Abstract
Papillary thyroid carcinoma is the most common type of thyroid cancer and accounts for almost 89.4% of all thyroid carcinomas. Hodgkin lymphoma is a heterogeneous group of neoplasms and represents 10% of lymphomas. These two cancers do not share the same risk factors. Some studies have reported the association of thyroid papillary carcinoma with lymphomas, mainly Hodgkin's lymphoma, treated with radiotherapy. However, to our knowledge less than 10 cases have illustrated synchronous papillary thyroid carcinoma and Hodgkin lymphoma with no history of radiotherapy. We present the case of a 49‐year‐old female patient, with no history of past exposure to radiation, who was incidentally diagnosed with Hodgkin lymphoma during the work up for papillary thyroid carcinoma. Our patient had total thyroïdectomy with cervical lymphadenectomy. The histopathologic examination concluded to a papillary thyroid carcinoma of classical variant. And the lymph node dissection enabled us to diagnose not only papillary thyroid carcinoma's lymph node metastasis, but also Hodgkin Lymphoma. This discovery of the Hodgkin lymphoma was totally incidental. The discovery of synchronous tumors in patients with papillary thyroid carcinoma has been reported in the literature. However, the diagnosis of Hodgkin through lymph node dissection for papillary thyroid carcinoma is extremely rare. This underlines the singularity and the importance of our case. The synchronous papillary thyroid carcinoma and Hodgkin lymphoma is a rare condition, which may pose significant diagnostic and treatment dilemmas. To date, there is no standardized approach due to lack of experience. The molecular mechanisms of this link are poorly understood and yet remain to be elucidated.
Keywords: case report, Hodgkin lymphoma, nodal metastasis, papillary thyroid carcinoma, secondary primary cancer, synchronous
The synchronous occurrence of DTC and HL is an extremely rare condition. It may pose significant diagnostic and treatment dilemmas. It should be kept in mind by both pathologists and clinicians so as not to miss the diagnosis and to properly plan the therapeutic strategy.
1. INTRODUCTION
Papillary thyroid carcinoma (PTC) represents the most common type of thyroid cancer accounting for almost 89,4% of all thyroid carcinomas (TC). 1 It usually presents with a high rate of loco‐regional lymph node metastases. 2
Whereas, Hodgkin lymphoma (HL) is a heterogeneous group of neoplasms with an incidence estimated in Europe around 2,2–2,7 per 100,000 cases per year. 3 It represents 10% of lymphomas. Some studies have reported the association of PTC with lymphomas, mainly HL, treated with radiotherapy. 4 However, to the best of our knowledge, less than 10 cases have illustrated synchronous PTC and HL with no history of radiotherapy. 5
We present the case of a 49‐year‐old female patient, with no history of past exposure to radiation, who was incidentally diagnosed with HL during the workup for PTC. This case exemplifies the case of two synchronous cancers, of which one (HL) was fortuitously discovered. The simultaneous association of these two cancers is extremely rare and singular. We aim to study its clinico‐pathological characteristics and try to identify common risk factors for these two cancers, which could explain their association.
2. CASE PRESENTATION
We report a case of a 49‐year‐old woman, with no medical history, in particular with no history of past exposure to radiation. She had a family history of thyroid goiter (aunts and sister). The patient presented to the Department of otorhinolaryngology with a basicervical lump growing for 2 years. She also complained of dysphagia to solids and intermittent dysphonia.
Physical examination was unremarkable other than an indurated, painless basicervical mass measuring 3 cm and a left supraclavicular lymphadenopathy. Cervical ultrasound revealed, in the right upper lobe of the thyroid, a solid hypoechoic nodule measuring 45 × 38 mm with irregular speculated outlines and few microcalcifications. It also showed five hypoechoic nodules in the left lobe, supraclavicular and jugulodigastric lymphadenopathies.
