Abstract
The aim of this study was to investigate the antibacterial interactions of pulegone and 1,8‐cineole with monolaurin ornisin against Staphylococcus aureus. The individual and combined antibacterial activities of the compounds were evaluated using minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), fractional inhibitory concentration index (FICi), and time‐kill methods. Furthermore, the mechanism of the antibacterial action of the compounds was tested by measuring the release of cell constituents. The MIC values of pulegone, 1,8‐cineole, nisin, and monolaurin were 5.85 µl/ml, 23.43 µl/ml, 6.25 µg/ml, and 0.031 mg/ml, respectively. A synergistic antibacterial activity (FICi = 0.5) was found between 1,8‐cineole and nisin. The time‐kill assay showed that the populations of S. aureus exposed to 1,8‐cineole, nisin, and their combination were decreased by 5.9, 5.3, and 7.1 log CFU (colony‐forming units)/mL, respectively. The combination of 1,8‐cineole and nisin also induced the highest release of cell constituents. It was concluded that the combination of 1,8‐cineole and nisin could be considered as a novel and promising combination which may reduce the required dose of each antibacterial compound.
Keywords: 1,8‐cineole; combination; monolaurin; nisin; pulegone; S. aureus
The antibacterial activity of pulegone and 1,8‐cineole combined with monolaurin or nisin was investigated against Staphylococcus aureus. A synergistic antibacterial effect was found between 1,8‐cineole and nisin. This combination also induced the highest release of cell constituents. Therefore, the combination of 1,8‐cineole and nisin could be considered as a novel and effective antibacterial combination to control S. aureus.
1. INTRODUCTION
Nowadays, the food‐borne disease has become a globally serious concern that is severely on the rise and has destructive effects on food security and human health. Based on the reports, an average of 600 million people in the world annually become infected with pathogens, or their toxins through the consumption of contaminated foods (Faour‐Klingbeil & CD Todd, 2020). Staphylococcus aureus, a Gram‐positive bacterium, is one of the most common food‐borne pathogens that cause food intoxication by its enterotoxins (Zhou et al., 2020). S. aureus is capable of surviving and growing in a wide range of temperatures (7–48.5°C), pH (4.0–10.0), and a high salt concentration (Guo et al., 2020). Consumers might be at risk for S. aureus contamination through the processing or manufacture of various foods such as ready‐to‐eat foods, cooked meat, dairy, egg, bean, aquatic products, and fresh vegetables (Ma et al., 2018).
Synthetic preservatives are used to inhibit bacterial growth and increase the shelf life and ensure food safety. Nevertheless, chemical preservatives may cause harmful effects on human health, such as headaches, palpitations, allergies, stomach cancer, asthma, skin rashes, and contact dermatitis (Sharma, 2015). In fact, due to the increasing awareness of consumers about the adverse effects of chemical antimicrobials, the tendency to use natural compounds to protect food and control food‐borne pathogens has increased significantly (Baygar, 2019).
Pulegone is a monoterpene ketone found in the leaves and flowers of several members of the mint family (Mkaddem et al., 2009). Terpenes are capable of penetrating into the bacterial cell wall, leading to denaturation of proteins and disintegration of the cell membrane, leading to cytoplasmic leakage, cell lysis, and eventually cell death (Oussalah et al., 2007). Based on the published reports, pulegone can effectively destroy S. aureus, S. typhimurium, and Escherichia coli (Mkaddem et al., 2009). 1,8‐cineole (eucalyptol) is a monoterpene that occurs in the essential oils of several aromatic plants and spices, including Origanum vulgare (oregano), Rosmarinus officinalis (rosemary), Thymus vulgaris (thyme), Zingiber officinale (ginger), Coriandrum sativum (coriander), and eucalyptus oil (Charalambous, 1994; Sebei et al., 2015). Generally, essential oils or their compounds possess the desired antibacterial effect at higher doses, but this can cause a negative sensory impact (Gutierrez et al., 2009). The combination of essential oils with other antimicrobial agents could reduce their doses and solve this problem. Some previous studies have investigated the antimicrobial interaction between 1,8‐cineol and other essential oil components such as limonene (van Vuuren & Viljoen, 2007), camphor (Viljoen et al., 2003), aromadendrene (Mulyaningsih et al., 2010), and carvacrol (De Sousa et al., 2012).
Nisin is a natural preservative used in the food industry to prevent the growth of microorganisms, especially Gram‐positive bacteria, such as S. aureus (Wang et al., 2020). However, it has been reported that some bacterial species, including S. aureus, may acquire resistance to nisin (Zhou et al., 2014).
Monolaurin, an ester of lauric acid, is used as an antimicrobial and emulsifier agent in the food industry. It possesses the strongest antimicrobial effect among fatty acid esters (Raeisi et al., 2016). Generally, this monoester is very effective against Gram‐positive bacteria such as Staphylococcus and Streptococcus, as well as effectively inhibits toxin production by Staphylococci (Lieberman et al., 2006). Higher concentrations of monolaurin may result in undesirable soapy aroma and taste (Bell & De Lacy, 1987). Antibacterial interaction between nisin and monolaurin has been previously reported (Mansour & Millière, 2001; Zhang et al., 2009).
Some previous studies have reported the individual antibacterial effect of pulegone, 1,8‐cineole, monolaurin, and nisin and their combination with other antimicrobial agents. However, there is no report on the antibacterial interactions of two essential oil components (pulegone and 1,8‐cineole) with monolaurin or nisin. Then, the objective of the current study was to evaluate the combined antibacterial activity of pulegone with monolaurin/nisin and 1,8‐cineole with monolaurin/nisin against Staphylococcus aureus.
