Abstract
We examined the longitudinal association between physical activity (PA) and the incidence of self-reported diagnosed depression in adults in southern Brazil during the COVID-19 pandemic. Data from the PAMPA (Prospective Study About Mental and Physical Health) cohort was used. Data collection for baseline was carried out on June–July 2020, with two follow-up assessments taking place six months apart. An online, self-reported questionnaire assessed depression and PA. Depression was assessed by asking participants whether they were ever diagnosed with depression. We included 441 participants (women: 75.9%; mean age [SD]: 38.0 [13.5]) in southern Brazil. Over the follow-up, 21.8% (95% confidence interval [CI]: 18.1%–25.9%) were diagnosed with depression. Insufficiently active (<150 min per week of physical activity) (Incidence rate [IR]: 61.9; 95%CI: 39.5–102.4; p = 0.047) and active (≥150 min per week of physical activity) (IR: 50.4; 95%CI: 31.9–84.0; p = 0.015) participants had reduced IR of depression per 1000 persons-year at risk compared to inactive ones (0 min per week of physical activity) (IR: 99.9; 95%CI: 79.7–126.8). In the adjusted analyses, participants in the insufficient active (hazard ratio [HR]: 0.58; 95%CI: 0.34–0.98) and active (HR: 0.53; 95%CI: 0.31–0.93) group had a lower risk of developing depression than the inactive group. PA both at and out of home reduced the risk of incident depression (HR: 0.49; 95%CI: 0.25–0.98) compared to no physical activity. Endurance (HR: 0.52; 95%CI: 0.28–0.97) and endurance plus strengthening (HR: 0.40; 95%CI: 0.17–0.95) PA reduced the risk of incident depression compared to none. Being physically active during pandemic, regardless of the amount of PA practiced, reduced the incidence of depression in adults in southern Brazil.
Keywords: Physical activity, Depression, COVID-19, Cohort studies
1. Introduction
Mental disorders are the main contributing factors to increased morbidity and disability globally (Prince et al., 2007; WHO, 2017). Depressive disorders are conditions associated with sadness, physical and mental fatigue, reduced function of cognitive abilities, and other symptoms (WHO, 2017). It is estimated that at least 15% of the world population may develop depression throughout the life course (Strakowski & Nelson, 2015). Between 2007 and 2017, the number of years lived with disability, an indicator of diseases morbidity, attributable to depression, increased by 14.3%, leading this condition to the third main cause of morbidity worldwide (James et al., 2018).
Although depression was already considered a public health emergency before 2020 (WHO, 2017), the COVID-19 pandemic raised the prevalence of people with moderate-to-severe depressive symptoms (Salari et al., 2020). In order to reduce the virus spread, social distancing strategies were adopted, including the closure of public spaces and fitness centers (Nussbaumer-Streit et al., 2020). Although these strategies were crucial to controlling virus transmission, some collateral effects were observed, including reduced physical activity level (Caputo & Reichert, 2020) and increased depressive symptoms (Salari et al., 2020). For example, an 6.6-fold increase in the prevalence of depressive symptoms (Feter et al., 2021) and a 17% decrease in the proportion of adults reaching the WHO recommendation of physical activity (i.e., 150 min of moderate-to-vigorous physical activity per week) was observed among adults in southern Brazil (Caputo et al., 2021).
As an alternative to remaining physically active, some people started to do home-based exercises, including muscle strengthen activities, running, and jumping rope (Caputo et al., 2021; Hammami, Harrabi, Mohr, & Krustrup, 2020). Although the protective effects of physical activity on depression are well established (Schuch et al., 2018), the association between physical activity in different places (e.g., at home) and the incidence of depression during the COVID-19 are scantly explored. Considering the non-pharmacological and low-cost features of physical activity and the sharp increase in depressive symptoms, especially in Brazil (Feter et al., 2021), this study aimed to examine the longitudinal association between physical activity and the incidence of depression in adults in southern Brazil during the COVID-19 pandemic. We also investigated how different physical activity parameters, including volume, type, place, and trajectory, were associated with the incidence of depression. We hypothesized that adults who reached the physical activity recommendation (i.e., 150 min per week) at the first three months of the COVID-19 pandemic may had reduced incidence of depression one year later (Schuch et al., 2018). Lack of professional guidance and equipment observed in home-based physical activity (Bland, Bigaran, Campbell, Trevaskis, & Zopf, 2020; Crochemore-Silva et al., 2020) may impair individuals to practice at proper intensity and volume, limiting the protective effects from these activities on mental health. Also, endurance (Lewis et al., 2010; Pedersen, 2019) and strength activities (Aidar et al., 2014; Moraes et al., 2020), either isolated or combined, were hypothesized to reduce the incidence of depression as both can trigger pathways associated with improved mental health including release of neurogenic factors and reduced oxidative stress and inflammation (Pedersen, 2019).
2. Methods
2.1. Study design
The present study used data from the first three waves of the PAMPA (Prospective Study About Mental and Physical Health) Cohort, a state-level, ambispective longitudinal study with adults from the Rio Grande do Sul, the southernmost state of Brazil. A full description of the PAMPA cohort can be found elsewhere (Feter et al., 2020). The study protocol was approved by the institutional research ethics board of the School of Physical Education of the Federal University of Pelotas, Brazil (protocol: 4.093.170).
2.2. Recruitment phase
Adults living in the Rio Grande do Sul State were recruited via personal networks, social and local media (Feter et al., 2020; Leite, Feter, Doring, Cassurriaga, & Caputo, 2020). Data were collected using an online-based, self-reported questionnaire. Wave 1 took place from June 22nd, 2020 to July 23rd, 2020 while the other two waves were performed six and 12 months later. Data collection was performed exclusively online. At the time of wave 1, the social distancing restrictions in the Rio Grande do Sul State due to the COVID-19 pandemic reduced by up to 75% the maximal capacity of social clubs, gyms, theaters, religious temples, commercial activities, and malls to prevent gatherings. However, such restrictions were eased throughout 2021 as the vaccine campaign started in February 2021, followed by decreased local virus transmission.
