Abstract
Background
Cerebral palsy is a disorder of movement and posture arising from a non‐progressive lesion in the developing brain. Spasticity, a disorder of increased muscle tone, is the most common motor difficulty and is associated with activity limitation to varying degrees in mobility and self care.
Oral baclofen, a gamma‐aminobutyric acid (GABA) agonist, has been used in oral form to treat spasticity for some time, but it has a variable effect on spasticity and the dose is limited by the unwanted effect of excessive sedation. Intrathecal baclofen produces higher local concentrations in cerebrospinal fluid at a fraction of the equivalent oral dose and avoids this excessive sedation.
Objectives
To determine whether intrathecal baclofen is an effective treatment for spasticity in children with cerebral palsy.
Search methods
We searched the CENTRAL, MEDLINE, EMBASE and CINAHL databases, handsearched recent conference proceedings, and communicated with researchers in the field and pharmaceutical and drug delivery system companies.
Selection criteria
We included studies which compared the effect of intrathecal baclofen treatment on spasticity, gross motor function or other areas of function with controls.
Data collection and analysis
Two authors selected studies, two authors extracted data and two authors assessed the methodological quality of included studies.
Main results
Six studies met the inclusion criteria. The data obtained were unsuitable for the conduct of a meta‐analysis; we have completed a qualitative summary.
All studies were found to have high or unclear risk of bias in some aspects of their methodology.
Five of the six studies reported data collected in the randomised controlled phase of the study. A sixth study did not report sufficient results to determine the effect of intrathecal baclofen versus placebo. Of these five studies, four were conducted using lumbar puncture or other short‐term means of delivering intrathecal baclofen. One study assessed the effectiveness of implantable intrathecal baclofen pumps over six months.
The four short‐term studies demonstrated that intrathecal baclofen therapy reduces spasticity in children with cerebral palsy. However, two of these studies utilised inappropriate techniques for statistical analysis of results. The single longer‐term study demonstrated minimal reduction in spasticity with the use of intrathecal baclofen therapy.
One of the short‐term studies and the longer term study showed improvement in comfort and ease of care. The longer term study found a small improvement in gross motor function and also in some domains of health‐related quality of life.
Some caution is required in interpreting the findings of the all the studies in the review due to methodological issues. In particular, there was a high risk of bias in the methodology of the longer term study due to the lack of placebo use in the control group and the absence of blinding to the intervention after randomisation for both participants and investigators.
Authors' conclusions
There is some limited short‐term evidence that intrathecal baclofen is an effective therapy for reducing spasticity in children with cerebral palsy. The effect of intrathecal baclofen on long‐term spasticity outcomes is less certain.
The validity of the evidence for the effectiveness of intrathecal baclofen in treating spasticity in children with cerebral palsy from the studies in the review is constrained by the small sample sizes of the studies and methodological issues in some studies.
Spasticity is a impairment in the domain of body structure and function. Consideration must also be given to the broader context in determining whether intrathecal baclofen therapy is effective. The aim of therapy may be, for example, to improve gross motor function, to increase participation at a social role level, to improve comfort, to improve the ease of care by others or to improve the overall quality of life of the individual. Intrathecal baclofen may improve gross motor function in children with cerebral palsy, but more reliable evidence is needed to determine this.There is some evidence that intrathecal baclofen improves ease of care and the comfort and quality of life of the individuals receiving it, but again small sample sizes and methodological issues in the studies mean that these results should be interpreted with caution.
Further evidence of the effectiveness of intrathecal baclofen for treating spasticity, increasing gross motor function and improving comfort, ease of care and quality of life is needed from other investigators in order to validate these results.
The short duration of the controlled studies included in this review did not allow for the exploration of questions regarding whether the subsequent need for orthopaedic surgery in children receiving intrathecal baclofen therapy is altered, or the safety and the economic implications of intrathecal baclofen treatment when long‐term therapy is administered via an implanted device. Controlled studies are not the most appropriate study design to address these questions, cohort studies may be more appropriate.
Plain language summary
Intrathecal baclofen for treating spasticity in children with cerebral palsy
Spasticity, which is an increase in muscle tone, is the most common difficulty with movement seen in children with cerebral palsy. Baclofen is a medication which acts on receptors in the brain and spinal cord to reduce abnormal muscle tone. It has been used as an oral medication for many years. The disadvantages of oral administration are that only a small amount of the medication crosses the blood‐brain barrier before it can exert an effect, and that the dose is limited by the unwanted effect of excessive sedation. The administration of baclofen into the fluid surrounding the spinal cord overcomes these problems. This treatment is called intrathecal baclofen therapy and it is administered via a pump placed under the skin connected to a catheter which enters the membranes covering the spinal cord to deliver the baclofen directly into the fluid surrounding the spinal cord and brain.
This review concludes that there is a small amount of evidence from studies performed to date that intrathecal baclofen is an effective treatment for reducing spasticity in children with cerebral palsy in the short‐term. The effect of intrathecal baclofen on spasticity in children with cerebral palsy over the long term is less clear.
Two short‐term studies (by the same investigators) demonstrate a reduction in spasticity, but a single, longer term study shows minimal evidence for reduced spasticity with the use of intrathecal baclofen. Two further short‐term studies showed reduction in spasticity with the use of intrathecal baclofen, but the authors used inappropriate methods of analysing the data, so it is uncertain whether these results are valid. All these studies had a small number of participants, making the findings less reliable.
The research also provides evidence from one short term study and one longer term study (again, by the same investigators), that intrathecal baclofen therapy improves the comfort, ease of care and some aspects of quality of life for children with cerebral palsy. However, the results of the longer term study may have been influenced by both the participants and the investigators being aware of whether they were receiving treatment or allocated to the control group. This same study found that intrathecal baclofen improves gross motor function a little in children with cerebral palsy, but again, these results may have been influenced by the way the study was conducted.
Further evidence of the effectiveness of intrathecal baclofen for treating spasticity is needed from other investigators in order to validate these results.
There is little evidence from the short‐term randomised controlled trials about the safety and economic implications of this treatment when long‐term therapy is administered through an implanted device. For the same reasons, we could not reach a conclusion on whether the subsequent need for orthopaedic surgery is altered in children receiving intrathecal baclofen therapy.
Background
Cerebral palsy is a term which describes a group of permanent disorders of the development of movement and posture, causing activity limitation. They are attributed to non‐progressive disturbances that occurred in the developing foetal or infant brain (Rosenbaum 2007). It has an incidence of approximately two per 1000 live births (Hagberg 1993; Himmelmann 2014; Oskoui 2013; Reid 2011b; Stanley 1984). Approximately 80‐90% of individuals with cerebral palsy manifest spasticity as the predominant motor disorder (Hagberg 2001; Reid 2011a).
Spasticity is generally defined as a form of hypertonia (increased muscle tone) where there is velocity‐dependent resistance of muscle to passive stretch due to a heightened stretch reflex (Lance 1980, as cited in Dietz 1999; Sheean 2002).
Spasticity is an impairment at the body structure and function level of the International Classification of Functioning, Disability and Health (WHO 2002). It can lead to activity limitation or participation restriction due to impairment of motor function and positioning to varying degrees, particularly in the functional domains of mobility and self‐care. The comfort of the child and ease with which others are able to care for the child may also be negatively affected by spasticity. Spasticity also has role in producing the musculoskeletal problems seen in individuals with cerebral palsy, such as muscle/tendon contractures, bony torsions and hip dysplasia.
A range of methods are currently used to treat spasticity, including physiotherapy, casting and splinting, orthopaedic and neurosurgery, botulinum toxin injections and oral medications. Baclofen, a gamma‐aminobutyric acid (GABA) agonist, acts selectively on GABA‐B receptors in the brain and layers II and III of the dorsal grey matter of the spinal cord (Davidoff 1985) to produce an inhibitory effect on presynaptic transmitter release via the restriction of calcium influx into presynaptic terminals, as well as an effect at postsynaptic terminals to decrease neuronal activity by increasing potassium conductance (Zieglgansberger 1988). The oral form of this drug has been in clinical usage for the past four decades, and has a variable degree of effect in reducing spasticity, but often at the cost of producing untoward side effects such as sedation, related to its relative inability to cross the blood brain barrier and the need for high dose levels to achieve substantial clinical effect (Knuttson 1974).
The use of intrathecal baclofen was first described in 1984 in the treatment of adults with spasticity from spinal cord injury (Penn 1984). Intrathecal baclofen is delivered via a drug delivery system consisting of a subcutaneously‐placed pump containing a drug reservoir, connected to a catheter running posteriorly into the subarachnoid space, to produce much higher local concentrations in the cerebrospinal fluid at a fraction of the equivalent oral dose. Early studies highlighted the ability of intrathecal baclofen to reduce tone and other signs of spasticity in adult recipients (Hugenholtz 1992; Loubser 1991). Over the past 20 years there has been an increasing use of this therapy in children with spasticity. This review summarises the evidence for the effectiveness of intrathecal baclofen in children with spastic cerebral palsy.
Objectives
To determine the effectiveness of intrathecal baclofen in treating spasticity in children with cerebral palsy
To determine whether intrathecal baclofen reduces the need for subsequent orthopaedic surgery in children with spasticity.
To determine the safety of intrathecal baclofen therapy.
To consider the economic implications of the therapy.
Methods
Criteria for considering studies for this review
Types of studies
This review focused on studies with the highest level of evidence (i.e. randomised controlled trials), but we also considered non‐randomised controlled trials.
Types of participants
Eligible studies included children aged 0 to 18 years diagnosed with spastic‐type cerebral palsy who had been treated with intrathecal baclofen. We considered studies where at least 90% of the population studied consisted of participants with this type of motor involvement and from this age group.
Types of interventions
Intrathecally‐administered baclofen, delivered either by continuous infusion or intermittent bolus, via an implanted or externally‐sited device.
Types of outcome measures
The primary outcome measures sought were measures of spasticity and gross motor function. The Ashworth scale and the modified Ashworth scale categorise the degree of increase in muscle tone during examination of the affected limb on a five‐point scale. They are commonly‐used measures of spasticity (Ashworth 1964; Bohannon 1987). Measures of gross motor function include, for example, the Gross Motor Function Measure (GMFM) (Russell 1989), a validated assessment tool administered by a physiotherapist.
We considered other measures of function, including the Pediatric Evaluation of Disability Inventory (PEDI), which measures functional capability and level of independence in the areas of self‐care, mobility and social function (Haley 1992).
We also sought studies where other outcome measures were used, including measures of ease of care, comfort and quality of life.
We also collected data regarding the subsequent need for orthopaedic surgery, safety and the economic implications of this therapy where these outcomes were reported.
Search methods for identification of studies
See: Cochrane Movement Disorders Group methods used in reviews.
We identified studies for inclusion in the review using the following sources:
the Cochrane Central Register of Controlled Trials (CENTRAL);
MEDLINE;
EMBASE;
CINAHL;
the Cochrane Movement Disorders Review Group Specialised Trials Register;
handsearches of recent conference proceedings;
contact with principal researchers in the field; and
contact with pharmaceutical and drug delivery system companies.
The terms used to search MEDLINE (1948 to week 38 2013), EMBASE 1980 to Week 38 2013 and CINAHL 1982 to Week 38 2013 are listed in Table 1, Table 2 and Table 3.
1. Search strategy ‐ MEDLINE.
| Search terms |
| 1. intrathecal.af. 2. infusion pump$.af. 3. infusion pumps/ 4. infusion pumps, implantable/ 5. infusion system$.af. 6. injections, spinal/ 7. injection$.af. 8. infusion.af. 9. infusions, parenteral/ 10. continuous infusion.af. 11. drug infusion.af. 12. drug infusion/ 13. chronic drug administration.af. 14. bolus.af. 15. or/1‐14 16. baclofen.af. 17. Baclofen/ 18. aminobutyric acid$.af. 19. 4 Aminobutyric Acid/ 20. GABA.af. 21. muscle relaxant$.af. 22. muscle relaxants, central/ 23. or/16‐22 24. spastic$.af. 25. exp Muscle Hypertonia/ 26. hypertoni$.af. 27. MUSCLE RIGIDITY/ 28. exp Muscle Spasm/ 29. exp muscle contraction/ 30. exp muscle tonus/ 31. muscle spasm$.af. 32. muscle stiff$.af. 33. muscle spasm$.af. 34. muscle contraction$.af. 35. muscle ton$.af. 36. muscle rigidity.af. 37. ashworth score.af. 38. PEDI.af. 39. GMFM$.af. 40. exp motor skills/ 41. functional assessment.af. 42. exp treatment outcome/ 43. exp "Activities of Daily Living"/ 44. exp ambulation/ 45. exp locomotion/ 46. or/24‐45 47. cerebral palsy.af. 48. Cerebral Palsy/ 49. 47 or 48 50. 15 and 23 and 46 and 49 51. ("randomi#ed controlled trial$" or "randomi#ed controled trial$").af. 52. Randomized Controlled Trial/ 53. randomization/ 54. (randomi#ation or "random allocation$").af. 55. exp controlled study/ 56. ("controlled stud$" or "controled stud$").af. 57. ("controlled clinical trial$" or "controled clinical trial$").af. 58. exp evidence based medicine/ 59. exp clinical trial/ 60. (clin$ adj5 trial$).af. 61. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).af. 62. Double Blind Procedure/ 63. Single Blind Procedure/ 64. Placebo/ 65. placebo$.af. 66. random$.af. 67. exp methodology/ 68. "research design$".af. 69. follow up/ 70. evaluation/ 71. exp comparative study/ 72. prospective study/ 73. retrospective study/ 74. exp prevalence/ 75. "cross sectional stud$".af. 76. (control$ or prospectiv$ or volunteer$).af. 77. or/51‐76 78. 50 and 77 79. limit 78 to (humans and ("all infant (birth to 23 months)" or "all child (0 to 18 years)" or "infant (1 to 23 months)" or "preschool child (2 to 5 years)" or "child (6 to 12 years)" or "adolescent (13 to 18 years)")) 80. limit 79 to (case reports or comment or "review") 81. 79 not 80 |
2. Search strategy ‐ EMBASE.
| Search Terms |
| 1. intrathecal.af. 2. exp intrathecal drug administration/ 3. infusion pump$.af. 4. exp infusion pump/ 5. infusion system$.af. 6. exp infusion system/ 7. exp injection/ 8. injection$.af. 9. infusion.af. 10. exp infusion/ 11. exp infusion system/ 12. continuous infusion.af. 13. exp continuous infusion/ 14. drug infusion.af. 15. drug infusion/ 16. chronic drug administration.af. 17. chronic drug administration/ 18. bolus.af. 19. exp bolus injection/ 20. or/1‐19 21. baclofen.af. 22. Baclofen/ 23. aminobutyric acid$.af. 24. 4 Aminobutyric Acid/ 25. 4 aminobutyric acid derivative/ 26. GABA.af. 27. muscle relaxant$.af. 28. exp Muscle Relaxant Agent/ 29. spasmolytic agent/ 30. or/21‐29 31. exp spasticity/ 32. spastic$.af. 33. exp Muscle Hypertonia/ 34. hypertoni$.af. 35. MUSCLE RIGIDITY/ 36. muscle stiffness/ 37. exp Muscle Spasm/ 38. exp muscle contraction/ 39. exp muscle tone/ 40. muscle spasm$.af. 41. muscle stiff$.af. 42. muscle spasm$.af. 43. muscle contraction$.af. 44. muscle ton$.af. 45. muscle rigidity.af. 46. exp muscle function/ 47. exp motor performance/ 48. exp motor control/ 49. exp motor dysfunction/ 50. exp treatment outcome/ 51. exp activities of daily living/ 52. exp ambulation/ 53. ashworth score.af. 54. PEDI.af. 55. GMFM$.af. 56. or/31‐55 57. cerebral palsy.af. 58. Cerebral Palsy/ 59. spastic paresis/ 60. 57 or 58 or 59 61. 20 and 30 and 56 and 60 62. ("randomi#ed controlled trial$" or "randomi#ed controled trial$").af. 63. Randomized Controlled Trial/ 64. randomization/ 65. (randomi#ation or "random allocation$").af. 66. exp controlled study/ 67. ("controlled stud$" or "controled stud$").af. 68. ("controlled clinical trial$" or "controled clinical trial$").af. 69. exp evidence based medicine/ 70. exp clinical trial/ 71. (clin$ adj5 trial$).af. 72. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).af. 73. Double Blind Procedure/ 74. Single Blind Procedure/ 75. Placebo/ 76. placebo$.af. 77. random$.af. 78. exp methodology/ 79. "research design$".af. 80. follow up/ 81. evaluation/ 82. exp comparative study/ 83. prospective study/ 84. retrospective study/ 85. exp prevalence/ 86. "cross sectional stud$".af. 87. (control$ or prospectiv$ or volunteer$).af. 88. or/62‐87 89. 61 and 88 90. limit 89 to (human and (infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)) 91. limit 90 to (letter or "review") 92. 90 not 91 |
3. Search strategy ‐ CINAHL.
| Search terms |
| 1. intrathecal.af. 2. Infusions, Intraspinal/ 3. Infusion Pumps, Implantable/ 4. infusion pump$.af. 5. injection$.af. 6. infusion$.af. 7. Infusion Devices, Intermittent/ 8. continuous infusion$.af. 9. Infusions, Parenteral/ 10. infusion rates/ 11. Injections, Intraspinal/ 12. Neurosurgery/ 13. bolus.af. 14. or/1‐13 15. baclofen.af. 16. baclofen/ 17. muscle relaxants, central/ 18. aminobutyric acid$.af. 19. Aminobutyric Acids/ 20. GABA.af. 21. GABA/ 22. or/15‐21 23. spastic$.af. 24. Muscle Spasticity/ 25. exp Muscle Hypertonia/ 26. hypertoni$.af. 27. "Range of Motion"/ 28. muscle contraction/ 29. Muscle Tonus/ 30. muscle spasm$.af. 31. Spasm/ 32. ashworth score.af. 33. PEDI.af. 34. Physical Therapy Assessment/ 35. Occupational Therapy Assessment/ 36. GMFM$.af. 37. Functional Assessment/ 38. Motor Skills/ 39. Treatment Outcomes/ 40. exp locomotion/ 41. exp "Activities of Daily Living"/ 42. or/23‐41 43. cerebral palsy.af. 44. Cerebral Palsy/ 45. 43 or 44 46. 14 and 22 and 42 and 45 47. randomized controlled trial$.mp. 48. exp randomized controlled trials/ 49. random allocation$.mp. 50. controlled clinical trials.mp. 51. clinical trial.pt. 52. exp clinical trials/ 53. (clin$ adj5 trial$).tw. 54. double blind method$.mp. 55. single blind method$.mp. 56. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).tw. 57. placebos/ 58. placebo$.tw. 59. random.tw. 60. exp research design/ 61. exp follow up studies/ 62. exp evaluation studies/ 63. exp prospective studies/ 64. retrospective design/ 65. retrospective panel studies/ 66. exp case control studies/ 67. comparative study/ 68. cross‐sectional studies/ 69. (control$ or prospectiv$ or volunteer$).tw. 70. or/47‐69 71. 46 and 70 72. limit 71 to (infant <1 to 23 months> or preschool child <2 to 5 years> or child <6 to 12 years> or adolescence <13 to 18 years>) 73. limit 72 to "review" 74. 72 not 73 |
We placed no limitations on language of publication for this search. We searched the Cochrane Central Register of Controlled Trials using the same subject terms as above. The Cochrane Movement Disorders Review Group Specialised Trials Register was not available at the time of this review.