2. MATERIALS AND METHODS
2.1. Materials
Brain heart infusion (BHI) broth, plate count agar (PCA), peptone water, dimethyl sulfoxide, and HCl were obtained from Merck (Darmstadt, Germany). Pulegone, 1,8‐cineole, and nisin were purchased from Sigma‐Aldrich. Monolaurin was obtained from Lauricidin Inc., Galena, IL, USA.
2.2. Preparation of the bacterial suspension
Staphylococcus aureus (PTCC1431) was procured from the microbial collection of the Department of Food Hygiene and Quality Control, Urmia University. The colonies of the bacterium grown on the PCA were transferred to BHI broth (10 ml) and incubated at 37°C for 24 h. Then, the broth containing bacteria was centrifuged at 9342 g for 10 min and washed with peptone water (0.1%) thrice. After that, 10 ml of peptone water (0.1%) was added to the bacterial pellet and resuspended. Finally, the turbidity of the bacterial suspension was adjusted to 0.1 at 600 nm (≈108 CFU/ml) using a spectrophotometer (Pharmacia LKB, Uppsala, Sweden) (Mortazavi & Aliakbarlu, 2019).
2.3. Preparation of the antimicrobial solutions
First, 600 μl pulegone or 1,8‐cineole was mixed with 1 ml dimethyl sulfoxide (10%) to prepare their stock solutions (375 μl/ml) and 1 ml of this solution was added to 9 ml BHI broth. Then, different twofold concentrations (187.5–0.73 μl/ml) of the compounds were prepared in BHI broth from the stock solutions. To prepare the stock solution of monolaurin, 500 mg of monolaurin was dissolved in 5 ml of ethanol (96%) and 0.1 ml of this solution was added to 9.9 ml BHI broth. Then, the solution was diluted to reach concentrations ranging from 1 to 0.0156 mg/ml (Razavi‐Rohani & Griffiths, 1994). The amount of 10 mg of nisin was also dissolved in 1 ml of HCl (0.02 M, pH = 1.6) to prepare its stock solution. Different concentrations from this stock solution (200 to 3.125 µg/ml) were subsequently prepared (Dufour et al., 2003). The stock solutions of all the antimicrobial compounds were initially sterilized using a syringe filter (0.45 μm).
2.4. Determination of the MIC and MBC of the antimicrobials
The microdilution method was used to determine the minimal inhibitory concentration (MIC) of the antimicrobial compounds used in this study. First, 95 μl of BHI broth, 5 μl of the bacterial suspension (approximately 106 CFU/ml), and 100 μl of different concentrations of the antimicrobial solution were dispensed in the wells of a 96‐well microplate. The microplate was vortexed (260 rpm (revolutions per minute), 30 s) and then incubated at 37°C for 24 h. After incubation, the bacterial growth was visually assessed and the lowest concentration of each antimicrobial in which bacterial growth (turbidity) was not observed, was recorded as the MIC (Mortazavi & Aliakbarlu, 2019). To determine the minimum bactericidal concentration (MBC), 5 μl from the wells with no visible growth (MIC and higher concentrations) was cultured on PCA plates and incubated at 37°C for 24 h. MBC was defined as the lowest concentration of an antimicrobial at which no bacterial colony was formed on PCA. Wells without bacterial suspension (100 μl broth + 100 μl antimicrobial) and wells without antimicrobials (195 μl broth + 5 μl of the bacterial suspension) were also designed as the sterility control and the growth control, respectively (Mortazavi & Aliakbarlu, 2019).
2.5. Determination of FIC (fractional inhibitory concentration)
The antibacterial interactions between pulegone/1,8‐cineole and monolaurin/nisin were examined by the FIC method. To determine the MIC individually, in the wells of the first row of a 96‐well microplate, 95 µl BHI broth, 5 µl bacterial suspension (≈106 CFU/ml), and 100 µl of different concentrations of pulegone or 1,8‐cineole were added. Similarly, the wells of the first column of the microplate were filled with 95 µl BHI broth, 5 µl bacterial suspension, and 100 µl of different concentrations of monolaurin or nisin. In the remaining wells, 95 µl BHI broth, 5 µl bacterial suspension, and 50 µl from each antimicrobial were added. The combinations were as follows: 50 µl pulegone + 50 µl monolaurin, 50 µl pulegone + 50 µl nisin, 50 µl 1,8‐cineole + 50 µl monolaurin, and 50 µl 1,8‐cineole + 50 µl nisin. The final volume of each well was 200 µl (Nafis et al., 2019). The FIC index (FICi) was calculated according to the following equations:
The antibacterial interactions were interpreted as total synergistic (FICi ≤ 0.5), partial synergistic (0.5 < FICi ≤ 0.75), indifferent (0.75 < FICi ≤ 2), and antagonistic (FICi > 2) (Nafis et al., 2019).
2.6. Time‐kill method
To investigate the lethal effects of pulegone and 1,8‐cineole combined with nisin or monolaurin, 50 μl of bacterial suspension (≈108 CFU/ml) was inoculated into tubes containing 4450 μl of BHI broth. Then, 500 µl from each antimicrobial solution was added to four tubes at the final concentration of MIC. In combined treatments, an amount of 250 µl of each antimicrobial at MIC concentration was also used as follows: 250 µl pulegone + 250 µl monolaurin, 250 µl pulegone + 250 µl nisin, 250 µl 1,8‐cineole + 250 µl monolaurin, and 250 µl 1,8‐cineole + 250 µl nisin. One tube was also considered as growth control, which contained BHI broth and bacterial culture. Subsequently, all of the tubes were incubated at 37°C. Serial dilutions from the tubes were prepared at 0, 2, 4, 8, and 24 h and cultured on the BHI agar. Finally, the colonies were enumerated after 24 h incubation at 37°C, and then the time‐kill curve was plotted (Avila et al., 1999; Lavigne et al., 2020).