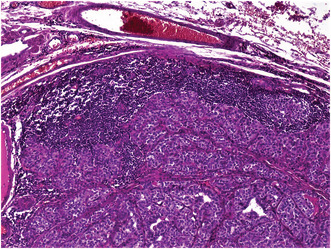
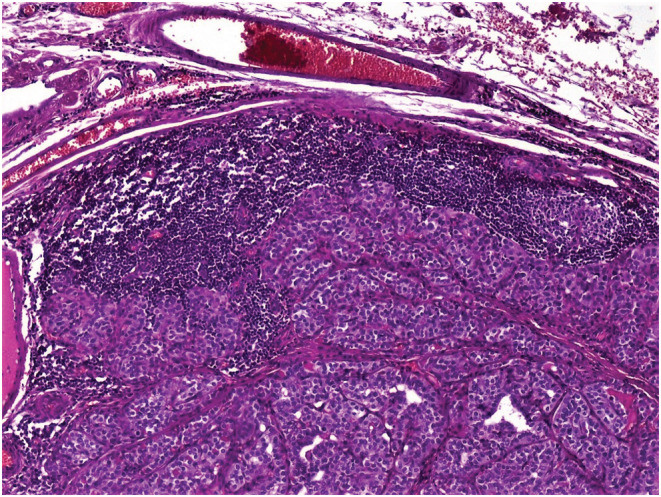
Total thyroidectomy with cervical lymphadenectomy was performed. Microscopic examination concluded to a 5 cm PTC of classical variant in the right lobe with gross extrathyroidal extension invading strap muscles and lymphovascular invasion. Nerve involvement was not detected. Cervical lymphadenectomy revealed metastases of PTC in one out of the seven lymph nodes dissected (Figure 1). Surprisingly, one of the cervical lymph nodes free of PTC metastases was engaged by classical HL sclerosis nodular type (Figure 2). The histopathological examination identified a partial destruction of the normal architecture of that lymph node, replaced by nodules surrounded by fibrosis. Reed Sternberg cells and a reactive lymphoid hyperplasia were noted. The Reed Sternberg cells were positive for CD30 and CD15 in immunohistochemical staining (Figure 3)and were negative for CD20, CD5, and LMP1.
FIGURE 1.
Lymph node metastasis of papillary thyroid carcinoma. Hematoxylin and Eosin. ×40
FIGURE 2.
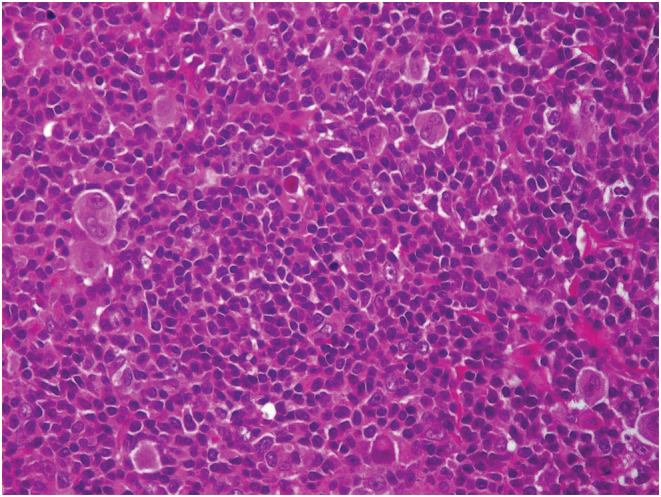
Nodular sclerosis Hodgkin's lymphoma: Reed Sternberg cells with a pale abundant eosinophilic cytoplasm. The nuclei are bilobed with owl‐eye inclusion‐like nucleoli. These cells are present in a rich inflammatory reactive background
FIGURE 3.
