Abstract
Cellular senescence is a ubiquitous process with roles in tissue remodelling, including wound repair and embryogenesis. However, prolonged senescence can be maladaptive, leading to cancer development and age-related diseases. Cellular senescence involves cell-cycle arrest and the release of inflammatory cytokines with autocrine, paracrine and endocrine activities. Senescent cells also exhibit morphological alterations, including flattened cell bodies, vacuolization and granularity in the cytoplasm and abnormal organelles. Several biomarkers of cellular senescence have been identified, including SA-βgal, p16 and p21; however, few markers have high sensitivity and specificity. In addition to driving ageing, senescence of immune and parenchymal cells contributes to the development of a variety of diseases and metabolic disorders. In the kidney, senescence might have beneficial roles during development and recovery from injury, but can also contribute to the progression of acute kidney injury and chronic kidney disease. Therapies that target senescence, including senolytic and senomorphic drugs, stem cell therapies and other interventions, have been shown to extend lifespan and reduce tissue injury in various animal models. Early clinical trials confirm that senotherapeutic approaches could be beneficial in human disease. However, larger clinical trials are needed to translate these approaches to patient care.
Subject terms: Senescence, Biomarkers, Chronic kidney disease
Cellular senescence has beneficial functions in embryonic development, wound healing and tumour suppression but can also be maladaptive, contributing to cancer development and disease. This Review describes the mechanisms, hallmarks and consequences of senescence, as well as the therapeutic potential of senescence-targeting interventions.
Key points
Cellular senescence regulates physiological and homeostatic processes, particularly during embryonic development and wound healing, but can also be a pathological process that contributes to ageing, various diseases and metabolic disorders.
Senescent cells are characterized by morphological alterations including large, flat bodies and organelle abnormalities, as well as loss of physiological functions, an inability to proliferate and the senescence-associated secretory phenotype.
SABG, p21 and p16 are the most commonly used senescence markers but have limitations; novel non-invasive approaches are needed to detect cellular senescence with high sensitivity and specificity in vitro.
Cellular senescence is involved in the pathogenesis of chronic kidney disease and acute kidney injury, but also seems to have a protective role in the early stages of acute kidney injury.
Senescence-targeting interventions, including senolytic drugs conjugated to antibodies against β2-microglobulin, chimeric antigen receptor T cells and anti-ageing vaccines, show promise for clinical application.
Clinical trials are needed to assess the safety and efficacy of senotherapeutic approaches, optimize treatment regimens and develop more individualized and standardized treatment strategies.
Introduction
The phenomenon of cellular senescence was discovered in the 1960s in human diploid cell strains that had exhausted their replicative potential1. Senescence is characterized by cell-cycle arrest in the G1 or possibly G2 phase, which prevents the proliferation of damaged cells2,3. By contrast, cellular quiescence, a reversible growth arrest state secondary to scarce nutrition and growth factors, takes place in the G0 phase4. Cellular senescence occurs during embryonic development and can be induced by cellular impairment, including DNA damage, telomere shortening or dysfunction, oncogene activation or loss of tumour suppressor functions, epigenetic changes and organelle damage5.
The principal cause of senescent stress is DNA damage6, which activates the DNA damage response (DDR) and the canonical p53–p21 pathway7 (Fig. 1). P21 (also known as cyclin-dependent kinase inhibitor 1) inhibits cyclin–cyclin-dependent kinase complexes that block the formation of the DREAM complex, which represses cell-cycle genes by binding their homology region8. Unlike DDR-induced senescence, epigenetic alterations cause senescence mainly via the p16–RB pathway9. p16 (also known as cyclin-dependent kinase inhibitor 2A) can inhibit the formation of cyclin D–CDK4/6 complexes and thereby prevent phosphorylation of RB and promote formation of the RB–E2F complex, which inhibits the transcription of cell-cycle genes8. Evidence suggests that p21 is mainly activated early during the evolution of senescence, whereas p16 maintains cellular senescence10.
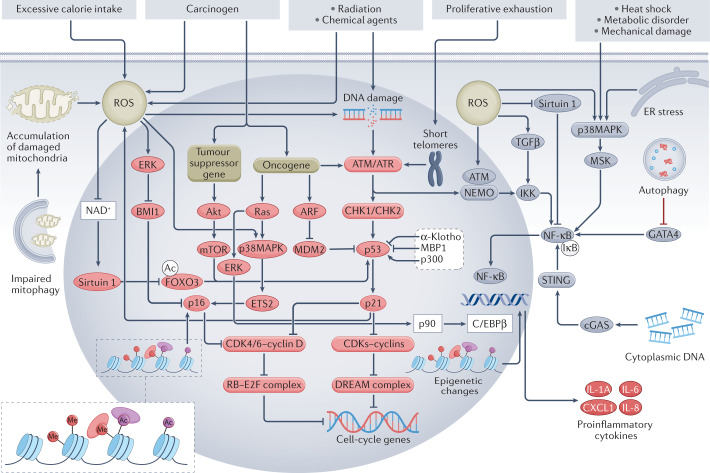
Fig. 1. Mechanisms of cellular senescence and the SASP.
DNA damage secondary to radiation, chemical agents or accumulation of reactive oxygen species (ROS) is the main cause of cellular senescence-inducing stress. Proliferation-induced telomere shortening can also activate the DNA damage response, which in turn leads to activation of the p53–p21 pathway, inhibition of cyclin-dependent kinase (CDK)–cyclin complexes and formation of the DREAM complex, which represses cell-cycle genes, leading to cell-cycle arrest and senescence. Activated p21 can also induce further ROS production, forming a vicious circle. In addition, impaired mitophagy can lead to mitochondrial dysfunction and excessive ROS production. ROS can induce senescence independently of the DNA damage response by activating the p16–RB pathway via activation of ERK, which inhibits BMI1 and thereby enables activation of p16, and by activating p38MAPK signalling, which upregulates ETS2 and in turn activates p16, and by inhibiting NAD+, which leads to reduced expression of sirtuin 1 and activation of FOXO3, which activates p53. P16 inhibits the formation of CDK4/6–cyclin D complexes and thereby promotes formation of the RB–2F complex, which inhibits the transcription of cell-cycle genes. Oncogene activation can activate the p53–p21 pathway not only via the DNA damage response, but also via the ARF–MDM2 signalling. In addition, oncogene activation can activate the p16–RB pathway via p38MAPK signalling. Loss of tumour suppressor genes induces senescence via Akt–mTOR signalling, which activates p53. Other factors that regulate the p53–p21 pathway include α-Klotho, MBP1 and p300. Epigenetic alterations such as methylation (Me) and acetylation (Ac) can induce senescence through the p16–RB pathway. NF-κB signalling regulates the senescence-associated secretory phenotype (SASP) and together with the transcription factor C/EBPβ, co-activates promoters of SASP genes, such as those that encode pro-inflammatory cytokines. The DNA damage response protein ATM together with NEMO activates the NF-κB–C/EBPβ signalling pathway. ROS can activate the SASP not only by promoting nuclear translocation of NEMO and activation of ATM, but also by inhibiting sirtuin 1 and activating p38MAPK and TGFβ, which in turn activate NF-κB. Heat shock, metabolic disorders, mechanical damage and endoplasmic reticulum (ER) stress can also activate the NF-κB–C/EBPβ pathway via p38MAPK signalling. Cytoplasmic DNA accumulation can trigger aberrant activation of cGAS-STING cytoplasmic DNA sensors and promote the SASP via activation of NF-κB. Impairment of autophagy hampers degradation of the transcription factor GATA4, which activates NF-κB and leads to initiation of the SASP. Notably, oncogenic Ras can activate C/EBPβ via ERK–p90 signalling and histone epigenetic changes can regulate the SASP independently of the NF-κB–C/EBPβ pathway. ARF, ADP-ribosylation factor; BMI1, B lymphoma Mo-MLV insertion region 1 homologue; cGAS, cyclic GMP–AMP synthase; CHK, checkpoint kinase; ERK, extracellular regulated protein kinases; ETS2, E26 transformation-specific proto-oncogene 2; E2F, early 2 factor; FOXO3, forkhead box protein O3; IKK, IκB kinase; MDM, mouse double minute 2; MSK, mitogen- and stress-activated protein kinase; NEMO, NF-κB essential modulator; STING, stimulator of interferon genes.
The senescence-associated secretory phenotype (SASP) is an important feature of senescent cells that comprises the release of numerous cytokines, chemokines, growth factors and proteases11, which are sometimes enclosed within microparticles, into the extracellular environment. Many cell types also release extracellular vesicles, which contain cellular contents, including proteins, lipids and nucleic acids. Extracellular vesicles have a role in inter-cellular communication, and altered extracellular vesicle cargoes are important components of the SASP12. Through the release of SASP factors, senescence can modulate pathways in neighbouring cells and tissues as well as at remote sites. Notably, senescent cells that are induced by different stress stimuli may manifest distinctive SASP components13.
Cellular senescence has beneficial biological functions in the regulation of embryonic development, wound healing, resolution of fibrosis and tumour suppression. However, prolonged senescence can result in deleterious sequelae, including tumour development, chronic inflammation, immune deficit and stem cell exhaustion. Interest in cellular senescence and in senescence-modulating interventions is increasing owing to observations that in addition to driving ageing, cellular senescence has important roles in the pathogenesis of chronic diseases, including osteoporosis, metabolic syndrome, type 2 diabetes mellitus, cancer, reproductive ageing, atherosclerosis, neurodegeneration, glaucoma and chronic kidney disease (CKD). In this Review, we describe the mechanisms, hallmarks and consequences of cellular senescence, as well as the therapeutic potential of senescence-targeting interventions.
Senescence in physiology and pathology
Diverse types of stimuli trigger senescence, reflecting its spectrum of roles under different conditions (Table 1). Developmental senescence and replicative senescence (which occurs secondary to telomere shortening) occur under physiological conditions during embryogenesis and ageing, respectively, whereas other types of senescence are often induced by pathological stressors, including tumorigenesis, diabetes mellitus, chemotherapy or radiation.
Table 1.
Inducers of cellular senescence
| Inducer | Senescence pathway |
|---|---|
| Replicative stress | Cell proliferation leads to telomere shortening, which hinders DNA copying and can activate the DNA damage response, resulting in replicative senescence. |
| Oncogene activation | Activation of oncogenes can directly induce the DNA damage response or activate MDM2–p53–p21 or p38AMPK–p16 signalling pathways, which lead to cellular senescence. |
| Loss of tumour suppressor gene | Loss of a tumour suppressor gene can induce cellular senescence via activation of Akt–mTOR–p53 signalling. |
| Development | Senescence markers can be observed in the limbs, nervous system, gut endoderm and mesonephros; p21 has an important role in development-associated senescence. |
| Epigenetic influences | Epigenetic modification of histones induces senescence by activating p16–RB signalling. |
| Mitochondrial dysfunction | Mitochondrial dysfunction leads to overproduction of reactive oxygen species, which cause DNA damage and activate the DNA damage response or ERK–p16–RB signalling. |
| Chemotherapeutic drugs or ionizing radiation | These therapies induce DNA damage and the DNA damage response, which lead to cellular senescence. |
Embryogenesis and development
In 2006, a transcript of INK4b, which encodes a cyclin-dependent kinase (CDK) inhibitor that blocks progression of the cell cycle beyond the G1 phase, was detected in the roof plate of the developing chicken hindbrain14, implicating senescence in the regulation of embryonic development. Subsequently, p66Shc, which regulates oxidative stress-induced senescence, was found to mediate early cleavage arrest in failed bovine embryonic development, suggesting that cellular senescence might fine-tune embryogenesis to prevent the continued development of poor-quality embryos15.
In the mammalian embryo, senescence occurs at multiple locations, including the limbs, nervous system and gut endoderm16. In the developing kidney, accumulation of senescent cells signals immune cells to facilitate mesonephros regression through macrophage-mediated phagocytosis of these senescent cells17. Markers of senescence, including senescence-associated β-galactosidase (SABG), p21, p27 (encoded by CDKN1B) and p15 (encoded by CDKN2B), were detected in mouse mesonephros at embryonic day 12.5 to 14.5 (ref.17). Knocking down p21 in mouse embryos results in developmental abnormalities16. Senescence has also been shown to regulate the development of multiple tissues in zebrafish embryos18.
Importantly, senescent decidual cells in the mammalian endometrium can secrete multiple canonical implantation factors and form a suitable environment for embryonic implantation19. Cellular senescence may therefore have roles in pruning and remodelling developing systems and modulating their microenvironment, and is thus required to ensure fetal integrity.
Wound healing
Wound healing is a physiological response for repairing tissue injury that involves inflammation, new tissue formation and tissue remodelling. Cellular senescence has an important role throughout the wound-healing process. In cutaneous wound healing, the matricellular protein CCN1 can induce fibroblast or myofibroblast senescence and thereby reduce fibrosis via activation of the DDR and ROS–p16 signalling20. Similarly, in corneal wound healing, fibroblast senescence manifests as an anti-fibrogenic phenotype, with reduced responses to fibroblast growth factor 2 (FGF2; also known as basic FGF) and platelet-derived growth factor-BB, and increased expression of MMP1, MMP3 and MMP13 (ref.21).
