Abstract
Sub-Saharan Africa still bears the greatest forms of malnutrition. Attention is shifting to the use of edible insects in forms which are acceptable to people irrespective of their social status and level of civilization in efforts to alleviate protein malnutrition. Gonimbrasia zambesina (Lepidoptera: Saturniidae) caterpillars emerge seasonally in the coastal part of Kenya and despite their rich nutritional profile, their consumption is low. This study was thus undertaken to evaluate the effect of substituting wheat flour with G. zambesina caterpillar flour at 0%, 5%, 10%, 15% and 20% substitution levels on the nutritional composition and sensory properties of wheat muffins. Substituting wheat flour with G. zambesina caterpillar flour resulted in significantly high protein, fat and fibre contents of enriched wheat muffins. There was also an increasing trend in the ash, minerals and tocopherol content. Invitro protein digestibility significantly decreased from 10 to 20% substitution levels. There was a significant difference (p < 0.05) in the carbohydrate contents of enriched wheat muffins. The sensory scores for colour, texture, aroma and the overall acceptability of wheat muffins decreased with increasing substitution levels. At 10% substitution level, wheat muffins had significantly higher nutritional content than control wheat muffins (0%) and were comparable to muffins enriched with 5% G. zambesina caterpillar flour in terms of overall acceptability. Thus, enriching wheat muffins with G. zambesina caterpillar flour at 10% substitution level has the potential to contribute to improved protein nutrition since they have a higher protein content than the control wheat muffin and are 88.8% digestible (in vitro).
Keywords: Malnutrition, Protein quality, Edible insects, Baking, Food acceptability, Enrichment
Introduction
Protein malnutrition and deficiencies of macro and micro elements are still of concern in middle and low-income countries (FAO 2019; Manditsera et al. 2019; Momanyi et al. 2019). Occasioned by the outbreak of COVID-19 pandemic and the slow rate of vaccine roll-out in the developing countries, there is uncertainty about the situation of food security. This is because the pandemic has interrupted the availability and accessibility of healthy diets among the vulnerable groups occasioned by the closure of markets, increased food prices and disrupted healthy food supply systems (FAO 2020).To bring protein malnutrition under control, it must be approached from both multi-sectoral and multi-level responses since malnutrition is the outcome of a combination of various determinants i.e. food, health and care (Gillespie & Bold 2017; Momanyi et al. 2019). In sub-Saharan Africa, nutrition-specific interventions such as micronutrient fortification, dietary diversification and dietary supplementation for children are championed in response to protein malnutrition (Gillespie & Bold 2017; Momanyi et al. 2019). Through these nutrition-specific interventions, households can access quality proteins from insects, leaves and fungi. (Asefa 2021; Chen et al. 2021). Recently, much focus has been on promoting the consumption and utilization of insects through incorporation into bakery products such as bread, cookies, buns, biscuits and muffins as a cheap alternative source of protein to alleviate protein malnutrition in middle and low-income countries (Alemu et al. 2017; Ayensu et al. 2019; Ayieko et al. 2010; Bawa et al. 2020; de Oliveira et al. 2017; Kinyuru et al. 2009; Osimani et al. 2018; Van Huis 2013).
The African emperor moth, G. zambesina (Lepidoptera: Saturniidae) larva(e) is/are known for its nutritional benefits and seasonal abundance in the coastal region of Kenya (Subramanian et al. 2017). Locally, they are referred to as ‘maungu ya mwembe’, in Swahili meaning ‘mango tree caterpillar’. This name originates from the fact that these caterpillars feed on mango (Mangifera indica) leaves. However, from personal observation and from talking to farmers, some feed on the leaves of Euclea natalensis (Ericales: Ebenaceae). These caterpillars are seasonal and emerge in large quantities during the on-seasons (September–December) during which members of the Agiryama community collect them for food due to their high nutritional composition (Subramanian et al. 2017). However, despite the high protein content of G. zambesina caterpillars (constituting more than 50% of their body weight), its consumption is mostly associated with the elderly members of the community and poor families as most young adults and ‘civilized’ individuals consider consuming caterpillars disgusting and primitive (Gillespie & Bold 2017).
To promote consumption and consumer acceptability of G. zambesina caterpillars, there is a need to transform them into flour which can then be incorporated into wheat products (muffins) such that the caterpillars are indirectly consumed (de Carvalho et al. 2020). This would ensure effective utilization of the caterpillars’ high protein, fat and mineral contents (Subramanian et al. 2017). Muffins are made from weak or moderate wheat flour with a protein content of 8-10% that needs to be improved (Purnomo et al. 2012). Changing the form of presentation of Lepidoptera larvae to consumers has shown to promote the consumption of Bunaea alcinoe (Lepidoptera: Saturniidae) which was made into flour and used to make pulp for children in West Africa (Dauda et al. 2014). Therefore, this study aimed at promoting the consumption of G. zambesina caterpillars as a source of protein by using the caterpillars’ flour to enrich wheat muffins.
Materials and methods
Sample collection and Preparation ofG. zambesinacaterpillar flour.
Approximately, 10 kg of mature G. zambesina caterpillars were obtained from Kipepeo Butterfly centre, Gede, Kenya. The sample was inactivated in a freezer at -25 °C for 30 min, degutted, washed and rinsed in tap water. The samples were then evenly spread on drying nets to drip and then dried to a constant weight at 55 °C for 3 days in a locally made wooden electrical dryer with a lid. The dryer was 0.46 m high, 0.62 m wide and 0.62 m long fitted with two 100 watts bulbs. On one opposite end of the dryer were 12 closely spaced 1 cm diameter holes. The caterpillars were milled into flour using a coffee bean grinder (DeLonghi KG-79, Wuhan, China) and sieved through a 250 μm sieve to obtain fine flour. The flour was frozen-stored at -25 °C in polyethylene sterile zip-lock bags (Vinayak Udyog, New Delhi, India) for 6 months.
