Abstract
Although HIV pre-exposure prophylaxis (PrEP) is free in Thailand, many transgender women discontinue taking it after initiation. We determined the loss to follow-up (LTFU) rate of transgender women who initiated PrEP at the Mplus Foundation, Chiang Mai, Thailand, and identified associated risk factors using Cox proportional hazard models. Of 235 participants who initiated PrEP, 59 (55%) out of 108 remaining participants had reactive syphilis. The LTFU rate at 6 months was 38% (95% confidence interval [CI]: 29–48%). Multivariable analysis indicates that LTFU is independently associated with age ≥ 26 years old (adjusted hazard ratio [aHR] = 2.09; 95% CI: 1.06–4.14) and reactive syphilis (aHR = 1.98; 95% CI:1.01–3.88). Delayed appointment scheduling by the PrEP providers and the syphilis clinic was associated with transgender women having reactive syphilis, and the lockdown policy during the COVID-19 pandemic might have influenced them to discontinue PrEP and their subsequent LTFU.
Keywords: Transgender women, PrEP, Loss to follow-up, Preventing HIV, Thailand
Introduction
Antiretroviral pre-exposure prophylaxis (PrEP) is a promising addition for HIV prevention, particularly for groups who are at substantial risk of contracting HIV such as men who have sex with men (MSM) and transgender people [1]. The World Health Organization (WHO), European AIDS Clinical Society, and the Thailand National Guidelines on Pre-Exposure Prophylaxis recommend offering either daily or on-demand PrEP composed of emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) [2–4]. In addition, the results from several clinical trials have shown that daily and on-demand dosing PrEP regimens are effective at preventing HIV infection among populations at high risk of HIV (MSM, transgender women (TGWs), and heterosexual discordant couples) [1, 5–11]. For the important key issue in ensuring PrEP effectiveness, the findings from an adherence sub-study conducted by the Partners PrEP Study in Africa found that people with high PrEP adherence (> 80%) did not contract HIV (PrEP efficacy: 100%; 95% confidence interval (CI): 83.7–100%) [12].
In Thailand, the PrEP project for HIV prevention started as a pilot study on MSM at the end of 2014 by the Thai Red Cross AIDS Research Center (TRCARC) in Bangkok [13]; this was a community-based test-and-treat study with the aim of improving HIV testing, care, and treatment. Even with PrEP projects being conducted at more sites in Thailand since then, approximately 50% of new infections from 2015 to 2019 were found in the MSM and transgender groups [14]. The authors of a progress report for Thailand to end AIDS reported that Chiang Mai City, which is the capital of Northern Thailand, has the highest HIV/AIDS epidemic burden for MSM, followed by Bangkok [15]. In addition, the outcomes of a cohort study on MSMs and TGWs in Thailand by Seekaew et al. [16] showed that participants living in Chiang Mai had higher discordance between the self-perceived and actual risk of contracting HIV than those from other areas. The authors suggested that the risk discordance for both MSMs and TGWs could result from inconsistent condom use when engaging in trusted, intimate, and/or stable relationships with their male partners. However, alternative HIV protection methods such as taking PrEP and/or having sex with a partner with an undetectable viral load were not accounted for due to these methods not being widespread and well known at the time of their study (2015 to 2016). In another previous study of Thai MSMs and TGWs [17], the authors also suggested that PrEP uptake was associated with a higher HIV risk perception. To reduce risk discordance and the actual risk of HIV transmission, PrEP uptake as an alternative HIV prevention has been promoted and is offered to the key population comprising MSMs, transgender people, and sex workers at high risk of contracting HIV in Thailand by the MPlus Foundation. This is a medical technology clinic that provides a comprehensive range of HIV services to the key population, including free testing for HIV and other sexually transmitted infections (STIs), community outreach, and sexual health counseling in Chiang Mai [18]. Moreover, PrEP sponsored by the “Princess PrEP” program of the TRCARC and the Nakornping PrEP project at Nakornping hospital in Chiang Mai is provided free of charge to the key population.
Based on the results of previous studies, approximately 4–46% of the key population comprising MSMs, transgender people, and people who inject drugs discontinue oral PrEP after 3–6 months [19–23]. The reasons for MSMs and transgender people discontinuing PrEP and LTFU were perceiving themselves not to be at risk of HIV infection, the side effects of PrEP, cost, insurance support, and sexual behavior [24–26]. As a result, there has been a relatively large number of HIV-infected cases in these groups after PrEP discontinuation [24, 27]. Moreover, several factors associated with PrEP discontinuation or LTFU have been reported, including testing for or diagnosing STIs at PrEP initiation, the different types of PrEP use, age at PrEP initiation, duration of PrEP use, and having a mental health disorder [20, 26, 28–30].
Although the prevalence of PrEP uptake in Thailand is increasing, data from the MPlus Foundation indicates that the loss to follow-up (LTFU) after PrEP initiation among TGWs has increased in recent years (36% in 2017 compared to 58% in 2018) [18]. A previous report from the Princess PrEP program that compared the retention of PrEP between MSMs and TGWs also found that PrEP use retention was significantly lower in the latter compared to the former at each visit (1, 3, 6, 9, and 12 months) [31]. Since the different retention rates might have resulted from the different characteristics and behavior factors of these populations, the current study was first focused on investigating LTFU after PrEP initiation in the TGW population.
In previous studies on the PrEP retention rate in Thailand [31–33], associated factors with the retention rate were only considered, compared, or investigated at specific time points (e.g., 3 or 6 months after initiation) rather than during the whole follow-up period. In contrast, the aim of the current study is to investigate the cumulative incidence of LTFU as the discontinuation of PrEP after initiation and also identify associated risk factors among TGWs who had received PrEP in Chiang Mai, Thailand. Our results will be used to develop a strategy to increase PrEP initiation and PrEP retention rates for the populations at high risk for HIV and to recommend prevention or reduction measures for the risk of contracting HIV infection.