Conversely, cellular senescence can interfere with wound healing. For example, inflammation-mediated cellular senescence decreases fibroblast proliferation and migration, which is vital during new tissue formation22. Tenovin-1 treatment induced senescence of cultured astrocytes and thereby impaired their wound-healing activity23. Moreover, senescent lung fibroblasts induced G2/M cell-cycle arrest of alveolar epithelial cells, leading to aberrant wound repair and re-epithelialization24. Senescent mesenchymal stem cell (MSC)-derived extracellular vesicles also inhibit wound healing via a mechanism that involves downregulation of miR-146a25. In diabetes, oxidative stress activates caveolin 1–PTRF signalling, which leads to induction of cellular senescence via the p53–p21 pathway26. The resulting diabetes-induced senescence26, together with a CXCR2-enriched SASP27, impair wound healing. These findings indicate that although transient cellular senescence can promote tissue repair, the prolonged presence of senescent cells can hamper this process.
Cancer
Cellular senescence can have beneficial and detrimental effects in cancer (Fig. 2). Oncogene activation, loss of anti-oncogenes and irreparable DNA damage not only induce apoptosis but also elicit cellular senescence to prevent tumour initiation. Transient insults may result in cell-cycle arrest, which can prevent carcinogenic mutations from being passed on to the next generation of cells and accelerate immune clearance.
Fig. 2. Cellular senescence in cancer.
Most carcinogenic mutations induce senescence through the p53–p21 and p16–RB pathways, although some mutations activate p21 directly. Reactive oxygen species (ROS)-induced senescence in cancer cells is mainly dependent on p16, p21 and/or p27. Senescence-induced cell-cycle arrest can prevent mutations from being passed on to the next generation of cells and accelerate immune clearance, resulting in suppression of tumurigenesis. However, the senescence-associated secretory phenotype (SASP) also contributes to a pro-inflammatory and growth-stimulatory microenvironment that can promote tumour development.
DDR signalling is the main mechanism of oncogene-induced senescence. Oncogene activation or irreparable DNA damage induce activation of ataxia telangiectasia mutated (ATM) and checkpoint kinase 2 (CHK2), leading to phosphorylation of histone H2AX and p53 and activation of p53–p21 signalling28. Additional pathways, including NLRP6–NF-κB–p14ARF–MDM2 and miR-203–ITPKA–MDM2, can also activate p53–p21 signalling. In breast carcinoma cells, overexpression of oncogenic ERBB2 elicits senescence by upregulating p21 independently of p53 (ref.29). Loss of anti-oncogenes such as PTEN can induce senescence through the Akt–mTOR–p53 and p19ARF–MDM2–p53 signalling pathways30.
Depletion of CSN5 induced senescence in p53-null cells, suggesting that alternative pathways of senescence exist31. Further studies demonstrated that the p16–RB and TAp63–p21–RB pathways are involved in oncogene-induced senescence and suppression of tumorigenesis32,33. In mice, inactivation of heat shock factor 4 (HSF4) enhanced senescence and suppressed tumorigenesis independently of p53 via a mechanism that is dependent on p21 and p27 (ref.34). Reactive oxygen species (ROS)-induced senescence in cancer cells is dependent on p16INK4A, p21Waf1/Cip1 and/or p27Kip1, but does not require p53 (ref.35).
The high-mobility group box-containing protein 1 (HBP1) transcription factor is a novel activator of p21 (and thereby senescence) that acts by attenuating degradation of p53 or by regulating Wnt–β-catenin–EZH2 signalling independently of p53 (ref.36). Wnt signalling also has a role in inducing the senescence of thyroid cancer cells37. In Ras-induced breast cancer, the growth arrest and DNA damage 45a protein was found to suppress tumorigenesis by inducing senescence and apoptosis via p38 MAPK signalling38. Other mechanisms, including IL-6–STAT3 signalling and autophagy, also have roles in tumorigenesis and senescence, but how they contribute requires further investigation.
Although senescence can prevent cancer by inducing cell-cycle arrest, evidence suggests that the SASP-evoked chronic inflammatory microenvironment can promote tumorigenesis. For example, knockdown of polypyrimidine tract-binding protein 1 (PTBP1) in a mouse model inhibited the SASP and prevented the growth of inflammation-driven advanced liver tumours39. The Ras oncogene-activated chemokine growth-regulated alpha protein can reprogram the stromal microenvironment by inducing senescence of fibroblasts and thereby promote ovarian tumorigenesis40. p38–MAPK and p44–p42 MAPK–ERK signalling also foster a pro-inflammatory and growth-stimulatory microenvironment41 by mediating post-transcriptional regulation of SASP mRNAs, including IL-6, IL-8, GM–CSF and CCL20 mRNA42. IL-6 induces increases in suppressive myeloid cells, which inhibit antitumour T cell responses43. In addition, the SASP factors CXCL1 and matrix metalloproteinase 14 (MMP14) stimulate ovarian and hepatocellular carcinoma by regulating p65 and facilitating invasion, respectively44, whereas IL-6, MMP3, MMP9 and CXCL10 contribute to the development of squamous cell skin carcinoma41. The pro-tumorigenesis SASP factor osteopontin is regulated by C/EBPβ and the transcription factor Myb45, and has roles in the regulation of processes that are critical for cancer progression, including immune responses, cell adhesion and cell migration. Moreover, colon tumour cells can secrete phospholipase-D2, which induces senescence of neighbouring fibroblasts46. Senescent fibroblasts in turn release the SASP factors HGF, MIF and CCL2, which activate Wnt signalling and increase the stem cell properties of colon tumour cells46.
The metalloproteinase inhibitor TIMP1 is an important molecular switch that determines the effects of senescence in prostate cancer. TIMP1 inactivation promotes metastasis through the activation of MMPs, which leads to upregulation of secreted factors that promote tumour progression, including FGF1, growth/differentiation factor 15 and insulin-like growth factor-binding protein 5 (ref.47). Other factors such as COX2 also regulate the SASP composition and contribute to tumorigenesis48.
Senescence also has important roles in the initiation, progression, chemoresistance and prognosis of kidney cancers. Repression of phospholipase A2 receptor (PLA2R1) by the oncogenic HIF2α–MYC pathway increases kidney cell resistance to senescence and accelerates the growth of renal cell carcinoma (RCC)49. Poly(C)-binding protein 4 (PCBP4) and p53-bound enhancer regions 2 (P53BER2) have been identified as renal tumour suppressors that induce senescence by upregulating p53 expression50,51. RCC is characterized by high expression of xeroderma pigmentosum complementation group-F (XPF) and endoglin, which increase its chemoresistance. Knockdown of XPF or endoglin increased the senescence and reduced the chemoresistance of renal cancer cells52,53. Conversely, inactivation of TP53 (which encodes p53) increased senescence and promoted an inflammatory microenvironment, which facilitates RCC aggressiveness and metastasis54. The mechanisms that tip the balance between the pro-tumorigenic and anti-tumorigenic functions of senescence are unclear and likely cell type specific. Moreover, different levels of oncogene activation cause disparate senescence trajectories such that transient senescence might be tumour suppressive55, whereas prolonged or permanent senescence might promote proliferation, stemness, migration and invasion of cancer cells56. Additional studies are needed to identify the stage at which senescence might become maladaptive and warrant inhibition.
Immunosenescence
In healthy individuals, immunosenescence begins at around the age of 50 years57 and results in impaired vaccine responses and increased susceptibility to infection, autoimmunity and cancer, as well as chronic inflammation. A reduction in autophagy is an important mechanism that contributes to immunosenescence. Impairment of autophagy hinders the degradation of damaged mitochondria, resulting in the accumulation of ROS and DNA damage58.
Patients with CKD show accelerated immunosenescence that mimics that of elderly individuals59. The resulting chronic inflammation is associated with loss of kidney function60 and complications such as cardiovascular disease and infections61. However, immunosenescence can be beneficial in settings where immune cell activation is undesirable. For example, immunosenescence of CD4+ T cells in transplant recipients facilitates acceptance of kidney allografts62.
In people aged >60 years, naive T cells decline, whereas memory T cells increase and show loss of CD28 and elevated expression of the senescence markers CD57 and KLRG1 (ref.63). Although age-related changes are more common in CD8+ T cells, an increased frequency of highly differentiated CD4+ T cells that express NKG2D has been reported in elderly people64. Senescent T cells express pro-inflammatory cytokines, exhibit shortened telomeres63 and might negatively impact human longevity. The offspring of centenarians, who presumably carry longevity-favouring genes, have fewer senescent T cells than age-matched controls65. Increased expression of T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain is associated with T cell exhaustion and senescence66.
Senescent B cells are also prevalent in the elderly. Memory B cells fill the immunological space, whereas naive B cells markedly decrease, resulting in a reduced ability to respond to new pathogens67. Senescent B cells also have a decreased capacity for somatic hypermutation68 and thus show blunted antibody responses to infectious agents. The decrease in naive B cells is caused mainly by a decline of B cell lymphopoiesis, secondary to increased apoptosis of B cell precursors, impaired responsiveness to IL-7 and decreased potency of haematopoietic stem cells69. Moreover, senescent B cells, characterized by reduced expression of CD23, CD21 and CD35, accumulate in the bone marrow and suppress B cell lymphopoiesis in aged mice70.
Although the number of natural killer (NK) cells does not necessarily decline with ageing, their function deteriorates owing to the altered expression of various cytokines71, which reduces their ability to recognize infected and malignant target cells.
Senescent macrophages express high levels of the senescence-related markers p16INK4a and SABG72. They release pro-inflammatory cytokines that promote chronic inflammation and have an overabundance of ROS, which reduces their propensity for efferocytosis73. Expression of activating transcription factor 3 (ref.74) and bromodomain-containing protein 4 (ref.75) may contribute to macrophage senescence. Intriguingly, mature macrophages share inherent phenotypic similarities with senescent cells in terms of their signalling pathways, gene expression, metabolism and levels of organelles such as lysosomes76. The suitability of p16INK4a and SABG as markers for macrophage senescence has been questioned because these markers are also upregulated in response to stimuli that induce macrophage polarization to a M2 phenotype, such as IL-4 and IL-13 (ref.77). Furthermore, activated macrophages in atherosclerotic lesions resemble senescent cells and show lipid accumulation, SASP and a persistent DDR75. Hence, a senescence-like phenotype in macrophages might constitute a physiological activation state adopted in response to challenge rather than true senescence. Notably, cancer cells with chemotherapy-induced senescence undergo transcriptional changes linked to phagocytosis and can engulf adjacent cells78. Clearly, the interplay between truly senescent cells and senescent-like macrophages warrants additional studies.
Stem cell senescence
Stem cells can release paracrine factors to repair injured tissues and organs and have an important role in regeneration. However, risk factors such as ageing, irradiation, and metabolic disorders can induce stem cell senescence (SCS) and dysfunction, which contributes to age-related diseases79. For example, senescent p16INK4a-positive MSCs contribute to osteoarthritis by secreting DKK1, which triggers premature bone remodelling and cartilage degradation80. Senescent MSCs also lose their cartilage regenerative properties.
The mechanisms that underlie SCS are complex. Stressors including ROS can promote MSC senescence induced by ageing, expansion and hypoxia81, and transforming growth factor β1 (TGFβ1) can induce SCS by increasing ROS production and upregulating p16INK4A and p21 (ref.82). In addition, non-coding RNAs regulate SCS. For example, miR-152 induces human dental pulp SCS83, whereas the long non-coding RNA Hnscr attenuates hypothalamic SCS84. Haematopoietic SCS is regulated by the endogenous Ewing sarcoma gene85. Low mitochondrial NAD+ levels contribute to bone-marrow MSC senescence, which may be associated with reduced expression of sirtuin 1 and sirtuin 3 (refs.86,87). Notably, ageing is linked to compensatory overexpression of sirtuin 6 in bone marrow MSCs, which decreases SCS and confers oxidative stress resistance88. Several transcription factors also regulate SCS of different origins. NANOG delays senescence in hair follicle-derived MSCs89, GATA6 and SOX11 regulate bone-marrow SCS90, MDM2–p53 signalling has a vital role in epidermal SCS91 and the ROR2–STK4–FOXO1–SMS1 pathway regulates dental pulp SCS92. Endometrial SCS, which is associated with recurrent pregnancy loss, may be mediated by SERPINB2–sonic hedgehog signalling93.
The role of autophagy in hypoxia and d-galactose-induced SCS is controversial. Some studies have shown that autophagy inhibits MSC senescence94; however, high glucose-induced autophagy increases bone-marrow SCS95. Thus, the role of autophagy in SCS likely depends on the context. We found that fat-tissue-derived MSC senescence was elevated in patients with obesity96 but not in patients with diabetic kidney disease compared with age-matched healthy individuals97. Hence, SCS is not necessarily a uniform finding in human disease.
Redox control of senescence
Oxidant stress caused by overproduction of ROS and/or deficiency of antioxidant defences can induce cellular senescence. Many factors can interrupt redox homeostasis and hence induce DNA damage, including high glucose, radiation and expression of oncogenes98. For example, autophagy-deficient melanocytes and keratinocytes with an overabundance of ROS become senescent99,100 and lactose and fumarate can induce fibroblast and renal cancer cell senescence by inducing oxidative stress101. Downregulation of cathepsin-D not only increases ROS generation, but also inhibits the activity of nuclear factor erythroid 2-related factor 2 (NRF2)102, which has an important role in antioxidant defence and prevention of senescence. The integral membrane protein caveolin 1 inhibits the activity of the antioxidant enzyme thioredoxin reductase 1, inducing ROS accumulation and cellular senescence103. In turn, oxidative stress has a positive feedback effect on caveolin 1 by activation of p38MAPK signalling, forming a vicious cycle104. Loss of the anti-apoptotic protein BCL-2 also disrupts redox homeostasis and impairs angiogenic function in senescent endothelial cells105. Interestingly, H2 produced by gut bacteria can scavenge the hydroxyl radical and relieve oxidative stress, resulting in suppression of cellular senescence106. Many other factors also modulate the senescence process by regulating cellular redox status.
