Abstract
Pyrazinamide (PZA) is an important antituberculosis drug. Unlike most antibacterial agents, PZA, despite its remarkable in vivo activity, has no activity against Mycobacterium tuberculosis in vitro except at an acidic pH. M. tuberculosis is uniquely susceptible to PZA, but other mycobacteria as well as nonmycobacteria are intrinsically resistant. The role of acidic pH in PZA action and the basis for the unique PZA susceptibility of M. tuberculosis are unknown. We found that in M. tuberculosis, acidic pH enhanced the intracellular accumulation of pyrazinoic acid (POA), the active derivative of PZA, after conversion of PZA by pyrazinamidase. In contrast, at neutral or alkaline pH, POA was mainly found outside M. tuberculosis cells. PZA-resistant M. tuberculosis complex organisms did not convert PZA into POA. Unlike M. tuberculosis, intrinsically PZA-resistant M. smegmatis converted PZA into POA, but it did not accumulate POA even at an acidic pH, due to a very active POA efflux mechanism. We propose that a deficient POA efflux mechanism underlies the unique susceptibility of M. tuberculosis to PZA and that the natural PZA resistance of M. smegmatis is due to a highly active efflux pump. These findings may have implications with regard to the design of new antimycobacterial drugs.
The antituberculosis drug pyrazinamide (PZA) is an analogue of nicotinamide, which is a vitamin B3 (nicotinic acid; also called niacin) precursor. The discovery of PZA in the late 1940s as a powerful drug against tuberculosis (TB) was based on the serendipitous observation that nicotinamide, curiously, had activity against tubercle bacilli in animal models (19). Subsequent synthesis of analogues of nicotinamide led to the identification of PZA as the most active derivative against Mycobacterium tuberculosis (12). PZA is an important component of the current 6-month short-course TB chemotherapy. This therapy, which consists of isoniazid, rifampin, PZA, and ethambutol and is also called DOTS (for directly observed treatment, short-course), is recommended by the World Health Organization for treatment of every TB patient (30). PZA plays a unique role in shortening the therapy from a period of 9 to 12 months down to 6 months, because PZA kills a population of semidormant tubercle bacilli, residing in an acidic environment (occurring during active inflammation), which are not killed by other TB drugs (6, 14). Unlike other TB drugs, PZA, despite its remarkable activity in vivo (10), has no activity against tubercle bacilli in vitro in normal culture medium (26) except under acidic-pH conditions (e.g., pH 5.5) (11).
M. tuberculosis is uniquely susceptible to PZA, whose MIC for this bacterium is about 16 to 50 μg/ml (11). In contrast, other mycobacteria, and all nonmycobacteria, are completely insensitive to PZA (5). In M. tuberculosis, the susceptibility to PZA correlates with the presence of a single enzyme with nicotinamidase and pyrazinamidase (PZase) activities (7). Strains of M. tuberculosis that are resistant to PZA are often defective in PZase activity (7, 9, 28), and we have recently cloned the M. tuberculosis PZase gene (pncA) (21) and shown that mutation of pncA is a major mechanism of PZA resistance in M. tuberculosis (22). However, the correlation between PZase activity and PZA susceptibility does not exist for nontuberculous mycobacteria, since they have ample PZase activity but are nevertheless intrinsically resistant to PZA (5, 25). Despite the performance of many studies, the mode of action of PZA in M. tuberculosis is unknown. Just as isoniazid requires activation by the M. tuberculosis catalase-peroxidase (32), PZA, as a prodrug, needs to be activated by the bacterial nicotinamidase-PZase into pyrazinoic acid (POA) (7, 21), the active form of the drug, in bacterial cells. Yet, the active derivative POA is not directly used to treat TB patients, because the bactericidal activity of POA, when given orally to mice infected with M. tuberculosis, was found to be not as significant as that of the prodrug PZA, presumably due to poor absorption through the gastrointestinal tract and to significant serum binding (7). However, the reasons why PZA requires an acidic environment to show activity and why M. tuberculosis is uniquely susceptible to PZA were unknown. In this study, we show that the role of acidic pH is to enhance the accumulation of POA and that M. tuberculosis has a defective efflux mechanism for POA whereas the naturally PZA-resistant bacterium M. smegmatis has a much more active POA efflux mechanism.
MATERIALS AND METHODS
Chemicals and radiochemicals.
Carbonyl cyanide m-chlorophenylhydrazone (CCCP), nigericin, valinomycin, reserpine, N,N′-dicyclohexyl carbodiimide (DCCD), sodium salicylate, [14C]benzoic acid, and [14C]salicylic acid were obtained from Sigma Chemical Co. [carbonyl-14C]PZA (specific activity, 52 mCi/mmol) was kindly supplied by the National Institutes of Health AIDS Reagents Program, Rockville, Md. The amount of [14C]PZA used in the various experiments was between 1 and 2 μCi/ml, which is equivalent to 2.4 to 4.8 μg of PZA/ml; these PZA concentrations are much lower than its MIC for M. tuberculosis and are thus unlikely to cause cidal effects. [14C]POA was generated by incubating [14C]PZA with purified M. tuberculosis PZase, overexpressed in Escherichia coli, for about 60 min at 37°C, conditions under which [14C]PZA was converted to [14C]POA completely as judged by thin-layer chromatography (TLC) analysis (see below).
