Abstract
It was reported that tuberculosis and BCG vaccination are potential tools for reducing the burden of COVID-19, mainly through the non-specific trained immunity. We have investigated whether BCG vaccination is able to induce cross-reacting antibodies against the SARS-CoV-2. We have tested the induced humoral immune responses against the SARS-CoV-2 Spike in the mouse model, after either BCG or rabies DNA-based vaccination alone or in Prime/Boost approach to COVID-19 DNA-based vaccination. We have demonstrated that BCG vaccination alone was able to induce cross-reacting antibodies to SARS-CoV-2 Spike. It can also boost the antibody response induced by a COVID-19 DNA-based vaccination. Hence, both BCG and latent tuberculosis infection can explain the lower burden of COVID-19 in developing countries, not only through the trained immunity but also by inducing cross-reacting antibodies. Furthermore, with the emergence of different COVID-19 variants, or eventually other Betacoronaviruses, the use of BCG vaccination can help against immune escapes of the current vaccines.
Introduction
The ongoing global pandemic of coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), identified for the first time in December 2019 in Wuhan, China. A first report [1] indicated that tuberculosis (TB) BCG vaccination is a potential tool for reducing the burden of COVID-19. BCG is currently one of the most widely used vaccines in the world (more than four billion BCG-vaccinated individuals). Since this initial report, many others came out with controversial results [2]. While many showed no statistical evidences [3–5], others strongly supported protective effects [6, 7]. Furthermore, patients with latent tuberculosis infection (LTBI) showed a low COVID-19 mortality [8, 9]. According to the World Health Organization (WHO) one quarter of the world's population may have LTBI [10]. Therefore, both LTBI and BCG may provide immunological protection against COVID-19, especially in low income countries.
Two possible mechanisms have been proposed to explain the beneficial effects of BCG. The non-specific trained immunity [11], and the heterologous lymphocyte responses. In recent reports, in silico investigations of T and B-cell epitope prediction methods of the BCG proteome, showed high similarities to immunogenic peptides of SARS-CoV-2 including the receptor binding domain of the spike [12, 13].
In our investigation, we have tested the induced humoral immune responses against the SARS-CoV-2 Spike in the mouse model, after either BCG or rabies DNA-based vaccination alone or in Prime/Boost approach to COVID-19 DNA-based vaccination.
Materials and Methods
Vaccines and Plasmids
The BCG vaccine is produced in the “Institut Pasteur de Tunis” using the strain “Pasteur 1173 P2”. The plasmid backbone pCMV3ISS and pCMV3ISS-GPV encoding to the PV strain glycoprotein of the rabies virus were constructed as described16. For DNA-based vaccine candidate encoding the full length SARS-CoV-2 Spike Glycoprotein we have used the following primers:
- S1BgLIIup
AAGATCTATGTTTGTTTTTCTTGTTTTATTGCC
- S1 BamHIStop rev
ATTAGGATCCTGTCTTGGTCATAGACACTGG
- S2BamHI Start up
AGGATCCATGTCAGTAGATTGTACAATGTACATTTGTGG
- S2rev
ATTATGTGTAATGTAATTTGACTCCTTTGAGCACTGGC
- TMBgLII up
AAGATCTAAATGGCCATGGTACATTTGGCTAGG
- S2-BamHIrev
AGGATCCTTATGTGTAATGTAATTTGACTCCTTTGAGC
The Spike gene was amplified by RT-PCR in different steps, using the viral RNA of a COVID-19 Tunisian patient as a matrix. A nasopharyngeal sample from a Covid-19 positif patient was used for RNA extraction via the Qiamp viral RNA mini kit (Qiagen Gmbh, Germany). We have amplified the S1 domain of the Spike using the set of primers (S1BgLIIup and S1 BamHIStop rev) giving pCMV3ISS-S1. Then, we have constructed pCMV3ISS-S2 encoding to the S2 domain with S2BamHI Start up and S2rev. To get rid of BamHI site inside the S2 domain, we have used the primers TMBgLII up and S2-BamHIrev and the matrix was pCMV3ISS-S2, to amplify the Transmembrane domain (from nucleotides 3752 to 3822 of the spike), giving pCMV3ISS-BgLII-TM. The plasmid pCMV3ISS-BgLII-TM was linearized with BgLII and Inserted the remaining of the S2 domain issued by the digestion of pCMV3ISS-S2 by BamHI. Therefore, we have the plasmid pCMV3ISS-S2ΔBamHI, which is identical to pCMV3ISS-S2 except that the site BamHI at position 3752 is eliminated (GGATCT instead of GGATCC). Then, we have extracted the S2 gene by digesting pCMV3ISS-S2ΔBamHI with BamHI and inserted it to pCMV3ISS-S1 after linearization with BamHI, inorder to obtain the plasmid pCMV3ISS-Cov2S, encoding to the full length of the Spike. In addition, to the full length S, the plasmid pCMV3ISS-Cov2S encodes to an insertion of three amino acids Glycine, Serine and Methionin (between Threonine734 and Serine735).
