Abstract
Purpose:
To describe the incidence, prevalence, and clinical characteristics of presumed ocular histoplasmosis syndrome (POHS) in a Histoplasma endemic region.
Methods:
The International Classification of Diseases, 9th and 10th Revision codes were used to search the Rochester Epidemiology Project, a record-linkage system for medical care provided in Olmsted County, MN. Medical records were reviewed to confirm POHS diagnoses in county residents from January 1, 2006 to December 31, 2015. Age- and sex-adjusted incidence rates were calculated and adjusted to the 2010 U.S. White population.
Results:
There were 18 incident cases (30 eyes) and 87 prevalent cases (131 eyes). The incidence rate was 1.35 per 100,000 per year. The mid-study prevalence rate was 0.064%. Choroidal neovascularization (CNV) occurred in 17.4% of affected eyes. At last follow-up, 16.8% of affected eyes had POHS-related decreased VA (<20/40).
Conclusion:
This study assesses the epidemiology and clinical features of POHS in a Midwestern U.S. county.
Keywords: Presumed ocular histoplasmosis syndrome, Epidemiology, Choroidal neovascularization
Introduction
Presumed ocular histoplasmosis syndrome (POHS) is a clinical syndrome characterized by a triad of multifocal chorioretinal scarring, peripapillary atrophy, and choroidal neovascularization (CNV) in the absence of anterior segment and vitreous inflammation. Although its precise pathogenesis remains controversial, POHS is thought to be caused by hematogenous dissemination of Histoplasma capsulatum to the choroidal circulation.1, 2 H. capsulatum, the most endemic mycosis in the United States (U.S.), is found predominantly in the Midwestern U.S. along the Ohio and Mississippi River valleys in soil contaminated by bat and bird droppings, and is primarily acquired through inhalation of fungal spores.2, 3 Symptomatic histoplasmosis occurs in only 1% of infections, typically manifesting as pulmonary disease; rarely, extrapulmonary organ involvement and systemic dissemination may occur, particularly among immunocompromised patients.4 In Minnesota, the incidence of systemic histoplasmosis is approximately 3 to 4 cases per 100,000 persons.4, 5
The majority of POHS cases are subclinical and asymptomatic, but approximately 5% develop CNV that may lead to acute visual symptoms and vision loss.6 Histopathologic studies of POHS chorioretinal scars demonstrate focal inflammation with disruption of Bruch’s membrane and the retinal pigment epithelium.3, 7 Macular chorioretinal lesions are a risk factor for the development of POHS-related CNV at sites of pre-existing chorioretinal lesions. Despite the availability of intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment for active POHS-related CNV, vision loss may still occur due to fibrovascular macular scarring.7, 8 Thus, POHS strongly contributes to irreversible vision loss in the Midwestern U.S.6
Most studies investigating POHS epidemiology were conducted nearly 50 years ago and estimated a prevalence ranging from 0.013% to 5.3%.9–13 To date, no studies have reported the incidence of POHS, thus current understanding of POHS epidemiology is limited. The purpose of this study was to investigate the population-based incidence, prevalence, and clinical characteristics of POHS over a 10-year period in a Midwestern U.S. county in which H. capsulatum is endemic.5, 14
Materials and Methods
The medical records were retrospectively reviewed for all patients who were diagnosed with POHS while residing in Olmsted County, Minnesota from January 1, 2006 to December 31, 2015. Patients were identified using the Rochester Epidemiology Project (REP), a medical records linkage system established in 1966 that uses diagnostic and surgical procedure codes to track medical care delivered to residents of Olmsted County, Minnesota.15 The REP includes medical care provided by Mayo Clinic, Olmsted Medical Group, and affiliated hospitals, encompassing virtually all care delivered to the residents of Olmsted County, Minnesota.16 Participants in the REP database are asked to give authorization for minimal risk research when they are first entered into the medical system in Olmsted County, Minnesota. The Institutional Review Boards (IRBs) of Mayo Clinic and Olmsted Medical Center approved this retrospective cohort study, and the research adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Since the current study was retrospective and considered minimal risk, the IRBs did not require additional informed consent from the patients included in this study and waiver of consent was granted by the IRBs.
