Abstract
Background
The efficacy and safety of upadacitinib in atopic dermatitis (AD) have been defined in clinical trials, but no real-world data are currently available. We aimed to assess the safety and effectiveness of upadacitinib in a real-world AD patient cohort that mostly included patients who failed the available systemic therapies, including dupilumab.
Methods
Prospective cohort study collecting data on upadacitinib-treated AD adult patients completing at least 16 weeks of therapy.
Results
Forty-three patients showed rapid and marked response to upadacitinib with significant reduction of all disease severity scores since the first follow-up visit. At week 16, Eczema Area and Severity Index (EASI) 75, EASI 90, and EASI 100 response was observed in 97.5%, 82.1%, and 69.2% of patients, respectively. EASI 90 response reflected the achievement of a clear or almost clear condition (POEM 0-2), self-evaluated by 79.5% of patients. Patients’ quality of life improved as suggested by the achievement of DLQI 0/1 by 38.5% of patients at week 4, and by 76.9% at week 16.
Conclusion
Elevated effectiveness and favorable safety of upadacitinib were confirmed in patients unresponsive to dupilumab, who were not included in upadacitinib trials.
Key Points
| Real-world data on upadacitinib in the treatment of atopic dermatitis are limited. |
| Upadacitinib demonstrated effectiveness in patients excluded from trials because of prior failure on dupilumab. |
Introduction
Atopic dermatitis (AD) is a chronic-relapsing, highly itchy, inflammatory skin disease that is associated with a negative impact on patients’ quality of life (QoL) [1]. It represents a common skin condition with an increasingly higher prevalence in adulthood that in Italy is estimated at 8.1% [2]. Because of the complex immune mechanism underlying AD pathogenesis, topicals and/or conventional systemic immunosuppressive/immunomodulant therapies (i.e., cyclosporine) are prescribed, though their use is often limited by the lack of effectiveness or safety risks related with their long-term use [3, 4]. Dupilumab, a subcutaneous monoclonal antibody inhibiting the signaling of two pathogenic cytokines, interleukin (IL)-4 and IL-13 [5], is now available in most European countries for the treatment of moderate-to-severe AD in adults and adolescents/children. Although many patients benefit from dupilumab therapy, persistence of AD was reported in a consistent proportion of dupilumab-exposed patients in pivotal trials and in real-world studies [5–8]. Moreover, about 31% of patients continued to have flares and 4–17% were unresponsive or showed an inadequate response to dupilumab [7–11]. In addition, dupilumab-related adverse events might be commonly observed and the eventual cause of discontinuation, such as conjunctivitis, which was reported in up to 40% of subjects treated in a real-world setting [8, 12–14], or dupilumab-associated facial and neck erythema, which occurred in 11% of cases [15]. Overall, these reports underline the need for therapeutic alternatives for the treatment of moderate-to-severe AD. New drugs are being investigated for the treatment of AD, in particular agents targeting Janus kinase (JAK)-1 represent a promising therapy to treat AD as multiple proinflammatory cytokines implicated in AD pathogenesis signal through this kinase [16]. Upadacitinib, an oral selective JAK inhibitor with greater inhibitory potency for JAK1 than JAK2, JAK3, or tyrosine kinase 2 [17], has been recently approved by the European Medicines Agency (EMA) for the treatment of both adults and adolescents with moderate-to-severe AD. Trials demonstrated high efficacy and a favorable safety profile of upadacitinib in treating moderate-to-severe AD across two doses (15 mg and 30 mg), either as monotherapy or combined with topical corticosteroids with a substantial steroid-sparing effect [18–20]. Moreover, a head-to-head trial comparing upadacitinib with dupilumab demonstrated superiority of upadacitinib in treating AD, with a significantly greater proportion of upadacitinib-treated patients achieving both primary and secondary endpoints [21]. Nevertheless, the trial setting implicates patient selection based on predetermined inclusion and exclusion criteria, which does not always reflect daily clinical practice. Thus, this study aimed to provide evidence of safety and effectiveness of upadacitinib in a heterogenous, real-world, AD patient cohort that mostly included patients failing available systemic therapies, in particular patients who discontinued dupilumab because of lack of efficacy or occurrence of adverse events.
