Abstract
The newly isolated bacterial strain GP1 can utilize 1,2-dibromoethane as the sole carbon and energy source. On the basis of 16S rRNA gene sequence analysis, the organism was identified as a member of the subgroup which contains the fast-growing mycobacteria. The first step in 1,2-dibromoethane metabolism is catalyzed by a hydrolytic haloalkane dehalogenase. The resulting 2-bromoethanol is rapidly converted to ethylene oxide by a haloalcohol dehalogenase, in this way preventing the accumulation of 2-bromoethanol and 2-bromoacetaldehyde as toxic intermediates. Ethylene oxide can serve as a growth substrate for strain GP1, but the pathway(s) by which it is further metabolized is still unclear. Strain GP1 can also utilize 1-chloropropane, 1-bromopropane, 2-bromoethanol, and 2-chloroethanol as growth substrates. 2-Chloroethanol and 2-bromoethanol are metabolized via ethylene oxide, which for both haloalcohols is a novel way to remove the halide without going through the corresponding acetaldehyde intermediate. The haloalkane dehalogenase gene was cloned and sequenced. The dehalogenase (DhaAf) encoded by this gene is identical to the haloalkane dehalogenase (DhaA) of Rhodococcus rhodochrous NCIMB 13064, except for three amino acid substitutions and a 14-amino-acid extension at the C terminus. Alignments of the complete dehalogenase gene region of strain GP1 with DNA sequences in different databases showed that a large part of a dhaA gene region, which is also present in R. rhodochrous NCIMB 13064, was fused to a fragment of a haloalcohol dehalogenase gene that was identical to the last 42 nucleotides of the hheB gene found in Corynebacterium sp. strain N-1074.
1,2-Dibromoethane is a synthetic organic chemical that was used primarily in an antiknock additive to gasoline. It is also one of the most effective and widely used pesticidal soil fumigants. Reduction in the use of leaded gasoline since the late 1970s and of 1,2-dibromoethane for agricultural applications in the late 1980s, owing to its cancer-causing potential and its detection in groundwater supplies, has reduced human exposure to this extremely toxic xenobiotic. However, it is still produced in large amounts for use as a lead scavenger in some countries; as a fumigant for stored grain; as a solvent for resins, gums, and waxes; and as an intermediate in the synthesis of dyes and pharmaceuticals (1).
Many years after its last known application as a soil fumigant, residual 1,2-dibromoethane is still found at remarkably high concentrations in soil because it strongly interacts with the soil matrix (37). 1,2-Dibromoethane can slowly leach from such contaminated soils to groundwater over exceedingly long periods, and because of its slow chemical conversion in aqueous milieu, it is a continuous source of contamination of water supplies.
For a better understanding of the fate and persistence of 1,2-dibromoethane in the environment and for the development of bioremediation techniques for the cleanup of polluted locations, it is important to study the physiology and ecology of bacteria that degrade this toxic compound. Although biodegradation of 1,2-dibromoethane in soil under aerobic and anaerobic conditions was demonstrated by different researchers (5, 7, 8, 26, 27, 37), little is known about the biology of the bacteria that catalyze these reactions, because attempts to obtain pure cultures of bacteria that can metabolize 1,2-dibromoethane have been unsuccessful up to now.
The first report concerning the enrichment and isolation of 1,2-dibromoethane-degrading organisms was published recently (14). In this report, Freitas dos Santos et al. described the enrichment of a mixed bacterial culture capable of complete aerobic mineralization of 1,2-dibromoethane. Here we describe the isolation of a pure bacterial culture that can utilize 1,2-dibromoethane as a sole carbon and energy source. It was obtained by using the mixed culture described by Freitas dos Santos et al. (14) as an inoculum in our further isolation experiments. The results demonstrate that the newly isolated organism belongs to the genus Mycobacterium and metabolizes 1,2-dibromoethane via ethylene oxide by the sequential action of a hydrolytic haloalkane dehalogenase and a haloalcohol dehalogenase. The haloalkane dehalogenase gene was isolated from a cosmid library, and its nucleotide sequence and deduced amino acid sequence were compared with sequences in different DNA and protein databases.
MATERIALS AND METHODS
Chemicals and enzymes.
All halogenated compounds were supplied by Acros Organics (Geel, Belgium) and were at least 97% pure according to the manufacturer. Ethylene oxide was obtained from Hoek Loos (Schiedam, The Netherlands). Restriction enzymes, T4 DNA ligase, Taq DNA polymerase, standard Taq amplification buffer, the DNA-packaging kit, and chemicals used for PCR amplification were purchased from Boehringer (Mannheim, Germany).
Bacterial strains, plasmids, and growth conditions.
Strain GP1 was isolated from the mixed bacterial culture, capable of aerobic degradation of 1,2-dibromoethane, described by Freitas dos Santos et al. (14). Samples of the mixed culture (5%, vol/vol) were transferred to mineral medium (MMY) supplemented with 2 mM 1,2-dibromoethane. Prolonged batch enrichment was carried out without shaking at room temperature, after which organisms were isolated on MMY agar plates that were incubated with 7.5 μl of 1,2-dibromoethane on a filter in the lid of each petri dish. After 5 and 10 days of incubation at 30°C, 7.5 μl of 1,2-dibromoethane was again added to each filter.
MMY contained (per liter) 5.4 g of Na2HPO4 · 12H2O, 1.4 g of KH2PO4, 0.5 g of (NH4)2SO4, 0.2 g of MgSO4 · 7H2O, 10 mg of yeast extract, and 5 ml of salts solution (16). Carbon sources were added up to 5 mM, calculated as if the compounds added were completely dissolved in the water phase. Cells of strain GP1 were grown in serum flasks that were filled to one-fifth of their volume and were closed gas-tight with Teflon-lined screw caps to prevent evaporation of volatile substrates. The cultures were incubated at 25°C under rotary shaking, and growth was monitored turbidimetrically at 450 nm.
Yeast extract-peptone-dextrose (YEPD) agar (containing 1% yeast extract, 2% peptone, 2% glucose, and 2% agar) and MMY supplemented with 5 mM n-propanol were used as multipurpose growth media for strain GP1. Escherichia coli JM101 and plasmid pCR2.1 (Invitrogen, Leek, The Netherlands) were used for cloning of PCR products. E. coli HB101 was used as the recipient in transduction experiments. Plasmids pLAFR3 (36) and pBluescript SK− (Stratagene, Leusden, The Netherlands) were used as cloning vectors. E. coli strains were grown at 37°C in Luria-Bertani (LB) medium (32). When required, Difco agar (15 g/liter) was added to the medium. LBZ medium, used for qualitative dehalogenase activity determination, was solid LB medium without NaCl. Ampicillin (100 μg/ml) and tetracycline (12.5 μg/ml) were added to the medium for detection of recombinant plasmids.