Conference proceedings handsearched included the American Academy for Cerebral Palsy and Developmental Medicine Annual Meeting Abstracts 1997‐2013 and the European Academy Childhood Disability Annual Meeting Abstracts 1998‐2013.
We contacted the authors of included studies and pharmaceutical and drug delivery system manufacturers by email, letter or both regarding whether they were aware of any studies, published or unpublished, which may be potentially eligible for inclusion in the review.
Data collection and analysis
Selection of studies
Two authors conducted an initial review of titles and abstracts of potentially eligible studies identified by the searches. Only those articles where there was sufficient information contained in the title and abstract to clearly determine that the study did not meet the selection criteria for the review were excluded at this stage.
Two authors then independently assessed the remaining trials in detail. We included studies if they were described as a randomised controlled trial or non‐randomised controlled trial (i.e. another group received the same care but without intrathecal baclofen), at least 90% of the participants were diagnosed with spastic‐type cerebral palsy, 90% of the participants were aged 0 to 18 years, the participants received intrathecal baclofen either by continuous infusion or intermittent bolus (either via an implanted or an externally‐sited device) and if participants were assessed using at least one measure of spasticity (e.g. Ashworth scale) or gross motor function (e.g. GMFM). We also considered studies using other outcome measures for inclusion in the review.
We resolved disagreements by conducting a joint review of the relevant studies.
Figure 1 summarises the process of selecting studies for inclusion in this review.
1.
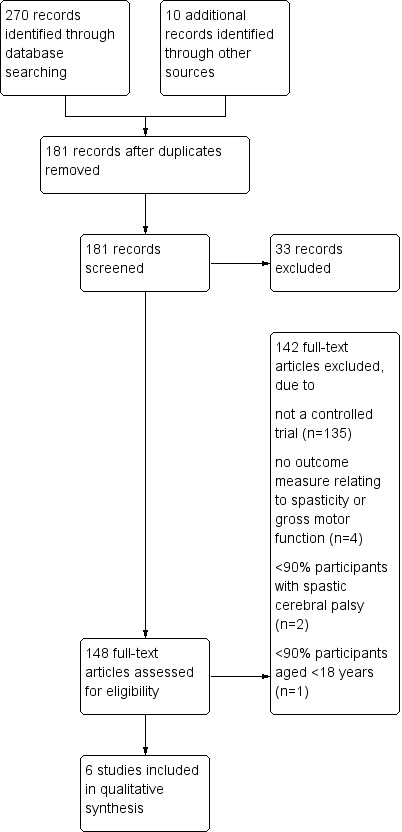
Study flow diagram.
Data extraction and management
Two authors extracted data from the published reports separately using a data collection form, resolving disagreements by joint review. We sought additional information, where required, through correspondence with the authors of the included studies.
Assessment of reporting biases
We assessed the included studies for methodological quality using the Cochrane Collaboration's tool for assessing bias, as described in Chapter 8 (section 8.5) of the Cochrane Handbook for Systematic Reviews of Interventions. We summarised the criteria for determining the risk of bias into an assessment form which contained the following domains:
selection bias: including sequence generation and allocation concealment:
performance bias: blinding of participants to assigned intervention;
detection bias: blinding of those assessing outcome to allocation:
attrition bias: incomplete outcome data
reporting bias: selective outcome reporting
other bias: potential for carry‐over effect in cross over study design
Two authors independently reviewed studies for methodological quality using these criteria. We both allocated a score for study quality in each of the domains above, rating the risk of bias as either low, medium or high. Disagreements were resolved by conducting a joint assessment.
Exploration of publication bias through assessment of funnel plot asymmetry was not possible due to unsuitability of the data obtained from the included studies.
Data synthesis (meta‐analysis)
Meta‐analysis was not performed, as data obtained from the included studies were unsuitable for inclusion in a meta‐analysis.This was for a number of reasons, including differing study designs, different outcome measures used and invalid statistical methods used in some included studies.
The studies were reasonably homogenous from a clinical and methodological viewpoint for the five studies utilising bolus administration of intrathecal baclofen. It is not possible to comment on statistical heterogeneity due to incomparable outcome data.
Results
Description of studies
Design of studies
Six published papers referring to randomised controlled trials were found to meet the inclusion criteria for the review.
The five earlier studies (Albright 1991, Armstrong 1997, Gilmartin 2000, Hoving 2006, Hoving 2007) were trials with a cross over design, utilising the same repeated outcome measures with escalating doses of intrathecal baclofen versus placebo, given via lumbar puncture or spinal catheter, in a paired manner in random order over a number of days. The Hoving 2009a study was a randomised trial of intrathecal baclofen therapy delivered via an implanted pump over 6 months versus a control group who did not receive a intrathecal baclofen pump.
The randomised controlled trial was only one component of the protocol in two studies (Armstrong 1997; Gilmartin 2000). In both these studies the double‐blind, randomised controlled trial was the first phase of the study. The code was broken at the end of this trial and the results used to determine which participants should receive implantable intrathecal baclofen pumps. The second phase of these studies involved an open‐label, long‐term intrathecal infusion of baclofen with no control group. The second phase of these studies is not considered in this review.
The references Hoving 2006 and Hoving 2007 refer to two studies performed on the same group of participants after the same intervention, using different outcome measures. It is the same group of participants which was then randomised to receive either an intrathecal baclofen pump immediately or remain as a control group for the subsequent study reported in Hoving 2009a, where continuous intrathecal baclofen therapy via implanted pump was evaluated against a control group who did not receive this therapy.
The key features of these studies are described in the Characteristics of included studies table.
Participants
The characteristics of the participants in the included studies are described in the table Characteristics of included studies and Table 4 ('Participant characteristics in included studies').
4. Participant characteristics in included studies.
| Study ID | Age range (mean) | Distribution of spasticity | Severity | M:F | Co‐morbidities |
| Albright 1991 | 5 to 27 years (12.2 years) | 17/17 quadriplegia | 'moderate' | 11:6 | ND |
| Armstrong 1997 | 4 to 16 years (8.9 years) | 10/10 quadriplegia | 'severe' | ND | ND |
| Gilmartin 2000 | 4 to 31 years (10.3 years) | 35/51 quadriplegia, 12/51 diplegia; 4/51 paraplegia | severe ‐ Ashworth score >= 3 | 29:22 | ND |
| Hoving 2006/Hoving 2007 | 7 to 16 years (13 years) | 14/17 quadriplegia; 3/17 diplegia | GMFCS 3 ‐ 1; GMFCS 4 ‐ 2; GMFCS 5 ‐ 13 | 8:8 | ND |
| Hoving 2009a | as above | as above | as above | as above | ND |
F: female GMFCS: Gross Motor Function Classification System M: male
The participants in the included studies had spastic‐type cerebral palsy, with a total of 76 children with quadriplegic distribution, 15 with diplegia and four described as paraplegic in distribution. The age range of the participants in the included studies ranged from 4 to 27 years. The method of classification of the severity of cerebral palsy varied between studies and included modes of classification based on degree of spasticity and gross motor function. Albright 1991 reports that participants in that study had moderate severity cerebral palsy, defined as those "whose gait and other movements might improve if their spasticity was alleviated and their muscles strengthened by physical therapy." Armstrong 1997 reports participants in that study had severe cerebral palsy defined as "interfering with daily care or function." Gilmartin 2000 included children with severe cerebral palsy defined as "an average Ashworth scale score of three or more in the four lower extremity measurements." The Gross Motor Function Classification System (GMFCS), developed in 1997 (Palisano 1997) (subsequent to the publication of the two earlier studies, Albright 1991 and Armstrong 1997), was used by Hoving 2006 and Hoving 2007 to classify the severity of cerebral palsy of participants, with the majority of participants in that study classified as GMFCS V (14 out of 17 participants), the most severe functional limitation category, where children have no means of independent mobility. One participant was classified as GMFCS III and two participants as GMFCS IV. 14 of 17 participants had quadriplegic distribution of spasticity and three had diplegic distribution. Hoving 2009a reports on the same group of 17 children enrolled in the earlier studies by the same author.
Albright 1991 undertook the randomised controlled trial in two groups of participants. Group one were 17 children with spastic cerebral palsy and group two were six participants with spasticity due to various disorders. The results obtained in these two groups were reported separately in the publication. Only data pertaining to group one are included in this review. In the Armstrong 1997 study a total 19 participants were included, 10 with spastic cerebral palsy. The author was contacted to provide results for this group from the RCT phase, as this information was not available in the published report. This information was not available at the time of publication of this review.
Interventions
Five included studies involved paired baclofen and placebo bolus intrathecal injection in the same individuals. Escalating doses of baclofen were given versus placebo in four studies (Albright 1991, Armstrong 1997, Hoving 2006, Hoving 2007). The dose range used was 10 to 100 µg. Gilmartin 2000 trialled 50 µg intrathecal injections of baclofen against placebo in a double‐blind fashion. The study blind was broken after the completion of this step. If no positive response had occurred, subsequent higher doses of baclofen (75 µg, 100 µg) were given as open‐label injections.
The mode of intrathecal baclofen delivery was via lumbar puncture or temporary devices (percutaneous spinal catheter or implanted port with spinal catheter) in all five of these studies. The only exception to this is in Armstrong 1997, where five children underwent a modified protocol to reduce the risk of infection by avoiding repeated injections through the access port. In these children infusion pumps were inserted and the double‐blind trial was performed on an outpatient basis.
The duration of the shorter‐term studies (Albright 1991, Armstrong 1997, Gilmartin 2000, Hoving 2006, Hoving 2007) ranged from 24 hours to eight days, however in the Armstrong 1997 study the duration of the double‐blind randomised controlled phase of the study is not clear from the published report. The children who underwent the regular protocol in this study had bolus injections on a daily basis, with a drug and placebo interval of four days and a washout period of at least two days. The five children who underwent the modified protocol in this study had a drug and placebo interval of 10 to 14 days with a washout period of four days, but the number of baclofen or placebo dosages trialled is not stated.
One study (Hoving 2009a) evaluated continuous intrathecal baclofen therapy via implanted pump (Synchromed, Medtronic Inc, Minneapolis) in comparison to a control group with no implanted pump. Standard treatment, including any physiotherapy, occupational therapy and speech therapy was continued in both groups. This study followed on from the previous studies (Hoving 2006, Hoving 2007) by the same author. The participants were administered intrathecal baclofen and placebo injections via lumbar puncture in the earlier studies. Given that all participants had a positive response, they were then randomised to receive an implanted programmable infusion pump after either one month (intervention group) or six months (control group). There was no placebo used in the control group and, after randomisation, treatment was open‐label with no blinding of participants or investigators. Participants were evaluated after six months treatment. In the intervention group, the mean daily dose of intrathecal baclofen was 67 µg immediately after pump implantation and 176 µg six months later.
Oral baclofen was used in three studies in some participants. There was no comment regarding its use in Armstrong 1997 (see Table 5 'Use of oral baclofen').
5. Use of oral baclofen during studies.
| Study ID | Oral baclofen use | Comments |
| Albright 1991 | No | |
| Armstrong 1997 | ND | |
| Gilmartin 2000 | Yes | 2/51 participants (in whom withdrawal was considered hazardous); withdrawn from other subjects |
| Hoving 2006/Hoving 2007 | Yes | 7/17 participants |
| Hoving 2009a | Yes | 3/9 participants in intervention group and 4/8 participants in control group. Withdrawn over period of 10 days from commencement continuous intrathecal baclofen therapy in intervention group |
Outcomes
Ashworth scale scores were used as a primary outcome measure for the majority of studies (Albright 1991; Armstrong 1997; Gilmartin 2000; Hoving 2007) and a further study (Hoving 2009a) used the Ashworth scale score as a secondary outcome measure . Hoving 2006 employed electrophysiological measures of spasticity the primary outcome measure. Hoving 2009a utilised the following primary outcome measures: Gross Motor Function Measure, 66 item (GMFM‐66 ‐ a measure of gross motor function, Russell 1989), the Pediatric Evaluation of Disability Inventory (PEDI ‐a measure of functional skills and need for caregiver assistance in daily life, Haley 1992) and the The Child Health Questionnaire Parent Form 50 (CHQ‐PF50 ‐ a quality of life measure, Landgraf 1996). Hoving 2007 and Hoving 2009a also utilised the Visual Analogue Scale (VAS) as a primary outcome measure for individually formulated problems.
Spasticity
Clinical measures of spasticity
Albright 1991 and Gilmartin 2000 compared mean Ashworth scale scores pre and post intrathecal baclofen and placebo, calculated from examining a number of upper and lower limb muscle groups. Hoving 2007 compared Ashworth scale scores pre and post intrathecal baclofen and placebo for individual muscle groups pre and post intrathecal baclofen and placebo administration. Armstrong 1997 has not reported the results of the randomised controlled trial of baclofen versus placebo performed prior to implantation of intrathecal baclofen pumps in the publication and these data have not been available from the author to date. Hoving 2009a included Ashworth scale scores for individual muscle groups as one of the secondary outcome measures.
Albright 1991 and Gilmartin 2000 compared mean Ashworth scores at baseline and after treatment with intrathecal baclofen and placebo. However, the Ashworth scale is an ordinal scale. It is not appropriate to produce mean Ashworth scores, as it is not a continuous variable and, as such, values which fall between the defined scoring numbers have no meaning, as the difference between the consecutive score values on the scale are not necessarily proportionate. For example, the distance between one and two on the Ashworth scale is not necessarily equivalent to the distance between two and three. Therefore, it is not appropriate to combine the outcome measures from the included studies into a summary statistic. Results of the individual studies are thus reported below without the mean Ashworth scale scores and without their associated statistical results.
Electrophysiological measures of spasticity
Hoving 2006 used electrophysiological measures of spasticity, the H‐reflex and the flexor reflex, as outcome measures.The H‐reflex can be used as a tool to measure the excitability of neural components of the stretch reflex arc. The ratio of the maximal H amplitude to maximal M amplitude (H/M ratio) is considered an index of spasticity (Hoving 2006). The flexor reflex is a polysynaptic and multisegmental spinal response which is influenced by spinal and supraspinal inputs. Lower limb flexor reflex excitability has been shown to be influenced by spasticity (Sandrini 2005).
Gross Motor Function
The GMFM‐66 (Russell 1989) was used as a secondary outcome measure in the Hoving 2009a study. The GMFM is an activity‐level measure, consisting of a standardised assessment of gross motor function in children with cerebral palsy, with items grouped into five dimensions; lying and rolling, sitting, crawling and kneeling, standing and walking.
Other Measures of Function
Subscales of the PEDI (Haley 1992) were used as outcome measures in Hoving 2009a. Specifically, the PEDI caregiver assistance scale of the self‐care domain was used as a primary outcome measure and the PEDI functional skills scale as a secondary outcome measure. The PEDI is an assessment of key functional capabilities and performance in children which is norm‐referenced from age six months to 7.5 years. It measures what children actually do in the daily life and, as such, is an ICF activity‐domain measure (Hayley 2010). The PEDI consists of two sections; functional skills and caregiver assistance. The functional skills section assess the child’s current repertoire of daily life skills in each of 3 domains (self‐care, mobility and social function), whereas the Caregiver Assistance section assesses the extent to which the child’s overall performance of complex daily tasks such as dressing or moving around is supported by help from a caregiver (Hayley 2010).
