Abstracts
Index of Oral Presentations
OP 01 Diet: from plants to cans
OP 02 SGLT2 inhibitors: promiscuous pleiotropy
OP 03 Risk for CVD and CKD
OP 04 Walking with diabetes
OP 05 How to become a fat cell
OP 06 Intracellular regulation of insulin release
OP 07 Finding a phenotype for diabetic kidney disease
OP 08 Cardiovascular disease mechanisms: something new on the table?
OP 09 Fighting diabetes with tubes, scanners, and catheters
OP 10 Beta cells: protecting what is precious
OP 11 Adipose tissue profiling and cardio-metabolic risk
OP 12 GWAS and more
OP 13 Beyond type 1 and type 2
OP 14 Exercising your tissues in shape
OP 15 Preserving kidney function
OP 16 Flames and scars in the liver
OP 17 Toying with monitoring: from Present Continuous to Future Perfect
OP 18 Cross-talk communication in the pancreas
OP 19 GLP1 agonists: from here to eternity
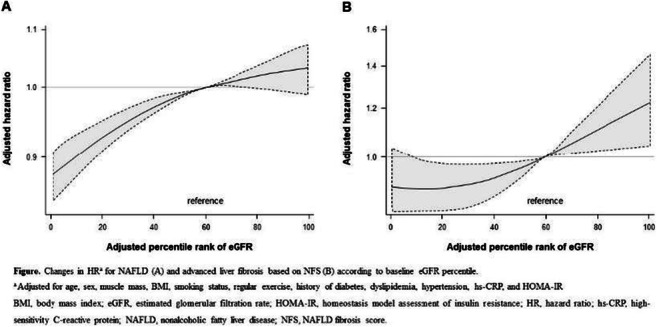
OP 20 NAFLD and treatment
OP 21 Retinopathy future vision
OP 22 Giving birth with diabetes
OP 23 Are we too slow to outlaw the low?
OP 24 How to burn energy
OP 25 Lipid in and out of the liver
OP 26 The dark side of diabetes
OP 27 Improving your insulin sensitivity: lessons from human studies
OP 28 Desirable diets
OP 29 Saving sweet souls
OP 30 Novel ways of beta cell replacement
OP 31 Diabetes: size matters
OP 32 Pain or no pain?
OP 33 Therapy outside the box
OP 34 Insulin signalling, novelties from the petri dish!
OP 35 Different pathways involved in killing the beta cell
OP 36 Central aspects of diabetes
OP 37 Insulin deficiencies and cardiovascular disease
OP 38 It is always a D-D-Day: diabetes, digital, device
OP 39 Too little sugar is also bad: understanding hypoglycaemia
OP 40 When the clock ticks
OP 41 Viruses and diabetes: more than COVID-19
OP 42 Moving towards the beta cell plasma membrane
OP 43 Microvascular cocktail
OP 44 Grading insulin therapy: simple, simpler, the safest
OP 45 Newer agents, better outcomes
OP 46 Profiling human diabetes risk
OP 47 Novel mechanistic insights in peripheral insulin sensitivity
OP 48 How we loose our beta cells
Index of Short Oral Discussions
SO 01 Epigenetics rules
SO 02 Pregnancy and diabetes
SO 03 Food, drinks and spices
SO 04 Starting with autoimmunity
SO 05 Genetics of type 2 diabetes
SO 06 Sometimes one gene is enough
SO 07 SARS-CoV2 and other viruses
SO 08 Benefits of a healthy lifestyle
SO 09 Type 1 diabetes: from molecules to treatment
SO 10 Diversity in diabetes
SO 11 Novel biomarkers and risk factors
SO 12 Different facets of type 2 diabetes treatment
SO 13 It's getting complicated
SO 14 COVID-19 around the globe
SO 15 Calcium signalling in the islet: we are still learning
SO 16 A new niche to replace beta cells
SO 17 Intracellular signalling balancing beta cell survival and death
SO 18 Stressing the beta cell into dysfunction
SO 19 How complicated is type 1 diabetes?
SO 20 The other diabetes and islet function
SO 21 The fat burning the islets
SO 22 Exercise and diabetes: much to learn!
SO 23 Gestational diabetes and pregnancy
SO 24 Life with diabetes from conception to delivery
SO 25 Insulin sensitivity: lessons from cellular and animal models
SO 26 Novel markers and omics signatures
SO 27 If you cannot measure it, you cannot improve it: novel methods in diabetes research
SO 28 Understanding insulin sensitivity: lessons from the clinic
SO 29 Glucose homeostasis regulation beyond insulin
SO 30 Non-classical regulators of metabolism
SO 31 Gut feelings are good
SO 32 Latest drug avenues to treatment
SO 33 Insulin in action
SO 34 The hepato-skeletal impact on metabolic control
SO 35 Metabolic inflexibility and complications in humans
SO 36 Modelling obesity and type 2 diabetes
SO 37 Dietary and nutritional interventions
SO 38 SGLT2 inhibitors and renal outcomes
SO 39 Newer agents - cardiovascular outcomes
SO 40 Incretins: impact on BMI
SO 41 Incretins: basic science
SO 42 Clinical epidemiology and pharmacotherapy
SO 43 Glucose lowering agents
SO 44 Lessons from trials
SO 45 Beta cell function and glucose control
SO 46 Incretins everywhere
SO 47 Treatments, molecules and outcomes: a smorgasbord
SO 48 Hypoglycaemia: hip hip hooray yet to come
SO 49 Is newer (insulin) always better?
SO 50 Is longer better? Looking for different basal insulin approaches
SO 51 Even "old dogs" can learn new tricks
SO 52 Money isn't everything?
SO 53 Pumping, looping, freeing
SO 54 Making sense out of sensors and data
SO 55 From low to high and back: the many faces of insulin therapy
SO 56 Diversity of life with diabetes
SO 57 Type 1 diabetes: still the challenge number one
SO 58 Autonomic rhythm
SO 59 Getting a grip on nerves
SO 60 Preventing microvascular complications
SO 61 Saving the feet
SO 62 Brain, nerve, and heart interaction
SO 63 Diversity of the diabetic kidney
SO 64 New treatment avenues for the diabetic kidney
SO 65 Translating signals in the diabetic kidney
SO 66 Mechanisms of diabetic kidney disease
SO 67 Flames and scars in NAFLD: pathogenesis and therapy
SO 68 From brain circulation to cognitive dysfunction
SO 69 Type 1 diabetes: new findings and complications
SO 70 Circulating markers of cardiovascular risk
SO 71 Prevention and treatment of cardiovascular complications
SO 72 Diabetes dysmetabolism dialogues with the cardiovascular component
SO 73 Diabetes in the vessels
SO 74 Weighing risks of cardiovascular complications
SO 75 Emerging comorbidities in diabetes: clinical associations and mechanisms
SO 76 Cancer and type 2 diabetes: interconnections and mortality
SO 77 Disclosing fatty liver disease mechanisms
SO 78 Screening tools, lipids and novel biomarkers
SO 79 Focus on the heart and beyond
OP 01 Diet: from plants to cans
1
Diet and all-cause mortality in individuals with type 2 diabetes: a systematic review and meta-analysis of prospective studies
J. Barbaresko1, A. Lang1, E. Szczerba1,2, C. Baechle1,2, L. Schwingshackl3, M. Neuenschwander1,2, S. Schlesinger1,2;
1Institute for Biometrics and Epidemiology, German Diabetes Center, Düsseldorf, 2German Center for Diabetes Research (DZD), Munich-Neuherberg, 3Institute for Evidence in Medicine, University of Freiburg, Freiburg, Germany.
Background and aims: Type 2 diabetes (T2D) is a major health concern associated with several comorbidities and mortality. Dietary factors may influence the progression of diabetes; however, high-quality systematic reviews are lacking. Therefore, the aim was to systematically summarise and evaluate the evidence on dietary factors and the risk of all-cause mortality in individuals with T2D from observational prospective studies.
Materials and methods: A systematic literature search was conducted in PubMed and Web of Science up to September 2021 to identify prospective observational studies investigating any dietary factor (dietary patterns, food groups, macro- and micronutrients, and secondary plant compounds) in association with all-cause mortality in individuals with T2D. We conducted pairwise (high vs. low intake) and dose-response meta-analyses to calculate summary risk ratios (SRR) with corresponding 95% confidence intervals (CI) using random effects models. The inconsistency between the study results was assessed using I2. The certainty of evidence of the associations was evaluated by applying a validated tool.
Results: In total, we identified 97 studies and performed 38 meta-analyses. Moderate certainty of evidence was found for decreased all-cause mortality with higher intakes of fish (SRR per serving/week: 0.95; 95% CI: 0.92, 0.99; I2=0%; n=6 studies), whole grain (SRR per 20 g/d: 0.84; 95% CI: 0.71, 0.99; I2=0%; n=2), fibre (SRR per 5 g/d: 0.86; 95% CI: 0.81, 0.91; I2=0%; n=3) and n-3 polyunsaturated fatty acids (SRR per 0.1 g/d: 0.87; 95% CI: 0.82, 0.92; I2=0%; n=2). There was low certainty of evidence for an inverse association of vegetable consumption (SRR per 100 g/d: 0.88; 95% CI: 0.82, 0.94; I2=0%; n=2), and plant protein intake (SRR per 10 g/d: 0.91; 95% CI: 0.87, 0.96; I2=42%; n=3), as well as positive associations of egg consumption (SRR per 10 g/d: 1.05; 95% CI: 1.03, 1.08; I2=56%; n=7), and cholesterol intake (SRR per 300 mg/d: 1.19; 95% CI: 1.13, 1.26; I2=0%; n=2). For other dietary factors such as dietary patterns, other food groups, macro- and micronutrients, evidence was limited.
Conclusion: This meta-analysis showed that intake of fish, whole grain, fibre and n-3 polyunsaturated fatty acids may be inversely associated with all-cause mortality in individuals with T2D. There is limited evidence for other dietary factors and all-cause mortality in individuals with T2D and thus, more research is needed.
Disclosure: J. Barbaresko: None.
2
Appropriate consumption of different animal-based foods to reduce type 2 diabetes risk: an umbrella review of meta-analyses of prospective studies
A. Giosuè, I. Calabrese, G. Riccardi, O. Vaccaro, M. Vitale;
Clinical Medicine and Surgery, "Federico II" University of Naples, Naples, Italy.
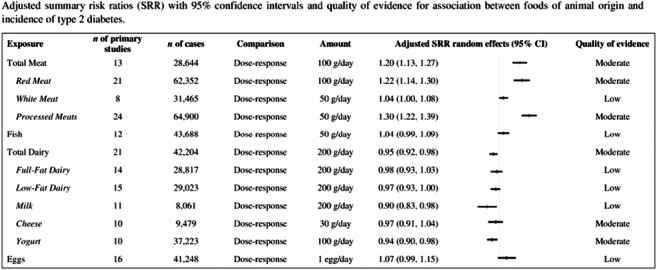
Background and aims: Dietary recommendations for the prevention of type 2 diabetes (T2D) clearly indicate the most appropriate choices for plant-based foods; as for foods of animal origin, a limited consumption of all items is generally recommended. However, not all animal protein sources are equal; moreover, they are largely used worldwide. Therefore, we have reviewed data on the relationship between the consumption of various foods of animal origin and the incidence of T2D to support dietary recommendations for T2D prevention with updated and reliable scientific evidence on the appropriate choices for animal-based foods.
Materials and methods: The study is an umbrella review of dose-response meta-analyses of prospective cohort studies. A systematic search of the literature was conducted in PubMed, Web of Science, Scopus and Embase according to PRISMA guidelines. The methodological quality of each meta-analysis was evaluated trough AMSTAR (A Measurement Tool to Assess Systematic Reviews). For each food group, we meta-analyzed the risk ratios (RR) for T2D incidence reported in the primary studies included in the available meta-analyses. The quality of evidence was evaluated with a modified version of NutriGrade.
Results: 13 meta-analyses met the criteria for inclusion in the review with 175 summary RR on consumption of total meat (n=13), red meat (n=21), white meat (n=8), processed meats (n=24), fish (n=12), total dairy (n=21), full-fat dairy (n=14), low-fat dairy (n=15), milk (n=11), cheese (n=10), yogurt (n=10) and eggs (n=16) in relation to T2D incidence. There was a substantial increase in T2D risk with the consumption of 100 g/day of total meat (RR 1.20, 95% CI 1.13-1.27) or red meat (RR 1.22, 95% CI 1.14-1.30) or 50/day of processed meats (RR 1.30, 95% CI 1.22-1.39), with a moderate quality of evidence; also 50 g/day of white meat showed a positive relationship with T2D risk (RR 1.04, 95% CI 1.00-1.08). As for dairy foods, we found an inverse association for T2D incidence with the intake of 200 g/day of total dairy (RR 0.95, 95% CI 0.92-0.98), low-fat dairy (RR 0.97, 95% CI 0.93-1.00) or milk (RR 0.90, 95% CI 0.83-0.98), as well as 100 g/day of yogurt (RR 0.94, 95% CI 0.90-0.98); conversely, a neutral relationship emerged for 200 g/day of full-fat dairy (RR 0.98, 95% CI 0.93-1.03) or 30 g/day of cheese (RR 0.97, 95% CI 0.91-1.04), with a quality of evidence scored between moderate and low. Finally, the consumption of 100 g/day of fish and 1 egg/day showed a neutral association with T2D risk (RR 1.04, 95% CI 0.99-1.09 and 1.07, 95% CI 0.99-1.15, respectively), with low quality of evidence.
Conclusion: The scientific evidence we have extensively reviewed shows that the habitual consumption of dairy foods in moderate amounts - especially low-fat types, milk and yogurt - could be appropriate for the optimization of T2D prevention. Within this context, moderate amounts of fish and eggs could represent suitable substitutes for red and processed meats in most eating occasions.
Clinical Trial Registration Number: PROSPERO CRD42022306145
Disclosure: A. Giosuè: None.
3
Ultra-processed food consumption and risk of type 2 diabetes: results from three prospective cohort studies in the US
Z. Chen1, N. Khandpur1, C. Monteiro2, S. Rossato2, T. Fung1, J.E. Manson1, W. Willett1, E.B. Rimm1, F.B. Hu1, Q. Sun1, J.-P. Drouin-Chartier3;
1Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, USA, 2Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil, 3Department of Nutrition, Institut sur la Nutrition et les Aliments Fonctionnels (INAF), Faculté de Pharmacie, Université Laval, Québec, Canada.
Background and aims: Prospective evidence on the association between ultra-processed food (UPF) consumption and type 2 diabetes (T2D) risk remains limited. We aimed to prospectively examine associations between UPF intake and T2D in US men and women.
Materials and methods: We prospectively followed 62,583 women in the Nurses’ Health Study (NHS, 1984-2014), 88,633 women in the Nurses’ Health Study II (NHSII, 1991-2017), and 38,837 men in the Health Professionals Follow-up Study (HPFS, 1986-2016). Diet was assessed using validated food frequency questionnaires every 2-4 years. UPF were categorized according to the Nova classification. Associations with T2D were assessed using Cox proportional hazards models with adjustments for demographics, dietary and lifestyle factors, and medical history.
Results: During 4,784,680 person-years of follow-up, we documented 17,432 T2D cases. In multivariable-adjusted analyses, higher UPF intake was associated with a higher risk of T2D: the pooled hazard ratio (HR) comparing extreme quintiles of intake in servings of UPF per day was 1.29 (95% confidence interval (CI): 1.21, 1.37; Ptrend<0.0001). The association remained significant after further adjustment for overall diet quality, assessed using the Alternative Healthy Eating Index (pooled HR comparing extreme quintiles: 1.21, 95% CI: 1.13, 1.28; Ptrend<0.0001). The results were consistent across subgroups in analyses stratified by age, sex, BMI, diet quality and physical activity, as well as when symptomatic diabetes at diagnosis was used at the outcome.
Conclusion: UPF consumption is associated with a higher risk of T2D, independent of overall diet quality. These findings provide further support for the current recommendations of limiting UPF consumption as part of a healthy diet for the prevention of type 2 diabetes.
Disclosure: Z. Chen: None.
4
The association between plant-based diet indices and obesity and metabolic diseases in Chinese adults: longitudinal analyses from the China Health and Nutrition survey
B. Chen1, J. Zeng1, M. Qin2, W. Xu1, Z. Zhang3, X. Li4, S. Xu1;
1Evidence-Based Medicine Centre, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, 2Department of Traditional Chinese Medicine, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, 3Evidence-Based Medicine Centre, Daxing Hospital, Xi’an, Shanxi, 4College of Medicine, Wuhan University of Science and Technology, Wuhan, Hubei, China.
Background and aims: A wide range of health benefits are associated with consuming a diet high in plant-based foods. Diet quality can be accurately assessed using plant-based diet indices, however there is inadequate evidence that plant-based diet indices are linked to obesity, hypertension, and type 2 diabetes (T2D), especially in Chinese cultures who have traditionally consumed plant-rich foods.
Materials and methods: The data came from the China Nutrition and Health Survey. Overall, 11,580 adult participants were enrolled between 2004 and 2006 and followed up until 2009 or 2015 (follow-up rate: 73.4%). Dietary intake was assessed across three 24-hour recalls, and two plant-based dietary indices (overall plant-based diet indice (PDI) and healthy plant-based diet indice (hPDI)) were calculated using China Food Composition Code and categorized into quintiles. The study's endpoints were overweight/obesity, hypertension, and T2D. The Hazard ratio (HR) and dose-response relationship were assessed using the Cox proportional risk model and restricted cubic splines.
Results: During the median follow-up period of more than ten years, 1270 (33.4%), 1509 (31.6%), and 720 (11.5%) participants developed overweight / obesity, hypertension, and T2D, respectively. The higher PDI score was linked with a reduced risk of overweight/obesity [HR: 0.71 (95% CI: 0.55-0.93), P-trend <0.001], hypertension [HR: 0.63 (95% CI: 0.51-0.79), P-trend <0.001], and T2D [HR: 0.79 (95% CI: 0.72-0.87), P-trend <0.001]. The hPDI score was inversely associated with overweight/obesity [HR: 0.79 (95% CI: 0.62- 0.98), P-trend = 0.02] and T2D [HR: 0.84 (95% CI: 0.75-0.93), P-trend = 0.001]. In the aged <55-year-old group, subgroup analysis indicated a significant negative association between PDI/hPDI and overweight/obesity, hypertension, and T2D.
Conclusion: The PDI and hPDI scores were very similar in application in Chinese populations, and our findings highlight that adherence to overall plant-based diet index helps to reduce the risk of T2D, obesity, and hypertension in Chinese adults who habitually consume plant-based foods, especially for those aged <55 year. Further understanding of how plant-based diet quality is associated with chronic disease will be needed in the future, which will help develop dietary strategies to prevent diabetes, hypertension, and related chronic diseases.
Supported by: The study was partly supported by the Young Talents Project of Hubei Provincial Health Commission, C
Disclosure: B. Chen: None.
5
Longitudinal serum branched-chain amino acids, lifestyle intervention and the risk of type 2 diabetes in the Finnish Diabetes Prevention study
J. Kivelä1, J. Meinilä2, M. Uusitupa3, J. Tuomilehto1,4, J. Lindström1;
1Department of Public Health and Welfare, Finnish Institute for Health and Welfare, Helsinki, Finland, 2Department of Food and Nutrition, University of Helsinki, Helsinki, Finland, 3Public Health and Welfare, University of Eastern Finland, Kuopio, Finland, 4Saudi Diabetes Research Group, King Abdulaziz University, Jeddah, Saudi Arabia.
Background and aims: Circulating branched-chain amino acids (BCAA) are associated with the risk of type 2 diabetes (T2D). We examined to what extent lifestyle intervention aiming to prevention of T2D interacts with this association and how BCAA concentrations change during the intervention.
Materials and methods: We comprised trajectory clusters by k-means clustering of serum fasting BCAA analysed annually during the four-year intervention by sandwich ELISA. We investigated whether the baseline BCAA, BCAA trajectories and BCAA change trajectories predict T2D in a median 11-year follow-up and whether BCAA predicts T2D differently in the intervention (n=198) and control group (n=196) participants of the Finnish Diabetes Prevention Study.
Results: Elevated baseline BCAA predicted the incidence of T2D in the entire study cohort (HR 1.04; 95% CI 1.01, 1.06) and control group (HR 1.06; 95% CI 1.03, 1.09), but not in the intervention group. BCAA concentration decreased during the first year in the whole cohort (-14.9 μmol/l [SD 58.5], p<0.001), with no significant difference between the intervention and control groups. We identified five BCAA trajectory clusters and five trajectory clusters for the change in BCAA. Trajectories with high mean BCAA levels were associated with an increased hazard ratio for T2D compared to a trajectory with low BCAA levels (trajectory with highest vs lowest mean BCAA, HR 3.99; 95% CI 1.46, 10.93). A trajectory with increasing BCAA levels had a higher hazard ratio for T2D compared with a decreasing trajectory in the intervention group only (HR 25.39; 95% CI 2.83, 227.62).
Conclusion: Lifestyle intervention modified the association of the baseline BCAA concentration and BCAA trajectories with the incidence of T2D. Our study adds to the accumulating evidence on the mechanisms behind the effect of lifestyle changes on the risk of T2D.
Clinical Trial Registration Number: NCT00518167
Supported by: Päivikki and Sakari Sohlberg f., Yrjö Jahnsson f., Juho Vainio f., the Academy of Finland
Disclosure: J. Kivelä: None.
6
Fasting ketone bodies and incident type 2 diabetes in the general population
T. Szili-Torok1, M.H. de Borst1, E. Garcia2, R.T. Gansevoort1, R.P.F. Dullaart1, M.A. Connelly2, S.J.L. Bakker1, U.J.F. Tietge3;
1Internal Medicine, University Medical Center Groningen (UMCG), Groningen, Netherlands, 2Laboratory Corporation of America Holdings (Labcorp), Morrisville, USA, 3Division of Clinical Chemistry, Karolinska Institutet, Stockholm, Sweden.
Background and aims: With a rising incidence and prevalence of type 2 diabetes, prevention strategies including identification of prospective biomarkers become increasingly relevant. Ketone bodies recently received a renewed interest in this respect; however, data on a relationship between these metabolites and diabetes risk are scarce. Therefore, we investigated in the present prospective study the association between fasting ketone bodies and type 2 diabetes incidence in the general population.
Materials and methods: This study from the PREVEND cohort included 3786 participants from the general population initially free of diabetes. Baseline fasting ketone body concentrations were measured by nuclear magnetic resonance spectroscopy.
Results: 276 participants (7.3%) developed type 2 diabetes during a median [IQR] follow-up of 7.3 [5.6-7.7] years. In Kaplan-Meier analysis sex-stratified ketone body levels were strongly positively associated with incident type 2 diabetes (log rank test, p<0.001), which was confirmed in Cox regression analyses adjusted for several relevant confounders including age, sex, BMI, diastolic and systolic blood pressure, hsCRP, HOMA-IR, total cholesterol, HDL cholesterol, triglycerides, serum creatinine, eGFR and urinary albumin concentrations (aHR per 1 SD increase [95 CI], 11.84 [5.55, 25.25], p<0.001). There was no significant interaction by sex. Further, individually 3-beta-hydroxybutyrate (13.27 [6.18, 28.53], p<0.001) and acetoacetate/acetone (3.87 [2.1, 7.13], p<0.001) were associated with incident type 2 diabetes. In sensitivity analyses including only metabolic syndrome-free individuals the conclusions did not change (9.18 [2.99, 28.22], p<0.001). The addition of ketone body levels to the Framingham diabetes risk score has resulted in an improved model fit (p<0.001).
Conclusion: Fasting plasma ketone body levels are strongly associated with incident type 2 diabetes in the general population independent of several other recognized risk factors. These results may have important implications for diabetes prevention including dietary strategies.
Clinical Trial Registration Number: MEC96/01/022
Supported by: This work was supported by the Center for Innovative Medicine (CIMED, FoUI-963234, to UJFT).
Disclosure: T. Szili-Torok: None.
OP 02 SGLT2 inhibitors: promiscuous pleiotropy
7
Effect of SGLT2 inhibitor dapagliflozin on skeletal muscle fatty acid metabolism in patients with type 2 diabetes
A. Gemmink1, Y.J.M. op den Kamp1, M. de Ligt1, B. Dautzenberg1, R. Esterline2, J. Hoeks1, V.B. Schrauwen-Hinderling1,3, S. Kersten4, B. Havekes5, T.R. Koves6, D.M. Muoio6, M.K.C. Hesselink1, J. Oscarsson7, E. Phielix1, P. Schrauwen1;
1Nutrition and Movement Sciences, Maastricht University, Maastricht, Netherlands, 2BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, USA, 3Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 4Human Nutrition and Health, Wageningen University, Wageningen, Netherlands, 5Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 6Medicine, Duke University, Durham, USA, 7BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Background and aims: SGLT2 inhibitors increase urinary glucose excretion and have beneficial effects on whole-body energy and substrate metabolism, which may be facilitated by altered muscle metabolism. This may be the consequence of the adaptive response to the loss of about 50-100g glucose per day in the urine, which can be regarded as a form of mild calorie restriction. Here, we investigated the effects of 5 weeks of dapagliflozin (10 mg orally once daily) treatment on skeletal muscle fat metabolism in type 2 diabetes patients.
Materials and methods: Twenty-six type 2 diabetes patients were randomized to a 5-week double-blind, cross-over study with 6-8 weeks of wash-out. 31P- and 1H-Magnetic resonance spectroscopy was used to determine intramyocellular lipid content (IMCL) and phosphocreatine (PCr) recovery rate. Muscle biopsies were analyzed for lipid droplet (LD) morphology, mitochondrial network integrity and mitochondrial-LD interaction with confocal microscopy. Furthermore, biopsies were analyzed for levels of acylcarnitines, amino acids and Krebs cycle intermediates, and gene expression levels of CPT1A and CPT1B. Results are presented as Least Squares Means (95% CI).
Results: IMCL content increased after dapagliflozin treatment (0.27 (0.21-0.34) vs. 0.33 (0.25-0.40)%, p<0.05) due to larger (0.25 (0.19-0.31) vs. 0.28 (0.20-0.36) μm2, p<0.05) and more LDs (0.015 (0.009-0.021) vs. 0.0018 (0.012-0.018) μm-2, p=0.09). Dapagliflozin increased levels of several long-chain acylcarnitine species, while acetylcarnitine levels (154.63 (131.24-178.03) vs. 114.56 (91.16-137.95) pmoles*mg tissue-1, p<0.001) were decreased. Dapagliflozin treatment reduced levels of several amino acids and Krebs cycle intermediates in skeletal muscle. PCr recovery rate (23.1 (20.7-25.5) vs. 23.1 (20.7-25.5) s, p=0.88), mitochondrial network integrity (1.59 (1.31-1.87) vs. 1.73 (1.33-2.12), p=0.44), and mitochondrial-LD interaction (13.45 (10.41-16.49) vs. 12.51 (9.70-15.32)%, p=0.20) were unaffected by dapagliflozin. CPT1A (1.33 fold, p<0.001) and CPT1B (1.13 fold, p<0.05) gene expression increased upon dapagliflozin treatment.
Conclusion: The increase in IMCL levels and changes in LD morphology mainly resemble changes induced by fasting. Changes in long-chain acylcarnitine and acetylcarnitine levels suggest enhanced fatty acid metabolism in skeletal muscle. Reduced amino acid levels and Krebs cycle intermediates suggest enhanced amino acid utilization for gluconeogenesis. Taken together, these findings indicate that dapagliflozin induces a change in skeletal muscle substrate metabolism favoring fatty acid oxidation and a reduced glycolytic flux without changes in mitochondrial function and mitochondrial-LD interaction.
Clinical Trial Registration Number: NCT03338855
Supported by: AstraZeneca
Disclosure: A. Gemmink: None.
8
The effect of SGLT2 inhibitor dapagliflozin on substrate metabolism in humans with prediabetes
A. Veelen1, C. Andriessen1, Y. Op den Kamp1, E. Erazo Tapia1, M. de Ligt1, J. Mevenkamp2, J. Jörgensen1, E. Moonen-Kornips1, G. Schaart1, B. Havekes3, J. Oscarsson4, V. Schrauwen-Hinderling2,1, E. Phielix1, P. Schrauwen1;
1Nutrition and Movement Sciences, Maastricht University, Maastricht, Netherlands, 2Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 3Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 4BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Background and aims: Metabolic flexibility is defined as the capacity to switch from fat oxidation in the overnight fasted state to glucose oxidation in the postprandial state. We recently showed that individuals with prediabetes have impaired 24-hour and nocturnal fat oxidation. Inhibition of sodium-glucose cotransporter 2 (SGLT2) results in glucosuria, causing an energy deficit, which could trigger a more fasted condition and reliance on hepatic glycogen. The objective of the current study was to determine whether dapagliflozin, a SGLT2 inhibitor, could elicit a more pronounced 24-hour and nocturnal fat oxidation, improve mitochondrial function and lead to enhanced overnight glycogen use in individuals with prediabetes.
Materials and methods: Fourteen individuals with prediabetes (BMI 30.3 ± 2.1kg/m2; age 66.3 ± 6.2 years) underwent 2-weeks of treatment with dapagliflozin (10mg/day) or placebo in a randomized, placebo-controlled, cross-over design. Outcome parameters include 24-hour respiratory exchange ratio (RER) and substrate oxidation measured by whole-room indirect calorimetry. Twenty-four-hour blood samples were collected to determine levels of several metabolites. Hepatic glycogen and hepatic lipid content and composition were measured in the morning by MRS, and ex vivo skeletal muscle mitochondrial oxidative capacity was measured by high-resolution respirometry.
Results: Dapagliflozin treatment resulted in a urinary glucose excretion of 36 gram/24-hour, leading to a negative energy balance. Twenty-four-hour blood glucose levels decreased upon dapagliflozin (AUC; p = 0.017), while 24-hour free fatty acids and nocturnal β-hydroxybutyrate were elevated (AUC; p = 0.002 and p = 0.012, respectively), indicating a more pronounced reliance on fat oxidation. Indeed, following dapagliflozin, 24-hour RER was lower (0.814 ± 0.006 versus 0.827 ± 0.004; p = 0.051), in line with an increased 24-hour fat oxidation (p = 0.033) and a reduced 24-hour carbohydrate oxidation (p = 0.041). Nocturnal fat oxidation was higher after dapagliflozin (p = 0.039). Coupled, and maximally uncoupled mitochondrial respiration upon lipid-derived substrates were higher after dapagliflozin (O2-flux: 68.2 ± 3.2 versus 64.6 ± 3.2 pmol/mg/s; p = 0.071 and 87.6 ± 5.4 versus 78.1 ± 5.5 pmol/mg/s; p = 0.007, respectively). No changes were observed in hepatic glycogen or lipid content and composition.
Conclusion: Dapagliflozin treatment for 2 weeks in humans with prediabetes improves 24h and nocturnal fat oxidation. Dapagliflozin treatment also had significant effects on 24h glucose and free fatty acid levels, and on nocturnal β-hydroxybutyrate levels. These data indicated a more pronounced fasted state, although no changes were observed in hepatic glycogen. In addition, dapagliflozin improved ex vivo skeletal muscle mitochondrial oxidative capacity. These results show that dapagliflozin in prediabetes individuals elicits metabolic health effects that may mimic the effects of calorie restriction.
Clinical Trial Registration Number: NCT03721874
Supported by: AstraZeneca
Disclosure: A. Veelen: None.
9
Metabolome analysis of the effects by SGLT2 inhibitor ipragliflozin and metformin on human metabolites, and relationship with clinical data in a randomised controlled study
M. Koshizaka, A. Tsukagoshi, R. Ishibashi, Y. Maezawa, K. Yokote;
Chiba University, Chiba, Japan.
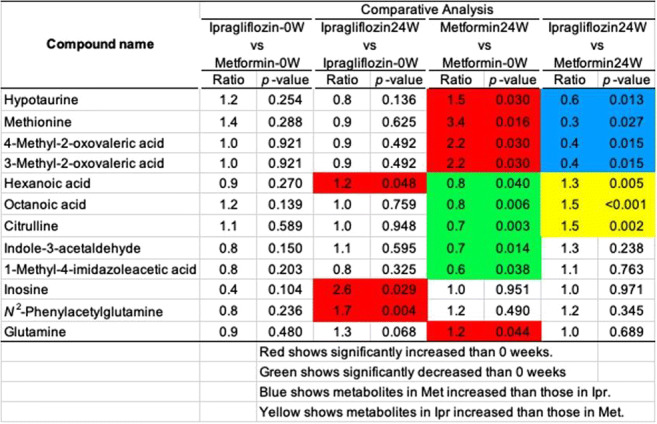
Background and aims: A clinical study comparing the effects of SGLT2 inhibitor ipragliflozin (Ipr) with those of metformin (Met) on visceral fat area and glucose and lipid metabolism revealed Ipr reduced visceral fat. To investigate the mechanism, metabolome analysis of the effects of Ipr and Met on human metabolites was performed with the samples obtained in the clinical study.
Materials and methods: In total 103 patients with type 2 diabetes, with HbA1c >=7% and < 10%, and BMI >=22 kg/m2 were randomly assigned to receive Ipr 50 mg or Met 1000 mg. Metabolome analysis using blood samples before and 24 weeks after administration was performed to identify changed metabolites, and analyzed their correlation with the changes of clinical data.
Results: Of them, 15 patients in the Ipr group and 15 in the Met group were analyzed, and there was no difference in the background between the groups.After 24 weeks, the visceral fat area significantly reduced in the Ipr group than in the Met group (-17.8% vs. -4.7%, P=0.015), as were subcutaneous fat area and body weight. Both HbA1c and blood glucose levels decreased by more than 7% and 14%, respectively. Red blood cell count and hemoglobin were increased in the Ipr group and decreased in the Met group. GOT, GPT and γGT were decreased in the Ipr group and increased in the Met group. Uric acid levels were decreased in the Ipr group. Triglycerides were decreased by more than 8% in both groups, total cholesterol (TC) was decreased in the Met group, LDL-C tended to be decreased in the Met group, and HDL-C tended to be increased in the Ipr group. The bone resorption marker TRACP5b was increased in the Ipr group and decreased in the Met group.As table, metabolome analysis showed that the increased metabolites after Met administration were methionine, glutamine, methyl-2-oxovaleric acid, hypotaurine, and the decreased metabolites were citrulline, indol-3-acetaldehyde, 1-methyl-4-imidazole acetic acid, octanoic acid, and hexanoic acid. Whereas inosine and N2-phenylacetylglutamine were the metabolites that increased after Ipr administration.Of them, methionine, methyl-2-oxovaleric acid, hypotaurine, citrulline, octanoic acid, and hexanoic acid were significantly different between the groups. Regarding to the correlation between metabolites and changes of clinical data, indole-3-acetaldehyde was correlated with HbA1c (r=-0.47), inosine with TRACP-5b (r=-0.75), muscle area (r=0.61) and HbA1c (r=-0.52), and N2-phenylacetylglutamine with BAP (r=0.79).
Conclusion: In Ipr, N2-phenylacetylglutamine, metabolite of phenylalanine, increased. Reportedly, phenylalanine reduced visceral fat. The patients treated with Ipr may reduce visceral fat by the mechanism of phenylalanine-N2-phenylacetylglutamine pathway. In Met, the changes in octanoic acid and hexanoic acid suggested that the β-oxidation of short-chain saturated fatty acids by mitochondria.
Clinical Trial Registration Number: UMIN000015170
Supported by: This study was funded by API. The funding source had no role in the study.
Disclosure: M. Koshizaka: Grants; Astellas Pharma Inc.
10
Effect of Dapagliflozin on renal and hepatic glucose kinetics in type 2 diabetes and NGT subjects
D. Tripathy1, C. Solis-Herrera2, X. Chen2, A. Hansis-Diarte2, R. Chilton2, R. DeFronzo2, E. Cersosimo2;
1Dept. of Medicine, University of Texas Health Science, STVHS, 2Dept. of Medicine, University of Texas Health Science, San Antonio, USA.
Background and aims: We previously have shown that both acute and chronic SGLT-2 inhibition increases endogenous glucose production (EGP). However, the relative contribution of liver versus kidney - responsible for the increase in EGP has not been identified.
Materials and methods: We assessed the effect of a single dose of Dapagliflozin or Placebo on renal glucose production in 13 T2DM (age=57.5±1.8 yrs, BMI=30±1.4 kg/m2) and 9 NGT (age 42±2 yrs, BMI= 30±1.1 kg/m2) subjects. Renal glucose production was measured using arteriovenous balance technique across the kidney combined with [3-3H] glucose infusion and PAH infusion (for determination of renal blood flow) before and 4 hours after administration of Dapagliflozin (10 mg) or Placebo; thus, each subject served as their own control.
Results: EGP increased following Dapagliflozin (DAPA) in both T2DM (2.00±0.11 to 2.43±0.15, P<0.05) and NGT (1.72±0.11 to 2.1±0.16, p<0.05), while it decreased after placebo in T2DM (2.02±0.12 vs 1.15±0.06) and NGT (2.10±0.2 vs 2.05±0.1) (both p<0.01, DAPA vs placebo). The fractional renal extraction of glucose (0.02± 0.004 vs 2.99±1.0, p=0.001 in T2DM, and 0.02± 0.004 vs 1.62± 1.4 in NGT, p=NS) and renal glucose uptake (0.067 ± 0.02 vs 0.347 ± 0.06 in T2DM and 0.08±0.02 vs 0.27 ± 0.08 mg/kg.min in NGT) were higher following DAPA vs placebo (p<0.05) and were entirely explained by the increase in glucosuria. There was a small, non-significant increase (0.065 & 0.032 mg/kg.min, respectively) in renal glucose production (RGP) following dapagliflozin in T2DM and NGT compared to the 0.45 mg/kg.min increase in total body EGP.
Conclusion: A single dose of Dapagliflozin significantly increases EGP which primarily is explained by an increase in hepatic glucose production.
Clinical Trial Registration Number: NCT02981966
Supported by: AstraZeneca
Disclosure: D. Tripathy: None.
11
Different effects of SGLT-2 inhibitors on subcutaneous and epicardial adipose tissue metabolome in severe heart failure subjects
B.J. Kasperova1, M. Mraz2,3, O. Kuda4, T. Cajka4, D. Hlavacek5, J. Mahrik5, S. Stemberkova-Hubackova1, I. Pleyerova1, K. Rosolova1, P. Svoboda1, P. Novodvorsky2,6, P. Ivak7, V. Melenovsky8, I. Netuka7, M. Haluzik2,3;
1Experimental Medicine Center, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2Department of Diabetes, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 3Department of Medical Biochemistry and Laboratory Diagnostics, 1st Faculty of Medicine, Prague, Czech Republic, 4Institute of Physiology, Academy of Science of the Czech Republic, Prague, Czech Republic, 5Anestehsiology and Resuscitation Department, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 6Department of Oncology & Metabolism, University of Sheffield, Sheffield, UK, 7Cardiovascular Surgery Department, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 8Cardiology Department, Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Background and aims: The exact mechanisms behind favorable metabolic and cardioprotective effects of SGLT-2 inhibitors (SGLT-2i) are still not fully understood. Here, we performed a complex metabolomic analysis of subcutaneous (SAT) and epicardial (EAT) adipose tissue of heart failure subjects treated with SGLT-2i in order to assess their impact on different fat depots and identify potential cardioprotective factors.
Materials and methods: Nine subjects with severe heart failure with reduced ejection fraction (NYHA III-IV) treated with SGLT-2i and 8 age-, BMI- and left ventricular ejection fraction-matched control subjects scheduled for heart transplantation or mechanical support implantation were included into the study. Eight SGLT-2i subjects and 5 control subjects had type 2 diabetes mellitus. A complex metabolomic analysis of SAT and EAT obtained during surgery was performed using liquid chromatography and mass spectrometry.
Results: SAT of SGLT-2i subjects showed marked increase in ketone bodies and a corresponding decrease of ketogenic triacylglycerols with medium-chain fatty acids suggesting enhanced ketogenesis typical for SGLT-2i use. In contrast, no such change was seen in EAT which, conversely, contained increased amount of long-chain triacylglycerols indicating significant differences in response to SGLT-2i treatment between these depots and a tendency to preserve EAT lipid content. Compared with control group, both SAT and EAT of SGLT-2i subjects consistently exerted surprisingly high levels of sphingolipids, especially sphingomyelins and ceramides, and ether-linked lipid species.
Conclusion: SGLT-2i treatment elicits different metabolic responses in SAT and EAT with SAT showing mainly accented ketogenesis, while the preservation of EAT suggests other functions including potential cardioprotection. The exact role of increased sphingolipids and ether-linked lipids in both adipose tissue depots remains to be elucidated.
Supported by: IKEM, IN 00023001, NV19-02-00118
Disclosure: B.J. Kasperova: None.
12
Empagliflozin-induced metabolic changes and cardiac function in patients with type 2 diabetes: a randomised cross-over MRI study with insulin as comparator
R. Thirumathyam1, G. Hall2, J.P. Gøtze2, J.J. Holst3, U. Dixen4, E.A. Richter5, N. Vejlstrup6, S. Madsbad1, P.L. Madsen7, N.B. Jørgensen1;
1Department of Endocrinology, Hvidovre Hospital, Hvidovre, 2Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, 3Department of Biomedical Sciences, University of Copenhagen, Copenhagen, 4Department of Cardiology, Hvidovre Hospital, Hvidovre, 5Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, 6Department of Cardiology, Rigshospitalet, Copenhagen, 7Department of Cardiology, Herlev Gentofte Hospital, Herlev, Denmark.
Background and aims: Empagliflozin reduces cardiovascular risk in type 2 diabetes (T2D), possibly through improved cardiac function. Metabolic effects of empagliflozin include lowered glucose and insulin concentrations, elevated free fatty acids (FFA) and ketone bodies and have been suggested to convey the cardiovascular benefits of empagliflozin treatment. We aimed to evaluate the influence of these metabolic changes on cardiac function in patients with T2D.
Materials and methods: 17 subjects (13 M) with T2D, aged 58±3 years (mean±sem), BMI 32.9±0.9 kg/m2, HbA1c 52.4±2.4 mmol/L, TD2 duration of 8.9±1.3 years were treated with empagliflozin (E) and NPH-insulin (I) for 5 weeks in a cross-over design with 3 weeks of washout between treatments. Insulin was titrated to produce similar glycemic control as during empagliflozin. Patients were studied before and at the end of each treatment period. Metabolic changes were evaluated with fasting glucose, insulin, FFAs and 3-OH butyrate concentrations. Cardiac function with cardiac MRI during rest and chronotropic stress on two separate days without and with acute lowering of FFAs with acipimox. Cardiac endpoints were changes between treatments and washout (E vs WO or I vs WO) and between treatments (E vs I) in left ventricular peak filling rate (∆LVPFR) and left ventricular ejection fraction (∆LVEF).
Results: Fasting glucose was reduced from 8.7±0.5 mM during washouts to 7.6±0.3 mM on both treatments, while serum insulin was lower (E: 103±14; I: 141±16 pM (p<0.01)) and FFAs (E: 0.60±0.03; I: 0.50±0.05 mM (p=0.02)) and 3-OH butyrate higher (E: 0.27±0.03; I: 0.23±0.02 mM (p=0.03)) on empagliflozin compared with insulin treatment. Cardiac function was largely unchanged with any treatment and was not improved with empagliflozin compared to insulin in rest or during stress (∆LVPFR Rest: E vs WO: -38±19 (p=0.08);I vs WO: 8±25 ml/s; E vs I: -55±19 ml/s (p<0.01); ∆LVEF Rest: E vs WO: 2 ±1 (p=0.11), I vs WO: 1±1%, E vs I: -0±2%; ∆LVPFR Stress: E vs WO:-4±45, I vs WO : -16±28 ml/s, E vs I: -11±47 ml/s; ∆LVEF Stress: E vs WO: -1±2; I vs WO: -1±1%; E vs. I: 1±1%). Acipimox reduced FFAs by ~35% at all visits, without affecting ketone body concentration. Cardiac diastolic function was unchanged by acipimox administration, but LVEF was similarly reduced during both treatments (E: -6±2% I: -5.4±1%) under resting condition.
Conclusion: Neither empagliflozin nor insulin treatment improve cardiac function in patients with T2D. Treatment specific metabolic effects play no role for cardiac function under the studied conditions.
Clinical Trial Registration Number: EudraCT no. 2017-002101-35
Supported by: Boehringer Ingelheim
Disclosure: R. Thirumathyam: None.
OP 03 Risk for CVD and CKD
13
CSII is associated with lower NAFLD indices in patients with type 1 diabetes
L. Bozzetto, G. Della Pepa, E. Raso, R. Boccia, S. Gianfrancesco, A.A. Rivellese, G. Annuzzi;
Federico II University, Naples, Italy.
Background and aims: NAFLD is a raising concern also in type 1 diabetes (T1D) and is associated with micro and macrovascular complication. This study evaluated whether different ways of insulin administration (multiple daily injections [MDI] or continuous subcutaneous insulin infusion [CSII]) may affect NAFLD indices.
Materials and methods: We performed a cross-sectional study on 658 patients with T1D (37±13 years, 51% male, HbA1c 7.8±1.2%, body mass index 25±4 kg/m2) who had no history of excessive alcohol consumption or other secondary chronic liver disease, regularly attending the Diabetes Unit of an University Teaching Hospital. NAFLD was assessed by the Fatty Liver Index (FLI) and Hepatic Steatosis Index (HSI). Anthropometric, biochemical, and clinical parameters were retrieved by electronic records. Differences in NAFLD indices between patients on MDI or CSII were evaluated by univariate analysis, adjusted for possible confounders.
Results: Patients on CSII (n=259), compared with those on MDI (n=399), differed for gender distribution (men: 47% vs 55%, p=0.046), diabetes duration (22±11 vs 18±12; p<;0.001), prevalence of retinopathy (26% vs 18%, p=0.018), and nephropathy (15% vs 10%, p=0.035), respectively. According to univariate analysis adjusted for gender and diabetes duration, patients on CSII had a significantly lower HSI (36±5 vs 37±6; p=0.003), FLI (20±21 vs 25±24; p=0.003), waist circumference (85±12 vs 87±14 cm; p=0.047), triglycerides (76±44 vs 85±60 mg/dl; p=0.035), and insulin daily dose (0.53±0.22 vs 0.64±0.25 UI/kg body weight; p<;0.001).
Conclusion: Patients with T1D on CSII had better NAFLD indices, despite a longer diabetes duration and a higher prevalence of diabetes microvascular complications. Lower and more physiologically distributed daily insulin doses may have contributed to a better regulation of lipogenic pathways.
Disclosure: L. Bozzetto: None.
14
Increased prevalence of NAFLD in adults with glomerular hyperfiltration: a 8 year cohort study based on 147,162 Koreans
D.-J. Koo1, S. Park2;
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Changwon Fatima Hospital, Changwon, 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Seoul, Republic of Korea.
Background and aims: Finding an indicator that can predict the development of nonalcoholic fatty liver disease (NAFLD) at an early stage is of clinical significance. Renal function and liver status are interconnected and share several physiological pathways. This study aimed to evaluate whether glomerular hyperfiltration (GHF) could predict the development of NAFLD and fibrosis progression.
Materials and methods: We performed a longitudinal cohort study of 147,479 participants who underwent comprehensive medical examinations annually or biennially from January 3, 2011 to December 31, 2018. Study subjects were the age group between 20 and 65 years of age without baseline kidney disease and NAFLD. Age- and sex-specific estimated glomerular filtration rate (eGFR) above the 95th percentile was defined as the GHF cutoff value, and eGFR values between the 50th and 65th percentiles were used as reference groups. The primary endpoint of this study was the development of NAFLD diagnosed by abdominal ultrasonography, and fibrosis status was assessed by the NAFLD fibrosis score (NFS).
Results: During 598,745 person-years of follow-up (median, 4.6 years), NAFLD occurred in a total of 29,410 subjects. Subjects with baseline GHF had the highest hazard ratio (HR) for NAFLD (HR 1.21; 95% CI 1.14-1.29) and fibrosis progression (HR 1.42; 95% CI 1.11-1.82) after adjusting for confounding factors. The persistent GHF group during follow-up had the highest HR for NAFLD compared to the persistent non-GHF group (HR 1.31; 95% CI 1.14-1.51). Higher baseline eGFR percentile maintained a higher risk of NAFLD and fibrosis progression. These results were consistent in all subgroups and statistically more prominent in participants without diabetes.
Conclusion: In conclusion, this study demonstrated that glomerular hyperfiltration was associated with the development of NAFLD and fibrosis progression in relatively healthy young adults. Glomerular hyperfiltration may be used as a clinical surrogate marker for the early diagnosis of NAFLD regardless of obesity, insulin resistance and hypertension, especially in subjects without diabetes.
Disclosure: D. Koo: None.
15
Increased risk for microvascular outcome in NAFLD: a nationwide, population-based cohort study
T. Ebert1, L. Widman2, P. Stenvinkel3, H. Hagström4;
1Medical Department III - Endocrinology, Nephrology, Rheumatology, University of Leipzig, Leipzig, Germany, 2Division of Biostatistics, Karolinska Institutet, Stockholm, Sweden, 3Division of Renal Medicine, Karolinska Institutet, Stockholm, Sweden, 4Karolinska Institutet, Stockholm, Sweden.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is considered a multisystemic disease, as it is bidirectionally linked to other cardiometabolic disorders, such as type 2 diabetes (T2D). However, the long-term risk for microvascular outcomes in NAFLD is unclear.
Materials and methods: Using the outpatient part of the nationwide Swedish Patient Register, we identified all individuals with a first NAFLD diagnosis (N=4,943) and matched these (age, sex, and municipality) with up to ten reference individuals from the general population (N=44,606). Using population-based registers, we ascertained the development of microvascular diseases. The primary outcome was defined as a composite outcome of any diagnosis representative of microvascular disease (chronic kidney disease, retinopathy, and neuropathy). As secondary outcomes, we separately examined the risk of each specific microvascular outcome. Hazard ratios (aHR, adjusted for cirrhosis, T2D, hypertension, hyperlipidemia, malignant tumors) for time-to-event analyses were investigated by Cox proportional-hazards models.
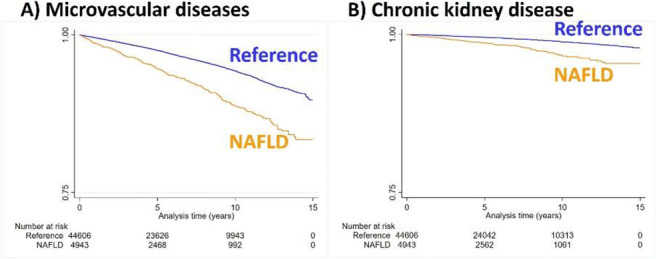
Results: Median follow-up times was 5.0 years. The incidence rate of microvascular diseases was twice as high in patients with NAFLD (11.6 per 1000 person-years [95% confidence interval (CI)=10.5-13.0]) vs. reference individuals (5.8 per 1000 person-years [95%CI=5.6-6.1]). Kaplan-Meier estimates for the development of (A) microvascular diseases and (B) chronic kidney disease are shown in Figure 1. NAFLD was independently and positively associated with the development of microvascular diseases compared to non-NAFLD subjects (aHR=1.41 [95%CI=1.23-1.61]). When stratifying the analysis by follow-up time, sex, or age categories, results remain virtually unchanged.
Conclusion: NAFLD is positively associated with the development of microvascular diseases, independent of available confounders, e.g. T2D. Individuals with NAFLD should be screened for microvascular diseases in addition to macrovascular and metabolic diseases.
Supported by: EFSD Future Leaders Mentorship Programme, Novo Nordisk postdoctoral fellowship, Swedish Research Council, CIMED,ALF
Disclosure: T. Ebert: Grants; EFSD Mentorship Programme supported by AstraZeneca, Novo Nordisk postdoctoral fellowship run in partnership with Karolinska Institutet, Stockholm, Sweden, Karolinska Institutet Research Foundation, Swedish Kidney Foundation, Stiftelsen Stig och Gunborg Westman. Lecture/other fees; Sanofi, CME-Verlag, Santis.
16
Fatty liver index is an independent risk factor for all-cause mortality and major cardiovascular events in type 1 diabetes: a 10-year observational study
G. Penno1, M. Garofolo1, D. Lucchesi1, E. Gualdani2, P. Falcetta1, M. Giambalvo1, P. Francesconi2, S. Del Prato1;
1Department of Clinical and Experimental Medicine, University of Pisa, Pisa, 2Epidemiology Unit, Regional Health Agency of Tuscany, Firenze, Italy.
Background and aims: Non-alcoholic fatty liver disease (NAFLD), also known as metabolic dysfunction-associated fatty liver disease (MAFLD), has been associated with increased risk of death, with CVD as the most common cause of death in people with and without type 2 diabetes. Whether this also applies to type 1 diabetes (T1D) has not been yet reported.
Materials and methods: We prospectively observed 774 T1D (males 52%, 30.3±11.1 years old, diabetes duration (DD) 18.5±11.6 years, HbA1c 7.8±1.2%) to assess whether fatty liver index (FLI, based on BMI, waist, GGT and triglycerides), a proxy of NAFLD, predicts the risk of all-cause death and major CVD (combined endpoint of myocardial infarction, stroke, ischemic amputation or coronary, carotid and peripheral revascularizations).
Results: Over a median follow-up of 11 years (IQR 9.9-13.0), 57 out of 774 subjects died (7.4%) while 49 major CV events (6.7%) occurred in 736 individuals for whom incidence data were retrieved (95.1%). FLI score was <30 in 515 subjects (66.5%), ≥30-60 in 169 (21.8%), and ≥60 in 90 (11.6%). Mortality rate increased across FLI scores: 3.9, 10.1 and 22.2%, K-M log-rank 40.367, p<0.0001. In unadjusted Cox, with score <30 as reference, the risk of death increased in score ≥30-60 (HR 2.85, [95% CI 1.49-5.45], p=0.002) and even more in score ≥60 (HR 6.07, [3.27-11.29], p<0.0001). After adjustment for the Steno Type 1 Risk Engine (ST1-RE, inclusive of age, sex, DD, systolic BP, LDL-cholesterol, HbA1c, albuminuria, GFR, smoking and exercise), that enters first, HRs for death was 1.52 (0.78-2.97, p=0.222) for FLI ≥30-60 and 3.041 (1.59-5.82, p=0.001) for FLI ≥60. Inclusion of prior CVD among covariates modified HRs only slightly. The effect of FLI was unchanged when analysis was restricted to 733 subjects without prior CVD. Adjustment for the EURODIAB PCS Risk Engine (EURO-RE: age, HbA1c, WHR, ACR and HDL-cholesterol) instead of ST1-RE, confirms the independent role of FLI (HRs of 1.24 [0.62-2.48] for FLI ≥30-60 and 2.54 [1.30-4.95], p=0.007) for FLI ≥60, also after inclusion of prior CVD as a confounder, or when analysis was restricted to subjects without prior CVD. Incidence of major CV events increased across FLI scores: 3.5, 10.5 and 17.2%, K-M log-rank 29.16, p<0.0001. In unadjusted Cox, with score <30 as reference, risk of CV events increased in score ≥30-60 (HR 3.24, [95% CI 1.65-6.34], p=0.001) and in score ≥60 (HR 5.41, [2.70-10.83], p<0.0001). After adjustment for the ST1-RE, that enters first, HRs for death was 1.80 (0.90-3.61, p=0.096) for FLI ≥30-60 and 2.98 (1.45-6.13, p=0.003) for FLI ≥60. Inclusion of prior CVD among covariates modified HRs only moderately. The effect of FLI became weaker when analysis was restricted to 733 subjects without prior CVD. Adjustment for the EURODIAB PCS risk engine instead of ST1-RE, confirms the independent role of FLI (HRs of 1.49 [0.73-3.03] for FLI ≥30-60 and 2.44 [1.17-5.09, p=0.017] for FLI ≥60), also after inclusion of prior CVD as a confounder.
Conclusion: This is the first observational prospective study to demonstrate that FLI is associated with higher all-cause mortality and increased risk of incident major CV events in T1D, independently of validated risk engines based on established CV risk factors and diabetes-related variables.
Disclosure: G. Penno: None.
17
Sub-optimal glycaemic control and insulin resistance in young adults with type 1 diabetes increases platelet expression of P-selectin and phosphatidylserine
R.C. Sagar, S.M. Pearson, N. Kietsiriroje, M. Hindle, K. Naseem, R. Ajjan;
Leeds Institute of Cardiovascular and Metabolic Medicine, Leeds, UK.
Background and aims: Patients with Type 1 Diabetes Mellitus (T1D) have increased risk of morbidity and mortality associated with earlier onset cardiovascular disease (CVD) and those with a combination of T1D and insulin resistance (IR) may be at even greater risk. Platelet hyperactivity has been described in diabetes and is associated with adverse cardiovascular complications. However, the factors driving pathological platelet function and the relative contribution of glycaemia and IR remain unclear. Our aim was to investigate the impact of glycaemic control and IR on platelet activation in young adults with T1D through use of four-colour multi-parameter flow cytometry.
Materials and methods: Patients aged 18-40 with T1D (>3 years since diagnosis) were recruited (n=23). Glycaemic control was evaluated using HbA1c and time in range (TIR) over 14 days using FreeStyle Libre Pro. Estimated glucose disposal rate (eGDR), a clinical marker of insulin resistance, was calculated using a validated equation. eGDR <8mg/kg/min confers IR status. Using whole blood, we applied 4-colour multiparameter flow cytometry to measure 3 distinct markers of platelet activation, both at rest and in response to activation with dual agonists Protease-activated receptor-1 (PAR-1) and Collagen related peptide XL (CRP-XL). Statistics were conducted via Prism v9.3.1.
Results: Mean age of participants was 24 (range 19-30) (69% males). All were on insulin therapy [1 with adjunctive metformin]. Mean HbA1c (SD) was 67mmol/mol (±14). Individuals were split into 2 equal groups based on 1) HbA1c (≥67 and <67 mmol/mol), 2) TIR (<50% vs >50%), 3) eGDR (<8 vs >8 mg/kg/min). Platelet data are expressed as mean(±SD) Mean Fluorescence intensity (MFI) of each marker. Those with HbA1c ≥67 expressed higher basal P-selectin MFI (641±290 vs 354±202; p=0.01) and following dual agonist stimulation (73345±62376 versus 25829±8607; p=0.02). Phosphatidylserine (PS) expression at the platelet surface was measured as a marker of procoagulant platelets. Basal PS expression appeared higher in those with HbA1c ≥67mmol/mol compared with <67 (1112±1037 vs 505±125; p=0.13). Following dual agonist stimulation, PS exposure was higher in those with HbA1c ≥67mmol/mol compared to those with lower HbA1c (21531±10214 vs 10858±3478; p=0.07). Patients were also stratified using TIR, with largely similar findings.Participants with eGDR <8mg/kg-1.min-1 showed a trend towards higher mean basal PS expression, but not P-selectin, compared with higher eGDR (PS 1216±1152 vs 458±163, p=0.09). Following dual agonist stimulation, those with eGDR <8mg/kg-1.min-1 had a greater activation induced PS exposure (23718±10200 vs 13649±6017; p=0.02), while P-selectin failed to show a statistical difference (57782±52949 vs 34991±30283; p=0.16).
Conclusion: In young adults with T1D, suboptimal glycaemic control appears to enhance platelet activation through increased expression of P-selectin and PS both basally, suggestive of elevated circulating platelet activity and following agonist stimulation, demonstrating a greater propensity to activate. The presence of low eGDR in this cohort may further exacerbate platelet activation, indicating both glycaemia and IR contribute to platelet reactivity in individuals with T1D.
Supported by: BHF Clinical Research Fellowship
Disclosure: R.C. Sagar: None.
18
Non-invasive fibrosis scores as prognostic biomarkers of liver and cardiovascular events and all-cause mortality in adults with type 2 diabetes in the UK: a longitudinal study
M. Jara1, Q.M. Anstee2,3,3, T.L. Berentzen1, L.M. Nitze1, M.S. Kjær1, K.K. Mangla1, J.M. Tarp1, K. Khunti4;
1Novo Nordisk A/S, Søborg, Denmark, 2Translational & Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle, UK, 3Newcastle NIHR Biomedical Research Centre, Newcastle upon Tyne Hospitals NHS Trust, Newcastle, UK, 4Diabetes Research Centre, Leicester General Hospital, Leicester, UK.
Background and aims: Non-alcoholic steatohepatitis is associated with type 2 diabetes (T2D), life-threatening liver-related complications, increased liver-specific and all-cause mortality and cardiovascular (CV) disease. Biopsy-confirmed liver fibrosis is an important predictor of severe outcomes but biopsies are not scalable for widespread use. This real-world study investigated the prognostic utility of six non-invasive fibrosis scores on clinical outcomes in patients with T2D seen in routine general practice.
Materials and methods: Using a longitudinal cohort design, patients ≥18 years with T2D, ≥1 fibrosis score calculable from the UK Clinical Practice Research Datalink (CPRD) after 1 January 2001, no alcohol-related disorders and/or other chronic liver diseases in Hospital Episodes Statistics (HES), and/or no prescriptions of drugs inducing liver disease in CPRD were included. Patients were followed from date of inclusion until time of first clinical outcome event (liver-related hospitalisation or death [liver event], CV-related hospitalisation or death [CV event] or all-cause death) recorded in HES or Office for National Statistics Death Registration; database migration; 10 years’ follow-up; or 1 January 2020, whichever came first. Fibrosis-4 Index (FIB4), the score of focus, was categorised as low (<1.30), indeterminate (1.30-2.67) or high (>2.67) risk according to established cut-offs previously shown to be associated with fibrosis. Cumulative incidence functions were calculated and hazard ratios (HRs) were estimated using Cox proportional hazard models with calendar time as underlying timescale.
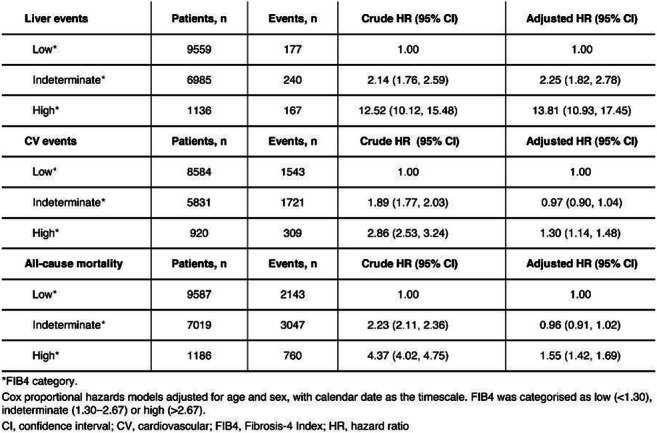
Results: In total, 17 793 eligible patients (55% male, median age 66.7 years) had T2D and measures available for FIB4 calculation. Among these patients there were 584 liver events, of which ascites (n=233), cirrhosis (n=113) and gastro-oesophageal varices (n=110) were the most common. Cumulative incidence proportions for an incident event after 10 years’ follow-up in the high FIB4 group were 16% (liver), 36% (CV) and 69% (death). HRs for patients in the indeterminate and high FIB4 groups vs the low-risk group indicated a significantly higher risk of all three event types (Table), also after adjustment for sex and age. For the other scores, HRs were also higher in patients with a high vs low score.
Conclusion: In this real-world population of patients with T2D and no other clinically recognised liver disease, the risk of a clinical event was significantly higher in patients with a high vs low FIB4 score, highlighting the prognostic potential of FIB4 (and other non-invasive fibrosis scores) in this population.
Supported by: Novo Nordisk
Disclsoure: M. Jara: Employment/Consultancy; Novo Nordisk A/S. Stock/Shareholding; Minority shareholder in Novo Nordisk A/S.
OP 04 Walking with diabetes
19
Prevalence, incidence and risk factors for Charcot foot in patients with diabetes: a nationwide Swedish study
G. Tsatsaris1, N. Rajamand Ekberg1, T. Fall2, S. Catrina1;
1Department for Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, 2Department of Medical Sciences, Molecular Epidemiology, Uppsala Universitet, Uppsala, Sweden.
Background and aims: Charcot foot (CF) is a serious complication of diabetes mellitus (DM) with potential disastrous consequences. Despite being reported for the first time in 1868, the epidemiology of this condition is still unknown since just a few studies are available. We report here the prevalence, incidence and risk factors for the CF in a nationwide Swedish retrospective register-based study of patients with DM.
Materials and methods: The prevalence and incidence of CF in patients with DM were established based on data from the Swedish National Patient Registry and the Swedish Prescribed Drug Register for the period 2006 - 2016. Risk factors of CF were assessed using logistic regression based on data from 2001 - 2016 from the Swedish National Diabetes Register in a matched design with a control diabetes group without CF (1:10). In order to examine the effect of HbA1c, diabetes duration, macro- and microalbuminuria, atherosclerosis and retinopathy on CF risk, direct acyclic graphs (DAGs) were used to select potential confounders to be included in the statistical analysis.
Results: 3449 patients with DM and CF were included in the study. The prevalence of CF in the total diabetic population increased during the observation period from 0.55% in 2006 to 0.79% in 2016, whereas the incidence remained stable under the same time period. The increase in prevalence was observed in both type 1 (T1D) and type 2 diabetes (T2D) patients, with higher prevalence in T1D (1.97% in 2016) than in T2D (0.60% in 2016). Subjects with T1D had a longer diabetes duration before developing CF compared to subjects with T2D (33.12 ± 13.39 years respective 14.64 ± 9.49 years). Female gender was identified as a risk factor for the development of CF in T1D (OR: 1.29, 95% CI: 1.14 - 1.45, p-value <0.001) but not in T2D (OR: 0.72, 95% CI: 0.64 - 0.81, p-value <0.001). Atherosclerotic disease was associated with increased risk for CF in both types of DM (T1D OR: 4.73, 95% CI: 3.54 - 6.32, p-value <0.001) (T2D OR: 6.14, 95% CI: 5.12 - 7.36, p-value <0.001). Diabetes duration, HbA1c, pre-existing diabetic microangiopathic complications, body mass index (BMI), osteoporosis and peripheral vascular disease were identified as risk factors of CF in both types of DM. Area under the curve in our prediction model was 0.78 in T1D and 0.71 in T2D.
Conclusion: The prevalence of CF was higher in patients with T1D than in patients with T2D with progressive increase in both types of diabetes during period of observation. This might reflect a longer survival time after CF diagnosis in patients with DM especially in T1D. Identification of risk factors for CF offer the possibility of a predictive model for the development of CF.
Supported by: Stockholm County Research Council, Bert von Kantzows Foundation, Frimurarestifelse
Disclosure: G. Tsatsaris: Grants; Stockholm County Research Council, Bert von Kantzows Foundation, Frimurarestifelse.
20
Genome wide association meta study of diabetic foot ulcers
S. Altintas1, G. Bouland2, A. Veluchamy3, M. Thangam4, E. Lindholm4, W. Meng3, J.A. Andersen1, C.S. Hansen1, L.T. Dalgaard5, C. Palmer3, E. Ahlqvist4, L.M.T. Hart6, A. Rasmussen1, P. Rossing1, T.S. Ahluwalia1;
1Steno Diabetes Center Copenhagen, Herlev, Denmark, 2Leiden University Medical Center, Leiden, Netherlands, 3University of Dundee, Dundee, Scotland, UK, 4Lund University Diabetes Center, Malmo, Sweden, 5Roskilde University, Roskilde, Denmark, 6Amsterdam University Medical Center, Amsterdam, Netherlands.
Background and aims: Diabetic foot ulcers (DFUs) are a severe complication of diabetes mellitus. Globally, a lower limb is amputated due to diabetes every 30 seconds; foot ulceration precedes 85% of diabetes-related amputations. While several risk factors are known - including sensorimotor peripheral neuropathy (DSPN), peripheral artery disease, foot deformities, and poor glycemic control - the genetics of DFUs are poorly understood. In this study, we conducted the first genome-wide association meta-study of diabetic foot ulcers with the aim of identifying genetic loci associated with DFU risk in diabetic (type 1 and type 2) individuals with DSPN.
Materials and methods: A meta-analysis of DFUs was conducted, comprising four independent genome-wide association studies from diabetes cohorts of European ancestry (AfterEU, Denmark; SDR, Sweden; GoShare, Scotland; DCS, Netherlands). This case-control study comprised a total of 980 cases (with DFU and DSPN) and 6196 controls (no history of DFU, but with DSPN). DSPN was defined as bilateral vibration sensation threshold ≥25V or absent sensation to monofilament. Logistic regression models were applied adjusting for sex, duration of diabetes and principal components. Summary statistics from the four European cohorts were meta-analysed using fixed effects inverse-variance based meta-analysis.
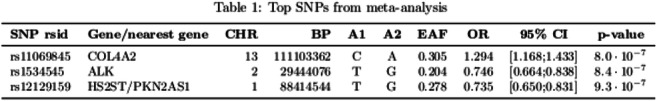
Results: In the GWAS meta-analysis, we identified three common single nucleotide polymorphisms (SNPs) that were suggestive (p-value<1×10-6), from three loci; an overview of these results is given in Table 1. Two common variants - rs11069845 (intronic), and rs1534545 (missense variant) - were located in COL4A2 and ALK. The third, rs12129159, was located within 1mb from HS2ST1 and PKN2-AS1.
Conclusion: Three suggestive loci associated with DFU risk were identified in the current ongoing study. Two loci were located in COL4A2 and ALK, which have known roles in small vessel disease and neuronal development. Additional GWAS data from other participating centers will be added to the current analyses towards identification of loci associated with diabetic foot ulcers of neuropathic origin.
Disclosure: S. Altintas: None.
21
Bone morphogenetic protein-7 promotes diabetic wound healing by decreasing inflammation and matrix metalloproteinase-9 expression
E.C. Leal1,2, J. da Silva1,2, A. Figueiredo1, Y.-H. Tseng3, E. Carvalho1,2;
1Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal, 2Institute of Interdisciplinary Research, University of Coimbra, Coimbra, Portugal, 3Joslin Diabetes Center, Boston, USA.
Background and aims: Diabetic foot ulcers is the leading cause of prolonged hospital admission, health-related costs, and reduced quality of life for diabetic patients. Bone morphogenetic protein-7 (BMP7) is a protein of the transforming growth factor beta (TGF beta) superfamily. Some known properties of BMP7, such as the modulation of inflammation, indicate that it may promote tissue regeneration. However, the role of BMP7 in the skin is not fully understood, particularly in conditions of diabetes during wound healing. Our main aim was to evaluate the role of BMP7 in diabetic wound healing and study the underlaying mechanisms.
Materials and methods: We used male C57BL/6 mice, and diabetes was induced with intraperitoneal administration of streptozotocin (50mg/kg) for 5 consecutive days. After six weeks of diabetes, two wounds of 6 mm in diameter, were induced on the back of diabetic mice and treated topically with BMP7 (0.5 μg per wound/day) or vehicle. The wound size was measured every day up to day 10 post wounding, and the wounded skin was collected. The number of inflammatory cells present at the wound site, macrophages (CD68) and lymphocytes (CD4), was determined by immunohistochemistry. The expression of inflammatory markers (IL6, keratinocyte-derived chemokine - CXCL1/KC, TNF alpha, IL1 beta), and matrix metalloproteinase-9 (MMP9) was measured by quantitative PCR. Finally, H&E and Masson's Trichrome were used to assess histology and collagen deposition.
Results: BMP7 accelerated wound closure by 20% (p<0.05) in diabetic mice when compared to vehicle-treated wounds. The number of lymphocytes in diabetic wounds did not change with BMP7 treatment, but the number of macrophages was reduced by 30% (p<0.05) when compared to vehicle-treated wounds. In addition, BMP7 significantly decreased (p<0.05) the expression of inflammatory mediators and MMP9 expression in diabetic wounds. We also observed that in diabetic wounds treated with BMP7, the area of granulation tissue was significantly increased by 50% (p<0.05), and the collagen deposition was 35% higher (p<0.05) when compared to vehicle-treated wounds, indicating that BMP7 promotes better wound healing progression and a decrease in extracellular matrix degradation.
Conclusion: BMP7 promotes wound healing in diabetes by decreasing local inflammation and, consequently, MMP9 expression, known to be increased in inflammatory conditions. This effect of BMP7 treatment in diabetic wounds prevented the high degradation of the extracellular matrix, evident in the diabetic condition. It also led to an increase in tissue regeneration and in collagen deposition, so a better progression of wound healing. This study suggests that BMP7 is a therapeutic agent of interest for the treatment of diabetic foot ulcers.
Supported by: EFSD/Lilly EXPAND Programme, FCT, SPD
Disclosure: E.C. Leal: None.
22
Novel topical esmolol hydrochloride for diabetic foot ulcer: phase 3, randomised, double-blind, placebo-controlled, multi-centre study
A. Rastogi1, S.A. Kulkarni2, S.K. Deshpande2, S. Agarwal3, V. Vishwanathan4, A.G. Unnikrishnan5, Galnobax Study Group;
1Endocrinology, Post Graduate Institute of medical Education and Research, Chandigarh, 2Novalead Pharma, Pune, 3Ruby Hall Clinic, Pune, 4Diabetes, MV Diabetes and Resaerch center, Chennai, 5Diabetes, Chellaram Diabetes center, Pune, India.
Background and aims: Pre-clinical and phase 2 study with esmolol suggest its potential role in treatment of diabetic foot (DFU). We aimed to study the efficacy of topical esmolol for DFU healing.
Materials and methods: This is the FIRST randomized, double-blind, placebo-controlled, parallel-group, multi-centre, phase-3 study done at 27 centers across India to evaluate efficacy of topical esmolol hydrochloride gel for uninfected diabetic foot ulcers (DFU) . Participants with non-infected full thickness (UTS 1B) DFU of duration>4 week, size 2cm2-15cm2 and ABI 0.7-1.3 were randomized after a run-in phase (1 week) to receive Esmolol + standard of Care (SoC), SoC only, or vehicle + SoC (3:3:1 proportion) for 12 week (treatment phase) and followed further till 24 week. Participants visited the investigational site once a week during the 12-week treatment phase for wound measurement and at week 14, 16, 20 and 24 during follow-up phase. SoC included debridement, moist wound environment and off-loading with modified insole and shoes. The primary outcome was proportion of wound closure within 12-week in Esmolol + SoC and SoC only groups. The secondary outcomes included proportion of participants achieving target ulcer closure (24-weeks), and time to ulcer closure during treatment phase. Target ulcer closure was defined as 100% re-epithelialization, confirmed on two consecutive site visits (two weeks apart). All analyses were performed for intention-to-treat (ITT) i.e., safety evaluable population.
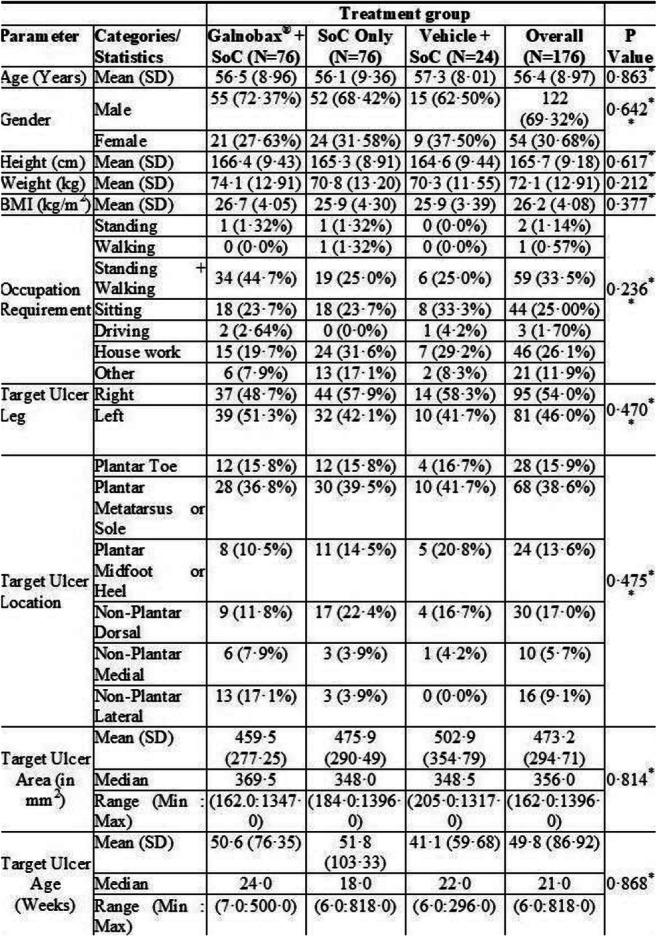
Results: Overall, 251 participants were screened and 176 were randomized to the three groups with baseline characteristics in Table 1. The proportion of participants who achieved target ulcer closure within 12 weeks was 41of 68 (60·3%) participants in Esmolol + SoC group compared to 30 of 72 (41·7%) participants in SoC only group (OR = 2·126, 95% CI 1·08-4·17, p =0·0276). Proportion of target ulcer closure by the end of study (week 24) was achieved in 44 of 57 (77·2%) participants in Esmolol + SoC group and 35 of 63 (55·6%) participants in SoC only group (OR = 2·708, 95% CI 1·22-5·99; p = 0·0126). The mean time for ulcer closure was similar (74·3 days for Esmolol + SoC group and 72·5 days for SoC only group). The Esmolol + SoC group showed significant advantage over SoC (p<0·05) in participants with BMI>25 kg/m2, high HbA1c, longer ulcer duration, hemoglobin<11 g/dl, low eGFR and ulcers that did not achieve 50% area reduction within initial 4 weeks of treatment. The percent ulcer area reduction from end-of-treatment to end-of study was 60·7% for Esmolol + SoC group compared to a negligible reduction of 2·7% in SoC only group (p = 0·021).
Conclusion: Topical Esmolol is a novel treatment that significantly improves healing of DFU compared to SoC.
Clinical Trial Registration Number: NCT03998436
Disclosure: A. Rastogi: None.
23
Flexor tendon tenotomy treatment of the diabetic foot: a multicentre randomised controlled trial
J. Askø Andersen1,2, A. Rasmussen3, S. Engberg3,4, J. Bencke5, M. Frimodt-Møller1, K. Kirketerp-Møller3,6, P. Rossing1;
1Complication Research, Steno Diabetes Center Copenhagen, Gentofte, 2Orthopedic Department, North Zealland Hospital, Hillerød, 3Foot Clinic, Steno Diabetes Center Copenhagen, Gentofte, 4Novo Nordisk A/S, Søborg, 5Human Movement Analysis Laboratory, Department of Orthopedic surgery,, Copenhagen University Hospital at Amager-Hvidovre, Hvidovre, 6Copenhagen Wound Healing Center, Bispebjerg Hospital, København, Denmark.
Background and aims: A fifth to a third of all individuals with diabetes will incur a diabetic foot ulcer during their life-time. There are several factors that influence the risk of incurring a diabetic foot ulcer with one being hammertoe deformities. The aim of this study was to evaluate effects on ulcer healing and prevention of needle flexor tendon tenotomy treatment of the diabetic hammertoe deformity.
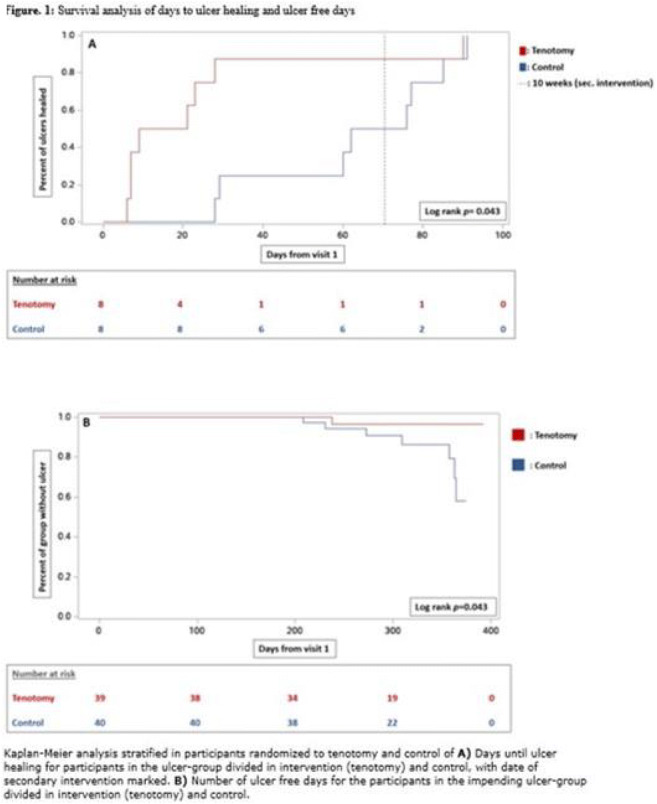
Materials and methods: A multicenter randomized controlled trial of individuals with diabetes and ulcers or impending ulcers associated with hammertoes, performed between 1st of November 2019 and 31st of March 2021. Participants were stratified on the presence of ulcer, into individuals with ulcers and individuals with impending ulcers. Participants were randomized to tenotomy and standard non-surgical treatment or standard non-surgical treatment alone. Primary outcomes were time to ulcer healing and progression from impending ulcer to active ulcer.
Results: Of 224 screened individuals with diabetes, 95 (59.0% male) were included. The mean follow-up was 291 (±70) days, 28 (29.5%) had type 1 diabetes, mean diabetes duration was 20 (13-26) years, and mean age was 67.7 (±9.8) years. Of the included participants 16 had ulcers, of whom eight were randomized to intervention. Of the remaining 79 individuals with impending ulcers, 39 were randomized to intervention. For participants with ulcers, healing rates favored tenotomy (100% vs 37.5%, p=0.026) as did time to ulcer healing (p=0.04). For individuals with impending ulcers, incidence of progression to an active ulcer was lower (1 vs 7, p=0.028) and number of ulcer-free days were higher (p=0.043) in the tenotomy group. No serious adverse events were recorded.
Conclusion: This randomized study showed that the simple procedure of needle flexor tendon tenotomies was effective and safe when treating and preventing ulcers associated with the diabetic hammertoe deformity.
Clinical Trial Registration Number: NCT04154020 & NCT04154046
Supported by: the Jascha Foundation and the Aase og Ejnar Danielsen Foundation
Disclosure: J. Askø Andersen: None.
24
Saving the foot: simple orthopaedic intervention to adjust the mechanics of the ulcerated neuropathic foot improves outcomes by reducing sepsis, amputation and mortality
J. Blong1, A. Sharpe2, J. Cairney-Hill1, A. Gorman2, M. Allen2, S. Haycocks2, M. Stedman3, A. Robinson4, E. Gee1, A. Heald4;
1Orthopaedic Surgery, Salford Royal NHS Foundation Trust, Salford, 2Podiatry, Salford Royal NHS Foundation Trust, Salford, 3Res Consortium, Andover, 4Endocrinology & Diabetes, Salford Royal NHS Foundation Trust, Salford, UK.
Background and aims: Ulceration of a neuropathic foot is a poor prognostic indicator for individuals with diabetes and presents a considerable financial burden for the health economy. Orthopaedic and vascular surgeons have become embedded within most specialist diabetes foot multidisciplinary teams (DF MDT) to offer reactive interventions in the context of acute diabetic foot sepsis, such as incision and drainage of abscess, or amputation. We describe how a day-case procedure list within a DF MDT has improved outcomes by performing proactive simple surgical procedures, with a view to expediting ulcer resolution and thus minimising subsequent morbidity and mortality.
Materials and methods: Patients with ulceration (without associated abscess) were offered a percutaneous procedure performed under local anaesthetic by an orthopaedic surgeon. Purpose of surgery was to adjust the mechanics of the foot and offload the ulcerated region, expediting healing. We anticipated improved rates of ulcer resolution and reduced complication rates including diabetic foot sepsis (£11,998 per admission), amputation (£9,221 per patient) and mortality. Percutaneous tenotomies (PT) performed for toe apex ulcers. Procedure cost £501. Patient able to mobilise full weight-bearing in normal footwear immediately post-procedure. Percutaneous tendoachilles lengthening (TAL) performed for plantar metatarsal head ulcers. Procedure cost £1140. Patient partial weight-bears for four weeks in total contact cast, followed by two weeks full weight-bearing in a walker boot.
Study period April 2019 - October 2021. Primary outcome: ulcer resolution. 12 month follow-up period.
Results: Results - see table. Key findings from the intervention cohorts: successful ulcer resolution achieved for all individuals. No admissions for diabetic foot sepsis. Reduced recurrence and amputation rates. No mortality within 12 months. One complication of Achilles tendon rupture following TAL (non-concordant with immobilisation instructions). *3 of the 15 conservative cohort achieved ulcer resolution (average 20 weeks). Conservative cohort average cost £9902. Intervention cohort average cost £1211, giving average savings of £8691 per patient. This demonstrates an 88% reduction in healthcare costs.
Conclusion: These simple daycase percutaneous procedures support accelerated ulcer healing, with reduced rates of recurrence, amputation and mortality. We have demonstrated significant patient benefit and cost savings for this simple intervention, which merits full evaluation in a clinical trial.
Disclosure: J. Blong: None.
OP 05 How to become a fat cell
25
Fas (CD95) activation inhibits browning of white adipose tissue in obesity
S. Wueest1,2, P.P. van Krieken1,2, N.K. Konrad1,3, C. Koch2,3, M.S.F. Wiedemann1, M. Borsigova1,2, S. Boettcher2,3, M. Blüher4,5, D. Konrad1,2;
1Children's Hospital, Zurich, Switzerland, 2University of Zurich, Zurich, Switzerland, 3University Hospital Zurich, Zurich, Switzerland, 4University of Leipzig, Leipzig, Germany, 5University Hospital Leipzig, Leipzig, Germany.
Background and aims: We previously observed that adipocyte-specific Fas (CD95) knockout mice were protected from the development of high-fat diet (HFD)-induced adipose tissue inflammation, hepatic steatosis and insulin resistance. In these mice, Cre recombinase was under the control of the Fabp4-promotor questioning adipocyte specificity of Fas knockdown. Herein, we aimed to reassess the adipocyte-specific role of Fas on glucose metabolism in newly generated mice expressing Cre recombinase controlled by the Adipoq-promotor.
Materials and methods: 6-week-old adipocyte-specific Fas knockout (FasΔadipo) and control (FasF/F) littermates were fed a regular chow or HFD (~60% kcal fat) for 20 weeks. Glucose metabolism was assessed by intraperitoneal glucose and insulin tolerance tests. After 20 weeks, liver and fat depots were analyzed using Western blotting, qPCR and histological staining. In vitro, p53 or Fas was knocked down in subcutaneous adipocytes using CRISPR-Cas9 and siRNA, respectively, and lysates were analyzed using Western blotting. Moreover, FAS expression was determined in human subcutaneous adipose tissue and correlated to p53 and UCP1.
Results: HFD-fed FasΔadipo mice displayed improved glucose and insulin tolerance (AUC in mmol/l*min: GTT 2474±879 in FasF/F vs. 2071±646 in FasΔadipo, p<0.05; ITT 1044±94 in FasF/F vs. 739±35 in FasΔadipo, p<0.01), reduced adipose tissue inflammation as well as decreased hepatic lipid content compared to control littermates. Importantly, HFD-induced weight gain was significantly reduced in FasΔadipo mice (23.1±1.7g in FasF/F vs. 16.7±1.6g in FasΔadipo, p<0.01). The latter was associated with increased expression of browning markers such as UCP1 in inguinal adipose tissue as well as highly reduced protein levels of the UCP1-repressor p53 in isolated adipocytes. Preliminary data in cultured subcutaneous adipocytes revealed that Fas knockdown increased and Fas ligand (FasL) stimulation decreased isoproterenol-induced UCP1 protein levels, respectively. Moreover, FasL-induced downregulation of UCP1 was blunted in p53-depleted cells, indicating that Fas activation reduces browning of white adipocytes in a p53-dependent manner. In agreement, FAS (CD95) expression correlated positively with p53 but negatively with UCP1 in human subcutaneous adipose tissue.
Conclusion: Fas activation in adipocytes contributes to HFD-associated adiposity through inhibition of adipose tissue browning.
Supported by: Swiss National Science Foundation (#310030-179344 to DK)
Disclosure: S. Wueest: None.
26
Aripiprazole, but not olanzapine, directly inhibits human adipocyte differentiation and glucose metabolism
M. Vranic1, V. Ferreira2, S. Hetty1, A. Sarsenbayeva3, F. Ahmed1, G. Fanni1, Á.M. Valverde2, J. Eriksson1, M.J. Pereira1;
1Department of Medical Sciences, Uppsala University, Uppsala, Sweden, 2IIBm Alberto Sols (CSIC-UAM); CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), ISCIII, Madrid, Spain, 3Department of Molecular and Clinical medicine, University of Gothenburg, Gothenburg, Sweden.
Background and aims: Second-generation antipsychotics (SGAs) are the cornerstone treatment of schizophrenia and other mental disorders. However, they can cause adverse metabolic effects, such as obesity and diabetes, and the exact mechanisms behind this are unknown. Different SGAs carry different propensities to induce metabolic adverse effects, e.g. olanzapine (OLA) is a high-risk drug associated with worse metabolic outcomes, while aripiprazole (ARI) is considered more neutral. We aimed to investigate the effects of SGAs on adipocyte differentiation and glucose and lipid metabolism.
Materials and methods: Human subcutaneous primary preadipocytes obtained from healthy subjects (n=6) were differentiated without or with therapeutic and supra-therapeutic concentrations of ARI (1, 2, and 10 μM), its active metabolite dehydroaripiprazole (DARI; 0.4 and 4 μM), and OLA (0.2 and 2 μM) for 14 days when differentiation rate and glucose uptake were assessed. Furthermore, the expression of genes related to cell differentiation and glucose and lipid metabolism were measured. In addition, adipose tissue samples obtained from mice treated in vivo without or with ARI or OLA for 7 months were used for gene expression analyses (n=9, control; n=9, OLA; n=8, ARI).
Results: Supra-therapeutic concentrations of ARI reduced preadipocyte differentiation rate (up to ~80%), assessed by the number of cells accumulating lipids and expression of adipogenic genes (PPARG, CEBPA, CD36; p<0.05). The expression of lipid storage genes (FASN, LPL, DGAT2, ATGL; p<0.05), and mitochondrial function genes (TFAM, PDK4, ACO1; p<0.05) were also reduced compared to control. Conversely, therapeutic levels of ARI and supra- and therapeutic levels of DARI increased the expression of mitochondrial biogenesis and lipid oxidation genes (TFAM, PDK4, CPT1B, ACO1; p<0.05), and LEP (p<0.05). Basal and insulin-stimulated glucose uptake was dose-dependently reduced (up to ~70%) in cultures exposed to ARI and DARI during differentiation (p<0.05), which corresponded to reduced expression of GLUT1 and GLUT4 (p<0.05). OLA, when present during differentiation, did not affect adipogenesis or lipid and glucose metabolism but increased LEP expression by ~2 fold (p<0.05). ARI- and OLA-treated mice had decreased expression of PPARG and GLUT4 (p<0.05), as well as increased expression of lipid oxidation genes PDK4 (p<0.05) in epididymal, but not inguinal adipose tissue. LEP expression was increased, while FASN expression was decreased in both inguinal and epididymal adipose tissues in ARI- and OLA-treated mice (p<0.05).
Conclusion: Therapeutic levels of ARI and DARI, but not OLA, negatively affected adipocyte glucose metabolism and increased the expression of lipid oxidation markers, suggesting a potential substrate switch from glucose to lipid oxidation in adipocytes. OLA, instead, increased leptin levels, which may contribute to the development of obesity in humans, potentially via CNS effects. Our results suggest that while ARI directly affects adipocyte metabolism, OLA might require long-term exposure as well as the involvement of other organs, such as the brain, in mediating its adverse metabolic effects.
Supported by: H2020 Marie Sklodowska Curie ITN TREATMENT; SSMF; SDF; EXODIAB; UU ALF
Disclosure: M. Vranic: None.
27
Senescent adipose stromal cells-derived CCL5 induces endothelial dysfunction, a key step in the onset of atheroslerosis
L. Le Pelletier1, K. Ngono Ayissi1, J. Gorwood1, F. Boccara1,2, M. Auclair1, M. Atlan1,3, B. Fève1,4, J. Capeau1, C. Lagathu1, V. Béréziat1;
1Sorbonne Université - INSERM - Centre de Recherche Saint Antoine, 2AP-HP, Saint-Antoine Hospital, Department of Cardiology, 3Tenon Hospital, Department of Plastic Surgery, 4AP-HP, Saint-Antoine Hospital, Department of Endocrinology, Paris, France.
Background and aims: Adipose tissue plays a major role in the regulation of metabolism through its storage/mobilisation and endocrine function. Aging is associated with adipose tissue redistribution, oxidative stress, and fibrosis. These alterations lead to decreased storage capacities and insulin-resistance, leading to the onset of cardiometabolic complication such as atherosclerosis. We recently demonstrated that adipose tissue aging is associated with an accumulation of senescent adipose stromal cells (ASCs) from which arise insulin-resistance and dysfunctional adipocytes. Here, we hypothesize that senescent ASCs can also contribute to the onset of endothelial dysfunction, early key step in the development of cardiometabolic disease. Our aims were firstly, to establish the proof of concept that senescent ASCs can induce endothelial dysfunction, secondly, to identify a potential candidate involved in the dialogue between ASCs and endothelial cells.
Materials and methods: ASCs were isolated from adipose tissue of young (mean ± error of the mean (SEM) age: 18.6 ± 2.6 years; mean ± SEM BMI: 24.4 ± 0.6 Kg/m2) or aged (mean ± SEM age: 60.3 ± 0.9 years; mean ± SEM BMI: 25.6 ± 0.4 Kg/m2) healhty female donors. The impact of senescent ASCs on Human Coronary Artery Endothelial Cells (HCAECs) from healthy donors was analysed trhough conditioned media experiments.
Results: We found that conditioned media from aged-donor ASCs, but not from young-donor ASCs, induced endothelial dysfunction, characterized by a decrease in Nitric Oxide (NO) production, increased senescence markers (Senescence-associated-β-galactosidase activity, and cell cycle arrest protein P16 and P21) and the acquisition of a pro-adherent phenotype (increased InterCellular Adhesion Molecule (ICAM) and Vascular Cell Adhesion Molecule (VCAM) expression). Preventing the onset of aged-donor ASCs senescence allowed to maintain the endothelial function. To identify molecules implicated in ASC - HCAEC cell communication, we compared the secretory profil of aged- versus young-donor ASCs. The secretion of CCL5 was found to be specifically increased in the conditioned media obtained from aged-donor ASCs. HCAECs exposed to CCL5 displayed endothelial dysfunction (decrease in NO production, senescence, pro-adherent phenotype, pro-migratory effect, and amplified vascular networks formation). Conversely, the addition of a CCL5 receptor antagonist was abble to prevent the deleterious effect of conditioned media from aged-donor ASCs and maintain the endothelial function of HCAECs.
Conclusion: Overall, our data demonstrate the key role of ASCs and CCL5 in age-related endothelial dysfunction. They allow to a better understanding of the contribution of adipose tissue to aged-related vascular complications, thus opening new therapeutic perspectives on the support of cardiometabolic diseases.
Disclosure: L. Le Pelletier: None.
28
Requirement of plakoglobin during the early stages of adipogenesis
F. Abou Azar1,2, S. Allali1,2, M. Abayomi1,2, S. Yuen1,2, S. Del Veliz1,2, Y. Mugabo1,2, F. Paré2, G. Lavoie3,1, P.P. Roux1,3, G.E. Lim1,2;
1Université de Montréal, 2Centre de Recherche du Centre hospitalier de l'Université de Montréal, 3Institut de recherche en immunologie et en cancérologie, Université de Montréal, Montréal, Canada.
Background and aims: Plakoglobin, also known as γ-catenin, is primarily involved in cell-cell adhesion; however, its additional roles in cellular processes have not been extensively studied. Its close homolog, β-catenin, is the main transcriptional coactivator of the Wnt signaling pathway. Previous studies have highlighted the Wnt/β-catenin signalling pathway’s anti-adipogenic properties and its ability to repress the expression of adipogenic genes to preferentially induce osteogenesis in vitro. While plakoglobin is capable of inhibiting β-catenin activity in a context-dependent manner, its role in adipogenesis remains unclear. Over-expression of the scaffold protein 14-3-3ζ in mice exacerbated high-fat diet-induced obesity. Following comparison of the 14-3-3ζ interactome in visceral adipose tissue between lean and obese mice, an enrichment of plakoglobin in the 14-3-3ζ interactome was detected, suggesting a contribution of plakoglobin to weight gain. Thus, we hypothesize that plakoglobin is an essential regulator of adipogenesis.
Materials and methods: In vitro assessment of plakoglobin’s role in adipogenesis was performed in murine 3T3-L1 pre-adipocytes and human adipose-derived stem cells, which were transfected with either overexpressing plasmids or siRNA targeting plakoglobin or β-catenin. RT-qPCR, immunoblotting, SuperTOPFlash, and Oil-Redo-O (ORO) staining were used to assess gene expression, protein abundance, Wnt/β-catenin transcriptional activity, and lipid content, respectively.
Results: Plakoglobin knockdown prior to adipocyte differentiation in 3T3-L1 pre-adipocytes reduces PPARγ2 protein abundance by 69% (p ≤ 0.05 based on two-way ANOVA and post-hoc tests), indicating an inhibition of adipogenesis. Conversely, β-catenin depletion upregulated PPARγ2 expression 180% (p ≤ 0.05 based on two-way ANOVA and post hoc tests). Rescue experiments via double knockdown of both plakoglobin and β-catenin did not affect PPARγ2 expression. In preliminary studies, plakoglobin depletion in human derived-adipose stem cells diminished PPARγ2 expression by 87% (n=2), while β-catenin knockdown led to a 270% (n=2) increase in PPARγ2 protein abundance. ORO staining showed that decreasing plakoglobin expression led to decreased lipid content in differentiated 3T3-L1 and human adipose-derived stem cells. Wnt transcriptional activity was diminished following knockdown of β-catenin by 46% (p ≤ 0.05 based on one-way ANOVA), while it was unaffected by plakoglobin depletion. Plakoglobin and β-catenin overexpression downregulated PPARγ2 abundance during adipogenesis by 63 and 34%, respectively (p ≤ 0.05 based on two-way ANOVA and post hoc tests).
Conclusion: Our study has revealed a novel and complex role for plakoglobin in adipogenesis. Comparable trends were observed in both 3T3-L1 pre-adipocytes and human adipose-derived stem cells, suggesting plakoglobin holds a species conserved effect. Additional in-depth studies are required to understand the mechanisms by which plakoglobin influences adipocyte differentiation.
Supported by: Canada Research Chairs, CIHR, Banting Research Foundation
Disclosure: F. Abou Azar: None.
29
Ampk γ2 is an essential player in adipogenesis and adipocyte function
Y. Cheng1, X. Hui2;
1Medicine, Hong Kong University, 2School of Biomedical Sciences, Chinese University of Hong Kong, Hong Kong, Hong Kong.
Background and aims: Adipogenesis is the process in which the multipotent mesenchymal stem cells (MSCs) are differentiated into mature adipocytes through the pre-adipocyte stage. Adenosine monophosphate-activated protein kinase (Ampk) is a heterotrimeric serine/threonine kinase composed of one catalytic α subunit and two regulatory subunits β and γ. Ampk γ2 subunit has been overlooked and has long been believed to simply serve as a binding partner of the nucleotide. Here we uncovered the unique function of Ampk γ2 in determining adipogenesis and adipocyte function.
Materials and methods: Ampk γ2 over-expression or knockdown stromal vascular cells (SVCs) were established. We also generate adipocyte-specific Ampk γ2 knockout mice (AKO) via the Cre-LoxP strategy to elucidate the physiological effects of Ampk γ2 in adipose tissue. SVCs were isolated from the inguinal subcutaneous adipose tissue of AKO and wildtype (WT) mice. Selective deletion of the Ampk γ2 gene was induced by Tamoxifen. SVCs were differentiated to white adipocytes in vitro.
Results: Ampk γ2 was abundantly expressed in mature adipocytes. The expression of Ampk γ2 was gradually increased during adipogenesis but selectively reduced in obese adipose tissue. Knockdown or deletion of Ampk γ2 significantly compromised the adipogenic differentiation of SVCs into lipid-laden adipocytes with reduced expression of Fas, Pparγ, C/ebpα, and Fabp4. The worst adipogenesis was shown if Ampk γ2 was deleted on the first two days of differentiation, suggesting that Ampk γ2 was mainly involved in the mitotic clonal expansion phase of adipogenesis. In addition, the deletion of Ampk γ2 in mature adipocytes mitigates beige cell biogenesis. By immunofluorescence staining, we found that Ampk γ2 harbors a unique nucleus-targeting sequence. We also identified the genes that are altered by Ampk γ2 in mature adipocytes by RNA sequencing.
Conclusion: Ampk γ2 plays an essential role in adipogenesis and holds potential as a therapeutic target for obesity and metabolic diseases.
Supported by: Health and Medical Research Fund (06172346)
Disclosure: Y. Cheng: None.
30
Estrogen receptor beta knockdown reduces differentiation of preadipocytes from postmenopausal women
F. Ahmed, S. Hetty, M. Vranic, G. Fanni, M.J. Pereira, J.W. Eriksson;
Medical Science, Uppsala University, Uppsala, Sweden.
Background and aims: Estrogen deficiency in postmenopausal women is linked to redistribution of body fat, insulin resistance, and T2D. E2 signaling occurs mainly through estrogen receptors alpha (ESR1) and beta (ESR2). We have previously shown that ESR2 expression, but not ESR1, is higher in subcutaneous adipose tissue (SAT) from postmenopausal compared to premenopausal women. The functional impact of this on adipose tissue metabolism is not fully known. This study investigates the association between ESR2 expression and lipid and glucose metabolism in human subcutaneous adipose tissue (SAT) in subjects with and without T2D and in in vitro differentiated adipocytes.
Materials and methods: SAT were obtained by needle biopsies from 20 control and 20 T2D subjects with T2D matched for sex (10M/10F per group), age (58±11 vs 58±9 years) and BMI (30.8±4.6 vs 30.7±4.9 kg/m2). ESR2 gene expression in SAT was correlated to markers of obesity and glucose metabolism. ESR2 knockdown (KD) was performed in preadipocytes from postmenopausal women (age: 63±13 years, BMI 30.3±6.2 kg/m2, n=4) using CRISPR/Cas9 gene editing. In vitro differentiated KD adipocytes were characterized for differentiation rate, lipid storage, and glucose uptake.
Results: Postmenopausal females express higher levels of ESR2 in SAT compared to premenopausal women and males (1.5-fold; p<0.05) whereas ESR1 expression was not dependent on sex or menopausal status. We found that ESR2 expression was lower in subjects with obesity (BMI≥30) compared to non-obese subjects, independent of sex (p<0.05). Additionally, ESR2 expression did not differ between control and subjects with T2D. ESR2 expression in SAT from females, but not males, was negatively correlated with weight, markers of central adiposity (e.g. waist-to-hip ratio, visceral adipose tissue volume), and markers of fatty acid oxidation (e.g. CPT1A, CPTIB), and positively correlated with subcutaneous adipocyte size and markers related to lipid storage (e.g. LPL, ACACA), and glucose transport (e.g. GLUT4, AKT2) (p<0.05 all). In preadipocytes, ESR2 KD reduced preadipocyte differentiation (10-30%, p<0.05) compared to wild-type cultures on day 7 and day 14 of differentiation. This corresponded to transient reductions in the expression of differentiation markers (e.g. PPARG), markers of lipogenesis (e.g. LPL, FAS), lipolysis (e.g. HSL, PLIN1), and adipokines (e.g. ADIPOQ) (p<0.05 all). Glucose uptake was reduced in adipocytes in KD cultures (p<0.05), however, this effect was lost after normalizing for preadipocyte differentiation rate.
Conclusion: Our results indicate that ESR2 is involved in regulating human adipocyte differentiation and lipid storage, and higher ESR2 expression may promote greater energy deposition into SAT as seen after menopause. This provides insight into a potential molecular target to promote a healthy obesity phenotype.
Supported by: Swedish Diabetes Foundation, EXODIAB, the Ernfors Foundation, the Swedish Society for Medical Research
Disclosure: F. Ahmed: None.
OP 06 Intracellular regulation of insulin release
31
Glucose metabolites upstream of glyceraldehyde 3-phosphate dehydrogenase trigger activation of the mechanistic target of rapamycin complex 1 pathway in diabetic islets
E. Haythorne, M. Lloyd, J. Walsby-Tickle, J. Sandbrink, G. Cyranka, J. McCullagh, F.M. Ashcroft;
University of Oxford, Oxford, UK.
Background and aims: Hyperglycaemia leads to impaired mitochondrial respiration and hyperactivation of the nutrient-sensing mechanistic target of rapamycin complex 1 (mTORC1) pathway in pancreatic islets. Recent studies in non-islet cells suggest that glycolytic intermediates upstream of glyceraldehyde 3-phoshate dehydrogenase (GAPDH), fructose-1,6-bisphosphate (F1,6BP) and dihydroxyacetone phosphate (DHAP), signal glucose availability to mTORC1, leading to its activation. Thus, the aims of this study were to determine if this mechanism exists in beta cells/islets and if mTORC1 hyperactivation mediates mitochondrial dysfunction in diabetes.
Materials and methods: We utilised the βV59M mouse model where hyperglycaemia/diabetes is initiated via a tamoxifen-inducible KATP channel activating mutation in pancreatic beta cells. Islets were studied after 2 weeks of diabetes (>20mmol/l). Additionally, INS1 832/13 insulinoma cells were cultured at high (HG:25mM) or low (LG:5mM) glucose +/- the GAPDH inhibitor, koningic acid (5μM:KA), for 48hrs. To examine the impact of mTORC1 hyperactivation on mitochondrial efficiency, islets were incubated with/without the S6 kinase inhibitor PF-4708671 (10μM:S6Ki) for 48 hours. Metabolomics was performed using anion exchange chromatography mass spectrometry. GAPDH activity was measured biochemically. mTORC1 signalling was determined by the ratio of phosphorylated (p-S6) and total ribosomal protein S6 (tot-S6) using standard western blotting methods. Oxygen Consumption Rate (OCR) was monitored using an extracellular flux analyser.
Results: mTORC1 signalling was increased in diabetic islets (p-S6/total-S6 ratio: control = 0.36±0.02 vs diabetic = 0.70±0.082 AU, p<0.05; n=6). Compared to control islets, diabetes led to an increase in the relative abundance of F1,6BP (8.23±1.93-fold, p<0.01, n=4) and DHAP (1.43±0.04-fold, p<0.01, n=3). INS1 cells cultured at LG + KA recapitulated the effects of hyperglycaemia, with increased relative abundances of F1,6BP and DHAP, activation of mTORC1 and impaired GSIS. Diabetic islets displayed an attenuated glucose-stimulated OCR, relative to control islets, but this was significantly improved by incubation with S6Ki (diabetic = 34.45±10.72 vs diabetic+S6Ki 203.94±39.24% increase in OCR above baseline, p<0.001; n=7-8). The ATP-synthase inhibitor, oligomycin, produced significantly less inhibition of OCR in diabetic islets, compared to control islets, an effect that was prevented by mTORC1 inhibition (diabetic = 55.01±9.44 vs diabetic+S6Ki = 240.32±9.71% decrease in OCR, p<0.001; n=7-8).
Conclusion: We provide evidence that diabetes/hyperglycaemia leads to a build-up of glycolytic metabolites upstream of GAPDH which chronically activates mTORC1 in pancreatic islets. Additionally, our results suggest that hyperactivation of mTORC1 signalling is partially responsible for mitochondrial dysfunction in diabetic islets and may be involved in regulating ATP-synthase activity.
Supported by: MRC, Wellcome trust, Novo Nordisk
Disclosure: E. Haythorne: None.
32
Deletion of Carboxypeptidase E in beta cells disrupts proinsulin processing and accelerates streptozotocin-induced hyperglycaemia in mice
Y.-C. Chen1,2, A.J. Taylor2, K. Fok2, M. Komba2, X.-Q. Dai3, J.M. Fulcher4, A. Swensen4, A.E. Patterson2, R.I. Klein Geltink2, W.-J. Qian4, P.E. MacDonald3, C.B. Verchere1,2;
1Department of Surgery, University of British Columbia, Vancouver, Canada, 2BC Children's Hospital Research Institute, Vancouver, Canada, 3Department of Pharmacology, University of Alberta, Edmonton, Canada, 4Pacific Northwest National Laboratory, Richland, USA.
Background and aims: Carboxypeptidase E (CPE) facilitates the conversion of prohormones into mature hormones, and is highly expressed in neuroendocrine tissues. Carriers of CPE mutations have elevated plasma proinsulin and develop severe obesity and hyperglycemia. In this study, we aimed to determine whether loss of Cpe in pancreatic beta cells is sufficient to disrupt proinsulin processing and accelerate development of diabetes in mice.
Materials and methods: Mice with Cpe deletion in pancreatic beta cells (βCpeKO; Cpefl/fl x Ins1Cre/+) and littermate control mice (Wt; Cpefl/fl and Cpefl/+) were treated with high fat diet (HFD) or multiple low dose streptozotocin (STZ), and body weight, plasma proinsulin, glucose tolerance, insulin sensitivity, and beta-cell area were assessed. Islets from βCpeKO mice were collected for analysis by electron microscopy, transcriptomics, peptidomics, proinsulin biosynthesis, dynamic insulin secretion, exocytosis, in vitro cell proliferation, metabolic flux, and live cell imaging. Mice with inducible beta-cell-specific Cpe deletion (iβCpeKO; Cpefl/fl x Pdx1CreER) were treated with HFD, and pancreatic beta-cell proliferation was analyzed.
Results: βCpeKO mice lack mature insulin granules in beta cells. Instead, they have 4.5-fold increased proinsulin protein in islets, and significantly elevated proinsulin immunoreactivity in plasma (37 vs. 1084 pM, Wt vs. βCpeKO, p < 0.05). However, glucose- and KCl-stimulated insulin secretion dynamics in βCpeKO islets, and insulin granule exocytosis in βCpeKO beta cells, remained largely intact. Upon HFD challenge, male and female βCpeKO mice showed comparable weight gain, glucose tolerance, and insulin sensitivity compared to Wt littermates. Interestingly, beta-cell area was increased in chow-fed βCpeKO mice (0.8 vs 1.6%, p < 0.05), and beta-cell EdU incorporation was elevated in HFD-fed iβCpeKO mice (2.6 vs 6.5%, p < 0.05). Consistent with these observations, islets from βCpeKO mice displayed increased proinsulin biosynthesis and elevated beta-cell proliferation (4.9 vs 7.4%, p < 0.05) upon high glucose treatment. In addition, transcriptomic analysis showed that βCpeKO beta cells have elevated glycolysis and HIF1α-target gene expression. Yet, high glucose treatment also led to mildly reduced mitochondrial membrane potential (1.53 vs. 1.27, p < 0.05), and increased mitochondrial (0.41 vs. 0.56, p < 0.05) and cytosolic (0.065 vs. 0.077, p < 0.05) reactive oxygen species in beta cells from βCpeKO mice. Lastly, after multiple low dose STZ treatment, βCpeKO mice had accelerated development of hyperglycemia (16.0 vs. 12.2 mM 7 days post-STZ, p < 0.05).
Conclusion: These findings confirm that Cpe mediates proinsulin processing in pancreatic beta cells. Lack of Cpe and impaired proinsulin processing is associated with beta-cell mass compensation via increased beta-cell proliferation. Under acute metabolic and oxidative stress challenges, Cpe and proper proinsulin processing are required to maintain beta-cell function and glucose homeostasis.
Supported by: This work is supported by JDRF and CIHR
Disclosure: Y. Chen: None.
33
Identification of TBL1 and TBLR1 as novel regulators of insulin transcription in beta cells
A.A. Walth1, R. Terron Exposito1, A.-C. König2, S.M. Hauck2, A. Feuchtinger3, P.E. MacDonald4, M. Rohm1;
1Institute for Diabetes and Cancer, Helmholtz Munich, Neuherberg, Germany, 2Research Unit Protein Science, Helmholtz Munich, Neuherberg, Germany, 3Core Facility Pathology & Tissue Analytics, Helmholtz Munich, Neuherberg, Germany, 4Department of Pharmacology, University of Alberta, Edmonton, Canada.
Background and aims: Insulin is a central regulator of glucose homeostasis and its synthesis by pancreatic β-cells is tightly controlled. On transcriptional level insulin synthesis is mediated by transcription factors and transcriptional cofactors such as TBL1 (transducin β-like 1) and TBLR1 (TBL-related 1). Originally identified as components of activator and repressor complexes such as the NCoR (nuclear receptor corepressor)/SMRT (silencing mediator for retinoid and thyroid receptors) repressor complex, TBL1 and TBLR1 gained interest as essential checkpoints of transcription by exchanging regulatory complexes ligand dependently. We here aimed to investigate the β-cell specific function of TBL1 and TBLR1.
Materials and methods: TBL1 and TBLR1 gene expression in islets was assessed by qPCR. To address the β-cell specific role of TBL1 and TBLR1 we generated and characterized mice with an Ins1-cre-driven double deletion of TBL1 and TBLR1 (TBL/RβKO). Islet morphology was examined through immunohistochemical analysis. TBL1 and TBLR1 interaction partners were identified in MIN6 cells using endogenous immunoprecipitation coupled to mass-spectrometry. Identified hits were verified in MIN6 and INS1 cells. Insulin promoter activity was assessed by a dual-luciferase reporter assay in INS1 cells upon siRNA mediated TBL1 and TBLR1 knock-down.
Results: We found TBL1 and TBLR1 levels to be dysregulated upon aging (n=4; p<0.05) and chronic hyperglycemia (n=6; p<0.05) in murine pancreatic islets. TBL/RβKO mice developed severe hyperglycemia (n=5; 600 mg/dl vs. 150 mg/dl at 12 weeks of age; p<0.0001) along with impaired insulin gene expression (n=6; 4.2-fold, p<0.0001). Alterations to islet architecture and reduced numbers of insulin positive cells (n=4; p<0.05) preceded the development of hyperglycemia. A transcriptome analysis revealed downregulation of β-cell identity genes, while disallowed genes - including genes from alternative islet cell types - were upregulated, suggesting dedifferentiation or impaired maturation of the β-cells. A subsequent interactome analysis identified components of the NCoR/SMRT repressor complex and PAX6 (paired box protein 6) as direct TBL1 and TBLR1 (n=3; p<0.01) interaction partners. We also found the components of the NCoR/SMRT complex to directly interact with the islet cell master regulator PAX6, suggesting TBL1 and TBLR1 as possible regulators of PAX6-mediated gene expression. Indeed, we found the insulin promoter, which is a PAX6 target, under direct TBL1 and TBLR1 control.
Conclusion: Our data support the notion that TBL1 and TBLR1 are novel regulators of insulin gene expression and β-cell functionality. Through interaction with NCoR /SMRT and PAX6, a critical regulator of β-cell maturity and identity, TBL1/TBLR1 may contribute to the maintenance of β-cell identity and its loss in pathologic conditions.
Disclosure: A.A. Walth: None.
34
Single-cell-resolution imaging of alpha/beta cell metabolic response to glucose stimulation in living human-derived Langerhans islets
F. Cardarelli1, F. Azzarello1, L. Pesce1, V. De Lorenzi1, G. Ferri1, M. Tesi2, S. Del Guerra2, P. Marchetti2;
1Scuola Normale Superiore, 2Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy.
Background and aims: A cascade of highly regulated biochemical processes connects glucose stimulation to hormone secretion in specialized cells within Langerhans islets of the mammalian pancreas, the α and β cells. Given the importance of this process for systemic glucose homeostasis, non-invasive and fast strategies capable to monitor quantitatively α- and β-cells metabolic responses in living islets are highly desirable. Despite the efforts, however, no report thus far was able to probe the specific signature of α- and β-cells response to glucose stimulation in living human islets (HIs).
Materials and methods: To tackle this issue we used here a combination of label-free Fluorescence Lifetime Imaging Microscopy (FLIM) in living HIs with post-fixation immunofluorescence. More in detail, by the phasor approach to FLIM we discriminated the free and protein-bound forms of NAD(P)H molecules in optical sections of living HIs and, by means of their ratio, we defined the tissue metabolic shift upon pulsed glucose stimulation. Then, by using post-fixation immunofluorescence, we identified α and β cells, finally matching single-cell identities with their metabolic response. A cohort of 4 healthy donor patients was included in this study.
Results: A total of 15 islets were measured from the above-mentioned cohort, with 312 α cells and 654 β cells identified. We observed a neat metabolic shift towards oxidative phosphorylation in the great majority of β cells, in keeping with previous results from rat/mouse β cells. By contrast, we observed a wide spectrum of metabolic shifts in α cells, from glycolysis- to oxidative-phosphorylation-oriented ones, apparently contradicting previous reports which assessed an univocal glycolytic response to glucose in rat/mouse α cells. Interestingly, at the patient level, the heterogeneous α-cell responses reveal an inverse proportionality with respect to the amount of insulin secreted (independently probed by an ELISA assay): the higher the insulin secreted, the more glycolysis-oriented the metabolic shift measured. Noteworthy, such inverse proportionality transforms into marked linear anti-correlation (Pearson’s correlation coefficient: -0,997) if α- and β-cell responses are summed and plotted against the amount of secreted insulin (see Graph).
Conclusion: The emerging picture indicates the synergistic action of α and β cells as key signature of HI metabolic response to glucose and, in turn, as a basic constituent for the regulation of systemic glycaemia. While demonstrating the effectiveness of an optical-microscopy-based protocol to measure the specific responses of α and β cells in a living human Langerhans islet, present results pave the way to similar investigations to be conducted in diabetes and/or using drugs to restore cell functionality.
Supported by: European Research Council (grant agreement N. 866127, project CAPTUR3D)
Disclosure: F. Cardarelli: Grants; ERC Consolidator grant.
35
Mitochondrial AASS-mediated l-lysine degradation is required to maintain beta cell function
L. Cataldo1,2, Q. Gao1, K. Trost1, M. Fex2, H. Mulder2, T. Moritz1;
1The Novo Nordisk Foundation Centre for Basic Metabolic Research, University of Copenhagen., Copenhagen, Denmark, 2Lund University Diabetes Center, Lund University., Malmo, Sweden.
Background and aims: Aminoadipate-Semialdehyde Synthase (AASS) is a mitochondrial-located bifunctional enzyme with lysine-ketoglutarate reductase and saccharopine dehydrogenase activities, involved in the first two steps in the catabolic pathway of L-lysine, an insulinotropic amino acid in humans. AASS-mediated L-lysine catabolism may contribute to fueling insulin secretion since Acetyl-CoA and L-glutamate are generated in the pathway; the former feeds the TCA cycle while L-glutamate acts as a metabolic coupling factor (MCF) for glucose-stimulated insulin secretion (GSIS). In addition, L-lysine cell accumulation is cytotoxic and a mutation in AASS was recently identified as responsible for familial hyperlysinemia, an autosomal recessive disorder. Thus, AASS-mediated degradation of L-lysine may play a dual role in β cell function by maintaining L-lysine levels at physiological levels and providing L-lysine-derived metabolites that amplify GSIS.
Materials and methods: AASS mRNA levels in human islets from non-diabetic (ND) and type 2 diabetes (T2D) subjects were assessed. Human islets and INS1 832/13 cells were transfected with scramble (control) and AASS siRNAs (AASS-KD) and GSIS was assessed by ELISA. Live cell cytosolic calcium was measured by Fluo4 dye and confocal microscopy. Glucose-stimulated mitochondrial metabolism was investigated in AASS-KD INS1 832/13 cells by extracellular flux analyzer, confocal microscopy and mass spectrometry-based metabolomics analysis.
Results: AASS mRNA levels were reduced in pancreatic islets from T2D vs ND donors (p=2.7e-5, n=188) and correlated negatively with hyperglycemia (HbA1c) (r=-0.158, p=0.009, n=169) but positively with GSIS stimulatory index (r=0.10, p=0.026, n=182). In vitro studies showed that AASS silencing reduced basal and GSIS in human islets. Accordingly, AASS silencing reduced insulin secretion in INS1 832/13 cells in response to basal and high glucose, as well as in response to pyruvate and cAMP-promoting agents (IBMX-FSK), but not in response to high K+. Increased cytosolic calcium responses were accompanied by reduced cytosolic ATP:ADP ratio and ATP-linked mitochondrial respiration in AASS-KD vs control cells. Metabolomics analysis indicated altered glutamate metabolism and mitochondrial TCA cycle activity, and a Warburg effect.
Conclusion: Our data suggest that AASS-mediated lysine degradation is an active metabolic pathway required to maintain normal glutamate production, mitochondrial TCA cycle and OXPHOS function, and cellular calcium homeostasis, as well as insulin secretion. Reduced AASS expression with consequent decreased AASS-mediated L-lysine degradation pathway may contribute to β cell dysfunction in T2D.
Disclosure: L. Cataldo: None.
36
Quantification of the dynamics of Ins2 gene activity
J.C.M. Chu1, H. Modi1, S. Skovsø1, C. Ellis1, N.A.J. Krentz2, Y.B. Zhao1, H. Cen1, N. Noursadeghi1, E. Panzhinskiy1, Y. Xia1, S. Xuan3, M.O. Huising4, T.J. Kieffer1, F.C. Lynn2, J.D. Johnson2;
1Department of Cellular and Physiological Sciences, University of British Columbia, Vancouver, Canada, 2Department of Surgery, University of British Columbia, Vancouver, Canada, 3Department of Medicine Hematology and Oncology, Columbia University, New York City, USA, 4Department of Neurobiology, Physiology and Behavior, UC Davis, Davis, USA.
Background and aims: In pancreatic β-cells, the insulin gene has been shown to be expressed in a wide distribution, with examples of “extreme” β-cells exhibiting >2 fold higher insulin gene activity. Pseudo-time analyses of human single cell RNA sequencing data have suggested that cells may transition between high and low expression states of the INS gene. However, the mechanisms of these dynamics have yet to be elucidated. The the phenomenon of switching between low and high INS gene activity states has also yet to be observed in real-time.
Materials and methods: Ins2GFP knock-in mice, with the second exon of the wild-type Ins2 gene being replaced with GFP, were used for monitoring of endogenous insulin gene expression. We investigated the temporal kinetics of endogenous insulin gene activity using live-cell imaging, with complementary experiments employing FACS and single-cell RNA sequencing analyses in β-cells isolated from Ins2GFP knock-in mice. We applied inhibitors and activators of pathways related to insulin production, endoplasmic reticulum stress, and insulin secretion to determine how perturbations to β-cell function may impact Ins2 gene behavior.
Results: Live-cell imaging captured Ins2 gene activity dynamics in single β-cells over time and revealed two states in GFP expression (Ins2GFP(high), Ins2GFP(low)), consistent with bimodal expression states observed with our FACS analysis and immunofluorescence staining experiments. Autocorrelation analysis identified cells with oscillating behavior, with oscillation periods of ~17 hours. RNA velocity and hierarchical clustering analyses on single cell RNA sequencing data on cells from Ins2GFP mice revealed that β-cells with higher levels of Ins2 gene activity had a more mature β-cell profile, with genes such as Pdx1, Ucn3, and Nkx6-1 being significantly correlated with high GFP mRNA (p<0.01). Perturbation of insulin gene activity with inhibitors and activators of insulin production were shown to influence Ins2 gene activity, with some small molecules, including GLP-1, arginine, and sodium butyrate inducing transitions from Ins2GFP(low) states to Ins2GFP(high) states.
Conclusion: In conclusion, we identify a subset of pancreatic β-cells with dynamic Ins2 gene activity. Our observations define a previously uncharacterized form of β-cell plasticity in both basal and induced conditions. Understanding the dynamics of insulin production has relevance for understanding the pathobiology of diabetes and for regenerative therapy research.
Supported by: CIHR operating grant
Disclosure: J.C.M. Chu: None.
OP 07 Finding a phenotype for diabetic kidney disease
37
Genome-wide analysis of treatment-resistant hypertension in individuals with and without diabetes
A.A. Antikainen1,2, H. Sánez Tähtisalo2,3, S. Mutter1,2, R. Lithovius1,2, P.-H. Groop1,2, K. Kontula2,3, T. Hiltunen2,3, N. Sandholm1,2;
1Folkhälsan Research Center, 2Research Program for Clinical and Molecular Metabolism, Faculty of Medicine, University of Helsinki, 3Department of Medicine, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Hypertension is a major risk factor for CVD. Almost half of individuals with hypertension fail to reach blood pressure (BP) targets despite pharmacological treatments. A subset of them have treatment-resistant hypertension (RHTN), which is defined as BP above the treatment target with at least three antihypertensive drugs, of which one is a diuretic, or controlled BP with four or more drugs. RHTN increases the risk for CVD, especially in diabetes. RHTN genetics has not been excessively studied, thus, we aimed to find genomic loci linked to RHTN in type 1 diabetes (T1D) and in the general population.
Materials and methods: We performed genome-wide association studies for RHTN in T1D in the Finnish Diabetic Nephropathy Study (606 individuals, 288 with RHTN) and in the general population in the LIFE-Fin Study (625 individuals, 297 with RHTN). Analyses were adjusted for age, sex and genomic data principal components in the minimal model, and additionally for BMI, albuminuria, and eGFR in the full model. We meta-analysed the cohorts and attempted replication in a third Finnish cohort, GENRES (N≈220), where standardized mean BP response to four different antihypertensive monotherapies was used as an indicator of drug treatment responsiveness. Finally, we performed gene and gene-set analyses with MAGMA on the FUMA platform.
Results: We discovered two variants in the minimal model with near genome-wide significance: rs1484486 (minor allele frequency (MAF)=32%, odds ratio (OR)=0.54, p=7.76×10-8) within LOC105369168, and rs11151487 (MAF=14%, OR=0.47, p=5.36×10-7) within an intron of CCDC102B. In the full model, we found also rs61009649 (MAF=38%, OR=1.81, p=4.34 ×10-7) close to a pseudogene. We were unable to replicate these in GENRES. Of note, we discovered further suggestive loci of which one from the full model (rs3138242, MAF=10%, OR=0.44, p=9.26×10-6) replicated for systolic office BP response in GENRES (β=-0.21, p=0.03, N=226). The variant is located within an intron of DCN, coding for decorin, which plays an important role in connective tissues. Interestingly, the variant is an expression QTL of DCN in fibroblasts (https://gtexportal.org/). Gene-set scoring revealed significant biological pathways (e.g., the SLRP pathway, p=5.0×10-9), and enrichment of genes with expression in heart tissues (Figure 1).
Conclusion: We studied the genetics of RHTN in individuals with and without diabetes and identified multiple candidate loci, including a variant linked to expression of decorin, and performed gene scoring revealing a significant enrichment of genes with heart tissue expression stressing the link between RHTN and CVD.
Supported by: The Finnish Foundation for Cardiovascular Research
Disclosure: A.A. Antikainen: None.
38
DNA methylation is a risk factor for kidney failure in individuals with type 1 diabetes
A. Syreeni1,2, E.H. Dahlström1, L.J. Smyth3, Y. Gupta3, C. Forsblom1,2, J. Kilner3, G.J. McKay3, A. Maxwell3, A. McKnight3,2, P.-H. Groop1, N. Sandholm1,2;
1Folkhälsan Research Center, Helsinki, Finland, 2Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 3Molecular Epidemiology Research Group, Centre for Public Health, Queen’s University Belfast, Belfast, UK.
Background and aims: We previously identified DNA methylation differences at multiple CpG loci in a cross-sectional study of individuals with or without diabetic kidney disease (DKD). Here, we aimed to study DNA methylation as a risk factor for the development of kidney failure in individuals with type 1 diabetes and DKD.
Materials and methods: The study included 397 individuals with type 1 diabetes and macroalbuminuria at baseline from the Finnish Diabetic Nephropathy (FinnDiane) Study. At baseline, the mean (SD) age was 43 (±10.8) years, and 38% were women. Macroalbuminuria (>200 μg/min or >300 mg/24h) was determined from two of three overnight or 24 h urine collections. The study participants were followed up until either kidney failure developed or December 31, 2017. Data on kidney failure requiring dialysis and/or a transplant was collected from the Finnish Care Register for Health Care, study visits, or medical files. Genome-wide blood-derived DNA methylation data was generated for the Infinium HD Methylation EPIC BeadChips (Illumina) in Belfast. After quality control, we extracted M-values (M = log2(β / (1-β)) for 763,064 CpG sites using RnBeads v.2.6.0. M-values for each CpG site were analysed separately with the Cox proportional-hazards model with sex, baseline age, and six estimated white blood cell counts as covariates.
Results: During a median of 7.2 (interquartile range: 2.9-14.0) years of follow-up, 196 individuals developed kidney failure. Eleven CpGs were associated with developing kidney failure with p < 6.6×10-8 - a p-value threshold corrected for the number of studied CpGs. The top CpG cg17944885 is located on chromosome 19 between genes ZNF788P and ZNF625-ZNF20. Higher methylation at this locus was a risk factor for kidney failure (HR [95%CI] = 2.32 [1.95, 2.76], p = 1.4×10-21). Seven significant CpGs were located in or near genes; cg23597162 in JAZF1, cg12272104 in DAZAP1, cg21871803 in AHCYL2, cg12065228 in PQLC2, cg26236214 in ARHGEF7, cg19942083 in the promoter of PTPN6, and cg03262246 <1500 bp from the transcription start site of CDKN2AIPNL. In an independent cohort look-up from Belfast of kidney failure vs controls with no evidence of kidney disease (n = 519), 10 of these CpGs were significantly associated (p < 10-8). Additionally, cg17944885 was strongly associated with DKD in our cross-sectional meta-analysis of the FinnDiane and Belfast cohorts (tot n = 1,304, p = 2.0×10-44). In a cohort of 473 individuals with diabetes from the Chronic Renal Insufficiency cohort, six of eleven significant CpGs were associated with eGFR in the whole blood DNA methylation analysis (3.7×10-13 ≤ p ≤ 0.05). Furthermore, all six of our eleven top CpGs that were available in the epigenome-wide meta-analysis for eGFR in 33,605 individuals from the Chronic Kidney Disease Genetics Consortium were significantly (p < 1.1×10-7; cg17944885 and CpGs in JAZF1 and PQLC2) or nominally (p < 0.05; CpGs in or near DAZAP1, AHCYL2, and PTPN6) associated with eGFR in their study.
Conclusion: DNA methylation at several CpGs show consistent associations with kidney function and the risk of developing kidney failure.
Supported by: NIH (1R01DK105154-01A1) GENIE II
Disclosure: A. Syreeni: None.
39
The genetic background predicts the type of renal lesions and the progression of fibrosis in patients with type 2 diabetes
P. Pontrelli, C. Cinefra, F. Conserva, M. Fiume, A. Gallone, F. Pesce, F. Giorgino, L. Gesualdo;
University of Bari Aldo Moro, Bari, Italy.
Background and aims: Diabetic Nephropathy (DN) is the major causes of end-stage renal failure worldwide. The occurrence of either real DN or non-diabetic renal disease (NDRD) in diabetic patients explains why kidney biopsy remains the gold standard for accurate diagnosis and treatment. Thus, there is immediate need for non-invasive biomarkers to discriminate DN and NDRD, and/or to predict DN onset. Our group has largely demonstrated that only those patients featuring specific DN lesions show an accumulation of Lys63-ubiquitinated proteins at tubular level. The aim of this project was to identify single nucleotide polymorphisms (SNPs) associated to genes involved in Lys63 ubiquitination, and asses whether these SNPs are able to predict the different type of renal damage as well as the progression of kidney disease in type 2 diabetic patients.
Materials and methods: We selected 10 HapMap SNPs within coding and regulatory sequences of both miR27b-3p, miR1228-3p and Ube2V1 gene, all involved in Lys63-ubiquitination, in order to evaluate their diagnostic and prognostic value. 201 patients were enrolled in this study, in particular: diabetic patients with a biopsy-proven diagnosis of DN (DN), diabetic patients with a biopsy-proven diagnosis of other nephropathy in the absence of DN (NDRD), diabetic patients without clinical signs of impaired renal function (T2D), diabetic patients with a biopsy-proven coexistence of both conditions (ND+NDRD), non-diabetic patients with glomerulonephritis (CKD) and non-diabetic patients without renal damage (CTRL). For each patient we analyzed: i) 10 selected HapMap SNPs using TaqMan Real-Time PCR; ii) the glomerular and tubulointerstitial fibrosis at the kidney level, quantified following Sirius Red staining of kidney biopsies using the Aperio ImageScope slide scanner; iii) relevant clinical parameters at the time of renal biopsy and at the follow-up.
Results: The analyzed SNPs showed a different genotype frequency among all the patients classes. Interestingly, SNPs rs4759275, rs4759277, rs4744422, rs3802456 showed a statistically significant difference in genotypes frequency comparing DN patients with CEU Population (p<0.04, 0.05, 0.002, 0.001 respectively) and a control cohort enclosing CTRL and T2D (p<0.02, 0.05, 0.001, 0.04 respectively). SNPs rs761214, rs10761364, rs2306692 genotypes frequency was statistically different among DN patients and the control cohort (p<0.001). The genotype frequencies of the SNPs rs10761364 (p<0.01) and rs7853195 (p<0.04) were significantly related to tubular fibrosis in DN patients, while the SNPs rs4744422 (p<0.03) and rs761214 (p<0.02) to the glomerular one. In order to evaluate the diagnostic power of the identified SNPs, we used a logistic regression model, and we observed that, when adjusted for age, sex, eGFR and glycaemic index, SNP rs10761364, discriminates DN from NDRD (p<0.05; OR=1.002-1.008; 95% CI).
Conclusion: Our data demonstrated that the allelic forms of the analyzed SNPs are linked to different renal lesions in diabetic patients. These results could provide the starting point for the creation of a new non-invasive diagnostic system based on clinical and genetic data.
Supported by: BeatDKD IMI2 Project
Disclosure: P. Pontrelli: None.
40
Loss of the transcription factor Tcf21 in adult podocytes leads to susceptibility in diabetic kidney disease
N. Teramoto1, Y. Maezawa1, T. Minamizuka1, M. Koshizaka1, Y. Endo2, Y. Akimoto3, K. Yokote1;
1Department of Endocrinology, Hematology and Gerontology, Chiba University Graduate School of Medicine, Inohana, Chuo-ku,Chiba, 2Kazusa DNA Research Institute Laboratory of Medical Omics Research, Kazusakamatari, Kisarazu, Chiba, 3Department of Microscopic Anatomy, Kyorin University School of Medicine, Mitaka city, Tokyo, Japan.
Background and aims: Diabetic kidney disease (DKD) is the most common cause of dialysis induction in Europe. Tcf21/Pod1 is a basic helix-loop-helix transcription factor that is essential for the development of the lungs, heart, and kidneys. Previously, we reported that Tcf21 regulates podocyte development. However, the precise role of Tcf21 in mature individuals and DKD remains unclear.
Materials and methods: We generated mice that lacked Tcf21 only in podocytes after birth (iKO) using a doxycycline induction system. We also constructed a diabetes mouse model using streptozotocin injections. In addition, we used cultured human podocytes to investigate the effects of Tcf21 overexpression and the factors that regulate Tcf21 expression.
Results: When the Tcf21 gene was deleted specifically in podocytes in 3-week-old mice, urinary protein was not observed. In contrast, when diabetes was induced in these mice, massive urinary protein and strong glomerulosclerosis was present in 60% of iKO mice. Ultrastructural analysis revealed glomerular basement membrane thickening and podocyte foot process effacement, suggesting that acquired podocyte-specific Tcf21 KO mice are susceptible to DKD. In addition, RNA-seq analysis of Tcf21-overexpressing podocytes showed accumulation of genes involved in the interferon pathway, extracellular matrix production, senescence, and autophagy in gene ontology analysis. Analysis of individual genes showed upregulation of interferon-related genes such as IFI6 and IFI27, increased expression of SERPINE1 (PAI-1), which is one of the senescence-associated secreted phenotypes, and decreased expression of HMGA2, whose SNIP is known to correlate with susceptibility to DKD. In addition, Tcf21 expression was suppressed by 58% and 27% after TGF-β and high glucose plus insulin stimulation, respectively.
Conclusion: Our results suggest that Tcf21 protects against DKD in mature individuals. Elucidation of the mechanism by which Tcf21 prevents DKD may lead to the identification of novel therapeutic targets.
Disclosure: N. Teramoto: None.
41
Empagliflozin attenuates obesity-related kidney dysfunction and NLRP3 inflammasome activity through the HO-1/adiponectin axis
T. Ye, J. Zhang, J. Shi, C. Kan, F. Han, N. Hou, X. Sun;
Department of Endocrinology and Metabolism, Affiliated Hospital of Weifang Medical University, Weifang, China.
Background and aims: Empagliflozin (EMPA) is a novel sodium-glucose cotransporter 2 inhibitor (SGLT2i) that produces protective cardiovascular-renal outcomes in patients with diabetes. The heme oxygenase-1 (HO-1)/adiponectin axis is an essential antioxidant system with anti-apoptotic and anti-inflammatory properties. This study explored whether EMPA improves obesity-related kidney disease by regulating the renal HO-1-mediated adiponectin axis.
Materials and methods: Four-week male C57BL/6J mice were randomly assigned to control, high-fat diet (HFD) and EMPA (10 mg/kg) groups. Mice in the control group were fed a regular diet, while mice in the other groups were fed an HFD. After receiving an HFD for 24 weeks, mice in the EMPA group were administered EMPA (10 mg/kg/day) by oral gavage for another 8 weeks. Blood biochemical and urinary albumin-to-creatinine ratios (UACR) were measured. The morphology and microstructure of the kidney were analyzed by histopathology and transmission electron microscopy. RNA-seq analysis of differential gene expression in kidneys was performed. Renal NLRP3 inflammasome with related cytokines and HO-1/adiponectin were determined.
Results: HFD mice showed significant metabolic abnormality and renal injury, including increased body weight, fat mass, urinary albumin excretion, morphologic changes, and lipid accumulation. EMPA treatment significantly decreased the final body weight (44.87 ± 1.42 g vs. 49.67 ± 1.48 g, P<0.05), fat mass (11.75 ± 0.78 g vs. 14.56 ± 0.43 g, P<0.05), and fat/weight (26.08 ± 1.09% vs. 29.37 ± 0.75%, P<0.05), compared with HFD alone. Besides, EMPA significantly improved glucose hemostasis, decreased FFA (746.30 ± 56.59 μmol/L vs. 1303.00 ± 81.14 μmol/L, P<0.05) but had no beneficial effects on triglyceride (29.31 ± 2.71 mg/dL vs. 32.87 ± 1.69 mg/dL, P>0.05). Furthermore, EMPA decreased UACR (21.01±1.99 μg/μmol vs. 45.24±4.71 μg/μmol, P<0.05), attenuated kidney injury, including reduced glomerular hypertrophy, renal fibrosis, mitochondria swell, and lipid accumulation. RNA-seq analysis showed that the differentially expressed genes shared in EMPA vs. HFD and HFD vs. control were enriched in GO and KEGG categories associated inflammation process. HFD mice showed increased renal NLRP3 activity and reduced HO-1/adiponectin axis, indicating excessive inflammation. However, EMPA significantly enhanced renal HO-1/adiponectin axis and decreased NLRP3 activity, recovering the anti-inflammation.
Conclusion: EMPA treatment improved metabolic disorders and protected against obesity-related kidney disease by activating the HO-1/adiponectin axis and reducing NLRP3 inflammasome activity. Kidney transcriptome analysis revealed that EMPA affects essential genes closely associated with inflammation. Our findings provide new knowledge concerning the mechanism for SGLT2i-mediated renal protection in obesity.
Supported by: NSFC (81870593, 82170865)
Disclosure: T. Ye: None.
42
Identification of markers for predicting the onset of chronic kidney disease in older people with type 2 diabetes by metabolomic profiling: Edinburgh Type 2 Diabetes Study
J. Krasauskaite1, B.R. Conway2, C.J. Weir1, Z. Huang1, J.F. Price1;
1Usher Institute, University Of Edinburgh, 2The Queen's Medical Research Institute, University Of Edinburgh, Edinburgh, UK.
Background and aims: Renal disease affects a large proportion of people with type 2 diabetes and it is associated with excess morbidity/ mortality. While well-established clinical biomarkers, namely estimated glomerular filtration rate (eGFR) and albuminuria are used in routine screening, these markers do not explain all of the risk. Hence, the search for new markers is a high priority. Metabolomics may reveal novel markers of chronic kidney disease (CKD) that could aid identification of patients at higher risk of renal impairment and improve risk prediction of incident CKD. We aimed to identify significant associations between metabolites and the clinically relevant outcome of incident CKD in a Scottish population of older people with type 2 diabetes and to evaluate the ability of metabolites to predict CKD onset.
Materials and methods: The Edinburgh type 2 diabetes Study (ET2DS) is a population-based cohort of 1,058 adults (49% female) with type 2 diabetes, aged 60-75 years. Nightingale metabolomic platform was used to measure 149 serum metabolite concentrations at baseline. Kidney function was determined by eGFR, calculated using the CKD-EPI equation. Incident CKD was defined as 2 of 3 eGFR records <60mL/min/1.73 m2 during follow-up. An initial multivariable-adjusted discovery screen considered the correlation between each metabolite and baseline eGFR (adjusted for age and sex). Metabolites that were significantly associated with eGFR were then related to incident CKD events in logistic regression analysis adjusted for known clinical risk factors. Risk prediction analysis involved refitting a published risk prediction model for incident CKD to evaluate the complementary value of significant metabolites.
Results: There were 823 participants in ET2DS with no CKD based on eGFR records at baseline and 217 (26%) experienced new onset CKD during follow-up (median= 6.8 years [IQR 0.9- 7.6]). Corrected for multiple testing, 68 metabolites were significantly associated with baseline eGRF (Bonferroni corrected p<0.00034). Of these, only amino acid phenylalanine (Phe) was significantly associated with incident CKD after adjustment for known clinical risk factors (OR 0.73 [95% CI 0.60- 0.89], p=0.002). Phe was added to the published risk prediction model containing the clinical variables (eGFR, age, sex, BMI, use of diabetes medications, cardiovascular disease history, smoking, hypertension, HbA1c, albumin-to-creatinine ratio). Phe remained significant in this model and higher levels of Phe reduced risk of CKD onset (HR 0.80, [95% CI 0.68-0.93] per unit of SD, p-value= 0.004). However, Phe yielded only a small improvement in risk prediction (original model concordance (c )-statistic 0.81 [95% CI 0.79- 0.84], model +Phe c-statistic 0.82 [95% CI 0.79-0.84].
Conclusion: Amino acid Phe was associated with incident CKD in people with type 2 diabetes, although, it did not improve an already well performing risk prediction model. It is possible to hypothesise that a more sophisticated multivariable analysis may reveal a combination of metabolites associated with CKD onset that together may improve the risk prediction.
Supported by: MRC
Disclosure: J. Krasauskaite: None.
OP 08 Cardiovascular disease mechanisms: something new on the table?
43
C-reactive protein, C-peptide, and risk of cardiovascual events and mortality after type 2 diabetes diagnosis: a Danish cohort study
A.D. Kjaergaard1,2, A. Gedebjerg1,3, M. Bjerre3,2, J. Nielsen4, J. Rungby5, I. Brandslund6, M. Maeng7, H. Beck-Nielsen4, A.A. Vaag8, H.T. Sørensen1,9, T.K. Hansen2,9, R.W. Thomsen1,9;
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, 2Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, 3Clinical Medicine, Aarhus University, Aarhus, 4Steno Diabetes Center Odense, Odense University Hospital, Odense, 5Endocrinology, Bispebjerg, Copenhagen University Hospital, Copenhagen, 6Department of Clinical Biochemistry, Lillebaelt Hospital, Vejle, 7Department of Cardiology, Aarhus University Hospital, Aarhus, 8Steno Diabetes Center Copenhagen, Copenhagen University Hospital, Copenhagen, 9Clinical Medicine, Aarhus University, Aarhus N, Denmark.
Background and aims: We investigated the relationship between high-sensitivity C-reactive protein (hsCRP, a marker of low-grade inflammation), alone or in combination with C-peptide (a marker of insulin resistance), and risk of cardiovascular events (CVEs) and mortality in patients with recent-onset type 2 diabetes (T2D) and no hospital history of CVEs.
Materials and methods: We measured serum hsCRP in 7,301 patients and C-peptide in 5,765 patients with recent-onset T2D and followed them for a first CVE, including myocardial infarction, stroke, coronary revascularization, and cardiovascular death, and death from any cause.
Results: High (>3 mg/L) versus low (<1 mg/L) hsCRP was associated with an increased CVE risk during a median follow-up of 4.8 years (adjusted hazard ratio: 1.45 [95% confidence interval: 1.08-1.96]), and with strongly increased all-cause mortality (2.49 [1.90-3.27]), mainly driven by cancer mortality. Compared to patients with low levels of both hsCRP (≤3 mg/L) and C-peptide (<1470 pmol/L), those with high levels of both biomarkers had highest risks of CVE (1.62 [1.11-2.36]) and all-cause mortality (2.42 [1.77-3.29]). The risk of CVE increased more with high C-peptide alone (1.54 [1.09-2.18]) than high hsCRP alone (1.37 [1.00-1.88]). In contrast, the risk of all-cause mortality increased much more with high hsCRP alone (1.90 [1.46-2.49]) than with high C-peptide alone (1.15 [0.82-1.61]).
Conclusion: In a contemporary cardiovascular prevention setting, elevated hsCRP is a much weaker predictor of future CVE than of all-cause mortality in patients with early T2D. C-peptide is a more accurate predictor of CVE risk than hsCRP, emphasizing the importance of targeting insulin resistance for prevention of CVE.
Supported by: Novo Nordisk Foundation
Disclosure: A.D. Kjaergaard: None.
44
Mechanistic insights into the effects of empagliflozin in patients with type 2 diabetes and heart failure
A. Elrakaybi1,2, K. Laubner1, Q. Zhou3,4, G. Päth1, H. Schmitt3, M.J. Hug5, J. Seufert1;
1Division of Endocrinology and Diabetology, Department of Medicine II, Medical Center – University of Freiburg, Freiburg, Germany, 2Department of Clinical Pharmacy, Ain Shams University, Cairo, Egypt, 3Department of Cardiology and Angiology I, Heart Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 4Department of Cardiology, University Hospital Basel, Basel, Switzerland, 5Pharmacy, Medical Center – University of Freiburg, Freiburg, Germany.
Background and aims: Sodium- glucose co-transporter (SGLT) 2 inhibitors were the first antidiabetic drugs to demonstrate remarkable reductions in CV mortality and hospitalisation for heart failure (HF) in patients with and without diabetes. However, the exact mechanisms are still under debate. We aimed to investigate the effect of empagliflozin on certain plasma biomarkers and potential cardiac remodelling parameters, while also determining their possible associations in diabetic patients with HF. In-vitro experiments aimed to evaluate potential anti-inflammatory effects of empagliflozin.
Materials and methods: Adult patients with type 2 diabetes (T2D) and HF with either left ventricular ejection fraction (LVEF) ≤ 45% (EFFORT-1) or LVEF > 45% (EFFORT-2) were recruited. The patients received either 25 mg empagliflozin or placebo and were followed up for 48 weeks. Endothelin-1, galectin-3, insulin-like growth factor binding protein (IGFBP)-7 and kidney injury molecule (KIM)-1 were measured using ELISA at baseline and after 2, 12, 24 and 48 weeks of randomization. Left ventricular end- diastolic diameter (LVEDD) and posterior wall diameter were evaluated at baseline, week 12, 24 and 48. Change from baseline and the corresponding differences between treatment groups were determined with a mixed effects repeated measure analysis using treatment, visit and treatment-by-visit interaction as fixed effects and baseline value as a covariate. The means and differences between log-transformed data were back transformed to original scale as ratios. Pearson or Spearman correlation coefficients were calculated. The expression of inflammatory proteins was measured in HUVECs after high glucose and TNF-α stimulation ± empagliflozin.
Results: 63 patients were recruited, 24 in EFFORT-1 and 39 in EFFORT-2. Empagliflozin significantly reduced KIM-1 levels by 38% at week 48 in EFFORT-2 compared to placebo (95% CI; -57%, -13%), with no significant differences observed in EFFORT-1. Empagliflozin improved cardiac remodelling parameters via reduction of LVEDD at all time points in EFFORT-1, reaching significance only at week 24 with difference -7.88 (95% CI; -12.2, -3.61) from placebo, whereas borderline non-significant differences were shown at week 48 [-4.40 (95% CI; -8.84, 0.042)]. In EFFORT-2, empagliflozin significantly reduced posterior wall diameter at week 48 [-2.29 (95% CI; -3.60, -0.99)]. No clinically meaningful correlations were observed between the plasma biomarkers and any of the measured parameters. In HUVECs, empagliflozin did not significantly impact the expression of inflammatory markers.
Conclusion: Empagliflozin demonstrated as shown by a decrease in the biomarker KIM-1 a renal tubular protective effect in HF patients, while it contributed to the reduction of adverse cardiac remodelling. These CV benefits of empagliflozin can most likely not be explained by anti-inflammatory actions.
Supported by: This trial was funded through an IIT Grant by Boehringer Ingelheim. A. E. was supported by DAAD
Disclosure: A. Elrakaybi: Grants; This trial was funded through an IIT Grant by Boehringer Ingelheim. A. E. was supported by the German Academic Exchange Service (DAAD) – German Egyptian Research Long-Term Scholarship (GERLS) Program.
45
Effect of semaglutide on MACE by baseline kidney function in participants with type 2 diabetes and high risk of cardiovascular disease: SUSTAIN 6 and PIONEER 6 post hoc analysis
P. Rossing1, S. Bain2, H. Bosch-Traberg3, O. Frenkel3, H.L. Heerspink4, S. Rasmussen3, L. Mellbin5;
1Steno Diabetes Center Copenhagen, Gentofte, Denmark, 2Swansea University Medical School, Swansea, UK, 3Novo Nordisk A/S, Søborg, Denmark, 4University Medical Center Groningen, Groningen, Netherlands, 5Karolinska Institutet, Stockholm, Sweden.
Background and aims: People with type 2 diabetes (T2D) are at increased risk of cardiovascular (CV) disease and chronic kidney disease (CKD). As shown in a previous post hoc analysis, semaglutide (pooled s.c. once-weekly [OW] and oral once-daily) reduces the risk of major adverse CV events (MACE) vs placebo. The current post hoc analysis investigated the association between baseline kidney function and risk of MACE (composite of CV death, nonfatal myocardial infarction and nonfatal stroke), and the effect of semaglutide on risk of MACE by baseline kidney function.
Materials and methods: Participants with T2D and at high CV risk (N=6,480) receiving semaglutide (s.c. OW 0.5 or 1.0 mg or oral 14 mg) or placebo in SUSTAIN 6 and PIONEER 6 were categorised according to baseline kidney parameters: eGFR <45 (CKD stage 3b or worse), ≥45-<60 (CKD stage 3a) and ≥60 mL/min/1.73 m2 (CKD stage 1 or 2) and urine albumin-to-creatinine ratio (UACR) <30, ≥30-≤300 and >300 mg/g. eGFR subgroup analyses used pooled SUSTAIN 6 and PIONEER 6 data; UACR subgroup analyses used SUSTAIN 6 data only (no PIONEER 6 data available). MACE risk by baseline kidney function was analysed with a Cox proportional hazards model (reference groups: eGFR ≥60 mL/min/1.73 m2 and UACR <30 mg/g). The effect of semaglutide on MACE across kidney function subgroups was assessed with unadjusted and adjusted (for important baseline predictors of CV and renal diseases) analyses.
Results: Most participants included in the SUSTAIN 6 and PIONEER 6 trials had normal or mildly decreased kidney function (eGFR <45, ≥45-<60 and ≥60 mL/min/1.73 m2; n=731, n=968 and n=4,762, respectively) and were normoalbuminuric (UACR <30, ≥30-≤300 and >300 mg/g; n=1,934, n=884 and n=420, respectively). Regardless of treatment, MACE risk was higher in participants with eGFR <45 mL/min/1.73 m2 (HR 1.52, 95% CI [1.15;1.99], p=0.0026) and ≥45-<60 mL/min/1.73 m2 (1.36, [1.04;1.76], p=0.022) vs those with ≥60 mL/min/1.73 m2 at baseline. Similarly, MACE risk was higher in participants with UACR ≥30-≤300 mg/g (HR 1.53, 95% CI [1.14;2.04], p=0.0043) and >300 mg/g (2.52, [1.84;3.42], p<0.0001) vs those with <30 mg/g at baseline. Semaglutide reduced the risk of MACE consistently across baseline kidney function subgroups in both the unadjusted and adjusted analyses vs placebo (pinteraction >0.05 for all analyses; Figure).
Conclusion: The risk of MACE was greater for participants with impaired kidney function than in those with normal kidney function. Semaglutide showed consistent reductions in MACE risk across eGFR and UACR subgroups. These findings indicate that semaglutide provides CV benefits in people with T2D and high CV risk across a broad spectrum of kidney function and damage.
Clinical Trial Registration Number: NCT01720446; NCT02692716
Supported by: Novo Nordisk A/S
Disclosure: P. Rossing: Employment/Consultancy; Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Vifor, Sanofi Aventis. Grants; AstraZeneca, Novo Nordisk. Lecture/other fees; Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi Aventis.
46
Overexpression of miR-210 attenuates endothelial dysfunction in a mouse model of obesity and type 2 diabetes
A. Collado1, T. Jiao1, G. Zaccagnini2, J. Yang1, M. Carlström3, F. Martelli2, J. Pernow1,4, Z. Zhou1;
1Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden, 2Laboratory of Molecular Cardiology, IRCCS Policlinico San Donato, Milan, Italy, 3Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 4Karolinska University Hospital, Stockholm, Sweden.
Background and aims: MicroRNA (miR)-210 plays a protective role in many cardiometabolic diseases, and its levels are decreased in whole blood, erythrocytes, and plasma in type 2 diabetes (T2D). We recently demonstrated that miR-210 levels are lower in carotid artery plaques from patients with T2D compared to non-diabetic patients. However, the role of miR-210 in the modulation of endothelial function is not fully understood. We test the hypothesis that overexpression of miR-210 has therapeutic potential by reversing endothelial dysfunction in mice with Western diet (WD)-induced obesity and T2D.
Materials and methods: Eight weeks old miR-210 transgenic mice were treated with a WD for 12 weeks. During the last 10 days of the diet regime, doxycycline or vehicle dissolved in drinking water was given to switch on miR-210 (miR-210/on, n=7) expression or keep it off (miR-210/off, n=7), respectively. Age-matched wild-type (WT, n=7) control mice received standard chow within the same period. At the end of the treatment, body weight and glucose levels were measured. Aortic segments were isolated, and endothelial function was determined by acetylcholine-induced endothelium-dependent relaxation (EDR) using wire myographs. The expression of the miR-210 target protein tyrosine phosphatase 1B (PTP1B) and the levels of the oxidative stress marker 4-hydroxynonenal (4-HNE) were quantified by immunohistochemistry. All animal experiments and procedures were performed according to the guidelines by the U.S National Institutes of Health (NIH publication no 85-23, revised 1996). Differences in concentration-dependent relaxations were analyzed using two-way ANOVA with repeated measures. Multiple comparisons between groups were performed with one-way ANOVA followed by the Bonferroni post hoc test. Data are shown as mean ± SD.
Results: miR-210/off mice fed with a WD had a significant increase in body weight (BW; 39.0±5.7 g miR-210/off vs. 25.5±2.2 g WT, p<0.001) and glucose levels (10.0±1.2 mM miR-210/off vs. 7.6±0.4 mM WT, p<0.01). A similar increase was found in miR-210/on mice (BW: 34.3±6.4 g, p=0.05 vs. WT; glucose: 9.8±1.9 mM, p<0.05 vs. WT). EDR in aortas from miR-210/off mice fed with WD was significantly impaired compared to vessels from WT fed with regular chow. Notably, EDR was markedly improved in aortas of miR-210/on mice fed with WD. Furthermore, the expression of PTP1B and the levels of 4-HNE were significantly elevated in the aortas from miR-210/off mice fed with WD when compared to WT (~4.3 fold, p<0.001 and 6 fold, p<0.01 increase of PTP1B and 4-HNE vs. WT, respectively). The expression was attenuated in aortas of miR-210/on mice fed with WD compared to miR-210/off mice (~2.2 fold, p<0.01 and 3 fold, p<0.05 decrease of PTP1B and 4-HNE vs. miR-210/off mice, respectively).
Conclusion: Overexpression of miR-210 ameliorates endothelial dysfunction in mice fed with WD without affecting BW or glucose levels. Increasing miR-210 levels may become a therapeutic strategy for T2D to attenuate cardiovascular dysfunction.
Supported by: the Hjärt-Lungfonden (20190341 and 20200326 to ZZ; 20190266 to JP, and 20210431 to MC).
Disclosure: A. Collado: None.
47
Inhibition of microRNA-181c rescues diabetes-impaired angiogenesis through activation of key angiogenesis mediators
E.L. Solly1,2, J. Mulangala3, B.A. Di Bartolo4, S.J. Nicholls5, P.J. Psaltis1,2, C.A. Bursill1,2, J.T.M. Tan1,2;
1Vascular Research Centre, Lifelong Health Theme, South Australian Health & Medical Research Institute, Adelaide, 2Adelaide Medical School, The University of Adelaide, Adelaide, 3Vascular Biology Program, Centenary Institute of Cancer and Inflammation, The University of Sydney, Sydney, 4Faculty of Medicine and Health, The University of Sydney, Sydney, 5Monash Cardiovascular Research Centre, Monash University, Melbourne, Australia.
Background and aims: Diabetic vascular complications are characterized by impaired angiogenic responses to ischemia. Many patients remain refractory to current therapies, highlighting the need to identify novel therapeutic targets. We recently identified an anti-angiogenic role for miRNA-181c. However, it’s role in diabetes-impaired angiogenesis was unknown. This study aimed to elucidate the role of miRNA-181c in diabetes-impaired angiogenesis.
Materials and methods: Human coronary artery endothelial cells were transfected with a miRNA-181c inhibitor (antimiR-181c) or negative control (antimiR-Neg), exposed to glucose (5mM or 25mM, 48h) then subjected to Matrigel tubulogenesis assay or Boyden Chamber migration assay. Protein levels of angiogenic mediators (VEGFA) and activation of angiogenesis pathways (p-ERK2, p-eNOS, p-p38 MAPK) was determined by Western Blot. In vivo, we assessed the effect of miR-181c inhibition on diabetes-impaired angiogenesis using murine models of hindlimb ischemia and wound healing. Hindlimb blood-flow reperfusion was measured longitudinally by Laser Doppler imaging and gene expression was assessed 3-days post-ischemic induction when angiogenesis is important. Wound area was calculated daily. Neovascularization was assessed by CD31 (capillaries) and smooth muscle α-actin (arterioles) immunostaining in hindlimbs and wounds.
Results: Inhibition of miRNA-181c increased tubule formation (antimiR-181c: 99.9±7.1 vs. antimiR-Neg: 74.7±3.9, n=11, P<0.01) and cellular migration (antimiR-181c: 115.7±23.4 vs. antimiR-Neg: 37.9±7.1, n=6, P<0.05) in high glucose. Mechanistically, this was associated with increased VEGFA levels (antimiR-181c: 112.8±7.9 vs. antimiR-Neg: 90.8±5.8, n=17, P<0.05) and activated ERK2 signalling (antimiR-181c: 113.9±11.9 vs. antimiR-Neg: 79.9±8.9, n=12, P<0.05) in high glucose. In diabetic mice, inhibition of miRNA-181c increased blood flow reperfusion to the ischemic hindlimb, compared to diabetic control mice (Diabetic antimiR-181c: 0.432±0.04 vs. Diabetic antimiR-Neg: 0.282±0.03, n=11, P<0.001), returning it back to non-diabetic levels (0.373±0.04). This was associated with improved early induction of the pro-angiogenic mediator Erk2 (Diabetic antimiR-181c: 93.8±10.1 vs. Diabetic antimiR-Neg: 60.9±9.3, n=8, P<0.05) and an increase in hindlimb arteriolar density (Diabetic antimiR-181c: 133.6±23.8% vs. Diabetic antimiR-Neg: 72.8±10.3%, n=11, P<0.05). Inhibition of miRNA-181c significantly increased the rate of wound closure (Diabetic antimiR-181c: 76.8±3.6% vs. Diabetic antimiR-Neg: 59.7±4.3%, n=11, P<0.01) and increased the number of CD31 wound neovessels (Diabetic antimiR-181c: 142.5±39.2% vs. Diabetic antimiR-Neg: 54.3±13.3%, n=11, P<0.05) in diabetic mice.
Conclusion: Inhibition of miRNA-181c rescues diabetes-impaired angiogenesis. This presents miRNA-181c as a novel therapeutic target for the prevention of diabetic vascular complications.I
Supported by: THRF, Diabetes Australia
Disclosure: E.L. Solly: None.
48
Association between microangiopathic complications and cardiac structure and function in asymptomatic patients with type 2 diabetes
M. Nguyen, S. Pinto, P. Poignard, P. Valensi;
Jean Verdier hospital - APHP, Bondy, France.
Background and aims: Diabetic retinopathy has been associated with an increased risk of cardiac events including heart failure and with echocardiographic alterations. The pathophysiology of diabetic cardiomyopathy is complex and plurifactorial. The present study aimed to examine the relationship between microangiopathic complications and these alterations in patients with type 2 diabetes (T2D).
Materials and methods: We included 699 patients (male/female 54%/46%) with T2D, free of cardiac history and symptom but with other cardio-vascular risk factors. They were separated in 4 groups according to the number (from 0 to 3) of microangiopathic complications among retinopathy, nephropathy and peripheral neuropathy (G0 to G3). An echocardiography was performed, with measurement of structural and functional parameters. Silent coronary disease was assessed by performing a stress myocardial scintigraphy to detect silent myocardial ischemia (SMI), and a coronary angiography in the patients with SMI. NT-proBNP was measured in 243 of them.
Results: A higher number of microangiopathic complications was associated with male gender, age, diabetes duration, hypertension and SMI (p<0.005 for all comparisons). Left ventricle systolic dysfunction (ejection fraction <50%), dilatation, hypokinesia and hypertrophy were detected in 3.9%, 8.4%, 7.6% and 34.1% of the population, respectively. The prevalence of hypokinesia and hypertrophy increased from G0 to G3 (p=0.02 and 0.03), as well as interventricular septal and posterior wall thickness and NT-proBNP levels (p<0.0001 for all comparisons). Multivariate analyses showed that hypertrophy, septal thickness and NT-proBNP were significantly associated with the number of microangiopathic complications, independently from gender, diabetes duration, hypertension and SMI.
Conclusion: The association between the number of microangiopathic complications and these left ventricle structural and functional alterations, independent from major potential confounding factors, particularly silent coronary disease, stands for a microvascular contribution to the development of diabetic cardiomyopathy.
Disclosure: M. Nguyen: None.
OP 09 Fighting diabetes with tubes, scanners, and catheters
49
Advances in diabetes management: has pregnancy glycaemic control in women with type 1 diabetes changed in the last decades?
F. Citro1, F. Nicolì1, M. Aragona2, L. Battini3, C. Bianchi2, S. Del Prato1, A. Bertolotto2;
1Department of Clinical and Experimental Medicine, University of Pisa, 2Department of Medicine, University Hospital of Pisa, 3Maternal-Infant Department, University Hospital of Pisa, Pisa, Italy.
Background and aims: Over the recent years, multiple therapeutic and management opportunities have been made available to treat pregnant women with type 1 diabetes (T1DM). However, analysis assessing whether these different approaches may have any specific advantage/disadvantage in metabolic control and outcomes is still limited.
Materials and methods: We performed a retrospective analysis on pregnant women with T1DM, managed between 2008 and 2020 at the Pisa University Hospital, to analyze metabolic data according to types of basal insulin (NPH, detemir or glargine), insulin administration ways [Multiple Daily Injections (MDI) or Continuous Subcutaneous Insulin Infusion (CSII)] and glucose monitoring systems [Self-Monitoring of Blood Glucose (SMBG) or Continuous/Flash Glucose Monitoring (CGM/FGM)] that were adopted during pregnancy.
Results: We identified 136 T1DM women (age: 32 [IQR 30-35] years old; preconception HbA1c: 58.1±11.6 mmol/mol). Of them, 103 (76%) were on MDI based on NPH (n: 53, 51%), detemir (n: 35, 34%) or glargine (n: 15, 15 %). The remaining 33 women (24%) were on CSII. A CGM/FGM system was used in 33 (24%) women (20 (19%) on MDI and 13 (39%) on CSII). HbA1c, fasting plasma glucose, lipid profile and weight gain at baseline and during pregnancy were comparable among women treated with different basal insulins. Pregnancy planning was more common in women on CSII (94% vs. 60%, p=0.001) and, as compared to women on MDI treatment, they had better pregestational HbA1c (54±5.4 vs. 60±13 mmol/mol; p=0.044), first trimester fasting plasma glucose (103±38 vs. 140 ± 59 mg/dL, p=0.004), lower pregnancy weight gain (10.7±4.0 vs. 13.8±6.2 kg, p=0.018) and lower pre-prandial insulin dose at first (0.25±0.09 vs. 0.38 ± 0.18 UI/kg, p= 0.002), second (0.30±0.11 vs. 0.43±0.20 UI/kg, p=0.003) and third (0.42±0.20 vs. 0.54±0.24 UI/kg; p=0.017) trimester. Women using CGM/FGM had significantly lower pregestational (54.1±8.0 vs. 59.9±13.1 mmol/mol; p=0.041) and first trimester (46.5±5.5 vs. 51.2±7.1 mmol/mol, p=0.034) HbA1c levels than those on SMBG. Mode of delivery (vaginal or caesarean section) and neonatal outcomes (birth weight, macrosomia, Large for Gestational Age (LGA), Small for Gestational Age (SGA), preterm birth, Apgar score at 5’, congenital malformations) were comparable in all groups. At logistic regression analysis, in the whole group, third trimester HbA1c level was associated with LGA risk [OR=2.596 (1.408-4.787)].
Conclusion: Treatment with NPH or long-acting insulin analogs didn’t significantly change pregnancy metabolic data of women with T1DM, although CSII and CGM/FGM can optimize preconception and first trimester pregnancy glycemic control. Nonetheless, irrespective of the therapeutic management, third trimester HbA1c remains the strongest risk factor for LGA.
Disclosure: F. Citro: None.
50
Fully automated closed-loop insulin delivery vs standard insulin therapy in adults with type 2 diabetes: an open-label, single-centre randomised crossover trial
C.K. Boughton1, A.B. Daly1, M. Nwokolo1, S. Hartnell2, M.E. Wilinska1, A. Cezar1, M.L. Evans1, R. Hovorka1;
1Box 289, University of Cambridge, 2Wolfson Diabetes and Endocrine Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Background and aims: Despite advances in oral and injectable therapies for type 2 diabetes (T2D), many adults with T2D requiring insulin do not reach the recommended target HbA1c levels and there is a clear need for novel therapeutic approaches. We evaluated the safety and efficacy of fully closed-loop insulin delivery, not requiring meal bolusing, compared with standard insulin therapy in adults with T2D.
Materials and methods: In an open-label, single-centre, randomised, crossover study, 26 adults with T2D (7 females, mean±SD age 59±11y, diabetes duration 18±8y, baseline HbA1c 75±15 mmol/mol [9.0±1.4%], BMI 35.3±8.6kg/m2) underwent two 8-week periods of unrestricted living comparing CamAPS HX fully closed-loop using Fiasp (CL), with standard insulin therapy and a masked continuous glucose monitor (control) in random order. There was a 2-4 week washout between intervention periods. Primary endpoint was time spent in target glucose range (3.9 to 10.0 mmol/L).
Results: The proportion of time spent in target glucose range was mean±SD 66.3±14.9% with CL vs. 32.3±24.7% with control (mean difference 35.3 percentage points [95%CI 28.0, 42.6]; P<0.001). The proportion of time spent above target glucose range (>10.0 mmol/L) was 33.2±14.8% with CL vs. 67.0±25.2% with control (mean difference 35.2 percentage points [95%CI -42.8, -27.5]; P<0.001). Mean glucose was lower during CL use (9.2±1.2 mmol/L vs. 12.6±3.0 mmol/L; mean difference 3.6 mmol/L [95%CI 2.5, 4.6]; P<0.001). There was a reduction in HbA1c at the end of the CL period at 57±9 mmol/mol [7.3±0.8%] compared to 72±13 mmol/mol [8.7±1.2%] at the end of the control period (mean-adjusted difference 15mmol/mol [95%CI 11, 20]; 1.4% [95%CI 1.0, 1.8]; P<0.001). The proportion of time in hypoglycaemia (<3.9 mmol/L) was low and similar between treatment periods (median [IQR] 0.43% [0.20, 0.77] in the CL period vs. 0.08% [0.00, 1.05] in the control period; P=0.751). There were no episodes of severe hypoglycaemia in either group.
Conclusion: Fully closed-loop insulin delivery improved glucose control without increasing the risk of hypoglycaemia compared to standard insulin therapy in adults with T2D. The treatment may represent a safe and effective method of achieving glycaemic target in this group.
Clinical Trial Registration Nubmer: NCT04701424
Supported by: The study was supported by the National Institute for Health Research Cambridge Biomedical Research
Disclosure: C.K. Boughton: None.
51
Important decrease of hospitalisations for acute diabetes events before and after FreeStyle Libre® system initiation in type 2 diabetes with basal insulin therapy in France
J.-P. Riveline1, F. Levrat-Guillen2, B. Detournay3, E. Vicaut4, G. De Pouvourville5, C. Emery3, B. Guerci6;
1Department of Endocrinology and Diabetology, Lariboisière Hospital, Assistance Publique - Hôpitaux de Paris, Paris, France, 2Abbott Diabetes Care, Maidenhead, UK, 3CEMKA, Bourg-La-Reine, France, 4Clinical Research Unit, Fernand Vidal Hospital, Assistance Publique - Hôpitaux de Paris, Paris, France, 5Department of Economics, ESSEC Business School, Cergy-Pontoise, France, 6Department of Endocrinology, Diabetology and Nutrition, Brabois Adult Hospital, Vandoeuvre-lès-Nancy, France.
Background and aims: Glycemic management in people with T2D (PwT2D) who are not on intensive insulin regimens is of considerable importance. Due to the progressive nature of T2D, initiation of insulin treatment may be necessary to achieve glycemic target. Initiation of basal-insulin therapy in PwT2DM is associated with serious diabetes-related acute events, such as severe hypoglycemia or diabetes ketoacidosis (DKA) that can be life-threatening. We assessed the impact of initiating FreeStyle Libre® glucose monitoring system (FSL) as compared to the usual self-glucose monitoring of glycemia on hospitalizations for acute diabetes events (ADEs) in PwT2D on basal insulin scheme +/- other hypoglycemiant agents.
Materials and methods: A retrospective study on the overall French national SNDS reimbursement claims database (≈66 million people) was conducted on all French PwT2D on basal insulin therapy receiving a first reimbursement of FreeStyle Libre between 01/08/2017 to 31/12/2018. The analysis looked at claims data for the 12 months before, and up to 24 months after FSL initiation. Hospitalizations for diabetes-related acute events were identified, using ICD-10 codes as main or related diagnosis: severe hypoglycemia (SH) events (E160, E161, E162 and T383); DKA events (ICD-10 codes E101 and E111), comas (ICD-10 codes E100, E110 and E140) and hyperglycemia related stays (ICD 10 code R739).
Results: We identified 5,933 PwT2D on a basal insulin therapy who initiated FSL during the selection period. Only 78.9% of patients were on both basal insulin and other hypoglycemiant agents, of which 40% were documented as receiving sulphonylurea. Amongst PwT2D on a basal insulin therapy, 2.01% experienced at least one hospitalization for any ADE in the year before FSL initiation compared to 0.75% (1 year after) and 0.60% (2 years after) (Fig. 1), and these results are similar independently of the sulfonylurea use or not. This reduction in ADEs was mainly driven by 75% fewer DKA admissions, with a 44% reduction in admissions for SH. These patterns of reduction in ADEs persisted after 2 years, with a further 43% reduction in DKA rates.
Conclusion: This sub-group analysis of our previous RELIEF study strongly suggests the value of the FSL system in a PwT2D population initially treated with basal insulin therapy in reducing diabetes related acute events and their long-term consequences.
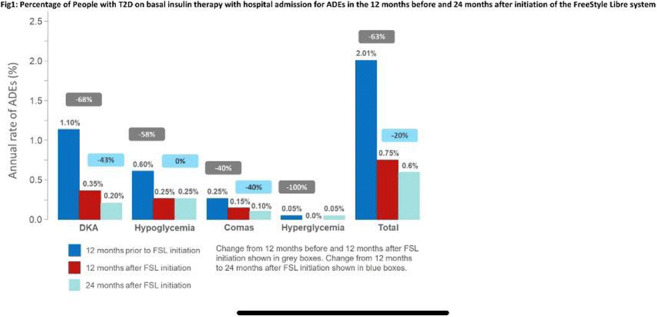
Supported by: Funded by Abbott Diabetes Care
Disclosure: J. Riveline: Honorarium; Abbott.
52
Accuracy of a real-time continuous glucose monitoring system during cardiac surgery with hypothermic extracorporeal circulation
D. Herzig1, M. Vettoretti2, D.P. Guensch3, A. Melmer1, A.C.K. Goerg1, A. Kadner4, A. Facchinetti2, A. Vogt3, L. Bally1;
1Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Bern University Hospital, University of Bern, Bern, Switzerland, 2Department of Information Engineering, University of Padova, Padova, Italy, 3Department of Anaesthesiology and Pain Medicine, Bern University Hospital, University of Bern, Bern, Switzerland, 4Department of Cardiac and Vascular Surgery, Bern University Hospital, University of Bern, Bern, Switzerland.
Background and aims: Frequent blood glucose testing is a key element of perioperative care. Continuous glucose monitoring (CGM) allows monitoring glucose levels in real-time and guide clinical decision making. Several studies have shown the feasibility of CGM in the inpatient setting with satisfactory performance. The accuracy of sensors, however, may be affected in extreme conditions that have not been well studied. Here we evaluated the accuracy of the Dexcom G6 CGM system during cardiac surgery using hypothermic extracorporeal circulatory circulation (ECC).
Materials and methods: Sixteen adults wore the DexcomG6 CGM sensor 22.8 [11.2, 79.4 hours] (median [min, max]) prior to cardiac surgery with hypothermic ECC until hospital discharge or a maximum of 10 days. A subset of 11 patient also underwent deep hypothermic cardiac arrest (DHCA). A calibration was performed with arterial blood at the time of anaesthesia induction using the Accu-Chek® Inform II meter. During surgery, blood for reference values was sampled every 20 minutes from the arterial line and the heart-lung machine during ECC/DHCA. Post-surgery reference values were obtained from capillary blood measurements using the Accu-Chek® Inform II meter. Core body temperature was monitored using an esophageal probe. The primary endpoint was the mean absolute relative difference (MARD) between sensor and reference method during surgery.
Results: Mean±SD surgery duration was 5.4±1.7 hours with body temperature decreasing to 25.2±4.1Co (range 20.9 to 32.0Co). Individual intraoperative reference glucose levels ranged between 5.5±1.7 and 16.1±2.6 mmol/l. MARD of 256 paired CGM/reference values was 23.8% during surgery. MARD was 29.1% during ECC (154 pairs) and 41.6% during DHCA (10 pairs), with a negative bias in all three periods (signed relative difference was -13.7%, -26.6% and -41.6, respectively). During surgery, a total of 86.3% pairs were in Clarke error grid Zones A or B (A, 51.6%) and 41.0% of intraoperative sensor readings were within the limits specified by the ISO 15197:2013 norm. Sensor readings were available for 91.8% [56.8%, 100.0%] of the intraoperative time. In the postoperative period (from end of surgery until hospital discharge), MARD was 15.0% (144 pairs).
Conclusion: Extreme conditions such as deep hypothermia in cardiac surgery challenge the performance of Dexcom G6 system. However, the accuracy of sensor recovered in most cases with adequate performance in the post-surgery period.
Supported by: Swiss Helmut Horten Foundation, Swiss Foundation of Anaesthesiology and Intensive Care, Dexcom
Disclosure: D. Herzig: None.
53
Evidence of significant reduction in pain and sensory symptoms of diabetic neuropathy with 10kHz spinal cord stimulation: 24-month RCT outcomes
E. Petersen1, T.G. Stauss2, J.A. Scowcroft3, J.L. White4, S.M. Sills5, K. Amirdelfan6, M.N. Guirguis7, J. Xu8, C. Yu9, A. Nairizi10, D. Patterson11, V. Galan12, R.S. Taylor13, D. Caraway14, N.A. Mekhail8;
1Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, USA, 2Advanced Pain Management, Greenfield, USA, 3Pain Management Associates, Lee's Summit, USA, 4Accelerated Enrolment Solutions, Orlando, USA, 5Touchstone Interventional Pain Center, Medford, USA, 6IPM Medical Group, Walnut Creek, USA, 7Ochsner Clinic Foundation, New Orleans, USA, 8Cleveland Clinic Foundation, Cleveland, USA, 9Swedish Pain & Headache Center, Seattle, USA, 10Reno Tahoe Pain Associates, Reno, USA, 11Nevada Advanced Pain Specialists, Reno, USA, 12Pain Care, Stockbridge, USA, 13University of Glasgow, Glasgow, UK, 14Nevro, Redwood City, USA.
Background and aims: Painful diabetic neuropathy (PDN) can lead to severe deterioration in quality of life, loss of function, and increased health care costs. A large randomized controlled study was undertaken to provide high level clinical evidence for the use of 10kHz spinal cord stimulation (SCS), which does not cause paresthesias like traditional SCS, for the treatment of PDN.
Materials and methods: Prospective, multicenter, RCT to document the impact of 10kHz SCS on PDN. Participants had PDN symptoms ≥12 months (M), refractory to medications, lower limb pain intensity ≥5cm (0-10cm visual analog scale [VAS]), and hemoglobin A1c ≤10%. Patients (N=216) were allocated 1:1 to 10kHz SCS (Nevro Corp.) plus conventional medical management (CMM) or CMM alone with optional crossover at 6 M.
Results: There were 90 patients implanted in the 10kHz arm after meeting 50% pain relief requirement in a temporary trial, and 84 (93%) remained in follow-up at 24 months. The 10kHz SCS patients maintained substantial pain relief from 3 M, averaging an 81.9% (95%CI 77.3 - 86.5) decrease at 24 M. At 6 M follow up, 0% of 10kHz SCS participants but 93% of eligible CMM patients elected to crossover. At 18 M follow-up after SCS, both groups (n=138) reported similar significant improvements in pain, sleep disturbance, and in pain interference with mood and daily activities (see Fig 1). In addition, the neurological assessment found a majority of patients treated with SCS experienced sensory improvements. In terms of health care utilization, hospitalizations were 38% less in the 10kHz group at 6 M. There were no stimulation-related neurological deficits and 6 total explants (3.9%), 5 due to procedure-related infections and 1 as a precaution for endocarditis.
Conclusion: The largest RCT to date of SCS management of PDN demonstrates safety, durable pain relief, and clinically meaningful improvement on neurological examination over 24 M with 10kHz SCS. These PDN patients with moderate to severe pain that was refractory to CMM achieved clinically important improvements in quality of life, and reduced health care utilization with a safe, reversible, minimally invasive procedure.
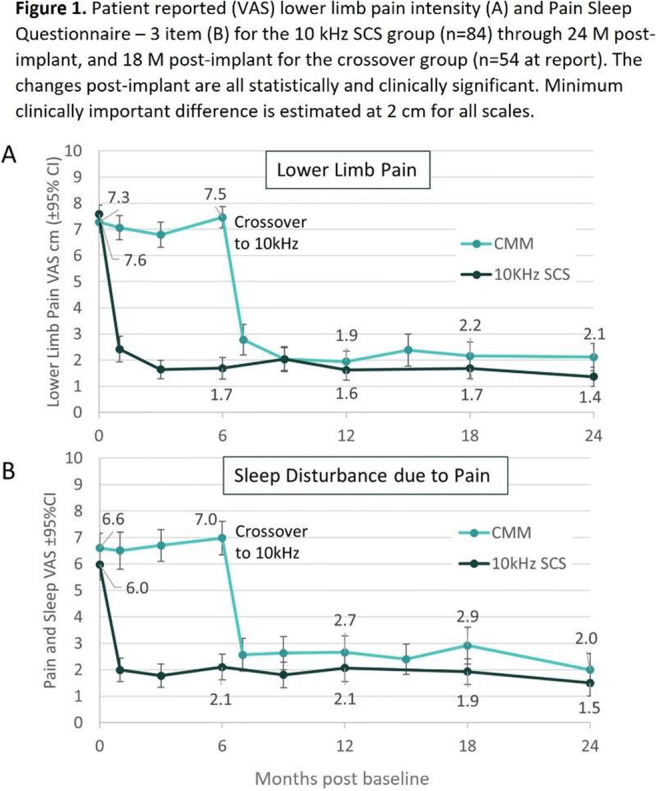
Clinical Trial Registration Number: NCT03228420
Supported by: Nevro
Disclosure: E. Petersen: Employment/Consultancy; Nevro, Medtronic Neuromodulation, Abbott Neuromodulation, Saluda.
54
Duodenal jejunal bypass liner (DJBL) treatment for type 2 diabetes and obesity: glycaemic and CVD risk factor improvements vs risks in patients treated worldwide
R.E.J. Ryder1, P. Sen Gupta2, T. Battelino3, P. Kotnik3, J. Teare4, A. Ruban4, H. Frydenberg5, L. Munro5, S. Fishman6, R. Cohen7, C. de Jonge8, J.-W. Greve9, H. Sourij10, K. Laubner11, J. Seufert11;
1City Hospital, Birmingham, UK, 2Guy's and St Thomas' Hospitals,, London, UK, 3University Medical Center, Ljubljana, Slovenia, 4Imperial College, London, UK, 5Epworth Hospital, Richmond, Australia, 6Sourasky Medical, Tel Aviv, Israel, 7Oswaldo Cruz Hospital, Sao Paulo, Brazil, 8Catharina Hospital, Eindhoven, Netherlands, 9Zuyderland MC, Heerlen, Netherlands, 10Medical University, Graz, Austria, 11Freiburg University, Freiburg, Germany.
Background and aims: There is uncertainty over the balance of benefits and risks of proximal intestinal exclusion with a temporarily inserted DJBL for treatment of type 2 diabetes and obesity. An online registry was established under the auspices of the Association of British Clinical Diabetologists (ABCD) in 2017.
Materials and methods: DJBL safety and efficacy data were entered into the ABCD registry from patients worldwide. To evaluate the glycaemic effectiveness of DJBL, we categorised patients into groups according to baseline HbA1c.
Results: As of March 2022, data had been submitted on 1022 patients (mean ± SD age 51.3 ± 11.4 years, 52.5% male, 84.9% type 2 diabetes, BMI 41.1 ± 8.7 kg/m2) from 34 centres in 10 countries (table). In those with both baseline and time of removal data, mean ± SD weight fell by 13.3 ± 9.7 kg from 120.2 ± 25.3 to 106.9 ± 23.8 kg (n=811, p<0.001), HbA1c by 13.7 ± 15.9 from 67.6 ± 19.8 to 54.0 ± 13.9 mmol/mol (by 1.3 ± 1.5, from 8.3 ± 1.8 to 7.1 ± 1.3 %) (n=646, p<0.001), systolic BP fell from 135.7 ± 18.0 to 129.5 ± 17.0 mmHg (n=448, <0.001) and cholesterol fell from 4.8 ± 1.3 to 4.2 ± 1.0 mmol/L (n=467, p<0.001). In those with baseline HbA1c ≥ 53 mmol/mol, HbA1c fell by 17.0 ± 16.3 from 74.6 ± 16.3 to 57.6 ± 12.9 mmol/mol (n = 506, p<0.001); with baseline HbA1c ≥ 64 mmol/mol, HbA1c fell by 20.7 ± 16.9 from 80.8 ± 15.0 to 60.1 ± 13.4 mmol/mol (n=365, p<0.001); with baseline HbA1c ≥ 75 mmol/mol , HbA1c fell by 27.0 ± 18.0 from 90.0 ± 13.9 to 63.0 ± 14.4 mmol/mol (n=207, p<0.001); with baseline HbA1c ≥ 86 mmol/mol , HbA1c fell by 34.9 ± 18.1 from 99.1 ± 13.2 to 64.1 ± 15.9 mmol/mol (n=111, p<0.001). There were 43 (4.2%) serious adverse events (SAE) and 139 (13.6%) less serious SAEs (table). All SAE patients made a full recovery.
Conclusion: The data demonstrate that, in response to DJBL, the higher the initial HbA1c, the greater its fall, with HbA1c fall 27.0 mmol/mol (2.5 %) when initial HbA1c ≥ 75 mmol/mol (≥ 9.0 %) and 34.9 mmol/mol (3.2 %) when initial HbA1c ≥ 86 mmol/mol (≥ 10.0 %). In view of impact on microvascular and macrovascular risk factors, the benefits of DJBL therapy could potentially reduce complications of diabetes. The SAE rate was not insignificant but acceptable. As all patients made a full recovery and many experienced significant benefit despite the SAE, this registry data from a large patient number suggests that the benefits of DJBL outweigh the risks. With monitoring during the time of DJBL and prompt removal if indicated, this treatment is a useful option.
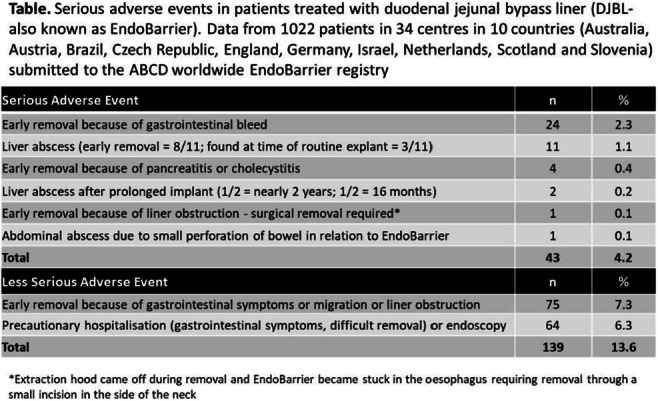
Supported by: ABCD
Disclosure: R.E.J. Ryder: None.
OP 10 Beta cells: protecting what is precious
55
Early effects of treatment with intralymphatic administration of rhGAD65 in LADA appear similar to those observed in type 1 diabetes
I. Hals1,2, A. Björklund3, C.N.D. Balasuriya2, R. Casas4, J. Ludvigsson4,5, V. Grill1;
1Dept of Clinical and Molecular Medicine, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2Clinic of Medicine, St Olavs hospital, Trondheim University Hospital, Trondheim, Norway, 3Dept. of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden, 4Dept of Biomedical and Clinical Sciences, Linköping university, Linköping, Sweden, 5Crown Princess Victoria Children's Hospital, University Hospital, Linköping, Sweden.
Background and aims: We perform an investigator-initiated open-labelled 12 months pilot study in which treatment with intralymphatic administration of the antigen specific immunotherapy Diamyd® (rhGAD65) is tested for the first time in patients with LADA. We aim to evaluate safety, immune response and insulin secretion after intralymphatic treatment with rhGAD65 in LADA.
Materials and methods: Fourteen LADA individuals, women/men: 7/7, with high anti-GAD titers (>190 RU/ml) and not on insulin therapy received 3 intralymphatic injections of 4 μg rhGAD65, one month apart. Safety parameters, immune responses and insulin secretion capacity were summarized in an interim analysis 5 months after baseline.
Results: Baseline values (mean ± SD) were: fasting C-peptide 0.65 ± 0.36 nmol/L, HbA1c 43 ± 7 mmol/mol and BMI 26.5 ± 5.2 kg/m2, diabetes duration 5.9 ± 3.8 months, age 47 ± 8 years. Half the study population was HLA-DR3DQ2 positive. Interim analysis at 5 months from baseline showed no treatment-related serious adverse events. There was a clear GAD-specific immune response both in terms of anti-GAD and T-cell reactions. At 2 and 5 months from baseline, median (Q1, Q3) GAD65 stimulation index of PBMC proliferation was 2.16 (1.25, 3.06) and 2.34 (1.81, 3.58) respectively, p< 0.01 for increase from baseline (corresponding findings in type 1 diabetes (n=56): 2.77 (1.96, 6.05) and 3.19 (2.02, 6.19), p< 0.0001). At 5 months from baseline, supernatant concentrations of cytokines from GAD65-stimulated secretion by PBMCs were increased for interleukin (IL) IL-1 beta, IL-4, IL-5, IL-6, IL-10, IL-13 and IL-17, tumor necrosis factor (TNF) alpha, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1 and interferon (IFN) gamma, p< 0.05 or less for difference. Insulin secretion assessed from meal stimulation tests showed a modest decline in terms of postprandial 2 hour levels of C-peptide: at baseline median (Q1, Q3) levels were 1.95 (1.35, 2.30) nmol/L and after 5 months 1.45 (1.25, 1.85) nmol/L, p< 0.025 for difference. Parameters of glucagon stimulation tests were not affected.
Conclusion: Intralymphatic treatment with rhGAD65 in LADA appears safe. It induces important immune responses that are similar to those observed after intralymphatic rhGAD65 in type 1 diabetes.
Clinical Trial Registration Number: NCT04262479
Supported by: Liaison committee between Central Norway RHA and NTNU
Disclosure: I. Hals: None.
56
Loss of beta cell Scn9a Na+ channel activity is protective in the context of type 1 diabetes while suppressing glucose stimulated insulin secretion
P. Overby, S. Provenzano, G. Sun, N. Nahirney, J. Kolic, S. Skovsø, J.D. Johnson;
Department of Cellular and Physiological Sciences, The University of British Columbia, Vancouver, Canada.
Background and aims: Type 1 diabetes is caused by β-cell death resulting in insulin insufficiency. Somewhat paradoxically, the recent clinical success of verapamil provides rationale for identifying protective therapeutic targets that suppress insulin secretion by limiting excitotoxicity. We previously used high-throughput screening to identify the FDA-approved Na+ channel inhibitor carbamazepine as a drug that protects mouse β-cells from multiple stressors. Follow-up studies demonstrated a decrease in spontaneous diabetes incidence in non-obese diabetic (NOD) mice treated with carbamazepine. Here, we aimed to characterize the effects of carbamazepine and related drugs on human islet survival and function, and to investigate the specific role of Scn9a (Nav 1.7), which is the most highly expressed voltage-depended Na+ channel gene in β-cells and the likely cellular target of carbamazepine.
Materials and methods: Human islet cell death in the presence of carbamazepine and its analogues (e.g. oxcarbazepine), structurally unrelated Nav1.7 inhibitors (e.g. ProTx-II), and a Nav1.7 agonist (e.g. veratridine) were investigated using high-content imaging. The dynamics of glucose-stimulated insulin secretion from human and mouse islets were assessed by perifusion. The specific role of Scn9a in β-cell function and type 1 diabetes was assessed in ‘floxed' NOD mice crossed with the Ins1Cre knockin allele or injected at 7 weeks with adeno-associated virus (AAV8) containing Cre under the control of an Ins1 promoter.
Results: Carbamazepine and oxcarbazepine protected human β-cells from cytokine- and ER stress-induced death, while veratridine increased cell death under these conditions. Glucose-stimulated insulin secretion was moderately reduced in perifused human islets in combination with carbamazepine (p=0.0044), while veratridine increased insulin secretion (p=0.035). We observed a significant reduction in glucose stimulated insulin secretion between NOD:Scn9afl/fl;Ins1Cre knockout mice and their wildtype littermate NOD:Scn9awt/wt;Ins1Cre controls (p<0.0001). NOD:Scn9afl/fl injected with AAV8-Ins1Cre showed reduced type 1 diabetes incidence relative to injected NOD:Scn9awt/wt controls.
Conclusion: Collectively, our studies to date point to Scn9a as a potential pre-clinical drug target to protect β-cells from excitotoxicity in type 1 diabetes.
Supported by: JDRF
Disclosure: P. Overby: None.
57
Tirzepatide improves multiple aspects of beta cell function
K. Mather1, A. Mari2, J. Li1, S. Urva1, T. Heise3, J. DeVries4, E. Pratt1, R. Heine1, M. Thomas1, Z. Milicevic1;
1Eli Lilly and Company, Indianapolis, USA, 2National Research Council Institute, Rome, Italy, 3Profil Institute for Clinical Research, Neuss, Germany, 4Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany.
Background and aims: In this study we investigated the effect of tirzepatide (TZP) 15 mg (novel GIP-GLP-1 dual receptor agonist), placebo and semaglutide 1 mg (SEMA) on beta-cell function.
Materials and methods: In a 28-week double-blind randomised controlled trial in people with type 2 diabetes the effect of TZP 15 mg (GIP-GLP-1 dual receptor agonist), placebo and SEMA 1 mg on beta-cell function was analysed using euglycaemic and hyperglycaemic glucose clamps (EGC, HGC) and Mixed Meal Tolerance Tests (MMTT).
Results: (Table): During EGC and HGC, TZP improved insulin sensitivity (M-value), first and second phase insulin secretion (ISR) and beta-cell glucose sensitivity (ratio of ISR and glucose increments) more than SEMA and placebo. During MMTT, both fasting and AUC glucose were reduced more with TZP than with SEMA or placebo, while incremental glucose AUC was reduced similarly with TZP and SEMA vs placebo. Despite a similar glucose response the total and incremental insulin AUC were reduced by TZP but not by SEMA. Model-derived beta-cell glucose sensitivity was similarly increased with TZP and SEMA from baseline. In contrast, ISR at 7.2 mmol/L glucose increased more with TZP than with placebo and SEMA.
Conclusion: The glucose-lowering effect of TZP is mediated through improvements in multiple aspects of beta-cell function, including beta-cell glucose sensitivity and ISR, and in insulin sensitivity, resulting in pronounced lowering of fasting and postprandial glucose. These effects help explain the superior improvement in glucose control seen with TZP vs comparators in clinical trials.
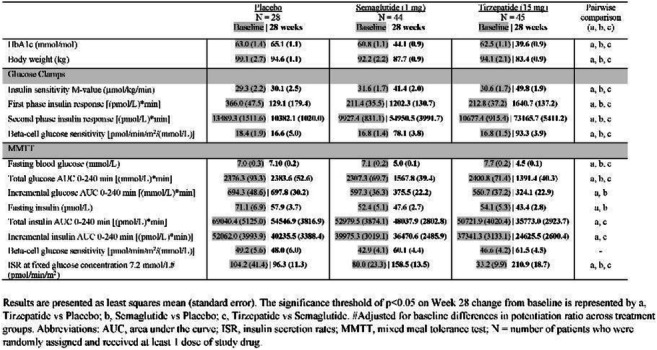
Clinical Trial Registration Number: NCT03882970
Disclosure: K. Mather: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
58
Shotgun proteomics unveils mechanisms behind metformin-induced protection against pro-inflammatory cytokine-induced human islet cell damage
M. Tesi1, L. Giusti2, F. Ciregia1, L. Marselli1, L. Zallocco1, M. Suleiman1, C. De Luca1, S. Del Guerra1, D.L. Eizirik3, M. Cnop3, M.R. Mazzoni1, P. Marchetti1, A. Lucacchini1, M. Ronci4;
1University of Pisa, Pisa, Italy, 2University of Camerino, Camerino, Italy, 3Université Libre de Bruxelles, Brussels, Belgium, 4University of Chieti-Pescara, Chieti, Italy.
Background and aims: Metformin (Met), a drug commonly used for the treatment of type 2 diabetes (T2D), has been shown to have direct protective actions on human β-cells exposed to gluco- and/or lipotoxic conditions and on islets from T2D donors. We presently assessed whether Met could relieve stress induced by pro-inflammatory cytokines in human β-cells and investigated the underlying mechanisms by shotgun proteomic analysis.
Materials and methods: Altogether, 14 human islet preparations were used. Islets were exposed to 50 U/ml interleukin-1β plus 1000 U/ml interferon-γ for 48 h, with or without 2.4 μg/ml Met, a therapeutical concentration of the drug. Glucose-stimulated insulin secretion studies, caspase 3/7 activity assay, shotgun label free proteomics and Western blots were performed.
Results: Met prevented the reduction of the insulin stimulation index (control islets, Ctrl: 5.4±1.7; cytokines: 3.4±1.1, p<0.05 vs Ctrl; cytokines plus Met: 4.7±1.6; mean±SEM) and the increase of caspase 3/7 activity (Ctrl: 1±1.1; cytokines: 1.5±1.1, p<0.001 vs Ctrl; cytokines plus Met: 1.2±0.1, p<0.01 vs cytokines and Ctrl) induced by cytokine exposure. Proteomics analysis identified 2,304±392 proteins. Cytokines significantly altered 244 proteins (145 up- and 99 downregulated), while in the presence of Met cytokines modified 231 proteins (128 up- and 103 downregulated). There were 212 differentially expressed proteins in common. Among the proteins regulated in opposite direction in the two conditions, were proteins involved in vesicle motility (e.g. transgelin, Ras-related protein Rab-14), defence from oxidative stress (e.g. peroxiredoxins, PRDX2 and PRDX5), metabolism (e.g. flavin reductase, mitochondrial ATP synthase subunit O), protein synthesis (e.g. 40S ribosomal proteins and eukaryotic translation initiation factor 4E), glycolysis and its regulation (e.g. triosephosphate isomerase and pyruvate kinase), cytoskeletal proteins and proteins interacting with the cytoskeleton (e.g. myosin light polypeptide 6, Ras-related protein Ral-A and coactosin-like protein). Met inhibited pathways linked to inflammation, immune reactions, mammalian target of rapamycin (mTOR) signaling and cell senescence. Some of the key changes were confirmed by Western blot.
Conclusion: Met prevents part of the deleterious actions of pro-inflammatory cytokines on human β-cells, which is accompanied by islet proteome modifications. These results suggest that Met, a widely used drug for the treatment of T2D, might be repurposed for other types of diabetes.
Supported by: INNODIA, INNODIA HARVEST, PRIN2017, CAPTUR3D
Disclosure: M. Tesi: None.
59
Irisin administration restores beta cell functional mass in a mouse model of type 2 diabetes
N. Marrano1, G. Biondi1, A. Borrelli1, M. Rella1, L. Roberto2, A. Cignarelli1, S. Perrini1, L. Laviola1, F. Giorgino1, A. Natalicchio1;
1Department of Emergency and Organ Transplantation, University of Bari Aldo Moro, Bari, 2Transgenic Mice Facility, Biogem S.c.a.r.l., Ariano Irpino (AV), Italy.
Background and aims: Irisin is a hormone secreted by skeletal muscle following physical activity or excess of saturated fatty acids (SFAs), able to improve metabolic homeostasis and promote energy expenditure. Serum irisin levels are reduced in type 2 diabetes (T2D), while exogenous irisin administration improves glycemic control in diabetic mice. We have previously demonstrated that irisin protects human and rodent beta-cells and pancreatic islets from SFAs-induced apoptosis, increases insulin byosinthesis and glucose-stimulated insulin secretion (GSIS), and promotes beta-cell proliferation, both in vitro and in vivo in healthy wild type mice. We have also demonstrated that irisin restores the defective GSIS and reduces the high rate of apoptosis characteristic of islets from T2D patients. The beta-cellular effects of irisin administration to T2D mice are still unknown.
Materials and methods: 6 weeks-old C57Bl/6 mice (n = 8) were fed a high-fat diet (HFD, 60% of energy deriving from fat) for 10 weeks and then intraperitoneally injected with streptozotocin (STZ, 100 mg/Kg) to induce diabetes. 4 standard diet (SD)-fed mice were used as control. HFD/STZ mice were treated with 0.5 μg/g irisin (n = 4) or vehicle (n = 4), for 14 days. Fasting glycemia, insulinemia, and body weight have been measured throughout the study. Glucose tolerance and pancreatic islets function have been assessed on the last day of the study through an intraperitoneal glucose tolerance test. Pancreatic islets architecture has been evaluated through immunofluorescence analyses.
Results: As expected, compared to SD mice, HFD/STZ mice showed higher glycemia and body weight, glucose intolerance, and reduced GSIS; in addition, HFD/STZ mice showed reduced islets volume (-78 %; p<0.001 vs SD), beta-cell area (-35 %; p<0.001 vs SD), and insulin content (-60 %; p<0.001 vs SD), while increased alpha-cell area (+54 %; p<0.01 vs SD). Irisin administration significantly restored glycemia (-31 %; p<0.05 vs HFD/STZ), body weight (-13 %; p<0.001 vs HFD/STZ), glucose tolerance (-27 % AUC; p<0.001 vs HFD/STZ), GSIS (+23 % AUC; p<0.005 vs HFD/STZ), islets volume (+61 %; p<0.01 vs HFD/STZ), beta-cell (+34 %; p<0.001 vs HFD/STZ) and alpha-cell (-49 %; p<0.01 vs HFD/STZ) area, and insulin content (+36 %; p<0.001 vs HFD/STZ). Of note, irisin induced a 9-fold increase in beta-cell proliferation rate (p<0.001 vs SD and HFD/STZ).
Conclusion: These results show for the first time that irisin restores beta-cell functional mass also when administered in vivo to diabetic mice, likely by promoting beta-cell proliferation. In conclusion, irisin could be considered a valid new anti-diabetes therapeutic strategy.
Supported by: “EU–ESF PON R&I 2014-20, AIM” and “Tecnopolo per la Medicina di Precisione CUP B84I18000540002”
Disclosure: N. Marrano: None.
60
Non-invasive evaluation of the preservation effect of imeglimin on pancreatic beta cell mass using 111In-labeled exendin-4 SPECT/CT imaging
M. Fauzi, T. Murakami, H. Fujimoto, A. Botagarova, K. Sakaki, S. Kiyobayashi, N. Inagaki;
Department of Diabetes, Endocrinology, and Nutrition, Kyoto University, Graduate School of Medicine, Kyoto, Japan.
Background and aims: Progressive loss of β-cell mass (BCM) is one of the hallmarks of type 2 diabetes. However, observing BCM requires an invasive method and can only provide cross-sectional data. Recently, a noninvasive approach for observing BCM on living subjects using indium 111-labeled exendin-4 derivative ([Lys12(111In-BnDTPA-Ahx)]exendin-4) (111In-Ex4) has been developed. Imeglimin, a novel antidiabetic agent, has been reported to increase insulin secretion by optimizing mitochondrial function, which lowers the blood glucose levels. However, the effect of imeglimin on BCM preservation has not been fully understood. The Leprdb/db (db/db) mouse is a diabetic mouse model with obesity, in which diminished mitochondrial number in pancreatic β-cells and rapidly decreased BCM were observed. Therefore, we investigated the effect of imeglimin on in-vivo BCM in db/db mice using 111In-Ex4 single-photon emission computed tomography/computed tomography (SPECT/CT) technique.
Materials and methods: Five-week-old male db/db mice were assigned to imeglimin- or vehicle-treated group. The drug was administered orally at a dose of 150 mg/kg B.I.D. for five weeks. 111In-Ex4 SPECT/CT scans were conducted to evaluate BCM changes longitudinally at 5 (baseline), 7, and 10 weeks of age. Oral glucose tolerance tests (OGTTs) were conducted with the same interval as the SPECT/CT scans. Following the longitudinal SPECT/CT scans, immunohistochemical analysis of BCM was conducted. Electron microscopy was also used for evaluating the mitochondrial structure of pancreatic β-cells. On islets harvested from 8-week-old db/db mice after 1-week imeglimin or vehicle treatment, mitochondrial membrane potentials were evaluated with JC-1 dye, a fluorescent lipophilic carbocyanine dye.
Results: Imeglimin-treated group demonstrated significantly lower random blood glucose levels compared to the vehicle-treated group during the age of 6-10 weeks (p < 0.05). Furthermore, OGTT results showed significantly better glucose tolerance at 10 weeks old (p < 0.05). Although the db/db mice in the vehicle-treated group showed progressive loss of RI intensities in the pancreas, based on SPECT/CT during 5-week treatment, the intensity loss was significantly attenuated in the imeglimin-treated group at 10 weeks (p < 0.01). This result was confirmed using the BCM calculation with immunohistochemistry (p < 0.05). In electron microscopy analysis, imeglimin-treated group showed significantly smaller numbers of mitochondria with abnormal structures, such as loss of proper cristae formation, compared with the vehicle-treated group (p < 0.01). This observation was in line with ex-vivo analysis of islet mitochondrial membrane potentials of db/db mice; islets of imeglimin-treated group displayed significantly higher red/green fluorescence intensity ratios (p < 0.05), which indicated functionally healthier mitochondria with preserved membrane potentials in the islet cells.
Conclusion: Imeglimin treatment on db/db mice demonstrated the preservation effect of BCM according to longitudinal observation with 111In-Ex4 SPECT/CT. This effect may be caused partly by preserved mitochondrial structures and mitochondrial membrane potentials of pancreatic β cells treated with imeglimin.
Disclosure: M. Fauzi: None.
OP 11 Adipose tissue profiling and cardio-metabolic risk
61
The effect of ovariectomy and estradiol substitution on metabolic parameters and transcriptomic profile of adipose tissue in a prediabetic model
I. Markova1, M. Huttl1, D. Miklankova1, L. Sedova2, O. Seda2, H. Malinska1;
1Centre for Experimental Medicine, Institute for Clinical & Experimental Medicine, 2Institute of Biology and Medical Genetics, the First Faculty of Medicine, Prague, Czech Republic.
