Abstract
Matcha tea has been used as an adjunct in weight loss programs. The weight loss effects of matcha tea were evaluated in a prospective non-randomized open-label comparative study of overweight and obese individuals who followed a specified low-calorie diet (LCD) plan. A total of 40 participants were enrolled and assigned to either matcha tea or control groups. The matcha tea group followed a LCD plan and received matcha tea once daily, whereas the control group followed only the LCD diet plan. The study lasted 12 weeks. The main outcome measures included anthropometric measurements, fasting blood glucose, hemoglobin A1c (HbA1c), lipid profile, obesity-related hormone peptides, pro-inflammatory and anti-inflammatory cytokines, and oxidative stress biomarkers. Thirty-four participants had completed the study. The matcha tea and control groups showed significant reductions in body weight, body mass index, waist circumference, water content, minerals, and fat mass at week 12. The post-treatment body composition and anthropometric measurements were not significantly different between the two groups. The matcha tea group showed a potential increase in HDL-C, a potential decrease in blood glucose, and a potential increase in HbA1c. Furthermore, the study indicated a potential decrease in insulin and leptin levels, a potential increase in the activity of superoxide dismutase, and a potential decreased activity of glutathione peroxidase. IL-10 was increased by matcha tea consumption. The data suggest that matcha tea may have some potential effect on weight loss, along with anti-inflammatory properties. The findings of this study will be used to design a multicenter randomized clinical trial to examine the potential weight loss benefits of matcha tea.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11130-022-00998-9.
Keywords: Matcha tea, Camellia sinensis, Obesity, Inflammation, Hypocaloric diet, Oxidative stress
Introduction
Obesity is a global health issue that is dramatically increasing worldwide [1, 2]. In 2016, it was estimated that about 13% of the world's adult population are obese indicating that the prevalence of obesity has tripled since 1975 [2]. Obesity has been recognized as a major risk factor for many diseases, including cardiovascular, metabolic, pulmonary, and musculoskeletal diseases, as well as psychological conditions and certain types of cancer [1, 3]. Obesity is a chronic, complex and multifactorial disease [1]. It is manifested by an excessive accumulation of fat that results from an imbalance between energy intake and expenditure [1].
Weight loss can be achieved using several approaches, including lifestyle management [1], pharmacotherapeutic approaches [1], and surgical procedures [3]. Although adopting a healthy lifestyle, including healthier food choices and frequent physical activity can result in weight loss, but long-term maintenance is challenging [1]. On the other hand, prescribed weight loss medications may not be effective for all cases and their use is associated with adverse effects [1]. Hence, there is a growing interest to test other weight loss choices that are commonly used by the public such as herbal products and dietary supplements. It has been estimated that about 15% of adults have used herbal products or dietary supplements for weight loss [4]. For most of natural and herbal products, there is a lack of controlled clinical trials to demonstrate their safety and efficacy [4].
Tea [Camellia sinensis L. Kuntze (Theaceae)] is one of the most popular drinking beverages all over the world [5]. Green tea consumption is reported to have beneficial effects on health [5]. Matcha tea, a special type of green tea, is traditionally used in Japanese tea ceremonies [6]. It is different from green tea in terms of growing conditions and manufacturing processing [6]. Matcha tea is different, as well, from other forms of tea in the way of preparation and consumption. While in most types of green teas the water extract of the leaves is consumed, in matcha tea, however, water is added to the finely ground leaves powder and the whole leaves are consumed [6]. A number of clinical studies have demonstrated a positive correlation between green tea consumption that is rich in caffeine and catechins particularly (-)-epigallocatechin-3-gallate, and weight reduction and glucose control [7]. However, such studies are lacking for matcha tea, which has a potent antioxidant activity and is a rich source of caffeine [8]. Moreover, there is a worldwide increased interest in matcha tea as a healthy nutrient with promising applications in the field of functional food or nutraceuticals [8], including Jordan where a number of diet centers have started recently adding matcha tea to their low caloric diet plans.
The major goal of this study was to examine the efficacy of matcha tea administration on weight reduction in a prospective non-randomized open-label comparative study of overweight and obese individuals who were following a specified low-calorie diet (LCD) plan. The goal of the study was achieved via evaluation of the effects of matcha tea administration on anthropometric measurements, lipid profile, metabolic profile and glycemic control, obesity-related hormones, anti-inflammatory cytokines, and oxidative stress biomarkers. The procedure and the results of this pilot study will be utilized in designing a future larger, randomized controlled trial.
Materials and Methods
The material and methods section are reported as a supplementary online resource.