Ease of Care, Comfort and Quality of Life
The Child Health Questionnaire, Parent Form 50 (CHQ‐PF50) was another primary outcome measure employed in Hoving 2009a, The Child Health Questionnaire is a multidimensional quality of life instrument that focuses upon two components: physical and psychosocial functioning and well‐being It is designed and normed for children 5 to 18 years of age (Landgraf 1996)
The Visual Analogue Scale (VAS) was used as an outcome measure in Hoving 2007 and Hoving 2009a for individually formulated problems. The VAS is a widely used tool which allows the quantification of subjective experiences, such as pain. Popularised by Aitken (Aitken 1969) for the measurement of subjective physical symptoms (dyspnoea and fatigue), it has been found to be a valid and reliable measurement tool for pain in adults and children over six years of age (Tyler 1993), as well as variables such as satisfaction (Singer 1998) and quality of life (de Boer 2004; Jaeschke 1990; Parkin 2004;).
Need for Subsequent Orthopaedic Surgery
None of the included studies in this review reported outcomes relating to the subsequent need for orthopaedic surgery with intrathecal baclofen therapy.
Safety
All studies in the review recorded information about safety or adverse effects following administration of intrathecal baclofen or placebo, but in two cases (Hoving 2006 and Hoving 2009a) these were not included in the same publication.
Albright 1991 states that changes in medical or neurological condition and in vital signs was recorded hourly for eight hours.
Armstrong 1997 describes that participants vital signs were monitored during the trial.
Gilmartin 2000 describes that participants were monitored on an apnoea monitor or pulse oximeter and their vital signs recorded at the same interval as the Ashworth scale score.
Hoving 2006 does not include information regarding adverse effects, this is included in the Hoving 2007 publication, where it is stated that "side effects and complications were recorded on standardised forms," which were completed twice every test day (p655‐6)
The Hoving 2009a report did not include information regarding the safety of the treatment and refers to a concurrent publication which contains this data (Hoving 2009b). However, the Hoving 2009b publication contains data regarding safety and complications for a mean duration of 18 months, a period beyond that of the initial randomised controlled trial. Adverse events were '"recorded on standardised forms" and were defined as "any undesirable experience occurring to a participant during the study, whether or not considered to be related to continuous intrathecal baclofen therapy" (Hoving 2009b). "Serious adverse events" were defined as those that resulted in death, were life‐threatening, resulted in hospitalisation, resulted in persistent or significant disability or incapacity (p248‐9).
Economic Implications
None of the studies included studies in this review reported outcomes relating to the economic implications of intrathecal baclofen therapy.
Risk of bias in included studies
The Albright 1991, Armstrong 1997, Gilmartin 2000, Hoving 2006 and Hoving 2007 studies were all assessed as having unclear risk of bias in at least two of the domains evaluated.
Hoving 2009a was assessed as having high risk of performance and detection bias due to lack of blinding of participants and those assessing outcome to treatment allocation.
Results of the assessment of potential bias in the included studies are summarised in the Risk of bias in included studies table below.
Effects of interventions
It was not possible or appropriate to pool data from the included studies for a number of reasons.
Firstly, it is not appropriate to combine results where study methodology differs significantly. Hoving 2009a studied continuous ITB using implanted pumps and it would not be valid to compare this with the other included studies which utilised short duration bolus doses of intrathecal baclofen via lumbar puncture or spinal catheter.
For the remaining five studies where ITB boluses were administered via lumbar puncture or lumbar catheter, the Ashworth scale, was used as an outcome measure in four, with one study (Hoving 2006) using electrophysiological measures of spasticity. One of these four studies (Armstrong 1997) did not report results for the randomised controlled phase of the study and two studies (Albright 1991; Gilmartin 2000) utilised inappropriate statistical methods for handling the Ashworth scale data.
The Ashworth scale is an ordinal rather than a continuous scale and therefore it is not statistically valid to calculate mean values. Two of the six included studies utilised mean Ashworth scores (Albright 1991; Gilmartin 2000) as the outcome measure .The raw data were not available from the authors to permit another type of meta‐analysis to be performed. A qualitative summary has been completed.
Spasticity
Clinical measures of spasticity
The reported results of all studies in this review suggest intrathecal baclofen is effective for reducing spasticity in children with cerebral palsy.
Albright 1991 reports that following intrathecal baclofen administration, "muscle tone in the lower extremities was significantly reduced but tone in upper extremities was not." There was no significant difference between the three doses (25 µg, 50 µg and 100 µg) in Ashworth score four hours after intrathecal baclofen bolus administration. Albright represented graphically the outcome of placebo and intrathecal baclofen injections. However, the placebo data are not published in the report. The report comments "there were significant differences (p<0.05) in tone in the lower extremities between the placebo values and each of the baclofen dose values."
Armstrong 1997 did not report results of the randomised controlled phase of the study, but reports that following this phase, the code was broken and six out of 10 participants with cerebral palsy subsequently went on to intrathecal baclofen pump implantation.
Gilmartin 2000 reports statistically significant differences in mean Ashworth score in the lower limbs between treatment and control groups at four hours following injection of 50 µg baclofen or placebo. Statistically significant alteration of mean Ashworth score from baseline was observed at each time point (two, four, six and eight hours). A small (less than one point) but statistically significant alteration in Ashworth score was also observed in the upper limbs at each time point.
Hoving 2007 reports that "all children showed a positive clinical effect in response to intrathecal baclofen at some day during the test treatment and subsequently received a pump for continuous delivery of intrathecal baclofen." The effective intrathecal baclofen dose was defined as the dose at which a "satisfying improvement" in the individual treatment goals was achieved and a reduction of at least one point in the Ashworth score compared with baseline on that day in at least three of six selected muscle groups. The effective dose was found to be 12.5 µg in one child, 20 µg in another, 25 µg in 10 children and 50 µg in five children. Ashworth scores assessed at two, four and six hours after the effective intrathecal baclofen dose significantly decreased in comparison with baseline for all muscle groups except for the left hip flexors two hours after intrathecal baclofen administration.
Hoving 2009a determined a statistically significant reduction in spasticity in four of the 22 muscle groups assessed in the treatment group compared to the control group at six months from baseline.
Electrophysiological measures of spasticity
Hoving 2006 reported reduced motorneurone excitability in response to the intrathecal baclofen boluses. A statistically significant difference was seen in response to injections of intrathecal baclofen compared to placebo in H‐max and H/M ratio. No statistically significant difference was seen following intrathecal baclofen bolus versus placebo for the flexor reflex threshold values. Statistically significant differences were seen, however, in the flexor reflex area in those children who were not also taking oral baclofen at the time of the study.
Gross Motor Function
GMFM‐66 showed a positive difference in favour of the treatment group (improvement of mean 1.2 points (SD 2.3) versus worsening of mean ‐1.3 points (SD 3.0) in the control group, p=0.028) in the Hoving 2009a study after six months of treatment.
No statistically significant improvement at six months was seen in the Hoving 2009a study in the GMFM lying and rolling dimension, GMFM sitting dimension or GMFM goal dimensions.
Other Measures of Function
Hoving 2009a found no improvement in the PEDI functional skills scale or caregiver assistance scale in the treatment versus control group after six months ITB treatment.
Ease of Care, Comfort and Quality of Life
Two studies in the review found that intrathecal baclofen may be an effective treatment for improving comfort, ease of care and quality of life in children with cerebral palsy.
Hoving 2007 assessed Visual analogue scale (VAS) scores for individually formulated goals were assessed pre and post placebo and intrathecal baclofen injections. The VAS was completed by caregivers for 15 out of 17 participants due to inability of the participant to either understand the task or to draw a vertical line. Ease of care and pain were the two most common goals nominated, and these showed statistically significant improvement with intrathecal baclofen administration and not with placebo administration.
Hoving 2009a found statistically significant (p=0.001) changes in VAS scores at six months from baseline in the treatment group (mean increase 4.0, SD 1.7) compared with the control group (mean ‐0.2 SD 1.3). The VAS scores were reported for individually formulated problems, with an average of three VAS scores per participant. In participants who nominated ease of care as a VAS goal (n=9 treatment group, n=7 control group), scores improved by a mean of 3.9 points (SD 2.2) in the treatment group and by a mean of 0.1 (SD 1.6) in the control group (p=0.008). In those who nominated pain as a goal area on the VAS (n=6 treatment group, n=6 control group), scores improved by a mean of 4.2 (SD 2.9) in the treatment group and worsened by in the control group with a mean of ‐1.3 (SD2.4) points (p=0.016)
Hoving 2009a reported statistically significant improvement in the CHQ‐PF50 psychosocial summary score (3.4 points, SD 7.9) versus control group (‐5.7 points, SD 8.8, p=0.027) at 6 months compared to baseline. Significant differences were also seen in the following sub‐domains: bodily pain/discomfort, with an improvement of mean 24.4 points (SD 20.7) in the treatment group versus worsening of ‐10.6 points (SD 26.8) in the control group (p=0.014); mental health, with an improvement of mean 9.1 points (SD 9.1) in the treatment group versus worsening in the control group of ‐3.5 points (SD 15.1) (p=0.045); parental impact‐time, with improvement of 5.2 points (SD 18.1) in treatment group versus worsening in the control group of ‐19.8 points (SD 15.1) (p=0.045).
Safety
Sedation or lethargy is the commonest adverse effect noted in all included studies which reported on this outcome (Albright 1991, Armstrong 1997, Gilmartin 2000, Hoving 2007).
Complications reported in these studies (in the randomised controlled phase of the study, where relevant) are summarised in Table 6.
6. Complications of intrathecal baclofen.
| Study | CNS | Cardiovascular | Respiratory | Nausea/vomiting | Other |
|
Albright 1991 (n = 23) NB children with cerebral palsy not differentiated from other participants |
Sedation: 1 treatment Disorientation/agitation/lethargy: 1 treatment Control results not reported |
NR | NR | NR | |
|
Armstrong 1997 (n = 19) NB patients with cerebral palsy not differentiated from other participants |
Sedation: 5 treatment Control results not reported |
Bradycardia: 2 treatment Hypotension: 2 treatment Control results not reported |
Respiratory depression: 1 treatment Apnoea: 1 treatment Control results not reported |
NR | 1 death due to respiratory arrest. Melatonin had been commenced concurrent with the appearance of apnoea |
| Gilmartin 2000 (n = 51) | Sedation: 5 treatment, 0 placebo Hypotonia: 1 treatment, 0 placebo Headache: 2 treatment, 3 placebo Dizziness: 1 treatment, 0 placebo |
NR | NR | 8 treatment, 2 placebo | |
| Hoving 2006 (n=16) | NR | NR | NR | NR | |
| Hoving 2007 (n = 17) | Symptoms reduced CSF pressure: 14 Lethargy: 7 treatment Hypotonia: 1 treatment Excessive perspiration: 1 treatment Radiculopathy: 1 participant |
NR | NR | gastroenteritis: 1 participant | |
| Hoving 2009a | Complications not discussed in this paper. See Hoving 2009b for discussion of complications of intrathecal baclofen (no data on complications experienced by control group). |
CNS: central nervous system CSF: cerebrospinal fluid
As previously discussed, Hoving 2009a does not report on adverse effects and refers to a concurrent publication, Hoving 2009b, which includes this data. The follow‐up periods between the studies differ, however (mean six months in Hoving 2009a, and mean 18 months in Hoving 2009b) and adverse effects for the control group are not reported. For these reasons, the findings reported in the Hoving 2009b paper are not included in the table of results and are instead considered in the discussion below.
Discussion
Main findings of the review
The randomised controlled trials in this review provide a little evidence that intrathecal baclofen is effective for reducing spasticity in children with cerebral palsy in the short‐term. The effect of intrathecal baclofen on spasticity in children with cerebral palsy in the longer term is less clear.
The evidence for the effectiveness of intrathecal baclofen for treating spasticity in children with cerebral palsy is limited by small sample sizes and methodological issues, including high or unclear risk of bias, in the studies in this review.
Spasticity is an impairment at the body structure and function level. The aim of reducing spasticity by the use of intrathecal baclofen therapy is often to prevent or reduce the limitation at the activity and participation level or to address quality of life goals. For example, the ultimate goal may be to improve mobility and/or self‐care or to increase participation at a social role level. The aim of reducing spasticity may also be directed at non‐functional goals, such as to improve comfort, to increase the ease of care by others and to improve the overall quality of life of the individual.
Two studies demonstrate evidence that intrathecal baclofen reduces pain and improves ease of care (Hoving 2007, Hoving 2009a).
There is a small amount of evidence from one study (Hoving 2009a) that gross motor function and certain domains of health‐related quality of life is improved with the use of intrathecal baclofen. Cautious interpretation of this result is required due to the small sample size and high risk of bias in the methodology in this study.
Many of the secondary questions of this review could not be addressed, as the short duration and temporary modes of intrathecal baclofen delivery utilised in the controlled trials included did not allow for the exploration of questions such as alteration in the subsequent need for orthopaedic surgery in children receiving intrathecal baclofen therapy, the rate of complications and the economic implications of this treatment when long‐term therapy is administered via an implanted device. Evidence relating to these questions is unlikely to be derived from randomised controlled trials due to the limited duration of this type of study. Data collection over a period of some years is required to assess these outcomes. Given this, a discussion of the evidence available from published studies regarding these outcomes is included below.
Outcome measures used to assess intrathecal baclofen therapy in children with cerebral palsy
Spasticity
The Ashworth scale (Ashworth 1964) and the modified Ashworth scale (Bohannon 1987) are commonly employed and accepted measures of spasticity in clinical practice and clinical research. The original Ashworth scale was the outcome measure used for all studies included in this review. The advantages of the Ashworth scales are that they can be performed rapidly (Scholtes 2006) and are therefore useful when serial assessments over several hours are performed, as in the studies included in this review. The disadvantages of utilising the Ashworth scale as a measure of spasticity relate to questions about its validity and reliability.
Spasticity is generally defined as a form of hypertonia where there is velocity‐dependent resistance of muscle to passive stretch due to a heightened stretch reflex (Lance 1980, as cited in Dietz 1999; Sheean 2002). The validity and reliability of the Ashworth score as a measure of spasticity has been questioned. The Ashworth scale was originally developed to measure the efficacy of an anti‐spasticity drug in adults with multiple sclerosis (Ashworth 1964). This scale was modified to the form now commonly used in clinical practice by the addition of a further category, 1+, with the aim of increasing its sensitivity (Bohannon 1987). The Ashworth scale and the modified Ashworth scale describe the resistance perceived by the examiner when moving a joint through its range of movement (except for category four, where the limb is rigid) at a single, unspecified speed. It is therefore poorly able to assess any velocity‐dependent alteration in muscle resistance to passive movement. Thus, the Ashworth and modified Ashworth scale are measures of passive resistance to movement, rather than of spasticity (Damiano 2002; Pandyan 1999). The contribution of spasticity versus other causes of increased muscle activity and tone (voluntary and reflex) and altered muscle, tendon and joint viscoelastic properties, to the passive resistance to movement cannot be differentiated from spasticity by the use of the Ashworth scales (Damiano 2002; Pandyan 1999).
The relevance of this theoretical limitation to the use of the Ashworth scale as a measure of spasticity for assessing the effectiveness of intrathecal baclofen therapy is not of great significance in practice, however. In the absence of other interventions that alter musculoskeletal viscoelastic properties, this component is likely to stay constant over the short term. Other causes of hypertonia, such as dystonia, may confound Ashworth scores in the context of assessing intrathecal baclofen therapy. In a clinical context, however, differentiating the relative contribution of spasticity and dystonia to the Ashworth score in a child with cerebral palsy is of uncertain importance as intrathecal baclofen addresses both these causes of hypertonicity. Intrathecal baclofen may be an effective treatment for the secondary dystonia seen in cerebral palsy, although high level evidence is not yet available (Albright 1998; Albright 2001; Dachy 2004; Motta 2004a; Woon 2007).
Studies investigating the reliability of the Ashworth scale have also shown mixed results. The interrater and intrarater (test‐retest) reliability of the modified forms of the scale have been variable and frequently poor in studies performed in children with cerebral palsy (Clopton 2005; Fosang 2003; Yam 2006). There are few data available regarding the reliability of the original form of the scale in children with cerebral palsy, but in studies in other patient groups, the interrater reliability of the original form of the Ashworth scale (as used by all studies in this review) is somewhat better than the modified scale (Brashear 2002; Haas 1996). Protocols which use a single rater, as did the Gilmartin 2000, Hoving 2006, Hoving 2007 and Hoving 2009a studies, for assessing Ashworth scores remove the issue of interrater reliability.
