Abstract
It is relatively well accepted that climate change can affect human pathogenic diseases; however, the full extent of this risk remains poorly quantified. Here we carried out a systematic search for empirical examples about the impacts of ten climatic hazards sensitive to greenhouse gas (GHG) emissions on each known human pathogenic disease. We found that 58% (that is, 218 out of 375) of infectious diseases confronted by humanity worldwide have been at some point aggravated by climatic hazards; 16% were at times diminished. Empirical cases revealed 1,006 unique pathways in which climatic hazards, via different transmission types, led to pathogenic diseases. The human pathogenic diseases and transmission pathways aggravated by climatic hazards are too numerous for comprehensive societal adaptations, highlighting the urgent need to work at the source of the problem: reducing GHG emissions.
Subject terms: Ecology, Social sciences, Environmental impact, Environmental health
A systematic review shows that >58% of infectious diseases confronted by humanity, via 1,006 unique pathways, have at some point been affected by climatic hazards sensitive to GHGs. These results highlight the mounting challenge for adaption and the urgent need to reduce GHG emissions.
Main
The ongoing emission of greenhouse gases (GHGs) is intensifying numerous climatic hazards of the Earth’s climate system, which in turn can exacerbate human pathogenic diseases1. The societal disruption caused by pathogenic diseases, as clearly revealed by the COVID-19 pandemic, provides worrisome glimpses into the potential consequences of looming health crises driven by climate change2–6. While the conclusion that climate change can affect pathogenic diseases is relatively well accepted2–6, the extent of human vulnerability to pathogenic diseases affected by climate change is not yet fully quantified. On one hand, it is increasingly recognized that the emission of GHGs has consequences on a multitude of climatic hazards of the Earth’s system (for example, warming, heatwaves, droughts, wildfires, extreme precipitation, floods, sea level rise and so on; Fig. 1)4,7. On the other hand, there is a broad taxonomic diversity of human pathogenic diseases (for example, bacteria, viruses, animals, plants, fungi, protozoa and so on), and transmission types (for example, vector-borne, airborne, direct contact and so on; glossary in Text Box 1) that can be affected by those hazards. The combination of numerous climatic hazards by the numerous pathogens reveals the potentially large number of interactions in which climatic hazards could aggravate human pathogenic diseases; with the set of ‘viable’ interactions, or interactions for which empirical data exists, approximating the full extent of human vulnerability to climate change as it relates to pathogenic diseases. Yet, with few exceptions2,8, past studies about the impact of climatic hazards on human pathogenic diseases have commonly focused on specific groups of pathogens (for example, bacteria9, viruses10), hazards (for example, warming11, precipitation12, floods13) or transmission types (for example, vector-14,15, food-16, waterborne16,17). This failure to integrate available information prevents the quantification of the full threat to humanity posed by climate change as it relates to pathogenic diseases. In this paper, we attempt to fill this gap by applying a systematic approach to screen the literature for the set of interactions in which climatic hazards have been linked to human pathogenic diseases.
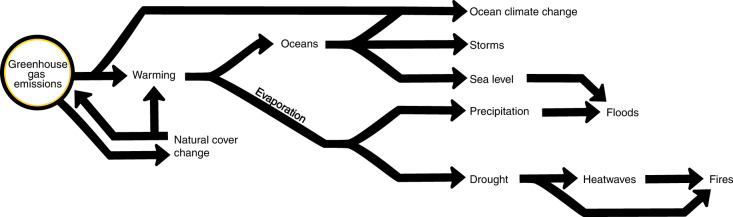
Fig. 1. Climatic hazards of the Earth’s system affected by the ongoing emission of GHGs.
We considered the following ten climate hazards. GHGs mediate the balance between incoming solar radiation and outgoing infrared radiation; thus, (1) their excess in the atmosphere causes warming. Compounded with an increased capacity of the air to hold water, warming accelerates soil water evaporation, leading to (2) drought in places that are commonly dry; excess drought can lead to (3) heatwaves when heat transfer from water evaporation ceases. Drought and heatwaves ripen the conditions for (4) wildfires. In moist places, the quick replenishment of evaporation strengthens (5) precipitation, which is prone to cause (6) floods as rain falls on moist places/saturated soils. Warming of the oceans enhances evaporation and wind speeds, intensifying downpours and the strength of (7) storms, whose surges can be aggravated by (8) sea level rise, which in turn can aggravate the impacts of floods. Uptake of CO2 in the oceans causes ocean acidification, whereas changes in ocean circulation and warming reduces oxygen concentration in seawater; these combined ocean physical–chemical changes are referred to as (9) ocean climate change in this paper. We included (10) changes in natural land cover as one of the hazards because it can be a direct emitter of GHGs via deforestation and respiration, modify temperature via albedo and evapotranspiration and because it can be a direct modifier in the transmission of pathogenic diseases59,84. This figure is intended as a justification for the hazards used and not as a full array of interactions between GHGs and hazards and feedback loops among hazards.
Box 1 Glossary.
Climate hazard: a climate-related event or trend or their impact on geophysical systems (for example, floods, droughts and sea level rise) that is linked to GHG emissions (paraphrased for brevity from Intergovernmental Panel on Climate Change AR5 synthesis report7; Fig. 1).
Disease: any disorder of structure or function in any organ of the human body.
Pathogen: any biological agent, regardless of size or taxa, capable of causing a disease.
Pathogenic disease: any disorder of structure or function in any organ of the human body caused by an organism (Methods provide additional details).
Transmission pathway: route by which one climatic hazard (for example, drought), via a transmission type (for example, waterborne), leads to the appearance of a pathogenic disease (for example, salmonellosis). Commonly, pathogenic disease emerged through alternative triggers (for example, floods, in the example above with salmonellosis) or transmission type (for example, direct contact). Each of such transmission pathways is referred as a ‘unique pathway’ in the paper. We report the number of unique pathways as they help to reveal the bulk of different adaptations required (for example, education campaigns or improved medical capacity in the pathways outlined above).
Aggravated diseases: refers to cases in which climatic hazards provoked or heightened the severity of a disease. The term is used only to refer to the nature of the relationship between a hazard and a disease, not the strength of such relationship.
Diminished diseases: refers to cases in which a given climatic hazard truncated or reduced the severity of a disease; the term is not meant to imply any strength of such relationship.