Background and aims: The postmenopausal period leads to the development of abdominal obesity, insulin resistance, hepatic steatosis, and other lipid and carbohydrate metabolism disorders. Adipose tissue, its metabolic and endocrine activity, partially regulated by sex hormones, may play a significant role in the development of these metabolic changes. The pathogenesis of these disorders and the possible effect of hormonal substitution are not precisely known. The study aimed to analyse the effect of ovariectomy and estradiol substitution in a prediabetic model with insulin resistance - females of the hereditary hypertriglyceridemic rat strain (HHTg).
Materials and methods: HHTg females underwent bilateral ovariectomy (HHTg-OVX) or sham surgery (SHAM) at 8 weeks. Two weeks after surgery half of the HHTg-OVX rats were substituted with 17-β estradiol (OVX-E) at a dose of 12.5 μg/kg b.wt./day for 12 weeks subcutaneously.
Results: Ovariectomy in HHTg females was accompanied by weight gain, increased serum leptin levels, impaired glucose tolerance, and decreased adipose tissue insulin sensitivity (p<0.05). Serum concentrations of glucose, insulin, cholesterol, NEFA, adiponectin and inflammatory cytokines MCP-1 and hsCRP did not differ between groups. In contrast, ovariectomy resulted in significant ectopic deposition of triacylglycerols (TAG) in the liver (p<0.001) and kidneys (p<0.001), although serum TAG levels were reduced after ovariectomy. Estradiol substitution alleviated the development of only some of the metabolic disorders associated with ovariectomy; in particular, it improved insulin sensitivity and reduced ectopic TAG deposition (liver: -16%, p<0.01; kidneys: -23%, p<0.001). Transcriptomic analysis of visceral adipose tissue revealed 813 differentially expressed transcripts in HHTg-OVX vs. SHAM, mostly pertaining to the regulation of lipid and glucose metabolism, cell cycle and oxidative stress (AMPK, FoxO, PI3-Akt signaling pathways). Estradiol substitution affected 1059 transcripts with significant overrepresentation in the signaling pathways of lipid metabolism, especially steroid biosynthesis, regulation of lipolysis in adipocytes and glycerol lipid metabolism. The principal component and hierarchical clustering analyses of transcriptome shifts corroborated the metabolic data, showing higher similarity of transcript clusters between OVX-E and SHAM groups, both contrasting with HHTg-OVX. Still, there was a clear distinction between OVX-E and SHAM with regard to transcript clusters related to steroid biosynthesis, glycerolipid or glutathione metabolism.
Conclusion: Ovariectomy in HHTg females worsens adipose tissue insulin resistance and significantly potentiates ectopic lipid deposition. Changes in the visceral adipose tissue transcriptome, especially in pathways relevant to lipid metabolism, oxidative stress, and insulin signaling, may contribute to metabolic abnormalities. Estradiol substitution may partially alleviate some of these disorders.
Supported by: MH CZ No. NU20-01-00083 and IKEM, IN00023001
Disclosure: I. Markova: None.
62
Mir-15b mediates the obesity-induced adipocyte insulin resistance by targeting insulin receptor
L. Xingjing;
Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, Medical School, Southeast Univ, Nanjing, China.
Background and aims: In recent years, the morbidity of obesity has been increasing rapidly worldwide, which is a major risk factor for type 2 diabetes mellitus (T2DM). Obesity, mainly characterized by abnormal and excessive white adipose tissue accumulation, is the most common cause of insulin resistance (IR), where the insulin target tissues fail to respond normally to circulating insulin. However, the precise mechanism by which obesity affects insulin resistance in the major insulin sensitive tissues remains unclear. Adipose glucose uptake plays a significant role in systemic insulin sensitivity, therefore clarifying the regulatory factors of adipose insulin sensitivity is of great significance to find effective therapeutic targets of obesity. Obesity causes the increase of hepatic miR-15b, which provokes hepatocyte insulin resistance, but has no effect in skeletal muscle. The upregulation of miR-15b induced by obesity causally resulted in an impairment of hepatocyte insulin signaling and the decrease of the insulin receptor (INSR) expression. However, no studies have explored whether miR-15b is involved in adipose tissue insulin resistance induced by obesity so far. Aim: To study the role of miR-15b in the adipose tissue of DIO mice and IR 3T3-L1 adipocytes
Materials and methods: We fed mice with high-fat diet (HFD) for 10 weeks to construct obese and insulin-resistant (IR) mice models, and treated3T3-L1 adipocytes with chronic hyperinsulinemia to establish IR adipocyte models. Cell transfection was performed using riboFECTTMCP or Lipofectamine2000. Insulin stimulated fluorescence labeled 2-NBDG uptake assay was used to detect the capacity of glucose uptake in adipocytes. The expression levels of miR-15b, the insulin receptor (INSR) and its downstream insulin signaling molecules were detected by real-time PCR and Western blot respectively.
Results: We found that expression of miR-15b was increased, while INSR expression was downregulated in adipose tissue of diet-induced-obese (DIO) mice. In IR 3T3-L1 adipocytes, the expression of miR-15b also ascended, accompanied by the decrease of INSR expression. Bioinformatics analysis and luciferase reporter analysis suggested that INSR was a potential target of miR-15b. Overexpression of miR-15b led to decreased INSR expression and impaired insulin signal transduction in adipocytes, and inhibition of endogenous miR-15b can reverse the downregulation of INSR and insulin resistance induced by high insulin. In addition, when miR-15b was overexpressed, the simultaneous overexpression of INSR partially alleviated the insulin resistance in adipocytes.
Conclusion: These results suggested that the impaired insulin signaling in adipocytes caused by obesity was at least partially mediated by the downregulation of INSR induced by elevated miR-15b.
Supported by: No. 81570734
Disclosure: L. Xingjing: None.
63
Impact of rare heterozygous mutations of PCSK1 on obesity: implication for treatment with MC4R agonists
L. Folon1, M. Baron1, M. Derhourhi1, B. Balkau2, G. Charpentier3, S. Franc3, R. Roussel4, M. Canouil1, P. Froguel1,5, A. Bonnefond1,5;
1Inserm UMR1283, CNRS UMR8199, Lille, France, 2Inserm U1018, Villejuif, France, 3CERITD, Evry, France, 4Inserm U1138, Paris, France, 5Imperial College London, London, UK.
Background and aims: Pathogenic mutations in key genes involved in the leptin-melanocortin pathway (critical for the control of food intake) are well-established causes of monogenic forms of obesity. Recently, the melanocortin-4 receptor (MC4R) agonist setmelanotide has been developed to efficiently treat obese patients with homozygous mutations in POMC and LEPR. Its relevance to other forms of monogenic obesity is currently discussed. PCSK1 encodes the PC1/3 enzyme that is involved in this pathway. Bi-allelic pathogenic mutations in PCSK1 lead to early-onset obesity associated with severe endocrinopathy, but the clinical impact of heterozygous pathogenic PCSK1 mutations on obesity is still elusive. We performed large-scale functional genetic studies to clarify the link between heterozygous PCSK1 mutations and obesity.
Materials and methods: All 14 coding exons of PCSK1 were sequenced in 10,000 individuals (including obese and/or diabetic patients and controls) by next-generation sequencing. The detected heterozygous variants were created by mutagenesis and inserted into plasmids. Functional assays of each variant were performed in HEK293 cells: enzymatic activity was analysed using a fluorescent PC1/3 substrate and protein expression in cell lysates was studied by western blotting. Then, we determined different pathogenicity groups of these rare variants by clustering them through machine learning (k=5). Finally, we compared the pathogenicity of PCSK1 variants predicted by in silico REVEL (rare exome variant ensemble learner) test with results of in vitro analyses.
Results: We identified 66 rare heterozygous variants of PCSK1 (minor allele frequency < 1%). The variants were classified into 5 groups (A-E) based on their enzymatic activity, compared to the activity of the wild-type protein. The 17 variants in group A had complete loss of enzymatic activity whereas group B included 11 variants with partial loss of enzymatic activity. These loss of function variants were located mostly in catalytic domain but also in the prodomain and P domain of PC1/3. They strongly affect the structure of the protein preventing the expression of the mature active form of PC1/3. We found that class A variants increased the risk of developing obesity by 6-fold in both children (P=0.097; OR=6.0) and adults (P=0.038; OR=5.6). In contrast, class B variants were not associated with obesity risk (P=0.86; OR=1.1). Importantly, we observed a lack of sensibility and specificity of the largely used in silico tools predicting putative heterozygous pathogenic PCSK1 mutations. Indeed, the pathogenic variants predicted by REVEL showed 4 false negatives and 11 false positives compared to the results of in vitro functional assays.
Conclusion: Our results strongly suggest that only obese individuals carrying PCSK1 heterozygous mutations proven pathogenic through in vitro functional testing (i.e. category A) may be eligible for setmelanotide treatment, but not the carriers of mutations with only intermediate in vitro deleterious effect. Therefore, in vitro tests are required following the molecular diagnosis of rare heterozygous PCSK1 mutations before initiating expensive medication.
Disclosure: L. Folon: None.
64
Obesity specific N6-methyladenosine (m6A) RNA modification in human adipose tissue
T. Rønningen1, Y. Zeng2, M. Visnovska1, M.B. Dahl3, J. Wang3, L. la Cour Poulsen1, J.A. Kristinsson4, T. Mala4, J.K. Hertel5, J. Hjelmesæth5, T.G. Valderhaug6, H.H. He2, Y. Böttcher3;
1Department of Clinical Molecular Biology (EpiGen), Akershus University Hospital, Lørenskog, Norway, 2Princess Margaret Cancer Centre, Toronto, Canada, 3Department of Clinical Molecular Biology (EpiGen), University of Oslo, Oslo, Norway, 4Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway, 5Morbid Obesity Centre, Vestfold Hospital Trust, Tønsberg, Norway, 6Department of Endocrinology, Akershus University Hospital, Lørenskog, Norway.
Background and aims: Omental visceral adipose tissue (OVAT) has distinct gene expression and epigenetic profiles compared to subcutaneous adipose tissue (SAT), contributing to metabolic dysfunction. Post-transcriptional RNA modifications such as N6-methyladenosine (m6A) play important roles in regulating RNA metabolism. The FTO gene, whose genetic variants associate with obesity, encodes one of two known m6A demethylases, suggesting a mechanistic role for m6A in obesity. The aim of this study was (i) to map m6A signatures genome wide in paired samples of human adipose tissue and primary adipocytes, (ii) to identify obesity specific signatures and (iii) to decipher whether m6A discriminates for its depot of origin.
Materials and methods: RNA was extracted from intra-individually paired biopsies of SAT and OVAT from patients with obesity (BMI >35, n=10) and normal-weight controls (BMI <25, n=3). RNA was also obtained from primary adipocytes from 5 patients with obesity. M6A signatures were mapped using methylated RNA immunoprecipitation combined with next generation sequencing (meRIP-seq). meRIP-seq data were analyzed using established bioinformatics pipelines; peak calling was performed using MeTPeak and differential methylation analysis was performed using RADAR.
Results: We identified an average of ~6000 mRNA transcripts containing m6A in OVAT and SAT. Of those, we identified a number of genes (n~100) with differential m6A methylation in patients with obesity compared to normal weight controls. Further, by intersecting gene lists with m6A data from primary adipocytes, we also identified whether the specific m6A signature originates from adipocytes or cells from the stromal vascular fraction. Whilst most of the ~6000 methylated transcripts were conserved between SAT and OVAT, we identified a catalogue of genes (n=338) showing depot specific methylation.
Conclusion: We here present the first genome wide mapping of m6A in paired samples of human adipose tissue, identifying several novel targets linking m6A to obesity. Functional analyses of target genes will further elaborate on their role in obesity and metabolic dysfunction.
Supported by: South-Eastern Norway Regional Health Authority
Disclosure: T. Rønningen: None.
65
Waist-to-hip ratio is a stronger, more consistent predictor of all-cause mortality than BMI
I. Khan1, M. Chong2, A. Le2, P. Mohammadi-Shemirani2, R. Morton2, C. Brinza3, M. Kiflen4, S. Narula2, L. Akhabir2, S. Mao2, K. Morrison2, M. Pigeyre2, G. Paré2;
1College of Medicine and Health, University College Cork, Cork, Ireland, 2Pathology and Molecular Medicine, McMaster University, Hamilton, Canada, 3School of Medicine, Queen's University, Kingston, Canada, 4Temerty Faculty of Medicine, University of Toronto, Toronto, Canada.
Background and aims: Current clinical guidelines recommend a body mass index (BMI) between 18.5-24.9 kg/m2. However, BMI does not consider inter-individual variation in body composition and is thus not consistent in predicting risk of disease or mortality. We sought to determine whether current BMI recommendations are valid when accounting for body composition, and which of BMI, waist-to-hip ratio (WHR), and fat mass index (FMI) is the strongest and most consistent predictor of all-cause mortality across the range of body composition.
Materials and methods: We partitioned the British Caucasian UK Biobank (UKB) population (N= 387,672) into two subsets: a discovery cohort (N = 337,078) and validation cohort (N = 50,594). The discovery cohort was used to derive genetically-determined adiposity measures while the validation cohort was used for all subsequent analyses. Observational relationships between BMI, WHR, and FMI with mortality from all-cause, cancer, cardiovascular disease (CVD), respiratory disease, or non-CVD, - were analysed. Mendelian randomization (MR) was then used to assess causality of observed associations and to investigate effects across percentiles of BMI, WHR, and FMI.
Results: The study population consisted of 25,297 deaths and 25,297 randomly selected age- and sex-matched controls from the UKB (mean (SD) age: 61.6 (6.2), 59.3% male). Observational relationships between BMI and FMI with all-cause mortality were J-shaped, whereas the relationship between WHR with all-cause mortality increased linearly. Genetically-determined WHR had a stronger association with all-cause mortality compared to BMI or FMI, and exhibited a stronger effect in males compared to females (OR per SD of genetically-predicted WHR (95% CI): 1.51 (1.32 - 1.72); genetically-predicted BMI (95% CI): 1.29 (1.20 - 1.38); genetically-predicted FMI (95% CI): 1.26 (1.20 - 1.32), P for all analyses and comparisons < 0.05). Unlike BMI or FMI, the relationship between genetically-determined WHR and all-cause mortality was consistent across all percentiles of BMI, FMI, or WHR (P > 0.05).
Conclusion: In contrast to BMI, WHR has the strongest causal effect on risk of mortality regardless of the levels of adiposity and body composition, but show evidence of differential effects according to sex. Clinical recommendations and interventions should prioritize setting healthy WHR targets for males and females rather than general BMI targets. More precise recommendations for body shape may make a significant difference in disease burden and deaths due to excess of adiposity.
Supported by: OGS-M
Disclosure: I. Khan: None.
66
Single-cell RNA sequencing of human visceral adipose tissue identifies new macrophage clusters in health and obese type 2 diabetes
Y. Yuan, H. Wang, H. Sun, Y. Bi;
Department of Endocrinology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China.
Background and aims: Adipose tissue macrophages are closely associated with type 2 diabetes mellitus (T2DM). However, in the development of obese T2DM, the atlas of human visceral adipose tissue macrophages and its evolvement remain unrevealed. This study aims to decipher the composition of human visceral adipose tissue macrophages and investigate their roles in T2DM.
Materials and methods: Non-obese non-T2DM, obese non-T2DM and obese T2DM subjects were enrolled for single-cell RNA sequencing of the stromal vascular fraction (SVF) of visceral adipose tissue. The sequencing data was analyzed to identify and annotate macrophage clusters and functional analysis was performed. Weighted gene correlation network analysis (WGCNA) was performed to reveal the distinct genetic and functional changes of macrophage clusters in obesity and T2DM. Flow cytometry was employed to validate the macrophage clusters, and to evaluate their abilities of lipid-buffering and tumor necrosis factor-α (TNF-α) production.
Results: Four macrophage clusters were identified in human visceral adipose tissue, including 1) perivascular macrophages (PVMs) with potential angiogenic and anti-inflammatory capacities. 2) Phagocytic macrophages (PMs) with endocytic potentials. 3) Monocyte-derived macrophages (MMs) that could regulate inflammatory response and the activation of T cells; 4) Lipid-associated macrophages (LAMs) that harbored lipid metabolic potentials. PVMs were located adjacent to blood vessels, while the other three clusters infiltrated between adipocytes, with PMs forming crown-like structures (CLSs). Among the four macrophage clusters, PVMs, MMs and LAMs have been reported previously, while PMs were discovered and defined for the first time. Flow cytometric analysis revealed that the levels of PMs raised significantly in T2DM patients, and their levels positively correlated with fasting blood glucose and HbA1c. Further functional experiments suggested that PMs had the highest lipid-uptake capacities and intracellular lipid content among the four macrophage clusters, which increased significantly in obese non-T2DM subjects and dropped in obese T2DM subjects. In addition, PMs had the highest levels of TNF-α+ cells, which were significantly higher in obese non-T2DM and obese T2DM subjects as compared to non-obese non-T2DM subjects.
Conclusion: The study analyzed the atlas of human visceral adipose tissue macrophages in non-obese non-T2DM, obese non-T2DM and obese T2DM states for the first time. Four distinct macrophage clusters were identified, among which PMs were newly discovered and defined. Flow cytometric analysis revealed that PMs may be closely involved in the pathogenesis of T2DM. The results deepened the understanding of human visceral adipose tissue macrophages and laid foundations for the understanding of the role of adipose tissue macrophages in obesity and T2DM, as well as the development of novel therapeutical strategies targeting obese T2DM.
Supported by: NSFC, China
Disclosure: Y. Yuan: None.
OP 12 GWAS and more
67
Genetics of serum C-peptide in type 1 diabetes
A.D. Paterson1, D. Roshandel1, A. Spiliopoulou2, S. McGurnaghan2, S.B. Bull3, P.M. McKeigue2, H.M. Colhoun2;
1The Hospital for Sick Children, Toronto, Canada, 2University of Edinburgh, Edinburgh, UK, 3Lunenfeld-Tanenbaum Research Institute, Toronto, Canada.
Background and aims: Heritability of serum C-peptide (CP) in type 1 diabetes (T1D) is 26%. Two independent genome-wide association studies (GWAS) of CP in T1D have identified multiple loci in the HLA region, insulin gene (INS), and a locus on chromosome 1. Although some of these loci overlap with those for T1D, some are independent. However, the identified loci together account for only a small proportion of CP variation. Here, we performed a large meta-GWAS of CP, including studies from the two prior GWAS, to improve the statistical power and detect new loci. We also investigated the association of T1D genetic risk score (GRS) at the HLA region with CP.
Materials and methods: 7,248 unrelated European subjects with T1D from four studies were included: the Scottish Diabetes Research Network Type 1 Bioresource (SDRNT1BIO) (n = 4,824, random CP), Diabetes Control and Complications Trial (DCCT) (n = 1,304, fasting CP), Coronary Artery Calcification in Type 1 Diabetes (CACTI) (n = 529, fasting CP) and Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) (n = 591, random CP). GWAS genotyping was performed with Illumina arrays. Ungenotyped variants were imputed using TOPMed, the largest imputation reference panel to date, which imputes lower frequency variants with better quality than previous panels. SNPs with minor allele frequency >0.01 and high imputation quality (INFO >0.80) present in both SDRNT1BIO and DCCT (the two large cohorts) were included in the meta-GWAS (n = 8,150,645). Classical HLA alleles, amino acid sequences and additional SNPs in the HLA region were imputed using multi-ethnic HLA reference panel on Michigan imputation server. Study-specific effect sizes and standard errors for scores and/or loci were estimated by multiple linear regressions of log (CP) with adjustment for covariates (sex, age at diagnosis, T1D duration). Meta-GWAS analysis was performed using METAL v1.5 weighting effect sizes using the inverse of the standard errors. We also tested association of T1D GRS at the HLA region with CP. T1D GRS at the HLA region was explored in total and separately for DR-DQ and non-DR-DQ haplotypes. Non-DR-DQ haplotypes were divided into class I, DPB1, and intergenic regions between DRB1 and DQA1 which do not track classic HLA alleles but regulate class II gene expression.
Results: We identified association at the HLA region (rs9271349, A>G, Chr6:32616053, β (SE) = 0.57 (0.08), p = 1.62E-12). In the HLA imputation, DRB1*06:02 (β (SE) = 0.90 (0.11), p = 1.18E-16) and DRB1*15:01 (β (SE) = 0.70 (0.10), p = 1.68E-12) haplotypes were associated. Five amino acid changes within HLA-DRB1, HLA-DQB1 and HLA-A reached the genome-wide significance threshold. DR-DQ GRS was not associated with CP whereas non-DR-DQ GRS was associated with lower CP levels in all four studies: SDRNT1BIO (β = -0.17, p = 2.00E-8), DCCT (β = -0.06, p = 0.045), CACTI (β = -0.08, p = 0.015) and WESDR (β = -0.10, p = 6.44E-4). The signal mostly came from class I haplotypes (A*2402, B*3906 and A*2902); and intergenic regions (rs9271346, rs116522341 and rs1281934). HLA-A*2402, HLA-B*3906 and intergenic GRSs were associated with lower CP whereas HLA-A*2902 GRS was associated with higher CP.
Conclusion: There is heterogeneity of T1D HLA variants in terms of their associations with CP. T1D DR-DQ GRS is not associated with CP. This could eventually provide insight into mechanisms and opportunities to preserve beta cell function.
Supported by: MRC: MR/T032340/1; CIHR: UCD-170583
Disclosure: A.D. Paterson: None.
68
Genome-wide association study on stroke in type 1 diabetes
E.H. Dahlstrom1,2, A. Antikainen1,2, L. Thorn1,2, A. Syreeni1,2, S. Hägg-Holmberg1,2, J. Putaala3, V. Harjutsalo1,2, P.-H. Groop1,4, N. Sandholm1,2;
1Folkhalsan Research Center, 2Research Program for Clinical and Molecular Metabolism, University of Helsinki and Helsinki University Hospital, 3Neurology, Helsinki University Hospital and University of Helsinki, 4Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: The risk of stroke is markedly increased in type 1 diabetes. In addition, individuals with type 1 diabetes experience stroke at a considerably younger age. Despite the elevated risk of stroke, few studies have assessed genetic factors influencing stroke risk in type 1 diabetes. Therefore, our aim was to identify genetic risk factors for stroke in a genome-wide association study (GWAS).
Materials and methods: This study included 4,916 individuals with type 1 diabetes from the Finnish Diabetic Nephropathy (FinnDiane) Study with data available on stroke. Cases had experienced a stroke based on Finnish nationwide registry data (ICD: I60, I61, I62, I63, I64), with majority of the events verified from medical records by a neurologist. Controls were required to be older than 35 years and have a diabetes duration of more than 20 years. Genotyping was performed with Illumina HumanCoreExome chips at the University of Virginia, US. Imputation was done with Beagle at the Finnish Institute for Molecular Medicine (FIMM), using a Finnish reference panel (SISu v.3). We performed GWAS with the score test adjusted for the calendar year of diabetes onset, sex, and genotyping batches using the RVTESTS software. We included 8,812,836 variants with minor allele frequency (MAF) >1% and good imputation quality (r2 >0.60).
Results: One locus, containing 8 variants, was significant at a genome-wide threshold (p<5×10-8) and associated with stroke in type 1 diabetes (lead variant: rs146444827, MAF=1.2%, OR=4.25, p=2.2×10-8). The variants were located on chromosome 16 near or in the intronic region of the ADAMTS18 gene—a member of the ADAMTS gene family containing genes important in angiogenesis and coagulation. Variants within or near this gene have previously been associated with human cortical structure in brain MRI, brain sulcal depth, and body mass index (BMI) in GWAS. Furthermore, two other variants were suggestively associated with stroke (p<5×10-7). These were located on chromosome 14 within the NRXN3 gene, which has also been associated with BMI and specifically expressed in the brain (rs77936402, MAF=1.8%, OR=2.91, p<3.7×10-7), and on chromosome 1 in the PTGFR gene (rs145968355, MAF=1.2%, OR=3.77, p<1.7×10-7).
Conclusion: We have performed the largest GWAS to date on stroke in type 1 diabetes, and identified one new locus near ADAMTS18 gene, and two additional loci suggestively associated with stroke.
Supported by: EFSD/Sanofi European Diabetes Research Programme in Macrovascular Complications
Disclosure: E.H. Dahlstrom: None.
69
Identification of novel type 1 and type 2 diabetes genes by colocalisation of human islet eQTL and GWAS variants
A. Piron1, M.L. Colli1, M. Defrance2, D.L. Eizirik1, J.M. Mercader3, M. Cnop1;
1ULB Center for Diabetes Research, Université Libre de Bruxelles, Brussels, Belgium, 2Interuniversity Institute of Bioinformatics in Brussels (IB2), Université Libre de Bruxelles, Brussels, Belgium, 3Programs in Metabolism and Medical and Population Genetics, Broad Institute of Harvard and MIT, Cambridge, USA.
Background and aims: Type 2 diabetes (T2D) results from progressive pancreatic beta cell failure. It is tightly related with glycemic traits, e.g. HbA1c, fasting and 2h post-challenge glucose and fasting insulin. Type 1 diabetes (T1D) results from an autoimmune destruction of beta cells. In both diseases genetic factors play a key role, but how genetic variants lead to beta cell failure remains poorly understood. Here, we analyzed the relationship between genetic variants associated with T2D, T1D, and glycemic traits (from genome-wide association studies, GWAS) and cis-expression quantitative trait loci (eQTL) in human islets. We developed a novel colocalization approach to assess whether genetic variants associated with traits share association signals with gene expression, leveraging a dataset of >400 samples.
Materials and methods: Colocalization analyses were performed between human islet eQTL from TIGER (http://tiger.bsc.es/) and GWAS from DIAMANTE (T2D), Type 1 Diabetes Genetics Consortium and MAGIC (glycemic traits). A novel colocalization method was developed that prioritizes candidate variants prior to testing with coloc R package. It shortlists candidate variants with specifically tailored rank-rank hypergeometric overlap analysis detecting linkage disequilibrium-induced overlap of variants. It identifies overlapping signals when multiple GWAS SNPs exist in the region of interest, a scenario in which coloc performs suboptimally. As a further improvement, the shortlisting method uses eQTL effect (up- or downregulation) and GWAS directions (increasing or decreasing risk) to further reduce the analyzed set of variants. The function of some of the novel genes was investigated in human islets and EndoC-bH1 cells.
Results: In total, 158 colocalizations were identified: 27 for T1D, 84 for T2D, 21 for HbA1c, 21 for fasting glucose, 4 for 2h post-challenge glucose, 1 for fasting insulin. The novel shortlisting method followed by coloc identified 58 new colocalizations of GWAS and eQTL SNPs that were not detected by coloc alone. Out of the 84 T2D eGenes, 16 also show colocalization with at least one additional glycemic trait. Five variants with low minor allele frequency (MAF<0.1) colocalized for T2D. Among them we discovered colocalization between MYO5C expression and T2D risk (MAF=0.05), with the protective allele being associated with increased MYO5C expression. A well-known eQTL for ADCY5 colocalized with T2D and all glycemic traits. T1D and T2D co-localizations were detected in the intronic region of GLIS3 that has been reported to modulate apoptosis in beta cells in both diseases. A FUT2 eQTL colocalized with a T1D GWAS signal; with the T1D protective allele being associated with increased FUT2 expression. Interestingly, infection of human beta cells by coxsackievirus B1 decreased FUT2 expression.
Conclusion: We identified 158 eQTLs colocalizing with T1D, T2D and glycemic traits, including 27 T1D, 37 glycemic and 30 T2D eGenes that are novel. This human islet expression regulatory variation sheds light on genes mediating genetic variant effects, representing an invaluable asset to understand glycemic traits and the pathophysiology of T2D and T1D.
Supported by: J.M.M. is supported by American Diabetes Association (1-19-ICTS-068) and by NHGRI, grant U01HG011723
Disclosure: A. Piron: None.
70
The impact of rare pathogenic variants of GLIS3 on type 2 diabetes
S. Meulebrouck1, M. Canouil1, M. Derhourhi1, B. Balkau2, G. Charpentier3, S. Franc3, M. Michel4, R. Roussel4, M. Vaxillaire1, P. Froguel1,5, A. Bonnefond1,5;
1Inserm UMR1283, CNRS UMR8199 - EGID, Lille, France, 2Inserm U1018, Villejuif, France, 3CERITD, Evry, France, 4Inserm U1138, Paris, France, 5Imperial College London, London, UK.
Background and aims: Type 2 diabetes (T2D) is due to a combination of environmental and genetic factors. There are monogenic forms, caused by a mutation in a single gene, that are usually (but not always) more severe and appear earlier than polygenic forms. Among the more than 30 genes responsible for monogenic diabetes, the biallelic pathogenic mutations of GLIS3, which encodes a key transcription factor for β cell lineage, are known to cause a rare autosomal recessive syndromic form of neonatal diabetes. We hypothesized that pathogenic heterozygous GLIS3 variants could also be associated with a dominantly transmitted form of monogenic diabetes or even contribute to common T2D.
Materials and methods: We sequenced GLIS3 in 5,471 individuals. The identified rare heterozygous variants were then functionally investigated through luciferase assays based on their ability to bind to the promoter region of the INS gene and activate its transcription. The pathogenicity of each variant was then assessed by combining our luciferase results with the other criteria from the American College of Medical Genetics and Genomics (ACMG). Finally, we performed adjusted association studies between the cluster of pathogenic or likely pathogenic (P/LP) variants and T2D risk, and we analyzed the phenotype of carriers and non-carriers.
Results: We identified 105 rare heterozygous variants of GLIS3. Through luciferase assays, we subsequently found 49 loss-of-function variants, presenting a decrease in luciferase activity compared to the wild-type GLIS3. By combining our functional results with the other ACMG criteria, we identified 18 P/LP variants. These variants were enriched at the C-terminus of the protein, containing its transactivation domain. Finally, the association studies showed that these P/LP variants were strongly associated with T2D risk (P = 0.003; OR = 3.91). Strikingly, all the T2D patients carrying a rare P/LP GLIS3 variant were treated with sulfonylureas, suggesting an early alteration of insulin secretion that responded to this class of medication. Importantly, both age of onset of diabetes and body mass index were similar between carriers and non-carriers.
Conclusion: We conclude that heterozygous P/LP variants of GLIS3 strongly increase the risk to develop T2D, and can not be clinically distinguished from the other common forms of T2D using classic monogenic diabetes clinical criteria. Genetic screening may be useful for precision medicine, as GLIS3 deficiency may severely impair insulin secretion and seem to respond to sulfonylureas as MODY1 and 3 do.
Disclosure: S. Meulebrouck: None.
71
Sequencing of 448 Greenlandic individuals uncovers a novel splice-affecting HNF1A variant with large population impact on diabetes
M.E. Jørgensen1,2, A.C.B. Thuesen3,4, F.F. Stæger5, A. Kaci6, M.H. Solheim6, I. Aukrust6, E. Jørsboe3, C.G. Santander7, A. Gilly8, M.L. Pedersen1,9, E. Zeggini8, L. Bjørkhaug10, A. Albrechtsen7, I. Moltke7, T. Hansen3;
1Steno Diabetes Center Greenland, Nuuk, Greenland, 2Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark, 3Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, 4Clinical Research, Steno Diabetes Center Copenhagen, Herlev, Denmark, 5Section for Computational and RNA Biology, Department of Biology,, University of Copenhagen, Copenhagen, Denmark, 6Center for Diabetes Research, Department of Clinical Science, University of Bergen, Bergen, Norway, 7Section for Computational and RNA Biology, Department of Biology, University of Copenhagen, Copenhagen, Denmark, 8German Research Center for Environmental Health, Neuherberg, Germany, 9Greenland Center for Health Research, Institute of Health and Nature, University of Greenland, Nuuk, Greenland, 10Department of Safety, Chemistry and Biomedical laboratory science, Western Norway University of Applied Sciences, Bergen, Norway.
Background and aims: The genetic disease architecture of Inuit includes a large number of common high-impact variants. Identification of such variants contributes to our understanding of the genetic aetiology of diseases and improves global equity in genomic personalised medicine. We aimed to identify and characterise novel variants in genes associated with Maturity Onset Diabetes of the Young (MODY) in the Greenlandic population.
Materials and methods: Using combined data from Greenlandic population cohorts of 4497 individuals, including 448 whole genome sequenced individuals, we screened 14 MODY genes for known and novel variants. We functionally characterised an identified novel variant and assessed its association with diabetes prevalence and cardiometabolic traits and population impact.
Results: We identified a novel HNF1A variant with an allele frequency of 1·9% in the Greenlandic Inuit and absent elsewhere. Functional assays indicate that it prevents normal splicing of the gene. The variant caused lower 30-min insulin (β=-232 pmol/L, βSD=-0·695, P=4·43×10-4) and higher 30-min glucose (β=1·20 mmol/L, βSD=0·441, P=0·0271) during an oral glucose tolerance test. Furthermore, the variant was associated with type 2 diabetes (OR 4·4, P= 7·24×10-6) and HbA1c (β=0·11 HbA1c%, βSD=0·21, P=0·0052). The variant explained 2·5% of diabetes variance in Greenland.
Conclusion: The reported variant has the largest population impact of any previously reported variant within a MODY gene. Together with the recessive TBC1D4 variant, we show that close to 1 in 5 cases of diabetes (18%) in Greenland has a monogenic diabetes aetiology compared to 1-3% in large populations.
Supported by: Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation.
Disclosure: M.E. Jørgensen: None.
72
A common variant in the ketohexokinase gene is associated with fructosuria and cardiometabolic outcomes
A.M. Buziau1, C.G. Schalkwijk1, J.L.J. Scheijen1, P.I.H. Simons1, C.J.H. van der Kallen1, S.J.P. Eussen1, N.C. Schaper2, P.C. Dagnelie1, M.M.J. van Greevenbroek1, A. Wesselius2, C.D.A. Stehouwer2, M.C.G. Brouwers2;
1Internal Medicine, Maastricht University, 2Maastricht University Medical Center+, Maastricht, Netherlands.
Background and aims: There is an ongoing discussion on whether fructose per se is disadvantageous for cardiometabolic health. Recently, pharmacological inhibition of ketohexokinase (KHK), the first enzymatic step of fructolysis, was shown to reduce intrahepatic lipid content in humans. The study of individuals carrying functional variants in the KHK gene will provide more insight into the lifelong effects of inhibition of KHK, as these individuals have been exposed from birth to a KHK protein with less enzymatic activity. The aims of the present study were: 1) to study whether a common missense variant in KHK (rs2304681) is a functional variant that affects fructosuria (similar to pharmacological inhibition of KHK); and 2) to study the association between rs2304681 and cardiometabolic outcomes.
Materials and methods: First, linear regression analyses were performed to study the association between rs2304681 and 24h urinary fructose levels (quantified by tandem mass spectrometry), with adjustment for age, sex, and type 2 diabetes (T2D), in the Maastricht Study, a population-based cohort. Second, we used summary-level data on the association of rs2304681 with non-alcoholic fatty liver disease, T2D, hypertension, and myocardial infarction, obtained from publicly available databases.
Results: First, the rs2304681 minor A allele (frequency: 0.36) was associated with higher 24h urinary fructose levels (unstandardized beta: 0.064; 95% CI: 0.027-0.100; n=1,471). Second, the rs2304681 minor A allele protected from hepatic steatosis (OR: 0.972; 95% CI: 0.957-0.988; n=36,703; UK Biobank), T2D (OR: 0.985; 95% CI: 0.975-0.99; n=1,331,670; fixed-effects meta-analysis in the AGEN and the European DIAMANTE cohorts) and myocardial infarction (OR: 0.976; 95% CI: 0.961-0.992; n=583,191; fixed-effects meta-analysis in the CARIoGRAMplusC4D and the UK Biobank cohorts). Two studies both showed a protective effect on the risk of hypertension (OR: 0.988; 95% CI: 0.976-0.999; n=440,285; UK Biobank; and Z-score: -2.59; p = 0.01; n=192,763; the combined CHD Exome+, ExomeBP, and GoT2D cohorts).
Conclusion: Lifelong impairment of KHK activity (reflected by rs2304681) is associated with fructosuria and protection from cardiometabolic disease. These findings suggest that fructose per se has harmful cardiometabolic effects, which may be mitigated by pharmacological inhibition of KHK.
Supported by: Dutch Diabetes Research Foundation
Disclosure: A.M. Buziau: None.
OP 13 Beyond type 1 and type 2
73
Clusters of prediabetes and type 2 diabetes stratify all-case mortality in a cohort of participants undergoing invasive coronary diagnostics
K. Prystupa1,2, G.E. Delgado3,4, A.P. Moissl5,3, M.E. Kleber3,6, M. Heni1,2, A.L. Birkenfeld1,2, A. Fritsche1,2, W. März3,7, R. Wagner1,2;
1Department of Internal Medicine IV, Division of Endocrinology, Diabetology and Nephrology, University of Tuebingen, Tübingen, 2Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Center Munich at the University of Tübingen, Tübingen, 3Vth Department of Medicine (Nephrology, Hypertensiology, Rheumatology, Endocrinology, Diabetology), Medical Faculty Mannheim, University of Heidelberg, Mannheim, 4Center for Preventive Medicine and Digital Health Baden-Württemberg (CPDBW), Medical Faculty Mannheim, Heidelberg University, Mannheim, 5Institute of Nutritional Sciences, Friedrich Schiller University Jena, Jena, 6SYNLAB MVZ für Humangenetik Mannheim GmbH, Mannheim, 7SYNLAB Academy, SYNLAB Holding Deutschland GmbH, Mannheim, Germany.
Background and aims: The risk for complications and mortality among patients with type 2 diabetes (T2D) is heterogeneous. Different trajectories can be identified in the prediabetic state, which comprises heterogeneous metabolic clusters. It is not known whether such pathophysiologic clusters of prediabetes and diabetes affect survival in at-risk persons being evaluated for coronary heart disease.
Materials and methods: The LURIC Study recruited patients referred for coronary angiography at a median age of 63(IQR 56-70) who have since been followed up for an overage of 20 years. Clustering of 1269 subjects without diabetes was performed with oGTT-derived glucose and insulin; fasting triglyceride, high-density lipoprotein, BMI, waist, and hip circumference. Patients with T2D (n=794) were clustered using age, BMI, glycemia, homeostasis model assessment, and islet autoantibodies. Associations of clusters with mortality were analyzed using Cox regression.
Results: Individuals without diabetes were classified into six subphenotypes, with 884 assigned to subjects at low-risk (cluster 1,2,4) and 385 at high-risk (cluster 3,5,6) for diabetes. We found significantly increased mortality in clusters 3 (hazard ratio (HR) 1.42), 5 (HR 1.43), and 6 (HR 1.46) age-, BMI-, HbA1c- and sex-adjusted. In the T2D group, 508 were assigned to mild age-related diabetes (MARD), 183 to severe insulin-resistant diabetes (SIRD), 84 to mild obesity-related diabetes (MOD), 19 to severe insulin-deficient diabetes (SIDD). Compared to the low-risk non-diabetes group, crude mortality was not different in MOD. Increased mortality was found for MARD (HR 2.2), SIRD (HR 2.2), and SIDD (HR 2.5).
Conclusion: Metabolic clustering successfully stratifies survival even among persons already undergoing invasive coronary diagnostics. Novel clustering approaches based on glucose metabolism can identify persons who require specific attention as they have an increased mortality risk.
Supported by: BMBF
Disclosure: K. Prystupa: None.
74
Novel clusters of prediabetes and their association with progression and regression: a 3-year follow-up study
Y. Liu, M. Sang, S. Qiu, Z. Sun;
Southeast University, Nanjing, China.
Background and aims: Cluster analysis may assist in stratifying heterogeneous clinical presentations of prediabetes. However, the association of cluster-based subgroups of prediabetic with its progression to diabetes and regression to normoglycemia remains unclear. This study was aimed to address this issue with novel clusters of prediabetes derived from four parameters.
Materials and methods: We developed a k-means clustering model in participants with prediabetes (N=4,138) from the SENSIBLE and SENSIBLE-Addition studies, based on body mass index (BMI), age, triglyceride-and-glucose (TyG) index, and hemoglobin A1c (HbA1c). TyG index was used to assess insulin resistance. Of the included participants with prediabetes at baseline, 1,629 were followed-up for 3 years. Prediabetes was defined as impaired fasting glucose and/or impaired glucose tolerance based on World Health Organization 1999 criteria. Logistic regression analyses were performed to obtain the odds ratios (ORs) and 95% confidence intervals (CIs).
Results: Three clusters were identified, with cluster 0, 1 and 2 accounting for 28.7%, 30.6% and 40.7%, respectively. Participants with prediabetes were featured by the youngest and the lowest HbA1c in cluster 0, the highest BMI and TyG index in cluster 1, and the oldest and the lowest BMI in cluster 2. After the adjustment for gender, ethnic groups, serum uric acid, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and estimated glomerular filtration rate, cluster 1 (OR 3.61, 95% CI: 2.21-5.90) and cluster 2 (OR 3.01, 95% CI: 1.87-4.84) were both associated with increased risk of progression to diabetes when compared with cluster 0. Moreover, cluster 1 and cluster 2 were both associated with decreased chance of regression to normoglycemia (OR 0.42, 95% CI: 0.31-0.57; and OR 0.48, 95% CI: 0.37-0.63, respectively).
Conclusion: Our cluster-based analysis showed that participants featured by older age and higher degree of insulin resistance, obesity, or insulin resistance had higher risk of progression to diabetes and lower risk of regression to normoglycemia. While young participants with sufficient attention to BMI and blood glucose management were more likely to regress to normoglycemia.
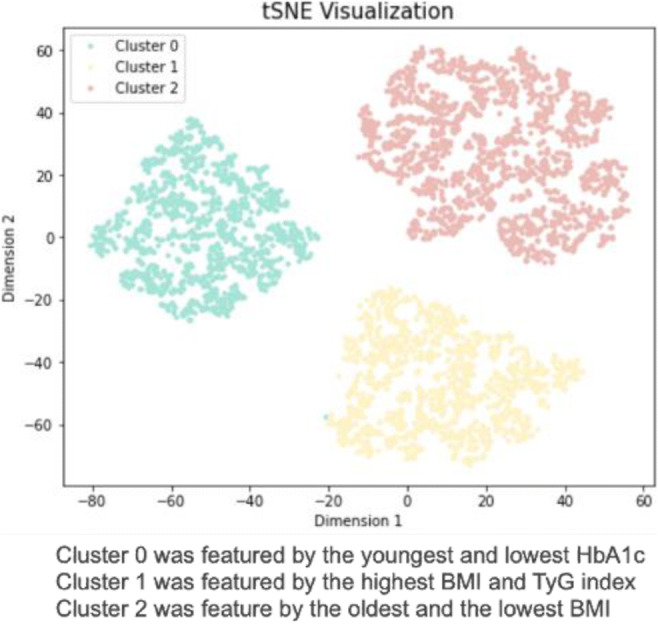
Supported by: the National Key R&D Program of China (2016YFC1305700)
Disclosure: Y. Liu: None.
75
Incidence of HbA 1c -defined prediabetes and progression to type 2 diabetes: a nationwide study with routine care laboratory data
S.K. Nicolaisen1, R.W. Thomsen1, D. Witte2, H.T. Sørensen1, L. Pedersen1;
1Department of Clinical Epidemiology, Aarhus University Hospital and Aarhus University, 2Steno Diabetes Center Aarhus and Department of Public Health, Aarhus University, Aarhus, Denmark.
Background and aims: Since glycated hemoglobin (HbA1c) became a diagnostic tool for diabetes in 2011, HbA1c testing has increasingly been used. Population-based routine clinical care laboratory databases may therefore be a valuable research tool in prediabetes research. We aimed to describe HbA1c measurement patterns in the entire Danish population, to identify and characterize individuals with incident HbA1c-defined prediabetes, and to examine their risk of progression to diabetes.
Materials and methods: All HbA1c measurements in the Danish nationwide laboratory databases during 2012-2018 were characterized. The general population incidence rate of prediabetes (HbA1c 42-47 mmol/mol) was calculated. Among individuals with prediabetes, the 5-year cumulative incidence of diabetes (HbA1c >=48 mmol/mol) was assessed.
Results: Among 5,483,467 Danish residents, 13,107,797 HbA1c measurements from 3,183,880 (58%) individuals were available in the database for the 2012-2018 period. A total of 373,628 individuals were identified with incident HbA1c-defined prediabetes, corresponding to an incidence rate of 15.5 (95% CI 15.5-15.6) per 1,000 person-years. The median HbA1c at prediabetes diagnosis was 43 mmol/mol (IQR 42-44), median age was 67.8 years (IQR 57.9-76.4), and 52.0% were women. During a median follow-up of 2.7 years, 46,821 (12.5%) developed HbA1c-defined diabetes, yielding an estimated cumulative 5-year diabetes incidence of 20.1% (95% CI 19.9-20.3).
Conclusion: More than half of the Danish entire nation had at least one HbA1c measurement registered during 2012-2018. The incidence rate of prediabetes was 15.5 per 1000 person-years, and likely due to extensive testing, persons were identified at an early stage of prediabetes. Within 5 years, one in five individuals with prediabetes will progress to diabetes.
Disclosure: S.K. Nicolaisen: None.
76
Antibody positive patients with type 2 diabetes who rapidly progress to insulin have similar characteristics and type 1 genetic risk scores as patients with type 1 diabetes
V. Simpson1,2, N. Thomas1,2, A.V. Hill1,2, B.M. Shields1,2, S. Deshmukh1, T.J. McDonald1,2, A.G. Jones1,2, StartRight Study Group;
1University of Exeter, 2Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.
Background and aims: New EASD/ADA guideline recommend testing islet autoantibodies in all clinically suspected adult-onset type 1 diabetes (T1D), including those treated as type 2 diabetes (T2D) with early progression to insulin, and advise that a positive islet antibody in this setting confirms type 1 diabetes. We assessed whether a positive islet autoantibody in the setting of initial treatment as type 2 diabetes and early progression to insulin confirmed the genetic and C-peptide progression characteristics of type 1 diabetes.
Materials and methods: In 1793 participants with recently diagnosed adult-onset diabetes (duration at recruitment <12 months, median 5 months) we compared the baseline characteristics, T1D genetic risk score (T1DGRS) and rate of loss of beta-cell function (annual urine c-peptide creatinine ratio (UPCR), over median 2 years) between definite T1D, defined as a clinical diagnosis of T1D, treated with insulin within 14 days of diagnosis and ≥2 positive islet-autoantibodies (of GAD, IA2, Znt8, n=305) and patients treated as T2D (diagnosed or suspected T2D without insulin for >2 weeks from diabetes diagnosis) with positive islet autoantibodies and insulin treatment within 3 years of diagnosis (n=94). We also compared these characteristics in antibody positive T2D not progressing to insulin within 3 years (n=79) and autoantibody negative tT2D (n=687).
Results: T1DGRS and C-peptide progression are shown in figure 1. Participants with positive autoantibodies and rapid progression to insulin treatment had genetic susceptibility consistent with confirmed T1D: T1DGRS 13.0 (95%CI 12.7-13.4), compared to 12.9 (95%CI 12.7-13.1) in multi-antibody positive T1D (p=0.63). These participants also had rapid loss of C-peptide, again consistent with confirmed T1D: annual change in UPCR -37% (-25,-47) in comparison to -36% (-30,-41) in multiple antibody positive T1D (p=0.8). In contrast, positive islet autoantibodies in those without early progression to insulin had T1D genetic susceptibility and C-peptide loss more similar to T2D than T1D: in antibody positive participants without progression to insulin and antibody negative T2D T1DGRS was 10.8 (95%CI 10.2-11.5) and 10.0 (95%CI 9.8-10.2) (p <0.001) respectively, annual change in UCPCR -10% (0,-25%) and -5% (0,-15%) (p<0.001) respectively. In a sub-group of those autoantibody positive (n = 40) and early insulin treatment, but without insulin within 6 months of diagnosis, T1DGRS and UCPCR remained consistent with classical type 1 diabetes (T1DGRS 12.9 (95% CI 12.5-13.4), UCPCR annual change -37% (95% CI -19%,-51%).
Conclusion: In people initially treated as T2D with early progression to insulin a positive islet autoantibody test confirms the genetic characteristics and rate of C-peptide loss of classical T1D. These patients should therefore be considered to have, and be treated as, T1D.
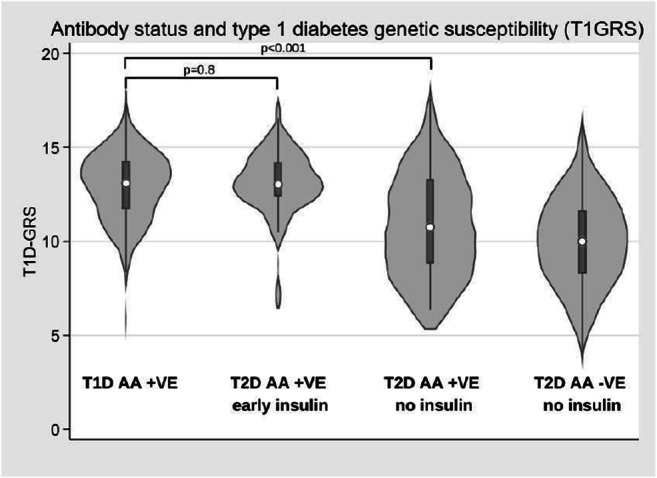
Clinical Trial Registration Number: NCT03737799
Supported by: Diabetes UK
Disclosure: V. Simpson: None.
77
Children with newly-diagnosed diabetes but no autoantibodies shoud be genetically tested
M. Harsunen1,2, J.L.T. Kettunen2,3, T. Härkönen1,2, P. Vähäsalo4,5, R. Veijola4,5, J. Ilonen6, P.J. Miettinen1,7, M. Knip1,2, T. Tuomi2,3;
1New Children's Hospital, Pediatric Research Center, University of Helsinki and Helsinki University Hospital, Helsinki, 2Research Program for Clinical and Molecular Metabolism, University of Helsinki, Helsinki, 3Abdominal Centre, Endocrinology, University of Helsinki and Helsinki University Hospital, Helsinki, 4Department of Pediatrics, PEDEGO Research Unit, Medical Research Center, University of Oulu, Oulu, 5Department of Children and Adolescents, Oulu University Hospital, Oulu, 6Immunogenetics Laboratory, Institute of Biomedicine, University of Turku, Turku, 7Translational Stem Cell Biology and Metabolism Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Background and aims: Patients with monogenic forms of diabetes may be misclassified and treated suboptimally. While the prevalence of type 1 diabetes (T1D) is high in children in Finland, that of monogenic diabetes is not known. We assessed the prevalence and clinical manifestations of monogenic diabetes in children, who were negative for T1D related autoantibodies (AAB) or positive only for low titer islet cell antibodies (ICA) at diagnosis.
Materials and methods: A next generation sequencing gene panel including 42 genes was used to identify monogenic diabetes in participants of the Finnish Pediatric Diabetes Register, covering approximately 90% of newly-diagnosed diabetes in Finland (<16 years). Five AAB (ICA, IAA, GADA, IA-2A, ZnT8A) and HLA class II genotypes were analysed at diagnosis.
Results: Out of 6482 participants initially registered with T1D, we sequenced DNA for 152 (2.3%) testing negative for all AAB and 49 (0.8%) positive only for ICA ≤10 JDFU (ICAlow). Monogenic diabetes was revealed in 19 (12,5%) of the AAB-negative and 2 (4%) of the ICAlow individuals. The genes involved were GCK, HNF1A, HNF4A, HNF1B, INS, KCNJ11, RFX6, LMNA and WFS1. None of these patients had ketoacidosis at diagnosis or carried an HLA genotype conferring high risk for T1D. The diagnosis of monogenic diabetes led to a change in the treatment of many patients. A switch from insulin treatment to oral medication has been successful in patients with variants in HNF1A, HNF4A or KCNJ11, significantly improving their glycemic control and quality of life. The figure illustrates continuous glucose monitoring before and after a transfer from insulin pump to sulfonylurea in a patient with KCNJ11 variant. Next, we will proceed to exome sequencing of the AAB-negative children without genetic diagnosis.
Conclusion: More than 10% of AAB-negative children had a genetic variant associated with monogenic diabetes. Because diagnosis of monogenic diabetes can lead to major changes in the treatment, we recommend referring all children with newly-diagnosed diabetes who are AAB-negative to genetic testing. Low-titer ICA in the absence of other AAB does not always indicate a diagnosis of T1D.
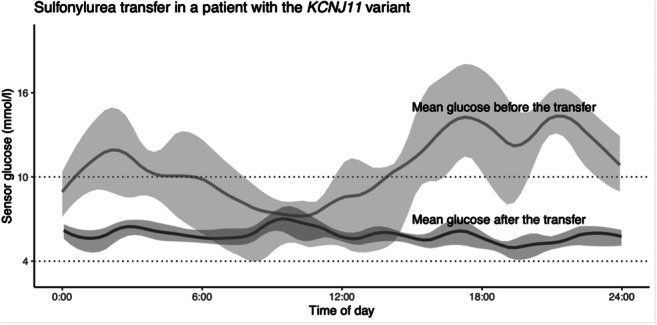
Disclosure: M. Harsunen: None.
78
Penetrance of MODY is substantially lower in clinically unselected cohort: important implications for opportunistic genomic testing
K. Patel1, U. Mirshahi2, K. Colclough3, C. Wright1, A. Wood1, R. Beaumont1, T. Laver1, R. Stahl2, A. Golden2, J. Goehringer2, Geisinger-Regeneron DiscovEHR Collaboration, T. Frayling1, A. Hattersley1, D. Carey2, M. Weedon1;
1University of Exeter, Exeter, UK, 2Geisinger Health System, Danville, USA, 3Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.
Background and aims: Accurate estimates of penetrance are needed for counselling of individuals with a mutation in common MODY (Maturity Onset Diabetes of the Young) genes. We aimed to comprehensively assess the penetrance of the three most common causes of MODY (HNF1A, HNF4A and GCK) in large genotype-first (clinically unselected) and phenotype-first (clinically selected) cohorts.
Materials and methods: We analysed clinical and genetic sequencing data from four different cohorts: 1742 probands referred for clinical MODY testing; 2194 family members of the MODY probands; clinically unselected 132,194 individuals from an American hospital-based cohort; and 198,748 individuals from a UK population-based cohort.
Results: Age-related penetrance of diabetes for pathogenic variants in HNF1A and HNF4A was substantially lower in the clinically unselected cohorts compared to clinically referred probands (ranging from 32% to 98% at age 40yrs for HNF1A, and 21% to 99% for HNF4A). The background rate of diabetes, but not clinical features or variant type, explained the reduced penetrance in the unselected cohorts. In contrast, the penetrance of mild hyperglycaemia for pathogenic GCK variants was similarly high across cohorts (ranging from 89 to 97%) despite substantial variation in the background rates of diabetes.
Conclusion: Penetrance of HNF1A/HNF4A-MODY but not GCK-MODY is substantially lower in the genotype-first approach and has important implications for opportunistic screening during genomic testing. Our finding suggests the need to tailor genetic interpretation and counselling for individuals based on the context in which a pathogenic variant was identified.
Supported by: Wellcome Trust, MRC
Disclosure: K. Patel: None.
OP 14 Exercising your tissues in shape
79
Does exercise matter? The effects of different volumes of exercise on beta cell function in patients with newly diagnosed type 2 diabetes
M. Lyngbaek1, G. Legaard1, N. Nielsen1, T. Almdal2, M. Ried-Larsen1, K. Karstoft2;
1Centre for Physical Activity Research, Copenhagen University Hospital - Rigshospitalet, 2Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark.
Background and aims: Dietary weight loss may lead to improved beta-cell function (disposition index: DI = insulin sensitivity x insulin secretion) in persons with type 2 diabetes (T2D). However, the role of exercise in adjunct to diet therapy remains unclear. Thus, we performed a study assessing beta-cell function both at supraphysiological conditions using the gold standard hyperglycemic clamp, and at physiological conditions using a mixed meal tolerance test. We expected to observe a dose-dependent improvement in beta-cell function when adding exercise to dietary weight loss.
Materials and methods: Persons with T2D duration < 7 years and obesity/overweight were randomized 1:1:1:1 (stratified by sex) to either 16 weeks of 1) Standard care 2) Dietary intervention 3) Dietary intervention + exercise 3 times/week 4) Dietary intervention + exercise 6 times/week. The exercise interventions consisted of both aerobic and resistance training. The dietary intervention aimed at a 25% caloric deficit and weight loss. The primary outcome was the change in beta-cell function assessed with clamp-derived late-phase disposition index. Major secondary outcomes included clamp- and mixed meal-derived changes in beta-cell function, insulin secretion, and insulin sensitivity from baseline to 16-weeks follow-up. The full protocol has been published.
Results: We randomized 82 participants (35% females, mean age (SD) of 58.2 (9.8) years, BMI 33.1 (3.7) kg/m2, median T2D duration (IQR) of 4.0 (1.9 to 5.5) years. Adherence to the diet (25-30% energy reduction) and exercise (>85%) was similar across intervention groups. Five persons (6%) were lost to follow-up. From the hyperglycemic clamp we observed improvement in late-phase disposition index in all three intervention groups. The mean difference (MD) from standard care (95% confidence intervals (CI)) was 58 (16 to 116)%, 105 (49 to 182)% and 137 (73 to 225)% for diet only, moderate volume exercise, and the high volume exercise, respectively. While the improvement was larger in the high volume exercise group compared to diet group (MD (95% CI): 50 (10 to 104)%), no difference where observed between the exercise groups (high volume exercise vs. moderate volume exercise MD (95% CI): 16 ( -16 to 59)%) or between moderate volume exercise and the diet group (MD (95% CI): 29 (-5 to 77)%). No differences in glucose-stimulated insulin secretion were observed between the groups. Improvements in insulin sensitivity followed a similar pattern as observed for the disposition index. The observations during the meal test confirmed the findings from the hyperglycemic clamp.
Conclusion: Sixteen weeks of high volume of exercise in combination with dietary weight loss improves beta-cell function more than standard care or diet alone in persons with T2D. However, there was no significant difference between moderate and high volume of exercise in beta-cell function, suggesting that increasing exercise volume from 3 to 6 times/week, may not amplify the beta-cell function. The improvement in beta-cell function was mainly mediated by improved insulin sensitivity, whereas adding exercise to the dietary intervention did not seem to increase glucose- or meal-stimulated insulin secretion beyond dietary weight loss alone.
Clinical Trial Registration Number: NCT03769883
Supported by: TrygFonden ID 124708 and Svend Andersen Fonden
Disclosure: M. Lyngbaek: Grants; Danish Diabetes Academy NNF17SA0031406.
80
Improved beta cell function following eight weeks high-intensity interval training combining rowing and cycling in type 2 diabetes
M.H. Petersen1, M.E. de Almeida2, E.K. Wentorf2, J.V. Stidsen1, N. Ørtenblad2, K. Højlund1;
1Steno Diabetes Center Odense, 2Department of Sports Science and Clinical Biomechanics, Odense, Denmark.
Background and aims: High-intensity interval training (HIIT) and recruitment of several muscle groups seem to enhance the effect of exercise training on glucose homeostasis in type 2 diabetes. However, the effect of these factors on beta-cell function in type 2 diabetes remains to be established. Here, we investigated the effect of a novel HIIT-protocol engaging upper and lower body muscles on the beta-cell function in individuals with type 2 diabetes compared with glucose-tolerant obese and lean controls.
Materials and methods: Fifteen obese men with type 2 diabetes, and age-matched, glucose-tolerant obese (n=15) and lean (n=18) men underwent an 8-week, supervised, HIIT-protocol combining rowing and cycling. Before and after the training period, the participants were evaluated by the Botnia clamp combining an IVGTT with a hyperinsulinemic-euglycemic clamp to measure first- and second-phase insulin secretion, insulin sensitivity and estimate beta-cell function adjusted for insulin sensitivity (disposition index).
Results: At baseline, insulin sensitivity was ~40% lower, and first-phase insulin secretion and the disposition index were >90% lower in men with type 2 diabetes compared with both lean and obese controls (all p<0.05). The HIIT-protocol induced a marked increase (~30-40%) in insulin sensitivity in all groups (all p<0.05). In patients with type 2 diabetes, this was accompanied by a large (>200%) but highly variable improvement in the disposition index (p<0.05), and a clinically relevant reduction in HbA1c (~4 mmol/mol). However, first-phase insulin secretion and the disposition index were still markedly reduced in men with type 2 diabetes (all p<0.05). In lean and obese controls, HIIT also improved (~30%) the disposition index (all p<0.05) and tended to reduce the second-phase insulin response (p<0.10). No group-differences were seen in the HIIT-induced responses on insulin secretion and insulin sensitivity.
Conclusion: HIIT combining rowing and cycling induces a large, but highly variable increase in beta-cell function adjusted for insulin sensitivity in men with type 2 diabetes, and to a numerically smaller extent in obese and lean glucose-tolerant men. However, after the HIIT-protocol, the beta-cell function was still severely impaired in men with type 2 diabetes, even when adjusted for the improvement in insulin sensitivity, suggesting that this component of type 2 diabetes is less reversible than insulin sensitivity.
Clinical Trial Registration Number: 17/31977
Supported by: Novo Nordisk Foundation and Sawmill owner Jeppe Juhl and wife Ovita Juhls Memorial Bursary
Disclosure: M.H. Petersen: None.
81
Exercise-responsive non-coding RNAs in the regulation of skeletal muscle metabolism
E. Caria1, I. Sen1, M. Katayama1, M. Savikj2, A.M. Abdelmoez2, J.A.B. Smith2, N.J. Pillon1, J.R. Zierath2, A. Krook1;
1Department of Physiology and Pharmacology, Karolinska Institutet, 2Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Background and aims: Exercise triggers profound structural and metabolic adaptations in skeletal muscle. While this largely accounts for the benefits of exercise in the treatment or prevention of type 2 diabetes (T2D), a detailed understanding of the molecular mechanisms involved remains unclear and warrants further research. Non-coding RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), have recently emerged as important regulators of skeletal muscle physiology, and their expression in skeletal muscle is modulated by different exercise training programs. miRNAs are also incorporated into exosomes and secreted upon exercise to exert autocrine, paracrine and/or endocrine effects on tissue-specific or whole-body glucose homeostasis. Here, we aimed to identify exercise-responsive serum exosomal miRNAs (study A) or skeletal muscle-derived lncRNAs (study B) that mediate skeletal muscle adaptations to exercise training or acute exercise in individuals with or without T2D.
Materials and methods: In study A, young healthy individuals (n=12) underwent three weeks of aerobic exercise training, and serum was collected before and after for exosome isolation. Differentially expressed exosomal miRNAs were identified using a miRNA focus PCR panel. Functional characterization of the identified candidates was performed by transfecting primary human skeletal muscle cells with miRNA mimics, and effects on glucose metabolism were assessed using radiolabelled substrate assays. In study B, a separate cohort of individuals with T2D (n=19) or normal glucose tolerance (NGT, n=17) underwent 30 minutes of cycling exercise at 85% of their maximum heart rate. Vastus lateralis muscle biopsies were taken at baseline, immediately after, and three hours after exercise, and RNA sequencing was performed to identify differentially expressed, skeletal muscle-derived lncRNAs.
Results: In study A, exosomal miR-136-3p and miR-139-5p were increased in the circulation following three weeks of aerobic exercise training. Overexpression of miR-136-3p or miR-139-5p in human primary skeletal muscle cells elicited exercise-like metabolic effects, in terms of increased glucose uptake (miR-136-3p) and glycogen synthesis (miR-139-5p). In study B, a single bout of exercise changed the expression of more than 200 lncRNAs in the T2D group, whereas this number was much lower in the NGT group (24). We identified exercise-responsive lncRNAs that were co-expressed with metabolic genes and shortened the candidate list to 41 lncRNAs for screening, according to their fold-changes versus baseline and expression level in human myotubes.
Conclusion: Exosomal miR-136-3p and miR-139-5p are increased in serum following exercise training. Increased miR-136-3p or miR-139-5p lead to metabolic adaptations in skeletal muscle. An acute exercise bout alters the expression of several lncRNAs specifically in skeletal muscle from subjects with T2D. Further functional characterization of these candidates could provide mechanistic insights into the regulation of skeletal muscle metabolism by exercise, particularly in the context of T2D.
Disclosure: E. Caria: None.
82
Exercise-induced crosstalk between immune cells and adipocytes in humans: role of oncostatin-M
L. Dollet1,2, L.L. Lundell2, A.V. Chibalin3, L.A. Pendergrast3, N.J. Pillon1, K. Caidahl3, A.S. Deshmukh2, T. De Castro Barbosa4, M. Rydén4, R. Barrès2, H. Wallberg-Henriksson1, J.R. Zierath2,3, A. Krook1;
1Department of Physiology and Pharmacology, Karolinska Institutet, Solna, Sweden, 2Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, 3Department of Molecular Medicine and Surgery, Karolinska Institute, Solna, Sweden, 4Department of Medicine (H7), Karolinska Institutet, Stockholm, Sweden.
Background and aims: The discovery of novel exercise-regulated circulatory factors has fueled interest in the role of organ-crosstalk, especially between skeletal muscle and adipose tissue in conferring the beneficial effects of exercise on insulin sensitivity and metabolism. We aimed to study the adipose tissue secretome in the context of exercise, and its potential role in inter- and intra-organ crosstalk in the response to exercise in individuals with normal glucose tolerance or type 2 diabetes.
Materials and methods: Men and women with normal glucose tolerance (NGT, n=20) or type 2 diabetes (T2D; n=28) performed an acute exercise bout (30 min cyclometer). Leg adipose tissue biopsies were collected from the participants at rest, immediately after exercise, and after 3h recovery for RNA sequencing analysis. Putative exercise-induced secreted proteins were selected, and cultured human adipocytes were exposed in vitro to recombinant candidate proteins. Phosphorylation arrays and immunoblot analysis were performed to track signalling events, and lipolytic responses were measured. Plasma measurements were performed using ELISA. THP1 human monocytes were differentiated in vitro, and gene expression of candidates were measured after acute exposure to an adrenergic agonist and conditioned media from contracted cultured myotubes.
Results: Transcriptomic analysis revealed time-dependent changes, with 712 and 893 genes altered post exercise, and 1353 and 4316 genes altered after recovery in adipose tissue from individuals with NGT or T2D respectively (FDR<0.05). The differential response in T2D adipose tissue was associated with a sustained activation of inflammatory pathways as shown by gene ontology analysis. Oncostatin-M was identified as one of the most upregulated putative secreted factors post exercise (logFC=3.35 and 2.95 for NGT and T2D). Oncostatin-M plasma level was increased after exercise in both groups (2-way ANOVA p<0.001). Exposure of isolated human adipocytes to recombinant oncostatin-M activated phospho-ERK and MAPK signalling and stimulated basal lipolysis (+47%, p<0.001). RNA sequencing in adipose tissue cell populations showed that oncostatin-M expression mainly arises from the macrophage fraction, while the corresponding receptors are expressed in adipocytes. Accordingly, oncostatin-M expression was induced in Thp1 macrophages in response to adrenergic stimuli (+620%, p<0.001), as well as in response to conditioned media from exercised myotubes (+ 79% at 3h, 2-way ANOVA p<0.001).
Conclusion: Collectively, our findings reveal that the adipose tissue transcriptome profile is robustly altered by exercise in a time-dependent manner, with a sustained increase in an inflammatory signature unique to Type 2 diabetes. Our results suggests that immune cells may be an important player in the crosstalk between skeletal muscle and adipose tissue during exercise, through the secretion of factors such as oncostatin-M to regulate adipocyte metabolism.
Supported by: NNF
Disclosure: L. Dollet: Grants; NNF20OC0060969.
83
High intensity interval training improves whole-body insulin sensitivity and skeletal muscle oxidative capacity and favourably affects hepatic fat storage
M. Bergman1, R. Mancilla1, P. Veeraiah2, V.H.W. de Wit-Verheggen1, Y.M.H. Bruls2, J. Hoeks1, V.B. Schrauwen-Hinderling1,2, M.K.C. Hesselink1;
1Department of Nutrition and Movement Sciences, Maastricht University, 2Department of Radiology and Nuclear Medicine, Maastricht University Medical Center+, Maastricht, Netherlands.
Background and aims: High intensity interval training (HIIT) is a time-efficient alternative for conventional exercise training and considered a promising exercise modality to counteract obesity-related metabolic impairments. We studied if HIIT prompts beneficial effects on insulin sensitivity, muscle oxidative capacity and intrahepatic lipid (IHL) content in overweight/obese individuals. Since carbohydrate-rich and insulinogenic sports drinks are frequently consumed after training and may impact the outcome of the training, we also explored if co-ingestion of a standardized glucose and casein hydrolysate post-exercise affects the anticipated HIIT-mediated metabolic improvements.
Materials and methods: Twenty-three overweight and obese adults (10 males and 13 females, mean age: 64 ± 7.5 years, BMI: 31.8 ± 3.3 kg/m2) completed 12 weeks of progressive HIIT on a stationary bike while consuming either water during/post exercise (HIIT+WAT) or with co-ingestion of a glucose and casein hydrolysate immediately post-exercise (HIIT+CHO/PRO). Before and after the HIIT program, muscle oxidative capacity (expressed as the PCr recovery rate constant) was assessed via phosphorus magnetic resonance spectroscopy (31P-MRS) and IHL content and composition were determined by proton magnetic resonance spectroscopy (1H-MRS). Whole-body insulin sensitivity was assessed by a 2-step hyperinsulinemic-euglycemic clamp (10 - 40 mU), along with maximal aerobic capacity (VO2max) on a cycle ergometer and body composition by BodPod.
Results: VO2max improved significantly from 41.0 ± 1.7 to 44.8 ± 1.8 ml/FFM/min in the HIIT+WAT group and from 42.8 ± 1.9 to 47.5 ± 2.0 ml/FFM/min in HIIT+CHO/PRO, whereas the PCr recovery rate constant improved in the HIIT+WAT group from 0.030 ± 0.002 to 0.041 ± 0.003 s-1 and in the HIIT+CHO/PRO group from 0.031 ± 0.002 to 0.038 ± 0.003 s-1. In the combined group, HIIT tended (P=0.06) to decrease IHL content significantly (P=0.048), increased the polyunsaturated fatty acid (PUFA) fraction and tended (P=0.08) to decrease the monounsaturated fatty acid (MUFA) hepatic fat content, whereas the saturated fatty acid fraction did not change. Whole-body insulin sensitivity, as reflected by the M-value, improved significantly upon HIIT, to a similar extent in both groups (HIIT+WAT: Δ 8.2 ± 3.7 and HIIT+CHO/PRO: Δ 6.9 ± 4.1 μmol/FFM/min, P = 0.01). No changes in body weight and body composition were observed upon HIIT in either of the groups.
Conclusion: HIIT promotes both insulin sensitivity and muscle oxidative capacity in overweight/obese individuals. Moreover, IHL composition changes slightly (a shift towards more PUFA and less MUFA) in overweight/obese individuals. These benefits can be attained regardless of co-ingesting glucose and proteins after exercise and occurred in the absence of changes in body weight or body composition.
Clinical Trial Registration Number: NCT03405545
Disclosure: M. Bergman: None.
84
The plasma and tissue metabolomics responses to high intensity interval training and its importance in insulin resistance in obesity and type 2 diabetes
P.M. Møller1, M.H. Petersen1, M.E. De Almeida2, N. Ørtenblad2, J. Havelund3, N.J.K. Færgeman3, K. Højlund1;
1Steno Diabetes Center Odense, Odense University Hospital, Odense C, 2Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense M, 3Biochemistry and Molecular Biology, Villum Center for Bioanalytical Sciences, University of Southern Denmark, Odense M, Denmark.
Background and aims: Insulin resistance (IR) links obesity with type 2 diabetes (T2D). Evidence suggests that IR is associated with a range of specific changes in circulating and inter-tissue metabolites. Physical activity improves insulin sensitivity in obesity and T2D. Recent data suggests that high-intensity interval training (HIIT) and recruitment of several muscle groups enhance the beneficial effects of exercise on glucose homeostasis. Here, we aimed to uncover the nearly undiscovered plasma and tissue metabolomics signatures of a novel HIIT protocol engaging upper and lower body muscles in obesity and T2D.
Materials and methods: Plasma samples and skeletal muscle and subcutaneous adipose tissue (SAT) biopsies were obtained from obese patients with T2D (n=15) and matched glucose-tolerant obese (n=15) and lean (n=18) individuals before and after their participation in an 8-week HIIT protocol combining rowing and cycling 3 times/week. Insulin sensitivity, VO2max, and body composition were assessed before and after the HIIT protocol. Plasma and tissue samples were investigated by untargeted metabolomics analyses using LC-MS. The metabolomics data will be used for biocomputational analyses, including clustering and correlation analyses.
Results: The HIIT protocol markedly increased insulin sensitivity (~30-40%) in all groups (p<0.05), and also improved VO2max (p<0.05), and reduced total body fat (p<0.001). These physiological changes in response to HIIT were accompanied by changes in the plasma metabolome, predominantly in T2D patients. The changes included the induction of lactic acid and amino acid derivatives (p<0.05), including BCAA degradation products, previously observed to increase glucose and fatty acid uptake in primary human adipocytes. The largest baseline differences were observed in the lipidome of T2D patients compared with the obese and lean groups (p<0.05). This included increased levels of sphingolipids, and diacylglycerols, previously coupled to tissue IR. The plasma data will be supplemented and compared to metabolomics and lipidomic analyses of fat and muscle biopsies, and further enrichment and correlation analyses with clinical data will be performed.
Conclusion: T2D patients display an altered lipidomic profile in plasma, whereas HIIT induces changes in the plasma metabolome, which could suggest a switch in energy metabolism and organ crosstalk.
Supported by: SDCO PhD grant, Region Syddanmark PhD grant
Disclosure: P.M. Møller: None.
OP 15 Preserving kidney function
85
Effects of treatment with semaglutide, empagliflozin and the combination on kidney function in type 2 diabetes: impact of oxygenation and perfusion
S. Gullaksen1,2, L. Vernstrøm1,2, S.S. Sørensen1,2, K.L. Funck2, P.L. Poulsen2, E.G. Laugesen2;
1Dept. of Clinical Medicine, Aarhus University, Aarhus, 2Aarhus University Hospital, Dept. of Endocrinology and Internal Medicine, Denmark.
Background and aims: Glucagon-like peptide-1 receptor agonists (GLP-1ra) and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) have each shown kidney protective effects in large clinical outcome-studies. The mechanisms behind these effects are sparsely elucidated and the effects of combination therapy remains obscure. In this randomized clinical trial, we compared kidney function in persons with type 2 diabetes receiving either the GLP1-ra semaglutide (SEMA), the SGLT2-i empagliflozin (EMPA) or the combination (COMBI). EMPA treatment was double blinded. Kidney oxygenation was primary outcome and regional (cortex and medulla) assessments were included.
Materials and methods: 80 participants with type 2 diabetes (age 68.1 ± 6.5 years, male 76.25 %, diabetes duration 8[4;12] years), were randomized to either placebo, EMPA, SEMA or COMBI for 32 weeks. Kidney excretory function was assessed by urinary albumine/creatinine ratio (UACR) and glomerular filtration rate (GFR). Magnetic resonance imaging was used to assess kidney oxygenation with R2*(Hz) (higher values indicating lower oxygenation) by a blood oxygen level depedent sequence (BOLD) and renal perfusion (arterial spin labelling (ASL)).
Results: UACR was reduced significantly for the COMBI group (-35% 95% CI [-46;-92]%, p=0.01), with the same tendency for each of the separate treatment arms. GFR decreased significantly from baseline and compared to placebo in all three treatment groups (SEMA -19 mL/min 95% CI [-7;-31]mL/min, p=0.001; EMPA -18 mL/min 95% CI [-6;-30]mL/min, p<0.001 and COMBI -26mL/min 95% CI [-14;-38]mL/min, p<0.001). Medullary renal oxygenation was reduced compared to placebo in both EMPA and the COMBI groups (figure 1A) (0.91s-1 95% CI [0.16;1.65]s-1, p=0.01 and 0.91s-1 95% CI [0.26;1.57]s-1, p< 0.01, respectively). EMPA reduced medullary oxygenation significantly compared to SEMA (1.03s-1 95% CI [0.22;1.84]s-1, p=0.01). No significant changes were observed in cortical oxygenation (figure 1A). Compared to placebo, cortical perfusion was reduced in SEMA and COMBI, whereas medullary perfusion was reduced across treatments, figure 1B.
Conclusion: Our data demonstrate joined effects of EMPA and SEMA on UACR and GFR and reveal important differential effects of EMPA and SEMA on renal physiology. As hypoxia is considered a main driver in kidney disease, we hypothesized that EMPA and/or SEMA would exert their beneficial effects through improved kidney oxygenation. Contrary to our hypothesis, EMPA treatment for 32 weeks was not associated with improved medullary oxygenation but even slightly reduced it. This is in line with recent rodent studies. SEMA treatment is associated with a substantial reduction in kidney perfusion, probably leading to reduced hyperfiltration. To our knowledge, these effects have not previously been demonstrated in humans.
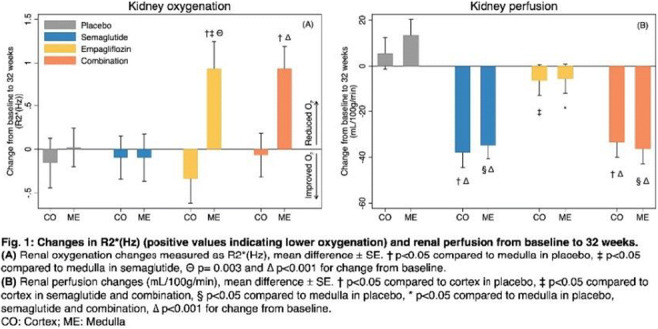
Clinical Trial Registration Number: EUDRACT 2019-000781-38
Supported by: NNF, DMA Research Foundation, Health Research Foundation of Central Denmark Region
Disclosure: S. Gullaksen: None.
86
Low blood oxygen saturation is associated with microvascular complications in individuals with type 1 diabetes
J.-C. Laursen, H.I. Mizrak, S.K. Heckquet, H. Kufaishi, M. Frimodt-Møller, C.S. Hansen, P. Rossing;
Steno Diabetes Center Copenhagen, København, Denmark.
Background and aims: Blood oxygen saturation (SpO2) is lower in both type 1 diabetes (T2D) and type 2 diabetes (T2D) as compared with non-diabetes (CON). Hypoxia is thought to play a role in the progression of diabetic complications, but it is unknown whether low SpO2 is associated with diabetic complications.
Materials and methods: Cross-sectional study in T1D and T2D separately. SpO2 was measured in the supine body position with pulse oximetry and patients were divided in low SpO2 (< 96%) and high SpO2 (≥ 96%) which has been proposed as a threshold. Outcomes were albuminuria (two out of three consecutive measurements ≥ 30 mg/g), any retinopathy (fundus photography), neuropathy (symmetric vibration perception threshold ≥ 25 V), and cardiovascular disease (CVD) (history). Odds ratios (OR) were adjusted for diabetes duration, gender, smoking, exercise, body mass index, and blood hemoglobin.
Results: We included 663 patients with T1D (23 with low vs. 640 with high SpO2) and 425 with T2D (43 with low vs. 382 with high SpO2). In T1D, the OR (95% CI, p-value) with low vs. high SpO2 was for albuminuria 3.3 (1.3 to 8.3, p < 0.01), for retinopathy 2.9 (1.1 to 7.8, p = 0.03), for neuropathy 5.3 (1.9 to 14.9, p < 0.01), and non-significant for CVD (1.0 (0.3 to 3.5, p = 0.95)). In T2D, there were no significant differences between low vs. high SpO2 in the odds of having albuminuria, retinopathy, neuropathy, or CVD (Figure 1).
Conclusion: SpO2 below 96% was associated with the microvascular complications, albuminuria, retinopathy, and neuropathy in T1D, but not with the macrovascular complication CVD. This could reflect that microvascular lung damage is related to hypoxia. No associations were observed in T2D.
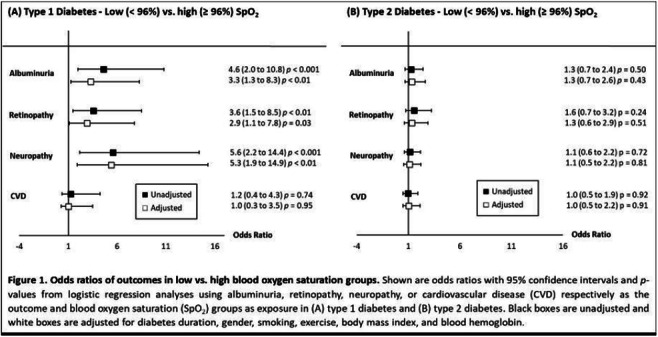
Supported by: NNF14OC0013659
Disclosure: J. Laursen: Lecture/other fees; For Boehringer Ingelheim, all fees donated to Steno Diabetes Center Copenhagen.
87
Development in eGFR trajectories in people with diabetic nephropathy
C.G. Poulsen1, K. Jesse1, B. Carstensen1, M. Frimodt-Moeller1, T.W. Hansen1, F. Persson1, D. Vistisen1,2, P. Rossing1,2;
1Steno Diabetes Center Copenhagen, 2University of Copenhagen, Copenhagen, Denmark.
Background and aims: Diabetic nephropathy (DN) is a frequent and serious complication to both type 1 diabetes (T1D) and type 2 diabetes (T2D) and is characterized by a progressive loss of kidney function, elevated blood pressure and increased risk of cardiovascular disease and death. The effect of advancing diabetes care over the past decades on progression and prognosis for persons diagnosed with DN requires updating. In this study we analyzed the development over calendar time in eGFR trajectories from the time of DN diagnosis.
Materials and methods: In a retrospective cohort study, data was collected from electronic health records from persons attending the outpatient clinic at Steno Diabetes Center Copenhagen, Denmark between 2001-2020. Inclusion criteria were: T1D/T2D and DN, defined as urine albumin to creatinine ratio (UACR) > 300mg/g or urine albumin excretion rate (UAER) > 300 mg/24hours in two separate measurements > 60 days apart. Individual eGFR trajectories were calculated separately for T1D and T2D, using mixed-effects models with fixed effects of age, sex, date of DN diagnosis, duration of DN, and random effects of person and duration of DN.
Results: The T1D cohort included 891 persons, 59.7% were male and median (IQR) age at DN diagnosis was 50 (38-62) years. Figure 1A shows the estimated trajectories for eGFR for a person diagnosed with T1D and DN at age 50 in 2000, 2005, 2010 and 2015. eGFR at the time of DN diagnosis increased over time with 1.8 ml/min/1.73m2 per calendar year. The T2D cohort included 1447 persons, 71.9% were male and median (IQR) age at DN diagnosis was 65 (58-72) years. Figure 1B shows the estimated trajectories for eGFR for a person diagnosed with DN at age 65 in 2000, 2005, 2010 and 2015. eGFR at the time of DN diagnosis increased with 0.7 ml/min/1.73m2 per calendar year. For both T1D and T2D, the trajectories depict a tendency towards an attenuating decline in eGFR over time, when diagnosed with DN in more recent years, but with a faster decline in the first five years after diagnosis of DN.
Conclusion: Kidney function at time of DN diagnoses has increased over the past 20 years, especially in T1D. Moreover, the eGFR decline after diagnosis seems attenuating when diagnosed in more recent years. This may be explained by improved awareness and treatment.
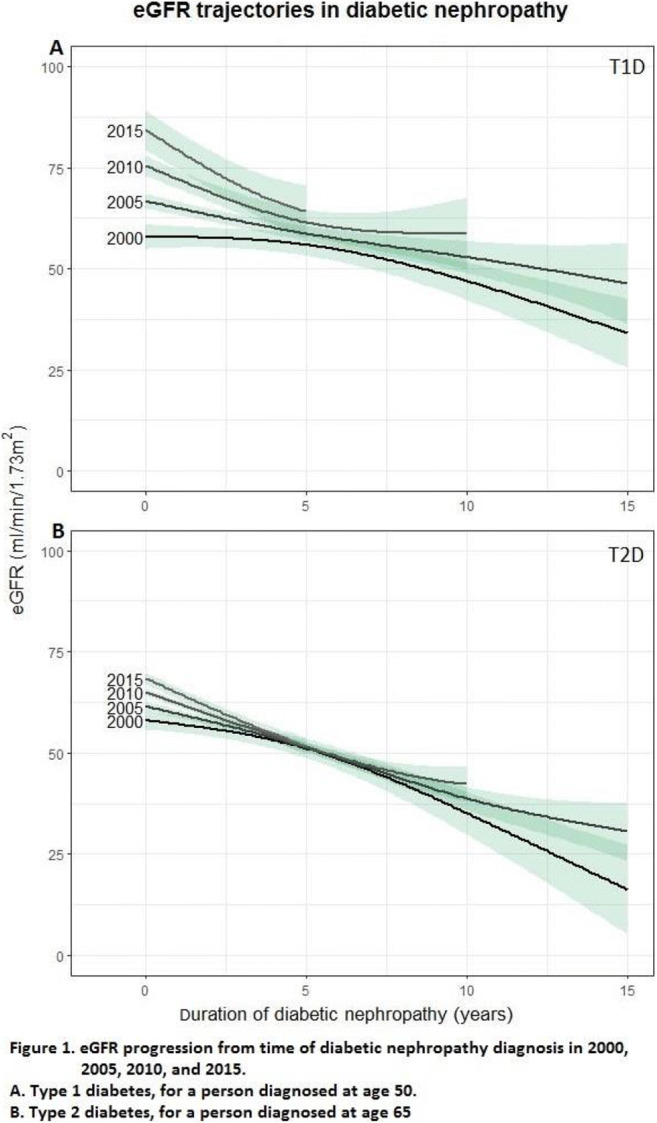
Supported by: Skibsreder Per Henriksen, R. og Hustrus Fond
Disclosure: C.G. Poulsen: None.
88
Shifts in KDIGO CKD risk groups with empagliflozin: Reno-protection from SGLT2 inhibition across the spectrum of risk
S.E. Inzucchi1, B. Zinman2, M. Mattheus3, D. Steubl3, A.P. Ofstad4, C. Wanner5;
1Yale School of Medicine, New Haven, USA, 2Mount Sinai Hospital, University of Toronto, Toronto, Canada, 3Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany, 4Boehringer Ingelheim Norway KS, Asker, Norway, 5Würzburg University Clinic, Würzburg, Germany.
Background and aims: Chronic kidney disease (CKD) is a common complication of type 2 diabetes (T2D), manifested by progressive decline in glomerular filtration rate (GFR) that can lead to end-stage kidney disease (ESKD) and need for renal replacement therapy. The impacts of CKD are increased cardiovascular (CV) and mortality risk, and healthcare costs. SGLT2 inhibitors reduce the progression of established CKD. The KDIGO ‘heat map’ has 18 categories of kidney function based on estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio. These categories are ordered into 4 groups with low, moderate, high and very high risk for progression to ESKD. The heat map is used clinically to track patients with or at risk for CKD and to encourage prompt subspecialty referral as needed. We sought to determine the effect of the SGLT2 inhibitor empagliflozin (empa) on changes in CKD risk by assessing worsening and improvement in KDIGO risk group in EMPA-REG OUTCOME.
Materials and methods: 7020 patients with T2D and established CVD were randomised and treated with empa 10, 25 mg (pooled for subsequent analyses) or placebo and followed for a median of 3.1 yrs. In this post hoc analysis, we categorised trial patients based on their KDIGO CKD risk group at baseline and their last value on-treatment. Worsening risk was defined as a shift to a more advanced group (e.g., low to moderate). Improvement in risk was defined as a shift to a less advanced group (e.g., high to moderate). We then compared the proportion (%) of patients who experienced worsening or improvement in risk status between empa and placebo by baseline KDIGO CKD risk groups. Odds ratios (OR) with 95% confidence intervals (CI) were calculated using logistic regression models, adjusting for study treatment, sex, region, baseline age, BMI, HbA1c and KDIGO risk.
Results: In the placebo group the % of patients with worsening in their KDIGO CKD risk were 32.1%, 31.5% and 36.3% in the low, moderate and high risk baseline groups, respectively. The corresponding values in the empagliflozin groups were lower: 26.0%, 24.4% and 23.3%, respectively. The OR (95% CI) for worsening risk across all baseline groups was 0.70 (0.62, 0.78) in favour of empa (Figure). Improvement in KDIGO CKD risk group was experienced by 18.7%, 16.8% and 18.5% of patients assigned to placebo in the moderate, high and very high baseline risk groups. The corresponding values in empagliflozin were higher: 24.6%, 27.1% and 26.2%, respectively. The OR (95% CI) for improved risk across all baseline groups was 1.56 (1.30, 1.86) in favour of empa (Figure).
Conclusion: The use of empa was associated with a 30% lower odds of worsening and >50% higher odds of improvement in KDIGO CKD risk groups. These data support greater use of SGLT2 inhibitors across a broad spectrum of CKD risk.
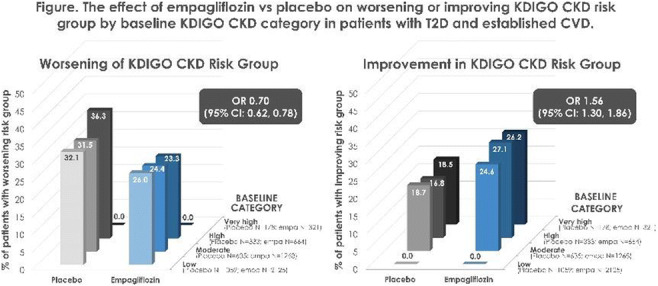
Clinical Trial Registration Number: EMPA-REG OUTCOME (NCT01131676)
Supported by: Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance
Disclosure: S.E. Inzucchi: Honorarium; AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Merck, Lexicon, Esperion, vTv Therapeutics, Abbott, Pfizer.
89
Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, reduces the risk of cardiovascular and renal disease as assessed by Steno Risk Engines in adults with type 1 diabetes
E.B. Stougaard1, P. Rossing1,2, D. Vistisen1,3, P. Banks4, M. Girard4, M.J. Davies4, F. Persson1;
1Steno Diabetes Center Copenhagen, Herlev, Denmark, 2Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 3Department of Public Health, University of Copenhagen, Copenhagen, Denmark, 4Lexicon Pharmaceuticals, Inc., The Woodlands, USA.
Background and aims: Sotagliflozin (SOTA) as an adjunct to insulin therapy improves glycemic control, without an increased risk of hypoglycaemia, reduces body weight and blood pressure (BP), and increases time in range in adults with type 1 diabetes (T1D). Treatment with SOTA is associated with an increased risk of diabetic ketoacidosis (DKA). SOTA demonstrated CV and renal benefits in high-risk adults with type 2 diabetes. The cardiorenal benefits along with the cardiometabolic effects may collectively outweigh the risk of DKA when evaluating SOTA for adults with T1D. The present analysis estimated the risk of CVD and end-stage kidney disease (ESKD) in adults with T1D treated with SOTA.
Materials and methods: Patient-level data were used from the three Phase 3 inTandem trials evaluating 2977 adults with T1D randomized to once-daily placebo, SOTA 200 mg, or SOTA 400 mg for 24 weeks. Analyses focused on 3 cohorts (1. SOTA 200 mg; 2. SOTA 400 mg; 3. SOTA pooled). A subgroup analysis was performed in patients with a BMI ≥27 kg/m2. For each patient, the cumulative risks of developing CVD and ESKD were estimated using the Steno T1 Risk Engines, which are validated prediction models for predicting 5- and 10-year risk of CVD and 5-year risk of ESKD. For CVD risk estimation, only patients without previous CVD (98% of overall cohort) at baseline were included. Baseline values for age and duration of diabetes were used in the model at Week 24. Missing albuminuria values were set to normal. Smoking and exercise history were not collected, and these variables were set to No. The estimated risk was calculated at baseline and Week 24 in both treatment groups. If a participant did not have a Week 24 assessment, a baseline observation carried forward approach was used. The difference in least-square mean percent change in estimated risk from baseline (95% CI and p-value) was compared between groups using a mixed model with percent change from baseline as dependent and including the treatment group as fixed effect, and the baseline value as covariate.
Results: SOTA significantly reduced 5- and 10-year CVD risk scores by approximately 4 to 7% compared to placebo at 24 weeks (Table). ESKD risk score was numerically reduced with SOTA 200 mg and significantly reduced with SOTA 400 mg relative to placebo. Similar results were observed with SOTA pooled and in patients with baseline BMI ≥27 kg/m2.
Conclusion: Using the Steno T1 Risk Engines, the estimated risk of CVD and ESKD was significantly reduced with SOTA compared to placebo in adults with T1D. This analysis provides additional clinical results that may positively enhance the benefit/risk assessment of SOTA use in T1D.
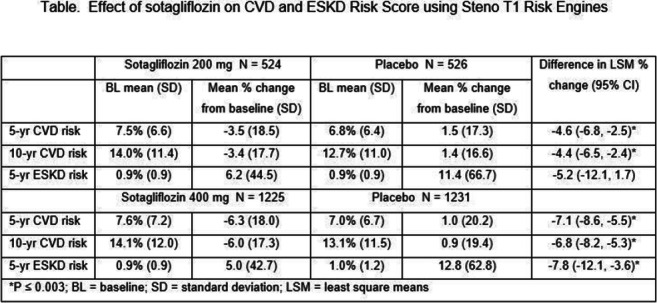
Disclosure: E.B. Stougaard: None.
90
Morphological and functional ultrasound features of diabetic kidney disease phenotypes in people with type 2 diabetes
M. Garofolo1, V. Napoli2, D. Lucchesi1, S. Accogli2, M. Mazzeo2, P. Rossi2, P. Falcetta1, M. Giambalvo1, E. Neri2, G. Penno1, S. Del Prato1;
1Department of Clinical and Experimental Medicine, University of Pisa, 2Department of Translational Research, Academic Radiology, University of Pisa, Pisa, Italy.
Background and aims: Diabetic kidney disease (DKD) develops in 40% of people with type 2 diabetes (T2D) and is the leading cause of end stage kidney disease. Non-albuminuric DKD has become the prevailing DKD phenotype (PH) and exhibits distinct clinico-pathological characteristics from those with albuminuria, including a higher proportion of females, better risk factors profile, and possibly distinct renal structural lesions on biopsy. Whether DKD PHs may also recognize differences in kidney morphological and vascular features has not been yet explored. We evaluated to which extent kidney ultrasonography (US) may differentiate DKD PHs in T2D subjects, in a cross-sectional, single-center study.
Materials and methods: DKD PHs were defined by KDIGO baseline eGFR strata and albuminuria categories: no-DKD (preserved eGFR and normoalbuminuria), albuminuria and preserved eGFR (DKD stages 1-2), decreased eGFR and normoalbuminuria (NA-DKD), and albuminuria with decreased eGFR (MA-DKD). Total and parenchymal renal volumes were calculated applying the ellipsoid formula for conventional (2D) US and with manual segmentation for 3D US (by X-matrix array technology); total and parenchymal renal volumes were adjusted for body surface area (aTRV, aPRV). Renal resistive index (RI) was also determined at the renal interlobar arteries. All US procedures were made by a radiologist blinded to patients’ characteristics.
Results: Out of 256 subjects, 26.2% had no-DKD, 24.6% DKD stages 1-2, 24.2% NA-DKD and 25.0% MA-DKD. No difference in albuminuria were observed between DKD stages 1-2 and MA-DKD (or between no-DKD and NA-DKD). Consistently, eGFRcreat levels did not differ in MA-DKD as compared to NA-DKD (or between no-DKD and DKD stages 1-2). Compared to no-DKD, RI was higher in all DKD PHs, being the highest in MA-DKD and with a significant trend of RI >0.70 prevalence to increase across PHs (p=0.022). aTRV3D and aPRV3D significantly differed across PHs (p<0.0001 for both). Compared to no-DKD (aTRV3D 180 ml/m2, [IQR 162-205], and aPRV3D 150 ml/m2, [132-162]), volumes were higher in DKD stages 1-2 (aTRV3D 198 ml/m2, [170-224] and aPRV3D 163 ml/m2, [140-184]) and reduced in both NA-DKD and MA-DKD, with significantly lower volumes in NA-DKD (aTRV3D 153 ml/m2, [136-168], and aPRV3D 127 ml/m2, [106-141]) as compared to MA-DKD (aTRV3D 164 ml/m2, [143-186], and aPRV3D 138 ml/m2, [122-150]; p=0.017 and 0.011 for aTRV3D and aPRV3D, respectively). These differences were confirmed for 2D volumes, and for DKD PHs established by GFR estimation based on creatinine and cystatin C CKD-EPI equation. In adjusted logistic regression models, compared to no-DKD, RI and aPRV3D were associated with DKD stages 1-2 and MA-DKD; only aPRV3D with NA-DKD. Compared to no-DKD, ROC curves, traced on top of conventional risk factors, showed that US parameters did not improve characterization of DKD stages 1-2 and MA-DKD, while aPRV3D significantly improved phenotyping of NA-DKD.
Conclusion: In type 2 diabetes, the emerging NA-DKD phenotype showed reduced TRVs and PRVs even when compared with MA-DKD with similar eGFR reduction. These findings support the hypothesis that different damage pathways underly the progression of different DKD phenotypes.
Disclosure: M. Garofolo: None.
OP 16 Flames and scars in the liver
91
Role of the constitutive androstane receptor CAR in sex- and gut microbiota-dependent non-alcoholic fatty liver disease
M. Huillet1, F. Lasserre1, A. Polizzi1, V. Alquier-Bacquie1, A. Fougerat1, C. Rives1, C. Martin1, J. Bruse1, J.H. Wan2, B. Chassaing3, S. Lotersztajn2, L. Gamet-Payrastre1, H. Guillou1, N. Loiseau1, S. Ellero-Simatos1;
1Toxalim, UMR 1331, INRAE, Université de Toulouse, Toulouse, 2INSERM-UMR 1149, Centre de Recherche sur l'Inflammation, Paris, 3INSERM U1016, CNRS UMR 8104, Université de Paris, Paris, France.
Background and aims: Non-alcoholic fatty liver diseases (NAFLD), including steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis and hepatocarcinoma, are sexually-dimorphic diseases, in which the gut microbiota seems to play a causal role. Mechanisms involved in disease progression remain incompletely understood. The constitutive androstane receptor (CAR, official name NR1I3) is a liver enriched-nuclear receptor, that regulates the transcription of genes involved in both xenobiotic detoxification and energy metabolism. In addition, CAR represents a potential candidate for the recognition of microbial signals by its ability to bind a large number of exogenous molecules. In this context, we aimed to investigate the potential role of CAR in the progression of NAFLD.
Materials and methods: First, we analyzed hepatic CAR expression using publicly available transcriptomic data from NAFLD human cohorts of the Gene Expression Omnibus (GEO) repository. Then, we used adult C57Bl6/J CARWT (WT) or CARKO (KO) female and male mice fed a chow diet (CD) or a sucrose and lipid enriched diet (western-diet, WD) for 14 weeks (n= 8-12/group). Gut microbiota composition, glucose and lipid metabolism and hepatic phenotype were studied using 16S-sequencing, glucose tolerance test, hepatic histology (H&E and sirus red staining), hepatic qPCR and plasma biochemical analysis.
Results: In clinical studies, CAR expression was lower in NASH patients with advanced fibrosis than in those with mild fibrosis (adjusted p-value (padj)=0.04 GSE163211, padj=0.01 GSE49541), and in patients with cirrhosis or hepatocarcinoma compared to healthy patients (padj =3x10-19 GSE10143, padj =10-40 GSE14520). Thus, hepatic expression of CAR seems to decrease during NAFLD progression in humans. In preclinical mouse studies, WD feeding increased body weight and induced liver triglyceride deposition (steatosis) in both males and females, with no significant effect of CAR deletion. KO WD fed males had significantly higher circulating levels of alanine aminotransferase (584±175 vs. 202±113 U.L-1, p=10-5) and alkaline phosphatase (242±48 vs. 113±37 U.L-1, p=10-5), indicative of liver damages, as well as increased liver fibrosis (% sirus red staining: 0.3±0.15 vs. 0.7±0.3, p=0.005; Col1a1 mRNA: 2.5 fold increase, p=10-5) , compared with WT males. In females, we observe no influence of genotype on WD-induced liver damages or fibrosis. Caecal microbiota composition of KO WD-fed males was significantly different from WT WD-fed males. To assess the role of gut microbiota in CAR-dependent liver dysfunctions, KO and WT male mice were co-housed in same cages and fed a WD. There were no differences in liver damages between WT and KO co-housed males, indicating that gut microbiota transfer by coprophagy attenuated WD-induced CAR-dependent liver damages.
Conclusion: CAR plays a sex- and gut microbiota-dependent role in WD-induced NAFLD in mice. We hypothesize that hepatic CAR is involved in the detoxification of gut microbial metabolites and we will further investigate this using portal vein metabolomics to unravel potential deleterious metabolites involved in sexually-dimorphic NAFLD.
Supported by: JPI HDHL ANR FATMAL
Disclosure: M. Huillet: None.
92
Hepatic fat and macrophages are increased in livers of diabetic patients without NAFLD
A. Korn1,2, C. Nadeem1, E. Bos1, H.W.M. Niessen1,2, S. Simsek3,4, P.A.J. Krijnen1,2;
1Pathology, Amsterdam UMC, Location VUmc, Amsterdam, 2Amsterdam Cardiovascular Sciences, Amsterdam, 3Internal Medicine, Northwest Clinics, Alkmaar, 4Internal Medicine, Amsterdam UMC, Location VUmc, Amsterdam, Netherlands.
Background and aims: Diabetes mellitus (DM) is strongly associated with non-alcoholic fatty liver disease (NAFLD), which increases risk of severe liver disease as well as extra-hepatic microvascular disorders. NAFLD is diagnosed in liver biopsies when fat accumulation, lymphocytes, ballooning of hepatocytes and/or fibrosis are found, but it is often diagnosed relatively late due to limitations of current diagnostic techniques and minor clinical manifestations in the early stages of development. This study aimed to analyse putative early changes in the liver of deceased DM patients that were without clinical diagnosis of NAFLD and did not have histopathological characteristics of NAFLD at autopsy, and analysed age/sex effects hereon.
Materials and methods: Liver tissue was obtained at autopsy from 24 DM patients and 66 non-diabetic control patients. All patients did not have histopathological characteristics of NAFLD. Hepatic fat (percentage and number of cells) and inflammatory cells (CD45-positive lymphocytes and CD68-positive macrophages) were studied through (immuno)histochemical analysis.
Results: We observed a 5-fold increase in percentage of fat (0.7±1.1, p=0.0007) and an 8-fold increase in amount of fat cells in DM patients (94.6±133.5, p<0.0001) compared with the non-diabetic controls (% fat: 0.4±0.9; fat cells: 19.9±47.8). The number of CD45-positive lymphocytes was not affected in the diabetic livers (p=0.06), whereas the number of CD68-positive cells was increased in DM patients (p=0.0002) compared to controls. Fat percentage and fat cells were significantly higher in DM2 patients compared to both DM1 (p=0.02 and p=0.04 respectively) and non-diabetic controls (p=0.0007 and p<0.0001 respectively), while the number of CD68-positive cells was significantly elevated in both DM groups, regardless of type (DM1: p=0.0008; DM2: p=0.007). We found no significant differences between men and women in the non-diabetic control groups nor in the diabetic patient group, regardless of age. No correlation was found between hepatic fat and inflammation.
Conclusion: Hepatic fat and the number of macrophages is increased in the liver in DM patients that are not yet diagnosed with NAFLD, warranting early clinical investigation for signs of NAFLD in patients with DM.
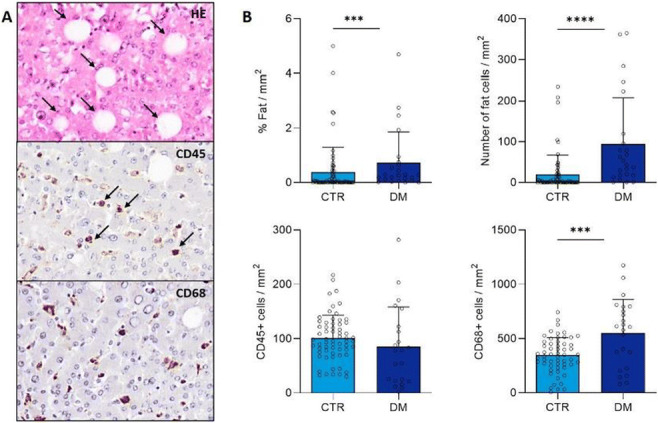
Supported by: EFSD/Sanofi European Diabetes Research Programme in Macrovascular Complications
Disclosure: A. Korn: None.
93
Ubiquitin-proteasome system dysfunction in the liver of severely obese men with and without type 2 diabetes
B. Stocks1, E. Näslund2, J.R. Zierath3, A.S. Deshmukh1;
1Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen N, Denmark, 2Department of Clinical Sciences, Karolinska Institutet, Stockholm, Sweden, 3Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Background and aims: Dysregulated liver metabolism, in particular insulin resistance, is a predominant factor in the pathogenesis of type 2 diabetes. Protein degradation via the ubiquitin-proteasome system has been implicated in the development of type 2 diabetes. Through a diversity of signals, ubiquitination regulates a range of cellular processes such as protein degradation, enzyme activation and sub-cellular localisation. Nonetheless, a global understanding of ubiquitin-proteasome system dysfunction in obesity and type 2 diabetes has yet to be established. As the human genome encodes more than 600 E3 ligases (which catalyse the transfer of ubiquitin to protein substrates) and over 100 deubiquitinases, a systems approach is required to truly understand ubiquitin-proteasome system dysfunction. We aimed to characterise the regulation of the ubiquitin-proteasome system in the liver of severely obese men with and without type 2 diabetes.
Materials and methods: Liver samples from 9 non-obese men, 8 severely obese men (Ob) and 10 severely obese men with type 2 diabetes (Ob-T2D) were collected during cholecystectomy (non-obese) or Roux-en-Y gastric bypass surgery (Ob and Ob-T2D). Samples were lysed in 4% SDS and digested using Lys-C and trypsin. Peptides were measured via liquid chromatography tandem mass spectrometry (LC-MSMS) using an EASY nanoLC coupled to an Exploris 480 Orbitrap on a 100-min gradient using data independent acquisition. Protein ubiquitination was assessed via immunoblotting in human and rodent liver.
Results: We identified the regulation of 4319 proteins in the livers of 9 non-obese, 8 severely obese non-diabetic men (Ob) and 10 severely obese men with type 2 diabetes (Ob-T2D), collected during cholecystectomy (non-obese) or Roux-en-Y gastric bypass surgery (Ob and Ob-T2D). We found 162 proteins that were differentially regulated in Ob and/or Ob-T2D compared to non-obese men. Of these, 11 ubiquitin-proteasome system proteins were regulated, including the E3 ligases COPB2, DCAF11, DCUN1D1, EML2, LRSAM1, SEC13, SEC31A, STRN and TBL2, the deubiquitinase UCHL1, and the 26S proteasome protein PSMD7. Furthermore, protein ubiquitination is dysregulated in the liver of Ob and Ob-T2D patients.
Conclusion: These data identify ubiquitin-proteasome system dysfunction in the liver of severely obese men with and without type 2 diabetes, providing protein targets for future mechanistic and therapeutic research.
Supported by: EFSD Rising Star Fellowship Programme
Disclosure: B. Stocks: Grants; EFSD Rising Star Fellowship Programme.
94
Liver inflammation increases the proliferation of oval cells during non-alcoholic fatty liver disease
S. Calero Pérez1,2, I. Barahona1,2, P. Valdecantos1,2, Á.M. Valverde1,2;
1IIBm Alberto Sols (CSIC-UAM), 2CIBERdem (ISCIII), Madrid, Spain.
Background and aims: Oval cells (OCs) are hepatic progenitor cells with an emerging role in repopulation capacity and differentiation towards hepatocytes or colangiocytes under conditions of liver damage. However, their susceptibility to an inflammatory environment in the context of obesity-associated insulin resistance and non-alcoholic liver disease (NAFLD) is unknown. On that basis, the aim of this study was, first, to identify the impact of an environment mimicking metainflammation in obesity and NAFLD in OC expansion in mice and, second, to study the interactome between OCs and immune cells of the liver at the molecular and cellular levels.
Materials and methods: To induce NAFLD, eight week-old male mice (C57Bl6j x 129 sv) were fed a Western Diet (WD) containing 21.1% fat, 41% sucrose, and 1.25% cholesterol and a high sugar solution (23.1 g/L d-fructose and 18.9 g/L d-glucose) for 12 weeeks. Additionally, CCl4 (0.32 μg/g body weight) was injected intra-peritoneally (i.p.) once per week, starting one week after the WD. OCs were isolated from mouse livers and established in culture. To generate a NAFLD-like proinflammatory environment, peritoneal macrophages or bone marrow-derived macrophages (BMDM) were treated with a mixture of palmitate (400 μM) and LPS (150 ng/ml) (PA/LPS) for 8 h, after which the culture medium was replaced with fresh medium for a further 16 h. The conditioned medium (CM) was used to stimulate OCs for different time-periods.
Results: NAFLD was evaluated in mice upon 12 weeks of intervention and histopathological analysis of liver sections showed features of non-alcoholic steatohepatitis (NASH) including steatosis, ballooning, inflammation and fibrosis, as revealed by the NAS score (p<0.001, n=6-11), serum transaminases (p<0.001, n=6-11), Col1a1 mRNA levels (p<0.001, n=6-11) and hydroxyproline (p<0.001, n=6-11). Importantly, a marked increase in A6/SOX9 positive cells (p<0.001, n=6-11), surrounded by inflammatory monocytes (Ly6c+) was found in all mice with NASH (p<0.01, n=3), together elevated mRNA levels of the progenitor markers Epcam and Krt19 (p<0.001, n=6-11), pointing to a major presence of OCs in livers with NASH. Stimulation of OCs with CM collected from PA/LPS-stimulated macrophages for 5-30 min triggered a rapid activation of proinflammatory signaling cascades including phosphorylation of STAT3 (p<0.001, n=3), p65-NFκB (p<0.05, n=3), degradation of IκBα (p<0.05, n=3) and nuclear translocation of p65-NFκB (p<0.001, n=3). Treatment for 24 h with PA/LPS-CM increased the proliferation of OCs as reflected by increased PCNA levels (p<0.05, n=3). Gene expression levels related to OCs were obtained from the NCBI Gene Expression Omnibus database based on the gene chips of fibrotic livers associated to NAFLD (GSE130970, GSE49541, GSE162694 and GSE48452). In line with data in mice, expression of the progenitor markers SOX9, EPCAM and KRT7, was significantly increased in NAFLD patients with advanced fibrosis (p<0.001 n=8-103).
Conclusion: Our results have demonstrated that OC expansion concurs with inflammatory features during NAFLD progression in mice. The molecular studies revealed that the interactome between liver inflammatory cells and OCs favors OCs proliferation, an effect that opens therapeutic perspectives to preserve OCs proliferation under liver damage associated to NAFLD, but favoring their plasticity.
Supported by: RTI2018-094052-B-100 (MICINN/AEI/FEDER, EU), S2017/BMD-3684 (Comunidad de Madrid, Spain)
Disclosure: S. Calero Pérez: None.
95
Phthalate exposure is associated with NAFLD, but not with liver fibrosis in the United States
S. Ciardullo1, E. Muraca2, R. Cannistraci1, G. Lattuada2, G. Perseghin1;
1University of Milano Bicocca, 2Policlinico di Monza, Monza, Italy.
Background and aims: Recent epidemiological observations have reported an association between phthalates exposure and insulin resistance and obesity while data are scarce on the association between these pollutants and nonalcoholic fatty liver disease (NAFLD) and liver fibrosis.
Materials and methods: To address this gap in knowledge we performed a population-based cross-sectional study using data from the 2017-2018 cycle of the National Health and Nutrition Examination Survey, a complex survey conducted to gain information on the health status of the general population of the United States. The target population consisted in all adult participants with available data on vibration controlled transient elastography (VCTE) and on urinary phthalate concentrations, with the exclusion of individuals with chronic viral hepatitis and/or significant alcohol consumption (>1 standard drink/day in women and >2 standard drink/day in men). Concentrations of mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) were measured in a spot morning urine sample using liquid chromatography-electrospray ionization-tandem mass spectrometry. Multivariable logistic regression analysis was used to evaluate the association between phthalate concentrations and liver steatosis (controlled attenuation parameter > 274 dB/m) and significant liver fibrosis (liver stiffness measurement > 8 kPa) after adjustment for confounders.
Results: A total of 1793 participants were included. Weighted prevalence of NAFLD and significant fibrosis were 40.3% and 6.8%, respectively. Patients with NAFLD showed higher levels of both MEOHP and MEHHP, while no difference was found in MEHP. Concentrations of all three metabolites did not differ significantly between participants with and without significant liver fibrosis. After adjustment for potential confounders including age, race-ethnicity, body mass index, glomerular filtration rate and diabetes, we found a significant positive association between both MEOHP (OR 1.01, 95% CI 1.00-1.02 per unit increase, p=0.014) and MEHHP (OR 1.01, 95% CI 1.00-1.03 per unit increase, p=0.031) and NAFLD, while no significant association was found for MEHP. None of the three molecules was associated with fibrosis in the multivariable model.
Conclusion: Our findings have revealed an association between phthalate exposure and NAFLD, but not liver fibrosis independently of diabetes and obesity. Further studies elucidating potential pathophysiological mechanisms are needed.
Disclosure: S. Ciardullo: None.
96
The fibrotic NASH index: a simple non-invasive score to screen for liver disease in individuals with metabolic risk factors
F. Tavaglione1,2, O. Jamialahmadi2, A. De Vincentis3, S. Qadri4, V. Bruni5, S. Carotti6, G. Perrone6, D. Tuccinardi7, S. Manfrini7, P. Pozzilli7, A. Picardi1, H. Yki-Järvinen4, L. Valenti8, U. Vespasiani-Gentilucci1, S. Romeo2,9;
1Clinical Medicine and Hepatology Unit, Campus Bio-Medico University, Rome, Italy, 2Department of Molecular and Clinical Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 3Internal Medicine Unit, Campus Bio-Medico University, Rome, Italy, 4Department of Medicine, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 5Bariatric Surgery Unit, Campus Bio-Medico University, Rome, Italy, 6Department of Pathology, Campus Bio-Medico University, Rome, Italy, 7Department of Endocrinology and Diabetes, Campus Bio-Medico University, Rome, Italy, 8Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milano, Italy, 9Clinical Nutrition Unit, Department of Medical and Surgical Sciences, University Magna Graecia, Catanzaro, Italy.
Background and aims: Type 2 diabetes is a key risk factor for non-alcoholic fatty liver disease (NAFLD), which is the major cause of chronic liver disease worldwide. Indeed, up to 65% of individuals with type 2 diabetes have NAFLD. Given the huge number of individuals at high risk of NAFLD, accurate and affordable non-invasive screening strategies for liver disease are urgently needed. Herein, we aimed to develop a simple non-invasive score using routine laboratory tests to identify, among individuals at high risk of NAFLD, those with fibrotic non-alcoholic steatohepatitis (NASH) defined as NASH, NAFLD activity score (NAS) ≥4, and fibrosis stage ≥2.
Materials and methods: The derivation cohort included 264 morbidly obese individuals undergoing intraoperative liver biopsy in Rome, Italy. The best predictive model was developed and internally validated using a bootstrapping stepwise logistic regression analysis (2000 bootstrap samples). Performance was estimated by the area under the receiver operating characteristic curve (AUROC). External validation was assessed in three independent European cohorts (Finland, n=370; Italy n=947; England n=5,368) of individuals at high risk of NAFLD, namely with type 2 diabetes, overweight/obesity, and/or metabolic syndrome. All cohorts received Local Research Ethics approval and all participants gave written informed consent to the study.
Results: The final predictive model, designated as Fibrotic NASH Index (FNI), combined aspartate aminotransferase (AST), high-density lipoprotein (HDL) cholesterol, and hemoglobin A1c (HbA1c). Performance for fibrotic NASH was satisfactory in both derivation and external validation cohorts (AUROCs 0.78 and 0.80-0.95, respectively). In the derivation cohort, rule-out and rule-in cut-offs were 0.10 for sensitivity ≥0.89 (negative predictive value [NPV] 0.93) and 0.33 for specificity ≥0.90 (positive predictive value [PPV] 0.57), respectively. In external validation cohorts, sensitivity ranged from 0.87 to 1 (NPV 0.99-1) and specificity from 0.73 to 0.94 (PPV 0.12-0.49) for rule-out and rule-in cut-off, respectively.
Conclusion: FNI is the first score for fibrotic NASH based on simple blood tests, namely AST, HDL cholesterol, and HbA1c. FNI may represent an accurate and affordable non-invasive tool to screen for liver disease individuals with metabolic risk factors in primary healthcare and diabetology clinics.
Supported by: VR; VINNOVA; Svenska Diabetesstiftelsen; Hjärt-Lungfonden; KAW; NNF; SSF; AZN
Disclosure: F. Tavaglione: None.
OP 17 Toying with monitoring: from Present Continuous to Future Perfect
97
Gaps remain in achieving target type 1 diabetes glycaemic goals despite advanced technologies
L. Laffel1, J. Liu2, L. Titievsky3, K. Hagan3, T. Liu3, K. Chandarana3, J. Gaglia3, W. Wolf2, J. Bispham4, K. Chapman2, D. Finan2, R. Bergenstal5;
1Dept of Pediatric & Adoles. Med., Joslin Diabetes Centre, Boston, 2T1D Exchange, Boston, 3Vertex Pharmaceuticals, Boston, 4Evidera PPD, Waltham, 5International Diabetes Center, HealthPartners Institute, Minneapolis, USA.
Background and aims: Continuous glucose monitoring (CGM) metrics and self-reported disease characteristics (severe hypoglycaemic events [SHEs], HbA1c) warrant further description in people with T1D using CGM and pumps, including hybrid closed-loop systems (HCLS).
Materials and methods: We conducted a one-time online survey of adults with T1D in the T1D Exchange registry in the USA or online communities, where ~50% of participants contributed up to 1 year of CGM data. Patients were asked about their medical history (SHEs, HbA1c) while glucose management indicator (GMI), prolonged hypoglycaemic events (<54 mg/dL), time in and below range (TIR/TBR), and coefficient of variation (CV) were derived from CGM data.
Results: Patients who completed the survey and contributed CGM data (N=926) had a mean age of 42y and T1D duration of 25y; 73% were female, 96% white; 94% had ≥1 year of CGM use. Mean HbA1c was 6.6% (69.0% had HbA1C <7%). While most patients met consensus glycaemic targets (HbA1c, GMI, TIR, TBR, and CV), with higher proportions observed in those using HCLS versus those using pump + CGM (not HCLS) and MDI + CGM (Table), patients continued to have significant hypoglycaemia based on CGM data and an average of 1.1 SHEs in the prior year.
Conclusion: Despite improvements in glycaemic control (TIR, TBR, and self-reported HbA1c) with advanced technologies, many patients are still unable to achieve clinical targets and experience significant hypoglycaemia, highlighting the unmet need for novel T1D treatments.
Supported by: Vertex Pharmaceuticals
Disclosure: L. Laffel: Other; Advisory boards/consulting for Medtronic, Dexcom, Janssen, Boehringer Ingelheim, Provention, Dompe, Lilly, Roche, and Insulet.
98
Intermittently scanned continuous glucose monitoring is associated with a long-term glucose-lowering effect for type 1 diabetes patients in poor glycaemic control
M.H. Jensen1,2, S.L. Cichosz2, P. Gustenhoff3, A. Nikontovic1, O. Hejlesen2, P. Vestergaard3,1;
1Steno Diabetes Center North Denmark, Aalborg University Hospital, 2Department of Health Science and Technology, Aalborg University, 3Department of Endocrinology, Aalborg University Hospital, Aalborg, Denmark.
Background and aims: Lowering glucose levels is a complex task for patients with type 1 diabetes and they often lack close contact to health care professionals. Several studies have shown that reduced HbA1c can be obtained from use of intermittently scanned continuous glucose monitoring (isCGM), but the studies are typically of a shorter duration of 3-6 months with strict inclusion/exclusion criteria or with patient populations with mixed indications. The aim of this study was to investigate the long-term effect of isCGM on HbA1c in type 1 diabetes patients with poor glycaemic control in a region-wide real-world setting.
Materials and methods: All type 1 diabetes patients receiving an isCGM device due to poor glycaemic control (≥70 mmol/mol) in the period of 2020-21 in Region North Denmark (“T1D-CGM”) were compared with all T1D patients without isCGM (“T1D-NOCGM”) in the same period. Patients in the T1D-NOCGM group inherited the date of initiation of isCGM as an index date from the T1D-CGM patient with the closest year of birth. A multiple linear regression model adjusted for age, sex, diabetes duration and use of continuous subcutaneous insulin infusion was constructed to estimate the difference in change from baseline HbA1c between the two groups. Change from baseline HbA1c was for each person calculated as the difference between the average of all values one year before initiation of isCGM (baseline HbA1c) and the average of all values two years after.
Results: The cohort consisted of 897 patients receiving isCGM and 1,630 without isCGM. Included T1D-CGM patients were 47% female, had an average age of 46 years (SD: 16 years), a diabetes duration of 12 years (SD: 8 years) and a baseline HbA1c of 80 mmol/mol (SD: 12 mmol/mol). T1D-NOCGM patients were 41% female, had an average age of 54 years (SD: 17 years), a diabetes duration of 12 years (SD: 10 years) and a baseline HbA1c of 64 mmol/mol (SD: 16 mmol/mol). The estimated adjusted difference in change from baseline HbA1cbetween T1D-CGM vs T1D-NOCGM was -5.9 mmol/mol (95% CI: -6.7 to -4.7 mmol/mol; p<0.0001) and the development in average HbA1c levels can be seen in the figure below.
Conclusion: Our results indicate that type 1 diabetes patients with poor glycaemic control from Region North Denmark benefit from using isCGM with a sustained 24-month improvement in HbA1c. However, results should be interpreted with care, as the study was not randomized. Results need to be further investigated, for example, with subgroup analysis to clarify if all subgroups benefit equally from CGM.
Disclosure: M.H. Jensen: Employment/Consultancy; Abbott Laboratories A/S. Stock/Shareholding; Novo Nordisk A/S.
99
Conversion from FreeStyle Libre to Freestyle Libre 2 is associated with improvements in continuous glucose monitoring metrics
F.W. Gibb, R.H. Stimson, R.J. Wright, S. Forbes, M.W.J. Strachan, A. Dover;
Edinburgh Centre for Endocrinology & Diabetes, Edinburgh, UK.
Background and aims: Real-time continuous glucose monitoring (rtCGM) is associated with improvements in glycaemic control in comparison to Freestyle Libre. The ALERTT1 study indicated improvements in both high and low glucose metrics favouring rtCGM over the original Freestyle Libre system. We sought to assess whether Freestyle Libre 2, with alarm functions, was associated with similar improvements.
Materials and methods: An observational study to assess changes in CGM metrics, at six months, in 415 adults with type 1 diabetes (≥ 1 year's duration) after converting from Freestyle Libre to Freestyle Libre 2 . Secondary outcomes included predictors of reduction in time below range (TBR) ≥ 0.5% (<3.9mmol/l) and increase in time in range (TIR) ≥5.0% (3.9 - 10 mmol/l). Participants were included if ≥70% CGM data (2 week block) was available both at baseline and six months.
Results: Low and high glucose alarms were used by 74.5% and 58.6%, respectively. In all users, TBR fell by a median of 1.0% (IQR -2.7 - 0.3, p <0.001) after 6 months but TIR was unchanged (p = 0.920) (table). Average duration of low glucose events (<3.9 mmol/l) fell by 14 minutes (-41 - 13, p <0.001) and the number of low glucose events per 2 weeks fell from 7 to 4 (p <0.001) . Low alarm thresholds were significantly correlated with baseline TBR (R -0.232, p < 0.001) and high alarm thresholds were significantly correlated with baseline time above range (R 0.168, p = 0.009). Alarm thresholds were not independently associated with glycaemic response, however low alarm use was independently associated with a fall in TBR of ≥0.5% (OR 2.9 [95% CI 1.5 - 5.6], p = 0.002) and high alarm use was associated with a rise in TIR of ≥5% (OR 2.17 [1.2 - 4.2], p = 0.013).
Conclusion: Freestyle Libre 2 was associated with modest but clinically relevant improvements in hypoglyaemia metrics but did not improve TIR or average glucose. Low and high glucose alarms were independently associated with improvements in TBR and TIR, respectively, and users of this system should be encouraged to use alarms at thresholds appropriate for the individual's circumstances.
Disclosure: F.W. Gibb: Honorarium; Abbott Diabetes, Insulet, Novo Nordisk, Lilly.
100
Efficacy and safety of real-time continuous glucose monitoring guiding insulin administration in the hospital: a randomised clinical trial
G.E. Umpierrez1, R.J. Galindo1, M.A. Urrutia1, P. Vellanki1, A.L. Migdal1, G.M. Davis1, F.J. Pasquel1, M. Fayfman1, T. Idrees1, L. Peng1, L.G. Singh2, E.K. Spanakis2;
1Div. of Endocrinology, Emory University School of Medicine, Atlanta, 2Div. of Endocrinology, University of Maryland Medical Center, Baltimore, USA.
Background and aims: Inpatient studies in insulin-treated patients have reported high accuracy and higher detection of hypo- and hyperglycaemic events with the use of real-time continuous glucose monitoring (RT-CGM) compared to capillary point-of-care (POC) glucose testing. In a randomised controlled study, we tested the efficacy and safety of RT-CGM in adjusting inpatient insulin therapy.
Materials and methods: A total of 173 general medicine and surgery patients with type 1 and type 2 diabetes treated with a basal bolus insulin regimen were randomised to a standard of care (POC group, n=85) wearing a blinded Dexcom G6 CGM for up to 10 days with insulin dose adjusted based on POC results, or to a RT-CGM group (n=88) who had their insulin adjustment based on daily Dexcom G6 RT-CGM profile. Primary endpoints were differences in time in range (TIR: 3.9-10 mmol/L) and hypoglycemia (<3.9 and <3.0 mmol/L).
Results: There were no significant differences in TIR (54.51%±27.72 vs 48.64%±24.25, p=0.14), mean daily glucose (10.2±2.2 mmol/L vs 10.4±2.2 mmol/L, p=0.36), percent of patients with CGM values <3.9 mmol/L (36% vs 39%, p=0.68) or <3 mmol/L (14% vs 24%, p=0.12) between RT-CGM-guided and POC group. Among patients with ≥ 1 hypoglycaemic event, compared to POC, the RT-CGM group experienced a significant reduction in hypoglycaemia reoccurrence (1.80±1.54 vs 2.94±2.76 events/patient, p=0.037), lower percentage of time below range (TBR) <3.9 mmol/L (1.89%±3.27 vs 5.47%±8.49, p=0.024), lower recurrent nocturnal hypoglycaemic events (1.21±0.43 vs 1.93±0.92, p=0.02) and lower incidence-rate ratio <3.9 mmol/L (0.53, 95% CI:0.31-0.92) and <3.0 mmol/L (0.37, 95% CI:0.17-0.83).
Conclusion: Our results indicate that the inpatient use of Dexcom G6 RT-CGM is safe and effective in guiding insulin adjustment resulting in a similar improvement in glycaemic control and in a significant reduction of recurrent hypoglycaemic events compared to POC-guided insulin adjustment.
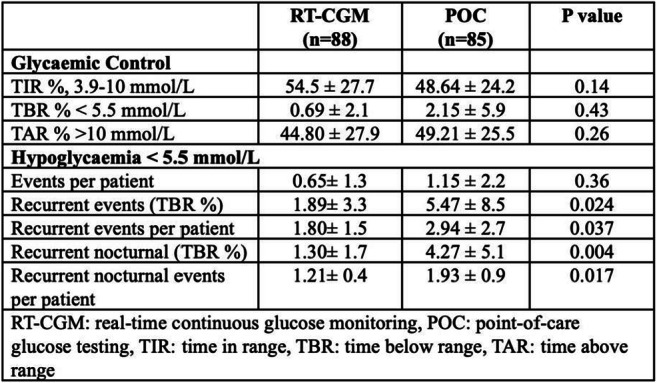
Clinical Trial Registration Number: NCT03877068
Supported by: Dexcom
Disclosure: G.E. Umpierrez: Grants; Dexcom, Baxter.
101
Cutaneous reactions associated with diabetes adhesives devices: results of the CutaDiab study
M. Diedisheim1, A. Sola-Gazagnes1, A. Carlier2, L. Potier2, A. Hartemann3, S. Jacqueminet3, C. Pecquet4, J.-B. Julla5, T. Vidal-Trecan5, J.-F. Gautier5, E. Larger1, D. Dubois Laforgue1, R. Roussel2, J.-P. Riveline5;
1Diabetology, Cochin Hospital, APHP, 2Diabetology, Bichat Hospital, APHP, 3Diabetology, Pitié-Salpêtrière Hospital, APHP, 4Dermatology, Tenon Hospital, APHP, 5Diabetology, Lariboisière Hospital, APHP, Paris, France.
Background and aims: The use of devices adhering to the skin for between 2 and 14 days is constantly increasing, as continuous glucose measurement systems (CGM) and insulin pumps. Skin reactions have been observed in patients with diabetes using these new technologies, sometimes leading to discontinuation. Prevalence and consequences of these skin intolerances are unknown. The objective of this study is to determine the prevalence and consequences of cutaneous reactions to CGM or pumps.
Materials and methods: This is an observational, cross-sectional, multicentre study involving four university hospital diabetology departments (Paris, France). A form with about fifty questions concerning diabetes evolution, use of pump and CGM, and description and consequences of possible cutaneous reaction was drawn up on touchpad. All adult patients with diabetes seen in consultation over a period of 6 months and using or having used in the last 10 years a system with skin adhesives were included. Included devices were insulin patch pump (e.g. OMNIPOD®, cell Novo®), pump with externalized catheter (e.g. PARADIGM®, ANIMAS®, MINIMED 640G®, YpsoPump®) and CGM system (Free Style®, DexCom® sensors, Enlite® sensors). Non-parametric tests were used for quantitatives values (Mann-Withney or Kruskal-Wallis tests), and chi-squared test for qualitative values.
Results: 851 patients were included. At least one cutaneous reaction was reported in 28% of CGM users (231/833) and in 29% of pump users (108/374). Patients with cutaneous reaction were more often women for CGM (63 vs 50% for patients with and without cutaneous reaction respectively, p<0.001) and for pump (73 vs 58%, p=0.006), and had more often type 1 diabetes than type 2 diabetes for CGM (84 vs 73%, p<0.001), without difference of type of diabetes for pump (91 vs 90% of type 1 diabetes). Symptoms were similar for CGM and pump reactions: erythema and pruritus in 70-75% of patients, pain in 20-25%, vesicle and peeling in 12-15%. The first symptom appeared within 24 hours of the first use of the device for 24% of CGM reactions, and 22% of pump reactions, or more than 6 months after the first use for 38% of CGM reactions, and 47% of pump reactions. Among patients with cutaneous reaction, this reaction does not modify the use of the device for 82% of reactions to CGM, and 80% for pump. Device use was definitely stopped for 12% of CGM reaction (3.2% of all users, 27/833), and for 7% of pump reaction (2.1% of all users, 8/374). The other reactions led to a decrease in use or to a change of device (6% for CGM, 13% for pump). Cutaneous reaction was more common for tubeless pumps than tubed pumps (31 vs 23%, p=0.03). These cutaneous reactions were no larger than the adhesive in 89% of cases for CGM and 93% for pump, suggesting skin irritation rather than an allergy.
Conclusion: Cutaneous reaction is a common reaction in pump or CGM users, but without consequences for the use of the device in the vast majority of cases, with very similar symptoms between CGMs and pump reactions. However, this reaction, which can appear more than 6 months after initiation of the device, leads to the interruption of its use in 2-3% of patients.
Clinical Trial Registration Number: NCT04853810
Disclosure: M. Diedisheim: None.
102
Impact of treatment costs on the cost-effectiveness of real-time continuous glucose monitoring vs self-monitoring of blood glucose in type 1 diabetes patients in the United Kingdom
H. Alshannaq1,2, G. Cogswell1, G.J. Norman1, P.M. Lynch1, S. Roze3;
1Dexcom, San Diego, USA, 2University of Cincinnati, Cincinnati, USA, 3Vyoo Agency, Lyon, France.
Background and aims: Previous studies have shown that real-time continuous glucose monitoring (rt-CGM) is cost-effective for patients with Type 1 diabetes (T1D) in multiple countries including the UK. However, rt-CGM technology is a rapidly evolving field with continued advancements in clinical efficacy and production efficiency. New generations of the technology are expected to be introduced into clinical practice, which will vary in price and budget impacts to global healthcare systems. The aim of this analysis was to examine the sensitivity of existing cost-effectiveness evidence to changes in treatment costs of rt-CGM technology from a UK health care system perspective.
Materials and methods: The IQVIA CORE diabetes model was used to conduct the treatment costs impact analysis. We used base case baseline data from a published cost-effectiveness analysis for rtCGM in patients with T1D in the UK compared to self-monitoring of blood glucose (SMBG). Clinical data were sourced from the DIAMOND trial of adults with T1D on multiple daily injections of insulin and adapted to the UK. The baseline mean age (SD) of the cohort was 47.6 years (12.7) and proportion of female 56%. Mean baseline HbA1c for the cohort was 8.6% (70 mmol/mol). Reduction in HbA1c was -1.0% and -0.4% for rt-CGM and SMBG, respectively. For treatment costs impact, we conducted sensitivity analyses for -20%, -30%, and -50% reduction from the published treatment cost of GBP 1850. The analysis was conducted from the UK National Health Service payer perspective over a lifetime horizon.
Results: Rt-CGM was associated with a 1.489 incremental gain in quality-adjusted life years (QALYs) compared with SMBG (mean [SD] 11.47 [2.04] versus 9.985 [1.84] QALYs). Variation in treatment costs had a significant impact on the incremental cost-effectiveness ratio (ICER). The ICER was reduced by 46% when treatment costs were reduced by 20% (ICER 9,558 to 5,140 GBP/QALY gained) and by 69% when treatment costs decreased by 30% (ICER 9,558 to 2,933 GBP/QALY gained). Rt-CGM became dominant compared to SMBG when treatment costs decreased by 50% (ICER -1490).
Conclusion: Our analysis shows that treatment cost for rt-CGM is a significant driver of cost-effectiveness against SMBG. Reductions in treatment cost will position rtCGM as a long-term economic strategy to manage diabetes for patients with T1D in the UK.
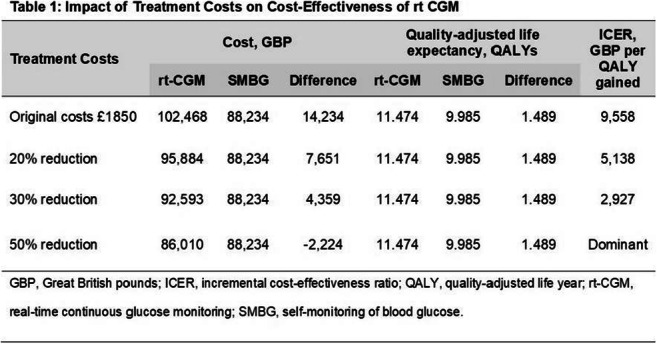
Disclosure: H. Alshannaq: Employment/Consultancy; Dexcom.
OP 18 Cross-talk communication in the pancreas
103
Chronic hyperglycaemia leads to dysregulated δ-cell metabolism and reduced glucose-stimulated somatostatin secretion from the pancreatic islets
T.G. Hill1, A.I. Tarasov2, M. Wallace3, E. Haythorne4, L.J.B. Briant1, G. Cyranka4, F.M. Ashcroft4;
1Oxford Centre for Diabetes, Endocrinology, and Metabolism, University of Oxford, Oxford, 2School of Biomedical Sciences, Ulster University, Coleraine, 3Wellcome Centre for Human Genetics, University of Oxford, Oxford, 4Department of Physiology, Anatomy, and Genetics, University of Oxford, Oxford, UK.
Background and aims: Reduced islet β-cell glucose metabolism-insulin secretion coupling and elevated postprandial α-cell glucagon secretion represent hallmark features of type-2 diabetes (T2D). Glucotoxicity has been extensively shown to trigger β-cell metabolic dysregulation, causing reduced glucose-stimulated insulin release. Chronic hyperglycaemia also leads to dysregulated glucagon secretion although the underlying causes remain largely obscure. We explored whether chronic hyperglycaemia alone also interferes with islet δ-cell function and glucose-stimulated somatostatin (Sst) secretion.
Materials and methods: Isolated WT (C57BL/6J), transgenic δ-cell-specific (δGCaMP6 and δRFP) mice, and non-diabetic cadaver donor human islets were chronically cultured under either normo-(Cont, control) or hyperglycaemic (Hyp, 25 mmol/l) glucose conditions in vitro for 14 days. Diabetic mouse islets were isolated from tamoxifen-inducible β-cell-specific Kir6.2-V59M (βV59M) mice subjected to 14 days of chronic in vivo hyperglycaemia (plasma glucose ≥ 25 mmol/l). δ-cell intracellular Ca2+ ([Ca2+]i) and ATP ([ATP]i) were imaged using GCaMP6 and Perceval sensors respectively and a wide-field Zeiss Axio Zoom V16. Sst-14 secretion from islets isolated from WT or βV59M mice, and non-diabetic human donors, was quantified by radioimmunoassay.
Results: (i) Mouse islet δ-cells exposed to chronic hyperglycaemia in vitro demonstrated a smaller increase in [Ca2+]i in response to stimulation with 20 mmol/l glucose (δGCaMP6 F/F0 AUC at 20 mmol/l glucose; Cont, 5.89 ± 0.20 vs Hyp, 1.74 ± 0.72, p < 0.05), when compared to normoglycaemia-exposed controls. (ii) Mouse islet δ-cells subjected to either chronic in vitro or in vivo hyperglycaemia also revealed impaired [ATP]i responses when subjected to acute glucose stimulation (δRFP islets transfected with Perceval: F/F0 AUC at 20 mmol/l glucose: Cont, 6.28 ± 0.21 vs Hyp, 2.06 ± 0.52, p < 0.01. βV59M islets transfected with Perceval F/F0 AUC at 20 mmol/l glucose: Cont, 2.87 ± 0.21 vs Hyp, 1.30 ± 0.17, p < 0.05); and (iii) lower glucose-stimulated Sst secretion (fold-change 20 mmmol/l glucose vs 1 mmol/l glucose; WT islets: Cont, 4.30 ± 0.13 vs Hyp, 2.36 ± 0.84; p < 0.05; βV59M islets: Cont, 24.75 ± 4.67 vs Hyp, 9.73 ± 3.49, p < 0.01), when compared to normoglycaemia-exposed controls. (iv) Similarly, human islets exposed to chronic in vitro hyperglycaemia also showed decreased glucose-stimulated Sst secretion when compared to normoglycaemic control islets.
Conclusion: These data demonstrate that chronic hyperglycaemia alone results in dysregulation of glucose-stimulated δ-cell function and reduced Sst secretion. This may, via loss of normal paracrine regulation of the α-cells, contribute to the abnormal increase in postprandial α-cell glucagon secretion seen in T2D patients.
Supported by: Novo Nordisk-Oxford Fellowship
Disclosure: T.G. Hill: None.
104
Paracrine signalling in delta cells is disturbed in human type 2 diabetes
L. Matuseviciene1, O.M. Hmeadi2, P.-E. Lund2, S. Barg2;
1Uppsala University, 2Uppsala Universitet, Uppsala, Sweden.
Background and aims: Pancreatic delta cells secrete somatostatin in response to elevated glucose, which in turn suppresses insulin and glucagon secretion from beta and alfa cells, respectively. In type 2 diabetes, this paracrine regulation is disturbed, but the underlying mechanisms remain unclear. Research on isolated delta cells is rare and relatively little is known about the cellular control of somatostatin release. The aim of this study was to quantify the effects of intra-islet signalling pathways on exocytosis and membrane potential in delta cells from non-diabetic and type 2 diabetic islets.
Materials and methods: Live cell imaging and electrophysiology were performed in dispersed pancreatic islets of non-diabetic (ND) or type 2 diabetic (T2D) cadaveric human donor islets. To identify delta cells and for imaging exocytosis by TIRF-microscopy, dispersed islet cells were transduced with adenovirus coding for fluorescent granular marker under control of somatostatin promoter. Exocytosis was evoked by elevated K+ in presence of 10mM glucose/200μM diazoxide (control) and either of the following: 100nM insulin, 10nM glucagon, 400nM somatostatin, 400nM GABA and 5μM adrenaline, 2μM forskolin, or 10nM exendin4. In addition, standard whole-cell voltage clamp and capacitance recordings were performed on single delta cells to study effects on exocytosis, ion channels. Somatostatin receptor 2 (SSTR2) expression was analysed by confocal microscopy of immunostained human pancreatic tissue sections.
Results: K+-stimulated exocytosis of somatostatin granules followed a biphasic timecourse, with a tendency towards reduced exocytosis in delta cells of T2D donors (-28%, p=0.08, 6 ND donors, n=50 cells; 4 T2DM donors, n=25 cell); this was strengthened by capacitance recordings (-40%, p=0.018; 10 ND donors, n=68 cells; 4 T2D donors, n=42 cells). There were no changes in Ca2+-currents that could explain the decrease in exocytosis, but Na+-currents were reduced in T2D cells, compared with ND (-31%, p=0.02; 10 ND donors, n=66 cells; 4 T2D donors, n=34 cells). K+-stimulated exocytosis decreased in presence of somatostatin when compared with diazoxide only (-39%, p=0.03, 4 ND donors, n=18 cells) and robustly increased by glucagon (+50%, p<0.0001; 5 ND donors, n=28 cells), exendin-4 (+61%, p<0.0001; 4 ND donors, n=17 cells), forskolin (+68%, p<0.0001, 5 ND donors, n=23 cells), and GABA (+66%, p<0.0001, 5 ND donors, n=22 cells). No significant difference in the compounds’ effects were detected between ND and T2D, or in 1 mM vs 10 mM glucose. In contrast, adrenaline inhibited exocytosis in ND delta cells (-59%, p<0.0001; 8 ND donors, n=41 cells), but significantly increased in T2D delta cells (+56%, p<0.0001; 2 T2D donors, n=13 cells; 6 ND donors, n=50 cells). In addition, short term exposure to GABA depolarized the membrane potential of delta cells, while somatostatin repolarized and inhibited electrical activity. Finally, immunostaining of human pancreas sections revealed internalization and reduced surface expression of SSTR2 in beta cells of T2D donors.
Conclusion: Human delta cells are responsive to autocrine inhibition by somatostatin, as well as a variety of paracrine signalling pathways. Type 2 diabetes is associated with decreased delta cell secretory capacity and a prominent reversal of adrenalin’s effect from inhibitory to stimulatory. The latter may indirectly contribute to hyperglycemia in type 2 diabetes by strengthening somatostatin-dependent inhibition of insulin secretion.
Supported by: NNF, DF, BDF, VR, MF-UU, EXODIAB, Ernfors, Rudbergs
Disclosure: L. Matuseviciene: None.
105
The requirement of beta cells to the glucose responsiveness of alpha cells depends on the depolarisation status of alpha cells
F. Khattab, P. Gilon;
IREC EDIN, UCLouvain, Brussels, Belgium.
Background and aims: The mechanisms by which glucose controls glucagon release are largely unknown. In particular, it is unclear whether glucose controls α-cell activity directly or indirectly via β- or δ-cells. In this study, we used mouse pseudo-islets (PI) of various compositions to investigate the role of β- and δ-cells in the control of glucagon secretion by glucose.
Materials and methods: Several mouse models expressing a fluorescent protein specifically in α-, β-, or δ-cells were used. Islets were dispersed and cells were FACS-sorted. Highly pure populations of α-, β-, and δ-cells were then reaggregated into various types of pseudo-islets by culturing them during 5-6 days. The effect of a change of the glucose (G) concentration between 1 (G1) and 10 mmol/l (G10) was tested on glucagon and insulin secretions from islets or PI in perifusion experiments. Perifusion media contained a 2.1 mmol/l mixture of amino acids present at physiological concentrations and containing (in mmol/l) 0.4 alanine, 0.5 glutamine, 0.2 lysine, 0.25 glycine, 0.15 leucine, 0.25 valine, 0.15 threonine, 0.1 serine, and 0.1 arginine.
Results: Dispersed islets cells, including pure α-cells, spontaneously reaggregated into pseudo-islets within 5-6 days. As expected, control islets showed an increase in glucagon secretion in response to a drop of glucose concentration from G10 to G1. The response was similar in pseudoislets obtained after reaggregation of all cell types. However, glucagon release from pseudo-islets made of pure α-cells (α-PI) was impaired in response to glucose. The addition of β-cells (α/β-PI), but not δ-cells (α/δ-PI) to α-cells restored normal glucagon secretion in response to G1. To determine whether this restoration was specifically due to the presence of β-cells or simply to the reestablishment of cell-to-cell contacts with another cell type, α-cells were reaggregated with mouse embryonic fibroblasts (α/MEF-PI). The presence of MEF did not restore a normal glucagon response to G1. We also generated α/β/MEF-PI to verify that MEF did not interfere with glucagon secretion. α/β/MEF-PI showed a normal simulation of glucagon secretion in response to G1, further confirming the importance of β-cells. Interestingly, in the presence of diazoxide (a KATP channel opener) and high potassium (30 mmol/l K+) which, together, clamp the membrane potential at a depolarized level, a drop of glucose concentration from G10 to G1 stimulated glucagon release from α-PI, suggesting that glucose modulates the efficacy of Ca2+ on exocytosis in α-cells independently of β and δ-cells.
Conclusion: We show, for the first time, that mouse α-cells can reaggregate into pseudo-islets. We demonstrate that, in conditions where the plasma membrane is not clamped, glucagon secretion in response to glucose from pure α-PI is impaired, and that the addition of β-cells, but not δ-cells, restores a normal control of glucagon secretion by α-cells. However, in conditions where the plasma membrane is clamped at a depolarized level, low glucose efficiently stimulates glucagon release from pure α-PI, i.e. independently of β- and δ-cells.
Supported by: FRIA, FNRS, Helmsley, ARC, SFD, EFSD/JDRF/Lilly Programme
Disclosure: F. Khattab: None.
106
Antecedent hypoglycaemia enhances intra-islet α-δ-cell paracrine feedback loop to impair α-cell glucagon secretion
R. Gao, S. Acreman, P. Rorsman, Q. Zhang;
OCDEM, University of Oxford, Oxford, UK.
Background and aims: Glucagon released by pancreatic α-cells is a principal glucose-elevating hormone that counter-regulates hypoglycaemia. Glucagon secretion can be regulated by inhibitory somatostatin released from neighbouring δ-cells. Appropriate somatostatin secretion maintains a balanced islet glucagon output but abnormal islet paracrine tone due to somatostatin hypersecretion often leads to defective secretion of glucagon and impairs glucose counter-regulation. However, how δ-cell releases somatostatin and how it becomes dysregulated remain obscure. Here we studied the crosstalk between α- and δ-cells, and how this cellular communication is affected by antecedent hypoglycaemia.
Materials and methods: Glucagon and somatostatin secretion was assessed using static hormone secretion assays. α- and δ-cell intracellular Ca2+ dynamics were monitored using live cell imaging with islets from reporter-expressing mice (GCaMP6f specifically expressed in α- or δ-cells, GCG-GCaMP6f or SST-GCaMP6f, respectively). Direct interaction between α-cell exocytosis and its adjacent δ-cell response was characterised using a combination of electrophysiology and high-speed Ca2+ imaging.
Results: To study α-δ-cell crosstalk, single α-cells within intact SST-GCaMP6f islets were stimulated with depolarisations from -70 to 0 mV using voltage-clamp technique. Stimulation of α-cell led to rapid increase of Ca2+ in neighbouring δ-cells (~900 ms delay). The magnitude of depolarisation-triggered α-cell exocytosis was positively correlated with the AUCs of δ-cell Ca2+ transients (R=0.56, P<0.001), suggesting δ-cells respond to paracrine factors released by α-cells. Blockade of glucagon receptor or glutamate receptor strongly reduced δ-cell Ca2+ activity, exocytosis and somatostatin secretion at low glucose (where α-cells’ activity is high). Glucagon-dependent δ-cell Ca2+ activity is originated from ER and mediated through both Gαs and Gαq. Antagonising Gαs or Gαq pathway reduced basal δ-cell activity by 48.7% (P=0.002) or 47.5% (P=0.004), respectively. This paracrine interaction is sensitive to metabolic cues and is strengthened following exposure to hypoglycaemia. In islets pretreated with hypoglycaemia (2 mM glucose, 2-hr, followed by overnight recovery in 5 mM glucose), basal somatostatin hypersecretion (P=0.026) and δ-cell Ca2+ hyperactivity (P=0.047) were observed. These were correlated with reduced glucagon secretion (P=0.006) and α-cell activity (P<0.001) in response to 1 mM glucose. At single cell level, δ-cells became more sensitive to α-cell exocytosis (R=0.69, P<0.001). Hypoglycaemic exposure also induced persistent δ-cell structural changes with protrusion extension by ~1.5 μm (P=0.007). These effects were reversed by including a glucagon receptor blocker during the hypoglycaemic pretreatments, indicating a key role of intensive glucagon release during antecedent hypoglycaemia in δ-cell plasticity.
Conclusion: Pancreatic α- and δ-cells are tightly coupled to form a reciprocal paracrine network, a mechanism that fine-tunes glucagon secretion to prevent glucagon overshoot. This coupling is plastic and sensitive to metabolic cues: δ-cell sensitivity to adjacent α-cell activity is enhanced by antecedent hypoglycaemia, leading to defective glucagon response to later hypoglycaemia. These hypoglycaemia-induced dysregulated α-δ-cell interaction may underlie the defective glucagon secretion in recurrent hypoglycaemia in diabetes.
Supported by: The Royal Society, Medical Research Council, Diabetes UK
Disclosure: R. Gao: None.
107
Insulin-like growth factor binding protein 7 reduces glucose stimulated insulin secretion and downregulates key beta cell transcription factors
E. Westholm, A. Karagiannopoulos, A. Wendt, L. Eliasson;
Islet Cell Exocytosis Group, Faculty of Medicine, Malmö, Sweden.
Background and aims: Cystic fibrosis (CF) is one of the most prevalent genetic diseases in the world and is caused by loss-of-function mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The most common non-pulmonary complication of CF is cystic fibrosis-related diabetes mellitus (CFRD), which is characterised by impaired insulin secretion. In a ferret CFTR-KO model, the secretome from pancreatic ductal epithelium showed an upregulation of transforming growth factor β1 (TGFβ1). In the same study TGFβ1 stimulated an increased expression of insulin-like growth factor binding protein 7 (IGFBP7) in the surrounding pancreatic stromal cells. IGFBP7 can bind insulin with high affinity and interact with the insulin receptor and IGF receptors. The aim of this project is to investigate if IGFBP7 affects β-cell physiology, especially insulin secretion.
Materials and methods: EndoC-βH1 cells were used in glucose stimulated insulin secretion (GSIS) experiments. In acute IGFBP7 treatment, the GSIS assay buffer was supplemented with 100 nM IGFBP7. C-peptide was measured as a proxy for secreted insulin using ELISA and the results were normalised to total protein (ng/mg prot/h). In long-term treatment, cells were cultured 72 h with 100 nM IGFBP7 prior to GSIS, and secreted insulin was normalised to total insulin (%/h). Gene expression after long-term treatment was assessed with RT-qPCR. RNA from EndoC-βH1 cells, 4 passages, and primary human islets from 4 donors was prepared using the TruSeq library kit and sequenced on an Illumina platform. Transcripts were quantified with Salmon v. 0.14 and results were processed using the DESeq2 tool and are reported as normalised counts. For GSIS and RT-qPCR data, statistical analysis was done using paired T-test.
Results: In acute treatment experiments, IGFBP7 reduced the secretion of C-peptide at 20 mM glucose stimulation (CTRL 111 ng/mg prot/h vs. IGFBP7 90 ng/mg prot/h, n=5, P=0.018). A similar effect was observed with long-term treatment of IGFBP7 (CTRL 5.2 %/h vs. IGFBP7 4.0 %/h, n=4, P=0.0085). Long-term treatment with IGFBP7 did not significantly change total insulin content of EndoC-βH1 cells. With long-term treatment, there was a decreased expression of genes encoding for important β-cell transcription factors such as PDX1, NKX6-1, PAX4, FOXO1 (n=4, P<0.05). We also saw a reduction of VAMP2 (n=4, P<0.05). In RNA sequencing data, there was similar expression of potential IGFBP7 binding receptor genes INSR, IGF1R, IGF2R as with TGFBR1 and KCNJ11, in EndoC-βH1 cells and primary human islets. However, the expression of IGFBP7 was almost 50 times higher in primary human islets in comparison to EndoC-βH1 cells.
Conclusion: Combined, these experiments show that IGFBP7 cause impaired β-cell function with reduced insulin secretion and decreased gene expression levels of important β-cell genes. In the context of cystic fibrosis and CFRD, we hypothesize that elevated IGFBP7 functions as a mediator of pathophysiological intra-pancreatic crosstalk between defect ductal cells and islet cells. Such cross talk likely plays a role in disease development.
Supported by: CF Trust International consortium SRC019 and RfCF
Disclosure: E. Westholm: None.
108
Role of tRNA-derived fragments in the cross-talk between immune cells and beta cells during type 1 diabetes pathogenesis
F. Brozzi1, C. Cosentino1, C. Jacovetti1, K. Wu1, V. Menoud1, M.B. Bayazit1, C. Guay1, R. Regazzi1,2;
1Department of Fundamental Neurosciences, University of Lausanne, 2Department of Biomedical Sciences, University of Lausanne, Lausanne, Switzerland.
Background and aims: Type 1 diabetes (T1D) is an autoimmune disease characterized by immune cell infiltration in the islets of Langerhans (insulitis). Immune cells release pro-inflammatory cytokines and extracellular vesicles (EVs) in the site of infiltration, causing β-cell death. This study focuses on decoding the dialogue between T-cells and β-cells during T1D pathogenesis, on the analysis of T-cell EV content and of their functional impact on β-cells. We have previously demonstrated that specific miRNAs are transferred from T-cells to β-cells via EVs during insulitis, causing β-cell inflammation and apoptosis. tRNA-derived fragments (tRFs) are also emerging as major components of T-cell EVs and may potentially be delivered to β-cells during the autoimmune attack. tRFs are fragments originating from transfer RNAs (tRNAs) and have several functions in the control of cellular processes, including apoptosis. Indeed, our preliminary results show an increase in tRF expression in islet cells of pre-diabetic NOD mice. The aim of the present study is to identify the tRFs that are transferred from T-cells to islet cells during T1D pathogenesis and affect β-cell function, inflammation, and survival.
Materials and methods: Small RNAseq analysis was performed in EVs isolated from NOD CD4+/CD25- T-cells and in pancreatic islet cells treated for 24h with NOD T-cell EVs. To prove the direct transfer of selected tRFs, the RNA in the CD4+/CD25- T-cells was tagged with a uridine analogue (EU), and then isolated form the β-cells treated with the EVs produced from EU-tagged T-cells. The tagged RNA was analyzed by qPCR to identify selected tRFs transferred to β-cells. RNA mimics were used to overexpress the candidate tRFs in β-cells, with the aim of reproducing the EV-dependent rise of the selected RNA and study its effect. Apoptosis was assessed with Propidium Iodide (PI)/ Hoechst staining and cleaved caspase-3 assay.
Results: The small RNA data sets from T-cell EVs and from islet cells treated with EVs were compared with the tRFs that are significantly up regulated in the islets of pre-diabetic NOD mice (265 tRFs in 8 weeks-old vs 4 weeks-old mouse islets, adjusted p-value=0.05, n=4). From this comparison we identified a subset of 7 tRFs that are transferred from T-cells to β-cells in the initial phases of T1D. Their expression increased in islets during insulitis (2.0 to 6.2 Fold Change (FC), in 8 weeks-old vs 4 weeks-old mouse islets, p-value <0.05, n=4, t-Test ) and after in-vitro incubation with T-cell EVs (2.0 to 4.8 FC in EV treated vs control islets, p-value<0.05, n=4, t-Test). Moreover, with an RNA-tagging strategy, we were able to recover in EV-treated β-cells some of the miRNAs and tRFs that were increased in NOD islet cells during insulitis. The overexpression of three tRFs candidates increased β-cell apoptosis in primary islet cells (1.6 to 1.8 FC, tRFs vs Ctrl, p-value <0.05, ANOVA).
Conclusion: We provide a direct “proof of concept” that tRFs can be shuttled between T-cells and β-cells via EVs, and identify a new mechanisms through which T-cells may trigger β-cell dysfunction and apoptosis in T1D pathogenesis. Mechanistic studies will be done to evaluate the mode of action of selected tRFs and to evaluate the potential design of new strategies for the treatment of diabetic patients.
Disclosure: F. Brozzi: None.
OP 19 GLP1 agonists: from here to eternity
109
Dulaglutide in youth with type 2 diabetes: results of the AWARD-PEDS randomised, placebo-controlled trial
S. Arslanian1, T. Hannon2, P. Zeitler3, L. Chao4, C. Boucher-Berry5, M. Barrientos-Pérez6, E. Bismuth7, S. Dib8, J. Cho9, D. Cox9;
1UPMC Children's Hospital of Pittsburgh, Pittsburgh, USA, 2Riley Hospital for Children, Indianapolis, USA, 3Children's Hospital Colorado, Aurora, USA, 4Children's Hospital of Los Angeles, Los Angeles, USA, 5Children's Hospital of the University of Illinois, Chicago, USA, 6Angeles Hospital of Puebla, Puebla, Mexico, 7University Robert Debré Hospital, Paris, France, 8Federal University of São Paulo State, São Paulo State, Brazil, 9Eli Lilly and Company, Indianapolis, USA.
Background and aims: AWARD-PEDS was a Phase 3 trial to assess the efficacy and safety of dulaglutide (DU), a once-weekly GLP-1 receptor agonist, in youth (10 to <18 years old) with type 2 diabetes (T2D) treated with lifestyle alone or on stable metformin with or without basal insulin. The primary aim was to demonstrate superiority of DU (pooled doses) vs placebo for change in HbA1c at 26 weeks.
Materials and methods: Participants (mean age, 14.5 yrs; mean BMI, 34.1 kg/m2) were randomised to placebo (N=51), DU 0.75 mg (N=51), or DU 1.5 mg (N=52). Analyses included all patients with ≥1 dose of study drug, excluding data after initiation of rescue therapy.
Results: DU was superior to placebo (figure) in improving glycaemic control measured by change in HbA1c, percent of patients with HbA1c <53 mmol/mol (7%), and change in fasting glucose at Week 26. No effect of DU was observed on BMI change (p=0.776). Fewer patients assigned to DU compared to placebo required rescue therapy (2.9% vs 17.6% respectively, p=0.003). Incidence of common GI adverse events was higher in DU group vs placebo [nausea (14.6% vs 7.8%), vomiting (15.5% vs 3.9%), diarrhoea (18.4% vs 13.7%)] but comparable to that observed in adults.
Conclusion: In conclusion, in youth with inadequately controlled T2D treated with or without metformin and/or basal insulin, once weekly DU 0.75 mg or 1.5 mg was superior to placebo in improving glycaemic control without an effect on BMI through 26 weeks, with a safety profile consistent with that established in adults.
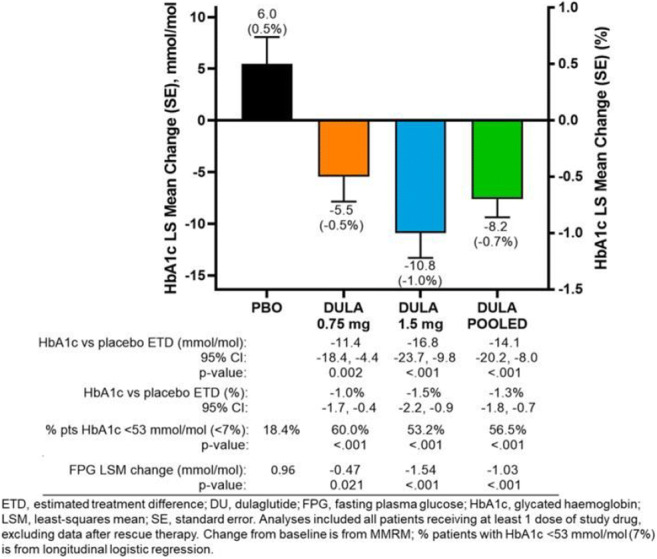
Clinical Trial Registration Number: NCT02963766
Supported by: Eli Lilly and Company
Disclosure: S. Arslanian: Grants; Eli Lilly and Company, Novo Nordisk. Other; Advisory Board: Eli Lilly and Company, Novo Nordisk; DMC member: AstraZeneca, Eli Lilly and Company.
110
Beta cell function and sensitivity to incretins before and after Roux-en-Y gastric bypass in patients with type 2 diabetes
M.S. Svane1, M. Hindsø1, C. Martinussen1, C. Dirksen1, N.B. Jørgensen1, B. Hartmann2, V.B. Kristiansen1, J.J. Holst2, K.N. Bojsen-Møller1, S. Madsbad1;
1Copenhagen University Hospital Hvidovre, Hvidovre, 2University of Copenhagen, Copenhagen, Denmark.
Background and aims: Roux-en-Y gastric bypass (RYGB) improves glucose tolerance in patients with type 2 diabetes (T2D), but whether RYGB affects beta-cells independently of signals from the gut is unclear. Also, the sensitivity of the beta-cell to glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) remains to be clarified. We aimed to investigate beta-cell function before and after RYGB, and particularly, the sensitivity of the beta-cell to the incretin hormones. We hypothesized that sensitivity to GIP would be increased after RYGB because of relieve of glucotoxicity.
Materials and methods: Nine patients with obesity and T2D (5 women, age: 45±3 years, weight: 127.4±8.1 kg, BMI: 42.7±2.3 kg/m2) were examined with a 75 g OGTT and three 90-min hyperglycemic clamps at ~15 mM plasma glucose (CV 2%) with primed continuous co-infusions of either GIP (1.5 pmol/kg/min), GLP-1 (1 pmol/kg/min) or saline before (pre) and 3 months (3mo) after RYGB. Beta-cell function was calculated separately for the OGTT (as beta-cell glucose sensitivity (β-GS): slope between insulin secretion rates and plasma glucose concentrations) and the intraveneous stimulation (first phase as the acute insulin response to glucose (AIR) (positive incremental (pi)AUC0-10 min) and second phase (piAUC 20-90 min) during clamps).
Results: After RYGB, fasting plasma glucose decreased (pre: 7.9±0.3 mmol/L, 3mo: 5.4±0.3, p<0.01) and insulin sensitivity improved (HOMA2 IR: pre 4.1±0.3, 3mo: 2.4±0.2, p<0.01). Weight loss was 19±0.7 kg (14.9%). During the OGTT, 2-hour plasma glucose decreased (pre: 13.6±0.7 mmol/L, 3mo: 7.3±0.8, p<0.01) and insulin secretion increased resulting in a two-fold increase in β-GS (pre: 0.9±0.1 (mmol/kg/min)/mmol/L, 3 mo: 1.8 ±0.1, p<0.01). GIP secretion decreased slightly after RYGB (piAUC pre: 5270±913 pmol/L· min, 3mo: 4310±339, p=0.01), whereas GLP-1 secretion increased markedly (pre: 1156±583 pmol/L· min, 3mo: 6007±691, p<0.01). During the saline clamp both first and second phase of insulin secretion increased after RYGB (AIR pre: 15±5 pmol/kg, 3mo: 58±15, p<0.01; piAUC 20-90 min pre: 296±54, 3mo: 445±54, p<0.05). GIP-infusion augmented second phase of insulin secretion equally before and after RYGB (pre: +96±16% vs saline-clamp, 3mo: +90±14%, p=0.57). GLP-1 infusions increased insulin secretion even more, with a small decline in the potentiating effect after RYGB (Pre: +399±84%, 3mo: +233±32%, p=0.03).
Conclusion: In patients with obesity and T2D, beta-cell function increases after RYGB both in response to oral and iv glucose. An insulinotropic effect of GIP was demonstrated before surgery and this effect was maintained postoperatively. The potentiating effect of GLP-1 on insulin secretion was pronounced both before and after surgery with a slight decline observed after RYGB. Thus, the improved beta cell function in response to an oral glucose stimulus after RYGB is the result of GLP-1 hypersecretion, rather than an increased insulinotropic action of the incretins.
Clinical Trial Registration Number: NCT04782999
Supported by: European Research Council, Horizon 2020, Grant: BYPASSWITHOUTSURGERY
Disclosure: M.S. Svane: Grants; European Research Council, Novo Nordisk Foundation, Danish Diabetes Association.
111
Ly3437943 (LY), a novel triple GIP/GLP-1/glucagon receptor agonist, provides glucose lowering and weight loss in patients with type 2 diabetes after 12 weeks of treatment
Z. Milicevic, S. Urva, M. Loh, T. Coskun, Y. Du, C.T. Benson, C. Loghin, A. Haupt;
Eli Lilly and Company, Indianapolis, USA.
Background and aims: Multi-receptor incretin agonists are being developed for several metabolic disorders. LY is an investigational triple agonist with potent activity on glucose-dependent insulinotropic polypeptide (GIP), glucagon-like polypeptide-1 (GLP-1), and glucagon receptors. LY was safely studied in a prior first-in-human study, and pharmacokinetic properties supported once weekly dosing. The primary objective of this randomized, double-blind, placebo-controlled, Phase 1 proof-of-concept study was to assess the safety and tolerability of multiple ascending doses of LY in patients with type 2 diabetes (T2D).
Materials and methods: Seventy-two patients were randomized (9:3:1) to 5 rising dose cohorts of subcutaneous LY, placebo, and dulaglutide 1.5mg, respectively. Within cohort, dose-escalation was implemented at highest 2 cohorts. Vital signs, laboratory data and adverse events (AEs) were monitored to assess safety and tolerability. Efficacy was assessed by monitoring change in glycated hemoglobin (HbA1c) and body weight at week 12.
Results: The most common treatment-emergent AEs were gastrointestinal (nausea and diarrhea), which were mostly mild in severity. By week 12, mean systolic and diastolic blood pressure decreased from baseline in LY compared to placebo group, while pulse and heart rate increased from baseline within most of the LY cohorts and the dulaglutide cohort, but not with placebo. By week 12, mean HbA1c decreased from baseline in all groups, with higher doses of LY showing statistically significant, placebo-adjusted decreases of up to 17.1 mmol/mol. Except at the initial cohort, dose-dependent decreases in mean placebo-adjusted body weight of up to 8.96 kg were observed with LY.
Conclusion: LY3437943 exhibits a safety and tolerability profile similar to other incretins. The promising glycemic and body weight loss efficacy within this study highlights the potential for LY to provide additional benefit versus existing therapies in treatment of T2D and obesity.
Clinical Trial Registration Number: NCT04143802
Disclosure: Z. Milicevic: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
112
Effect of liraglutide on muscle fat infiltration in adults with overweight or obesity: a randomised clinical trial
K.V. Patel1, A. Pandey2, C. Ayers2, J. Linge3, O.D. Leinhard3, P.H. Joshi2, I.J. Neeland4;
1Houston Methodist Hospital, Houston, USA, 2University of Texas Southwestern Medical Center, Dallas, USA, 3AMRA Medical and Linköping University, Linköping, Sweden, 4University Hospitals Harrington Heart and Vascular Institute and Case Western Reserve University School of Medicine, Cleveland, USA.
Background and aims: Excess muscle fat is observed in obesity and associated with greater burden of cardiovascular risk factors and higher risk of mortality. Liraglutide reduces total body weight and visceral fat but its effect on muscle fat infiltration (MFI) is unknown.
Materials and methods: This study is an analysis of a randomized, double-blind, placebo-controlled trial that examined the effects of liraglutide plus a lifestyle intervention on visceral adipose tissue among adults with body mass index ≥30 kg/m2 or ≥27 kg/m2 and metabolic syndrome without diabetes. Participants were randomly assigned to a once-daily subcutaneous injection of liraglutide (target dose 3.0 mg) or matching placebo for 40 weeks. Body composition was assessed by magnetic resonance imaging at baseline and follow-up. MFI was measured as the average proportion of fat in viable muscle tissue (muscle tissue with fat fraction <50%) of the bilateral anterior thighs. Treatment effects and 95% confidence intervals of liraglutide and placebo on MFI were calculated by means of generalised linear mixed models with random effects for participants. The associations of changes in MFI with changes in measures of body composition and cardiometabolic biomarkers were assessed using Spearman correlation coefficients.
Results: Among the 128 participants with follow-up imaging (92.2% women, 36.7% Black), median MFI at baseline was 7.8%. The percent change in MFI over follow-up was greater among participants randomized to liraglutide (n = 73) compared with placebo (n = 55) (-2.81% vs. 0.29%, p-value = 0.001) (Figure). Longitudinal change in MFI was significantly correlated with change in body weight (r = 0.30), visceral adipose tissue (r = 0.41), and abdominal subcutaneous adipose tissue (r = 0.32) but not with change in total body lean tissue or total thigh muscle. Change in MFI was significantly associated with change in N-terminal pro-B-type natriuretic peptide (r = 0.28) and high-sensitivity C-reactive protein (r = 0.29) but not change in fasting plasma glucose or insulin.
Conclusion: Among adults with overweight or obesity free of diabetes, once daily subcutaneous liraglutide reduced mean anterior thigh MFI compared with placebo independent of changes in thigh muscle volume. The contribution of MFI improvement to the cardiometabolic benefits of liraglutide require further study.
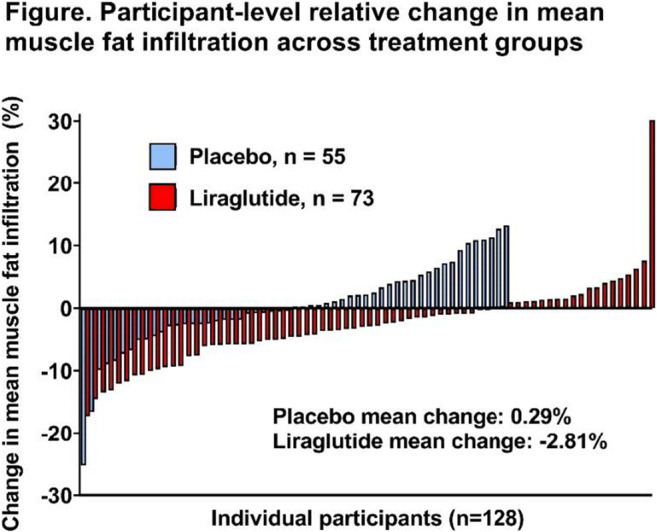
Clinical Trial Registration Number: ClinicalTrials.gov Identifier: NCT03038620
Supported by: Funding: Novo Nordisk
Disclosure: K.V. Patel: None.
113
Glucagon-like peptide 1 receptor agonists and gallbladder or biliary diseases: data from the FDA Adverse Event Reporting System
H. Zhang, L. He, J. Wang, N. Yang, W. Li, L. Xu, Y. Li, F. Ping;
Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, Peking U, Beijing, China.
Background and aims: Associations between glucagon-like peptide 1 receptor agonists (GLP-1RAs) and gallbladder or biliary diseases remains controversial in real-world settings. We aimed to make a detailed analysis of the gallbladder or biliary diseases reports for GLP-1RAs versus sodium-glucose cotransporter-2 inhibitors (SGLT-2i) or non-GLP-1RAs in the United States Food and Drug Administration Adverse Event Reporting System (FAERS).
Materials and methods: The FAERS database was mined from the first quarter of 2013 to the fourth quarter of 2020. Disproportional analyses with proportional reporting ratios (PRR) and 95% confidence intervals (CI) and multiple logistic analyses with odds ratios (OR) and 95% CI were performed.
Results: 1,109 cases of gallbladder or biliary diseases were extracted from the FAERS database. Significant associations between GLP-1RAs and increased reporting of gallbladder or biliary diseases were observed in the disproportional analyses (PRR: 2.366 [95% CI: 2.057-2.721]) and multiple-adjusted analyses (OR: 2.992 [95% CI: 2.533-3.534], P<0.0001), which remained consistent across the subcategories. Liraglutide (PRR: 5.001 [95% CI: 4.277-5.847]) and semaglutide (PRR: 6.267 [95% CI: 5.143-7.635]) showed significant signals of gallbladder or biliary diseases; exenatide and dulaglutide showed significant signals of only cholelithiasis and biliary obstruction, respectively. More gallbladder or biliary diseases reports with GLP-1RAs were observed in males than females (P=0.0134) and groups with indications of weight reduction than glycemic control (P<0.0001).
Conclusion: GLP-1RAs was associated with increased reporting of not only gallbladder disorders but also biliary diseases in a large pharmacovigilance database, particularly semaglutide and liraglutide, adding to available evidence from trials through real-word data.
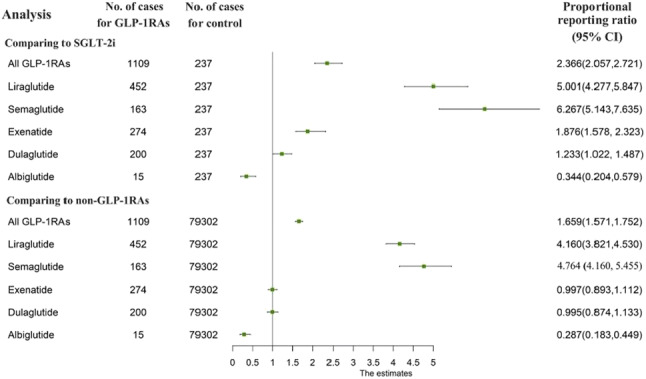
Supported by: Natural Science Foundation of Beijing Municipality Beijing (M22014)
Disclosure: H. Zhang: None.
114
Once-daily oral small molecule GLP-1R agonist PF-07081532 robustly reduces glucose and body weight within 4-6 weeks in adults with type 2 diabetes and non-diabetic adults with obesity
C. Buckeridge1, N. Tsamandouras1, S. Carvajal-Gonzalez1, L.S. Brown2, K.L. Chidsey1, A.R. Saxena1;
1Pfizer Worldwide Research and Development, Cambridge, 2Pfizer Worldwide Research and Development, Collegeville, USA.
Background and aims: PF-07081532 is a small molecule glucagon-like peptide-1 receptor (GLP-1R) agonist for once-daily oral administration. This Phase 1, randomised, double-blind, placebo-controlled, multiple ascending dose study investigated the safety, tolerability, pharmacokinetics and pharmacodynamics of PF-07081532 administered for 28 or 42 days in adults with type 2 diabetes mellitus (T2DM) inadequately controlled on metformin, and in non-diabetic adults with obesity.
Materials and methods: The study enrolled 51 participants with T2DM who received study drug once-daily for 28 or 42 days, and 15 participants with obesity who received study drug once-daily for 42 days. Participants were randomised to PF-07081532 or placebo in a 4:1 ratio. The daily dose was titrated over the dosing period up to a target dose; 61 participants completed inpatient dosing.
Results: In participants with T2DM, the mean baseline in fasting plasma glucose (FPG) and mean daily glucose (MDG) were 192 mg/dL and 212 mg/dL, respectively, and were similar across treatment groups. Mean baseline body weight for participants with T2DM and participants with non-diabetic obesity were 90 kg and 98 kg, respectively. PF-07081532 administered orally once-daily resulted in robust reductions in glucose and weight. Modelled mean reductions in MDG in participants with T2DM ranged up to 91 mg/dL over 28 days and 99 mg/dL over 42 days, compared with 29 mg/dL with placebo. Decreases in FPG of up to 79 mg/dL were observed over 28 days and 102 mg/dL over 42 days. Observed body weight reduction in participants with T2DM ranged up to 5 kg over 28 to 42 days, compared with 2 kg for placebo, with a similar magnitude of decrease in participants with non-diabetic obesity. Most adverse events were mild and consistent with the mechanism of action. No clinically significant, adverse trends in laboratory measures, electrocardiogram or vital sign abnormalities were apparent.
Conclusion: In adults with T2DM, once-daily oral administration of the GLP-1R agonist, PF-07081532 robustly reduced plasma glucose and body weight with a safety and tolerability profile consistent with the GLP-1R agonist class. Similar changes in body weight were observed in participants with non-diabetic obesity.
Clinical Trial Registration Number: NCT04305587
Supported by: Sponsored by Pfizer Inc.
Disclosure: C. Buckeridge: Employment/Consultancy; Pfizer Inc. Stock/Shareholding; Pfizer Inc.
OP 20 NAFLD and treatment
115
One night of prolonged fasting improves nocturnal substrate oxidation without modulating hepatic glycogen in individuals with NAFL and healthy age-matched individuals
K.H.M. Roumans1, A. Veelen1, C. Andriessen1, J. Mevenkamp1,2, P. Veeraiah1,2, B. Havekes3, H.P.F. Peters4, L. Lindeboom1,2, P. Schrauwen1, V.B. Schrauwen-Hinderling1,2;
1Department of Nutrition and Movement Sciences, Maastricht University, Maastricht, 2Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, 3Department of Internal Medicine, Maastricht University Medical Center, Maastricht, 4Unilever Food Innovation Center, Wageningen, Netherlands.
Background and aims: Increasing overnight fasting time, as with time-restricted eating, has been shown to improve metabolic health. The mechanisms underlying these beneficial metabolic effects remain inconclusive, but may be related to larger fluctuations in hepatic glycogen which in turn could induce a higher fat oxidation during the night. Increasing fat oxidation may especially be an interesting therapeutic strategy for people with a high amount of ectopic fat accumulation, as with non-alcoholic fatty liver (NAFL). Here, we investigated whether acutely prolonging an overnight fast from 9.5-hours to 16-hours, without changing total daily energy intake, reduces overnight hepatic glycogen and improves substrate metabolism in individuals with NAFL compared to age-matched healthy lean individuals.
Materials and methods: Eleven participants with NAFL and ten control participants participated in a randomized cross-over trial, restricting food intake to either a 14.5- or 8-hour time period, thereby creating an overnight period of 9.5-hours of fasting or 16-hours of fasting. Hepatic glycogen was measured with 13C-MRS after a standardized lunch at 2 pm and the next morning at 6.30 am in both arms. Nocturnal substrate oxidation was measured with whole-room indirect calorimetry (respiration chamber) and a meal test was performed in the morning after the overnight fast to assess the metabolic response to a meal using indirect calorimetry (ventilated hood) and assessment of plasma metabolites from blood draws.
Results: Hepatic glycogen levels were not affected by extending overnight fasting time in the NAFL or control group. Extending fasting time led to a lower nocturnal carbohydrate oxidation and higher fat oxidation in both groups (intervention * time, p < 0.005; for carbohydrate and fat oxidation). However, in both arms, the respiratory exchange ratio measured during the night remained higher in the NAFL group compared with the control group (population p < 0.001, figure 1). During the meal test, the AUC of triglycerides appeared higher with a 16-hour versus 9.5-hour fast in both the NAFL and control group (intervention, p = 0.014), but no other postprandial differences between short and extended fasting were observed.
Conclusion: These results suggest that a prolonged overnight fast can improve nocturnal substrate oxidation, and that these improvements are not mediated by changes in hepatic glycogen depletion.
Clinical Trial Registration Number: NCT03593343 and NCT04510155
Supported by: PPP Allowance by top Sector Life Sciences & Health and by Unilever R&D Wageningen
Disclosure: K.H.M. Roumans: None.
116
Therapeutic benefits of 17a-estradiol in hepatic fibrosis
S. Ali Mondal, R. Sathiaseelan, B. Miller, M. Stout;
Aging & Metabolism, Oklahoma Medical Research Foundation, Oklahoma City, USA.
Background and aims: Diabetes is an independent risk factors for the development of non-alcoholic steatohepatitis (NASH), the leading cause of liver transplant in the world. Moreover, liver fibrosis is the best determinant of mortality in NASH, therefore the reversal of liver fibrosis is a major focus in the field. There is currently no approved therapy for treating liver fibrosis. Men and postmenopausal women are at a higher risk of developing liver fibrosis compared to premenopausal women, thereby indicating a role of endogenous estrogens in controlling the progression of liver fibrosis. In this study, we sought to determine if 17α-E2 can prevent and/or reverse collagen deposition and/or increase collagen degradation in a CCl4-induced liver fibrosis mice model.
Materials and methods: 12-14 weeks old C57BL/6Jmale mice were used and randomized into five groups: (a) Vehicle group (n=15): mice were injected with olive oil intraperitoneally(i.p.) twice weekly for 8 weeks. (b) CCl4 group (n=15): mice were injected with 40% CCl4 (at a dose of 1ul/g bodymass)i.p. weekly twice for 8 weeks. Mice were maintained on Chow TestDiet 58YP (c) CCl4-17α-E2-Preventive group: Chow+17α-E2 (TestDiet 58YP + 17α-E2,14.4ppm) treatment started concomitantly with 40% CCl4 injection (1ul/g bodymass) i.p., weekly twice for 8 weeks. (d) CCl4-17α-E2-Therapeutic group: Chow+17α-E2 (TestDiet 58YP + 17α-E2,14.4ppm) treatment started on day 29 (after week 4) of CCl4(1ul/g bodymass, i.p.) injections to find out the effect of 17α-E2 in treating already developed fibrotic liver. (e) A short-term liver injury group (CCl4 -Baseline; n=18) was introduced using 40% CCl4 injected (i.p.), weekly twice at a dose of 1μl/g bodymass for 4 weeks to check the baseline development of liver fibrosis. The preventive and therapeutic effects of 17α-E2 treatment on collagen turnover rates were evaluated using stable isotope (D2O) labeling techniques.
Results: Compared with control mice, mice receiving CCl4 displayed a robust upregulation of hepatic collagen synthesis rates (P<0.0001)and declines in collagen degradation(P<0.0001) in parallel with significant elevations in TGFꞵ1(P<0.0001) and lysyl oxidase like-2 protein (LOXL2)(P<0.0001), which are responsible for hepatic stellate cell activation and collagen crosslinking, respectively. Conversely, mice receiving 17α-E2 demonstrated significantly reduced collagen synthesis rates(P<0.0001) and greater collagen degradation rates(P<0.001), which was mirrored by declines in TGFꞵ1(P<0.05) and LOXL2 protein(P<0.05), especially in the therapeutic group. These improvements were associated with increased matrix metalloproteinases-2 activity(P<0.05) and elevated levels of PPARγ(P<0.05) in both the preventive and therapeutic groups, which are established mechanisms related to the regression of liver fibrosis.
Conclusion: These findings indicate that 17α-E2 acts in a multimodal fashion to reduce fibrotic burden in the liver. Future studies will be needed to determine the cell-type-specific mechanisms by which 17α-E2 affects collagen deposition and degradation in the liver.
Supported by: NIH [R00 AG051661; R01 AG070035]
Disclosure: S. Ali Mondal: None.
117
The effect of a 16-week diet intervention with or without differing amounts of exercise volume on hepatic fat content in people with type 2 diabetes
C. Durrer1, M. Lyngbæk1, B. Liebetrau1, G.E. Legård1, T.P. Almdal2, M.A.V. Lund3, M. Ried-Larsen1;
1Centre for Physical Activity Research, Rigshospitalet, 2Hormone and Metabolic Diseases, Rigshospitalet, 3Department of Biomedical Sciences, Copenhagen University, Copenhagen, Denmark.
Background and aims: Diet and exercise with the goal of weight loss continue to be the first line treatment for non-alcoholic fatty liver disease (NAFLD) and represent a means to treat the disease which otherwise has no approved pharmacological treatment. Exercise, even independent of weight loss, can relieve hepatic insulin resistance and decrease fat liver content but its effectiveness in addition to dietary changes is unclear. The aim of this project was to test the hypothesis that an energy-reduced diet, with or without concomitant exercise training (at two different levels of exercise volume) in people with type 2 diabetes (T2D) will reduce liver fat in a dose-dependent manner.
Materials and methods: The study was a secondary analysis of a 16-week parallel group, 4-arm assessor blinded randomized controlled trial. People with T2D aged 18-80 years, diabetes duration <7 years, absence of severe comorbidities were eligible for the study. Participants were randomly allocated (1:1:1:1, stratified by sex) to either 1) No intervention control (CON), 2) Dietary intervention (DIET), 3) DIET + moderate volume exercise (MVE), or 4) DIET + high volume exercise (HVE). Medications were managed using a pre-defined algorithm by a study endocrinologist blinded to participant allocation. Hepatic fat content was quantified via magnetic resonance imaging T1-minimized signal fat fraction. Briefly, the liver was segmented based on vascular anatomy and the fat fraction was assessed in each segment. The values were then averaged to give a mean fat fraction for the entire liver. Statistical analysis was performed using a constrained baseline approach via a mixed effects linear model. Data were log-transformed to meet model assumptions and estimated marginal means were back-transformed to their original scale. Mean differences are presented as ratio (percentage) differences with associated 95% confidence intervals.
Results: Following the 16-week intervention there were reductions in liver fat by -53% [CI95: -75 to -10]; P=0.022 in the DIET group, -72% [CI95: -85 to -47]; P<0.001 in the MVE group, and -83% [CI95: -91 to -68]; P<0.001 in the HVE group compared to CON. There were no significant differences in liver fat reduction between the DIET and MVE group or the MVE and HVE group, but there was a larger reduction in liver fat in the HVE group compared to the DIET group (-63% [CI95: -79 to -35]; P=0.001). Marginal means and associated 95% confidence intervals at follow-up are presented in Figure 1, the dashed red line represents the constrained baseline.
Conclusion: A 16-week intervention with a 25% caloric deficit is able to reduce liver fat in people with T2D. There is an additional reduction in liver fat only when a high-volume of exercise training is added to the diet intervention.
Clinical Trial Registration Number: NCT03769883
Supported by: TrygFonden, Svend Andersen fonden, CIHR
Disclosure: C. Durrer: None.
118
Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: a randomised controlled trial (ESSENTIAL study)
Y. Kim;
Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Republic of Korea.
Background and aims: The effect of ezetimibe, Niemann-Pick C1-Like 1 inhibitor, on liver fat is not clearly elucidated. Our primary objective was to evaluate the efficacy of ezetimibe plus rosuvastatin versus rosuvastatin monotherapy to reduce liver fat using magnetic resonance imaging-derived proton density-fat fraction (MRI-PDFF) in patients with nonalcoholic fatty liver disease (NAFLD).
Materials and methods: A randomized controlled, open-label trial of 70 participants with NAFLD confirmed by ultrasound were assigned to receive either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks. Liver fat change was measured as average values in each of nine liver segments by MRI-PDFF. Magnetic resonance elastography (MRE) was used to measure liver fibrosis change.
Results: Combination therapy significantly reduced liver fat compared with monotherapy by MRI-PDFF (mean difference: 3.2%; p=0.020). There were significant reductions from baseline to study completion by MRI-PDFF for both the combination and monotherapy groups, respectively (18.1% to 12.3%; p<0.001 and 15.0% to 12.4%; p=0.003). Individuals with higher body mass index, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to treatment with ezetimibe. MRE-derived change in liver fibrosis was not significantly different (both groups, p>0.05). CAP (controlled attenuation parameter) by transient elastography was significantly reduced in the combination group (321dB/m to 287 dB/m; p=0.018), but not in the monotherapy group (323 dB/m to 311 dB/m; p=0.104).
Conclusion: Ezetimibe and rosuvastatin were found to be safe to treat participants with NAFLD. Further, ezetimibe combined with rosuvastatin significantly reduced liver fat in this population.
Clinical Trial Registration Number: NCT03434613
Supported by: Yuhan Corporation
Disclosure: Y. Kim: None.
119
Time-restricted feeding and obeticholic acid/semaglutide drug combination have a different impact on non-alcoholic steatohepatitis in diet-induced obese mice
F. Briand, N. Breyner, E. Grasset, T. Sulpice;
PHYSIOGENEX, Escalquens, France.
Background and aims: Combination of therapies is a promising strategy for the treatment of NASH and liver fibrosis. Here we evaluated whether the Farnesoid X Receptor agonist obeticholic acid (OCA) combined with the glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (SEMA) would show superior effects than weight loss induction with time-restricted feeding (TRF) in mice.
Materials and methods: Mice were fed a 60% high fat/2% cholesterol diet with 10% fructose supplemented drinking water (HFCF diet) for 25 weeks to induce obesity and NASH/liver fibrosis. After the diet-induction period, mice were kept on HFCF diet and treated with vehicle (control) or OCA 30mg/kg p.o. QD + SEMA 0.06mg/kg s.c. QD, or placed on TRF from the last 3 hours of the dark cycle till the end of the light cycle, without access to food, but free access to normal drinking water (i.e. without fructose) every day, for 6 weeks.
Results: OCA+SEMA induced a 20% lower caloric intake, which led to a 27% lower body weight (p<0.001 vs. control). TRF had a weaker effect on caloric intake (-9%) but also reduced body weight (-11%, p<0.05 vs. control). Both OCA+SEMA and TRF reduced the HOMA-IR index of insulin resistance and significantly reduced plasma ALT and AST levels, but these effects were more pronounced with OCA+SEMA. TRF, and to a greater extent OCA+SEMA, significantly reduced liver weight, hepatic fatty acids, triglycerides, and total cholesterol levels. Both OCA+SEMA and TRF led to significantly lower NAFLD activity score. However, OCA+SEMA did not alter liver fibrosis, while TRF showed a clear anti-fibrotic effect in the liver, with lower % Sirius Red labelling (p<0.05 vs. control).
Conclusion: OCA+SEMA combination reduced body weight and NAFLD activity score but did not improve hepatic fibrosis, while TRF improved both NASH and liver fibrosis. These TRF benefits should be further investigated in obese NASH patients.
Disclosure: F. Briand: Employment/Consultancy; PHYSIOGENEX. Stock/Shareholding; PHYSIOGENEX.
120
Cellular resistance to reactive metabolites is reversely affected by acute glucose stress and caloric restriction in type 2 diabetes patients with complications
A. Sulaj1,2, E. von Rauchhaupt1,2, C. Rodemer1, R. Bulkescher1, E. Kliemank1, S. Kopf1,2, P.P. Nawroth1,2, J. Szendroedi1,2, J. Zemva1;
1University Hospital of Heidelberg, Heidelberg, 2German Center for Diabetes Research, Neuherberg, Germany.
Background and aims: Reactive oxygen (ROS) and reactive dicarbonyl species (RCS) such as methylglyoxal (MG), are reactive metabolites (RM) known to contribute to the progression of diabetic complications. Depending on the dose, RM can also act protective by inducing hormetic reactions rendering cells more resistant to potentially harmful RM. This study investigated the cellular resistance to ROS and RCS in patients with type 2 diabetes with (T2D+) and without (T2D-) diabetic complications compared to healthy subjects (Ctrl) under acute glucose stress as well as under caloric restriction, to test whether these conditions have an impact on hormesis.
Materials and methods: An oral glucose tolerance test (oGTT) was performed before and after 5 days of fasting-mimicking diet (FMD) in T2D+ and T2D- patients and in healthy subjects (n=10: 6 males, 4 females per group). Study participants were matched for age, BMI, diabetes duration, HbA1c and antihyperglycemic medication. Peripheral blood mononuclear cells (PBMCs) were isolated before and 2h after the oGTT. PBMCs were immediately plated on a 96-well plate. To test resistance to ROS and RCS, cells were incubated with increasing levels of H2O2 and MG respectively for 24hrs. Afterwards, cell viability was measured with a CellTiter-Glo assay to calculate the corresponding EC50-value. Differences in EC50-value before and after oGTT were expressed as percentage changes (ΔEC50).
Results: Age and BMI were as expected comparable between study groups (T2D+: 66.3 [61.6, 71.0] yrs, 29.0 [26.5, 31.5] kg/m2; T2D-: 63.8 [60.2, 67.4] yrs, 27.9 kg/m2 [25.7, 30.2]; Ctrl: 63.8 [58.6, 69.0] yrs, 28.8 [25.5, 32.2] kg/m2. HbA1c was comparable between T2D+ and T2D- patients, but significantly higher in T2D+ (7.4 [6.9, 7.9] %, p < 0.0001) and T2D- (7.0 [6.2, 7.7] %, p < 0.001) compared to Ctrl (5.5 [5.1, 5.8] %). ΔEC50 of MG increased in healthy controls before (+12.9 %) and after (+12.6 %) FMD, whereas ΔEC50 of MG was unaffected in T2D- (before FMD: +1.8 %; after FMD: -1.7 %). In T2D+ ΔEC50 of MG significantly decreased after FMD (before FMD: -3.9 %; after FMD: -19.0 %, p = 0.005) and was reduced compared to T2D- (-1.7 % vs. -19.0 %, p = 0.027) and healthy controls (+12.6 % vs. -19.0 %, p = 0.035). ΔEC50 of H2O2 showed the same trend in all groups as for MG, but did not reach significance. However, EC50 of H2O2 before oGTT was significantly higher in T2D+ as compared to T2D- (before FMD: 134.3 μM vs. 91.8 μM, p = 0.028; after FMD: 134.0 μM vs. 98.6 μM, p= 0.032) and Ctrl (before FMD: 134.3 μM vs. 116.5 μM, p = 0.030; after FMD: 134.0 μM vs. 125.8 μM, p = 0.038). These results could mainly be attributed to higher EC50 values in female study participants.
Conclusion: Acute glucose stress induces cellular resistance to ROS and RCS in a hormetic manner in healthy subjects, a response which in diabetic patients seems to be lost during progression into diabetic complications. This response could not be restored after 5 days of caloric restriction. Furthermore, our data suggest for different capacities in handling of RM between female and male diabetic patients.
Supported by: German Research Foundation DFG (SFB 1118), German Center for Diabetes Research DZD (82DZD07C2G)
Disclosure: A. Sulaj: None.
OP 21 Retinopathy future vision
121
An early window of opportunity: risk factors for diabetic retinopathy are associated with early retinal neurodegenerative changes: The Maastricht Study
F. van der Heide1, S. Mokhtar1, R. Henry1, A. Kroon1, P. Dagnelie1, T. Berendschot1, S. Eussen1, J. Schouten2, M. Schram1, C. van der Kallen1, M. van Greevenbroek1, H. Savelberg1, N. Schaper1, C. Webers1, C. Stehouwer1;
1University of Maastricht, Maastricht, 2Canisius ziekenhuis, Nijmegen, Netherlands.
Background and aims: If determinants of retinal neurodegeneration, which precedes diabetic retinopathy, can be identified, there may already be an opportunity for the early prevention of diabetic retinopathy in individuals who are at risk for type 2 diabetes (e.g. individuals with prediabetes or obesity). We investigated the associations of risk factors for diabetic retinopathy with retinal neurodegenerative changes, using population-based data from an observational cohort study.
Materials and methods: We used cross-sectional data from The Maastricht Study (up to 5,666 participants, 50.5% men, mean ± SD age 59.7±8.7 years, and 22.6% with type 2 diabetes [the latter oversampled by design]). We investigated the associations of risk factors for diabetic retinopathy with retinal sensitivity, an index of retinal function, and retinal nerve fibre layer (RNFL) thickness, an index of retinal neural structure. We used linear regression analyses (results expressed as standardized betas) and adjusted for potential confounders (age, sex, educational level and key cardiovascular risk factors). We tested for interaction by sex and type 2 diabetes status.
Results: After full adjustment, greater HbA1c and lower healthy diet score (quantified with the Dutch healthy Diet index) were associated with lower retinal sensitivity (standardized betas [95%CI] -0.05 [-0.08; -0.02] and -0.06 [-0.09; -0.03], respectively) and lower RNFL thickness (-0.05 [-0.08; -0.02] and -0.03 [-0.06; -0.00]); high versus light alcohol consumption was associated with lower RNFL thickness (-0.08 [-0.16; -0.01],respectively), but not with retinal sensitivity (0.04 [-0.03; 0.10]); current versus never smoking was associated with lower retinal sensitivity (-0.14 [-0.22; -0.06]), but not with RNFL thickness (0.09 [-0.00; 0.18]); in individuals with, but not in individuals without, type 2 diabetes greater 24-hour ambulatory systolic blood pressure was associated with lower retinal sensitivity and lower RNFL thickness (in individuals with type 2 diabetes, -0.06 [-0.12; -0.04] and -0.06 [-0.13; 0.00], respectively); and, higher total cholesterol level was associated with greater retinal sensitivity (0.05 [0.02; 0.08]) and greater RNFL thickness (the latter only in individuals with type 2 diabetes; in individuals with type 2 diabetes, 0.09 [0.03; 0.16]). Sex did not modify any of the associations under study.
Conclusion: This population-based study found that most risk factors for diabetic retinopathy were independently associated with retinal neurodegenerative changes. Hence, early prevention of risk factors for diabetic retinopathy, already in individuals with prediabetes or obesity, may contribute to the prevention of diabetic retinopathy.
Supported by: ERDF, PL, DMEA, SW, PSID, CVCM, CARIM, CAPHRI, NUTRIM, SA, Perimed, DF, OF, HE, JC, NNF, SAN
Disclosure: F. van der Heide: None.
122
Transcriptome analysis reveals that retinal neuromodulation is the main underlying mechanism of the neuroprotective effect of sitagliptin in diabetic retina
H. Ramos, P. Bogdanov, J. Huerta, A. Deàs-Just, C. Hernández, R. Simó;
Diabetes and Metabolism Research Unit, Vall d'Hebron Research Institute (VHIR), Barcelona, Spain.
Background and aims: The neurovascular unit (NVU) is a functional coupling between neurons, glial cells and blood vessels that integrates vascular flow with metabolic activity. Its impairment is an early event in the pathogenesis of diabetic retinopathy (DR), which participates in the neurodegeneration and the early microvascular impairment of the diabetic retina. Consequently, NVU becomes an emergent therapeutic target of DR. The reduction of synaptic protein expression, the impairment of neurotransmission and alterations in neuronal morphology have been described as underlying mechanisms of NVU impairment. We previously reported that topical administration (eye drops) of sitagliptin, a dipeptidyl peptidase-4 inhibitor (DPP-4i), prevented retinal neurodegeneration induced by diabetes in db/db mice. To further explore the mechanisms involved in the beneficial effects of DPP-4i on diabetes-induced retinal neurodegeneration, we have compared by transcriptomic analysis the retinal expression patterns of mRNA in vehicle-treated db/db mice (an experimental model of DR) vs. db/db mice treated with sitagliptin.
Materials and methods: Ten db/db mice, aged 10 weeks, were topically treated with sitagliptin eye drops (5 μL/eye; concentration: 10 mg/mL) for 2 weeks twice per day, while other ten db/db received a topical administration of vehicle (5 μL/eye). Ten db/+ mice (non-diabetic mice) were assigned as control group. Before euthanasia, full-field electroretinogram recordings were used to address retinal functionality. At 12 weeks, after euthanasia, one eye was used for a transcriptome analysis and the other for its validation through RT-PCR and for protein assays through Western Blotting (WB) and Immunohistochemistry (IHC).Biological relevance of the transcriptome analysis was assessed through gen set enrichment analyses (GSEA) on two different annotation databases [Gene Ontology and Reactome Pathway Knowledge base].
Results: Diabetic mice topically treated with sitagliptin presented different expression patterns of mRNA in the retina in comparison to those treated with vehicle. GSEA revealed a positive enrichment, after sitagliptin treatment, of multiple candidates linked to synaptic transmission. Additionally, we observed in both, RT-PCR and WB/IHC assays, that presynaptic proteins involved in vesicle biogenesis, mobilization, docking, fusion and recycling, were down-regulated in db/db mice retinas in comparison with non-diabetic controls. Topical administration of sitagliptin inhibits this diabetes-induced down-regulation and improves the functionality of diabetic retinas without any effect on blood glucose levels.
Conclusion: Sitagliptin exerts neuroprotective effects in db/db mice retinas by inhibiting the down-regulation of key presynaptic proteins induced by diabetes. Notably, this effect is unrelated to the improvement of metabolic control. This finding opens up a new strategy for treating not only DR but also other retinal diseases in which synaptic abnormalities/neurodegeneration play a crucial role.
Supported by: UEB-VHIR, UAT-VHIR, VHIR, MICINN, CIBERDEM, UAB
Disclosure: H. Ramos: None.
123
Presence of retinopathy and kidney and cardiovascular events in type 2 diabetes and normoalbuminria: a post-hoc analysis of The PRIORITY study
T. Hansen1, V. Rotbain Curovic1, N. Tofte1, M.K. Lindhardt1,2, C. Delles3, M. Frimodt-Møller1, H. Mischak4, F. Persson1, H. von der Leyen5, P. Rossing1, on the behalf of the PRIORITY Study Group;
1Steno Diabetes Center Copenhagen, Herlev, Denmark, 2Department of Medicine, Holbæk Hospital, Holbæk, Denmark, 3Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK, 4Mosaiques Diagnostics, Hannover, Germany, 5Hannover Clinical Trial Center, Hannover Medical School, Hannover, Germany.
Background and aims: To evaluate the association between diabetic retinopathy and development of albuminuria, impaired kidney function and cardiovascular events in persons with type 2 diabetes and normoalbuminuria.
Materials and methods: Post-hoc analysis of the prospective observational PRIORITY study including 1756 persons with type 2 diabetes and normoalbuminuria followed for three years. The study was originally designed to investigate the prediction of a urinary proteomic risk classifier (CKD273) for development of albuminuria. Diabetic retinopathy included information from medical records on non-proliferative and proliferative changes, presence of macular oedema and history of laser treatment. Cox proportional hazard models were fitted to investigate baseline retinopathy status to development of 1) microalbuminuria (urinary albumin-creatinine ratio >30mg/g on ≥ 2 out of 3 urine samples); 2) chronic kidney disease (eGFR <60 ml/min/1.73m2); and 3) cardiovascular events (myocardial infarction, stroke, coronary intervention, and hospitalization for heart failure). Adjustment included sex, baseline age, diabetes duration, HbA1c, systolic blood pressure, eGFR, urinary albumin-creatinine rate and urinary proteomic risk classifier status. Baseline LDL cholesterol, body mass index and history of cardiovascular disease were also included in the adjustment for cardiovascular events.
Results: At baseline, 287 (16.3%) had retinopathy. Compared to persons without retinopathy, they were older (mean ±SD: 62.7±7.7 vs 61.4±8.3 years, p=0.019), had longer diabetes duration (17.9±8.4 vs. 10.6±7.0 years, p<0.001) and higher HbA1c (62±13 vs. 56±12 mmol/mol, p<0.001). The adjusted hazard ratios of retinopathy at baseline for development of albuminuria (n=197), chronic kidney disease (n=166) and cardiovascular events (n=64) were: 1.54 (95%CI: 1.06, 1.73), 0.89 (95%CI: 0.57, 1.38), and 2.56 (95%CI: 1.40, 4.66), compared to persons without retinopathy.
Conclusion: Individuals with normoalbuminuric type 2 diabetes and retinopathy had higher risk of developing albuminuria, but not impaired kidney function, and had a markedly higher risk of cardiovascular disease during the 3-year follow-up, compared to individuals without retinopathy.
Clinical Trial Registration Number: NCT02040441
Supported by: European Union Seventh Framework Programme (FP7/20072-013)
Disclosure: T. Hansen: None.
124
Stroke incidence increases with severity of diabetic retinopathy and maculopathy in people with type 1 diabetes
M.I. Eriksson1,2, K. Hietala1,3, P. Summanen4,1, C. Forsblom1,5, J. Putaala6, A. Ylinen1,2, S. Hägg-Holmberg1,2, P.-H. Groop1,5, L.M. Thorn1,7, on behalf of the FinnDiane Study;
1Folkhälsan Institute of Genetics, Folkhälsan Research Center, Helsinki, 2Research Program in Clinical and Molecular Metabolism, University of Helsinki, Helsinki, 3Department of Ophthalmology, Central Finland Hospital, Jyväskylä, 4Department of Ophthalmology, University of Helsinki and Helsinki University Hospital, Helsinki, 5Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, 6Department of Neurology, University of Helsinki and Helsinki University Hospital, Helsinki, 7Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Although the retinal vessels are suggested to mirror the brain’s vasculature, there are limited data on this association in type 1 diabetes. We therefore aimed to study the link between severity of diabetic retinopathy (DR) and stroke in type 1 diabetes.
Materials and methods: We included 1,327 participants with type 1 diabetes from the Finnish Diabetic Nephropathy Study (mean age 38.9±10.9 years, men 51.9%, kidney disease 35.9%). Exclusion criteria were stroke before baseline or missing DR data at baseline. Baseline visits were conducted in 1994-2006. Strokes were identified from registers, until the end of 2017, and verified from medical files. DR was graded according to the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. Data on macular oedema were available for 637 (48.0%) participants, and any clinically relevant macular oedema was considered as maculopathy.
