ABSTRACT
Overreliance on short-acting β2-agonists (SABA) has been a common feature of asthma management globally for at least 30 years. However, given the evidence against the long-term use of SABA, including potentially increased risk of exacerbations, emergency room visits, overall healthcare resource utilization, and mortality, the latest Global Initiative for Asthma report no longer recommends SABA only therapy. Since 2014, we implemented an ICS-containing reliever strategy at our asthma center at the G Baigorria Hospital in Argentina; we only administered budesonide/formoterol via a single inhaler device across the spectrum of asthma severity and completely eliminated the use of SABA therapy. In this article, we compare hospitalization data from our center, previously reported in the EAGLE study (when inhaled corticosteroids plus as-needed SABA was administered) for the years 1999 and 2004 with data from 2017 to 2018 (when budesonide/formoterol in a single inhaler device was administered as maintenance and/or anti-inflammatory reliever therapy [MART/AIR] without any SABA) from our center, to assess the impact of two distinct asthma management strategies on asthma-related hospitalizations. MART/AIR regimens in our SABA-free center reduced asthma hospitalizations from 9 (1999 and 2004) to 1 (2017 and 2018) (Fisher’s exact test, p = 0.031; odds ratio = 0.11; 95% confidence interval [CI] = 0.013–0.98); the hospitalization rate was reduced by 92% (1.47% in 1999 and 2004 to 0.12% in 2017 and 2018). Our data provide preliminary real-world evidence that MART/AIR with budesonide/formoterol simultaneously with SABA elimination across asthma severities is an effective asthma management strategy for reducing asthma-related hospitalizations.
KEYWORDS: Asthma management, exacerbations, hospitalizations, Short-acting beta2 agonists
Introduction
Asthma affects approximately 339 million patients worldwide [1]; an analysis of 46 countries reported a reduction in asthma mortality rates from 0.44 deaths/100 000 people in 1993 to 0.19 deaths/100 000 patients in 2006 [2]. However, despite improvements in clinical care and the adoption of policy changes in some countries, there was no appreciable change in global asthma mortality rates from 2006 to 2012 [2]. Therefore, novel strategies may be required to substantially reduce asthma-related mortality [2]. Since 2006, the Global Initiative for Asthma (GINA) has recommended the use of low-dose inhaled corticosteroids (ICS)/formoterol as a reliever option for patients at steps 3–5 (evidence A) [3]. This key strategy reduced exacerbation rates by 32%, as demonstrated in a meta-analysis of 16 randomized controlled trials in over 20 000 patients with persistent asthma [4]. Additionally, results from a systematic review and meta-analysis reported that as-needed ICS/formoterol was associated with a significantly prolonged time to first severe exacerbation compared with ICS plus as-needed short-acting β2-agonist (SABA) regimens in patients with mild asthma [5]. Consequently, since 2019, GINA has recommended ICS/formoterol administration at steps 1 and 2 as the preferred reliever [6].
Since 2014, we have implemented an anti-inflammatory reliever strategy at our asthma center at the G Baigorria Hospital in Argentina. We only administer budesonide/formoterol via a single inhaler device across the spectrum of asthma severity [7]. Herein, we compare hospitalization data previously reported in the EAGLE study [8] (when ICS plus as-needed SABA was administered) for years 1999 and 2004 with data from 2017 to 2018 (when budesonide/formoterol in a single inhaler device was administered as maintenance and/or anti-inflammatory reliever therapy) from our center. To test the hypothesis that by eradicating SABA and replace with budesonide/formoterol we would reduce asthma hospitalization, we assessed the impact of these two distinct asthma management strategies on asthma-related hospitalizations.
