Abstract
The objective of this single-center cohort study was to characterize the frequency, clinical characteristics, and molecular epidemiology of pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after vaccination. Between May 15, 2021, and January 1, 2022, 171 children experienced SARS-CoV-2 infection postvaccination, 146 (86%) following the Omicron variant predominance. Outcomes were generally mild and comparable before and after Omicron predominance.
Keywords: COVID-19, SARS-CoV-2, breakthrough, vaccine, pediatric
Abbreviations: COVID-19, Coronavirus disease 2019; Ct, Cycle threshold; GISAID, Global Initiative on Sharing Avian Influenza Data; I-CARE, Illinois Comprehensive Automated Immunization Registry Exchange; mRNA, Messenger RNA; RT-PCR, Reverse-transcription polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VOC, Variant of concern; WGS, Whole-genome sequencing
The rollout of highly effective vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reduced the incidence of coronavirus disease 2019 (COVID-19) and the burden of the illness on health care systems.1, 2, 3 However, the emergence of variants of concern (VOCs), particularly those with vaccine-evading mutations, such as Omicron,4 has led to a greater incidence of COVID-19, especially among individuals who have completed the primary vaccine series.5 Although the durability of SARS-CoV-2 vaccines has not been characterized in all pediatric age groups, anti-spike antibodies in adolescents wane significantly by 6 months postvaccination,6 and booster doses increase protection.7 Although COVID-19 in vaccinated adults is relatively mild,8 little is known about the frequency and clinical characteristics of COVID-19 in children who have completed the primary SARS-CoV-2 vaccine series. As of summer 2022, among Chicago residents, nearly 50% of children aged 5-11 years and 75% of those aged 12-17 years old had completed the primary series of SARS-CoV-2 vaccines.9 Children are increasingly returning to congregate settings, such as schools and childcare facilities, with variable enforcement of COVID-19 mitigation measures. With the risk of COVID-19 potentially increasing, a better understanding of pediatric COVID-19 after vaccination is needed.
In the spring of 2021, the US experienced a surge of COVID-19 during which the alpha and gamma VOCs predominated. These were largely supplanted by the Delta VOC, which predominated through summer and fall 2021. In December 2021, the Omicron VOC rapidly became the predominant strain, leading to a steep increase in COVID-19 cases and hospitalizations nationwide.10 , 11 In children, the effectiveness of messenger RNA (mRNA) vaccines in preventing COVID-19 was notably diminished for the Omicron VOC.12 , 13 Consistent with these data, the incidence of pediatric COVID-19 surged substantially in both vaccinated and unvaccinated children during this period.
The objective of this single-center cohort study was to characterize the frequency and clinical characteristics of pediatric COVID-19 cases among those previously vaccinated against SARS-CoV-2. Additionally, we delineated pre-Omicron and Omicron periods using community molecular epidemiology and whole-genome sequencing (WGS) to examine the prevalence of VOCs in vaccinated children and the association between VOCs with clinical outcomes. These data are essential for informing ongoing public health strategy for vaccination and nonpharmacologic mitigation interventions for children.
Methods
This retrospective cohort study was conducted at the Ann & Robert H. Lurie Children's Hospital of Chicago. The hospital's Institutional Review Board approved this study (IRB 2020-3792). Vaccine records for the state of Illinois, including SARS-CoV-2 vaccines, were available through the Illinois Comprehensive Automated Immunization Registry Exchange (I-CARE) and autopopulated into the hospital's electronic medical record. We considered a person to be fully vaccinated as of 14 days after receipt of the second dose of Pfizer-BioNTech (Pfizer) or Moderna mRNA vaccine (Moderna), or 14 days after receipt of a single dose of the J&J/Janssen viral vector vaccine (Janssen Vaccines).
