ABSTRACT
Background
Observational studies suggest that blood concentrations of 25-hydroxyvitamin D [25(OH)D] are associated with morbidity, viral suppression, and mortality among adults living with HIV.
Objectives
We evaluated the effect of cholecalciferol (vitamin D3) supplementation on the risk of HIV disease progression, HIV-1 viral suppression, comorbidities, weight change, and depression among HIV-infected individuals that were initiating antiretroviral therapy (ART) in Dar es Salaam, Tanzania.
Methods
We conducted a randomized, double-blind, placebo-controlled trial of vitamin D3 supplementation among 4000 HIV-infected adult men and nonpregnant women initiating ART with insufficient serum 25(OH)D concentrations (<30 ng/mL). Participants were randomly assigned to receive either weekly 50,000-IU doses for 4 wk followed by daily 2000 IU vitamin D3 until 1 y or a matching placebo regimen given in weekly followed by daily doses until 1 y. Participants were followed up at weekly visits for the first month followed by monthly visits thereafter. We conducted intent-to-treat analyses to assess the effect of vitamin D3 supplementation on the secondary trial outcomes of HIV progression or death, viral suppression, comorbidities, change in BMI, >10% weight loss, incident wasting, and depression.
Results
During follow-up, 345 participants (17.2%) in the vitamin D3 group and 371 participants (18.6%) in the placebo group experienced HIV disease progression or death and there was no difference in risk between groups (RR: 0.91; 95% CI: 0.79, 1.06). Vitamin D3 supplementation did not affect the risk of an unsuppressed HIV-1 viral load (>1000 copies/mL) after 6 mo (RR: 1.10; 95% CI: 0.87, 1.41) and there was also no effect on change in BMI, risk of >10% weight loss, wasting, comorbidities, and depression (P values >0.05).
Conclusions
Vitamin D supplementation did not affect the risk of HIV progression, viral suppression, common morbidities, weight-related indicators, or depression among adults initiating ART in Tanzania.
This trial was registered at clinicaltrials.gov as NCT01798680.
Keywords: vitamin D, dietary supplements, micronutrients, HIV, antiretroviral therapy, body weight, depression, clinical trial, sub-Saharan Africa
Introduction
Vitamin D deficiency is estimated to affect ∼1 billion people globally with an additional 50% of the global population being vitamin D insufficient (1). In sub-Saharan Africa, it is estimated that 34.2% of the population is vitamin D deficient as defined by serum or plasma 25-hydroxyvitamin D [25(OH)D] concentrations < 20 ng/mL; however, there is a high degree of heterogeneity in the prevalence of deficiency among countries and populations within the region (2). Although vitamin D deficiency is common in the general population, people living with HIV may be at higher risk than the general population owing to factors related to HIV infection, including inflammation and comorbidities, as well as exposure to antiretroviral medications (3, 4). In a prior observational study in Tanzania, we found that 9.2% of adults initiating antiretroviral therapy (ART) had vitamin D deficiency [serum 25(OH)D < 20 ng/mL] and 43.6% were vitamin D insufficient [serum 25(OH)D = 20–30 ng/mL] (5).
Vitamin D is important for calcium homeostasis and bone health; however, more recent evidence suggests it may play a role in multiple extraskeletal conditions, including infectious and noncommunicable diseases (6). Observational studies have found that low vitamin D status is associated with an increased risk of acute respiratory infections (ARIs) and randomized trials have identified beneficial effects of vitamin D supplementation on the incidence of ARIs (7–9). In the context of HIV, observational studies have found vitamin D deficiency to be associated with an increased risk of HIV disease progression, increased risk of comorbidities, and mortality (6, 10, 11). A previous observational study conducted in HIV-infected individuals in Tanzania found that vitamin D deficiency at ART initiation was associated with an increased risk of mortality, pulmonary tuberculosis (PTB), and oral thrush along with wasting and weight loss (5, 12). Observational data have also shown associations between low vitamin D and increased risk of depression in the general population (13, 14) and depression is a common comorbidity among people living with HIV in sub-Saharan Africa (15, 16). To the best of our knowledge, no randomized trials to date have evaluated the effect of vitamin D supplementation on HIV disease progression, viral suppression, common comorbidities related to HIV or ART, weight change, and depression in sub-Saharan Africa.
We conducted a randomized, double-blind, placebo-controlled trial of cholecalciferol (vitamin D3) supplementation in HIV-infected adults initiating ART in Tanzania. We previously reported no effect of vitamin D3 supplementation on the coprimary trial outcomes of mortality and incidence of PTB (17). We also found that the vitamin D3 supplementation regimen increased serum 25(OH)D concentrations as expected (17). In this study, we present the effect of vitamin D supplementation on secondary trial outcomes including HIV progression or death, viral suppression, morbidity, weight change, and depression.