Results: During a median of 17.7 (14.0-19.2) follow-up-years, 133 (10.4%) had a stroke (97 ischemic, 27 haemorrhagic, 9 unclassified). At baseline, 45.1% had no or mild DR (ETDRS 10-30), 16.0% moderate to severe DR (ETDRS 40-55), and 39.0% proliferative DR (ETDRS 60-80). Of the participants with available macular oedema data, 74.9% had maculopathy. Table 1 presents incidence rates and HR for stroke by severity of DR and maculopathy. In Cox regression analysis, adjusted for diabetes duration, sex, systolic and diastolic blood pressure, BMI, LDL cholesterol, triglycerides, and smoking, ETDRS 40-55 and ETDRS 60-80 were predictors of stroke (Table 1). Additionally, maculopathy was a predictor of stroke when analysed separately in a similar model (Table 1). After further adjustment for kidney disease, retinopathy severity (p=0.081 and p=0.171), and maculopathy (p=0.063) were no longer significant for any stroke. ETDRS 60-80 remained a significant predictor of haemorrhagic stroke (HR 10.2, 95% CI 1.2-86.2, p=0.033) in the final Cox model.
Conclusion: In our study, the incidence of stroke increased by the severity of DR and prevalence of maculopathy. Proliferative DR was a predictor of haemorrhagic stroke even in the presence of kidney disease.
Supported by: Folkhälsan Research Foundation, EVO governmental grant, Academy of Finland, Stockmann Foundation
Disclosure: M.I. Eriksson: Grants; Medical Society of Finland, Stockmann Foundation.
125
Fenofibrate use and diabetic retinopathy progression in patients with type 2 diabetes: a propensity-matched cohort study
N. Kim, J. Kim, K. Kim, J. Bae, K. Kim, S. Kim;
Internal Medicine, Korea University College of Medicine, Seoul, Republic of Korea.
Background and aims: To determine the association between fenofibrate use and diabetic retinopathy progression in patients with type 2 diabetes (T2D) and metabolic syndrome undergoing statin therapy in a real-world database.
Materials and methods: In this propensity-matched cohort study, patients with T2D and metabolic syndrome (≥ 30 years) receiving statin therapy were matched 1:2 by propensity score into the statin plus fenofibrate group (n=23,692) and statin-only group (n=46,223). The primary outcome was a composite of diabetic retinopathy progression including vitreous hemorrhage, vitrectomy, laser photocoagulation, intravitreous injection therapy and retinal detachment.
Results: For the primary outcome, the incidence rate per 1,000 person year was 14.25 in the statin-only group and 12.65 in the statin plus fenofibrate group. The risk of the primary outcome was significantly lower (hazard ratio [HR], 0.89; 95% confidence interval [CI], 0.83 to 0.96; p=0.001) in the statin plus fenofibrate group than in the statin-only group. Patients with retinopathy at baseline showed marked benefits of fenofibrate treatment (HR, 0.86; 95% CI, 0.78 to 0.94; p=0.001). In addition, the statin plus fenofibrate group exhibited significantly lower risks of vitreous hemorrhage (HR, 0.87; 95% CI, 0.78 to 0.96; p=0.008), laser photocoagulation (HR, 0.89; 95% CI, 0.81 to 0.98, p=0.022) and intravitreous injection therapy (HR, 0.78; 95% CI, 0.66 to 0.92; p=0.003) than those in the statin-only group. There was no significant interaction between the different characteristics at baseline and the treatment effect.
Conclusion: In this propensity-weighted cohort study, the addition of fenofibrate to statins was associated with significantly lower risk of diabetic retinopathy progression than statin therapy alone in patients with T2D and metabolic syndrome.
Supported by: Abbott Laboratories, Korea
Disclosure: N. Kim: None.
126
Blocking haemopexin with specific antibodies: a new experimental strategy for treating diabetic retinopathy
R. Simó1, P. Bogdanov1, A. Duarri2, A. Salas2, D. Sabater1, H. Ramos1, H. Isla2, C. Hernández1;
1Diabetes and Metabolism Research Unit, Vall d'Hebron Research Institute, CIBERDEM, 2Ophthalmology Research Group, Vall d'Hebron Research Institute, Barcelona, Spain.
Background and aims: By means of a proteomic analysis we identified hemopexin (Hpx) as a genuine and abundant protein in the vitreous fluid of patients with diabetic macular edema (DME). In addition, we provided first evidence that Hpx is overexpressed in the retina of diabetic patients, and induces hyperpermeability due to the breakdown of the blood-retinal barrier (BRB), an essential pathogenic mechanism for the development of DME. The aims of the present study are: 1) To evaluate whether the inhibition of Hpx by specific antibodies (intravitreally administered) is able to arrest vascular leakage in two experimental models of diabetes 2) To explore in in vitro models whether Hpx antibodies are able to inhibit microvascular angiogenesis.
Materials and methods: In vivo studies: An intravitreal (IVT) injection of 1.5 μl of anti-Hpx antibody (1 mg/ml), (n=10) or saline (n=10) in each eye was administered to db/db mice at age of 12 weeks. Ten non-diabetic mice (db/+) matched by age served as a control group. Retinal vascular permeability was examined 5 days after intravitreal injection using the Evans Blue albumin method. HPX expression (mRNA) was determined by RT-PCR. The same protocol and assessments were performed using Long-Evans rats without diabetes (n=9) and with STZ-induced diabetes (n=9). In vitro studies: The microvascular angiogenesis was tested by using the scratch wound healing technique and sprouting assay, both stimulated by VEGF treatment (25 ng/mL). For the former we used human retinal endothelial cells (HRECs) and the inhibitory effect of anti-Hpx antibodies (0.36 mcg/mL) was tested. To examine sprouting we measured the area (mm2) of sprouted cells using choroidal explants from Long-Evans rats which were cultured under different conditions: 5.5 mM glucose, 30 mM glucose, 30 mM + bevacizumab (125 and 250 mcg/mL), 30 mM glucose + 0.1 microM dexamethasone, 30 mM glucose + anti-Hpx antibodies (0.36 mcg/mL). In addition, explants from Long-Evans rats with STZ-induced diabetes treated with IVT injection of anti-Hpx antibodies (1mg/ml) vs. treated with vehicle were compared.
Results: A higher expression of Hpx was detected in retina from diabetic animals in comparison with non-diabetic animals (p<0.05). IVT injections of anti-Hpx antibodies significantly reduced vascular leakage in the two diabetic models in comparison with vehicle (p<0.01). Treatment with Hpx antibodies was able to significantly reduce the HRECs migration induced by high glucose concentration with and without VEGF after scratch (p<0.01). In addition, Hpx neutralization inhibited the vessel outgrowth from choroidal explants in non-diabetic rats (60% of reduction of the sprouting area; p<0.01) and STZ-induced diabetic rats (75% reduction; p<0.01 of the sprouting area). Notably, this effect was similar to obtained with bevacizumab or dexamethasone.
Conclusion: The blockade of Hpx-induced permeability by using IVT injections of anti-Hpx antibodies can be envisaged as a new strategy for treating DME. The in vitro and ex-vivo antiangiogenic effect also suggest proliferative retinopathy as a potential target, but in vivo experiments are lacking. Further research is needed to confirm Hpx not only as a therapeutic target of advanced diabetic retinopathy but also to explore its role as an alternative to anti-VEGF agents.
Supported by: ISCiii (DTS19/00171)
Disclosure: R. Simó: None.
OP 22 Giving birth with diabetes
127
Glycaemic control and complication rate through pregnancy and thirteen-years post-partum in women with microalbuminuria (type 1 diabetes)
N. Asatiani, R. Kurashvili, E. Inashvili, E. Shelestova, T. Akhobadze;
Diabetes in Pregnancy, National Center for Diabetes Research, Tbilisi, Georgia.
Background and aims: The aim of the present work was to assess the degree of glycemic control and complications rate through pregnancy and thirteen-years post-partum in women with microalbuminuria (T1DM).
Materials and methods: In total 191 patients with T1DM were enrolled in the study. Based on albuminuria levels in the 1st trimester women were separated into 2 groups (Gr.). Gr.1 - 116 women with normoalbuminuria, Gr.2 - 75 women with microalbuminuria. Preconception care was performed in 46.5%- Gr.1 and 57.3% -Gr.2. Strict metabolic control was maintained and fetal surveillance was performed throughout the pregnancy. Repeated examinations were performed 13 years’ post-partum.
Results: At entry HbA1c(%) levels for Gr.1 and 2 were: 7.42 (0.15) and 7.25 (0.14); by the end of the pregnancies they statistically decreased in both groups (Gr.1- P=0.000, Gr.2 - P=0.000). At entry percent (%) of retinopathy for Gr.1- 8.6 and Gr.2 - 20.0; by term the percent has not increased. The percent of women with macroalbuminuria increased, together with the growth of gestational age. By term macroalbuminuria was observed in 2.5% (Gr.1) and in 14.6% (Gr.2) of patients (P=0.0094, OR-5.67). In Gr.1 percent of pre-eclampsia and preterm deliveries before 37 weeks of gestation was lower, than in Gr.2 (pre-eclampsia - P = 0.0064, OR -4.64; preterm deliveries P = 0.048; OR -2.8). Perinatal mortality was observed in Gr.1 - 0.8% and in Gr.2 - 6.6% of women (P - 0.006, OR - 7.73). Repeated examinations 13 years post-partum showed that HbA1c levels were statistically higher, than at the end of pregnancy: Gr.1-7.7 (0.41) (P=0.0002), Gr.2 - 8.05 (0.26) (P=0.000). Repeated examinations showed that percent of retinopathy, neuropathy, micro and macroalbuminuria, increased in both groups. Besides, patients from Gr.2 had statistically higher complications rate, than patients from Gr.1 (retinopathy - P=0.0002, OR - 3.45; microalbuminuria - P<0.0001, OR - 10.2; macroalbuminuria -P=0.0032, OR- 3.03). Chronic kidney disease were observed in 36 patents. Nine patients from Gr.2 are on regular hemodialysis, and in two patient kidney transplantation was performed.
Conclusion: If microalbuminuria was detected in the 1st trimester, the risk of preeclampsia increased 4.6 times, the risk of preterm delivery increased 2.8 times and risk of perinatal mortality increased 7.3 times, compared to patients with normoalbuminuria. In both groups glycemia control deterioration was observed thirteen years post-partum. The higher complication percent was found if pregnancy proceeded with microalbuminuria, the risk of retinopathy increased 3.4 times and the risk of CKD increased 3 times compared to patients with normoalbuminuria.
Disclosure: N. Asatiani: None.
128
Glucose response patterns based on 75g OGTTs during pregnancy and their association with the risk of macrosomia: a latent class analysis of three cohort studies
L. Fritsche1, A. Hulman2, K. Prystupa3, M. Heni4, S.L. White5, A.L. Birkenfeld6, A. Peter4, A. Fritsche3, A. Kun7, L. Poston5, A. Tabak8, R. Wagner9;
1Institute for Diabetes Research and Metabolic Diseases, Helmholtz Center Munich, Tuebingen, Germany, 2Steno Diabetes Center, Aarhus, Denmark, 3Department of Internal Medicine, Division of Endocrinology, Diabetology and Nephrology, University Hospital Tuebingen, Tuebingen, Germany, 4Institute for Clinical Chemistry and Pathobiochemistry, Department for Diagnostic Laboratory Medicin, University Hospital Tuebingen, Tuebingen, Germany, 5Department of Women and Children’s Health, King's College London, London, UK, 6. Department of Internal Medicine, Division of Endocrinology, Diabetology and Nephrology, University Hospital Tuebingen, Tuebingen, Germany, 7Department of Obstetrics & Gynaecology, Tolna County Balassa János Hospital, Szekszárd, Hungary, 8Department of Public Health, Faculty of Medicine, Semmelweis University, Budapest, Hungary, 9German Center for Diabetes Research, Neuherberg, Germany.
Background and aims: Studies in the general population show that different glucose response patterns based on oral glucose tolerance tests (OGTT) are associated with different clinical characteristics and long-term outcomes. We aimed to identify comparable glucose response patterns in pregnancy and their association with maternal and fetal outcomes.
Materials and methods: We used latent class trajectory modelling to identify glucose response patterns using a 5-point 75g OGTT in 470 pregnant women at 27.3±2.2 weeks of gestation. We assessed these classes and examined pregnancy outcomes in two independent European cohorts with 3-point OGTTs: a population based Hungarian cohort with 7073 pregnant women and the control group of the UK Pregnancies Better Eating and Activity Trial comprising of 610 obese women. Pregnancy outcomes in the different classes were analysed using generalized estimating equations (GEE).
Results: We identified five different glucose response patterns (classes, Fig.1). Rate of gestational diabetes (GDM) was lowest in class 1 (< 7%) and highest in class 5 (100%) for all three cohorts. Class 3 was characterized by transient hyperinsulinemia at 30 minutes (Fig.1) but the prevalence of GDM was only 25-36%. Compared to class 1, women in class 3 had higher gestational weight gain (GWG, β=0.76 SE: 0.28kg, p=0.0076, adjusted for study, age, gestational age, pre-gestational BMI, GDM treatment and AUCGlucose). New-borns in class 3 had the highest risk of macrosomia (OR 1.49 95%CI: [1.17, 1.90] vs. class 1) after adjustment for study, maternal age, parity and smoking, but this was attenuated by additional adjustment for pre-pregnancy BMI and GWG.
Conclusion: We found an easily identifiable group of pregnant women who have an increased risk of macrosomia often without formally meeting GDM criteria. These women would most likely benefit from a therapy (e.g. nutritional counselling) targeting excessive glucose excursions and GWG.
Clinical Trial Registration Number: NCT04270578, ISRCTN89971375
Supported by: BMBF (01GI0925) to DZD
Disclosure: L. Fritsche: None.
129
Changes in body fat partitioning and insulin resistance between preconception and postpartum in Singaporean women: the SPRESTO study
S. Sadananthan1, N. Michael1, Y. Manuel1,2, K. Thirumurugan1, J. Yaligar1, M. Tint1,2, K. Tan3, K.M. Godfrey4, P.D. Gluckman1, Y. Chong1,2, Y. Lee1,2, J.K.Y. Chan3, S.-Y. Chan1,2, J.G. Eriksson1,2, S. Velan1;
1Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore, Singapore, 2National University of Singapore, Singapore, Singapore, 3KK Women’s and Children’s Hospital, Singapore, Singapore, 4MRC Lifecourse Epidemiology Unit & NIHR Southampton Biomedical Research Centre, University of Southampton & University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Background and aims: Pregnancy is a period when the body experiences dynamic changes in body composition, hormone levels, insulin resistance, and a build-up of energy reserves in the form of fat to support lactation. While postpartum weight retention has been linked to increased metabolic risk, the role of changes in body fat partitioning has not been well investigated. In the Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO), we characterized the changes in body weight, body fat partitioning, and insulin resistance between preconception and 3 months postpartum.
Materials and methods: 1039 women (aged 18-45 years, Chinese, Malay, and Indians) who intended to get pregnant within the next 12 months were recruited; those who conceived were followed through their pregnancy and at 3 months postpartum. 68 participants underwent MRI at both preconception and postpartum time points; volumetric abdominal MRI was used for the segmentation and quantification of deep subcutaneous (DSAT), superficial subcutaneous (SSAT), and visceral adipose tissue (VAT) volumes. Ectopic fat accumulation within the liver and muscle (intramyocellular lipids (IMCL)) was determined using magnetic resonance spectroscopy. Insulin resistance was assessed using homeostatic model assessment for insulin resistance 2 (HOMA2-IR) preconception, at week 24-28 of gestation, and at 3 months postpartum.
Results: As expected, both body weight and HOMA2-IR significantly increased during pregnancy. At 3 months postpartum, HOMA2-IR returned to preconception levels, while body weight was higher than preconception (Table 1); subcutaneous fat depots (DSAT and SSAT) and IMCL at postpartum were similar to the preconception levels, but VAT (p = 0.001) and liver fat (p = 0.027) were increased 3 months after delivery. There were no associations between the changes in fat depots and the change in HOMA2-IR.
Conclusion: Our findings indicate that subcutaneous fat depots may be preferentially mobilized in the early postpartum period, relative to VAT and ectopic fat in the liver. While elevated VAT and liver fat have been generally linked to elevated insulin resistance, increases in both these depots were not accompanied by an increase in HOMA2-IR in the early postpartum period. Further assessments are required to evaluate whether the dissociation between these pathogenic fat depots and HOMA2-IR is a transient phenomenon.
Clinical Trial Registration Number: NCT03531658
Supported by: NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014
Disclosure: S. Sadananthan: Grants; National Medical Research Council, Singapore National Research Foundation (NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014), Singapore Institute for Clinical Sciences.
130
Maternal diabetes and attention-deficit/hyperactivity disorder in childhood
C.E. Cesta1, K.K.C. Man2,3, L.J. Kjerpeseth4, L. Gao2, Nordic Pregnancy Drug Safety Studies (NorPreSS) consortium, A.Y.L. Chan2,5, M.H.C. Hsieh6, E.C.C. Lai6, H. Zoega7,8, I.C.K. Wong3,2;
1Karolinska Institutet, Stockholm, Sweden, 2The University of Hong Kong, Hong Kong, Hong Kong, 3UCL School of Pharmacy, London, UK, 4Norwegian Institute of Public Health, Oslo, Norway, 5University of Groningen, Groningen, Netherlands, 6National Cheng Kung University, Tainan, Taiwan, 7University of Iceland, Reykjavik, Iceland, 8UNSW Sydney, Sydney, Australia.
Background and aims: Recent studies suggest an increased risk of attention-deficit/hyperactivity disorder (ADHD) in the children born to mothers with diabetes mellitus during pregnancy, including pregestational diabetes (PGDM) and gestational diabetes mellitus (GDM). However, current evidence remains inconclusive. This study aims to assess the association between prenatal exposure to maternal diabetes mellitus (MDM) and the risk of ADHD in childhood.
Materials and methods: This is a multinational cohort study with linked mother-child pairs using healthcare databases from Asia (Hong Kong (HK), Taiwan) and Northern Europe (NorPreSS: Finland, Iceland, Norway, Sweden) including children born between 2001-2018 with follow-up through 2020 (subject to data availability). Cox proportional hazard regression models and propensity score fine stratification, including demographic, comorbidity, and comedication covariates, were used to calculate hazard ratios (HR) with a 95% confidence interval (CI) for each comparison.
Results: We included 4,554,325 million mother-child pairs (HK: 535,924; Taiwan: 887,120; NorPreSS: 3,131,281) in the analyses, of which 6.1% of children were prenatally exposed to MDM (71,232 to PGDM; 207,171 to GDM). A total of 158,154 children had ADHD (HK: 16,453; Taiwan: 85,471; NorPreSS: 56,230). Children born to mothers with MDM were at a higher risk of developing ADHD (HK: PS-weighted HR 1.18, 95%CI 1.11-1.26; Taiwan: 1.13, 1.09-1.18; NorPreSS: 1.22; 1.18-1.26). The elevated risk was present separately for PGDM (HK=1.20, 0.98-1.46; Taiwan=1.63, 1.45-1.83; NorPreSS=1.31, 1.24-1.38) and for GDM (HK=1.18, 1.10-1.25; Taiwan=1.11, 1.07-1.15; NorPreSS=1.17, 1.12-1.22). Study sized allowed for a sibling-matched GDM analysis, which showed no association between prenatal exposure to GDM and the risk of ADHD (HK: 1.00, 0.86-1.16; Taiwan: 0.96, 0.85-1.07).
Conclusion: Our findings suggest that children prenatally exposed to maternal diabetes in general, and specifically to PGDM and GDM, have an increased risk of ADHD in childhood. However, for at least GDM exposure the risk is largely due to unmeasured familial confounding. Further investigation will assess the role of pharmacological treatment of MDM during pregnancy and glycaemic control on the association between maternal diabetes and child ADHD.
Supported by: Hong Kong Research Council (no.1711202), NordForsk (no.83539), MSCA (no.844728)
Disclosure: C.E. Cesta: Grants; EU Horizon 2020 MSCA global fellowship (no. 844728).
131
Pregestational diabetes and risk of congenital heart defects in the offspring: French nationwide study using the French PMSI-MCO database
M. Lemaitre, G. Bourdon, A. Bruandet, X. Lenne, D. Subtil, T. Rakza, A. Vambergue;
CHRU Lille, Lille, France.
Background and aims: Congenital Heart defects (CHD) are the most common type of congenital malformations in offspring of women with pregestational diabetes. Our aim was first to estimate the incidence of CHDs in the offspring of mothers with type 1 diabetes and those with type 2 diabetes using data from the national French Medical Information System Program in Medicine, Surgery and Obstetrics database (PMSI-MCO), compared to the general population. Secondly, we investigated if the association between maternal diabetes and CHD varied with type of diabetes (Type 1 diabetes (T1D) or type 2 diabetes (T2D) in a large national cohort in France.
Materials and methods: The presence of CHDs and maternal diabetes were screened for according to the International Classification of Diseases, 10th [Revision], in the PMSI-MCO database, from 2012 to 2020. A logistic model was used to estimate risk factors for maternal-fetal prognostic indicators in women with T1D, in women with T2D, and in the control population by adjusting for maternal age, gender of the newborn, prematurity, Small for gestational Age (SGA), Large for gestational Age (LGA) and, mode of delivery.
Results: 6,076,251 mother-infant pairs were included. The rate of congenital malformations was 6.2% in the control group, 8.0% in women with T1D and 8.4% in women with T2D (p<0.001). The incidence of CHD was 8.0 per 1000 births in the control group, 29.6/1000 and 27.4/1000 in women with T1D and those with T2D, respectively (p<0.001). The risk of CHD was 2.07 times higher in women with type 1 diabetes [CI 95% [1.91-2.24], p<0.001] and 2.20 times higher in women with type 2 diabetes [CI 95% [1.99-2.44]], p<0.001] with no difference found between T1D and T2D (p=0.336). Cesarean section, SGA, LGA, and prematurity were also associated with an excess risk of CHD.
Conclusion: Pregestational diabetes is a risk factor for the development of CHD in the offspring without a significant difference between the two types of diabetes. Metabolic control is a modifiable risk factor that can be addressed to reduce the risk of CHD.
Disclosure: M. Lemaitre: None.
132
Beta cell function, hepatic insulin clearance and insulin sensitivity in South Asian and Nordic women after gestational diabetes
A. Sharma1,2, I. Nermoen1,2, E. Qvigstad3,2, C. Sommer2, N. Sattar4, J. Gill4, H. Gulseth5, S. Sollid6, K. Birkeland2,3, S. Lee-Ødegård2,3;
1Ahus, Lorenskog, Norway, 2University of Oslo, Oslo, Norway, 3Oslo University Hospital, Oslo, Norway, 4University of Glasgow, Glasgow, UK, 5Norwegian Institute of Public Health, Oslo, Norway, 6Vestre Viken Drammen Hospital, Drammen, Norway.
Background and aims: The risk of developing type 2 diabetes (T2D) after gestational diabetes mellitus (GDM) is twice as high in South Asian compared to Nordic women. We aimed to assess and compare ethnic differences in β-cell function and insulin sensitivity (IS) in women with normal glucose tolerance (NGT), prediabetes and T2D, 1-3 years after a GDM pregnancy.
Materials and methods: We performed an OGTT in South Asian (n=179) and Nordic (n=108) women living in Norway. Based on the OGTT, we calculated the pre-hepatic insulin secretion rate (ISR) by deconvolution of C-peptide kinetics, and hepatic insulin clearance (HIC) as the ratio of ISR to peripheral insulin levels. First phase insulin secretion was assessed by the insulinogenic index (IGI). Fasting insulin secretion was represented by the homeostasis model assessment (HOMA)2-β. β-cell glucose sensitivity (βC-GS) was approximated from cross-correlation of ascending glucose and ISR levels during the OGTT. Hepatic insulin sensitivity (IS) was estimated by HOMA2-S, peripheral IS by the muscle IS index (muscle-ISI) and whole-body IS by the Matsuda index (Matsuda-ISI). β-cell function adjusted for insulin resistance was estimated by the disposition index (DI); estimated by IGI and either HOMA2-IR (DI-HOMA2-IR) or Matsuda-ISI (DI-Matsuda).
Results: Compared to Nordic women, South Asians had higher levels of glucose, insulin, C-peptide and ISR during the OGTT. HOMA2-β and βC-GS (p<0.05) were higher in South Asian vs. Nordic women. HIC was lower, and so were HOMA2-S, muscle-ISI, Matsuda-ISI and both DI-HOMA2-IR and DI-Matsuda. In NGT women, our results were similar, expect for a higher IGI, but similar DI-HOMA2-IR and DI-Matsuda between South Asian and Nordic women. In women with prediabetes or T2D, both ethnicities displayed similar OGTT glucose levels, but South Asian exhibited higher OGTT insulin and ISR levels than Nordic women (Table 1).
Conclusion: In women examined 1-3 years after a GDM pregnancy, South Asians displayed a ‘stressed’ β-cell function compared to Nordic women. South Asians also displayed lower IS and HIC across all glucose tolerance categories. In spite of NGT, South Asian women exhibited higher fasting and first-phase insulin secretion. Hence, a more ‘stressed’ β-cell function in addition to lower IS and HIC in South Asian women may increase their propensity to develop T2D after GDM.
Supported by: The Research Council of Norway, grant number 273252.
Disclosure: A. Sharma: None.
OP 23 Are we too slow to outlaw the low?
133
Severe hypoglycaemia presenting to a hospital emergency department: clinical characteristics and mortality outcomes
S.H. Song1, B.M. Frier2;
1Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, 2The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK.
Background and aims: The prevalence of young-onset type 2 diabetes (YOT2D), diagnosed below age 40, is increasing with many requiring insulin. Severe hypoglycaemia (SH) is common in insulin-treated diabetes and may be a marker of underlying illness. The frequency of SH in YOT2D was ascertained in all people requiring treatment in a hospital emergency department (ED), and clinical characteristics and mortality outcomes were examined.
Materials and methods: Patients with SH who attended ED at Northern General Hospital, Sheffield, UK between January 2019 and March 2022 were identified. Complete collection of SH events was ensured by interrogating several sources: ambulance service, hospital ED, inpatient and general practice records. Data included diabetes type, clinical characteristics and comorbidities, and were analysed by age of diabetes onset, below and above age 40. Charlson score quantified the comorbidity burden.
Results: A total of 537 episodes of SH occurred in 442 patients, of whom 155 (35.1%) had type 1 diabetes (T1D), 184 (41.6%) had type 2 diabetes (T2D), 7 (1.6%) had insulin-treated secondary pancreatic diabetes and 96 (21.7%) were non-diabetic, with mean blood glucose concentrations at presentation of 1.8±0.7, 2.0±0.7, 1.8±0.8 and 2.0±0.7 mmol/L respectively. SH events recurred in 61 (13.8%) patients (2-10 ED visits per person), totalling 153 (28.5%) attendances. Forty-five (24.5%) patients with T2D were taking neither sulfonylureas nor insulin. T1D had more impaired hypoglycaemia awareness (51.0% vs 14.6%, p<0.005) and history of SH (56.1% vs 15.8%, p<0.005) than T2D, while cognitive impairment was less prevalent (7.1% vs 19.6%, p=0.001). T2D had higher Charlson score (8.5±3.1 vs 4.3±3.1, p<0.005) and greater socioeconomic deprivation than T1D (20% most deprived decile; 53.3% vs 38.1%, p=0.006). More T2D required hospital admission than T1D (74.4% vs 60.3%, p=0.002). Of those diagnosed age <40, 131 (84.5%) had T1D (YOT1D) and 24 (13.0%) had T2D (YOT2D) with median (range) age of diabetes onset 18 (1-39) and 35 (18-39) years respectively. YOT2D had more cardiorenal disease (66.7% vs 42.7%, p=0.04) and heart failure (41.7% vs 13.7%, p=0.003) than YOT1D. Mental illness was equally common in both types of diabetes (YOT1D vs YOT2D; 50.4% vs 45.8%, p=NS). Of those diagnosed age >40, T2D had more cardiorenal disease (84.0% vs 47.8%, p<0.005), heart failure (30.8% vs 8.7%, p=0.026) and liver disease (20.5% vs 4.3%, p=0.046) than T1D. Most SH occurred in YOT1D (188 episodes) and older-onset T2D (179 episodes). Only 28 episodes occurred in YOT2D. Mortality after index presentation to ED was higher in T2D than T1D (38.0% vs 14.2%, p<0.005) with shorter mean time to death (239.8±290.6 vs 470.0±318.2 days, p=0.002). Compared to T1D, the T2D cohort had more cancer (34.3% vs 9.1%, p=0.029), respiratory disease (40.0% vs 9.1%, p=0.008) and cardiorenal disease (87.1% vs 63.6%, p=0.024). The mortality was similar in non-diabetics to T2D (37.5% vs 38.0%, p=NS) but death occurred significantly earlier (113.7±218.4 vs 239.8±290.6 days, p=0.014). Charlson score was elevated (non-diabetic vs T2D; 7.7±2.8 vs 9.9±2.7, p<0.005) and cardiorenal disease was prevalent in both groups (non-diabetic vs T2D; 94.4% vs 87.1%, p=NS).
Conclusion: SH is uncommon in YOT2D except when certain comorbid conditions co-exist. SH may be a biochemical marker of severe underlying medical disorders that predicts a poor prognosis.
Disclosure: S.H. Song: None.
134
Determining the incidence and clinical predictors of severe hypoglycaemia in patients receiving insulin and sulphonylureas for type 2 diabetes
R.L.M. Cordiner, K. Bedair, L. Donnelly, G. Leese, E.R. Pearson;
Level 5, Mailbox 12, University of Dundee, Dundee, UK.
Background and aims: The most serious adverse effect of sulphonylureas (SU) and insulin treatment of patients with type 2 diabetes (T2DM) is hypoglycaemia, which may result in significant morbidity and mortality. We aimed to investigate the incidence and clinical predictors of severe hypoglycaemia (SH) requiring paramedic intervention in patients treated with SU or insulin for T2DM in real-world data from patients in Tayside and Fife regions of Scotland
Materials and methods: We undertook a retrospective cohort study of n=23016 patients with T2DM treated with insulin or SU in NHS Tayside and Fife between 2008 and 2016. Data were from a SCI-diabetes extract linked to unique Scottish Ambulance Service data in the region. We calculated the incidence rates of severe hypoglycaemia (SH) per 1000 person years. Chi-Squared tests were conducted to evaluate clinical and biochemical characteristics of patients who had experienced SH episodes within the region. A fixed effect Poisson regression model was developed to identify predictors of SH. We then developed and validated a clinical prediction tool to predict individual risk for SH.
Results: The incidence of SH in SU treated T2DM was 0.5% per year, and 1.8% in those treated with insulin. SH was less common in males treated with SU (0.47%-year male; 0.66% female, Chi-Squared Test p<0.0001), but more common in males treated with insulin (1.9%/year male, 1.7 %/year female, Chi-Squared Test p<0.0001). The incidence of SH in females treated with gliclazide was almost double that of males (0.41% male, 0.75% female, Chi-Squared Test p<0.0001). There was increased risk of SH with increasing age, duration of diabetes and creatinine, and lower HBA1c and a BMI <30 kg/m2. Modified release gliclazide was the SU with lowest risk of SH, with incidence of 0.02% per year versus 1.3% with glibenclamide. The model was trained on the observation period data from the Tayside population (n=8903) with replication in Fife (n=8737), the mean model error was 0.037 per 1000 person years, suggesting that the model performed well with little error and could have potential clinical utility.
Conclusion: The incidence of SH associated with SU is low, more than a third lower than that of insulin. However, some individuals are at high risk even when treated with SU - e.g. older women with long duration of diabetes and low HbA1c. Our validated prediction tool has the clinical potential to identify those at high risk of SH and optimise their treatment to minimise risk (by switching of insulin or SU, reducing dose, or changing to Gliclazide MR).
Supported by: Wellcome Trust New Investigator Award
Disclosure: R.L.M. Cordiner: None.
135
Dishabituation with high intensity exercise improves hormonal and symptom responses to hypoglycaemia in people with type 1 diabetes and impaired awareness of hypoglycaemia
C.M. Farrell1, A.D. McNeilly1, S.M. Hapca2, T.W. Jones3, P.A. Fournier3, D. West4, R.J. McCrimmon1;
1Clinical Research Centre, Ninewells Hospital, Dundee, UK, 2Computing Science, University of Stirling, Stirling, UK, 3University of Western Australia, Perth, Australia, 4Faculty of Medical Sciences, Newcastle University, Newcastle, UK.
Background and aims: Impaired awareness of hypoglycaemia (IAH) in Type 1 diabetes (T1D) is a major risk factor for severe hypoglycaemia. We recently proposed that IAH develops as a form of adaptive memory (Habituation) to repeated hypoglycaemia. Consistent with this, we found that introduction of a single novel stress stimulus [high intensity exercise (HIE)] partially restored counterregulatory (CRR) hormonal and symptom responses to hypoglycaemia in people with T1D and IAH; a process referred to as Dishabituation. For HIE to prove to be an effective therapeutic intervention, it is important to demonstrate sustained efficacy in individuals with TID who suffer from IAH.
Materials and methods: To address this question, we conducted a 4-week pilot, single centre, randomised parallel-group study using HIE in people with T1D and IAH: HIT4HYPOS. Individuals (n=18) underwent a 4-week run-in period of insulin optimisation, before randomisation to HIE (3 session per week [4 x 30 s cycle sprints separated by 2 min of recovery] to reach an intensity corresponding to ≥ 90% peak heart rate achieved during VO2peak assessment) for 4 weeks plus use of real-time continuous glucose monitoring (rtCGM) or Control (rtCGM alone). At baseline and following the intervention they underwent a 90-minute hyperinsulinaemic hypoglycaemic clamp at 2.5 mmol/l with measurement of hormonal CRR and symptom scores.
Results: Change from baseline was compared between groups using a generalised estimated equation, adjusted for baseline and euglycaemia. In comparison to Control, HIT significantly increased glucagon (Δ mean [SEM] 0.9 [3.89] vs 16.2 [6.21] ng/l, p < 0.01) and maintained noradrenaline (-987.71 [447.37] vs 514.03 [731.73] pmol/l, p < 0.01), but not adrenaline responses (298.1 [687.42] vs 1129.7 [747.40] pmol/l; p = 0.11) during equivalent hypoglycaemia. Post intervention total symptom scores during hypoglycaemia were greater in the HIT group than the Control group (Edinburgh Hypoglycaemia scale; 25.0 +/- 2.0 vs 20.4 +/- 2.4 vs, p <0.05).
Conclusion: These findings suggest that 4 weeks of HIE provide sustained benefits on the hormonal and symptomatic CRR to hypoglycaemia in people with T1D and IAH. Of note there was a small but significant increase in hypoglycaemia-induced glucagon. Therefore, HIT may represent a novel therapeutic intervention for people with T1D and IAH.
Clinical Trial Registration Number: ISRCTN15373978
Supported by: Diabetes UK and JDRF
Disclosure: C.M. Farrell: Grants; Diabetes UK 17/0005591, JDRF 3-SRA-2017-485-S-B.
136
Decreased branched-chain amino acids and elevated fatty acids during antecedent hypoglycaemia in type 1 diabetes
R. She1,2, N. Al-Sari3, I. Mattila3, P. Henriksen3, J. Pedersen4, A.-S. Sejling1,5, C. Legido-Quigley3,6, U. Pedersen-Bjergaard1,2;
1Department of Endocrinology and Nephrology, Nordsjællands Hospital, Hillerød, Denmark, 2Institute of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 3Steno Diabetes Center Copenhagen, Herlev, Denmark, 4Department of Internal Medicine, Herlev and Gentofte Hospital, Herlev, Denmark, 5Novo Nordisk A/S, Bagsværd, Denmark, 6King's College London, Institute of Pharmaceutical Science, UK.
Background and aims: Hypoglycaemia is a major limiting factor in achieving recommended glycaemic targets for individuals in insulin treatment and can lead to late diabetes complications, incremental morbidity, and mortality. While counterregulatory hormonal responses have been studied extensively in patients with type 1 diabetes, a more comprehensive assessment of the metabolic responses has not been done previously. This post hoc study explored potential responses of the metabolome to hypoglycemia.
Materials and methods: A post hoc study examining the metabolome from participants with euglycemia and hypoglycemia during hyperinsulinemic clamps. Setting and Participants: Twenty-one outpatients with type 1 diabetes were recruited in the outpatient clinic. Intervention: On two consecutive days participants underwent a period of euglycemia (5.0-5.5 mmol/l) and a period of hypoglycemia (2.0-2.5 mmol/l) during the hyperinsulinemic glucose clamp. Primary Outcome Measure: Plasma samples were taken 40 min after reaching euglycaemia and 20 min and 40 min after reaching hypoglycaemia. Non-targeted plasma metabolomic analyses were conducted using two-dimensional gas chromatography/time-of-flight mass spectroscopy. Metabolites were analyzed by a linear mixed effect model, adjusting for age and sex. P-values were adjusted for multiple testing using the Benjamini-Hochberg method.
Results: In total, 79 metabolites were identified. Concentrations of the branched-chain amino acids, leucine, and isoleucine, decreased during hypoglycaemia at day 1: isoleucine (β±SE: -0.72±0.16, p = 2.2x10-3), leucine (β±SE: -0.78±0.18, p = 3.8x10-3). At day 2, five amino acids including all three branched-chained amino acids decreased: Leucine (β±SE: -0.62±0.13, p = 0.002), isoleucine (β±SE: -0.59±0.19, p = 0.026), valine (β±SE: -0.52±0.12, p =0.002), methionine (β±SE: -0.62±0.19, p = 0.026) and phenylalanine (β±SE: -0.65±0.20, p = 0.026). Two fatty acids were increased, oleic acid (β±SE: 0.50±0.14, p = 0.016) and tetradecanoic acid (β±SE: 0.63±0.17, p = 0.013). More metabolites responded to hypoglycemia on day 2; however, metabolic responses were not significantly different between the two days. Hormonal counter-regulatory responses to hypoglycemia were not different between the two days.
Conclusion: In conclusion, this study found that the metabolome alternates in response to insulin-induced hypoglycemia, resulting in decrement of several amino acids and increment of two fatty acids. The hormonal counterregulatory response was indifferent between the first and subsequent hypoglycemic episode, and while we also could not find a significant change in metabolic responses, the additional significant changes in metabolites during day 2 suggest an altered metabolic response to a subsequent hypoglycemic episode, which is not a result of an attenuation of the hormonal counterregulatory response.
Clinical Trial Registration Number: NCT01337362
Supported by: Steno Collaborative Grants research programme
Disclosure: R. She: None.
137
Recurrent low glucose exposure (RLG) induces intrinsic metabolic adaptations in pancreatic alphaTC1.9 cells
K.M. Partridge, N.G. Morgan, K.L.J. Ellacott, C. Beall;
RILD Building, Institute of Biomedical and Clinical Sciences, Exeter, UK.
Background and aims: Recurrent hypoglycaemia is a severe complication associated with insulin-treated diabetes. Over time, pancreatic alpha cells fail to release glucagon in response to recurrent bouts of hypoglycaemia for poorly defined reasons. Previous studies have focused on potential changes in the brain-pancreas axis as a mechanism to explain this diminished alpha cell hypoglycaemia counter-regulation. However, little is known about whether or how alpha cell metabolism/function is altered following recurrent hypoglycaemia. We therefore sought to determine the intrinsic alpha cell metabolic changes after recurrent bouts of low glucose (RLG) in a murine alpha cell line, alphaTC1.9.
Materials and methods: To mimic recurrent hypoglycaemia seen in diabetes, alphaTC1.9 cells were treated with between zero - four bouts of RLG (0.5 mmol/l) then allowed to recover overnight in euglycaemic-like levels of glucose (5.5 mmol/l). Cell glycolytic rate was measured using a Seahorse XFe96 flux analyser by calculating proton efflux rates derived from glycolysis (glycoPER), the extracellular acidification rate (ECAR) and oxygen consumption rates (OCR) - an indicator of mitochondrial metabolism. Acute glucose utilisation (ΔECAR) was measured after injection of between 0.1 - 11.7 mmol/l glucose. Data were analysed using unpaired two tailed t-test or Two-Way ANOVA with multiple comparisons test.
Results: RLG augmented basal glycolysis compared to acute low glucose (ALG) (pmol/min/μg) (ALG; 5.095 ± 0.539, RLG; 10.801 ± 0.687, p < 0.0001; n = 33 - 35). After mitochondrial inhibition, RLG increased compensatory glycolysis (pmol/min/μg) (ALG; 12.494 ± 0.701; RLG; 22.088 ± 1.038; p < 0.0001, n = 33 - 35). RLG enhanced baseline OCR (ALG; 32.543 ± 0.717, RLG; 39.248 ± 1.278, p < 0.0001, n = 33 - 35). After four prior bouts of low glucose, RLG enhanced acute glucose utilisation (mpH/min/μg) in a concentration-dependent manner (0.1 mmol/l; ALG 0.089 ± 0.025, RLG 0.159 ± 0.039; 0.5 mmol/l; ALG 0.566 ± 0.051, RLG 0.703 ± 0.065, 5.5 mmol/l; ALG 1.148 ± 0.077, RLG 1.383 ± 0.059, p < 0.05, 11.7 mmol/l; ALG 1.204 ± 0.104, RLG 1.476 ± 0.077, p < 0.05, n = 20 - 24).
Conclusion: We demonstrate for the first time that intrinsic metabolic adaptations in an alpha cell line are induced by prior bouts of antecedent low glucose. RLG increased alpha cell basal glycolytic flux and glucose utilisation, even at low/hypoglycaemic levels of glucose. RLG also enhanced basal oxidative metabolism suggesting that mitochondria are working harder at baseline. These data suggest that enhanced glucose utilisation may play a role in driving the alpha cell dysfunction associated with defective counterregulatory responses to hypoglycaemia.
Supported by: DUK PhD studentship
Disclosure: K.M. Partridge: None.
138
The impact of CGM with a predictive hypoglycaemia alert function on hypoglycaemia in physical activity for people with type 1 diabetes: PACE study
S. Rilstone1, N. Oliver1, B. Tanushi1, N. Hill2;
1Imperial College London, 2Imperial College Healthcare NHS Trust, London, UK.
Background and aims: The benefits of exercise for people Type 1 Diabetes (T1D) are significant, and include improvement in cardiovascular risk factors. Uptake of exercise is low in people with T1D, with fear of hypoglycaemia cited as the main barrier to exercise. Insulin and fuelling strategies are complex, and varying insulin sensitivity associated with different exercises regimens can make diabetes management challenging, resulting in hypoglycaemia. Technology has great potential to improve self-management of diabetes during exercise, offering real time glucose feedback with trend arrows, and alarms to alert the user of impending hypoglycaemia during exercise when close observation of glycaemia may be challenging. The aim of the study is to assess the impact of Dexcom G6 real-time CGM (RT-CGM) with a predictive hypoglycaemia alert function on the frequency, duration and severity of hypoglycaemia occurring before, during and after regular physical activity in people with type 1 diabetes.
Materials and methods: People with type 1 diabetes on multiple daily injections or insulin pump therapy who exercised regularly (150min/week or more) have been recruited to this observational, within subject crossover study. Participants undertook 10 days of blinded CGM, whilst living their normal lives including their usual activity which was recorded using a sports watch. At the end of the 10 days, the RT-CGM was unblinded so that participants were able to see their glucose data. Participants were randomised 1:1 to use the “urgent low soon” alert switched on or off. The urgent low soon alert is an alarm that sounds when the algorithm predicts that the wearer will be 3.1mmol/l or less in the next 20 minutes. Participants continued to undertake and record their usual exercise. After 40 days the participants crossed over, so the participants that had the alarm off now switched it on, and vice versa for a further 40 days. Participants recorded their carbohydrate and insulin doses throughout the study.
Results: 24 participants were randomised. The participants (8 men, 16 women) had a mean (SD) age of 35 (11) years, duration of diabetes of 14 (7) years, BMI 26 (4.7)kg/m2 and HbA1c of 58 (12) mmol/mol]. 1 person was excluded from the analysis due to incomplete data. The baseline median percentage time < 3.0mmol/l was 0.25 (0.0.5-0.89)%. At the end-point the percentage time <3.0mmol/l was significantly lower with urgent low soon alerts on (median treatment difference 0.12% (95% CI 0.04-0.2, p=0.0036), compared to the urgent low soon off period. Time spent in hypoglycaemia below 2.8mmol/L was significantly different in the 24 hours after activity favouring the urgent low soon (p=0.035).
Conclusion: A CGM device with a an urgent low soon alert reduces exposure to level 2 hypoglycaemia compared to real time continuous glucose monitoring with a threshold alarm. Additionally an urgent low soon alert reduces exposure to disabling hypoglycaemia below 2.8mmol/l in the 24 hours after exercise compared to a threshold alert. These data suggest people with T1D undertaking regular exercise benefit most from a predictive alert monitoring system.
Clinical Trial Registration Number: NCT04142944
Supported by: Dexcom
Disclosure: S. Rilstone: Grants; Dexcom.
OP 24 How to burn energy
139
The oxidative phenotype of abdominal and femoral adipose tissue in women with normal weight or obesity
I.G. Lempesis1,2, N. Hoebers2, Y. Essers2, J.W.E. Jocken2, L.J. Dubois3, E.E. Blaak2, K.N. Manolopoulos1,4, G.H. Goossens2;
1Institute of Metabolism and Systems Research (IMSR), College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK, 2Department of Human Biology, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, Netherlands, 3The M-Lab, Department of Precision Medicine, GROW School for Oncology and Reproduction, Maastricht University Medical Centre+, Maastricht, Netherlands, 4Centre for Endocrinology, Diabetes and Metabolism, Birmingham Health Partners, Birmingham, UK.
Background and aims: Body fat distribution is closely associated with the risk for developing obesity-related cardiometabolic diseases. Rodent and human studies suggest that mitochondrial dysfunction in adipose tissue (AT) contributes to obesity-related metabolic complications. However, whether mitochondrial function differs between AT depots remains unknown. Here, we aimed to explore whether the oxidative phenotype differs between upper and lower body subcutaneous AT in humans.
Materials and methods: We investigated the abdominal and femoral AT oxidative machinery in 21 postmenopausal women (age 50-65 yrs) with normal weight (PwNW; BMI 18-25 kg/m2) versus obesity (PwOB; BMI 30-40 kg/m2). In vivo fractional extraction (FE) and release (FR) of oxygen, carbon dioxide, and several metabolites across abdominal and femoral AT were determined using the arterio-venous balance technique. Oxidative phosphorylation (OXPHOS) protein levels were determined in abdominal and femoral AT biopsies and differentiated adipocytes derived from the same donors. Finally, we assessed ex vivo mitochondrial respiration in abdominal and femoral adipocytes (Seahorse XF96 analyzer).
Results: The FE of oxygen (22.6 ± 2.1 vs. 35.8 ± 3.1%, p=0.009) and the FR of carbon dioxide (4.1 ± 0.8 vs. 7.0% ± 1.2, p=0.076) across abdominal AT were lower compared to femoral AT. Adipocyte size (68.5 ± 1.9 vs. 68.4 ± 1.6 μm, p= 0.791) and blood flow (9.3 ± 2.1 vs. 5.8 ± 1.8 mL/min, p= 0.296) were not different between abdominal and femoral AT. OXPHOS protein expression was lower both in AT (complexes I and III) and in adipocytes (complexes III and V) in PwOB than PwNW but did not differ between depots. Basal respiration (p = 0.020), maximal respiration (p= 0.001) and spare respiratory capacity (p=0.001) were lower in abdominal compared to femoral adipocytes derived from both PwNW and PwOB.
Conclusion: These findings demonstrate for the first time that AT oxygen extraction and adipocyte oxygen consumption are lower in ABD than FEM AT in postmenopausal women with normal weight or obesity, already at the preclinical stage, independent of adipocyte size.
Supported by: EFSD/Lilly Research Programme, EFSD Anniversary Programme
Disclosure: I.G. Lempesis: None.
140
Pdgfrα-specific deletion of Alms1 in mice recapitulates the obesity and insulin resistance of global Alms1 KO and this cannot be explained by discoordinated ciliary dynamics
E.J. McKay1, I.H. Luijten1, D. McCormick1, P. Mill2, R.K. Semple1;
1Centre for Cardiovascular Science, University of Edinburgh, 2Institute for Genetics and Cancer, University of Edinburgh, Edinburgh, UK.
Background and aims: Alström Syndrome (AS) is a rare autosomal recessive disease featuring early onset, severely insulin resistant diabetes, fatty liver and heart failure among other features. These cardiometabolic complications occur in the face of only moderate obesity in many patients. AS is caused by biallelic loss-of-function mutations in the ALMS1 gene, encoding a large centrosomal protein. The precise derangement of centrosomal and/or primary ciliary function caused by loss of ALMS1 is unknown, as most studies have reported primary cilia to be morphologically normal. Abnormal cell cycle kinetics have been suggested to play a role in some facets of the syndrome. Several global knockout (KO) mouse models have been described to recapitulate key metabolic components of AS, but none have used tissue-specific KO to tease out contributions of different cell types to pathology. Hypotheses: 1) 1) The metabolic profile of AS closely resembles that of lipodystrophy. We thus hypothesised that loss of Alms1 function in mesenchymal stem cell populations, such as adipose precursor cells, would recapitulate the metabolic derangement in AS. 2) Alms1 loss leads to disco-ordination of ciliary and cell cycles, and it is such discoordination rather than abnormal ciliary structure, that compromises tissue homeostasis.
Materials and methods: 1) A novel global KO mouse was generated by crossing the EUCOMM Tm1c Alms1 line with global CAG-Cre mice. A Pdgfrα-Cre driver was used to abrogate Alms1 function only in mesenchymal progenitor cells and their descendants including preadipocytes and adipocytes. We undertook metabolic phenotyping of global and Pdgfrα+ Alms1-KO mice on a 45% high-fat diet. 2) 2) NIH-3T3 flp-in cells were edited using CRISPR-Cas9 to cause biallelic loss-of-function mutations of Alms1. The cycle and ciliary biosensor, Arl13bCerulean-Fucci2a, was stably expressed in Alms1-/- and WT control cells using the flp-in system. Live cell imaging of these cells was then performed, followed by analysis of cell cycle and ciliary coordination dynamics.
Results: 1) Consistent with previous models and the human disease, global Alms1 KO mice were hyperphagic, obese, insulin resistant, and had severe hepatosteatosis. KO of Alms1 only in MSCs and their descendants recapitulated key metabolic phenotypes of global KO animals including obesity and insulin resistance. Interestingly hyperphagia was also seen despite preservation of neuronal Alms1. 2) No significant difference in the time between deciliation and cytokinesis, or the time between cytokinesis and ciliation was observed.
Conclusion: 1. MSC-derived lineages are critical in driving the severe metabolic syndrome in AS 2. Hyperphagia in AS does not depend on neuronal Alms1 deficiency 3. Discoordinated ciliary and cell cycles do not appear to explain the critical role of MSCs in the metabolic syndrome of AS.
Supported by: BHF, Wellcome Trust
Disclosure: E.J. McKay: None.
141
A larger brown fat volume and lower mean radiodensity are related to a greater cardiometabolic risk, especially in young men
F. Acosta1,2, G. Sanchez-Delgado2,3, B. Martinez-Tellez2,4, F. Osuna-Prieto2,5, A. Mendez-Gutierrez6,7, C. M. Aguilera6,8, A. Gil6,8, J. Llamas Elvira9, J. Ruiz Ruiz2,8;
1Turku PET Centre, University of Turku, Turku, Finland, 2PROFITH research group, University of Granada, Granada, Spain, 3Pennington Biomedical Research Center, Baton Rouge, USA, 4Department of Medicine, Division of Endocrinology, Einthoven Lab for Experimental Vascular Medicine, Leiden University Medical Center, Leiden, Netherlands, 5Department of Analytical Chemistry, University of Granada, Granada, Spain, 6Department of Biochemistry and Molecular Biology II, Biomedical Research Centre, Granada, Spain, 7Biohealth Research Institute in Granada, Granada, Spain, 8Biohealth Research Institute, Granada, Spain, 9University Hospital Virgen de las Nieves, Granada, Spain.
Background and aims: Brown adipose tissue (BAT) is important in the maintenance of cardiometabolic health in rodents. Recent reports appear to suggest the same in humans, although if this is true remains elusive, partly because of the methodological bias that affected previous research. The present cross-sectional work reports the relationships of cold-induced BAT volume, activity (peak standardized uptake, SUVpeak) and mean radiodensity (an inverse proxy of the triacylglycerols content) with the cardiometabolic and inflammatory profile of 131 young adults, and how these relationships are influenced by sex and body weight.
Materials and methods: This was a cross-sectional study carried out in Granada (south of Spain) during October-December 2015 and 2016. Subjects underwent personalized cold exposure for 2 h to activate BAT, followed by static 18F-fluorodeoxyglucose positron emission tomography-computed tomography scanning to determine BAT variables. Information on cardiometabolic risk (CMR) and inflammatory markers was gathered, and a CMR score and fatty liver index (FLI) calculated. Anthropometry, body composition and lifestyle behaviors (potential confounders) were also measured.
Results: In men, BAT volume was positively related to homocysteine and liver damage markers concentrations (independently of BMI and seasonality) and the FLI (all P≤0.05). In men too, BAT mean radiodensity was negatively related to the glucose and insulin concentrations, alanine aminotransferase activity, insulin resistance, total cholesterol/HDL-C, LDL-C/HDL-C, the CMR score, and the FLI (all P≤0.02). In women it was only negatively related to the FLI (P<0.001). These associations were driven by the results for the overweight and obese subjects (see figure below). No relationship was seen between BAT and inflammatory markers (P>0.05).
Conclusion: These findings show that a larger BAT volume and a lower BAT mean radiodensity are related to a higher CMR, especially in young men, which may support that BAT acts as a compensatory organ in states of metabolic disruption.
Clinical Trial Registration Number: NCT02365129
Supported by: Potential support (not decided yet) - Turku Foundation, University of Turku
Disclosure: F. Acosta: None.
142
Brown adipose tissue content of triglycerides, but not SUV, is associated with cardiovascular risk markers in volunteers from a tropical region
M. Monfort-Pires1, G.R. Silva2, P. Dadson1, G.A. Nogueira3, M. U-Din1, K.A. Virtanen1, L.A. Velloso4;
1Turku PET Centre, Turku PET Centre, Turku University Hospital, Turku, Finland, 2Nutrition, School of Public Health, University of Sao Paulo, Sao Paulo, Brazil, 3Clinical Medicine, University of Campinas, Campinas, Brazil, 4Medicine, University of Campinas, Campinas, Brazil.
Background and aims: Since the discovery of active brown adipose tissue (BAT) in human adults, the tissue has been considered a potential target for the treatment of obesity, diabetes, and cardiovascular diseases. A detailed characterization of BAT structure and function could help understand its role in metabolism, but most studies evaluating BAT structure and function were performed in temperate climate regions, while 40% of the world’s population are living in tropical areas.
Materials and methods: We used 18F-fluorodeoxyglucose positron emission tomography - combined with magnetic resonance imaging to evaluate BAT activity (FDG standardized uptake value - SUV), BAT volume and BAT content of triglycerides (TG) in 30 lean and 15 subjects with overweight or obesity living in a tropical area in Southeast Brazil. Body composition was analyzed using DXA and magnetic resonance imaging and fasting blood samples were withdrawn for several determinations. The study was approved by the Local Ethics Committee, and it has been performed in accordance with the ethical standards laid down in the Helsinki Declaration.
Results: We observed that BAT activity (FDG SUV) and BAT volume are not correlated with leanness (p>0.05 for all); instead, BAT triglyceride content is correlated with waist circumference (correlation coefficient r=0.71; p<0.01) and markers of cardiovascular risk, such as Castelli Index I (correlation coefficient r=0.51; p<0.01) and II (correlation coefficient r=0.47; p<0.01). Also, it was inversely associated with HDL (correlation coefficient r= -0.48; p<0.05). To further investigate this association, we performed an analysis of variance (ANOVA) comparing BMI, waist circumference, total fat mass, visceral adipose tissue, HDL, Castelli I and Castelli II indexes against BAT activity, volume, and TG content. BAT mean SUV and volume did not differ across categories of BMI or waist circumference, or across tertiles of total fat mass and visceral adipose tissue, HDL, Castelli I and II indexes (p>0.05 for all). However, we detected a difference between low and high BMI (68.8 ± 6.1 versus 77.8 ± 5.1 % of TG) and waist circumference (68.7 ± 4.9 versus 77.6 ± 5.3 % of TG) and between the first and 3rd tertile of visceral fat mass (68.3 ± 4.2 versus 78.4 ± 4.3 % of TG) for the BAT TG content (p<0.05 for all). In addition, differences between tertiles were observed for HDL cholesterol, Castelli I and Castelli II indexes (p<0.05 for all).
Conclusion: This study expands knowledge regarding the structure and function of BAT in people living in tropical areas. In addition, we provide evidence that BAT triglyceride content could be a marker of cardiovascular risk.
Supported by: FAPESP: #2016/10616-7/#2017/22586-8/2019/02055-3
Disclosure: M. Monfort-Pires: None.
143
Humans with metabolically active brown fat demonstrate higher capacity to catabolise branched chain amino acids
M. U-Din1, V. de Mello Laaksonen2, K. Hanhineva2, J. Newman3, K. Kristiansen4, M. Klingenspor5, T. Fromme5, T. Niemi6, M. Taittonen6, T. Saari1, J. Raiko1, P. Nuutila1, K.A. Virtanen1;
1Turku PET Centre, Turku, Finland, 2University of Eastern Finland, Kuopio, Finland, 3University of California, Davis, USA, 4University of Copenhagen, Copehagen, Denmark, 5Technical University Munich, Freising, Germany, 6Turku University Hospital, Turku, Finland.
Background and aims: Branched chain amino acids (BCAA: valine, leucine, isoleucine) may synergize with circulatory lipids to induce insulin resistance. Metabolically active brown adipose tissue (BAT) may be a potent site for BCAA catabolism that participates in systemic clearance of BCAA. The study aimed to investigate the systemic and BAT-specific metabolism of BCAA in healthy human adults.
Materials and methods: Seventy-nine (25M/54F) humans with BMI 27 ± 6 kg/m2 and whole-body insulin sensitivity (M-value) of 39 ± 20 μmol/kg/min participated. M-value was determined with hyperinsulinemic-euglycemic clamp. Circulatory BCAA were determined with non-targeted metabolomics on serum samples. Tissue BCAA were determined with non-targeted metabolomics, and tissue transcriptomics were determined with RNA-sequencing on human supraclavicular BAT samples. BAT metabolism was determined with PET imaging with either [18F]-FDG or [18F]-FTHA radiotracers to measure glucose or NEFA uptake, respectively, under cold stimulation. The subjects manifesting BAT glucose uptake ≥ 3.0 μmol/100g/min or NEFA uptake ≥ 0.7 μmol/100g/min were classified as subjects possessing a high metabolically active BAT (High-BAT).
Results: Circulatory levels of BCAA were directly related to obesity markers and inversely related to M-value. The subjects with high BAT metabolism (High-BAT) demonstrated lower levels of circulatory BCAA compared to Low-BAT subjects (valine p = 0.009, leucine p = 0.056, isoleucine p = 0.09); the differences persisted whilst adjusting for whole-body fat percentage (ANCOVA, valine p = 0.01, leucine p = 0.06, isoleucine p = 0.095). In BAT, there was a trend of lower levels of valine and isoleucine in High-BAT compared to Low-BAT (p=0.07 and p=0.07, respectively) while there was no difference in the mRNA expression of the cellular transporters of BCAA uptake (SLC7A5, SLC38A7, SLC38A9, SLC6A15, SLC3A2) between High-BAT and Low-BAT. There was no relationship between the circulatory and BAT levels of BCAA (all p > 0.19) indicating that the differences in tissue levels of BCAA (High-BAT vs Low-BAT) are likely due to the differences in catabolic capacity. In line with this, the mRNA expression of the genes in BAT regulating BCAA catabolism BCKDHA, ACADSB, and HIBADH, was higher in High-BAT in comparison to Low-BAT (all p < 0.05). The expression of the gene encoding BCAA mitochondrial transport-mediator (SLC25A44), BCKD complex (BCKDHA, BCKDHB, DBT, DLT), and several others involved in the catabolism of BCAA (ACAD8, AUH, MCCC1, ACAT1, MCEE) was in direct relationship with the expression of the thermogenic gene, UCP1 (all p < 0.05).
Conclusion: These results suggest that humans with metabolically active BAT have a greater capacity for systemic clearance of BCAA in comparison to those with lower metabolically active BAT. In BAT, the genes encoding proteins responsible for the catabolism of BCAA are linked with thermogenic capacity indicating BCAA catabolism may participate to fuel BAT thermogenesis. An enhancement of BAT metabolism may prove to be a useful target for combating systemic BCAA induced metabolic disruptions in insulin resistance.
Supported by: AoF
Disclosure: M. U-Din: None.
144
Hepatocyte PPARα is required for the sensing of adipose-derived fatty acids and for full brown adipose tissue activation during lipolysis
A. Fougerat1, G. Schoiswohl2, A. Polizzi1, M. Régnier1, C. Wagner2, B. Tramunt3, S. Ellero-Simatos1, L. Payrastre1, C. Postic4, W. Wahli5, N. Loiseau1, A. Montagner3, D. Langin3, A. Lass2, H. Guillou1;
1Toxalim, INRAE, Toulouse, France, 2Institute of Molecular Biosciences, Graz, Austria, 3Institute of Metabolic and Cardiovascular Diseases, Toulouse, France, 4INSERM U1151/CNRS UMR 8253, Institut Necker-Enfants Malades (INEM), Paris, France, 5Center for Integrative Genomics, Lausanne, Switzerland.
Background and aims: Peroxisome proliferator-activated receptor α (PPARα) is a nuclear receptor and a member of the PPAR family, which also includes PPARβ/δ and PPARγ. In hepatocytes, PPARα acts as a lipid sensor that controls the expression of genes involved in whole-body energy homeostasis during fasting, including fatty acid oxidation and ketogenesis. During fasting, PPARα is also required for the expression of the hormone fibroblast growth factor 21 (FGF21), a hepatokine with systemic metabolic effects. In this work, we investigated the role of hepatocyte PPARα activity in the dialogue between the liver and adipose tissues.
Materials and methods: We used mouse models of selective deletion of PPARα in hepatocytes and of adipose triglyceride lipase (ATGL) in adipocytes as a model of defective lipolysis. First, we performed liver whole genome expression analysis in fasted mice upon cell-specific deletion of adipocyte ATGL or hepatocyte PPARα. Second, we tested the consequences of hepato-specific PPARα deficiency during pharmacological induction of adipocyte lipolysis with a β3-adrenergic receptor agonist.
Results: In the absence of ATGL in adipocytes, ketone body and FGF21 production is impaired in response to starvation. Liver transcriptome analysis reveals that adipocyte ATGL is critical for regulation of hepatic gene expression during fasting, with a strong induction of PPARα target gene expression. Adipose tissue lipolysis, induced by acute activation of the β3-adrenergic receptor, also triggers PPARα-dependent responses in the liver, including gene expression, FGF21 production and ketogenesis. Hepatic PPARβ/δ is dispensable for these β3-adrenergic responses. In addition, the absence of hepatocyte PPARα alters brown adipose tissue (BAT) morphology and reduces the expression of BAT markers upon stimulation of β3-adrenergic signaling while the deletion of FGF21 in hepatocytes does not affect BAT activation under this condition.
Conclusion: Altogether, our results support a dominant role for adipose ATGL in generating fatty acids that trigger hepatocyte PPARα activity and underscore the critical role of hepatic PPARα not only in the sensing of lipolysis-derived lipids but also in triggering BAT activation independent of hepatocyte FGF21 production. Intact PPARα activity in hepatocytes is required for cross-talk between adipose tissues and the liver during fat mobilization.
Supported by: ANR Hépadialogue/Région Occitanie/Agreenskills
Disclosure: A. Fougerat: None.
OP 25 Lipid in and out of the liver
145
Skeletal muscle derived myokine affects insulin sensitivity and lipogenesis in a human hepatocyte spheroid model
J.-B. Potier1,2, A. Dumond2, A. Fardellas3, M. Pinget2, M. Aouadi3, K. Bouzakri2,1;
1Ilonov, Strasbourg, France, 2Université de Strasbourg, Centre européen d'étude du diabète, Strasbourg, France, 3Karolinska Institutet, Center for Infectious Medicine, Huddinge, Sweden.
Background and aims: Inter-organ crosstalk has recently emerged as a crucial aspect of body homeostasis, especially in metabolic diseases such as type 2 diabetes (T2D) and NAFLD. Skeletal muscle is highly implied in this crosstalk, through the secretion of specifics cytokines called myokines. Among those, our laboratory has focused on the myokine X (MyoX). Due to our previous results on the prevention of diabetes and to the pathophysiological link between this disease and NAFLD, we chose to investigate the effect of the MyoX on the liver. Primary human hepatocytes (PHH) spheroids, the gold standard model for the in vitro study of liver diseases, were used for our experiments.
Materials and methods: PHH spheroids were cultured in lean and metabolic syndrome (MetS) conditions treated, or not, with different concentrations of MyoX: 0.1, 1 and 10 μg/ml. Spheroids were then stimulated with insulin and protein lysates were collected. Effects of MyoX on the expression of proteins related to glucose (Akt, ERK1/2, GLUT2) and lipid (SREBP1c, FAS, CPT1A) metabolism, two pathways implied in NAFLD development, were assayed by western blot. Furthermore, Nile Red staining was performed for the measurement of triglyceride accumulation.
Results: Concerning glucose metabolism, noticeable effects were observed for the phosphorylation of Akt, which was stronger at a dose of 10 μg/ml in lean condition, suggesting that the MyoX might enhance the intensity of the insulin downstream. Likewise, in the same condition, the slight increase in triglyceride accumulation, observed with the Nile Red staining, could be explained by an insulindependent de novo lipogenesis mechanism confirmed by the increase of SREBP1c phosphorylation (an insulin-dependent transcription factor responsible for the storage of fatty acid as triglycerides). Concerning MetS conditions, beneficial effects were observed at the lower dose of MyoX, with a decrease in triglyceride amount related to the reduction of SREBP1c phosphorylation, associated with no detrimental effect on p-Akt.
Conclusion: Taken together, our analysis suggest that high doses of MyoX might act as a sensitizer and exacerbates the effects of insulin in PHH in lean condition, enhancing the de novo lipogenesis. In MetS condition, potential beneficial effects were noticed for the lower dose of MyoX, suggesting that our protein might be of an interest for the prevention of NAFLD. Further studies should beperformed to determine the optimal dose of the myokine.
Disclosure: J. Potier: None.
146
The PNPLA3 I148M variant increases intrahepatic lipolysis and beta oxidation and decreases de novo lipogenesis and hepatic mitochondrial function in vivo in humans
P.K. Luukkonen1,2, K. Porthan2, N. Ahlholm2, F. Rosqvist3,4, S. Dufour1, X.-M. Zhang1, J. Dabek2, T.E. Lehtimäki5, W. Seppänen5, M. Orho-Melander6, L. Hodson4, K.F. Petersen1, G.I. Shulman1, H. Yki-Järvinen2;
1Yale University, New Haven, USA, 2Minerva Foundation Institute for Medical Research, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 3Uppsala University, Uppsala, Sweden, 4University of Oxford, Oxford, UK, 5University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 6Lund University, Malmö, Sweden.
Background and aims: The PNPLA3 I148M variant is the strongest genetic risk factor of non-alcoholic fatty liver disease (NAFLD) but the underlying mechanisms remain unknown. We studied the effect of this variant on hepatic metabolism in vivo under multiple physiological conditions by combining a recruit-by-genotype approach with state-of-the-art stable isotope techniques.
Materials and methods: We recruited 93 healthy participants (mean age 53±1 yrs, BMI 30±1 kg/m2, 19% men; 37 homozygous carriers [148MM] and 56 non-carriers [148II]). Hepatic de novo lipogenesis (DNL) was assessed after an overnight fast using D2O (148MM, n=19; 148II, n=36). Hepatic fate of exogenous fatty acids (FA) was determined using a mixed meal enriched in 13C-labeled FA (148MM, n=12; 148II, n=14). Endogenous glucose production, ketogenesis and hepatic mitochondrial citrate synthase flux (VCS) were assessed before and after a 6-day ketogenic diet by Positional Isotopomer NMR Tracer Analysis (PINTA) by infusing [3-13C]-lactate, [13C4]-β-hydroxybutyrate (β-OHB) and [2H7]-glucose (148MM, n=6; 148II, n=6). Intrahepatic triglycerides (IHTG) were assessed by NMR spectroscopy and hepatic mitochondrial redox state by plasma [β-OHB]/[acetoacetate].
Results: After an overnight fast, the 148MM group had higher plasma [β-OHB] (+104%, p<0.05) and lower DNL (-47%, p<0.01) than the 148II group, independent of plasma [NEFA] and [insulin]. After a mixed meal, exogenous 13C-labeled FAs in the 148MM group were channeled more to ketogenesis (+265%, p<0.001), which associated with an increased hepatic mitochondrial redox state (+36%, p<0.05). During a ketogenic diet, the 148MM group had greater intrahepatic lipolysis as reflected by a larger reduction in IHTG (77%, p<0.05) than the 148II group, which associated with: 1) increased plasma [β-OHB] (+90%, p<0.05), 2) increased mitochondrial redox state and 3) decreased VCS (-31%, p<0.01). Plasma [GDF-15], a mitochondrial stress marker, was increased by 40% (p<0.01) in the 148MM group compared to the 148II group.
Conclusion: The PNPLA3 I148M carriers have alterations in both intrahepatic anabolic/catabolic processes and mitochondrial function as reflected by increased intrahepatic lipolysis, decreased DNL and increased hepatic mitochondrial β-oxidation/ketogenesis. These changes associated with an increased mitochondrial redox state and reductions in hepatic mitochondrial VCS. These results provide new insights in the mechanisms by which the PNPLA3 I148M promotes NAFLD (Figure).
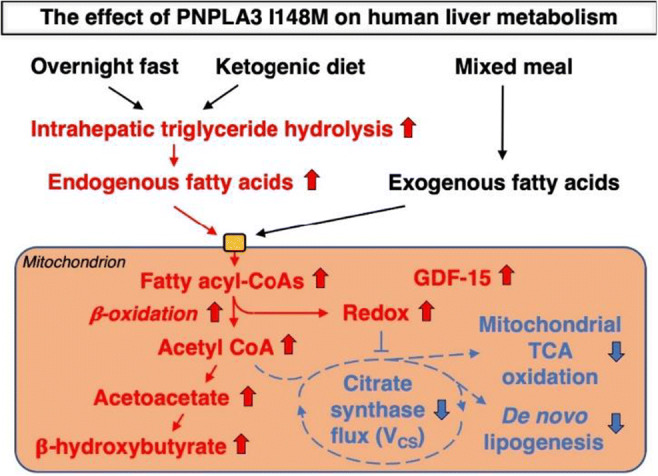
Clinical Trial Registration Number: NCT03737071
Supported by: Novo Nordisk Foundation, Sigrid Jusélius Foundation
Disclosure: P.K. Luukkonen: Grants; Novo Nordisk Foundation, Sigrid Jusélius Foundation.
147
Effect of tirzepatide on fasting lipids in patients with type 2 diabetes: meta-analysis of randomised controlled trials
T. Karagiannis1, I. Avgerinos1, A. Tsapas1,2, E. Bekiari1;
1Clinical Research and Evidence-Based Medicine Unit, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2Harris Manchester College, Oxford, UK.
Background and aims: Tirzepatide is a novel dual glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that has been shown to reduce HbA1c and body weight in patients with type 2 diabetes. We aimed to assess whether the salutary metabolic effects of tirzepatide extend to improvements in patients’ lipid profile.
Materials and methods: We searched Pubmed, Embase and the Cochrane Library up to January 2022 for randomised controlled trials (RCTs) of at least 12 weeks’ duration that compared tirzepatide 5, 10 or 15 mg once weekly with placebo or other glucose lowering drugs in adults with type 2 diabetes, and reported results on fasting lipids. Outcomes included change from baseline in the concentrations of triglycerides, total cholesterol (T-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C) and high-density lipoprotein cholesterol (HDL-C). We conducted random effects meta-analyses pooling mean differences (MDs) with 95% confidence intervals (CIs).
Results: We included data from six RCTs (n = 6527 participants). Comparator was placebo, GLP-1 RA and basal insulin in two, one and two trials respectively, while one trial had both a placebo and a GLP-1 RA control arm. Duration of intervention was 26, 40 and 52 weeks in one, three and two trials respectively. Compared to placebo, tirzepatide induced dose-dependent reductions in fasting triglycerides, T-C, LDL-C and VLDL-C (Table), and had no effect on HDL-C. Compared to GLP-1 receptor agonists (dulaglutide and semaglutide), tirzepatide reduced triglycerides (MDs ranging from -12.51 mg/dL with tirzepatide 5 mg to -24.98 mg/dL with tirzepatide 15 mg) and VLDL-C (MDs ranging from -1.89 mg/dL to -4.08 mg/dL). Compared to basal insulin, tirzepatide improved participants’ lipid profile by reducing triglycerides, T-C, LDL-C and VLDL-C, and by increasing HDL-C.
Conclusion: Based on pooled data from RCTs, treatment with tirzepatide dose-dependently decreased levels of triglycerides, T-C, LDL-C and VLDL-C versus placebo. As such, in addition to its glucose lowering effect, tirzepatide could be used to improve the atherogenic lipoprotein profile in patients with type 2 diabetes by complementing the effect of lipid lowering drugs.
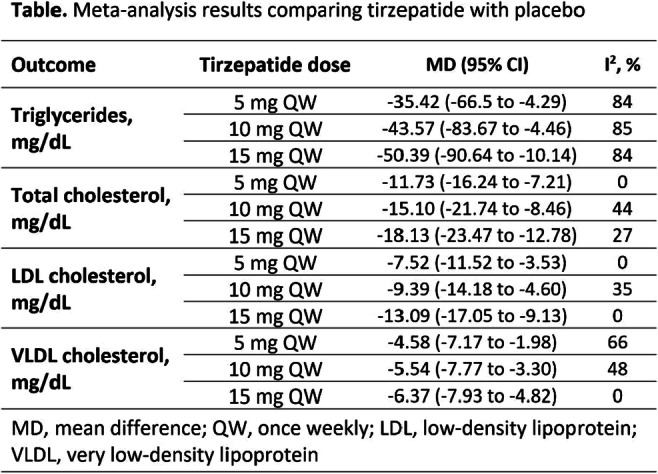
Disclosure: T. Karagiannis: None.
148
Lipidomic and metabolomics profiling in low birth weight men reveals a dysmetabolic phenotype associated with increased liver fat
L. Elingaard-Larsen1, C. Brøns1, S. Villumsen1, L. Justesen1, A. Thuesen2, M. Ali1, M. Kim1, C. Legido-Quigley1, G. van Hall3, E. Rubæk Danielsen3, T. Hansen2, A. Vaag1;
1Steno Diabetes Center Copenhagen, Herlev, 2University of Copenhagen, Copenhagen, 3Rigshospitalet, Copenhagen, Denmark.
Background and aims: Being born with a low birth weight (LBW) is associated with an increased risk of developing Type 2 Diabetes (T2D) in adulthood. The subcutaneous adipose tissue (SAT) plays a central role in T2D pathophysiology. We hypothesized, that LBW individuals have an impaired SAT expandability, resulting in ectopic lipid deposition in the liver, thereby contributing to the pathophysiological events linking LBW with T2D development. Using untargeted metabolomics and lipidomics analyses, as well as state-of-the-art magnetic resonance (MR) spectroscopy for liver fat determinations, we aimed to assess the extent to which increased liver fat is associated with dysmetabolic traits in early middle-aged, non-obese LBW men.
Materials and methods: Forty-eight healthy, non-obese males aged 35-39 years, born at term with a LBW (BW<10th percentile, n=26) and age- and BMI-matched NBW controls (BW: 50-90th percentile, n=22) were included in the study. We measured body composition, hepatic fat content using 1H MR spectroscopy and hepatic glucose production determined by isotopic tracers. Untargeted serum metabolomics and lipidomics were performed using mass-spectrometry.
Results: LBW subjects had a significantly increased hepatic fat content compared to NBW controls (P=0.014). Interestingly, 5 of the LBW subjects (20%) and none of the NBW subjects, fulfilled the diagnostic criteria of non-alcoholic fatty liver disease (NAFLD), displaying a median hepatic fat content of 9.45%. This subgroup showed several metabolic derangements compared with NBW and LBW men without NAFLD, including hepatic insulin resistance (P=0.02) and increased fasting levels of triglycerides (TG) (P=0.03). Untargeted serum metabolomics of 65 distinct metabolites, identified 7 metabolites which showed different levels between LBW subjects with NAFLD and NBW controls (P<0.05). This included ornithine and citrulline, suggesting an upregulation of the urea cycle in LBW subjects with NAFLD. Furthermore, a pathway analysis highlighted tRNA charging as the top canonical pathway, driven by differences in amino acid levels between NBW and LBW subjects, in which LBW subjects with NAFLD exhibited elevated levels of nearly all the amino acids. Lipidome profiling included 279 lipids and revealed increased levels of phosphatidylcholines and TGs (P<0.05) in the LBW subjects with NAFLD compared to both LBW without NAFLD and NBW subjects. Both lipid species had differential structural composition in the LBW subjects with NAFLD, including an increased amount of long chain fatty acids and fewer double bonds (0-7 bonds).
Conclusion: Increased liver fat content may play a key role linking LBW with increased risk of developing T2D. The most adverse metabolic phenotype was seen in the LBW subgroup with NAFLD, displaying hepatic insulin resistance and dyslipidemia. Metabolomics and lipidomics analysis emphasize this phenotype by revealing an altered amino acid profile as well as changes in the composition and structural organization of lipids known to be associated with overt T2D.
Clinical Trial Registration Number: NCT02982408
Supported by: NNF, EFSD Organ Crosstalk Program, Trygfonden, Augustinus Fonden and Aase and Ejnar Danielsens Fond, EFSD/Boehringer Ingelheim Research Programme
Disclosure: L. Elingaard-Larsen: None.
149
3-hydroxybutyrate infusion suppresses free fatty acid concentrations in type 1 diabetes patients and healthy men
M. Bangshaab1,2, M.V. Svart3,2, N. Rittig3,2, M.G.B. Pedersen1,2, N. Møller1,3;
1Medical Research Laboratory, Department of Clinical Medicine, Aarhus University, 2Steno Diabetes Center Aarhus, Aarhus University Hospital, 3Department of Internal Medicine and Endocrinology, Aarhus University Hospital, Aarhus, Denmark.
Background and aims: Diabetic ketoacidosis (DKA) remains the most important cause of premature death in younger persons with type 1 diabetes (T1DM). Recent data have revealed the existence of a feedback loop whereby the most prominent ketone body 3-hydroxybutyrate (3-OHB) inhibits adipose tissue release of free fatty acids (FFA), which are the major ketogenic precursors. The current study was designed to test whether this feedback mechanism is disrupted in men with T1DM, as a potential pathophysiological mechanism in DKA.
Materials and methods: We used a 2x2 crossover design to study 10 men with T1DM and 10 age-matched healthy men (CTR) randomly receiving 3-hours intravenous infusion of i) Na-D/L-3-OHB and ii) NaCl, separated by a 1-hour wash-out period. Participants were fasting during examinations. Two-way repeated measures ANOVA analyses were performed to evaluate the effect of time, group and time x group interactions.
Results: During the 3-OHB infusion blood D-3-OHB concentrations rose from 0.2±0.06 to 1.2±0.12 mmol/l in T1DM, a slightly higher concentration compared to CTR, 0.2±0.03 to 1.0±0.1 mmol/l (time, p<0.001 and time x group, p=0.031). 3-OHB infusion decreased circulating FFA concentrations from 0.68±0.12 to 0.15±0.03 mmol/l in T1DM and from 0.48±0.09 to 0.21±0.04 mmol/l in CTR (time, p <0.001 and time x group, p=0.06) while during NaCl infusion, FFA were slowly increasing (time, p=0.002) in both groups. Plasma glucose concentrations were 8.3±0.1 mmol/l in T1DM and 4.7±0.04 mmol/l in CTR (group, p<0.001). Peripheral plasma insulin concentrations were 59.2±2.8 pmol/l in T1DM and 16.3±1.1 pmol/l in CTR (group, p<0.001) and plasma glucagon concentrations were 5.5±0.1 pmol/l in T1DM and 7.8±0.3 pmol/l in CTR (group, p=0.01).
Conclusion: In conclusion our data show similar suppression of blood FFA concentrations by 3-OHB in T1DM compared with CTR, thus strongly suggesting that the inhibition of lipolysis (the negative feedback) by 3-OHB is intact in men with T1DM.
Clinical Trial Registration Number: NCT04656236
Supported by: Novo Nordisk Foundation
Disclosure: M. Bangshaab: None.
150
Inhibition of VEGF-B signalling prevents non-alcoholic fatty liver disease development and progression
A. Falkevall1, A. Mehlem1, E. Folestad1, M. Zeitelhofer1, P. Scotney2, U. Eriksson1;
1Dept of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden, 2CSL Innovations Ltd, Melbourne, Australia.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is a common co-morbidity of type 2 diabetes entailing a range of pathologies from hepatic steatosis to hepatocellular carcinoma. We aimed to explore the role of VEGF-B signaling in this disease complex.
Materials and methods: Mice with adipocyte specific over-expression of VEGF-B, or adipocyte-specific deletion of Vegfb were used to investigate the role of VEGF-B signaling in adipose tissue lipolysis and the development of NAFLD.
Results: We describe a novel function of Vascular Endothelial Growth Factor B (VEGF-B) and show by using mice with adipocyte specific over-expression of VEGF-B, or adipocyte-specific deletion of Vegfb that VEGF-B signaling in adipose tissue regulates lipolysis and directly control hepatic steatosis and NAFLD development in diabetic mice. Inhibiting VEGF-B signaling, using both pharmacological and genetic strategies, in mouse models of NAFLD lowers hepatic steatosis and reduces NAFLD associated-pathologies. Mechanistically we show that VEGF-B signaling in the adipose tissue ameliorates NAFLD by resolving adipose tissue insulin resistance and the activity of the hormon sensitive lipase, a rate-limiting enzyme in lipolysis.
Conclusion: We conclude that VEGF-B antagonism represents a novel approach to combat NAFLD by targeting steatosis through suppressing adipose tissue lipolysis.
Supported by: VR Distinguished Professor Award, EFSD/Boehringer Ingelheim Research Programme
Disclosure: A. Falkevall: Employment/Consultancy; CSL Innovations Ltd. Melbourne.
OP 26 The dark side of diabetes
151
Risk of incident diabetes after hysterectomy: results from the E3N cohort study
F. Bonnet1,2, P. Vaduva1, N. Laouali3,2, G. Fagherazzi4,2, M. Kvaskoff2;
1CHU de Rennes, Rennes, France, 2Centre for Research in Epidemiology and Population Health, Inserm U1018, Villejuif, France, 3Department of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts at Amherst, Amherst, USA, 4Deep Digital Phenotyping Research Unit. Department of Population Health, Luxembourg, Luxembourg.
Background and aims: Hysterectomy has been associated with the risk of hypertension and cardiovascular disease but few studies have examined the relation between hysterectomy and the risk of type 2 diabetes. These studies mostly included post-menopausal women. Furthermore, the potential influence of diet and physical activity has not been addressed in previous reports. The aim of our study was to investigate whether hysterectomy was associated with an increased risk of incident diabetes in a large French cohort of women. In addition, we aimed to examine whether an unhealthy lifestyle influenced the risk of diabetes among women who had a hysterectomy.
Materials and methods: We studied 81 144 women from the E3N cohort who were free of diabetes at baseline; they were followed-up for a mean of 16.4 years. Assessment of diet and physical activity was by questionnaire. Women with gynecological cancers were excluded from the analyses. Cox proportional hazard models with age as the timescale were used to estimate Hazard Ratios (HR) and 95% confidence intervals (CI). Covariates included educational level, physical activity, type of diet, body mass index, smoking status, family history of diabetes, age at menarche, menopausal status, age at menopause, use of oral contraceptives.
Results: A total of 4 367 women developed type 2 diabetes during the follow-up. Women with a history of hysterectomy had an increased risk of incident diabetes, which persisted after adjustment for the main confounding factors (adjusted HR= 1.18, 95% CI 1.10-1.27, p<0.0001). The association was not altered after further adjustment for reproductive factors or hormonal treatments. The type of diet and the level of physical activity did not modify the association with incident diabetes.An increased risk of diabetes was observed irrespective of the cause of hysterectomy; for women who had a hysterectomy for endometriosis or fibroids (adjusted HR= 1.19, 95% CI 1.11-1.28). There was no interaction between the presence of overweight at baseline (BMI≥25 kg/m2) and an increased risk of diabetes. Hysterectomy with (HR: 1.23, 95% CI 1.13-1.35, p<0.001) and without oophorectomy (HR: 1.13, 95% CI 1.03-1.25, p=0.013) were both associated with an increased risk of diabetes. The risk of incident diabetes was greater for women who had a hysterectomy before the age of 50: before 40 years (adjusted HR: 1.27, 95% CI 1.07-1.50, p=0.006); between the age of 40 and 50 years (adjusted HR: 1.27, 95% CI 1.16-1.40, p<0.0001); after age 50 (adjusted HR: 1.06, 95% CI 0.95-1.18, p=0.28) as compared to those without a hysterectomy in these age groups.
Conclusion: Our findings show that women who had a hysterectomy before the age of 50 had an increased risk of developing incident diabetes. This elevated risk appears to be independent of oophorectomy and is not explained by an unhealthy diet or physical inactivity. The underlying mechanisms leading to diabetes among these women remain to be elucidated.
Disclosure: F. Bonnet: None.
152
Association between endometriosis and risk of type 2 diabetes: results of the E3N prospective cohort study
P. Vaduva1, N. Laouali2,3, G. Fagherazzi4, F. Bonnet1,2, M. Kvaskoff2;
1Department of Endocrinology, Diabetes and Nutrition, Rennes University Hospital, Rennes, France, 2Exposome and Heredity Team, Centre for Research in Epidemiology and Population Health, Inserm U1018, Villejuif, France, 3Department of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts at Amherst, Amherst, USA, 4Deep Digital Phenotyping Research Unit. Department of Population Health, Luxembourg Institute of Health, Luxembourg, Luxembourg.
Background and aims: Endometriosis is a chronic inflammatory disease estimated to occur in 10% of women of reproductive age, with an unknown etiology. It is associated with signs and symptoms such as chronic pelvic pain, chronic fatigue and infertility. Several studies suggest an association between this disease and the risk of chronic cardio-metabolic conditions such as hypertension, hypercholesterolaemia, atherosclerosis, and myocardial infarction, but its association with the risk of type 2 diabetes (T2D) is poorly documented. The aim of this study is to analyze the relationship between endometriosis and occurrence of T2D.
Materials and methods: E3N (Etude Epidemiologique après de femmes de l’Education Nationale) is a prospective cohort involving French women born in 1925-1950 followed-up since 1990. Were considered here both prevalent (diagnosed before the initial questionnaire in 1992, retrospectively reported) and incident cases (diagnosed after the initial questionnaire, prospectively reported) of endometriosis. Data on laparoscopically confirmed endometriosis were collected every 2-3 years through self-report. T2D cases were identified using diabetes-specific questionnaires and drug reimbursement insurance databases. Statistical analysis used Cox models, and stratified analyses examined the influence of age, body mass index (BMI) (<25 or ≥25kg/m²), infertility, and menopausal status on the associations between endometriosis and T2D risk.
Results: Among the 83,582 women, free of T2D at baseline, followed up for nearly 17 years, 4606 reported a diagnosis of endometriosis. Age at inclusion was 51 ± 6 years. Compared with prevalent cases, incident cases were younger at inclusion and more likely to be parous and to have ever used hormonal treatments, while they had similar height, BMI, age at menarche, and menstrual cycle length. Women with endometriosis were more likely to have a family history of diabetes (p<0.0001), personal history of elevated cholesterol (p=0.0007) or hypertension (p=0.001), younger age of menarche (p=0.0002), more frequent bilateral oophorectomy (p<0.0001), more frequent oral contraceptives use (p<0.0001) and a higher education level (p=0.017). In a model adjusted for age, BMI, physical activity, smoking, education, age at menarche, and oral contraceptive use, endometriosis was not associated with the risk of T2D (HR=1.09; 95% CI=0.92-1.29). This relationship was similar after further adjustment for family history of diabetes, hypertension, and menopausal status (HR=0.99; 95% CI=0.83-1.19). The relationship between endometriosis and T2D risk did not differ by age at inclusion, BMI, use of infertility treatments, or menopausal status.
Conclusion: Laparoscopically confirmed endometriosis was not associated with risk of type 2 diabetes in this large French cohort. These results are consistent with a recent prospective American study, suggesting that endometriosis is neither a marker nor a risk factor for type 2 diabetes.
Disclosure: P. Vaduva: None.
153
Cardiovascular and metabolic morbidity in women with previous gestational diabetes: a nationwide register-based cohort study
M.H. Christensen1,2, K.H. Rubin3,4, T.G. Petersen4, E.A. Nohr2,3, C.A. Vinter1,2, M.S. Andersen3,5, D.M. Jensen1,2;
1Steno Diabetes Center Odense, Odense University Hospital, 2Department of Gynecology and Obstetrics, Odense University Hospital, 3Department of Clinical Research, University of Southern Denmark, 4OPEN - Odense Patient data Explorative Network, Odense University Hospital, 5Department of Endocrinology, Odense University Hospital, Odense, Denmark.
Background and aims: Gestational diabetes mellitus (GDM) is associated with increased risk of cardiovascular disease (CVD). To our knowledge, the metabolic component of dyslipidemia has not been addressed as an outcome after GDM before, and nor has it been investigated whether the severity of morbidity is associated with previous GDM. Also, insulin treatment during GDM pregnancy and development of manifest diabetes mellitus (as proxies for impaired β-cell function) may further increase the risk of cardiovascular and metabolic morbidity (CVMM). Aims of this study were to investigate incidence and severity of CVMM in women with previous GDM in a Danish population and to study whether proxies of impaired β-cell function influence the incident CVMM risk.
Materials and methods: This study is a nationwide register-based cohort study on the complete cohort of 700,648 women delivering in Denmark during 1997-2018. Exposure was GDM based on ICD-10 diagnosis code. Primary outcome was an overall CVMM outcome consisting of ICD-10 diagnosis code for ischemic heart disease, heart failure, stroke/transient cerebral ischemia (TCI), hypertension, dyslipidemia, venous thrombosis, cardiac arrhythmia, and/or redemption of prescribed medication within antihypertensive, antithrombotic and/or lipid modifying agents. Secondary outcomes were: major CVD (ischemic heart disease, heart failure, stroke/TCI), hypertension (diagnosis code and/or medication), dyslipidemia (diagnosis code and/or medication), and venous thrombosis. Insulin treatment during GDM pregnancy was defined as diagnosis code of insulin treated GDM and/or redemption of insulin during GDM pregnancy. The associations were studied using multiple cox regression models with GDM entering as a time-varying exposure. Severity of morbidity was investigated as number of CVMM related hospital contacts and of redemptions of prescribed CVMM related medication in women with incident CVMM.
Results: The median follow-up period was 10.2-11.9 years depending on specific outcome (total range 0-21.9 years). GDM was associated with significantly increased risk of any CVMM (adjusted hazard ratio (aHR) 2.13 [95% CI 2.07-2.20]), major CVD (aHR 1.69 [95% CI 1.55-1.84]), hypertension (aHR 1.89 [95% CI 1.82-1.96], dyslipidemia (aHR 4.48 [95% CI 4.28-4.69]), and venous thrombosis (aHR 1.32 [95% CI 1.16-1.50]). Insulin treatment during pregnancy and development of manifest diabetes exacerbated risk estimates. Previous GDM was associated with significantly more hospital contacts with CVMM diagnosis codes and more redeemed prescriptions of CVMM related medication in women with incident CVMM within 3 years after initial hospital contact/redemption (p<0.001).
Conclusion: Previous GDM was associated with significantly higher risk of cardiovascular and metabolic morbidity, especially for incident dyslipidemia where a 4.5-fold increased risk was found. The risk was exacerbated by proxies of β-cell impairment. Severity of morbidity was significantly worse, if GDM preceded CVMM.
Supported by: Research grant from the Danish Diabetes Academy which is funded by the Novo Nordisk Foundation
Disclosure: M.H. Christensen: Grants; Danish Diabetes Academy, Region of Southern Denmark, University of Southern Denmark.
154
Association of type 2 diabetes, according to the number of risk factors within target range, with incident major depressive disorder and incident depressive symptoms
A.C.E. van Gennip1, T.T. van Sloten1, A.A. Kroon1, S. Köhler2, A. Koster3, S.J.P. Eussen4, B.E. de Galan1,5, M.T. Schram6, C.D.A. Stehouwer1;
1Internal Medicine, MUMC+, CARIM, Maastricht, 2Psychiatry and Neuropsychology, MUMC+, MHENS, Maastricht, 3CAPHRI, Social Medicine, Maastricht, 4Epidemiology, MUMC+, CARIM, Maastricht, 5Internal Medicine, Radboud UMC, Nijmegen, 6Internal Medicine, Heart and Vascular Centre, MUMC+, CARIM, MHENS, Maastricht, Netherlands.
Background and aims: Type 2 diabetes is associated with increased risks of major depressive disorder (MDD) and depressive symptoms. The extent to which risk factor modification can mitigate these excess risks is unclear. We investigated the associations between incident MDD and clinically relevant depressive symptoms among individuals with type 2 diabetes, according to the number of risk factors on target, compared to controls without diabetes.
Materials and methods: Prospective data were from UK Biobank of 77,786 individuals (n=9,047 type 2 diabetes; n=68,739 controls; baseline 2006-2010; follow-up for MDD until February, 2022). Incident depressive symptoms, defined as a Patient Health Questionnaire (PHQ-9) score of ≥10, were determined on average 7 years after baseline. Analysis was replicated using data from the Netherlands (the Maastricht Study; cohort with oversampling of type 2 diabetes; examination 2010-2017, incident depressive symptoms (PHQ-9 ≥10) assessed annually until 2017). Individuals with type 2 diabetes were categorized according to the number of seven selected risk factors within target range (nonsmoking; guideline-recommended levels of HbA1c, blood pressure, BMI, albuminuria, physical activity and diet).
Results: In UK Biobank, after a mean follow-up of 12 years, 493 (5.5%) individuals with type 2 diabetes and 2,574 (3.7%) controls had incident MDD. Compared to controls, individuals with type 2 diabetes had a higher risk of MDD (HR 1.61 (95% CI, 1.46; 1.77)). Among individuals with type 2 diabetes, excess risk of MDD decreased stepwise for a higher number of risk factors on target (Figure). Similarly, risk of depressive symptoms decreased stepwise for a higher number of risk factors on target (Figure). Individuals with type 2 diabetes who had 5-7 risk factors on target had no excess risk of depressive symptoms (OR for depressive symptoms 1.12 (0.76; 1.66)). These results were replicated in the Maastricht Study. In the Maastricht Study, compared to controls, among individuals with type 2 diabetes, the HR for depressive symptoms was 3.53 (2.55; 4.87) for individuals who had 0-2 risk factors on target, 2.19 (1.61; 2.98) for individuals who had 3 risk factors on target, 1.71 (1.25; 2.35) for individuals who had 4 risk factors on target and 1.03 (0.63; 1.68) for individuals who had 5-7 risk factors on target, respectively.
Conclusion: Among individuals with type 2 diabetes, excess risk of MDD and depressive symptoms compared to controls without diabetes decreased stepwise for a higher number of risk factors on target.
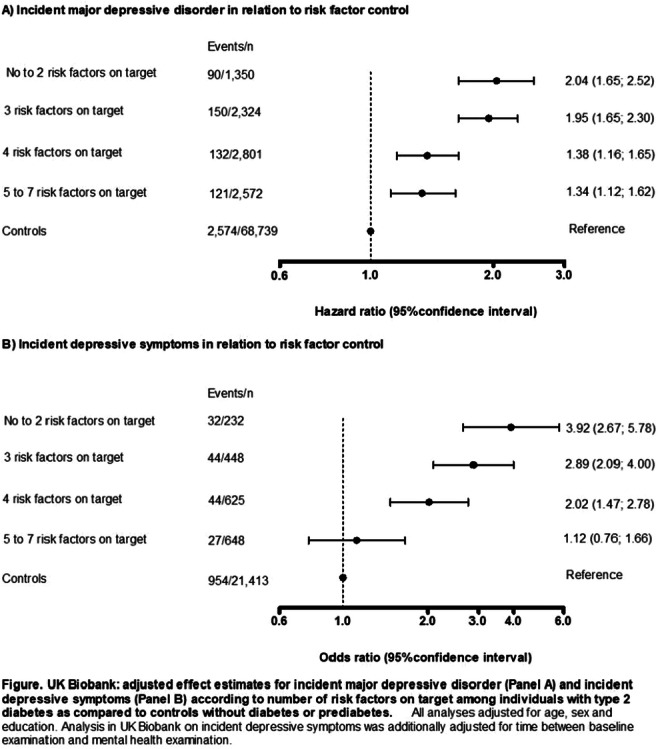
Supported by: ERDF, PoL, SDW, PSID, CVC, CARIM, CAPHRI, NUTRIM, SA, HFL, JCBV, NNF, SAN, ZonMW, NCDC, MP, DHF
Disclosure: A.C.E. van Gennip: None.
155
Pre(diabetes) and a higher level of glycaemia are continuously associated with corneal neurodegeneration: The Maastricht Study
S.B.A. Mokhtar1,2, F.C.T. van der Heide1,2, K.A.M. Oyaert1, C.J.H. van der Kallen1,2, T.T.M. Berendschot3,4, F. Scarpa5, A. Colonna5, B.E. de Galan1,2, M.M.J. van Greevenbroek1,2, C.G. Schalkwijk1,2, R.M.A. Nuijts3, M.T. Schram1,2, C.A.B. Webers3,4, C.D.A. Stehouwer1,2;
1Department of Internal Medicine, Maastricht University Medical Center+ (MUMC+), Maastricht, Netherlands, 2CARIM School for Cardiovascular Diseases, Maastricht, Netherlands, 3University Eye Clinic Maastricht, Maastricht University Medical Center+ (MUMC+), Maastricht, Netherlands, 4MHeNS School of Mental Health and Neuroscience, Maastricht, Netherlands, 5Information Engineering, University of Padova, Padova, Italy.
Background and aims: Diabetic neuropathy is a hallmark complication of type 2 diabetes. In the cornea, early morphological changes of small nerve fibers are demonstrable using corneal confocal microscopy. We aim to assess the association of glucose metabolism status and different continuous measures of glycemia with corneal nerve fiber measures.
Materials and methods: Population-based cross-sectional data from The Maastricht Study of N= 3,471 participants (mean age 59.4 years, 48.4% were men, 14.7% with prediabetes, and 21% with type 2 diabetes). We studied the associations, after adjustment for demographic, cardiovascular risk, and lifestyle factors, between glucose metabolism status (prediabetes and type 2 diabetes vs normal glucose metabolism status) and measures of glycemia (fasting plasma glucose, 2-hour post-load glucose, HbA1c, skin autofluorescence, and duration of diabetes) with a Z-score of corneal nerve fiber measures or with individual corneal nerve fiber measures (corneal nerve branch density, fiber density, fiber length, and fractal dimension). We used linear regression analyses and, for glucose metabolism status, P for trend analyses.
Results: After full adjustment, a more adverse glucose metabolism status was associated with a lower Z-score of corneal nerve fiber measures (beta [95%CI], prediabetes vs normal glucose metabolism status -0.08 [-0.17; 0.03]; type 2 diabetes vs normal glucose metabolism status -0.14 [-0.25; -0.04], P for trend = 0.001). Analogously, higher levels of fasting plasma glucose (per SD, -0.09 [-0.13; -0.05]), 2-hour post-load glucose (-0.07 [-0.11; -0.03]), HbA1c (-0.08 [-0.11; -0.04]), skin autofluorescence (-0.05 [-0.08; -0.01]), and duration of diabetes (-0.09 [-0.17; -0.00]) were all significantly associated with a lower Z-score of corneal nerve fiber measures. In general, directionally similar associations were observed for individual corneal nerve fiber measures.
Conclusion: This is the first population-based study to show that a more adverse glucose metabolism status and higher levels of glycemia are linearly and independently associated with corneal neurodegeneration; These data suggest that glycemia-associated corneal neurodegeneration is a continuous process that already starts before the onset of type 2 diabetes. Further research is needed to investigate whether early reduction of hyperglycemia can prevent corneal neurodegeneration.
Supported by: ERDF, PoL, DMoEA, SDW, PSID, CVC, CARIM, MHeNS, CAPHRI, NUTRIM, SA, HFL, PERIMED,UGfJ-C, NNF, SAN
Disclosure: S.B.A. Mokhtar: None.
156
Multimorbidity in type 1 diabetes is common and associated with increased mortality
A. Ylinen1,2, S. Hägg-Holmberg1,3, S. Mutter1,3, S. Satuli-Autere1,2, V. Harjutsalo1,3, C. Forsblom1,4, P.-H. Groop1,4, L.M. Thorn1,2, The FinnDiane Study Group;
1Folkhälsan Institute of Genetics, Folkhälsan Research Center, 2Department of General Practice and Primary Health Care, University of Helsinki, 3Research Program for Clinical and Molecular Metabolism, Faculty of Medicine, University of Helsinki, 4Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Multimorbidity is commonly defined as the coexistence of two or more chronic conditions or diseases, and it is associated with increased mortality and decreased quality of life. Type 1 diabetes is an example of a disease accompanied by other chronic conditions at a relatively young age. The number of chronic conditions correlates with the probability of depression and severe hypoglycemic episodes, but otherwise, data on multimorbidity in type 1 diabetes are scarce. Our aim was, therefore, to study the prevalence of multimorbidity and its impact on mortality in type 1 diabetes.
Materials and methods: This study included 4,069 adults (51.4% men) with type 1 diabetes from the Finnish Diabetic Nephropathy Study, mean age at baseline 38.1±12.0 and duration of diabetes 22.2±12.4 years. The accumulation of diseases was defined based on the number of chronic conditions at baseline from a list of 32 conditions. Conditions were grouped into three subcategories: vascular comorbidities, autoimmune disorders, and other conditions. Data on conditions were collected from clinical records, questionnaires, and registers. Data on mortality were retrieved until the end of 2017. Hazard ratios for all-cause mortality were calculated in sex and age adjusted Cox regression models.
Results: The prevalence of multimorbidity was 60.4% (n=2,458) at baseline. We did not observe a difference between women and men, but an evident association with age: 31.1% in those below 30 years, 59.8% in the 30-40 years age group, 74.8% in the 40-50 group, 84.3% in the 50-60 group, and 93.2% in those above 60 years (p<0.001). We observed vascular comorbidities in 49.2% (n=2,000), autoimmune disorders in 12.7% (n=515), and other conditions in 19.8% (n=807). During a median follow-up of 16.7 (IQR 13.6-18.8) years, 784 (19.3%) participants died. Multimorbidity clearly increased mortality (HR 6.0 [95% CI 4.6-7.9], p<0.001). The figure (showing baseline prevalence of different disease combinations and their mortality HR [95% CI], compared to those with diabetes alone) highlights the increased mortality risk in those with vascular comorbidities from a HR of 5.9 (4.4-7.9) when only vascular comorbidities are present to 11.0 (7.2-16.8) when all three are present.
Conclusion: The prevalence of multimorbidity in individuals with type 1 diabetes is high and increases with age. The increased mortality related to multimorbidity is largely driven by vascular comorbidities, and the risk is further attenuated by the presence of other chronic conditions, but not by autoimmune disorders.
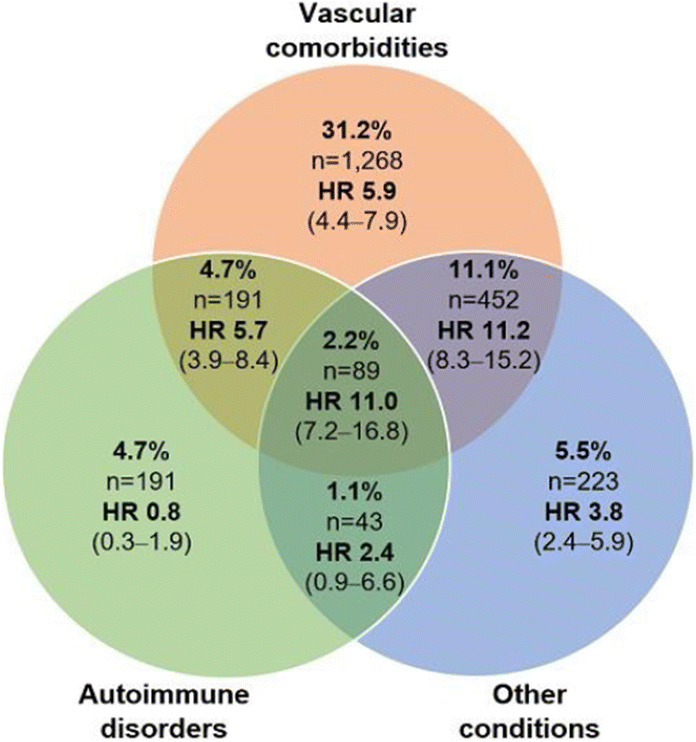
Supported by: Folkhälsan Research Foundation, EVO governmental grant, Academy of Finland, Stockmann foundation
Disclosure: A. Ylinen: Grants; Biomedicum Helsinki Foundation, Stiftelsen Dorothea Olivia, Karl Walter och Jarl Walter Perkléns Minne, Finska läkaresällskapet.
OP 27 Improving your insulin sensitivity: lessons from human studies
157
Circulating microRNA signatures in prepubertal children with obesity and insulin resistance
D. Santos1,2, P. Porter-Gill3, S. Bennuri3, G. Good3, L. Delhaey3, A.E. Sørensen4, R. Shannon3,5, L.T. Dalgaard4, B. Elisabet3,5, E. Carvalho1,6;
1Obesity, Diabetes and Complications, CNC - Center for Neurosciences and Cell Biology, University fo Coimbra, Coimbra, Portugal, 2PhD program in Experimental Biology and Biomedicine, Institute for Interdisciplinary Research, Coimbra, Portugal, 3Arkansas Children’s Research Institute, Little Rock, USA, 4Department of Science and Environment, Roskilde University, Roskilde, Denmark, 5Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, USA, 6Department of Geriatrics, University of Arkansas for Medical Sciences, Little Rock, USA.
Background and aims: The higher rates of diabetes in childhood reflect unhealthy life-style habits, observed by the high prevalence of obesity and related complications. Circulating microRNAs (miRs) have been suggested as biomarkers and predictors of insulin resistance. We aimed to identify an insulin resistance miR profile, in prepubertal children before development of obesity-induced glucose intolerance.
Materials and methods: Sixty-three prepubertal children (5-9 years of age) were included in a cross-sectional study. Peripheral blood was collected, and plasma miRs were evaluated using TaqMan Advanced miRNA Human Serum/Plasma plates and then were validated by RT-qPCR. Subjects were first divided into normal weight (n=20, NW) and overweight or obese (n=43, OW/OB) according to the BMI z-score. The OW/OB were subdivided into insulin sensitive or metabolically healthy obese (n=26, MHO) and insulin resistant or metabolically unhealthy obese (n=17, MUO), based on HOMA-IR≥1.95.
Results: Fasting plasma glucose levels were normal across the groups [NW: 4.92 ± 0.35 vs OW/OB: 5.07 ± 0.65 vs MHO: 4.98 ± 0.52 vs MUO: 5.23 ± 0.81, p>0.05]. Insulin levels were significantly decreased in the NW when compared with OW/OB [NW: 3.49 (2.38 - 4.71) vs OW/OB: 7.27 (4.91 - 11.10), p ≤ 0.001] and with MHO groups [NW: 3.65 ± 1.55 vs MHO: 5.43 ± 1.77, p ≤ 0.001]. Insulin levels were also different among the OW/OB group when stratified by HOMA-IR≥1.95 [MHO: 5.43 ± 1.77 vs MUO:18.16 ± 10.38, p ≤ 0.001]. Higher levels of miR-146a-5p and miR-18a-5p were observed in the OW/OB group [NW:0.03 (0.01- 0.13) vs OW/OB: 0.04 - 0.30, p = 0.003 and NW: 0.04 (0.04 - 0.07) vs OW/OB: 0.08 (0.04 - 0.18), p = 0.017]. Both miR-18a-5p and miR-146-a were positively correlated with BMI z-score (r = 0.32, p = 0.010 and r = 0.30, p = 0.018). miR-146a-5p was also correlated with the HOMA-IR (r = 0.26, p = 0.038). However, only miR-18a-5p was correlated with BMI z-score independently of the degree of insulin sensitivity [B = 0.42 (0.19), p = 0.032], while miR-146-5p [B = 0.16 (0.14), p = 0.28], miR-423-3p [B = -0.01 (0.11), p = 0.94] and miR-152-3p [B = -0.14 (0.12), p = 0.26] were associated with insulin resistance. Twenty genes, regulators of key metabolic pathways in insulin resistance development, with a positive enrichment by more than 3-fold, p ≤ 0.001, including Metal Regulatory Transcription Factor 1 (MTF1), RAR Related Orphan Receptor A (RORA) and Nuclear Receptor Coactivator 1 (NCOA1), were identified as predicted targets from at least two or more of the identified miRs.
Conclusion: We identified four miRs associated with obesity and insulin resistance, providing evidence of key alterations that occur early in prepubertal obesity. These miRs could potentially be used as biomarkers of insulin resistance.
Clinical Trial Registration Number: NCT03323294
Supported by: FCT, NIH/NIGMS, USDA/ARS, DDA
Disclosure: D. Santos: None.
158
Insulin resistance and visceral or ectopic fat deposition may independently determine metabolic and cardiovascular complications in type 1 diabetes
J.R. Snaith1,2, D.J. Holmes-Walker3,4, J.R. Greenfield1,2;
1Diabetes and Metabolism, Garvan Institute of Medical Research, Darlinghurst, 2Faculty of Medicine and Health, St Vincent's Health Care Campus, Sydney, 3Diabetes and Endocrinology, Westmead Hospital, Westmead, 4Western Clinical School, University of Sydney, Sydney, Australia.
Background and aims: Insulin resistance is an under-recognised cardiovascular risk factor in type 1 diabetes (T1D). The mechanisms linking insulin resistance to increased cardiovascular risk in T1D remain unknown. Our aim was to determine if insulin resistance is associated with adverse body composition in T1D. We hypothesised that adults with type 1 diabetes would display insulin resistance, increased visceral adipose tissue (VAT) and hepatic steatosis.
Materials and methods: Forty adults with T1D (age 37.4±8.8 years, HbA1c 7.5±0.9%, diabetes duration 22.9±8.8 years, BMI 26.4±0.9 kg/m2) and 20 age- gender- and BMI-matched non-diabetic controls (age 37.0±8.4 years, HbA1c 5.1±0.3%, BMI 26.2±4.3 kg/m2) participated in the cross-sectional component of INTIMET (Insulin Resistance in Type 1 Diabetes Managed with Metformin), a randomised double-blind placebo-controlled study. Insulin sensitivity was determined by the hyperinsulinaemic-euglycaemic clamp (60 mUm2 per min, 5.5 mmol/L). Body composition was assessed by dual-energy x-ray absorptiometry (DXA) and abdominal and anterior thigh magnetic resonance imaging (MRI). Hepatic steatosis was estimated by transient elastography and arterial stiffness by radial artery applanation tonometry. Data was expressed as mean ± standard deviation. Skewed data was log-transformed prior to analysis. T-tests and Mann-whitney U tests were used to detect between group differences. Pearson correlation was used to determine linear correlations.
Results: Participants with T1D had 29.5% lower insulin sensitivity than adults without diabetes (glucose infusion rate [GIR] 62±20 vs 88±18 μmol/min·kgFFM respectively; p=0.0004). Despite the difference in insulin sensitivity, there was no difference in DXA VAT (593±530 vs 665±590g T1D vs controls; p=0.6) or degree of hepatic steatosis (controlled attenuation parameter 229±37.0 vs 231±35.7 dB/m, respectively; p=0.9). MRI-determined liver fat (2.8±5.8 vs 2.5±2.3%, respectively; p=0.9) and anterior thigh muscle fat infiltration (4.0±1.0 vs 4.5±1.5%, respectively; p=0.3) were also comparable. T1D had increased arterial stiffness (augmentation index [AIx] 11.2±11.4 vs 4.7±13.1%, respectively; p=0.05). AIx did not correlate significantly with GIR. However, AIx correlated with VAT mass (r=0.281, p=0.03) and anterior thigh fat infiltration (r=0.473, p=0.004), largely driven by controls (r=0.716, p=0.013 vs T1D: r=0.391, p=0.059).
Conclusion: Adults with T1D demonstrated 1) a unique phenotype of insulin resistance, without the expected features of increased visceral adiposity, ectopic liver and muscle fat; 2) increased arterial stiffness; and 3) an association between aberrant fat distribution and arterial stiffness. These findings raise the possibility that insulin resistance and altered fat distribution exert independent effects on cardiometabolic complications in T1D, with visceral and arterior thigh fat being possible important drivers of cardiovascular risk. Future analyses in INTIMET will determine the relative effects of muscle vs liver insulin resistance on these metabolic parameters.
Clinical Trial Registration Number: ACTRN12619001440112
Supported by: Diabetes Australia, St Vincent's Clinic Foundation, NHMRC, UNSW
Disclosure: J.R. Snaith: None.
159
Circulating succinate response is associated with insulin sensitivity and glucose tolerance
B.D. Astiarraga1, L.M. Guasch1, M. Arnoriaga-Rodriguez2, J. Fernandez-Real2, J. Vendrell1, S. Fernandez-Veledo1;
1Diabetes and associated metabolic diseases research group - DIAMET, Hospital Universitari de Tarragona Joan XXIII, Tarragona, 2Nutrition, Eumetabolism and Health Group, Dr. Josep Trueta University Hospital, Girona, Spain.
Background and aims: Succinate emerged as a key player in metabolic processes. Fasting and meal-stimulated succinate levels are altered in obesity and type 2 diabetes (T2D) and recovery after weight loss. Moreover, circulating succinate response depends on the route of glucose administration in an incretin-like manner. We aim to explore metabolite behavior in prediabetes and its dependence on insulin resistance (IR) status.
Materials and methods: Cohort I, twenty-six volunteers received a 3-h OGTT and, on a separate day, an isoglycemic variable i.v. glucose infusion (ISO) mimicking the glycemia recorded during OGTT. Subjects were classified according to ADA criteria in normal glucose tolerance (NGT - 39 ± 11 years, BMI 28.4 ± 3.1 kg/m2, HbA1c 5.2 ± 0.1%) or impaired glucose tolerance (IGT - 48 ± 10 years, BMI 30.4 ± 3.1 kg/m2, HbA1c 5.5 ± 0.4%) groups, matched by sex-age-BMI (n=13 for both). Cohort II, ten lean (CT - 45 ± 12 years, BMI 22.1 ± 2.2 kg/m2, HbA1c 5.3 ± 0.3%) and ten sex-age matched subjects with obesity (OB - 48 ± 10 years, BMI 36.6 ± 3.0 kg/m2, HbA1c 5.5 ± 0.2%) received a 2-h euglycemic hyperinsulinemic clamp (EHC, 240 pmol.min-1.m-2). Anthropometric and clinical data were collected at baseline, and glucose, insulin, and succinate were determined during the tests for both cohorts.
Results: Cohort I, FPG was 5.4 ± 0.3 vs 6.0 ± 0.6 mmol/L, p=0.002, and 2-h glycemia was 7.1 ± 1.3 vs 8.8 ± 2.5, p=0.04, respectively for NGT and IGT groups. Further, the IGT group was hyperinsulinemic and insulin resistant (OGIS, 392.5 ± 33.5 vs 316.2 ± 49.3 ml.min-1.m-2) in comparison to NGT group (p<0.0001 for both). Fasting succinate was 52 ± 2% higher in the IGT group (p=0.01) as well as, the AUC of succinate during OGTT (50.8 ± 40.0%, p=0.003). I.V. glucose elicits a smaller succinate response by 19.6 ± 11.8% in both groups (Panel A). Cohort II, no differences were found for FPG or HbA1c between groups. At fasting, the OB group showed high insulinemia 70.0 ± 29.1 vs 22.5 ± 8.9 pmol/L (p=0.0001), and succinate levels from 66.9 ± 23.1 vs 44.9 ± 9.0 μmol/L (p=0.03), for OB and CT groups, respectively. During EHC, glucose, and insulin levels were similar between groups whereas, the OB group displayed diminished rates of glucose uptake (M/I) from 38 ± 11.6 vs 22.5 ± 13.0 mg.min-1.kgffm-1.pmol.L-1, for CT and OB groups respectively (p=0.02). Succinate levels rose in parallel to insulin in both groups (Panel B), the fold increase was doubled in CT group (p=0.003). In the pooled data, the M/I was associated with BMI (r=-0.639, p=0.002), fasting succinate (r=-0.579, p=0.007), and succinate response during EHC (r=0.504, p=0.02).
Conclusion: In prediabetes, succinate levels paralleled hyperinsulinemia and IR status. These findings support the role of succinate as an early marker of IR before beta-cell failure in T2D.
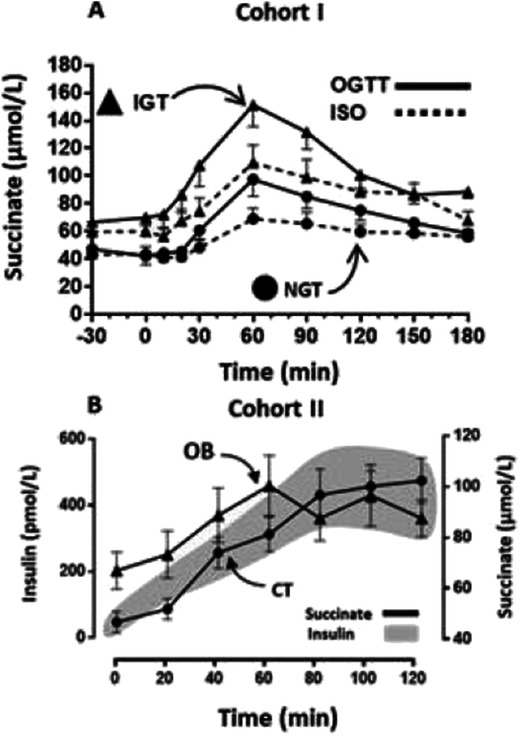
Supported by: ISCIII, MINECO, Ciberdem, FEDER, ThinkGut, ERDF
Disclosure: B.D. Astiarraga: None.
160
Repeated exposure to cold-induced shivering thermogenesis improves glucose homeostasis in overweight and obese adults
A.J. Sellers1, S.M.M. van Beek1, D. Hashim1, H. Pallubinsky1, E. Moonen-Kornips1, G. Schaart1, A. Gemmink1, T. van de Weijer2, M.K.C. Hesselink1, P. Schrauwen1, J. Hoeks1, W. van Marken Lichtenbelt1;
1Department of Nutrition and Movement Sciences, Maastricht University, 2Department of Radiology and Nuclear Medicine, Maastricht University, Maastricht, Netherlands.
Background and aims: Previously, we demonstrated that 10 days of mild cold acclimation (14-15°C, 6h/day) robustly improved insulin sensitivity in patients with type 2 diabetes, partly via an enhanced glucose transporter 4 (GLUT4) translocation in skeletal muscle, as assessed in the overnight fasted state. Although non-shivering thermogenesis is involved in mild cold acclimation, a follow-up study indicated that some level of (mild) muscle activity/shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation. Therefore, we here investigated the effects of repeated bouts of cold-induced shivering thermogenesis on glucose homeostasis.
Materials and methods: In a single-arm intervention study, 15 overweight/obese men and (postmenopausal) women (n = 11 and 4, respectively, 40-75 years, BMI: 27-35 kg/m²) were exposed to 10 consecutive days of intermittent cold-induced shivering thermogenesis (10°C with 1h of shivering per day) via a water-perfused suit. Shivering thermogenesis was confirmed, by surface electromyography and visual observation, and the 1h of shivering was started when resting energy expenditure increased by 50%. Before and after the intervention, a 2-hour oral glucose tolerance test (OGTT) was performed in the overnight fasted state, under thermoneutral conditions. Prior to the OGTTs, heart rate and blood pressure were measured and muscle biopsies were taken.
Results: Repeated exposure to cold-induced shivering thermogenesis significantly reduced fasting plasma glucose concentrations (5.84 ± 0.38 vs 5.67 ± 0.32 mmol/L, p=0.013) and improved glucose tolerance during the OGTT by 6% (total area under the glucose curve: p=0.041). Plasma insulin concentrations at baseline and during the OGTT were unaffected. Interestingly, fasting plasma triglyceride and free-fatty acid concentrations were robustly decreased by 32% and 11% (p=0.001 and p=0.036, respectively). Additionally, repeated cold exposure markedly reduced systolic and diastolic blood pressure by 7.4% (p<0.001) and 8.1% (p<0.001), respectively, and tended to decrease resting heart rate (p=0.062) when measured at thermoneutrality. Analyses of muscle GLUT4 translocation are currently ongoing and will be presented during the meeting.
Conclusion: Repeated exposure to cold, leading to cold-induced shivering thermogenesis, improved glucose homeostasis and other clinically relevant metabolic health parameters in overweight/obese individuals, and hence presents an alternative strategy for the treatment and prevention of type 2 diabetes.
Clinical Trial Registration Number: NCT04516018
Supported by: ZonMW PTO
Disclosure: A.J. Sellers: None.
161
Thromboxane is elevated in men after exercise and improves skeletal muscle glucose uptake and whole-body glucose homeostasis
A.M. Abdelmoez1, M. Borg2, L. Dollet2,3, J.A.B. Smith2, A. Chibalin1, A. Krook2, J.R. Zierath1,3, N.J. Pillon2;
1Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden, 2Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 3Center for Basic Metabolic Research, Copenhagen, Denmark.
Background and aims: Prostanoids (thromboxane and prostaglandins) are lipid mediators that signal through cell surface receptors expressed in multiple cell types, including skeletal muscle. In men, exercise is associated with increased levels of prostanoids in the circulation and in skeletal muscle. Additionally, subjects with type 2 diabetes show altered levels of prostanoids in the circulation compared to healthy controls. However, the role of prostanoids in the adaptive response of skeletal muscle to exercise or metabolic disease is unknown. We hypothesized that prostanoids play a role in skeletal muscle remodelling and metabolism.
Materials and methods: The exercise response of genes involved in the synthesis of prostanoids was assessed using the MetaMEx database (metamex.eu). Blood samples were obtained from men and women with type 2 diabetes and their matched healthy controls before and after an oral glucose tolerance test. Later, the subjects underwent an acute (30 minutes) aerobic exercise bout, and blood samples were collected before and after exercise. Levels of prostanoids in plasma were measured using ELISA and LC-MS. Primary human myotubes were incubated with the thromboxane receptor agonist I-BOP and levels of glucose uptake, oxidation, and incorporation into glycogen were measured using radiolabelled substrates. Western blot was performed to track signalling events. EDL and soleus muscles from male mice were incubated ex-vivo with I-BOP, and glucose oxidation was measured using [14C]-glucose. Glucose tolerance test in mice was performed after an acute administration of I-BOP.
Results: Skeletal muscle levels of cyclooxygenase-2 (PTGS2), the enzyme required for the first step in prostanoids synthesis from arachidonic acid, was higher in women after exercise but not in men. The thromboxane synthase (TBXAS1) mRNA was elevated after exercise in men but not women. Concomitantly, levels of thromboxane B2 in plasma were higher after exercise only in men (+58%, p=0.033, n=5). Activating the thromboxane receptor with I-BOP in skeletal muscle cells resulted in increased glucose uptake (+53%, p=0.0019, n=5), oxidation (+28%, p=0.017, n=5), and incorporation into glycogen (+398%, p<0.001, n=4). This coalesced with signalling events indicative of active actin cytoskeleton remodelling and GLUT4 translocation to the plasma membrane. In isolated mouse skeletal muscle, I-BOP increased ex-vivo skeletal muscle glucose oxidation (+118%, p<0.001, n=7-8). Finally, acute administration of the thromboxane receptor agonist in mice improved glucose tolerance (AUC -34%, p=0.009, n=7-8).
Conclusion: Endogenous production of prostanoids represents a novel sex-dependent physiological adaptation to exercise. Activating the thromboxane receptor in skeletal muscle improves whole body glucose tolerance, suggesting a potential role of prostanoid in promoting the metabolic health benefits of physical activity. Our findings implicate that drugs targeting the production of prostanoids and sex-specific exercise regimens have potential to improve glucose control and overall metabolic health in individuals with type 2 diabetes.
Supported by: EFSD/Novo Nordisk Foundation Future Leaders Award NNF21SA0072747, Diabetes Wellness Sverige PG21-6524
Disclosure: A.M. Abdelmoez: None.
162
High dietary fat intake increases glucagon levels and the glucagon-to-insulin-ratio in healthy lean subjects
B. Schuppelius1, R. Schüler2, O. Pivovarova-Ramich3,4, S. Hornemann2, A. Busjahn5, J. Machann4,6, M. Kruse2, A.F.H. Pfeiffer1;
1Department of Endocrinology and Metabolism, Charité - University Medicine Berlin, Berlin, 2Department of Clinical Nutrition, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, 3Reseach Group Molecular Nutritional Medicine, Dept. of Molecular Toxicology, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, 4German Center for Diabetes Research (DZD), München-Neuherberg, 5HealthTwiSt GmbH, Berlin, 6Institute of Diabetes Research and Metabolic Diseases (IDM) of the Helmholtz Centre Munich, Tübingen, Germany.
Background and aims: Emerging data support an essential role of glucagon (GCGN) for lipid metabolism. However, data on the role of dietary fat intake on GCGN secretion is limited, particularly in humans. We analyzed whether altering nutritional fat intake affects glucagon levels in healthy lean subjects.
Materials and methods: 92 twins (age: 31 ± 14 years BMI: 22.8 ± 2.7 kg/m2) consumed two 6-weeks diets: a low fat healthy diet (LFD: 30 %Energy (%E) fat, 15 %E protein, 55 %E carbohydrate) followed by an isocaloric high fat diet (HFD: 45 %E fat, 15 %E protein, 40 %E carbohydrate). 24 twins additionally continued with a high protein diet (HPD: 30 %E protein, 30 %E fat, 40 %E carbohydrate). Clinical investigation days were performed after 6 weeks of LFD, after 1 and 6 weeks of HFD and after 6 weeks of HPD. GCGN and insulin were measured with specific Mercodia sandwich assays. Liver fat content was determined by MR spectroscopy. For estimation of heritability, the “ACE” structural equation model was applied.
Results: The LFD caused a significant decrease of basal GCGN (-27 %, p ≤ 0.001) compared to screening. After 6 weeks of HFD and minimal weight gain (0,6 %, p = 0.124) GCGN increased significantly (117 %, p ≤ 0.001) while triglycerides remained similar and FFA decreased. 6 weeks of HPD further increased GCGN levels ~72 % (p = 0.502). Meal tolerance tests in a subgroup of 14 twins showed increased postprandial GCGN responses after 1 week of HFD that further increased after 6 weeks. Fasting insulin and HOMA-IR increased moderately after one week of HFD, while 6 weeks of HPD significantly decreased both. The fasting GCGN-to-insulin ratio decreased through LFD (p ≤ 0.001) but increased after HFD (p ≤ 0.001) and even further after HPD (p = 0.018). Liver fat content did not increase during the HFD and did not correlate with GCGN levels after the different diets. Heritability of GCGN was 45% after the LFD. The change of GCGN through the diets was not heritable at any time point.
Conclusion: Under isocaloric conditions high fat intake strongly increases basal and postprandial GCGN levels in healthy young subjects. The increased GCGN-to-insulin ratio suggests a reduced insulin-mediated inhibition of GCGN secretion as one potential mechanism. Nevertheless, the postprandial elevated GCGN levels indicate an additional effect of dietary fat intake. As body weight, liver fat and insulin resistance showed no clinically relevant increases, the observed rise in glucagon may represent a protective metabolic response to high dietary fat intake which increases fat oxidation to prevent fatty liver.
Clinical Trial Registration Number: NCT01631123
Supported by: BMBF (NUGAT 0 315 424 OP-R, MK, AB, AFHP), DFG grant (KFO218 PF164/16–1 OP-R, AK, AFHP)
Disclosure: B. Schuppelius: None.
OP 28 Desirable diets
163
Undesired side effects of a formula diet on erythropoetic parameters: data from two randomised controlled trials
S. Kabisch1,2, S.L. Jahn1, U. Dambeck3, M. Kemper3,2, C. Gerbracht3, C. Honsek3, N.M.T. Meyer1,2, M.A. Osterhoff3, A.F.H. Pfeiffer1,2, J. Spranger1,2;
1Charité University Hospital Berlin, Berlin, 2German Center of Diabetes Research e.V., Neuherberg, 3Clinical Nutrition, German Institute of Human Nutrition, Nuthetal, Germany.
Background and aims: Prevention and therapy of type 2 diabetes are based on lifestyle interventions. Apart from classic low-fat or low-carb dietary concepts, formula diets have been proven to be very effective due to rapid and strong weight loss, even supporting diabetes remission. These drastic dietary approaches elicit widespread benefits on various fat depots, lipid profile, glycemia and blood pressure, depending on the achieved weight loss. Thus, long-term meal replacement of up to three months is proposed to allow rapid weight reductions of >15 kg. However, the spectrum of undesired side effects of formula diets is still insufficiently described. We intended to assess detrimental effects on iron metabolism and erythropoesis in two comparable dietary RCTs using a formula diet and/or conventional food products.
Materials and methods: 269 subjects with with high-risk prediabetes and 177 patients with overt type 2 diabetes participated in our two studies on DIabetes Nutrition Algorithms for Prediabetes / Diabetes“ (DiNA-P/DiNA-D). Both trials started with a 3-week 1:1-randomised hypocaloric diet phase (1200-1500 kcal/d), being characterised as either low-carb (< 40 g/d) or low-fat (< 30 kcal%). DiNA-P used conventional food products for both diets, while in DiNA-D the low-fat approach was designed as formula diet (MODIFAST/Vitalkost Nr. 1) with small amounts of vegetables. Clinical tests included a full metabolic assessment with oGTT/MMTT, body imaging and the analysis of blood counts and iron parameters as safety outcomes. In both studies, blood sampling amounts were 180 ml before and after the diet. The statistical analysis was done by two-sided paired (within-group) and unpaired (between-group) comparisons according to the respective data distribution pattern (t-test or Mann-Whitney-U test). P values below 0.05 were considered statistically significant.
Results: In DiNA-P, both diet groups showed significant, but clinically possibly irrelevant reductions of hemoglobin, hematocrit, MCV and iron after 3 weeks, among which the effect on iron was stronger for the low-carb diet. MCH and ferritin decreased under low-fat regime, only. Erythrocyte counts remained unchanged. No patient fulfilled criteria of anaemia. In DiNA-D, both diets led to a significant reduction of erythrocyte count, hematocrit, iron and transferrin saturation. Additionally, the low-fat formula diet markedly reduced hemoglobin levels and RDW-CV, while only the low-carb diet decreased ferritin. The low-fat formula diet had a significantly stronger impact on erythrocyte count (-0.14±0.26 vs. -0.05±0.24 Tpt/L, p=0,032), hematocrit (-1.4±2.5 vs. -0.6±2.1 %, p=0,044), hemoglobin (-0.44±0.77 vs. -0.16±0.69 g/dL, p=0,020) and RDW-CV (-0.3±0.5 vs. -0.0±0.7 %, p<0,001).
Conclusion: To our knowledge, this is the first report of a consistent, qualitative alteration of erythropoetic parameters as a specific, early-onset side effect of a formula diet. In prolonged regimes - as done in trials and clinical practice for up to three months - this could lead to anaemia. Rheologically desired hemodilution and a potential impact on the validity of HbA1c measurements in these patients are additional aspects worth considering. Further clinical trials are warranted.
Clinical Trial Registration Number: NCT02459496
Supported by: German Center of Diabetes Research (DZD), German Diabetes Association (DDG), California Walnut Comm.
Disclosure: S. Kabisch: Employment/Consultancy; German Center of Diabetes Research e.V. (DZD). Grants; German Center of Diabetes Research e.V. (DZD), German Diabetes Association (DDG). Lecture/other fees; Sanofi, Lilly Deutschland, Berlin Chemie. Non-financial support; California Walnut Commission.
164
An isoenergetic multifactorial diet reduces pancreatic fat and increases postprandial insulin response in patients with type 2 diabetes: a randomised controlled trial
G. Della Pepa1, G. Costabile1, V. Brancato2, D. Salamone1, A. Corrado1, M. Vitale1, C. Cavaliere2, M. Mancini3, M. Salvatore2, D. Luongo3, G. Riccardi1, A.A. Rivellese1, G. Annuzzi1, L. Bozzetto1;
1Federico II University, 2IRCCS Synlab SDN, 3Institute of Biostructure and Bioimaging of National Council of Research, Naples, Italy.
Background and aims: Very little is known about the effect of diet composition per se, indipendently of body weight reduction, on pancreatic fat (PF). We evaluated the effect of an isocaloric multifactorial diet with a diet rich in monounsaturated fatty acids (MUFA) and similar macronutrient composition on PF and postprandial insulin response in Type 2 Diabetes (T2D).
Materials and methods: According to a randomized controlled parallel group design, 39 individuals with T2D, 35-75 years-old, in satisfactory blood glucose control, were assigned to an 8-week isocaloric intervention with a multifactorial diet rich in MUFA, polyunsaturated fatty acids, fibre, polyphenols, and vitamins (n=18) or a MUFA rich diet (n=21). Before/after the intervention, PF content was measured by the proton-density fat fraction using a 3D mDixon MRI sequence, plasma insulin and glucose concentrations were measured over a 4h test-meal with a similar composition as the assigned diet.
Results: After 8 weeks, PF significantly decreased after the multifactorial diet (15.7±6.5% vs. 14.1±6.3%, p=0.024) while it did not change after the MUFA diet (17.1±10.1% vs. 18.6±10.6%, p=0.139) with a significant difference between diets (p=0.014). Postprandial glucose response was similar in the two groups. Early postprandial insulin response (iAUC0-120) significantly increased with the multifactorial diet (36340±34954 vs. 44138±31878 pmol/L·min, p=0.037), while it did not change significantly in the MUFA diet (31754±18446 vs. 26976±12265 pmol/L·min, p=0.178), with a significant difference between diets (p=0.023). Changes in PF inversely correlated with changes in early postprandial insulin response (r=-0.383, p=0.023).
Conclusion: In T2D patients, an isocaloric multifactorial diet including several beneficial dietary components markedly reduced PF. This reduction was associated with an improved postprandial insulin response.
Clinical Trial Registration Number: NCT03380416
Disclosure: G. Della Pepa: None.
165
The role of glucagon in type 2 diabetes remission by weight loss
E. Lalama1, K. Ruether1, J. Zhang1, B. Schuppelius1, N. Kraenkel2, M. Csanalosi1, S. Kabisch1, E. Latz3, A. Christ3, A.F.H. Pfeiffer1;
1Department of Endocrinology and Metabolic Diseases, Charité, Berlin, 2Medizinische Klinik für Kardiologie, Charité, Berlin, 3University of Bonn, Bonn, Germany.
Background and aims: Remission of type 2 diabetes (T2D) was achieved by weight loss of 15kg in over 80% of patients within 6 years after diagnosis in the DIRECT study due to improvements of insulin sensitivity and insulin secretion. The role of Glucagon (GCG) has not been evaluated although GCG is thought to play a central role in the early development of diabetes by increasing hepatic glucose production and thereby insulin secretion resulting in a feed forward cycle of hyperinsulinemia and insulin resistance. The opposite view emphasizes that intra-islet alpha- to beta-cell cross talk is essential for intact insulin secretion and GCG determines hepatic fat oxidation, thus supporting intact metabolism. We investigated the role of GCG in diabetes remission within our “FAIR” study - Fasting-Associated Immune-metabolic Remission of Diabetes.
Materials and methods: Participants (n=36) with overt T2D and BMI over 27 kg/m² were studied before (V1) and 3 months after (V3) consuming an 800 (males) or 600 kcal/day (females) formula diet. Mixed Meal Tests (MMT) were done at V1 and V3, GCG, insulin, and C-peptide (all Mercodia Assays) and glucose as well as clinical routine and anthropometric values were collected. We compared tertiles of low, middle and high GCG levels and areas under the curve (AUC) in the MMTs regarding GCG, insulin, glucose, as well as HbA1c and calculated insulin sensitivity and secretion.
Results: The body weight loss of 16 ± 0.8 kg of 34 participants was associated with strong improvements of insulin sensitivity (HOMA, Matsuda and PREDIM indices) and insulin secretion (disposition index, DIO2). GCG baseline levels did not change in the lowest tertile (V1: 3.5 ± 0.4 to V3: 3.0 ± 0.5 pmol/L, ns) but decreased significantly in the middle (V1: 7.1 ± 0.3 to V3: 5.1 ± 0.6, p=0.016) and highest tertile (V1: 13.5 ± 1.1 to V3: 8.0 ± 1.2 pmol/L, p=0.003). Insulin secretion (DIO2) improved significantly in the middle (V1: 63.2 ± 15 to V3: 172.3 ± 40.2, p=0.001) and higher tertile (V1: 44 ± 7.4 to V3: 109.3 ± 18.1, p= 0.006) but not in the lower tertile. The fasting and MMT-AUC glucose, HbA1c, HOMA and Matsuda index significantly improved in all groups and insulin decreased throughout the cohort, although the changes were more pronounced in the higher GCG tertiles. Of note, we identified a subgroup of 9 participants who showed a small to no change in MMT-AUC insulin (-50 to 50 mU/L) secretion but large decreases of fasting and MMT-AUC GCG, HbA1c, HOMA-IR, Matsuda-index and fasting and MMT-AUC glucose.
Conclusion: GCG responses to weight loss-induced diabetes remission vary extensively. Higher GCG before intervention is associated with greater decreases of fasting and postprandial GCG and greater improvements of insulin secretion while improvements of insulin sensitivity were independent of GCG dynamics. Diabetes remission may be primarily GCG-dependent in a subgroup of patients which will be further characterized. Overall, our data support a protective role of GCG in early diabetes.
Clinical Trial Registration Number: NCT05295160
Supported by: EFSD/Boehringer Ingelheim Research Programme
Disclosure: E. Lalama: Grants; EFSD Boehringer Ingelheim European Research Programme.
166
Determinants of blood glucose concentrations after a high carbohydrate meal in type 2 diabetes: a multiple linear regression analysis
C. Xie, R.J. Jalleh, W. Huang, Y. Sun, K.L. Jones, M. Horowitz, C.K. Rayner, T. Wu;
Adelaide medical school, The University of Adelaide, Adelaide, Australia.
Background and aims: Postprandial glycaemia is a key determinant of overall glycaemic control in type 2 diabetes (T2D), particularly when glycated haemoglobin (HbA1c) is < 8.5%. Understanding the determinants of postprandial hyperglycaemia in T2D is therefore of major importance to optimising management. We have employed a multiple linear regression model to determine the association of blood glucose concentrations over 4 hours after a high carbohydrate meal with fasting blood glucose concentrations, the rate of gastric emptying, insulin sensitivity, and postprandial insulin, glucagon and glucagon-like peptide-1 (GLP-1) responses, in T2D patients with relatively good glycaemic control.
Materials and methods: 71 patients with T2D managed by diet and/or metformin monotherapy (39 male, age 64.7 ± 0.8 years, BMI 30.1 ± 0.6 kg/m2, HbA1c 6.6 ± 0.1 %, duration of known diabetes 5.4 ± 0.6 years) consumed a mashed potato meal (1541.8 kJ, labelled with 100 μL 13C-octanoic acid) between t = 0 to 5 min, after an overnight fast. Venous blood was sampled at t = 0, 15, 30, 60, 90, 120, 180 and 240 min for the measurement of blood glucose and plasma insulin, glucagon and total GLP-1 concentrations. Gastric emptying (expressed in kcal/min) was measured by a breath test, and insulin sensitivity by the Matsuda index. Multiple linear regression analysis was performed to examine relationships of blood glucose concentrations at each time point with fasting blood glucose, gastric emptying, insulin sensitivity, and changes from baseline in plasma insulin, glucagon and total GLP-1, with adjustment for age, sex and BMI. Data are mean values ± SEM. P < 0.05 was considered statistically significant.
Results: Postprandial blood glucose concentrations were positively associated with fasting blood glucose between t = 15 to 240 min (P < 0.001 each), and the rate of gastric emptying between t = 30 to 60 min (P < 0.001 each), and inversely associated with the increments in plasma GLP-1 between t = 90 to 180 min (P = 0.04 at t = 90 min, 0.07 at t = 120 min and < 0.001 at t = 180 min, respectively). In contrast, blood glucose was negatively associated with the rate of gastric emptying at t = 180 min (P = 0.03). At t = 240 min, blood glucose was directly associated with the Matsuda index and change in plasma insulin, but inversely with the change in plasma glucagon (P < 0.05 each).
Conclusion: In relatively well-controlled T2D, postprandial glycaemia is predictably related to fasting blood glucose concentrations, but also to the rate of gastric emptying in the early postprandial phase (within the first hour), whereas in the late phase blood glucose is dependent on GLP-1 concentrations. These observations support the concept of slowing gastric emptying and stimulating GLP-1 secretion, to minimise postprandial excursion in T2D.
Clinical Trial Registration Number: ACTRN12614001131640)
Supported by: NHMRC
Disclosure: C. Xie: None.
167
The 'early' postprandial glucagon response to a mixed meal is dependent on the rate of gastric emptying in type 2 diabetes
W. Huang1, C. Xie1, N.J.W. Albrechtsen2, K.L. Jones1, M. Horowitz1, C.K. Rayner1, T. Wu1;
1Adelaide Medical School, The University of Adelaide, Adelaide, Australia, 2Department of Clinical Biochemistry, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark.
Background and aims: Gastric emptying (GE), which exhibits a substantial inter-individual variation, is a major determinant of postprandial glycaemic and insulinaemic responses in both health and type 2 diabetes (T2D). T2D is characterised by attenuated suppression of plasma glucagon after meals, which contributes to postprandial hyperglycaemia. The relationship between the postprandial glucagon response and GE has not been reported. We examined the relationship between plasma glucagon and GE of a standardised mixed meal in well-controlled T2D.
Materials and methods: 94 patients with T2D managed by diet and/or metformin monotherapy (61 male, age 64.6 ± 0.7 years, BMI 29.8 ± 0.5 kg/m2, HbA1c 6.6 ± 0.1% and duration of known diabetes 5.3 ± 0.5 years) were evaluated on a single study day. After an overnight fast, participants consumed a mashed potato meal (1541.8 kJ: 61.4g carbohydrate, 7.4g protein and 8.9g fat, labelled with 100 μL 13C-octanoic acid) between 0-5 min. Venous blood was sampled at t = 0, 15, 30, 60, 90, 120, 180, 240 min for measurements of blood glucose (glucometer) and plasma glucagon (radioimmunoassay). Gastric emptying was assessed by breath test. Data are mean values ± SEM. P < 0.05 was considered statistically significant.
Results: After the meal, blood glucose concentrations increased progressively from 8.2 ± 0.1 mmol/L to the peak of 14.0 ± 0.31 mmol/L at t = 90 min, followed by a decline towards baseline. Plasma glucagon increased from a fasting level of 76.1 ± 2.1 pg/ml to a peak of 92.7 ± 2.6 pg/ml at t = 30 min and then decreased to a nadir of 65.6 ± 1.9 pg/ml at t = 180 min. The gastric half-emptying time (T50) was 68.2 ± 1.4 min (range 39-116 min). The incremental area under the plasma glucagon curve between t = 0-30min (glucagon iAUC0-30min) was inversely related to the T50 (r = -0.3, P = 0.007). The magnitude of increases in blood glucose from baseline at t = 30 (r = -0.3, P = 0.0003), 60 (r = -0.5, P < 0.0001) and 90 min (r = -0.3, P = 0.004) were related inversely to the T50. The increase in blood glucose at t = 30 min was related directly to the glucagon iAUC0-30min (r = 0.3, P = 0.008).
Conclusion: In well-controlled T2D, the early postprandial glucagon response to a mixed meal is related to the rate of gastric emptying, and predictive of the initial glycaemic response. These observations support the concept of slowing of gastric emptying to minimise postprandial glycaemic excursions in T2D.
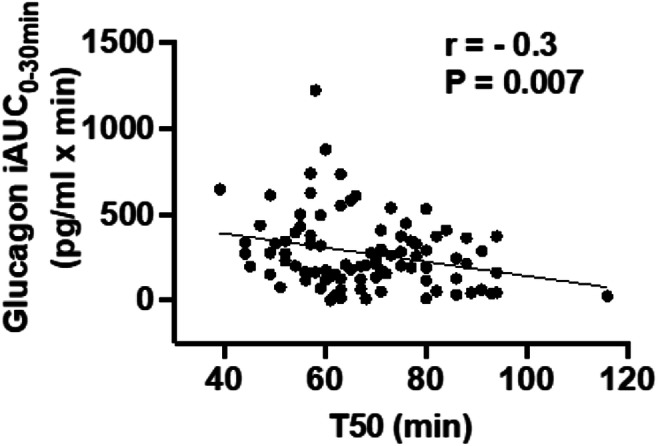
Clinical Trial Registration Number: ACTRN12614001131640
Supported by: NHMRC GNT1147333
Disclosure: W. Huang: None.
168
Disparities in blood glucose and incretin responses to intraduodenal glucose infusion in healthy young males and females
T. Wu, C. Xie, W. Huang, Y. Sun, M. Horowitz, K.L. Jones, C.K. Rayner;
Adelaide medical school, The University of Adelaide, Adelaide, Australia.
Background and aims: Premenopausal women are at lower risk of type 2 diabetes compared to men, but the underlying mechanism(s) remain elusive. The incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) released from the small intestine, modulate insulin and glucagon secretion and play a key role in the regulation of glucose homeostasis. Incretin hormone responses to small intestinal nutrient stimulation are known to vary substantially between individuals, but the potential influence of sex has not been evaluated. We have compared blood glucose and plasma insulin, GIP and GLP-1 responses to intraduodenal glucose infusions in healthy young males and females.
Materials and methods: Data from two independent studies were included in the analysis. In Study 1, 9 females and 20 males, matched for age (female vs. male: 25.0 ± 1.5 vs. 26.3 ± 2.5 years, P = 0.2) and BMI (21.6 ± 0.6 vs. 23.5 ± 0.8 kg/m2, P = 0.3), received an intraduodenal glucose infusion at 2 kcal/min between t = 0 to 60 min. In Study 2, 10 females and 26 males, matched for age (26.4 ± 2.3 vs. 25.2 ± 1.2 years, P = 0.5) and BMI (25.2 ± 2.0 vs. 26.3 ± 1.3, P = 0.5), received an intraduodenal glucose infusion at 3 kcal/min between t = 0 to 60 min. Blood was sampled at t = 0, 15, 30, 45 and 60 min for measurements of blood glucose and plasma insulin, total GLP-1 and GIP levels. Data are means ± SEM. P < 0.05 was considered significant.
Results: Fasting blood glucose, and plasma insulin, GLP-1 and GIP, did not differ between male and female subjects in either study. In Study 1, the incremental areas under the curve between t = 0 to 60 min (iAUCs0-60min) for blood glucose (females vs. males: 135.7 ± 13.1 vs. 140.6 ± 8.5 mmol/L*min, P = 0.8) and plasma GIP (females vs. males: 1937.9 ± 159.5 vs. 1946.2 ± 98.7 pmol/L*min, P = 0.9) during intraduodenal glucose infusion (2kcal/min) did not differ between males and females. However, the iAUC0-60min for plasma GLP-1 (females vs. males: 524.8 ± 89.7 vs. 264.3 ± 57.2 pmol/L*min, P = 0.03) and insulin (females vs. males: 1787.4 ± 229.5 vs. 1030.0 ± 153.4 mU/L*min, P = 0.005) were ~2-fold higher in females than males. In Study 2, the iAUCs0-60min for blood glucose (females vs. males: 99.8 ± 11.7 vs. 110.4 ± 8.0 mmol/L*min, P = 0.5), plasma GIP (females vs. males: 1006.2 ± 124.8 vs. 1030.3 ± 67.3 pmol/L*min, P = 0.9) and plasma insulin (females vs. males: 2829.8 ± 746.1 vs. 2739.9 ± 433.8 mU/L*min, P = 0.9) during intraduodenal glucose infusion (3kcal/min) did not differ between males and females. However, the iAUC0-60min for plasma GLP-1 was 2.5-fold higher in females than males (645.3 ± 135.7 vs. 262.4 ± 54.2 pmol/L*min, P = 0.01).
Conclusion: Healthy young females exhibit comparable GIP but a markedly greater GLP-1 response to small intestinal glucose infusion than males. This disparity warrants further studies to delineate the underlying mechanisms and may also be of relevance to the reduced risk of diabetes in premenopausal women than men.
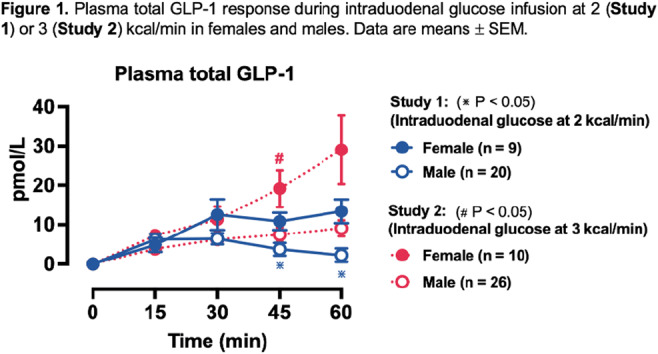
Supported by: NHMRC
Disclosure: T. Wu: None.
OP 29 Saving sweet souls
169
Psychological resilience is predictive of future HbA1c and mental health status in adults with new onset type 1 diabetes
S.M. Brackley1,2, N. Thomas1,2, A. Hill1,2, B. Shields1, T. McDonald1, C. Fox3, J. Huber4, A. Jones1,2;
1College of Medicine & Health, National Institute for Health Research (NIHR) Exeter Clinical Research Facility, Exeter, 2Research and Development, Royal Devon and Exeter NHS Foundation Trust, Exeter, 3Leicester Diabetes Centre, University of Leicester, Leicester, 4School of Sport and Health Sciences, University of Brighton, Brighton, UK.
Background and aims: Psychological resilience is the successful adaptation in the face of adversity. It is thought to be important for effective self-management of Type 1 diabetes, and is associated with glycaemic control in cross-sectional studies. We aimed to determine the relationship between baseline resilience following a recent diagnosis of diabetes with future glycaemic control and perceived health status, in Type 1 and 2 diabetes.
Materials and methods: We prospectively assessed the relationship between resilience with future HbA1c and health status in 1267 participants with recently diagnosed Type 1 and 2 diabetes (duration <12 months, age >=18 years) in the StartRight Study. Psychological resilience was measured using the Connor-Davidson Resilience Scale (CD-RISC) 10-item questionnaire and health status was measured using the Short Form 12-item (SF12) questionnaire. These questionnaires and HbA1c were assessed annually for up to 3 years.
Results: Mean resilience was 30.3 (SD 7.3, scale 0-40) at the first study visit (median 4 months diabetes duration) and reduced by one point over a median 24 months of follow-up [p<0.001]. At baseline, resilience was strongly correlated with the mental health component of the SF12 [Pearson’s r=0.57] and weakly correlated to the physical health component [Pearson's r=0.20]. In all participants, a difference of +10 points in baseline CD-RISC was associated with a 1.8mmol/mol lower HbA1c at 2 years [95% CI 0.7-2.8, p<0.01]. In type 1 diabetes (n=462), a difference of +10 points associated with a 4.1mmol/mol lower 2-year HbA1c [95% CI 2.0-5.7, p<0.001] and in the subgroup developing insulin deficiency (urinary c-peptide : creatinine ratio <0.2nmol/mmol, n=91) tended towards a numerically stronger association of 6.6mmol/mol lower HbA1c [95% CI -0.5 to 9.8, p=0.07]. In type 2 diabetes (n=573), CD-RISC was associated with a 1.1mmol/mol lower follow-up HbA1c per +10pts CDRISC [95% CI 0.0-2.2, p=0.06]. In all subgroups, lower baseline resilience was associated with greater decline in SF-12 assessed mental health (Standardised beta = +0.38, 95% CI 0.32-0.45, p<10-15) but not physical health (Standardised beta = +0.07, 95% CI 0.00-0.14, p=0.06).
Conclusion: A short, validated psychological resilience questionnaire close to diagnosis of type 1 diabetes is predictive of future glycaemic control and decline in perceived mental health status. This supports a need for studies to determine whether targeted interventions may improve outcomes for people with type 1 diabetes and low resilience.
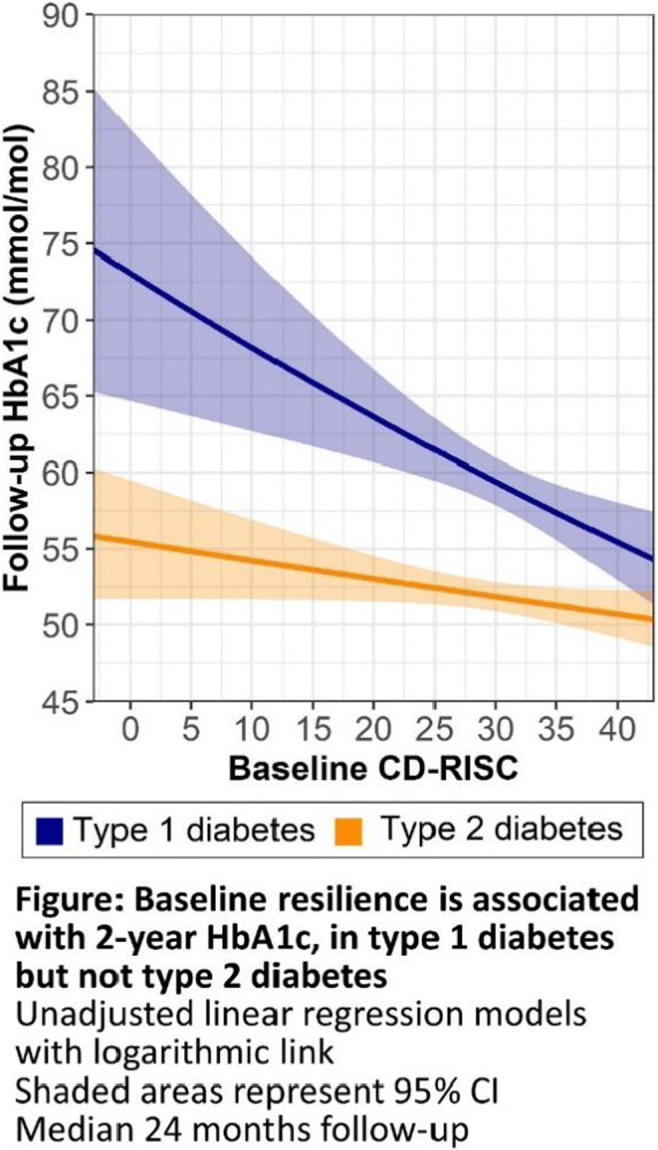
Clinical Trial Registration Number: NCT03737799
Supported by: Diabetes UK, NIHR
Disclosure: S.M. Brackley: None.
170
Caregiver-report adherence in diabetes questionnaire is predictive of 10-year HbA1c trajectories in children and adolescents with type 1 diabetes: a population-based study
K.P. Marks1,2, N.H. Birkebæk3,2, F. Pouwer4,5, E.H. Ibfelt6, M. Thastum7, M.B. Jensen8;
1Department of Clinical Medicine - Paediatrics, Aarhus University, Aarhus N, 2Steno Diabetes Center Aarhus, Aarhus N, 3Department of Paediatrics, Aarhus University Hospital, Aarhus N, 4Department of Psychology, University of Southern Denmark, Odense, 5Steno Diabetes Center Odense, Odense, 6Steno Diabetes Center Copenhagen, Copenhagen, 7Department of Psychology and Behavioural Sciences, Aarhus University, Aarhus N, 8Department of Economics, Aarhus University, Aarhus N, Denmark.
Background and aims: Adherence or self-care skills comprise of all child/adolescent or family’s efforts that are required to fulfill an array of diabetes-specific recommendations, in collaboration with health care providers. We seek to identify distinct 10-year HbA1c trajectories in children/adolescents with type 1 diabetes (T1D) and determine whether the caregiver- and/or child/adolescent-reported answers to Adherence in Diabetes Questionnaire (ADQ) predict membership to these HbA1c trajectories, controlling for sex, age of T1D diagnosis, and insulin pump status.
Materials and methods: We analyzed longitudinal, population-based data from a 2009 Danish national survey cohort (N=672, ages 10-17 years). The survey included the caregiver- and child/adolescent-report on ADQ. HbA1c levels were obtained annually (2010-2020) from the Danish Registry of Childhood and Adolescent Diabetes and Danish Adult Diabetes Registry. First, four distinct HbA1c trajectories from early adolescence to emerging adulthood (12-27 years) were identified with group-based trajectory modeling. Second, associations of baseline ADQ scores with HbA1c trajectory/group membership were investigated by use of a multinomial logit model. We controlled for sex, age at T1D onset, and insulin pump status.
Results: Baseline participant characteristics were: n= 672 (321 [47.8%] males); mean (SD) age 14.4 (2.2) years; mean (SD) age of T1D diagnosis 8.46 (3.50) years; n= 282 (42%) insulin pump users. Four HbA1c trajectories/groups were identified: group 1 (“stable on target [HbA1c 53 mmol/mol (7.0 %)], gradual decrease”, 30% of the sample), group 2 (“above target, small wave”, 40% of the sample), group 3 (“well above target, moderate wave”, 22% of the sample), and group 4 (“well above target, large wave”, 8% of the sample). A higher caregiver-reported ADQ score was predictive of less likely membership to group 2 (coeff.= -0.68, SE= 0.24, p= 0.005), group 3 (coeff.= -1.27, SE= 0.24, p< 0.001) and group 4 (coeff.= -1.74, SE= 0.29, p< 0.001) as compared to group 1. The child/adolescent-reported ADQ score was not a significant predictor once the caregiver-reported ADQ was included in the model. Males were less likely than females to be members of group 2 (coeff.= -0.45, SE= 0.23, p< 0.05) compared to group 1, whereas age of T1D diagnosis, and insulin pump status were not predictive of any group membership.
Conclusion: About 30% of the children and adolescents had a very unfavorable HbA1c trajectory. Caregiver-reported ADQ should be used to identify children at risk of a high or increasing HbA1c trajectory across adolescence and young adulthood.
Supported by: KPM: grant # NNF17SA0031406, Danish Diabetes Academy. NHB: grant from Poul and Erna Sehested's Fond
Disclosure: K.P. Marks: Grants; KPM’s PhD scholarship and work was supported by a research grant from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation, grant number NNF17SA0031406. Other; MT and NHB are coauthors of the Adherence in Diabetes Questionnaire, which is freely available and in the public domain. MT and NHB have no financial conflicts of interest.
171
Associations between generalised anxiety disorder, glycaemic management, and demographic factors among adults with diabetes in Europe
E. Cox, E. Ye, R. Wood, C. Pang;
dQ&A, San Fransisco, USA.
Background and aims: Previous research shows that people with diabetes (PWD) have higher rates of mental health disorders, such as generalized anxiety, compared to the general population. While mental health support, such as therapy or psychiatric medication, has been shown to effectively address mental health concerns among PWD, extant research on the relationship between diabetes management and generalized anxiety is limited. Thus, the present study aims to investigate the relationship between diabetes management metrics and anxiety among PWD in Europe.
Materials and methods: From October to November 2021, 3,077 adults living with diabetes in France, Germany, Italy, Netherlands, Sweden, and the United Kingdom took an online survey in which they reported their most recent HbA1c, if they knew it (n=2,561). Glucose sensor users (n=2,011) also reported the percentage of time in a typical day spent in the target range (70-180 mg/dl), otherwise known as Time in Range (TIR). All respondents completed the Generalized Anxiety Disorder diagnostic tool (GAD-7), a 7-item validated measure assessing the severity of generalized anxiety disorder (GAD). The subsequent responses (66% type 1, 52% female) were scored and analyzed. Statistical testing was conducted using two-proportion Z-tests.
Results: PWD in Italy and the UK report the highest rates of anxiety (63% and 51%, respectively), while PWD in the Netherlands report the lowest rates (39%). Across all European countries studied, women with diabetes are more likely to report experiencing anxiety than men with diabetes (57% vs. 39%, p<0.001). Anxiety is more prevalent among adults with diabetes under 45 years of age than those 45 and above (59% and 34%, p<0.001). PWD with HbA1c levels greater than 7% are significantly more likely than those with HbA1c levels less than or equal to 7% to have moderate (13% vs. 10%, p=0.03) or severe anxiety (6% vs. 4%, p=0.04). PWD using glucose sensors with TIR under 70% have significantly higher rates of moderate or severe anxiety relative to those who spend 70% or more of time in the target range (22% vs. 14%, p<0.001).
Conclusion: This research reveals mental health disparities in nationality, gender, and age among PWD in Europe. Further, these findings highlight a link between the glycemic management of diabetes and anxiety severity. To both minimize anxiety and improve diabetes management, this study emphasizes the need for an integrated approach to mental health support and diabetes management, targeted specifically at at-risk demographic groups.
Disclosure: E. Cox: Employment/Consultancy; EC, EY, RW, and CP are employees of dQ&A, a company that provides research services for a fee to several clients (>10) in the diabetes field.
172
Relationship between elevated diabetes distress and DSM-5 personality traits: evidence from the Czech validation sample
J. Konecna1, D. Lacko2, K.D. Riegel3;
13rd Department of Medicine - Department of Endocrinology and Metabolism, General University Hospital in Prague, Praha, 2Czech Academy of Sciences, Institute of Psychology, Brno, 3Department of Addictology, 1st Faculty of Medicine, General University Hospital in Prague, Praha, Czech Republic.
Background and aims: The level of subjectively experienced Diabetes Distress has an impact on the diabetes management and treatment outcomes. Howerer, there are more psychosocial factors other than DD which could also increase the experienced burden. An example could be the lack of ability to regulate emotions or to regulate negative emotional experiences which is one of the personality traits. Personality traits are also discussed in the association of treatment outcomes or adherence to the diabetes mellitus treatment. The level of Diabetes Distress (DD) captured with a Czech version of the Diabetes Distress Scale (DDS) is observed through the prism of the DSM-5 personality traits according to the Alternative model for personality disorders (AMPD).
Materials and methods: The sample comprised 358 participants with diabetes mellitus (DM) (56.2% female, age M = 42.33, SD = 14.33 years). The subjects have completed both the Czech version of the DDS and the shortened 160-item version of the Personality Inventory for DSM-5 (PID-5). The association between the DDS and PID-5 was analyzed with multiple regressions. The DDS psychometric properties were analyzed in a structural equation modeling framework with a set of confirmatory factor analyses.
Results: Our findings in the matter of the relationship between the levels of DD and personality traits suggest a high association between the PID-5 Negative Affectivity domain and the Emotional burden DDS subscale (β = .852, pHolm < .001), and also between Negative Affectivity and the Regimen Distress DDS subscale (β = .435, pHolm = .006). Furthermore, the Czech version of the DDS showed satisfactory psychometric properties in its factor structure, internal consistency, and measurement invariance between genders and across age. The McDonald’s omega values of subscales varied from .81 in case of Regimen distress up to .92 in case of Emotional distress.
Conclusion: Several specific personality traits according to the AMPD deserve attention in the relation of the subjectively experienced levels of DD. The level of Negative affectivity among the patients with DM could affect their emotional burden level and perception of regimen distress. The DDS is a reliable scale for measuring DD in terms of research and clinical practice within Czech samples.
Supported by: Ministry of Health, Czech Republic [GJIH-1599-04-1-180] [64165]
Disclosure: J. Konecna: Grants; This work was supported by the Ministry of Health, Czech Republic [GJIH-1599-04-1-180] – conceptual development of research organization [64165] General University Hospital in Prague, Czech Republic.
173
High burden of depression, anxiety and severe obesity in young women with newly diagnosed type 2 diabetes: reports from a Swedish multicentre study of 1027 patients
E.O. Melin1, P. Wanby2, T. Neumark3, S. Holmberg2, A.-S. Nilsson Neumark4, K. Johansson5, M. Landin-Olsson1, H. Thulesius6, M. Hillman7, M. Thunander1;
1Diabetology and Endocrinology, Lund University, Lund, 2Medicine and Optometry, Linnaeus University, Kalmar, 3RaD, Region Kronoberg, Region Kalmar, Regional Executive Office - Coordination of Health Care, Kalmar, 4Department of Research, Region Kalmar County, Kalmar, 5Department of Health and Caring Sciences, Linnaeus University, Växjö, 6Linnaeus University, Kalmar, 7Lund University, Lund, Sweden.
Background and aims: Depression is a risk factor for type 2 diabetes mellitus and cardiovascular disease. The aims were to explore the prevalence of depression, anxiety, antidepressant use, obesity, Hemoglobin A1c, life-style factors, and pre-existing cardiovascular disease, in patients with newly diagnosed T2D; to explore associations with depression; and to compare with general population data.
Materials and methods: Multicentre, cross-sectional study. Inclusion criteria: adults with serologically verified newly diagnosed type 2 diabetes mellitus. Data collection and analyses included age, sex, current depression and anxiety (Hospital Anxiety and Depression Scale), previous depression, antidepressant use, body mass index (BMI), Hemoglobin A1c, and pre-existing myocardial infarction and stroke.
Results: In all 1027 participants, aged 18-94 years, the depression prevalence was 12%, and depression was associated with age (per year) (inversely) (odds ratio (OR) 0.97), anxiety (OR 12.2), previous depression (OR 7.1), antidepressant use (OR 4.2), obesity (BMI ≥30 and ≥40 kg/m2) (OR 1.7 and OR 2.3 respectively), smoking (OR 1.9), physical inactivity (OR 1.8), and women (OR 1.6) (all p ≤0.013). Younger, ≤59 years, women (n=113) compared to younger men (n=217) had higher prevalence of current depression (31% vs 12%), previous depression (43 vs 19%), anxiety (42% vs 25%), antidepressant use (37% vs 12%), obesity (BMI ≥ 30 and ≥ 40 kg/m2) (73% vs 60% and 18% vs 9% respectively), and smoking (26% vs 16%) (all p ≤0.029). Older, ≥60 years, women (n=297) compared to older men (n=400) had higher prevalence of previous depression (45% vs 12%), anxiety (18% vs 10%), antidepressant use (20% vs 8 %), and obesity (BMI ≥30 and ≥40 kg/m2) (55% vs 47% and 7% vs 3% respectively) (all p ≤0.048). Compared to the Swedish general population (prevalence of depression (women 11.2%/men 12.3%) and antidepressant use (women 9.8%/men 5.3%)), younger women with T2D had higher prevalence of current depression, and all patients had higher prevalence of antidepressant use.
Conclusion: Three risk factors for cardiovascular disease, obesity, smoking, and physical inactivity, were associated with depression in patients with newly diagnosed type 2 diabetes mellitus. The younger women had the highest prevalence of depression, anxiety, and severe obesity. All patients had higher prevalence of antidepressant use than people in the Swedish population.
Supported by: FORSS, Region Kronoberg
Disclosure: E.O. Melin: None.
174
Let the patient choose! Patient preferences for type 2 diabetes therapy in the trimaster double-blind three-way randomised crossover trial
B. Shields1, C. Angwin1, A.G. Jones1, R.R. Holman2, N. Sattar3, N. Britten1, E. Pearson4, M. Shepherd1, A.T. Hattersley1;
1University of Exeter Medical School, Exeter, 2University of Oxford, Oxford, 3University of Glasgow, Glasgow, 4University of Dundee, Dundee, UK.
Background and aims: Determining optimal glucose lowering drugs for a person with type 2 diabetes requires balancing the benefits and side effects of alternative therapies. No randomised trials have allowed patients to compare their own experience on different drugs to decide their own preference. We aimed to examine patient preference to second/third line glucose-lowering drugs in a three-way double blind randomised crossover trial.
Materials and methods: People with poorly controlled type 2 diabetes (HbA1c >58mmol/mol) on stable metformin +/- sulfonylurea therapy received, in randomly assigned order, 16 weeks each of pioglitazone 30mg, sitagliptin 100mg, and canagliflozin 100mg. At the end of each treatment period, HbA1c, and weight were measured, and patient-reported benefits, side effects, and willingness to take the drug long-term were recorded. At the end of the study, patients were asked to rank the three drugs in order of preference and state why. Reasons were coded, by 2 independent observers, as “feeling better” or “lack of side effects” compared with the other drugs.
Results: 457 participants tried all three study drugs. For each drug, achieved HbA1c was similar (pioglitazone 59.5, sitagliptin 59.9 canagliflozin 60.5 mmol/mol, p=0.19) and similar proportions of participants stated they would take it long term (54%, 58%, 59% respectively, p=0.48). 448/457 stated a drug preference. 115 (25%) preferred pioglitazone, 158 (35%) sitagliptin, 175 (38%) canagliflozin. The drug chosen as preferred, compared with the other two, was associated with a lower HbA1c (4.8[95%CI 4.1, 5.5]mmol/mol lower, p<0.001) and less side effects (0.5[0.36, 0.65] fewer, p<0.001). Regardless of preference, pioglitazone was associated with the highest weight on therapy (2.6 [2.3, 2.9]kg higher, p<0.001). Overall, 51% chose the preferred drug because of “feeling better”, whereas 39% chose it due to “lack of side effects”, with 10% unclassifiable. Canagliflozin was less often chosen by patients because of lack of side effects (27%) compared to pioglitazone (50%) and sitagliptin (42%). However, it was most often chosen due to “feeling better” (68%) compared with sitagliptin (48%) or pioglitazone (39%).
Conclusion: After trying all three drugs, that were equally effective overall, patients preferred the drug that gave them lowest HbA1c and least side effects. We propose that in the absence of a specific indication for a particular drug, patients should be offered a brief trial of potential alternative therapies to allow them to decide which they prefer.
Clinical Trial Registration Number: NCT02653209
Supported by: MRC
Disclosure: B. Shields: None.
OP 30 Novel ways of beta cell replacement
175
A highly oxygenated hydrogel enhanced the survival of human islets encapsulated within macroencapsulation devices
D.A. Domingo-Lopez1, D. Brandhorst2, E. O’Cearbhaill3, F. Coulter3, L. McDonough4, H. Brandhorst2, S. Deotti3, P. Johnson2, H. Kelly4, G.P. Duffy1;
1College of Medicine, Nursing and Health Sciences, Anatomy and Regenerative Medicine Institute, National University of Ireland Galway, Galway, Ireland, 2Nuffield Department of Surgical Sciences, John Radcliffe Hospital, Research Group for Islet Transplantation, University of Oxford, Oxford, UK, 3School of Mechanical Engineering, Centre for Biomedical Engineering, University College of Dublin, Dublin, Ireland, 4School of Pharmacy & Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland.
Background and aims: Islet transplantation aims to reverse type 1 diabetes by restoring insulin production. Post-transplantation islet survival is limited by the lack of suitable support matrix and insufficient oxygen supply (hypoxia), which is aggravated when using macroencapsulation devices. Graft failure can be overcome by cell encapsulation in highly oxygenated biomaterials, like Oxygel (OxG), able to provide O2 and extracellular matrix (ECM) support when incorporated in macroencapsulation devices.
Materials and methods: OxG was formulated by the shear mixing of Hyaluronic acid hydrogel (HA) with a Perfluorocarbon nanoemulsion and oxygenated by simultaneous O2 and gel infusion using a customized set-up (n=3). Mechanical properties; viscosity (n), storage (G’) and loss (G”) modulus, were characterized using oscillatory rheology and creep recovery test. O2 levels of oxygenated OxG (O2-OxG) and controls were monitored by oxygen microsensors. Oxygen diffusion coefficient (Dv) was estimated by experimentally fitting the O2 release to a Fickian diffusion model. Human islets were isolated (n = 7) and mixed with different matrices: (A) supplemented CMRL; (B) HA; (C) OxG or (D) O2-OxG. Afterwards, silicone macrodevices were loaded with 600 IEQ and cultured for 5 days. Islets were recovered and islet death was characterized by FDA-PI staining. Data was normalized to IEQ and related to preculture (PC) data.
Results: OxG mechanical properties showed a shear thinning behavior with a transition from viscoelastic solid to viscoelastic liquid behavior at 3-4 Pa. OxG recovered its initial viscosity after 4 min of stress removal. High O2 tension was achieved in O2-OxG and O2-PFD emulsion (475-641 torr) that was released for 90 h compared to a fast oxygen release found in O2-PBS (14 hours). Significantly smaller Dv was found in OxG (2.71 ± 0.04 x 10-10 m2 s-1) and PFD emulsion (2.75 ± 0.05 x 10-10 m2 s-1, p > 0.05 vs OxG) compared to PBS (3.93 ± 0.17 x 10-9 m2 s-1, p < 0.001 vs OxG and PFD emulsion). Human islets loss was reduced when encapsulated in O2-OxG (20.10 ± 6.36%, p > 0.05 vs PC) compared to OxG (36.80 ± 4.87 %, p < 0.001 vs PC), HA (65.40 ± 7.80 %. p < 0.001 vs PC) and CMRL (89.07 ± 2.88%, p < 0.001 vs PC). A decrease in cell death was also observed in O2-OxG (37.57 ± 1.96%, p > 0.05 vs PC [31.71 ± 2.14%]) compared to OxG (42.79 ± 1.37 %, p < 0.001 vs PC), HA (43.23 ± 2.07 %, p < 0.001 vs PC) and CMRL (49.19 ± 1.57%, p < 0.001 vs PC).
Conclusion: Oxygel was developed as an ECM-based hydrogel with optimal mechanical properties (shear-thinning and self-healing), high O2 loading capacity and slow O2 diffusion ability. The preliminary in vitro evaluation of Oxygel’s influence on human islets indicates that the incorporation of a suitable ECM within macrodevices helps maintaining the integrity of encapsulated islets/cells over time. Additionally, O2 delivery within macroencapsulation devices appears to be highly beneficial in overcoming hypoxia-mediated islet death.
Supported by: DELIVER EU Horizon 2020 MSCA programme (812865)
Disclosure: D.A. Domingo-Lopez: None.
176
Microsphere-based bioartificial islet with beta cells and mesenchymal stem cells co-encapsulation for diabetes treatment
J. Sun, L. Li;
Department of Endocrinology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China.
Background and aims: Recent therapeutic approaches of type 1 diabetes mellitus (T1DM) target restoration of endogenous insulin production rather than the traditional insulin injection therapy. Thus, β-cell replacement therapy is the practical cell treatment option for the management of T1DM. However, the broad application of β-cell transplantation is greatly limited by donor shortage, side effects of immunosuppressants and persistent post-transplantation loss of β-cells due to hypoxia. Co-transplantation with mesenchymal stem cells (MSCs) could contribute to enhancing function and survival of β-cells. Biocompatible hydrogels have potential to facilitate implantation, which could eliminate direct contact of β-cells with immunocompetent cells. This study aimed to develop porous hydrogel microspheres to co-encapsulate β cells and MSCs to construct novel biological artificial islets for hyperglycemia treatment.
Materials and methods: We used microfluidic technology to co-encapsulate β-cells and MSCs in porous hydrogel microspheres based on the hybrid solution of gelatin methacrylate (GelMA) and poly (ethylene oxide) (PEO) within fast response time. The β-cell viability and insulin secretory function was examined in vitro. Naked β cells and MSCs, microspheres encapsulated with β cells alone and microspheres co-encapsulated with β cells and MSCs were implanted into the omentum of diabetic mice, and blood glucose and body weight changes were monitored. The glucose tolerance test was performed at 4 weeks of implantation and cell-laden microspheres were removed at six weeks post-transplantation to assess β-cell function.
Results: Three dimensional (3D) porous structure in microspheres facilitated β-cell proliferation in clusters. β-cells co-encapsulated with MSCs increase glucose-stimulated insulin secretion and content in vitro. The transplantation results suggested that bioartificial islets significantly reversed diabetes in diabetic mice within a week post-transplantation and continuously regulate the dynamic balance of blood glucose levels in vivo. Fluorescence microscopy showed distinct insulin-expressing cells in bioartificial islets removed after 6 weeks of implantation.
Conclusion: The bioartificial islet could be endowed with the high glucose responsiveness and ability of insulin secretion, achieving highly simulation of islet survival environment. The results of transplantation further confirmed the therapeutic potential of bioartificial islets to significantly improve hyperglycemia and achieve stable blood glucose control in diabetic mice. Overall, the novel bioartificial islet has a distinctive function in the treatment of diabetes, and could suggest a suitable platform for β cell transplantation in clinical application.
Supported by: National Natural Science Foundation of China (No 6590009658)
Disclosure: J. Sun: None.
177
Parallel single cell RNA sequencing and spatial transcriptomics analysis of the developing human pancreas
O. Olaniru1, U. Kadolsky2, S. Kannambath2, H. Vaikkinen2, K. Fung2, P. Dhami2, S.J. Persaud1;
1Department of Diabetes, King's College London, 2BRC, King's College London, London, UK.
Background and aims: There is a global research effort to generate unlimited amounts of human beta-cells in vitro for type 1 diabetes transplantation therapy but current stem cell differentiation protocols are mostly derived from mouse pancreas development, and they do not generate fully functional beta cells. We have therefore generated and integrated single cell RNA sequencing (scRNA-seq) and spatial transcriptomics of the developing human pancreas at multiple timepoints to provide detailed transcriptomic analysis of the various pancreatic cell types.
Materials and methods: Using scRNA-seq, we determined the transcriptome of over 53,000 human fetal pancreatic cells at 8, 10, 12, 13, 14, 15, 18, 19 and 20 post conception weeks. We performed 10x Visium spatial transcriptomics on 8 pancreas sections at four developmental time points covering over 10,000 barcoded spots to spatially localise the pancreatic cells. scRNA-seq and spatial transcriptomics data were integrated by canonical correlation analysis. Using time-series and pseudotime trajectory inferences including a deep-learning based spatial trajectory analysis, we uncovered differentiation transitions occurring in temporal and spatial contexts. We validated novel genes driving endocrine differentiation by smFISH and analysed the influence of pancreas microenvironment on endocrine progenitor differentiation by a connectome-based network analysis.
Results: scRNA-seq revealed distinct clusters of acinar, ductal, endocrine progenitors, alpha, beta, delta, immune, endothelial, Schwann and mesenchymal cells. Spatial transcriptomics samples were sequenced to a median depth of 177.5 × 106 reads (interquartile range 116.9-294.4 × 106), which yielded a mean of 1692 genes and 3395 unique molecular identifiers per spot. We identified spatially correlated genes and spatially proximal cells and found that spatial neighbourhoods were shared by acinar and endocrine cells, by endocrine, ductal, acinar and pancreatic progenitors, and also by endothelial and mesenchymal cells, suggesting that these neighbouring cell populations are more likely to interact together. Cell trajectory inference identified three endocrine progenitor populations and novel branch-specific genes as the endocrine progenitors differentiate towards alpha and beta cells, which were confirmed by smFISH. By integrating scRNAseq with spatial transcriptomics, we showed that mesenchymal cells undergo transition in the presence of immune cells to increase acinar cell number, with upregulation of CTRB2, SYCN, CEL and CPA1 (p<2.8x10e-4) and downregulation of COL3A1, EEF1A1, SNX3, COL1A2, RPL9 (p<3.9 x10e-5). Spatial differentiation trajectories in situ indicated that Schwann precursor cells are spatially co-located with endocrine progenitors and contribute to beta cell maturation via the L1CAM-EPHB2 pathway.
Conclusion: We have characterised and spatially resolved multiple human pancreatic cell populations at multiple developmental stages. We have identified sub-populations of human endocrine progenitors, novel genes that may direct their differentiation to beta or alpha cell lineage, and the influence of pancreas microenvironment on endocrine progenitor differentiation. Our data identified the roles of Schwann precursor cells and mesenchymal cells in the differentiation of endocrine progenitors and acinar cells, respectively.
Supported by: Novo Nordisk UK Research Foundation and NC3Rs
Disclosure: O. Olaniru: None.
178
Elucidating the role of TFB1Mgenetic variants in diabetogenic mechanisms using genome-edited stem cell models
F. Roberts, S. Hladkou, T. Singh, R. Prasad, M. Fex, H. Mulder;
Clinical Research Center, Malmö, Sweden.
Background and aims: Mitochondria play a pivotal role in linking the metabolism of nutrients and insulin release from pancreatic beta (β)-cells via metabolic coupling factors. It is established that Type 2 Diabetes (T2D) arises when insulin secretion fails due to β-cell dysfunction. Transcription Factor B1 Mitochondrial (TFB1M) is a nuclear-encoded methyltransferase, essential for ribosomal stability and translation of mitochondrially encoded genes. In this context, TFB1M function is important for physiological insulin release via proper mitochondrial function. We previously described the human TFB1M gene intronic SNP rs950994 (A/G) as an expression quantitative trait locus (eQTL). The risk (AA) allele is associated with reduced islet TFB1M expression, reduced β-cell mass, metabolic coupling and impaired insulin secretion. The rs950994 SNP is also prognostically associated with T2D traits. Therefore, we aimed to understand the pathogenetic effects of the rs950994 SNP specifically on β-cell dysfunction in T2D.
Materials and methods: We created a humanized β-cell model by utilizing human induced pluripotent stem cells (hIPSCs) differentiated into β-like cells. Fibroblasts in skin biopsies from one female and one male TFB1M rs950994 risk (AA) carrier were reprogrammed to hIPSCs and subsequently differentiated into β-like cells. The efficiency of differentiation was determined by stage-specific RNA/protein marker expression via FACS, qPCR, Western and ICC. Genome editing by CRISPR/Cas12a was performed in donor hIPSCs via homology-directed repair and the risk (AA) alleles were successfully edited to non-risk (GG) alleles. Insulin-expressing β-like cells from risk and non-risk donors were FACS-sorted using a lentivirus GFP reporter and analyzed for TFB1M mRNA expression. Insulin secretion was quantified by insulin ELISA.
Results: The hIPSCs from both donors were differentiated to insulin-expressing β-like cells with 3-5% efficiency. Rs950994 risk (AA) to non-risk (GG) allele correction was 41%, and 59% efficient in male and female hIPSCs, respectively. Compared to non-risk (GG) allele β-like cells, the insulin-producing β-like cells from a heteroclonal allele-corrected population of risk (AA) background exhibited decreased IBMX-induced insulin secretion (3.76/fold change over basal versus a 4.66-fold change) and total insulin content (486.3 versus 1297.5 mU/g total protein) and a 22% decreased TFB1M mRNA expression. Insulin data was derived from β-like cells differentiated from one female donor.
Conclusion: These data demonstrate a reduced expression of TFB1M mRNA in risk (AA) allele β-like cells coupled with reduced insulin secretion capacity and insulin content compared to non-risk (GG) variants. Thus, we have provisionally shown that theTFB1M risk variant (AA) is indeed an eQTL, which may underlie the increased risk of T2D conferred by rs950994. Further mechanistic explanations are warranted to explain this effect at the β-like cell level, including analysis of additional donors
Disclosure: F. Roberts: None.
179
Stem cell-derived islets display functional and metabolic maturation post-engraftment
T. Barsby, E. Vähäkangas, H. Montaser, J. Ustinov, J. Saarimäki-Vire, T. Otonkoski;
University of Helsinki, Helsinki, Finland.
Background and aims: Pluripotent stem cell-derived islets (SC-islets) are a promising tool for the development of translational therapies for diabetes. Following murine engraftment, SC-islets respond dynamically to glucose challenge, humanize blood glucose levels, and display gene network expression patterns closely related to those of primary human islets. However, it is unknown to what extent SC-islets maintain immature features of beta cell function post-engraftment and little is known about how nutrient-sensitive metabolic pathways develop during in vivo maturation. Here we generate SC-islets and follow their functional acquisition in parallel with metabolite tracing analyses and mitochondrial morphology throughout in vivo engraftment in mice.
Materials and methods: SC-islets were differentiated in vitro using an optimised 7-stage protocol and engrafted into the kidney capsules of NOD-SCID-Gamma mice for up to 4 months. Dynamic graft function assays as well as blood glucose and C-peptide measurements were conducted throughout the engraftment time course. Grafts were retrieved at 1- and 4-months post-implantation for immunohistochemical and electron microscopic assays of insulin granule and mitochondrial morphology. Retrieved grafts were also acutely exposed to basal and stimulatory concentrations of [U-13C6] glucose for LC-MS based metabolite flux analyses.
Results: SC-islets demonstrated increasing levels of C-peptide release and improved dynamic function during 4 months of murine engraftment. Metabolite tracing assays of SC-islets (pre- and post- humanization of murine blood glucose levels) showed that engrafted SC-islets progressively acquired glucose-responsive TCA metabolite flux patterns more closely resembling those of primary human islets. However, the labelling patterns of other TCA-derived metabolites (such as glutamine and proline) still differed from primary islets even after extended engraftment times. Furthermore, the directionality and flux of the TCA cycle in basal glucose conditions was also aberrant in SC-islet derived grafts. From this data we found that de novo aspartate production (derived from glucose metabolism) correlated with the acquisition of improved graft function. We further correlated these metabolic findings with assays of mitochondrial morphology and candidate marker proteins as proxies of functional maturity.
Conclusion: Engrafted SC-islets display progressive functional maturation within the first 4 months following implantation. Functional improvements correlated with enhanced glucose-responsive TCA-metabolite flux, although differences in the production of TCA-derived metabolites and directionality within the TCA cycle still differed between late stage grafted SC-islets and primary adult islets. The profiling of SC-islets post-engraftment is a key step in gauging the efficacy and safety of utilizing SC-islets in cell replacement therapies.
Supported by: STEMM Research Programs Unit and the Academy of Finland
Disclosure: T. Barsby: None.
180
MicroRNAs predictive of islet graft function in islet transplant recipients
W.K.M. Wong1, M.V. Joglekar1, V. Saini1,2, C.X. Dong1, P.S. Kunte3, B.L. Anderson4, K.Z. Dajani4, A.M. Simpson2, P.E. MacDonald4, P.A. Senior4, K.K. Danielson5, A.M.J. Shapiro4, A.A. Hardikar1;
1School of Medicine, Western Sydney University, Campbelltown, Australia, 2School of Life Sciences and the Centre for Health Technologies, University of Technology Sydney, Sydney, Australia, 3KEM Hospital and Research Center, Pune, India, 4University of Alberta, Edmonton, AB, Canada, 5University of Illinois, Chicago, USA.
Background and aims: Human islet transplantation is the only approved cell-based therapy for type 1 diabetes in some countries. However, several pancreatic islets die immediately after transplantation. The success of islet transplantation depends on the number of surviving and functional islet cells, which have been difficult to estimate using current methodologies. We aimed to assess microRNAs as surrogate markers of beta-cell function/death, and identify their potential to predict subsequent graft function in islet transplant recipients.
Materials and methods: Plasma samples were collected from islet transplant recipients (n=15) before and at 1-hour, 24-hours (in Edmonton) or from n=18-23 recipients at 1-week and 20-weeks (in Chicago) after transplantation of allogeneic human islets. Real-time qPCR was used to measure a panel of microRNAs that we had identified to be associated with insulin gene transcription. Significantly altered circulating microRNAs were identified using appropriate statistical methods based on data distribution and variance. We also employed machine-learning (ML) workflows to identify important microRNAs associated with fasting C-peptide levels and exogenous insulin requirement at one month post-transplant.
Results: Nineteen of the insulin transcript-associated microRNAs demonstrated an overall increase in abundance at 1-hour post-transplantation, compared to their pre-transplant levels. Two of the measured microRNAs (miR-375-3p, and miR-216b-5p) had significant increase (p<0.05) at the 1-hour post-transplant stage and then reduced at 24-hours post-transplant. Interestingly, these two microRNAs continued to remain low at 1-week and 20-weeks post-transplant. ML workflows (penalised regression with bootstrapping) identified microRNAs measured at 1-hour post islet transplant that could predict post-transplantation C-peptide levels at 1-month (spearman R=0.79, p=0.0008). Insulin requirement at 1-month post-transplant was determined with microRNAs measured at 1-hour post islet transplant using ML methodologies. The important microRNAs for determining insulin requirement at 1-month yielded an area under the curve of 0.85 (n=15, Sensitivity=0.60, specificity=0.83) in a Receiver Operating Characteristics curve analysis using leave one out cross-validation ML technique.
Conclusion: Upregulation of insulin transcript-associated microRNAs in just 1-hour post-transplant, indicates that they may be released as a result of islet cell death and could be used as biomarkers to assess beta-cell function in islet transplant recipients. Validation of these microRNAs in other sample sets will confirm the utility of these microRNAs in predicting islet graft function within an hour post-transplantation.
Supported by: JDRF International, JDRF Australia and Helmsley Trust.
Disclosure: W.K.M. Wong: None.
OP 31 Diabetes: size matters
181
IDF diabetes atlas: global prevalence estimates of prediabetes for 2021 and projections for 2045
D.J. Magliano1, M. Fang2, M. Rooney2, K. Ogurtsova3, E. Boyko4, E. Selvin2;
1Baker Heart and Diabetes Institute, Melbourne, Australia, 2Epidemiology, John Hopkins University, Baltimore, USA, 3German Diabetes Center of the Leibniz Association, Dusseldorf, Germany, 4Epidemiology, University of Washington, Seattle, USA.
Background and aims: Persons with prediabetes—impaired glucose tolerance (IGT) or impaired fasting glucose (IFG)—are at high risk for developing diabetes and microvascular and macrovascular complications. We aimed to estimate the global prevalence of IGT and IFG.
Materials and methods: We systematically reviewed 7,014 articles and reports published after 2000 to identify reliable estimates of IGT (2-hour glucose concentration of 7.8-11.0 mol/L ) and IFG (fasting glucose of 6.1-6.9 mmol/L) for each country. Studies were assessed for quality using pre-established criteria. Extracted data were modelled using logistic regression to produce smoothed age-specific prevalence estimates of IGT and of IFG for 2021, and for 2045. For countries without in-country data, estimates were extrapolated from countries with similar economies, ethnicity, geography, and language.
Results: There were 51 high-quality studies for IGT (from 43 countries) and 42 high-quality studies for IFG (from 39 countries). Approximately 80% of countries did not have high quality IFG or IGT data. The global prevalence of IGT in 2021 was 9.1% (464 million adults) and is projected to increase to 10.0% (640 million) in 2045 (Figure A). The global prevalence of IFG in 2021 was 5.6% (286 million) and is projected to increase to 6.2% (397 million) in 2045 (Figure B). The prevalence of IGT and IFG was highest in high income countries.
Conclusion: The global burden of prediabetes is substantial and growing. There is pressing need to implement interventions which will halt this increase, and to avoid the future diabetes epidemic that currently threatens to overwhelm global healthcare systems.
Disclosure: D.J. Magliano: None.
182
Body size change from childhood to adulthood and type 2 diabetes risk
G.D. Carrasquilla, T.O. Kilpeläinen, R.J.F. Loos;
NNF Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Obesity prevalence is rising worldwide among children and adults. The high prevalence is particularly alarming among children, given the long-term health consequences and risk for chronic diseases, including type 2 diabetes (T2D). Little is known as to whether excess adiposity in childhood affects people’s risk of T2D in adulthood, independent of adult BMI or obesity status. Here, we examine whether relative body size in childhood affects T2D, independent of body size in adulthood. We also examine whether a change in relative body size from childhood to adulthood independently affects T2D risk.
Materials and methods: We used data from 378,873 individuals of European ancestry from the UK Biobank who did not have T2D in adulthood at baseline. Based on self-reported relative body size at age 10 (comparative body size higher, average, or lower than peers), we classified individuals in three sex-stratified groups: low (LowC), average (AverageC), or high (HighC). We created similar-sized sex-stratified groups for adult body size based on BMI: LowA, AverageA, and HighA. By combining childhood and adulthood body size categories, the population was divided in nine categories (Figure 1A), with most individuals being average in both childhood and adulthood. However, a substantial number of individuals changed categories between childhood and adulthood. Over an average follow up of 8.38 years, 7,042 individuals developed T2D. We applied multivariate Cox proportional regression models to obtain hazard ratios and 95% confidence intervals of incident T2D risk across the nine body size groups. Individuals with an average body size during childhood and adulthood were defined as the reference population. Analyses were adjusted for sex, age, principal components, socioeconomic factors, and various lifestyle factors including diet, physical activity, smoking and alcohol.
Results: Above average body size in adulthood (HighA) was a major risk factor for T2D (Figure 1B). Interestingly, among the “above average” (HighA) adults, those whose body size was below average during childhood (LowC-HighA) had a higher risk (HR: 4.9, 95%CI 4.4, 5.4) than those whose body size during childhood was average (HR: 3.6 95%CI 3.3, 4.0) or even above average (HR: 3.7, 95%CI 3.4, 4.2) (Figure 1B). A similar trend, although less pronounced, was observed in the other adult body size categories as well. Specifically, a below average body size during childhood increased risk of T2D in adulthood at each level of the adult body size (Figure 1B).
Conclusion: We show that below average body size in childhood increases risk of T2D in adulthood at each level of adult body size. The increase in risk is most evident among those who gain above average body size in adulthood. We speculate that lean children may have an increased susceptibility for T2D because of a limited capacity for triglyceride storage in the adipose tissue, which may lead to metabolic complications upon weight gain due to lipotoxicity in adipose tissue and systematically.
Supported by: Novo Nordisk Foundation (NNF18CC0034900, NNF17OC0026848 and NNF17SA0031406) and MSCA (846502)
Disclosure: G.D. Carrasquilla: None.
183
Type 2 diabetes, and its modulating effect on the aging body, studied using voxel-wise analysis of whole-body MR images
J. Kullberg1,2, A. Martinez Mora1, N. Ahmad1, F. Malmberg1,3, R. Strand1,3, L. Johansson2, T. Fall4, S.C. Larsson1,5, L. Lind6, H. Ahlström1,2;
1Department of Surgical Sciences, Uppsala University, Uppsala, 2Antaros Medical, Mölndal, 3Department of Information Technology, Uppsala University, Uppsala, 4Department of Medical Sciences, Molecular Epidemiology and Science for Life Laboratory, Uppsala University, Uppsala, 5Unit of Cardiovascular and Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, 6Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Background and aims: Morphology of multiple organs and tissues have been associated with type 2 diabetes (T2D) and aging. Magnetic Resonance Imaging (MRI) can be used for detailed studies of body composition. Studies are typically performed after explicit quantification of targets of interest. Here we studied perturbations in body composition in T2D and aging at the voxel-level using a statistical image analysis approach (Imiomics) that utilizes all image data collected.
Materials and methods: Neck-to-knee water-fat MRI from participants in the UK Biobank imaging study (18 778 males, age 65.0±7.7, T2D 6.5% and 20 120 females, age 63.7±7.4, T2D 2.8%) were investigated. T2D was classified using a previously validated algorithm. Image registrations of the water-fat MRI scans were used for sex-stratified voxel-wise analysis of tissue volume and fat content throughout the body. Multiple regression was used to model voxel-wise association to T2D and age using what can be described as association imaging. Interactions between T2D and age were also studied. Key findings were confirmed using explicit measurements. This research used the UK Biobank resource (applic. no. 14237) and SNIC (sens2019016).
Results: Volume and fat content of multiple tissues were associated with both T2D (Figure 1) and age. Findings of particular interest included findings in liver, skeletal muscle, and bone marrow as well as regional differences within the subcutaneous adipose tissue (SAT), including differences in the upper vs lower body and in the anterior vs posterior lower abdomen. A lower fat content in long bones was found in T2D, especially in females and was confirmed by explicit measurements. Most tissue volumes correlated negatively with age. However, visceral adipose tissue (VAT) and lower anterior SAT show positive correlations. Liver and muscle showed higher fat contents in T2D and muscle fat fraction increased with age. T2D patterns were relatively similar in both sexes except for lower thigh muscle volumes in males. T2D and age interactions were found in upper body SAT and VAT, especially in females, as well as in liver volume, liver fat and bone marrow fat content in long bones in both sexes. Most deviations seen in T2D in middle aged subjects tended to normalize during aging.
Conclusion: This voxel-wise study of T2D and age confirmed many previous reported findings but also identified and visualized multiple findings that would be very difficult to study using other techniques.
Supported by: Swedish Research Council, Heart-Lung Foundation, EXODIAB
Disclosure: J. Kullberg: Employment/Consultancy; Antaros Medical. Stock/Shareholding; Antaros Medical.
184
Low birth weight is associated with lower age and BMI at the time of type 2 diabetes diagnosis in the Danish DD2 cohort
A.L. Hansen1, R.W. Thomsen2, C. Brøns1, H.M.L. Svane2, J.S. Nielsen3, P. Vestergaard4, K. Højlund3, N. Jessen5, M.H. Olsen6, T. Hansen7, H.T. Sørensen2, A.A. Vaag1;
1Steno Diabetes Center Copenhagen, Herlev, 2Dept. of Clinical Epidemiology, Aarhus University, Aarhus, 3Steno Diabetes Center Odense, Odense, 4Steno Diabetes Center North, Aalborg, 5Steno Diabetes Center Aarhus, Aarhus, 6Steno Diabetes Center Zealand, Holbæk, 7Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Low birthweight (LBW) is a risk factor for type 2 diabetes (T2D), but the extent to which LBW influences the clinical presentation of T2D is unknown. We examined whether LBW is associated with a range of key clinical characteristics, including age at diagnosis, family history of diabetes, obesity, hypertension, subclinical inflammation, HBA1c, insulin secretion and action, as well as comorbidities in patients with newly diagnosed T2D in the nationwide Danish Centre for Strategic Research in Type 2 diabetes (DD2) cohort.
Materials and methods: Our analysis included 7,297 newly diagnosed T2D patients enrolled in the DD2 cohort by general practitioners and outpatient hospital clinics in Denmark during 2010-2018. We retrieved birthweight data from midwife records in the Danish National Archives. Birthweight was divided into three groups: the lowest 25% (<3000g = LBW), the middle 50% (3,000-3,700 g = normal birthweight (NBW)), and the highest 25% (>3,700g = high birthweight (HBW). Log-binomial and Robust Poisson regression analyses were used to calculate sex- and age-adjusted prevalence ratios (PRs) with 95% confidence intervals (95% CIs) of T2D characteristics associated with LBW and HBW exposure, using NBW as the reference category.
Results: In total, 1,556 (21.3%) patients had LBW, 3,279 (44.9%) NBW, and 1,544 (21,1%) HBW. Compared with NBW (median age at T2D onset = 62.3 years), LBW was associated with a lower age (59.6 years) and HBW conversely with a higher age at T2D onset (64.8 years), as reflected by opposite directed PR´s in LBW versus HBW in patients with onset before age 45 versus after 75 years (LBW: PR 1.36 (95% CI, 1.13-1.64) at age <45 versus 0.74 (95% CI, 0.59-0.92) at age >75 years. HBW: PR 0.65 (95% CI, 0.52-0.83) at age < 45 versus 1.48 (95% CI, 1.24-1.76) at age > 75 years). At T2D onset, patients with LBW were leaner (BMI <25: PR 1.11 (95% CI, 1.03-1.21)), less often severely obese (BMI >40: PR 0.55 (95% CI, 0.42-0.72), and had lower waist circumference (<94/80 cm for men/women: PR 1.34 (95% CI, 1.13-1.59)). After adjustments for sex, age and BMI, LBW patients had a higher Charlson Comorbidity Index score (≥3: PR 1.36 (95% CI, 1.06-1.75)), used more antihypertensive medications (≥3 antihypertensive drugs: PR 1.33 (95% CI, 1.06-1.67)), had increased subclinical inflammation (hsCRP >3 mg/L: PR 1.09 (95% CI, 1.00, 1.19)), and were less likely to have a family history of diabetes (no affected first-degree relatives: PR 1.08 (95% CI, 1.02-1.15)) versus 3 or more affected relatives: PR 0.68 (95% CI, 0.52-0.90)).
Conclusion: LBW was associated with younger age, less obesity and less genetic predisposition at time of T2D diagnosis. Furthermore, LBW was associated with increased subclinical inflammation, hypertension and overall comorbidity burden. There is a need for prospective studies of disease trajectories, comorbidities, complications, and mortality in T2D patients with LBW.
Supported by: Novo Nordisk Foundation
Disclosure: A.L. Hansen: Grants; Novo Nordisk Foundation.
185
Four weeks of carbohydrate overfeeding induce widespread dysmetabolic traits in men born with a low birth weight compared to matched normal birth weight controls
C. Brøns1, A.B. Thuesen2, S.O. Villumsen1, L. Justesen1, L.O. Elingaard-Larsen1, J. Størling1, M. Kim1, C. Legido-Quigley1, G. van Hall3, E.R. Danielsen3, T. Hansen2, A.A. Vaag1;
1Steno Diabetes Center Copenhagen, Herlev, 2University of Copenhagen, Copenhagen, 3Rigshospitalet, Copenhagen, Denmark.
Background and aims: Low birth weight (LBW) is a well-known risk factor for development of type 2 diabetes (T2D) later in life. This is particularly apparent when exposed to an affluent dietary lifestyle. Data from our group has indicated that impaired subcutaneous adipose tissue expandability, associated with increased ectopic (hepatic) fat deposition, may be on the critical path of T2D development in LBW subjects. We aimed to study whether a 4-week overfeeding (+25% energy) challenge, consisting of simple carbohydrates (COF), would result in differential dysmetabolic effects, including increased hepatic fat content, in LBW men compared with normal birth weight (NBW) controls.
Materials and methods: We included 22 healthy, early middle-aged, and non-obese LBW men (mean BW 2797±175 g) as well as 21 NBW controls (mean BW 3807±176 g) matched for age and BMI. We measured body composition by DXA, hepatic fat content by magnetic resonance spectroscopy, glucose and insulin metabolism, hepatic glucose production (HGP) by deuterium glucose, energy metabolism by indirect calorimetry, selected plasma biomarkers by multiplex Mesoscale Discovery technology as well as untargeted serum metabolomics and lipidomics by mass spectrometry before and after 4 weeks COF.
Results: In response to COF LBW, but not NBW subjects, displayed increased fasting plasma levels of glucose (P=0.03), C-peptide (P=0.01), leptin (P=0.0002) and FGF21 (P=0.005), as well as increased energy expenditure (P=0.0002) and fat oxidation rates (P=0.02) compared to baseline. COF resulted in similar significant increases in body weight in both groups. Hepatic fat content increased slightly in both LBW (P=0.023) and NBW (P=0.018) subjects after COF, however, with the LBW group having significantly increased hepatic fat content before and after COF compared with NBW controls. Fasting plasma adiponectin was significantly reduced in LBW compared with NBW subjects both before and after COF. Among 65 metabolites, 8 were differentially abundant between the groups (P<0.05) after COF. Ingenuity pathway analyses (IPA) revealed accumulation and increased lipid peroxidation in LBW subjects after COF. Further, IPA showed activation of the PPARGC1A pathway in LBW subjects contrasting a minor downregulation of the pathway in NBW subjects after COF. Finally, among 279 lipids identified by lipidomics, the LBW subjects exhibited a significantly (PFisher=0.0004) higher number of lipids to be increased after COF (n=26) compared to NBW subjects (n=6). This finding was further supported by a network analysis showing a general upregulation of the lipid profile in LBW subjects opposing a slight downregulation in NBW controls after COF.
Conclusion: LBW subjects at increased risk of T2D respond to COF with differential increases in plasma glucose, C-peptide, leptin and FGF21 levels, as well as with increased fat oxidation rates and severe differential perturbations of lipid metabolism, compared to NBW controls. The findings stress the importance of LBW individuals refraining from overeating simple carbohydrates. Additional studies are needed to further understand the role of increased hepatic fat accumulation in LBW subjects in T2D development.
Clinical Trial Registration Number: NCT02982408
Supported by: EFSD/Lilly Research Programme, Organ Cross-talk, NNF, Augustinus Foundation, Aase and Ejnar Danielsen Foundation, Trygfonden
Disclosure: C. Brøns: Grants; European Foundation for the Study of Diabetes, Novo Nordisk Foundation, Aase and Ejnar Danielsens Fond, Augustinus Foundation, TrygFonden.
186
Efficacy of metformin in preventing progression to diabetes in Chinese subjects with impaired glucose regulation: results from a multicentre, open-label RCT
L. Zhang1, Y. Zhang2, S. Shen3, X. Wang4, Q. Li5, L. Ji6, N. Sun6, G. Li7, CDPP study group;
1The Second Hospital of Hebei Medical University, Shijiazhuang, 2Baoding First Central Hospital, Baoding, 3Yanji Hospital, Yanji, 4Jinzhou Central Hospital, Jinzhou, 5Kailuan General Hospital, Tangshan, 6Peking University People’s Hospital, Beijing, 7Chinese Association of Geriatric Research, Beijing, China.
Background and aims: Impaired glucose regulation (IGR) is an important risk factor for the development of diabetes, and includes impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). Pharmacological agents can decrease the rate of progression from IGT to diabetes, with the ADA stating that metformin currently has the strongest body of evidence. The objectives of this study were to determine whether lifestyle intervention (LSI) plus metformin was superior to LSI alone in preventing diabetes, and to assess the effect of metformin on weight loss and blood pressure in Chinese subjects with IGR.
