Abstract
Patient: Male, 19-year-old
Final Diagnosis: Odontogenic keratocyst
Symptoms: Swelling
Medication:—
Clinical Procedure: Enucleation
Specialty: Otolaryngology
Objective:
Challenging differential diagnosis
Background:
Odontogenic keratocysts are odontogenic cysts that increase in dimension based on growth factors and have a high recurrence rate. The radiological features of odontogenic keratocysts can be confusing owing to their similarity with other intraosseous cysts. The aim of treatment is to minimize patient morbidity and to reduce the risk of recurrence, along with complete surgical excision.
Case Report:
We report a case of a young man who presented to our hospital for a cystic lesion located in the posterior left mandible with clinical and radiological features of a dentigerous cyst. The lesion was treated accordingly for this diagnosis by enucleation. During surgery, a thick and firm cystic membrane was identified. Histopathological examination of the specimen established the final diagnosis of odontogenic keratocyst by identifying squamous epithelium with focal parakeratosis and ulceration and a diffuse inflammatory lymphoplasmacytic infiltrate. The patient’s evolution was favorable, with no sign of recurrence on cone beam computed tomography examination at the 6-month follow-up and with healing of the surgical defect.
Conclusions:
The diagnosis of odontogenic keratocyst is challenging, requiring preoperative 3-dimensional imaging and biopsy for extensive lesions. Adjuvant biochemical and immunological examination of cystic aspirate could sometimes be helpful for making a correct diagnosis. The treatment needs to be individualized according to the patient’s age and the tumor’s histopathological type and features. If the histopathological examination of surgical specimen indicates a more aggressive lesion than expected, a careful and individualized follow-up is imperative. No reintervention is needed if the patient does not present evidence of recurrence.
Keywords: Dentigerous Cyst, Diagnostic Errors, Odontogenic Cysts
Background
Odontogenic keratocysts (OKCs) are benign intraosseous lesions with a high recurrence rate [1]. Men are more commonly affected than women, with most OKCs occurring in White populations [1–3]. The age distribution is wide, ranging from 8 to 82 years, with the peak of incidence reported in the second and third decade of life [4]. OKCs occur in tooth-bearing regions. The mandible is more prone to develop OKCs than the maxilla, with the posterior region of mandible, the angle and ascending ramus, being involved most commonly [1–5].
The pathogenesis of OKCs is based on the mechanism of intraluminal hyperosmolarity, on the active epithelial proliferation, on the collagenolytic activity of the cyst wall and on the synthesis of interleukin-1 and interleukin-6 by keratinocytes [6]. This mechanism, different from other cysts (eg, dentigerous cysts), which grow purely by intraluminal hyperosmolarity, dictates a particular growth along the cancellous channels with a reduced cortical expansion, especially in large lesions [6,7].
OKCs are characterized by a cystic wall with a uniform parakeratosis squamous epithelium that has a well-defined basal layer of palisaded columnar or cuboidal cells. Also, a flat epithelium-connective tissue interface with unnoticeable rete ridge formation is observed. The histopathological features can be influenced by a history of local infection, with an inflammatory cell infiltrate being seen in this case. The cystic wall has small satellite cysts, cords, or islands of odontogenic epithelium that can be present beyond the fibrous wall into the adjacent intramedullary spaces [7,8].
Accurate clinical diagnosis is challenging owing to the similar radiological features of OKCs with bony cysts and benign bony tumors [4,9]. The radiological image of OKCs is usually described as a well-defined unilocular or multilocular radiolu-cent lesion with few intralesional bony septa, scalloped borders, and a clear peripheral radiopaque rim. The mandibular canal is usually marked by large lesions, and the roots of adjacent teeth are displaced with uncommon resorption of the roots [3,9]. Approximately 30% of OKCs are associated with an impacted tooth [3,9–11].
The choice of conservative or radical treatment is based on multiple variables: size, location of the lesion, unilocular or multilocular form, soft tissue management, presence of cortical bone perforation, and patient age [3,12]. There are many surgical options, starting with conservative enucleation alone or associated with additional measures (ostectomy, Carnoy’s solution, and cryotherapy), marsupialization and decompression, and ending with a radical treatment by marginal or segmental resection [3,12]. The included tooth is always extracted with the cystic lesion.