2.7. Estimation of the cell constituents’ release
The effect of the antimicrobial compounds on the cell membrane integrity of S. aureus was evaluated by determining the release of cell constituents to the supernatant. The overnight culture of S. aureus in the BHI broth was centrifuged at 4000 g for 15 min, and the supernatant was discarded. The bacterial cells were resuspended in phosphate‐buffered saline (PBS) and centrifuged three times again at 4000 g for 15 min. Then, each individual antimicrobial compound (at 2 × MIC concentration) and its combination (at MIC concentration of each antimicrobial) were added to the bacterial suspension in PBS. A tube containing bacteria suspended in PBS was considered as a control. All tubes were incubated for 1 h in a shaker incubator at 250 rpm and 37°C. Then, the tubes were centrifuged at 13,400 g for 20 min. Eventually, the concentration of cell constituents in the supernatants was measured using a spectrophotometer at 260 nm. The PBS buffer was considered as a blank to zero the spectrophotometer (Mutlu‐Ingok & Karbancioglu‐Guler, 2017). To calculate cell constituent release, the optical density (OD) of the tube containing antimicrobial solution and the bacterial suspension was subtracted from that of the antimicrobial solution.
2.8. Statistical analysis
The experiments of MIC, MBC, and FIC were carried out three times, while time‐kill and cell constituents’ release experiments were conducted two times. The results of colony count were converted to log CFU/ml using Microsoft Excel 2013 and plotted using GraphPad Prism 9. The data were analyzed with SPSS 22.0 software (SPSS Inc., Chicago, LA) by analysis of variance (ANOVA) procedure, and Duncan test at 5% significant level.
3. RESULTS AND DISCUSSION
3.1. MIC and MBC of the antimicrobial compounds
The results of the MIC and MBC values of pulegone, 1,8‐cineole, monolaurin, and nisin against S. aureus are presented in Table 1. The MIC values of pulegone and 1,8‐cineole against S. aureus were 5.85 and 23.43 µl/ml, respectively. Meanwhile, the MBC values of these compounds were found to be 11.71 and 23.43 µl/ml, respectively. Therefore, pulegone was more active against S. aureus than 1,8‐cineole. Similar to our results, it was reported that the MIC value of 1,8‐cineole (eucalyptol) against S. aureus was 20 µl/ml (Zengin & Baysal, 2014). However, another study showed that MIC and MBC values of 1,8‐cineole against S. aureus were 1.25% and 5% (v/v), respectively (W. Wang et al., 2012). It has also been shown that the MIC values of pulegone and 1,8‐cineole against S. aureus were 1.8 and 3.6 mg/ml, respectively (Sonboli et al., 2006). Another work showed that the MIC value of 1,8‐cineole was 10 μl/mL against S. aureus (Honório et al., 2015). Meanwhile, the MIC value of pulegone was found to be 2.8 μl/ml against S. aureus (Amalich et al., 2016). According to the reported results, the MIC values could be varied. The antimicrobial performance of essential oils in vitro depends on various factors such as antimicrobial components, type of microorganism, culture medium, amount of inoculum, pH, temperature, and food composition (Tajkarimi et al., 2010; H. Zhou et al., 2014).
TABLE 1.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of pulegone, 1,8‐cineol (µl/ml), monolaurin (mg/ml), and nisin (µg/mL) against Staphylococcus aureus
| Antimicrobial | MIC | MBC |
|---|---|---|
| Pulegone | 5.85 | 11.71 |
| 1,8‐Cineol | 23.43 | 23.43 |
| Monolaurin | 0.031 | 0.031 |
| Nisin | 6.25 | 6.25 |
| Erythromycin a | 8 | 8 |
µg/ml.
The MIC value of monolaurin was 0.031 mg/ml. This finding is similar to the results reported by other researchers (Raeisi et al., 2016). Evidence exists that monolaurin destroys the cell membrane by its lipophilic characteristics and inhibits the growth of Gram‐positive bacteria, while it has no effect on Gram‐negative bacteria (Delamare et al., 2007; Tajik et al., 2014). In one study, the MIC of monolaurin against S. aureus was 0.0625 mg/ml (Preuss et al., 2005), while Tangwatcharin and Khopaibool (2012) reported that the MIC value of monolaurin against S. aureus ATCC 25923 was 0.1 mg/ml. The other study demonstrated that monolaurin alone could inhibit S. aureus at a level of 128 μg/ml (pH = 7) and 16 μg/ml (pH = 5) but had no effect on E. coli. Therefore, the most potent antibacterial activity against S. aureus was observed at the lower pH (Aminzare et al., 2014). The MIC values of monolaurin against S. aureus ATCC 25923 and ATCC 1885 were 100 and 250 μg/ml, respectively (Sadiq et al., 2016; Tajik et al., 2014).
In this study, the MIC value of nisin was determined to be 6.25 µg/ml. In agreement with our finding, other researchers showed that the MIC value of nisin against S. aureus ranged from 2 to 32 µg/ml (Dosler et al., 2012). It was shown that the MIC value of nisin against S. aureus was 8 µg/ml (Zhao et al., 2014). However, another study showed that the MIC value of nisin against S. aureus strains was in the range of 16–32 µg/ml (Shi et al., 2017).