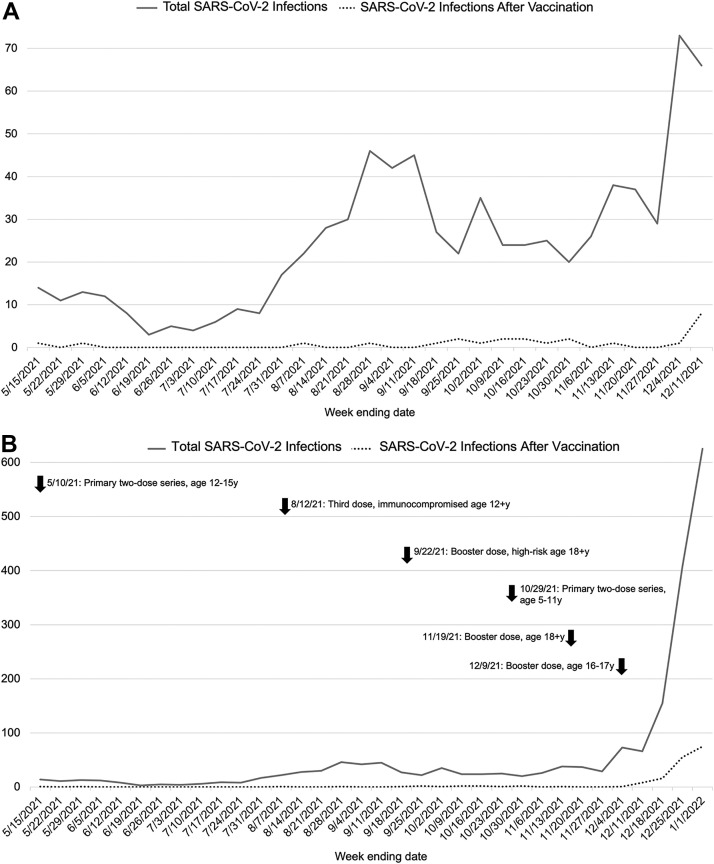
All patients aged 0-20 years with a positive reverse-transcription polymerase chain reaction (RT-PCR) test for SARS-CoV-2 after being fully vaccinated, irrespective of COVID-19 symptoms, were identified as having COVID-19 after vaccination via an electronic medical record query. In cases where multiple infections were suspected, only the first infection was included. Dates of Pfizer-BioNTech vaccine Emergency Use Authorization by the US Food and Drug Administration for individuals aged 0-20 years are listed in Figure 1 (available at www.jpeds.com).
Figure 1.
Incidence of total SARS-CoV-2 infection and SARS-CoV-2 infection after vaccination at Lurie Children's during the A, pre-Omicron study period (May 15, 2021, to December 11, 2021, prior to the substantial surge in SARS-CoV-2 infection activity) and B, the complete study period (May 15, 2021, to January 1, 2022). The solid line represents the total incidence of pediatric SARS-CoV-2 infection, and the dashed line represents the total incidence of SARS-CoV-2 infection after vaccination. In B, arrows indicate date of Food and Drug Administration Emergency Use Authorization of Pfizer-BioNTech SARS-CoV-2 mRNA vaccine in children and young adults aged 0-20 years. Not shown is the initial authorization of SARS-CoV-2 vaccine in adolescents aged 16+ years on December 11, 2020.
The first COVID-19 infection in a fully vaccinated child at our center was diagnosed on May 15, 2021, which we defined as the first day of the study period; our study period continued through January 1, 2022. The COVID-19 incidence at our center during this period was included as well. We stratified the study period into pre-Omicron and Omicron periods. We defined the beginning of the Omicron period as the date after which 50% of our medical center surveillance samples undergoing WGS were identified as the Omicron VOC, which occurred during the week of December 6-12, 2021.
SARS-CoV-2 WGS
After identifying our patient cohort, all residual diagnostic specimens with sufficient volume for RNA extraction and a sufficiently low quantitative RT-PCR cycle threshold (Ct) value (ie, sufficiently high viral load) were subject to SARS-CoV-2 WGS at the Center for Pathogen Genomics and Microbial Evolution at Northwestern University. A small subset of samples were also sequenced at the Regional Innovative Public Health Laboratory at the Chicago Department of Public Health and Rush University Medical Center through their COVID-19 surveillance program. Laboratory methods for viral RNA extraction, complementary DNA synthesis, viral genome amplification, sequencing library preparation, Illumina sequencing, genome assembly, and phylogenetic analysis are described in the Appendix (available at www.jpeds.com). Consensus genome sequences were deposited in the Global Initiative on Sharing Avian Influenza Data (GISAID) public database (Table I; available at www.jpeds.com).
Study Data and Definitions
Vaccination dates and type, as well as positive SARS-CoV-2 PCR test date, were extracted directly from the electronic medical record. Manual chart review was completed to extract demographic data (ie, age, sex, race, and ethnicity) and pertinent clinical characteristics for each patient infection. The clinical data included comorbid conditions (specifically diabetes, respiratory disease, cardiac disease, immunocompromising conditions, and obesity, defined as body mass index ≥95th percentile), presence of a viral coinfection by a multiplex PCR test (BioFire Respiratory 2.1 Panel; BioFire Diagnostics), symptom data (fever, rhinorrhea, congestion, loss of taste/smell, cough, shortness of breath, sore throat, gastrointestinal symptoms, headache), hospitalization for COVID-19, and receipt of therapies for COVID-19 (eg, monoclonal antibodies, remdesivir, corticosteroids, tocilizumab, baricitinib, supplemental oxygen). Of note, corticosteroids were recorded as COVID-19 treatment if given specifically as treatment for COVID-19 at the provider's discretion; for example, if corticosteroids were given solely for croup symptoms in a child who was SARS-CoV-2–positive, that was not considered COVID-19 treatment. Each patient was assigned a World Health Organization COVID-19 Severity Score.14
Statistical Analyses
Demographic, clinical, and outcome categorical variables for the entire cohort, as well as for patient subgroups infected during the pre-Omicron and Omicron periods, were summarized and reported as frequency and percentage or as median and IQR. The frequencies of these variables were compared between subgroups using the χ2 test or Fisher exact test; continuous variables were compared using the Wilcoxon rank-sum test. A 2-sided P value < .05 was considered statistically significant. Statistical analyses were performed using Stata/IC 16.0 (StataCorp).