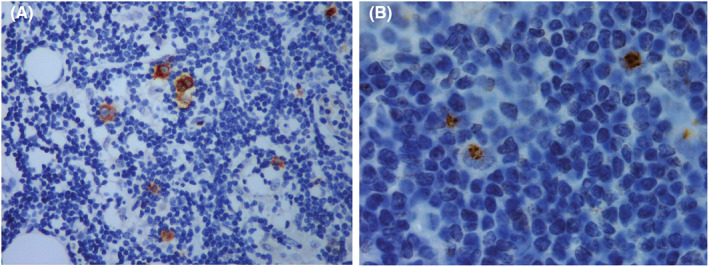
Immunohistochemical study's results: (A) Reed Sternberg cells showing strong CD 30 positivity (membranous and golgi Zone positivity); (B) Reed Sternberg cells showing strong CD 15 positivity (golgi zone positivity)
PTC was considered as T3bN1bM0 and Stage I according to the TNM 2016/AJCC prognostic stage grouping eight edition.
The patient started to receive thyroid hormone replacement and was referred to hematology department to undergo treatment for HL and to nuclear medicine department for radio‐iodine therapy.
The patient underwent four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD). Then, she had radio‐iodine treatment and 54 Gy of external beam radiotherapy targeting PTC. The radiation field included the tumoral bed and the cervical area. At last, she had two additional cures of radio‐iodine therapy. The follow‐up scintigraphy performed 18 months after the thyroidectomy did not show any abnormality. The patient was discharged and advised for close follow‐up.
3. DISCUSSION
Differentiated thyroid carcinoma (DTC) is the most frequent endocrine malignancy and its incidence is ever‐increasing worldwide. 6 PTC represents the most common histologic subtype of DTC.
Regional lymph node metastases are present in up to 50% of patients with PTC at the time of diagnosis. 7 In this case report, lymph node dissection enabled us to diagnose not only PTC's lymph node metastasis, but also HL. This discovery of the HL was totally incidental.
The identification of synchronous tumors in patients with PTC has been reported in the literature. However, the diagnosis of HL through lymph node dissection for PTC is extremely rare. This underlines the singularity and the importance of our case.
In fact, several studies have shown an increased incidence of secondary primary cancer (SPC) in patients with PTC. 8 , 9 In most of the cases, PTC and the second primary tumor are diagnosed synchronously within the same year. Indeed, according to a large Italian cross‐sectional study comprising 6386 females, PTC was associated with increased risk for extra‐thyroid malignancy with an odds ratio (OR) of 3.2. 10 The incidence of the concomitant hematological malignancy was 7%. 10 In addition, CARLES 11 reported that among 184 patients with PTC, there were 33 patients with second primary carcinoma (17.9%). In one‐third of patients, both neoplasms were diagnosed within the same year. Lympho‐hematological malignancies (including HL, chronic lymphatic leukemia, acute lymphatic leukemia, and acute myeloid leukemia) were observed in five cases (2.7%).
In such cases, concomitant PTC and extra‐thyroid malignancy have been attributed to exposure to a common risk factor, genetic background, treatment effect, or surveillance bias. Some studies have reported the association of PTC with lymphomas essentially HL treated with radiotherapy. 4 In a previous study, 12 6 out of 1677 HL patients who underwent radiotherapy developed thyroid malignancy after a latent period of 9–19 years. They were probably radiation‐induced cancers.
However, less than 10 cases of synchronous PTC and HL with no history of radiotherapy had been reported in the literature. 5 Abboud and al. have reported the case of a 24‐year‐old woman who was diagnosed with PTC after 10 years of disease‐free HL. This patient had undergone six cycles of ABVD for stage IA HL with no external radiotherapy. 13
In our review of literature, we report only two cases of synchronous PTC and HL in Tunisia, both diagnosed in Farhat Hached Hospital of Sousse, respectively, in 2017 and 2020. 14 , 15 The two patients, women aged of 50 and 51 years old with no history of exposure to radiation, had total thyroidectomy, adjuvant chemotherapy, and radio‐iodine treatment.