2.3. Sample
A full description of sample size calculation can be found elsewhere (Feter et al., 2020). Briefly, we used the prevalence of depression in the Rio Grande do Sul State in 2013 (13.2%; 95% confidence interval [IC]: 11.8%–15.0%]) to calculate the required sample size. Based on the State's population according to the latest census (10,693,929 inhabitants)(IBGE, 2010), a 95%CI with 1.8 percentage points of margins of error, and a possible lost-to-follow-up of up to 30%, the required sample size at baseline was set at 1767 participants. Further, the Rio Grande do Sul State was divided for the state's government social distancing plan into seven macroregions of health, as follows (names are in Portuguese): Serra, Norte, Nordeste, Centro-Oeste, Vales, Metropolitana, and Sul. Using the latest national census, we divided the required sample size proportionally to the number of people living in each region. Participants aged 18 years and older living in the Rio Grande do Sul State were included in wave 1. From these individuals, those who were still living in the State and provided contact information (e.g., phone number, social media) were contacted in waves 2 and 3.
2.4. Outcome
Self-reported medical diagnostic of depression (henceforth referred as depression) was defined as the primary outcome for this study. Participants answered the following question in all waves: “Has any doctor already told you that you have depression?“. Only participants who answered no for this question at wave 1 were included in the present study. Then, the incidence of depression was defined as any new cases during waves 2 and 3. This question was previously used in the Brazilian Telephone-based Surveillance System for Noncommunicable Diseases (VIGITEL)(Enes & Nucci, 2019).
2.5. Exposure
In wave 1 physical activity before social distancing and during the current week were determined. The following validated question (Milton, Bull, & Bauman, 2011) was used to assess physical activity in the current week: “In the last seven days, on how many days have you done physical activity, which was enough to raise your breathing rate. This may include sport, exercise, and brisk walking or cycling for recreation or to get to and from places, but should not include housework or physical activity that may be part of your job”. This question was previously validated. To assess physical activity before the COVID-19 pandemic, the reference period was the changed: “Before social distancing restrictions, on how many days have you done physical activity, which was enough to raise your breathing rate. This may include sport, exercise, and brisk walking or cycling for recreation or to get to and from places, but should not include housework or physical activity that may be part of your job”.
If participants indicated a positive answer (i.e., “Yes”), then the number of days and minutes were asked. Respondents with zero minutes of physical activity were classified as inactive, between one and 149 min per week classified as insufficiently active, and those with 150 min and over were considered physically active according to the latest guidelines of physical activity provided by the World Health Organization (WHO)(Bull et al., 2020). To investigate the effects of changing physical activity on incident depression, we further classified participants based on the physical activity status (inactive: <150 min per week; active: 150+ minutes per week) in both periods, as follows: remained inactive, became inactive, became active, or remained active.
Participants were also asked to identify the types of physical activity they performed before and during social distancing and whether these activities were performed at home or out-of-home. Home-based (or at home) physical activity was defined as activities performed within the participant's household (those activities performed in shared gyms inside residential condominiums were not considered to be at-home activities). Out-of-home activities included all physical activities practiced external to participants' household, including shared gyms and running/walking. An initial list of physical activities was provided (running/walking, rope jump, cycling, strength exercises, stretching exercises, stair use). Also, a blank space was offered so participants could report other types of activities practiced. The main activities reported were running/walking (47.8%), cycling (19.6%), strength exercises (22.3%), stretching exercises (13.8%), and stair use (8.9%). Running/walking, cycling, and stair use were merged as ‘endurance’ activities. Stretching activities was combined with Yoga and Pilates as ‘stretching and mind-body’ activities category. All other activities summed up less than 1% and, thus, were not analyzed.
2.6. Possible confounders
Sociodemographic characteristics, including age, ethnic group, conjugal situation, and educational level, were assessed in wave 1. We also asked participants in wave 1 how social distancing affected their monthly income (i.e., decreased, unchanged, increased). Self-reported weight and height were reported to calculate body mass index (BMI). BMI was further categorized into normal (BMI<25 kg/m2), overweight (BMI ≥25 and < 30 kg/m2), and obese (BMI ≥30 kg/m2). Other chronic diseases diagnosed by a physician were also reported during wave 1 using a question previously used in the VIGITEL (Enes & Nucci, 2019).
2.7. Data analyses
All analyzes were performed using STATA/MP 14.2 (Stata Corp, College Station, Texas). Due to the overrepresentation of respondents from one macroregion in the State (Sul, N = 436, 64.6%), all analyzes were weighted by the respondents' proportion in each macroregion. Normality of data distribution was defined as Skewness between −1 and 1 and thorough visual inspection of histograms. Continuous data were reported as mean and 95% confidence interval (CI), while categorical variables were shown as proportions and 95%CI. Differences on sociodemographic, behavioral, and health-related factors between participants and those were tested using the chi-square test.
The incidence rate of depression was reported as incidence rate per 1000 persons-year at risk and respective 95%CI. Differences in incidence rates among physical activity status, type, setting, and change in physical activity status were tested using the stir command. Proportional Cox regression models were used to investigate the effect of physical activity status (inactive, insufficiently active, active), place (none, at home, out of home, both), type (endurance, strength, combined, mind/body), and change in status (remained or became inactive; remained or became active) on the incidence of depression over the follow-up period. Crude analyzes were reported as model 1. Model 2 included the variables age, sex, educational level, ethnicity, and conjugal status as potential confounders. Model 3 included variables from the previous model plus change in monthly income, daily routine during the COVID-19 pandemic, BMI, and diagnosed chronic diseases. Values were reported as hazard ratio (HR) and 95%CI. We adopted a p-value lower than 0.05 as the level of significance. Proportional-hazards assumptions were confirmed using proportional hazards global tests and by plotting Schoenfeld residuals against time. Sensitivity analysis was performed using inverse probability weights for successful follow-up to correct the analyses for losses to follow-up (Seaman & White, 2013).