Materials and methods: In this study, 1706 subjects with IGR, including IFG (fasting plasma glucose 6.1-6.9 mmol/L and 2-h postprandial glucose <7.8 mmol/L) and IGT (fasting plasma glucose <7.0 mmol/L and 2-h postprandial glucose 7.8-11.1 mmol/L) by OGTT, were randomly assigned (1:1) to LSI plus metformin (n=848) or to LSI only (n=858) . The primary endpoint was the incidence of diabetes based on OGTT. Secondary endpoints were weight and blood pressure reduction. Safety was assessed by the incidence of adverse events (AEs).
Results: The mean follow-up time was 1.99±0.62 and 1.94±0.60 years in the LSI plus metformin and LSI groups, respectively ; incidence of diabetes was 18.23 and 20.72 per 100 person-years. The risk of diabetes was 17% lower in the LSI plus metformin group vs the LSI group (HR 0.83; 95% CI 0.70-0.99); however, in a prespecified analysis of subjects with IFG alone, the risk of diabetes was not significantly different between two groups (HR 0.84; 95% CI 0.55-1.30). Progression to diabetes was significantly delayed in the LSI plus metformin group (Figure ). The LSI plus metformin group showed a greater weight reduction after 2 years (1.03 kg) than the LSI group (−2.43±0.28 vs −1.40±0.27 kg respectively, p=0.0002). In subjects who were hypertensive at baseline but not receiving an anti-hypertensive agent, the mean change in systolic/diastolic BP was not significantly different between groups: −9.4/−3.9 mmHg (LSI plus metformin group) and −8.97/−3.4 mmHg (LSI group). The incidences of AEs and serious AEs were 34.55% and 1.71% in the LSI plus metformin group, and 22.68% and 1.29% in the LSI group, respectively.
Conclusion: This study demonstrated that LSI plus metformin is more effective than LSI alone in reducing the risk of diabetes, delaying progression to diabetes, and reducing body weight in Chinese adults with IGR. The effects for subjects with IFG alone remain uncertain.
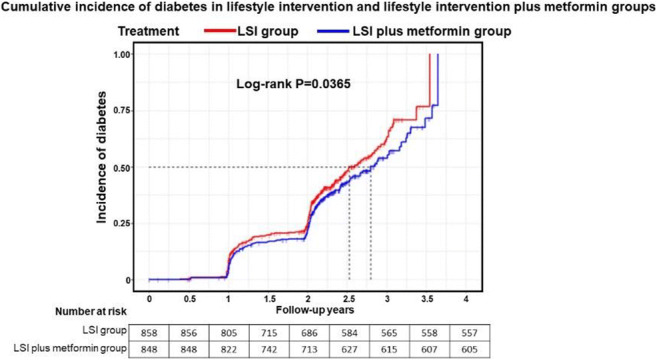
Clinical Trial Registration Number: ClinicalTrials.gov ID: NCT03441750
Supported by: Chinese Association of Geriatric Research (CAGR) and Merck Serono China Co. Ltd.
Disclosure: L. Zhang: Other; This study was sponsored by the Chinese Association of Geriatric Research (CAGR) and was funded by Merck Serono China Co. Ltd., an affiliate of Merck KGaA, Darmstadt, Germany.
OP 32 Pain or no pain?
187
Painful diabetic peripheral neuropathy in type 1 diabetes in the Epidemiology of Diabetes Interventions and Complications (EDIC) study
B.H. Braffett1, L. El ghormli1, J.W. Albers2, E.L. Feldman2, R.A. Gubitosi-Klug3, W. Herman2, C.L. Martin2, T.J. Orchard4, B.A. Perkins5, N.H. White6, J.M. Lachin1, R. Pop-Busui2, DCCT/EDIC Research Group;
1George Washington University, Rockville, USA, 2University of Michigan, Ann Arbor, USA, 3Case Western Reserve University, Cleveland, USA, 4University of Pittsburgh, Pittsburgh, USA, 5University of Toronto, Toronto, Canada, 6Washington University St. Louis, St Louis, USA.
Background and aims: We evaluated the prevalence and incidence as well as risk factors associated with painful diabetic peripheral neuropathy (DPN) in participants with type 1 diabetes (T1D) enrolled in the Epidemiology of Diabetes Interventions and Complications (EDIC) study, an observational follow-up of the Diabetes Control and Complications Trial (DCCT).
Materials and methods: Data were obtained from 1,401 participants followed for 26 years during EDIC. The Michigan Neuropathy Screening Instrument (MNSI) questionnaire, which includes specific neuropathic pain questions (Q2: “Do you ever have any burning pain in your legs and/or feet?” and Q6: “Does it hurt when the bed covers touch your skin?”), was administered annually. Painful DPN was defined as a positive response to Q2 and/or Q6 plus a MNSI examination score >2. Sustained painful DPN was defined as occurring on 2 or more consecutive annual visits. In addition, Kaplan-Meier estimates were used to describe the 26-year cumulative incidence of the first occurrence of any sustained painful DPN.
Results: Over the 26-year duration in EDIC, the prevalence of neuropathic pain (Q2 and/or Q6) increased from 8.5% to 19.8% while the prevalence of a MNSI examination score >2 increased from 22.9% to 43.5%. During EDIC, 889 (63.5%) participants did not experience any painful DPN while 512 (36.5%) experienced painful DPN at least once, of which 263 (18.8%) met the criteria for sustained painful DPN. After adjusting for age at DCCT closeout and DCCT treatment group, participants with sustained painful DPN had significantly higher BMI, waist circumference, systolic blood pressure, pulse pressure, heart rate, total and LDL cholesterol, triglycerides and HbA1c levels over time versus participants without any painful DPN during the study (p<0.0005 for all). Among the 1,348 participants without painful DPN at EDIC year 1, the 26-year cumulative incidence of sustained painful DPN was 18% (Figure).
Conclusion: In this large cohort of well-characterized T1D participants, the prevalence of neuropathic pain and DPN increased steadily over the 26-year follow-up. These data also suggest that differences in several cardiometabolic risk factors, in addition to glucose control, may contribute to differences in painful DPN risk.
Clinical Trial Registration Number: NCT00360893
Supported by: NIH/NIDDK
Disclosure: B.H. Braffett: None.
188
Increased functional connectivity of the Thalamus and primary Somatosensory cortex and insular cortex following treatment withdrawal: a potential biomarker of painful-DPN
G. Sloan1, D. Selvarajah2, K. Teh3, I. Wilkinson3, S. Tesfaye1;
1Sheffield Teaching Hospitals, 2Department of Oncology and Human Metabolism, 3Academic Unit of Radiology, Sheffield, UK.
Background and aims: Altered functional connectivity has been identified in key brain regions involved in somatosensory perception in patients with painful diabetic peripheral neuropathy (painful-DPN), using resting state functional-magnetic resonance imaging (fMRI). However, these studies have not looked at the impact of neuropathic pain treatments. A greater understanding of treatment upon these pain processing areas might lead to greater understanding of the mechanisms of these pharmacotherapeutic agents and also development of new treatments that target these brain regions.
Materials and methods: A total 15 participants (Age, 62.1 ± 9.0; HbA1c 65.4 ± 16.2 mmol/mol; 13 type 2 diabetes, 1 type 1 diabetes and 1 MODY; 13% female) enrolled in the OPTION-DM clinical trial (ISRCTN17545443) underwent neuroimaging. All participants had clinical and neurological assessments, including the modified Toronto Clinical Neuropathy Score, Doleur Neuropathique 4 and Neuropathic Pain Symptom Inventory. Participants underwent fMRI imaging using 3T (Achieva, Phillips Healthcare) when the participants were on maximum tolerated medication (Treatment Scan) and one week after washout of these medications (Washout Scan). The data was analysed using Conn Functional Connectivity Toolbox in SPM.
Results: There was a significant increase in Pain Numeric Rating Scale (NRS) from Treatment Scan (4.0 ± 2.1) to the Washout Scan (6.1 ± 2.4, p=0.044). There was a significantly greater functional connectivity between the Primary Somatosensory Cortex (S1) and the Thalamus, and the Insular Cortex and Thalamus (p false discovery rate [FDR] = 0.041) during the Treatment Scan compared with the Washout Scan. Moreover, there was a significant difference in the change between scans in S1 - Thalamic functional connectivity in participants with Severe-Pain (NRS ≥8 at baseline: Age, 64.5 ± 10.1; 10% Female; -0.372 ± 0.275) compared to participants with Moderate-Pain (NRS ≤7: Age, 57.4 ± 3.0; 20% Female; -0.051 ± 0.180, p=0.035). The change in S1-Thalamic connectivity also correlated with a number of variables including baseline pain (r -0.585, p=0.022), NPSI (r -0.597, p=0.019) and the difference in NRS at the Treatment Scan and Baseline pain (r -0.513, p=0.050).
Conclusion: This is the first study to look at the impact of neuropathic pain medication withdrawal on functional connectivity of pain matrix brain regions. On neuropathic pain medication withdrawal there is an increase S1 - Thalamus and Insular Cortex - Thalamus functional connectivity. Moreover, the change in S1 - Thalamus functional connectivity from the Treatment Scan to Withdrawal Scan differentiated participants with high and low baseline neuropathic pain and correlated with baseline pain. This study further demonstrates that the thalamus is a key area for the central mechanisms of painful-DPN and its functional connectivity to the S1 and Insular Cortex has the potential to act as a biomarker of painful-DPN.
Disclosure: G. Sloan: None.
189
Better target engagement of opioid receptor systems in responders to neuropathic pain treatment: a neurotransmitter-enriched functional connectivity mapping study
D. Selvarajah1, K. Teh2, J. Mcallister1, A. Anandhanarayanan1, J. Fan1, G. Sloan3, S. Tesfaye3;
1Department of Oncology and Metabolism, University of Sheffield, 2Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, 3Diabetes Research Department, Sheffield Teaching Hospitals, Sheffield, UK.
Background and aims: The neurobiological mechanisms underlying treatment response in painful diabetic neuropathy are poorly understood. We have demonstrated that responders to neuropathic pain treatment have greater functional connectivity between the insula cortex and the corticolimbic system compared to non-responders. Activity within these networks is mediated by endogenous opioid receptor systems and may hold clues to possible future treatment targets.
Materials and methods: 43 painful-DN subjects [responders (VAS<4; n=29) and non-responders (VAS>4; n=14)] underwent detailed clinical and neurophysiological assessment, and RS-fMRI. Data analysis was performed using the NITRC Functional ConnectivityToolbox and SPM8 in MATLAB. RS-fMRI data was masked and binarised using an opioid receptor atlas to restrict the analysis to the voxels with high receptor density. Subject-specific spatial maps of responders and non-responders were compared.
Results: Compared to painful-DN non-responders, responders had greater functional connectivity between the corticolimbic system with the opioid receptor networks [F(2)(41)=43.53;intensity=128.7; R-amygdala beta=0.48; p-FDR<0.0001; R-putamen beta=0.3; p-FDR<0.0001; dorsal lateral prefrontal cortex beta=0.25; p-FDR=0.0002 and the posterior parietal cortex networks beta0.25; p-FDR=0.0002].
Conclusion: Painful DN treatment responders have better target engagement of opioid receptors systems compared to non-responders. Whilst further clinical validation in larger cohorts is warranted, our findings suggests that a functioning/intact descending pain inhibition network is crucial for a better pain response. Interventions targeted at this network could provide better pain relief in non-responders to neuropathic pain treatment.
Supported by: EFSD/Lilly Research Programme
Disclosure: D. Selvarajah: None.
190
Declining incident rates of distal polyneuropathy in a cohort study, but with distinct age-related patterns between diabetes types in the period 1996-2018
H. Mizrak, H. Amadid, P. Rossing, D. Vistisen, C. Hansen;
Steno Diabetes Center Copenhagen, Herlev, Denmark.
Background and aims: Distal symmetric sensorimotor polyneuropathy (DSPN) is irreversible and debilitating for people with diabetes and imposes a considerable burden on health care systems. The incidence of major diabetic complications, such as cardiovascular disease, renal disease, blindness and amputation, has been decreasing in many countries over the last few decades. However, it is unknown if this applies to diabetic neuropathy. The aim of this study was to estimate temporal changes in the incidence rates of DSPN in individuals with type 1 diabetes (DM1) and type 2 diabetes (DM2) in a longitudinal cohort at a tertiary diabetes treatment facility. In addition, differences in age-related incidence rates were investigated.
Materials and methods: In the period between 1996-2018, we identified 13,650 individuals with DM1 and DM2, with repeated measures of vibration perception threshold (VPT) and a height measure. Individuals with prevalent neuropathy at the first performed foot exam were excluded. In total 2783 individuals, 1071 individuals with DM1 and 1712 individuals with DM2 were included in the further incidence analysis. VPT was measured with Bio-Thesiometer and age- sex- and height-specific cut-off values were used to assess the presence of DSPN. DSPN was defined as decreased VPT in both feet. Incidence rates of DSPN, sex, age and calendar time were modelled by Poisson regression for time-split data and separate for DM1 and DM2.
Results: From 1996 to 2018, 737 cases of incident DSPN occurred during 18,677.5 person-years (PY) among individuals with DM1 corresponding to an overall incidence rate of 19.5/100 PY, and 709 cases of incident DPSN occurred during 18,677.5 PY among individuals with DM2 corresponding to an overall incidence rate of 15.6/100PY. For both DM1 and DM2 we observe a decreasing incidence rate in the period 2007-2017, similar in all ages (data not shown). For DM1 decreasing incidence rate occurs with increasing age, and there are no sex differences (figure 1A). However, we observe an increasing incidence rate with increasing age for DM2, and we see no sex differences (figure 1B).
Conclusion: This cohort study shows that the incidence rate for DSPN in both DM1 and DM2 has been declining from 2007 to 2017. With increasing age, individuals with DM1 and DM2 have, respectively, declining and increasing incidence rates of DSPN.
Disclosure: H. Mizrak: None.
191
Type 2 diabetes phenotypes and polyneuropathy: a prevalence study in the DD2 Cohort
F.P. Kristensen1, D.H. Christensen1, B.C. Callaghan2, J. Stidsen3, J.S. Nielsen3, K. Højlund3, T.S. Jensen4, P. Vestergaard5, N. Jessen6, T. Hansen7, R.W. Thomsen1;
1Dept. of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark, 2Dept. of Neurology, University of Michigan, Ann Arbor, USA, 3Steno Diabetes Center Odense, Odense University Hospital, Odense, Denmark, 4Dept. of Neurology, Aarhus University Hospital, Aarhus, Denmark, 5Steno Diabetes Center North Denmark, Aalborg University Hospital, Aalborg, Denmark, 6Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, Denmark, 7Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Hyperinsulinemia may cause diabetic polyneuropathy (DPN), but large-scale studies are scarce. Three phenotypes of type 2 diabetes (T2D) have recently been proposed: hyperinsulinemic (low insulin sensitivity, high beta cell function), classical (low insulin sensitivity, low beta cell function), and insulinopenic (high insulin sensitivity, low beta-cell function). We aimed to investigate the association of hyperinsulinemia with DPN.
Materials and methods: We included 3,397 recently diagnosed T2D patients prospectively enrolled from general practitioners and outpatient hospital clinics in Denmark, 2010-2015. Insulin sensitivity and beta-cell function were quantified with the HOMA2 model based on fasting serum C-peptide and plasma glucose levels measured at enrollment. DPN was defined as a score of ≥4 on the Michigan Neuropathy Screening Instrument questionnaire sent out median 3 years after study enrollment. We imputed missing values of potential confounders and applied Poisson regression to calculate adjusted prevalence ratios (PR) of DPN.
Results: We identified 900 (27%) hyperinsulinemic, 2,150 (63%) classical, and 347 (10%) insulinopenic T2D patients. Hyperinsulinemic patients had the highest prevalence of central obesity (waist circumference ≥88/102 cm [F/M]; 89% of hyperinsulinemic, 75% of classical, and 36% of insulinopenic) and had more dyslipidemia and hypertension, but less dysregulated HbA1c (≥53 mmol/L; 9%, 16%, 22%). The age-, sex-, and diabetes duration adjusted PR of DPN was 1.44 (95% CI 1.23-1.68) for hyperinsulinemic T2D patients, compared with the classical phenotype. The prevalence remained elevated (1.32 [1.13-1.55]) after further adjustment for waist circumference, dyslipidemia, hypertension, and HbA1c. For the insulinopenic patients, the adjusted PRs of DPN were 0.87 (0.65-1.15) and 1.16 (0.86-1.55), respectively. In spline analyses, both hyperinsulinemia (ie., higher beta-cell function) and lower insulin sensitivity associated with increased prevalence of DPN. The association between increasing hyperinsulinemia and increasing DPN prevalence persisted among patients with low insulin sensitivity (classical or hyperinsulinemic patients). In contrast, among hyperinsulinemic (high beta-cell function) patients, decreasing insulin sensitivity was not associated with DPN.
Conclusion: The prevalence of DPN is increased in T2D patients with the hyperinsulinemic phenotype. Hyperinsulinemia per se is associated with DPN, irrespectively of insulin resistance and other metabolic factors.
Supported by: Novo Nordisk Foundation.
Disclosure: F.P. Kristensen: None.
192
Environmental risk factors of incident distal sensorimotor polyneuropathy: results from the population-based KORA F4/FF4 study
C. Herder1,2, S. Zhang3, K. Wolf3,2, H. Maalmi1,2, G.J. Bönhof1,4, W. Rathmann1,2, L. Schwettmann3,5, B. Thorand3,2, M. Roden1,4, A. Schneider3, D. Ziegler1,4, A. Peters3,2;
1German Diabetes Center, Düsseldorf, 2German Center for Diabetes Research, München-Neuherberg, 3Helmholtz Zentrum München, Neuherberg, 4Heinrich Heine University Düsseldorf, Düsseldorf, 5Martin Luther University Halle-Wittenberg, Halle (Saale), Germany.
Background and aims: Distal sensorimotor polyneuropathy (DSPN) is a common condition in older populations with high prevalences of obesity and type 2 diabetes. We hypothesised that the risk of DSPN is affected by ubiquitous environmental risk factors, particularly in people with obesity.
Materials and methods: The study was based on 423 participants aged 62-81 years without DSPN who participated in the population-based Cooperative Health Research in the Region of Augsburg (KORA) F4 survey (2006-2008) in Southern Germany. During a mean follow-up of 6.5 years, 188 participants developed DSPN, which was defined using the Michigan Neuropathy Screening Instrument. Environmental exposures, including annual and seasonal mean air temperature, greenness (assessed with the normalised difference vegetation index [NDVI]), road traffic noise and annual mean air pollution, were assessed at participants’ residences. The cumulative risk index (CRI) was calculated to evaluate the joint effects of co-occurring exposures on the risk of DSPN based on effect estimates from multi-exposure Poisson regression models. The models were adjusted for age, sex, height, waist circumference, smoking, alcohol consumption, physical activity, education and neighbourhood socioeconomic status.
Results: In the total study sample, the co-occurrence of an interquartile range (IQR) decrease in temperature of the warm season and NDVI in a 100-m buffer and an IQR increase in night-time average traffic noise and particle number concentration (PNC) was associated with an increased risk of DSPN (CRI [95% CI] 1.39 [1.02, 1.91]). Additional adjustment for HbA1c and other covariables did not change associations between the exposures and DSPN. Effect estimates for exposure combinations were generally higher in individuals with obesity (CRI between 1.34 and 2.01) than in those without obesity (CRI between 0.90 and 1.33). The four-exposure model showed a twofold increased risk of DSPN among obese (CRI [95% CI] 2.01 [1.10, 3.67], but not among non-obese individuals (CRI [95% CI] 1.18 [0.83, 1.67], P for interaction=0.13).
Conclusion: Ubiquitous environmental exposures modulate the risk of DSPN in the older population. The joint effects of lower air temperature in the warm season, less greenness close to participants‘ residences, and higher noise and air pollution levels identified people with obesity as a particularly vulnerable subgroup.
Supported by: DZD
Disclosure: C. Herder: None.
OP 33 Therapy outside the box
193
Discovery of malonic acid as a novel adipocyte beiging molecule
C. Luk, N.J. Haywood, K.I. Bridge, K.J. Simmons, N.Y. Yuldasheva, P. Sukumar, L.D. Roberts, S.B. Wheatcroft, R.M. Cubbon, M.T. Kearney;
Leeds Institute of Cardiovascular and Metabolic Medicine, Faculty of Medicine and Health, University of Leeds, Leeds, UK.
Background and aims: Pilot data from our group show that endothelial insulin-like growth factor 1 receptor knockdown (ECIGF-1RKD) mice are protected from diet-induced insulin resistance. In vitro, we found their endothelial cells secrete a small molecule capable of inducing adipocyte beiging and used metabolomics to identify candidate small molecule metabolites. With the hypothesis that adipose remodelling is the primary contributor to the improved metabolic phenotype and is mediated by an endothelial-derived small molecule, we aim to screen the adipocyte beiging effect of these hits.
Materials and methods: Mature murine 3T3-L1 adipocytes were stimulated with the different metabolites before measuring gene expression of various white and beige adipocyte markers by qPCR. To probe the beiging mechanism, adipocytes were stimulated with malonic acid at different doses or for different duration, with or without pre-treatment with relevant inhibitors. To begin to examine functional readouts, the concentration of adiponectin secreted by stimulated adipocytes was measured by ELISA. Finally, to determine the translational potential, mature human white adipocytes were stimulated with malonic acid and its derivative, di-tert-butyl malonate (DBM). To determine the dose-dependent effect of DBM, different doses of DBM were applied to human adipocytes. Mitochondrial respiration of stimulated adipocytes was measured by Seahorse respirometry.
Results: Among 28 screened metabolites, only malonic acid induced gene expression of beige adipocyte markers in mature 3T3-L1 adipocytes. 24-hour treatment with 10mM malonic acid induced gene expression of various beige adipocyte markers including Ucp1 (15-fold, n=6-7, p<0.05), Cited1 (12-fold, n=5, p<0.01) and Fgf21 (by 6-fold, n=5, p<0.05). Time-course study showed malonic acid-induced Fgf21 gene upregulation (first determined at 6-hour time-point, p<0.05) preceded Ucp1 gene upregulation (first determined at 8-hour time-point, p<0.05). Lower doses of malonic acid did not induce beige marker gene expression. Malonic acid-induced Ucp1 gene upregulation is partly attenuated in adipocytes pre-treated with either 100nM MitoQ (-59.3%, n=5, p<0.001), 20nM PD173074 (-50.0%, n=5, p<0.05) or 3μM A485 (-47.7%, n=5, p<0.01), suggesting malonic acid-induced beiging is partly mediated by mitochondrial oxidative stress, FGF receptors and Cbp/p300 signalling, respectively. In addition, malonic acid enhanced adipocyte secretion of adiponectin (+21.5%, n=3, p<0.05). 24-hour treatment with 10mM DBM recapitulated malonic acid-induced UCP1 gene upregulation in human adipocytes (13-fold, n=5, p<0.05). Dose-response study showed 100μM DBM was sufficient to induce UCP1 gene upregulation in human white adipocytes (+143%, n=5, p<0.05). Preliminary data from respirometry study suggest DBM may increase basal respiration in adipocytes (+78.75%, n=3, p=ns).
Conclusion: Malonic acid can induce adipocyte beiging and adiponectin secretion in vitro. Malonic acid-induced beiging is partly mediated by mitochondrial oxidative stress, FGF receptors and Cbp/p300 signalling. DBM may have therapeutic potential in diet-induced diabetes. Future work will focus on examining the pathway by which IGF-1R deficiency leads to increased endothelial malonic acid secretion and the translational relevance of DBM in treating diabetes.
Supported by: BHF 4-year PhD studentship
Disclosure: C. Luk: Grants; British Heart Foundation 4-year PhD studentship.
194
Hepatic S100A9-TLR4-MTORC1 axis normalises diabetic ketogenesis
G. Ursino1, G. Ramadori1, A. Höfler2, S. Odouard1, P. Teixeira1, F. Visentin1, C. Veyrat-Durebex1, G. Lucibello1, R. Firnkes1, S. Ricci1, J. Elmquist3, T. Vogl4, A. Boland5, R. Coppari1;
1Physiology & Cell Metabolism, University of Geneva, Geneva, Switzerland, 2Molecular Biology, University of Geneva, Geneva, Switzerland, 3Centre of Hypothalamic Research, University of Texas Southwestern Medical Centre, Dallas, USA, 4Institute of Immunology, University of Munster, Munster, Germany, 5Molecular Biology, Universtiy of Geneva, Geneva, Switzerland.
Background and aims: Type 1 Diabetes (T1D) afflicts millions of people and is usually diagnosed in children and young adults with an incidence that has been increasing at an alarming annual rate of ~3%. T1D is mainly characterized by hyperglycaemia, however β-cell loss also leads to other serious metabolic derangements such as hypertriglyceridemia, hyperglucagonemia and diabetic ketoacidosis (DKA). DKA is often life threatening (accounting for up to 263 yearly hospitalization events for each 1000 patients) and occurs due to unrestrained ketogenesis, whose incidence is high in diabetic pateints. While insulin therapy reduces ketogenesis this approach is sub-optimal and currently available insulin adjuvants actually increase the risk of developing DKA. To this end, we have identified a ketone normalizing action of S100A9 in T1D. The aims of our study were to uncover where (cell types and tissues) and how (molecular mechanism) S100A9 was exerting this beneficial effect on ketogenesis and to assess its therapeutic potential and clinical relevance.
Materials and methods: We generated insulin deficient mice lacking or re-expressing Toll-Like Receptor 4 (TLR4) only in liver or hepatocytes and mice with hepatic-restricted and hepatocyte restricted loss of Tuberous Sclerosis Complex 1 (TSC1, an mTORC1 inhibitor) in the context of liver overexpression of S100A9. We assessed cellular signaling pathways and circulating metabolic parameters in-vivo and ex-vivo fatty acid oxidation in these mice to determine the mechanism of action of S100A9. We tested the translational feasibility and safety of an S100A9 based therapeutic in insulin deficient mice through administration of recombinant mouse and human S100A9 (acute and chronic treatment)We analyzed plasma samples of decompensated diabetic patients to determine clinical feasibility of S100A9 treatment.
Results: S100A9 suppresses diabetic ketogenesis via activating mTORC1 signaling downstream of the TLR4-Akt pathway in hepatic non-parenchymal cells. This activation suppresses fatty acid oxidation in the liver. Extracellular S100A9 activates mTORC1 signaling in a cell-autonomous fashion. Administration of recombinant mouse and human S100A9 suppresses DKA in insulin deficient rodents.Chronic treatment of recombinant S100A9 suppresses DKA and displays a promising safety profile in insulin deficient rodents. Plasmatic S100A9 content was only modestly increased in decompensated diabetic patients (possibly indicating a compensatory mechanism to reduce ketogenesis) therefore providing scope for further increasing plasmatic S100A9 as a potential therapeutic clinical avenue in diabetes.
Conclusion: Our results indicate that it acts through the hepatic TLR4-mTORC1 axis in non-parenchymal cells; hence suggesting the existence of an insulin-independent intra-hepatic network able to regulate lipid and ketone metabolism in TID. S100A9 shows promise as a therapeutic,holding potential to reduce insulin needs and consequently diminish unwanted side effects of insulin treatment while improving metabolic control.
Supported by: B&KH, GVM, ERC, JDRF, INNOGAP, SNF
Disclosure: G. Ursino: None.
195
Validation of duodenal targeting by oral pharmacologic duodenal exclusion therapy for treatment of type 2 diabetes
T. Carlson1, A. Nimgaonkar2, T. Guerina1, K. Colbert1, S. Polomoscanik1, J. Petersen1, T. Jozefiak1, M. Fineman1;
1Glyscend Inc, Lowell, 2Glyscend Inc, Baltimore, USA.
Background and aims: Metabolic surgery is the most successful long-term therapy for T2DM and obesity, reducing macro, microvascular complications, and mortality. The acute and chronic metabolic improvements observed with these surgeries are believed to be, at least in part, a direct consequence of preventing nutrient exposure to the proximal small intestine (duodenum and proximal jejunum exclusion). The use of metabolic surgery and duodenal exclusion devices is limited, however, due to their invasive nature. We developed oral, non-absorbed polymers (GLY100, GLY200) designed to mimic the effects of metabolic surgery non-invasively by augmenting the natural mucus lining of the proximal small intestine to create a temporary barrier. In chronic rodent models of T2DM (GK-Rat and ZDF-Rat), these polymers produced progressive reductions in post-prandial glucose of up to 70% following standardized caloric loads. Improvements in fasting plasma glucose, HOMA-IR, and bodyweight were also observed. The aim of this study was to visualize the retained duodenal barrier in rats using appropriate imaging modalities.
Materials and methods: Imaging of GLY200 after gavage administration to fasted Sprague-Dawley rats was performed using 2 methods: Computed Tomography (CT), and an In Vivo Imaging System (IVIS). For CT imaging, GLY200 was covalently conjugated with diatrizoate via an efficient amide forming reaction and unconjugated diatrizoate was administered as a control. CT images were obtained after transient isoflurane administration 1, 2, and 4 hours after gavage. For IVIS imaging, GLY200 was reacted with FITC to produce a fluorescein-GLY200 conjugate, and FITC-dextran70 was administered as a control. Rats were euthanized 0.5, 1.0, 2, 4, and 8 hours after dosing and the GI-tracts were immediately removed for imaging.
Results: CT imaging (see figure) clearly demonstrated retention of GLY200-diatrizoate within the proximal small intestine 1- and 2-hours after administration, with an intense signal only in the duodenum. In comparison, the diatrizoate control was broadly distributed over the small intestine distal to the duodenum by 1-hour. In IVIS imaging, GLY200-FITC showed intense fluorescence in the proximal small intestine that persisted for 4-hours after administration and was undetectable by 8-hours. In comparison, FITC-dextran70 was distributed broadly throughout the distal intestine and cecum by 30 minutes with no retention in the duodenum.
Conclusion: Our imaging studies confirm that GLY200 conjugate is effectively retained in the duodenum after oral administration. These observations, along with previously reported glycemic improvements in chronic rodent models of T2DM, support the hypothesis that orally administered polymers designed to target the proximal small intestinal gut wall could potentially be used to non-invasively recapitulate the glycemic and weight effects observed with metabolic surgery and duodenal exclusion devices.
Disclosure: T. Carlson: None.
196
Human engineered skeletal muscle to treat insulin resistance in type 2 diabetes
H. Shoyhet1, Y. Herman1, M. Beckerman1, E. Karnieli2, S. Levenberg1;
1Biomedical engineering, Technion- Israel Technion Israel of Technology, 2Medicine, Technion- Israel Technion Israel of Technology, Haifa, Israel.
Background and aims: Diabetes Mellitus type II (DM2) is the most common type of diabetes, widely spread in developed countries and responsible for more than 1 million deaths every year. It is characterized by skeletal and adipose tissues insulin resistance and is usually non-insulin dependent. However, progression of the disease can cause various complications, including insulin impairment. Glucose transporter type 4 (GLUT4), mainly expressed in skeletal muscle tissue, is the key factor in glucose homeostasis overall and in DM2 specifically; Many studies have shown reduction in GLUT4 expression and translocation levels in DM2 patients. Therefore, we hypothesize GLUT4 can be part of the solution. Previous study in our group showed that implantation of GLUT4 over-expressing (GLUT4 OE) mouse skeletal muscle tissue in diabetic mice can improve blood glucose levels. To bring the technology closer to the clinic, we aim to prove that the concept works in human cells as well. Moreover, to improve patient’s compliance, less invasive delivery methods must be developed. In this study we have two aims: 1. Engineer GLUT4 OE human skeletal muscle tissue and implant it in diabetic mice. 2. Develop a new delivery method of the engineered skeletal muscle tissue using minimally invasive procedure
Materials and methods: Human myoblasts are genetically modified to overexpress GLUT4 via viral transduction. The cells are seeded on 3D PLLA/PLGA scaffolds for differentiation and maturation. The 3D tissue is characterized using immunostaining and glucose uptake assays to assess their anti-diabetic properties. The scaffolds are implanted in diabetic mice, and their blood glucose levels are monitored prior and following implantation. We have also developed a novel scaffold to deliver the functional muscle tissue using non-invasive procedure. The scaffold is characterized using the same methods listed above. We are also assessing the scaffolds mechanical properties and injectability.
Results: We have genetically modified human myoblasts to overexpress GLUT4. The modified cells can be grown on 3D PLLA/PLGA scaffolds and differentiated to functional skeletal muscle tissue. Immunostaining and molecular assays are used to characterize and quantify the GLUT4 expression. Glucose uptake assays show the engineered tissue has ~ 50% (p>0.05) enhanced uptake capacity compared to WT cells. We also developed new scaffold to deliver functional skeletal muscle tissue via injection. The novel injectable scaffold was fine-tuned to enable skeletal muscle growth and differentiation which was confirmed via immunostaining for myogenic markers. Tissue survival and functionality following injection were assessed using Alamar-Blue and glucose uptake assays showing no singnificant differences prior and following injecion. Glucose uptake assays also confirm that GLUT4 OE skeletal muscle tissue can develop on the scaffold.
Conclusion: New routes to treatment for type II diabetes are needed to fight this global issue. We present a concept of therapy based on GLUT4 and skeletal muscle tissue engineering. Preliminary results indicate this approach has great potential to tackle one of the key issues in type II diabetes and insulin resistance.
Supported by: RAS fund, TEVA fellowship
Disclosure: H. Shoyhet: None.
197
Oral long-acting menin inhibitor normalises type 2 diabetes in two rat models
T. Butler, S. Mourya, W. Li, B. Law, T. Archer, T. Kinoshita, P. Somanath;
Biomea Fusion, Inc., Redwood City, USA.
Background and aims: Menin is a scaffold protein that has been recognized for its role in T2DM as a key regulator of β-cell proliferation. Menin inhibition has previously been shown to improve glycemic control in diabetic mice. Herein, we report the first evidence that BMF-219, an orally bioavailable, selective, covalent menin inhibitor, restores glycemic control in Zucker Diabetic Fatty (ZDF) Rat and Streptozotocin-induced (STZ) Rat models of T2DM.
Materials and methods: Rats were treated daily with BMF-219, vehicle, or pioglitazone for 16 days and analyzed for fasting and non-fasting blood glucose levels, insulin, c-peptide, and blood lipemic levels. Oral Glucose Tolerance Test (OGTT) was conducted up to Day 15 in both models and two-weeks post-treatment in the ZDF model. Body weight of all rats was also monitored.
Results: BMF-219 was well tolerated throughout the conduct of the study. BMF-219 treatment resulted in a significant 50% reduction in fasting and non-fasting blood glucose levels, reduced serum insulin and c-peptide levels (p<0.05), and reduced HOMA-IR (p<0.001) after two weeks of treatment in ZDF rats. BMF-219 decreased glucose levels at all timepoints during an OGTT at Day 15 (AUC reduction of 54%, p<0.001) and at Day 29 (AUC reduction of 40%, p<0.05, ~2 weeks after the last dose in the ZDF model, indicating prolonged glycemic control. Strikingly, BMF-219, but not pioglitazone, reduced blood glucose levels during an OGTT in STZ animals (AUC reduction of 41%, p<0.05, see figure). Significant reductions in blood lipemic levels (p<0.01) and body weight were observed in both models.
Conclusion: Collectively, our data indicate the novel and marked potential of BMF-219 as an oral, long-acting treatment for T2DM.
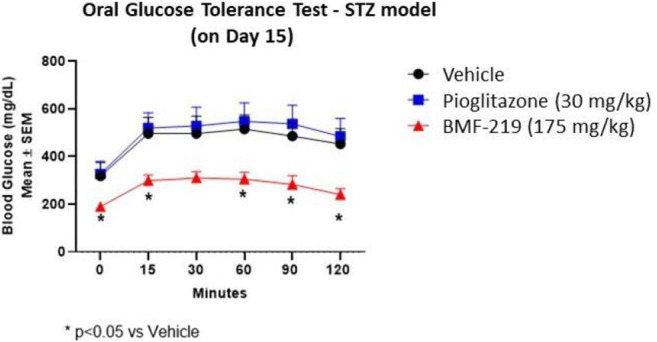
Disclosure: T. Butler: Employment/Consultancy; Biomea Fusion, Inc. Stock/Shareholding; Biomea Fusion, Inc.
198
Glp-1/gdf15 dual agonist to treat obesity and hyperglycaemia in mice and non-human primates
Y.Y. Zhang, A. Kharitonenkov, X.Y. Zhao, X.N. Dong, Y.Y. Zhang, H.X. Zou, Y.G. Jin, W. Guo, P. Zhai, X. Chen;
Beijing QL Biopharmaceutical Co., Ltd., Beijing, China.
Background and aims: Obesity and its associated co-morbidities such as Type 2 Diabetes, cardiovascular disease and NASH are considerable health concerns that are exponentially rising worldwide. Only limited medicinal options to treat these diseases are currently available. Recent data is suggestive that polypharmacy-based molecules are capable to provide augmented efficacy. Utilizing protein engineering strategies we designed here long-acting GLP-1/GDF15 fusion protein, QL1005, and explored its therapeutic potential in animals.
Materials and methods: The in vitro assays in this report were conducted in the absence and presence of human serum albumin (HSA) to demonstrate the impact of fatty acid-protraction on molecules’ potencies. In vivo tests were carried in DIO and db/db mice, and obese cynomolgus monkeys to demonstrate the pharmacological efficacy of QL1005 on body weight, food intake, plasma glucose, circulating lipids and other metabolic parameters as compared to the benchmark molecules.
Results: QL1005 is an engineered dual agonist protein, designed via fusing GLP-1 and GDF15 analogs by a peptide linker conjugated with a fatty acid for time-action extension. In cell-based assays QL1005 is superior in potency to GLP-1 analog semaglutide with its pharmacokinetic parameters are comparable to this GLP-1 agonist. When tested in obese mice, QL1005 demonstrated dose-dependent lowering of body weight, food intake, fasting glucose and triglyceride with all these effects being greater in magnitude to the efficacy of semaglutide or long-acting GDF15. Of importance, QL1005-induced metabolic improvements come as the result of activity emanating from both therapeutic components within this dual agonist molecule, GLP-1 and GDF15. Indeed, the individual pathway-inactivated QL1005 analogs are approximately 50% efficacious than parental QL1005 suggestive that this co-agonist in animals is balanced in activation of both GLP-1 and GDF15 receptors. When dosed to cynomolgus obese monkeys, QL1005 is more efficacious in body weight and glucose lowering but similar in the incidence of GI side-effects as compared to semaglutide treatment.
Conclusion: Altogether, this novel GLP-1/GDF15 fusion protein in animals reveals itself therapeutically efficacious to treat obesity, diabetes and related co-morbidities thus demonstrating the promise of the poly-pharmaceutical approach in metabolic drug discovery and developments.
Disclosure: Y.Y. Zhang: None.
OP 34 Insulin signalling, novelties from the petri dish!
199
Finnish-specific AKT2 gene variant leads to impaired insulin signalling in human myotubes
S. Mäkinen1,2, N. Datta1,2, Y.H. Nguyen1,2, V.M. Olkkonen1,3, A. Latva-Rasku4, P. Nuutila4, M. Laakso5, H.A. Koistinen1,2;
1Minerva Foundation Institute for Medical Research, Helsinki, 2Department of Medicine, University of Helsinki and Helsinki University Hospital, Helsinki, 3Department of Anatomy, Faculty of Medicine, University of Helsinki, Helsinki, 4Turku PET Centre, University of Turku and Turku University Hospital, Turku, 5Institute of Clinical Medicine, Internal Medicine, University of Eastern Finland, Kuopio, Finland.
Background and aims: Finnish-specific gene variant p.P50T/AKT2 (MAF=1.1%), is associated with higher fasting insulin concentrations, in vivo insulin resistance, and increased predisposition to type 2 diabetes. Here, we have investigated in vitro the impact of the gene variant on glucose metabolism and intracellular signaling in human primary myotubes, that were established from 14 male p.P50T/AKT2 variant carriers and 14 controls.
Materials and methods: Insulin-stimulated glucose uptake and its incorporation into glycogen were detected with [3H]-2-Deoxy-D-glucose and [14C]-D-Glucose, respectively, and the rate of glycolysis was measured with Seahorse XFe96 analyzer. Insulin signaling was investigated with western blotting. Binding of the p.P50T/AKT2 and control-form PH domains to phosphatidylinositol (3,4,5)-trisphosphate ((PI(3,4,5)P3)) was assayed using PIP Strips™ Membranes.
Results: Insulin increased glucose uptake and glycogen synthesis in human primary myotubes, with no difference between p.P50T/AKT2 variant carriers (n=10) and controls (n=8) (2-way ANOVA with repeated measures). Insulin-stimulation led to a significant increase in glycolytic rate (p<0.001) and in compensatory glycolysis (p<0.01) in control myotubes (n=14), but not in p.P50T/AKT2 variant myotubes (n=14, p=NS). Comparison between the genotypes revealed a significant reduction in insulin-stimulated glycolysis and compensatory glycolysis in myotubes from p.P50T/AKT2 variant carriers (p<0.05, 2-way ANOVA). Insulin-stimulated phosphorylation of AKT-Thr308 (p<0.05), AS160-Thr642 (p<0.05) and GSK3β-Ser9 (p<0.05) was reduced in p.P50T/AKT2 variant carriers (n=10) compared to controls (n=8) (2-way ANOVA with repeated measures). Binding of the variant form of p.P50T/AKT2-PH domain to PI(3,4,5)P3 was reduced when compared to the control protein (p<0.001, n= 4 repeated assays, unpaired t-test).
Conclusion: p.P50T/AKT2 leads to impaired insulin signaling in human primary myotubes, which may be a result of a defective PI(3,4,5)P3-binding capacity of the p.P50T/AKT2-PH domain.
Supported by: Diabetes Wellness Sverige, Finnish Diabetes Research Foundation, Finska Läkaresällskapet
Disclosure: S. Mäkinen: None.
200
Role of Diacylglycerol kinase δ in skeletal muscle glucose metabolism in type 2 diabetes
F. Tramontana, M. Kuefner, A. Krook, J.R. Zierath;
Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden.
Background and aims: Reduced Diacylglycerol Kinase δ (DGKδ) protein abundance and activity have been observed in skeletal muscle from people with type 2 diabetes (T2D), resulting in increased diacylglycerol (DAG) levels - a widely recognized lipotoxic mediator of insulin resistance. Thus, downregulation of DGKδ may play a role in the regulation of DAG and the development of insulin resistance in type 2 diabetes. However, the underlying mechanisms by which reduction of DGKδ levels lead to the development of insulin resistance are still unknown. We hypothesized that lower of DGKδ in skeletal muscle impairs glucose homeostasis by disrupting the canonical insulin signaling pathway, thus contributing to skeletal muscle insulin resistance.
Materials and methods: Primary human skeletal muscle cells were isolated from vastus lateralis biopsies obtained from people with type 2 diabetes (n=6) or normal glucose tolerance (NGT) (n=6). Cells were differentiated to mature myotubes and transfected with DGKδ siRNA (siDGKδ). Cells were harvested 48 hours after transfection, under basal and insulin-stimulated (120nM) conditions. Western blot, qPCR analysis, glycogen synthesis assay, and lactate measurements were performed to assess glucose homeostasis and insulin signaling.
Results: Cells transfected with DGKδ were characterized. DGKδ gene expression was reduced 80% after silencing. Further characterization revealed that siDGKδ silencing in myotube cultures derived from people with type 2 diabetes showed impaired abundance of proteins involved in the insulin signaling cascade as compared to myotube cultures derived from people with NGT. Insulin signal transduction was also impaired, including reduced pAKTT308 (-53.8%, p=0.03) and its downstream substrate, pAS160T642 (-47.5%, p=0.02), along with a trend towards increased levels of the inactive form of glycogen synthase (pGSS641) (+121%, p=0.07). Moreover, insulin-stimulated levels of PKC substrates were reduced in myotube cultures from people with T2D transfected with siDGKδ as compared to myotube cultures from people with NGT (+17.6%, p=0.07), suggesting increased PKC activity. DGKδ silencing decreased insulin-stimulated glucose incorporation into glycogen as compared to control cells (-8.9%, p=0.05), although no differences were seen between diagnosis groups. siDGKδ treatment of myotube cultures from people with type 2 diabetes displayed a 75% increase in lactate production under basal conditions as compared to myotube cultures from people with NGT (p<0.001).
Conclusion: Reduced levels of DGKδ may contributes to dysregulation of glucose metabolism in skeletal muscle through impairments in insulin signaling. Therefore, reductions in DGKδ abundance may further aggravate glycemic control and insulin sensitivity in type 2 diabetes. Level and activity of DGKδ may influence cellular processes as diverse as insulin signaling, glucose metabolism and energy homeostasis.
Supported by: EFSD/Novo Nordisk Programme for Diabetes Research in Europe
Disclosure: F. Tramontana: None.
201
Pharmacological inhibition of Integrin a5b1 improves insulin sensitivity in H9C2 cells and cardiac performance in obese mice
A.K. Banah, V. Musale, C.K. Hennayake, C.E. Murdoch, L. Kang;
University of Dundee, Dundee, UK.
Background and aims: Extracellular matrix (ECM) remodelling has recently emerged as a key contributor to the development of insulin resistance (IR). Inhibition of ECM collagen and hyaluronan deposition by clinical and preclinical antifibrotic drugs attenuated cardiac IR and cardiac dysfunction in diet-induced obese mice. Fibronectin is a multifunctional glycoprotein in the ECM known to communicate with cells mainly via the integrin α5β1 receptor. The present study investigated the effects of CLT-28643, an α5β1 integrin antagonist, on insulin sensitivity in H9C2 cardiomyocytes, and cardiac function in high-fat-high-sucrose (HFHS) fed obese mice.
Materials and methods: In vitro effect of ECM on insulin signalling was investigated by culturing H9C2 cells on fibronectin coated plate for 5 days followed by 15 min treatment with insulin (10nM). Furthermore, insulin resistant H9C2 cells, induced by high glucose (33.3 mM) and palmitic acid incubation (100 μM) for 24-h, were subjected to CLT-28643 treatment for 3 hrs followed by 15 min incubation with insulin (100nM). Insulin sensitivity of treated cells was examined by phosphorylation of Akt using western blot. In vivo, male C57BL/6 mice fed 45% high-fat diet and 30% sucrose water for 10 weeks received twice-daily oral gavage of either vehicle or CLT-28643 (75 mg/kg body weight) for 4 weeks. A separate group of mice fed with chow diet for 14 weeks were used as lean controls (n=8). Cardiac function was measured by Pressure-Volume (PV) loop analysis (Transonic) using PV conductance catheter in closed-chest preparation. Immunohistochemical analysis was performed to assess changes in collagen and alpha-smooth muscle actin (α-SMA) expression in the left ventricle (LV) of the heart.
Results: In H9C2 cells, FB coating decreased insulin-stimulated phosphorylation of Akt (Ser473); CLT-28643 prevented high glucose & palmitate-induced IR as indicated by an increased ratio of the phosphorylated Akt (Ser473) to total AKT compared to vehicle-treated cells (2.67±1.55 fold increase, P<0.05). In mice, HFHS diet resulted in significant increases in body weight (29.05 ± 0.82 (chow) vs 40.86 ± 1.34 (HFHS) gram, P<0.0001), %fat mass (15.52 ± 0.80 vs. 51.30 ± 5.89%, P<0.0001) and decreases in %lean mass (78.29 ± 0.98 vs. 58.61 ± 1.01%, P<0.0001) compared to chow-fed mice. CLT-28643 displayed a trend of decrease in %fat mass, despite no changes in body weight or %lean mass. HFHS diet impaired cardiac function in mice as evidenced by a decrease in end-systolic elastance (31.06 ± 6.23 (chow) vs 10.88 ± 1.47 (HFHS) mmHg/μL, P<0.01), ejection fraction (86.14 ± 4.24 vs 72.82 ± 3.60%, P<0.05) and an increase in heart rate (554.3 ± 21.41 vs 603.7 ± 9.72bpm, P<0.05) and ventricular arterial coupling (VAC) index (0.19 ± 0.06 vs 0.46 ± 0.07, P<0.05) compared to lean controls. Interestingly, elevated heart rate was reversed by CLT-28643 treatment, suggesting an improved cardiac performance; while the slope of the end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relation (EDPVR) were not different among groups. Immunohistochemical analysis revealed no significant changes in LV collagen and α-SMA expression in experimental mice.
Conclusion: Pharmacological inhibition of integrin α5β1 improved insulin signalling in H9C2 cells and cardiac performance in obese mice. These findings suggest that therapies targeting FB-α5β1 interaction may be beneficial to obesity-associated cardiometabolic complications.
Supported by: BHF
Disclosure: A.K. Banah: None.
202
Impact of protein kinase D2 in the regulation of hepatic insulin sensitivity
P. Rada1,2, A.B. Hitos1,2, E. Rey3, E. Carceller-López1, J. Pose-Utrilla1,4, C. García-Monzón3, G. Sabio5, T. Iglesias1,4, Á. González-Rodríguez2,3, Á.M. Valverde1,2;
1Instituto de Investigaciones Biomédicas Alberto Sols (CSIC/UAM), 2Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), 3Instituto de Investigación Sanitaria Princesa, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), 4Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), 5Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain.
Background and aims: Protein Kinase D2 (PKD2) is a Ser/Thr kinase of the Ca2+-Calmodulin kinase superfamily. Recently, emerging evidences indicate that PKD2 regulates glucose homeostasis in several tissues. Two previous studies, one in global PKD2-null mice, and other in mice lacking PKD2 in intestine, reported opposite results with metabolic dysfunction or protection against High Fat Diet (HFD)-induced obesity, respectively. Given that insulin resistance is a key initial trigger for NAFLD progression, unravelling PKD2 contribution to the initial stages of this disease will add fundamental knowledge to metabolic liver dysfunction with potential therapeutic value.
Materials and methods: PKD pharmacological and genetic inhibition was analyzed in primary hepatocytes and in Huh7 cells. Huh7 cells were transfected with EGFP-PKD2-CA, a constitutively active PKD2 fused to EGFP. Insulin signalling was examined in hepatocytes stimulated with insulin (10 nM, 5-15 min). As an in vivo model of hepatic insulin resistance, mice with a liver-specific PKD2 depletion (PKD2∆Hep) were fed HFD. Parameters assessing glucose homeostasis and hepatic insulin sensitivity were analyzed. PKD2 was overexpressed in liver by an injection of AAV-EGFP-PKD2-CA and insulin sensitivity was evaluated. PKD signature was analyzed in liver biopsies from NAFLD patients.
Results: PKD pharmacological inhibition resulted in higher AKT phosphorylation upon insulin stimulation in both primary mouse hepatocytes (p<0.001, n=6) and Huh7 cells (p<0.01, n=3). Similar results were obtained in PKD2∆Hep hepatocytes (p<0.05, n=5) in which IRS1 was upregulated (p<0.001, n=6). Moreover, PKD2 knocking down by shRNA enhanced the insulin response (p<0.01, n = 8-10). Alternatively, EGFP-PKD2-CA overexpression reduced insulin-stimulated AKT phosphorylation compared to EGFP-transfected cells (p<0.01 t=5 min; p<0.001 t=15 min; n=4-10). In this line, in vivo injection of AAV-EGFP-PKD2-CA resulted in moderate impairment of glucose homeostasis and reduced IR and AKT phosphorylation in liver (p<0.05 pIR, pAKT(Ser473); p<0.001 pAKT(Thr308), n = 5-6 mice/group). Importantly, HFD-fed PKD2∆Hep mice displayed a tendency to improve glucose tolerance and insulin sensitivity compared to control mice, being statistically significant at different time points (GTT: p<0.05 at 15 min; ITT: p<0.05 at 30, 60 and 90 min; PTT: p<0.05 at 60 min) (n=7-10 mice/group). Our preliminary data showed an increase in insulin-induced AKT phosphorylation in livers from HFD-fed PKD2∆Hep mice compared to controls. Moreover, PKD immunostaining showed that PKD2 was increased in NAFLD patients.
Conclusion: Results herein strongly suggest a role of PKD2 in the control of hepatic insulin signalling and point PKD2 as a new therapeutic target in the progression of hepatic insulin resistance-related pathologies.
Supported by: MICINN RTI2018-094052-B-100; CAM S2017/BMD-3684; CIBERDEM (ISCIII)
Disclosure: P. Rada: None.
203
Alpha-melanocyte stimulatory hormone (αMSH): a novel and potent regulator of glucose tolerance in humans
N.G. Docherty1, P. Swan1, B. Johnson2, S. Samarasinghe3, M. Cowley4, C.W. le Roux1,5, A.D. Miras2;
1School of Medicine & Medical Science, University College Dublin, Dublin, Ireland, 2Department of Metabolism, Digestion and Reproduction Imperial College London, London, UK, 3School of Medicine & Medical Science, Department of Metabolism, Digestion and Reproduction Imperial College London, London, UK, 4Monash Biomedicine Discovery Institute, Melbourne, Australia, 5Centre for Diabetes, Coleraine, UK.
Background and aims: Intravenous administration of exogenous αMSH lowers glucose excursions during oral glucose tolerance testing (OGTT) in pre-clinical studies in mice. We set out to interrogate whether this action is preserved in human physiology, both in vivo and in vitro.
Materials and methods: Two cohorts, each of fifteen healthy volunteers received infusions of physiological saline, 15, 150, and 1500 ng/kg/hr αMSH initiated 30 minutes prior to the administration of a standard OGTT. Plasma glucose and insulin were measured during the OGTT. To assess the effect of αMSH on skeletal muscle glucose disposal, subjects in cohort 1 underwent sequential hyperinsulinaemic-euglycaemic clamp studies with saline and 150ng/kg/hr αMSH infusion. In a separate cohort of healthy volunteers (n=6), primary human myotube cultures were generated from vastus lateralis muscle biopsies and used to directly assess glucose uptake in response to αMSH.
Results: Infusion of αMSH (1500ng/kg/hr) led to a 45% reduction in the 2-hour incremental area under the curve (iAUC) for plasma glucose (p<0.001). Accordingly in cohort 1, the iAUC for plasma insulin was reduced by 20% (p=0.006). In clamp studies, αMSH increased glucose requirements for the maintenance of euglycaemia. Primary human myotube cultures expressed melanocortin receptor subtypes (MC1R>MC3R≈MC4R). A sixty-minute incubation of myotube cultures with 10nM αMSH increased glucose uptake by two-fold versus vehicle (p=0.001), this being equipotent to the effect obtained with insulin. The glucose uptake promoting effects of insulin and α-MSH in myotubes were additive.
Conclusion: These findings substantiate a role for peripheral αMSH as a hitherto undescribed component of the endocrine control of glycaemia in human physiology. The αMSH-skeletal muscle axis offers a novel target for the development of diabetes pharmacotherapy.
Clinical Trial Registration Number: ISRCTN26265036
Supported by: Health Research Board Ireland and EFSD Albert Renold Travel Fellowship
Disclosure: N.G. Docherty: None.
204
Abrogation of circular dorsal ruffles by hyper insulinemia impairs insulin receptor internalisation and recycling
A. Teshima1, M.A. Correia1, L.M. Oliveira1, D.C. Barral1, R.M. de Oliveira1, M.P. Macedo1,2;
1Chronic Diseases Research Centre (CEDOC), NOVA Medical School, Faculdade de Ciencias Medicas, Universidade NOVA de Lisboa, Lisbon, 2Departament of Medical Science, Departamento de Ciencias Medicas, Universidade de Aveiro, Aveiro, Portugal.
Background and aims: Insulin resistance is a hallmark for type 2 diabetes mellitus (T2DM) and occurs when the body cannot respond appropriately to circulating insulin levels. Hyperinsulinemia is both a trigger and a consequence of insulin resistance. Insulin mediates its physiological effects through binding to the extracellular subunit of the insulin receptor (lnsR) at the plasma membrane. Impairing the activity of any component of this complex, from insulin binding to InsR internalization, trafficking and signal propagation, can lead to insulin resistance. However, how altered InsR internalization contributes to the development of insulin resistance and T2DM is understudied. Ligand-activated InsR is internalized and trafficked to early endosome (EE), where InsR is dephosphorylated and sorted. InsR can be subsequently conducted to lysosome for degradation or recycled back to the plasma membrane. Previous results from our group showed that lnsR is internalized through actin-rich ring-shaped structures circular dorsal ruffles (CDRs), which form exclusively at the dorsal surface of hepatocytes, upon insulin stimulation. Our group also showed that chronic exposure to elevated insulin concentration inhibits CDRs formation. Having these findings in mind, we hypothesized that inhibition of CDRs formation by exposing hepatocytes to hyper insulin concentration compromises InsR internalization and subsequent trafficking to EE and recycling to membrane.
Materials and methods: Hepa 1-6 mouse hepatoma cells were used to study lnsR internalization and subsequent trafficking. Cells were maintained in normal medium or, to mimic hyperinsulinemia condition, they were maintained in medium supplemented with 200 nM of insulin for 48h. Cells were serum-starved for 3 hours, then stimulated with 100nM insulin for 1, 5, 15 and 30 mins. To observe EE and recycling endosomes (RE), cells were fixed and processed for immunofluorescence, being labeled with EEA1, a marker for EE, and Rab11a a marker for RE and colocalization was measured respectively.
Results: We observed that in normal condition, InsR colocalizes with EEA1 peaking at 5 minutes, then we observed a decrease of colocalization along time, suggesting that the vesicles containing InsR are maturating along the endocytic route. On the other hand, hyper insulin incubated cells, mimicking prediabetes, showed significant decrease of InsR colocalization with EEA1 in every timepoint, indicating an abrogation of the internalization in early endocytosis. Subsequently, we looked at recycling endosomes by staining for Rab11a. While in control condition Rab11a co-localizes with InsR at 15 minutes upon insulin stimulation, when CDR formation is blocked in cells exposed to chronic hyperinsulin, recycling to the plasma membrane is impaired.
Conclusion: Here we show for the first time that hyperinsulinemia inhibits lnsR internalization through CDRs and subsequent trafficking to EE and RE. In toto, our results suggest that abrogation of CDRs formation in a condition mimicking prediabetes impairs InsR internalization and thus contributing to the onset and/or exacerbation of insulin resistance.
Supported by: PTDC/MEC-MET/29314/2017, UIDB/Multi/04462/2020, Programa Gilead GÉNESE─Edição de 2019
Disclosure: A. Teshima: None.
OP 35 Different pathways involved in killing the beta cell
205
IER3IP1 mutations lead to ER stress-induced neonatal diabetes
H. Montaser, S. Leppänen, H. Ibrahim, S. Eurola, J. Saarimäki-Vire, T. Otonkoski;
University of Helsinki, Helsinki, Finland.
Background and aims: IER3IP1, a highly conserved protein in humans, is highly expressed in the developing brain cortex and β-cells. The endoplasmic reticulum-transmembrane protein is thought to play a major role in regulating ER secretion and cell apoptosis. Homozygous mutations in IER3IP1 have been associated with neonatal diabetes and microcephaly. The aim of this study is to characterize the role of IER3IP1 in the development, function, and survival of human β-cells using human embryonic stem cell (hESC)-derived pancreatic islets (SC-islets).
Materials and methods: Utilizing CRISPR/Cas9, we deleted the first exon of IER3IP1 in hESCs to generate a knockout (KO) model. Alongside their wild-type (WT) counterparts, the KO clones were differentiated into SC-islets using an optimized 7-stage protocol. The effect of IER3IP1-KO on β-cell development was characterized using flow cytometry, qPCR, and immunohistochemistry for β-cell and ER stress markers. Additionally, the survival of IER3IP1-KO β-cells was tested by treating the cells with chemical ER-stress inducers and measuring their apoptosis using TUNNEL assay. Furthermore, the functionality of SC-islets was tested in-vitro through glucose-stimulated insulin secretion assay, and in-vivo after implanting the SC-islets under the kidney capsule of immunodeficient mice.
Results: We confirmed the successful generation of IER3IP1-KO hESC clones using Sanger sequencing and qPCR. Intriguingly, IER3IP1-KO SC-islets showed significantly lower percentage of β-cells (41% in WT vs 21% in KO, p= 0.001, n= 3), reduced INS transcript levels, and drastically diminished insulin content (766 ng hINS/μg DNA in WT vs 111 ng hINS/μg DNA, p= 0.02, n= 3). Additionally, immunostaining of IER3IP1-KO β-cells showed notably higher accumulation of proinsulin, and significantly higher levels of the ER stress marker BiP when compared to their wild-type controls. Furthermore, IER3IP1-KO β-cells were more vulnerable to apoptosis upon inducing ER stress using chemical stressors. Testing their functionality, IER3IP1-KO SC-islets were able to respond to high glucose treatment by increasing their insulin secretion; however, their total insulin secretion was substantially lower than WT SC-islets. Moreover, the levels of circulating human C-peptide detected in mice implanted with WT SC-islets were significantly higher than those detected in the mice with KO implants, since the first month of implantation (606 pmol/L human C-peptide in WT-grafted mice vs 82 pmol/L human C-peptide in KO-grafted mice, p= 0.009, n= 3). After three months of implantation, the WT grafts were able to regulate the blood glucose during an intraperitoneal glucose tolerance test, while the KO grafts failed to upregulate their C-peptide secretion and the mice showed impaired glucose tolerance.
Conclusion: Our results indicate the essential role of IER3IP1 in maintaining normal ER homeostasis and regulating the function and survival of human beta cells.
Supported by: ILS doctroal program, UH and Orion research foundation.
Disclosure: H. Montaser: None.
206
Inhibition of the type 1 diabetes candidate gene PTPN2 aggravates tumor necrosis factor alpha-induced human beta cell dysfunction and death
A. Roca Rivada, S. Marín Cañas, M.L. Colli, D.L. Eizirik;
ULB Center for Diabetes Research, Université Libre de Bruxelles, Brussels, Belgium.
Background and aims: Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine produced by macrophages/monocytes during acute inflammation. The cytokine plays a role in insulitis and pancreatic beta-cell loss in type 1 diabetes mellitus (T1D) and a phase 2 clinical study showed that TNF-α inhibition in newly diagnosed T1D patients preserves C-peptide production (PMID: 33207093). In this study, we evaluated the effects of TNF-α (as compared to type I IFNs, previously shown by us to be affected by PTPN2) in human pancreatic beta-cells silenced for the protein tyrosine phosphatase PTPN2, a candidate risk gene for T1D.
Materials and methods: EndoC-βH1 cells, dispersed human islets, and iPSC-derived beta-like cells were transfected with siCTRL, siPTPN2, siJNK1, or siBIM. After 24h of recovery cells were left untreated (vehicle) or treated for 48h with IFN-α (2000 units/mL), TNF-α (1000 units/mL), or the combination IFN-α + TNF-α (2000 and 1000 units/mL respectively). Apoptosis was evaluated using Hoechst and propidium iodide staining; mRNA levels were assessed by RT-PCR. PTPN2, pJNK1, pP38, pBIM, and GAPDH protein expression were examined by immunoblot. Results are the mean of 6-7 independent experiments; statistical analysis was done by one-way ANOVA with Bonferroni correction.
Results: 48 hours treatment with IFN-α or TNF-α in EndoC-βH1, dispersed human islets or iPSC-derived β-like cells silenced for PTPN2 induced a significant increase in cellular apoptosis (17.7-50.5% for IFN-α, and 26.5-50.5% for TNF-α; P < 0.01 compared to cells exposed to the cytokines without PTPN2 silencing); a similar result was observed with the combination of IFN-α + TNF-α (24.7-43.8%; P < 0.01 in iPSC-derived β-like cells, and P < 0.001 in EndoC-βH1 cells and dispersed human islets). There was no potentiation between IFN-α + TNF-α suggesting that these cytokines may act through a similar intracellular pathway. To test this hypothesis, we co-silenced PTPN2 and JNK1 or BIM, known to be involved in IFN-induced apoptosis, in EndoC-βH1 and iPSC-derived β-like cells (>50-60% silencing each). This abolished the proapoptotic effects of IFN-α, TNF-α, or the combination of both cytokines with (P < 0.05) or without PTPN2 inhibition (P < 0.01). Interestingly, silencing PTPN2 increased by 2-fold TNFα-induced phosphorylation of JNK1 (P < 0.05), P38 (P < 0.01) and BIM (P < 0.01), suggesting a critical and protective role for PTPN2 against TNF-α toxic effect in beta-cells. These findings also point to an unexpected common pathway for signaling between IFNα and TNFα.
Conclusion: TNF-α has been shown to play a role in the pathogenesis of human T1D. We presently show that PTPN2 is a key regulator of the deleterious effects of TNF-α in human beta-cells. PTPN2 is a candidate risk gene for T1D, and it is conceivable that patients carrying risk-inducing PTPN2 polymorphisms may particularly benefit from therapies targeting TNF-α.
Disclosure: A. Roca Rivada: None.
207
GDF11 improve age-dependent beta cell deterioration in mouse and man
R. Wu;
Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden.
Background and aims: GDF11 is a member of the transforming growth factor (TGFβ) superfamily. An age-dependent decline in serum GDF11 levels can be observed in multiple mammalian species. In mice, GDF11 treatment protects glucotoxicity-induced beta-cell dysfunction and apoptosis. GDF11 deficient mice exhibit decreased beta-cell mass and reduced expression of MafA in beta cells during embryonic development. However, the roles of GDF11 in the regulation of ageing-related cell functions are rather controversial. Here we aim to explore the underlying function of GDF11 in controlling beta-cell deterioration in ageing rodents and islets from human donors.
Materials and methods: All human islets were collected from the Nordic Network for Islet Transplantation (Uppsala University, Sweden) via EXODIAB Human Tissue Lab in Lund University Diabetes Centre. In vitro insulin secretion experiments and immunostaining were performed in human islets from 7 non-diabetic and 3 diabetic donors with > 50 years of age. Aged diabetic db/db mice (24 weeks) and GK rats were used in the study. The db/db mice were intraperitoneally injected with GDF11 (100 ug/kg body weight) or vehicle (12 mice in each group) every other day for 30 days. Blood samples were collected from vena in the mice, and both blood glucagon and insulin were analyzed by ELISA. Blood glucose was also measured during the period of GDF11 injection and continued for 5 months. A 100 ng/ml GDF11 was used for in vitro experiments in the insulin-secreting INS-1 832/13 cells, primary rodent and human islets for three days.
Results: In our study, we found GDF11 expression was reduced by more than 80% in the aged human donors (~66 years old) with diabetes in comparison with non-diabetic donors (n=3, p<0.01). Similarly, GDF11 expression was 50% lower in diabetic GK rats than Wistar rats (n=3, p<0.01). After GDF11 treatment, Pdx1, the transcription factor indicating pancreatic beta cell identity, significantly increased (n=3, p<0.01). Notably, injection of GDF11 in aged db/db mice decreased both blood glucose and blood insulin significantly, while the blood glucose continued to be improved even 150 days after withdrawal of treatment (n=3, p<0.05). GDF11 treatment elevated insulin content in both diabetic human islets (n=3, p<0.01) and INS1 832/13 cells (n=3, p<0.005). Notably, glucose-stimulated insulin secretion appeared to be affected differentially in the GFD11-treated isolated islets from db/db mice: increased at low secretion rates (n=4, p<0.05) but decreased at high secretion rates (n=4, p<0.01)
Conclusion: Our findings suggest a novel role of GDF11 in reversing the age-dependent decline in beta-cell function and therefore hold promise for therapy of age-related diabetes.
Supported by: The Swedish Research Council (2019-01567, 2018-03258); Clinical research (ALF); Crafoord foundation
Disclosure: R. Wu: None.
208
Insulin, proinsulin and PC1/3 expression heterogeneity and beta cell function in diabetes
P.S. Apaolaza Gallegos1, Y.C. Chen2, K. Grewal2, Y. Lurz1, B. Verchere3, T. Rodriguez-Calvo1;
1Helmholtz Zentrum München, Munich, Germany, 2Department of Surgery, University of British Columbia, Vancouver, Canada, 3Department of Surgery and Pathology & Laboratory Medicine, University of British Columbia, Vancouver, Canada.
Background and aims: The detection of proinsulin (PI), insulin (INS), or C-peptide in the blood may help predict the decay in beta-cell mass and function during diabetes progression. However, their expression in the pancreas is still not well-understood. Thus, we aimed to provide an in-depth characterization of INS, PI, and the prohormone convertase PC1/3 (PC1) expression in the islets of non-diabetic (ND), autoantibody-positive (AAb+), type 1 diabetic (T1D) and type 2 diabetic (T2D) donors using confocal imaging and state-of-the-art image analysis. Moreover, we investigated islet beta-cell phenotype and islet morphology during disease progression.
Materials and methods: FFPE-Pancreatic sections from 20 age-matched ND, 7 AAb+ (4 single (s) and 3 double (d)), 8 T1D with short disease duration (<5y), 9 with long-duration T1D (>15y), and 6 T2D donors were included in the study. Confocal microscopy images for INS, PI, and PC1 from up to 30 islets/donor were analyzed using the software QuPath. Different cell populations were defined as follows: 1) INS+PI+PC1+, triple+, 2) INS+PI+PC1-, INS+PI-PC1+, INS-PI+PC1+ double+ and 3) INS+PI-PC1-, INS-PI+PC1-, INS-PI-PC1+ single+ cells. Proportion and density (number of cells/islet area) of each population as well as islet cellularity/ morphology were analyzed. The sum of triple+, double+, and single+ cells was used to determine total INS+, PI+, and PC1+ cells.
Results: In dAAb+, in comparison with the ND group, the density, and proportion of INS+PI+PC1- and INS-PI+PC1- cells was increased, while there was a decrease in INS+PI-PC1+ cells, suggesting active PI and insulin cellular accumulation without high PC1 expression. After onset, the proportion and density of total+ and triple+ (INS+PI+PC1+) cells were low in most T1D donors due to beta-cell loss. Conversely, INS+PI-PC1+ doubled, and INS-PI-PC1+ increased between 16% and 40% in T1D, and 5% in T2D compared to ND donors. No differences were observed in INS-PI+PC1+ cells among groups, with the exception of T1D (>15y) donors (8 times lower values). In general, single PI+ or INS+ cells were rare. The study of islet morphology revealed that the number of cells per islet was comparable in all groups while endocrine cell density was reduced in dAAb+ donors, yet increased in all T1D and T2D donors compared to ND donors. Last, no differences were found between ND and sAAb+ donors in all categories.
Conclusion: Beta-cells undergo functional and morphological changes as diabetes progresses. We observed a decrease in cells expressing INS, PI, and PC1 from dAAb+ to T1D, reflecting the ongoing beta cell loss. Changes in distinct double+ cell subpopulations may represent different stages of beta-cell dysfunction, as suggested by the increase in cells lacking PC1 in the islets of dAAb+ donors. Conversely, the increase in single PC1+ cells in T1D and T2D could be due to an increase in PC1+ alpha cells, possibly driven by dedifferentiation or to the presence of beta cells that cannot produce PI and INS. Lastly, islet morphological analysis suggests that beta-cell hypertrophy first and then atrophy might occur during disease progression. Our findings reveal intriguing dynamics of proinsulin/insulin production and conversion and could contribute to our understanding of diabetes pathogenesis at a beta-cell level.
Disclosure: P.S. Apaolaza Gallegos: None.
209
FFA1/Gq - Gi interaction determines glucose-induced insulin secretion of neonatal beta cells
F. Gerst1,2, E. Lorza-Gil1,2, G. Kaiser1,2, T. Ulven3, E. Kostenis4, H.-U. Häring2, A.L. Birkenfeld1,2, S. Ullrich1,2;
1IDM, Helmholtz Center Munich, Tübingen, Germany, 2German Center for Diabetes Research (DZD e.V.), Tübingen, Germany, 3Department of Drug Design and Pharmacology, University of Copenhagen, Copenhagen, Denmark, 4Institute of Pharmaceutical Biology, Bonn University, Bonn, Germany.
Background and aims: Adequate functional maturation of neonatal beta cells determines the efficacy of glucose metabolism during adult life. Insulin secretion of newborn islets relies on aminoacids and fatty acids metabolism, while glucose-responsive insulin secretion progressively arises days to weeks later after birth. Long chain fatty acids (LCFA) are major components of breastmilk and insulin secretagogues. LCFA are endogenous ligands of FFA1, a Gq/PLC coupled receptor acting on Ca2+ mobilization and actin network in beta cells. Whether FFA1 recruits Gα12/13 and impact on GSIS via downstream RhoA/ROCK-mediated actin remodelling is unknown. We assessed the role of FFA1 in postnatal maturation of neonatal beta cells in regard to the regulation of insulin secretion and beta cell mass.
Materials and methods: WT and Ffar1 KO BL6NCrl mice harboring a RIP-Cre driven EGFP expression were bred and 1, 6, 11 and 26d old offspring (P1, P6, P11, P26) were used for analysis. Pancreatic insulin content was measured in P1-P26 pancreata. Beta cell proliferation and mass were determined in P1-P26 pancreatic sections by estimating the number of Ki67-stained/EGFP-positive islet cells and the EGFP-positive islet area, respectively. Insulin secretion was performed in static incubations with WT and Ffar1 KO P6 islets using palmitate (600 and 60 μM), TUG469 (FFA1 agonist, 10 μM), exendin-4 (GLP-1R agonist, 100 nM), FR900359 (Gq inhibitor, 1 μM), pertussis toxin (PTX, Go/Gi inhibitor, 100 ng/ml), CT3 toxin (RhoA inhibitor, 2 μg/ml) and H1152 (ROCK inhibitor, 1μM).
Results: WT islets displayed glucose (12 mM) stimulated insulin secretion (GSIS, 6-fold change (FC) over 2.8G) mirroring the ongoing functional maturation. WT islets showed also a robust secretion in response to palmitate (600 μM) and exendin-4 (5-FC over 12G). In KO islets both glucose- and palmitate- stimulated insulin secretion were impaired, indicating that FFA1 signalling is critical for both secretagogues. As expected, palmitate (60 μM)- and TUG469-stimulated (2.25- and 3.4-FC over 12G, respectively) insulin secretion was inhibited by FR900359 (0.34- and 0.13-FC over 12G, respectively) in WT islets. Surprisingly, Gq inhibitor canceled also GSIS (from 10.6- to 1.7-FC over 2.8G) of WT islets. When the inhibitory Go/Gi signalling was blocked with PTX, GSIS was massively increased (100-FC over 2.8G), an effect that was up to 90% inhibited by FR900359 in WT islets. Noteworthy, PTX rescued GSIS in KO islets (from 1.6- to 11-FC over 2.8G). In addition, inhibition of RhoA and ROCK improved GSIS in KO islets. The secretory defects of KO islets do not originate in insufficient insulin production, since pancreatic insulin content increased with offspring’s age irrespective of Ffar1 genotype. Deletion of Ffar1 augmented beta cell proliferation in P6 offspring. Nevertheless, we found no genotype-driven differences in beta cell mass of P1-P26 offspring.
Conclusion: These findings indicate that (i) glucose responsiveness of the neonatal islets fully depends on active Gq and (ii) FFA1/Gq inactivation unlocks Go/Gi- and G12/13/RhoA/ROCK-mediated signals with negative impact on GSIS. Thus, FFA1/Gq signaling is essential for the gain of function of neonatal beta cells.
Supported by: 01GI0925
Disclosure: F. Gerst: None.
210
Oral administration of the small molecule cathepsin S inhibitor LY3000328 accelerates disease onset and impairs glucose tolerance in non-obese diabetic mice
T. Fløyel1, C. Frørup1, M. Haupt-Jørgensen2, L.J. Holm2, K. Pedersen2, M.Ø. Mønsted2, K. Buschard2, J. Størling1,3, F. Pociot1,4;
1Translational Type 1 Diabetes Research, Clinical Research, Steno Diabetes Center Copenhagen, Herlev, 2Bartholin Institute, Department of Pathology, Rigshospitalet, Copenhagen, 3Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, 4Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Accumulating data suggest a role for lysosomal cathepsin proteases in β-cell destruction and development of type 1 diabetes (T1D). We recently showed that cathepsin S (CTSS) is upregulated by proinflammatory cytokines in human pancreatic islets and β-cell lines. CTSS is increased in plasma from individuals with T1D compared to healthy individuals. Previous studies have investigated the involvement of CTSS in autoimmune diabetes and regulation of blood glucose, however, whether CTSS has protective or deleterious effects is not clear. The aim of this study was to confirm that circulating CTSS is increased in individuals with T1D and to examine if oral administration of the CTSS inhibitor LY3000328 affects autoimmunity, glucose tolerance, and diabetes incidence in non-obese diabetic (NOD) mice.
Materials and methods: CTSS was measured in serum from 148 children with T1D and 147 healthy siblings by ELISA (CV%<10). NOD mice were fed chow diet with LY3000328 ~10 mg/kg/day (CTSSi) or a standard diet (CTRL) from 3 weeks of age throughout life. Mice were euthanized at diabetes diagnosis (defined as two blood glucose measurements >12 mmol/l with an interval of two days) or at 310 days of age. 13-weeks-old mice were used for investigation of glucose tolerance and insulitis. Three days post intravenous glucose tolerance test, mice were anaesthetized for collection of heart blood (serum), pancreas, and spleen. Insulitis was evaluated from H&E stainings of pancreatic sections. Serum concentrations of IFNγ, IL-1β, IL-2, IL-4, IL-6, IL-10, TNFα, IP10, KC/GRO, MCP-1, MIP-1α, and MIP-2 were measured by MSD multiplex immunoassays. The effect of LY3000328 on CTSS activity was examined in serum using CTSS activity assay and in spleen by immunoblotting of the CTSS substrate invariant chain p10 (Iip10).
Results: Serum CTSS was increased by 8% in children with T1D compared to healthy siblings (p<0.01). Oral administration of LY3000328 led to increased diabetes incidence (CTRL, n=20; CTSSi, n=20; p<0.01) and lower glucose tolerance (CTRL, n=7; CTSSi, n=6; p<0.05) in NOD mice. There were no differences in weight, food intake, water intake, or insulitis score (p>0.05). Oral administration of LY3000328 correlated with lower serum levels of IL-6 (CTRL, n=8; CTSSi, n=4; p<0.05). CTSS inhibition was confirmed by detecting lower CTSS activity in serum (CTRL, n=8; CTSSi, n=5; p<0.001) and accumulation of Iip10 in spleen from mice treated with LY3000328 (CTRL, n=8; CTSSi, n=5; p<0.0001).
Conclusion: Our results demonstrate that LY3000328-mediated inhibition of CTSS accelerates onset of autoimmune diabetes and impairs glucose tolerance, suggesting that CTSS may have a protective role during development of T1D. CTSS may regulate β-cell sensitivity via IL-6 which previously has been shown to have a protective effect on β cells.
Supported by: JDRF, DFF/FSS
Disclosure: T. Fløyel: None.
OP 36 Central aspects of diabetes
211
Involvement of hypothalamic de novo ceramide synthesis in central resistin-induced glucose intolerance: impact on hypothalamic and hepatic inflammation
J. Guitton1, C. Alexandre1, M. Taouis1, M. López2, Y. Benomar1, H. Le Stunff1;
1Institut des Neurosciences Paris-Saclay (NeuroPSI), Saclay, France, 2Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Santiago de Compostela, Spain.
Background and aims: In obesity, ectopic lipid accumulation in non-adipose tissues causes functional impairments in several metabolic pathways leading to lipotoxicity that promotes peripheral inflammation and insulin resistance (IR). Recently, the hypothalamus, a brain area involved in energy homeostasis, has also been reported as a target of lipotoxicity. Interestingly, it has been shown that accumulation of reactive lipid species, such as ceramide, in the hypothalamus induces central IR and impaired glucose homeostasis. Besides, in an over-nutrition environment, the hypothalamus is also subjected to changes in circulating factors such as resistin, a key mediator linking obesity with IR and type 2 diabetes. Recently, we have reported that resistin induces whole body IR and promotes hypothalamic inflammation. Moreover, several studies reported resistin as a regulator of peripheral lipid metabolism impairing insulin sensitivity through a central mechanism. In this context, we investigated the potential involvement of hypothalamic de novo ceramide synthesis in central resistin-induced hypothalamic inflammation and dysregulation of glucose homeostasis.
Materials and methods: C57Bl6J mice (n=5/group) were intracerebroventricularly (ICV) infused with resistin for 15 days. To investigate the role of hypothalamic de novo ceramide synthesis in ICV resistin-induced hypothalamic inflammation and glucose intolerance we selectively knocked down the serine palmitoyltransferase (SPT) 1, a de novo ceramide synthesis limiting enzyme, in the hypothalamus through stereotaxic adenovirus injection (shRNA-SPT1) into the third ventricle. In the hypothalamus, we evaluated microgliosis and astrogliosis by immunohistochemistry. Hypothalamic and hepatic inflammation as well as enzymes driving gluconeogenesis were evaluated by RTqPCR. Glucose homeostasis was assessed by an intraperitoneal glucose tolerance test (IP-GTT).
Results: Injection of shRNA-SPT1 adenovirus in the third ventricle decreases hypothalamic SPT1 mRNA expression by 62% (p<0,001). Resistin ICV infusion induces hypothalamic inflammation as evidenced by increased IL-1β expression (p<0,01) in association with significant changes in microglia and astrocyte morphology in the hypothalamus as a sign of reactive gliosis. Concomitantly, ICV resistin-treated mice exhibit marked glucose intolerance and increased hepatic expression of key proinflammatory markers IL-6 (p<0,05), TNFα (p<0,05), IL-1β (p<0,01) and NF-Κβ (p<0,01) in addition to the upregulation of hepatic glucose 6-phosphatase (p<0,05) as a sign of increased hepatic gluconeogenesis. Importantly, the selective deletion of SPT1 in the hypothalamus prevents ICV resistin-induced hypothalamic inflammation. At the periphery, hypothalamic down-regulation of SPT1 improves glucose tolerance by counteracting hepatic inflammation and gluconeogenesis induced by resistin.
Conclusion: These findings reveal hypothalamic de novo ceramide synthesis as a new regulatory pathway of resistin-induced hypothalamic inflammation and dysregulation of glucose homeostasis. Targeting this signaling pathway may constitute a significant breakthrough to overcome obesity-induced hypothalamic inflammation and related metabolic dysfunction.
Supported by: SFD Société Francophone du Diabète
Disclosure: J. Guitton: None.
212
Effect of high fat diet and exendin 4 on inhibitory neurotransmission markers and insulin signalling in the brain in high fat fed mice
V. Sancho1, A. Faraone2, A. Dardano1, S. Del Prato1, M. Mainardi3,2, G. Daniele1;
1Clinic and Experimental Medicine, Pisa University, 2Laboratory of Biology, Scuola Normale Superiore, 3National Research Council, Pisa, Italy.
Background and aims: Brain plasticity (induced by visual deprivation) is modulated by the tone of GABAergic neurotransmission. In a previous study, we have shown that visual cortical plasticity was altered in morbidly obese patients, and it is completely rescued 6 months after bariatric surgery. Brain plasticity improvement was related to increased GLP-1 levels post-RYGB. To shed light on the molecular mediators of this effect, we explored the impact of high-fat diet (HFD) on GABA and insulin signaling in the visual cortex and hippocampus of HFD mice. We also have assessed Exendin-4 (Ex4; a GLP-1 agonist) effects on the same molecular pathways.
Materials and methods: 60-days old male C57BL/6J mice were fed HFD (23% proteins; 42% CHO - 28% starch, 9% sucrose, 5% maltodextrin; 34% fats; 60% fat caloric content) or standard chow diet (SD: 18.5% proteins; 46% CHO - 42% starch, 4% sucrose; 3% fats; 6.55% fat caloric content) for 8 weeks followed by Ex4 (25ng/kg; N=XX) or vehicle (VC; N=XX) intraperitoneal injection administration and blood samples collection 1h post-injection, before animals were sacrifice for hippocampus and visual cortex dissection. Glycemia was determined by YLS 2300 STAT Plus glucometer and insulinemia by ELISA. Western blotting was used to assess expression levels of total and phosphorylated Akt and ERK kinases, and GABAergic markers GAD2 and vGAT, aall djusted for β-Actin expression.
Results: HFD was associated with body weight gain compared to SD (35.2±0.7 vs 32.4±0.4 g; p≤0.003), hyperglycemia (319±18 vs 238.4±16 mg/dl, p≤0.02) and hyperinsulinemia (72.2±19.5 vs 28.4±7.0 pmol/l, p≤0.05). In HDF mice, GAD2 levels were increased by 156±15% as compared to SD (p=0.03) in the visual cortex and reduced in the hippocampus (79±4% of SD, p=0.027) with no difference in vGAT expression. As far as insulin signaling is concerned, in HFD mice, phospho-Akt expression was reduced in the visual cortex (HFD 71±9% of SD, p=0.048) and increased in the hippocampus (HFD-vehicle 139±7% of SD-vehicle, p=0.004). One-hr after Ex-4, both glucose (99.8±12.0 vs 70.8±23.9 mg/dl) and insulin levels 7.9±6.5 vs 33.5±9.3 pmol/l) were decreased in HFD mice as compared to VC (both p≤0.001). Ex4 did not affect Akt phosphorylation in the visual cortex of both SD and HFD mice, while a significant reduction was observed in the hippocampus of HFD mice (HFD-Ex4 99±7 vs HFD-vehicle 139±7% of SD-vehicle, p=0.002). ERK phosphorylation in the visual cortex was not affected by Ex4, but it was increased in SD mice (175±17% of SD-vehicle, p=0.015)
Conclusion: HFD differentially affects the expression of GABAergic markers and activation of insulin-related signaling in the visual cortex and hippocampus. HFD is followed by increased Akt phosphorylation in the hippocampus and Exendin-4 acutely normalizes this alteration, suggesting a potential beneficial effect in rescuing altered insulin-related signaling in the brain caused by high-fat diets.
Disclosure: V. Sancho: None.
213
Central action of FGF19 improves energy homeostasis in diet-induced obese mice
L. Zangerolamo, M. Carvalho, C. Solon, G.M. Soares, L.A. Velloso, H.C.L. Barbosa;
University of Campinas, Campinas, Brazil.
Background and aims: Fibroblast growth factor-19 (FGF19) is a gut-derived hormone released postprandially, which is emerging as a potential therapeutic agent for metabolic disorders, including diabetes and obesity. It is known that the central (hypothalamic) action of FGF19 reduces body weight (BW) in diet-induced obese (DIO) mice, however, the mechanisms involved in this phenomenon remain poorly understood. Considering the crucial role of the hypothalamus in activating thermogenic mechanisms in brown adipose tissue (BAT), we aimed to investigate the central actions of FGF19 upon the energy homeostasis of DIO mice, focusing on the thermogenic capacity of BAT.
Materials and methods: In this study, C57BL/6 lean and DIO (8 weeks of high-fat diet) mice were used. For chronic (10 days) central FGF19 administration (2 μg/day), mice received a cannula into the lateral ventricle of the brain. A micro-osmotic pump (Alzet) filled with recombinant FGF19 or saline was subcutaneously implanted in mice and were connected to the newly implanted brain cannula via a catheter. Food intake and BW were monitored throughout the treatment. Energy expenditure (EE) and respiratory quotient (RQ) were measured by indirect calorimetry. Fat depots weights were obtained at the time of euthanasia and the adipocytes area was evaluated by histological analysis. Insulin sensitivity was measured by insulin tolerance test and through the homeostasis model assessment-estimated insulin resistance (HOMA-IR). BAT temperature was acquired by infrared thermography (FLIR Systems) and gene expression was evaluated by qPCR. Data were analyzed by one-way ANOVA and are displayed as mean ± SEM. The difference between the groups was considered statistically significant if P ≤ 0.05.
Results: DIO mice treated with FGF19 displayed reduced BW gain (g) (0.30 ± 0.10 Lean x 2.03 ± 0.08 DIO x 1.18 ± 0.22 DIO+FGF19) during the treatment, which was associated with lower food intake (kcal) (158.50 ± 5.67 Lean x 210.00 ± 16.84 DIO x 152.40 ± 7.11 DIO+FGF19), diminished fat depots (% BW) (epididimal: 0.38 ± 0.04 Lean x 2.81 ± 0.27 DIO x 1.95 ± 0.30 DIO+FGF19; inguinal: 0.40 ± 0.04 Lean x 1.89 ± 0.34 DIO x 0.99 ± 0.16 DIO+FGF19), and smaller adipocyte size (μm2) (1606 ± 213 Lean x 11685 ± 1016 DIO x 6572 ± 179 DIO+FGF19). The central action of FGF19 also improved peripheral insulin sensitivity, kITT (%/min) (4.95 ± 0.22 Lean x 3.10 ± 0.20 DIO x 4.63 ± 0.24 DIO+FGF19), as well as HOMA-IR (0.77 ± 0.07 Lean x 5.61 ± 1.10 DIO x 2.04 ± 0.89 DIO+FGF19) in treated mice. Furthermore, central administration of FGF19 ameliorated EE (Kcal/day/kg^0.75) (191.24 ± 2.56 Lean x 164.19 ± 4.12 DIO x 182.46 ± 2.61 DIO+FGF19) and RQ (0.92 ± 0.01 Lean x 0.79 ± 0.01 DIO x 0.87 ± 0.02 DIO+FGF19) in mice during the dark cycle. This improvement observed in FGF19 treated DIO mice was also associated with higher BAT temperature (°C) (35.58 ± 0.33 Lean x 34.60 ± 0.14 DIO x 36.76 ± 0.35 DIO+FGF19), as well as increased expression of thermogenic marker genes in the BAT (UCP1: 0.27 ± 0.06 DIO x 0.64 ± 0.09 DIO+FGF19; DIO2: 0.25 ± 0.03 DIO x 0.68 ± 0.18 DIO+FGF19; PPARGC1α: 0.52 ± 0.09 DIO x 1.39 ± 0.30 DIO+FGF19; PRDM16: 0.33 ± 0.05 DIO x 0.73 ± 0.15 DIO+FGF19, and CIDEA: 0.35 ± 0.06 DIO x 0.79 ± 0.11 DIO+FGF19, fold change of Lean).
Conclusion: Our results show that central delivery of FGF19 improves energy metabolism in DIO mice, and the increased thermogenic capacity of BAT must be involved, at least in part, in the observed beneficial effects.
Supported by: FAPESP
Disclosure: L. Zangerolamo: Grants; FAPESP - 2020/14020-7.
214
Modulation of hypothalamic AMPK phosphorylation by Olanzapine controls energy balance and body weight
V. Ferreira1, C. Folgueira2, M. Guillén1, P. Zubiaur3, D. Grajales1, A. Sarsenbayeva4, P. López Larrubia1, J.W. Eriksson4, M.J. Pereira4, F. Abad-Santos3, G. Sabio2, P. Rada1, Á.M. Valverde1;
1IIBm Alberto Sols (CSIC-UAM), Madrid, Spain, 2Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain, 3Instituto de Investigación Sanitaria La Princesa, Madrid, Spain, 4Clinical Diabetes and Metabolism, Uppsala University, Uppsala, Sweden.
Background and aims: Second-generation antipsychotics (SGAs) are mainstay therapy for psychiatric disorders. SGA-treated patients present risk for weight gain and insulin resistance. Since overweight/obesity favors insulin resistance and Type 2 Diabetes, we evaluated the effect of olanzapine (OLA), a widely prescribed SGA, in mice, focusing on body weight, energy balance and insulin sensitivity. We also explored OLA effects in protein tyrosine phosphatase-1B deficient (PTP1B-KO) mice, a preclinical model of insulin and leptin hypersensitivity.
Materials and methods: Wild-type (WT) and PTP1B-KO mice were fed an OLA-supplemented diet (5 mg/kg/day, 7 months) or treated via intraperitoneal (i.p.) injection (10 mg/kg/day, 8 weeks). Readouts of hypothalamus-periphery crosstalk were assessed by OLA intrahypothalamic administration with or without adenoviruses expressing a constitutive active AMPKα1 mutant.
Results: Although both genotypes of mice treated orally with OLA presented hyperphagia (p<0.001, n=19-33), weight gain was enhanced only in the WT (p<0.001, n=9-10). Unexpectedly, all mice receiving OLA via i.p. lost weight (p<0.001, n=6-11) without changes in food intake, but with an increase in energy expenditure (p<0.05, n=4-6). Elevation in UCP-1 levels (p<0.05, n=5-6) and brown adipose tissue (BAT) temperature (p<0.05, n=7-17) appeared concomitantly with lower hypothalamic phospho-AMPK (p<0.01, n=8-13). Likewise, OLA central injection reduced phospho-AMPK (p<0.05, n=3) and increased BAT UCP-1 (p<0.05, n=5-6), effects abolished by hypothalamic AMPK activation (n=6-7). OLA i.p. treatment was associated with enhanced Tyrosine Hydroxylase (TH)-positive innervation (p<0.05, n=10-14) and less sympathetic neuron-associated macrophages in subcutaneous white adipose tissue (iWAT). Both central and i.p. OLA injections increased UCP-1 (p<0.05, n=8-11) and TH (p<0.05, n=5-11) in iWAT, effects prevented by hypothalamic AMPK activation. Contrarily, BAT thermogenesis was unaltered in dietary-treated WT mice while increased in PTP1B-KO mice (p<0.05 n=6-7). Notably, OLA treatment induced markers of insulin resistance in WT mice by both administration routes. Specifically, after i.p. OLA treatment, WT mice presented insulin (p<0.05, n=5-9) and pyruvate (p<0.05, n=17-24) intolerance, effects absent in PTP1B-KO mice. The insulin signaling analysis showed a reduction insulin receptor (p<0.05, n=4-5) and Akt (p<0.05, n=4-5) phosphorylation in liver, muscle and primary hepatocytes from OLA-treated WT mice concomitantly with inflammatory hallmarks (p<0.05, n=5). Importantly, PTP1B-KO mice were protected against OLA-induced insulin resistance.
Conclusion: Mechanistically, we found that OLA reduces hypothalamic phospho-AMPK inducing weight loss through thermogenesis activation. In conclusion, we unraveled an unexpected metabolic rewiring controlled by hypothalamic AMPK that avoids weight gain in male mice treated i.p. with OLA by activating BAT thermogenesis and iWAT browning, as well as a therapeutic benefit of PTP1B inhibition against OLA-induced insulin resistance and weight gain.
Supported by: ITN-TREATMENT (721236); FCT/FEDER (2020.08388.BD); MICINN/AEI/FEDER, EU (RTI2018-094052-B-100)
Disclosure: V. Ferreira: None.
215
Effect of interesterified fat and its metabolites on inflammation and insulin resistance in hypothalamus
J.E. Miyamoto1,2, R.M.A. Santos1, B.P. Siqueira1, A. Reginato3, J. Guitton4, P.L.R. Menta1, T.G. Ramalheira1, A.S. Torsoni1, L.M. Ignácio-Souza1, M.A. Torsoni1, H. Le Stunff4, C. Magnan2, M. Milanski1;
1Laboratory of Metabolic Disorders, University of Campinas, Limeira, Brazil, 2Biologie Fonctionnelle et Adaptative (BFA) - CNRS UMR 8251, Université Paris Cité, Paris, France, 3Medicine/Endocrinology, Albert Einstein College of Medicine, Bronx, USA, 4Neuroscience Paris-Saclay Institute (NeuroPSI) - UMR CNRS 9197, Université Paris Saclay, Paris, France.
Background and aims: The overconsumption of dietary fats leads to inflammation in peripheral tissues, which is implicated in the development of obesity and insulin resistance (IR). More recently, hypothalamus has become a key target of dietary fat in the development of central and peripheral IR. Nevertheless, the role of different types of dietary lipids, particularly the nature of triacylglycerols (TAGs) in the development of IR are still poorly understood. TAGs occur naturally or they can be manufactured from vegetable oils and animal fats by interesterification. This latter process leads to an enrichment of saturated fatty acids (SAFA) on the sn-2 position of TAGs. Recently, we provided evidence that a normocaloric interesterified lipid diet impaired glucose tolerance in mice. In the present study, we evaluated the role of interesterified palm oil and their metabolites on insulin signaling and inflammation in mice and in hypothalamic neuronal cells.
Materials and methods: Adult male Swiss mice were divided into four experimental groups: nomorcaloric palm oil diet (PO), nomorcaloric interesterified palm oil diet (IPO), PO high fat diet (POHFD) or IPO high fat diet (IPOHFD) during 8 weeks. Glucose homeostasis was assessed by GTT. Hypothalamus were processed and RNA-seq was performed. For in vitro analysis, mHypoA 2/28 hypothalamic cell was treated for 16h with TAG derivatives namely monoacylglycerol either linked to palmitate on sn-2 position (2-palmitoylglycerol (2-PG)) or to oleic acid (Oleic) on sn-2 position (2-oleylglycerol (2-OG)). Free palmitic acid (Palm) or oleic acid were used as controls. Pro-inflammatory mediators expression were assessed by RTqPCR. Insulin signaling pathway was evaluated by quantifying Akt phosphorylation. Involvement of de novo ceramide synthesis was determined by the use of the serine palmitoyl-transferase inhibitor, myriocin.
Results: The interesterification generates new TAGs, which is characterized by increased SAFA on sn-2, induced glucose intolerance. In vivo, normocaloric IPO diet induced the regulation of various genes in hypothalamus including cellular stress markers. In vitro studies with hypothalamic cell lines (mHypoA-2/28) evidenced that the treatment with 2-PG as free palmitate impaired insulin signaling by decreasing AKT phosphorylation. 2-PG also promoted inflammation by increasing IL-6 levels. Interestingly, treatment 2-OG or free oleic acid did not induced inflammation. Importantly, lipid profile analysis revealed that both Palm and 2-PG treatment increased intracellular levels of palmitate in mHypo 2/28 cells. Finally, myriocin treatment counteracted the impairment of insulin signaling induced by 2-PG.
Conclusion: Altogether, our data showed that interesterified fats targets hypothalamus to mediate their metabolic effect. This preliminary data also allowed us to assert that SAFA on sn-2 position of TAG could as free fatty acids to alter insulin signaling and inflammation in neuronal cells and contributed therefore to the development of obesity and IR.
Supported by: FAPESP 2016/24768-3 and 2020/16040-5 (Fellowship), 2017/15925-0 and 2019/26538-3 (Grant)
Disclosure: J.E. Miyamoto: None.
216
A role for GIP in the regulation of food intake and body weight in mice
J.E. Lewis, T. Darwish, F. Reimann, F.M. Gribble;
Institute of Metabolic Science, University of Cambridge, Cambridge, UK.
Background and aims: Hormones from the gut that signal nutrient uptake and availability to the brain are key elements in the control of appetite. Glucagon-like peptide-1 (GLP-1) based pharmacotherapies are licensed for the treatment of type 2 diabetes and obesity and dual agonist peptides stimulating both GLP-1R and glucose-dependent insulinotropic peptide receptors (GIPR) have been shown to promote greater weight reduction. GIP is well established to regulate blood glucose via its insulinotropic and glucagonotropic action on the pancreas, however, the role of GIP in the regulation of food intake and body weight remains controversial. This study addressed the metabolic roles of endogenous GIP by directly activating the GIP-secreting cell population.
Materials and methods: Utilising a novel rodent model (GIP-Cre x Dq), targeting the Designer Receptor Activated by Designer Drugs (Dq-DREADD) to GIP expressing cells, we measured intraperitoneal glucose tolerance, acute food intake and whole-body physiology/feeding behaviour in metabolic cages in the lean and diet induced obese state with and without activation of cells through Dq with the DREADD-ligand CNO in a cross-over design (n=14).
Results: In lean GIP-Cre x Dq mice, CNO resulted in an increase in plasma GIP, akin to that in the postprandial state in mice. In lean and diet induced obese mice, the increase in GIP was associated with improved glucose tolerance (p<0.0001) and a reduction in food intake both in the ad lib fed state at the onset of the dark phase (p<0.01) and in a fast-refeed paradigm (p<0.001). The reduction in food intake was a consequence of reduced feeding time (p<0.05) and an increase in the time between meals (p<0.001). No change in energy expenditure or activity was detected following GIP cell activation, however, a transient reduction in body weight was apparent (p<0.05). In lean mice, the effects on glucose tolerance and food intake were blocked by pre-treatment with a GIPR antagonistic antibody, suggesting the effects were specific to GIP and mediated through an antibody accessible receptor site.
Conclusion: These studies provide new insights into the physiological roles of GIP, suggesting that it contributes to the control of appetite as well as blood glucose levels.
Supported by: EFSD/Lilly European Diabetes Research Programme
Disclosure: J.E. Lewis: None.
OP 37 Insulin deficiencies and cardiovascular disease
217
Incidence of cardiovascular disease in latent autoimmune diabetes in adults
Y. Wei, E. Ahlqvist, T. Andersson, T. Tuomi, S. Carlsson;
Karolinska Institutet, Stockholm, Sweden.
Background and aims: Latent autoimmune diabetes in adults (LADA) is a slowly progressing form of autoimmune diabetes with an adult onset. Our aim was to contribute to the limited knowledge on the risk of CVD in LADA with varying degrees of autoimmunity.
Materials and methods: This population-based Swedish study followed individuals with newly diagnosed LADA (n= 587) and type 2 diabetes (T2D, n=2013) and matched diabetes-free controls (n=2385) between 2007 and 2019 through National Diabetes, Patient and Cause-of-Death Registers. HR (95% CI) for a first occurrence of CVD (ischemic heart disease, stroke, and heart failure) in LADA and T2D compared to controls were estimated by Cox regression and adjusted for age, sex, calendar year and lifestyle factors. LADA patients were stratified based on the median level (250 IU/ml) of glutamic acid decarboxylase antibodies (GADA) into LADAhigh and LADAlow.
Results: During a median follow-up of 6.05 years, we recorded 328 CVD events (37 in LADA and 173 in T2D). The risk of a first occurrence of CVD was increased in T2D (HR: 1.58, 95% CI: 1.22, 2.06) but not in LADA (HR: 1.25, 95% CI: 0.86, 1.83) when compared to people without diabetes. However, stratification by GADA levels revealed that patients with LADAhigh had excess risk of CVD (HR: 1.72, 95% CI: 1.09, 2.71) that was on par with that observed in T2D (Figure 1). LADAhigh patients had worse beta-cell function (median HOMA-B 30.9 vs 46.8, p<0.001) and glycemic control (median HbA1c during follow-up: 49.7 vs 55.3 mmol/mmol, p<0.001), but lower prevalence of CVD (6.7% vs 14.8%, p=0.002) at baseline compared to LADAlow patients.
Conclusion: Among LADA patients free of CVD at the time of diabetes diagnosis, those with high GADA levels have higher future risk of CVD, which might be driven by worse β cell function and glucose control. However, attention should also be paid to LADA patients with low GADA given their much higher CVD prevalence at diabetes diagnosis.
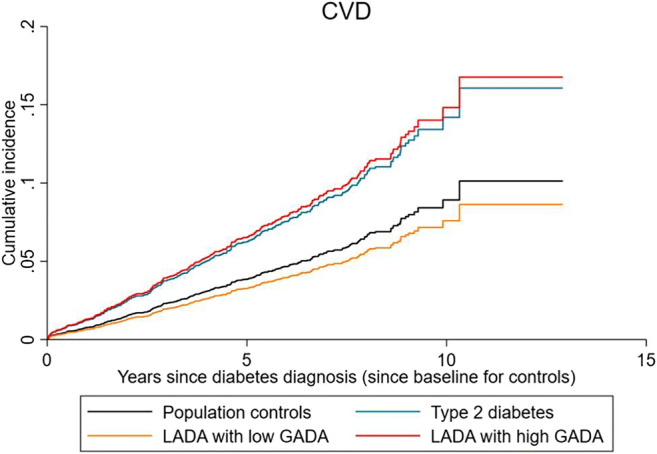
Supported by: CSC, Novo Nordisk foundation, Swedish Research Council, FORTE.
Disclosure: Y. Wei: None.
218
Endotrophin is a risk marker of complications in a type 1 diabetes cohort
A.L. Møller1,2, N.H. Tougaard3, P.F. Rønn3, T.W. Hansen3, F. Genovese1, M.A. Karsdal1, D.G.K. Rasmussen1, P. Rossing3,4;
1Nordic Bioscience, Herlev, 2Department of Biomedical Sciences, University of Copenhagen, Copenhagen, 3Steno Diabetes Center Copenhagen, Herlev, 4Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Persons with diabetes have a high risk of complications related to the micro- and macrovascular circulation. Hyperglycemia can trigger pathological pathways leading to fibrosis, an overreaction to tissue injury caused by accumulation of extracellular matrix components. In this study, we investigated, the potential of endotrophin, a pro-fibrotic molecule generated during collagen type VI (COL6) formation, as a risk marker for development of complications and mortality in an unselected type 1 diabetes population.
Materials and methods: We measured endotrophin in serum and urine in 1468 individuals with type 1 diabetes (49% females, mean±SD age of 51±16 years) recruited from a Diabetes Center between 2012-2016. Urine endotrophin values were normalized to urinary creatinine levels. Participants were followed for a median of up to 6.4 years, and information on endpoints was extracted from national registers. Endpoints included: a composite renal endpoint, major adverse cardiovascular event (MACE), all-cause mortality, progression of albuminuria, incident heart failure (HF), and sight-threatening eye disease. Cox proportional hazards models adjusted for conventional risk factors and prevalent disease (endpoint of interest) at baseline were applied.
Results: A doubling of serum endotrophin was independently associated with the composite renal endpoint (n=36, HR: 3.69, 95% CI: 2.24-6.07), MACE (n=140, HR: 1.46, 95% CI: 1.12-1.89), all-cause mortality (n=93, HR: 1.44, 95% CI: 1.03-2.0), and progression of albuminuria (n=80, HR: 1.69, 95% CI: 1.22-2.35), but not with incident HF or sight-threatening eye disease after adjustment for conventional risk factors. The associations remained significant after further adjustment for prevalent disease at baseline: composite renal endpoint (HR: 3.27, 95% CI: 1.89-5.65) and MACE (HR: 1.43, 95% CI: 1.06-1.93). A doubling of urine endotrophin was not associated with any of the endpoints after adjustment.
Conclusion: Serum endotrophin released during COL6 formation is an independent risk marker of mortality, cardiovascular and renal complications in persons with type 1 diabetes.
Supported by: This work was supported by the Innovation Fund Denmark (0172-00270B)
Disclosure: A.L. Møller: None.
219
Predictors of arterial stiffness in patients with type 1 diabetes: importance of long-term glycaemic control and physical activity
S. Helleputte1,2, P. Calders1, J. Marlier3, T. De Backer4,1, B. Lapauw3,1;
1Faculty of Medicine and Health Sciences, Ghent University, Gent, 2Fonds Wetenschappelijk Onderzoek (FWO) Vlaanderen, Brussels, 3Endocrinology, Ghent University Hospital, Gent, 4Cardiology, Ghent University Hospital, Gent, Belgium.
Background and aims: In patients with type 1 diabetes, arterial stiffness can add value in estimating cardiovascular disease (CVD) risk. Traditional CV risk factors and glycaemic control as reflected by HbA1c, are main determinants of arterial stiffness. However, the relationship with other markers of glycaemic control, as advanced glycation end products (AGEs) and continuous glucose monitoring (CGM)-derived parameters, and with physical activity levels, is less well explored. This study aimed to examine the relationship of arterial stiffness with short- and long-term parameters of glycaemic control, physical performance and activity level in patients with type 1 diabetes free from overt CVD.
Materials and methods: Cross-sectional study in patients with a type 1 diabetes duration of at least ten years and still free from known CVD. Current level and 10-years history of HbA1c was evaluated, as well as skin AGEs. Arterial stiffness was assessed with carotid-femoral pulse wave velocity (cf-PWV). CGM for 7 days was used to determine time in range (TIR), time in hyper- and hypoglycaemia, and glycaemic variability. Levels of physical activity and exercise capacity were evaluated. Pearson (r) and Spearman (rs) correlations, and multiple linear regression was used to investigate associations with cf-PWV.
Results: 54 patients (M/F: 32/22; age: 46 ± 9.5 yrs; type 1 diabetes duration: 27 ± 8.8 yrs; HbA1c: 7.8 ± 0.83%) were included. cf-PWV showed significant associations with traditional risk factors age (rs= +0.69), type 1 diabetes duration (rs= +0.41) and 24-hours mean arterial pressure (rs= +0.45); cf-PWV was significantly associated with current HbA1c (rs= +0.28), mean 10-years HbA1c (rs= +0.36) and AGEs (rs= +0.40), but not with any of the CGM-derived parameters; and negatively associated with VO2max (rs= -0.41) and physical activity level (rs= -0.60). Multiple linear regression for cf-PWV showed that the model with the best fit included age, type 1 diabetes duration, 24-hour mean arterial pressure and mean 10-years HbA1c (adjusted R2= 0.645); and that VO2max had independent predictive value.
Conclusion: This study demonstrated that long-term glycaemic exposure as reflected by mean 10-years HbA1c and AGEs is a main predictor of arterial stiffness in patients with type 1 diabetes, while no relationship was found with any of the CGM-parameters. Importantly, physical activity level and exercise capacity were inversely associated with arterial stiffness. Our findings stress the importance of early and sustained good glycaemic control and of engagement in physical activity to prevent premature CVD in patients with type 1 diabetes.
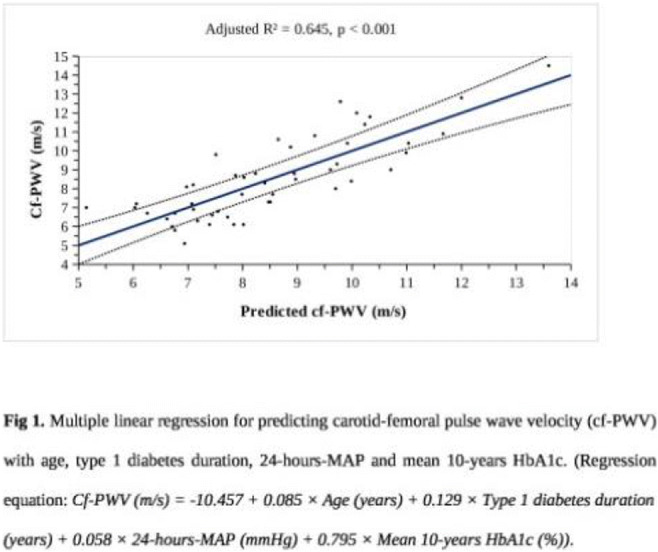
Supported by: The first author (S.H.) is supported by a PhD grant Fundamental Research from FWO Vlaanderen.
Disclosure: S. Helleputte: Grants; Fonds Wetenschappelijk Onderzoek (FWO) Vlaanderen PhD fellowship grant.
220
Hyperglycaemia and hypoglycaemia exposure are differentially associated with micro- and macrovascular complications in adults with type 1 diabetes
A. Mesa1, M. Giménez1,2, I. Pueyo1, V. Perea3, C. Viñals1, J. Blanco1,2, I. Vinagre1,2, T. Serés-Noriega1, L. Boswell1,4, E. Esmatjes1,2, I. Conget1,2, A.J. Amor1;
1Endocrinology and Nutrition Department, Hospital Clínic de Barcelona, Barcelona, 2IDIBAPS (Institut d’investigacions biomèdiques August Pi i Sunyer), Barcelona, 3Endocrinology and Nutrition Department, Hospital Mútua de Terrassa, Terrassa, 4Endocrinology and Nutrition Department, Althaia – Xarxa Assistencial Universitària de Manresa, Manresa, Spain.
Background and aims: Information on the association between continuous glucose monitoring (CGM) data and chronic complications in type 1 diabetes (T1D) is scarce. We explored the relationship between high and low glucose exposure glucometrics obtained by CGM and micro- and macrovascular complications in this population.
Materials and methods: Cross-sectional study in T1D patients without cardiovascular disease (CVD) and with at least one of the following: ≥40 years, diabetic nephropathy or ≥10 years of T1D duration with CVD risk factors. CGM data were obtained from a CGM sensor regularly used by each patient (Dexcom G5, Guardian Sensor 3 or FreeStyle Libre). CGM-derived glucometrics from 14 consecutive days were collected: glucose management indicator (GMI), coefficient of variation and proportion of time (%) <54 (time below range; TBR<54), <70, 70-180 (time in range; TIR), >180 (time above range; TAR). Carotid plaque (intima-media thickness ≥1.5 mm) was evaluated by standardized ultrasonography protocol. Logistic regression models adjusted for CGM sensor, age, sex, diabetes duration and other CVD risk factors were constructed to test the independent association between CGM-derived glucometrics and chronic complications.
Results: We included 152 patients (54.6% men, mean age 48.7±10.0 years, T1D duration 28.6±11.3 years, 5-year mean HbA1c 7.31% (6.86-7.89), insulin pump therapy 36.2%, TIR 61.3±15.3%, TBR<54 1.0±1.5%, TAR 33.3±15.7%, GMI 7.11±0.71%). Sixty-seven patients showed carotid plaque and n=71 microvascular complications (retinopathy and/or nephropathy). TAR (OR 1.28 [1.09-1.51], p=0.003; 5% increase) and GMI (OR 3.05 [1.46-6.36], p=0.003; 1% increase) were directly associated with the presence of microvascular complications, while TIR had an inverse relationship (OR 0.79 [0.66-0.93], p=0.005; 5% increase). TBR<54 was directly associated with the presence of plaques, even after adjusting for 5-year mean HbA1c (p<0.05; Figure).
Conclusion: Glucometrics related to high glucose exposure were independently associated with microvascular complications. Only low glucose exposure glucometrics was significantly associated with preclinical atherosclerosis. Our data supports the role of hypoglycemia in the development of CVD in this population.
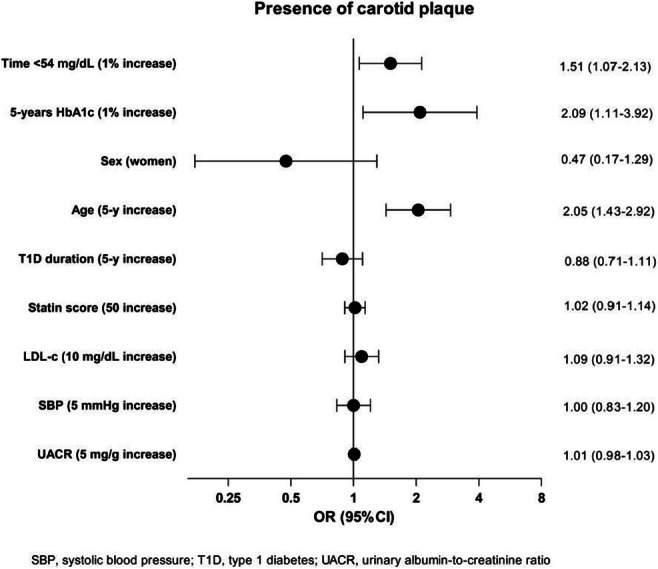
Supported by: Contracte Clínic Recerca "Josep Font-Emili Letang" and Ajut programa doctorat ACD
Disclosure: A. Mesa: Grants; Contracte Clínic de Recerca "Josep Font-Emili Letang", Ajut per a la realització d'un programa de doctorat de l'Associació Catalana de Diabetis.
OP 38 It is always a D-D-Day: diabetes, digital, device
221
Long term weight loss in a primary care-anchored eHealth lifestyle coaching programme in Denmark: a randomised controlled trial
L. Hesseldal1, J.R. Christensen1, T.B. Olsesen2, M.H. Olsen3, P.R. Jakobsen4, J.T. Lauridsen5, D.H. Laursen6, J.B. Nielsen1, J. Søndergaard1, C.J. Brandt1;
1Research Unit for General Practice,, Research Unit for General Practice, Institute of Public Health; University of Southern Denmark, Odense, 2Steno Diabetes Center Odense, Steno Diabetes Center Odense, Odense University Hospital (OUH), Odense, 3Department of Internal Medicine, Holbaek Hospital and Steno Diabetes Center Zealand, Holbaek, 4Research Unit for General Practice, Research Unit for General Practice, Institute of Public Health; University of Southern Denmark, Odense, 5Department of Business and Economics, University of Southern Denmark, Odense, 6Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Long-term weight loss among subjects with obesity can reduce the risk and progression of noncommunicable diseases (NCDs) such as cardiovascular disease, respiratory disease, and type 2 diabetes (T2D). Unfortunately, long-term weight loss has been historically difficult for patients with obesity and T2D to achieve and maintain. Observational studies suggest that digital coaching can lead to long-term weight loss and potentially reduce the risk of developing NCDs. To assess whether an eHealth lifestyle coaching program (LIVA) for motivated subjects with obesity with or without T2D leads to significant long-term (more than 6 months) weight loss compared to usual care.
Materials and methods: In an open, randomized controlled trial, 340 subjects with obesity with or without T2D were enrolled from March 2018 to March 2019 and randomized to the intervention (200) and control (140) groups. The digital lifestyle intervention comprised an initial one hour face-to-face motivational interview followed by digital coaching using behavioral change techniques enabled by individual live monitoring.
Results: At 6 and 12 months, data were assessed for 235 participants, 149 from the intervention group and 86 from the control group. After 12 months mean body weight and body mass index were reduced significantly in both groups but significantly more in the intervention group (-4.6(95%CI, -5.7; -3,4) kg vs. -1.4(95%CI,-2.6;-0.1) kg, P<0.005 and -1.5(95%CI,-1.9;-1.2) kg/m2 vs. -0.5(95%CI,-0.9;-0.1) kg/m2, P < 0.005). HbA1c was significantly reduced in both the intervention (-6.0(95%CI,-7.7;-4.3)mmol/mol) and control group (-4.9(95%CI,-7.4;-2.4)mmol/mol) without significant group difference. Blood pressures and lipid profiles did not change significantly.
Conclusion: Compared to usual care, digital lifestyle coaching can induce significant weight loss in obese subjects with or without T2D.
Clinical Trial Registration Number: NCT03788915
Disclosure: L. Hesseldal: None.
222
Telehealth versus in-person standard care in patients with type 1 diabetes treated with multiple doses of insulin: an open-label randomised controlled trial
S. Ballesta1,2, J.J. Chillarón1,2, Y. Inglada1, A. Rodriguez3, E. Climent2, G. Llauradó2, H. Camell4, J.A. Flores1,2, D. Benaiges1,2;
1Endocrinology and Nutrition, Hospital Comarcal de l´Alt Penedès, Vilafranca del Penedès, 2Endocrinology and Nutrition, Hospital del Mar, Barcelona, 3Research department, Hospital Comarcal de l´Alt Penedès, Vilafranca del Penedès, 4Internal Medicine, Hospital Comarcal de l´Alt Penedès, Vilafranca del Penedès, Spain.
Background and aims: Increasing evidence indicates that telehealth (TH) is non-inferior to in-person approach regarding metabolic control in type 1 diabetes (T1D) patients, and offers advantages such as a decrease in travelling-time to outpatient clinic and increased accesibility for shorter and more frequent visits. This point has been even more important during the COVID-19 pandemic in rural zones, where distances are longer and time spent travelling are greater. The primary aim of our study was to compare, in T1D patients assisted in a rural area, the change in HbA1c at 6-month between TH and in-person visits. As secondary objectives we compared glucometric parameters, hypoglycemic events, EsDQoL questionnaire, direct and indirect costs, and patient’s satisfaction.
Materials and methods: Randomized controlled study, open-label, parallel arms, among T1D subjects. In the conventional group, patients were submitted to standard in-person visits (30 minutes) in outpatient clinic (baseline, 3 and 6 months). In the TH group, patients were submitted to teleconsultation (10 minutes) in month 1, 2, 3 and 4; baseline and 6-month visits were in-person. Mean-differences in change of included variables during follow-up were analyzed.
Results: 55 subjects were included (29 conventional, 26 TH). Mean age was 50.6 years ± 12.0, 48% were females, T1D evolution was 21.5 years + 11.6 and mean HbA1c was 7.56 % ± 0.7. No differences in baseline characteristics between groups were observed. At 6-month follow-up, the mean change in HbA1c was 0.2% (range: -1.3 - 1.2) in the control group and -0.05% (range: -0.8 - 1.7) in the TH group (mean difference 0.013, P = 0.932). Changes in EsDQoL questionnaire, glucometry parameters, mild hypoglycemic events and costs are shown in Table 1. No severe hypoglycemia was detected. Total time spent by endocrinologist was 93.2 minutes + 12.0 in conventional group vs 102.9 minutes + 7.6 in TH group (P= 0.001). 46% of patients in TH group preferred alternation between both types of visits. The main concern about telehealth was non-compliance with visiting hours, and main advantages were no need to travel to hospital and time saving.
Conclusion: TH is comparable to in-person visits regarding HbA1c levels at 6-month follow-up among T1D subjects, with significant improvement in time in range (TIR) and EsDQoL. Despite increased costs for national care system, teleconsultations imply a decrease in direct and indirect costs for patients. Further studies about telemedicine in T1D are necessary to evaluate a more efficient timing of the TH visits, as well as long-term cost analysis studies.
Clinical Trial Registration Number: ClinicalTrials.gov identifier NCT04758884
Disclosure: S. Ballesta: None.
223
Association between HbA1c and time in range in adults with type 1 diabetes using sensor-based glucose monitoring: a Swedish National Diabetes Register population-based study
E. Kjölhede1,2, J. Nåtman3, J. Ekelund3, S. Salö4, N.F. Nielsen5, B. Eliasson1, K. Eeg-Olofsson1,3;
1Department of Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden, 2Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, Gothenburg, Sweden, 3Centre of Registers, Gothenburg, Sweden, 4Novo Nordisk Skandinavia AB, Malmö, Sweden, 5Novo Nordisk A/S, Søborg, Denmark.
Background and aims: New sensor-based continuous glucose monitoring (CGM) systems used in combination with modern insulin treatment (either multiple daily injections or insulin pump) offer potential to improve glycaemic control and provide new glycaemic measures. HbA1c is an important measure of overall glycaemic control but not of glucose variability. In Sweden today, >85% of type 1 diabetes (T1D) patients use sensor-based glucose monitoring, making it possible to assess new measures of glucose variability and time in range (TIR) in routine diabetes care. This study aimed to describe the relationship between HbA1c and TIR (percentage of time in the range of 4-10 mmol/L).
Materials and methods: This cross-sectional nationwide study included people aged ≥18 years with T1D using CGM (intermittent-scanning or real-time) with at least one registration of TIR in the Swedish National Diabetes Register (NDR) from 1 January 2020 to 23 December 2021. Each TIR value was matched to the HbA1c value closest in time, maximum ±90 days. If a patient had more than one TIR and HbA1c pairing, one observation was selected at random. Other sensor data variables were collected from the same registration as the selected TIR value. For all other characteristics in the NDR, the latest registered value within 365 days before the TIR registration was selected. The linear association was assessed using Pearson correlation coefficient. Linear regression models were estimated between HbA1c and TIR, with and without adjustment for time below range (TBR; <4 mmol/L), diabetes duration, sex and insulin delivery method.
Results: The analysis included 27,980 people with T1D, 46% women, 30% on insulin pump, 7% with previous coronary heart disease and 64% with retinopathy. Mean (±SD) values were: age 48±18 years, diabetes duration 25±16 years, HbA1c 59±13 mmol/mol, TIR 59±19%, TBR 5±5%, sensor glucose 9.2±2.0 mmol/L and sensor glucose SD 3.3±1.0 mmol/L (mean coefficient of variation 36±7%). The distribution of TIR was marginally right-shifted in women compared with men. Figure 1 shows the association between HbA1c and TIR with a correlation coefficient −0.71 (−0.71 in men, −0.71 in women, −0.72 in pen users, −0.69 in pump users). In the crude linear regression, R2 was 0.51 and, in the adjusted model, R2 was 0.57.
Conclusion: This study with real-world data showed a clear association between HbA1c and TIR in adults with T1D, suggesting that TIR may be a relevant complement to HbA1c in everyday practice. Future studies are needed to evaluate if TIR is also associated with risk of complications and costs.
Supported by: Novo Nordisk A/S
Disclosure: E. Kjölhede: Honorarium; Novo Nordisk: consulting fee for reviewing and revising survey content.
224
Accuracy of continuous glucose monitoring systems during exercise-related hypoglycaemia in patients with type 1 diabetes
K. Maytham1, P.G. Hagelqvist1, U. Pedersen-Bjergaard2, F.K. Knop3, A. Andersen1, T. Vilsbøll1;
1Clinical Research, Steno Diabetes Center Copenhagen, University of Copenhagen, Herlev, 2Department of Endocrinology and Nephrology, Nordsjællands Hospital Hillerød, University of Copenhagen, Hillerød, 3Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark.
Background and aims: Hypoglycaemia is common in patients with type 1 diabetes, especially during exercise. Continuous glucose monitoring (CGM) accuracy is clinically important since low sensor precision may lead to undetected events of hypoglycaemia or unnecessary meal intake resulting in hyperglycaemia. We investigated sensor accuracy in available CGM devices during exercise-related hypoglycaemia.
Materials and methods: Fifteen patients with type 1 diabetes participated in two separate euglycaemic-hypoglycaemic clamp days (Clamp-exercise and Clamp-rest) with five different phases: 1) a baseline-euglycemic phase, 2) a plasma glucose decline phase ± exercise, 3) a 15-minute hypoglycaemic phase ± exercise, 4) a 45-minute hypoglycaemic phase (at rest), and 5) a plasma glucose incline phase. Plasma glucose was determined every five minutes, using Dexcom G6 and FreeStyle Libre 1. Yellow Spring Instruments 2900 (YSI) was used as a plasma glucose reference method, enabling mean absolute relative difference (MARD) assessment during each phase and Clarke error grid analysis during each day. We defined ΔMARD = MARDe (Clamp-exercise) - MARDr (Clamp-rest).
Results: We observed an overall higher MARD for Dexcom G6 compared to FreeStyle Libre 1 throughout both clamp days. CGM accuracy during phase 2 was more accurate during exercise compared to rest for Dexcom G6 (ΔMARD = -6.2 percentage points) while FreeStyle Libre 1 showed opposite results (ΔMARD = +5.3 percentage points) (Figure 1). During phase 3, exercise had no effect on Dexcom G6 accuracy, whereas FreeStyle Libre 1 was more accurate during rest (ΔMARD = +13.5 percentage points). During phase 4 (post-exercise versus post-rest) Dexcom G6 was more accurate after exercise than after rest (ΔMARD = -8.4 percentage points), whereas FreeStyle Libre 1 showed no differences between days. Clarke error grid analysis showed a decrease in clinically acceptable treatment decisions during Clamp-exercise for FreeStyle Libre 1 while a corresponding increase was observed for Dexcom G6.
Conclusion: Dexcom G6 had an overall lower accuracy compared to FreeStyle Libre 1. Exercise negatively impacted FreeStyle Libre 1 sensor performance during both declining plasma glucose and hypoglycaemia, whereas it improved Dexcom G6 sensor performance. Thus, physical exercise can affect the accuracy of different sensor systems in different manners, which may be of clinical importance, especially in detecting episodes of hypoglycaemia.
Clinical Trial Registration Number: NCT04650646
Supported by: DFF
Disclosure: K. Maytham: None.
OP 39 Too little sugar is also bad: understanding hypoglycaemia
225
Tmem117 in AVP neurons regulates the counterregulatory response to hypoglycaemia
S. Gaspari, G. Labouèbe, A. Picard, A. Rodriguez Sanchez-Archidona, B. Thorens;
University of Lausanne, Lausanne, Switzerland.
Background and aims: The counterregulatory response to hypoglycemia (CRR), which ensures a sufficient glucose supply to the brain, is an essential survival function. It is orchestrated by incompletely characterized glucose-sensing neurons, which trigger a coordinated autonomous and hormonal response that restores normoglycemia. A genetic-genomic screen for insulin-induced glucagon (GCG) secretion was conducted using a panel of 36 BXD recombinant inbred mouse strains. Tmem117, located on chromosome 15, was identified as a potential regulator of GCG secretion. Here, we aimed at characterizing the role of Tmem117 in hypoglycemia-induced GCG secretion.
Materials and methods: Immunofluorescence microscopy (IF); physiological characterization of mice with conditional, cell-specific gene inactivation; electrophysiological and in vivo fiber photometry measurements were used.
Results: We found that Tmem117 expression in the hypothalamus of the BXD mice was negatively correlated with the GCG response (Pearson’s R: -0.55; p=0.0003). IF found Tmem117 to be expressed in vasopressin (AVP) magnocellular neurons of the paraventricular and the supraoptic nuclei of the hypothalamus. Inactivation of Tmem117 specifically in AVP neurons (AVPTM117KO) led to higher hypoglycemia-induced copeptin (CPP; surrogate for AVP) secretion and higher plasma GCG levels in male mice [CPP: 89±5 vs 121±13 pg/ml, GCG: 124±19 vs 186±21 pg/ml, in Ctrl and AVPTM117KO mice, respectively; p<0.01; 2way-ANOVA RM]. In female mice this secretory phenotype was present only during the proestrus phase [CPP: 66±9 vs 107±16 pg/ml, GCG: 224±30 vs 377±22 pg/ml, in Ctrl and AVPTM117KO mice, respectively; p<0.05; t-test]. c-Fos IF and electrophysiological recordings performed on acute brain slices revealed that a large proportion (50%) of AVP magnocellular neurons were activated by hypoglycemia (glucose-inhibited, GI neurons). Inactivation of Tmem117 did not affect the glucose responsiveness of the AVP neurons. However, it led to their progressive elimination (number of AVP neurons in the SON: 91±9 vs 54±3 in Ctl and AVPTM117KO mice, respectively; 35 days after Tmem117 inactivation; p<0.05; t-test) and a loss of the increased secretory phenotype (CPP: 55±14 vs 83±6 pg/ml, GCG: 81±11 vs 119±19 pg/ml in Ctl and AVPTM117KO mice, respectively; 34 days after Tmem117 inactivation; p>0.05; 2way-ANOVA RM). Overexpression of Tmem117 in the insulin secreting MIN6B1 cells reduced K+-induced [Ca++]i and insulin secretion (Fluo-4: 35452±1030 vs 30888±2513 RFU, insulin: 145±11 vs 88±11 μg/L in Ctl and Tmem117 overexpressing cells, respectively; p<0.01; t-test). Inversely, fiber photometry experiments showed that Tmem117 inactivation increased [Ca++]i both at baseline (GCaMP7: 15±2 vs 42±10 z-score in Ctl and AVPTM117KO mice, respectively; p<0.01; 2way-ANOVA RM) and during insulin-induced hypoglycemia (GCaMP7: 1961±54 vs 2491±64 AUC in Ctl and AVPTM117KO mice, respectively; p<0.01; 1way-ANOVA).
Conclusion: Our study shows that AVP magnocellular neurons are activated by hypoglycemia to secrete AVP, leading to enhanced GCG secretion. It identifies Tmem117 as a regulator of hypoglycemia-induced CPP and GCG secretion, whose effect depends on the modulation of intracellular calcium dynamics. Finally, as these data were based on an unbiased genetic screen, they highlight the physiologically important contribution of AVP neuroendocrine cells to the counterregulatory response.
Supported by: INTEGRATE 694798, SNSF 310030-182496,
Disclosure: S. Gaspari: None.
226
Molecular investigations of defective hypoglycaemia counter-regulation in a murine model of type-2 diabetes: a multi-omics study
J. Castillo-Armengol1,2, A. Rodriguez Sanchez-Archidona2, C. Fledelius1, F. Marzetta3, B. Thorens2;
1Novo Nordisk A/S, Måløv, Denmark, 2Center for Integrative Genomics (CIG), University of Lausanne, Lausanne, Switzerland, 3Vital-IT Group, SIB Swiss Institute of Bioinformatics, Lausanne, Switzerland.
Background and aims: Repeated exposures to insulin-induced hypoglycaemia in diabetic patients progressively impair the counter-regulatory response (CRR), characterised by reduced secretion of glucagon and other counter-regulatory hormones. Increasing evidence indicates that glucose responsive neuronal networks, in particular located in the hypothalamus, orchestrate the CRR. However, how these neuronal networks become dysfunctional remains to be elucidated. Here we characterised the hypothalamus transcript profiles and chromatin structures in a mouse model of impaired CRR.
Materials and methods: Type-2 diabetes mellitus (T2DM) was induced in C57BL6N mice by high fat diet feeding and low dose streptozotocin injections. Mice were exposed to one (acute hypoglycaemia, AH) or multiple (recurrent hypoglycaemia, RH) insulin-induced hypoglycaemic episodes and plasma glucagon levels were measured. Single nuclei RNA sequencing (snRNAseq) and ATAC sequencing (snATACseq) data were obtained from the hypothalamus and cortex of AH and RH mice.
Results: A defective counter-regulatory response was observed in the T2DM mice exposed to RH as shown by the significant impairment of glucagon secretion in response to insulin injection (AH, n=33, 94.52±9.20 pg/mL vs RH, n=37, 59±pg/mL, p<0.001). Analysis of snRNAseq data revealed identical hypothalamic cell subpopulations in the hypothalamus of AH and RH mice. However, in the neuronal population, a total of 2373 genes were differentially expressed (FDR<0.05) between both mouse groups. Functional enrichment analysis revealed that these genes were strongly associated to the organisation of the synapses and cell projections. Interestingly, numerous genes related to the mitochondrial electron transport chain were also affected. The analysis of open chromatin regions confirmed the observations of the transcriptomic profiling. A subsequent analysis of the cortex of these animals showed that the effects observed in the hypothalamus were also identified in the cortex, suggesting that other brain regions might be similarly affected by the RH. A proteomic profiling of the synaptosomes from the hypothalami of these animals is currently being investigated and will provide additional characterisation of the synaptic proteome.
Conclusion: The present study provides a model of T2DM and defective counter-regulation in mice. Moreover, we show that the impairment of the gluco-regulatory response is related to genes controlling synaptic function and organisation. These changes in synaptic gene expression were observed not only in the hypothalamus, but also in the cortex suggesting that repeated exposure to hypoglycaemia impacts not only glucose sensing cells but may also induce neuronal dysfunctions in multiple brain regions.
Supported by: Novo Nordisk A/S, Innovative Medicines Initiative 2 Joint Undertaking (JU)
Disclosure: J. Castillo-Armengol: Employment/Consultancy; Novo Nordisk A/S. Grants; It has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 777460.
227
Heart rate variability, an index of autonomic function, is associated with C-peptidogenic index, an index of beta cell function: The Maastricht study
E. Rinaldi1, F. van der Heide2, R. Henry3, A. Kroon2, C. van der Kallen2, S. Eussen2, E. Bonora1, R. Bonnadonna4, M. Trombetta1, C. Zusi1, C. Schalkwijk2, M. van Greevenbroek2, C. Stehouwer2;
1Department of Medicine, University Hospital of Verona, Verona, Italy, 2Maastricht University, Maastricht, Netherlands, 3University of Maastricht, Maastricht, Netherlands, 4Department of Medicine, University Hospital of Parma, Parma, Italy.
Background and aims: Beta cell dysfunction is an important contributor to the early pathobiology of type 2 diabetes and its causes are matter of intensive investigation. Autonomic dysfunction may contribute to the pathobiology of beta cell dysfunction. We investigated, using population-based data, whether autonomic function is associated with beta cell function.
Materials and methods: We used cross-sectional data from The Maastricht Study, a population-based cohort study (N=2,007; mean ± SD age 60 ± 8 years; 52% men; and 24% with type 2 diabetes [the latter oversampled by design]). We assessed autonomic function from 24-hour ECGs as time- and frequency-domain heart rate variability (HRV); and we, using data from a 7-point oral glucose tolerance test, estimated beta cell function as C-peptidogenic index, beta cell glucose sensitivity, beta cell potentiation factor, overall insulin secretion, and beta cell rate sensitivity. Then, we used linear regression analyses to study the associations of standardized indices of HRV with standardized indices of beta cell function; and adjusted for age, sex, educational level, insulin sensitivity and major cardiovascular risk factors. In addition, we tested for interaction by sex and glucose metabolism status (type 2 diabetes; prediabetes; normal glucose metabolism) to investigate whether associations differed in strength by these factors.
Results: After full adjustment, greater time and frequency domain HRV were significantly associated with a higher C-peptidogenic index (standardized beta [95%CI], 0.05 [0.00;0.09] and 0.05 [0.00;0.09], respectively) and numerically similarly, though not statistically significantly, associated with higher beta cell glucose sensitivity (0.04 [0.00;0.08] and 0.04 [-0.00;0.08]), higher beta cell potentiation factor (0.04 [-0.00;0.08] and 0.03 [-0.01;0.08]), and higher overall insulin secretion (0.04 [-0.00;0.08] and 0.03 [-0.01; 0.07]). However, neither time nor frequency domain HRV were associated with beta cell rate sensitivity. Last, sex did not modify associations and glucose metabolism did not consistently modify associations.
Conclusion: The present population-based study found that better autonomic function, estimated from greater HRV, was associated with better beta cell function, estimated from C-peptidogenic index. Hence, autonomic dysregulation may contribute to the beta cell dysfunction and, ultimately, to deterioration of glucose metabolism.
Supported by: ERDF, PL, DMEA,SD,PSID,CVM,CARIM, CAPHRI,NUTRIM, SA, HFL, DF, OF, JC, NNF, SA,
Disclosure: E. Rinaldi: None.
228
Agpat5 in AgRP neurons is required for hypoglycaemia-induced glucagon secretion
A. Strembitska1, G. Labouebe1, A. Picard1, X.P. Berney1, D. Tarussio1, M. Jan2, B. Thorens1;
1University of Lausanne, 2Vital-IT, Lausanne, Switzerland.
Background and aims: The counter-regulatory response to hypoglycemia restores blood glucose levels by stimulating glucagon secretion and hepatic glucose release. This response is coordinated by still poorly characterized brain hypoglycemia sensing mechanisms, which control autonomic nervous activity and the hypothalamus-pituitary-adrenal axis. In a previous genetic and genomic screen for hypothalamic regulators of hypoglycemia-induced glucagon secretion we identified Agpat5, located on mouse chromosome 8, as a candidate gene. Here we aimed at characterizing the role of Agpat5, a mitochondrial membrane-associated enzyme that uses fatty acyl-CoAs and lysophosphatidic acid to produce phosphatidic acid.
Materials and methods: We generated mice lacking Agpat5 expression in AgRP neurons of the arcuate nucleus of the hypothalamus (AgRPAgpat5KO). Glucagon secretion was assessed following insulin-induced hypoglycemia (IIH). AgRP neurons activation was assessed by c-Fos immunostaining, patch clamp analysis and fiber photometry. Mitochondrial respiration experiments were conducted using a Seahorse instrument.
Results: We found that AgRPAgpat5KO (KO) mice had reduced glucagon secretion upon IIH as compared to control mice (29.0±4.1pM (Ctrl) vs 16.1±1.7pM (KO), n=18-22, p<0.001, two-way ANOVA, Tukey’s post hoc). c-Fos immunostaining showed reduced number of AgRP neurons activated by hypoglycemia in KO vs. Ctrl mice. Patch clamp analysis performed on acute brain slices revealed that inactivation of Agpat5 reduced by half the percentage of glucose inhibited (GI) AgRP neurons (72.2% (Ctrl) vs 33.3% (KO), n=18 neurons/group, p<0.05, Fisher’s exact test). In vivo fiber photometry revealed that AgRP neurons of KO mice were less activated by hypoglycemia than those of Ctrl mice. These hypoglycemia sensing defects were associated with defective activation of the vagal nerve. To find how Agpat5 could interfere with hypoglycemia sensing, we firs used a hypothalamic cell line. We found that silencing Agpat5 expression increased Cpt1a-dependent mitochondrial fatty acid β-oxidation (FAO), oxygen consumption rate (OCR), and ATP production (49.8% increase in mitochondrial OCR with Agpat5 silencing (p<0.05) vs 3.4% decrease in OCR with Agpat5/CPT1a silencing (p>0.05) when compared to Ctrl, n=5, two-way ANOVA, Tukey’s post hoc). In mice, inactivating Cpt1a in AgRPAgpat5KO mice (AgRPAgpat5KO/CPT1aKO) restored the number of hypoglycemia-activated AgRP neurons as determined by c-Fos immunostaining and patch clamp analysis. This suggests that limiting FAO in AgRP neurons is necessary for effective hypoglycemia detection.
Conclusion: Collectively, these data indicate that Agpat5 is required to partition acyl-CoA away from mitochondrial FAO and ATP generation. This is required to ensure that the hypoglycemia-dependent fall in ATP production, which triggers AgRP firing through inhibition of the Na+/K+ATPase, is not prevented by increased FAO. This mechanism is especially important during the fasted state when circulating free fatty acid concentrations increase. Our study thus describes a so far unknown protective mechanism that ensures proper hypoglycemia sensing by AgRP neurons and the control of glucagon secretion.
Supported by: FNS, ERC-INTEGRATE, Hypo-Resolve
Disclosure: A. Strembitska: None.
OP 40 When the clock ticks
229
Time-of-day effects of exercise training on multi-tissue metabolome and skeletal muscle proteome profiles in men with type 2 diabetes
M. Savikj1, S. Ben2, S. Shogo3, K. Caidahl1,4, A. Krook5, A.S. Deshmukh2,6, J.R. Zierath1,2, H. Wallberg-Henriksson5;
1Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden, 2Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, 3Center for Epigenetics and Metabolism, INSERM U1233, Department of Biological Chemistry, School of Medicine, University of California, Irvine, USA, 4Department of Clinical Physiology, Karolinska University Hospital, Stockholm, Sweden, 5Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 6The Novo Nordisk Foundation Center for Protein Research, Clinical Proteomics, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Metabolic effects of exercise may partly depend on the time-of-day when exercise is performed. We tested the hypothesis that exercise timing affects the adaptations in multi-tissue metabolome and skeletal muscle proteome profiles in men with type 2 diabetes.
Materials and methods: Men fitting the inclusion (type 2 diabetes, age 45-68 years and body mass index 23-33 kg/m2) and exclusion criteria (insulin treatment, smoking, and concurrent systemic disease) were included in a randomized crossover trial (n=15). Participants that fully completed all exercise sessions (n=8) were analysed. The trial consisted of two weeks of high-intensity interval training (HIT) (three sessions/week) either in the morning (08:00, n=5) or afternoon (16:45, n=3), a two-week wash-out period, and an additional two weeks of HIT at the opposing time. Blood, skeletal muscle and subcutaneous adipose tissue were obtained before the first, and after each training period. Broad-spectrum, untargeted proteomic analysis was performed on skeletal muscle, and metabolomic analysis was performed on all biosamples. Differential content was assessed by linear regression and pathway set enrichment analyses were performed. Coordinated metabolic changes across tissues were identified by Spearman correlation analysis.
Results: Metabolic and proteomic profiles remained stable after two weeks of HIT, and individual metabolites and proteins were not altered. However, coordinated changes in relevant metabolic pathways and protein categories were identified. Morning and afternoon HIT similarly increased plasma diacylglycerols, skeletal muscle acyl-carnitines, and subcutaneous adipose tissue sphingomyelins and lysophospholipids. Acyl-carnitines were central to training-induced metabolic cross-talk across tissues. Plasma carbohydrates, via the penthose phosphate pathway, were increased and skeletal muscle lipids were decreased after morning compared to afternoon HIT. Skeletal muscle lipoproteins were higher, and mitochondrial complex III abundance was lower after morning compared to afternoon HIT.
Conclusion: We provide a comprehensive analysis of a multi-tissue metabolomic and skeletal muscle proteomic responses to training at different times of the day in men with type 2 diabetes. Increased circulating lipids and changes in adipose tissue lipid composition were common between morning and afternoon HIT. However, afternoon HIT increased skeletal muscle lipids and mitochondrial content to a greater degree than morning training. This diurnal component in the metabolomic and proteomic training response may be clinically relevant and warrants further investigation.
Clinical Trial Registration Number: Pilot study for: NCT03553524
Supported by: KAW, Swedish Diabetes and HLF Foundations, Vetenskapsrådet, SLL, EFSD/Lilly and SRP Programmes
Disclosure: M. Savikj: None.
230
Beta cell functional heterogeneity underpinning coordinated oscillatory activity is not fixed
K. Suba1, B. Hansen1, Y. Patel2, B.M. Owen3, W. Distaso4, V. Salem1;
1Department of Bioengineering, Imperial College London, London, 2PerkinElmer, Newport, Wales, 3Department of Metabolism, Digestion and Reproduction, Imperial College London, London, 4Imperial College London, London, UK.
Background and aims: There is increasing recognition that pancreatic islets work as functional units. Electrically coupled beta-cells subserve pulsatile insulin release, co-ordinated by sub-populations of leader cells in vivo. It remains unknown whether this beta-cell functional heterogeneity is associated with a fated or fixed identity of these highly connected hubs. We identified the same cross-section of pancreatic islets in vivo, at single-cell resolution, in repeated imaging sessions over months to longitudinally track the activity of leader cells.
Materials and methods: We implanted Ins1CreGCaMP6ffl/fl-expressing islets into the anterior eye chamber of wild-type C57BL/6 syngeneic recipients. Calcium dynamics of reporter islets were imaged over a period of 4months; at baseline (normal chow), after high-fat diet and following daily injections of gut-hormone analogues or weight-matching intervention. The same islet cross-section was re-identified during each imaging session and calcium oscillations of individual beta-cells were recorded. First responder cells during a wave of calcium activity were identified and all beta-cell calcium traces were subjected to independent Granger causality analysis
Results: We found that first responder cells were among the top 25% of cells with the highest number Granger links. Granger leaders emerged from the same area in the islet cross-section during our recordings (<4minutes) however their identity changed dynamically over a longer period of time (months) in vivo.
Conclusion: The identity of Granger leader cells remains stable in the acute setting (minutes to hours) but changes dynamically over longer periods of time (months). Investigating the mechanisms underlying these changes will improve our understanding of pulsatile insulin release and how to sustain it.
Disclosure: K. Suba: None.
231
Time-of-day influences post-exercise metabolism in mouse adipose tissue
L.A. Pendergrast1, L. Dollet2,3, L.S. Lundell3, A.M. Ehrlich3, S.P. Ashcroft3, J.T. Treebak3, A. Krook2, J.R. Zierath1,3;
1Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden, 2Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 3Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Exercise is an effective approach to reduce the risk of type 2 diabetes, as well as to improve glucose uptake and mitochondrial activity in adipose tissue. Physiological events such as hormonal secretion, feeding behavior, and tissue metabolism follow a diurnal pattern regulated by central and peripheral circadian clocks. We hypothesized that the circadian clock may interact with exercise in regulating adipose tissue function and thus investigated the time-of-day response to exercise on the adipose tissue transcriptome and metabolism.
Materials and methods: Mice performed a 60-minute exercise bout (or sham) during the early rest (day, ZT3) or early active (night, ZT15) phase. Tissue and serum samples were collected at 0, 4, 8, 12, 16, and 20h post-exercise (n=6 per group), and RNA sequencing was performed on inguinal adipose tissue at t=0h after exercise. An additional group of mice was exercised (or sham) during the early rest phase in either an ad libitum or 10h-fasted state, with samples collected immediately following intervention (n=7 per group). Adipose tissue response and whole-body metabolic adaptation were evaluated by measuring gene expression, hepatic and skeletal muscle glycogen, serum lipid, and hormone levels.
Results: The exercise intervention did not modulate rhythmic expression of core clock genes Arntl and Nr1d1 in adipose tissue. Exercise-mediated adrenaline and corticosterone levels were similar between the early rest and early active phase, yet plasma non-esterified fatty acids (NEFA) were increased with early active phase exercise only (two-way ANOVA; p<0.05). Transcriptomic data revealed a time-of-day specific exercise response in adipose tissue, with changes specific to active phase only (79 transcripts altered in active phase versus 0 in rest phase, FDR<0,05). Among genes altered by early active phase exercise were those related to stress and thermogenesis, such as Nr4a3 and Ucp1. To decipher if feeding status and differential substrate availability are responsible for these time-of-day specific effects, we replicated the “unfed” state of the early active phase mice by fasting mice 10-hours prior to the early rest phase. Fasted early rest mice showed an increase in plasma NEFA (0.94± 0.06 mmol/L versus 0.66± 0.05 mmol/L; p=0.0001) and a depletion of liver glycogen (26.12±9.5 μmol/g versus 98.31±14.3 μmol/g; p=0.0001) similar to early active phase mice. However, fasted early rest phase exercise did not recapture expression patterns of early active phase exercise-responsive genes.
Conclusion: Exercise in the active phase influences adipose tissue gene expression and increases exercise-induced lipolysis. However, fasted early rest phase exercise does not replicate the time-of-day profile in gene expression observed with early active phase exercise, and implicates a role of the circadian clock in the adipose tissue post-exercise transcriptome. Our results provide evidence to suggest that exercise timing can fine-tune the metabolic benefits of exercise—particularly within adipose tissue. Time of day is a central variable to consider in prescribing exercise interventions for humans with metabolic syndrome.
Supported by: KID funding from Karolinska Institutet. Novo Nordisk Foundation Challenge Program
Disclosure: L.A. Pendergrast: None.
232
The effects of hyperglycaemia on the circadian clock in TALLYHO/JngJ mice
S.P. Ashcroft1, A.M. Ehrlich1, T.S. Nielsen1, S. Larsen2, J.T. Treebak1, J.R. Zierath3,1;
1Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, 2Center for Healthy Aging Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark, 3Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Background and aims: Perturbed circadian rhythms have been associated with metabolic dysfunction and type 2 diabetes (T2D) however, little is known regarding how hyperglycemia influences the circadian clock in peripheral tissues. To investigate this further, we utilised the TALLYHO/JngJ mouse which is a polygenic rodent model of T2D characterised by hyperglycemia, hyperinsulinemia, peripheral insulin resistance and hepatic steatosis. Interestingly, the hyperglycemic phenotype is not 100% penetrant allowing us to segregate these mice into normoglycemic (TALLY-Low) and hyperglycemic (TALLY-High) groups. Therefore, the aim of the study is to determine how the degree of hyperglycemia influences the circadian clock in TALLY-Low and TALLY-High mice. To do this, we performed a multi-tissue transcriptomic analyses of skeletal muscle, adipose tissue and liver samples collected across a circadian cycle.
Materials and methods: Male TALLY-Low and TALLY-High mice were studied between the ages of 6 to 18 weeks. Age and sex matched C57BL/6NTac mice were utilised as a healthy control mouse in all experiments. To characterise the development of hyperglycaemic phenotype, body weight, blood glucose and insulin were monitored on a weekly basis from 6 to 18 weeks of age (n=10/group). To assess tissue specific alterations to the circadian clock, liver, quadricep and white adipose tissue samples were collected across a 24-hour period at zeitgeber times (ZT) 0, 4, 8, 12, 16 and 20 for RNA-sequencing (n=4/group) in 10-week-old mice. Finally, metabolic caging was utilised to assess daily rhythms in whole-body metabolism (n=12/group).
Results: TALLY-High mice displayed overt hyperglycaemia by 9 weeks of age (20.9 ± 7.2 mmol) in comparison to TALLY-Low (10.9 ± 1.1 mmol) and C57BL/6NTac (8.9 ± 0.5 mmol) mice (P < 0.05). At 8 weeks of age, both TALLY-Low (1.4 ± 0.4 ng/ml) and TALLY-High (1.0 ± 0.4 ng/ml) display hyperinsulinemia in comparison to C57BL/6NTac mice (0.6 ± 0.1 ng/ml) (P < 0.05). Insulin production is, however, almost completely reduced in TALLY-High mice by 18 weeks of age (0.5 ± 0.4 ng/ml) whereas hyperinsulinemia is maintained in TALLY-Low mice of the same age (1.6 ± 0.3 ng/ml). Both TALLY-Low and TALLY-High mice display reduced activity, energy expenditure and RER in comparison to C57BL/6NTac mice (P < 0.05). Finally, preliminary data revealed time-of-day dependent alterations in the expression of circadian clock genes Bmal1, Cry1 and Cry2 in TALLY-High liver samples collected at ZT4 and ZT16. Further analyses will determine the effects of hyperglycemia on the circadian clock in skeletal muscle and adipose tissue.
Conclusion: In conclusion, the TALLYHO/JngJ mouse is characterised by the divergent development of the hyperglycemic phenotype. This divergence is exacerbated by a reduction in insulin production in TALLY-High mice and preliminary analyses have revealed disruptions to the circadian transcriptome in the liver of TALLY-High mice.
Disclosure: S.P. Ashcroft: None.
OP 41 Viruses and diabetes: more than COVID-19
233
SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children
H.L. Gulseth1, P.L.D. Ruiz1,2, K. Størdal3, Ø. Karlstad1, N. Gunnes1,2, N.A. Lund-Blix1, H. Bøås1, L.C. Stene1, G. Tapia1;
1Norwegian Institute of Public Health, 2Oslo University Hospital, 3University of Oslo, Oslo, Norway.
Background and aims: Viral infections, including respiratory viruses, are hypothesized to increase the risk of type 1 diabetes. Several case reports suggest an association between new onset type 1 diabetes and SARS-CoV-2 infection. In this nationwide register-based study we aimed to test whether SARS-CoV-2 infection was associated with an increased risk of developing type 1 diabetes in children and adolescents.
Materials and methods: We linked individual-level data from national health registries for all children and adolescences in Norway (1.2 million individuals). Data were obtained from the Norwegian preparedness register that is updated daily with individual-level data on PCR-confirmed SARS-CoV-2 infections, COVID-19 vaccinations and disease diagnoses from primary and secondary health care. Children were followed from March 1st 2020 (the start of the pandemic) until diagnosis of type 1 diabetes, age 18 years, death, or March22th 2022, whichever occurred first. In a full population cohort and a test-negative design we used Cox regression with SARS-CoV-2 PCR positivity as a time-dependent exposure to estimate hazard ratios (HR) with 95% confidence intervals (CI), both unadjusted and adjusted for age, sex, non-Nordic country of origin, geographical area and socio-economic factors. Analyses were done separately for first type 1 diabetes diagnosis within or after 30 days post-SARS-CoV2 infection.
Results: We identified a total of 424,354 children with SARS-CoV-2 infection (of 1,202,174 children included at study start) and 990 incident cases of type 1 diabetes. The adjusted HR for type 1 diabetes at least 31 days after SARS-CoV-2 infection was 1.63 (95% CI 1.08, 2.47) in a test-negative design and 1.57 (95% CI 1.06, 2.33) in the full-cohort (figure).
Conclusion: Our findings suggest that SARS-CoV-2 infection is associated with increased risk of subsequent type 1 diabetes. Future studies should include long-term follow-up and SARS-CoV2 virus variants.
Disclosure: H.L. Gulseth: None.
234
Relation of incident type 1 diabetes to recent COVID-19 infection: cohort study using e-health record linkage in Scotland
H.M. Colhoun1, S. McGurnaghan1, L. Blackbourn1, L.E. Bath2, D.A. McAllister3, T.M. Caparrotta1, S.H. Wild4, S.N. Wood4, D. Stockton5, P.M. McKeigue4;
1Institute of Genetics & Cancer, University of Edinburgh, Edinburgh, 2Royal Infirmary of Edinburgh, Edinburgh, 3University of Glasgow, Glasgow, 4University of Edinburgh, Edinburgh, 5Public Health Scotland, Edinburgh, UK.
Background and aims: Studies using claims databases reported that SARS-CoV-2 infection >30 days earlier increased the incidence of type 1 diabetes. Using exact dates of diabetes diagnosis from the national register in Scotland linked to virology laboratory data we sought to replicate this finding.
Materials and methods: A cohort of 1849411 individuals aged <35 years without diabetes, including all those in Scotland who subsequently tested positive for SARS-CoV-2, was followed from 1 March 2020-22 November 2021. Incident type 1 diabetes was ascertained from the national registry. Using Cox regression we tested the association of time-updated infection with incident diabetes. Trends in incidence of type 1 diabetes in the population from 2015-2021 were also estimated in a generalised additive model.
Results: There were 365080 in the cohort with at least one detected SARS-CoV-2 infection during follow-up and 1074 who developed type 1 diabetes. The rate ratio for incident type 1 diabetes associated with first positive test for SARS-CoV-2 (reference category: no previous infection) was 0.88 (95% CI 0.63 to 1.23) for infection more than 30 days earlier and 2.62 (95% CI 1.81 to 3.79) for infection in the previous 30 days. However negative and positive SARS-CoV-2 tests were more frequent in the days surrounding diabetes presentation. In those aged 0-14 years incidence of type 1 diabetes during 2020-2021 was 20% higher than the 7-year average.
Conclusion: Type 1 diabetes incidence in children increased during the pandemic. However the cohort analysis suggests that SARS-CoV-2 infection itself was not the cause of this increase.
Supported by: DiabetesUK 17/0005627
Disclosure: H.M. Colhoun: None.
235
Modifiable risk factors including HbA1c and BMI are consistently associated with severe influenza, pneumonia, and Covid-19 infection outcomes in people with type 2 diabetes
R. Hopkins, K.G. Young, J. Godwin, D. Raja, N.J. Thomas, B.M. Shields, J.M. Dennis, A.P. McGovern;
Institute of Biomedical & Clinical Science, University of Exeter Medical School, Exeter, UK.
Background and aims: Previous UK population-based research has identified risk factors for severe Covid-19 outcomes in people with type 2 diabetes, but it is unclear whether these are specific to Covid-19 or general to respiratory infections. We aimed to compare risk factors for hospitalisation from Covid-19 (pre-vaccination roll-out) to those for pneumonia and influenza.
Materials and methods: UK routine primary care data were accessed from the Clinical Practice Research Datalink (CPRD) and linked to Hospital Episode Statistics (HES). We followed adults with type 2 diabetes from 01/09/2018-31/05/2019 (influenza and pneumonia hospitalisation cohort, n = 655,677) and from 01/02/2020-31/10/2020 (Covid-19 hospitalisation cohort, n = 583,185). We used multivariable Cox proportional hazard models to identify sociodemographic risk factors (sex, age, ethnicity, index of multiple deprivation quintile) and modifiable risk factors (HbA1c and BMI) for hospitalisation in each cohort. Models were adjusted for macrovascular and microvascular complications, and other key comorbidities.
Results: We observed 6,061 (1.04%) hospitalisations for Covid-19, 1,358 (0.21%) for influenza, and 13,987 (2.13%) for pneumonia. When assessing sociodemographic risk factors, for Covid-19 we replicated previously reported associations between male sex, older age, greater deprivation, non-white ethnicities, and severe outcomes. However, these differed from the associations found for the other respiratory infections. We observed a differential effect of ethnicity, where compared to people of white ethnicity, black and south Asian groups had increased Covid-19 hospitalisation (adjusted hazard ratio [aHR] 1.84 [95%CI 1.67-2.03], p<0.001, and 1.42 [1.30-1.54], p<0.001, respectively), but lower hospitalisation for pneumonia (aHR 0.74 [95%CI 0.68-0.82], p<0.001, and 0.87 [0.81-0.93], p<0.001, respectively). There was a stronger association between age and hospitalisation for pneumonia (aHR per 10 year increase in age: 1.51 [95%CI 1.48-1.54], p <0.001) than for Covid-19 (aHR 1.23 [1.19-1.26], p<0.001) and influenza (aHR 1.15 [1.08-1.22], p<0.001). Assessing modifiable risk factors, high HbA1c (>86 mmol/mol) was a consistent risk factor for all three respiratory infections: aHR for HbA1c >86 vs 48-53 mmol: 1.48 [95%CI 1.33-1.65], p<0.001 for Covid-19, 1.62 [1.29-2.05], p<0.001 for influenza, 1.33 [1.23-1.44], p<0.001 for pneumonia. Similarly, marked obesity (BMI>40) was a consistent risk factor: BMI >40 vs 25-29.9 kg/m² aHR 1.50 [1.36-1.67], p<0.001 for Covid-19, 1.48 [1.21-1.81], p<0.001 for influenza, 1.34 [1.24-1.44], p<0.001 for pneumonia.
Conclusion: In people with type 2 diabetes, sociodemographic risk factors, such as ethnicity and age, are differentially associated with hospitalisation for Covid-19, influenza, and pneumonia. Therefore existing Covid-19 risk models cannot be assumed to be entirely applicable to new respiratory infections. However, higher HbA1c and BMI are consistently associated with hospitalisation for all three infections studied. This supports that good glycaemic and weight control may lower the risk of severe respiratory infection outcomes.
Supported by: Diabetes UK (20/0006220)
Disclosure: R. Hopkins: None.
236
Enterovirus infection and risk of islet autoimmunity and type 1 diabetes: systematic review and meta-analysis of molecular studies
S.R. Isaacs1,2, A. Roy3, B. Dance1, D.B. Foskett1,2, A.J. Maxwell1,2, W.D. Rawlinson1,2, K.W. Kim1,2, M.E. Craig1,4;
1School of Women's and Children's Health, Faculty of Medicine, University of New South Wales, 2Serology and Virology Division, NSW Health Pathology, Virology Research Laboratory, Prince of Wales Hospital, 3School of Medical Sciences, Faculty of Medicine, University of New South Wales, 4Institute of Endocrinology and Diabetes, Children's Hospital at Westmead, Sydney, Australia.
Background and aims: Molecular methods of enterovirus (EV) detection have been widely adopted in recent years, especially within large prospective cohort studies of islet autoimmunity (IA) and type 1 diabetes (T1D). Here, we present a major update to our previous systematic review of molecular detection of EV infection and (i) IA or (ii) T1D.
Materials and methods: A systematic search of controlled observational human studies was performed using PubMed and Embase databases until August 2021 (PROSPERO #CRD42021236044), with no language restrictions. Eligible studies included cohort or case-control studies measuring EV RNA or protein in blood, stool, or tissue of individuals with IA or T1D, and in controls. Studies were assessed for bias using the Newcastle-Ottawa Scale and NHMRC levels of evidence, with meta-analysis performed in RevMan v5.4 using the Mantel-Haenszel method and random effects models, producing ORs with 95% CIs and p-values for each outcome.
Results: The initial search returned 3266 publications; removal of 570 duplicates and 1799 irrelevant studies left 897 relevant articles for full-text screening. After consolidation of participant overlap, the 113 records meeting eligibility criteria were assigned to 61 study units (50 case-control/NHMRC-III, 11 nested case-control/NHMRC-II), where 41 examined T1D, nine IA, and 11 both outcomes. NOS scores ranged from 2 to 8, with 34 studies scoring ≥6, indicating good methodological quality. For 25 studies, cases could be further separated into multiple groups, resulting in 91 case sub-groups overall (24 for IA, 67 for T1D). There were 11975 participants altogether, including 6003 cases (900 with IA, 5103 with T1D) and 5972 controls. Study design, quality, and method of EV detection demonstrated high levels of statistical heterogeneity. Meta-analysis of 56 studies demonstrated a significant association between EV infection and IA (17 studies, OR 2.16; 1.28-3.64; p=0.004; χ2/df=2.77) and T1D (48 studies, OR 8.41; 5.16-13.69; p<0.00001; χ2/df=6.59); within one month of T1D onset (27 studies, OR 18.01; 9.37-34.60; TBC p<0.00001; χ2/df=3.50).
Conclusion: There is a clinically significant association between EV infection, and both IA and T1D. This further supports the rationale for development of EV-targeted vaccines and antiviral therapy to prevent and reduce the impact of T1D. Large prospective studies commencing from pregnancy (e.g., ENDIA and DIPP-novum) will provide insight into the temporal relation between EV infection and the development of IA and T1D in early life.
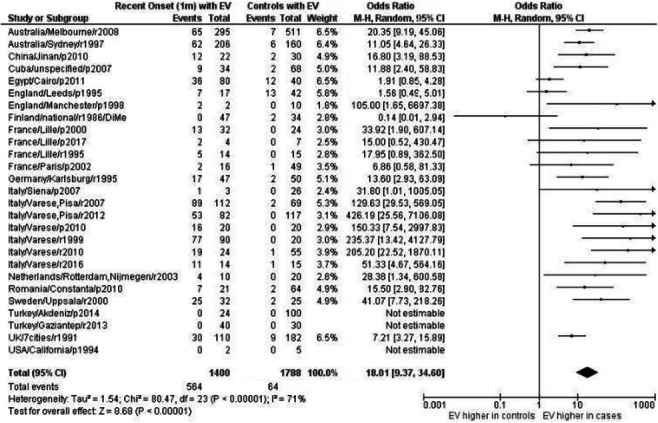
Disclosure: S.R. Isaacs: None.
OP 42 Moving towards the beta cell plasma membrane
237
OSBP-mediated PI(4)P-cholesterol exchange at endoplasmic reticulum-secretory granule contact sites controls insulin secretion
S. Panagiotou1, P.M. Nguyen1, A. Wendt2, L. Eliasson2, A. Tengholm1, O. Idevall-Hagren1;
1Medical Cell Biology, Uppsala University, Uppsala, 2Clinical science, Lund University Diabetes Centre, Malmö, Sweden.
Background and aims: Insulin granule biogenesis is a multi-stage process that involves cargo loading and processing and intracellular transport. Mature granules dock with the plasma membrane and undergo exocytosis to release insulin. We recently identified phosphatidylinositol-4 phosphate (PI[4]P) dephosphorylation by Sac2 on the surface of insulin granules as a key step in granule docking and found that Sac2 levels were reduced in islets from type-2 diabetics. The aim of this study was to determine how Sac2 and its lipid substrate PI(4)P contribute to the regulation of insulin secretion from β-cells.
Materials and methods: Clonal MIN6 β-cells expressing fluorescently tagged proteins were imaged by spinning disk confocal and TIRF microscopy. Cellular cholesterol distribution was determined using fluorescently labeled cholesterol (TopFluor-chol; live cells) or Filipin (fixed cells). Insulin secretion from MIN6 cells and mouse islets was determined by ELISA. Ultrastructural analysis of mouse and human islet cells was performed by transmission electron microscopy. For visualization of ER-granule contact sites in living cells, we developed a method based on dimerization-dependent red fluorescent protein monomers.
Results: Sac2 knockdown resulted in pronounced cholesterol accumulation on insulin granules, seen as increased TopFluor-chol fluorescence (0.117 for control, 0.161 for Sac2-KD, n = 25-26 cells, P < 0.05) and enhanced Filipin staining (control = 98 ± 3, Sac2-KD = 136 ± 5, n = 54, P < 0.001). Oxysterol-binding protein (OSBP) can exchange PI(4)P for cholesterol at membrane contact sites, and we found that OSBP knockdown normalized insulin granule cholesterol levels in Sac2 knockdown cells. OSBP localized to the trans-Golgi compartment under resting conditions but redistributed to insulin granules following inhibition of lipid exchange with 20 nM OSW-1 (n=6, P<0.001 for comparison to control). Similar redistribution was seen in mouse islets immunostained for OSBP and insulin. Depolarization of MIN6 cells also resulted in enrichment of OSBP at insulin granules (0.02±0.02 vs 0.13±0.03, n=30, P=0.0008) through a mechanism involving acidification of the cytosol, since it was prevented by the addition of 20 mM NH4Cl (-0.01±0.03, n=30, P=0.003). Ultrastructural examination showed many insulin granules in close proximity to the ER, and expression of an ER-insulin granule proximity reporter in mouse islets confirmed the existence of physical contacts between these two organelles and revealed that OSBP resides at these locations. siRNA-mediated knockdown of OSBP in MIN6 cells caused a 16±2% (n=4, P<0.05) reduction in GSIS and acute inhibition of OSBP with OSW-1 impaired second phase insulin secretion from mouse islets (n=6, P<0.05) without affecting cytosolic Ca2+.
Conclusion: Sac2 controls granule PI(4)P levels, which in turn fuel cholesterol transport to granules through the action of OSBP at ER-insulin granule contact sites. Defects in this lipid exchange result in impaired insulin secretion. This study identifies ER-granule contacts as important reaction centers in β-cells where lipid exchange occurs, but these sites are likely involved in additional processes, and identifying these is an important future goal.
Supported by: Swedish Research Council, NNF
Disclosure: S. Panagiotou: None.
238
Presynaptic scaffold protein, liprin, regulates glucose stimulated insulin secretion and the spatial organisation of exocytosis in pancreatic beta cells
K. Deng, N. Hallahan, P. Thorn;
Charles Perkins Centre, The University of Sydney, Sydney, Australia.
Background and aims: A key feature of insulin secretion is that it is targeted towards specialised regions (or hot spots) of the β-cell membrane. Recent evidence has shown that these exocytotic hotpots lie at the interface between β-cells and extracellular matrix (ECM) proteins of the islet capillaries. However, how insulin secretion is targeted towards these regions of cell-capillary contact remains elusive. Analogous to targeted insulin exocytosis in the β-cell, neurotransmitter release in neurons is confined to a specialized region of the presynaptic membrane known as the active zone; here, exocytosis is spatially regulated by a large multiprotein complex consisting of presynaptic scaffolds which dock and tether synaptic vesicles to specific sites for fusion. Interestingly, several of these presynaptic scaffold proteins (liprin, ELKS, RIM and piccolo) that are typically associated with the neuronal active zone have also been identified in β-cells and are enriched at the β-cell-capillary interface where secretion is targeted; however, whether these scaffold proteins facilitate targeted exocytosis in β-cells, as they do in neurons, is unknown. This research investigates presynaptic-like mechanisms for the spatial regulation of exocytosis in the β-cell, particularly, the role of liprin in positioning sites of β-cell insulin exocytosis.
Materials and methods: β-cells were isolated from humanely sacrificed C57BL/6 mice (approved by local and national ethics). We performed 3D live-cell two-photon imaging on β-cells expressing GFP-tagged liprin, to visualise the precise location of liprin with respect to sites of insulin exocytosis. We then optimised a protocol for the knockdown of liprin using an adenoviral-mediated shRNA system. We measured glucose-stimulated insulin secretion (GSIS) in control (scramble shRNA) and knockdown (liprin shRNA) β-cells using homogenous time resolved fluorescence and performed immunofluorescence staining. Finally, we expressed various liprin mutant constructs (designed to specifically interfere with binding to other presynaptic scaffold proteins) in β-cells to test whether liprin interacts or assembles with other scaffold proteins in a β-cell ‘presynaptic-like’ complex.
Results: We show that liprin assembles in small ‘islands’ or microdomains at the β-cell-capillary interface, where insulin granules preferentially fuse near (<0.3 μm) liprin structures (p<0.01) but are excluded from regions directly overlapping with liprin (p<0.01), suggesting that liprin may be involved in tethering insulin granules to specific sites for fusion. Western blot confirmed shRNA-knockdown of liprin (42.1%±4.0) in β-cells. Knockdown of liprin abolishes GSIS (p<0.05) and disrupts localisation of ELKS at the β-cell-capillary interface. Rescue of liprin knockdown using adenoviral-mediated overexpression rescues secretory phenotype. Finally, we show that expression of the N-terminus of liprin (ELKS- and RIM- binding domains) alone is sufficient for normal GSIS. Deletion of N-terminus significantly reduces GSIS (p<0.05), suggesting that liprin may function through interactions with ELKS and RIM in an ‘presynaptic-like’ complex at the β-cell-capillary interface.
Conclusion: We conclude that liprin is involved in spatially regulating insulin exocytosis through mechanisms analogous to a neuronal synapse.
Supported by: NHMRC, The Bioscientifica Trust
Disclosure: K. Deng: Grants; National Health and Medical Research Council, The Bioscientifica Trust.
239
Vamp8 is an endosomal v-SNARE involved in GLP1R trafficking and inhibits insulin exocytosis
L. Liu, M. Marshall, J. Saras, S. Barg;
Medical Cell Biology, Uppsala University, Uppsala, Sweden.
Background and aims: Insulin is released by Ca2+-dependent-dependent exocytosis of secretory granules, while many receptors, ion channels and transporters reach the membrane by constitutive exocytosis of vesicles that belong to the endosomal system. Both processes rely on distinct sets of SNARE proteins for membrane fusion. Beta cells express both VAMP2 on insulin granules and VAMP8 (endobrevin), which is an endosomal vesicular SNARE that has been proposed to have an additional role in “newcomer” exocytosis of insulin granules. The differential role of VAMP isoforms in insulin secretion is not well understood.
Materials and methods: Live cell TIRF-microscopy was performed in INS1 cells and in dispersed beta cells of cadaveric human donors. Vesicle behavior, exocytosis, and protein location was studied by expressing EGFP- or mCherry-tagged versions. Exocytosis was evoked by elevated K+ in presence of 10mM glucose/200μM diazoxide. Association of proteins with vesicular compartments was analyzed by quantitative image analysis. Gene expression was studied in publicly available expression data.
Results: VAMP8-EGFP was found on rab5-, rab7, and rab11-positive endosomal vesicles, but not on rab3-or NPY-labeled insulin granules. Depolarization with elevated K+ promoted exocytosis of the VAMP8/rab11-positive vesicles; these vesicles were distinct from insulin granules since exocytosis proceeded with a slower monophasic time course, was insensitive to tetanus toxin cleavage of VAMP2, and was accelerated by somatostatin but not exendin-4. VAMP8-positive vesicles underwent exocytosis within seconds of their arrival at the plasma membrane, in contrast to minutes for secretory granules. These vesicles also contained GLP1-receptor, GLUT2, and the autophagosome marker LC3. Expression of full-length VAMP8, VAMP8 lacking its transmembrane domain (but not VAMP2) inhibited exocytosis of insulin granules. The latter is consistent with negative correlation between VAMP8 gene expression and secretory index (SI) in human donor islets (puncorr.=0.00016, n=125).
Conclusion: We conclude that VAMP8 associates with early, late and recycling endosome compartment, but not insulin containing secretory granules. VAMP8 traffics to the plasma membrane with, and likely promotes, exocytosis of a rab11-positive endosomal recycling compartment. While Ca2+-dependent exocytosis of these vesicles controls surface expression of important membrane proteins (GLP1R and GLUT2), the associated release of VAMP8 may also regulate insulin secretion. Finally, the data suggest that the previously described “newcomer” exocytosis reflects release from an endosomal compartment, rather than exocytosis of insulin containing secretory granules.
Supported by: VR, NNF, DiabF, Ernfors, Rudberg
Disclosure: L. Liu: None.
240
Villin interacts with Snap25 and its expression increases in tamoxifen-treated EndoC-βH3 cells
H. Mziaut1, I. Kalaidzidis1, J. Dehghany2, A. Gheisari3, M. Herbig4, J.-C. Escolano4, A. Sönmez1, M. von Bülow5, M. Lohmann5, Y. Kalaidzidis6, M. Meyer-Hermann2, J. Guck4,7, A. Schulte5, R. Scharfmann8, M. Solimena1,6;
1Paul Langerhans Institute Dresden, Dresden, Germany, 2Helmholtz Centre for Infection Research, Braunschweig, Germany, 3Biopolis Dresden Imaging Platform (BioDIP), TU Dresden, Dresden, Germany, 4Biotechnology Center, Center for Molecular and Cellular Bioengineering, Dresden, Germany, 5Diabetes, Sanofi-Aventis Deutschland GmbH, Frankfurt, Germany, 6Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany, 7Max Planck Institute for the Science of Light & Max-Planck-Zentrum für Physik und Medizin, Erlangen, Germany, 8INSERM, U1016, Institut Cochin, Paris, France.
Background and aims: Ica512/Ptprn is a transmembrane cargo of the insulin secretory granules (ISGs). In Ica512-/- mouse islets, ISG stores as well as mRNA and protein levels of the F-actin modifier villin are strongly reduced. In rat insulinoma INS-1 cells, villin dynamically modulates the size of actin cages surrounding cortical ISGs, hence regulating their mobility and exocytosis. Evidence that villin acts downstream of Ica512 suggests that ISGs directly influence the remodelling of the cortical cytoskeleton for tight control of glucose-stimulated insulin secretion (GSIS). In the present studies we tested this hypothesis further.
Materials and methods: ISG motility in siRNA villin-depleted INS-1 cells was analyzed by total internal reflection fluorescence microscopy and in silico modelling. Protein-protein interactions in INS-1 cells were investigated by Förster-resonance energy transfer (FRET) and microscale thermophoresis (MST). Gene expression and protein localization in tamoxifen (TAM)-treated and untreated EndoC-βH3 cells, a surrogate model of human pancreatic β-cells, were assessed by next generation sequencing and confocal microscopy, respectively. Stiffness of TAM-treated and untreated EndoC-βH3 cells was quantified by atomic force microscopy (AFM) and real-time deformability cytometry (RT-DC).
Results: In silico modelling of siRNA villin-depleted insulinoma INS-1 cells suggested that increased ISG motility could not account alone for enhanced basal insulin release unless more docking sites for ISG exocytosis were accessible. Overexpression in INS-1 cells of villin and Snap25-mCherry for MST, and villin-TQ2, Snap25-Venus and positive and negative control reporters for FRET indicated that villin interacts with the SNARE Snap25. This finding is reminiscent of previous studies indicating that the villin paralogue gelsolin interacts with the SNARE Syntaxin4. Accordingly, in siRNA villin-depleted INS-1 cells, Snap25 was less restricted at the plasma membrane. Reduced proliferation as well as enhanced ISG stores and GSIS of TAM-treated EndoC-βH3 cells correlated with increased mRNA and protein expression of ICA512 and villin. Furthermore, TAM-treated EndoC-βH3 cells, as measured by RT-DC and AFM were 32% larger and had significantly higher apparent elastic modulus, while the processive mobility of ISGs was increased.
Conclusion: Based on these data we propose that: a) villin-induced remodelling of the F-actin cytoskeleton modulates ISG exocytosis by regulating their mobility and access to Snap25; b) upon β-cell differentiation villin expression is upregulated through Ica512 to adapt the plasticity of the cortical cytoskeleton to the enlarged ISG stores, thus enabling a tight control of GSIS.
Supported by: RHAPSODY, EFPIA, SERI, BMBF, SMWK and DZD
Disclosure: H. Mziaut: None.
OP 43 Microvascular cocktail
241
Prognostic value of diabetes microvascular complication for 21-years all-cause mortality
L. Sacchetta1, M. Chiriacò1, G. Forotti1, S. Leonetti2, L. Nesti1, A. Natali1, A. Solini1, D. Tricò1;
1University-Hospital of Pisa, 2Sant'anna School of advanced studies, Pisa, Italy.
Background and aims: Diabetes mellitus is associated with microvascular complications that impair patients’ prognosis and quality of life. Here we aimed to evaluate the impact of directly-measured diabetes microvascular complications, alone or in combination, on long term all-cause mortality.
Materials and methods: 497 subjects were screened for microvascular complications from 1999 to 2001. Diabetic kidney disease (DKD) was defined as glomerular filtration rate (GFR) <60 ml/min measured by dynamic renal scintigraphy and/or overnight albuminuria >20 μg/min; diabetic retinopathy (DR) by presence of pre-proliferative or proliferative retinopathy at dilated fundus oculi examination; and cardiac autonomic neuropathy (CAN) by presence of at least 2 cardiovascular test abnormalities and/or postural hypotension. Mortality data were retrieved from administrative databases for 303 (61.0%) patients in April 2021. Multivariate models were adjusted for age, sex, BMI, HbA1c, type and duration of diabetes.
Results: After 5,244 person-years of follow-up among 303 participants (median follow-up 21.0 [14.0-21.0] years, age 55.5±13.8 years, 155 [51%] women, BMI 28.6±5.9 kg/m2, HbA1c 9.0±2.1%, 218 [71.9%] with type 2 diabetes and 85 [28.1%] with type 1 diabetes), a total of 133 (43.9%) deaths occurred. The prevalence of DKD, DR, and CAN at baseline was 23.1%, 33.3%, and 24.1%, respectively. The presence of DKD reduced the mean overall survival (OS) by 2.6 years (-14.4%; log-rank test p<0.0001) and increased the adjusted all-cause mortality risk by 117% (aHR 2.17 [1.45-3.26]) (panel A). Patients with CAN showed 8.1% reduction in mean OS (-1.4 years; log-rank test p=0.046) and 54% increase in the adjusted risk for all-cause mortality (aHR 1.54 [1.01-2.36]). DR showed a mean OS reduction of 1.3 years (-7.4%; log-rank test p=0.02) but only numerically higher mortality risk (HR 1.23 [0.82-1.84]). A significant interaction emerged between CAN and type of diabetes (p=0.04), with CAN increasing all-cause mortality risk only in type 2 diabetes patients (HR 1.78 [1.32-2.81]). Patients with one, two, or three complications showed, respectively, a reduction in mean OS of 0.48 years (-2.7%), 2.52 years (-14%), and 2.96 years (-16.4%) (log-rank test p=0.0016). The presence of two or three concomitant microvascular complications increased the adjusted risk for all-cause mortality by 203% (HR 3.03 [1.62-5.68]) and 692% (HR 7.92 [2.93-21.37]), respectively (panel B).
Conclusion: Chronic microvascular complications significantly increase the 21-year all-cause mortality risk in patients with diabetes, regardless of age, sex, BMI, glycaemic control, diabetes type and duration. Multiple microvascular complications reduce life expectancy and have additive effects on the incidence of all-cause death in a real-life population with diabetes.
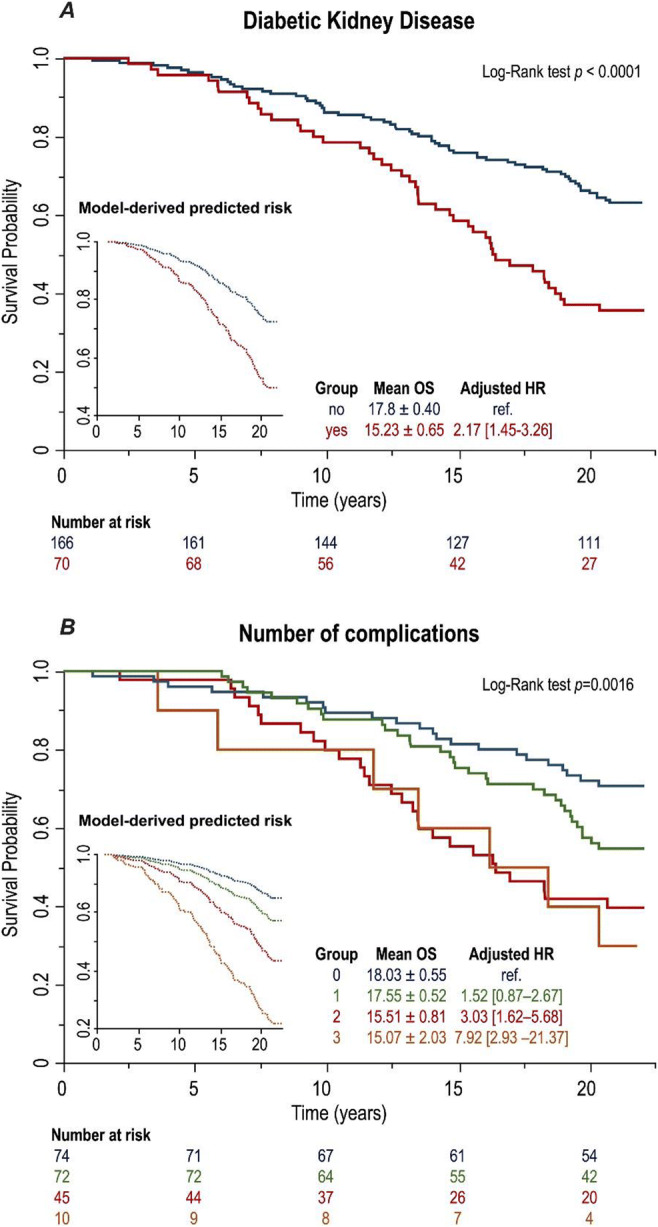
Supported by: EFSD Rising Star Fellowship
Disclosure: L. Sacchetta: None.
242
Three-years follow up of retinal neurodegeneration and neuropathic characteristics in pediatric type 1 diabetic patients
M. Menduni1, F. Picconi1, M.C. Parravano2, B. Russo1, A. Maiorino1, L. Chioma3, S. Cianfarani3, D. Ylli4, P.I. Patera3, S. Frontoni1;
1Unit of Endocrinology, Diabetes and Metabolism, S. Giovanni Calibita Fatebenefratelli Hospital, Rome, Italy, 2IRCCS-G.B. Bietti Foundation, Rome, Italy, 3Diabetes Unit, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy, 4Division of Endocrinology, MedStar Washington Hospital Center, MedStar Health Research Institute, Washington DC, USA.
Background and aims: Retinal neurodegeneration (RN) is considered an early marker of diabetic retinopathy. Few data are available on the possible association between RN and diabetic neuropathy (DN) and the predictive role of glycemic variability (GV) and lipid profile on early RN signs in the pediatric population with type 1 diabetes mellitus (T1DM). We previously demonstrated, in adult T1DM patients, a significant association between GV and triglyceride (TG) with early RN and between TG and the risk for early peripheral DN, independent of glucose control. The aim of our study is to evaluate the 3-year progression of structural alteration of neuroretina, the possible association between these early alterations and DN and the predictive role of GV and lipids on these precocious changes in pediatric T1DM subjects.
Materials and methods: 25 patients with T1DM (ages 10-20 years), using continuous glucose monitoring and continuous subcutaneous insulin infusion, without any complication, and 18 healthy controls (C), comparable in age and gender, were enrolled and followed for 3 years. All subjects underwent an Optical Coherence Tomography Heidelberg Spectralis, with analysis of macular neuroretinal layers. In T1DM, metabolic parameters, GV indexes, peripheral (Michigan Neuropathy Screening Instrument-MNSI, thermal threshold and vibration perception threshold-VPT) and autonomic assessment (using cardiovascular autonomic reflex tests) were investigated. Data were collected at baseline (V0) and after 12 (V1), 24 (V2) and 36 months (V3).
Results: During tthe 3-year follow up, among neuroretinal layers, Retinal Nerve Fiber Layer (RNFL), Outer Plexiform Layer (OPL) and Inner Retinal Thickness (IRT) were significantly thinner in T1DM versus C and a progressive reduction in OPL was observed in T1DM (p<0.05). In T1DM patients, negative correlations were observed between GV and neuroretinal layers, specially between continuous overall net glycemic action-1h and RNFL at V0 (r=-0.4, p=0.05), between mean absolute glucose (MAG) and OPL (r=-0.5, p=0.04) at V2 and between IRT delta thickness (V2-V1) and Lability Index (r=-0.6, p=0.01). No significant correlation between HbA1c and macular layer thickness was observed (p>0.05). Among metabolic parameters, a negative correlation between TG and IRT delta thickness (V2-V1) r=-0.5, p<0.01) was found. Among neuropathic characteristics, at V3, a negative correlation between the systolic blood pressure fall and OPL (r= -0.9 p=0.04) and a positive correlation between the VPT and RNFL (r=0.2 p=0.05) were observed. Moreover, there was a positive correlation between low blood glucose index and VPT (r=0.7 p=0.01) and between MAG and MNSI (r=0.7 p=0.02).
Conclusion: Very early morphological alterations of neuroretina are already present in pediatric T1DM patients without both vascular retinopathy and DN, and there is a possible association between these variations and early signs of peripheral and autonomic DN. These data corroborate the hypothesis that RN is, in fact, an early sign of DN and GV and TG should be efficaciously addressed in the early stage of T1DM.
Disclosure: M. Menduni: None.
243
Progression of diabetic retinopathy in a prospective randomised trial comparing Everolimus versus Mycophenolate therapy in pancreas and kidney transplant recipients
B. Hagerf (Voglova)1, Z. Hladikova1, L. Nemetova1, M. Zahradnicka1, K. Kesslerova2, T. Sosna2, F. Saudek1;
1Institute for Clinical and Experimental Medicine, 2Ophtalmology Clinic of Thomayer Hospital, Prague, Czech Republic.
Background and aims: Successful pancreas and kidney transplantation (SPK) in type 1 diabetic patients does not initially halt progression of diabetic retinopathy (DR), but tends to stabilize it in the long run. mTOR inhibitors are known for their antiangiogenic effect, but the impact of long-term systemic immunosuppression on DR had not yet been prospectively studied. Therefore we initiated a prospective randomized trial comparing the effect of either everolimus (E) or mycophenolic acid (MPA) on the course of DR in SPK recipients.
Materials and methods: Waitlisted Type-1 diabetic subjects were randomized to treatment with MPA or E together with tacrolimus, 6 weeks steroids and ATG induction. Eye examination including optical coherence tomography was done at the baseline, 6, 12 and 24 months. The composite primary endpoint comprised new need for laser therapy, newly diagnosed proliferation, clinically significant macular edema (CSME), best corrected visual acuity (BCVA) worsening. For statistical evaluation we used t-test, Mann-Whitney test, Fisher’s exact test, Kaplan-Meier test and log-rank test. Endpoints were evaluated per patient and per eye.
Results: Out of 64 enrolled patients, 55 (MPA 29, E 26) completed the follow-up. Most of these had proliferative DR (82.4% eyes in MPA and 78.2% in E group) with previous laser treatment. 2-year patient and death-censored pancreas and kidney graft survival rates did not differ between the groups. 59% of the patients in the MPA group and 50% in E group met the primary endpoint (p=0.6), mostly due to new proliferation, need for laser treatment and BCVA worsening. When analyzed per eye, the need for laser therapy was more frequent in the MPA group, reaching statistical significance in the second year post-transplant (22.8% vs 5.8% eyes, p=0.015), which corresponded with BCVA worsening (33.3% and 15.7%, p=0.045). Retinopathy-related BCVA worsening rate was significantly higher in the MPA than E group (22.8% vs 5.8%, p=0.015). Other causes of vision worsening were cataract and glaucoma. Total occurrence of bleeding was 19.3% in MPA group vs 3.9% in the E group (p=0.0154). There was no case of new blindness. Central retinal thickness increased in the 6th month post-transplant in both groups with subsequent restoration at 12 and 24 months. CSME rate was almost identical in both groups (MPA 7%, E 7.8%, p=0.9). Patients treated with laser less than 12 months pre-transplant were more likely to suffer from further DR progression meeting the primary endpoint in 72.7% (MPA) and 66.7% (E).
Conclusion: We observed retinopathy progression in both groups, with slightly more favorable outcomes in the everoliumus-treated group. We did not observe typical early worsening, rather ongoing natural course of advanced diabetic retinopathy in both groups.
Clinical Trial Registration Number: EUDRACT No 2013-004934-14
Supported by: Ministry of Health of Czech Republic, grant no.15-26746 A
Disclosure: B. Hagerf (Voglova): None.
244
Circulating proteins associated with risk of progression to ESKD in type 1 diabetes: results of multi-cohort study
J.K. Haukka1,2, Z.M. Dom3,4, V. Curovic5, K. Ihara3, E. Satake3,4, S. Mutter2, C. Forsblom1,2, A. Doria3,4, N. Sandholm1,2, P. Rossing5, P.-H. Groop1, A. Galecki3,6, A. Krolewski3,4;
1Folkhälsan Research Center, Helsinki, Finland, 2Abdominal Center, Nephrology, Helsinki, Finland, 3Research Division, Joslin Diabetes Center, Boston, USA, 4Harvard Medical School, Boston, USA, 5Steno Diabetes Canter Center, Copenhagen, Herlev, Denmark, 66Department of Biostatistics, School of Public Health, University of Michigan, Ann Arbor, USA.
Background and aims: Recently, many circulating proteins were found to be associated with the development of diabetic kidney disease (DKD) and its progression to ESKD. To facilitate research on the development of prognostic tests and on the role of circulating proteins in the etiology of DKD, a small set of 21 important proteins, referred to as the Joslin Kidney (JK) Panel, was developed. The assays to quantify these proteins were developed by OLINK Inc (Uppsala Sweden). The aim of this study was to examine whether the proteins included in the JK Panel were associated with progression to ESKD.
Materials and methods: Three international cohorts of patients with T1D, macro-albuminuria and eGFR >45 ml/min had been assembled and followed for 7 - 20 years to ascertain onset of ESKD: at Joslin (Boston) 387 patients (127 ESKD), at Steno (Denmark) 435 patients (104 ESKD), and at FinnDiane (Finland) 387 patients (127 ESKD). Methods of the assembly and follow-up of these cohorts were described previously (Skupien et al, 2019). Serum concentrations of the 21 proteins were measured with the OLINK platform. Association with DKD progression to ESRD was analyzed with Cox regression adjusted for baseline urinary albumin excretion rate, eGFR, and HbA1c.
Results: On average, patients who progressed to ESKD in comparison with those who did not progress had shorter duration of diabetes (26 vs. 28 years), higher baseline HbA1c (9.5 % vs. 8.9 %) and lower baseline eGFR (69 vs. 89 ml/min/1.73m2). Across the 3 cohorts all 21 proteins with nominally significant P-values show positive association with DKD progression (Figure 1). Strikingly, in the Joslin and the FinnDiane Cohorts almost all circulating proteins included in the JK Panel had strong association with progression to ESKD except for TNFRSF6B and PI3. In the Steno cohort, examined proteins showed weaker association with progression to ESKD compared to the two other cohorts. We are presently using the above data to develop a prognostic algorithm to estimate time to onset of ESKD in patients with DKD.
Conclusion: The JK Panel of circulating proteins measured using a custom-made OLINK platform showed extremely similar findings in two international cohorts. The weaker association with progression to ESKD of the majority proteins examined in Steno Cohort is difficult to explain at present. The JK Panel should be examined further not only for prediction of time to ESKD but also for identification of patients who will respond to specific reno-protective therapies and for monitoring the effectiveness of such therapies.
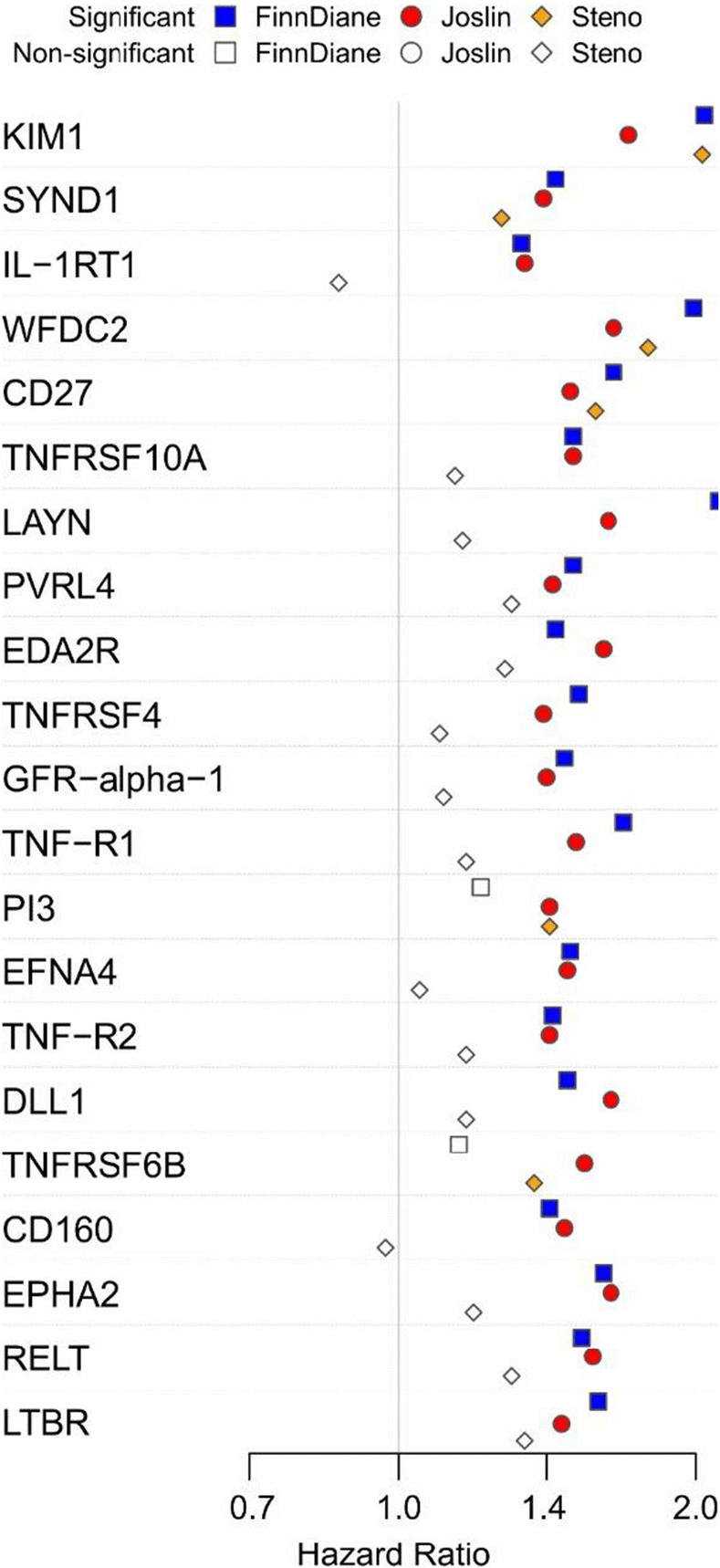
Disclosure: J.K. Haukka: None.
OP 44 Grading insulin therapy: simple, simpler, the safest
245
Delayed prandial insulin boluses are frequent and associated with glucose control in type 1 diabetes patients on advanced technologies
R. Triggiani, R. De Angelis, L. Bozzetto, G. Annuzzi;
Federico II University, Naples, Italy.
Background and aims: Advanced technologies (Hybrid Closed Loop Systems (HCLS) and Sensor Augmented Pump (SAP)) significantly improve blood glucose control in patients with type 1 diabetes. However, the optimization of their performances requires patient’s compliance to proper pre-meal insulin bolus administration. We explored how timely patients on HCLS or SAP inject the pre-meal bolus and whether delayed boluses may affect blood glucose control.
Materials and methods: One-hundred fifty-three type 1 diabetes patients, 79 women and 54 men, aged 41.9±14.2 on HCLS (n=121) or SAP (n=32) were consecutively recruited for this study. Two diabetologists (RT, RDA) independently reviewed two-week CGM and pump reports. Delayed boluses were assigned when a prandial bolus was preceded on CGM by a steep increase in blood glucose clearly indicating a postprandial response. An online questionnaire on Fear of Hypoglycemia was completed by participants Two-week CGM metrics were evaluated in relation to the number of delayed boluses by Pearson correlation
Results: At least one delayed bolus was identified in all patients, with a mean total number over two weeks of 9.2±4.4 delayed boluses. The number of delayed boluses was directly associated with GMI (r= 0.356, p<0.001), Coefficient of variation (r= 0.290, p<0.001), TAR>250 (r= 0.412, p<0.001), TAR>180 (r= 0.303, p<0.001), TBR<70 (r= 0.063, p=0.436), TBR<54 (r= 0.082, p=0.311), and inversely associated with TIR (r= -0.441, p<0.001). The number of delayed boluses was directly associated with fear of hypoglycemia at work, during night and being alone.
Conclusion: Delayed insulin boluses in patients on advanced technologies are very common (on average 1 out of 5 meals) and are associated with a significant worsening of blood glucose control. Adequate attention should be given to the timing of bolus injection also in patients on advanced technologies, mainly acting on fear of hypoglycemia.
Disclosure: R. Triggiani: None.
246
With which dose of NPH insulin to start at bedtime? Study in a cohort of 1006 patients with type 2 diabetes and failure of oral antidiabetic drugs
B. Mertes1, S. Gödde1, G. Egidi2, T. Uebel3, G. Kramer4, N. Kuniß4,5;
1Abteilung Diabetes, Neuropathie, Fußsyndrom, Cardioangiologisches Centrum Bethanien, Frankfurt am Main, 2Hausarztpraxis, Bremen, 3Hausarztpraxis, Neckargemünd, 4FB Endokrinologie und Stoffwechsel, Universitätsklinikum Jena, Klinik für Innere Medizin III, Jena, 5Dr. med. Kielstein Ambulante Medizinische Versorgung GmbH, Erfurt, Germany.
Background and aims: In the updated German National Health Care Guideline “Therapy of diabetes type 2”, for the first time, NPH insulin (Neutral Protamine Hagedorn, isophane insulin) at bedtime is recommended, if the HbA1c therapy target is missed after failure of oral medication. As a starting dose, various research groups worldwide recommend 0.1-0.2 IU/kg body weight. However, systematic studies investigating the starting dose of an insulin therapy with NPH insulin do not exist.
Materials and methods: A cohort of 1006 patients with diabetes type 2 and failure of oral antidiabetic drugs was treated additionally with NPH insulin at bedtime (63% male, age 59.5±11.5 years, weight 91.8±19.1 kg, body mass index 31.4±5.9 kg/m², diabetes duration 7.5±5.7 years, HbA1c 8.6±1.1%, number of different oral antidiabetic agents 1.2±0.5/patient). Blood glucose levels were checked twice during the night (22:00, 2:00, 5:00, and 7:00 h) to detect hypoglycaemia from a dose of 12 IU. All patients participated in structured education programme for individuals with insulin therapy at baseline. The combination of oral antidiabetic agents remained unchanged. Multiple linear regression was used to determine which factors influence final insulin dose.
Results: The NPH insulin dose of initially 9.4±2.0 IU was adapted within 20.2±24.9 days to 9.8±2.7 IU. This corresponds to a final insulin dose of 0.11±0.03 IU/kg body weight. In 77 patients (7.7%) the initial insulin dose had to be reduced by 2.44±1.09 IU due to low blood glucose levels during the night. Severe hypoglycaemia did not occur. Body weight and HbA1c were significantly associated with the final insulin dose. The lower the BMI (p<0.001) and the higher the HbA1c (p<0.001), the higher the insulin dose.
Conclusion: The NPH insulin dose of up to 0.2 IU/kg recommended by various research groups worldwide is too high. For example, a patient with body weight of 90 kg would need to inject 18 IU NPH insulin at baseline, but only 10 IU according to our study. A too high dose can be associated with nocturnal hypoglycaemia. A limitation of this study is the retrospective setting. If oral medication fails in type 2 diabetes, NPH insulin should be started at night with 0.1 IU/kg body weight. At a NPH insulin dose of 12 IU and above, monitoring blood glucose levels during the night is recommended.
Disclosure: B. Mertes: None.
247
Comparison of the basal insulin analogues Gla-300 and IDeg 100 using continuous glucose monitoring in people with type 1 diabetes: the InRange randomised controlled trial
T. Battelino1, T. Danne2, S. Edelman3, P. Choudhary4, E. Renard5, J. Westerbacka6, B. Mukherjee6, P. Picard7, V. Pilorget6, R. Bergenstal8;
1UMC–University Children’s Hospital, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 2Diabetes Centre for Children and Adolescents, Children’s and Youth Hospital ”Auf Der Bult”, Hannover, Germany, 3University of California, San Diego, USA, 4Diabetes Research Centre, University of Leicester, Leicester, UK, 5Department of Endocrinology, Diabetes and Nutrition, Montpellier University Hospital, University of Montpellier, Montpellier, France, 6Sanofi, Paris, France, 7IviData LIFE SCIENCES, Levallois-Perret, France, 8International Diabetes Center at Park Nicollet, Minneapolis, USA.
Background and aims: InRange is the first large randomised controlled trial to use continuous blood glucose monitoring (CGM) time-in-range (TIR) as a primary efficacy endpoint to compare second-generation basal insulin (BI) analogues, insulin glargine 300 U/mL (Gla-300) and insulin degludec 100 U/mL (IDeg-100) in adults with type 1 diabetes (T1D).
Materials and methods: Multicentre, randomised, active-controlled, parallel-group, 12-week open-label study comparing efficacy of Gla-300 and IDeg-100 using 20-day CGM profiles (≥10 days evaluable) at Week 12. Inclusion: adults with T1D treated with multiple daily injections, using BI analogues once daily and rapid-acting insulin analogues for ≥1 year; HbA1c ≥7 (≥53 mmol/mol) and ≤10% (≤86 mmol/mol) at screening; not requiring CGM for routine care during study.
Results: In total, 343 participants were randomised (172 Gla-300, 171 IDeg-100): mean (SD) age was 42.8 (13.3) years, BMI 27.3 (4.8) kg/m2, T1D duration 20.5 (12.8) years, 33.8% had ≥1 diabetic complication. Non-inferiority of Gla-300 versus IDeg-100 was demonstrated on percent TIR 3.9-10 mmol/L (primary endpoint, Table). Non-inferiority was demonstrated on glucose total coefficient of variation (CV, main secondary endpoint) with lower CV for Gla-300. Following demonstration of non-inferiority on TIR and glucose CV, superiority of Gla-300 over IDeg-100 on TIR 3.9-10 mmol/L was tested and not demonstrated. Rates of hypoglycaemia at <3.9 to ≥3.0 mmol/L and <3.0 mmol/L thresholds did not differ between groups. Safety profiles were consistent with known profiles of Gla-300/IDeg-100 and no unexpected safety findings were identified. No treatment-emergent adverse event (TEAE) leading to permanent treatment discontinuation and no death due to TEAE was reported.
Conclusion: Using clinically relevant CGM metrics, the InRange study shows that Gla-300 is non-inferior to IDeg-100 in people with T1D, with similar hypoglycaemia and safety profiles.
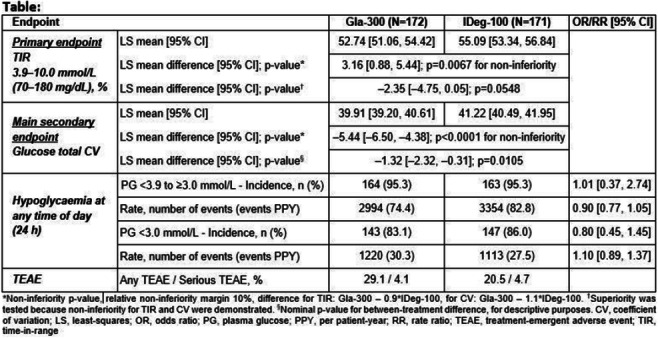
Clinical Trial Registration Number: NCT04075513
Supported by: Sanofi
Disclosure: T. Battelino: Grants; Abbott Diabetes Care, Medtronic, Novo Nordisk, GluSense, Sanofi, Sandoz, Novartis and Zealand. Honorarium; Participation on advisory boards for Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, Indigo and Medtronic. Stock/Shareholding; DreaMed Diabetes. Other; Speaker for AstraZeneca, Eli Lilly, Novo Nordisk, Medtronic, Pfizer, Sanofi, Dexcom, Abbott and Roche.
