Abstract
Handgrip strength (HGS), a simple tool for the evaluation of muscular strength, is independently associated with negative prognosis in many diseases. It is unknown whether HGS is prognostically relevant in COVID-19. We evaluated the ability of HGS to predict clinical outcomes in people with COVID-19-related pneumonia. 118 patients (66% men, 63 ± 12 years), consecutively hospitalized to the “Santa Maria” Terni University Hospital for COVID-19-related pneumonia and respiratory failure, underwent HGS measurement (Jamar hand-dynamometer) at ward admission. HGS was normalized to weight2/3 (nHGS) The main end-point was the first occurrence of death and/or endotracheal intubation at 14 days. Twenty-two patients reached the main end-point. In the Kaplan–Meyer analysis, the Log rank test showed significant differences between subjects with lower than mean HGS normalized to weight2/3 (nHGS) (< 1.32 kg/Kg2/3) vs subjects with higher than mean nHGS. (p = 0.03). In a Cox-proportional hazard model, nHGS inversely predicted the main end-point (hazard ratio, HR = 1.99 each 0.5 kg/Kg2/3 decrease, p = 0.03), independently from age, sex, body mass index, ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2 ratio), hypertension, diabetes, estimated glomerular filtration rate and history of previous cardiovascular cardiovascular disease. These two latter also showed independent association with the main end-point (HR 1.30, p = 0.03 and 3.89, p < 0.01, respectively). In conclusion, nHGS measured at hospital admission, independently and inversely predicts the risk of poor outcomes in people with COVID-19-related pneumonia. The evaluation of HGS may be useful in early stratifying the risk of adverse prognosis in COVID-19.
Keywords: COVID-19, Respiratory failure, Handgrip strength, Sarcopenia, Muscular wasting, Obesity, Sarcopenic obesity
Introduction
The current impact of global outbreak of the coronavirus disease 2019 (COVID-19) on all-cause mortality and morbidity excess remains highly significant across all age spans [1, 2]. To improve risk stratification, set healthcare priorities and allow optimal allocation of resources, the evaluation of early prognostic markers of disease severity is of high importance.
Muscular involvement is a clinical manifestation of the multisystem COVID-19 syndrome. In COVID-19 patients, several reports described higher rates of muscle fatigue, weakness, and myalgias (often associated with increases in serum creatine phosphokinase (CPK), with an extent proportional to the gravity of the systemic inflammation [3–5]. This link is also suggested by the close correlation between serum CPK levels and inflammatory markers of disease such as c-reactive protein (CRP) [6].
Handgrip strength (HGS) is a simple, reproducible, and inexpensive tool for the evaluation of muscular strength in clinical practice through the measurement of the maximum static force that a hand can squeeze using a dynamometer. It is well acknowledged that HGS is a marker of global sarcopenia and muscular impairment and it is independently associated with increased risk of all-cause mortality in large-scale population-based studies [7, 8] as well as in critical illnesses [9]. Initial reports suggested that HGS was lower in COVID-19 patients, as compared to seronegative subjects [10]. Among COVID-19 hospitalized patients, HGS was consistently found to be closely correlated to indexes of disease severity even after accounting for age and gender differences [11]. However, the hypothesis that low HGS could predict future adverse clinical outcomes in patients hospitalized for severe COVID-19 has not been investigated to date.
The aim of the present study is to test the hypothesis that handgrip strength, a simple measure of global muscle strength, predicts short-term adverse outcomes in subjects with COVID-19-associated pneumonia independently from the potential effect of other clinical confounders.
Methods
Patients
All consecutive patients with COVID-19-related interstitial pneumonia and respiratory failure admitted to the “Santa Maria” Terni University Hospital, during the period between February and April 2021, except those who were directly admitted to intensive care units (ICU), were enrolled in the present study. The diagnosis of COVID-19 was based on positivity of viral RNA at the RT-PCR on the nasopharyngeal swab performed at Hospital admission, according to standardized procedures [12]. COVID-19-related interstitial pneumonia was diagnosed by radiological findings at the chest-X-ray or high-resolution computerized tomography (HRCT) scan. Respiratory failure was defined as standard partial oxygen pressure (PaO2) < 60 mmHg, evaluated through arterial blood gas analysis at hospital admission (Radiometer ABL90 FLEX) or need for oxygen support.
