Abstract
Background:
Few studies have investigated the relationship of seminal L-Carnitine (LC) with male infertility associated with varicocele. The purpose of this prospective cross-sectional study was to assess seminal plasma LC levels in infertile oligoathenoteratozoospermic (OAT) men with varicocele.
Methods:
Overall, 86 men were investigated. They were divided into infertile OAT men with varicocele (n=45), infertile OAT men without varicocele (n=21), and fertile men (n=20) as a control group. According to WHO guidelines, these men were subjected to history taking, clinical examination, and semen analysis. Seminal LC levels were evaluated by the colorimetric method. Statistical comparisons were done using Kruskal-Wallis and Mann-Whitney U tests and correlations were verified by the Pearson test. P-value<0.05 was set to be statistically significant.
Results:
The mean seminal plasma LC levels were significantly lower in infertile OAT men with varicocele (216.3±57.1 ng/ml) compared to infertile OAT men without varicocele (252.9±62.9 ng/ml, p=0.01), or fertile men (382.8±63.6 ng/ml, p=0.001). Besides, the mean seminal plasma LC level exhibited statistically significant decreases in infertile OAT men of varicocele grade III compared to varicocele grade II cases, and in infertile OAT men with bilateral varicocele compared with unilateral varicocele cases. Collectively, there was a statistically significant positive correlation between seminal LC levels with sperm concentration, motility, and normal morphology.
Conclusion:
Seminal LC levels are expressively reduced in infertile OAT men with varicocele and are influenced by an increase in varicocele grade and laterality.
Keywords: Oligoasthenoteratozoospermic, Infertility, L-Carnitine, Men, Semen, Sperm, Varicocele
Introduction
Approximately, in 50% of infertile couples, nonstandard semen parameters point to male factor infertility (1). Various reasons have been offered for male factor infertility, the most important of which are hormonal imbalance, congenital anamolies, inflammatory problems, environment/lifestyle, as well as varicocele (2–5).
Varicocele has been reported in about 20% of adults and adolescents and 19–41% of males are suffering from infertility problems (6). Varicocele is habitually linked to decreased sperm count, total sperm motility, and normal morphology percentages (7–9). Many concepts were raised to elucidate how varicocele has a negative influence on male fertility. Those concepts comprise scrotal hyperthermia, Leydig cell dysfunction, metabolites retrograde flow, hypoxia, oxidative stress (OS), and apoptosis (10, 11).
L-Carnitine (LC) is an endogenous branched-chain non-essential amino acid derivative that is manufactured in multiple organs such as liver, kidneys, and testes, originating from L-lysine and L-methionine (12). Intracellular LC takes part in the lipid metabolism by transporting fatty acids from the cytoplasm to the mitochondria for β-oxidation (13, 14).
Higher concentrations of LC were reported in the human epididymis than in the peripheral blood (15, 16). Seminal LC was shown to play its role by improving the environment in the epididymal lumen and affecting β-oxidation of fatty acids in the mitochondria. Besides, seminal LC was demonstrated to imitate the effects of glucocorticoid that suppresses the macrophages protecting sperm membrane and chromatin from the damage of free oxygen radicals (17).
Numerous studies showed the valuable effects of LC intake on sperm motility, and sperm DNA fragmentation concluding that combining metabolic and micro-nutritive aspects is helpful for male factor infertility treatment (18–22). Tsampoukas et al. (23) pointed out that although the evidence regarding the role of LC as a primary or adjuvant treatment of varicocele is sparse, the pathophysiological significance of LC implicates a potential role of the molecule in the management of varicocele.
In this context, scarce studies investigated the relationship of seminal LC with male factor infertility associated with varicocele (24–26). Therefore, there is a gap in the literature and clinical practice regarding association of LC deficiency with both the grade as well as severity of varicocele. Therefore, this work was designed to evaluate seminal plasma LC levels in infertile OAT men with varicocele.
