Abstract
Introduction:
Unregulated e-cigarette devices and their nicotine content have amplified the potential of e-cigarettes as addictive agents. Several e-cigarette-related parameters have been identified altering nicotine’s absorption profile, so their potential effects on addiction should be considered. Of these factors, nicotine forms (protonated and free base) play a significant role in the addiction potential yet their impact on nicotine’s absorption has been studied with limited research.
Areas covered:
Current review aims to emphasize on the possible mechanism behind different absorption profiles of nicotine forms considering their physical states (droplet and vapor phase) and the aerosol particle size, their analysis in e-cigarette research and the regulatory attention warranted by them to combat nicotine addiction in the population due to e-cigarettes.
Expert opinion:
The protonated form of nicotine is being correlated with the smooth sensory effects and high nicotine absorption as compared to free base nicotine. With the introduction of nicotine salts, which yield mostly the protonated form, the youth popularity of e-cigarettes has spiked exponentially. While it is important to control nicotine levels in e-cigarette products, attention should also be given to the nicotine forms present in these products in order to address nicotine addiction in the population.
Keywords: Electronic cigarettes, nicotine delivery, free base nicotine, nicotine salt, aerosol, battery power, addiction, e-liquids
1. Introduction: electronic cigarettes
Electronic cigarettes (e-cigarettes) are one of the most controversial products in the tobacco world. Unlike conventional cigarettes, these battery-powered devices deliver nicotine without combustion. The vehicle of nicotine in these products is e-liquid, which is a mixture of propylene glycol (PG) and vegetable glycerin (VG) in varying ratios. The nicotine concentration in these e-liquids can vary from 0 to >50 mg/mL with actual content often deviating from the label claim [1]. Along with nicotine, PG and VG, these e-liquids contain a variety of flavoring chemicals, ethanol and water in varying proportions. As of 2014, more than 7700 flavoring combinations have been available in the e-cigarette market [2]. This number might have gone up since then. Since Aug 2016, the U.S. Food and Drug Administration (U.S. FDA) has been regulating these products; however, the limit on nicotine concentration and list of permissible ingredients in these products has not yet been specified [3].
For over a decade, e-cigarettes have evolved rapidly. When e-cigarettes were introduced in the U.S. market around 2007 [4], their design was simple and they were known as first generation e-cigarettes. These were ‘cig-a-like’ devices with fixed and low-voltage batteries. The second-generation devices, known as clearomizers, have larger and variable voltage batteries. The third-generation devices are known as Mod devices. These Mods allow users to vary voltage, wattage, temperature, and the type of coil. Mods usually come in a variety of shapes with large fluid tanks that can be disassembled to provide more customizability in terms of volume of e-liquid [5]. With the evolution of e-cigarette technologies, new devices are continuously being introduced in to the market. In 2017, JUUL Labs, Incorporated (JUUL, San Francisco, USA) introduced its POD-based device, which became a popular device among the youth [6]. These POD-based devices are USB-shaped and contain nicotine salts, specifically nicotine benzoate. The initial ban by the U.S. FDA on some flavored pods of closed system e-cigarettes such as JUUL mango flavor, inspired e-cigarette manufacturers to introduce other similar devices through the loopholes of the ban. These devices included Puff bars (Puff Bar, CA, USA) which were one time use, multiflavored, disposable e-cigarettes (recently banned in Jul 2020) [7]. Such a continuously evolving market of not well-regulated e-cigarette devices pose a great danger due to a lack of quality control of these products. Inadequate quality control can impact the nicotine delivery, released toxicants, and safety features of the devices [8,9]. Nevertheless, despite maximal efforts by the U.S. FDA robust guidelines for e-cigarette device regulations have not yet been established.
While the cigarettes use among middle school and high school students has dropped significantly (from 4.3% in 2011 to 2.3% in 2019 for middle school and from 15.8% in 2011 to 5.8% in 2019 for high school students), the e-cigarette use among them has increased dramatically so as to reverse the progress toward reducing overall tobacco use [10]. With more than 5 million middle and high school students having used e-cigarettes in the United States, it is important to address the concerns of nicotine addiction in youth [11]. Consideration should be given to nicotine delivery and the factors affecting it. Several studies have identified the factors which can affect the nicotine delivery, either alone or in combination. These interlinked factors include the nicotine concentration, ratio of propylene glycol (PG) and vegetable glycerin (VG), type of e-cigarette device, battery power, puffing profile and flavors [12–17]. As stated earlier [1], the unregulated nicotine levels in e-liquids can expose users to high nicotine levels. Additionally, Kosmider et al. [12] found out that the nicotine delivery increased with higher PG content at low power settings. Baassiri et al. [13] also found out that decreasing the PG/VG ratio decreases the nicotine delivery. Furthermore, DeVito et al. [15] has summarized multiple studies [18–21 in their review article which shows that higher powers of advanced e-cigarettes can increase the amount of nicotine aerosolized and increase nicotine delivery. Flavors also play an important role in the choice and addiction of e-cigarettes, especially in youth population [15]. In the study carried out by Helen et al. [17] flavors were found to affect the nicotine delivery through change in puffing behavior as well as e-liquid pH. Some authors [22,23] have also studied the stability of nicotine in e-liquids over time as nicotine can undergo degradation to form products such as nicotine-N-oxide, cotinine, and myosmine. Based on these stability studies, the nicotine breakdown products were not found to be more than 2% of nicotine content of e-liquids. Thus, stability may not act as major factor affecting nicotine delivery.
In the last few years, extensive research has been carried out for identifying the effects of above-mentioned factors on nicotine delivery. However, there is limited research available on the effect of nicotine form(s) i.e., protonated and free base on nicotine delivery. This review aims to summarize the potential effects of nicotine form on nicotine delivery, possible mechanism of these effects, methods for determining the free base nicotine yield of e-liquids and e-cigarette regulations considered for nicotine forms.
2. Nicotine: physical and chemical properties
Nicotine, a major component of cigarettes and e-cigarettes, is a naturally occurring plant alkaloid. It is a pale-yellow liquid with a density of 1.01 g/cm3. It is a bicyclic compound of a molecular weight 162.23 g/mol with one pyridine and pyrrolidine ring (Figure 1).
Figure 1.