Materials and methods
The EAGLE study [8] assessed patients with asthma at three time points: 1994, 1999, and 2004. Our asthma center was included in the study. For this analysis, we used hospitalization data from our cohort (from the EAGLE study) from 1999 to 2004 and compared it with data from 2017 to 2018 from our center, further stratifying patients based on whether they were treated at our center or at another center prior to hospitalization. Clinical records of admitted patients (aged 15–69 years) from our center with a primary diagnosis of acute asthma exacerbations were described according to the International Classification of Diseases (Ninth Revision, code 493.01 and Tenth Revision, codes J45 and J46) if patients were hospitalized for at least 24 hours between 2017 and 2018. Patients with other diagnoses, such as chronic obstructive pulmonary disease and bronchiectasis; those with scheduled surgeries; and those who were smokers or ex-smokers with a smoking history of >10 pack-years were excluded. During both time periods (1999 and 2004, and 2017 and 2018), patients with asthma treated at our center had unrestricted access to the center and free asthma medications [9], with SABA elimination in the latter period being the major difference between the two time periods. An unpaired Student’s t-test was used for continuous variables. The odds ratio was calculated by a two-tailed Fisher’s exact test that was used to analyze the contingency table, comparing the absolute number of hospitalizations for patients previously treated at our center with those who were not, using the GraphPad Prism 7 software (San Diego, CA, USA).
Results
The demographic and clinical characteristics of patients included in this analysis during both periods are presented in Table 1 [Table 1 near here]. In 1999 and 2004, 26 patients with asthma who were being treated at other centers were admitted to our hospital due to asthma exacerbations; nine patients treated at our asthma center were also admitted to hospital during that same time period. Only one of the nine patients were admitted twice and she died due to breast cancer 5 years later. Four further patients were lost for follow-up in the next study period. During the 2-year period 2017–2018, 18 patients who were being treated and followed up at other centers had asthma exacerbation-related hospitalizations, while only one patient treated at our asthma center was admitted to hospital during that same time period. This patient had a history of asthma and hyperventilation syndrome that resembled a severe asthma exacerbation. Notably, the only patient that died from this 2017 and 2018 group was a 53-year-old male patient with asthma who was being treated at one of the other centers and receiving SABA regularly following discontinuation of ICS plus long-acting β2-agonist (LABA) therapy. Our asthma patients in the 2017 and 2018 time period received: only budesonide/formoterol as needed in a 22%; while 75% were on regular maintenance budesonide/formoterol plus as needed. Twenty-two patients were under high ICS doses plus tiotropium and only two patients received omalizumab of the 869 patients.
Table 1.
General demographic and clinical characteristics of patients during the two study periods.
| Hospitalized patients followed up at other centers |
Hospitalized patients followed up in our center |
|||
|---|---|---|---|---|
| 1999 and 2004 | 2017 and 2018 | 1999 and 2004 | 2017 and 2018 | |
| Total number of hospitalized patients | 26 | 18 | 9 | 1a |
| Overall asthma patients | – | – | 611 | 869 |
| Age (years) | 42.0 ± 14.6 | 34.8 ± 17.0 | 42.2 ± 13.2 | 41 |
| Female | 14 | 13 | 7 | 1 |
| Male | 12 | 5 | 2 | 0 |
| Treatments | ||||
| SABA | 26 | 18 | 9 | 0 |
| Bud/form only PRN | – | – | – | 193 (22%) |
| Regular Middle dose plus PRN | – | 652 (75%) | ||
| Regular High dose+tiotropium | 22 | |||
| Omalizumab | 2 | |||
| Positive smoking history | 5 | 4 | 2 | 0 |
| Spirometry in the past year | 1 | 3 | 6 | 1 |
| Intubation | 2 | 6 | 1 | 1 |
| ICU admissions | 9 | 12 | 2 | 1 |
| Prior hospitalization | 21 | 5 | 8 | 1 |
| Deaths | 0 | 1 | 0 | 0 |
| Hospitalization rates | Unknown | Unknown | 1.47%b | 0.12%b |
Note: Data are presented as the number of patients unless stated otherwise. Hospitalization rates of the pooled patient population from other centers were unknown because the asthma population number (denominator) was not known.
Only one patient who followed up at our SABA-free center was hospitalized during 2017 − 2018.
Age is expressed in years as mean ± standard deviation.
Smoking history referred to current smokers or ex-smokers (all patients smoked less than 10 pack-years), while intubation refers to endotracheal intubation due to asthma.
aFisher’s exact test, p = 0.031; odds ratio = 0.11 (95% confidence interval = 0.013–0.98).