Methods
This prospective cross-sectional study comprised 66 infertile OAT men who were recruited from the University Hospital, in addition to 20 healthy fertile men as controls, after obtaining the ethics committee approval in addition to informed consents. Sample size calculation was based on a single proportion formula (27). The cases were divided into 3 groups of fertile males without varicocele (n=20), infertile OAT men without varicocele (n=21), and infertile OAT men with varicocele (n=45). Fertile controls fulfilled the criteria for normozoospermia (sperm concentration >15 million/ml, total sperm motility >40%, and sperm normal forms >4%) and having offspring in the previous two years. Inclusion criteria of the patients’ group were OAT (sperm concentration <15 million/ml, total sperm motility <40%, and sperm normal forms <4%), lack of initiating pregnancy within a year of unguarded sexual relation, and normal female factor. Exclusion criteria were azoospermia, secondary varicocele, congenital anomalies, smoking, and leukocytospermia.
All participants were subjected to history taking, genital examination, and semen analysis. The ejaculates were collected after 4–5 days of sexual abstinence and were inspected according to WHO guidelines (28). Clinical examination was carried out in the standing position with/without the Vals-alva maneuver. Color Doppler was carried out to diagnose varicocele (29). Varicocele was classified into grade I (only palpable during Valsalva maneuver), grade II (palpable distension on standing upright), and grade III (visible through scrotal skin) (30). LC levels in the seminal plasma were estimated utilizing a colorimetric assay kit (Bio-Vision, USA). The range for L-Carnitine is ∼1–200 μM with a detection sensitivity of 10 μM (colorimetric).
Statistical analysis: SPSS software version 23 (IBM, USA) was used for the statistical analysis. Kolmogorov-Smirnov test demonstrated that the data were not normally distributed. Comparisons amongst the investigated groups were carried out using Kruskal-Wallis and Mann-Whitney U tests for non-parametric data. Correlations between the variables were verified by the Pearson test. The p-value <0.05 was set to be statistically significant.
Results
The participants demonstrated matched age and no statistically significant difference was found between all investigated groups (p=0.173). Clinically, the group of infertile OAT men with varicocele consisted of 20 cases that had grade II varicocele and 25 cases that had grade III varicocele. Additionally, 10 cases had unilateral varicocele and 35 cases had bilateral varicocele. The mean seminal plasma LC levels were lower in infertile OAT men with varicocele compared to infertile OAT men with no varicocele as well as fertile controls and the differences were statistically significant (p=0.001) (Table 1). In infertile OAT men with varicocele, the mean seminal LC levels were lower in infertile OAT men with grade III varicocele (n=21) compared to infertile OAT men with grade II (n=25) (p=0.001) (Table 2), and in infertile OAT men with bilateral varicocele (n=35) compared with infertile OAT men with unilateral varicocele (n=10); the obtained differences were statistically significant (p=0.036) (Table 3). Collectively, seminal LC levels exhibited statistically significant positive correlations with sperm concentration (r=0.638, p=0.001), total motility percentages (r=0.705, p=0.001), and normal forms percentages (r=0.690, p=0.001) (Figure 1).
Table 1.
Baseline characteristics of the investigated groups [mean±SD (median)]
| Fertile men (n=20) | Infertile OAT men without varicocele (n=21) | Infertile OAT men with varicocele (n=45) | p-value | |
|---|---|---|---|---|
| Age (years) | 29.5±5.5 (30) | 29.4±5.4 (28) | 30.8±3.8 (32) | 0.173 |
| Semen volume (ml) | 2.5±1.0 (2.25) | 2.6±1.7 (2.3) | 2.3±0.97 (2.2) | 0.813 |
| Sperm concentration (106/ml) | 49.9±20.2 (53.3) | 6.7±4.1 (6.3) | 5.7±2.7 (6.0) | 0.001 * |
| Sperm total motility (%) | 57.5±8.0 (60) | 26.2±8.8 (30) | 16.9±7.7 (15.0) | 0.001 * |
| Sperm normal morphology (%) | 5.5±0.9 (5.0) | 2.4±0.9 (2.0) | 2.1±0.8 (2.0) | 0.001 * |
| Seminal LC (ng/ml) | 382.8±63.6 (386.9) | 252.9±62.9 (262.2) | 216.3±57.1 (221.7) | 0.001 * |
Statistical analysis using Kruskal-Wallis test
LC: L-Carnitine, OAT: Oligoasthenoteratozoospermia
Table 2.