Preparation of wheat muffins
Five different wheat muffins with substitution levels of 0%, 5%, 10%, 15% and 20% of wheat flour with G. zambesina caterpillar flour were made. The wheat muffins were prepared according to the procedure by Purnomo et al. (2012) with slight modifications in the quantities of ingredients. The quantities of ingredients used for the control wheat muffins (0% G. zambesina caterpillar flour) and other formulations (5%, 10%, 15% and 20% G. zambesina caterpillar flour) are shown in Table 1. Sieved wheat and G. zambesina caterpillar flour(s) were mixed for 3 min with a hand mixer (Model: RM/382, Guangdong, China) at high speed (as written on the equipment) during which, melted margarine, water and whisked eggs were added at 30-sec interval, respectively. The mixer speed was adjusted to medium (as written on the equipment) upon which, baking powder and refined sugar were added, mixed for 2 min and the batter was poured into standard 6-well muffin pans. Baking was done in preheated oven (Electrolux AR 85, Italy) at 180 °C for 20 min. After which, the wheat muffins were cooled for 20 min at room temperature. Wheat muffin samples for chemical analysis were stored in polyethylene zip-lock bags (Vinayak Udyog, New Delhi, India) by deep freezing at -25 °C.
Table 1.
Quantities of raw materials used in wheat muffin formulation
| Ingredient | Treatment levels (Level of G. zambesina flour) | ||||
|---|---|---|---|---|---|
| 0% | 5% | 10% | 15% | 20% | |
| Wheat flour (g) | 150.00 | 142.50 | 135.00 | 127.50 | 120.00 |
| G. zambesina flour (g) | 0.00 | 7.50 | 15.00 | 22.50 | 30.00 |
| Margarine (g) | 98.57 | 98.57 | 98.57 | 98.57 | 98.57 |
| Refined sugar (g) | 91.43 | 91.43 | 91.43 | 91.43 | 91.43 |
| Beaten eggs (g) | 85.71 | 85.71 | 85.71 | 85.71 | 85.71 |
| Baking powder (g) | 2.14 | 2.14 | 2.14 | 2.14 | 2.14 |
| Water (g) | 47.14 | 47.14 | 47.14 | 47.14 | 47.14 |
Proximate analysis
Proximate composition of wheat flour, G. zambesina caterpillar flour and wheat muffins samples was determined according to AOAC (2000). Moisture and dry matter contents were determined as moisture loss on drying in an oven (Memmert, Schwabach, Germany) at 130 °C for 1 h 15 min (Method 930.15). Crude ash was determined in a muffle furnace at 550 °C for 6 h (Method 923.03). Crude protein was determined in the Kjeldahl apparatus (Gerhardt, Königswinter, Germany) after digestion with concentrated H2SO4 (Method 955.04). A conversion factor of 6.25 was used to convert nitrogen to protein (Kinyuru 2020). Fat content was determined by Soxhlet extraction (Method 920.39). The crude fibre was determined as a loss on ignition following acid-base sample digestion (Method 962.09). The total carbohydrate content of wheat muffins was determined by difference method (Dauda et al. 2014).
In vitroprotein digestibility (IVPD).
A pepsin-trypsin digestion method according to Wang et al. (2010) was adopted for G. zambesina caterpillar flour and wheat muffins samples with modification. Briefly, 0.5 g sample was suspended in 9.5 ml 0.1 M HCl and mixed with 5 mg pepsin (1:3000; activities 0.8 Anson units/mg, Mumbai, India) in 0.5 ml 0.1 M HCl in the digestion tubes and covered. The tubes were gently shaken in a water bath at 37 °C for 2 h. The solution was neutralized by 10 ml 1.0 M phosphate buffer (pH 8.0). Trypsin (Pankreasprotease; activity min 200 FIPU/g, Merck Darmstadt, Germany) (100:1 ratio of substrate/enzyme ratio, w/w) was added to the neutral solution and covered. The tubes were incubated at 37 °C in a water bath for 2 h then enzyme activity was terminated by adding equal volumes of trichloroacetic acid (10% w/v). Samples were frozen at -25 °C for 20 min, vortexed (Henry Troemner, LLC, USA) for 15 min (2500 g, 20 °C) and left to settle overnight. The supernatant was discarded and the residue oven-dried (Memmert, Schwabach, Germany) at 105 °C for 3 h. About 0.2 g residue was taken and analysed for residual protein using the Kjeldahl method. Protein digestibility was calculated by the formula:
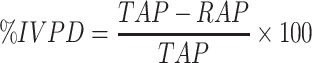
where TAP is the total amount of protein and RAP is the residual amount of protein.
Mineral content
The mineral contents concentration of G. zambesina caterpillar flour and wheat muffins samples were determined by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) quantitation according to Series (1992) and Phan-thien et al. (2012). Briefly, samples (0.5 g each) were mixed with 8.0 ml HNO3 and 1 ml 0f 30% H2O2 in digestion tubes and subjected to temperature digestion (Multiwave Go Plus, Anton Par, Graz, Austria) of 10 min ramp to 100 °C, 10 min hold followed by, 10 min ramp to 180 °C, 10 min hold. The digests were transferred into 50 ml falcon tubes and diluted to the mark using 2% nitric acid. The solutions were then quantified in Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) equipment (Model: Optima 2100DV PerkinElmer, Massachusetts, USA). Mixed standard CatNo.43,843 (Sigma Aldrich, USA) was serial diluted using 2% nitric acid to obtain calibration standards of 400, 800, 2000 and 4000 µg/L and an external standard calibration method was applied. Perkin Elmer Winlab 32 software was used to perform the calibration. To calculate the final concentration for each mineral, the data obtained was used in the formula:
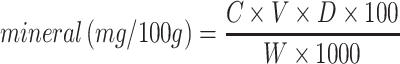
where C is the concentration of the sample in µg/L, V is the total dilution volume in L, D is the dilution factor, W is the weight of sample in g, 100 is the conversion factor to report results in mg/100 g, and 1000 is the conversion of µg to mg.