Methods
Study design and population
This was a longitudinal study with follow-up at 1, 2, 3, and 6 months after PrEP initiation of TGWs who were ≥ 18 years old and had initiated PrEP between January 2016 and December 2020 at the Mplus Foundation run by expert providers in Chiang Mai, Thailand. Exclusion criteria were a history of renal dysfunction, HIV, or a history of chronic hepatitis B virus infection. The study information was revalidated before being compiled and used for statistical analysis.
Data collection
The participants who had initiated PrEP and attended 1-, 2-, 3-, and 6-month follow-up visits after PrEP initiation were enrolled in the study. Before having PrEP initiation, each individual underwent laboratory screening, including serum creatinine, hepatitis B surface antigen, syphilis infection by using the SD BIOLINE Syphilis 3.0 rapid test kit, and HIV testing by using the Determine HIV-1/2 rapid test kit, both of which provide results within 20 minutes. Individuals were assessed for a history of hepatitis B virus infection and/or renal disease. Individuals who had tested positive were double-checked with other rapid test kits (Colloidal Gold Device and SD Bioline HIV 1/2 3.0). Individuals who tested HIV positive underwent counseling by MPlus advisers and were transferred to government hospitals.
For those who had completed 6 months of follow-up, the counselors contacted via phone calls to inquire about adverse effects after taking PrEP and to address their concerns about the long-term adverse effects of taking PrEP. Similarly, participants who were LTFU were contacted by the counselors to inquire about any adverse effects after taking PrEP, to address their concerns about the long-term adverse effects of taking PrEP, and to determine the reason for their LTFU.
The variables assessed for association with the risk of LTFU included certain demographics (age, occupation, and relationship status), behavioral characteristics at the baseline (a history of taking PrEP before participation in the study, a history of drug use before sex, a history of injected drug use, sex work experience), and testing positive for syphilis at PrEP initiation. Adverse effects and behavioral characteristics (i.e., hormone use, condom use, and the ability to schedule a timely PrEP follow-up appointment) after receiving PrEP were also considered. Although information concerning adverse effects such as feeling anxious and condom use during sexual intercourse was collected at each follow-up visit, it was not available for those who only initiated PrEP (n = 127). Whether PrEP initiation occurred during the lockdown policy to prevent the spread of COVID-19 in Thailand (one month before the start of lockdown (3 April 2020) to the end of lockdown (3 May 2020)) was also considered in this study.
Sample size
The sample size was calculated based on the following formula developed by Schoenfeld to determine the minimum number of participants required for the study when the size of the study population is unknown [34). This was calculated based on the power of the test being equal to 85% as follows:
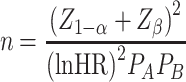 |
,
where 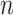 is the required sample size;
is the required sample size; 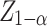 was set as 1.96 for α = 0.05;
was set as 1.96 for α = 0.05; 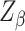 (the standard normal deviate for the power of the test) was set as 1.04. Following the procedure of [35], who performed a study of risk factors associated with LTFU after PrEP initiation among TGWs and MSMs in which age was evaluated as a potential risk factor, we set the proportions of participants < 25 (
(the standard normal deviate for the power of the test) was set as 1.04. Following the procedure of [35], who performed a study of risk factors associated with LTFU after PrEP initiation among TGWs and MSMs in which age was evaluated as a potential risk factor, we set the proportions of participants < 25 (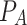 ) and ≥ 25 (
) and ≥ 25 (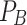 ) years old who were LTFU after PrEP initiation to be 0.45 and 0.55, respectively, and the hazard ratio (
) years old who were LTFU after PrEP initiation to be 0.45 and 0.55, respectively, and the hazard ratio (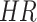 ) for LTFU to be 1.5. According to the formula with a power of the test of 85% and a 95% confidence level, the required sample was 222 participants. However, we considered that some attrition of participants could occur during follow-up (participants omitted from the study because of reasons not related to LTFU, such as death and HIV infection). Thus, the attrition rate was set as 6% in this calculation according to that used in a previous study in which around 6% of MSMs and TGWs living in Chiang Mai were found to be HIV positive [36]. Therefore, at least 235 participants were required for the current study.
) for LTFU to be 1.5. According to the formula with a power of the test of 85% and a 95% confidence level, the required sample was 222 participants. However, we considered that some attrition of participants could occur during follow-up (participants omitted from the study because of reasons not related to LTFU, such as death and HIV infection). Thus, the attrition rate was set as 6% in this calculation according to that used in a previous study in which around 6% of MSMs and TGWs living in Chiang Mai were found to be HIV positive [36]. Therefore, at least 235 participants were required for the current study.
Statistical analysis
Participants were considered LTFU when they discontinued PrEP offered by MPlus for more than one month after one of the follow-up visits at 1, 2, 3, and 6 months despite repeated attempts to contact them. Continuous variables are presented as medians and interquartile ranges (IQRs) and categorical variables as frequencies and percentages. Characteristic variables were compared between groups of participants who came to follow-up and participants who came only at the initiation by using Fisher’s exact tests for discrete variables and Wilcoxon rank-sum (Mann-Whitney) tests for continuous ones. Cumulative LTFU rates at 1, 2, 3, and 6 months after PrEP initiation were estimated by using Kaplan-Meier curves. Participants who did not attend PrEP follow-up visits (n = 127) were excluded from the analysis of the cumulative LTFU rate. In addition, Log-rank tests were used to compare LTFU rates between groups of variables. Risk factors associated with LTFU were identified by using Cox proportional hazard regression models. Clinically relevant variables or potential associated factors with P-values of less than 0.25 in the univariable analyses [37] (i.e., age, syphilis infection, history of taking PrEP before participation in the study, feeling anxious after taking PrEP, hormone use, condom use, and being able to schedule a timely PrEP follow-up appointment) were included in a multivariable analysis with backward elimination. In addition, since the frequency of PrEP use (daily or on-demand) presented with a higher P-value, it was also included in the multivariable analysis due to our hypothesis that TGWs using on-demand PrEP may use it less often than those who use PrEP daily (as suggested by Koppe et al. [29]). Covariances that were collinear were excluded from the multivariable model. All analyses were performed using Stata version 15 (StataCorp, College Station, Texas, USA).