Baseline characteristics according to varicocele grade [mean ± SD (median)]
| Grade II varicocele (n=25) | Grade III varicocele (n=20) | p-value | |
|---|---|---|---|
| Age (years) | 30.6±3.4 (32.0) | 31.2±4.3 (31.0) | 0.810 |
| Semen volume (ml) | 2.0±0.8 (2.0) | 2.4±0.81.1 (2.7) | 0.067 |
| Sperm concentration (106/ml) | 6.1±2.9 (6.5) | 5.1±2.1 (5.4) | 0.150 |
| Sperm total motility (%) | 18.6±7.7 (20.0) | 14.8±7.3 (15.0) | 0.114 |
| Sperm normal forms (%) | 2.3±0.8 (2.0) | 1.8±0.6 (2.0) | 0.026 * |
| Seminal LC (ng/ml) | 237.3±29.3 (234.7) | 174.0±62.3 (182.5) | 0.001 * |
Statistical analysis using Mann-Whitney U test
LC: L-Carnitine, OAT: Oligoasthenoteratozoospermia
Table 3.
Baseline characteristics in OAT men with unilateral versus bilateral varicocele [mean±SD (median)]
| Unilateral varicocele (n=10) | Bilateral varicocele (n=35) | p-value | |
|---|---|---|---|
| Age (years) | 31.7±2.8 (32.0) | 30.6±4.1 (31.0) | 0.308 |
| Semen volume (ml) | 2.3±0.7 (2.4) | 2.2±1.00.8 (2.0) | 0.653 |
| Sperm concentration (106/ml) | 4.6±2.6 (4.8) | 6.0±2.6 (6.0) | 0.158 |
| Sperm total motility (%) | 14.0±5.2 (15.0) | 17.7±8.17.7 (20.0) | 0.215 |
| Sperm normal forms (%) | 2.3±0.7 (2.0) | 2.0±0.8 (2.0) | 0.363 |
| Seminal LC (ng/ml) | 242.5±37.0 (255.0) | 199.3±57.8 (211.5) | 0.036 * |
Statistical analysis using Mann-Whitney U test
LC: L-Carnitine, OAT: Oligoasthenoteratozoospermia
Figure 1.
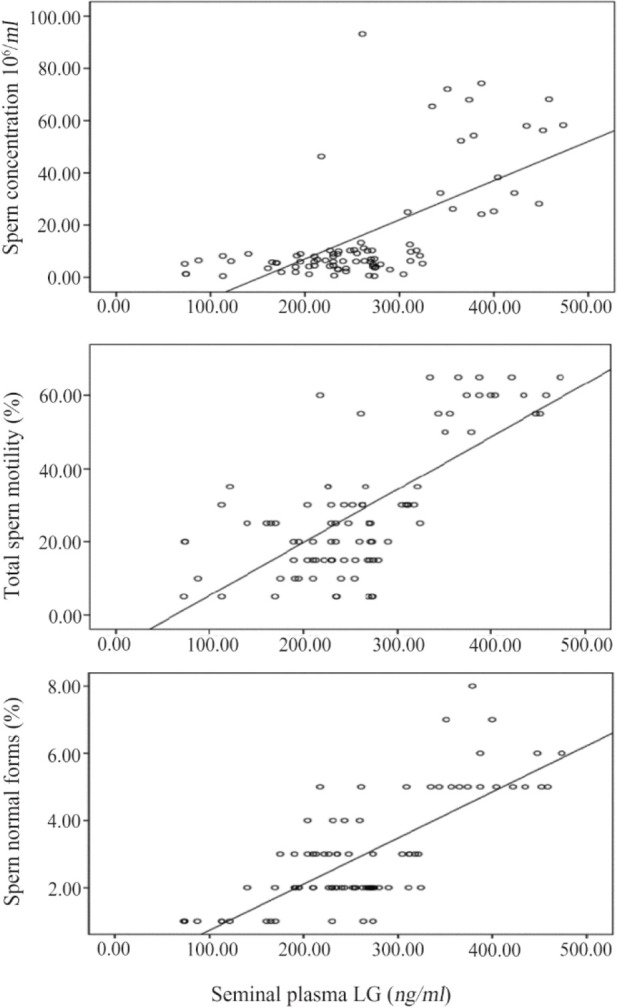
Significant positive correlations between seminal plasma LC (ng/ml) levels with sperm concentration, total sperm motility percentages, and sperm normal forms percentages
Discussion
Varicocele is one of the common correctable causes of male infertility. It has been demonstrated that varicocelectomy in men with abnormal semen parameters was associated with better fertility outcome. The adjuvant drug therapy, especially different types of antioxidants, seems to be associated with better fertility outcome, in comparison with no medical treatment (31, 32). Therefore, assessment of some antioxidants such as LC could be beneficial for clinical purposes.