Results
Figure 1 illustrates changes in overall COVID-19 incidence at our center during the study period of May 15, 2021, to January 1, 2022. In late December 2021, there was a >4-fold increase in incidence, jumping from 221 total cases between December 5 and December 18 to 1032 cases between December 19 and January 1, corresponding to the emergence and predominance of Omicron. During the study period, 171 patients in our center experienced COVID-19 after SARS-CoV-2 vaccination (Table II ). There were 25 (14.6%) such infections during the 30-week pre-Omicron period and 146 (85.4%) during the 3-week Omicron period. The proportion of COVID-19 cases in vaccinated children increased when Omicron became predominant, accounting for 12.3% of all infections during our Omicron period, compared with 3.3% of all cases in our pre-Omicron period, during which time the Delta VOC was largely predominant. Of the samples with measured SARS-CoV-2 RT-PCR Ct values, 10 of 21 samples (47.6%) in the pre-Omicron period and 95 of 123 (77.2%) samples in the Omicron period had a Ct <30 and were eligible for WGS. Residual samples from 98 patients (57%) were available and successfully sequenced, and all were identified as a VOC, including 93 (94.9%) Omicron, 4 (4.1%) Delta, and 1 (1%) was Gamma. The patient population was significantly younger during the Omicron period (median, 14 [IQR, 12-16] years vs 16 [IQR, 13-17] years; P = .002).
Table II.
Demographics, clinical characteristics, and outcomes of 171 children with COVID-19 following vaccination
| Variables | Total infections following vaccination, n (%) (N = 171) | Pre-Omicron, n (%) (N = 25) | Omicron, n (%) (N = 146) | P value |
|---|---|---|---|---|
| Age, y, median (IQR) | 14 (12-16) | 16 (13-17) | 14 (12-16) | .0022 |
| Days between vaccination∗ and infection, median (IQR) | 160 (76-186) | 116 (95-163) | 167.5 (40-187) | .0382 |
| Sex (male), n (%) | 90 (52.6) | 15 (60) | 75 (51.4) | .517 |
| Race (Black), n (%) | 31 (18.1) | 0 (0) | 31 (21.2) | .071 |
| Ethnicity (Hispanic), n (%) | 56 (32.8) | 10 (40) | 46 (31.5) | .676 |
| Comorbid condition for COVID-19 complications (any), n (%) | 89 (52.1) | 11 (44) | 78 (53.4) | .397 |
| Cardiac | 10 (5.9) | 0 (0) | 10 (6.9) | .361 |
| Respiratory | 38 (22.2) | 3 (12) | 35 (24) | .296 |
| Immunodeficiency | 18 (10.5) | 2 (8) | 16 (10.9) | 1.00 |
| Diabetes | 4 (2.3) | 0 (0) | 4 (2.7) | 1.00 |
| Obesity | 35 (20.5) | 6 (24) | 29 (19.9) | .163 |
| COVID vaccine type (Pfizer-BioNTech), n (%) | 167 (97.7) | 24 (96) | 143 (98) | .472 |
| Received booster dose, n (%) | 5 (2.9) | 0 (0) | 5 (3.4) | 1.00 |
| Received third dose because of immunodeficiency, n (%) | 1 (0.6) | 1 (4) | 0 (0) | .146 |
| Viral coinfection†, n (%) | 1 (0.6) | 0 (0) | 1 (0.7) | 1.00 |
| Symptoms of COVID-19 (any), n (%) | 151 (88.3) | 17 (68) | 134 (91.8) | .003 |
| Fever | 50 (29.2) | 7 (28) | 43 (29.5) | 1.00 |
| Upper respiratory infection symptoms | 60 (35.1) | 9 (36) | 51 (34.9) | 1.00 |
| Loss of taste and/or smell | 3 (1.8) | 0 (0) | 3 (2.1) | 1.00 |
| Cough | 54 (31.6) | 5 (20) | 49 (33.6) | .245 |
| Shortness of breath | 10 (5.9) | 0 (0) | 10 (6.9) | .361 |
| Sore throat | 56 (32.8) | 5 (20) | 51 (34.9) | .171 |
| Gastrointestinal symptoms | 20 (11.7) | 0 (0) | 20 (13.7) | .047 |
| Headache | 30 (17.5) | 4 (16) | 26 (17.8) | 1.00 |
| Other | 45 (26.3) | 3 (12) | 42 (28.8) | .089 |
| Hospitalized for COVID-19‡, n (%) | 4 (2.3) | 0 (0) | 4 (2.7) | 1.00 |
| COVID-19 pharmacologic treatment, n (%) | 2 (1.2) | 1 (4) | 1 (0.7) | .272 |
| Monoclonal antibodies§, n (%) | 1 (0.6) | 1 (4) | 0 (0) | .146 |
| Remdesivir | 1 (0.6) | 0 (0) | 1 (0.7) | 1.00 |
| Corticosteroids | 1 (0.6) | 0 (0) | 1 (0.7) | 1.00 |
| COVID-19 severity score, n (%) | ||||
| Mild (1-2) | 165 (96.5) | 25 (100) | 140 (95.9) | .594 |
| Moderate (3-5) | 6 (3.5) | 0 (0) | 6 (4.1) |
Bold P values denote statistical significance.