3. Results
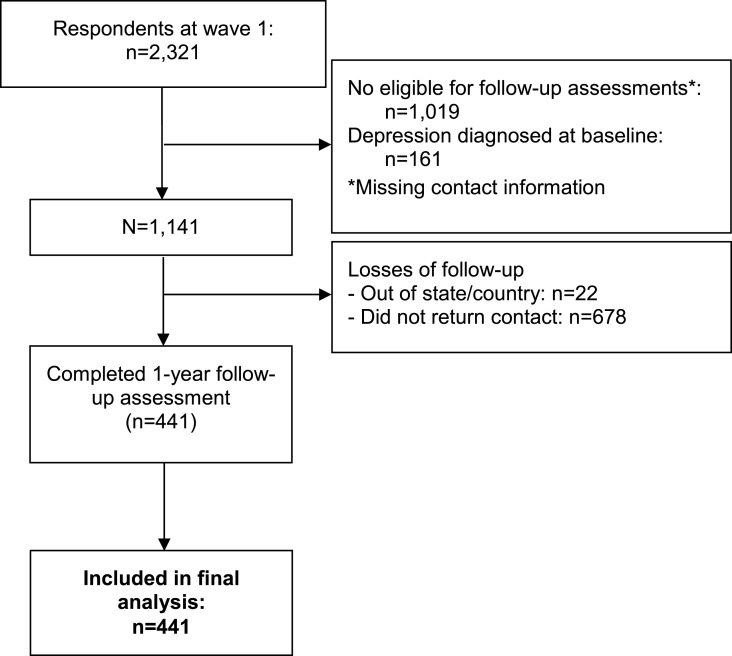
From eligible participants at baseline (n = 1141), 441 (38.7%) were followed in either wave 2 or 3, as shown in Fig. 1 . Participants who were more schooled and with no chronic diseases were more likely to be followed up when compared to their respective peers (Supplementary Table 1). Table 1 describes sociodemographic, health-related, and behavioral characteristics of the included sample. Most of the participants were women, white, lived with a partner, and had a university degree or above. Over one-year period, 21.8% (95%CI: 18.1%–25.9%) received medical diagnosis of depression. The incidence rate of depression was 82.9 (95%CI: 65.2, 106.8) per 1000 persons-year at risk from June 2020 to June 2021. Those who reported having received a medical diagnosis of depression over the follow-up were more likely to be inactive and remained inactive during the pandemic than non-diagnosed participants.
Fig. 1.
Study workflow.
Table 1.
Sociodemographic and health-related characteristics of participants and the association with self-reported diagnoses of depression. N = 441.
| N | Total | Depression |
P-value | ||
|---|---|---|---|---|---|
| No (n = 345) | Yes (n = 96) | ||||
| Age, years (mean [95%CI]) | 441 | 37.7 (36.5, 39.0) | 37.8 (36.4, 39.2) | 37.4 (34.3, 40.4) | 0.797a |
| Gender (% [95%CI]) | 0.292b | ||||
| Male | 101 | 23.8 (19.9, 28.2) | 88.8 (80.8, 93.7) | 11.2 (6.3, 19.2) | |
| Female | 340 | 75.9 (71.5, 79.8) | 82.4 (77.7, 86.2) | 17.6 (13.8, 22.3) | |
| Ethnicity (% [95%CI]) | 0.056b | ||||
| White | 400 | 91.2 (88.3, 93.5) | 77.5 (73.1, 81.2) | 22.5 (18.8, 26.8) | |
| Black | 21 | 4.6 (3.0, 6.9) | 83.3 (58.2, 94.7) | 16.7 (5.3, 41.8) | |
| Mixed | 19 | 3.9 (2.5, 6.2) | 95.2 (71.7, 99.4) | 4.8 (0.1, 28.2) | |
| Other | 1 | 0.2 (0.0, 1.5) | – | – | |
| Conjugal status (% [95%CI]) | 0.236b | ||||
| With partner | 274 | 62.5 (57.7, 67.1) | 85.6 (80.7, 89.4) | 14.4 (10.6, 19.3) | |
| Living alone | 167 | 37.5 (32.9, 42.3) | 81.2 (74.2, 86.6) | 18.8 (13.4, 25.8) | |
| Educational achievement (% [95%CI]) | 0.722b | ||||
| High school or below | 119 | 27.0 (22.9, 31.5) | 82.9 (74.6, 88.8) | 17.1 (11.5, 25.3) | |
| University degree or above | 322 | 73.0 (68.5, 77.1) | 84.3 (79.7, 88.0) | 15.7 (12.0, 20.3) | |
| Body mass index (% [95%CI]) | 0.344c | ||||
| Normal | 209 | 47.6 (42.7, 52.4) | 85.6 (79.9, 89.1) | 14.4 (10.1, 20.0) | |
| Overweight | 151 | 34.4 (29.9, 39.1) | 84.4 (77.4, 89.5) | 15.6 (10.5, 22.6) | |
| Obese | 80 | 18.1 (14.6, 22.1) | 78.4 (67.5, 86.4) | 21.6 (13.6, 32.6) | |
| Chronic diseases (% [95%CI]) | 0.195b | ||||
| No | 237 | 54.3 (49.4, 59.0) | 86.1 (80.8, 90.1) | 13.9 (9.9, 19.1) | |
| Yes | 204 | 45.7 (41.0, 50.6) | 81.4 (75.1, 86.3) | 18.6 (13.7, 24.9) | |
| Daily routine during social distancing (% [95%CI]) | 0.034b | ||||
| Stayed at home all the time or leaving home only to essential activities | 240 | 54.7 (49.9, 59.5) | 80.4 (74.7, 85.1) | 19.5 (14.9, 25.3) | |
| Leaving home every day to non-essential activities | 201 | 45.3 (40.5, 50.1) | 88.2 (82.7, 92.1) | 11.8 (7.9, 17.3) | |