Methods
The Trial of Vitamins—4 (ToV4) was a randomized, double-blind, placebo-controlled trial (NCT01798680) of vitamin D3 supplementation conducted among 4000 HIV-infected adults that were initiating ART in Dar es Salaam, Tanzania. The trial protocol and the efficacy results for the primary trial outcomes of all-cause mortality and incidence of PTB have been reported elsewhere (17, 18). The trial was approved by the Harvard TH Chan School of Public Health Institutional Review Board (IRB13-0231), the Tanzanian National Health Research Ethics Sub-Committee (National Institute for Medical Research/HQ/R.8a/Vol. IX/1658), and the Tanzania Food and Drug Authority (Tanzania Medicines and Medical Devices Authority 13/CTR/0005/3). Written informed consent was obtained from all participants.
Briefly, the trial was conducted from February 2014 to March 2018 at 4 large HIV care and treatment centers. Participants were eligible for enrollment if they met the following inclusion criteria: 1) adult men or women aged ≥18 y, 2) documented HIV diagnosis, 3) initiating ART at the time of randomization, 4) insufficient serum 25(OH)D concentration <30 ng/mL at the screening visit, 5) intended to stay in Dar es Salaam for ≥1 y after enrollment, and 6) provided written informed consent. Exclusion criteria were 1) pregnant women and 2) enrolled in any other clinical trial. All study participants were provided with HIV care and treatment that adhered to Tanzanian national guidelines (18). Efavirenz–lamivudine–tenofovir was the first-line ART regimen during the trial. Participants received co-trimoxazole prophylaxis if their CD4 T-cell count was <200 cells/μL. Participants diagnosed with tuberculosis received directly observed therapy with a 6-mo short-course regimen comprising a 2-mo intensive phase of daily rifampicin–isoniazid–pyrazinamide–ethambutol followed by a 4-mo continuation phase of daily rifampicin and isoniazid. All participants also received psychological counseling and general nutritional counseling on diet for people living with HIV as the standard of care at each clinic visit. No additional food or nutritional supplements were provided as standard of care.
Screening and randomization
The screening visit for ToV4 was integrated into the HIV care and treatment program ART eligibility visit. CD4 T-cell count was measured at the ART eligibility visit (FACS-Calibur System, Becton Dickinson). Serum 25(OH)D concentrations were measured with a commercial enzyme immunoassay (Immunodiagnostics) and test procedures have been presented in the trial protocol (18). Participants who were eligible for randomization and consented to enrollment were randomly assigned in a 1:1 ratio to the vitamin D3 supplementation or placebo group. The randomization list was computer-generated and stratified by study clinic. Participant allocation was completely concealed through the use of prelabeled, sequential participant identification numbers. The trial participants, study staff, and investigators were blinded to the randomly assigned groups.
The vitamin D3 supplementation group received oral supplements containing 50,000 IU vitamin D3 to be taken under direct observation at the randomization visit and once a week for the next 3 wk at study clinic visits (total of 4 doses, each 50,000 IU), followed by daily oral supplements containing 2000 IU vitamin D3 to be taken at home starting at the fourth week until trial discharge at 1 y postrandomization. The trial protocol presents complete details on the rationale for the selection of the vitamin D3 supplementation regimen (18). Briefly, the regimen was intended to increase 25(OH)D concentrations quickly and safely with the weekly 50,000 IU supplements during the first month of ART followed by maintenance of 25(OH)D concentrations thereafter with the daily 2000-IU supplements until trial discharge at 1 y. The placebo group received matching oral placebo supplements taken at identical times under direct observation for the first month and then daily from 1 mo until trial discharge. Both the vitamin D3 and the placebo supplements were manufactured by Tishcon Corporation and were identical in appearance, taste, smell, and weight.
At the randomization visit, study participants received a full clinical examination and tuberculosis screening from study physicians and were assessed for WHO HIV disease stage. Participants suspected of having PTB had a chest X-ray and provided a sputum sample on the day PTB was suspected, were given a container for an early-morning sputum sample collected the next day, and had a third sputum sample collected at the clinic the following day. Sputum smears were stained using the Ziehl–Nielsen technique and examined for acid-fast bacilli. PTB was diagnosed when ≥1 sputum smear had acid-fast bacilli detected or if there were radiological features suggestive of PTB when sputum smears were negative. Study nurses assessed participant height and weight, then collected information on sociodemographic characteristics and self-reported symptoms during the last 28 d, and administered the Hopkins Symptom Checklist (HSCL-25) to assess depressive symptoms (19).
