Abstract
Background
Chronic total occlusion (CTO) of the coronary artery is a difficult problem in clinical practice. The triglyceride–glucose (TyG) index is an effective risk predictor of cardiovascular risk. However, the relationship between the TyG index and the prognosis of CTO patients remains unstudied. Thus, the present study aimed to investigate the relationship between the TyG index and cardiovascular risk in CTO patients.
Methods
This was a single-centre, retrospective cohort study. We retrospectively enrolled 652 patients with CTO lesions diagnosed by angiography and who underwent revascularization through PCI. Patients were routinely followed up for 24 months unless meeting the endpoint. The primary endpoint was the composite of all-cause death, nonfatal myocardial infarction, unplanned revascularization, and nonfatal ischaemic stroke. To test the association of the TyG index with cardiovascular risk, the categorized TyG index and Cox proportional hazards regression models were utilized.
Results
A total of 652 patients were enrolled in the final analysis (male: 83.7%, age: 58.2 ± 10.49 years). The average TyG index was 8.8 ± 0.57. CTO PCIs were procedurally successfully completed in 503 (77.15%) patients. During the follow-up period of 22.8 ± 3.84 months, 73 (11.19%) major adverse cardiovascular and cerebral events (MACCEs) occurred. When fully adjusted, there was a 2.09-fold risk for MACCEs among patients with the highest TyG index compared with those with the lowest TyG index [T2 vs. T1: hazard ratio (HR) 1.24, 95% confidence interval (CI) 0.65–2.38, P = 0.057; T3 vs. T1: HR 2.09, 95% CI 1.14–3.86, P = 0.018; P for trend = 0.036]. The restricted cubic spline (RCS) analysis showed that the HR for MACCEs increased as the TyG index increased over 8.71 [HR per standard deviation (SD) 1.740, 95% CI 1.23–2.46, P = 0.002]. The risk of MACCEs increased with increasing tertiles of TyG index in successful CTO PCI patients and nondiabetes mellitus (DM) patients (P < 0.05) but not in patients with failed CTO PCI and DM patients.
Conclusion
The study revealed that the TyG index had significant relevance to cardiovascular risk in CTO patients and suggests that the TyG index is feasible for predicting cardiovascular risk in CTO patients.
Keywords: Triglyceride-glucose index, Chronic total occlusion, Cardiovascular events
Background
CTO of the coronary artery is difficult to address in clinical practice. With the update of clinical guidelines and the innovation of technology and instruments, the success rate of CTO PCI has been increased to 70%–95.9% [1, 2]. Successful PCI treatment could alleviate angina symptoms, improve quality of life and decrease cardiovascular events [3, 4]. However, there is still a 10.4% 10-year cardiac mortality rate in patients who received successful PCI for CTO lesions [4]. Thus, it is necessary to explore a target that can identify high-risk patients with cardiovascular events after CTO PCI to prescribe more active treatment to reduce events.
Insulin resistance (IR) is considered to be closely associated with cardiovascular risk. Studies have shown that the TyG index is reliable in reflecting IR status [5]. TyG index, which only needs to acquire the TG and fasting blood glucose levels of patients, has become an attractive choice for reflecting IR levels because of its high availability and low cost. Studies have shown a high correlation between the TyG index and previous standard IR indices, including the hyper insulinemic-euglycemic glucose clamp and HOMA-IR [6].
In some aspects, TyG is even more effective than the HOMA-IR model, especially in predicting metabolic syndrome, and arterial stiffness [7, 8]. And several studies have demonstrated that the TyG index can predict myocardial infarction, stroke or other adverse cardiovascular events [9–11]. However, no studies have investigated the relationship between the TyG index and cardiovascular events in CTO patients. Thus, we aimed to explore whether the TyG index could predict adverse cardiovascular events in CTO patients.
Methods
Study population
This was a single-centre, retrospective cohort study. A total of 738 patients who met the inclusion criteria were included. 69 patients were excluded due to incomplete key variables (n = 22) and the exclusion criteria (n = 47). Finally, 669 patients were enrolled in the final follow-up. The inclusion criteria consisted of age > 18 years, and diagnosis of CTO lesions of at least one major epicardial coronary artery by coronary angiography from January 1st 2019 to 31st December 2019 in Beijing Anzhen Hospital, Capital Medical University. The main exclusion criteria for this study were: ST-elevation myocardial infarction (STEMI), suspected familial hypertriglyceridaemia or current use of triglyceride-lowering medications, type 1 diabetes mellitus, acute infection, severe renal failure (estimated glomerular filtration rate < 30 mL/min), including those receiving renal replacement therapy, alanine aminotransferase or aspartate aminotransferase ≥ 5 times the normal upper limit, left ventricular ejection fraction < 30% or New York Heart Association (NYHA) ≥ Grade III. We also excluded patients with incomplete key information, including triglyceride (TG) and fasting blood glucose (FBG) levels. Patients were classified into categories by TyG index tertiles. The levels of the TyG index in each tertile were as follows: T1: ≤ 8.50, T2: > 8.50, ≤ 8.95, T3: > 8.95. The institutional review board of Beijing Anzhen Hospital of Capital Medical University approved the study design and implementation. The waiver of informed consent was granted and the personal information of enrolled patients was concealed.