In addition to directly inducing DDR-mediated senescence signalling, ROS can regulate Akt signalling, an important pathway to cellular senescence, by modulating the expression of several microRNAs including miR-181a and miR-182 (ref.107). Redox homeostasis is also key to maintaining the integrity of telomeres108. Ultimately, telomere shortening and loss hinder DNA copying and lead to replicative cellular senescence.
Elevated oxidative stress can also induce senescence accompanied by nuclear shape alteration (and thereby DDR) through overexpression of lamin B1 (ref.109) and can modulate the SASP by regulating the homeostasis of the SASP initiators, IL-1α and Ca2+ (refs.110,111). In patients with systemic sclerosis, oxidative stress induces fibroblast senescence and contributes to pro-fibrotic and pro-inflammatory phenotypes112. By contrast, senescent fibroblasts exhibit antifibrotic phenotypes during normal wound healing. This discrepancy might be the result of the intrinsic characteristics of dermal fibroblasts in patients with systemic sclerosis, which have high intracellular ROS levels, reduced proliferation rate, impaired bioenergetic functions and increased DNA damage and DDR compared with healthy fibroblasts112. Conceivably, differing features such as ROS levels may determine the propensity of fibroblast senescence to be linked to a pro-fibrogenic or anti-fibrogenic phenotype.
Clearly, increased oxidative stress has a key role in the development of cellular senescence. The ability of exogenous antioxidant strategies to attenuate senescence in selected cells without impairing fibroblast function remains to be explored.
Cellular alterations in senescence
Morphological alterations
Cellular senescence is characterized by morphological alterations that are controlled by molecular changes such as increased p53 expression and inhibition of RB phosphorylation. Generally, senescent cells have a larger and flatter body than healthy cells with a spread shape113. These changes might be due to cell-cycle arrest in the G1/G2 phase following the generation of intracellular content for cell division and its accumulation in the cytoplasm and nuclei. Enlarged and flat cells are associated with enhanced formation of actin stress fibres owing to upregulation of cofilin 1 (ref.114).
Senescent cells are also characterized by large nuclei, multi-nuclei115 and chromatin reorganization, including chromosome condensation, redistribution and the formation of heterochromatic foci, possibly secondary to loss of lamin B1 from the inner surface of the nuclear envelope116. Vacuolization, granularity and intracellular debris in the cytoplasm of senescent cells have also been reported117,118. Some organelles show abnormal morphological changes, such as swollen mitochondria, increased lysosome numbers119 and endoplasmic reticulum expansion, which is regulated by the transcription factor ATF6α120. Senescent morphology correlates with enhanced actin stress fibres and redistribution of focal adhesion plaques, which may permit the enlarged cells to adhere and spread and contribute to their capacity for tumour suppression121.
In addition, specialized senescent cells may exhibit distinct morphological characteristics. For example, senescent auditory cells are ramified122, senescent microglia have large bodies with a reduced number of processes123 and senescent bone marrow-derived MSCs have a polygonal phenotype124. Kidney cells seem to share typical phenotypic characteristics of senescence. In aged kidneys, tubular cells, and to a lesser degree glomerular and interstitial cells, show as a diminished proliferative response that correlates with markers of cellular senescence, including p16INK4a, SABG and telomere shortening125. Senescent glomerular endothelial cells drive podocyte injury, inducing reorganization of the F-actin cytoskeleton and a decreased number of focal adhesion processes126. Cellular morphological characteristics in different organ systems likely reflect prevailing local mechanisms of cellular damage.
Functional alterations
Senescent cells have abnormal function and are unable to proliferate owing to their stable cell-cycle arrest in the G1/G2 phase. Senescence thereby reduces the number of cells that are available to execute normal tissue functions. An increased rate of senescent cell production (due to increased exposure to stressors) and a reduced rate of senescent cell removal (due to deregulated immunosurveillance together with pro-survival signals and resistance to apoptosis) lead to the accumulation of these cells in organs with ageing127. In turn, senescent cells promote degeneration, ageing, systemic chronic inflammation — termed inflammaging — and age-related diseases due to the release of SASP factors and the resulting spread of senescence to previously non-senescent cells128. Congruently, senescent cells accumulate at the sites of age-related degenerative or preneoplastic pathology129. In addition to the SASP, the complement system contributes to inflammaging by recruiting macrophages and increasing the release of ROS, TNF and IL-6 (ref.130), which are important inducers of senescence.
SASP factors can exert both beneficial and deleterious functions. Beneficial effects of SASP factors include the roles of CXCL5 in embryonic development131, PDGF-AA in tissue repair132, IL-6 in cellular reprogramming133, MMP1 and MMP33 in fibrosis resolution20 and the anti-tumorigenic properties of CCL2 (ref.134). Notably, SASP factors also recruit immune cells to clear pre-malignant senescent cells. This process of senescence surveillance is an important contributor to the anti-tumorigenic properties of senescent cells135. Deleterious effects of SASP factors include the roles of IL-8, IL-10 and TNF in inflammation136, PDGF-BB in fibrogenesis137 and MMP1 and MMP3 in tumorigenesis138. Some SASP cytokines such as IL-6, IL-8 and CCL2 also promote vascular smooth muscle cell calcification and impair insulin sensitivity139,140.
The SASP is regulated via complex DDR-dependent and DDR-independent mechanisms (Fig. 1). Activated NF-κB and the transcription factor, CCAAT/enhancer-binding protein-β (C/EBPβ) co-activate promoters of SASP genes141. However, NF-κB-independent SASP regulation mechanisms also exist. For example, cytoplasmic DNA accumulation can cause aberrant activation of cGAS-STING cytoplasmic DNA sensors and promote SASP by induction of interferon-β142. Downregulation of the lamin B receptor, which regulates heterochromatin organization, is necessary for the generation of cytoplasmic DNA143. Factors that are linked to infections, such as lipopolysaccharide and SARS-CoV-2 S antigen, can increase the release of pro-inflammatory, pro-fibrotic and pro-apoptotic SASP factors by senescent cells144.
Hallmarks and markers of senescence
SABG activity is a widely used marker of senescence in tissues and cell cultures. The activity of SABG can be detected at pH 6 (the intra-lysosomal pH of senescent cells), whereas the activity of acidic β-galactosidase can be detected at pH 4 (the intralysosomal pH of non-senescent cells)145. However, SABG has some limitations as a marker of senescence. For example, many apoptotic non-senescent cells in developing limbs and healthy neurons during early embryonic development are SABG positive146 and cell density might influence SABG staining regardless of cell proliferation status147. These findings suggest that SABG is not a sufficiently robust and specific marker of senescent cells.
Gene and protein components of senescence-related signalling pathways such as p16 and p21 are canonical markers of senescence148,149. Expression of Met is also considered to be a marker of cellular senescence150 and the accumulation of nuclear globular actin accumulation has been reported to be a more sensitive marker of cellular senescence than SABG activity151. Other markers of senescence include telomere shortening152, the DNA double-strand-break marker H2AX153, typical chromatin changes such as senescence-associated heterochromatin foci154, cytosolic double-stranded DNA and miR-146a155,156. Decreased cellular proliferation potential (which can be measured using BrdU or EdU incorporation assays) and increased apoptosis resistance (for example, as a result of upregulation of BCL proteins) also occur in some types of senescent cells157.
Potential blood or urine biomarkers of senescence include plasma angiopoietin-like 2, growth/differentiation factor 15, stanniocalcin 1 and serine protease inhibitors, serum T-kininogen and urinary 8-oxoguanosine11,158–160. Activin A is normally only expressed during embryonic development, but is copiously expressed in injured and senescent kidneys and can be detected in the plasma and urine of patients with kidney disease161. SASP signatures enable the detection and characterization of cellular senescence in vivo but need to be defined in specific biological contexts as they might overlap with inflammatory profiles that are not associated with senescence. Extracellular vesicles can be isolated from urine or peripheral blood and analysed as an index of parent cell senescence; for example, increased levels of urinary URAT1+p16+ extracellular vesicles reflect increased proximal tubular cell senescence162.
Several markers characterize specific senescent tissues or cells. For example, loss of EPC1 expression is a marker of senescence in human dermal fibroblasts163. In T cells, expression of NKG2D, KLRG1, CD57 and CD28 might reflect senescence64,164,165. NKG2D166 and activin A161 are potential markers of senescence in kidney tissue. Spectrin–phaemoglobin crosslinking is an important feature of senescence in red blood cells167, SRSF1 has been postulated to be a key marker of endothelial senescence168 and maspin is a marker of senescence in oral premalignant lesions169.
Endogenous autofluorescence of MSCs is considered to be a useful marker to rapidly determine their senescent status in vitro170. However, the small number of senescent cells relative to quiescent cells, robust tissue autofluorescence, and low penetration of fluorescence signals hamper the detection of senescent cells in vivo using fluorescence-based imaging. Near-infrared imaging methods are not clinically applicable because of the limited availability of high-performance second near-infrared region (NIR-II) fluorophores with high brightness and biocompatibility as well as the long-term health risks of using non-biodegradable quantum dots and lanthanide-doped nanoparticles. Positron emission tomography-based methods of detecting senescent cells are being investigated in animal studies but will require the development of sensitive clinical probes171. Advances in non-invasive imaging methods may enable the detection and spatial allocation of senescence-prone regions that warrant intervention.
Senescence in kidney diseases
Kidney cell senescence was first described in 1992 (ref.172). In addition to its role in physiological kidney ageing, senescence has important roles in the development of CKD and acute kidney injury (AKI) (Fig. 3). Interventions that clear senescent cells, including senolytic drugs, are therefore promising novel treatments for kidney diseases.
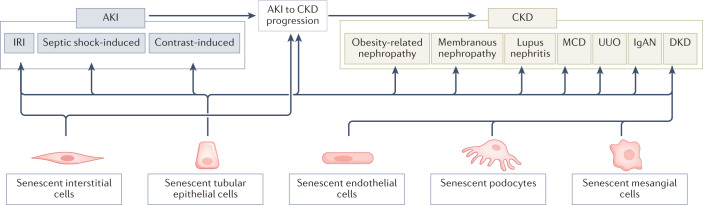
Fig. 3. Cellular senescence in kidney diseases.
Cellular senescence is involved in the pathogenesis of acute kidney injury (AKI) and many types of chronic kidney disease (CKD). Data from animal models suggest that senescent interstitial and tubular epithelial cells contribute to ischaemia–reperfusion injury (IRI), septic shock-induced AKI and contrast-induced AKI, as well as to AKI-to-CKD progression. Tubular epithelial cell senescence has also been detected in many forms of CKD, including obesity-related nephropathy, membranous nephropathy, lupus nephritis, minimal change disease (MCD), unilateral ureteral obstruction (UUO), IgA nephropathy (IgAN) and diabetic kidney disease (DKD). Endothelial cell, podocyte and mesangial cell senescence might also contribute to DKD.
Acute kidney injury
Emerging evidence indicates that senescence contributes to the progression of AKI and that senescence inhibition can promote kidney recovery. For example, inhibition of tubular cell senescence using lipoxin A4 restored renal function in a rat model of septic shock-induced AKI173. Similarly, in a rat model of contrast-induced AKI, pre-treatment with paricalcitol before contrast medium administration reduced cellular senescence (that is, expression of SABG and p16INK4A) and tissue damage and prevented kidney dysfunction174. Notably, patients aged over 70 years have a 3.5-fold higher incidence of AKI than younger individuals175. This increased incidence has been linked to immunosenescence, amplification of the SASP176, and/or downregulation of the geroprotective protein α-Klotho177. Urine and plasma levels of p21 correlate with renal cortical expression of this protein and could be a useful non-invasive biomarker of AKI and kidney ageing178.
The complement system (C5a), DNA methylation, Wnt4–β-catenin signalling and ROS have been implicated in the development of cellular senescence in AKI179,180. Senescence has been shown to reduce the regenerative capacity of tubular, glomerular and interstitial cells181 and to delay recovery from AKI induced by ischaemia–reperfusion injury (IRI) in mice182. Following IRI, markers of kidney cell senescence (Bax and p16 mRNA) peaked at day 12, suggesting an increase in senescent cells in the chronic phase after AKI that might contribute to maladaptive repair and progression to CKD183. Treatment with nicotinamide mononucleotide reduced tubular cell DNA damage and senescence and attenuated renal fibrosis in mouse models of ischaemic AKI184. Increasing evidence suggests that cellular senescence might also have beneficial effects in AKI. A small-molecule inhibitor of CDK4 and CDK6 induced proximal tubule cell-cycle arrest and ameliorated kidney injury in a mouse IRI model185. Moreover, we found that inhibition of senescence within the first week after induction of renal ischaemia in a mouse model impeded functional recovery (measured using renal perfusion and plasma cystatin-c levels)186, suggesting a protective role early in the process of AKI. Delineating the time course of kidney injury and the role of cellular senescence during each phase might identify a therapeutic time window to target senescence and interrupt the development of AKI.
Chronic kidney disease
CKD is increasingly recognized to mimic age-related diseases and senescence and the SASP are important drivers of CKD progression187 (Fig. 4). CKD can accelerate the senescence of immune, endothelial and vascular smooth muscle cells via a process known as uraemia-associated ageing188,189, potentially constituting a feed-forward mechanism of cellular damage. Immunosenescence in CKD manifests as an increased proportion of terminally differentiated T cells, telomere shortening of mononuclear cells, low thymic output189 and reduced immune-mediated clearance of senescent kidney cells, which promotes CKD progression.