Materials and Methods
Study Design and Population
In this study, we prospectively collected data on adult patients affected by moderate-to-severe AD treated with upadacitinib from October 2020 to June 2021. Patients were referred to nine Italian dermatological centers in the context of a national compassionate use program authorized by the Italian Medical Agency (named, AIFA). AIFA recommendation allowed the use of either 15 mg or 30 mg upadacitinib based on the physician’s decision. Accordingly, physicians could prescribe both upadacitinib dosages within the compassionate use program, though in this study all patients were treated with 30 mg upadacitinib. The objective of the compassionate use program was to provide upadacitinib to adult patients with moderate-to-severe AD who were non-responders, intolerant, or had a contraindication to drugs approved for the treatment of AD. Patients were eligible if aged between 18 and 75 years, and had completed the prespecified washout from prior treatments, according to criteria applied for upadacitinib trials [17, 18].
Prior to upadacitinib initiation, a washout period of at least 4 weeks was recommended in patients using systemic immunosuppressive agents that included corticosteroids, methotrexate, cyclosporine, azathioprine, phosphodiesterase type 4 (PDE-4) inhibitors, interferon-γ, and mycophenolate mofetil. A washout period of five half-lives or within 12 weeks (whichever was longer) was considered in case of targeted biologic treatments [17, 18].
All patients were encouraged to use emollients daily, while topical corticosteroids of different potencies or topical calcineurin inhibitors were applied during the study according to the physician’s recommendations.
Follow-up visits were scheduled according to an appointment timetable at each center and patient availability, optionally after 4 weeks and, as required, after 16 weeks from baseline. Signed informed consent was obtained from patients in order to extract data from their clinical records. Approval of this study was obtained by the Local Ethics Committee—Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Prot N.: 4434.
Data Collection
Patients who achieved at least 16 weeks of treatment and those who discontinued treatment prior to week 16 were included in the study. Physicians were asked to report data derived from the medical records in an electronic case report form. Although the intermediate timepoint at week 4 was additionally considered, both baseline and week 16 information were required for each patient. Baseline characteristics included age, gender, occupation, smoking habits (smoker, former smoker, or non-smoker), AD history and severity, prior treatments, atopic and non-atopic comorbidities, and concomitant therapies. At baseline and at each follow-up visit, Eczema Area and Severity Index (EASI) and Body Surface Area (BSA) were used to assess disease severity and skin involvement, respectively, whilst itch severity was assessed by a 0–10 Numeric Rating Scale (itch-NRS), sleep disturbances/sleeplessness by a 0–10 NRS scale (sleep-NRS), pain intensity by a 0–10 Numeric Rating Scale (pain-NRS), patient’s QoL by the Dermatology Life Quality Index (DLQI) and global patient-oriented disease severity by the Patient-Oriented Eczema Measure (POEM).
Safety was assessed by physical examination and laboratory tests (i.e., complete blood count, transaminases, creatinine, blood glucose, prothrombin time, activated partial thromboplastin time, international normalized ratio, creatine phosphokinase). Adverse events (AEs) were defined as any abnormal physical condition or blood test alteration collected by the physicians throughout the study period every 16 weeks or more tightly based on clinical needs.