Search strategy and selection criteria
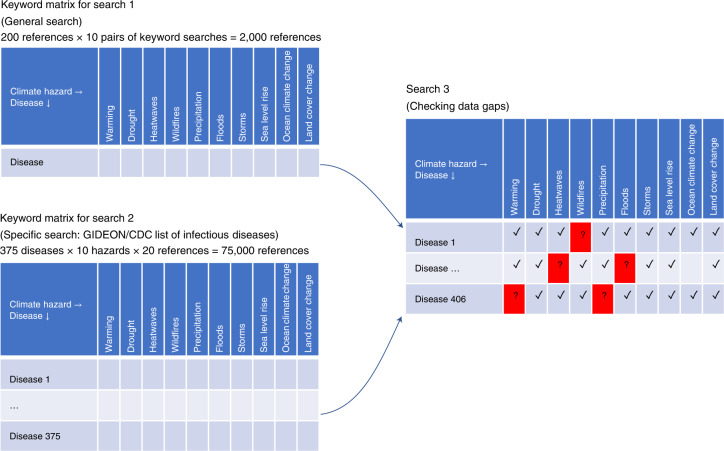
We carried out three complementary literature searches (Fig. 2) to find case examples of pathogenic diseases affected by climatic hazards. For the first search, we performed independent queries for each combination of the keyword ‘disease’ with each of ten climatic hazards known to be sensitive to GHG emissions (Fig. 1). For the second search, we carried out independent queries for scientific papers combining each disease name listed in two authoritative databases of infectious diseases (Supplementary Table 1) with each of the ten climatic hazards. Then, we created a table listing all diseases resulting from searches one and two as rows and each climatic hazard as columns; and subsequently, we carried out additional searches for combinations of diseases and climatic hazards in which the first two searches did not return any case examples. For this third search, we used alternative names of the diseases and pathogens. All searches were done in Google Scholar. We scrutinized >77,000 titles in total across these different searches. For inclusion, papers needed to report an explicit climatic hazard (for example, heatwaves, floods; Fig. 1) affecting an explicit pathogenic disease (for example, malaria, dengue; Supplementary Table 1) in an implicit place and/or time (Methods). A total of 830 references contained case examples of diseases affected by climatic hazards (Supplementary Table 2).
Fig. 2. Literature search strategy.
We carried out three complementary literature searches about case examples of diseases affected by climatic hazards. Search 1 combined as keywords ‘disease’ by each of the ten climatic hazards analysed. Search 2 combined each of the ten climatic hazards analysed by each disease name listed in two authoritative databases of infectious diseases [i.e., GIDEON (Global Infectious Disease and Epidemiology Network) and CDC (Center for Disease Control and Prevention)]. Search 3 was a data gap confirmation, and in it, we looked for all combinations (disease names by climatic hazards) in which the first two searches did not return any case example, using alternative names of the diseases, pathogens and hazards. For this latest search, an approximation of the number of references scrutinized cannot be calculated because this latter search was variable in the number of papers scrutinized until data were found or 200 citations reviewed (Methods).
Whereas pathogenic diseases are commonly associated with transmissible microbes (for example, bacteria and viruses18), here we took a broader definition of pathogens to ensure that we include non-microbial and non-transmissible agents that are causative of human illness (Methods, also ref. 18). For instance, reducing the scope to just microbes would have excluded plant19 and fungal20 allergens, which are aggravated by warming, floods and storms and are becoming a serious health problem for non-communicable outbreaks of asthma19, skin21 and respiratory20,22 illness. We found 40 diseases not listed in authoritative lists of infectious diseases (Supplementary Table 1 and Fig. 3), highlighting the number of diseases caused by biological agents and affected by climate change that would be overlooked by reducing the focus to microbes alone (Methods).
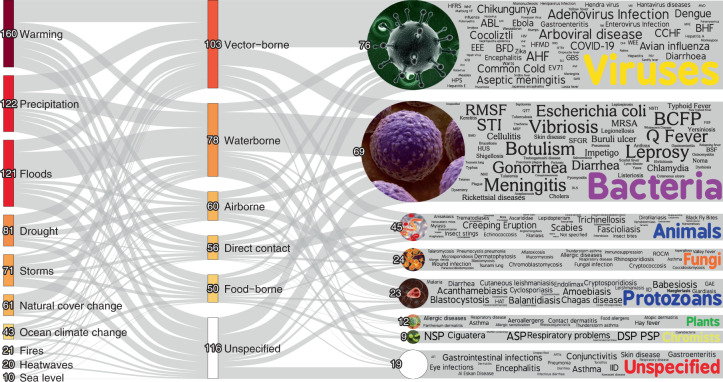
Fig. 3. Pathogenic diseases aggravated by climatic hazards.
Here we display the pathways in which climatic hazards, via specific transmission types, result in the aggravation of specific pathogenic diseases. The thickness of the lines is proportional to the number of unique pathogenic diseases. The colour gradient indicates the proportional quantity of diseases, with darker colours representing larger quantities and lighter colours representing fewer. Numbers at each node are indicative of the number of unique pathogenic diseases (caveats in Supplementary Information 1). An interactive display of the pathways and the underlying data are available at https://camilo-mora.github.io/Diseases/. Several disease names were abbreviated to optimize the use of space in the figure; their extended names are provided in Supplementary Table 1. Credits: word clouds, WordArt.com; bacteria, Wikimedia Commons (www.scientificanimations.com); other images, istockphoto.
Results and discussion
We found 3,213 empirical case examples in which climatic hazards were implicated in pathogenic diseases. All empirical case examples were related to 286 unique pathogenic diseases (Supplementary Table 1), of which 277 were aggravated (glossary in Text Box 1) by at least one climatic hazard (Fig. 3). Although 63 diseases were diminished (glossary in Text Box 1) by some climatic hazards, 54 of them were at times also aggravated by other climatic hazards; only nine pathogenic diseases were exclusively diminished by climatic hazards (Fig. 4a and Supplementary Table 1). Hereafter, we report diseases that were aggravated by climatic hazards, unless otherwise indicated. The compilation of pathogenic diseases aggravated by climatic hazards represent 58% of all infectious diseases reported to have impacted humanity worldwide (that is, out of an authoritative list of 375 infectious diseases documented to have impacted humanity (Methods), 218 were found to be aggravated by climatic hazards; Fig. 4b and Supplementary Table 1). We found 1,006 unique pathways in which climatic hazards, via different transmission types, resulted in cases of pathogenic diseases (an interactive display of the diseases is available at https://camilo-mora.github.io/Diseases/). Warming (160 unique diseases), precipitation (122), floods (121), drought (81), storms (71), land cover change (61), ocean climate change (43), fires (21), heatwaves (20) and sea level (10) were all found to influence diseases triggered by viruses (76), bacteria (69), animals (45), fungi (24), protozoans (23), plants (12) and chromists (9). Pathogenic diseases were primarily transmitted by vectors (103 unique diseases), although case examples were also found for transmission pathways involving waterborne (78), airborne (60), direct contact (56) and foodborne (50 unique diseases) (Fig. 3). Among all case examples of pathogenic diseases impacted negatively by climatic hazards, there were 19 general disease names (for example, gastrointestinal infections) that lacked information on the causal pathogen (Fig. 3 and Supplementary Table 1); for 116 diseases, there was no information provided on the transmission pathway (caveats in Supplementary Information 1).
Fig. 4. Diseases affected by climatic hazards.
