Abstract
Background.
Mental health disorders commonly co-occur, even between conceptually distinct syndromes, such as internalizing and externalizing disorders. The current study investigated whether phenotypic, genetic, and environmental variance in negative emotionality and behavioral control account for the covariation between major depressive disorder (MDD) and alcohol use disorder (AUD).
Method.
A total of 3623 members of a national twin registry were administered structured diagnostic telephone interviews that included assessments of lifetime histories of MDD and AUD, and were mailed self-report personality questionnaires that assessed stress reactivity (SR) and behavioral control (CON). A series of biometric models were fitted to partition the proportion of covariance between MDD and AUD into SR and CON.
Results.
A statistically significant proportion of the correlation between MDD and AUD was due to variance specific to SR (men = 0.31, women = 0.27) and CON (men = 0.20, women = 0.19). Further, genetic factors explained a large proportion of this correlation (0.63), with unique environmental factors explaining the rest. SR explained a significant proportion of the genetic (0.33) and environmental (0.23) overlap between MDD and AUD. In contrast, variance specific to CON accounted for genetic overlap (0.32), but not environmental overlap (0.004). In total, SR and CON accounted for approximately 70% of the genetic and 20% of the environmental covariation between MDD and AUD.
Conclusions.
This is the first study to demonstrate that negative emotionality and behavioral control confer risk for the co-occurrence of MDD and AUD via genetic factors. These findings are consistent with the aims of NIMH’s RDoC proposal to elucidate how transdiagnostic risk factors drive psychopathology.
Keywords: Alcohol use disorder, behavior genetics, behavioral control, co-morbidity, dual diagnosis, externalizing, internalizing, major depressive disorder, negative emotionality, research domain criteria
Introduction
Co-occurring mental health disorders are common. Epidemiological studies estimate that 17% of US adults meet past-year criteria for multiple diagnoses, representing half of those with any psychiatric disorder (Kessler et al. 2005). Even conceptually distinct disorders frequently co-occur, such as internalizing (e.g. related to anxiety, mood) and externalizing (e.g. related to substance use, impulse control) disorders. For example, individuals with a past-year alcohol use disorder (AUD), relative to those without, are 2.3 times more likely to meet criteria for major depressive disorder (MDD; Grant et al. 2004). Further, individuals with multiple mental health diagnoses receive more treatment but have greater disability after treatment than those with one diagnosis (Burns et al. 2005). Therefore, understanding the common mechanisms underlying diverse forms of psychopathology is of great public health importance. The current study investigated whether phenotypic and genetic variance in negative emotionality and behavioral control account for the covariation between two conceptually distinct disorders: MDD and AUD.
Factor analytic work has consistently demonstrated that internalizing and externalizing factors capture a large degree of covariation among common mental health disorders (see Krueger & Markon, 2006 for a review). A similar two-factor model also fits genetic covariation among mental health disorders (Kendler et al. 2011). These two distinct classes of psychopathology have been consistently identified across research groups, samples, and analytic approaches. It is often overlooked, however, that these two factors demonstrate moderate phenotypic (r = 0.41–0.66; Gjone & Stevenson, 1997; Lahey et al. 2004; Krueger & Markon, 2006; Lahey et al. 2012) and genetic (rG = 0.52–0.53; Kendler & Myers, 2014) correlation, suggesting that common risk processes may be involved in both. Focusing on transdiagnostic risk factors, including personality and genetic factors, is consistent with the aim of NIMH’s Research Domain Criteria (RDoC) Project to identify fundamental risk factors that span multiple disorders (Insel et al. 2010; Sanislow et al. 2010). Identifying these common risk factors is pertinent to improving etiologic models of how distinct disorders co-occur, as well as their diagnostic classification and criteria. Further, such risk factors may inform intervention efforts aimed at more effectively addressing co-occurring problems.
Empirical work on the internalizing and externalizing disorders suggests at least two mechanisms that may underlie their co-occurrence. First, negative emotionality, or general distress, is often linked to internalizing psychopathology (Andrews et al. 1990; Clark & Watson, 1991). For example, neuroticism loads onto an internalizing spectrum factor (Hettema et al. 2006; Eaton et al. 2011), and it has substantial genetic overlap with major depression (rG = 0.49–0.67; Fanous et al. 2002). Further, neuroticism is correlated with substance use disorders (Malouff et al. 2007), and affect regulation models suggest that individuals high in trait neuroticism may use alcohol to regulate emotions (Cooper et al. 1995). In addition to being associated with internalizing and externalizing disorders, negative emotionality explains a substantial proportion of the phenotypic covariation among these disorders (Khan et al. 2005; Ellingson et al. 2015). Notably, this risk factor resembles the negative valence system, highlighted in the RDoC proposal, which includes subconstructs related to anxiety (e.g. potential threat) and sadness (e.g. loss).
Second, theoretical work suggests that trait disinhibition, defined as behavior ‘arising from lessened controls on response inclinations’, broadly confers risk for externalizing disorders (Gorenstein & Newman, 1980, p. 302, Sher & Trull, 1994; Nigg, 2003). Specifically, disinhibition is attributable to an imbalance between reward sensitivity and behavioral (under)control, resulting in greater consideration being given to proximal outcomes (e.g. getting drunk/high) over more distal outcomes (e.g. social or occupational goals). Reward sensitivity maps onto appetitive motivation (e.g. for getting drunk/high; Gray, 1990) and may be specific to externalizing disorders. In contrast, behavioral undercontrol maps onto executive functioning (Miyake et al. 2000), or executive control (Nigg, 2003), and appears to more broadly underlie psychopathology.
Behavioral control (reverse-scored) loads onto an externalizing spectrum factor and is related to phenotypic and genetic liability for externalizing psychopathology (Krueger et al. 2002). Similarly, control-based risk factors appear to be involved in internalizing disorders, with conscientiousness being the second-strongest personality correlate of MDD, after neuroticism (Kendler & Myers, 2010). The parallels between conscientiousness and behavioral control have been highlighted in the impulsivity literature, with both loading onto the construct of lack of planning (Whiteside & Lynam, 2001). Therefore, the definition of behavioral control in the current study was adapted from Whiteside & Lynam’s (2001) definition of lack of planning (the tendency to act on the spur of the moment and at the cost of long-term goals). Empirical work suggests mechanisms by which behavioral control may underlie both MDD and AUD, including attentional biases (Hankin et al. 2010; Sharbanee et al. 2014), delay discounting (Bickel & Marsch, 2001; Pulcu et al. 2014), and effortful/inhibitory control (Field et al. 2010; Kanske & Kotz, 2012). Consistent with this literature, prior work by our group has shown that trait effortful control (comprised of attentional, activational, and inhibitory control) explains phenotypic covariation between MDD and AUD, beyond what can be explained by negative emotionality (Ellingson et al. 2015). Further, behavioral control fits within the RDoC cognitive system, which includes constructs related to attention, response selection, and response inhibition.