Crude extracts and enzyme assays.
Cells of strain GP1 were harvested in the late exponential growth phase by centrifugation (10 min at 10,000 × g), washed with 1 volume of 50 mM Tris-sulfate buffer (pH 8.2), and disrupted at 4°C in an appropriate amount of this buffer by sonication (15 s per ml of suspension at a 70-W output in a Vibra cell sonicator). A crude extract was obtained by centrifugation (45 min at 16,000 × g).
Haloalkane and haloalcohol dehalogenase activities were measured by incubating an appropriate amount of cell extract with 3 ml of 5 mM substrate in 50 mM Tris-sulfate buffer (pH 8.2) at 30°C. Halide liberation was monitored colorimetrically as described previously (19). All dehalogenase activities are expressed as units per miligram; 1 U was defined as the amount of enzyme required to produce 1 μmol of halide per min. Protein concentrations were estimated with Coomassie brilliant blue by using bovine serum albumin as the standard. Most enzyme assays were carried out twice, and the differences in specific activity were less than 10%.
Epoxide hydrolase and epoxide carboxylase activities were measured by monitoring the time-dependent depletion of ethylene oxide by gas chromatography. Cell extracts were prepared in 50 mM Tris-sulfate buffer (pH 8.2) containing 10% glycerol. Assays were performed in sealed 30-ml flasks by mixing 1 ml of cell extract (3 mg of total protein) with 0.2 mM substrate in 50 mM Tris-sulfate buffer (pH 8.2). For epoxide carboxylase assays, the reagents and reaction conditions were those described previously (2, 3).
Coenzyme A (CoA)-dependent conversion of ethylene oxide by cell extracts was also measured by monitoring the time-dependent depletion of ethylene oxide by gas chromatography. Cell extracts were prepared in 50 mM sodium phosphate buffer (pH 7.2) containing 2 mM cysteine. Assays were performed in sealed 30-ml flasks by mixing 1 ml of cell extract with 0.2 mM substrate in 50 mM Tris-sulfate buffer (pH 8.2), with reagents and reaction conditions as described previously (10). Chemical hydrolysis of ethylene oxide was negligible under the conditions used.
Gas chromatography.
Ethylene oxide, 2-bromoethanol, and 2-chloroethanol were analyzed by capillary gas chromatography. Samples (1 ml) were extracted with 1 ml of diethyl ether containing 0.05 mM 1-bromohexane or 1-chlorohexane as an internal standard. Extracts were analyzed by split injection of 4-μl samples into a type HP-5 column (model HP 19091J-413; Hewlett-Packard) with helium as the carrier gas. The column was installed in a model 6890 gas chromatograph (Hewlett-Packard) equipped with a flame ionization detector. The oven was temperature programmed as follows: 3 min isothermal at 30°C followed by an increase at 10°C/min to 120°C. The retention times for ethylene oxide, 2-bromoethanol, and 2-chloroethanol were 2.2, 5.9, and 4.2 min, respectively.
Haloalkanes and (halo)alcohols were analyzed by capillary gas chromatography as described previously (34).
Construction and screening of a genomic library.
General procedures for cloning and DNA manipulation were performed essentially as described by Sambrook et al. (32). Total genomic DNA was isolated from 1-propanol-grown cells of strain GP1 by a previously described procedure (29). A partial Sau3A DNA genomic library of strain GP1 was constructed in the cosmid vector pLAFR3. Cosmid cloning was performed by the strategy described by Staskawicz et al. (36). The vector was isolated from E. coli HB101 by the alkali lysis method and was purified by cesium chloride gradient centrifugation (32). Ligation mixtures were packaged in vitro with a DNA-packaging kit. E. coli HB101 was transduced with these packaging mixtures (32), and colonies were selected on LBZ agar plates containing tetracycline.
Restriction analysis of plasmids isolated from transduced HB101 clones showed that 8 of 10 clones tested had plasmids with inserts. Tetracycline-resistant colonies were screened for dehalogenase activity by monitoring halide production upon incubation with halogenated compounds (19). For this, a small amount of cells were incubated in a microtiter plate with 150 μl of a mixture of 5 mM 1,2-dibromoethane and 2-bromoethanol in 50 mM Tris-sulfate buffer (pH 8.2). After overnight incubation of the plate at 30°C, 100 μl of 0.25 M NH4Fe(SO4)2 in 6 M HNO3 followed by a drop of saturated Hg(SCN)2 in ethanol were added. A red color indicated the presence of dehalogenase activity.
Subcloning of the haloalkane dehalogenase gene.
Cosmid pGP1-4B5, containing the haloalkane dehalogenase gene, was digested with PstI, and its fragments were ligated into the PstI site of pBluescript SK−. The ligation mixture was used to transform CaCl2-competent cells of E. coli JM101, and transformants were plated on LBZ plates containing ampicillin (100 μg/ml), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 40 μg/ml), and isopropyl-β-d-thiogalactopyranoside (IPTG; 0.4 mM). Ampicillin-resistant white colonies displaying haloalkane dehalogenase activity with 1,2-dibromoethane were selected. Plasmid DNA (pBS4E11) of one of these colonies was isolated, and the complete 1.7-kb PstI insert was sequenced.
Cloning of the 16S rRNA gene.
To clone the 16S rRNA gene of strain GP1, biomass of a single colony was directly used for PCR amplification. The synthetic oligonucleotide primers used were described by Marchesi et al. (22): 63f, CAGGCCTAACACATGCAAGTC-3′; 1387r, 5′-GGGCGG(A/T)GTGTACAAGGC-3′ (numbering based on the E. coli 16S rRNA gene [9]). The amplification reaction mixture contained standard Taq amplification buffer, 250 μM (each) deoxyribonucleotide triphosphate, 0.5 μM (each) primer, biomass of strain GP1, and 2.5 U of Taq DNA polymerase. The cycling parameters were 95°C for 10 min followed by 30 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 60 s, with a final elongation step of 72°C for 7 min.
PCR products were cloned into plasmid pCR2.1 as specified by the manufacturer (Invitrogen). The ligation mixture was used to transform CaCl2-competent cells of E. coli JM101, and ampicillin-resistant white colonies were selected from X-Gal plates. Plasmid DNA was isolated and checked by restriction analysis.
Nucleotide sequencing.
Inserts of pBluescript SK− and pCR2.1 were cycle sequenced with the Amersham Thermo Sequenase cycle-sequencing kit with 7-deaza-dGTP and Cy5 labelled fluorescent primers. Sequencing reaction mixtures were run on the Pharmacia ALF-Express automatic sequencing machine. Both strands were sequenced to ensure accuracy.