The Tardieu scale, as originally described by Held in 1969 (Scholtes 2006), and the modified Tardieu scale (Boyd 1999) may be a more valid and reliable measure of spasticity in children with cerebral palsy. The original Tardieu scale assesses passive movement of the joints at three specified velocities, noting the duration of muscle reaction to stretch and the joint angle at which the muscle reaction is first felt. It is therefore very time‐consuming (Scholtes 2006). The modified Tardieu scale defines only the joint angle at which muscle resistance is first encountered ('the catch') during rapid velocity movement (as fast as possible, faster than the natural drop of the limb segment under gravity (Boyd 1999).This examination is accompanied by measurement of joint range of motion using a slow speed of movement (Boyd 1999), thereby identifying the velocity‐dependant component of resistance to passive movement. The construct validity of the Tardieu scales as a measure of spasticity is greater than that of the Ashworth scales and this has been supported by experimental studies of its validity (Boyd 1999; Patrick 2006). The reported interrater reliability of the modified Tardieu scale, however, has been found to be somewhat variable in studies involving children with cerebral palsy (Fosang 2003; Mackey 2004; Yam 2006).
A number of instrumented biomechanical and electrophysiological measures of spasticity have also been developed. These include the H‐reflex and flexor reflex, as used by Hoving (Hoving 2006), instrumented torque and EMG measurements during passive motion at preset velocities, ramp and hold tests, pendulum tests and others (Damiano 2002). A complete review of these measures will not be discussed as part of this systematic review. None of these are in common use in the clinical setting.
As discussed previously, the issue of whether measures of spasticity are the most relevant outcome measure for assessing the efficacy of intrathecal baclofen therapy needs to be considered. The question of whether reduction of spasticity maximises function at the activity and participation levels, or improves the quality of life of the individual, must be addressed. For this reason, the use of outcome measures which evaluate function at the activity and participation level and those which address comfort and quality of life of the individual and ease of care by others are required to assess the effectiveness of intrathecal baclofen in addition to measures of spasticity.
Gross Motor Function and Other Measures of Function
Changes in the ICF dimensions of activity and participation with the use of intrathecal baclofen therapy were examined by one of the randomised controlled trials included in this review, Hoving 2009a. The GMFM‐66 is a well‐validated and in widely used as a measure of gross motor function in clinical settings and in research (Wang 2006). The PEDI is similarly well‐validated activity‐level measure in wide use across clinical and research settings (Hayley 2010).
Ease of Care, Comfort and Quality of Life
Care and comfort outcome measures were used by two studies in this review (Hoving 2007, Hoving 2009a). It may be argued that these measures are of the greatest importance in assessing the effect of intrathecal baclofen in individuals with severe cerebral palsy (GMFCS IV and V). The visual analogue scale (VAS) for individually formulated problems was the outcome measure used in both these studies, with a majority of participants nominating pain and ease of care as problem areas.
Health‐related quality of life is a construct which encompasses the impact of health status, including disease and treatment, on physical, psychological and social functioning (de Lissovoy 2007).The Child Health Questionnaire is a generic (rather than disease‐specific) measure of health‐related quality of life. The advantage of generic measures is that they are appropriate for use in all children and normative values have been generated which provide a baseline with which to make comparisons of children with disabilities, such as cerebral palsy (McCullough 2008). Evaluation of the CHQ in studies to date has demonstrated acceptable validity and reliability (Davis 2010; McCullough 2008). However, when assessing the effect of an intervention in children with cerebral palsy, a cerebral palsy‐specific measure may have been a more appropriate tool (Davis 2010; McCullough 2008).
Effectiveness of intrathecal baclofen in treating children with cerebral palsy
Spasticity
The majority of the studies included in this systematic review indicate that intrathecal baclofen may be effective in reducing spasticity in children with cerebral palsy, but the evidence is limited by the small sample sizes used and inappropriate statistical methods used in two of the studies.
Six studies met the inclusion criteria for this review, five of which used spasticity as a primary outcome measure and a sixth used spasticity as a secondary outcome measure (Hoving 2009a). One of these studies had not reported the results of the randomised controlled phase of the study (Armstrong 1997) and therefore did not contribute any data to this review. The incorrect statistical handling of Ashworth scores by a further two studies (Albright 1991, Gilmartin 2000), introduces difficulty in determining the validity of the results of these studies.
Of the other studies included in the review, two were short‐term (Hoving 2006, Hoving 2007) and one was conducted over 6 months (Hoving 2009a). The short term studies demonstrated reduction of spasticity with the use of intrathecal baclofen therapy. Hoving 2009a reported that reduction in spasticity with continuous intrathecal baclofen therapy was demonstrated in only a minority of muscle groups at 6 months compared to baseline.
In summary, therefore, reduction in spasticity was seen in four short‐term randomised controlled trials with adequate methodology and of small size, but two of these studies have utilised questionable statistical analysis. A fifth randomised controlled study, which examined the use of intrathecal baclofen via implanted pump over six months demonstrated minimal reduction of spasticity.
The magnitude of the treatment effect of intrathecal baclofen on spasticity cannot be determined by this review due to the inability to calculate a summary statistic from the outcome measures used in the studies. The Ashworth scale is an ordinal measure and therefore it is not statistically valid to average or summate scores. It was also not possible to discuss statistical heterogeneity in this instance.
In future, meta‐analysis of Ashworth scale data may be possible if outcome data from future controlled studies can be dichotomised into two groups (following treatment with either intrathecal baclofen or placebo), as: 1. No improvement in Ashworth score 2. Improvement in Ashworth score by one or more points.
Gross Motor Function
Hoving 2009a utilised the GMFM to investigate the effect of intrathecal baclofen therapy on mobility and self‐care. A positive impact was seen on gross motor function with the use of intrathecal baclofen reported in Hoving 2009a. The treatment group demonstrated a small, statistically significant improvement in the 66 item GMFM score, with a mean improvement of 1.2 points (SD 2.3) in the treatment group and a mean worsening of ‐1.3 points (SD 2.4)in the control group. No statistically significant difference was seen in the individual dimensions of the 88 Item GMFM (lying and rolling; sitting; crawling and kneeling; standing; walking, running and jumping) between the treatment and control groups. Although the change seen in GMFM‐66 is small, it is probably of clinical significance (Oeffinger 2008; Wang 2006).
However, as mentioned previously, this result should be viewed with some caution due to the small sample size and risk of bias in the methodology, as there was no use of placebo in the control group and no blinding of either participants or investigators after randomisation. The deterioration in various outcome measures in the control group from baseline over the six months of the study may be explained by the participants and investigators knowing that the control had been selected to receive intrathecal baclofen treatment at a delayed time‐point to the treatment group.
Other Measures of Function
No change in self‐care or other functional abilities, as measured by the PEDI functional skills and caregiver assistance scales, was seen with intrathecal baclofen treatment in the Hoving 2009b study.
Ease of Care, Comfort and Quality of Life
Health‐related quality of life, as measured by the CHQ‐PF50, in the Hoving 2009a study was found to improve in children treated with intrathecal baclofen therapy in several domains of the measure: psychosocial summary, bodily pain/discomfort, parental impact‐time and mental health. This is an important finding given that the ultimate goals of intrathecal baclofen treatment may often encompass improvement in heath‐related quality of life and the lack of evidence in the literature about the impact of this intervention upon this objective. Again, however, the lack of a placebo or blinding of either participants or investigators to allocation after randomisation introduces the risk of bias and these results should therefore be interpreted with caution.
Need for Subsequent Orthopaedic Surgery
It has been proposed that reducing spasticity by treating children with intrathecal baclofen leads to a reduced rate of muscle contracture and bony deformity and hence reduces the need for orthopaedic surgery (Hagglund 2005). There is also concern that intrathecal baclofen may increase the rate of spinal deformity.These questions cannot be addressed by the short‐term, controlled trials included in this review, as data collection over a longer period would be required to ascertain this.
In relation to this question, Gerszten 1998 completed retrospective analysis of 48 children who had received intrathecal baclofen pumps with a mean follow up period of 53 months found that orthopaedic surgery was planned at the time of pump insertion for 28 participants, but was subsequently required in only 10 children. In all participants who underwent surgery after intrathecal baclofen pump placement, this surgery had been planned prior to the pump insertion and the most commonly performed procedure was femoral osteotomy.
Krach 2003 studied hip status in children with cerebral palsy one year after pump implantation (following on from the Gilmartin 2000 study). The rate of change of migration percentage from baseline was 1.3%, a rate statistically significantly less than the 5% per year reported as the expected natural rate of progression in children with cerebral palsy.
Scoliosis is common in children with cerebral palsy, with a wide variation in reported incidence of 6.5‐76% (Loeters 2010; Shilt 2008), with the highest incidence occurring in non‐ambulatory children (Saito 1998; Lonstein 1983). Interpreting studies which seek to address the question of the influence of intrathecal baclofen upon spinal deformity is made more complex by issues relating to the high (but not precisely defined) background incidence of scoliosis in children with cerebral palsy and the large number of factors which can influence its development and progression. Also, the timing of intrathecal baclofen pump insertion in relation to skeletal maturity might also influence the rate of scoliotic curve progression, but again there is little evidence in the published literature to confirm this.
There are a number of published retrospective case series (Burn 2010; Ginsburg 2007; Sansone 2006) and case‐control studies ( Krach 2005; Motta 2002; Seneran 2007), with the majority of these suggesting an increased incidence or rate of progression of scoliosis with the use of intrathecal baclofen therapy. Evidence from Shilt 2008 prospective cohort study determined that there was no statistically significant difference in the rate of progression of Cobb angles (6.6 degrees per year in the ITB group versus 5.0 degrees per year in the control group). The study compared individuals with cerebral palsy receiving ITB with a control group of children with cerebral palsy matched for age, sex, topographic motor involvement and initial Cobb angle (but not for GMFCS level). However, it should be noted that the method used to identify control participants may have been biased towards selection of patients with more significant scoliotic curves or curve progression, given that these participants were identified retrospectively from a clinic database, and only those individuals with at least two spinal radiographs on record were eligible for inclusion in the study.
Safety of Intrathecal Baclofen Therapy
The frequency and nature of complications is an important consideration in assessing the risk‐benefit ratio of intrathecal baclofen therapy, particularly given that intrathecal baclofen therapy has the potential to cause adverse events of a life‐threatening nature.
The studies included in this review are unable to answer this question as they were conducted over a short time period and most utilised temporary modes of intrathecal drug delivery, with one study (Hoving 2009a) utilising implantable pumps versus standard care for six months.
The Hoving 2009a article does not report on the safety of the continuous intrathecal baclofen therapy studied, but rather reports on this in a separate paper (Hoving 2009b). Hoving 2009b reports on adverse events recorded prospectively for a mean of 18.4 months from the insertion of an intrathecal baclofen pump (range 12‐24 months). Eighty adverse events were recorded for the study population of 17 children, eight of which were serious, but not life‐threatening in nature. Three of these serious adverse events were device‐related complications requiring further surgery and four were drug‐related (dysphagia, dysarthria, excessive hypotonia). One participant had new onset of seizures, although it is not clear that this was related to the use of intrathecal baclofen.
Elsewhere in the literature, exploration of the evidence available regarding the long‐term safety of intrathecal baclofen in children with cerebral palsy reveals a large number of case series (Albright 1993; Albright 2003; Borowski 2010; Hoving 2009b; Krach 1999a; Motta 2007; Rippe 2005; Ward 2009). Several of these report on large number of paediatric participants (63‐200 individuals) followed for significant periods of time (mean durations of 50‐70 months) (Albright 2003; Borowski 2010; Motta 2007). Overall, these series reveal the rate of significant complications to be around 30% over the long term in those receiving intrathecal baclofen therapy. Motta 2007 calculated an incidence of one significant complication every 11.3 years of treatment
Of the serious complications of ITB therapy, common adverse events include device related complications, with catheter‐related complications (disconnection, obstruction, migrations, tears and fractures) the most frequent complications seen in long‐term intrathecal baclofen therapy, occurring in 10% to 30% of individuals (Albright 1993; Albright 2003; Borowski 2010; Krach 1999a; Motta 2007; Rippe 2005; Ward 2009;). Pump‐related infections are also common, occurring in around 8% to 11% of individuals(Albright 1993; Albright 2003; Borowski 2010; Krach 1999a; Motta 2007; Rippe 2005; Ward 2009;). Cerebrospinal fluid leaks have been reported in 3% to 17% of individuals receiving intrathecal baclofen therapy (Albright 2003; Krach 1999a; Gilmartin 2000; Motta 2007; Ward 2009).
In relation to life expectancy, the best available published evidence does not show any increased mortality from the use of intrathecal baclofen therapy. Krach 2010 reports on a matched cohort study comparing survival in 359 individuals with cerebral palsy receiving intrathecal baclofen treatment with individuals without ITB pumps, matched for age, sex GMFCS level, the presence of epilepsy and feeding tube use. This study found no significant difference in survival curves between the two groups.
Economic implications
The cost‐effectiveness of ITB was not addressed by the randomised controlled trials included in this review, but Dutch Study Group on Child Spasticity conducted a cost‐effectiveness analysis alongside their randomised study (Hoving 2008). A number of other studies have examined the economic costs versus the benefits of intrathecal baclofen therapy via mathematical modelling (Bensmail 2009; de Lissovoy 2007; Sampson 2002).
Hoving 2008 determined in their cost‐utility analysis that the cost per quality‐adjusted life‐year (QALY) for ITB was ϵ32,737 (US $36,665) in the year 2003. This was determined by prospective recording via a questionnaire and cost diary kept by the participants of the intervention and other health care costs in comparison with a retrospective questionnaire for the year preceding the ITB treatment.This cost per QALY is similar to US $42,000 per QALY modelled by de Lissovoy 2007 in the context of the US health care system, but higher than the cost per QALY modelled by Sampson 2002 in the United Kingdom of US $10,550‐$19,570. All these costs are well within the range that is widely accepted as offering good value for money (de Lissovoy 2007; Hoving 2008).
Potential limitations of the review
Risk of bias in review outcome
There is a clear risk of bias in the findings of this review.
A high risk of performance and detection bias due to lack of blinding of participants and those assessing outcome to treatment allocation in the Hoving 2009a study. These biases may have influenced the findings of worsening of the visual analogue scale scores, GMFM‐66,and a number of the CHQ‐PF50 domains in the control group from baseline to reassessment after six months. These are variables that would not be expected to alter over six months duration in the control group. This may have lead to an incorrect finding of a statistically significant difference between the intrathecal baclofen treatment and control groups.
All studies in the review had small sample sizes, which can be more likely to be biased towards positive findings.
A particular limitation of this review is that three of the six included studies were from the same group of researchers and the studies performed on the same group of participants. Any biases inherent in these studies may therefore have been similar for all three studies, thus biasing the review outcome.
Potential bias in the conduct of the review
A thorough search of the published literature has been conducted in this review and it is likely that all published studies from journals indexed in the databases searched and trial registers have been identified.
Publication bias is difficult to overcome, although attempts were also made to identify unpublished studies via handsearching of conference proceedings and contact with researchers and with drug and device manufacturing companies. No unpublished studies were able to be identified.
Authors' conclusions
Implications for practice.
Intrathecal baclofen may be an effective therapy for reducing spasticity in children with cerebral palsy, but the evidence for this is limited to date.
At present, there is a small amount of evidence from randomised controlled trials which suggest that that intrathecal baclofen is effective for reducing spasticity in children with cerebral palsy in the short‐term. The effect of intrathecal baclofen on spasticity outcomes in children with cerebral palsy in the long‐term is less certain. The validity of the evidence is constrained by the small sample sizes of the studies and methodological issues in the studies.
Spasticity is a impairment in the domain of body structure and function and consideration must also be given to the broader context in determining whether intrathecal baclofen therapy is effective. The aim of therapy may be, for example, to improve motor function, to increase participation at a social role level, to improve comfort, to improve the ease of care by others or to improve the overall quality of life of the individual. There is some evidence that intrathecal baclofen improves ease of care and the comfort and quality of life of the individuals receiving it, but again small sample sizes and methodological issues in the studies mean that these results should be interpreted with caution. Intrathecal baclofen therapy may improve gross motor function in children with cerebral palsy, but more reliable evidence is required.
The short duration of the studies included in this review did not allow for the exploration of questions regarding whether the subsequent need for orthopaedic surgery in children receiving intrathecal baclofen therapy is altered, or the safety and the economic implications of intrathecal baclofen treatment when long‐term therapy is administered via an implanted device.
Implications for research.
Further evidence of the effectiveness of intrathecal baclofen for treating spasticity, increasing gross motor function, improving comfort, ease of care and quality of life is required from other investigators in order to validate these results. In particular, evidence of the effectiveness of intrathecal baclofen for treating spasticity over longer durations of therapy is needed.
It is unlikely that randomised controlled trials will provide answers to the questions of whether the subsequent need for orthopaedic surgery in children receiving intrathecal baclofen therapy is altered, the long‐term safety of the therapy and its economic implications. Cohort studies may be more an appropriate study design to answer these questions.