Bone resection is considered the best option for radical curative treatment and is associated with the lowest recurrence rates. Segmental resection (removal of a bone segment without maintaining bone continuity) and marginal resection (removal of the lesion and a margin of disease-free bone, maintaining osseus continuity) are associated with recurrence rates ranging from 0% to 8.4% in different studies [6,13,14]. However, bone resection has an increased morbidity and, therefore, it is not recommended as a primary treatment modality and should be reserved for treatment of multiple or recurring lesions [6,13,14]. However, there is still no consensus about the best treatment modality, especially of large tumors in young patients.
The recurrence of OKCs can be explained by many factors, such as young age (14–17 years), increased dimension, posterior mandible location, exitance of satellites and daughter cysts, or epithelial residues into the surgical field, as well as by OKCs associated with nevoid basal cell carcinoma syndrome (Gorlin Syndrome), which is prone to transform into local aggressive tumors or neoplasms such as ameloblastoma and squamous cell carcinoma [15,16]. Also, OKCs are defined by a higher mitotic activity compared with other cysts of odontogenic origin, a factor that can influence the recurrence rate [5,17].
We report the case of a young male patient who presented to our service for a mandibular dentigerous cyst-like lesion that had an unexpected histopathological examination diagnosis of OKC.
Case Report
A 19-year-old White male patient presented to the Oral and Maxillofacial Department of Cluj-Napoca County Hospital for left mandibular region swelling. The patient reported a cystic lesion incidentally identified by a routine orthopantomography 6 months prior to the medical consultation. Moreover, he indicated an acute inflammatory episode 5 months before this presentation, which he self-treated with antibiotics (amoxicillin clavulanate).
On physical examination, the patient presented mild facial asymmetry, a painless and hard ballooned bone on the left inferior vestibule with the disruption of the mandible bone contour in the retromolar trigone region.
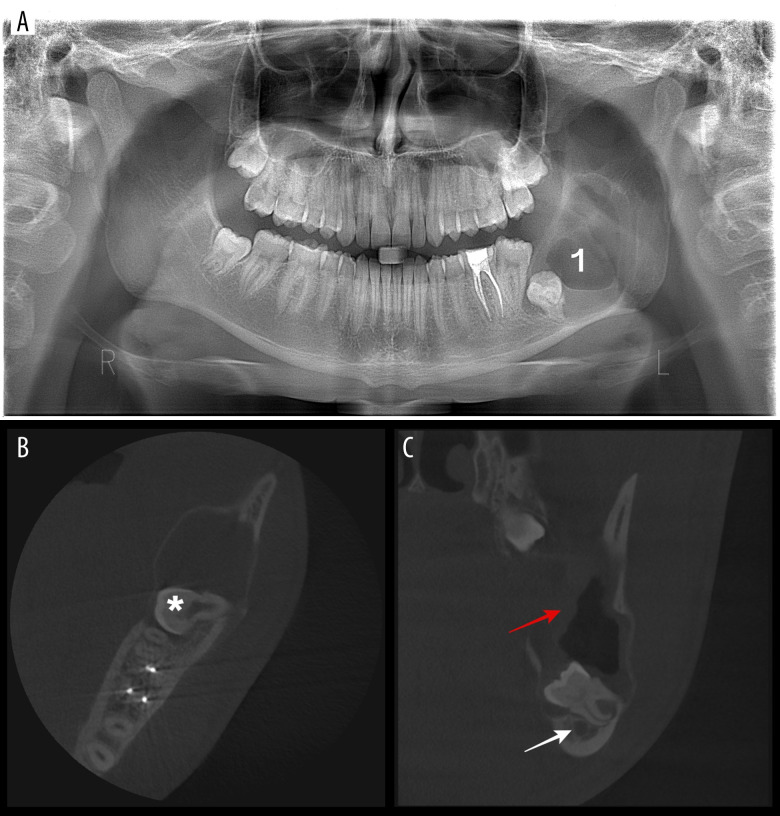
Orthopantomography illustrated an unilocular radiolucent lesion of the left mandibular posterior region and ascending ramus that was associated with a low-impacted third molar (Figure 1A). A cone beam computed tomography (CBCT) scan was then performed to better evaluate the lesion, illustrating a hypodense single cystic cavity with a well-defined contour, extended from the apical region of 3.7 to the sigmoid notch and measuring 40×24×20 mm. The cyst presented a membrane thickness of 8 mm. The lesion displaced the inferior alveolar nerve to the basilar and posterior margins of the mandible and mildly ballooned the buccal and lingual plates (Figure 1B, 1C). A stage diagnosis of dentigerous cyst was established, and enucleation conservative treatment under sedation was then chosen. The patient had COVID-19 infection at the time of the surgical appointment, and the procedure was delayed for 3 weeks.