Results and Discussion
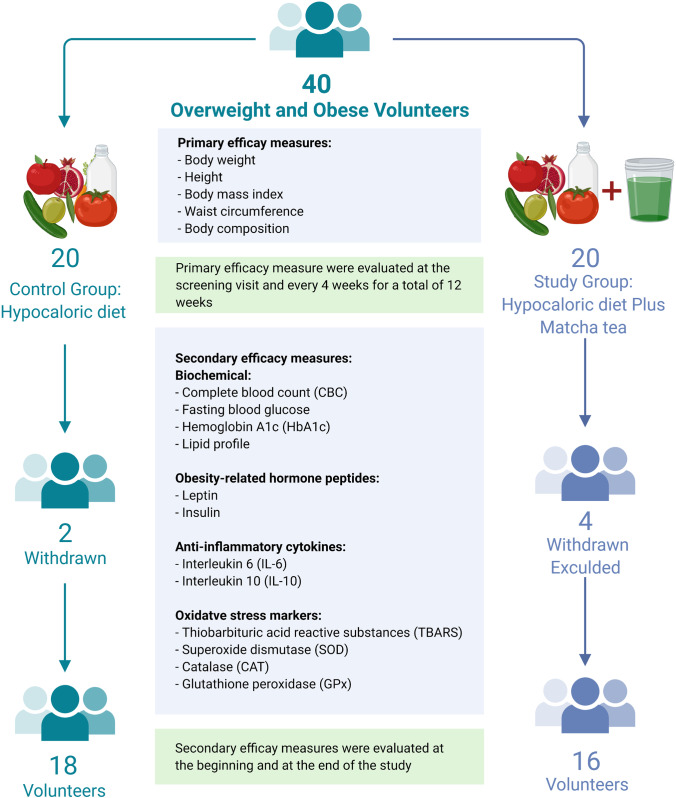
Over 100 people were screened for their eligibility and those who met the inclusion criteria were invited to participate in the study. Despite meeting the inclusion criteria, some individuals refused to participate, including those with a history of sensitivity to green tea, those who prefer to avoid herbal products, and women planning to become pregnant in the next three months. Therefore, 40 participants were enrolled and were allocated into either matcha tea or control groups (Fig. 1). There were 6 males and 14 females in each group. Participants in the matcha tea group were asked to follow a specified LCD plan in addition to an oral intake of a beverage of matcha tea [2 g in a cup of water once daily]. However, participants in the control group were asked to follow the same specified diet plan as in the study group (Fig. 1). Participants were required to visit the diet center once a week to ensure compliance with the diet plan and matcha tea consumption. On day 0, after fasting overnight, each volunteer undergoes blood and a body composition test. During the study, four female participants were excluded from the matcha tea group based on the following reasons: changing their living place, testing positive for COVID-19 with severe symptoms, being under quarantine due to family members testing positive for COVID-19, and not complying with the study protocol. Moreover, two female participants from the control group were excluded from the study due to failing to comply with the study protocol and refusing the blood withdrawal due to needle fear. Hence, a total of 34 participants completed the study (matcha tea group: 6 males and 10 females and control group: 6 males and 12 females). During the period of this pilot study, there were no reported adverse effects in the matcha tea group.
Fig. 1.
Study design and methodology
Demographics, Composition Analysis, and Anthropometric Measurements
Baseline demographics, including gender and age as well as baseline anthropometric measurements including, weight, height, body mass index (BMI), and waist circumference (WC) showed no significant differences between the two groups (p > 0.05) (Table S1, Online Resource). A total of 27.8% of the control group and 37.5% of the matcha tea group were obese class I. Furthermore, 33.3% of the control group and 18.8% of the matcha tea group were obese class II. Likewise, 33.3% of the control group and 31.3% of the matcha tea group were classified as obese class III. Finally, overweight individuals make up 5.6 and 12.5% of the control and matcha tea groups, respectively. However, there was a significant difference between the two groups (p < 0.05) in terms of LDL-C and leptin levels (Table S1, Online Resource).
After 12 weeks of matcha tea intake, the body weight, BMI, and WC of the matcha tea group significantly decreased (Table 1). Moreover, the body content of water, protein, minerals, and fat mass were significantly reduced (Table 1). On the other hand, a significant difference was detected among all variables in the control group (p < 0.05), except for the protein content of the body (Table 1). Based on the between-group analysis of the post-treatment data, no significant differences were detected between the two groups for body weight, BMI, WC, water content, protein, minerals, or fat mass (p > 0.05) (Table 1). The results are consistent with previous studies on green tea for 12 weeks [9]. However, the protein content of the body was significantly reduced in participants who received a LCD plan plus matcha tea for 12 weeks compared to baseline protein content. This could be explained by the effect of matcha tea on increasing weight loss that affects all tissue types, leading not only to a reduction of fat mass but also decreased lean tissue [10]. Slentz et al. [11] reported a significant reduction in the weight and fat mass (abdomen, waist, hip circumference) for overweight participants who did not follow a diet but did an exercise at different intensities. Such reduction was increased as the physical activity increased [11].