For plasmid purification, the Qiagen kit (Qiagen Gmbh, Germany) was used according to the manufacturer recommendations.
Animal Experimentation in Mice
Each group of BALB/c mice was composed of five female animals. Mice were anaesthetized with sodium pentobarbital, then immunized with 50 μg or 100 μg of Qiagen purified pCMV3ISS-CoV2S plasmid intramuscularly in 100 μl of PBS. For Prime/Boost Experiments mice were administered either 200 µl of BCG (1/10th of the human dose) or 50 μg of pCMV3ISS-GPV and two weeks later 50 μg of pCMV3ISS-CoV2S, in the same conditions as above. Mice were identified individually and bled at days 0, 28 and 90 post-vaccination.
ELISA Assay for Antibody Detection
For the assay of antibodies against the SARS-CoV-2, we have developed an in house ELISA. The plating was with the extracellular domain of the spike protein expressed on Baculovirus-Insect Cells (SinoBiological, China). Sera were tested after an initial dilutions to 1/50 or 1/20. Given results are optical density absorptions at 450 nm (OD).
Statistical Analysis
EpiCalc 2000 version 1.02 was used for statistical analysis. We calculated p-values for each comparison (Significant at the 95% confidence interval when lower than 0.05).
Results and Discussion
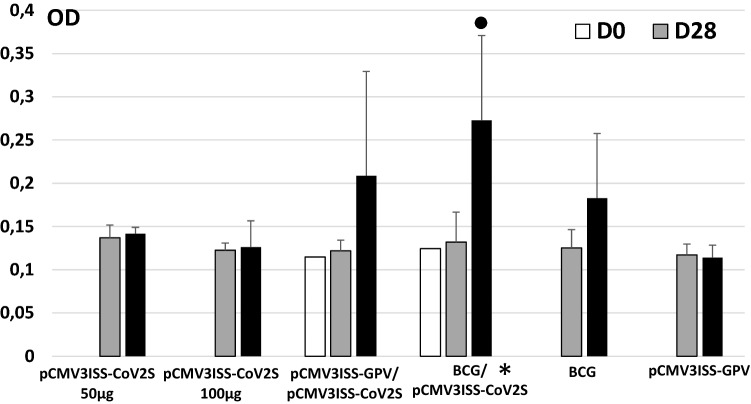
Mice immunized with only pCMV3ISS-CoV2S (Group 1 50 µg, Group 2 100 µg) raised only baseline antibody titers against the SARS-CoV-2 spike when compared to the PBS Group. When mice were primed with either BCG vaccine (Group 4) or with pCMV3ISS-GPV (Group 3) then boosted with pCMV3ISS-CoV2S, high antibody titers were raised (Fig. 1). Mice of Groups 3 and 4 compared to those of Group 1 (administered only pCMV3ISS-CoV2S) showed increases of the induced antibody titers at day 90 post-priming, the difference was statistically not significant between Groups 3 and 1 (p-value 0.227) and significant between Groups 4 and 1 (p-value 0.00136). Mice administered only BCG (Group 5) induced a higher level of cross-reacting antibodies to SARS-CoV-2 spike at day 90 compared to day 28, optical density absorption at 450 nm (OD) of 0.183 and 0.125, respectively (p-value: 0.189 not significant). In a previous experiment, we have shown almost the same trend in inducing cross-reacting antibodies to SARS-CoV-2 spike by the administration of BCG alone (Table 1). We have compiled the results of both experiments and the comparison between day 90 (OD 0.200) and day 28 (OD 0.117) was statistically significant (p-value: 0.018).
Fig. 1.
Kinetics of humoral immune responses in mice. Mice of Group 1 and 2 were administered at Day0 50 µg or 100 µg of pCMV3ISS-CoV2S, respectively. Mice of Group 3 were administered at Day 0 50 µg of pCMV3ISS-GPV and 2 weeks later 50 µg of pCMV3ISS-CoV2S. Mice of Group 4 were administered at Day 0 the 1/10th of BCG human dose ant 2 weeks later 50 µg of pCMV3ISS-CoV2S. Mice of Group 5 were administered only at Day 0 the 1/10th of BCG human dose. Mice of Group 6 were administered only at Day0 50 µg of pCMV3ISS-GPV. Given results are optical density absorptions at 450 nm (OD). * Statistically significant compared to Group 1, filled circle statistically significant between days 90 and 28 in the same Group, both when p-values are lower than 0.05
Table 1.