Exclusion criteria were: (1) absence of respiratory failure or any clinical or radiological sign of interstitial pneumonia; (2) moderate or advanced cognitive impairment or any other psychiatric disorder (e.g. delirium) resulting in an inability to execute simple verbal orders; (3) known neuromuscular degenerative or inflammatory diseases (such as amyotrophic lateral sclerosis, multiple sclerosis); (4) any other upper extremity musculoskeletal disease (e.g. polymyalgia rheumatica, rheumatoid arthritis, statin-related myopathy or known abnormal limitations of strength) or hand or wrist surgery within the previous 3 months; (5) advanced frailty (Clinical Frailty Scale [13] ≥ 7) due to cachexia or malnutrition.
All patients signed a written informed consent form to voluntarily participate in this study. A formal ethical approval was obtained from the local Ethics committee (acceptance protocol number: 908/2020). The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines of the International Conference of Harmonization (ICH GCP).
Clinical evaluation
Clinical, demographic, and anthropometric parameters were evaluated at ward admission in all patients. Data about previous diseases and drug treatment were collected through direct medical interview, contact with members of the family or family doctors, and medical records. Previous cardiovascular disease was defined if a positive history of heart failure, coronary artery disease, stroke or transitory ischemic attack, aortic aneurysm or peripheral artery disease was present. A detailed clinical history related to SARS-CoV-2 infection was also taken, including potential sources of contagion, preceding symptoms, time from beginning of symptoms and hospital access. Weight and height were measured and body mass index (BMI) was calculated as weight (kg)/height2 with height expressed in (m2). Obesity was diagnosed if BMI was > 30 kg/m2. Complete laboratory assessment and arterial blood gas examination were performed at the time of hospital admission. Fraction of inspired oxygen (FiO2) was annotated and expressed as PaO2/FiO2 ratio. Estimated glomerular filtration rate (eGFR) was derived from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.
All patients received an immediate bolus of dexamethasone (6 mg i.v.) according to current treatment guidelines for COVID-19-related respiratory failure [14]. Respiratory support was also immediately delivered according to respiratory failure severity through standard oxygen therapy (low flow nasal cannula oxygen system, reservoir system and high flow VenturiMask oxygen system), continuous positive airway pressure (cPAP), or bi-level non-invasive ventilation (NIV).
Handgrip strength (HGS)
HGS measurement was performed at the time of the first clinical re-evaluation, occurring about 30 min after the beginning of immediate treatment procedures, upon achievement of a clinical steady state. HGS was measured through a calibrated Jamar hand dynamometer according to the American Society of Hand Therapists protocol [15]. All the patients were placed in seated position taking advantage, if needed, of electric adjustable beds. HGS measurement was taken with shoulder adducted, elbow flexed to 90 degrees, and forearm and wrist neutral. The operator placed the dynamometer in the patient’s dominant hand while lightly supporting the base of the dynamometer, he instructed the patient to squeeze as hard as possible for 2–3 s three times with 15 s of rest between measures on the same hand and encouraged the subject during the procedure. The test was performed by two trained medical staff (MDA, AA). The mean of three measurement at the dominant arm was used for the analysis.
Values of HGS were obtained in kilograms (Kg) and allometrically scaled to weight2/3 (normalized HGS, nHGS), according to previous literature, to exclude the confounding impact of obesity on handgrip strength [16]. Values of HGS were also compared, for each individual, with reference values derived from a cohort of 502,713 people from the general population, corresponding to the 50th percentile according to sex, age, height and arm lateralization [15]. In a test cohort of 25 healthy individuals (mean age 42 ± 13 years, 45% women), the coefficient of variation of HGS measurements performed by the same medical staff within the study protocol was 5.3%.
Outcome measures and sample size
The study design was prospective observational. The study hypothesis was that patients with higher than mean nHGS, compared to subjects with lower than mean nHGS were at increased risk of reaching the main endpoint. The main endpoint was the first occurrence of a composite outcome including death and/or need for endotracheal intubation within the acute phase of the disease, fixed at 14 days from the time of first HGS measurement. Those patients who were early discharged were contacted by phone by medical staff. Need for endotracheal intubation, based on the clinical course of the COVID-19-related respiratory failure, was judged on each patient together with the emergency physician/critical care consult team. This was mainly based on failure of non-invasive ventilation to maintain an adequate peripheral blood oxygenation or on the presence of clinical findings, e.g. fatigue of respiratory muscles. Potential contraindications to endotracheal intubation (e.g. upper way obstruction requiring surgical airway), or do-not-intubate (DNI) orders did not affect endpoint adjudication (e.g. if a patient was deemed to receive endotracheal intubation but was not intubated based on a DNI order, he was judged as reaching the main endpoint).