Table 1.
Within-group body composition, anthropometric, and biochemical measurements data at baseline and after 12 weeks
| Variable | Matcha tea (n = 16) | Control (n = 18) | Post-treatment p-value |
||||
|---|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | p-value | Baseline | After 12 weeks | p-value | ||
| Body composition and anthropometric measurements | |||||||
| Weight, kg | 97.4 (22.9) | 86.8 (20.3) | < 0.0001 | 100.6 (19.4) | 90.3 (17.6) | < 0.0001 | 0.600 |
| Body mass index, kg/m2 | 35.7 (5.5) | 31.8 (4.7) | < 0.0001 | 36.9 (4.4) | 33.2 (4.3) | < 0.0001 | 0.386 |
| Waist circumference, cm | 114.3 (16.4) | 103.7 (16.1) | < 0.0001 | 116.4 (13.2) | 104.9 (12.1) | < 0.0001 | 0.800 |
| Water, L | 39.4 (9.9) | 38.1 (9.4) | 0.002 | 40.4 (9.2) | 39.2 (9.2) | 0.002 | 0.738 |
| Protein, kg | 10.4 (2.8) | 10.2 (2.6) | 0.014 | 10.3 (2.6) | 10.2 (2.5) | 0.263 | 0.991 |
| Minerals, kg | 4.1 (1.1) | 3.8 (1.0) | 0.001 | 4.5 (1.2) | 4.2 (1.1) | < 0.0001 | 0.286 |
| Fat mass, kg | 43.4 (11.9) | 34.7 (10.7) | < 0.0001 | 45.3 (10.3) | 36.7 (9.6) | < 0.0001 | 0.568 |
| Lipid profile of study participants | |||||||
| Total cholesterol, mg/dL | 176.6 (39.2) | 175.6 (39.6) | 0.817 | 194.6 (28.3) | 183.4 (25.5) | 0.107 | 0.490 |
| Triglycerides, mg/dL | 115.4 (77.4) | 101.2 (42.7) | 0.265 | 101.9 (32.5) | 99.8 (38.1) | 0.717 | 0.920 |
| Low-density lipoprotein cholesterol, mg/dL | 113.5 (34.8) | 112.4 (35.1) | 0.753 | 136.3 (26.6) | 125.3 (24.9) | 0.047 | 0.221 |
| High-density lipoprotein cholesterol, mg/dL | 43.9 (13.1) | 46.1 (11.3) | 0.105 | 41.3 (9.5) | 41.4 (7.5) | 0.980 | 0.155 |
| Complete blood count with the differential of study participants | |||||||
| Red blood cells, million/mm3 | 5.1 (0.7) | 5.0 (0.7) | 0.519 | 5.0 (0.5) | 5.0 (0.6) | 0.722 | 0.961 |
| Hemoglobin, g/dL | 14.3 (1.9) | 13.7 (1.9) | 0.065 | 13.7 (2.6) | 13.7 (2.3) | 0.810 | 0.730 |
| Hematocrit, % | 41.5 (4.8) | 41.6 (5.3) | 0.877 | 40.6 (6.7) | 41.0 (6.0) | 0.526 | 0.748 |
| Mean corpuscular volume, fL | 80.3 (10.1) | 83.2 (5.4) | 0.233 | 81.7 (8.7) | 81.7 (7.8) | 0.925 | 0.534 |
| Mean corpuscular hemoglobin, pg | 28.4 (2.7) | 27.8 (2.2) | 0.060 | 27.3 (3.7) | 27.4 (3.4) | 0.774 | 0.647 |
| Mean corpuscular hemoglobin concentration, % | 34.3 (1.3) | 33.5 (0.7) | 0.025 | 33.5 (1.4) | 33.4 (1.1) | 0.466 | 0.747 |
| Red cell distribution width, % | 14.1 (1.7) | 14.2 (1.4) | 0.656 | 14.6 (2.1) | 15.0 (2.6) | 0.104 | 0.260 |
| White blood cells, thousand/mm3 | 7.6 (1.7) | 7.2 (1.4) | 0.386 | 7.1 (2.0) | 6.9 (1.7) | 0.655 | 0.610 |
| Neutrophils, cells/mm3 | 58.2 (11.4) | 55.3 (6.3) | 0.113 | 58.8 (7.2) | 54.2 (7.9) | 0.065 | 0.648 |
| Lymphocytes, cells/mm3 | 32.4 (9.4) | 35.7 (5.7) | 0.046 | 32.2 (6.7) | 37.2 (7.7) | 0.052 | 0.515 |
| Monocytes, cells/mm3 | 7.1 (1.8) | 6.7 (1.3) | 0.383 | 6.8 (1.8) | 6.6 (1.4) | 0.675 | 0.867 |