3.2. FIC
The results of FIC indices for the antimicrobial compounds (combination of pulegone with monolaurin/ nisin and 1,8‐cineole with monolaurin/nisin) are presented in Table 2. It was found that the combination of 1,8‐cineole and nisin had a synergistic effect against S. aureus with a FIC index of 0.5. Besides, the combination of pulegone and monolaurin resulted in a partial synergistic effect (FICi = 0.75). However, the combined use of 1,8‐cineole with monolaurin and pulegone with nisin was ineffective against S. aureus. Several studies have reported the antimicrobial interactions between nisin and other antimicrobials. For example, the combination of nisin and lactoperoxidase system showed a synergistic effect against S. typhimurium and S. aureus (Dufour et al., 2003). Furthermore, a synergistic effect between nisin and coenzyme Q has been reported against S. aureus isolates (Zhao et al., 2014). Another similar study showed a synergistic effect between nisin and cinnamaldehyde against S. aureus strains with the FIC value of 0.3 (Shi et al., 2017). It has been reported that the combined use of 1,8‐cineole and carvacrol could synergistically inhibit Listeria monocytogenes, Aeromonas hydrophila, and Pseudomonas fluorescens (De Sousa et al., 2012). In another study, synergy was found between 1,8‐cineole and aromadendrene against S. aureus (Mulyaningsih et al., 2010). Meanwhile, 1,8‐cineole in combination with limonene showed a synergistic effect against S. aureus (van Vuuren & Viljoen, 2007). However, it has been reported that the antibacterial effects of 1,8‐cineole in combination with α‐terpineol or linalool were additive (FICi = 1) against S. aureus (Zengin & Baysal, 2014).
TABLE 2.
The fractional inhibitory concentration index (FICi) of the antimicrobials against Staphylococcus aureus
| Antimicrobial combination | FIC | FICi | Function |
|---|---|---|---|
| Pulegone | 0.25 | 0.75 | Partial synergistic |
| Monolaurin | 0.5 | ||
| Pulegone | 1 | 1.5 | Indifferent |
| Nisin | 0.5 | ||
| 1,8‐Cineol | 1 | 1.122 | Indifferent |
| Monolaurin | 0.122 | ||
| 1,8‐Cineol | 0.25 | 0.5 | Synergistic |
| Nisin | 0.25 |
3.3. Time‐kill assay
The antibacterial interaction between pulegone and monolaurin against S. aureus during 24 h incubation is shown in Figure 1. Within the first 8 h, monolaurin showed stronger antibacterial activity than pulegone against S. aureus. However, at the end of 24 h incubation, there was no significant difference between their effects. Within the first 2 h, no significant difference was detected between the antibacterial performance of monolaurin and the combination of pulegone and monolaurin (P+ML). Nevertheless, at subsequent incubation times, monolaurin had surprisingly stronger antibacterial activity than the combination of pulegone and monolaurin. The combination of pulegone and monolaurin had a significantly stronger antibacterial effect than pulegone alone at the first 8 h. After 24 h incubation, however, there was no significant difference between their effects. Furthermore, all treatments displayed significant effects on S. aureus at the end of incubation, and approximately a 6‐log reduction in bacteria counts was induced by the treatments compared to control.
FIGURE 1.
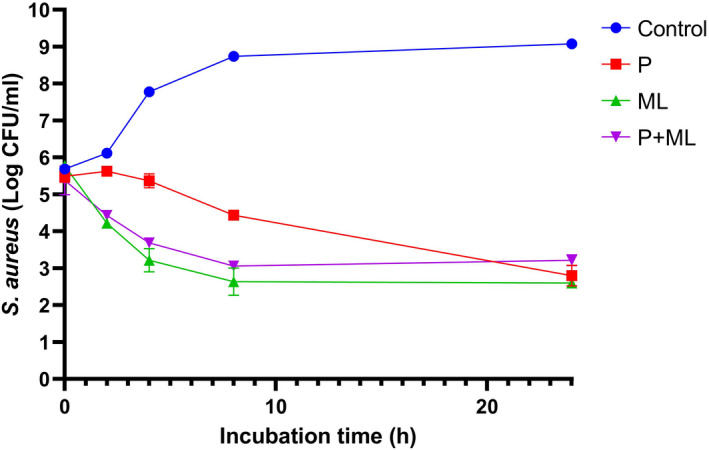
The antibacterial effects of pulegone, monolaurin, and their combination against Staphylococcus aureus (log CFU/ml) during 24 h incubation at 37°C. P: pulegone; ML: monolaurin
Figure 2 shows the antibacterial performance of pulegone, nisin, and their combination. Within the first 8 h, the antibacterial activity of nisin and the combination of pulegone and nisin (P + N) were similar, and their effects were significantly stronger than pulegone alone. After 24 h, a weak regrowth was observed in nisin treatment.
FIGURE 2.
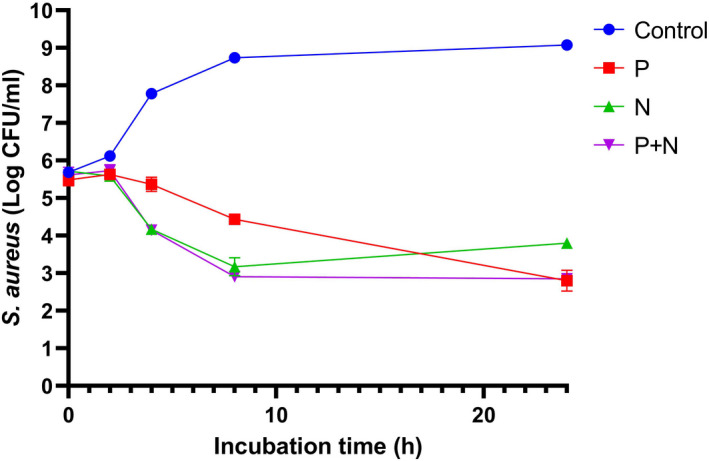
The antibacterial effects of pulegone, nisin, and their combination against Staphylococcus aureus (log CFU/ml) during 24 h incubation at 37°C. P: pulegone; N: nisin
The antibacterial interaction between 1,8‐cineol and monolaurin is illustrated in Figure 3. Within the first 8 h, the antibacterial effect of 1,8‐cineol was equal to that of the combination of 1,8‐cineol and monolaurin (C+ML). However, monolaurin alone showed stronger antibacterial activity than other treatments. At the end of 24 h incubation, the antibacterial effect of monolaurin was similar to that of the combination of 1,8‐cineol and monolaurin, and the effects of these two treatments were stronger than that of 1,8‐cineol alone.