History
Protocol first published: Issue 1, 2004 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 14 November 2008 | Amended | Converted to new format. |
Acknowledgements
Dr Maureen O'Donnell for contributing to the writing of the protocol for this review.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albright 1991.
| Methods | Randomised, double‐blind, paired placebo or intrathecal baclofen bolus treatment | |
| Participants | Group 1: 17 patients with spastic cerebral palsy, 5 to 27 years Group 2: 6 patients with various disorders, 7 to 31 years |
|
| Interventions | Lumbar puncture; intrathecal baclofen bolus 25 mcg, 50 mcg or 100 mcg on different days or 0.9% saline | |
| Outcomes | Ashworth score upper and lower limbs | |
| Notes | Ashworth scores after placebo treatment not reported in published manuscript, but are represented graphically. Author unable to supply these data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p1419 "The order of each pair of injections was randomised according to a coin toss by the pharmacist." |
| Allocation concealment (selection bias) | Unclear risk | No relevant detail recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p1420 Table 2 records all results following ITB administration |
| Selective reporting (reporting bias) | Unclear risk | Placebo results not in report. Limited data in relation to upper limbs in report. |
| Blinding (performance bias and detection bias) | Low risk | Study described as "double blind." |
| Potential for carry‐over effect in cross‐over study design | Low risk | p1419 24 hours between injections |
Armstrong 1997.
| Methods | Phase 1: trial dose 25 mcg baclofen to determine whether beneficial and whether excessive sedation produced. A further trial with 10 mcg dose performed if the patient sedated with 25 mcg dose. If evidence of benefit without significant side effects, patients proceeded to next phase Phase 2: double‐blind, placebo‐controlled trial of baclofen or saline Phase 3: code broken, pump implantation in individuals who benefited, open‐label, long‐term infusion of intrathecal baclofen |
|
| Participants | 19 children with spasticity, of whom 10 had spastic cerebral palsy | |
| Interventions | Subarachnoid catheter with subcutaneous access port; escalating dose of intrathecal baclofen (dose and bolus versus continuous infusion not specified), or intrathecal saline | |
| Outcomes | Ashworth score upper and lower limbs | |
| Notes | Ashworth score results of randomised phase not reported. Author unable to supply this data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation or methods relating to this not described. |
| Allocation concealment (selection bias) | Unclear risk | No relevant detail recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant detail recorded. |
| Selective reporting (reporting bias) | Unclear risk | No relevant detail recorded. |
| Blinding (performance bias and detection bias) | Low risk | Study described as "double blind." |
| Potential for carry‐over effect in cross‐over study design | Low risk | Washout period of at least two to four days |
Gilmartin 2000.
| Methods | Phase 1: double‐blind, placebo‐controlled trial of intrathecal baclofen versus saline Phase 2: open‐label, long‐term intrathecal baclofen infusion |
|
| Participants | 51 patients with spastic cerebral palsy | |
| Interventions | Lumbar puncture, percutaneous spinal catheter or spinal catheter with subcutaneous port; intrathecal baclofen bolus 50 mcg or 0.9% saline | |
| Outcomes | Ashworth score lower limbs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p72 Study described as "randomised." No further details in relation to this |
| Allocation concealment (selection bias) | Unclear risk | No relevant details recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p73 Table 2 records all results |
| Selective reporting (reporting bias) | Low risk | p73 Table 2 records all results |
| Blinding (performance bias and detection bias) | Low risk | p72 Study described as "randomised, double‐blind." |
| Potential for carry‐over effect in cross‐over study design | Low risk | p72 "48‐hour washout period between injections" |
Hoving 2006.
| Methods | Randomised, double‐blind, placebo‐controlled dose escalation trial | |
| Participants | 16 patients with spastic cerebral palsy, 7 to 16 years | |
| Interventions | External lumbar catheter; intrathecal baclofen bolus of 25 mcg, 50 mcg, 75 mcg or 100 mcg or placebo (not specified) | |
| Outcomes | Electrophysiological changes ‐ H‐reflex, H/M ratio, flexor reflex threshold, flexor reflex threshold area | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p1510 "Randomization lists were generated by an independent statistician" |
| Allocation concealment (selection bias) | Low risk | p1510 "the numbered study medication was delivered to the investigator....except for the pharmacist, all involved people were blinded to the contents of the study medication bolus" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p1512 Table 3 and p1514 Table 4 records all results apart from two children who "received ITB at their first test day and showed a clear positive clinical response at once" |
| Selective reporting (reporting bias) | Unclear risk | p1512 Table 3 and p1514 Table 4 records all results apart from two children who "received ITB at their first test day and showed a clear positive clinical response at once" |
| Blinding (performance bias and detection bias) | Low risk | p1510 "except for the pharmacist, all involved people were blinded to the contents of the study medication bolus" |
| Potential for carry‐over effect in cross‐over study design | Low risk | p1510 24 hours between injections |
Hoving 2007.
| Methods | Randomised, double‐blind, placebo‐controlled dose escalation trial | |
| Participants | 17 patients with spastic cerebral palsy, 7 to 16 years | |
| Interventions | External lumbar catheter; intrathecal baclofen bolus of 25 mcg, 50 mcg, 75 mcg or 100 mcg or placebo (not specified) | |
| Outcomes | Ashworth score in lower limbs and visual analogue scale for individually formulated problems | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p655 "An independent statistician generated the randomisation lists" |
| Allocation concealment (selection bias) | Low risk | p655 "All other involved people were blinded to the contents of the study medication boluses" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p656. Results of one child not included in statistical analysis as protocol and blinding were not adhered to due to adverse effects of the test treatment |
| Selective reporting (reporting bias) | Unclear risk | Not all results included in report |
| Blinding (performance bias and detection bias) | Low risk | p655 "All other involved people were blinded to the contents of the study medication boluses" |
| Potential for carry‐over effect in cross‐over study design | Low risk | p655 24 hours between injections |
Hoving 2009a.
| Methods | Randomised controlled trial. Randomisation by random sequence generation, investigator blinded to next assignment in sequence. After randomisation, treatment was open‐label and non‐blinded | |
| Participants | 17 patients with spastic cerebral palsy, 7 to 16 years | |
| Interventions | Intrathecal baclofen via implanted pump | |
| Outcomes | Evaluated after 6 months of treatment. Primary outcome measures: PEDI (Pediatric Evaluation of Disability Inventory) ‐ caregiver assistance scale of the self‐care domain and visual analogue scale for individually formulated problems. Other outcome measures Ashworth score, PEDI ‐ functional skills scale and caregiver assistance scale within self‐care domain, GMFM (Gross Motor Function Measure), CHQ‐PF50 (Child Health Questionnaire ‐Parent Form 50) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p241 "An independent statistician generated the allocation schedule with an unpredictable sequence of assignments." |
| Allocation concealment (selection bias) | Low risk | p241 "The investigator who enrolled the children has no entry to this list and was, at the time of each enrolment, not aware of the next assignment in the sequence." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p242 Figure 1 documents no attrition. |
| Selective reporting (reporting bias) | Low risk | p243 Table 3 records all results |
| Blinding (performance bias and detection bias) | High risk | p241 "After randomisation, the treatment was open‐label and non‐blinded." |
| Potential for carry‐over effect in cross‐over study design | Low risk | Not relevant to this study as not cross‐over design |
H/M ratio: ratio of the maximal H amplitude to maximal M amplitude (an index of spasticity)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albright 1993 | Not a controlled trial |
| Albright 1995 | Not a controlled trial ; intrathecal baclofen versus selective dorsal rhizotomy |
| Albright 1998 | Not a controlled trial ; patients with dystonia |
| Albright 2000 | Not a controlled trial ; patients with dystonia |
| Albright 2001 | Not a controlled trial ; patients with dystonia |
| Albright 2003 | Not a controlled trial |
| Albright 2004 | Not a controlled trial |
| Albright 2006 | Not a controlled trial |
| Aldohondo 2013 | Not a controlled trial |
| Aleton 2012 | Not a controlled trial |
| Almeida 1997 | Case report |
| Anonymous 1996 | News item |
| Awaad 2000 | Not a controlled trial |
| Awaad 2003 | Not a controlled trial |
| Ayyangar 2002 | Not a controlled trial |
| Ayyangar 2005 | Not a controlled trial |
| Ayyangar 2007 | Not a controlled trial. |
| Ayyangar 2011 | Not a controlled trial. |
| Baker 2012 | Not a controlled trial |
| Barry 2001 | Not a controlled trial. |
| Bensmail 2006 | Not a controlled trial. |
| Bensmail 2009 | Cost‐effectiveness modelling study. |
| Bjornson 2003 | Not a controlled trial |
| Bleichman 2001 | Not a controlled trial |
| Bleichman 2002 | Not a controlled trial |
| Bleyenheuft 2007 | Not a controlled trial |
| Bollo 2012 | Not a controlled trial |
| Borowski 2008 | Not a controlled trial. |
| Borowski 2010 | Case series. |
| Bourg 2005 | Not a controlled trial. |
| Bragg 2013 | Not a controlled trial |
| Brochard 2009a | Case series. |
| Brochard 2009b | Case series. |
| Broseta 1990 | Not a controlled trial. |
| Burn 2010 | Case series |
| Cabrera 2004 | Not a controlled trial |
| Caird 2008 | Retrospective case‐control study. |
| Campbell 1999 | Case series. |
| Campbell 2002 | Retrospective review. |
| Charles 2010 | Not a controlled study. |
| Church 2008 | Not a controlled study. |
| Concalves 1994 | Not a controlled trial. |
| Creedon 1997 | Meta‐analysis. |
| Dachy 2004 | Not a controlled trial ; patients with dystonia. |
| Damiano 2005 | Not a controlled trial. |
| Dickey 2013 | Not a controlled trial |
| Eldridge 2012 | Not a controlled trial |
| Emery 2003 | Review article. |
| Ethans 2005 | Not a controlled trial. |
| Fares 1999 | Case series. |
| Fares 2004 | Case series. |
| Farfara 2007 | Not a controlled trial |
| Filipetti 2006 | Review. |
| Fitzgerald 2004 | Not a controlled trial. |
| Gerszten 1997 | Not a controlled trial. |
| Ginsburg 2002 | Not a controlled trial. |
| Ginsburg 2007 | Case series. |
| Giordano 2011 | Not a controlled trial. |
| Gooch 2004 | Case series |
| Gray 2007 | Not a controlled trial. |
| Gutierrez‐Solana 2003 | Case series |
| Hagglund 2005 | Not a controlled trial. |
| Haranhalli 2011 | Case series. |
| Henry‐Socha 2012 | Not a controlled trial |
| Hester 2012 | Not a controlled trial |
| Hoving 2008 | Cost‐effectiveness analysis. |
| Hoving 2009b | Not a controlled trial. |
| Kashii 2012 | Not a controlled trial |
| Kolaski 2007 | Review. |
| Krach 1997 | Not a controlled trial. |
| Krach 1999 | Case series. |
| Krach 1999a | Case series |
| Krach 2003 | Case series |
| Krach 2005 | Not a controlled trial |
| Krach 2005a | No outcome measure relating to spasticity or gross motor function |
| Krach 2005b | Not a controlled trial |
| Krach 2005c | Not a controlled trial |
| Krach 2006 | No outcome measure relating to spasticity or gross motor function |
| Krach 2009 | Review article. |
| Krach 2010 | Not a randomised trial |
| Latash 1996 | < 90% participants with spastic cerebral palsy |
| Lazorthes 1990 | Not a controlled trial |
| Leslie 1999 | Treatment guideline |
| Martinez Moreno 2013 | Not a controlled trial |
| McCoy 2006a | Not a controlled trial |
| McCoy 2006b | Not a controlled trial |
| McMahon 2003 | Case series |
| Meythaler 2001 | Case series |
| Moosa 2008 | Not a controlled trial. |
| Morton 2011 | Controlled trial in subset of patients meeting study criteria, as participants were excluded if they did not respond to an initial test dose of ITB |
| Motta 2000 | Not a controlled trial |
| Motta 2001 | Not a controlled trial |
| Motta 2002 | Not a controlled trial |
| Motta 2004 | Not a controlled trial |
| Motta 2004a | Not a controlled trial |
| Motta 2007 | Case series |
| Motta 2008 | Case series |
| Motta 2009 | Not a controlled trial. |
| Motta 2011 | Not a controlled trial. |
| Mukherjee 2002 | Not a controlled trial |
| Muquit 2012 | Not a controlled trial |
| Murphy 2002 | Case series |
| Najarian 2012 | Not a controlled trial |
| Ochs 1996 | Not a controlled trial |
| Penn 1995 | Not a controlled trial |
| Piccinini 1999 | Case series |
| Piccinini 2001 | No outcome measurement relating to spasticity or gross motor function |
| Pin 2011 | Not a controlled trial |
| Pritula 2012 | Not a controlled trial |
| Ramstad 2010 | No a controlled trial |
| Rawicki 1999 | Not a controlled trial |
| Revivo 2001 | Not a controlled trial |
| Revivo 2002 | Not a controlled trial |
| Rippe 2005 | Not a controlled trial |
| Rippe 2005a | Not a controlled trial |
| Saltuari 1992 | Not a controlled trial |
| Sansone 2006 | Retrospective review. |
| Saval 2008 | Case series |
| Scheinberg 2001 | Not a controlled trial |
| Seneran 2007 | Retrospective case‐control study |
| Sgouros 2002 | Not a controlled trial |
| Shilt 2008 | No measure of spasticity or gross motor function utilised |
| Silva 2012 | Not a controlled trial |
| Simpson 1999 | Review article |
| Sivakumar 2010 | Not a controlled trial |
| Staal 2003 | Retrospective review |
| Steinbok 1995 | Not a controlled trial |
| Taira 2006 | Not a controlled trial |
| Tasseel Ponche 2010 | Not a controlled trial |
| Turner 2012 | Not a controlled trial |
| Ughratadar 2012 | Not a controlled trial |
| Ushewokunze 2012 | Not a controlled trial |
| van Hulst 2009 | Not a controlled trial |
| van Schaeybroeck 2000 | < 90% patients aged 18 years or less |
| van Schie 2001 | Patients with dystonia |
| Vitztum 2000 | Treatment guideline |
| Vloeberghs 2005 | Study yet to be completed ; protocol and pre‐intervention baseline comparisons published only |
| von Koch 2001 | Review article |
| Ward 2009 | Not a controlled trial |
| Wunderlich 2006 | Not a controlled trial |
| Zdolsek 2011 | Not a controlled trial |
| Zierski 1988 | Not a controlled trial |
Contributions of authors
Protocol written by Dr James Rice and Dr Maureen O'Donnell. Review conducted by Dr Monika Hasnat and Dr James Rice.
Paper written by Dr Monika Hasnat.
Declarations of interest
Nil known.
New
References
References to studies included in this review
Albright 1991 {published data only}
- Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA 1991;265(11):1418‐22. [PubMed] [Google Scholar]
Armstrong 1997 {published and unpublished data}
- Armstrong RW, Steinbok P, Cochrane DD, Kube SD, Fife SE, Farrell K. Intrathecally administered baclofen for treatment of children with spasticity of cerebral origin. Journal of Neurosurgery 1997;87:409‐14. [DOI] [PubMed] [Google Scholar]
Gilmartin 2000 {published and unpublished data}
- Gilmartin R, Bruce D, Storrs BB, Abbott R, Krach L, Ward J, et al. Intrathecal baclofen for management of spastic cerebral palsy: multicentre trial. Journal of Child Neurology 2000;15(2):71‐7. [DOI] [PubMed] [Google Scholar]
Hoving 2006 {published data only}
- Hoving MA, Kranen‐Mastenbroek VHJM, Raak EPM, Spincemaille GHJJ, Hardy ELM, Vles JSH. Placebo controlled utility and feasibility study of the H‐reflex and flexor. Clinical Neurophysiology 2006;117:1508‐17. [DOI] [PubMed] [Google Scholar]
Hoving 2007 {published and unpublished data}
- Hoving MA, Raak EPM, Spincemaille GHJJ, Palmans LJ, Sleypen FAM, Vles JSH. Intrathecal baclofen in children with spastic cerebral palsy: a double‐blind, randomised, placebo‐controlled dose finding study. Developmental Medicine and Child Neurology 2007;49:654‐9. [DOI] [PubMed] [Google Scholar]
Hoving 2009a {published data only}
- Hoving MA, Raak EPM, Spincemaille GHJJ, Palmans LJ, Becher JG, Vles JSH. Efficacy of intrathecal baclofen therapy in children with intractable cerebral palsy: a randomised controlled trial. European Journal of Neurology 2009;13:240‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albright 1993 {published data only}
- Albright AL, Barron WB, Fasick MP, Polinko P, Jonosky J. Continuous intrathecal baclofen for spasticity of cerebral origin. JAMA 1993;270(20):2475‐7. [PubMed] [Google Scholar]
Albright 1995 {published data only}
- Albright LA, Barry MJ, Fasick MP, Janosky J. Effects of continuous intrathecal baclofen infusion and selective posterior rhizotomy on upper extremity spasticity. Pediatric Neurosurgery 1995;23:82‐5. [DOI] [PubMed] [Google Scholar]
Albright 1998 {published data only}
- Albright LA, Barry MJ, Painter MJ, Shultz B. Infusion of intrathecal baclofen for generalized dystonia in cerebral palsy. Journal of Neurosurgery 1998;88:73‐6. [DOI] [PubMed] [Google Scholar]
Albright 2000 {published data only}
- Albright AL, Shafron DH, Barry MJ. Intrathecal baclofen for the treatment of severe generalised dystonia. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2000 . 2000; Vol. 83.