Participant follow-up procedures
All participants were followed at weekly clinic visits for 3 wk (weeks 1, 2, and 3) followed by monthly clinic visits starting at 1 mo until discharge at 12 mo postrandomization. At all clinic visits, physicians performed a full clinical examination, screened for PTB, and assessed WHO HIV disease stage. In addition, at all visits research nurses administered a questionnaire that included symptoms reported within the last 28 d, and measured participant weight. Nurses administered the HSCL-25 to assess depressive symptoms at the 6- and 12-mo visits. Nurses directly observed participants taking the trial regimen during the 3 weekly clinic visits and conducted pill counts at the monthly clinic visits when participants took the regimen at home. Adherence was quantified separately for weekly and monthly supplement doses. The adherence percentage for the directly observed weekly doses was calculated as the number of weekly doses taken by the participant divided by the number of weekly doses expected. The adherence percentage for the daily doses that was assessed by pill count was calculated as the number of daily doses taken by the participant divided by the number of daily doses expected.
Outcome definitions
HIV disease progression was defined as any increase in WHO HIV disease stage during follow-up from the WHO HIV stage at randomization, or death (20). Participant BMI (in kg/m2) was calculated at randomization and all follow-up visits. We examined the effect of vitamin D3 supplementation on mean BMI over follow-up, the incidence of wasting (BMI <18.5), and weight loss of >10% from randomization based on the definition of HIV-related wasting (21). At every study visit, participant symptoms in the past 28 d were assessed based on self-report of a list that included nausea or vomiting, cough, fever, diarrhea, oral thrush, skin rashes or lesions, neuropathy, and genital discharge or sores. An unsuppressed HIV-1 viral load was defined as >1000 copies/mL (22). The HSCL-25 was used to assess symptoms of depression and anxiety at 6 and 12 mo of follow-up. The HSCL-25 has previously been validated among Tanzanian women living with HIV and, based on the validation study results, we defined symptoms consistent with depression as an average score >1.06 for 8 questions (23). In the validation study, this cutoff demonstrated 88% sensitivity and 89% specificity compared with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of major depressive disorder. Nevertheless, this cutoff has not been validated among men living with HIV in Tanzania and therefore we also assessed HSCL-25 scores continuously and conducted a sensitivity analysis among all participants that defined symptoms consistent with depression using the standard HSCL-25 cutoff of 1.75 (19).
Statistical analysis
An intent-to-treat analysis was used as the analytic strategy for all analyses. For time-to-event analysis of HIV disease progression, >10% weight loss from ART initiation, and incident wasting, the log-rank test was used to evaluate differences between the treatment groups. Proportional hazard regression models were also used to produce HRs. The effect of vitamin D3 supplementation on BMI at monthly clinic visits was examined using linear mixed-effects models with a random intercept, a compound symmetric covariance structure, and robust SEs. We examined the effect of vitamin D3 supplementation on postrandomization symptoms reported to nurses at monthly clinic visits (repeated events) using generalized estimating equations with an exchangeable working covariance and the log link to produce population-averaged RR estimates (24). Log-binomial regression models were used to assess the RRs of the binomial outcomes of symptoms consistent with depression at 6 and 12 mo of follow-up and unsuppressed (>1000 copies/mL) and detectable viral load (>50 copies/mL) after 6 mo of ART. Linear regression models with a robust empirical variance structure were used to analyze continuous HSCL-25 scores. All models included a fixed effect for study clinic to account for stratified randomization. Sensitivity analyses that adjusted for baseline 25(OH)D concentration and CD4 T-cell count, which showed some degree of imbalance between treatment groups based on a P < 0.20, were conducted (17). All statistical analyses were done with SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Figure 1 presents the trial flow diagram. A total of 6250 HIV-infected individuals initiating ART consented to serum 25(OH)D screening, of which 4848 (77.7%) had 25(OH)D concentrations <30 ng/mL and were eligible for randomization. Among the eligible individuals, 4000 participants were enrolled and randomly assigned in the trial (2001 to vitamin D3 and 1999 to placebo). The baseline characteristics were similar between the randomly assigned groups (Table 1). The percentage of trial participants with baseline 25(OH)D concentrations that indicated vitamin D insufficiency (20–30 ng/mL) was 48.2%, 44.6% of participants had mild vitamin D deficiency [25(OH)D = 10–20 ng/mL], and only 7.2% of participants had 25(OH)D concentrations that indicated moderate or severe vitamin D deficiency (<10 ng/mL).
FIGURE 1.
