Abstract
Purpose of Review
West Nile virus (WNV) is an arbovirus transmitted by mosquitos of the genus Culex. Manifestations of WNV infection range from asymptomatic to devastating neuroinvasive disease leading to flaccid paralysis and death. This review examines WNV epidemiology and ecology, with an emphasis on travel-associated infection.
Recent Findings
WNV is widespread, including North America and Europe, where its range has expanded in the past decade. Rising temperatures in temperate regions are predicted to lead to an increased abundance of Culex mosquitoes and an increase in their ability to transmit WNV. Although the epidemiologic patterns of WNV appear variable, its geographic distribution most certainly will continue to increase. Travelers are at risk for WNV infection and its complications. Literature review identified 39 cases of documented travel-related WNV disease, the majority of which resulted in adverse outcomes, such as neuroinvasive disease, prolonged recovery period, or death.
Summary
The prediction of WNV risk is challenging due to the complex interactions of vector, pathogen, host, and environment. Travelers planning to visit endemic areas should be advised regarding WNV risk and mosquito bite prevention. Evaluation of ill travelers with compatible symptoms should consider the diagnosis of WNV for those visiting in endemic areas as well as for those returning from destinations with known WNV circulation.
Keywords: Flavivirus, Neuroinvasive, One Health, Culex mosquito, Emerging infection, Epidemiology, Imported
Introduction
West Nile virus (WNV), deriving its name from the West Nile district of Uganda [1], is a mosquito-borne arbovirus belonging to the Flaviviridae family. Now commonly found in Africa, Europe, the Middle East, North America, and West Asia, WNV epidemiology has been evolving in the past decades [2–4]. The virus itself has undergone adaptive genetic changes while expanding its geographic areas [5]. The epidemic of 1999 in the New York City area is a reminder of the ability of such viruses to leap from one hemisphere to another via migratory birds [6].
With birds and mosquitos as its natural hosts, the virus can infect a wide variety of other vertebrates as dead-end hosts, causing severe disease in some, notably humans and horses [4, 6]. In humans, the clinical course of WNV infection ranges from asymptomatic or mild febrile illness to severe neurological manifestations (West Nile neuroinvasive disease; WNND) and fatalities [2, 4, 6]. The elderly and immunocompromised hosts are at increased risk for disseminated WNV infection and for developing fatal encephalitis [7]. In animal models, an overactive inflammatory response can lead to increased blood–brain barrier (BBB) permeability for the viruses, leading to neuronal death [7]. Despite the significance of neurological involvement in severe disease, the exact mechanism is not clear. To date, the primary treatment of WNV infection has remained supportive with potential benefits from corticosteroids and IVIG [8, 9]. Multiple outbreaks have occurred in the past two decades in different parts of the world, including in travelers [10, 11]. With the ongoing SARS-CoV-2 pandemic complicating the differential diagnosis of febrile illnesses, it is timely to review WNV outbreaks and understand its evolving epidemiology. The objective of this article is to review the epidemiology, transmission, and clinical course of WNV with an emphasis on travel-related infections. We conducted a PubMed search using the syntax (west nile) AND (travelers) or (imported) to obtain the cases identified in travelers.
Epidemiology
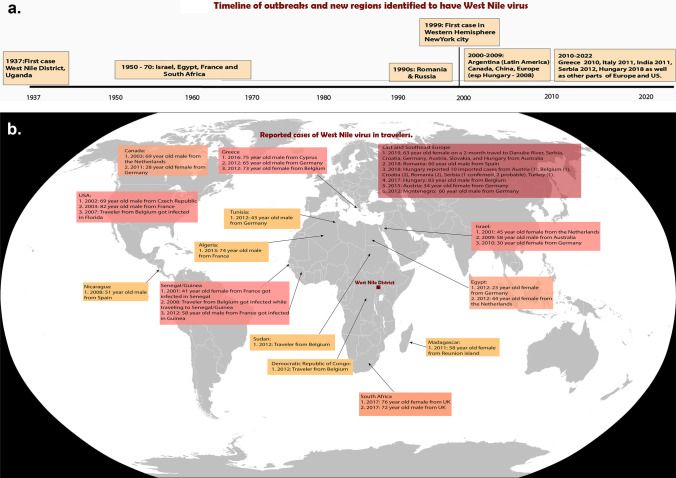
The first case of WNV was diagnosed in a woman presenting with a febrile illness in 1937 in Uganda [1]. After that, cases were reported in Israel, Egypt, France, and South Africa throughout the 1950s to 1970s, followed by large outbreaks in Romania and Russia in the 1990s [12–14]. WNV infections were reported for the first time in Algeria and Morocco in 1994 and 1996, respectively [15•]. In 1999, the first WNV cases in the Western Hemisphere were identified in New York City, where 59 patients were hospitalized, among whom 7 died (12%) [16]. Since this outbreak, it has spread throughout North America, with many reported cases in humans, while sporadic cases as well as several outbreaks have been reported in Europe. To explain the difference between the two regions, various hypotheses have been suggested, including the difference in virulence, susceptibility of the bird species, the competence of mosquitoes, and partial protection due to cross-reactivity with other flaviviruses in Europe, such as the tick-borne encephalitis virus [17].
In the twenty-first century, major WNV outbreaks occurred in Argentina, Canada, China, and throughout Europe, notably in Hungary in 2008, Greece in 2010, Italy in 2011, and Serbia in 2012 [18–25]. In Europe, there were, on average, 18 newly affected areas annually between 2011 and 2017, and 45 additional areas reported in 2018, with most infections occurring from early summer to early autumn and peaking in August [26•]. Until 2017, the countries with the highest number of cases were Greece, Italy, Romania, and Hungary [27••]. In 2018, Hungary reported 215 locally acquired and 10 imported human infections [28]. In Turkey, where Europe and Asia converge, WNV exposure in humans has been documented in all main regions of Anatolia and Thrace over a relatively long period (1973–2019) [29], while cases have been exported to other countries, such as Hungary, through returning travelers [28]. The European CDC reported a 7.2-fold increase in cases from 2017 to 2018, especially in Bulgaria (15-fold), France (13.5-fold), and Italy (10.9-fold), which is partially attributed to the unusually hot summers. In 2020, a major outbreak was reported in Spain with 77 confirmed cases of WNV, of which 72 developed neuroinvasive disease and 7 died, partly attributed to reduced activities for vector control during that season, among other factors [30–33]. In 2021, 54 infections were reported through October 7, 2021 in Greece, 52 in Italy, 7 in Hungary, 7 in Romania, 6 in Spain, 3 in Austria, 3 in Germany, and 17 in Serbia, with cases reported for the first time in Spree-Neiße in Germany and La Spezia in Italy [34].
In the USA, WNV was the most common cause of neuroinvasive arboviral disease in 2018; however, neuroinvasive disease occurred at an incidence of 0.51 per 100,000 in 2018, approximately 25% higher than the median incidence of 0.41 during 2008–2017 [35]. According to the CDC, between 2009 and 2018, the highest number of total cases in the USA occurred in California (n = 2819), Texas (n = 2043), Illinois (n = 728), and Arizona (n = 632), with North Dakota recording the highest average annual incidence of neuroinvasive disease (3.16 cases per 100,000 population), followed by South Dakota (3.06), Nebraska (1.95), and Mississippi (1.17) [35]. In 2019, WNV continued to be the most common cause of domestic arboviral neuroinvasive disease in the USA; however, an annual incidence of 0.19 per 100,000 was 53% lower than the median annual incidence during 2009–2018. The Midwestern and South Central states, particularly Texas, saw the widest decrease, as opposed to the Mountain region, where the number of cases exceeded the annual median incidence during 2009–2018 [36]. For 2021 through January 11, 2022, 2695 cases were reported in the USA, of which 1855 presented with WNND (69%), 840 presented with non-neuroinvasive disease, and 191 died (7%) [37]. Most cases were reported in Arizona (1645), Colorado (174), and California (115) [37].