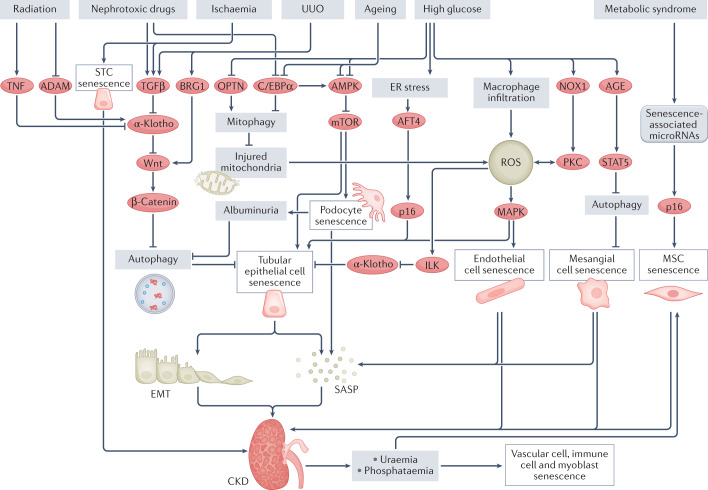
Fig. 4. Mechanisms of cellular senescence in chronic kidney disease.
Several stimuli can trigger the senescence of various kidney cell types through different pathways. High glucose levels result in macrophage infiltration into the kidney, mitochondrial dysfunction and activation of NOX1–PKC signalling, which lead to an increase in reactive oxygen species (ROS) and senescence of tubular epithelial cells and endothelial cells. High glucose can also induce senescence of tubular epithelial cells by provoking endoplasmic reticulum (ER) stress, which activates ATF4–p16 signalling. In addition, high glucose induces mesangial cell senescence via AGE–STAT5 signalling, which leads to the inhibition of autophagy and therefore results in the accumulation of injured mitochondrial and ROS (not shown). Nephrotoxic drugs and ageing can induce podocyte and tubular epithelial cell senescence via inhibition of C/EBPα, which leads to a reduction in AMPK–mTOR signalling. Ageing and high glucose can also induce tubular epithelial cell senescence by inhibiting AMPK. Nephrotoxic drugs, ischaemia, radiation and unilateral ureteral obstruction (UUO) activate Wnt–β-catenin signalling, which inhibits autophagy and therefore induces the senescence of tubular epithelial cells. Notably, ischaemia and metabolic syndrome can induce the senescence of kidney scattered tubule-like cells (STCs) and mesenchymal stem cells (MSCs), respectively, resulting in impairment of kidney repair, which promotes progression to chronic kidney disease (CKD). Chronic kidney cell senescence promotes epithelial-to-mesenchymal transition (EMT) and results in the senescence-associated secretory phenotype (SASP), which increases inflammation, eventually leading to the development of fibrosis and CKD. Furthermore, CKD can lead to uraemia-induced senescence of immune cells, endothelial cells, vascular smooth muscle cells and MSCs. CKD is also associated with hyperphosphataemia, which induces senescence in myoblasts, endothelial cells and vascular smooth muscle cells and thereby contributes to sarcopenia and vascular calcification. ADAM, A-disintegrin and metalloproteinase; ATF4, activating transcription factor 4; BRG1, brahma-related gene 1; ILK, integrin-linked protein kinase; NOX1, NADPH oxidase 1; OPTN, optineurin.
The mechanisms that underlie CKD-induced senescence include hyperphosphataemia, a common complication of CKD that elicits senescence in myoblasts190, endothelial cells191 and aorta smooth muscle cells192 and contributes to sarcopenia and vascular calcification. Furthermore, uraemic toxins have been implicated in the senescence of proximal tubular cells193. Tubular epithelial cell senescence can be induced by inhibition of AMPK–mTOR signalling194, activation of the Wnt–β-catenin pathway195 or overexpression of Wnt9a196, and promotes epithelial to mesenchymal transition and consequent fibrosis. α-Klotho, an endogenous antagonist of Wnt–β-catenin signalling, was downregulated in unilateral ureteral obstruction, adriamycin nephropathy, and IRI models of fibrotic kidney disease197. Senolytic combination therapy with dasatinib and quercetin alleviated kidney fibrosis in mouse models of chronic renal ischaemia186 and abrogated the progression of AKI to CKD in mouse models of IRI and cisplatin-induced injury198.
Podocyte senescence induces glomerulosclerosis in the ageing kidney via AMPK–mTOR signalling199, whereas DKD or overfeeding can induce renal cellular senescence by decreasing sirtuin 1 expression200. In patients with DKD, the circulating senescence marker activin A is elevated161 and p16 is upregulated in tubular epithelial cells201. In mice with streptozotocin-induced diabetes, glomerular endothelial senescence is driven by M1 macrophages and largely dependent on intracellular ROS202. High blood glucose also induces mesangial cell senescence by activating RAGE–STAT5 signalling, which inhibits autophagy203 and therefore leads to accumulation of injured mitochondria and ROS, which are important inducers of senescence.
We detected cellular senescence (upregulation of p16, p19 and p21) in the kidneys of mice186, pigs204 and patients186 with renal artery stenosis and observed that ischaemic renovascular disease induces senescence of renal scattered tubular-like cells, which impairs their reparative capacity205. Senescence of renal tubular epithelial cells promotes progression of immunoglobulin A (IgA) nephropathy206 and the presence of p16INK4a-positive cells in kidney biopsy samples from patients with lupus nephritis is associated with renal injury and a worse prognosis207. Elevated gene expression of the senescence markers Tp16, Tp19 and Tp53 was also observed in mice with obesity-induced kidney injury208 and p16 expression was strikingly increased in biopsy samples from patients with glomerular disease209. Exposure of kidney cells to nephrotoxic factors including radiation125, TNF210, hypoxia or glucose oxidase211,212 can induce cell-cycle arrest in vitro. Replicative senescence is also involved in the development of chronic allograft nephropathy213. The complement factor H-related genes CFHR1 and CFHR3 have been implicated in tubular cell senescence in allografts in transplant recipients with IgA nephropathy214.
Extensive basic and clinical studies are required to elucidate the complex mechanisms of cellular senescence in CKD. Although these mechanisms are shared across many forms of kidney disease, the extent and unique features of cellular senescence likely vary with the severity and underlying aetiology of CKD.
Interventions targeting senescence
As cellular senescence has key roles in many age-related diseases, interventions targeting senescence, known as senotherapeutics, are potential therapies for these diseases (Table 2). The promise of such approaches is underscored by the observation that genetic animal models of senescent cell deficiency show improved recovery from kidney injury215 and extended lifespan128. Existing senotherapeutic approaches include drugs that selectively eliminate senescent cells, known as senolytics, drugs that inhibit the SASP, known as senomorphics, exogenous cell-based products and non-pharmacological therapies.
Table 2.
Senotherapeutic approaches
| Senotherapeutic approach | Type | Examples | Refs. |
|---|---|---|---|
| Senolytic interventions | Apoptosis inducers | Quercetin, AP20187, navitoclax, A-1331852, A-1155463, EF24 and venetoclax, antibody-engineered toxic drugs, ginsenoside | 6,186,217,225,230,231,235 |
| Immunotherapies | Chimeric antigen receptor T cells, activator of invariant natural killer T cells, vaccines targeting GPNMB (which is overexpressed in senescent cells) | 236–238 | |
| Senomorphic drugs | SASP regulators | Metformin, ruxolitinib, rapamycin, resveratrol, melatonin, androgen, oestrogen, oestradiol, glucocorticoids | 239,243,244,247,250,254–257 |
| Stem cells and their products | Stem cells | Bone marrow MSCs, pluripotent stem cells, umbilical cord-derived MSCs | 258,259,261 |
| Stem cell-derived extracellular vesicles | MSC-derived extracellular vesicles, dental pulp stem cell-derived extracellular vesicles, antler stem cell-derived extracellular vesicles | 262,264,282 | |
| Non-pharmacological therapies | Lifestyle interventions | Habitual moderate exercise, healthy diet, calorie restriction | 268,269,271,273 |
| Others | Fractional micro-needling radiofrequency treatment, radio-electric asymmetric conveyer technology | 234,275 |
MSC, mesenchymal stem cell; SASP, senescence-associated secretory phenotype.
Senolytic interventions
Senolytic drugs were developed to overcome the characteristic resistance to apoptosis of senescent cells by inducing pre-programmed cell death216. Quercetin, a natural flavonoid found in some fruit and vegetables, has been shown to eliminate senescent vascular smooth muscle and endothelial cells in animal models by inducing apoptosis through activation of AMPK217,218, sirtuin 1–PINK1-mediated mitophagy219 and NRF2–NF-κB220 signalling. We found that quercetin blunted the expression of senescence markers in the kidneys of obese mice208 and had beneficial, senescence-independent effects on cardiac function in mice fed a high-fat diet owing to pro-angiogenic activity221, highlighting the multiple modes of action of senolytic drugs.
The plant flavonoid fisetin222,223 and procyanidin C1, which is a flavonoid found in grape seeds, are also senolytics224. Herbal extracts can also have senolytic activity. For example, one of the best-known anti-ageing drugs, ginsenoside, is an extract of ginseng that prevents senescence of bone marrow MSCs by activating NRF2 and PI3K–Akt signalling225. Ginsenoside can also modulate the SASP, reduce inflammation, balance redox status and attenuate organ ageing226,227. These observations suggest that selected dietary interventions with senolytic effects could potentially halt the progression of senescence-associated CKD.
AP20187, an FK1012 analogue, selectively induced apoptosis of p16Ink4a‐expressing senescent cells in various mouse models, resulting in improvements in age‐related brain inflammation, cognitive function and stenotic kidney function186,228. Navitoclax (also known as ABT-263) is an inhibitor of anti-apoptotic BCL-2 family proteins that induces apoptosis and exerts potent senolytic effects in ageing animal models and in some types of senescent cells in vitro229,230. Other BCL-2 family inhibitors, including A-1331852, A-1155463, EF24 and venetoclax, are also effective senolytics6,231,232. In addition, heat shock protein 90 inhibitors have senolytic activity233 and radio-electric asymmetric conveyer technology was shown to be effective in reducing the senescence of cultured stem cells234. Notably, these interventions do not selectively target senescent cells, and thus can be associated with off-target adverse effects, such as abnormal embryo development, dysregulated wound healing and tumorigenesis.
Organ-targeted or cell-targeted approaches using protein-based or peptide-based carriers, nanoparticles, extracellular vesicles or other vehicles could increase the specificity, decrease the off-target effects and facilitate the clinical translation of senolytic interventions. Current strategies to selectively target senescent cells include conjugating toxic drugs to antibodies against the senescent membrane marker β2-microglobulin235, which is upregulated via a p53-dependent mechanism, suggesting that it is a marker of stress-induced senescence. Another strategy involves activation of invariant NK T cells to improve immunosurveillance and removal of senescent cells236. Moreover, chimeric antigen receptor T cells that were engineered to specifically recognize the urokinase-type plasminogen activator receptor on the surface of senescent cells had senolytic effects in vitro and in vivo237. Finally, anti-ageing vaccines have been developed to target CD153+ senescent T cells or GPNMB+ senescent endothelial cells with promising results in obese mouse models238.
Senomorphic drugs
One of the best-studied senomorphic drugs is metformin, which reduces the incidence of age-related diseases and expands the lifespan of Caenorhabditis elegans, mice and patients with type 2 diabetes mellitus239. Metformin also enhances the anticancer efficacy of CDK4 and CDK6 inhibitors by modulating the SASP240, inhibits endothelial senescence caused by high glucose-induced metabolic memory by regulating the sirtuin 1–p300–p53–p21 pathway241, triggers immune‐mediated clearance of senescent cells and restores tumour immunosurveillance242.
Other senomorphic drugs include ruxolitinib, a JAK inhibitor that reduces inflammation and alleviates frailty in aged mice by suppressing inflammatory SASP factors243. mTOR inhibitors, such as rapamycin, inhibit senescence and suppress the SASP in endothelial cells244 and fibroblasts245 by inducing autophagy, thereby reducing the accumulation of damaged organelles. Activation of mTOR leads to peroxisome proliferator-activated receptor-γ coactivator 1β-dependent mitochondrial biogenesis, ROS production and persistent activation of the DDR246. Thus, inhibition of mTOR ameliorates cellular senescence.
Several herb extracts, such as resveratrol247, also show anti-SASP activity. Furthermore, the small molecule ML324 that inhibits KDM4, which is involved in the epigenetic regulation of SASP genes, was shown to decrease the SASP in senescent tumour stromal cells248. However, this approach needs to be used cautiously to avoid adverse effects given the sometimes incongruent behaviours of tumour and stromal microenvironments249 and possibly other cellular niches.
Some hormones also have senomorphic effects. For example, melatonin suppresses SASP gene expression by interrupting the recruitment of CREB-binding protein (CBP) by poly-(ADP-ribose) polymerase 1 (PARP1), which is a sensor of DNA damage250. Melatonin improves senescent T cell activity251, alleviated cardiac mitochondrial dysfunction in a mouse model of accelerated senescence252, and rescued MSCs from uraemic toxin-induced senescence in CKD253. Other hormones, including androgens254, oestrogens255, oestradiol256 and glucocorticoids257, can also modulate the release of inflammatory cytokines. However, glucocorticoids need to be used with caution as they can induce senescence in primary human tenocytes257.