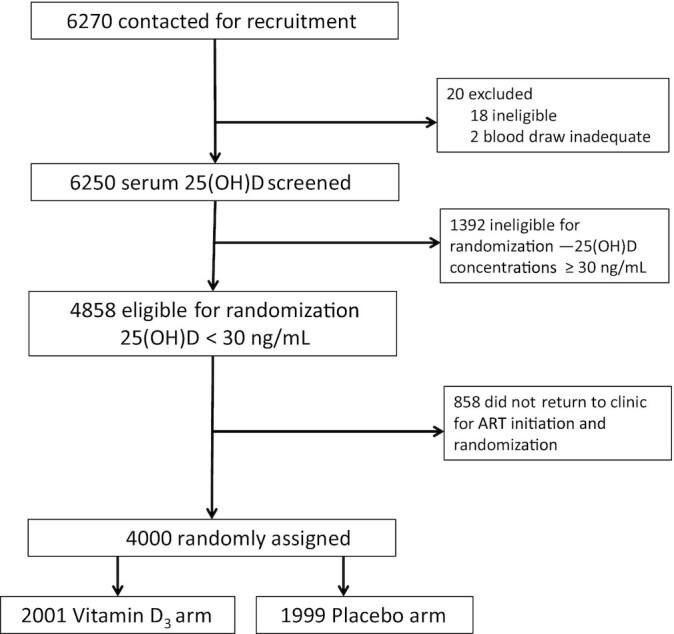
Trial flow diagram for recruitment of HIV-infected adult men and nonpregnant women with insufficient serum 25(OH)D concentrations (<30 ng/mL) at ART initiation. ART, antiretroviral therapy; vitamin D3, cholecalciferol; 25(OH)D, 25-hydroxyvitamin D.
TABLE 1.
Baseline characteristics of HIV-infected adult men and nonpregnant women with insufficient serum 25(OH)D concentrations (<30 ng/mL) at ART initiation enrolled in the trial, by vitamin D3 and placebo treatment groups1
| Vitamin D3 (n = 2001) | Placebo (n = 1999) | |
|---|---|---|
| Sex | ||
| Female | 1367 (68) | 1368 (68) |
| Male | 634 (32) | 631 (32) |
| Age, y | 38.6 ± 9.8 | 38.8 ± 10.0 |
| Education | ||
| No formal education | 308 (15) | 327 (16) |
| Primary | 1294 (64) | 1294 (65) |
| Secondary/advanced | 398 (20) | 377 (19) |
| BMI, kg/m2 | ||
| <18.5 | 440 (22) | 404 (20) |
| 18.5–24.9 | 1038 (52) | 1064 (53) |
| ≥25.0 | 521 (26) | 531 (27) |
| CD4 T-cell count, cells/μL | ||
| <200 | 866 (43) | 845 (42) |
| 200–349 | 461 (23) | 445 (22) |
| 350–499 | 300 (15) | 333 (17) |
| ≥500 | 284 (14) | 178 (14) |
| Missing | 90 (5) | 98 (5) |
| WHO HIV disease stage | ||
| I/II | 744 (37) | 760 (38) |
| III | 1161 (58) | 1143 (57) |
| IV | 96 (5) | 96 (5) |
| Baseline pulmonary tuberculosis | 189 (10) | 175 (9) |
| HSCL-25 score consistent with depression | 982 (49) | 953 (48) |
| ART regimen | ||
| Efavirenz/lamivudine/tenofovir | 1940 (97) | 1943 (97) |
| Other ART regimen | 61 (3) | 56 (3) |
| 25(OH)D concentrations at screening visit, ng/mL | ||
| Insufficient (20.0–30.0) | 955 (48) | 972 (49) |
| Moderate deficiency (10.0–19.9) | 920 (46) | 865 (43) |
| Severe deficiency (0–9.9) | 126 (6) | 162 (8) |
n = 4000. Values are n (%) or mean ± SD. ART, antiretroviral therapy; HSCL-25, Hopkins Symptom Checklist-25; vitamin D3, cholecalciferol; 25(OH)D, 25-hydroxyvitamin D.
During the trial, 415 deaths (10.4%) were recorded, 19 (0.5%) participants withdrew consent to continue participating, and 94 (2.4%) women became pregnant and were censored. Loss to follow-up was low in both arms (2.1% in the vitamin D3 group and 1.7% in the placebo group). Adherence to the trial supplements was high in both groups; 81.6% and 81.4% of participants took all the weekly doses in the vitamin D3 and placebo groups, respectively. The mean adherence for the daily regimen supplements was 80.8% for the vitamin D3 group and 80.6% for the placebo group.
HIV progression or death
During the trial, 345 of 2001 participants (17.2%) in the vitamin D3 and 371 of 1999 participants (18.6%) in the placebo group experienced HIV disease progression or death and there was no difference in risk between the randomly assigned groups (P = 0.22) (Table 2).
TABLE 2.