This investigation was part of a larger study of the overall ocular inflammatory disease epidemiology in Olmsted County, Minnesota. A total of 90 International Classification of Diseases, 9th Revision (ICD-9) and 234 ICD-10 codes for ocular inflammation were used to query the REP database to identify all residents of Olmsted County diagnosed with ocular inflammatory disease from January 1, 2006 to December 31, 2015. Olmsted Country residence during the study period and research authorizations were confirmed through the database, and patients who were non-residents or did not grant authorization were excluded from the study. The medical records of 4,253 potential cases identified via the ICD code search were reviewed to verify diagnoses on the basis of documented ophthalmologic diagnoses and examinations.
The diagnosis of POHS was retrospectively confirmed via review of the medical records and ophthalmic examination findings by an ocular inflammation specialist (WMS). POHS cases were identified by the documentation of small atrophic chorioretinal scars with or without peripapillary atrophy or CNV. CNV was diagnosed through clinical findings and confirmed with optical coherence tomography imaging and fluorescein angiography. Cases were excluded if there was a CNV without chorioretinal scars, if the CNV could be attributed to other etiologies (i.e. myopic degeneration or exudative age-related macular degeneration) and there were no chorioretinal scars, and if the clinical course was more consistent with multifocal choroiditis, (inflammatory reactivation of lesions or the development of new lesions). Incident cases were defined as patients who were first diagnosed with POHS during the study period from January 1, 2006 to December 31, 2015 and had no previously documented ophthalmic examination findings consistent with POHS. Prevalent cases were defined as patients who were diagnosed with POHS prior to the study period and seen by an ophthalmologist or optometrist at least once during the study period. If patients had documented ophthalmic examination features consistent with POHS prior to the study period, they were considered prevalent. Demographic data such as age, sex, and self-reported race were collected and compared to the general mid-study Olmsted County, Minnesota population (2010). Clinical data included presenting ophthalmic symptoms, ophthalmologic examination findings, smoking status, POHS-related treatments, presence of CNV, and Snellen visual acuity (VA) at presentation and last follow-up. Active smoking was defined as active tobacco use at any time during the study period, while former smoking was defined as any tobacco use prior the study period (prior to January 1, 2006). VA loss attributable to POHS was defined as eyes with Snellen VA worse than 20/40 and documented vision-threatening POHS-related examination findings (i.e., macular scarring due to atrophy or subretinal fibrosis). Longitudinal findings were ascertained from ophthalmology follow-up visits, and the medical records were reviewed through April 15, 2020.
Overall incidence was estimated using the age- and sex-specific population figures for Olmsted County census data from 2006 to 2015. Prevalence rates were estimated using the population census figures in 2010. Age was stratified into the following groups by years: 0 to 14, 15 to 24, 25 to 44, 45 to 64, and 65 to 110. Population estimates for individual years between census years were determined using linear interpolation. Because the Olmsted County, Minnesota population is approximately 85% White, incidence and prevalence rates were also age- and/or sex-adjusted to the 2010 census figures for the U.S. White population so data could be compared to national estimates. Incident and prevalent cases were combined for prevalence calculations. The 95% confidence intervals (95% CI) for the rates were calculated assuming a Poisson error distribution.
Results
The mid-study population of Olmsted County, Minnesota in 2010 was 144,248. After review of the medical records, 108 POHS cases were identified. Eleven cases were excluded due to lack of Olmsted County residency at time of diagnosis. Of the remaining 97 POHS patients, there were 18 confirmed incident cases and 79 prevalent cases. The demographic characteristics of the Olmsted County, Minnesota population and all confirmed POHS cases at the mid-study point (January 1, 2010) are described in Table 1. The mean age of POHS cases was 58.8 years (range: 18 to 97 years), compared to 36.1 years in the general population. There were 54 (55.7%) POHS cases who were female, the majority (79.3%) were 45 years or older, and 96 (99.0%) were White.