Sporadic cases have also been reported from Central and South America from equines, birds, and humans [38, 39]. In 2003, a human case of WNND was diagnosed in the Bahamas, followed by 6 human cases (3 with encephalitis) in Northern Mexico in 2004 [39, 40]. In 2005, WNND cases were announced in central Cuba.
In Asia, very few human cases have been reported. Even though WNV has been isolated from pigeons and migratory birds in Korea, the first reported human case was from a traveler on a business trip to Guinea, Africa [41]. In India, initial WNV antibodies were first detected in Mumbai in 1952. In contrast to western countries, there were frequent reports of children succumbing to WNV infection in the 1980s. Similarly, serological evidence of WNV infection was reported from Pakistan in the 1970s. However, neuroinvasive disease cases from India, Pakistan, and Sri Lanka were not reported until 2011–2015. In 2011, an outbreak was reported in the southern State of India, Kerala [42]. In China, outbreaks of viral encephalitis caused by WNV infection have been recorded locally since 2013 [43]. In Australia, Kunjin virus lineage (1b) is common in the Northern region. However, clinical cases are found to be rare [44].
The epidemiological situation in Africa is less clear. Challenges lie in the similarity of WNV symptoms with those of other arboviruses and tropical diseases such as malaria and typhoid fever, poor public health data sharing, and potential cross-reactivity resulting from the use of non-specific WNV neutralization assays, and inconsistent surveillance [15•].
The Virus
WNV is an enveloped, 11-kilobase, positive sense, single-stranded RNA virus in the family Flaviviridae, phylogenetically related to other mosquito-borne flaviviruses [45], and most closely related to the neurotropic flaviviruses, Japanese encephalitis virus, and St. Louis encephalitis virus. Like other flaviviruses, the virions are spherical particles, 45–50 nm in diameter, with icosahedral symmetry. The E protein lies flat over the lipid envelope, forming a nearly complete shell around it. The E protein binds to multiple receptors on the surface of diverse cell types, facilitates virion entry via receptor-mediated endocytosis, and is the primary target of neutralizing antibodies. Host ribosomes translate the ( +) sense viral RNA into a single, long polypeptide, which is subsequently cleaved by viral and host proteases into the mature viral structural and non-structural proteins. The viral RNA-dependent RNA polymerase replicates viral genomes. Progeny genomes are packaged within nascent viral capsids, which assemble into virions on the endoplasmic reticulum, transit through the Golgi apparatus, and are secreted via exocytic vesicles that bud through the plasma membrane after a furin-mediated cleavage of the viral prM protein[45].
Up to 9 lineages of WNV have been recognized (Table 1) with varying degrees of neuroinvasiveness; most symptomatic outbreaks are attributed to lineages 1 and 2 [46, 47]. Lineage 1 is subclassified into the more severe clade 1a, which originated from Egypt and currently circulating in Europe, Africa, and the Americas, and clade 1b (Kunjin virus) isolated in Oceania and rarely causes a neurological disease [48–52]. Lineage 2 originated from Uganda and was initially contained in Africa but emerged later in 2004 in Europe, especially in Hungary, Greece, and Italy [22, 23, 51–54]. The other lineages are less widespread; lineage 3 (Rabensburg virus) was isolated in the Czech Republic, lineage 4 in Russia, lineage 5 (also considered clade 1c of lineage 1) in India, lineage 6 originally from Malaysia and then in Spain, lineage 7 (Koutango virus) in Senegal without any reported natural human infection yet, lineage 8 in Senegal, and lineage 9 (sub-lineage of lineage 4) in Austria [51, 55–60]. Interestingly, a lower frequency of neuroinvasive disease was seen in the African outbreaks, compared to the American ones, despite the circulation of lineages 1, 2, 7, and 8 and the high antibody seroprevalence in sampled humans [46]. In the 2011 India outbreak, most cases were found to be infected with lineage I. However, lineage V (initially subclassified as 1c) is now prevalent in the Northeast region of India [61•].
Table 1.
West Nile virus lineages
| Lineages | Geographic distribution |
|---|---|
| 1a | Europe, Africa, and Americas |
| 1b | Oceania |
| 2 | Africa and Europe (Hungary, Greece, and Italy) |
| 3 | Czech Republic |
| 4 | Russia |
| 5 | India (Also considered 1c) |
| 6 | Spain |
| 7 | Senegal (no reports of human infection) |
| 8 | Senegal |
| 9 | Austria (considered sub-lineage of 4) |
Vector and Transmission
The primary vector of WNV are the mosquitos of the Culex genus. In the USA, WNV is transmitted mainly through C. pipiens, C. tarsalis, and C. quinquefasciatus [62], while in Europe, the predominant vector is C. pipiens; regardless, over 50 mosquito species have been found to carry the virus in North America alone[63]. Furthermore, although the presence of WNV has also been reported in Aedes spp., these mosquitoes are not regarded as significant vectors of the virus in the wild [64, 65]. Culex mosquitos are mostly night biters, with their feeding activity peaking between dusk and dawn [66]. The infection of migratory species of birds can also enable the virus to travel much further, and introduce WNV to new locations [67]. In addition to mosquito transmission, WNV transmission has also occurred through other routes, although rarely, such as organ transplantation, blood transfusions, and breast milk, or even vertically [4].
The primary vertebrate host of WNV is birds, with the virus maintained in an enzootic cycle between birds and Culex spp. mosquitos. In the USA, the American robin is considered the primary host for WNV, as vectors display a feeding preference for their species [64, 68]. The transmission cycle begins when a female mosquito takes a blood meal from a viremic host. The ingested viral particles end up in the midgut of the insect. After infecting and replicating within the midgut epithelium, they are released into the mosquito hemolymph, the cavity containing the insect’s circulatory fluid. From there, the virus disseminates throughout the insect’s body, reaching the salivary glands, where it accumulates and can be transmitted to a new host during the next blood meal [69]. Mammals are considered “dead-end” hosts for the virus, as the levels of viremia achieved in mammals are not adequate for transmission to other mosquitos, terminating the cycle of WNV transmission[2].
After the ingestion of the blood meal, the female Culex spp. mosquito searches for a water source, where it will lay between 100 and 300 eggs. The eggs hatch into larvae that mature into pupae, from which the adult mosquito finally emerges [70]. This process is highly temperature-dependent, and the duration of each stage varies significantly depending on temperature. Eggs cannot hatch in temperatures under 7 °C, require 10 days of incubation at 10 °C or 3 days at 20 °C, while they can hatch after a single day at 30 °C. Similarly, larvae can evolve into adult mosquitos in a week or less at 30 °C. Still, at 15 °C, they may require 3 weeks or more in order to reach adulthood [71].
Additionally, temperature plays a significant role in the external incubation period (EIP) of WNV, defined as the time between ingesting an infectious blood meal until the moment the mosquito can transmit the virus to a new host. EIP is measured in degree-days, the product of the average daily temperature multiplied by days with a temperature over the minimum developmental threshold, i.e., the minimum temperature at which the virus develops. The EIP for WNV in Culex mosquitos has been estimated to be 109 degree-days for temperatures over 14.3 °C; therefore, at 30 °C, Culex mosquitos can transmit the virus in just 7 days after being exposed[72]. However, this comes at a cost, as the survival of the mosquito decreases as the temperature increases [62]. Transmission peaks at temperatures between 23 and 26 °C [73]. An increase in temperature has shown a positive association with the circulation of WNV in Southern Europe, with higher temperature lengthening the vector’s season [27••].
Consequently, as global warming progresses, the rate at which Culex populations multiply and become infected with WNV may increase in temperate regions. Evidence of this is already present in the Americas, where increasing temperatures have permitted Culex species to expand northward, with C. pipiens reaching southern Canada [74]. In Southern Europe, this phenomenon has been observed with an increase in temperature, showing positive association with the circulation of WNV and lengthening the vector’s season [27••]. In addition, changes in precipitation patterns will create additional habitats for these vectors, enabling them to colonize new areas and expand their populations [74].