Assuming, from previous literature, an intubation rate of 20% in non-ICU patients with severe COVID-19 [17], it was estimated that enrollment of 114 patients would provide a ± 10% difference in the rate of composite endpoint occurring in patients with higher than mean nHGS compared to patients with lower than mean nHGS, with adequate power (70%) to rule out a Type I error with an alpha level of 0.05%.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation or standard error where expressly reported. The assumption of satisfactory normal distribution was tested for all the examined variables by the Kolmogorov–Smirnov Z test. Pearson’s and Spearman’s correlation coefficients were used to assess the strength of correlation between variables. Differences between groups were tested with univariate comparisons and multivariate general linear models. The 14-day outcome of the two groups separated by the median value of the variable of interest (nHGS) were compared using Kaplan–Meier survival analysis. Univariate comparisons and multivariate Cox-proportional hazard models tested the association between nHGS and the main endpoint, with nHGS introduced as a continuous variable. In the multivariate model, age, sex, BMI, PaO2/FiO2 ratio, eGFR, and comorbidities such as hypertension, diabetes and previous cardiovascular (CV) disease were also included. The software used for statistical analysis was Statistical Package for Social Sciences (SPSS) version 26. A p value less than 0.05 was considered statistically significant.
Results
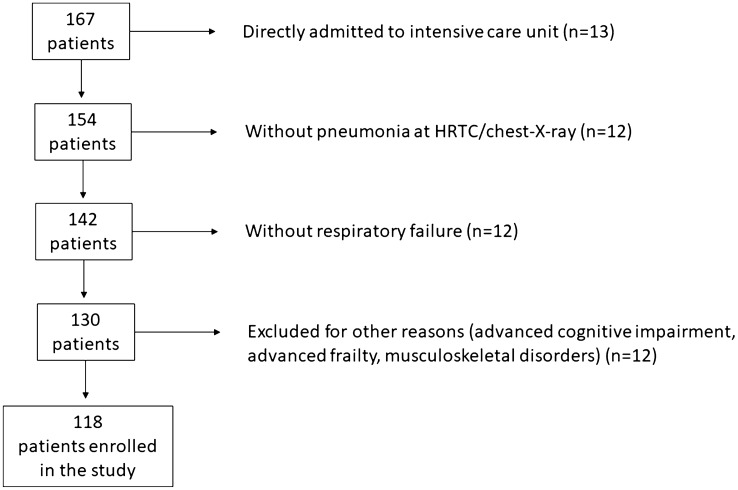
From an unselected population of 154 patients hospitalized for COVID-19, 13 patients were directly admitted to ICU, 12 patients were excluded for absence of radiological signs of pneumonia at the chest-X-ray or HRCT scan, 12 patients were excluded for the absence of respiratory failure, and further 12 patients were excluded for the following reasons: 9 for advanced cognitive impairment, 2 for muscular disorders, 1 for cachexia and frailty. The remaining 118 patients (78 males, 40 females) were considered for the subsequent analysis. The trial profile is displayed in Fig. 1.
Fig. 1 .
Trial profile of the study
The main clinical and anthropometric characteristics are reported in Table 1. Patients were admitted to the hospital after 8 ± 6 days from the beginning of symptoms. Hypertension was present in the 46% of the study population, obesity was present in the 34%, 12% of patients reported a positive history of previous CV disease, 12% were diabetics. 13% of patients had an eGFR < 60 mL/min/1.73m2. After clinical stabilization, 15 patients (13%) were on treatment with NIV, 38 (32%) required cPAP, 65 (55%) were on conventional oxygen support. "Trial profile of thestudy".
Table 1.
