Abstract
To evaluate soluble CD147 levels in COVID-19 and identify whether these are associated with hyperinflammation and disease severity. One-hundred and nine COVID-19 patients and 72 healthy blood donors were studied. Levels of CD147, matrix metalloproteases (MMP) and inflammatory markers were measured on hospital arrival, while the need for mechanical ventilation and the occurrence of death during hospitalization were recorded. CD147 levels were higher in COVID-19 (1.6, 1.0–2.3 vs 1.3, 1.0–1.6 ng/ml; P = 0.003) than controls. MMP-2 (9.2, 4.5–12.9 vs 4.2, 3.7–4.6 ng/ml; P < 0.001), MMP-3 (1.1, 0.9–1.3 vs 0.9, 0.7–1.0 ng/ml; P < 0.001) and MMP-9 (0.9, 0.5–1.2 vs 0.4, 0.2–0.6 ng/ml; P < 0.001) were also higher in COVID-19, while MMP-1 (0.6, 0–1.4 vs 0.6, 0.3–0.7 ng/ml; P = 0.711) was not different. Significant correlations were found between CD147 and MMP-2 (ρ = 0.34), MMP-3 (ρ = 0.21), interleukin 6 (ρ = 0.21), and the neutrophil/lymphocyte ratio (ρ = 0.26). Furthermore, CD147 levels were higher in patients who required mechanical ventilation (1.8, 1.4–2.4 vs 1.2, 0.8–1.9 ng/ml; P < 0.001) and in those who ultimately died (1.9, 1.4–2.7 vs 1.4, 0.9–1.9 ng/ml; P = 0.009). CD147 is elevated in COVID-19 and appears to contribute to hyperinflammation and disease severity.
Keywords: CD147, COVID-19, Matrix Metalloproteases, Inflammation, Mechanical Ventilation
Introduction
Almost two years of the first recorded cases of atypical pneumonia in Wuhan, China, the disease is known as Coronavirus 19 (COVID-19) has affected more than 400 million people and killed nearly six million worldwide (World Health Organization 2019; Coronavirus Resource Center (2022). Aside from glucocorticoids, the first really effective drugs to treat COVID-19 are only recently emerging, many of which were originally designed and developed for other infections (Khoo et al. 2021; Pfizer 2021). This may in part be due to the lack of identification of optimal therapeutic targets. Therefore, research aimed at elucidating the mechanisms of SARS-CoV-2 infection is required, to provide promising targets for the development of specific drugs against COVID-19.
The major determinant of cellular tropism shown by SARS-CoV-2 is the spike protein, which directs infection by binding to membrane receptors in host cells. The main receptor for cellular infection is the angiotensin-converting enzyme 2 (ACE2) (Lan et al. 2020). ACE2 is an enzyme (carboxypeptidase) located mainly in the cell membrane; it is homologous to ACE1 but acts inversely on the renin–angiotensin–aldosterone axis as it is an angiotensin II deactivator, which is an active peptide that causes vasoconstriction, promotes hypertrophy, fibrosis, and inflammation (Gheblawi et al. 2020). In addition to ACE2, some molecules that can function as cofactors in ACE2-mediated SARS-CoV-2 infection have recently been identified, such as neuropilin-1 and transmembrane serine protease-2 (Cantuti-Castelvetri et al. 2020; He et al. 2021). In parallel, it has been shown that CD147, a glycoprotein of the immunoglobulin superfamily also known as basigin or extracellular matrix metalloprotease inducer (EMMPRIN), plays an important functional role in facilitating infection by SARS-CoV-2 (Wang et al. 2020). Indeed, CD147 mediates the entry of SARS-CoV-2 into host cells by endocytosis, and its blockage by anti-CD147 antibodies inhibits virion amplification in a dose-dependent manner, at least in cellular models (Wang et al. 2020).
Apart from its function as a putative receptor used by SARS-CoV-2 for its entry into host cells, CD147 is the main tissue inducer of matrix metalloproteases (MMPs). These MMPs are zinc-dependent endopeptidases responsible for cleaving the immediate components of the extracellular matrix. The degradation of different components of the extracellular matrix mediated by MMPs is an important component in tissue damage associated with COVID-19. Altered plasma concentrations of MMPs have been found in COVID-19 patients, and increased levels of MMP-2 and MMP-9 appear to be associated with an increased risk of in-hospital mortality (D Avila-Mesquita et al. 2021).