Effect of pH on [14C]PZA conversion and [14C]POA accumulation in M. tuberculosis.
Late-log-phase M. tuberculosis H37Ra cultures (2 to 3 week old), grown in 7H9 liquid medium supplemented with albumin-dextrose-catalase, were centrifuged, and the cells were resuspended to a density of about 5 × 109/ml in 7H9 medium adjusted to various pH values. [14C]PZA was added to a concentration of 2.5 μCi/ml. Following incubation at 37°C for about 16 h, both the supernatant fluids and bacterial lysates, prepared by sonication of concentrated bacterial cells washed with phosphate-buffered saline, were analyzed by TLC followed by autoradiography (see below). To test the effect of pH on POA accumulation in the M. tuberculosis cells, [14C]POA was added to the bacterial suspensions, at various pH values, at a concentration of 1 μCi/ml. At various times, 50-μl portions were removed, filtered through 0.45-μm-pore-size nitrocellulose membranes, and washed with 0.1 M potassium phosphate buffer (pH 7.0) containing 0.1 M LiCl. In this as well as other experiments, the radioactivity associated with cells was measured by scintillation counting. The intracellular concentration of POA was calculated by assuming that 1 mg of dry cells is equivalent to 3 μl of internal water (31).
TLC.
For TLC, 2-μl portions of radioactive supernatants or lysates were spotted onto a 0.25-mm-thick silica G gel 60 plate with an aluminum backing (Whatman). The TLC plate was developed in 1-butanol–10% ammonia (5:1). The plate was then air dried and exposed to X-ray film for autoradiography.
Determination of intracellular pH.
M. tuberculosis H37Ra cells were grown in 7H9 liquid medium (pH 6.6) to late log phase. The culture was centrifuged, and the cells were resuspended in Sauton’s medium (pH 5.0) at a density of 5 × 109/ml. POA was added to 0.4 or 4 mM (about 500 μg/ml). Salicylate, used as a positive control, was also added to 4 mM. Each sample was tested in triplicate. [14C]benzoic acid or [14C]salicylic acid (Sigma Chemical Co.), as a pH probe, was added to a final concentration of 1 μCi/ml at time zero, 50-μl portions were removed at various times and filtered through 0.45-μm-pore-size nitrocellulose membranes, and the membranes were washed twice with 2 ml of 7H9 medium. Scintillation cocktail (3 ml) was then added to each filter in a counting vial, and the radioactivity was measured with a scintillation counter. The internal cell volume was measured by using 3H2O and [14C]taurine, and the internal pH was calculated according to the method of Rottenberg (18).
Isolation of membrane and cytoplasmic fractions from M. tuberculosis.
A late-log-phase culture of M. tuberculosis H37Ra (50 ml) was harvested, and the cells were washed twice with 40 mM potassium phosphate buffer (pH 6.5) containing 1 mM EDTA and then resuspended in 5 ml of 40 mM potassium phosphate buffer (pH 6.5) containing 0.3 mM phenylmethylsulfonyl fluoride, 1,000 U of DNase I, 0.5 mg of RNase A, and 2 mM magnesium chloride. The cell suspension (5 ml) was sonicated for 10 to 15 min on ice and then centrifuged at 13,000 rpm for 15 min to remove large cellular debris and unbroken cells. The supernatant was then spun at 32,000 rpm for 1 h to separate the membrane and cytosolic fractions. The supernatant fraction (cytosolic fraction) was saved. The pellet (membrane fraction) was washed with 40 mM potassium phosphate buffer (pH 6.5) containing 0.3 mM phenylmethylsulfonyl fluoride and 1 mM EDTA and then dissolved in 40 mM potassium phosphate buffer (pH 6.5) containing 1% Triton X-100. Both the supernatant and pellet fractions were tested for PZase activity by incubating them with [14C]PZA (1 to 2 μCi) in a volume of 30 μl for 7 h, and the degree of conversion of [14C]PZA to [14C]POA was monitored by TLC as described above.
PZA accumulation and conversion in PZA-susceptible and -resistant M. tuberculosis complex organisms.
Two- to 3-week-old M. tuberculosis H37Ra and M. bovis BCG cultures, grown in Sauton’s medium, were harvested and then washed with Sauton’s medium, and the cell pellets were resuspended in Sauton’s medium (pH 6.6) at 5 × 109 cells/ml. [14C]PZA was added to these cell suspensions to a concentration of 1 μCi/ml, and the cell mixtures were incubated at 37°C. At different time points, 50-μl portions were removed and washed with Sauton’s medium by filtration on 0.45-μm-pore-size nitrocellulose filters by the use of a vacuum pump. The amount of radioactivity associated with the bacterial cells was determined by scintillation counting.