Phylogenetic analysis.
The 16S rRNA gene sequence of strain GP1 was compared with those in the GenBank database (6) by using FASTA3 (25) and with those in the Ribosomal Database Project by using the SIMILARITY RANK program (21), to determine its most similar sequences. Similar 16S rRNA gene sequences were downloaded and aligned by using CLUSTALW (38). Evolutionary distances were calculated by using the Jukes-Cantor algorithm (18), and the phylogenetic tree was determined by the neighbor-joining method (33) with TREECON for Windows (40). A sequence from Rhodococcus rhodochrous was used as the outgroup. Tree topologies were also compared between trees constructed by the methods of maximum likelihood and maximum parsimony by using PHYLIP version 3.5c (13) and the neighbor-joining method. Bootstrap analysis (12) of up to 500 replicates was performed on the phylogeny.
The secondary structure of the 16S rRNA molecule was analyzed with RNAVIZ (11) and the secondary-structure information at the small-subunit rRNA database at Antwerp (41) and was used to manually edit the alignment.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequence and the sequence of the haloalkane dehalogenase gene region of strain GP1 have been deposited at the EMBL Nucleotide Sequence Database (accession no. AJ012626 and AJ012627, respectively).
RESULTS
Isolation and characterization of strain GP1.
Recently, Freitas dos Santos et al. (14) described aerobic mineralization of 1,2-dibromoethane by a mixed bacterial culture. To isolate a pure culture capable of growth with 1,2-dibromoethane as the sole carbon and energy source, samples from this mixed culture were transferred to flasks containing MMY and scored for growth on 2 mM 1,2-dibromoethane. Initially, it took 2 weeks before growth on 1,2-dibromoethane was observed. This period was reduced to 1 day during repeated subculturing over a 12-month period, after which cells were streaked on MMY-agar plates incubated in the presence of 1,2-dibromoethane in air. Individual colonies were restreaked on the same medium and tested for growth on 1,2-dibromoethane in liquid cultures. In this way, a pure culture of strain GP1 was obtained.
Strain GP1 was a gram-positive, nonmotile, oxidase-negative, and catalase-positive rod with a yellow pigmentation. The organism showed optimal growth on YEPD agar at 25 to 30°C but no growth at 37 or 45°C. Incubations at 30°C yielded visible colonies after 36 to 48 h. No growth was observed on LB medium, and growth on nutrient broth and brain heart infusion medium was very poor.
Strain GP1 was able to grow on 1,2-dibromoethane, both in liquid cultures and on plates. The organism could be maintained on MMY-propanol plates for 10 serial transfers without loss of its 1,2-dibromoethane degrading capacity. Apart from halogenated compounds, a number of organic chemicals could also support growth: ethanol, 1-propanol, 1-butanol, 1-hexanol, glycerol, pyruvate, glucose, fructose, and ethylene oxide. The latter compound could serve as a growth substrate up to a concentration of at least 2.5 mM. The organism did not utilize methanol, 2-propanol, 2-butanol, ethylene glycol, 1,2-propanediol, 1,3-propanediol, 1,2-butanediol, citrate, galactose, sucrose, maltose, acetone, acetaldehyde, n-pentane, n-hexane, benzene, or toluene.
16S rRNA gene sequence analysis.
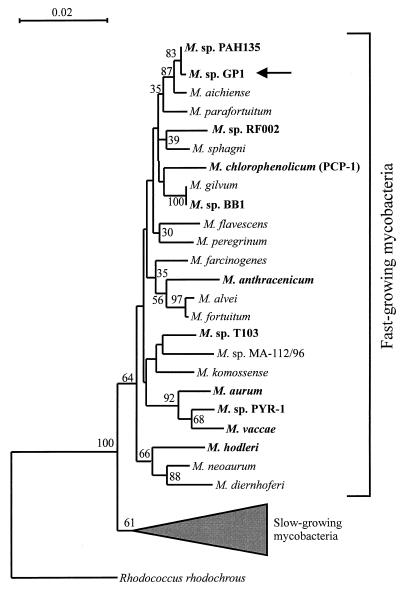
The newly determined sequence of the 1,314-bp DNA segment of the 16S rRNA gene of strain GP1 was compared with other 16S rRNA gene sequences available in the GenBank database and in the Ribosomal Database Project. From this initial screening, it was evident that strain GP1 is a Mycobacterium species. Optimal linear alignment results showed that strain GP1 was most similar (2 bp were different in the 1,314-bp overlap region) to Mycobacterium sp. strain PAH135 (15, 43). The phylogenetic tree for the 16S rRNA gene sequences showed that strain GP1 is a member of the subgroup which contains the fast-growing mycobacteria (Fig. 1). The similarity values between strain GP1 and the fast-growing mycobacteria range from 0.9452 to 0.9985. Comparison of the trees constructed by the method of maximum likelihood and maximum parsimony and by the neighbor-joining method showed the same topology.
FIG. 1.
Phylogenetic tree based on 16S rRNA gene sequence analysis, illustrating the relationships of strain GP1 to the most closely related bacteria. Base positions 54 to 1368 (numbering based on the E. coli 16S rRNA gene) were included in the analysis. The scale bar represents 0.02 fixed mutation per site. Bootstrap values were derived from 500 analyses. All organisms with known biodegradative capabilities are shown in boldface.
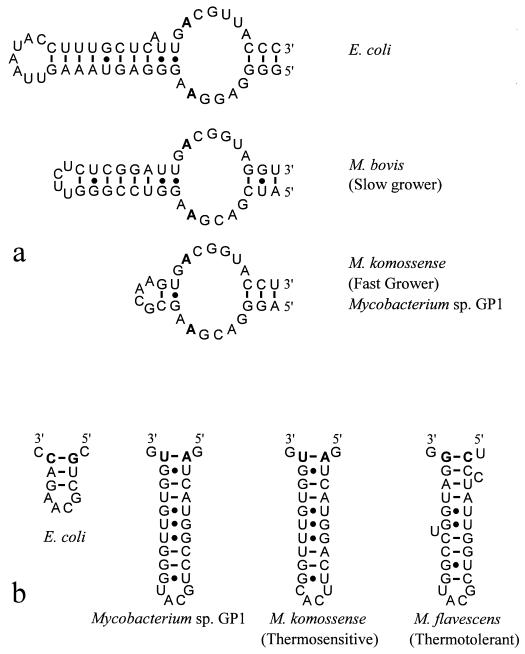
Secondary-structure analysis of 16S rRNA molecules led to the identification of structural signatures, based on the length and sequence variations of two specific 16S rRNA regions, that have been proposed as identifiers for subgroups of mycobacteria (28, 31, 35). The helix flanked by positions 451 to 482 (E. coli numbering) differentiates the fast-growing mycobacteria from the slow-growing mycobacteria. Mycobacterium sp. strain GP1 has a signature identical to that of M. komossense (Fig. 2a), which is a representative fast grower. A second signature flanked by positions 184 to 193 (E. coli numbering) differentiates the thermosensitive strains from the thermotolerant strains among the fast-growing mycobacteria. Mycobacterium sp. strain GP1 shared the signature proposed for thermosensitive strains, which is in agreement with the physiological data obtained for strain GP1. Its signature is similar in secondary structure, but not in primary structure, to M. komossense (Fig. 2b).