In this study, the mean seminal plasma LC levels were significantly lower in infertile OAT men with varicocele compared to infertile OAT men without varicocele and fertile controls. Besides, seminal plasma LC levels exhibited statistically significant positive correlations with sperm concentration, motility, and normal forms percentages. Those aforementioned outcomes show the association of normal levels of seminal LC with normozoospermic semen parameters.
Matalliotakis et al. (33) reported that seminal LC levels differ significantly between fertile men and infertile men and there is statistically significant positive correlation between the levels and sperm count, motility percentage, and normal forms percentage. Moreover, several researchers reported statistically significant positive correlations between seminal LC levels with total sperm count and sperm normal morphology percentage (34–36). Furthermore, the role of seminal free LC in the preservation of normal sperm features was validated in previous research (37–39).
Reviewing the literature, most studies that linked varicocele with male infertility focused mainly on the role of testis. Yet, it was suggested that varicocele can also disturb the epididymis that plays a central role in sperm maturation, sperm motility, and is the place where the sperm nucleus alters chromatin condensation (15, 40, 41).
Previously, Lenzi et al. (42) pointed out that the effects of LC on the male genital function are connected mainly with its high concentration in the epididymis where the uptake of the LC from the blood is active. Moreover, epididymal sperm can concentrate LC during their passage from the caput to the cauda to provide patterns of energetic substrate. This function is of great importance since epididymal sperm employ fatty acid oxidation for their energy metabolism; on the contrary, the ejaculated sperm employ the glycolytic process. Besides, carnitines also possess both antioxidant as well as anti-apoptotic properties (43, 44) that could counteract the accentuated burden of the seminal OS as well as apoptotic markers in infertile men associated with varicocele (8, 45, 46).
These effects were perceived with the statistically significant decreases in seminal LC levels in infertile OAT men of grade III varicocele compared to infertile OAT men of grade II, and in men with bilateral varicocele compared with unilateral one. This relation could be explained due to the increased OS effect associated with these conditions on the antioxidant LC. Lehtihet et al. (47) concluded that left-sided grade III varicocele could induce a reversible suppression of the epididymal function where treatment of varicocele results in improved semen quality as well as epididymal function. Sofimajidpour et al. (48) reported statistically significant decreases in sperm total motility in grade III varicocele compared to grade II varicocele. Also, Mostafa et al. (49) showed that different seminal miRNA levels are lowered in infertile OAT men with varicocele which is linked to elevated varicocele grade and its bilaterality is negatively correlated with OS (apoptotic markers). Besides, Alkan et al. (50) and Ashrafzade et al. (51) linked ROS overproduction to elevated varicocele grade, reduced semen concentration, and normal sperm morphology.
However, this study still has some limitations due to the relatively low number of participants, the need to investigate fertile men with varicocele, and the necessity to assess seminal LC after varicocele surgical repair in infertile OAT men with varicocele.
Conclusion
The aforementioned results show that seminal plasma LC levels are significantly reduced in infertile OAT men with varicocele and are influenced by an increase in its grade and laterality. This finding indicates the benefits of assessing the seminal plasma LC in clinical practice among these men. Additionally, LC supplementation should be evaluated in such cases with/without varicocele surgical repair.