Albright 2001 {published data only}
- Albright AL, Barry MJ, Shafron DH, Ferson SS. Intrathecal baclofen for generalised dystonia. Developmental Medicine and Child Neurology 2001;43:652‐7. [DOI] [PubMed] [Google Scholar]
Albright 2003 {published data only}
- Albright AL, Gilmartin R, Swift D, Krach LE, Ivanhoe CB, McLaughlin JF. Long‐term intrathecal baclofen therapy for severe spasticity of cerebral origin. Journal of Neurosurgery 2003;98:291‐5. [DOI] [PubMed] [Google Scholar]
Albright 2004 {published data only}
- Albright AL, Awaad Y, Muhonen MB, Boydston WR, Gilmartin R, Krach LE, et al. Performance and complications associated with the SynchroMed 10‐ml infusion pump for intrathecal baclofen administration in children. Journal of Neurosurgery 2004;101:64‐8. [DOI] [PubMed] [Google Scholar]
Albright 2006 {published data only}
- Albright AL, Ferson SS. Intrathecal baclofen therapy in children. Neurosurgical Focus 2006;21(2):e3. [DOI] [PubMed] [Google Scholar]
Aldohondo 2013 {published data only}
- Aldahondo N, Krach LE. Priapism as a sign of intrathecal baclofen withdrawal: a case series. Developmental Medicine and Child Neurology. 2013; Vol. 55, issue s3:61‐2.
Aleton 2012 {published data only}
- Aleton E, Decavel P, Michel F, Bevalot J, Godard J, Parratte B. Intrathecal baclofen for spasticity management: A comparative analysis of complications in a series of 88 pumps for adults and children [Intrathecal baclofen for spasticity management: A comparative analysis of complications in a series of 88 pumps for adults and children]. Annals of Physical and Rehabilitation Medicine. October 2012; Vol. 55:e336‐9.
Almeida 1997 {published data only}
- Almeida GL, Campbell SK, Girolami GL, Penn RD, Corcos DM. Multidimensional assessment of motor function in a child with cerebral palsy following intrathecal administration of baclofen. Physical Therapy 1997;77(7):751‐64. [DOI] [PubMed] [Google Scholar]
Anonymous 1996 {published data only}
- Anonymous. Intrathecal baclofen for cerebral spasticity. American Family Physician 1996;54(5):1808. [Google Scholar]
Awaad 2000 {published data only}
- Awaad Y, Ham S, Chinarian J, Tayem H, Munoz S. Intrathecal baclofen therapy for the treatment of spasticity in children with cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2000. 2000; Vol. 84.
Awaad 2003 {published data only}
- Awaad Y, Tayem H, Munoz S, Ham S, Michon AM, Awaad R. Functional assessment following intrathecal baclofen therapy in children with spastic cerebral palsy. Journal of Child Neurology 2003;18:26‐34. [DOI] [PubMed] [Google Scholar]
Ayyangar 2002 {published data only}
- Ayyangar R, Bukrey SL, Warschausky SA, Hurvitz EA. Use of the modified Ashworth score as a selection criterion for intrathecal baclofen therapy: is it a useful predictor of long‐term functional gain?. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2002. 2002; Vol. 91.
Ayyangar 2005 {published data only}
- Ayyangar R, Fox M, McCoy A. Weight gain in children receiving intrathecal baclofen therapy for management of cerebral origin spasticity. Developmental Medicine and Child Neurology ‐ Supplementum. Abstract of the 59th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2005. 2005; Vol. 102.
Ayyangar 2007 {published data only}
- Ayyangar E, Enebak L, Garton H, Fox M. Relationshp between health‐related quality of life, spasticity and function in children with cerebral palsy undergoing intrathecal baclofen therapy. Developmental Medicine and Child Neurology. 2007; Vol. 49, issue s111:2.
Ayyangar 2011 {published data only}
- Ayyangar R, Fox MA, Warschausky SA, Giordani B, Garton H, Damodaran A. Cognitive effects of intrathecal baclofen therapy in adolescents with cerebral palsy. Developmental Medicine and Child Neurology. 2011; Vol. 53, issue s5:7‐8.
Baker 2012 {published data only}
- Baker L, Eldridge B, Randall M, Petersen S, Wray A, Antolovich G. Management of acute intrathecal baclofen withdrawal. Developmental Medicine and Child Neurology. 2012; Vol. 54, issue s5:75.
Barry 2001 {published data only}
- Barry MJ. Predictors of success of intrathecal baclofen for ease of care and comfort. Dissertation, School of Health and Rehabilitation Sciences, University of Pittsburgh 2001.
Bensmail 2006 {published data only}
- Bensmail D, Quera Salva MA, Roche N, Benyahia S, Bohic M, Denys P, et al. Effect of intrathecal baclofen on sleep and respiratory function in patients with spasticity. Neurology 2006;67:1432‐6. [DOI] [PubMed] [Google Scholar]
Bensmail 2009 {published data only}
- Bensmail D, Ward Ab, Wissel J, Motta F, Saltuari L, Lissens J, et al. Cost‐effectiveness modeling of intrathecal baclofen therapy versus other interventions for disabling spasticity. Neurorehabilitation and Neural Repair 2009;23(6):546‐52. [DOI] [PubMed] [Google Scholar]
Bjornson 2003 {published data only}
- Bjornson KF, McLaughlin JF, Loeser JD, Nowak‐Cooperman KM, Russel M, Bader K, et al. Oral motor, communication and nutritional status of children during intrathecal baclofen therapy: a descriptive pilot study. Archives of Physical Medicine and Rehabilitation 2003;84:500‐6. [DOI] [PubMed] [Google Scholar]
Bleichman 2001 {published data only}
- Bleichman J, Bode R, Gaebler‐Spira D, Tann B. Weight gain in patients with cerebral palsy following implantation of intrathecal baclofen infusion pumps. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 55th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2001. 2001; Vol. 88.
Bleichman 2002 {published data only}
- Bleichman J, Bode R, Gaebler‐Spira D, Tann B. Weight gain in patients with cerebral palsy following implantation of intrathecal baclofen infusion pumps. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2002. 2002; Vol. 91.
Bleyenheuft 2007 {published data only}
- Bleyenheuft C, Filipetti P, Caldas C, Lejeune T. Experience with external pump trial prior to implantation for intrathecal baclofen in ambulatory patients with spastic cerebral palsy. Neurophysiologie Clinique 2007;37(1):23‐8. [DOI] [PubMed] [Google Scholar]
Bollo 2012 {published data only}
- Bollo RJ, Gooch JL, Walker ML. Sterotactic endosccopic placement of third ventricle catheter for long term infusion of intrathecal baclofen in patients with secondary generalised dystonia. Journal of Neurosurgery Pediatrics 2012;10:30‐3. [DOI] [PubMed] [Google Scholar]
Borowski 2008 {published data only}
- Borowski A, Littleton AG, Dabney KW, Miller F. Baclofen pump implantation and spinal fusions in children: does order or timing influence complications?. Developmental Medicine and Child Neurology. 2007; Vol. 49:2‐3.
- Borowski A, Shah SA, Littleton AG, Dabney KW, Miller F. Baclofen pump implantation and spinal fusion in children. Spine 2008;33:1995‐2000. [DOI] [PubMed] [Google Scholar]
Borowski 2010 {published data only}
- Borowski A, Littleton AG, Borkhuu B, Presedo A, Shah S, Dabney KW, et al. Complications of intrathecal baclofen pump therapy in pediatric patients. Journal of Pediatric Orthopedics 2010;30:76‐81. [DOI] [PubMed] [Google Scholar]
Bourg 2005 {published data only}
- Bourg V, Lazorthes Y, Sallerin B. Implantation of an intrathecal baclofen pump in children with cerebral palsy: The results from a two year follow up of a multicentre study. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the European Academy of Childhood Disability Annual Meeting 2005. 2005; Vol. 103.
Bragg 2013 {published data only}
- Bragg TM, Meyer E. Intraventricular baclofen for the treatment of secondary dystonia and spasticity: clinical outcomes, dosing and side effects. Developmental Medicine and Child Neurology. 2013; Vol. 55, issue s3:9.
Brochard 2009a {published data only}
- Brochard S, Remy‐Neris O, Filipetti P, Bussel B. Intrathecal baclofen infusion for ambulant children with cerebral palsy. Pediatric Neurology 2009;40:265‐70. [DOI] [PubMed] [Google Scholar]
Brochard 2009b {published data only}
- Brochard S, Lempereur M, Filipetti P, Remy‐Neris O. Changes in gait following continuous intrathecal baclofen infusion in ambulant children and young adults with cerebral palsy. Developmental Neurorehabilitation 2009;12(6):397‐405. [DOI] [PubMed] [Google Scholar]
Broseta 1990 {published data only}
- Broseta J, Garcia‐March G, Sanchez‐Ledesma MJ, Anaya J, Silva I. Chronic intrathecal baclofen administration in severe spasticity. Stereotactic and Functional Neurosurgery 1990;54:147‐53. [DOI] [PubMed] [Google Scholar]
Burn 2010 {published data only}
- Burn S, Zeller R, Drake JM. Do baclofen pumps influence the development of scoliosis in children?. Journal of Neurosurgery 2010;5:195‐9. [DOI] [PubMed] [Google Scholar]
Cabrera 2004 {published data only}
- Cabrera MN, Snow S, Smith BP, Koman LA, Shilt JS. Acute effects of intrathecal baclofen on spinal deformity in patients with cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 58th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2004. 2004; Vol. 99.
Caird 2008 {published data only}
- Caird MS, Palanca AA, Garton H, Hensinger RN, Ayyangar RN, Drongowski A, et al. Outsomes of posterior spinal fusion and instrumentation in patients with continuous intrathecal baclofen infusion pumps. Spine 2008;33:E94‐9. [DOI] [PubMed] [Google Scholar]
Campbell 1999 {published data only}
- Campbell W, Grant G, McLaughlin J, Bjornson K, Loeser J, Ferrel A, et al. Continuous intrathecal baclofen infusion in children and adolescents with spasticity of cerebral origin. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 1999. 1999; Vol. 80.
Campbell 2002 {published data only}
- Campbell WM, Ferrel A, McLaughlin JF, Grant GA, Loeser JD, Graubert C, et al. Long‐term safety and efficacy of continuous intrathecal baclofen. Developmental Medicine and Child Neurology 2002;44(10):660‐5. [DOI] [PubMed] [Google Scholar]
Charles 2010 {published data only}
- Charles DP, Gill CE, Taylor HM, Putman MS, Blair CL, Roberts AG, et al. Spasticity treatment facilitates direct care delivery for adults with profound intellectual disability. Movement Disorders 2010;25(4):466‐73. [DOI] [PubMed] [Google Scholar]
Church 2008 {published data only}
- Church C, Quaile K, Lyons S, Miller F. The effect of intrathecal baclofen on ambulation. Developmental Medicine and Child Neurology. 2008; Vol. 50, issue s5:37.
Concalves 1994 {published data only}
- Concalves J, Garcia‐March G, Sanchez‐Ledesma MJ, Onzain I, Broseta J. Management of intractable spasticity of supraspinal origin by chronic cervical intrathecal infusion of baclofen. Sterotactic and Functional Neurosurgery 1994;62:108‐12. [DOI] [PubMed] [Google Scholar]
Creedon 1997 {published data only}
- Creedon SD, Dijkers PJM, Hinderer SR. Intrathecal baclofen for severe spasticity: a meta‐analysis. International Journal of Rehabilitation and Health 1997;3(3):171‐85. [Google Scholar]
Dachy 2004 {published data only}
- Dachy B, Dan B. Electrophysiological assessment of intrathecal baclofen in dystonic children. Clinical Neurophysiology 2004;115:774‐8. [DOI] [PubMed] [Google Scholar]
Damiano 2005 {published data only}
- Damiano DL, Gilgannon MD, Abel MF. Responsiveness and uniqueness of the pediatric outcomes data collection instrument compared to the gross motor function measure for measuring orthopaedic and neurosurgical outcomes in cerebral palsy. Journal of Pediatric Orthopaedics 2005;25(5):641‐5. [DOI] [PubMed] [Google Scholar]
Dickey 2013 {published data only}
- Dickey MP, Rice M, Kinnet DG, Lambert R, Donauer S, Gerber MA, Staat MA. Infectious complications of intrathecal baclofen pumps in a paediatric population. The Pediatric Infectious Diseases Journal 2013;32(7):715‐22. [DOI] [PubMed] [Google Scholar]
Eldridge 2012 {published data only}
- Eldridge B, Pearce L, Petersen S, Antolovich G, Wray A, Randall M. The efficacy of intrathecal baclofen therapy on children and adolescents with complex movement disorders of neurological origin. Developmental Medicine and Child Neurology. 2012; Vol. 54:65.
Emery 2003 {published data only}
- Emery E. Intrathecal baclofen. Literature review of the results and complications [Baclofene intrathecal. Analyse de la literature des resultats et des complications]. Neurochirurgie 2003;49(2‐3):276‐88. [PubMed] [Google Scholar]
Ethans 2005 {published data only}
- Ethans KD, Schryvers OI, Nance PW, Casey AR. Intrathecal drug therapy using Codman model 3000 constant flow implantable infusion pumps: experience with 17 cases. Spinal Cord 2005;43:214‐8. [DOI] [PubMed] [Google Scholar]
Fares 1999 {published data only}
- Fares Y, Barrio ER, Burzaco JA. El uso intratecal de baclofen en el tratamiento de las espasticidad rebelde, moldes y resultados. Dolor 1999;14:291‐9. [Google Scholar]
Fares 2004 {published data only}
- Fares Y, Kasim RM, Barrio ER, Burzaco JA. Dosage of intrathecal baclofen maintenance therapy in the spastic syndromes. Lebanese Medical Journal 2004;52(1):13‐8. [PubMed] [Google Scholar]
Farfara 2007 {published data only}
- Farfara A, Kwiatkowski S, Kawecka J, Grzegorzewski P, Kawecki Z. The parents expectations after baclofen pump implantation in child with severe spasticity in physical rehabilitation and psychological aspects [Oczekiwania rodzicow po wszczepieniu pompy baclofenowej u dziecka ze spastycznoscia w aspekcie rehabilitacyjynum i psychologicznym]. Przeglad lekarski 2007;64(Suppl 2):18‐21. [PubMed] [Google Scholar]
Filipetti 2006 {published data only}
- Filipetti P, Caldas C, Delpierre Y. Spasticity management and progress in ambulatory cerebral palsy [Avancees dans le traitement de la spasticite et enfant IMOC]. Archives de Paediatrie 2006;13:614‐20. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2004 {published data only}
- Fitzgerald JJ, Tsegaye M, Vloeberghs MH. Treatment of childhood spasticity of cerebral origin with intrathecal baclofen: a series of 52 cases. British Journal of Neurosurgery 2004;18(3):240‐5. [DOI] [PubMed] [Google Scholar]
Gerszten 1997 {published data only}
- Gerszten PG, Albright AL, Barry MJ. Effect on ambulation of continuous intrathecal baclofen infusion. Pediatric Neurosurgery 1997;27:40‐4. [DOI] [PubMed] [Google Scholar]
Ginsburg 2002 {published data only}
- Ginsburg GM, Lauder AJ. Progression of scoliosis in spastic quadriplegic patients after insertion of an intrathecal baclofen pump. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2002. 2002; Vol. 91.
Ginsburg 2007 {published data only}
- Ginsburg GM, Lauder AJ. Progression of scoliosis in patients with spastic quadriplegia after the insertion of an intrathecal baclofen pump. Spine 2007;32:2745‐50. [DOI] [PubMed] [Google Scholar]
Giordano 2011 {published data only}
- Giordano F, Gentileschi P. The multidisciplinary network on dystonia and spasticity in children: presentation and first results. Developmental Medicine and Child Neurology. 2011; Vol. 53, issue s3:3‐4.
Gooch 2004 {published data only}
- Gooch JL, Oberg WA, Grams B, Ward LA, Walker ML. Care provider assessment of intrathecal baclofen in children. Developmental Medicine and Child Neurology 2004;46(8):548‐52. [DOI] [PubMed] [Google Scholar]
Gray 2007 {published data only}
- Gray N, Vloeburghs M, Morton R. Monitoring intrathecal baclofen ‐ choosing the right outcome measure. Developmental Medicine and Child Neurology. 2007; Vol. 49, issue s111:11‐2.
Gutierrez‐Solana 2003 {published data only}
- Gutierrez‐Solana LG, Ruiz‐Falco ML, Perez‐Diaz C. Intrathecal baclofen therapy: the selection of patients and short term results in five patients [Terapia con baclofen intratecal: Seleccion de pacientes y resultados a corto plazo en cinco pacientes]. Revista de Neurolgia 2003;37(1):83‐5. [PubMed] [Google Scholar]
Hagglund 2005 {published data only}
- Hagglund G, Anderssson S, Duppe H, Pedertsen HL, Nordmark E, Westborn L. Prevention of severe contractures might replace multilevel surgery in cerebral palsy: results of a population based health care programme and new techniques to reduce spasticity. Journal of Pediatric Orthopaedics 2005;14:269‐73. [DOI] [PubMed] [Google Scholar]
Haranhalli 2011 {published data only}
- Haranhalli N, Anand D, Wisoff JH, Harter DH, Weiner HL, Blate M, Roth J. Intrathecal baclofen therapy: complication avoidance and management. Childs Nervous System 2011;27:421‐7. [DOI] [PubMed] [Google Scholar]
Henry‐Socha 2012 {published data only}
- Henry‐Socha N, Aldahondo N, Kim PD, Krach E. Unusual complication of intrathecal baclofen pump and catheter placement in a child with cerebral palsy: a case report. 2012 American Academy of Physical Medicine and Rehabilitation, AAPM&R Annual Assembly. Conference Publication. 2012; Vol. 4:S343.