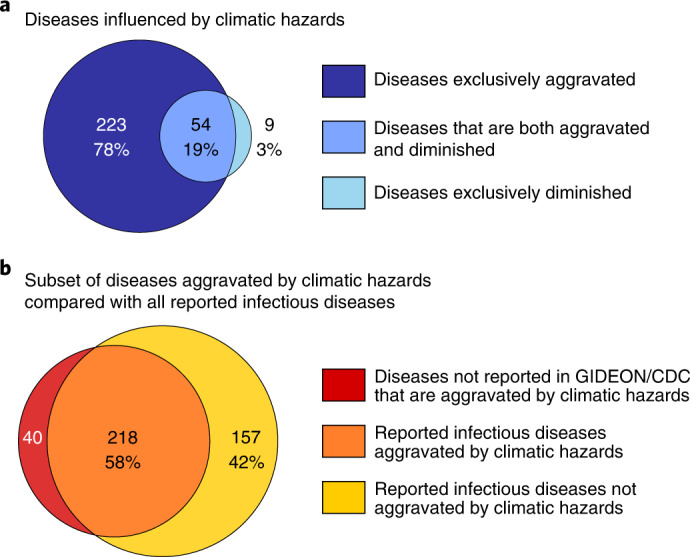
a, Discrimination of pathogenic diseases between those aggravated and diminished by climatic hazards. b, Set of diseases aggravated by climatic hazards in comparison to all reported ‘infectious’ diseases known to have affected humanity (that is, an authoritative compilation of diseases known to have affected humanity in recent history by GIDEON and CDC; Methods).
Pathogenic diseases affected by climatic hazards
While numerous biological, ecological, environmental and social factors contribute to the successful emergence of a human pathogenic disease23, at the most basic level, it depends on a pathogen and a person coming into contact, and the extent to which peoples’ resistance is diminished, or the pathogen is strengthened, by a climatic hazard. We outline empirical case examples to reveal how climatic hazards can affect these aspects in the emergence of pathogenic diseases. Case examples were grouped under given subheadings for the purpose of better presenting our results and not as an attempt to outline a contextual model about the emergence of diseases. We caution that while empirical cases indicate an effect of climatic hazards on the emergence of pathogenic disease, their relative contribution was not quantified in this study (caveats in Supplementary Information 1). The complete list of cases, transmission pathways and associated papers can be explored in detail at https://camilo-mora.github.io/Diseases/. At this website, users can navigate an interactive Sankey plot displaying how climatic hazards lead to pathogenic diseases via given transmission modes and click on any disease named in this paper to see the case example, citation and a copy of the paper. For the purpose of transparency, the web tool and background data are public. We also provide a supplement listing the papers from which case examples were obtained (Supplementary Table 2).
Climatic hazards bringing pathogens closer to people
Shifts in the geographical range of species are one of the most common ecological indications of climate change24. Warming25 and precipitation changes25, for instance, were associated with range expansion of vectors such as mosquitoes25, ticks26, fleas27, birds28 and several mammals29 implicated in outbreaks by viruses25, bacteria25, animals25 and protozoans25, including dengue25, chikungunya25, plague29, Lyme disease25, West Nile virus28, Zika25, trypanosomiasis30, echinococcosis31 and malaria25 to name a few. Climate-driven expansions were also observed in aquatic systems, including cases of Vibrio species (for example, cholera32), anisakiasis33 and envenomizations by jellyfish34. Warming at higher latitudes allowed vectors and pathogens to survive winter, aggravating outbreaks by several viruses (for example, Zika, dengue)35. Habitat disruptions caused by warming, drought, heatwaves, wildfires, storms, floods and land cover change were also associated with bringing pathogens closer to people. Spillovers from viruses (for example, Nipah virus36 and Ebola37), for instance, were associated with wildlife (for example, bats38, rodents39 and primates38) moving over larger areas foraging for limited food resources caused by drought or finding new habitats following wildfires. Likewise, reductions in snow cover caused by warming forced voles to find shelter in human inhabitations, triggering hantavirus outbreaks40. Drought also caused the congregation of mosquitoes and birds around remaining water sources facilitating the transmission of West Nile virus41. Floods and storms were commonly associated with wastewater overflow, leading to the direct and foodborne transmission of noroviruses16, hantavirus42, hepatitis43 and Cryptosporidium44. Warming was also related to melting ice and thawing permafrost exposing once-frozen pathogens45. For instance, genetic analyses of an anthrax outbreak in the Arctic circle suggest that the bacterial strain may have been ancient and emerged from an unearthed animal corpse as the frozen ground thawed46. The successful emergence of pathogens frozen in time could be regarded as a ‘Pandora’s box’, given the potentially large pool of pathogens accumulated over time and the extent to which these pathogens may be new to people45.
Climatic hazards bringing people closer to pathogens
Climatic hazards also facilitated the contact between people and pathogens by moving people closer to pathogens. Heatwaves, for instance, by increasing recreational water-related activities, have been associated with rising cases of several waterborne diseases such as Vibrio-associated infections47, primary amoebic meningoencephalitis48 and gastroenteritis49. Storms, floods and sea level rise caused human displacements implicated in cases of leptospirosis50, cryptosporidiosis51, Lassa fever52, giardiasis53, gastroenteritis54, Legionnaires’ diseases53, cholera55, salmonellosis56, shigellosis56, pneumonia57, typhoid58, hepatitis58, respiratory disease50 and skin diseases50 among others. Land use changes facilitated human encroachment into wild areas and created new ecotones that brought people into closer proximity to vectors and pathogens59, leading to numerous disease outbreaks such as Ebola60, scrub typhus61, Queensland tick typhus61, Lyme disease62, malaria63 and so on. Drought and heavy precipitation were involved in the movement of livestock to suitable areas, which in turn led to pathogen exposure and disease outbreaks (for example, anthrax64, haemorrhagic fever29). Changes in precipitation and temperature were also noted to affect human social gatherings and the transmissibility of viruses such as influenza65 and COVID-1966. Kappor et al66. suggested that heavy rainfall could exogenously induce social isolation, helping to explain lower COVID-19 cases after heavy rainfall; however, increased cases of COVID-19 were associated with increases in precipitation in Indonesia67, perhaps reflecting different behavioural responses to extreme rain. Higher temperatures have been associated with increased COVID-19 cases in some instances67, and although a mechanism was not outlined, it is possible that extreme heat forces people indoors, which can increase the risk of virus transmission, especially when combined with poor or reduced ventilation; in a related mechanism, increased transmission of coronaviruses during cool spells may be related to increased social gatherings, among other factors68.