Results
Characteristics
The 235 transgender women who initiated PrEP during the study period and took part in the study were divided into 127 participants who did not attend PrEP follow-up visits and 108 participants who attended at least one. The socio-demographic characteristics, syphilis testing, and behavioral data for these two groups are summarized in Table 1. Specifically, the median age of participants who did not attend a PrEP follow-up visit was lower than those who attended at least one (23 vs. 26 years old; P-value = 0.001). More than half of those who did not attend a PrEP follow-up visit (53%) were students or unemployed, while 45% who attended at least one were employees (P-value < 0.001). In terms of syphilis diagnosis, transgender women who did not attend PrEP follow-up visits were more likely to have reactive syphilis at PrEP initiation compared to those who attended at least one PrEP follow-up visit (83% vs. 55%; P-value < 0.001). Transgender women who attended at least on PrEP follow-up were more likely to have had sex worker experience than those who did not attend any (12% vs. 3%; P-value = 0.011). In addition, transgender women who attended at least one PrEP follow-up visit were more likely to be able to schedule a PrEP follow-up appointment than those who did not attend any (77% vs. 43%; P-value < 0.001).
Table 1.
The characteristics of transgender women utilizing PrEP
| Characteristic Frequency (Percentage) or Median [Interquartile range] |
Overall (N = 235) |
Follow-up After PrEP Initiation | Statistical Value | P-value | ||
|---|---|---|---|---|---|---|
| At Least Once (n = 108) |
Did Not Attend (n = 127) |
|||||
| Age (years old) | 24 [21-31] | 26 [22-33] | 23 [20-28] | 3.47a | 0.001** | |
| Relationship status | 0.83b | 0.402 | ||||
| Single | 120 (71%) | 58 (67%) | 62 (74%) | |||
| With a partner | 50 (29%) | 28 (32%) | 22 (26%) | |||
| Missing | 65 | 22 | 43 | |||
| Occupation | 16.15b | < 0.001** | ||||
| College student/unemployed | 52 (38%) | 15 (24%) | 37 (53%) | |||
| Freelance/self-employed | 41 (31%) | 20 (31%) | 21 (30%) | |||
| Employee | 41 (31%) | 29 (45%) | 12 (17%) | |||
| Missing | 101 | 44 | 57 | |||
| Syphilis infection | 22.85b | < 0.001** | ||||
| Non-reactive | 70 (30%) | 49 (45%) | 21 (17%) | |||
| Reactive | 164 (70%) | 59 (55%) | 105 (83%) | |||
| Missing | 1 | 0 | 1 | |||
| History of taking PrEP before participation in the study | 0.01b | 1.000 | ||||
| Yes | 2 (1%) | 1 (1%) | 1 (1%) | |||
| No | 233 (99%) | 107 (99%) | 126 (99%) | |||
| History of taking PEP before participation in the study | 3.22b | 0.105 | ||||
| Yes | 37 (16%) | 22 (20%) | 15 (12%) | |||
| No | 198 (84%) | 86 (80%) | 112 (88%) | |||
| History of drug abuse | 6.03b | 0.018* | ||||
| Yes | 16 (7%) | 12 (11%) | 4 (3%) | |||
| No | 217 (93%) | 94 (89%) | 123 (96%) | |||
| Missing | 2 | 2 | 0 | |||
| History of injected drug use | 0.20b | 1.000 | ||||
| Yes | 3 (1%) | 1 (1%) | 2 (2%) | |||
| No | 232 (99%) | 107 (99%) | 125 (98%) | |||
| Sex worker experience | 6.87b | 0.011* | ||||
| Yes | 17 (7%) | 13 (12%) | 4 (3%) | |||
| No | 218 (93%) | 95 (88%) | 123 (97%) | |||
| Frequency of PrEP use | 0.08b | 0.869 | ||||
| Daily | 189 (80%) | 86 (80%) | 103 (81%) | |||
| On-demand | 46 (20%) | 22 (20%) | 24 (19%) | |||
| Hormone use | 0.12b | 0.840 | ||||
| Yes | 94 (74%) | 48 (73%) | 46 (75%) | |||
| No | 33 (26%) | 18 (27%) | 15 (25%) | |||
| Missing | 108 | 42 | 66 | |||
| Able to schedule a PrEP follow-up appointment | 13.91b | < 0.001** | ||||
| Yes | 73 (62%) | 50 (77%) | 23 (43%) | |||
| No | 45 (38%) | 15 (23%) | 30 (57%) | |||
| Missing | 117 | 53 | 64 | |||
Abbreviations: PrEP, pre-exposure prophylaxis; PEP, post-exposure prophylaxis
aWilcoxon rank-sum test; bFisher’s exact test
*P-value < 0.05; **P-value < 0.01
Adverse effects of PrEP and condom use
From a total of 108 participants who attended at least one PrEP follow-up visit, 34% had felt anxious. After PrEP initiation, the majority of transgender women (63%) used a condom during sexual intercourse. This information was collected during each visit. Therefore, information on the adverse effects of PrEP and condom use could not be obtained from those who only initiated PrEP (n = 127).
Reasons for discontinuing PrEP
After participants became LTFU, we contacted them to inquire about the reason for not following up; the results are reported in Table 2. Of those who did not attend PrEP follow-up visits (n = 127), 28% thought they were no longer at risk, 6% moved away from Chiang Mai, 6% had travel or appointment scheduling difficulties, and 6% could not attend due to the COVID-19 situation. Of the 38 participants who attended follow-up at least once (n = 108) and then became LTFU, 18% had direct side effects from PrEP or they were afraid of reduced hormone efficacy due to taking PrEP, 16% thought that they were not at risk, and 16% had travel or appointment schedule difficulties.
Table 2.