Table 1:
Demographics of the general Olmsted County, Minnesota population and confirmed presumed ocular histoplasmosis (POHS) cases at the study period mid-point, January 1, 2010.
| General Olmsted County, Minnesota Population | All confirmed POHS cases | |
|---|---|---|
| Total number | 144,248 | 97 |
| Mean age (years) | 36.1 | 58.8 (Range: 18 to 97 years) |
| Female | 73,763 (51.1%) | 54 (55.7%) |
| Age category (years) | ||
| 0–14 | 30,682 (21.3%) | 0 (0%) |
| 15–24 | 17,065 (11.8%) | 2 (2.1%) |
| 25–44 | 40,200 (27.8%) | 18 (18.6%) |
| 45–64 | 38,168 (26.5%) | 44 (45.4%) |
| ≥65 | 18,133 (12.5%) | 33 (34.0%) |
| Race | ||
| Asian | 7,771 (5.4%) | 1 (1.0%) |
| Black | 6,751 (4.7%) | 0 (0%) |
| Hispanic | 6,081 (4.2%) | 0 (0%) |
| White | 120,348 (83.4%) | 96 (99.0%) |
| Other | 3,297 (2.3%) | 0 (0%) |
The overall age- and sex-adjusted incidence of POHS was 1.35 per 100,000 per year (95% CI: 0.72 to 1.97) adjusted for the 2010 U.S. White population, or 1 in 74,074 patients (Table 2). In females, the age-adjusted POHS incidence was 1.56 per 100,000 per year (95% CI: 0.64 to 2.48), and in males the age-adjusted incidence was 1.18 per 100,000 per year (95% CI: 0.30 to 2.06). The highest incidence was observed in males ≥65 years old, with an incidence rate of 3.85 per 100,000 per year (95% CI: 1.24 to 11.93). Due to the low number of cases, analysis of trends between years in the study period, age groups, and sexes could not be performed.
Table 2:
Incidence rate (per 100,000 per year) of presumed ocular histoplasmosis in Olmsted County, Minnesota from 2006 to 2015 by age and sex.
| Total | Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | N | Rate | 95% CI | N | Rate | 95% CI | N | Rate | 95% CI |
| Total (crude) | 18 | 1.25 | 0.79–1.98 | 11 | 1.49 | 0.83–2.69 | 7 | 0.99 | 0.47–2.08 |
| Total (adjusted) | 18 | 1.35* | 0.72–1.97 | 11 | 1.56+ | 0.64–2.48 | 7 | 1.18+ | 0.30–2.06 |
| 0–14 | 0 | - | - | 0 | - | - | 0 | - | - |
| 15–24 | 2 | 1.16 | 0.29–4.64 | 2 | 2.30 | 0.57–9.18 | 0 | - | - |
| 25–44 | 4 | 0.98 | 0.37–2.61 | 3 | 1.46 | 0.47–4.53 | 1 | 0.49 | 0.07–3.50 |
| 45–64 | 7 | 1.86 | 0.89–3.90 | 4 | 2.07 | 0.78–5.51 | 3 | 1.64 | 0.53–5.09 |
| 65+ | 5 | 2.79 | 1.16–6.70 | 2 | 1.97 | 0.49–7.88 | 3 | 3.85 | 1.24–11.93 |
N = Number of cases, 95% CI = 95% confidence interval.
Age- and sex-adjusted,
Age-adjusted.
The overall age- and sex-adjusted mid-study (2010) prevalence of POHS was 0.064% (95% CI: 0.050% to 0.077%) adjusted for the 2010 U.S. White population (Table 3). The age-adjusted prevalence was similar in females (0.063%) and males (0.066%). Males who were ≥65 years had the highest prevalence rate (0.227%). The mid-study prevalence (2010) was similar to the prevalence at the beginning and the end points of the study: 0.065% (95% CI: 0.050% to 0.079%) in 2006 and 0.065% (95% CI: 0.051% to 0.079%) in 2015.