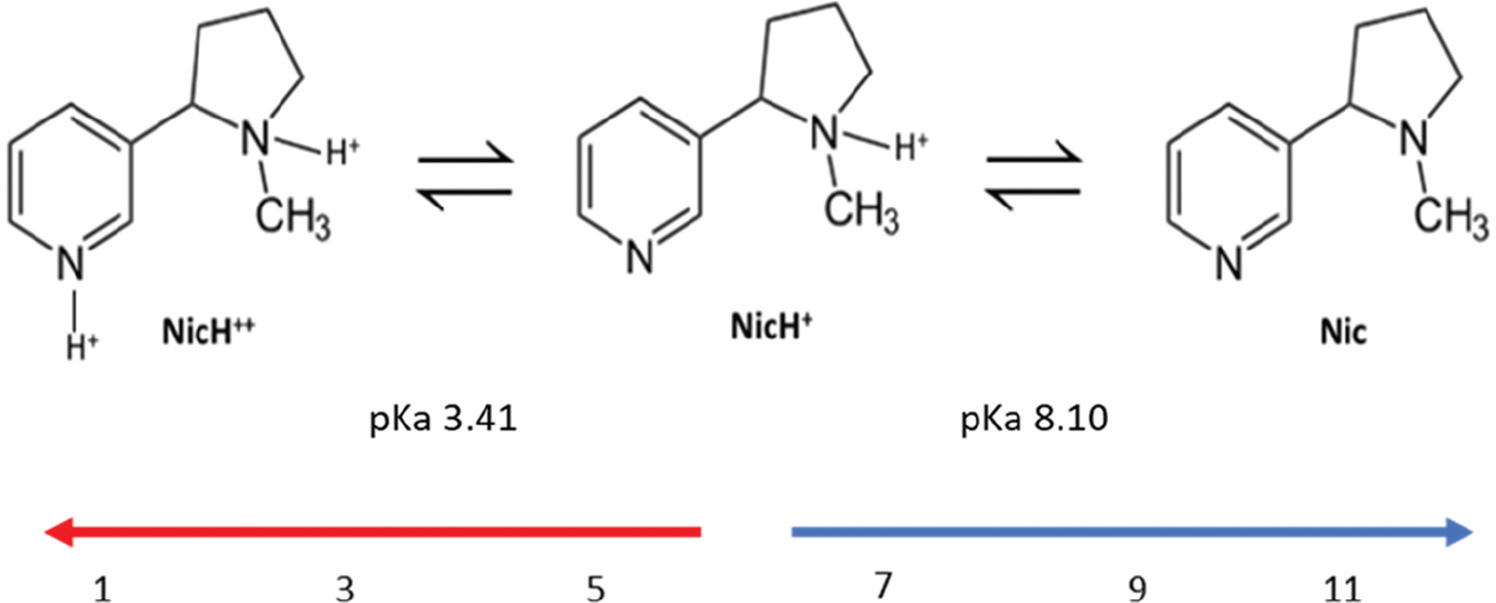
Different forms of nicotine based on the pH of a solvent (water).
With two nitrogen’s, one on each ring, nicotine exhibits two pKa’s. The nitrogen of the pyrrolidine ring is more basic (pka = 8.10 at 25°C) than that of the pyridine ring (pka = 3.41 at 25°C) [24]. Although nicotine is a lipophilic molecule with a log P of 1.17, it is water miscible [25]. Based on the two pKa’s, nicotine can exist in three forms depending on the pH of the solvent (Figure 1). These three forms are diprotonated, mono-protonated, and free base (un-protonated) nicotine. The contribution of the pyridine ring toward nicotine ionization can be neglected as its pKa is very low and the diprotonated form can exist only at pH < 5.5. For studying the effect of nicotine form on the absorption profile of nicotine from cigarettes and e-cigarettes, only mono-protonated and free base forms play relevant roles. In e-cigarettes, e-liquids are the vehicle of nicotine. Composition of these e-liquids determines the ratio of free base to protonated nicotine. Recently, a variety of nicotine salts have been introduced into the e-cigarette market. These salts include lactate, benzoate, malate, salicylate, levulinate, and tartrate [26]. In all nicotine salts, the pyrrolidine nitrogen is converted to a protonated form. This shift in the form of free base nicotine to protonated nicotine has become a matter of interest due to their different sensory and absorption effects. Sensory effects could be multifactorial, but some studies have suggested the free base nicotine has harsher sensory effects in throat as compared to salt-based protonated nicotine [27]. Similarly, the absorption profiles of these two forms of nicotine have been found to be different in many studies [28–32]. Taking into consideration these distinct properties of nicotine forms, it becomes important to study their effect on nicotine delivery which eventually affects nicotine’s addiction potential.
3. Addiction potential of nicotine
Nicotine is well known for its addictive properties through its action on the central nervous system (CNS). The major target of nicotine binding is nicotinic acetylcholine receptors which are primarily located in the central and peripheral nervous systems, and muscles [33]. Of these, receptors in the brain play a critical role in nicotine addiction. There are number of subunits of nicotine acetyl choline receptors in the mammalian brain. The high affinity nicotine binding receptors are composed of α4 and β2 subunits [34,35]. After inhalation from cigarettes or e-cigarettes, nicotine enters into the pulmonary venous blood circulation and reaches the brain through arterial circulation in 10–20 seconds [36]. Once in the brain, it binds to nicotinic acetylcholine receptors and releases a number of neurotransmitters such as dopamine. This dopamine plays a critical role in pleasurable feelings and reward mechanism to initiate nicotine addiction [35]. With an increase in nicotine exposure, the number of nicotine binding sites increases in the brain and the neurological system adapts to pharmacological effects of nicotine, thus further potentiating nicotine addiction [34,37] In youth (<25 years old), the prefrontal cortex area of the brain is not fully developed [38] and high absorption of nicotine can upregulate the nicotine binding sites and affect the prefrontal cortex development leading to lack in attention and lasting effects on cognitive functions [39]. Following the addiction, a sudden cessation of nicotine exposure leads to nicotine withdrawal symptoms which are difficult to manage. Thus, it becomes extremely critical to manage the nicotine exposure, especially in the youth to prevent them from a potential lifetime addiction and to avoid the withdrawal symptoms [4].
Currently, the e-cigarette market is not well regulated in the United States. Although, the FDA has started taking appropriate measures [4], youth in the United States are still at risk of getting addicted to nicotine due to e-cigarettes. In a 2019 survey, more than 5 million middle and high school students stated that they have used e-cigarettes in the past 30 days [11]. Along with the social and environmental factors, there are several e-cigarettes associated parameters which can influence the nicotine delivery to vapers. These factors include unregulated nicotine concentrations in e-liquids, PG:VG ratio, flavors, unregulated e-cigarette devices with potential to change the nicotine delivery to users by varying the power outputs, puffing behavior and lastly, but importantly, the nature of nicotine form in e-cigarette aerosol.