The present study aimed to extend these findings using a national sample of adult twins. First, we attempted to replicate work showing that trait measures of negative emotionality and behavioral control account for unique covariation between MDD and AUD (Ellingson et al. 2015). Second, behavior genetic models were used to investigate whether negative emotionality and behavioral control account for the genetic covariation between MDD and AUD. Given that no prior research has investigated the role of these personality measures in explaining the genetic covariation between internalizing and externalizing psychopathology, these analyses were largely exploratory. We proposed three hypotheses for these models. First, we hypothesized that genetic factors would comprise most of the phenotypic covariation between MDD and AUD, as is typically the case for phenotypic covariance among disorders (e.g. Krueger et al. 2002; Kendler et al. 2011). Second, given the large body of evidence implicating negative emotionality as a risk factor for internalizing and externalizing disorders (e.g. Khan et al. 2005; Tackett et al. 2013), we expected that it would explain a significant proportion of the covariation among MDD and AUD. Finally, mounting evidence suggests that behavioral control is involved in risk for internalizing and externalizing psychopathology (e.g. Field et al. 2010; Kanske & Kotz, 2012), and we hypothesized that it would explain some degree of covariance, beyond what is accounted for by negative emotionality. The degree to which these factors account for covariation among these conceptually distinct disorders will help determine the extent to which they may be important targets for broad intervention efforts.
Method
Participants
Participants were 3623 members of the Australian Twin Registry (ATR) Cohort II. In 1980–1982, a sample of 4268 twin pairs born during 1964–1971 were registered with the ATR, in response to appeals though the media and Australian school systems. They were first surveyed in 1989–1992. Data in the current study were collected in 2004–2007, for a study primarily focused on gambling (Slutske et al. 2009), via structured diagnostic telephone interview and mailed self-report questionnaire [interview: n = 4764 twins, mean age = 37.7 years (range = 32–43), response rate = 80%; questionnaire: n = 4369 twins, response rate = 92%]. The current study was based on data from same-sex monozygotic (MZ) and dizygotic (DZ) twins. There were 1461 complete twin pairs [867 MZ (347 male, 520 female), 594 DZ (227 male, 367 female)] and 701 twins from incomplete pairs [304 MZ (153 male, 151 female), 397 DZ (216 male, 181 female)].
Procedures
Interviews were conducted by trained lay-interviewers who were blind to the psychiatric status (e.g. diagnoses) of the co-twin. Informed consent was obtained from all participants.
Retest data were collected for a small subsample of the participants (n = 166) several months after their initial interview [mean interval = 3.4 months (standard deviation (S.D.) = 1.4 months, range = 1.2–9.5 months]. These data were used to evaluate the test-retest reliability of the diagnostic measures, using the kappa (κ) (Cohen, 1968) and Yule’s Y (Spitznagel & Helzer, 1985) coefficients.
Measures
Personality
Participants who completed the diagnostic interview were also administered the 196-item Multidimensional Personality Questionnaire (MPQ; Tellegen, 1982), a Big Three measure of personality. The MPQ consists of three higher-order scales that are each composed of 3–4 lower-order subscales: Constraint (Control, Traditionalism, and Harm Avoidance), Negative Emotionality (Stress Reaction, Alienation, and Aggression), and Positive Emotionality (Wellbeing, Social Potency, Social Closeness, and Achievement). The current study used the 14-item Stress Reaction subscale (SR; e.g. ‘I am too sensitive for my own good’) and the 20-item Control subscale (CON; e.g. ‘I often act without thinking’). As a subscale of negative emotionality, SR correlates strongly with other indicators of the tendency to experience negative affective states, such as Neuroticism from the NEO Five-Factor Inventory (NEO-FFI; McCrae & Costa, 1987) (r = 0.73; Tellegen & Waller, 2008). In contrast, the CON subscale was developed to assess self-regulation and correlates moderately with Conscientiousness from the NEO-FFI (r = 0.52). Adequate internal consistency was demonstrated for both SR (α = 0.86) and CON (α = 0.81) in the current sample.
AUD
The AUD section from the WHO Composite International Diagnostic Interview (CIDI) was used to assess lifetime AUD symptoms (Robins et al. 1988). These symptoms were used to create an approximate DSM-5 AUD diagnosis based on a cut-off of endorsing two or more symptoms (excluding ‘craving,’ which was not included in this version of the CIDI). About one-third of participants met criteria for AUD (30.5%). The estimated test–retest reliability for this measure of AUD was 0.57 based on κ [95% confidence interval (CI) 0.42–0.71] and 0.63 based on Y (95% CI 0.47–0.79).
MDD
The assessment of MDD was a modified version of the depression assessment from the Alcohol Use Disorder and Associated Disabilities Interview Schedule – IV used in the National Epidemiologic Survey of Alcohol and Related Conditions (Grant et al. 2004). To be administered all MDD criteria, individuals had to endorse having experienced a period of at least 2 weeks during which depressed mood or loss of interest/pleasure in daily activities was present. These symptoms were used to create a DSM-5 MDD diagnosis based on a cut-off of endorsing five or more symptoms. Distress, impairment, and exclusionary criteria (e.g. depression due to physical causes) were not assessed, resulting in a broad diagnosis of MDD. About one-third of participants met criteria for MDD (33.0%), and the estimated test–retest reliability was 0.71 based on κ (95% CI 0.59–0.84) and 0.73 based on Y (95% CI 0.60–0.85).
Statistical analyses
A series of biometric models was fitted directly to the raw twin data by the method of robust weighted least squares (for the analysis of categorical phenotypes) or maximum likelihood (for the analysis of continuous phenotypes) using the Mplus program version 7 (Muthén & Muthén, 1998–2012). To account for missing data, including incomplete twin pairs, analyses were conducted using full information maximum likelihood (FIML; Enders & Bandalos, 2001). Models were iteratively fit to all phenotypes to determine the best-fitting reduced models to carry forward for the final parameter estimates. As described below, variance due to the shared family environment was negligible in univariate models (i.e. for each phenotype) and in a quadrivariate model (i.e. including all phenotypes), resulting in reduced models estimating only genetic and unique environmental contributions. Bivariate model fitting was conducted with (1) MDD and CON, (2) MDD and SR, (3) AUD and CON, and (4) AUD and SR, in order to partition the covariation between each pair of variables into genetic and environmental factors.