Clinical characteristics of the study population (n = 118)
| Age, years | 63 ± 12 |
|---|---|
| Men, % | 66 |
| Height, cm | 170 ± 10 |
| Weight, Kg | 85 ± 17 |
| BMI, Kg/m2 | 29.1 ± 4.8 |
| Previous medical history | |
| Hypertension, % | 46 |
| Obesity, % | 34 |
| T2DM, % | 12 |
| Current smoking, % | 14 |
| Previous CV disease, % | 12 |
| Respiratory function | |
| PaO2/FiO2 ratio | 201 ± 77 |
| NIV, n (%) | 15 (13) |
| cPAP, n (%) | 38 (32) |
| Conventional oxygen treatment, n (%) | 65 (55) |
| Laboratory assessment | |
| eGFR < 60 mL/min/1,73m2, % | 13 |
| Serum CRP, mg/dL | 9.1 ± 6.1 |
| Serum ferritin, ng/L | 806 ± 680 |
| Serum LDH, U/L | 329 ± 111 |
| Absolute lymphocyte count/mm3 | 0.84 ± 0.41 |
| Serum CPK, U/L | 148 ± 232 |
| Serum albumin, g/dL | 3,3 ± 0.4 |
| Handgrip strength | |
| Handgrip strength, Kg | 27.1 ± 10 |
| Handgrip strength/weight2/3, Kg/Kg2/3 | 1.32 ± 0.5 |
| Measured handgrip strength/reference handgrip strength, % | 70 ± 16 |
| Measured—reference handgrip strength*, Kg | − 8.1 ± 7 |
*Reference handgrip strength was derived in each individual according to sex, age, height and arm lateralization (see Ref. 47). BMI body mass index, T2DM type 2 diabetes mellitus, COPD chronic obstructive pulmonary disease, CV cardiovascular, PaO2/FiO2 partial oxygen pressure/fraction of inspired oxygen, NIV non-invasive ventilation, cPAP continuous positive airway pressure, eGFR estimated glomerular filtration rate, CRP c-reactive protein, LDH: lactate dehydrogenase, CPK creatine phosphokinase
Mean HGS was 27.1 ± 10 kg, mean nHGS was 1.32 ± 0.5 kg/Kg2/3. HGS was, on average, 70 ± 16% of the expected reference values (− 8.1 ± 7 kg lower than reference HGS, p < 0.001). Lower HGS values compared to reference were found in 103 out of 118 patients (87%).
During the following 14 days from study admission, 22 patients reached the main end-point, which was endotracheal intubation in all patients. nHGS was lower in subjects reaching the main end-point than in the remaining population (1.21 ± 0.5 kg/Kg2/3 vs 1.49 ± 0.4 kg/Kg2/3, p = 0.01). These subjects were also more frequently diabetics and had more often a positive history of CV disease (Table 2). They also differed in terms of eGFR and PaO2/FiO2 ratio, but not in terms of age, sex, BMI, serum CRP, lactate dehydrogenase (LDH), CPK, ferritin levels, and absolute lymphocyte count at hospital admission. The percentage of measured/reference HGS was 26% lower in subjects reaching the main end-point vs those not reaching the main end-point (60% ± 16% vs 83% ± 15%, p < 0.01).
Table 2.
Clinical findings in patients reaching vs not reaching the main endpoint according to the study protocol
| Patients reaching the main endpoint (n = 22) | Patients not reaching the main endpoint (n = 96) | P | |
|---|---|---|---|
| Age, years | 66.4 ± 12 | 62.4 ± 9 | 0.15 |
| Sex M, % | 68 | 67 | 0.91 |
| BMI, Kg/m2 | 29.8 ± 4 | 29.0 ± 5 | 0.50 |
| Hypertension, % | 69 | 49 | 0.16 |
| T2DM, % | 31 | 7 | < 0.01 |
| Previous CV disease, % | 31 | 6 | < 0.01 |
| eGFR, mL/min/1.73m2 | 75 ± 25 | 90 ± 25 | 0.01 |
| Serum CRP, mg/dL | 11.6 ± 4 | 8.9 ± 7 | 0.09 |
| Serum LDH, U/L | 357 ± 98 | 321 ± 115 | 0.21 |
| Serum ferritin, ng/L | 1054 ± 871 | 751 ± 620 | 0.16 |
| Serum CPK, U/L | 190 ± 265 | 145 ± 238 | 0.45 |
| Absolute lymphocyte count/mm3 | 0.75 ± 0.27 | 0.82 ± 0.43 | 0.47 |
| PaO2/FiO2 ratio | 161 ± 58 | 208 ± 84 | 0.03 |
| Handgrip strength/weight2/3, Kg/Kg2/3 | 1.21 ± 0.09 | 1.49 ± 0.06 | 0.01 |
| Measured handgrip strength/reference handgrip strength*, % | 60 ± 16 | 83 ± 15 | < 0.01 |
| Measured handgrip strength < reference handgrip strength*, % | 100 | 85 | 0.04 |