4. Effect of nicotine form on nicotine’s absorption profile
Like e-cigarettes, effect of nicotine form on nicotine’s absorption profile is one of the controversial topics with contradictory research outcomes. Earlier, tobacco companies have been accused of allegedly using alkaline chemical substances such as ammonia or its related basic compounds in manufacturing of cigarettes [40,41]. Their intended purpose was to shift the balance from protonated (NicH+) to free base form (Nic) of nicotine in tobacco for a rapid and efficient absorption of nicotine in consumers [41]. Based upon the pH partition hypothesis of drug absorption, any drug molecule easily penetrates biological membrane (except specific barriers such as Blood Brain Barrier) in an unionized form [42]. Due to its unionized form, free base nicotine is believed to easily cross the biological membrane of the respiratory tract leading to nicotine’s rapid absorption. Additionally, the rapid deposition of free base nicotine in the upper respiratory track was found to have sharp and sudden sensory effects [40,43,44]. This sensory effect was termed as harshness or impact [44].
To explore the reasons behind these practices followed by tobacco companies, many researchers carried out in vitro [45,46] and in vivo [28–30] studies to explain the effect of nicotine form on nicotine’s absorption. Takano et al. [45,46] have carried out in vitro studies of nicotine uptake in alveolar cells (rat primary cultured alveolar epithelial cells) and showed that nicotine transport is by simple diffusion as well as carrier mediated transport. Based on their experimental results, the proposed transporter of nicotine is proton coupled antiporter. These in vitro studies of nicotine uptake in primary cultured alveolar epithelial cells have shown higher uptake of nicotine at higher extracellular pH. Shao et al. [28] performed a study where nicotine was delivered to rats in the form of an aerosol. The pharmacokinetic patterns of nicotine in a ‘human smoking a cigarette’ scenario were simulated by nicotine aerosol inhalation in rodents. The authors showed that LC50 values of nicotine significantly changed from pH 6.8 (higher) to 8.0 (lower). Adrian et al. [29] carried out in vivo buccal permeability studies of nicotine which showed that nicotine absorption through buccal mucosa is pH dependent. Buccal permeability as well as systemic absorption of nicotine was increased as amount of nonionized nicotine was increased. Papp values obtained from in vivo permeability studies on buccal mucosa indicated simple passive diffusion as a predominant mechanism of nicotine transport across buccal mucosa. Burch et al. [30] carried out the study of effect of pH on nicotine absorption in healthy smokers. Nicotine solutions with pH ranging from 5.6 to 11 were used for aerosol inhalation. The authors have reported higher mean rise in plasma nicotine concentration with increasing pH.
In summary, the above studies (see Table 1) showed that the rate of nicotine absorption is higher for basic than acidic nicotine solutions and aerosols. In other words, free base nicotine was found to exhibit higher absorption than protonated nicotine.
Table 1.
Studies showing free base nicotine has higher absorption than protonated nicotine.
| Study | Authors | Significant findings | Comment |
|---|---|---|---|
|
| |||
| Nicotine uptake in rat primary cultured alveolar epithelial cells (in vitro) | Takano et al. [45,46] | Higher uptake of nicotine at higher extracellular pH | Absorption across a biological membrane in direct contact |
| Pharmacokinetic study of inhalation of the aerosol of nicotine solution in water in rodents. (in vivo) | Shao et al. [28] | LC50 values of nicotine was found to be higher at pH 6.8 than that at pH 8.0. | Particle size of the aerosol (MMAD) ranges from 1.95 to 3.55 μm. |
| Human buccal permeability studies of nicotine (in vivo, clinical) | Adrian et al. [29] | Buccal permeability as well as systemic absorption of nicotine was increased as amount of nonionized nicotine was increased. | Absorption across a biological membrane in direct contact |
| Effect of pH of nicotine solution on its absorption in healthy smokers (in vivo, clinical) | Burch et al. [30] | Higher mean rises in plasma nicotine concentration with increasing pH | Particle size of the aerosol (MMAD) is 8.5 μm. |
In contrast, clinical studies carried out recently have shown that the protonated nicotine causes higher and rapid nicotine absorption than the free base form. Pax Labs’ patent has shown nicotine salts, which give mostly protonated nicotine, give higher plasma nicotine concentrations (Cmax) than free base nicotine of the same concentration at a given puff profile [31]. As per the clinical study carried out by PAX labs, 2% nicotine benzoate results in three times higher Cmax than the 2% free base nicotine. Similarly, a study carried out by O’Connell et al. [32]showed that nicotine lactate gives higher plasma nicotine concentration (Cmax) than the free base nicotine of the same concentration using the same device and puff profile conditions such as puff duration, puff interval, and number of puffs. As summarized in Table 2, these recent clinical studies provide strong evidence that protonated nicotine is responsible for higher and faster nicotine absorption than free base nicotine. With these contradictory results (Tables 1 and Tables 2), some authors have also argued earlier that due to the large surface area of lungs and buffering capacity of the fluid lining in the lungs, the pH of nicotine aerosol would not impact the absorption of nicotine [47].
Table 2.
Studies showing protonated nicotine has higher absorption than free base nicotine.
| Study | Authors | Significant findings | Comment |
|---|---|---|---|
|
| |||
| Nicotine salt formulations for aerosol devices and methods thereof | Bowen et al. (Pax Labs, Inc.) [31] | Nicotine salts gives three times higher plasma nicotine concentration (Cmax) than free base nicotine of the same concentration at a given puff profile | Device used is JUUL e-cigarette which is similar to 1st generation e-cigarette |
| Pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers | O’Connell et al [32]. | Nicotine lactate gives higher plasma nicotine concentration (Cmax) than the free base nicotine of the same concentration at a given puff profile | Device used is myblu e-cigarette which is similar to 1st generation e-cigarette |
The question arises here as how shall we explain the outcomes of these studies which showed contradictory results of absorption profiles of the two different forms of nicotine? A possible explanation can be given by considering two important properties which determine site of e-cigarette aerosol deposition: I) The particle size distribution of the aerosol and, II) physical states (i.e. droplets or vapor phase) of free base and protonated nicotine forms in the aerosol.
5. Nicotine form, the size distribution, and physical forms of the resulting aerosol
The particle size analysis of e-cigarette aerosol is a widely studied topic yet lacks standardization. Care should be taken to avoid confusion in terms of the units commonly used to report the size distribution of the aerosolized e-liquids. The sizing done with count-based instruments such as Scanning Mobility Particle Sizer (SMPS) and Aerodynamic Particle Sizer (APS) is typically reported using count median diameter (CMD). On the other hand, the size measured with impactors is usually provided in terms of mass median aerodynamic diameter (MMAD), which can be related to volumetric median aerodynamic dimeter (VMAD) with the knowledge of the density of e-liquid. In any case, the other important aspect of the size distribution, which is the geometric standard deviation (GSD) is often neglected. This parameter is important to be noted to allow conversion of various size modalities to each other. A group of conversion equations for size distribution statistics were initially derived by Hatch and Choate [48], and their use has further been described by Hinds for comparisons [49]. In the future efforts that may be made in the standardization direction, aerodynamic diameters are preferred in the context of respiratory deposition and health effects of inhaled aerosols.