Fig. 1 depicts one of two quadrivariate Cholesky models (Neale & Cardon, 1992; Loehlin, 1996) that were fitted to quantify the extent to which (a) the phenotypic covariation between MDD and AUD was explained by SR and CON, and (b) the genetic and environmental covariation between MDD and AUD was explained by additive genetic and unique environmental factors due to SR and CON. In this first model, factor A1 accounts for all of the genetic variation in SR (the variable entered first) and the genetic variation in CON (path a12), AUD (a13), and MDD (a14) that is explained by SR. Factor A2 then accounts for the residual genetic variation (i.e. after accounting for variation in SR) in CON (a22), and any residual genetic variation in AUD (a23) and MDD (a24) that is due to CON. Factor A3 accounts for any residual genetic variation (i.e. after accounting for variation in SR and CON) in AUD (a33), and any residual genetic variation in MDD (a34) due to AUD. Finally, factor A4 accounts for any genetic variation in the risk for MDD, unexplained by SR, CON, and AUD. This same approach was applied to decompose the unique environmental variation using factors E1, E2, E3, and E4.
Fig. 1.
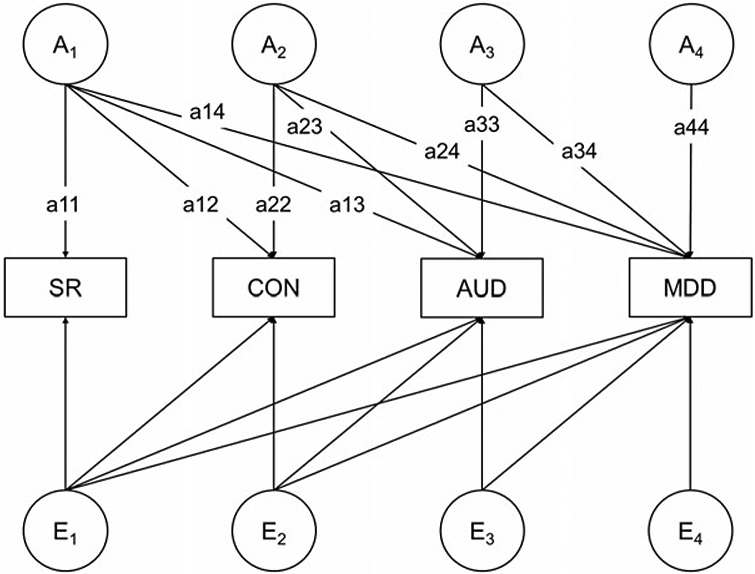
A model quantifying the extent to which the genetic and environmental covariation between major depressive disorder (MDD) and alcohol use disorder (AUD) is explained by (1) additive genetic and unique environmental factors that influence stress reaction (SR), (2) additive genetic and unique environmental factors that influence behavioral control (CON), and (3) additive genetic and unique environmental factors that influence both SR and CON. For simplicity, this model is illustrated for only one individual from a twin pair. In this model, SR is entered first; thus, the proportion of genetic covariation between MDD and AUD that is attributable to CON (after accounting for genetic variation shared with SR) is [(a23 × a24)/(a13 × a14 + a23 × a24 + a33 × a34)]. To determine the proportion of genetic covariation attributable to SR (after accounting for genetic variation shared with CON), this model was re-run with CON entered first.
The total genetic covariation between MDD and AUD is the sum of pathways (a13 × a14), (a23 × a24), and (a33 × a34). The proportion of genetic covariation between MDD and AUD that is attributable to CON (after accounting for genetic variation shared with SR) is [(a23 × a24)/(a13 × a14 + a23 × a24 + a33 × a34)] and the covariation not explained by CON or SR is [(a33 × a34)/(a13 × a14 + a23 × a24 + a33 × a34)]. The proportion of phenotypic covariation between MDD and AUD that is explained by variance specific to CON is [(a23 × a24) + (e23 × e24)/(a13 × a14 + a23 × a24 + a33 × a34) + (e13 × e14 + e23 × e24 + e33e34)]. To determine the proportion of phenotypic, genetic, and environmental covariation that was unique to SR (i.e. after accounting for CON), the quadrivariate model was re-run with CON entered first and SR entered second. Therefore, the quadrivariate models estimated the proportion of phenotypic, genetic, and environmental covariation between MDD and AUD due to (1) SR only, (2) CON only, (3) both SR and CON, and (4) neither SR nor CON.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The Institutional Review Board at the University of Missouri and the Human Research Ethics Committee at the Queensland Institute of Medical Research approved all data collection.
Results
Phenotypic analyses
Phenotypic correlations were estimated using proc corr in SAS version 9.4 (SAS Institute, 2002–2010; see Table 1). Subsequently, phenotypic structural equation models (similar to the biometric models) were fitted to determine the proportions of covariance between MDD and AUD that were attributable to SR and CON. Estimates from the quadrivariate model suggested that MDD and AUD were comparably correlated among men (r = 0.34, 95% CI 0.20–0.48) and women [r = 0.32, 95% CI 0.22–0.42)]†,1. The correlation between SR and CON was higher among men [0.14 (0.08–0.19)] than women [0.06 (0.01–0.10)]. Among men, a statistically significant proportion of the correlation between MDD and AUD was accounted for by variance specific to SR [0.31 (0.18–0.44)] and CON [0.20 (0.07–0.33)]. Similar estimates were obtained among women [SR = 0.27 (0.14–0.40); CON = 0.19 (0.06–0.33)]. Therefore, there was evidence that CON explains unique covariation between MDD and AUD, above what is explained by SR. A small proportion of the correlation was also due to variance common to SR and CON. In total, variance unique to and shared by SR and CON explained approximately half of the phenotypic correlation between MDD and AUD. Biometric analyses were conducted to determine the degree to which this covariation was explained by genetic and/or environmental influences on SR and CON.
Table 1.
Phenotypic correlations between major depressive disorder, alcohol use disorder, stress reaction, and behavioral control
| Phenotype | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Major depressive disorder | – | 0.31 | 0.39 | 0.08 |
| 2. Alcohol use disorder | 0.33 | – | 0.20 | 0.29 |
| 3. Stress reaction | 0.42 | 0.24 | – | 0.06 |
| 4. Behavioral control | 0.13 | 0.22 | 0.14 | – |
Estimates for men are on the lower diagonal, women on the upper diagonal.