Figure 1.
Preoperative radiological examination. (A) Orthopantomography examination (“1” indicates the cyst). (B) Cone bean computed tomography (CBCT) axial view illustrating the ballooned buccal and lingual plates and the buccal-lingual orientation of the impacted tooth (* indicates the crown of the tooth). (C) CBCT coronal view illustrating the displaced inferior alveolar nerve and the relationship between the tooth and the mandibular canal (white arrow indicates the mandibular canal and red arrow indicates the 8 mm-thick cystic membrane).
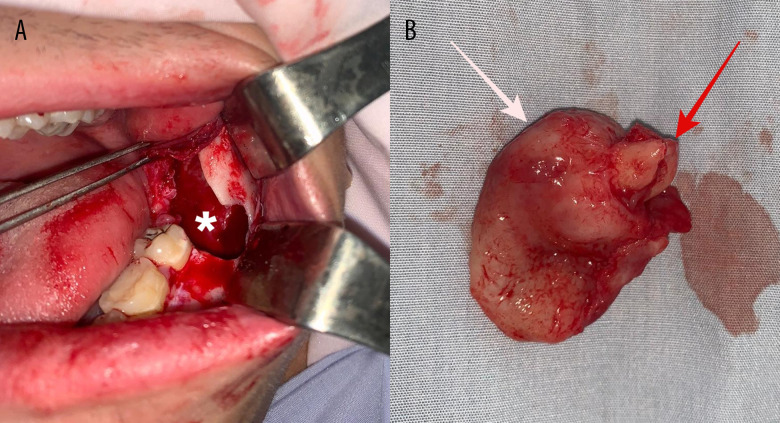
After elevating a full-thickness flap, a very thin cortical bone with small perforation on the retromolar trigone was identified. The cyst was punctured to deflate it, and then enucleation was conducted simultaneously with the 3.8 extraction (Figure 2A). The cyst was removed with the crown of the impacted 3.8 tooth inside the lesion (Figure 2B). During surgery, the cyst presented a completely thick, firm membrane with relative adherence to the bony cavity. Rigorous mechanical curettage and cavity irrigation solutions of Betadine 1% and chlorhexidine digluconate 0.2% were performed before closure with non-resorbable “U” sutures. The specimen was sent for histopathological examination. The patient was discharged after 2 days, and the swelling remitted 1 week after surgery.
Figure 2.
Clinical and paraclinical view of the tumor. (A) Intraoperative view after cyst removal (* indicates the remnant osseous geode). (B) Specimen of excision (white arrow indicates the cystic membrane and red arrow indicates the roots of the 3.8 tooth).
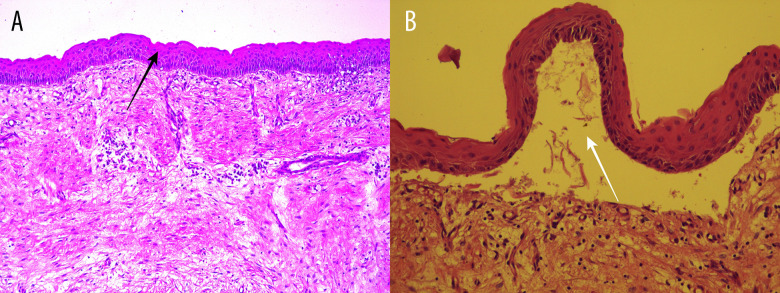
Histopathological examination illustrated a 35×20×12-mm cyst. A fibrous wall with stratified squamous epithelium, focal parakeratosis, and ulceration was identified on microscopic analysis. Also, edema and diffuse inflammatory lymphoplasmacytic infiltrate were observed (Figure 3A). No budding areas were identified, and the lining was thick throughout without the formation of rete ridges. Separation of the epithelium from underlying connective tissue was focally present (Figure 3B), and no satellite cysts were identified. The final diagnosis established by histopathological examination was OKC. The patient was informed about the diagnosis, and the follow-up program was planned for every 3 months in the first year and every 6 months after that, for a total of 10 years.