There are multiple studies which have reported particle size in various modalities. Zhang et al. [50] reported e-cigarette particle size as volumetric median aerodynamic diameter (VMAD) of 250–440 nm with GSD 1.3–1.6. Studying the effect of power levels on the particle size, Pourchez et al. [51] have reported a positive correlation between power levels and particle size of e-cigarettes aerosol. They reported a particle size as mass median aerodynamic diameter (MMAD) of 613–949 nm over the range of 7–22 W of power. Aldreman et al. [52] studied three e-cigarettes (cartomizers and disposable) and reported aerosol particle size (MMAD) ranging from 534–631 nm with GSD 1.50–1.52. Oldham et al. [53] analyzed 20 different cartridges using unspecified e-cigarette device and reported the particle size (MMAD) close to 1 mm or higher (e.g. 0.9–1.2 mm, GSD 1.7–2.2). Son et al. [54] found the mass median diameter (MMD) of the aerosol for a range of device and e-liquid parameters to be close to 3 μm. A number of other studies have reported the particle size as count median diameter (CMD) ranging from 18 to 386 nm [55–57]. Such a wide range and disagreements in different modalities of size measurements mainly rise from the lack of controlled or consistent sizing conditions such as varying dilution ratio (i.e. air added to the original concentration of the aerosol from the device), power output, propylene glycol-based e-liquids vs vegetable glycerin-based e-liquids, different flavors of e-liquids, puff volume, temperature, and relative humidity in different studies [54]. Therefore, in the process of standardization, efforts should be made to carry out controlled experiments of particle size measurements with consistent conditions through which results can be reported in comparable modalities.
As summarized in Table 2, the clinical studies by Pax Labs [31] and O’Connell et al. [32] have used JUUL and myblu™ e-cigarettes, respectively. These devices are similar to 1st generation e-cigarettes with low power outputs and do not have the power variability options. The size of aerosol generated from these devices is expected to mostly be in the submicron range. Recently Clapp et al. [58] presented a dataset at Society of Toxicology reporting particle size (MMAD) of JUUL e-cig aerosol as 0.53 μm. On the other hand, the study carried out by Shao et al. (Table 1) using the aerosol of nicotine solution in water reported the MMAD of aerosol in the range of 1.95–3.55 μm. The nicotine solution was delivered with a collision atomizer (BGI Inc.) instead of an e-cigarette and the increase in size was associated with increasing nicotine concentration in the solution [28]. Similarly, in a clinical study carried out by Burch et al., the particle size (MMAD) of aerosol was 8.5 μm [30]. Such larger particles mainly deposit in the oropharyngeal cavity and upper respiratory tract due to the impaction filtration at the peak of inhalation and gravitational settling during breath hold, so the amount of aerosol reaching the alveoli is reduced [59,60]. Here, the nicotine absorption is more through the oropharyngeal and respiratory tract membrane in direct contact rather than through alveolar deposition in the lungs, in contrast to the absorption of submicron size aerosol particles. Similar to the in vitro studies carried out earlier by Takano et al. [45,46] and the buccal permeability study carried out by Adrian et al. [29], the pH partition hypothesis holds true in such cases where, free base nicotine shows higher absorption than protonated nicotine through the biological membrane in direct contact. Further research to verify the effect of nicotine form on aerosol size distribution is still warranted to explain the resulting enhancement in absorption based on regional deposition pattern.
With a particle size distribution in submicron range, e-cigarette aerosol particles have a higher probability of reaching smaller airways and alveoli where absorption rate is higher than the upper respiratory tract [61]. The fate of e-cigarette aerosol deposition is further affected by physical states of aerosol mixtures resulting from free base and protonated nicotine forms. As per Pankow theory, nicotine in aerosol exists in a protonated or free base form depending on the chemical composition of the aerosol. Free base nicotine can exist in particulate or gaseous state while the protonated nicotine can exist only in particulate state [44]. Based on the aerosol deposition theory, one possible explanation for higher and faster absorption of nicotine from its salt form (protonated) could be that protonated nicotine in aerosol has a higher chance of reaching the lungs since protonated nicotine is less volatile as compared to free base nicotine [44]. When e-cigarette aerosol is a dynamic mixture of droplet and vapor phases, for example when nicotine is in free base form, the two phases continuously go through conversion between each other as a result of condensation or evaporation while each phase being removed due to vapor uptake and droplet deposition on the walls [62]. While droplet deposition can be expected in both free base form as well as protonated form, vapor uptake is expected only in free base form. Thus, free base form contributes to more losses in the oropharyngeal region, explaining the harsh feeling and less delivery to the lungs.
Furthermore, the fraction of the gaseous form of free base nicotine reaching the lower respiratory tract has a higher chance of being exhaled out, than that of the particulate form. Jabbal et al. [61] have argued that although the submicron particles also have a high probability of being exhaled, this is counterpoised by their ability to be distributed deeply throughout the lungs. Nevertheless, to address the exhalation concern, the charged nature of the protonated nicotine particles should be taken in to account. The submicron particles with charged surface has higher propensity toward the walls of the alveoli and thus show higher deposition as compared to uncharged particles [60,63,64].
Thus, nicotine salts result in higher Cmax than free base nicotine. Considering the large surface area of lungs and buffering capacity of the lining fluid, it can be argued that once in lungs, nicotine in any charge state can easily get absorbed in blood [47]. This argument puts the not well understood absorption mechanism of nicotine across cell membrane as nonrelevant. Instead, it is the amount of nicotine reaching to lungs that affects the plasma nicotine concentration and this amount of nicotine reaching lungs does get affected by the nicotine form in the aerosol.
Having said that, an independent clinical study aimed to understand protonated vs free base nicotine absorption profile under various vaping conditions is still warranted to conclude this explanation.
6. Methods to determine free base nicotine: pre- and postvaporization of e-cigarette
As described above, nicotine delivery to vapers is affected by free base or protonated nicotine yield of e-cigarette. Therefore, it becomes necessary to determine the free base or protonated nicotine yield of e-liquids and classify them based on the yield. This yield can be calculated both in prevaporization and post-vaporization conditions and has been reported as fraction or percent of total nicotine [65,66].
Currently there are two complementary approaches being followed for calculating the free base nicotine yield in pre-vaporization condition of e-liquids [65,67]. The first is Dilution approach and another is Without Dilution approach. The Dilution approach is followed by two methods which are Henderson-Hasselbalch method and Liquid-Liquid Extraction method. While, the Without Dilution approach is followed by 1H NMR method [65].