Stem cells and extracellular vesicles
In addition to many other salutary effects, stem cells and their extracellular vesicles can exert senolytic activity. For example, bone-marrow-derived MSCs decreased senescence and improved cardiac function in aged mice258, pluripotent stem cells prevented stress-induced senescence in cardiomyocyte-derived cells259 and human umbilical cord-derived MSCs protected rat kidneys from AKI-induced senescence180. We observed relatively modest senolytic efficacy of adipose-derived MSCs in post-stenotic mouse and human kidneys260. In contrast to these senolytic effects, human umbilical cord-derived MSCs increased splenic CD4+ T cell senescence and alleviated symptoms of lupus in mice261. These differing findings suggest that the effects of MSCs are likely cell type and context dependent.
Stem cell extracellular vesicles have robust anti-senescence activity and might be less prone to rejection or tumour formation than their parent stem cells. MSC-derived extracellular vesicles inhibited oxidative stress-induced senescence in endothelial cells, promoted wound closure in ageing diabetic mice262 and decreased myocardial senescence, possibly by improving the systemic inflammatory profile, in a pig model of metabolic renovascular disease263. Dental pulp stem cell-derived extracellular vesicles prevented irradiation-induced senescence in submandibular cells by reducing inflammation and oxidative stress264 and extracellular vesicles from antler progenitor cells alleviated MSC senescence in aged mice265.
The anti-senescence functions of stem cells and extracellular vesicles have been mainly attributed to their contents, which can regulate the SASP, repair damaged organelles and rescue the function of senescent cells. However, stem cells and their extracellular vesicles likely also have indirect effects on senescence as a result of restoring the tissue microcirculation, parenchymal cells and cellular functions and/or inhibiting immune cell activation. Their application as primary anti-senescence interventions might therefore be premature. To date, clinical trials using stem cells or their extracellular vesicles to target senescence have not been reported.
Lifestyle interventions
Several lifestyle factors might accelerate senescence. For example, sleep deprivation activates the DDR and promotes the SASP in humans266, whereas a healthy lifestyle, such as habitual exercise and caloric restriction, retards ageing. Animal and human studies have shown that lifelong exercise or habitual moderate exercise in aged individuals have beneficial effects on immunosenescence and age-related diseases (for example, metabolic disorders and liver steatosis) by modulating mitochondrial function, inflammation, the SASP and lipolysis267–269. Overfeeding accelerates senescence in mice270, whereas caloric restriction alleviates senescence in dogs271 and in murine white adipose tissue272. These findings might constitute a key mechanism by which caloric restriction extends lifespan and retards age-related chronic diseases. Dietary interventions can also influence age-related health through epigenetic alterations and modification of the gut microbiota. For example, nutrients such as betaine, choline and folate promote epigenetic changes that blunt age-related changes and CKD by targeting methylome or chromatin, whereas excessive intake of sugar promotes progression of age-related diseases by decreasing microbial diversity in animal models273.
Cumulative lifetime exposure to external stressors, such as temperature, oxygen levels and inadequate nutrition, elicit adaptive homeostatic mechanisms, including antioxidant and anti-inflammatory responses, by activating the NRF2–KEAP1 signalling pathway274. Modifying these exposures might offer new strategies for improving the health span and combatting CKD and could potentially have beneficial effects on cellular senescence.
Clinical trials
Clinical studies of senolytic therapies are relatively scarce compared with the numerous animal and in vitro studies, but have shown some success. For example, fractional micro-needling radiofrequency treatment effectively removed senescent keratinocytes and improved hyperpigmentation of aged skin275 and treatment with dasatinib and quercetin reduced the levels of circulating SASP factors and the abundance of senescent cells in adipose tissue and skin in patients with diabetic kidney disease276. Raltegravir277 decreased senescent T lymphocytes in treatment-suppressed people with HIV. A psychosocial intervention with horticultural therapy involving park visits and gardening activities over a 6-month period alleviated immunosenescence and chronic inflammation in older adults (aged 61–77 years), assessed as having reduced IL-6 levels and T cell exhaustion278. Hence, non-invasive and cost-effective strategies for improving well-being should be vigorously pursued.
Importantly, senotherapeutic approaches can have adverse effects. Some senolytic drugs, such as navitoclax, can induce neutropenia, trabecular bone loss and osteoprogenitor dysfunction279,280. Moreover, as SASP factors have many physiological roles, including immunosurveillance in tumorigenesis, the potential adverse effects of senomorphic drugs might outweigh their benefits and reduce the success of clinical trials. Furthermore, genes may dictate traits that provide survival benefits in one stage of life but become maladaptive in another phase, a phenomenon known as antagonistic pleiotropy. The effect of senolytic drugs on tumorigenesis might therefore be age-dependent and, given the role of cellular senescence in development, the effects of senolytic drugs might differ between early and later stages of life281. Notably, we found that premature delivery reduced the efficacy of senolytic drugs in a renal artery stenosis model186, consistent with temporal (rather than trait dependent) antagonistic pleiotropy. Thus, administration of these drugs needs to be carefully timed relative to both the instigating insult and the developmental stage of the patient, as these factors might dictate potential sequelae.
Given that chronic conditions continually generate new senescent cells, the frequency of senotherapy also requires further exploration. A mouse study suggested that intermittent administration of senolytics could be particularly effective at alleviating physical dysfunction and increasing survival128. Combination approaches that use senolytics and anti-SASP interventions to target a broad range of cell types might be particularly powerful.
Finally, the success of clinical trials of senotherapeutics could potentially be maximized by screening participants prior to enrolment to enable selection of those showing evidence of cellular senescence. However, sensitive screening tools need to be developed and validated to enable selection of those participants who are most likely to benefit from the intervention.
Conclusions
Cellular senescence drives various pathological processes but also has important roles in physiology and homeostasis, particularly in embryonic development and wound healing. Although senescence contributes to the development of age-related diseases, immune system dysfunction and tumour progression, it is also an important mechanism that protects against tumorigenesis. Evidently, cellular senescence is a double-edged sword, emphasizing the importance of discerning physiological from pathological senescence and developing therapies together with context and time-dependent treatment strategies to suppress its harmful effects while permitting its beneficial functions. Targeted delivery of senolytic compounds to senescent cells might also help to minimize their adverse effects and enhance their benefits. Although many senolytic therapies have been investigated in animal models and in vitro, clinical trials of targeted treatment strategies are needed to assess their safety and efficacy in patients.
As existing senescence indices may differ among conditions and organs, non-invasive methods of detecting senescence with high sensitivity and specificity are needed. Many inflammatory factors are also SASP components; therefore, their expression is not sufficient to identify cellular senescence. Urinary exosomes bearing specific markers are useful for detecting kidney senescence162, but methods for their harvesting and characterization require standardization. Clinical end-points and indices of the therapeutic success of senotherapeutics are required for clinical trials. In the future, novel biomarkers of senescence could potentially be used to direct the management of patients who might benefit from attenuation of cellular senescence.
Acknowledgements
The authors’ work was supported by National Institutes of Health grant numbers DK120292, DK122734, AG062104, AG013925 and AG61456, the Connor Fund, Robert P. and Arlene R. Kogod, Robert J. and Theresa W. Ryan, and the Noaber Foundation.
Glossary
- DREAM complex
A multisubunit complex formed by the assembly of p130 and p107 with their dimerization partner, E2F4/5, and a multi-vulva class-B core complex.
- Somatic hypermutation
A programmed process of adaptation to new foreign elements (such as microbes) whereby changes are introduced to the nucleotide sequences of immunoglobulin genes during B cell development.
- Efferocytosis
The process by which apoptotic cells are removed by phagocytic cells.
- Ramified
Used to describe cells that have long branch-like processes.
- Geroprotective protein
A protein that has anti-ageing effects.
- Radio-electric asymmetric conveyer technology
A technology delivering very low-intensity radio-electric emission to generate microcurrents in tissues or culture media and thereby produce biological effects.
- Metabolic memory
The durable effect of prior hyperglycaemia on the initiation and progression of diabetic complications.
- Tenocytes
Elongated fibroblasts and fibrocytes that reside between collagen fibres.
Author contributions
W.H. researched the data for the article and wrote the text. W.H. and L.O.L contributed substantially to discussion of the content. All authors reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Nephrology thanks Giuseppe Castellano and the other, anonymous, reviewers for their contribution to the peer review of this work.
Competing interests
L.O.L. is an adviser to AstraZeneca, CureSpec, Beren, Ribocure Pharmaceuticals and Butterfly Biosciences. Patents on senolytic drugs and their uses are held by the Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. The other authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 3.Gire V, Dulic V. Senescence from G2 arrest, revisited. Cell Cycle. 2015;14:297–304. doi: 10.1080/15384101.2014.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun D, Buttitta L. States of G0 and the proliferation-quiescence decision in cells, tissues and during development. Int. J. Dev. Biol. 2017;61:357–366. doi: 10.1387/ijdb.160343LB. [DOI] [PubMed] [Google Scholar]
- 5.Herranz N, Gil J. Mitochondria and senescence: new actors for an old play. EMBO J. 2016;35:701–702. doi: 10.15252/embj.201694025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossiello F, Herbig U, Longhese MP, Fumagalli M, d’Adda di Fagagna F. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 2014;26:89–95. doi: 10.1016/j.gde.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrova NV, Velichko AK, Razin SV, Kantidze OL. Small molecule compounds that induce cellular senescence. Aging Cell. 2016;15:999–1017. doi: 10.1111/acel.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulić V, Beney GE, Frebourg G, Drullinger LF, Stein GH. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol. Cell Biol. 2000;20:6741–6754. doi: 10.1128/MCB.20.18.6741-6754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basisty N, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terlecki-Zaniewicz L, et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging. 2018;10:1103–1132. doi: 10.18632/aging.101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özcan S, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging. 2016;8:1316–1329. doi: 10.18632/aging.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, et al. Upregulation of chicken p15INK4b at senescence and in the developing brain. J. Cell Sci. 2006;119:2435–2443. doi: 10.1242/jcs.02989. [DOI] [PubMed] [Google Scholar]
- 15.Favetta LA, et al. The oxidative stress adaptor p66Shc is required for permanent embryo arrest in vitro. BMC Dev. Biol. 2007;7:132. doi: 10.1186/1471-213X-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storer M, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz-Espín D, et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva-Álvarez S, et al. Developmentally-programmed cellular senescence is conserved and widespread in zebrafish. Aging. 2020;12:17895–17901. doi: 10.18632/aging.103968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawlings TM, et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife. 2021;10:e69603. doi: 10.7554/eLife.69603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, et al. Induction of fibroblast senescence during mouse corneal wound healing. Invest. Ophthalmol. Vis. Sci. 2019;60:3669–3679. doi: 10.1167/iovs.19-26983. [DOI] [PubMed] [Google Scholar]
- 22.Younis LT, Abu Hassan MI, Taiyeb Ali TB, Bustami TJ. 3D TECA hydrogel reduces cellular senescence and enhances fibroblasts migration in wound healing. Asian J. Pharm. Sci. 2018;13:317–325. doi: 10.1016/j.ajps.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang M, et al. Tenovin-1 induces senescence and decreases wound-healing activity in cultured rat primary astrocytes. Biomol. Ther. 2019;27:283–289. doi: 10.4062/biomolther.2018.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blokland KEC, et al. Senescence of IPF lung fibroblasts disrupt alveolar epithelial cell proliferation and promote migration in wound healing. Pharmaceutics. 2020;12:389. doi: 10.3390/pharmaceutics12040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu M, et al. Hydrogen peroxide-induced senescence reduces the wound healing-promoting effects of mesenchymal stem cell-derived exosomes partially via miR-146a. Aging Dis. 2021;12:102–115. doi: 10.14336/AD.2020.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitar MS, Abdel-Halim SM, Al-Mulla F. Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. Am. J. Physiol. Endocrinol. Metab. 2013;305:E951–E963. doi: 10.1152/ajpendo.00189.2013. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson HN, et al. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J. Invest. Dermatol. 2019;139:1171–1181. doi: 10.1016/j.jid.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 29.Trost TM, et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005;65:840–849. doi: 10.1158/0008-5472.840.65.3. [DOI] [PubMed] [Google Scholar]
- 30.Alimonti A, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsujimoto I, Yoshida A, Yoneda-Kato N, Kato JY. Depletion of CSN5 inhibits Ras-mediated tumorigenesis by inducing premature senescence in p53-null cells. FEBS Lett. 2012;586:4326–4331. doi: 10.1016/j.febslet.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Yasaei H, et al. Carcinogen-specific mutational and epigenetic alterations in INK4A, INK4B and p53 tumour-suppressor genes drive induced senescence bypass in normal diploid mammalian cells. Oncogene. 2013;32:171–179. doi: 10.1038/onc.2012.45. [DOI] [PubMed] [Google Scholar]