Definitions
The TyG index was calculated by the formula: ln [TG (mg/dl) × glucose (mg/dl)/2] [12]. Diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L, random blood glucose ≥ 11.1 mmol/L, and/or blood glucose ≥ 11.1 mmol/L 2 h after the oral glucose tolerance test or current use of anti-diabetic medication [13]. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, and/or in-use of antihypertensive drugs [14]. Unstable angina was defined as myocardial ischaemia at rest or during minimal exercise without cardiac enzyme elevation [15]. CTO was defined as a coronary angiographically demonstrated lesion with thrombolysis in myocardial infarction (TIMI) grade 0 blood flow, no thrombus, an unstained proximal fibrous cap, and mature collateral circulation, with a duration of occlusion for more than 3 months [16].
Perioperative management
All PCI procedures and medical treatments were in accordance with current guidelines on myocardial revascularization [17]. The detailed PCI strategy was determined by experienced cardiologists. Coronary angiogram data were analysed by at least two experienced cardiologists. Multivessel diseases were defined as more than two main epicardial coronary artery stenoses ≥ 50%. Diffuse lesions were referred to as single stenotic lesions ≥ 20 mm in length. Procedurally successful CTO PCI was defined as achievement of TIMI grade 2 or greater antegrade flow in all ≥ 2.5-mm distal branches without any in-hospital major adverse cardiovascular events, including death, acute myocardial infarction, or clinically driven target vessel revascularization.
Data collection
Demographic, medical history, PCI procedure information and medical treatment at discharge were collected from the medical information recording system. Blood samples were collected in the morning after overnight fasting and tested on the same day using standard laboratory methods in the central laboratory. Patients included in the study were routinely followed up by telephone communication, medical history system, or outpatient service.
Outcomes
All enrolled patients were followed up for 24 months except when lost to follow-up or if they reached an endpoint of observation. The primary endpoint was the composite of MACCEs, which included all-cause death, nonfatal myocardial infarction, unplanned revascularization and nonfatal ischaemic stroke. Nonfatal stroke was defined as acute cerebral infarction diagnosed by typical symptoms or imaging [18]. Myocardial infarction (MI) was defined according to the fourth general definition of MI [19]. Unplanned repeat revascularization was defined as any accidental PCI or coronary artery bypass grafting (CABG) after indexing procedures.
Statistical analysis
Continuous variables are presented as the mean ± SD or median (interquartile range). One-way ANOVA was used for the comparison of normally distributed data and the Kruskal‒Wallis test was used for the comparison of nonnormally distributed data. The categorical variables are presented as the number of cases with percentages and were tested by the χ2 test (Fisher’s exact test). A Cox proportional hazard regression model was used to analyse the relationship between the TyG index and cardiovascular events and adjusted for multiple models: Model 1: adjusted for age, sex, and body mass index (BMI); Model 2: Model 1 + current smoker, hypertension, diabetes mellitus, dyslipidaemia, previous PCI, and previous CABG; Model 3: Model 2 + acute myocardial infarction (AMI), FBG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), successful CTO treatment, and use of statins, ezetimibe, or antidiabetic medication. A stepwise regression method was used. The survival curve for each group was plotted by using the Kaplan–Meier method and analysed by the log-rank test. RCS analysis was conducted to explore correlations between the TyG index level and cardiovascular risk. Sensitivity analysis was performed according to whether the CTO PCI was procedurally successful and the diabetes status. We used SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) for data analysis. P < 0.05 was considered statistically significant (double tails).