248
Switching from multiple insulin injections to a fixed combination of degludec and liraglutide in patients with type 2 diabetes: Simplify study: results after 3 months
E. Martinka1, I. Dravecka2, I. Tkac3;
1Diabetology, National institute of endocrinology and diabetology, Lubochna, 2Department of Internal Medicine 1, Faculty of Medicine, P.J. Safarik University, Faculty of Medicine, L. Pasteur University Hospital, Kosice, 3Department of Internal Medicine 4, Faculty of Medicine, P.J. Safarik University, Faculty of Medicine, L. Pasteur University Hospital, Kosice, Slovakia.
Background and aims: A non-randomized, open-label, multicenter, single-arm prospective study was performed with the aim to evaluate whether switching from basal insulin + prandial insulin boluses (IIT) to fixed-combination of the basal insulin analog degludec and the GLP-1 receptor agonist liraglutide (IDegLira) in patients with type 2 diabetes mellitus (DM2T) is at least as effective as previous IIT treatment in terms of glycemic control, body weight, blood pressure, and blood lipid levels. The analysis was performed after the first 3 months.
Materials and methods: The study enrolled 147 patients with DM2T with duration > 5 years, on IIT treatment > 12 months, with HbA1c > 7%, with preserved insulin secretion and total daily insulin dose (TDDI) <0.7 U/kg body weight or <70 U/day. Parameters of glycemic control (HbA1c, glycemia in glycemic profiles) were compared during the treatment of IIT and subsequently after 3 months of treatment after switching to IDegLira. In a subgroup (n=31) of patients, continuous glucose monitoring (CGM) was performed and parameters time in range (TIR), time above range (TAR), and time below range (TBR) were evaluated. Other endpoints were body weight (BW), body mass index (BMI), blood pressure, lipids, the prevalence of hypoglycemia, and insulin doses. Switching from IIT to IDegLira was performed in a single ambulatory session with an initial dose of IDegLira up to 16 U followed by titration of ± 2-4 U/3 days.
Results: Analysis of the results after the first 3 months of treatment showed that switching from IIT to IDegLira was associated with a significant improvement both with HbA1c (8.6±0.9 vs 7.7±1.2, p<0,0001) and plasma glucose levels in glycemic profiles. TIR and TAR values were also improved, but the difference was not statistically significant. Change of treatment has also been associated with significant reductions in BW (97.7±18.3 vs 94.2±17.9 kg, p<0,0001), BMI, systolic blood pressure, triglycerides, total cholesterol, and LDL-cholesterol. There was also a significant reduction in frequency of hypoglycaemia both in self-monitoring (0.52 vs 0.03 episodes per patient, 19,7% vs 3,4% of patients) and TBR (2.8% vs 0.8%, p<0,05 in CGM), and a significant reduction in insulin doses (55.6±14.3 vs 30.9±9.4 U, p<0,0001).
Conclusion: Switching from IIT to IDegLira in DM2T with HbA1c> 7%, preserved insulin secretion and TDDI <70U was a safe, effective, and less demanding form of treatment, the benefit of which manifests already after three months of treatment.
Disclosure: E. Martinka: Lecture/other fees; Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi.
OP 45 Newer agents, better outcomes
249
Effect of empagliflozin on left ventricular contractility and peak oxygen uptake in subjects with type 2 diabetes without heart disease: results of the EMPA-HEART trial
L. Nesti1, N.R. Pugliese1, P. Sciuto1, D. Tricò1, A. Dardano1, I. Fabiani2, A. Natali1;
1Università di Pisa, 2Fondazione Toscana G. Monasterio, Pisa, Italy.
Background and aims: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are effective in primary prevention of hospitalization for heart failure through mechanisms that still are uncertain. The EMPA-HEART trial is a phase III, active-controlled, parallel groups, exploratory study aiming at verifying whether the SGLT2i empagliflozin is associated with improved myocardial contractility and/or cardiopulmonary fitness in patients with type 2 diabetes (T2D) without heart disease, in comparison with sitagliptin as an active control, an equally effective glucose lowering agent that has been shown to be neutral on the prevention of heart failure-related events.
Materials and methods: Patients with T2D of either sex, age 40-80 years, on stable therapy with metformin and/or basal insulin, suboptimal glycaemic control (HbA1c 7.0-8.5%), and normal biventricular systo-diastolic function, no ischemic or valvular heart disease, and devoid of micro- or macrovascular complications were randomized to either empagliflozin 10 mg/die or sitagliptin 100 mg/die for 6 months and were subjected to resting and exercise echocardiography with speckle-tracking technology (left ventricle global longitudinal strain, LV-GLS, a more sensitive measure of systolic function than 2D-left ventricular ejection fraction), cardiopulmonary exercise testing (measurement of peak oxygen uptake, VO2peak) , and an extensive vascular, endothelial, autonomic, and biohumoral characterization.
Results: Forty-four patients completed the study, 22 per arm. While glycaemic control similarly improved in both groups, a relative reduction in body weight (-1.6; [-2.7/-0.5] kg, p=0.03) and plasma uric acid (-1.5; [-2.3/-0.6], p=0.002), as well as an increase in haemoglobin (+0.7; [+0.2/+1.1] g/dL, p=0.0003) were evident with empagliflozin. No difference between the treatments was detectable in the absolute changes of either LV-GLS at 1 month (empagliflozin vs sitagliptin: +0.44; [-0.10/+0.98] %, p=0.11) and 6 months of therapy (+0.53; [-0.56/+1.62] %), or in VO2peak (+0.43; [-1.4/+2.3] ml/min/kg, p=0.65), as well as in vascular function and plasma biomarkers of ventricular strain and damage, extracellular matrix remodeling, and inflammation. Nevertheless, with empagliflozin, the subgroup with baseline LV-GLS below the median experienced a significantly greater increase (time*drug p<0.05) in LV-GLS at both 1 month (+1.22; [+0.31/+2.13] %) and at 6 months (+2.05; [+1.14/+2.96] %), while sitagliptin only induced a modest improvement in LV-GLS at 6 months (+0.92; [+0.21/+0.62] %).
Conclusion: Empagliflozin has neutral impact on both LV-GLS and exercise tolerance in subjects with T2D and normal left ventricular function. Nonetheless, in patients with subclinical systolic dysfunction (baseline LV-GLS <16.5%) it produces a rapid and persistent amelioration of LV contractility.
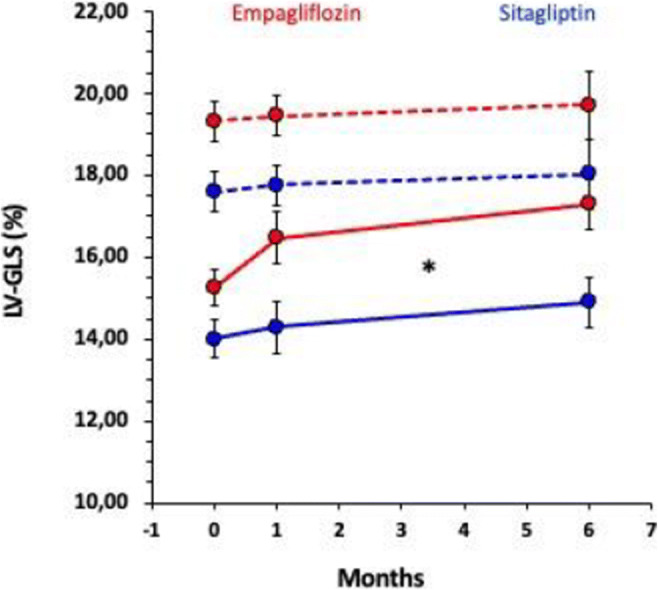
Clinical Trial Registration Number: EUDRACT Code 2016-0022250-10
Supported by: Investigator-initiated study supported at 49% by an unrestricted grant from Boehringer Ingelheim
Disclosure: L. Nesti: None.
250
Novel subgroups of patients with type 2 diabetes show differential cardiovascular and kidney benefits with canagliflozin: a data-driven proteomic cluster analysis
M. Schechter1, A. Koshino1, N. Jongs1, B. Neal2, C. Arnott2, V. Perkovic2, M.K. Hansen3, H.J. L. Heerspink1,2;
1Dept of Clinical Pharmacy and Pharmacology, University of Groningen, Groningen, Netherlands, 2The George Institute for Global Health, UNSW Sydney, Sydney, Australia, 3Janssen Research and Development, LLC, Spring House, USA.
Background and aims: Canagliflozin (CANA) improves cardiovascular (CV) and kidney outcomes in patients with type 2 diabetes (T2D) at high CV or kidney risk. By applying a data-driven approach, we tested whether a combination of novel protein biomarkers could identify patients that are more likely to experience CV or kidney benefits with CANA treatment.
Materials and methods: The CANVAS trial randomized 4330 participants with T2D at high CV risk to receive CANA or placebo. The primary CV outcome was a composite of non-fatal myocardial infarction or stroke or CV death. The prespecified kidney outcome was a composite of sustained ≥40% estimated glomerular filtration rate (eGFR) decline, end-stage kidney disease, or death due to kidney failure. K-mean clustering, relying on eight novel blood (IL-6, TNFR1, TNFR2, KIM1, GDF15, MMP7) and urine (EGF, MCP1) protein biomarkers, was used to classify patients into subgroups. The elbow, silhouette, and gap statistic methods were applied to determine the optimal clusters’ number. Treatment effects of CANA on CV and kidney outcomes across biomarker clusters were assessed using Cox proportional hazard models.
Results: Complete biomarkers values at baseline were available in 3381 (78.1%) participants, followed for a median [IQR] of 6.1 [5.9, 6.4] years. K-mean clustering, based on the eight novel biomarkers, discovered two different patients’ clusters (Cluster 1, N=1957; Cluster 2, N=1424). Compared with Cluster 1, participants in Cluster 2 had lower baseline eGFR (mean [SD]= 69.6 [16.6] vs 86.2 [13.8] mL/min/1.73 m2), higher urine albumin to creatinine ratio (UACR; median [IQR]= 19.6 [8.3,82.7] vs 8.9 [5.8,18.5] mg/g), and were more likely to have established CV disease (61.4% vs. 57.5% of patients). All biomarkers’ levels were higher in Cluster 2, except for urine EGF which was higher in Cluster 1. Within each cluster, baseline characteristics were balanced between the CANA and placebo groups. Compared with placebo, CANA improved the cardiovascular outcome in Cluster 2 but not in Cluster 1 (P-interaction =0.011; table). In contrast, its effect on the kidney outcome was statistically significant in Cluster 1 but not in Cluster 2 (P-interaction =0.004). The interaction remained statistically significant after model adjustment for other baseline clinical characteristics.
Conclusion: This data-driven analysis, relying on a combination of novel biomarkers, suggested two separate clusters among participants in the CANVAS trial - in one cluster CANA improves the CV outcome, and in the other CANA improves the kidney outcome. These hypothesis-generating findings warrant validation in a separate clinical trial.
Clinical Trial Registration Number: NCT01032629
Supported by: Janssen Research & Development
Disclosure: M. Schechter: None.
251
SGLT-2 inhibitors and GLP-1 receptor agonists are associated with lower mortality in younger people with type 2 diabetes and a first myocardial infarction between 2016 and 2020
M. Löndahl1, S. Puvaneswaralingam2, E. Uddman1, K. Filipsson1;
1Endocrinology, Clinical Sciences, Lund University, 2Endocrinology, Skane University hospital, Lund, Sweden.
Background and aims: Type 2 diabetes is a significant risk factor for cardiovascular disease and death. In people with type 2 diabetes and higher risks of cardiovascular disease, several large randomised controlled trials have identified a reduction in cardiovascular mortality and non-fatal cardiovascular complications after initiating treatment with sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP1-RA). Although the first data were published in 2015, the prescription of these drugs in people with high-cardiovascular risk has been modest. This study aimed to evaluate the effect of prescription of SGLT-2 inhibitors and /orGLP1-RA on all-cause mortality in people younger than 70 years with type 2 diabetes having their first myocardial infarction.
Materials and methods: This study is an observational, population-based cohort study of individuals with type 2 diabetes, aged between 40 and 69 years, and the first diagnosis of myocardial infarction between January 2016 and December 2020 in Sweden using data from national registries. People with type 2 diabetes were identified in the National Drug Prescription Registry and defined as people who at least twice had been prescribed an anti-diabetic drug. Prescription of anti-diabetic and antihypertensive drugs and date of the collection were registered. Data on comorbidities and cardiovascular events were collected from the National Patient Registry, which contains nationwide hospital discharge information since 1987. Information on the time of death was retrieved from the Swedish Cause of Death Register. Patients were grouped according to the use of SGLT-2 inhibitors, GLP1-RA, or no prescription of any of these drugs. The primary endpoint, all-cause mortality, is presented as Kaplan-Meier curves, and statistical differences were evaluated using the Log-Rank test.
Results: Of the 9117 identified patients below 70 years of age with a history of type 2 diabetes and a first myocardial between 2016 and 2020, 4584 (50.3%) had been prescribed neither an SGLT-2 inhibitor nor a GLP1-RA, while 26.8% was prescribed an SGLT-2 inhibitor, 9.4% a GLP-1 RA and 13.6% drugs from both these classes. During the follow-up period (median three years), 1001 events (11.0%) occurred. The five-year mortality is given in Figure 1.
Conclusion: In this population of younger people with type diabetes and a first myocardial infarction between 2016 and 2020, prescription of an SGLT-2 inhibitor or a GLP1-RA was associated with lower five-year mortality. The number of high-risk patients with access to the drugs still seems too low.
Supported by: Unrestricted grant from Boehringer-Ingelheim.
Disclosure: M. Löndahl: Grants; Unrestricted grant from Boehringer-Ingelheim. Lecture/other fees; AstraZeneca, Boehringer-Ingelheim, EliLilly, NnovNordisk.
252
Efficacy of ertugliflozin (ERTU) on hospitalisation for heart failure (HHF) across the spectrum of pre-trial ejection fraction (EF): post hoc analyses of VERTIS CV
A. Pandey1, A.A. Kolkailah1, F. Cosentino2, C.P. Cannon3, R. Frederich4, D.Z.I. Cherney5, S. Dagogo-Jack6, R.E. Pratley7, N.B. Cater8, I. Gantz9, J.P. Mancuso10, D.K. McGuire1;
1Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, USA, 2Unit of Cardiology, Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden, 3Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA, 4Pfizer Inc., Collegeville, USA, 5University of Toronto, Toronto, Canada, 6University of Tennessee Health Science Center, Memphis, USA, 7AdventHealth Translational Research Institute, Orlando, USA, 8Pfizer Inc., New York, USA, 9Merck & Co., Inc., Kenilworth, USA, 10Pfizer Inc., Groton, USA.
Background and aims: There is controversy over whether SGLT2 inhibitors have efficacy in high-risk patients (pts) with HF and EF >60% with or without T2D. VERTIS CV studied pts with T2D and atherosclerotic CVD (ASCVD); 23% had a history of HF. In VERTIS CV, ERTU significantly reduced risk of first and total HHF vs placebo (PBO). Whether efficacy in VERTIS CV is consistent across the spectrum of pre-trial EF is unknown, particularly for pts with EF >60%. These post hoc analyses explored the effects of ERTU vs PBO on time to first and total HHF in VERTIS CV across pre-trial EF.
Materials and methods: ERTU 5 and 15 mg groups were combined for all analyses. Treatment effects of ERTU vs PBO on risk of first and total HHF were analysed using adjusted Cox models for first and Andersen-Gill models for total (i.e., first + recurrent) events. Data on pre-trial EF were from medical records at trial entry. Multiplicative interaction terms (EF * treatment arm) were used to determine if ERTU efficacy was modified by pre-trial EF.
Results: 8246 pts were randomised to ERTU 5 or 15 mg or PBO (mean follow up 3.5 years). Overall, 5006 pts had pre-trial EF data available; 959 had EF ≤45%, 2860 had EF >45-60%, and 1187 had EF >60%. Overall, the event rate for first HHF was lower with ERTU vs PBO (HR 0.70; 95% CI 0.54-0.90). The findings were generally consistent across pre-trial EF (P-interaction=0.26; Figure), including pts with pre-trial EF >60% (HR 0.72; 95% CI 0.34-1.55). Overall, the event rate for total HHF was lower with ERTU vs PBO (HR 0.70; 95% CI 0.56-0.87). A significant interaction was observed between pre-trial EF and treatment arm for the risk of total HHF events (P-interaction=0.02), with a greater magnitude of risk reduction in pts with a low pre-trial EF (≤45%; HR 0.39; 95% CI 0.26-0.57). However, the 95% CIs for the HR for total HHF for those with EF >45-60% and >60% nearly entirely or entirely contained the 95% CI of the overall population, respectively (Figure).
Conclusion: In the VERTIS CV trial of pts with T2D and ASCVD, the efficacy of ERTU in preventing first HHF was generally comparable across the spectrum of pre-trial EF. The trend for greater benefit at lower EF was statistically significant for total HHF events. Findings for pts with EF >45-60% and >60% appeared quantitively consistent with the overall findings for both first and total HHF.
Clinical Trial Registration Number: NCT01986881
Supported by: Merck Sharp & Dohme Corp., Kenilworth, NJ, USA; Pfizer Inc., New York, NY, USA
Disclosure: A. Pandey: Grants; Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, National Institute of Aging GEMSSTAR Grant (1R03AG067960-01), Applied Therapeutics. Non-financial support; A.P. has served on the advisory board of Roche Diagnostics.
OP 46 Profiling human diabetes risk
253
Clustering analysis based on parameters of glucose metabolism shows different lipidomic profile among men and women
A. Pina1, M.J. Meneses1,2, R. Ribeiro2, R. Patarrão1, F. Carli3, J. Boavida2, J.F. Raposo2, A. Gastaldelli3, M.P. Macedo1,2;
1MEDIR, CEDOC, Lisbon, Portugal, 2APDP - Associação Protectora dos Diabéticos de Portugal, Lisbon, Portugal, 3National Research Council (CNR), Pisa, Italy.
Background and aims: Clustering analysis has been used to identify different phenotypes and to identify subjects at risk of developing Type 2 diabetes (T2D) and related comorbidities.Not only glucose but also lipids have a relevant role in the development and progression of T2D. Lipids (such as fatty acids, diacylglycerol (DAG) and sphingolipids) can act as signaling molecules and impact insulin action and metabolism through the regulation of intracellular pathways. Thus, we hypothesized that subjects stratified according to parameters related to insulin and glucose metabolism might have a different lipidomic profile.
Materials and methods: We studied 953 subjects from the PREVADIAB2 cohort (follow up of subjects that were without diabetes 5 years before, PREVADIAB1) and performed hierarchical clustering analysis based on fasting insulin secretion (fISR), resistance (HOMA-IR) and clearance (fIC) and insulinogenic index (IGI) during OGTT. The resulting clusters were profiled according to insulin resistance in different tissues (liver, muscle or adipose tissue) and insulin secretion, and the lipidome of a subset (n=488, 273 women and 215 men) was assessed by LC/MS-QTOF.
Results: Among the 488 subjects of PREVADIAB2 here reported, 41% did not change their glucose tolerance status while 19% had a worsening status(with prediabetes or T2D). We identified four clusters (Figure), that were named according to their main metabolic features: Liver Sensitive (LS); Pancreas Glucose Sensitive (PGS); Insulin Deficient (ID); and Insulin Resistant (IR). The LS cluster had the most advantageous lipid profile, whereas the other clusters presented lipid associated to glucose dysmetabolism. Although the pattern of glucose metabolism parameters’ were similar in men and women among clusters, the lipidomic profiles were different. Overall, women presented lower lysophosphatidylcholine (LPC) and long chain ceramides (CER 26:1) and higher sphingomyelins (SM) compared to men. Women presented lower TG in the LS group but slightly higher TG in the IR group. CER with less than 18 Carbons were higher in women than men in PGS, ID and IR groups (Figure).
Conclusion: In conclusion, our work shows that not only glucose but also lipidomic profile should be included in the analysis of phenotypes, but furthermore differentiated by gender for the increased risk of T2D.
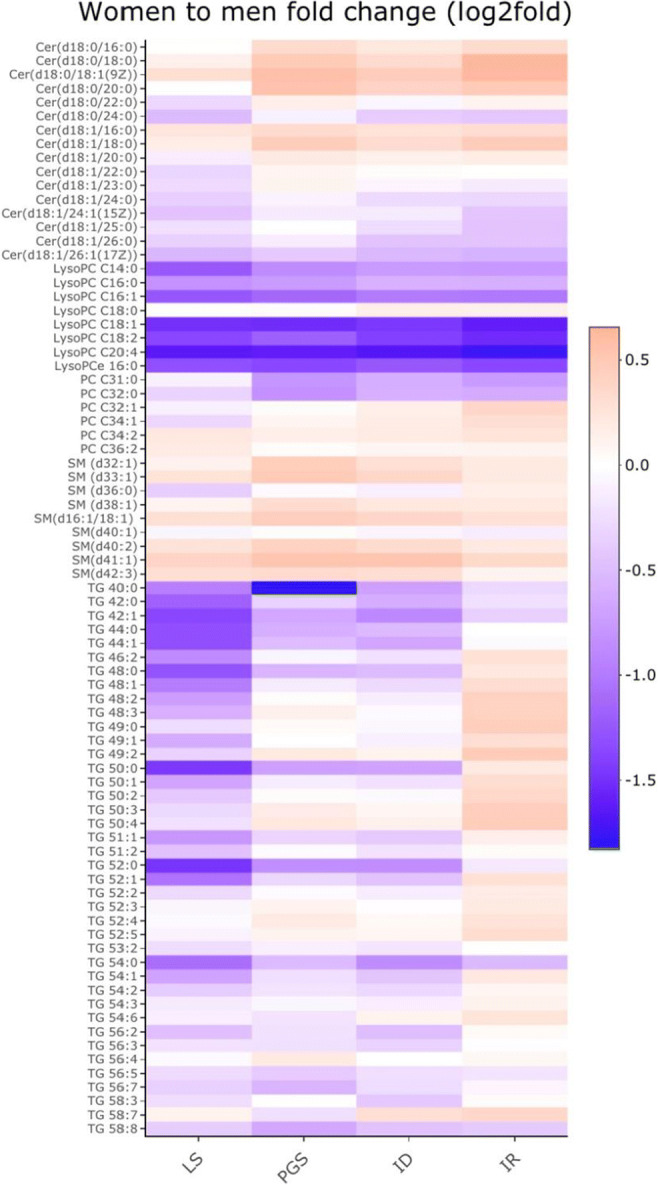
Supported by: mtFoieGras-RISE No. 734719; SPD iNOVA4Health UIDB/Multi/04462/2020;FCT PTDC/MEC-MET/29314/2017
Disclosure: A. Pina: Grants; mtFoieGras (Marie Sklodowska-Curie RISE Grant Agreement No. 734719), SPD iNOVA4Health UIDB/Multi/04462/2020, FCT PTDC/MEC-MET/29314/2017.
254
Dynamics of intramyocellular lipid content during the initial course of type 1 and type 2 diabetes
M. Schön1,2, K. Strassburger3,2, Y. Kupriyanova1,2, O.P. Zaharia1,4, K. Bódis1,4, I. Yurchenko1,2, C. Möser1,2, M. Huttasch1,2, M. Bombrich1,2, V. Schrauwen-Hinderling1,2, V. Burkart1,2, M. Roden1,4;
1Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, 2German Center for Diabetes Research (DZD), München-Neuherberg, 3Institute for Biometrics and Epidemiology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, 4Department of Endocrinology and Diabetology, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany.
Background and aims: Ectopic fat accumulation in skeletal muscle associates with insulin resistance and is frequently higher in both type 1 (T1D) and type 2 (T2D) diabetes. Recent studies also suggest an important role of intramyocellular lipid content (IMCL) for the development of diabetes-related comorbidities, such as non-alcoholic fatty liver disease (NAFLD). However, the dynamics of IMCL over the course of diabetes remain unknown. The aim of this study was (i) to compare IMCL in humans with recent-onset (known diabetes duration <1 year) T1D and T2D, (ii) to monitor the dynamics of IMCL during the initial 5 years of diabetes, and (iii) to examine whether worsening of insulin resistance and increase in liver fat is associated with accelerated IMCL accumulation.
Materials and methods: Participants of the German Diabetes Study (GDS) with recent-onset T1D (n=132, male/female=72/60) and T2D (n=139, m/f=95/44), and glucose-tolerant humans (CON; n=128, m/f=87/41) underwent 1H magnetic resonance spectroscopy for the assessment of IMCL in tibialis anterior muscle and liver fat content. Whole-body insulin sensitivity (WBIS) was assessed by hyperinsulinemic-euglycemic clamps, and body composition and skeletal muscle mass by bioelectrical impedance. All measurements were repeated after 5 years in subgroups of T1D (n=27, m/f=12/15), T2D (n=29, m/f=24/5) and CON (n=20, m/f=16/4).
Results: At diabetes diagnosis, humans with T2D had lower WBIS, but higher IMCL than persons with T1D and CON (all p<0.01), even after adjustments for age, sex and body mass index (BMI). Adults with T1D had lower IS, but similar IMCL when compared with CON (p<0.01). IMCL were positively correlated with BMI in all groups (CON/T1D/T2D; ß=2.12/2.16/2.74, p=0.04/0.03/0.01) and negatively with WBIS in T1D and T2D (ß=-3.03/-4.12, both p<0.01). In T2D, IMCL also correlated negatively with skeletal muscle mass (ß=-3.61, p<0.01), but positively with liver fat content (ß=2.47, p=0.01). After 5 years of overt diabetes, WBIS decreased in T1D and T2D (both p<0.01) and liver fat increased in T2D (p<0.01) without any change in IMCL.
Conclusion: In conclusion, ectopic fat accumulation is elevated already at diagnosis of T2D. However, IMCL remain largely unchanged within 5 years after diagnosis demonstrating that muscle triglyceride content does not necessarily mirror progression of insulin resistance and NAFLD in T2D.
Clinical Trial Registration Number: NCT01055093
Supported by: BMFB, DZD
Disclosure: M. Schön: None.
255
Diabetes remission by weight loss in 'normal' weight people with type 2 diabetes: the ReTUNE study
R. Taylor1, A.C. Barnes2, K.M. Irvine2, L. Clark3, A. Al-Mrabeh4, A. Solovyova5, T.L. Kelly2, K.G. Hollingsworth3, C. Martin-Ruiz6, R.R. Holman7, D. Romeres8, C. Cobelli9;
1Newcastle University, Newcastle upon Tyne, UK, 2Human Nutrition Research Centre, Newcastle University, Newcastle upon Tyne, UK, 3Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, UK, 4Centre for Cardiovascular Science, Edinburgh University, Edinburgh, UK, 5Professional Services, Newcastle University, Newcastle upon Tyne, UK, 6Biosciences, Newcastle University, Newcastle upon Tyne, UK, 7OCDEM, University of Oxford, Oxford, UK, 8Department of Endocrinology, University of Virginia, Charlottesville, USA, 9Department of Women and Child's Health, University of Padova, Padova, Italy.
Background and aims: Pathophysiological changes underlying weight loss-induced remission of type 2 diabetes (T2DM) have been reported only in people with BMI >27kg/m2. The Personal Fat Threshold hypothesis predicted that the same pathophysiological mechanisms would operate in people with BMI <27kg/m2, but at a lower individual threshold for overflow of fat from subcutaneous to intraorgan sites. ReTUNE aimed to test the hypothesis in this population.
Materials and methods: People with T2DM (mean±SD) 59.3±7.1 years, BMI 24.8±1.7kg/m2, 13/20 female) were studied before and after up to three induced 5% weight loss cycles, each comprising a 2-4 week low energy diet (800kcal/day formula meal replacements plus non starchy vegetables), followed by 4-6 weeks of weight maintenance. All hypoglycaemic agents were stopped after baseline tests and before weight loss. Studies were repeated at 12 months. Outcomes were compared to normoglycaemic controls matched for post-weight loss (n=20; data shown in square brackets). Intrahepatic and intrapancreatic fat was quantified by magnetic resonance, plasma very low density lipoprotein triglyceride (VLDL-1TG) by ultracentrifugation and insulin secretion by Disposition Index (DI) after standard meal. Data are shown as mean±SE or median (interquartile range).
Results: 70% (14/20) achieved sustained remission (HbA1c <48mmol/mol off all hypoglycaemic agents). After the 1st weight loss cycle 10 were in remission, 3 after the 2nd cycle and 1 after the 3rd cycle of weight loss, with median time to remission of 8 (IQR 8-14) weeks. There was no weight regain from the the final weight loss visit to 12 months (64.1±2.9 to 64.11±2.6kg p=0.86). Weight loss was accompanied by decrease in some signals of adipose tissue distress (PAI-1 8.64 (6.97-11.01) to 5.99 (3.94-7.73) p<0.02 [control 4.87(3.49-7.03] ng/ml; GDF-15 591(439-872) to 444(395-554) [446 (383-614] pg/ml p<0.05. Between baseline and 12 months: BMI fell 24.8±0.4 to 22.5±0.4 kg/m2 (p<0.0001) [21.5±0.5]; total body fat 32.1±1.5 to 27.6±1.8% (p<0.0001) [24.6±1.5]; liver fat 4.1(2.0-6.1) to 1.5(1.4-1.8% (p<0.001)[1.3(1.0-2.0)]; VLDL-1TG 0.32(0.28-0.57) to 0.22(0.15-0.35)mmol/l (p<0.05) [0.21(0.15-0.29)] and pancreas fat 6.1±0.5 to 5.0±0.7% (p<0.01) [4.1±0.3]. Fasting plasma insulin decreased from 47±6 to 24±6pmol/l (p<0.001) [23±2] and DI increased from 289 (183-373) to 774 (486-1709) dl/kg/min per pmol/l (p<0.05) but remained subnormal [2751 (1524-3526].
Conclusion: The mechanistic changes underpinning weight loss-induced remission of T2DM in people with BMI <27 are the same previously observed in those with BMI >27kg/m2. T2DM occurs if a person exceeds their personal fat threshold and becomes too heavy for their own constitution, irrespective of BMI.
Clinical Trial Registration Number: ISRCTN15177113
Supported by: Diabetes UK 17/0005645
Disclosure: R. Taylor: None.
256
Machine-learning prediction of obesity-related multi-morbidities
L.E. Lund1, R.L. Nielsen1, Z. McVey1, R.R. Kitchen1, L.G. Leal1, A.T.H. Schreyer2, T. Monfeuga1, K.S. Matthiessen3, M.L. Wolden3, R. Gupta1;
1Computational Biology, Novo Nordisk Research Centre Oxford, Oxford, UK, 2Technical University of Denmark, Kgs. Lyngby, Denmark, 3Payer Evidence Generation, Novo Nordisk, Søborg, Denmark.
Background and aims: Obesity is a risk factor for the onset of several clinical complications. The clinical trajectory of a person with obesity varies; although some patients do not develop any obesity-related complications (ORCs), others develop multi-morbidities with fast progression. There is a clinical need to understand which ORCs frequently co-occur as well as the time of progression to these, and to identify prognostic biomarkers of multi-morbidities for early patient identification and intervention. We developed a predictive risk model to assess the progression of ORCs. Our focus in this study is early diagnosis and optimising treatment opportunities.
Materials and methods: Participants in UK biobank (N=~500K) with linked electronic health records data from primary and secondary care data were used in this study to identify progression of ORCs. Individual patients’ journeys were mapped in the context of first clinical obesity indication (BMI ≥ 30 or obesity diagnosis; N=~145K) to map risk of developing ten of the more commonly occurring ORCs. These ORCs are cardiovascular disease, chronic back pain, chronic respiratory diseases, dyslipidaemia, gastroesophageal reflux disease, hypertension, knee osteoarthritis, mental disorders, obstructive sleep apnoea, and type 2 diabetes (T2D). Machine-learning models (Extreme gradient boosting; XGB) were trained to predict the risk among the subpopulation with obesity to develop multi-morbidity (two or more ORCs) within 10 years of the first indication of obesity with no previous history of the selected ORCs. The XGB models integrated multi-faceted data including blood biomarker data as well as anthropometrics, lifestyle and nutrition, questionnaires, socioeconomic status, and genetic data to estimate individual risk.
Results: 81,504 people with obesity had no diagnosis of the ORCs before obesity onset. 42% of these people (N=34,629) developed no complications, 25% developed one, 15% developed two (time of onset 6.2 ± 7.3 years), and 18% developed ≥3. Hypertension was most common (39%), followed by dyslipidaemia (12%), and T2D (11%). When predicting ORCs within 1-10 years following first obesity indication, XGB models performed slightly better when predicting risk of developing 3+ ORCs [Receiver operating characteristic (ROC) AUC: 95% CI 0.72-0.76] compared to 2+ ORCs [ROC AUC: 95% CI 0.70-0.72]. The most predictive model identified 69% of the non-progressors and 68% of the progressors where age, HbA1c, albuminuria, and self-reported health ratings were signatures of ORC archetypes.
Conclusion: Sub-groups of individuals with obesity progress to multi-morbidity. Predictive models help distinguish people with obesity at high risk of developing ORCs from non-progressors. Such predictive models can potentially assist in identification of appropriate intervention and treatment strategies that are tailored to meet the needs of the individual.
Disclosure: L.E. Lund: None.
OP 47 Novel mechanistic insights in peripheral insulin sensitivity
257
Proteome-wide mechanisms of hyperinsulinemia and sucrose-induced, tissue-specific insulin resistance
H.H. Cen1, V.R. Richard2, Y.H. Xia1, S. Skovsø1, J.D. Botezelli1, X. Hu1, J. Collier3, R.P. Zahedi2, C.H. Borchers2,4, J.D. Johnson1;
1Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, Vancouver, Canada, 2Segal Cancer Proteomics Centre, Lady Davis Institute, Jewish General Hospital, McGill University, Montreal, Canada, 3Pennington Biomedical Research Center, Baton Rouge, USA, 4Gerald Bronfman Department of Oncology, Jewish General Hospital, McGill University, Montreal, Canada.
Background and aims: Hyperinsulinemia has been shown to play a causal role in obesity and insulin resistance during the progression to type 2 diabetes, challenging the long-held dogma that it is solely an adaptive response. Added sucrose in food and beverages also has been shown to influence obesity and diabetes in human and pre-clinical models, but the mechanisms are not well defined. We aim to study the roles of hyperinsulinemia in sucrose-induced insulin resistance and obesity and to dissect the tissue-specific molecular mechanisms involved.
Materials and methods: Genetically modified mice that produce less insulin (Ins1+/-;Ins2-/- vs Ins1+/+;Ins2-/-) were fed with water or 30% liquid sucrose in the context of a chow diet (Cat. # 5LJ5, PicoLab) and phenotyped for 26 weeks. To assess molecular changes in signaling pathways in multiple tissues, the total proteome and phosphoproteome of the liver, skeletal muscle and inguinal white adipose tissue were measured by label-free quantitative mass spectrometry. Pathways enriched from differentially expressed proteins and phospho-sites were analyzed using multiple bioinformatic tools.
Results: Ten weeks of sucrose-feeding increased body weight and fasting glucose while impairing glucose tolerance and insulin sensitivity in female mice. These effects were abrogated in mice with reduced insulin gene dosage. Proteomics and phosphoproteomics revealed distinct molecular changes in the liver, muscle and adipose tissue. The liver had the highest number of altered proteins and phospho-sites (>1000), followed by adipose tissue, while muscle had the least changes. The changes induced by sucrose were largely attenuated in mice with reduced insulin in all tissues. In the liver, pathways related to EIF2/4 and p70S4K signaling and glucose metabolism were downregulated by sucrose, while pathways related to amino acid metabolism and fatty acid oxidation were upregulated. Total and phospho-proteomics data were compared to transcriptomic data for a multi-omics analysis that allowed us to infer tissue-specific effects of hyperinsulinemia in the context of sucrose supplementation.
Conclusion: Sucrose induced weight gain and insulin resistance, which were rescued by preventing hyperinsulinemia. A large number of sucrose-induced changes in protein expression and phosphorylation status were identified in the peripheral tissues, especially in the liver. These data will offer new insights into the underlying molecular changes leading to obesity and type 2 diabetes.
Supported by: CIHR
Disclosure: H.H. Cen: None.
258
Impact of human Kallistatin on the metabolic phenotype of diet induced obese mice
L. Sandforth1, J. Reinke2, S. Brachs2, D. Willmes3, J.D. McBride4, A. Peter1, J. Spranger2, J.-X. Ma4, G.I. Shulman5, J. Jordan6, S. Haufe7, A.L. Birkenfeld1;
1University Hospital Tuebingen, Tuebingen, Germany, 2Charité University School of Medicine, Berlin, Germany, 3University Clinic Dresden, TU Dresden, Dresden, Germany, 4University of Oklahoma Health Sciences Center, Oklahoma City, USA, 5Yale University School of Medicine, New Haven, USA, 6German Aerospace Center, Cologne, Germany, 7Hannover Medical School (MHH), Hannover, Germany.
Background and aims: Kallistatin (KST), also known as SERPIN A4, is a circulating, broadly acting human plasma protein with anti-angiogenic and anti-inflammatory properties. Clinical studies in humans revealed increased levels of KST in poor glycemic control and microvessel disease. Whether or not KST has a direct effect on glucose homeostasis in the setting of insulin resistance and Type 2 diabetes is currently unknown.
Materials and methods: To address this question, the expression of human KST in subcutaneous white adipose tissue (sWAT) of obese patients (n=47) before and after 6 months of dietary intervention (B-SMART study) was measured. Additionally, transgenic mice systemically overexpressing human KST (hKST-TG) and littermate-control wildtype mice (WT) were studied under chow (ND) and high fat diet (HFD) conditions. Metabolic phenotyping was performed using 1H nuclear magnetic resonance (NMR), intraperitoneal glucose tolerance test (IPGTT), hyperinsulinemic-euglycemic clamp, measurement of membrane and cytosolic diacylglycerides (DAGs) in liver and muscle, triglycerides (TAGs) in liver lysates, mRNA expression of inflammatory marker genes in liver and muscle and inflammatory markers in plasma. Furthermore, protein level of total β-catenin and β-actin protein level in liver tissue was analyzed using Western Blot Analysis.
Results: In human subjects, there was a more than 1.5-fold increase in KST with a body weight loss of more than 6% after intervention. Mice body weights were similar between hKST-TG and WT mice up to an age of 24 weeks on both diets. Intraperitoneal glucose tolerance tests (IPGTT) yielded similar glucose and insulin excursion curves in ND animals. In the weight matched HFD cohort, an IPGTT revealed an improvement in insulin sensitivity in hKST-TG mice. Additionally, the HOMA-IR was significantly lower in hKST-TG on HFD (2.2±0.27, hKST-TG vs. 4.42±0.87, WT, p<0.05), also indicating improved insulin sensitivity in hKST-TG mice. To better understand the tissue specific contribution to the protective effect of hKST, hyperinsulinemic euglycemic clamp studies with tracer labelled glucose were performed. Glucose infusion rates were higher in hKST-TG mice (31.5±3.7 mg/kg/min, hKST-TG vs. 18.1±3.5 mg/kg/min, WT, p<0.05), validating the insulin sensitizing effect of hKST in HFD fed mice. Specifically, hKST overexpression protected against HFD induced hepatic insulin resistance (clamp hepatic glucose output: 7.7±1.9 mg/kg/min, hKST-TG vs 12.1±0.8 mg/kg/min, WT, p=0.05). To further gain mechanistic insight, body composition, energy metabolism, tissue lipid and inflammatory markers, with and without LPS stimulation, were assessed but did not reveal differences between genotypes. In contrast, changes in the β-catenin pathway were observed, potentially explaining the phenotype.
Conclusion: These data show that human KST protects against diet induced insulin resistance in mice. We speculate that increased KST levels in the setting of poor glycemic control and microvascular complications are rather protective than harmful.
Clinical Trial Registration Number: NCT00956566
Disclosure: L. Sandforth: None.
259
Down-regulated FXR signalling in the gut in subjects with prediabetes and type 2 diabetes
F. De Vito1, E. Suraci1, R. Marasco1, F. Andreozzi1, M.L. Hribal1, F. Luzza1, G. Sesti2, T.V. Fiorentino1;
1Department of Medical and Surgical Sciences, University Magna Graecia of Catanzaro, Catanzaro, 2Department of Clinical and Molecular Medicine, University Sapienza of Rome, Rome, Italy.
Background and aims: The bile acids farnesoid X receptor (FXR) is emerging to be implicated in the regulation of energy homeostasis. Treatment with obeticholic acid (OCA), a FXR agonist, has been found to improve glucose metabolism in subjects with type 2 diabetes (T2DM) with mechanisms not completely elucidated. In the gut, FXR is mainly expressed in the ileum and promotes transcription of fibroblast growth factor-19 (FGF19), a hormone with positive effects on energetic and glucose homeostasis. Additionally, preclinical evidence suggests that FXR regulates expression of the tight-junction (TJ) proteins, which control intestinal barrier permeability. Altered gut permeability has been associated with cardiometabolic disorders. Herein, we examined whether subjects with altered glucose tolerance have reduced ileal FXR abundance along with a downregulation of FGF19 and TJ expression, and whether these aberrations are reverted by treatment with OCA.
Materials and methods: Study population includes 32 subjects subdivided on the basis of their glucose tolerance in: normal glucose tolerance (NGT) (n=12), prediabetes (n=10) and T2DM (n=10). All subjects underwent to a colonoscopy with terminal ileum endoscopy and collection of ileum mucosa biopsies. Intestinal levels of FXR, FGF19 and TJ protein were assessed by WB and RT-PCR. Serum levels of FGF-19 were measured by a specific ELISA kit. To investigate the effect of OCA treatment, ileal mucosa specimens collected from subjects with T2DM were cultured in absence or presence of OCA.
Results: Ileal levels of FXR were progressively reduced in individuals with prediabetes (-15%, P=0.01) and T2DM (-25%, P=0.01) as compared to those with NGT. The reduced ileal FXR abundance observed in subjects with dysglycemic conditions was paralleled by a reduction in ileal levels of FGF-19 mRNA, with subjects with prediabetes exhibiting a 25% reduction, and those with T2DM a 50% reduction (both P<0.05). These changes were accompanied by a similar reduction in serum FGF-19 concentration (-25% and -45% in subjects with prediabetes and T2DM, respectively, as compared to NGT group, P<0.05). Additionally, we observed a progressive decrease in ileal mRNA and protein levels of the TJ zonulin (ZO)-1, claudin-1 and occludin in subjects with prediabetes and T2DM (P<0.05). Next, in order to explore whether FXR stimulation by OCA treatment may revert dysglycemic related aberrations in ileal FXR/FGF-19/TJ integrity axis we performed intestinal organ culture experiments. Ileal mucosa specimens collected from subjects with T2DM were cultured in presence or absence of OCA (20 μM) for 6 hours. We found that OCA treatment resulted in an upregulation of FGF-19 mRNA and release in the culture media by 10-fold and 1.4-fold, respectively, and in 6-fold increased TJ protein ZO-1 and occludin expression (P<0.01).
Conclusion: Our findings demonstrate that a dowregulation of FXR/FGF-19/TJ axis occurs in subjects with prediabetes and T2DM, indicating intestinal FXR signaling as a novel target in prevention and/or treatment of T2DM.
Supported by: EFSD/Lilly Young Investigator Research Award Programme
Disclosure: F. De Vito: None.
260
Expression, phosphorylation, and oligomerisation of TBC1D1 splice variants from human skeletal muscle cells
A.D. Bedou1,2, T. Stermann1,2, S. Eickelschulte1,2, A. Chadt1,2, H. Al-Hasani1,2;
1Institute for Clinical Biochemistry and Pathobiochemistry, German Diabetes Center, Heinrich Heine University, Leibniz Center for Diabetes Research, Düsseldorf, 2German Center for Diabetes Research, Munich-Neuherberg, Germany.
Background and aims: The 160kDa RabGTPase-activating protein TBC1D1 is a crucial factor in skeletal muscle insulin- and contraction-stimulated glucose uptake. Phosphorylation of the 600kDa oligomeric TBC1D1 by AKT or AMPK leads to increased translocation of the glucose transporter GLUT4 to the plasma membrane and enhanced glucose uptake. Genetic variation in TBC1D1 confers risk for severe obesity. For the human TBC1D1 gene, 3 major isoforms are annotated in the NCBI RefSeq database, however, the properties and function of these splice variants are not clear. We aim to determine the expression profile of the 3 different TBC1D1 variants in human primary skeletal muscle cells (HSMCs) and to analyze their function in cell-free in vitro systems.
Materials and methods: Expression levels of TBC1D1 were determined in un- and differentiated HSMCs by RT-PCR. TBC1D1 isoforms were cloned in mammalian expression vectors, overexpressed in HEK-293 cells and purified via co-immunoprecipitation. Oligomerization of TBC1D1 isoforms was assessed using pulldown assays and Western Blot analysis. AKT2 and AMPK were applied to analyze the kinase-dependent phosphorylation kinetics of purified recombinant full-length His6-TBC1D1 variants expressed in Sf9 insect cells via the Baculovirus system.
Results: HSMCs displayed expression of two TBC1D1 variants, encoding for a SHORT 1168 aa and a LONG 1262 aa TBC1D1 isoform. Interestingly, the third annotated variant missing 103 amino acids in the TBC-RabGAP domain (GAP-VAR) was not detected. Expression of the LONG variant was 11-times higher in differentiated than in undifferentiated HSMCs (p < 0.0001). Combined co-immunoprecipitation and immunoblotting analysis showed interconvertible oligomerization of all three isoforms: LONG vs. GAP-VAR 1.17% (p = 0.03) and SHORT 4.29% (p = 0.0005), respectively, as well as GAP-VAR and SHORT 0.37% (p = 0.08). While phosphorylation of all TBC1D1 isoforms was detected in both homo- and heterooligomers, TBC1D1 heterooligomerization resulted in reduced phosphorylation levels compared to the homodimers. Both AKT2 and AMPK showed a strong preference for Ser660 in the LONG variant (Km pSer660: ~8 μM; Km pSer660: ~ 3 μM), whereas corresponding Km values for AKT2 and AMPK were about 14-times higher in the GAP-VAR variant (Km pSer660: ~117 μM; Km pSer660: ~ 42 μM). Interestingly, Km values for the phosphorylation sites Thr596 and Ser700 were in the same order of magnitude for AKT2 and AMPK for the LONG and the GAP-VAR variant. Moreover, AMPK Ser700 phosphosite showed Km values about 5-times higher in the GAP-VAR variant compared to the LONG variant (Km pSer700: ~ 1.5 μM; Km pSer700: ~8 μM).
Conclusion: Our findings suggest that the abundance of TBC1D1 splice variants switches from predominant expression of the SHORT variant in undifferentiated myoblasts to the LONG variant as predominant isoform in differentiated myotubes. Moreover, TBC1D1 protein isoforms generate both homo- and heterooligomers independent of phosphorylation by AKT2 and AMPK. Phosphorylation levels of the main AKT target site Thr596 are not different between the LONG and the GAP-VAR variant. In contrast, both AMPK target sites Ser660 and Ser700 show a stronger affinity for the LONG variant compared to the GAP-VAR TBC1D1 variant.
Disclosure: A.D. Bedou: None.
OP 48 How we loose our beta cells
261
Glutamate decarboxylase autoantibody characteristics can stratify those at risk of early insulin requirement in adult-onset type 2 diabetes
S.L. Grace1, A.E. Long2, K.M. Gillespie2, A.J.K. Williams2, V. Lampasona3, P. Achenbach4, E.R. Pearson5, T.J. McDonald1, A.G. Jones1;
1University of Exeter, Exeter, UK, 2University of Bristol, Bristol, UK, 3Istituto Scientifico San Raffaele, Milan, Italy, 4Helmholtz Zentrum München, Munich, Germany, 5University of Dundee, Dundee, UK.
Background and aims: Autoantibodies to full length glutamate decarboxylase 65 autoantibodies (GADA(1-585)), are the most commonly detected islet autoantibodies in adults diagnosed with type 2 diabetes (T2D), but progression to insulin therapy within this group is highly variable. We aimed to investigate whether analysis of GADA characteristics can improve identification of early insulin requirement in adult-onset GADA positive diabetes.
Materials and methods: Adult-onset patients diagnosed with T2D (n=6814) were screened for GADA(1-585) positivity using the RSR ELISA; 198 tested positive. Positive sera were subsequently tested for the truncated (t)GADA(96-585) epitope, GADA(1-585) affinity and GADA(1-585) IgG subtype analysis (using specialised luciferase/radio immunoprecipitation assays). GADA characteristics were compared between those diagnosed with T2D with (n=77) and without (n=121) early insulin requirement (<5 years from diagnosis), and with GADA(1-585) positive type 1 diabetes (T1D) (n=144). We compared the performance of full length GADA(1-585), tGADA(96-585) positivity, high/low GADA(1-585) affinity and presence of GADA(1-585) IgG subtypes in predicting early insulin requirement using survival analysis.
Results: Positivity for tGADA(96-585) was similar between individuals with T1D and those with early insulin requiring T2D (91% vs. 95% respectively), compared with those without early insulin requirement (75%, p<0.00001). tGADA(96-585) positivity identified a group diagnosed younger (median 55 years vs. 64 years, p<0.002), with a higher type 1 diabetes genetic risk score (median 0.256 vs. 0.216, p=2.9x10-5), lower c-peptide levels (1037 pmol/L vs. 3300 pmol/L, p=0.006) and increased positivity for IA-2A (23% vs. 5%, p=0.01) than those positive only to GADA(1-585). In survival analysis, positivity for tGADA(96-585) stratified those at higher risk of early insulin requirement (HR 2.22 [95% CI 1.32-3.74] p=0.003). High GADA(1-585) affinity did not stratify those with early insulin requirement (HR 1.68 [95% CI 0.91-3.1], p=0.09) but the presence of the IgG1 GADA(1-585) subtype was associated with early insulin requirement when compared with IgG unrestricted subtypes (HR 2.15 [95% CI 1.16-4.01], p=0.016).
Conclusion: tGADA(96-585) epitope specificity can distinguish those with GADA(1-585) positive clinically diagnosed T2D who have the genetic and C-peptide characteristics of T1D and rapid progression to insulin therapy. Strategic testing for GADA epitopes and subclasses will predict those with adult-onset diabetes at risk of rapid progression to insulin therapy.
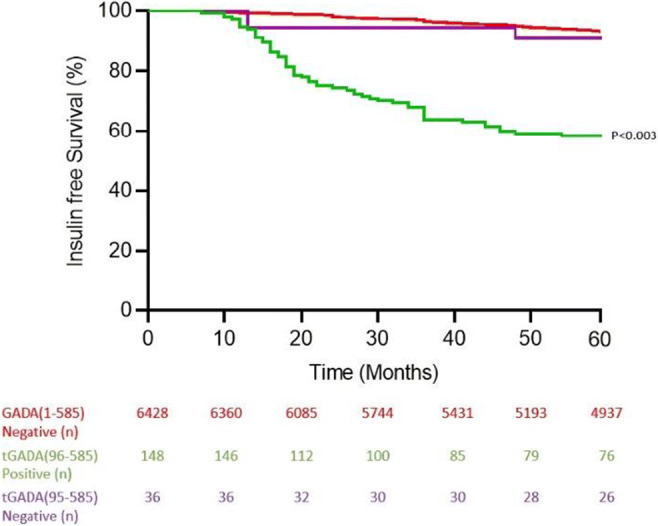
Supported by: The Novo Nordisk UK Research Foundation
Disclosure: S.L. Grace: None.
262
GLP-1R modulates the alloimmune response as a T cells negative costimulatory molecule
V. Usuelli1, M. Ben Nasr1, A. Seelam1, V. Fiorentino2, F. D'Addio1, C. Loretelli1, E. Assi1, G. Zuccotti1, F. Folli3, P. Fiorina1;
1Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, DIBIC, University of Milan, Milan, 2University of Catanzaro, Catanzaro, 3University of Milan, ASST Santi Paolo e Carlo, Milan, Italy.
Background and aims: Glucagon Like Peptide-1 (GLP-1) regulates glucose metabolism by activating its receptor (GLP-1R), which is expressed by pancreatic beta-cells, gastric mucosa and the hypothalamic appetite center. A GLP-1R agonist, Exendin-4 (Exe-4), has reduced the incidence and severity of severe diabetes complications. Beside these metabolic effects, an increasing body of evidences suggests that GLP-1R may have a role in the immune system. GLP-1R expression has been detected in various populations of immune cells, including dendritic cells and T lymphocytes. However, there is no conclusive evidence about a possible immune role of GLP-1R and its signaling. In this present study, we seek to clarify the role of GLP-1R in T lymphocytes during the alloimmune response using murine models of islet and cardiac allotransplantation.
Materials and methods: Cardiac allografts from BALB/c mice were transplanted intra-abdominally in C57BL/6 mice. Rejection was determined as cessation of cardiac contractility. Pancreatic islets from BALB/c mice were inoculated under the renal capsule of each C57BL/6 recipient rendered diabetic with streptozotocin. Rejection of islet allografts was defined as blood glucose levels >250 mg/dl. Transplanted mice were intraperitoneally injected according to the following protocols: 1) Exe-4 0.2μg; 3) Exe-4 2μg; 4) Exe-4 2μg + Exe-9-39 2μg; 5) Exe-4 2μg + Rapamycin 0.1mg; 6) saline. Kaplan-Meier analysis was used for analysis of survival. When two groups were compared, a two-sided unpaired Student t test or a Mann-Whitney test was used.
Results: Our data showed that CD4 and CD8 T lymphocytes expressed GLP-1R (human T cells: CD4+=6%, CD8+=28%; murine T cells: CD4+=6%, CD8+=4,5%). The presence of a discrete GLP-1R+ population was mainly confined to CD4+, CD8+ effector T cells and to CD4+ regulatory T cells. When C57BL/6 mice received islet or cardiac allotransplantation from a fully mismatched BALB/c donors, an expansion of GLP-1R+CD4+ and GLP-1R+CD8+ T cells occurred in the spleen and were found to infiltrate the graft at high percentage. The treatment with Exendin-4 significantly prolonged islet (p≤0.001) and cardiac allograft survival (p<0.01), and reduced graft T lymphocytes infiltrates. A strong synergism in mitigating alloimmunity was observed between Exendin-4 and Rapamycin showing a prolongation of graft survival (p≤0.0001). The beneficial effects of Exendin-4 were partly abolished by the co-administration of its antagonist Exendin 9-39. To get further insight into the molecular mechanism responsible for T cell modulation by GLP-1R, we observed that mitogen stimulation of splenocytes rendered CD4+GLP-1R+ and CD8+GLP-1R+ T cells more apoptotic as compared to their negative counterparts. Notably, our transcriptomic profiling on CD4+GLP-1R+ and CD8+GLP-1R+ T cells revealed their tendency toward an apoptotic and anergic profile as compared to their counterparts CD4+GLP-1R- and CD8+GLP-1R- T cells.
Conclusion: Altogether, our findings suggested that GLP-1R appeared to act as a negative costimulatory molecule and the GLP-1R agonist Exendin-4 prolongs allograft survival, mitigates alloimmunity during alloimmune response, reduces T lymphocytes graft infiltration and skews the effector/regulatory T cell balance.
Disclosure: V. Usuelli: None.
263
Targeting pancreatic islet NLRP3 improves islet graft revascularisation in an insulin-dependent manner
S. Wrublewsky, T. Speer, L. Nalbach, A.S. Boewe, M. Pack, L.P. Roma, M.D. Hoffmann, B.M. Schmitt, A. Weinzierl, M.D. Menger, M.W. Laschke, E. Ampofo;
Universität des Saarlandes, Homburg, Germany.
Background and aims: Hypoxia-induced cell death, which is caused by an insufficient revascularization of the grafts, is a major problem of pancreatic islet transplantation. It has been reported that the loss of the nucleotide-binding oligomerization domain (NOD)-like receptor protein (NLRP)3 inflammasome protects islets against hypoxia-induced cell death and enhances insulin secretion. Therefore, we hypothesized that the inhibition of NLRP3 improves pancreatic islet transplantation.
Materials and methods: Islets were isolated from wild type (WT), Nlrp3-/- and caspase (Casp)1-/- mice and cultured for 24 hours. Moreover, isolated WT islets were cultivated with or without the NLRP3 inhibitor CY-09 for 24 hours. The viability and cellular composition of the islets were analyzed by flow cytometry and immunohistochemistry. The effect of NLRP3 on angiogenesis was studied by tube formation, aortic ring and spheroid sprouting assays. Insulin secretion was determined by an enzyme-linked immunosorbent assay (ELISA) and insulin expression by quantitative real-time (qRT)-PCR, Western blot and co-immunoprecipitation. In vivo, islets were transplanted into mouse dorsal skinfold chambers and their revascularization was assessed by intravital fluorescence microscopy during a 14-day observation period. Moreover, we used the streptozotocin-induced diabetic model for evaluating the endocrine function of the grafts.
Results: We found that Nlrp3 deficiency and loss of NLRP3 activity do not affect the viability and cellular composition of the islets. WT islets exposed to CY-09 as well as transplanted Nlrp3-/- islets exhibited a higher functional microvessel density (442 ± 18 cm/cm2 and 400 ± 9 cm/cm2) when compared to controls (413 ± 21 cm/cm2 and 365 ± 8 cm/cm2). The fraction of intra-islet endothelial cells within the Nlrp3-/- and WT exposed to CY-09 grafts was significantly increased (21 ± 2% and 20 ± 3%) when compared to their corresponding controls (10 ± 2% and 9 ± 2%). Additional in vitro analyses revealed that NLRP3-dependent insulin release stimulates angiogenesis. We could further shown, that the loss of NLRP3 in hypoxic islets triggers insulin gene expression by inducing the shuttling of MafA and pancreatic and duodenal homeobox (PDX)-1 into the nucleus. This was mediated by a reduced interaction of NLRP3 with the thioredoxin-interacting protein (TXNIP). Transplantation of Nlrp3-/- islets or WT islets exposed to CY-09 under the kidney capsule of diabetic mice accelerated the restoration of normoglycemia (165 ± 23 mg/dL and 165 ± 21 mg/dL) when compared to controls (314 ± 28 mg/dL and 308 ± 38 mg/dL). In contrast, transplantation of Casp1-/- islets did not restore physiological blood glucose levels (217 ± 37 mg/dL).
Conclusion: This study demonstrates that suppression of NLRP3 in isolated islets markedly improves their revascularization after transplantation in an insulin-dependent manner.
Disclosure: S. Wrublewsky: None.
264
Metabolic stress by high fat diet increased diabetes incidence in the LEW.1AR1-IDDM rat model of autoimmune diabetes without trigger by systemic inflammation
J.-N. Dahmen1,2, T. Schöppe2, M. Tiedge2;
1Dept. of Internal Medicine and Cardiology | CVK, Charité - Universitätsmedizin Berlin, Berlin, 2Dept. of Medical Biochemistry & Molecular Biology, Rostock University Medical Center, Rostock, Germany.
Background and aims: Metabolic stress and systemic inflammation are not only considered factors in the pathogenesis of non-insulin dependent diabetes mellitus (NIDDM) but may also play a role in the activation of autoaggressive T-cells in autoimmune diabetes. The crosstalk between metabolism, immune system and beta cells may affect the trigger period of autoimmunity. To clarify the mechanisms of metabolic stress on beta cell autoimmunity we fed a hypercaloric high fat diet (HFD) to LEW.1AR1-iddm rats. This strain develops spontaneous autoimmune diabetes beginning at about 60 days of age. Infiltration of pancreatic islets starts within a narrow time frame between 40 and 45 days of life. We investigated the effect of the HFD high fat diet on the inflammatory and metabolic state during the development of beta cell autoimmunity. We also tested whether a HFD increases the incidence of diabetes and diabetes onset occurs earlier in life.
Materials and methods: LEW.1AR1-iddm rats and diabetes-resistant LEW.1AR1 rats received a high fat diet (HFD; 60 % of energy from fat) or a standard diet (SD; 9 % of energy from fat). Key parameters of metabolism (indirect calorimetry; body weight; blood glucose) and systemic inflammation (gene expression patterns in peripheral blood mononuclear cells -PBMCs) were determined at different ages between day 35 and 60. Animals were monitored for hyperglycemia until the age of 120 days.
Results: Under standard diet LEW.1AR1-iddm rats showed higher gain of body weight and higher respiratory quotients than the diabetes-resistant background strain. Gene expression signatures in PBMCs were characterized by proinflammatory cytokines IL-1β and IFN-γ at the beginning of insulitis. After day 50 low levels of FOXP3 expression and an increase of T-cell markers could be observed. The HFD lowered the respiratory quotient to 0.7 and resulted in a higher energy uptake, metabolic rate and activity scores in both strains. Prediabetic LEW.1AR1-iddm rats showed significantly lower blood glucose concentrations and a lower gain of body weight under HFD. Interestingly, expression of pro-inflammatory (IFN-γ, IL-1β, IL-6, IL-17) and anti-inflammatory (IL-4; FOXP3) genes of PBMCs were significantly lower under HFD. The median diabetes-free survival was 79 days in LEW.1AR1-iddm rats under SD (n = 49) and 71 days under HFD (n = 22). LEW.1AR1-iddm rats on HFD had a higher incidence of diabetes in comparison to the SD (95 % vs. 65 %). Log rank test showed significant differences of diabetes free survival between the two diets (p = 0.019).
Conclusion: A HFD increased the incidence of diabetes with earlier onset in LEW.1AR1-iddm rats. In contrast to the situation in T2D, an increase in systemic inflammation does not seem to be responsible for the increased beta cell autoimmunity. However, possible mechanisms may be due to the functional overload of beta cells by metabolic stress, which promotes the recruitment of autoaggressive T-cells via enhanced release of antigens.
Supported by: DDG (German Diabetes Foundation)
Disclosure: J. Dahmen: Grants; Supported by German Diabetes Foundation (DDG).
SO 01 Epigenetics rules
265
Sorting of human pancreatic islets by flow cytometry into alpha and beta cells reveals unique novel genes expressed in the different cell types
J.K. Ofori1, K. Bacos1, A. Perfilyev1, A. Wendt2, U. Krus3, L. Eliasson2, C. Ling1;
1Dept. of Clinical Sciences, Epigenetic and Diabetes Unit, Lund University, 2Dept. of Clinical Sciences, Islet Cell Exocytosis Unit, Lund University, 3Dept. of Clinical Sciences, Lund University, Malmö, Sweden.
Background and aims: Human islets have several distinct cell types that regulate glucose homeostasis. The two main cell types are α-cells, which secrete glucagon to raise plasma glucose levels in the fasted state, and β-cells that release insulin to decrease plasma glucose levels in the fed state. Dysfunction in these cell types contributes to pathogenesis of type 1 diabetes and type 2 diabetes. Here, we aimed to sort α- and β-cells from human pancreatic islets and use RNA sequencing to identify unique and abundant transcripts expressed in respective cell type.
Materials and methods: Human islets from 16 multi-organ donors were sorted by flow cytometry into α- and β-cells subsequent RNA isolation was performed according to the MARIS protocol. We performed RNA sequencing on the sorted α- and β-cells using NextSeq 500 (Illumina). Downstream analysis was done using R, and the data was filtered to only include protein-coding genes. A transcript was considered expressed in a particular cell type if it has at least 20 reads in 40% of the samples. Furthermore, using WEB-based Gene Set Analysis Toolkit (http://www.webgestalt.org/), we analyzed the enrichment for KEGG pathways or biological processes for unique transcripts expressed in these two cell types.
Results: We identified 13,001 and 12,875 genes expressed in human α- and β-cells, respectively. Interestingly, genes expressed in our α- and β-cell cohort significantly overlapped (93-94%) with previous published work by Blodgett et. al, 2015 when we reanalysis their RNA sequencing data (N= 6 donors) with the same settings as ours (N=16 donors). The number of unique transcripts identified to be expressed in either α- and β-cells were 617 and 497, respectively. Transcripts uniquely expressed in α-cells show significant enrichment in biological processes such as cAMP-mediated signaling (ADCY4, ADGRB2, ADRB1 etc.), regulation of ion transport ( ANO6, FXYD3, KCNIP1 etc.), cell proliferation (FAM83B, FAP, KLB etc.), homeostatic process ( F7, PLAUR, FGA etc.), exocytosis (DOC2A, GRIN3A, SYT3 etc.) and regulation of apoptotic process (VIL1, LPAR1, AR etc.). The only significant KEGG pathway identified for unique transcripts expressed in α-cells was Protein digestion and absorption pathway (DPP4, SLC7A7, SLC3A1 etc.). Moreover, we identified significant enrichment of transcript uniquely expressed in β-cells to be involved in gland development (IGF2, NRG1, NKX3-1 etc.), cell-cell signaling (GAD1, GLP1R,, SIX3 etc.), regulation of hormone levels (SLC2A2, SPP1, FOXE1 etc.), ion transport (KCNJ16, GLRA1, STEAP3 etc.) and synaptic signaling(P2RX2, TMEM108, LRFN2 etc.).Next, we looked at abundantly expressed transcripts in α- and β-cells. Interestingly, ~40% of top 20 most highly expressed transcripts in both cell types are mitochondrially encoded genes (MT-CO1, MT-ND4, MT-CO3, MT-CYB, MT-ND2, MT-ATP6, MT-ND5).
Conclusion: We identified unique genes regulating for example exocytosis and gland development to be expressed in either α- or β-cells. Our data also reveal that mitochondrially encoded genes are among the most abundantly expressed transcripts in both α- and β-cells.
Supported by: SRC, Fysiografen, EXODIAB
Disclosure: J.K. Ofori: None.
266
Impact of type 2 diabetes-associated variants at the SLC30A8 locus on transcriptional activity and function of human b cells and super-enhancer at SLC30A8 gene locus
M. Hu1, I. Kim1, M. Canouil2, S. Bonas-Guarch3, A.A. Khamis2, J. Ferrer3, P. Froguel1, G.R. Rutter1;
1Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK, 2Inserm U1283, CNRS UMR 8199, Institut Pasteur de Lille, Lille, France, 3Regulatory Genomics and Diabetes, The Barcelona Institute of Science and Technology, Barcelona, Spain.
Background and aims: Genome-wide association studies (GWAS) and subsequent epigenomic mapping of human islets have identified many genetic variants and dissected the pathways through which these influence the β cell function to alter type 2 diabetes (T2D) risk. SLC30A8, which encodes zinc transporter ZnT8, has been extensively studied and shown to impact insulin granule structure and regulate insulin secretion. Whilst affecting the primary sequence of ZnT8, the lead variant at this locus, rs13266634, and two others, lie within active enhancers which are the components of an islet-specific super-enhancer. Here, we aimed to elucidate (1) how these variants affect the transcriptional activity of super-enhancer and the expression of downstream candidate genes and (2) may in turn influence β cell function.
Materials and methods: Human pancreas-derived EndoC-βH3 cells were used throughout. Promoter-luciferase reporter, qRT-PCR, and glucose-stimulated insulin secretion (GSIS) assays were deployed according to standard protocols to identify and assess the function of variants, enhancers, and genes. CRISPR/Cas9 genome editing was used to create the required mutations or deletions.
Results: Using published epigenomic maps, we found that 3 out of 11 GWAS-identified variants at the SLC30A8 locus lay within two active enhancers of the super-enhancer. eQTL analyses suggested that these variants were nominally associated with the expression of genes encoding EIF3H, UTP23, and RAD21 at the 5’ end of the locus. Both promoter-HiC and HiChIP analyses indicated that the super-enhancer made spatial contacts with these three genes through chromatin looping. The variant region, which is embedded in the super-enhancer, also contacted the promoter region of the RAD21 gene. These findings suggest that the variants could influence the expression of these remotely located genes. We first analyzed the transcriptional activity of three variants using promoter-luciferase assays. The protective variants of rs3802177 and rs35859536 displayed lower luciferase activity compared with their risk variants. No change was observed for rs1326634. We next assessed the role of variant-bearing enhancers in β cell function. Deletion of variant-bearing enhancers led to 20-40% reduced expression of SLC30A8 and other genes but increased insulin secretion upon stimulation of glucose and IBMX. Finally, we individually knocked out these remote genes potentially under the control of the super-enhancer in EndoC-βH3 cells. Deletion of RAD21 or UTP23 severely reduced the viability of cells (up to 90%) suggesting that these genes are indispensable for β cell survival.
Conclusion: Taken together, these data demonstrate that modification of super-enhancer activity at the SLC30A8 locus affects β cell function. The risk variants are likely to exert their pathogenic effects by affecting the transcriptional activity of the super-enhancer and the expression levels of SLC30A8 as well as the nearby genes EIF3H, RAD21, and UTP23.
Supported by: MRC
Disclosure: M. Hu: None.
267
Genomic enhancers regulations in type 2 diabetes
E. Carcarino1, M. Didisheim1, C. Vandiedonck1, D. Girard1, R. Nielsen2, H.-J. Garchon3, H. Mambu-Manbueni3, J.-B. Julla1, L. Potier1, R. Roussel1, S. Mandrup2, J.-F. Gautier1, N. Venteclef1;
1Institut National de la Santé et de la Recherche Médicale (INSERM) U1151, Paris, France, 2Department of Biochemistry and Molecular Biology, University of Southern Denmark, Odense, Denmark, 3Institut National de la Santé et de la Recherche Médicale (INSERM) U1173, Montigny le Bretonneux, France.
Background and aims: A recent classification has established 4 subgroups of type 2 diabetes (T2D) categorised according to insulin resistance, insulin secretion deficiency, age, body mass index and glycated hemoglobin. Interestingly, these T2D subtypes seem to be associated with inflammatory markers such as leukocytes and monocytes blood counts. Moreover, emerging studies revealed that genomic regulatory regions, named enhancers, are crucial to modulate immune cell gene transcription in response to environmental stimuli. In this study we aimed at 1) investigating whether immuno-inflammatory profiles of blood immune cells can define new T2D endotypes; 2) evaluating genomic enhancers regulatory impacts on T2D distinction.
Materials and methods: We recruited 723 type 2 diabetic subjects (33% females; median age: 61 years old; median diabetes duration: 13 years; median BMI: 28.3 kg/m2; median HbA1c: 7.7 %) in Lariboisière and Bichat University Hospitals (Paris, France). Isolation of venous blood from patients allowed to 1) perform immunophenotyping analysis based on antibodies-coupled flow cytometry, in order to measure patients monocytes frequencies and analyze them by k-means clustering approach; 2) perform patients monocytes immunoselection, followed by RNA-sequencing (RNA-seq) and Assay for Transposase-Accessible Chromatin (ATAC-seq) analysis, in order to combine the study of patients transcriptome and chromatin accessibilities and then evaluate the enhancers dynamics in the T2D subgroups.
Results: 3 main clusters were defined by applying k-means clustering according to blood monocyte subpopulations frequencies: classical, intermediate, and non-classical monocytes. RNA-seq analyses identified specific immune responses associated with each cluster, with an enrichment of interleukin-1B (IL-1B) expression in one of the clusters. ATAC-Seq analyses revealed that higher levels of IL-1B transcription are specifically promoted by increased chromatin accessibility of a specific enhancer, at IL-1B locus, in this sub-mentioned cluster (p<0.05). Interestingly, a common Single Nucleotide Polymorphism (SNP), already associated to regulation of IL-1B and inflammatory conditions, is located within this enhancer which may impact IL-1B transcription.
Conclusion: All together, our results provide evidence that dysregulation of a specific enhancer associated with IL-1B gene expression could contribute to the inflammatory profile of a subgroup of T2D patients.
Clinical Trial Registration Number: NCT02671864
Supported by: EFSD/Lilly Young Investigator Research Award Programme
Disclosure: E. Carcarino: None.
268
Hyperglycaemia induces endothelial dysfunction via epigenetic regulation driven by histone methyltransferase EZH2
J. Sánchez-Ceinos1, S. Hussain1, A. Waheed-Khan1,2, L. Zhang1, J. Pernow1, F. Cosentino1;
1Medicine, Solna, Cardiology Unit, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden, 2Diabetes, Central Clinical School, Monash University, Melbourne, Australia.
Background and aims: Post-translational modifications (PTMs) of histones are crucial epigenetic mechanisms regulating gene expression in response to environmental changes. However, in pathological conditions, this regulation may determine maladaptive molecular disturbances contributing to the development of diseases. The trimethylation of histone 3 at lysine 27 (H3K27me3) is a PTM linked to the repression of genes involved in various biological events, such as oxidative stress. The addition or removal of this PTM to the promoter of certain genes are dynamically modulated by methyltransferases EZH1 and EZH2, and demethylases KDM6A, B, and C, respectively. Since hyperglycemia-induced endothelial dysfunction is closely related to oxidative stress and inflammation, herein we investigated the role of H3K27me3 in the regulation of these processes and whether targeting chromatin-modifying enzymes may represents a valuable strategy to safeguard endothelium function.
Materials and methods: We employed human aortic endothelial cells (HAECs) exposed to either normal or high glucose and endothelial cells from diabetic individuals (D-HAECs) to test the effect of EZH2 targeting, through pharmacological inhibition with GSK126 or siRNA. Gene expression levels of H3K27me3 and related chromatin-modifying enzymes (EZH1, EZH2 and KDM6A, B and C), as well as genes involved in oxidative stress and inflammation were determined by RT-PCR, western blotting, and/or confocal studies. The binding of H3K27me3 to the promoter region of candidate genes was evaluated by chromatin immunoprecipitation (ChIP). Reactive oxygen species (ROS) production and NF-κB binding activity were measured using electron spin resonance spectroscopy and protein assay, respectively. Endothelium-dependent and -independent relaxations were assessed in aortas from male mice incubated with normal and high glucose (5 and 25 mM, respectively) by myograph.
Results: H3K27me3 levels were significantly increased in HAECs exposed to high glucose and in D-HAECs as compared to HAECs cells under normal glucose conditions. ChIP assays revealed increased binding of H3K27me3 to the promoters of antioxidant genes (SOD1, SOD2, and JunD) in these cells. RT-PCR and immunoblotting studies confirmed the repression of these genes. By contrast, NOX4, a major source of oxidative stress, was upregulated. Along with an increase in ROS, HAECs exposed to high glucose and D-HAECs exhibited pro-inflammatory changes, namely activation of NF-κB and its downstream target genes. Gene expression of EZH2 was upregulated in these cells, whereas KDM6A and KDM6B were downregulated. Interestingly enough, treatment with either EZH2-specific inhibitor GSK126 or EZH2 silencing was able to promote a favourable transcriptional program rescuing the observed molecular derangements. Finally, GSK126 treatment of murine aortas incubated with high glucose restored endothelium-dependent relaxations.
Conclusion: Our results unravel adverse epigenetic signatures underlying endothelial dysfunction in diabetes. Targeting EZH2 may serve as a potential novel anti-atherosclerotic treatment in this setting to prevent endothelial dysfunction through attenuation of oxidative stress and proinflammatory mediators.
Supported by: Swedish Research Council, the Swedish Heart & Lung Foundation, and King Gustav V and Queen Victoria
Disclosure: J. Sánchez-Ceinos: None.
269
Altered H3K4me3 profile on TFAM promoter causes preadipocytes and mitochondrial alterations in first-degree relatives of type 2 diabetics
F. Zatterale1, M. Longo1, A. Desiderio1, R. Spinelli1,2, L. Parrillo1, M. Campitelli1, P. Florese1, U. Smith2, G.A. Raciti1, F. Beguinot1;
1DISMET & URT of IEOS-CNR, Federico II University of Naples, Naples, Italy, 2Department of Molecular and Clinical Medicine, University of Gothenburg, Gothenburg, Sweden.
Background and aims: First-Degree Relatives of type 2 diabetics (FDR) are characterized by enlarged subcutaneous adipocytes and inappropriate subcutaneous adipose tissue (SAT) hypertrophy, independent of obesity and fat distribution. FDR have a risk of developing type 2 diabetes (T2D) up to 10-fold higher than age-matched subjects without a family history of the disease (CTRL). In FDR, dysfunctional adipose tissue and T2D risk are contributed by impaired adipocyte precursor cells (APCs) recruitment into the adipogenic pathway. Epigenetic regulatory mechanisms are directly involved in several steps of adipocyte differentiation biology and play an important role in this abnormality. The present study explores changes in the genome-wide tri-methylation of lysine 4 on histone H3 (H3K4me3) profile in APCs from SAT of healthy FDR and matched control individuals. H3K4me3 profile is linked to transcriptional consistency and cell identity and plays a fundamental role in stem cell biology.
Materials and methods: Deep chromatin-immunoprecipitation sequencing (ChIP-Seq) for H3K4me3 was applied in APCs isolated from FDR (n=9) and CTRL (n=11) SAT biopsies. mRNA levels were measured by Real-time PCR (qPCR), while methylation levels by bisulfite sequencing. The mitochondrial DNA (mtDNA) amount was assessed by qPCR. Histone modifications enrichment was measured by ChIP-qPCR.
Results: ChIP-seq data analysis revealed 2644 differentially H3K4me3 enriched regions in CTRL and FDR. Cellular component analysis of annotated genes revealed significant enrichment in mitochondrial-related genes. TFAM gene, one of the most H3K4me3 differentially enriched genes that regulates mitochondrial content and stability, was chosen for further investigation. In FDR APCs, a significant reduction in H3K4me3 enrichment at the TFAM promoter was paralleled by TFAM mRNA and protein levels reduction (p<0.05). FDR APCs also exhibited reduced mtDNA content (p<0.01) and mitochondrial-encoded gene transcription (p<0.05). TFAM promoter showed no differences in DNA methylation between FDR and CTRL, while H3K27ac enrichment was detected in CTRL APCs (p<0.05). TFAM-siRNA transfection of CTRL APCs reduced TFAM mRNA (p<0.001) and protein levels (p<0.001) as well as mtDNA content (p<0.01) and mitochondrial DNA-encoded genes transcription (p<0.01), indicating a direct involvement in inducing the mitochondrial alterations observed in the FDR APCs. FDR APCs exhibited decreased adipogenic potential (p<0.001) and impaired TFAM induction during adipogenesis. Furthermore, FDR APCs show a significant decrease in mtDNA at all stages of adipogenesis (p<0.05). To test the TFAM effect on APCs differentiation capacity, CTRL APCs were differentiated and silenced throughout adipogenesis. We found that TFAM-silencing impairs APCs differentiation (p<0.05), mtDNA content (p<0.001), and mitochondrial encoded genes transcription (p<0.001), establishing a new insight into TFAM regulation of APCs differentiation.
Conclusion: We determined an epigenetic signature in FDR preadipocytes associated with increased T2D risk. Histone modification events regulate APC ability to differentiate into mature adipocyte by modulating the TFAM expression and affecting mitochondrial function.
Disclosure: F. Zatterale: None.
270
Could miR-295-3p be a novel epigenetic regulator of GLUT4?
J.V. Esteves1, L.S. Amendola2, M.M. Okamoto1, U.F. Machado1;
1Departament of Physiology and Biophysics, Institute of Biomedical Sciences, University of São Paulo, 2Laboratório de Lípides (LIM 10), Hospital das Clínicas (HCFMUSP) da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Background and aims: The loss of glycemic homeostasis is directly related to the reduction of GLUT4 protein expression (encoded by the SLC2A4 gene) in peripheral tissues, but participation of epigenetic regulators like microRNAs (miRNAs) in this process is poorly understood. The aim of this study was to evaluate the expression of miRNAs potentially involved in the Slc2a4/GLUT4 repression in muscle/adipose tissues of obese type 2 diabetic (T2D) mice.
Materials and methods: Mice were rendered T2D by neonatal subcutaneous injection of monosodium glutamate from day 1 to day 5 (2 mg/kg body weight) and day 7 (4 mg/kg body weight). Control mice received only the vehicle (0.9% NaCl). In 27-week-old animals, blood was collected for metabolic analysis, and gastrocnemius muscle and periepididymal adipose tissue were harvested for Slc2a4 mRNA and miRNAs (RT-qPCR) and GLUT4 (Western blotting) measurement. In silico analysis was used to select predicted miRNAs for validation. The comparison among groups was performed by unpaired t-test or Mann-Whitney test, as suitable.
Results: T2D mice developed increasing concentrations of plasma glucose (21%, P<0.05), fructosamine (33%, P<0.05), triglycerides (65%, P<0.01) and insulin (223%, P<0.0001). The Slc2a4/GLUT4 expression was reduced in muscle (~20%, P<0.01) and adipose (-80%, P<0.0001) tissues. In silico analysis revealed hundreds of miRNAs as potential regulators of Slc2a4 expression; from these, increased miR-295-3p expression was confirmed in muscle (110%, P<0.05) and adipose tissue (203%, P<0.01).
Conclusion: T2D represses the Slc2a4/GLUT4 expression in peripheral tissues, and that may be related to enhanced miR-295-3p content. Next steps will be taken to determine that miR-295-3p is a direct epigenetic regulator of GLUT4, establishing its true role in glycemic homeostasis.
Supported by: FAPESP (#2016/15603-0; #2017/19449-9)
Disclosure: J.V. Esteves: None.
271
Hyperglycaemia induces Toll-like receptor activation in macrophages
L. Badillo Garcia1, K. Moganti1, M. Mossel1, V. Ryabov1, M. Harmsen2, H. Klüter1, J. Kzhyshkowska1;
1Heidelberg University, Mannheim, Germany, 2Dept. Pathology and Medical Biology, University of Groningen, Groningen, Netherlands.
Background and aims: Hyperglycaemia (HG) is a hallmark of diabetes critical for the development of diabetic vascular complications. Macrophages are key innate immune cells responsible for inflammatory reaction in diabetes and development of micro- and macrovascular complications. Inflammatory programming of macrophages can be regulated and maintained by epigenetic mechanisms. The aim of our study was to identify the effect of hyperglycemia on the Toll-like receptors gene expression.
Materials and methods: CD14+ monocytes were isolated out of buffy coats using magnetic cell sorting. M0 (non-activated), M1 (pro-inflammatory) and M2 (healing) macrophages were differentiated in normal and hyperglycemic (HG) conditions for 6 days. Bisulfite sequencing was used to identify specific methylation profile of Toll-like receptor gene promoters in monocytes. After monocyte to macrophage differentiation the cells were stimulated with TLR agonists (LPS, FSL-1 and Pam3CSK4). The effect of HG on Toll-like receptor gene expression was quantified using RT-PCR. The secretion product of macrophages was assessed by ELISA.
Results: Provisional data from the bisulfite sequencing showed methylation of the promoters of TLR1, TLR4 and TLR6 before differentiation to macrophages. Hyperglycemia induced upregulation in expression of the following Toll-like receptors: TLR1 in M0 and M1, TLR4 in M2, TLR6 in M1 and TLR7 and TLR8 in M0. The highest effect of HG was on TLR7 expression in M0 (fold change = 2.83), TLR1 in M1 (fold change= 1.59) and TLR4 in M2 (fold change= 1.54). Results of ELISA revealed increased production, in presence of HG and TLR agonists, of IL1beta in M0, and TNFα in M1 macrophages. The significance of difference between groups of experimental data in ELISA and RT-PCR analysis was determined using Wilcoxon matched-pairs rank test.
Conclusion: Our data demonstrates that hyperglycemia induces gene expression of Toll-like receptors in macrophages. Changes TLR expression profile on pro-inflammatory and healing macrophages that can enhance macrophage sensitivity for the metabolic unwanted-self products in diabetes and can provide conditions for metabolic inflammation.
Supported by: GRK 1874: DIAMICOM (DFG)
Disclosure: L. Badillo Garcia: None.
SO 02 Pregnancy and diabetes
272
The impact of multiple deprivation on gestational diabetes incidence
S. Jeyaparam;
Faculty of Medicine, Imperial College London, London, UK.
Background and aims: Disparities in social class have been shown to affect a number of health outcomes, including life expectancy, recovery from myocardial infarctions and recovery from hip fractures. Importantly, deprivation has previously been shown to correlate with perinatal mortality and the incidence of type 2 diabetes. However, to date, no study has explored the relationship between deprivation and the incidence of gestational diabetes (GD). We aimed to identify the presence of any relationship between postcode Index of Multiple Deprivation (IMD) rank and incidence of GD, after accounting for age, BMI and specific ethnicity (amongst 13 different ethnic groups). Furthermore, we also examined for relationships between IMD rank and perinatal outcomes: birthweight centile; stillbirths; special care baby unit (SCBU) admissions and severe (<32 week) prematurity.
Materials and methods: We conducted a retrospective cohort analysis of 23490 pregnancies followed in a major hospital in Northwest London. The 2019 English Indices of Multiple Deprivation was used to identify the deprivation rank and decile for each maternal postcode. Of those that received an oral glucose tolerance test (OGTT) (n= 17844), 1854 were diagnosed with GD. Birthweight centile was calculated from absolute birthweight after adjusting for ethnic origin, maternal height, maternal weight, parity, sex and outcome (live birth/stillbirth). Multiple logistic regression and correlation analyses were used to identify correlations between variables.
Results: After controlling for age, BMI & ethnicity, there was no correlation between which deprivation decile a woman's postcode was and their odds of developing gestational diabetes. (Kendall's Tau b = 0.000, significance 0.932). After adjusting for the previously mentioned confounders, every standard increase in age increased the odds of developing GD by 7.6% (OR: 1.076, p<0.001); BMI similarly increased odds by 5.9% (OR: 1.059, p<0.001). After adjusting for age, BMI and IMD rank, ethnic origin greatly affected odds of developing GD, particularly for Asian women who were all over three-fold more likely to develop GD compared to white British women (ORs: Pakistani 3.332; Indian 4.675; Bangladeshi 5.824; other Asian 3.756; mixed white and Asian 3.075, p<0.001 in all cases). There was no significant correlation between IMD rank and perinatal outcomes.
Conclusion: Our study demonstrates that after adjusting for age, BMI and ethnicity, multiple deprivation did not correlate with incidence of gestational diabetes or perinatal outcomes. We have also shown the effect of age, BMI and individual ethnicity on odds of developing gestational diabetes.
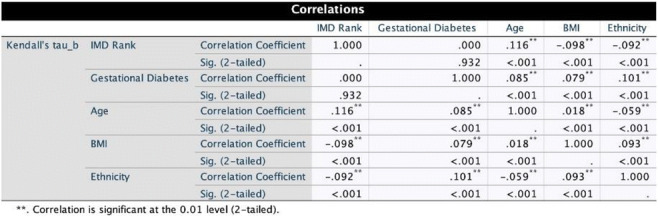
Disclosure: S. Jeyaparam: None.
273
Exposure to non-insulin 2nd-line glucose-lowering medications in early pregnancy and risk of major congenital malformations
S. Hernandez Diaz1, R. Rotem1, K.F. Huybrechts2, B.T. Bateman3, K. Furu4, G. Chodick5, E.W. Seely6, E. Patorno2, C.E. Cesta7;
1Epidemiology, Harvard T.H. Chan School of Public Health, Boston, USA, 2Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA, 3Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Palo Alto, USA, 4Norwegian Institute of Public Health, Oslo, Norway, 5Maccabitech Institute for Research and Innovation, Maccabi Healthcare Services, Tel Aviv, Israel, 6Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA, 7Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden.
Background and aims: The use of 2nd line non-insulin glucose-lowering drugs is increasing overall and in women of reproductive age which may result in fetal exposure in early pregnancy. However, the teratogenic risk of these drugs is unknown. We evaluated if exposure to these drugs in early pregnancy is associated with an increased risk of major congenital malformations (MCM) in the infant.
Materials and methods: We conducted a cohort study of pregnant women with type 2 diabetes and their liveborn infants in the IBM MarketScan Research Database in the US (2011-2019), Maccabi Health Services (MHS) in Israel (2009-2020), and pooled population health register data from Finland, Iceland, Norway, and Sweden (2009-2020). Exposure was defined based on ≥1 prescription fills from 90 days before the first day of the last menstrual period (LMP) to 90 days after LMP of insulin or any non-insulin 2nd line glucose-lowering drugs including sulfonylureas, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) analogues, sodium-glucose co-transporter 2 (SGLT2) inhibitors, or others. Crude prevalence ratios (PR) of MCM at birth and 95% confidence intervals (CI) were estimated comparing exposure to a non-insulin 2nd line glucose-lowering drugs with exposure to insulin in women with type 2 diabetes. Pregnancies with no pregestational diabetes were used as a secondary reference group. Pregestational diabetes, type 2 diabetes, and MCMs were identified using validated algorithms.
Results: Among 5,747,733 pregnancies, 6435 women with type 2 diabetes received insulin and 3190 a non-insulin 2nd line glucose-lowering drugs, with sulfonylureas (n=1488) and GLP-1 analogues (n=490) being the most common. The risk of MCM was 3.1% among infants born to mothers with no pregestational diabetes, 7.2% in those exposed to insulin, and 7.2% in those exposed to a non-insulin 2nd line glucose-lowering drug. The crude PR of MCM for women exposed to non-insulin 2nd line glucose-lowering drugs was 2.34 (95%CI 2.07-2.66) compared with those without pregestational diabetes, and 0.99 (95%CI 0.85-1.16) compared with those with type 2 diabetes exposed to insulin. Results were similar in all three cohorts.
Conclusion: Preliminary results show a higher prevalence of MCM among infants born to mothers using non-insulin 2nd line glucose-lowering drugs in early pregnancy compared with pregnant women with no pregestational diabetes. However, the PR moves to the null when using a comparison group with the same indication (type 2 diabetes) and using the standard treatment in pregnancy (insulin). Further exploration will focus on individual 2nd-line non-insulin glucose-lowering drugs and on specific MCM groups and adjust for diabetes severity.
Supported by: NICHD (R01HD097778), Nordforsk (no.83539), Research Council of Norway (no.273366), and EU Horizon MS
Disclosure: S. Hernandez Diaz: Grants; NICHD R01HD097778.
274
Gestational weight gain in women with pregestational diabetes and risk of small for gestational age newborns
V. Davila-Batista1, B. Vega Guedes2, A. González-Lleó1, A. Wägner1;
1Endocrinology Department, Complejo Hospitalario Universitario Insular Materno Infantil, Las Palmas de Gran Canaria, 2Gynecology and Obstetrics Department, Complejo Hospitalario Universitario Insular Materno Infantil, Las Palmas de Gran Canaria, Spain.
Background and aims: Maternal diabetes are associated with an increased risk of large newborns. On the other hand, gestational weight gain (GWG) is related to offspring weight. However, the effect of low GWG on the risk of low birth weight is not clear in women with pre-existing type 1 or type 2 diabetes. Our objective is to evaluate the association between GWG and the risk of low birth weight in pre-gestational diabetes
Materials and methods: A retrospective cohort study was conducted in all the women with pregestational diabetes (type 1 or type 2) who delivered after 34 weeks of pregnancy at a public reference hospital between 2011 and 2019, using their clinical records. Absolute GWG was calculated as weight in the third trimester (t) minus weight at index date. GWG was classified by the Institute of Medicine (IOM) guidelines 2009, which include recommended cut-off points according to pre-pregnancy body mass index (BMI) categories. Small for gestational age newborns were defined as weight below the 10th percentile (10p) adjusted for gestational age and sex of the newborn in the reference population and a birth weight below 3kg was considered low. Odds ratios (OR) and their 95% confidence intervals (CI) were estimated using conditional logistic regression controlling for age at conception, parity, BMI, Glycosylated haemoglobin (HbA1c) at first (t1) and third (t3) trimesters, gestational days at birth, delivery type, Priscilla White classification and type of diabetes. Separate analyses by type of diabetes, BMI and glycaemic control were performed.
Results: 455 women with pregestational diabetes (46.6% type 1) were evaluated, 27.5% with insufficient GWG according to the IOM. 7.7% of the offspring were small for gestational age. Each 1kg increase in GWG was inversely associated with low birth weight (OR 0.90; 95%CI 0.84-0.97). Low GWG was associated with an increased risk of low birth weight (ORIOM 4.62; 95%CI 1.24-17.16). A suggestive effect modification was shown by HbA1c >6% in 3t (ORIOM 7.90; 1.55-40.23), BMI < 30 kg/m2 (ORIOM 8.33; 95%CI 1.82- 38.11) and Type 1 diabetes (ORIOM 131.21; 4.37-3930.54); and might the association be not significant by HbA1c >6%, BMI >30 kg/m2 or Type 2
Conclusion: In women with pre-gestational diabetes, low GWG is associated with small for gestational age newborns. In particular, low GWG was positively associated in better glycaemic control, non-obesity and Type 1 diabetes
Supported by: ISCIII (Sara Borrell CD21/00025; PI16/00587)
Disclosure: V. Davila-Batista: None.
275
Prevalence of gestational diabetes in women with a family history of type 2 diabetes in first- and second-degree relatives
C. Monod1,2, G. Kotzaeridi1, T. Linder1, D. Eppel1, I. Rosicky1, V. Filippi2, A. Tura3, I. Hoesli2, C.S. Göbl1;
1Department of Obstetrics and Gynecology, Medical University of Vienna, Vienna, Austria, 2Department of Gynecology and Obstetrics, University Hospital Basel, Basel, Switzerland, 3Metabolic Unit, CNR Institute of Neuroscience, Padova, Italy.
Background and aims: A family history of type 2 diabetes mellitus (T2DM) markedly increases an individual's lifetime risk of developing the disease. For gestational diabetes (GDM), this risk factor is less well characterised but may play an essential role. This study aimed to investigate the relationship between family history of T2DM in first- and second-degree relatives in women with a diagnosis of GDM. It also aimed to assess the differences in metabolic characteristics at early gestation in women with and without a family history of T2DM.
Materials and methods: This prospective cohort study included 1164 pregnant women. A broad risk evaluation was performed before 16+0 weeks of gestation, including assessment of maternal characteristics, detailed family history of the different types of diabetes, and a laboratory examination of glucometabolic parameters. First degree relatives were defined as parents or siblings of the pregnant woman, second degree relatives were defined as grandparents, aunts and uncles. Participants were followed-up until delivery and GDM assessed according to the latest World Health Organisation diagnostic criteria. Statistical analysis Continuous variables were summarized by mean ± standard deviation or as median and IQR (in case of skewed distribution). These were compared by analysis of variance or rank based inference. Categorical variables were summarized by counts and percentages, and compared by binomial logistic regression. Odds ratios and 95% confidence intervals (95%CI) were additionally calculated for binary outcomes. Tukey’s honestly significant difference (HSD) test was used for all subgroup (k=4) comparisons to achieve a 95% coverage probability. Statistical analysis was performed with R (version 4.0.2) and contributing packages (especially “multcomp” and “nparcomp”). A two-sided p-value of ≤0.05 was considered statistically significant.
Results: We showed that pregnant women with first- (FHD1) (n=51, 26.6% (OR 1.91, 95%CI 1.16 - 3.16, p=0.005), second- (FHD2) (n=57, 26.3% (OR 1.88, 95%CI 1.16 - 3.05, p=0.005) or both first- and second degree relatives with T2DM (FHD1+D2) (n=31, 33.3% (OR 2.64, 95%CI 1.41 - 4.94, p<0.001) had a markedly increased risk of GDM compared to those with negative family history (FHN) (n=100, 15.9%). The association was the strongest if both parents were affected (OR 4.69, 95%CI 1.33 - 16.55, p=0.009). Women with FHD1 and FHD1+D2 had adverse glucometabolic profiles for almost all parameters, except for HDL-cholesterol, already in early pregnancy, compared to those with FHN, including impaired insulin action, as well as higher glucose concentrations during the diagnostic OGTT.
Conclusion: We confirmed that a family history of T2DM is an important risk factor for GDM, also by applying the current diagnostic criteria. Furthermore, we showed that the degree of kinship plays an essential role in quantifying the risk. Future research should develop further available tools for prediction of GDM and integrate the degree of kinship when considering the family history of T2DM in the risk stratification.
Disclosure: C. Monod: None.
276
Role of histone methyltransferase MLL1-driven H3K4me3 in the transmission of oxidative and inflammatory phenothypes to the offspring of women with gestational diabetes
N. Di pietrantonio1,2, M. Shumliakivska1, J. Sánchez-Ceinos1, D. Mandatori2, A. Rakow3, P. Di Tomo2, M.P.A. Baldassarre4, M. Sennström5, G. Formoso4, T. Bonfini6, A. Pandolfi2, F. Cosentino1;
1Department of Medicine, Unit of Cardiology, Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden, 2Department of Medical, Oral and Biotechnological Sciences, CAST (ex CeSI-MeT), University G. d’Annunzio, Chieti, Italy, 3Department of Neonatology, Karolinska University Hospital, Stockholm, Sweden, 4Department of Medicine and Aging Sciences, University G. d’Annunzio, Chieti, Italy, 5Department of Women’s and Children’s Health, Karolinska University Hospital, Stockholm, Sweden, 6Department of Oncology Hematology, Hospital Spirito Santo, Pescara, Italy.
Background and aims: Gestational diabetes (GD) is characterized by chronic hyperglycemia during pregnancy. Hyperglycemia-induced oxidative stress and inflammation are potent drivers of atherosclerotic cardiovascular disease (ASCVD). Recently, it is emerging that histone modifications are key players in hyperglycemia-induced ASCVD and putative mediators of the transmission of GD phenotypes to the offspring. The present study was designed to investigate the link between Mixed Lineage Leukemia 1 (MLL1)-dependent trimethylation of histone 3 at lysine 4 amino residue (H3K4me3) and oxidative and inflammatory phenotypes. Additionally, we aim to investigate whether such GD-induced epigenetic mark is transmitted and persist in the offspring born to GD women.
Materials and methods: We obtained peripheral blood mononuclear cells (PBMC) from GD and control women, endothelial cells from human umbilical cord vein (HUVEC) and cord blood mononuclear cells (CBMC) at birth, as well as PBMC from adolescents born to control and GD women. NF-kB p65 and its downstream target Vascular Cell Adhesion Molecules (VCAM-1) gene and protein expression were evaluated by RT-qPCR, immunofluorescence and flow-cytometry, respectively. NADPH oxidase isoform 4 (NOX4) protein expression was evaluated by immunofluorescence. H3K4me3 on NF-kB p65 and (NOX4) promoter regions was assessed by chromatin immunoprecipitation (ChIP) and RT-qPCR. NF-kB p65 DNA binding activity was measured by ELISA and a HUVEC-monocytes adhesion assay was performed to assess the adhesion of monocytes to endothelial cells.
Results: We observed increased gene expression of NF-kB p65 and its downstream target VCAM-1 in GD- as compared to control-HUVEC. For the first time, we demonstrated that MLL1-driven H3K4me3 on NF-kB p65 promoter is upstream to the activation of such inflammatory pathway. Indeed, treatment with MLL1 inhibitor (MM-102, 10 μM) blunted upregulation of both NF-kB p65 and VCAM-1 genes, as well as TNFα-induced NF-kB p65 DNA binding activity and VCAM-1 protein expression. Furthermore, inhibition of histone methyltransferase MLL1 abolished upregulation of pro-oxidant NOX4 gene. Interestingly enough, the MLL1-induced H3K4me3 was present not only in PBMC from GD women and CBMC at birth but also in PBMC from adolescent born to GD women.
Conclusion: Our results suggest that MLL1-induced epigenetic mark is responsible for the maternal inflammatory and oxidative phenotypes in GD women, and it is transmitted to the offspring. The deciphering of epigenetic-induced chromatin remodeling opens the perspective for pharmacological reprogramming of adverse histone modifications to reduce the burden of early abnormal phenotypes in the offspring potentially leading to ASCVD.
Disclosure: N. Di pietrantonio: None.
277
Differential epigenetic profile related to carbohydrates metabolism pathways in pregnant women with gestational diabetes
T.M. Linares-Pineda1,2, N. Peña-Montero1, A. Fernández-Valero1, M. Suarez3,2, F. Lima1, M. Molina-Vega1, M.J. Picón1, S. Morcillo1;
1Endocrinology, Hospital Universitario Virgen de la Victoria de Málaga, 2IBIMA, 3Endocrinology, Hospital Universitario Regional de Málaga, Málaga, Spain.
Background and aims: Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance with onset during pregnancy. These women and their offspring have higher risk of develop metabolic disorders. Factor such as nutrition or the intrauterine environment may play an important role, through epigenetic mechanisms, in the development of GDM as well as in the treatment selection. The aim of this work is to identify epigenetics marks involved in mechanism or pathways related to gestational diabetes outcome.
Materials and methods: Gestational diabetes mellitus was diagnosed by a two-step approach: O`Sullivan test at 24-28 weeks, and those pregnant women with a positive test were carried out an OGTT-100 g between 24-30 weeks. A total of 32 pregnant women were selected, 16 of them with GDM and the other 16 non-GDM from EPI-DG cohort (Málaga, Spain). DNA methylation pattern were obtained from Illumina Methylation EPIC BeadChip, with samples from diagnostic visit. Raw data were analysed with ChAMP Package 2.9.10 in R. Also, the statistic and differentially methylation positions (DMPs) was obtained with limma package in R, using False Discovery Rate (FDR) <0.05 and deltaBeta>|5|%. A functional enrichment using STRING, DAVID (2021 Update) and Cytoscape was performed with those DMPs with annotated genes. Finally, a correlation analysis was made with those DMPs related to insulin and glucose metabolism (p<0.05).
Results: Epigenetic profile of pregnant women with GDM was compared with non-GDM group at diagnosis visit. The data were adjusted by age, previous BMI, sex of the foetus, gestational age, HOMAIR and pharmacological treatment. We obtained 1.141 DMPs. A total of 751 DMPs annotated to genes were included in a functional enrichment analysis. Results from STRING were clustered in Cytoscape and enrichment in DAVID. A total of 4 cluster were related to carbohydrate metabolism pathways. Among these cluster, we found 7 genes directly related to signalling insulin pathways, Type II diabetes mellitus, Type I diabetes mellitus, cardiopathy pathways, insulin resistance and carbohydrates absorption (GAD1, GAD2, AKT2, SOS2, CACNAID, mTOR and PRKAA2). GAD1 and GAD2 are related to Type 1 diabetes. SOS2, PRKAA2 and AKT2 are implicated in insulin signalling pathways, also AKT2 is related to diabetic cardiopathy, carbohydrates absorption and other inflammation mechanism. CACNAID has a role in the T2DM development. And finally, mTOR is implicated in many pathways related to inflammation, and insulin resistance. 4 CPGs from these 7 genes were significantly correlated with biochemical variables (Figure 1).
Conclusion: In conclusion, we observed a differential epigenetic profile in genes related to carbohydrate metabolism that could be involved in the development of gestational diabetes.
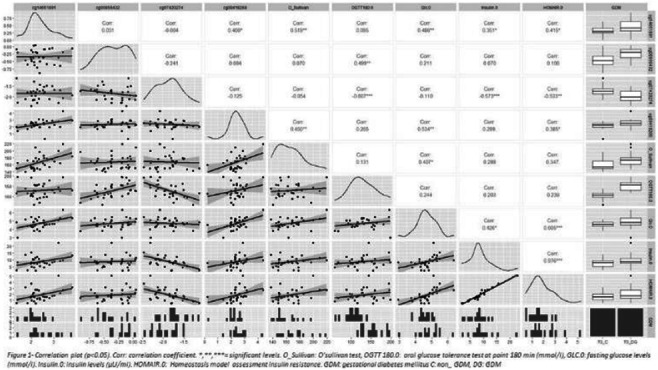
Supported by: ISCIII
Disclosure: T.M. Linares-Pineda: None.
278
A genotype-informed gestational diabetes prediction algorithm highlights importance of unified diagnostic criteria
L. Mendizabal1, M. Arregui1, J. Valerio2, A. Ramos2, N. De la Torre2, A. Barabash2, I. Urrutia3, A. Cortazar3, S. Gaztambide3, L. Castaño3, A. Martinez-Martinez4, E. Camarillo4, L. Simon1, M. Zulueta1, A. Calle-Pascual2;
1Patia, San Sebastián, Spain, 2Hospital Clínico Universitario San Carlos, Madrid, Spain, 3Cruces University Hospital, Bizkaia, Spain, 4Universidad Autonoma de Mexico, Ciudad de Mexico, Mexico.
Background and aims: Early risk detection might offer opportunities to improve care for women at high risk of developing GDM. Predictive models that include genetic susceptibility factors are becoming attractive, and the genetic architecture of GDM predisposition is progressively being considered in the development of predictive algorithms. However, to expand their clinical use, the importance of unified diagnostic criteria should be emphasized. Our aim was to develop a risk assessment algorithm that integrates genetic and clinical variables and test it in women diagnosed with GDM according to different criteria.
Materials and methods: We analyzed a cohort of 711 women from Hospital Clínico San Carlos (HCSC, Madrid, Spain), with 425 control pregnancies and 286 GDM cases diagnosed per IADPSG criteria. The HCSC cohort was randomly divided into a training/development dataset (70% of cohort) for algorithm development and a test dataset (30% of cohort) for validation. We tested the model in a cohort of 157 women (89 controls, 68 cases diagnosed per NDDG criteria from Hospital Cruces (Bilbao, Spain) and in a cohort of 416 women (346 controls, 70 cases per IADPSG criteria from HMPMPS, México). A total of 114 single nucleotide polymorphisms (SNPs) were selected for this analysis after exhaustive exploration of the databases published to date of SNPs associated with GDM. Discrimination and calibration of risk scores were assessed using the receiver operating characteristic (ROC) curve in the internal and the external validation groups.
Results: The algorithm provided a risk score for GDM integrating 10 genetic variants, maternal age, and pre-gestational BMI. In the training dataset the AUC was 0.74, sensitivity of 77% and specificity of 64%. AUCs in the HCSC, HMPMPS and Cruces validation sets were 0.71, 0.70 and 0.62 respectively. The model performance was moderate/high in the cohorts in which GDM had been diagnosed according to the IADPSG criteria; in contrast, the robustness of the model was significantly reduced in the cohort in which the NDDG criteria had been used.
Conclusion: A new algorithm for GDM risk assessment was developed, indicating that the use of genetic markers in combination with clinical characteristics may improve early GDM risk evaluation. Our study highlights the importance of applying consensus criteria for the diagnosis of GDM.
Disclosure: L. Mendizabal: None.
SO 03 Food, drinks and spices
279
The association between pre-Ramadan diabetes education and metabolic parameters in Muslims with type 2 diabetes: a retrospective study
P.J.M. Elders1, A.A.A. van der Heijden1, M. Hassanein2, S. Bouchareb1;
1Amsterdam UMC, location VU, Amsterdam, Netherlands, 2Endocrine department, Dubai Hospital, Dubai, United Arab Emirates.
Background and aims: Ramadan fasting might improve metabolic parameters, such as HbA1c, blood pressure and weight in people with type 2 diabetes (T2D). But, Ramadan fasting is also associated with an increased risk of acute complications such as hypo- and hyperglycaemie. Pre-Ramadan Education (PRE) has been shown to reduce the risk of hypoglycaemia and prevent weight gain. We aimed to: (1) compare metabolic parameters during Ramadan with periods outside Ramadan in Muslims with T2D and (2) assess the association between PRE and metabolic parameters in patients who had received PRE.
Materials and methods: For aim 1 we performed a retrospecitive longitudinal observational study of routine care GP data in Amsterdam in practises in which religious background was routinely noted in the medical file. Inclusion criteria were : age ≥ 18 years, a T2DM diagnosis and the indication that the patient was a muslim. Routine data of patients over a periode of five years were extracted. Generalized Estimating Equations (GEE) models were used to analyse the difference of the outcome parameters measured during the period of Ramandan and the three months thereafter (Ramadan period) and the remaing 8 months of the year (non Ramadan period). For aim 2 we included people with T2D who received PRE and gave informed consent for this study. From these patients the same routine care GP data were extracted as described for aim 1. Using GEE we examined the association between PRE and metabolic parameters during the Ramadan periods before and after PRE. The GEE analyses were adjusted for age, time since baseline and diabetes duration.
Results: The characteristics of the participants is shown in table 1. Aim 1: During Ramadan significant lower systolic blood pressure (-1.7 mmHg; p= 0.000), weight (-0.32 kg/m; p= 0.000) and BMI (-0.23 kg/m2; p= 0.000) were observed compared to outside Ramadan. Fasting glucose increased with 0.19 mmol/L (p= 0.003) during Ramadan. Hba1c (-0.61 mmol/mol; p.08), LDL-C 9 (-0.03 mmol/l ;p=0.53) and weight (-0.55 kg; p=0.051) were not statistically significant lower during Ramadan. Aim 2: PRE was only associated with a significant decrease in weight (-0.4 kg/m2 ; p= 0.048) compared to previous Ramadans.
Conclusion: Our study showed that Ramadan fasting was not associated with major metabolic dysregulation. Fasting glucose level during Ramadan is probably explained by the late eating that occurs during this period. Ramadan Education seems to have positive effects and might provide an opportunity to empower Mulims with T2D to improve their diabetes selfmanagement
Clinical Trial Registration Number: NTR7690
Supported by: This study was supported by a grant of ZonMW
Disclosure: P.J.M. Elders: Grants; ZONmw Goed Gebruik Geneesmiddelenn.
280
A beneficial effect of cinnamon and red capsicum intake on postprandial changes in plasma metabolites evoked by a high-carbohydrate meal in men with overweight/obesity
M. Ciborowski1, A. Hameed1, E. Adamska-Patruno1, J. Godzien1, P. Czajkowski1, U. Krasowska1, K. Pietrowska1, J. Fiedorczuk1, K. Miniewska1, M. Moroz1, W. Bauer1, J. Sieminska1, M. Gorska2, A. Kretowski2;
1Clinical Research Centre, Medical University of Bialystok, 2Department of Endocrinology, Diabetology and Internal Medicine, Medical University of Bialystok, Bialystok, Poland.
Background and aims: It has been reported that a high-carbohydrate (HC) intake might be associated with an increased risk of type 2 diabetes (T2DM) development, especially among obese people. A diet rich in highly processed carbohydrates exerts unfavourable effects on lipid profile, which may have implications for metabolic syndrome and T2DM development. It has also been reported that consumption of cinnamon and capsicum can prevent or slow the development of T2DM by improving glycemic control, hyperlipidemia, and insulin resistance. In the present study postprandial metabolomics was performed to indicate differences in plasma metabolites affected by the HC or normo-carbohydrate meal between humans with normal weight (NW) or overweight/obesity (OW/OB). Moreover, we have also evaluated if the change in the level of plasma metabolites evoked by HC meal in OW/OB individuals can be lifted by the cinnamon and capsicum intake.
Materials and methods: The first part of the study was performed on a group of 24 male participants (12 with NW: BMI 24±2, 35±9 y.o. and 12 with OW/OB: BMI 31±5, 38±6 y.o.). The volunteers participated in two meal-challenge-tests visits within 2-3 weeks intervals. After fasting blood collection, subjects received a standardized HC-meal or NC-meal. During the meal, test blood was collected at 30, 60, 120, and 180 minutes after the meal. In the second study, 20 male OW/OB individuals (BMI 32±4, 46±8 y.o.) were enrolled on a double-blind, placebo-controlled trial consisting of two visits during which they received an HC meal with the capsule containing 2 g of cinnamon and 200 mg of capsicum or placebo capsule. Blood samples were collected as previously. LC-MS-based untargeted metabolomics was performed on collected plasma samples. Based on the relation between time points and metabolite intensity for each metabolite AUC was calculated. Obtained AUCs were forwarded for partial least square discriminant analysis (PLS-DA) modelling. Based on the PLS-DA model volcano plots were built to select significant metabolites according to multivariate statistics (variable importance in projection value, VIP).
Results: Significant difference in metabolites’ AUC between NW and OW/OB individuals were mainly observed after the HC-meal. Among other metabolites, a substantial increase of AUC was mainly observed for lysophospholipids (+35-274%, VIP=1.06-1.88), fatty acids (+54-129%, VIP=1.21-1.77), fatty acid amides (+69-682%, VIP=1.68-3.61), sphingomyelin (SM 33:2) (+37%, VIP=1.1) or uric acid (+145%, VIP=1.4). A decrease in AUC was observed for sphingosine 16:0 (-40%, VIP=1.34), sphinganine 16:0 (-60%, VIP=1.52), and lauroyldiethanolamide (-71%, VIP=2.44). The intake of cinnamon and capsicum significantly reduced the AUC of lysophospholipids (-9-34%, VIP=1.12-1.99), uric acid (-100%, VIP=1.18) and SM 33:2 (-54%, VIP=1.3).
Conclusion: Several metabolic pathways were found dysregulated after an HC meal in people from the OW/OB group but not the NW group. An HC meal may induce an unfavourable postprandial metabolic response in OW/OB individuals, but it can be partially lifted by the cinnamon and capsicum intake.
Clinical Trial Registration Number: NCT03792685
Supported by: EU Horizon 2020 MSC Action Grant No 754432
Disclosure: M. Ciborowski: None.
281
Tea consumption and the risk of type 2 diabetes: a cohort study and updated systematic review and dose-response meta-analysis
X. Li1, J. Zeng2, B. Chen2, Q. Yan2, Y. Cui2, W. Xu2, X. Zhang3, S. Xu4,2;
1College of Medicine, Wuhan University of Science and Technology, Wuhan, 2Evidence-Based Medicine Centre, Office of Academic Research, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, 3Department of Nephrology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, 4Department of Endocrinology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China.
Background and aims: The relationship between tea-drinking and the risk of type 2 diabetes mellitus (T2DM) has been of long-term interest, but the results of published cohort studies and meta-analyses were inconsistent. The aim of this study was to define the relationship between tea consumption and future T2DM risk by a cohort study and an updated dose-response meta-analysis.
Materials and methods: The cohort study was based on the China Health and Nutrition Survey (CHNS). A total of 5199 adult participants without diabetes were involved in 1997 and followed up in 2009. Tea consumption data was collected using structured questionnaires, and T2DM was diagnosed according to the American Diabetes Association’s criteria. Cox proportional hazard regression models were carried out with adjustment for characteristics, diet, and lifestyle factors. Stratification analyses (e.g. according to age or sex) and sensitivity analyses (e.g. by excluding participants who developed diabetes during the first 3 years of follow-up) were conducted. A systematic literature search was performed in PubMed, Web of Science and EMBASE through September 2021. A total of 19 cohort studies (1,076,311 participants) were included in the updated dose-response meta-analysis regarding tea drinking and the risk of T2DM. In addition, we conducted subgroup analyses according to tea-drinking frequency (< 1 cup/d, 1-3 cups/d, and ≥ 4 cups/d), sex (male and female), type of tea (green tea, Oolong tea, and black tea), and the location of the study (Europe and America, or Asia).
Results: In cohort study, the incidence of T2DM was 10.04%, and 45.76% participants were tea-drinkers. Cox proportional hazard regression showed the hazard ratio (HR) of T2DM in tea-drinkers was 1.02 (95% confidence interval [CI]: 0.82-1.28) compared with no-tea-drinkers. In the stratification analyses and sensitivity analyses, the results did not change significantly. However, the dose-response meta-analysis showed that there was a significant linear association between tea consumption and T2DM risk (P for linear trend: 0.003), and the RR (95% CI) for an increment of 1 cup of tea per day was 0.986 (0.977-0.995), shown in Figure 1. Compared with the 0 cup/d tea-drinking group, the risks of diabetes in the < 1 cup/d, 1-3 cups/d tea-drinking, and ≥ 4 cups/d tea-drinking groups were 1.00 (95% CI: 0.98-1.02), 0.96 (95% CI: 0.91-1.00), and 0.83 (95% CI: 0.76-0.90), respectively. Tea-drinking was found not to be associated with the development of diabetes in sex, location, or type of tea subgroups.
Conclusion: Drinking tea was beneficial in reducing the risk of T2DM, but only at high doses (e.g. ≥ 4 cups/d). Tea is thought to be a component of a good diet, and daily adequate tea consumption seems to have clinically significant effect to reduce the risk of T2DM developing.
Supported by: the Young Talents Project of Hubei Provincial Health Commission, China
Disclosure: X. Li: None.
282
Habitual intake of dietary dicarbonyls is associated with greater insulin sensitivity and lower prevalence of type 2 diabetes: the Maastricht Study
K. Maasen1, S.J.P. Eussen2, P.C. Dagnelie2, A. Opperhuizen3,4, C.D.A. Stehouwer1, M.M.J. van Greevenbroek1, C.G. Schalkwijk1;
1CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, 2CAPHRI Care and Public Health Research Institute/CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, 3NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, 4Netherlands Food and Consumer Product Safety Authority, Utrecht, Netherlands.
Background and aims: Dicarbonyls are highly reactive compounds and major precursors of advanced glycation endproducts (AGEs). Dicarbonyls are formed endogenously but also during food processing. Circulating dicarbonyls are positively associated with insulin resistance and type 2 diabetes, but consequences of dietary dicarbonyls are unknown. Therefore, this study examined the associations of dietary intake of dicarbonyls with insulin sensitivity, β-cell function, and prevalence of prediabetes or type 2 diabetes.
Materials and methods: In 6282 participants (60 ± 9 yrs, 50% men, 23% type 2 diabetes [oversampled]) of the population-based cohort The Maastricht Study, we estimated habitual intake of the dicarbonyls methylglyoxal (MGO), glyoxal (GO), and 3-deoxyglucosone (3-DG). Food Frequency Questionnaires were linked to our food composition database, including MGO, GO, and 3-DG concentrations in >200 foods and drinks measured via UPLC-MS/MS. Insulin sensitivity, β-cell function, and glucose metabolism status were measured by a seven-point oral glucose tolerance test (available for n=2390, 2336, and 6282 respectively). Insulin sensitivity was assessed as the Matsuda index. β-cell function was assessed as C-peptidogenic index, overall insulin secretion, glucose sensitivity, potentiation factor, and rate sensitivity. Additionally, insulin sensitivity was measured as HOMA2-IR (n=2611). Cross-sectional associations of dietary dicarbonyls with insulin sensitivity, β-cell function, and glucose metabolism status were investigated using linear or logistic regression adjusting for age, sex, BMI, smoking, alcohol intake, physical activity, total energy intake, educational level, history of cardiovascular diseases, triglycerides, LDL, total cholesterol/HDL ratio, use of antihypertensive- or lipid-modifying medication and insulin sensitivity (models with β-cell function as outcome only).
Results: Higher dietary MGO and 3-DG intakes were associated with greater insulin sensitivity after full adjustment, indicated by both a higher Matsuda index (MGO: Std. β [95% CI] = 0.08 [0.04, 0.12] and 3-DG: 0.09 [0.05, 0.13]) and a lower HOMA2-IR (MGO: Std. β = -0.05 [-0.09, -0.01] and 3-DG: -0.04 [-0.08, -0.01]). Moreover, higher MGO and 3-DG intakes were associated with lower prevalence of type 2 diabetes, after excluding individuals with previously diagnosed diabetes (OR [95% CI] = 0.78 [0.65, 0.93] and 0.81 [0.66, 0.99]). There were no consistent associations of MGO, GO, and 3-DG intakes with β-cell function.
Conclusion: Higher habitual consumption of the dicarbonyls MGO and 3-DG was associated with better insulin sensitivity and with lower prevalence of type 2 diabetes, after excluding individuals with known diabetes. This suggests that food-derived dicarbonyls may play a protective role in type 2 diabetes. These novel observations warrant further exploration in prospective cohorts and intervention studies.
Supported by: NVWA
Disclosure: K. Maasen: Grants; The Netherlands Food and Consumer Product Safety Authority.
283
Circulating short chain fatty acids as potential new biomarkers for the prediction of type 2 diabetes risk
G. Llaurado1,2, A. Junza3,2, E. Rubinat4,2, O. Yanes3,2, X. Correig5,2, D. Mauricio6,2, J. Vendrell7,2, S. Fernández-Veledo7,2;
1Department of Endocrinology and Nutrition, Hospital del Mar, Institut Hospital del Mar d'Investigacions Mèdiques, Universitat Pompeu Fabra, Barcelona, 2Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), ISCIII, Madrid, 3Department of Electronic Engineering, Institut d'Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Tarragona, 4Faculty of Nursing and Physiotherapy, University of Lleida, Lleida, 5Metabolomics Platform, Institut d'Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Reus, 6Department of Endocrinology, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma Barcelona, Barcelona, 7Department of Endocrinology, Hospital Universitari Joan XXIII, Institut d'Investigació Sanitària Pere Virgili, Rovira i Virgili University, Tarragona, Spain.
Background and aims: Common diabetes risk factors offer limited ability to identify subjects at risk of developing type 2 diabetes (T2D). It becomes essential to find novel reliable biomarkers to improve the identification of subjects at risk. The aim of the study was to evaluate the role of circulating short chain fatty acids (SCFA) as potential novel biomarkers to predict T2D risk.
Materials and methods: The Di@bet.es study is a prospective population-based study from a random cluster sampling in the Spanish population over 18 years (n=5,072). Of these, 4,347 were free of T2D in the cross-sectional study. Follow-up losses were about 45%. The final sample for re-screening finally comprised 2,408 subjects. A metabolomics-driven analyses of SCFA (acetate, propionate, butyrate and isobutyrate) was carried out using gas chromatography-mass spectrometry in samples obtained from the Di@bet.es cohort to identify subjects who developed T2D after 7 years (median) of follow-up.
Results: At baseline, the circulating concentrations of propionate (3.4 [2.2-4.1] mcmol/L vs. 3.6 [1.0-4.3] mcmol/L vs. 3.6 [2.4-4.5] mcmol/L; p<0.001) and isobutyrate (0.90 [0.59-1.09] mcmol/L vs. 0.93 [0.64-1.17] mcmol/L vs. 0.94 [0.59-1.19] mcmol/L; p=0.001) were increased in parallel with the carbohydrate metabolism status (normal vs. prediabetes vs. T2D). Subjects who developed T2D (incident cases) after a median follow-up of 7 years had higher baseline circulating concentrations of butyrate (11.4 [1.6-14.1] mcmol/L vs. 12.3 [6.9-15.2] mcmol/L; p=0.040) and isobutyrate (0.90 [0.58-1.09] mcmol/L vs. 0.96 [0.72-1.23] mcmol/L, p=0.004). In the multivariate analysis, baseline circulating concentrations of butyrate (OR 1.03 [95%CI: 1.01-1.07]; p=0.037) and isobutyrate (OR 1.9 [95%CI: 1.2-3.1]; p=0.009) were independently associated with development of T2D after adjusting for age, sex, family history of T2D, prediabetes, obesity, hypertension and dyslipidemia. The C-statistics for predicting T2D was 0.777 (95%CI: 0.757-0.797) and 0.774 (95%CI: 0.754-0.794) for butyrate and isobutyrate respectively, offering a good ability to predict the development of T2D, although similar to the reference model based on traditional clinical factors (0.781 [95%CI: 0.762-0.800]).
Conclusion: Circulating concentrations of SCFA increase in parallel with alterations in carbohydrate metabolism and are independently associated with the development of T2D at 7 years of follow-up. However, they offer limited ability to improve risk prediction compared to traditional risk factors.
Supported by: PI20/00312 - ISCIII
Disclosure: G. Llaurado: None.
284
Interaction between dairy product intake or phospholipid odd chain fatty acids and GAD65 autoantibodies on the incidence of adult onset diabetes
A.-M. Lampousi, S. Carlsson, J.E. Löfvenborg, on behalf of the EPIC-InterAct consortium;
Karolinska Institutet, Stockholm, Sweden.
Background and aims: Islet autoimmunity is associated with an increased risk of adult-onset diabetes. We investigated whether high dairy product intake or plasma phospholipid odd chain fatty acids may modify the excess risk conferred by antibodies against the 65 kDa isoform of GAD (GAD65).
Materials and methods: We analysed data from the EPIC-InterAct case-cohort study including 11,124 incident cases of adult-onset diabetes and a subcohort of 14,866 individuals collected across eight European countries. Adjusted Prentice-weighted Cox regression models were used to estimate the hazard ratios (HRs) of adult-onset diabetes comparing tertiles of dairy product intake (total dairy, milk, fermented dairy) or plasma phospholipid fatty acids 15:0 and 17:0, stratified by GAD65 autoantibody status assessed in plasma samples at baseline. We evaluated additive interactions using the proportion attributable to interaction (AP), as well as interactions on the multiplicative scale, between GAD65 antibody positivity (≥65 units/mL) or high positivity (≥167.5 units/mL) and low fatty acids or dairy intake.
Results: Overall, 3.5% of incident diabetes cases and 1.9% of non-cases were GAD65 antibody positive at baseline. High concentrations of the fatty acids, in particular 17:0, were inversely associated with adult-onset diabetes in both antibody negative (HR: 0.38, 95% confidence intervals (CI) 0.35-0.42) and positive individuals (HR: 0.42, 95% CI 0.27-0.64). The combination of low 17:0 fatty acid levels and high GAD65 antibody positivity compared to antibody negativity and high or moderate 17:0 fatty acid levels was associated with a HR of 6.08 (95% CI 3.92-9.43) with indication of additive (AP: 0.25, 95% CI: 0.08-0.43) but not multiplicative (p=0.532) interaction. There were no clear associations between total dairy or milk intake and adult-onset diabetes. Fermented dairy was inversely associated with diabetes but only in antibody negative individuals (HR: 0.86, 95% CI 0.79-0.94).
Conclusion: Higher levels of plasma phospholipid 17:0 fatty acid may partly impede the progression from GAD65 antibody positivity to adult-onset diabetes.
Supported by: FORTE, VR and Novo N supported AML, SC and JEL
Disclosure: A. Lampousi: Grants; Swedish research council, Swedish reasearch council for health working life and welfare, Novo nordisk foundation.
285
GABA and sitagliptin prevent impairment of beta cell function caused by high-fat diet
Z. Wang1, L. Fan1, Y. Ni1, A. Ma1, D. Wu1, Y. Zhao1, J. Li1, Q. Cui1, Y. Zhou1, L. Zhang1, Y.-R. Lou2, G. Prud’homme3, Q. Wang1;
1Department of Endocrinology and Metabolism, Huashan Hospital, Fudan University, Shang Hai, China, 2Department of Clinical Pharmacy and Drug Administration, School of Pharmacy, Fudan University, Shang Hai, China, 3Keenan Research Center for Biomedical Science, Division of Endocrinology and Metabolism, Unity Health Toronto (St. Michael’s site), Toronto, Canada.
Background and aims: Excessive loss of islet beta cells is a major cause for the development of type 2 diabetes (T2D), and seeking an effective method to enhance beta-cell proliferation is a promising therapeutic strategy for T2D. In this study, we examined whether combined therapy of GABA and sitagliptin is effective in promoting beta-cell proliferation and ameliorating the impairment of beta-cell function caused by high-fat diet (HFD) feeding in mice.
Materials and methods: Male C57BL/6J mice were fed with normal chow diet, HFD, or HFD combined with GABA, sitagliptin, or both drugs.Daily oral drug administration was initiated one week before HFD and maintained for two weeks.
Results: After two weeks of intervention, we found that GABA or sitagliptin administration ameliorated the impairment of glucose tolerance induced by HFD. This was associated with improved insulin secretion in vivo. Notably, combined administration of GABA and sitagliptin significantly enhanced these effects as compared to each of the monotherapies. Combined GABA and sitagliptin was superior at increasing beta-cell mass and associated Ki67+ and PDX-1+ beta-cell counts. In addition, we found that HFD-induced compensatory beta-cell proliferation was associated with increased activation of unfolded protein response (UPR), as indicated by BiP expression.
Conclusion: Increased activation of unfolded protein response (UPR) could be an important mechanism of compensatory beta-cell proliferation, and beta cells treated with GABA and sitagliptin showed greater UPR activation. Our results suggest that the combined use of these agents produces superior therapeutic outcomes.
Supported by: the National Natural Science Foundation of China (No.81630020, 81570518, and 81800751)
Disclosure: Z. Wang: None.
SO 04 Starting with autoimmunity
286
Childhood screening and interception strategies: an economic cohort simulation model
J.L. Dunne1, L. Ferrat2, R.B. McQueen3, R. Neusner1, R.A. Oram2, M. Rewers4, M.R. Trusheim5;
1Janssen Research and Development, LLC, Raritan, USA, 2Institute of Biomedical and Clinical Science, Exeter, UK, 3Department of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, USA, 4Barbara Davis Center for Diabetes, Aurora, USA, 5Co-Bio Consulting LLC, Acton, USA.
Background and aims: Type 1 diabetes (T1D) screening and monitoring studies suggest opportunities for scaling up to public health population-based programs. Genetic Risk Scores (GRS) for T1D are also demonstrating the ability to identify those at higher risk of progressing to T1D as well as an increased opportunity for implementation into public health settings potential as a result of practical considerations, including falling gene sequencing costs. Previous studies have shown that screening and monitoring children for T1D progression significantly decreases diabetic ketoacidosis (DKA) events at diagnosis, which has been shown to have both short- and long-term benefits. Furthermore, identification of those at higher risk of T1D provides an opportunity to intervene and delay T1D onset.
Materials and methods: A non-stochastic, state transition cohort simulation projected 2019 US births through adolescence. Data from previously collected natural history studies, including TEDDY, DIPP & DAISY were used to model the patient journey. Children seroconverting to islet autoantibodies (IAB) each year and children with lower T1D risk were simulated as separate cohorts with distinct progressions. Interception strategies were layered over this natural history projection. Success of newborn GRS screening followed by annual IAB and glucose monitoring to intercept T1D progression and reduce DKA events was explored. An IAB screening strategy at ages 2 and 5 was also simulated. Therapeutic intervention to delay T1D onset was also simulated.
Results: Real-world benchmarks for newborn screening (85%) and patient follow-up (80%) as well as rapid T1D progression (<12 months) for some children limited maximum interception at dysglycemia to 44% of children progressing to T1D by age 15 using a GRS screening strategy in the DAISY-driven (US) population progression. The IAB screening strategy simulation, assuming that 60% of the children in the US will receive IAB screening, resulted in a 32% dysglycemia interception rate. A DIPP-driven (Finnish) population progression with younger skewed seroconversion results in a higher 48% dysglycemia interception rate for the newborn GRS screening strategy and a 43% dysglycemia interception rate for the IAB screening strategy.
Conclusion: Short-term net incremental costs in the simulated US population reach $636M at current test costs or $158K per T1D case interception. Breakeven costs could be achieved with $10 IAB test cost and $5 GRS costs with a therapeutic to delay T1D onset by 3 years. Alternatively, breakeven costs could be achieved by extending the T1D onset delay to 5 years with $25 IAB test costs and $5 GRS costs. Including potential long-term benefits of early intervention and improved glycemic control would further improve the breakeven levels.
Disclosure: J.L. Dunne: Employment/Consultancy; Janssen Research and Development, LLC. Stock/Shareholding; Johnson & Johnson.
287
Family history of type 1 diabetes is associated with incidence of multiple islet autoimmunity but not with progression to clinical type 1 diabetes: the MIDIA study
L. Stene;
Norwegian Inst. of Public Health, Oslo, Norway.
Background and aims: Type 1 diabetes (T1D) is characterized by a preclinical period of islet autoimmunity (IA) of varying length from months to over 10 years. Genetic susceptibility of T1D is mainly determined by HLA-DQ genotype with highest risk in those with the HLA DQ8/2 genotype. HLA mainly influence islet autoimmunity (IA) and to a lesser degree progression to clinical disease. We aimed to investigate whether family history of T1D was differentially associated with incidence IA and progression to T1D in children with the HLA DQ8/2 genotype
Materials and methods: The MIDIA study HLA tested over 48000 newborns throughout Norway during 2001-2007 and followed 908 children with the HLA DQ8/2 genotype. Blood samples were scheduled at ages 3, 6, 9 and 12 months and annually thereafter (more frequent if positive for autoantibodies). Islet autoantibodies (anti-insulin, GAD65 and IA-2) were measured in all blood samples using radiobinding assays. IA was defined as repeated positivity for at least two of three autoantibodies. Date of diagnosis of T1D by the 31 Dec, 2016 was obtained from the Norwegian Childhood Diabetes Registry. T1D a first degree relative (FDR) was ascertained by a parental questionnaire when the child was 3 months old. We estimated risk (of IA or T1D) by family history, as 1 minus the Kaplan-Meier survival estimate.
Results: The cumulative incidence of IA was much higher in those with an affected FDR (n=65) vs. those without (n=843), approximately 30% and 6% at age 10 years, respectively (Figure a). The probability of progressing from IA to clinical T1D was very similar in both groups, at approximately 60% ten years after seroconversion (Figure b).
Conclusion: Family history, probably mediated by genetics other than HLA DQ, primarily influence the incidence of islet autoimmunity and not the progression from islet autoimmunity to clinical type 1 diabetes.
Disclosure: L. Stene: None.
288
The incidence of childhood-onset type 1 diabetes, time trends and association to population composition in Sweden: a 40-year follow-up
I. Waernbaum1, T. Lind2, A. Möllsten2, G. Dahlquist2;
1Department of Statistics, Uppsala University, Uppsala, 2Department of Clinical Sciences, Pediatrics, Umeå University, Umeå, Sweden.
Background and aims: During the 1980s and 1990s, the incidence of childhood-onset type 1 diabetes more than doubled in Sweden, followed by a plateau. In the present 40-year follow-up, we investigated if the incidence remained stable and whether this could be explained by increased migration from countries reporting lower incidences.
Materials and methods: We used 23 143 incident cases reported 1978-2019 to the nationwide, population-based, Swedish Childhood Diabetes Register and population data from Statistics Sweden. Generalized additive models and ANOVA were applied to analyze effects of onset-age, sex, time-trends, parental country of birth and interaction effects between these factors.
Results: The flattening of the incidence increase seems to remain over the period 2000-2019. When comparing the incidence for all children in Sweden with that for children with both parents born in Sweden, trends were parallel but at higher level for the latter. Comparing incidence trends between individuals with Swedish (high diabetes trait) and Asian backgrounds (low diabetes trait) showed the Asian subpopulation having a stable increase in incidence over time.
Conclusion: In Sweden, the increase in incidence seen in the late 20th century has stabilized at a high level over the last two decades. Increased immigration from countries with lower incidence does not explain the observed levelling-off.
Supported by: This study was supported by the Swedish Research Council grant number 2018-02565. I.W. was supported
Disclosure: I. Waernbaum: Grants; Swedish Research Council grant number 2018-02565, Swedish Research Council grant number 2016-00703.
289
Prevalence of positivity for diabetes-associated autoantibodies in patients classified and treated as type 2 diabetes and their further characterisation
M. Rončáková1, A. Davani1, V. Mikušová2, P. Novodvorsky3,4, E. Martinka1;
1National Endocrinology and Diabetology Institute, Lubochna, Slovakia, 21st Dept. of Internal Medicine, University Hospital, Martin, Slovakia, 3Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 4Human Oncology and Metabolism, University of Sheffield, Sheffield, UK.
Background and aims: Classification of diabetes is not always straightforward. Patients with insulin resistance and overweight/obesity initially diagnosed with type 2 diabetes (T2D) might exhibit presence of autoimmune insulitis with positivity for diabetes-associated autoantibodies (AA). We sought to establish the prevalence of autoimmune insulitis in patients with poorly controlled T2D referred to a tertiary diabetes centre for further management. We aimed to characterise these patients in detail and compare them to patients without AA positivity in order to establish the characteristics linked with the presence of autoimmune insulitis.
Materials and methods: This was a prospective observational study. All patients with T2D diagnosis admitted to the NEDÚ, Ľubochňa, Slovakia within the first 6 months of 2016 were included in the study. Data on age, sex, anthropometric measures, BP, age at diagnosis and diabetes duration, HbA1c, c-peptide levels, biochemistry, lipid profile, hypoglycaemia, antidiabetic medication, insulin dose and time to insulin initiation, drug history, presence of diabetic complications and other comorbidities were collected. All patients were tested for anti-GAD, islet antigen-2 autoantibody (IA-2A) and insulin autoantibody (IAA). Patients with positivity for at least one AA (≥1 IU/ml) (AA+) were compared with those with negative AA (AA-).
Results: Six hundred and ninety-two individuals (387, 55.6% female) with mean (range) age 61.5 (24-83) years, mean (SD) HbA1c 9.1 (1.8)% and mean (SD) duration of diabetes 14.3 (7.9) years were analysed. One hundred and sixty-four patients (164/692, 23.7%) tested positive for at least 1 AA: 136/692 (19.6%) were positive for anti-GAD, 21/692 (3.0%) were positive for IA-2A, 28/692 (4.0%) were positive for IAA, 16/692 (2.3%) were double-positive for anti-GAD/IA-2A and 5/692 (0.7%) were double-positive for anti-GAD/IAA. Table 1 shows the comparisons between the AA+ and AA- patients in which significant differences were detected. Eighty-nine (89/692, 12.9%) patients met the current diagnostic criteria for latent autoimmune diabetes of the adults (LADA) (>30 years at diagnosis, presence of AA and absence of insulin requirement for ≥6 months after diagnosis).
Conclusion: Nearly 1 in 4 individuals in this group of patients with poorly controlled T2D were shown to have positivity in at least one of the tested diabetes-associated autoantibodies. AA+ patients differed from AA- patients in several characteristics largely confirming previously published data. We conclude that several pathological processes linked with distinct types of diabetes can develop in parallel including insulin resistance and autoimmune insulitis.
Disclosure: M. Rončáková: Lecture/other fees; NovoNordisk, Merck, Sanofi, Eli Lily, Boehringer Ingelheim, Abbot, Novartis, Janssen, Medtronic, Roche, Berlin Chemie Menarini. Other; NovoNordisk, Pfizer, Johnson & Johnson, Merck, Sanofi, Eli Lily, Boehringer Ingelheim, Abbot, Novartis, Janssen, Medtronic, Roche, Berlin Chemie Menarini.
290
Interaction between tobacco use and genetic susceptibility in the risk of LADA and type 2 diabetes
J. Edstorp1, E. Ahlqvist2, L. Alfredsson1,3, V. Grill4, B. Rasouli1, E.P. Sørgjerd4,5, T. Tuomi2,6, B. Åsvold7,5, S. Carlsson1;
1Karolinska Institutet, Stockholm, Sweden, 2Department of Clinical Sciences in Malmö, Clinical Research Centre, Lund University, Malmö, Sweden, 3Center for Occupational and Environmental Medicine, Region Stockholm, Stockholm, Sweden, Stockholm, Sweden, 4Norwegian University of Science and Technology, Trondheim, Norway, 5HUNT Research Centre, Department of Public Health and Nursing, NTNU, Norwegian University of Science and Technology, Trondheim, Norway, 6Institute for Molecular Medicine Finland, Helsinki University, Helsinki, Finland, 7Department of Endocrinology, Clinic of Medicine, St. Olavs Hospital, Trondheim, Norway.
Background and aims: Smoking is associated with type 2 diabetes and latent autoimmune diabetes in adults (LADA). Our aim was to investigate whether interaction with genetic susceptibility to type 2 diabetes, insulin secretion, and insulin resistance aggravates this association.
Materials and methods: We used case-control data with incident cases (LADA n=593, type 2 diabetes n=2038) and controls (n=3036). Weighted genetic risk scores were calculated for type 2 diabetes (T2D-GRS), insulin secretion (IS-GRS), and insulin resistance (IR-GRS). We derived odds ratios (OR) with 95% confidence intervals by conditional logistic regression adjusted for lifestyle factors. Additive interaction was estimated by attributable proportion due to interaction (AP).
Results: Smoking increased the risk of type 2 diabetes and LADA (OR 1.70 [1.37-2.09], 1.52 [1.18-1.96], respectively). In type 2 diabetes, the risk associated with smoking was further increased in individuals with genetic susceptibility to type 2 diabetes (OR 3.25 [2.06−5.12]), IS (OR 3.33 [2.12−5.25]), and IR (OR 2.49 [1.58−3.93]), compared with non-smokers with low-intermediate genetic susceptibility. In LADA, corresponding associations were OR 2.00 [1.16−3.45] for genetic susceptibility to T2D, OR 2.65 [1.62−4.33] to IR, and OR 2.01 [1.15−3.51] to IS. There was additive interaction between smoking and IR-GRS in the risk of type 2 diabetes (AP 0.36 [0.02−0.70]) and LADA (AP 0.59 [0.31−0.86]). We aim to replicate these findings using data from the Norwegian HUNT study.
Conclusion: Our findings suggest that smoking confers a stronger effect on type 2 diabetes and LADA risk in individuals with genetic susceptibility to type 2 diabetes and related traits.
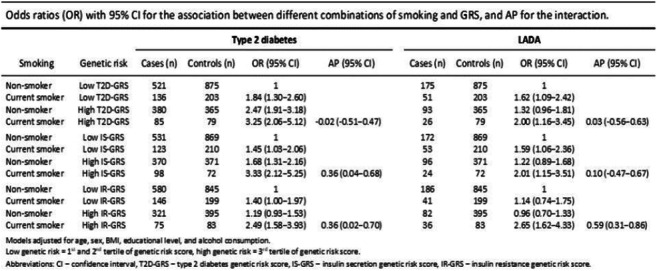
Supported by: Swedish Research Council, FORTE, Novo Nordisk Foundation, Swedish Diabetes Foundation
Disclosure: J. Edstorp: None.
291
Routine islet autoantibody testing in clinically diagnosed adult-onset type 1 diabetes can help identify misclassification and guide successful insulin cessation
R.J. Eason1,2, N.J. Thomas1,2, A.V. Hill2, B.A. Knight1,2, A. Carr1, A.T. Hattersley1,2, T.J. McDonald1,2, B.M. Shields1, A.G. Jones1,2, StartRight Study Group;
1University of Exeter College of Medicine & Health, 2Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.
Background and aims: Recent joint American and European diabetes association guidelines recommend routine islet autoantibody testing in all adults newly diagnosed with type 1 diabetes. We aimed to assess the impact of routine islet autoantibody testing in this population.
Materials and methods: We prospectively assessed the characteristics and progression (annual change in Urine C-peptide Creatinine Ratio (UCPCR)) associated with islet autoantibody status (GAD, IA-2 and ZNT8) in 722 adults (≥ 18 years old) with clinically diagnosed type 1 diabetes and duration <12 months. We also evaluated changes in treatment and glycaemic control over 2 years after informing participants and their clinicians of islet autoantibody results. We then performed a similar analysis in a further group of 91 participants in the same study with clinically suspected type 1 diabetes, defined by insulin from diagnosis, and a clinical diagnosis indicating possible type 1 diabetes (e.g., ‘uncertain type’ ‘possible type 1’). Findings were compared to 731 participants with islet antibody negative type 2 diabetes in the same cohort.
Results: 24.8% (179/722) of participants diagnosed with type 1 diabetes were islet autoantibody negative. This group had genetic and C-peptide characteristics suggestive of a high prevalence of non-autoimmune diabetes: lower mean type 1 diabetes genetic risk score (islet autoantibody negative versus positive: 10.85 vs 13.09 (p<0.001) (type 2 diabetes 10.12)) and a lower annual change in C-peptide (UCPCR) -24% vs -43% (p<0.001) (type 2 diabetes -6%). Both results are presented in Figure 1. After median 24-months follow up, treatment change was seen in 36.6% (60/164) of islet autoantibody negative participants: 22.6% (37/164) discontinued insulin, with a HbA1c similar to those continuing insulin (57.5 vs 60.8mmol/mol [7.4 vs 7.7%] (p=0.4)) and 14.0% (23/164) added adjuvant agents to insulin. In participants with clinically suspected type 1 diabetes, islet autoantibody negativity was associated with the characteristics of T2D: higher baseline C-peptide (islet autoantibody negative versus positive: 1159.4 vs 747.8pmol/L (p<0.05) (type 2 diabetes 1792.3pmol/L)) and lower mean type 1 diabetes genetic risk score (9.98 vs 12.60 (p<0.001)). 52.7% (29/91) of islet autoantibody negative participants in this group successfully discontinued insulin treatment.
Conclusion: In adult-onset clinically diagnosed or suspected type 1 diabetes, negative islet autoantibodies should prompt careful consideration of other diabetes subtypes and, when available to clinicians, are associated with successful insulin cessation. These findings support recent recommendations for routine islet autoantibody assessment in adult-onset type 1 diabetes.
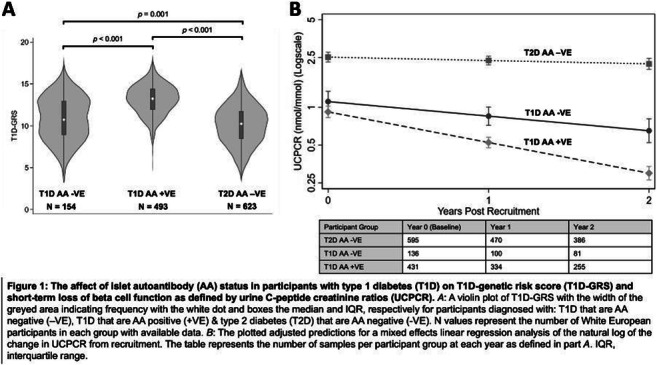
Clinical Trial Registration Number: NCT03737799
Supported by: NIHR and Diabetes UK
Disclosure: R.J. Eason: None.
292
Progression of robustly defined adult onset type 1 diabetes is unaffected by onset age suggesting their inclusion in intervention studies is possible
N. Thomas, A. Hill, R. Bolt, P. Tippett, B. Shields, T. McDonald, A. Jones, StartRight Study group;
Level 3, Exeter University, Exeter, UK.
Background and aims: Type 1 diabetes diagnosed in older adults purportedly progresses more slowly than younger onset disease. This could be explained by difficulties with diabetes classification, with some older adults clinically diagnosed with type 1 diabetes misclassified. Older adults with type 1 diabetes are rarely eligible for intervention studies due to the uncertain progression in this age group. However, recruitment is a key barrier to therapy study completion and nearly half of type 1 diabetes occurs after 30 years. We therefore aimed to determine the impact of age at diagnosis on progression in robustly defined adult onset type 1 diabetes.
Materials and methods: We assessed the relationship between age of onset and C-peptide loss in 1793 participants with newly diagnosed type 1 diabetes in the prospective STARTRIGHT study. Islet autoantibodies (GADA, IA-2A, ZNT8A) and stimulated serum C-peptide were measured in all participants at a mean 5 months duration. A post meal urine sample for C-peptide Creatinine Ratio (UCPCR) measurement was collected annually for two years. Type 1 diabetes was defined in two ways: ≥2 positive islet-autoantibodies (regardless of clinical diagnosis)(n=385) and clinically diagnosed type 1 diabetes with a single positive islet autoantibody(n=180). Mixed-effects models were used to model annual log UCPCR split by median diagnosis age.
Results: Age at diagnosis was not associated with mean starting C-peptide for both type 1 diabetes definitions. When defined by ≥2 islet autoantibodies mean C-peptide was 433pmol/l (95%CI 383-480) and 432pmol/l (361-503) in those diagnosed ≤35 and >35 years respectively, when defined by clinical diagnosis and a single positive antibody 418pmol/l (347-503) vs 425pmol/l (350-515). Age of onset was not associated with annual decline in UCPCR (Figure 1). When T1D was defined by 2 positive islet antibodies annual decline in UCPCR was 42%(34-50%) and 44%(37-51%) in those diagnosed ≤35 and >35 years respectively[p=0.7]. When defined by clinical diagnosis and a single positive islet antibody the decline was 41%(28-52%) vs 43%(34-51%) in those diagnosed ≤35 and >35 years respectively[p=0.8]. Genetic predisposition to type 1 diabetes, captured by a type 1 diabetes genetic risk score, did not differ by definition or age of diagnosis suggesting a high specificity for type 1 diabetes identification throughout (mean risk score ranging from 12.9 to 13.4)[all comparisons p>0.1](type 2 diabetes mean 10.4).
Conclusion: Our results suggest that when defined robustly late onset type 1 diabetes progression is rapid and does not differ from young adult onset disease. This suggests that older adults developing type 1 diabetes could be enrolled in intervention studies as long as initial clinical diagnosis is confirmed by at least one positive islet antibody or in those without an initial clinical diagnosis where two islet antibodies are positive. This would markedly increase the available study recruitment pool.
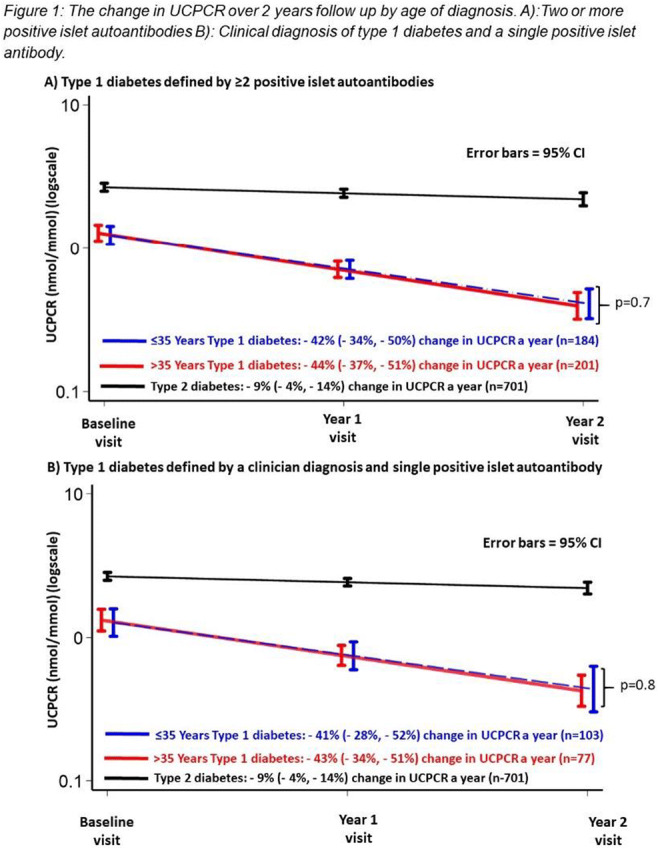
Clinical Trial Registration Number: NCT03737799
Supported by: DUK NIHR
Disclosure: N. Thomas: None.
293
Use of HOMA-IR and Matsuda Index as indicators of insulin resistance in autoantibody positive relatives of patients with type 1 diabetes
A. Petrelli1, A. Bichisecchi2, D. Carnovale1, F. Cugnata2, A. Stabilini1, R. Bonfanti1,3, E. Bosi1, L. Piemonti1, J. Sosenko4;
1Diabetes Research Institute, IRCCS Ospedale San Raffaele, Milan, Italy, 2Vita-Salute San Raffaele University, Milan, Italy, 3Pediatric Department, IRCCS Ospedale San Raffaele, Milan, Italy, 4Diabetes Research Institute, University of Miami, Miami, USA.
Background and aims: HOMA-IR (H) and Matsuda Index (MI) are used to measure insulin resistance (IR) and insulin sensitivity (IS) in certain populations; however, their use in autoantibody positive (Aab+) relatives of patients with type 1 diabetes (T1D) is problematic, since insulin and glucose, which can be abnormal in at-risk individuals, are basic to their calculations. Here we tested whether H or MI had value for measuring IR and IS in a cohort of n=153 relatives of patients with T1D who tested positive for at least 1 Aab.
Materials and methods: At-risk subjects were stratified in 3 groups based on their H and MI levels, and BMI percentile (BMIp) was calculated for each group. H and MI were correlated with BMIp and the Insulinogenic Index (IGI), a measure of insulin deficiency. Linear mixed-effect model (LME) analysis was performed on a total of n=512 observations (including repeated measures of follow-up visits) to test the effect of age, sex, Aab≥2, fasting glucose ≥100 mg/dl, high-risk HLA-DR types, Index60, presence of anti-insulin antibodies and BMIp on H or MI.
Results: There was an inverse curvilinear association (Spearman r=-0.892) between H and MI (Figure), similar to associations described in Ab negative cohorts. BMIp was greater in the highest tertile of H than the lowest (67.8 ± 26.3 vs. 43.5 ± 32.1, p<0.01), with an inverse pattern for MI tertiles (63.6 ± 31.3 vs. 42.2 ± 31.1, p<0.01). In linear regression models H or MI were strongly associated with BMIp (p<0.001 for both) and this association was maintained also when IGI was added to the models (p<0.001 for both). Furthermore, confirming previous observations, linear mixed-effect models analysis showed that individuals with ≥ 2Aab had higher H and lower MI compared to relatives with <2 Aab.
Conclusion: Among Aab+ relatives of patients with T1D, H and MI could be used as relative indicators of IR and IS.
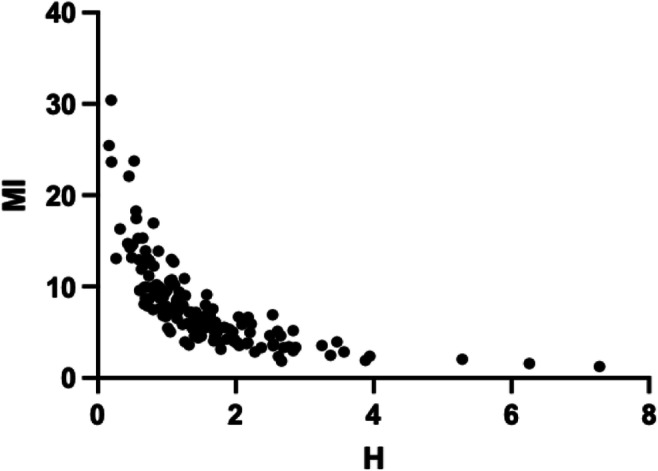
Supported by: JDRF
Disclosure: A. Petrelli: None.
SO 05 Genetics of type 2 diabetes
294
Role of GWAS associated purinergic receptor P2RY1 gene in insulin secretion and the impact of rare variants on type 2 diabetes risk
A. Dance1, M. Derhourhi1, M. Canouil1, M. Marre2, G. Charpentier3, A. Bonnefond1, P. Froguel1,4;
1Inserm UMR1283, CNRS UMR8199, European Genomic Institute for Diabetes (EGID), Institut Pasteur de Lille, Lille University Hospital, Lille, France, 2Inserm U1138, Centre de Recherche des Cordeliers, Paris, France, 3CERITD (Centre d’Étude et de Recherche pour l’Intensification du Traitement du Diabète), Evry, France, 4Department of Metabolism, Imperial College, London, UK.
Background and aims: ATP and ADP play a key role in insulin secretion by activating in β cells the ATP-dependent potassium channels through the purinergic receptors. Genome-wide association studies (GWAS) have shown that the P2RY1 locus is associated with glucose levels variation. P2YR1 belongs to the Gq Protein Coupled Receptor (Gq GPCR) family and is highly expressed in pancreatic islets, particularly in β cells. We performed a large-scale functional genetic study in order to decipher the putative impact of rare P2YR1 variants on the risk of type 2 diabetes (T2D). We also characterized the role of P2RY1 in insulin secretion.
Materials and methods: P2YR1 coding regions were sequenced in 6,348 adults included in the French RaDiO study. To assess the functional activity of each variant, we perform 1/ NFAT-RE (Nuclear factor of activated T-cell Response Element) luciferase assays on HEK293 cells overexpressing each variant, followed by P2YR1 activation by its agonists MRS2365 (methanocarba-2MeSADP) during 16 hours, but also 2/ western blots and 3/ immunofluorescence assays on these cells to assess expression and cellular localization of each mutant. In human pancreatic EndoCβH5 cells, we are also performing glucose-stimulated insulin secretion assays coupled to P2YR1 activation by MRS2365.
Results: We identified 16 rare missense variants in P2RY1 (including 6 novel variants) carried by 19 participants. Most of the carriers presented with T2D (0.46% of carriers among cases with T2D versus 0.17% of carriers among normal glucose controls). In 200k exome data from the UK Biobank we found 10 loss-of-function variants (Lof, nonsense or frameshift). There were three times more P2RY1 LoF carriers with T2D than carriers with normal glucose (assessed by HbA1c), confirming the association with T2D identified in the French cohort. Functional analyses of each variant found in Radio are in progress.
Conclusion: Our association studies suggest a putative role of rare coding variants of P2RY1 in T2D risk. We hypothesize that P2RY1 may play a key role in insulin secretion and the ongoing functional studies will respond to this question. P2RY1 potent and selective agonists that cannot cross the blood-brain barrier are available, and could be tested as a potential new class of insulin secretagogues.
Disclosure: A. Dance: None.
295
Effects of risk gene variants of type 2 diabetes and fatty liver disease on plasma amino acid levels in middle aged women prone to diabetes
A. Nadasdi1, Z. Kukor2, V. Gál3, T. Masszi1, A. Guttman4, A. Patócs5, P. Igaz5,6, A. Somogyi1, G. Firneisz5,1;
1Dept. of Internal Medicine and Haematology, Semmelweis University, Budapest, 2Dept. of Molecular Biology; Institute of Biochemistry and Molecular Biology, Semmelweis University, Budapest, 3Brain Imaging Centre, Research Centre for Natural Sciences, ELKH, Hungarian Academy of Sciences, Budapest, 4Research Institute of Biomolecular and Chemical Engineering, University of Pannonia, Veszprém, 5MTA-SE Molecular Medicine Research Group, ELKH, Hungarian Academy of Sciences, Budapest, 6Dept. of Internal Medicine and Oncology, Semmelweis University, Budapest, Hungary.
Background and aims: Recently circulating amino acids (AAs) were implicated in the regulation of the liver-alpha cell axis contributing to inappropriate glucagon secretion in type 2 diabetes and metabolic (dysfunction) associated fatty liver disease (MAFLD). We studied the plasma levels of AAs and hepatic (HTGC) and pancreatic (PTGC) triacylglycerol contents in a type 2 diabetes prone population stratified by PNPLA3 rs738409 and TCF7L2 rs7903146 genotypes.
Materials and methods: Post hoc analysis of a genotype-based recall study on MAFLD (n=39 young-middle aged women, n=21 with history of gestational diabetes [GDM], median BMI=26.2kg/m2, age=37.0 years, targeted enrolment with rs738409 CC and GG genotypes only) was performed. TCF7L2 rs7903146 genotypes were also obtained from the prior GDM - genetic study. Routine laboratory parameters, HbA1c, glucose, insulin and glucagon values and indices of HOMA2-IR and HOMA2-B were assessed during a 75g OGTT. Protein-depleted blood plasma was lyophilized. Amino acids were conjugated with Pacific Blue dye and concentrations were determined by capillary electrophoresis. HTGC and PTGC values were measured using magnetic resonance (MR) based methods. Internationally proposed MAFLD and standard prediabetes/diabetes diagnostic criteria were used. Between-groups differences and correlations were assessed.
Results: Inverse correlation was found between plasma alanine and PTGC (R=-0.32, p=0.051). Plasma levels of gluconeogenetic precursor AAs (glutamate p=0.059, and aspartate p= 0.056) and glucagon-alanine index were also increased (p= 0.039) in PNPLA3 risk homozygotes (GG). Among ketogenic AAs a significant correlation was found between phenylalanine plasma levels and HTGC in PNPLA3 risk genotype group (R=0.64, p=0.026). Surprisingly phenylalanine was inversely correlated with PTGC in TCF7L2 risk (T) allele carriers (R=-0.57 p=0.013). In contrast the tyrosine levels were inversely correlated with PTGC in TCF7L2 risk allele non-carriers (CC, R=-0.47, p=0.036). Plasma citrulline levels correlated inversely with fasting serum insulin levels (R=-0.41, p=0.01) and consistently also with HOMA2-IR (R=-0.41, p=0.009) and HOMA2-B (only in TCF7L2 rs7903146 CC group: R=-0.47, p=0.037). The ornithine/citrulline ratio as a suggested surrogate marker of mitochondrial dysfunction directly correlated with fasting serum insulin (R=0.63, p=0.005), and consistently with HOMA2-IR (R=0.63, p=0.005), and HOMA2-B (R=0.55, p=0.019) in TCF7L2 risk allele carriers.
Conclusion: Genetic risk factors for both type 2 diabetes and fatty liver disease significantly contribute to the regulation of certain plasma AA levels and potentially also associated with suggested markers of hepatic glucagon effects and mitochondrial dysfunction early in the course of type 2 diabetes development.
Supported by: Woerwag Pharma Doctoral Fellowship Award
Disclosure: A. Nadasdi: Grants; Woerwag Pharma Doctoral Fellowship Award.
296
Kdelr3 mutation is associated with higher risk for diabetes development in NZO mice
D. Altenhofen1,2, J. Khuong1,2, K. Kaiser1,2, T. Kuhn1,2, S. Görigk1,2, S. Lebek1, T. Stermann1, B. Knebel1, A. Schürmann3,2, A. Chadt1,2, H. Al-Hasani1,2;
1Pathobiochemistry, Institute for Clinical Biochemistry and Pathobiochemistry, German Diabetes Center, Leibniz Center for Diabetes Research, Heinrich-Heine-University, Düsseldorf, 2German Center for Diabetes research (DZD), München-Neuherberg, 3German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Potsdam, Germany.
Background and aims: With the aim to discover novel diabetogenic risk genes, we previously identified a quantitative trait locus (QTL) for blood glucose on mouse chromosome 15 (Chr.15) in a crossbreeding of type 2 diabetes (T2D) -prone New Zealand Obese (NZO) and T2D-resistant (C3H) mice, designated “Nbg15” (NZO blood glucose on Chr.15). C3H-allele carriers of the Nbg15 locus had strong protection from T2D development compared to NZO-allele carriers. We therefore investigated underlying genetic variants in this locus for possible diabetes-protective functions.
Materials and methods: Mouse recombinant congenic strains (RCS) were generated carrying the full (consomic, Nbg15conC3H/C3H), proximal (Nbg15proxC3H/C3H) and distal (Nbg15disC3H/C3H) Nbg15 locus from C3H and compared to a control carrying Nbg15-NZO alleles (Nbg15NZO/NZO). All RCS were fed a high-fat diet (45% fat/cal) and monitored for blood glucose and plasma insulin levels. Next, phenotypic data was combined with microarray gene expression analyses and in silico predictions for DNA polymorphisms impact in gene/protein function. Pancreas morphometry was determined using hematoxylin/ eosin staining. Statistical differences were calculated with one-way ANOVA followed by Bonferroni pos-hoc-test or Students ttest and considered as p<0.05.
Results: Nbg15conC3H/C3H and Nbg15disC3H/C3H but not Nbg15proxC3H/C3H mice displayed significantly reduced 6h fasted blood glucose (190.5 ± 23 mg/dL, 173.6 ± 13 mg/dL, 284.9 ± 40 mg/dL vs. 358.3 ± 32 mg/dL; p<0.001) and increased plasma insulin levels when compared to Nbg15NZO/NZO controls (11.4 ± 1.2 μg/L, 15.2 ± 2.3 μg/L, 4.1 ± 0.5 μg/L vs. 5.9 ± 0.8 μg/L; p<0.01). Histological analysis of the pancreata of these mice revealed an increased number of Langerhans islets only in the Nbg15conC3H/C3H and Nbg15disC3H/C3H groups (Con: 25.8 ± 4.5, Dis: 22.3 ± 1.2, Prox: 19.6 ± 0.6 vs. Control: 17.8 ± 2.6, islet size 0-10000 μm²; p<0.01). These results pointed towards a molecular mechanism protecting Nbg15-C3H mice from pancreatic beta cell dysfunction, mainly displayed by genetic factors located in the distal sublocus. Investigation of potentially causal genes for Nbg15dis locus in pancreatic Langerhans islets of the parental mouse strains led to the identification of a potentially disruptive E96V mutation in the ER lumen protein-retaining receptor 3 (Kdelr3) gene from NZO mice (SIFT: 0.08). Of note, Kdelr3 mRNA levels were higher in NZO islets of Langerhans compared to the C3H strain (2(-ΔCt): 1.2 ± 0.08 vs 1.3 ± 0.06). Furthermore, knockdown of Kdelr3 (-74%) in the cultured beta cell line Min6 led to a downregulation of genes involved in processing of insulin granules (Pcsk1: -35%, Pcsk2: -28%, Znt8: -44%).
Conclusion: We mapped susceptibility for diabetes to a region on Chr. 15 in NZO mice. In this locus, a NZO-specific coding mutation in the Kdelr3 gene is associated with hyperglycemia, hypoinsulinemia and reduced islet size. Kockdown experiments in cultured beta cells provide functional evidence that Kdelr3 is involved in regulation of beta cell function.
Supported by: German Center for Diabetes research (DZD)
Disclosure: D. Altenhofen: None.
297
Allelic editing of the PPARGC1A Gly482Ser shows allele-dependent causal effects on cold-induced UCP-1 expression and PGC-1α protein stability in human brown adipocytes
M. Huang, H. Mulder, S. Kalamajski, P. Franks;
Clinical Research Center, Lund University, Malmö, Sweden.
Background and aims: PPARGC1A (PGC-1α) is a master regulator of adaptive thermogenesis in brown adipose tissue. A common polymorphism in PPARGC1A (rs8192678, C/T, Gly482Ser) has become a robust candidate for obesity in the context of gene and environment interaction. Experimental studies are needed to investigate allele-specific effects on human brown adipocytes.
Materials and methods: We used CRISPR-Cas9 to edit the rs8192678 locus to C/C and T/T genotypes in an immortalized human brown adipocyte cell line; we then used these cells to evaluate allele-dependent effects on UCP1 expression after mild cold exposure. We also used CRISPR-Cas9 to generate adipocytes with C-terminally luciferase-tagged PPARGC1A to study the effects of rs8192678 and cold exposure on PGC-1α stability.
Results: In both T/T and C/C adipocytes, the UCP1 expression increased after 6-hour cold exposure (32 oC vs 37 oC), but this effect was lower in C/C adipocytes (p=0.026, n=6). Furthermore, PGC-1α protein was more stable in C/C than in T/T adipocytes at 37 oC (protein half-life: 116 vs 72 minutes). At 32 oC, PGC-1α half-life increased to 277 minutes in C/C cells, but remained unchanged in T/T cells.
Conclusion: Our data shows gene x environment interaction between the different rs8192678 alleles and cold exposure, manifested through differential UCP1 expression. The C allele confers lower UCP1 expression increase after mild cold exposure, which coincides with the increased stability of the 482Gly PGC-1α protein variant. Our study may provide the molecular basis behind epidemiological studies that links rs8192678 polymorphism with obesity and metabolic disorders.
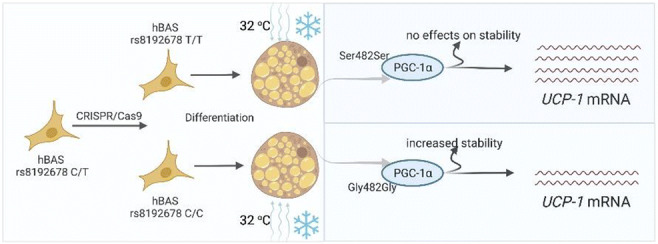
Supported by: ERC; CSC; VR; LUDC-IRC; The Albert Påhlsson Foundation and The Hjelt Diabetes Foundation
Disclosure: M. Huang: None.
298
IPSCs derived from insulin resistant offspring of type 2 diabetes patients harbor genetic defects
B. Memon1, A. Elsayed1, I. Bettahi2, N. Suleiman2, I. Younis3, E. Wehedy1, F. El-Ejeh4, A. Abou-Samra2, E. Abdelalim1;
1Diabetes Research Center, Hamad Bin Khalifa University, 2Hamad Medical Corporation, 3Carnegie Mellon Institute in Qatar, 4Cancer Research Center, Hamad Bin Khalifa University, Doha, Qatar.
Background and aims: Insulin resistance (IR) is caused by both genetic and acquired factors, however, the genetic factors are not well understood. Few clinical studies on first-degree relatives of type 2 diabetic (T2D) patients, which have the highest genetic predisposition to T2D, have given insights into the role of IR in T2D pathogenesis; without fractionating the effect of acquired factors. Induced pluripotent stem cells (iPSCs) are excellent tools for disease modeling as they can retain the genetic imprint of the disease. Therefore, in this study, we aimed to investigate the genetic perturbations associated with IR in the offspring of T2D patients, by using iPSC technology.
Materials and methods: iPSCs were generated from lean, IR offspring of T2D patients as well as from insulin-sensitive (IS) subjects, who were categorized based on hyperinsulinemic-euglycemic clamp. Transcriptomics was performed, followed by functional validation of target hits. IS and IR iPSCs were then differentiated to mature hepatocytes and differentially expressed proteins were assessed using the Olink platform for proteomics.
Results: The IS- (3 subjects) and IR-iPSCs (4 subjects) were fully characterized for pluripotency and had normal karyotyping. Transcriptomics on IR-iPSCs revealed dysregulated gene networks such as; L1TD1, RIF1, and the ZNF family members, ZNF195, ZNF770 and ZNF208 were downregulated in all IR-iPSCs whereas MFGE8, EGR1 and the lactate exporter SLC16A3 were upregulated. Our results highlight that the IR-iPSCs have increased oxidative stress and a heightened response to hypoxia indicated by accumulation of reactive oxygen species and a high susceptibility to H2O2-induced apoptosis. Moreover, the IR-iPSCs also had augmented levels of lactate secretion compared to IS-iPSCs under normal conditions. Furthermore, glucose uptake assay on mature hepatocytes derived from IR-iPSCs showed that they had a diminished capacity for glucose uptake in response to insulin compared to the IS-hepatocytes. O-Link assay for IR-hepatocytes (n=6 per IR) indicated that markers regulating hepatic function, glucose metabolism, glycosylation, autophagy, inflammation and stress or those associated with IR and T2D were differentially expressed compared to IS-hepatocytes (n=6 per IS). Our results also identified that several protective genes like p53, GALNT2, DNAJB1 and PREB had high levels under serum-starved conditions in the IR-hepatocytes. However, IR-hepatocytes failed to upregulate the above genes upon stimulation with insulin, unlike the IS-hepatocytes.
Conclusion: Overall, our IR-iPSC model can be employed for T2D modeling and drug screening studies that target genetic perturbations associated with IR in individuals with a high risk for T2D.
Supported by: NPRP10-1221-160041
Disclosure: B. Memon: None.
299
Strong association between variants in MiRNA genes and risk of type 2 diabetes in a Greek population
X. Tsekmekidou1, M. Grammatiki1, G. Karaliolios1, E. Melidou1, P. Rakitzi1, T. Koufakis1, F. Tsetsos2, M. Georgitsi2, A. Roumeliotis3, N. Papanas4, P. Paschou2, J. Yovos1, K. Kotsa1;
1Division of Endocrinology and Metabolism, First Internal Medicine Clinic, AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, 2Department of Molecular Biology and Genetics, Democritus University of Thrace, Alexandroupolis, 3Division of Nephrology and Hypertension, First Internal Medicine Clinic, AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, 4Diabetes Centre-Diabetic Foot Clinic, Second Department of Internal Medicine, Democritus University of Thrace, Alexandroupolis, Greece.
Background and aims: MicroRNAs (miRNAs) are small non-coding RNAs that modulate gene expression at the post-transcriptional level. They have recently emerged as attractive diagnostic and predictive biomarkers. Some miRNAs are involved in insulin secretion and others in the development of pancreatic β-cells, and therefore in the pathogenesis of Type 2 Diabetes (T2D). Our objective was to investigate a possible association between variants in miRNA genes and the risk of T2D in a case-control study design.
Materials and methods: The study population included 1,285 participants consisting of 716 individuals with T2D and 569 normoglycemic controls. In the control group, individuals over 65 years of age were included to minimize the probability that T2D will present at a later stage in the course of their life. The absence of T2DM was documented by both HbA1c<6.5% and FPG<126mg/dl. DNA was obtained from whole blood and the samples were analyzed using the Illumina Infinium PsychArray. Variants within upstream and downstream of MiR124a, MiR27a, MiR146a, MiR34a, MIRLET7A2, MiR128a, MiR196a2, MiR499a, MiR4513, MiR149 were genotyped and allele frequencies were compared between the groups. All statistical analyses were undertaken within PLINK and SPSS packages utilizing permutation analysis tests.
Results: A strong association with T2D was identified for rs1531212 (OR=1.375, p=0.018) in MiR23aHG. This is a host gene that includes MiR27a, among others. Rs6120777 in MYH7B, located upstream of MiR499a, was strongly associated with T2D (OR=1.27, p=0.018). A protective role was established against T2D for rs2425012 (OR=0.794, p=0.018), located upstream of MiR27a, and for rs883517 (OR=0.788, p=0.024) and rs2961920 (OR=0.80, p=0.041), both located upstream of MiR146a. Implementing the dominant model of analysis, two more strong associations were identified: Rs3746435 (OR=1.239, p=0.025) in MYH7B, located upstream of MiR499a and rs3746444 (OR=1.235, p=0.046) in MiR499a. No other variants reached the level of significance. The recessive model analysis produced insignificant results. No differences between male and female participants were observed.
Conclusion: Our findings suggest three new genetic indicators of risk for T2D, located near MiR27a and MiR499a. Rs1531212 is a non-coding transcript variant and rs6120777 is an intron variant in MHY7B which encodes the heavy chain 7B of myosin II. Although they both cause no direct alteration on the protein produced, they could have an indirect effect. Rs3746435 in MYH7B is a missense variant, resulting in an altered heavy chain. Larger population studies are needed to replicate our results and further clarify the role of MiRNAs and MYH7B in the pathogenesis of T2D.
Supported by: THALES. Investing in knowledge society through the European Social Fund (MIS 380273)
Disclosure: X. Tsekmekidou: None.
300
Investigating the effect of the HNF-1a G319S variant on liver and pancreas function under different physiological states
M. Sebastian, T. Morriseau, C. Doucette;
Department of Physiology and Pathophysiology, University of Manitoba, Winnipeg, Canada.
Background and aims: Genetic testing in Anishininew communities in central Canada led to the discovery of the HNF-1aG319S variant, which may contribute to youth-onset type 2 diabetes. HNF-1a is a transcription factor that controls glucose and lipid metabolism in the liver, and maintenance of pancreatic beta cell identity and function. Currently, it is unclear how the G319S variant influences these pathways. Given the metabolic demand associated with traditional lifestyle practices in central Canada, the G319S variant may instead confer an advantage to prolonged fasting. Here, we examine the impact of prolonged fasting in G319S expressing mice compared to control mice fed a standard chow diet.
Materials and methods: CRISPR/Cas9 was used to knock in the G319S variant in C57BL/6 mice, creating male and female wildtype (G/G), heterozygous (G/S), and homozygous (S/S) mice. At 3 months, mice were sacrificed either under ad libitum condition or after 24 hours fasting. Liver tissues were collected for gene expression and assessment of triglyceride contents. Islets were isolated to assess insulin secretion capacity, insulin content, and for electron micrography (EM) used to investigate morphological differences.
Results: A statistically significant reduction in liver triglycerides was observed in G/S (p=0.0237) and S/S (p=0.0185) mice compared to G/G. In addition, increased expression of genes involved in cholesterol synthesis and ketogenesis was observed, including HMGCR in G/S (p=0.0140) and S/S (P=0.0073) mice, as well as increased expression of genes involved in gluconeogenesis, including G6PT-1 in S/S mice (p=0.0290). Once fasted, a decrease in blood glucose was observed in G/S (P=<0.0001), and S/S (P=0.0385) mice compared to G/G, and a trend towards increased blood ketones was also seen. In pancreatic islets, a reduction in insulin content was seen in G/S (p=0.0175) and S/S (p=0.0065) mice compared to G/G. EM images showed an increase percentage of immature insulin granules in male S/S (p=0.0157) compared to G/G.
Conclusion: Our findings indicate that the G319S variant alters fatty acid and glucose metabolism in the liver as there is a shift toward ketogenesis and gluconeogenesis, and a propensity toward insulin depletion in the islets, which may indicate that the G319S variant provides a metabolic advantage during extended periods of fasting.
Supported by: CIHR
Disclosure: M. Sebastian: None.
SO 06 Sometimes one gene is enough
301
Monogenic diabetes in Slovakia: mutational spectrum and HNF1A functional studies
D. Gasperikova1, M. Skopkova1, T. Valkovicova1, Z. Dobiasova1, M. Sklenar1, V. Rambani1, S. Borecka1, Slovak Monogenic Diabetes Study Group, J. Stanik1,2;
1Institute of Experimental Endocrinology, Biomedical Research Center SAS, 2Department of Paediatrics, National Institute of Children's Diseases and Faculty of Medicine, Comenius University, Bratislava, Slovakia.
Background and aims: Monogenic diabetes is a heterogeneous condition with broad mutation spectrum of various genes including large portion of identified variants with unknown significance. The aim of the study was to describe the mutational spectrum of monogenic diabetes in Slovakia and to perform functional studies of variants identified in one of the monogenic diabetes genes - HNF1A.
Materials and methods: Since 2004, DNA samples from 637 patients with suspicion on monogenic diabetes and from 704 relatives were collected throughout Slovakia. The relevant genes were analysed using Sanger sequencing, MLPA or NGS approaches. The identified variants were categorised using the ACMG guidelines. Variants in the HNF1A gene encoding transcription factor HNF1α were functionally analysed using luciferase assay, EMSA, and immunofluorescence staining for nuclear localization.
Results: Pathogenic or probably pathogenic variants were identified in 198 out of 637 probands (31%). 125 had causal variant in GCK, 39 in HNF1A, 8 in HNF4A, 6 in mtDNA (m.3243A>G), 5 in HNF1B, 2 in ABCC8, 5 in KCNJ11, 4 in the INS gene, and single cases in RFX6, WFS1, TRIM37, and EIF2AK4 genes. Subsequent analysis of family members identified further 221 patients. One third of all identified variants in the GCK, HNF1A and HNF4A genes were novel. Interestingly, the founder mutation c.-557G>C in the promoter of the GCK gene represented 26 % of all identified GCK-MODY cases. Next, we functionally tested 8 HNF1A variants that were newly described or classified as variants of unknown significance (VUS) to assess their effect. The pathogenicity was proved in 5 tested variants, as they showed reduced transactivation activity (<40%) and/or decreased DNA-binding ability of HNF1α (<40% of the wild-type). Nuclear localization was not altered in any of the examined mutated proteins. As a result, we were able to reclassify 4 tested variants as likely pathogenic, and 2 variants as likely benign/benign.
Conclusion: The main cause of monogenic diabetes in Slovakia are mutations in the GCK gene (63% of probands) with founder mutation in 26% cases, followed by HNF1A (20%). Functional studies allowed correct classification of studied variants, which lead to molecular genetic diagnosis of HNF1A-MODY and subsequently to correct treatment.
Supported by: VEGA 2/0131/21, APVV-20-0236, VEGA 1/0572/21, APVV-17-0296
Disclosure: D. Gasperikova: None.
302
Genetic screening for monogenic diabetes using whole exome sequencing in a single hospital cohort in Japan
S. Tanaka1, H. Akagawa1, M. Ogata2, N. Iwasaki3;
1Tokyo Women’s Medical University Institute for Integrated Medical Sciences (TIIMS), 2Department of Nutrition and Dietetics, Faculty of Nutrition, Tokyo Kasei University, 3Institute of Geriatrics, Tokyo Women’s Medical University, Tokyo, Japan.
Background and aims: Maturity onset diabetes of the young (MODY) is the most common type among diabetes mellitus caused by a single gene disorder. Recent genetic analyses are moved to whole exome sequencing (WES) from Sanger sequencing or panel sequencing. Results of genetic diagnosis for monogenic diabetes using WES were found in Caucasian, Korean and Chinese, though such study has not been reported in Japan. The aim of this study was to elucidate the pathogenic variants in clinically diagnosed with MODY in Japanese by using WES.
Materials and methods: Patients who were clinically diagnosed with MODY were consented for participation in this study. Inclusion criteria were (1) age at diagnosis of diabetes < 30 years, (2) negative for GAD autoantibodies if tested, and (3) BMI < 30 m2/kg. Family history was not considered. Seventy-two patients (22 men and 50 women), including 62 subjects who were negative for previous sanger sequencing or panel sequencing for MODY1, MODY2, MODY3 and MODY5, were analyzed. Genomic DNA samples were subjected to exome enrichment using a SureSelect Human All Exon V6 kit. Enriched DNA libraries were sequenced using 150-bp paired-end reads on a NovaSec6000® (Illumina Inc, San Diego, CA). Variants were annotated with 1000 genome, PolyPhen2, SIFT, CADD, ClinVar and gnomAD. Copy number variation (CNV) was evaluated by eXome Hidden Markov Model (XHMM) and confirmed by Multiplex Ligation-dependent Probe Amplification (MLPA). Pathogenicity was evaluated according to ACMG guideline 2015. This study was performed under the permission of Ethical Board for the human Genome study in the University.
Results: The mean age at diagnosis of diabetes was 16.8 ± 5.3 (m ± SD) years, BMI at first visit was 21.6 ± 2.7 m2/kg. Among 10 subjects whose analyses was started by WES, pathogenic variants were found in seven subjects (three in HNF-1α, two in HNF-4α, one each in GCK and HNF-1β), and the detection rate was 70% in this group. In the second group consisted with subjects who were negative for previous sanger sequencing and panel sequencing, we found pathogenic variant each in PDX1, ABCC8, INSR, and WFS1. In addition, a large genomic deletion was found in HNF-4α in one subject by XHMM and confirmed by MLPA. Thus, the tentative detection rate was 8.1% (5/62) in the second group. The WFS case was associated with compound heterozygous pathogenic variants, however, the patient did not develop diabetes insipidus nor optic nerve atrophy, indicating that diagnosis in very early phase was made.
Conclusion: Overall, 12/72(16.7%) clinically suspected MODY subjects were found to have pathogenic variants in known monogenic diabetes genes , including a large genomic deletion in HNF-4α and WFS. At the same time, a low diagnostic rate (8.1%) was disclosed in 62 subjects who were negative in previous sanger or panel sequencing. Further systematic study including MLPA screening for exonic aberrations, and challenging for identifying unknown variants for Asian monogenic diabetes are still needed.
Supported by: Manpei Suzuki Diabetes Foundation, KAKENHI(21K06281)
Disclosure: S. Tanaka: None.
303
Clinical spectrum associated with variants in the INS gene in patients with suspected monogenic diabetes
M. Gomes Porras1, R. García Moreno2, M. Güemes Hidalgo3, R. Vallejo Mora1, M. Ruiz De Adana1, J. Gómez Zumaquero4, Á. Campos Barros5;
1Endocrinology, Hospital Universitario Regional de Málaga, Málaga, 2Endocrinology, Hospital Universitario La Paz, Madrid, 3Pediatric Endocrinology, Hospital Infantil Universitario Niño Jesús, Madrid, 4Molecular Endocrinology, IBIMA, Málaga, 5Molecular Endocrinology, INGEMM, IdiPAZ, Madrid, Spain.
Background and aims: The INS gene encodes for preproinsulin, the precursor of insulin. INS mutations can cause MODY-INS (MODY10; OMIM #613370) and have been described more frequently in cases of neonatal DM. Opposite to what might be expected, the phenotypic expression reported is variable. The main objective was to clinically, biochemically and molecularly characterize patients with MODY-INS diabetes attending the Endocrinology Department of two Spanish tertiary level hospitals during the 2009-2022.
Materials and methods: Multicenter cross-sectional study that included 121 pediatric patients with suspected monogenic diabetes analyzed by targeted NGS with a custom panel including up to 482 genes associated with dysglycemia. The variants detected were classified according to ACMG criteria and prioritized using criteria of confidence and quality, coverage (20x/pb >95%), allele frequency in control population <1% (gnomAD), impact ("missense", "nonsense", "frameshift", "splicing effect") and in silico prediction of pathogenicity (CADD V1.6 score >15). Quantitative data were expressed as mean±standard deviation and qualitative data as absolute frequencies and percentages.
Results: 3/121 patients (2.5%), 2 males and 1 female, aged 15±4.58 years and BMI 21.8±3.5Kg/m2, presented with heterozygous INS deleterious variants. While segregation analysis revealed that the pathogenic variants NM_000207.3:c.140G>A,p.(Gly47Asp) and NM_000207.3:c.163C>T,p.(Arg55Cys) in exon 2, were de novo, no allelic segregation study could be performed for the NM_000207.3:c.62C>T,p.(Pro21Leu) exon 1 variant (VUS). The two pathogenic variants are registered in HGMD, associated with MODY-INS and DM1, respectively, and none of the 3 is registered in gnomAD(V3.1.2). Diabetic debut at 12±2.6 years in the form of simple hyperglycemia, negative pancreatic autoimmunity, preserved C-peptide at diagnosis and 2/3 after 4 years from diagnosis. Initial HbA1c was 6.4±0.5% (currently 6.7±2.1%) and their lipoproteic profile was normal. None developed acute or chronic complications of diabetes after 4±2 years of follow-up. The patient with the INS p.(Pro21Leu) variant presented additional, predicted deleterious VUS in GPR183 and NRXN3. He was overweight and insulin resistance, treated with metformin. The patient with the INS p.(Gly47Asp) variant presented poor metabolic control due to poor adherence to low-dose insulin therapy, his DM was initially classified as type 1b, delaying the molecular diagnosis for about 3 years. The patient with the INS p.(Arg55Cys) variant presented good metabolic control with hygienic-dietary measures.
Conclusion: The prevalence of MODY-INS is higher than reported. Phenotypic expression occurred in early childhood, varying from mild to severe hyperglycemia with insulinopenia up to insulin resistance in relation to the association with other predicted deleterious variants in candidate genes (MODY-X). Suspicion of MODY-INS should be raised even in the absence of a family history of DM. The analysis of INS sequences should be included in the molecular diagnostic routine of suspected monogenic diabetes.
Supported by: Fundación SEEP
Disclosure: M. Gomes Porras: None.
304
Loss of FOXA2 induces ER stress and hepatic steatosis and alters developmental gene expression in human iPSC-derived hepatocytes
E.M. Abdelalim1, M. Aghadi1, R. Elgendy2;
1Diabetes Research Center, Hamad Bin Khalifa University, Doha, Qatar, 2Discovery Biology, AstraZeneca, Gothenburg, Sweden.
Background and aims: FOXA2 has been known to play important roles in liver functions in rodents. However, its role in human hepatocytes is not fully understood. Recently, we generated FOXA2 mutant induced pluripotent stem cell (FOXA2-/-iPSC) lines and illustrated that deficiency of FOXA2 results in developmental defects in iPSC-derived pancreatic islet cells, which may lead to monogenic diabetes. Here, we used FOXA2-/-iPSC lines to understand the role of FOXA2 on development and function of human hepatocytes.
Materials and methods: TTwo FOXA2-/-iPSC lines and their WT controls were differentiated into hepatic progenitors (HP) and mature hepatocytes (MH). The developmental and functional hepatic genes were examined using immunostaining, qRT-PCR, and Western blotting at different stages. RNA-sequencing was performed on iPSC-derived HP and MH and functional assays were performed on iPSC-derived MH. Also, FOXA2 was overexpressed in HP lacking FOXA2 at HP stage and its effect was evaluated at MH stage.
Results: Lack of FOXA2 resulted in significant alterations in the expression of key developmental and functional genes in HP and MH as well as an increase in the expression of ER stress markers. Functional assays demonstrated an increase in lipid accumulation, bile acid synthesis and glycerol production, while a decrease in the glucose uptake, glycogen storage, and Albumin secretion. RNA-sequencing analysis further validated the findings by showing a significant increase in genes associated with lipid metabolism, bile acid secretion, and suggested the activation of hepatic stellate cells and hepatic fibrosis in MH lacking FOXA2. Overexpression of FOXA2 in HP reversed the defective phenotypes and improved hepatocyte functionality in iPSC-derived MH lacking FOXA2.
Conclusion: These results indicate that the appropriate expression of FOXA2 in human hepatocytes is essential for normal hepatocyte development and protects hepatic cells from ER stress, hepatic steatosis, and bile acid toxicity. Furthermore, the in vitro human hepatocyte model presented here can be used to identify novel therapeutic targets for associated liver disorders.
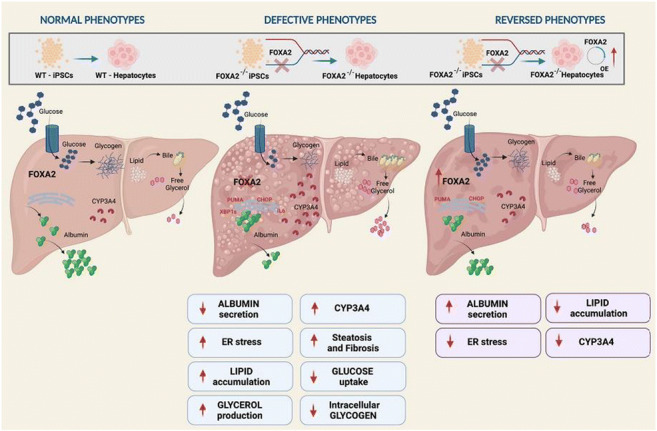
Supported by: NPRP10-1221-160041
Disclosure: E.M. Abdelalim: None.
305
Single cell transcriptomics highlights impaired differentiation in stem cell-derived beta cells from a Wolfram syndrome patient
S. Torchio1,2, R. Chimienti2, F. Manenti2, M.T. Lombardo2, V. Zamarian2, S. Pellegrini2, V. Sordi2, L. Piemonti1,2;
1Università Vita-Salute San Raffaele, 2IRCCS Ospedale San Raffaele, Milano, Italy.
Background and aims: Wolfram Syndrome (WS) is a rare genetic disease presenting with diabetes mellitus, optic atrophy and progressive neurodegeneration. It is caused by mutations affecting the protein Wolframin, which is implicated in ER stress response, autophagy and Ca++ handling: its loss provokes apoptosis in β cells. Understanding of molecular mechanisms ongoing in the disease is lacking, but it could help to develop effective therapies. Taking advantage of WS-affected, patient-derived iPSCs and the CRISPR/Cas9-corrected counterpart, we sought to investigate what could be the primary molecular defect in the deriving β cells: to do so, we employed Single Cell Transcriptomics (SCT).
Materials and methods: iPSCs were generated from a WS patient carrying novel heterozygous mutations in the Wolframin gene; gene correction was achieved in heterozygosity through CRISPR/Cas9-directed recombination using a ssODN as donor template. Differentiation into β cells was performed by using a standardized in vitro protocol, and the endpoints were analyzed by FACS, Western blot, RT-qPCR and by SCT. Ca++ flux measurements were performed using Fluo-4 acquired at a fluorescence microscope. Stress induction was forced by Thapsigargin (TG) or inflammatory cytokines (IL) administration. Apoptosis was measured by FACS staining with AnnexinV/PI.
Results: We evaluated the two iPSC-derived differentiated cell lines by SCT. We found that both could efficiently commit to the pancreatic endocrine lineage, but while the corrected line presented the expected hormone secreting cell types, WS cells showed a severe impairment in the proportion of α (1.5% vs 12%, WS vs corrected), γ (0% vs 1%) and δ cells (0.3% vs 1%), unbalancing the ratio towards β cell production (95% vs 76%). Such disruption of the endocrine niche leads to an impaired secretory ability of the residual β cells, with a loss in GSIS of 40% compared to the corrected counterpart. Secretory impairment was explained by reduced amounts of PCSK1 and SNAP25 genes (p<0.0001) and by asynchronous, poorly time-controlled Ca++ fluxes. Again, Ca++ handling impairment stems from low expression of voltage-gated channels CACNA1C and CACNA1D (p<0.0001) and increased RGS4 (p<0.0001), a known inhibitor of Ca++-induced insulin release. At the molecular level, WS β cells in basal conditions display poor activation of the PERK and IRE1α pathways of ER stress response, as seen through ATF4 and XBP-1s downregulation (p<0.05), but a significant upregulation of proapoptotic DDIT3 gene in response to TG (p<0.05). In parallel, stress induction also triggers autophagy in both β cell lines, but while corrected ones properly process and degrade p62, WS ones have abnormally high levels of p62 gene and protein even at longer timepoints (8h and 16h, p<0.05 and p<0.01 respectively), indicating an incomplete autophagic flux. Furthermore, both TG and IL triggered a higher degree of cell death in WS β cells than the corrected counterpart (p<0.0001), suggesting a greater susceptibility to apoptosis.
Conclusion: Our data delineate a new paradigm in understanding the multiple known impairments seen in WS stem-derived β cells: these come from a defective differentiation process, which leads to the production of fragile β cells that are characterized by molecular impairments, low functionality and poor survival.
Disclosure: S. Torchio: None.
306
Dach1 associated with familial diabetes decreases insulin secretion if silenced
V. Schwitzgebel1, C. Jimenez Sanchez1, R. Dusaulcy1, J.-L. Blouin1, I. Stankute2, R. Verkauskiene3, F. Reimann4, F. Gribble4, P. Maechler1;
1Diabetes Center Faculty of Medicine, Dept. of Pediatrics, Gynecology and Obstetrics, University of Geneva, Geneva, Switzerland, 2Department of Pediatric Endocrinology, University of Kaunas, Kaunas, Lithuania, 3Department and Institute of Endocrinology, University of Kaunas, Kaunas, Lithuania, 4University of Cambridge, Cambridge, UK.
Background and aims: Genetic variants in Dachshund family transcription factor 1 (DACH1) have been associated with familial young-onset diabetes. Mouse studies showed that Dach1 plays a role in the perinatal generation of most of the beta cell mass. As the knockout mice shortly die after birth, the function of Dach1 has not been studied in mature beta cells. Our aims were to establish the role of Dach1 in mature beta cells and to identify the regulatory network of Dach1.
Materials and methods: We used primary FACS-sorted beta cells of 10-week-old transgenic GLU-Venus x INS-Cherry male mice. Beta cells were knocked-down for Dach1 using SiRNA-mediated technology and used for glucose- and KCl-stimulated insulin secretion tests. Human islets were isolated at the Geneva University Hospital (Switzerland). Total RNA was extracted from isolated mouse beta cells or cultured isolated human islets for RNA-sequencing. Quantitative RT-PCR was performed. Immunohistochemistry and image analysis were used for the assessment of cell proliferation or apoptosis detection.
Results: We first confirmed the expression of DACH1/Dach1 in adult human and mouse beta cells before silencing Dach1 using siRNA (si-Dach1). We achieved a silencing efficiency of more than -4 log2 fold change in comparison to control cells (si-Control), analyzed by qPCR and confirmed on the protein level by immunofluorescence. Glucose-induced insulin secretion (GSIS) showed a significantly (**p=<0.01) blunted response in si-Dach1 cells (0.4 ± 0.21) in comparison to control cells (si-Control, 1.3 ± 0.55), but the same cells responded to the subsequent addition of KCl, indicating an intact triggering pathway. Results are means ± SD of 4-8 independent experiments. Apoptosis and proliferation were not modified by siDach1 in comparison to control cells. To further elucidate the molecular changes leading to a blunted insulin secretion, we performed RNA sequencing analysis of siDach1 and control primary mouse beta cells. We identified nine differentially regulated genes (downregulated: Itgam, Pcsk1; upregulated: Fkbp9, Crk, Rhpn2, Enpp2, Etv5, and Matn2). Exposure of human islets to different metabolic stressors, such as high glucose, oleate and palmitate, alone or in combination diminished Dach1 expression.
Conclusion: Our study shows for the first time that glucose-stimulated insulin secretion is completely blunted in beta cells silenced for Dach1 expression, demonstrating a crucial role for Dach1 in normal beta cell function. However, insulin release upon KCl stimulation was unchanged, possibly pointing to a metabolism-secretion coupling effect. Further, we identified the genetic regulatory network of Dach1, implicated in the integrin-mediated signaling pathway, cell adhesion, and differentiation. We also report the significant downregulation of DACH1 expression in isolated human islets following in vitro chronic exposure to different metabolic stressors. Therefore, DACH1 may participate in the islet adaptive response elicited by metabolic stress, further pointing towards a crucial role of this gene in islet-cell functionality.
Supported by: SNF
Disclosure: V. Schwitzgebel: None.
307
Aetiology and pathogenesis of HNF1A-MODY using human islets deficient of HNF1A and a new heterozygous mouse model (Hnf1a Δe4-10 ) of the human disease
A. Acosta Montalvo1,2, C. Saponaro1, J. Thévenet1, M. Chiral3, A. Piron4, N. Delalleau1, G. Pasquetti1, M. Moreno Lopez1, V. Gmyr1, M. Cnop4, J. Kerr-Conte1, F. Pattou1, M. Pontoglio3, A. Liston2, C. Bonner1;
1Univ. Lille, Inserm, CHU Lille, Institut Pasteur, France, Lille, France, 2VIB center for brain and disease research KU Leuven, Leuven, Belgium, 3Institute Necker-Enfants-Malades, Paris, France, 4ULB Center for Diabetes Research, Brussels, Belgium.
Background and aims: Heterozygous mutations in the HNF1A gene cause pancreatic beta cell dysfunction, insulin secretory defects, and Maturity-onset-diabetes-of-the-young (HNF1A-MODY). Mice with heterozygous mutations in Hnf1a do not phenocopy the human disease as of yet. However, Hnf1a KO mice have reduced renal SGLT2 expression, increased glycosuria and endogenous glucose production, similar to HNF1A-MODY patients. Since SGLT2 is also expressed in alpha cells and is required for glucagon regulation, the goal of this study was (1) to investigate HNF1A regulation of SGLT2 and hormone secretion in human islets; and (2) to create the first heterozygous mouse model expressing a mutant Hnf1a protein with a dominant mode of action similar to the human frameshift mutation P291fsincC, in order to monitor disease-onset and progression.
Materials and methods: Adult normoglycemic human islets were transfected with chemically synthesized small interfering RNAs (siRNAs) targeting HNF1A (h-HNF1Asi) vs. scrambled controls. Glucose transporters and hormone protein expression and secretion was analyzed by Western blotting and ELISA techniques. The Cre-LoxP system was used to create the Hnf1a-MODY mice. CRISPR/Cas-mediated genome editing was used to insert the loxp sequences into intron 4 and after exon 10. Hnf1aloxp/loxp mice were bred with CMV-CRE mice to produce heterozygous Hnf1a+/Δe4-10 mice. To obtain littermate controls, Hnf1a+/Δe4-10 mice were bred with Hnf1a+/+ mice. The measured parameters in this study were weight, fasting glucose, insulin, glucagon, glycosuria, and oral glucose tolerance tests (OGTT).
Results: We first observed that HNF1A is heterogeneously expressed in human alpha, beta, and delta cells. h-HNF1Asi islets displayed reduced insulin secretion and content (51%), which was rescued by GLP1 treatment. HNF1A deficiency reduced SGLT2 protein expression (72%), correlating with increased glucagon content (104%) and secretion, which was not suppressed by high-glucose stimulation. Hnf1a+/Δe4-10 mice (n = 4) were born normoglycemic, but after 18 weeks developed fasting hyperglycemia (****p< 0.0001). Postprandial glucose enrichments peaked within 30 minutes of the OGTT (***p< 0.001), but rapidly declined thereafter. Plasma insulin levels were slightly lower (ns) while fasting, but increased after the OGTT (*p< 0.05). There are no significant differences in fasting (0’) and post-glucose glucagon levels, despite a reduction in renal Sglt2 expression (**p< 0.1) and postprandial glycosuria (> 50 mg/dL). The latter which has been suggested to precede diabetes in human HNF1A mutation carriers.
Conclusion: Collectively, these findings indicate that HNF1A deficiency downregulates renal and alpha cell SGLT2 protein expression, which is associated with an alteration in glucagon secretion in response to glucose stimulation. The heterozygous Hnf1a+/Δe4-10 mice will also be advantageous to evaluate the efficacy of anti-diabetic drugs present and future.
Supported by: EFSD/Lilly Research Programme 2016; ISITE 2018; FWO
Disclosure: A. Acosta Montalvo: None.
308
Novel modification of monogenic diabetes gene panel test improves diagnosis of 6q24 methylation related diabetes
R. Kumar, M. Wakeling, E.D. Franco, E.J. Self, S.E. Flanagan, A.T. Hattersley, K.A. Patel;
Institute of Biomedical and Clinical Science, University of Exeter Medical School, Exeter, UK.
Background and aims: Methylation defects at chr6q24 are a common cause of neonatal diabetes. Currently, their diagnosis requires dedicated methylation-specific assay (MS-MLPA) testing. The majority (>81%) of cases are due to paternal uniparental disomy (UPD), paternal duplication, or ZFP57 mutations. We aimed to assess if novel modification of the existing monogenic diabetes gene panel can identify chr6q24 methylation defects due to these mechanisms, and thus reduce the need for methylation specific testing.
Materials and methods: We performed chr6q24 MS-MLPA (gold-standard test) and our modified gene panel on 291 individuals with diabetes due to suspected 6q24 defects. Our modified panel included coverage of ZFP57 and common variants in a 200kb region on chr6q24. This allowed identification of individuals with suspected UPD (homozygous on 6q24), duplications (skewed variant allele fraction), and ZFP57 mutations.
Results: Gold-standard testing identified specific methylation defects in 28/291 (9.6%) cases (8 with UPDs, 9 with duplications and 11 with ZFP57 mutations). Our modified panel identified 27/28 of individuals with specific methylation defects (sensitivity 96.4%, 95%CI 81.7-99.9). 235/263 were correctly identified as negative (specificity 89.4%, 95%CI 85.0-92.8%). The positive predictive value was 49.1% (95%CI 35.4-62.9%) and the negative predictive value was 99.6% (95%CI 97.7-100.0%). All false positive cases had coincidental homozygosity on 6q24, which could not be distinguished from UPD.
Conclusion: Novel modification of the existing monogenic diabetes gene panel can identify the majority of 6q24 methylation defects. These modifications are easy to implement in existing gene panels worldwide and will greatly reduce the need to perform expensive methylation testing.
Supported by: University of Exeter Expanding Excellence in England (E3) UKRI; Wellcome Trust; Diabetes UK
Disclosure: R. Kumar: None.
309
Genome-wide CRISPR screens identify therapeutic targets for HNF1A-deficient diabetes
M. Cuenca-Ardura1, M. De Vas2, D. Balboa1, J. Ferrer1;
1Centre for Genomic Regulation (CRG), Barcelona, Spain, 2Imperial College London, London, UK.
Background and aims: HNF1A encodes a transcription factor required for glucose-stimulated insulin secretion in human pancreatic β-cells. Heterozygous mutations in HNF1A cause haploinsufficiency and lead to monogenic early-onset diabetes. Moreover, hypomorphic variants in HNF1A and altered HNF1A function have been associated with increased risk of type 2 diabetes. Therefore, stimulating the expression of the wild-type HNF1A allele represents a potential therapeutic strategy in such patients. However, upstream mechanisms that regulate HNF1A in human adult β-cells remain unclear. We aim to identify candidate genes and pathways that control the expression of HNF1A.
Materials and methods: We performed two genome-wide loss-of-function and gain-of-function CRISPR screens in a human β-cell line carrying a tdTomato reporter knocked into HNF1A (EndoC-bH3 HNF1A/tdTomato). This reporter line allows modelling of clinical HNF1A haploinsufficiency as well as measuring the endogenous levels of HNF1A using a fluorescent readout. EndoC-bH3 HNF1A/tdTomato were transduced with lentiviral human CRISPR KO and CRISPRa libraries targeting all human genes with several sgRNAs. After selection, cells were sorted according to their tdTomato intensity of fluorescence. MAGeCK was used to calculate enrichment of sgRNAs in the Tomato-Low and -High populations and to select candidate genes that regulate HNF1A.
Results: We observed a high concordance between the two genetic screens. The CRISPRa screen identified 56 candidate activators and 26 candidate repressors of HNF1A at high confidence thresholds (P<0.001). The CRISPR KO screen uncovered 31 putative activators and 12 repressors of HNF1A (P<0.001). Several top candidates were validated individually in EndoC-bH3 cells by qPCR of HNF1A and some of its transcriptional targets. Functional analyses revealed an enrichment of selected pathways among candidate HNF1A activators and repressors.
Conclusion: Using an unbiased genetic approach, we have interrogated the whole genome of human pancreatic β-cells to find genes and pathways that potentially regulate HNF1A. Validations in stem-cell-derived islet models and in primary human islets are ongoing to confirm these findings. Integration of these genetic screens with two high-throughput chemical screens will further advance our understanding on core genetic pathways critical for human β-cell function, and pave the way for new precision medicine strategies to treat monogenic diabetes.
Supported by: Boehringer Ingelheim Fonds (BIF)
Disclosure: M. Cuenca-Ardura: None.
310
Low-dose treatment with sulfonylureas improves hyperglucagonemia after a glucose challenge in HNF1A-MODY individuals and Hnf1a-/- mice
C. Saponaro1, I. Spiliotis2, A. Acosta-Montalvo1, L. Anguelova2, J. Thevenet1, M. Chiral3, V. Gmyr1, J. Kerr-Conte1, F. Pattou1, M. Pontoglio3, K. R. Owen2, C. Bonner1;
1Inserm U1190 - Translational Research for Diabetes, Lille, France, 2Oxford Centre for Diabetes, Endocrinology & Metabolism, Oxford, UK, 3Institute Necker-Enfants-Malades, Paris, France.
Background and aims: HNF1A is a transcription factor expressed in several metabolic organs involved in the maintenance of glucose homeostasis. A single mutation of HNF1A results in Maturity-onset-diabetes-of-the-Young (HNF1A-MODY), characterized by insulin secretory defects and hyperglycemia. Although endogenous glucose production has been reported, less is known about glucagon physiology in HNF1A-MODY. The first-line treatment for HNF1A-MODY patients is Sulfonylureas (SU), which increases insulin secretion by acting directly on the ATP-sensitive K+ channels of beta cells, independently of glucose. Patients on SU treatment achieve good glycaemic control, however, whether SU affect glucagon secretion in this population is unknown. Here, we aimed to determine the role of HNF1A on glucagon secretion and to establish whether low-dose gliclazide (SU) had an effect in HNF1A-MODY patients.
