Abstract
The growing literature demonstrating air pollution associations on COVID-19 mortality contains studies predominantly examining long-term exposure, with few on short-term exposure, and rarely both together to estimate independent associations. Because mechanisms by which air pollution may impact COVID-19 mortality risk function over timescales ranging from years to days, and given correlation among exposure time windows, consideration of both short- and long-term exposure is of importance. We assessed the independent associations between COVID-19 mortality rates with short- and long-term air pollution exposure by modeling both concurrently. Using California death certificate data COVID-19-related deaths were identified, and decedent residential information used to assess short- (4-week mean) and long-term (6-year mean) exposure to particulate matter <2.5µm (PM2.5), nitrogen dioxide (NO2), and ozone (O3). Negative binomial mixed models were fitted on weekly census tract COVID-19 mortality adjusting for potential confounders with random effects for county and census tract and an offset for population. Data were evaluated separately for two time periods March 16, 2020–October 18, 2020 and October 19, 2020–April 25, 2021, representing the Spring/Summer surges and Winter surge. Independent positive associations with COVID-19 mortality were observed for short- and long-term PM2.5 in both study periods, with strongest associations observed in the first study period: COVID-19 mortality rate ratio for a 2-μg/m3 increase in long-term PM2.5 was 1.13 (95%CI:1.09,1.17) and for a 4.7-μg/m3 increase in short-term PM2.5 was 1.05 (95%CI:1.02,1.08). Statistically significant positive associations were seen for both short- and long-term NO2 in study period 1, but short-term NO2 was not statistically significant in study period 2. Results for long-term O3 indicate positive associations, however, only marginal significance is achieved in study period 1. These findings support an adverse effect of long-term PM2.5 and NO2 exposure on COVID-19 mortality risk, independent of short-term exposure, and a possible independent effect of short-term PM2.5.
Keywords: Air pollution, COVID, Mortality, Particulate matter, Nitrogen dioxide, Ozone
Abbreviations: COVID-19, coronavirus disease 2019; NO2, nitrogen dioxide; PM10, particulate matter < 10 µm; PM2.5, particulate matter < 2.5 µm; SD, standard deviation
1. Introduction
There is growing literature in support of a relationship between ambient air pollution exposure and increased risk of mortality related to the coronavirus disease 2019 (COVID-19) (Ali et al., 2021; Ali and Islam, 2020; Bourdrel et al., 2021; Katoto et al., 2021; Zang et al., 2022). It is hypothesized that air pollution could affect risk of COVID-19 mortality through a variety of different mechanisms operating over timeframes ranging from years to weeks/days, including via increasing risk of underlying medical conditions, reducing biological defenses against the virus, and severing as a pathway for viral exposure (Bourdrel et al., 2021; Woodby et al., 2021). Exposure to air pollution adversely contributes to the development and exacerbation of several comorbid conditions, including cardiopulmonary and metabolic diseases and risk factors (Bourdrel et al., 2021; Munzel et al., 2017; Nawrot et al., 2011; Yang et al., 2019), which have been shown to increase risk of COVID-19 mortality (Bourdrel et al., 2021; Wu and McGoogan, 2020; Yang et al., 2019; Zhou et al., 2020). Air pollution can contribute to chronic rhinitis and rhinosinusitis, which could increase airway mucosal permeability and make it easier for SAR-CoV-2 to pass through the airway (Annesi-Maesano et al., 2012; Kesic et al., 2012; London et al., 2018; Sungnak et al., 2020). Air pollution may lower immune response and promote replication of respiratory virus (Bourdrel et al., 2021; Ciencewicki and Jaspers, 2007; Noah et al., 2012; Rebuli et al., 2019; Zhang et al., 2019a; Zhang et al., 2019b). There is also some evidence that SAR-CoV-2 virus can interact with air pollutants, including being carried by fine particles (Bourdrel et al., 2021; Setti et al., 2020).