Results
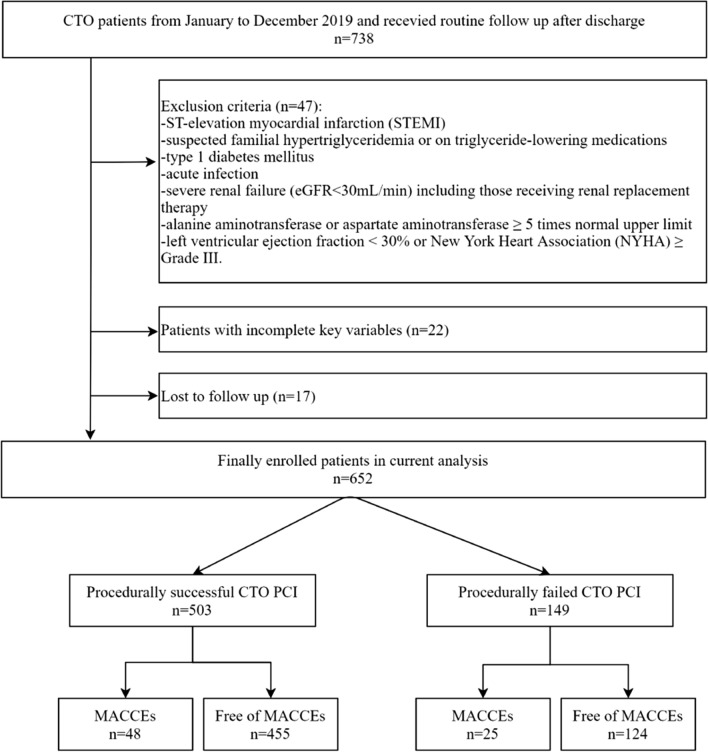
As shown in Fig. 1, 17 patients were lost during follow-up and 652 patients were enrolled in the final analysis. During the 22.8 ± 3.84-month follow-up period, 73 (11.19%) MACCEs occurred. The average time interval of MACCEs was 13.7 ± 6.15 months.
Fig. 1.
Flow diagram of the study. CTO chronic total occlusion, eGFR estimated glomerular filtration rate, MACCEs major adverse cardiac and cerebrovascular events, PCI percutaneous coronary intervention
Baseline characteristics
The baseline characteristics are shown in Table 1. Male patients comprised 83.7% of the patients in the study, and the mean age of the patients was 58.2 ± 10.49 years. The mean TyG index was 8.8 ± 0.57. Among the 652 patients, procedurally successful CTO PCIs were performed in 503 (77.15%) patients. Patients in the higher TyG index tertiles tended to have hypertension, diabetes and dyslipidaemia; and to higher FBG, TC, TG and LDL-C. Patients with high TyG index levels were more likely to take antidiabetic agents. In terms of angiography results, an increased prevalence of multivessel and diffuse lesions was found as the TyG index increased.
Table 1.
Baseline characteristics of patients according to TyG index levels
| Variable | Total | TyG index level | P value | ||
|---|---|---|---|---|---|
| T1 (≤ 8.5) |
T2 (> 8.5, ≤ 8.95) |
T3 (> 8.95) |
|||
| N (%) | 652 | 217 | 223 | 212 | - |
| Age, y | 58.2 ± 10.49 | 59.1 ± 10.78 | 58.5 ± 10.33 | 56.9 ± 10.29 | 0.069 |
| Male, n (%) | 546(83.7) | 189(87.1) | 188(84.3) | 169(79.7) | 0.113 |
| BMI, kg/m2 | 26.0 ± 3.19 | 25.2 ± 3.06 | 26.2 ± 3.06 | 26.7 ± 3.30 | < 0.001 |
| Heart rate, bpm | 72.22 ± 10.60 | 71.11 ± 10.04 | 72.43 ± 11.37 | 73.13 ± 10.26 | 0.133 |
| SBP, mmHg | 128.34 ± 17.32 | 126.41 ± 17.49 | 128.02 ± 17.60 | 130.66 ± 16.66 | 0.037 |
| Medical history and risk factors, n (%) | |||||
| Current smoker | 238(36.5) | 84(38.7) | 75(33.6) | 79(37.3) | 0.522 |
| Hypertension | 431(66.1) | 121(55.8) | 155(69.5) | 155(73.1) | < 0.001 |
| Diabetes | 240(36.8) | 53(24.4) | 71(31.8) | 116(54.7) | < 0.001 |
| Dyslipidemia | 481(73.8) | 153(70.5) | 163(73.1) | 165(77.8) | 0.217 |
| Previous MI | 108(16.6) | 33(15.2) | 39(17.5) | 36(17.0) | 0.797 |
| Previous Stroke | 37(5.7) | 14(6.5) | 17(7.6) | 6(2.8) | 0.081 |
| Previous PCI | 172(26.4) | 61(28.1) | 57(25.6) | 54(25.5) | 0.778 |
| Previous CABG | 32(4.9) | 9(4.1) | 14(6.3) | 9(4.2) | 0.505 |
| Laboratory Tests | |||||
| eGFR, ml/min/1.73m2 | 126.53 ± 32.44 | 132.30 ± 39.25 | 122.95 ± 25.59 | 124.38 ± 30.49 | 0.005 |
| FBG, mmol/L | 6.16 ± 1.36 | 5.5979 ± 0.93 | 6.0504 ± 1.03 | 6.86 ± 1.69 | < 0.001 |
| TC, mmol/L | 4.01 ± 1.05 | 3.72 ± 0.98 | 3.86 ± 0.86 | 4.45 ± 1.15 | < 0.001 |
| TG, mmol/L | 1.28(0.93, 1.76) | 0.84(0.70, 0.97) | 1.30(1.15, 1.50) | 2.05(1.74, 2.54) | < 0.001 |
| LDL-C, mmol/L | 2.39 ± 0.86 | 2.21 ± 0.85 | 2.33 ± 0.75 | 2.62 ± 0.93 | < 0.001 |
| TyG index | 8.8 ± 0.57 | 8.2 ± 0.25 | 8.7 ± 0.13 | 9.4 ± 0.43 | < 0.001 |
| ACS type, n (%) | |||||