Reasons for discontinuing PrEP after initiation and attending/not attending follow-up visits
| Reason for Discontinuing PrEP | Participants who Did Not Attend a Follow-up PrEP Visit (n = 127) | Participants who LTFU After Attending at Least One Follow-up Appointment (n = 38) |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 1. Not recorded | 64 | 50 | 18 | 47 | |
| 2. Changed sexual behavior (abstained from sexual relations) | 36 | 28 | 6 | 16 | |
| 3. Obtaining PrEP difficult because of travel and/or appointment schedule mismatch | 8 | 6 | 6 | 16 | |
| 4. Moved away from Chiang Mai | 8 | 6 | 1 | 3 | |
| 5. Covid situation | 8 | 6 | 0 | 0 | |
| 6. Side effects/afraid of interaction with sex hormones | 2 | 2 | 7 | 18 | |
| 7. Little knowledge of PrEP | 1 | 1 | 0 | 0 | |
Abbreviations: PrEP, pre-exposure prophylaxis; LTFU, loss to follow-up
Cumulative LTFU after PrEP initiation
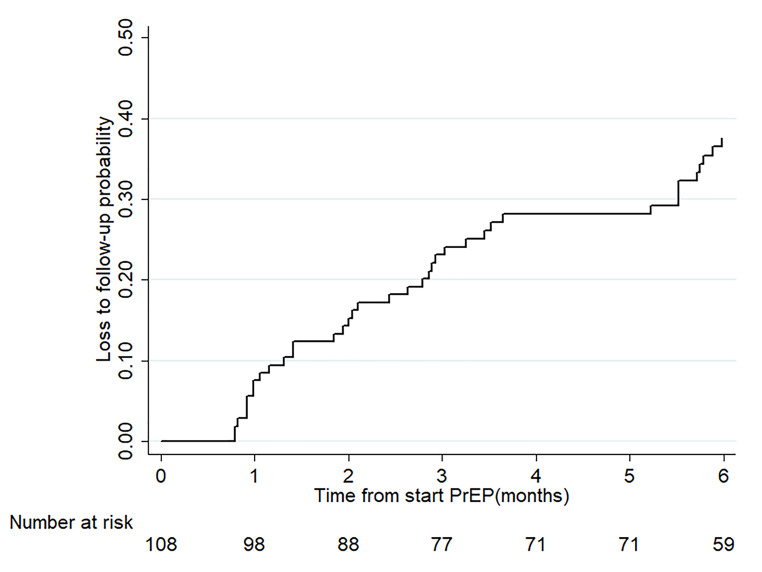
The 127 participants who did not attend PrEP follow-up visits were excluded from the analysis of the cumulative LTFU rate. The cumulative LTFU rates after PrEP initiation of the 108 participants who attended at least one PrEP follow-up visit are shown in Fig. 1. The LTFU rates at 1, 2, 3, and 6 months after PrEP initiation were 8% (95% CI: 4–14%), 14% (95% CI: 9–23%), 23% (95% CI: 16–32%), and 38% (95% CI: 29–48%), respectively.
Fig. 1.
Cumulative loss to follow-up rate within 6 months after pre-exposure prophylaxis initiation. The lost to follow-up in both groups was increasing after 1 month from start pre-exposure prophylaxis
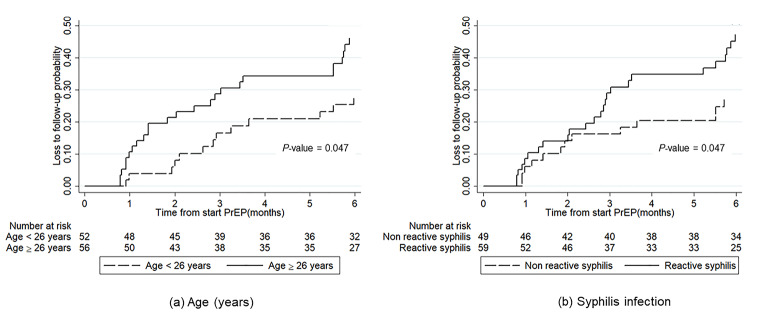
Fig. 2a and b show the cumulative LTFU rates within 6 months after PrEP initiation by age group and syphilis infection status, respectively. The results in Fig. 2a suggest that older transgender women (age ≥ 26 years old) had a significantly higher follow-up rate than younger ones (P-value = 0.047). In addition, transgender women infected with syphilis had a significantly higher follow-up rate than those who were not infected (P-value = 0.047).
Fig. 2.
Cumulative loss to follow-up rate up to 6 months after PrEP initiation by (a) age group and (b) syphilis infection status. LTFU was significantly higher in the older transgender women group (solid line) than the younger group (long-dashed line). LTFU was significantly higher in the transgender women living with syphilis (solid line) than those with non-reactive syphilis (long-dashed line)
Factors associated with LTFU
In the univariable analysis, age ≥ 26 years old (Hazard ratio [HR] = 1.95; 95% CI = 1.00–3.81; P-value = 0.045), reactive syphilis (HR = 1.95; 95% CI = 1.00–3.81; P-value = 0.046), experience of taking PrEP before participating in the study (HR = 2.65; 95% CI = 1.00–7.49; P-value = 0.036), and hormone use (HR = 2.56; 95% CI = 1.18–5.56; P-value = 0.020) were associated with LTFU (Table 3). In the multivariable analysis, LTFU was independently associated with age ≥ 26 years old (adjusted hazard ratio [aHR] = 2.09; 95% CI: 1.06–4.14; P-value = 0.029) and reactive syphilis (aHR = 1.98; 95% CI: 1.01–3.88; P-value = 0.042) after adjusting for the frequency of PrEP use (daily or on-demand).
Table 3.