Thus, the objective of this proof-of-concept study was to measure the soluble levels of CD147 in the serum of patients with COVID-19 and to identify whether they are associated with markers of tissue damage and inflammation. Furthermore, the possible association between CD147 levels and disease severity was also evaluated.
Materials and Methods
Study Design
The present study was carried out at the Instituto Nacional de Cardiología Ignacio Chávez, Mexico City, a third-level academic center dedicated to the study and management of cardiovascular diseases and related conditions. Our Institute received only seriously ill patients for hospital management, while those with milder forms of the disease were referred to less specialized medical centers or sent home.
In this study, we included patients older than 18 years who were admitted to the respiratory triage with a diagnosis of COVID-19 and who met the WHO case definition of SARS-CoV-2 infection. Upon admission, disease severity was classified as moderate or severe as follows: moderate illness was defined based on clinical signs of pneumonia such as fever, cough, dyspnea, or tachypnea; meanwhile, severe disease was defined as the presence of pneumonia and at least one of the following: respiratory rate > 30 breaths/minute, severe respiratory distress, or oxygen saturation < 90% on room air. In addition, a nasopharyngeal swab was performed, and the SARS-CoV-2 positivity was evaluated in an RT-PCR test; no serial tests were performed to assess viral clearance. All patients included had a negative result in the influenza rapid test.
Clinical and laboratory data were collected from the electronic records by two independent investigators, according to a previously designed proforma file. Each database was reviewed by a third investigator and discrepancies were solved by reviewing the discordant data directly from the medical record. It should be noted that treatments instituted, laboratory and imaging studies requested, admission to the Intensive Care Unit, and the decision to provide invasive ventilatory support were made at the absolute discretion of the treating physicians. Similarly, the decision to discharge each patient was made solely by the attending physician, based on the clinical status. Consequently, the present study did not influence medical decision-making or clinical course.
Upon arrival at respiratory triage, patients authorized the use of their clinical data for research purposes. Approval was granted by the Bioethics Committee of the Instituto Nacional de Cardiología Ignacio Chávez (protocol number 20–1186). All procedures were carried out in accordance with the ethical standards of the 2013 Declaration of Helsinki, its addenda and local regulations.
Laboratory Procedures
Blood samples were obtained at hospital admission and immediately processed for the measurement of inflammatory (C-reactive protein (CRP), interleukin (IL)-6) and thrombotic (D-dimer, antiphospholipid antibodies) markers, as part of the routine diagnostic and prognostic approach established in our Institute (Amezcua-Guerra et al. 2021a, 2021b). A remnant of these samples was used to carry out the measurements of CD147, MMP-1, MMP-2, MMP-3 and MMP-9 by enzyme-linked immunosorbent assays with commercially available reagents (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s instructions. As a reference, sera from healthy blood donors obtained during 2019 (pre-pandemic) and stored at –70 °Celsius were used; control individuals were matched for age and sex. Although they may have suffered some type of degradation due to prolonged storage (up to two years), sera from healthy individuals obtained before the pandemic were selected as a reference since this guaranteed us that no control individual had suffered from a mild or asymptomatic form of SARS-CoV-2 infection.
Statistics
Data distribution was evaluated using the D'Agostino-Pearson test. Frequencies and percentages were used to describe categorical variables, while medians with interquartile range were used to describe numerical variables. Differences between medians were evaluated using the Mann–Whitney U test. Correlations were evaluated using Spearman’s rho (ρ) coefficient with 95% confidence intervals. Finally, the ability of CD147 to predict the need for invasive mechanical ventilation and the occurrence of death was evaluated using the area under the receiver operating characteristic curve (AUC) with 95% confidence intervals.
All statistical analyses were performed under the two-tailed principle and a P < 0.05 value was established for significance. The GraphPad Prism v.9 (GraphPad Software, La Jolla, CA, USA) package was used for the calculations.