Materials and methods: An Oral glucose tolerance test (OGTT) was performed with 75 g of anhydrous glucose in solution in 7 HNF1A-MODY patients before and 72h after stopping gliclazide, and glycemia, c-peptide, and glucagon levels were measured. Hnf1a-/- mice compared to WT controls were fasted overnight, treated acutely with 40 mg/kg of gliclazide or vehicle 1h before the OGTT (2g/kg glucose), and glycemia, insulin, and glucagon levels were measured.
Results: In HNF1A-MODY patients glucose rose significantly off gliclazide (p=0.003), however, this was not reflected by significant changes in c-peptide or glucagon levels. However, when glucose levels were taken into account the change from baseline glucagon levels (Δ glucagon /glucose ratio) showed a significant decrease on, but not off, gliclazide (p<0.05), unlike c-peptide. Of note, HNF1A-MODY individuals, both on and off treatment, showed glucagon levels significantly below the normal range expected in healthy individuals. Hnf1a-/- mice compared to wild-type mice displayed glucose intolerance, associated with decreased plasma insulin levels and hyperglucagonemia that was not suppressed after a glucose challenge. The acute treatment with gliclazide significantly decreased glycemia after fasting and a glucose challenge compared to vehicle-treated animals. Moreover, after the OGTT insulin secretion was increased and glucagon over-secretion was suppressed in Hnf1a-/- mice treated with gliclazide compared to vehicle.
Conclusion: Collectively, these findings indicate that HNF1A is also involved in the regulation of glucagon secretion, and low-dose treatment with gliclazide improves hyperglucagonemia after glucose challenge. However, whether long-term treatment with gliclazide affects alpha cell function and glucagon secretion needs further investigations.
Supported by: EFSD/Lilly Research Programme 2016;Societe Francophone du Diabète
Disclosure: C. Saponaro: None.
SO 07 SARS-CoV2 and other viruses
311
Long and short isoforms of SARS-CoV-2 receptor Angiotensin I-Converting Enzyme type 2 (ACE2) are increased in pancreatic islets of type 2 diabetes patients
D. Fignani1, G. Licata1, E. Aiello1, G.E. Grieco1, C. Formichi1, L. Nigi1, L. Marselli2, P. Marchetti2, G. Sebastiani1, F. Dotta1,3;
1Diabetes Unit, Dept. of Medicine, Surgery and Neuroscience, University of Siena; Umberto di Mario Foundation c/o TLS, Siena, 2Dept. of Endocrinology, University of Pisa, Pisa, 3Tuscany Region Centre for Precision Medicine (CReMeP), Siena, Italy.
Background and aims: SARS-CoV-2 can infect human pancreatic islets through β cell-specific expression of ACE2 receptor. We and others have previously shown that β cells do express the novel short-ACE2 (s-ACE2) isoform alongside with S-protein binding long ACE2 (l-ACE2). It is reported that type 2 diabetes (T2D) is a major comorbidity of COVID-19, and T2D patients are predisposed to poor prognosis and exacerbated altered glycometabolic control after infection. Considering the expression of ACE2 in pancreatic islets, we should take into account the hypothesis of a partial insulin deficiency due to direct damage and/or dysfunction of β cells during SARS-CoV-2 infection in T2D patients. Here, we aimed at elucidating the in-situ expression pattern of ACE2 in T2D respect to non-diabetic donors (CTR) which may account for a higher susceptibility to SARS-CoV-2.
Materials and methods: We take advantage from the large biorepository of T2D donor tissues from University of Pisa. We analyzed multiple FFPE sections from n=20 CTR (F=9 M=11; age mean±S.D.: 70.6 ±7 yrs; BMI: 26.2 Kg/m2±4.1 Kg/m2) and from n=20 T2D (F=6 M=14; age mean±S.D.: 71.7±7.6 yrs; BMI: 27.1 Kg/m2±2.7 Kg/m2), subjected to triple IFA using monoclonal mouse anti-human ACE2 (MAB933; detecting s-ACE2), polyclonal rabbit anti-human ACE2 (ab15348; detecting both ACE2 isoforms) and with polyclonal insulin. Stained sections were analyzed using confocal microscopy; Additionally, previous bulk RNA sequencing datasets of T2D and CTR islets were interrogated to evaluate ACE2 expression and other molecules involved in its upregulation.
Results: The analysis of n=1082 islets across all donors confirmed the expression of s-ACE2 and l-ACE2 in pancreatic β cells, both in T2Ds and CTRs. A higher prevalence of s-ACE2 respect to l-ACE2 in β cells was detected (mean colocalization rate in all donors s-ACE2: 10.8%; l-ACE2: 8.2%). We observed a significantly increased colocalization rate (%) between s-ACE2 [MAB933] and INS in T2D compared to CTR (T2D mean±S.D.: 12.5 ± 7.6% and CTR 9.23 ± 7.0%, p<0.001) and of l-ACE2 [ab15348] and INS in T2D compared to CTR (T2D mean±S.D.: 11.45 ± 13.7% and CTR 5.3% ± 5.3%; p<0.001). Moreover, the normalized signal intensity analysis (s-ACE2 and l-ACE2 mean pixel intensity/islet area) confirmed the upregulation of both ACE2 isoforms in T2D (grey scale values of s-ACE2 in T2D=52.5±34.6 and in CTR=37.1±28.1, p<0.001; grey scale values of l-ACE2 in T2D=53.2±63.5 and in CTR=27.3±22, p<0.001). RNA sequencing of isolated T2D islets vs CTRs confirmed the upregulation of ACE2 mRNA in T2D vs age-matched controls. Moreover, we observed an increased expression of the inflammatory signalling molecules STAT1 and NFAT in T2D vs CTRs, both involved in the transcriptional regulation of ACE2, thus implying the existence of active signalling pathways contributing to ACE2 hyperexpression in T2D.
Conclusion: We observed the upregulation of s-ACE2 and l-ACE2 in pancreatic islets of T2D donors. Higher ACE2 expression in T2D islets might increase their susceptibility to SARS-CoV-2 infection during COVID-19 disease, thus exacerbating glycometabolic demise due to a potential direct damage or dysfunction of β cells.
Disclosure: D. Fignani: None.
312
Pre-vaccination glucose time in range positively correlates with antibody response to SARs-CoV2 mRNA vaccine BNT162b2 in patients with type 1 diabetes
S. Briganti1, G. Alhamar1,2, V. Viola1, M. Faraj1, C. Zannella3, G. Franci4, C. Fusco5,6, C. Isgrò7,8, G. Leanza1, A. Spinelli9, S. Pieralice1, G. Matarese10,11, P. Pozzilli1, M. Galgani10,12, R. Strollo9;
1Department of Endocrinology and Diabetes, Campus Biomedico University of Rome, Rome, Italy, 2Dasman Diabetes Institute, Al-Asimah, Kuwait, 3Dipartimento di Medicina Sperimentale, Universita degli Studi della Campania, Naples, Italy, 4Dipartimento di Medicina Chirurgia e Odontoiatria 'Scuola Medica Salernitana', Università degli Studi di Salerno, Baronissi, Italy, 5Consiglio Nazionale delle Ricerche Area di Ricerca di Napoli 1, Naples, Italy, 6Unita di Neuroimmunologia, IRCCS, Fondazione Santa Lucia, Rome, Italy, 7Department of Medicine, Campus Biomedico University of Rome, Rome, Italy, 8Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari, Bari, Italy, 9Department of Science and Technology for Humans and the Environment, Campus Biomedico University of Rome, Rome, Italy, 10Istituto per l'Endocrinologia e l'Oncologia Sperimentale "G. Salvatore", Consiglio Nazionale delle Ricerche Area di Ricerca di Napoli 1, Naples, Italy, 11I Dipartimento di Medicina Molecolare e Biotecnologie Mediche, Università degli Studi di Napoli Federico II, Naples, Italy, 12Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, Naples, Italy.
Background and aims: Poor glucose control has been associated with increased mortality in COVID-19 patients with type 1 diabetes (T1D). The aim of this study was to assess the effect of glucose control on antibody response to the SARS-CoV2 vaccine BNT162b2 in T1D.
Materials and methods: We studied 26 T1D patients scheduled to receive two doses, 21 days apart, of the BNT162b2 mRNA vaccine to SARs-CoV2. Patients were followed prospectively for six months with regular evaluation of SARS-CoV2 antibodies and glucose control. IgG to spike glycoprotein were assessed by ELISA, and serum neutralization by a live SARS-CoV2 assay (Vero E6 cells system). Continuous glucose monitoring, including time in range (TIR) and above range (TAR), and HbA1c were collected. The primary exposure and outcome measures were pre-vaccination glucose control, and antibody response after vaccination, respectively. IgG area under the curve (AUC) assessed the overall antibody response along the six-months study timeframe.
Results: Baseline TIR and TAR strongly correlated with peak-IgG, as well as with the IgG-AUC (TIR: r=0.75; p=0.02; TAR: r=-0.81; p=0.008). Furthermore, pre-vaccination TIR was associated with serum neutralization potency (r=0.49; P=0.042). Glucose control along the study timeframe was also associated with IgG response as showed by the correlation between time-dependent mean of TIR and TAR and IgG-AUC (TIR: r=0.93, P<0.0001; TAR: r=-0.84, P<0.0001). Pre-vaccination HbA1c was inversely related to peak-IgG, although the relationship did not reach statistical significance (r= -0.33; P=0.14).
Conclusion: Our findings indicate a strong relationship between glucose control and antibody response after SARS-CoV2 vaccination, highlighting the importance of achieving well-controlled blood glucose for COVID-19 prevention.
Supported by: EFSD/JDRF/Lilly Programme in T1D Research
Disclosure: S. Briganti: None.
313
Association between SARS-CoV-2 infection and presymptomatic type 1 diabetes or celiac autoimmunity in general population children: Autoimmunity Screening for Kids (ASK)
M. Rewers, C. Geno Rasmussen, F. Dong, M. Stahl, L. Yu, B.I. Frohnert, K. Simmons, E. Liu, A.K. Steck, for ASK Study Group;
University of Colorado, Aurora, USA.
Background and aims: Early detection of presymptomatic type 1 diabetes (T1D) or celiac disease (CD) can prevent significant morbidity and cost associated with diagnoses that are delayed until significant clinical symptoms develop. We report outcomes of combined screening for presymptomatic T1D and CD in general population children and explore potential association of autoimmunity with SARS-CoV-2 infection.
Materials and methods: In 2017-2022, the Autoimmunity Screening for Kids program screened 29,441 children aged 1-17 y in Colorado, USA. Study participants’ race/ethnicity represented the Colorado population; only 4.9% had a first-degree relative (FDR) with T1D. Standard radiobinding assays (RBA) and electrochemiluminescence (ECL) assays were applied to detect autoantibodies to insulin, GAD, IA-2, and ZnT8 - markers of T1D autoimmunity as well as tissue transglutaminase autoantibodies (TGA) - a marker of CD. Screening positive children were invited to confirm results with repeat testing; if confirmed positive for islet autoantibodies (IA), they were monitored for development of diabetes (home BG testing, HbA1c, OGTT, CGM) and offered diabetes education, psychological support and participation in prevention trials. Those persistently TGA positive were referred to clinical services including intestinal biopsy and/or additional celiac autoantibody testing. Since July 2020, history of SARS-CoV-2 infection and immunization was collected from all study participants. In addition, antibodies to SARS-CoV-2 spike protein receptor binding domain and nucleocapsid protein were measured by ECL to distinguish response to infection from that to immunization.
Results: Presymptomatic T1D was detected in 0.97% of children (95%CI 0.86-1.09%), including 0.44% with multiple IA and 0.53% with a single high-affinity (ECL+) IA predicting, respectively, a 44% and 29% 5-y risk of clinical T1D. Multiple IA were more frequent in non-Hispanic whites than Hispanics (OR=1.81; 1.25-2.62, p=0.001) and in FDRs than non-FDRs (OR=3.64; 2.29-5.79, p<0.0001), adjusting for age, sex, and family history. However, only 14% of children with presymptomatic T1D were FDRs. During follow-up 44 children who initially screened positive progressed to clinical T1D; only 2/41 (5%) of those who completed a confirmation and enrolled in monitoring developed DKA. Overall, 764 children 2.60% (2.42-2.78%) of children without a previous diagnosis of CD screened positive for TGA. Of those, 81% were confirmed persistently positive and 197 have been diagnosed with CD, so far. Among 4717 children screened in 7/2020-12/2021,1524 had serologic evidence of SARS-CoV-2 infection. The infection was not associated with presymptomatic T1D (OR 1.50; .80-2.78) or TGA (OR=1.02, .63-1.59; p=0.95) adjusting for age, sex, race/ethnicity, and family history of, respectively T1D or CD.
Conclusion: This population-based screening program found a high prevalence of presymptomatic T1D and CD in Colorado children. There was no association between SARS-CoV-2 infection and the presence of autoantibodies. Clinical monitoring and education of children detected by screening reduced DKA at diagnosis to <5% and facilitated diagnosis of CD.
Supported by: JDRF 2-SRA-2021-1065-M-N
Disclosure: M. Rewers: Grants; JDRF 2-SRA-2021-1065-M-N.
314
Coxsackie virus protease 2A alters the sorting of insulin secretory granule cargoes and induces the premature activation of cathepsins
K.-P. Knoch1, A. Kosok1, Z. Marinicova1, I. Kalaidzidis1, Y. Kalaidzidis2, A. Müller1, A. Sönmez1, C. Wegbrod1, K. Ganss1, M. Solimena1;
1Paul Langerhans Institute Dresden of the Helmholtz Center Munich at the Univ. Hospital and Medical F, 2Max Planck Institute of Molecular Cell Biology, Dresden, Germany.
Background and aims: Enteroviruses (EVs), including Coxsackieviruses B5 (CVB5), have been implicated in the pathogenesis of type 1 diabetes (T1D). CVB5-infection of β-cells inhibits the cap-dependent translation of host proteins, but not the cap-independent translation of secretory granule (SG) cargoes such as insulin, ICA512/IA-2/PTPRN, PC1/3, PC2 and CgA. Several of these proteins are major targets of humoral and/or T-cell autoimmunity in T1D. Nonetheless, in CVB5-infected β-cells mature SG cargoes are depleted due to intracellular degradation. Previous studies showed that CVB5 protease 2A is responsible for this phenotype. Proteomic analysis of CVB5 2A-transfected MIN6 insulinoma cells revealed the reduction of several factors involved in post-Golgi vesicular transport, such as Arf1, GGA2, Rab3b and Sytl4. However, only the knockdown of Rab3b and GGA2 generated the phenotype observed upon overexpression of CVB5 2A. Here we investigated the impact of GGA2 depletion on the sorting and maturation of SG proteins and Cathepsins.
Materials and methods: Mouse insulinoma MIN6 cells were transfected with either CVB5 protease 2A or 3C or infected with CVB5. Levels of insulin and other SG cargoes in control and CVB expressing cells were assessed by western blotting, ELISA, immunostaining and quantitative proteomics. Gga2-/- MIN6 cells were generated using CRISPR/Cas9 technology. Cathepsin B and D activity was measured by fluorometric assay. Golgi enriched fractions were isolated from cell extracts by discontinuous sucrose density gradients. The luminal pH in different compartments along the secretory pathway was measured with CFP-reporters and fluorescence life imaging microscopy.
Results: Confocal and live-time microscopy showed that in Gga2-/- MIN6 cells exit of SG cargoes and cathepsins from the TGN is delayed. GGA2 depletion was also associated with a significant drop of the pH in the lumen of the Golgi complex, and especially in the late Golgi and the TGN. Similar phenotypic changes were observed in CVB5 2A-overexpressing MIN6 cells. Furthermore, the activity of cathepsin B and D in Golgi fractions isolated from Gga2-/- MIN6 cells was increased.
Conclusion: Our data suggests the following scenario: CVB 2A-induced acidification of the late Golgi and TGN results in the premature activation of cathepsins already in these compartments. This could foster the degradation of incoming SG cargoes and the generation of new SG cargoes-derived antigenic peptides. The abundance of the latter together with the altered pH could favour their competitive displacement of peptides already preloaded on in-transit MHC class I molecules, thus altering the antigen repertoire presented by CVB5/EV-infected β-cells.
Supported by: IMI-INNODIA, DZD
Disclosure: K. Knoch: None.
315
No association between incidence of type 1 diabetes and rotavirus vaccination in Swedish children
A. Rangert1, C. Oldin2, M. Golsäter2, J. Ludvigsson1, K. Åkesson1;
1Department of Biomedical and Clinical Sciences, Linköping university, Linköping, 2Jönköping County council, Jönköping, Sweden.
Background and aims: Rotavirus may trigger type 1 diabetes (T1D) and rotavirus vaccination is hypothesized to decrease the incidence of T1D. In Sweden, rotavirus vaccination was introduced in 2014 in two regions, in some regions 2016-2018 and from 2019 in all Sweden. The aim of the study was to investigate whether rotavirus vaccination is associated with a change in incidence and clinical manifestation of T1D.
Materials and methods: All Swedish children <15 years of age (n= 7893) diagnosed with T1D between 2009 and 2019 were retrieved from the SWEDIABKIDS database. Vaccine coverage was collected from Child Health Services. Three vaccine groups were created: I: Start 2014; II: Gradual start 2016-2018; III: No vaccination. Incidence of T1D before rotavirus vaccine (2009-2014) was compared to incidence rates after (2015-2019).
Results: The mean incidence of type 1 diabetes for children <15 years was 42·61 cases per 100 000 persons during the observed period. The incidence rate ratio (IRR) was 0·86 for children <5years in group I (received vaccine), (p=0·10) when comparing the years before and after 2014. IRR was 0·88 (p=0·14) respectively 0·85 (p=0·02) for children in the same age in two other groups II, III. A similar IRR reduction was also seen among older children who received no vaccine in both group I (children 10-14·9 years: IRR =0·84, p=0·02) and in group III (children 5-9·9 years: IRR=0·85, p=<0·01). We found no differences in clinical manifestation at onset associated with rotavirus vaccination.
Conclusion: There is no association between incidence or clinical manifestation of type 1 diabetes and rotavirus vaccination in children.
Disclosure: A. Rangert: None.
316
The influence of glucocorticoid therapy on glucose metrics monitored by continuous glucose monitoring in hospitalised patients with diabetes and infection
M.T. Olsen1, C.K. Klarskov1, A.K. Jensen2, N.A. Windum1, B. Lindegaard3, P.L. Kristensen1;
1Department of Endocrinology and Nephrology, Copenhagen University Hospital – North Zealand, Hilleroed, 2Department of Public Health, University of Copenhagen, Biostatistics, Copenhagen, 3Department of Pulmonary- and Infectious Diseases, Copenhagen University Hospital – North Zealand, Hilleroed, Denmark.
Background and aims: Infections and glucocorticoids (GCs) might induce hyperglycemia, however, it is unclear if the severity of infection influences glucocorticoid-induced hyperglycemia or vice versa. This study aimed to assess the effect of infection severity and GC treatment on glucose levels in hospitalized patients with diabetes mellitus (DM) using continuous glucose monitoring (CGM).
Materials and methods: Data were collected in a randomized controlled trial, CGM-ISO, which included hospitalized patients with DM and infection from May 2020 until February 2021. The primary endpoint was to describe daytime and nighttime glucose trajectories during treatment with prednisolone, dexamethasone, or no GC treatment when infection severity was low defined by C-reactive protein (CRP) <43.5mg//L or high CRP≥43.5mg/L. Glucose data were obtained by the Dexcom G6 CGM. The CGM data were divided into days with and without GC treatment. A hierarchical Gaussian Process regression analysis was built to determine the influence of GCs and CRP levels on glucose levels adjusted for glucose-lowering medication (daily insulin dose). Postprandial glucose excursions (PPGE) were also assessed for days with and without GCs.
Results: Eighty-seven subjects were included in the analysis and 63% received GCs. In total, 210 days without GCs and 169 days with GCs (19 with prednisolone and 150 with dexamethasone) were analyzed. GC treatment was associated with increased glucose levels, especially during the daytime. High CRP levels increased glucose levels independently of GC treatment by approx. 1 mmol/L (18 mg/dL). On days with dexamethasone treatment, high CRP levels increased glucose levels by approx. 1 mmol/L (18 mg/dL) compared to days with dexamethasone treatment and low CRP levels. On days with prednisolone treatment, high CRP levels increased glucose levels by approx. 6 mmol/L (110 mg/dL) compared to days with prednisolone treatment and low CRP levels. Mean PPGE (95% CI) after lunch were significantly higher during prednisolone treatment of 5.2 (3.5 to 6.9) mmol/L (94 (63 to 124) mg/dL) and dexamethasone treatment of 3.6 (3.1 to 4.1) mmol/L (65 (56 to 74) mg/dL) compared to no GC treatment of 2.0 (1.7 to 2.4) mmol/L (36 (31 to 43) mg/dL), but not at breakfast or dinner. See Figure 2.
Conclusion: GC treatment and high CRP levels are associated with increased daytime glucose levels, especially during prednisolone treatment. However, the effect of high CRP on glucose levels was surprisingly low during dexamethasone treatment. PPGEs were significantly higher during GC treatment at lunch (compared to no GC treatment), but not at breakfast and dinner.
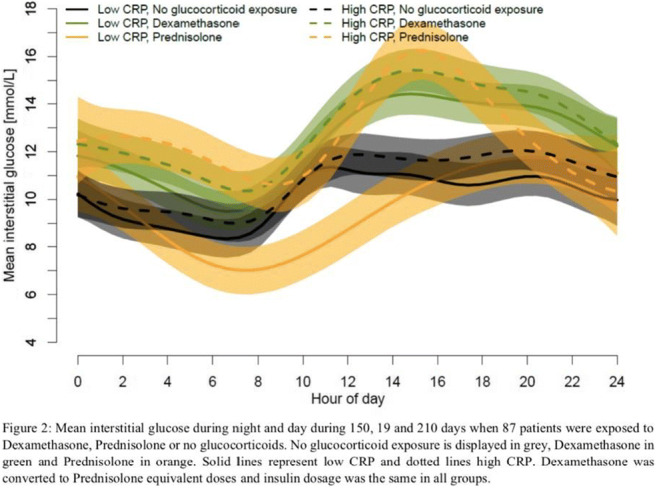
Clinical Trial Registration Number: #NCT04430608
Supported by: NNF and GLFFF
Disclosure: M.T. Olsen: None.
SO 08 Benefits of a healthy lifestyle
317
Incidence of type 2 diabetes and factors associated with it in the PPP-Botnia Study
V. Ahuja1, L. Hakaste1,2, O.P. Dwivedi1, M. Lehtovirta1, B. Isomaa2,3, K. Lahti4, T. Tuomi1,5;
1Institute for Molecular Medicine Finland, Helsinki, 2Folkhälsan Research Center, Helsinki, 3Jakobstad Hospital, Jakobstad, 4Vasa Central Hospital, Vasa, 5Abdominal Center, Endocrinology, Helsinki University Central Hospital, Helsinki, Finland.
Background and aims: Our objective was to estimate the incidence of type 2 diabetes (T2D) and associated risk factors in the PPP-Botnia Study (Prevalence, Prediction, Prevention).
Materials and methods: The PPP-Botnia Study is a population-based prospective cohort study in Western Finland with 18-75-year-old participants randomly invited from the Finnish Population Registry in 2004-2008 (baseline study), first follow-up was conducted in 2011-2014 (the second study was started in 2018). Of 5208 baseline participants, 4844 were non-diabetic [mean age (SD) 48.97 (14.66) years; 54% women] with 24360 person-years of follow-up until the first follow-up visit (N=3850; 188 died before it). There were 153 incident cases of T2D. The subjects participated in a 2-hour oral glucose tolerance test. We used Poisson regression to obtain the crude and adjusted incidence of T2D and to evaluate the associated baseline risk factors.
Results: The crude and age-sex adjusted incidence rate ratio (IRR) for T2D with 95% CI were 6.28 (5.34-7.33) and 7.61 (4.53-12.51) per 1000 person-years. In multivariable analysis (Table) using standardized predictors, impaired fasting glycaemia/glucose tolerance (IFG/IGT) compared with normal tolerance (IRR 3.35, 95% CI 2.23-5.08), age (IRR 1.60, 95% CI 1.27-2.02), and BMI (IRR 1.30, 95% CI 1.09-1.55) significantly associated with incident T2D. Protection against T2D was independently associated with good insulin secretion evaluated by the disposition index (IRR 0.61, 95% CI 0.53-0.72) and insulin sensitivity evaluated by the Matsuda index (IRR 0.81, 95% CI 0.66-0.99). Male sex (IRR 1.41, 95% CI 1.00-1.99), family history of diabetes (IRR 1.41, 95% CI 0.99-1.98), and glutamic acid decarboxylase antibodies (IRR 1.34, 95% CI 0.40-3.30) did not reach statistical significance as predictors of incident T2D.
Conclusion: Besides abnormal glucose tolerance at baseline, age was the strongest predictor of T2D, and good beta-cell function was strongly associated with protection. While recorded family history was not a significant risk factor, in the future we will substitute it with polygenic risk scores for T2D, T1D, and the contributing traits.
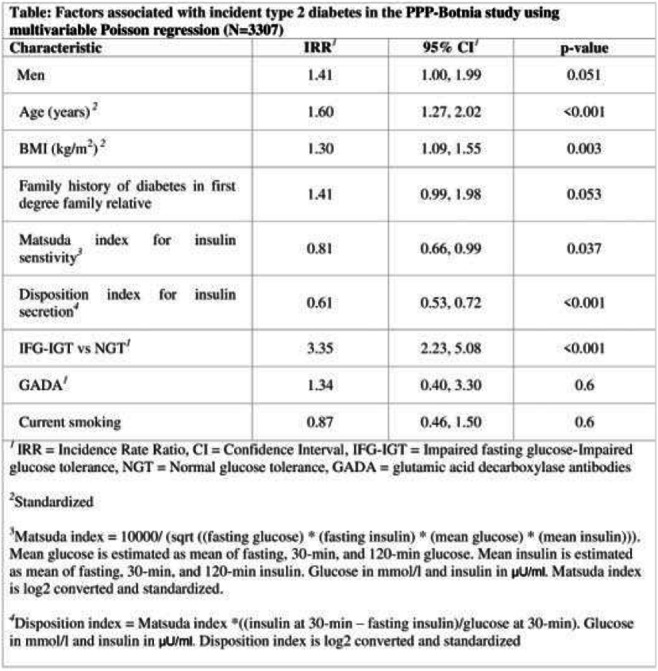
Supported by: SJF, FRF, EXGENESIS, SAGF, SCFF, FDRF, FLHF, FMS, PNF, HUCHRF, PF, OF, NHCF,
Disclosure: V. Ahuja: None.
318
Lifestyle risk factors and metabolic markers of cardiovascular diseases in Bangladeshi rural-to-urban male migrants compared with their non-migrant siblings
S.J. Mumu1,2, D. Merom1;
1Western Sydney University, 2Torrens University Australia, Sydney, Australia.
Background and aims: The increasing prevalence of cardiovascular diseases (CVDs) in developing countries like Bangladesh has been linked to progressive urbanisation. Comparisons of rural and urban populations often find a higher prevalence of CVD risk factors in the urban population, but rural-to-urban migrants might have different CVD risk profiles than either rural or urban residents. This study aimed to describe differences in CVD risk factors between migrants and non-migrants siblings and to determine whether acculturation factors were associated with CVD risk factors among migrants.
Materials and methods: Using a sibling-pair comparative study, 164 male migrant who migrated from Pirganj rural areas to Dhaka City of Bangladesh and their rural siblings (total n=328) were assessed by interview, anthropometric measurement, blood pressure and blood samples for OGTT and lipid profile. Comparisons were made using linear or logistic mixed effects models. The association between length of urban residence, categorised into tertiles, and CVD risk factors were assessed by logistic regression model.
Results: Physical inactivity, inadequate intake of fruit and vegetables and possible existence of a mental health disorder had 3.3 (1.73; 6.16), 4.3 (2.32; 7.92) and 2.9 (1.37; 6.27) times higher odds among migrants than their rural siblings, respectively. Migrants watched television on average 20 minutes (95% CI 6.17-35.08 min/day) more per day than the rural sibling group whereas PUFA intake, fruit and vegetable and fish intake of the migrants were -5.31 gm/day (-6.91; -3.70), -21.64 serving/week (-28.20; -15.09), -14.10 serving/week (-18.32; -9.87) lower than that of the rural siblings. No significant difference was observed for other variables. For acculturation, only one-third migrants use local dialect to communicate with their spouse, children and friends. When migrants were asked if their dietary habits changed since migrating the city, most of them reported increasing consumption of different unhealthy foods (60% to 76%) and more than one-third believed they were less active than before migration. After adjusting, the risk of physical inactivity (p for trend, 0.001), inadequate fruit and vegetable intake (p for trend, <0.001), a mental health disorder (p for trend, 0.009) and low HDL (p for trend, <0.001) were tended to be higher for each increasing tertile of urban life exposure in migrants. However, diabetes was 5.5 times higher in migrants than rural siblings in the first tertile of urban life exposure, but no clear pattern of higher diabetes risk was found in the following tertiles.
Conclusion: The findings suggest that migration from rural-to-urban environment increases CVD risk which exacerbate with time spent in urban area due to acculturation.
Supported by: MoE, Bangladesh and WSU
Disclosure: S.J. Mumu: None.
319
Does health-related lifestyle modify the association between type 2 diabetes and dementia risk? Finding from UK Biobank prospective cohort study
J. Boonpor1, J. Xing1, S.R. Gray1, F.K. Ho2, C. Celis-Morales1,3;
1Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK, 2Institute of Health and Wellbeing, University of Glasgow, Glasgow, UK, 3Human Performance Lab, Education, Physical Activity and Health Research Unit, Universidad Católica del Maule, Talca, Chile.
Background and aims: Unhealthy lifestyle and type 2 diabetes (T2D) accounts for a sizable increased risk of dementia. However, whether lifestyle could modify the association between T2D and dementia risk remains unclear. Therefore, this study aimed to investigate to what extent the association between T2D and dementia risk is modified by lifestyle.
Materials and methods: 369,627 participants of UK Biobank (mean age 55.6 years; 54.6% women) were included in this prospective study. All-cause dementia was the outcome. T2D was self-reported. A lifestyle was derived from television viewing time, sleep duration, physical activity, alcohol consumption, and dietary intake of processed and red meat, fruit and vegetable, oily fish, and smoking status. The score was then categorised into most-, moderate-and least healthy. Cox-proportional hazard models investigated the association between lifestyle scores, T2D and dementia.
Results: The median follow-up was 9.1 (IQR: 8.3-9.7) years after excluding the first two years of follow-up. Compared to non-T2D, T2D was associated with a 28% higher risk of all-cause dementia (HR 1.28 [95% CI 1.11, 1.47]). Those with the least healthy lifestyle had a 91% (HR 1.91 [95% CI 1.25, 2.91]) higher risk of all-cause dementia compared to those with the healthiest lifestyle. Compared to individuals without T2D and the healthiest lifestyle, those without and with T2D but who have the least healthy lifestyle score had 36% (HR 1.36 [95% CI: 1.09, 1.21]) and 142% (HR: 2.42 [95% CI 1.67, 3.50]) higher risk of all-cause dementia, respectively.
Conclusion: The associations between T2D and dementia risk are accentuated by poor lifestyle behaviours. This highlights that maintaining a healthy lifestyle, especially in people with T2D, could reduce dementia risk.
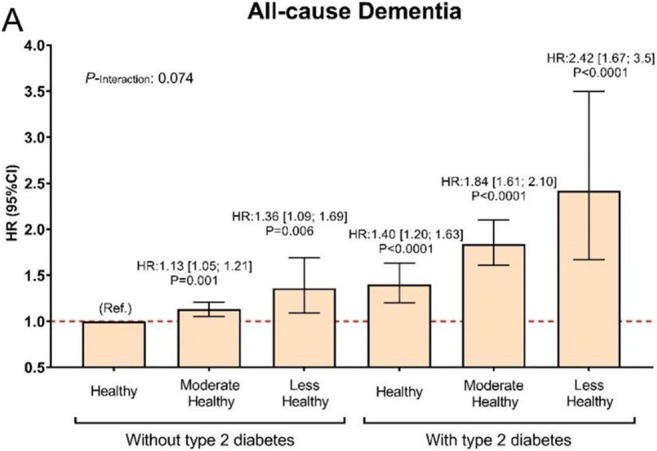
Clinical Trial Registration Number: Ref 11/NW/0382 on June 17, 2011
Disclosure: J. Boonpor: Other; The Royal Thai Government Scholarship for JB's PhD study.
320
Lifestyle behaviour patterns and incident type 2 diabetes in the Dutch Lifelines cohort study
M.F. Duan1, L.H. Dekker1,2, J.-J. Carrero3, G. Navis1;
1Internal Medicine, University Medical Center Groningen, Groningen, Netherlands, 2National Institute for Public Health and the Environment, Bilthoven, Netherlands, 3Medical Epidemiology and Biostatistics, Karolinska Institutet, Solna, Sweden.
Background and aims: It has been suggested that lifestyle factors tended to cluster in certain behavioural patterns. However, evidence is lacking how these lifestyle factors cluster in the general population, and how these behavioural patterns are related to incident type 2 diabetes. We therefore aimed to investigate how lifestyle behaviours clustered in the general population and how these lifestyle patterns were associated with incident type 2 diabetes.
Materials and methods: Using data from the Dutch Lifelines cohort study, latent class analysis was performed to derive lifestyle patterns on five lifestyle factors, i.e. smoking, diet quality, TV watching, physical activity level and risk drinking. Associations between lifestyle patterns and incident type 2 diabetes were estimated.
Results: Among 61,869 participants analysed, we identified 900 cases of type 2 diabetes during follow-up (205,696 person-years; incidence rate 4.38 per 1000 person-years). Five groups of individuals with distinct lifestyle behavioural patterns were revealed. Using the “healthy lifestyle group” (characterised by minimal lifestyle risk behaviours) as reference, the “unhealthy lifestyle group” (characterised by maximal risk behaviours) had the highest risk for type 2 diabetes (HR 1.63 [95%CI 1.37, 1.93]), followed by the “poor diet and low physical activity group” (HR 1.21 [95%CI 1.00, 1.46]) and the “risk drinker group” (HR 1.11 [95%CI 0.83, 1.47]), while the “couch potato group” (characterised by excessive TV watching) showed no elevated risk. These models were adjusted for age, sex, total energy intake, education, BMI and family history of diabetes.
Conclusion: Our study shows that lifestyle factors tended to cluster in unique behavioural patterns within the heterogeneous population. These behavioural patterns were differentially associated with incident type 2 diabetes. Our findings provide the important evidence basis for designing more tailored intervention strategies for diabetes prevention, which can be manifested through targeting multiple lifestyle risk factors simultaneously at an intermediate level.
Supported by: European Union Horizon 2020
Disclosure: M.F. Duan: None.
321
DNA methylation patterns reflect individual lifestyle independent from obesity
I. Klemp1, A. Hoffmann2, L. Müller1, T. Hagemann2, K. Horn3, J. Thiery4, M. Löffler3, R. Burkhardt5, M. Blüher1,2, K. Krohn6, M. Scholz3,7, R. Baber6,7, P.W. Franks8, P. Kovacs1, M. Keller1,2;
1Medical Department III – Endocrinology, Nephrology, Rheumatology, University of Leipzig, Leipzig, Germany, 2University of Leipzig and University Hospital Leipzig, Helmholtz Institute for Metabolic, Obesity and Vascular Research (HI-MAG), Leipzig, Germany, 3Institute for Medical Informatics, Statistics and Epidemiology, Leipzig, Germany, 4Medical Faculty, Kiel, Germany, 5Institute of Clinical Chemistry and Laboratory Medicine, Regensburg, Germany, 6Medical Faculty, Leipzig, Germany, 7LIFE Leipzig Research Center for Civilization Diseases, Leipzig, Germany, 8Department of Clinical Sciences, Genetic and Molecular Epidemiology Unit, Malmö, Sweden.
Background and aims: Obesity is driven by modifiable lifestyle factors whose effects may be mediated by epigenetics. Therefore, we investigated lifestyle effects on blood DNA methylation in participants of the LIFE-Adult study, a well-characterized population-based cohort from Germany.
Materials and methods: Lifestyle Scores (LS) based on diet, physical activity, smoking and alcohol intake were calculated in 4107 participants of the LIFE-Adult study. Fifty subjects with an extremely healthy and 50 with an extremely unhealthy lifestyle (5th and 95th pct. LS) were selected for genome-wide DNA methylation analysis in blood samples employing Illumina EPIC technology
Results: Differences in DNA methylation pattern between BMI groups (<25 vs. >30kg/ m2) were rather marginal compared to inter-lifestyle differences (0 vs. 145 DMPs), which identified 4682 differentially methylated regions (DMRs; FDR<5%) annotated to 4426 unique genes. A DMR annotated to the Glutamine-Fructose-6-Phosphate Transaminase 2 (GFPT2) locus showed the strongest hypo-methylation (~6.9%) and one annotated to Glutamate Rich 1 (ERICH1) the strongest hyper-methylation (~5.4%) in healthy compared to unhealthy lifestyle individuals. Intersection analysis showed that diet, physical activity, smoking and alcohol intake are equally contributing to the observed differences, which affects, amongst others, particularly pathways related to glutamatergic synapse (adj.P<0.01) and axon guidance (adj.P<0.05). We showed that methylation age correlates with chronological age and waist-to-hip ratio with lower DNAmAge acceleration distances in participants with healthy lifestyle. Finally, two identified top DMPs for the Alanyl Aminopeptidase (ANPEP) locus also showed the strongest expression quantitative trait methylation (eQTM) in blood.
Conclusion: DNA methylation patterns help discriminate individuals with a healthy vs. unhealthy lifestyle, which may mask subtle methylation differences derived from obesity.
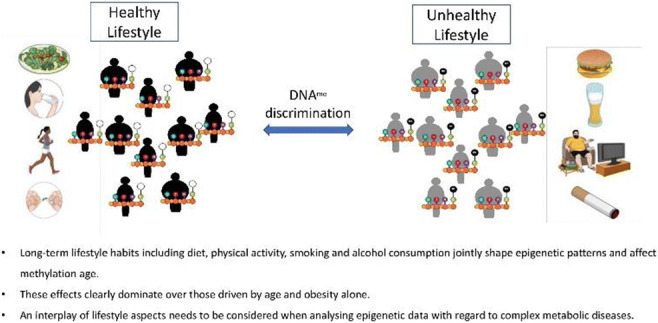
Supported by: DFG, DDG, medical faculty UKL, DZD, Free state of Saxony
Disclosure: I. Klemp: None.
322
Associations of physical activity and sedentary time with indices of beta cell function and insulin resistance in type 2 diabetes
S.L. Domazet1, J. Tarp2, J.V. Stidsen1, R.W. Thomsen2, K. Højlund1, A. Groentved3, J.C. Broend3, J.S. Nielsen1;
1Clinical Research, Steno Diabetes Center Odense, Odense, 2Aarhus University, Department of Clinical Epidemiology, Aarhus, 3Department of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark.
Background and aims: The aim of this study was to investigate the associations between levels of total and intensity-specific physical activity and sedentary time with HOMA indices of beta-cell function and insulin resistance in persons with newly diagnosed type 2 diabetes (<2 years). To study the associations further, we divided the study sample into predefined pathophysiological sub-phenotypes according to beta-cell function and insulin resistance.
Materials and methods: We measured physical activity and sedentary time by dual monitor accelerometry in 765 persons with type 2 diabetes. We calculated HOMA indices and defined groups of sub-phenotypes according to low HOMA-β/high HOMA-S (insulinopenic), low HOMA-β/low HOMA-S (classic) and high HOMA-β/low HOMA-S (hyperinsulinaemic).
Results: We conducted multiple linear regression analysis with measures of time spent in total, light, moderate and vigorous physical activity and time being sedentary as explanatory variables while controlling for age and sex. An additional 10 minutes/day of light and moderate to vigorous physical activity was associated with a 0.6% (95% CI 0.3%; 0.9%) and 1.9% (95% CI 1.3%; 2.6%) higher insulin sensitivity, respectively. For each additional 1,000 steps/day insulin sensitivity was 2% (95% CI 1.0%; 3.0%) higher. Conversely, a higher physical activity level was associated with lower beta-cell function (-1.2%;-2.9%, p<0.01), while more sedentary time was associated with higher beta-cell function (0.7%, 95% CI 0.3%; 1.0%). We further regressed our predefined groups of sub-phenotypes on physical activity and sedentary time. Compared with the classic group (reference), the hyperinsulinaemic group was significantly more sedentary (24.0 min/day 95% CI 8.8; 39.2) and spent less time in light (-14.6 min/day, 95% CI -23.5; -5.8) and moderate to vigorous physical activity (-6.1 min/day, 95% CI -10.4; -1.8). The insulinopenic group was significantly more active than the other two groups (p<0.01). When we further adjusted our regression models for awake time, physical and mental well-being, comorbidities, smoking and BMI, we found that the hyperinsulinaemic group was no longer different from the classic group with respect to activity level, whereas the insulinopenic group remained less sedentary and more active than both the classic and the hyperinsulinaemic group (p<0.01).
Conclusion: These findings suggest that a higher degree of insulin resistance/hyperinsulinemia is associated with more sedentary time and lower physical activity levels independent of physical and mental health, current smoking and BMI. The observed inverse association between physical activity and beta-cell function might support evidence stressing that beta-cell function is less affected by exercise. Although, a different disease etiology or genetic profile in combination with healthier lifestyle might as well explain the high physical activity level in persons with very low beta-cell function.
Clinical Trial Registration Number: NCT02015130
Supported by: Danish Agency for Science. The Novo Nordisk Foundation.
Disclosure: S.L. Domazet: None.
323
Cluster randomised trial to investigate the impact of applying a diabetes risk score in primary care on change in physical activity
E. Seidel-Jacobs1,2, F. Kohl3, M. Tamayo4, J. Rosenbauer1,2, M.B. Schulze2,5, O. Kuss1,2, W. Rathmann1,2;
1Institute for Biometrics and Epidemiology, German Diabetes Center (DDZ), Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf, Düsseldorf, 2German Center for Diabetes Research (DZD), München-Neuherberg, 3Institute for Occupational, Social and Environmental Medicine, Centre for Health and Society, Faculty of Medicine, Heinrich Heine University Düsseldorf, Düsseldorf, 4The Association of Statutory Health Insurance Physicians North Rhine, Düsseldorf, 5Department of Molecular Epidemiology, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany.
Background and aims: Diabetes risk scores are an adequate tool for identifying people at increased risk of diabetes, but little evidence exists on the impact of diabetes risk scores on health behaviour in clinical practice. The aim of this study was to investigate the effect of applying a non-invasive diabetes risk score in primary care as component of routine health checks on self-reported physical activity.
Materials and methods: A cluster randomised trial was conducted, in which primary care physicians (PCPs), randomised (1:1) by minimisation to an intervention and control group, enrolled participants without known diabetes, ≥35 years of age with a BMI ≥27.0 kg/m2 in North-Rhine-Westphalia (Western Germany). The intervention was the application of the non-invasive validated German Diabetes Risk Score as add-on to a standard routine health check. Primary outcome was the difference in participant’s physical activity (International Physical Activity Questionnaires (IPAQ)) after 12 months between the intervention and control group. Secondary outcomes were differences in BMI, waist circumference, perceived health, anxiety, depression, and motivation for lifestyle change. Linear (for continuous outcomes) or generalized linear with a logit link (for binary outcomes) mixed models including a random intercept to adjust for the cluster effect were fitted to assess the intervention effects.
Results: Three hundred fifteen participants (intervention: n=153, mean age 54 ± 8.1, 50% men; controls: n= 162, mean age 49 ± 8.0, 64% men) were recruited by 30 PCPs (intervention: n=16; control: n=14). A slight increase in physical activity was observed in the intervention group with an adjusted mean change of 388 (95% CI: -235; 1,011) metabolic equivalents minutes per week. There were only minimal differences in BMI, waist circumference, and motivation for lifestyle change. In both groups, constant levels of perceived health, anxiety and depression were observed over 12 months.
Conclusion: The use of a diabetes risk scores alone is not effective in promoting physical activity under real life routine practice conditions. Therefore, more support is needed for people at risk to develop type 2 diabetes to increase their motivation, to change lifestyle, and to increase physical activity.
Clinical Trial Registration Number: NCT03234322, ClinicalTrials.gov
Supported by: German Federal Ministry of Education and Research, BMBF to German Center for Diabetes Research, DZD
Disclosure: E. Seidel-Jacobs: None.
324
Accelerometer-derived physical activity and sedentary time and cardiac biomarkers: the Maastricht Study
E.J. Vandercappellen1, A. Koster2, R.M.A. Henry1, C.J.H. van der Kallen1, N.C. Schaper1, H.H.C. Savelberg3, S.J.P. Eussen4, P.C. Dagnelie1, M.T. Schram1, M.M.J. van Greevenbroek1, A. Wesselius5, S.J.R. Meex6, J.P. Kooman1, A.A. Kroon1, C.D.A. Stehouwer1;
1Internal Medicine, Maastricht University Medical Centre, 2Social medicine, Maastricht University, 3Nutrition and movement science, Maastricht University, 4Epidemiology, Maastricht University, 5Complex genetics and epidemiology, Maastricht University, 6Clinical chemistry, Maastricht University Medical Centre, Maastricht, Netherlands.
Background and aims: Cardiac troponins and NT-proBNP are biomarkers of cardiac injury that are used clinically in the diagnosis of myocardial infarction and heart failure. Type 2 diabetes is associated with chronically elevated cardiac biomarkers, which in turn are associated with adverse outcomes like lower event-free survival. It is not known whether the amount, types and patterns of physical activity and sedentary behaviour are associated with levels of cardiac biomarkers. We investigated these relationships in people without and with type 2 diabetes.
Materials and methods: In the population-based Maastricht Study (n=2370, 51.3% male, 28.3% T2D, 15.2% prediabetes) we determined cardiac biomarkers (hs-cTnI, hs-cTnT and NT-proBNP). Physical activity and sedentary time were measured by the ActivPAL activity monitor and divided into quartiles (quartile 1 (Q1) served as reference). The weekly pattern of moderate-to-vigorous physical activity and coefficient of variation (CV) was calculated. Linear regression analyses were conducted with adjustment for demographic, lifestyle, and cardiovascular risk factors.
Results: Higher amounts of total physical activity were associated with lower levels of hs-cTnI (Q2) and hs-cTnT (Q2). Higher levels of light intensity physical activity were associated with lower levels of hs-cTnI (Q2 and Q3) and with higher levels of hs-cTnT (Q4). Additionally, those with the highest levels of vigorous intensity physical activity had significantly higher levels of hs-cTnI and lower levels of NT-proBNP. Compared to the least sedentary, those in Q3 had significantly lower levels of hs-cTnI and individuals in Q2 and Q3 had lower levels of hs-cTnT. With regard to physical activity patterns, so-called weekend warriors and regularly actives (both groups had ≥150min of moderate-to-vigorous physical activity/week) had lower levels of NT-proBNP but not with hs-cTnI and hs-cTnT compared with inactives. A higher weekly moderate-to-vigorous physical activity CV (indicating more irregular activity) was associated with lower levels of hs-cTnI and higher levels of NT-proBNP, but not with hs-cTnT. Similar associations were found in those with and without type 2 diabetes.
Conclusion: Physical activity and sedentary time had complex associations with cardiac biomarkers. However, a minimum of 150 minutes of moderate-to-vigorous physical activity was associated with less NT-proBNP. Additionally, there was an association between the CV and NT-proBNP, implying that more regularity of moderate-to-vigorous physical activity during the week associates with lower levels of NT-proBNP. Our data suggest that performing at least 150min of moderate-to-vigorous physical activity equally divided over the week reduces NT-proBNP in individuals with and without type 2 diabetes.
Supported by: This project was partly funded through an EFSD award supported by AstraZeneca.
Disclosure: E.J. Vandercappellen: Grants; This project was partly funded through an EFSD award supported by AstraZeneca.
SO 09 Type 1 diabetes: from molecules to treatment
325
Automatising immune phenotyping of type 1 diabetes samples using a machine learning workflow
J.A. Vera-Ramos1, B. Prietl1,2, L. Herbsthofer2, P. López-García2, V. Pfeifer2, T. Pieber1,2;
1Institute of Endocrinology and Diabetology, Medical University of Graz, 2CBmed GmbH - Center for Biomarker Research, Graz, Austria.
Background and aims: Autoimmune diseases share common mechanisms leading to a self-tolerance breakdown, suggesting the possibility of finding similar patterns in different diseases. These patterns can be helpful to understand disease progression and the recovery of patients. We aimed to find such patterns by inspecting the cell population distributions of samples from patients of different autoimmune diseases.
Materials and methods: We used machine learning (ML) to perform deep immune phenotyping of type 1 diabetes (T1D) and healthy controls to identify common patterns and dissimilarities among different autoimmune diseases. To this end, PBMCs were isolated from patients with T1D (n=69) and healthy donors (n=69). A flow cytometry approach was applied, using two different marker panels, focusing on CD4pos T cell populations. Then, a manual gating analysis was compared to a ML method implemented in R. The ML pipeline includes unsupervised pre-gating of lymphocytes using the R package flowCore, a data normalization step to improve the performance of the model, and FlowSOM clustering to group cells based on similarities in their marker expression using Self Organizing Maps (SOM). An intermediate step is done to filter samples that have a low recount of cells after filtering for CD3+CD4+ T cells using flowCore. Cell population distributions were tested for significant differences between T1D samples and controls using MANOVA.
Results: After applying our automated workflow on two of the panels focusing on T cells we could identify 14 cell clusters on the first panel and 16 clusters on the second one. The MANOVA test on panel 1 revealed several clusters significantly different on T1D samples: (i) a group of clusters defined by CD4pos T cells expressing high amounts of CD25 and FoxP3 and low amounts of Ki67 (p=0.02, p=0.003, p=0.01); and (ii) CD4pos T cells expressing high amounts of Ki67 and CD15s (p=0.005, p=0.002). On the second panel, we found a trending cluster defined by CD4pos T cells with high expression of CD127 and CD307c, median amounts of CD366, LAP, PI-16, and CD45RA, and low amounts of CD95, CD147, CD25, and HLA-DR (p=0.05).
Conclusion: In conclusion, our high-throughput ML workflow is able to determine the cell population profile of many samples efficiently, and identify differences in the cell population abundance of different groups. This unsupervised analysis approach enables the discovery of new biomarkers complementing traditional workflows and streamlines the profiling of large datasets, for which conventional analysis is unfeasible. In the future, we plan to apply this workflow to large clinical studies to automatically classify and compare cell populations of not only T1D patients but other autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus.
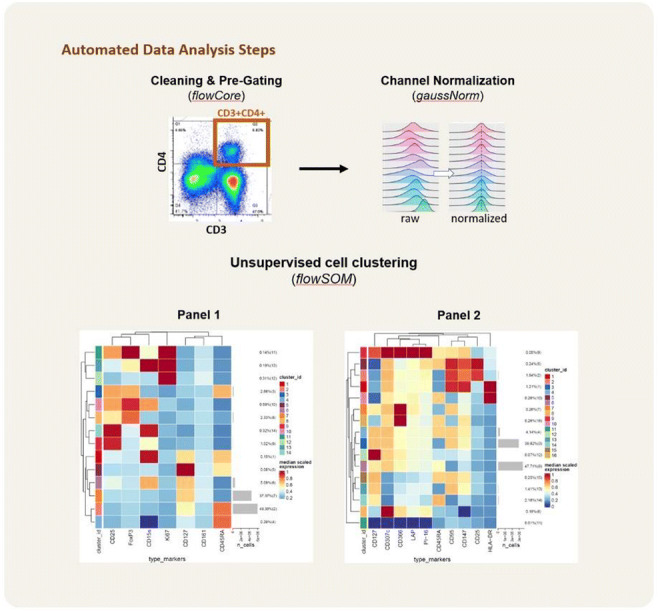
Supported by: JDRF
Disclosure: J.A. Vera-Ramos: Grants; JDRF.
326
Transcription and splicing regulation by NLRC5 shape the interferon response in human pancreatic beta cells
F. Szymczak1, M. Alvelos1, S. Marín-Cañas1, Â. Castela1, S. Demine2, M.L. Colli1, A. Op de Beeck1, L. Marselli3, P. Marchetti3, D.L. Eizirik1;
1ULB Center for Diabetes Research, Université Libre de Bruxelles, Anderlecht, Belgium, 2Indiana Biosciences Research Institute, Indianapolis, USA, 3Islet Cell Laboratory, University of Pisa, Pisa, Italy.
Background and aims: IFNα is a key regulator of the dialogue between pancreatic beta-cells and the immune system in early type 1 diabetes (T1D) via up-regulation of HLA class I and neoantigen presentation, but the molecular mechanisms regulating HLA class I expression in beta-cells remain to be clarified. To answer this question, we exposed islet-like cells derived from induced pluripotent stem cells (iPSC) - akin to the cells of a neonate - to IFNα and performed bulk and single-cell RNA-sequencing (RNA-seq) to unveil IFNα’s effect on global gene expression during the early stages of T1D. In follow up experiments, we focused on NLRC5, identified in this initial analysis as a key IFNα-regulated gene with a potential regulatory role on HLA class I expression.
Materials and methods: RNA-seq was performed in human iPSC-derived islet-like cells (Bulk/single-cell) and EndoC-βH1 after transfection with control siRNA or a siRNA targeting NLRC5 (Bulk), both exposed to IFNα (2,000 U/mL). The validation experiments were performed on EndoC-βH1, dispersed human islets and iPSC-derived islet-like cells. Cells were left untreated or treated with IFNα for 8 or 24h. Gene expression levels were measured by qPCR. NLRC5 protein expression was assessed by western blot. The HLA-ABC expression at the beta-cell surface was measured by flow cytometry. Alternative splicing (AS) events were also evaluated.
Results: RNA-seq analysis from islet-like cells exposed to IFNα evidenced induction of HLA class I expression, along with other genes involved in antigen presentation (i.e., B2M, TAP1/2 and PSM8/9 and the transcriptional activator NLRC5; fold change >1.5; n=5; P value adjusted <0.05). This overexpression was detected in all of the islet cell types (fold change >1.5, n=5, P value adjusted < 0.05). NLRC5 depletion by siRNAs decreased IFNα-induced HLA Class I (by 41%, n=6, P value adjusted < 0.01) and genes related to antigen presentation, such as TAP2 (n=6; P value adjusted < 0.001), a key peptide transporter that facilitates the loading of antigens and stabilizes HLA at the cell surface, as well as the chemokine CXCL10 (32%; n=6; P value adjusted < 0.0001). KD of NLRC5 prevented by > than 80% IFNα-induced changes in splicing regulators (n=6; P value adjusted <0.05), including changes in NOVA1 and 2, and downstream splicing events.
Conclusion: The transcriptional activator NLRC5 regulates IFNα-induced expression of HLA class I expression and related genes, and of chemokines in human beta-cells. NLRC5 also mediates the effects of IFNα on AS, a potential generator of beta-cell neoantigens, suggesting that it is a central mediator of the effects of IFNα on beta-cells that may contribute to trigger and amplify autoimmunity in T1D.
Supported by: FRS-FNRS, WELBIO, Dutch Diabetes Research Fundation, INNODIA, EFPIA, JDRF
Disclosure: F. Szymczak: None.
327
Identification of circulating isomiRNAs as predictive biomarkers of beta cell decline in type 1 diabetes
G.E. Grieco1,2, S. Auddino1,2, M. Bruttini1,3, D. Fignani1,2, A. Mori1,3, E. Aiello1,2, G. Licata1,2, G. Sebastiani1,2, F. Dotta1,3;
1Diabetes unit, Dept. of Medicine, Surgery and Neuroscience, University of Siena, 2Umberto Di Mario Foundation, c/o Toscana Life Sciences, 3Tuscany Region Centre for Precision Medicine (CreMeP), Siena, Italy.
Background and aims: Circulating biomarkers in type 1 diabetes (T1D) remain urgently needed to stratify T1D patients in the early stages of the disease and predict their progression. Iso-microRNAs (isomiRs) are post-transcriptional sequence variants of microRNAs (miRNAs) and represent an underestimated proportion of circulating RNAs in plasma. Standard bioinformatic sequencing pipelines and qPCR methods cannot efficiently distinguish circulating isomiRs from canonical miRNAs. Therefore, in this study, we aim to analyse circulating isomiRs using small RNA seq and advanced bioinformatics methods in plasma from T1D patients and control subjects to discover novel disease biomarkers associated with baseline or follow-up clinical conditions.
Materials and methods: We extracted RNA from 200 μl plasma from n=16 control subjects (age=28±5.4 yrs; BMI=21.3±2.3 Kg/m2) and n=16 newly diagnosed (< 6 weeks) T1D patients (age=28.4±7.2 yrs; BMI=23.1±2.8 Kg/m2; ≥2 Aab+) followed for 12 months with scheduled visits every 3 months. Small RNA seq was performed using QIAseq miRNA library kit and sequenced using Illumina NOVASeq6000. Sequences of isomiRs and miRNAs were extracted using two bioinformatics pipelines (sRNAbench, miRge 3.0). Reads expressed in less than 40% of the samples with less than 6 counts per million (CPM) were removed. After normalisation with DESeq2 (Median of Ratios method), sequences shared by both bioinformatics pipelines were retained. Differential expression and association with clinical outcomes at baseline or follow-up were performed using a p-value < 0.05 (FDR corrected), absolute log2 fold-change > 1 and Spearman Rho test.
Results: In plasma, isomiRs account for 93% of all mapped sequences and 41% of all reads, indicating that plasma contains a higher number of isomiRNAs compared to miRNAs. We found n=179 canonical miRNAs, and n=2009 isomiRs derived from n=208 canonical miRNAs. Of note, n=75 sequences were identified as isomiRs only. We identified n=66 canonical miRNAs and n=52 isomiRNAs differentially expressed between T1D and CTRs. Interestingly, n=9 sequences identified exclusively as isomiRs resulted differentially expressed. IsomiRs of miR-222-3p and miR-320a-3p, but not their canonical sequences, correlated negatively with fasting c-peptide at 3 (isomiR-222-3p: p=0.02; isomiR-320a-3p: p=0.04), 6 (isomiR-222-3p:, p=0.0005; isomiR-320a-3p: p=0.003) and 12 months (isomiR-222-3p: p=0.01; isomiR-320a-3p: p=0.01) of follow-up, and also inversely correlated with MMTT c-peptide AUC at 3 (isomiR-222-3p: p=0.01; isomiR-320a-3p: p=0.01), 6 (isomiR-222-3p: p=0.03; isomiR-320a-3p: p=0.04) and 12 months (isomiR-222-3p: p=0.04; isomiR-320a-3p: p=0.02) post-diagnosis, thus predicting beta-cell functional decline during follow-up.
Conclusion: IsomiRs represent a large proportion of circulating RNAs. Their differentiation from canonical miRNA is essential to identify reproducible and consistent disease biomarkers. Using an unprecedented approach, we identified several isomiRs differentially expressed in T1D able to predict beta-cell loss of function.
Supported by: No.115797-INNODIA and No.945268 INNODIA HARVEST
Disclosure: G.E. Grieco: None.
328
Plasma levels of the miR-193b/365a cluster in combination with clinical parameters predict response to Lactococcus lactis - based antigen-specific combination therapy
G. Sassi1, G. Licata2, G. Ventriglia2, D. Ellis1, N. Van Damme3, S. Caluwaerts4, A. Mori5, P. Rottiers4, C. Mathieu1, F. Dotta2,5, C. Gysemans1, G. Sebastiani2;
1Clinical and Experimental Endocrinology, CHROMETA, KU Leuven, Leuven, Belgium, 2Diabetes Unit, Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy, 3Data Mining and Modeling for Biomedicine, VIB Center for Inflammation Research, Ghent, Belgium, 4Precigen Actobio, Zwijnaarde (Ghent), Belgium, 5Tuscany Centre for Precision Medicine (CReMeP), Siena, Italy.
Background and aims: Combining systemic immunomodulation with disease-relevant antigens could provide longer-term solutions for preventing and even reversing autoimmune type 1 diabetes (T1D). Our team established that a combination therapy (CT), composed of a short-course low-dose anti-CD3 treatment with oral delivery of genetically-modified Lactococcus lactis (L. lactis) bacteria secreting full proinsulin plus the anti-inflammatory cytokine IL-10 (LL-PINS+IL-10), was effective in reversing T1D in the non-obese diabetic (NOD) mouse model. Here, we aimed to identify robust peripheral biomarkers for prediction of CT response using circulating cell-free microRNAs (miRNAs). Furthermore, we exploited droplet-based CITE-sequencing (CITE-seq), a multimodal phenotyping method that simultaneously measures RNA and cell surface proteins at single cell level, to investigate the immune cell types regulated by the identified miRNA signature.
Materials and methods: New-onset diabetic NOD mice were injected intravenously with anti-CD3 (d0-5) and inoculated with LL-PINS+IL-10 for 6 weeks. TaqMan™ miRNA array followed by single-assay Q-PCR was performed on plasma samples taken from responder (R) and non-responder (NR) mice before CT initiation. CITE-seq was used to profile FACS-sorted circulating and pancreas-infiltrated CD45+ immune cells of new-onset diabetic NOD mice.
Results: Overall disease remission was 45% by the CT (n=110) compared to 0% in untreated controls (n=13; P<0.01) that remained hyperglycaemic. Taqman™ miRNA analysis identified six miRNAs that were upregulated in plasma of NR compared to R mice before CT initiation. Of those, the miR-193b/365a cluster, composed of two paralogs, was significantly overexpressed in plasma of NR compared to R mice after different validation steps. Combining plasma expression of the miR-193b/365a cluster with clinical parameters (i.e., age and glycaemia at CT initiation) allowed prediction of CT outcome with 83% specificity and 89% sensitivity. Finally, we selected the high-confidence miR-193b/365a target genes from the Molecular Signatures Database (MSigDB) and scored their expression in CITE-seq profiled immune cells. Interestingly, increased levels of miR-365-3p and miR-193-3p in NR mice could influence several of the identified lymphoid and myeloid cell types.
Conclusion: The miR-193b/365a cluster may serve as a novel circulating biomarker that provides additional support for individualized therapy with the L. lactis-based CT.
Disclosure: G. Sassi: None.
329
The effect of type 1 diabetes on lumbar spinal cord transcriptome
K. Zglejc-Waszak1, A. Korytko1, M. Banach2, J. Juranek1;
1Department of Human Physiology and Pathophysiology, University of Warmia and Mazury in Olsztyn, Olsztyn, 2Department of Neurology, Jagiellonian University, Cracow, Poland.
Background and aims: Diabetic peripheral neuropathy (DPN) is characterized by numbness, reduced ability to feel pain or temperature changes and by progressive loss of neurons. The mechanism of DPN remains still unknown. However, studies in the 1960’s revealed that pathological changes may be present already in spinal cord neurons of diabetic patients before DPN symptoms occurs. Next-generation sequencing (NGS) allows for study and characterization of the transcriptomic alternations as well as molecular pathways in diabetic spinal cord. The aim of this study was to identify altered (up- or down-regulated) genes with known biological function, the most significantly altered biological pathway, gene ontology (GO) terms combined with functional annotation and interaction network of the selected set of genes in spinal cord harvested from mice with type 1 diabetes (T1D).
Materials and methods: All experiments were approved by the Local Ethical Committee in Olsztyn, Poland and were carried out in accordance with the 3R principles (Replacement, Reduction and Refinement). The data from T1D model was originally generated from male C57BL/6 wild type mice (Medical University of Bialystok, Poland). Eight weeks old mice (n=10) were injected intraperitoneally with streptozotocin (n=5; STZ, 50mg/kg) or vehicle (n=5; PBS, pH=7.4) for five consecutive days and were sacrificed 24 weeks after the last injection (six months of living as a diabetic). Animals with a blood glucose level ≥ 13 mmol/L (260 mg/dL) were considered diabetic. Illumina NovaSeq 6000 system was used for sequencing.
Results: Analysis revealed, that among 538 differentially expressed genes (DEGs; P ≤ 0.05), 330 (208 up- and 122 down-regulated) DEGs had known biological function, according to DAVID database. The GO enrichment analysis demonstrated that DEGs were involved in biological process (BP), molecular function (MF) as well as cellular component (CC) GO terms. The analysis demonstrated that the most enriched biological pathway in diabetic spinal cord was PI3K-Akt pathway (mmu04151). The qPCR expression patterns of hydroxyacid oxidase 1 (HAO1), neuroepithelial cell transforming 1 (NET1), ras homolog family member J (RHOJ), thioredoxin interacting protein (TXNIP) and cathepsin E (CTSE) aligned with NGS results. The GeneMANIA analysis of the validation genes, indicated that all of these genes as well as 20 additional related genes are connected in one network. In silico analysis revealed that CTSE, cathepsin D (CTSD), renin 1 structural (REN1), pepsinogen A5 (PGA5) and progastricsin (PGC) genes building a interactions network were involved in aspartic-type peptidase activity term, category MF.
Conclusion: Our results showed that T1D alters the transcriptomic profile of mouse spinal cord. Moreover, it may affect PI3K-Akt metabolic signaling pathway in lumbar spinal cord. Thus, regulation of this pathway and its downstream molecules may be a potential therapeutic target for the treatment of diabetic neurological complications. Our results may serve as a guide for further studies on the effect of hyperglycemic milieu on central nervous system function.
Clinical Trial Registration Number: 57/2019
Supported by: National Science Centre in Poland, grant no. UMO-2018/30/E/NZ5/00458
Disclosure: K. Zglejc-Waszak: None.
330
Submicron ultrasound contrast agents as therapeutic vehicles in type 1 diabetes
M. Ciccaglione1, E. Abenojar2, K. McDaniel1, M. Sticco-Ivins1, A. Michels1, A. Exner2, R. Benninger1;
1University of Colorado, Aurora, 2Case Western Reserve University, Cleveland, USA.
Background and aims: Type 1 diabetes (T1D) is an autoimmune disorder characterized by immune-mediated destruction of insulin-producing beta cells resulting in severe hyperglycemia if untreated. A promising approach for T1D prevention is antigen-specific immune therapy, which aims to promote the expansion of anti-inflammatory immune cells, such as regulatory T cells (Tregs), near the disease site. However, there has been limited investigation into optimizing the efficacy of many of these therapeutics using innovative therapeutic delivery vehicles. We investigated the usage of submicron ultrasound contrast agents (nanobubbles (NBs)) as delivery vehicles for antigen-specific peptide therapeutics in order to optimize therapeutic efficacy. NBs are small lipid-shelled bubbles that can be imaged and burst with ultrasound and have been shown to accumulate specifically in immune-infiltrated islets in mice because of high vascular permeability. We will assess if tolerogenic peptides can be incorporated into NBs, if NBs can deliver peptides specifically to islets, and if doing so can expand insulin-reactive Tregs and prevent diabetes onset.
Materials and methods: NBs were synthesized encapsulating an insulin B:9-23 peptide mimotope and peptide/NB colocalization was assessed with confocal microscopy. NB echogenicity was assessed with contrast enhanced ultrasound (CEUS). Peptide bioactivity was assessed by pulsing a primary culture of antigen presenting cells with peptide and culturing with an insulin-reactive T cell hybridoma. Resulting IL-2 production was measured via ELISA. Biodistribution of peptide-NBs was assessed by infusing fluorescently-labeled NBs intravenously in NOD (T1D model) or SCID (no immune infiltration) mice and dissecting abdominal organs after 30 min. Tissue was sectioned and imaged with confocal microscopy. Immune infiltration was assessed by scoring H&E-stained sections. Expansion of insulin-reactive Tregs is determined by isolating islet lymphocytes of NOD Foxp3-EGFP mice, performing surface antibody stains and insulin-I-A(g7) tetramer stains, and analyzing with flow cytometry.
Results: Peptide palmitoylation enhanced retention in NBs: colocalization between palm-peptide and the NB shell was 60% higher than with plain peptide (P<0.001). Incorporation of palm-peptide into the NB did not disrupt the echogenic properties of the NB compared to empty NBs. In vitro bioactivity, indicated by T cell IL-2 production, was substantially higher for palm-peptide-NBs compared to empty NBs (P<0.0001), with much of the peptide's bioactivity retained. Peptide-NBs accumulated specifically in the NOD islets compared to other abdominal organs and SCID islets (P<0.0001), peptide was only retained in NBs in vivo if palmitoylated, and accumulation of NB-delivered palm-peptide in NOD islets was correlated with islet immune infiltration (P<0.05). Initial experiments show a decrease in proportion of islet CD4+ T cells that are insulin-reactive Tregs.
Conclusion: Antigen-specific therapeutics have great potential, but very few studies have optimized their efficacy using innovative routes of administration. This project serves as an important proof-of-principle for the utility of NBs for peptide delivery and has the potential to improve therapeutic intervention in T1D.
Supported by: NIH TL1 TR002533, NIH T32 DK120520, JDRF 1-INO-2019-783-S-B, AB Nexus | University of Colorado
Disclosure: M. Ciccaglione: None.
331
Reprogramming gastrointestinal cells: A new strategy to restore endogenous insulin production for diabetes?
C. Ayachi1,2, T. Napolitano1, S. Silvano1, M. Plaisant1, J. Becam1,2, A. Sousa de veiga1,2, E. Pichery1,2, P. Collombat1,2;
1Institut de Biologie Valrose (iBV) - Diabetes Genetics Team, 2Université Cote d’Azur (UCA), Nice, France.
Background and aims: Type 1 Diabetes (T1D) arises as a result of a cell-mediated autoimmune loss of pancreatic insulin-producing β-cells. β-cell replacement represents a promising avenue of research for an alternative therapeutic strategy, whether it be through cell differentiation or reprogramming of different cell sources. However, to withstand a putative autoimmune attack of neo-generated β-cells, a renewable source of β-cells could be crucial. Interestingly, the gastrointestinal (GI) epithelium is a highly regenerative tissue with the potential to provide a continuous source of insulin-producing cells upon cellular reprogramming. Previous studies in the pancreas demonstrated the in vivo conversion of pancreatic glucagon-producing α-cells into functional β-like cells upon the ectopic expression of Pax4. Acknowledging that glucagon-producing cells are highly represented in the GI tract, we sought to determine whether these cells could also be turned into β-like cells.
Materials and methods: We made use of transgenic mice misexpressing Pax4 in glucagon-producing cells (Gcg_CreERT2::Pax4OE) to evaluate a putative Pax4-mediated gastrointestinal L-to-β-like cell conversion. Additionally, we implemented an ex vivo approach based on mice-derived colonoids to assess the functionality of these neo-generated β-like cells.
Results: Our results demonstrate that the in vivo ectopic expression of Pax4 in gastrointestinal glucagon-producing L-cells results in their conversion into functional insulin-producing β-like cells. Accordingly, the sole misexpression of Pax4 was found sufficient to induce a dramatic decrease in glucagon transcript contents in Gcg_CreERT2::Pax4OE GI tract (up to 98.89% depending on the GI segment analyzed, ****P value < 0,0001), in favor of the acquisition of a β-cell phenotype. Indeed, gastrointestinal insulin-expressing cells were detected in Gcg_CreERT2::Pax4OE animals (no insulin-expressing cells were identified in control animals). Accordingly, quantitative analyses by RT-qPCR outlined a massive increase in insulin transcript contents in Gcg_CreERT2::Pax4OE GI tract (up to 5435.5% depending on the GI segment analyzed, ****P value < 0,0001). Lineage-tracing approaches showed that all insulin-expressing cells arose from previous glucagon-producing L-cells, indicative of a glucagon-to-insulin cell conversion upon Pax4 misexpression. The characterization of newly formed gastrointestinal insulin-expressing cells revealed that these cells displayed most features of pancreatic β-cells. Among others, these include the expression of key β-cell factors, the production and processing of insulin, and the expression of components of the glucose-sensing machinery as outlined by an improved glucose responsiveness (*P value = 0,0135). Lastly, functional tests on mice-derived colonoids established the ability of gastrointestinal β-like cells to release insulin upon glucose stimulation (***P value = 0,000878).
Conclusion: From this study, we conclude that the sole expression of Pax4 appears sufficient to convert the gastrointestinal glucagon-producing L-cells into functional β-like cells in vivo, thus highlighting the potential of the GI epithelium as an innovative renewable source of insulin-producing cells to replenish the β-cell mass in T1D patients.
Supported by: The authors are supported by the JDRF.
Disclosure: C. Ayachi: None.
332
Continuous glucose monitoring identifies early dysglycaemia in very young children at risk of type 1 diabetes being followed in the Australian ENDIA pregnancy cohort study
A. Haynes1, A. Tully1, G.J. Smith1, M.A.S. Penno2, M.E. Craig3,4, J.M. Wentworth5,6, M. Harris7,8, P.G. Colman5, G. Soldatos9,10, A.J. Anderson2, K.J. McGorm2, H. Oakey2, J. Couper11, E.A. Davis1,12, on behalf of the ENDIA Study Group;
1Level 7, Northern Entrance, Telethon Kids Institute, Nedlands, 2Robinson Research Institute, Adelaide, 3School of Women's and Children's Health, Faculty of Medicine, University of New South Wales, Sydney, 4Institute of Endocrinology and Diabetes, The Children's Hospital at Westmead, Sydney, 5Department of Diabetes and Endocrinology, Royal Melbourne Hospital, Melbourne, 6Walter and Eliza Hall Institute of Medical Research, Melbourne, 7The University of Queensland Diamantina Institute, Faculty of Medicine, The University of Queensland, Translational Research Institute, Woolloongabba, 8Queensland Children’s Hospital, South Brisbane, 9Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Melbourne, 10Diabetes and Vascular Medicine Unit, Monash Health, Melbourne, 11Department of Diabetes and Endocrinology, Women’s and Children’s Hospital, Adelaide, Australia, 12Department of Diabetes and Endocrinology, Perth Children's Hospital, Nedlands, Australia.
Background and aims: Prospective longitudinal studies of individuals at risk of type 1 diabetes (T1D) have shown that impaired glucose homeostasis starts months to years before symptomatic disease. No such studies have yet been conducted in young pre-school aged children, who may represent a rapidly progressive T1D phenotype. This study aims to investigate early dysglycaemia using continuous glucose monitoring (CGM) in young children with, and without, persistent islet autoimmunity being longitudinally followed in the Australian Environmental Determinants of Islet Autoimmunity (ENDIA) study.
Materials and methods: ENDIA is prospectively following 1,473 children with a first degree relative with T1D. Islet autoantibody testing (IAA, IA2A, GADA, ZnT8) is conducted 3-monthly from birth to 2 years, and 6-monthly thereafter. ENDIA children with persistent islet autoimmunity, and an age and sex matched autoantibody negative ENDIA participant, are invited to wear a blinded Dexcom G6 CGM for ≥ 14-days. CGM metrics evaluated include mean sensor glucose (SGL), standard deviation (SD) and coefficient of variation of sensor glucose (CEV) and % time in range (3.9 - 7.8 mmol/mol).
Results: 33 (19 autoantibody positive, 14 autoantibody negative) participants (mean age 4.9(±1.7) years) have completed a CGM session. Higher glycaemic variability was observed in children with persistent autoimmunity (Table).
Conclusion: Early dysglycemia is detectable using CGM in young children with persistent islet autoimmunity and may be relevant for characterising metabolic progression and staging of pre-clinical T1D in this population.
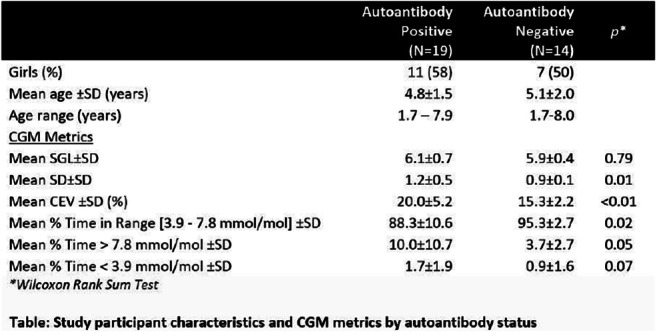
Clinical Trial Registration Number: ACTRN12620000947909
Supported by: JDRF, HCT, Raine Medical Research Foundation, DRWA, WCHF, APEG
Disclosure: A. Haynes: None.
SO 10 Diversity in diabetes
333
Variation in the relationship between fasting glucose and HbA 1c : implications for the diagnosis of diabetes in different age and ethnic groups
Y. Ram1, Y. Xu1, T.C. Dunn1, R. Ajjan2;
1Abbott Diabetes Care, Alameda, USA, 2The University of Leeds, Leeds, UK.
Background and aims: Glycated hemoglobin (HbA1c) remains the benchmark biomarker for glycemic management. However, HbA1c can vary based on factors other than glycemia, including conditions that affect red blood cell (RBC) lifespan. The aim of this study is to examine the role of personal non-glycemic factors on the relationship between fasting plasma glucose (FPG) and HbA1c. The impact of race, age, and gender on HbA1c at FPG Diabetes Diagnosis Criteria (DDC), was analyzed by accounting for personal RBC factors with a recently-described kinetic model.
Materials and methods: The relationship between FPG and HbA1c, calibrated for National Glycohaemoglobin Standardization Program, was assessed in 12,531 individuals from the 2001-2018 National Health and Nutrition Examination Survey. FPG and HbA1c were used to calculate Apparent Glycation Ratio (AGR) for different subgroups of individuals based on age, race, and gender. AGR accounts for individual RBC glucose uptake and lifespan and characterizes a personalized relationship between glucose and HbA1c.
Results: Black individuals had higher HbA1c at FPG DDC [mean: 6.7% (95% CI: 6.7-6.8)] compared with White individuals [6.5%, (6.5-6.5); p<0.001]. Their AGRs were 72.7 mL/g (72.4-72.9) and 69.8 mL/g (69.6-70.0) respectively. Younger individuals <21 years had lower HbA1c at FPG DDC and AGR compared to >50 years of age [6.5% (6.5-6.6) and 6.57% (6.6-6.6), respectively; p<0.001)] [AGR: 70.3 mL/g (70.1-70.5) and 70.8 mL/g, (70.6-71) respectively; p<0.001). Women and men also showed differences in HbA1c at FPG DDC [6.7% (6.6-6.7) and 6.5% (6.4-6.5), respectively; p<0.001] [AGR: 71.7 mL/g (71.5-71.8) and 69.3 mL/g (69.2-69.5) respectively; P<0.001). Although there are differences between the subgroup means, there was 30 times more variation in AGR values within each group compared to differences between groups.
Conclusion: The relationship between FPG and HbA1c is affected by nonglycemic factors, which show wide inter-individual variability and are affected by race, age and gender. Assessment of AGR helps to understand individual relationship between glucose levels and HbA1c, thus increasing accuracy of HbA1c for screening and diagnosis of diabetes.
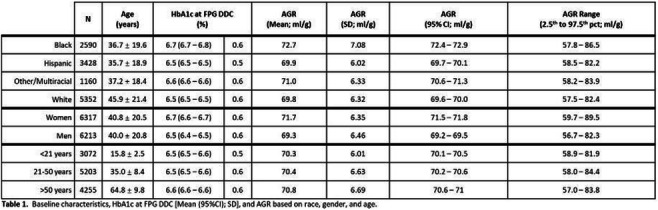
Disclosure: Y. Ram: Employment/Consultancy; Abbott Diabetes Care.
334
Subclassification of 3,529 individuals with type 2 diabetes
D.H. Christensen1, S.K. Nicolaisen1, E. Ahlqvist2, J.V. Stidsen3, J.S. Nielsen3, K. Højlund3, S. García-Calzón4,5, C. Ling5, I. Brandslund6, P. Vestergaard7, C. Brøns8, R.W. Thomsen1, A. Vaag8;
1Depart. of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark, 2Depart. of Clinical Sciences, Genomics, Diabetes and Endocrinology unit, Lund University Diabetes Centre, Lund, Sweden, 3Steno Diabetes Center Odense, Odense University Hospital, Odense, Denmark, 4Depart. of Nutrition, Food Science and Physiology, University of Nevarra, Pamplona, Spain, 5Depart. of Clinical Sciences, Epigenetics and Diabetes unit, Lund University Diabetes Centre, Malmö, Sweden, 6Depart. of Biochemistry, University Hospital of Southern Denmark, Vejle, Denmark, 7Steno Diabetes Center North Jutland, Aalborg University Hospital, Aalborg, Denmark, 8Steno Diabetes Center Copenhagen, Gentofte Hospital, Gentofte, Denmark.
Background and aims: A Swedish data-driven cluster study identified four distinct type 2 diabetes (T2D) clusters based on HbA1c level, BMI, age at diagnosis, and HOMA2 estimates of insulin resistance and beta-cell function. A Danish study suggested three T2D phenotypes (insulinopenic, classical, and hyperinsulinemic) based only on HOMA2 measures. We investigated and compared these two new T2D classifications using the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort.
Materials and methods: In 3,529 individuals recently diagnosed with T2D, we first did a k-means cluster analysis with a forced k-value of four in order to replicate the Swedish clusters: severe insulin deficient (SIDD), severe insulin resistant (SIRD), mild obesity-related (MOD), and mild age-related (MARD) diabetes. Next, we performed an analysis open to alternative k-values (i.e. data determined the optimal number of clusters). Lastly, we compared the data-driven clusters with the three Danish T2D phenotypes.
Results: Compared with the Swedish results, the replicated SIDD cluster in DD2 comprised individuals with lower mean HbA1c (86 mmol/mol vs. 101 mmol/mol) and the MOD cluster individuals in DD2 were less obese (mean BMI 32 kg/m2 vs. 36 kg/m2). Our alternative k-value analysis suggested the most optimal number of T2D clusters in the DD2 cohort to be three (i.e. k-value of three), not identifying a MOD-like cluster (Figure, upper panel). When comparing the four replicated Swedish clusters in the DD2 to the three Danish HOMA2-based phenotypes, 81%, 79%, and 69% of the SIDD, MOD, and MARD individuals, respectively, fitted the classical T2D phenotype, whereas 70% of SIRD individuals fitted the hyperinsulinemic phenotype (Figure, lower panel). Among the three clusters identified with the alternative k-value analysis, 60% of individuals in the most insulin-resistant cluster constituted 76% of individuals with a hyperinsulinemic phenotype.
Conclusion: Different classification approaches did not classify T2D patients in a consistent manner. The T2D classes characterized by hyperinsulinemia/high insulin resistance appeared most distinct.
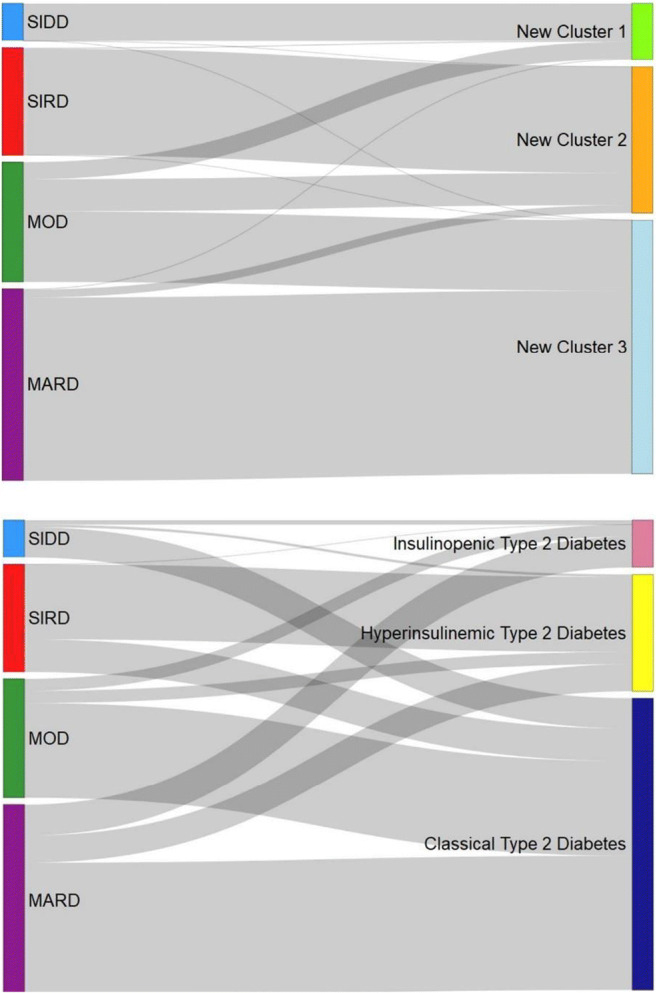
Supported by: Novo Nordisk Foundation
Disclosure: D.H. Christensen: Grants; The DD2 study was supported by the Novo Nordisk Foundation.
335
Socio-economic inequalities in the prevalence and incidence of pharmacologically-treated diabetes in France in 2020
M. Guion1, L. Mandereau-Bruno1, E. Cosson2, S. Fosse-Edorh1;
1Santé Publique France, Saint-Maurice, 2Department of Endocrinology-Diabetology-Nutrition, Jean-Verdier Hospital, University of Paris 13, Sorbonne Paris Cité, CRNH-IdF, CINFO, AP-HP, Bondy, France.
Background and aims: Social inequalities in health (SIH) may impact the prevalence of diabetes and diabetes-related complications. The aim of this study was to describe the association between SIH and prevalence/incidence of pharmacologically-treated diabetes in 2020 in France including French overseas territories (FOT).
Materials and methods: Pharmacologically-treated people with diabetes were identified using a validated algorithm in the National health data system (Système National des Données de Santé: SNDS). SIH were measured via the French Deprivation index (FDep) of the 2015 version available for the Metropolitan France. Individuals living in FOT (excluding Mayotte) were considered separately. Age-standardized (2013 European standard population) prevalence and incidence rates were stratified by sex. Denominators were the French health consumers.
Results: Data were available for 65,580,975 health consumers (97.3% of the French population). Age-standardized diabetes prevalence and incidence rates were lower in women than in men in metropolitan France, but prevalence was higher in women than men in FOT. Both prevalence and incidence were increasing by FDep quintiles (Q1 less deprived area, Q5 most deprived area) in metropolitan France and were even higher in FOT, in both genders. The Q5/Q1 ratio of age-standardized prevalence and incidence were higher in women (1.8 and 1.7 respectively) than in men (1.4 and 1.5 respectively).
Conclusion: SIH are positively associated with prevalence and incidence of pharmacologically-treated diabetes in metropolitan France. The association is stronger in women than in men. Diabetes prevalence and incidence are higher in FOT regardless of sex. Future studies will investigate whether the influence of SIH on diabetes epidemiology varies over years.
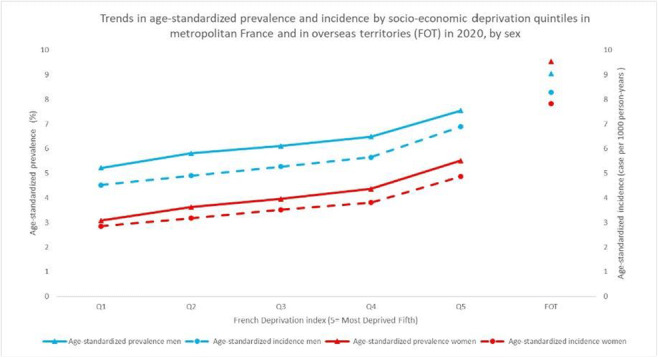
Supported by: These results are produced in the context of a doctoral project funded by the Fondation de France.
Disclosure: M. Guion: None.
336
Gender disparities in time-to-initiation of cardioprotective glucose-lowering drugs in patients with type 2 diabetes and cardiovascular disease: a nationwide cohort study
K.L. Funck1, L. Bjerg1, A.A. Isaksen1, A. Sandbæk1, E.L. Grove2;
1Steno Diabetes Center Aarhus, Aarhus University Hospital, 2Department of Cardiology, Aarhus University Hospital, Aarhus N, Denmark.
Background and aims: Initiation of cardioprotective glucose-lowering drugs (GLDs) is often delayed in patients with type 2 diabetes (T2DM) and cardiovascular disease (CVD). We aimed to examine the impact of gender and specific CVD diagnosis on time-to-initiation of either an SGLT2 inhibitor or GLP1 analogue (collectively cardioprotective GLD) after a dual diagnosis of T2DM and CVD.
Materials and methods: In a registry-based nationwide Danish cohort study, we identified patients with a new dual diagnosis of T2DM and CVD (ischaemic heart disease, stroke, peripheral arterial disease [PAD] or heart failure) between January 1, 2012 and December 31, 2018. Index date was defined as the latest of the diagnosis of T2DM or CVD. Patients were followed until initiation of a cardioprotective GLD, death or the censor-date, whichever came first. Poisson models were used to estimate the initiation rate of cardioprotective GLD after the index date. We investigated if there was effect modification by gender on the association between CVD type and initiation rate using a Chi-Square test and estimated the initiation rate for men and women according to the type of cardiovascular disease. Final analysis was adjusted for age, HbA1c and low-density lipoprotein (LDL) cholesterol.
Results: We included 67,647 patients with new-onset T2DM and CVD (61% male, mean age 70±12 years, mean HbA1c 54±16 mmol/mol). During 230,000 person-years (PY) 6,344 people (9.4%) redeemed a prescription of a cardioprotective GLD-1. Initiation rates differed considerably according to the type of CVD (range 41-82 initiation during 1000 PY, Table 1) and we found effect modification by gender (p<0.05). Table 1 shows the initiation rate during 1000 PY for a man and a woman at age 70 years, with a diabetes duration of 3 years, HbA1c of 50 mmol/mol and LDL cholesterol of 2.2 mmol/L. The adjusted initiation rate ratio for men compared with women was 1.13 (95% CI: 1.03-1.24), 0.91 (95% CI: 0.78-1.06), 1.14 (95% CI: 0.92-1.42), and 1.30 (95% CI: 1.07-1.57) for ischaemic heart disease, stroke, PAD, and heart failure, respectively.
Conclusion: Overall, a small fraction of patients with a dual diagnosis of T2DM and CVD are initiated in cardioprotective GLD treatment. The initiation rate for men and women is comparable after diagnosis of stroke and PAD, however for heart failure and ischaemic heart disease, men are prescribed GLDs faster than women.
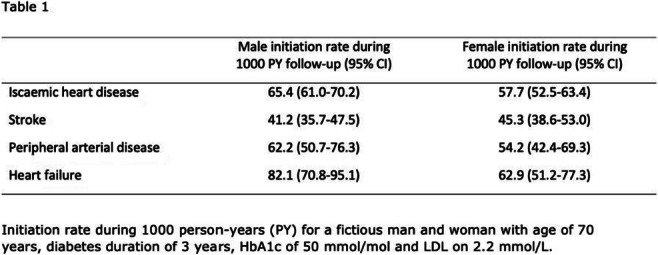
Supported by: Unrestricted research grant Boehringer Ingelheim Danmark A/S
Disclosure: K.L. Funck: Grants; Non-restricted research grant Boehringer Boehringer Ingelheim A/S.
337
About one-in-three new onset diabetic ketosis cases is caused by ketosis-prone type 2 diabetes in adults: a systematic review and meta-analysis
A. Kovacs1,2, S. Bunduc2, D.S. Veres3,2, D. Palinkas4,2, E.B. Gagyi2, K. Marta2, J.P. Hegyi2, B. Eross2, E. Mihaly1,2, E. Sipter1, P. Panczel1, P. Hegyi2, N. Hosszufalusi1,2;
1Department of Internal Medicine and Hematology, Semmelweis University, 2Center for Translational Medicine, Semmelweis University, 3Department of Biophysics and Radiation Biology, Semmelweis University, 4Military Hospital – State Health Centre, Budapest, Hungary.
Background and aims: Ketosis-prone type 2 diabetes mellitus (KPD2) has been previously described in the literature. Nevertheless, only since 2019 was it defined by the World Health Organization as a hybrid form of diabetes characterized by unprovoked keto(acido)sis onset with preserved insulin secretion and absence of diabetes related autoantibodies. It mainly affects non-Caucasian ethnicity patients but the exact prevalence is unknown. Insulin treatment, although necessary at onset, may be waived in some of the KPD2 cases during disease course. We conducted a systematic review and meta-analysis to assess the prevalence and to describe the clinical characteristics of KPD2 among patients presenting with diabetic ketoacidosis or ketosis.
Materials and methods: The systematic search was performed in the five main databases - MEDLINE, Embase, Web of Science, Scopus and CENTRAL on 15th October, 2021 without any filters applied. For eligibility assessment we used the following definition of KPD2 (exposed group): newly diagnosed autoantibody-negative diabetic keto(acido)sis cases with preserved beta cell function (C-peptide in the given reference range). The comparison group comprised typical type 1 diabetic keto(acido)sis patients. The random effect model was used in meta-analysis to calculate the pooled prevalence, odds ratios (ORs), mean differences (MDs) and the 95% confidence intervals (CIs). The I2 value and chi-square test assessed statistical heterogeneity.
Results: Out of 16.962 articles 11 were eligible for the meta-analysis counting 2010 mainly adult patients. Among new onset diabetes patients presenting with diabetic keto(acido)sis 35% (95%CI:0.24-0.49; I2=94%) belonged to the KPD2 group. When including in the analysis also the diabetic patients admitted for diabetic keto(acido)sis regardless of the moment of diagnosis, the prevalence of KPD2 was 25% (95%CI:0.14-0.42; I2=97%). Patients with KPD2 were older (MD=11.55 years, 95%CI:5.5-17.6; I2=88%) and had a significantly higher BMI (MD=5.48 kg/m2, 95%CI:3.25-7.72; I2=92%) by comparison with type 1 diabetes patients. HbA1c and triglyceride levels at admission, gender distribution, and family history of diabetes did not differ significantly between the two groups.
Conclusion: Ketosis-prone type 2 diabetes is a common type of diabetes among adult patients with diabetic ketosis. Testing for C-peptide and islet cell autoantibodies in all newly diagnosed patients with diabetic ketoacidosis or ketosis at onset is essential for a proper classification and will allow an adequate long-term treatment.
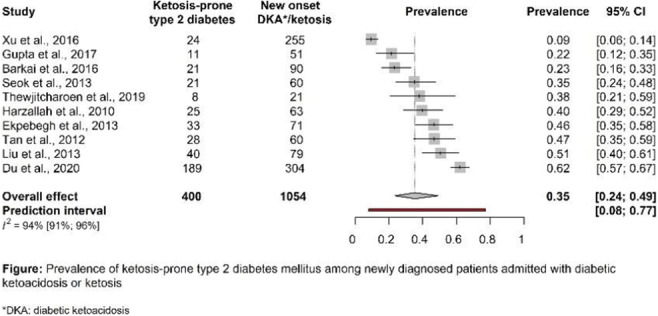
Disclosure: A. Kovacs: None.
338
Metabolically healthy obese is actually not healthy but at a risk for developing diabetes: a retrospective study
S. Mizushiri, M. Daimon;
Department of Endocrinology and Metabolism, 5 Zaifu-cho, Hirosaki, Japan.
Background and aims: Although obese individuals without obesity-related metabolic abnormalities are often defined as metabolically healthy, risks for developing such abnormalities have not been evaluated well. Recently, a prospective study showed that metabolically healthy obese subjects were at higher risk of diabetes, stroke, and cardiovascular disease than metabolically healthy subjects without obesity in the UK. We here analyzed the association between metabolically healthy obesity and risk for developing diabetes in a Japanese population.
Materials and methods: The participants were recruited from the Iwaki study, a health promotion study of Japanese people aimed to prevent lifestyle-related disease. Among 1167 participants of the Iwaki study held in 2014, 931 individuals attended at least one time from 2015 to 2019. We excluded 7 and 75 individuals without complete data sets and who were diagnosed with diabetes in 2014 respectively, thus 849 subjects were enrolled in this study. Obesity was defined as BMI >25 kg/m2. We used the National Cholesterol Education Program ATP III Guideline to categorize metabolically unhealthy as participants meeting at least two criteria of metabolic abnormalities.
Results: In 2014, the number of metabolically healthy subjects without and with obesity were 606 and 101 respectively. Cox proportional hazard model showed metabolically healthy subjects with obesity were at higher risk of diabetes compared with those without obesity (Hazard ratio 3.70, p=0.002), even if after adjusted by age and sex (Hazard ratio 3.32, p=0.005).
Conclusion: Our analysis suggest an importance of encouraging people with obesity to reduce body weight even though they are metabolically healthy. We are extending such analyses in association with other lifestyle-related diseases.
Disclosure: S. Mizushiri: None.
SO 11 Novel biomarkers and risk factors
339
Sphingolipids in type 2 diabetes: a meta-analysis
M. Muilwijk1, T. Wong1, G. Burchell2, S. Remmelzwaal1,3, J.W.J. Beulens1;
1Epidemiology and Data Science, Amsterdam UMC, 2Faculty of Earth and Life Sciences, Vrije Universiteit Amsterdam, 3General Practice/Family Medicine, Amsterdam UMC, Amsterdam, Netherlands.
Background and aims: Evidence suggests that some sphingolipids are associated with type 2 diabetes (T2D). Past years, studies investigating sphingolipids and incident T2D rapidly increased. Therefore a meta-analysis on the association of sphingolipids and T2D is warranted.
Materials and methods: Medline, Web of Science, Embase and the Cochrane library were searched through January 2022. Prospective studies that reported associations between plasma or serum sphingolipids and incident T2D were selected amongst humans. The multivariable-adjusted relative risks of T2D were calculated analyses per study-specific SD difference in a given sphingolipid by random effects meta-analyses.
Results: A total of 18 prospective studies were identified, which included in total 18,529 individuals of whom 3,447 participants developed type 2 diabetes. Meta-analysis was performed for 19 sphingolipids, of which 10 were statistically significantly associated with type 2 diabetes. Ceramides showed consistent associations with type 2 diabetes risk with a pooled relative risk per study-specific SD increase of 1.18 [95% confidence interval 1.12-1.24]; I2=0.0%]) for ceramide d18:1/22:0, and similar results for other ceramides. The results for sphingomyelins were mixed, e.g. the pooled relative risk per study-specific SD increase was 1.14 [95% confidence interval 1.05-1.24]; I2=50.4%] for sphingomyelin 36:1 and 0.86 [0.79-0.93]; I2=44%] for sphingomyelin 36:3. We were not able to meta-analyze complex sphingolipids such as lactosylceramides, due to limited data. [BJ(1]Kun je gemiddelde duur van follow-up geven?
Conclusion: Several sphingolipids are consistently associated with the risk of T2D. Sphingolipids may potentially be used as biomarkers for T2D.
Supported by: EXPOSOME-NL (NWO grant number 024.004.017)
Disclosure: M. Muilwijk: None.
340
Role of soluble leptin receptor in diabetes prevention? Combination of cross-sectional, clinical intervention and Mendelian randomisation studies
C. Sommer1, K.G. Vangberg2, G.-H. Moen3,4, D.M. Evans4, S. Lee-Ødegård3, I.K. Blom-Høgestøl1, L. Sletner5, A.K. Jenum3, C.A. Drevon3, H.L. Gulseth6, K.I. Birkeland3;
1Oslo University Hospital, Oslo, Norway, 2Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway, 3University of Oslo, Oslo, Norway, 4University of Queensland, Brisbane, Australia, 5Akershus University Hospital, Lørenskog, Norway, 6Norwegian Institute of Public Health, Oslo, Norway.
Background and aims: Leptin is mainly produced by adipocytes, inhibits appetite, and is associated with bone metabolism, fertility, and insulin sensitivity. Signaling depends on membrane-bound leptin receptors, which via ectodomain shedding form soluble leptin receptors (sOb-R) which can be quantified in blood. High plasma sOb-R is inversely associated with gestational (GDM) and type 2 diabetes (T2D) in longitudinal observation studies. We explored associations between sOb-R and relevant metabolic markers, how sOb-R levels respond to hyperglycemia, hyperinsulinemia, acute exercise, food intake, and tested for causality using two-sample Mendelian Randomization (MR).
Materials and methods: In five independent clinical studies (n = 24 to 823), sOb-R was quantified in serum or plasma by commercial ELISA kits using monoclonal antibodies. We performed two-sample MR using publicly available summary data from genome-wide association studies of fasting glucose, fasting insulin, T2D and BMI as exposures, and sOb-R as the outcome. We performed multivariable MR to assess independent causality.
Results: In pooled, cross-sectional data (n=973), sOb-R was negatively associated with percent body fat and fasting C-peptide, a byproduct of insulin production. sOb-R decreased in response to acute hyperinsulinemia during glucose clamp in two independent clinical studies (n=61 and n=39), and immediately increased in response to both intensive exercise (n=26) and food intake (n=39). In two-sample MR, both higher fasting insulin and higher BMI were causally linked to lower sOb-R levels (Table 1). The causal effect of higher insulin on lower sOb-R persisted independently of BMI in multivariable MR (beta -1.6, p<0.001). The effect of higher BMI on lower sOb-R was unchanged (beta -0.21, p = 0.058) independently of fasting insulin. The cross-sectional study of fasting glucose and sOb-R (n=973), or effect of hyperglycemia on sOb-R (n= 61) were inconclusive, in line with two-sample MR which indicated no causal relationship of fasting glucose on sOb-R.
Conclusion: Both BMI and insulin seem to causally and independently decrease sOb-R levels in blood. Conversely, intensive exercise and food intake acutely increased sOb-R. Our results may suggest that sOb-R is involved in short term regulation of leptin signaling, either directly or indirectly.
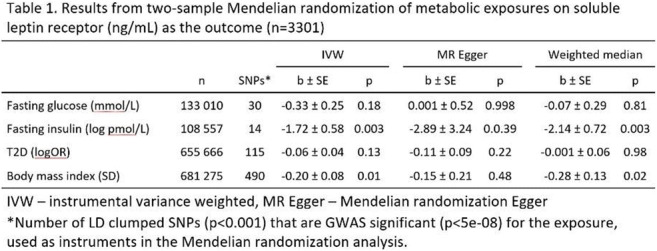
Clinical Trial Registration Number: NCT00992797, NCT01803568,
Supported by: Norwegian Diabetes Association
Disclosure: C. Sommer: None.
341
Plasma concentrations of complement C3 explain part of the associations of several measures of adiposity with type 2 diabetes: the Maastricht study
S. Jin1, C.G. Schalkwijk1, S.J.P. Eussen1, A. Koster2, E. Kooi1, M.C.G. Brouwers1, C.D.A. Stehouwer1, M.M.J. Van Greevenbroek1;
1CARIM School for Cardiovascular Disease, Maastricht University, 2CAPRHI Care and Public Health Research Institute, Maastricht University, Maastricht, Netherlands.
Background and aims: A wealth of clinical and epidemiological evidence links obesity to type 2 diabetes (T2D), but the underlying causal pathways are not yet fully elucidated. Plasma concentration of complement C3, the central hub in complement activation, has been identified as an independent risk factor for incident T2D. Moreover, complement C3 is expressed in adipose tissue and the plasma concentration of C3 is higher in individuals with obesity. In this study, we investigated whether and how much of the association between obesity and T2D was explained by C3.
Materials and methods: In 3544 population-based participants (51.7% men, 59.9±8.3 years, 28% T2D, oversampled), we measured C3 by ELISA in fasting EDTA plasma, body mass index (BMI) and waist circumference by standard methods, visceral (VAT) and subcutaneous adipose tissue (SAT) by MRI, insulin resistance as HOMA-IR, disturbed glucose metabolism as fasting glucose concentration and the diagnosis T2D by an oral glucose tolerance test. We conducted regression analyses to investigate the association of adiposity (all measures of adiposity were standardized) with insulin resistance, glucose metabolism and T2D, and evaluated the mediating effect of C3 in these associations. These regression analyses were adjusted for age, sex, socioeconomic status, and smoking habits. The 95% confidence intervals of the mediating effects were obtained by bootstrapping.
Results: All measures of adiposity (BMI, waist, VAT and SAT) were positively associated with insulin resistance, glucose metabolism and T2D (all P<0.001). These positive associations could be partly mediated by plasma C3. All 95% confidence intervals indicated significant mediated effects. C3 explained 26.2%, 22.3%, 25.3% and 27.5%, respectively, of the associations of BMI, waist, VAT and SAT with T2D. The strongest mediating effect of C3 in the association between adiposity and insulin resistance was seen for SAT (28.4% of the association between SAT and HOMA2-IR was explained by C3). Also in the association between adiposity and glucose metabolism, the strongest mediating effect of C3 was seen for SAT (44.6% of the association between SAT and fasting glucose was explained by C3). Additional adjustment for dietary habits and physical activity did not materially alter these observations (n=2951).
Conclusion: In middle-aged to elderly participants, the strong and positive association of several measures of adiposity with insulin resistance, glucose metabolism and T2D could for a substantial part be explained by the plasma concentration of complement C3. Our data thus suggest direct or indirect effects of C3 on insulin resistance and glucose metabolism contributing to T2D, starting from expanded and dysfunctional fat depots.
Supported by: CSC, EFSD Research Programme
Disclosure: S. Jin: None.
342
Identifying biomarkers of psoriasis-driven metabolic disease
V.S. Gesheva1,2, S. Sayers1, E. Evans1, G. Bewick1, R. Andres-Ejarque3, I. Tosi3,4, P. DiMeglio3,4, R. Hannen2, P. Caton1;
1Department of Diabetes, King's College London, 2Keratify Ltd., London, UK, 3St John’ Institute of Dermatology, King's College London, 4NIHR Biomedical Research Centre, Guys & St Thomas NHS Foundation Trust & King's College London, London, UK.
Background and aims: Immune-mediated skin diseases such as psoriasis instigate changes in the skin secretome, which consequently result in dysfunction of key metabolic tissues and increased risk of psoriasis co-morbidities, such as type 2 diabetes (T2D). However, the potential skin secretome factors, which may induce metabolic and inflammatory effects in psoriasis remain poorly characterised. Proteomic analysis or existing gene expression datasets identified vimentin, parathymosin, prothymosin-alpha, dermcidin, desmin, interleukin 19 and interleukin 36-alpha as candidate skin secretome proteins. This project aimed to investigate the underlying events occurring in pancreatic islets and subcutaneous adipose tissue (sAT) which mediate psoriasis metabolic co-morbidities and to identify novel prognostic biomarkers for early identification of psoriasis patients at increased risk of developing metabolic disorders, such as T2D.
Materials and methods: Full-thickness human skin was obtained from psoriasis patients before (baseline) and at week 12 of therapy with the anti-tumour necrosis factor (TNF) drug adalimumab and healthy controls, total RNA was extracted and expression of genes encoding for candidate biomarkers was measured using qRT-PCR. Pancreatic islets were isolated from 2-4-month-old CD1 male mice. sAT was collected from male 6-month-old C57BL/6 mice fed either standard or high-fat-high-fructose diet (HFHFD). Islets and sAT were treated with recombinant proteins at a range of (patho)physiological concentrations, and with cocktail treatment (vimentin;500 ng/ml, dermcidin;1000 ng/ml, prothymosin-alpha;1000 ng/ml, parathymosin;1000 ng/ml, desmin;500 ng/ml). Glucose-stimulated insulin secretion (GSIS; radioimmunoassay) was used to assess pancreatic islet health. sAT function was determined by qRT-PCR measurements of functional and inflammatory gene expression.
Results: Expression of VIM, DCD, PTMA, PTMS, and DES was significantly (p<0.05) reduced in baseline psoriasis lesional skin compared to either healthy controls or psoriasis non-lesional skin samples. As previously shown, expression of IL19 and IL36A was increased in psoriasis lesional skin samples in comparison to healthy controls and psoriasis non-lesional skin. Treatment with the anti-TNF drug adalimumab resulted in increased expression of VIM, DCD, PTMA, PTMS in lesional skin samples, but the same change was not observed in non-lesional skin. Vimentin, prothymosin-alpha, interleukin 19, interleukin 36-alpha and cocktail islet treatments decreased GSIS, whereas parathymosin, dermcidin, and desmin increased GSIS. Cocktail treatment of mouse sAT induced a significantly higher expression of inflammatory genes IL-1β and IL6 (p<0.01), along with a significant reduction in the expression of functional markers GLUT4 and PPARγ (p<0.05) both in standard and HFHFD samples.
Conclusion: The disruption of metabolic function observed in psoriasis could be promoted by changes in the expression of distinct factors present in the skin secretome which in turn affect the functional and secretory profiles of sAT and islets thus facilitating systemic effects on key metabolic organs.
Supported by: MRC Medical Research Council
Disclosure: V.S. Gesheva: None.
343
Night blindness and risk of diabetes in the Chinese population: a multi-centre, cross-sectional study
J. Wang1, Y. Liu1, Y. Zhou2, Y. Ding3, S. Qiu3, Z. Sun3;
1Southeast University, 2Nanjing Medical University, Nanjing First Hospital, 3Zhongda Hospital of Southeast University, Nanjing, China.
Background and aims: Night blindness (NB) is a disease caused by vitamin A deficiency. It is characterized by impaired vision at sunset and at night while normal vision during daylight. Previous studies have shown that vitamin A deficiency is associated with increased risk of diabetes, but it remains unknown whether NB is related to diabetes. The aim of the present study was to investigate their relationship in the Chinese population.
Materials and methods: This multi-centre, cross-sectional study was conducted in 8 sites from south, east, north, west and middle regions in China and enrolled 5,491 subjects aged 20-70 years. NB was diagnosed through the standardized interview validated by the WHO. Diabetes was ascertained by a standard 75g oral glucose tolerance test, the use of antidiabetic medications, and/or the self-reported history. Participants with overweight or obesity were defined as those with body mass index ≥24 kg/m2. Binary logistics model was applied to assess the association of NB with the presence of diabetes.
Results: There were 411 participants diagnosed with NB. Compared with the participants without NB, the prevalence of diabetes was higher in those with NB (16.1% vs. 12.1%, p = 0.020). The crude odds ratio (OR) of diabetes for participants with NB was 1.386 (95% CI 1.051, 1.828) compared with those without NB. After multiple adjustments (sex, age, BMI, TG, TC, HDL, LDL), the association still remained significant (OR 1.352, 95% CI 1.016, 1.800 ). Participants with NB had a larger waist circumference (86.83±10.15 vs. 85.05±10.58, p=0.001) and a higher prevalence of overweight or obesity than those without NB (65.8% vs. 65.0%, p = 0.056).
Conclusion: Participants with NB had higher odds of diabetes and overweight/obesity in the Chinese population.
Supported by: NSFC-81870534
Disclosure: J. Wang: None.
344
Association between periodontitis and undiagnosed glucose intolerance status in apparently healthy young adults aged <40 years
Y. Lyu, J. Kim, S. Kim;
Chosun university hospital, Gwangju, Republic of Korea.
Background and aims: Periodontal disease is prevalent disease in diabetic patients and untreated periodontal disease lead to raise the blood sugar and make it harder to control diabetes. Several studies report that diabetes screening of patients with periodontal disease is effective strategy, but this screening in young adults remains unclear. Thus, the aim of this study was to evaluate between periodontitis and undiagnosed glucose intolerance status in apparently healthy young adults aged <40 years.
Materials and methods: This study was based on the data from the Korean National Health and Nutrition Examination Survey (KNHANES), conducted by the Korean Ministry of Health and Welfare in 2010-2019. This survey is a cross-sectional and nationally representative study of noninstitutionalized civilians using a stratified, multistage, clustered probability sampling design. Of 10,654 participants, data for 1,864 subjects were included in the analysis. Multivariate logistic regression analyses were conducted separately to evaluate association with periodontal disease and undiagnosed glucose intolerance status in groups stratified by sex.
Results: The prevalence of undiagnosed prediabetes and undiagnosed diabetes was 20.1% and 2.7% in male, 12.4% and 0.8% in female, respectively. After fully adjusting for confounding factors, including age, smoking, alcohol, family income, education, place of residence, exercise and number of tooth brushing, Multivariate logistic regression analysis showed that periodontal disease was significantly associated with increased risk of diabetes (OR=2.595 (95% CI 1.201-5.606) in male, but, no significant associations were found in female.
Conclusion: Increased attention and implementation of precise strategies for identifying diabetes in young adults with periodontal disease would allow for increased wellbeing as well as reduced healthcare burdens associated with diabetes.
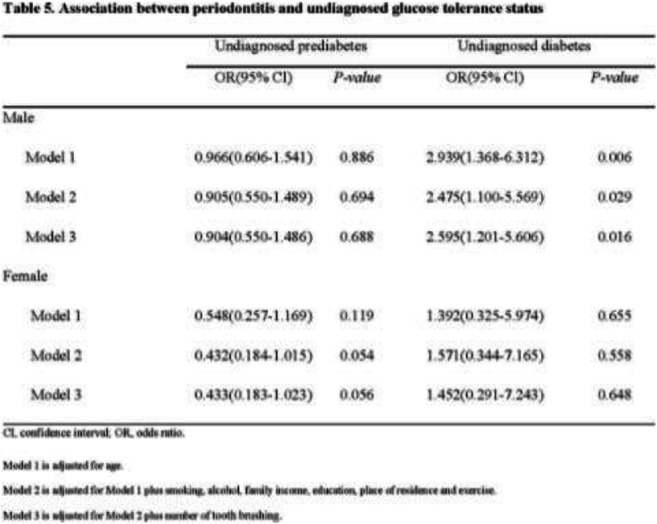
Disclosure: Y. Lyu: None.
345
The association between social jetlag and the metabolic syndrome and type 2 diabetes: a systematic review and meta-analysis
E.J. Bouman1, J.W.J. Beulens1,2, L. Groeneveld1, R.S. de Kruijk1, L. Schoonmade3, S. Remmelzwaal1, P.J.M. Elders4, F. Rutters1;
1Epidemiology and Data Science, Amsterdam Public Health Research Institute, Amsterdam UMC, Amsterdam, 2Julius Centre for Health Science and Primary Care, University Medical Centre Utrecht, Utrecht, 3University Library, Vrije Universiteit, Amsterdam, 4General practice, Amsterdam Public Health Research Institute, Amsterdam UMC, Amsterdam, Netherlands.
Background and aims: We aimed to determine the association between social jetlag and the Metabolic Syndrome and Type 2 Diabetes (T2D) in a systematic review and meta-analysis.
Materials and methods: A systematic literature search was conducted in PubMed/Embase/Scopus until September 2020. Included studies described an association between social jetlag and the Metabolic Syndrome and/or T2D, were available full text and were written in English or Dutch. Data extraction and quality assessment was performed on pre-piloted forms independently by two reviewers. If three or more studies reported the same outcome, results were meta-analyzed using random-effects analysis.
Results: 5434 titles/abstracts were screened, 133 papers were read full-text, and 46 studies were included. One study was rated as low quality, 22 were of moderate quality, and 23 were of high methodological quality. The high quality studies showed that having social jetlag compared to no social jetlag was significantly associated with higher levels of BMI (0.39kg/m2, 95% confidence interval 0.01;0.76;I2=100%) in 12 studies, higher odds of obesity (1.13, 1.07;1.18;I2=0%) in seven studies, higher waist circumference (0.78cm, 0.22;1.35;I2=7%) in six studies, higher levels of HbA1c (0.65%, 0.12;1.18;I2=100) in five studies, and lower odds of hypertension (0.56, 0.17;0.95;I2=53%) in four studies. No statistically significant associations were observed for abdominal obesity, High Density Lipoprotein (HDL) levels, triglycerides, blood pressure, fasting glucose levels, Metabolic Syndrome or T2D. Sensitivity analyses on health status and statistical quality did not reduce heterogeneity.
Conclusion: Social jetlag is associated with factors of the Metabolic Syndrome and T2D. However, high heterogeneity for some parameters complicate the interpretation of our results. Future studies should use prospective study designs to further assess cause and effect of the observed associations and try to elucidate the pathways and mechanisms that underlie this association.
Supported by: Diabetes Fonds, Senior Fellowship Grant
Disclosure: E.J. Bouman: None.
346
The association of vital exhaustion and burnout with (measures of) the metabolic syndrome: a systematic review and meta-analysis
S.H.M. Kremers1, J.W.J. Beulens1,2, M. Strikwerda1, S. Remmelzwaal1, P.J.M. Elders3, F. Rutters1;
1Epidemiology and Datascience, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, 2Julius Centre for Health Sciences and Primary Care, Utrecht, 3General Practice, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Background and aims: Metabolic syndrome strongly increases the risk of developing type 2 diabetes. Vital exhaustion and burnout have been associated with metabolic syndrome. However, current literature shows inconsistent results. Therefore, we aim to investigate the association of vital exhaustion and burnout with (measures of) Metabolic Syndrome in a systematic review and meta-analysis.
Materials and methods: PubMed, EMBASE and PsycINFO were searched from inception to April 2, 2020. Studies that investigated adult populations (>18 years), vital exhaustion or burnout as determinants and (measures of) the Metabolic Syndrome as outcomes were included. Data extraction and quality assessment were performed using the Effective Public Health Practice Project tool. When possible, results were meta-analysed using random-effects analyses.
Results: 5317 titles/abstracts were screened, 140 papers were read full text, of which 70 studies were included. 48 studies were cross-sectional, 13 prospective, 4 case-control studies, 2 randomized controlled trials, and 3 had alternative designs. Preliminary results show that burnout and vital exhaustion were significantly correlated with BMI (correlation coefficient: 0.1, 95% CI: 0.0-0.1,I2=61.6%, 13 studies) and diastolic blood pressure (correlation coefficient: 0.1, 95% CI: 0.0-0.2, I2=49.9%, 9 studies). Burnout, but not vital exhaustion, was associated with elevated triglycerides, with a standardized mean difference of 0.22 mmol/L (95% CI: 0.0-0.4, I2=0%, 3 studies). Burnout and vital exhaustion were associated with hypertension, with a pooled odds ratio of 1.2 (95% CI: 1.1-1.4, I2=0.01%, 3 studies). Lastly, vital exhaustion was significantly associated with a slightly higher pooled odds of metabolic syndrome (odds ratio: 1.1, 95% CI: 1.0;1.1, I2=57.1%, 2 studies). This meta-analysis did not show significant associations of burnout and vital exhaustion with systolic blood pressure, HDL, waist-to-hip ratio, fasting glucose, and metabolic syndrome incidence, and we were unable to meta-analyse waist circumference. Most studies were considered of moderate methodological quality and have a moderate to low level of GRADE evidence, due to having cross-sectional designs.
Conclusion: Our meta-analysis indicates that burnout and vital exhaustion are associated with certain parameters of metabolic syndrome. However, no conclusions can be drawn about the incidence of metabolic syndrome. The results should be interpreted with caution due to moderate to low methodological quality and high heterogeneity of the included studies.
Disclosure: S.H.M. Kremers: None.
347
Association between cholecystectomy and dysglycaemia: cross-sectional and prospective analyses
M. Sang1, C. Xie2, Y. Liu1, S. Qiu3, X. Wang4, Z. Sun1, T. Wu2;
1Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China, 2Centre of Research Excellence in Translating Nutritional Science to Good Health, Adelaide Medical School, The University of Adelaide, Adelaide, Australia, 3Department of General Practice, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China, 4Department of Clinical Nutrition, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China.
Background and aims: A handful of cross-sectional studies have reported that cholecystectomy is associated with increased risk of diabetes. However, the relationship between cholecystectomy and the risk of prediabetes has not been assessed. Moreover, the association between cholecystectomy and variations in blood glucose has not been evaluated prospectively. In the current study, we conducted both cross-sectional and prospective analyses to examine the association between cholecystectomy and dysglycaemia in Chinese community-dwelling adults.
Materials and methods: A total of 1612 Chinese community-dwelling adults (n = 1564 without cholecystectomy and n = 48 with cholecystectomy) were surveyed for previous history of cholecystectomy and then followed up over ~3.2 years. Glycaemic status at baseline and follow-up was defined according to the World Health Organization (WHO) 1999 criteria. Percent changes (∆) in fasting blood glucose and HbA1c from baseline at the follow-up visit were calculated to assess fluctuations in blood glucose and defined as stable (-10% ≤ ∆ < 10%), improved (∆ < -10%), or worsened (∆ ≥ 10%) glycaemic control.
Results: The cross-sectional analyses at baseline revealed that participants with cholecystectomy were associated with a 1.9-fold increase in the risk of prediabetes (P = 0.003) and a 4-fold increase in the risk of diabetes (P < 0.001) compared to those without cholecystectomy after adjusting for age, gender, body mass index, current smoking, current drinking, hypertension, triglycerides, high-density lipoprotein, and low-density lipoprotein. The prospective analyses revealed that participants with cholecystectomy were associated with higher risk of deterioration in glycaemic control (∆FPG ≥ 10%: 25.0% vs. 8.8%, P = 0.001 and ∆HbA1c ≥ 10%: 45.8% vs. 23.5%, P < 0.001, for participants with vs without cholecystectomy). These associations remained significant after adjusting for age, gender and baseline glycaemic status.
Conclusion: In Chinese community-dwelling population, individuals undergone cholecystectomy are at increased risk of prediabetes and diabetes and deterioration in glycaemic control. Therefore, cholecystectomized patients should be regularly monitored to avoid the risk of dysglycaemia.
Supported by: National Key R&D Program of China (2016YFC1305700)
Disclosure: M. Sang: None.
SO 12 Different facets of type 2 diabetes treatment
348
Prolonged use of proton pump inhibitors and risk of type 2 diabetes: results from a large population-based nested case-control study
G. Perseghin1, F. Rea2, L. Savaré2, G. Morabito2, G. Corrao2, S. Ciardullo1;
1University of Milano-Bicocca, Monza, 2University of Milano-Bicocca, Milan, Italy.
Background and aims: It is still debated whether prolonged use of proton pump inhibitors (PPIs) might affect metabolic health. The aim of the present study is to investigate the relationship between prolonged use of PPIs and the risk of developing diabetes.
Materials and methods: We performed a case-control study nested into a cohort of 777,420 patients newly treated with PPIs between 2010 and 2015 in Lombardy, Italy. A total of 50,535 cases diagnosed with diabetes until 2020 were matched with an equal number of controls that were randomly selected from the cohort members according to age, sex, and clinical status. Exposure to treatment with PPIs was assessed in case-control pairs based on time of therapy. A conditional logistic regression model was fitted to estimate the odds ratios (OR) and 95% confidence intervals (CI) for the exposure-outcome association, after adjusting for several covariates. Sensitivity analyses were performed to evaluate the robustness of our findings.
Results: Compared to patients who used PPIs for <8 weeks, increased odds of diabetes of 19% (95% CI, 15-24%), 43% (38-49%), and 56% (49-64%) were observed among those who used PPIs for between 8 weeks and 6 months, 6 months and 2 years, and >2 years, respectively. The results were consistent when analyses were stratified according to age, sex and clinical profile, with higher ORs being found in younger patients and those with worse clinical complexity. Sensitivity analyses revealed that the association was consistent and robust.
Conclusion: Regular and prolonged use of PPIs is associated with a higher risk of diabetes. Physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use.
Disclosure: G. Perseghin: None.
349
Risk factors for development of de novo post transplantation diabetes from 8 weeks after kidney transplantation
R. Carlsen1, A. Åsberg1,2, M. Svensson3, K.I. Birkeland1, I. Bressendorff4, H.L. Gulseth5, K. Midtvedt1, T.G. Jenssen1,6;
1Department of Transplantation Medicine, Oslo University Hospital, Oslo, Norway, 2Department of Pharmacy, University of Oslo, Oslo, Norway, 3Department of Nephrology, Akershus University Hospital, Lørenskog, Norway, 4Department of Renal Medicine, Herlev and Gentofte Hospital, Herlev, Denmark, 5Department of Transplantation Medicine, Norwegian Institute of Public Health, Oslo, Norway, 6Faculty of Health Sciences, University of Tromsø, Tromsø, Norway.
Background and aims: Between 10 to 30% of kidney transplant recipients develop post-transplant diabetes mellitus (PTDM). PTDM is a major risk factor for cardiovascular events and death in kidney transplant recipients. At our center prednisolone doses are tapered to a low maintenance dose eight weeks after transplantation. In a retrospective single-center cohort study we investigated risk factors for development of de novo PTDM from eight weeks after kidney transplantation.
Materials and methods: Inclusion criteria were kidney transplantation, age > 18 years, no known diabetes prior to, or at the eight-week control after transplantation and with at least one year of follow up. At the eight-week control an oral glucose tolerance test (OGTT) was performed for PTDM diagnosis. De novo PTDM was defined as fasting glucose ≥ 7 mmol/L, 2-hours glucose ≥ 11.1 mmol/L at OGTT performed routinely at the one-year visit, or at least two registered dispensations of glucose lowering medication in the Norwegian Prescription Database (NorPD).
Results: In total 632 patients (median age 53 years, 62 % males) with a median follow-up of 2.7 years [1-4] were included of which 54 (8.5%) developed de novo PTDM (31 diagnosed by the OGTT at 1 year and 23 identified by glucose lowering dispensation). De novo PTDM patients were older than non-PTDM patients at transplantation (median age 63 vs 52 years), were more often males (81 % vs 61%), and had higher fasting blood glucose (mean 5.8 vs 5.3 mmol/L), 2-hour glucose (mean 8.3 vs 6.6 mmol/L) and HbA1c (median 38 vs 32 mmol/mol) at the eight-week post-transplantation control. In a multiple logistic regression analysis, significant associated factors of PTDM were age (years), OR 1.04 (CI 95%: 1.01;1.06), triglycerides (mmol/L), OR 1.42 (CI 95%: 1.10;1.81) and tacrolimus (μg/L) OR 1.23 (CI 95%: 1.03;1.46) concentrations measured at the one-year control visits, and CMV serostatus (positive donor to negative recipient), OR 2.12 (CI 95%: 1.04;4.28). Plasma magnesium, gender, BMI and plasma creatinine were not associated with development of PTDM.
Conclusion: Development of de novo PTDM from eight weeks after kidney transplantation was associated with increased age, higher triglycerides, higher tacrolimus concentrations and D+/R- CMV-serostatus.
Disclosure: R. Carlsen: None.
350
Glycated haemoglobin and diabetes treatment trajectories in type 2 diabetes: the Fremantle Diabetes Study Phase II
W.A. Davis, T.M.E. Davis;
Medical School, University of Western Australia, Fremantle, Australia.
Background and aims: No studies have examined combined HbA1c and diabetes treatment trajectories. Our aim was to explore the relationship between glycaemia and diabetes treatment complexity over 6 years in a prevalent cohort of community-based people with type 2 diabetes using group-based trajectory modelling (GBTM), and identify baseline predictors of trajectories.
Materials and methods: Sixty-one percent (899) of the 1482 participants in the Fremantle Diabetes Study Phase II (FDS2) type 2 diabetes cohort attended ≥3 biennial comprehensive assessments in the 6 years following recruitment between 2008 and 2011. Blood glucose-lowering therapy and HbA1c were documented at each assessment. GBTM identified combined HbA1c and diabetes treatment trajectory groups. To assist model selection, the Bayesian Information Criterion determined the optimum number of groups and their functional form. Other selection criteria included average posterior probabilities of group membership >0.70 and odds of correct classification >5. Multinomial regression identified plausible baseline predictors of group membership.
Results: At baseline, participants had a mean±SD age of 65.1±10.4 years, 54.4% were males, their diabetes duration was a median [inter-quartile range] 8 [2-15] years, and their HbA1c was 6.7 [6.2-7.5]% (50 [44-58] mmol/mol); 27.5% were managed with lifestyle alone, 29.3% with single non-insulin therapy, 23.6% with multiple non-insulin therapies, and 19.6% with insulin (alone or combined with other therapies). GBTM revealed three trajectory groups (low (19.3%), medium (54.0%), high (26.8%)) corresponding to increasing HbA1c and treatment complexity (see Figure). For all groups, the average posterior probabilities were >0.98 and the odds of correct classification were >64. Over 6 years, the low group maintained an HbA1c ≤6.5% with progression from 1.7% to 17.7% on single/multiple non-insulin therapy, the medium group showed a modest increase in HbA1c (6.9% to 7.2%) with an increase from 33.1% to 61.2% on multiple non-insulin therapy/insulin, while the high group maintained an HbA1c at 8.0% with insulin treatment progressing from 68.6% to 98.5%. Multinomial regression revealed age at diabetes diagnosis (negatively), diabetes duration, indices of obesity, and retinopathy significantly predicted membership of both the medium and high groups compared to the low group with increasing magnitude from the medium to high group.
Conclusion: These data show that, regardless of baseline HbA1c, glycaemic trajectories in community-based type 2 diabetes changed minimally over time despite treatment intensification. This includes the high group (>25% of the cohort) which had prolonged poor glycaemic control and experienced therapeutic inertia even though almost all progressed to insulin. People diagnosed young with long duration disease and obesity may not be managed proactively enough to maintain appropriate glycaemic targets and thus prevent microangiopathy.
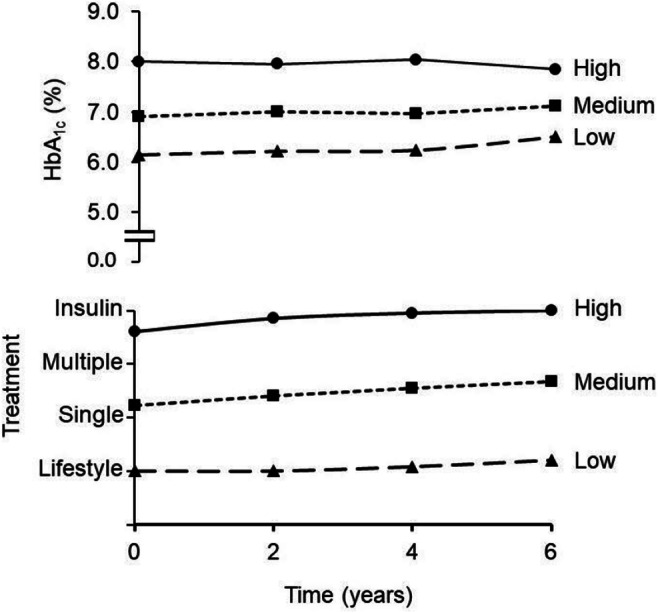
Supported by: Australian NHMRC Project Grants
Disclosure: W.A. Davis: Grants; Australian NHMRC Project Grants.
351
Fracture risk in treatment with GLP-1RA compared to DPP-4i: a Danish nationwide cohort study
Z.K. Al-Mashhadi1,2, R. Viggers3,4, R. Fuglsang-Nielsen2,5, P. Vestergaard3,4, S. Gregersen1,2, J. Starup-Linde2,5;
1Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, 2Department of Clinical Medicine, Aarhus University, Aarhus, 3Steno Diabetes Center North Jutland, Department of Endocrinology, Aalborg University Hospital, Aalborg, 4Department of Clinical Medicine, Aalborg University, Aalborg, 5Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Background and aims: Type 2 diabetes mellitus is associated with increased risk of fracture. The evidence regarding glucagon-like peptide 1 receptor agonists (GLP-1RA) and fracture risk is unsettled. We investigated the risk of major osteoporotic fractures (MOF) when comparing GLP-1RA to dipeptidyl peptidase 4 inhibitors (DPP-4i).
Materials and methods: We conducted a Danish population-based cohort study. Discharge diagnosis codes (ICD-10 and ICD-8-system) and redeemed drug prescriptions (ATC codes) were obtained from the Danish National Patient Registry and the Danish National Prescription Registry, respectively. We identified subjects treated with metformin in combination with either GLP-1RA or DPP-4i between 2007 and 2018. Subjects were propensity-score matched 1:1 on age, sex, and index date. Hazard rate ratios (HR) for MOF were calculated using a Cox proportional hazards model. In addition, additive rather than relative hazard effects were investigated using Aalen’s additive hazards model.
Results: We identified 42,816 individuals treated with either combination. After matching, 32,266 individuals remained. Median follow-up times in the GLP-1RA and DPP-4i group were 642 days and 529 days, respectively. The crude HR for MOF was 0.89 [95% CI 0.76-1.05] with GLP-1RA compared to DPP-4i. In the fully adjusted model, an unaltered HR of 0.86 [95% CI 0.73-1.03] was obtained. For hip fractures, we found a crude HR of 0.68 [95% CI 0.49-0.96] and a similar adjusted HR.
Conclusion: The risk of MOF tended to be slightly lower with GLP-1RA than with DPP-4i. The lower fracture risk became significant when examining hip fractures and when allowing follow-up to continue after medication change.
Supported by: Steno Collaborative Grant, NNF
Disclosure: Z.K. Al-Mashhadi: Grants; Steno Collaborative Grant, Novo Nordisk Foundation, Denmark (Grant no. NNF18OC0052064).
352
Metformin affects DNA methylation patterns in newly-diagnosed patients with type 2 diabetes
S. Garcia-Calzon1,2, S. Schrader1, A. Perfilyev1, C. Ling1;
1Epigenetics and Diabetes Unit, Lund University Diabetes Centre, Malmö, Sweden, 2Food Science and Physiology, University of Navarra, Pamplona, Spain.
Background and aims: Metformin is the first line pharmacological treatment for type 2 diabetes (T2D). However, the molecular mechanisms involved in metformin action are not yet totally understood. Therefore, the aim of this study was to elucidate whether metformin therapy affects epigenetic mechanisms in newly-diagnosed individuals with T2D.
Materials and methods: 171 individuals with T2D from the ANDIS cohort were included. Cases were considered those subjects on metformin therapy (n=43), whereas controls (n=128) were randomly selected and matched to cases by age, sex, BMI, HbA1c, statins and antihypertensives. Genome-wide DNA methylation was analysed using the MethylationEPIC array and multiple linear regression models were run to assess differences in methylation between cases and controls.
Results: We found 1,451 sites (>2% absolute difference in methylation, FDR<0.05) differentially methylated between the two groups after adjusting for potential clinical confounders and cell composition using the reference-free Houseman method. The majority of these sites presented higher methylation levels in those individuals on metformin therapy. Notably, 13 sites showed >10% absolute difference in DNA methylation between cases and controls, and include annotated genes involved in glucose metabolism such as PFKP (19% absolute difference in methylation, cases 55.6 ± 30.4, controls 74.5 ± 22.9, q=0.015). KEGG pathway analyses of the genes annotated to the 1,451 sites yielded 6 overrepresented pathways (FDR<0.05) among which were cAMP signalling pathway, calcium signalling pathway and circadian entrainment.
Conclusion: Metformin therapy associates with differential DNA methylation genome-wide in newly-diagnosed T2D individuals, suggesting that metformin’s effects might involve epigenetic mechanisms.
Supported by: EFSD Research Programme, Novo Nordisk Foundation, ERC, H2020-MSCA, IRC15-0067, SDF
Disclosure: S. Garcia-Calzon: None.
353
Transcriptional and epigenetic changes in skeletal muscle of persons with diabetes after bariatric surgery
L. Kovac1, M. Ouni1, S. Gancheva2, S. Kahl2, M. Jähnert1, M. Roden2, A. Schürmann1;
1Experimental Diabetology, German Institute of Human Nutrition, Nuthetal, 2German Center for Diabetes Research, München-Neuherberg, Germany.
Background and aims: Bariatric surgery is known to be the most effective intervention to treat metabolic abnormalities associated with obesity and type 2 diabetes (T2D). In our recent study, it was shown that bariatric surgery alters the skeletal muscle transcriptome of obese people, in part through changes in DNA methylation. Next, we were interested in corresponding changes that occur in T2D individuals in response to bariatric surgery, and how they compare to effects detected in non-diabetic obese individuals.
Materials and methods: RNA-sequencing and DNA methylation analyses were done on skeletal muscle of 13 obese non-diabetic and 13 obese diabetic individuals, before and one year after bariatric surgery. Several in silico methods were combined for data analysis, including pathway enrichment and integrating phenotypes of knockout mice.
Results: In the muscle of participants with diabetes, 2012 genes were differentially expressed before and one year after the surgery (p<0.05). Among these, 803 genes were differentially methylated (p<0.05) and enriched in pathways linked to skeletal muscle development, hypoxia response and cell adhesion (p<0.05). The expression of 69 genes, including candidates involved in insulin signaling, lipid metabolism and glucose homeostasis, correlated with whole-body insulin sensitivity. Specifically, seven genes were previously described as T2D susceptibility genes in GWAS (e.g. ACHE, ABCC5, CAVIN1) and 26 genes associated with glucose metabolism, lipid levels and skeletal muscle function (e.g. IGFBP4, TXLNA, LDB3), according to the phenotype data of corresponding knockout mice. Interestingly, we observed striking differences between obese and T2D transcriptional response to surgery. Prior to surgery, obese and T2D persons exhibit over 2700 differentially expressed genes(q<0.05), of which approximately 70% remain significantly different one year after surgery. Only a third of the affected genes was commonly regulated between the two groups, indicating distinctive responses of skeletal muscle to the surgery. The differences in expression were mirrored in the affected biological pathways, with insulin signaling and fatty acid metabolism genes significantly enriched among those altered in obese but not T2D participants.
Conclusion: First, we generated a comprehensive overview of transcriptional and epigenetic changes in skeletal muscle, providing molecular insights into the long-term response of T2D people to bariatric surgery. Second, our comparison of skeletal muscle transcriptome from obese and T2D individuals suggests that these two groups are significantly different before and exhibit divergent responses to the bariatric surgery.
Supported by: BMBF
Disclosure: L. Kovac: Grants; 82DZD00302.
SO 13 It's getting complicated
354
Causes and determinants of mortality in community-based people with type 1 diabetes: The Fremantle Diabetes Study Phase I
T.M.E. Davis, W.A. Davis;
University of Western Australia, Fremantle, Australia.
Background and aims: Few studies have examined the causes of death and their predictors in community-based people with type 1 diabetes (T1D). Our aim was to determine cause-specific mortality and baseline determinants in the longitudinal observational Fremantle Diabetes Study Phase I (FDS1) cohort.
Materials and methods: FDS1 participants with T1D were followed from the time of study entry between 1993 and 1996 to end-2017 through the validated Western Australian Data Linkage System. All deaths were ascertained and their causes adjudicated based on UK Prospective Diabetes Study categories. Cox with or without Fine and Gray competing risk regression with age as the timeline were used to identify aetiological/prognostic risk factors.
Results: The mean±SD age of the 125 FDS1 participants with T1D at entry was 42.0±16.0 years, 59.2% were male and their median diabetes duration [inter-quartile range] was 11 [3-21] years. During 2,231 person-years (mean+SD 17.9+7.4 years) of follow-up, 55 (44.0%) died (mortality rate (MR) (95% CI) 24.7 (18.6, 32.1) /1,000 person-years). The cause of death was cardiovascular disease (CVD) in 25 (45.5%; MR 11.2 (7.3, 16.5) /1,000 person-years), cancer in 5 (9.1%), renal disease in 5 (9.1%), and other less frequent causes including infection, trauma or unknown cause in 20 (36.4%). Independent predictors of all-cause mortality were diabetes diagnosis in young adulthood (age 18-27 years or 2nd quintile; hazard ratio (HR) (95% CI) 3.57 (1.61, 7.96) vs other quintiles), HbA1c (1.37 (1.18, 1.60) per 1% (11 mmol/mol) increase), ln(urinary albumin:creatinine ratio) (1.21 (1.04, 1.40), where a 2.72 fold increase in ACR equates to an increase of 1 in ln (ACR)), and presence of retinopathy (2.26 (1.05, 4.09)). CVD death was predicted by age at diagnosis 18-27 years (6.71 (2.08, 21.7)), HbA1c (1.43 (1.11, 1.86) per 1% (11 mmol/mol) increase), and a body shape index (1.013 (1.003, 1.023) per increase of 0.001 m11/6/kg2/3); after adjustment for the competing risk of non-CVD death, the predictors were ln(ACR) (1.42 (1.19, 1.71) and a past history of coronary artery disease (CAD; 3.02 (1.32, 6.90)).
Conclusion: These data show that diagnosis of T1D in young adulthood carries a relatively poor prognosis, but also show that poor glycaemic control and its consequences (albuminuria and retinopathy) predict premature mortality. CVD death in T1D remains relatively common; it shares some aetiological risk factors with all-cause death (diagnosis in young adulthood and HbA1c), but albuminuria and past CAD are prognostically important.
Supported by: Raine Foundation University of Western Australia
Disclosure: T.M.E. Davis: Grants; Raine Foundation University of Western Australia.
355
Relationships between CGM-derived metrics and risk factors for hypoglycaemia among patients with type 2 diabetes with TIR more than 70%
J. Lu1, D. Chen2, W. Xu2, D. Yang2, Z. Liu2, B. Lin2;
1The First Municipal Hospital of Guangzhou, 2The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Background and aims: To explore the risk factors associated with hypoglycemia by using continuous glucose monitoring (CGM) in Type 2 Diabetes Mellitus (T2DM) patients with TIR (time in range) >70%.
Materials and methods: This was a retrospective study. We analyzed the data of 111 T2DM patients with TIR>70% who received 48-72h CGM in Department of Endocrinology and Metabolic Disease from January 2018 to October 2019. The mean blood glucose (MBG) and glucose variability (GV) including the mean amplitude of glucose excursion (MAGE), the largest amplitude of glycemic excursion (LAGE), coefficient of variation of blood glucose (CV), mean of daily difference (MODD) and low blood glucose index (LBGI) were measured on admission using CGM. Univariate and multivariate logistic regression were used to analyze the relationship between hypoglycemia (defined as blood glucose below 70mg/dL, detected by CGM) and MBG, MAGE, LAGE, CV, MODD and LBGI. The receiver operator characteristic (ROC) curve was drawn to evaluate the ability of predicting hypoglycemia.
Results: (1) Of 111 subjects, 53 patients experienced 278 events with a glucose level<3.9 mmol/l and 63 events with a glucose level<3.0 mmol/L. The highest incidence of hypoglycemia was presented from 10pm. to 6am. (40.6%). (2) Compared to nonhypoglycemia group, MAGE, LAGE, MODD, CV and LBGI were significantly higher (t=-7.46 to -3.176, all P<0.005) while MBG was lower than those in hypoglycemia group (t=2.944, P=0.004). (3) Multivariate logistic regression analysis showed that MBG (OR=0.51,P=0.007) and GV including MAGE (OR=1.79,P=0.002), LAGE (OR=1.90,P<0.001), MODD (OR=7.59,P<0.001), CV (OR=1.23,P<0.001), LBGI (OR=5.39,P=0.001) were significant and independent risk factors of hypoglycemia. (4) The ROC curve for predicting hypoglycemic events indicated that the cutoff points for MBG, MAGE, LAGE, MODD, CV and LBGI were 7.26mmol/L, 2.85mmol/L, 9.25mmol/L, 1.35mmol/L, 22.66% and 1.31. Hypoglycemia was observed only 27.4% of patients in the MBG≥7.26mmol/l group but a higher proportion of 61.2% in the MBG<7.26mmol/l group (P<0.05), meanwhile 15.9% patients developed hypoglycemia in the LBGI<1.31mmol/l group but a larger proportion 89.6% in the LBGI≥1.31mmol/l group(P<0.05). Using MBG and LBGI as hypoglycemia risk factors, the proportion of hypoglycemia patients was 3.4% without hypoglycemia risk factors, 34.8% with one risk factor, and 100% with two risk factors (P<0.001).
Conclusion: The incidence of hypoglycemia is high in T2DM patients with TIR>70%. MBG and GV including MAGE, MODD, CV, LBGI and LAGE are the significant and independent risk factors for hypoglycemia. Low MBG and large glucose excursion may possibly develop hypoglycemia. To achieve good glycemia control without inducing hypoglycemia, maintaining glucose in a euglycemia level and minimizing the glucose excursion are of great importance.
Clinical Trial Registration Number: [2020]02-107-01
Disclosure: J. Lu: None.
356
Incidence and characteristics of hyperosmolar hyperglycaemic state: a Danish cohort study
E.V. Rosager1, A.L.K. Heltø1, L. Friis-Hansen2, J. Petersen3,4, F.E. Nielsen1, S.B. Haugaard5,6, R. Gregersen1,3;
1Department of Emergency Medicine, Copenhagen University Hospital - Bispebjerg and Frederiksberg, 2Department of Clinical Biochemistry, Copenhagen University Hospital - Bispebjerg and Frederiksberg, 3Center for Clinical Research and Prevention, Copenhagen University Hospital - Bispebjerg and Frederiksberg, 4Department of Public Health, Section of Biostatistics, 5Department of Endocrinology, Copenhagen University Hospital - Bispebjerg and Frederiksberg, 6Institute of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Background and aims: The hyperosmolar hyperglycemic state (HHS) is the most rare and life-threatening diabetic complication, consisting of hyperosmolarity, hyperglycemia, clinical dehydration, absence of significant ketoacidosis, and a degree of encephalopathy. We aimed to find the incidence of HHS among patients with diabetes, describe the clinical and biomarker profiles of HHS in Denmark, and describe subgroups of HHS patients with acidosis and/or acute kidney injury (AKI).
Materials and methods: In this national register-based cohort study, we identified acutely admitted patients from Danish hospitals with HHS (defined as hyperglycemia ≥33 mmol/L and hyperosmolarity (plasma-sodium x2 + glucose) ≥320 mmol/L) based on blood samples taken 2 h before till 6 h after hospital arrival. We included patients of age ≥18 years from the four out of five regions in Denmark where laboratory data were available in the period from 2016-2018. Only the first hospitalization with HHS was included. Subgroups of patients with acidosis defined by pH < 7.35, and/or AKI (relative to last creatinine result, defined by the Kidney Disease improving Global Outcome guidelines) were described.
Results: We included 541 patients who met the HHS criteria at hospital admission. This was from a population of approximately 3.7 million adult inhabitants of whom 223,224 people had a known diabetes diagnosis. Of the HHS patients, 68% had known diabetic comorbidity and their median (Q1;Q3) duration since diabetes onset was 12 (6;15) years. The 3-year incidence proportion of HHS among patients with diabetes was 0.15%. The included patients had a median age of 70 (58;80) years and 60% were male. Diabetes with complications (as defined by the multimorbidity index M3) was the most frequent comorbidity (32%), and 75% had prescriptions of more than 5 different drugs. The median plasma glucose and sodium levels were 50 (42;62) and 142 (137;149) mmol/L, respectively. Median levels of potassium (4.7 [4.2;5.4] mmol/L), lactate (3.1 [2.5;5.2] mmol/L), C-reactive protein (23 [8;81] mg/L), leukocytes (14.7 [11,19] 109/L), creatinine (147 [106;209] mmol/L) and carbamide (19 [14;27] mmol/L) were elevated. A proportion of 63% of HSS patients had AKI (AKI data available for 430 patients) and 67% had acidosis (pH data available for 478 patients).
Conclusion: We identified 541 individuals with HHS who were acutely admitted to Danish hospitals during a 3-year period. The 3-year incidence of HHS among patients with diabetes was 0.15%. One third of the patients with HHS had not known diabetes at the time of admission. The HHS patients were old (median age of 70 years), often male sex, had prescribed more than 5 drugs, and presented with nephropathy, but a relatively low C-reactive protein.
Supported by: BFH Research Commitee Fund
Disclosure: E.V. Rosager: None.
357
Stress hyperglycaemia is associated with an increased risk of post-acute pancreatitis diabetes
J. Zhang, Y. Lv, L. Li;
Department of Endocrinology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China.
Background and aims: Acute pancreatitis is the most common cause of Pancreatic exocrine diabetes and its prevalence has increased dramatically worldwide. However, the key risk factors for diabetes secondary to acute pancreatitis remain unclear. Stress hyperglycemia has been shown to be associated with an increased risk of diabetes in acute or critically ill patients. Therefore, we investigated whether patients with acute pancreatitis without known diabetes have a higher risk of developing subsequent diabetes compared to patients who remain normoglycemic.
Materials and methods: This retrospective observational study was conducted on 3575 in-patients with acute pancreatitis from 2016 to 2020. Among 946 patients met the inclusion criteria. Patients were stratified by stress hyperglycemia and normoglycemic counterparts. The association between stress hyperglycemia and evidence of diabetes was evaluated using regression modeling.
Results: There were 817 (86.4%) normoglycemic and 129 (13.6%)stress hyperglycemia patients. 41 patients (31.8%) with stress hyperglycemia had evidence of diabetes compared with 25 patients (3.1%) without stress hyperglycemia (P < 0.001). After multivariable adjustment including age, sex, history of diabetes, alcohol consumption, smoking, hypertension, obesity, disease severity, fatty liver disease and serological markers, Stress hyperglycemia was significantly associated with subsequent diabetes odds ratio 4.407 (95%, CI 2.377-8.172). Further adjustment of diet, exercise and recurrence of pancreatitis, stress hyperglycemia remained a predictor of diabetes mellitus.
Conclusion: Stress hyperglycemia was independently associated with diabetes secondary to acute pancreatitis. Accordingly, a follow-up diabetes-screening programs for AP with stress hyperglycemia is an important part of identifying the disease as soon as possible, delaying islet damage, avoiding adverse outcomes and improving the prognosis of post-acute pancreatitis diabetes mellitus.
Disclosure: J. Zhang: None.
358
Fasting proinsulin independently predicts incident type 2 diabetes in the general population, particularly in subjects with hypertension or kidney dysfunction
S. Sokooti Oskooei1, W.A. Dam1, T. Szili-Torok1, J. Gloerich2, A.J. van Gool2, A. Post1, M.H. de Borst1, R.T. Gansevoort1, H.J.L. Heerspink1, R.P.F. Dullaart1, S.J.L. Bakker3;
1University Medical Center Groningen, Groningen, 2Radboud University Medical Center, Nijmegen, 3Internal Medicine, University Medical Center Groningen, Groningen, Netherlands.
Background and aims: Fasting proinsulin levels may serve as a marker of β-cell dysfunction and predict type 2 diabetes development. Moreover, kidneys have been found to be a major site for degradation of proinsulin. We aimed to evaluate the predictive value of proinsulin compared to insulin and C-peptide for the risk of incident type 2 diabetes added to a base model of clinical predictors and examined potential effect-modification by variables related to kidney function.
Materials and methods: We included 5001 subjects of the Prevention of Renal and Vascular End-Stage Disease study without type 2 diabetes at baseline. Proinsulin was measured in plasma with U-PLEX platform using ELISA immunoassay. Cox proportional hazards regression and Harrel’s C-statistics were used to evaluate the association between fasting proinsulin and incident type 2 diabetes. Interaction was tested to assess potential effect-modification by hypertension, eGFR and urinary albumin excretion (UAE).
Results: Median fasting proinsulin was 6.89 (5.15-9.55) pmol/L. During a median of 7.2 (6.0-7.7) years follow up, 271 individuals developed type 2 diabetes. Higher levels of proinsulin were associated with an increased risk of incident type 2 diabetes independent of glucose, insulin, C-peptide and clinical factors [HR 1.28; per 1 SD increase 95% CI: 1.08-1.52]. Moreover, Harrell’s C-index and IDI were significantly improved with the addition of proinsulin to the Framingham Offspring (FOS) risk score (Table 1). Furthermore, we found significant effect modification by hypertension (p=0.019), eGFR (p=0.020) and UAE (p=0.034), consistent with a particularly strong association in subjects with hypertension or kidney dysfunction (eGFR<90 mL/min/1.73 m2 or urinary albumin excretion ≥15 mg/24 hours).
Conclusion: Higher fasting proinsulin level is an independent predictor of incident type 2 diabetes in the general population, particularly in subjects with hypertension or kidney dysfunction.
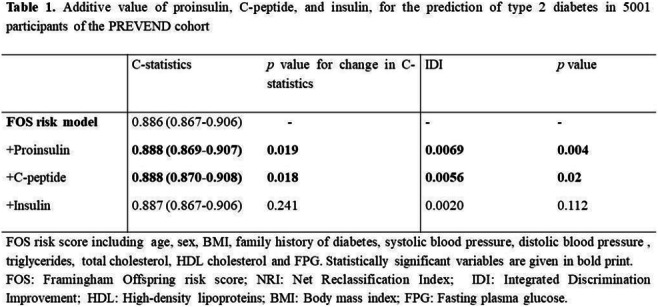
Supported by: European Union’s Horizon 2020 research and innovation programm Marie Sklodowska-Curie
Disclosure: S. Sokooti Oskooei: None.
359
Differential proportion trends of primary lower limb amputations associated with diabetes or peripheral arterial disease between age groups in Quebec, Canada
S. O'Connor1, C. Blais2, E.A.L. Sidi2, J. Leclerc3,1, P. Poirier1;
1Pharmacy, Institut universitaire de cardiologie et pneumologie de Québec - Université Laval, 2Bureau d’information et d’études en santé des populations, Institut national de santé publique du Québec, 3Nursing, Université du Québec à Trois-Rivières, Québec, Canada.
Background and aims: Lower limb amputations (LLA) represent an important complication of both diabetes and patients with peripheral arterial disease (PAD). Given the increasing risk of LLA with age, demographic changes in Quebec and recent increasing incidence of LLA in other Canadian provinces, our objective was to document proportion trends of primary LLA according to age groups in the province of Quebec, Canada, from 2006 to 2019.
Materials and methods: Using the Quebec Integrated Chronic Disease Surveillance System (n=4,526,798 in 2019), we identified all primary LLA among adults ≥40 years using codes from the Canadian Classification of Health Interventions and the International Classification of Diseases 10th edition. We calculated annual proportions (per 100,000) with primary cases of LLA as the numerator and the population with diabetes / PAD at risk as the denominator, with 99% CI. Proportions were stratified by age groups: (40-64, 65-79 and ≥80 years) and by type of LLA (Minor: distal to tibia; Major: tibia, knee and thigh).
Results: In 2019, among adults with a primary LLA associated with diabetes and / or PAD, 46.0% had diabetes alone, while 13.8% had PAD alone and 40.1% had both diabetes and PAD. In 2019, cases were on average 70±12 (SD) years old and 66.6% were ≥65 years old. Between 2006 to 2019, while the proportion of all primary LLA remained stable for the 40-64 years (2019: 115 per 100,000 [99% CI: 97-132] [n=284]) and ≥80 years age groups (2019: 160 per 100,000 [99% CI: 132-189] [n=206]), we observed a relative decline of 27% among the 65-79 years age group, despite an increase in the number of incident LLA (2006: 167 per 100,000 [99% CI: 142-192] [n=290]; 2019: 121 per 100,000 [99% CI: 105-137] [n=359]). Between 2006 and 2019, annual proportion of minor LLA remained stable in all age groups (2019: 40-64 years: 96 per 100,000 [99% CI: 80-112] [n=238]; 65-79 years: 94 per 100,000 [99% CI: 80-109] [n=280]; ≥80 years: 126 per 100,000 [99% CI: 101-152] [n=162]). While the proportion of major LLA remained stable among the 40-64 years (2019: 19 per 100,000 [99%CI: 12-26] [n=46]) and ≥80 years age groups (2019: 34 per 100,000 [99% CI: 21-48] [n=44]), we observed a 56% relative reduction in the proportion of major LLA among adults from 65-79 years (Figure 1).
Conclusion: Given the aging population in Quebec, stable proportion trends and reduction of major LLA among adults between 65 and 79 years suggest that the progress in preventive care may have favorably influenced the severity of LLA among age groups at risk. However, our results also depicted the importance to further improve preventive care in order to fully prevent unnecessary LLA.
Supported by: Canadian Institutes for Health Research (CIHR)
Disclosure: S. O'Connor: None.
360
Cardiovascular outcomes in patients with both diabetes and phenotypic familial hypercholesterolaemia: a nationwide register-based cohort study
J. Brinck1, E. Hagström2, J. Nåtman3, S. Franzén4, K. Eeg-Olofsson5, D. Nathanson1, B. Eliasson5;
1Department of Medicine Huddinge, Karolinska Institute, Stockholm, 2Department of Medical Sciences, Uppsala university, Uppsala, 3National Diabetes Register, Centre of Registers, Region Västra Götaland, Gothenburg, 4Department of Public Health and Community Medicine, University of Gothenburg, Gothenburg, 5Department of Molecular and Clinical Medicine, University of Gothenburg, Gothenburg, Sweden.
Background and aims: Patients with diabetes have increased mortality and cardiovascular morbidity compared to the general population. The hereditary trait, familial hypercholesterolemia (FH), also leads to the premature development of atherosclerotic cardiovascular disease. Whether cardiovascular risk is exacerbated in patients with combined traits is not yet known.
Materials and methods: In this nationwide, register-based cohort study, patients with diabetes were included between January 1st, 2002, and December 31st, 2020. Phenotypic FH was identified in the patients with diabetes using the Dutch Lipid Clinic Network criteria. Adjusted Cox-proportional hazards models were used to assess the risk of mortality and cardiovascular outcomes in patients with or without phenotypic FH, using matched controls from the general population as the reference.
Results: A total of 45,585 patients with type 1 diabetes (227,923 controls) and 655,250 patients with type 2 diabetes (655250 controls) were followed for a median of 14·1 and 7·9 years, respectively. Of those, 153 and 7197, respectively, had phenotypic FH. Compared to controls, patients with diabetes were more at risk of cardiovascular mortality (Type 1: hazard ratio [HR] 21·3, 95% confidence interval [CI] 14·6-31·0; Type 2: HR 2·40, 95%CI 2·19-2·63) and of a cardiovascular event (Type 1: HR 15·1, 95%CI 11·1-20·5; Type 2: HR 2·73, 95%CI 2·58-2·89). Patients with phenotypic FH consistently had higher annual estimated mean LDL cholesterol levels throughout the whole study period (p<0·05) and were more at risk for all major cardiovascular outcomes (p<0·0001) than patients without FH. The proportion of patients receiving any lipid-lowering treatment, a high intensity statin, or at least two classes of drugs was higher in patients with phenotypic FH (p<0·0001).
Conclusion: Patients with both diabetes and phenotypic FH in routine clinical care are more at risk of adverse cardiovascular outcomes and have higher LDL cholesterol levels despite receiving intensified lipid-lowering therapy.
Disclosure: J. Brinck: Grants; City councils (ALF 83620).
361
Costs of major complications in people with and without diabetes in Tasmania, Australia
N.T.T. Dinh1,2, B. de Graaff1, J.A. Campbell1, M.D. Jose3,4, J. Burgess3,5, T. Saunder3, A. Kitsos3, N. Wiggins1, A.J. Palmer1;
1Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia, 2Thai Nguyen University of Medicine and Pharmacy, Thai Nguyen, Viet Nam, 3School of Medicine, University of Tasmania, Hobart, Australia, 4Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia, 5Department of Endocrinology, Royal Hobart Hospital, Hobart, Australia.
Background and aims: Diabetes is a costly disease that places a huge burden on the Australian healthcare system. Most of the costs related to diabetes are due to management of complications. In order to reduce the economic burden of diabetes, it is essential to identify complications that are the key cost drivers. Additionally, estimating medical costs of complications in people with diabetes will provide essential input data for economic models to identify cost-effective interventions. Our study aimed to estimate costs of diabetes complications in the year of first occurrence and the second year, and to quantify the incremental costs of diabetes versus non-diabetes related to each complication.
Materials and methods: In this matched retrospective cohort study, people with diabetes (n=45,378) were identified based on either diabetes diagnostic criteria or diagnostic codes from a linked dataset in Tasmania, Australia between 2004-2017. Using propensity score matching, each of them was matched on age, sex, and residential areas with two matched non-diabetes people obtained from the same dataset. Direct costs (including hospital, emergency room visits and pathology costs) were calculated from the healthcare system perspective and expressed in 2020 Australian dollars. The average-per-patient costs and the incremental costs in people with diabetes were calculated for each complication. The confidence interval was derived by a bias-corrected bootstrapping method with 1,000 resamples.
Results: For people with diabetes, first-year costs of complications were: dialysis $87,460 (95% CI 79,818, 95,366), lower extremity amputations $71,258 (65,969, 76,427), kidney transplant $50,031 (34,619, 68,791), non-fatal myocardial infarction $33,196 (31,761, 34,458), foot ulcer/gangrene $32,628 (29,671, 35,606), heart failure $30,752 (29,235, 32,517), ischemic heart disease $30,538 (28,219, 32,922), non-fatal stroke $30,035 (28,606, 31,750), kidney failure $28,534 (23,398, 35,349), nephropathy $23,990 (18,471, 31,485), vitreous haemorrhage $21,630 (14,655, 29,259), neuropathy $20,347 (18,577, 22,555), retinopathy $20,290 (15,368, 26,711), angina pectoris $17,730 (16,416, 19,187), transient ischemic attack $17,638 (15,929, 19,850), blindness/low vision $15,607 (10,584, 21,668). The second-year costs ranged from 19% (ischemic heart disease) to 73% (dialysis) of first-year costs. Complication costs were 115%-259% higher than in people without diabetes.
Conclusion: Costs of treating complications are higher for people with diabetes versus people without diabetes. Diabetes complication treatment required substantial healthcare resources, even after the first year of occurrence. Costly complications included renal complications (especially dialysis), foot complications (especially lower extremity amputation), and macrovascular complications (especially non-fatal myocardial infarction). Our results can be used to populate diabetes simulation models and will support policy analyses to reduce the burden of diabetes.
Supported by: This study was made possible through funding from the Royal Hobart Hospital Research Foundation
Disclsoure: N.T.T. Dinh: Other; MJ is a member of the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA).
362
Cause-specific excess risk of hospitalisation in people with type 2 diabetes compared to the general population in Australia, 2010-2017
D. Tomic, A. Salim, J.I. Morton, J.E. Shaw, D.J. Magliano;
Baker Heart and Diabetes Institute, Melbourne, Australia.
Background and aims: As diabetes management has improved, we are beginning to see a diversification of diabetes complications. Hospitalisation rates provide one measure of the burden of diabetes complications, but this has mostly been described in terms of broad disease categories. We sought to quantify the excess risk of cause-specific hospitalisations at a narrow category level amongst Australians with type 2 diabetes (T2DM) compared to the general population.
Materials and methods: We linked Australians with T2DM registered on the National Diabetes Services Scheme (Australian Diabetes Registry) to hospitalisation datasets to determine the incidence of hospitalisations at a narrow category level for the years 2010-2017. We used the general Australian population, sourced from a National Morbidity Database, as a comparator group. Poisson regression was used to model incidence of hospitalisations. The model was sex- and cause-specific and was adjusted for age and calendar-year effects. We identified the leading causes of excess hospitalisation amongst those with T2DM. Causes were ranked according to excess admissions in the diabetes population, calculated as observed minus expected admissions after standardisation for age. Hospitalisation for T2DM (E11), including admissions for glucose disturbances such as hypoglycaemia in those with T2DM, was excluded from calculations.
Results: People with T2DM were at increased risk for hospitalisation for most diagnoses compared to the general population. The leading causes for excess hospitalisation amongst males with T2DM were stress-related disorders, iron deficiency anaemia, and pneumonia (Table). In females with T2DM, the leading causes were iron deficiency anaemia, urinary tract infections, and depressive episodes. In both sexes, there was a substantial excess risk of admissions for infections, mental health disorders, and gastrointestinal conditions amongst those with T2DM. While heart failure and other traditional diabetes complications were amongst the leading causes of excess risk in males with T2DM, not a single classical microvascular or macrovascular complication of diabetes featured in the top ten causes of excess hospitalisation for females with T2DM.
Conclusion: Diabetes management strategies may require revision to account for the substantial burden of a wide set of lesser-acknowledged diabetes complications, especially in females, such as mental health disorders, anaemia, and infection.
Disclosure: D. Tomic: None.
SO 14 COVID-19 around the globe
363
The role of glycated haemoglobin, body mass index and waist-to-hip ratio in determining the risk of COVID-19 and long COVID
A. Knuppel1, S.V. Eastwood1, R.J. Silverwood2, K.M. Tilling3, A.D. Hughes1, D.M. Williams1, N. Chaturvedi1;
1MRC Unit for Lifelong Health and Ageing, University College London, London, 2Centre for Longitudinal Studies, University College London, London, 3MRC Integrative Epidemiology Unit, University of Bristol, Bristol, UK.
Background and aims: Both diabetes and obesity are associated with severity of and risk of death from COVID-19. The underlying mechanisms, and their role in risk of prolonged post-COVID-19 symptoms (long COVID), is unclear. We therefore aimed to investigate the association of HbA1c, body mass index (BMI) and waist-to-hip ratio (WHR) measured pre-pandemic with a) self-reported COVID-19 and b) long COVID in UK prospective cohort studies.
Materials and methods: In preliminary analyses we used data from the 1958 National Child Development Study (NCDS) and the 1970 British Cohort Study (BCS70). Both are ongoing cohort studies following nationally representative samples of all births in Great Britain. HbA1c was measured in 2002 in NCDS participants, and 2016-18 in BCS70 participants. Weight, height, waist and hip circumference were also measured at this time, and questionnaires on health and lifestyle were completed. All eligible participants (NCDS, N=4793; BCS70, N=3419) completed three questionnaires during the Covid-19 pandemic (May 2020 to February 2021), of which the last one included questions on ongoing COVID-19-related symptoms. COVID-19 was self-reported based on test confirmation or strong suspicion, long COVID was defined as having symptoms that affected functioning for 4+ weeks after COVID-19 and was compared to those reporting symptoms for <4 weeks. Multivariable logistic regression was used to determine associations of HbA1c, self-reported diabetes, BMI, and WHR with presence of COVID-19 and long COVID in each study. Study-level results were meta-analysed using random-effects models.
Results: Between May 2020 and February 2021, NCDS participants were aged 63 years, BCS70 51 years. In NCDS 572 (12%) participants reported ever having COVID-19 and in BCS70 599 (18%). In February 2021, 73 participants reported having long COVID (17% of COVID-19 cases) in NCDS and 64 in BCS70 (14% of COVID-19 cases). A BMI of ≥25kg/m2 was associated with higher odds of COVID-19. There was no association of WHR with COVID-19. There may be a positive association of WHR with long COVID, but this result lacked precision. There was no association of categorical or continuous HbA1c with either outcome (see Figure 1).
Conclusion: In prospective analyses of two nationally representative UK cohorts a high pre-pandemic BMI was associated with a higher risk of COVID-19, while a high WHR appeared more strongly associated with a higher risk of suffering from long COVID. We found little evidence for associations of HbA1c with either COVID-19 or long COVID. We suggest that obesity-related mechanisms are responsible for the excess risks of COVID-19 in association with diabetes, rather than hyperglycaemia per se. Further research is needed to explore mechanisms underlying these associations.
Supported by: CONVALESCENCE funded by NIHR (COV-LT-0009)
Disclosure: A. Knuppel: None.
364
Hyperglycaemia, but not previous diagnosis of diabetes, is an independent indicator of poor outcome in patients hospitalised for severe COVID 19
E. Eletto1, R. Aldigeri2, A. Dei Cas3, A. Ticinesi3, A. Nouvenne3, B. Prati1, E. Balestreri1, A. Vazzana1, M. Antonini1, V. Spigoni2, S. Schirò2, N. Sverzellati3, T. Meschi3, R. Bonadonna3;
1Azienda Ospedaliero-Universitaria of Parma, 2University of Parma, 3University of Parma and Azienda Ospedaliero-Universitaria of Parma, Parma, Italy.
Background and aims: Diabetes mellitus (DM) and hyperglycemia are strong risk factors for poor outcome(s) in patients hospitalized for COVID-19. However, their relative role in affecting patient prognosis is still under debate. Furthermore, it is unknown whether they are only proxies of organ, especially lung, damage. Aim of this study is to evaluate the predictive power of known DM and hyperglycemia, as well as indicators of lung damage, on death/admission to intensive care unit (ICU) in patients hospitalized for COVID-19 during the first wave of SARS-CoV-2 pandemic.
Materials and methods: We retrieved the clinical data/records of the patients admitted with COVID-19 between 23rd February 2020 and 31st March 2020 in the Covid-1 macro-unit of the University Hospital of Parma. Known diabetes was defined by self-reported history, electronic medical records or medications. Fasting plasma glucose, inflammatory markers and main biochemical variables were collected at admission. Patients underwent computed tomography (CT) chest and arterial blood gas analysis to calculate the SpO2/FiO2 (P/F ratio). The primary outcome was a composite of ICU admission or death. Logistic regression analysis was used to identify independent risk indicators of the primary end-point by univariable and multivariable models. We used Receiver Operating Characteristic (ROC) curves to assess the overall predictive power of the different regression models.
Results: A total of 757 subjects were included, 143 of whom (19.2%) had known diabetes. Patients with DM were older and showed higher comorbidity rate. The primary outcome occurred in 61.5% of patients with DM compared to 43.1% in those without (log-rank test p<0.001). Among variables associated with COVID-19 severity, age, obesity, arterial hypertension, previous cardiovascular (CV) event, eGFR, glucose levels at admission (but not known diabetes), C-reactive protein, SpO2/FiO2 and HR-CT visual score of pneumonia extension, were significantly associated with risk indicators of poor outcome. In a multivariate logistic regression model older age (p<0.001), previous CV disease (p<0.001), poor eGFR (p<0.04), glucose levels ≥126 mg/dl (p<0.048) and a lower P/F ratio (p<0.001) resulted significant and independent negative prognostic factors. The ROC curve showed a very good accuracy (UC=0.91) of the model in predicting the primary composite end-point.
Conclusion: Known diabetes indicated poor COVID-19 outcomes, but not when adjusted for other baseline clinical variables and comorbidities, suggesting that its impact was mostly driven by concomitant factors and complications. Fasting hyperglycemia was an independent predictor of poor outcomes, even after taking into account the impairment of lung function. The molecular mechanism(s) underlying the tight association between high glucose and poor COVID-19 outcome remain(s) to be elucidated.
Clinical Trial Registration Number: NCT04550403
Supported by: Parma University "Bando straordinario per ricerche in ambito SARS-CoV-2 and COVID-19"
Disclosure: E. Eletto: None.
365
Prevalence and incidence of treated diabetes during the COVID-19 pandemic: real-world data from the Greek national electronic prescription database
C. Siafarikas1, G. Karamanakos1, K. Makrilakis1, K. Mathioudakis2, A. Tsolakidis2, S. Liatis1;
1First Department of Propaedeutic Internal Medicine, Laiko General Hospital, 2e-Government for Social Security Services (IDIKA), Athens, Greece.
Background and aims: It has been suggested that the COVID-19 pandemic significantly affected the access of patients with diabetes (DM) to health care facilities. The aim of this study was to record and evaluate the change in prevalence and incidence of treated DM, the year before (2019) and the years during SARS-CoV2 pandemic (2020 and 2021).
Materials and methods: Data derived from the electronic prescription database of the e-Government Center for Social Security Services (IDIKA) from 01/01/2019 to 31/12/2021 were analyzed. To calculate the prevalence of DM, individuals with unique active social security registration number (AMKA), who were dispensed at least one prescription of a glucose-lowering medication with an ICD-10 code relevant to diabetes, during each of the years under study, were included, while, to calculate the incidence, only those who had not been dispensed such a prescription before the index year were taken into account.
Results: The total study population was 10,289,140 in 2019, 10,630,726 in 2020 and 11,246,136 in 2021. The number of patients that received glucose-lowering medication was 821,428 in 2019 (prevalence 7.98%), 724,714 in 2020 (6.81%) and 880,115 in 2021 (7.82%). The incidence of total treated DM was 1.67 per 1000 persons in 2019, 1.05 per 1000 persons in 2020 and 2.48 per 1000 persons in 2021.
Conclusion: The prevalence of treated DM decreased in 2020 and returned to pre-pandemic levels in 2021, while its incidence decreased in 2020 and increased up to higher than pre-pandemic levels in 2021. These data suggest that restrictive measures of social distancing and self-isolation may have led to reduced accessibility to health care providers, explaining the fewer drug prescriptions during the first year of the pandemic. On the other hand, the implementation of a virtual electronic drug prescription system and the relaxation of restrictions during the second year may explain the rise in both incidence and prevalence of DM during the second year.
Disclosure: C. Siafarikas: None.
366
Impacts of the COVID-19 outbreak on glycaemic control and body composition in individuals with glucose intolerance in Japan
S. Yoshiji, T. Murakami, R. Tsukaguchi, M. Ogura, K. Shide, N. Inagaki;
Kyoto University, Kyoto, Japan.
Background and aims: During the coronavirus disease 2019 (COVID-19) outbreak, European countries imposed the strict lockdown, raising concerns for negative impacts on glycaemic control and body composition. In Japan, voluntary-based restrictions were imposed as a state of emergency (SoE). However, metabolic consequences of the “soft” lockdown are yet to be clarified. Here we aim to evaluate the effects of the SoE on glycaemic control and directly measured body composition in individuals with glucose intolerance (GI) in Japan.
Materials and methods: We retrospectively evaluated individuals with GI who had (i) longitudinal assessment of body composition, (ii) dietary follow-up by nutritionists and (iii) glycaemic measurement before and after the SoE in Kyoto University Hospital from January 1 2018 to May 31 2021. The SoE was declared on April 16 2020 and lasted until May 31 2021 with intermittent lifting. We evaluated whether the metabolic profiles of these patients changed after the SoE declaration by assessing the body composition and glycaemic control changes. Body composition was assessed by InBody 720 bioelectrical impedance analyzer, and measurements included body weight, fat mass, fat-free mass, and skeletal muscle mass. Nutritionists collected daily diet and exercise information.
Results: We identified 415 eligible individuals with GI (type 2 diabetes: 364, impaired glucose tolerance: 31, type 1 diabetes: 12). Baseline (January 2019 - December 2019) mean ± SD age was 64.8 ± 12.4 years, HbA1c 7.3 ± 1.2%, body mass index (BMI) 26.1 ± 4.9 kg/m2, body weight 70.0 ± 16.5 kg, fat mass 22.1 ± 10.2 kg, and skeletal mass 26.2 ± 5.9 kg. Forty percent were female. After SoE (June 2020 - May 2021), BMI (Mean ± SE change: 0.13 ± 0.05 kg/m2, P = 0.018), body weight (0.32 ± 0.15 kg, P = 0.034), and fat mass (0.45 ± 0.14 kg, P = 0.001) significantly increased, whereas skeletal mass (-0.12 ± 0.05 kg, P = 0.016) significantly decreased. Of note, these measurements did not significantly change from 2018 to 2019 in these patients. Although there was no overall increase in HbA1c (P = 0.197), HbA1c change significantly correlated with change in BMI (r = 0.36: P < 0.001), body weight (r = 0.37: P < 0.001), and fat mass (r = 0.41: P < 0.001). Further, there were 128 individuals whose HbA1c significantly increased by ≥0.3% after the SoE. These individuals had no significant HbA1c change from 2018 to 2019. Thus, we performed a subgroup analysis of individuals with and without HbA1c worsening of ≥ 0.3% (worsening group [n = 128] vs. non-worsening group [n = 287]). In the worsening group, changes in BMI (0.66 ± 0.13 vs. -0.11 ± 0.06 kg/m2, P < 0.001), body weight (1.76 ± 0.95 kg vs. -0.33 ± 0.16 kg, P < 0.001), and fat mass (1.92 ± 0.61 vs. -0.20 ± 0.16 kg, P < 0.001) were significantly higher than in the non-worsening group. Regarding the dietary habits, snack intake significantly increased in the worsening group (P = 0.036), whereas exercise frequency didn’t significantly change (P = 0.880).
Conclusion: Overall, the SoE conferred unfavourable metabolic consequences in individuals with GI, highlighted by increased body weight, body fat, and decreased skeletal muscle. Individuals whose glycaemic control worsened during the SoE had increased body weight and body fat compared to those without glycaemic worsening. This can be partially due to increased snack intake. Our study indicates that even “soft” social restrictions are associated with significant metabolic worsening, which warrants clinical attention.
Clinical Trial Registration Number: R2377
Disclosure: S. Yoshiji: None.
367
The clinical course of Covid-19 in diabetes patients: a nation-wide multistate modelling cohort study
H. Amadid, P. Rønn, L. Diaz, B. Carstensen, P. Rossing, D. Vistisen;
Steno Diabetes Center Copenhagen, Herlev, Denmark.
Background and aims: The higher morbidity and mortality of Coronavirus disease 2019 (COVID-19) among diabetes patients may be influenced by their diabetes status and clinical profile. We aimed to model the clinical course of COVID-19 from positive test of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to hospitalization, admission to intensive care unit (ICU), need of mechanical ventilation and/or in-hospital death and to evaluate possible prognostic factors related to diabetes status and clinical profile.
Materials and methods: From nation-wide registers in Denmark, we identified 5078 individuals (2827 men and 2258 women) with diabetes and a positive test for SARS-CoV-2 from 27 January 2020 until 11 December 2020. The median age at time of the positive test was 61.6 years. A 6-state multistate model was built, and 10 transitions were analyzed using Poisson regression with linear effects of diabetes duration, age, as well as categorical effect of diabetes types (T1D/T2D), sex, diabetes-related complication status and medications. Cardiometabolic factors, lifestyle factors and vaccination status are prognostic factors to be evaluated.
Results: During 29,181 person-weeks, 1102 (21.7%) were hospitalized, 139 (2.6%) needed ICU treatment, 110 (2.2%) needed mechanical ventilation and 171 (3.4%) died while in hospital.For the transition from “positive test” to “hospitalization”, the incidence rate ratio (IRR) for women vs. men was 0.64, 95% CI (0.56;0.72), a one-year increase in age 1.05, 95% CI (1.05;1.06), T2D vs T1D 1.02, 95% CI (0.76;1.37), a one-year increase in diabetes duration 1.00, 95% CI (1.00;1.01), co-existing cardio-kidney complications 1.60, 95% CI (1.35;1.89) (end-stage kidney disease, chronic kidney disease, cardiovascular disease and heart failure were associated with 60-255% increased risk, respectively) and use of cardio-kidney-protective medications 0.74, 95% CI (0.65;0.85). Treatment with SGLT-2i, GLP-1, metformin, RAS-blocking agents and lipid-lowering drugs conferred a 10-30% lower risk, respectively. HbA1c was associated with a 7% increased risk (1.07, 95% CI 1.02;1.13).
Conclusion: Male sex, age, cardio-kidney complications and increase in Hba1c was associated with a higher risk of hospitalization. Treatment with SGLT-2i, GLP-1, metformin, RAS-blocking agents and lipid-lowering drugs conferred a protective association for hospitalization. Longer diabetes duration slightly increased the risk of hospitalization while type of diabetes showed no association with hospitalization. Analyses on updated data (longer follow-up) and including the remaining prognostic factors will be performed also for the states ICU and need of mechanical ventilation.
Supported by: NNF20SA0063090
Disclosure: H. Amadid: None.
368
Temporal trends in risk of severe COVID-19 infection in persons with diabetes
J.V. Stidsen1, A. Green1, L. Rosengaard2, K. Højlund1,3;
1Steno Diabetes Center Odense, Odense University Hospital, 2Open Patient data Explorative Network, Odense University Hospital, 3Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Background and aims: Coronavirus disease-2019 (COVID-19) affects admission risk and mortality in diabetes and diabetes-related conditions. We examined the temporal trends in admission risk and mortality in the total Danish population by diabetes and diabetes-related conditions in the two first waves of COVID-19 in Denmark.
Materials and methods: We identified all persons with diabetes in the whole Danish population using national registries. COVID-19-related risks of admission and death were assessed using Cox regression analysis in wave 1 (1 March-31 August 2020) and wave 2 (1 September 2020-28 February 2021) of the pandemic for persons with and without diabetes. Analysis were stratified according to status of hypertension, obesity, cardiovascular disease and microvascular disease.
Results: For persons without diabetes (n=5,479,755) the crude 6 month cumulative incidence of COVID-19 admission increased from 4 to 10 per 10,000 from wave 1 to 2, compared with a corresponding increase from 16 to 54 per 10,000 for persons with diabetes (n=321,933). The hazard ratio (HR) of COVID-19 admission for persons with diabetes, compared to persons without diabetes, after adjustment for co-morbidity and treatment, increased from 1.40 (95% CI 1.27, 1.55) to 1.76 (1.65, 1.87) from wave 1 to wave 2 (p<0.001 for interaction with wave). The crude 6 month mortality rate increased from 0.63 to 1.5 in 10,000 persons without diabetes and from 4.3 to 10 in 10,000 persons with diabetes. The increased HR for mortality in persons with diabetes compared to persons without, did not change according to wave (HR 1.65 [1.34, 2.03] and 1.64 [1.43, 1.88]). However, when expressed according to the hospitalized persons only, the crude mortality fell from 26.8% to 19.6% in persons with diabetes, while only a minor decrease was seen in persons without diabetes (16.7% to 15.5%). The HR for mortality, expressed according to the hospitalized persons only, in persons with diabetes compared to persons without, decreased from HR 1.27 (1.03, 1.57) in the first wave, to HR 1.07 (0.93, 1.23) in the second wave (P=0.17 for interaction with wave). Compared to persons without both diabetes and cardiovascular disease (CVD), persons with diabetes without CVD had increased mortality (HR 2.06 [1.68, 2.54]) and persons with both conditions even more (HR 5.87 [4.96, 6.96], p<0.001 for difference between diabetes with and without CVD). Similar results were seen for hypertension, obesity and microvascular disease. The results were similar for the two waves.
Conclusion: The risk of COVID-19 admission increased in persons with diabetes from wave 1 to wave 2 of the COVID-19 pandemic in the Danish population. However, the risk of mortality in persons with diabetes compared to the whole population did not change, most likely due to a reduced mortality among hospitalized persons with diabetes. For persons with diabetes, CVD conferred increased mortality risk.
Supported by: Danish Agency for Higher Education and Science (0237-00012B)
Disclosure: J.V. Stidsen: None.
369
New-onset hyperglycaemia in nondiabetic patients hospitalised for COVID-19: prevalence, risk factors and post-discharge outcomes
E. Falbo1, M. Ferrante1, S. Damanti2, M. Cilla2, J. Castellani1, R. De Lorenzo1, G. Vitali2, S. Martinenghi2, C. Magnaghi2, E. Bosi1, P. Rovere-Querini1,2, C. Conte3;
1Vita-Salute San Raffaele University, Milan, 2IRCCS San Raffaele Hospital, Milan, 3San Raffaele Roma Open University, Rome, Italy.
Background and aims: New-onset hyperglycemia (NOH) is seen in about 50% of patients hospitalized for COVID-19 despite only 7% having diabetes, suggesting that COVID-19 negatively impacts glucose metabolism. Factors associated with NOH, especially glucocorticoid (GC) therapy, have not been deeply investigated. Systemic GCs, which may cause may hyperglycemia, are routinely used in the treatment of COVID-19 requiring oxygen support. We assessed the prevalence of NOH (defined as hyperglycemia requiring insulin therapy) in patients without DM hospitalized for COVID-19, investigated factors associated with NOH and described in-hospital and post-discharge outcomes in these patients.
Materials and methods: This was a post-hoc analysis of data prospectively collected for a large observational study. Data from patients hospitalized for COVID-19 pneumonia without DM at the beginning of the second pandemic wave (between August and November of 2020) who returned for a 1-month follow-up visit were analyzed. For each patient, we calculated the cumulative GC dose administered during hospitalization, which was converted into methylprednisolone dose equivalent (MPD), in addition to anthropometric data, comorbidities, laboratory tests and outcomes during the hospital stay and one month after discharge.
Results: A total of 156 patients were included in the analysis (median age 61 [IQR 52-76] years, 43% females, median BMI 26 kg/m2 [IQR 24-30]). One hundred and forty-three (92%) patients received GC therapy during hospitalization. We compared patients with and without NOH. Thirty-seven (23.7 %) patients developed NOH, all of them received GC therapy. NOH patients had significantly higher systolic blood pressure (SBP) and inflammatory markers. NOH patients received significantly higher GC doses, were more likely to need non-invasive ventilation, and had a greater risk of admission to the intensive care unit, but death rates were similar. Baseline BMI was not associated with NOH. At univariable linear regression analyses, higher plasma glucose (PG) on admission (OR 1.04 [95% CI 1.019; 1.060]) and total MPD dose per kg (OR [95% CI 1.018 - 1.330]) increased the risk of NOH. Ninety patients returned for the 1-month follow-up assessment (17 with NOH). NOH patients had significantly higher random capillary blood glucose (110 [98.2; 128.5] vs. 99.5 [ 90; 107] mg/dL, p<0.007) and persistently higher SBP (130 [122; 143] vs. 126 [ 115; 140] mmHg, p<0.048). BMI was similar between groups. However, the proportion of patients with obesity was greater in the NOH group (58% vs 12%, p=0.037).
Conclusion: PG upon admission and total cumulative MPD per kg were associated with increased risk of NOH during hospital stay, suggesting that GC therapy contributes to and may aggravate the derangements in glucose metabolism seen in COVID-19 patients. After discharge, patients with NOH had persistently higher glucose and SBP levels as compared with patients without NOH, and were more likely to have obesity. Glucose values should be monitored during GC therapy, especially in non-diabetic patients with hyperinflammation and elevated SBP. Those who develop NOH during hospital stay should be monitored for alterations in glucose metabolism and cardiometabolic risk factors after discharge.
Clinical Trial Registration Number: NCT04318366
Disclosure: E. Falbo: None.
370
Covid-19 associated hyperglycaemia relates to both insulin resistance and beta cell dysfunction
J. Gojda1, A. Lang2, M. Krbcová1, A. Ouřadová1, K. Koudelková1, V. Šebo3, A. Gvozdeva1, L. Slagmolen4, J. Potočková1, F. Karpe5, S. Schlesinger2;
1Internal Medicine Dept., Charles University in Prague, Prague, Czech Republic, 2Institute for Biometrics and Epidemiology, Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, 3Dept. of Patophysiology, Charles University in Prague, Prague, Czech Republic, 4Exercise Physiology Research Group, Katholieke Universiteit Leuven, Prague, Belgium, 5University of Oxford, Oxford, UK.
Background and aims: Hyperglycaemia is a common feature of severe acute COVID-19 and it impacts morbidity and mortality. Several mechanisms were suggested to contribute to its development: inflammation-driven hypermetabolism and insulin resistance (IR), beta-cell damage, and/or corticosteroid administration. The study aimed to compare indices of glucose homeostasis and metabolism in patients with acute severe COVID-19 and its course over 6 months of follow-up.
Materials and methods: Patients without a known history of diabetes were enrolled in the ‘Prospective cohort study into metabolic derangements associated with COVID-19’ (COMETA study), where patients with acute severe COVID-19 were examined at baseline (within 4 weeks from the onset of bilateral pneumonia with respiratory failure) and after 6 months of follow-up: clinical examination, anthropometry, bioimpedance, fasting indirect calorimetry, and OGTT. Insulin secretion and sensitivity were expressed as Matsuda insulin sensitivity index (ISI), insulinogenic Index (IGI), and disposition index (DI). Resting energy expenditure (REE) was calculated using the Weir equation and substrate preference using the respiratory quotient (RQ) and nitrogen losses.
Results: Thirty-seven patients (11 women) were available for the analyses: baseline examination was within 21±6.5 days after COVID-19 diagnosis, high-dose corticosteroids were administered in all. Patients were hypermetabolic at baseline (30.6 ± 4.3 kcal/kg lean mass/day, ~120% predicted values) and REE declined over the 6 months (ΔT6-T0: MD (95% CI)-5.5 (-6.9, -4.1) kcal/kg lean mass/day, p<0.0001). Prevalence of newly diagnosed pre/diabetes was 59% (22 pts.) at baseline and remained as high as 14 patients (45%) at 6 months, none had positive islet autoantibodies. When comparing patients with (H=20) and without (C=12) hyperglycaemia at baseline, we found that insulin sensitivity was comparable among groups (ISIH=3.07 ± 1.18, ISIC=3.23 ± 1.72, p=0.77), whereas beta cell dysfunction was present in the hyperglycaemic group only (IGIH=1.04 ± 0.55, IGIC=2.94 ± 1.82, p<0.001; DIH=3.19 ± 2.16 vs DIC=8.78 ± 6.37, p<0.01). Over the 6 months, insulin sensitivity improved only in the hyperglycaemic group (0m_ISIH=3.07 ± 1.18 vs. 6m_ISIH=4.52 ± 2.75, p=0.01). IGI and DI did not improve in any group.
Conclusion: Acute severe COVID-19 was associated with hypermetabolism that did not persist over six months. A high incidence of hyperglycaemia was observed. While hyperglycaemia in the acute phase related to both insulin resistance and beta-cell dysfunction, it was improved insulin sensitivity that determined the normalization of glycaemic indices over the follow-up. Maladapted beta cell response on hyper inflammation and exogenous corticosteroids were possible causes of hyperglycaemia.
Supported by: Charles University Cooperatio METD and SVV 260531/SVV/2020: EFSD/AZ Mentorship Programme
Disclosure: J. Gojda: Grants; Charles University Cooperatio METD, SVV 260531/SVV/2020, EFSD mentorship programme supported by AstraZeneca.
SO 15 Calcium signalling in the islet: we are still learning
371
A-cell Ca2+ oscillations and glucagon secretion depend on Ca2+release from the endoplasmic reticulum
S. Acreman1, G. Denwood2, R. Gao1, J. Ma1, P. Rorsman1, Q. Zhang1;
1OCDEM, University of Oxford, 2University of Oxford, Oxford, UK.
Background and aims: Glucagon is the principal glucose counter-regulator hormone secreted by the pancreatic α-cells. It is increasingly clear that dysregulation of glucagon secretion contributes to both hyper- and hypo-glycaemia in diabetes. Precisely how glucagon secretion is regulated remains unclear but most studies focus on glucose-dependent α-cell electrical activity and subsequent transmembrane Ca2+ (Ca2+t) influx. However, how α-cells handle intracellular Ca2+ ([Ca2+]i) and the role of Ca2+ release from the endoplasmic reticulum (ER) is not fully understood. Using transgenic animal models, we investigated membrane potential-independent regulatory mechanisms of glucagon secretion, with a specific focus on α-cell ER Ca2+ release.
Materials and methods: A-cell electrical activity and transmembrane Ca2+ current were monitored using the patch-clamping technique. A-cell [Ca2+]i dynamics were recorded using live cell Ca2+ imaging of islets from mice expressing a genetically encoded Ca2+ sensor GCaMP6f specifically in α-cells. Hormone secretion was conducted using static incubation followed by ELISA.
Results: The relative contributions of voltage sensitive Ca2+ channels (Cavs) in α-cells were dissected using voltage-clamp experiments and specific blockers. In response to membrane depolarisation (-70 to 0 mV), ~60% of α-cell Ca2+t current flows through L-type Cavs, and the remaining Ca2+ current is carried by N- (~20%) and P/Q-type Cavs (~20%). L-type Cavs are likely to contribute to α-cell action potential generation and 10 μM isradipine (L-type Cav blocker) completely abolished α-cell electrical activity in islets in 1 mM glucose. However, isradipine was without apparent effect on low glucose-induced glucagon secretion (0.060±0.005% vs. 0.069±0.005% of content; p>0.1) and only had marginal effect on α-cell [Ca2+]i oscillation frequency (0.79±0.09 vs. 0.68±0.08 spikes/min, P<0.005); and glucose remained inhibitory to both α-cell [Ca2+]i oscillation frequency (P<0.0001) and glucagon secretion (P<0.05). Similarly, α-cell [Ca2+]i oscillations and glucagon secretion was unaffected by repolarising α-cell membrane using 100 μM diazoxide, a KATP channel opener, in the presence of 1 or 6 mM glucose. The membrane potential-independent [Ca2+]i activity is sensitive to intracellular ATP. In α-cells voltage-clamped at resting membrane potential (-70 mV), intracellular application of 1 mM ATP stimulated α-cell [Ca2+]i activity (AUC=2424±411), and increasing the cytosolic ATP concentration to 3 mM was inhibitory (P<0.05). Blockade of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) with cyclopiazonic acid (CPA, 10 μM), to deplete ER Ca2+, suppressed α-cell [Ca2+]i oscillations, and glucagon secretion (0.028±0.006% vs. 0.009±0.0005% of content; p<0.005) at 1 mM glucose. In comparison, in the presence of 6 mM glucose, CPA was without effect on α-cell [Ca2+]i oscillations or glucagon secretion (0.012±0.003 vs. 0.013±0.003% of content, p>0.99). 100 μM ryanodine, a blocker of intracellular Ca2+-releasing channel ryanodine receptor, like CPA, abolished changes in α-cell [Ca2+]i oscillations in response to glucose.
Conclusion: The data collectively show a significant component of glucagon secretion is independent to α-cell electrical activity. α-cell sensitivity to glucose metabolism (ATP levels) is likely to be exerted at the level of the ER, with SERCA and ryanodine receptor likely playing major roles in glucagon secretion.
Supported by: EFSD Research Programme, DUK
Disclosure: S. Acreman: None.
372
Focal adhesion kinase and the functional consequences of beta cell polarity
D. Jevon, K. Deng, N. Hallahan, N. Flores-Rodriguez, W.J. Gan, P. Thorn;
University of Sydney, Sydney, Australia.
Background and aims: The Islets of Langerhans are complex micro-organs, the 3-dimensional characteristics of which have been so far underappreciated due to limitations and difficulties in sample preparation and data collection techniques. These 3-dimensional characteristics inform spatial polarity to each β cell, and β cells form structural and secretory domains in response to their contacts with capillaries and other cells. Along beta cell capillary contacts, focal adhesion kinase is localised and activated. This research investigates the broad role of beta cell capillary contacts and the local activation of focal adhesion kinase to beta cell calcium influx and insulin secretion.
Materials and methods: Pancreatic slices were gathered from humanely sacrificed male FAKflox-Inscre, GCaMP6s-Inscre, or Bl6/C57 mice in accordance with ethical research standards. Slices were fixed and stained for whole islet 3D imaging by Z stacks (100-300 optical sections per islet), or cultured overnight for live cell assays. Glucose stimulated insulin secretion was measured from whole slices by HTRF assay or single cells by 2 photon microscopy. Subcellular calcium influx was measured in GCaMP6s-Inscre mice and FAKflox-Inscre or Bl6/C57 mice incubated with the fluorescent calcium reporter, fura-2 AM imaged by 2 photon microscopy. The focal adhesion kinase inhibitor, Y15 was used in calcium influx and glucose stimulated insulin secretion assays. Controls and experimental groups contain minimum n=3 and significance was measured by t-test and ANOVA where applicable.
Results: β cells contact capillaries with 8.8%+/-1.5% of their total surface area and 32%+/-1.0% of β cells form functionally equivalent secretory domains with multiple capillaries. Increased β cell capillary contact surface area is associated with higher insulin secretion (R2=0.37, p<0.0001) and characterized by local focal adhesion kinase phosphorylation. Calcium influx into β cells begins at the capillary interface and is visualised as a wave with an average velocity of 50.6+/-6.1μm/s. Focal adhesion kinase inhibition with Y15 reduces the peak amplitude of calcium waves after glucose stimulation by 46%+/-4.8% and glucose stimulated insulin secretion by 53%+/-6.5%. Low levels of calcium activity are observed at 2.8mM glucose in some β cells in pancreatic slices of C57/Bl6 mice and not in Y15 treated slices or slices from FAKflox-Inscre mice. In Bl6/C57 slices, a significant increase (p<0.001) in insulin secretion is observed between 2.8mM and 5.0mM glucose, an increase which is not observed in Y15 treated Bl6/C57 slices (p>0.5) and FAKflox-Inscre slices (p>0.5).
Conclusion: Islet β cells form polar and functional secretory domains with capillary contacts and focal adhesion kinase is active in these domains. Insulin secretion is localised to these contacts, as is calcium inflow, which travels as a wave across the cell. Focal adhesion kinase inhibition or constitutive knockout reduces β cell calcium activity, glucose sensitivity and glucose stimulated insulin secretion.
Disclosure: D. Jevon: None.
373
The beta cell primary cilium is an autonomous Ca 2+ organelle involved in GABA signalling
T.C. Incedal, G. Sanchez, O. Idevall-Hagren;
BMC box 571, Uppsala University, Uppsala, Sweden.
Background and aims: The primary cilium is an organelle present in most of the mammalian cells. It has its base at the centriole, from which microtubule bundles extend and push on the plasma membrane, generating an antenna like structure of that protrudes from the cell surface. Primary cilia orchestrate signal transduction independent from the cell body. The presence of specific ciliary receptors and effector proteins enables the generation of local ciliary signals and provides a platform for crosstalk between different pathways, often involving Ca2+ and cyclic adenosine monophosphate (cAMP). Islet cells are equipped with prominent primary cilia that are important for the regulation of β-cell function, but how these small structures influence cell function is not known. In the current study, we aim to identify local signalling pathways in the primary cilium of intact islets.
Materials and methods: We used intact mouse pancreatic islets of Langerhans expressing a novel cilia targeted Ca2+ sensor and total internal reflection fluorescence microscopy to record ciliary Ca2+ dynamics under different physiological conditions.
Results: Mouse islet cells were equipped with long primary cilia (9±0.5μm, n=249 cilia, 8 islets, 4 animals) that often clustered in the islet interstitium and exhibited diverse morphologies that included dilated tips and bifurcations. Immunofluorescence staining revealed strong enrichment of metabotropic GABAB1 receptors at the cilium base that were mobilized to more distal parts upon agonist binding. Addition of the GABAB1-selective agonist baclofen or inhibition of GABA breakdown with vigabatrin caused pronounced Ca2+ increases in the primary cilia of mouse islet cells expressing a cilia-targeted Ca2+indicator (4.1-fold, P=0.002, n=11 islets; P=0.007, n=7 islets), but was without effect on cytosolic Ca2+. The response to baclofen was abolished by GABAB1 receptor knockdown and L-type channel inhibition. Cyclic nucleotides have been suggested to crosstalk with Ca2+ in the primary cilium. Elevation of cAMP in mouse islets expressing the light-activated adenylyl cyclase bPAC was without effect on cilia Ca2+, while elevation of cGMP using a light-activated guanyl cyclase (RhGC) or addition of atrial natriuretic peptide (whose receptors is a cell membrane-bound guanylyl cyclase) triggered Ca2+ increases. Immunofluorescence staining revealed the presence of the cGMP-selective cyclic nucleotide-gated channel CNGA3 in primary cilia of mouse islet cells. When Ca2+ influx (20 mM glucose or 30 mM KCl) or release from the ER (carbachol) was triggered, the ensuing rise of cytosolic Ca2+ did not propagate beyond the first two μm of the cilia base. This insulation against cytosolic Ca2+ contrasts earlier work in non-excitable cells.
Conclusion: We show that the primary cilium of islet β-cells is a unique Ca2+ compartment that is isolated against cytosolic Ca2+, likely via a combination of efficient extrusion and buffering. Both GABA and cGMP, important regulators of β-cell function, selectively trigger Ca2+ increases in the primary cilium through distinct mechanisms. We propose that the primary cilium should be considered an important signalling centre in β-cells that can receive and transmit signals of relevance for cell function, the cellular implications of ciliary calcium signalling remain to be elucidated.
Supported by: Swedish Research Council, NNF, EFSD/Lilly Research Programme, EXODIAB, Diabetesfonden
Disclosure: T.C. Incedal: None.
374
Optogenetic stimulation of pancreatic beta cells to investigate gap junction coupling among heterogenous subpopulations
C.H. Levitt;
Bioengineering, University of Colorado Anschutz, Denver, USA.
Background and aims: Insulin secreting β-cells of the Islets of Langerhans are functionally heterogenous. Distinct spatially organized subpopulations of β-cells have been shown to mechanistically drive insulin secretion and glucose homeostasis. Electrical coupling between β-cells via gap junction protein Connexin-36 (Cx36) promotes the synchronization of electrical activity. In diabetes, it has been shown that gap junction coupling is disrupted and results in a loss of coordinated calcium influx ([Ca2+]i) and insulin release. The cell-specific influence that gap junction coupling has whole islet electrical dynamics, however, is not clear. Here we will assess the degree to which disruptions in functional subpopulations of β-cells are a result of disruptions to gap junction coupling.
Materials and methods: To directly influence the electrical properties of single cells in the islet, β-cell specific channelrhodopsin-2 (Chr2) was expressed in transgenic mice. Chr2 is a light sensitive cationic channel that causes cell depolarization upon stimulation of blue light. A Ca2+ sensitive fluorescent dye was used to visualize calcium dynamics upon single cell stimulation in isolated islets.To mimic diabetogenic conditions where Cx36 coupling is disrupted, islets were treated with a 0.1X cocktail of pro-inflammatory cytokines (IL-1β, TNF- α, and IFN- γ). Recovery of gap junction function was achieved by co-treatment with a mimetic peptide (S293) that competitively inhibits cytokine mediated Cx36 phosphorylation. Islets were subject to cytokine treated, and cytokine + peptide co-treatment under conditions of basal (5mM) and elevated (11mM) glucose.
Results: Pro-inflammatory cytokines disrupt Cx36 gap junction coupling through nitric oxide regulated PKCδ activation which causes a loss of synchronized [Ca2+]i. At basal glucose, we demonstrate that cytokine treatment impairs electrical recruitment of neighboring cells following single-cell ChR2 stimulation. In untreated islets, >50% of cells could recruit an area larger than the mean recruitment area of an untreated islet whereas only ~7% of cells could accomplish the same in cytokine treated. When gap junction coupling was recovered with co-treatment of cytokines and S293 peptide, the proportion of recruited cells tripled (~30%).At elevated glucose, single cell stimulation drove coordinated electrical dynamics across the islet. Under cytokine treated the driving of coordinated dynamics was decreased (decreasing in spectral power at the driving frequency) compared to co-treatment with S293.
Conclusion: At both low and high glucose conditions, pro-inflammatory cytokines induced disruptions to the coordinated activity induced by ChR2-stimulation of a single β -cell. Recovery of Cx36 by S293 and the subsequent increase in the recruitment area suggests that gap junction coupling is sufficient for coordinated regulation of [Ca2+]i and overall insulin release. Taken together, these finding support the critical role of gap junction coupling allowing coordinated electrical activity driven by heterogenous subpopulations, and thus the proper regulation of insulin release.
Disclosure: C.H. Levitt: None.
375
Quantifying the relationship between emergent islet function, gap junctions, and beta cell dynamics: a network theory approach
J. Briggs, J. Dwulet, V. Kravets, D. Albers, R. Benninger;
University Of Colorado Anschutz Medical Campus, Aurora, USA.
Background and aims: Coordinated insulin release from functionally heterogeneous beta cells in the pancreatic islet is essential for proper glucose homeostasis and is disrupted in type 1 and type 2 diabetes. Network theory is a helpful tool in understanding this islet coordination and the mechanisms driving it. Coordinated insulin secretion results from the heterogeneous dynamics of individual beta cells and the intercellular interactions through the gap junctions that connect them. This emergent insulin secretion can be studied using a functional network representation and the gap junctions can be studied using a structural network.In diabetic conditions, beta-cell death or gap junction dysfunction alters the structural network. However, because the relationship between the structural and functional networks has not been well quantified, we cannot predict how specific types of disruption in structure will impact the islet function. In this presentation, we combine experimental and computational techniques to quantify the relationship between the structural and functional networks of the beta cells in the pancreatic islets.
Materials and methods: We use a computational model to simulate islet dynamics with healthy and decreased coupling conditions. This approach allows us to compare the structural and functional networks using traditional and network theory-based statistics. We then validated the findings from this simulation using concurrent calcium imaging to quantify the functional network, fluorescence recovery after photobleaching to quantify gap junction permeability to quantify the structural network, and NAD(P)H autofluorescence to quantify metabolic activity. Finally, we used Connexin36 knockout mice to quantify the functional network response to structural network changes that can also occur under diabetic conditions.
Results: Our computational and experimental data indicate that the functional network is more reflective of the metabolism than structural gap junctions. We show that functional subpopulations of beta cells, including beta cell hubs, are statically differentiated by their intrinsic metabolic activity rather than structural connections (P < 0.001). Functionally connected cells are more likely to share metabolic qualities than a gap junction (P < 0.01). Using weighted network analysis, we show that short and medium-range functionally connected cell pairs (1-5 gap junction separation) are more likely to share similar metabolic rates (P < 0.001) while long-range functionally connected pairs (6-7 gap junctions) are more likely to share a chain of highly conductive gap junctions (P < 0.01). This indicates that metabolic similarities are primarily responsible for synchronizing cells over short distances, while gap junctions are required for synchronizing cells over long distances. Last, we show that while loss of physical connections changes functional network properties, removing gap junctions is not enough to explain islet dynamics fully.
Conclusion: Altogether, our results suggest that the emergent functional network of the pancreatic islet is more reflective of variations in beta-cell metabolic activity than structural gap junction connections. These results provide insight into how to interpret the relationship between structure, function, and intrinsic cell dynamics in the pancreatic islet and how insulin secretion dynamics may be altered in diabetes.
Supported by: NSF DGE-1938058_Briggs (to JKB); R01 DK102950, R01 DK106412 (to RKPB); HIRN 25B1104 (to VK)
Disclosure: J. Briggs: None.
376
Illuminating the mechanism for Gi/o protein-coupled receptor inhibition of beta cell Ca2+ influx and insulin secretion
M.T. Dickerson, P.K. Dadi, K.E. Zaborska, A.Y. Nakhe, C.M. Schaub, J.R. Dobson, N.M. Wright, J.C. Lynch, C.F. Scott, D.A. Jacobson;
Molecular Physiology and Biophysics, Vanderbilt University, Nashville, USA.
Background and aims: Pancreatic β-cell insulin secretion depends on Ca2+ entry and is essential for maintaining glucose homeostasis. It is well-established that Gi protein-coupled receptors (Gi-GPCRs) such as somatostatin (SST) receptors (SSTRs) and α2A-adrenergic receptors (ADRs) limit excessive insulin secretion in-part by reducing β-cell electrical excitability; however, the mechanisms underpinning Gi-GPCR control of β-cell function are poorly understood. Therefore, due to the importance of Gi-GPCRs to islet function and dysfunction, we set out to provide a clear understanding of how Gi-GPCRs control β-cell Ca2+ handling and hormone secretion.