*Reference handgrip strength was derived in each individual according to sex, age, height and arm lateralization (see Ref. 47). BMI body mass index, T2DM type 2 diabetes mellitus, COPD chronic obstructive pulmonary disease, CV cardiovascular, PaO2/FiO2 partial oxygen pressure/fraction of inspired oxygen, NIV non-invasive ventilation, cPAP continuous positive airway pressure, eGFR estimated glomerular filtration rate, CRP c-reactive protein, LDH lactate dehydrogenase, CPK creatine phosphokinase
The Kaplan–Meyer curve showed the probability of the main end-point stratified by mean nHGS, corresponding to 1.32 kg/Kg2/3 (Logrank p value = 0.03, Fig. 2). In a multivariate Cox-proportional hazard model, nHGS (adjusted hazard ratio -aHR- for each nHGS decrease of 0.5 kg/Kg2/3 = 1.99, 95% CI 1.01–3.91, p = 0.03) inversely predicted the occurrence of the main end-point independently from age, sex, BMI, PaO2/FiO2 ratio, eGFR, and comorbidities such as hypertension, diabetes and previous CV disease. eGFR (aHR for each 10 mL/min/1.73 m2 decrease 1.30, 95% CI 1.03–1.64, p = 0.03) and history of previous CV disease (aHR 3.89, 95% CI 1.14–13.24, p < 0.01) were two other independent predictors of adverse outcome in the study population (Table 3).
Fig. 2.
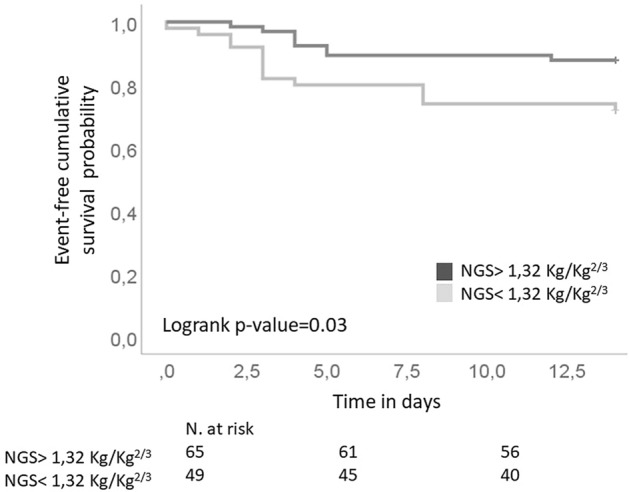
Kaplan–Meyer curve showing the probability of the main end-point stratified by mean normalized Handgrip Strength (nHGS), corresponding to 1.32 kg/Kg.2/3
Table 3.
Univariate and multivariate Cox-proportional hazard models exploring the role of independent predictors for the time to reach the main outcome (first occurrence of death and/or endotracheal intubation) during the acute phase of the disease
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | Lower | Upper | p value | HR | Lower | Upper | p value | |
| nHGS (each 0.5 kg/Kg2/3 decrease) | 2.47 | 1.06 | 5.79 | 0.03 | 1.99 | 1.01 | 3.91 | 0.03 |
| Age (years) | 1.28 | 0.88 | 1.86 | 0.19 | – | – | – | – |
| Male sex | 1.02 | 0.42 | 2.51 | 0.95 | – | – | – | – |
| BMI (Kg/m2) | 1.03 | 0.95 | 1.11 | 0.48 | – | – | – | – |
| Previous CV disease | 6.63 | 2.47 | 17.85 | < 0.01 | 3.89 | 1.14 | 13.24 | < 0.01 |
| eGFR (each 10 mL/min/1.73m2 decrease) | 1.32 | 1.08 | 1.71 | 0.01 | 1.30 | 1.03 | 1.64 | 0.03 |
| Hypertension | 2.06 | 0.72 | 5.95 | 0.18 | – | – | – | – |
| Diabetes mellitus | 4.63 | 1.60 | 13.4 | < 0.01 | – | – | – | – |
| PaO2/FiO2 ratio (each 10 unit decrease) | 1.08 | 1.01 | 1.17 | 0.035 | – | – | – | – |
All the listed variables were included in the multivariate model. The adjusted hazard ratio of variables independently associated with the main outcome were reported in terms of hazard ratio (HR), 95% confidence intervals (lower, upper), and p value. HR of variables not significantly associated with the main outcome in the multivariate model were not reported. nHGS handgrip strength normalized to weight2/3, eGFR estimated glomerular filtration rate, BMI body mass index. CV cardiovascular, PaO2/FiO2 partial oxygen pressure/fraction of inspired oxygen
Discussion
Results from our study showed that in a cohort of patients hospitalized in non-ICU wards for COVID-19-related interstitial pneumonia and respiratory failure, HGS normalized to weight 2/3 inversely predicted the risk of future short-term adverse clinical events, such as endotracheal intubation. Such risk was independent from the presence of confounders such as age, sex, BMI, other comorbidities, and indexes related to respiratory failure. Given the remarkable negative impact of endotracheal intubation on overall survival in severe COVID-19 [18], our observations raise the hypothesis that in patients with COVID-19-related respiratory failure, measurement of HGS could represent an easy, fast and portable tool to early identify patients at higher risk of complications and adverse prognosis and, therefore, might help in stratifying the risk of future events beyond classical risk factors. Indeed, in our population, each decrease in 10 kg/Kg2/3 upper arm muscle strength was associated to a nearly twofold risk of endotracheal intubation. We also found, in line with previous reports [19], that a positive history of CV diseases and reduced eGFR also independently predicted adverse clinical outcomes in COVID-19-related pneumonia.