Table 3:
Prevalence rate of presumed ocular histoplasmosis in Olmsted County, Minnesota at the mid-study period (January 1, 2010) by age and sex.
| Total | Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | N | % | 95% CI | % | Rate | 95% CI | % | Rate | 95% CI |
| Total (crude) | 84 | 0.058 | 0.047–0.072 | 45 | 0.061 | 0.046–0.082 | 39 | 0.055 | 0.040–0.076 |
| Total (adjusted) | 84 | 0.064* | 0.050–0.077 | 45 | 0.063+ | 0.045–0.083 | 39 | 0.066+ | 0.045–0.087 |
| 0–14 | 0 | - | - | 0 | - | - | 0 | - | - |
| 15–24 | 2 | 0.012 | 0.003–0.047 | 2 | 0.023 | 0.006–0.093 | 0 | - | - |
| 25–44 | 14 | 0.035 | 0.021–0.006 | 10 | 0.049 | 0.027–0.092 | 4 | 0.020 | 0.008–0.053 |
| 45–64 | 39 | 0.102 | 0.075–0.140 | 22 | 0.111 | 0.074–0.170 | 17 | 0.092 | 0.057–0.148 |
| 65+ | 29 | 0.160 | 0.111–0.230 | 11 | 0.108 | 0.060–0.194 | 18 | 0.227 | 0.143–0.361 |
N = Number of cases; % = percent of population; 95% CI = 95% Confidence interval.
Age- and sex-adjusted,
Age-adjusted.
The clinical characteristics of incident POHS cases are described in Table 4. There were 30 affected eyes from 18 patients; 12 (66.7%) cases had bilateral involvement. The mean age at POHS diagnosis was 52.5 years (range: 24 to 76 years). At initial presentation, visual symptoms were present in 5 (27.8%) patients, including decreased VA (3 patients) and blurred vision (2 patients). None of the patients received a histoplasmin skin test or urine antigen test. Two patients (11.1%) were current smokers, while 7 (38.9%) were former smokers. At baseline, chorioretinal scarring was observed in 28 (93.3%) incident POHS-affected eyes, peripapillary atrophy in 15 (50.0%), and CNV in 4 (13.3%). Only one patient demonstrated all three cardinal features of POHS at initial diagnosis. (Figure 1) All 4 of the eyes with CNV had visual symptoms at presentation. One CNV-affected eye was treated with laser photocoagulation and intravitreal anti-VEGF injections. The other 3 eyes received intravitreal anti-VEGF injections. The 4 eyes with CNV were treated with anti-VEGF received an average of 16 injections (range: 4 to 47) during the follow-up period. All 4 eyes were initially managed with bevacizumab. One was switched to aflibercept due to refractory neovascular activity and subsequently had good response. During the follow-up period (mean: 5.0 years, range: 0.7 to 14.0 years), none of the fellow eyes developed CNV, and the 6 unilateral cases did not develop clinical evidence of POHS in the contralateral eye.
Table 4:
Clinical characteristics of incident presumed ocular histoplasmosis (POHS) cases.
| Clinical characteristics of incident POHS cases | |
|---|---|
| By patient | |
| Number of affected patients | 18 |
| Mean age at diagnosis (years) | 52.5 (Range: 24 to 76) |
| Visual symptoms at baseline presentation * | 5 (27.8%) |
| Current smokers | 2 (11.1%) |
| Former smokers | 7 (38.9%) |
| Mean follow-up from initial presentation (years) | 5.0 (Range: 0.7 to 14.0 years) |
| By eye | |
| Number of affected eyes | 30 |
| Chorioretinal scarring | 28 (93.3%) |
| Peripapillary atrophy | 15 (50.0%) |
| Choroidal neovascularization | 4 (13.3%) |
Decreased visual acuity in 3 patients, blurred vision in 2 patients.