Effect of reserpine and valinomycin on accumulation of POA in M. smegmatis and M. tuberculosis.
[14C]PZA was added to a concentrated bacterial suspension (5 × 109 cells/ml) in 7H9 liquid medium at pH 6.6 to a final concentration of 2 μCi/ml. A sublethal concentration of reserpine (20 μM) was added to the M. smegmatis cells after they had been incubated with [14C]PZA for 1 min, allowing a substantial amount of PZA to be taken up by the cells and converted to POA. At various times after the addition of reserpine, 50-μl portions were removed and spotted onto 0.45-μm-pore-size nitrocellulose membranes under a vacuum. Because washing the M. smegmatis cells with buffer tends to remove [14C]POA associated with the cells, the supernatant was removed by vacuum filtration without washing. The membrane area where the cell suspension was spotted was cut out, and the radioactivity was determined. The effect of valinomycin (1 μM; sublethal concentration) was determined in the same manner, using 10 mM potassium. The effect of reserpine and valinomycin on [14C]POA accumulation in M. tuberculosis was examined in a similar manner with the following modifications. Reserpine (50 μM) and valinomycin (1 μM) were added 2 h after addition of [14C]PZA to allow sufficient conversion of PZA to POA. Portions (50 μl) of suspension were filtered, and the radioactive cells on the membrane were washed twice with 2 ml of 0.1 M potassium phosphate buffer (pH 7.0) containing 0.1 M LiCl.
RESULTS
Acidic pH enhances accumulation of POA in M. tuberculosis.
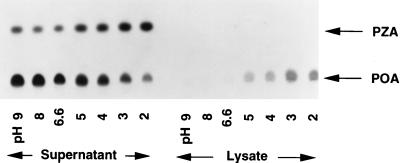
We examined the conversion of PZA to POA and the accumulation of POA in M. tuberculosis H37Ra by incubating bacterial cells with [14C]PZA for 16 h under various pH conditions. Culture supernatants and bacterial lysates were analyzed by TLC followed by autoradiography (Fig. 1). [14C]PZA was converted to POA, which was not further converted into other components (Fig. 1). At neutral or alkaline pHs, there was little POA associated with the bacterial cells and POA was found mainly in the supernatant. In contrast, at acidic pHs, there was much more [14C]POA associated with the bacterial cells (Fig. 1), although the conversion of [14C]PZA was somewhat reduced, judging from the increased amount of unaltered [14C]PZA in the supernatant. This was apparently due to acid inhibition of the PZase (data not shown).
FIG. 1.
Effect of pH on [14C]PZA conversion in M. tuberculosis. [14C]PZA was incubated with M. tuberculosis H37Ra cells for 16 h under various pH conditions. [14C]PZA conversion and accumulation of [14C]POA by H37Ra in both supernatant and cell lysate were analyzed by TLC.
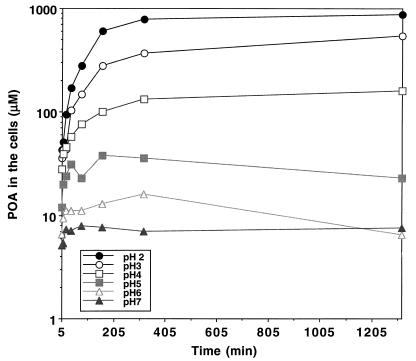
We then tested whether the increased accumulation of POA in the M. tuberculosis cells under acidic-pH conditions was due to passive distribution of POA between the bacterial cells and the medium. Using [14C]POA, we found that the effect of acidic pH was indeed to enhance the accumulation of POA (a weak acid with a pKa of 2.9 [3]) in M. tuberculosis cells and that the POA accumulation was more pronounced at lower pH values (Fig. 2). This observation is consistent with the expectation that weak acids are accumulated intracellularly under acidic-pH conditions because an equilibrium is reached when the concentration of the uncharged form or conjugate acid, which permeates through the membrane by passive diffusion, becomes equal on both sides of the membrane (16, 18). It is noteworthy that there was a major difference in between POA accumulation at pH 6.6 and that at pH 2 to 5 (Fig. 1 and 2). At pH 6.6, conditions under which PZA has no antituberculosis activity (26), the POA concentration in the M. tuberculosis cells was similar to the external concentration; by contrast, the POA concentrations in the cells at acidic pH values, conditions under which PZA shows activity, were several-hundred-fold higher than the external concentration, as expected from the passive distribution theory (Fig. 1 and 2).
FIG. 2.
Effect of pH on [14C]POA accumulation in M. tuberculosis. The accumulation of [14C]POA by strain H37Ra at various pH values was monitored at different time points (5, 10, 20, 40, 80, 160, 320, and 1,320 min) after [14C]POA addition at 19 μM (1 μCi/ml). The concentrations of POA in the cells were determined as described in Materials and Methods and are expressed on a log scale.