Under the Dilution approach, El-Hellani et al. established a Liquid-Liquid Extraction method for calculating the free base nicotine yield in e-liquids [68]. A study carried out by Gholap et al. [65] and Duell et al. [66] have shown that Liquid-Liquid Extraction method suffers a drawback of inaccurate quantification of free base nicotine yield in e-liquids. The extraction method is not specific for nicotine and can be affected by flavoring chemicals in the e-liquids. Additionally, e-liquids diluted in water can be a mixture of weak acids or bases and according to Le Chatelier’s principle, after a single extraction of free base nicotine (Nic) in organic solvent, there can be a change in equilibrium between Nic and NicH+ before the second extraction. Thus, results obtained in Liquid-Liquid Extraction method can be overestimated [65].
Another method under the Dilution approach is Henderson-Hasselbalch method. This method has been used by many authors by diluting e-liquids in fixed amount of water followed by pH measurement [1,69–71]. This dilution method has also been used for analytical characterization of smokeless tobacco [72]. This is a pH relevant approach which is considered to give a relative scale for free base nicotine measurement and classify e-liquids based on it. The rationale behind the Henderson Hasselbalch method of the Dilution approach could be that after inhalation of the e-cigarette vapor/aerosol, it undergoes a rapid condensation process in the oral cavity. This multi component process is characterized by steps such as nucleation, condensation of surrounding vapor, coagulation, and hygroscopic absorption of water in the oral cavity [73]. In short, the condensed aerosol droplets are a complex mixture of propylene glycol, vegetable glycerin, nicotine (both free base and protonated forms), flavoring chemicals, possible degradants after vaporization and water which predominates during hygroscopic growth of particles in the humid environment of oral cavity [62,73]. Additionally, the droplets undergo deposition in the respiratory tract followed by further dilution. The Dilution approach is based on the similar principle of condensation and deposition of the e-cigarette aerosol in oropharyngeal tract.
Although this method can provide a valuable characterization of e-liquids in terms of the free base nicotine yield, the method should be used with caution considering some concerns. After dilution, a solvent system is a mixture of water, nicotine, PG, VG, and flavoring chemicals. Such mixture, especially PG and VG, can affect the autoprotolysis constant of water and change the pKa of nicotine [74]. As per the mathematical model of inhaled e-cigarette aerosol presented by Asgharian et al. [56], the vapor concentration of each constituent of the e-cigarette aerosol in the oral cavity plays an important role in droplet formation by condensation. Among all the constituents, water vapor has the highest concentration considering the highly humid oral cavity. Thus, water content predominates in particle condensation (growth) as well as deposition process. Considering such high content of water vapor, it is important to study the dilution factor under the dilution approach such that it will negate the effect of PG and VG on the autoprotolysis constant of water. As described by Gholap et al. [65], a fixed dilution, for example 10X or higher, can smooth out the effect of PG, VG, and flavoring chemicals on autoprotolysis constant of water and pKa of nicotine. Having said that, the dilution factor remains an arbitrary number since the actual fraction of water in the condensation process may not be determined.
| Equation 1. Extended Henderson Hasselbalch Equation |
One more concern in this method is the effect of ionic concentration on the ionization of nicotine (NicH+) after dilution. As of now, the contents of e-liquids are not displayed on packaging or e-liquid containers and it is not possible to calculate ionic strength of solvent after dilution. The activity coefficient (γ) of H+ ions (required for protonation of nicotine) may get affected (0.96–0.83) over the range of 0.001–0.1 M of ionic strength [75]. This small change in the activity coefficients is not expected to affect the free base nicotine calculation to a large extent due to log function of the Henderson-Hasselbalch equation (Equation 1). Nevertheless, this remains a limitation of the Henderson Hasselbalch method of Dilution approach. Therefore, an alternative approach to Henderson-Hasselbalch method has been warranted by some scientists.
One of the major critiques of the Dilution approach is the change in the solvent system of e-liquids after dilution. This critique holds true if the intention of the free base nicotine measurement is prior to vaporization in e-liquid native solution [67].
One such alternative approach is Without Dilution approach which is followed by 1H NMR method as described by Duell et al. [66]. This method gives an absolute scale of free base nicotine content in e-liquids by analyzing e-liquids in their original solvent. Several e-liquids have been analyzed by Duell et al. [66] using this approach. This novel approach is promising yet, considering the complexity of analysis, the method shall be tested in detail for parameters such as overlap of nicotine’s -CH3 peak region with that of flavoring chemicals, selectivity, resolution, limit of detection, baseline drift, and alternate validating method to overcome these concerns [65].
The post vaporization yield of free base nicotine is dependent on its prevaporization yield. Additionally, the postvaporization yield of free base nicotine can be affected by several factors such as flavor, PG:VG ratio, nicotine form, puffing topography, and battery power. Thus, it is also necessary to measure free base nicotine yield in post vaporization condition.
The post vaporization aerosol is a highly unstable phase to analyze as it is. Therefore, it is necessary to collect the aerosol on a stable medium before analysis. Henderson Hasselbalch method of the Dilution approach provides a stable medium for collecting the aerosol for analysis. However, one of the major challenges of the 1H NMR method of the Without Dilution approach is that it does not provide a medium to collect e-cigarette aerosol. Earlier research has analyzed post vaporized aerosol by collecting it in NMR sample tube [66]. Such collection can lead to loss of free base form of nicotine as it is more volatile. Additionally, the aerosol can deposit on the upper region of the tube. Such analysis can lead to overestimation or underestimation of free base nicotine [76]. Therefore, although promising, the Without Dilution approach by 1H NMR needs to be studied further to address to this concern.
7. Conclusion
As the popularity of e-cigarettes is growing, especially in the youth population, the nicotine exposure should be regulated to fight nicotine addiction among youth. There are several factors which affect the nicotine exposure to a user. The e-cigarettes-related factors include ratio of propylene glycol (PG) and vegetable glycerin (VG), type of e-cigarette device, battery power, puffing profile, flavors, nicotine concentration and last but important, nicotine forms. The review summarizes the impact of nicotine forms on nicotine delivery. Nicotine forms, protonated (salt based), and free base form, are found to have different sensory and absorption effects, as detailed in section 4. The limited research available on nicotine forms (Tables 1 and Tables 2) has contradictory outcomes. Limitations to such studies include lack of controls, consistent conditions and standardized approaches in terms of analysis such as e-cigarette particle size measurement techniques, puff profiles and testing devices. Section 5 of the review explains the possible reasons for such contradictory outcomes of the studies and also recommends important factors to be considered for a future independent clinical study aimed to understand protonated vs free base nicotine absorption profile. Finally, the review summarizes the different analytical approaches being studied for determination of fractions of free base or protonated nicotine forms in e-liquids in pre and post vaporization conditions. Although complementary and promising, these analytical approaches need further research to address their limitations. Such a study will eventually help in standardization of e-cigarette analysis and help in establishing a strong relationship between different nicotine forms and their impact on nicotine delivery.