Risk factors for LTFU among 108 transgender women taking PrEP
| Variable | n/N | (%) | Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | LR | P-value | aHR | 95% CI | LR | P-value | ||||
| Age | 4.00 | 0.045* | 4.77 | 0.029* | |||||||
| < 26 (ref.) | 13/52 | 25 | 1.00 | – | 1.00 | – | |||||
| ≥ 26 | 25/56 | 45 | 1.95 | 1.00–3.81 | 2.09 | 1.06–4.14 | |||||
| Syphilis infection | 3.98 | 0.046* | 4.14 | 0.042* | |||||||
| Non-reactive (ref.) | 13/49 | 27 | 1.00 | – | 1.00 | – | |||||
| Reactive | 25/59 | 42 | 1.95 | 1.00–3.81 | 1.98 | 1.01–3.88 | |||||
| Frequency of PrEP use | 1.13 | 0.287 | 1.59 | 0.207 | |||||||
| Daily (ref.) | 28/86 | 33 | 1.00 | – | 1.00 | – | |||||
| On-demand | 10/22 | 45 | 1.50 | 0.73–3.09 | 1.63 | 0.78–3.41 | |||||
| Receiving PrEP within lockdown period | 0.20 | 0.652 | |||||||||
| No (ref.) | 35/101 | 35 | 1.00 | – | |||||||
| Yes | 3/7 | 43 | 1.33 | 0.41–4.31 | |||||||
| Relationship status | < 0.01 | 0.999 | |||||||||
| Single (ref.) | 19/58 | 33 | 1.00 | – | |||||||
| With a partner | 10/28 | 36 | 1.00 | 0.47–2.15 | |||||||
| Occupation | 0.45 | 0.800 | |||||||||
| College student/unemployed | 7/15 | 47 | 1.43 | 0.50–4.08 | |||||||
| Freelance/self-employed (ref.) | 7/20 | 35 | 1.00 | – | |||||||
| Employee | 11/29 | 38 | 1.18 | 0.46–3.04 | |||||||
| History of taking PrEP before participation in the study | 4.37 | 0.036* | |||||||||
| Yes (ref.) | 4/22 | 18 | 1.00 | – | |||||||
| No | 34/86 | 40 | 2.65 | 1.00–7.49 | |||||||
| Sex worker experience | 0.77 | 0.379 | |||||||||
| Yes (ref.) | 3/13 | 23 | 1.00 | – | |||||||
| No | 35/95 | 37 | 1.64 | 0.50–5.33 | |||||||
| Adverse effects after taking PrEP | 0.17 | 0.677 | |||||||||
| Yes | 8/19 | 42 | 1.20 | 0.52–2.78 | |||||||
| No (ref.) | 17/46 | 37 | 1.00 | – | |||||||
| Feeling anxious after taking PrEP | 2.80 | 0.094 | |||||||||
| Yes | 12/22 | 55 | 1.97 | 0.90–4.33 | |||||||
| No (ref.) | 13/43 | 30 | 1.00 | – | |||||||
| Hormone use | 5.37 | 0.020* | |||||||||
| Yes (ref.) | 14/48 | 29 | 1.00 | – | |||||||
| No | 12/18 | 67 | 2.56 | 1.18 − 5.56 | |||||||
| Condom use after taking PrEP | 2.99 | 0.084 | |||||||||
| Yes | 19/41 | 46 | 2.15 | 0.86–5.38 | |||||||
| No (ref.) | 6/24 | 25 | 1.00 | – | |||||||
| Able to schedule a timely PrEP follow-up appointment | 2.35 | 0.126 | |||||||||
| Yes (ref.) | 17/50 | 34 | 1.00 | – | |||||||
| No | 8/15 | 53 | 2.00 | 0.86–4.68 | |||||||
Abbreviations: ref., reference group; HR, hazard ratio; aHR, adjusted hazard ratio; 95%CI, 95% confidence interval; n, number of LTFU participants; N, number of participants; PrEP, pre-exposure prophylaxis; LR, Likelihood Ratio test
*P-value < 0.05
Discussion
We conducted a longitudinal study with follow-up at 1, 2, 3, and 6 months after PrEP initiation among TGWs in Chiang Mai, Thailand. We found an increased rate of LTFU for follow-up visits over time (23% at 3 months to 38% at 6 months after PrEP initiation), which is similar to the trend previously reported for MSMs and TGWs in community-based clinics in the US [20, 21]; the researchers found that the rate of discontinuing PrEP services (defined as individuals who did not return within 30 days of a scheduled follow-up visit after PrEP initiation) was 4–16% at 3–4 months and increased to 21–38% at 6–7 months after PrEP initiation. The most prevalent reason for discontinuing PrEP reported by those who did not attend any follow-up visits after initiation or were LTFU after attending at least once was that they were no longer at risk of HIV transmission (i.e., abstaining from sexual relations).
Other reasons for discontinuation found in the current study included moving away from the city, the side effects of PrEP, and relationship factors (e.g., changing partner, changing sexual behavior, and/or entering a monogamous relationship), which are consistent with the findings from previous studies on PrEP use by MSMs and TGWs [24–26]. The findings from several similar studies in the US and Canada point toward two common reasons for discontinuing PrEP services: cost and lack of health insurance support [20, 24]. Although the TGWs in Thailand can access PrEP for free, we also found that some of them had a problem with travel costs when accessing PrEP services. This is in accordance with the finding from previous studies suggesting that low annual income is associated with poor attendance of PrEP follow-up appointments [23, 38]. Specifically, from research into examining potential barriers to accessing PrEP in individuals who initiated PrEP when it was free, Kamis et al. [38] still found that after adjusting for age, race/ethnicity, and health insurance status, low income was the major finding associated with poor attendance for PrEP care. Thus, a vending service for the PrEP program could increase the opportunity to access PrEP and lead to a reduction in PrEP LTFU.
We found that fear of interaction between sex hormones and PrEP is one of the reasons for PrEP. Although many researchers have reported that PrEP does not affect the concentration of transition-related hormones [39, 40], we found that 18% of TGWs discontinued PrEP after attending at least one follow-up visit and 2% of those did not attend because they believed that PrEP use could affect their hormone therapy outcomes. In addition, the Thailand National Guidelines on Pre-Exposure Prophylaxis in 2018 suggest that healthcare providers should appoint hormone specialists for TGWs who are concerned with potential PrEP and hormone interactions [4]. More clarity about both the advantages and disadvantages of PrEP should be available to potentially increase the number of PrEP users and continuation among TGWs in Thailand.