Since air pollution can potentially impact COVID-19 mortality risk through various mechanisms operating over a range of time frames, both short- and long-term exposure periods may be relevant for study. Additionally, the correlation between short- and long-term air pollution exposures may be high, thus including examination of both exposure windows in analyses will serve to better understand their independent effects. Among papers examining air pollution effects on COVID-19 mortality, many examine associations with long-term exposure and only a few have examined short-term associations—even fewer studies have evaluated the effects of both short- and long-term air pollution exposure. A review and meta-analysis including studies published up to August 2021 on outdoor air pollution and COVID-19 risk, found 14 articles related to outdoor pollution and risk of COVID-19 mortality: 12 investigated associations for long-term air pollution, 2 for short-term air pollution, and no study investigated both exposures (Zang et al., 2022). Results of the meta-analysis indicated positive associations for long-term exposure to nitrogen dioxide (NO2) and particulate matter < 2.5 µm (PM2.5) and null association for long-term particulate matter < 10 µm (PM10), ozone (O3), and nitrogen oxides, and for short-term exposure to PM2.5 and O3. There are a couple of studies that have investigated effects of both short- and long-term air pollution on case fatality rates (as opposed to population-based mortality rates). In a study based in Mexico City, researchers reported a positive association between PM2.5 and mortality among cases that seemed to be driven by the effects of long-term rather than short-term air pollution exposure (Lopez-Feldman et al., 2021). Similarly, a study across several cities in China found both short- and long-term PM2.5 and PM10 to be related to COVID-19 case fatality rate, with stronger associations observed for long-term (2015-2019) exposure (Yao et al., 2020). Consideration of both short- and long-term air pollution exposures is important to better understand the driver of observed associations.
In this brief communication, we present a study on the independent associations of short- and long-term air pollution exposure on risk of COVID-19 mortality. This is an update to our prior study which only examined the effects of long-term pollution exposure (Garcia et al., 2022). We used mortality data for the state of California through April 25, 2021 and investigated the relationship in two separate time periods of the pandemic, reflecting the first set of Spring and Summer surges and the Winter surge.
2. Presentation of the Analysis and Concerns
Relationship between risk of COVID-19 mortality and short- and long-term exposure to PM2.5 (μg/m3), NO2 (ppb), and O3 (ppb) was examined using California death certificate data and ambient air quality monitoring data from the U.S. Environmental Protection Agency. Details on data sources and statistical approach have been previously published (Garcia et al., 2022). In brief, an internally developed algorithm was used in complement with International Statistical Classification of Diseases and Related Health Problems, Tenth Revision to identify COVID-19-related deaths from death certificates and assign residential census tracts for decedents. COVID-19 weekly deaths were aggregated by census tract starting March 16, 2020—with introduction of the pandemic in California—and ending April 25, 2021. Inverse distance-squared weighting of air monitoring data from up to four monitoring sites nearby (<50 km) the centroid of each census tract was used to estimate 24-hour average PM2.5 and NO2 and daily maximum 8-hour average O3 concentrations. In addition to the long-term exposure assessed in our prior study (six-year annual average from 2014 to 2019) (Garcia et al., 2022), the current analyses included exposure to short-term air pollution using average pollutant concentration in the four weeks prior to index week of mortality (ensuring exposure always preceded death). One month (≈ 4 weeks) average air pollution exposure has been shown to be associated with COVID-19 mortality (Chen et al., 2022). To identify the separate effects of short- and long-term air pollution, short-term exposure was defined as deviation from long-term average (short-term deviation = long-term average pollution – short-term air pollution), thus creating orthogonal pollution variables. Correlation coefficients between long-term average and short-term deviation were -0.01, -0.12, and -0.01 for PM2.5, NO2, and O3, respectively. Exposure effect estimates were scaled to provide mortality rate ratios associated with a one standard deviation (SD) increase during the relevant exposure window. For each pollutant, there was a single SD value for long-term exposure and two SDs for short-term exposure, one for each study period. We used the smaller of these latter two SDs to scale the short-term air pollution effects. Covariates for the current analysis were selected based on our prior long-term air pollution study (Garcia et al., 2022) as well as the potential for confounding the short-term air pollution and COVID-19 mortality relation. Covariates included census tract sociodemographic characteristics from the American Community Survey 2014–2018 5-year Estimates, including race/ethnicity (percent non-Hispanic Black, percent non-Hispanic Asian, and percent Hispanic any race), age (percent over age 65 years), total population, population density (persons per square mile), as well as Social Deprivation Index (Robert Graham Center, 2015). County-level variables included COVID-19 test positivity (weekly means lagged by two weeks) and time-varying cumulative percent population vaccinated (population percent with at least one dose); these were used only for sensitivity analyses to control for potential time-varying confounding. Continuous covariates were mean-centered and scaled to a one standard deviation change, except Social Deprivation Index (left as-is) and population density (log-transformed).