| Monthly income reduced since COVID-19 (% [95%CI]) | 0.267b | ||||
| No | 246 | 56.2 (51.3, 60.9) | 85.7 (80.6, 89.7) | 14.3 (10.3, 19.4) | |
| Yes | 195 | 43.8 (39.1, 48.7) | 81.7 (75.3, 86.7) | 18.3 (13.3, 24.7) | |
| Physical activityd(% [95%CI]) | 0.010c | ||||
| Categories | |||||
| None | 220 | 48.0 (43.5, 52.6) | 72.7 (66.4, 78.2) | 27.3 (21.8, 33.6) | |
| Insufficiently active | 106 | 26.9 (23.0, 31.1) | 82.1 (74.3, 88.0) | 17.9 (12.0, 25.7) | |
| Active | 115 | 25.0 (21.3, 29.3) | 86.1 (78.4, 91.3) | 13.9 (8.7, 21.6) | |
| Place | 0.056b | ||||
| None | 215 | 47.6 (43.0, 52.2) | 73.4 (67.1, 78.9) | 26.6 (21.1, 32.9) | |
| At home | 85 | 19.9 (16.5, 23.8) | 80.2 (70.7, 87.2) | 19.8 (12.8, 29.3) | |
| Out of home | 17 | 4.1 (2.7, 6.4) | 84.2 (60.0, 95.0) | 15.8 (5.0, 40.0) | |
| Both | 124 | 28.4 (24.4, 32.7) | 85.4 (78.2, 90.5) | 14.6 (9.5, 21.8) | |
| Type | 0.116b | ||||
| None | 215 | 47.6 (43.0, 52.2) | 73.4 (67.1, 78.9) | 26.6 (21.1, 32.9) | |
| Endurance | 103 | 25.1 (21.3, 29.3) | 83.5 (75.5, 89.2) | 16.5 (10.8, 24.5) | |
| Strength | 38 | 8.3 (6.1, 11.2) | 78.9 (62.9, 89.2) | 21.1 (10.8, 37.1) | |
| Endurance + strength | 64 | 14.0 (11.1, 17.5) | 85.9 (75.0, 92.6) | 14.1 (7.4, 25.0) | |
| Mind/body | 21 | 5.0 (3.4, 7.5) | 82.6 (61.1, 93.5) | 17.4 (6.5, 38.9) | |
| Change in physical activity | 0.046b | ||||
| Remained inactiv | 181 | 41.0 (36.5, 45.7) | 72.4 (65.4, 78.4) | 27.6 (21.6, 34.6) | |
| Became inactive | 145 | 32.9 (28.6, 37.4) | 79.3 (71.9, 85.2) | 20.7 (14.8, 28.1) | |
| Became active | 33 | 7.5 (5.4, 10.4) | 87.9 (71.4, 95.5) | 12.1 (4.5, 28.6) | |
| Remained active | 82 | 18.6 (15.2, 22.5) | 85.4 (75.9, 91.5) | 14.6 (8.5, 24.1) | |
t-test for independent sample [mean (95%CI)].
Chi-squared [% (95%CI)] to compare the difference in the proportion of participants among groups.
Linear trend test.
Inactive: 0 min per week of physical activity; Insufficient active: 1–149 min per week of physical activity; active: ≥150 min per week of physical activity.
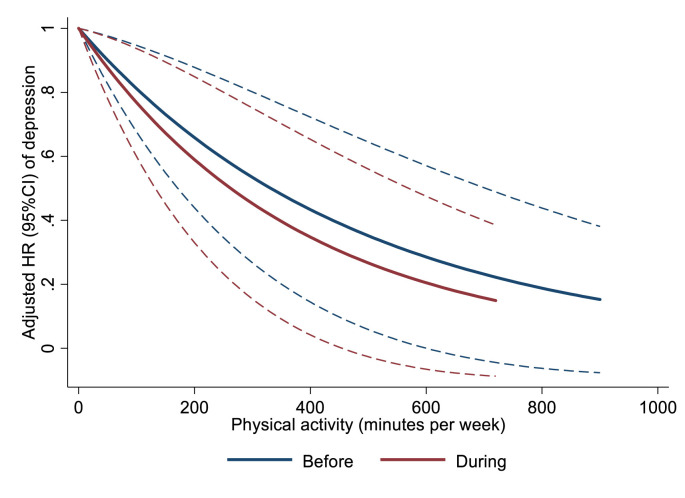
Fig. 2 illustrates the effects of weekly volume of physical activity before and during the early stages of the COVID-19 pandemic in southern Brazil on the risk of depression. In both moments, increasing the time spent on physical activities reduced the risk of depression. Table 2 showed the incidence rate and hazard ratio of incident depression stratified by different physical activity parameters. Active and insufficiently active participants had a reduced incidence rate and risk of depression compared to inactive ones, although this effect was only observed considering the current physical activity. Including confounders, such as age, sex, education, and daily routine during the early stage of the COVID-19 pandemic, did not change this effect. No difference was observed between active and insufficiently active participants regarding the incidence rate of depression.
Fig. 2.
Association between volume of physical activity (minutes per week) and the incidence of depression in adults in southern Brazil. N = 441.
Table 2.