ICU, intensive care unit; SABA, short-acting β2-agonist.
bOverall, 611 patients with asthma were treated at our center in 1999 and 2004 and 869 in 2017 and 2018, the hospitalization rate was reduced from 1.47% in 1999 and 2004 to 0.12% in 2017 and 2018, marking a 92% reduction. PRN: as needed. Bud/form: budesonide 160 µg/formoterol 4.5 µg in a single inhaler. The middle dose of Budesonide/formoterol was 2 inhalations bid. High doses incorporated another inhaled corticosteroid.
During both study periods, all admitted patients who were being treated at other centers received high doses of as-needed SABA in the days prior to hospitalization. Many patients did not receive controller medications, and among those who did, adherence to controller medication in the past 2 weeks could not be verified because many patients had run out of ICS/LABA inhalers and did not claim or renew their prescriptions. This reflects a common patient behavior in which patients discontinue ICS medication and do not attend physician appointments, instead over relying on their SABA therapy. These patients had a SABA canister available that was increasingly used before admission, suggesting potential adverse clinical outcomes associated with high SABA use. Our analysis indicates that implementation of the Maintenance and Reliever Therapy (MART) or the Anti-Inflammatory Reliever (AIR) regimens and SABA-free strategy at our center significantly reduced asthma hospitalizations from nine in 1999 and 2004 to one in 2017 and 2018 (Fisher’s exact test, p = 0.031; odds ratio = 0.11; 95% confidence interval [CI] = 0.013–0.98; Figure 1) [Figure 1 near here]. Considering the 611 patients with asthma treated at our center in 1999 and 2004 and 869 in 2017 and 2018, the hospitalization rate was reduced from 1.47% in 1999 and 2004 to 0.12% in 2017 and 2018, marking a 92% reduction.
Figure 1.
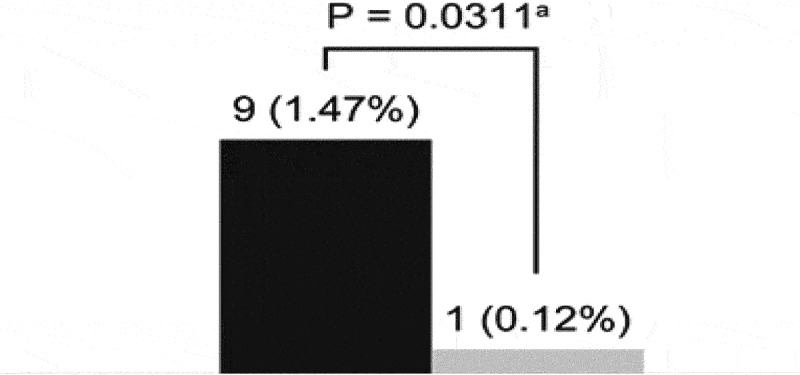
Asthma hospitalizations at our center in the two studied periods.
Discussion
Overreliance on SABA has been a common underlying feature of hospital admissions for asthma in Argentina for at least 30 years. Until GINA 2019 [6], SABA was consistently included as the reliever option in most asthma guidelines despite an insufficient, and often inconsistent, evidence base. For instance, SABA was cited in GINA recommendations with an anonymous reference in the appendix [10]. Moreover, initial recommendations for SABA were based on a short 5-hour trial published more than 50 years ago [11] that enrolled only 24 patients with asthma. Evidence favoring long-term SABA use in asthma is scarce, while there are multiple arguments against its use. SABA has the potential for long-term adverse effects, including an increase in asthma severity through enhanced bronchial hyperresponsiveness and reduced lung function [12]. Furthermore, SABA overuse potentially increases the risk of exacerbations [13–15], emergency room (ER) visits [16], overall healthcare resource utilization [13,16,17], and mortality [14,18]. Consequently, the GINA report no longer recommends SABA-only therapy [6].