Pathogens strengthened by climatic hazards
In addition to facilitating contacts between people and pathogens, climatic hazards also enhanced specific aspects of pathogens, including improved climate suitability for reproduction, acceleration of the life cycle, increasing seasons/length of likely exposure, enhancing pathogen vector interactions (for example, by shortening incubations) and increased virulence. Warming, for instance, had positive effects on mosquito population development, survival, biting rates and viral replication, increasing the transmission efficiency of West Nile virus69. Ocean warming accelerated the growth of harmful algal blooms and diseases caused by Pseudonitzschia sp70., blue–green cyanobacteria70 and dinoflagellates70. Ocean warming and heavy precipitation, which reduces coastal water salinity, appear to provide fertile conditions for Vibrio vulnificus32 and Vibrio cholerae71, this being a leading explanation for Vibriosis outbreaks in areas where this disease is rare72. In other cases, warming and intense precipitation increased food and habitat resources, which caused surges in rodent populations associated with cases of plague73 and hantaviruses74. Storms, heavy rainfall and floods created stagnant water, increasing breeding and growing grounds for mosquitoes and the array of pathogens that they transmit (for example, leishmaniasis75, malaria75, Rift Valley fever73, yellow fever15, St. Louis encephalitis54, dengue75 and West Nile fever76). Climatic hazards were also implicated in the increasing capacity of pathogens to cause more severe illness (that is, virulence). Heat, for instance, was related to upregulated gene expression of proteins affecting transmission, adhesion, penetration, survival and host injury by Vibrio spp77,78. Heatwaves were also suggested as a natural selective pressure towards ‘heat-resistant’ viruses, whose spillover into human populations results in increased virulence as viruses can better cope with the human body’s main defence (that is, fever)79,80. Food shortages due to drought were implicated in reduced bat autoimmune defence, which increased virus shedding, favouring outbreaks by Hendra virus81,82.
People impaired by climatic hazards
Climatic hazards have also diminished human capacity to cope with pathogens by altering body condition; adding stress from exposure to hazardous conditions; forcing people into unsafe conditions; damaging infrastructure, forcing exposure to pathogens and/or reducing access to medical care. Body malnutrition and condition, for instance, affect immunocompetence to disease83. As such, the broad effects of climatic hazards on land84 and marine85 food supply4,86, and the reduced concentration of nutrients in crops under high CO287, can directly cause human malnutrition, helping to explain the increased risk of food-deprived populations to disease outbreaks (for example, Cryptosporidium88, measles89 and cholera90). Cases of reduced resistance to various diseases were also found in relation to rapid weather variability known to be aggravated by GHG emissions65. For instance, failure of the human immune system to adjust to large changes in temperature was suggested as a likely mechanism to explain outbreaks of influenza65. Likewise, stress, via changes in cortisol and down-regulation of inflammatory response, can reduce the body’s capacity to cope with diseases91. Exposure to life-threatening conditions such as floods and hurricanes, extraneous conditions during heatwaves and depression from lost livelihood due to drought4 are a few examples in which climatic hazards are inducive to stress and cortisol variations and a likely mechanism by which climatic hazards reduce the body’s capacity to deal with pathogens. Climatic hazards also forced people into unsafe situations that facilitated the risk of disease outbreaks. In some instances, drought, by reducing water availability, forced the use of unsafe drinking water, causing outbreaks of diarrhoea, cholera and dysentery92. Reduced water resources were also conducive to poor sanitation responsible for cases of trachoma42, chlamydia42, cholera93, conjunctivitis42, Cryptosporidium26, diarrhoeal diseases42, dysentery94, Escherichia coli93, Giardia95, Salmonella93, scabies42 and typhoid fever94. Climatic hazards also affected the risk of disease by damaging critical infrastructure. For instance, floods, heavy rain and storms were related to damages in sewage systems and disrupted potable water involved in outbreaks of cholera96, diarrhoea96, hepatitis A96, hepatitis E96, leptospirosis96, acanthamoebiasis96, cryptosporidiosis96, cyclosporiasis96, giardiasis96, rotavirus96, shigellosis96 and typhoid fever96. By reducing access to medical health, basic supplies or reducing income, these hazards were associated with outbreaks of gonorrhoea97 and other venereal diseases98.
Diseases diminished by climatic hazards
Whereas the great majority of diseases were found to be aggravated by climatic hazards, some were found to be diminished (63 out of 286 diseases; Fig. 4a). Warming, for instance, appears to have reduced the spread of viral diseases probably related to unsuitable conditions for the virus or because of a stronger immune system in warmer conditions (for example, influenza65, SARS99, COVID-19100, rotaviral and noroviral enteritis101). However, we also found that most diseases that were diminished by at least one hazard were at times aggravated by another and sometimes even the same hazard. For instance, in some cases, schistosomiasis infections were reduced by floods, limiting habitat suitability of the snail host102. However, in other cases, floods increased human exposure and broadened the dispersal of the host103. Droughts also reduced the prevalence of malaria and chikungunya via reduction of breeding grounds104, but in others, drought led to increased mosquito density in reduced water pools74,105.
Concluding remarks
The global distress caused by the emergence of COVID-19 clearly revealed the considerable human vulnerability to pathogenic diseases. Such types of disease have the capacity to not only cause illness and death in large numbers of people but can also trigger broader socioeconomic consequences (for example, the cumulative financial costs of the COVID-19 pandemic could mount to US$16 trillion for the United States alone106). It should be noted that this was not an isolated event; the burden of diseases such as human immunodeficiency virus, Zika, malaria, dengue, chikungunya, influenza, Ebola, MERS and SARS cause millions of deaths each year107 and an inexplicable amount of human suffering. As demonstrated in this review, 277 human pathogenic diseases can be aggravated by the broad array of climatic hazards triggered by our ongoing emission of GHGs and include 58% of all infectious diseases known to have impacted humanity in recorded history. Furthermore, we found over 1,000 different pathways in which the array of climatic hazards, via different transmission types, resulted in disease outbreaks by a taxonomic diversity of pathogens. The sheer number of pathogenic diseases and transmission pathways aggravated by climatic hazards reveals the magnitude of the human health threat posed by climate change and the urgent need for aggressive actions to mitigate GHG emissions.
Methods
Diseases analysed
To provide a comprehensive assessment for diseases, we define ‘pathogenic diseases’ broadly as any disorder of structure or function in any organ of the human body caused by an organism, regardless of size or taxa. We used ‘pathogen’ in the broader definition of the term (that is, from the Greek pathos, which means ‘suffering’ and implies disease, and gen, which means ‘producer of’. Pathogen: producer of a disease18). Cunliffe18 reviewed the origin and roots of the term ‘pathogen’ and concluded that technically the term ‘pathogen’ refers to any agent, regardless of its nature, that causes a disease; but this original meaning is often ‘distorted’ to refer to only microorganisms (for example, viruses and bacteria)18; that was not the case in this study. Here we adopt the original definition of pathogen as any organism that can harm the human body (we avoided the use of the term ‘infectious’ disease because such a definition commonly implies microorganisms). This was done to ensure we include non-microbial and non-transmissible agents that are causative of human illness. For instance, reducing the scope to just microbes could have led to the exclusion of plant and fungal allergens, which are aggravated by warming, floods and storms and which are becoming a serious health problem for non-communicable outbreaks of asthma, skin and respiratory allergies. These allergens include not only pollen and spores but also gases. For instance, under increasing temperatures, certain tree species can increase the production of biogenic isoprenes, which in turn are precursors for the formation of ozone near the ground22, which in turn can aggravate respiratory allergies. We also found numerous reports of harm caused by animals, such as increased snake75 and insect108 envenomizations after floods75, droughts109 and heatwaves108, as these disturbances can reduce, expand or shift suitable habitats, forcing these animals into closer contact with people. These cases were included in our review for the sake of comprehensiveness. We found 40 diseases not listed in the authoritative Global Infectious Diseases and Epidemiology Network (GIDEON) or US Centers for Disease Control and Prevention (CDC) database of infectious diseases (Supplementary Table 1), highlighting the number of diseases caused by biological agents and affected by climate change that would be overlooked by reducing the focus to microbes alone.