Incidence rate per 1000 person-time, hazard ratio, and respective 95% confidence interval of depression in adults during the COVID-19 pandemic in southern Brazil. N = 441.
| Incidence rate (95%CI) | Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Physical activity | |||||||
| Categories | |||||||
| Before | 0.038a | 0.045a | 0.058a | ||||
| None | 98.8 (70.2, 136.2) | Ref | Ref | Ref | |||
| Insufficiently active | 85.0 (55.4, 130.4) | 0.87 (0.51, 1.49) | 0.88 (0.51, 1.51) | 0.90 (0.52, 1.55) | |||
| Active | 60.7 (43.8, 84.2) | 0.61 (0.39, 0.98) | 0.62 (0.38, 0.99) | 0.64 (0.39, 1.02) | |||
| During | 0.008a | 0.010a | 0.012a | ||||
| None | 99.8 (77.2, 129.1) | Ref | Ref | Ref | |||
| Insufficiently active | 59.2 (37.3, 94.0)† | 0.59 (0.35, 1.00) | 0.57 (0.34, 0.97) | 0.58 (0.34, 0,98) | |||
| Active | 51.1 (31.3, 83.4)† | 0.51 (0.29, 0.88) | 0.53 (0.30, 0.92) | 0.53 (0.31, 0.93) | |||
| Setting | 0.138b | 0.159b | 0.242b | ||||
| None | 99.1 (76.5, 128.5) | Ref | Ref | Ref | |||
| At home | 73.2 (46.1, 116.1) | 0.88 (0.49, 1.61) | 0.85 (0.46, 1.55) | 0.66 (0.34, 1.28) | |||
| Out of home | 62.5 (20.2, 193.8) | 0.87 (0.27, 2.83) | 0.86 (0.27, 2.87) | 0.66 (0.16, 2.79) | |||
| Both | 45.3 (27.3, 75.2)† | 0.47 (0.24, 0.93) | 0.45 (0.23, 0.90) | 0.49 (0.25, 0.98) | |||
| Type | 0.363b | 0.362b | 0.438b | ||||
| None | 99.1 (76.5, 128.5) | Ref | Ref | Ref | |||
| Endurance | 50.7 (30.5, 84.1)† | 0.52 (0.28, 0.96) | 0.52 (0.28, 0.97) | 0.52 (0.28, 0.97) | |||
| Strength | 80.8 (40.4, 161.1) | 0.85 (0.38, 1.87) | 0.78 (0.35, 1.74) | 0.78 (0.35, 1.74) | |||
| Endurance + strength | 52.9 (27.5, 101.7)† | 0.43 (0.19, 1.01) | 0.40 (0.17, 0.95) | 0.40 (0.17, 0.95) | |||
| Mind/body | 66.7 (25.0, 177.6) | 0.61 (0.19, 1.96) | 0.59 (0.18, 1.90) | 0.59 (0.18, 1.90) | |||
| Change in physical activity | 0.079b | 0.100b | 0.113b | ||||
| Remained inactive | 104.8 (79.5, 138.3) | Ref | Ref | Ref | |||
| Became inactive | 66.1 (44.3, 98.6) | 0.68 (0.40, 1.13) | 0.63 (0.37, 1.07) | 0.67 (0.39, 1.13) | |||
| Became active | 48.2 (18.1, 128.4)† | 0.53 (0.19, 1.48) | 0.49 (0.17, 1.38) | 0.49 (0.17, 1.37) | |||
| Remained active | 52.2 (29.6, 91.9)† | 0.45 (0.22, 0.92) | 0.47 (0.23, 0.98) | 0.48 (0.23, 0.99) | |||
Model 1: Crude analysis
Model 2: Adjusted for age, sex, ethnicity, educational achievement, and conjugal status.
Model 3: Model 2 plus change in monthly income, daily routine during the COVID-19 pandemic, body mass index, and diagnosed chronic diseases
Note: Boldface indicates statistical significance (p<0
†p < 0.05 compared to none or inactive group.
p for linear trend.
p for heterogeneity.
In adjusted analysis, participants engaging in physical activity both at-home and out-of-home had reduced incidence rate and risk of incident depression compared to those who did not practice physical activity regardless the setting (Table 2). Endurance (e.g., running, cycling) and combined (i.e., endurance plus strength) physical activity reduced the risk of incident depression compared to none. Strength only and mind/body exercises were not associated with the incidence of depression over the follow-up. No differences were observed among other physical activity places (p for heterogeneity = 0.242) and types (p for heterogeneity = 0.438), as shown in Supplementary Table 2. Finally, participants who remained active from before to during the early stages of the COVID-19 pandemic had a reduced incidence rate and risk of incident depression compared to those who remained inactive. Sensitivity analyses accounting for losses of follow-up did not change our findings.
4. Discussion
This is one of the few longitudinal studies carried out outside rich countries investigating the effects of physical activity on the risk of depression during the COVID-19 pandemic. One in five people developed depression across the one-year follow-up period. Physical activity practice during the early stages of the COVID-19 reduced the incidence of depression in Brazilian adults. Specific conditions regarding the type of activity and where it is practiced seem to mediate this association. These findings provide timely relevant data on non-pharmacological strategies to reduce this indirect effect of the COVID-19 pandemic in the Brazilian population.
