Abstract
Background
Achondroplasia is the commonest skeletal dysplasia of autosomal dominant inheritance caused by “gain of function” mutations in the fibroblast growth factor receptor 3 (FGFR3) gene. Foramen magnum compression due to accelerated ossification and spinal canal stenosis secondary to reduced interpedicular distance is a hallmark of achondroplasia, driven by G380R nucleotide pair substitution. In severe cases, limb weakness and neurogenic claudication will require surgical decompression. Rarely, a neurological condition may mimic the compressive spinal dysfunction and therefore, non-surgical causes must also be considered in cases of acute neurological deterioration in children with achondroplasia. Myasthenia gravis (MG) is an autoimmune condition resulting in fatigable muscle weakness. There are no reported cases of myasthenia gravis in achondroplasia in the literature.
Results
We report a child with achondroplasia scheduled for decompressive surgery for severe lumbar canal stenosis presenting with neurological claudication and knee weakness. While waiting for surgery during the COVID-19 pandemic, she developed generalized fatigability and severe weakness raising concerns of acute worsening of cord compression. Urgent investigations ruled out spinal cord compression but identified an unexpected concurrent myasthenia gravis with positive antibodies to acetylcholine receptors. The surgical intervention was postponed averting the potential risk of life-threatening anaesthetic complications. She was successfully managed with a combination of pyridostigmine, steroids, azathioprine, and plasma exchange.
Conclusion
We report the first case of myasthenia gravis in achondroplasia and review implications in the management.
Keywords: Achondroplasia, Dwarfism, Myasthenia gravis, Lumbar stenosis
Introduction
The term “achondroplasia” (without cartilage formation in Greek) was first used in 1878 to distinguish it from rickets. Achondroplasia is the commonest skeletal dysplasia resulting in marked short stature accounting for 90% of such cases [1]. The inheritance is autosomal dominant with a 100% penetrance. Over 80% of cases arise from a spontaneous mutation, with virtually all mutations in FGFR3 arising in the same nucleotide pair and resulting in the same glycine to arginine substitution (G380R) in the FGFR3 protein [2, 3].
This specific mutation is at least 500- or 1000-fold more frequent than expected [4, 5]. The reduction in interpedicular distance is driven by the G80R nucleotide pair substitution.
The characteristic phenotype includes rhizomelic shortening of the extremities and it is categorized as a physeal (growth plate) dysplasia with accelerated ossification of the cartilage. It is distinct from other skeletal dysplasias and advanced paternal age is a known risk factor [6]. Pathological features include small foramen magnum and cervicomedullary junction compression, a straight thoracic spine, a thoraco-lumbar kyphosis particularly due to vertebral wedging, and lumbosacral hyperlordosis. The neural arch malformation, decreasing interpedicular distance from L1 down to S1, shortened and thickened pedicles with narrow vertical laminae, and posteriorly protruding discs can all contribute to spinal canal stenosis. The achondroplastic spinal canal can be narrowed up to one-third or less of a normal spine [7–9].
The consequences of FGFR3 activation in achondroplasia include increased mortality and morbidity, particularly deaths from spinal cord compression and sleep apnoea in infants and small children [2]. The bony abnormalities associated with achondroplasia—i.e., foramen magnum and spinal canal stenosis—may have a significant effect on mortality at all ages but particularly in children. Efforts to minimize these complications are recommended.
Clinical monitoring in achondroplasia patient is needed to allow timely detection and intervention to prevent neurological sequel which can develop in a proportion of children.
Myasthenia gravis (MG) is an autoimmune condition secondary to antibodies to neuromuscular junction components leading to weakness. There are no reported cases of myasthenia gravis in achondroplasia. We hereby report the first case of myasthenia in achondroplasia to raise awareness about this concurrent combination and review the clinical assessment and how to deal with this rare presentation.
Exemplary case
An 11-year-old girl with known achondroplasia presented to our neurosurgical unit with a 6-month history of intermittent episodes of lower limb weakness described as “legs giving way.”