Literature search
No single search of the literature would probably yield the full array of cases in which climatic hazards have affected pathogenic diseases. For instance, authors could name diseases by their (i) generic names or (ii) the pathogen’s names, and thus the use of one name or the other could lead to a biased assessment of the literature. To ensure a comprehensive assessment of the scientific literature, we carried out a systematic mapping of the literature. Specifically, we carried out three different systematic searches to capture the multitude of forms in which diseases may have been used/named in scientific papers (Fig. 2). We started with a general search combining as keywords ‘disease’ by each of the ten climatic hazards analysed. We reviewed the first 200 references from each search (that is, 200 references × 10 pairs of keyword searches = 2,000 references). Then, we carried out a second more specific search combining as keywords each disease name from an authoritative list of disease names by each of the ten climatic hazards analysed. The list of disease names was obtained from GIDEON and the CDC, which combined have 375 disease names. We reviewed the first 20 references from each search (that is, 375 diseases × 10 hazards × 20 references = 75,000 references). In the third literature search, we used alternative disease and pathogen names for the cases in which no data were collected in the first and second search; we also used ‘hurricanes’ and ‘typhoons’ as alternative names for storms. In this third search, for each cell in our matrix of hazards by diseases for which we were not able to find case examples, we used all alternative names for the diseases and species names of the pathogens causing the disease as listed in the GIDEON and CDC databases. In practical terms, this third search ensured that data gaps were real and not related to a limitation with the use of keywords. For this third search verifying data gaps, we looked over the first 200 references returned or until a case example was found, whichever occurred first (caveats in Supplementary Information 1). All searches were done in Google Scholar between January 2020 and May 2020. Searches were not filtered by the date in which papers were published; however, >90% of the papers with case examples were published after 2000 (Supplementary Table 2). We scrutinized >77,000 titles in total across these different searches. For inclusion, papers had to report case examples of diseases affected by climatic hazard. Our definition of a case example was any excerpt in a scientific paper in which an explicit climatic hazard was claimed to affect an explicit disease in an implicit place and time. The purpose of these criteria was to ensure that we collected examples with traceable evidence. For instance, a claim such as ‘warming could affect the spread of malaria’ was not included as it lacks evidence to a place and time of where and when the incident occurred. In turn, a claim such as ‘warmer than normal summers have resulted in increasing malaria cases in Kenya’ was included as this claim provides traceable evidence that an explicit climate hazard (that is, warming) impacted an explicit human disease (that is, malaria) in a given place (that is, Kenya). We excluded opinion-based papers that claimed a relationship between a disease and a hazard existed but did not include a case example. For instance, a paper claiming that ‘malaria is greatly affected by precipitation’ was not included; while maybe true, it fails to provide case examples of where and when the evidence is. We selected only empirical evidence-based papers (caveats in Supplementary Information 1). The search for relevant papers was done only once and manually by 11 people, including professors, post-doctoral fellows, graduate students and one undergraduate student. Participants were allocated equally and randomly the list of keywords to use in Google Scholar. Depending on the search (Fig. 2), each user scrutinized a given number of returned titles and summaries, and any reference deemed as relevant of having empirical data was added to a web database of relevant references. The database of relevant references was maintained online to avoid entering duplicated references, which may appear during alternative keyword searches. A total of 3,200 relevant references were compiled. Portable document formats (PDFs) were secured for each relevant reference. The papers with PDFs were allocated randomly to each participant, who was in charge of reading the given paper and entering in an online database any case of empirical data and/or if the paper lacked it. Once all PDFs were read and empirical data collected, each entry was read independently by at least one alternative participant to ensure the entry met the criteria implemented (that is, each entry was read by at least two people, the person who entered and an additional participant). Papers came primarily from peer-reviewed journals and scientific books; a total of 830 references contained case examples of diseases affected by climatic hazards (the complete list of references used is shown in Supplementary Table 2). Not all papers reported how given pathogens were transmitted, but when such information was provided, we also collected it. We followed the checklist for systematic review protocols suggested in Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) (Supplementary Table 3).
The list of diseases used as search criteria came from the GIDEON database (www.gideononline.com), which we complemented with the CDC National Notifiable Diseases Surveillance System (https://www.cdc.gov/nndss/). The two databases have 375 disease names combined (Supplementary Table 1). GIDEON claims to be a compilation of infectious disease cases dating back to 1348 AD, although it claims to be particularly accurate of reported infectious disease cases worldwide after 1920. GIDEON was developed in consultation with the World Health Organization and has been operational for 25 years, compiling human disease cases through dedicated searches over a broad array of public and governmental data sources. Case examples of diseases affected by climatic hazards were stored in an online database, which included the excerpts as copied directly from the paper, the given hazard, transmission type and disease. We created an interactive webpage that allows anyone to visualize the numerous pathways of transmission and the underlying data (https://camilo-mora.github.io/Diseases/).
To quantify the fraction of infectious human diseases aggravated by climate change, we paired the list of pathogenic diseases found to be aggravated by climatic hazards with the authoritative list of infectious diseases compiled in the GIDEON and CDC databases. In most cases, diseases could be paired by a direct match in the disease’s name; but in other cases, we paired diseases using disease synonyms and/or the pathogen’s scientific name (the resulting pairing of climate-aggravated diseases to the GIDEON and CDC databases is provided in Supplementary Table 1). In this paper, we conservatively reported the percentage of diseases aggravated by climate change (58%) based on direct and/or pathogen species match only. When matches were done to include the pathogen’s genus name, 74% of diseases in the GIDEON and CDC databases were found to be aggravated by climate change (Supplementary Table 1). This indicates that the numbers we report probably underestimate the total impact of climatic hazards on pathogenic diseases.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41558-022-01426-1.
Supplementary information
Supplement 1.
Three workbooks for Tables S1, S2 and S3 were combined into this file.
Acknowledgements
We thank M. Kantar for his help in the early literature search. We are also very thankful to the GIDEON and CDC databases for their extensive work compiling disease cases. The paper was developed as part of the graduate course on ‘Methods for Large Scale Analyses’ in the Department of Geography, University of Hawai‘i at Manoa.
Author contributions
All authors conceived the paper. C.M., T.M., I.M.G., J.M.D., H.v.H., T.A.K., R.O.S., C.Z.S., K.M.W. and E.C.F. carried out the literature searches and reading of papers. C.M. and T.M. analysed the data. C.M., T.M. and I.M.G. made the figures. C.M. wrote the first draft of the paper; all authors helped revise the final version of the article and were responsible for the decision to submit the article. All authors have verified the data and confirm to have full access to all the data in the study and accept responsibility for this publication.
Peer review
Peer review information
Nature Climate Change thanks Sotiris Vardoulakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All data collected as part of the literature search are publicly available at: https://github.com/Camilo-Mora/Diseases. List of diseases affected by climatic hazards is provided in Supplementary Table 1. The list of references with empirical cases is provided in Supplementary Table 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41558-022-01426-1.