Figure 3.
Histopathological examination. (A) The fibrous wall with stratified squamous epithelium, focal parakeratosis, and ulceration (black arrow indicates the stratified squamous epithelium). (B) Epithelium under higher magnification (white arrow indicates the separation of the epithelium from the underlying connective tissue).
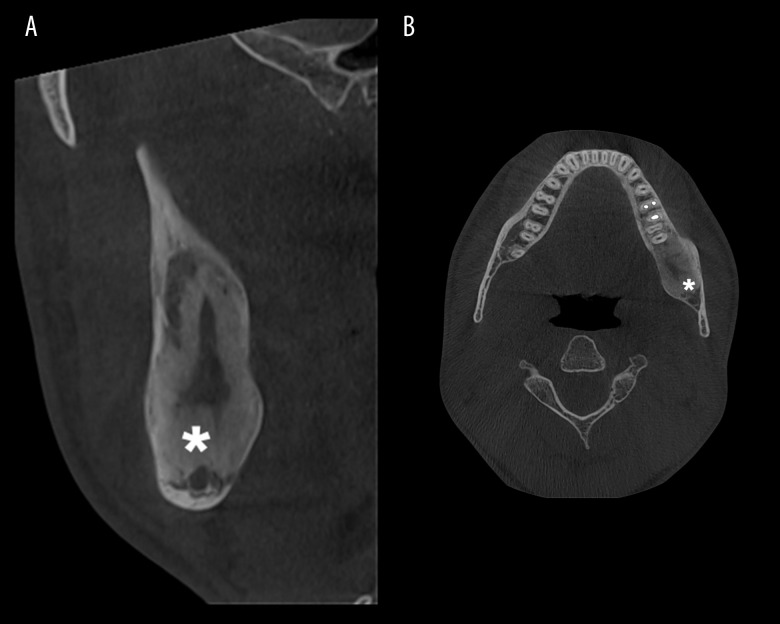
At the 3-month follow-up, we observed a small dehiscence in the retromolar region, and the CBCT indicated a small reduction of osseous geode by cortical bone deposition. An iodoform (triiodomethane)-impregnated hemostatic gelatine sponge (Hygitech) was placed in the dehiscence, and the patient was instructed to carefully clean the healing wound with chlorhexidine digluconate (0.12%). We considered that chemical cauterization at this time was not necessarily owing to the lack of evidence of local recurrence as well as the deposition of new healthy bone at the margins of the surgical defect. At the 6-month follow-up, the dehiscence was smaller than at the previous examination, and the CBCT indicated a 14×12×10-mm cavity, with deposition of cortical bone twice as abundant compared with the previous examination (Figure 4A, 4B).
Figure 4.
Postoperative cone beam computed tomography examination at the 6-month follow-up. (A) Cross-section view (* indicates the new bone deposition). (B) Axial view (* indicates the new bone deposition).
Discussion
The radiological features of the lesion in the presented case were more similar to a dentigerous cyst: single radiolucent lesion associated with a crown of an impacted tooth, ballooned cortical bone, and displaced mandibular canal [18]. The unilocular radiological image of OKCs is usually seen in small lesions [3]. In these cases, the septa within the lesion are incomplete and difficult to identify.
According to the radiological image and clinical examination of our patient, we considered that no biopsy was needed owing to the specific features of a dentigerous cyst. Moreover, the inflammation induced by the biopsy can influence the histo-pathological result of the postsurgical specimen [19].