FIGURE 3.
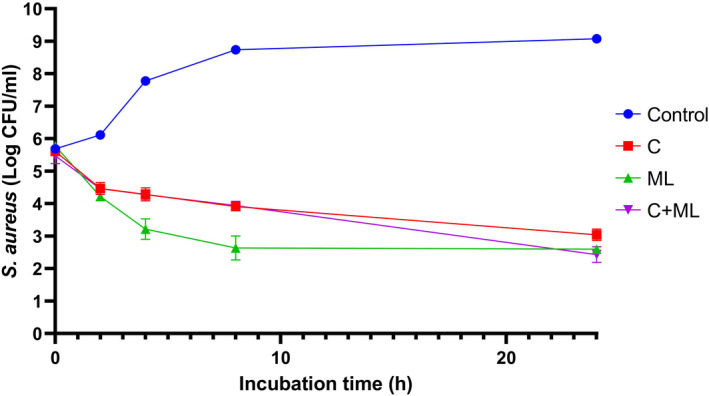
The antibacterial effects of 1,8‐cineol, monolaurin, and their combination against Staphylococcus aureus (log CFU/ml) during 24 h incubation at 37°C. C: 1,8‐cineol; ML: monolaurin
The antibacterial effect of 1,8‐cineole and nisin, individually and in combination, on S. aureus is depicted in Figure 4. During the first 4 h of incubation, the combination of 1,8‐cineole and nisin (C + N) showed significantly (p < .05) stronger antibacterial activity than the individual treatment of 1,8‐cineole or nisin. At the end of 24 h incubation, the combination of 1,8‐cineole and nisin also exhibited the greatest antibacterial performance against S. aureus, and more than 7‐log reduction in the bacterial count was induced by this treatment compared to control. Meanwhile, the antibacterial effect of 1,8‐cineole (5.9 log reduction) was higher than that of nisin (5.3 log reduction). Generally, the results of the time‐kill assay were in agreement with the FIC method.
FIGURE 4.
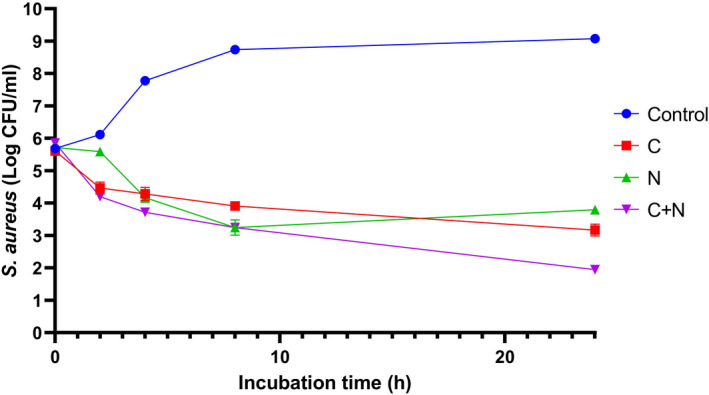
The antibacterial effects of 1,8cineol, nisin, and their combination against Staphylococcus aureus (log CFU/ml) during 24 h incubation at 37°C. C: 1,8‐cineol; N: Nisin
Many studies have shown that some antimicrobials, such as nisin and monolaurin alone, are more effective against Gram‐positive bacteria but have no effect on Gram‐negative ones (Raeisi et al., 2016; Wang et al., 2020). However, it has been proven that the combined use of the antimicrobials can increase not only the antibacterial effect but also reduce the required dose of each antimicrobial (Wang et al., 2020). It has been reported that the combination of nisin and cell‐free supernatant of Bacillus licheniformis showed synergistic bactericidal activity against S. aureus after 4 h (He & Chen, 2006). The results of the time‐kill test in broth and pasteurized milk showed that nisin in combination with cinnamaldehyde had a synergistic antibacterial effect against S. aureus (Shi et al., 2017).
3.4. Cell constituents’ release
The cell membrane integrity was evaluated by measuring the release of cell constituents such as nucleic acids and proteins into the supernatant at 260 nm. The internal cell constituents were released into the supernatant following cell membrane disruption, and then the absorbance of the supernatant at 260 nm was increased. Table 3 shows the cell constituents’ release in S. aureus induced by individual antimicrobials and their combinations. It was found that the combination of 1,8‐cineole with nisin had the greatest effect on cell constituents’ release (OD = 1.231), followed by the combination of 1,8‐cineole with monolaurin (OD = 0.860). However, monolaurin alone showed the lowest effect on cell constituent's release. Compared to control, the combination of 1,8‐cineole with nisin and 1,8‐cineole with monolaurin increased the absorbance of the supernatant by 7.4 and 5.2 times, respectively.
TABLE 3.