Day 1 starts 14 days after completing primary vaccine series.
The single viral coinfection was rhinovirus/enterovirus.
No patients required respiratory support or intensive care.
Specific monoclonal antibody unavailable; patient received at outside location.
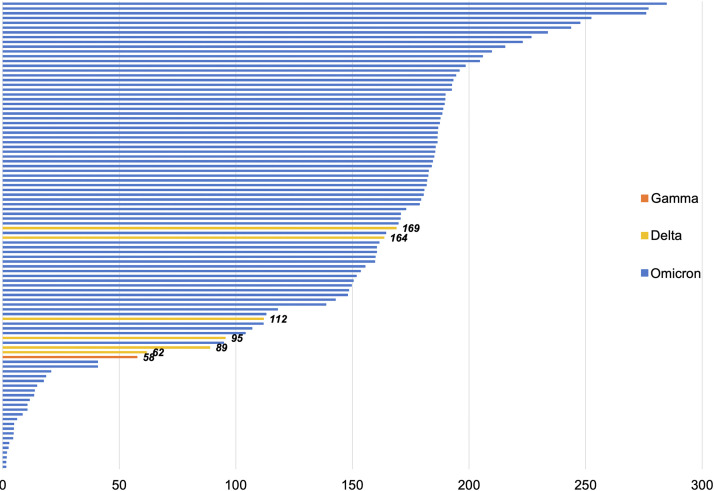
All demographic, clinical, and outcome data for the entire cohort, and during the pre-Omicron (May 15, 2021, to December 11, 2021) and Omicron (December 12, 2021, to January 1, 2022) periods, are listed in Table II. Among the 171 patients with COVID-19 after vaccination, 167 (97.7%) had received the Pfizer-BioNTech mRNA vaccine. Six patients had received a third dose of vaccine. Five patients received a booster dose, a median 200 (IQR, 198-204) days after receipt of second dose, and 1 patient received a third dose because of underlying immunodeficiency at 106 days after the second dose. The median time elapsed between the date of full vaccination and date of a positive SARS-CoV-2 test was greater during the Omicron period (168 [IQR, 40-187] days vs 116 [IQR, 95-163] days; P = .039), and Figure 2 illustrates these data for successfully sequenced samples. Among the entire cohort, 89 patients (52.1%) had a comorbid condition, most commonly a respiratory condition (n = 38; 22.2%) or obesity (n = 35; 20.5%). There were no significant differences in the frequency of comorbid conditions between the time periods. During the Omicron period, patients with COVID-19 after vaccination were significantly more likely to experience symptoms of COVID-19 (91.8% vs 68%; P = .003), particularly gastrointestinal symptoms (13.7% vs 0%; P = .047). No children with multisystem inflammatory syndrome in children after vaccination were identified in our cohort.
Figure 2.
Days between full vaccination and positive SARS-CoV-2 test for 98 patients with a successfully sequenced SARS-CoV-2 nasopharyngeal sample. Samples are organized from longest to shortest period between being fully vaccinated (ie, day 0 is 14 days after receiving dose 2) and infection date. Among the 98 samples, 93 (94.9%) were Omicron (blue), 4 (4.1%) were Delta (yellow), and 1 (1%) was Gamma (red). For the 5 non-Omicron samples, the number of days between vaccination and a positive SARS-CoV-2 test is labeled beside the line.
In total, 9 patients with COVID-19 after vaccination were hospitalized (all during the Omicron period), but only 4 (2.3%) were hospitalized because of their COVID-19 infection; the remainder were asymptomatic or mild infections in children who were hospitalized for a different reason (eg, psychiatric care, treatment for an alternative medical problem). Each of these cases was in children with a complex medical history (Table III; available at www.jpeds.com). None of these patients experienced a concomitant infection with a different respiratory virus. None of the hospitalized patients required supplemental oxygen. One of the hospitalized patients received corticosteroids and remdesivir as treatment. One other patient reportedly received monoclonal antibodies as early directed therapy for mild infection (specific monoclonal antibody received at outside infusion center by verbal self-report; unable to be confirmed); this patient did not require hospitalization. Two of the 4 samples were successfully sequenced from the hospitalized participants and identified as the BA.1 lineage of the Omicron VOC.