- 33.Guo X, et al. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat. Cell Biol. 2009;11:1451–1457. doi: 10.1038/ncb1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X, et al. Inactivation of heat shock factor Hsf4 induces cellular senescence and suppresses tumorigenesis in vivo. Mol. Cancer Res. 2012;10:523–534. doi: 10.1158/1541-7786.MCR-11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osanai M, et al. Occludin-mediated premature senescence is a fail-safe mechanism against tumorigenesis in breast carcinoma cells. Cancer Sci. 2007;98:1027–1034. doi: 10.1111/j.1349-7006.2007.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, et al. HBP1-mediated regulation of p21 protein through the Mdm2/p53 and TCF4/EZH2 pathways and its impact on cell senescence and tumorigenesis. J. Biol. Chem. 2016;291:12688–12705. doi: 10.1074/jbc.M116.714147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong J, Jiang P, Zhong L, Wang Y. The novel tumor suppressor gene ZNF24 induces THCA cells senescence by regulating wnt signaling pathway, resulting in inhibition of THCA tumorigenesis and invasion. Front. Oncol. 2021;11:646511. doi: 10.3389/fonc.2021.646511. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–8454. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- 39.Georgilis A, et al. PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell. 2018;34:85–102. doi: 10.1016/j.ccell.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alimirah F, et al. Cellular senescence promotes skin carcinogenesis through p38MAPK and p44/42MAPK signaling. Cancer Res. 2020;80:3606–3619. doi: 10.1158/0008-5472.CAN-20-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alspach E, et al. p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov. 2014;4:716–729. doi: 10.1158/2159-8290.CD-13-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruhland MK, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016;7:11762. doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamura K, Suzuki T, Nohara K. Gestational arsenite exposure augments hepatic tumors of C3H mice by promoting senescence in F1 and F2 offspring via different pathways. Toxicol. Appl. Pharmacol. 2020;408:115259. doi: 10.1016/j.taap.2020.115259. [DOI] [PubMed] [Google Scholar]
- 45.Flanagan KC, et al. c-Myb and C/EBPβ regulate OPN and other senescence-associated secretory phenotype factors. Oncotarget. 2018;9:21–36. doi: 10.18632/oncotarget.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz-Galván S, et al. Tumor cell-secreted PLD increases tumor stemness by senescence-mediated communication with microenvironment. Oncogene. 2019;38:1309–1323. doi: 10.1038/s41388-018-0527-2. [DOI] [PubMed] [Google Scholar]
- 47.Guccini I, et al. Senescence reprogramming by TIMP1 deficiency promotes prostate cancer metastasis. Cancer Cell. 2021;39:68–82. doi: 10.1016/j.ccell.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Gonçalves S, et al. COX2 regulates senescence secretome composition and senescence surveillance through PGE2. Cell Rep. 2021;34:108860. doi: 10.1016/j.celrep.2021.108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vindrieux D, et al. Repression of PLA2R1 by c-MYC and HIF-2alpha promotes cancer growth. Oncotarget. 2014;5:1004–1013. doi: 10.18632/oncotarget.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan W, et al. Mice deficient in poly(C)-binding protein 4 are susceptible to spontaneous tumors through increased expression of ZFP871 that targets p53 for degradation. Genes Dev. 2016;30:522–534. doi: 10.1101/gad.271890.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie H, et al. Cell-cycle arrest and senescence in TP53-wild type renal carcinoma by enhancer RNA-P53-bound enhancer regions 2 (p53BER2) in a p53-dependent pathway. Cell Death Dis. 2021;12:1. doi: 10.1038/s41419-020-03229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, et al. Higher expression of XPF is a critical factor in intrinsic chemotherapy resistance of human renal cell carcinoma. Int. J. Cancer. 2016;139:2827–2837. doi: 10.1002/ijc.30396. [DOI] [PubMed] [Google Scholar]
- 53.Hu J, et al. Endoglin is essential for the maintenance of self-renewal and chemoresistance in renal cancer stem cells. Stem Cell Rep. 2017;9:464–477. doi: 10.1016/j.stemcr.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamal Y, Cheng C, Frost HR, Amos CI. Predictors of disease aggressiveness influence outcome from immunotherapy treatment in renal clear cell carcinoma. Oncoimmunology. 2019;8:e1500106. doi: 10.1080/2162402X.2018.1500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 56.Ou HL, et al. Cellular senescence in cancer: from mechanisms to detection. Mol. Oncol. 2021;15:2634–2671. doi: 10.1002/1878-0261.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubelt F, et al. Onset of immune senescence defined by unbiased pyrosequencing of human immunoglobulin mRNA repertoires. PLoS One. 2012;7:e49774. doi: 10.1371/journal.pone.0049774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Puleston DJ, Simon AK. Autophagy and immune senescence. Trends Mol. Med. 2016;22:671–686. doi: 10.1016/j.molmed.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Sato Y, Yanagita M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 2019;15:625–640. doi: 10.1038/s41581-019-0185-9. [DOI] [PubMed] [Google Scholar]
- 60.Chiu YL, et al. Emergence of T cell immunosenescence in diabetic chronic kidney disease. Immun. Ageing. 2020;17:31. doi: 10.1186/s12979-020-00200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lioulios G, Fylaktou A, Papagianni A, Stangou M. T cell markers recount the course of immunosenescence in healthy individuals and chronic kidney disease. Clin. Immunol. 2021;225:108685. doi: 10.1016/j.clim.2021.108685. [DOI] [PubMed] [Google Scholar]
- 62.Trzonkowski P, et al. Immunosenescence increases the rate of acceptance of kidney allotransplants in elderly recipients through exhaustion of CD4+ T-cells. Mech. Ageing Dev. 2010;131:96–104. doi: 10.1016/j.mad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez IJ, et al. Immunosenescence study of T cells: a systematic review. Front. Immunol. 2020;11:604591. doi: 10.3389/fimmu.2020.604591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alonso-Arias R, et al. NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age. 2011;33:591–-605. doi: 10.1007/s11357-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pellicanò M, et al. Evidence for less marked potential signs of T-cell immunosenescence in centenarian offspring than in the general age-matched population. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:495–504. doi: 10.1093/gerona/glt120. [DOI] [PubMed] [Google Scholar]
- 66.Song Y, et al. T-cell immunoglobulin and ITIM domain contributes to CD8+ T-cell immunosenescence. Aging Cell. 2018;17:e12716. doi: 10.1111/acel.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Listì F, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann. NY Acad. Sci. 2006;1089:487–495. doi: 10.1196/annals.1386.013. [DOI] [PubMed] [Google Scholar]
- 68.de Bourcy CF, et al. Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proc. Natl Acad. Sci. USA. 2017;114:1105–1110. doi: 10.1073/pnas.1617959114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lescale C, et al. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 70.Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFα and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging. 2012;4:535–546. doi: 10.18632/aging.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadhu S, et al. Radiation-induced macrophage senescence impairs resolution programs and drives cardiovascular inflammation. J. Immunol. 2021;207:1812–1823. doi: 10.4049/jimmunol.2100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, et al. Using ROS as a second messenger, NADPH oxidase 2 mediates macrophage senescence via interaction with NF-κB during Pseudomonas aeruginosa infection. Oxid. Med. Cell Longev. 2018;2018:9741838. doi: 10.1155/2018/9741838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Q, et al. Activating transcription factor 3 involved in Pseudomonas aeruginosa PAO1-induced macrophage senescence. Mol. Immunol. 2021;133:122–127. doi: 10.1016/j.molimm.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging. 2020;12:9240–9259. doi: 10.18632/aging.103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Behmoaras J, Gil J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 2021;220:e202010162. doi: 10.1083/jcb.202010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall BM, et al. p16Ink4a and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging. 2017;9:1867–1884. doi: 10.18632/aging.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tonnessen-Murray CA, et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 2019;218:3827–3844. doi: 10.1083/jcb.201904051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 2016;89:767–778. doi: 10.1016/j.kint.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malaise O, et al. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging. 2019;11:9128–9146. doi: 10.18632/aging.102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen PM, et al. c-Maf regulates pluripotency genes, proliferation/self-renewal, and lineage commitment in ROS-mediated senescence of human mesenchymal stem cells. Oncotarget. 2015;6:35404–35418. doi: 10.18632/oncotarget.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan C, et al. TGF‑β induces periodontal ligament stem cell senescence through increase of ROS production. Mol. Med. Rep. 2019;20:3123–3130. doi: 10.3892/mmr.2019.10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu S, Ran S, Liu B, Liang J. miR-152 induces human dental pulp stem cell senescence by inhibiting SIRT7 expression. FEBS Lett. 2016;590:1123–1131. doi: 10.1002/1873-3468.12138. [DOI] [PubMed] [Google Scholar]
- 84.Xiao YZ, et al. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. 2020;31:534–548. doi: 10.1016/j.cmet.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Cho J, et al. Ewing sarcoma gene Ews regulates hematopoietic stem cell senescence. Blood. 2011;117:1156–1166. doi: 10.1182/blood-2010-04-279349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pi C, et al. Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD+-Sirt1 signaling. Aging. 2019;11:3505–3522. doi: 10.18632/aging.101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Son MJ, Kwon Y, Son T, Cho YS. Restoration of mitochondrial NAD+ levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cell. 2016;34:2840–2851. doi: 10.1002/stem.2460. [DOI] [PubMed] [Google Scholar]
- 88.Zhai XY, et al. Knockdown of SIRT6 enables human bone marrow mesenchymal stem cell senescence. Rejuvenation Res. 2016;19:373–384. doi: 10.1089/rej.2015.1770. [DOI] [PubMed] [Google Scholar]
- 89.Liu F, et al. NANOG attenuates hair follicle-derived mesenchymal stem cell senescence by upregulating PBX1 and activating AKT signaling. Oxid. Med. Cell Longev. 2019;2019:4286213. doi: 10.1155/2019/4286213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwata T, et al. Functional regulatory mechanisms underlying bone marrow mesenchymal stem cell senescence during cell passages. Cell Biochem. Biophys. 2021;79:321–336. doi: 10.1007/s12013-021-00969-y. [DOI] [PubMed] [Google Scholar]
- 91.Gannon HS, Donehower LA, Lyle S, Jones SN. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev. Biol. 2011;353:1–9. doi: 10.1016/j.ydbio.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong XY, et al. Downregulation of ROR2 promotes dental pulp stem cell senescence by inhibiting STK4-FOXO1/SMS1 axis in sphingomyelin biosynthesis. Aging Cell. 2021;20:e13430. doi: 10.1111/acel.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho A, et al. An endogenous anti-aging factor, sonic hedgehog, suppresses endometrial stem cell aging through SERPINB2. Mol. Ther. 2019;27:1286–1298. doi: 10.1016/j.ymthe.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang D, et al. Autophagy inhibits the mesenchymal stem cell aging induced by D-galactose through ROS/JNK/p38 signalling. Clin. Exp. Pharmacol. Physiol. 2020;47:466–477. doi: 10.1111/1440-1681.13207. [DOI] [PubMed] [Google Scholar]
- 95.Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE. 2015;10:e0126537. doi: 10.1371/journal.pone.0126537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conley SM, et al. Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front. Cell Dev. Biol. 2020;8:197. doi: 10.3389/fcell.2020.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hickson LJ, et al. Diabetic kidney disease alters the transcriptome and function of human adipose-derived mesenchymal stromal cells but maintains immunomodulatory and paracrine activities important for renal repair. Diabetes. 2021;70:1561–1574. doi: 10.2337/db19-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang CF, et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J. Invest. Dermatol. 2015;135:1348–1357. doi: 10.1038/jid.2014.439. [DOI] [PubMed] [Google Scholar]
- 100.Song X, et al. Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biol. 2017;11:219–230. doi: 10.1016/j.redox.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xing S, et al. Lactose induced redox-dependent senescence and activated Nrf2 pathway. Int. J. Clin. Exp. Pathol. 2019;12:2034–2045. [PMC free article] [PubMed] [Google Scholar]
- 102.Su S, et al. Lowering endogenous cathepsin d abundance results in reactive oxygen species accumulation and cell senescence. Mol. Cell Proteom. 2017;16:1217–1232. doi: 10.1074/mcp.M115.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Volonte D, Galbiati F. Inhibition of thioredoxin reductase 1 by caveolin 1 promotes stress-induced premature senescence. EMBO Rep. 2009;10:1334–1340. doi: 10.1038/embor.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uraoka M, et al. Loss of Bcl-2 during the senescence exacerbates the impaired angiogenic functions in endothelial cells by deteriorating the mitochondrial redox state. Hypertension. 2011;58:254–263. doi: 10.1161/HYPERTENSIONAHA.111.176701. [DOI] [PubMed] [Google Scholar]
- 106.Sakai T, Kurokawa R, Hirano SI, Imai J. Hydrogen indirectly suppresses increases in hydrogen peroxide in cytoplasmic hydroxyl radical-induced cells and suppresses cellular senescence. Int. J. Mol. Sci. 2019;20:456. doi: 10.3390/ijms20020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu X, et al. Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma. J. Mol. Med. 2018;96:1095–1106. doi: 10.1007/s00109-018-1682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurz DJ, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 109.Barascu A, et al. Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence. Nucleus. 2012;3:411–417. doi: 10.4161/nucl.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chandrasekaran A, et al. Redox and mTOR-dependent regulation of plasma lamellar calcium influx controls the senescence-associated secretory phenotype. Exp. Biol. Med. 2020;245:1560–1570. doi: 10.1177/1535370220943122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. Redox control of the senescence regulator interleukin-1α and the secretory phenotype. J. Biol. Chem. 2013;288:32149–32159. doi: 10.1074/jbc.M113.493841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mancini OK, et al. Oxidative stress-induced senescence mediates inflammatory and fibrotic phenotypes in fibroblasts from systemic sclerosis patients. Rheumatology. 2021;61:1265–1275. doi: 10.1093/rheumatology/keab477. [DOI] [PubMed] [Google Scholar]
- 113.Bourdens M, et al. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Sci. Rep. 2019;9:8671. doi: 10.1038/s41598-019-45191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsai CH, et al. Up-regulation of cofilin-1 in cell senescence associates with morphological change and p27kip1-mediated growth delay. Aging Cell. 2021;20:e13288. doi: 10.1111/acel.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oliva JL, Caino MC, Senderowicz AM, Kazanietz MG. S-Phase-specific activation of PKC alpha induces senescence in non-small cell lung cancer cells. J. Biol. Chem. 2008;283:5466–5476. doi: 10.1074/jbc.M707576200. [DOI] [PubMed] [Google Scholar]