Her past medical history included achondroplasia and was under clinical and radiological follow-up for her spinal lumbar stenosis. Her past surgical history included lumbar L1–L2 augmentation laminoplasty (and undercutting of T12 and L3) for cauda equina syndrome and bladder dysfunction at age of 5 years old with full recovery postoperatively.
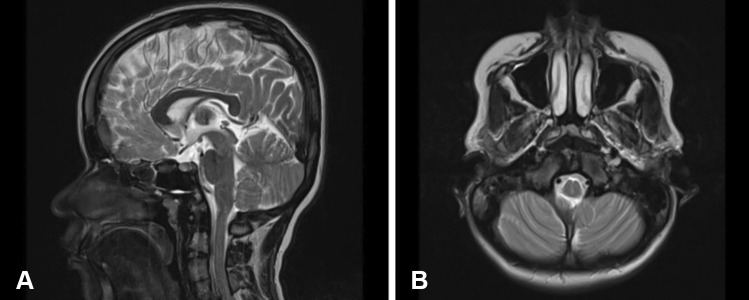
On examination, she was found to have normal power, tone and sensation bilaterally in the upper and lower limbs. She had no sphincter dysfunction. As part of investigations, she underwent MRI whole spine which demonstrated previously known multiple segmentation anomalies but worsening of the lumbar canal stenosis at the segment below the operated level. There was no overt spinal cord compression at the foramen magnum or other levels (Fig. 1). A decision to operate on the further stenosed levels was made following discussion of benefits, risks, and complications.
Fig. 1.
Sagittal (A) and axial (B) MRI brain T2 sequence showing no evidence of compression at the level of foramen magnum
Symptomatic children with MRI evidence of progression of spinal stenosis but without overt neurological deficits are often listed for surgery electively.
In this case, while waiting for surgery, over the course of a few weeks, she had a significant clinical deterioration with difficulty swallowing and chewing, slurred speech, and weakness of her upper and lower limbs. In light of rapid and new constellation of symptoms, urgent repeat brain and whole spine MRI scan were carried out. When no obvious compressive explanation for her clinical state could be identified, paediatric and neurology input was sought, and she was found to have evidence of fatigability of muscles involving both upper and lower limbs.
She underwent electromyography with evidence of decremental response on repetitive muscle stimulation. Repetitive nerve stimulation and single-fibre EMG studies were abnormal, showing increased jitter and conduction block consistent with a post-synaptic neuromuscular junction transmission disorder and in the clinical context most likely myasthenia gravis.
Autoantibody screening for myasthenia gravis demonstrated positive acetylcholine receptor antibodies (anti AChR) confirming diagnosis of myasthenia gravis. She also underwent an MRI of the thorax which excluded a thymoma.
She was treated initially with corticosteroids and pyridostigmine with a significant improvement in symptoms. On weaning steroids, her symptoms worsened significantly 3 months later, and she required therapeutic plasma exchange for six courses. She was re-started on high dose steroids as well as azathioprine as a long-term steroid sparing agent. She underwent elective thymomectomy with histology demonstrating an atrophic thymic tissue.
Her lumbar stenosis was managed expectantly and non-operatively. Follow-up at 16 months demonstrates stable myasthenic symptoms on azathioprine, pyridostigmine, and low dose maintenance steroids. Follow-up clinical surveillance for her lumbar canal stenosis reveals worsening bilateral sciatica and numbness on sitting for long periods of time and she is currently awaiting further lumbar surgery.
Clinical presentation
As exemplified by rare case above which represents first case of concurrent myasthenia and achondroplasia, spinal canal stenosis symptoms may worsen over time slowly. Surgical intervention is normally reserved for loss of limb movement or sensation, deteriorating mobility with neurological claudication, and critically, sphincteric disturbances. The goal of surgery is to augment the spinal canal to relieve spinal cord compression and pressure on the cauda equina nerves.
In achondroplasia, the genetically driven abnormally narrow spinal bony canal causes symptomatic spinal stenosis often at multiple sites sometimes simultaneously or progressively over time despite previous decompressions.