Discussion
At the Ann & Robert H. Lurie Children's Hospital of Chicago, COVID-19 incidence increased sharply in mid to late December 2021. The incidence of COVID-19 in children who completed the primary vaccine series also increased during this time; 12.3% of all infections between December 12, 2021, and January 1, 2022, were in previously vaccinated children. Among all COVID-19 infections occurring after vaccination in this time period in this study, nearly 95% were caused by the Omicron VOC, which has been shown to have increased transmissibility and vaccine evasion.4 , 15 COVID-19 after vaccination generally occurred at least 5 months after completion of the primary vaccination series, supporting the currently recommended booster schedule in children aged 12 years and older that recommends a single booster 5 months after the primary series in all children aged 12 and older; a third dose 1 month after the initial 2-dose series, a first booster 3 months later, and then a second booster 4 months later is currently recommended for immunocompromised children aged 12 years and older.16 Although patients with COVID-19 after vaccination during the Omicron period were younger than those in the pre-Omicron period, this is biased by the fact that children younger than 12 years were not eligible for the vaccine until late in the pre-Omicron period. Along those lines, during the Omicron period, children aged 12 years and older might have concomitantly experienced waning immunity several months after becoming eligible for vaccine,6 complicating the ability to discern waning immunity from Omicron vaccine escape, although both likely contributed to the rise in COVID-19 in this age group during the Omicron period.
We found that COVID-19 after vaccination was generally mild and only rarely required hospitalization or treatment. The severity of COVID-19 caused by the Omicron VOC in children after vaccination, characterized by proportion of infections requiring hospitalization, treatment, respiratory support, or intensive care, was low and did not differ between the pre-Omicron and Omicron periods. This supports recent data showing that although current vaccine regimens are less effective at preventing COVID-19 caused by Omicron, they were largely protective against hospitalization and severe disease in children.10 , 11 Hospitalization events observed in vaccinated patients experiencing COVID-19 were driven by the medical complexity of patients who would benefit from close monitoring by their care teams, not by the severity of their infection. Of the 4 hospitalization events for COVID-19, 3 were only 1-day stays for observation and did not require any intervention. During the Omicron period, COVID-19 after vaccination was more frequently associated with symptoms, with a greater prevalence of gastrointestinal symptoms during this period. Differences in symptomatology may represent strain-specific differences, but this requires further investigation.
This study has several limitations. As a single-center study, our data might not be externally generalizable. Based on the availability and Ct values of our samples, we were able to collect WGS data for only 57% of our samples. Furthermore, a greater proportion of samples during the Omicron period (77.2% vs 47.6% in the pre-Omicron period) had a Ct <30 and were eligible for sequencing. The reasons for this are unknown but could be explained by reported immune evasion by Omicron,4 leading to higher nasopharyngeal viral loads. Furthermore, the proportion of infections with symptoms was higher during the Omicron period; a previous study in children reported higher nasopharyngeal viral loads in children with COVID-19 symptoms.17 To account for this, we also used time as a proxy for variant trends, as was done in prior studies,10 , 11 although we acknowledge that this is an imperfect delineator. Additionally, the pre-Omicron and Omicron periods were distinguishable not only by their most prevalent VOC, but also by the ages of those eligible for vaccination against SARS-CoV-2. This could have impacted our data by skewing the age group younger or potentially by misrepresenting outcomes as Omicron-related as opposed to age-related. As an additional confounder, there was an increase in the number of children vaccinated against SARS-CoV-2 over time, which might have played a role in the increasing frequency of COVID-19 among vaccinated children in the later time periods. Furthermore, although we used an electronic state registry to identify vaccine history in our patients, and we consider this more reliable than report of vaccine history by the historian and documentation in the medical record, it is possible that some vaccinated children were not captured by this registry. Finally, the patients presenting at our hospital may have changed over time given the general change in testing patterns between the pre-Omicron and Omicron periods; during the latter, there was greater access to at-home SARS-CoV-2 tests, which could have impacted the number and medical complexity of patients who would have been tested previously at our hospital.
In summary, cases of COVID-19 after completion of a primary vaccine series against SARS-CoV-2 are increasingly likely to occur because of waning immunity and emerging VOCs with vaccine escape. Nevertheless, our data strongly suggest that vaccines are an effective and vital tool for reducing severe outcomes as a vast majority of COVID-19 cases after vaccination in our study were mild. As new VOCs arise, investigations of their vaccine-evading characteristics will be needed to monitor and quickly identify when additional nonpharmacologic risk mitigation tools should be used. This information will be particularly helpful to inform public health strategy in pediatric populations, as the safety of schools and childcare centers remains a priority. Continued investigation of COVID-19 in the vaccinated population also will be important to help guide booster shot priorities for children.
Acknowledgments
We thank the staff at the Clinical Microbiology and Special Infectious Diseases laboratories at Lurie Children's Hospital for their assistance in saving and processing specimens for WGS and the Regional Innovative Public Health Laboratory at the Chicago Department of Public Health and Rush University Medical Center for their sequencing of some samples included in this study. WGS data collection was supported in part through the computational resources and staff contributions provided for the Quest high-performance computing facility at Northwestern University, which is jointly supported by the Office of the Provost, Office for Research, and Northwestern University Information Technology.