Results
During the study period, serum samples were collected from 109 patients with COVID-19 (67% male) aged 54 years (46–65 years); in addition, serum from 72 healthy blood donors (56% male; median age: 52, 42–60 years) without any comorbidity was analyzed as a reference control. The main clinical and demographic characteristics are presented in Table 1. It is worth noting the high frequency of comorbidities, especially diabetes (39%), hypertension (46%) and chronic renal failure (11%), for a median Charlson comorbidity index of 2 (1–4). There was a 7 day delay from the onset of symptoms to hospital admission. At hospital arrival, polypnea and tachycardia were found in most of the patients, with an oxygen saturation in ambient air of 82% (70–88%). A total of 88 patients (80%) were classified as having severe COVID-19. On admission, laboratory studies showed leukocytosis with neutrophilia and lymphopenia, for a neutrophil/lymphocyte ratio of 10 (6–17). In addition, hypoalbuminemia and an intense inflammatory and cellular injury response characterized by elevated CRP, IL-6, D-dimer, and ferritin were found. Laboratory findings are summarized in Table 1.
Table 1.
Clinical and laboratory data at hospital admission
| COVID-19 Patients (n = 109) | |
|---|---|
| Age, years | 54 (46–65) |
| Male, n | 73 |
| Body mass index, kg/m2 | 27.7 (25.7–30.8) |
| Current smoking, n | 24 |
| Comorbidities, n | |
| Diabetes | 43 |
| Hypertension | 51 |
| Dyslipidemia | 15 |
| Coronary artery disease | 8 |
| Stroke | 3 |
| Chronic heart failure | 7 |
| Chronic kidney disease | 12 |
| Organ transplant | 5 |
| Cancer | 1 |
| Clinical data, at admission | |
| Temperature, °C | 36.9 (36.5–37.5) |
| Respiratory rate, breaths/min | 24 (20–30) |
| Cardiac rate, beats/min | 97 (83–110) |
| Systolic artery pressure, mmHg | 126 (110–137) |
| Diastolic artery pressure, mmHg | 76 (70–84) |
| Oxygen saturation (%), at room air | 82 (70–88) |
| Laboratory data, at admission | |
| Leucocytes × 103 per mm3 | 10.1 (7.2–12.4) |
| Neutrophils × 103 per mm3 | 8.5 (6–11) |
| Lymphocytes × 103 per mm3 | 0.8 (0.6–1.1) |
| Platelets × 103 per mm3 | 222 (167–288) |
| Hemoglobin, g/dl | 14.7 (13.1–16.1) |
| Albumin, g/dl | 3.5 (3.1–3.8) |
| Creatinine, mg/dl | 1.04 (0.85–1.54) |
| Troponin I, ng/ml | 18.4 (8.3–89.5) |
| Creatine kinase, U/l | 111 (53–199) |
| D-dimer, ng/ml | 439 (247–820) |
| Fibrinogen, mg/dl | 5.4 (4.5–6.2) |
| C-reactive protein, mg/l | 157 (72–264) |
| Ferritin, μg/l | 654 (326–1103) |
| Interleukin-6, pg/ml | 16.2 (4.5–85.2) |
Data are presented as median (interquartile range) unless otherwise specified
According to the extent of respiratory failure, the patients sequentially received supplemental oxygen through a nasal cannula, high-flow oxygen therapy, non-invasive ventilation, and finally endotracheal intubation with mechanical ventilatory support. The prone position was part of the standard medical treatment. In addition, a total of 95 patients (87%) received some type of heparin (in particular, low molecular weight heparin), 33 (30%) received dexamethasone and 26 (23%) received biological therapies with IL-6 inhibitors (tocilizumab) or the Jak-STAT signaling pathway inhibitors (baricitinib or ruxolitinib). No patient received convalescent plasma. At the time of the study, there were no SARS-CoV-2 vaccines or remdesivir in pre-clinical/clinical use and typing of viral variants was not yet available. During hospital follow-up, 25 patients (22%) had at least one thromboembolic event, while 61 (55%) required invasive mechanical ventilation and 38 patients (34%) eventually died. The hospital stay was 14 days (11–25 days). Table 2 summarizes the hospital management and the main clinical outcomes.
Table 2.