Correlations between major depressive disorder (MDD) and alcohol use disorder (AUD) are tetrachoric coefficients. Correlations between MDD and stress reaction (SR), MDD and behavioral control (CON), AUD and SR, and AUD and CON are biserial coefficients. Correlations between SR and CON are Pearson coefficients.
Twin correlations
Table 2 displays the within-trait and cross-trait twin correlations. Inspection reveals that the within-trait MZ correlations were larger than the DZ correlations for MDD, AUD, SR, and CON, which implicates genetic influences for all of the phenotypes studied. In addition, the cross-trait MZ correlations were larger than the DZ correlations for MDD, AUD, SR, and CON, which implicates genetic influences on the covariation between these traits.
Table 2.
Within-trait and cross-trait twin correlations between major depressive disorder, alcohol use disorder, stress reaction, and control
| Twin 1 phenotype | Twin 2 phenotype |
|||||||
|---|---|---|---|---|---|---|---|---|
| Monozygotic men (500 pairs) |
Monozygotic women (671 pairs) |
|||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 1. Major depressive disorder | 0.34 | 0.43 | ||||||
| 2. Alcohol use disorder | 0.19 | 0.52 | 0.25 | 0.53 | ||||
| 3. Stress reaction | 0.25 | 0.15 | 0.44 | 0.24 | 0.11 | 0.43 | ||
| 4. Behavioral control | 0.19 | 0.22 | 0.09 | 0.43 | 0.10 | 0.26 | −0.02 | 0.36 |
| Dizygotic men (443 pairs) |
Dizygotic women (548 pairs) |
|||||||
| Twin 1 phenotype | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| 1. Major depressive disorder | −0.01 | 0.30 | ||||||
| 2. Alcohol use disorder | −0.01 | 0.28 | 0.06 | 0.26 | ||||
| 3. Stress reaction | −0.09 | 0.07 | 0.08 | 0.15 | 0.01 | 0.19 | ||
| 4. Behavioral control | 0.004 | 0.15 | −0.01 | 0.16 | 0.04 | 0.16 | −0.05 | 0.20 |
There were also 701 twins from incomplete pairs (153 monozygotic men, 151 monozygotic women, 216 dizygotic men, 181 dizygotic women).
Biometric model fitting
Results from the bivariate biometric models are displayed in Table 3. These models fit very well (root mean square error of approximation <0.02). Notably, additive genetic factors explained nearly all of the covariation between CON and AUD (0.96) and a substantial proportion of the covariation between CON and MDD (0.83). Genetic contributions to the covariation between SR and the two disorders were less substantial (AUD: proportion A = 0.56, MDD: proportion A = 0.59). Genetic and environmental correlations were also estimated between the psychiatric and personality measures. Whereas SR demonstrated genetic and environmental overlap with psychiatric outcomes, CON demonstrated only genetic overlap.
Table 3.
Bivariate behavior genetic model estimates of the proportion of covariation for psychiatric diagnoses [major depressive disorder (MDD), alcohol use disorder (AUD)] and personality measures (behavioral control, stress reactivity) attributed to genetic and environmental factors, and genetic and environmental correlation estimates
| Phenotype | Proportion of covariation due to biometric factors |
|||
|---|---|---|---|---|
| Behavioral control and diagnosis |
Stress reactivity and diagnosis |
|||
| Additive genetic | Unique environment | Additive genetic | Unique environment | |
| Full sample | ||||
| DSM-5 AUD | 0.96 (0.92–0.99 ) | 0.04 (0.01 to 0.08) | 0.56 (0.30–0.82) | 0.44 (0.18–0.70) |
| DSM-5 MDD | 0.83 (0.73–0.93) | 0.17 (0.07 to 0.27) | 0.59 (0.45–0.73) | 0.41 (0.27–0.55) |
| Men | ||||
| DSM-5 AUD | 0.93 (0.84–1.00) | 0.08 (−0.01 to 0.16) | 0.63 (0.26–1.00) | 0.37 (0.00–0.74) |
| DSM-5 MDD | 0.77 (0.61–0.93) | 0.23 (0.07 to 0.39) | 0.50 (0.26–0.75) | 0.50 (0.26–0.74) |
| Women | ||||
| DSM-5 AUD | 0.96 (0.93–1.00) | 0.04 (−0.002 to 0.08) | 0.49 (0.12–0.86) | 0.51 (0.14–0.88) |
| DSM-5 MDD | 0.86 (0.73–0.99) | 0.14 (0.01 to 0.27) | 0.64 (0.47–0.82) | 0.36 (0.18–0.53) |
| Biometric factor correlations |
||||
| Behavioral control and diagnosis |
Stress reactivity and diagnosis |
|||
| Phenotype | r G | r E | r G | r E |
| Full sample | ||||
| DSM-5 AUD | 0.57 (0.44–0.69) | −0.01 (−0.11 to 0.10) | 0.26 (0.13–0.39) | 0.18 (0.08–0.29) |
| DSM-5 MDD | 0.32 (0.17–0.47) | −0.03 (−0.12 to 0.07) | 0.56 (0.44–0.69) | 0.27 (0.18–0.36) |
| Men | ||||
| DSM-5 AUD | 0.48 (0.29–0.68) | −0.01 (−0.18 to 0.15) | 0.32 (0.12–0.52) | 0.16 (0.00–0.32) |
| DSM-5 MDD | 0.48 (0.22–0.75) | −0.04 (−0.18 to 0.10) | 0.58 (0.32–0.83) | 0.30 (0.16–0.45) |
| Women | ||||
| DSM-5 AUD | 0.62 (0.44–0.79) | 0.00 (−0.14 to 0.14) | 0.21 (0.04–0.38) | 0.20 (0.06–0.34) |
| DSM-5 MDD | 0.24 (0.05–0.43) | −0.02 (−0.15 to 0.10) | 0.57 (0.43–0.71) | 0.24 (0.13–0.35) |
AUD, Alcohol use disorder; MDD, major depressive disorder.
Values in parentheses are 95% confidence intervals. Some confidence intervals exceeded possible values and were bounded at 1.00.