The cell constituents’ release in Staphylococcus aureus induced by individual (at 2 × MIC (minimum inhibitory concentration)) and combined antimicrobials (at MIC of each antimicrobial)
| Antimicrobial treatments | ODa | ODb | ODa–ODb |
|---|---|---|---|
| Control | 0.165 ± 0.014 | – | 0.165 ± 0.014 |
| Pulegone | 3≤ | 2.140 | – |
| 1,8‐Cineol | 1.690 ± 0.018 | 1.100 | 0.590 ± 0.018 |
| Monolaurin | 0.368 ± 0.011 | 0.022 | 0.346 ± 0.011 |
| Nisin | 0.611 ± 0.031 | 0.156 | 0.455 ± 0.031 |
| Pulegone + Monolaurin | 3≤ | 1.938 | – |
| Pulegone + Nisin | 3≤ | 2.112 | – |
| 1,8‐Cineol + Monolaurin | 1.710 ± 0.024 | 0.850 | 0.860 ± 0.024 |
| 1,8‐Cineol + Nisin | 1.971 ± 0.016 | 0.740 | 1.231 ± 0.016 |
ODa = optical density of PBS + antimicrobial + bacterial suspension.
ODb = optical density of PBS + antimicrobial.
A previous study indicated that the combination of α‐terpineol with eucalyptol (1,8‐cineole) caused the highest release of 260 nm absorbing materials from S. aureus (Zengin & Baysal, 2014). The results of another study showed that the combination of nisin and cinnamaldehyde induced more damage on the cell membrane of S. aureus compared to nisin or cinnamaldehyde alone (Shi et al., 2017). The combination of nisin and Zataria multiflora essential oil significantly increased the cell constituents’ release from S. aureus (Moosavy et al., 2008).
4. CONCLUSIONS
The present study investigated the antibacterial effects of pulegone and 1,8‐cineole alone and combined with monolaurin or nisin against S. aureus. The results showed that there was a synergistic effect between 1,8‐cineole and nisin against S. aureus. This combination also caused the highest release of cell constituents. Therefore, the combination of 1,8‐cineole and nisin could be considered as a novel and promising antibacterial combination. The combined use may reduce the required dose of each antibacterial compound and decrease the development of antibacterial resistance. However, further researches are required to evaluate the effectiveness of this combination in food models.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENT
The authors would like to thank for financial support of Urmia University.
Farhanghi, A. , Aliakbarlu, J. , Tajik, H. , Mortazavi, N. , Manafi, L. , & Jalilzadeh‐Amin, G. (2022). Antibacterial interactions of pulegone and 1,8‐cineole with monolaurin ornisin against Staphylococcus aureus . Food Science & Nutrition, 10, 2659–2666. 10.1002/fsn3.2870
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Amalich, S. , Zerkani, H. , Cherrat, A. , Dédianhoua, N. , Soro, K. , Bourakhouadar, M. , Mahjoubi, M. , EL Hilali, F. , & Zair, T. (2016). Study on Mentha pulegium L. from M’rirt (Morocco): Antibacterial and antifungal activities of a pulegone‐rich essential oil. Journal of Chemical and Pharmaceutical Research, 8, 363–370. [Google Scholar]
- Aminzare, M. , Razavi Rohani, S. M. , Raeisi, M. , Javadi Hosseini, S. , & Hashemi, M. (2014). Antibacterial effects of monolaurin, sorbic acid and potassium sorbate on Staphylococcus aureus and Escherichia coli . Journal of Food Quality and Hazards Control, 1, 52–55. [Google Scholar]
- Baygar, T. (2019). The inhibition effects of eugenol and pulegone on Stenotrophomonas maltophilia: An opportunistic pathogen. Erciyes Üniversitesi Veteriner Fakültesi Dergisi, 16(1), 23–29. 10.32707/ercivet.538021 [DOI] [Google Scholar]
- Bell, R. G. , & De Lacy, K. M. (1987). The efficacy of nisin, sorbic acid and monolaurin as preservatives in pasteurized cured meat products. Food Microbiology, 4(4), 277–283. 10.1016/S0740-0020(87)80001-1 [DOI] [Google Scholar]
- Charalambous, G. (1994). Spices, herbs and edible fungi (p. 764). Elsevier Science. [Google Scholar]
- De Sousa, J. P. , De Azerêdo, G. A. , De Araújo Torres, R. , Da Silva Vasconcelos, M. A. , Da Conceição, M. L. , & De Souza, E. L. (2012). Synergies of carvacrol and 1,8‐cineole to inhibit bacteria associated with minimally processed vegetables. International Journal of Food Microbiology, 154(3), 145–151. 10.1016/j.ijfoodmicro.2011.12.026 [DOI] [PubMed] [Google Scholar]
- Delamare, A. P. L. , Moschen‐Pistorello, I. T. , Artico, L. , Atti‐Serafini, L. , & Echeverrigaray, S. (2007). Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chemistry, 100(2), 603–608. [Google Scholar]
- Dosler, S. , & Chemotherapy, A.‐G.‐J. (2012). In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram‐positive bacteria. Journal of Chemotherapy, 24(3), 137–143. 10.1179/1973947812Y.0000000007 [DOI] [PubMed] [Google Scholar]
- Dufour, M. , Simmonds, R. S. , & Bremer, P. J. (2003). Development of a method to quantify in vitro the synergistic activity of “natural” antimicrobials. International Journal of Food Microbiology, 85(3), 249–258. 10.1016/S0168-1605(02)00544-5 [DOI] [PubMed] [Google Scholar]