Effect of vitamin D3 supplementation on HIV disease progression or death, viral suppression, nutritional outcomes, and depression as compared with placebo among HIV-infected adult men and nonpregnant women with insufficient serum 25-hydroxyvitamin D concentrations (<30 ng/mL) at ART initiation enrolled in the trial1
| Vitamin D3n events/ n participants at risk (%) | Placebo n events/ n participants at risk (%) | HR (95% CI) | P value | |
|---|---|---|---|---|
| HIV disease progression or death2 | 345 / 2001 (17.2) | 371 / 1999 (18.6) | 0.91 (0.79, 1.06) | 0.22 |
| Unsuppressed viral load >1000 copies/mL after 6 mo of ART3 | 123 / 993 (12.4) | 107 / 951 (11.3) | 1.10 (0.87, 1.41) | 0.43 |
| >10% weight loss from ART initiation4 | 213 / 1844 (11.6) | 204 / 1847 (11.0) | 1.05 (0.87, 1.28) | 0.60 |
| Incident wasting (BMI < 18.5 kg/m2)5 | 113 / 1559 (7.3) | 137 / 1595 (8.6) | 0.84 (0.66, 1.08) | 0.17 |
| HSCL-25 scores consistent with depression at 6 mo of ART6 | 368 / 1385 (26.6) | 395 / 1387 (28.5) | 0.94 (0.83, 1.06) | 0.29 |
| HSCL-25 scores consistent with depression at 12 mo of ART6 | 228 / 1232 (18.5) | 242 / 1261 (19.2) | 0.96 (0.82, 1.12) | 0.60 |
ART, antiretroviral therapy; HSCL-25, Hopkins Symptom Checklist-25; vitamin D3, cholecalciferol.
HIV disease progression defined as any increase in WHO HIV disease stage or death.
Among participants who had HIV-1 viral load assessed after 6 mo of ART.
Among participants who had baseline weight assessed and ≥1 postbaseline weight assessment.
Among participants who had baseline weight assessed, a baseline BMI ≥18.5 kg/m2, and ≥1 postbaseline weight assessment.
Tanzania-adapted HSCL-25 cutoff used to define depression.
HIV-1 viral suppression
The proportion of participants with an unsuppressed viral load (>1000 copies/mL) after 6 mo of ART was 12.4% in the vitamin D3 group and 11.3% in the placebo group and there was no difference in risk between the randomly assigned groups (RR: 1.10; 95% CI: 0.87, 1.41) (Table 2).
Morbidities
Table 3 presents the effect of vitamin D3 supplementation on participant-reported morbidities at monthly follow-up visits. There was no effect of vitamin D3 on the risk of nausea or vomiting, cough, fever, diarrhea, oral thrush, skin rashes or lesions, neuropathy, and genital discharge or sores during follow-up (P values >0.05).
TABLE 3.
Effect of vitamin D3 supplementation on participant-reported morbidities during the prior month collected at monthly follow-up clinic visits as compared with placebo among HIV-infected adult men and nonpregnant women with insufficient serum 25-hydroxyvitamin D concentrations (<30 ng/mL) at antiretroviral therapy initiation enrolled in the trial1
| Vitamin D3n visits morbidity reported/ n follow-up clinic visits (%) | Placebo n visits morbidity reported/ n follow-up clinic visits (%) | RR (95% CI) | P value | |
|---|---|---|---|---|
| Weakness or fatigue | 593 / 15,049 (3.9) | 598 / 14,996 (4.0) | 0.98 (0.85, 1.11) | 0.71 |
| Nausea or vomiting | 343 / 15,049 (2.3) | 305 / 14,996 (2.0) | 1.11 (0.92, 1.34) | 0.27 |
| Cough | 489 / 15,049 (3.3) | 490 / 14,996 (3.3) | 0.99 (0.85, 1.16) | 0.89 |
| Fever | 460 / 15,049 (3.1) | 436 / 14,996 (2.9) | 1.04 (0.89, 1.21) | 0.65 |
| Diarrhea | 181 / 15,049 (1.2) | 157 / 14,996 (1.1) | 1.13 (0.87, 1.47) | 0.35 |
| Oral thrush | 59 / 15,049 (0.4) | 48 / 14,996 (0.3) | 1.21 (0.81, 1.80) | 0.35 |
| Skin rashes or lesions | 527 / 15,049 (3.5) | 479 / 14,996 (3.2) | 1.09 (0.93, 1.27) | 0.29 |
| Neuropathy | 262 / 15,049 (1.7) | 242 / 14,996 (1.6) | 1.06 (0.84, 1.34) | 0.61 |
| Genital discharge or sores | 260 / 15,049 (1.7) | 245 / 14,996 (1.6) | 1.05 (0.83, 1.31) | 0.70 |
Vitamin D3, cholecalciferol.