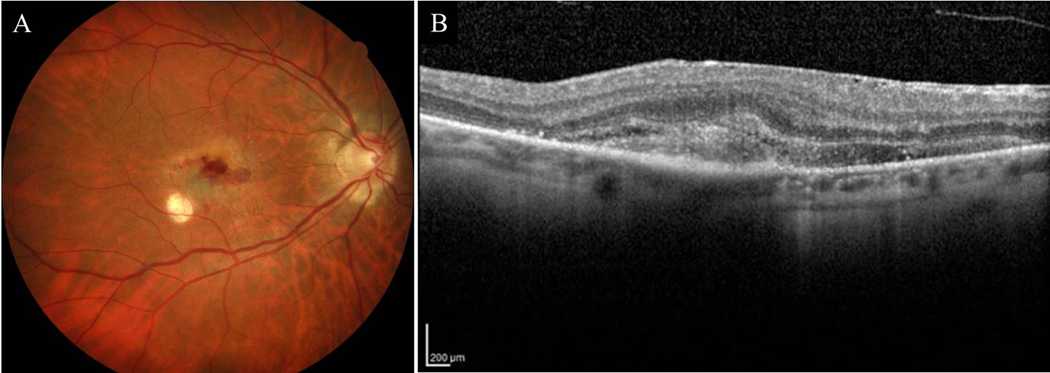
Figure 1A:
Color fundus photograph of a confirmed POHS case who presented with CNV, peripapillary atrophy, and chorioretinal scars. 1B: OCT showing subretinal hyperreflective material and fluid consistent with active CNV
The clinical characteristics of incident and prevalent POHS cases are compared in Table 5. Of the 79 prevalent cases, 52 (65.8%) were bilateral, for a total of 131 affected eyes. Among the prevalent cases, 24 (18.3%) eyes had a history of CNV compared to 4 (13.3%) incident eyes. At last follow-up, 84 (64.1%) of prevalent POHS-affected eyes had Snellen VA of 20/40 or better compared to 86.7% of incident eyes. No incident eyes had VA worse than 20/40 due to POHS (including those with CNV), whereas 27 (20.6%) prevalent eyes experienced POHS-related vision loss with final VA worse than 20/40. Combining all of the POHS-affected eyes, 28 (17.4%) had CNV, 110 (68.3%) had a final Snellen VA of 20/40 or better, and 27 (16.8%) had VA loss (worse than 20/40) attributable to POHS.
Table 5:
Clinical characteristics of incident versus prevalent presumed ocular histoplasmosis (POHS) cases.
| Incident POHS cases (N=18) |
Prevalent POHS cases (N=79) |
All POHS cases (N=97) |
|
|---|---|---|---|
| Number of affected eyes | 30 | 131 | 161 |
| Choroidal neovascularization | 4 (13.3%) | 24 (18.3%) | 28 (17.4%) |
| Snellen visual acuity at last follow-up | |||
| 20/20 to 20/40 | 26 (86.7%) | 84 (64.1%) | 110 (68.3%) |
| 20/50 to 20/150 | 4 (13.3%) | 22 (16.8%) | 26 (16.1%) |
| 20/200 or worse | 0 (0%) | 25 (19.1%) | 25 (15.5%) |
| Visual acuity <20/40 due to POHS | 0 (0%) | 27 (20.6%) | 27 (16.8%) |
N = number of patients.
Discussion
There are few studies of POHS epidemiology, and none have investigated POHS incidence. In this retrospective population-based cohort study, the overall incidence of POHS was 1.35 per 100,000 per year, or 1 in 74,074 patients, in a Midwestern U.S. county in which the state-specific incidence of systemic histoplasmosis is 3 to 4 cases per 100,000.5 The overall mid-study prevalence was 0.064%. Females accounted for 55.7% of all POHS cases, similar to the general population (51.1% female). The highest rates of incidence and prevalence were observed in males ≥65 years old. CNV was observed in 17.4% of all POHS-affected eyes and 16.8% of affected eyes had decreased VA attributable to POHS.
Most of the previous studies investigating POHS epidemiology were performed nearly 50 years ago and reported prevalence ranging from 0.013% to 5.3%, in comparison to the prevalence of 0.064% in this study.9, 10, 12, 13, 17 Differences in study design may account for the variability in prevalence rates. In 1970, Ganley et al. reported a POHS prevalence of 2.6% in a small Maryland community of 1,144 individuals. Since 74% of the community was examined, the study likely captured a substantial number of asymptomatic patients who would not have sought an eye examination outside of the study. 9 In contrast, a more recent study by Benedict et al. utilized medical claims data to estimate a POHS prevalence of 0.013%, or 13 cases per 100,000 patients, within an insurance-based cohort.13 However, the cases were identified through the use of ICD-9 and ICD-10 codes, and the medical records were not reviewed to confirm diagnoses. Accuracy rates of ICD-9 codes for capturing ocular inflammatory diagnoses are highly variable and may have under-ascertained the true number of POHS cases in the population.18, 19 The current study would have missed asymptomatic patients who had no other need for an eye examination, so the true POHS prevalence in Histoplasma endemic regions may be closer to the results from Ganley et al. Nonetheless, the results of the present study are likely still representative of patients who comprise a community ophthalmology or optometry practice.