| Unstable angina | 567(87.0) | 185(85.3) | 194(87.0) | 188(88.7) | 0.574 |
| AMI | 78(12.0) | 29(13.4) | 26(11.7) | 23(10.8) | 0.714 |
| Medication at discharge, n (%) | |||||
| Aspirin | 640 (98.2) | 214(98.6) | 219(98.2) | 207(97.6) | 0.752 |
| clopidogrel | 426(65.3) | 142(65.4) | 147(65.9) | 137(64.6) | 0.960 |
| ticagrelor | 226(34.7) | 75(34.6) | 76(34.1) | 75(35.4) | 0.960 |
| statin | 639(98.0) | 214(98.6) | 216(96.9) | 209(98.6) | 0.321 |
| Ezetimibe | 134(20.6) | 37(17.1) | 44(19.7) | 53(25.0) | 0.117 |
| Any antidiabetic agents | 167(25.6) | 31(14.3) | 49(22.0) | 87(41.0) | < 0.001 |
| Angiographic Coronary anatomy, n (%) | |||||
| Any left main disease | 150(23.0) | 53(24.4) | 53(23.8) | 44(20.8) | 0.629 |
| Multivessel disease | 392(60.1) | 125(57.6) | 121(54.3) | 146(68.9) | 0.005 |
| Lesions > 20 mm | 295(45.2) | 81(37.3) | 104(35.3) | 110(51.9) | 0.009 |
| Ostial lesion | 70(10.7) | 28(12.9) | 23(10.3) | 19(9.0) | 0.406 |
| Bifurcation | 20(3.1) | 8(3.7) | 6(2.7) | 6(2.8) | 0.808 |
| Treated vessel, n (%) | |||||
| LM | 61(9.4) | 24(11.1) | 21(9.4) | 16(7.5) | 0.458 |
| LAD | 415(63.7) | 148(68.2) | 141(63.2) | 126(59.4) | 0.166 |
| LCX | 264(40.5) | 96(44.2) | 90(40.4) | 78(36.8) | 0.291 |
| RCA | 316(48.5) | 91(41.9) | 115(51.6) | 110(51.9) | 0.062 |
| Mean stent diameters, mm | 2.9 ± 0.40 | 2.8 ± 0.38 | 2.9 ± 0.38 | 2.9 ± 0.42 | 0.490 |
| Total length of stents, mm | 50(29,71) | 50(28,69.5) | 51(29,71) | 50(20,74) | 0.872 |
| Treatment of CTO lesions, n (%) | |||||
| LAD | 251(38.5) | 92(42.4) | 77(34.5) | 82(38.7) | 0.237 |
| LCX | 186(28.5) | 72(33.2) | 66(29.6) | 48(22.6) | 0.049 |
| RCA | 227(34.8) | 57(26.3) | 84(37.7) | 86(40.6) | 0.004 |
| Successful CTO PCI | 503(77.1) | 169(77.9) | 165(74.0) | 169(79.7) | 0.346 |
| Mean stent diameters at CTO lesion, mm | 2.8 ± 0.38 | 2.8 ± 0.37 | 2.8 ± 0.38 | 2.8 ± 0.40 | 0.340 |
| Total length of stents at CTO lesion, mm | 40(26,66) | 40(28,65) | 38.5(25.25,64.75) | 45(28.5,68) | 0.768 |
TyG index triglyceride-glucose index, BMI body mass index, SBP systolic blood pressure, MI myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, eGFR estimated glomerular filtration rate, FBG fasting blood glucose, HbA1c glycosylated haemoglobin, TC total cholesterol, TG triglyceride, LDL-C low-intensity lipoprotein-cholesterol, ACS acute coronary syndrome, AMI acute myocardial infarction, LM left main coronary artery, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery, CTO chronic total occlusion
Relationship between the TyG index and cardiovascular events in CTO patients
The relationship between the TyG index and cardiovascular events in CTO patients is shown in Table 2. As the TyG index tertiles increased, the risk of cardiovascular events increased significantly after being adjusted by different models. Model 3 revealed a 2.09-fold risk for MACCEs among those with the highest TyG index compared with those with the lowest TyG index (T2 vs. T1: HR 1.24, 95% CI 0.65–2.38, P = 0.057; T3 vs. T1: HR 2.09, 95% CI 1.14–3.86, P = 0.018; P for trend = 0.036).
Table 2.