| Eosinophils, cells/mm3 | 2.1 (1.9) | 1.8 (1.5) | 0.352 | 1.9 (1.6) | 1.9 (1.7) | 0.816 | 0.889 |
| Basophils, cells/mm3 | 0.7 (2.0) | 0.6 (0.6) | 0.827 | 0.2 (0.4) | 0.1 (0.3) | 0.668 | 0.012 |
| Platelets, thousand/mm3 | 288.0 (59.2) | 276.8 (42.0) | 0.205 | 314.4 (103.3) | 286.9 (89.0) | 0.009 | 0.682 |
| Mean platelet volume, fL | 9.0 (1.2) | 9.0 (0.8) | 0.718 | 9.2 (1.3) | 8.9 (0.9) | 0.262 | 0.839 |
| Metabolic profile | |||||||
| Fasting blood glucose, mg/dL | 91.0 (9.9) | 85.2 (9.2) | 0.004 | 88.1 (13.2) | 83.8 (8.5) | 0.211 | 0.659 |
| Glycemic control | |||||||
| Hemoglobin A1c, % | 5.0 (0.4) | 5.2 (0.4) | 0.004 | 5.1 (0.4) | 5.2 (0.2) | 0.134 | 0.845 |
| Obesity-related hormones | |||||||
| Insulin, mIU/L | 13.8 (8.9) | 11.3 (7.8) | 0.163 | 10.2 (6.9) | 10.7 (4.3) | 0.698 | 0.785 |
| Leptin, ng/mL | 3.9 (1.7) | 3.0 (1.2) | 0.079 | 2.3 (1.1) | 2.0 (0.8) | 0.285 | 0.005 |
| Anti-inflammatory cytokines | |||||||
| Interleukin-6, pg/mL | 5.2 (2.6) | 5.1 (3.0) | 0.761 | 5.4 (3.0) | 7.2 (6.5) | 0.152 | 0.236 |
| Interleukin-10, pg/mL | 8.0 (0.8) | 10.2 (0.7) | < 0.0001 | 7.8 (1.3) | 7.7 (1.30) | 0.589 | < 0.0001 |
| Oxidative stress biomarkers | |||||||
| Superoxide dismutase, inhibition % | 63.03 (17.8) | 72.0 (11.8) | 0.086 | 66.6 (11.5) | 73.7 (9.0) | 0.108 | 0.644 |
| Catalase, nmol/min/mL | 22.6 (9.7) | 24.7 (12.0) | 0.524 | 19.8 (8.8) | 23.4 (11.7) | 0.305 | 0.755 |
| Thiobarbituric acid reactive substances, µM | 115.4 (32.0) | 107.3 (32.3) | 0.482 | 112.5 (18.3) | 96.6 (21.6) | 0.030 | 0.269 |
| Glutathione peroxidase, nmol/min/mL | 8.8 (5.0) | 6.4 (1.4) | 0.098 | 9.3 (4.2) | 8.5 (4.4) | 0.367 | 0.168 |
Data are presented as a mean with a standard deviation in parenthesis. Bold values indicate significant differences (p < 0.05)
Lipid Profile Analysis
No statistically significant differences (p > 0.05) were detected in the lipid profile data after 12 weeks of matcha tea consumption (total cholesterol, triglycerides, LDL, or HDL) (Table 1). The level of HDL-C increased after 12 weeks of matcha tea in comparison to baseline levels, although the difference was not statistically significant. HDL-C exerts a protective effect on the development of cardiovascular diseases as it reverses the transport of cholesterol and exerts antioxidant, and anti-inflammatory effects [12]. The results are inconsistent with Yuan and colleagues [13] finding that green tea administration reduced total cholesterol and LDL-C [13]. The control group, on the other hand, showed a significant reduction in LDL levels (p < 0.05) (Table 1). This might be attributed by higher baseline levels of LDL-C in the control group as more participants were categorized as obese classes I and II and hence the effect on LDL-C was observed.