References
- 1.Pörtner, H. O. et al. Climate Change 2022: Impacts, Adaptation and Vulnerability (IPCC, 2022).
- 2.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- 3.Smith, K. et al. in Climate Change 2014: Impacts, Adaptation, and Vulnerability (eds Field, C. B. et al.) 709–754 (Cambridge Univ. Press, 2014).
- 4.Mora C, et al. Broad threat to humanity from cumulative climate hazards intensified by greenhouse gas emissions. Nat. Clim. Change. 2018;8:1062–1071. doi: 10.1038/s41558-018-0315-6. [DOI] [Google Scholar]
- 5.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- 6.Epstein P. The ecology of climate change and infectious diseases: comment. Ecology. 2010;91:925–928. doi: 10.1890/09-0761.1. [DOI] [PubMed] [Google Scholar]
- 7.IPCC Climate Change 2014: Synthesis Report (eds Core Writing Team, Pachauri, R. K. & Meyer, L. A.) (IPCC, 2014).
- 8.Jaenisch T, Patz J. Assessment of associations between climate and infectious diseases: a comparison of the reports of the Intergovernmental Panel on Climate Change (IPCC), the National Research Council (NRC), and United States Global Change Research Program (USGCRP) Glob. Change Hum. Health. 2002;3:67–72. doi: 10.1023/A:1019625332705. [DOI] [Google Scholar]
- 9.Hellberg RS, Chu E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: a review. Crit. Rev. Microbiol. 2016;42:548–572. doi: 10.3109/1040841X.2014.972335. [DOI] [PubMed] [Google Scholar]
- 10.Tabachnick WJ. Climate change and the arboviruses: lessons from the evolution of the dengue and yellow fever viruses. Ann. Rev. Virol. 2016;29:125–145. doi: 10.1146/annurev-virology-110615-035630. [DOI] [PubMed] [Google Scholar]
- 11.Khasnis AA, Nettleman MD. Global warming and infectious disease. Arch. Med. Res. 2005;36:689–696. doi: 10.1016/j.arcmed.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 12.McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6:543–547. doi: 10.4161/21505594.2014.975022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahern M, Kovats RS, Wilkinson P, Few R, Matthies F. Global health impacts of floods: epidemiologic evidence. Epidemiol. Rev. 2005;27:36–46. doi: 10.1093/epirev/mxi004. [DOI] [PubMed] [Google Scholar]
- 14.Hunter PR. Climate change and waterborne and vector‐borne disease. J. Appl. Microbiol. 2003;94:37–46. doi: 10.1046/j.1365-2672.94.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 15.Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vector borne diseases. Am. J. Prev. Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Semenza JC, et al. Climate change impact assessment of food- and waterborne diseases. Crit. Rev. Environ. Sci. Technol. 2012;42:857–890. doi: 10.1080/10643389.2010.534706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols G, Lake I, Heaviside C. Climate change and water-related infectious diseases. Atmosphere. 2018;9:385. doi: 10.3390/atmos9100385. [DOI] [Google Scholar]
- 18.Cunliffe J. A proliferation of pathogens through the 20th century. Scand. J. Immunol. 2008;68:120–128. doi: 10.1111/j.1365-3083.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 19.Cecchi L, et al. Projections of the effects of climate change on allergic asthma: the contribution of aerobiology. Allergy. 2010;65:1073–1081. doi: 10.1111/j.1398-9995.2010.02423.x. [DOI] [PubMed] [Google Scholar]
- 20.Demain JG. Climate change and the impact on respiratory and allergic disease: 2018. Curr. Allergy Asthma Rep. 2018;18:22. doi: 10.1007/s11882-018-0777-7. [DOI] [PubMed] [Google Scholar]
- 21.Andersen LK, Davis MD. The effects of the El Niño Southern Oscillation on skin and skin-related diseases: a message from the International Society of Dermatology Climate Change Task Force. Int. J. Dermatol. 2015;54:1343–1351. doi: 10.1111/ijd.12941. [DOI] [PubMed] [Google Scholar]
- 22.Guenther AB, Zimmerman PR, Harley PC, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. J. Geophys. Res. Atmos. 1993;98:12609–12617. doi: 10.1029/93JD00527. [DOI] [Google Scholar]
- 23.Metcalf CJE, Lessler J. Opportunities and challenges in modeling emerging infectious diseases. Science. 2017;357:149–152. doi: 10.1126/science.aam8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 25.Nava A, Shimabukuro JS, Chmura AA, Luz SLB. The impact of global environmental changes on infectious disease emergence with a focus on risks for Brazil. ILAR J. 2017;58:393–400. doi: 10.1093/ilar/ilx034. [DOI] [PubMed] [Google Scholar]
- 26.Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009;2009:593232. doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngongeh LA, Idika IK, Ibrahim Shehu AR. warming and its impacts on parasitology/entomology. Open Parasitol. J. 2014;5:1–11. doi: 10.2174/1874421401405010001. [DOI] [Google Scholar]
- 28.LaDeau SL, Calder CA, Doran PJ, Marra PP. West Nile virus impacts in American crow populations are associated with human land use and climate. Ecol. Res. 2011;26:909–916. doi: 10.1007/s11284-010-0725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gale P, Drew T, Phipps LP, David G, Wooldridge M. The effect of climate change on the occurrence and prevalence of livestock diseases in Great Britain: a review. J. Appl. Microbiol. 2009;106:1409–1423. doi: 10.1111/j.1365-2672.2008.04036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancien J, Muguwa J, Lannes C, Bouvier JB. Tsetse and human trypanosomiasis challenge in south eastern Uganda. Int. J. Trop. Insect Sci. 1990;11:411–416. doi: 10.1017/S1742758400012832. [DOI] [Google Scholar]
- 31.Karesh WB, et al. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vezzulli L, Colwell RR, Pruzzo C. Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb. Ecol. 2013;65:817–825. doi: 10.1007/s00248-012-0163-2. [DOI] [PubMed] [Google Scholar]
- 33.Arriaza BT, Reinhard KJ, Araújo AG, Orellana NC, Standen VG. Possible influence of the ENSO phenomenon on the pathoecology of diphyllobothriasis and anisakiasis in ancient Chinchorro populations. Mem. Inst. Oswaldo Cruz. 2010;105:66–72. doi: 10.1590/S0074-02762010000100010. [DOI] [PubMed] [Google Scholar]
- 34.Kaffenberger BH, Shetlar D, Norton SA, Rosenbach M. The effect of climate change on skin disease in North America. J. Am. Acad. Dermatol. 2017;76:140–147. doi: 10.1016/j.jaad.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Coates SJ, Enbiale W, Davis MD, Andersen LK. The effects of climate change on human health in Africa, a dermatologic perspective: a report from the International Society of Dermatology Climate Change Committee. Int. J. Dermatol. 2020;59:265–278. doi: 10.1111/ijd.14759. [DOI] [PubMed] [Google Scholar]
- 36.Patz JA, et al. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy, G. J. et al. in Climate Change and Health (ed Leal, W) 475–514 (Springer, 2016).