Not shown are model estimates for the proportion of covariation between AUD-MDD [A = 0.63 (0.41–0.85), E = 0.37 (0.15–0.59)], and the biometrical factor correlations among AUD and MDD [full sample: rG = 0.44 (0.28–0.60), rE = 0.22 (0.09–0.36); men: rG = 0.40 (0.11–0.68), rE = 0.31 (0.11–0.50); and women: rG = 0.47 (0.28–0.66), rE = 0.15 (−0.03 to 0.34)].
A quadrivariate biometric model was constructed to decompose the genetic and environmental covariance between AUD and MDD into CON and SR (see Supplementary material for model-fitting results). An initial model included all three biometric factors (ACE), and a nested model fixed variance due to C at zero without a significant decrement in fit. Given the negligible influence of C, AE models were carried forward. We first fit an AE model in which SR was entered first, in order to derive genetic and environmental estimates specific to CON. Within this model, constraining parameters to be the same across men and women did not provide a significant decrement in fit, suggesting that there were no sex differences in the sources of covariation between MDD and AUD (see Supplementary material). The phenotypic correlation between MDD and AUD in the constrained model was 0.33, of which approximately two-thirds was explained by genetic influences [A = 0.63 (0.41–0.85)] and one-third was explained by unique environmental influences [E = 0.37 (0.15–0.59)].
The standardized estimate for the genetic parameter from SR to CON (path a12) was low and non-significant [0.002 (−0.01 to 0.02)], and constraining it to zero did not significantly worsen model fit. Further, after accounting for SR, the proportion of environmental covariance explained by environmental influences on CON [(e23 × e24)/(e13 × e14 + e23 × e24 + e33 × e34)] was negligible [0.01 (−0.03 to 0.05)], and paths e23 and e24 could be constrained to zero without a significant decrement in fit (see online Supplementary material). Therefore, CON explained the covariation between MDD and AUD solely via genetic mechanisms: a moderate proportion of the genetic covariance was explained by genetic influences on CON (0.32; see Table 4). In this model, genetic influences both specific to and shared between CON and SR accounted for 66% of the genetic covariation [A = 0.66 (0.38–0.94)], and unique environmental influences explained 20% of the unique environmental covariation [E = 0.20 (0.03–0.38)].
Table 4.
Proportions of the genetic and environmental covariation between major depressive disorder and alcohol use disorder accounted for by genetic and environmental influences specific to stress reaction and behavioral control
| Trait | Additive genetic covariance |
Unique environmental covariance |
|---|---|---|
| Full sample | ||
| Stress reaction | 0.33 (0.14–0.52) | 0.23 (0.04 to 0.41) |
| Behavioral control | 0.32 (0.16–0.48) | 0.00 (0.00 to 0.00) |
| Men | ||
| Stress reaction | 0.46 (0.05–0.87) | 0.17 (−0.04 to 0.37) |
| Behavioral control | 0.42 (0.08–0.77) | 0.00 (0.00 to 0.00) |
| Women | ||
| Stress reaction | 0.26 (0.03–0.48) | 0.32 (−0.11 to 0.74) |
| Behavioral control | 0.26 (0.07–0.45) | 0.00 (0.00 to 0.00) |
Values in parentheses are 95% confidence intervals.
The proportion of environmental covariance explained by environmental influences on Control was negligible (0.01) and was constrained to 0.00 for model-fitting.
We subsequently fit a model in which CON was entered first. Results indicated that both genetic and environmental influences unique to SR accounted for a significant proportion of the genetic (0.33) and environmental (0.23) covariance between MDD and AUD (Table 4). In this model, 71% of the genetic and 23% of the unique environmental covariation between AUD and MDD was explained by both CON and SR [A = 0.71 (0.42–0.995), E = 0.23 (0.04–0.41)].
Discussion
The current study investigated whether transdiagnostic factors, negative emotionality and behavioral control, account for the covariation between MDD and AUD. Consistent with prior work, results suggest that both risk factors account for a statistically significant proportion of the covariation between MDD and AUD (Ellingson et al. 2015). Further, nearly all of the covariation explained by control was due to genetic factors, and negative emotionality explained the covariation via genetic and environmental factors. These findings are of particular interest with regard to NIMH’s RDoC initiative, which involves ‘shifting the central research focus of the field away from clinical description to more squarely examine aberrant mechanisms. RDoC first aims to identify reliable and valid psychological and biological mechanisms and their disruptions, with an eventual goal of understanding how anomalies in these mechanisms drive psychiatric symptoms’ (p. 631, Sanislow et al. 2010). As outlined by the RDoC proposal, the trait measures of negative emotionality and behavioral control used in the current study map onto the negative valence (e.g. potential threat) and cognitive (e.g. cognitive/effortful control) systems, respectively (Morris & Cuthbert, 2012).
The extant literature suggests that negative emotionality may increase risk for MDD and other internalizing psychopathology, as distress and negative affect are central to these disorders (Clark & Watson, 1991; Eaton et al. 2011). In contrast, evidence suggests that negative emotionality increases risk for AUD by way of coping motives and alcohol use as a means of affect regulation (Cooper et al. 1995), which may then increase liability for alcohol-related consequences. Supporting this relation, longitudinal research has found that changes in coping-related drinking motives mediate the association between changes in neuroticism and alcohol-related problems (Littlefield et al. 2010), and genetically-informed research has shown that coping-related drinking motives mediate the genetic overlap between neuroticism and alcohol-related problems (Littlefield et al. 2011).
With respect to behavioral control, measures such as disinhibition have been linked with AUD and other externalizing psychopathology, as these disorders are characterized by impulsive behavior (Gorenstein & Newman, 1980; Sher & Trull, 1994). There has been considerably less work concerning behavioral control as a risk factor for MDD and other internalizing disorders, but a lack of self-control has been linked to general, negative outcomes (e.g. health behaviors, mortality; Bogg & Roberts, 2004). There are several processes by which control-based risk may underlie MDD and AUD. Prior work by our group found that the Effortful Control Scale (subscales of inhibitory control, activational control, attentional control; Derryberry & Rothbart, 1988) explained a substantial proportion of the covariation between MDD and AUD (Ellingson et al. 2015). Given the content of the Effortful Control and MPQ Control scales, low behavioral control may be a risk mechanism via diminished ability to inhibit urges to drink, thus increasing liability for AUD (Sharbanee et al. 2014). Similarly, low behavioral control may decrease one’s tendency to initiate adaptive activities when a depressed mood is present, thus increasing liability for MDD (Dimidjian et al. 2011). Alternatively, cognitive/attentional control may increase attentional biases toward stimuli high in emotional valence, such as fixations on potential reward (e.g. craving; Field et al. 2006) or loss/threat (e.g. rumination; Ouimet et al. 2009). Finally, behavioral control may confer risk broadly via maladaptive decision making, resulting in problematic alcohol use, as well as negative life circumstances that make depressive episodes more likely. It will be important for future research to elucidate how control-based processes are associated with MDD and AUD, and potentially general psychopathology (Caspi et al. 2013).