Determination of vitamin A and E
Retinol, α-tocopherol, and γ-tocopherol of G. zambesina caterpillar flour and wheat muffins samples were analysed by HPLC according to the method described by Bhatnagar-panwar and Bhatnagar-mathur (2013) and Hosotani and Kitagawa (2003). Briefly, 0.5 g of powdered product sample was weighed in a 25 ml tube in triplicate. Ethanol (6 ml) with 0.1% (BHT) was added, homogenized for 1 min together with 120 µl potassium hydroxide 80% (w/v) and mixed by vortexing. The tubes were incubated for 5 min at 85 °C in a water bath, removed and immediately ice-cooled. Deionized water (4 ml) was added to each tube and vortexed. Hexane (5ml) was added, mixed by vortexing and the samples centrifuged (Eppendorf model: centrifuge 5810, Hamburg, Germany) at 1791 g for 5 min. The upper phase (hexane) was transferred to centrifuge tubes, extraction done 3 more times with 4 × 3 × 3 ml hexane pooling the extract into the 25 ml tube. Deionized water (5 ml) was added to the extract, vortexed for 1 min and centrifuged (Eppendorf model: centrifuge 5810, Hamburg, Germany) for 5 min at 1791 g. The hexane layer was recovered into a clean test tube and evaporated under nitrogen in the N-Evap (Organomotion, Massachusetts, USA), reconstituted into 1 ml methanol: tetrahydrofuran (85:15v/v) vortexed and sonicated for 30 s. The reconstituted residue (0.8 ml) was transferred to HPLC vials for quantification in the HPLC system (Shimadzu Nexera UPLC, Kyoto, Japan) with a reverse-phase gradient, YMC C30, carotenoid column (3 μm, 150 × 3.0 mm, YMC Wilmington, NC) and an injection volume of 10 µl. The HPLC has two-phase; mobile phase A: methanol/tert-butyl methyl ether/water (85:12:3, v/v/v, with 1. % ammonium acetate in the water) and mobile phase B: methanol/tert-butyl methyl ether/water (8:90:2, v/v/v, with % ammonium acetate in the water) at a flow rate of 0.4 ml/min. Vitamin A and E were monitored at 290 nm wavelength on an SPD -M2A detector. Standards were compared to the extracts for vitamin A and E concentrations determination.
Sensory evaluation
The sensory evaluation study was authorized by Jaramogi Oginga Odinga University of Science and Technology ethical review committee (ERC). Participants selected on a volunteer basis were presented with consent forms to carefully read through before accepting to undertake the study. The panel was made up of 30 semi-trained members of age 18–27 years. The number of females to males was in the ratio of 2:1. Each participant was presented with five coded wheat muffins EM-4 (0% G. zambesina caterpillar flour: 100% wheat flour), EM-3 (5% G. zambesina caterpillar flour: 95% wheat flour), EM-1 (10% G. zambesina caterpillar flour: 90% wheat flour), EM-5 (15% G. zambesina caterpillar flour:85% wheat flour) and EM-2 (20% G. zambesina caterpillar flour: 80% wheat flour) in random order, clean water for rinsing their palates before and after tasting the sample and a sensory score card. Panelists were asked to rate each wheat muffin in terms of aroma, colour, taste, texture and overall acceptability on a 5-point hedonic scale (1: extremely dislike, 2: dislike, 3: neither like nor dislike, 4: like and 5: extremely like).
Statistical analysis
Data were analysed using Statistical Analysis System (SAS) version 8.3. Data were arranged in an excel sheet as per the variables and tested for normality using PROC UNIVARIATE and for homogeneity using Levene’s method. T-test was used to compare the proximate components of raw materials while one-way analysis of variance (ANOVA) with multiple comparisons using Tukey’s honest significance difference (Tukey’s HSD) test was used to determine the significance of difference among treatment levels at a significance level of p ≤ 0.05. The results were presented as means ± standard deviations.
Results
Raw material characterization
There was a significant difference (p < 0.05) in the chemical composition of G. zambesina flour and wheat flour (Table 2). The result showed that G. zambesina caterpillar flour was higher in ash, crude protein, fat and crude fibre than wheat flour. However, the carbohydrate content of G. zambesina caterpillar flour was lower than wheat flour. The major component of G. zambesina caterpillar flour on a dry matter basis was protein (59.33 ± 0.30%). The IVDP of G. zambesina caterpillar flour was 64.05%. The potassium, sodium and phosphorus concentrations of the caterpillar flour were 1740.0 mg/100 g, 49.1 mg/100 g and 555.2 mg/100 g, respectively. Zinc concentration was 17.8 mg/100 g and iron was 8.0 mg/100 g. Retinol, α-tocopherol and ɣ-tocopherol concentrations of G. zambesina caterpillar flour were 0.00 mg/100 g, 4.32 mg/100 g and 0.16 mg/ 100 g, respectively.
Table 2.
Proximate composition of Gonimbrasia zambesina caterpillar flour and wheat flour
| Flour | Proximate components (%) | |||||
|---|---|---|---|---|---|---|
| Protein | Fat | Carbohydrate | Fibre | Ash | Dry matter | |
| CF | 59.33 ± 0.30a | 14.87 ± 0.26a | 1.17 ± 0.39b | 15.50 ± 0.25a | 3.46 ± 0.20a | 94.33 ± 0.06a |
| WF | 13.11 ± 0.45b | 0.65 ± 0.09b | 75.56 ± 0.69a | 0.88 ± 0.01b | 0.53 ± 0.10b | 90.73 ± 0.17b |
| t-value | 85.57 | 52.11 | -93.74 | 59.16 | 13.24 | -20.11 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0002 | < 0.0001 |
Key: CF = Caterpillar flour; WF = Wheat flour; Values are mean ± standard deviation of triplicate analysis. Means with the same superscript letter along the column are not significantly different (p < 0.05)
Proximate composition of wheat muffins enriched withG. zambesinacaterpillar flour.
As shown in Table 3, there was a significant difference (p < 0.05) in crude protein, crude fat and carbohydrate and crude fibre contents of wheat muffins enriched with G. zambesina caterpillar flour. The wheat muffins showed a significant increase (p < 0.05) in protein content on substitution with up to a 73.6% increase from the reference wheat muffins. However, there was no significant difference (p < 0.05) in the ash and dry matter content of the wheat muffins.
Table 3.