Since March 2020, the incidence of depression has increased dramatically worldwide (Pappa et al., 2020; Salari et al., 2020). In the United Kingdom, the annual incidence rate of general practitioners-recorded depression has fallen from 22.5 per person-years in 1996 to 14.0 per person-years in 2006 (Rait et al., 2009). However, an upsurge in depressive symptoms was observed during the early stages of the COVID-19 pandemic, which was followed by a fairly rapid decline after lockdown policies were implemented (Fancourt, Steptoe, & Bu, 2021). In a sample of Spanish university graduates (N = 14,907) with similar age than the present study (mean age: 36.7; standard deviation: 11.7 years), the authors reported 774 (5.2%) incident cases of depression after a median of 10.3 years of follow-up, value 4.2 times lower than the 21.8% observed in our study (Gómez-Donoso et al., 2020). In Brazil, the number of adults diagnosed with depression increased 34.2% from 2013 (7.9%) to 2019 (10.8%), affecting 16.3 millions in 2019 (de Souza Lopes, Gomes, Junger, & Menezes, n. d.). Specific groups such young adults (18–24 years old) who were not working showed a 3-fold increase in the prevalence in the same period, what may be attributable to the economic recession over these years in the country (de Souza Lopes et al., n. d.). Likewise, the proportion of adults with moderate-to-severe symptoms of depression had a 6.6-fold increase during the first three months of the pandemic in southern Brazil (Feter et al., 2021). Curiously, young adults and those with reduced monthly income since social distancing restrictions were implemented also showed a greater risk for aggravated depressive and anxiety symptoms (Feter et al., 2021).
On the other hand, physical activity has been proved an effective, low-cost strategy to reduce the incidence of depression regardless of age (Dishman, McDowell, & Herring, 2021; Gianfredi et al., 2020; Schuch et al., 2018). In a previous meta-analysis of prospective cohort studies with 266,939 individuals, physical activity reduced the risk of incident depression in 22% (95%CI: 13%, 30%) regardless of age and sex (Schuch et al., 2018). For example, individuals who became or remained physically active during the first three months of the COVID-19 pandemic had lower risk of aggravated depressive symptoms than those who remained inactive (Feter et al., 2021). Our findings indicate that physical activity may protect against the pandemic-related incident depression, confirming our initial hypothesis. In addition, we showed that even in people who did physical activity below the recommended weekly volume (i.e., 150 min), this protective effect was observed. Further, the inverse, linear dose-response relationship between current physical activity and the risk of incident depression observed in our study supports the “every move count” movement, leading by the WHO(Bull et al., 2020).
Although endurance (Stanton & Reaburn, 2014), strength (Carneiro et al., 2020), and mind/body exercises (Miller et al., 2020) are strongly recommended for people with depression, only endurance activities, either alone or combined with strength activities, were associated with reduced risk of developing the disease, refusing our initial hypothesis. Previous studies have shown that the protective effect triggered by endurance exercise may be related to the kynurenine metabolism (Pedersen, 2019). In people with depression, kynurenine, a neurotoxic tryptophan metabolic associated with symptoms of depression, is found in high concentration at the hippocampus (Parrott et al., 2016; Pedersen, 2019). On the other hand, endurance exercises are capable to increase expression of skeletal muscle kynurenine aminotransferase (Metcalfe, Koliamitra, Javelle, Bloch, & Zimmer, 2018; Schlittler et al., 2016), which shifts the concentration of the neurotoxic kynurenine to kynurenic acid and consequently reduces symptoms of depression (Pedersen, 2019; Schlittler et al., 2016).
Besides the biological mechanism, the absent of effect from other activities may be related to lack of proper settings and professional guidance, as initially hypothesized. As social distancing had restricted the access to fitness facilities, some people chose to do physical activity at home using especially fitness content from social medias. However, these activities may be impaired by different factors, including lack of professional support, adequate space and equipment, technology literacy, and adherence (Bland et al., 2020; Crochemore-Silva et al., 2020). Such issues may be more explicit in strengthening activities where the lack of professional guidance and setting may prevent the training to reach target zones of volume and intensity to trigger the well-known benefits of strength training on individuals’ health.
Participants who remained active from before to during the pandemic showed lower risk and incidence of depression. Physical activity can promote long-term brain plasticity (Erickson et al., 2019; Stimpson, Davison, & Javadi, 2018) especially through the release of neurogenic factors including brain-derived neurotrophic factor (Pilc, 2010). Besides protecting the brain against the deleterious impact of the COVID-19 pandemic on mental health (Wolf et al., 2021), previous experience with physical activity may facilitate practice at home during a more restrictive stage of the pandemic. As previously stated, physical activity has myriad positive effects on physical and mental health, including reducing the risk of more severe forms of the COVID-19 (Nigro et al., 2020). Although social distancing restrictions have been eased in some countries, including Brazil, the prevalence of people with symptoms with depression are still elevated (Feter et al., 2021). Large-scale, low-cost, and non-pharmacological strategies, including physical activity, must be promoted to reduce the long-term impact of this indirect effect of the pandemic in the population, especially in the moment the world is facing two pandemics concomitantly - physical inactivity and the COVID-19 pandemic (Hall, Laddu, Phillips, Lavie, & Arena, 2021). Furthermore, given the current characteristics (unsupervised, prescribed by non-exercise professionals, videos from social media) and the increasing demand of physical activity at home, robust randomized controlled trials to test and deliver telehealth exercise intervention through telephone calls, messages, video conferencing, and other web-based platforms remain warranted.
There are a few limitations on this study. First, although retention rate was lower than expected (52% vs 70% (Feter et al., 2020)), other population-based cohort studies found similar or lower (e.g., 28%) response rates using online-based questionnaires (Brown et al., 2020). Also, no significant difference was observed in participants by follow-up assessments (Supplementary Table 1). In addition, sensitivity analysis accounting for losses of follow-up showed low influence of selection bias in our findings. Second, we used self-reported measures of diagnosis of depression, physical activity, and other variables. However, in-person interviews or assessments were not allowed by the national research ethics committee. Third, the proportion of participants with an academic degree was overrepresented in the sample. This sampling bias was expected since less educated people might have limited internet access and therefore lower likelihood to participate in online-based researches. However, the COVID-19 has a deeper impact in lower economic groups, so sampling bias is likely to underestimate our occurrence measurements (Stopa et al., 2015). Fourth, although the longitudinal design, reverse causality might not be ruled out due to the short follow-up period. Future waves of the PAMPA cohort will allow the investigation of the long-term effects of the COVID-19 pandemic on mental health and the effect of physical activity on these symptoms. Fifth, the question used to assess physical activity in the present study does not allow to discriminate between moderate and vigorous physical activity.