Statistical Analyses
Data were summarized as mean and standard deviation, median and interquartile range, or as absolute number and percentage, as appropriate. The within-group comparison of study outcomes (between baseline with week 4 and week 16) was performed listwise by the Wilcoxon-rank test for dependent observations. Different endpoints of response were considered: the achievement of EASI 50, EASI 75, and EASI 90 at weeks 4 and 16 as compared to baseline; an absolute DLQI value of 0/1 at the follow-up visit, meaning no impact of the disease on the patient’s QoL; and an absolute POEM value of 0–2 corresponding to patient-assessed clear or almost clear condition. As identified by an international expert consensus on the treat-to-target approach in AD, we also considered other treatment goals, though the timepoints identified by the consensus to assess the achievement of these therapeutic targets differed from our real-world setting [19]. At week 4, as an early timepoint, we evaluated the number of patients achieving a reduction of at least 3 points in the absolute itch-NRS score, a reduction of absolute DLQI score of at least 4 points, and a reduction of absolute POEM score of at least 4 points. At week 16, as a late timepoint, we evaluated the number of patients achieving an absolute EASI score ≤ 7, an absolute itch-NRS score ≤ 4, an absolute DLQI score ≤ 5, and an absolute POEM score ≤ 7. A p value < 0.05 was considered statistically significant. Data are reported and analyzed “as observed”. Thus, no missing imputation was performed. Data analysis was performed by STATA 13 for Windows (College Station, TX, USA).
Results
Forty-three patients (15 females and 28 males) were treated with 30 mg upadacitinib daily for an observation period of 16 weeks. Baseline demographic and clinical characteristics of the study population are illustrated in Table 1. All patients had been previously treated with at least one systemic agent. In particular, dupilumab was prescribed in 42/43 patients, while in one case it was not prescribed as it was considered not indicated (severe conjunctivitis with marked facial involvement). The most common reason for dupilumab discontinuation was inefficacy (39 cases, 92.9%), followed by the occurrence of AEs (three cases: two for recalcitrant conjunctivitis and one for head/neck eczema).
Table 1.
Demographic characteristics of the study population
| Characteristics | |
|---|---|
| Total population | 43 patients |
| M/F, n (%) | 28 (65.1)/15 (34.9) |
| Mean age, years (± SD) | 45.91 (± 15.8) |
| Mean BMI, kg/m2 (± SD) | 24.6 (± 3.5) |
| Median age at the onset of disease (25–75 percentile) | 6 (1–27) |
| Allergic rhinitis, n (%) | 15/43 (34.9) |
| Asthma, n (%) | 12/43 (27.9) |
| Allergic conjunctivitis, n (%) | 10/43 (23.3) |
| Chronic rhinosinusitis with nasal polyposis, n (%) | 1/43 (2.3) |
| Food allergy, n (%) | 10/43 (23.3) |
| Patients previously treated with CsA, n (%) | 42/43 (97.7) |
| Patients previously treated with dupilumab, n (%) | 42/43 (97.7) |
| Patients previously treated with methotrexate, n (%) | 20/43 (46.5) |
| Patients previously treated with oral corticosteroids, n (%) | 14/43 (42.4) |
| Patients previously treated with tralokinumab, n (%) | 2/43 (4.6) |
BMI body mass index, CsA cyclosporine, n number, SD standard deviation
Thirty-nine of 43 patients completed 16 weeks of treatment, whereas two withdrew from treatment due to Aes, while two were lost to follow-up.
Upadacitinib Improves Clinical Manifestations and Symptoms
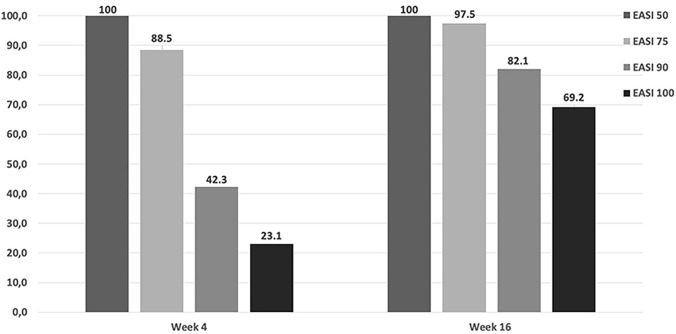
The EASI score significantly reduced over time, and, similarly, symptoms (itch and pain) as well as sleeplessness and QoL improved throughout the study period. The baseline median EASI score (26.0; interquartile range (IQR) 23.0–28.0) rapidly dropped (3.5; IQR 1.0–5.0) at week 4 and a further reduction was observed at the following visits (0.0; IQR 0.0–2.0; p for changes at different timepoints compared to baseline < 0.001; Table 2). Skin involvement (BSA score) reduced similarly to the EASI score (Table 2). Symptoms, both itch and pain, and sleep disturbances improved from week 4. Response rates in terms of EASI 50, EASI 75, EASI 90, and EASI 100 were high from week 4 (Fig. 1). After 4-week treatment, all patients achieved EASI 50, whilst EASI 75, EASI 90, and EASI 100 were reached in 88.5%, 42.3%, and 23.1% of patients, respectively. Further improvements were observed at week 16 with 97.5%, 82.1%, and 69.2% of patients achieving EASI 75, EASI 90, and EASI 100 responses, respectively (Fig. 1).