Hester 2012 {published data only}
- Hester SM, Fisher JF, Lee MR, Macomsen S, Vender JR. Evaluation of salvage techniques for infected baclofen pumps in paediatric patients with cerebral palsy. Journal of Neurosurgery Pediatrics 2012;10:548‐54. [DOI] [PubMed] [Google Scholar]
Hoving 2008 {published data only}
- Hoving MA, Evers SMA, Ament AJHA, Raak EPM, Vles JSH. Intrathecal baclofen therapy in children with intractable cerebral palsy: a cost‐effectiveness analysis. Developmental Medicine and Child Neurology 2008;50:450‐5. [DOI] [PubMed] [Google Scholar]
Hoving 2009b {published data only}
- Hoving MA, Raak EPM, Spincemaille GHJJ, Kranen‐Masternbroek VHJM, Kleef M, Gorter JW, et al. Safety and one‐year efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy. European Journal of Paediatric Neurology 2009;13:247‐56. [DOI] [PubMed] [Google Scholar]
Kashii 2012 {published data only}
- Kashii H, Ando A, Terashima H, Ota S, Kubota M, Morota N. Current status of intrathecal baclofen therapy for children with severe spasticity and/or dystonia. Developmental Medicine and Child Neurology. 2012; Vol. 54:117‐8.
Kolaski 2007 {published data only}
- Kolaski K, Logan LR. A review of the complications of intrathecal baclofen in patients with cerebral palsy. NeuroRehabilitation 2007;22:383‐95. [PubMed] [Google Scholar]
Krach 1997 {published data only}
- Krach LE, Gilmartin R, Bruce D, Storrs B, Abbott R, Ward J, et al. Functional changes noted following treatment of individuals with cerebral palsy with intrathecal baclofen. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 1997. 1997; Vol. 75.
Krach 1999 {published data only}
- Krach LE. Management of intrathecal baclofen withdrawal: A case series. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 1999. 1999; Vol. 80.
Krach 1999a {published data only}
- Krach LE. Complications associated with continuous infusion of intrathecal baclofen. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 1999. 1999; Vol. 80.
Krach 2003 {published data only}
- Krach LE, Kriel RL, Gilmartin RC, Swift DM, Storrs BB, Abbott R, et al. Hip status in cerebral palsy after one year of continuous intrathecal baclofen infusion. Pediatric Neurology 2004;30:163‐8. [DOI] [PubMed] [Google Scholar]
Krach 2005 {published data only}
- Krach LE, Walker K, Kapp L. Comparison of mortality in case‐matched individuals with cerebral palsy receiving versus not receiving intrathecal baclofen. Developmental Medicine and Child Neurology ‐ Supplementum. Abstract of the 59th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine. 2005; Vol. 102.
Krach 2005a {published data only}
- Krach L, Nugent A, Rapp L, Kempla B, Nettleton A. Use of medication after orthopaedic surgery: comparison of children with cerebral palsy with and without intrathecal baclofen. Neurorehabilitation and Neural Repair. 2005; Vol. 19, issue 4:362.
Krach 2005b {published data only}
- Krach LE, Kriel RL, Gilmartin RC, Swift DM, Storrs BB, Abbot R, et al. GMFM 1 year after continuous intrathecal baclofen infusion. Pediatric Rehabilitation 2005;8(3):207‐13. [DOI] [PubMed] [Google Scholar]
Krach 2005c {published data only}
- Krach LE, Walker K, Rapp L. The effect of intrathecal baclofen treatment on the development of scoliosis in individuals with cerebral palsy: a retrospective, case‐matched review. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 59th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2005. 2005; Vol. 102.
Krach 2006 {published data only}
- Krach LE, Nettleton A, Klempka B. Satisfaction of individuals treated long‐term with continuous infusion of intrathecal baclofen by implanted programmable pump. Pediatric Rehabilitation 2006;9(3):210‐8. [DOI] [PubMed] [Google Scholar]
Krach 2009 {published data only}
- Krach LE. Intrathecal baclofen use in adults with cerebral palsy. Developmental Medicine and Child Neurology 2009;51(Supplement 4):106‐12. [DOI] [PubMed] [Google Scholar]
Krach 2010 {published data only}
- Krach LE, Kriel R, Day SM, Strauss DJ. Survival of individuals with cerebral palsy receiving continuous intrathecal baclofen treatment: a matched cohort study. Developmemental Medicine and Child Neurology 2010;52:672‐6. [DOI] [PubMed] [Google Scholar]
- Krach LE, Kriel RL, Day SM. Survival following intrathecal baclofen pump implantation. Developmental Medicine and Child Neurology. 2008; Vol. 50:36‐7. [DOI] [PubMed]
Latash 1996 {published data only}
- Latash ML, Penn RD. Changes in voluntary motor control induced by intrathecal baclofen in patients with spasticity of different etiology. Physiotherapy Research International 1996;1(14):229‐46. [DOI] [PubMed] [Google Scholar]
Lazorthes 1990 {published data only}
- Lazothes Y, Sallerin‐Caute B, Verdie JC, Bastide R, Carillo JP. Chronic intrathecal baclofen administration for control of severe spasticity. Journal of Neurosurgery 1990;72:393‐402. [DOI] [PubMed] [Google Scholar]
Leslie 1999 {published data only}
- Leslie DP, Ahrendt L. Managing severe spasticity with intrathecal baclofen (ITB) therapy. Journal of Care Management 1999;Special Edition:21‐6. [Google Scholar]
Martinez Moreno 2013 {published data only}
- Martinez Moreno M, Moraleda‐Perez S, Deiana A, Pascual‐Pascual S. Psychotic symptoms associated to intrathecal baclofen infusion. Developmental Medicine and Child Neurology. 2013; Vol. 55, issue s2:44.
McCoy 2006a {published data only}
- McCoy RN, Blasco PA, Russman BS, O'Malley JP. Validation of a care and comfort hypertonicity questionnaire. Developmental Medicine and Child Neurology 2006;48(3):181‐7. [DOI] [PubMed] [Google Scholar]
McCoy 2006b {published data only}
- McCoy AA, Fox MA, Schaubel DE, Ayyangar RN. Weight gain in children with hypertonia of cerebral origin receiving intrathecal baclofen therapy. Archives of Physical Medicine and Rehabilitation 2006;87:1503‐8. [DOI] [PubMed] [Google Scholar]
McMahon 2003 {published data only}
- McMahon MA, Kinnett DG, Bailes AF, Bean J. Intrathecal baclofen therapy effects on health‐related quality of life, gross‐motor function and caregiver assistance in children with spasticity of cerebral origin. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 57th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2003. 2003; Vol. 96.
Meythaler 2001 {published data only}
- Meythaler JM, Guin‐Renfroe S, Law C, Grabb P, Hadley MN. Continuously infused intrathecal baclofen over 12 months for spastic hypertonia in adolescents and adults with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2001;82:155‐61. [DOI] [PubMed] [Google Scholar]
Moosa 2008 {published data only}
- Moosa A, Ghosh D, Khera J, Luciano M. Intrathecal baclofen pump in children with CSF shunting. Developmental Medicine and Child Neurology. 2008; Vol. 50, issue s4:37‐8.
Morton 2011 {published data only}
- Morton RE, Gray N, Vloeburghs M. Controlled study of the effects of continuous intrathecal baclofen infusion in non‐ambulant children with cerebral palsy. Developmental Medicine and Child Neurology 2011;53(8):736‐41. [DOI] [PubMed] [Google Scholar]
Motta 2000 {published data only}
- Motta F, Galli M, Sibella F, Crivellini M. The effect of intrathecal baclofen therapy on gait in children with cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2000. 2000; Vol. 83.
Motta 2001 {published data only}
- Motta F, Buonaguro V, Carletti T, Galli E. Intrathecal baclofen therapy (ITBT) in children affected by spastic diplegia. A comparison of results among gait analysis, functional and subjective patient evaluations. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 55th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2001. 2001; Vol. 88.
Motta 2002 {published data only}
- Motta F, Buonaguro V, Galli E. Intrathecal baclofen therapy in children affected by spastic diplegia. A comparison of results among gait analysis, functional and participative patient evaluations. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2002. 2002; Vol. 91.
Motta 2004 {published data only}
- Motta F, Stignani, Buonaguro V, Abramini M. Effect of intrathecal baclofen on upper limb function in cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 58th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2004. 2004; Vol. 99.
Motta 2004a {published data only}
- Motta F, Buonaguro V, Stignani C, Condurso F. Functional assessment of children with dystonia before and after intrathecal baclofen therapy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 58th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2004. 2004; Vol. 99.
Motta 2007 {published data only}
- Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen in children and adolescents: safety and complications in 200 consecutive cases. Journal of Neurosurgery 2007;107:32‐5. [DOI] [PubMed] [Google Scholar]
Motta 2008 {published data only}
- Motta F, Stignani C, Antonello CE. Upper limb function after intrathecal baclofen treatment in children with cerebral palsy. Journal of Pediatric Orthopedics 2008;28(1):91‐6. [DOI] [PubMed] [Google Scholar]
Motta 2009 {published data only}
Motta 2011 {published data only}
- Motta F, Antonello C, Stignani C. Intrathecal baclofen and motor function in cerebral palsy. Developmental Medicine and Child Neurology 2011;53:443‐8. [DOI] [PubMed] [Google Scholar]
Mukherjee 2002 {published data only}
- Mukherjee S, Taylor S, Gaebler‐Spira D. Changes in seating needs and complications after intrathecal baclofen pump therapy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2002. 2002; Vol. 91.
Muquit 2012 {published data only}
- Muquit S, Ugratdhar I, Ingale H, Vloeberghs. Cervical catheter placement for intrathecal baclofen test dose: is it safe?. Childs Nervous System 2012;28:919‐22. [DOI] [PubMed] [Google Scholar]
Murphy 2002 {published data only}
- Murphy NA, Irwin MCN, Hoff C. Intrathecal baclofen therapy in children with cerebral palsy: efficacy and complications. Archives of Physical Medicine and Rehabilitation 2002;83:1721‐5. [DOI] [PubMed] [Google Scholar]
Najarian 2012 {published data only}
- Najarian CR, Taniguchi M, Krach LE, Stansbury J, Bonau T, Partington M. Catheter access port aspiration to assess intrathecal baclofen withdrawal. Developmental Medicine and Child Neurology. 2012; Vol. 54, issue s6:3‐4.
Ochs 1996 {published data only}
- Ochs Ga, Tonn JC. Functional outcome and clinical significance of long‐term intrathecal baclofen therapy for severe spasticity. Journal of Neurological Rehabilitation 1996;10:159‐66. [Google Scholar]
Penn 1995 {published data only}
- Penn RD, Gianino JM, York MM. Intrathecal baclofen for motor disorders. Movement Disorders 1995;10(5):675‐7. [DOI] [PubMed] [Google Scholar]
Piccinini 1999 {published data only}
- Piccinini L, Krach LE, Gage J, Novacheck T. Gait and functional evaluation pre and post intrathecal baclofen pump implantation. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 1999. 1999; Vol. 80.
Piccinini 2001 {published data only}
- Piccinini L, Maghini C, Germiniasi C, Beretta E. EEG anomalies after intrathecal baclofen vs oral treatment in children affected by cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 55th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2001. 2001; Vol. 88.
Pin 2011 {published data only}
- Pin TW, McCartney L, Lewis J, Waugh MC. Use of intrathecal baclofen in ambulant children and adolescents with spasticity and dystonia of cerebral origin: a systematic review. Developmental Medicine and Child Neurology 2011;53:885‐95. [DOI] [PubMed] [Google Scholar]
Pritula 2012 {published data only}
- Pritula SL, Fox MA, Ayyangar R. Weight changes in children receiving intrathecal baclofen for the treatment of spasticity. Journal of Pediatric Rehabilitation Medicine 2012;5(3):197‐201. [DOI] [PubMed] [Google Scholar]
Ramstad 2010 {published data only}
- Ramstad K, Jahnsen R, Lofterod B, Skjeldal OH. Continuous intrathecal baclofen therapy in children with cerebral palsy ‐ when does improvement emerge?. Acta Paediatrica 2010;99:1661‐5. [DOI] [PubMed] [Google Scholar]
Rawicki 1999 {published data only}
- Rawicki B. Treatment of cerebral origin spasticity with continuous intrathecal baclofen delivered via an implantable pump: Long‐term follow‐up review of 18 patients. Journal of Neurosurgery 1999;91:733‐6. [DOI] [PubMed] [Google Scholar]
Revivo 2001 {published data only}
- Revivo G, Tann B, Peng M, Grant J, Gaebler‐Spira D. Impact of intrathecal baclofen pump placement on care and comfort measures in children and adults with cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 55th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2001. 2001; Vol. 88.
Revivo 2002 {published data only}
- Revivo G, Tann B, Peng M, Grant J, Gaebler‐Spira D. Impact of intrathecal baclofen pump placement on care and comfort measures in children and adults with cerebral palsy. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2002. 2002; Vol. 91.
Rippe 2005 {published data only}
- Rippe DM, Tann B, Gaebler‐Spira DJ, Krach LE, Gooch J, Dabrowski E. Complications of intrathecal baclofen pump therapy for severe hypertonia in children: a long‐term follow‐up review of 785 patients from four centres. Developmental Medicine and Child Neurology ‐ Supplementum. Abstract of the European Academy of Childhood Disability Annual Meeting 2005. 2005; Vol. 103.
Rippe 2005a {published data only}
- Rippe D, Tann B, Gaebler‐Spira D, Krach L, Gooch J, Dabrowski E. Complications of intrathecal baclofen pump therapy for severe hypertonia in children: A long‐term follow‐up review of 785 patients from four centres. Developmental Medicine and Child Neurology ‐ Supplementum. Abstracts of the 59th Annual Meeting of the American Academy for Cerebral Palsy and Developmental Medicine 2005. 2005; Vol. 102.
Saltuari 1992 {published data only}
- Saltauri L, Kronenberg M, Marosi MJ, Kofler M, Russegger L, Rifici C, et al. Long‐term intrathecal baclofen treatment in supraspinal spasticity. Acta Neurologica 1992;47(3):195‐207. [PubMed] [Google Scholar]
Sansone 2006 {published data only}
- Sansone JM, Mann D, Noonan K, Mcleish D, Ward M, Iskandar BJ. Rapid progression of scoliosis following insertion of intrathecal baclofen pump. Journal of Pediatric Orthopedics 2006;26(1):125‐8. [DOI] [PubMed] [Google Scholar]
Saval 2008 {published data only}
- Saval A, Chiodo AE. Effect of intrathecal baclofen concentration on spasticity control: case series. Journal of Spinal Cord Medicine 2008;31:394‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheinberg 2001 {published data only}
- Scheinberg AM, O'Flaherty S, Chaseling R, Dexter M. Continuous intrathecal baclofen infusion for children with cerebral palsy: a pilot study. Journal of Paediatrics and Child Health 2001;37:283‐8. [DOI] [PubMed] [Google Scholar]
Seneran 2007 {published data only}
- Seneran H, Shah SA, Presedo A, Dabney KW, Glutting JW, Miller F. The risk of progression of scoliosis in cerebral palsy patients after intrathecal baclofen therapy. Spine 2007;32:2348‐54. [DOI] [PubMed] [Google Scholar]
Sgouros 2002 {published data only}
- Sgouros S, Seri S. The effect of intrathecal baclofen on muscle co‐contraction in children with spasticity of cerebral origin. Pediatric Neurosurgery 2002;37(5):225‐30. [DOI] [PubMed] [Google Scholar]
Shilt 2008 {published data only}
- Shilt JS, Lai LP, Cabrera MN, Frino J, Smith BP. The impact of intrathecal baclofen on the natural history of scoliosis in cerebral palsy. Journal of Pediatric Orthopedics 2008;28(6):684‐7. [DOI] [PubMed] [Google Scholar]
Silva 2012 {published data only}
- Silva S, Nowicki P, Caird MS, Hurvitz EA, Ayyangar RN, Farley FA, et al. A comparison of hip dislocation rates and hip containment procedures after selective dorsal rhizotomy versus intrathecal baclofen pump insertion in nonambulatory cerebral palsy patients. Journal of Pediatric Orthopedics 2012;32:853‐56. [DOI] [PubMed] [Google Scholar]
Simpson 1999 {published data only}
- Simpson RK. Neurological management of chronic pain and spasticity. Critical Reviews in Physical and Rehabilitation Medicine 1999;11:207‐27. [Google Scholar]
Sivakumar 2010 {published data only}
- Sivakumar G, Yap Y, Tsegaye M, Vloeberghs M. Intrathecal baclofen therapy for spasticity of cerebral origin ‐ does the position of the intrathecal catheter matter?. Childs Nervous System 2010;26:1097‐102. [DOI] [PubMed] [Google Scholar]
Staal 2003 {published data only}
- Staal C, Arends A, Ho S. A self‐report of quality of life of patients receiving intrathecal baclofen therapy. Rehabilitation Nursing 2003;28(5):159‐63. [DOI] [PubMed] [Google Scholar]
Steinbok 1995 {published data only}
- Steinbok P, Daneshvar H, Evans D, Kestle JRW. Cost analysis of intrathecal baclofen versus selective functional posterior rhizotomy in the treatment of spastic quadriplegia associated with cerebral palsy. Pediatric Neurosurgery 1995;22:255‐65. [DOI] [PubMed] [Google Scholar]
Taira 2006 {published data only}
- Taira T, Ochiai T, Goto S, Hori T. Fifteen year experience of intrathecal baclofen treatment in Japan. Acta Neurochiriguria Supplement 2006;99:61‐3. [DOI] [PubMed] [Google Scholar]
Tasseel Ponche 2010 {published data only}
- Tasseel Ponche S, Ferrapie A‐L, Chenet A, Menei P, Gambart G, Menegalli Bogeli D, et al. Intrathecal baclofen in cerebral palsy. A retrospective study of 25 wheelchair‐assisted adults [Baclofene intrathecal dans le paralysie cerebrale. Etude retrospective de 25 patients adultes non marchants]. Annals of Physical and REhabilitation Medicine 2010;53:483‐98. [DOI] [PubMed] [Google Scholar]
Turner 2012 {published data only}
- Turner M, Nguyen HS, Cohen‐Gadol Aaron. Intraventricular baclofen as an alternative to intrathecal baclofen for intractable spasticity or dystonia: outcomes and technical considerations. Journal of Neurosurgery Pediatrics 2012;10:315‐9. [DOI] [PubMed] [Google Scholar]
Ughratadar 2012 {published data only}
- Ughratadar I, Muquit I, Ingale H, Moussa A, Ammar A, Vloeberghs M. Cervical implantation of intrathecal baclofen pump catheter in children with severe scoliosis. Journal of Neurosurgery Pediatrics 2012;10:34‐8. [DOI] [PubMed] [Google Scholar]
Ushewokunze 2012 {published data only}
- Ushewokunze S, Antolovich G, Eldridge B, Randall M, Petersen S, Wray A. Intrathecal baclofen use in children with hemidystonia ‐ a series of four cases. Developmental Medicine and Child Neurology. 2012; Vol. 54:65.