The conservative treatment by enucleation can be sufficient for particular OKCs, with or without any adjuvant application of Carnoy’s solution [19,20]. Moreover, our patient was young, and, in general, a more radical treatment of the lesion could reduce the quality of life by increasing morbidity and risk of local complication (eg, pathological bone fracture) [21,22]. Pereira Santana et al used the same protocol in a similar posterior mandible lesion. Additionally, they placed a drain tube into the surgical defect and instructed the patient to perform local irrigation with chlorhexidine digluconate (0.12%) 3 times a day for 1 month to favor the healing by secondary intention [23]. As was done by Pereira Santana et al, we instructed our patient to lavage the dehiscence with chlorhexidine digluconate until the lesion was perfectly healed. Sarfi et al and Carvalho et al similarly treated OKCs lesions of the mandible. However, they used Carnoy’s solution during surgery owing to the preoperative histopathological diagnosis of OKCs, based on biopsy fragments [24,25].
Moreover, during surgery we identified a thick, firm cystic membrane easily elevated from the bone geode, as opposed to the particularly thin and friable membrane of OKCs [8]. The thick and firm membrane facilitated the 1-piece excision of the cyst, with macroscopic healthy bone margins. This finding was similar to that described by Vallejo-Rosero et al [26]. The intra-operative aspect of lesions can modify the further treatment. In our presented case, at the time of surgery we did not suspect any features of OKCs or any signs that could orientate the surgeon to reconsider the treatment [12]. Contrary to our clinical diagnosis and intraoperative findings, the histopatho-logical examination result of the removed specimen presented the specific features of OKCs.
In addition to the histopathological features of the cystic wall of OKCs, the cystic space of OKCs is filled with a clear liquid or a cheesy substance containing keratinaceous debris [8]. Biochemical and immunological markers of OKCs are present in the cystic fluid. These biomarkers can improve the diagnosis and, further, they will help the surgeon to treat the bone pathology appropriately. The cystic fluid can be harvested by aspiration, and the levels of total protein, albumin, prealbumin, inorganic phosphorous, and epithelial debris are biomarkers of OKC diagnosis [27].
The follow-up needs to be individualized according to the recurrence rate associated with the histopathological diagnosis and treatment method [3]. Enucleation has higher recurrence rates (27.8–30.8%) than bone resection (0–8.4%) [6,13,14]. Large lesions also have an increased risk of recurrence [28]. Most OKC recurrences reported in literature are within the first 5 to 7 years after treatment, with a shorter time (2 years) in cases in which CBCT was used for examination [3]. We planned a follow-up program by CBCT examination for 10 years in the present case, in which the OKC had a high risk of recurrence [28].
The mechanism of recurrence is still insufficiently understood [15,29,30]. First, there are some mechanisms related to the surgical technique. Incomplete enucleation or multiple-piece excision of the cyst lining, due to its thin and friable membrane, can let satellite microcysts, retained mural islands, or a portion of the lining into position. Also, the attempt to save the adjacent teeth or the curettage of the scalloped margins are difficult maneuvers that can lead to persistence of cells within the surgical field. These remnant structures have a great intrinsic growth potential that facilitates the development of the recurrence [15,29,30]. Second, there are some mechanisms related to the features of the lesions. Lesions located in the posterior mandibular region are prone to recur owing to the difficult access to remove all the cystic membrane pieces hidden within bone. Aggressive OKCs perforate the cortical bone and further involve the adjacent mucosa, which will represent the external lining of the resulted osseous geode. Also, multiple OKCs of Gorlin syndrome present high mitotic activity of the lining epithelium. However, inherent genetic potential for proliferation of the odontogenic epithelium of some patients cannot be treated but can be kept under close observation [15,29,30]. We enucleated the cyst in 1 piece, together with the tooth extraction, and the bone defect was rigorously cleaned by mechanical curettage and abundant Betadine 1% and chlorhexidine digluconate 0.2% irrigation.
At the 3-month follow-up, a dehiscence was observed along with healthy cortical bone deposition at the borders of the bone defect. We considered that reintervention and application of Carnoy’s solution at the surgical defect was not appropriate owing to the lack of any recurrence signs. Moreover, Carnoy’s solution is commonly used intraoperatively in conjunction with local conservative treatment, and we thought that the use of the Carnoy’s solution on the new bone deposition would bring no benefit in terms of recurrence risk [31]. Furthermore, the healthy bone deposition increased by the time of the next follow-up appointment, and Carnoy’s solution can hide the possible remnants of the OKC, which can make the use of the chemical surface treatment useless. Similar to our case of conservative treatment with Carnoy’s solution, Pereira Santana et al, who did not used chemical curettage, found no signs of recurrence at 8 years after surgery [23–25].