FIG. 2.
Comparison of secondary structures of the 16S rRNA molecule. (a) Signatures in the V3 variable region which differentiate between the fast- and slow-growing mycobacteria. Representatives of both classes are shown. Bases 451 and 482 (from the 5′ end) are shown in boldface. (b) Signatures in the V2 variable region which differentiate between the thermotolerant and thermosensitive mycobacteria. Representatives of both classes are shown. Bases 184 and 193 (from the 5′ end) are shown in boldface.
Utilization of halogenated compounds.
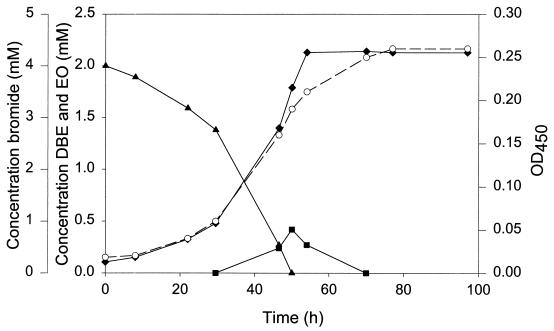
Growth of strain GP1 with 2 mM 1,2-dibromoethane as the substrate was monitored in batch culture (Fig. 3). Growth resulted in disappearance of the substrate and simultaneous formation of biomass and inorganic bromide, with no indication of the accumulation of brominated intermediates. Ethylene oxide, however, transiently accumulated in the medium, indicating that this is an intermediate formed during the degradation of 1,2-dibromoethane. Concentrations of 1,2-dibromoethane above 2.5 mM were toxic for strain GP1 and completely inhibited its growth on pyruvate or fructose.
FIG. 3.
Growth of strain GP1 on 2 mM 1,2-dibromoethane in batch culture. Symbols: ▴, 1,2-dibromoethane (DBE) concentration; ■, ethylene oxide (EO) concentration; ⧫, bromide concentration; ○, optical density at 450 nm (OD450).
We determined whether strain GP1 could utilize halogenated compounds that are structurally related to 1,2-dibromoethane and its possible degradation products. The results showed that besides 1,2-dibromoethane, only a few halogenated compounds support growth (Table 1). 1-Bromopropane, 1-chloropropane, and 2-chloroethanol could serve as growth substrates up to a concentration of at least 5 mM, whereas concentrations of 2-bromoethanol above 1.5 mM were very toxic for strain GP1 and completely inhibited growth. No growth was observed with 1 mM dibromomethane, dichloromethane, diiodomethane, bromochloromethane, bromoform, 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2-trichloroethane, 1,1,2,2-tetrachloroethane, 1,3-dibromopropane, 1,3-dichloropropane, 1,2,3-trichloropropane, 1,2,3-tribromopropane, 1,2-dibromo-3-chloropropane, 2-bromo-1-chloropropane, 1-bromo-3-chloropropane, 1-chlorobutane, 1-chloropentane, 1-chlorohexane, 1-bromohexane, 1-bromodecane, 1,3-dichloropropene, 1,2-dichloroethylene, bromoacetate, chloroacetate, dibromoacetate, dichloroacetate, 2-chloropropionic acid, 3-chloropropionic acid, 3-chloro-1,2-propanediol, 1,3-dichloro-2-propanol, 2,3-dichloro-1-propanol, and 1-chloro-2-propanol.
TABLE 1.
Utilization of halogenated compounds by strain GP1
| Carbon source | Concn (mM) | Generation timeb (h) | Halide productiona (mM) in:
|
|
|---|---|---|---|---|
| Inoculated medium | Sterile control | |||
| Bromopropane | 2 | 42 | 2.1 | 0.1 |
| 1-Chloropropane | 2 | 50 | 2.0 | <0.1 |
| 2-Bromoethanol | 1 | 18 | 1.0 | 0.15 |
| 2-Chloroethanol | 2 | 20 | 2.0 | <0.1 |
Halide levels were determined after 2 weeks of cultivation in liquid MMY at 25°C.
Generation times were determined after inoculation (1%, vol/vol) of fresh medium with cells from a culture grown on the same carbon source.
Metabolism of 1,2-dibromoethane.
Activities of enzymes that may be involved in 1,2-dibromoethane metabolism were tested with crude extracts prepared from cells grown on either 1,2-dibromoethane or 1-propanol (Table 2). Extract of 1,2-dibromoethane-grown cells converted 1,2-dibromoethane, bromochloroethane, 1-chloropropane, and 1-bromobutane to the corresponding monoalcohols and halide ions, which indicates that dehalogenation of haloalkanes is a hydrolytic reaction in this organism. There was no significant difference in haloalkane dehalogenase activities in extracts prepared from 1-propanol-grown cells and those in extracts prepared from 1,2-dibromoethane-grown cells, indicating that expression of the haloalkane dehalogenase is constitutive and independent of the growth substrate used. The highest level of haloalkane dehalogenase activity was observed with 1,2-dibromoethane, whereas low dehalogenase activity (less than 30 mU/mg) was measured with the analog 1,2-dichloroethane.
TABLE 2.
Dehalogenase activities in crude extracts from strain GP1
| Substrate | Dehalogenase sp acta (mU/mg of protein)
|
Product | |
|---|---|---|---|
| 1,2-DBE | 1-Propanol | ||
| 1,2-Dibromoethane | 220 | 230 | 2-Bromoethanol |
| 1,2-Dichloroethane | <30 | <30 | |
| Bromochloroethane | 180 | NDb | 2-Chloroethanol |
| 1-Chloropropane | 50 | ND | 1-Propanol |
| 1-Bromobutane | 30 | ND | 1-Butanol |
| 1,3-Dibromopropane | 80 | 80 | ND |
| 2-Bromoethanol | 5,200 | 8,110 | Ethylene oxide |
| 2-Chloroethanol | 70 | 110 | Ethylene oxide |
| 3-Chloro-1,2-propanediol | ND | 300 | ND |
| 2,3-Dichloro-1-propanol | ND | <30 | |
| 1,3-Dichloro-2-propanol | ND | 5,220 | Epichlorohydrin |
| 1-Chloro-2-propanol | ND | 3,260 | Propylene oxide |
| Bromoacetate | <30 | <30 | |
| Chloroacetate | <30 | <30 | |
| Dibromoacetate | <30 | <30 | |
| Dichloroacetate | <30 | <30 | |
Specific activities with various substrates (5 mM) were determined with extracts prepared from cells grown with either 1,2-dibromoethane or 1-propanol.