Therefore, an integrative approach to address human, animal, and environmental health (One Health approach) will be critical to understanding and limiting the impact on humans. One such example is observing several birds, such as crows and jays succumb to WNV infection. This has led to the use of dead-bird surveillance programs to track WNV emergence and permit the preemptive allocation of mosquito control resources in areas of increased risk [75, 76].
Clinical Manifestations, Diagnosis, and Management
Clinical manifestations include asymptomatic infection, WN fever, or neuroinvasive disease (WNND). The majority of the patients (> 80%) remain asymptomatic, with only 1% of patients presenting with neurological manifestations [77].
In uncomplicated WN fever, most patients present with acute onset of fever, chills, nausea, weakness, fatigue, myalgia, arthralgia, and headache [77, 78]. Around –-50% of patients may develop lymphadenopathy associated with rash lasting approximately 7 day s[2, 6]. These symptoms are usually mild and resolve in less than a week, but prolonged fatigue is common [6]. Patients’ history plays a vital role in diagnosing these cases, such as a history of travel to endemic regions, especially during the summer months, outdoor activities, mosquito bites, and other environmental conditions. A thorough physical examination is necessary to identify any rash, mosquito bite, or lymphadenopathy. Specific laboratory tests on serum and CSF are warranted to confirm the diagnosis. Serological tests include WNV-specific antibody tests on serum or CSF samples (IgM-specific ELISA) [77, 78]. In immunocompromised hosts, antibody development might be delayed. In such cases, PCR detection for WNV can be helpful. The plaque reduction neutralization test (PRNT) is the most specific antibody test for flaviviruses. To date, the mainstay of treatment for WNV infection is supportive care [2], which includes managing nausea and vomiting with anti-emetic and rehydration, analgesics, and antipyretics.
In rare and severe cases, patients have neurological manifestations leading to WNND. WNND includes syndromes of meningitis, encephalitis, acute flaccid paralysis (AFP)/poliomyelitis, and transverse myelitis [77, 79]. In rare cases, cranial nerve palsies, movement disorders, and parkinsonian features have also been reported [79]. The exact mechanism of neuro-invasion is unclear. Possibly, an overactive immune response leads to increased permeability of the BBB to the virus; however, if the immune system lags in clearing the virus, neuronal cell death can occur, associated with severe manifestations [7]. Risk factors for encephalitis include older age, diabetes, alcohol abuse, and immunocompromised states [80]. Amongst neuroinvasive cases in the USA, hospitalization rates were > 85% in all age groups but were highest among patients aged ≥ 70 years (98%) [35]. Clinical and laboratory evaluation, along with neuro-imaging, play a pivotal role in diagnosing these cases. Patients frequently have an altered mental status, cranial nerve abnormalities, and generalized weakness. In patients with AFP, asymmetric lower motor neuron weakness with preserved sensations and abnormal deep tendon reflexes might be noted [79]. Laboratory investigations on serum samples mainly reveal leukocytosis, anemia, hyponatremia, transaminitis, and rarely elevated creatinine kinase levels. CSF studies show neutrophil predominant pleocytosis and elevated protein levels, and the serological and viral tests are crucial in confirming these cases.
Despite the relative insensitivity, with only 20 to 70% of patients with acute WNND having associated abnormalities, MRI is the key study in neuro-imaging [77]. When present, lesions preferentially affect deep gray matter structures, including the basal ganglia, thalami, brainstem, and cerebellum [79]. In the case of AFP, MRI can sometimes show abnormalities in the anterior horns. However, electrophysiological studies (electromyography and nerve conduction velocities) can help in further characterization [79].
Management for WNND is symptomatic with supportive care. Patients at risk of airway compromise require urgent intubation and ventilation support. Even though a small randomized control trial of 18 patients did not show any significant benefits from high-dose corticosteroids [81], several case reports with neurological manifestations observed clinical improvement [8, 9]. Animal models have also shown potential benefits with IVIG [82, 83] but a randomized clinical trial failed to show any benefits in humans [84]. Similarly, in vitro studies have shown promising results for interferon and ribavirin [85, 86]. However, these compounds have shown mixed evidence when used in humans [2, 87, 88]. There is also some ongoing research for newer therapeutics [89•]. Patients with WNND, especially those with AFP, require long-term acute rehabilitation and physiotherapy [2, 77]. Long-term complications for a year or more after infections are common in patients recovering from WNV infection.
Travel-Related WNV—Case Compilation
Our PubMed search identified 39 reports of travel-related WNV infection in published literature[90]. (Table 2 and Fig. 1). Of these 39 cases, eighteen were reported from East and Southeast Europe, eight from Eastern Mediterranean and North Africa, seven from Sub-Saharan Africa including Madagascar, five from North America, and one from Central America. Nearly all travelers (35) were from Europe. Socio-demographic and past medical history was available in 25 cases. In these 25 cases, the age range varied from 23 to 83 years, with 10 patients (40%) having an age of ≥ 65 years. Five patients reported a current or past history of immunosuppression (4 patients had a history of cancer, and 1 patient had a renal transplant). Most of these patients (19/29 cases with details available, 65.5%) were reported to have neurologic involvement. An adverse outcome, such as death, neurological sequela, or prolonged recovery was reported in 15 patients.
Table 2.
Published reports of West Nile virus in travelers
| Year of occurrence | Patient, significant PMH | Origin/residence country | Destination | Reason for travel | Duration and city of stay in the endemic areas | Lineage | Clinical course and outcome |
|---|---|---|---|---|---|---|---|
|
2001 [90] (Estiva et al.) |
41-year-old female, previously healthy | France | Senegal | Tourism | NA | NA | Acute febrile rash, rapid recovery as inpatient |
|
2001 [10] (Meeuse et al.) |
45-year-old female, previously healthy | The Netherlands | Israel | Tourism | NA | NA | Fever and altered behavior, rapid recovery as inpatient |
|
2002 [91] (Hubalek et al.) |
69-year-old male, previously healthy | Czech Republic | USA | Tourism | 2-month trip | NA | Fever, blurred speech, marked bradypsychism, and hydrocephalus, gradual recovery |
|
2003 [92] (Charles et al.) |
82-year-old male | France | USA | Tourism | Atlanta, Georgia, | NA | Encephalitis, inpatient rapid recovery |
|
2003 [93] (Prick et al.) |
69-year-old male | The Netherlands | Canada | Tourism | Ontario | NA | Encephalitis, rapid recovery as inpatient with a residual short-term memory dysfunction |
|
2007 [94] (D VDB et al.) |
NA | Belgium | USA | NA | Florida | NA | Neuroinvasive disease |
|
2008 [94] (D VDB et al.) |
NA | Belgium | Senegal/Guinea | NA | NA | NA | Confirmed WNV fever case without neuro involvement |
|
2008 [95] (Monge Maillo B et al.) |
51-year-old, previously healthy male | Spain | Nicaragua | Missionary | 2-year stay in Managua | NA | Meningo-encephalitis with acute flaccid paralysis, residual right upper limb paraparesis and muscular atrophy |
|
2009 [96] (Rogers et al.) |
58-year-old male | Australia | Israel | Visiting friends and relatives | Tel Aviv | 1 (New York 99 strain) | Encephalitis, rehabilitation for persisting lethargy and mild ataxia |
|
2010 [97] (Aboutaleb et al.) |
30-year-old female, previously healthy | Germany | Israel | Tourism | 10-day trip at the Sea of Galilee | NA | Fever, retro-orbital headache, and a macular rash, inpatient rapid recovery |
|
2011 [98] (Schultze-Amberger et al.) |
28-year-old female, previously healthy | Germany | Canada | Tourism | 2-week holiday trip to Ottawa mainly, and Ontario | NA | Severe encephalitic syndrome, discharged after 2 months in a rehabilitation center with full recovery |
|
2011 [99] (Larrieu et al.) |
58-year-old female, history of hypertension | Reunion island | Madagascar | Tourism | 2 weeks in the province of Mitsinjo | NA | Encephalitis, death after 1 month due to cardiogenic shock |
|
2012 [100] (Kropman et al.) |