In-hospital management and major outcomes
| COVID-19 Patients (n = 109) | |
|---|---|
| Main drug therapies, n | |
| Glucocorticoids | 33 |
| Hydroxychloroquine | 8 |
| Azithromycin | 17 |
| Lopinavir/ritonavir | 66 |
| Interleukin-6 or Jak-STAT inhibitors | 26 |
| Heparins | 95 |
| Main clinical outcomes, n | |
| Vascular thrombosis (any) | 25 |
| Pulmonary thromboembolism | 3 |
| Stroke | 1 |
| Myocardial infarction | 4 |
| Severe bleeding | 14 |
| Invasive mechanical ventilation | 61 |
| Death | 38 |
| Days of hospital stay, median (interquartile range) | 14 (11–25) |
A total of 21 patients (19%) had moderate COVID-19, while the remaining 88 patients had severe disease on hospital arrival. No differences were found in serum CD147 levels between patients with moderate and severe COVID-19 (1.9, 1.3–2.3 ng/ml vs 1.5, 0.9–2.2 ng/ml; P = 0.190). Likewise, no significant differences were found in the concentration of MMPs according to the disease severity (data not shown). Regarding soluble levels of CD147 in serum, we found a significantly higher concentration in patients with COVID-19 (1.6, 1.0–2.3 ng/ml) compared to that observed in healthy controls (1.3, 1.0–1.6 ng/ml; P = 0.003). Similarly, the levels of MMP-2 (9.2, 4.5–12.9 ng/ml vs 4.2, 3.7–4.6 ng/ml; P < 0.001), MMP-3 (1.1, 0.9–1.3 ng/ml vs 0.9, 0.7–1.0 ng/ml; P < 0.001) and MMP-9 (0.9, 0.5–1.2 ng/ml vs 0.4, 0.2–0.6 ng/ml; P < 0.001) were also higher in COVID-19 patients. In contrast, MMP-1 levels (0.6, 0–1.4 ng/ml vs 0.6, 0.3–0.7 ng/ml; P = 0.711) were not different between groups. These data are presented in Fig. 1.
Fig. 1.
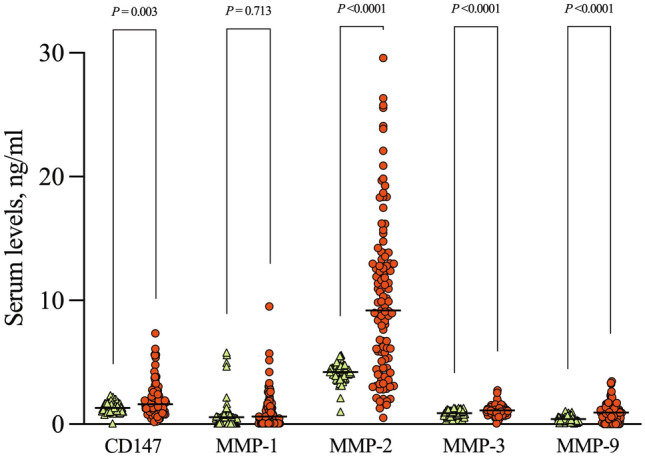
Soluble CD147 and matrix metalloproteinase (MMP) concentrations. Significantly higher levels of CD147, MMP-2, MMP-3, and MMP-9 were found in sera from COVID-19 patients (red circles) than in healthy controls (green triangles). MMP-1 levels were not different between groups. Horizontal lines indicate median values
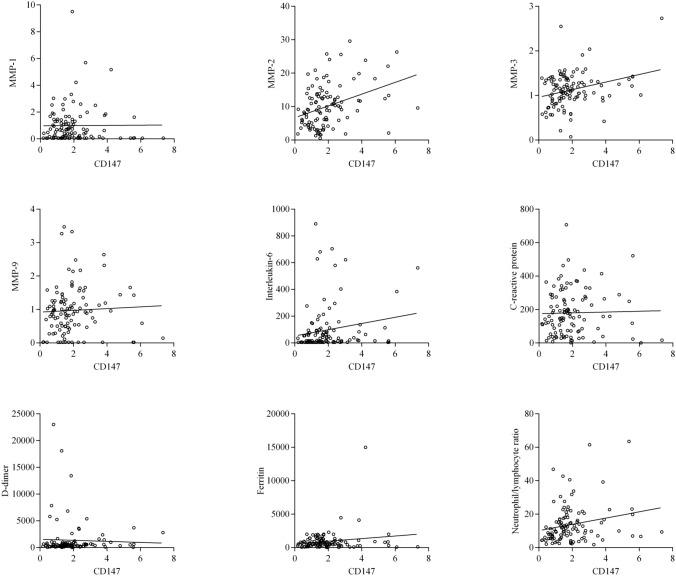
Once the abnormality in serum levels of CD147 in COVID-19 was demonstrated, we evaluated the potential associations of CD147 with MMPs and markers of inflammation and tissue damage in patients with COVID-19. As can be seen in Fig. 2, CD147 levels showed a direct correlation with MMP-2 levels, with a ρ coefficient of 0.34 (0.16–0.50; P = 0.0003). Similarly, a direct correlation was found with the levels of MMP-3, with a ρ coefficient of 0.21 (0.02–0.39; P = 0.028). In contrast, we did not observe an association with the levels of MMP-1 (ρ = –0.002, –0.19 to 0.19; P = 0.979) or MMP-9 (ρ = –0.14, –0.05 to 0.33; P = 0.130). Additionally, CD147 levels correlated directly with IL-6 (ρ = 0.21, 0.02–0.39; P = 0.026), as well as with the neutrophil/lymphocyte ratio (ρ = 0.26, 0.07–0.43; P = 0.006). However, we did not find an association with levels of CRP (ρ = 0.05, –0.14 to 0.24; P = 0.603), D-dimer (ρ = 0.15, –0.04 to 0.33; P = 0.117) or ferritin (ρ = 0.03, –0.16 to 0.22; P = 0.736).