8. Expert opinion
Nicotine exposure is an important factor to be addressed while regulating e-cigarette products. The FDA has initiated the process to reduce the nicotine concentration in cigarettes [77]. However, it has not yet taken any step to limit the nicotine levels in e-cigarettes. Nicotine exposure via e-cigarettes is not only affected by nicotine concentration but also by several factors such as PG:VG ratio, battery power outputs, flavors, and puff profile. To address this nicotine exposure as a function of these factors, Shihadeh et al. [78] have used a term ‘nicotine flux’ which is the nicotine emitted per puff second by a given e-cigarette design under given use conditions.
The term ‘nicotine flux’ describes total nicotine emitted at given conditions but does not take in to account the fraction of different forms of nicotine emitted. As described earlier in sections 4 and 5, the nicotine form in e-cigarette aerosol is one of the critical factors affecting nicotine absorption. Additionally, the fraction of the nicotine form (free base or protonated) in the e-cigarette aerosol can be affected by PG:VG ratio, battery power outputs, and flavors. Earlier studies have shown that at high power settings, flavors and increase in VG content increases carbonyl products in e-cig aerosol [79]. Such carbonyl products include formaldehyde, acetaldehyde, acrolein, acetals to name a few [80–84]. Aldehydes can be easily oxidized to acids in the presence of moisture, oxygen and a heated coil surface. Acetic acid and formic acid are also well-known thermal decomposition products of both propylene glycol and vegetable glycerin in e-liquids [85]. These decomposition products are well known for their inflammatory and carcinogenic properties [86]. Additionally, the acidic nature of these products can change the ratio of nicotine forms in the aerosol, thus affecting the nicotine absorption. Therefore, considering the flux variabilities provided by 3rd and 4th generation of e-cigarettes and increasing popularity of salt based protonated nicotine e-cigarettes such as JUUL, it is important to address nicotine form as important factor along with nicotine flux while regulating the nicotine exposure due to e-cigarettes.
A two-step approach would be helpful in studying nicotine forms. First, the determination of free base or protonated form of nicotine in pre- and postvaporization of e-liquids can help in classifying the e-liquids based on their yield of the nicotine form. As mentioned in section 6, promising and complementary approaches are being studied for measurement of nicotine forms in pre and post vaporization of e-liquids. However, considering the complexities and limitations of each of these approaches, a further research is still warranted which would help in the standardization process of the analysis.
Second, an independent clinical study measuring plasma nicotine concentration-time profile as a function of nicotine form with various puff profile conditions is required. As described in Table 2, different forms of nicotine (salt vs free base) are claimed to show different plasma nicotine concentration-time profiles [31,32]. However, with limited data and study conditions, a robust relationship between nicotine form and plasma nicotine concentration levels cannot be established yet. Section 5 describes the potential reasons for varied outcomes of these studies considering critical parameters such as particle size distribution and the physical state of nicotine in aerosol. Furthermore, an independent detailed clinical study should be conducted with controlled and consistent conditions (standardization) in the use of e-cigarette to establish a robust relationship between nicotine forms and plasma nicotine concentration levels.
This two-step approach would be complementary in better understanding of the effect of nicotine forms on nicotine exposure and help in better regulation of e-cigarettes. Currently, the regulation of e-cigarettes is a multifaceted problem. With a greater concern of addressing a lifetime nicotine addiction in the youth population, it is also critical to offer adults less harmful alternatives to tobacco products for fighting the addiction. As described above, a nicotine exposure is affected by not just nicotine concentration, device settings, e-liquids ingredients but also nicotine forms. As stated by Shihadeh et al. [87], regulating one factor at a time may lead to unintended health effects. For example, limiting only the nicotine concentration may drive users to use high power settings to obtain a desired nicotine yield. Such high-power settings can change the ratio of nicotine forms and may expose a person to toxic compounds generated from e-liquid ingredients (PG, VG and flavors). Therefore, multidimensional regulations should be sought for e-cigarettes to fight the nicotine exposure and serve them as a safer alternative over combustible cigarettes.
Article highlights.
Nicotine delivery by e-cigarettes has become a matter of concern due to increasing addiction of the youth to these products.
Nicotine delivery by e-cigarettes is affected by several factors. Of these factors, nicotine form, i.e. free base or protonated, is one of the important factors which has shown to have different sensory and absorption effects.
The research on nicotine forms is limited and studies have reported contradictory results about absorption profiles of nicotine forms.
Various analytical approaches are being studied for the determination of free base or protonated fractions of total nicotine in these products.
Regulatory attention to nicotine forms is warranted to address nicotine addiction caused by e-cigarettes.
This box summarizes key points contained in the article.
Funding
This work was supported by the Virginia Youth Tobacco Projects Small Grants Program for research funding and [2P30DA033934-06] (The Central Virginia Center on Drug Abuse Research, NIDA), and additional funding through the Virginia Foundation for Healthy Youth, Youth Tobacco Prevention grants program. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. Laleh Golshahi acknowledges the support of Center for the Study of Tobacco Products (CSTP) in the form of a pilot grant under
Abbreviations
- NA
Not Applicable
- Nic
Free base nicotine
- NicH+
Protonated nicotine
- e-cig
electronic cigarette
- PG
Propylene Glycol
- VG
Vegetable Glycerin
- MMAD
Mass Median Aerodynamic Diameter
- VMAD
Volumetric Median Aerodynamic Diameter
- MMD
Mass Median Diameter
- CMD
Count Median Diameter
Footnotes
Declaration of interest
M Halquist is funded by NIDA (2P30DA033934-06) and received a small grant from the Virginia Youth Tobacco Projects Small Grants Program. L Golshahi received a pilot study grant from the Center for the Study of Tobacco Products under the main grant: U54DA036105. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.The National Academies of Sciences Engineering and Medicine. Public health consequences of E-cigarettes. Washington, DC: National Academies Press; 2018. Available from: https://www.nap.edu/catalog/24952/public-health-consequences-of-e-cigarettes [PubMed] [Google Scholar]
- 2.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Adminstration. Premarket tobacco product applications for electronic nicotine delivery systems guidance for industry [Internet]; [cited 2020 Jun 6]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/premarket-tobacco-product-applications-electronic-nicotine-deliverysystems-ends
- 4. Gholap V, Halquist MS. Historical perspective of proactive and reactive regulations of E-cigarettes to combat nicotine addiction. J Addict Med. 2020;00:1. • Timeline of e-cigarette epidemic and FDA’s limited regulatory response.