Myasthenia gravis, as a complicating factor in this case, is characterized by muscle weakness and activity dependent fatigability. Presentation can range from ptosis, extra-ocular, neck, facial, proximal limb, bulbar, and respiratory muscle weakness. MG patients can present in myasthenic crisis due to respiratory muscle involvement requiring urgent ventilatory support.
Droopy eyelids or double vision is the most common symptom at initial presentation of MG, with more than 75% of patients. Less commonly, MG may also present with prominent oropharyngeal symptoms early in the disease course, including dysarthria, dysphagia, and difficulty chewing and slurred speech in as many as 20% of cases [10].
Investigations and diagnosis
Approximately 85% of myasthenia gravis patients have autoantibodies against the acetylcholine receptor, whereas about 5% of patients have autoantibodies against the muscle-specific kinase (MUSK). Antibodies have also been identified to proteins located at the neuromuscular junction including to low-density lipoprotein receptor-related protein 4 (LRP4), agrin, collagen Q, and cortactin [11]. In the remaining about 10% of patients, no autoantibodies can be found despite typical neurophysiological and clinical features suggestive of myasthenia.
Electrophysiology in patients with MG typically demonstrates a decremental response (a decrement in the compound motor action potential of > 10% by the 4th or 5th stimulation) on repetitive nerve stimulation or increased jitter on single-fibre study [12].
CT or MRI thorax is of paramount importance as 15% of patients with autoimmune MG have thymomas. Thymectomy has also been shown to be effective in patients with generalized myasthenia without thymoma who are AChR antibody positive [13].
Management
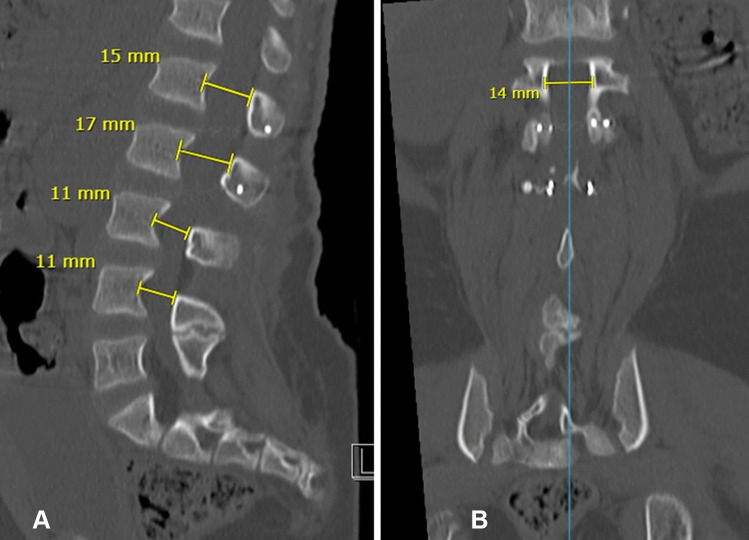
The standard technique of decompression for lumbar stenosis in achondroplasia is a laminectomy of compressed levels. In a large series of cases, the need for revisional spinal surgery was 22.5% and additional spinal fusion to the decompressive laminectomy was needed in as many as 72% of cases [14]. In our institution, we have been performing an augmentation laminoplasty of the compressed segments, as a means to augment the spinal canal while preserving the posterior column (Fig. 2). In our series, we have noted a significant reduction in the need to perform an additional spinal fusion procedure over a 15-year follow-up period [15, 16].
Fig. 2.
(A) Sagittal CT lumbar spine demonstrating augmentation laminoplasty at L1 and L2 with widened canal dimensions (in mm) compared to non-augmented levels at L3 and L4. (B) Coronal view CT lumbar spine corresponding to upper L1 level canal (14 mm) with screws visible inferiorly
First-line treatment in myasthenia gravis is symptomatic management with pyridostigmine. Early use of immunosuppression, where good control is not achieved with pyridostigmine, is important [17]. Oral prednisolone is used as first-line immunosuppression with appropriate prevention and monitoring of side effects. Second-line therapies including azathioprine and mycophenolate may be considered where there is no response to steroids, inability to wean to a reasonable minimum effective dose, or if side effects are intolerable [17].