PTC and HL do not share the same risk factors. A genetic origin could explain the simultaneous occurrence of these cancers, but no common mutation had been identified so far. Many mutations have been involved in the development of these tumors separately. For example, the BRAF mutation described in the PTC and numerous non‐Hodgkin's lymphomas had never been identified in the HL. 16
It is well known that the Epstein–Barr virus (EBV) infection is a common risk factor of HL. 17 In our seeking for common risk factors to HL and PTC, we raised inquiries whether EBV could play a role in the tumorigenesis of PTC. In our review of literature, the results of several studies were discordant. The detection of EBV in PTC has been proven controversial. 18 Few studies had demonstrated that EBV was also associated to PTC. 19 , 20 , 21 In fact, Homayouni and al found that EBNA1 was detected in 65.8% of patients with PTC and the frequency of the positive samples was significantly higher at the younger age. 18 However, other studies concluded to no correlation between EBV and PTC. 22
In our case, we performed immunostaining with LMP1 for the involved lymph node by HL, but it was negative. Further studies are needed to evaluate the role of EBV in the tumorigenesis of PTC.
Treatment of PTC includes surgery, with total thyroidectomy in most cases, radio‐iodine ablation of remnant thyroid tissue, external radiotherapy for advanced stages carcinomas (T3, T4, extra‐thyroid involvement) and long‐term thyrotropin suppressive therapy. 7 , 11 HL's treatment is based on chemotherapy, mainly the ABVD protocol, followed by external involved‐field radiation treatment. 17
Due to the small number of reported cases and the lack of data on the post‐treatment outcome of patients, there is currently no consensus regarding the treatment of concomitant PTC and HL. Some authors suggest treating HL first, as it is the cancer with the most aggressive malignancy potential and the most guarded prognosis, then treating PTC after hematological stabilization. 3 In our case, HL was diagnosed fortuitously during the workup for PTC. Therefore, chemotherapy for HL was started after surgery. Then, after the end of the chemotherapy protocol and the stabilization of the patient, we completed the PTC's treatment with external radiotherapy and radio‐iodine therapy.
4. CONCLUSION
We presented the case of a 49‐year‐old female patient who was incidentally diagnosed with HL during the workup for PTC. This case underlines the importance of careful lymph node dissection and microscopic examination of the lymph nodes, not only to detect lymph node metastases from a previously diagnosed cancer but also to diagnose other pathologies, especially that may have a worse prognosis.
The synchronous occurrence of PTC and HL is an extremely rare condition, which may pose significant treatment dilemmas. It should be kept in mind by both pathologists and clinicians so as not to miss the diagnosis and to properly plan the therapeutic strategy. To date, molecular mechanisms of this link and the common risk factors of these two tumors are poorly understood and yet remain to be elucidated.
AUTHOR CONTRIBUTIONS
MBT and FK involved in conceptualization, drafting, writing, preparing the figures, revising and editing all aspects of the manuscript. DC involved in clinical assessment, obtaining the consent of the patient and revising the manuscript. IH, KBL, RH, EBB, RJ involved in data collection, interpretation of submitted material and revising the manuscript. ACD reviewed and approved the final version of the paper.