Being physically active during pandemic, regardless of the amount of physical activity practiced, reduced the incidence of depression in adults in southern Brazil. The type of physical activity does not seem to matter as both at and out of home PA and endurance and endurance plus strengthening were favorable. Our data indicate that increasing population-level physical activity may aid in the control of the burden of social distancing on mental health as a consequence of COVID-19 pandemic in Brazil.
Declaration of competing interest
None.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mhpa.2022.100468.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Aidar F.J., de Matos D.G., de Oliveira R.J., Carneiro A.L., Cabral B.G. de A.T., Dantas P.M.S., et al. Relationship between depression and strength training in survivors of the ischemic stroke. Journal of Human Kinetics. 2014;43:7–15. doi: 10.2478/hukin-2014-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland K.A., Bigaran A., Campbell K.L., Trevaskis M., Zopf E.M. Exercising in isolation? The role of telehealth in exercise oncology during the COVID-19 pandemic and beyond. Physical Therapy. 2020;100(10):1713–1716. doi: 10.1093/ptj/pzaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Goodman A., Peters A., Ploubidis G.B., Sanchez A., Silverwood R., et al. 2020. COVID-19 survey in five national longitudinal studies: Wave 1 user guide (version 1) (London) [Google Scholar]
- Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British Journal of Sports Medicine. 2020;54(24):1451. doi: 10.1136/bjsports-2020-102955. LP – 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo E.L., Feter N., Doring I.R., Leite J.S., Cassuriaga J., Rombaldi A.J., et al. How has COVID-19 social distancing impacted physical activity patterns? Data from the PAMPA cohort, Brazil. Journal of Exercise Science & Fitness. 2021 doi: 10.1016/j.jesf.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo E.L., Reichert F.F. Studies of physical activity and COVID-19 during the pandemic: A scoping review. Journal of Physical Activity and Health. 2020;1(aop):1–10. doi: 10.1123/jpah.2020-0406. [DOI] [PubMed] [Google Scholar]
- Carneiro L., Afonso J., Ramirez-Campillo R., Murawska-Ciałowciz E., Marques A., Clemente F.M. The effects of exclusively resistance training-based supervised programs in people with depression: A systematic review and meta-analysis of randomized controlled trials. International Journal of Environmental Research and Public Health. 2020;17(18):6715. doi: 10.3390/ijerph17186715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochemore-Silva I., Knuth A.G., Wendt A., Nunes B.P., Hallal P.C., Santos L.P., et al. Prática de atividade física em meio à pandemia da COVID-19: Estudo de base populacional em cidade do sul do Brasil. Ciência & Saúde Coletiva. 2020;25:4249–4258. doi: 10.1590/1413-812320202511.29072020. [DOI] [PubMed] [Google Scholar]
- Dishman R.K., McDowell C.P., Herring M.P. Customary physical activity and odds of depression: A systematic review and meta-analysis of 111 prospective cohort studies. British Journal of Sports Medicine. 2021;55(16):926. doi: 10.1136/bjsports-2020-103140. LP – 934. [DOI] [PubMed] [Google Scholar]
- Enes C.C., Nucci L.B. Public Health Reports; 2019. A telephone surveillance system for noncommunicable diseases in Brazil. 0033354919848741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson I.K., Hillman C., Sullman C.M., Ballard R.M., Bloodgood B., Conroy D.E., et al. Guidelines, 2018 Phys Activity Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Medicine & Science in Sports & Exercise. 2019;51(6):1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancourt D., Steptoe A., Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in england: A longitudinal observational study. The Lancet Psychiatry. 2021;8(2):141–149. doi: 10.1016/S2215-0366(20)30482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feter N., Caputo E.L., Doring I.R., Leite J.S., Cassuriaga J., Reichert F.F., et al. medRxiv. Cold Spring Harbor Laboratory Press; 2020. Longitudinal study about low back pain, mental health, and access to healthcare system during COVID-19 pandemic: Protocol of an ambispective cohort short title: PAMPA cohort: Study protocol. [DOI] [Google Scholar]
- Feter N., Caputo E.L., Doring I.R., Leite J.S., Cassuriaga J., Reichert F.F., et al. Sharp increase in depression and anxiety among Brazilian adults during the COVID-19 pandemic: Findings from the PAMPA cohort. Public Health. 2021;190:101–107. doi: 10.1016/j.puhe.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfredi V., Blandi L., Cacitti S., Minelli M., Signorelli C., Amerio A., et al. Depression and objectively measured physical activity: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health. 2020;17(10):3738. doi: 10.3390/ijerph17103738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Donoso C., Sánchez-Villegas A., Martínez-González M.A., Gea A., Mendonça R. de D., Lahortiga-Ramos F., et al. Ultra-processed food consumption and the incidence of depression in a mediterranean cohort: The SUN project. European Journal of Nutrition. 2020;59(3) doi: 10.1007/s00394-019-01970-1. [DOI] [PubMed] [Google Scholar]
- Hall G., Laddu D.R., Phillips S.A., Lavie C.J., Arena R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Progress in Cardiovascular Diseases. 2021;64:108–110. doi: 10.1016/j.pcad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami A., Harrabi B., Mohr M., Krustrup P. Physical activity and coronavirus disease 2019 (COVID-19): Specific recommendations for home-based physical training. Managing Sport and Leisure. 2020:1–6. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE) 2010. Censo demográfico 2010.https://censo2010.ibge.gov.br/ from. [Google Scholar]