Effect of substituting wheat flour with G. zambesina caterpillar flour on the proximate composition of wheat muffins
| Level of Substitution |
Proximate components (%) | |||||
|---|---|---|---|---|---|---|
| Protein | Fat | Carbohydrate | Fibre | Ash | Dry matter | |
| 0% | 19.67 ± 0.70d | 28.83 ± 0.64c | 47.00 ± 1.16a | 0.89 ± 0.00c | 0.93 ± 0.09a | 97.32 ± 0.18a |
| 5% | 25.47 ± 0.50bc | 29.75 ± 0.38bc | 39.96 ± 0.37b | 1.14 ± 0.00c | 1.11 ± 0.11a | 97.44 ± 0.14a |
| 10% | 28.23 ± 1.55b | 30.68 ± 0.64b | 35.40 ± 2.22bc | 1.82 ± 0.07b | 1.18 ± 0.01a | 97.31 ± 0.08a |
| 15% | 31.58 ± 0.44ab | 31.52 ± 0.51ab | 30.83 ± 0.49c | 2.04 ± 0.06b | 1.23 ± 0.26a | 97.19 ± 0.19a |
| 20% | 34.15 ± 0.54a | 32.62 ± 0.22a | 26.09 ± 0.96c | 2.67 ± 0.22a | 1.35 ± 0.20a | 96.87 ± 0.19a |
| F(4,10) value | 43.64 | 8.65 | 43.36 | 42.46 | 0.91 | 1.78 |
| P-value | < 0.0001 | 0.0028 | < 0.0001 | < 0.0001 | 0.4924 | 0.2096 |
Key: Values are mean ± standard deviation of triplicate analysis. Means with different superscript letters along the column are significantly different (p < 0.05)
IVPD of wheat muffins enriched with G. zambesina caterpillar flour
There was significant difference (p < 0.05) in the IVPD of wheat muffins enriched with G. zambesina caterpillar flour (Fig. 1). The IVPD was high in wheat muffins enriched with G. zambesina caterpillar flour at 5%, 10% and 15% substitution levels. The 20% wheat muffins showed a similar IVPD to that of the control wheat muffins
Fig. 1.
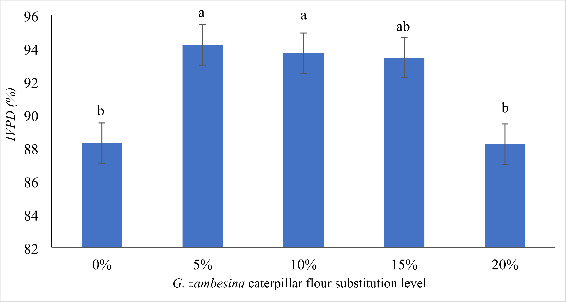
Effect of substituting wheat flour with G. zambesina caterpillar flour on in vitro protein digestibility of wheat muffins
Note: Bars with different alphabet letters are significantly different (p < 0.05); Error bars represents Standard Error
The minerals concentration of wheat muffins enriched withG. zambesinacaterpillar flour.
Table 4 shows that potassium, phosphorus and sodium concentrations of G. zambesina caterpillar flour enriched wheat muffins at different substitution levels were significantly different (p < 0.05). However, there was no significant difference (p < 0.05) in the iron and zinc concentrations. The mineral content concentrations increased with increase in substitution level of wheat flour with G. zambesina caterpillar flour.
Table 4.
Minerals content of wheat muffins enriched with G. zambesina caterpillar flour
| Level of substitution | Mineral concentration (mg/100 g) | ||||
|---|---|---|---|---|---|
| K | P | Na | Fe | Zn | |
| 0% | 156.10 ± 2.40c | 172.93 ± 3.00b | 202.83 ± 1.82b | 4.49 ± 0.19a | 2.31 ± 0.01a |
| 5% | 161.59 ± 5.16c | 174.77 ± 1.06b | 213.12 ± 12.83ab | 4.67 ± 0.31a | 2.90 ± 0.06a |
| 10% | 197.36 ± 4.35b | 177.91 ± 3.89ab | 222.16 ± 1.93ab | 4.76 ± 0.03a | 3.34 ± 0.13a |
| 15% | 242.11 ± 4.32a | 185.28 ± 4.51ab | 234.79 ± 11.16ab | 4.91 ± 0.04a | 3.37 ± 0.40a |
| 20% | 273.14 ± 9.25a | 194.19 ± 1.20a | 255.50 ± 5.68a | 4.98 ± 0.27a | 3.40 ± 0.14a |
| F(4,5) value | 83.13 | 8.12 | 6.33 | 0.89 | 5.66 |
| P-value | < 0.0001 | 0.0206 | 0.0341 | 0.5304 | 0.0424 |
Key: Values are mean ± standard deviation of triplicate analysis. Means with different superscripts (alphabet) along the column are significantly different (p < 0.05)
Retinol, α-tocopherol and ɣ-tocopherol content of wheat muffins enriched withG. zambesinacaterpillar flour.
As shown in Fig. 2, α-tocopherol concentration slightly increased with a corresponding increase in substitution level. The increase in concentrations of ɣ-tocopherol with increase in substitution level was non-significant. Retinol was present in the control wheat muffins and those enriched with G. zambesina caterpillar flour wheat muffins at different substitution levels.
Fig. 2.
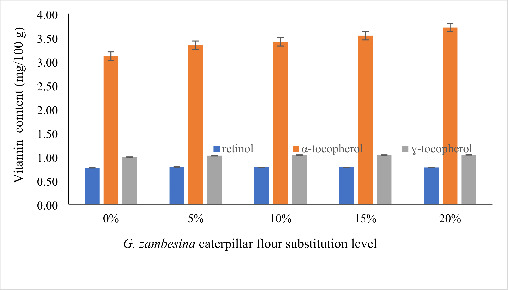
Retinol, α-tocopherol and ɣ-tocopherol contents of wheat muffin enriched with G. zambesina caterpillar flour
Note: Error bars represent Standard Error.
Sensory properties of wheat muffins enriched with G. zambesina caterpillar flour
There was significant difference (p < 0.05) in aroma, colour, texture and overall acceptability of wheat muffins enriched with G. zambesina caterpillar flour (Table 5). However, there was no significant difference (p < 0.05) in taste. The least score for aroma, colour, taste, texture and overall acceptability was obtained for EM-2. A decline in sensory scores was observed for aroma, colour and overall acceptability with increase in substitution level. Generally, all the sensory attributes of the enriched wheat muffins were acceptable among panelists since all had above average sensory scores.
Table 5.