44-year-old female | The Netherlands | Egypt | Tourism | NA | NA | Poliomyelitis-like, lower extremity weakness/paralysis, with complete recovery on the right and partial recovery on the left |
|
2012 [101] (Gabriel et al.) |
65-year-old male, history of reduced cardiac output (ejection fraction 25%), coronary artery bypass graft surgery in 1990, and an implantable cardioverter-defibrillator several years ago | Germany | Greece | Tourism | several weeks in the village of Leskimi in Corfu | NA | Neuroinvasive disease, impaired motor and cognitive capacity, death after 3 months of neurological rehabilitation due to septic shock |
|
2012 [102] (Cnops et al.) |
73-year-old female, history of lymphoma | Belgium | Greece | Tourism | Kavala city in Macedonia | 2 | Encephalitis, death |
|
2012 [94] (D VDB et al.) |
NA | Belgium | Democratic Republic of Congo | NA | NA | NA | Neuroinvasive disease present |
|
2012 [94] (D VDB et al.) |
NA | Belgium | Sudan | NA | NA | NA | WNV fever without neuroinvasive disease |
|
2012 [101] (Gabriel et al.) |
60-year-old male, history of kidney transplantation in 1999 due to nephrosclerosis | Germany | Montenegro | Tourism | Bijela, and traveled back by road, via Bosnia, Serbia, and Hungary | NA | Neuroinvasive disease with several complications including seizures, kidney transplant failure, and gastrointestinal bleeding. Severe residual speech and motor impairment |
|
2012 [101] (Gabriel et al.) |
23-year-old female, previously healthy | Germany | Egypt | Study | 7 months in Cairo | NA | Influenza-like illness, inpatient rapid recovery |
|
2012 [101] (Gabriel et al.) |
43-year-old male, previously healthy | Germany | Tunisia | Tourism | NA | NA | Fever, asthenia, and hematemesis, inpatient rapid recovery |
|
2012 [103] (Hwang et al.) |
58-year-old male, medical history of diabetes and tobacco smoking |
Korea (first reported case of WNV in Korea) |
Guinea | Business trip | Was in Guinea, West Africa 7 months ago | NA | Headache since 2 months, cognitive impairment with mild memory disturbance, Leg weakness, arachnoiditis, myelitis |
|
2013 [104] (Quatresous et al.) |
74-year-old male, history of hypercholesterolemia and asymptomatic carotid stenosis, | France | Algeria | Tourism | NA | NA | Orchi-epididymitis and meningo-encephalitis |
|
2015 [105] (Pietsch et al.) |
34‐year‐old female, previously healthy | Germany | Austria | Tourism | 4‐week trip to the Barrier Lake of Ottenstein and Vienna | 2 | Influenza-like illness and exanthema, outpatient, rapid recovery |
|
2016 [106] (Paphitou et al.) |
75-year-old male, history of coronary artery disease and localized prostate cancer | Cyprus | Greece | Tourism | NA | NA | Meningo-encephalitis and flaccid paralysis, successfully weaned from mechanical ventilation and able to move his limps against resistance on day 60 of rehabilitation |
|
2017 [11] (Parkash et al.) |
72-year-old male, CKD stage 3 | UK | South Africa | Visiting friends and relatives | 3-week in Johannesburg, except for a 5-day safari in Kruger National Park region | 2 | Influenza-like illness, inpatient rapid recovery |
| 2017 [107] (Wollants et al.) | 83-year-old male, history of moderate chronic kidney disease | Belgium | Hungary | NA | 8-month trip | 2 | Multiple organ failure and loss of consciousness, residual memory loss 3 months after rehabilitation |
|
2017 [11] (Prakash et al.) |
76-year-old female, previously healthy | UK | South Africa | Visiting friends and relatives | 3-week in Johannesburg, except for a 5-day safari in Kruger National Park region | 2 | Meningo-encephalitis, residual mild cognitive impairment, ongoing balance disorder |
|
2018 [108] (Velasco et al.) |
60-year-old male, history of meningioma (treated with radiotherapy years ago) and thymoma in clinical remission | Spain | Romania | NA | Ajdud | 2 | Neuroinvasive disease, acalculous cholecystitis, pancreatitis, Takotsubo syndrome, full recovery after 1 year of rehabilitation |
|
2018 [28] (Nagy et al.) |
10 imported cases noted in Hungary | Cases were related to travel to following countries: Austria (1), Belgium (1), Croatia (2), Romania (2), Serbia (1 confirmed, 2 probable), Turkey (1) | |||||
|
2019 [109] (Whyler et al.) |
63-year-old female, history of follicular lymphoma in remission, with rituximab-induced hypogammaglobulinemia | Australia | Southeast Europe | Tourism | 2-month travel to Danube River, Serbia, Croatia, Germany, Austria, Slovakia, and Hungary | 2 (West Nile virus Bulgaria 2015) | Meningo-encephalitis with severe acquired brain injury, death after 32 days |
Fig. 1.
a Timeline of outbreaks and new regions identified to have West Nile virus. b Reported cases of West Nile virus in travelers (area of acquisition)
Discussion
The majority of imported WNV cases in our literature review manifested neurologic disease or severe disease, and a sizable proportion of cases were 65 years or older. The increased risk for severe disease in persons with advanced age or comorbidities calls for heightened caution for these populations when visiting endemic regions, especially in the months of high transmission [110, 111]. Since no antiviral is available to treat WNV infection, and no vaccine is available, prevention in travelers rests on avoiding bites by the vectors, primarily Culex mosquitoes. Wearing clothing such as long sleeves, trousers, and socks provides an essential layer of protection.
Furthermore, several public health measures have been deployed to limit the number of infections caused by the virus. One Health approaches, through surveillance of outbreaks in birds and horses act as early warning systems [4, 75, 76]. In addition, WNV has long been a reportable disease, which permits active surveillance data to be collected, leading to early recognition of outbreaks [112]. Lastly, the screening of donated blood for WNV acts both as a surveillance tool as well as a preventive measure, as blood products that test positive are discarded [113, 114].
The main vector mosquitoes Culex spp. are night biters and have peak biting activity at dusk and dawn. Travelers to WNV-endemic areas should be advised to apply EPA-approved repellent during peak biting activity times [115]. An additional bite prevention strategy is to treat outer clothing with permethrin (or another pyrethroid) when traveling in an area with a high incidence of WNV [116]. Similar to other vector-borne diseases, insecticide-resistant genes have been identified in Culex mosquitoes and appear to be associated with enhanced vector competence for WNV [117]. Travelers likely need to use multiple bite prevention measures to reduce the risk of WNV infection. Mosquito avoidance strategies still need improvement since the vector avoidance practice by travelers to malaria-infested regions remains suboptimal despite receiving advice and educational material by healthcare professionals prior to travel [118].
Despite WNV-focused mosquito surveillance and control efforts such as insecticide spraying undertaken by health authorities, forecasting human WNV risk level at destinations remains challenging due to the complex interactions between the virus, the reservoir hosts, mosquito vectors, and environmental factors such as temperature, precipitation, population density, immunity, and land use. [119••, 120] The exposure destinations from our case reviews were widely distributed and included most of the known areas with WNV activity. In regions with suboptimal WNV surveillance, such as Africa, travel-related WNV infections may serve as sentinels of virus circulation [121].
While our review sought reports on patients that acquired WNV infection at their travel destination, travelers from a WNV-endemic area can also import the infection to their destination. For instance, a 59-year-old male traveler from Philadelphia, the USA, to London, UK, developed confusion, and serology and MRI imaging were consistent with his diagnosis of neuroinvasive WNV[122]. Although Culex mosquitoes have been detected in the UK, this case, fortunately, did not lead to further spread.
As the global spread of the WNV continues, the need for the development of an effective vaccine is increasing. Despite several vaccines being available for horses, a suitable human vaccine is yet to be approved [123]. However, there are currently multiple human vaccines candidates in development, with some entering phase II trials [124]. Furthermore, in vivo studies have shown that there is some degree of cross protection against WNV in animals that have been immunized against other flaviviruses, such as Japanese encephalitis virus. It remains to be determined whether such an effect is observed in humans as well [125]. Continued research in developing an effective human vaccine will be essential to prevent future outbreaks.