In the post-treatment data, there were no significant differences in lipid profiles between the two groups (p > 0.05) (Table 1).
Complete Blood Count with Differential Analysis
In the matcha tea group, no statistically significant differences (p > 0.05) were detected in the complete blood count and differential data after 12 weeks of matcha tea consumption, except for mean corpuscular hemoglobin concentration (MCHC) and lymphocyte count. Where MCHC significantly decreased and lymphocyte count significantly increased (Table 1). Further studies are needed to examine the underlying cause of increased lymphocyte levels after matcha tea consumption in a larger clinical study. A previous study on rats revealed unaffected complete blood count by green tea consumption [14], inconsistent with the current human study. Measuring the levels of serum ferritin, total iron-binding capacity, and transferrin should be considered in future studies to confirm iron deficiency anemia. The control group, on the other hand, showed a significant reduction in platelet count (Table 1). A previous study revealed a decrease in platelet count, though not significant, in participants who followed a very low-calorie diet for eight weeks [15]. Platelets are the main players in the formation of thrombus as well as regulation of hemostasis. Low platelet count increased the risk of bleeding. However, it has been shown that it is important to assess the function of platelets when their count is low, especially for making clinical decisions [16]. An analysis of the between-group data revealed no significant differences between matcha tea and control groups in terms of complete blood count and differential (p > 0.05) except for basophils count which was significantly higher in the matcha tea group (Table 1). Basophils play a role in host defense against invading microorganisms, especially parasites, as well as for the development of allergic response [17]. Increased levels of basophils could be due to infection or hypothyroidism. Therefore, future studies should examine the levels of thyroid hormones as well as thyroid-stimulating hormones to withdraw conclusions. Of interest, EGCG decreased the expression of vascular cell adhesion molecule-1 (VCAM-1) [18]. VCAM-1 regulates the migration of basophils from blood to the tissue [19]. The reduced expression of VCAM-1, by EGCG, attenuated the migration of basophils from blood to tissues and this might explain the increased level of basophils in blood by matcha tea [20]. Therefore, examining the level and expression of VCAM-1 is of outstanding importance in future studies.
Metabolic Profile and Glycemic Control
A significant reduction in the levels of fasting blood glucose was observed in the matcha tea group. However, the HbA1c level for the matcha tea group was unexpectedly increased from 5.0 ± 0.4% to 5.2 ± 0.4% (p = 0.004), although it remained within normal limits. In contrast, no significant differences were detected in blood glucose or HbA1c among those in the control group (p > 0.05) (Table 1). Liu and colleagues [21] suggested, in their meta-analysis, that green tea reduced fasting glucose as well as HbA1c [21]. The increased HbA1c in the current study could be due to lower hemoglobin levels that affect HbA1c at a given fasting glucose [22]. The effect of matcha tea on HbA1c should be studied longitudinally for longer durations to support the current argument. Furthermore, the level of insulin was reduced after 12 weeks of matcha tea consumption, although statistical analysis did not detect a significant difference. Thus, this suggests that the increase in HbA1c was not caused by matcha tea. The previous meta-analysis showed a significant reduction in insulin by green tea [21]. Increasing the number of enrolled participants is needed in future studies. An analysis of the between-group data indicated that there were no significant differences between matcha tea and control groups in terms of blood glucose and HbA1c levels (p > 0.05) (Table 1).
Obesity-Related Hormones
Neither the matcha tea nor control groups showed any significant differences in insulin and leptin levels (p > 0.05) (Table 1). Matcha tea consumption in addition to a LCD for 12 weeks reduced leptin levels, though it did not reach a significant level (p > 0.05). However, matcha tea administration for 12 weeks resulted in a higher leptin concentration compared to control. The reason for this could be that participants who consumed match tea already had high levels of leptin at baseline. Auvichayapat and colleagues [23] showed that leptin levels were reduced by supplementation with green tea in obese participants in a randomized clinical trial [23]. A previous study proposed that EGCG and theaflavins of tea polyphenols suppress the gene expression of fatty acid synthase [24]. However, there are other determinants of the concentration of leptin, such as ghrelin and resistin, that should be examined in future studies. The between-group data showed that there was a significant difference in leptin levels between the matcha tea group (3.0 ± 1.2 ng/mL) and the control group (2.0 ± 0.8 ng/mL) with a p-value of 0.005 (Table 1).