Sensory scores for wheat muffins enriched with G. zambesina caterpillar flour on a 5-point hedonic scale
| Coded sample | Aroma | Colour | Taste | Texture | Overall acceptability |
|---|---|---|---|---|---|
| EM-4 | 4.63 ± 0.49a | 4.29 ± 0.91a | 4.17 ± 0.87a | 4.40 ± 0.86a | 4.47 ± 0.97a |
| EM-3 | 4.17 ± 0.59ab | 4.17 ± 0.59ab | 3.90 ± 0.66a | 4.17 ± 0.70ab | 4.17 ± 0.75ab |
| EM-1 | 3.57 ± 0.77bc | 3.93 ± 0.83b | 4.00 ± 0.69a | 3.83 ± 0.79bc | 3.83 ± 0.75bc |
| EM-5 | 3.20 ± 1.06 cd | 3.53 ± 1.01b | 4.00 ± 0.87a | 3.37 ± 1.33c | 3.67 ± 0.96c |
| EM-2 | 2.80 ± 1.13d | 3.97 ± 1.07b | 3.90 ± 0.66a | 3.63 ± 1.07c | 3.53 ± 0.90c |
| F(4, 145) value | 22.60 | 2.98 | 0.62 | 5.37 | 5.74 |
| P-value | < 0.0001 | 0.0212 | 0.6498 | 0.0005 | 0.0003 |
Key: Values are mean ± standard deviation of analysis. Means with different superscripts (alphabet) along the column are significantly different (p < 0.05)
Discussion
In this study, the results for raw material characterization demonstrated that G. zambesina caterpillar flour has got high protein content. This is in agreement with the findings by Subramanian et al. (2017) which show more than 50% of the body weight of G. zambesina caterpillar (dry weight) is protein. The high crude fibre content of G. zambesina caterpillar flour might be attributed to the presence of chitin which forms part of certain insects’ exoskeleton (Dauda et al. 2014). Contrariwise, wheat flour had low crude fibre content and this might be attributed to the removal of wheat bran during milling (Chen et al. 2021). Gonimbrasia zambesina caterpillar flour had higher fat content than wheat flour. Fat is important in the human diet since it makes food more palatable (Igbabul et al. 2014). In comparison to other closely related Lepidoptera species, the fat content of G. zambesina was higher than that of Bunaea alcinoe (10.85 ± 0.65%) and Cirina forda (Lepidoptera: Saturniidae) (4.68 ± 0.01%) (Dauda et al. 2014; Omotoso 2006). The lower fat content observed for wheat flour might be attributed to the grain fat store (germ) which is only 3% of the grain (Kumar et al. 2011). Gonimbrasia zambesina caterpillar flour was lower in carbohydrate content in comparison to wheat flour and this might be attributed to degutting which reduces carbohydrates originating from the host plant (Lautenschläger et al. 2017).
As was expected, increasing the substitution level of wheat flour with G. zambesina caterpillar flour led to a progressive increase in crude protein, crude fat, crude fibre, and ash content of the wheat muffins. The percentage crude protein content of wheat muffins increased by 29.5 − 73.6% from 5 − 20% substitution levels. This increase in crude protein content in the enriched wheat muffins might be attributed to the high protein content of G. zambesina caterpillar flour used for enrichment (Table 2). This finding is in agreement with that by Ayensu et al. (2019) on the nutritional composition of biscuits fortified with orange-fleshed potato and palm weevil larvae (Rhynchophorus phoenicis). Kinyuru et al. (2009) aslso observed a similar trend in their research on process development, nutritional composition of wheat buns enriched with edible termites (Macrotermes subhylanus). The decrease in the carbohydrate content of wheat muffins with increase in the substitution level of G. zambesina caterpillar (47.00 ± 1.16–26.09 ± 0.96) flour might be attributed to the low contents of carbohydrate in G. zambesina caterpillar flour. Wheat flour, which is the major component of the wheat muffins is higher in carbohydrate content than G. zambesina caterpillar flour (Table 2). Thus, substituting it with G. zambesina caterpillar flour (low in carbohydrate) during wheat muffins formulation lowers its carbohydrate content. This findings corroborates with that by Ayensu et al. (2019) who reported a decrease in the carbohydtrate content of biscuits fortified with orange-fleshed potato and R. phoenicis larve flour. Similarly, de Oliveira et al. (2017) also reported a decreasing trend in the carbohydtate content of bread enriched with cockroach (Nauphoeta cinerea) flour with increase in substitution levels.
Increase in fibre content of wheat muffins with corresponding increase in substitution levels (0.89 ± 0.00-2.67 ± 0.22) might be ascribed to the high crude fibre content of G. zambesina caterpillar flour used to substitute wheat flour (low fibre content) in wheat muffins formulation. This finding is in agreement with those by Ayensu et al. (2019) and Osimani et al. (2018) in their research on biscuits fortified with R. phoenicis larvae flour and bread enriched with cricket powder (Acheta domesticus) respectively. Fibre aids in bowel movement and plays a role in maintaining the health of the digestive system (Nantanga and Amakali 2020). The European food safety authority (EFSA) suggests that for a food product to be considered as a high fibre food, it should provide 3 mg/100 g of dietary fibre (EFSA 2010; Osimani et al. 2018). Thus, wheat muffins enriched with G. zambesina caterpillar flour might contribute toward meeting the fibre daily intake threshold.
Despite increase in crude fat content of wheat muffins enriched with G. zambesina caterpillar flour with increase in substitution levels (28.83 ± 0.64–32.62 ± 0.22), the crude fat contents of the wheat muffins were generally high. This observation might be ascribed to margarine used as an ingredient in the formulation of wheat muffins. A similar observation of increasing fat contents with increase in substitution level was made by Ayensu et al. (2019) in his research on biscuits fortified with R. phoenicis larvae flour and orange-fleshed sweet potato. A non-significant increase in the ash content of wheat muffins enriched with G. zambesina caterpillar flour with increase in substitution level was observed. The ash content of a product is an indication of the mineral concentration of that particular product (Chen et al. 2021; Cheng and Bhat 2016). Thus, the trend indicated that enriching wheat muffins with G. zambesina caterpillar flour could improve the mineral concentration of the wheat muffins. Other previous research studies on biscuit and bread making (Ayensu et al. 2019; de Oliveira et al. 2017; Osimani et al. 2018) have also shown similar findings of linear correlation between ash content and the substitution level of wheat flour with insects flour.