Relationship between TyG index and cardiovascular events in CTO patients
| TyG Tertiles | HR (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2a | Model 3a | |
| T1 | 1 (reference)# | 1 (reference)# | 1 (reference)# | 1 (reference)# |
| T2 | 1.29 (0.67–2.47) | 1.29 (0.67–2.47) | 1.32 (0.69–2.52) | 1.24 (0.65–2.38) |
| T3 | 2.44 (1.35–4.39) | 2.44 (1.35–4.39) | 2.46 (1.37–4.44) | 2.09 (1.14–3.86) |
aThe log-rank test and backward stepwise selection methods in a Cox proportional hazards regression model were performed; Model 1: adjusted for age, sex, BMI; Model 2: Model 1 + current smoker, hypertension, diabetes mellitus, dyslipidemia, previous PCI, previous CABG; Model 3: Model 2 + acute myocardial infarction, FBG, TC, LDL-C, successful CTO treatment, using of statin, ezetimibe, or antidiabetic medication. TyG index tertiles: T1: ≤ 8.50, T2: > 8.50, ≤ 8.95, T3: > 8.95
#P < 0.05
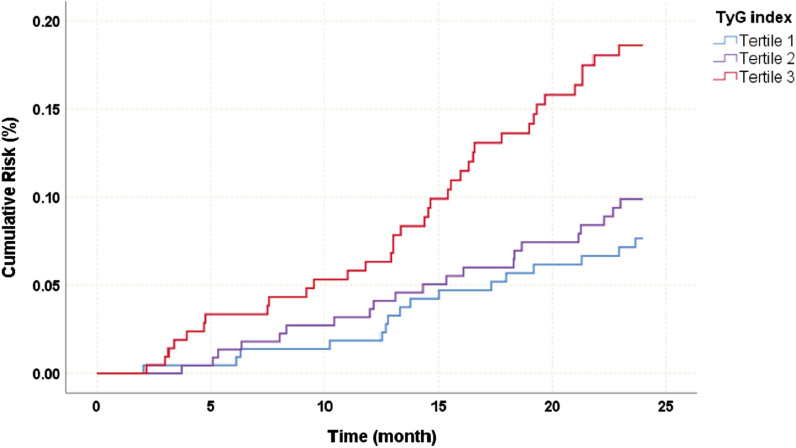
The Kaplan‒Meier curve was used to show the cumulative risk of cardiovascular events by TyG index tertiles (Fig. 2). As TyG index tertiles increased, the cumulative risk of cardiovascular events became significantly higher (log-rank P = 0.004).
Fig. 2.
Kaplan–Meier curve of cumulative risk of cardiovascular events according to different TyG index levels. TyG index triglyceride-glucose index
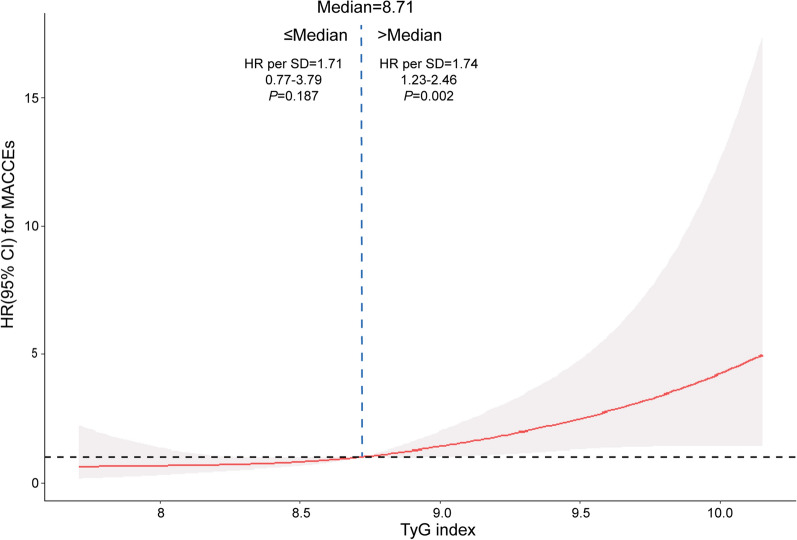
To visualize the relationship between the TyG index and cardiovascular risk, we fitted a RCS model (Fig. 3). The RCS curve appears to be J-shaped, with the HR for MACCEs significantly increasing as the TyG index increased over 8.71 (HR per SD 1.74, 95% CI 1.23–2.46, P = 0.002). For a TyG index ≤ 8.71, the HR per SD was 1.709 (95% CI 0.77–3.79, P = 0.187).
Fig. 3.