Materials and methods: Electrophysiology, fluorescence imaging (Ca2+, Na+, cAMP), and hormone secretion assays were employed to study Gi-GPCR regulation of islet function. A pharmacological approach and transgenic mice with pancreatic GIRK2 knockout (KO) were used to assess contributions of G protein-gated inwardly rectifying K+ (GIRK) channels and Na+/K+ ATPases (NKAs) to β-cell Gi signaling. Islets with β-/δ-cell-restricted Gi-coupled Designer Receptors Exclusively Activated by Designer Drugs (Gi-DREADDs) were used to examine islet-cell-specific Gi signaling effects. NKA phosphorylation was probed using immunoblotting.
Results: Gi-GPCR activation (SSTRs or α2A-ADRs) stimulated β-cell Ca2+ oscillations and out-of-phase cAMP oscillations. SST-induced Ca2+ oscillations were unaffected by GIRK channel inhibition (tertiapin-Q) or GIRK2 KO; NKA inhibition (ouabain or extracellular K+ removal) terminated Gi-GPCR-mediated Ca2+ oscillations in 98±2% of islets (P<0.001). NKA inhibition also reduced SST-induced decreases in human islet Ca2+ by 80±16% (P<0.05). This was a β-cell-specific effect as selective activation of β-cell Gi-DREADDs stimulated Ca2+ oscillations in 88±9% of islets (P<0.01). δ-cell Gi-DREADD activation decreased glucose-stimulated (9mM) SST secretion by 63±4% (P<0.01); as a result of diminished SST secretion, islet Ca2+ oscillations sped up by 42±8% (P<0.01) and glucose-stimulated insulin secretion increased by 336±66% (P<0.001). This was reversed by exogenous SST, suggesting that intra-islet SSTR signaling tunes islet Ca2+ oscillations through NKA activation. SST-induced β-cell NKA activity depended on Src tyrosine kinases (STKs) that when inhibited (dasatinib) terminated SST-induced Ca2+ oscillations in 62±10% of islets (P<0.01). Moreover, SST increased NKA Tyr10 phosphorylation by 65±12%, indicating that this effect was mediated by NKA phosphorylation (P<0.05). SST-induced NKA activity was also inhibited by increasing cAMP. For example, elevation of cAMP with forskolin terminated SST-induced Ca2+ oscillations in 84±6% of islets (P<0.0001), which depended on PKA activity. Furthermore, Gs signaling-induced (liraglutide) cAMP increases decreased SST-induced islet Na+ efflux by 76±11% (P<0.01).
Conclusion: Therefore, these findings reveal that NKA-mediated β-cell Vm hyperpolarization is the primary mechanism for Gi-GPCR control of β-cell Ca2+ handling and insulin secretion. Gi-GPCRs activate β-cell NKAs through phosphorylation by STKs as well as by decreasing cAMP levels and PKA activity. Intra-islet SSTR signaling regulates β-cell NKA activity, which tunes glucose-stimulated Ca2+ oscillations and presumably pulsatile insulin secretion.
Supported by: NIH NIDDK, Helmsley Charitable Trust, ADA, and JDRF
Disclosure: M.T. Dickerson: None.
377
Pancreatic delta cells switched islet Ca 2+ oscillation modes by inhibiting endogenous glucagon from alpha cells
H. Ren, Y. Li, Y. Yu, T. Chang, W. Qian, X. Yang, L. Chen, C. Tang;
Lvzhihe building, Peking University, Beijing, China.
Background and aims: Glucose-stimulated pancreatic islets show different patterns of Ca2+ signaling with fast, slow and mixed fast/slow oscillations. However, it is not clear whether they are characteristic features of individual islets. And how much the paracrine perturbation switch the Ca2+ oscillation modes.
Materials and methods: Microfluidic chips for islet Ca2+ imaging; Optoinhibition of δ cells using transgenic mice (Sst-Cre+;Ai35f/+); Pharmacologically blocking Sstr2 using CYN.
Results: We report islets spontaneous slowdown from fast to slow/mixed Ca2+ oscillation under high glucose stimulation. The fast oscillation stage typically lasts for several hours and intrinsic to the islet. The modes switch is reversible. Low glucose resting for 30 minutes fully restored the fast oscillation. Microfluidic perfusion delays and static incubation brings forward the mode switch, which suggests the critical role of the paracrine environment. Correspondingly, exogenous glucagon delays and somatostatin brings forward the mode switch. Pharmacologically blocking Sstr2 on α cells triggers endogenous glucagon secretion and switches islets back to fast oscillations. Optoinhibition and relief of δ cells cause islets to repeatably show fast and slow oscillations.
Conclusion: Our study highlights the importance of δ-α cell interactions to generate tunable islet Ca2+ oscillation patterns. The design principle of pancreatic islet was revealed, as the minority of δ cells command the majority of α and β cells.
Disclosure: H. Ren: None.
378
Three-dimensional imaging reveals conserved patterns of calcium wave in the pancreatic islet
Y. Yu1, H. Ren1,2, Y. Li3, X. Yang1, L. Chen3, C. Tang1,2;
1Center for Quantitative Biology, Peking University, 2Peking-Tsinghua Center for Life Sciences, Peking University, 3College of Future Technology, Peking University, Beijing, China.
Background and aims: Global calcium wave within pancreatic islets is important to the pulsatile secretion of insulin. Recent studies focused on wave propagation in fast oscillation mode, but few of them documented the wave pattern of slow oscillation, and how fast and slow waves interact in mixed mode is almost obscure.
Materials and methods: We developed a 3D time-lapse imaging pipeline based on two-photon microscopy, microfluidic chips, and quantitative analysis to record high spatial and temporal resolution calcium waves within pancreatic islets. In brief, islets isolated from Ins1-Cre+; GCaMP6ff/+ mice were loaded into microfluidic chips with sustained 10 mM glucose stimulation and 3D images were acquired by two-photon microscopy. Each 3D image in time series is composed of several 2D images in a z-stack, which step size was between 3.7-6.5 μm and whose imaging depth was around 150 μm-200 μm, according to the sample size. (Figure A) The time resolution could stably reach 1 Hz.
Results: We showed conserved calcium wave propagation patterns of fast (period < 1 min) and slow (period > 1 min) oscillation modes upon high glucose stimulation. Fast waves behaved like traveling waves, which could be initiated by multiple conserved β cell clusters in the periphery. In contrast, the slow oscillation wave propagation pattern was similar to “diffusion wave”, which was sharply activated by β cells in the periphery, and the signaling gradually propagated into the core. (Figure B) The mean velocity, calculated from the average distance of the wave to propagate per second, of the fast and slow waves is 61.4 ± 22.5 μm/s and 2.1 ± 0.7 μm/s, respectively. Both wave velocity distributions of fast and slow modes and their propagation patterns implied hidden mechanisms, other than electrical coupling via gap junction, to account for β cell synchronization. What’s more, it was striking that the wave propagation pattern of a mixed oscillation could be decomposed into a slow wave and several fast waves, with the same pattern observed in pure fast/slow oscillations.
Conclusion: Our study showed that β cells synchronized at fast and slow time scales and their complex interplay, which might imply the unknown mechanism of multicellular systems in general.
Supported by: CMST,NNSFC,NKRDPC
Disclosure: Y. Yu: None.
SO 16 A new niche to replace beta cells
379
Stem cell-derived, fully differentiated islet cells for type 1 diabetes
C. Ricordi1, A. Naji2, M.R. Rickels3, B. Bruinsma4, G. Marigowda4, L. Ross4, C. Wang4, F. Pagliuca4, B. Sanna4, L.S. Kean5, A. Peters6, P. Witkowski7, J. Markmann8;
1University of Miami Diabetes Research Institute and Cell Transplant Center, Miami, 2University of Pennsylvania, Philadelphia, 3University of Pennsylvania Perelman School of Medicine, Philadelphia, 4Vertex Pharmaceuticals, Boston, 5Boston Children's Hospital, Boston, 6University of Southern California, Los Angeles, 7University of Chicago, Chicago, 8Massachusetts General Hospital, Boston, USA.
Background and aims: Cadaveric islet transplantation can achieve glycemic control in T1D, but cadaveric islet quantity and quality are limiting. We report the first patient administered VX-880, an investigational allogeneic stem cell-derived, fully differentiated, pancreatic islet cell replacement therapy.
Materials and methods: Following a single infusion of VX-880 at half the target dose, the patient was monitored for safety and tolerability, as assessed by adverse events (AEs) and clinical laboratory assessments, fasting and stimulated C-peptide, HbA1c, glycemic variability, interstitial glucose by continuous glucose monitoring, and exogenous insulin dose.
Results: A 64yo male with a 40y history of T1D complicated by impaired awareness of hypoglycemia with 5 severe hypoglycemic events (SHEs) the year before VX-880 was receiving 34U insulin/day at baseline (HbA1c 8.6%; undetectable fasting and stimulated C-peptide). After a single VX-880 infusion at half target dose, fasting C-peptide was detected by Day 29 and increased rapidly; HbA1c and daily insulin decreased in parallel. At Day 90, robust increases in fasting and stimulated C-peptide, improved glycemic control, and a substantial reduction in exogenous insulin administration were observed and continued to improve through last time point assessed (Table). VX-880 was generally safe and well tolerated; most AEs were mild or moderate and consistent with immunosuppression. The most common AEs were SHEs (not serious or related to VX-880) which occurred in the perioperative period. There was 1 serious AE of rash (mild, not related to VX-880) which resolved.
Conclusion: These unprecedented results are the first evidence that stem cell-derived islets can restore insulin production and glucose control in T1D. The study continues to enroll and dose patients.
Clinical Trial Registration Number: NCT04786262
Supported by: Vertex Pharmaceuticals
Disclosure: C. Ricordi: Other; C. Ricordi serves as a scientific advisor to Vertex Pharmaceuticals Incorporated.
380
An improved approach for generation of beta cell like insulin-producing cells from induced pluripotent stem cells
B. Cetin, A.D. Sanlioglu;
Gene and Cell Therapy, Akdeniz University, Antalya, Turkey.
Background and aims: The necessity for increasing the effectiveness of current treatment modalities, as well as developing new therapeutic approaches for diabetes management is well acknowledged. Induced Pluripotent Stem Cells (iPSCs) hold great promise for regenerative medicine applications, including the treatment of diabetes, due to their low immunogenicity besides their high potential for self-renewability and differentiation. Studies aiming for the generation of new beta cell sources from pluripotent stem cells have paved the way for direct methodologies for differentiation of iPSCs into beta cell-like insulin-producing cells (IPCs) via small molecules activating key signaling pathways throughout beta-cell development. Yet there are still hurdles to overcome for the development of protocols with optimal efficiency and safety.
Materials and methods: We present an improved approach for the generation of IPCs from IPSCs via use of key small molecules. TNF-Related Apoptosis-Inducing Ligand (TRAIL) molecule was included in our protocol based on its yet not-well explained protective role in diabetes development shown by the work of our group and other groups, along with possible unknown mechanisms we hypothesize to be in action on beta cells. In correlation with this, a proliferative effect of TRAIL on rodent beta cells was evident in a recent study by our group
Results: In our study, stepwise differentiation of IPCs from human fibroblast-originated iPSCs was confirmed by the expression of Sox17 and FoxA2 (definite endoderm); PDX-1, and insulin (pancreatic differentiation); and PDX-1, insulin, NeuroD1, Pax6 and Islet-1 (differentiation into IPCs). The inclusion of TRAIL in our protocol displayed differentiated IPCs with increased insulin and c-peptide secretion.
Conclusion: We believe that our combined approach may be useful as an improved strategy, besides contributing to revealing of the potential of TRAIL as a candidate therapeutic molecule in diabetes.
Supported by: Akdeniz University, BAP
Disclosure: B. Cetin: None.
381
Endoc-βH5 human beta cells: a unique thaw and go model for accelerating diabetes research with highly functional and ready-to-use human beta cells
H. Olleik, B. Blanchi;
Human Cell Design, Toulouse, France.
Background and aims: In 2021, 537 million adults were living with diabetes worldwide (90% T2D), a number that is predicted to rise to 643 million by 2030. The need for physiologically relevant human cellular models to study human beta cell function, insulin secretion and diabetes is thus greater than ever. To date, human pancreatic islets present many drawbacks related to lack of availability and reproducibility that limit their use. While animal models remain far from human reality.
Materials and methods: Different assays were used for the validation of EndocBH5. For example, microscopy and FACS analysis were performed to examine the morphology of the cells and detect the beta cell identity markers. Furthermore, GSIS and incretin pharmacology analysis were performed in 96 well plates to study the functional capacity of EndocBH5. Also siRNA, using lipofectamine, was used to know down genes of interest for disease modeling.
Results: Specifically, EndocBH5 is a pure population of insulin expressing cells (100% INS/92.3% PDX1/90% NKX6.1). Insulin content is close to that of native pancreatic beta cells (> 5μg/million cells). EndocBH5 cells dose dependently respond to physiological concentrations of glucose (highest potentiation of insulin secretion between 2.8 and 8 mM glucose) with elevated absolute insulin secretion values (hundreds of ng/hr/106 cells) similar to native islets. Furthermore, they dose dependently respond to GLP1R agonists, GIP agonist D-ALA-2-GIP and dual agonist tirzepatide with reproducible EC50 and increased insulin secretion >3-fold over high glucose. In addition, EndocBH5 cells can be assayed in 384-well plates paving the way for HTS and have been validated as 3D spheroids with extremely robust functional data. Finally, expression of diabetes related genes can be modified to produce relevant disease models.
Conclusion: Overall, EndocBH5 represent a novel human pancreatic beta cell solution with very high potential for developing human diabetes models, unraveling diabetes mechanisms in human cells and developing anti-diabetic drugs.
Disclosure: H. Olleik: None.
382
Exendin-4 enhances GSIS through upregulation of MAFA and mitochondrial oxidative phosphorylation machinery genes in hPSC-derived beta cells
A. Diane, R. Bin Abdul Mu-u-min, H.H. Al-Siddiqi;
Diabetes Research Center, Qatar Biomedical Research Institute, Hamad Bin Khalifa University, Doha, Qatar.
Background and aims: Impaired insulin secretion contributes to the pathogenesis of diabetes mellitus type 1 (T1DM) through autoimmune destruction of pancreatic β-cells and the pathogenesis of diabetes mellitus type 2 (T2DM) through β-cell dedifferentiation and other mechanisms. Emerging beta-cell replacement with human pluripotent stem cell (hPSC)-derived β-cells may provide remedial cell therapy. Most protocols to differentiate hPSCs to insulin-expressing β-cells in vitro have generated hPSC-derived β-cells with either immature phenotype such as impaired or weakened glucose-stimulated insulin secretion (GSIS) relative to primary β-cells. Evidence has shown that β-cell mitochondria play a central role in coupling glucose metabolism to insulin exocytosis. Therefore, the impairment of GSIS in hPSC-derived β-cells may be attributed to mitochondrial dysfunction. The aim of this study is to investigate the effect of Exendin-4 (GLP-1 receptor analog), known to promote mitochondrial function, on enhancing maturation and functionality of hPSC-derived ß-cells.
Materials and methods: Differentiation of hPSC into β-cells was carried out in a stepwise 3D differentiation protocol using 30 ml spinner flask adapted from the protocol of Veres et al. (2019). This consists of 6 stages: S1, definitive endoderm; S2, gut tube endoderm; S3, pancreatic progenitors 1; S4, pancreatic progenitors 2; S5, endocrine precursors; and S6, beta-like cell formation. The efficiency of relevant stage-specific marker expression was tested using flow cytometry. Finally, to further test the effect of GLP-1 receptor analog in promoting the maturation of β-cell phenotype, 50 nM Exendin-4 was added to the suspension culture during the last three days of differentiation. To determine the functionality of hPSC-derived β-cells, GSIS was performed. Gene expression in cell clusters was determined by RT-PCR. Data are mean ± SEM (n=3)
Results: Flow cytometry data for relevant stage-specific markers showed 90 % OCT4 positive, 90% SOX17 and 70% PDX1 positive cells, indicating good pluripotency, high definite endoderm and pancreatic progenitor induction, respectively. As for ß-cell markers, we found 40.7% NKX6.1 positive cells during stage 6, indicating a generation of insulin-expressing β-cells. Expression profiling during differentiation confirmed the generation of insulin-expressing β-cells. However, GSIS data showed no difference in c-peptide secretion between low (2.8mM) and high (20 mM) glucose but high response to direct cellular depolarization-mediated c-peptide by KCl; suggesting a lack of functional ß-cells. Interestingly, addition of Exendin-4 (50 nM) during the last 3 days of the differentiation significantly enhanced GSIS associated with increased expression of MAFA and genes encoding mitochondrial oxidative phosphorylation machinery.
Conclusion: Our data demonstrated for the first time in 3D differentiation of hPSC-derived β-cells using spinner flasks that addition of Exendin-4 enhances GSIS through upregulation of MAFA and mitochondrial oxidative phosphorylation machinery genes.
Supported by: QBRI intramural grant (BR01) to Dr. Heba H. Al-Siddiqi
Disclosure: A. Diane: None.
383
Co-transplantation of pancreatic islets and microvascular fragments effectively restores normoglycaemia in diabetic mice
E. Ampofo, S. Wrublewsky, A. Weinzierl, I. Hornung, M. Menger, M. Laschke;
Saarland University, Homburg, Germany.
Background and aims: Insufficient revascularization of pancreatic islets is one of the major obstacles impairing the success of islet transplantation. To overcome this problem, we introduce in the present study a straightforward strategy to accelerate the engraftment of isolated islets. For this purpose, we combined adipose tissue-derived microvascular fragments (MVF) with pancreatic islets and co-transplanted this mixture in streptozotocin (STZ)-induced diabetic mice.
Materials and methods: Islets and MVF were isolated from C57/BL6N mice and their viability was assessed by calcein/propidium iodide stainings. Two hundred fifty islets and 20,000 MVF as well as 500 islets and 40,000 MVF were co-transplanted under the kidney capsule or into the subcutaneous space of diabetic mice, respectively. The revascularization and endocrine function of the grafts were determined by the determination of blood glucose levels twice a week. An intraperitoneal glucose tolerance test (IPGTT) was performed at the end of the entire observation period. The grafts’ morphology, viability and cellular composition were analyzed using immunohistochemistry.
Results: We found that the co-transplantation of islets and MVF markedly accelerates the restoration of normoglycemia in diabetic recipients when compared to the transplantation of islets alone. In fact, the co-transplantation of 250 islets and 20,000 MVF under the kidney capsule reversed diabetes in 88%. The subcutaneous transplantation of 500 islets and 40,000 MVF leads to normoglycemia in all STZ-treated mice when compared to controls. Additional immunohistochemical analyses of the grafts revealed a significantly higher number of islet cells and microvessels in the co-transplantation groups at the end of the observation period.
Conclusion: Our findings clearly demonstrate that the co-transplantation of islets and MVF significantly accelerates the revascularization of the grafts. This not only improves the success rates of islet transplantation, but could also be easily translated into clinical practice.
Disclosure: E. Ampofo: None.
384
Comparison of different hyaluronic acid-based matrices to maintain human islet survival in macroencapsulation devices
D. Brandhorst1, H. Brandhorst1, D.A. Domingo-Lopez2, E. O'Cearbhaill3, L. McDonough4, F.B. Coulter3, S. Deotti3, H. Kelly4, G. Duffy2, P.R.V. Johnson1;
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK, 2Anatomy and Regenerative Medicine Institute, National University of Ireland, Galway, Ireland, 3School of Mechanical Engineering, University College of Dublin, Dublin, Ireland, 4School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland.
Background and aims: Encapsulation of isolated islets has the potential to enable transplantation without the requirement for life-long immunosuppression. However, to date, insufficient oxygen supply has meant that long-term graft survival of macroencapsulated islets has been challenging. The aim of this study was to investigate the efficiency of different bio-compatible matrices that can deliver oxygen, thereby facilitating early graft survival and providing a replacement for the collagenase-digested extracellular matrix.
Materials and methods: Isolated human islets (n = 7) were subjected to quality assessment (yield, counted as islet particle number [IN] and islet equivalents [IEQ]; viability [FDA-PI]; early apoptosis [Annexin-V] and reactive oxygen species [ROS] production [DCFH-DA]) before mixed with different matrices: (A) supplemented CMRL (control); (B) native Hyaluronic-acid (HA)-based Gel; (C) Beta (ß)-Gel (HA-Gel+Perfluorodecalin-emulsion) or (D) pre-oxygenated ß-Gel (ß-Gel+O2). Afterwards, silicone-based 3D-printed macrodevices were loaded with 600 matrix-immersed IEQ and cultured for 4-5 d in normoxia. Subsequently, islet characterisation was performed as described above. Parameters were related to IEQ and normalised to preculture (PC) data (mean ± SEM).
Results: Following culture a massive loss of islets was noted when embedded in CMRL (11±3% recovery, p<0.001 vs PC, vs ß-Gel+O2 [81±7%]; p<0.01 vs ß-Gel [63±4%]) or HA-Gel (35±8%, p<0.01). Reduction of islet yield correlated with substantially enhanced fragmentation (ratio of IN over IEQ) (421±70%, p<0.001 vs PC; 283±80%, p<0.01; 158±11%, p<0.05; 152±22%, NS) in CMRL, HA-Gel, ß-Gel or ß-Gel+O2, respectively. Accumulation of cell fragments was associated with increased islet ROS production (733±403%; 497±293%, p<0.05 vs CMRL; 177±89%, p<0.01; 140±64%, p<0.01) after encapsulation in CMRL, HA-Gel, ß-Gel and ß-Gel+O2, respectively. Compared with CMRL (75±4%, p<0.001 vs PC, vs ß-Gel+O2) and HA-Gel (84±2%, p<0.05 vs PC, vs ß-Gel+O2) viability was significantly better preserved in ß-Gel (84±3%, p<0.05 vs PC, vs CMRL) and ß-Gel+O2 (92±5%).Early apoptosis was highest in CMRL (1095±228%, p<0.001 vs PC, vs ß-Gel+O2) and HA-Gel (716±193%, p<0.01 vs PC, p<0.05 vs CMRL) but lower in ß-Gel (345±62%, p<0.05 vs PC, vs CMRL) or ß-Gel+O2 (337±63%).Overall survival, defined as survival of viable cells only, was marginal in CMRL (8±2%, p<0.001 vs PC, vs ß-Gel+O2), low in HA-Gel (29±7%, p<0.001 vs PC) but higher in ß-Gel (54±6.3%, p<0.05 vs PC, p<0.01 vs CMRL) and ß-Gel+O2 (75±7%, p<0.01 vs HA-Gel).
Conclusion: Our findings demonstrate that the use of a suitable bio-compatible matrix is essential to reduce inflammation and to protect the integrity of macro-encapsulated human islets. As hypoxia is the most decisive factor for islet survival, the efficient delivery of oxygen, even for a limited time, helps to promote islet survival within macrodevices.
Supported by: European Union’s Horizon 2020 grant # 645991
Disclosure: D. Brandhorst: None.
385
Proteome profiling of whole plasma and plasma-derived extracellular vesicles facilitates the detection of tissue biomarkers in the NOD mouse
V.M. Stone1, I. Diaz Lozano1, H. Sork1,2, M. Eldh3, M. Pernemalm4, S. Gabrielsson3, M. Flodström-Tullberg1;
1Department of Medicine Huddinge, Karolinska Institutet, Stockholm, Sweden, 2Institute of Technology, Tartu, Estonia, 3Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden, 4Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden.
Background and aims: Type 1 diabetes (T1D) results from a destruction of the insulin-producing pancreatic β-cells. The discovery of protein biomarkers that are measurable in serum or plasma during the pre-diabetic period could facilitate personalised disease interventions. Recent studies have shown that extracellular vesicles (EVs) found in liquid biopsies are a source of proteins derived from different tissues. The aim of this study was to address if combined analysis of whole plasma samples and plasma-derived EV fractions increases the number of unique proteins identified by mass spectrometry (MS) compared to the analysis of whole plasma samples alone.
Materials and methods: Plasma (up to 650 μl per animal) was collected from non-diabetic NOD mice (approximately 8-10 weeks old) and either saved as whole plasma or filtered (0.8 μm) prior to enrichment of EVs by size exclusion chromatography (SEC; qEV original, Izon) or membrane affinity (MA; exoEasy, Qiagen). Particle size, structure and number were analysed by nano particle tracking analysis (NTA; NanoSight) and transmission electron microscopy (TEM). LC-MS/MS analysis was performed using EV-enriched plasma samples and high-resolution isoelectric focusing (HiRIEF) LC-MS/MS using whole plasma. For some analyses, samples were depleted of abundant plasma proteins prior to MS analysis using the Multiple Affinity Removal Spin Cartridge Mouse-3 column (Agilent Technologies). Bioinformatics analysis was performed using DAVID v6.8 and R (version 4.0.4).
Results: EVs enriched by SEC (qEV) were on average around 93 ± 9 nm in size with a mean number of 3.2 x 108 ± 1.6 x 108 particles isolated per μl of plasma. In contrast fewer particles were enriched by exoEasy (MA; 3.6 x 107 ± 2.5 x 107 particles per μl plasma) and they were on average bigger in size (mean 201 ± 24 nm). TEM analysis of exoEasy enriched fractions revealed a greater fraction of larger particles with a less-defined shaped whilst EVs with cup-shaped morphology indicative of exosomes were found in the qEV enriched fractions. HiRIEF LC-MS/MS analysis of plasma samples depleted of abundant proteins and subjected to peptide fractionation identified more than 2300 proteins, while the LC-MS/MS analysis of EV-enriched plasma samples enriched using qEV identified more than 600 proteins. Of the proteins detected in EV-enriched samples, more than a third were not identified in whole plasma samples and many were classified as either tissue-enriched or of tissue-specific origin.
Conclusion: Parallel profiling of EV-enriched plasma fractions and whole plasma samples increases the overall proteome depth and facilitates the discovery of tissue-enriched proteins in plasma from pre-diabetic NOD mice. This integrated proteome profiling scheme may be useful for the discovery of new biomarkers in T1D.
Supported by: Barndiabetesfonden, J&J & KI Partnership Program, KI, Novo Nordisk Foundation, EDF, Mobilitas Plus
Disclosure: V.M. Stone: None.
SO 17 Intracellular signalling balancing beta cell survival and death
386
Differential spatiotemporal GLP-1 receptor signalling in primary pancreatic beta cells
M.A. Ravier, N. Zaïmia, G. Bertrand;
Institute for Functional Genomics, Univ Montpellier, CNRS, INSERM, Montpellier, France.
Background and aims: Increasing the efficacy of GLP-1 receptor (GLP-1R) agonists is currently the main challenge in type 2 diabetes research. GLP-1R is a G protein-coupled receptor (GPCR) known to be positively coupled to cAMP production and PKA/EPAC2 activations and to recruit the scaffold protein beta-arrestin2 (ARRB2), which may activate new signaling pathways such as the kinases ERK1/2. Most of the studies have investigated GLP-1R signaling in recombinant clonal non-beta cell lines. Consequently, a better understanding of the mechanisms involved in GLP-1R internalization/desensitization and signaling in primary pancreatic beta cells is necessary. In addition, special attention should be paid to the effect of therapeutic pharmacological concentrations found in type 2 diabetes patients treated with GLP-1R agonists (nM) versus circulating physiological concentrations (pM) of GLP-1.
Materials and methods: Experiments were performed in beta cells from 4-month-old Arrb2-/- and Arrb2+/+ male mice. PKA (AKAR3) and ERK1/2 (EKAR) activities, and EPAC2 recruitment underneath the plasma membrane (EPAC2-GFP), were assessed by live-cell microscopy in mouse pancreatic beta cells after genetic expression of the sensors of interest by adenoviral infection. ERK1/2 (P-ERK1/2) and CREB (P-CREB) activation were assessed by immunofluorescence. GLP-1R internalization was determined by immunofluorescence from 4% formaldehyde fixed and non-permeabilised beta cells.
Results: In beta cells, GLP-1 (10pM to 10nM) caused a rapid activation of PKA, which remained sustained during stimulation (>40min). This activation persisted (~25 min) after cessation of GLP-1 stimulation at pharmacological concentrations (slope -0.4649±0.04) but not at physiological concentrations (slope -0.8826±0.11, p=0.001). Interestingly, the recovery was faster with a direct activator of the adenylate cyclase (1μM forskolin; slope: -2.673±0.10; p<0.001) that trigger larger activation of PKA. In parallel, EPAC2 is activated at pharmacological (10nM) and not physiological (10pM) concentrations of GLP-1. This was associated with massive GLP-1R internalization (~60%, p<0.001), which remained 25 min after cessation of stimulation (~40% p<0.001) at pharmacological concentrations. In contrast, a lack of GLP-1R internalization was observed at physiological GLP-1 concentrations (10-100pM). ARRB2, known to uncouple GPCRs from G protein and induce their internalization, is not involved in this process. Finally, pharmacological concentrations led to sustained activation of ERK1/2 kinases and nuclear activation of CREB only in the presence of ARRB2 (p<0.001). In contrast, physiological concentrations of GLP-1 caused transient ARRB2-independent activation of ERK1/2 and do not activate CREB.
Conclusion: This study reports for the first time that physiological and pharmacological concentrations of GLP-1 resulted in distinct cellular spatiotemporal responses in primary beta cells. Special attention should be paid to cell signaling when generating new GLP-1R agonists to treat type2 diabetes.
Supported by: SFD reaserch grant
Disclosure: M.A. Ravier: None.
387
The expression and function of CLEC11A in human pancreatic islets and human EndoC-βH1 cells
J. Cen1, R. Shi2, G. Westermark1, Z. Sun3, N. Welsh1, J. Lau1;
1Department of Medical Cell Biology, Uppsala University, Uppsala, Sweden, 2Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, China, 3Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, Medical School, Southeast University, Nanjing, China.
Background and aims: Beta-cell dysfunction is a hallmark of disease progression in patients with diabetes. The aim of this study was to explore the expression and function of CLEC11A, a secreted sulfated glycoprotein that initially identified as a growth factor, in human islets and human beta-cell line, and to investigate if CLEC11A can protect against beta-cell dysfunction caused by chronic palmitate exposure.
Materials and methods: Human islets and human EndoC-βH1 cells were treated with different concentrations of recombinant human CLEC11A protein (rhCLEC11A) ( 0, 10, 100, and 1000 ng/ml, respectively) for 2 days to test the effect of rhCLEC11A on beta-cell function and cell proliferation. EndoC-βH1 was treated with or without palmitate (1.5mM with 2% BSA) for 2 , 4, or 5 days, respectively, and the effect of rhCLEC11A on palmitate-treated beta-cells was explored. The expression levels of the proteins was assessed by western blot and immunofluorescence. The gene mRNA expression levels were measured by RT-qPCR. Beta-cell function was assessed by glucose-stimulated insulin secretion (GSIS) and insulin content. The proliferation rate of EndoC-βH1 cells was measured by WST-1 assay and Ki67 staining.
Results: CLEC11A was expressed in beta-cells and alpha-cells in human islets but not in EndoC-βH1 cells; whereas the receptor of CLEC11A called ITGA11, was found in both human islets and EndoC-βH1 cells. The dose-dependent effects of rhCLEC11A on beta-cell function revealed that after 2 days’ culture, rhCLEC11A (1000 ng/ml) significantly increased the insulin stimulation index (ISI, calculated from GSIS) around 50% (p<0.05) and doubled insulin content (p<0.05), when compared with the control human islets. Similar effects were found in EndoC-βH1 cells cultured with rhCLEC11A (100 ng/ml) for same period. In EndoC-βH1 cells, 2-day rhCLEC11A (10 ng/ml) treatment induced cell proliferation rate around 10% (p<0.05) and elevated the expression levels of transcription regulators MAFA (p<0.05) and PDX-1 (p<0.05) about 50%, respectively. Palmitate alone reduced ISI in EndoC-βH1 cells in a time-dependent manner, with 20% (p<0.05) and 30% (p<0.05) of the control levels at 2-day and 4-day culture, respectively. When rhCLEC11A (100 ng/ml) was introduced in palmitate-treated cells, ISI was not affected after 2-day culture (p>0.05) but was improved 25% after 4-day culture (p<0.05) compared with the palmitate-cultured cells at the respective time points. Palmitate alone reduced the mRNA expression levels of INS and MAFA in EndoC-βH1 after 5-day culture , with 10% (p<0.05) and 50% (p<0.05) of the control levels, respectively. Compared with palmitate-exposed cells, the administration of rhCLEC11A (100 ng/ml) significantly improved INS mRNA expression, reaching 60% of the control level (p<0.05), but had no significant impact on the MAFA expression (p>0.05) in palmitate-exposed EndoC-βH1 cells.
Conclusion: CLEC11A is expressed in the insulin-secreting beta-cells and glucagon-secreting alpha-cells in human islets but not in EndoC-βH1 cells. The finding that rhCLEC11A induced the mRNA expression of transcription factors MAFA and PDX1 is associated with the accentuated insulin secretion, insulin content and proliferation in human beta-cells.
Supported by: Swedish Child Diabetes Foundation and Family Ernfors Foundation
Disclosure: J. Cen: None.
388
CD47/SIRP-alpha: a critical axis that may contribute to beta cell loss in type 1 diabetes
K. Afi Leslie, S.J. Richardson, N.G. Morgan, M.A. Russell;
University of Exeter Medical School, Exeter, UK.
Background and aims: Interleukin (IL)-13 enhances the expression of the transmembrane glycoprotein, Signal Regulatory Protein Alpha (SIRPα) in human β-cells via a Signal Transducer and Activation of Transcription factor (STAT)-6 dependent mechanism. SIRPα interacts with CD47 to transmit “don’t eat me” signals to macrophages but it is also expressed on both α- and β-cells in human islets suggesting a role for the CD47/SIRPα pathway in control of islet cell viability. Therefore, we have studied the expression of both CD47 and SIRPα in human pancreas sections, isolated human islets and human EndoC-βH1 cells to gain insights into their functional roles.
Materials and methods: Human pancreas sections from control subjects or those with recent-onset type 1 diabetes from within the Exeter Archival Diabetes Biobank were used for immunostaining studies. In cultured EndoC-βH1 cells, CD47 or SIRPα were knocked down using interference RNA. qRT-PCR and western blotting were employed to monitor changes in gene and protein expression, respectively in isolated islets or cell lines.
Results: Selective knockdown of either SIRPα or CD47 in EndoC-βH1 cells led to reduced viability (scrambled control: 23.6±1.4% cell death, siSIRPα 45.0±1.2%; p<.001, siCD47: 32.23±1.7% p,0.05). Immunostaining and quantification of human pancreas sections revealed higher levels of CD47 expression in β- than in α-cells of normal individuals. By contrast, in type 1 diabetes, a significant increase in α-cell expression of CD47 was seen in each of the previously described T1D endotypes (T1DE1 & 2); (control T1DE1 53.6±2.5AU, T1DE1 69.7±2.1AU p<0.001; control T1DE2 52.5±1.7AU, T1DE2 66.7±2.4AU p<0.001). Importantly, however, CD47 was differentially expressed by the β-cells of subjects with TIDE1 when compared to T1DE2. In T1DE1, β-cell CD47 was further increased above that of age matched controls (control: 63.1±1.9AU, T1DE1 beta cells 77.2±2.7AU; P<0.001), whereas, in the β-cells of people with T1DE2, the staining intensity did not exceed background levels. SIRPα was predominantly expressed in human islet β-cells in control subjects α-cells - 84.0±3.6AU, β-cells - 147.0±3.5AU, p<0.01). A significant loss of SIRPα was observed in the β-cells of subjects with T1DE1 or T1DE2 (p<0.001) while its expression was unchanged in α-cells (p=0.99).
Conclusion: Changes in the expression patterns of CD47 and SIRPα may contribute to β-cell loss in T1D but these changes occur differentially between the two disease endotypes, T1DE1 & 2. Our results imply that targeting of the CD47/SIRPα signalling pathway may offer the potential to reduce the rate of β-cell loss during progression to type 1 diabetes. However, they also suggest that care should be exercised when using therapeutic checkpoint inhibitors interacting via this pathway (e.g. anti-CD47 reagents for cancer therapy) to ensure that β-cell viability is not compromised inadvertently.
Supported by: Diabetes UK
Disclosure: K. Afi Leslie: None.
389
Peptide X, a promising inhibitor of MP001 protein, which improves human pancreatic islet survival and function
A. Langlois1, M. Kanzaki2, G. Bechtluft1, M. Pinget1, K. Bouzakri1;
1Centre Europeen d'Etude du Diabete, Strasbourg, France, 2Department of Biomedical Engineering, Graduate School of Biomedical Engineering, Sendaï, Japan.
Background and aims: Previously, our team identified by proteomics that several proteins are up-regulated in human pancreatic islets upon exposure with conditioned medium derived from insulin-resistant skeletal muscle cell culture induced by incubation with TNF-α. Among them, the MP001 protein which has been identified as being specific for the β-cell. Recently, we demonstrated that MP001 is a key protein involved in insulin granule exocytosis via an impact on the dynamics of actin filaments and cellular remodeling of β-cells. Moreover, its inhibition using siRNA technology improved human pancreatic islet survival and function. The aim of this project was to develop a pharmacological strategy of MP001 inhibition and to evaluate it effect on human islet survival and function. For that, we have tested the Peptide X.
Materials and methods: Studies were carried out on human pancreatic islet. These were incubated for 24h in presence of Peptide X 0.1, 1 or 10μM versus its Peptide control. Islet functionality was assessed using glucose stimulation insulin secretion test. Data were expressed in percentage toward total insulin content. Moreover, the effect of Peptide X on islet survival and function was studied using western blotting targeting Akt, p-Akt, NFκBp65, p-NFκBp65, PGC1α, IRS1, p-IRS1, IRS2, p-IRS2, Tbc1d1, p-Tbc1d1 and AS160. Protein expression was reported as a percentage versus Peptide control. Statistics were performed using non parametric test (Mann Whitney) for molecular study and ordinary one-way ANOVA followed to multiple comparison test for functionality evaluation. Data were reported as mean ± SEM for the indicated number of replicates and a p value of <0.05 was considered statistically significant.
Results: Firstly, we showed that Peptide X potentiates insulin secretion from 0.1μM in comparison to control with no improvement for higher concentrations (Peptide X 0.1μM 16.7mM glucose = 4.35±0.52%, Peptide X 0.1μM 2.8mM glucose = 1.24±0.33%, p< 0.001 n=13 versus Peptide control 0.1μM 16.7mM glucose = 3.30±0.39%, Peptide X 0.1μM 2.8mM glucose = 1.25±0.28%, p< 0.01 n=13). Thus, for the molecular study we used 0.1μM. Secondly, proteomic demonstrated that Peptide X improves human islet survival increasing Akt activation (p-AKT: 206±67% versus control, p<0.001, n=7), NFkBp65 (139±28% versus control, n=9) and PGC-1α expressions (146±10% versus control, p<0.01, n=5). Finally, Peptide X has a positive action on islet function up-regulating proteins involved in insulin molecular pathway as IRS1 (283±83% versus control, p<0.001, n=10), p-IRS2 (179±42% versus control, p<0.05, n=9), p-Tbc1d1 (179±39% versus control, p<0.05, n=7) and AS160 (185±36% versus control, p<0.05, n=9).
Conclusion: Our study has demonstrated for the first time that Peptide X, inhibiting MP001, improves human pancreatic islet survival and function. Consequently, based on our data, we propose that Peptide X could be a promising therapeutic approach for diabetic patients.
Disclosure: A. Langlois: None.
390
Islet macrophage depletion does not prevent amyloid-induced upregulation of tissue plasminogen activator (tPA)
N. Esser1,2, M.F. Hogan1, A.T. Templin1, R. Akter1, J.J. Castillo1, D.P. Raleigh3, S. Zraika1, R.L. Hull1, S.E. Kahn1;
1Division of Metabolism, Endocrinology & Nutrition, Department of Medicine, University of Washington, Veterans Affairs Puget Sound Health Care System, Seattle, USA, 2Laboratory of Immunometabolism and Nutrition, GIGA-I3, University of Liège, Liège, Belgium, 3Department of Chemistry, Stony Brook University, Stony Brook, USA.
Background and aims: Islet amyloid deposition and islet inflammation are pathogenic hallmarks of type 2 diabetes. Aggregation of human islet amyloid polypeptide (hIAPP) increases pro-inflammatory gene expression in islet macrophages and results in beta-cell loss. We previously found the fibrinolysis activator tissue plasminogen activator (tPA) is specifically upregulated in islets with amyloid deposition and reduces amyloid fibril formation through plasmin generation. Since macrophages can produce tPA, we determined whether they contribute to hIAPP-induced tPA upregulation.
Materials and methods: To determine whether hIAPP increases tPA expression in macrophage, tPA mRNA levels were quantified in rat bone-marrow-derived macrophages (BMDMs) treated for 24 h with 10 μM hIAPP, non-amyloidogenic 10 μM mouse IAPP (mIAPP) or vehicle. Further, to determine the contribution of islet macrophages to tPA upregulation by amyloid deposition, isolated islets from hIAPP transgenic mice (that have the propensity to form islet amyloid) and wild-type mIAPP mice (that do not form islet amyloid) were treated for 48 h with clodronate-containing liposomes (CLOD-lip) to deplete macrophages or PBS-containing liposomes (PBS-lip) as control. Islets were then cultured for 48 h in 16.7 mM glucose, which induces amyloid deposition in hIAPP, but not mIAPP islets. Islet macrophage markers (Emr1 and Itgam) and tPA mRNA levels were quantified.
Results: hIAPP but not mIAPP increased tPA gene expression in BMDMs (4.2±1.0 vs 1.5±0.6 vs 1.0±0.5 fold, hIAPP vs mIAPP vs vehicle respectively; n=4; p<0.05 hIAPP vs mIAPP and vehicle; p=0.25 mIAPP vs vehicle). As expected, islet tPA gene expression significantly increased by 2.1-fold in PBS-lip-treated hIAPP islets vs mIAPP islets (Table 1). In hIAPP and mIAPP islets, CLOD-lip treatment significantly abrogated Emr1 and Itgam gene expression vs PBS-lip treatment, confirming islet macrophage depletion in both genotypes (Table 1). CLOD-lip treatment also significantly reduced tPA expression (vs PBS-lip) in both hIAPP and mIAPP islets; however, tPA expression was still increased by 2.3-fold in CLOD-lip treated hIAPP islets vs mIAPP islets (Table 1).
Conclusion: hIAPP treatment induces tPA expression in macrophages and islet macrophage depletion decreases, but does not prevent, upregulation of tPA in amyloid-laden islets. Thus, our data suggest that, in addition to macrophages, other islet cell types produce tPA in response to amyloid deposition.
Supported by: US Department of Veterans Affairs; National Institutes of Health; UW McAbee Fellowship; SFD
Disclosure: N. Esser: None.
391
Pancreatic beta cells are resistant to RIPK1 kinase-mediated death
T. Takiishi1, P. Xiao1, E.A. Gurzov1, M. Bertrand2, A.K. Cardozo1;
1Experimental Gastroenterology Laboratory and Endotools, Universite Libre Bruxelles, Brussels, 2VIB-UGent Center for inflammation Research, Ghent, Belgium.
Background and aims: Type 1 diabetes (T1D) is caused by progressive immune-mediated loss of insulin-producing β-cells. Inflammation is very detrimental to β-cell function and survival, moreover, both apoptosis and necrosis have been implicated as a mechanism of β-cell loss in T1D. The protein receptor interacting serine/threonine kinase 1 (RIPK1) promotes inflammation either directly by serving as a scaffold for NF-κB and MAPK activation, or instead indirectly by acting as a kinase that triggers the dismantlement of the cell by apoptosis or necroptosis. To investigate if RIPK1 kinase activity is involved in T1D pathology, we evaluated whether RIPK1 kinase is activated in β-cells and whether this activation would affect cell survival. Furthermore, we investigated if mice lacking RIPK1 kinase activity would present glucose abnormalities in homeostasis or different susceptibility to immune-mediated diabetes in the multiple low-dose streptozotocin (MLDSTZ) model.
Materials and methods: The RIPK1 knockin mouse line carrying a mutation mimicking serine 25 phosphorylation (Ripk1S25D/S25D), which abrogates RIPK1 kinase activity, was utilized to assess the in vivo role of RIPK1 in β-cells both in physiological conditions and after MLDSTZ induced diabetes. Weekly glycemia and bodyweight of Ripk1S25D/S25D mice and wild type littermates (Ripk1+/+) were followed. IpGTT, iTT and pancreas insulin content measurements were performed. In vitro, β-cell death and RIPK1ser166 phosphorylation (a marker of activation) was analysed in mouse islets and EndoC-βH1 cells exposed to conditions known to induce RIPK1-dependent apoptosis/necroptosis: TNF + BV6 (a cIAP inhibitor), with or without zVAD.fmk (pan caspase inhibitor) or Nec-1 (RIPK1 kinase inhibitor).
Results: Under physiological conditions Ripk1S25D/S25D mice did not display any overt abnormalities and showed normal weight gain, glycemia and body mass composition, presenting normal glucose metabolism and β-cell function when compared to control littermates. Moreover, disease progression was not different between Ripk1S25D/S25D and Ripk1+/+ mice when rendered diabetic by MLDSTZ. Both Ripk1S25D/S25D and Ripk1+/+ displayed hyperglycaemia, glucose intolerance and significant loss of pancreatic insulin content, without any significant alterations caused by absence of RIPK1 kinase function. In vitro, RIPK1 enzymatic activity was highly induced by TNF+BV6+zVAD both in mouse islets and human β-cells. However, RIPK1 activation did not lead to β-cell death.
Conclusion: RIPK1 kinase does not affect glucose homeostasis nor diabetes presentation in mice. Contrary to what is described on most cell types, pancreatic β-cells are resistant to RIPK1-dependent cell death. This may reflect an adaptation to the inability of β-cells to proliferate and self-renewal.
Supported by: FNRS-EOS; CDR J.0109.22
Disclosure: T. Takiishi: Grants; FNRS-EOS.
392
Identification of a human-specific alteration of beta cell function after prolonged culture of pancreatic islets under glucotoxic conditions
M. Tariq1, A.H. de Souza1, H. Chae1, M. Jaffredo2, A. Wojtusciszyn3, M. Raoux2, P. Gilon1, C. Broca3, J.-C. Jonas1;
1Pole of Endocrinology, Diabetes and Nutrition, Université Catholique de Louvain, Brussels, Belgium, 2UMR 5248, University of Bordeaux, CNRS, Pessac, France, 3Laboratoire de Thérapie Cellulaire du Diabète, Hopital St Eloi, CHU Montpellier, Montpellier, France.
Background and aims: The rapid reversibility of recent type 2 diabetes (T2D) by very low-calorie diet in some patients correlates with a marked improvement of glucose-stimulated insulin secretion (GSIS), emphasizing the role of β-cell dysfunction in the early stages of the disease. In search of human-specific mechanisms of β-cell dysfunction, we extensively characterized the glucotoxic alterations of insulin secretion and upstream coupling events in human pancreatic islets cultured for 1 to 3 weeks at ~5, 8, 10 or 20 mmol/l glucose and acutely stimulated by a stepwise increase in glucose concentration.
Materials and methods: Islets from 46 non-diabetic (ND) and 6 type 2 diabetic (T2D) donors were obtained from 5 isolation centers over the last 10 years. The islets were precultured 3-7 days in RPMI containing 5 mmol/l glucose and 10% FBS. They were then cultured for 1-3 weeks in the same medium containing 5.5, 8.5, 10.5 or 20.5 mmol/l glucose before measurements of insulin secretion during culture, islet insulin/DNA content ratio, β-cell apoptosis, cytosolic and mitochondrial thiol redox state, and assessment of dynamic insulin secretion and upstream coupling events during stepwise stimulation with glucose (NAD(P)H autofluorescence, ATP/(ATP+ADP) ratio, electrical activity, cytosolic Ca2+ concentration ([Ca2+]c)).
Results: Culture of ND-islets for 1 to 3 weeks at ~8, 10 or 20 vs 5 mmol/l glucose did not increase β-cell apoptosis or oxidative stress but concentration-dependently decreased insulin content and increased the β-cell sensitivity to subsequent stimulation with glucose. The islet glucose responsiveness (max amplitude of GSIS per islet) was larger after culture at 8 or 10 vs 5 mmol/l glucose but was markedly reduced after culture at 20 vs 5 mmol/l glucose. In the latter islets, [Ca2+]c and insulin secretion responses to acute stepwise stimulation with glucose were bell-shaped, with a maximal stimulation at 5 to 10 mmol/l glucose followed by a rapid sustained inhibition above that concentration. This glucotoxic alteration was a robust characteristic of human islets. It resulted from long-term stimulation of glucose metabolism and was fully reversible after culture at 5 mmol/l glucose. Finally, acute activation/inhibition of glucokinase during perifusion of islets cultured at 20 mmol/l glucose indicated that the acute reduction of [Ca2+]c and insulin secretion above 10 mmol/l glucose was due to overstimulation rather than inhibition of glucose metabolism. Similar results were obtained in islets from T2D-donors.
Conclusion: Long-term exposure of human islets to mildly elevated glucose concentrations markedly increases their glucose sensitivity and reveals a bell-shaped glucose response curve for changes in [Ca2+]c and insulin secretion. This human-specific glucotoxic alteration may contribute to β-cell dysfunction in T2D independently from a detectable increase in β-cell apoptosis and oxidative stress.
Supported by: DiaType Project Innoviris, FRS-FNRS CDR J.0087.19 and ARC18/23-094-CfB, Brussels; SFD, Paris
Disclosure: M. Tariq: None.
393
Identifying factors linking hyperglycaemia-induced metabolic dysregulation to loss of insulin biosynthesis in pancreatic beta cells
M. Lloyd, E. Haythorne, F. Ashcroft;
Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK.
Background and aims: Diabetes/chronic hyperglycaemia alters glucose metabolism and reduces insulin biosynthesis in pancreatic beta cells. However, the underlying molecular mechanism(s) are poorly understood. The aim of this study was to investigate how changes in beta cell metabolism are linked to the downregulation of insulin biogenesis.
Materials and methods: INS-1 insulinoma cells were cultured at low (LG: 5 mM) or high (HG: 25 mM) glucose, ± selective glycolytic or pentose phosphate pathway enzyme inhibitors, for 48 hrs. For experiments with mitochondrial fuels, methyl pyruvate or methyl succinate was added to the media to 20 mM. Gene expression was measured by RT-qPCR. Cellular insulin content was quantified by ELISA.
Results: Chronic hyperglycaemia markedly reduced mRNA expression of the insulin genes (Ins1 and Ins2) and the associated transcription factors MafA, NeuroD1, Nkx6-1, Pax6, and Pdx1. 10 mM mannoheptulose, a glucokinase inhibitor, prevented downregulation of these genes in HG cells, but the mitochondrial substrates methyl pyruvate and methyl succinate did not cause their downregulation. LG cells cultured with 5 μM koningic acid, an irreversible inhibitor of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), showed reduced Ins1 and Ins2 expression and a 75% reduction in insulin content. These data suggest a metabolite upstream of GAPDH triggers changes in insulin gene expression. The pentose phosphate pathway is upregulated in hyperglycaemia; however, 100 μM 6-aminonicotinamide, an inhibitor of the oxidative pentose phosphate pathway, did not affect insulin gene expression or content in HG cells. 24 hr culture of LG cells with 25 mM 2-deoxyglucose (which is metabolised to 2-deoxyglucose 6-phosphate but does not undergo further glycolysis) reduced Ins1 and Ins2 expression by >60%. Time course experiments showed Ins1 and Ins2 expression declined by >80% in HG cells after 24 hrs, and was not fully restored by subsequent transfer to LG for 24 hrs.
Conclusion: These results suggest that chronic hyperglycaemia downregulates insulin gene expression in pancreatic beta cells via changes in glycolytic metabolites that lie downstream of glucokinase and upstream of GAPDH. Experiments with 2-deoxyglucose implicate glucose 6-phosphate as a major driver of insulin loss. The data also suggest that repression of insulin expression is not readily reversible following reversal of hyperglycaemia; recurrent episodes of hyperglycaemia could thereby contribute to a progressive impairment of beta cell function.
Supported by: BBSRC, Novo Nordisk, MRC and Wellcome Trust
Disclosure: M. Lloyd: None.
SO 18 Stressing the beta cell into dysfunction
394
Gliclazide protects against oxidative stress and mitochondrial toxicity in beta cells exposed to the antiretroviral agent rilpivirine
S.C. Maandi, J.G. Mabley;
Huxley Building, University of Brighton, Brighton, UK.
Background and aims: Due to the increased incidence of type 2 diabetes in the ageing HIV population, it is necessary to optimise the management of diabetes in this population. Antiretroviral therapy has been linked to increased risk of developing type 2 diabetes through insulin resistance, as well as β-cell dysfunction. Rilpivirine is a common antiretroviral drug used for the treatment of HIV, and is a potential candidate for HIV preexposure prophylaxis. However, rilpivirine has been shown to impair ß-cell function through increased oxidative stress and mitochondrial toxicity. The common anti-diabetic drug gliclazide, a second-generation sulphonylurea, has been shown to exhibit secondary anti-oxidant and cell protective properties. Therefore, this study investigates whether the damaging effects of rilpivirine in β-cells can be attenuated by gliclazide.
Materials and methods: The rat β-cell line INS-1E was used in this study. INS-1E cells were subject to a 24-hour exposure to clinical concentrations of gliclazide (0 or 1 μM) alone or in addition to concentrations of rilpivirine (10 or 20 μM) that were shown to impair β-cell function and viability, respectively. Then, glucose-stimulated insulin secretion (GSIS) was assessed by ELISA and cell viability was measured using an MTT assay. INS-1E cells were stained with the dichloro-dihydro-fluorescein diacetate (DCFH-DA) fluorogenic dye and then imaged by confocal microscopy to evaluate oxidative stress. To determine changes in mitochondrial membrane potential (ΔΨm), INS-1E cells were stained with the TMRE probe and then analysed by flow cytometry. Data is expressed as mean ± SEM and statistical analysis was carried out using one-way ANOVA and Bonferroni correction. P < 0.05 was considered as significant.
Results: A 24-hour exposure to gliclazide attenuated the damaging effects of rilpivirine on GSIS and cell viability in INS-1E cells. Gliclazide improved GSIS from 26.7 ± 5% (rilpivirine 10 μM) to 63.0 ± 3% (gliclazide 1 μM in combination with rilpivirine 10 μM) (p < 0.05 vs. rilpivirine alone treatments). Gliclazide 1 μM increased cell viability from 70.1 ± 2% (rilpivirine 20 μM alone) to 81 ± 1% (gliclazide 1 μM in combination with rilpivirine 20 μM) (p < 0.05 vs. rilpivirine alone treatments). Gliclazide (1 μM) also protected against rilpivirine-mediated INS-1E apoptosis. Furthermore, gliclazide reduced rilpivirine (10 μM)-mediated ROS generation from 2.82 ± 0.32 RFU (rilpivirine 10 μM alone) to 0.49 ± 0.13 RFU (gliclazide 1 μM in combination with rilpivirine 10 μM) (p < 0.05 vs. rilpivirine alone treatments). The rilpivirine (10 μM)-mediated increase in ΔΨm (133.6 ± 11%) was prevented by 1 μM gliclazide (95.2 ± 5%) (p < 0.05 vs. rilpivirine alone treatments).
Conclusion: Our results show that gliclazide protected against the damaging effects of rilpivirine in β-cells, likely via the reduction of oxidative stress and restoration of mitochondrial function. From a clinical perspective, gliclazide may be used as anti-diabetic of choice in the management of type 2 diabetes in the ageing HIV population and individuals on HIV preexposure prophylaxis.
Disclosure: S.C. Maandi: None.
395
Alpha4 contributes to the metabolic dysfunction of the pancreatic beta cell under metabolic stress
A. Kowluru, M. Hali;
Wayne State University, Detroit, USA.
Background and aims: Extant data in several cell types have implicated alpha4 (also referred to as IGBP1) in the biogenesis, stability and activation of protein phosphatases (e.g., PP2A). Mechanistically, alpha4 binds to the catalytic subunit of PP2A (PP2Ac), thus affecting its substrate specificity. Earlier studies have also demonstrated that deletion of alpha4 leads to decreased stability of PP4c, PP6c and PP2Ac to promote loss of their corresponding phosphatase activities. Interestingly, increased expression of alpha4 has been reported in human diseases, including lung and breast cancers. Recent studies from our laboratory have identified alpha4 as one of the interacting partners of PP2Ac, and suggested that deletion of alpha4 results in suppression of high glucose (metabolic stress) induced hyperactivation of PP2A in pancreatic beta cells. The current study is undertaken to decipher the roles of alpha4 in the regulation of acute (insulin secretion) and chronic effects (cell dysfunction) of glucose in pancreatic beta cells.
Materials and methods: Rat islets were isolated by the collagenase digestion method. Human islets were from Prodo Laboratories. INS-1 832/13 cells were cultured in the presence of low (2.5 mM; LG) or high (20 mM; HG) glucose for 24 hrs. Nuclear/non-nuclear fractions and membrane/cytosolic fractions were isolated from LG and HG exposed INS-1 832/13 cells using commercially available NE-PER Nuclear and Cytoplasmic Extraction Kit and Mem-PER Plus Kit (both from Thermo Scientific), respectively. Cell death was quantified using Cell Death Detection ELISA kit from Roche. Expression of proteins involved in the alpha4 signaling axis and phosphorylation status of various stress kinases were quantified by western blotting and densitometry.
Results: Alpha4 is expressed in human islets, rat islets and INS-1 832/13 cells. Incubation of INS-1 832/13 cells with HG significantly increased the expression of alpha4 (~1.7 fold). C2-Ceramide, a biologically active sphingolipid (50 μM; 24 hrs.) also increased the expression of alpha4 (~1.8 fold). Subcellular distribution studies of alpha4 in LG and HG exposed INS-1 832/13 cells revealed that it is predominantly soluble, and its expression is significantly increased in the non-nuclear (~2.3 fold)/cytosolic (~1.45 fold) fractions in cells exposed to metabolic stress. siRNA-mediated knockdown of alpha4 (~ 80 % knockdown) exerted minimal effects on glucose- or KCl-induced insulin secretion. Deletion of alpha4 significantly increased p38MAPK and JNK1/2 phosphorylation under LG conditions, comparable to the degree seen under HG conditions. Paradoxically, a significant potentiation of HG-induced p38MAPK and JNK2 phosphorylation was noted following alpha4 deletion. HG-induced CHOP expression (ER stress marker) and Caspase-3 activation were significantly attenuated in cells following alpha4 knockdown. Lastly, depletion of endogenous alpha4 significantly reduced HG-induced cell death in INS-1 832/13 cells.
Conclusion: Based on these findings we conclude that alpha4 contributes to HG-induced metabolic dysfunction of the islet beta cell. Our data also indicate that it plays critical roles in maintaining cell function under basal conditions since suppression of its expression promotes stress kinase activation. Additional studies are needed to further decipher roles of alpha4 and the identity of its target proteins (e.g., PP2A) in the cascade of events leading to beta cell dysregulation and demise under metabolic stress.
Supported by: Department of VA and NIH
Disclosure: A. Kowluru: None.
396
Glutamate dehydrogenase activation elicits biphasic insulin secretion and implicates a metabolic pathway with proline and glutamate in beta cell stimulus-secretion coupling
S. Gheibi;
Clinical Research Center, Lund University, Malmö, Sweden.
Background and aims: Insulin is secreted by β-cells in a biphasic manner. The rapid first phase is thought to be provoked by an influx of Ca2+ termed the triggering pathway. For insulin release to be sustained, further metabolic events in β-cells account for the amplifying pathway. Here, several pathways and metabolites have been proposed to be involved in metabolic coupling. In view of this, glutamate and glutamate dehydrogenase (GDH) have been implicated in control of insulin secretion. Therefore, we asked which metabolic changes in β-cells are unique upon stimulation with glucose or activation of GDH.
Materials and methods: Insulin secretion was assessed in clonal INS-1 832/13 cells stimulated with 16.7 mM glucose or 20 mM 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), a non-metabolizable analog of leucine, which allosterically activates GDH. In addition, plasma membrane potential and cytosolic Ca2+ levels were measured by the dyes PMPI and Fluo4, respectively. Mitochondrial membrane potential was measured by the dye TMRM, and ATP/ADP ratio determined by the probe Perceval HR. Mitochondrial oxygen consumption rate (OCR) was determined with the Seahorse Extracellular Flux Analyzer XF24. Metabolic signatures were determined by gas chromatography/mass spectrometry.
Results: Similar to glucose, BCH provoked a robust biphasic insulin response in INS-1 832/13 cells, which was associated with elevated cytosolic Ca2+-levels and ATP/ADP ratio, plasma membrane depolarization, mitochondrial OCR, and hyperpolarization of the inner mitochondrial membrane. Metabolomics analyses revealed profound stimuli-dependent differences in metabolite profiles; stimulation with BCH increased levels of α-ketoglutarate while those of glutamate decreased. Glucose increased levels of glutamate and α-ketoglurate as well as those of fumarate, malate, citrate and pyruvate. Interestingly, BCH enhanced formation of proline. Inhibition of this process by silencing proline 5-carboxylate (P5C) synthase, abrogated formation of proline and perturbed insulin secretion in response to BCH.
Conclusion: Activation of GDH provokes a robust biphasic insulin response. Stimulation of insulin secretion by GDH is associated with proline formation. This suggests that P5C synthase controls a metabolic pathway in β-cells that is important for stimulus-secretion coupling.
Supported by: Fysiografen
Disclosure: S. Gheibi: None.
397
The role of luminal H2O2 in ER Ca2+ dysregulation and toxicity of palmitate in insulin-secreting INS-1E cells
I. Mehmeti, S. Sharifi, M. Böger, S. Lortz;
Hannover Medical School, Hannover, Germany.
Background and aims: The endoplasmic reticulum (ER) lumen is not only the major site for assembling and folding newly synthesized proteins but also the main intracellular Ca2+ store. Ca2+ ions are involved in versatile biochemical processes, including posttranslational processing and protein folding. Disruption of ER Ca2+ homeostasis is usually accompanied by an ER stress response that can ultimately lead to apoptosis if unresolved. Abnormal ER Ca2+ depletion has been linked to pancreatic β-cell dysfunction and death under lipotoxic conditions. However, the underlying mechanism of how β-cell toxic saturated free fatty acids palmitate (PA) perturbs the ER Ca2+ homeostasis and its interplay with other organelles is not fully understood. Previously, we showed that an accumulation of H2O2 in the ER is associated with PA-mediated ER stress induction. Therefore, the aim of the present study was to investigate the role of luminal H2O2 and the ER-resident H2O2-inactivating glutathione peroxidase-8 (GPx8) in the regulation of Ca2+ dynamics in insulin-secreting INS-1E cells under lipotoxic conditions. In addition, the importance of GPx8 in maintaining mitochondrial function, insulin content, and oxidative protein folding under prolonged PA exposure was investigated.
Materials and methods: Live cell imaging of Ca2+ dynamics was performed using the Ca2+ indicator Fura-2/AM and ER or mitochondrial-targeted FRET-based Ca2+ biosensor cameleon. To determine the Ca2+-ATPase activity, an ER-Ca2+store refilling assay was performed. The mitochondrial membrane potential was determined using the JC-1 dye (flow cytometry) and the ATP content by a luciferase-based assay before and after treatment with PA (0.5 mM and 1.0 mM). Insulin content was assessed by RIA and oxidative protein folding by using ER-roGFP2 probe upon a reductive pulse with DTT and subsequent washout.
Results: Treatment of INS-1E control cells with PA resulted in a massive decrease in ER luminal and elevated cytosolic/mitochondrial Ca2+ content. Sustained abnormal ER-mitochondria Ca2+ fluxes were accompanied by a severe depolarization of mitochondrial membrane potential (0.5 mM PA: 178%; 1.0 mM PA: 211%) and significant reduction of ATP content (0.5 mM PA: 45%; 1.0 mM PA: 47%) compared to untreated cells. In addition, INS-1E control cells pre-incubated with PA revealed a significantly retarded ER Ca2+ reuptake rate (t1/2 0.3 ± 0.002 min (untreated) vs. 0.6 ± 0.1 min after 24 h PA incubation). Importantly, detoxification of luminal H2O2 by GPx8 expression abrogated the lipotoxic effects of PA. Moreover, GPx8 expressing INS-1E cells successfully maintained ER Ca2+ levels, revealed a significantly better ER Ca2+ reuptake rate in response to PA (t1/2 0.4 ± 0.02 min after 24 h PA exposure), and supported oxidative protein folding and preserved insulin content under lipotoxic conditions.
Conclusion: These data point out the importance of luminal H2O2 in PA-induced dysregulation of ER Ca2+ homeostasis and its interplay with mitochondrial function in insulin-secreting β-cells. Depletion of ER Ca2+ stores appears to be achieved through redox-dependent inactivation of Ca2+-ATPase upstream of mitochondrial dysfunction. Elimination of H2O2 in the ER can thus be a promising strategy to mitigate beta-cell dysfunction and destruction in the pre-diabetic phase of T2D.
Supported by: This research has received support from the Deutsche Forschungsgemeinschaft (German Research Foundat
Disclosure: I. Mehmeti: None.
398
Vimentin as a regulator of cell survival and endoplasmic reticulum stress in pancreatic alpha and beta cells
L. Marroqui1,2, A. Pérez-Serna1,2, R.M. Medina-Gali1,2, R.S. dos Santos1,2;
1Universidad Miguel Hernandez, Elche (Alicante), 2CIBERDEM, Madrid, Spain.
Background and aims: Vimentin, an intermediate filament protein, is considered a marker of mesenchymal cells. In pancreas, vimentin expression has been detected in epithelial ductal cells as well as in α- and β-cells. Recent studies suggested that vimentin-expressing endocrine cells might be undergoing dedifferentiation, which could contribute to islet cell dysfunction and/or the onset of diabetes. Thus, our aim was to investigate whether vimentin could also be involved in α- and β-cell survival.
Materials and methods: Small interfering RNAs (inhibition >80%) were used to inhibit vimentin expression, while a plasmid encoding human vimentin was used to overexpress vimentin in mouse α-cells (αTC1-9) and human β-cells (EndoC-βH1). Cell viability was evaluated by Hoechst/Propidium iodide staining. mRNA and protein expression were measured by quantitative RT-PCR and western blot, respectively. A combination of interleukin-1β plus interferon-γ (“cytokines”) was used to model the proinflammatory milieu in diabetes.
Results: In FACS-purified rat cells, vimentin expression in α-cells was 285-fold higher than in β-cells (n=7; p<0.001). In αTC1-9 cells, vimentin silencing induced a 2-fold increase in basal apoptosis (n=3-8, p<0.01), but did not sensitize cells to cytokine-induced apoptosis. Vimentin silencing also increased expression levels of the ER stress markers CHOP and BiP under basal condition (1.5-2.5-fold change; n=4-6, p<0.01), while no changes were observed upon cytokine treatment. In a mirror experiment, overexpression of vimentin in αTC1-9 cells slightly decreased cytokine-induced apoptosis and prevented cytokine-induced expression of CHOP and BiP (n=3, p<0.05). In EndoC-βH1 β-cells, vimentin overexpression also prevented cytokine-induced apoptosis.
Conclusion: These findings suggest that, besides its putative role in dedifferentiation, vimentin may play an important role in α- and β-cell survival at basal condition and against proinflammatory assaults.
Supported by: Generalitat Valenciana (SEJI/2018/023) and Agencia Estatal de Investigación (PID2020-117569RA-I00)
Disclosure: L. Marroqui: None.
399
The IGFBP3/TMEM219 pathway regulates homeostasis of pancreatic endocrine progenitors
E. Assi, F. D'addio, A. Maestroni, V. Usuelli, M. Ben Nasr, C. Loretelli, A. Abdelsalam, A. Seelam, L. Loreggian, A. Petrazzuolo, G. Zuccotti, P. Fiorina;
Biomedical and Clinical Sciences, University of Milan, Milan, Italy.
Background and aims: Reduction in beta cell mass, mainly due to increased beta cell apoptosis and/or a failure to replenish beta cells, is a common feature of both type 1 (T1D) and type 2 (T2D) diabetes. We and other have demonstrated that targeting beta cell death is key mechanism to preserve beta cells and prevent or delay diabetes onset. Recently, the IGFBP3/TMEM219 axis has been shown to control beta cell homeostasis. Here, we will demonstrate whether TMEM219 is expressed also in beta cell endocrine progenitors thus representing a marker for beta cell in their early stage of maturation. We will also prove that rescuing TMEM219-expressing endocrine progenitors is associated with a preservation of beta cells.
Materials and methods: TMEM219 expression was evaluated by flow cytometry, immunoassay and immunofluorescence in undifferentiated human embryonic stem cells (h-ESCs), pancreatic progenitors, human islets and fetal pancreata. Levels of ALDH and PDX1 were assessed by qRT-PCR. Then, ALDH, PDX1, caspase 8 and HNF6 expression was evaluated by qPCR in endocrine progenitors as well as human islets after 72h treatment with IGFBP3 alone or in combination with the recombinant protein, ecto-TMEM219 (the extracellular domain of TMEM219 receptor). All experiments were run in triplicate.
Results: Using targeted immunoassay and flow cytometry we first discover TMEM219 protein expression in endocrine progenitors obtained from in vitro-differentiated h-ESCs. In particular, almost 50% (p<0.001) of endocrine progenitors were positive for TMEM219 expression. Of note, TMEM219 was found to be highly expressed in human fetal pancreata (5-months-old) by immunostaining and therefore may be involved in fetal remodeling of the pancreas. Moreover, addition of IGFBP3 to TMEM219-expressing h-ESC-derived endocrine progenitors in vitro induced a 2-fold (p<0.05) increase of Caspase 8 expression (2-fold) and reduce the mRNA of the progenitor markers HNF6 and PDX1. Expression of the three genes was next restored to normal in the presence of ecto-TMEM219 suggesting that IGFBP3/TMEM219 blockade in vitro acts to preserve beta cells at their early stages of maturation. Interestingly, we showed that expression of ALDH and PDX1 is still retain in TMEM219-positive cells of human mature islets suggesting the existence, even in the adult pancreas, of cells with endocrine progeniture features and the potential of renewing the beta cell mass. Here again, in the presence of IGFBP3 we observed an almost 50% (p<0.001) reduction of ALDH and PDX1 mRNA expression which was re-established to normal by ecto-TMEM219 addition (p<0.01), ultimately confirming that IGFBP3/TMEM219 signaling may also exert an effect on early-stage beta cells.
Conclusion: Our data demonstrate that endocrine progenitors as well as beta cells in their early stages of maturation express TMEM219, which is then retained by islet beta cells, and that these cells similarly suffer from IGFBP3-mediated Caspase 8 damage. The discovery of an axis that controls beta cells in their early stage of maturation may be relevant in understanding pathogenesis of diabetes and in designing therapeutic aimed at preserving the residual beta cells.
Disclosure: E. Assi: None.
400
Mining the transcriptome of target tissues of autoimmune and degenerative pancreatic beta cell and brain diseases to identify novel therapies
X. Yi1, B.M. De Souza1, T. Sawatani1, F. Szymczak1, L. Marselli2, P. Marchetti2, M. Cnop1,3, D.L. Eizirik1,4;
1Faculty of Medicine, ULB (Université Libre De Bruxelles) Center for Diabetes Research, Brussels, Belgium, 2Department of Clinical and Experimental Medicine, and AOUP Cisanello University Hospital, PISA, Italy, 3Division of Endocrinology, Erasmus hospital, Brussels, Belgium, 4WELBIO, Université Libre De Bruxelles, Brussels, Belgium.
Background and aims: Target tissues of autoimmune and degenerative diseases show signals of inflammation. Here we used publicly available RNA-seq data of pancreatic β-cells in type 1 (T1D) and type 2 diabetes (T2D) and neuronal tissue in multiple sclerosis (MS) and Alzheimer’s disease (AD) to identify shared inflammatory gene signatures and then mined these signatures to search for new therapies to revert them (“perturbagens”) and protect these target tissues.
Materials and methods: The target tissue transcriptomic data (cases vs controls for T1D: 4 vs 12; T2D: 28 vs 183; MS: 5 vs 5; AD: 122 vs 80) were re-analyzed in our pipeline, using GENCODE V36 (GRCh38.p13) as reference, Salmon (V1.4.0) for transcript quantification and DESeq 2 (V1.28.1) for differential expression. Rank-rank hypergeometric overlap (RRHO) analysis was applied to search for similar gene expression signatures in diseases pairs. The top 150 commonly up- or downregulated genes were submitted to Connectivity Map libraries containing transcriptomes of human cells exposed to chemical perturbagens, in order to reposition drugs. Drugs were tested on human islets (at least 5 preparations) exposed for 48 h to 50 U/ml interleukin-1β + 1000 U/ml interferon-γ to mimic inflammation in T1D and 0.5 mmol/l palmitate to mimic metabolic stress in T2D.
Results: Concordantly upregulated genes in pairs of diseases were related to inflammation (e.g., signaling by interleukins and interferons, cytokine-cytokine receptor interaction, chemokine signaling), particularly when comparing T1D vs AD. We identified bromodomain inhibitors (the most consistently ranked chemical perturbagen with |median tau scores| >90) as perturbagens that potentially revert the transcriptional signatures among the 4 diseases. The bromodomain inhibitors I-BET151 and GSK046 reduced the pro-inflammatory effects of cytokines in human islets (reduced CXCL10expression by ~30-60%, IL-6 by ~40% and IL-8 by ~40%; P<0.05) and lowered expression of the endoplasmic reticulum stress marker CHOP (by ~30-40%; P<0.05), but they did not prevent apoptosis. In palmitate-exposed islets the beneficial effects of the bromodomain inhibitors were less marked, with only a significant decrease of CXCL1mRNA expression (~40%; P<0.005).
Conclusion: Key inflammation-induced molecular mechanisms are shared between β-cells and brain in autoimmune and degenerative diseases. We identify bromodomain inhibitors as agents that may be repositioned to decrease islet inflammation.
Supported by: Welbio-FNRS(WELBIO-CR-2019C-04); INNODIA (115797); INNODIA HARVEST (945268)
Disclosure: X. Yi: None.
401
Sorla is an endocytic receptor for pro-islet amyloid polypeptide and is protective against islet amyloid formation
A.Z.L. Shih1,2, Y.C. Chen3,4, C.B. Verchere3,4, T.E. Willnow1,2;
1Max-Delbrück-Center for Molecular Medicine, Berlin, Germany, 2Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 3BC Children's Hospital Research Institute and Centre for Molecular Medicine and Therapeutics, Vancouver, Canada, 4Department of Pathology & Laboratory Medicine, University of British Columbia, Vancouver, Canada.
Background and aims: The neuronal sorting receptor SORLA has an established function in reducing amyloid burden in the brain by preventing accumulation of amyloid-beta peptides, the main amyloidogenic agent of amyloid plaques in Alzheimer disease. Recent single-cell transcriptomic analyses show that SORLA transcripts are expressed in pancreatic islet beta cells. However, the function of SORLA in islets have not been studied previously. We hypothesized that SORLA is also involved in amyloid formation in the pancreas through regulating islet amyloid polypeptide (IAPP).
Materials and methods: We studied the effects of SORLA deficiency on islet histopathology, beta cell function and glucose homeostasis in human IAPP transgenic mice. We tested binding interactions between SORLA ectodomain with pro- and mature mouse IAPP peptides using microscale thermophoresis. Using established cell lines and primary islet cells, we further examine the mechanisms in which SORLA participates in the accumulation of IAPP.
Results: SORLA deficiency in human IAPP transgenic mice significantly increased the amount of islet amyloid deposits compared to wildtype controls (WT 0.56±0.16% vs KO 6.9±1.8% Thioflavin S positive islet area, n=9). This was accompanied with increased islet cell death (WT 1.4±0.6% vs KO 3.3±1.4% TUNEL positive cells, n=9). However, overall islet area, proportions of alpha and beta cells per islet remained comparable between SORLA genotypes. Interestingly, SORLA ectodomain showed stronger binding affinities to the two pro-forms of IAPP (pro-IAPP1-70 Kd ~ 268±43 nM, pro-IAPP1-51 Kd ~ 329±29 nM) as compared to mature IAPP (Kd ~ 921±177 nM). Additional studies on SORLA-overexpressing cell line demonstrated that SORLA mediated endocytosis of proIAPP peptides, but not mature IAPP, towards the endolysosomal pathway.
Conclusion: These findings demonstrated a protective role of SORLA against islet amyloid deposition, likely through clearance of extracellular proIAPP secreted by beta cells.
Supported by: ERC, Novo Nordisk Foundation, CIHR, JDRF
Disclosure: A.Z.L. Shih: None.
SO 19 How complicated is type 1 diabetes?
402
Trace positive GAD65 antibodies in adult onset type 1 diabetes re-associated a wide variety of metabolic phenotypes
K. Wyne;
The Ohio State University, Columbus, USA.
Background and aims: Adult onset Type 1 Diabetes Mellitus (T1D) is frequently misdiagnosed as type 2 diabetes mellitus (T2DM). Common reasons for the misdiagnosis include that the person was considered "too old" to have T1D or had a detectable c-peptide. We also have patients who were told that they have "antibody negative T1D" because their GAD65 was the only detectable antibody and it was not strongly positive. The T1D Clinic at our State University now routinely measures all 5 antibodies (GAD65, ICA (islet cell antibodies), IAA (insulin autoantibody), IA-2 (insulinoma-associated antigen-2) and ZnT8 (Zinc transporter 8)) in all adults either newly diagnosed with T1D, with newly diagnosed diabetes in which there is any suspicion for T1D, or characteristics that are not typical for T2DM, with a goal of determining therapeutic options for each individual patient.
Materials and methods: Charts were reviewed of all adults age 18 years or above who were newly diagnosed with T1D between July 2016 to July 2019 at our State Medical Center. The majority were identified when they presented with diabetic ketoacidosis (DKA). Adults who were originally thought to have T2DM and were negative for all 5 antibodies were not included in the analysis. Noninsulin injectables and oral glucose management agents were not used in these patients at the time of evaluation. 43 individuals (15%; age range 18.0-74 years) for whom the GAD65 level was trace positive were identified. Trace positive was defined as 0.03-2.0 with reference range of <0.02 nmol/L. GAD65 was measured by Mayo Clinic Department of Lab Med & Pathology. ICA, IAA, IA-2 and ZnT8 were measured by Quest Diagnostics Nichols Institute.
Results: The overall prevalence of antibody positivity in the adults age 18.0 years and above was: GAD65 81%, ICA 17%, IA-2 52%, and ZnT8 51%. IAA is evaluated separately as many patients are on insulin before having their antibodies drawn. 15% were found to have a trace positive GAD65 and in all cases it was their only antibody, except for 6 of them who had been on insulin therapy and had an IAA present. BMI range was 20-48 kg/m2. Metabolic evaluation of these patients found a wide range of insulin dosing from 0.2-2.0 IU/kg/day along with some who only required 10-20% of total daily dose as basal insulin.
Conclusion: Individuals with trace positive GAD65 should be treated with insulin, at least as the foundation of their therapy, to prevent DKA. While the presence of the GAD65 antibody defines the T1D, these low levels of antibody are an indicator that a traditional approach to managing T1D will probably not attain/maintain target A1c nor will it optimize metabolic control. Once a trace positive GAD65 is identified, management needs to be personalized and often requires unexpectedly high basal dosing.
Disclosure: K. Wyne: None.
403
Glycaemic parameters measured by CGM alter hypoglycaemia-induced inflammatory responses in people with type 1 diabetes
M. Ajie1, C. Verhulst1, J. van Heck1, T.W. Fabricius2, U. Pedersen-Bjergaard2,3, C. Tack1, R. Stienstra1,4, B. de Galan1,5;
1Department of Internal Medicine, Radboudumc, Nijmegen, Netherlands, 2Department of Endocrinology and Nephrology, Nordsjællands Hospital, Hillerød, Denmark, 3Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Hillerød, Denmark, 4Department of Nutrition, Metabolism and Genomics Group, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands, 5Department of Internal Medicine, Maastricht University Medical Centre, Maastricht, Netherlands.
Background and aims: Hypoglycaemia causes activation of the immune system and is linked to an increased risk for cardiometabolic disease. We set out to determine how glycaemic parameters based on continuous glucose monitoring (CGM) affect the immune response to a hypoglycaemic event.
Materials and methods: 38 individuals with type 1 diabetes (M:F ratio 21:17, age 51±28 years, BMI 26±6 kg/m2, diabetes duration 22±25 years, HbA1c 8.0±1.3%) were recruited. Participants underwent a hyperinsulinaemic-normoglycaemic (5.2±0.1 mmol/L) hypoglycaemic (2.7±0.0 mmol/L) glucose clamp. Venous blood was drawn during the normoglycaemic phase, during the hypoglycaemic phase, and one day after the clamp to measure circulating immune cell composition and inflammatory markers. CGM data from 6 days prior to the clamp experiment were used to assess the relation between glucose variance (CV), time above range (TAR), and time below range (TBR) with immune responses to experimental hypoglycaemia.
Results: At baseline, glucose variability (CV) was positively associated with circulating granulocyte counts (p < 0.05). Participants with TBR above median had lower circulating immune cell counts as compared with those with low TBR (p < 0.05). Hypoglycaemia induced by the clamp caused inflammatory responses in all participants, as illustrated by increased levels of circulating monocytes (p < 0.01), lymphocytes (p < 0.01), and inflammatory proteins like CRP (all p < 0.05), which were sustained until one day after the clamp. Post clamp, CV was associated with increased inflammatory responses to hypoglycaemia, as reflected by the levels of circulating inflammatory proteins CD5 (p < 0.01), TNFRSF9 (p < 0.05), and IL-17C (p < 0.05). TAR was positively correlated with increased responses of circulating granulocytes (p < 0.01) and lymphocytes (p < 0.05). TBR was negatively associated with the increase in circulating granulocytes (p < 0.05), lymphocytes (p < 0.05), and IL-6 (p < 0.01) in response to hypoglycaemia.
Conclusion: Glycaemic parameters based on CGM impact on the immune response to hypoglycaemia, with the TBR having an attenuating effect and the TAR a stimulating effect on hypoglycaemia-induced inflammation. Future studies are needed to identify drivers of these altered responses.
Clinical Trial Registration Number: NCT 03 97 62 71
Supported by: This work was funded by the Innovative Medicines Initiative 2 Joint Undertaking nr 777460
Disclosure: M. Ajie: None.
404
Evidence of atypical non-autoimmune diabetes amongst young people diagnosed with type 1 diabetes in Cameroon: results from young-onset diabetes in sub-Saharan Africa study
J.C. Katte1, E. Sobngwi2, M.Y. Dehayem2, S. Deshmukh1, K. Patel1, B. Shields1, A.T. Hattersley1, T.J. McDonald1, A.G. Jones1;
1Institute of Biomedical and Clinical Science, University of Exeter Medical School, Exeter, UK, 2Department of Internal Medicine and Specialities, Faculty of Medicine and Biomedical Sciences, Yaounde, Cameroon.
Background and aims: Previous research has suggested that type 1 diabetes in sub-Saharan Africa may have an atypical phenotype, with lower levels of islet autoantibodies. We aimed to determine whether clinically diagnosed type 1 diabetes in young people in Cameroon is of autoimmune aetiology.
Materials and methods: We assessed islet autoantibodies (GAD, IA-2, ZnT8), type 1 diabetes genetic susceptibility (genetic risk score (T1DGRS) and imputed HLA) and plasma C-peptide in 295 participants with a clinical diagnosis of type 1 diabetes, insulin treatment and diabetes onset before 30 years in Cameroon.
Results: The median age at diagnosis was 15 (IQR 12, 18) years, and median diabetes duration was 4.5 (1.4, 8.3) years. 71.0% of participants were negative for islet autoantibodies (<3 years diabetes duration 65.1%, n=106), with antibodies associated with other autoimmune diseases (TPO, TTG) rare (2.3%). Participants with islet autoantibody-negative diabetes had similar age of diabetes onset and BMI as islet autoantibody-positive diabetes participants; median age of diabetes onset 15 (IQR 13, 17) years vs 15 (IQR 11, 18), p=0.84 and median BMI SD z score -0.008(IQR -0.579, 0.561) vs -0.074 (IQR -0.857, 0.493), p=0.14. Participants with islet autoantibody-negative diabetes had substantially lower T1DGRS, lower prevalence of type 1 diabetes-associated HLA, and higher C-peptide levels compared to those with islet autoantibody-positive diabetes (Figure 1): T1DGRS 8.52 (6.70, 10.31) vs 11.61 (9.93, 12.83), p<0.001 (published median T1DGRS in black American type 2 diabetes 8.97 , HLA DR3 and/or DR4 28.5% (95%CI 22.2-35.7) vs 69.0% (95%CI 57.3-78.6), p<0.001 (reference population of Cameroonians without diabetes 23.2%), and C-peptide 124 (36, 284) vs 48 (3-166)pmol/L, p=0.0006. Severe insulin deficiency (C-peptide <200 pmol/L) occurred in 66.3% (95%CI 59.1-72.7) and 78.7% (95%CI 67.9-86.5) of islet autoantibody-negative diabetes and positive participants respectively. Iset autoantibody-negative diabetes was not associated with markers of potential malnutrition such as low socioeconomic status or reduced height.
Conclusion: The majority of young people diagnosed with type 1 diabetes in Cameroon do not have features suggestive of either autoimmune type 1 diabetes or type 2 diabetes. These findings suggest that an alternative non-autoimmune diabetes form must be considered in this population.
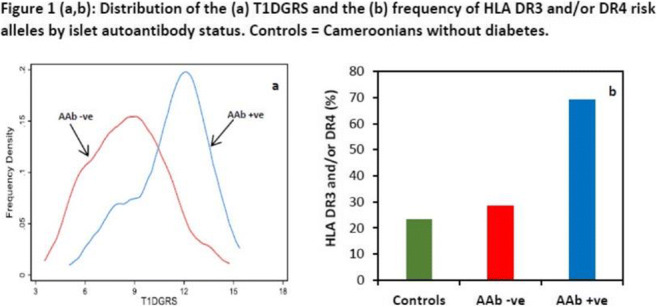
Supported by: NIHR
Disclosure: J.C. Katte: Grants; This research was funded by the NIHR (17/63/131) via the University of Exeter, UK.
405
Net proteome and bioenergetic profile of PMA- and ionomycin-stimulated neutrophils point to metabolic alterations in neutrophils of people with longstanding type 1 diabetes
S. Bissenova1, D. Ellis1, A. Callebaut1, R. Derua2, G. Eelen3, M. Buitinga4, C. Mathieu1, C. Gysemans1, L. Overbergh1;
1Clinical and Experimental Endocrinology, KU Leuven, Leuven, Belgium, 2Laboratory of Protein Phosphorylation and Proteomics, KU Leuven, Leuven, Belgium, 3Laboratory of Angiogenesis and Vascular Metabolism, VIB, Leuven, Belgium, 4Diabetes and Metabolism Research Group, Maastricht University, Maastricht, Netherlands.
Background and aims: Neutrophils can be detected in the pancreatic immune cell infiltrates of people with type 1 diabetes (T1D), but how they initiate and exacerbate the autoimmune destructive process remains to be clarified. Among the various neutrophil functions, the process of neutrophil extracellular trap formation (NETosis) has been implicated in the pathogenesis of several autoimmune diseases. Our aim was to study the NET proteome (NETome) and bioenergetics profile of T1D neutrophils.
Materials and methods: Peripheral blood neutrophils isolated from people with longstanding T1D (n=4; 14±7 years at T1D onset; 12±10 years disease duration; 133±37 mg/ml random glycaemia) and sex- and age-matched healthy controls (HC, n=3) were stimulated with phorbol-myristate acetate (PMA, 100 nM) or ionomycin (20 μM) for 3 hours. NETome was studied by LC-MS/MS analysis, while metabolic changes during NETosis were explored by a Seahorse extracellular flux analyzer.
Results: Levels of PMA- and ionomycin-stimulated NETosis were comparable in HC and T1D neutrophils (PMA: 85% vs 90%; ionomycin: 63% vs 77% respectively), as well as plasma levels of NET markers. In the PMA-stimulated NETs, a total of 370 proteins were identified in both subjects, with 70 and 22 unique to HC and T1D subjects, respectively. Upon ionomycin stimulation, we identified 321 proteins, 53 and 21 of which were unique to HC and T1D subjects, respectively. In both stimulated conditions, the NET proteins of HC and T1D subjects were found to be involved in identical biological processes and similar molecular functions. However, upon quantification with Progenesis QI software, a total of 44 proteins were differentially expressed in the NETomes of HC and T1D subjects when stimulated with PMA. Ionomycin-induced NETomes contained 27 differentially expressed proteins (1% FDR, P<0.05). Reactome analysis revealed that the proteins enriched in HC NETomes in PMA- and ionomycin- stimulated conditions were involved in neutrophil degranulation and innate immunity. In both stimulated conditions, proteins enriched in T1D NETomes were involved in glucose metabolism, such as glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, fructose-bisphosphate aldolase A, and UTP-glucose-1-phosphate uridylyltransferase. Supernatants from unstimulated and PMA- or ionomycin-stimulated T1D neutrophils contained higher levels of lactate, a byproduct of glycolysis, than HC subjects. PMA induced a similar increase in mitochondrial respiration (reported as oxygen consumption rate, OCR) in both HC and T1D neutrophils, which demonstrated little responsiveness to ionomycin stimulation. On the other hand, both PMA and especially ionomycin induced an increase in the extracellular acidification rate (ECAR), an approximate measure for lactate production by anaerobic glycolysis, with higher levels in T1D compared to HC neutrophils.
Conclusion: Together these data suggest possible alterations in the metabolic profile of T1D neutrophils, which can provide vital insight into their role in the pathophysiology of the disease.
Supported by: This work was supported by IMI-JU INNODIA & INNODIA HARVEST
Disclosure: S. Bissenova: None.
406
The ELSA Study: UK paediatric general population screening for type 1 diabetes: "sooner we screen, sooner we can intervene"
L.M. Quinn1, D. Shukla2, S.M. Greenfield2, T. Barrett3, P. Narendran1,4;
1Institute of Immunology and Immunotherapy, University of Birmingham, 2Institute of Applied Health Research, University of Birmingham, 3Institute of Cancer and Genomic Sciences, University of Birmingham, 4Department of Diabetes, University Hospitals of Birmingham, Birmingham, UK.
Background and aims: Multiple autoantibody testing identifies children with presymptomatic type 1 diabetes and is able to stratify those at risk over a 10-15-year period. Identifying children at risk reduces the rates of diabetic ketoacidosis at presentation and allows them to participate in clinical trials for type 1 diabetes prevention. Type 1 diabetes general population testing programmes have been trialled in the US and Germany and found a presymptomatic rate of 0.3%, achieved a reduction in diabetic ketoacidosis rates from 20% to 5%, and were considered acceptable to families. Here we outline our plans for developing a UK based system for testing for pre-type 1 diabetes in the general population.
Materials and methods: To assess acceptability, we will undertake qualitative interviews with families and stakeholders, to understand perspectives on screening for type 1 diabetes and to discuss the barriers and solutions to implementation of a UK screening programme for autoantibody testing. Qualitative interviews will be thematically analysed and we will recruit until thematic saturation is met. To assess feasibility of a UK general population screening programme for type 1 diabetes, we will recruit 20,000 children aged 3-13 years for autoantibody testing. This will initially be performed using dried blood spot testing, and will be undertaken in community settings including home-testing, schools, and general practices. Children with positive dried blood spot samples will be recalled for a confirmatory serum sample. Stage of type 1 diabetes will be determined using an oral glucose tolerance test. Families of autoantibody positive children will be offered an education session to explain the symptoms of type 1 diabetes and what their child’s risk means. Autoantibody positive children will then be offered entry into a monitoring programme for earlier identification of overt type 1 diabetes, and to facilitate entry into trials for type 1 diabetes prevention. Following completion of the screening programme, we will hold interviews with families and stakeholders to understand their experiences and feedback from the screening study.
Results: For feasibility, we will report on recruitment, relative uptake by demographics and by recruitment modality, frequencies of autoantibody positive and negative children, sensitivity and specificity of the dried blood spots, and distress experienced from a positive screening result. For acceptability, we will report on emerging themes from the qualitative interviews. Long-term follow-up will reveal type 1 diabetes incident cases and episodes of diabetic ketoacidosis following the screening study.
Conclusion: The outcomes of the ELSA study will determine feasibility and acceptability of autoantibody testing for type 1 diabetes in the UK. The qualitative interviews will provide insights from families and stakeholders to optimise the processes for autoantibody testing, and the screening study will identify at-risk individuals who could benefit from preventative immunotherapy trials. The ELSA study supports progress towards establishment of UK national screening for type 1 diabetes.
Supported by: The ELSA study is funded by NIHR, DUK and JDRF and alumni of The Birmingham General Hospital, UK.
Disclosure: L.M. Quinn: None.
407
Phase I study investigating PK and PD of highly concentrated insulin aspart AT278 U500
E. Svehlikova1, V. Hoeller1, M. Urschitz1, M. Wolf1, B. Lackner2, C. Magnes2, M. Ratzer2, D. Gerring3, J. Jezek3, S.J. Howell3, L. Zakrzewski3, V. Pillai3, N. Ashcroft3, F. Lawrence3, T.R. Pieber1,2;
1Medical University of Graz, Graz, Austria, 2HEALTH - Institute of Biomedicine and Health Sciences, Graz, Austria, 3Arecor Limited, Cambridge, UK.
Background and aims: Concentrated insulins allow administration of high insulin doses in a smaller volume. This enables a reduction in the number of injections for people with diabetes with high insulin needs and supports miniaturisation of insulin delivery devices. The aim of the study was to compare the PK/PD profile of a novel formulation of insulin aspart (AT278 U500/mL) with that of standard insulin aspart (IAsp U100/mL).
Materials and methods: Serum insulin aspart and plasma glucose concentrations were measured in 38 adult male subjects with type 1 diabetes following a single s.c. dose (0.3 U/kg) of AT278 and Iasp in a randomised, double-blind, crossover, euglycaemic clamp study.
Results: AT278 showed a faster onset of insulin exposure compared with IAsp, as demonstrated by an earlier onset of appearance (-6.0 min, P<0.0001), earlier tEarly50%Cmax (-23.0 min, P<0.0001), and 4.0 times higher insulin exposure within the first 30 min after dosing (AUCInsulin,0-30min; 95% CI: 3.29; 4.90). AT278 showed a more rapid onset of glucose-lowering effect compared with IAsp, as demonstrated by an earlier onset of action (-9.5 min, P<0.0001) and earlier tEarly50%GIRmax (-20.0 min, P<0.0001) and 8.9 times higher insulin action within the first 30 min after dosing (AUCGIR,0-30min; 95% CI: 5.96;17.46). Overall insulin exposure and action were comparable between both insulins (AUCInsulin,0-8h treatment ratio 0.98 [95% CI: 0.92; 1.00]; AUCGIR,0-8h treatment ratio 1.02 [95% CI: 0.95; 1.09]).
Conclusion: AT278, the novel U500 insulin aspart, maintains rapid-acting characteristics in a reduced dose volume. AT278 has the potential to enable dosing at the start of a meal, to reduce injection burden and thus to improve blood glucose management and convenience for people on high-dose insulin therapy.
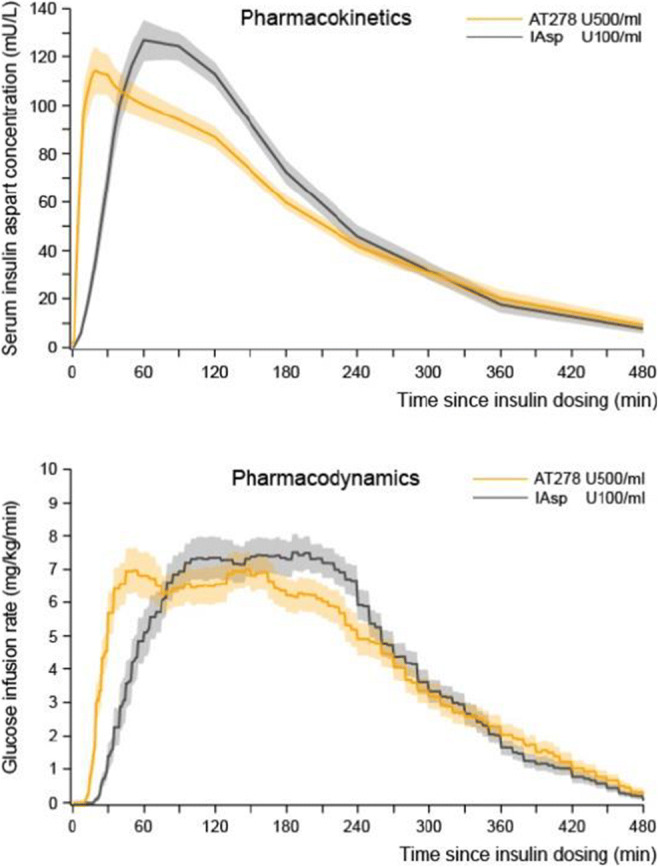
Clinical Trial Registration Number: NCT04660305
Disclosure: E. Svehlikova: None.
408
Relationships between cytokine storm and stress hyperglycaemia in hospitalised patients with COVID-19
A. Da Porto1, G. Colussi1, C. Tascini2, M. Fabris3, E. Sozio2, M. Peghin2, F. Curcio3, C. Catena1, L. Sechi1;
1Internal Medicine, Clinica Medica, University of Udine, 2Medicine, Infectious Disease, University of Udine, 3Medicine, Laboratory Medicine, University of Udine, Udine, Italy.
Background and aims: An unusual high prevalence of stress hyperglicemia (SH) has been reported in hospitalized patients with COVID-19. Cytokine storm has been supposed to have a pivotal role in worsening insulin resistance, damaging beta-cells thus causing stress hyperglycemia. Further SH is associated with worse outcomes. With this study we aimed to evaluate the existing relationships between main actors of the “cytokine storm” and SH in 150 hospitalized patients with Covid-19.
Materials and methods: This is a cross- sectional analysis of GIRA-COVID database that was a prospective, observational study conducted in 2021 at the University of Udine. For assessment of SH, we calculate Stress Hyperglycemia ratio (SHR) defined by the ratio of admission glucose top the estimated average glucose derived from glycated hemoglobin. Patients were considered to have SH when SHR were >1.14. Values of normally distributed variables are expressed as mean ± standard deviation or median and interquartile range. Normality of distribution was assessed by the Kolmogorov-Smirnov test. Skewed variables were analyzed in the regression model after logarithmic conversion. Pearson’s chi-squared test was used to compare frequency distributions. Association between stress hyperglycemia and outcomes were evaluated by multivariate logistic regression model. Multivariate logistic regression analysis was performed to identify what cytokines were independent predictors of stress hypeglycemia.
Results: Main clinical and laboratory data are summarized in table 1. As expected patients with SH had an increased risk of mechanical ventilation and dead (OR 2.453 IC 1.078-6.010 and OR 2,781 IC 1.049 7.369) independently from the presence of diabetes and after correction for age, sex, BMI and severity of disease. Univariate analysis showed that SH were directly associated with CXCL10 (beta = 0.486; P < 0.001), IL-10 (beta = 0.409; P 0.000), IL10/TNF alfa (beta 0.330 P 0.012). In multivariate logistic regression model log CXCL10 (OR 14.5 IC 2.5-77) log IL-10 (OR 6.7 IC 1.9-24) and IL10/TNF alfa (OR 1,5 IC 1.1-2.2) resulted independent predictors of stress hyperglycemia after correction for Age, Sex, Diabetes status, CPR and Severity of disease.
Conclusion: In our study SH was associated with poor outcomes independently from diabetes. Among Cytokines, CXCL10, IL-10 and IL10/TNFα were associated with SH. CXCL10 have shown to be an important mediator of beta-cell apoptosis in experimetal models, thus our data support that CXCL10 may have an important role in the pathogenesis of COVID-19 related stress hyperglicemia. Further SH was associated with high IL10/TNFα ratio, well know marker of immunoparalysis. This association could partially explain the excess of mortality seen in patients with COVID-19 and stress hyerglycemia.
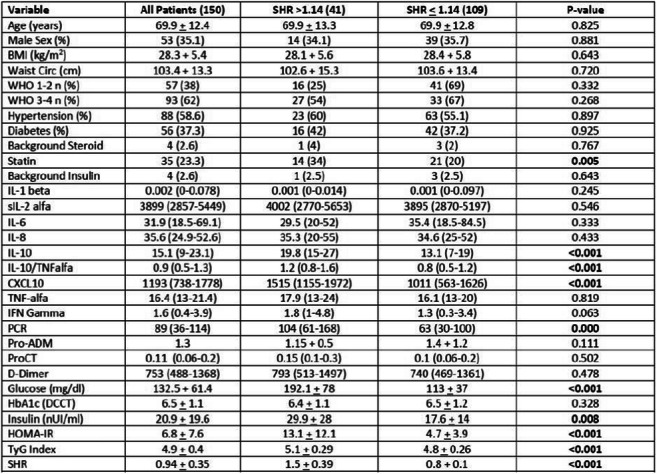
Disclosure: A. Da Porto: None.
409
Impact of the COVID-19 lockdown on behaviour, stress, anxiety and glycaemic control in patients with beta cell transplantation for type 1 diabetes
C.P. Landstra1, M.M. Ruissen1, H. Regeer1, M.F. Nijhoff1, B.E.P. Ballieux2, P.J.M. van der Boog1, A.P.J. de Vries1, S.D. Huisman1, E.J.P. de Koning1;
1Internal Medicine, Leiden University Medical Centre, 2Clinical Chemistry and Laboratory Medicine, Leiden University Medical Centre, Leiden, Netherlands.
Background and aims: Patients with severely complicated type 1 diabetes (T1D) who receive β-cell transplantation (Tx) have multiple risk factors for a severe course of coronavirus disease, including the use of immunosuppressive agents (IS). Lockdown strategies implemented due to the COVID-19 pandemic are known to impact both mental and physical health, but this impact is expected to be even greater in patients at high risk for a severe course of COVID-19. We therefore aimed to investigate the behavioural, mental and physical implications of the nationwide lockdown in islet and pancreas transplant recipients (referred to in this abstract as β-cell Tx).
Materials and methods: In order to study the effect of the lockdown on glycaemic control, all patients with T1D and an islet transplantation or pancreas transplantation with non-optimal graft function according to the Igls criteria using IS were eligible. As a control group, patients with T1D without IS were included. Lockdown behaviour and self-reported changes in anxiety, stress, physical activity, weight, and glycaemic control were assessed using questionnaires. HbA1c and continuous glucose monitoring (CGM) metrics during lockdown were compared to measurements before lockdown.
Results: Islet and pancreas (β-cell) Tx recipients (n=51, age 55 (48 - 59) years, BMI 23.3 (20.9 - 27.4) kg/m2, diabetes duration 42 (34 - 48) years) adhered more stringently to lockdown measures compared to patients with T1D alone (n=272, age 53 (37 - 62) years, BMI 25.2 (23.0 - 28.0) kg/m2, diabetes duration 27 (15 - 39) years). In β-cell Tx recipients as compared to T1D, 52.1% vs 18.3% (p <0.001) reported not going out for groceries and 45.8% vs 14.0% (p <0.001) reported not leaving the house at all. Fear of coronavirus infection was higher in β-cell Tx recipients (VAS 5.0 (3.0-7.0) vs 3.0 (2.0 - 5.0), p=0.004) and glycaemic control worsened during lockdown as assessed by HbA1c (ΔHbA1c +1.67±8.74 vs -1.72±6.15 mmol/mol, p=0.006) as well as CGM (Δ time in range β-cell Tx -4.5% (-6.0% - 1.5%) vs T1D 3.0% (-2.0% - 6.0%), p=0.038; Δ time above range β-cell Tx 5.5% (-0.5% - 7.5%) vs T1D -3.0% (-7.5% - 3.0%), p=0.025). Among β-cell Tx recipients, 29.2% self-reported more difficulty with glycaemic control, 26.8% increased insulin use, 40.0% less physical activity, 41.7% weight gain, 29.2% increased anxiety and 33.3% increased stress since the start of lockdown. Having had a β-cell Tx was the most important predictor of not leaving the house during the COVID-19 lockdown.
Conclusion: Islet or pancreas transplantation (β-cell Tx) in patients with type 1 diabetes leads to additional fear of infection, more stringent social isolation behavior and deterioration of glycemic control during the COVID-19 pandemic and the subsequent lockdown. In fact, having had a β-cell transplantation was the most important determinant of social isolation during the COVID-19 lockdown. In addition, these patients experience high rates of stress and anxiety, decreased physical activity and weight gain which requires continuous awareness amongst health care professionals.
Disclosure: C.P. Landstra: None.
SO 20 The other diabetes and islet function
410
Single-cell sequencing reveals preserved pancreatic islet cell identity by administration of metabolite-based diet in autoimmune diabetes
V. Vandenbempt1, S.E. Eski2, M.K. Brahma1, A. Li1, S. Demine1, P. Xiao3, A.K. Cardozo3, S.P. Singh2, E. Mariño4, C. Gysemans5, E.N. Gurzov1;
1Signal transduction and metabolism laboratory, Université Libre de Bruxelles, Anderlecht, Belgium, 2IRIBHM, Université Libre de Bruxelles, Anderlecht, Belgium, 3Inflammatory and Cell Death Signaling in Diabetes, Université Libre de Bruxelles, Anderlecht, Belgium, 4Infection and Immunity Program, Biomedicine Discovery Institute, Monash University, Melbourne, Australia, 5Clinical and Experimental Endocrinology, Department of Chronic Diseases, KU Leuven, Leuven, Belgium.
Background and aims: An altered gut bacterial composition is associated with the pathogenesis of type 1 diabetes (T1D) and short-chain fatty acids (SCFA) are known play a pivotal role in maintaining gut homeostasis. A special diet based on high-amylose maize-resistant starch modified with acetate and butyrate metabolites (HAMSAB) provided protection from autoimmune diabetes in the NOD mouse model. We recently tested the HAMSAB diet in patients with established T1D showing improvement in glucose control. Based on these findings, we studied the molecular mechanisms and effects of SCFA in pre-diabetic pancreatic islets.
Materials and methods: EndoC-βH1 and INS-1E β-cell lines were treated with acetate (250μM), butyrate (10μM) and/or IFN-γ (1,000U/mL) + IL-1β (50U/mL). The cell viability was analyzed using SYTOX™ Green Nucleic Acid Stain assay. five-week-old female NOD mice were fed with HAMSAB or HAMS control diet for five consecutive weeks. The pancreata were harvested, islets isolated using collagenase, and dispersed into single cells by trypsin. Single-cell RNA (scRNA)-sequencing was performed with 10x Chromium. The raw counts were analyzed using RStudio with the Seurat package. The cells were filtered based on RNA features, counts, and mitochondrial percentage and annotated by their principal component analysis using UMAP.
Results: Physiological concentrations of acetate and/or butyrate showed minimal effects on pro-inflammatory cytokine-induced cell death in EndoC-βH1 and INS-1E β-cell lines, suggesting that improved β-cell function is not due to SCFA-induced β-cell survival. To study the effect of the gut metabolites in the endocrine cells, we performed scRNA-seq in pancreatic islets isolated from pre-diabetic NOD mice fed HAMSAB or HAMS diets for five weeks. scRNA-seq analysis mapped the gene expression profiles of 4,301 and 4,113 individual islet cells from HAMSAB or HAMS fed mice, respectively. Cells were annotated into 12 clusters: 5 immune and 7 pancreatic endocrine cell types. The scRNA-seq dataset indicated that T-cells, B-cells, macrophages, and dendritic cell subsets infiltrated the islets of Langerhans from both HAMSAB and HAMS-fed mice. Interestingly, HAMSAB reduced the number of CD8+ cytotoxic cells, in line with previously described tolerogenic effects. Moreover, subclustering and differential gene expression analysis indicated that HAMSAB enhances β-cell function and decreases their stress response. In addition, the HAMSAB preserved the identity of endocrine cells evaluated by decreased dedifferentiated poly-hormonal (Ins+Gluc, Ins+Sst) cells expressing endocrine progenitor genes (MafA, Nfix) in mice fed this diet.
Conclusion: The HAMSAB diet prevents diabetes development in NOD mice, at least in part, by enhancing β-cell function and preserving cell identity of endocrine cells under inflammatory-mediated autoimmune stress.
Clinical Trial Registration Number: ACTRN12618001391268
Supported by: F.R.S-FNRS PhD Aspirant Scholarship, ERC Consolidator Grant, JDRF Career Development Award
Disclosure: V. Vandenbempt: None.
411
Immunoreactivities against different tyrosine-phospfatase 2 (IA-2)(256-760) epitopes characterise distinct clinical phenotypes in LADA
L. D'Onofrio, C. Tiberti, F. Panimolle, R. Amendolara, S. Zampetti, E. Maddaloni, R. Buzzetti;
Department of Experimental Medicine, “Sapienza” University of Rome, Rome, Italy.
Background and aims: Antibodies (Abs) against intracellular epitopes of the tyrosine-phosphatase 2 (IA-2) protein are detected in type 1 diabetes (T1D). In people with latent autoimmune diabetes in adults (LADA) IA-2 Abs are mainly directed against the IA-2(256-760) portion, which contains both intra- and extracellular amino acidic sequences. We aimed to evaluate if Abs against intra- and extracellular domains of IA-2 differ between LADA and T1D and whether they might intercept different immunoreactivities related to different clinical features in people with LADA.
Materials and methods: Subjects with LADA (n=101, male 55%) and T1D (n=61, male 59%), all testing positive for IA-2(256-760)Abs, were enrolled. The evaluation of immunoreactivity for three different IA-2 epitopes (IA-2(256-760), IA-2JM(601-630) and IA-2IC(605-979) fragments) allowed to identify whether the immunoreactivity against the IA-2(256-760) was limited to the extracellular domain of the protein (group A: positive for IA-2(256-760) and negative for IA-2IC(605-979) and IA-2JM(601-630)) or involved also the intracellular domain (group B: positive for IA-2(256-760) and positive for IA-2IC(605-979) or IA-2JM(601-630) or both of them). Clinical and biochemical features were also collected in subjects with LADA.
Results: Immunoreactivity limited to the extracellular portion of IA2-protein was found in 59.4% of people with LADA and in 14.8% of people with T1D (p<0.001). Conversely, intracellular immunoreactivity was more frequent among people with T1D (85.2%) compared to LADA (40.6%). As shown in table 1, among people with LADA, those in group A were older (p=0.01), with higher age at onset of diabetes (p=0.008), higher BMI (p=0.005), waist circumference (p=0.008) and triglycerides (p=0.006), and lower fasting blood glucose (p=0.05) and HDL cholesterol levels (p=0.005) compared to people in group B. They were also more frequently affected by hypertension (group A 57.6% Vs group B 26.8%, p=0.002) and less frequently by other autoimmune diseases (group A 14.6% Vs group B 57.7%, p<0.001). GAD Abs positivity was more frequent among participants with LADA in group B (p<0.001).
Conclusion: In the majority of people with LADA immunoreactivity against IA-2 involves only the extracellular domain of the protein, the clinical phenotype of these individuals resembles those of type 2 diabetes. Further studies are needed to investigate the significance and clinical impact of this heterogeneity.
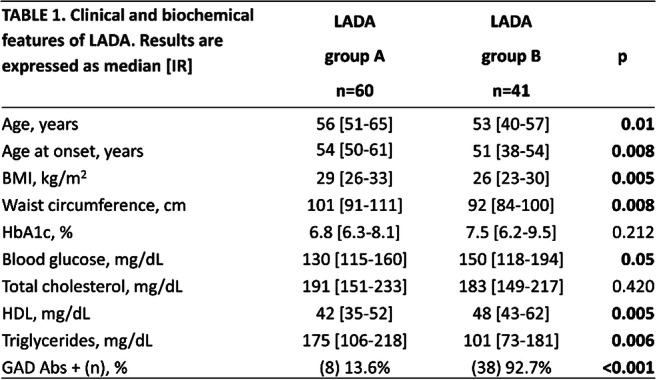
Supported by: PRIN 2017: 20175L9H7H
Disclosure: L. D'Onofrio: None.
412
Progressive pancreatic morphological changes and pro-inflammatory cell density in cystic fibrosis is associated with islet atrophy
Y. Al-Selwi1, O. Govaere1, Y. Bury2, R. Coulthard2, D. Tiniakos1,3, G. Klöppel4, J.A.M. Shaw1,5, N. Kattner1;
1Cellular and Pathology, Newcastle University, Newcastle, UK, 2Cellular and Pathology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK, 3Department of Pathology, National and Kapodistrian University of Athens, Athens, Greece, 4Institute of Pathology TUM School of Medicine, Technical University of Munich, Munich, Germany, 5Institute of Transplantation, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK.
Background and aims: Cystic fibrosis (CF) is the outcome of mutations in the CF transmembrane conductance regulator gene, which leads to impaired fluid secretions in the lungs and pancreas. CF-related diabetes (CFRD) is a common secondary complication of CF, yet the underlying pathogenesis of CFRD is not fully understood. This study aimed to analyse pancreatic histopathological changes in post-mortem CF tissue and to identify possible cellular players associated with these changes.
Materials and methods: Paraffin-embedded tissue blocks were obtained from the body of post-mortem CF pancreata (age range: premature-27 years old, n=8) and normal pancreas donors (age range: 18-29 years old, n=7). All tissue slides were stained for H&E, picro sirius red, CD45 and α-smooth muscle actin (α-SMA) to identify immune cells and activated fibroblasts respectively.
Results: The CF cohort displayed 3 different abnormal morphologies where early-stage CF represented a fibrotic pattern (Group 1, 7 blocks) and the end-stage CF demonstrated lipoatrophic (Group 2, 10 blocks) and fibrotic & lipoatrophic (Group 3, 8 blocks) patterns. Histopathological scoring (0: normal, 1: mild, 2: moderate and 3: severe) was performed for inflammation, acinar atrophy, ductal dilation/loss, insular atrophy and exocrine pancreas fibrosis, followed by statistical analysis using one-way ANOVA. Group 1 pancreata demonstrated the absence of duct dilation and adipocytes replacing the exocrine tissue, but there was evidence of some α-SMA+ activated fibroblasts in areas of increased collagen deposition and surrounding some acinar cells (not significantly different from controls). Presence of some inflammatory cells was confirmed (Group 1: mean ± SD inflammation score: 0.7 ± 0.5 control: 0 ± 0, p>0.05). Compared to the controls, acinar atrophy in Group 1 was not significant (Group 1: 1.4 ± 0.5, control: 0.4 ± 0.5, p>0.05), However, as CF progresses into the end-stage there was a significant increase in acinar atrophy (Group 1: 1.4 ± 0.5 vs Group 2: 3.0 ± 0.0, p<0.001 and vs Group3: 3.0 ± 0.0, p=0.01). Correspondingly, the number of islets decreased and surviving islets embedded in a pool of fatty or fibrotic tissue appeared more atrophic with irregular morphology and abnormal size and evidence of peri-islet activated fibroblasts as disease pathology progressed. In end-stage CF, activated fibroblasts were observed in the fibrotic regions of the tissue rather than regions with infiltrating adipocytes and this correlated with significant presence of inflammatory cells (Group 2: 2.2 ± 1.0; Group 3: 1.9 ± 1.0; control: 0.0 ± 0.0, p<0.001). Additionally, ductal dilation and exocrine fibrosis in Group 2 were not observed but extensive ductal loss was more apparent. Greatest duct dilation was observed in Group 3.
Conclusion: As CF progresses, pancreata undergo architectural remodelling including acinar and insular atrophy. Fibrotic changes are associated with inflammation and activated fibroblasts, possibly impacting on islet morphology.
Disclosure: Y. Al-Selwi: None.
413
Insulin and glucagon doublepositive cells in islets from pancreatic resections of children with congenital hyperinsulinism
J. Schultz1, C. Wolke2, R. Waterstradt1, F. Dombrowski3, W. Bartlen4, M. Tiedge1, U. Lendeckel2, S. Baltrusch1;
1Institute of Medical Biochemistry and Molecular Biology, Rostock, 2Institute of Medical Biochemistry and Molecular Biology, Greifswald, 3Institute of Pathology, University Medicine, Greifswald, 4Clinic of Pediatric Surgery, University Medicine, Greifswald, Germany.
Background and aims: Congenital hyperinsulinism (CHI) is a rare disorder characterized by severe postnatal hypoglycemia. The disease can lead to cerebral damage to the child due to uncontrolled insulin secretion, making rapid diagnosis and treatment necessary. Mutations in the ABCC8 and KCNJ11 genes have been associated with the disease but cannot causally explain the alteration. In pancreatic sections, the focal area appears as a region in which individual islets are barely recognizable and insulin-positive cells predominate. Therefore, the aim of this study was to take the next step and examine isolated islets of children from resection specimens in vivo in whom partial pancreatic resection was required because of failure of drug therapy.
Materials and methods: Pancreatic islets were isolated by collagenase digestion from focally presenting resection samples of four children who were between 2 and 12 months of age at the time of surgery. Islets were cultured on PCA slides for 4 days, resulting in outgrowth of cells. Insulin and glucagon were determined in the supernatant every 2 days by ELISA. The islets were then analysed for glucose-dependent intracellular calcium response using FURA. Finally, islets were fixed and investigated for insulin and glucagon localisation and content by immunofluorescence. Gene expression of insulin and glucagon was determined by RT-PCR.
Results: Insulin secretion was significantly 5-fold higher in islets from the focally resected area than in islets from the healthy peripheral area. While islets from the healthy peripheral area responded with a calcium increase to the glucose stimulus like adult human islets, islets isolated from the focus showed only oscillations. However, islets of children who were younger at the time of surgery showed an overall lower calcium and insulin response. These islets exhibited a high degree of heterogeneity with respect to alpha and beta cells. In addition to a varying proportion of insulin and glucagon positive cells, cells producing both hormones were detected.
Conclusion: Thus, the severity of the manifestation of CHI seems to be largely dependent on the stage of development of the endocrine pancreas. Thus, markers of early and late pancreatic development should be further investigated. Our results may also explain the large differences between CHI patients in response to drug and surgical therapy. The investigation of older and cured CHI patients, especially those after pancreatectomy, would thus also provide information on new therapeutic approaches for type 2 diabetes mellitus.
Disclosure: J. Schultz: None.
414
Investigating the role of a novel MODY-associated gain-of-function mutation in TALK-1 in islet dysfunction and glucose intolerance
A.Y. Nakhe1, J. Kim2, P.K. Dadi1, K.E. Zaborska1, D.A. Jacobson1;
1Molecular Physiology and Biophysics, Vanderbilt University, 2Chemistry, Vanderbilt University, Nashville, USA.
Background and aims: TALK-1, the most islet-restricted and abundantly expressed K+ channel of the human ꞵ-cell is a key regulator of electrical activity and glucose-stimulated insulin secretion (GSIS). Recently we identified a gain-of-function (GOF) mutation near the pore domain of TALK-1 (Leu114Pro) that segregates with Maturity-Onset Diabetes of the Young (MODY) in an autosomal dominant manner, but only in a single family. The present study aimed to confirm TALK-1-Leu114Pro as the mutation that causes the MODY phenotype and investigate the impact of TALK-1 GOF on glucose homeostasis.
Materials and methods: A mouse model containing the TALK-1-Leu114Pro mutation was generated utilizing CRISPR-SpCas9 editing in the C57BL/6J background. Whole-cell currents and β-cell membrane potential (Vm) were recorded using patch-clamp electrophysiology. Intracellular Ca2+ handling was monitored using fluorescence microscopy, islet hormone secretion was measured using ELISAs and islet composition was studied by immunofluorescence staining.
Results: Interestingly, heterozygous TALK-1-Leu114Pro C57BL/6J mice and homozygous TALK-1-Leu114Pro CD-1(ICR) mice exhibit neonatal lethality; this may be due to alterations in islet hormone secretion during neonatal development, as homozygous TALK-1-Leu114Pro CD-1(ICR) neonates were severely hyperglycemic. However, the neonatal hyperglycemia was transient and eventually followed in adults by glucose intolerance. We next determined how TALK-1-Leu114Pro affects electrical activity and found blunted glucose-induced β-cell action potential firing due to a GOF in whole-cell TALK-1 currents. The hyperpolarizing TALK-1-Leu114Pro current also resulted in blunted ꞵ-cell glucose-stimulated Ca2+ entry and Ca2+ oscillations (Ca2+ AUC (a.u.): Wildtype (WT) islets=213; N=3 mice and TALK-1-Leu114Pro+/-islets=10.01; N=3 mice, P=0.0008). On the endoplasmic reticulum (ER) membrane, TALK-1-Leu114Pro decreased basal [Ca2+]ER levels and enhanced IP3-induced Ca2+ release (Acetylcholine-stimulated [Ca2+]ER release AUC (a.u.): WT=26.83; N=4 mice and TALK-1-Leu114Pro+/-=37.84; N=4 mice, P<0.0001). Islets with TALK-1-Leu114Pro showed decreased GSIS which resulted in reduced plasma insulin levels in TALK-1-Leu114Pro+/- mice. This suggests that the defect in islet Ca2+ handling and GSIS in TALK-1-Leu114Pro+/- mice results in an impairment in glucose homeostasis (ip GTT AUC: WT=12,721; N=8 mice and TALK-1-Leu114Pro+/-=25,974; N=4 mice, P=0.0038). However, TALK-1 activity in other islet cell types may also contribute to glucose dyshomeostasis in TALK-1-Leu114Pro+/- mice. For example, pancreatic α-cell area and islet glucagon secretion are increased TALK-1-Leu114Pro+/- mice (α-cell area/islet (a.u.): WT=22.17; N=3 mice and TALK-1-Leu114Pro+/- =9.562; N=3 mice, P=0.0072 and glucagon secretion at 2mM glucose in pg/ml/islet: WT=3.58; N=3 mice and TALK-1-Leu114Pro+/- =10.16; N=3 mice, P=0.007).
Conclusion: Taken together, this study shows that the MODY-associated TALK-1-Leu114Pro mutation disrupts glucose homeostasis in adult mice (which resembles a MODY phenotype) and causes neonatal lethality by potentially altering islet hormone secretion during development. Overall, these data strongly suggest that TALK-1 is an islet-restricted target for the treatment for diabetes.
Supported by: R01DK115620
Disclosure: A.Y. Nakhe: None.
415
The glucocorticoid dexamethasone strongly induces ZBTB16 expression in human insulin-secreting beta cells
A. Karagiannopoulos1,2, E. Westholm1,2, J. Ofori3,2, E. Cowan1,2, J. Esguerra1,2, L. Eliasson1,2;
1Islet Cell Exocytosis, Department of Clinical sciences, Lund University, 2Lund University Diabetes Centre, Skåne University Hospital, 3Epigenetics, Department of Clinical sciences, Lund University, Malmö, Sweden.
Background and aims: Glucocorticoids (GC) are widely prescribed anti-inflammatory and immunosuppressant drugs. However, chronic or high-dose administration has been associated with diabetes onset. GCs have been found to contribute to β-cell dysfunction via activation of the Glucocorticoid Receptor (GR), and subsequent regulation of target genes, though the exact molecular mechanisms are still unclear. Previous RNA-seq data and bioinformatics analysis has shown the transcription factor zinc finger and BTB domain-containing protein 16, ZBTB16, to be the most potent target gene of GR upon glucocorticoid treatment in β-cells. Here, we aim to investigate the conditions under which the expression of ZBTB16 is regulated with GC treatment and its potential role in β-cell function.
Materials and methods: All experiments were performed in human EndoC-βH1 cells with the synthetic glucocorticoid dexamethasone (Dexa). Gene and protein expression were measured with RT-qPCR and Western Blot analysis, respectively. Insulin secretion stimulated with glucose (1 or 20 mM) and 500μM IBMX was analyzed with ELISA. Overexpression (OE) and knockdown (KD) of the ZBTB16 gene was induced via appropriate expression plasmid vector or siRNA. Chromatin immunoprecipitation (ChIP) and PCR were performed to validate GR binding sites on the ZBTB16 using an anti-GR antibody. All experimental data were analyzed by unpaired t-test, 1- or 2-way ANOVA.
Results: ZBTB16 expression was elevated dose-dependently with increasing Dexa concentration after treatment for 24h (0.1-1000nM). Moreover, when applying a time-dependent protocol (100 nM Dexa; 2, 8, 24, 48h) ZBTB16 expression was significantly induced within 8h (N=4, p=0.006), with a tendency towards elevated expression after 2h. The induction remained stable also after 48h. At the same time point (48h) insulin secretion at high glucose was significantly reduced compared to the treated control (N= 4, p = 0.002), but overexpression of ZBTB16 alone did not affect insulin secretion or cellular apoptotic processes. To demonstrate a potential protective role of ZBTB16, we measured the expression of a key β-cell transcription factor, PDX-1 upon ZBTB16 KD in presence of Dexa. Dexa alone have earlier been shown to reduce the expression of PDX-1. Interestingly, combining ZBTB16 KD and Dexa further reduced PDX-1 expression as compared to the Dexa treated control (N=3, p=0.03). Moreover, by integrating publicly available ChIP-seq data, we identified 10 intronic sites on the ZBTB16 gene and performed validation by ChIP assay (N=3). Despite displaying a stronger binding pattern in the majority of the positions, there was no clear differential binding on the ZBTB16 gene between control and GC-treated EndoC-βH1cells.
Conclusion: Although ZBTB16 expression is strongly and rapidly induced in EndoC-βH1 cells upon dexamethasone treatment, ZBTB16 is not likely directly implicated in GC-mediated β-cell dysfunction. Consequently GC-ZBTB16 co-treatment may have potential as a future strategy to alleviate diabetes associated with GC treatment.
Supported by: Swedish Research Council, SRA-Exodiab, Swedish Foundation for Strategic Research (LUDC-IRC)
Disclosure: A. Karagiannopoulos: None.
416
Differential expression of phosphorylated Tau forms in the brain, nerves and pancreas in health and disease
C. Lekka1, S. Dhayal1, H. Tulmin2, C.S. Flaxman1, M.L. Zeissler1, N.G. Morgan1, J.A. Todd2, S. Richardson1, I.M. Stefana2;
1University of Exeter, Exeter, 2University of Oxford, Oxford, UK.
Background and aims: The microtubule associated protein Tau is a marker of neurodegenerative tauopathies in brain, such as Alzheimer’s Disease (AD). Neuronal Tau can be hyperphosphorylated (pTau) to yield aggregates of poorly-soluble paired helical filaments (PHFs) which have been associated with disease pathology. Tau expression has also been reported outside the brain and we find that it is expressed in the pancreas , predominantly in beta cells. We, therefore, aimed to characterise the expression of Tau in human islets, focussing particularly on presumed pathological pTau variants.
Materials and methods: A panel of 46 anti-Tau antibodies (16 total, 10 isoform-specific, 16 phosphorylation-specific and 4 PHF-specific) was validated by Western blot and immunohistochemistry. The requirement for protein phosphorylation of relevant variants was verified by treatment with lambda phosphatase. Co-staining with Thioflavin S, a beta-sheet binding dye, was employed to identify aggregated Tau. Antibodies that met our validation criteria were used to stain human brain (normal and tauopathies, Oxford Brain Bank) and human pancreas sections (Exeter Archival Diabetes Biobank and nPOD).
Results: Several pTau forms were detected in both human brain and pancreas (e.g. Tau-pSer199, Tau-pSer396) whereas others were detected only in brain (e.g. Tau-pThr181, Tau-pSer238). Interestingly, analysis of staining patterns in individual beta cells showed that islet Tau was immunoreactive with phospho-peptide-raised antibodies (pSer202/pThr205, pThr231, pSer396) but not with those targeting PHF-raised antibodies (AT8, AT180, PHF-13) thought to recognise the same phosphorylated regions within Tau. Both classes of antibody detected pTau in intrapancreatic nerves and brain.
Conclusion: Tau is phosphorylated at several residues in human beta cells, including some that are considered pathological. However, unlike the equivalent variants present in tauopathy brain, beta cell variants were not immunoreactive with antibodies raised against PHF-Tau implying that PHF formation is not a typical feature of islet Tau. These results highlight the importance of the use of fully validated antibodies to study Tau forms and provide the basis for more detailed characterisation of the expression and functional role of beta-cell pTau.
Supported by: E3
Disclosure: C. Lekka: None.
417
Mechanistic investigation into the role of type 2 diabetes-associated STARD10 in insulin secretion and pancreatic beta cells
W. Tan1,2, N. Amirruddin1,2, M. Hu3, S. Muralidharan2, P. Banke2, F. Torta2, N.H.J. Ng1, G.A. Rutter3,4, A.K.K. Teo1,2;
1Institute of Molecular and Cell Biology, A*STAR, Singapore, Singapore, 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, 3Imperial College London, London, UK, 4CHUM Research Centre, University of Montreal, Montreal, Canada.
Background and aims: Type 2 diabetes (T2D) risk-associated variants have been identified at the STARD10 locus and these non-coding variants are linked to poorer β cell function and lower STARD10 expression in human islets. STARD10 is a lipid transfer protein that has been shown to mediate intracellular trafficking of various phospholipids between lipid vesicles. Notably, global StarD10 knockout (KO) mice had defective glucose tolerance and glucose-stimulated insulin secretion (GSIS). However, the mechanisms of how STARD10 regulates insulin secretion in β cells are still unclear. Hence, in this study, we aim to investigate the role of STARD10 in pancreatic β cells.
Materials and methods: The STARD10 gene was deleted in MEL-1 human embryonic stem cells (hESCs) using the CRISPR-Cas9 system and these cells were differentiated into β-like cells. Flow cytometry, transcriptomic and lipidomic analyses were carried out on these cells.
Results: Compared to the isogenic control, STARD10 KO β-like cells expressed lower levels of INS and had a lower percentage of insulin+ cells (p < 0.05). Transcript analysis revealed that STARD10 KO β-like cells also had lower expression of cell cycle genes such as MKI67, CCNA2, CCNB1, CCNB2, CCNE1 and CCNE2 (p < 0.05). Consistently, flow cytometry analysis then demonstrated that STARD10 KO β-like cells had lower percentage of Ki67+ cells (p < 0.05). Our findings suggest that STARD10 could be regulating β cell proliferation. To identify lipid targets of STARD10 in β cells, lipidomic analysis was performed and we found that STARD10 KO β-like cells had more than twice the amount of intracellular triglyceride levels as compared to the isogenic control (p < 0.05). In line with this, we also observed an almost complete loss of ETFB expression (p < 0.05), a mitochondrial protein that transfers electrons to the mitochondrial respiratory chain for fatty acid oxidation, in STARD10 KO β-like cells.
Conclusion: As triglyceride accumulation in β cells could affect GSIS, our findings indicate that the loss of STARD10 may impair GSIS by increasing intracellular triglyceride levels with ETFB as one of the downstream mediators. The loss of STARD10 can therefore impair β cell function and expansion, contributing to increased T2D risk.
Supported by: NMRC OF-YIRG
Disclosure: W. Tan: None.
SO 21 The fat burning the islets
418
Islet autotransplantation/total pancreatectomy vs pancreatoduodenectomy in patients at high risk for POPF: a prospective randomised trial
L. Piemonti1,2, R. Melzi2, R. Nano2, A. Mercalli2, F. Aleotti3, G. Capretti4,5, A. Zerbi5,4, G. Balzano3;
1Università Vita Salute San Raffaele, Milan, 2Diabetes Research Institute, IRCCS Ospedale San Raffaele, Milan, 3Pancreas Translational & Clinical Research Center, IRCCS Ospedale San Raffaele, Milan, 4Pancreatic Surgery, IRCCS Humanitas Research Hospital, Rozzano, 5Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy.
Background and aims: The benefit and safety of total pancreatectomy (TP) as alternative to pancreatoduodenectomy (PD) for the treatment of cephalic pancreatic diseases remain controversial. TP does avoid anastomosis-associated morbidity, but is burdened by severe diabetes. The Islet Auto Transplantation in combination with TP (TPIAT) may mitigate this problem. The aim of this trial was to compare the outcomes of TPIAT with those of PD, when performed in patients at high risk for postoperative pancreatic fistula (POPF)
Materials and methods: In this randomized, open-label, controlled trial conducted in two high-volume pancreatic surgery centers in Italy, we recruited patients with a benign, premalignant, or malignant indication for PD and at high risk for POPF (i.e., soft pancreas and pancreatic duct diameter ≤3 mm). Eligible patients were randomly assigned (1:1) to undergo either PD or TPIAT. The primary endpoint of the study was the incidence of complications occurring within 90 days after surgery.
Results: Between October 2010 and September 2019, 61 patients were assigned to either PD (n=31) or TPIAT (n=30). In the intention-to-treat analysis, morbidity rate was 90·3% in PD and 60% in TPIAT (p=0·008). PD was associated with an increased risk of complications requiring medical [OR 7·64 (95% CI 1·35-43·3), p=0·022] or surgical treatment [OR 2·82 (95% CI 0·86-9·24), p=0·086]. The postoperative length of stay was shorter for TPIAT than for PD [median 10·5 vs 16·0 days; p<0·001) and a trend toward a reduced mortality was evident. TPIAT was associated with a higher risk to develop diabetes [HR 9·1 (95% CI 3·76-21·9); p<0·0001], but patients maintained good metabolic control and showed sustained C-peptide production over time
Conclusion: TPIAT is safe, feasible and efficient and may become standard treatment in patients candidates for PD who are at high-risk for POPF.
Clinical Trial Registration Number: NCT01346098
Supported by: Italian Ministry of Heath
Disclosure: L. Piemonti: None.
419
Alterations of human islet G protein-coupled receptor expressomes in response to obesity
Z. Lyu, M. Zhao, D. Hopkins, P. Atanes, S.J. Persaud;
Department of Diabetes, King's College London, London, UK.
Background and aims: G protein-coupled receptors (GPCRs) are key regulators of islet function and energy homeostasis, and they are the targets of many pharmacotherapies. Here, we have quantified GPCR mRNA expression in islets isolated from lean and obese human organ donors to determine whether islet GPCR expression is altered in obesity.
Materials and methods: RT-qPCR was used to quantify 384 non-olfactory GPCR mRNAs relative to five reference genes (ACTB, GAPDH, PPIA, TBP, and TFRC) in human islets isolated from lean (BMI=22.6±0.5 kg/m2, n=7) and obese (BMI=32±0.8 kg/m2, n=8) donors.
Results: GPR56 was the most highly expressed GPCR mRNA in islets from both obese and lean donors (obese: 0.136±0.024; lean: 0.106±0.020). Most of the abundant GPCR mRNAs displayed similar expression levels in islets from both obese and lean donors: e.g. FZD6 (obese: 0.047±0.007; lean: 0.031±0.003, NS), CXCR4 (obese: 0.040±0.003; lean: 0.037±0.006, NS), F2RL1 (obese: 0.039±0.006; lean: 0.046±0.008, NS), TAS2R45 (obese: 0.037±0.006; lean: 0.025±0.005, NS), F2R (obese: 0.038±0.010; lean: 0.027±0.011, NS). Overall, 197 and 256 GPCRs, accounting for 51.3% and 66.7% of 384 non-olfactory GPCRs, were expressed above trace level in human islets from lean and obese donors respectively, while 191 GPCR mRNAs were common to both lean and obese donor groups. 40.9% (n=157) and 27.1% (n=104) of the 384 GPCR mRNAs quantified were expressed at trace level (0.001% to 0.0001% expression compared to the five reference genes) in lean and obese donor islets. We also found that 7.8% (n=30) and 6.3% (n=24) of all quantified GPCRs were absent in islets from lean and obese donors, respectively. 117 GPCR mRNAs were upregulated at least 2-fold in islets from obese donors, and there was >2-fold downregulation of 21 GPCR mRNAs (Figure 1a). Of particular interest, as shown in Figure 1b, several receptors signalling via Gαs or Gαq showed significant upregulation in islets from obese donors (RXFP1: 8.49±2.12-fold increase, p=0.021; PROKR2:3.90±0.69-fold increase, p=0.0025); GPR110: 3.90±1.21-fold increase, p=0.041).
Conclusion: Under conditions of obesity human islets show significant alterations in mRNAs encoding numerous GPCRs. The increased expression of three Gαs- or Gαq-coupled receptors, which have not previously been investigated in β-cells, may lead to potentiation of insulin secretion and/or β-cell mass to regulate glucose homeostasis, which could circumvent the increased insulin resistance that occurs in obesity.
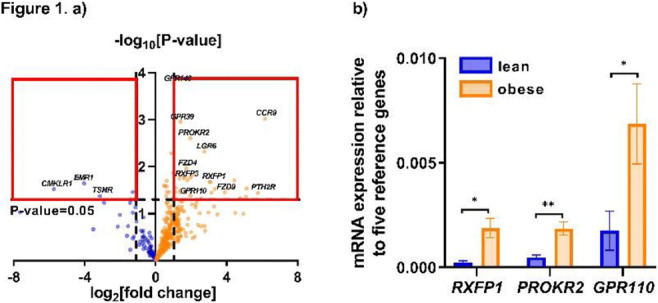
Disclosure: Z. Lyu: None.
420
Islet mRNA expression of GRKs, β-arrestins and RGS proteins in humans and mice under conditions of obesity
J. Starikova, P. Atanes, D. Hopkins, M. Zhao, S.J. Persaud;
Department of Diabetes, King's College London, London, UK.
Background and aims: G protein-coupled receptors (GPCRs) regulate key β-cell functions, including insulin secretion, proliferation, and survival. GPCR activation and downstream signal transduction is tightly regulated by GPCR kinases (GRKs), β-arrestins, and regulator of G protein signalling (RGS) proteins, but little is known about their expression in islets and the influence of obesity on their expression. This study aimed to quantify mRNA expression of these regulatory proteins in MIN6 β-cells, and in mouse and human islets, and determine whether they are altered in obesity.
Materials and methods: Human islets were provided from the King’s Human Islet Isolation Laboratory from lean (BMI 22.2 ± 0.74 kg/m2; n=4) and obese (BMI 30.6 ± 0.61 kg/m2; n=4) non-diabetic cadaveric heart-beating donors. Mouse islets were isolated from 16-week-old C57BL/6 male mice that had been fed for 10-weeks on either standard chow (10% fat; weight: 30.6 ±0.7 g; n=4) or a high fat diet (60% fat; weight: 47.6 ± 1.9 g; n=4). GRK, β-arrestin and RGS protein mRNA expression was determined by two-step SYBR-Green qPCR using validated primers and data are expressed relative to mRNA levels of the housekeeping gene β-actin.
Results: As expected, the phototransduction GRK mRNAs 1 and 7 were absent in human islets. GRK2, 3, 4 and 5 mRNAs were significantly upregulated in islets from obese donors (fold change, GRK2: 11.88 ± 0.29, GRK3: 9.87 ± 0.84, GRK4: 3.90 ± 0.40, and GRK5: 4.25 ± 0.49, p<0.05). Human islets expressed detectable levels of mRNAs encoding RGS1, 2, 4, 5, 7, 9, 10, 11, 12 16, 17, 19 and 22, and RGS1 and RGS2 mRNAs were significantly decreased in obesity (fold change, RGS1: 4.12 ± 0.66, and RGS2: 3.12 ± 0.83, p<0.05). GRK6 (0.0025 ± 0.00054) and RGS2 (0.052 ± 0.0048) were the most abundantly expressed GPCR regulator mRNAs in islets from lean donors, but this shifted to GRK2 (0.010 ± 0.00025) and RGS4 (0.030 ± 0.0064) in islets from obese donors. Mouse islets expressed mRNAs encoding Grk3, 5 and 6 and Rgs1, 2 3, 4, 5 7, 9, 10, 11 12, 16 and 17, and there were no statistically significant differences in Grk or Rgs mRNA expression in islets from lean and obese mice. Grk3 was the most abundantly expressed Grk mRNA in islets from both lean (0.12 ± 0.032) and obese (0.15 ± 0.018) mice, as well as in MIN6 cells (0.027 ± 0.0023). Rgs11 was the most highly expressed Rgs mRNA in lean (0.30 ± 0.011) and obese (0.30 ± 0.023) mouse islets, but Rgs4 was the most abundant (0.12 ± 0.010) Rgs mRNA in MIN6 cells. Mouse and human islets and MIN6 cells expressed β-arrestin 1 and 2 mRNAs and there were no significant differences in expression under conditions of obesity. However, while βARR1 and βARR2 mRNAs were expressed at similar levels in human islets, βarr2 mRNA was more abundant than barr1 in both mouse islets and MIN6 cells (p<0.05).
Conclusion: We have identified alterations in GRK and RGS mRNA expression in human islets related to obesity and have revealed differences between human and mouse islet responses to obesity. We have also demonstrated preferential expression of βarr2 mRNA in mouse islets and MIN6 cells, but not in human islets.
Supported by: MRC
Disclosure: J. Starikova: None.
421
Perilipin 2 protects against lipotoxicity-induced islet fibrosis by restoring islet stellate cells quiescence
Y. Zhou1, H. Wang1, Y. Wang1, Z. Sun2, J. Ma1;
1Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, 2Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China.
Background and aims: We explored whether and how perilipin 2 protected islets function through against lipotoxicity-induced islet dysfunction by regulating islet stellate cell (ISC) activation.
Materials and methods: Six-week-old male rats were given a high-fat diet or a control diet for 28 weeks. Glucose metabolic phenotypes were assessed using glucose/insulin tolerance tests, masson and immunohistochemical staining. ISC activation levels were assessed from rats and palmitic acid (PA)-treated cultured ISCs by immunofluorescence, Oil red O staining, electron microscopy, quantitative PCR, and western blotting. Changes in ISCs phenotype of activation degree and its underlying mechanisms were assessed by target gene lentiviral infection, high performance liquid chromatography (HPLC), and western blotting.
Results: Obese rats showed glucose intolerance, decreased endocrine hormone profiles, and elevated expression of α-smooth muscle actin (α-SMA), a polygonal appearance without cytoplasmic lipid droplets (LDs) of ISCs in rats and isolated islets. PA-treated cultured ISCs exhibited faster proliferation and migration abilities with the induction of mRNA levels of lipid metabolism proteins, especially perilipin 2 (Plin2). The overexpression of Plin2 resulted in an ISC “re-quiescent” phenotype associated with inhibition of the Smad3-TGF-β signaling pathways.
Conclusion: Our observations suggest a protective role of Plin2 in weakening ISC activation. It may serve as a novel therapeutic target for preventing islet fibrosis for T2DM.
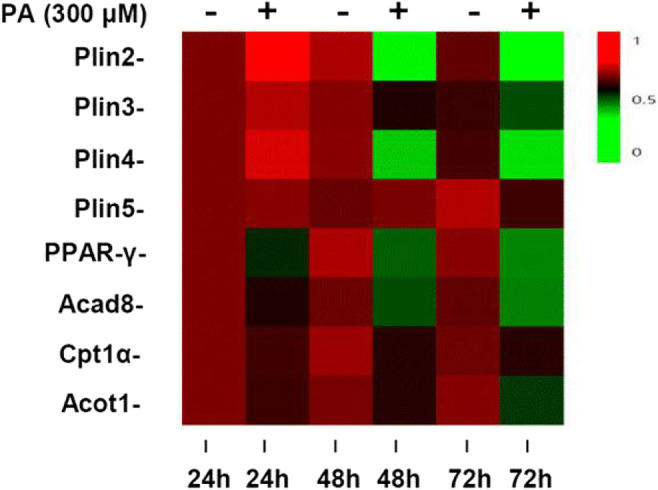
Disclosure: Y. Zhou: None.
422
Short-chain fatty acids receptors modulate the function and plasticity of pancreatic alpha cells
A. Sánchez-Roncero1,2, T. Fernández-Marcelo2, P. Martínez-Oca1,2, R. Sánchez-Domínguez3,4, J.C. Segovia3,4, F. Escrivá1,2, C. Álvarez1,2, E. Fernández-Millán1,2;
1Bioquímica y Biología Molecular, Facultad de Farmacia, Universidad Complutense de Madrid, 2CIBERDEM, ISCIII, 3Differentiation and Cytometry Unit, Hematopoietic Innovative Therapies Division, CIEMAT, 4CIBERER, ISCIII, Madrid, Spain.
Background and aims: The intestinal microbiome can regulate the development of metabolic diseases, such as obesity or type 2 diabetes, through several metabolites. Among these, short-chain fatty acids (SCFAs) are the most abundant. In addition to acting as metabolic substrates, they can also signal through GPR41 (FFAR3) and GPR43 (FFAR2), G-protein-coupled receptors. Previous studies demonstrated that both receptors are expressed mainly in white adipocytes, immune cells and pancreatic β-cells. In the present study, we focused on the role of GPR41 and GPR43 on growth and function of pancreatic α-cells under both physiological and pro-obesogenic conditions.
Materials and methods: The experiments were carried out in the α-TC1.9 cell line and in isolated pancreatic islets of male Wistar rats fed a standard or a high-fat (HF) diet for 12 weeks. The presence of GPR41 and GPR43 in pancreatic islet α-cells was determined by immunofluorescence and flow cytometry. The effect of specific agonists of GPR41 (AR420626) and GPR43 (PA) on genes underpinning α-cell identity was determined by RT-qPCR. GPR-mediated glucagon secretion was measured by ELISA and α-cell proliferation by in toto immunofluorescence of Ki67.
Results: Histological and flow cytometry analysis of rat islets confirmed that GPR41 and GPR43 colocalize with glucagon in α-cells. The treatment of α-TC1.9 cells with agonists of GPR41 and GPR43 increased the expression levels of Gcg (<0.001) and Pcsk2 (<0.05) but decreased Ffar2 and Pcsk1 levels (<0.001). Additionally, both agonists increased glucagon secretion at low glucose levels. These results were confirmed in isolated islets from animals fed a standard diet. HF-fed animals showed hyperglucagonaemia (<0.01) and increased expression (<0.01) and secretion of glucagon, under low (<0.05) and high glucose concentrations (<0.01). When GPR41 and GPR43 receptors were activated, Gcg expression was further increased in islets from HF-fed animals but failed to potentiate glucagon release.
Conclusion: Our results suggest that both receptors, GPR41 and GPR43, are able to modulate function of α-cells becoming potential targets for the treatment of diabetes.
Supported by: CIBERDEM (ISCIII), PID2020-116134RB-I00 (MICINN), B2017/BMD-3684 (CAM), Spain.
Disclosure: A. Sánchez-Roncero: None.
423
Characterisation of visceral adipose tissue-derived Ccl4 on beta cell function
T. Ashik, P. Atanes, N. Haq, G.A. Bewick, S.J. Persaud;
Department of Diabetes, King's College London, London, UK.
Background and aims: White adipose tissue is a highly active source of secretory products, known as adipokines, which can have endocrine effects to regulate cell function through binding to cell surface receptors. Little is known about adipokine action at β-cells, but several adipokines have actions that are transduced by binding to islet G protein-coupled receptors (GPCRs), and levels of some adipokines are altered in obesity. We have previously shown that Ccl4 mRNA expression is significantly upregulated in mouse visceral adipose tissue with high-fat feeding. Here, we aimed to identify its cognate GPCR and determine its functional effects on MIN6 β-cells and mouse islets.
Materials and methods: PRESTO-Tango β-arrestin reporter assays were performed to interrogate potential Ccl4-activating GPCRs and their mRNA expression in MIN6 β-cells and mouse islets was quantified by RT-qPCR. The effects of recombinant Ccl4 on apoptosis, proliferation and insulin secretion were quantified in MIN6 β-cells and/or mouse islets.
Results: 100ng/mL Ccl4 significantly activated Cxcr1 and Cxcr5 in PRESTO-Tango assays and Cxcr5, but not Cxcr1, mRNA was expressed in MIN6 β-cells and mouse islets. In MIN6 β-cells, Ccl4 induced concentration-dependent protection against cytokine-induced apoptosis (% control: -cytokines: 100±7.9; +cytokines: 595.2±37.0; +10ng/mL Ccl4: 502.8±18.0; +50ng/mL Ccl4: 497.8±19.5; +100ng/mL Ccl4: 469.1±24.5; p<0.01, n=3) and reduction in serum-stimulated proliferation (% control: 0% FBS: 100.0±4.9; 10% FBS: 176.1±6.5; +10ng/mL Ccl4: 162.0±7.9; +50ng/mL Ccl4: 156.7±9.5; +100ng/mL Ccl4: 129.8±5.3; p<0.0001, n=3). Conversely, in mouse islets, Ccl4 did not affect cytokine-induced apoptosis (% control: -cytokines: 100.0±11.1; +cytokines: 210.0±21.7; +10ng/mL Ccl4: 278.3±21.2; +50ng/mL Ccl4: 231.3±19.1; +100ng/mL Ccl4: 242.9±15.9; n=3; ns), insulin secretion (% control: 2mM glucose: 100.0±14.9; 20mM glucose: 337.7±34.6; +10ng/mL Ccl4: 308.8±27.1; +50ng/mL Ccl4: 338.6±28.0; +100ng/mL Ccl4: 365.3±29.8; n=5; ns) or proliferation (% Ki67 +ve control: RPMI: 100.0±29.5; +100ng/mL Ccl4: 70.4±22.9; n=14-18 single mouse islets; ns).
Conclusion: Ccl4 demonstrated anti-apoptotic and anti-proliferative actions on MIN6 β-cells that were not replicated in mouse islets. The absence of any apoptotic, insulin secretory and proliferative effects in primary mouse islets suggests that Ccl4 is unlikely to play a role in regulating β-cell function via cross-talk between adipocytes and islets. These data have contributed increased understanding of the role of adipose tissue-derived GPCR-targeting ligands in regulating β-cell function in obesity. Furthermore, while MIN6 cells are a useful primary β-cell surrogate for some studies, primary islets should always be used to confirm physiological relevance.
Supported by: MRC
Disclosure: T. Ashik: None.
424
Oxidative ER stess in beta cells: the role of ERO-1α in palmitate-mediated toxicity
S. Sharifi, I. Mehmeti;
Institute of Clinical Biochemistry, Hannover Medical School, Hannover, Germany.
Background and aims: Prolonged exposure of pancreatic β-cells to the unsaturated long-chain fatty acid palmitate (PA), leads to β-cell dysfunction and loss in human and rat β-cells, a process known as lipotoxicity. A possible mechanism of lipotoxicity is considered to be an excessive production of H2O2. In particular, the endoplasmic reticulum (ER) as a major site of oxidative protein folding could represent a significant source of H2O2. ER oxidoreductin 1 (ERO-1) isoenzymes, ERO-1α and ERO-1β, both catalyse oxidative protein folding and generate H2O2 as a byproduct. However, whether the ERO-1-derived H2O2 is involved in lipotoxicity is still unknown. Our preliminary data revealed that both isoforms are expressed in pancreatic β-cells, but interestingly, only ERO-1α expression was significantly induced by PA. Therefore, the aim of this study was to uncover the role of ERO-1α in PA-mediated toxicity in insulin-secreting INS-1E cells.
Materials and methods: An ERO-1α knockout (KO) insulin-secreting INS-1E cell line was generated by CRISPR/Cas9 technique. The KO of ERO-1α was confirmed by Western blot. Insulin secretion and insulin content were determined by radioimmunoassay, while proinsulin was quantified by a mouse/rat proinsulin ELISA kit in the presence of 3, 10, and 30 mM glucose. For further experiments, cells were treated with increasing concentrations of PA (0.1-1.5 mM) for 24 h. Thereafter, cell viability, caspase activity, and overall oxidative stress were assessed via MTT-assay, flow cytometry, and oxidation of 2′,7′-dichlorofluorescein-diacetate (DCF-DA), respectively. Transcriptional changes were detected by RT-qPCR.
Results: Exposure of insulin-secreting INS-1E cells to PA resulted in a significant induction of ERO-1α gene expression (0.5 mM PA: 1.6-fold and 1.0 mM PA: 2-fold; p<0.01 vs untreated cells; n=4), whereas the expression of ERO-1β remained unaffected. The RT-qPCR data could also be verified on protein level. Knockout of ERO-1α had no negative effect on glucose-stimulated insulin secretion, insulin and proinsulin content compared to wild-type cells. However, KO of ERO-1α led to a significantly higher resistance of INS-1E cells against PA-mediated toxicity. ERO-1α KO cells showed a 58% residual cell viability at highest PA concentration of 1.5 mM tested, whereas wild-type cells revealed a residual cell viability of only 42% (p<0.001; n=10). Moreover, the PA-mediated overall ROS generation in ERO-1α KO cells was significantly lower compared to wild-type cells. The oxidation rate of DCFDA-H2 comprised only 143% in ERO-1α KO, while it was 228% in identically treated wild-type cells (p<0.01, p<0.001; n=6). In addition, ERO-1α KO cells exhibited lower caspase-9 and -3 activity upon PA treatment compared to wild-type cells (p<0.05; n=4).
Conclusion: The observed protection against PA-mediated toxicity by specific ERO-1α KO suggests that H2O2 formed in the ER lumen by ERO-1α is substantially involved in the induction of ER oxidative stress and subsequent β-cell death. Thus, specific KO of ERO-1α could increase the resistance of β-cells against lipotoxicity without affecting insulin folding and its glucose-stimulated insulin secretion.
Supported by: German Research Foundation (DFG, ME5567/2-1)
Disclosure: S. Sharifi: None.
425
Intrapancreatic adipose tissue accumulation is associated to beta cell dedifferentiation in humans
F. Cinti1, T. Mezza1, C. Cefalo1, S. Moffa1, F. Impronta1, G. Di Giuseppe1, U. Capece1, G. Ciccarelli1, L. Soldovieri1, I. Severi2, S. Cinti2, A. Giaccari1;
1IRCCS Fondazione Policlinico Gemelli, Rome, 2Università Politecnica delle Marche, Ancona, Italy.
Background and aims: the role of intrapancreatic fat accumulation in the development of Type 2 diabetes (T2D) is still on debate. β cell dedifferentiation is one of the mechanisms responsible of β cells failure in T2D but its role in the prediabetes state is unknown. The aim of our study was to investigate the intrapancreatic fat and beta cell dedifferentiation process before diabetes onset.
Materials and methods: 8 pancreatic samples from non diabetic patients undergoing pancreatic surgery, in whom an oral glucose tolerance test (OGTT) has been performed, have been morphologically surveyed. Based on the pre-operative OGTT, the subjects were divided into 3 glucose tolerance groups: normal (NGT, n = 3), altered (IGT, n = 3) or newly diagnosed diabetes (DM, n = 2). Dedifferentiation score has been assessed as the percentage of cells retaining endocrine features (synaptophysin immunoreactivity) but no longer containing the four endocrine hormones. The intraparenchymal white adipose tissue (WAT) score as been calculated as the % of pancreatic tissue occupied by white adipose tissue.
Results: WAT score resulted significantly higher in DM patients compared to NGT and IGT (WAT score DM 43.79 ± 20.83%, IGT 10.67 ± 8.5%, NGT 4.43 ± 4.37%; p=0.03). We observed a progressive and significant increase in dedifferentiation score, paralleling worsening glucose tolerance (from NGT through IGT to T2D; 4.8±3.8; 32,37±7.4; 40,38±19 respectively; p=0.02). Finally, we found a positive correlation between WAT score and dedifferentiation score (r=0.7; p=0.0052).
Conclusion: We observed a progressive increase in WAT and dedifferentiation score paralleling the worsening of glucose tolerance. Our data suggest that, before diabetes onset, the accumulation of WAT might be responsible for dedifferentiation process and, therefore, it might become a new potential target to curb diabetes onset.
Supported by: EFSD/AZ Mentorship Programme
Disclosure: F. Cinti: None.
SO 22 Exercise and diabetes: much to learn!
426
Positive effects of physical activity on prescription on glycaemic control, fitness and quality of life in newly diagnosed type 2 diabetic patients in a randomised control trial
T.N. Nguyen1,2, I. van der Ploeg3, C.J. Sundberg3,4, H.T.T. Vu1,2;
1Department of Geriatrics, Hanoi Medical University, Hanoi, Viet Nam, 2Scientific Research Department, National Geriatric Hospital, Hanoi, Viet Nam, 3Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden, 4Department of Learning, Informatics, Management and Ethics, Karolinska Institutet, Stockholm, Sweden.
Background and aims: Type 2 diabetes (T2D) can be both prevented and treated cost-effectively with lifestyle improvements, including increasing physical activity (PA). PA on prescription (PAP) has proven to be a feasible way to increase an individual’s PA levels. The aim of the study was to compare the effects of PAP with standard care (SC) in T2D patients in Vietnam.
Materials and methods: A randomized control trial was conducted with 87 drug-naïve T2D patients. Participants were randomly assigned (1:1, similarity in demographic, anthropometric and clinical characteristics) to the PAP group (n = 44) or the SC group (n = 43). The PAP group received individualized recommendation for PA, intensive face-to-face training every two weeks and were instructed on how to use the pedometer (Yamax Corporation, Tokyo, Japan) and to record the step-count daily using diaries. The SC group received the standard recommendations according to WHO guidelines for newly diagnosed T2D patients. Primary outcomes were evaluated as changes in HbA1c, fasting blood glucose (FPG) levels, insulin resistance (Homeotasis Model Assessment - Insulin Resistance, HOMA-IR), aerobic fitness levels (VO2-max measured by using a submaximal exercise test) and health related quality of life (HRQoL) including two domains on physical health and mental health (SF-36 questionnaire) from baseline to 12-week follow-up. Secondary outcomes were self-reported PA levels assessed by using Global Physical Activity Questionnaire, BMI, waist circumference, blood pressure and lipid profile.
Results: The mean step-count daily of the PAP group was 6942 (SD: 1254) steps/day. Self-reported PA levels of the PAP group (3649 ± 3258 METs/week) were significantly higher than those of the SC group (2241 ± 1751 METs/week). The FPG levels of the PAP group decreased significantly compared with the SC group (difference between groups at week 12: -0.65, 95% CI: -1.02, -0.28 mmol/l), this reduction started at week 2 and maintained throughout the intervention period. The mean HbA1c levels decreased from 63.0 to 52.3 mmol/mol in the PAP group (p < 0.001) and from 60.5 to 58.1 mmol/mol in the SC group (p > 0.05). Change in HOMA-IR values over 12 weeks in the PAP group was higher than that in the SC group (difference between groups, 11.7, 95%CI: 6.8, 16.6). The VO2-max increased in PAP group (from 2.75 ± 0.70 to 3.14 ± 0.73 L/min) as compared to SC group (from 2.51 ± 0.72 to 2.68 ± 0.71 L/min). PAP had promising effects on HRQoL: Physical health (from 62.9 to 74.6 scores) and mental health (from 60.0 to 70.2 scores) in the PAP group were significantly improved in comparison to the SC group, (from 60.7 to 63.5 scores, and from 60.2 to 62.1 scores, respectively). BMI, waist circumference, total cholesterol, LDL cholesterol and triglycerid were significantly more decreased in the intervention group than in the control group, p<0.05. There was no significant difference between the two groups in blood pressure.
Conclusion: PAP had positive effects on glucose control, insulin resistance, aerobic fitnness, BMI, waist circumference, lipid profile and HRQoL in newly diagnosed T2D patients.
Supported by: VR/SIDA 348-2011-7246
Disclosure: T.N. Nguyen: None.
427
Blood glucose response to cycling and running in individuals with type 1 diabetes: a systematic review and meta-analysis
M.L. Eckstein1, F. Aziz2, F. Aberer1, S. Böckel1, R.T. Zimmer1, M. Erlmann1, H. Sourij2, O. Moser1;
1Division of Exercise Physiology and Metabolism, University of Bayreuth, Bayreuth, Germany, 2Department of Endocrinology and Diabetology, Medical University of Graz, Graz, Austria.
Background and aims: Running and cycling represent the most common types of endurance exercise in individuals with type 1 diabetes (T1D). Recommendations for glycemic management prior to those exercises are the same even though different musculature is involved. The risk of exercise-induced hypoglycemia is evident in both exercise types, it remains unclear to which extent the blood glucose (BG) decrease differs in response to these exercise types. The aim of this systematic review and meta-analysis was to assess how running and cycling influence the magnitude of BG in individuals with T1D.
Materials and methods: A systematic literature search was conducted in EMBASE, PubMed, Cochrane Central Register of Controlled Trials, and ISI Web of Knowledge for publications from January 1950 until February 2021. Parameters included for analysis were population (adults and adolescents), exercise type, intensity, duration, and insulin preparation. The meta-analysis was performed to estimate the pooled mean with a 95% confidence interval (CI) of delta BG levels. In addition, sub-group and meta-regression analyses were performed to assess their influence on delta BG.
Results: The database search identified 3192 studies of which 71 articles were included in the meta-analysis. Due to crossover designs within each article, 153 different study results were included for analysis. Data from 1901 exercise tests from individuals with T1D with a mean age of 29 ± 4 years were included. Independent of the type of exercise, the overall pooled delta BG was -53.73 mg/dL [-59.73;-47.74] and a mean exercise duration of 46 ± 21 minutes. In cycling studies (n=111), the pooled BG decrease was -48.95 mg/dL [-54.04;-43.09] in comparison to a pooled BG decrease of -65.89 mg/dL [-80.69;-51.08] (p=0.03) in running studies (n=42). During cycle exercise, low intensity led to a BG decrease of -33.51 mg/dL [-53.27;-13.74], during moderate intensity to -55.30 mg/dL [-62.28;-48.32] and during vigorous intensity to -40.62 mg/dL [-51.56;-29.68] (p=0.021). During running, low intensity exercise led to a BG decrease of -11.85 mg/dL [-48.81;+25.10], moderate intensity to -59.01 mg/dL [-81.10;-36.93] and vigorous intensity to -82.19 mg/dL [-97.06;-67.29] (p=0.002). Cycling studies with no insulin reduction pre-exercise led to a BG decrease of -46.87 mg/dL [-54.40;-39.34] compared to studies with insulin reductions and a delta BG of -55.44 mg/dL [-61.88;-48.99] (p=0.090). Running studies with no insulin reduction led to a BG decrease of -84.54 mg/dL [-98.57;-70.51] compared to insulin reductions with a delta BG of -26.23 mg/dL [-52.32;-0.13] (p<0.001). Meta-regression analysis in cycling studies revealed that exercise duration >60 minutes (-18.07 [-34.43;-1.71]) (p=0.03) and moderate exercise intensity (-20.33 [-38.79;-1.86]) (p=0.03) were significantly influencing delta BG levels. Adults (-119.98 [-170.53;-69.44]) (p<0.001), no reduction of insulin (+57.70 [+25.38;+90.02]) (p<0.001) and high-intensity interval exercise (+86.75 [+55.51;+117.99]) (p<0.001) significantly influenced delta BG levels in running studies.
Conclusion: Running led to a larger decrease in BG in comparison to cycling. Active individuals with T1D should be aware that current recommendations for cycling might not be transferable to running.
Clinical Trial Registration Number: CRD42021239671
Disclosure: M.L. Eckstein: None.
428
Pulmonary function in uncomplicated type 1 diabetes: blunted during exercise even though normal at rest
I. Jlali1, E. Heyman1, R. Matran2, G. Marais1, A. Descatoire3, R. Rabasa-Lhoret4, M. Pawlak-Chaouch1, P. Mucci1, P. Fontaine5, G. Baquet1, S. Tagougui1;
1Univ. Lille, Univ. Artois, Univ. Littoral Côte d’Opale, ULR 7369 - URePSSS - Unité de Recherche Pluridisciplinaire Sport Santé Société, F-59000, Lille, France, 2Department of Physiology, EA 2689 & IFR 22, Lille, France, 3RegionalHospital Center of Roubaix, Roubaix, France, 4Montreal Clinical Research Institute, Montreal, Canada, 5Department of Diabetology, University hospital; EA 4489, Lille, France.
Background and aims: Type 1 diabetes (T1D) is associated with increased risk of impaired lung function and this may contribute to the impairment in aerobic fitness, a powerful marker of health status with prognostic value. We aimed to examine the pulmonary function at rest and the ventilatory response during maximal exercise in individuals with T1D free from microangiopathy.
Materials and methods: Seventeen adults with T1D free from clinically detectable microangiopathy (glycated hemoglobin ‘HbA1c’:8.0±1.3%), and 17 adults without T1D, matched on gender, age, level of physical activity and body composition were included. Lung function was assessed by spirometry and measurements of the combined diffusing capacity for nitric oxide (DLNO) and carbon monoxide (DLCO) at rest. Subjects performed a maximal exercise test during which the respiratory parameters (maximal oxygen uptake ‘VO2max’, breathing frequency, ventilation, tidal volume) were measured.
Results: At rest, DLCO, DLNO and its 2 components Dm (membrane diffusion capacity) and VC (pulmonary capillary volume), as well as spirometry parameters (forced vital capacity=4.8±1.0L vs. 5.4±1.0L; forced expiratory volume in 1 second=4.1±0.7L vs. 4.4±0.7L) were comparable among T1D and control group. During exercise, ventilatory response was different between the two groups: tidal volume ‘VT’ was lower in T1D vs. control group (P<0.05, VT 2.15±0.53L vs 2.36±0.45L, respectively). Individuals with T1D showed a reduced VO2max (P=0.04) in comparison with control group. There was a negative correlation between VO2max and HbA1c level (r=-0.75, P=0.032).
Conclusion: At rest before exercise, alveolar-capillary diffusion capacity is normal in individuals with T1D. Yet, the decrease in VO2max and tidal volume during exercise in individuals with T1D seems to be associated with an alteration of the pulmonary function even before the appearance of micro-macrovascular complications.
Clinical Trial Registration Number: NCT02051504
Supported by: société francophone du diabète
Disclosure: I. Jlali: None.
429
Barriers to exercise in type 1 diabetes individuals with insulin resistance
A.M. Alobaid1, M.A. Zulyniak1, M. Hopkins1, M.D. Campbell2,3;
1School of Food Science and Nutrition, University of Leeds, Leeds, 2School of Health Sciences and Wellbeing, University of Sunderland, Sunderland, 3Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK.
Background and aims: Insulin resistance (IR) is prevalent in Type 1 Diabetes (T1D), and increases risk of macro and microvascular complications. Regular exercise participation improves IR, and may reduce risk of diabetes complications developing and progressing. However, there is a lack of evidence regarding the perceived benefits and barriers to exercise in those with T1D, and whether these differ between individuals with and without IR. In this study, we used estimated glucose disposal rate (eGDR) - a validated tool for assessing IR status - and the Exercise Benefits/Barriers Scale (EBBS), to examine whether perceived benefits and barriers to exercise differ among T1D individuals with and without IR.
Materials and methods: A total of eighty-six individuals with T1D (age 29±6 years; BMI 26.24±4.20 kg/m2) were included in the present analysis, with baseline pre-treatment data aggregated from two clinical studies with common experimental procedures. The cohort was categorised according to IR status using a previously published eGDR cut-point of <6 mg.kg.ml (41.9%) for IR and ≥6 mg.kg.ml (58.2%) for non-IR. Group differences were examined using independent t-tests for normally distributed variables, Mann-Whitney U test was applied for non-normally distributed variables, and fisher exact tests for categorical data. Pearson correlations were used to assess interrelationships between variables and explore potential confounders. The relationship between EBBS scores and IR was also assessed using general linear regression, with sequential adjustment for age, length of diagnosis, and exercise participation. Data analysis was performed using SPSS (IBM SPSS Statistics 27), with a p-value of <0.05 deemed statistically significant.
Results: Individuals with IR tended to be older, with a longer duration of diabetes, and presented with higher HbA1c, and increased prevalence of hypertension (p<0.05; Table 1). Self-report exercise participation levels were lower in those with IR (P=0.017; Table 1), although exercise intensity (moderate vs. vigorous exercise) was comparable (p>0.05; Table 1). eGDR was positively associated with total EBBS score in the unadjusted regression model (β=4.975, 95%CI:4.070 to 5.880, p<0.001); this association remained robust following adjustment for age, length of diagnosis, and exercise participation (β=3.990, 95%CI:2.977 to 5.003, p<0.001).
Conclusion: eGDR is positively associated with total EBBS scores in individuals with T1D, meaning that those with IR demonstrate significantly lower EBBS scores. These findings suggest that habitual physical activity or exercise interventions should be adapted to consider T1D and IR-specific target behaviours.
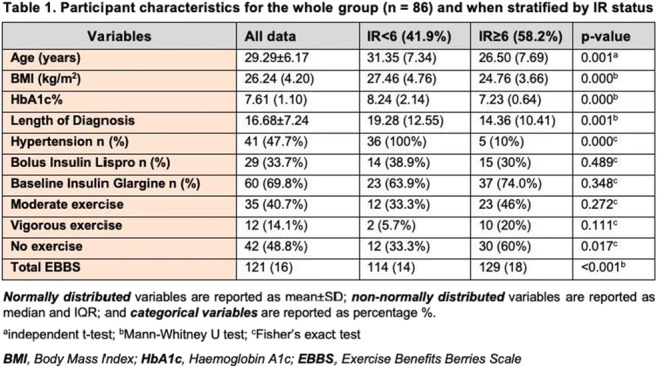
Disclosure: A.M. Alobaid: None.
430
Insulin glargine u300 versus insulin degludec around spontaneous exercise sessions in people with type 1 diabetes: the Ultraflexi-1 study
H. Sourij1, A. Mueller1, F. Aberer1, F. Aziz1, H. Kojzar1, C. Sourij2, A. Obermayer1, F. Abbas1, M.L. Eckstein3, J. Lenz1, C. Sternad1, P.N. Pferschy1, N.J. Tripolt1, H. Ziko1, O. Moser1,3;
1Division of Endocrinology and Diabetology, Interdisciplinary Metabolic Medicine Trials Unit, Medical University Graz, Graz, Austria, 2Division of Cardiology, Medical University Graz, Graz, Austria, 3Exercise Physiology & Metabolism, Institute of Sports Science, University of Bayreuth, Bayreuth, Germany.
Background and aims: Regular physical activity and exercise represent a corner stone in the treatment of type 1 diabetes (T1D), however, exercise-induced hypoglycemia remains the major barrier to a physically active lifestyle. Therefore, the ULTRAFLEXI-1 study compared two basal insulin analogues, Glargine U-300 (IGlar U300) and Degludec (IDeg), in two different doses (100% and 75% of the regular dose) when used around spontaneous exercise sessions in adults with T1D.
Materials and methods: We performed a randomized, single-center, four-period, cross-over trial and included adults with T1D treated with multiple daily insulin injections and an HbA1c ≤10% (≤86 mmol/mol). In each of the four 2-weeks-periods, participants attended six spontaneous evening cycling sessions (60 minutes, moderate intensity). The days of exercising were randomized and announced at 8 A.M. to the participants. The basal insulin used on the exercise days during the four periods were: IGlar U300 100% or 75% of the regular dose or IDeg 100% or 75%, respectively. 100% of the regular basal insulin dose was used at all non-exercise days. Continuous glucose monitoring was performed using a blinded Dexcom G6 device. The primary outcome was the time spent in hypoglycemia (<70 mg/dl, time below range (TBR)) during the six 24-hour post-exercise periods in the four trial arms. The difference in TBR between 100% IGlar and 100% IDeg or 75% IGlar and 75% IDeg was analyzed in hierarchical order using the repeated measures linear mixed model.
Results: 25 people were enrolled (14 male), aged 41.4±11.9 years, with a mean diabetes duration of 16.8±10.4 years and a mean HbA1c of 7.5±0.8%. (59±9 mmol/mol). Mean TBR during the 24-hour periods following the exercise sessions was 2.71±2.56% for IGlar U300 (100%) and 4.37%±3.43% for IDeg (100%) (p=0.025) as well as 2.28±2.67% for IGlar U300 compared to 2.55±2.87% with IDeg when using the 75% dose on exercise days (p=0.720).
Conclusion: Time spent in hypoglycemia after spontaneous exercise sessions was significantly lower in people with type 1 diabetes receiving IGlar U300 as compared to IDeg when the 100% dose was used.
Clinical Trial Registration Number: EudraCT: 2019-003209-89
Supported by: IIS grant to HS from Sanofi-aventis
Disclosure: H. Sourij: Grants; Böhringer Ingelheim, MSD, NovoNordisk, Sanofi. Lecture/other fees; Amgen, AstraZeneca, Bayer, Böhringer Ingelheim, Daiichi Sankyo, Eli Lilly, Kapsch, MSD, NovoNordisk, Sanofi.
SO 23 Gestational diabetes and pregnancy
431
Impaired pregravid beta cell function is associated with adipose tissue insulin resistance and increased risk of gestational diabetes
N. Michael1, S.A. Sadananthan1, J. Yaligar1, S.L. Loy2, L.J. Li3, K.H. Tan2, K. Godfrey4, P.D. Gluckman1, Y.S. Chong3, Y.S. Lee3, M. Wlodek1, S.Y. Chan3, J.K.Y. Chan2, S. Velan1, J.G. Eriksson1,3;
1Singapore Institute for Clinican Sciences, Singapore, Singapore, 2KK Women’s and Children’s Hospital, Singapore, Singapore, 3National University of Singapore, Singapore, Singapore, 4NIHR Southampton BRC & MRC LEC, University of Southampton, Southampton, UK.
Background and aims: Gestational diabetes mellitus (GDM) is hypothesized to partly reflect inadequate pancreatic β-cell responses to the increased insulin resistance seen in pregnancy. However, it is unclear if inadequate β-cell function in women with GDM emerges only during pregnancy, or could be present before pregnancy. To understand the aetiology of β-cell dysfunction in GDM, we first investigated the cross-sectional association of pregravid β-cell function with adipose tissue insulin resistance in the The Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO) study. Elevated adipose tissue insulin resistance could potentially expose β-cells to chronically elevated NEFA and NEFA-induced lipotoxicity. Next, we studied the temporal relationship between pregravid β-cell function and the risk of GDM.
Materials and methods: The S-PRESTO study recruited 1039 women (18-45y, Chinese, Malay or Indian ethnicities), planning to conceive within 1 year. The current analysis focuses on 326 women who conceived successfully within 1 year of recruitment and underwent GDM screening. OGTT was performed pregravid and at 28 weeks’ gestation, with GDM diagnosed using the International Association of Diabetes and Pregnancy Study Group criteria. At both visits, the insulinogenic index (ratio of the change in insulin between 0-30 min to the change in glucose between 0-30 min), and hepatic insulin resistance (measured using HOMA2-IR) were recorded. β-cell compensation for hepatic insulin resistance was assessed using the disposition index (DI; ratio of insulinogenic index to HOMA2-IR). Pregravid adipose tissue insulin resistance (Adipo-IR) was defined as the product of fasting plasma insulin and NEFA levels. DI and Adipo-IR were log-transformed to reduce skewness.
Results: At the pregravid visit, a 10% increase in Adipo-IR predicted a 3.5% reduction (95%CI: -5.4% to -1.5%, p = 0.001) in DI, adjusted for age, ethnicity, educational attainment, parity and pregravid BMI. 16% of the women were diagnosed with GDM. DI was consistently lower in women who were diagnosed with GDM (relative to normoglycemic women), in both the pregravid (Geometric mean (95%CI): 20.2 (15.6 to 26.1) vs 32.4 (28.9 to 36.2), p = 0.001) and the antenatal (14.3 (11.6 to 17.7) vs 27.2 (25.0 to 29.6), p<0.001) phases. Women in the lowest tertile of pregravid DI (relative to middle and upper tertiles) had a 4-fold higher odds of developing GDM (Odds Ratio (95%CI): 4.0 (1.6 to 10.3), p=0.003), adjusted for age, ethnicity, educational attainment, parity and pregravid BMI.
Conclusion: Impaired pregravid β-cell compensation for hepatic insulin resistance (as measured by lower DI) was associated with elevated Adipo-IR and characterized women who subsequently developed GDM during pregnancy. It is plausible that chronic exposure of β-cells to elevated NEFA caused by high Adipo-IR may be involved in the blunting of β-cell responses to hepatic insulin resistance. Our findings point to a potential role for pregravid interventions targeting adipose tissue and β-cell functions for improving women’s metabolic health during pregnancy.
Clinical Trial Registration Number: NCT03531658
Supported by: NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014.
Disclosure: N. Michael: Grants; This work was supported by Singapore National Medical Research Council: NMRC/TCR/004-NUS/2008, NMRC/TCR/012-NUHS/2014, Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore, Godfrey is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group (17/63/154) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP), and the British Heart Foundation (RG/15/17/3174). Lecture/other fees; Chong, Lee, Chan & Godfrey have received reimbursement for speaking at conferences sponsored by nutrition companies. Other; Godfrey, Chong & Chan are part of an academic consortium that has received research funding from nutrition companies.
432
Analysis of factors determining neonatal birth weight in patients with type 1 diabetes treated with personal insulin pump
M. Zurawska-Klis, M. Kosinski, K. Cypryk;
Department of Internal Diseases and Diabetology, Medical University of Lodz, Lodz, Poland.
Background and aims: Diabetes complicating pregnancy increases the risk of fetal growth acceleration (LGA - Large for Gestational Age). The aim of the study was to evaluate the factors influencing birth weight in patients with DM1 treated with a personal insulin pump (IP).
Materials and methods: The study included 93 pregnant women with DM1 treated with IP, 54% used continuous glucose monitoring. Medical history, data on the course of DM1, comorbidities, insulin requirements, weight gain and glycemic outcomes were analyzed.
Results: The mean age of women was 31.2±4.33 years, diabetes duration 15.9±7.43 years, pre-pregnancy BMI 23.8 (95% CI: 23.0-24.6) kg / m2, pre-pregnancy HbA1c 7.12±1.28, 23% had HbA1c below 6.5%. In the analyzed cohort, there were 40 LGA cases and 1 SGA case, which was excluded from the analysis. The patients were divided into 2 groups - the study group consisted of 40 patients with LGA newborns, the control group consisted of 52 patients. The patients did not differ in terms of anthropometric data, comorbidities or diabetes complications. Patients in the LGA group were less frequently primiparous (45% vs 67%, p=0.03), and had earlier miscarriages (25% vs 9.4%, p=0.05). Only in the LGA group, earlier deliveries of children with macrosomia (birth weight >4000g) were observed (12.5% vs 0%, p=0.012). There were no differences in insulin requirements, HbA1c, or the percentage of patients achieving the HbA1c target in consecutive trimesters (T). Mean glycemia was slightly higher in the LGA group in individual Ts, p>0.05. Only the mean glycemia before pregnancy and the time spent above 140mg/dL in the III T were higher in the LGA group - 143±27 vs 124±26 mg/dL, respectively, p=0.05 and 37.3±19.5% vs 29.7±13.6%, p=0.05. Apart from an increased percentage of macrosomia and birth weight, no differences in obstetric outcomes were observed. In the LGA group, 23 newborns (57.5%) had macrosomia, and in the control group 5 (9.4%) newborns. Correlations between the incidence of LGA and the history of miscarriage (r=0.214), macrosomia (r=0.28) and parity (r=0.22) were observed, all p<0.005. There were correlations between the occurrence of LGA and mean glycaemia before pregnancy, in II and III T (r: 0.396, 0.220, 0.299, respectively), time spent in the range (TIR) in II and II T (r=0.214 and 0.221), maternal body weight in II and III T (r=0.20 and 0.264) and maternal weight gain in pregnancy (p=0.222), all p<0.05. There was no correlation between LGA frequency and insulin doses.
Conclusion: In women with type 1 diabetes treated with IP, the incidence of LGA is associated with the observed pre-pregnancy glycaemia, in II and III T, and TIR in these trimesters. The burden of obstetric medical history also influences the incidence of LGA.
Disclosure: M. Zurawska-Klis: None.
433
Prolactin levels associate with insulin secretion in pregnancy and are reduced in multi- vs nulliparous women
D. Löffler1,2, J. Hummel3,2, L. Sandforth1,2, A.L. Birkenfeld1,3, H. Preissl3,2, A. Peter4,3, A. Fritsche1,3, R. Wagner2, M. Heni4,3, L. Fritsche3,2;
1University Hospital Tübingen, 2German Center for Diabetes Research (DZD e.V.), 3Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Center Munich at the University of Tübingen, 4Institute for Clinical Chemistry and Pathobiochemistry, University Hospital Tübingen, Tuebingen, Germany.
Background and aims: Pregnancy is a state of physiological insulin resistance and gestational diabetes (GDM) develops when this cannot be compensated by sufficient increase in insulin secretion. To meet the increased insulin requirements, β-cells adapt during the course of pregnancy. Animal models suggest that this process is regulated in part via the hormone prolactin. While GDM risk increases with age and parity, impact of prolactin in GDM pathogenesis is currently unclear. We aimed to investigate the association of prolactin with insulin secretion and the effect of parity on prolactin levels in the last trimester of pregnancy in a cohort of deeply phenotyped women.
Materials and methods: We used data from the ongoing PREG study. Women with a singleton pregnancy had a 5-point OGTT with 75 g glucose. GDM was diagnosed according to the IADPSG criteria. Insulin sensitivity was assessed with NEFA-insulin sensitivity index and insulin secretion with AUCC-peptide 0-30/ AUCGlucose 0-30. Fasting prolactin was measured in serum by a commercial chemiluminescence assay. Associations of prolactin with insulin sensitivity and parity were analyzed with multivariable linear regression models adjusted for age, BMI and gestational age. Models for the outcome insulin secretion were additionally adjusted for insulin sensitivity. Post hoc comparisons of level contrasts were corrected with Bonferroni method.
Results: We examined 503 women during gestational week 27.5 (SD 2.4), of these 137 were diagnosed with GDM. Women with GDM had lower insulin secretion (p<0.001), lower insulin sensitivity (p<0.001) and lower prolactin levels (131 (54) vs. 144 (61) μg/l, punivariate=0.027). Prolactin associated negatively with insulin sensitivity (p=0.0108) and positively with insulin secretion (p=0.019). We found a significant negative association of parity with prolactin levels (p<0.001). Nulliparous women had the highest prolactin levels, while women with one or more previous pregnancies had significantly lower prolactin levels (post hoc tests 0 vs 1 pregnancy p<0.001, 0 vs 2 and more pregnancies p<0.001, 1 vs. 2 and more pregnancies p=0.0373). The negative association of number of parities with prolactin was independent of age, BMI and GDM status and was also observed in a subgroup of 209 nulli- and 209 multiparous women matched for age, BMI and gestational age.
Conclusion: Our data indicate that with each subsequent pregnancy, the physiological prolactin levels are lower. Since animal data and our correlative findings suggest a stimulatory effect on beta-cells, this phenomenon could contribute to reduced insulin secretion and higher GDM risk with increasing number of pregnancies.
Clinical Trial Registration Number: NCT04270578
Supported by: BMBF(01GI0925) to DZD
Disclosure: D. Löffler: None.
434
Hypoglycaemia increases during pregnancy after gastric bypass: the Bariatric surgery And consequences for Mother and Baby In pregnancy (BAMBI) study
L.L. Stentebjerg1,2, L.R. Madsen3,4, R.K. Støving5,2, L.T. Andersen6,2, C.A. Vinter6,1, C.B. Juhl7,8, D.M. Jensen1,6;
1Steno Diabetes Center Odense, Odense University Hospital, Odense, 2Department of Clinical Research, University of Southern Denmark, Odense, 3Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, 4Danish Diabetes Academy, Odense University Hospital, Odense, 5Department of Endocrinology, Odense University Hospital, Odense, 6Department of Gynecology and Obstetrics, Odense University Hospital, Odense, 7Department of Endocrinology, Hospital of South West Jutland, Esbjerg, 8Department of Regional Health Research, University of Southern Denmark, Odense, Denmark.
Background and aims: Roux-en-Y Gastric bypass surgery (RYGB) is complicated by subsequent alterations in glucose profiles partly due to an enforced incretin response. The increased insulin resistance during pregnancy poses an additional challenge to glucose metabolism in women treated by RYGB. However, the magnitude and impact of this has only been sparsely investigated. The aims of the BAMBI study were to investigate the interstitial glucose (IG) profiles during the course of pregnancy and the incidence of hypoglycemic events in pregnant women previously treated by RYGB compared to matched controls.
Materials and methods: In the prospective BAMBI study, 23 pregnant women with RYGB and 23 BMI- and parity-matched pregnant controls were studied with continuous glucose monitoring (CGM) in the 1st, 2nd, and 3rd trimester as well as 4-6 weeks postpartum. Time in range (TIR) was defined as time with IG of 3.5-7.8 mmol/l. The primary outcomes were time below range (TBR) (%), and the incidence of hypoglycemic events (< 3.0 and 3.5 mmol/l) quantified by CGM.
Results: Pregnancies occurred 30 (IQR: 15.4-98.1) months following RYGB with a reduction of BMI from 45.2 kg/m2 (IQR: 42.0-54.3) pre-surgery to 31.5 kg/m2 (IQR: 26.5-39.1) pre-pregnancy. TIR was significantly lower throughout pregnancy and postpartum for the RYGB group compared to controls (87.3-89.5% vs. 93.7-96.1%, p<0.01). TBR increased from the 1st trimester (1.0%, SD 1.5) to the 2nd (2.1%, SD 3.2) and 3rd trimester (2.0%, SD 2.7) and returned to a reduced TBR postpartum (0.6%, SD 0.7). Similarly, the median weekly number of hypoglycemic events increased during pregnancy for the RYGB group with 0, 0.8, 0.4 and 0 weekly events < 3.0 mmol/l, and 1.6, 2.4, 2.9 and 1.1 weekly events < 3.5 mmol/l in the 1st, 2nd, and 3rd trimester and postpartum, respectively. The median weekly number of hypoglycemic events for the controls was 0 for both the 3.0 and 3.5 cut-off.
Conclusion: The results of the BAMBI study show that women treated by RYGB pre-pregnancy spend more time in TBR and are more exposed to hypoglycemia than matched controls. Further research is warranted investigating if the increased risk of fetal growth restriction among pregnant women treated with gastric bypass is associated with exposure to hypoglycemia during pregnancy.
Clinical Trial Registration Number: NCT03713060
Supported by: LLS received funding from the Region of Southern Denmark
Disclosure: L.L. Stentebjerg: Grants; Region of Southern Denmark.
435
Body composition and effect of insulin treatment during pregnancy in Socs2-/- mice with gestational diabetes and macrosomia
L. Hernandez-Baraza1, C. Valverde-Tercedor1, M. Díaz1, A.M. Wägner1,2, L. Fernández-Pérez1, B. Guerra1, Y. Brito-Casillas1;
1Instituto Universitario en Investigaciones Biomédicas y Sanitarias, 2Complejo Hospitalario Universitario Insular Materno Infantil, Las Palmas de Gran Canaria, Spain.
Background and aims: The Suppressor of Cytokine Signaling 2 (SOCS2) protein modulates cytokine response, growth, inflammatory processes, and cytokine-mediated metabolism of lipids and carbohydrates. Thus, its ablation in mice (Socs2-/-) generates gigantism and insulin-resistance as well as gestational diabetes (GDM) and macrosomia with high mortality rates (>88%). Our aim is to evaluate the body composition variations in pregnant Socs2-/- as a potential early diagnostic tool for macrosomia. Additionally, we assess the potential use of insulin to prevent foetal macrosomia.
Materials and methods: Body Weight (BW) and composition (lean, fat and fluid) were evaluated in 7 Socs2-/- and 4 C57BI/6J pregnant females (age: 196.3±28 days,) using an NMR-TD spectrometer (Minispec+ LF90II). Basal glucose was measured at days 7 and 14 of pregnancy (first and second gestational thirds) using a glucometer (Glucomen Areo Menarini). Besides, Socs2-/- mothers and offspring were retrospectively analyzed and compared for the presence of macrosomia, based on whether the mother was insulin-treated (0.5U/kg, Glargine) or not during pregnancy (3 females with 22 neonates vs 21 females with 137 neonates, respectively). Macrosomia in this strain was previously defined as >1.43 g birth weight. Mann-Whitneys’s U, Student’s test and Chi2 test were used for comparisons.
Results: Basal blood glucose did not differ between groups for the whole evaluation (Socs2-/-: 151±2.19 mg/dL; control: 144±13.63 mg/dL) (p=0.46). BW of Socs2-/- tended to be greater at day 7 compared to controls (29.5±2.2 vs 25.7±2.1g, respectively; p=0.063) and was significantly higher at day 14 of pregnancy (36±3.2 vs 28.2±2.3g, respectively; p=0.006). Fat percentage was higher in controls (7d: 26.2±3.1%; 14d: 21.3±2.8%) than in Socs2-/- (7d: 10.6±2.1%; 14d: 11±2.9%) (p=0.016; p=0.012). Fluid content was reduced in Socs2-/- compared to controls at 7d (6.06±1.16 vs 9.3±0.7%, p=0.016), but not at 14d (10.3±1.0 vs 11.7±1.5%; p=0.16). Finally, differences were no significantly (p>0.05) in lean percentage on days 7 and 14 between control (7d: 79.9±2.9%; 14d: 80.5±3.0%) and Socs2-/- (7d: 63.3±16.3%; 14d: 71.9±6.9%). Neonates from untreated mothers were heavier than offspring of mice receiving insulin (1.5±0.2g vs 0.8±0.4g, respectively; p<0.001) and the prevalence of macrosomia was higher, too (65.9 ±24.01% vs 20 ±34.6%, respectively; p=0.007).
Conclusion: Although it is necessary to extend the analysis of body composition to more animals and later pregnancy periods to assesses if these variables indeed predict macrosomia, actual results show weight, fat and fluid as parameters with potential noninvasive pre-diagnostic interest for macrosomia. Insulinization of pregnant Socs2-/- resulted in almost 50% decreased occurrence of macrosomia, reinforcing a novel in vivo model for GDM. New specific insulin evaluations will be conducted to define its potential to ameliorate the severity of macrosomia. Further studies should be performed to fully elucidate the potential role of SOCS2 in the development of GDM.
Disclosure: L. Hernandez-Baraza: None.
436
Insulin sensitivity, growth differentiation factor-15 and fetuin-B during pregnancy after assisted reproductive therapy in the STORK cohort
E. Qvigstad1,2, M.-C.P. Roland3, K. Godang1, T. Lekva4;
1Dept of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, 2Institute of Clinical Medicine, University of Oslo, 3Dept. of Obstetrics and Gynaecology, Oslo University Hospital, 4Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway.
Background and aims: Assisted reproductive therapy (ART) may increase the risk of gestational diabetes (GDM) and glucose intolerance after pregnancy. We have previously shown reduced insulin sensitivity (IS) in pregnant women after ART. To gain more insight in this association we have investigated fetuin-B (fet-B) and growth differentiation factor-15 (GDF-15), that both increase with low IS such as in GDM, comparing pregnant women after ART with controls (CTR) with normal glucose tolerance from the STORK cohort.
Materials and methods: In STORK, 1031 healthy pregnant Norwegian women attended OGTTs at gestational week (GW) 14-16 and 30-32. 75 women had conceived after ART, and 847 women were glucose tolerant (WHO 2013 criteria), and served as CTR. From the 5-point OGTT glucose and insulin results, we calculated IS (Matsuda). In a representative subsample, we analyzed fasting plasma levels of fet-B and GDF-15 (ELISA). In addition we explored if BMI at GW 14-16 (BMI below/above 25 kg/m2) influenced these biomarkers and IS levels.
Results: The ART women were 32.8 years (SD 3.5), ie. 1.8 years older than the CTR women (p<0,001). IS was significantly lower in ART women in GW 14-16 and GW 30-32. In addition, Fet-B differed between the groups and was 11% lower in ART at GW 30-32, p<0,05. However, by stratifying the ART women by BMI below/above 25 kg/m2, we found improved IS at GW 14-16 and 30-32 in the normal weight ART women (26 and 33 % resp., both p <0,01). The effect of ART on IS remained significant after adjustment for maternal age, BMI, family history of diabetes and smoking in early pregnancy, but not at GW 30-32. The regression analysis demonstrated BMI as the main variable for IS levels at both time points, GDF-15 was also a significant factor for IS at GW 30-32 (p<0,018).
Conclusion: The reduced IS in pregnant women after ART, was not paralleled by the expected increases in the measured biomarkers in pregnancy, when compared to CTR women. Our data suggest that ART treatment exerts a negative influence on insulin sensitivity during gestation. However, as BMI remains the most important variable to explain IS, also after ART, IS might be improved by lifestyle change, similarly to proven interventions for GDM.
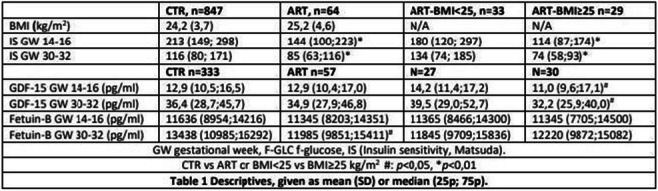
Supported by: JS Kvanes endowment, Norway
Disclosure: E. Qvigstad: Grants; 4200 euros for biomarker analysis from J.S.Kvanes' endowment for diabetes research, Norway.
437
Metabolic syndrome prevalence in women with gestational diabetes
V. Bartakova1, K. Chalasova1, L. Pacal1, P. Janku2, K. Kankova1;
1Dept. of Pathological Physiology, Masaryk University, 2Dept. of Obstetric and Gynaecology, University Hospital Brno, Brno, Czech Republic.
Background and aims: Gestational diabetes mellitus (GDM) is one of the most common complications in pregnancy. Women with GDM have a higher occurrence of peripartal complication as well as an increased risk of type 2 diabetes mellitus, metabolic syndrome (MS) and subsequent cardiovascular diseases after delivery or anytime later. However, MS could precede gravidity and GDM could represent manifestation of one of its components. The aims of the study were (i) to detect the prevalence of MS in women in the time of GDM diagnosis, (ii) to detect the prevalence of MS in the sub-group of GDM patients with any form of impaired glucose tolerance after delivery, and (iii) to find whether GDM women with MS have a higher risk of peripartal adverse.
Materials and methods: Study comprised n=455 women with GDM enrolled during 2013-2019. Following GDM diagnosis, subjects were followed in Diabetology centre in Faculty Hospital Brno, 65% (n=295) of them delivered in Faculty Hospital Brno and data on delivery were thus retrievable. 48% (n=219) GDM patients underwent repeated oGTT test up to 1 year after delivery with 11.4% (n=25) manifesting persistence or early conversion of GDM to permanent glucose intolerance (diabetes or prediabetes, evaluated according to WHO criteria). GDM was diagnosed according to IADPSG criteria. IDF criteria for MS definition were modified to pregnancy situation as a presence of a minimum of 3 of 5 criteria: GDM, BMI before pregnancy ≥30 kg/m2, blood pressure >130/85mmHg, triacyl-glyceroles (TAG) >1.7mmol/l, high density lipoproteins (HDL) <1.3mmol/l (with exception of BMI all parameters were evaluated in the second trimester of pregnancy). Following perinatal data were analysed: offspring weight (macrosomia), length of delivery, necessity of delivery induction or instrumental delivery, unplanned Caesarean section, Apgar score value.
Results: Fully developed MS was detected in 22.6% (n=103) of GDM patients at 24th-28th week of pregnancy. In those with any form of persistent glucose intolerance after delivery the prevalence of MS was as high as 40%. Presence of MS in GDM patients was statistically significantly associated with two peripartal outcomes: higher incidence of pathologic Apgar score and macrosomia (P=0.01 resp. P= 0.0004, chi-square test).
Conclusion: Presence of MS in GDM patients is a statistically significant risk factor (P= 0.04 chi-square test) for persistence of impaired glucose tolerance after delivery and selected adverse peripartal outcomes.
Supported by: grant nr. NV18-01-00046, Ministry of Health, Czech Republic
Disclosure: V. Bartakova: None.
SO 24 Life with diabetes from conception to delivery
438
Pregnancy in women with hyperglycaemia in pregnancy: impact of multiples vulnerabilities on fetal growth
H. Bihan1, C. Nachtargaele2, E. Vicaud2, M. Sal1, N. Berkane1, S. Pinto3, S. Tatulashvili1, M. Fermaut4, L. Carbillon4, E. Cosson1;
1Endocrinology, Diabetology, Nutrition, Avicenne Hospital, Bobigny, 2Université Denis Diderot, 75009 Paris, AP-HP, Unité de Recherche Clinique St-Louis-Lariboisière, Paris, 3Diabetology, Jean Verdier Hospital, Bondy, 4Department of Obstetrics and Gynecology, Jean Verdier Hospital, Bondy, France.
Background and aims: In women with hyperglycemia in pregnancy (HIP), psychosocial deprivation could be associated with earlier and higher exposure to hyperglycemia and a poorer maternofetal prognosis. Other vulnerabilities, such as food insecurity and low French proficiency, could influence prenatal care adherence and outcomes.
Materials and methods: We analyzed the links between individual deprivation, food insecurity, national language proficiency and fetal growth (main criterion), a composite HIP-related outcomes, and other core outcomes in a multi-ethnic cohort of women who were cared for HIP (childbirth between 01/01/2012 and 31/12/2018) in a specific program in a deprived area.
Results: Among 1,168 women (36% from North Africa, 15% from sub-Saharan Africa, 17.3% from South Asia), 55.4% suffered from deprivation, 17.9% from food insecurity, and 27.5% from low national language proficiency. Insulin therapy was prescribed to 43.3% of women. The fetal growth categories (small- or adequate- or large-for-gestational-age infants: SGA/AGA/LGA, respectively) were similar regardless of the level of deprivation or food insecurity, with 11.4% of SGA, 76.5% of AGA, and 12.2 % of LGA infants. Paradoxically, women with a low National language proficiency had fewer events for composite criteria, mediated by fewer LGA (8.3%) but higher SGA infants (16.7%).
Conclusion: Optimized single-center care with specialized follow-up could contribute to reduce health inequalities in women with HIP. The higher risk of SGA in women with low National language proficiency suggests paying special attention to possible dietary restrictions by misunderstanding dietary advice.
Disclosure: H. Bihan: None.
439
The relationship between clinically significant neonatal hypoglycaemia and cord blood C-peptide levels in neonates of mothers with type 1 diabetes
G. Aharon-Hananel1, K. Levi1, R. Hemi1, E. Barhod1, O. Kordi-Patime1, S. Mazaki-Tovi2, R. Yoeli-Ullman2, T. Cukierman-Yaffe1;
1Division of Endocrinology, Diabetes and Metabolism, The Chaim Sheba Medical Center, Tel Hashomer, 2Department of Obstetrics and Gynecology, The Chaim Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel.
Background and aims: Neonate of patients with type I diabetes (T1D) are at increased risk for neonatal hypoglycemia. It is hypothesized that this is a result of maternal hyperglycemia and subsequent fetal hyperinsulinemia. The aim of this study was to determine the relationship between clinically significant neonatal hypoglycemia (CS-hypo) and cord-blood c-peptide (CBCP) concentrations in patients with T1D.
Materials and methods: This was a prospective cohort study including patients with T1D followed at a single tertiary center. Clinical variables and glucose control data during pregnancy were prospectively recorded. Cord-blood of neonates was collected, and CBCP concentration was determined. The correlation between CS-hypo (neonatal hypoglycemia requiring IV glucose treatment) and CBCP concentrations was determined.
Results: This analysis pertains to 54 pregnancies. Mothers to neonates that experienced CS-hypo had longer diabetes duration (19 vs. 13 years, p=0.023), higher HbA1c at conception (7.3 [6.3-8.8] vs. 6.5 [6.0-7.0], p=0.042) and higher rates of caesarian section (73.3% vs. 28.2%, p=0.005) than mothers to neonates who did not. No differences were observed between the groups in BMI, age, and other maternal complications, nor in glucose control indices. CBCP levels were significantly higher in neonates with CS-hypo than in those who did not (3.3 mcg/L vs 1.9 mcg/L, p=0.002). After adjustment for age at conception, BMI, diabetes duration, neonatal birth weight and 3rd trimester HbA1c, every 1 unit higher in CBCP level was associated with a 1.46 (1.02-2.09, p=0.035) fold greater risk for CS-hypo.
Conclusion: In neonates of patients with T1D, higher CBCP levels are associated with a higher risk for neonatal hypoglycemia.
Disclosure: G. Aharon-Hananel: None.
440
The contribution of maternal glucose to birth weight is less in Uganda (sub-Saharan Africa) compared to white and black ethnic groups in the HAPO study
W.P. Nakanga1, I. Sekitoleko2, R.C. Andrews1, T. Salome2, A.G. Jones1, B.M. Shields1, A.T. Hattersley1, M.J. Nyirenda2;
1University of Exeter, Exeter, UK, 2Medical Research Council/ Uganda Virus and Research Institute and LSHTM Uganda Research Unit, Entebbe, Uganda.
Background and aims: Maternal glycaemia has a significant influence on fetal birth weight and maternal and fetal morbidity. Glycaemia measured in a third-trimester oral glucose tolerance test shows a linear relationship with birth weight and other birth complications. The Hyperglycaemia Adverse Pregnancy Outcomes (HAPO) study established a similar relationship between glucose and birth weight in multiple ethnic groups, and they proposed cut-off criteria where the risk of large for gestational age (LGA) was 1.75 times greater than the population risk. However, the HAPO study did not include any cohorts from sub-Saharan Africa (SSA), where 17% of the world population lives. No studies have examined the relationship between maternal glycaemia and fetal birth weight in SSA. This study aims to address this.
Materials and methods: We compared the relationship between oral glucose tolerance test (OGTT) glucose measures and obstetric outcomes in participants from Uganda (n=2544), black participants in HAPO (n=1224) and white participants in HAPO (n=7679). We used multivariable linear regression to assess the correlation between birth weight corrected for gestational age and sex with maternal glucose concentration. Logistic regression was used to determine the association of LGA (defined as birthweight > 90th percentile) and maternal fasting glucose. We compared the relationship between oral glucose tolerance test (OGTT) glucose measures and obstetric outcomes in participants from Uganda (n=2544), black participants in HAPO (n=1224) and white participants in HAPO (n=7679). We used multivariable linear regression to assess the correlation between birth weight corrected for gestational age and sex with maternal glucose concentration. Logistic regression was used to determine the association of LGA (defined as birthweight > 90th percentile) and maternal fasting glucose.
Results: The contribution of maternal fasting glucose to birth weight is lower in Uganda than in other settings (β-coefficient (95%CI) 104(58.6 to 149)g/mmol/L in Uganda, 203(137 to 269) HAPO-black, and 239(214 to 265) HAPO-white. Likewise, the risk of LGA with higher fasting glucose was smaller in Uganda compared to the other studies (adjusted odds ratio (95%CI) 1.07 (0.94 to 1.23) in Uganda, 1.29 (1.22 to 1.38) HAPO-black, and 1.34(1.24 to 1.45) HAPO-white. Analysis using 1-hr and 2-hr post glucose load concentrations produced similar results.
Conclusion: We found that associations between maternal glucose and fetal birth weight are substantially lower in our SSA cohort than in countries recruited into the HAPO study. This suggests that cut-offs for pregnancy generated from other study populations need to be confirmed in SSA before they are adopted.
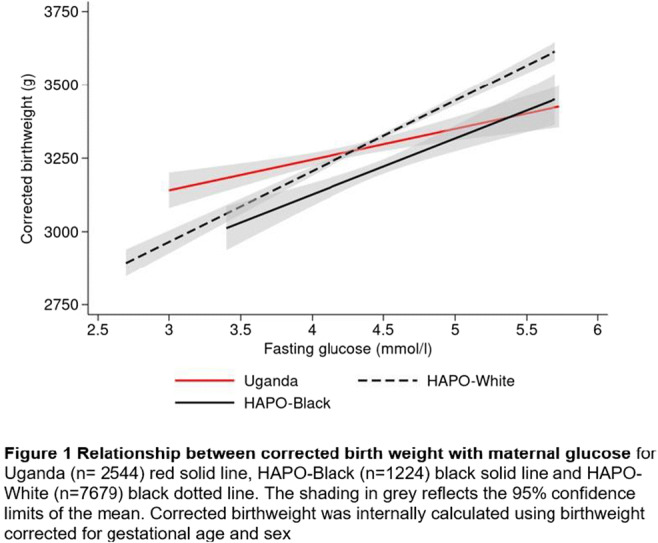
Supported by: MRC
Disclosure: W.P. Nakanga: None.
441
The use of intermittently scanned CGM vs sensor augmented pump in a cohort of pregnant women with type 1 diabetes: efficacy on glycaemic control and pregnancy outcomes.
C. Font1, N. Seguí1, V. Perea2, M. Rabassa3, D. Roca1, J. Bellart1, M. Giménez1, I. Conget1, I. Vinagre1;
1Hospital Clínic de Barcelona, 2Hospital Mutua Terrassa, 3Universitat de Barcelona, Barcelona, Spain.
Background and aims: SAP (sensor augmented pump) therapy has been shown to improve metabolic control and perinatal morbidity in pregnant women with type 1 diabetes (T1D.) Few studies have compared the use of SAP with treatment with multiple doses of insulin (MDI) and intermittently scanned CGM (isCGM) in pregnant women with T1D. The objective of this study is to evaluate possible differences in metabolic control and maternal-fetal outcomes between both systems in these pregnant women.
Materials and methods: Cohort study in pregnant women with T1D controlled in two tertiary hospitals. The use of the SAP system (640G pump) was compared with FSL monitoring and MDI treatment in the period 2018-2021. The glucometric indicators for 14 days per trimester in the SAP group were obtained through the Carelink platform and those corresponding to the FSL group through Libreview.