Analyses were separated into two study periods reflecting the COVID-19 surges in Spring and Summer (March 16 - October 18, 2020) and Winter (October 19, 2020 – April 25, 2021), which will be referred to as study period 1 and study period 2, respectively. Study periods were separated such that study period 1 ended on the week with the fewest occurring COVID-19 deaths (determined via the week with the most frequent minimum weekly deaths by county) and study period 2 began the following week as mortality rates started to rise. A separation of study periods was greatly motivated by the introduction of vaccine administration and differences in COVID-19 mortality between Spring/Summer 2020 and Winter 2021. Assessment of residential air pollution effects on COVID-19 mortality were implemented through Negative Binomial Mixed Models. Single pollutant models included census tract covariates as an adjustment for potential confounding and population totals as an offset to model a mortality rate outcome (e.g., weekly deaths/population). Census tracts with a total population of zero were excluded. Short-term (4-week average deviation) and long-term (2014-2019 average) air pollution were included together in the models. Calendar week was included as a natural cubic spline centered on the week with the most fatalities (July 27 - Aug 2, 2020 and Jan 4 - Jan 10, 2021 for the two study periods, respectively), with degrees of freedom selected based on AIC/BIC (6 df and 7 df, respectively). Random intercepts were included for county and census tract, as well as uncorrelated random slopes on time by county via spline basis functions. Sensitivity analyses included models additionally adjusting for (i) county-level COVID-19 test positivity (2-week lag), (ii) county-level percent population vaccinated, and (iii) a fixed effect of state region. The fixed effect for state region was included to more directly control for confounding bias by regional differences in COVID-19 mortality and pandemic-related policies and behaviors. Regions were defined based on contiguous counties with similar mortality rates with the exceptions of the four counties with the highest mortality rates, which were each considered their own separate regions (Los Angeles, Orange, San Diego, and Imperial). Random slopes were removed in instances of non-convergence—specifically, in region adjustment models for O3 in study period 1 and NO2 in study period 2, and vaccination adjustment model for O3 in study period 2.
There were 16,135 COVID-19 related deaths observed in study period 1 and 44,370 COVID-19 deaths in study period 2. The mean concentration for short-term PM2.5 was 11.9 μg/m3 (standard deviation = 8.9) in study period 1 and 11.6 μg/m3 (4.7) in study period 2. Mean long-term PM2.5 concentration was 10.2 μg/m3 (2.0). Short-term NO2 pollution exhibited a mean concentration of 7.1 ppb (3.7) and 13.5 ppb (5.5), respectively in the two study periods. The mean long-term NO2 pollution was 11.4 ppb (3.9). Mean short-term O3 concentration in study period 1 was 47.5 ppb (11.8) and 39.9 ppb (9.9) in study period 2. Mean long-term O3 pollution was 42.9 ppb (6.9). The highest correlation among short-term air pollution in study period 1 was 0.52 for NO2 and O3. PM2.5 had slightly higher correlation with NO2 (r = 0.43) than with O3 (r = 0.36) in study period 1. In study period 2, the highest correlation was exhibited between PM2.5 and NO2 (r = 0.55). O3 had minimal positive correlation with PM2.5 (r = 0.15), and minimal negative correlation with NO2 (r = -0.12).