Anti-Inflammatory Cytokines
Interleukin (IL)-6 possesses pro- and anti-inflammatory properties [25]. In within-group analysis, the matcha tea group showed no significant differences in the levels of the anti-inflammatory cytokine IL-6 (p = 0.761) after matcha tea consumption. Previous studies revealed that green tea decreased the level of IL-6 [26]. A future study with a larger matcha tea dosage or a larger sample size may confirm the beneficial effects of matcha tea on lowering the levels of IL-6. However, a significant increase was observed in the IL-10 levels (Table 1). IL-10 is a potent anti-inflammatory cytokine as it inhibits the synthesis of several inflammatory mediators such as TNF-α, GM-CSF, IL-5 [27], IFN-γ, IL-2, and IL-4 [28]. Consistent with the current finding, a previous study showed that green tea increased the level of IL-10 [26]. Nevertheless, no significant differences were observed in the levels of IL-6 or IL-10 among the control group (Table 1). In the post-treatment data, the level of IL-10 was significantly higher in the matcha tea group compared to the control group (Table 1).
Oxidative Stress Biomarkers
Oxidative stress is a phenomenon caused by an imbalance between the production of reactive species and accumulation in cells and tissues, and the ability of a biological system to detoxify these reactive products [29]. The levels of oxidative stress enzymes, superoxide dismutase (SOD), catalase, or glutathione peroxidase (GPx), were not significantly different between groups or within groups, except for thiobarbituric acid reactive substances (TBARS), which was significantly decreased from 112.5 ± 18.3 to 96.6 ± 21.6 µM, p = 0.003 (Table 1). However, the study showed an increased activity of SOD and decreased activity of GPx by matcha tea consumption for 12 weeks in comparison to baseline levels. The increased activity of SOD is a compensatory mechanism for the increased oxidative stress. A previous study showed that the consumption of green tea did not affect the serum levels of GPx and catalase activities [30]. However, in the current study, the activity of GPx was measured rather than its level. Another study on rats showed that green tea increased SOD activity [31]. Moreover, healthy women who consumed green tea extract had reduced levels of TBARS [32]. The variations in the results could be due to the dose differences of consumed matcha tea or the concentration of antioxidants in matcha tea itself that is affected by the storage conditions. Future studies should examine the activity of other antioxidant enzymes such as glutathione reductase. TBARS decreased significantly within the control group, and this could be due to the significant reduction of LDL levels. It has been shown that TBARS concentration is correlated with the levels of cholesterol and LDL-C in plasma [33]. In addition, it might be due to the increased levels of smoking in the matcha group than in the control group which could result in an oxidant/antioxidant imbalance as was shown previously, where TBARS levels were significantly higher (p < 0.05) in smokers than in nonsmokers [34]. It was shown in another study that cigarette smoking increased oxidative stress, affecting lipid peroxidation and diminishing both enzymatic and non-enzymatic antioxidant status that reduce SOD, catalase, and GPx [35].
Endpoints Percentage Change
Percentage changes between the two groups of all data were analyzed (Fig. 2 and Table S2, Online Resource). There was a significant difference between matcha and control groups in terms of percentage insulin concentration change (p = 0.024). The percentage reduction in insulin reached 16.5% in the matcha group. However, the control group demonstrated a percentage increase of 25.9%. Regarding the anti-inflammatory cytokines, there was a significant difference between matcha and control groups in terms of percentage IL-10 concentration change (p < 0.0001). The percentage increase in IL-10 reached 27.7% in the matcha group. However, the control group demonstrated a percentage reduction of 1.0%. Moreover, IL-6 demonstrated a decrease of 0.5% in the matcha group and an increase of 34.2% in the control group, but their changes were not statistically significant (p = 0.056).
Fig. 2.
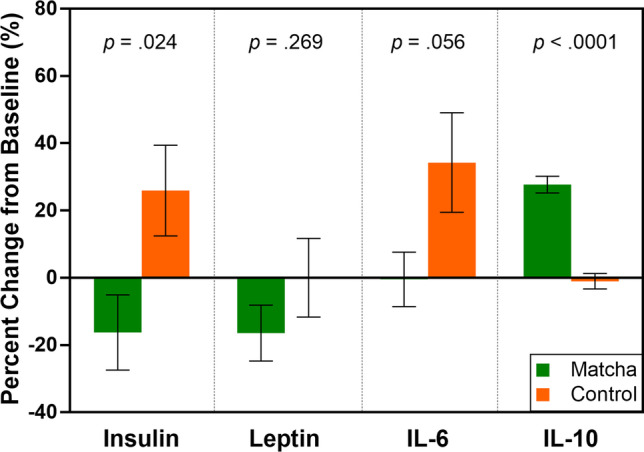
Percentage change from baseline for the levels of insulin, leptin, IL-6 and IL-10 of study participants
There are some limitations to this study, such as (1) The small sample size as this is a pilot study, a larger-scale trial should be conducted. (2) Restrictions and lockdowns associated with COVID-19 affected participant compliance during the study. (3) The lack of regular follow-up with the participants during the period of the study to identify trends in biochemical data and other obesity-related hormones. (4) Liver function tests were not performed to assess the effect of matcha tea on the liver. (5) Lack of bioavailability and pharmacokinetics analysis of matcha components in plasma. (6) Randomization of participants was not implemented. Nevertheless, all the limitations of this study, as well as its design, measures, procedures, recruitment criteria, and operational strategies will be taken into account in designing a future randomized controlled trial.