The internal pH.
We measured the internal pH of M. tuberculosis H37Ra cells at an external pH of 5.0, using [14C]benzoic acid or [14C]salicylic acid as a probe. The internal pH of H37Ra cells was close to 7 and did not decrease upon exposure to an acidic pH of 5.0 for 5 to 6 h. Addition of salicylate (4 mM) lowered the internal pH quickly, as expected. POA at 4 mM (500 μg/ml), however, did not significantly lower the internal pH of the M. tuberculosis cells, probably because at pH 5.0, according to the Henderson-Hasselbach equation, only 0.8% of the POA is uncharged and therefore POA crosses the membrane too slowly. When M. tuberculosis H37Ra cells were incubated with PZA at a concentration of 50 μg/ml for 2 h at pH 5.0, the drug did not have any significant effect on the internal pH. These data suggest that the internal pH of living M. tuberculosis cells is actively maintained at close to 7.
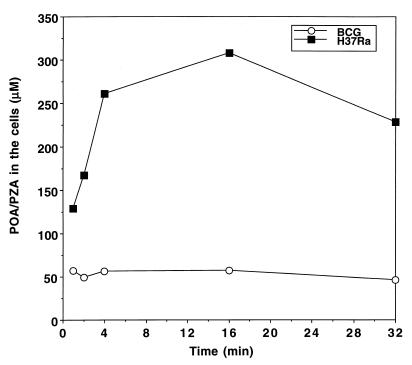
PZA-resistant M. tuberculosis complex organisms do not accumulate PZA.
Two strains, M. bovis BCG (which is naturally resistant to PZA due to a mutation at nucleotide position 169 of the pncA gene [21]) and a PZA-resistant M. tuberculosis clinical isolate (with a nucleotide deletion at position 391 of the pncA gene) (22), were incubated with [14C]PZA. Neither strain was able to convert [14C]PZA into POA, as demonstrated by TLC analysis (data not shown). Importantly, the intracellular concentration of PZA remained similar to the external concentration (Fig. 3), indicating that PZA had not accumulated. In contrast, PZA-susceptible M. tuberculosis H37Ra, with a functional PZase, was able to convert PZA and retained a significant amount of radioactivity (mostly POA in the cells, as determined by TLC) in the cells even at an external pH of 6.6 at early time points (Fig. 3).
FIG. 3.
Comparison of PZA accumulation in PZA-susceptible and -resistant M. tuberculosis complex organisms. [14C]PZA was added to 5 × 109 M. tuberculosis H37Ra or M. bovis BCG cells at a concentration of 1 μCi/ml at pH 6.6. At various time points after PZA addition, portions of bacterial cells were removed and washed by filtration, using phosphate buffer (pH 6.6). The results of a representative experiment are shown here.
However, when BCG was transformed with a functional M. tuberculosis or M. avium pncA gene, the recombinant BCG strains expressed PZase activity, converted PZA to POA, accumulated POA in bacterial cells at an acidic pH, and became susceptible to PZA (data not shown). Furthermore, both BCG and the PZA-resistant M. tuberculosis isolate accumulated [14C]POA from the external medium at acidic pHs (data not shown). This result is consistent with the finding that both BCG and PZA-resistant M. tuberculosis strains are still susceptible to POA (7).
Localization of PZase in M. tuberculosis.
We tested the PZase activity in the isolated membrane fraction, the cytosolic fraction, and the culture supernatant of M. tuberculosis H37Ra. The conventional PZase assay (29) was very insensitive and failed to detect PZase activity in these fractions. However, with the more sensitive method using radioactive [14C]PZA, PZase activity was mainly found in the cytosolic fraction (data not shown). Similarly, most PZase activity was located in the cytosolic fraction in M. smegmatis (data not shown). No PZase activity was detected in the supernatant fluids of either M. tuberculosis or M. smegmatis cultures. These data suggest that most PZA conversion occurs in the cytoplasm.
Differences in PZA transport and conversion for PZA-susceptible M. tuberculosis and naturally resistant mycobacteria or other bacteria.