Children experiencing a myasthenic crisis with respiratory failure or severe bulbar dysfunction require urgent access to the intensive care unit. Plasma exchange may be preferred over intravenous immunoglobulin in children with respiratory failure requiring ventilation due to more rapid onset of action [17].
Perioperative anaesthetic considerations
Surgery and anaesthesia in myasthenic gravis patients are associated with an increased risk of death and severe complications. The higher sensitivity to muscle relaxants even in periods of complete remission is the main source of the risk [18]. Prolonged ventilation in the ICU setting and, less commonly, a perioperative cholinergic crisis also add to the risk [19, 20].
When operating on a child with autoimmune MG, it is important for the surgical and anaesthetic teams to be aware of medications that may worsen myasthenia.
Patients with myasthenia may be very sensitive to non-depolarizing neuromuscular blocking agents (such as rocuronium/vecuronium/cisatracurium). They could also be resistant to depolarizing blocking agents like succinylcholine [21].
Antibiotics are another group of drugs with serious interactions. The aminoglycosides affect neuromuscular transmission by both inhibiting acetylcholine release pre-synaptically and blocking the acetylcholine receptor post-synaptically. They have been associated with aggravating pre-existing MG and postoperative respiratory distress [21]. The fluoroquinolone antibiotics (including ciprofloxacin, gemifloxacin, levofloxacin, moxifloxacin, and ofloxacin) have been known to cause neuromuscular blockade, and should also be avoided [22]. Prior to major surgery, a single 1 g/kg dose of intravenous immunoglobulin should be considered to prevent worsening of myasthenic symptoms perioperatively [23].
There are no known molecular or genetic links between achondroplasia and MG with no reports of concurrence of these two rare conditions.
Conclusion
We report the first case of myasthenia gravis in a child with achondroplasia.
Had surgery been expedited on consideration of worsening cord compression without appropriate investigations, it is likely the child could have suffered potentially life-threatening perioperative complications from MG. This case highlighted the need for careful clinical assessment and consideration of neurological symptoms and attention to non-surgical pathology that may mimic or add further complexity to the compressive presentation in achondroplasia.
Abbreviations
- FGFR
Fibroblast growth factor receptor
- MG
Myasthenia gravis
- MUSK
Muscle-specific kinase
- MRI
Magnetic resonance imaging
Author contribution
FA has written the article with input from AP, PD, and GS.
Availability of data and material
Not applicable.
Declarations
Ethics approval
Appropriate consent has been obtained.
Consent to participate
Appropriate consent has been obtained.