Individual kinetics of induced antibodies against SARS-CoV-2 after BCG vaccination in mice
| Mice | 1 | 2 | 3 | 4 | 5 | Means | Standard deviations |
|---|---|---|---|---|---|---|---|
| Day 14 | 0.100 | 0.089 | 0.090 | 0.146 | 0.075 | 0.100 | 0.027 |
| Day 28 | 0.070 | 0.175 | 0.100 | 0.121 | 0.089 | 0.110 | 0.041 |
| Day 90 | 0.130 | 0.323 | 0.330 | 0.197 | 0.098 | 0.215 | 0.107 |
We have followed the kinetics of the induced humoral immune responses individually (Table 2). At least 4 mice out of 5 primed with BCG (Group 4) and 2 out of 5 primed with pCMV3ISS-GPV (Group 3) seroconverted by day 90. Two mice out of 4 administered only BCG (Group 5), showed seroconversions. In a previous experiment 3 mice out of 5 have seroconverted (Table 1). Hence, we can confirm that mice administered only BCG are able to induce cross-reacting antibodies towards the SARS-CoV-2 Spike. Mice administered only the rabies pCMV3ISS-GPV did not show significant seroconversions towards the SARS-CoV-2 Spike. However, when sera were diluted to 1/20 and tested by ELISA, mouse 3, has shown most likely a titer that can be considered as a seroconversion (Table 2). Further investigations are needed to confirm that rabies Glycoprotein is able to induce cross-reacting antibodies to SARS-CoV-2 Spike.
Table 2.
Individual kinetics of induced antibodies against SARS-CoV-2 in mice
| Mouse 1 | Mouse 2 | Mouse 3 | Mouse 4 | Mouse 5 | Means | Standard deviations | |
|---|---|---|---|---|---|---|---|
| Group 1: pCMV3ISS-CoV2S 50 µg | |||||||
| Day 28 | 0.124 | 0.156 | 0.143 | 0.12 | 0.141 | 0.137 | 0.015 |
| Day 90 | 0.136 | 0.153 | 0.145 | 0.136 | 0.138 | 0.142 | 0.007 |
| Group 2: pCMV3ISS-CoV2S 100 µg | |||||||
| Day 28 | 0.13 | 0.125 | 0.119 | 0.129 | 0.11 | 0.123 | 0.008 |
| Day 90 | 0.173 | 0.114 | 0.14 | 0.101 | 0.103 | 0.126 | 0.030 |
| Group 3: Prime pCMV3ISS-GPV/Boost pCMV3ISS-CoV2S | |||||||
| Day 0 | 0.130 | 0.111 | 0.140 | 0.117 | 0.077 | 0.115 | 0.024 |
| Day 28 | 0.140 | 0.124 | 0.110 | 0.130 | 0.105 | 0.122 | 0.012 |
| Day 90 | 0.170 | 0.134 | 0.410 | 0.213 | 0.116 | 0.209 | 0.120 |
| Group 4: Prime BCG/Boost pCMV3ISS-CoV2S | |||||||
| Day 0 | 0.150 | 0.094 | 0.150 | 0.114 | 0.120 | 0.124 | 0.023 |
| Day 28 | 0.190 | 0.098 | 0.130 | 0.110 | 0.131 | 0.132 | 0.035 |
| Day 90 | 0.150 | 0.420 | 0.290 | 0.280 | 0.227 | 0.273 | 0.098 |
| Group 5: BCG | |||||||
| Day 28 | 0.110 | 0.110 | 0.121 | 0.156 | 0.125 | 0.021 | |
| Day 90 | 0.282 | 0.130 | 0.117 | 0.198 | 0.183 | 0.075 | |
| Group 6: pCMV3ISS-GPV | |||||||
| Day 28 | 0.140 | 0.109 | 0.110 | 0.125 | 0.105 | 0.117 | 0.012 |
| Day 90a | 0.110 (0.116) | 0.098 (0.111) | 0.130 (0.204) | 0.124 (0.115) | 0.104 (0.100) | 0.114 (0.129) | 0.014 (0.042) |
ELISA results when sera were diluted to 1/20th
aBetween brackets
To our knowledge this is a first demonstration that BCG vaccination alone was able to induce cross-reacting antibodies to SARS-CoV-2 Spike. It can also boost the antibody response induced by a COVID-19 DNA-based vaccination. Although, the effect of BCG vaccination on the fate of Covid-19 is still controversial, many reports consider the trained immunity as the main mechanism of protection [14]. Others through in silico investigations have hypothesized that BCG may carry similar T-and B-cell epitopes with SARS-CoV-2 [12, 13]. They have found numerous BCG MHC-I-restricted T-cell epitopes similar to MHC-I-restricted T-cell SARS-CoV-2 epitopes and some BCG B-cell epitopes similar to SARS-CoV-2 B-cell epitopes, including the receptor binding domain of the spike glycoprotein.