Fig. 2.
Association between CD147 levels and different inflammatory and tissue damage analytes in COVID-19 patients. Significant correlations were found between CD147 and MMP-2 (rho = 0.342), MMP-3 (rho = 0.210), IL-6 (rho = 0.212) and the neutrophil/lymphocyte ratio (rho = 0.258). No other significant associations were found (see main text)
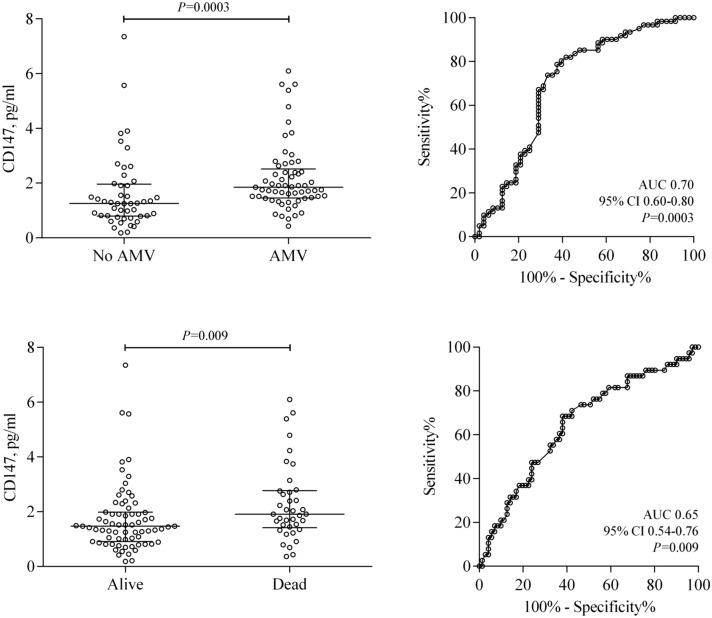
In a final group of analyzes, we evaluated whether serum CD147 levels, measured at the time of hospital admission, were different between COVID-19 patients who required invasive ventilatory support and those who eventually died. As seen in Fig. 3, CD147 levels were higher in COVID-19 patients who required assisted mechanical ventilation (1.8, 1.4–2.4 ng/ml vs 1.2, 0.8–1.9 ng/ml; P < 0.001) than in their counterparts that did not require it. The discriminative ability of CD147 levels to identify patients at risk for invasive ventilatory assistance was significant, with an AUC of 0.70 (0.60–0.80; P = 0.0003). Similarly, CD147 concentration was higher in patients who died (1.9, 1.4–2.7 ng/ml vs 1.4, 0.9 to 1.9 ng/ml; P = 0.009) compared to survivors, for an AUC of 0.65 (0.54–0.76; P = 0.009).
Fig. 3.
Soluble CD147 in COVID-19 patients according to the occurrence of major adverse outcomes. Serum CD147 levels were significantly higher both in patients who required assisted mechanical ventilation (AVM) and in those who eventually died; horizontal lines indicate median values with an interquartile range. Furthermore, CD147 levels at hospital admission effectively discriminated against patients who developed adverse outcomes during hospitalization (see areas under the ROC curve [AUC])
Discussion
In this study, the potential association of soluble CD147 with different markers of extracellular matrix turnover, tissue damage, and inflammation was evaluated in COVID-19. Our results show that serum CD147 levels are elevated in COVID-19 and are directly associated with levels of MMPs and prototypical markers of inflammation and tissue injury. Interestingly, CD147 serum levels, measured at the time of arrival at respiratory triage, predict both the requirement for invasive ventilation and the occurrence of death during hospitalization, establishing this molecule as a marker of disease severity, as well as a potential therapeutic target in COVID-19 patients.