Acknowledgement
None.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Funding: None.
References
- 1.Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mintziori G, Nigdelis MP, Mathew H, Mousiolis A, Goulis DG, Mantzoros CS. The effect of excess body fat on female and male reproduction. Metabolism. 2020;107(6):154193. [DOI] [PubMed] [Google Scholar]
- 3.Henkel R, Offor U, Fisher D. The role of infections and leukocytes in male infertility. Andrologia. 2021; 53(1):e13743. [DOI] [PubMed] [Google Scholar]
- 4.Mintziori G, Kita M, Duntas L, Goulis DG. Consequences of hyperthyroidism in male and female fertility: pathophysiology and current management. J Endocrinol Invest. 2016;39(8):849–53. [DOI] [PubMed] [Google Scholar]
- 5.Vander-Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018; 62:2–10. [DOI] [PubMed] [Google Scholar]
- 6.Roque M, Esteves SC. Effect of varicocele repair on sperm DNA fragmentation: a review. Int Urol Nephrol. 2018;50(4):583–603. [DOI] [PubMed] [Google Scholar]
- 7.D’Andrea S, Micillo A, Barbonetti A, Giordano AV, Carducci S, Mancini A, et al. Determination of spermatic vein reflux after varicocele repair helps to define the efficacy of treatment in improving sperm parameters of subfertile men. J Endocrinol Invest. 2017;40(10):1145–53. [DOI] [PubMed] [Google Scholar]
- 8.Pallotti F, Paoli D, Carlini T, Vestri AR, Martino G, Lenzi A, et al. Varicocele and semen quality: a retrospective case-control study of 4230 patients from a single centre. J Endocrinol Invest. 2018;41(2):185–92. [DOI] [PubMed] [Google Scholar]
- 9.Mostafa T, Nabil N, Rashed L, Abo-Sief AF, Eissa HH. Seminal SIRT1-oxidative stress relationship in infertile oligoasthenoteratozoospermic men with varicocele after its surgical repair. Andrologia. 2020; 52(1):e13456. [DOI] [PubMed] [Google Scholar]
- 10.Mostafa T, Rashed LA, Nabil NI, Osman I, Mostafa R, Farag M. Seminal miRNA relationship with apoptotic markers and oxidative stress in infertile men with varicocele. Biomed Res Int. 2016;2016: 4302754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen CFS, Østergren P, Dupree JM, Ohl DA, Sønksen J, Fode M. Varicocele and male infertility. Nat Rev Urol. 2017;14(9):523–33. [DOI] [PubMed] [Google Scholar]
- 12.Ringseis R, Keller J, Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur J Nutr. 2012;51(1):1–18. [DOI] [PubMed] [Google Scholar]
- 13.Ribas GS, Vargas CR, Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014;533(2):469–76. [DOI] [PubMed] [Google Scholar]
- 14.Bene J, Hadzsiev K, Melegh B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr Diabetes. 2018;8(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potts RJ, Jefferies TM, Notarianni LJ. Antioxidant capacity of the epididymis. Hum Reprod. 1999;14(10):2513–6. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online. 2004;8(4):376–84. [DOI] [PubMed] [Google Scholar]
- 17.Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the treatment of idiopathic asthenozoospermia: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2006;85(5):1409–14. [DOI] [PubMed] [Google Scholar]
- 18.Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, et al. Placebo controlled double blind randomized trial on the use of L-carnitine and L-acetyl-carnitine combined treatment in asthenozoospermia. Fertil Steril. 2004;81(6):1578–84. [DOI] [PubMed] [Google Scholar]
- 19.Mongioi L, Calogero AE, Vicari E, Condorelli RA, Russo GI, Privitera S, et al. The role of carnitine in male infertility. Andrology. 2016;4(5):800–7. [DOI] [PubMed] [Google Scholar]