Previous studies have found that POHS typically affects patients in early- to mid-adulthood (20 to 50 years old) with no sex predilection.10, 20, 21 In contrast, in the medical claims database study by Benedict et al., 81% of POHS patients were age 45 years or older and 64% were female.13 The age demographics in this study were similar to those in the Benedict study: 79.4% of all POHS cases were ≥45 years old, but only 58.8% were female. Since the majority (72.2%) of incident cases was asymptomatic in this study, the age at presentation may have been influenced by selection bias. Older individuals may be more likely to seek ophthalmic care for age-related ocular complaints such as symptomatic presbyopia or cataract progression that typically manifest over the age of 45 years. Thus, we could have missed POHS cases in individuals who were younger than 45 years old without visual symptoms or ophthalmic disease diagnoses or risk factors that warranted an eye examination. In other words, the actual age of POHS onset might be younger than reported in this retrospective study, but patients were not diagnosed until they sought ophthalmic care at an older age.
In previous studies, CNV was estimated to occur in approximately 5% of POHS patients.6 In the current study, CNV was observed in 28.9% of all patients (17.4% of affected eyes). These results are more similar to those reported by Benedict et al., who noted that 25% of patients with a POHS-related ICD-9 code also had a CNV diagnostic code.13 The higher CNV rates in this cohort could also represent selection bias since patients with visual symptoms or other comorbidities may be more likely to be evaluated by eye care providers at Mayo Clinic or Olmsted Medical Center rather than the small number of private practice optometrists in Olmsted County, Minnesota, who are not included in the REP study database. A patient who had chorioretinal scars and peripapillary atrophy without any other ophthalmic pathology may not have been referred to an ophthalmologist within the medical system that participates in the REP. Therefore, this study may have missed asymptomatic, younger patients. Nevertheless, the majority of eye care delivered in Olmsted County is captured by the REP, thus it is unlikely that this study missed a significant number of POHS cases.16
The limitations of this study included its retrospective study design, which contributed to non-standardized diagnostic criteria, diagnostic workup, data collection, and follow-up. None of the patients received a histoplasmin skin test or urine antigen test. Although these tests are not required to diagnose POHS, these data may have been informative in further elucidating the association between POHS and H. capsulatum. It was not possible to assess for incidence trends in age at diagnosis, sex, or race due to the low number of cases and the predominately White study cohort and population. Another limitation was related to the use of diagnostic codes to identify potential cases. Patients with diagnostic coding for CNV but not POHS may have been missed. However, since the POHS cohort was identified as part of a larger ocular inflammation epidemiology study, other codes for inflammation such as chorioretinal scars were included and may have helped identify cases.
In summary, this study provides a population-based estimate of POHS epidemiology and highlights its clinical course. The overall age- and sex-adjusted incidence of POHS in a predominately White U.S. county population was 1.35 per 100,000 per year, or approximately 1 in 74,000 patients. The overall age- and sex-adjusted prevalence rate was 0.064%. No apparent sex difference was observed between POHS cases and the general population, and highest incidence and prevalence rates were observed among males ≥65 years old. CNV was a relatively uncommon complication, though higher than previously reported. Most POHS-affected eyes had good visual acuity outcomes (≥20/40) at last follow-up, but nearly one-fifth had vision loss related to POHS.
Acknowledgments
Funding/Support:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source had no role in conduct of the research and preparation of the article.
Financial Support:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The sponsor had no role in the design or conduct of this research.
Abbreviations/Acronyms:
- POHS
Presumed ocular histoplasmosis syndrome
- ICD
International Classification of Diseases
- CI
Confidence interval
- VA
Visual acuity
- Anti-VEGF
Anti-vascular endothelial growth factor
- REP
Rochester Epidemiology Project
- CNV
Choroidal neovascularization
Footnotes
Declaration of Interest: The following authors report no conflict of interest: Timothy T. Xu, Margaret M. Reynolds, David O. Hodge, and Wendy M. Smith.