Table 2.
Disease severity reported as median scores (and interquartile range) for EASI, BSA, itch-NRS, sleep-NRS, pain-NRS, POEM, and DLQI, at baseline, at week 4, and at week 16
| Baseline | Week 4 | Week 16 | p value* | |
|---|---|---|---|---|
| Patients | n = 43 | n = 26 | n = 39 | |
| EASI score | 26.0 (23.0–28.0) | 3.5 (1.0–5.0) | 0.0 (0.0–2.0) | < 0.001 |
| BSA score | 24.0 (20.0–30.0) | 2.0 (0.0–4.0) | 0.0 (0.0–2.0) | < 0.001 |
| Itch-NRS score | 9.0 (7.0–10.0) | 2.0 (0.0–2.0) | 0.0 (0.0–0.0) | < 0.001 |
| Sleep-NRS score | 8.0 (5.0–9.0) | 1.0 (0.0–2.0) | 0.0 (0.0–0.0) | < 0.001 |
| Pain-NRS score | 7.0 (0.0–8.0) | 1.0 (0.0–1.0) | 0.0 (0.0–0.0) | < 0.001 |
| POEM score | 18.0 (15.0–23.0) | 3.5 (0.0–6.0) | 0.0 (0.0–2.0) | < 0.001 |
| DLQI score | 16.0 (10.0–20.0) | 2.5 (0.0–5.0) | 0.0 (0.0–1.0) | < 0.001 |
*Wilcoxon rank test for dependent observations comparing baseline values with both week 4 and week 16 scores. The comparison was done list-wise
BSA Body Surface Area, DLQI dermatology life quality index, EASI eczema area severity index, NRS numeric rating scale, POEM patient-oriented eczema measure
Fig. 1.
Treatment response to upadacitinib in terms of Eczema Area and Severity Index (EASI) 50, EASI 75, EASI 90, and EASI 100 responses. Percentage of patients achieving EASI 50, EASI 75, EASI 90, and EASI 100 responses at the follow-up visits
This significant (p < 0.001) and rapid decrease in disease severity was associated with an improvement of patients’ QoL. A significantly lower median value of DLQI scoring was detected at visit 16 (0.0; IQR 0.0–1.0) compared to baseline (16.0; IQR 10.0–20.0). The achievement of DLQI 0/1 (no impact on QoL) was reported in 38.5% and 76.9% at weeks 4 and 16, respectively. Based on the POEM criteria, patients considered their skin condition as clear or almost clear (POEM 0–2) in 42.3% and 79.5% of cases after 4 and 16 weeks of therapy, respectively. According to the recently proposed treatment goals, the vast majority of patients could be considered as responsive to upadacitinib therapy. More than 88% of patients achieved early treatment goals after 4 weeks of treatment and similar response rates, above 92%, for late therapeutic goals were detected after 16 weeks of treatment (Table 3).
Table 3.