Limitations
Several limitations are present in this review. A systemic search of gray literature was not conducted. While some cases can be identified as an imported cases based on travel history and endemicity of the region (e.g., ex. Korea), many imported WNV infections may be under-recognized due to the wide geographic distribution of WNV. In areas known to have WNV, confirmation of an imported case may be difficult unless molecular characterization can be performed [126, 127]. Furthermore, mild cases may not undergo testing for specific diagnosis if patients recover quickly; therefore, the published travel-related cases may be biased towards a high prevalence of neuroinvasive manifestations.
Conclusion
WNV is an emerging arbovirus that can cause disease ranging from asymptomatic to devastating. Because the presence of its vectors is increasingly identified, cases of WNV disease will likely be reported from new areas. Improved surveillance is needed in many regions. Travelers are potentially at risk, especially if they are unaware of the presence of competent mosquitoes at their destination. Health care providers should advise their patients planning to travel to endemic areas regarding WNV risk and mosquito bite prevention methods. Evaluation of ill travelers with compatible symptoms should consider the diagnosis of WNV for those visiting in endemic areas as well as for those returning from destinations with known WNV circulation.
Compliance with Ethical Standards
Ethics Approval
This review article is in compliance with ethical standards.
Conflict of Interest
Chinmay Jani, Loukas Kakoullis, Nour Abdallah, Christian Mouchati, Stephanie Page, and Robert Colgrove report no conflict of interest. Lin Chen reports honoraria from Shoreland Inc, Valneva Inc, Takeda, Sanofi-Pasteur, Emergent BioSolutions, and Merck, not related to this work.
Research Involving Human and Animal Participants
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of Topical Collection on Tropical, Travel and Emerging Infections
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chinmay Jani, Email: Chinmay.jani@mah.harvard.edu, Email: ctjani1494@gmail.com.
Loukas Kakoullis, Email: Loukas.kakoullis@mah.harvard.edu.
Nour Abdallah, Email: nour.abdallah29@gmail.com.
Christian Mouchati, Email: cmouchati@gmail.com.
Stephanie Page, Email: spage1@mah.harvard.edu.
Robert Colgrove, Email: robert_colgrove@hms.harvard.edu.
Lin H. Chen, Email: lchen@hms.harvard.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Smithburn KC, Hughes TP, Burke AW, Paul JH. A Neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med. 1940;s1–20(4):471–92.
- 2.Rossi SL, Ross TM, Evans JD. West Nile virus. Clin Lab Med. 2010;30(1):47–65. doi: 10.1016/j.cll.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monath TP, Arroyo J, Miller C, Guirakhoo F. West Nile virus vaccine. Curr Drug Targets Infect Disord. 2001;1(1):37–50. doi: 10.2174/1568005013343254. [DOI] [PubMed] [Google Scholar]
- 4.West Nile Virus Fact Sheets. WHO. https://www.who.int/news-room/fact-sheets/detail/west-nile-virus. Accessed on 18 Apr 2022
- 5.Grubaugh ND, Ebel GD. Dynamics of West Nile virus evolution in mosquito vectors. Curr Opin Virol. 2016;21:132–138. doi: 10.1016/j.coviro.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2(9):519–529. doi: 10.1016/S1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 7.Lim SM, Koraka P, Osterhaus AD, Martina BE. West Nile virus: immunity and pathogenesis. Viruses. 2011;3(6):811–828. doi: 10.3390/v3060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyrgos V, Younus F. High-dose steroids in the management of acute flaccid paralysis due to West Nile virus infection. Scand J Infect Dis. 2004;36(6–7):509–512. doi: 10.1080/00365540410020659. [DOI] [PubMed] [Google Scholar]
- 9.Karagianni P, Alexopoulos H, Sourdi A, Papadimitriou D, Dimitrakopoulos AN, Moutsopoulos HM. West Nile virus infection triggering autoimmune encephalitis: pathophysiological and therapeutic implications. Clin Immunol. 2019;207:97–99. doi: 10.1016/j.clim.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Meeuse JJ, ter Borg F, Lohmann HJ, Groen J. Patient with West Nile fever in the Netherlands. Ned Tijdschr Geneeskd. 2001;145(43):2084–2086. [PubMed] [Google Scholar]
- 11.Parkash V, Woods K, Kafetzopoulou L, Osborne J, Aarons E, Cartwright K. West Nile Virus infection in travelers returning to United Kingdom from South Africa. Emerg Infect Dis. 2019;25(2):367–369. doi: 10.3201/eid2502.172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murgue B, Murri S, Triki H, Deubel V, Zeller HG. West Nile in the Mediterranean basin: 1950–2000. Ann N Y Acad Sci. 2001;951:117–126. doi: 10.1111/j.1749-6632.2001.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. The Lancet. 1998;352(9130):767–771. doi: 10.1016/S0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 14.Platonov AE, Shipulin GA, Shipulina OY, Tyutyunnik EN, Frolochkina TI, Lanciotti RS, et al. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg Infect Dis. 2001;7(1):128–132. doi: 10.3201/eid0701.010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.• Mencattelli G, Ndione MHD, Rosa R, Marini G, Diagne CT, Diagne MM, et al. Epidemiology of West Nile virus in Africa: an underestimated threat. PLoS Negl Trop Dis. 2022;16(1). Comprehensive review of the incidence of WNV in each African country, circulating lineages, and clades. [DOI] [PMC free article] [PubMed]
- 16.Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344(24):1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 17.Beck C, Jimenez-Clavero MA, Leblond A, Durand B, Nowotny N, Leparc-Goffart I, et al. Flaviviruses in Europe: complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int J Environ Res Public Health. 2013;10(11):6049–6083. doi: 10.3390/ijerph10116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XL, Fu SH, Liu WB, Wang HY, Lu Z, Tong SX, et al. West nile virus infection in Xinjiang. China Vector Borne Zoonotic Dis. 2013;13(2):131–133. doi: 10.1089/vbz.2012.0995. [DOI] [PubMed] [Google Scholar]
- 19.Khan E, Barr KL, Farooqi JQ, Prakoso D, Abbas A, Khan ZY, et al. Human West Nile Virus disease outbreak in Pakistan, 2015–2016. Front Public Health. 2018;6:20. doi: 10.3389/fpubh.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artsob H, Gubler DJ, Enria DA, Morales MA, Pupo M, Bunning ML, et al. West Nile Virus in the New World: trends in the spread and proliferation of West Nile Virus in the Western Hemisphere. Zoonoses Public Health. 2009;56(6–7):357–369. doi: 10.1111/j.1863-2378.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 21.Sambri V, Capobianchi M, Charrel R, Fyodorova M, Gaibani P, Gould E, et al. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect. 2013;19(8):699–704. doi: 10.1111/1469-0691.12211. [DOI] [PubMed] [Google Scholar]
- 22.Papa A, Bakonyi T, Xanthopoulou K, Vazquez A, Tenorio A, Nowotny N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg Infect Dis. 2011;17(5):920–922. doi: 10.3201/eid1705.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagnarelli P, Marinelli K, Trotta D, Monachetti A, Tavio M, Del Gobbo R, et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro Surveill. 2011;16(43). [PubMed]
- 24.Bakonyi T, Ferenczi E, Erdelyi K, Kutasi O, Csorgo T, Seidel B, et al. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. 2013;165(1–2):61–70. doi: 10.1016/j.vetmic.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Popović N, Milošević B, Urošević A, Poluga J, Lavadinović L, Nedelijković J, et al. Outbreak of West Nile virus infection among humans in Serbia, August to October 2012. Euro Surveill. 2013;18(43). [DOI] [PubMed]
- 26.• Young JJ, Haussig JM, Aberle SW, Pervanidou D, Riccardo F, Sekulic N, et al. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill. 2021;26(19). Epidemiological description of WNV in the European Union, highlighting the significance of transmission intensity. [DOI] [PMC free article] [PubMed]
- 27.•• Brugueras S, Fernandez-Martinez B, Martinez-de la Puente J, Figuerola J, Porro TM, Rius C, et al. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: a systematic review. Environ Res. 2020;191:110038. Review of the impact of climate change on emerging infectious diseases. [DOI] [PubMed]
- 28.Nagy A, Mezei E, Nagy O, Bakonyi T, Csonka N, Kaposi M, et al. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Euro Surveill. 2019;24(28). [DOI] [PMC free article] [PubMed]
- 29.Ergunay K, Polat C, Ozkul A. Vector-borne viruses in Turkey: a systematic review and bibliography. Antiviral Res. 2020;183:104934. doi: 10.1016/j.antiviral.2020.104934. [DOI] [PubMed] [Google Scholar]
- 30.Garcia San Miguel Rodriguez-Alarcon L, Fernandez-Martinez B, Sierra Moros MJ, Vazquez A, Julian Paches P, Garcia Villacieros E, et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill. 2021;26(19). [DOI] [PMC free article] [PubMed]
- 31.Epidemiological Update: West Nile Virus transmission season in Europe, 2018.” European Centre for Disease Prevention and Control, 14 Dec. 2018, https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018. Accessed on 27 April 2022.