van Hulst 2009 {published data only}
- Hulst BM, Tel PA, Groot V, Ouwerkerk WJR, Vermeulen RJ, Becher JG, Peerdeman SM. Complications of intrathecal baclofen therapy in children and related caregiver satisfaction [Complicaties bij kinderen met intrathecale baclofentherapie en (gerelateerde) verzorgertevredenheld]. Tijdschrift voor Kindergeneeskunde 2009;77(5):191‐7. [Google Scholar]
van Schaeybroeck 2000 {published data only}
- Schaeybroek P, Nuttin B, Lagae L, Schrijvers E, Borghgraef C, Feys P. Intrathecal baclofen for intractable cerebral spasticity: a prospective placebo‐controlled double‐blind study. Congress of Neurological Surgeons 2000;46(3):603‐12. [DOI] [PubMed] [Google Scholar]
van Schie 2001 {published data only}
- Schie PEM, Becher JG, Vermeulen RJ. The influence of intrathecal baclofen therapy (ITB) on the need for care of children with general dystonia. Developmental Medicine and Child Neurology ‐ Supplementum. European Academy of Child Disability Annual Meeting Abstracts 2001. 2001; Vol. 89.
Vitztum 2000 {published data only}
- Vitztum C, Olney B. Intrathecal baclofen therapy and the child with cerebral palsy. Orthopaedic Nursing 2000;19(1):43‐8. [DOI] [PubMed] [Google Scholar]
Vloeberghs 2005 {published data only}
- Vloeberghs M, Keetley R, Morton R. Intrathecal baclofen in the management of spasticity due to cerebral palsy. Pediatric Rehabilitation 2005;8(3):172‐9. [DOI] [PubMed] [Google Scholar]
von Koch 2001 {published data only}
- Koch CS, Park TS, Steinbok P, Smyth M, Peacock WJ. Selective posterior rhizotomy and intrathecal baclofen for the treatment of spasticity. Pediatric Neurosurgery 2001;35:57‐65. [DOI] [PubMed] [Google Scholar]
Ward 2009 {published data only}
- Ward A, Hayden S, Dexter M, Scheinberg A. Continuous intrathecal baclofen for children with spasticity and/or dystonia: Goal attainment and complications associated with treatment. Journal of Paediatrics and Child Health 2009;45:720‐6. [DOI] [PubMed] [Google Scholar]
Wunderlich 2006 {published data only}
- Wunderlich CA, Krach LE. Gram‐negative meningitis and infections in individuals treated with intrathecal baclofen for spasticity: a retrospective study. Developmental Medicine and Child Neurology 2006;48:450‐5. [DOI] [PubMed] [Google Scholar]
Zdolsek 2011 {published data only}
- Zdolsek HA, Olesch C, Antolovich G, Reddihough G. Intrathecal baclofen therapy: benefits and complications. Journal of Intellectual and Developmental Disability 2011;36(3):207‐13. [DOI] [PubMed] [Google Scholar]
Zierski 1988 {published data only}
- Zierski J, Dralle D, Wurdinger T. Implanted pump systems for treatment of spasticity. Acta Neurochirurgica 1988;94(Suppl 43):93‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Aitken 1969
- Aitken RCB. Measurement of feelings using visual analogue scales.. Proceedings of the Royal Society of Medicine 1969;62:989‐93. [PMC free article] [PubMed] [Google Scholar]
Ashworth 1964
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964;192:540‐3. [PubMed] [Google Scholar]
Bohannon 1987
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy 1987;67(2):206‐7. [DOI] [PubMed] [Google Scholar]
Boyd 1999
- Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. European Journal of Neurology 1999;6(Suppl 4):S23‐35. [Google Scholar]
Brashear 2002
- Brashear A, Zafonte R, Corcoran M, Galvez‐Jiminez N, Gracies JM, Gordon, et al. Inter‐ and intrarater reliability of the Ashworth scale and the disability assessment scale in patients with upper limb poststroke spasticity. Archives of Physical Medicine and Rehabilitation 2002;83:1349‐54. [DOI] [PubMed] [Google Scholar]
Clopton 2005
- Clopton N, Dutton J, Featherston T, Grigsby A, Mobley J, Melvin J. Interrater and intrarater reliability of the modified Ashworth scale in children with hypertonia. Pediatric Physical Therapy 2005;17:268‐74. [DOI] [PubMed] [Google Scholar]
Damiano 2002
- Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, Abel MF. What does the Ashworth scale really measure and are instrumented measures more valid and precise?. Developmental Medicine and Child Neurology 2002;44:112‐8. [DOI] [PubMed] [Google Scholar]
Davidoff 1985
- Davidoff RA. Antispasticity drugs: Mechanisms of action. Annals of Neurology 1985;17:107‐16. [DOI] [PubMed] [Google Scholar]
Davis 2010
- Davis E, Shelly A, Waters E, Davern M. Measuring the quality of life of children with cerebral palsy: comparing the conceptual differences and psychometric properties of three instruments. Developmental Medicine and Child Neurology 2010;52:174‐80. [DOI] [PubMed] [Google Scholar]
de Boer 2004
- Boer AG, Lanschot JJ, Stalmeier PF, Sandick JW, Hulscher JBF, et al. Is a single‐item visual analogue scale as valid, reliable and responsive as multi‐item scales in measuring quality of life?. Quality of Life Research 2004;12(2):311‐20. [DOI] [PubMed] [Google Scholar]
de Lissovoy 2007
- Lissovoy G, Matza LS, Green H, Werner M, Edgar T. Cost‐effectiveness of intrathecal baclofen therapy for the treatment of severe spasticity associated with cerebral palsy. Journal of Child Neurology 2007;22(1):49‐59. [DOI] [PubMed] [Google Scholar]
Dietz 1999
- Dietz V. Supraspinal pathways and the development of muscle‐tone dysregulation. Developmental Medicine and Child Neurology 1999;41:708‐15. [DOI] [PubMed] [Google Scholar]
Fosang 2003
- Fosang AL, Galea MP, McCoy AT, Reddihough DS, Story I. Measures of muscle and joint performance in the lower limb of children with cerebral palsy. Developmental Medicine and Child Neurology 2003;45:664‐70. [DOI] [PubMed] [Google Scholar]
Gerszten 1998
- Gerszten PC, Albright AL, Johnstone GF. Intrathecal baclofen infusion and subsequent orthopaedic surgery in patients with spastic cerebral palsy. Journal of Neurosurgery 1998;88:1009‐13. [DOI] [PubMed] [Google Scholar]
Haas 1996
- Haas BM, Bergstrom E, Jamous A, Bennie A. The inter rater reliability of the original and of the modified Ashworth scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord 1996;34(9):560‐4. [DOI] [PubMed] [Google Scholar]
Hagberg 1993
- Hagberg B, Hagberg G, Olow I. The changing panorama of cerebral palsy in Sweden: VI. Prevalence and origin during birth year period 1983‐1986. 1993 82;4:387‐93. [DOI] [PubMed] [Google Scholar]
Hagberg 2001
- Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991‐94. Acta Paediatrica 2001;90:271‐7. [PubMed] [Google Scholar]
Haley 1992
- Haley SM, Coster WJ, Ludlow LH, et al. Paediatric Evaluation of Disability Inventory (PEDI): Version 1.. New England Medical Centre ‐ PEDI Research Group 1992.
Hayley 2010
- Hayley SM, Coster, WI, Kao YC, Dumas HM, Fragala‐Pinkham MA, Kramer JM, et al. Lessons from Use of the PediatricEvaluation of Disability Inventory:Where Do We Go from Here. Pediatric Physical Therapy 2010;22:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Himmelmann 2014
- Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden. XI. Changing patterns in the birth‐year period 2003–2006. Acta Pædiatrica 2014;103:618–624. [DOI] [PubMed] [Google Scholar]
Hugenholtz 1992
- Hugenholtz H, Nelson RF, Dehoux E, Bickerton R. Intrathecal baclofen for intractable spinal spasticity: a double‐blind cross‐over comparison with placebo in 6 patients. Canadian Journal of Neurological Sciences 1992;19:188‐95. [PubMed] [Google Scholar]
Jaeschke 1990
- Jaeschke R, Singer J, Guyatt GH. A comparison of seven‐point and visual analogue scales.. Controlled Clinical Trials 1990;11(1):43‐51. [DOI] [PubMed] [Google Scholar]
Knuttson 1974
- Knuttson E, Lindblom U, Beissinger RL, Martensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimum therapeutic responses in spastic paresis. Journal of Neurological Sciences 1974;23:473‐84. [DOI] [PubMed] [Google Scholar]
Landgraf 1996
- Landgraf JM, Abetz L, Ware JE. Child Health Questionnaire (CHQ): A User's Manual. Health Institute, New England Medical Center, 1996. [Google Scholar]
Loeters 2010
- Loeters MJB, Maathuis CGB, Hadders‐Algrra M. Risk factors for emergence of scoliosis in children with cerebral palsy: a systematic review. Developmental Medicine and Child Neurology 2010;52:605‐11. [DOI] [PubMed] [Google Scholar]
Lonstein 1983
- Lonstein JE, Akbarnia A. Operative treatment of spinal deformities in patients with cerebral palsy or mental retardation. Journal of Bone and Joint Surgery, America 1983;65(1):43‐55. [PubMed] [Google Scholar]
Loubser 1991
- Loubser PG, Narayan RK, Sandin KJ, Donovan WH, Russell KD. Continuous infusion of intrathecal baclofen: long term effects on spasticity in spinal cord injury. Paraplegia 1991;29:48‐64. [DOI] [PubMed] [Google Scholar]
Mackey 2004
- Mackey AH, Walt SE, Lobb, G, Stott S. Intraobserver reliability of the modified Tardieu scale in the upper limb of children with hemiplegia. Developmental Medicine and Child Neurology 2004;46:267‐72. [DOI] [PubMed] [Google Scholar]
McCullough 2008
- McCullough N, Parkes J. Use of the Child Health Questionnaire in children with cerebral palsy: A systematic review and evaluation of psychometric properties. Journal of Paediatric Psychology 2009;33(1):80‐90. [DOI] [PubMed] [Google Scholar]
Oeffinger 2008
- Oeffinger D, Bagley A, Rogers S, Gorten G, Kryscio R, Abel M, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimally clinically important differences. Developmental Medicine and Child Neurology 2008;50:918‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oskoui 2013
- Oskoui M, Coutinho F, Dykeman J, Jette N. An update on the prevalence of cerebral palsy: a systematic review and meta‐analysis. Developmental Medicine & Child Neurology 2013;55:509–519. [DOI] [PubMed] [Google Scholar]
Palisano 1997
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology 1997;39:214‐23. [DOI] [PubMed] [Google Scholar]
Pandyan 1999
- Pandyan AD, Johnson GR, Price CIM, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitation of the Ashworth and modified Ashworth scales as measures of spasticity. Clinical Rehabilitation 1999;19:373‐83. [DOI] [PubMed] [Google Scholar]
Parkin 2004
- Parkin D, Rice N, Jacoby A, Doughty J. Use of a visual analogue scale in daily patient diary: Modelling cross‐sectional time‐series data on health‐related quality of life.. Social Science and Medicine 2004;59(2):351‐60. [DOI] [PubMed] [Google Scholar]
Patrick 2006
- Patrick E, Ada L. The Tardieu scale differentiates contracture from spasticity whereas the Ashworth scale is confounded by it. Clinical Rehabilitation 20;2:173‐82. [DOI] [PubMed] [Google Scholar]
Penn 1984
- Penn Rd, Kroin JS. Intrathecal baclofen alleviates spinal cord spasticity. Lancet 1984;8385:1078. [DOI] [PubMed] [Google Scholar]
Reid 2011a
- Reid SM, Carlin JB, Reddihough DS. Distribution of motor types in cerebral palsy: how do registry data compare?. Developmental Medicine & Child Neurology 2011;53:233–238. [DOI] [PubMed] [Google Scholar]
Reid 2011b
- Reid SM, Carlin JB, Reddihough DS. Rates of cerebral palsy in Victoria, Australia, 1970 to 2004: has there been a change?. Developmental Medicine & Child Neurology 2011;53:907–912. [DOI] [PubMed] [Google Scholar]
Rosenbaum 2007
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M. A report: the definition and classification of cerebral palsy. April 2006. Developmental Medicine and Child Neurology 2007;49(s109):8‐14. [PubMed] [Google Scholar]
Russell 1989
- Russell D, Rosenbaum P, Cadman DT, Gowland C, Hardy S, Jarvis S. The Gross Motor Function Measure: a means to evaluate the effects of physical therapy. Developmental Medicine and Child Neurology 1989;31:341‐52. [DOI] [PubMed] [Google Scholar]
Saito 1998
- Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet 1998;351(9117):1687‐92. [DOI] [PubMed] [Google Scholar]
Sampson 2002
- Sampson FC, Hayward A, Evans G, Morton R, Collett B. Functional benefits and cost/benefit analysis of continuous intrathecal baclofen infusion for the management of severe spasticity. Journal of Neurosurgery 2002;96:1052. [DOI] [PubMed] [Google Scholar]
Sandrini 2005
- Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Wiler JC. The lower limb flexor reflex in humans. Progress in Neurobiology 2005;77(6):353‐95. [DOI] [PubMed] [Google Scholar]
Scholtes 2006
- Scholtes VB, Becher JG, Beelen A, Lankhorst GJ. Clinical assessment of spasticity in children with cerebral palsy: a critical review of available instruments. Developmental Medicine and Child Neurology 2006;48:64‐73. [DOI] [PubMed] [Google Scholar]
Sheean 2002
- Sheean G. The pathophysiology of spasticity. European Journal of Neurology 2002;9(Suppl 1):3‐9. [DOI] [PubMed] [Google Scholar]
Singer 1998
- Singer AJ, Thode HC. Determination of minimally clinically significant difference on patient visual analogue satisfaction scale.. Academic Emergency Medicine 1998;5(10):1007‐11. [DOI] [PubMed] [Google Scholar]
Stanley 1984
- Stanley FJ, Alberman E. The epidemiology of the cerebral palsies. Philadelphia: JB Lippincott, 1984. [Google Scholar]
Tyler 1993
- Tyler DC, Tu A, Douthit J, Chapman CR. Towards validation of pain measurement tools for children: A pilot study. Pain 1993;52(3):301‐9. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang HY, Yang YH. Evaluating the responsiveness of 2 versions of the Gross Motor Function Measure for children with cerebral palsy. Archives of Physical Medicine and Rehabilitation 2006;87:51‐6. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Towards a common language for functioning, disability and health: ICF. www.who.int/classifications/ICF 2002.
Woon 2007
- Woon K, Tsegaye M, Vloeburghs MH. The role of intrathecal baclofen in the management of primary and secondary dystonia in children. British Journal of Neurosurgery 2007;21(4):355‐8. [DOI] [PubMed] [Google Scholar]
Yam 2006
- Yam WKL, Leung MSM. Interrater reliability of modified Tardieu scale in children with spastic cerebral palsy. Journal of Child Neurology 2006;21:1031‐5. [DOI] [PubMed] [Google Scholar]
Zieglgansberger 1988
- Zieglgansberger W, Howe JR, Sutor B. The neuropharmacology of baclofen. Muller H, Zierski J, Penn RD (Eds) Local Spinal Therapy of Spasticity. Berlin: Springer, 1988. [Google Scholar]