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocr Rev. 2000;21(1):23–39. doi: 10.1210/edrv.21.1.0387. [DOI] [PubMed] [Google Scholar]
- 2.Bellus GA, Hefferon TW, Ortiz de Luna RI, Hecht JT, Horton WA, Machado M, Kaitila I, McIntosh I, Francomano CA. Achondroplasia is defined by recurrent G380R mutations in FGFR3. Am J Hum Genet. 1995;56:368–373. [PMC free article] [PubMed] [Google Scholar]
- 3.Shiang R, Thompson IM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 4.Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90:175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnheim N, Calabrese P. Germline stem cell competition, mutation hot spots, genetics disorders, and older fathers. Annu Rev Genom Hum Genet. 2016;17:219–243. doi: 10.1146/annurev-genom-083115-022656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg LE, Rosenberg DD (2012) Birth defects in human genes and genomes. eBook ISBN: 9780123852137
- 7.Carlisle ES, Ting BL, Abdullah MA, Skolasky RL, Schkrohowsky JG, Yost MT, Rigamonti D, Ain MC (2011) Laminectomy in patients with achondroplasia. Spine (Phila. Pa. 1976). 36:886–892 [DOI] [PubMed]
- 8.Solanki G, Mallucci C, Cinalli G, Le Merrer M, Renier D, Pierre-Kahn A, Zerah M, Sainte-Rose C. Cranio-cervical compression under 2 years of age in achondroplasia—a 23 year review. 33rd Annual Meeting of the International Society for Pediatric Neurosurgery. Vancouver, Canada, Childs Nerv Syst. 2005;21:825–857. [Google Scholar]
- 9.King JA, Vachhrajani S, Drake JM, Rutka JT. Neurosurgical implications of achondroplasia. J Neurosurg Pediatr. 2009;4:297–306. doi: 10.3171/2009.3.PEDS08344. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Choundry MA, Mohammad Y et al (2004) Respiratory failure as a first presentation of myasthenia gravis. Med Sci Monit 10(12):CR684–9 [PubMed]
- 11.Lazaridis K, Tzartos SJ. Myasthenia gravis: autoantibody specificities and their role in MG management. Front Neurol. 2020;11:596981. doi: 10.3389/fneur.2020.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnis MF, Jayawant S. Juvenile myasthenia gravis: a paediatric perspective. Autoimmune Dis. 2011;2011:404101. doi: 10.4061/2011/404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrager JB. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375:2005–2007. doi: 10.1056/NEJMc1611704. [DOI] [PubMed] [Google Scholar]
- 14.Sciubba DM, Noggle JC, Marupudi NI, Bagley CA, Bookland MJ, Carson BS, Sr, Ain MC, Jallo GI. Spinal stenosis surgery in pediatric patients with achondroplasia. J Neurosurg. 2007;106(5 Suppl):372–378. doi: 10.3171/ped.2007.106.5.372. [DOI] [PubMed] [Google Scholar]
- 15.Slator N, Fayeye O, Solanki GA (2017) Spinal stenotic compression in young achondroplasia children — first report of treatment with augmentation laminoplasty. 45th Annual Meeting of International Society for Pediatric Neurosurgery, Denver, USA, 8–12 October. Childs Nerv Syst 33:1785–1853. 10.1007/s00381-017-3557-0
- 16.Afshari FT, Slator T, Ramakrishnan PR, Solanki GA (2022) Spinal canal stenosis in achondroplasia: the role of augmentation laminoplasty — a 15 year single institution experience. Childs Nerv Syst. accepted for publication [DOI] [PubMed]
- 17.O’Connell K, Ramdas S, Palace J. Management of juvenile myasthenia gravis. Front Neurol. 2020;24(11):743. doi: 10.3389/fneur.2020.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon FX. Anesthesia issues in the perioperative management of myasthenia gravis. Semin Neurol. 2004;24:83–94. doi: 10.1055/s-2004-829587. [DOI] [PubMed] [Google Scholar]
- 19.Haroun-Bizri S, Maalouli J, Deeb P, Baraka A. Anesthetic management for a patient with myasthenia gravis undergoing coronary artery bypass graft. Middle East J Anesthesiol. 2003;17:299–305. [PubMed] [Google Scholar]
- 20.Heliopoulos I, Patlakas G, Vadikolias K, et al. Maximal voluntary ventilation in myasthenia gravis. Muscle Nerve. 2003;27:715–719. doi: 10.1002/mus.10378. [DOI] [PubMed] [Google Scholar]
- 21.Barrons RW. Drug-induced neuromuscular blockade and myasthenia gravis. Pharmacotherapy. 1997;17(6):1220–1232. [PubMed] [Google Scholar]
- 22.Jones SC, Sorbello A, Boucher RM. Fluoroquinolone-associated myasthenia gravis exacerbation: evaluation of postmarketing reports from the US FDA adverse event reporting system and a literature review. Drug Saf. 2011;34(10):839–847. doi: 10.2165/11593110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Neuman A, Granlund B (2022) Anesthesia for patients with myasthenia gravis. In: Treasure island (FL): StatPearls Publishing [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.