Conclusion
This is a pilot study designed to shed light on the effects of matcha tea on overweight and obesity. Our findings suggest that matcha tea can be beneficial for weight loss. Matcha tea is considered a functional food and its consumption has been linked to an anti-obesity effect. Consequently, matcha tea can be viewed as a healthier nutraceutical choice that provides multiple health benefits to the human body. As a result, a thorough investigation of the chemical composition of matcha tea, in vitro studies, in vivo clinical trials, as well as animal studies are needed to confirm the positive effects of matcha tea. A deeper investigation of the bioavailability and pharmacokinetics of matcha tea components in humans is also warranted.
Considering the findings of this study, a future large-scale randomized controlled study is recommended. The study should be a randomized, multicenter study where different doses of matcha tea should be tested for at least 24 weeks. Moreover, the future study should include participants with BMI ≥ 30 kg/m2. Secondary efficacy measures should be evaluated monthly and include, in addition to those in the pilot study, the following: hemoglobin, ghrelin, resistin, adiponectin, TNF-α, IL-5, IL-2, IL-4, GPx, glutathione reductase levels, liver function tests, heart rate, systolic and diastolic blood pressure. Moreover, pharmacokinetics profiling of the main components of matcha tea, functional studies on gastrointestinal motility and basal metabolic rate are highly recommended.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BMI
Body mass index
- GPx
Glutathione peroxidase
- IL
Interleukin
- LCD
Low-calorie diet
- MCHC
Mean corpuscular hemoglobin concentration
- SOD
Superoxide dismutase
- TBARS
Thiobarbituric acid reactive substances
- VCAM-1
Vascular cell adhesion molecule-1
- WC
Waist circumference
Author Contributions
Conceptualization: Tamam El-Elimat, Nour A. Al-Sawalha; Methodology: Wala’a M. Qasem, Ramzi T. Munaiem, Reema Al‐Qiam; Formal analysis and investigation: Tamam El-Elimat, Nour A. Al-Sawalha, Mahmoud M. AbuAlSamen; Writing—original draft preparation: Tamam El-Elimat, Wala’a M. Qasem; Writing—review and editing: All authors; Funding acquisition: Tamam El-Elimat; Resources: Tamam El-Elimat, Ahmed H. Al Sharie; Supervision: Tamam El-Elimat, Nour A. Al-Sawalha.
Funding
This research was supported by the Deanship of Research, Jordan University of Science and Technology, Irbid, Jordan (Grant No. 572/2020).
Data Availability
All data generated during this study are included in this published article and its Online Resource files.
Declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of King Abdullah University Hospital affiliated with the Jordan University of Science and Technology (Date 4/10/2020/No. 8/135/2020).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Not applicable.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCafferty BJ, et al. Obesity: Scope, lifestyle interventions, and medical management. Tech Vasc Interv Radiol. 2020;23:100653. doi: 10.1016/j.tvir.2020.100653. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous (2020) Obesity and overweight. World Health Orgnization (WHO). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 May 2020
- 3.Wolfe Bruce M, et al. Treatment of obesity. Circul Res. 2016;118:1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich M, et al. Fundamentals of pharmacognosy and phytotherapy. London: Elsevier Health Sciences; 2018. [Google Scholar]
- 5.Chacko SM, et al. Beneficial effects of green tea: A literature review. Chin Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saijo R (1999) Japanese green tea – Characteristics and manufacture. In: Jain NK (ed) Global advances in tea science. Aravali Books International, New Delhi, p 761–766
- 7.Janssens PLHR, et al. Nutraceuticals for body-weight management: The role of green tea catechins. Physiol Behav. 2016;162:83–87. doi: 10.1016/j.physbeh.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Koláčková T, et al. Matcha tea: Analysis of nutritional composition, phenolics and antioxidant activity. Plant Foods Hum Nutr. 2020;75:48–53. doi: 10.1007/s11130-019-00777-z. [DOI] [PubMed] [Google Scholar]
- 9.Haghighatdoost F, Hariri M. The effect of green tea on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Phytother Res. 2019;33:2274–2287. doi: 10.1002/ptr.6432. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, et al. Effects of catechin enriched green tea on body composition. Obesity. 2010;18:773–779. doi: 10.1038/oby.2009.256. [DOI] [PubMed] [Google Scholar]
- 11.Slentz CA, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med. 2004;164:31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Fisher EA, et al. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Atertio Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/atvbaha.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan F, et al. Effects of green tea on lipid metabolism in overweight or obese people: A meta-analysis of randomized controlled trials. Mol Nutr Food Res. 2018;62:1601122. doi: 10.1002/mnfr.201601122. [DOI] [PubMed] [Google Scholar]
- 14.Essex K, et al. Green tea consumption does not adversely affect kidney function and haematological parameters. Food and Public Health. 2019;9:79–83. doi: 10.5923/j.fph.20190903.01. [DOI] [Google Scholar]
- 15.Toplak H, Wascher TC. Influence of weight reduction on platelet volume: Different effects of a hypocaloric diet and a very low calorie diet. Eur J Clin Invest. 1994;24:778–780. doi: 10.1111/j.1365-2362.1994.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 16.Boknäs N, et al. Platelet function testing at low platelet counts: When can you trust your analysis? Res Pract Thromb Haemostasis. 2019;3:285–290. doi: 10.1002/rth2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone KD, et al. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig A, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- 19.Cook-Mills JM. VCAM-1 signals during lymphocyte migration: Role of reactive oxygen species. Mol Immunol. 2002;39:499–508. doi: 10.1016/s0161-5890(02)00206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Żak A, Pokora I. Effect of long-term green tea extract supplementation on peripheral blood leukocytes in crossfit-trained and untrained men. Cen Eur J Sport Sci Med. 2017;10:67–76. doi: 10.18276/cej.2017.3-06. [DOI] [Google Scholar]
- 21.Liu K, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340–348. doi: 10.3945/ajcn.112.052746. [DOI] [PubMed] [Google Scholar]
- 22.Bae JC, et al. Hemoglobin A1c values are affected by hemoglobin level and gender in non-anemic Koreans. J Diabetes Invest. 2014;5:60–65. doi: 10.1111/jdi.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auvichayapat P, et al. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial. Physiol Behav. 2008;93:486–491. doi: 10.1016/j.physbeh.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006;50:211–217. doi: 10.1002/mnfr.200500138. [DOI] [PubMed] [Google Scholar]
- 25.Scheller J, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Molina N, et al. Green tea polyphenols change the profile of inflammatory cytokine release from lymphocytes of obese and lean rats and protect against oxidative damage. Int Immunopharmacol. 2015;28:985–996. doi: 10.1016/j.intimp.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Pretolani M, Goldman M. IL-10: a potential therapy for allergic inflammation? Immunol Today. 1997;18:277–280. doi: 10.1016/S0167-5699(97)80023-0. [DOI] [PubMed] [Google Scholar]
- 28.Del Prete G, et al. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 29.Pizzino G, Irrera N. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu A, et al. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res. 2013;33:180–187. doi: 10.1016/j.nutres.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirjatmadi B, Purwanto B. Effect of green tea (Camellia sinensis) on activity of superoxide dismutase (SOD) Int J Public Health Clin Sci. 2018;5:95–103. doi: 10.32827/ijphcs.5.6.95. [DOI] [Google Scholar]
- 32.Tinahones F, et al. Green tea reduces LDL oxidability and improves vascular function. J Am Coll Nutr. 2008;27:209–213. doi: 10.1080/07315724.2008.10719692. [DOI] [PubMed] [Google Scholar]
- 33.Schimke I, et al. Concentration of thiobarbituric acid-reactive substances (TBARS) in the plasma of patients with atherosclerosis with different localizations and different degrees of severity. Z Med Laboratoriumsdiagn. 1990;31:176–180. [PubMed] [Google Scholar]
- 34.Isik B, et al. Oxidative stress in smokers and non-smokers. Inhalation Toxicol. 2007;19:767–769. doi: 10.1080/08958370701401418. [DOI] [PubMed] [Google Scholar]
- 35.Pasupathi P, et al. Oxidative stress bio markers and antioxidant status in cigarette smokers compared to nonsmokers. J Pharm Sci Res. 2009;1:55. doi: 10.1080/08958370701401418. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this published article and its Online Resource files.