The restricted cubic spline of MACCEs and the TyG index. HR hazard ratio, CI confidence interval, MACCEs major adverse cardiovascular and cerebral events, TyG index triglyceride-glucose index
We performed sensitivity analyses in this study. First, we tested the effect of successful CTO PCI on the association between the TyG index and incident MACCEs. As shown in Table 3, the prognostic value of the TyG index in successful CTO PCI patients was similar to the findings of the main analysis (T2 vs. T1: HR 1.33, 95% CI 0.58–3.04, P = 0.495; T3 vs. T1: HR 2.69, 95% CI 1.29–5.60, P = 0.008, P for trend = 0.013), but not in patients with failed CTO PCI. Second, we stratified the patients by diabetes status. We found that high TyG index tertile was related to cardiovascular risk in nondiabetes patients (T2 vs. T1: HR 1.01, 95% CI 0.41–2.51, P = 0.979; T3 vs. T1: HR 2.35, 95% CI 1.06–5.20, P = 0.036, P for trend = 0.040), but not in diabetes patients.
Table 3.
Sensitivity analysis of the relationship between the TyG index and cardiovascular events stratified by diabetes status and successful or failed CTO PCI
| T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| DM# | 1 (reference) | 0.356 | 1.81 (0.68–4.86) | 0.237 | 2.11 (0.74–5.98) | 0.162 |
| Non-DM# | 1 (reference) | 0.040 | 1.01 (0.41–2.51) | 0.979 | 2.35 (1.06–5.20) | 0.036 |
| Successful CTO PCI* | 1 (reference) | 0.013 | 1.33 (0.58–0.04) | 0.495 | 2.69 (1.29–5.60) | 0.008 |
| Failed CTO PCI* | 1 (reference) | 0.373 | 0.52 (0.15–1.79) | 0.299 | 1.32 (0.37–4.67) | 0.672 |
#Adjusted for age, sex, BMI, current smoker, hypertension, dyslipidemia, previous PCI, previous CABG, AMI, FBG, TC, LDL-C, using of statin, ezetimibe, or antidiabetic medication, successful CTO PCI. TyG index Tertiles in diabetes mellitus patients: T1: ≤ 8.65, T2: > 8.65, ≤ 9.26, T3: > 9.26; TyG index Tertiles in non-diabetes mellitus patients: T1: ≤ 8.43, T2: > 8.43, ≤ 8.83, T3: > 8.83
*Adjusted for age, sex, BMI, current smoker, hypertension, DM, dyslipidemia, previous PCI, previous CABG, AMI, FBG, TC, LDL-C, using of statin, ezetimibe, or antidiabetic medication. TyG index Tertiles in successful CTO PCI patients: T1: ≤ 8.50, T2: > 8.50, ≤ 8.96, T3: > 8.96; TyG index Tertiles in failed CTO PCI patients: T1: ≤ 8.53, T2: > 8.53, ≤ 8.91, T3: > 8.91
Discussion
In this study, we demonstrated the correlation between the TyG index and the risk of cardiovascular events in patients with CTO lesions for the first time. The risk of cardiovascular adverse events in CTO patients increased significantly with the increase in the TyG index. A higher TyG index (> 8.71) was associated with significant increases per SD of the TyG index, and no such significant association was found for a lower TyG index (≤ 8.71). The findings of our study extend our understanding of TyG as a tool for cardiovascular events, especially for patients with CTO lesions. This suggests that the TyG index may be helpful in identifying more patients at high risk who need more aggressive treatment and follow‐up strategies.
Our observations that an elevated TyG index was associated with a increased cardiovascular risk are consistent with several recent studies. Among patients with different pathological processes of atherosclerosis, including arterial stiffness, coronary artery calcification, in-stent restenosis and STEMI, ACS, and nonobstructive MI, the TyG index appears to be effective in predicting cardiovascular events [10, 20–22]. However, few researchers have studied the relationship between the TyG index and cardiovascular risk in CTO patients. A study has shown the an elevated TyG index is closely related to the underdevelopment of collateral circulation in CTO lesions, and its risk assessment performance is better than that of a single metabolic abnormality index [23]. Similar to the current study, HOMA-IR, an index of IR, has been reported to predict cardiovascular events in patients with non-ST-elevation myocardial infarction (NSTEMI) combined with type 2 diabetes mellitus (T2DM) and CTO lesions, but the sample size was small and highly selective [24]. Our findings are consistent with these studies.
Insulin resistance, which is represented by the TyG index, is correlated with CTO. Animal research has proven that IR can affect the expression of vascular endothelial growth factor (VEGF) and its receptors, which are major angiogenic factors expressed in response to hypoxia [25]. Insufficient response of the myocardium to angiogenesis under ischaemia may lead to poor collateral circulation. In addition, increased production of reactive oxygen species (ROS) and persistent endothelial dysfunction are also the potential mechanisms of the poor collateral development in patients with IR [26, 27]. Besides, when insulin resistance occurs in adipose tissue, the level of circulating free fatty acids (FFAs) increases. A high concentration of FFAs increases TG-rich very-low-density lipoprotein (VLDL), which leads to the clearance of high-density lipoprotein (HDL) and reduces its concentration [28, 29]. All of these changes contributed to atherogenic dyslipidaemia and plague progression caused by insulin resistance. A clinical study demonstrated that IR is associated with poor collateral vessel formation [30]. A high TyG index is also reported to be strongly associated with poor coronary collateralization in CTO patients [23]. However, well-developed collateral circulation of the coronary artery can improve the survival and prognosis of patients with coronary artery disease [31, 32]. These results indicated that IR, which is represented by the TyG index, may play a key role in the development of collateral circulation and could explain why a high TyG index could predict cardiovascular events in CTO patients.