These findings do not identify specific genetic or environmental factors related to SR or CON that contribute to MDD and AUD. Molecular genetic research is needed to further the progress in this area. Notably, the RDoC proposal suggests that transdiagnostic risk factors, such as the negative valence and control systems, are more likely to map onto the biological systems that drive psychopathology. That being said, the search for genetic markers of personality traits has not yet yielded robust findings, and the current understanding is that any genes identified will be of extremely small effect and require very large samples to be discovered (e.g. Genetics of Personality Consortium et al. 2015, n > 50 000, 100 + significant genetic associations; Schizophrenia Working Group of the Psychiatric Genomics Consortium et al. 2014). Notably, de Moor et al. (2015) used polygenic risk scores (i.e. a composite of the top risk alleles) to explain 1% of the variance in neuroticism from the top markers via meta-analysis of 27 studies and over 63 000 participants.
The twin correlations in the current study also suggest potential sex differences in how behavioral control and negative emotionality confer risk for psychopathology. In particular, there was a notable difference in DZ correlations involving MDD for men and women, but MZ correlations were similar for men and women. Future research may benefit from including opposite-sex twin pairs to investigate whether quantitative or qualitative sex differences confer risk for psychopathology via behavioral control and negative emotionality (Neale & Maes, 1999; Neale et al. 2006).
Limitations
There are at least three limitations that should be considered when interpreting the findings of the current study. Given that the sample was a cohort of middleaged Australian adults, the results may not generalize to other ages or samples, particularly those with different degrees of risk for alcohol or mood-related problems (e.g. adolescence, emerging adulthood). In addition, self-report measures of personality were used. Much of the work on ‘bottom-up’ emotional reactivity and ‘top-down’ cognitive control uses laboratory-based measures and/or neuroimaging methods. Applying these alternative measurement methods to similar cognitive and affective factors will be important for understanding how these factors confer risk for both MDD and AUD. Regarding the application of these findings to constructs described in RDoCs, the use of categorical diagnoses rather than symptom counts may be viewed as a limitation. These concerns may be allayed by the analytic approach, however, which assumes that the distributions of liability for MDD and AUD are continuous and normal, and diagnoses represent cases in which liability crosses a threshold (i.e. liability-threshold model; Neale & Cardon, 1992). Finally, and as noted above, the current analytic approach allows important inferences to be made about the aggregate genetic liability to these disorders, but molecular genetic research will be important for identifying specific genes that contribute to the risk for internalizing and externalizing psychopathology related to cognitive and negative valence systems. In addition, the cross-twin, cross-trait correlations in the current study suggest the presence of non-additive genetic influences (i.e. dominance or epistasis), with MZ twin estimates more than twice that of DZ twin estimates. The current sample was underpowered to adequately investigate these possibilities, but future behavior and molecular genetic studies could address this possibility.
Conclusions
The current study shows that trait measures of behavioral control account for the covariation between MDD and AUD (Li & Sinha, 2008; Field et al. 2010; Kanske & Kotz, 2012; Ellingson et al. 2015) beyond what is accounted for by negative emotionality (Khan et al. 2005). Importantly, this is the first study to demonstrate that both behavioral control and negative emotionality confer risk for this covariation via genetic factors, and that negative emotionality also confers risk for this covariation via environmental factors. Given the distinct role that behavioral control appears to play in the covariation between these two distinct disorders, future research could investigate whether behavioral control underlies risk for general psychopathology, as has been shown for negative emotionality (Tackett et al. 2013). Additional research will also be needed for identifying specific risk factors that comprise this genetic and environmental risk. In addition, longitudinal research will be important for disentangling the temporal relation between MDD, AUD, behavioral control, and negative emotionality. Finally, these findings suggest that behavioral control and negative emotionality may be important intervention targets to address a wide range of mental health problems.
Supplementary Material
Acknowledgements
Thanks to Bronwyn Morris and Megan Fergusson for coordinating the data collection for the twins, and to David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
Funding for this study was provided by the National Institutes of Health grants R01MH66206 (W.S.S.), F31AA022294 (J.M.E.), and F31AA023419 (L.S.R.).
Footnotes
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291716001525.
Declaration of Interest
None.
These correlations were estimated in Mplus. They differ slightly from the correlations presented in Table 1 as full information maximum likelihood procedures were employed to account for missing data.
The notes appear after the main text.