An earlier publication from our center [19] reported that patients with asthma who did not use SABA as a reliever showed significantly better asthma control and improved adherence to maintenance treatment and had a higher ICS daily dose than those who used SABA as a reliever [19]. Our results attempted to demonstrate that eliminating SABA from asthma management is feasible when the combination inhaler with budesonide/formoterol is prescribed. The efficacy and safety of budesonide/formoterol as MART for moderate-to-severe asthma [4] and as an AIR therapy for mild-to-moderate asthma [5] are firmly established. Importantly, in addition to its well-known anti-inflammatory action, the ICS component has a nongenomic bronchodilator effect that may potentiate the effects of SABA on the airway smooth muscle [20].
Although our study sample is small, our asthma-related ER visit/hospitalization rate of 0.12% in 2017–2018 after adopting a SABA-free strategy was considerably lower than that reported in a large database study which analyzed >400 000 patients in the USA and UK (2.32% in 2010–2011 and 1.37% in 2009–2011, respectively) [21]. Furthermore, results of a nationwide telephone survey in 2014 reported hospitalization in 23% of patients (aged 20–44 years) with asthma who reported exacerbations in Argentina’s urban areas [22]. This finding indicates an urgent need for both patient and physician education and effective implementation of treatment strategies in Argentina per global evidence-based guidelines.
This retrospective observational study has some inherent limitations in terms of design, such as a small sample size, not matched comparison, potential selection/information bias, and lack of evaluation of secular trends and direct causality. Nonetheless, we have successfully shown that SABA reliance was the most common reason for patient hospitalization and that a significant reduction in hospitalizations is possible by simultaneously implementing an AIR or MART regimens and eliminating SABA, as documented at our center. Since our center is the referral unit for the region, it is highly unlikely that any of our asthma patients sought assistance at other sites during the study period under investigation; however, that possibility cannot be entirely discounted. Another potential limitation from a health policy viewpoint is that the inhaler cost for budesonide/formoterol treatment may be an initial obstacle to implementing MART in Argentina. However, published studies have shown that as-needed budesonide/formoterol and MART regimens are more cost-effective in the long-term compared with conventional treatment regimens, which should encourage physicians and policy makers to adopt MART regimens and eliminate SABA from asthma management in Argentina and globally [23–25]. Furthermore, Papi et al. [26] published that the risk of severe asthma exacerbation was significantly lower with as-needed use of a fixed-dose combination of 180 μg of albuterol and 160 μg of budesonide than with as-needed use of albuterol. These findings also clearly highlight the risk of SABA monotherapy.
Conclusion
The use of MART/AIR with budesonide/formoterol simultaneously with the elimination of SABA across asthma severities could be an effective strategy to prevent asthma-related hospitalizations.
Acknowledgments
This study was not funded by any entity. Although writing support was funded by AstraZeneca, the company had no intellectual input in the article.
Funding Statement
This study was not funded by any entity. Although writing support was funded by AstraZeneca, the company had no intellectual input in the article.
Author contributions statement
LJN, NSN, and NB contributed to the original survey design, drafted the analysis plan, carried out the statistical analysis and drafted and edited the manuscript. OMF and DMF contributed to the analysis plan and edited the manuscript. All authors approved the final manuscript for submission.
Disclosure statement
LJN has received personal fees as a speaker for Novartis and AstraZeneca, fees for participating in an advisory board from Sanofi Genzyme and AstraZeneca, and personal fees to travel to meetings from Boehringer Ingelheim and AstraZeneca. OMF has received personal fees as a speaker for AstraZeneca and fees to travel to meetings from Teva Pharmaceuticals and AstraZeneca. NSN, NB, and DMF do not have any conflicts of interest.