A limitation of our case report was the lack of patient’s medical history prior the detection of the cyst. Also, a particularity of the case was the COVID-19 infection at the time of the first treatment appointment, which required 3 weeks of treatment delay.
Conclusions
The diagnosis of OKC is challenging, requiring preoperative 3-dimensional imaging and biopsy for extensive lesions. Adjuvant biochemical and immunological examination of cystic aspirate could be sometimes helpful for making a correct diagnosis. The treatment needs to be individualized according to the patient’s age and the tumor’s histopathological type and features. Chlorhexidine digluconate irrigation of the surgical site can help the healing process. If the histopathological examination of the surgical specimen indicates a more aggressive lesion than expected, a careful and individualized follow-up is imperative. No reintervention is needed if the patient does not present evidence of recurrence.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Avril L, Lombardi T, Ailianou A, et al. Radiolucent lesions of the mandible: A pattern-based approach to diagnosis. Insights Imaging. 2014;5(1):85–101. doi: 10.1007/s13244-013-0298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boffano P, Cavarra F, Agnone AM, et al. The epidemiology and management of odontogenic keratocysts (OKCs): A European multicenter study. J Craniomaxillofac Surg. 2022;50(1):1–6. doi: 10.1016/j.jcms.2021.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Borghesi A, Nardi C, Giannitto C, et al. Odontogenic keratocyst: Imaging features of a benign lesion with an aggressive behaviour. Insights Imaging. 2018;9(5):883. doi: 10.1007/s13244-018-0644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilodeau EA, Collins BM. Odontogenic cysts and neoplasms. Surg Pathol Clin. 2017;10(1):177–222. doi: 10.1016/j.path.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald D. Lesions of the jaws presenting as radiolucencies on cone-beam CT. Clin Radiol. 2016;71(10):972–85. doi: 10.1016/j.crad.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Menon S. Keratocystic odontogenic tumours: Etiology, pathogenesis and treatment revisited. J Maxillofac Oral Surg. 2015;14(3):541. doi: 10.1007/s12663-014-0734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarfe WC, Toghyani S, Azevedo B. Imaging of benign odontogenic lesions. Radiol Clin North Am. 2018;56(1):45–62. doi: 10.1016/j.rcl.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.de Lima JL, Dias-Ribeiro E, Honfi ES, et al. Odontogenic keratocyst of mandible. Indian J Otolaryngol Head Neck Surg. 2006;58(4):373–76. doi: 10.1007/BF03049599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veena KM, Rao R, Jagadishchandra H, Rao PK. Odontogenic keratocyst looks can be deceptive, causing endodontic misdiagnosis. Case Rep Pathol. 2011;2011:159501. doi: 10.1155/2011/159501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirapathomsakul D, Sastravaha P, Jansisyanont P. A review of odontogenic keratocysts and the behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(1):5–9. doi: 10.1016/j.tripleo.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Burgos R, González-Martín-Moro J, Pérez-Fernández E, Burgueño-García M. Clinical, radiological and therapeutic features of keratocystic odontogenic tumours: A study over a decade. J Clin Exp Dent. 2014;6(3):e259–64. doi: 10.4317/jced.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendes RA, Carvalho JFC, van der Waal I. Characterization and management of the keratocystic odontogenic tumor in relation to its histopatho-logical and biological features. Oral Oncol. 2010;46(4):219–25. doi: 10.1016/j.oraloncology.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kaczmarzyk T, Mojsa I, Stypulkowska J. A systematic review of the recurrence rate for keratocystic odontogenic tumour in relation to treatment modalities. Int J Oral Maxillofac Surg. 2012;41(6):756–67. doi: 10.1016/j.ijom.