Unlike our findings, it was reported that BCG and other common childhood vaccines in the mouse model, have failed to induce cross-reacting antibodies against SARS-CoV-2 [15]. However, they have followed the induced antibodies only up to the 7th week post-immunization. We were able to detect significant antibody titers only at day 90 post-vaccination. In COVID-19 patients it was reported that neutralizing SARS-CoV-2 RBD-specific clones accumulated with time and largely contributed to the late memory B-cell pool [16]. Also, they have used seroneutralization test and we have used ELISA for the detection of antibodies, which results might be not correlated. In a recent paper [17], it was shown in their figures that mice administered only BCG, induced a detectable antibody response by ELISA but not by virus neutralization test, even though the authors did not mention that.
Our findings are very important for several reasons. First, it can be used as a tangible proof to stop the controversy about the correlation between the National policy of BCG vaccination and the lower burden of the COVID-19. It can also explain in high endemic regions of TBLI, Mycobacterium may stimulate the immune system against the COVID-19 [14]. Both, BCG and LTBI can explain the lower burden of COVID-19 in developing countries; Second, the mechanism of BCG vaccination for reducing COVID-19 burden is not only through the trained immunity but also by the induction of cross-reacting antibodies (adaptive immunity); Third, even though specific COVID-19 vaccines have been released, they are still in limited supply and many of them induced suboptimal immune responses. BCG can be used as a prime vaccination to enhance the induced humoral immune responses by a COVID-19 booster vaccination [18]; Last, and with the emergence of different COVID-19 variants, or eventually other Betacoronaviruses, the use of BCG vaccination can help against immune escapes of the current vaccines [14, 19].
When the rabies valence was used instead of the BCG for prime vaccination with pCMV3ISS-GPV, then boosted with a COVID-19 DNA-based vaccination, enhanced humoral immune response was shown but statistically not significant. Furthermore, when pCMV3ISS-GPV was administered alone, there is at least in one mouse a seroconversion. Although, further investigations are needed, we think that the rabies glycoprotein is able to induce cross-reacting antibodies with the SARS-CoV-2 Spike. It was recently suggested that the SARS-CoV-2 spike protein may interact with nicotinic acetylcholine receptors through the spike Y674-R685 region, homologous to that of snake neurotoxins and to the rabies virus glycoprotein [20] and even to the region 157–172 of the HIV-1 gp120, which cross-reacted with serum antibodies from rabies vaccinated patients [21]. Hence, we can speculate that DNA-based vaccination has induced cross-reacting antibodies to the SARS-CoV-2 Spike.
Conclusion
It was reported that BCG vaccination is a potential tool for reducing the burden of COVID-19, mainly through the non-specific trained immunity. Here we have demonstrated that BCG vaccination alone was able to induce cross-reacting antibodies to SARS-CoV-2 Spike. In addition, we think that with the emergence of different COVID-19 variants, the use of BCG may increase the induced immune responses by the current vaccines and further prevent the immune escapes.
Acknowledgements
We would like to thank Prof Henda Triki and Dr Mariem Gdoura for giving us the viral RNA of a COVID-19 Tunisian patient and helping us in the set up of the home made ELISA for antibody assays. We would like to thank Dr Nizar Laabidi for giving us the BCG vaccine.
Author Contributions
NR constructed the plasmid pCMV3ISS-CoV2S. CB realized the conception of the investigation, the animal experimentation, antibody assays, statistical analysis and paper drafting.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data Availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Research on mice has been performed with the approval of a National Ethics Committee “Animal Ethics Committee—National School of Veterinary Medicine, IACUC, ENMV- Sidi Thabet, Tunisia” (Approval Number: CEEA-ENMV 37/21, Date the 9th of Décembre 2021).