CONFLICT OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Ben Thayer M, Khanchel F, Helal I, et al. Incidental discovery of a Hodgkin lymphoma synchronous to a papillary thyroid carcinoma. Clin Case Rep. 2022;10:e06246. doi: 10.1002/ccr3.6246
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Arrangoiz R, Llano JGD, Mijares MF, et al. Current understanding of papillary thyroid carcinoma. Int J Otolaryngol Head Amp Neck Surg. 2021;10(3):184‐221. [Google Scholar]
- 2. Park JH, Lee KS, Bae KS, Kang SJ. Regional lymph node metastasis in papillarythyroid cancer. Korean Thyroid Assoc. 2014;7(2):129‐135. [Google Scholar]
- 3. Cotoi L, Borlea A, Petrescu C, et al. Papillary thyroid carcinoma and Hodgkin lymphoma in young patients ‐ case report. Endocr Abstr. 2019:ea0067gp9. doi: 10.1530/endoabs.67.GP9 [DOI] [Google Scholar]
- 4. Weshler Z, Krasnokuki D, Peshin Y, Biran S. Thyroid carcinoma induced by irradiation for Hodgkin's disease. Report of a case. Acta Radiol Oncol Radiat Phys Biol. 1978;17(5):383‐386. [DOI] [PubMed] [Google Scholar]
- 5. Krishnatreya M, Rahman T, Kataki AC, Lahkar K. Synchronous primary cancers in the head and neck region and upper aero digestive tract: Role of triple endoscopy. Indian J Cancer. 2015;52(1):53‐56. [DOI] [PubMed] [Google Scholar]
- 6. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973‐2002. JAMA. 2006;295(18):2164‐2167. [DOI] [PubMed] [Google Scholar]
- 7. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subramanian S, Goldstein DP, Parlea L, et al. Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta‐analysis. Thyroid. 2007;17(12):1277‐1288. [DOI] [PubMed] [Google Scholar]
- 9. AaronP B, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, JonathanD T. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93(2):504‐515. [DOI] [PubMed] [Google Scholar]
- 10. Prinzi N, Sorrenti S, Baldini E, et al. Association of thyroid diseases with primary extra‐thyroidal malignancies in women: results of a cross‐sectional study of 6,386 patients. PloS ONE. 2015;10(3):e0122958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zafon C, Obiols G, Mesa J. Second primary cancer in patients with papillary thyroid carcinoma. Anticancer Res. 2013;33(1):337‐340. [PubMed] [Google Scholar]
- 12. Hancock SL, Cox RS, McDougall IR. Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med. 1991;325(9):599‐605. [DOI] [PubMed] [Google Scholar]
- 13. Abboud B, Yazbeck T, Daher R, Chahine G, Ghorra C. Papillary thyroid carcinoma after chemotherapy for Hodgkin's disease. Am Surg. 2010;76(11):1316‐1317. [PubMed] [Google Scholar]
- 14. 34e Congrès de la Société Française d'Endocrinologie ‐ du 11 au 14 octobre 2017, Poitiers | [Internet]. Accessed July 25, 2022. https://www.congres‐sfe.com/2017/getabstract!fr!!!!33bda641‐39b7‐11e7‐a5f1‐e8c2100b0703
- 15. Ahlem B, Nozha M, Marwa BN, Moncef M. Concomitant of hodgkin lymphoma and papillary thyroid carcinoma. Otorhinolaryngol‐Head Neck Surg. 2020;5:2. https://www.oatext.com/concomitant‐of‐hodgkin‐lymphoma‐and‐papillary‐thyroid‐carcinoma.php [Google Scholar]
- 16. Hussain MRM, Baig M, Mohamoud HSA, et al. BRAF gene: from human cancers to developmental syndromes. Saudi J Biol Sci. 2015;22(4):359‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ansell SM. Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90(11):1574‐1583. [DOI] [PubMed] [Google Scholar]
- 18. Homayouni M, Mohammad Arabzadeh SA, Nili F, Razi F, Amoli MM. Evaluation of the presence of Epstein‐Barr virus (EBV) in Iranian patients with thyroid papillary carcinoma. Pathol Res Pract. 2017;213(7):854‐856. [DOI] [PubMed] [Google Scholar]
- 19. Shimakage M, Kawahara K, Sasagawa T, et al. Expression of Epstein‐Barr virus in thyroid carcinoma correlates with tumor progression. Hum Pathol. 2003;34(11):1170‐1177. [DOI] [PubMed] [Google Scholar]
- 20. Pandya D, Mariani M, He S, et al. Epstein‐Barr virus microRNA expression increases aggressiveness of solid malignancies. Pfeffer S, editor. PLoS ONE. 2015;10(9):e0136058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu ST, Ge JN, Li RC, et al. Is Epstein‐Barr virus infection associated with thyroid tumorigenesis?‐A southern China cohort study. Front Oncol. 2019;9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kijima Y, Hokita S, Takao S, et al. Epstein‐Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol. 2001;64(4):513‐518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