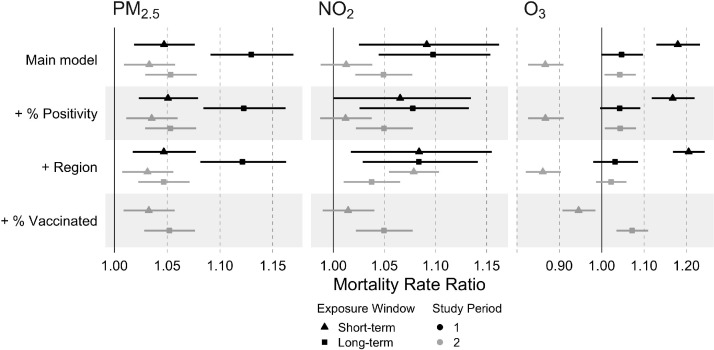
Long-term air pollution was positively associated with COVID-19 mortality (Fig. 1 ). Statistically significant effects were seen for long-term exposure to PM2.5 and NO2 in both study periods; O3 effects were marginally significant in study period 1 and statistically significant in study period 2. Stronger pollution effects were observed in the first study period compared with the second study period. In the first study period, a one SD increase in long-term air pollution was associated with: a 13% (95% Confidence Interval: 9%, 17%) higher COVID-19 mortality rate for PM2.5 (SD = 2.0 μg/m3), a 10% (4%, 15%) higher COVID-19 mortality rate for NO2 (SD = 3.9 ppb), and a 5% (-0.1%, 10%) higher COVID-19 mortality rate for O3 (SD = 6.9 ppb). In comparison, in the second study period, a one SD increase in long-term air pollution was associated with: a 5% (3%, 8%) higher COVID-19 mortality rate for PM2.5 (SD = 2.0 μg/m3), a 5% (2%, 8%) higher COVID-19 mortality rate for NO2 (SD = 3.9 ppb), and a 4% (0.6%, 8%) higher COVID-19 mortality rate for O3 (SD = 6.9 ppb). Positive associations for long-term exposure to PM2.5 and NO2 were robust in sensitivity analyses, although some effect estimates were slightly attenuated. O3 results maintained statistical significance in second study period sensitivity models that adjusted for test-positivity and vaccination level, but not when adjusted for geographical region.
Fig. 1.
Mortality Rate Ratios and 95% confidence intervals associated with short- and long-term air pollution by study period. Main models were fitted by study period through Negative Binomial Mixed regression adjusting for census tract race/ethnicity, age, population density, Social Deprivation Index, and calendar week, with random effects for county and census tract, and an offset for census tract total population. Sensitivity models included separate additional adjustment for COVID-19 test positivity, geographical region, and percent population vaccinated (study period 2 only). Effect estimates for a pollutant-specific standard deviation increase in concentration. Standard deviations for short- and long-term effects, respectively, are: 4.7 and 2.0 μg/m3 for PM2.5, 3.7 and 3.9 ppb for NO2, 9.9 and 6.9 ppb for O3. PM2.5: Particulate matter < 2.5 μm; NO2: Nitrogen dioxide; O3: Ozone.