- 20.Hasson MA. The relationship between the use of L-Carnitine supplements and the male sub-infertility. Int J Pharm Qual Assur. 2019;10(4):735–40. [Google Scholar]
- 21.Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, et al. The effect of anti-oxidants on male factor infertility: the males, anti-oxidants, and infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113(3):552–60.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Cui Y, Dong L, Sun M, Zhang Y. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: a systematic review and meta-analysis. Andrologia. 2020;52(2):e13470. [DOI] [PubMed] [Google Scholar]
- 23.Tsampoukas G, Khan MF, Katsouri A, Akhter W, Moussa M, Deliveliotis K, et al. L-carnitine as primary or adjuvant treatment in infertile patients with varicocele. a systematic review. Arch Ital Urol Androl. 2020;92(3). [DOI] [PubMed] [Google Scholar]
- 24.Abbaticchio G, Giagulli VA, Defini M, Micale FM, Giorgino R. Free l-carnitine in human semen. Arch Androl. 1985;15(2–3):137–42. [DOI] [PubMed] [Google Scholar]
- 25.Andó S, Carpino A, Buffone M, Maggiolini M, Sisci D. The evaluation of free-L-carnitine, zinc and fructose in the seminal plasma of patients with varicocele and normozoospermia. Andrologia. 1989; 21(2):155–60. [PubMed] [Google Scholar]
- 26.Pajovic B, Dimitrovski A, Radojevic N, Vukovic M. A correlation between selenium and carnitine levels with hypo-osmotic swelling test for sperm membrane in low- grade varicocele patients. Eur Rev Med Pharmacol Sci. 2016;20(4):598–604. [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson B, Trapp R. Basic and clinical biostatistics. 4th ed. USA: McGraw-Hill; 2004. 416 p. [Google Scholar]
- 28.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5th ed. Switzerland: Geneva: WHO Press; 2010. 271 p. [Google Scholar]
- 29.Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21(1):56–83. [DOI] [PubMed] [Google Scholar]
- 30.Kim YS, Kim SK, Cho IC, Min SK. Efficacy of scrotal Doppler ultrasonography with the Valsalva maneuver, standing position, and resting-Valsalva ratio for varicocele diagnosis. Korean J Urol. 2015;56(2):144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YW, Niu YH, Wang DQ, Li H, Pokhrel G, Xu H, et al. Effect of adjuvant drug therapy after varicocelectomy on fertility outcome in males with varicocele-associated infertility: systematic review and meta-analysis. Andrologia. 2018;50(8):e13070. [DOI] [PubMed] [Google Scholar]
- 32.Majzoub A, ElBardisi H, Covarrubias S, Mak N, Agarwal A, Henkel R, et al. Effect of microsurgical varicocelectomy on fertility outcome and treatment plans of patients with severe oligozoospermia: an original report and meta-analysis. Andrologia. 2021;53(6):e14059. [DOI] [PubMed] [Google Scholar]
- 33.Matalliotakis I, Koumantaki Y, Evageliou A, Matalliotakis G, Goumenou A, Koumantakis E. L-carnitine levels in the seminal plasma of fertile and infertile men: correlation with sperm quality. Int J Fertil Womens Med. 2000;45(3):236–40. [PubMed] [Google Scholar]
- 34.Gürbüz B, Yalti S, Fiçicioğlu C, Zehir K. Relationship between semen quality and seminal plasma total carnitine in infertile men. J Obstet Gynaecol. 2003;23(6):653–6. [DOI] [PubMed] [Google Scholar]
- 35.De Rosa M, Boggia B, Amalfi B, Zarrilli S, Vita A, Colao A, et al. Correlation between seminal carnitine and functional spermatozoal characteristics in men with semen dysfunction of various origins. Drugs R D. 2005;6(1):1–9. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh N, Goodarzi M, Bab Al-Havaejee H, Safari M, Amiri I, Najafi R, et al. L-carnitine level in seminal plasma of fertile and infertile men. J Res Health Sci. 2007;7(1):43–8. [PubMed] [Google Scholar]