Footnotes
This work was supported by funding from the Walder Foundation's Chicago Coronavirus Assessment Network (Chicago CAN) Initiative. Additional funding for whole-genome sequencing and variant identification was provided by National Institutes of Health Grants R21 AI163912 (to J.H.) and U19 AI135964 (to E.O.). The authors declare no conflicts of interest.
Supplementary Data
Appendix
Table I.
GISAID accession numbers, lineages, and VOC classification of 98 SARS-CoV-2 sequences included in the study
| ID | GISAID accession | Pango lineage | NextClade clade | Variant classification |
|---|---|---|---|---|
| LCH-0984 | EPI_ISL_9513520 | BA.1.1 | 21K | Omicron |
| LCH-0985 | EPI_ISL_9513521 | BA.1 | 21K | Omicron |
| LCH-0986 | EPI_ISL_9513522 | BA.1 | 21K | Omicron |
| LCH-0987 | EPI_ISL_9513523 | BA.1 | 21K | Omicron |
| LCH-0988 | EPI_ISL_9513524 | BA.1 | 21K | Omicron |
| LCH-0990 | EPI_ISL_9513525 | BA.1 | 21K | Omicron |
| LCH-0991 | EPI_ISL_9513526 | BA.1.1 | 21K | Omicron |
| LCH-0992 | EPI_ISL_9513527 | BA.1 | 21K | Omicron |
| LCH-0993 | EPI_ISL_9513528 | BA.1 | 21K | Omicron |
| LCH-0994 | EPI_ISL_9513529 | BA.1 | 21K | Omicron |
| LCH-0995 | EPI_ISL_9513530 | BA.1 | 21K | Omicron |
| LCH-0997 | EPI_ISL_9513531 | BA.1 | 21K | Omicron |
| LCH-0998 | EPI_ISL_9513532 | BA.1 | 21K | Omicron |
| LCH-0999 | EPI_ISL_9513533 | BA.1 | 21K | Omicron |
| LCH-1000 | EPI_ISL_9513534 | BA.1 | 21K | Omicron |
| LCH-1001 | EPI_ISL_9303283 | BA.1 | 21K | Omicron |
| LCH-1003 | EPI_ISL_9303285 | BA.1.1 | 21K | Omicron |
| LCH-1004 | EPI_ISL_9303286 | BA.1 | 21K | Omicron |
| LCH-1005 | EPI_ISL_9303287 | BA.1.1 | 21K | Omicron |
| LCH-1006 | EPI_ISL_9303288 | BA.1 | 21K | Omicron |
| LCH-1007 | EPI_ISL_9303289 | BA.1 | 21K | Omicron |
| LCH-1008 | EPI_ISL_9303290 | BA.1 | 21K | Omicron |
| LCH-1009 | EPI_ISL_9513535 | BA.1 | 21K | Omicron |
| LCH-1012 | EPI_ISL_9513536 | BA.1 | 21K | Omicron |
| LCH-1013 | EPI_ISL_9513537 | BA.1.1 | 21K | Omicron |
| LCH-1014 | EPI_ISL_9513538 | BA.1 | 21K | Omicron |
| LCH-1015 | EPI_ISL_9513539 | BA.1.1 | 21K | Omicron |
| LCH-1016 | EPI_ISL_9513540 | BA.1.1 | 21K | Omicron |
| LCH-1017 | EPI_ISL_9513541 | BA.1 | 21K | Omicron |
| LCH-1018 | EPI_ISL_9513542 | BA.1 | 21K | Omicron |
| LCH-1020 | EPI_ISL_9513543 | BA.1 | 21K | Omicron |
| LCH-1021 | EPI_ISL_9513544 | BA.1 | 21K | Omicron |
| LCH-1022 | EPI_ISL_9513545 | BA.1 | 21K | Omicron |
| LCH-1023 | EPI_ISL_9513546 | BA.1 | 21K | Omicron |
| LCH-1024 | EPI_ISL_9513547 | BA.1 | 21K | Omicron |
| LCH-1025 | EPI_ISL_9513548 | BA.1 | 21K | Omicron |
| LCH-1029 | EPI_ISL_9513549 | BA.1 | 21K | Omicron |
| LCH-1030 | EPI_ISL_9513550 | BA.1 | 21K | Omicron |
| LCH-1032 | EPI_ISL_9513551 | BA.1.1 | 21K | Omicron |
| LCH-1033 | EPI_ISL_9303291 | BA.1.1 | 21K | Omicron |
| LCH-1034 | EPI_ISL_9303292 | BA.1 | 21K | Omicron |
| LCH-1035 | EPI_ISL_9303293 | BA.1 | 21K | Omicron |
| LCH-1036 | EPI_ISL_9303294 | BA.1 | 21K | Omicron |
| LCH-1037 | EPI_ISL_9303295 | BA.1.1 | 21K | Omicron |