The relationship between HGS, SARS-CoV-2 infection and severity of COVID-19 has been the object of previous research. In a population of older adults undergoing longitudinal HGS assessment, incident SARS-CoV-2 infection was independently associated with a more marked age-related HGS decline in comparison to seronegative individuals [20]. In the presence of SARS-CoV-2 infection, low HGS was also associated with an increased risk of hospitalization for COVID-19 [21]. Finally, among COVID-19 hospitalized patients, HGS was consistently found to be closely correlated to indexes of disease severity even after accounting for age and gender differences [11]. All these observations are concordant in generating the hypothesis that SARS-CoV-2 infection and related COVID-19 could impair muscular strength. However, to the best of our knowledge, our study was the first, to date, to unveil the potential of HGS to predict future adverse clinical outcomes in the hospital setting. This could be of high importance, especially in low-resource settings, to improve risk stratification at the time of first clinical stabilization after hospital admission, to set healthcare priorities and rationalize the allocation of medical resources.
It is well acknowledged that HGS is marker of global sarcopenia and muscular impairment and its association with increased risk of all-cause mortality has been previously described in population-based studies [7, 8]. However, the pathophysiological mechanisms through which low HGS could affect short-term clinical outcomes in COVID-19-associated pneumonia remain to be demonstrated. Indeed, a low HGS, evaluated at Hospital admission, could not only reflect the impact of COVID-19 but also be influenced by pre-existing chronic conditions known to affect global muscular impairment and sarcopenic status. Therefore, a certain loss of muscular strength might already have been supposed in people with chronic diseases [22, 23]. The evidence, however, that a very high number of patients showed HGS values far below expected reference values [15] also suggest that COVID-19 and related consequences might have an independent role in determining reduced HGS in the early phases of the disease. Our study was not designed to distinguish the impact of pre-existing sarcopenia and muscle wasting on future clinical outcomes, therefore, further studies are needed to better describe this aspect. Nevertheless, our results demonstrate the ability of HGS as a useful and inexpensive tool to predict risk of future adverse outcomes in patients hospitalized for COVID-19 independently from the etiology of muscular impairment.
In obese individuals, the evaluation of sarcopenia through the measurement of muscular strength is often masked by larger muscle size and for this reason, it frequently goes unnoticed [24, 25]. This is of importance, given that obesity is highly prevalent among patients with severe COVID-19, and it is also an independent risk factor of adverse outcomes [26]. Of note, in our population, we found reduced HGS values even after accounting for allometrical normalization of HGS for weight, and also that the association with nHGS with adverse outcomes remained significant in the multivariate model even after considering the potential impact of BMI on the outcome measure.