References
- Andrews G, Stewart G, Morris-Yates A, Holt P, Henderson S (1990). Evidence for a general neurotic syndrome. British Journal of Psychiatry 157, 6–12. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96, 73–86. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW (2004). Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin 130, 887–919. [DOI] [PubMed] [Google Scholar]
- Burns L, Teesson M, O’Neill K (2005). The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction 100, 787–796. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE (2013). The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science 2, 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology 100, 316–336. [DOI] [PubMed] [Google Scholar]
- Cohen J (1968). Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychological Bulletin 70, 213–220. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P (1995). Drinking to regulate positive and negative emotions: a motivational model of alcohol use. Journal of Personality and Social Psychology 69, 990–1005. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK (1988). Arousal, affect, and attention as components of temperament. Journal of Personality and Social Psychology 55, 958–966. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Martell C, Muñoz RF, Lewinsohn PM (2011). The origins and current status of behavioral activation treatments for depression. Annual Review of Clinical Psychology 7, 1–38. [DOI] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, Oltmanns TF (2011). Aging and the structure and long-term stability of the internalizing spectrum of personality and psychopathology. Psychology and Aging 26, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson JM, Richmond-Rakerd LS, Slutske WS (2015). Brief report: cognitive control helps explain comorbidity between alcohol use disorder and internalizing disorders. Journal of Studies on Alcohol and Drugs 76, 89–94. [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling 8, 430–457. [PubMed] [Google Scholar]
- Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS (2002). Neuroticism, major depression and gender: a population-based twin study. Psychological Medicine 32, 719–728. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP (2006). Attention to drugrelated cues in drug abuse and addiction: Component processes. In Handbook of Implicit Cognition and Addiction (ed. R. W. Wiers and A. W. Stacy), pp. 151–163. Sage Publications Inc.: Thousand Oaks, CA, USA. [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC (2010). Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research 34, 1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetics of Personality Consortium, de Moor MH, Verweij KJ, van den Berg SM, Krueger RF, Fuciano M, Arias Vasquez A, Matteson LK, Derringer J, Esko T, Amin N, Gordon SD, Hansell NK, Hart AB, Seppälä I, Huffman JE, Konte B, Lahti J, Lee M, Miller M, Nutile T, Tanaka T, Teumer A, Viktorin A, Wedenoja J, Abecasis GR, Adkins DE, Agrawal A, Allik J, Appel K, Bigdeli TB, Busonero F, Campbell H, Costa PT, Davey Smith G, Davies G, de Wit H, Ding J, Engelhardt BE, Eriksson JG, Fedko IO, Ferrucci L, Franke B, Giegling I, Grucza R, Hartmann AM, Heath AC, Heinonen K, Henders AK, Homuth G, Hottenga JJ, Iacono WG, Janzing J, Jokela M, Karlsson R, Kemp JP, Kirkpatrick MG, Latvala A, Lehtimäki T, Liewald DC, Madden PA, Magri C, Magnusson PK, Marten J, Maschio A, Medland SE, Mihailov E, Milaneschi Y, Montgomery GW, Nauck M, Ouwens KG, Palotie A, Pettersson E, Polasek O, Qian Y, Pulkki-Råback L, Raitakari OT, Realo A, Rose RJ, Ruggiero D, Schmidt CO, Slutske WS, Sorice R, Starr JM, St Pourcain B, Sutin AR, Timpson NJ, Trochet H, Vermeulen S, Vuoksimaa E, Widen E, Wouda J, Wright MJ, Zgaga L, Porteous D, Minelli A, Palmer AA, Rujescu D, Ciullo M, Hayward C, Rudan I, Metspalu A, Kaprio J, Deary IJ, Räikkönen K, Wilson JF, Keltikangas-Järvinen L, Bierut LJ, Hettema JM, Grabe HJ, van Duijn CM, Evans DM, Schlessinger D, Pedersen NL, Terracciano A, McGue M, Penninx BW, Martin NG, Boomsma DI. (2015). Meta-analysis of genome-wide association studies for neuroticism, and the polygenic association with major depressive disorder. JAMA Psychiatry 72, 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjone H, Stevenson J (1997). The association between internalizing and externalizing behavior in childhood and early adolescence: genetic of environmental common influences? Journal of Abnormal Child Psychology 25, 277–286. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP (1980). Disinhibitory psychopathology: a new perspective and a model for research. Psychological Review 87, 301–315. [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry 61, 807–816. [DOI] [PubMed] [Google Scholar]
- Gray JA (1990). Brain systems that mediate both emotion and cognition. Cognition & Emotion 4, 269–288. [Google Scholar]
- Hankin BF, Gibb BE, Abela JRZ, Flory K (2010). Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. Journal of Abnormal Psychology 119, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS (2006). A population-based twin study of the relationship between neuroticism and internalizing disorders. American Journal of Psychiatry 163, 857–864. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA (2012). Effortful control, depression, and anxiety correlate with the influence of emotion on executive attentional control. Biological Psychology 91, 88–95. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Rnysamb E, Neale MC, Reichborn-Kjennerud T (2011). The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. American Journal of Psychiatry 168, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J (2010). The genetic and environmental relationship between major depression and the five-factor model of personality. Psychological Medicine 40, 801–806. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J (2014). The boundaries of the internalizing and externalizing genetic spectra in men and women. Psychological Medicine 44, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS (2005). Personality and comorbidity of common psychiatric disorders. British Journal of Psychiatry 186, 190–196. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M (2002). Etiologic connections among substance dependence, antisocial behavior and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology 111, 411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE (2006). Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology 2, 111–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ (2012). Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology 121, 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Waldman ID, Loft JD, Hankin BL, Rick J (2004). The structure of child and adolescent psychopathology: generating new hypotheses. Journal of Abnormal Psychology 113, 358–385. [DOI] [PubMed] [Google Scholar]
- Li C-sR, Sinha R (2008). Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and Biobehavioral Reviews 32, 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Agrawal A, Ellingson JM, Kristjansson S, Madden PA, Bucholz KK, Slutske WS, Heath AC, Sher KJ (2011). Does variance in drinking motives explain the genetic overlap between personality and alcohol use disorder symptoms? A twin study of young women. Alcoholism: Clinical and Experimental Research 35, 2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, Wood PK (2010). Do changes in drinking motives mediate the relation between personality change and “maturing out” of problem drinking? Journal of Abnormal Psychology 119, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC (1996). The Cholesky approach: a cautionary note. Behavior Genetics 26, 65–69. [Google Scholar]