Matrix metalloproteinases belong to a family of endopeptidases that play an important role in homeostatic processes of tissue remodeling, by facilitating the degradation of the interstitium. Furthermore, MMPs are implicated in various pathological conditions, including lung diseases, cardiovascular disease, obesity, and different neoplasms (Kapoor et al. 2016). However, for these MMPs to acquire enzymatic activity they need to be activated, which is achieved through the CD147 molecule, a glycoprotein of the immunoglobulin superfamily, also known as basigin or EMMPRIN. In addition to its important role as an inducer of MMPs activity, CD147 has been identified as a putative receptor or co-receptor of the ACE2 molecule for SARS-CoV-2 cellular infection (Wang et al. 2020). The ACE2 has been widely recognized as the main receptor in infection by different coronaviruses, and the replication of SARS-CoV-1 in Vero E6 cells is specifically inhibited by an anti-ACE2 antibody (Li et al. 2003). In parallel, an interaction between the host cell CD147 receptor and the SARS-CoV-2 spike protein has been demonstrated, while the loss of CD147 or the blockade of CD147 inhibits the amplification of SARS-CoV-2 in Vero E6 and BEAS-2B cell lines. Furthermore, CD147 is a functional alternative receptor for entry of SARS-CoV-2 in ACE2-deficient cellular models (Wang et al. 2020).
The above data, obtained in cell and animal models, clearly suggested that CD147 has a relevant pathogenic role in SARS-CoV-2 infection and the immune-mediated tissue damage typically seen in COVID-19. In our study, we found that COVID-19 patients who require hospitalization have higher levels of soluble CD147 compared to healthy individuals. Although this finding is interesting, it is further enhanced by observing that CD147 levels are closely associated with the serum concentration of MMP-2, MMP-3 and MMP-9, as well as early markers of inflammation including IL-6 and the neutrophil/lymphocyte ratio. Regarding IL-6, it is important to highlight that this cytokine is a leader in guiding the cytokine release syndrome that characterizes the hyperinflammatory phase of COVID-19; accordingly, therapeutic blocking of IL-6 signaling with tocilizumab or sarilumab is one of the few strategies that have been shown to decrease mortality in COVID-19 (Amezcua-Guerra 2020). Similarly, the neutrophil/lymphocyte ratio is an acute phase index that effectively predicts the occurrence of thrombosis, requirement for mechanical ventilation, and death in COVID-19 (González-Flores et al. 2021). In our study, we also observed that patients with greater organ damage and who eventually required invasive ventilatory support or who died were also those with the highest concentrations of soluble CD147 at the time of hospital admission, which suggests that early measurement of serum CD147 levels can be a marker of severity and prognosis in these patients. It is important to emphasize that the identification of useful and easily accessible markers in a real-world setting is still a pending task (Amezcua-Guerra et al. 2021a; González-Flores et al. 2021).
In further support for an important role of CD147 in COVID-19, a phase 1/2 study recently showed that the administration of meplazumab, a humanized anti-CD147 monoclonal antibody, is safe and well tolerated in healthy volunteers while suggesting that meplazumab could accelerate the recovery of COVID-19 patients with pneumonia (Bian et al. 2021). Finally, in consideration of viral variants of concern or medical interest, the administration of meplazumab specifically and effectively inhibits SARS-CoV-2 infection and the associated cytokine release syndrome in a murine model transgenic for human CD147; it is noteworthy that efficacy against SARS-CoV-2 infection included delta, alpha, beta, and gamma variants (Geng et al. 2021). Now, CD147 is already being proposed as a therapeutic target to limit the cellular entry of SARS-CoV-2 (Behl et al. 2022).
COVID-19 has been a fantastic challenge for today’s medicine. One of the pending tasks is the development of effective therapies against SARS-CoV-2 infection, partly due to the limited information still available on the biology of the virus-host cells interaction. Our results suggest that CD147 plays an important role in COVID-19, not only as a putative receptor for SARS-CoV-2 cellular infection but also that high levels of its soluble form are associated with higher levels of MMPs, favoring systemic hyperinflammation and contributing to severe organ damage, demonstrated by the requirement for invasive ventilation and the occurrence of death in patients with COVID-19.