ND, not determined.
Conversion of the hydrolytic product of 1,2-dibromoethane, 2-bromoethanol, was initially expected to proceed via two oxidation steps to bromoacetate, which may then be dehalogenated by a haloacid dehalogenase. This degradation route is known to occur in 1,2-dichloroethane-degrading Xanthobacter autotrophicus and Ancylobacter aquaticus strains (17). However, extracts of 1,2-dibromoethane- or 1-propanol-grown cells showed no dehalogenase activity toward bromoacetate or other halogenated carboxylic acids tested (Table 2).
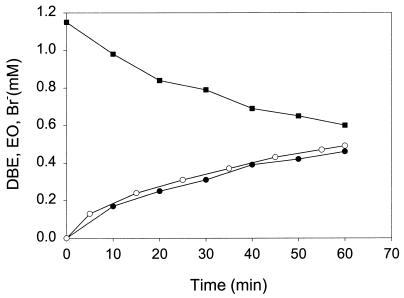
Surprisingly, rapid halide production was observed upon incubation of extracts with 2-bromoethanol and several other haloalcohols tested, indicating that direct dehalogenation of haloalcohols does occur (Table 2). The product formed from 2-bromoethanol (Fig. 4) and 2-chloroethanol was identified as ethylene oxide, whereas 1,3-dichloro-2-propanol and 1-chloro-2-propanol were converted to epichlorohydrin and propylene oxide, respectively. This haloalcohol dehalogenase activity was slightly elevated in extracts prepared from 1-propanol-grown cells compared to that in extracts prepared from 1,2-dibromoethane-grown cells. The highest level of haloalcohol dehalogenase activity was observed with 2-bromoethanol, indicating that this toxic intermediate was rapidly converted to the less toxic ethylene oxide.
FIG. 4.
Dehalogenation of 2-bromoethanol (■) in crude extract of 1,2-dibromoethane (DBE)-grown cells, with the production of ethylene oxide (EO) (●) and inorganic bromide (Br−) (○).
The formation of different products during the conversion of haloalkanes and haloalcohols suggests that at least two different dehalogenating enzymes are present in strain GP1: a hydrolytic haloalkane dehalogenase, which converts haloalkanes to the corresponding (halo)alcohols and halide ions, and a haloalcohol dehalogenase (also called halohydrin hydrogen-halide lyase [23, 24]), which converts haloalcohols to the corresponding epoxides and halide ions. Conversion of haloalkanes and haloalcohols to different products by a single enzyme is unlikely. Enzymes which are involved in hydrolytic conversion of haloalkanes (17, 20, 29) and haloalcohol dehalogenases that show lyase activity toward haloalcohols (23, 39) have been described previously, but strain GP1 is the first organism found to produce both dehalogenating enzymes. Together, these enzymes convert 1,2-dibromoethane to ethylene oxide.
Further metabolism of ethylene oxide is likely to proceed via malonate semialdehyde (2, 3) or via acetyl-CoA (10). However, no epoxide carboxylase activity or CoA- and NAD+-dependent conversion of ethylene oxide could be demonstrated. The possibility that ethylene glycol was an intermediate in ethylene oxide metabolism in strain GP1 is unlikely on the basis of two observations: no epoxide hydrolase activity toward ethylene oxide was detected in cell extracts, and strain GP1 does not utilize ethylene glycol as a growth substrate.
Isolation of dehalogenase genes involved in 1,2-dibromoethane metabolism.
As discussed above, the first step in 1,2-dibromoethane metabolism in strain GP1 appears to be catalyzed by a hydrolytic haloalkane dehalogenase. The activities that we measured in crude extract suggested that this enzyme resembled the haloalkane dehalogenase DhaA that was found in the gram-positive, haloalkane-degrading bacterium R. rhodochrous NCIMB 13064 (20). However, attempts to amplify the haloalkane dehalogenase gene from strain GP1 with primers designed on the 5′ and 3′ ends of the reported sequence of the dhaA gene (20) did not result in an amplification product. These primers were successfully used to amplify the haloalkane dehalogenase gene from Pseudomonas pavonaceae 170 (formerly known as P. cichorii 170), which is 100% identical to the dhaA gene of strain NCIMB 13064 (29). This result indicated that strain GP1 contains a haloalkane dehalogenase that is different from the previously identified DhaA in R. rhodochrous NCIMB 13064 and P. pavonaceae 170.
To identify the haloalkane dehalogenase and to confirm that two different dehalogenating enzymes are involved in 1,2-dibromoethane metabolism, a gene bank of strain GP1 was constructed in the cosmid vector pLAFR3. Cosmid clones were screened for dehalogenase activity with a mixture of 1,2-dibromoethane and 2-bromoethanol, which are the best substrates for the haloalkane and haloalcohol dehalogenase, respectively. Of 1,000 clones tested, 20 dehalogenase-positive clones were found. To determine whether these expressed haloalkane or haloalcohol dehalogenase activity, clones were incubated separately with 1,2-dibromoethane, 2-bromoethanol or 1,3-dichloro-2-propanol. All 20 clones showed halide production upon incubation with 1,2-dibromoethane, whereas none showed dehalogenase activity toward 1,3-dichloro-2-propanol. However, slow halide release was observed upon incubation of the clones with 2-bromoethanol. From these results, we concluded that all 20 dehalogenase-positive clones expressed the haloalkane dehalogenase and that this enzyme also exhibited low dehalogenase activity toward 2-bromoethanol. Hydrolytic conversion of both 1,2-dibromoethane and 2-bromoethanol was also observed for the haloalkane dehalogenase DhaA that we purified from P. pavonaceae 170 (unpublished data). The low dehalogenase activity toward 2-bromoethanol and the fact that the haloalkane dehalogenase clone does not convert 1,3-dichloro-2-propanol is in agreement with the presence in strain GP1 of a second dehalogenase, which rapidly converts 2-bromoethanol and other haloalcohols to the corresponding epoxides. To exclude the possibility that haloalcohol dehalogenase activity was not found in the initial screening because of inhibition of the enzyme by 1,2-dibromoethane, the complete gene library was also screened for haloalcohol dehalogenase activity with 1,3-dichloro-2-propanol. E. coli HB101 clones that expressed the haloalcohol dehalogenase were also not found in this screening.