Response to upadacitinib therapy evaluated through the achievement of additional treatment goals proposed by De Bruin Weller et al. [19]
| Therapeutic goals | Week 4 | Week 16 |
|---|---|---|
| Patients achieving absolute EASI score ≤ 7, n (%) | 38/39 (97.4) | |
| Patients achieving the reduction of at least 3 points in the absolute itch-NRS score, n (%) | 24/26 (92.3) | |
| Patients achieving the absolute itch-NRS score ≤ 4, n (%) | 37/39 (94.9) | |
| Patients achieving the reduction of absolute DLQI score of at least 4 points, n (%) | 24/26 (92.3) | |
| Patients achieving the absolute DLQI score ≤ 5, n (%) | 38/39 (97.4) | |
| Patients achieving the reduction of absolute POEM score of at least 4 points, n (%) | 23/26 (88.5) | |
| Patients achieving the absolute POEM score ≤ 7, n (%) | 36/39 (92.3) |
DLQI dermatology life quality index, EASI eczema area severity index, NRS numeric rating scale, POEM patient-oriented eczema measure
Upadacitinib Safety
Sixteen of 43 patients (37.2%) reported at least one AE during the study period. A total of 19 AEs was recorded. Most of them were evaluated as mild and they did not cause treatment interruption, except for two cases: one case of metastatic pancreatic carcinoma and one case of thrombophlebitis, permanently discontinuing treatment after 4 and 8 weeks, respectively. The case of metastatic pancreatic carcinoma occurred within the first 4 weeks of upadacitinib treatment in a 72-year-old male subject without any known comorbid condition, whilst the case of thrombophlebitis was reported after 8 weeks of upadacitinib therapy in a 73-year-old male subject affected by two comorbid conditions (hypertension and hypothyroidism), both pharmacologically well controlled. The most common AEs were blood test abnormalities, detected in 16 cases (84.2% of all AEs), that included blood creatine phosphokinase elevation, blood cell count alterations, and decreased hemoglobin levels. AEs of special interest consisted of two cases of papulopustular, facial acne and two cases of Coronavirus Disease 2019 (COVID-19), these latter causing treatment suspension for a period of 4 weeks, with successful re-treatment thereafter. In addition, one case of viral warts was reported.
Discussion
Safety and effectiveness of upadacitinib have been shown in clinical trials, but real-world data are currently limited [18, 20–24]. This prospective, multicentric study provides insights into the short-term safety and effectiveness of 30 mg upadacitinib in the treatment of moderate-to-severe adult AD patients in a real-world setting. In particular, our patient population consisted of difficult-to-treat patients who were unresponsive or had contraindications to several lines of systemic therapies including dupilumab, which in 92.9% of cases failed to obtain a satisfactory clinical response. Notably, patients previously exposed to dupilumab or JAK inhibitors were not included in the upadacitinib clinical trials [18, 20–22].
Rapid and marked reduction of disease severity was observed from the first follow-up visit, corresponding to 4 weeks of treatment. Being a real-world study, intermediate follow-up visits (visit after 4 weeks of treatment, for instance) could be differently scheduled, thus, clinical data might result missing. In those patients evaluated after 4 weeks’ treatment, the clinical response obtained during the first weeks of treatment was maintained over time with further reductions in disease severity scores thereafter. According to therapeutic goals identified by an international expert consensus seeking to define treat-to-target recommendations for AD [19], we also detected the achievement of both early and late therapeutic goals in a large percentage of patients (above 88%). These goals were obtained in a shorter timeframe (within 4 months of treatment) compared to those timepoints (3 and 6 months) proposed by the international expert consensus [19], suggesting an eventual revision of the treat-to-target strategy and goals in the future that considers the upcoming therapeutic options available for the management of AD. At week 4 and week 16, our study revealed elevated percentages of patients achieving EASI 75, EASI 90, and EASI 100, which were higher than clinical trial outcomes [20, 21]. In the Measure Up 1 and Measure Up 2 trials, EASI 75 was obtained by 79.7% and 72.9% of patients treated with 30 mg upadacitinib, respectively, and the association with topical low-to-medium potency corticosteroids did not enhance upadacitinib efficacy in a phase III trial, AD Up (77.1% of EASI 75 response at week 16) [20, 21]. In our cohort, almost all subjects (97.5%) achieved EASI 75 after 16 weeks of treatment. Differences in clinical outcomes between trial setting and our real-world experience were more marked in terms of EASI 90 and EASI 100. In our study, EASI 90 at week 16 was reached by 82.1% of patients, reflecting a self-assessed clear or almost clear condition (POEM 0–2) reported in 79.5% of treated cases.