Animal proteins are 90–95% digestible (in vitro) which is superior to plant proteins IVPD (75–80%) (Sá et al. 2020). However, the observed low IVPD for G. zambesina caterpillar flour (64.05%) might be attributed to the presence of chitin fibre and certain anti-nutrients in the caterpillar (Mba et al. 2019; Moyo et al. 2019). Wheat muffins with 5% G. zambesina caterpillar flour had higher IVPD than the control wheat muffins (0%). This might be attributed to the exposure of additional extra proteins (insect) to what is already available in wheat flour to the digestive enzymes when wheat flour is substituted with G. zambesina caterpillar flour. It might also be attributed to an opinion that, at 5% substitution level, minute quantities of chitin fibre in G. zambesina flour were introduced into wheat flour. Hence its effect of binding to proteins was very minimal. The general declining trend in IVPD of the wheat muffins from 5 to 20% substitution levels might be ascribed to corresponding increase in quantities of chitin fibre and anti-nutrients of G. zambesina caterpillar flour with increase in substitution level. Chitin fibre can reduce protein digestibility of insects protein by binding to proteins and is non-absorbable by the small intestine (Guerreiro et al. 2021; Marono et al. 2015; Mba et al. 2019). It also hampers hydrolysis of proteins as it successfully eludes the digestive enzymes rendering it non-absorbable by the small intestine (Moyo et al. 2019) .
Zinc and iron are of concern among pregnant women, vegans and people suffering from malnutrition (Ayensu et al. 2019). Zinc is essential in the human diet as it regulates the growth of cells, improves the quality of sperm, plays a vital role in the expression of genes and acts as an enzyme cofactor (Khan et al. 2013). Caterpillar (G. zambesina) flour with a zinc concentration of 17.8 mg/100 g might be recommended for people suffering from zinc deficiency as it has the potential to contribute to zinc daily recommended intake. The approximate RDA for zinc in adults is 12–15 mg/day (Khan et al. 2013). Dietary iron is essential for premenopausal women for haemoglobin formation. The approximate RDA for iron in adults is 10–15 mg/100 g (Khan et al. 2013). Gonimbrasia zambesina caterpillar thus has the potential to contribute to iron RDA. There was a linear relationship between the mineral concentration of wheat muffins and the substitution levels of wheat flour with G. zambesina caterpillar flour. Higher substitution levels resulted in wheat muffins with higher mineral concentrations (Table 4). This finding was in agreement with those by Ayensu et al. (2019) and Bawa et al. (2020) in their research on biscuits fortified with R. phoenicis larvae flour and the nutritional quality of bread and cookie enriched with A. domesticus powder, respectively. However, the iron and zinc concentrations of enriched wheat muffins were still below the iron and zinc RDA despite increase in the substitution level of wheat flour with G. zambesina caterpillar flour. The wheat muffins might be recommended to premenopausal women and people suffering from zinc deficiency together with other sources of iron and zinc (Khan et al. 2013). They might also be recommended to hypertensive people considering they are low in sodium. The high potassium concentrations of the wheat muffins are capable of contributing to potassium RDA (2500 mg/day) (Igbabul et al. 2014; Khan et al. 2013). The enriched wheat muffins also have the potential to reduce hypophosphatemia due to high phosphorus concentrations (Bawa et al. 2020).
Gonimbrasia zambesina caterpillar flour and wheat flour have a deficiency of vitamin A (Klemm et al. 2010). Therefore, the presence of low concentrations of retinol in wheat muffins enriched with G. zambesina caterpillar flour might be attributed to margarine (vitamin A fortified) used as an ingredient and not the caterpillar flour (Dary & Mora 2018). The increase in α-tocopherol and ɣ-tocopherol with increase in substitution level of wheat flour with G. zambesina caterpillar flour might be attributed to 4.32 mg/100 g α-tocopherol and 0.16 mg/100 g ɣ-tocopherol concentrations, respectively in G. zambesina caterpillar flour. Hence, the consumption of wheat muffins enriched with G. zambesina caterpillar flour has the potential to contribute to the recommended daily intake of vitamin E.
Sensory evaluation of wheat muffins provides vital information that can be used to improve their nutritional value. The colour of wheat muffins became more dark brown with increase in substitution level of wheat flour with G. zambesina caterpillar flour. This might be attributed to the Maillard reaction (Kinyuru et al. 2009; Purlis 2010). Gonimbrasia zambesina caterpillar flour is high in protein which is a requisite reactant for the Maillard reaction. (Kouřimská and Adámková 2016; Purlis 2010). The relatively low mean rating for aroma for wheat muffins at 20% substitution level (2.80 ± 1.13) might be attributed to the unique aroma associated with certain edible insects. These aromas tend to be more intense at higher substitution levels (Ayieko et al. 2010). The fact that there was no significant difference (p < 0.05) in taste might be attributed to egg and sugar which were constant ingredients in all the wheat muffins. Wheat muffins enriched with G. zambesina caterpillar flour at 5% and 10% substitution levels had a high mean rating for texture in comparison to those at 15% and 20% substitution levels (Table 5). This might be ascribed to the fact that at lower substitution levels, small quantities of G. zambesina caterpillar fibre is introduced into the wheat muffins. Fibre and the exoskeletion of lepidotera influences the texture of its flour (Kouřimská and Adámková 2016; Mishyna et al. 2020). Generally, Wheat muffins enriched with G. zambesina caterpillar flour were acceptable.
Conclusion
The research demonstrates that enriching wheat muffins with G. zambesina caterpillar flour improves their crude protein, crude fat, crude fibre, ash and mineral contents. The IVPD of wheat muffins enriched with G. zambesina caterpillar flour remains high at 5%, 10% and 15% substitution levels. Moreover, the vitamin E content in the G. zambesina caterpillar flour is maintained in wheat muffins enriched with G. zambesina caterpillar flour. Wheat muffins enriched G. zambesina caterpillar flour at 5% and 10% substitution levels are generally acceptable to consumers. This study shows 10% wheat muffins as the best formulation. This is due to the increase in 43.5% protein compared to the control wheat muffins. Furthermore, the protein digestibility (in vitro) of 10% wheat muffins is higher than that of the control and 20% wheat muffins and the product is also acceptable to the consumers.
Acknowledgements
This research was funded by Jaramogi Oginga Odinga University of Science and Technology, through the African Centre of Excellence in sustainable use of insects as feed and food (INSEFOODS).
Ethical approval
This study was approved by the Ethics Review Committee (ERC) of Jaramogi Oginga Odinga University of Science and Agriculture and the National Commission for Science Technology and Innovation (NACOSTI).