- 5.Williams M, Talbot P. Design features in multiple generations of electronic cigarette atomizers. Int J Environ Res Public Health. 2019;16:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control. 2019;28:115–116. [DOI] [PubMed] [Google Scholar]
- 7.Truth Initiative. What are puff bars [Internet]; [cited 2020 Jun 6]. Available from: https://truthinitiative.org/research-resources/emerging-tobacco-products/what-are-puff-bars
- 8.Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. 2017;1394:5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voos N, Goniewicz ML, Eissenberg T. What is the nicotine delivery profile of electronic cigarettes? Expert Opin Drug Deliv. 2019;16:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Adminstration. Youth tobacco use: results from the national youth tobacco survey [Internet]; [cited 2020 Aug 8]. Available from: https://www.fda.gov/tobacco-products/youth-andtobacco/youth-tobacco-use-results-national-youth-tobacco-survey
- 11.Centers for Disease Control and Prevention. About electronic cigarettes (E-cigarettes) [Internet]; [cited 2020 Aug 8]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- 12.Kosmider L, Spindle TR, Gawron M, et al. Nicotine emissions from electronic cigarettes: individual and interactive effects of propylene glycol to vegetable glycerin composition and device power output. Food Chem Toxicol. 2018;115:302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baassiri M, Talih S, Salman R, et al. Clouds and “throat hit”: effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols. Aerosol Sci Technol. 2017;51:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yingst JM, Hrabovsky S, Hobkirk A, et al. Nicotine absorption profile among regular users of a pod-based electronic nicotine delivery system. JAMA Netw Open. 2019;2:e1915494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of E-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2017;15:438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St.Helen G, Shahid M, Chu S, et al. Impact of e-liquid flavors on e-cigarette vaping behavior. Drug Alcohol Depend. 2018;189:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St.Helen G, Dempsey DA, Havel CM, et al. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug Alcohol Depend. 2017;178:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hellani A, Salman R, El-Hage R, et al. Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob Res. 2018;20:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Gómez D, Gaisl T, Barrios-Collado C, et al. Real-time chemical analysis of E-cigarette aerosols by means of secondary electrospray ionization mass spectrometry. Chem A Eur J. 2016;22:2452–2457. [DOI] [PubMed] [Google Scholar]
- 20.Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havel CM, Benowitz NL, Jacob P, et al. An electronic cigarette vaping machine for the characterization of aerosol delivery and composition. Nicotine Tob Res. 2017;19:1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennani I, Alami Chentoufi M, El Karbane M, et al. E-cigarette quality control: impurity and nicotine level analysis in electronic cigarette refill liquids. Sci World J. 2020;2020:3050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flora JW, Wilkinson CT, Sink KM, et al. Nicotine-related impurities in e-cigarette cartridges and refill e-liquids. J Liq Chromatogr Relat Technol. 2016;39:821–829. [Google Scholar]
- 24.Banyasz J The Physical Chemistry of Nicotine. Anal Determ Nicotine Relat Compd Metab. 1999; 152–154. Elsevier Science B.V. [Google Scholar]
- 25.U.S. National Library of Medicine. Nicotine [Internet]. PubChem. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Nicotine
- 26.Harvanko AM, Havel CM, Jacob P, et al. Characterization of nicotine salts in 23 electronic cigarette refill liquids. Nicotine Tob Res. 2020;22:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omaiye E, McWhirter KJ, Luo W, et al. Toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem Res Toxicol. 2019;32:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao XM, Xu B, Liang J, et al. Nicotine delivery to rats via lung alveolar region-targeted aerosol technology produces blood pharmacokinetics resembling human smoking. Nicotine Tob Res. 2013;15:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adrian CL, Olin HBD, Dalhoff K, et al. In vivo human buccal permeability of nicotine. Int J Pharm. 2006;311:196–202. [DOI] [PubMed] [Google Scholar]
- 30.Burch SG, Gann LP, Olsen KM, et al. Effect of pH on nicotine absorption and side effects produced by aerosolized nicotine. J Aerosol Med Depos Clear Eff Lung. 1993;6:45–52. [Google Scholar]
- 31. Xing ABC. United States Patent US9,215,895 B2. 2015. •• Pax lab patent claiming higher Cmax for nicotine salts vs free base nicotine at same concentration.
- 32.O’Connell G, Pritchard JD, Prue C, et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern Emerg Med. 2019;14:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor P Nicotinic receptors. Prim Auton Nerv Syst. 2012; 79–82. Elsevier. [Google Scholar]
- 34.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:3–10. [DOI] [PubMed] [Google Scholar]
- 38.Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb Perspect Med. 2012;2:a012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henningfield JE, Pankow JF, Garrett BE. Ammonia and other chemical base tobacco additives and cigarette nicotine delivery: issues and research needs. Nicotine Tob Res. 2004;6:199–205. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services. Chemistry and toxicology of cigarette smoke and biomarkers of exposure and harm. In: How tob smoke causes dis biol behav basis smoking-attributable dis a rep surg gen. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. p. 27–80. [Google Scholar]
- 42.Martin A. Diffusion and dissolution. Phys Pharm. Lea and Febiger. 1993;324–355. [Google Scholar]
- 43.Creighton DE The significance of pH in tobacco and tobacco smoke [Internet]. Internal document of Brown and Williamson Tobacco Corp.; 1987. Available from: Bates nos. 403605816/5832. [Google Scholar]
- 44.Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14:1465–1481. [DOI] [PubMed] [Google Scholar]
- 45.Takano M, Nagahiro M, Yumoto R. Transport mechanism of nicotine in primary cultured alveolar epithelial cells. J Pharm Sci. 2016;105:982–988. [DOI] [PubMed] [Google Scholar]
- 46.Takano M, Kamei H, Nagahiro M, et al. Nicotine transport in lung and non-lung epithelial cells. Life Sci. 2017;188:76–82. [DOI] [PubMed] [Google Scholar]
- 47.Seeman JI, Carchman RA. The possible role of ammonia toxicity on the exposure, deposition, retention, and the bioavailability of nicotine during smoking. Food Chem Toxicol. 2008;46(6):1863–1881. [DOI] [PubMed] [Google Scholar]
- 48.Hatch T, Choate SP. Statistical description of the size properties of non uniform particulate substances. J Franklin Inst. 1929;207:369–387. [Google Scholar]
- 49. Hinds WC. Particle size statistics. aerosol technol prop behav meas airborne part. Wiley; 1999. p. 75–110. • Aerosol particle size measurement: Methods and different modalities.