- 116.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 2012;23:2066–2075. doi: 10.1091/mbc.e11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang H, et al. Reactive oxygen species and nitric oxide induce senescence of rudimentary leaves and the expression profiles of the related genes in Litchi chinensis. Hortic. Res. 2018;5:23. doi: 10.1038/s41438-018-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem. Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 119.Liu J, et al. Senescence as a consequence of ginsenoside rg1 response on k562 human leukemia cell line. Asian Pac. J. Cancer Prev. 2012;13:6191–6196. doi: 10.7314/APJCP.2012.13.12.6191. [DOI] [PubMed] [Google Scholar]
- 120.Druelle C, et al. ATF6α regulates morphological changes associated with senescence in human fibroblasts. Oncotarget. 2016;7:67699–67715. doi: 10.18632/oncotarget.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen QM, et al. Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. J. Cell Sci. 2000;113:4087–4097. doi: 10.1242/jcs.113.22.4087. [DOI] [PubMed] [Google Scholar]
- 122.Rivas-Chacón LDM, et al. Role of oxidative stress in the senescence pattern of auditory cells in age-related hearing loss. Antioxidants. 2021;10:1497. doi: 10.3390/antiox10091497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shaerzadeh F, et al. Microglia senescence occurs in both substantia nigra and ventral tegmental area. Glia. 2020;68:2228–2245. doi: 10.1002/glia.23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ridzuan N, Al Abbar A, Yip WK, Maqbool M, Ramasamy R. Characterization and expression of senescence marker in prolonged passages of rat bone marrow-derived mesenchymal stem cells. Stem Cell Int. 2016;2016:8487264. doi: 10.1155/2016/8487264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berkenkamp B, et al. In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS ONE. 2014;9:e88071. doi: 10.1371/journal.pone.0088071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cohen C, et al. Glomerular endothelial cell senescence drives age-related kidney disease through PAI-1. EMBO Mol. Med. 2021;13:e14146. doi: 10.15252/emmm.202114146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karin O, Alon U. Senescent cell accumulation mechanisms inferred from parabiosis. Geroscience. 2021;43:329–341. doi: 10.1007/s11357-020-00286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu M, et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang AS, Dreesen O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018;9:247. doi: 10.3389/fgene.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Netti GS, et al. Role of complement in regulating inflammation processes in renal and prostate cancers. Cells. 2021;10:2426. doi: 10.3390/cells10092426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kawagoe Y, et al. CXCL5-CXCR2 signaling is a senescence-associated secretory phenotype in preimplantation embryos. Aging Cell. 2020;19:e13240. doi: 10.1111/acel.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell. 2018;17:e12711. doi: 10.1111/acel.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 136.Alimbetov D, et al. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology. 2016;17:305–315. doi: 10.1007/s10522-015-9610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rana T, et al. PAI-1 regulation of TGF-β1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2020;62:319–330. doi: 10.1165/rcmb.2019-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 139.Barinda AJ, et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 2020;11:481. doi: 10.1038/s41467-020-14387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zuccolo E, et al. The microRNA-34a-induced senescence-associated secretory phenotype (SASP) favors vascular smooth muscle cells calcification. Int. J. Mol. Sci. 2020;21:4454. doi: 10.3390/ijms21124454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Salotti J, Johnson PF. Regulation of senescence and the SASP by the transcription factor C/EBPβ. Exp. Gerontol. 2019;128:110752. doi: 10.1016/j.exger.2019.110752. [DOI] [PubMed] [Google Scholar]
- 142.Takahashi A, et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 2018;9:1249. doi: 10.1038/s41467-018-03555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.En A, Takauji Y, Ayusawa D, Fujii M. The role of lamin B receptor in the regulation of senescence-associated secretory phenotype (SASP) Exp. Cell Res. 2020;390:111927. doi: 10.1016/j.yexcr.2020.111927. [DOI] [PubMed] [Google Scholar]
- 144.Tripathi U, et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging. 2021;13:21838–21854. doi: 10.18632/aging.203560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 146.de Mera-Rodríguez JA, et al. Is senescence-associated β-galactosidase a reliable in vivo marker of cellular senescence during embryonic development? Front. Cell Dev. Biol. 2021;9:623175. doi: 10.3389/fcell.2021.623175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp. Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 148.Palmer A, et al. Expression of p16 within myenteric neurons of the aged colon: a potential marker of declining function. Front. Neurosci. 2021;15:747067. doi: 10.3389/fnins.2021.747067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.López-Domínguez JA, et al. Cdkn1a transcript variant 2 is a marker of aging and cellular senescence. Aging. 2021;13:13380–13392. doi: 10.18632/aging.203110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boichuck M, Zorea J, Elkabets M, Wolfson M, Fraifeld VE. c-Met as a new marker of cellular senescence. Aging. 2019;11:2889–2897. doi: 10.18632/aging.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kwak IH, Kim HS, Choi OR, Ryu MS, Lim IK. Nuclear accumulation of globular actin as a cellular senescence marker. Cancer Res. 2004;64:572–580. doi: 10.1158/0008-5472.CAN-03-1856. [DOI] [PubMed] [Google Scholar]
- 152.Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging. 2016;8:3–11. doi: 10.18632/aging.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015;43:2489–2498. doi: 10.1093/nar/gkv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corpet A, Stucki M. Chromatin maintenance and dynamics in senescence: a spotlight on SAHF formation and the epigenome of senescent cells. Chromosoma. 2014;123:423–436. doi: 10.1007/s00412-014-0469-6. [DOI] [PubMed] [Google Scholar]
- 155.Olivieri F, et al. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age. 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou R, et al. Cytosolic dsDNA is a novel senescence marker associated with pyroptosis activation. Tissue Cell. 2021;72:101554. doi: 10.1016/j.tice.2021.101554. [DOI] [PubMed] [Google Scholar]
- 157.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 158.Thorin-Trescases N, Labbé P, Mury P, Lambert M, Thorin E. Angptl2 is a marker of cellular senescence: the physiological and pathophysiological impact of Angptl2-related senescence. Int. J. Mol. Sci. 2021;22:12232. doi: 10.3390/ijms222212232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Walter R, Murasko DM, Sierra F. T-kininogen is a biomarker of senescence in rats. Mech. Ageing Dev. 1998;106:129–144. doi: 10.1016/S0047-6374(98)00107-9. [DOI] [PubMed] [Google Scholar]
- 160.Gan W, et al. Age-dependent increases in the oxidative damage of DNA, RNA, and their metabolites in normal and senescence-accelerated mice analyzed by LC-MS/MS: urinary 8-oxoguanosine as a novel biomarker of aging. Free Radic. Biol. Med. 2012;52:1700–1707. doi: 10.1016/j.freeradbiomed.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 161.Bian X, et al. Senescence marker activin A is increased in human diabetic kidney disease: association with kidney function and potential implications for therapy. BMJ Open. Diabetes Res. Care. 2019;7:e000720. doi: 10.1136/bmjdrc-2019-000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Santelli A, et al. Senescent kidney cells in hypertensive patients release urinary extracellular vesicles. J. Am. Heart Assoc. 2019;8:e012584. doi: 10.1161/JAHA.119.012584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tresini M, Pignolo RJ, Allen RG, Cristofalo VJ. Effects of donor age on the expression of a marker of replicative senescence (EPC-1) in human dermal fibroblasts. J. Cell Physiol. 1999;179:11–17. doi: 10.1002/(SICI)1097-4652(199904)179:1<11::AID-JCP2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 164.Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J. Clin. Immunol. 2010;30:798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henson SM, Akbar AN. KLRG1-more than a marker for T cell senescence. Age. 2009;31:285–291. doi: 10.1007/s11357-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Günther J, et al. Identification of the activating cytotoxicity receptor NKG2D as a senescence marker in zero-hour kidney biopsies is indicative for clinical outcome. Kidney Int. 2017;91:1447–1463. doi: 10.1016/j.kint.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 167.Snyder LM, et al. Irreversible spectrin-haemoglobin crosslinking in vivo: a marker for red cell senescence. Br. J. Haematol. 1983;53:379–384. doi: 10.1111/j.1365-2141.1983.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 168.Blanco FJ, Bernabéu C. The splicing factor SRSF1 as a marker for endothelial senescence. Front. Physiol. 2012;3:54. doi: 10.3389/fphys.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bascones-Martínez A, et al. Differences in the expression of five senescence markers in oral cancer, oral leukoplakia and control samples in humans. Oncol. Lett. 2012;3:1319–1325. doi: 10.3892/ol.2012.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bertolo A, Baur M, Guerrero J, Pötzel T, Stoyanov J. Autofluorescence is a reliable in vitro marker of cellular senescence in human mesenchymal stromal cells. Sci. Rep. 2019;9:2074. doi: 10.1038/s41598-019-38546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamagishi S, et al. Upregulation of cannabinoid receptor type 2, but not TSPO, in senescence-accelerated neuroinflammation in mice: a positron emission tomography study. J. Neuroinflammation. 2019;16:208. doi: 10.1186/s12974-019-1604-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Khandjian EW, Salomon C, Léonard N, Tremblay S, Türler H. Fibronectin gene expression in proliferating, quiescent, and SV40-infected mouse kidney cells. Exp. Cell Res. 1992;202:464–470. doi: 10.1016/0014-4827(92)90100-M. [DOI] [PubMed] [Google Scholar]
- 173.Chen C, et al. Lipoxin A4 restores septic renal function via blocking crosstalk between inflammation and premature senescence. Front. Immunol. 2021;12:637753. doi: 10.3389/fimmu.2021.637753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bae E, et al. Paricalcitol attenuates contrast-induced acute kidney injury by regulating mitophagy and senescence. Oxid. Med. Cell Longev. 2020;2020:7627934. doi: 10.1155/2020/7627934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Khan S, Loi V, Rosner MH. Drug-induced kidney injury in the elderly. Drugs Aging. 2017;34:729–741. doi: 10.1007/s40266-017-0484-4. [DOI] [PubMed] [Google Scholar]
- 176.Marquez-Exposito L, et al. Acute kidney injury is aggravated in aged mice by the exacerbation of proinflammatory processes. Front. Pharmacol. 2021;12:662020. doi: 10.3389/fphar.2021.662020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li Y, Lerman LO. Cellular senescence: a new player in kidney injury. Hypertension. 2020;76:1069–1075. doi: 10.1161/HYPERTENSIONAHA.120.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnson AC, Zager RA. Plasma and urinary p21: potential biomarkers of AKI and renal aging. Am. J. Physiol. Renal Physiol. 2018;315:F1329–F1335. doi: 10.1152/ajprenal.00328.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Castellano G, et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging. 2019;11:4382–4406. doi: 10.18632/aging.102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rodrigues CE, et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res. Ther. 2017;8:19. doi: 10.1186/s13287-017-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Westhoff JH, et al. Telomere shortening reduces regenerative capacity after acute kidney injury. J. Am. Soc. Nephrol. 2010;21:327–336. doi: 10.1681/ASN.2009010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng H, Fan X, Lawson WE, Paueksakon P, Harris RC. Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 2015;88:85–94. doi: 10.1038/ki.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sari FT, Sari FT, Sari FT, Arfian N, Sari DCR. Effect of kidney ischemia/reperfusion injury on proliferation, apoptosis, and cellular senescence in acute kidney injury in mice. Med. J. Malays. 2020;75:20–23. [PubMed] [Google Scholar]
- 184.Jia Y, et al. Nicotinamide mononucleotide attenuates renal interstitial fibrosis after AKI by suppressing tubular DNA damage and senescence. Front. Physiol. 2021;12:649547. doi: 10.3389/fphys.2021.649547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.DiRocco DP, et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 2014;306:F379–F388. doi: 10.1152/ajprenal.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim SR, et al. Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J. Am. Soc. Nephrol. 2021;32:1987–2004. doi: 10.1681/ASN.2020091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schroth J, Thiemermann C, Henson SM. Senescence and the aging immune system as major drivers of chronic kidney disease. Front. Cell Dev. Biol. 2020;8:564461. doi: 10.3389/fcell.2020.564461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carracedo J, et al. Mechanisms of cardiovascular disorders in patients with chronic kidney disease: a process related to accelerated senescence. Front. Cell Dev. Biol. 2020;8:185. doi: 10.3389/fcell.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Crépin T, et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transpl. 2020;35:624–632. doi: 10.1093/ndt/gfy276. [DOI] [PubMed] [Google Scholar]
- 190.Sosa P, et al. Hyperphosphatemia promotes senescence of myoblasts by impairing autophagy through Ilk overexpression, a possible mechanism involved in sarcopenia. Aging Dis. 2018;9:769–784. doi: 10.14336/AD.2017.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Olmos G, et al. Hyperphosphatemia induces senescence in human endothelial cells by increasing endothelin-1 production. Aging Cell. 2017;16:1300–1312. doi: 10.1111/acel.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Troyano N, et al. Hyperphosphatemia induces cellular senescence in human aorta smooth muscle cells through integrin linked kinase (ILK) up-regulation. Mech. Ageing Dev. 2015;152:43–55. doi: 10.1016/j.mad.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 193.Okada A, et al. D-serine, a novel uremic toxin, induces senescence in human renal tubular cells via GCN2 activation. Sci. Rep. 2017;7:11168. doi: 10.1038/s41598-017-11049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dong D, et al. Alleviation of senescence and epithelial-mesenchymal transition in aging kidney by short-term caloric restriction and caloric restriction mimetics via modulation of AMPK/mTOR signaling. Oncotarget. 2017;8:16109–16121. doi: 10.18632/oncotarget.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gong W, et al. Brahma-related gene-1 promotes tubular senescence and renal fibrosis through Wnt/β-catenin/autophagy axis. Clin. Sci. 2021;135:1873–1895. doi: 10.1042/CS20210447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Luo C, et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 2018;29:1238–1256. doi: 10.1681/ASN.2017050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li C, Shen Y, Huang L, Liu C, Wang J. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J. 2021;35:e21229. doi: 10.1096/fj.202001855RR. [DOI] [PubMed] [Google Scholar]