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fedinand Opondo Ouma, Email: fediopondo@gmail.com.
Alice Nakhumicha Muriithi, Email: muriithi.alice@gmail.com.
Joseph Ochieng’ Anyango, Email: ajochieng@egerton.ac.ke.
References
- Alemu MH, Olsen SB, Vedel SE, Kinyuru JN, Pambo KO. Can insects increase food security in developing countries? An analysis of Kenyan consumer preferences and demand for cricket flour buns. Food Secur. 2017;9(3):471–484. doi: 10.1007/s12571-017-0676-0. [DOI] [Google Scholar]
- AOAC (2000) Official methods of analysis, 18th edn. Association of Official Analytical Chemist,Washington, DC
- Asefa BG. Nutritional and Techno-Functional Properties of Fish Protein Powder (FPP) from Underutilized Small Fish (Barbus paludinosus) Species. J Food Nutr Sci. 2021;9(3):84–88. doi: 10.11648/j.jfns.20210903.13. [DOI] [Google Scholar]
- Ayensu J, Lutterodt H, Annan RA, Edusei A, Loh SP. Nutritional composition and acceptability of biscuits fortified with palm weevil larvae (Rhynchophorus phoenicis Fabricius) and orange-fleshed sweet potato among pregnant women. Food Sci Nutr. 2019;7(5):1807–1815. doi: 10.1002/fsn3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayieko M, Oriaro V, Nyambuga I (2010) Processed products of termites and lake flies: improving entomophagy for food security within the lake victoria region. Afr J Food Agric Nutr Dev 10(2). 10.4314/ajfand.v10i2.53352
- Bawa M, Songsermpong S, Kaewtapee C, Chanput W(2020) Nutritional, sensory, and texture quality of bread and cookie enriched with house cricket (Acheta domesticus) powder.Journal of Food Processing and Preservation, 44(8), e14601
- Bhatnagar-panwar M, Bhatnagar-mathur P. Rapid, accurate and routine HPLC method for large-scale screening of pro-vitamin A carotenoids in oilseeds. J Plant Biochem Biotechnol. 2013;24(1):84–92. doi: 10.1007/s13562-013-0239-1. [DOI] [Google Scholar]
- Chen C, Han Y, Li S, Wang R, Tao C. Nutritional, antioxidant, and quality characteristics of novel cookies enriched with mushroom (Cordyceps militaris) flour. CyTA - Journal of Food. 2021;19(1):137–145. doi: 10.1080/19476337.2020.1864021. [DOI] [Google Scholar]
- Cheng YF, Bhat R. Functional, physicochemical and sensory properties of novel cookies produced by utilizing underutilized jering (Pithecellobium jiringa Jack.) legume flour. Food Bioscience. 2016;14:54–61. doi: 10.1016/j.fbio.2016.03.002. [DOI] [Google Scholar]
- Dauda BE, Mathew J, Paiko YB, Ndamitso M(2014) Nutritive and Anti-nutritive Composition of Locust Bean Tree Emperor Moth Larvae Bunaea alcinoe (Lepidoptera-saturniidae Stoll 1780) from Gurara Local Government Area, Niger State, Nigeria.Journal of Scientific Research and Reports,1771–1779
- de Carvalho NM, Madureira AR, Pintado ME. The potential of insects as food sources–a review. Crit Rev Food Sci Nutr. 2020;60(21):3642–3652. doi: 10.1080/10408398.2019.1703170. [DOI] [PubMed] [Google Scholar]
- de Oliveira LM, da Silva Lucas AJ, Cadaval CL, Mellado MS. Bread enriched with flour from cinereous cockroach (Nauphoeta cinerea) Innovative Food Science and Emerging Technologies. 2017;44:30–35. doi: 10.1016/j.ifset.2017.08.015. [DOI] [Google Scholar]
- EFSA EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA); Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010;8(3):1462. doi: 10.2903/j.efsa.2010.1462. [DOI] [Google Scholar]
- FAO, IFAD, UNICEF, WFP, WHO (2019) Food Security and Nutrition in the World 2019. Safeguarding against economic slowdowns and downturns. Rome, FAO
- FAO, IFAD, UNICEF, WFP, WHO (2020) The State of Food Security and Nutrition in the World 2020. Transforming food systems for affordable healthy diets. Rome, FAO. 10.4060/ca9692en
- Gillespie S, Bold M, Van Den Agriculture, food systems, and nutrition : meeting the challenge. Global Challanges. 2017;1(3):1600002. doi: 10.1002/gch2.201600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro I, Serra CR, Coutinho F, Couto A, Castro C, Rangel F, Peres H, Pousão-ferreira P, Matos E, Gasco L, Gai F, Enes P. Digestive enzyme activity and nutrient digestibility in meagre (Argyrosomus regius) fed increasing levels of black soldier fly meal (Hermetia illucens) Aquacult Nutr. 2021;27(1):1–11. doi: 10.1111/anu.13172. [DOI] [Google Scholar]
- Hosotani K, Kitagawa M. Improved simultaneous determination method of β-carotene and retinol with saponification in human serum and rat liver. J Chromatogr B. 2003;791(1–2):305–313. doi: 10.1016/S1570-0232(03)00233-2. [DOI] [PubMed] [Google Scholar]
- Igbabul BD, Agude C, Inyang CU. Nutritional and Microbial Quality of Dried Larva of Cirina forda. Int J Nutr Food Sci. 2014;3:602–606. doi: 10.11648/j.ijnfs.20140306.28. [DOI] [Google Scholar]
- Khan N, Sultana A, Tahir N, Jamila N (2013) Nutritional composition, vitamins, minerals and toxic heavy metals analysis of Trianthema portulacastrum L., a wild edible plant from Peshawar, Khyber Pakhtunkhwa, Pakistan. Afr J Biotechnol 12(42). 10.5897/AJB2013.12972
- Kinyuru J, Kenji G, Njoroge M (2009) Process Development, Nutrition and Sensory Qualities of Wheat Buns Enriched with Edible Termites (Macrotermes subhylanus) from Lake Victoria Region, Kenya. Afr J Food Agric Nutr Dev 9(8). 10.4314/ajfand.v9i8.48411