| LCH-1038 | EPI_ISL_9303296 | BA.1 | 21K | Omicron |
| LCH-1039 | EPI_ISL_9303297 | BA.1 | 21K | Omicron |
| LCH-1040 | EPI_ISL_9303298 | BA.1 | 21K | Omicron |
| LCH-1041 | EPI_ISL_9303299 | BA.1 | 21K | Omicron |
| LCH-1042 | EPI_ISL_9303300 | BA.1 | 21K | Omicron |
| LCH-1043 | EPI_ISL_9303301 | BA.1 | 21K | Omicron |
| LCH-1044 | EPI_ISL_9303302 | BA.1.1 | 21K | Omicron |
| LCH-1045 | EPI_ISL_9303303 | BA.1 | 21K | Omicron |
| LCH-1046 | EPI_ISL_9303304 | BA.1 | 21K | Omicron |
| LCH-1050 | EPI_ISL_9513552 | BA.1 | 21K | Omicron |
| LCH-1051 | EPI_ISL_9513553 | BA.1.1 | 21K | Omicron |
| LCH-1053 | EPI_ISL_9513554 | BA.1.1 | 21K | Omicron |
| LCH-1055 | EPI_ISL_9513555 | BA.1 | 21K | Omicron |
| LCH-1057 | EPI_ISL_9513556 | BA.1 | 21K | Omicron |
| LCH-1058 | EPI_ISL_9513557 | BA.1 | 21K | Omicron |
| LCH-1059 | EPI_ISL_9513558 | BA.1.1 | 21K | Omicron |
| LCH-1060 | EPI_ISL_9513559 | BA.1 | 21K | Omicron |
| LCH-1062 | EPI_ISL_9513560 | BA.1 | 21K | Omicron |
| LCH-1064 | EPI_ISL_9513561 | BA.1 | 21K | Omicron |
| LCH-1067 | EPI_ISL_9513562 | BA.1 | 21K | Omicron |
| LCH-1068 | EPI_ISL_9513563 | BA.1.1 | 21K | Omicron |
| LCH-1069 | EPI_ISL_9513564 | BA.1 | 21K | Omicron |
| LCH-1070 | EPI_ISL_9513565 | BA.1 | 21K | Omicron |
| LCH-1071 | EPI_ISL_9513566 | BA.1.1 | 21K | Omicron |
| LCH-1072 | EPI_ISL_9513567 | BA.1 | 21K | Omicron |
| LCH-1075 | EPI_ISL_9513569 | BA.1 | 21K | Omicron |
| LCH-1076 | EPI_ISL_9513570 | BA.1 | 21K | Omicron |
| LCH-1078 | EPI_ISL_9513571 | BA.1 | 21K | Omicron |
| LCH-1079 | EPI_ISL_9513572 | BA.1 | 21K | Omicron |
| LCH-1080 | EPI_ISL_9513573 | BA.1 | 21K | Omicron |
| LCH-1081 | EPI_ISL_9513574 | BA.1 | 21K | Omicron |
| LCH-1082 | EPI_ISL_9513575 | BA.1 | 21K | Omicron |
| LCH-1084 | EPI_ISL_9513576 | BA.1 | 21K | Omicron |
| LCH-1087 | EPI_ISL_9303305 | BA.1 | 21K | Omicron |
| LCH-1088 | EPI_ISL_9303306 | BA.1 | 21K | Omicron |
| LCH-1138 | EPI_ISL_9912753 | P.1 | 20J | Gamma |
| LCH-1139 | EPI_ISL_9912754 | AY.26 | 21I | Delta |
| LCH-1141 | EPI_ISL_9912757 | AY.103 | 21J | Delta |
| LCH-1142 | EPI_ISL_9912758 | AY.103 | 21J | Delta |
| LCH-1145 | EPI_ISL_9912759 | AY.25 | 21J | Delta |
| LCH-1146 | EPI_ISL_9912760 | AY.100 | 21J | Delta |
| LCH-1147 | EPI_ISL_9912761 | AY.44 | 21J | Delta |
| LCH-1148 | EPI_ISL_9912762 | BA.1 | 21K | Omicron |
| LCH-1149 | EPI_ISL_9912764 | BA.1 | 21K | Omicron |
| LCH-1150 | EPI_ISL_9912806 | BA.1.1 | 21K | Omicron |
| LCH-1151 | EPI_ISL_9912808 | BA.1.1 | 21K | Omicron |
| LCH-1152 | EPI_ISL_9912811 | BA.1 | 21K | Omicron |
| LCH-1153 | EPI_ISL_9912813 | BA.1.1 | 21K | Omicron |
| LCH-1154 | EPI_ISL_9912815 | BA.1 | 21K | Omicron |
| LCH-1155 | EPI_ISL_9912817 | BA.1 | 21K | Omicron |
| LCH-1156 | EPI_ISL_9912818 | BA.1 | 21K | Omicron |
| LCH-1158 | EPI_ISL_9912819 | BA.1.1 | 21K | Omicron |
| LCH-1159 | EPI_ISL_9912820 | BA.1 | 21K | Omicron |
| 20 157 | EPI_ISL_9648695 | BA.1 | 21K | Omicron |
GISAID, Global Initiative on Sharing Avian Influenza Data.