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Johnson NR, Batstone MD, Savage NW. Management and recurrence of keratocystic odontogenic tumor: A systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(4):e271–76. doi: 10.1016/j.oooo.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Tarakji B, Baroudi K, Hanouneh S, et al. Possible recurrence of keratocyst in nevoid basal cell carcinoma syndrome: A review of literature. Eur J Dent. 2013;7(Suppl.1):S126. doi: 10.4103/1305-7456.119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reisner KR, Riva RD, Cobb RJ, et al. Treating nevoid basal cell carcinoma syndrome. J Am Dent Assoc. 1994;125(7):1007–11. doi: 10.14219/jada.archive.1994.0202. [DOI] [PubMed] [Google Scholar]
- 17.Aragaki T, Michi Y, Katsube KI, et al. Comprehensive keratin profiling reveals different histopathogenesis of keratocystic odontogenic tumor and orthokeratinized odontogenic cyst. Hum Pathol. 2010;41(12):1718–25. doi: 10.1016/j.humpath.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Koenig LJ, Tamimi DF, Petrikowski CG, Perschbacher SE. Diagnostic imaging: Oral and maxillofacial. 2nd ed. Elsevier; 2017. [Google Scholar]
- 19.Yuri Slusarenko da S, Maria da Graça N-H. Conservative treatment of primary and nonsyndromic odontogenic keratocyst: An overview of the practice. Int J Oral Dent Heal. 2018;4(2):070. [Google Scholar]
- 20.Dave M, Clarke L, Grindrod M, et al. Adapting treatment approaches for dentigerous cysts in paediatric and adult patients: A case series. Oral Surg. 2021;14(3):277–84. [Google Scholar]
- 21.Jia J, R-f L, Y-f S, et al. Pathological fractures of the mandible: A report of 27 cases. 2017;2:1839. [Google Scholar]
- 22.Fidele NB, Bing L, Sun Y, et al. Management of mandibular odontogenic keratocyst through radical resection: Report of 35 cases. Oncol Lett. 2019;18(1):733–41. doi: 10.3892/ol.2019.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira Santana CD, Garcia JJ, Lins Kusterer EFL, et al. Odontogenic kerato-cyst: Eight-year follow-up after conservative treatment. Int J Odontostomat. 2021;15(2):520–25. [Google Scholar]
- 24.Sarfi D, Bouya M, Ben Yahya I. Conservative management of a large odontogenic keratocyst: A case report. Adv Oral Maxillofac Surg. 2022;5:100238. [Google Scholar]
- 25.Carvalho HMP, De Oliveira MV, Albuquerque GC, et al. Conservative treatment of odontogenic keratocyst tumor in the symphysis/parasymphysis region and mandible body: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126(3):e80. [Google Scholar]
- 26.Vallejo-Rosero KA, Camolesi GV, de Sá PLD, Bernaola-Paredes WE. Conservative management of odontogenic keratocyst with long-term 5-year follow-up: Case report and literature review. Int J Surg Case Rep. 2020;66:8–15. doi: 10.1016/j.ijscr.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patidar M, Shetty P, Patidar N, et al. Biochemical and cytological comparison of keratocystic odontogenic tumours to nonkeratinising odontogenic cysts fluid. J Clin Diagn Res. 2015;9(7):ZC34. doi: 10.7860/JCDR/2015/12501.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung HD, Lim JH, Kim HJ, et al. Appropriate follow-up period for odontogenic keratocyst: A retrospective study. Maxillofac Plast Reconstr Surg. 2021;43(1):16. doi: 10.1186/s40902-021-00301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinard BE, Chuang SK, August M, Dodson TB. How well do we manage the odontogenic keratocyst? J Oral Maxillofac Surg. 2013;71(8):1353–58. doi: 10.1016/j.joms.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Myoung H, Hong SP, Hong SD, et al. Odontogenic keratocyst: Review of 256 cases for recurrence and clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(3):328–33. doi: 10.1067/moe.2001.113109. [DOI] [PubMed] [Google Scholar]
- 31.Ecker J, Horst R Ter, Koslovsky D. Current role of Carnoy’s solution in treating keratocystic odontogenic tumors. J Oral Maxillofac Surg. 2016;74(2):278–82. doi: 10.1016/j.joms.2015.07.018. [DOI] [PubMed] [Google Scholar]