According to our investigation, we found that the median age of TGWs who did not attend PrEP follow-up visits was 23 years old and the majority of them were students or unemployed. Similarly, unemployed TGWs and MSMs in a PrEP cohort study in the US were independently associated with LTFU within the first 12 weeks [23]. This may be due to unemployed TGWs’ inability to attend PrEP follow-up visits due to the cost of transportation and other related requirements.
We found that the TGWs aged 26 years old or more had a 2-fold higher risk of LTFU after attending at least one PrEP follow-up visit, which is consistent with the findings from a recent study on TGWs and MSMs in Thailand indicating that TGWs < 25 years old were more likely to attend PrEP follow-up visits than those aged ≥ 25 years old (83.9% vs. 39.4%) [32]. In contrast to a previous study on MSMs and transgender people in the US, our finding indicates that younger persons (aged 18–29 years old) were more likely to be LTFU (stopped returning for PrEP visits) than those aged 30–39 years old [26]. However, the findings from a recent study on TGWs and MSMs in Thailand indicate that more TGWs < 25 years old attended PrEP follow-up after one month than those aged ≥ 25 years old (83.9% vs. 39.4%) [32]. This may be due to the difference in cut-off age for the groups in the studies and the duration of PrEP retention. Moreover, Krakower et al. [26] found that PrEP discontinuation was more likely to occur among persons who identified as TGWs, which is supported by the findings from our study on investigating LTFU after PrEP initiation in the TGW population.
According to the Thailand National Guidelines on Pre-Exposure Prophylaxis in 2018, people who had an STI infection within the last 6 months were part of one of the key population-led PrEP initiatives in Thailand, and regular STI testing before PrEP initiation and every 3 months thereafter was recommended for people taking PrEP [4]. Moreover, physicians referred people who had STIs at PrEP initiation and afterward to sexually transmitted disease (STD)/STI clinics according to the Thailand National Guidelines for the treatment of STIs [41]. However, our findings show that TGWs with reactive syphilis at the baseline had a 2-fold higher risk of LTFU from the PrEP program compared to those with non-reactive syphilis. This is consistent with a long-term study of MSMs at a nurse-led community-based clinic in the US, in which MSMs diagnosed with STIs at the baseline were less likely to be retained in the PrEP program (aHR = 0.56, 95% CI: 0.33–0.95) [20]. In addition, 31% of MSM participants reported that they had sexual intercourse with partners with unknown HIV serostatus while 95% of them reported that they engaged in condomless sexual intercourse [20]. Individuals with reactive syphilis or STIs have a low HIV risk perception [42], which was identified as the most common reason for PrEP discontinuation [26, 43]. However, the results from a large survey of MSMs across six regions of the world using different methodologies and study designs signify that a recent STI test or treatment is associated with increased PrEP use [28]. Some possible explanations for this association may be because people who had an STI test and/or treatment have concerns about HIV transmissibility, the prevention of which is the key role of PrEP use [44]. Although we cannot ensure that reactive syphilis is one of the key factors for LTFU and we do not know of any underlying connection between the PrEP providers and the STD/STI clinics, investigation of this issue in a future study could be interesting.
Although receiving PrEP during the COVID-19 lockdown period was not statistically significantly associated with LTFU in our study, we found that some TGWs who did not attend PrEP follow-up visits (6%) reported a problem with accessing PrEP services because of the COVID-19 crisis. This small proportion agreed with the result from the following previous studies. The findings from a survey on PrEP use by MSMs during the COVID-19 lockdown indicate that 22.5% of PrEP users who used daily PrEP before the COVID-19 lockdown stopped doing so during the COVID-19 lockdown, the most common reasons being decreased sexual activity, concern about catching COVID-19, and having difficulties in accessing PrEP services [45]. Moreover, in a recent study on PrEP uptake during the COVID-19 crisis in 21 PEPFAR-funded countries (including Thailand) [46], the authors reported a 13% decrease in PrEP use by the key populations in Thailand compared with pre-COVID-19. Thus, the COVID-19 crisis could have reduced the retention rate due to limited access to PrEP. Accordingly, telemedical consultation services and PrEP delivery may increase the retention rate of PrEP use.
This study had several limitations. First of all, it is only based on the available data on TGWs utilizing PrEP from the MPlus foundation in Chiang Mai, which might not have produced generalizable results for the whole country. Second, the majority of participants did not report the reasons for PrEP discontinuation or LTFU, and so since these are only based on the participants who responded, we might not have captured the true reasons for discontinuing PrEP. To address this issue, we need to develop a process to contact all TGWs who missed a PrEP follow-up visit sooner than a month, e.g. within a week. It has recently been found that TGWs and persons with multiple mental health disorders are statistically significantly associated with PrEP discontinuation [26, 47], which is interesting given that mental health diagnoses have been associated with lower PrEP adherence and PrEP awareness/willingness/use. Furthermore, we did not include some potentially associated factors of LTFU after PrEP initiation, such as cost, insurance support, and the self-perceived risk of HIV infection in our analysis. Cost and insurance support were not included because PrEP is offered for free to the key population in Thailand. Although a low self-perceived risk of HIV infection was stated as the most common reason for discontinuing PrEP in previous studies [26, 43], we were unable to obtain this information since the MPlus Foundation did not include it in their questionnaire prior to providing PrEP to their clients. We will recommend to them to evaluate clients’ HIV risk perception as it could be useful for investigating the efficacy of PrEP use and related information concerning HIV prevention.
Conclusions
The LTFU rates at 1, 3, and 6 months after PrEP initiation among TGWs who had received PrEP in Chiang Mai, Thailand, were 8%, 23%, and 38%, respectively. After adjusting for the frequency of PrEP use, TGWs aged ≥ 26 years old and who had reactive syphilis at PrEP initiation were more likely to LTFU from the PrEP program. According to the Thailand National Guidelines for the treatment of STIs, physicians refer patients diagnosed with any STI to STD/STI clinics, and there is an inevitable time lag due to appointment scheduling of TGWs identified with reactive syphilis between the PrEP providers and the syphilis clinic. In addition, some participants reported a problem with accessing PrEP services during the COVID-19 lockdown period. Accordingly, telemedical consultation services and PrEP delivery may increase the retention rate of PrEP use.