- Malouff JM, Thorsteinsson EB, Rooke SE, Schutte NS (2007). Alcohol involvement and the five-factor model of personality: a meta-analysis. Journal of Drug Education 37, 277–294. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT (1987). Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology 52, 81–90. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN (2012). Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience 14, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (1998–2012). Mplus User’s Guide. Muthén & Muthén: Los Angeles, CA. [Google Scholar]
- Neale MC, Cardon LR (1992). Methodology for Genetic Studies of Twins and Families. Springer: Netherlands. [Google Scholar]
- Neale MC, Maes HH (1999). Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers B.V.: Dordrecht, Netherlands. [Google Scholar]
- Neale MC, Røysamb E, Jacobson K (2006). Multivariate genetic analysis of sex limitation and G × E interaction. Twin Research and Human Genetics 9, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2003). Response inhibition and disruptive behaviors. Annals of the New York Academy of Sciences 1008, 170–182. [DOI] [PubMed] [Google Scholar]
- Ouimet AJ, Gawronski B, Dozois DJA (2009). Cognitive vulnerability to anxiety: a review and an integrative model. Clinical Psychology Review 29, 459–470. [DOI] [PubMed] [Google Scholar]
- Pulcu E, Trotter PD, Thomas EJ, McFarquhar M, Juhasz G, Sahakian BJ, Deakin JFW, Zahn R, Anderson IM, Elliott R (2014). Temporal discounting in major depressive disorder. Psychological Medicine 44, 1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, Sartorius N, Towle LH (1988). The composite international diagnostic interview: an epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry 45, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS-E, Cuthbert BN (2010). Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology 119, 631–639. [DOI] [PubMed] [Google Scholar]
- SAS Institute (2002–2010). SAS Software, Version 9.3. SAS Institute Inc., Cary: NC. [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium, Ripke S, Neale BM, Corvin A, Walters JT, Farh K, Holmans PA, Lee P, Bulik-Sullivan B, Collier DA, Huang H, Pers TH, Agartz I, Agerbo E, Albus M, Alexander M, Amin F, Bacanu SA, Begemann M, Belliveau RA Jr, Bene J, Bergen SE, Bevilacqua E, Bigdeli TB, Black DW, Bruggeman R, Buccola NG, Buckner RL, Byerley W, Cahn W, Cai G, Campion D, Cantor RM, Carr VJ, Carrera N, Catts SV, Chambert KD, Chan RC, Chan RY, Chen EY, Cheng W, Cheung EE, Chong SA, Cloninger CR, Cohen D, Cohen N, Cormican P, Craddock N, Crowley JJ, Curtis D, Davidson M, Davis KL, Degenhardt F, Favero JD, Demontis D, Dikeos D, Dinan T, Djurovic S, Donohoe G, Drapeau E, Duan J, Dudbridge F, Durmishi N, Eichhammer P, Eriksson J, Escott-Price V, Essioux L, Fanous AH, Farrell MS, Frank J, Franke L, Freedman R, Freimer NB, Friedl M, Friedman JI, Fromer M, Genovese G, Georgieva L, Giegling I, Giusti-Rodríguez P, Godard S, Goldstein JI, Golimbet V, Gopal S, Gratten J, Haan LD, Hammer C, Hamshere ML, Hansen M, Hansen T, Haroutunian V, Hartmann AM, Henskens FA, Herms S, Hirschhorn JN, Hoffmann P, Hofman A, Hollegaard MV, Hougaard DM, Ikeda M, Joa I, Julià A, Kahn RS, Kalaydjieva L, Karachanak-Yankova S, Karjalainen J, Kavanagh D, Keller MC, Kennedy JL, Khrunin A, Kim Y, Klovins J, Knowles JA, Konte B, Kucinskas V, Kucinskiene ZA, Kuzelova-Ptackova H, Kähler AK, Laurent C, Lee J, Lee SH, Legge SE, Lerer B, Li M, Li T, Liang K, Lieberman J, Limborska S, Loughland CM, Lubinski J, Lönnqvist J, Macek M, Magnusson PK, Maher BS, Maier W, Mallet J, Marsal S, Mattheisen M, Mattingsdal M, McCarley RW, McDonald C, McIntosh AM, Meier S, Meijer CJ, Melegh B, Melle I, Mesholam-Gately RI, Metspalu A, Michie PT, Milani L, Milanova V, Mokrab Y, Morris DW, Mors O, Murphy KC, Murray RM, Myin-Germeys I, Müller-Myhsok B, Nelis M, Nenadic I, Nertney DA, Nestadt G, Nicodemus KK, Nikitina-Zake L, Nisenbaum L, Nordin A, O’Callaghan E, O’Dushlaine C, O’Neill FA, Oh S, Olincy A, Olsen L, Os JV, Psychosis Endophenotypes International Consortium, Pantelis C, Papadimitriou GN, Papiol S, Parkhomenko E, Pato MT, Paunio T, Pejovic-Milovancevic M, Perkins DO, Pietiläinen O, Pimm J, Pocklington AJ, Powell J, Price A, Pulver AE, Purcell SM, Quested D, Rasmussen HB, Reichenberg A, Reimers MA, Richards AL, Roffman JL, Roussos P, Ruderfer DM, Salomaa V, Sanders AR, Schall U, Schubert CR, Schulze TG, Schwab SG, Scolnick EM, Scott RJ, Seidman LJ, Shi J, Sigurdsson E, Silagadze T, Silverman JM, Sim K, Slominsky P, Smoller JW, So H, A Spencer CC, Stahl EA, Stefansson H, Steinberg S, Stogmann E, Straub RE, Strengman E, Strohmaier J, Stroup TS, Subramaniam M, Suvisaari J, Svrakic DM, Szatkiewicz JP, Söderman E, Thirumalai S, Toncheva D, Tosato S, Veijola J, Waddington J, Walsh D, Wang D, Wang Q, Webb BT, Weiser M, Wildenauer DB, Williams NM, Williams S, Witt SH, Wolen AR, Wong EH, Wormley BK, Xi HS, Zai CC, Zheng X, Zimprich F, Wray NR, Stefansson K, Visscher PM, Wellcome Trust Case-Control Consortium, Adolfsson R, Andreassen OA, Blackwood DH, Bramon E, Buxbaum JD, Børglum AD, Cichon S, Darvasi A, Domenici E, Ehrenreich H, Esko T, Gejman PV, Gill M, Gurling H, Hultman CM, Iwata N, Jablensky AV, Jönsson EG, Kendler KS, Kirov G, Knight J, Lencz T, Levinson DF, Li QS, Liu J, Malhotra AK, McCarroll SA, McQuillin A, Moran JL, Mortensen PB, Mowry BJ, Nöthen MM, Ophoff RA, Owen MJ, Palotie A, Pato CN, Petryshen TL, Posthuma D, Rietschel M, Riley BP, Rujescu D, Sham PC, Sklar P, Clair DS, Weinberger DR, Wendland JR, Werge T, Daly MJ, Sullivan PF, O’Donovan MC (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharbanee JM, Stritzke WGK, Jamalludin ME, Wiers RW (2014). Approach-alcohol action tendencies can be inhibited by cognitive load. Psychopharmacology 231, 967–975. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ (1994). Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology 103, 92–102. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG (2009). The Australian Twin Study of Gambling (OZ-GAM): rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics 12, 63–78. [DOI] [PubMed] [Google Scholar]
- Spitznagel EL, Helzer JE (1985). A proposed solution to the base rate problem in the kappa statistic. Archives of General Psychiatry 42, 725–728. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Lahey BB, van Hulle C, Waldman I, Krueger RF, Rathouz PJ (2013). Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. Journal of Abnormal Psychology 122, 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A (1982). Brief manual for the multidimensional personality questionnaire (unpublished manuscript). University of Minnesota: Minneapolis. [Google Scholar]
- Tellegen A, Waller NG (2008). Exploring personality through test construction: development of the Multidimensional Personality Questionnaire. In The SAGE Handbook of Personality Theory and Assessment (ed. Boyle GJ, Matthews G and Saklofske DH), pp. 261–292. Sage Publications Inc: Thousand Oaks, CA, USA. [Google Scholar]
- Whiteside SP, Lynam DR (2001). The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences 30, 669–689. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