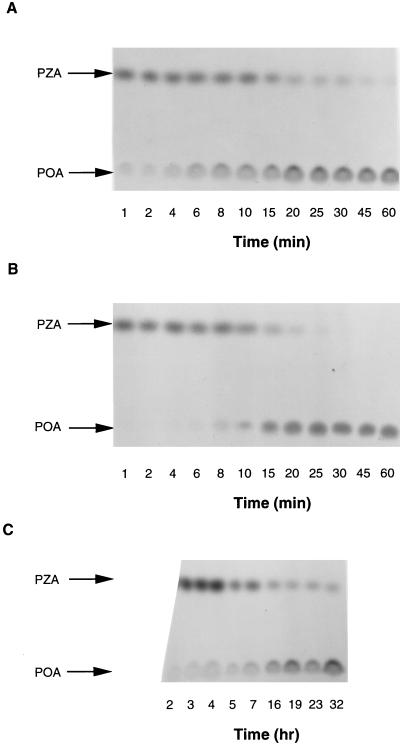
The basis for the natural resistance of nontuberculous mycobacteria to some antituberculosis drugs has often been suggested to be the impermeability of their cell walls (4). We compared conversion of [14C]PZA by susceptible M. tuberculosis with that by the fast-growing mycobacteria M. smegmatis, and M. vaccae, as well as a slow-growing mycobacterium, M. avium, at pH 6.6. Both M. smegmatis and M. vaccae are highly resistant to PZA (MICs, >2,000 μg/ml), whereas M. avium has an intermediate level of susceptibility to PZA (MIC, 500 μg/ml) (23). These nontuberculous mycobacteria did convert PZA to POA, indicating that their natural PZA resistance is not due to a defective PZase. Surprisingly, M. smegmatis and M. vaccae behaved very differently from the susceptible species M. tuberculosis. Conversion of [14C]PZA to POA occurred very rapidly in M. smegmatis, probably due to a higher PZase activity, as was recently shown by another laboratory (2). However, POA was released into the culture supernatant as soon as [14C]PZA was added to the culture (Fig. 4A). The same rapid release of POA was also found with M. vaccae (data not shown). We examined whether the rapid appearance of POA in the supernatant of M. smegmatis cultures was due to a secreted PZase. The culture supernatant did not contain any PZase activity (data not shown). E. coli, which is also naturally resistant to PZA (MIC, >2,000 μg/ml) (23), behaved similarly to M. smegmatis and M. vaccae, and all of the [14C]PZA was converted to and released from the cells as [14C]POA within 45 min (Fig. 4B). In contrast, M. tuberculosis converted [14C]PZA slowly, and POA began to appear in the supernatant only after 60 min; even after 32 h of incubation there was still unaltered [14C]PZA in the supernatant (Fig. 4C).
FIG. 4.
Comparison of [14C]PZA conversion and [14C]POA release among M. smegmatis, E. coli, and M. tuberculosis. Bacterial cell suspensions (about 5 × 109 cells/ml) were prepared from early-stationary-phase cultures and resuspended in an appropriate medium at pH 6.6. [14C]PZA (1 μCi/ml) was added to the bacterial suspensions, and the radioactive cell mixtures were incubated at 37°C for various periods of time up to 1 h for M. smegmatis (A) and E. coli (B) and up to 32 h for M. tuberculosis H37Ra (C). The extent of [14C]PZA conversion to [14C]POA in the supernatant fluids was monitored by TLC.
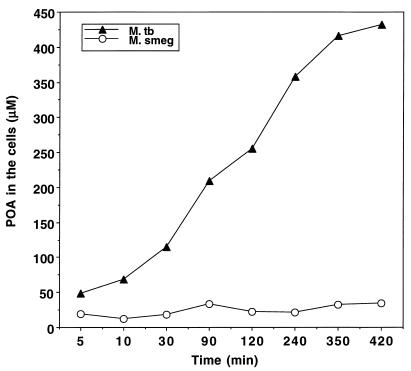
In similar [14C]PZA conversion experiments as in Fig. 1, the nonsusceptible species M. smegmatis and M. vaccae did not accumulate POA in the cells, even under acidic-pH conditions (data not shown). Consistent with this observation, when [14C]POA was added to cells at pH 5.5, little POA was found to be associated with the M. smegmatis cells, whereas increasing amounts of externally added [14C]POA entered into M. tuberculosis over time (Fig. 5). In the case of M. avium, both the rate of [14C]PZA conversion and the amount of [14C]POA associated with the M. avium cells under acidic pH conditions were intermediate between the values for M. tuberculosis and M. smegmatis (data not shown). This finding is consistent with the intermediate level of susceptibility of M. avium to PZA (23).
FIG. 5.
Differences in POA accumulation between M. tuberculosis (M. tb) and M. smegmatis (M. smeg). [14C]POA (2.5 μCi/ml) was added to bacterial suspensions with a density of 5 × 109 cells/ml at an acidic pH of 5.5, and the cells were incubated at 37°C for various lengths of time up to 7 h. Portions of the bacterial cell suspensions (50 μl) were removed and washed with Sauton’s medium by filtration under a vacuum. The internal POA concentrations in the bacterial cells were determined as described in Materials and Methods.
POA efflux in M. smegmatis and M. tuberculosis.