Sequence of the haloalkane dehalogenase region.
Plasmid pGP1-4B5 encoding haloalkane dehalogenase activity was isolated from an HB101 clone. PstI fragments of this plasmid were ligated into pBluescript SK−, and the ligation mixture was used to transform E. coli JM101. By using a colony assay, transformants could be tested quickly for the presence of the dehalogenase gene. Three dehalogenase-positive transformants were selected, and the corresponding plasmids all contained a single 1.75-kb PstI insert. One such plasmid (pBS4E11) was used for sequencing of the dehalogenase gene.
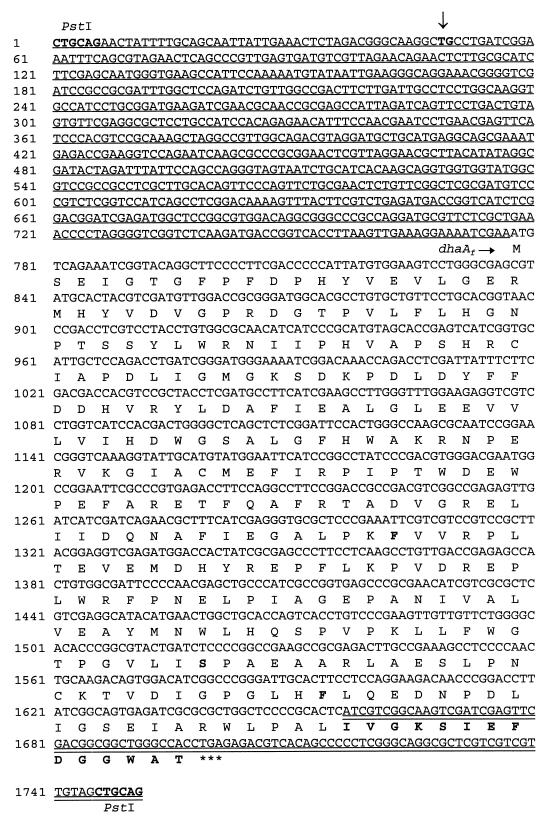
The nucleotide sequence of the entire 1.75-kb PstI fragment was determined independently in both directions (Fig. 5). An open reading frame coding for a protein of 307 amino acids was found. A search with the BLASTP program (4) in different protein databases showed that the first 293 amino acids of the deduced amino acid sequence were identical to the haloalkane dehalogenase DhaA from R. rhodochrous NCIMB 13064 (20), except for three amino acid substitutions (Fig. 5), whereas the last 14 amino acids were identical to the C-terminal sequence of the haloalcohol dehalogenase HheB (halohydrin hydrogen-halide [H]-lyase B) from Corynebacterium sp. strain N-1074 (44).
FIG. 5.
Complete nucleotide sequence of the 1.75-kb PstI fragment containing the haloalkane dehalogenase gene. The deduced amino acid sequence of the dehalogenase open reading frame is indicated below the corresponding DNA sequence. The three amino acid substitutions and the 14-amino-acid extension at the C terminus, which are the differences compared to the DhaA amino acid sequence, are shown in boldface. The sequence upstream of dhaAf identical to the sequence upstream of the dhaA gene in R. rhodochrous NCIMB 13064 is underlined. The position of the deletion of 12 nucleotides, compared to the dhaA gene region in strain NCIMB 13064, between nucleotides 49 (T) and 50 (G) is indicated by an arrow. The sequence identical to the hheB gene region in Corynebacterium sp. strain N-1074 is indicated by a double underline.
To examine the apparent fusion between DhaA- and HheB-encoding sequences in more detail, the complete sequence of the haloalkane dehalogenase gene region was compared with DNA sequences in different databases by using the BLASTN search program (4). The results of this search showed that a large segment of the dhaA gene region, which is also present in R. rhodochrous NCIMB 13064 (accession no. AF060871, AF017179, and L49435), was fused to a fragment of the haloalcohol dehalogenase gene hheB (accession no. D90350), which was first found in Corynebacterium sp. strain N-1074. The fusion of the dhaA gene to the last 42 nucleotides of the hheB gene gave rise to a new open reading frame coding for a novel dehalogenase of 307 amino acids. This haloalkane dehalogenase was named DhaAf. The sequence upstream of the haloalkane dehalogenase gene (dhaAf) from strain GP1 was identical to the sequence upstream of the dhaA gene from R. rhodochrous NCIMB 13064 except for a small deletion of 12 nucleotides (Fig. 5). The sequence downstream of dhaAf was 100% identical to the reported sequence downstream of hheB (44). To eliminate the possibility that the fusion of the dhaA gene region to the hheB gene occurred upon (sub)cloning, the fusion was confirmed by PCR analysis with total DNA of strain GP1 as the template (results not shown).
DISCUSSION
The fate and persistence of many pesticidal soil fumigants in the environment is largely dependent on the ability of microorganisms to metabolize these compounds. Although slow biodegradation of the soil fumigant and priority pollutant 1,2-dibromoethane by soil bacteria has been observed (5, 7, 8, 26, 27, 37), little is known about the intermediates in the process and the enzymes involved in biodegradation. In this paper, we describe the properties of the newly isolated strain GP1, which is capable of aerobic degradation and utilization of 1,2-dibromoethane. To our knowledge, this is the first report that describes the utilization of 1,2-dibromoethane as a growth substrate by a pure bacterial culture.
We were able to isolate strain GP1 from a mixed bacterial culture capable of aerobic mineralization of 1,2-dibromoethane (14) by prolonged batch enrichment. Attempts to isolate single 1,2-dibromoethane-degrading organisms from the mixed culture, without any adaptation procedure, were unsuccessful. The long period of selection (15 to 20 subcultivations) necessary to obtain a pure culture of strain GP1 suggests that this strain obtained or improved its 1,2-dibromoethane-degrading capacity during batch enrichment.
Strain GP1 was identified as a member of the subgroup which contains the thermosensitive, fast-growing mycobacteria. The physiological data obtained for strain GP1 and its fatty acid profile (LMG culture collection, Gent, Belgium [data not shown]) support this identification. Strain GP1 is the first member of the genus Mycobacterium that is capable of degrading short-chain haloalkanes.