Acknowledgements
We acknowledge the transgender women respondents and staffs of the MPlus Foundation, Chiang Mai, Thailand. This study was supported by Chiang Mai University.
Author contribution
NT, NattM, RA, PT, and NH were involved in the conception or design of the study. NattM, RA, and NR coordinated the operations. RA and NR contributed to data collection. NH is primary responsibility for literature search. NT, NatnM, WB, BP, NN, ST, PS, and KW contributed in literature search. NR, NatnM, WB, BP, NN, ST, PS, KW, and PT contributed in reviewing of the manuscript. NT, PT, and NH contributed to data analysis and data interpretation. NT, and NH wrote the first draft of the manuscript and all authors approved the final version of the manuscript.
Funding
Not applicable.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
There are no conflicts of interest in the conduct and reporting of the research.
Ethical approval
This study was approved and monitored by the Ethics Committee of the Faculty of Medicine, Chiang Mai University.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent for publication
Written informed consent was obtained from all participants included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European AIDS Clinical Society. Guideline version 10.0 November 2019. Available at: https://www.eacsociety.org/media/2019_guidelines-10.0_final.pdf. Published November 2019. Accessed July 2, 2021.
- 3.World Health Organization. What’s the 2 + 1 + 1? Event-driven oral pre-exposure prophylaxis to prevent HIV for men who have sex with men: Update to WHO’s recommendation on oral PrEP. Available at: https://www.who.int/publications/i/item/what-s-the-2-1-1-event-driven-oral-pre-exposure-prophylaxis-to-prevent-hiv-for-men-who-have-sex-with-men. Accessed July 15, 2021.
- 4.Department of Disease Control, Ministry of Public Health, Thailand. Thailand national guidelines on pre-exposure prophylaxis in 2018 [in Thai]. Available at: http://aidssti.ddc.moph.go.th/medias/view/126/549. Published July 2018. Accessed July 10, 2021.
- 5.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Heffron R, Kidoguchi L, et al. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. doi: 10.1371/journal.pmed.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV. 2018;5(2):e68–78. doi: 10.1016/S2352-3018(17)30156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the human immunodeficiency virus prevention trials network 067/ADAPT study. Clin Infect Dis. 2018;66(11):1712–21. doi: 10.1093/cid/cix1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thigpen MC, Rose CE, Paxton LA. Antiretroviral preexposure prophylaxis for HIV prevention. N Engl J Med. 2013;368(1):82–3. doi: 10.1056/NEJMc1210464. [DOI] [PubMed] [Google Scholar]
- 12.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colby D, Kongkabpan M, Teeratakulpisarn S, et al. PrEP or Peril: rolling out PrEP in the private sector without subsidy or government support. The 22nd International AIDS Conference (AIDS 2018). Amsterdam, 2018. [abstract THSY0206].
- 14.Thailand National AIDS Committee. Thailand national operational plan accelerating ending AIDS, 2015–2019. Available at: https://www.aidsdatahub.org/sites/default/files/resource/thailand-national-operational-plan-accelerating-ending-aids-2015-2019.pdf. Published November 2014. Accessed August 8, 2021.
- 15.National Monitoring and Evaluation Unit Bureau of AIDS, TB and STIs, Department of Disease Control, Minitry of Public Health, Thailand. Progress report for Thailand ending AIDS 2018. Available at: https://hivhub.ddc.moph.go.th/Download/Report/APR/2018/EN_GAM%202018.pdf. Published September 2018. Accessed August 1, 2021.
- 16.Seekaew P, Pengnonyang S, Jantarapakde J, et al. Discordance between self-perceived and actual risk of HIV infection among men who have sex with men and transgender women in Thailand: a cross-sectional assessment. J Int AIDS Soc. 2019;22(12):e25430. doi: 10.1002/jia2.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotzker R, Seekaew P, Jantarapakde J, et al. Importance of risk perception: predictors of PrEP acceptance among Thai MSM and TG women at a community-based health service. J Acquir Immune Defic Syndr. 2017;76(5):473–81. doi: 10.1097/QAI.0000000000001536. [DOI] [PubMed] [Google Scholar]
- 18.MPlus Foundation. About Mplus [in Thai]. Available at: https://www.mplusthailand.com. Accessed July 10, 2021.