In the sensitivity analysis, the TyG index appeared to be significantly correlated with cardiovascular risk only in patients who received successful CTO PCI but not in patients whose CTO lesion was not opened successfully. We speculated that several reasons might be responsible for this. In our opinion, the main reason for the result of the sensitivity analysis was the insufficient sample size of the failed CTO PCI subgroup. In addition, stents were implanted in most of the patients with successful CTO PCI but not in patients with failed PCI. Studies have shown that the TyG index appears to be associated with cardiovascular events in patients who undergo PCI [12, 33]. Furthermore, failed PCI may have damaged a significant amount of collateral circulation during the opening of the occluded lesion, and this may obscure the relationship between the TyG index and collateral circulation, as shown in a previous study [23]. Similarly, the TyG index only appeared to be associated with cardiovascular risk in nondiabetes patients. Some previous studies have indicated that the TyG index could predict cardiovascular events effectively both in patients with and without DM [33–35], but a study has found similar associations in diabetic populations but not in nondiabetic populations [36]. In this study, we assumed that this was mainly because the insufficient sample size in the DM group led to decreased test efficiency. To resolve this problem, larger randomized controlled trials are needed in the future to verify this study.
The current study has several limitations that need to be considered. First, the sample size and qualities were limited because of the single-centre retrospective study design. Second, we only included the baseline of the TyG index and the change in the TyG index in the follow-up period was missed, which may have an impact on the prognosis [37]. Third, although we adjusted the models according to the use of antilipemic and antidiabetic drugs, the results may be biased because we did not account for the type, intensity, and changes of these drugs. Finally, the present results may not be applicable to other ethnic groups because the participants in our study were only Chinese.
Conclusion
The study indicated that the TyG index has significant relevance to cardiovascular risk in CTO patients and suggested that the TyG index may be a reliable tool to predict cardiovascular events in CTO patients. The findings of our study extend our understanding of the TyG index as a tool for cardiovascular events, especially for patients with CTO lesions.
Acknowledgements
Not applicable.
Abbreviations
- ACS
Acute coronary syndrome
- AMI
Acute myocardial infarction
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CTO
Chronic total occlusion
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- FBG
Fasting blood glucose
- FFA
Free fatty acids
- HDL
High-density lipoprotein
- HIEC
Hyper insulinemic-euglycemic glucose clamp
- HR
Hazard ratio
- IR
Insulin resistance
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- LDL-C
Low-density lipoprotein cholesterol
- LM
Left main coronary artery
- MACCEs
Major adverse cardiovascular and cerebral events
- MI
Myocardial infarction
- NSTEMI
Non-ST-elevation myocardial infarction
- NYHA
New York Heart Association
- PCI
Percutaneous coronary intervention
- RCA
Right coronary artery
- RCS
Restricted cubic spline
- ROS
Reactive oxygen species
- SBP
Systolic blood pressure
- SD
Standard deviation
- STEMI
ST-elevation myocardial infarction
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TG
Triglyceride
- TIMI
Thrombolysis in myocardial infarction
- TyG index
Triglyceride-glucose index
- VEGF
Vascular endothelial growth factor
- VLDL
Very-low-density lipoprotein
Author contributions
YL: designed the study, analyzed the data and wrote the article; JL: substantively revised manuscript. All authors contributed to collecting and analyzing data. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundations of China (No. 81970291, No. 82170344), the Major State Basic Research Development Program of China (No. 2015CB554404).