Short-term air pollution results present statistically significant positive effects for first study period models (Fig. 1). Winter models showed attenuated results for both PM2.5 and NO2—however, NO2 results were no longer statistically significant. O3 short-term air pollution results demonstrate divergent relationships between study periods, with positive effects in study period 1 and negative effects in study period 2, both statistically significant. In the first study period, a one SD increase in short-term air pollution, within census tract, was associated with: a 5% (2%, 8%) higher COVID-19 mortality rate for PM2.5 (SD = 4.7 μg/m3), a 9% (2%, 16%) higher COVID-19 mortality rate for NO2 (SD = 3.7 ppb), and a 18% (13%, 23%) higher COVID-19 mortality rate for O3 (SD = 9.9 ppb). In comparison, in the second study period, a one SD increase in short-term air pollution, within census tract, was associated with: a 3% (1%, 6%) higher COVID-19 mortality rate for PM2.5 (SD = 4.7 μg/m3), a 1% (-1%, 4%) higher COVID-19 mortality rate for NO2 (SD = 3.7 ppb), and a 13% (-17%, -9%) lower COVID-19 mortality rate for O3 (SD = 9.9 ppb). Because NO2 is a component of the photochemical process that produces O3, we fitted additional O3 models adjusting for NO2. In the NO2-adjusted models, the short-term O3 effect estimate in the first study period remained similar (mortality rate ratio = 1.19 [1.13, 1.25]), while the association in the second study period was null (mortality rate ratio = 0.99 [0.94, 1.03])—indicating that inverse O3-mortality associations observed for the second study period were confounded by NO2. Sensitivity analyses showed short-term air pollution results were robust for all three pollutants in first study period models; PM2.5 results were robust in second study period models.
3. Conclusions
This study found positive relationships between weekly COVID-19 mortality and long-term exposure to PM2.5, NO2, and O3 across both study periods examined, in models adjusting for short-term air pollution exposure. Among short-term air pollutant exposures, only PM2.5 displayed a consistent positive association with COVID-19 mortality across the study periods. Results for short-term NO2 exposure are suggestive of an association, but effect estimates were attenuated and no longer statistically significant in the second study period. The results found in the present study further support our previously published findings (Garcia et al., 2022). After adjusting for short-term air pollutant effects, effects of long-term exposure to PM2.5, NO2, and O3 were consistent with the positive associations with COVID-19 mortality previously reported. This provides additional confidence that the estimated long-term pollution associations are robust and independent from short-term air pollution effects.
In comparison to other studies investigating COVID-19 mortality and both short- and long-term air pollutant exposure, our results similarly found stronger positive associations for long-term PM2.5 exposures when compared with the short-term exposure windows. However, while the Mexico City study reported no statistically significant positive association between short-term PM2.5 exposure and COVID-19 mortality when modeling both short- and long-term exposure together (Lopez-Feldman et al., 2021), our results indicated a small statistically significant positive relationship. A study modeling COVID-19 case fatality rate and short- and long-term exposures to particulate matter in cities across China (Yao et al., 2020) reported similar findings as those presented here, with both studies finding statistically significant positive associations for both short- and long-term PM2.5 exposures, and short-term exposures exhibiting attenuated results. However, results from our study indicated effects of a higher magnitude for both exposures; although direct comparison is difficult given that we examined population-based mortality rates while this other study examined mortality among cases (e.g., case-fatality).
This study has some limitations. First, because this is an ecological study, we are not able to control for potential individual-level confounders. If an individual-level confounder were positively related to air pollution and increased risk of COVID-19 mortality (e.g., lack of access to quality healthcare), pollution effect estimates could be biased upward and we may be overestimating positive associations. While we adjusted for many potential area-level confounders both in main models and in sensitivity analyses, these are not a substitute for individual-level confounders as they might not fully represent corresponding individual-level features. Second, there is a concern that major group facilities (e.g., institutionalized populations such as correctional facilities or nursing homes) may result in clusters of COVID-19 deaths which could bias the analyses. In order for confounding bias to occur, the locations of such facilities must be associated with air pollution levels. We found no correlation between census tract institutionalized populations (total percent institutionalized, percent in skilled nursing homes, or percent in adult correctional facilities) and either short- or long-term air pollution (correlation coefficient range: -0.03, 0.03), thus indicating this is likely not a major source of bias in our study. Third, there is a concern that without individual adjustment for age results could be biased due to confounding given that age is a strong risk factor for COVID-19 mortality. While we could not directly adjust for individual age, we were able to evaluate whether decedent's age was associated with air pollution, which is required element for confounding to occur. We observed no correlation between decedent's age and either short- or long-term air pollution (correlation coefficient range: -0.07, 0.005), thus indicating this is likely not a major confounding variable in our study. Fourth, we evaluated only one window of short-term exposure (4-week average prior to index week of deaths), but there may be other more sensitive time windows of exposure, and may even vary by pollutant. We do not have data on when individuals were infected or began exhibiting symptoms of COVID-19, thus we were unable to construct exposures estimates relative to specific periods in disease progression. Without knowing the most influential time of exposure, we decided to include all air pollution an individual was recently exposed to prior to death. Finally, we assessed pollutant effects on COVID-19 mortality, which is not the sole endpoint of COVID-19 adverse outcomes. There may be more sensitive endpoints related to ambient pollution, such as infection, severity, and symptoms related to “long COVID”.