- 32.Epidemiological Update: West Nile Virus transmission season in Europe, 2017.” European Centre for Disease Prevention and Control, 15 Dec. 2017, https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2017. Accessed on 27 April 2022.
- 33.Watts MJ, Sarto IMV, Mortyn PG, Kotsila P. The rise of West Nile virus in Southern and Southeastern Europe: a spatial-temporal analysis investigating the combined effects of climate, land use and economic changes. One Health. 2021;13:100315. doi: 10.1016/j.onehlt.2021.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West Nile virus in Europe in 2021 - human cases compared to previous seasons, updated 7 October 2021. https://www.ecdc.europa.eu/en/publications-data/west-nile-virus-europe-2021-human-cases-compared-previous-seasons-updated-7. Accessed on 27 April 2022.
- 35.McDonald E, Mathis S, Martin SW, Staples JE, Fischer M, Lindsey NP. Surveillance for West Nile virus disease - United States, 2009–2018. MMWR Surveill Summ. 2021;70(1):1–15. doi: 10.15585/mmwr.ss7001a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahey GM, Mathis S, Martin SW, Gould CV, Staples JE, Lindsey NP. West Nile virus and other domestic nationally notifiable arboviral diseases - United States, 2019. MMWR Morb Mortal Wkly Rep. 2021;70(32):1069–1074. doi: 10.15585/mmwr.mm7032a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West Nile Virus disease cases by state 2021. https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2021/disease-cases-state-2021.html. Accessed on 27 April 2022.
- 38.Bolfa P, Jeon I, Loftis A, Leslie T, Marchi S, Sithole F, et al. Detection of West Nile virus and other common equine viruses in three locations from the Leeward Islands. West Indies Acta Trop. 2017;174:24–28. doi: 10.1016/j.actatropica.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Komar N, Clark GG. West Nile virus activity in Latin America and the Caribbean. Rev Panam Salud Publica. 2006;19(2):112–117. doi: 10.1590/S1020-49892006000200006. [DOI] [PubMed] [Google Scholar]
- 40.Ramos C, Falcon Lezama JA. West Nile fever: an emerging disease in Mexico. Salud Publica Mex. 2004;46(5):488–490. doi: 10.1590/s0036-36342004000500013. [DOI] [PubMed] [Google Scholar]
- 41.Im JH, Kim TS, Chung MH, Baek JH, Kwon HY, Lee JS. Current Status and a Perspective of mosquito-borne diseases in the Republic of Korea. Vector Borne Zoonotic Dis. 2021;21(2):69–77. doi: 10.1089/vbz.2019.2588. [DOI] [PubMed] [Google Scholar]
- 42.Anukumar B, Sapkal GN, Tandale BV, Balasubramanian R, Gangale D. West Nile encephalitis outbreak in Kerala, India, 2011. J Clin Virol. 2014;61(1):152–155. doi: 10.1016/j.jcv.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Cao L, Fu S, Lu Z, Tang C, Gao X, Li X, et al. Detection of West Nile virus infection in viral encephalitis cases. China Vector Borne Zoonotic Dis. 2019;19(1):45–50. doi: 10.1089/vbz.2018.2275. [DOI] [PubMed] [Google Scholar]
- 44.Knope K, Doggett SL, Jansen CC, Johansen CA, Kurucz N, Feldman R, et al. Arboviral diseases and malaria in Australia, 2014–15: annual report of the National Arbovirus and Malaria Advisory Committee. Commun Dis Intell. 2018;2019:43. [PubMed] [Google Scholar]
- 45.Lindenbach, Brett D., Murray, Catherine L, Thiel, Heinz-Jürgen, Rice, Charles M. “Flaviviridae.” Fields Virology, 6th edition. Wolters Kluwer - Lippincott. p. 712–746.
- 46.Fall G, Di Paola N, Faye M, Dia M, Freire CCM, Loucoubar C, et al. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLoS Negl Trop Dis. 2017;11(11):e0006078. doi: 10.1371/journal.pntd.0006078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296(1):17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- 48.Hall RA, Scherret JH, Mackenzie JS. Kunjin virus: an Australian variant of West Nile? Ann N Y Acad Sci. 2001;951:153–160. doi: 10.1111/j.1749-6632.2001.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 49.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 50.Murray KO, Mertens E, Despres P. West Nile virus and its emergence in the United States of America. Vet Res. 2010;41(6):67. doi: 10.1051/vetres/2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez A, Herrero L, Negredo A, Hernandez L, Sanchez-Seco MP, Tenorio A. Real time PCR assay for detection of all known lineages of West Nile virus. J Virol Methods. 2016;236:266–270. doi: 10.1016/j.jviromet.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Rizzo C, Napoli C, Venturi G, Pupella S, Lombardini L, Calistri P, et al. West Nile virus transmission: results from the integrated surveillance system in Italy, 2008 to 2015. Euro Surveill. 2016;21(37). [DOI] [PMC free article] [PubMed]
- 53.Fall G, Diallo M, Loucoubar C, Faye O, Sall AA. Vector competence of Culex neavei and Culex quinquefasciatus (Diptera: Culicidae) from Senegal for lineages 1, 2, Koutango and a putative new lineage of West Nile virus. Am J Trop Med Hyg. 2014;90(4):747–754. doi: 10.4269/ajtmh.13-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakonyi T, Ivanics E, Erdelyi K, Ursu K, Ferenczi E, Weissenbock H, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12(4):618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubalek Z, Rudolf I, Bakonyi T, Kazdova K, Halouzka J, Sebesta O, et al. Mosquito (Diptera: Culicidae) surveillance for arboviruses in an area endemic for West Nile (Lineage Rabensburg) and Tahyna viruses in Central Europe. J Med Entomol. 2010;47(3):466–472. doi: 10.1603/ME09219. [DOI] [PubMed] [Google Scholar]
- 56.Lvov DK, Butenko AM, Gromashevsky VL, Kovtunov AI, Prilipov AG, Kinney R, et al. West Nile virus and other zoonotic viruses in Russia: examples of emerging-reemerging situations. Arch Virol Suppl. 2004;18:85–96. doi: 10.1007/978-3-7091-0572-6_7. [DOI] [PubMed] [Google Scholar]
- 57.Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, Murri S, Meyer R, et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298(1):96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- 58.Vazquez A, Sanchez-Seco MP, Ruiz S, Molero F, Hernandez L, Moreno J, et al. Putative new lineage of west nile virus. Spain Emerg Infect Dis. 2010;16(3):549–552. doi: 10.3201/eid1603.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shope RE. Epidemiology of other arthropod-borne flaviviruses infecting humans. Adv Virus Res. 2003;61:373–391. doi: 10.1016/S0065-3527(03)61009-2. [DOI] [PubMed] [Google Scholar]
- 60.Pachler K, Lebl K, Berer D, Rudolf I, Hubalek Z, Nowotny N. Putative new West Nile virus lineage in Uranotaenia unguiculata mosquitoes, Austria, 2013. Emerg Infect Dis. 2014;20(12):2119–2122. doi: 10.3201/eid2012.140921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.• Chowdhury P, Khan SA. Global emergence of West Nile virus: threat & preparedness in special perspective to India. Indian J Med Res. 2021;154(1):36–50. Comprehensive review of the history, epidemiology, and clinical presentation of WNV, which also examines its co-circulation with other flaviviruses in India. [DOI] [PMC free article] [PubMed]
- 62.Rochlin I, Faraji A, Healy K, Andreadis TG. West Nile Virus Mosquito Vectors in North America. J Med Entomol. 2019;56(6):1475–1490. doi: 10.1093/jme/tjz146. [DOI] [PubMed] [Google Scholar]
- 63.Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12(3):468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25(4):635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camp JV, Nowotny N. The knowns and unknowns of West Nile virus in Europe: what did we learn from the 2018 outbreak? Expert Rev Anti Infect Ther. 2020;18(2):145–154. doi: 10.1080/14787210.2020.1713751. [DOI] [PubMed] [Google Scholar]
- 66.Gingrich JB, Casillas L. Selected mosquito vectors of West Nile virus: comparison of their ecological dynamics in four woodland and marsh habitats in Delaware. J Am Mosq Control Assoc. 2004;20(2):138–145. [PubMed] [Google Scholar]
- 67.Reed KD, Meece JK, Henkel JS, Shukla SK. Birds, migration and emerging zoonoses: West nile virus, Lyme disease, influenza A and enteropathogens. Clin Med Res. 2003;1(1):5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciota AT. West Nile virus and its vectors. Current Opinion in Insect Science. 2017;22:28–36. doi: 10.1016/j.cois.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Vogels CBF, Göertz GP, Pijlman GP, Koenraadt CJM. Vector competence of European mosquitoes for West Nile virus. Emerging Microbes & Infections. 2017;6(1):1–13. doi: 10.1038/emi.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CDC. 2020. "Life cycle of culex species mosquitoes." 2020. https://www.cdc.gov/mosquitoes/about/life-cycles/culex.html.