Weight change, >10% weight loss, and incident wasting
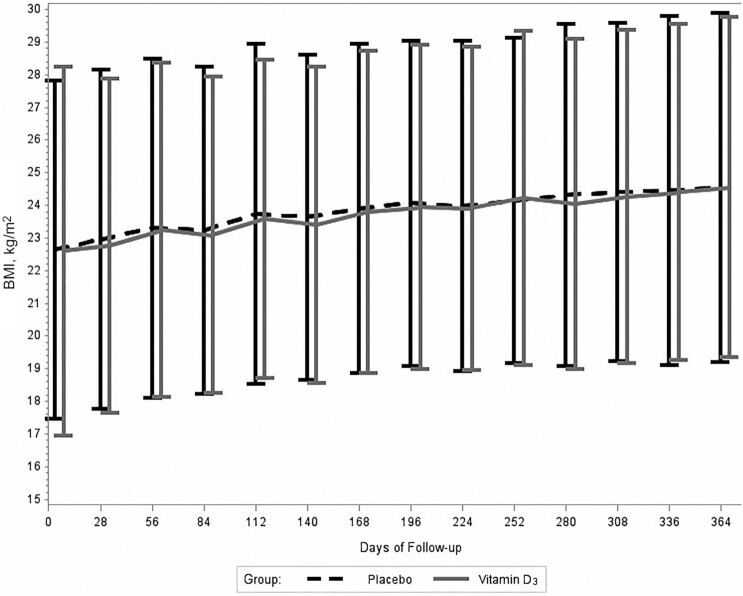
The mean BMI of participants increased from ART initiation to 12 mo of treatment in both randomly assigned groups (Figure 2). The mean ± SD BMIs at baseline and the 12-mo endline visit were 22.6 ± 5.7 and 24.6 ± 5.2 in the vitamin D3 group and 22.6 ± 5.2 and 24.6 ± 5.3 in the placebo group, respectively. There was no difference in change in BMI over the course of follow-up between the randomly assigned groups (P value for difference in trajectory = 0.99). There was also no difference in the risk of >10% weight loss from baseline (HR: 1.05; 95% CI: 0.87, 1.28) or incident wasting (HR: 0.84; 95% CI: 0.66, 1.08) between the randomly assigned groups (Table 2).
FIGURE 2.
Effect of vitamin D3 supplementation on participant BMI at monthly follow-up clinic visits as compared with placebo among HIV-infected adult men and nonpregnant women with insufficient serum 25-hydroxyvitamin D concentrations (<30 ng/mL) at antiretroviral therapy initiation enrolled in the trial. Values presented are means with bars indicating SDs. P value for difference in BMI trajectory between treatment groups = 0.99. Vitamin D3, cholecalciferol.
Depression
There was no effect of vitamin D3 on the risk of symptoms consistent with depression at 6 and 12 mo of follow-up using the Tanzania-adapted HSCL-25 cutoffs (P values >0.05) (Table 2). In a sensitivity analysis, there was also no effect on symptoms consistent with depression using the standard HSCL-25 cutoff at 6 mo (RR: 1.07; 95% CI: 0.94, 1.23) or 12 mo of follow-up (RR: 0.83; 95% CI: 0.61, 1.12).
Sensitivity analyses
In sensitivity analyses, there was no change in the findings after adjusting for potential baseline imbalances in 25(OH)D and CD4 T-cell count between the treatment groups (Supplemental Tables 1 and 2).
Discussion
In this randomized, double-blind, placebo-controlled trial, vitamin D3 supplementation did not affect secondary trial outcomes of HIV disease progression or death, viral load suppression, the incidence of morbidities, weight change, wasting, or depressive symptoms among adults living with HIV that were initiating ART in Tanzania.
We found no effect of vitamin D3 supplementation on the composite outcomes of HIV disease progression or death. These findings are consistent with our null findings on the coprimary trial outcomes of all-cause mortality and PTB (17). Overall, our trial findings are not consistent with our prior observational cohort studies in Tanzania as well as studies from high-income settings, which have found low concentrations of vitamin D to be associated with increased risk of mortality, disease progression, and poor treatment outcomes among people living with HIV (5, 6, 11). In a prior observational cohort study in Tanzania in adults initiating ART, we found that vitamin D deficiency defined by 25(OH)D concentrations <20 ng/mL at ART initiation was associated with twice the risk of mortality as compared with individuals who were vitamin D sufficient [25(OH)D concentrations >30 ng/mL] (5). Further, in the EuroSIDA cohort study in people living with HIV in Europe, Israel, and Argentina, individuals with 25(OH)D concentrations <12 ng/mL had an increased risk of mortality or AIDS events as compared with individuals with 25(OH)D concentrations >12 ng/mL (11). In addition, we found no effect of vitamin D3 supplementation on viral suppression, which is an important risk factor for HIV disease progression or death. This finding is also in contrast to several observational studies that have found an association between low vitamin D and greater HIV-1 viral load (25, 26). In addition, a small randomized trial of vitamin D3 supplementation among 58 children and young adults living with HIV in the United States found no effect on HIV-1 RNA detection but found some indication that vitamin D reduced absolute viral concentrations among individuals with detectable viral loads (27). As a result, our randomized trial results are not aligned with evidence from observational studies and may be due to low concentrations of vitamin D in blood in observational studies acting as a marker of disease severity or other factors rather than indicating a causal effect of vitamin D on HIV disease progression or viral suppression. Therefore, the association of vitamin D status with HIV disease progression and viral load suppression in observational studies may be attributable to unmeasured or residual confounding or other biases.