Availability of data and materials
The data used in the current study are available on reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
The study scheme was approved by the institutional review board of Beijing Anzhen Hospital of Capital Medical University with a waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vanhaverbeke M, Eertmans W, Holvoet W, Hendrickx I, McCutcheon K, Dubois C, et al. Contemporary strategies and outcomes of dedicated chronic total occlusion percutaneous coronary intervention programs: a prospective multicentre registry. J Interv Cardiol. 2021;2021:8042633. doi: 10.1155/2021/8042633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu EB, Tsuchikane E, Ge L, Harding SA, Lo S, Lim ST, et al. Retrograde versus antegrade approach for coronary chronic total occlusion in an algorithm-driven contemporary asia-pacific multicentre registry: comparison of outcomes. Heart Lung Circ. 2020;29(6):894–903. doi: 10.1016/j.hlc.2019.05.188. [DOI] [PubMed] [Google Scholar]
- 3.Hirai T, Grantham JA, Sapontis J, Cohen DJ, Marso SP, Lombardi W, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12(3):e007558. doi: 10.1161/CIRCINTERVENTIONS.118.007558. [DOI] [PubMed] [Google Scholar]
- 4.Park TK, Lee SH, Choi KH, Lee JM, Yang JH, Song YB, et al. Late survival benefit of percutaneous coronary intervention compared with medical therapy in patients with coronary chronic total occlusion: a 10-year follow-up study. J Am Heart Assoc. 2021;10(6):e019022. doi: 10.1161/JAHA.120.019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong S, Lee JH. The verification of the reliability of a triglyceride-glucose index and its availability as an advanced tool. Metabolomics. 2021;17(11):97. doi: 10.1007/s11306-021-01837-9. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. 2020;2020:4678526. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. 2021;20(1):82. doi: 10.1186/s12933-021-01274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604. doi: 10.1016/j.numecd.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46. doi: 10.1186/s12933-021-01238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19. doi: 10.1186/s12933-020-01210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo JW, Duan WH, Yu YQ, Song L, Shi DZ. Prognostic significance of triglyceride-glucose index for adverse cardiovascular events in patients with coronary artery disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:774781. doi: 10.3389/fcvm.2021.774781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. 2020;19(1):116. doi: 10.1186/s12933-020-01091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 15.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 16.Ybarra LF, Rinfret S, Brilakis ES, Karmpaliotis D, Azzalini L, Grantham JA, et al. Definitions and clinical trial design principles for coronary artery chronic total occlusion therapies: CTO-ARC consensus recommendations. Circulation. 2021;143(5):479–500. doi: 10.1161/CIRCULATIONAHA.120.046754. [DOI] [PubMed] [Google Scholar]
- 17.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 18.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 20.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao S, Ma W, Huang S, Lin X, Yu M. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab Cardiovasc Dis. 2021;31(11):3184–3192. doi: 10.1016/j.numecd.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao A, Liu J, Hu C, Liu Y, Zhu Y, Han H, et al. Association between the triglyceride glucose index and coronary collateralization in coronary artery disease patients with chronic total occlusion lesions. Lipids Health Dis. 2021;20(1):140. doi: 10.1186/s12944-021-01574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Z, Zhang L, Liu Z, He P, Yang Y, Wulasihan M. Prognostic value of glucose metabolism for non-ST-segment elevation infarction patients with diabetes mellitus and single concomitant chronic total occlusion following primary percutaneous coronary intervention. Medicine (Baltimore) 2017;96(45):e8362. doi: 10.1097/MD.0000000000008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 26.Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18(1):1. doi: 10.1007/s11906-015-0615-4. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, et al. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161–164. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 28.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 29.Murakami T, Michelagnoli S, Longhi R, Gianfranceschi G, Pazzucconi F, Calabresi L, et al. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler Thromb Vasc Biol. 1995;15(11):1819–1828. doi: 10.1161/01.ATV.15.11.1819. [DOI] [PubMed] [Google Scholar]
- 30.Mouquet F, Cuilleret F, Susen S, Sautière K, Marboeuf P, Ennezat PV, et al. Metabolic syndrome and collateral vessel formation in patients with documented occluded coronary arteries: association with hyperglycaemia, insulin-resistance, adiponectin and plasminogen activator inhibitor-1. Eur Heart J. 2009;30(7):840–849. doi: 10.1093/eurheartj/ehn569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias J, Hoebers LPC, van Dongen IM, Claessen B, Henriques JPS. Impact of collateral circulation on survival in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention with a concomitant chronic total occlusion. JACC Cardiovasc Interv. 2017;10:906–914. doi: 10.1016/j.jcin.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Hara M, Sakata Y, Nakatani D, Suna S, Nishino M, Sato H, et al. Impact of coronary collaterals on in-hospital and 5-year mortality after ST-elevation myocardial infarction in the contemporary percutaneous coronary intervention era: a prospective observational study. BMJ Open. 2016;6:e011105. doi: 10.1136/bmjopen-2016-011105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride-glucose index as a surrogate marker of insulin resistance for predicting cardiovascular outcomes in nondiabetic patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2021;28(11):1175–1194. doi: 10.5551/jat.59840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124. doi: 10.1186/s12933-022-01546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Tang YD, Zheng Y, Li C, Zhou Q, Gao J, Meng X, Zhang K, Wang W, Shao C. The impact of the triglyceride-glucose index on poor prognosis in nondiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol (Lausanne) 2021;12:710240. doi: 10.3389/fendo.2021.710240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui H, Liu Q, Wu Y, Cao L. Cumulative triglyceride-glucose index is a risk for CVD: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):22. doi: 10.1186/s12933-022-01456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the current study are available on reasonable request from the corresponding author.