Disclosures:
No conflicting relationship exists for any author.
Data Availability Statement:
The data supporting the results are available upon reasonable request to the senior author.
References
- 1.Chu JH, Feudtner C, Heydon K, et al. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis. 2006;42(6):822–5. [DOI] [PubMed] [Google Scholar]
- 2.Baddley JW, Winthrop KL, Patkar NM, et al. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis. 2011;17(9):1664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Surv Ophthalmol. 2015;60(4):279–95. [DOI] [PubMed] [Google Scholar]
- 4.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong PA, Jackson BR, Haselow D, et al. Multistate Epidemiology of Histoplasmosis, United States, 2011–2014. Emerg Infect Dis. 2018;24(3):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thuruthumaly C, Yee DC, Rao PK. Presumed ocular histoplasmosis. Curr Opin Ophthalmol. 2014;25(6):508–12. [DOI] [PubMed] [Google Scholar]
- 7.Meredith TA, Green WR, Key SN, et al. Ocular histoplasmosis: Clinicopathologic correlation of 3 cases. Surv Ophthalmol. 1977;22(3):189–205. [DOI] [PubMed] [Google Scholar]
- 8.Orlando RG, Davidorf FH. Spontaneous Recovery Phenomenon in the Presumed Ocular Histoplasmosis Syndrome. Int Ophthalmol Clin. 1983;23(2):137–49. [DOI] [PubMed] [Google Scholar]
- 9.Ganley JP, Smith RE, Knox DL, Comstock GW. Presumed ocular histoplasmosis. 3. Epidemiologic characteristics of people with peripheral atrophic scars. Arch Ophthalmol. 1973;89(2):116–9. [DOI] [PubMed] [Google Scholar]
- 10.Smith RE, Ganley JP. An epidemiologic study of presumed ocular histoplasmosis. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(5):994–1005. [PubMed] [Google Scholar]
- 11.Prasad AG, Van Gelder RN. Presumed ocular histoplasmosis syndrome. Curr Opin Ophthalmol. 2005;16(6):364–8. [DOI] [PubMed] [Google Scholar]
- 12.Asbury T. The status of presumed ocular histoplasmosis: including a report of a survey. Trans Am Ophthalmol Soc. 1966;64:371–400. [PMC free article] [PubMed] [Google Scholar]
- 13.Benedict K, Shantha JG, Yeh S, et al. Presumed ocular histoplasmosis syndrome in a commercially insured population, United States. PLoS One. 2020;15(3):e0230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnesota Department of Health. Average Annual Incidence of Histoplasmosis in Minnesota. 2017–2018. https://www.health.state.mn.us/diseases/histoplasmosis/histocasesannual.pdf; 2019. Accessed 2020.04.12.
- 15.Melton LJ 3rd., History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–74. [DOI] [PubMed] [Google Scholar]
- 16.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RE, Ganley JP, Knox DL. Presumed Ocular Histoplasmosis: II. Patterns of Peripheral and Peripapillary Scarring in Persons With Nonmacular Disease. Arch Ophthalmol. 1972;87(3):251–7. [DOI] [PubMed] [Google Scholar]
- 18.Stein JD, Lum F, Lee PP, et al. Use of health care claims data to study patients with ophthalmologic conditions. Ophthalmology. 2014;121(5):1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pimentel MA, Browne EN, Janardhana PM, et al. Assessment of the Accuracy of Using ICD-9 Codes to Identify Uveitis, Herpes Zoster Ophthalmicus, Scleritis, and Episcleritis. JAMA Ophthalmol. 2016;134(9):1001–6. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers JP, Hawkins BS, Schachat AP. Ocular histoplasmosis. In: Ryan S, Wilkinson C, Schachat A, et al. Retina Fifth Edition: Elsevier Inc., 2012: 1274–1284. [Google Scholar]
- 21.Chheda LV, Ferketich AK, Carroll CP, et al. Smoking as a Risk Factor for Choroidal Neovascularization Secondary to Presumed Ocular Histoplasmosis Syndrome. Ophthalmology 2012;119(2):333–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the results are available upon reasonable request to the senior author.