This high rate of effectiveness could be related to the characteristics of our patient cohort, which consisted of high-need patients with moderate-to-severe AD, who had shown multifailure to systemic therapies, and had no other valid therapeutic options than upadacitinib. Additional inclusion/exclusion criteria of the compassionate use program could possibly have selected a sub-population of great responders. Another factor that might contribute to the better responses documented in this real-world study is the allowed combination of topical potent-ultrapotent corticosteroids based on the physician’s choice. Similarly, real-world outcomes related to other targeted therapies used in AD, such as dupilumab, showed higher response rates in comparison with clinical trials [25–27]. As is commonly seen, in our study the improvement of skin manifestations was associated with a decrease of itch, an amelioration of sleep disturbances, as well as better QoL. In particular, at week 4 about one-third of patients achieved DLQI 0/1, which was obtained by a greater number of patients (76.9% of cases) at week 16. A significant improvement in patients’ QoL was also reported in the Measure Up 1 trial, with 41.1% of patients reaching DLQI 0/1 at week 16 [20]. No upadacitinib-treated patients withdrew from therapy because of ineffectiveness; the two cases of discontinuation were due to the occurrence of AEs. Overall, the safety profile was favorable and in line with clinical trials, showing plasma creatine phosphokinase elevation, anemia, and acne as the most frequently reported treatment-emergent AEs, the majority of which were mild and transient and did not cause treatment withdrawal.
Our study describing the safety and effectiveness of upadacitinib in patients unresponsive to dupilumab provides evidence with meaningful clinical implications as no data are currently available on dupilumab-exposed patients [5, 28, 29]. Long-term data on a larger patient population will be of great interest to confirm the persistence of treatment response and safety in upadacitinib-treated patients [30].
Acknowledgements
The authors received no external funding to perform this study. The study drug (upadacitinib) was provided by the manufacturer (AbbVie) through an expanded pre-approval access program. AbbVie was provided courtesy pre-submission proof reading with final content that was determined by the authors.
ACCURATE Group: Alberto Maria Bertoldi, Gabriella Fabbrocini, Maria Concetta Fargnoli, Giampiero Girolomoni, Aurora Parodi, Pietro Quaglino.
Declarations
Funding
None.
Conflict of interest
None declared for the authors with the exception of Ketty Peris who has served on the advisory board and received honoraria for lectures and/or research grants for Abbvie, Almirall, Lilly, Galderma, Leo Pharma, Pierre Fabre, Novartis, Sanofi, Sun Pharma, Janssen; Andrea Chiricozzi who served as advisory board member and consultant and has received fees and speaker's honoraria or has participated in clinical trials for AbbVie, Almirall, Leo Pharma, Lilly, Janssen, Novartis, and Sanofi Genzyme; Antonio Costanzo who served as advisory board member and consultant and has received fees and speaker's honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, Fresenius Kabi, Leo Pharma, Lilly, Janssen, Novartis, Sanofi Genzyme and UCB Pharma.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Not applicable.
Footnotes
The members of ACCURATE Group are listed in Acknowledgements.
Ketty Peris and Antonio Costanzo contributed equally to this manuscript.
Contributor Information
Andrea Chiricozzi, Email: chiricozziandrea@gmail.com.