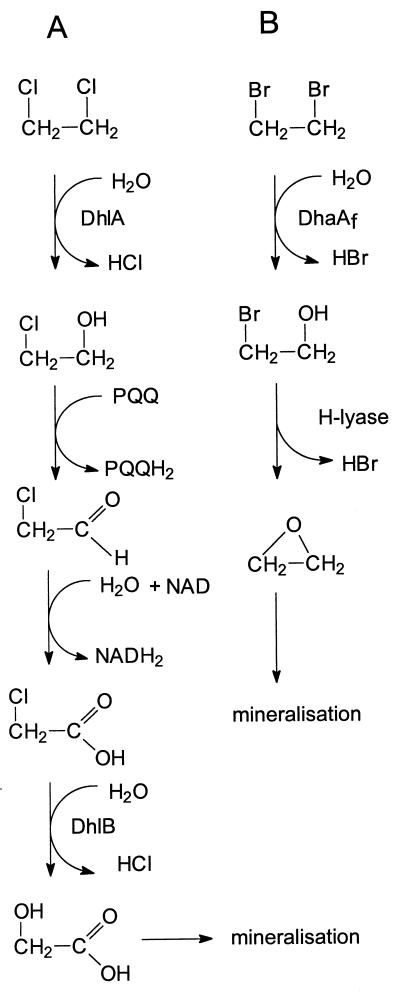
1,2-Dibromoethane is an excellent substrate for the haloalkane dehalogenase (DhlA) present in the 1,2-dichloroethane-degrading Xanthobacter autotrophicus and Ancylobacter aquaticus strains (17). The resulting 2-bromoethanol is expected to be oxidized in two steps to bromoacetate, which can be rapidly hydrolyzed by a haloacid dehalogenase (Fig. 6). However, these organisms cannot utilize 1,2-dibromoethane, because both 1,2-dibromoethane and 2-bromoethanol are toxic for these strains in the micromolar range. The absence of a functional aldehyde dehydrogenase, which results in the accumulation of the highly reactive bromoacetaldehyde, seems to be the cause of the lack of utilization of 1,2-dibromoethane for growth by 1,2-dichloroethane-degrading bacteria (42).
FIG. 6.
(A) Proposed route of the metabolism of 1,2-dichloroethane in X. autotrophicus and A. aquaticus strains. (B) Proposed route of the metabolism of 1,2-dibromoethane in Mycobacterium sp. strain GP1.
Mycobacterium sp. strain GP1 metabolizes 1,2-dibromoethane via ethylene oxide by the sequential action of a hydrolytic haloalkane dehalogenase and a haloalcohol dehalogenase (Fig. 6). The latter enzyme was highly active toward 2-bromoethanol and rapidly converted this intermediate to ethylene oxide. In this way, the organism prevents the accumulation of the toxic intermediates 2-bromoethanol and 2-bromoacetaldehyde, which were shown to be lethal for 1,2-dichloroethane-degrading strains (17, 42). Complete metabolism of 1,2-dibromoethane thus necessitates the sequential use of two dehalogenating enzymes to prevent the formation of toxic brominated intermediates.
The pathway(s) by which the epoxide intermediate, ethylene oxide, is further metabolized in Mycobacterium sp. strain GP1 is still unclear. Well-known strategies for epoxide conversion, such as epoxide carboxylation (2, 3), epoxide hydration (30), or transformation to acetyl-CoA (10), could not be demonstrated for the conversion of ethylene oxide in crude extracts of strain GP1. Hydrolysis of ethylene oxide to ethylene glycol is also unlikely, since strain GP1 does not utilize ethylene glycol as a growth substrate.
Isolation of the haloalkane dehalogenase gene of strain GP1 from a cosmid library was possible by screening recombinant E. coli HB101 clones for dehalogenase activity toward 1,2-dibromoethane. The dehalogenase gene appeared to encode a 307-amino-acid polypeptide, which is the result of a fusion between two known genes which encode dehalogenating enzymes. The first 293 amino acids were identical to the complete haloalkane dehalogenase DhaA (20), except for three amino acid substitutions, whereas the last 14 amino acids of the deduced amino acid sequence were identical to the C-terminal sequence of the haloalcohol dehalogenase HheB from Corynebacterium sp. strain N-1074 (44). Whether the fusion of the dhaA gene region to the hheB gene region, giving rise to a new open reading frame of 307 amino acids, occurred during prolonged enrichment because of the selective advantage provided by the new haloalkane dehalogenase or whether this is coincidental is unclear. The enzymatic activities of DhaAf and DhaA seem very similar, indicating that the three amino acid substitutions and the 14-amino-acid extension have no important influence on dehalogenating capacity. In either case, it is surprising that the haloalkane dehalogenase gene was fused exactly to the hheB gene, which normally encodes an enzyme that could be involved in the metabolism of the compound used for selection. This could reflect a preference for recombination events in the hheB gene region, which is under selective pressure.
The cofactor-independent debromination of 2-bromoethanol in strain GP1 may be catalyzed by an enzyme similar to the haloalcohol dehalogenases (H-lyases) present in Corynebacterium sp. strain N-1074 (HheB) (23, 24) and in Arthrobacter sp. strain AD2 (39). These enzymes also catalyze the conversion of haloalcohols (halohydrins) to the corresponding epoxides and halide ions. However, crude extract of strain GP1 has a very high specific dehalogenase activity toward 2-bromoethanol (5,000 to 8,000 mU/mg of protein), which is comparable to the specific dehalogenase activities measured for the purified haloalcohol dehalogenases from strains N-1074 and AD2 (11,000 and 2,000 mU/mg of protein, respectively). Surprisingly, no abundant protein band was observed when a crude extract of 1,2-dibromoethane-grown cells was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These results suggest that haloalcohol dehalogenase of strain GP1 is capable of degrading 2-bromoethanol much faster than are the dehalogenases found in strains N-1074 and AD2. This is in agreement with the observation that the rapid dehalogenation of 2-bromoethanol is an important step in the metabolism of 1,2-dibromoethane.
ACKNOWLEDGMENTS
This study was supported by the Life Sciences Foundation (SLW), which is subsidized by the Netherlands Organization for Scientific Research (NWO), and by EC Environmental and Climate Research Program contract ENV4-CT95-0086.