Table III.
Demographic and clinical characteristics of patients hospitalized because of COVID-19 following vaccination
| Age, y | Sex | Patient medical history | Hospital course | Hospital length of stay | COVID-19 therapies |
|---|---|---|---|---|---|
| 18 | Female | Premature ventricular contractions | Symptoms: fever, cough, dyspnea, chest pain, vomiting, diarrhea Supportive care: admitted for observation and intravenous fluids because of dehydration; no supplemental oxygen requirement |
24 h | No specific COVID-19 therapies |
| 18 | Female | Heart transplantation for congenital heart disease complicated by heterotaxy and polysplenia Patient has history of COVID-19 complicated by MIS-C in late 2020 |
Symptoms: fatigue, dyspnea, cough, sore throat Supportive care: no supplemental oxygen requirement |
4 d | Remdesivir × 4 doses∗ Dexamethasone × 2 doses∗ |
| 20 | Female | Connective tissue disorder requiring methotrexate | Symptoms: congestion, cough, diarrhea Supportive care: admitted for observation and intravenous fluids because of dehydration; no supplemental oxygen requirement |
48 h | No specific COVID-19 therapies |
| 17 | Male | Inherited endocrinopathy complicated by adrenal insufficiency | Symptoms: fever, cough Supportive care: in Emergency Department, developed hypotension and treated with stress-dose corticosteroids; no supplemental oxygen requirement |
48 h | No specific COVID-19 therapies |
MIS-C, multisystem inflammatory syndrome in children. None of these patients experienced a coinfection caused by another respiratory virus.
Despite the lack of oxygen requirement, the patient was hospitalized for observation and received targeted COVID-19 treatment at the provider's discretion because of immunocompromising conditions and history of COVID-19 complicated by MIS-C.
References
- 1.Klein N.P., Stockwell M.S., Demarco M., Gaglani M., Kharbanda A.B., Irving S.A., et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years—VISION Network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. doi: 10.15585/mmwr.mm7109e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenforde M.W., Self W.H., Gaglani M., Ginde A.A., Douin D.J., Talbot H.K., et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459–465. doi: 10.15585/mmwr.mm7112e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson M.G., Natarajan K., Irving S.A., Rowley E.A., Griggs E.P., Gaglani M., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glatman-Freedman A., Bromberg M., Hershkovitz Y., Sefty H., Kaufman Z., Rita Dichtiar R., et al. Effectiveness of BNT162b2 vaccine booster against SARS-CoV-2 infection and breakthrough complications. Israel. Emerg Infect Dis. 2022;28:948–956. doi: 10.3201/eid2805.220141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns M.D., Boribong B.P., Bartsch Y.C., Loiselle M., St Denis K.J., Sheehan M.L., et al. Durability and cross-reactivity of SARS-CoV-2 mRNA vaccine in adolescent children. Vaccines (Basel) 2022;10:492. doi: 10.3390/vaccines10040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming-Dutra K.E., Britton A., Shang N., Derado G., Link-Gelles R., Accorsi E.K., et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA. 2022;327:2210–2219. doi: 10.1001/jama.2022.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juthani P.V., Gupta A., Borges K.A., Price C.C., Lee A.I., Won C.H., et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21:1485–1486. doi: 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chicago Department of Public Health COVID-19 dashboard. https://www.chicago.gov/city/en/sites/covid-19/home/covid-dashboard.html
- 10.Iuliano A.D., Brunkard J.M., Boehmer T.K., Peterson E., Adjei S., Binder A.M., et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks K.J., Whitaker M., Anglin O., Milucky J., Patel K., Pham H., et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271–278. doi: 10.15585/mmwr.mm7107e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowlkes A.L., Yoon S.K., Lutrick K., Gwynn L., Burns J., Grant L., et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 years and adolescents aged 12-15 Years—PROTECT cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:422–428. doi: 10.15585/mmwr.mm7111e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price A.M., Olson S.M., Newhams M.M., Halasa N.B., Boom J.A., Sahni L.C., et al. BNT162b2 Protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araf Y., Akter F., Tang Y.D., Fatemi R., Parvez Md S.A., Zheng C., et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94:1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Use of COVID-19 vaccines in the US: interim clinical considerations. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html [PubMed]
- 17.Kociolek L.K., Muller W.J., Yee R., Dien Bard J., Brown C.A., Revell P.A., et al. Comparison of upper respiratory viral load distributions in asymptomatic and symptomatic children diagnosed with SARS-CoV-2 infection in pediatric hospital testing programs. J Clin Microbiol. 2020;59:e02593-20. doi: 10.1128/JCM.02593-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.