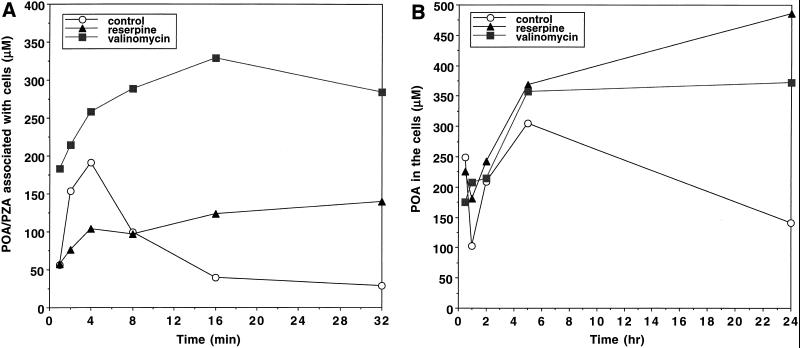
POA is expected to be accumulated passively under acidic-pH conditions, but it did not accumulate in M. smegmatis (Fig. 5); we suspected that this was due to an active efflux mechanism pumping POA out of the cells. To prove this, we tested various inhibitors of energy metabolism, including CCCP (8, 27), nigericin (17), valinomycin (17), and DCCD (1), as well as reserpine, a plant alkaloid that inhibits the Bacillus subtilis multidrug efflux pump (15), by incubating M. smegmatis cells with [14C]PZA at pH 6.6. Interestingly, reserpine at a sublethal concentration (20 μM) allowed significant accumulation of POA in M. smegmatis cells (Fig. 6A), although it inhibited PZA conversion to some degree because of its inhibition of PZase activity (data not shown). M. smegmatis cells extruded POA very rapidly (Fig. 6A). Since at the internal pH of 7 only 0.01% of the POA is expected to be in the membrane-permeable uncharged form, this rapid efflux strongly suggests that M. smegmatis has an active efflux mechanism that pumps out POA, a conclusion also supported by the reserpine inhibition data. Among the energy inhibitors, valinomycin at a sublethal concentration (0.85 to 1 μM) caused significant retention of [14C]POA in M. smegmatis cells (Fig. 6A).
FIG. 6.
Effect of reserpine and valinomycin on accumulation of POA in M. smegmatis (A) and M. tuberculosis (B). The experiments were performed by adding [14C]PZA to cells at pH 6.6 as described in Materials and Methods. Shown are data from a representative experiment.
To determine whether there is an efflux mechanism for POA in M. tuberculosis, we tested the effects of reserpine and valinomycin on the accumulation of [14C]POA in M. tuberculosis H37Rv by adding [14C]PZA at pH 6.6. Reserpine caused POA accumulation in H37Rv cells (Fig. 6B), suggesting that M. tuberculosis also has an active POA efflux mechanism. Valinomycin also caused significant retention of POA in M. tuberculosis cells (Fig. 6B). However, the rate of POA extrusion (about 0.3 pmol/mg/min [Fig. 6B]) was more than 2 orders of magnitude lower than that found in M. smegmatis (about 70 pmol/mg/min [Fig. 6A]).
DISCUSSION
Despite the importance of PZA in shortening the length of tuberculosis therapy, its mode of action is unknown. An unusual aspect of PZA is the requirement of an acidic pH for activity against M. tuberculosis (11). However, an acidic pH alone does not directly activate PZA (11), nor does it induce the M. tuberculosis PZase and hence lead to more efficient PZA conversion to POA (23). We have shown in this study that an acidic pH does not cause increased accumulation of PZA itself (Fig. 1) and that it results in decreased PZA conversion to some extent through inhibition of PZase activity. Instead, we found that an acidic pH enhanced the accumulation of POA (the active derivative of PZA) in M. tuberculosis (Fig. 1 and 2). This acid-facilitated accumulation of POA and the degree of POA accumulation in the cells correlate well with the previous finding that the MICs of PZA for M. tuberculosis decrease at lower pH values (20).
The increased accumulation of POA under acidic-pH conditions could be due to active transport of POA into the cells. However, a similar accumulation of POA took place when the compound was generated inside the cytoplasm (Fig. 1). We believe, therefore, that this is due to the passive equilibration of POA across the membrane. Any lipophilic, weak acids spontaneously diffuse through the membrane in their protonated forms and thus come to equilibrium when the concentrations of protonated forms become equal on both sides of the cytoplasmic membrane (16, 18). Since the cytosol in living M. tuberculosis cells is maintained at a pH close to 7 (see Results), most of POA in the cytoplasm will be in the dissociated form, and the total concentration of POA here will be more than 1,000-fold higher than the concentration of protonated POA. In contrast, when the external pH is acidic—say, pH 2.9—the total concentration of POA in the external medium will be only twice that of the protonated form. At equilibrium, the concentrations of protonated POA on both sides will be equal, with the result being that the total POA concentration in the cytoplasm is several orders of magnitude higher than that in the medium at an acidic pH, precisely as observed in Fig. 2. When the pH of the medium is close to 7, there is little difference in the pH across the membrane, and there should be no passive accumulation of POA, again as observed in this study (Fig. 2). This hypothesis predicts that a decrease of external pH by 1 unit should increase POA accumulation by a factor of about 10. In Fig. 2, however, the observed increase was only three- to fourfold. One possible reason for this discrepancy is the slow, active efflux of POA by M. tuberculosis cells (see below).