References
- [1].Global Asthma Network (GAN) . The global asthma report. 2018. cited2021 Aug 3 http://www.globalasthmareport.org
- [2].Ebmeier S, Thayabaran D, Braithwaite I, et al. Trends in international asthma mortality: analysis of data from the WHO mortality database from 46 countries (1993-2012). Lancet. 2017;390(10098):935–5. [DOI] [PubMed] [Google Scholar]
- [3].Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. [DOI] [PubMed] [Google Scholar]
- [4].Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting beta-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hatter L, Bruce P, Braithwaite I, Holliday, M, Fingleton, J, Weatherall, M, Beasley, R . ICS-formoterol reliever versus ICS and SABA reliever in asthma: a systematic review and meta-analysis. ERJ Open Res. 2021; 7(1):00701–2020. doi: 10.1183/23120541.00701-2020 PMID: 33532465; PMCID: PMC7836558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention. 2021. cited 2021 May 27 https://ginasthma.org/
- [7].Nannini LJ. Asthma paradoxes: time for a new approach across the spectrum of asthma severity. Eur Respir J. 2019;53(4):1802329. [DOI] [PubMed] [Google Scholar]
- [8].Rodrigo GJ, Neffen H, Nannini JA, et al. Hospitalizaciones por asma aguda durante el período 1994-2004 en Argentina y Uruguay: el estudio EAGLE. Rev Am Med Resp. 2009;9:41–48. [Google Scholar]
- [9].Nannini Jr LJ. Asthma educational program including free treatment reduced hospitalizations for asthma. Eur Respir J. 1997;10(25):101S. [Google Scholar]
- [10].Anonymous . Using beta 2-stimulants in asthma. Drug Ther Bull. 1997;35(1):1–4. [DOI] [PubMed] [Google Scholar]
- [11].Choo-Kang YF, Simpson WT, Grant IW. Controlled comparison of the bronchodilator effects of three beta-adrenergic stimulant drugs administered by inhalation to patients with asthma. Br Med J. 1969;2(5652):287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnston SL, Edwards MR. Mechanisms of adverse effects of β-agonists in asthma. Thorax. 2009;64(9):739–741. [DOI] [PubMed] [Google Scholar]
- [13].Bloom CI, Cabrera C, Arnetorp S, et al. Asthma-related health outcomes associated with short-acting β2-Agonist inhaler use: an observational UK study as part of the SABINA global Program. Adv Ther. 2020;37(10):4190–4208. [DOI] [PubMed] [Google Scholar]
- [14].Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stanford RH, Shah MB, D’Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403–407. [DOI] [PubMed] [Google Scholar]
- [16].FitzGerald JM, Tavakoli H, Lynd LD, et al. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–140. [DOI] [PubMed] [Google Scholar]
- [17].Silver HS, Blanchette CM, Kamble S, et al. Relationship between short-acting β2-adrenergic agonist use and healthcare costs. Am J Manag Care. 2011;17(1):19–27. [PubMed] [Google Scholar]
- [18].Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe (Sheff). 2015;11(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nannini LJ, Neumayer NS, Fernandez OM, et al. Outpatient asthma management without rescue bronchodilators? J Pulm Med Respir Res. 2019;5:024. [Google Scholar]
- [20].Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma: an evidence-based evaluation. Chest. 2006;130(5):1301–1311. [DOI] [PubMed] [Google Scholar]
- [21].Suruki RY, Daugherty JB, Boudiaf N, et al. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].NH ASJ, Bossio JC, Calabrese CA, et al. Prevalence and features of asthma in young adults in urban areas of Argentina. Arch Bronconeumol. 2018;54(3):134–139. [DOI] [PubMed] [Google Scholar]
- [23].FitzGerald JM, Arnetorp S, Smare C, et al. The cost-effectiveness of as-needed budesonide/formoterol versus low-dose inhaled corticosteroid maintenance therapy in patients with mild asthma in the UK. Respir Med. 2020;171:106079. [DOI] [PubMed] [Google Scholar]
- [24].Price D, Wirén A, Kuna P. Cost-effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy. 2007;62(10):1189–1198. [DOI] [PubMed] [Google Scholar]
- [25].Ställberg B, Ekström T, Neij F, et al. A real-life cost-effectiveness evaluation of budesonide/formoterol maintenance and reliever therapy in asthma. Respir Med. 2008;102(10):1360–1370. [DOI] [PubMed] [Google Scholar]
- [26].Papi A, Chipps BE, Beasley R, et al. Albuterol-budesonide fixed-dose combination rescue inhaler for asthma. N Engl J Med. 2022. Jun 2;386(22):2071–2083. Epub 2022 May 15. PMID: 35569035 [DOI] [PubMed] [Google Scholar]