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Consent for Participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042937. [DOI] [Google Scholar]
- 2.Aspatwar A, Gong W, Wang S, Wu X, Parkkila S. Tuberculosis vaccine BCG: the magical effect of the old vaccine in the fight against the COVID-19 pandemic. Int. Rev. Immunol. 2021;7:1–14. doi: 10.1080/08830185.2021.1922685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shivendu S, Chakraborty S, Onuchowska A, Patidar A, Srivastava A. Is there evidence that BCG vaccination has non-specific protective effects for COVID-19 infections or is it an illusion created by lack of testing? SSRN Electronic J. 2020 doi: 10.2139/ssrn.3579847. [DOI] [Google Scholar]
- 4.Patella V, Sanduzzi A, Bruzzese D, Florio G, Brancaccio R, et al. A survey among Italian physicians during COVID-19 outbreak. Could Bacillus Calmette-Guérin vaccine be effective against SARS-CoV2? Front Pharmacol. 2021;12:646570. doi: 10.3389/fphar.2021.646570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Fuente J, Armas O, Sánchez-Rodríguez L, Gortázar C, Lukashev AN. Citizen science initiative points at childhood BCG vaccination as a risk factor for COVID-19. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amirlak I, Haddad R, Hardy JD, Khaled NS, Chung MH, et al. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. medRxiv. 2020 doi: 10.1101/2020.08.10.20172288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marín-Hernández D, Nixon DF, Hupert N. Anticipated reduction in COVID-19 mortality due to population-wide BCG vaccination: evidence from Germany. Hum. Vaccin. Immunother. 2021 doi: 10.1080/21645515.2021.1872344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi H. Role of latent tuberculosis infections in reduced COVID-19 mortality: evidence from an instrumental variable method analysis. Med. Hypotheses. 2020;144:110214. doi: 10.1016/j.mehy.2020.110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madan M, Pahuja S, Mohan A, Pandey RM, Madan K, et al. TB infection and BCG vaccination: are we protected from COVID-19? Public Health. 2020;185:91–92. doi: 10.1016/j.puhe.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen A, Dahl Mathiasen V, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 2019;54(3):19006552019. doi: 10.1183/13993003.00655-2019. [DOI] [PubMed] [Google Scholar]
- 11.Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita Y, Sato R, Ikeda T, Sakagami T. BCG vaccine may generate cross-reactive T cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38(41):6352–6356. doi: 10.1016/j.vaccine.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbán S, Paragi G, Burián K, McLean GR, Virok DP. Identification of similar epitopes between severe acute respiratory syndrome coronavirus-2 and Bacillus Calmette-Guérin: potential for cross-reactive adaptive immunity. Clin. Transl. Immunol. 2020 doi: 10.1002/cti2.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marín-Hernández D, Nixon DF, Hupert N. Heterologous vaccine interventions: boosting immunity against future pandemics. Mol. Med. 2021;27(1):54. doi: 10.1186/s10020-021-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandeil A, Gomaa MR, El Taweel A, Mostafa A, Shehata M, et al. Common childhood vaccines do not elicit a cross-reactive antibody response against SARS-CoV-2. PLoS One. 2020;15(10):e0241471. doi: 10.1371/journal.pone.0241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokal A, Chappert P, Barba-Spaeth G, Roeser A, Fourati S, et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. 2021;184(5):1201–1213. doi: 10.1016/j.cell.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Counoupas C, Johansen MD, Stella AO, Nguyen DH, Ferguson AL, et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines. 2021;6:143. doi: 10.1038/s41541-021-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debisarun PA, Patrick S, Jorge D-A, Simone JCFM, Moorlag ET, et al. The effect of influenza vaccination on trained immunity: impact on COVID-19. MedRxiv. 2020 doi: 10.1101/2020.10.14.20212498. [DOI] [Google Scholar]
- 19.Malheiros Borges KC, da Costa AC, de Souza Barbosa LC, Ribeiro KM, Borges Dos Anjos LR, Kipnis A, Junqueira-Kipnis AP. Tuberculosis, BCG vaccination and COVID-19: are they connected? Mini Rev Med Chem. 2022 doi: 10.2174/1389557522666220104152634. [DOI] [PubMed] [Google Scholar]
- 20.Sofia A, Oliveira F, Ibarra AA, Bermudez I, Casalino L, et al. A potential interaction between the SARS-CoV-2 spike protein and nicotinic acetylcholine receptors. Biophys. J. 2021;120(6):983–993. doi: 10.1016/j.bpj.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracci L, Ballas SK, Spreafico A, Neri P. Molecular mimicry between the rabies virus glycoprotein and human immunodeficiency virus-1 GP120: cross-reacting antibodies induced by rabies vaccination. Blood. 1997;90:3623–3628. doi: 10.1182/blood.V90.9.3623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Not applicable.