- 50.Zhang Y, Sumner W, Chen D-R. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res. 2013;15:501–508. [DOI] [PubMed] [Google Scholar]
- 51.Pourchez J, Parisse S, Sarry G, et al. Impact of power level and refill liquid composition on the aerosol output and particle size distribution generated by a new-generation e-cigarette device. Aerosol Sci Technol. 2018;52:359–369. [Google Scholar]
- 52.Alderman SL, Song C, Moldoveanu SC, et al. Particle size distribution of E-cigarette aerosols and the relationship to cambridge filter pad collection efficiency. Beiträge zur Tab Int to Tob Res. 2015;26:183–190. [Google Scholar]
- 53.Oldham MJ, Zhang J, Rusyniak MJ, et al. Particle size distribution of selected electronic nicotine delivery system products. Food Chem Toxicol. 2018;113:236–240. [DOI] [PubMed] [Google Scholar]
- 54.Son Y, Mainelis G, Delnevo C, et al. Investigating E-cigarette particle emissions and human airway depositions under various e-cigarette-use conditions. Chem Res Toxicol. 2020;33:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sosnowski TR, Kramek-Romanowska K. Predicted deposition of E-cigarette aerosol in the human lungs. J Aerosol Med Pulm Drug Deliv. 2016;29:299–309. [DOI] [PubMed] [Google Scholar]
- 56.Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24:976–984. [DOI] [PubMed] [Google Scholar]
- 57.Zhao T, Shu S, Guo Q, et al. Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos Environ. 2016;134:61–69. [Google Scholar]
- 58.Clapp PW, Jedalis CT, Kemanl KLWJ An inexpensive in vitro exposure system for uniform sedimentation of liquid aerosols generated by new and emerging tobacco products (NETPs). Society of Toxicology; 2020. [Internet]. Available from: https://www.toxicology.org/groups/ss/IVSS/docs/2020_SOT_Meeting_Poster_Phillip_Clapp.pdf [Google Scholar]
- 59.Floyd EL, Queimado L, Wang J, et al. Electronic cigarette power affects count concentration and particle size distribution of vaping aerosol. PLoS One. 2018;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng YS. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech. 2014;15:630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jabbal S, Poli G, Lipworth B. Does size really matter?: relationship of particle size to lung deposition and exhaled fraction. J Allergy Clin Immunol. 2017;139:2013–2014.e1. [DOI] [PubMed] [Google Scholar]
- 62. Asgharian B, Price OT, Rostami AA, et al. Deposition of inhaled electronic cigarette aerosol in the human oral cavity. J Aerosol Sci. 2018;116:34–47. •• Mathematical model describing e-cigarette aerosol particle growth and factors affecting the particle size in mouth.
- 63.Melandri C, Prodi V, Zaiacomo TDE, et al. Deposition of charged particles in human airways. J Aerosol Sci. 1983;14:657–669. [Google Scholar]
- 64.Yu CP, Chandra K, Bq L, et al. Deposition of charged particles from laminar flows in rectangular and cylindrical channels by image force. J Aerosol Sci. 1978;9:175–180. [Google Scholar]
- 65. Gholap VV, Heyder RS, Kosmider L, et al. An analytical perspective on determination of free base nicotine in E-liquids. J Anal Methods Chem. 2020;2020:1–12. •• Critique on different analytical approaches being followed in determination of fractions of nicotine forms.
- 66.Duell AK, Pankow JF, Peyton DH. Free-base nicotine determination in electronic cigarette liquids by 1 H NMR spectroscopy. Chem Res Toxicol. 2018;31:431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pankow JF, Duell AK, Peyton DH. Free-base nicotine fraction αfb in non-aqueous vs. aqueous solutions : electronic cigarette fluids without vs. with dilution with water. Chem Res Toxicol. 2020;33:1729–1735. •• Critique on different analytical approaches being followed in determination of fractions of nicotine forms.
- 68.El-Hellani A, El-Hage R, Baalbaki R, et al. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chem Res Toxicol. 2015;28:1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lisko JG, Tran H, Stanfill SB, et al. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in E-Cigarette cartridges and refill solutions. Nicotine Tob Res. 2015;17:1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stepanov I, Fujioka N. Bringing attention to e-cigarette pH as an important element for research and regulation Research letter. Tob Control. 2015;24:413–414. [DOI] [PubMed] [Google Scholar]
- 71.Etter J-F, Bugey A. E-cigarette liquids: constancy of content across batches and accuracy of labeling. Addict Behav. 2017;73:137–143. [DOI] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention. Notice regarding requirement for annual submission of the quantity of nicotine contained in smokeless tobacco products manufactured, imported, or packaged in the United States. Fed Regist. 1999;64:14086–14096. [PubMed] [Google Scholar]
- 73. Sosnowski TR, Odziomek M. Particle size dynamics : toward a better understanding of electronic cigarette aerosol interactions with the respiratory system. Front Physiol. 2018;9:1–8. •• Review based on the mathematical model and mechanisms involved in the steps of e-cigarette aerosol particle interaction in oral cavity.
- 74.Kütt A, Selberg S, Kaljurand I, et al. pKa values in organic chemistry – making maximum use of the available data. Tetrahedron Lett. 2018;59:3738–3748. [Google Scholar]
- 75.Kielland J Individual activity coefficients of ions in aqueous solutions. J Am Chem Soc. 1937;59:1675–1678. [Google Scholar]
- 76.Meehan-Atrash J, Duell AK, McWhirter KJ, et al. Free-base nicotine is nearly absent in aerosol from IQOS heat-not-burn devices, as determined by 1H NMR spectroscopy. Chem Res Toxicol. 2019;32:974–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Food and Drug Adminstration. FDA’s comprehensive plan for tobacco and nicotine regulation [Internet]; [cited 2020 Jun 6]. Available from: https://www.fda.gov/tobacco-products/ctpnewsroom/fdas-comprehensive-plan-tobacco-and-nicotineregulation
- 78.Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res. 2015;17:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ooi BG, Dutta D, Kazipeta K, et al. Influence of the E-cigarette emission profile by the ratio of glycerol to propylene glycol in E-liquid composition. ACS Omega. 2019;4:13338–13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gillman IG, Kistler KA, Stewart EW, et al. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol. 2016;75:58–65. [DOI] [PubMed] [Google Scholar]
- 81.Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, Benzaldehyde. Thorax. 2016;71:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sleiman M, Logue JM, Montesinos VN, et al. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol. 2016;50:9644–9651. [DOI] [PubMed] [Google Scholar]
- 83.Pierce JS, Abelmann A, Finley BL. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail- flavored e-cigarettes. Environ Health Perspect. 2016;124:A100–A101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salamanca JC, Meehan-Atrash J, Vreeke S, et al. E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci Rep. 2018;8:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jensen RP Thermal decomposition of electronic cigarette liquids [Internet]. Portland State University; 2016. Available from: https://pdxscholar.library.pdx.edu/open_access_etds/3081/. [Google Scholar]
- 86.Farsalinos KE, Gillman G. Carbonyl emissions in E-cigarette aerosol: a systematic review and methodological considerations. Front Physiol. 2017;8:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Talih S, Salman R, El-Hage R, et al. Might limiting liquid nicotine concentration result in more toxic electronic cigarette aerosols? Tob Control. 2020:tobaccocontrol–2019–055523. DOI: 10.1136/tobaccocontrol-2019-055523 [DOI] [PMC free article] [PubMed] [Google Scholar]