Acknowledgements
None to acknowledge.
Author Contributions
Conceived and designed the work that led to the submission: RS, LMAG. Acquired data and played an important role in interpreting the results: RS, JGF, CGA, YJV, AH-D, RMV, SCA, FSM, EBG, MC-S, MABV, MBP, JLSG, CTA, JS, HGP, LMAG. Formal analysis: LMAG. Drafted the manuscript: LMAG. Review and editing: RS, JGF, CGA, YJV, AHD, RMV, SCA, FSM, EBG, MCS, MABV, MBP, JLSG, CTA, JS, HGP, LMAG. All the authors revised and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Funding for publication costs for this article was supported by the Instituto Nacional de Cardiología Ignacio Chávez.
Declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amezcua-Guerra LM. Brief Annotations on Cytokine Release Syndrome and Interleukin-6 Therapeutic Blockage in SARS-CoV-2/COVID-19. Arch Cardiol Mex. 2020;90(Suppl):84–87. doi: 10.24875/ACM.M20000067. [DOI] [PubMed] [Google Scholar]
- Amezcua-Guerra LM, Audelo K, Guzmán J, et al. A Simple and Readily Available Inflammation-Based Risk Scoring System on Admission Predicts the need for Mechanical Ventilation in Patients with COVID-19. Inflamm Res. 2021;70:731–742. doi: 10.1007/s00011-021-01466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua-Guerra LM, Rojas-Velasco G, Brianza-Padilla M, et al. Presence of Antiphospholipid Antibodies in COVID-19: a Case Series Study. Ann Rheum Dis. 2021;80:e73. doi: 10.1136/annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- D Avila-Mesquita C, Couto AES, Campos LCB, et al. MMP-2 and MMP-9 Levels in Plasma are Altered and Associated with Mortality in COVID-19 Patients. Biomed Pharmacother. 2021;142:112067. doi: 10.1016/j.biopha.2021.112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl T, Kaur I, Aleya,, et al. CD147-Spike Protein Interaction in COVID-19: Get the Ball Rolling with a Novel Receptor and Therapeutic Target. Sci Total Environ. 2022;808:152072. doi: 10.1016/j.scitotenv.2021.152072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H, Zheng ZH, Wei D, et al. Safety and Efficacy of Meplazumab in Healthy Volunteers and COVID-19 Patients: a Randomized Phase 1 and an Exploratory Phase 2 Trial. Signal Transduct Target Ther. 2021;6:194. doi: 10.1038/s41392-021-00603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Chen L, Yuan Y, et al. CD147 Antibody Specifically and Effectively Inhibits Infection and Cytokine Storm of SARS-CoV-2 and its Variants Delta, Alpha, Beta, and Gamma. Signal Transduct Target Ther. 2021;6:347. doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Flores J, García-Ávila C, Springall R, et al. Usefulness of Easy-to-Use Risk Scoring Systems Rated in the Emergency Department to Predict Major Adverse Outcomes in Hospitalized COVID-19 Patients. J Clin Med. 2021;10:3657. doi: 10.3390/jcm10163657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Hua X, Sun S, et al. Integrated Bioinformatic Analysis of SARS-CoV-2 Infection Related Genes ACE2, BSG and TMPRSS2 in Aerodigestive Cancers. J Inflamm Res. 2021;14:791–802. doi: 10.2147/JIR.S300127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Resource Center (2022) Available at: https://coronavirus.jhu.edu. Accessed on 10 Feb 2022
- Kapoor C, Vaidya S, Wadhwan V, et al. Seesaw of Matrix Metalloproteinases (MMPs) J Cancer Res Ther. 2016;12:28–35. doi: 10.4103/0973-1482.157337. [DOI] [PubMed] [Google Scholar]
- Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal Dose and Safety of Molnupiravir in Patients with Early SARS-CoV-2: A Phase I, Open-Label, Dose-Escalating, Randomized Controlled Study. J Antimicrob Chemother. 2021;76:3286–3295. doi: 10.1093/jac/dkab318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, et al. Angiotensin-Converting Enzyme 2 is a Functional Receptor for the SARS Coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer (2021) Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate
- Wang K, Chen W, Zhang Z, et al. CD147-Spike Protein is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduct Target Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2019) WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int. Accessed on 10 Feb 2022