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. The Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J.S., Feter N., Doring I.R., Cassurriaga J., Caputo E.L. Using social media for research during COVID-19 pandemic in a cohort in Rio Grande do Sul state, Brazil. Rev. Bras. Ativ. Fís. Saúde. 2020;1–5 [Google Scholar]
- Lewis G.D., Farrell L., Wood M.J., Martinovic M., Arany Z., Rowe G.C., et al. Metabolic signatures of exercise in human plasma. Science Translational Medicine. 2010;2(33) doi: 10.1126/scitranslmed.3001006. 33ra37-33ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe A.J., Koliamitra C., Javelle F., Bloch W., Zimmer P. Acute and chronic effects of exercise on the kynurenine pathway in humans – a brief review and future perspectives. Physiology & Behavior. 2018;194:583–587. doi: 10.1016/j.physbeh.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Miller K.J., Gonçalves-Bradley D.C., Areerob P., Hennessy D., Mesagno C., Grace F. Comparative effectiveness of three exercise types to treat clinical depression in older adults: A systematic review and network meta-analysis of randomised controlled trials. Ageing Research Reviews. 2020;58 doi: 10.1016/j.arr.2019.100999. [DOI] [PubMed] [Google Scholar]
- Milton K., Bull F.C., Bauman A. Reliability and validity testing of a single-item physical activity measure. British Journal of Sports Medicine. 2011;45(3):203–208. doi: 10.1136/bjsm.2009.068395. [DOI] [PubMed] [Google Scholar]
- Moraes H.S., Silveira H.S., Oliveira N.A., Mello Portugal E.M., Araujo N.B., Vasques P.E., et al. Is strength training as effective as aerobic training for depression in older adults? A randomized controlled trial. Neuropsychobiology. 2020;79(2):141–149. doi: 10.1159/000503750. [DOI] [PubMed] [Google Scholar]
- Nigro E., Polito R., Alfieri A., Mancini A., Imperlini E., Elce A., et al. Molecular mechanisms involved in the positive effects of physical activity on coping with COVID-19. European Journal of Applied Physiology. 2020:1–14. doi: 10.1007/s00421-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer‐Streit B., Mayr V., Dobrescu A.I., Chapman A., Persad E., Klerings I., et al. Quarantine alone or in combination with other public health measures to control COVID‐19: A rapid review. Cochrane Database of Systematic Reviews. 2020;(4) doi: 10.1002/14651858.CD013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa S., Ntella V., Giannakas T., Giannakoulis V.G., Papoutsi E., Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain, Behavior, and Immunity. 2020 doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott J.M., Redus L., Santana-Coelho D., Morales J., Gao X., O’connor J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Translational Psychiatry. 2016;6(10) doi: 10.1038/tp.2016.200. e918–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K. Physical activity and muscle-brain crosstalk. Nature Reviews Endocrinology. 2019;15(7):383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- Pilc J. The effect of physical activity on the brain derived neurotrophic factor: From animal to human studies. Journal of Physiology & Pharmacology. 2010;61(5):533–541. [PubMed] [Google Scholar]
- Prince M., Patel V., Saxena S., Maj M., Maselko J., Phillips M.R., et al. No health without mental health. The Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Rait G., Walters K., Griffin M., Buszewicz M., Petersen I., Nazareth I. Recent trends in the incidence of recorded depression in primary care. The British Journal of Psychiatry. 2009;195(6):520–524. doi: 10.1192/bjp.bp.108.058636. [DOI] [PubMed] [Google Scholar]
- Salari N., Hosseinian-Far A., Jalali R., Vaisi-Raygani A., Rasoulpoor S., Mohammadi M., et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Globalization and Health. 2020;16(1):1–11. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlittler M., Goiny M., Agudelo L.Z., Venckunas T., Brazaitis M., Skurvydas A., et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. American Journal of Physiology - Cell Physiology. 2016 doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Vancampfort D., Firth J., Rosenbaum S., Ward P.B., Silva E.S., et al. Physical activity and incident depression: A meta-analysis of prospective cohort studies. American Journal of Psychiatry. 2018;175(7):631–648. doi: 10.1176/appi.ajp.2018.17111194. [DOI] [PubMed] [Google Scholar]
- Seaman S.R., White I.R. Review of inverse probability weighting for dealing with missing data. Statistical Methods in Medical Research. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- de Souza Lopes, C., Gomes, N. L., Junger, W. L., & Menezes, P. R. (n.d.). Trend in the prevalence of depression and correlates in Brazil: Results from the national health surveys 2013 and 2019. [DOI] [PubMed]
- Stanton R., Reaburn P. Exercise and the treatment of depression: A review of the exercise program variables. Journal of Science and Medicine in Sport. 2014;17(2):177–182. doi: 10.1016/j.jsams.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Stimpson N.J., Davison G., Javadi A.-H. Joggin’ the noggin: Towards a physiological understanding of exercise-induced cognitive benefits. Neuroscience & Biobehavioral Reviews. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Stopa S.R., Malta D.C., Oliveira M. M. de, Lopes C. de S., Menezes P.R., Kinoshita R.T. Prevalence of self-reported depression in Brazil: 2013 national health survey results. Revista Brasileira de Epidemiologia. 2015;18:170–180. doi: 10.1590/1980-5497201500060015. [DOI] [PubMed] [Google Scholar]
- Strakowski S., Nelson E. Oxford University Press; 2015. Major depressive disorder. [Google Scholar]
- Wolf S., Seiffer B., Zeibig J.-M., Welkerling J., Brokmeier L., Atrott B., et al. Is physical activity associated with less depression and anxiety during the COVID-19 pandemic? A rapid systematic review. Sports Medicine. 2021 doi: 10.1007/s40279-021-01468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) World Health Organization; 2017. Depression and other common mental disorders: Global health estimates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.