We also found no effect of vitamin D3 supplementation on participant-reported morbidities. In a prior cohort study in Tanzania, we found that vitamin D deficiency at ART initiation was associated with increased risk of oral thrush, but there was no association with participant-reported cough, diarrhea, rash, neuropathy, nausea or fatigue, or genital ulcers (12). As a result, the null findings in the trial are partially aligned with the observational data. Nevertheless, randomized trials of vitamin D supplementation, conducted among individuals not living with HIV, have found an overall protective effect on the incidence of ARIs (9). As a result, the effect of vitamin D on ARIs and other comorbidities may differ for people living with HIV, whose risk of ARIs and other opportunistic infections is largely associated with the degree of immunosuppression (28).
We also found no effect of vitamin D3 supplementation on nutritional outcomes including change in BMI and incidence of >10% weight loss or wasting. In a prior observational cohort study in Tanzania, we found that vitamin D deficiency at ART initiation was associated with increased risk of >10% weight loss from ART initiation and wasting. It was hypothesized that vitamin D supplementation may reduce the incidence of comorbidities, including PTB, which are in turn associated with weight loss. In our randomized trial, we previously reported no overall effect of vitamin D supplementation on the incidence of PTB and we also found no effect on the incidence of other comorbidities or weight change in this study. Nevertheless, there is some observational evidence that vitamin D supplementation may play a role in weight loss or obesity in populations not affected by HIV, but randomized trial evidence is needed (29).
In addition, we found no effect of vitamin D3 supplementation on the risk of depression at 6 or 12 mo after ART initiation. Similar to other outcomes, prospective observational studies have found low serum 25(OH)D to be a risk factor for depression but this relation has not been replicated in randomized trials (14). Our null findings are aligned with the results of the recent large VITAL-DEP (VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention) trial conducted among a general population of US men and women over the age of 50 y that found no effect of vitamin D supplementation on the incidence or recurrence of depression as well as depressive symptoms (30). Our findings are also consistent with other randomized trials of vitamin D3 supplementation that have found no effect on depressive symptoms (31, 32). Therefore, the role of vitamin D supplementation in depression remains unclear, but the current evidence from randomized trials suggests limited to no effect.
There are several limitations to our study. Less than 5% of participants had 25(OH)D concentrations <10 ng/mL at baseline and therefore our findings may not be generalizable to populations with a greater prevalence of severe vitamin D deficiency. In addition, there was likely some degree of misclassification of participant-reported morbidities that are likely nondifferential to the randomly assigned treatment group and therefore would bias estimates toward the null. Last, we used the Tanzania-adapted HSCL-25 cutoff which was validated among pregnant women living with HIV, and as a result there may have been some degree of misclassification for men and nonpregnant women in our study (23). Nevertheless, there was no effect of vitamin D3 on depression in a sensitivity analysis of depression defined by the standard HSCL-25 cutoff.
In conclusion, vitamin D3 supplementation did not affect the risk of HIV progression or death, viral suppression, comorbidities, weight change, or depression outcomes. Taken together with the null findings of the trial on the primary outcomes of death and incidence of PTB, our findings do not support the routine use of vitamin D3 supplementation for adults living with HIV initiating ART during the first year of ART. Alternative strategies are needed to improve treatment outcomes and mental health for adults living with HIV in Tanzania and similar settings.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—CRS, FM, and WWF: designed the research; AM, WWF, FM, NU, TJN, SA, and CRS: conducted the research; CRS and MW: conducted the statistical analysis; AM and CRS: drafted the manuscript and had primary responsibility for the final content; WWF, FM, NU, TJN, SA, and MW: critically reviewed and revised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK098075 (to WWF).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ARI, acute respiratory infection; ART, antiretroviral therapy; HSCL-25, Hopkins Symptom Checklist-25; PTB, pulmonary tuberculosis; ToV4, Trial of Vitamins—4; vitamin D3, cholecalciferol; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Alfa Muhihi, Management and Development for Health, Dar es Salaam, Tanzania; Department of Community Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Wafaie W Fawzi, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Said Aboud, Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Tumaini J Nagu, Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Nzovu Ulenga, Management and Development for Health, Dar es Salaam, Tanzania.
Molin Wang, Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Biostatistics, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Ferdinand Mugusi, Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Christopher R Sudfeld, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Department of Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, USA.
Data Availability
Reasonable requests for de-identified individual participant data described in this article may be directed to the corresponding author. Data sharing requests will be subject to ethical approval and data transfer agreements.