Other limitations should be acknowledged. Results from our study should be conceived only as hypothesis-generating and need further confirmation in large-scale studies. The relatively small sample size translates into a lack of adequate power to correctly assess if HGS has discriminative ability or added value in terms of net reclassification improvement when combined to classical severity scores currently proposed in COVID-19-related respiratory failure to stratify the risk [27]. The evaluation of HGS was performed only at study admission and results do not take into account of the potential impact of HGS changes during the subacute phase of COVID-19, during early phases of hospital stay, and also in response to targeted treatments. Other measures of muscular mass, physical performance, or indirect signs of muscle loss as indicators of sarcopenic status were not accounted in the present analysis, therefore, their potential impact on prognosis cannot be compared with that of HGS. Finally, given the lack information about previous nutritional status and degree of habitual physical exercise, their relative contribution on measured HGS and on subsequent clinically relevant events could not be precisely assessed.
Our results are in favor of the hypothesis that screening of wasting disorders such as sarcopenia and muscular impairment in people hospitalized for COVID-19, through easy to perform, highly reproducible and clinically relevant tools such as HGS, may be useful to improve risk prediction. Our results could also inspire further interventional studies targeted to evaluate the potential role of treatment with high-protein diets and nutritional supplements during hospital stay in mitigating muscle energy loss and possibly to reduce the incidence of adverse clinical outcomes in patients with severe COVID-19-related respiratory failure.
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement.
Declarations
Conflict of interest
The authors declare the absence of any financial or non-financial interest that are directly or indirectly related to the work submitted for publication. The authors also declare the absence of competing interests directly or indirectly tied to this research, or professional interests or personal beliefs that may influence this research. All authors contributed to the study conception and design. The first draft of the manuscript was written by GP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute for Health Metrics and Evaluation. Estimation of total mortality due to COVID-19. http://www.healthdata.org/special-analysis/estimation-excess-mortality-due-covid-19-andscalars-reported-covid-19-deaths (accessed April 16, 2022).
- 2.Phillips MC, Sarff L, Banerjee J, Coffey C, Holtom P, Meurer S, Wald-Dickler N, Spellberg B. Effect of mortality from COVID-19 on inpatient outcomes. J Med Virol. 2022;94:318–326. doi: 10.1002/jmv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, Wei J, Gong Z, Zhou C, Yu H, Yu M, Lei H, Cheng F, Zhang B, Xu Y, Wang G, Dong W. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan. China Clin Microbiol Infect. 2020;26:767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan KH, Farouji I, Abu Hanoud A, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am J Emerg Med. 2020;38:1548.e1–1548.e3. doi: 10.1016/j.ajem.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 7.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S; Prospective Urban Rural Epidemiology (PURE) Study investigators (2015) Prognostic value of grip strength: findinHGS from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 386:266-273. 10.1016/S0140-6736(14)62000-6
- 8.Kim Y, Wijndaele K, Lee DC, Sharp SJ, Wareham N, Brage S. Independent and joint associations of grip strength and adiposity with all-cause and cardiovascular disease mortality in 403,199 adults: the UK Biobank study. Am J Clin Nutr. 2017;106:773–782. doi: 10.3945/ajcn.117.156851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali NA, O'Brien JM Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, Connors AF Jr, Marsh CB; Midwest Critical Care Consortium (2008) Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 178:261-268. 10.1164/rccm.200712-1829OC [DOI] [PubMed]
- 10.Tuzun S, Keles A, Okutan D, Yildiran T, Palamar D (2021) Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur J Phys Rehabil Med. 57:653–662. 10.23736/S1973-9087.20.06563-6 [DOI] [PubMed]
- 11.Kara Ö, Kara M, Akın ME, Özçakar L. Grip strength as a predictor of disease severity in hospitalized COVID-19 patients. Heart Lung. 2021;50:743–747. doi: 10.1016/j.hrtlng.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kevadiya BD, Machhi J, Herskovitz J, Oleynikov MD, Blomberg WR, Bajwa N, Soni D, Das S, Hasan M, Patel M, Senan AM, Gorantla S, McMillan J, Edagwa B, Eisenberg R, Gurumurthy CB, Reid SPM, Punyadeera C, Chang L, Gendelman HE. Diagnostics for SARS-CoV-2 infections. Nat Mater. 2021;20:593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed April 16, 2022. [PubMed]