- 38.Kontra JM. Zombie infections and other infectious disease complications of global warming. J. Lancaster Gen. Hosp. 2017;12:12–16. [Google Scholar]
- 39.Charron, D., Fleury, M., Lindsay, L. R., Ogden, N. & Schuster, C. J. in Human Health in a Changing Climate (ed Séguin, J) 173–210 (Health Canada, 2008).
- 40.Butler CD, Harley D. Primary, secondary and tertiary effects of eco-climatic change: the medical response. Postgrad. Med. J. 2010;86:230–234. doi: 10.1136/pgmj.2009.082727. [DOI] [PubMed] [Google Scholar]
- 41.Quarles W. Global warming means more pathogens. IPM Pract. 2017;35:1–8. [Google Scholar]
- 42.Patz JA, Engelberg D, Last J. The effects of changing weather on public health. Ann. Rev. Public Health. 2000;21:271–307. doi: 10.1146/annurev.publhealth.21.1.271. [DOI] [PubMed] [Google Scholar]
- 43.Yavarian J, Shafiei-Jandaghi NZ, Mokhtari-Azad T. Possible viral infections in flood disasters: a review considering 2019 spring floods in Iran. Iran. J. Microbiol. 2019;11:85–89. [PMC free article] [PubMed] [Google Scholar]
- 44.Boxall ABA, et al. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ. Health Perspect. 2009;117:508–514. doi: 10.1289/ehp.0800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu R, Trubl G, Taş N, Jansson JK. Permafrost as a potential pathogen reservoir. One Earth. 2022;5:351–360. doi: 10.1016/j.oneear.2022.03.010. [DOI] [Google Scholar]
- 46.Gross M. Permafrost thaw releases problems. Curr. Biol. 2019;29:R39–R41. doi: 10.1016/j.cub.2018.12.045. [DOI] [Google Scholar]
- 47.Baker-Austin C, et al. Heat wave-associated vibriosis, Sweden and Finland, 2014. Emerg. Infect. Dis. 2016;22:1216. doi: 10.3201/eid2207.151996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghanchi NK, et al. Case series of Naegleria fowleri primary ameobic meningoencephalitis from Karachi, Pakistan. Am. J. Trop. Med. Hyg. 2017;97:1600–1602. doi: 10.4269/ajtmh.17-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waits A, Emelyanova A, Oksanen A, Abass K, Rautio A. Human infectious diseases and the changing climate in the Arctic. Environ. Int. 2018;121:703–713. doi: 10.1016/j.envint.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 50.Oskorouchi HR, Nie P, Sousa-Poza A. The effect of floods on anemia among reproductive age women in Afghanistan. PLoS ONE. 2018;13:e0191726. doi: 10.1371/journal.pone.0191726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector‐borne diseases. Ann. N. Y. Acad. Sci. 2019;1436:157. doi: 10.1111/nyas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clegg J. Influence of climate change on the incidence and impact of arenavirus diseases: a speculative assessment. Clin. Microbiol. Infect. 2009;15:504–509. doi: 10.1111/j.1469-0691.2009.02847.x. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen HQ, Huynh TTN, Pathirana A, Van der Steen P. Microbial risk assessment of tidal-induced urban flooding in Can Tho City (Mekong Delta, Vietnam) Int. J. Environ. Res. Public. Health. 2017;14:1485. doi: 10.3390/ijerph14121485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivers LC, Ryan ET. Infectious diseases of severe weather-related and flood-related natural disasters. Curr. Opin. Infect. Dis. 2006;19:408–414. doi: 10.1097/01.qco.0000244044.85393.9e. [DOI] [PubMed] [Google Scholar]
- 55.Cornell, K. Climate change and infectious disease patterns in the United States: public health preparation and ecological restoration as a matter of justice. MSc thesis, Goucher College (2016).
- 56.Mishra V, et al. Climate change and its impacts on global health: a review. Pharma Innov. 2019;8:316–326. [Google Scholar]
- 57.Lemonick DM. Epidemics after natural disasters. Am. J. Clin. Med. 2011;8:144–152. [Google Scholar]
- 58.Khan AE, Xun WW, Ahsan H, Vineis P. Climate change, sea-level rise, and health impacts in Bangladesh. Environ. Sci. Policy Sustain. Dev. 2011;53:18–33. doi: 10.1080/00139157.2011.604008. [DOI] [Google Scholar]
- 59.Jones BA, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl Acad. Sci. USA. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zell R, Krumbholz A, Wutzler P. Impact of global warming on viral diseases: what is the evidence? Curr. Opin. Biotechnol. 2008;19:652–660. doi: 10.1016/j.copbio.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McFarlane RA, Sleigh AC, McMichael AJ. Land-use change and emerging infectious disease on an island continent. Int. J. Environ. Res. Public. Health. 2013;10:2699–2719. doi: 10.3390/ijerph10072699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White RJ, Razgour O. Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land‐use change. Mammal. Rev. 2020;50:336–352. doi: 10.1111/mam.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myers SS, et al. Human health impacts of ecosystem alteration. Proc. Natl Acad. Sci. USA. 2013;110:18753–18760. doi: 10.1073/pnas.1218656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munang’andu HM, et al. The effect of seasonal variation on anthrax epidemiology in the upper Zambezi floodplain of western Zambia. J. Vet. Sci. 2012;13:293–298. doi: 10.4142/jvs.2012.13.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Q, et al. Changing rapid weather variability increases influenza epidemic risk in a warming climate. Environ. Res. Lett. 2020;15:044004. doi: 10.1088/1748-9326/ab70bc. [DOI] [Google Scholar]
- 66.Kapoor R, et al. God is in the rain: the impact of rainfall-induced early social distancing on COVID-19 outbreaks. J. Health Econ. 2020;81:102575. doi: 10.1016/j.jhealeco.2021.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raza A, Khan MTI, Ali Q, Hussain T, Narjis S. Association between meteorological indicators and COVID-19 pandemic in Pakistan. Environ. Sci. Pollut. Res. 2021;28:40378–40393. doi: 10.1007/s11356-020-11203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nichols, G. L. et al. Coronavirus seasonality, respiratory infections and weather. BMC Infect. Dis.21, 1101 (2021). [DOI] [PMC free article] [PubMed]
- 69.El-Sayed A, Kamel M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020;27:22336–22352. doi: 10.1007/s11356-020-08896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruszkiewicz JA, et al. Brain diseases in changing climate. Environ. Res. 2019;177:108637. doi: 10.1016/j.envres.2019.108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrador BRG, et al. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: a review. Environ. Health. 2015;14:29. doi: 10.1186/s12940-015-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burge CA, et al. Climate Change influences on marine infectious diseases: implications for management and society. Ann. Rev. Mar. Sci. 2014;6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- 73.Mills JN, Gage KL, Khan AS. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 2010;118:1507–1514. doi: 10.1289/ehp.0901389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gubler DJ, et al. Climate variability and change in the United States: potential impacts on vector-and rodent-borne diseases. Environ. Health Perspect. 2001;109:223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dayrit JF, Bintanjoyo L, Andersen LK, Davis MDP. Impact of climate change on dermatological conditions related to flooding: update from the International Society of Dermatology Climate Change Committee. Int. J. Dermatol. 2018;57:901–910. doi: 10.1111/ijd.13901. [DOI] [PubMed] [Google Scholar]