References
- 1. Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mogire RM, Mutua A, Kimita W, Kamau A, Bejon P, Pettifor JMet al. . Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2020;8(1):e134–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15(3):425–9. [DOI] [PubMed] [Google Scholar]
- 4. Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17(4):513–20. [DOI] [PubMed] [Google Scholar]
- 5. Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7(6):e40036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Havers F, Smeaton L, Gupte N, Detrick B, Bollinger RC, Hakim Jet al. . 25-Hydroxyvitamin D insufficiency and deficiency is associated with HIV disease progression and virological failure post-antiretroviral therapy initiation in diverse multinational settings. J Infect Dis. 2014;210(2):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–9. [DOI] [PubMed] [Google Scholar]
- 8. Laaksi I, Ruohola J-P, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki Het al. . An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–17. [DOI] [PubMed] [Google Scholar]
- 9. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman Pet al. . Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vescini F, Cozzi-Lepri A, Borderi M, Re MC, Maggiolo F, De Luca Aet al. . Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58(2):163–72. [DOI] [PubMed] [Google Scholar]
- 11. Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso Met al. . Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25(10):1305–15. [DOI] [PubMed] [Google Scholar]
- 12. Sudfeld CR, Giovannucci EL, Isanaka S, Aboud S, Mugusi FM, Wang Met al. . Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2013;207(3):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H, Sun D, Wang A, Pan H, Feng W, Ng CHet al. . Serum 25-hydroxyvitamin D levels and depression in older adults: a dose–response meta-analysis of prospective cohort studies. Am J Geriatr Psychiatry. 2019;27(11):1192–202. [DOI] [PubMed] [Google Scholar]
- 14. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56–61. [DOI] [PubMed] [Google Scholar]
- 15. Bernard C, Dabis F, de Rekeneire N. Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0181960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regan M, Muhihi A, Nagu T, Aboud S, Ulenga N, Kaaya Set al. . Depression and viral suppression among adults living with HIV in Tanzania. AIDS Behav. 2021;25(10):3097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sudfeld CR, Mugusi F, Muhihi A, Aboud S, Nagu TJ, Ulenga Net al. . Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2020;7(7):e463–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sudfeld CR, Mugusi F, Aboud S, Nagu TJ, Wang M, Fawzi WW. Efficacy of vitamin D3 supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial. Trials. 2017;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1–15. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach: 2010 revision. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 21. Polsky B, Kotler D, Steinhart C. HIV-associated wasting in the HAART era: guidelines for assessment, diagnosis, and treatment. AIDS Patient Care STDs. 2001;15(8):411–23. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- 23. Kaaya SF, Fawzi MC, Mbwambo JK, Lee B, Msamanga GI, Fawzi W. Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatr Scand. 2002;106(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diggle PJ, Heagerty P, Liang K-Y, Zeger S. Analysis of longitudinal data. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 25. Flauzino T, Simao ANC, de Almeida ERD, Morimoto HK, Oliveira SR, Alfieri DFet al. . Association between vitamin D status, oxidative stress biomarkers and viral load in human immunodeficiency virus type 1 infection. Curr HIV Res. 2017;15(5):336–44. [DOI] [PubMed] [Google Scholar]
- 26. Bearden A, Abad C, Gangnon R, Sosman JM, Binkley N, Safdar N. Cross-sectional study of vitamin D levels, immunologic and virologic outcomes in HIV-infected adults. J Clin Endocrinol Metab. 2013;98(4):1726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stallings VA, Schall JI, Hediger ML, Zemel BS, Tuluc F, Dougherty KAet al. . High-dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo-controlled trial. Pediatr Infect Dis J. 2015;34(2):e32–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benito N, Moreno A, Miro JM, Torres A. Pulmonary infections in HIV-infected patients: an update in the 21st century. Eur Respir J. 2012;39(3):730–45. [DOI] [PubMed] [Google Scholar]
- 29. Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc. 2015;74(2):115–24. [DOI] [PubMed] [Google Scholar]
- 30. Okereke OI, Reynolds CF 3rd, Mischoulon D, Chang G, Vyas CM, Cook NRet al. . Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324(5):471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Koning EJ, Lips P, Penninx B, Elders PJM, Heijboer AC, den Heijer Met al. . Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am J Clin Nutr. 2019;110(5):1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bot M, Brouwer IA, Roca M, Kohls E, Penninx B, Watkins Eet al. . Effect of multinutrient supplementation and food-related behavioral activation therapy on prevention of major depressive disorder among overweight or obese adults with subsyndromal depressive symptoms: the MooDFOOD randomized clinical trial. JAMA. 2019;321(9):858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reasonable requests for de-identified individual participant data described in this article may be directed to the corresponding author. Data sharing requests will be subject to ethical approval and data transfer agreements.