ACCURATE Group:
Alberto Maria Bertoldi, Gabriella Fabbrocini, Maria Concetta Fargnoli, Giampiero Girolomoni, Aurora Parodi, and Pietro Quaglino
References
- 1.Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- 2.Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 3.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 4.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 5.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab versus placebo in AD. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 6.Thaçi D, Simpson EL, Deleuran M, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2) J Dermatol Sci. 2019;94:266–275. doi: 10.1016/j.jdermsci.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 7.De Wijs LEM, Bosma AL, Erler NS, et al. Effectiveness of dupilumab treatment in 95 patients with atopic dermatitis: daily practice data. Br J Dermatol. 2020;182:418–426. doi: 10.1111/bjd.18179. [DOI] [PubMed] [Google Scholar]
- 8.Faiz S, Giovannelli J, Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019;81:143–151. doi: 10.1016/j.jaad.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Kraus CN, Patel KG, et al. Real-world experience of dupilumab treatment for atopic dermatitis in adults: a retrospective analysis of patients' records. Int J Dermatol. 2020;59:253–256. doi: 10.1111/ijd.14573. [DOI] [PubMed] [Google Scholar]
- 10.Gori N, Chiricozzi A, Malvaso D, et al. Successful combination of systemic agents for the treatment of atopic dermatitis resistant to dupilumab therapy. Dermatology. 2021;237:535–541. doi: 10.1159/000512890. [DOI] [PubMed] [Google Scholar]
- 11.Chiricozzi A, Gori N, Di Nardo L, et al. Therapeutic impact and management of persistent head and neck atopic dermatitis in dupilumab-treated patients. Dermatology. 2021 doi: 10.1159/000519361. [DOI] [PubMed] [Google Scholar]
- 12.Olesen CM, Holm JG, Nørreslet LB, et al. Treatment of atopic dermatitis with dupilumab: experience from a tertiary referral centre. J Eur Acad Dermatol Venereol. 2019;33:1562–1568. doi: 10.1111/jdv.15609. [DOI] [PubMed] [Google Scholar]
- 13.Armario-Hita JC, Pereyra-Rodriguez J, Silvestre JF, et al. Treatment of moderate-to-severe atopic dermatitis with dupilumab in real clinical practice: a multicentre, retrospective case series. Br J Dermatol. 2019;181:1072–1074. doi: 10.1111/bjd.18041. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Villaverde R, Dominguez-Cruz J, Armario-Hita JC, et al. Fifty-two week follow-up safety and effectiveness results of dupilumab treatment of moderate-to-severe atopic dermatitis from a retrospective, multicentric series. Dermatol Ther. 2019;32:e12931. doi: 10.1111/dth.12931. [DOI] [PubMed] [Google Scholar]
- 15.Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84:1339–1347. doi: 10.1016/j.jaad.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 16.He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20:181–192. doi: 10.1007/s40257-018-0413-2. [DOI] [PubMed] [Google Scholar]
- 17.Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) MC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 19.De Bruin-Weller M, Biedermann T, Bissonnette R, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402. doi: 10.2340/00015555-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 21.Reich K, Teixeira HD, De Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169–2181. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 22.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao DX, Kahn JS, Cohen SR, et al. Results of switching from tofacitinib to upadacitinib in patients with atopic dermatitis: a retrospective medical record review. Dermatitis. 2021;32:e165–e166. doi: 10.1097/DER.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 24.Licata G, Gambardella A, Tancredi V, et al. Face atopic dermatitis resistant to dupilumab: a case series of three patients successfully treated with upadacitinib. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17705. [DOI] [PubMed] [Google Scholar]
- 25.Nettis E, Ferrucci SM, Ortoncelli M, et al. Use of dupilumab for 543 adult patients with moderate-to-severe atopic dermatitis: a multicenter, retrospective study. J Investig Allergol Clin Immunol. 2020 doi: 10.18176/jiaci.0641. [DOI] [PubMed] [Google Scholar]
- 26.Fargnoli MC, Esposito M, Ferrucci S, et al. Real-life experience on effectiveness and safety of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J Dermatolog Treat. 2021;32:507–513. doi: 10.1080/09546634.2019.1682503. [DOI] [PubMed] [Google Scholar]
- 27.Tavecchio S, Angileri L, Giuffrida FP, et al. Efficacy of dupilumab on different phenotypes of atopic dermatitis: one-year experience of 221 patients. J Clin Med. 2020;9:2684. doi: 10.3390/jcm9092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40–52. doi: 10.1016/S0140-6736(15)00388-8. [DOI] [PubMed] [Google Scholar]
- 29.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTYAD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg JI, De Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week-52 AD Up study results. J Allergy Clin Immunol. 2021;149(3):977–987.e14. doi: 10.1016/j.jaci.2021.07.036. [DOI] [PubMed] [Google Scholar]