- 19.Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. doi: 10.7448/IAS.19.1.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV pre-exposure prophylaxis (PrEP) uptake and retention among men who have sex with men in a community-based sexual health clinic. AIDS Behav. 2018;22(4):1096–9. doi: 10.1007/s10461-017-2009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for pre-exposure prophylaxis to prevent HIV-1 acquisition in young adult men who have sex with men and transgender women of color using a urine adherence assay. J Acquir Immune Defic Syndr. 2018;79(2):173–8. doi: 10.1097/QAI.0000000000001772. [DOI] [PubMed] [Google Scholar]
- 22.Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. doi: 10.1093/ofid/ofz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doblecki-Lewis S, Liu AY, Feaster DJ, et al. Patterns and correlates of participant retention in a multi-city pre-exposure prophylaxis demonstration project. J Acquir Immune Defic Syndr. 2018;79(1):62–9. doi: 10.1097/QAI.0000000000001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwald ZR, Maheu-Giroux M, Szabo J, et al. Cohort profile: l’actuel pre-exposure prophylaxis (PrEP) cohort study in Montreal, Canada. BMJ Open. 2019;9(6):e028768. doi: 10.1136/bmjopen-2018-028768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hevey MA, Walsh JL, Petroll AE. PrEP continuation, HIV and STI testing rates, and delivery of preventive care in a clinic-based cohort. AIDS Educ Prev. 2018;30(5):393–405. doi: 10.1521/aeap.2018.30.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi: 10.1002/jia2.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shover CL, Shoptaw S, Javanbakht M, et al. Mind the gaps: prescription coverage and HIV incidence among patients receiving pre-exposure prophylaxis from a large federally qualified health center in Los Angeles, California. AIDS Behav. 2019;23(10):2730–40. doi: 10.1007/s10461-019-02493-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayala G, Santos GM, Arreola S, Garner A, Makofane K, Howell S. Blue-Ribbon Boys: factors associated with PrEP use, ART use and undetectable viral load among gay app users across six regions of the world. J Int AIDS Soc. 2018;21(Suppl 5):e25130. doi: 10.1002/jia2.25130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koppe U, Marcus U, Albrecht S, et al. Barriers to using HIV pre-exposure prophylaxis (PrEP) and sexual behaviour after stopping PrEP: a cross-sectional study in Germany. BMC Public Health. 2021;21(1):159. doi: 10.1186/s12889-021-10174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landovitz RJ, Beymer M, Kofron R, et al. Plasma tenofovir levels to support adherence to TDF/FTC preexposure prophylaxis for HIV prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr. 2017;76(5):501–11. doi: 10.1097/QAI.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phanuphak N, Sungsing T, Jantarapakde J, et al. Princess PrEP program: the first key population-led model to deliver pre-exposure prophylaxis to key populations by key populations in Thailand. Sex Health. 2018;15(6):542–55. doi: 10.1071/SH18065. [DOI] [PubMed] [Google Scholar]
- 32.Ramautarsing RA, Meksena R, Sungsing T, et al. Evaluation of a pre-exposure prophylaxis programme for men who have sex with men and transgender women in Thailand: learning through the HIV prevention cascade lens. J Int AIDS Soc. 2020;23(Suppl 3):e25540. doi: 10.1002/jia2.25540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Songtaweesin WN, Kawichai S, Phanuphak N, et al. Youth-friendly services and a mobile phone application to promote adherence to pre-exposure prophylaxis among adolescent men who have sex with men and transgender women at-risk for HIV in Thailand: a randomized control trial. J Int AIDS Soc. 2020; 23 Suppl 5:e25564. [DOI] [PMC free article] [PubMed]
- 34.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. doi: 10.2307/2531021. [DOI] [PubMed] [Google Scholar]
- 35.Wahome EW, Graham SM, Thiong’o AN, et al. Risk factors for loss to follow-up among at-risk HIV negative men who have sex with men participating in a research cohort with access to pre-exposure prophylaxis in coastal Kenya. J Int AIDS Soc. 2020; 23 Suppl 6:e25593. [DOI] [PMC free article] [PubMed]
- 36.Nanthaprut P, Manojai N, Chanlearn P, et al. Comparison of HIV-positive incidence among transgender women and men who have sex with men at stand-alone and mobile voluntary counseling and testing facilities in Chiang Mai Province, Thailand. AIDS Patient Care STDS. 2021;35(4):116–25. doi: 10.1089/apc.2020.0258. [DOI] [PubMed] [Google Scholar]
- 37.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 38.Kamis KF, Marx GE, Scott KA, et al. Same-day HIV pre-exposure prophylaxis (PrEP) initiation during drop-in sexually transmitted diseases clinic appointments is a highly acceptable, feasible, and safe model that engages individuals at risk for HIV into PrEP care. Open Forum Infect Dis. 2019;6(7):ofz310. doi: 10.1093/ofid/ofz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant RM, Pellegrini M, Defechereux PA, et al. Sex hormone therapy and tenofovir diphosphate concentration in dried blood spots: primary results of the interactions between antiretrovirals and transgender hormones study. Clin Infect Dis. 2021;73(7):e2117–23. doi: 10.1093/cid/ciaa1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiransuthikul A, Janamnuaysook R, Himmad K, et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc. 2019;22(7):e25338. doi: 10.1002/jia2.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Monitoring and Evaluation Unit Bureau of AIDS, TB and STIs, Department of Disease Control, Minitry of Public Health, Thailand. Thailand national guideline on the treatment of sexually transmitted diseases in 2015 [in Thai]. Published September 2015. Available at: https://www.pidst.or.th/A437.html. Accessed July 10, 2021.
- 42.Li Y, Detels R, Lin P, et al. Prevalence of HIV and STIs and associated risk factors among female sex workers in Guangdong Province, China. J Acquir Immune Defic Syndr. 2010;53(Suppl 1):48–53. doi: 10.1097/QAI.0b013e3181c7d72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitfield THF, John SA, Rendina HJ, Grov C, Parsons JT. Why I quit pre-exposure prophylaxis (PrEP)? A mixed-method study exploring reasons for PrEP discontinuation and potential re-initiation among gay and bisexual men. AIDS Behav. 2018;22(11):3566–75. doi: 10.1007/s10461-018-2045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agha S. The impact of a mass media campaign on personal risk perception, perceived self-efficacy and on other behavioural predictors. AIDS Care. 2003;15(6):749–62. doi: 10.1080/09540120310001618603. [DOI] [PubMed] [Google Scholar]
- 45.Chow EPF, Hocking JS, Ong JJ, et al. Changing the use of HIV pre-exposure prophylaxis among men who have sex with men during the COVID-19 pandemic in Melbourne, Australia. Open Forum Infect Dis. 2020;7(7):ofaa275. doi: 10.1093/ofid/ofaa275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerzner M, De AK, Yee R, et al. Pre-exposure prophylaxis (PrEP) uptake and service delivery adaptations during the first wave of the COVID-19 pandemic in 21 PEPFAR-funded countries. PLoS ONE. 2022;17(4):e0266280. doi: 10.1371/journal.pone.0266280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Brown L, Przybyla S, Bleasdale J, Mitchell J, Zhang C. Characterizing racial differences of mental health burdens, psychosocial determinants, and impacts on HIV prevention outcomes among young men who have sex with men: a community-based study in two U.S. cities. J Racial Ethn Health Disparities. 2021 doi: 10.1007/s40615-021-01052-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.