- 76.Myaing TT. Climate change and emerging zoonotic diseases. KKU Vet. J. 2011;21:172–182. [Google Scholar]
- 77.Kimes NE, et al. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J. 2012;6:835–846. doi: 10.1038/ismej.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh MH, Lee SM, Lee DH, Choi SH. Regulation of the Vibrio vulnificus hupA gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect. Immun. 2009;77:1208–1215. doi: 10.1128/IAI.01006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casadevall A. Climate change brings the specter of new infectious diseases. J. Clin. Invest. 2020;130:553–555. doi: 10.1172/JCI135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beyer RM, Manica A, Mora C. Shifts in global bat diversity suggest a possible role of climate change in the emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total Environ. 2021;767:145413. doi: 10.1016/j.scitotenv.2021.145413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warburton EM, Pearl CA, Vonhof MJ. Relationships between host body condition and immunocompetence, not host sex, best predict parasite burden in a bat–helminth system. Parasitol. Res. 2016;115:2155–2164. doi: 10.1007/s00436-016-4957-x. [DOI] [PubMed] [Google Scholar]
- 82.Plowright RK, et al. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus) Proc. R. Soc. B. 2008;275:861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beldomenico PM, Begon M. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 2010;25:21–27. doi: 10.1016/j.tree.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 84.Mora C, et al. Suitable days for plant growth disappear under projected climate change: potential human and biotic vulnerability. PLoS Biol. 2015;13:e1002167. doi: 10.1371/journal.pbio.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mora C, et al. Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biol. 2013;11:e1001682. doi: 10.1371/journal.pbio.1001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thiault L, et al. Escaping the perfect storm of simultaneous climate change impacts on agriculture and marine fisheries. Sci. Adv. 2019;5:eaaw9976. doi: 10.1126/sciadv.aaw9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myers SS, et al. Increasing CO2 threatens human nutrition. Nature. 2014;510:139–142. doi: 10.1038/nature13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tirado MC, Clarke R, Jaykus L, McQuatters-Gollop A, Frank J. Climate change and food safety: a review. Food Res. Int. 2010;43:1745–1765. doi: 10.1016/j.foodres.2010.07.003. [DOI] [Google Scholar]
- 89.Greene M. Impact of the Sahelian drought in Mauritania, West Africa. Lancet. 1974;303:1093–1097. doi: 10.1016/S0140-6736(74)90568-6. [DOI] [PubMed] [Google Scholar]
- 90.Cabrol J-C. War, drought, malnutrition, measles—a report from Somalia. N. Engl. J. Med. 2011;365:1856–1858. doi: 10.1056/NEJMp1111238. [DOI] [PubMed] [Google Scholar]
- 91.Cohen S, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl Acad. Sci. USA. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calow RC, MacDonald AM, Nicol AL, Robins NS. Ground water security and drought in Africa: linking availability, access, and demand. Groundwater. 2010;48:246–256. doi: 10.1111/j.1745-6584.2009.00558.x. [DOI] [PubMed] [Google Scholar]
- 93.Salvador C, Nieto R, Linares C, Díaz J, Gimeno L. Effects of droughts on health: diagnosis, repercussion, and adaptation in vulnerable regions under climate change. Challenges for future research. Sci. Total Environ. 2020;703:134912. doi: 10.1016/j.scitotenv.2019.134912. [DOI] [PubMed] [Google Scholar]
- 94.Alhoot, M. A., Tong, W. T., Low, W. Y. & Sekaran, S. D. in Climate Change and Human Health Scenario in South and Southeast Asia (ed Akhtar, R) 243–268 (Springer, 2016).
- 95.Yusa A, et al. Climate change, drought and human health in Canada. Int. J. Environ. Res. Public Health. 2015;12:8359–8412. doi: 10.3390/ijerph120708359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ligon BL. Infectious Diseases that Pose Specific Challenges After Natural Disasters: A Review. Semin. Pediatr. Infect. Dis. 2006;17:36–45. doi: 10.1053/j.spid.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 97.Nsuami MJ, Taylor SN, Smith BS, Martin DH. Increases in gonorrhea among high school students following hurricane Katrina. Sex. Transm. Infect. 2009;85:194–198. doi: 10.1136/sti.2008.031781. [DOI] [PubMed] [Google Scholar]
- 98.Jochelson K. HIV and syphilis in the Republic of South Africa: the creation of an epidemic. Afr. Urban Q. 1991;6:20–34. [Google Scholar]
- 99.Sobral MFF, Duarte GB, da Penha Sobral AIG, Marinho MLM, de Souza Melo A. Association between climate variables and global transmission of SARS-CoV-2. Sci. Total Environ. 2020;729:138997. doi: 10.1016/j.scitotenv.2020.138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J, et al. Impact of meteorological factors on the COVID-19 transmission: a multi-city study in China. Sci. Total Environ. 2020;726:138513. doi: 10.1016/j.scitotenv.2020.138513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chua PL, et al. Global projections of temperature-attributable mortality due to enteric infections: a modelling study. Lancet Planet. Health. 2021;5:e436–e445. doi: 10.1016/S2542-5196(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 102.McCreesh N, Booth M. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 2013;29:548–555. doi: 10.1016/j.pt.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Wu X, Tian H, Zhou S, Chen L, Xu B. Impact of global change on transmission of human infectious diseases. Sci. China Earth Sci. 2014;57:189–203. doi: 10.1007/s11430-013-4635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moreno AR. Climate change and human health in Latin America: drivers, effects, and policies. Reg. Environ. Change. 2006;6:157–164. doi: 10.1007/s10113-006-0015-z. [DOI] [Google Scholar]
- 105.McCann DG, Moore A, Walker M-E. The water/health nexus in disaster medicine: I. drought versus flood. Curr. Opin. Environ. Sustain. 2011;3:480–485. doi: 10.1016/j.cosust.2011.10.011. [DOI] [Google Scholar]
- 106.Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324:1495–1496. doi: 10.1001/jama.2020.19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vos T, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsiao M-H, et al. Environmental factors associated with the prevalence of animal bites or stings in patients admitted to an emergency department. J. Acute Med. 2012;2:95–102. doi: 10.1016/j.jacme.2012.09.002. [DOI] [Google Scholar]
- 109.Jones NE, Baker MD. Toxicologic exposures associated with natural disasters: gases, kerosene, ash, and bites. Clin. Pediatr. Emerg. Med. 2012;13:317–323. doi: 10.1016/j.cpem.2012.09.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1.
Three workbooks for Tables S1, S2 and S3 were combined into this file.
Data Availability Statement
All data collected as part of the literature search are publicly available at: https://github.com/Camilo-Mora/Diseases. List of diseases affected by climatic hazards is provided in Supplementary Table 1. The list of references with empirical cases is provided in Supplementary Table 2.