- 71.European Centre for Disease Prevention and Control. n.d. "Culex Pipiens - Factsheet for Experts." Accessed 28 Jan 2022. https://www.ecdc.europa.eu/en/all-topics-z/disease-vectors/facts/mosquito-factsheets/culex-pipiens-factsheet-experts.
- 72.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2014;43(2):309–317. doi: 10.1093/jmedent/43.2.309. [DOI] [PubMed] [Google Scholar]
- 73.Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, El Moustaid F, et al. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23 degrees C and 26 degrees C. Elife. 2020;9. [DOI] [PMC free article] [PubMed]
- 74.Gorris ME, Bartlow AW, Temple SD, Romero-Alvarez D, Shutt DP, Fair JM, et al. Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasit Vectors. 2021;14(1):547. doi: 10.1186/s13071-021-05051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Troupin A, Colpitts TM. Overview of West Nile virus transmission and epidemiology. In: Colpitts TM, editor. West Nile Virus: methods and Protocols. New York, NY: Springer New York; 2016. p. 15–8. [DOI] [PubMed]
- 76.Mostashari F, Kulldorff M, Hartman JJ, Miller JR, Kulasekera V. Dead bird clusters as an early warning system for West Nile virus activity. Emerg Infect Dis. 2003;9(6):641–646. doi: 10.3201/eid0906.020794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jani C, Walker A, Al Omari O, Patel D, Heffess A, Wolpow E, et al. Acute transverse myelitis in West Nile Virus, a rare neurological presentation. IDCases. 2021;24:e01104. doi: 10.1016/j.idcr.2021.e01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patnaik JL, Harmon H, Vogt RL. Follow-up of 2003 human West Nile virus infections, Denver. Colorado Emerg Infect Dis. 2006;12(7):1129–1131. doi: 10.3201/eid1207.051399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60(3):286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 80.Bode AV, Sejvar JJ, Pape WJ, Campbell GL, Marfin AA. West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin Infect Dis. 2006;42(9):1234–1240. doi: 10.1086/503038. [DOI] [PubMed] [Google Scholar]
- 81.Murray KO, Baraniuk S, Resnick M, Arafat R, Kilborn C, Shallenberger R, et al. Clinical investigation of hospitalized human cases of West Nile virus infection in Houston, Texas, 2002–2004. Vector Borne Zoonotic Dis. 2008;8(2):167–174. doi: 10.1089/vbz.2007.0109. [DOI] [PubMed] [Google Scholar]
- 82.Planitzer CB, Modrof J, Kreil TR. West Nile virus neutralization by US plasma-derived immunoglobulin products. J Infect Dis. 2007;196(3):435–440. doi: 10.1086/519392. [DOI] [PubMed] [Google Scholar]
- 83.Agrawal AG, Petersen LR. Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis. 2003;188(1):1–4. doi: 10.1086/376871. [DOI] [PubMed] [Google Scholar]
- 84.Gnann JW, Jr, Agrawal A, Hart J, Buitrago M, Carson P, Hanfelt-Goade D, et al. Lack of Efficacy of high-titered immunoglobulin in patients with West Nile virus central nervous system disease. Emerg Infect Dis. 2019;25(11):2064–2073. doi: 10.3201/eid2511.190537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15(2):101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- 86.Gea-Banacloche J, Johnson RT, Bagic A, Butman JA, Murray PR, Agrawal AG. West Nile virus: pathogenesis and therapeutic options. Ann Intern Med. 2004;140(7):545–553. doi: 10.7326/0003-4819-140-7-200404060-00015. [DOI] [PubMed] [Google Scholar]
- 87.Chan-Tack KM, Forrest G. Failure of interferon alpha-2b in a patient with West Nile virus meningoencephalitis and acute flaccid paralysis. Scand J Infect Dis. 2005;37(11–12):944–946. doi: 10.1080/00365540500262690. [DOI] [PubMed] [Google Scholar]
- 88.Kalil AC, Devetten MP, Singh S, Lesiak B, Poage DP, Bargenquast K, et al. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin Infect Dis. 2005;40(5):764–766. doi: 10.1086/427945. [DOI] [PubMed] [Google Scholar]
- 89.• Yu Y, Si L, Meng Y. Flavivirus entry inhibitors. Adv Exp Med Biol. 2022;1366:171–97. Clear descriptions of the pathways utilised by Flaviviridae to infect host cells, as well as molecules that may be used to block these processes. [DOI] [PubMed]
- 90.Estival JL, Skowron F, Dupin M, Combemale P. Primary infection with West-Nile virus. Ann Dermatol Venereol. 2001;128(5):656–658. [PubMed] [Google Scholar]
- 91.Hubalek Z, Lukacova L, Halouzka J, Sirucek P, Januska J, Precechtelova J, et al. Import of West Nile virus infection in the Czech Republic. Eur J Epidemiol. 2006;21(4):323–324. doi: 10.1007/s10654-006-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charles PE, Zeller H, Bonnotte B, Decasimacker AL, Bour JB, Chavanet P, et al. Imported West Nile virus infection in Europe. Emerg Infect Dis. 2003;9(6):750. doi: 10.3201/eid0906.020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prick JJ, Kuipers S, Kuipers HD, Vliegen JH, van Doornum GJ. Another case of West Nile fever in the Netherlands: a man with encephalitis following a trip to Canada. Ned Tijdschr Geneeskd. 2003;147(20):978–980. [PubMed] [Google Scholar]
- 94.D VDB, Cnops L, Meersman K, Domingo C, A VANG, M VANE. Chikungunya virus and West Nile virus infections imported into Belgium, 2007–2012. Epidemiol Infect. 2015;143(10):2227–36. [DOI] [PMC free article] [PubMed]
- 95.Monge Maillo B, Lopez-Velez R, Norman F, de Ory F, Sanchez-Seco MP, Giovanni FC. Importation of West Nile virus infection from Nicaragua to Spain. Emerg Infect Dis. 2008;14(7):1171–1173. doi: 10.3201/eid1407.071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rogers BA, Hueston L, Ratnam I. Imported West Nile virus encephalitis in an Israeli tourist. Med J Aust. 2009;191(4):232–234. doi: 10.5694/j.1326-5377.2009.tb02763.x. [DOI] [PubMed] [Google Scholar]
- 97.Aboutaleb N, Beersma MF, Wunderink HF, Vossen AC, Visser LG. Case report: West-Nile virus infection in two Dutch travellers returning from Israel. Euro Surveill. 2010;15(34). [DOI] [PubMed]
- 98.Schultze-Amberger J, Emmerich P, Gunther S, Schmidt-Chanasit J. West Nile virus meningoencephalitis imported into Germany. Emerg Infect Dis. 2012;18(10):1698–1700. doi: 10.3201/eid1810.120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larrieu S, Cardinale E, Ocquidant P, Roger M, Lepec R, Delatte H, et al. A fatal neuroinvasive West Nile virus infection in a traveler returning from Madagascar: clinical, epidemiological and veterinary investigations. Am J Trop Med Hyg. 2013;89(2):211–213. doi: 10.4269/ajtmh.12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kropman E, Bakker LJ, de Sonnaville JJ, Koopmans MP, Raaphorst J, Carpay JA. West Nile virus poliomyelitis after a holiday in Egypt. Ned Tijdschr Geneeskd. 2012;155(35):A4333. [PubMed] [Google Scholar]