- Faour‐Klingbeil, D. , & C. D. Todd, E. (2020). Prevention and control of foodborne diseases in middle‐east north African countries: Review of national control systems. International Journal of Environmental Research and Public Health, 17(1), 70. 10.3390/ijerph17010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermo Avila, J. , de Liverant, J. G. , Martínez, A. , Martínez, G. , Muñoz, J. L. , Arciniegas, A. , & Romo de Vivar, A. (1999). Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus . Journal of Ethnopharmacology, 66(1), 75–78. 10.1016/S0378-8741(98)00203-7 [DOI] [PubMed] [Google Scholar]
- Guo, L. , Wang, Y. , Bi, X. , Duo, K. , Sun, Q. , Yun, X. , Zhang, Y. , Fei, P. , & Han, J. (2020). Antimicrobial activity and mechanism of action of the Amaranthus tricolor crude Extract against Staphylococcus aureus and potential application in cooked meat. Foods, 9(3), 359 10.3390/foods9030359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, J. , Bourke, P. , Lonchamp, J. , & Barry‐Ryan, C. (2009). Impact of plant essential oils on microbiological, organoleptic and quality markers of minimally processed vegetables. Innovative Food Science & Emerging Technologies, 10(2), 195–202. 10.1016/J.IFSET.2008.10.005 [DOI] [Google Scholar]
- He, L. , & Chen, W. (2006). Synergetic activity of nisin with cell‐free supernatant of Bacillus licheniformis ZJU12 against food‐borne bacteria. Food Research International, 39(8), 905–909. 10.1016/J.FOODRES.2006.05.008 [DOI] [Google Scholar]
- Honório, V. G. , Bezerra, J. , Souza, G. T. , Carvalho, R. J. , Gomes‐Neto, N. J. , Figueiredo, R. C. B. Q. , Melo, J. V. , Souza, E. L. , & Magnani, M. (2015). Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1, 8‐cineole. Frontiers in Microbiology, 6, 1223. 10.3389/fmicb.2015.01223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne, J.‐P. , Ranfaing, J. , Dunyach‐Rémy, C. , & Sotto, A. (2020). Synergistic effect of propolis and antibiotics on uropathogenic Escherichia coli . Antibiotics, 9(11), 739. 10.3390/antibiotics9110739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, S. , Enig, M. G. , & Preuss, H. G. (2006). A review of monolaurin and lauric acid: Natural virucidal and bactericidal agents. Alternative & Complementary Therapies, 12(6), 310–314. [Google Scholar]
- Ma, Y. , Zhao, Y. , Tang, J. , Tang, C. , Chen, J. , & Liu, J. (2018). Antimicrobial susceptibility and presence of resistance & enterotoxins/enterotoxin‐likes genes in Staphylococcus aureus from food. CyTA‐Journal of Food, 16(1), 76–84. [Google Scholar]
- Mansour, M. , & Millière, J. B. (2001). An inhibitory synergistic effect of a nisin–monolaurin combination on Bacillus sp. vegetative cells in milk. Food Microbiology, 18(1), 87–94. 10.1006/FMIC.2000.0379 [DOI] [Google Scholar]
- Mkaddem, M. , Bouajila, J. , Ennajar, M. , Lebrihi, A. , Mathieu, F. , & Romdhane, M. (2009). Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. Journal of Food Science, 74(7), M358–M363. [DOI] [PubMed] [Google Scholar]
- Moosavy, M. H. , Basti, A. A. , Misaghi, A. , Salehi, T. Z. , Abbasifar, R. , Mousavi, H. A. E. , Alipour, M. , Razavi, N. E. , Gandomi, H. , & Noori, N. (2008). Effect of Zataria multiflora Boiss. Essential oil and nisin on Salmonella typhimurium and Staphylococcus aureus in a food model system and on the bacterial cell membranes. Food Research International, 41(10), 1050–1057. 10.1016/j.foodres.2008.07.018 [DOI] [Google Scholar]
- Mortazavi, N. , & Aliakbarlu, J. (2019). Antibacterial effects of ultrasound, cinnamon essential oil, and their combination against Listeria monocytogenes and Salmonella typhimurium in milk. Journal of Food Science, 84(12), 3700–3706. 10.1111/1750-3841.14914 [DOI] [PubMed] [Google Scholar]
- Mulyaningsih, S. , Sporer, F. , Zimmermann, S. , Reichling, J. , & Wink, M. (2010). Synergistic properties of the terpenoids aromadendrene and 1,8‐cineole from the essential oil of Eucalyptus globulus against antibiotic‐susceptible and antibiotic‐resistant pathogens. Phytomedicine, 17(13), 1061–1066. 10.1016/J.PHYMED.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Mutlu‐Ingok, A. , & Karbancioglu‐Guler, F. (2017). Cardamom, cumin, and dill weed essential oils: Chemical compositions, antimicrobial activities, and mechanisms of action against campylobacter spp. Molecules (Basel, Switzerland), 22(7), 1191 10.3390/molecules22071191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafis, A. , Kasrati, A. , Jamali, C. A. , Mezrioui, N. , Setzer, W. , Abbad, A. , & Hassani, L. (2019). Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Industrial Crops and Products, 137(March), 396–400. 10.1016/j.indcrop.2019.05.032 [DOI] [Google Scholar]
- Oussalah, M. , Caillet, S. , Saucier, L. , & Lacroix, M. (2007). Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes . Food Control, 18(5), 414–420. [Google Scholar]
- Preuss, H. G. , Echard, B. , Dadgar, A. , Talpur, N. , Manohar, V. , Enig, M. , Bagchi, D. , & Ingram, C. (2005). Effects of essential oils and monolaurin on Staphylococcus aureus: In vitro and in vivo studies. Toxicology Mechanisms and Methods, 15(4), 279–285. [DOI] [PubMed] [Google Scholar]