This concept of passive equilibration of POA explains the observation that PZA is active against M. tuberculosis only at an acidic pH, not at neutral and alkaline pH values. When M. tuberculosis cells were incubated with PZA at neutral pH, radioactive POA was found inside the cells at early time points (Fig. 3 and 6B). This was expected, because POA was generated by the hydrolysis of PZA, a lipophilic, uncharged compound that should diffuse rapidly into the cells. Indeed, a comparison of the H37Ra data in Fig. 3 and 5 shows that PZA diffuses into cells much more rapidly than does POA. The intracellular POA thus generated then diffuses spontaneously and also by a slow, active efflux (see below) out into the medium, because there is no passive retention of POA under conditions of neutral external pH and because the volume of external medium is many orders of magnitude larger than the intracellular volume.
The finding that PZA-resistant TB complex organisms without PZase activity failed to accumulate or fix [14C]PZA was somewhat unexpected. Even when closely spaced time points were used, no accumulation of PZA could be demonstrated inside the PZase-defective M. bovis BCG cells. The most plausible explanations for this observation are (i) that PZA, as a neutral amide, is not accumulated in the cell regardless of the external pH and (ii) that lipophilic, uncharged PZA passively diffuses out of the cells during the filtration washing step. In contrast, in PZase-positive, PZA-susceptible M. tuberculosis, PZA will be converted to POA as it enters the cells and POA will be trapped inside the cells due to its negative charge. Thus, the PZA uptake experiment probably does not reflect the actual PZA uptake per se but rather represents POA accumulation in the cells. In fact, there was no difference between BCG and H37Ra with regard to PZA accumulation when the bacterial cells were spun through silicone oil (data not shown). Our observation that PZA-resistant, pncA-defective M. tuberculosis complex strains did not accumulate PZA is in keeping with the previous finding that E. coli mutants defective in pncA (encoding nicotinamidase) also failed to accumulate nicotinamide (13).
The data presented in Fig. 4 show the kinetic differences in PZA uptake, conversion to POA, and potential POA efflux among various bacteria. We believe that the differences reflect differences in PZase enzyme activity, POA efflux, and, to a much lesser extent, cell wall permeability, because PZA is a neutral amide and should passively diffuse into different cells relatively easily.
In this study, we found that the naturally PZA-resistant M. smegmatis has a highly active efflux mechanism for POA which can be inhibited by reserpine and valinomycin (Fig. 6A). Although nigericin and CCCP did not show inhibition, this could be due to the inadequate entry of these agents through the rather impermeable cell wall or to their active efflux. The valinomycin effect thus suggests that the pump is energized by the proton motive force or one of its components. M. tuberculosis also has a POA efflux mechanism, as demonstrated by increased accumulation of POA at neutral pH in the presence of reserpine and valinomycin (Fig. 6B). The M. tuberculosis efflux mechanism is much weaker than that of M. smegmatis, as evidenced by the orders-of-magnitude-slower kinetics of POA extrusion (compare Fig. 6B and A). Thus, in M. tuberculosis, at an acidic external pH, the rate of passive transmembrane equilibrium of POA apparently overwhelms that of active efflux, resulting in a huge accumulation of POA in the cells. The POA efflux mechanism in M. smegmatis appears to be different from the recently identified M. smegmatis MDR pump LfrA (24), since insertion of the lfrA gene into M. tuberculosis H37Ra did not cause enhanced efflux of POA or increased resistance to PZA or POA (unpublished data). Studies designed to identify the POA efflux mechanisms in M. smegmatis and M. tuberculosis are under way.
While both susceptible M. tuberculosis and other nonsusceptible mycobacteria have PZases to convert PZA to POA, the specificity of PZA for M. tuberculosis appears to be conferred at the stage of POA efflux, which is much weaker in M. tuberculosis than in the nonsusceptible M. smegmatis. It is noteworthy that the two types of PZA resistance, the acquired PZA resistance found in susceptible M. tuberculosis and the intrinsic PZA resistance found in nontuberculous mycobacteria, are caused by very different mechanisms. Acquired PZA resistance in susceptible M. tuberculosis is caused by mutations in the pncA gene which render the organisms unable to convert the prodrug PZA to bactericidal POA. In contrast, the intrinsic PZA resistance in M. smegmatis, and probably in many other nontuberculous mycobacteria, is due to a much more active POA efflux mechanism which does not allow accumulation of POA in the cells.
ACKNOWLEDGMENTS
We thank Peter Maloney for helpful discussions; Diane Griffin, Barbara Laughon, and Denis Mitchison for encouragement; and the National Institutes of Health (NIH) AIDS Reagents Program for [14C]pyrazinamide.
This work was supported by research grants from the American Lung Association, the Potts Memorial Foundation, and NIH (RO1AI40584) to Y.Z.