- 101.Gabriel M, Emmerich P, Frank C, Fiedler M, Rashidi-Alavijeh J, Jochum C, et al. Increase in West Nile virus infections imported to Germany in 2012. J Clin Virol. 2013;58(3):587–589. doi: 10.1016/j.jcv.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 102.Cnops L, Papa A, Lagra F, Weyers P, Meersman K, Patsouros N, et al. West Nile virus infection in Belgian traveler returning from Greece. Emerg Infect Dis. 2013;19(4):684–685. doi: 10.3201/eid1904.121594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hwang J, Ryu HS, Kim H, Lee SA. The first reported case of West Nile encephalitis in Korea. J Korean Med Sci. 2015;30(3):343–345. doi: 10.3346/jkms.2015.30.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quatresous I, Salomon B, Noel D. Orchitis and West Nile encephalitis. Med Mal Infect. 2013;43(11–12):481–482. doi: 10.1016/j.medmal.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 105.Pietsch C, Trawinski H, Lubbert C, Liebert UG. Short communication: West Nile fever imported from Austria to Germany. Transbound Emerg Dis. 2019;66(2):1033–1036. doi: 10.1111/tbed.13079. [DOI] [PubMed] [Google Scholar]
- 106.Paphitou NI, Tourvas A, Floridou D, Richter J, Tryfonos C, Christodoulou C. The first human case of neuroinvasive West Nile virus infection identified in Cyprus. J Infect Public Health. 2017;10(6):891–893. doi: 10.1016/j.jiph.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 107.Wollants E, Smolders D, Naesens R, Bruynseels P, Lagrou K, Matthijnssens J, et al. Use of next-generation sequencing for diagnosis of West Nile virus infection in patient returning to Belgium from Hungary. Emerg Infect Dis. 2018;24(12):2380–2382. doi: 10.3201/eid2412.180494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Velasco M, Sanchez-Seco MP, Campelo C, de Ory F, Martin O, Herrero L, et al. Imported Human West Nile virus lineage 2 infection in Spain: Neurological and gastrointestinal complications. Viruses. 2020;12(2). [DOI] [PMC free article] [PubMed]
- 109.Whyler NC, Teng JC, Brewster DJ, Chin R, Cox I, Druce J, et al. Diagnosis of West Nile virus encephalitis in a returned traveller. Med J Aust. 2019;211(11):501–2 e1. [DOI] [PubMed]
- 110.Humphreys JM, Young KI, Cohnstaedt LW, Hanley KA, Peters DPC. Vector Surveillance, host species richness, and demographic factors as West Nile Disease risk indicators. Viruses. 2021;13(5). [DOI] [PMC free article] [PubMed]
- 111.Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, et al. Prevalence of chronic comorbidities in dengue fever and West Nile virus: a systematic review and meta-analysis. PLoS ONE. 2018;13(7):e0200200. doi: 10.1371/journal.pone.0200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Statistics & Maps. CDC. https://www.cdc.gov/westnile/statsmaps/index.html. Accessed on 10 July 2022.
- 113.Pisani G, Cristiano K, Pupella S, Liumbruno GM. West Nile virus in Europe and safety of blood transfusion. Transfus Med Hemother. 2016;43(3):158–167. doi: 10.1159/000446219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blood Transfusion and organ donation. West Nile Virus. CDC. https://www.cdc.gov/westnile/transmission/blood-organ.html. Accessed on 10 July 2022.
- 115.Find the repellent that is right for you. United states environmental protection agency. https://www.epa.gov/insect-repellents/find-repellent-right-you. Accessed on 26 April 2022.
- 116.Londono-Renteria B, Patel JC, Vaughn M, Funkhauser S, Ponnusamy L, Grippin C, et al. Long-Lasting permethrin-impregnated clothing protects against mosquito bites in outdoor workers. Am J Trop Med Hyg. 2015;93(4):869–874. doi: 10.4269/ajtmh.15-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Atyame CM, Alout H, Mousson L, Vazeille M, Diallo M, Weill M, et al. Insecticide resistance genes affect Culex quinquefasciatus vector competence for West Nile virus. Proc Biol Sci. 1894;2019(286):20182273. doi: 10.1098/rspb.2018.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goodyer L, Song J. Mosquito bite-avoidance attitudes and behaviors in travelers at risk of malaria. J Travel Med. 2014;21(1):33–38. doi: 10.1111/jtm.12053. [DOI] [PubMed] [Google Scholar]
- 119.•• Kramer LD, Ciota AT, Kilpatrick AM. Introduction, spread, and establishment of West Nile virus in the americas. J Med Entomol. 2019;56(6):1448–55. Comprehensive review of the spread of WNV in the Americas, as well as of the factors that enabled to virus to persist in these continents. [DOI] [PMC free article] [PubMed]
- 120.Esser HJ, Mogling R, Cleton NB, van der Jeugd H, Sprong H, Stroo A, et al. Risk factors associated with sustained circulation of six zoonotic arboviruses: a systematic review for selection of surveillance sites in non-endemic areas. Parasit Vectors. 2019;12(1):265. doi: 10.1186/s13071-019-3515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamer DH, Rizwan A, Freedman DO, Kozarsky P, Libman M. GeoSentinel: past, present and futuredagger. J Travel Med. 2020;27(8). [DOI] [PMC free article] [PubMed]
- 122.Lewis R, Khan N, Lynn W, Sandhu G, Papineni P. Neuroinvasive West Nile virus in a traveller from the United States to England. J Travel Med. 2021;28(3). [DOI] [PubMed]
- 123.Kaiser JA, Barrett ADT. Twenty years of progress toward west nile virus vaccine development. Viruses. 2019;11(9). [DOI] [PMC free article] [PubMed]
- 124.West Nile virus vaccines. NIAID. https://www.niaid.nih.gov/diseases-conditions/wnv-vaccines. Accessed on 10 July 2022.
- 125.Chowdhury P, Khan S, Borah J, Dutta P. Cross-protective immunity against circulating Japanese encephalitis virus and West Nile virus by live attenuated Japanese encephalitis vaccine SA 14–14-2. Int J Infect Dis. 2016;45:434. doi: 10.1016/j.ijid.2016.02.922. [DOI] [Google Scholar]
- 126.Rizzo C, Salcuni P, Nicoletti L, Ciufolini MG, Russo F, Masala R, et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012;17(20). [PubMed]
- 127.Gobbi F, Barzon L, Capelli G, Angheben A, Pacenti M, Napoletano G, et al. Surveillance for West Nile, dengue, and chikungunya virus infections, Veneto Region, Italy, 2010. Emerg Infect Dis. 2012;18(4):671–673. doi: 10.3201/eid1804.110753. [DOI] [PMC free article] [PubMed] [Google Scholar]