- Kinyuru JN. Nutrient content and lipid characteristics of desert locust (Scistoscerca gregaria) swarm in Kenya. Int J Trop Insect Sci. 2020;41(3):1993–1999. doi: 10.1007/s42690-020-00308-3. [DOI] [Google Scholar]
- Klemm RDW, West KP, Palmer AC, Johnson Q, Randall P, Ranum P, Northrop-clewes C. Vitamin A fortification of wheat flour: Considerations and current recommendations. FoodNutr Bull. 2010;31(1suppl1):S47–S61. doi: 10.1177/15648265100311S105. [DOI] [PubMed] [Google Scholar]
- Kouřimská L, Adámková A. Nutritional and sensory quality of edible insects. NFS J. 2016;4:22–26. doi: 10.1016/j.nfs.2016.07.001. [DOI] [Google Scholar]
- Kumar P, Yadava RK, Gollen B, Kumar S, Verma RK, Yadav S. Nutritional Contents and Medicinal Properties of Wheat: A Review. Life Sci Med Res. 2011;22(1):1–10. [Google Scholar]
- Lautenschläger T, Neinhuis C, Kikongo E, Henle T, Förster A. Impact of different preparations on the nutritional value of the edible caterpillar Imbrasia epimethea from northern Angola. Eur Food Res Technol. 2017;243(5):769–778. doi: 10.1007/s00217-016-2791-0. [DOI] [Google Scholar]
- Manditsera FA, Luning PA, Fogliano V, Lakemond CMM. Effect of domestic cooking methods on protein digestibility and mineral bioaccessibility of wild harvested adult edible insects. Food Res Int. 2019;121:404–411. doi: 10.1016/j.foodres.2019.03.052. [DOI] [PubMed] [Google Scholar]
- Marono S, Piccolo G, Loponte R, Di Meo C, Attia YA, Nizza A, Bovera F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Italian J Anim Sci. 2015;14(3):3889. doi: 10.4081/ijas.2015.3889. [DOI] [Google Scholar]
- Mba ARF, Kansci G, Viau M, Rougerie R, Genot C(2019) Journal of Food Composition and Analysis Edible caterpillars of Imbrasia truncata and Imbrasia epimethea contain lipids and proteins of high potential for nutrition. Journal of Food Composition and Analysis, 79, 70–79. 10.1016/j.jfca.2019.03.002
- Mishyna M, Chen J, Benjamin O. Sensory attributes of edible insects and insect-based foods – Future outlooks for enhancing consumer appeal. Trends Food Sci Technol. 2020;95:141–148. doi: 10.1016/j.tifs.2019.11.016. [DOI] [Google Scholar]
- Momanyi DK, Owino WO, Makokha A, Evang E, Tsige H. Gaps in food security, food consumption and malnutrition in households residing along the baobab belt in Kenya. Nutr Food Sci. 2019 doi: 10.1108/NFS-11-2018-0304. [DOI] [Google Scholar]
- Moyo S, Masika PJ, Muchenje V. The potential of Imbrasia belina worm as a poultry and fish feed. A review. J Anim Feed Sci. 2019;28(3):209–219. doi: 10.22358/jafs/112156/2019. [DOI] [Google Scholar]
- Nantanga KKM, Amakali T. Diversification of mopane caterpillars (Gonimbrasia belina) edible forms for improved livelihoods and food security. J Arid Environ. 2020;177:104148. doi: 10.1016/j.jaridenv.2020.104148. [DOI] [Google Scholar]
- Omotoso OT. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae) J Zhejiang Univ Sci B. 2006;7(1):51–55. doi: 10.1631/jzus.2006.B0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimani A, Milanović V, Cardinali F, Roncolini A, Garofalo C, Clementi F, Pasquini M, Mozzon M, Foligni R, Raffaelli N, Zamporlini F, Aquilanti L. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innovative Food Science and Emerging Technologies. 2018;48:150–163. doi: 10.1016/j.ifset.2018.06.007. [DOI] [Google Scholar]
- Phan-thien K, Wright GC, Lee NA. Inductively coupled plasma-mass spectrometry (ICP-MS) and -optical emission spectroscopy (ICP-OES) for determination of essential minerals in closed acid digestates of peanuts (Arachis hypogaea L.) Food Chem. 2012;134(1):453–460. doi: 10.1016/j.foodchem.2012.02.095. [DOI] [Google Scholar]
- Purlis E. Browning development in bakery products - A review. J Food Eng. 2010;99(3):239–249. doi: 10.1016/j.jfoodeng.2010.03.008. [DOI] [Google Scholar]
- Purnomo EH, Sitanggang AB, Agustin DS, Hariyadi P. Formulation and Process Optimization of Muffin Produced from Composite Flour of Corn, Wheat and Sweet Potato [Formulasi dan Optimasi Proses Produksi Mufin dari Tepung Komposit Jagung, Gandum dan Ubi Jalar] Jurnal Teknologi dan Industri Pangan. 2012;23(2):165–165. doi: 10.6066/jtip.2012.23.2.165. [DOI] [Google Scholar]
- Sá AGA, Moreno YMF, Carciofi BAM. Food processing for the improvement of plant proteins digestibility. Crit Rev Food Sci Nutr. 2020;60(20):3367–3386. doi: 10.1080/10408398.2019.1688249. [DOI] [PubMed] [Google Scholar]
- Series SC(1992) Plant analysis reference procedures for the southern region of the United States
- Subramanian S, Tanga CM, Kusia E, Cerretti P, Khamis F, Copeland RS, Borgemeister C, Ekesi S(2017) Poster 10: Diversity of Edible Saturniids (Lepidoptera: Saturniidae) and Their Parasitoids in Kenya. In Symposium on Biological Control of Arthropods, (p 260)
- Van Huis A. Potential of Insects as Food and Feed in Assuring Food Security. Ann Rev Entomol. 2013;58(1):563–583. doi: 10.1146/annurev-ento-120811-153704. [DOI] [PubMed] [Google Scholar]
- Wang X, Gao W, Zhang J, Zhang H, Li J, He X, Ma H. Subunit, amino acid composition and in vitro digestibility of protein isolates from Chinese kabuli and desi chickpea (Cicer arietinum L.) cultivars. Food Res Int. 2010;43(2):567–572. doi: 10.1016/j.foodres.2009.07.018. [DOI] [Google Scholar]