- 199.Zhang L, et al. C/EBPα deficiency in podocytes aggravates podocyte senescence and kidney injury in aging mice. Cell Death Dis. 2019;10:684. doi: 10.1038/s41419-019-1933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Juvet C, et al. Renal programming by transient postnatal overfeeding: the role of senescence pathways. Front. Physiol. 2020;11:511. doi: 10.3389/fphys.2020.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu J, et al. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am. J. Physiol. Cell Physiol. 2015;308:C621–C630. doi: 10.1152/ajpcell.00096.2014. [DOI] [PubMed] [Google Scholar]
- 202.Yu S, et al. M1 macrophages accelerate renal glomerular endothelial cell senescence through reactive oxygen species accumulation in streptozotocin-induced diabetic mice. Int. Immunopharmacol. 2020;81:106294. doi: 10.1016/j.intimp.2020.106294. [DOI] [PubMed] [Google Scholar]
- 203.Shi M, et al. The RAGE/STAT5/autophagy axis regulates senescence in mesangial cells. Cell Signal. 2019;62:109334. doi: 10.1016/j.cellsig.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 204.Kim SR, Eirin A, Zhang X, Lerman A, Lerman LO. Mitochondrial protection partly mitigates kidney cellular senescence in swine atherosclerotic renal artery stenosis. Cell Physiol. Biochem. 2019;52:617–632. doi: 10.33594/000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen XJ, et al. Renovascular disease induces senescence in renal scattered tubular-like cells and impairs their reparative potency. Hypertension. 2021;77:507–518. doi: 10.1161/HYPERTENSIONAHA.120.16218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu J, et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl. Res. 2012;159:454–463. doi: 10.1016/j.trsl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 207.Tilman G, et al. High p16INK4a, a marker of cellular senescence, is associated with renal injury, impairment and outcome in lupus nephritis. RMD Open. 2021;7:e001844. doi: 10.1136/rmdopen-2021-001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim SR, et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res. 2019;213:112–123. doi: 10.1016/j.trsl.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sis B, et al. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int. 2007;71:218–226. doi: 10.1038/sj.ki.5002039. [DOI] [PubMed] [Google Scholar]
- 210.Kim DY, Lee M, Kim EJ. Involvement of Klotho, TNF‑α; and ADAMs in radiation‑induced senescence of renal epithelial cells. Mol. Med. Rep. 2021;23:22. doi: 10.3892/mmr.2020.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eleftheriadis T, Pissas G, Filippidis G, Liakopoulos V, Stefanidis I. The role of indoleamine 2,3-dioxygenase in renal tubular epithelial cells senescence under anoxia or reoxygenation. Biomolecules. 2021;11:1522. doi: 10.3390/biom11101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Troyano-Suárez N, et al. Glucose oxidase induces cellular senescence in immortal renal cells through ILK by downregulating Klotho gene expression. Oxid. Med. Cell Longev. 2015;2015:416738. doi: 10.1155/2015/416738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ferlicot S, et al. The role of replicative senescence in chronic allograft nephropathy. Hum. Pathol. 2003;34:924–928. doi: 10.1016/S0046-8177(03)00340-X. [DOI] [PubMed] [Google Scholar]
- 214.Pesce F, et al. DelCFHR3-1 influences graft survival in transplant patients with IgA nephropathy via complement-mediated cellular senescence. Am. J. Transpl. 2021;21:838–845. doi: 10.1111/ajt.16350. [DOI] [PubMed] [Google Scholar]
- 215.Lee DH, Wolstein JM, Pudasaini B, Plotkin M. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2012;302:F183–F191. doi: 10.1152/ajprenal.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Baar MP, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim SG, Sung JY, Kim JR, Choi HC. Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway. Korean J. Physiol. Pharmacol. 2020;24:69–79. doi: 10.4196/kjpp.2020.24.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jiang YH, Jiang LY, Wang YC, Ma DF, Li X. Quercetin attenuates atherosclerosis via modulating oxidized LDL-induced endothelial cellular senescence. Front. Pharmacol. 2020;11:512. doi: 10.3389/fphar.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu T, et al. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 2020;257:118116. doi: 10.1016/j.lfs.2020.118116. [DOI] [PubMed] [Google Scholar]
- 220.Shao Z, et al. Senolytic agent quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthritis Cartilage. 2021;29:413–422. doi: 10.1016/j.joca.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 221.Yu S, et al. Quercetin reverses cardiac systolic dysfunction in mice fed with a high-fat diet: role of angiogenesis. Oxid. Med. Cell Longev. 2021;2021:8875729. doi: 10.1155/2021/8875729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yousefzadeh MJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhu Y, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging. 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xu Q, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021;3:1706–1726. doi: 10.1038/s42255-021-00491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang Z, et al. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic. Biol. Med. 2021;174:182–194. doi: 10.1016/j.freeradbiomed.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 226.Yokozawa T, Satoh A, Cho EJ. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J. Pharm. Pharmacol. 2004;56:107–113. doi: 10.1211/0022357022449. [DOI] [PubMed] [Google Scholar]
- 227.Hou J, Cui C, Kim S, Sung C, Choi C. Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype. Chem. Biol. Interact. 2018;283:75–83. doi: 10.1016/j.cbi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 228.Ogrodnik M, et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20:e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhu Y, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mylonas KJ, et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl Med. 2021;13:eabb0203. doi: 10.1126/scitranslmed.abb0203. [DOI] [PubMed] [Google Scholar]
- 233.Fuhrmann-Stroissnigg H, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017;8:422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rinaldi S, et al. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci. Rep. 2014;4:6373. doi: 10.1038/srep06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Poblocka M, et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 2021;11:20358. doi: 10.1038/s41598-021-99852-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Arora S, et al. Invariant natural killer T cells coordinate removal of senescent cells. Med. 2021;2:938–950. doi: 10.1016/j.medj.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Amor C, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mendelsohn AR, Larrick JW. Antiaging vaccines targeting senescent cells. Rejuvenation Res. 2022;25:39–45. doi: 10.1089/rej.2022.0008. [DOI] [PubMed] [Google Scholar]
- 239.Soukas AA, Hao H, Wu L. Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol. Metab. 2019;30:745–755. doi: 10.1016/j.tem.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moiseeva O, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 241.Zhang E, et al. Metformin and resveratrol inhibited high glucose-induced metabolic memory of endothelial senescence through SIRT1/p300/p53/p21 pathway. PLoS ONE. 2015;10:e0143814. doi: 10.1371/journal.pone.0143814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Menendez JA, et al. Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer. Aging. 2011;3:1063–1077. doi: 10.18632/aging.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xu M, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA. 2015;112:E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sasaki N, Itakura Y, Toyoda M. Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy. Cell Commun. Signal. 2020;18:43. doi: 10.1186/s12964-020-00533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang R, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16:564–574. doi: 10.1111/acel.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Correia-Melo C, et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Menicacci B, et al. Chronic resveratrol treatment inhibits MRC5 fibroblast SASP-related protumoral effects on melanoma cells. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:1187–1195. doi: 10.1093/gerona/glw336. [DOI] [PubMed] [Google Scholar]
- 248.Zhang B, et al. KDM4 orchestrates epigenomic remodeling of senescent cells and potentiates the senescence-associated secretory phenotype. Nat. Aging. 2021;1:454–472. doi: 10.1038/s43587-021-00063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pazolli E, et al. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012;72:2251–2261. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yu S, et al. Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J. Pineal Res. 2017;63:12405. doi: 10.1111/jpi.12405. [DOI] [PubMed] [Google Scholar]
- 251.Yoo YM, Jang SK, Kim GH, Park JY, Joo SS. Pharmacological advantages of melatonin in immunosenescence by improving activity of T lymphocytes. J. Biomed. Res. 2016;30:314–321. doi: 10.7555/JBR.30.2016K0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rodríguez MI, et al. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic. Res. 2007;41:15–24. doi: 10.1080/10715760600936359. [DOI] [PubMed] [Google Scholar]
- 253.Han YS, Kim SM, Lee JH. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrPC-dependent enhancement of the mitochondrial function. J. Pineal Res. 2019;66:e12535. doi: 10.1111/jpi.12535. [DOI] [PubMed] [Google Scholar]
- 254.Rais M, Wilson RM, Urbanski HF, Messaoudi I. Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. Geroscience. 2017;39:373–384. doi: 10.1007/s11357-017-9979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Toillon RA, et al. Estrogens decrease gamma-ray-induced senescence and maintain cell cycle progression in breast cancer cells independently of p53. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:1187–1200. doi: 10.1016/j.ijrobp.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 256.Engelmann F, et al. Accelerated immune senescence and reduced response to vaccination in ovariectomized female rhesus macaques. Age. 2011;33:275–289. doi: 10.1007/s11357-010-9178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Poulsen RC, et al. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence. Ann. Rheum. Dis. 2014;73:1405–1413. doi: 10.1136/annrheumdis-2012-203146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang M, et al. Bone marrow mesenchymal stem cell transplantation retards the natural senescence of rat hearts. Stem Cell Transl Med. 2015;4:494–502. doi: 10.5966/sctm.2014-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang Y, et al. Rat induced pluripotent stem cells protect H9C2 cells from cellular senescence via a paracrine mechanism. Cardiology. 2014;128:43–50. doi: 10.1159/000357423. [DOI] [PubMed] [Google Scholar]
- 260.Kim SR, et al. Increased cellular senescence in the murine and human stenotic kidney: effect of mesenchymal stem cells. J. Cell Physiol. 2021;236:1332–1344. doi: 10.1002/jcp.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cheng T, Ding S, Liu S, Li Y, Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics. 2021;11:893–905. doi: 10.7150/thno.48080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xiao X, et al. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal. Transduct. Target. Ther. 2021;6:354. doi: 10.1038/s41392-021-00765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang L, et al. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic. Res. Cardiol. 2020;115:16. doi: 10.1007/s00395-019-0772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dong J, et al. Dental pulp stem cell-derived small extracellular vesicle in irradiation-induced senescence. Biochem. Biophys. Res. Commun. 2021;575:28–35. doi: 10.1016/j.bbrc.2021.08.046. [DOI] [PubMed] [Google Scholar]
- 265.Lei J, et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2021;13:220–226. doi: 10.1007/s13238-021-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Carroll JE, et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav. Immun. 2016;51:223–229. doi: 10.1016/j.bbi.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sun L, et al. Alterations in mitochondrial biogenesis and respiratory activity, inflammation of the senescence-associated secretory phenotype, and lipolysis in the perirenal fat and liver of rats following lifelong exercise and detraining. FASEB J. 2021;35:e21890. doi: 10.1096/fj.202100868R. [DOI] [PubMed] [Google Scholar]
- 268.Tylutka A, Morawin B, Gramacki A, Zembron-Lacny A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 2021;21:200. doi: 10.1186/s12877-021-02128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bianchi A, et al. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol. 2021;206:904–916. doi: 10.4049/jimmunol.2001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yzydorczyk C, et al. Transient postnatal overfeeding causes liver stress-induced premature senescence in adult mice. Sci. Rep. 2017;7:12911. doi: 10.1038/s41598-017-11756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Greeley EH, Spitznagel E, Lawler DF, Kealy RD, Segre M. Modulation of canine immunosenescence by life-long caloric restriction. Vet. Immunol. Immunopathol. 2006;111:287–299. doi: 10.1016/j.vetimm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 272.List EO, et al. Diet-induced weight loss is sufficient to reduce senescent cell number in white adipose tissue of weight-cycled mice. Nutr. Healthy Aging. 2016;4:95–99. doi: 10.3233/NHA-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mafra D, et al. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021;17:153–171. doi: 10.1038/s41581-020-00345-8. [DOI] [PubMed] [Google Scholar]
- 274.Shiels PG, et al. Manipulating the exposome to enable better ageing. Biochem. J. 2021;478:2889–2898. doi: 10.1042/BCJ20200958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lee YI, Kim E. Synergistic effect of 300 μm needle-depth fractional microneedling radiofrequency on the treatment of senescence-induced aging hyperpigmentation of the skin. Int. J. Mol. Sci. 2021;22:7480. doi: 10.3390/ijms22147480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hickson LJ, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Torres B, et al. Impact of switching to raltegravir and/or adding losartan in lymphoid tissue fibrosis and inflammation in people living with HIV. A randomized clinical trial. HIV Med. 2021;22:674–681. doi: 10.1111/hiv.13114. [DOI] [PubMed] [Google Scholar]
- 278.Wong GCL, et al. Horticultural therapy reduces biomarkers of immunosenescence and inflammaging in community-dwelling older adults: a feasibility pilot randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76:307–317. doi: 10.1093/gerona/glaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sharma AK, et al. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front. Cell Dev. Biol. 2020;8:354. doi: 10.3389/fcell.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Puglisi M, et al. A phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol. 2021;17:2747–2758. doi: 10.2217/fon-2021-0140. [DOI] [PubMed] [Google Scholar]
- 281.Rodríguez JA, et al. Antagonistic pleiotropy and mutation accumulation influence human senescence and disease. Nat. Ecol. Evol. 2017;1:55. doi: 10.1038/s41559-016-0055. [DOI] [PubMed] [Google Scholar]
- 282.Lei J, Jiang X, Li W, Ren J. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13:220–226. doi: 10.1007/s13238-021-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]