bissar S, Zamel IA, Warren KN, et al. Serological assays for assessing postvaccination SARS-CoV-2 antibody response. Microbiology Spectrum 2021;9(2):e00733-21. [DOI: 10.1128/Spectrum.00733-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PubMed] [Google Scholar]
MS Excel [Computer program]
- Microsoft Excel. Microsoft Corporation, 2018. Available at office.microsoft.com/excel.
Muecksch 2020
- Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal serological analysis and neutralizing antibody levels in Coronavirus disease 2019 convalescent patients. Journal of Infectious Diseases 2020;223(3):389-98. [DOI: 10.1093/infdis/jiaa659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osmanodja 2022
- Osmanodja B, Ronicke S, Budde K, Jens A, Hammett C, Koch N, et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. Journal of Clinical Medicine 2022;11(9):2565. [DOI: 10.3390/jcm11092565] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandey 2020
- Pandey SC, Pande V, Sati D, Upreti S, Samant M. Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sciences 2020;256:117956. [DOI: 10.1016/j.lfs.2020.117956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2021
- Patel MK, Bergeri I, Bresee JS, Cowling BJ, Crowcroft NS, Fahmy K, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine 2021;39(30):4013-24. [DOI: 10.1016/j.vaccine.2021.05.099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkmann 2021
- Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Marculescu R, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiology Spectrum 2021;9(1):e00247-21. [DOI: 10.1128/Spectrum.00247-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piechotta 2022
- Piechotta V, Mellinghoff SC, Hirsch C, Brinkmann A, Iannizzi C, Kreuzberger N, et al. Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: a systematic review. Blood Cancer Journal 2022;12(5):86. [DOI: 10.1038/s41408-022-00684-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Public Health Ontario 2021
- Ontario Agency for Health Protection and Promotion. COVID-19 real-world vaccine effectiveness – what we know so far. www.publichealthontario.ca/-/media/documents/ncov/covid-wwksf/2021/04/wwksf-vaccine-effectiveness.pdf?sc_lang=en (accessed 16 March 2022).
Ramos 2022
- Ramos A, Cardoso MJ, Ribeiro L, Guimarães JT. Assessing SARS-CoV-2 neutralizing antibodies after BNT162b2 vaccination and their correlation with SARS-CoV-2 IgG anti-S1, anti-RBD and anti-S2 serological titers. Diagnostics 2022;12(1):205. [DOI: 10.3390/diagnostics12010205] [DOI] [PMC free article] [PubMed] [Google Scholar]
R Core Team [Computer program]
- R Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team. Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at R-project.org.
Rubio‐Acero 2021
- Rubio-Acero R, Castelletti N, Fingerle V, Olbrich L, Bakuli A, Wölfel R, et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infectious Diseases and Therapy 2021;10:1505-18. [DOI: 10.1007/s40121-021-00475-x. Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2021
- Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of a heterologous COVID-19 prime-boost vaccination compared with homologous vaccine regimens. Nature Medicine 2021;27:1530-5. [DOI: 10.1038/s41591-021-01464-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2022
- Schmidt F, Muecksch F, Weisblum Y, Da Silva J, Bednarski E, Cho A. Plasma neutralization of the SARS-CoV-2 Omicron variant. New England Journal of Medicine 2022;386:599-601. [DOI: 10.1056/NEJMc2119641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrezenmeier 2022
- Schrezenmeier E, Rincon-Arevalo H, Jens A, Stefanski AL, Hammett C, Osmanodja B, et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022;7(9):e157836. [DOI: 10.1172/jci.insight.157836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 2021
- Schwarz T, Tober-Lau P, Hillus D, Helbig ET, Lippert LJ, Thibeault C, et al. Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerging Infectious Diseases 2021;27(8):2174-8. [DOI: 10.3201/eid2708.211145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sette 2021
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021;184:861-80. [DOI: 10.1016/j.cell.2021.01.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sholukh 2021
- Sholukh AM, Fiore-Gartland A, Ford ES, Miner MD, Hou YJ, Tse LV, et al. Evaluation of cell-based and surrogate SARS-CoV-2 neutralization assays. Journal of Clinical Microbiology 2021;59(10):e0052721. [DOI: 10.1128/JCM.00527-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrotri 2021
- Shrotri M, Swinnen T, Kampmann B, Parker E. An interactive website tracking COVID-19 vaccine development. Lancet Global Health 2021;9(5):e590-2. [DOI: 10.1016/ S2214-109X(21)00043-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thakkar 2021
- Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cell Press 2021;39(8):1081-90. [DOI: 10.1016/j.ccell.2021.06.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tober‐Lau 2021
- Tober-Lau P, Schwarz T, Vanshylla K, Hillus D, Gruell H, Suttorp N, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respiratory Medicine 2021;9(11):e104-5. [DOI: 10.1016/S2213-2600(21)00456-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tricco 2018
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine 2018;169(7):467-73. [DOI: 10.7326/M18-0850] [DOI] [PubMed] [Google Scholar]
Viana 2022
- Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022;603:679-86. [DOI: 10.1038/s41586-022-04411-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2020
- World Health Organization. Establishment of the WHO international standard and reference panel for anti-SARS-CoV-2 antibody. www.who.int/publications/m/item/WHO-BS-2020.2403 (accessed 31 May 2022).
WHO 2022a
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. www.covid19.who.int (accessed 15 May 2022).
WHO 2022b
- World Health Organization. Tracking SARS-CoV-2 variants. www.who.int/activities/tracking-SARS-CoV-2-variants (accessed 31 May 2022). [PubMed]
WHO 2022c
- World Health Organization. COVAX - Working for global equitable access to COVID-19 vaccines. www.who.int/initiatives/act-accelerator/covax (accessed 15 May 2022).
Wouters 2021
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 2021;397(10278):1023-34. [DOI: 10.1016/s0140-6736(21)00306-8] [DOI] [PMC free article] [PubMed] [Google Scholar]