- Raeisi, M. , Tajik, H. , Aminzare, M. , Sangin Abadi, S. , Yarahmadi, A. , Yarahmadi, E. , & Tepe, B. (2016). The role of nisin, monolaurin, and EDTA in antibacterial effect of Rosmarinus officinalis L. and Cinnamomum zeylanicum Blume essential oils on foodborne pathogens. Journal of Essential Oil Bearing Plants, 19(7), 1709–1720. [Google Scholar]
- Razavi‐Rohani, S. M. , & Griffiths, M. W. (1994). The effect of mono and polyglycerol laurate on spoilage and pathogenic bacteria associated with foods. Journal of Food Safety, 14(2), 131–151. 10.1111/j.1745-4565.1994.tb00590.x [DOI] [Google Scholar]
- Sadiq, S. , Imran, M. , Habib, H. , Shabbir, S. , Ihsan, A. , Zafar, Y. , & Hafeez, F. Y. (2016). Potential of monolaurin based food‐grade nano‐micelles loaded with nisin Z for synergistic antimicrobial action against Staphylococcus aureus . LWT‐Food Science and Technology, 71, 227–233. 10.1016/j.lwt.2016.03.045 [DOI] [Google Scholar]
- Sebei, K. , Sakouhi, F. , Herchi, W. , Khouja, M. L. , & Boukhchina, S. (2015). Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biological Research, 48, 7 10.1186/0717-6287-48-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. (2015). Food preservatives and their harmful effects. International Journal of Scientific and Research Publications, 5(4), 1–2. [Google Scholar]
- Shi, C. E. , Zhang, X. , Zhao, X. , Meng, R. , Liu, Z. , Chen, X. , & Guo, N. A. (2017). Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control, 71, 10–16. 10.1016/j.foodcont.2016.06.020 [DOI] [Google Scholar]
- Sonboli, A. , Mirjalili, M. H. , Hadian, J. , Ebrahimi, S. N. , & Yousefzadi, M. (2006). Antibacterial activity and composition of the essential oil of Ziziphora clinopodioides subsp. bungeana (Juz.). Rech. f. from Iran. Zeitschrift Für Naturforschung C, 61(9–10), 677–680. 10.1515/znc-2006-9-1011 [DOI] [PubMed] [Google Scholar]
- Tajik, H. , Raeisi, M. , Razavi Rohani, S. M. , Hashemi, M. , Amin Zare, M. , Naghili, H. , Rozbani, D. , & Ben Ammar, D. (2014). Effect of monolaurin alone and in combination with EDTA on viability of Escherichia coli and Staphylococcus aureus in culture media and Iranian white cheese. Journal of Food Quality and Hazards Control, 1(4), 108–112. [Google Scholar]
- Tajkarimi, M. M. , Ibrahim, S. A. , & Cliver, D. O. (2010). Antimicrobial herb and spice compounds in food. Food Control, 21(9), 1199–1218. 10.1016/j.foodcont.2010.02.003 [DOI] [Google Scholar]
- Tangwatcharin, P. , & Khopaibool, P. (2012). Activity of virgin coconut oil, lauric acid or monolaurin in combination with lactic acid against Staphylococcus aureus . Southeast Asian Journal of Tropical Medicine & Public Health, 43(4), 969–985. [PubMed] [Google Scholar]
- van Vuuren, S. F. , & Viljoen, A. M. (2007). Antimicrobial activity of limonene enantiomers and 1,8‐cineole alone and in combination. Flavour and Fragrance Journal, 22(6), 540–544. 10.1002/ffj.1843 [DOI] [Google Scholar]
- Viljoen, A. , Van Vuuren, S. , Ernst, E. , Klepser, M. , Demirci, B. , Başer, H. , & Van Wyk, B. E. (2003). Osmitopsis asteriscoides (Asteraceae)‐the antimicrobial activity and essential oil composition of a Cape‐Dutch remedy. Journal of Ethnopharmacology, 88(2–3), 137–143. 10.1016/S0378-8741(03)00191-0 [DOI] [PubMed] [Google Scholar]
- Wang, H. , Niu, Y. , Pan, J. , Li, Q. , & Lu, R. (2020). Antibacterial effects of Lactobacillus acidophilus surface‐layer protein in combination with nisin against Staphylococcus aureus . LWT, 124, 109208– 10.1016/j.lwt.2020.109208 [DOI] [Google Scholar]
- Wang, W. , Li, N. , Luo, M. , Zu, Y. , & Efferth, T. (2012). Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules, 17(3), 2704–2713. 10.3390/molecules17032704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin, H. , & Baysal, A. H. (2014). Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage‐forming bacteria and cell structure‐activity relationships evaluated by SEM microscopy. Molecules, 19(11), 17773–17798. 10.3390/molecules191117773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Wei, H. , Cui, Y. , Zhao, G. , & Feng, F. (2009). Antibacterial interactions of monolaurin with commonly used antimicrobials and food components. Journal of Food Science, 74(7), M418–M421 10.1111/J.1750-3841.2009.01300.X [DOI] [PubMed] [Google Scholar]
- Zhao, X. , Liu, Z. , Li, W. , Li, X. , Shi, C. , Meng, R. , Cheng, W. , Jin, K. , Yang, Z. , Shi, X. , Guo, N. , & Yu, L. (2014). In vitro synergy of nisin and coenzyme Q0 against Staphylococcus aureus . Food Control, 46, 368–373. 10.1016/j.foodcont.2014.05.051 [DOI] [Google Scholar]
- Zhou, H. , Fang, J. , Tian, Y. , & Lu, X. Y. (2014). Mechanisms of nisin resistance in Gram‐positive bacteria. Annals of Microbiology, 64(2), 413–420. 10.1007/s13213-013-0679-9 [DOI] [Google Scholar]
- Zhou, J. , Yin, L. , Dong, Y. , Peng, L. , Liu, G. , Man, S. , & Ma, L. (2020). CRISPR‐Cas13a based bacterial detection platform: Sensing pathogen Staphylococcus aureus in food samples. Analytica Chimica Acta, 1127, 225–233. 10.1016/j.aca.2020.06.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