- 15.Spruit MA, Sillen MJ, Groenen MT, Wouters EF, Franssen FM. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc. 2013;14:775.e5–e11. doi: 10.1016/j.jamda.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32:615–631. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed] [Google Scholar]
- 17.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, Carrier M, Reynolds HR, Kumar A, Turgeon AF, Kornblith LZ, Kahn SR, Marshall JC, Kim KS, Houston BL, Derde LPG, Cushman M, Tritschler T, Angus DC, Godoy LC, McQuilten Z, Kirwan BA, Farkouh ME, Brooks MM, Lewis RJ, Berry LR, Lorenzi E, Gordon AC, Ahuja T, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Contreras A, Costantini TW, de Brouwer S, Detry MA, Duggal A, Džavík V, Effron MB, Eng HF, Escobedo J, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Froess JD, Fu Z, Galanaud JP, Galen BT, Gandotra S, Girard TD, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Haniffa R, Hegde SM, Hendrickson CM, Higgins AM, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Huang DT, Hudock K, Hunt BJ, Husain M, Hyzy RC, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski A, King AJ, Knudson MM, Kornblith AE, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Gallego Lima F, Linstrum K, Litton E, Lopez-Sendon J, Lother SA, Marten N, Saud Marinez A, Martinez M, Mateos Garcia E, Mavromichalis S, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nicolau JC, Nunez-Garcia B, Park JJ, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pompilio M, Quigley JG, Rosenson RS, Rost NS, Rowan K, Santos FO, Santos M, Santos MO, Satterwhite L, Saunders CT, Schreiber J, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Singhal AB, Slutsky AS, Solvason D, Stanworth SJ, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Widmer RJ, Wilson JG, Yuriditsky E, Zhong Y, Berry SM, McArthur CJ, Neal MD, Hochman JS, Webb SA, Zarychanski R (2021). Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 385:777-789. 10.1056/NEJMoa2103417
- 18.Boscolo A, Pasin L, Sella N, Pretto C, Tocco M, Tamburini E, Rosi P, Polati E, Donadello K, Gottin L, Vianello A, Landoni G, Navalesi P; FERS, for the COVID-19 VENETO ICU Network (2021) Outcomes of COVID-19 patients intubated after failure of non-invasive ventilation: a multicenter observational study. Sci Rep 11:17730. 10.1038/s41598-021-96762-1 [DOI] [PMC free article] [PubMed]
- 19.Levy D, Gur E, Topaz G, Naser R, Kitay-Cohen Y, Benchetrit S, Sarel E, Cohen-Hagai K, Wand O. (2022) Mortality prediction using a modified R2CHA2DS2-VASc score among hospitalized COVID-19 patients. Intern Emerg Med. 25:1–7. 10.1007/s11739-022-02993-z. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 20.Del Brutto OH, Mera RM, Pérez P, Recalde BY, Costa AF, Sedler MJ. Hand grip strength before and after SARS-CoV-2 infection in community-dwelling older adults. J Am Geriatr Soc. 2021;69:2722–2731. doi: 10.1111/jgs.17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheval B, Sieber S, Maltagliati S, Millet GP, Formánek T, Chalabaev A, Cullati S, Boisgontier MP. Muscle strength is associated with COVID-19 hospitalization in adults 50 years of age or older. J Cachexia Sarcopenia Muscle. 2021;12:1136–1143. doi: 10.1002/jcsm.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur P, Bansal R, Bhargava B, Mishra S, Gill H, Mithal A. Decreased handgrip strength in patients with type 2 diabetes: a cross-sectional study in a tertiary care hospital in north India. Diabetes Metab Syndr. 2021;15:325–329. doi: 10.1016/j.dsx.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Hao YT. Effect of handgrip on coronary artery disease and myocardial infarction: a Mendelian randomization study. Sci Rep. 2017;7:954. doi: 10.1038/s41598-017-01073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch AA, Hayhoe RPG, Cameron D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc Nutr Soc. 2020;79:158–169. doi: 10.1017/S0029665119001150. [DOI] [PubMed] [Google Scholar]
- 25.Gingrich A, Volkert D, Kiesswetter E, Thomanek M, Bach S, Sieber CC, Zopf Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019;19:120. doi: 10.1186/s12877-019-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietri L, Giorgi R, Bégu A, Lojou M, Koubi M, Cauchois R, Grangeot R, Dubois N, Kaplanski G, Valéro R, Béliard S. Excess body weight is an independent risk factor for severe forms of COVID-19. Metabolism. 2021;117:154703. doi: 10.1016/j.metabol.2021.154703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley P, Frost F, Tharmaratnam K, Wootton DG; NW Collaborative Organisation for Respiratory Research. (2020) Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respir Res. 7(1):e000729. 10.1136/bmjresp-2020-000729 [DOI] [PMC free article] [PubMed]