This study has several strengths. First, our analysis was conducted on an outcome identified from state death certificate data. Because all deaths occurring in California were recorded in these data, we expect to have captured the vast majority of COVID-19 related deaths for our analysis, thus there is little concern about possible selection bias impacting our effect estimates. Second, our modeling approach allowed for a great deal of flexibility in geographically related county-specific trajectories using cubic splines in our random effects. Further adding to the flexibility, by examining two distinct study periods of the pandemic we allowed for time-period-specific nuances to be modeled independently of each other. Finally, as previously mentioned, the joint modeling of short- and long-term exposures allow us to quantify an isolated effect for short-term exposure, while further reinforcing the robustness previous long-term pollution results.
This study contributes to the small literature supporting an adverse effect of long-term air pollution exposure on COVID-19 mortality risk, independent of short-term air pollution exposure, as well as a possible independent effect of short-term PM2.5.
Funding Sources
This research was supported by the National Institute of Environmental Health Sciences (grant #s P30ES007048 and P30ES007048-25S1), the Hastings Foundation, and by the Keck School of Medicine of USC COVID-19 Research Fund through a generous gift from the W. M. Keck Foundation.
CRediT authorship contribution statement
Brittney Marian: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Ying Yan: Methodology, Formal analysis, Investigation, Data curation, Writing – review & editing. Zhanghua Chen: Methodology, Investigation, Writing – review & editing. Fred Lurmann: Investigation, Data curation, Writing – review & editing. Kenan Li: Investigation, Writing – review & editing. Frank Gilliland: Conceptualization, Investigation, Writing – review & editing, Funding acquisition. Sandrah P. Eckel: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition. Erika Garcia: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ali N., Fariha K.A., Islam F., Mishu M.A., Mohanto N.C., Hosen M.J., Hossain K. Exposure to air pollution and COVID-19 severity: A review of current insights, management, and challenges. Integr Environ Assess Manag. 2021;17:1114–1122. doi: 10.1002/ieam.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Islam F. The Effects of Air Pollution on COVID-19 Infection and Mortality—A Review on Recent Evidence. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.580057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annesi-Maesano I., Hulin M., Lavaud F., Raherison C., Kopferschmitt C., de Blay F., André Charpin D., Denis C. Poor air quality in classrooms related to asthma and rhinitis in primary schoolchildren of the French 6 Cities Study. Thorax. 2012;67:682–688. doi: 10.1136/thoraxjnl-2011-200391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdrel T., Annesi-Maesano I., Alahmad B., Maesano C.N., Bind M.-A. The impact of outdoor air pollution on COVID-19: a review of evidence from in vitro, animal, and human studies. European Respiratory Review. 2021;30 doi: 10.1183/16000617.0242-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Sidell M.A., Huang B.Z., Chow T., Eckel S.P., Martinez M.P., Gheissari R., Lurmann F., Thomas D.C., Gilliland F.D., Xiang A.H. Ambient Air Pollutant Exposures and COVID-19 Severity and Mortality in a Cohort of COVID-19 Patients in Southern California. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202108-1909OC. [DOI] [PubMed] [Google Scholar]
- Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Garcia E., Marian B., Chen Z., Li K., Lurmann F., Gilliland F., Eckel S.P. Long-term air pollution and COVID-19 mortality rates in California: Findings from the Spring/Summer and Winter surges of COVID-19. Environ Pollut. 2022;292 doi: 10.1016/j.envpol.2021.118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoto P.D.M.C., Brand A.S., Bakan B., Obadia P.M., Kuhangana C., Kayembe-Kitenge T., Kitenge J.P., Nkulu C.B.L., Vanoirbeek J., Nawrot T.S., Hoet P., Nemery B. Acute and chronic exposure to air pollution in relation with incidence, prevalence, severity and mortality of COVID-19: a rapid systematic review. Environmental Health. 2021;20:41. doi: 10.1186/s12940-021-00714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesic M.J., Meyer M., Bauer R., Jaspers I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS One. 2012;7:e35108. doi: 10.1371/journal.pone.0035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N.R., Lina I., Ramanathan M. Aeroallergens, air pollutants, and chronic rhinitis and rhinosinusitis. World Journal of Otorhinolaryngology - Head and Neck Surgery. 2018;4:209–215. doi: 10.1016/j.wjorl.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Feldman A., Heres D., Marquez-Padilla F. Air pollution exposure and COVID-19: A look at mortality in Mexico City using individual-level data. Sci Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T., Sorensen M., Gori T., Schmidt F.P., Rao X., Brook F.R., Chen L.C., Brook R.D., Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J. 2017;38:557–564. doi: 10.1093/eurheartj/ehw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot T.S., Perez L., Kunzli N., Munters E., Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- Noah T.L., Zhou H., Zhang H., Horvath K., Robinette C., Kesic M., Meyer M., Diaz-Sanchez D., Jaspers I. Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers. Am J Respir Crit Care Med. 2012;185:179–185. doi: 10.1164/rccm.201103-0465OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli M.E., Speen A.M., Martin E.M., Addo K.A., Pawlak E.A., Glista-Baker E., Robinette C., Zhou H., Noah T.L., Jaspers I. Wood Smoke Exposure Alters Human Inflammatory Responses to Viral Infection in a Sex-Specific Manner. A Randomized, Placebo-controlled Study. Am J Respir Crit Care Med. 2019;199:996–1007. doi: 10.1164/rccm.201807-1287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert Graham Center . Robert Graham Center; 2015. 2015 SDI at the County Level, 2015. ed. [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., Talavera-Lopez C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., Network H.C.A.L.B. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodby B., Arnold M.M., Valacchi G. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: What is the connection? Ann N Y Acad Sci. 2021;1486:15–38. doi: 10.1111/nyas.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang B.-Y., Guo Y., Markevych I., Qian Z., Bloom M.S., Heinrich J., Dharmage S.C., Rolling C.A., Jordan S.S., Komppula M., Leskinen A., Bowatte G., Li S., Chen G., Liu K.-K., Zeng X.-W., Hu L.-W., Dong G.-H. Association of Long-term Exposure to Ambient Air Pollutants With Risk Factors for Cardiovascular Disease in China. JAMA Netw Open. 2019;2:e190318. doi: 10.1001/jamanetworkopen.2019.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J., Wang W., Liu Z., Kan H., Qiu Y., Meng X., Wang W. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang S.T., Luan J., Li L., Yu H.X., Wu Q.J., Chang Q., Zhao Y.H. Ambient air pollution and COVID-19 risk: Evidence from 35 observational studies. Environ Res. 2022;204 doi: 10.1016/j.envres.2021.112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Huo X., Zhang Y., Huang Y., Zheng X., Xu X. Ambient fine particulate matter inhibits innate airway antimicrobial activity in preschool children in e-waste areas. Environ Int. 2019;123:535–542. doi: 10.1016/j.envint.2018.12.061. [DOI] [PubMed] [Google Scholar]
- Zhang S., Huo X., Zhang Y., Lu X., Xu C., Xu X. The association of PM2.5 with airway innate antimicrobial activities of salivary agglutinin and surfactant protein D. Chemosphere. 2019;226:915–923. doi: 10.1016/j.chemosphere.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]