REFERENCES
- 1.Azzi A, Casey R P, Nalecz M J. The effect of N,N′-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984;768:209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff H I M, Mizrahi V. Purification, gene cloning, targeted knockout, overexpression, and biochemical characterization of the major pyrazinamidase from Mycobacterium smegmatis. J Bacteriol. 1998;180:5809–5814. doi: 10.1128/jb.180.22.5809-5814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budavari S. The Merck index. 11th ed. Rahway, N.J: Merck & Co., Inc.; 1989. [Google Scholar]
- 4.David H. Basis for lack of drug susceptibility of atypical mycobacteria. Rev Infect Dis. 1981;3:878–884. doi: 10.1093/clinids/3.5.878. [DOI] [PubMed] [Google Scholar]
- 5.Good R C, Silcox V A, Kilburn J O, Plikaytis B D. Identification and drug susceptibility test results for Mycobacterium spp. Clin Microbiol Newslett. 1985;7:133–136. [Google Scholar]
- 6.Heifets L, Lindholm-Levy P. Pyrazinamide sterilizing activity in vitro against semi-dormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis. 1992;145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 7.Konno K, Feldman F M, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95:461–469. doi: 10.1164/arrd.1967.95.3.461. [DOI] [PubMed] [Google Scholar]
- 8.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClatchy J K, Tsang A Y, Cernich M S. Use of pyrazinamidase activity in Mycobacterium tuberculosis as a rapid method for determination of pyrazinamide susceptibility. Antimicrob Agents Chemother. 1981;20:556–557. doi: 10.1128/aac.20.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCune R M, Tompsett R, McDermott W. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott W, Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environment in vitro. Am Rev Tuberc. 1954;70:748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie D, Malone L, Kushner S, Oleson J J, Subbarow Y. The effect of nicotinic acid amide on experimental tuberculosis of white mice. J Lab Clin Med. 1948;33:1249–1253. [PubMed] [Google Scholar]
- 13.McLaren J, Ngo D T C, Olivera M. Pyridine nucleotide metabolism in Escherichia coli. J Biol Chem. 1973;248:5144–5159. [PubMed] [Google Scholar]
- 14.Mitchison D A. The action of antituberculosis drugs in short course chemotherapy. Tubercle. 1985;66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 15.Neyfakh A A, Bidnenko V E, Chen L B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikaido H, Thanassi D G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob Agents Chemother. 1993;37:1393–1399. doi: 10.1128/aac.37.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos S, Schuldiner S, Kaback H R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci USA. 1976;73:1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rottenberg H. The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 19.Ryan F. The forgotten plague. Boston, Mass: Little, Brown and Company; 1993. pp. 345–347. [Google Scholar]
- 20.Salfinger M, Heifets L B. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob Agents Chemother. 1988;32:1002–1004. doi: 10.1128/aac.32.7.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 22.Scorpio A, Lindholm-Levy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, Zhang Y. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1997;41:540–543. doi: 10.1128/aac.41.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun A, Scorpio A, Zhang Y. The pncA gene from naturally pyrazinamide-resistant Mycobacterium avium encodes pyrazinamidase and confers pyrazinamide susceptibility to resistant M. tuberculosis complex organisms. Microbiology. 1997;143:3367–3373. doi: 10.1099/00221287-143-10-3367. [DOI] [PubMed] [Google Scholar]
- 24.Takiff H E, Cimino M, Musso M C, Weisbrod T, Martinez R, Delgado M B, Salazar L, Bloom B R, Jacobs W R. Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc Natl Acad Sci USA. 1996;93:362–366. doi: 10.1073/pnas.93.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarnok I, Rohrscheidt E. Biochemical background of some enzymatic tests used for the differentiation of mycobacteria. Tubercle. 1976;57:145–150. doi: 10.1016/0041-3879(76)90052-0. [DOI] [PubMed] [Google Scholar]
- 26.Tarshis M S, Weed W A. Lack of significant in vitro sensitivity of Mycobacterium tuberculosis to pyrazinamide on three different solid media. Am Rev Tuberc. 1953;67:391–395. doi: 10.1164/art.1953.67.3.391. [DOI] [PubMed] [Google Scholar]
- 27.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trivedi S S, Desai S G. Pyrazinamidase activity of Mycobacterium tuberculosis—a test of sensitivity to pyrazinamide. Tubercle. 1987;68:221–224. doi: 10.1016/0041-3879(87)90058-4. [DOI] [PubMed] [Google Scholar]
- 29.Wayne L G. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974;109:147–151. doi: 10.1164/arrd.1974.109.1.147. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO report on the tuberculosis epidemic: stop TB at the source. Geneva, Switzerland: Tuberculosis Programme, World Health Organization; 1995. [Google Scholar]
- 31.Youatt J, Tham S H. Radioactive content of Mycobacterium tuberculosis after exposure to 14C-isoniazid. Am Rev Respir Dis. 1969;100:77–78. doi: 10.1164/arrd.1969.100.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase and isoniazid resistance in Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]