- 37.Tang LF, Jiang H, Shang XJ, Zhao LM, Bai Q, Hong K, et al. [Seminal plasma levocarnitine significantly correlated with semen quality]. Zhonghua Nan Ke Xue. 2008;14(8):704–8. Chninese. [PubMed] [Google Scholar]
- 38.Haseen Ahmed SD, Ahsan S, Iqbal T, Ahmed Burney SI. Relationship of seminal free L-Carnitine with functional spermatozoal characteristics: results from an observational study conducted in a tertiary care hospital of Karachi, Pakistan. J Pak Med Assoc. 2017;67(2):280–4. [PubMed] [Google Scholar]
- 39.Xu Y, Lu H, Wang Y, Zhang Z, Wu Q. Comprehensive metabolic profiles of seminal plasma with different forms of male infertility and their correlation with sperm parameters. J Pharm Biomed Anal. 2020;177:112888. [DOI] [PubMed] [Google Scholar]
- 40.Roaiah MM, Mostafa T, Salem D, El-Nashar AR, Kamel II, El-Kashlan MS. alpha-1,4-Glucosidase activity in infertile oligoasthenozoospermic men with and without varicocele. Andrologia. 2007;39(1):28–32. [DOI] [PubMed] [Google Scholar]
- 41.Vivas-Acevedo G, Lozano-Hernández R, Camejo MI. Varicocele decreases epididymal neutral α- glucosidase and is associated with alteration of nuclear DNA and plasma membrane in spermatozoa. BJU Int. 2014;113(4):642–9. [DOI] [PubMed] [Google Scholar]
- 42.Zhu B, Zheng YF, Zhang YY, Cao YS, Zhang L, Li XG, et al. Protective effect of L-carnitine in cyclophosphamide-induced germ cell apoptosis. J Zhejiang Univ Sci B. 2015;16(9):780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Modanloo M, Shokrzadeh M. Analyzing mitochondrial dysfunction, oxidative stress, and apoptosis: potential role of L-carnitine. Iran J Kidney Dis. 2019;13(2):74–86. [PubMed] [Google Scholar]
- 44.Naderi Noreini S, Malmir M, Ghafarizadeh A, Faraji T, Bayat R. Protective effect of L-carnitine on apoptosis, DNA fragmentation, membrane integrity and lipid peroxidation of spermatozoa in the asthenoteratospermic men. Andrologia. 2021;53(2):e13932. [DOI] [PubMed] [Google Scholar]
- 45.Mostafa T, Anis T, Imam H, El-Nashar AR, Osman IA. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia. 2009;41(2):125–9. [DOI] [PubMed] [Google Scholar]
- 46.Mostafa T, Rashed L, Nabil N, Amin R. Seminal BAX and BCL2 gene and protein expressions in infertile men with varicocele. Urology. 2014;84(3): 590–5. [DOI] [PubMed] [Google Scholar]
- 47.Lehtihet M, Arver S, Kalin B, Kvist U, Pousette A. Left-sided grade 3 varicocele may affect the biological function of the epididymis. Scand J Urol. 2014;48(3):284–9. [DOI] [PubMed] [Google Scholar]
- 48.Sofimajidpour H, Ghaderi E, Ganji O. Comparison of the effects of varicocelectomy and oral L-carnitine on sperm parameters in infertile men with varicocele. J Clin Diagn Res. 2016;10(4):PC07–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostafa T, Rashed L, Taymour M. Seminal cyclooxygenase relationship with oxidative stress in infertile oligoasthenoteratozoospermic men with varicocele. Andrologia. 2016;48(2):137–42. [DOI] [PubMed] [Google Scholar]
- 50.Alkan İ, Yüksel M, Canat H, Atalay HA, Can O, Özveri H, et al. Superoxide anion production by the spermatozoa of men with varicocele: relationship with Varicocele grade and semen parameters. World J Mens Health. 2018;36(3):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashrafzade A, Sadighi-Gilani M, Topraggaleh T, Khojasteh M, Sepidarkish M, Borjian Boroujeni P, et al. Oxidative stress-related miRNAs in spermatozoa may reveal the severity of damage in grade III varicocele. Andrologia.2020;52(9):e13598. [DOI] [PubMed] [Google Scholar]