REFERENCES
- 1.Alexeeff G V, Kilgore W W, Li M Y. Ethylene dibromide: toxicology and risk assessment. Berlin, Germany: Springer-Verlag KG; 1990. pp. 49–122. [DOI] [PubMed] [Google Scholar]
- 2.Allen J R, Ensign S A. Carboxylation of epoxides to β-keto acids in cell extracts of Xanthobacter strain PY2. J Bacteriol. 1996;178:1469–1472. doi: 10.1128/jb.178.5.1469-1472.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J R, Ensign S A. Identification and characterization of epoxide carboxylase activity in cell extracts of Nocardia corallina B276. J Bacteriol. 1998;180:2072–2078. doi: 10.1128/jb.180.8.2072-2078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belay N, Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987;53:1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson D A, Boguski M S, Lipman D J, Ostell J. GenBank. Nucleic Acids Res. 1997;25:1–6. doi: 10.1093/nar/25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower E J, McCarthy P L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983;45:1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower E J, McCarthy P L. Ethylene dibromide transformations under methanogenic conditions. Appl Environ Microbiol. 1985;50:527–528. doi: 10.1128/aem.50.2.527-528.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosius J, Palmer J L, Kennedy H P, Noller H F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bont J A M, Harder W. Metabolism of ethylene by Mycobacterium E 20. FEMS Microbiol Lett. 1978;3:89–93. [Google Scholar]
- 11.De Rijk P, de Wachter R. RNAVIZ, a program for the visualisation of RNA secondary structure. Nucleic Acids Res. 1997;25:4679–4684. doi: 10.1093/nar/25.22.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein J. PHYLIP (phylogeny inference package) version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 14.Freitas dos Santos L M, Leak D J, Livingston A G. Enrichment of mixed cultures capable of aerobic degradation of 1,2-dibromoethane. Appl Environ Microbiol. 1996;62:4675–4677. doi: 10.1128/aem.62.12.4675-4677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govindaswami M, Feldhake D J, Kinkle B K, Mindell D P, Loper J C. Phylogenetic comparison of two polycyclic aromatic hydrocarbon-degrading mycobacteria. Appl Environ Microbiol. 1995;61:3221–3226. doi: 10.1128/aem.61.9.3221-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen D B, Scheper A, Witholt B. Biodegradation of 2-chloroethanol and 1,2-dichloroethane by pure bacterial cultures. In: Houwink E H, van der Meer R R, editors. Innovations in biotechnology. Amsterdam, The Netherlands: Elsevier; 1984. pp. 169–178. [Google Scholar]
- 17.Janssen D B, van der Ploeg J R, Pries F. Genetics and biochemistry of 1,2-dichloroethane degradation. Biodegradation. 1994;5:249–257. doi: 10.1007/BF00696463. [DOI] [PubMed] [Google Scholar]
- 18.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press, Inc.; 1969. pp. 21–132. [Google Scholar]
- 19.Keuning S, Janssen D B, Witholt B. Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol. 1985;163:635–639. doi: 10.1128/jb.163.2.635-639.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulakova A N, Larkin M J, Kulakov L A. The plasmid-located haloalkane dehalogenase gene from Rhodococcus rhodochrous NCIMB 13064. Microbiology. 1997;143:109–115. doi: 10.1099/00221287-143-1-109. [DOI] [PubMed] [Google Scholar]
- 21.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–111. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchesi J R, Sato T, Weightman A J, Martin T A, Fry J C, Hiom S J, Dymock D, Wade W G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura T, Nagasawa T, Yu F, Watanabe I, Yamada H. Resolution and some properties of enzymes involved in enantioselective transformation of 1,3-dichloro-2-propanol to (R)-3-chloro-1,2-propanediol by Corynebacterium sp. strain N-1074. J Bacteriol. 1992;174:7613–7619. doi: 10.1128/jb.174.23.7613-7619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Nagasawa T, Yu F, Watanabe I, Yamada H. Characterization of a novel enantioselective halohydrin hydrogen-halide-lyase. Appl Environ Microbiol. 1994;60:1297–1301. doi: 10.1128/aem.60.4.1297-1301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignatello J J. Ethylene dibromide mineralization in soils under aerobic conditions. Appl Environ Microbiol. 1986;51:588–592. doi: 10.1128/aem.51.3.588-592.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pignatello J J. Microbial degradation of 1,2-dibromoethane in shallow aquifer materials. J Environ Qual. 1987;16:307–312. [Google Scholar]
- 28.Pitulle C, Dorsch M, Kazda J, Wolters J, Stackebrandt E. Phylogeny of rapidly growing members of the genus Mycobacterium. Int J Syst Bacteriol. 1992;42:337–343. doi: 10.1099/00207713-42-3-337. [DOI] [PubMed] [Google Scholar]
- 29.Poelarends G J, Wilkens M, Larkin M J, van Elsas J D, Janssen D B. Degradation of 1,3-dichloropropene by Pseudomonas cichorii 170. Appl Environ Microbiol. 1998;64:2931–2936. doi: 10.1128/aem.64.8.2931-2936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rink R, Fennema M, Smids M, Dehmel U, Janssen D B. Primary structure and catalytic mechanism of the epoxide hydrolase from Agrobacterium radiobacter AD1. J Biol Chem. 1997;272:14650–14657. doi: 10.1074/jbc.272.23.14650. [DOI] [PubMed] [Google Scholar]
- 31.Rogall T, Wolters J, Flohr T, Bottger E C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 34.Schanstra J P, Kingma J, Janssen D B. Specificity and kinetics of haloalkane dehalogenase. J Biol Chem. 1996;271:14747–14753. doi: 10.1074/jbc.271.25.14747. [DOI] [PubMed] [Google Scholar]
- 35.Stahl D A, Urbance J W. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990;172:116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg S M, Pignatello J J, Shawhney B L. Persistence of 1,2-dibromoethane in soils: entrapment in intraparticle micropores. Environ Sci Technol. 1987;21:1201–1208. [Google Scholar]
- 38.Thompson J D, Higgens D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van den Wijngaard A J, Reuvekamp P T W, Janssen D B. Purification and characterization of haloalcohol dehalogenase from Arthrobacter sp. strain AD2. J Bacteriol. 1991;173:124–129. doi: 10.1128/jb.173.1.124-129.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van de Peer Y, de Wachter R. TREECON for windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 41.Van de Peer Y, Caers A, de Rijk P, de Wachter R. Database on the structure of small ribosomal subunit RNA. Nucleic Acids Res. 1998;26:179–182. [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Ploeg J R, Kingma J, de Vries E J, van der Ven J G M, Janssen D B. Adaptation of Pseudomonas sp. GJ1 to 2-bromoethanol caused by overexpression of an NAD-dependent aldehyde dehydrogenase with low affinity for halogenated aldehydes. Arch Microbiol. 1996;165:258–264. doi: 10.1007/s002030050324. [DOI] [PubMed] [Google Scholar]
- 43.Wang R-F, Cao W-W, Cerniglia C E. Phylogenetic analysis of polycyclic aromatic hydrocarbon degrading mycobacteria by 16S rRNA sequencing. FEMS Microbiol Lett. 1995;130:75–80. doi: 10.1016/0378-1097(95)00186-9. [DOI] [PubMed] [Google Scholar]
- 44.Yu F, Nakamura T, Mizunashi W, Watanabe I. Cloning of two halohydrin halogen-halide lyase genes from Corynebacterium sp. strain N-1074 and structural comparison of the genes and gene products. Biosci Biotechnol Biochem. 1994;58:1451–1457. doi: 10.1271/bbb.58.1451. [DOI] [PubMed] [Google Scholar]