Results: 97 pregnant women (n=22 with SAP) were included. Pre-pregnancy control was higher in the SAP group, with no other significant differences between the two groups. Regarding glycemic control, in the FSL group, the % of time in range (63-140 mg/dl) was greater in the 1st (62 vs. 58%, p=0.036), 2nd (64 vs. 52%, p =0.001) and 3rd trimester (73 vs. 68%, p=0.027), and mean glycemia was lower in the 1st (117 vs. 132mg/dl, p=0.007), 2nd (118 vs. 133mg/dl, p =0.001) and 3rd trimester (109 vs. 120mg/dl, p=0.001), while the % of time in hypoglycemia (<63mg/dl) was higher in the 2nd (7 vs. 4%, p=0.015) and 3rd trimester (6 vs. 4%, p=0.028). There were no differences in HbA1c or in the rate of severe hypoglycemia (Table). Regarding neonatal complications, a higher incidence of hyperbilirubinemia was observed in the SAP group (50 vs. 24.32%, p=0.021), which was not confirmed when adjusting for confounding factors (maternal age, body mass index and gestational age at first visit). There were no significant differences in the incidence of macrosomia, neonatal hypoglycemia, malformations, and perinatal mortality, or in the rate of maternal complications between the two groups.
Conclusion: Despite some increase in hypoglycemia exposure, MDI+isCGM is equally effective and safe in terms of management and neonatal/maternal complications in comparison with the use of SAP in pregnant women with T1D.
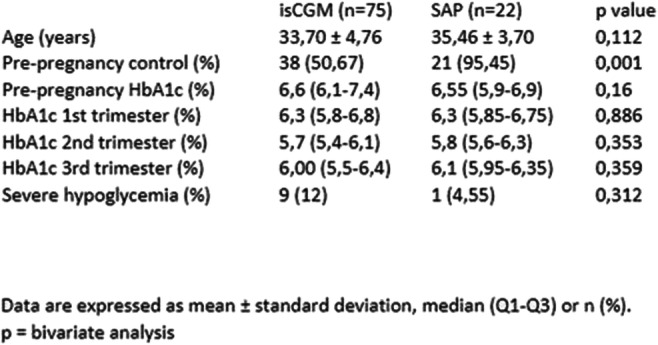
Disclosure: C. Font: None.
442
Are pre-pregnancy body mass index and gestational weight gain associated with maternal and infant adverse outcomes in French women with type 1 diabetes?
A. Vambergue, V. Laurent, M. Lemaitre, C. Ternynck, J. Bourry, F. Baudoux, D. Subtil;
CHRU, Lille, France.
Background and aims: Pregestational body mass index (BMI) and excessive gestational weight gain are associated with several adverse events during pregnancy. Our objective was to investigate the associations between prepregnancy BMI, gestational weight gain (GWG) and maternal-infant adverse outcomes in a french cohort of women with Type 1 diabetes (TD1).
Materials and methods: 771 women with T1D who delivered singleton infant at University Medical Center from 1997 to 2021 were included in this observational study. Regarding pregestational BMI, we defined 3 classes: normo-weight (BMI < 25 kg/m2), overweight (BMI ≥ 25 and < 30 kg/m2) and, obesity (BMI ≥ 30 kg/m2) according to WHO criteria. GWG were defined (low; adequate or high) according to the 2009 Institute of Medicine guidelines. We studied the following outcomes: maternal composite criterion (at least the presence of one among the appearance or aggravation of retinopathy, proteinuria, gravidic hypertension, pre-eclampsia, post-partum hemorrhage or ketoacidosis decompensation), fetal composite criterion (at least the presence of one among prematurity, shoulder dystocia, severe malformations, respiratory distress or neonatal ICU admission) and LGA. We assessed the association of BMI and GWG with pregnancy outcomes using logistic regression models before and after adjustment in confounding factors. The results were expressed in odds ratios (OR) and their 95% confidence intervals (CI).
Results: 61.1% of women with T1D were normo-weight, 24.4% with overweight and, 13.5% with obesity. We observed no association of BMI classes with maternal or fetal composite criteria or with LGA, before or after adjustment for confounding factors. 18.3% women had a low GWG, 31.9% an adequate GWG and, 49.8% a high GWG. We didn’t find any association between GWG and maternal or fetal composite criteria. But we observed a significant association between GWG and LGA. Using adequate GWG as reference, high GWG were associated with an increased rate of LGA (OR, 1.47; 95% CI, (1.02-2.11); p = 0.038) after adjustment in confounding factors.
Conclusion: In our study, pregestational BMI classes were not associated higher risk of adverse and infant outcomes. However, gestational weight gain greater than guidelines recommendations within recommended levels was associated with LGA.
Disclosure: A. Vambergue: None.
443
Does the prognosis of gestational diabetes differ whether it is diagnosed through fasting and/or post-load high glucose values?
E. Cosson1, M. Sal1, N. Berkane1, S. Pinto2, C. Nachtergaele3, S. Tatulashvili1, J.-J. Portal3, E. Vicaut3, L. Carbillon4, H. Bihan1;
1Diabetology-Endocrinology-Nutrition, AP-HP, Université Sorbonne Paris Nord, Bobigny, 2Diabetology-Endocrinology-Nutrition, AP-HP, Université Sorbonne Paris Nord, Bondy, 3Fernand Widal - URC, AP-HP, Université Sorbonne Paris Nord, Paris, 4Gynecology-Obstetrics, AP-HP, Université Sorbonne Paris Nord, Bondy, France.
Background and aims: Prognosis of gestational diabetes mellitus (GDM) may differ according to whether HIP has been diagnosed by high fasting glucose values only (isolated fasting GDM), post-OGTT glucose values only (isolated post-OGTT GDM) or both (fasting and post-OGTT GDM).
Materials and methods: From a multiethnic prospective study, we included women screened for GDM by a complete OGTT after 22 weeks of gestation (IADPSG/WHO criteria). We evaluated the risk for adverse pregnancy outcomes by GDM categories.
Results: We included 7,190 women, 30.1±5.5 years-old, body mass index 24.7±4.9 kg/m². There were 6,535 without GDM, 217 (3.0%) women with isolated fasting GDM, 310 (4.3%) with isolated post-OGTT GDM and 128 (1.8%) with fasting and post-OGTT GDM. The rates of Caesarean section were 19.4, 18.0, 27.7 and 24.2%; preeclampsia 1.8, 0.9, 1.9 and 2.3%; large-for-gestational-age (LGA) infants 8.5, 18.4, 12.3 and 20.3%; neonatal hypoglycemia 0.6, 1.0, 1.4 and 4.2% in the four groups, respectively. After adjusting for confounders (age, maternal body mass index, maternal height, ethnicity, smoking during pregnancy, family history of diabetes, parity, sex of the infant, hospitalization during pregnancy), as compared to no GDM, there was no increase in Caesarean section and preterm delivery in women with the three GDM categories. The adjusted odds ratio [95% confidence interval] for LGA infant were increased for isolated fasting GDM (1.98 [1.23-3.19]) and fasting and post-OGTT GDM (1.84 [1.25-2.71]) but not isolated post-OGTT GDM (1.32 [0.92-1.90]).
Conclusion: Despite glucose-lowering care (goal for fasting glucose < 5.3 mmol/l; 2-hour post prandial glucose < 7.8 mmol/l), GDM is associated with more LGA infants than no GDM, especially when fasting plasma glucose is high during diagnostic OGTT. As GDM is diagnosed when fasting plasma glucose is 5.1 mmol/l or higher, it might be beneficial to lower fasting glucose value under this threshold throughout pregnancy.
Disclosure: E. Cosson: None.
444
Polyhydramnios in early pregnancy with normal OGTT is an indicator for further investigation regarding suboptimal glucose patterns in In Vitro Fertilisation pregnancies
P. Thomakos1, C. Barreto1, A. Korantzis2, O. Kepaptsoglou1, I. Sklavounos3, E. Faros4, C.S. Zoupas1;
1Diabetes Center & Clinic Hygeia General Hospital, 2Iaso Maternity Hospital, 3Mitera Maternity Hospital, 4Lito Maternity Hospital, Athens, Greece.
Background and aims: In Vitro Fertilization (IVF) has become a popular method of assisted reproduction. IVF has an increased incidence of complications, including polyhydramnios in early pregnancy. Polyhydramnios is associated with adverse pregnancy outcomes and high risk of perinatal mortality. Increased amniotic fluid is an indicator for impaired glucose metabolism. Maternal blood glucose (BG) levels have shown a strong continuous association with pregnancy complications. The aim of this study was to identify the presence of suboptimal BG patterns in IVF pregnancies with normal OGTT complicated by polyhydramnios before the 24th week of gestation. In addition, the impact of intensive glucose management on increased amniotic fluid was examined.
Materials and methods: This study presents 86 IVF pregnancies diagnosed with polyhydramnios without congenital malformations and normal OGTT before the 24th week. In this group the presence of suboptimal BG patterns (BG levels >90 fasting [FBG] or >120 md/dL 1hour postprandial [PPBG]) were detected using SMBG (70 individuals) and/or blind CGMS (16 individuals) for 7 days. Polyhydramnios was diagnosed using the 4 quadrant Amniotic Fluid Index (AFI) ≥24cm and/or Deepest Vertical Pocket (DVP)>8cm. The CGMS BG levels were as follows Mean Sensor BG: 96.2±23 mg/dL, time>120 mg/dL: 295±38 min/24h. Clinical characteristics include the following age: 36±4 years, BMI: 24.1±3.7 kg/m2, OGTT: 0’=85±5, 60’=154±23, 120’=128±22 mg/dL, HbA1c: 5.4±0.2%, Hadlock (BPD-HC-AC-FL) percentile: 55.8±12%, AFI:25.1±2.8 cm, DVP: 8.7±1.2 cm. U/S polyhydramnios confirmation: 18.7±4 week. Insulin therapy was initiated to obtain tight glycaemic control and was adjusted to reach the BG target <90 FBG and <120 mg/dL PPBG. Mean dosage required over the pregnancy duration was 42±20 IU. Polyhydramnios was monitored at least every 2 weeks by U/S after the initiation of insulin treatment.
Results: After insulin treatment (mean duration 30±18 days) the AFI was reduced to less than the 24cm threshold (21.7±1.3 cm - delta 3.5±3 cm) and the DVP was reduced to less than the 8cm threshold (7.3±1 cm - delta 1.4 ±1 cm). Fasting BG: 81.9±5 mg/dL, 1h-PPBG: 107.9±8 mg/dL. Pregnancy outcome Weight gain: 11±2 kg, Week of delivery: 37.6±1.8, birth weight: 2980±410 g, Small for Gestational age: 6.9%, Large for Gestational age: 4.6%, RDS: 13.9%, Neonatal Hypoglycemia: 5.8%, Preeclampsia rate: 4.6%, Caesarean Section (CS): 80.2%. The rate of CS was affected subjectively by the obstetricians' and mothers’ preference.
Conclusion: Polyhydramnios is an indication of suboptimal BG patterns in IVF pregnancies with normal OGTT and no congenital malformations. In IVF pregnancies with polyhydramnios this study suggests a need to further screen for abnormal BG patterns before the 24th week of gestation even with normal OGTT. SMBG and/or CGMS could be used to identify suboptimal BG patterns before the 24th week of gestation. Additional investigation of the efficacy of the current GDM screening and the target range for BG levels in IVF pregnancies must be considered. Intensive insulin therapy plays an important role in optimizing glycemic control and reducing polyhydramnios.
Disclosure: P. Thomakos: None.
445
Free fatty acid-induced alterations in hepatic glucose metabolism during gestation in prediabetic mice
M. Liebmann, M. Asuaje Pfeifer, S. Scherneck;
Institute of Pharmacology, Toxicology and Clinical Pharmacy, Technische Universität Braunschweig, Braunschweig, Germany.
Background and aims: Gestational diabetes (GDM) is a complex metabolic disease that is typically diagnosed for the first time during pregnancy. The main characteristic of GDM is impaired glucose tolerance rather than persistent hyperglycemia, as in type 2 diabetes (T2DM). Further, GDM depends on different underlying pathomechanisms, these include adaptation defects in both beta cell mass and insulin secretion, severity of insulin resistance, and the presence of preconceptional (pc.) prediabetes. New Zealand obese (NZO) mice exhibit impaired glucose tolerance (IGT) before and during pregnancy, pc. hyperinsulinemia and develop hyperlipidemia with elevated plasma free fatty acids (FFA) during gestation, but no overt diabetes. In this regard, plasma FFA can be used as a marker, but they may also play a functional role in the development of GDM. Therefore, the aim of this study was to investigate the modulatory effects of FFA on the hepatic glucose metabolism in prediabetic mice during gestation.
Materials and methods: Female NZO mice and NMRI controls were examined pc. and on day 14.5 of gestation (d14.5). Animals were sacrificed at 9-10 weeks of age, livers were harvested and primary hepatocytes (PH) were cultured and examined untreated and after stimulation with insulin and co-stimulation with FFA (palmitic acid, 250 μM). AKT activity was then determined by ELISA (Ser473 phosphorylation after insulin stimulus). Hepatic glucose production was determined by fluorometric assay after overnight fasting with and without insulin stimulus over a period of 8 hours. Glucose uptake in PH was measured by fluorometric 2-deoxyglucose (2-DG) uptake assay, after initial glucose deprivation.
Results: Compared to the NMRI control, NZO-PH showed reduced AKT activation (pAKT/AKT ratio) after insulin stimulus both pc. (0.41 vs. 0.30 a.u., p < 0.05) and at d14.5 (0.38 vs. 0.23 a.u., p < 0.05). Stimulation with FFA reduced AKT activation in both strains (p < 0.001). The hepatic glucose production was significantly increased by FFA in NZO-PH compared to NMRI controls at both time points, pc. and at d14.5 (AUC pc.: 201.36 vs. 324.11, p < 0.01; d14.5: 219.21 vs. 319.57 a.u., p < 0.01), thus exhibiting a diminished response towards insulin stimulation. After insulin stimulation, NZO-PH showed decreased 2-DG uptake compared to controls at d14.5 (13.26 vs. 8.43 pmol/μg, p < 0.01). Co-stimulation with FFA and insulin significantly decreased 2-DG uptake in both strains, both pc. and at d14.5 (p < 0.0001), thus no apparent differences were observed between NZO-PH and NMRI controls.
Conclusion: FFA impaired AKT activation and the suppression of hepatic glucose production by insulin, while glucose uptake was still significantly reduced in NZO-PH compared with metabolic healthy NMRI controls. Since an increase in plasma FFA in vivo occurred only in pregnant NZO mice, this suggests an exacerbation of this effect. Thus, FFA are critical mediators of the prediabetic phenotype of NZO mice both pc. and during gestation. Elevated FFA during gestation could thus indicate a preexisting glucose tolerance disorder and require increased monitoring regarding future pregnancies.
Disclosure: M. Liebmann: None.
SO 25 Insulin sensitivity: lessons from cellular and animal models
446
Development of a 3D innervated human muscle-on-a chip model for diabetes research
J. Son1, J. Ahn2, O.-K. Hong1, S. Lee3, S. Chung2;
1Internal Medicine, The Catholic University of Korea, Seoul, 2Mechanical Engineering, Korea University, Seoul, 3Life Sciences, Gwangju Institute of Science and Technology, Gwangju, Republic of Korea.
Background and aims: A bioengineered three-dimensional (3D) human skeletal muscle construct that mimics structural and functional characteristics of native skeletal muscle is a promising in vitro model to evaluate muscle physiology and metabolism. In this study, we developed an innervated muscle-on-a-chip model using 3D human skeletal muscle bundles with motor neuron (MN) innervation, and then determined whether there are differences in the pattern of muscle metabolism-related gene expression and calcium flux between muscle bundles cultured with MN and those without MN.
Materials and methods: Muscle bundles was cultured with or without MN in custom-made microfluidic device. Briefly, human myoblasts were seeded into the muscle culture chamber of the device with 0.4% collagen/Matrigel mixture. After differentiation, human MN spheroid was seeded into the MN culture chamber and collagen/Matrigel was injected into the connecting channel. Muscle bundles with or without MN were cultured additional 2-3 weeks.
Results: Muscle bundles and MNs showed the features of differentiated states. Peripheral location of the nuclei and cross-striated sarcomere pattern was showed within the cells. MNs expressed Isl1 and ChAT and projected their axons to muscle bundles. Neuromuscular junction was confirmed by co-localization of BTX+ acetylcholine receptors and NF-H+ axon. The expression levels of glucose transporter 4, PGC-1α, Myosin Heavy Chain 1 (MYH1), MYH2 and creatine kinase were significantly increased in muscle bundles cultured with MN compared to those without MN. When L-glutamate (100 μM for every 12hrs), a neurotransmitter, was treated to muscle-MN unit to mimic an exercise intervention, spontaneous muscle contraction and a time-dependent increase in intracellular calcium flux were observed in muscle bundles.
Conclusion: This 3D innervated human skeletal muscle-on-a-chip model provides an advanced in vitro model of human skeletal muscle with improved structure, function, and metabolic flux. Our model can be potential platform for studying metabolic changes of human skeletal muscle in various pathophysiological conditions, including diabetes.
Supported by: This work was supported by the NRF grant, 2021RIF1A1061077 and research fund, BMCMC21LC03.
Disclosure: J. Son: None.
447
Epithelial insulin signalling: A gatekeeper of the gut barrier function?
S. Guilmeau1, D. Gueddouri1, M. Cauzac1, F. Sicherre1, G. Boudry2, A.-F. Burnol1;
1Cochin Institute, INSERM U1016, Paris, 2Institut NuMeCan INRAE, INSERM, Rennes, France.
Background and aims: A common feature of obesity, associated insulin resistance and T2D, is their association with chronic low-grade inflammation and higher risk of infection. An early increase of intestinal permeability and subsequent translocation of bacterial endotoxins into circulation have been suggested to pave the road to the metaflammation that accompanies the diabesity cascade. While a comprehensive mapping of the mechanisms that elicit or sustain such defective epithelial integrity remains poorly understood, microbial imbalance, food composition and hyperglycemia have been proposed to be at play. Because hyperglycemia and loss of insulin action are often two sides of the same coin, we hypothesize that, beyond hyperglycemia, defective intestinal insulin signaling could directly impair epithelial integrity.
Materials and methods: To address the potential role of intestinal insulin signaling per se in the regulation of gut permeability, we developed a mouse model of insulin receptor deficiency in the intestinal epithelium (IRΔGUT), that does not display parallel hyperglycemia or obesity, thereby allowing specifically the analysis of the functional consequences of local insulin resistance on gut permeability and gut barrier components.
Results: We first demonstrate that gut epithelial cells are highly responsive to insulin, and that intestinal insulin signaling is reduced in high fat diet-induced obese mice, suggesting that insulin-resistance directly impacts gut epithelium homeostasis. Of note, IRΔGUT mice display enhanced gut permeability and deeper analyses of gut barrier components reveal a significant deterioration of antimicrobial defense by Paneth cells (PC) in IRΔGUT mice, as illustrated by the drop in antimicrobial peptide expression, PC granules number and fecal lysozyme activity. Defective antimicrobial defenses upon loss of intestinal insulin action are accompanied by the onset of a significant gut microbial imbalance, that is characterized by a bloom of pro-inflammatory Proteobacteria. Moreover, the reduced expression of ISC markers and PC-derived ISC niche factors in IRΔGUT mice suggests that insulin may also regulate intestinal stem cells (ISC) homeostasis. Accordingly, functional assays through quantification of intestinal organoids survival upon genetic blockade of IR signaling and media supplementation with PC-derived niche factors pinpoint insulin signaling as a potential intrinsic mechanism of gut epithelial renewal. Further evaluations of the adaptive response to pathological conditions following intestinal IR loss show that IRΔGUT mice display higher gut colonization by enteropathogens upon C. rodentium or S. Typhimurium infections. Finally, intestinal IR deficiency is associated with a greater susceptibility to chemical-induced gut inflammation, as shown by higher and sustained weight loss, thereby reflecting inefficient re-epithelialization.
Conclusion: Together, our data indicate that intestinal insulin signaling is decreased upon obesity and acts as a gatekeeper of two essential components of the epithelial gut barrier: bactericidal and renewal capacities. Further investigations will help (i) to evaluate the mechanisms underlying functional defects of anti-microbial defense upon gut insulin resistance, and (ii) to assess whether insulin signaling represents an ISC intrinsic mechanism for gut barrier integrity maintenance.
Supported by: ANR, IDEX
Disclosure: S. Guilmeau: None.
448
Dietary sugar intake contributes to poor metabolic health and increased NASH-fibrosis risk in mice, regardless of glycaemic index
S.G. Lucic Fisher1, A.E. Brandon2, M. Huang3, S.M. Solon-Biet2, K.S. Bell-Anderson1;
1School of Life and Environmental Science, The University of Sydney, 2School of Medical Sciences, The University of Sydney, 3Department of Anatomical Pathology, St. Vincent's Hospital, Sydney, Australia.
Background and aims: Carbohydrates are the most abundant macronutrient in the diet, their quality is paramount to good metabolic health. The glycaemic index (GI) measures carbohydrate quality based on postprandial blood glucose levels, with lower GI foods associated with better metabolic health. However, the GI has been criticised due to its characterisation of fructose as Low GI regardless of its links to metabolic disease. In this study we fed mice diets containing sucrose (High-GI) or isomaltulose (Low-GI), two disaccharides both made up of glucose and fructose, to determine if GI could reduce metabolic dysfunction and Non-alcoholic steatohepatitis (NASH) fibrosis risk after lifelong sugar exposure.
Materials and methods: C57BL/6J female mice were fed a sucrose, isomaltulose or AIN93-G diet and mated with chow males. Pups (n=10 litters/diet) continued their mother’s diet until 30 weeks. Body composition was determined by EchoMRI, feeding behaviour by BioDAQ and oral glucose tolerance tests performed. Livers were fixed in formalin, stained with H&E and Picrosirius red (PSR), and assessed for pathology. Plasma was analysed for liver enzymes. Statistical analysis was performed using a linear mixed effects model adjusted for litter effect.
Results: Results: Both female and male pups fed sugar diets were fatter by 30-weeks compared to controls, but no difference in weight. Female sugar pups secreted more insulin in response to glucose and insulin peak was delayed in mice fed isomaltulose (AIN93-G 50.1 AUC vs sucrose 100.3 AUC p<0.05; vs 90.8 AUC isomaltulose p<0.01). At both 12- and 24-weeks, sugar fed mice ate for shorter periods of time (AIN93-G 141s/bout vs sucrose 81s/bout p<0.05; vs 88s/bout isomaltulose p<0.001) compared to AIN93-G mice but ate more frequently (AIN93-G 55 bouts/24hrs vs 75 bouts/24hrs sucrose p<0.001; vs 81 bouts/24hrs isomaltulose p<0.001) and had increased food intake over 24 hours (AIN93-G 2g vs sucrose 3.2g p<0.05; vs isomaltulose 3.4g p<0.05). Interestingly, at 24-weeks food intake was reduced, compared to 12-weeks in sugar-fed mice, but remained higher than AIN93-G mice. Utilising NAS fibrosis scoring, liver fibrosis (F3/F4) was observed in 42.8% of sucrose- and 24% of isomaltulose-fed mice compared to 0% of AIN93-G mice (AIN93-G vs sucrose p<0.01). This was corroborated by the % of red detected by PSR staining, with 3% detected in AIN93-G liver while sucrose and isomaltulose mice displayed 9% and 6.7%, respectively, at 200x magnification (AIN93-G vs sucrose p<0.01; vs isomaltulose p<0.05). Liver enzymes ALT (AIN93-G 143.2 U/L, sucrose 184.8 U/L, isomaltulose 191.5 U/L) and AST (AIN93-G 368.8 U/L, sucrose 448.5 U/L, isomaltulose 542.6 U/L) were also elevated in sugar mice. At 30-weeks AIN93-G mice had larger caecum’s than their sugar-fed counterparts (AIN93-G vs sucrose p<0.01; vs isomaltulose p<0.05).
Conclusion: These findings show that lifelong exposure to dietary sugars, regardless of their GI, can induce negative changes in metabolic health and increase NASH-fibrosis risk as confirmed by histology and liver enzymes.
Disclosure: S.G. Lucic Fisher: None.
449
A new role of endoplasmic reticulum-mitochondria calcium coupling in nutrient-induced Glucagon-Like Peptide 1 (GLP-1) secretion by L cells
A. Humbert, J. Rieusset;
CarMeN Laboratory, INSERM UMR-1060, Lyon 1 University, INRA U1397, Pierre-Bénite, France.
Background and aims: Postprandial GLP-1 secretion by enteroendocrine L cells plays a major role in the regulation of glucose homoeostasis, thus representing a therapeutic option of ever-growing significance for type 2 diabetes. However, the precise mechanisms linking nutrient sensing and GLP-1 secretion are incompletely elucidated. In this study, we focused on a potential new role of endoplasmic reticulum (ER)-mitochondria communication, at contact sites called MAMs (Mitochondria-Associated ER Membranes), as they are dynamically regulated by nutrients, they control cellular calcium homoeostasis crucial for hormone secretion, and their miscommunication was implicated in alterations of glucose homeostasis in several tissues. This study aimed at evaluating the implication of MAMs in nutrient-induced GLP-1 secretion by L cells and at deciphering the mechanisms involved.
Materials and methods: The action of nutrients [5 mmol/l glucose, 30μmol/l deoxycholic acid (DCA), 100 μmol/l palmitoleic acid (PA) or 10 mmol/l glutamine-alanine dimer (Gln-Ala)] on MAMs (measured by in situ Proximity Ligation Assay targeting VDAC1 and IP3R1) and GLP-1 secretion (ELISA, expressed as a ratio secreted GLP-1/total GLP-1) was studied in the STC-1 cell line. Treatments of 1 hour with nutrients and/or various inhibitors diluted in HEPES buffer enabled the characterisation of signalling pathways implicated in nutrient sensing. The causal role of MAMs in GLP-1 secretion has been evaluated by adenoviral expression of the organelle spacer protein FATE1, which disrupts MAMs. All results are expressed in % of effect (mean ± SEM, p-value, n) using appropriate statistical tests.
Results: In STC-1 cells, we confirmed that all known GLP-1 secretagogues induced GLP-1 secretion: glucose (+54 ± 20%; p<0.01; n=4 experiments (exp.)); DCA (+111 ± 18%; p<0.001; n=4 exp.); PA (+112 ± 31%; p=0.18; n=3 replicates (rep.)); Gln-Ala; +36 ± 1%; p=0.08; n=3 rep.). All of these nutrients also induced MAMs: glucose (+33 ± 1%; p<0.001; n=4 exp.); DCA (+36 ± 1.6%; p<0.001; n=4 exp.); PA (+53 ± 9%; p<0.01; n=6 rep.); Gln-Ala (+26 ± 2%; p<0.01; n=3 exp.). Adenoviral-mediated FATE1 expression, which reduced MAMs (-24% ± 4%; p<0.01; n=3 exp.), prevented glucose- and DCA-induced GLP-1 secretion. The inhibition of IP3R calcium release by 2APB (50 μmol/l) also prevented glucose- and DCA-induced GLP-1 secretion, suggesting calcium coupling effects. Mechanistically, we demonstrated that the glucose action on MAMs is electrogenic through SGLT1, as it is prevented by phloridzin (SGLT1 inhibitor, 1 mmol/l), diazoxide (KATP channel opener, 100 μmol/l) and the removal of extracellular calcium; those treatments also affected GLP1 secretion. Concerning DCA, its effect on MAMs is mediated through the TGR5-cAMP-PKA (Protein Kinase A) pathway as INT-777 (TGR5 agonist, 30μmol/l) and forskolin (cAMP formation inducer, 10 μmol/l) mimicked DCA effects on both contacts and secretion when H89 (PKA inhibitor, 10 μmol/l) prevented them.
Conclusion: Altogether, these results demonstrate a new role of ER-mitochondria calcium coupling in nutrient-induced GLP-1 secretion in L cells. The signalling pathways involved in the regulation of MAMs vary between nutrients, with an electrogenic effect for glucose and an effect mediated through TGR5-cAMP-PKA pathway for DCA. Confirmation of these data in more physiological models is currently underway.
Supported by: EFSD Grant - ANR TriMAMvirate
Disclosure: A. Humbert: None.
450
Hexokinase-2 linked scheduled glycolysis as a driver of insulin resistance linked dysglycaemia: evidence in the murine C2C12 myotube model in vitro
M. Xue1, N. Rabbani2, P. Thornalley1;
1Diabetes Research Center, Hamad Bin Khalifa University, 2College of Medicine, Qatar University, Doha, Qatar.
Background and aims: Insulin resistance in skeletal muscle is the major metabolic defect leading to the development of type 2 diabetes (T2DM) and represents a target of pharmacotherapy for diabetes prevention. There is currently no drug treatment addressing insulin resistance. The initiating metabolic dysfunction driving development of insulin resistance has not been disclosed. Recently, stabilization of hexokinase-2 (HK2) to proteolysis by increased glucose concentration, increasing flux through early-stage glycolysis without increase in expression and activity of other glycolytic enzymes, was identified as the initiator of metabolic dysfunction in hyperglycemia in vascular cells. This creates a wave of increased glycolytic intermediates activating hexosamine and protein kinase C pathways and increasing formation of methylglyoxal. Increased glucose-6-phosphate displaces HK2 from mitochondria, producing mitochondrial hyperpolarization and dysfunction. This is termed HK2-linked unscheduled glycolysis. Skeletal muscle exposed to increased fasting plasma glucose may also stimulate this. To test this, we studied HK2 protein abundance in murine C2C12 myotubes incubated with 5.5 and 25 mM glucose for 4 days and studied the effect of glyoxalase 1 inducer, trans-resveratrol and hesperetin in combination (tRES-HESP). Protein abundances of HK2 and glycolytic enzymes in C2C12 myotube cultures incubated with low, medium and high glucose were mined from published supplementary data.
Materials and methods: Murine C2C12 myotubes were incubated in culture for 4 days with 5.5, 25 mM glucose and 25 mM glucose with 5 μM tRES-HESP. Thereafter, HK2 protein abundance was determined by Western blotting, normalized to ß-actin. Protein abundances of HK2 and glycolytic enzymes in C2C12 myotube cultures incubated with 5.5, 11.1 and 25.0 mM glucose determined by label-free quantitative high resolution Orbitrap mass spectrometry proteomics were mined from published supplementary data - Kato et al., Appl. Sci. 2022, 12, 1553. Data are mean ± SD (n = 3).
Results: In C2C12 myotubes, HK2 protein increased 46 ± 5 % in 25 mM glucose, compared to cultures with 5 mM glucose (P<0.001, n = 3; Western blotting). tRES-HESP reversed this increase. Published proteomics studies corroborated this with 1.5 and 1.8 fold increase of HK2 in 11.1 and 25.0 mM glucose cultures, compared to 5.5. mM glucose control (P<0.01, n = 3). Importantly, data mining of abundances of other glycolytic enzymes, hexokinase-1, glucose-6-phosphate isomerase, phosphofructokinase, aldolase, triosephosphate isomerase and glyceraldehyde-3-phosphate dehydrogenase, showed that abundances of all other glycolytic enzymes were unchanged.
Conclusion: We conclude that high glucose concentration in C2C12 myotubes in vitro exhibits conditions producing HK2-linked unscheduled glycolysis. Increased HK2 protein without increase of other glycolytic enzymes in myotubes with high glucose concentration supports the hypothesis of HK2-linked unscheduled glycolysis as a driver of insulin resistance. tRES-HESP may correct this. Previously in a randomized double blind, placebo controlled crossover study in overweight and obese subjects (Hats-off study), tRES-HESP corrected insulin resistance - which may be mediated by countering HK2-linked unscheduled glycolysis.
Supported by: QF funded project
Disclosure: M. Xue: None.
451
Loss of insulin-degrading enzyme function leads to impaired glucagon signalling and enhanced mitochondrial respiration in hepatocytes
C.M. González-Casimiro1, J. Santo-Domingo1, I. Cózar-Castellano1,2, G. Perdomo1;
1Unidad de Excelencia Instituto de Biología y Genética Molecular (Consejo Superior de Investigaciones Científicas- Universidad de Valladolid), 47003, Valladolid, 2Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), 28029, Madrid, Spain.
Background and aims: Insulin-degrading enzyme (IDE) is a ubiquitous metalloprotease that degrades insulin and glucagon among other substrates. By decades, its main function has been attributed to hepatic insulin clearance, a process that regulates availability of insulin levels. Recent studies indicate a more important role of this protein in insulin sensitivity and glucose homeostasis. Much less attention has been dedicated to its role on regulating other hormones action and sensitivity, particularly glucagon and its control in hepatic mitochondrial function. In this work we aim to elucidate the effect of IDE on glucagon signalling and its impact on energy metabolism in hepatocytes.
Materials and methods: Liver specific IDE-KO (L-IDE-KO) mice were obtained by breeding homozygous mice for a floxed Ide allele with albumin-Cre mice. L-IDE-KO and WT livers were used to obtain tissue extracts and primary hepatocytes for culture. The mouse hepatocyte cell line (AML12) was transduced with an shRNA-Ide by means of a lentiviral vector and obtaining a stable line (AML12-shRNA-Ide). L-IDE-KO primary hepatocytes and AML12-shRNA-Ide with their respective controls were stimulated with glucagon and the signalling pathway was analysed by western blot and ELISA. Mitochondrial function and energy metabolism of AML12-shRNA-Ide and control cells were assessed by Seahorse XFe24 Analyzer with a Mito Stress Assay.
Results: Liver extracts and primary hepatocytes from L-IDE-KO mice, compared to WT, showed decreased expression of glucagon receptor (~60%), CREB protein (~40%), and diminished phosphorylation of CREB (~50%) upon glucagon stimulation. Ide gene expression and IDE protein levels were reduced by ~50% in AML12-shRNA-Ide cells. At basal state, glucagon receptor, FoxO1 and CREB protein were significantly lower in AML12-shRNA-Ide cells than in control cells, (~40%, ~75% and ~75%, respectively). Glucagon stimulation resulted in less (~30%) cAMP levels and changes in the kinetic of glucagon-mediated phosphorylation of CREB and other PKA substrates in AML12-shRNA-Ide. Seahorse analyses showed that both oxygen consumption and extracelluar acidification rates increased 2-fold in AML12-shRNA-Ide with a 2-fold increment of mitochondrial and glycolytic ATP production.
Conclusion: Reduced IDE expression in mouse hepatocytes has a deleterious effect on glucagon signalling, affecting this intracellular pathway in parallel with a shift to a more energetic phenotype. These findings suggest that IDE is necessary for a correct glucagon signalling and energy regulation in hepatocytes.
Supported by: LCF/PR/PR18/51130007 to G.P., MCIN/AEI/10.13039/501100011033 and CCVC8485 to I.C.-C., and G.P.
Disclosure: C.M. González-Casimiro: Employment/Consultancy; Fellowship from the Junta de Castilla y León and the European Social Fund (ORDER EDU/574/2018).
452
Effects of protein restriction on glucose tolerance and large intestine morphofunction in C57Bl/6 mice
K.M. de Oliveira1, B.L. Alves1, J.A. Silva Junior1, G.M. Soares1, F. Mousovich-Neto1, R.A. Ribeiro2, E.M. Carneiro1;
1Universidade Estadual de Campinas, Campinas, 2Universidade Estadual de Ponta Grossa, Ponta Grossa, Brazil.
Background and aims: Protein undernutrition induces damages in the gastrointestinal system and endocrine pancreas which have been correlated with the development of metabolic diseases such as type 2 diabetes mellitus. However, there is a lack of information regarding protein malnutrition actions on gut morphofunction as well as their impacts on glycemic homeostasis. Herein, we evaluated glucose homeostasis and large intestine morphofunctional parameters of protein-restricted mice.
Materials and methods: C57Bl/6 male mice from 30 to 120 days-old were distributed into control (C) group, which fed on a 14% protein diet; or protein restricted group (R), which fed on a 6% protein diet. Data were analyzed by Shapiro Wilk, followed by parametric (Student t) or non-parametric tests (Mann-Whitney U test); P <0.05.
Results: R mice had higher total food (364.2 ± 2.1 g.weeks-1) intake during the experimental period when compared to C mice (298.8 ± 7.1 g.weeks-1). Despite that, R group exhibited lower body weight (BW; 24.6 ± 0.6 g), feed efficiency (29.3 ± 0.3%) and Lee Index (304.6) when compared to C (27.2 ± 0.5 g, 41.4 ± 0.2%, 312.6 ± 1.5, respectively). Furthermore, protein restriction induced higher oral glucose tolerance during glucose (12175 ± 536.7mg/dL.min-1) and mixed meal (1924 ± 149.2) tolerance tests, increased insulin sensitivity (2.8 ± 0.1%min) and pancreas hypotrophy (8.0 ± 0.3 mg/g BW) in R group when compared to C group (14291 ± 698.0 and 2970 ± 350.6 mg/dL.min-1, 2.1 ± 0.2%min and 10.4 ± 0.7 mg/g BW, respectively). R mice exhibited a reduction in feces excretion (0.14 ± 0.03 g) and lower fecal energy content (3613 ± 33.0kcal/g) in comparison to C (0.25 ± 0.03 g and 3698 ± 20.3 kcal/g, respectively). Also, intestinal permeability was 60% higher and claudin 2 protein expression in proximal colon was 43% lower in R group (0.3 ± 0.03 FITC μg/mL) than C group (0.5 ± 0.1 FITC μg/mL). Protein deprivation decreased cecum weight (5.3±1.3 mg/g BW) and length (0.2 ± 0.01 cm/NAL), and diminished alpha diversity in caecal microbiota in R mice (Shannon Index = 5,77) in comparison to C mice (8.3 ± 1.4 mg/g BW and 0.3 ± 0.01 cm/NAL). Proximal colon morphologic evaluation showed that R group had higher height (35.7 ± 1.9 μm) and number of colonocytes (15.0 ± 0.5 μm) than C group (21.3 ± 1.0 μm and 10.3 ± 0.3, respectively). In colonic crypts, protein restriction decreased the diameter (37.0 ± 0.8 μm), depth (138.6 ± 2 μm), and distance among crypts (57.0 ± 1.2 μm), besides that, goblet cells showed increased number (17.2 ± 0.4), but reduced area (123.3 ± 1.8 μm2) in R mice when compared to C mice (41.7 ± 1.2 μm, 143.6 ± 2.7, 60.0± 1.3 μm, 16.3 ± 0.4 and 215.0 ± 2.5 μm2, respectively). Finally, R mice proximal colon had a 45% decrease in mucin 2 mRNA expression, lower neutral (14.8 ± 1.0%) and acid (24.0 ± 0.5%) mucins positive areas in colonic crypts than C group (20.7 ± 1.0 and 27.5 ± 0.7%).
Conclusion: Protein undernutrition induced hypotrophy both in pancreas and cecum. Despite that, R mice had higher glucose tolerance due to increased insulin sensitivity, while in the cecum, the hypotrophy was associated with reduced microbiota diversity. Decreased mucin production, mucin 2 gene expression, and claudin 2 protein expression are probably linked with increased intestinal permeability, impairing intestinal barrier function in undernourished mice models.
Supported by: FAPESP (2020/03956-1, 2013/07607-8 and 2018/26080-4), CAPES and CNPq
Disclosure: K.M. Oliveira: None.
453
A hepatic vesicular glucose production dependent on Caveolin-1 controls energy and glucose metabolism
L. Da Costa, C. Saint-Béat, C. Bron, A. Duchampt, G. Mithieux, A. Gautier-Stein;
INSERM U1213, Claude Bernard Lyon 1 University, Lyon, France.
Background and aims: The use of anti-diabetic drugs such as metformin to reduce hepatic gluconeogenesis is the first-line treatment for type 2 diabetes patients. Targeting hepatic gluconeogenesis is thus an efficient strategy to counteract the development of type 2 diabetes. Hepatic glucose production into the bloodstream depends on the GLUT2 transporter and a vesicular pathway. Our previous results suggested that the vesicular pathway of glucose production hinges on Caveolin-1 (Cav1). The objective of this project is to determine whether the vesicular pathway of glucose production controls energy and glucose metabolism.
Materials and methods: Based on a Cre-lox strategy inducible by tamoxifen, we developed a mouse model harboring a deletion of Cav1specifically in the liver. We used three different protocols. Gene deletion was performed in 8 week-old mice by tamoxifen injections in protocols 1 and 2, then mice were fed either a standard chow diet (STD) for 20 weeks (protocol 1, L.Cav1-/-.STD) or a high-fat, high-sugar diet (HFHS) for 15 weeks (protocol 2, L.Cav1-/-.HFHS).In protocol 3, mice were fed a HFHS diet for 12 weeks. Then, gene deletion was performed and the HFHS diet was followed for 12 weeks (HFHS.L.Cav1-/-). Wild-type mice were used as controls and received the same tamoxifen treatment.In vitro (cultured hepatocytes) and in vivo (pyruvate tolerance test) hepatic glucose production and insulin sensitivity were measured in protocol 1. Energy and carbohydrate metabolism (body weight, glucose and insulin tolerance tests, liver glycogen and enzymatic activities) were analyzed in protocols 2 and 3 and compared to wild-type mice.
Results: After 16 hours of fasting, hepatocytes from L.Cav1-/-.STD mice produce less glucose than wild-type hepatocytes. In vivo, pyruvate tolerance test confirms the lower fasting hepatic glucose production of L.Cav1-/-.STD compared to wild-type mice. This reduction in glucose production is not due to a decrease in hepatic glucose-6-phosphatase expression or activity. Insulin sensitivity is increased in L.Cav1-/-.STD compared to wild-type mice. Consistently, L.Cav1-/-.HFHS mice are protected from hyperinsulinemia (2.2ug/L ± 0.3 vs 3.5ug/L ± 0.5, p=0.05), impaired glucose tolerance and insulin sensitivity induced by HFHS diet. Moreover, L.Cav1-/-.HFHS mice gain less weight (final body weight : 46.3g ± 1.2 vs 52.5g ± 1.0, p=0.01) and accumulate less hepatic triglycerides and cholesterol than wild-type mice, without differences in food intake or energy expenditure. The lower accumulation of lipids in the liver of L.Cav1-/-.HFHS mice was parallel to an increase in the expression of genes involved in hepatic lipid oxidation. In mice previously fed with a HFHS diet (HFHS.L.Cav1-/-), deletion of Cav-1 specifically in the liver corrected hyperinsulinemia (2.2ug/L ± 0.4 vs 4.4ug/L ± 0.8, p=0.03), glucose intolerance and insulin resistance but not weight gain or hepatic steatosis induced by the diet.
Conclusion: Decreasing hepatic gluconeogenesis by specifically targeting the vesicular pathway of glucose production is sufficient to increase insulin sensitivity on a standard diet. This decreased hepatic glucose production prevents and corrects the deregulations of carbohydrate and energy metabolism induced by a HFHS diet, independently of a reduction in body weight. Our results highlight that the vesicular pathway of glucose production represents a promising therapeutic target in the prevention of type 2 diabetes.
Supported by: ANR TransGlucoPase
Disclosure: L. Da Costa: None.
SO 26 Novel markers and omics signatures
454
Increased methylglyoxal formation in plasma and tissues during a glucose tolerance test originates from exogenous glucose and is enhanced in diabetes
X. Zhang1, J. Scheijen1, G. Duchateau2, M. Alssema2,3, M. Teunissen1, D. Sun1, T. Kusters1, G. Dole1, C. Stehouwer1, K. Wouters1, C. Schalkwijk1;
1Internal Medicine, Maastricht University, Maastricht, 2Unilever R&D, Wageningen, 3Health Council: current affiliation, The Hague, Netherlands.
Background and aims: The dicarbonyl compound methylglyoxal (MGO) is linked to diabetes and its vascular complications. We have previously shown that plasma MGO is increased postprandially and that individuals with type 2 diabetes (T2D) display higher postprandial MGO levels. We investigated whether postprandial MGO formation in plasma and tissues originates from exogenous glucose, and whether these changes are affected by T2D.
Materials and methods: In humans, we performed a stable isotope labeled oral glucose tolerance test (OGTT) in 12 healthy males (age 25 yrs, range 21-30 yrs), BMI 22.5 kg/m2 (range 19.2-24.7 kg/m2). The treatment was a solution of 50 g glucose of which 2% is universally labeled D(+)13C6 glucose. Concentrations of MGO and glucose were measured in plasma at eleven time points with ultra- performance liquid chromatography-tandem mass spectrometry. In C57BL/6J and db/db mice, we performed an intraperitoneal GTT (IPGTT) with universally labeled D-13C6 glucose. Blood and pancreas, liver, spleen, kidney, visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) were collected at time 0, 30 min, 60 min, and 120 min for the measurement of concentrations of MGO at different time points of IPGTT.
Results: During the OGTT, plasma 12C MGO levels increased rapidly and reached a peak at 45 min, after which levels started to decline with the lowest level at 180 min post-load. After 180 min, plasma concentration of 12C MGO showed a slight increase. The plasma 13C3 MGO curve showed the same pattern as for 12C MGO, but the increase after 180 min was not observed. Plasma 12C MGO and 13C3 MGO followed the same pattern as for glucose. We calculated that newly formed MGO in the early phase of the OGTT is completely derived from exogenous glucose, while the increase of MGO after 180 min is not. In mice, the concentration of MGO in plasma and in tissues were 2 to 4.5-fold higher in db/db mice than lean mice. During IPGTT with D-13C6 glucose administration led to a fast increase of plasma 13C3 MGO concentrations and reached a peak at 30 min in control mice (189.7 ± 23.5 nmol/L) and with higher levels of increased plasma 13C3 MGO in db/db mice (397.8 ± 64.4 nmol/L; p<0.001). In tissues, concentrations of 13C3 MGO were increased in control mice for the first 30 min of the IPGTT and this was further increased in db/db mice. After 30 min, 13C3 MGO levels started to decrease in all tissues from control mice, while they continued to rise in pancreas, liver, spleen, and kidney or stayed high in VAT and SAT in db/db mice.
Conclusion: Exogenous glucose contributes to MGO formation both in plasma and in tissues during a glucose tolerance test and this was further increased in type 2 diabetes.
Clinical Trial Registration Number: ISRCTN42106325
Disclosure: X. Zhang: None.
455
Skeletal muscle creatine metabolism is altered in type 2 diabetes
D. Rizo-Roca1, L.A. Pendergrast1, A.V. Chibalin1, H.K.R. Karlsson1, M. Rydén2, E. Näslund3, J.R. Zierath1,4, A. Krook4;
1Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, 2Medicine (H7), Karolinska Institutet, Huddinge, 3Clinical Sciences, Karolinska Institutet, Stockholm, 4Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden.
Background and aims: High plasma creatine levels are associated with an increased risk of developing type 2 diabetes. Skeletal muscle is the primary site of both glucose and creatine disposal; however, the mechanism controlling the crosstalk between skeletal muscle creatine metabolism and type 2 diabetes has not been clarified. Here, we investigated 1) whether intramuscular creatine and phosphocreatine (P-creatine) and related genes are altered in plasma and skeletal muscle from people with type 2 diabetes, and 2) if creatine supplementation or depletion modulates glucose metabolism in obese mice.
Materials and methods: Plasma and vastus lateralis biopsies were obtained from fasted men with normal glucose tolerance (NGT, n=25) or type 2 diabetes (n=25). Creatine and P-creatine were assessed by LC-MS (NGT, n=15; T2D, n=25). Gene expression was obtained from a microarray analysis (n=25 both groups). Further experiments were performed on 5-week-old C57BL/6 male mice fed a chow (n=10) or a high fat diet (HFD, 60% kcal from fat, n=30) for 8 weeks. During the last 2 weeks of the dietary intervention, HFD mice received a daily intraperitoneal injection of either vehicle (PBS; n=10), creatine (0.3 mg/g) or a creatine kinase competitive inhibitor (βGPA; 0.4 mg/g). Mice were randomly assigned to groups. Body composition and GTT glucose tolerance was assessed. Ex-vivo skeletal muscle glucose transport was assessed using radiolabeled glucose. Statistical difference was determined using Student’s t test or Mann-Whitney U test in human studies, and one-way ANOVA or Kruskal-Wallis in animal studies.
Results: Compared to individuals with NGT, plasma creatine was increased in people with type 2 diabetes (+49%, p=0.015), while skeletal muscle P-creatine was reduced (-32%, p=0.02). These changes were associated with reduced mRNA of the creatine transporter SLC6A8 (-18%, p=0.022) and mitochondrial creatine kinase CKMT2 (-47%, p=0.011). Plasma creatine was inversely correlated with SLC6A8 mRNA (Spearman's ρ; r=-0.63, p<0.001) and skeletal muscle P-creatine was correlated with CKMT2 mRNA (Spearman's ρ; r=0.33, p=0.03). Fasting blood glucose was inversely associated with skeletal muscle P-creatine level in people with type 2 diabetes (Spearman's ρ; r=-0.43, p=0.04). HFD-fed mice treated with creatine had a greater increase in body weight (+4.5%, p=0.004) and lean mass (+9%, p=0.03) as compared to HFD-fed mice treated with PBS. GTT Glucose tolerance was impaired in all HFD-fed mice (p<.0001). Glucose uptake was decreased in extensor digitorum longus (basal: -35%, p=0.02 vs. chow) and soleus (basal: -40%, p=0.01; insulin: -44%, p=0.002) muscle of obese HFD-fed mice, while βGPA restored glucose uptake to control levels (p>0.05 vs chow, except in insulin-stimulated soleus). HFD-fed mice (n=8-10 per group) had reduced expression of Ckm ([log2 Fold-change vs Chow-PBS ±SD] -0.53±0.25, p=0.004), Ckmt2 (-0.55±0.06, p=0.0002), Hk2 (-0.41±0.13, p=0.024) and Igf1 (-0.55±0.06, p=0.02), while creatine treatment prevented the downregulation of gene expression.
Conclusion: Our data suggest that increased plasma creatine and reduced skeletal muscle P-creatine are a consequence, rather than a cause of insulin resistance in type 2 diabetes.
Disclosure: D. Rizo-Roca: None.
456
Reduced incretin effect is an early sign of diabetes appearence. A study in a human model of beta cell mass reduction
G. Di Giuseppe1, C.M.A. Cefalo1, S. Moffa1, F. Cinti1, G. Ciccarelli1, L. Soldovieri1, U. Capece1, F. Impronta1, A. Mari2, R.N. Kulkarni3, J.J. Holst4, A. Giaccari1, T. Mezza1;
1Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy, 2CNR Institute of Neuroscience, Padua, Italy, 3Joslin Diabetes Center - Harvard University, Boston, USA, 4University of Copenaghen, Copenaghen, Denmark.
Background and aims: In type 2 diabetes (T2D) patients, the incretin effect is impaired and contributes to only 20-35% of insulin response to oral glucose. One major reason appears to be impairment of the insulinotropic effect of the incretin hormones, GIP and GLP-1. However, the cause of the defective incretin effect and whether its impairment is a consequence of reduced incretin hormone secretion or reduced β-cell activity (defective beta-cell receptor expression/post-receptor defects secondary to diabetes) is still debated. Here, we evaluate changes in GLP-1 levels and differential β-cell responses to oral vs intravenous stimuli in nondiabetic humans before and after partial pancreatectomy. We also investigate protein expression related to modulation of GLP-1-induced insulin secretion in surgical pancreas biopsies.
Materials and methods: To investigate changes in in vivo GLP-1 and incretin effect, we enrolled 33 nondiabetic patients (M/F: 15/18; Age: 62±15; BMI: 26.5±4.3) scheduled for pancreatoduodenectomy (PD). All subjects underwent a 2-h hyperglycemic clamp (HC), an 830 kcal mixed meal test (MMT), and an oral glucose tolerance test (OGTT) before and after surgery. β-cell glucose sensitivity (βCGS) was calculated as the ratio of insulin secretion and glucose increments, during both the hyperglycemic clamp and the mixed meal test. We applied high performance liquid chromatography-mass spectrometry (HPLC-MS) analyses to islets isolated by laser capture microdissection (LCM) from samples obtained during surgery. We performed qualitative and quantitative analyses to detect differential protein expression among the 3 groups.
Results: Based on post-surgery OGTT, we divided subjects into 3 groups based on glucose tolerance after PD: normal glucose tolerance (post-NGT), impaired glucose tolerance (post-IGT), or diabetes (post-DM). Before surgery, post-IGT subjects exhibited increased GLP-1 secretion during MMT compared to other 2 groups (GLP-1 AUC: post-IGT 9795±1119 vs post-NGT 5319±1022 vs post-DM 5419±1440, P<0.05). A similar βCGS was observed in the 3 groups following i.v. glucose administration during HC stimulation (post-NGT 97.2±17.7 vs post-IGT 76.4±9.12 vs post-DM 72.7±11.8 pmol·min-1m-2·mM-1, P=0.2). However, a scaled reduction in βCGS was observed in post-IGT and post-DM following MMT (post-NGT 218±45.0 vs post-IGT 102±20.2 vs post-DM 92.3±22.8 pmol·min-1m-2·mM-1, P=0.01). Proteomic analysis revealed reduced expression of proteins regulating GLP-1-stimulated insulin secretion in IGT and DM subjects: Rap1 was expressed only in post-NGT group (p<0.01), while IQGA1 was significantly reduced in IGT and DM, compared to NGT (p=0.03) and correlated inversely with glucose AUC during OGTT (r=-0.57, P=0.03) and HOMA-IR (r=-0.78, P<0.001).
Conclusion: Our data indicate that GLP-1 secretion tends to increase in early stage beta-cell dysfunction, possibly as a sign of compensating for the impaired function in subjects at risk of impaired glucose tolerance and diabetes. Our findings suggest that poor glucose metabolism and insulin resistance are linked to in-situ impairment of islet sensitivity to GLP-1.
Supported by: EFSD/AZ Mentorship Programme
Disclosure: G. Di Giuseppe: None.
457
Ex vivo and in vivo experimental investigation of miR-148a-3p as a novel therapy for insulin secretion and type 2 diabetes
E. Cowan, C. Luan, J.K. Ofori, A. Karagiannopoulos, J.L.S. Esguerra, C. Ling, E. Zhang, L. Eliasson;
Clinical Sciences, Lund University, Malmö, Sweden.
Background and aims: Several microRNAs are differentially expressed in diabetes and essential for β-cell function. We previously described how miR-152 influences insulin production and release, recent preliminary data from our other studies confirm miR-148a-3p as the most abundant miR-148/152 family member in islets, and several others have pinpointed miR-148a as perturbed in diabetes. Together these findings led us to identify miR-148a-3p as a novel target for modulating β-cell insulin secretion. Here, we evaluate its role on insulin secretion and glucose metabolism in ex vivo and in vivo models of diabetes.
Materials and methods: MiR-148a-3p expression was quantified in islets from non-obese diabetic Goto-Kakizaki (GK) rats (6, 11 & 13.5 weeks) and obese diabetic db/db mice (10-12 weeks), as well as in glucolipotoxicity treated islets from standard NMRI (Naval Medical Research Institute) mice (12 weeks). LNA (locked nucleic acid) mediated miR-148a-3p silencing (50 nM) and subsequent insulin secretion and content assessments were performed in islets extracted from 7-week-old GKs. Thereafter, in vivo studies were performed for further metabolic investigations, where 5- and 11-week-old GKs were treated with LNA (two bolus injections at 20 mg/kg (Day 2 and 7) over 10 days) to silence miR-148a-3p. TargetScan (http://www.targetscan.org/) was also used to predict miR-148a-3p targets. All experimental data were analysed by unpaired t-test, 1- or 2-way ANOVA.
Results: MiR-148a-3p expression was significantly upregulated in islets from GK rats and an increasing trend was observed with increasing GK age (GK (11.5 weeks) vs GK (6 weeks): p<0.01, n≥3, and GK (13 weeks) vs W(Wistar) or GK (6 weeks): p<0.0001, n≥5). Contrastingly, miR-148a-3p expression was unaltered in islets of db/db vs wild type mice and in glucolipotoxicity treated vs untreated NMRI mouse islets. LNA silencing of miR-148a-3p in islets extracted from 7-week-old GKs (56% knockdown: p<0.0001, n=4) significantly increased the insulin secretion response to 16.7 mM glucose (p<0.01, n=4) while having no effect on insulin content. In vivo assessments revealed 15% (p=0.25, n=4) and 28% (p<0.01, n≥5) reductions in miR-148a-3p expression in extracted islets from 5- and 11-week-old miR-148a-3p LNA treated GKs respectively. IpGTT glucose area under the curve was unchanged with treatment in both cohorts, however, the response in islets extracted from the control GKs of the 11-week-old cohort to 16.7 vs 2.8 mM glucose (p<0.05, n=4) was further potentiated in islets extracted from miR-148a-3p LNA treated 11-week-old GKs (p<0.01, n=3). Moreover, TargetScan predicted ATP6AP2, MEOX2, GLRX5, SNN and GADD45A as the top 5 miR-148a-3p targets in humans, and both ATP6AP2 and GLRX5 have previously been implicated in insulin secretion.
Conclusion: MiR-148a-3p expression is influenced by increasing diabetic phenotype independent of obesity. Adequate and specific silencing of miR-148a-3p in the islets of the GK model improves insulin secretion. Furthermore, the potency of miR-148a-3p LNA mediated silencing in the GKs in vivo as well as the effects on the insulin secretory capacity in the islets of treated animals appears to increase with diabetic status. Finally, miR-148a-3p effects on insulin secretion may potentially occur via regulation of ATP6AP2 and/or GLRX5 in rodents and humans.
Supported by: EFSD/MSD Research Programme, Physiological Society Lund, SSF(LUDC-IRC), SRC, SRA grant SFO-EXODIAB & project grants
Disclosure: E. Cowan: None.
458
Transcriptional abnormalities in adipose tissue of humans with inherited insulin resistance
R. Kruse1, A.B. Hansen1, N. Sahebekhtiari1, K. Brusgaard2, K. Højlund1;
1Steno Diabetes Center Odense, Odense University Hospital, 2Department of Clinical Genetics, Odense University Hospital, Odense C, Denmark.
Background and aims: Insulin resistance in common metabolic disorders such as obesity and type 2 diabetes is linked to several molecular markers of adipose tissue dysfunction. However, it remains to be clarified whether insulin resistance is the cause or consequence of adipose tissue dysfunction in humans. Loss-of-function mutations in the insulin receptor gene (INSR) causes severe insulin resistance with molecular defects in skeletal muscle similar to that observed in obesity and type 2 diabetes. Here, we aimed to investigate the molecular consequences of inherited insulin resistance in human adipose tissue.
Materials and methods: In a case-control study, subcutaneous abdominal adipose tissue biopsies from carriers (n=6) of a dominant-negative mutation (Arg1201Gln) in the tyrosine kinase domain of the (INSR) and gender and weight-matched control individuals (n=18) were used for unbiased, discovery-mode, micro array-based transcriptional profiling using a human Clariom D assay from Affymetrix. The bioinformatic platforms DAVID and GSEA were used to identify changes in expression of biological pathways and genes/proteins for further validation by qPCR and Western blotting.
Results: The INSR mutation carriers were characterized by severe insulin resistance (Matsuda: 1.8±0.4 vs 13.3±1.3 AU; p<0.001) and fasting hyperinsulinemia (352±95 vs 51±5 pmol/l; p<0.001), but normoglycemia (HbA1c: 37±1 vs 34±1 mmol/mol; p=0.14). Transcriptional profiling and pathway analysis showed significant (FDR<0.05) downregulation of genes representing branched-chain amino acid degradation, fatty acid metabolism and mitochondrial oxidative phosphorylation in adipose tissue of INSR mutation carriers, whereas genes involved in interferon signaling were significantly (FDR<0.05) up-regulated in the INSR mutation carriers. Validation of selected markers are ongoing.
Conclusion: Our results demonstrate that a loss-of-function mutation in the INSR, which causes severe insulin resistance, is accompanied by transcriptional downregulation of molecular processes in the mitochondria and upregulation of interferon signaling genes in adipose tissue. These data suggest that at least some suggested markers of adipose tissue dysfunction could be the consequence of insulin resistance.
Disclosure: R. Kruse: None.
459
Oral D-xylose plasma appearance as a biomarker for intestinal glucose absorption in minipigs
R. Goutchtat1, C. Marciniak1, G. Baud1, M. Gobert1, V. Vangelder1, A. Quenon1,2, A. Dive2, M. Pottier2, T. Rabier2, S. Lapière2, V. Raverdy1, R. Caiazzo1, T. Hubert1,2, F. Pattou1;
1Université de Lille, Inserm, CHU Lille, Institut Pasteur Lille, 2Université de Lille, CHU Lille, Département Hospitalo-Universitaire de Recherche et d’Enseignement (DHURE), Lille, France.
Background and aims: Increasing evidences suggest that intestinal glucose absorption (IGA) plays a role in T2D pathophysiology, independently from insulin secretion and sensitivity. Challenging methods using multiple glucose tracer are classically used to measure IGA in humans. The use of D-xylose which is absorbed by SGLT1 but only marginally metabolized has been proposed. In this preclinical study, we evaluated the performances of D-xylose plasma appearance as a proxy of IGA.
Materials and methods: A total of 82 healthy adult minipigs (44.0 ± 14.2 kg; 72% female) were submitted to vigil 180 mn standardized mixed meal tests (MMT) containing 30 g of D-xylose. First, we analyzed the association between post prandial blood glucose and serum D-xylose appearance in 74 minipigs (cross sectional study). Second, we conducted longitudinal studies to analyze the changes of post prandial blood glucose excursion and serum D-xylose appearance following MMT induced in healthy minipigs by three specific interventions: 70% intestinal resection (n = 5) (to decrease IGA), 70% pancreatectomy (n = 15) (to decrease insulin secretion), and high fat diet for 2 months (HFD) (n = 8) (to decrease insulin sensitivity). Third, to evaluate the potential bias induced by gastric emptying during MMT, we measured serum D-xylose appearance when the meal was administered in the same animals orally or directly administered in the proximal jejunum via a surgically implanted jejunal tube.
Results: Blood glucose excursion and serum D-xylose appearance during MMT are illustrated in Fig.1a. Incremental 30 min postprandial glucose was correlated with 180 mn-area under curve of D-xylose appearance (r = 0.65; r2 = 0.42; p < 0.0001) (Fig.1b). In multivariate analysis this association remained significant following adjustment to insulin secretion (insulinogenic index) and insulin sensitivity (Matsuda index) (β = 0.62 ± 0.09; r = 0.61; r2 = 0.45; p < 0.0001). Longitudinal studies showed that D-xylose plasma appearance was significantly reduced after intestinal resection (55.5 ± 18.7 before vs 31.5 ± 4.8 after; p < 0.05), but remained unchanged after pancreatectomy (58.2 ± 24.4 before vs 55.5 ± 30.1 after; p = 0.35), and after HFD (43.1 ± 16.7 before vs 45.6 ± 12.6 after; p = 0.38). Finally, serum D-xylose appearance was not significantly different following oral (59.1 ± 15.3) or intrajejunal (59.0 ± 12.8) (p > 0.99).
Conclusion: In the Minipig, early postprandial glucose during MMT was correlated with D-xylose appearance, independently of insulin secretion, insulin sensitivity and gastric emptying. The results of our longitudinal experiments suggest that D-Xylose appearance could be used as a simple and reproducible proxy to evaluate IGA in large mammals.
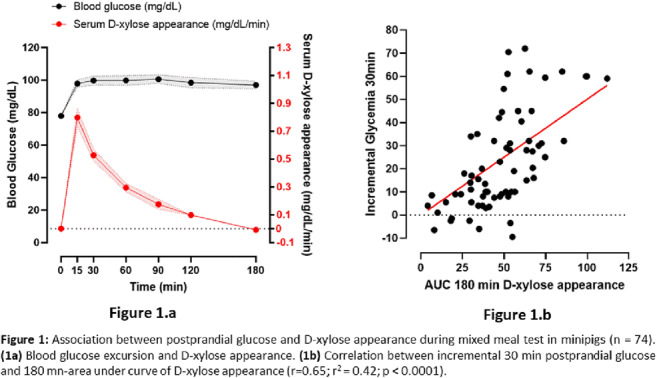
Supported by: The authors want to thank the CHU of Lille, EGID and the ANR for their respective contributions.
Disclosure: R. Goutchtat: None.
460
Single-cell transcriptomic analysis of human enteroendocrine cells in primary ileal and duodenal organoid culture
R. Bany Bakar, C.A. Smith, V.B. Lu, N. Guccio, D. Goldspink, E. Miedzybrodzka, F.M. Gribble, F. Reimann;
Wellcome Trust - MRC Institute of Metabolic Science, Addenbrooke's Hospital, University of Cambridge, Cambridge, UK.
Background and aims: Enteroendocrine cells (EECs) are hormone-secreting cells within the intestinal epithelium that play an important role in regulating food absorption, insulin secretion and appetite. Based on the success of glucagon-like peptide 1-based therapies for type 2 diabetes and obesity, EECs are themselves candidate targets for drug discovery programs to improve gut hormone secretion. The somatostatin (SST)-producing D-cell is an EEC of particular interest due to the profound inhibition exerted by SST over other EECs, highlighting D-cells as a critical regulator of the enteroendocrine axis. The aim of this study was to compare the transcriptome of human EECs subtypes found in intestinal organoids with a particular focus on the D-cell transcriptome.
Materials and methods: To label the full spectrum of human EECs in organoids from adult proximal small intestine (duodenum) and distal small intestine (ileum), CRISPR-Cas9 followed by homology-directed repair was used to insert a P2A ribosomal stutter sequence, followed by the fluorescent protein Venus sequence and neomycin resistant cassette, at the 3’ of the chromogranin A (CHGA) coding sequence, a general marker of EECs. To label D-cells in duodenal organoids, internal ribosome entry site sequence, followed by the fluorescent protein tdTomato sequence and puromycin resistant cassette, was inserted under the control of the endogenous somatostatin promoter. Single-cell RNA-sequencing of FACS-purified CGHA-venus positive cells and Bulk RNA sequencing of FACS-purified SST-tdTomato positive and negative cells were performed to characterise the D-cell transcriptome.
Results: The transcriptional profiles of 10,016 and 5,911 cells from duodenal and ileal organoids were analysed, respectively. Cluster analysis identified six major EECs populations from the ileal organoids and ten in the duodenal organoids. Gut hormones were amongst the top differentially expressed genes for each of the labelled clusters, including SST-expressing D cells, MLN/GHRL expressing M/X cells, and TPH1-expressing enterochromaffin cells in both regions, while GAST/ GIP/CCK expressing G/K/l cells were detected in the duodenum and GCG-expressing L cells in the ileum. Interestingly, multiple clusters of mature EECs were detected in both duodenum and ileum that do not express known hormonal markers. The expression of G-protein coupled receptors (GPCRs) differed between clusters, suggesting that EECs are regulated differentially. D-cells bulk RNA sequencing identified enriched expression of several GPCRs, including short-chain fatty acids receptor (FFAR2), bile acids receptor (GPBAR1), amino acids receptor (GPR142), trace amines receptor (TAAR1) and the vasopressin receptor (AVPR1B). The expression of voltage-gated ion channels was also examined; several voltage-gated ion channels are enriched in D-cells, including CACNA1A (P/Q type Ca+2 channel) and the L-type Ca+2 channel subunit CACNA1C that is broadly implicated in Ca2+2 -dependent vesicular exocytosis.
Conclusion: In this study, we have generated a high-resolution transcriptomic profile of human EECs from the duodenal and ileal organoids and the first in-depth transcriptomic analysis of human intestinal D-cells. Our results provide an important foundation to guide future genomics-based interrogation of EECs functions and their sensory apparatus.
Supported by: Welcome Trust
Disclosure: R. Bany Bakar: None.
SO 27 If you cannot measure it, you cannot improve it: novel methods in diabetes research
461
Do indices from mixed meal tolerance test follow the hyperbolic relationship between insulin sensitivity and insulin secretion?
N. Nielsen, K. Karstoft, H. Ellingsgaard, L. Lehrskov, N. Pilmark, M. Ried-Larsen;
Centre for Physical Activity Research, Copenhagen University Hospital - Rigshospitalet, Copenhagen, Denmark.
Background and aims: There is a paucity of studies investigating whether indices of insulin secretion and sensitivity derived from a mixed meal tolerance test (MMTT) follow the hyperbolic relationship and can be used to discriminate between persons with normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and overt type 2 diabetes (T2D), thus we aimed to investigate this.
Materials and methods: Data were collected from seven clinical trials including persons with NGT (n=49, HbA1c <42 mmol/mol), IGT (n=59, HbA1c 42-47 mmol/mol) or T2D (n=113, HbA1c >47 mmol/mol). Prior to the investigation, the participants were fasting >10 hours and glucose-lowering medication was discontinued >48 hours prior to test. Participants consumed a 735-kcal liquid meal (25E% fat, 63E% carbohydrate and 12E% protein). Samples were collected before and until 120 min post ingestion. Indices for insulin secretion and sensitivity were calculated based on plasma glucose and insulin values. The Matsuda index0-120min was calculated as a measure of insulin sensitivity (SI), and two different indices for insulin secretion were calculated; the early phase (ΔInsulin0-30min/ΔGlucose0-30min, insulinogenic index, IGI) and the total secretion (ratio of the area under the curve of insulin to area under the curve of glucose from 0-120 min, AUC). The association between SI and indices of insulin secretion were investigated to identify the satisfaction of the hyperbolic criteria (regression coefficient (r-value) approximately equal to −1 and the 95% confidence interval of the regression coefficient excluding 0).
Results: Mean (SD) HbA1c, age and sex were 37 (9) mmol/mol, 44 (12) years, 23 % female for NGT, 44 (5) mmol/mol, 49 (9) years, 49 % female for IGT and 50 (7) mmol/mol, 58 (10) years and 29 % female for T2D, respectively. The r-values (95%CI) for the hyperbolic relationship between SI and IGI or AUC were -0.97IGI (-1,52 to -0.43) and -1.02AUC (-1.19 to -0.82) for NGT, -0.12IGI (-0.43 to 0.18) and -0.56AUC (-0.71 to -0.41) for IGT and -0.45IGI (-0.71 to -0.18) and -0.86AUC (-1.07 to -0.65) for T2D. SI was 16 (1), 7 (2) and 5 (1) arbitrary units (a.u.) for NGT, IGT and T2D, respectively (P<0.01 for all between-group differences). IGI and AUC were -71% and -32% lower for T2D vs. NGT (p<0.01). For IGT vs. NGT, IGI was lower by -31%, but AUC was higher by 39% (P<0.01). DIIGI was 6408 (1290), 2170 (241) and 596 (65), and DIAUC was 905 (38), 616 (33) and 195 (12) a.u. for NGT, IGT and T2D, respectively. Both the DI and SI indices discriminated between all the classifications of NGT, IGT and T2D (all P<0.001) (figure 1).
Conclusion: The hyperbolic relationship between insulin sensitivity and insulin secretion can be established from a MMTT with a better model fit using AUC over IGI. DI derived from combinations of SI and both AUC and IGI, discriminated between stages of glucose tolerance.
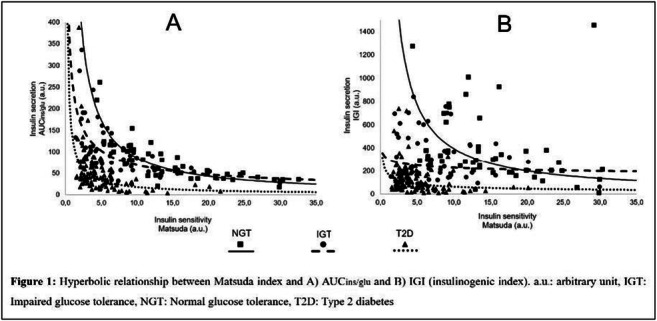
Supported by: Trygfonden ID 124708. Svend Andersen fonden. Swiss National Science Foundation. Novo Nordisk Fonden
Disclosure: N. Nielsen: None.
462
Tissue-specific and whole-body insulin sensitivity by integrated imaging and hyperinsulinaemic euglycaemic clamp: a repeatability study in type 2 diabetes subjects
I. Laitinen1, H. Litorp2, F. Sjöberg2, H. Haraldsson1, S. Ekström3, S. Pierrou1, S. Southekal4, M. Lu4, T. Coskun5, Z. Milicevic5, L. Johansson1;
1Antaros Medical, Mölndal, Sweden, 2Clinical Trial Consultants AB, Uppsala, Sweden, 3Antaros Medical, Uppsala, Sweden, 4Avid Radiopharmaceuticals Inc, Philadelphia, USA, 5Eli Lilly, Indianapolis, USA.
Background and aims: Assessment of glucose tissue uptake by positron emission tomography (PET) imaging under hyperinsulinemic euglycemic clamp (HEC) is a powerful tool for quantification of tissue-specific insulin resistance. An integrated whole-body PET and MRI (magnetic resonance imaging) dynamic assessment provides quantitative readout in several tissues simultaneously. The aim of the study was to investigate the repeatability of both whole-body glucose utilisation and tissue-specific insulin sensitivity with combined dynamic PET/MRI and HEC in T2D subjects.
Materials and methods: The study included overweight or obese T2DM subjects (n=16 completers) for two repeated examinations in standardised conditions one week apart. After overnight fasting, HEC was established and maintained (Insulin 56 mU/m2/min). In steady state, fluorine-18-deoxyglucose (18F-FDG) was injected and series of PET and MRI scans were repeated. Whole-body glucose utilisation (M-value) was calculated from the duration of the PET/MRI scan (1 hour). Tissue-specific glucose uptake (GU) were calculated from merged whole-body PET images using Patlak model, in skeletal muscle, subcutaneous and visceral adipose tissue (SAT, VAT), myocardium, and brain. The repeatability was assessed by calculating the intraclass correlation coefficient (ICC method 2,1) point estimates and the 95 % confidence intervals (CI). ICC levels were defined as the following: lower 95 % CI of ICC ≥ 0.75 indicates excellent, 0.6 - 0.74 good, 0.4 - 0.59 fair and <0.4 poor reliability.
Results: Repeatability was assessed in 16 subjects (full analysis set, FAS) and in a subset of 12 subjects (per protocol set, PPS, defined by a consistent HEC over both visits). The measured M-values and tissue GU displayed varying levels of insulin resistance (Table 1). ICC of M-value was 0.84 (95% CI 0.63-0.94) for the FAS, and 0.95 (95% CI 0.86-0.99) for PPS, indicating a good to excellent reliability of the M value as an assessment of whole-body insulin sensitivity during HEC and PET/MRI experimental setup in T2DM subjects. Tissue-specific GU reliability was fair to excellent of the GU in skeletal muscle in FAS and PPS analysis sets (ICC 0.78 and 0.94 with lower 95% CI 0.50 and 0.83, respectively). For the other measured tissues, the ICC indicated fair to good reliability for SAT, VAT, myocardium, and brain (Table 1).
Conclusion: 18F-FDG-PET/MRI provides selective information on tissue-specific insulin sensitivity during hyperinsulinemic euglycemic clamp with a reliability comparable to standard global M-value calculation. The method has potential to provide benefits in monitoring and evaluating T2DM treatment effects in interventional trials.
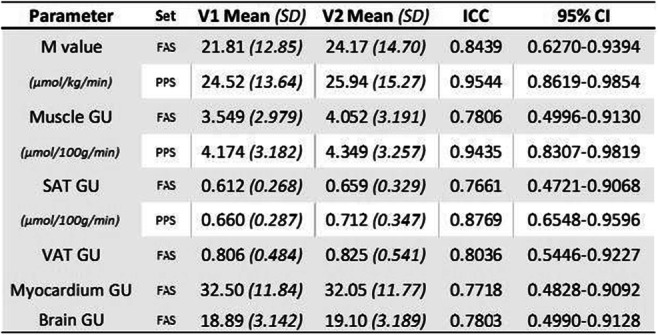
Clinical Trial Registration Number: 2020-04140 (EC Sweden)
Supported by: Antaros Medical was supported by Eli Lilly for the conduct of the study
Disclosure: I. Laitinen: Employment/Consultancy; Employed by Antaros Medical. Other; Study financed by Eli Lilly.
463
The insulin sensitivity index derived from euglycaemic clamps is correlated to liver fat content determined by magnetic resonance spectroscopy in type 1 diabetes
J. Mertens1,2, S. Francque2, M. Spinhoven3, F. Vanhevel3, C. De Block1;
1Endocrinology, Diabetology and Metabolism, University Hospital Antwerp, 2Gastroenterology and Hepatology, University Hospital Antwerp, 3Radiology, University Hospital Antwerp, Edegem, Belgium.
Background and aims: Nonalcoholic fatty liver disease (NAFLD) is strongly associated with type 2 diabetes and obesity. Insulin resistance (IR) is proposed as the driving factor. NAFLD is increasingly reported in type 1 diabetes (T1D), but the link between IR and NAFLD in this population needs elucidation. Furthermore, people with T1D are an excellent theoretical model to investigate IR in relation to NAFLD without confounders. This study aimed to assess whether IR is correlated with liver fat content (LFC) in adult people with T1D without causes of secondary liver disease.
Materials and methods: A hyperinsulinemic-euglycemic clamp was performed in fasting conditions according to DeFronzo et al. LFC was measured by magnetic resonance spectroscopy (MRS) and calculated as the mean of three independent regions of interest. NAFLD was defined as an LFC > 6.0 % and people with NAFLD were matched 1:1 to no-NAFLD control participants based on age and sex.
Results: 20 individuals with mean age of 48 ± 17 years and mean HbA1c of 7.3 ± 0.8 % were included. Mean BMI was 27.3 ± 4.3 kg/m2. Mean M-index (clamp-derived index of IR) was 5.12 ± 3.03 mg/kg/min. Mean LFC in the NAFLD group was 15.6 ± 8.3 % versus 2.8 ± 1.4 % in the control group (p < 0.001). Mean M-index differed significantly between groups (NAFLD: 2.88 ± 1.58 vs. 6.74 ± 2.11 mg/kg/min, p < 0.001). A low M-index means an increased IR. BMI was significantly higher in the NAFLD group (29.6 ± 3.6 vs. 25.1 ± 3.8 kg/m2, p = 0.014). Spearman correlation showed a strong correlation between the M-index and LFC (r = -0.85, p < 0.001). Multiple linear regression analysis showed a significant association between LFC as dependent variable and the M-index (B = -1.504, 95% CI: (-2.906 to -0.101), p = 0.037) and BMI (B = 0.879, 95% CI: (0.047 to 1.711), p = 0.040) but not with age nor HbA1c level as independent variables.
Conclusion: Euglycaemic clamp-derived measures of IR are strongly correlated to LFC in people with T1D, independently from BMI. These data support the pivotal role of IR in NAFLD pathophysiology and its therapeutic potential.
Disclosure: J. Mertens: None.
464
Evaluation of renal glucose uptake with [ 18 F]FDG-PET: methodological advancements and clinical outcomes
E. Rebelos1,2, A. Mari3, V. Oikonen1, H. Iida1, P. Nuutila1, E. Ferrannini2;
1Turku PET Centre, University of Turku, Turku, Finland, 2Institute of Clinical Physiology, CNR, Pisa, Italy, 3Institute of Neuroscience, CNR, Padova, Italy.
Background and aims: Renal metabolism has been previously assessed in humans using the arterial-venous (AV) differences technique, reporting whole-kidney glucose uptake rates ~38 μmol.min-1.100g-1 in healthy subjects. On the contrary, studying renal glucose metabolism non-invasively in humans has been thus far an unmet need. Positron emission tomography (PET) is the current gold standard for measuring regional tissue glucose uptake rates (GU), but the most widely used glucose analogue ([18F]FDG) is not a good substrate for sodium-glucose cotransporters (SGLTs). As a consequence, [18F]FDG spills over into the urine and [18F]FDG-PET considerably underestimates published rates of whole renal glucose uptake obtained using the arterial-venous differences technique.
Materials and methods: 16 morbidly obese and 9 lean subjects were studied with [18F]FDG-PET under fasting and insulin clamp (1mU/kg/min) conditions. Abdominal radioactivity was acquired ~90 min from tracer injection. At the end of the studies, participants voided their bladders and radioactivity in the urine was measured and decay-corrected. Based on the amount of [18F]FDG excreted in the urine we estimated intraluminal [18F]FDG radioactivity concentration. Thin regions of interest (ROI) were drawn in the renal cortex and in the renal medulla (Figure A), and the PET data were analysed using fractional uptake rate (FUR). FUR were multiplied by plasma glucose values to obtain cortical and medullary GU rates. The estimates of intraluminal [18F]FDG radioactivity were then used to correct the GU values.
Results: We found that the corrected glucose uptake is consistently higher in the medulla than cortex (3.30 [4.67] vs 1.15 [3.43] μmol.min-1.100ml-1 in fasting (p=0.0006), and 3.32 [2.43] vs 2.05 [2.99] μmol.min-1.100ml-1 in insulin clamp (p<0.0001)). Both cortical and medullary glucose uptake are higher in lean than obese participants under both fasting (cortical GU: 2.2 [1.6] vs -0.1[3.8] μmol.min-1.100ml-1, p=0.008; medullary GU: 6.6 [6.9] vs 2.4 [3.2], p=0.008) and insulinized conditions (cortical GU: 3.8 [0.9] vs 1.2 [1.6] μmol.min-1.100ml-1 p=0.0008; medullary GU: 5.0 [2.9] vs 2.9 [1.3], p=0.002). Moreover, cortical but not medullary glucose uptake is increased from fasting to the insulinized condition (Figure B).
Conclusion: Compared to the existing literature based on the AV differences technique, [18F]FDG-PET underestimates considerably renal GU rates. However, the data show for the first time that [18F]FDG-PET can still provide relevant physiological information on regional renal glucose uptake on the condition that [18F]FDG uptake is corrected for tubular radioactivity. Based on this study, we conclude that the human renal cortex is an insulin sensitive tissue.
Clinical Trial Registration Number: NCT00793143
Supported by: EFSD/MSD Research Programme
Disclosure: E. Rebelos: None.
465
Zinc-dependent insulin multimerisation: a study using FRET
R. Fritzen1, A.J. Gourley1, C.A. Blindauer2, J. Penedo3, A.J. Stewart1;
1School of Medicine, University of St Andrews, St Andrews, UK, 2Department of Chemistry, University of Warwick, Coventry, 3School of Biology, University of St Andrews, St Andrews, UK.
Background and aims: Both physiological insulin and insulin analogues used in the treatment of diabetes, exist in different multimeric states. Physiological insulin is stored in pancreas as a hexamer but is thought to only be “active” (able to bind to cognate receptors) in its monomeric form. Formation of insulin multimers requires the coordination of Zn2+and Ca2+ ions, where initially dimers form, before coming together to form the hexamer. Decomplexation of the hexamer after pancreatic release and the factors that control this process at physiologically relevant insulin concentrations (high pM/low nM range) have not been extensively studied. This is mainly due to a lack of sensitivity in the tools and approaches that have, up until now, been used to examine this process. Förster Resonance Energy Transfer (FRET) is a physical phenomenon occurring between a donor and an acceptor fluorophore. When in the vicinity of one another (1 to 10 nm), some of the energy received by the donor upon excitation is transferred to the acceptor which emits a photon at a characteristic wavelength allowing detection. When FRET occurs, the intensities of the donor and acceptor decrease and increase respectively proportionally to the distance separating the two fluorophores. Here we have generated insulin molecules labelled with the FRET-compatible dyes, Atto532 and Atto647N to study insulin complexation and decomplexation.
Materials and methods: Human insulin was crosslinked to Atto532 and Atto647N NHS esters through bioconjugation, where covalent linkage is targeted to the LysB29 residue of insulin. Labelling efficiency was determined using time-of-flight mass spectrometry and absorbance spectroscopy. Insulin complexes/multimers were examined by FRET using a fluorescence spectrophotometer under different conditions (varying metal ion concentrations, temperature and pH).
Results: We obtained >70% mono-labelled insulins with each respective dye. The insulins when mixed readily formed multimeric complexes that produced characteristic FRET signals. Mass spectrometric analysis of samples provided confirmation (and characterisation) of respective multimeric forms of insulin.
Conclusion: Here we have developed sensitive fluorescence-based tools to study insulin multimerization and decomplexation at physiologically relevant concentrations. These can be used to better understand insulin dynamics in vivo. Furthermore, the same approach may be applied to insulin analogues to study the pharmacodynamics of insulin-based therapeutics under different conditions.
Supported by: This project is supported by BBSRC (grant no. BB/V014684/1).
Disclosure: R. Fritzen: None.
466
Mechanisms underlying nutrient sensing in human glucose-dependent insulinotropic polypeptide secreting cells
N. Guccio, E.L. Miedzybrodzka, C.A. Smith, R. Bany Bakar, F. Reimann, F.M. Gribble;
Wellcome-MRC Institute of Metabolic Science-Addenbrooke's hospital, University of Cambridge, Cambridge, UK.
Background and aims: Glucose-dependent insulinotropic peptide (GIP) is a hormone secreted by a subset of enteroendocrine cells, namely K cells, found in the duodenum and proximal jejunum. GIP has long been neglected in comparison to its sister incretin glucagon-like peptide-1 (GLP-1), partly because in early studies GIP was less efficient than GLP-1 in stimulating insulin secretion during hyperglycaemic clamps in diabetic volunteers. The superior weight reduction seen with dual GIPR/GLP-1R agonist in comparison to GLP-1R-only agonist in recent clinical studies has, however, rekindled interest in GIP and the aim of this study was to identify mechanisms of GIP secretion from human K-cells.
Materials and methods: CRISPR-Cas9-mediated homology directed repair (HDR) was used to insert a yellow-fluorescent protein (Venus) transgene behind a ribosomal stutter sequence (P2A) to enable bicistronic expression from the GIP locus in human duodenum-derived organoids. The newly generated organoid reporter line was then used to perform fluorescence-activated cell sorting of Venus-expressing K cells for bulk RNA-sequencing, and live single-cell imaging experiments after loading with the Ca2+-sensitive fluorescent indicator fura2-AM. GIP secretion was assessed by ELISA of cell supernatants.
Results: Comparison of the transcriptome of Venus positive K-cells and fluorescent negative cells collected in parallel demonstrated strong enrichment in GIP mRNA (103-fold), confirming correct targeting of the fluorescent protein. Whilst the sodium dependent glucose transporter SGLT1 (SLC5A1), previously implicated in glucose sensing, was similarly expressed in both cell populations, a number of G-protein coupled receptors potentially involved in nutrient sensing were enriched in K-cells (n=4). These include amino acid-sensing receptors CASR (p<0.05) and GPR142 (p<0.05), long-chain fatty acids (LCFAs) receptor FFAR1 (p<0.05), monoacylglycerol-sensing receptor GPR119 (p<0.05) and bile acid-sensing receptor GPBAR1 (p<0.05). K-cell intracellular calcium levels were elevated following stimulation with 10 mM glucose (p<0.01; n=8), 20 mM L-phenylalanine (p<0.001, n=13), 20 mM L-tryptophan (p<0.0001; n=15) and 10 μM AM1638, a FFAR1 agonist (p<0.0001; n=22). Ca2+-responses correlated broadly with secretory responses, with AM1638 (10 μM) and glucose (10 mM) stimulating GIP-secretion 2- (p<0.01, n=8) and 1.8-fold (p<0.001; n=12), respectively.
Conclusion: This study has generated the first in-vitro model for studying identified human K cells. We identified a number of nutrient sensing pathways underlying human GIP secretion and the model will be employed to investigate strategies for future modulation of K-cell secretion in diabetes and/or obesity therapy.
Supported by: Wellcome Trust
Disclosure: N. Guccio: None.
SO 28 Understanding insulin sensitivity: lessons from the clinic
467
Comparison of predictive value of insulin resistance indices in young women with polycystic ovary syndrome
S. Hong1, Y.-A. Sung1, Y. Hong1, H. Lee1, K. Jeong2, H. Chung2, D. Song1, H. Jung1;
1Internal Medicine, Ewha Womans University College of Medicine, 2Obstetrics and Gynecology, Ewha Womans University College of Medicine, Seoul, Republic of Korea.
Background and aims: Polycystic ovary syndrome (PCOS) is a reproductive-metabolic disorder and insulin resistance is the main pathophysiology of PCOS. We aimed to investigate the superiority of four indices of insulin resistance in young women with PCOS and controls.
Materials and methods: This cross-sectional analysis included 667 women with PCOS, diagnosed by the National Institute of Child Health and Human Disease criteria, and 289 controls regular cycling control women. Triglyceride/glucose (TyG) index, lipid accumulation product (LAP), visceral adiposity index (VAI), and triglyceride/HDL cholesterol (TG/HDL) were measured, and insulin resistance was defined as a value of homeostatic model assessment-insulin resistance (HOMA-IR) ≥ 2.5.
Results: In women with PCOS, the one-standard deviation (SD) increment of TyG index, LAP, VAI, and TG/HDL significantly increased insulin resistance (all P <0.05). Similar trends were observed in control women, but TyG index was not statistically significant. In both groups, LAP constituted the strongest impact on detecting insulin resistance than TyG index, VAI, and TG/HDL (OR = 4.85 in PCOS, OR = 1.91 in control, respectively). All ROC curves of TyG index, LAP, VAI, TG/HDL for detecting insulin resistance were located statistically above the diagonal nondiscrimination line. The optimal cutoff values for TyG index, LAP, VAI, and TG/HDL for detecting insulin resistance were 4320, 24.4, 1.88, and 1.85 in PCOS women and 3344, 25.9, 1.34, and 1.70 in control women, respectively.
Conclusion: In women with PCOS, TyG index, LAP, VAI, and TG/HDL showed similar potential in detecting insulin resistance and LAP was the strongest discriminator.
Disclosure: S. Hong: None.
468
Identification of myokines potentially involved in the improvement of glucose homeostasis after bariatric surgery
L. Orioli1,2, M. Canouil3,4, K. Sawadogo5, L. Ning3,4, L. Deldicque6, P. Lause2, M. de Barsy1, P. Froguel7,3, A. Loumaye1, Y. Deswysen8, B. Navez8, A. Bonnefond3,4, J.-P. Thissen1,2;
1Department of Endocrinology and Nutrition, Cliniques Universitaires Saint-Luc, Brussels, Belgium, 2Institute of Experimental and Clinical Research (IREC), UCLouvain, Brussels, Belgium, 3European Genomic Institute for Diabetes (EGID), Institut Pasteur de Lille, Lille, France, 4University of Lille, Lille University Hospital, Lille, France, 5King Albert II Cancer and Hematology Institute, Cliniques Universitaires Saint-Luc, Brussels, Belgium, 6Institute of NeuroScience (IONS), UCLouvain, Louvain-La-Neuve, Belgium, 7Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK, 8Department of Oeso-gastro-duodenal and Bariatric Surgery, Cliniques Universitaires Saint-Luc, Brussels, Belgium.
Background and aims: Our study aims to identify myokines potentially involved in the improvement of glucose homeostasis after bariatric surgery.
Materials and methods: The MYDIASECRET Study is a prospective, longitudinal, and interventional study. Obese insulin-resistant patients were evaluated before and 3 months after bariatric surgery (n=62). Glucose homeostasis was assessed using the HOMA test. Muscle biopsies were taken from the vastus lateralis (n=39). Changes in muscle transcriptome were determined by RNA-sequencing (n=12). Differentially expressed genes (FDR≤0.10) encoding myokines regulating glucose homeostasis were identified based on the prediction of a signal peptide, annotations, and literature. Changes identified by RNA-sequencing were confirmed by real-time quantitative PCR (RT-qPCR) (n=39). Changes in plasma levels of myokines were measured by ELISA (n=62). A linear regression analysis was used to predict changes in glucose homeostasis parameters induced by bariatric surgery from changes in myokines gene expression (n=39) or circulating levels (n=62).
Results: Insulin sensitivity was significantly increased (HOMA-S, +34 %, p<0.001). Among the 1,363 differentially expressed genes, 4 up-regulated genes (BDNF, CX3CL1, ADAMTS9, ANG, fold-change ≥ 1.3) and 2 down-regulated genes (FDNC5, MSTN, fold-change ≤ 0.7) encoded myokines known to regulate glucose homeostasis. CX3CL1 (encoding fractalkine, +73 %, p<0.001) and MSTN (encoding myostatin, -45 %, p<0.001) were the most up- and down-regulated genes based on RT-qPCR results. Accordingly, the plasma levels of fractalkine were increased (+7 %, p=0.001) and myostatin levels decreased (-32 %, p<0.001). However, improved glucose homeostasis was not correlated with changes in gene expression or circulating levels of fractalkine or myostatin. In contrast, increased muscle expression of BDNF encoding brain-derived neurotrophic factor was significantly associated with decreased insulin resistance after surgery (HOMA-IR, adjusted estimate -0.58 [-0.96; -0.19], p=0.004).
Conclusion: Our study shows that improved glucose homeostasis after bariatric surgery is associated with changes in the expression of genes encoding myokines known to regulate glucose homeostasis. Changes in fractalkine and myostatin expression are reflected into changes in their circulating levels, but do not correlate with changes in glucose homeostasis. In contrast, the association between increased expression of BNDF and improved insulin sensitivity suggests that BDNF is a myokine potentially improving glucose homeostasis after bariatric surgery.
Clinical Trial Registration Number: NCT03341793
Supported by: FNRS, Fondation Saint-Luc, Novo Nordisk, AstraZeneca, Sanofi
Disclosure: L. Orioli: None.
469
Do sex differences in pediatric type 1 diabetes exist? A systematic review
S.A.G. de Vries1, C.L. Verheugt1, D. Mul2, M. Nieuwdorp1, T.C.J. Sas3,2;
1Vascular Medicine, Amsterdam University Medical Centers, Amsterdam, 2Diabeter, Rotterdam, 3Pediatrics, Erasmus University Medical Center, Amsterdam, Netherlands.
Background and aims: Sex differences are present in cardiovascular care and outcomes among adult patients with type 1 diabetes mellitus (T1DM), which typically commences in childhood. Younger age at diagnosis and longer disease duration increase risks of adverse outcomes. It is not known if sex influences care and outcomes in childhood. This systematic review provides an overview of sex differences in children with T1DM with a focus on patient and disease characteristics, treatment, comorbidities and complications.
Materials and methods: Literature in MEDLINE up to June 15, 2021 was searched, using the terms diabetes mellitus, sex characteristics, sex distribution, children and/or adolescents. Articles reporting sex differences were identified and assessed on quality with Joanna Briggs Institute critical appraisal tools. Narrative synthesis and an adapted Harvest plot were used to summarize evidence by category.
Results: A total of 8,640 articles were identified, rendering 86 studies for review. Selected studies showed higher HbA1c for girls, at diagnosis, during treatment and HbA1c increase over time. Studies reported higher prevalence of being overweight or obese, BMI and more dyslipidemia in girls. Hypoglycemia and partial remission occurred more in boys, but ketoacidosis and hospitalization in girls. Girls used pump therapy more often and needed higher insulin doses. Most comorbidities were more common in girls. All studies reported lower quality of life in adolescent girls.
Conclusion: Sex disparities were observed in children with T1DM. Several outcomes were disadvantageous to girls, especially during adolescence. Attention for the cause and treatment of these differences may provide an opportunity for better outcomes.
Disclosure: S.A.G. de Vries: None.
470
Effects of Lisosan-G on postprandial hypoglycaemia after bariatric surgery
D. Moriconi1, S. Baldi1, L. Antonioli1, L. Pucci2, S. Taddei1, M. Nannipieri1;
1Department of Clinical and Experimental Medicine, University of Pisa, 2IBBA, CNR, Pisa, Italy.
Background and aims: Postprandial hypoglycemia (PPHG) is a known late complication of bariatric surgery that increase the risk of cardiac arrhythmias and persistent cognitive deficits. Lisosan G (LG) is a fermented powder obtained from whole grains (Triticum aestivum) with beneficial effects on metabolic parameters. The aims of the study were: 1) to evaluate the effectiveness of using Lisosan G added to the diet in reducing PPHG events in patients undergoing RYGB, 2) to investigate the mechanism by which LG acts on the gut-pancreas axis.
Materials and methods: Seventeen patients self-reporting symptoms/signs of PPHG, who had undergone gastric bypass between 2015 and 2018, were enrolled. Each patients underwent clinical examination, blood test and 5-hour oral glucose load test (OGTT). Then, subjects were kept on a free diet with 15 days of continuous glucose monitoring (CGM, Freestyle Libre, Abbot). The diet was recorded in a food diary. At the end of the period LG was added to the same dietary regimen (5 g LG-powder, bid) and CGM was repeated for another 15 days. Finally, another 5-hours OGTT was performed at the end of the treatment period. Plasma insulin and C-peptide were measured by electrochemiluminescence on a Cobas e411 instrument (Roche Diagnostics S.p.A.). Plasma total GLP-1 concentrations were measured by ELISA (Millipore Corporation). Insulin sensitivity was assessed by the oral glucose insulin sensitivity index (OGIS). PPHG was defined as a plasma glucose level of ≤60 mg/dl.
Results: The mean age was 41±7 and BMI was 43.2±5.7 kg/m2 at the time of the surgery. PPHG was developed 29±8 months after RYGB and BMI was 25.8±3.7 Kg/m2. During the first CGM period, PPHG episodes were 4.9[3.2-8.1] and decreased with LG administration as recorded by second CGM period 1.9[0.9-3.3], pre vs post LG respectively, p=0.008. In addition, no episodes of severe hypoglycemia (blood glucose <50 mg/dl) occurred under LG treatment. Furthermore, during LG treatment, the overall length of time with glycemia <60 mg/dl during CGM, was reduced (210 [150-510] vs 25 [10-130], minutes, p=0.003). A marked increase in the blood glucose nadir (44±11 vs 55±10, mg/dl, pre vs post respectively, p= 0.033) was find during OGTT before and after administration of LG. Conversely, no difference was found in fasting glycemia and in the time-to-nadir (142±21 vs 154±34, minutes, p=ns). There were no differences in fasting GLP-1 (42±20 vs 37±17, pM/l, p=ns) with or without LG, while GLP-1 AUCtot significantly reduced during LG treatment (12.1±4.9 vs 8.9±4.2, nmol/L×min, p= 0.032); contextually, the insulin output during the first 2-hours of the OGTT (Insulin AUC0-120) significantly decreased (57±12 vs 48±10 nmol/m−2, p= 0.039). Finally, insulin sensitivity remained stable before and after LG administration (514±55 vs 534±44 mL/min-1· m-2).
Conclusion: LG is effective on reducing PPHG episodes, probably by attenuating GLP-1 and insulin release in response to the glucose load. This effect leads to a reduction in the severity of hypoglycemia
Disclosure: D. Moriconi: None.
471
Rabson-mendenhall syndrome: a rare cause of severe insulin resistance
R. Helal, T. Muammar, E.G. Fojas, N. Lessan;
Imperial College London Diabetes Centre, Abu Dhabi, United Arab Emirates.
Background and aims: Rabson-Mendenhall syndrome (RMS) is a rare autosomal recessive disorder marked by severe insulin resistance, associated with various phenotypic manifestations.
Materials and methods: We report the case of a 15 year old female with poorly-controlled diabetes who was referred to our paediatric diabetes clinic. She was diagnosed with RMS at the age of 50 days; genetic study showed homozygous mutation for R141W in INSR gene known to cause RMS. A strong family history of Rabson-Mandhall syndrome in her mother’s cousins (one affected and two carriers) was noted.
Results: She was born full-term through normal vaginal delivery; birth weight was 2.19 kg. She required admission to the neonatal unit for 1 week. She was re-admitted on day 50 with diabetic ketoacidosis (DKA); insulin level was high at 737 μIU/mL, IA2 and GAD antibodies negative, and C-peptide > 18 ng/ml. After recovery, patient’s blood glucose level was stabilized on high concentrated insulin dose 600-800 IU/day through continuous subcutaneous insulin infusion (CSII); Oral rosiglitazone was added after 2 months. At the age of 6 years, she was continued on CSII, with Rosiglitazone replaced by oral Mecasermin.At age 12, she had three further episodes of DKA, associated with acute infections- right lobar pneumonia, cellulitis of the abdominal wall, and acute gastroenteritis respectively. In each episode, she required even higher doses of intravenous insulin infusion (up to 2 IU/kg/hour). CSII treatment led to development of severe lipodystrophy at the cannula insertion sites. Therefore, on two occasions her treatment was temporarily changed to multiple daily injection therapy (MDI); Glargine 300 IU daily, Lispro 20 IU TID along with Mecasermin and SGLT2 inhibitor. Her fifth and latest episode of DKA in 2020 was precipitated by severe MRSA septicaemia, was managed with very high doses of intravenous insulin infusion, and resolved within a few hours.Currently, the patient is on combined therapy with Mecasermin 25 units subcutaneously twice daily, oral Dapagliflozin 10 mg once daily along with CSII (Medtronic 640G) with insulin lispro (total daily dose is 261 units /day (4.6U/kg/day) with an insulin to carbohydrate ratio of 1:3 gm, and insulin sensitivity factor of 20 mg/dL. Despite patient compliance, family support, and the intensified therapy, her glycaemic control remains poor with an average blood glucose of 304 ± 86 mg/dL and HbA1c of 10.8 ± 1.2 %. She has recently started a trial of recombinant human leptin and shifted to CSII closed-loop system (Medtronic 780G), with the aim of improving her insulin resistance, glycaemic control, and quality of life.
Conclusion: In conclusion, we have presented the challenges in management of RMS. RMS is very rare, but life expectancy is short with early mortality related to diabetes-related complications, resulting from severe insulin resistance and poor glycaemic control. Using a closed-loop insulin pump together with recombinant human leptin therapy to improve insulin sensitivity and reduce hyperinsulinemia, may offer new potentially useful lines of management.
Disclosure: R. Helal: None.
472
Metformin has dual effects on insulin hypersecretion: opportunity to improve glucose metabolism in children with obesity
Q. Wen1,2, R. Stenlid1,2, A.I. Chowdhury1, I. Ciba2, B.K. Aydin2, S.Y. Cerenius1, A. Forslund2, P. Bergsten1,2;
1Medical cell biology, Uppsala University, 2Women's and Children's health, Uppsala University, Uppsala, Sweden.
Background and aims: In children with obesity, insulin hypersecretion is proposed to precede insulin resistance. In attempts to attenuate such dysfunctional insulin secretion we reported that metformin prevented insulin hypersecretion from human isolated islets of Langerhans. In the present study, we aimed to investigate how metformin affects insulin hypersecretion with a translational approach and the window when metformin has more beneficial effects in children with obesity.
Materials and methods: Participants from the Uppsala Longitudinal Study of Childhood Obesity (ULSCO) cohort, who fulfilled the criteria of hyperinsulinemia and metformin treatment for at least six months (n=21), were evaluated retrospectively, using anthropometric measurements, OGTT, HbA1c, cholesterol and triglycerides before and after metformin treatment (mean duration 1.2 years). Beta-cell function and insulin resistance were estimated by insulinogenic index, oral disposition index and insulin AUC0-120/glucose AUC0-120 and HOMA-IR. Patients were classified as either “reducing” (n=9) or “increasing” (n=12) based on their insulin levels in OGTT before and after metformin treatment. Reduced or increased insulin AUC0-120 was used to define the groups.Isolated human islets were cultured in the presence of elevated palmitate levels, which induces insulin hypersecretion, for 0.5, 1, 2 or 3 days. Metformin was introduced after 0.5 or 1 day of palmitate exposure. After culture, islets were perfused and insulin secretion measured dynamically in the presence of 5.5 and 11mM glucose.
Results: In the insulin “reducing” group, 2-h glucose was reduced from 8.9±1.2 to 7.7±1.4 mmol/L (p=0.03) and triglycerides from 1.5±0.8 to 1.2±0.5 mmol/L (p=0.02). There was no change in these parameters in the insulin “increasing” group. No changes were observed in HOMA-IR in either group. Change in BMI was positively associated with alteration in insulin AUC 30-120 (r2=0.21, p=0.03) when investigated in 21 metformin treated individuals.Accentuated glucose-stimulated insulin secretion (GSIS), 44% (n=5, p=0.03), was observed in perfused human isles cultured 1 day in palmitate compared to islets cultured in absence of palmitate. GSIS then declined during the following two days of culture. This decline in GSIS was counteracted and increased, 66% (p=0.02), when metformin was introduced after 1 day’s culture in palmitate compared to islets exposed to palmitate alone for three days. When metformin was added to islets after 0.5 day of initial culture in the presence of palmitate, insulin secretion was reduced, 18% (n=7, p=0.01), during the subsequent 0.5 day compared to islets cultured for 1 day in palmitate only.
Conclusion: Metformin has dual effects on beta-cell hypersecretion. If introduced in the early stage of insulin hypersecretion metformin attenuates insulin hypersecretion and thereby may attenuate beta-cell hyperactivity. On the other hand, if metformin is introduced in the late stage, it increases insulin secretion. Clinical implications are that early introduction of metformin to children with obesity is preferable, which may restore normal insulin responses coupled to reduction in BMI and improved glycemic control.
Supported by: European Commission's Seventh Framework Programme, Swedish Diabetes Association
Disclosure: Q. Wen: None.
473
Changes in insulin sensitivity and the presence of partial clinical remission in type 1 diabetes: Prospective InLipoDiab1 study
A. Grzelka-Wozniak1, A.S. Januszewski2, P. Niedzwiecki1, A.J. Jenkins2, D. Zozulinska-Ziolkiewicz1, A. Uruska1;
1Department of Internal Medicine and Diabetology, Poznan University of Medical Sciences, Poznan, Poland, 2NHMRC Clinical Trials Centre, The University of Sydney, Camperdown NSW, Australia.
Background and aims: The phenomenon of insulin resistance is recognised in Type 1 diabetes (T1D), especially in patients with long-term T1D and is aggravated by exogenous insulin, body weight gain and ageing. Shortly after T1D onset a transitory period (‘honeymoon’) of clinical remission may occur, which is characterized by regained beta cell function, advantages of decreased exogenous insulin need and reduced risk of chronic diabetes complications. Factors affecting the clinical remission (CR) duration are physical activity, adjusted insulin dosing and non-smoking. Good insulin sensitivity (IS) may be also a CR-promoting factor, however there are little data on IS changes after T1D onset. Whilst the gold standard of IS is a euglycaemic hyperinsulinaemic clamp study this is not a practical clinical tool. Based on such clamps several formulae exist to estimate IS. The aim was to describe relationships between estimated IS and remission status during the first year of T1D.
Materials and methods: In a longitudinal observational study we analysed data from 164 patients (110 men, 67%) with newly diagnosed T1D aged (median (LQ, UQ) 26 (22, 31) years at T1D-onset, which was confirmed by autoantibodies. This IS analysis was a part of the Insulin Therapy and Lipoproteins' Profile in Type 1 Diabetes (InLipoDiab1) Study, and was performed at 3- time-points: baseline (at T1D diagnosis), and 3- and 12-months later. The primary endpoint is presence of partial CR (pCR) at 12-months, defined as IDAA1c≤9 calculated according to Mortensen et al.: A1c(%)+[4xinsulin dose(U/kg/day)]. The study group was divided by pCR presence or absence. IS was estimated by formulae validated with euglycemic hyperinsulinemic clamps by Januszewski et al.: eLog10M/I = -1.299 + 0.295(sex, M=1) + 0.546(HDL-C mM) - 0.030(BMI) - 0.057(HbA1c %) - 0.503(WHR) and eGDR = exp(0.861 + 0.705(sex, M=1) + 0.012(age yrs) - 0.101(HbA1c %) + 1.204(HDL-C mM) - 0.017(PP mmHg)).
Results: At 1-year post-T1D diagnosis pCR was observed in 92 patients (69%). During the 1-year observation IS in the whole group improved (median eGDR increased from 3.53 to 10.34 mg/kg/min, p<0.01). Subgroup comparison revealed greater improvement in the pCR subgroup for eLog10M/I and eGDR: (mean±SD) T0: -2.13±0.32, 3-months: -1.62±0.39, and 12-months: -1.57±0.44, p for trend<0.001 and median (LQ, UQ) T0: 2.79 (0.85, 4.74) mg/kg/min, 3-months 6.61 (1.50, 11.72) mg/kg/min, and 12-months 10.47 (4.46, 16.48) mg/kg/min, Friedman ANOVA p <0.001, respectively. In univariate logistic regression, IS assessed by eLog10M/I and eGDR was associated with pCR ORs of 5.88 (95% CI 1.53, 20.00), p<0.01 and 1.09 (95% CI 1.01, 1.16), p=0.04 respectively.
Conclusion: During the first year of T1D IS improvement was associated with higher chance of partial clinical remission. Improving IS could be a treatment target in T1D.
Clinical Trial Registration Number: NCT02306005
Disclosure: A. Grzelka-Wozniak: None.
SO 29 Glucose homeostasis regulation beyond insulin
474
Carotid body: a new intervenient of GLP-1 effects in cardiometabolic homeostasis
D. Sampaio-Pires, F.O. Martins, B.F. Melo, J.F. Sacramento, S.V. Conde;
CEDOC - CHRONIC DISEASES RESEARCH CENTER, Lisboa, Portugal.
Background and aims: GLP-1 is released by the gut in response to food intake. It binds to GLP-1 receptors (GLP-1R) stimulating insulin secretion and decreasing glucagon secretion in a glucose-dependent manner. Additionally, GLP-1 promotes insulin sensibility, decreases gastric emptying, improves cardiac function, and decreases weight by promoting satiety. As such, GLP-1R agonists are used in type 2 diabetes (T2D) treatment for their effects in glycemic control but also for their cardiovascular and weight benefits. The carotid bodies (CBs) are peripheral chemoreceptors, defined as O2 sensors, whose activation by hypoxia induce respiratory reflexes to normalize blood gases, also regulating blood pressure. These organs are also metabolic sensors, being its overactivation involved in the development of metabolic disorders. Herein, we tested the hypothesis that GLP1 acts on the CB, being involved in the cardiorespiratory responses elicited by this organ.
Materials and methods: Experiments were performed in male Wistar rats submitted to 10 weeks of a standard (NC) or 60% lipid-rich diet (HF) followed by a sham or carotid sinus nerve (CSN) resection surgery. The effect of liraglutide, a GLP-1R agonist (200 μg/Kg, i.c.), on basal and ischemic hypoxia CB-mediated cardiorespiratory responses were evaluated. Ischemic hypoxia was induced by occlusion of the common carotid artery (OCC) during 5 and 15 sec. Minute volume (MV), heart rate (HR) and blood pressure (BP) were recorded. The presence of GLP-1R on the CB was analyzed by immunohistochemistry and the activation of its signalling pathway by Western Blot. CBs neurotransmitters release and content in the response to liraglutide were evaluated. Significance between the mean values was calculated by t-student test and ANOVA and p < 0.05 was considered as significant.
Results: GLP-1R is expressed in the CB colocalizing with tyrosine hydroxylase, a marker for type 1 cells. Liraglutide increased baseline MV in NC and HF rats by 30% and 40% respectively, while decreased BP by 20% and 15% in the same groups. Liraglutide did not modify HR in any of the groups. CSN resection abolished liraglutide effects on ventilation, decreasing its effects on MV in NC (42%, p<0.05) and HF (43%, p=0.06) animals. CSN resection attenuated the effects of liraglutide on BP. HF animals exhibited a higher ventilatory response to OCC compared to NC animals, an effect abolished in CSN resected animals. Liraglutide decreased the effect of OCC5 on MV by 39% and 49% in NC and HF animals and the effect of OCC15 by 68% (p<0.05) in HF animals. BP in response to OCC5 decreased by 14% and 13% in NC and HF animals and Liraglutide further decreased BP in response to OCC5s by 72% and 52% (p<0.05) in the same groups. Liraglutide effects on MV and BP were abolished by CSN resection.
Conclusion: GLP-1 is present in the CB and modulates differentially the spontaneous and ischemic hypoxic cardiorespiratory responses mediated by this organ. Under basal conditions, Liraglutide acts on the CB to increase ventilation and decrease BP while under ischemic hypoxia, it blunts the respiratory reflex and heightens the decrease in blood pressure. Modulation of GLP-1 function in the CB might be important for the treatment of cardiometabolic comorbidities in obesity and T2D.
Supported by: Portuguese Foundation for Science and Technology contract reference CEECIND/04266/2017
Disclosure: D. Sampaio-Pires: None.
475
Insulin resistance is associated with an elevated glucose-dependent cortisol axis response: Impact in type 2 diabetes?
M.H. Lundqvist, M.J. Pereira, J.W. Eriksson;
Department of Medical Sciences, Clinical Diabetes and Metabolism, Uppsala University, Uppsala, Sweden.
Background and aims: The importance of insulin-antagonistic counterregulatory responses for glucose regulation and pathogenesis of type 2 diabetes (T2D) has been increasingly emphasized. Recently, our research group has demonstrated an augmented ACTH and cortisol response to hypoglycemia and a static elevation of glucagon levels in overweight vs lean subjects. Moreover, insulin resistance but not obesity per se was independently associated with those hormonal alterations. The aim of this study was to explore counterregulatory responses across a wide glycemic range in subjects with early T2D in comparison with control subjects without diabetes (C).
Materials and methods: Subjects with T2D; n=14, 6 males, median age 50 years (range 22-62), BMI 38.0 kg/m2 (27.4-48.8), HbA1c 48 mmol/mol (37-71), diabetes duration 18 months (0-57), 10 on metformin, 4 drug naïve; were matched (gender, age, BMI) to control subjects (C) without diabetes; n=14, 5 males, age 51 years (23-57), BMI 36.5 kg/m2 (24.1-49.5), HbA1c 35 mmol/mol (30-41). They underwent one stepwise hypoglycemic clamp (glucose nadir 2.7 mM) and one stepwise hyperglycemic clamp (max +9mM) with repeated cortisol axis measurements (cortisol, ACTH). Spearman correlations between hormonal responses and M-value, BMI and HbA1c were assessed in an extended cohort (total n=48) including additional subjects without diabetes; n= 20, 5 males, age 37 years (22-60), BMI 25.2 kg/m2 (19.9-31.4), HbA1c 34 mmol/mol (27-40). Multilinear regressions (MLR) were performed with M-value, BMI and HbA1c as independent variables in every model.
Results: In the hypoglycemic clamp, neither cortisolΔAUC nor ACTHΔAUC differed significantly in T2D vs C. In the extended cohort (n=48), cortisolΔAUC (Fig) and ACTHΔAUC correlated inversely with M-value (p<0.01 and <0.001, respectively) and positively with BMI (p<0.05, <0.01). In MLR, only M-value remained significantly associated with cortisolΔAUC (std beta -0.49, p <0.05; model R2 0.193, p <0.05) and ACTHΔAUC (std beta -0.56, p<.0.01, model R2=.290, model p<0.01). In the hyperglycemic clamp, the cortisol axis response did not differ in T2D vs C. In the extended cohort, ACTHAUC correlated positively with BMI (p<0.05), but the association did not remain significant in MLR.
Conclusion: Neither early T2D nor long-term glycemia per se was independently associated with glucose-dependent cortisol axis response. Instead, only insulin resistance was independently associated with elevated cortisol axis response to hypoglycemia, and this was seen across normoglycemic and T2D subjects. We propose that a gradual cortisol axis dysregulation can contribute to development of insulin resistance and, secondarily, T2D. Furthermore, the elevated ACTH response suggests a CNS origin behind this dysregulation.
Supported by: ALF, Swedish Diabetes Foundation, The Ernfors foundation, TREATMENT, EXODIAB, NovoNordisk Foundation
Disclosure: M.H. Lundqvist: None.
476
The ghrelin-NPY system in adipose tissue as a potential therapeutic target in type 2 diabetes
D. Rosendo-Silva1, H. Eickhoff2, P. Gomes3, T. Monteiro-Alfredo1, S. Viana4, A.S. Pires5, M. Abrantes5, F. Reis4, F. Botelho5, R. Seiça2, P. Matafome1;
1Institute for Clinical and Biomedical Research (iCBR), Institute of Physiology, Faculty of Medicine, University of Coimbra, 2Institute of Physiology, Faculty of Medicine, University of Coimbra, 3Surgery Department, Hospitalar Centre University of Coimbra, 4Institute for Clinical and Biomedical Research (iCBR), Institute of Pharmacology, Faculty of Medicine, University of Coimbra, 5Institute for Clinical and Biomedical Research (iCBR), Institute of Biophysics, Faculty of Medicine, University of Coimbra, Coimbra, Portugal.
Background and aims: Adipose tissue dysfunction and dysregulation of gut hormones secretion are two of the main hallmarks of type 2 diabetes. Both mechanisms are modulated by metabolic surgery, namely the normalization of ghrelin secretion, a hormone that promotes feeding by activating neuropeptide Y (NPY)-expressing neurons in the hypothalamus and that has been suggested as a nutrient sensor. Our hypothesis is that the ghrelin/NPY system regulates adipose tissue, being altered in subjects with obesity and metabolic syndrome, and modulated through metabolic surgery.
Materials and methods: To characterize the alterations of the ghrelin/NPY system in the human visceral adipose tissue samples, gene expression profile of the ghrelin/NPY system was evaluated through RT-PCR (ghrelin and NPY receptors). The effect of metabolic surgery on this system, was studied in Goto-Kakizaki rats, maintained in a caloric diet and submitted to sleeve gastrectomy (SG). The direct effects of ghrelin in adipocytes were explored in the 3T3-L1 cell line.
Results: We found a decreased expression of Y1 and Y5 receptors with the development of type 2 diabetes, in the visceral adipose of patients with obesity versus the prediabetics (p<0.05). On the other hand, the Y2 receptor was downregulated right after insulin-resistance establishment when compared to the insulin-sensitive subjects (p<0.05). While Y1 and Y5 receptors were highly positively correlated with PPARγ expression (r= 0.679 and r=0.779 with p<0.001, respectively), Y2 was correlated with UCP-1 instead (r=0.711, p<0.001). The ghrelin/NPY system was altered in the adipose tissue of obese rats that underwent SG, with a significant increase in Y1, Y2 and ghrelin receptors compared to high fat-fed littermates (p<0.01, p<0.05 and p<0.05, respectively). Moreover, ghrelin induced lipid accumulation and adipogenesis in adipocytes in vitro and increased Y1 receptor levels.
Conclusion: The ghrelin/NPY system is downregulated with the progression of metabolic dysregulation in obesity. We demonstrate a dichotomous association of Y1/Y5 and Y2 receptors regarding energy balance regulation in adipose and ghrelin treatment induced lipid accumulation, thus supporting its role as a nutrient sensor. The ghrelin/NPY system was restored by SG, in the visceral adipose tissue of an animal model of obesity and type 2 diabetes, further highlighting the potential as a therapeutic target to metabolic syndrome.
Disclosure: D. Rosendo-Silva: None.
477
Effect of ghrelin infusion on glucose tolerance and appetite after sleeve gastrectomy
N. Hedbäck1, M.-L. Dichman1, C. Dirksen1, K.N. Bojsen-Møller1, N.B. Jørgensen1, M. Hindsø1, V.B. Kristiansen1, J.J. Holst2, M.S. Svane1, S. Madsbad1;
1Hvidovre University Hospital, 2University of Copenhagen, Copenhagen, Denmark.
Background and aims: Ghrelin is an appetite stimulating peptide hormone secreted from the gastric mucosa in the fasting state with decreases in response to food intake. After sleeve gastrectomy (SG), plasma levels of ghrelin decrease dramatically. Whether this influences appetite and glucose tolerance is unknown. We therefore investigated the effects of ghrelin infusions on glucose tolerance and appetite in individuals with obesity before and three months after SG.
Materials and methods: Ten participants with no history of diabetes, scheduled for SG at Copenhagen University Hospital Hvidovre, Denmark, were enrolled in the study. Two four-hour standard mixed-meal tests with concomitant patient-blinded infusions of either acyl-ghrelin (1 pmol/kg/min) or placebo (saline) were performed in randomized order before and 3 months after surgery. Ghrelin or placebo infusions were initiated 60 min prior to meal intake and continued throughout the test day. Appetite was evaluated via Visual Analogue Scale (VAS)-scores of hunger and satiety through the test days. All values are mean ± SEM.
Results: Before SG, fasting plasma glucose was unaffected by ghrelin infusion, whereas both peak (ghrelin: 7.93 ± 0.3 mmol/L versus placebo: 6.98 ± 0.2, p<0.001) and total area under the curve (tAUC) of plasma glucose concentrations (1874 ± 61 min*mmol/L versus 1702 ± 42, p=0.03) increased during ghrelin infusion compared with placebo. Three months after SG, fasting plasma glucose was again unaffected by ghrelin infusion, and peak (8.47 ± 0.5 mmol/L versus 7.80 ± 0.3 mmol/L, p=0.04) and tAUC of plasma glucose concentrations (1796 ± 69 min*mmol/L versus 1611 ± 46 min*mmol/l, p=0.04) increased during ghrelin infusions compared with placebo. Before SG, scores of satiety and hunger were unaffected by ghrelin infusion compared with placebo (tAUC VAS satiety p=0.47; tAUC VAS hunger p=0.31). This was also observed three months after surgery (tAUC satiety p=0.89; tAUC hunger p=0.75).
Conclusion: We found that ghrelin infusions increase postprandial plasma glucose concentrations in individuals with obesity both before and following SG. In contrast, ghrelin infusion did not change visual analogue scale scores of hunger or satiety. This finding suggests a role of ghrelin in the regulation of postprandial glucose metabolism. The response appears to be maintained following SG. The decreased ghrelin secretion might be important for the glucose control after SG.
Clinical Trial Registration Number: NCT04055025
Supported by: European Research Council
Disclosure: N. Hedbäck: None.
478
Pentose phosphate pathway utilisation of gluconeogenic glucose-6-phosphate is increased in mice fed a high-fat/high-fructose diet compared to high-fructose alone
G. Belew1, A. Costa1, M. Meneses2, I. Viegas3, L. Tavares1, P. Oliveira4, M. Macedo2, J. Jones1;
1Metabolic Control Group, Center for Neuroscience and Cell biology, University of Coimbra, Coimbra, 2Chronic Diseases Research Centre (CEDOC), NOVA University of Lisbon, Lisbon, 3Center for Functional Ecology, University of Coimbra, Coimbra, 4Mitochondrial Toxicology and Experimental Therapeutics, Center for Neuroscience and Cell biology, University of Coimbra, Coimbra, Portugal.
Background and aims: In hepatocytes, the pentose phosphate pathway (PPP) is an important generator of NADPH for de novo lipogenesis (DNL) and for the maintenance of reduced glutathione in antioxidant defense. The development of non-alcoholic fatty liver disease (NAFLD) secondary to high sugar and/or high fat intake is characterized by elevated DNL rates and increased oxidative stress. It is not known whether PPP fluxes are modified in these settings. We developed a double tracer method that provides information on both DNL and PPP fluxes in feeding mice. We applied this to compare DNL and PPP fluxes in mice fed a normal chow diet supplemented with high-fructose corn syrup (HFCS-55) with a more obesogenic diet composed of high-fat chow supplemented with HFCS-55.
Materials and methods: Twelve C57BL/6 mice were provided with standard chow (SC) with the drinking water supplemented with 30% (w/v) of HFCS-55 (55/45 mixture of fructose and glucose) for 18 weeks. Eleven mice were placed on high-fat chow (HFC) with the same HFCS-55 supplement over the same period. At the beginning of the final evening, all mice were administered with 99% deuterated water containing and the HFCS-55 fructose component was enriched to 20% with [U-13C6]fructose. On the following morning, mice were deeply anesthetized with ketamine/xylazine and sacrificed by cardiac puncture. Arterial blood was collected and centrifuged to isolate plasma, livers were freeze-clamped, and the samples were stored at -80 °C until further processing. Liver triglyceride and glycogen were purified and analyzed for 2H and 13C-enrichments by NMR. DNL rates were quantified from lipid 2H-enrichment and adjusted for liver triglyceride levels. Whole-body adiposity was estimated from body water 2H enrichment. The fraction of gluconeogenic glucose-6-phosphate (G6P) utilized by the PPP was quantified from glycogen 13C-isotopomer analysis.
Results: Mice fed HFC + HFCS-55 has greater body weight (50 ± 1 vs 37 ± 1 g, p <0.0001), higher whole-body adiposity (29 ± 1 vs 16 ± 2%, p <0.0001), and higher liver triglyceride levels (15 ± 2 vs 8 ± 2 g/100 g liver, p < 0.025) compared to those fed SC + HFCS-55. DNL rates were not significantly different between the two groups although the high-fat chow mice had a tendency for higher rates (1.02 ± 0.1 vs 0.75 ± 0.1 g/100 g liver, p = 0.2109). However, the fraction of gluconeogenic G6P utilized by the PPP was significantly higher for the high-fat chow mice (17.0 % versus 13.8%, p = 0.034).
Conclusion: A diet high in both fat and sugar is more obesogenic than a diet high in sugar alone. In the presence of high fat, DNL rates were sustained while PPP utilization of G6P derived from gluconeogenesis was significantly increased. This may be explained by additional demand for NADPH above and beyond that consumed by DNL, possibly in response to increased oxidative stress.
Supported by: Marie Sklodowska-Curie Action-ITN Grant Agreement No. 722619; FCT
Disclosure: G. Belew: Grants; European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 722619, Portuguese Foundation for Science and Technology (FCT-FEDER-02/SAICT/2017/028147.
SO 30 Non-classical regulators of metabolism
479
Extracellular miRNAs related to obesity and type 2 diabetes as potential biomarkers for adipose tissue dysfunction
M. Clemente-Postigo1,2, C. Tercero-Alcazar1,2, M. Blasco-Montolio1, R. Guzmán-Ruíz1,2, M. Malagon1,2;
1Department of Cell Biology, Physiology, and Immunology, University of Córdoba; Maimónides Biomedical Research Institute of Córdoba (IMIBIC)., Cordoba, 2CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III., Madrid, Spain.
Background and aims: Adipose tissue (AT) dysfunction is a key player in obesity-related metabolic comorbidities and even might precede type 2 diabetes (T2D) onset. microRNAs (miRNAs), small RNAs that regulate gene expression, are released to the circulation and have emerged as potential tools to identify tissue dysfunction. AT has been proposed as a main contributor of circulating miRNAs (c-miRNAs), but it has not yet been characterized those c-miRNAs associated with obesity and/or T2D that come from AT and then, may also serve as biomarkers for AT dysfunction. Early AT dysfunction diagnosis may be crucial for obesity-related co-morbidities prevention and treatment. Thus, we aimed at identifying AT-derived extracellular miRNAs related to obesity and T2D that can serve as biomarkers for AT dysfunction.
Materials and methods: Public available databases interrogation for candidate c-miRNAs selection (i.e. c-miRNAs previously related to obesity and/or T2DM but also expressed in adipose tissue or cells) was performed. An in silico analysis of candidate c-miRNAs target genes and canonical pathway prediction analyses were also performed to confirm their putative role in relevant biological processes for AT function. In vitro preadipocyte and mature adipocyte 3T3-L1 models of obesity-related conditions [inflammation (TNFα) and insulin resistance (hyperglycaemia and hyperinsulinemia-HGHI)] were performed. In vitro AT fibrosis models with 3D cell cultures based on collagen I (COLI) hydrogels alone (control) or mixed with other AT extracellular matrix components altered in insulin resistance and obesity (i.e. COLVI and/or Lumican) were implemented. Extracellular miRNAs released in cell culture supernatant were measured and the expression of miRNA machinery related-genes were determined.
Results: Database interrogation and in silico analysis highlighted miR-15a-5p, miR-15b-5p and miR-24-3p as potential c-miRNA candidates that can be related to AT functionality and differentially released from AT in obesity and/or T2DM. Our in vitro models confirmed that adipocyte functional state affects the secretion of our candidate miRNAs with a significant decrease in extracellular levels of the three miRNAs analyzed (p<0.05) concomitant with a significant drop in the expression of genes involved in miRNA processing and secretion (Ago and Cd63) (p<0.05) under HGHI conditions compared to control in mature adipocytes. miRNA secretion was dependent on adipocyte differentiation stage. AT 3D environment also affected miRNA release compared to 2D cell cultures, especially for miR-24-3p (p<0.05). Specific fibrotic conditions (i.e. COLVI increase) significantly and specifically augmented miR-24-3p secretion from mature adipocytes compared to control (p<0.05).
Conclusion: Our candidate miRNAs are potential extracellular biomarkers of the physiological state of mature adipocytes, and miR-24-3p emerges as a specific biomarker of AT fibrosis.
Supported by: PID2019-108403RB-I00;PI-0092-2017;PI-0235-2021; FJCI-2017-32194; DOC_00448 (CECEU, PAIDI2020); FEDER
Disclosure: M. Clemente-Postigo: None.
480
Investigation of small non-coding RNA modulation in islet macrophages during obesity and diabetes
C. Cosentino1, A. Galli2, F. Brozzi1, R. Regazzi1;
1Université de Lausanne, Lausanne, Switzerland, 2Università di Milano, Milano, Italy.
Background and aims: Islet-resident macrophages (iMACs) play an important role in pancreatic islet development and homeostasis but their expansion and activation in obesity triggers islet inflammation, resulting in β-cell dysfunction. The gene expression profile of obese iMACs reflects a high complexity, with functional properties common to both pro- and anti-inflammatory macrophages. The activation of iMACs in obesity is likely to be elicited by unknown modulatory molecules highly sensitive to environmental changes. MicroRNAs (miRNAs) hold a key role in the control of gene expression. Moreover, the pool of transfer RNAs (tRNAs) is strictly regulated by nutrient availability and under stress conditions tRNAs can be cleaved, generating tRNA fragments (tRFs) with regulatory functions. The aim of the present work was to characterize the miRNA and tRF signatures of iMACs in obesity.
Materials and methods: Pancreatic islets were isolated from 8 weeks old overweight and hyperglycemic db/db and lean wild type (wt) mice (40.03±2.03 grams db/db vs 19.33±1.81 wt; 15.73±2.56 mmol/L blood glucose db/db vs 6.35±1.00 wt, p<0.001 t-test, n=8-12). CD45+, F4/80+, CD11b+ and CD11c+ iMACs were purified from dispersed islet cells using fluorescence-activated cell sorting (FACS). Bone marrow derived macrophages (BMDMs) from C57BL/6JRj mice were polarized in vitro to pro- and anti-inflammatory macrophages (M1 and M2, respectively). MiRNAs and tRFs differentially expressed in iMACs and BMDMs were identified by small RNA sequencing.
Results: Comparison of iMACs isolated from db/db and wt mice led to the identification of 14 differentially expressed miRNAs (≥1.5 fold change (FC), adjusted pvalue ≤ 0.05, n=4). This list was compared to the 198 differentially expressed miRNAs between M1 and M2 BMDMs. Interestingly, the anti-inflammatory miR-149-5p was the most upregulated miRNA both in obese iMACs (11.65 FC vs wt) and M2 BMDMs (37.29 FC vs M1). MiR-342-3p, previously associated with obesity, was also upregulated in db/db iMACs (2.27 FC vs wt) and more expressed in M2 compared to M1 BMDMs (2.76 FC). However, the pro-inflammatory miR-155 which is more abundant in M1 BMDM (249.3 FC vs M2) was also increased in obese iMACs (2.29 FC vs wt). tRFs analysis identified 28 of them differentially expressed in polarized BMDM and 7 in db/db iMACs (≥1.5 FC, adjusted p-value ≤ 0.05, n=4). Among the tRFs upregulated in obese iMACs, three were also enriched in M2 BMDM. The tRFs derived from tRNAAsp(GTC) were the most modulated in both datasets (34.05 FC increase in db/db iMACs vs wt ; 10.2 FC increase in M2 vs M1).
Conclusion: Small RNA profiling of obese iMACs highlighted the overexpression of anti-, but also, pro-inflammatory miRNAs, indicating that these molecules may contribute to the complex obese iMAC phenotype. Our study provide the first complete profiling of tRFs in macrophages. tRFs analysis in BMDM suggests that during macrophage polarization tRNAs undergo different cleavage pathways. Fragments derived from tRNAAsp(GTC) were highly modulated in obese iMACs and also strongly induced during M2 polarization. Functional investigation of the role of small RNAs, such as miR-342-3p and tRFs, in macrophage metabolism may contribute to better characterize the initial events that lead to islet inflammation and β-cell demise in obesity.
Supported by: MSCA
Disclosure: C. Cosentino: None.
481
Cxcl12/sdf-1 regulates skeletal muscle immunometabolism in type 2 diabetes
N.J. Pillon1, L. Lehtonen1, A. Marica1, J.A.B. Smith1, A.V. Chibalin2, A. Krook1, J.R. Zierath2;
1Physiology and Pharmacology (FYFA), 2Molecular Medicine and Surgery (MMK), Solna, Sweden.
Background and aims: The immune system is activated acutely during exercise and chronically in obesity and type 2 diabetes. Inflammatory responses are required for the beneficial adaptations to exercise training, but low-grade inflammation associated with metabolic diseases contributes to the development of insulin resistance and disease progression. Insight into the molecular events by which the immune system contributes to skeletal muscle adaptations are lacking, particularly in the context of type 2 diabetes. Here we hypothesized that an acute bout of exercise would trigger a distinct inflammatory response in skeletal muscle from individuals with type 2 diabetes compared to normal glucose tolerance. We aimed to test the role of cytokines selectively produced in individuals with type 2 diabetes in the regulation of skeletal muscle cell remodelling and metabolism.
Materials and methods: Men with type 2 diabetes (n=20) or normal glucose tolerance (n=17) performed an acute bout of exercise on a cycle ergometer (30 min, 85% of individual maximum heart rate). Skeletal muscle biopsies and plasma were taken before and after the exercise bout and analysed by RNA sequencing and immunoassays. In vitro experiments were performed in human primary or mouse C2C12 skeletal muscle cells. Glucose uptake was measured using radiolabelled 2-deoxyglucose and signalling pathways assessed by immunoblot analysis. Calcium signals were measured using the fluorescent probe Fluo4 and cyclic AMP levels determined with commercially available ELISA kits. Proliferation of myoblasts was estimated by 2-bromouridine incorporation and gene expression was measured by quantitative PCR.
Results: Acute exercise caused an inflammatory response in skeletal muscle, concomitant with an infiltration of immune cells detected by both RNA sequencing and immunostaining. Inflammation was potentiated in skeletal muscle from people with type 2 diabetes and triggered a differential production of cytokines. CXCL12 (aka SDF-1) was induced in individuals with type 2 diabetes, but not in individuals with normal glucose tolerance. In vitro experiments revealed that CXCL12 can be produced by endothelial cells in response to hypoxia or by macrophages in response to conditioned medium from skeletal myotubes exposed to electrical pulse stimulation. Myocytes treated with recombinant CXCL12 (100 μg/mL, 24h) exhibited increased proliferation and impaired differentiation capacity. CXCL12 inhibited cAMP production, suggesting activation of the receptor CXCR4 (coupled to Gq) and ERK signalling. Exposure to recombinant CXCL12 (100 μg/mL, 20 min) increased glucose uptake in myotubes independently of the insulin signalling pathway.
Conclusion: CXCL12/SDF-1 is induced skeletal muscle from individuals with type 2 diabetes as part of a non-canonical inflammatory response to acute aerobic exercise. Signalling of CXCL12 alters the phenotype and metabolic response of skeletal muscle cells, suggesting a role for atypical production of exerkines in regulating skeletal muscle remodelling and metabolism. Pharmacological interventions targeting selective aspects of inflammation such as CXCL12 signalling could help promote the benefits of exercise in individuals with type 2 diabetes.
Supported by: EFSD/Novo Nordisk Foundation Future Leaders Award, Diabetes Wellness Sverige
Disclosure: N.J. Pillon: None.
482
Bax Inhibitor 1 protects against pancreatic beta cell death and metabolic disorders
M. Blanc1, P. Xiao2, J. Gilleron1, M. Janona1, S. Lacas-Gervais3, L. Yvan-Charvet1, P. Gual1, A. K Cardozo2, B. Bailly-Maitre1;
1Centre Méditerranéen de Médecine Moléculaire, Nice, France, 2Inflammation and Cell Death Signalling group, Experimental Gastroenterology Laboratory and Endotools - Medical Faculty, Bruxelles, Belgium, 3Centre Commun de Microscopie Electronique Appliquée, Faculté des Sciences, Nice, France.
Background and aims: Hyperglycaemia, hyperlipidaemia, and the high demand in secretory protein leads to Endoplasmic Reticulum (ER) stress in pancreatic β cell. In response to the ER stress, the Unfolded Protein Response (UPR) is mediated by three transmembrane proteins located in the ER: ATF6, PERK and IRE1α. Bax Inhibitor-1 (BI-1) is a negative regulator of inositol-requiring enzyme 1 (IRE1α), an UPR sensor endowed with kinase and endoribonuclease (RNase) activities. BI-1 can interact with IRE1α and restrains its RNase activity signalling. Taking advantage of BI-1 deficient mice, we observed that BI-1 deletion leads to a hyperglycaemic profile, with an aggravation of the phenotype under HFD challenge. We hypothesized that BI-1 deficiency could sensitize the pancreatic β cell death and favor the onset of type 2 diabetes.
Materials and methods: Taking advantage of BI-1 deficient mice, we monitored systemic inflammation and pancreatic lipases levels in sera, glycemia and insulinemia. We performed GTT (Glucose Tolerance Test) /ITT (Insulin Test Tolerance) and GSIS (Glucose Stimulated Insulin Secretion) in fasted and fed conditions. From pancreatic sections and pancreatic islets isolated from WT and transgenic mice, we analysed pancreatic β cell death and ER stress markers by combining several approaches at structural (TEM: transmission electronic microscopy) and molecular & biochemical levels (H&E, TUNEL, MPO, qPCR and Western Blot). Statistical analyses were obtained by Mann-Whitney or Two-Way ANOVA (p-value < 0,05).
Results: BI-1 deficient mice present a glucose intolerance and a poor insulin response in steady state and under an HFD, as revealed after performing GTT and an ITT. Serum insulin levels were statistically reduced in the BI-1 KO mice. When challenged with glucose (GSIS), BI-1 deficient mice secrete significantly less insulin than BI-1 WT mice. Increased levels of TNFα, IFNγ and pancreatic lipases were statistically observed in the serum of BI-1 KO mice compared to WT mice, indicating respectively significant inflammation and pancreatic injuries in BI-1 KO mice. We observed less pancreatic islets in BI-1 KO correlating with significant increased β cell death and more infiltrated neutrophiles (MPO staining). Furthermore, BI-1 deficient mice present higher levels of inflammasome, and ER stress markers, especially IRE1α signaling in pancreatic β cells. Finally, the pharmacological inhibition of IRE1 RNase activity in HFD-BI-1 KO mice rescues pancreatic injuries, improves glucose homeostasis, and normalizes insulin levels.
Conclusion: Our data suggests that BI-1 acts as a guardian of β cells homeostasis by counteracting IRE1α dependent-inflammation (inflammasome) activation, -cell death and -metabolic disorders. We propose that normalizing BI-1 expression or pharmacologically targeting IRE1α could be effective in treating type 2 diabetes mellitus, but also in metabolic diseases associated with excessive ER stress.
Supported by: This work was supported by ANR (2019), SFD (2020) and FRM (2022).
Disclosure: M. Blanc: None.
483
A potential role of liver-expressed ASK1 in body weight regulation
A. Goergen1, T.D. Challa1, S. Wueest1, D. Konrad1,2;
1University Children's Hospital Zurich, University of Zurich, 2Zurich Center for Integrative Human Physiology, University of Zurich, Zurich, Switzerland.
Background and aims: The stress kinase apoptosis signal regulating kinase 1 (ASK1) has been linked to obesity-associated metabolic disorders. ASK1 is involved in the cellular stress response and confers the signal to downstream mediators, which in turn generate an appropriate response to deal with stress stimuli. Since the role of hepatic ASK1 remains controversial, we aimed to investigate the impact of liver-specific ASK1 overexpression on the development of high fat diet (HFD)-induced obesity and impaired glucose metabolism.
Materials and methods: 6 week-old male liver-specific ASK1 overexpressing (ASK1+hep) and control (ASK1F/F) mice were fed a regular chow or HFD for 20 weeks. Intraperitoneal insulin tolerance test was performed to assess insulin sensitivity and indirect calorimetry was used to measure energy expenditure. After 20 weeks of diet intervention, organs and systemic blood were sampled and analyzed using Western blotting, qPCR, histology or ELISA. In cultured hepatocytes, ASK1 was knocked down and overexpressed using siRNA and adenovirus, respectively, and lysates were analyzed using Western blotting and qPCR.
Results: After 20 weeks of diet feeding, insulin sensitivity was similar between the genotypes in chow-fed mice, while HFD-fed ASK1+hep mice displayed significantly improved insulin sensitivity compared to control mice (AUC in mmol/l*min: 720±163 in ASK1+hep vs. 1001±118 in ASK1F/F, p<0.01). Moreover, HFD-fed ASK1+hep mice gained significantly less body weight in comparison to littermate control mice (44.1±6.3g in ASK1+hep vs. 34.8±3.6g in ASK1F/F, p <0.001). There was no difference in bodyweight in chow-fed mice. In line with decreased body weight gain, energy expenditure was elevated in HFD-fed ASK1+hep mice (8.1±0.5 kcal/h*min in ASK1+hep vs. 7.3±0.6 kcal/h*min in ASK1F/F, p<0.02). Of note, hepatic fibroblast growth factor 21 (Fgf21) mRNA expression as well as circulating FGF21 levels (6904±1233 pg/ml in ASK1+hep vs. 3188±1944 pg/ml in ASK1F/F, p<0.001) were significantly increased in ASK1+hep mice. Moreover, FGF21 plasma levels negatively correlated with body weight. In vitro, preliminary data from gain and loss of function studies suggest that ASK1 regulates FGF21 production in hepatocytes.
Conclusion: We suggest a yet undescribed ASK1-mediated FGF21 induction in the liver, which positively affects body weight and metabolism. Given the prominent role of FGF21 in whole-body metabolism and body weight control, the identification of ASK1 as a potential cell-intrinsic Fgf21 enhancer may contribute to the development of new drugs counteracting obesity and associated diseases.
Supported by: Children's research center Grant 2020
Disclosure: A. Goergen: None.
484
Effect of maternal age and metabolic syndrome on early embryo development in a mouse model
J. Lilao-Garzon1, Y. Brito-Casillas1, S. Munoz-Descalzo1, A. Wägner1,2;
1University Institute of Biomedical and Healthcare Research, 2Servicio Endocrinología y Nutrición. Complejo Hospitalario Universitario Insular Materno-Infantil de Gran Canaria, Las Palmas de Gran Canaria, Spain.
Background and aims: Diabetes and advanced maternal age have a negative effect on reproductive success. However, there is limited knowledge about their effect on early embryo development. Before embryo implantation in the uterus, cells differentiate into the cells that will give rise to the placenta, the yolk sack (primitive endoderm, PrE) and the embryo proper (epiblast, Epi). Here we aim to evaluate how maternal age and metabolic syndrome induced by high fat diet (HFD) affect fecundity and embryo development in a mouse model.
Materials and methods: A diet induced model based on 60% HFD for 8 weeks was chosen and normal diet (ND) was used as control. To evaluate the effect of age, mice were classified into: young adults (Y= 12 weeks old; n= 33ND y 38HFD) and mature adults (M= 9 months old, n= 41ND y 48HFD). Oral glucose and insulin tolerance tests, weight and whole body composition were used to validate the phenotype. Their fecundity was established by descriptive methods like mating and pregnancy rates together with the number and quality of early embryos (E3.5) per female in each group. Embryo staging was established by the number of cells and by the proportion of Epi and PrE cells through quantitative immunofluorescence of confocal images. Markers expression (NANOG for Epi and GATA6 for PrE) was automatically quantified with specific software (MINS). Z-test and Mann Whitney-test were used to compare different groups.
Results: The presence of mild hyperglycaemia and obesity before pregnancy was confirmed by the phenotypic analysis. No statistical differences were observed on mating, pregnancy rates, or number of embryos per female. However, HFD early embryos showed lower proportion of PrE cells: YND= 34%, YHFD= 23%, MND=41%, MHFD= 28%, (Z-test: YND vs YHFD, p= 0.0045; MND vs MHFD, p= 0.0065; YND vs MND, p= 0.158). Moreover, GATA6 levels were significantly lower in embryos from HFD groups independently of the age in ICM cells (Mann Whitney-test: YND vs YHFD, p= 0.0002; MND vs MHFD, p= <0.0001; YND vs MND, p= p= <0.0001). Fewer PrE cells and lower levels of GATA6 indicate a defect in PrE formation.
Conclusion: The presence of mild hyperglycaemia and obesity before pregnancy leads to fewer PrE cells and alteration in cell fate decision makers that can be associated with a delay in early embryo development in a mouse model. Our results help to explain the underlying cause of the decreased reproductive success associated with diabetes and advanced maternal age.
Supported by: PhD Fellowship ULPGC (pif2017)
Disclosure: J. Lilao-Garzon: Grants; PhD Fellowship ULPGC (pif2017).
485
Effect of lipotoxic hepatocyte-derived extracellular vesicles in pancreas inflammation and beta cell functionality
R. Alén1,2, N. Cobo-Vuilleumier2,3, B.R. Gauthier2,3, I. Garcia-Martinez1,2, Á.M. Valverde1,2;
1"Alberto Sols" Biomedical Research Institute (CSIC-UAM), Madrid, 2Biomedical Research Network on Diabetes and Related Metabolic Diseases-CIBERDEM, Madrid, 3Andalusian Center for Molecular Biology and Regenerative Medicine (CABIMER), Sevilla, Spain.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is a common feature of obesity and type 2 diabetes mellitus (T2DM). Lipotoxic hepatocytes increase extracellular vesicles (Hep-EVs) release which can act locally and contribute to non-alcoholic fatty liver disease (NAFLD) progression. Hep-EVs can also enter the circulation and reach other metabolic tissues such as the pancreas and affect beta cell function. Thus, Hep-EVs could be involved in T2DM pathogenesis.
Materials and methods: Primary hepatocytes were isolated from C57BL6j male mice. Hep-EVs were obtained by differential ultracentrifugation from control hepatocytes (EVC) or stimulated with palmitic acid (EVPA). INS-1 rat insulinoma cells were incubated with Hep-EVs or with conditioned medium (CM) collected from Hep-EVs-treated macrophages. Mouse pancreatic islets were treated ex-vivo with Hep-EVs. Endoplasmic reticulum (ER) stress and inflammation pathways were examined by RT-qPCR, immunofluorescence, and Western-Blot. Beta cell gene expression was assessed by RT-qPCR and glucose-stimulated insulin secretion (GSIS) capacity by ELISA. C57BL/6J male mice were injected intravenously with Hep-EVs twice a week for four-weeks to study chronic effects. Inflammation in liver and pancreas was analyzed by flow cytometry and immunofluorescence.
Results: Hep-EVs were internalized by INS-1 cells cultured alone or co-cultured with macrophages. In mouse pancreatic islets, Hep-EVs were taken up by both macrophages and beta cells. EVPA triggered ER stress and increase Pdx-1 (n=4, p<0.05) and Ins1 (n=4, p<0.01) mRNA expression concomitant to GSIS impairment (n=3, p<0.001) in INS-1 cells compared to EVC. CM from EVPA-treated macrophages activated the NF-κB proinflammatory pathway (n=4, p<0.001) and reduced GSIS (n=4, p<0.05) in INS-1 cells compared to EVC. In mouse pancreatic islets, EVPA promoted NF-κB nuclear translocation in macrophages and elevated Il1b mRNA levels (n=3, p<0.05) which decreased GSIS (n=4, p<0.01) compared to EVC. In vivo, Hep-EVs reached mouse pancreas targeting both exocrine and islet macrophages at 24 h post-injection. Chronic injection of EVPA produced inflammation in the liver characterized by enhanced monocyte (n=6, p<0.01), neutrophil (n=6, p<0.05) and macrophage (n=6, p<0.05) recruitment with a decrease in M2 macrophage polarization (n=6, p=0.0649) compared to EVC. Pancreas inflammation was also observed in EVPA-injected mice. CD4+ T cells (n=6, p<0.01) recruitment was enhanced (n=6, p<0.01) whereas the number of macrophages did not change compared to EVC. However, a reduction in M2 polarization was detected (n=12, p<0.05).
Conclusion: In vitro exposure to lipotoxic Heps-EV and lipotoxic Hep-EVs-mediated inflammation results in an impairment of GSIS in beta cells. In vivo chronic injection of lipotoxic Heps-EVs in mice augmented proinflammatory infiltrates of immune cells in both liver and pancreas. In summary, our results identified a novel liver-pancreas axis mediated by lipotoxic Hep-EVs which seems to contribute to low-grade tissue inflammation, a key feature in the beta-cell dysfunction that characterizes T2DM.
Supported by: MICINN/AEI/FEDER, EU-Fundación Ramón Areces - CIBERdem - MINECO - MECD
Disclosure: R. Alén: None.
486
Neurometabolic and behavioural alterations in the adolescent offspring upon maternal glycation
A. Amaro1, D. Sousa1, M. Sá-Rocha1, M.F. Júnior2, C. Barra1, T. Monteiro1, R. Mello-Gomes2, F.I. Baptista1, P. Matafome1;
1Institute of Clinical and Biomedical Research (iCBR), Institute of Physiology, Faculty of Medicine, University of Coimbra, Coimbra, Portugal, 2Department of Physiological Sciences, Institute of Biological Sciences, University Federal of Goiás, Goiânia, Brazil.
Background and aims: Lactation period is an important programming window for the offspring predisposition to metabolic syndrome at adulthood but also to the modulation of neuronal alterations and behaviour later in life The mechanisms of this intricate relation between metabolic and behavioural alterations may rely on maternal diet and metabolic conditions. Our aim was to study the effects of maternal glycation during breastfeeding on offspring metabolism, neurodevelopment, and behaviour.
Materials and methods: Experimental groups (3): male offspring of lactating females treated with S-p-bromobenzylglutathione cyclopentyl diester (BBGC, 5mg/kg, day 1-6 of lactation) - a selective inhibitor of glyoxalase-1 (maternal glycation); a control group; a vehicle group (treated with dimethyl sulfoxide). Between postnatal day (P) 5 and P17, offspring were subjected to neurodevelopmental tests. After weaning (P21) maternal milk was collected to measure triglycerides and total antioxidant capacity; at P43 offspring were tested in the Elevated Plus Maze and Open Field tests; at P45, the glycaemic profile and triglycerides were evaluated, and the hippocampus was collected.
Results: Maternal glycation reduced milk triglyceride levels and total antioxidant capacity, also impairing offspring body weight gain. No glycation-induced changes were detected in offspring triglycerides levels, whereas in the insulin tolerance test, there was a significant decay of glucose rate over the time when compared with the controls. In the offspring hippocampus, it was observed a higher content of advanced glycation end products, as well as decreased glyoxalase 1 levels and increased total insulin receptor levels. Maternal glycation impairs offspring vestibular and olfactive system development and anticipates offspring eye opening. At adolescence, maternal glycation induces a disinhibition and anxiolytic-like effects in the offspring, together with upregulation of GABAa levels in hippocampus, which can underpin behavioural alterations.
Conclusion: The early exposure to glycation changes breastfeeding milk composition that are associated with offspring metabolic and neurodevelopment alterations, modulating its behaviour at adolescence.
Disclosure: A. Amaro: None.
487
Exposure to obesogenic environments during perinatal development modulates nutrient-sensing pathaways in adipose tissue
D. Sousa1, A. Amaro1, M.F. Junior2, S. Pereira3, M. Rocha1, C. Barra1, R. Mello-Gomes2, P. Oliveira3, P. Matafome1;
1Institute for Clinical and Biomedical Research (ICBR), Institute of Physiology, Faculty of Medicine, University of Coimbra, 2Department of Physiological Sciences, Institute of Biological Sciences, University Federal of Goiás, 3Centre for Neuroscience and Cell Biology, Coimbra, Portugal.
Background and aims: Obesogenic environments such as maternal obesity and westernized diets can impair the central and peripheral nutrient-sensing mechanisms of offspring during embryonic development and lactation. The dysregulation of energy balance regulators can compromise the insulin pathway and energy storage and therefore contributing to the development of metabolic syndrome at adulthood. Evaluate the insulin sensitivity in offspring exposed to maternal obesity and maternal glycation during perinatal period. With the second aim we intend to address the impact of these two obesogenic environments on peripheral and central signaling that regulate energy balance in newborn rats.
Materials and methods: Two animal models were studied: 1) Offspring (42 days) of Sprague-Dawley dams submitted to a hypercaloric diet during pregnancy and lactation. 2) Offspring (45 days) of Wistar dams treated with S-p-bromobenzylglutathione cyclopentyl diester (BBGC, selective inhibitor of Glyoxalase-1, 5mg/kg, 6 days after delivery). DMSO was used as vehicle. Besides lipid and glycaemic profiles, NPY, ghrelin, dopamine and insulin pathways in white adipose tissue (WAT) and liver of offspring were analysed.
Results: The male offspring submitted to maternal obesogenic diet presented higher WAT levels of lipogenic [NPY receptor-1 (NPY1R)], but also lipolytic and catabolic mechanisms [dopamine-1 receptor (D1R) and p-AMPK], whereas phosphorylated insulin receptor was decreased. However, exposure to maternal glycation has the opposite effects on offspring’s energy balance pathways, decreasing NPY1R levels, which is in accordance with the observed lower body weight and food intake in the descendance of BBGC -treated females. Regarding the liver, both NPY1R and D1R levels were decreased in both models.
Conclusion: Obesogenic environments induce compensatory mechanisms in WAT, increasing simultaneously lipid storage and oxidation. On the other hand, such mechanisms may be disrupted by exposure to glycotoxins during lactation, contributing to a greater predisposition for metabolic diseases later in life.
Disclosure: D. Sousa: None.
SO 31 Gut feelings are good
488
Gut-hormone ghrelin regulates female reproductive function in healthy and high-fat fed rodents
D. Khan, A. Sridhar, P.R. Flatt, R.C. Moffett;
School of Biomedical Sciences, Ulster University, Coleraine, UK.
Background and aims: Ghrelin, a 28 amino acid peptide predominantly secreted by endocrine cells in stomach modulates insulin secretion/action and regulates glucose metabolism, food intake and gastrointestinal motility through binding to the ghrelin receptor. Women affected by obesity and polycystic ovary syndrome (PCOS) exhibit significant reduced circulating ghrelin concentrations. Previous work from our laboratory and others indicates that high-fat diet (HFD) causes obesity and infertility in female rodents. To elucidate possible involvement of ghrelin, the present study has evaluated the effects ghrelin on reproductive function and altered reproductive hormone status in normal and high-fat fed mice.
Materials and methods: In the first study, effects of ghrelin on metabolic parameters and estrous cycling were evaluated in 8-10 weeks old healthy female TO-mice (n=9) administered twice daily i.p injections for 16 days of saline or ghrelin (25 nmol/kg body weight). In a second series, female TO mice (8 weeks-old) were fed with high fat diet (HFD) for 12-weeks with regular monitoring. Mice were then administered twice daily i.p injections for 21 days of saline or ghrelin (25 nmol/kg body weight). Estrous cycling and reproductive hormones were measured.
Results: Treatment for 16-days with ghrelin significantly (p<0.05 to p<0.01) reduced food intake by 18% and body weight by 7% with no change in blood glucose. Starting after 3-days of treatment, estrous cycling was monitored for the following 13 days. Treatment with ghrelin did not change number of estrous cycles (1.25 cycles/13 days) or cycle length (9.4 days cycle length in 13 days) compared to saline controls (1.8 cycles/13 days and 7.2 days cycle length). However, percentage of the total time spent in the various stages of estrous cycle showed reduced (p<0.05) time in metestrus (10±2%) and proestrus (10±2%) stages compared to 20±3% and 18±2% for controls. After 12 weeks of HFD, female TO mice exhibited significantly (p<0.001) 1.5-fold increased body weight without obvious change of blood glucose levels but hyperinsulinaemia, indicative of insulin resistance. 70% of the high-fat fed mice had irregular cycle lengths compared to 40% in the normal diet control group (p<0.001). Ghrelin administration in HFD fed mice reduced (p<0.05) food intake by 18% compared to saline controls but interestingly had no effect on body weight. Percentage time spent in time in metestrus was significantly (p<0.05) increased by 9% by ghrelin compared to HFD controls. Circulating levels of LH were significantly (p<0.05 to p<0.01) 3-times higher in HFD group irrespective of ghrelin administration and progesterone levels in plasma were decreased (p<0.01) by 5-times compared to normal-diet controls. However, ghrelin did not alter reproductive hormone levels when compared to the HFD group.
Conclusion: These data demonstrate that ghrelin affects reproductive function in females, highlighting the importance of a gut-reproductive axis in both health and high fat diet-induced obesity. Taken together with previous research, these observations suggest that modulation of key gut hormone actions in the reproductive axis could represent a novel means for treating diet induced reproductive disorders.
Supported by: DUK RD Lawrence Fellowship
Disclosure: D. Khan: None.
489
Relation between intestinal acetate production and plasma glucose levels in healthy subjects
M. Wijdeveld;
Internal medicine - vascular medicine, Amsterdam UMC, locatie AMC, Amsterdam, Netherlands.
Background and aims: Human obesity and glycemic dysregulation are increasing worldwide. Mounting evidence suggests a prominent role for the gut microbiome in pathophysiological pathways that influence the central nervous system and glucose homeostasis. In this regard, short-chain fatty acids (SCFA) have been reported to mediate host glucose metabolism, insulin secretion and central regulation of appetite. The SCFA acetate is one of the major metabolites produced by gut microbiota. In animal studies, acetate has been reported to function as a beneficial substrate in mediating glucose metabolism and appetite regulation. However, the effects of intestinal acetate production on plasma glucose levels and postprandial glucose metabolism have not yet been studied.
Materials and methods: We studied 60 individuals who were selected to either have a microbiota with either high or low intestinal acetate production from a sample of 429 individuals from the HELIUS cohort study. Postprandial glucose and insulin responses were compared between the two groups during a high-fiber standardized mixed meal test (SMMT). Additionally, hunger and satiety were assessed using visual analogue scale questionnaires. Finally, central craving and reward responses to virtual food cues and the receipt of palatable food were measured using fMRI.
Results: We observed a significant difference in microbiota composition between the groups. Applying a linear mixed model, we found a significant difference in glucose and insulin excursions upon the SMMT between the two groups (p = 0.0088 and 0.0025 respectively), with the low acetate producing group showing higher amplitude and lower baseline levels for both glucose and insulin compared to the high acetate producing group. There were no differences in insulin resistance or insulin secretion. Finally, high-calorie food stimuli invoked a higher CNS response in de caudate nucleus in the high acetate producing group, whereas anticipation of receiving palatable food invoked a higher CNS response in the putamen in the low acetate producing group (p = 0.041 and 0.011 respectively).
Conclusion: High acetate production appeared to be related to a change in postprandial glucose and insulin response, resulting in a lower amplitude for the high acetate producing group, however not paralleled by a change in insulin sensitivity. Our data suggest that acetate may provide a novel target for various non-invasive intervention strategies to promote metabolic health by increasing intestinal acetate. Nevertheless, more clinical intervention studies are needed to confirm this.
Clinical Trial Registration Number: 2019_211
Supported by: M. Wijdeveld is supported by the AMC MD/PhD fellowship 2017.
Disclosure: M. Wijdeveld: None.
490
Mechanisms underlying the beneficial effects of bariatric surgery on cardiometabolic risk in a ten-year long cohort study
S. Simati1, D. Tsilingiris1, A. Angelidi2, K. Stefanakis2, N. Tentolouris1, I. Anastasiou1, A. Alexandrou3, C. Mantzoros2, A. Kokkinos1;
1First Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Athens, Greece, 2Department of Internal Medicine, Boston VA Healthcare system and Beth Israel Deaconess Medical Center, Boston, USA, 3First Department of Surgery, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece.
Background and aims: The long-term effects of bariatric surgery on cardiovascular and metabolomic profile and underlying mechanisms mediating the observed improvements in body composition and overall health remain poorly investigated.
Materials and methods: A cohort of 28 patients with morbid obesity who underwent bariatric surgery [11 Roux-en-Y gastric bypass (RYGB), 17 sleeve gastrectomy (SG)] was studied preoperatively and at 3, 6, 12 months and 10 years postoperatively. Anthropometric and body composition data, biochemical and hematological parameters, echocardiographic indices, as well as circulating traditional and exploratory markers of insulin resistance, cardiovascular risk and inflammation were obtained. We additionally examined panels of adipokines, activins/follistatins, metabolomics/lipidomics, and key gastrointestinal hormones during a mixed-meal test (MMT).
Results: Significant reductions in weight and body mass index were found in both groups, reaching a nadir at the first postoperative year and remaining significantly lower up to 10 years (mean weight difference vs baseline: -33 kg, p<0.001 for both groups). Fat mass percentage decreased and lean mass increased, remaining unchanged at 10 years after a nadir 12-month postoperatively (p<0.001 vs. baseline). Fasting glucose, triglycerides, cholesterol fractions, ApoA1, and liver function tests were significantly improved up to study completion. Significant reductions were observed in the levels of c-reactive protein, up to 1 year, and inflammation marker GlycA, up to 10 years. Indices of insulin resistance (HOMA-IR, Matsuda Index, ISSI-2, DRI), and the non-invasive Fatty Liver Index, were all improved and persisted up to 10 years (p<0.05 vs. baseline for all variables and groups). Atrial and ventricular diameters were improved and epicardial fat thickness reduced [(-0.40 cm, 95% C.I. -0.56 to -0.23) at 10 years; p<0.001 vs. baseline, 6 and 12 months for both groups]. During the MMT, durable improvements of the areas-under-the-curve (AUCs) of glucose, insulin, and all proglucagon-derived products, as well as PYY were apparent up to study completion to a similar degree in both groups. Glicentin and oxyntomodulin increases were more pronounced in RYGB. Adiponectin increased and leptin reduced for the whole duration of follow-up. Activin A, B and AB were markedly and persistently ameliorated. Combined metabolomic/lipidomic panels demonstrated sustained improvements in numerous aspects of the circulating metabolite profile, mainly reductions of cholesterol- and triglyceride- rich particle species.
Conclusion: Bariatric surgery exerts beneficial effects on the profiles of postprandial gastrointestinal hormone secretion, improves adipokine levels and metabolipidomic profile, thus driving a series of profound and long-lasting improvements in body weight and composition and overall cardiometabolic health.
Clinical Trial Registration Number: NCT04170010
Disclosure: S. Simati: None.
491
Analysis of intestinal morphology and incretin-producing cells using tissue optical clearing and 3-D imaging
T. Hatoko1, N. Harada1, S. Tokumoto1, S. Yamane1, E. Ikeguchi-Ogura1, T. Kato1, T. Yasuda1, H. Tatsuoka1, S. Shimazu-Kuwahara1, D. Yabe2, Y. Hayashi3, N. Inagaki1;
1Department of Diabetes, Endocrinology and Nutrition, Kyoto University Graduate School of Medicine, Kyoto, 2Department of Diabetes and Endocrinology, Gifu University Graduate School of Medicine, Gifu, 3Department of Endocrinology, Division of Stress Adaptation and Protection, Research Institute of Env, Nagoya University, Nagoya, Japan.
Background and aims: Intestine has various functions such as nutrient absorption, intestinal barrier, and hormone secretion. The intestine is composed of intestinal epithelial cells (IECs) such as enteroendocrine cells (ECs) including incretin-producing L cells and K cells. Immunohistological analysis of intestinal tissue sections has generally been used for evaluating the number and localization of target cells in intestine. However, it is difficult to envision the three-dimensional (3-D) structure of villi and crypts and to evaluate the number and localization of IECs and ECs under two-dimensional intestinal tissues. Tissue optical clearing methods including CUBIC protocol permits detailed evaluation of organ 3-D structure as well as that of individual cells by tissue staining and autofluorescence. The aim of our study is to evaluate intestinal morphology, IECs, L cells and K cells in reporter mice by a new intestinal evaluation method using CUBIC protocol and 3-D imaging.
Materials and methods: Villin1-Cre and Ai14 mice were cross bred and villin1 reporter mice were generated to enable visualization of IECs by tdTomato fluorescence. Analysis of intestinal morphology and IECs was performed by using villin1 reporter mice. Next, villin1 reporter mice and glucagon-GFP knock-in mice or GIP-GFP knock-in mice were cross bred to evaluate both IECs (tdTomato fluorescence) and incretin-producing cells (GFP fluorescence). Cell number and localization of incretin-producing cells in intestine were evaluated. We got transparency samples of small intestine (SI) and colon using these mice and tissue optical clearing. 3-D fluorescence images of all samples were acquired with confocal microscopy and were analyzed.
Results: The cross section of villi was spindle-shaped in the upper SI (USI) and became closer to round shape toward the lower SI (LSI). Villus length and IEC number per villus were greater in the USI than those in the LSI. Crypt shape, crypt depth, and IEC number per crypt were similar in any part of intestine. L cell number per villus was lowest in duodenum and similar in all other areas. L cell number per crypt was similar in intestine. L cells were expressed mainly in the lower villi and crypts. K cell number per villus were higher in the USI than that in the LSI. K cells were expressed mainly in the upper villi and lower crypts. Next, intestinal morphology and cell number were evaluated in high-fat diet-fed obese mice (HFD) and control-fat diet-fed lean mice (CFD). Villus length of HFD was longer in the LSI than that of CFD. IEC number per villus of HFD was higher in the USI than that of CFD. K cell number per villus of HFD was higher in the USI than that of CFD, but K cell number per IEC number in villus of HFD was similar than that of CFD.
Conclusion: 3-D evaluation of the intestine enabled us to clarify intestinal morphology and the precise number and localization of IECs and incretin-producing cells in intestine.
Disclosure: T. Hatoko: Grants; Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan Society for the Promotion of Science (JSPS), Ministry of Health, labour, and Welfare, Ministry of Agriculture, Forestry and Fisheries, Japan Diabetes Foundation, Japan Association for Diabetes Education and Care, Merck Sharp & Dohme (MSD) Life Science Foundation, Public Interest Incorporated Foundation, Suzuken Memorial Foundation.
492
Gut derived extracellular vesicles have high affinity to the liver and their enrichment in lipids is associated to the development of metabolic diseases
R.M. de Oliveira1, I. Ferreira1, I. Coelho1, F. Carli2, B. Costa-Silva3, A. Gastaldelli2, P. Macedo1;
1CEDOC Chronic Disease FCM Nova, Lisbon, Portugal, 2Institute of Clinical Physiology (IFC), National Research Council (CNR), Pisa, Italy, 3Champalimaud Foundation, Lisbon, Portugal.
Background and aims: Metabolic homeostasis results from the interaction among all organs, highlighting inter-organ communication central for healthy energy balance. In particular, type 2 diabetes is a multi-organ disease and risk factor for other comorbidities. Gut-liver axis malfunction, which includes gut dysbiosis, alteration in gut hormone release, and nutrient absorption, suggest that the gut plays an important role in the etiology of diabetes. Extracellular vesicles (EVs), which transport specific cargo (proteins, DNA, microRNAs and lipids) along the circulation, are implicated in the development of metabolic diseases. Importantly, we unveiled that gut-derived EVs’ (GDE) protein content reflects the metabolic state of the organisms. Considering the established role of EVs in communication and our findings, we hypothesize that GDE act as mediators of gut-liver axis contributing to the development of metabolic diseases like fatty liver disease and type 2 diabetes. Our aim was to clarify how the cargo transported by the GDE is translate into metabolic alterations.
Materials and methods: For this study we used two groups of C57Bl/6J mice fed with normal chow diet (NCD) or high fat diet (HFD) for 12 weeks. Upon confirmation of prediabetic phenotype by glucose tolerance test (GTT), body weigh gain and liver histology, EVs were isolated by ultracentrifugation. EVs lipidic content and proteomics were profiled by LC/MS. Labeled GDEs were retro orbital injected to evaluate which organs were targeted by GDEs. Then, GDEs were injected into normoglycemic animals for six weeks and their biological impact on glucose and lipid metabolism was studied.
Results: Chronic exposure of healthy mice to GDEs revealed that GDEs have affinity to the liver and are preferentially uptaken by the resident hepatic macrophages, Kupffer cells (KC), which are involved in hepatic inflammatory response under metabolic stress. We observed that control mice, after receiving HFD-GDEs, had significantly higher weight gain in comparison with the control group. Moreover, we observed a positive correlation of the area under the curve (AUC) for the GTT of donor mice with AUC of the correspondent recipient mice, i.e. injected with GDE. Lipidomics studies of HFD-GDE vs NCD-GDE revealed an enrichment in ceramides, i.e, biologically active pro-inflammatory sphingolipids associated with insulin resistance, diabetes and fatty liver disease. Concomitantly, proteomic analysis showed that proteins related to lipid metabolism were more abundant in HFD-GDE compared to NCD-GDE. Interestingly, we observed that normoglycemic mice that received HFD-GDEs manifested increased levels of hepatic triglycerides, further suggesting that the altered GDEs content is translated into hepatic biological alteration.
Conclusion: In toto, GDEs are vehicles of communication in the gut-liver axis by carrying lipids and proteins. While in normoglycemic/normolipidemic state GDEs work as a detoxifying system, by carrying dietary toxic lipids and targeting them to KC; under HFD KC metabolism and their detoxification ability is impaired, thus promoting hepatic insulin resistance and prediabetes. Our results suggest that GDEs when rich in lipids are involved in the development of metabolic diseases.
Supported by: FCT ( PTDC/MEC-MET/29314/2017), iNOVA4Health UIDB/Multi/04462/2020
Disclosure: R.M. de Oliveira: None.
493
Intestinal dysbiosis and serum metabolites suggest an early progression to type 2 diabetes in patients with extreme obesity
A. Penesova1, L. Kubanova2,1, I. Hric2, K. Šoltýs3, Ž. Rádiková1, M. Kolisek4, V. Bielik2;
1Institute of Clinical and Translational Research, Biomedical Research Center, Bratislava, 2Department of Biological and Medical Science, Faculty of Physical Education and Sport, Comenius University, Bratislava, 3Department of Microbiology and Virology, Faculty of Natural Sciences, Comenius University, Bratislava, 4Biomedical Center Martin, Jessenius Faculty of Medicine, Comenius University, Martin, Slovakia.
Background and aims: The development of Type 2 DM in obese individuals is characterized by dysregulated glucose metabolism and insulin resistance, could be related to progressive disruption of the gut microbiome associated with obesity. The purpose of this prospective cross-sectional study was to compare the composition of the gut microbiota and energy metabolites between non-diabetic individuals with extreme obesity (EO) and healthy lean controls (CL).
Materials and methods: We recruited 19 non-diabetic EO (mean ± SD; age: 35.4 ± 7.0 years, BMI: 48.8 ± 6.7 kg.m-2) and 23 CL (age: 31.7 ± 14.8 years, BMI: 22.2 ± 1.7 kg.m-2). Their fecal microbiota was categorized using specific primers targeting the V1-V3 region of 16S rDNA, whereas serum metabolites were characterized by nuclear magnetic resonance spectroscopy. Multivariate statistical analysis and Random Forest models were applied to discriminate predictors with the highest variable importance.
Results: Impaired glucose regulation or prediabetes (according HbA1c between 42-47 mmol/l) was found only in 4 EO patients. We observed a significantly lower microbial α-diversity (Shannon index EO: 5,1±08 vs CL: 6,5±0,8; p<0,001 ), a relative abundance of beneficial bacterium Akkermansia (p<0,001) and the SCFA-producing bacteria Eubacterium hallii (p=0,02), Butyrivibrio (p=0,02), Marvinbryantia (p=0,01), and Coprococcus (p=0,05), and a higher abundance of the pathogenic bacteria Bilophila (p=0,02) and Fusobacterium (p=0,04) in EO. We observed a significantly negative association between the Shannon index and each of the following: glucose levels (rs = -0.355, p = 0.021), insulin levels (rs = -0.618, p = 0.001), IR HOMA (rs = -0.644, p = 0.001), HbA1C (rs = -0.400, p = 0.013), ALT (rs = -0.404, p = 0.008), serum citrate (rs = -0.615, p = 0.001), and serum acetate (rs = -0.544, p = 0.001). The machine learning algorithm identified citrate, acetate, alanine, IR HOMA, and insulin as important predictors and gave a ROC curve with an AUC of 0.991, indicating that their ability to discriminate probands from the EO and the CL was good.
Conclusion: Our results suggest that the shifts in gut microbiota and in serum acetate and citrate might be additional promising biomarkers of early progression to Type 2 diabetes in patients with obesity. Therefore, the non-invasive manipulation of gut microbiota composition in EO through a healthy lifestyle offers a new approach for managing extreme obesity and associated disorders.
Supported by: Grant No. APVV-17-0099 and APVV-19-0222 and VEGA 2/0129/20 and VEGA 1/0260/21
Disclosure: A. Penesova: Grants; APVV-17-0099 and APVV-19-0222 and VEGA 2/0129/20 and VEGA 1/0260/21.
494
Bile acids after bariatric surgery: a gateway to mitochondrial activation in human adipocytes
S. Heinonen1, E. Herbers1, B. Van der Kolk1, T. Saarinen2, A. Perino3, J. Kuula4, A. Hakkarainen4, H. Peltoniemi5, P.-H. Groop6, E. Pirinen1, K. Schoonjans3, M. Neuvonen7, M. Niemi7, A. Juuti2, K. Pietiläinen1;
1Research Program of Clinical and Molecular Metabolism, University of Helsinki, Helsinki, Finland, 2Gastrointestinal Surgery Abdominal Center, Helsinki University Hospital, Helsinki, Finland, 3Institute of Bioengineering, Faculty of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 4HUS Imaging, Helsinki University Hospital, Helsinki, Finland, 5Tilkka Hospital, Helsinki, Finland, 6Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 7Clinical Pharmacology and Individualized Drug Theraphy Research Program, University of Helsinki, Helsinki, Finland.
Background and aims: Bariatric surgery is an effective therapy for morbid obesity, with significant improvements in the peripheral tissue metabolism. We previously found that an underlying event of these improvements is likely the activation of adipose tissue (AT) mitochondrial pathways. We aimed to investigate whether circulating bile acids mediate the favorable effect on AT mitochondria, potentiating weight loss and a healthy metabolism of the tissues after the surgery.
Materials and methods: We examined 45 bariatric surgery patients (25 Roux-en-Y gastric bypass, 20 One-anastomosis gastric bypass) at baseline and at 6 and 12 months post-surgery. We measured body weight, amounts of intra-abdominal-, subcutaneous- and liver fat (DEXA, MRI), circulating plasma lipids, insulin sensitivity (HOMA-index, Hba1C), inflammation (hs-CRP), and plasma bile acids (BAs, AUC mean, 95%CI) during a mixed meal test, and AT mitochondrial mtDNA and mitochondria-related transcripts (mean±SE). Additionally, we examined mitochondrial activation in human primary preadipocytes treated during differentiation with an agonist (INT-777) targeting TGR5 BA-receptor and with DCA, a secondary BA, by cell respiration (Seahorse extracellular flux analyzer), mtDNA mass and mitochondrial transcripts (COX1, ND5, 12S-RNA; mean±SE).
Results: Significant weight loss, improvement of intra-abdominal and liver fat, insulin sensitivity and inflammation were observed at 6 and at 12 months after the surgery. Increased levels of plasma postprandial total, primary, conjugated, hydrophobic and non-hydroxylated BAs were detected at 6 and 12 months (AUC, p<0.05 all; total BA 1400uM [1200, 1600] vs. 1900uM [1500, 2400] vs. 1800uM [1400, 2400], respectively). Fasting plasma BA levels increased from 0 to 6 and 12 months (2.2uM [1.8, 2.5] vs. 4.6uM [3.1, 5.9] vs. 5.6uM [4.1, 7.0], p<0.001). AT mtDNA amount (1.1±0.03 vs. 1.27±0.07; p<0.01) and transcription of one of the main regulators of mitochondrial biogenesis in AT, PGC1α, (0.52±0.03 vs. 0.75±0.04; p<0.001) were increased at 12 months compared with baseline. Differentiated human primary preadipocytes treated 10d or 24hrs with TGR5 bile acid receptor agonist INT-777 or 24hrs with bile acid DCA showed increased mtDNA mass for INT-777 (1.1±0.04 vs. 1.32±0.1, p<0.05), and increased mitochondrial respiration (OCR) and levels of mitochondria-related transcripts (p<0.05) for INT-777 and DCA.
Conclusion: Weight loss by surgery leads to increased levels of postprandial total, primary, conjugated, hydrophobic, non-hydroxylated and fasting BAs. AT shows signs of increased mitochondrial biogenesis after the surgery. Compounds mimicking the effects of BAs and TGR5 inducers in human adipocytes may have potential as mitochondrial activators.
Clinical Trial Registration Number: NCT02882685
Supported by: HUS, AF, SJF, DRF, ORF, PRF, PNF, NNF
Disclosure: S. Heinonen: None.
495
Different impact of dietary fructose and galactose on metaflammation in rats: protective effects of prebiotic fructooligosaccharydes supplementation
R. Mastrocola1, D. Collotta2, F. El-Masri3, C. Murzio2, E. Aimaretti1, M. Aragno1, J. Frank3, M. Collino2;
1Dept. of Clinical and Biological Sciences, University of Turin, Turin, Italy, 2Dept. of Neurosciences “Rita Levi Montalcini”, University of Turin, Turin, Italy, 3Dept. of Food Biofunctionality, University of Hohenheim, Stuttgart, Germany.
Background and aims: We previously demonstrated that chronic consumption of fructose leads to tissue accumulation of advanced glycation endproducts (AGEs) in mice, inducing a state of low-grade chronic inflammation known as “metaflammation”. This was due to the ability of fructose-derived AGEs to deeply affect lipid metabolism in liver and skeletal muscle, evoking lipotoxicity. Galactose, as well as fructose, is one of the most consumed sugars, mainly in the form of lactose in dairy products. However, the impact of galactose on AGEs accumulation, related metaflammation and de novo lipogenesis has not yet been investigated. We thus aimed to investigate, in a strictly controlled in vivo environment, the intrinsic ability of the two sugars to exacerbate the deleterious effects of a chronic-fat diet and to identify differences in the activation of inflammatory and lipogenic pathways in target organs of metabolic derangements. We further tested the potential efficacy of supplementation with complex carbohydrates, namely the fermentable dietary fiber fructooligosaccharides (FOS), to counteract these effects.
Materials and methods: Male Sprague-Dawley rats (6/group) were fed 8 weeks as follows: 1) Control 5% fat diet (CNT), 2) 20% fat diet (FAT), 3) FAT+10% FOS, 4) FAT+25% galactose (GAL), 5) GAL+10% FOS, 6) FAT+25% fructose (FRU), 7) FRU+10% FOS. Inflammation markers and lipid metabolism pathways have been analysed at both systemic and tissue level.
Results: Chronic exposure to 20% fat in the presence or absence of simple carbohydrates did not significantly affect body weight gain, blood glucose or markers of systemic inflammation (TNF-alpha, CRP), whereas an increase in plasma triglycerides and cholesterol levels and in AST and ALT concentrations was detected in both FRU and GAL groups compared to CNT and, most notably, FOS administration counteracted those alterations. At local level (liver and skeletal muscle) we documented a sugar-induced significant increase in markers of inflammation and activities of the lipogenic pathways regulated by the sterol regulatory element-binding proteins (SREBPs) and the protection evoked by FOS.
Conclusion: We demonstrated that exposure to fructose or galactose evokes significant changes in plasma lipid profile and in local expression of early markers of inflammation and lipogenic pathways activation in liver and skeletal muscle, thus confirming the role of simple carbohydrates as main triggers of diet-induced metabolic derangements. The concurrent administration of the prebiotic FOS dampened the sugar-induced local metaflammation and lipid metabolism impairment.
Supported by: HDHL INTIMIC METADIS Carb-Q-4-Health 2020
Disclosure: R. Mastrocola: None.
496
Altered glucose tolerance conditions are associated with an activation of ER stress related responses in the gut
T.V. Fiorentino1, F. De Vito1, R. Marasco1, E. Suraci1, M.L. Hribal1, F. Andreozzi1, F. Luzza1, G. Sesti2;
1Department of Medical and Surgical Sciences, University Magna Graecia of Catanzaro, Catanzaro, 2Department of Clinical and Molecular Medicine, University Sapienza of Rome, Rome, Italy.
Background and aims: Preclinical evidence indicated that high glucose levels affect intestinal expression of tight-junction (TJ) proteins, thus leading to a disruption of intestinal epithelial integrity. Activation of endoplasmic reticulum (ER) stress plays an important role in cellular damage associated to glucotoxicity, and it has been reported to affect intestinal barrier integrity in animal studies. However, whether subjects with dysglycemic conditions have an activation of ER stress related responses in the colon has not been explored. In this study, we aimed to evaluate whether subjects with prediabetes or T2DM exhibit an activation of ER stress along with a disruption of mucosal integrity in the gut.
Materials and methods: We studied 38 Caucasian adults undergoing to a complete clinical characterization including OGTT, and colonoscopy with collection of colonic mucosa biopsies. Study participants were classified as having NGT (n=15), prediabetes (n=11) and T2DM (n=12) in accordance to the current American Diabetes Association criteria. Colonic levels of the ER stress activation markers Bip, inositol requiring kinase (IRE)-1, C/EBP homologous protein (CHOP), and phosphorylation levels of eukaryotic translation initiation factor 2 (eIF2)-α and c-Jun N-terminal kinase (JNK) were assessed by Western blot (WB). Expression of TJ proteins was assessed by RT-PCR and WB. Activation of pro-inflammatory pathway was evaluated by measuring nuclear factor κB (NF-kB) activity, and expression of the cytokines IL-1β, TNFα and IL-6 by RT-PCR.
Results: Subjects with prediabetes and T2DM displayed progressively increased levels of the ER stress markers Bip, IRE-1α, phosphorylated eIF2α and CHOP in the colonic mucosa as compared to those with NGT (P<0.05). Moreover, we found a 1.5- and 2-fold increase in phosphorylation levels of the stress kinase JNK in subjects with prediabetes and T2DM as compared to NGT group (P<0.05). The intestinal upregulation of ER stress found in subjects with altered glucose tolerance was paralleled by an altered intestinal mucosa integrity. We found that subjects with prediabetes and T2DM have significantly reduced expression levels of the TJ protein zonulin (ZO)-1, claudin-1 and occludin in the colonic mucosa (-30% and -50% respectively, P<0.05), coupled with increased circulating levels of ZO-1, a surrogate indicator of increased intestinal permeability (+15% and +20% in prediabetes and T2DM, respectively). Additionally, we found an up-regulation of the pro-inflammatory NF-kB activity (P<0.01) along with increased levels of IL-1β, TNF-α and IL-6 (P<0.05) in colonic mucosa of subjects with prediabetes and T2DM as compared to those with NGT.
Conclusion: These results demonstrate that both T2DM and prediabetes are linked to an activation of ER stress along with a compromised TJ integrity, and inflammation in the colonic mucosa.
Supported by: EFSD/Lilly Young Investigator Research Award Programme
Disclosure: T.V. Fiorentino: None.
SO 32 Latest drug avenues to treatment
497
Effect of the early and postponed statin therapy after menopause on metabolic and inflammatory factors in rat model of prediabetes
M. Hüttl1, I. Markova1, H. Malinska1, D. Miklankova1, T. Hlinka2, J. Pitha3, H. Bartuskova2;
1Centre for Experimental Medicine, Institute for Clinical and Experimental Medicine, 2Department of Physiology, Charles University, 3Department of Cardiology, Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Background and aims: Metabolic associated fatty liver disease (MAFLD), recently discussed important risk factor for cardiovascular disease (CVD) exerts significant sex dimorphism. In MAFLD, women after menopause are affected by faster progression of liver fibrosis, higher levels of hepatocytes damage and inflammation. Early intervention of metabolic risk factors of CVD after menopause, including statin therapy, might be more effective approach. We studied whether early vs. later statin therapy after menopause changes metabolic and inflammatory parameters associated with CVD more favorably in experimental model of prediabetes.
Materials and methods: Female Hereditary Hypertriglyceridemic rats (representing a non-obese model of prediabetes with features of insulin resistance, dyslipidemia, oxidative stress and strong hepatic lipid storage) were ovariectomized at 8th week of age. Two groups treated by atorvastatin (5 mg/kg) from 2nd (early therapy) and from 14th week (later therapy) after ovariectomy were studied together with untreated control group. At the 25th week after ovariectomy the experiment was terminated.
Results: Both early and later therapy affected neither whole body weight nor the food consumption in comparison with controls. At the end of experiment, early therapy group had higher index of visceral adiposity than later therapy group (p<0.01). This corresponded with serum leptin which was also higher in early therapy group (p<0.001). In later therapy group, liver enzymes, and total cholesterol decreased more than in early therapy group and also than in controls. Serum triglycerides (TG) were significantly lower in early therapy group (- 42%) and in later therapy group (-59%) than in controls; between group differences (p<0.05). In addition, early therapy surprisingly decreased fasting glucose levels and improved glucose tolerance, more than later therapy (p<0.01). Moreover, in early therapy group significantly lower HOMA-IR was observed than in later therapy group (p<0.05). Furthermore, early therapy ameliorated hepatic oxidative stress, i.e. increased the activity of antioxidant enzymes glutathione peroxidase and superoxide dismutase more than in later therapy group (p<0.001). Nevertheless, relative expressions of transcriptional factor Nrf2, a key regulator of the cell defense against oxidative stress, did not differ among study groups. In both statin-treated groups, the therapy considerably decreased hepatic TG (p<0.001). Pro-inflammatory marker (MCP-1) significantly decreased in both statins groups than in controls (p<0.01).
Conclusion: Early statin therapy vs. later statin therapy after menopause did not have favorable effects on most of metabolic and inflammatory factors associated with CVD in the experimental model of prediabetes. Moreover, statin therapy after menopause led to improved parameters of glucose metabolism irrespectively of the timing in this particular model. The definition of therapeutic window for optimal cardiovascular prevention in women after menopause seems to be more difficult than expected.
Supported by: MH CZ – DRO grant NU20-01-00083 and IKEM, IN 00023001
Disclosure: M. Hüttl: None.
498
Impact of once-weekly subcutaneous semaglutide 2.4 mg on metabolic syndrome in the 2-year, randomised controlled STEP 5 trial
R.L. Batterham1, M. Bhatta2, T. Holst-Hansen2, K. Kandler2, G. Rigas3, W.T. Garvey4;
1Centre for Obesity Research, Division of Medicine, University College London, London, UK, 2Novo Nordisk A/S, Søborg, Denmark, 3Department of Bariatric Metabolic Surgery, St George Private Hospital, Kogarah, Australia, 4Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, USA.
Background and aims: Metabolic syndrome (MetS) is associated with an increased risk of cardiovascular disease and type 2 diabetes. Obesity is a key driver of MetS. In the STEP 5 trial, treatment with once-weekly subcutaneous semaglutide 2.4 mg resulted in 15.2% weight loss after 104 weeks vs 2.6% with placebo. This post hoc analysis of STEP 5 investigated the effect of 2 years of treatment with semaglutide on MetS.
Materials and methods: 304 adults with overweight/obesity (BMI ≥30 kg/m2, or ≥27 kg/m2 with ≥1 weight-related comorbidity), without diabetes, were randomised 1:1 to semaglutide 2.4 mg or placebo (both plus diet and physical activity) for 104 weeks. We assessed MetS prevalence at baseline and weeks 52 and 104, and weight loss from baseline to week 104 (<10%/≥10%). MetS was defined as the presence of ≥3 National Cholesterol Education Program Adult Treatment Panel III criteria. Results are based on observed data from the in-trial period. P values were from a chi-square test of proportions. Analyses were not adjusted for multiplicity.
Results: There were 89 participants with MetS at baseline in the semaglutide group and 79 in the placebo group. Significantly greater proportions of participants had remission of MetS at weeks 52 and 104 with semaglutide vs placebo (p<0.01; Figure), and significantly lower proportions developed MetS (1.7% vs 23.2%, week 52; 7.1% [2 of these 4 participants were off drug] vs 25.9%, week 104; both p<0.01). When considering the degree of weight loss (<10%/≥10%) in both the semaglutide and placebo groups, semaglutide led to a higher rate of MetS remission in participants with <10% weight loss than placebo at both 52 weeks (57.9% vs 25.0%) and 104 weeks (39.5% vs 15.3%), with fewer participants developing MetS (6.3% vs 23.9%, week 52; 23.5% vs 28.9%, week 104). Weight loss of ≥10% led to higher MetS remission rates, which were similar in semaglutide- and placebo-treated participants (63.0% vs 66.7%, week 52; 71.1% vs 83.3%, week 104); no semaglutide-treated participants developed MetS with ≥10% weight loss (0.0% vs 22.2%, week 52; 0.0% vs 11.1%, week 104).
Conclusion: A greater proportion of participants treated with once-weekly subcutaneous semaglutide 2.4 mg achieved remission of MetS, and fewer developed incident MetS, compared with placebo. These benefits were maintained over 2 years of semaglutide treatment. These results suggest that the positive effects of semaglutide on MetS could potentially impact the progression to type 2 diabetes and cardiovascular disease while patients are taking the drug. However, these data should be interpreted with caution due to the small participant numbers involved.
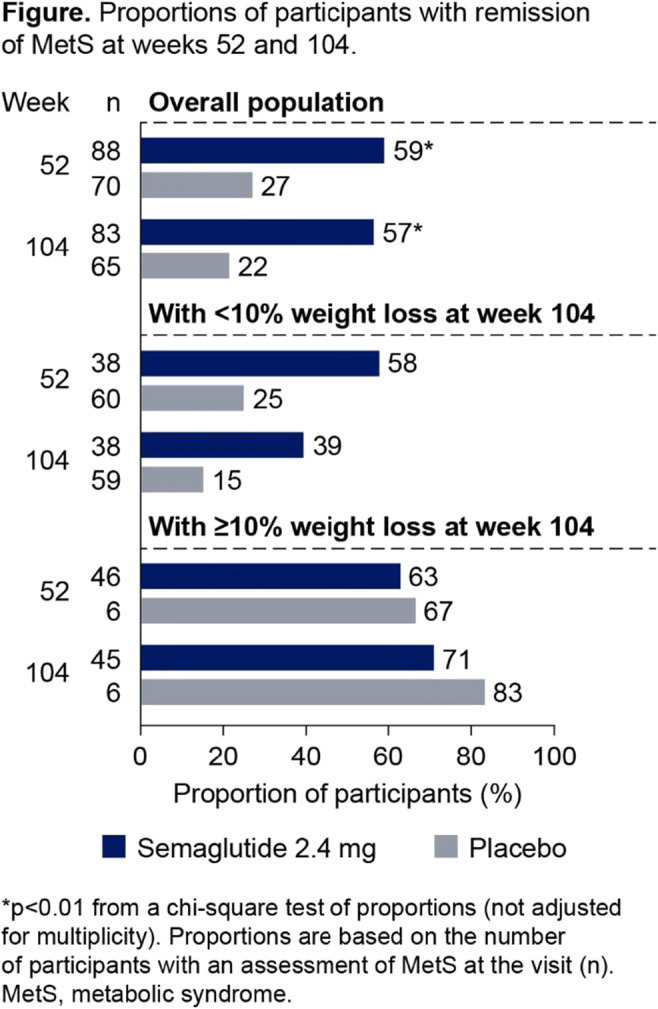
Clinical Trial Registration Number: NCT03693430
Supported by: Funded by Novo Nordisk A/S.
Disclosure: R.L. Batterham: Employment/Consultancy; Consultancy fees from Boehringer Ingelheim. Honorarium; Advisory board fees from Eli Lilly, Gila Therapeutics Inc, Novo Nordisk and Pfizer. Lecture/other fees; Lecture fees from Eli Lilly, International Medical Press, Novo Nordisk and ViiV Healthcare.
499
Associations between weight loss and glycaemic control with once-weekly semaglutide 1.0 mg and 2.4 mg in the STEP 2 trial
M. Davies1, L. Færch2, B. Goldman2, A. Pakseresht2, S. Pedersen3, J. Rosenstock4, I. Lingvay5;
1Diabetes Research Centre, Leicester, UK, 2Novo Nordisk A/S, Søborg, Denmark, 33C-ENDO Diabetes & Endocrinology Clinic Calgary, Calgary, Canada, 4Dallas Diabetes Research Center at Medical City, Dallas, USA, 5Departments of Internal Medicine/Endocrinology and Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, Dallas, USA.
Background and aims: Weight loss achieved via lifestyle intervention or bariatric surgery has been shown to improve glycaemic control in people with type 2 diabetes (T2D) and may help to achieve normoglycaemia. We aimed to assess the relationship between body weight loss and glycaemic control in participants with overweight/obesity and T2D receiving semaglutide pharmacotherapy.
Materials and methods: In the STEP 2 trial, 1210 adults with overweight/obesity (BMI ≥27 kg/m2), with T2D, were randomised 1:1:1 to semaglutide 2.4 mg (n=404), semaglutide 1.0 mg (n=403) or placebo (n=403), plus a reduced calorie diet and increased physical activity, for 68 weeks. Exploratory evaluations of data from the STEP 2 trial included mean change in body weight (%) from baseline to week 68 for participants achieving HbA1c <5.7% at week 20 and at week 68, and the proportions of participants with HbA1c <5.7%, <6.5% or <7.0% at week 68 by categories of body weight loss (≥5%, ≥10%, ≥15%), for semaglutide 2.4 mg, semaglutide 1.0 mg and placebo. Results are based on observed data for the on-treatment period.
Results: Mean body weight reductions at week 68 were 14.6% (semaglutide 2.4 mg [n=50]), 9.5% (semaglutide 1.0 mg [n=35]) and 7.8% (placebo [n=10]) in participants who achieved HbA1c <5.7% at week 20 vs 10.0% (n=308), 7.2% (n=323) and 3.2% (n=344), respectively, for those who did not achieve HbA1c <5.7% at week 20. Mean body weight reductions at week 68 were 16.7% (semaglutide 2.4 mg [n=87]), 13.5% (semaglutide 1.0 mg [n=63]) and 11.6% (placebo [n=7]) in participants who achieved HbA1c <5.7% at week 68 vs 8.8% (n=263), 6.2% (n=287) and 2.9% (n=330), respectively, for those who did not achieve HbA1c <5.7% at week 68. The proportions of participants with HbA1c <5.7% at week 20/week 68 were 14%/25% (semaglutide 2.4 mg), 10%/18% (semaglutide 1.0 mg) and 3%/2% (placebo). In addition, the proportions of participants achieving categories of body weight loss (≥5%, ≥10%, ≥15%) and HbA1c (<5.7%, <6.5%, <7.0%) were higher with semaglutide 2.4 mg and 1.0 mg than with placebo (Figure).
Conclusion: In people with overweight/obesity and T2D, greater weight loss was associated with a greater chance of achieving HbA1c <5.7% at both 20 and 68 weeks, with further benefits seen at the latter time point. Greater reductions in body weight were associated with higher proportions of participants achieving HbA1c thresholds of <5.7%, <6.5% and <7.0% across all treatment groups.
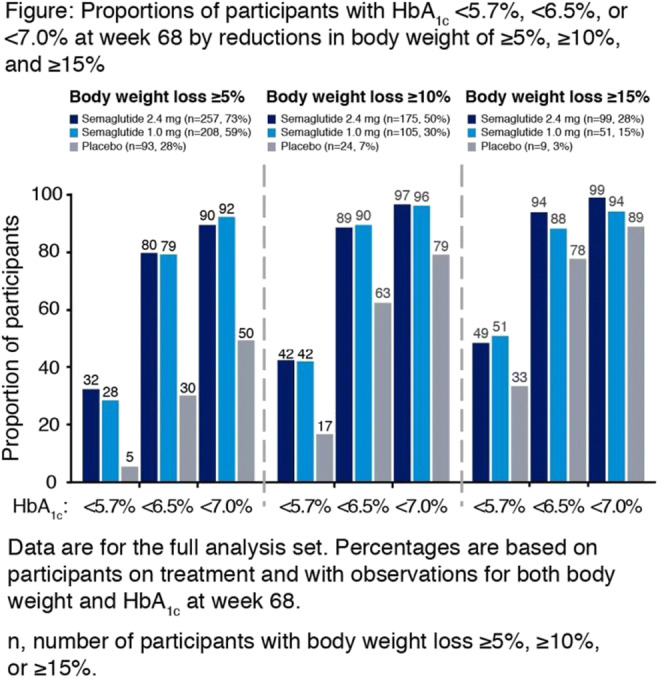
Clinical Trial Registration Number: NCT03552757
Disclosure: M. Davies: Employment/Consultancy; onsultancy fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi-Aventis. Honorarium; Advisory board fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi-Aventis, AstraZeneca, Gilead Sciences Ltd, Janssen, and Lexicon. Lecture/other fees; Lecture fees from Napp Pharmaceuticals and Takeda Pharmaceuticals International Inc. Other; Research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, and Sanofi-Aventis. Co-funded by the NIHR Leicester Biomedical Research Centre.
500
Dapagliflozin protects human cardiac progenitor cells from deleterious effects of the secretome from visceral adipose cells of obese subjects
G. Palma1, C. Caccioppoli1, R. D'Oria1, V.A. Genchi1, I. Calderoni1, A. Braun2, G. Santarpino2, A.D. Milano1, A. Cignarelli1, A. Natalicchio1, L. Laviola1, A. Pezzolla1, F. Giorgino1, S. Perrini1;
1University of Bari Aldo Moro, 2General Surgery GVM Bari, Bari, Italy.
Background and aims: Dysfunction of visceral and epicardial adipose tissue may alter heart performance and promote adverse cardiovascular outcomes in obesity and diabetes. The aim of this study is to assess whether the secretome from abdominal visceral (AV) and epicardial (E) adipose stem cells (ASC) and from AV mature adipocytes might affect the viability of human cardiac progenitor cells (CPC) in human obesity, as well as to evaluate the effects of dapagliflozin (DAPA), a selective SGLT-2 inhibitor.
Materials and methods: ASC and mature adipocytes were isolated from AV adipose tissue biopsies of 27 obese (Ob) and 13 non-Ob subjects undergoing abdominal surgery, and from E adipose tissue biopsies of 8 Ob and 8 non-Ob subjects undergoing cardiac surgery. Human CPC were isolated from right auricle biopsies of non-Ob, non-diabetic donors undergoing cardiac surgery. Human CPC were pretreated with 10 uM DAPA for 24 h or left untreated, and then exposed to the conditioned media (CM) of AV-ASC, E-ASC, and AV mature adipocytes. Apoptosis was evaluated by ELISA assay for cytoplasmic oligonucleosomes. Impairment of organization of actin filaments was assessed by indirect immunofluorescence. JNK activation was studied by immunoblotting of c-Jun phosphorylation.
Results: The CM of AV-ASC, E-ASC, and AV mature adipocytes from Ob subjects induced apoptosis in human CPC after 24 h (p<0.05). Exposure of human CPC to the CM of AV-ASC, E-ASC, and AV mature adipocytes for 24 h also resulted in impaired organization of the human CPC actin filaments. In addition, the CM of AV-ASC, E-ASC, and AV mature adipocytes induced c-Jun phosphorylation after 4 h and 8 h (p<0.05). Importantly, these effects were not observed when the human CPC were exposed to the CM of AV-ASC, E-ASC, and AV mature adipocytes from non-Ob subjects. Pretreatment of human CPC with DAPA resulted in reduced apoptosis (p<0.05), and less impairment of actin filaments following exposure to the CM of AV-ASC, E-ASC, and AV mature adipocytes, as well as in reduced c-Jun phosphorylation (p<0.05).
Conclusion: In human obesity, the secretome of both AV and E ASC and mature adipocytes induces stress kinase activation in human CPC and impairs their viability and functionality, and these effects can be counteracted by SGLT-2 inhibitors directly acting on the human CPC.
Disclosure: G. Palma: None.
501
The effects of MS-275, HDAC Class I inhibitor, in diabetic skeletal muscle atrophy
J. Jeon1, S.-E. Choi2, J. Jeong1, S. Park2, Y. Kang2, T. Kim3, S.-Y. Ahn4, H. Kin1, S. Han1, N. Lee1, D. Kim1, K.-W. Lee1;
1Department of Endocrinology and Metabolism, Ajou university, Suwon, 2Department of Physiology, Ajou university, Suwon, 3Division of Endocrinology and Metabolism, Seoul Medical Center, Seoul, 4Department of Internal Medicine, Busan Bumin Hospital, Busan, Republic of Korea.
Background and aims: Histone Deacetylase (HDAC) is considered one of the pathogenic factors that induces muscle atrophy. HDAC inhibitor, which regulates gene expressions by inhibiting the deacetylation of histones and non-histone proteins, plays an important role in many physiological processes, including muscle contraction, mitochondrial metabolism, and decision of cell fate. Recently it was reported that some of HDAC inhibitor prevented muscle atrophy, insulin resistance, and diabetes. However, the effects of MS-275 in diabetic skeletal muscle atrophy have not been well studied. In this study, we investigated the preventive effects of MS-275 and molecular mechanisms in diabetic muscle atrophy using db/db mice.
Materials and methods: To investigate the effects of MS-275 on diabetic muscle atrophy, db/db mice were randomly divided into three groups: control group, db/db group, and db/db plus MS-275 group. The mice were administered MS-275(7 mg/kg/ i.p)) for 4 weeks. The body weight of the mice was checked. After the mice were sacrificed, muscle tissue was excised, dissected, and weighed. Each muscle tissue was stained with hematoxylin and eosin. The H&E stained sections were measured for cross-sectional area analyses using Image J software. To investigate the molecular mechanisms of MS-275, several factors related with muscle atrophy were tested using RT-PCR.
Results: Administration of MS-275 to db/db mice suppressed muscle weight loss and insulin resistance. As a result of examining muscle mass and fat accumulation in the hindlimb by MRI, we found MS-275 reduced fat accumulation and improved muscle loss. Tibialis anterior (TA) muscle and gastrocnemius (GA) muscle had significantly induced muscle atrophy in db/db mice. However, treatment with MS-275 prevented weight loss of TA and GA muscles and it also recovered reduction of muscle fiber size. Interestingly, MS-275 dramatically reduced atrophy related gene expressions such as MURF1 and Atrogin-1 in TA muscle.
Conclusion: In this study, we investigated the effects of MS-275 on diabetic muscle atrophy using db/db mice. MS-275 ameliorated muscle atrophy through a reduction of atrophy related gene expressions in db/db mice. These results suggest MS-275 may be a basis for the development of therapeutic drugs as applied to atrophy-related muscular or metabolic diseases.
Disclosure: J. Jeon: None.
502
Svep1-based peptide as a potential therapeutic agent against adipose tissue dysfunction
N. Kislev, D. Benayahu;
Developmental and Cell Biology, Tel Aviv University, Tel Aviv, Israel.
Background and aims: Adipose tissue dysfunction lies at the center of obesity pathogenesis. It is associated with surpassing the storage capacity of adipose cells, which leads to metabolic impairment and systemic meta-inflammation. Proteins involved in this process have drawn much attention due to their ability to affect the inflammatory response and alter the metabolic state. Sushi, von Willebrand factor type A, EGF, and pentraxin 1 (SVEP1) is a multidomain protein with potential metabolic and immune functions. It is an adhesion molecule found mainly in the extracellular matrix of mesenchymal tissues and was previously shown to play a role in several metabolic conditions such as diabetes, obesity, and cardiovascular diseases. SVEP1 has only one known receptor, an integrin molecule that also plays a role in adhesion and migration; their interaction potentially affects the inflammatory status found in the center of these metabolic conditions. The previously studied binding site of SVEP1 to the integrin is a nine amino acids long peptide with potential inhibitory capabilities over the integrin's axes. In this study, we characterized the function of SVEP1 and its integrin receptor in adipose tissue and identified the SVEP1-based peptide as a possible therapeutic agent against adipose tissue inflammation.
Materials and methods: Fluorescently labeled and unlabeled peptides were generated based on the SVEP1 binding site to its receptor. RAW264.7 cells were used to study the peptide's binding affinity and functional effect using extensive morphological and molecular phenotyping. In order to understand the function and impact of the peptide in adipose tissue, as well as its binding affinity, High fat and normal fat diet-fed C57BL/6 mice were used.
Results: We found that the integrin receptor is expressed on specific adipose tissue macrophages subpopulations upregulated in high fat diet mice with the ability to promote inflammation. We then generated a peptide based on the SVEP1 binding site and characterized its binding properties in silico using a structural protein-protein interaction prediction. In- vitro accumulation assay indicated time and dose-dependent binding properties, while in vivo assays showed a specific binding capacity to immune subpopulations in adipose tissue. Our results, using the peptide, showed that it significantly inhibited the migration and adhesion of the cell, with a reduction of 30% in adhesiveness and motility. The peptide also affected the activation of the cells as co-incubation of the peptide with activated cells resulted in reduced nitrite production and inflammatory maker's expression levels compared to lipopolysaccharide activated macrophage-like cells.
Conclusion: This project will provide a valuable opportunity to advance the understanding of potential metabolic and immune mediators in adipose tissue and may serve as the foundation for future SVEP1-based therapeutics.
Disclosure: N. Kislev: None.
503
Effects of the bile acid TUDCA on glucose metabolism and skeletal muscle loss in senile mice
M. Carvalho, L. Zangerolamo, L.B. Santos, H.C.L. Barbosa;
University of Campinas, Campinas, Brazil.
Background and aims: Aging is often associated with the development of metabolic disorders and body changes, such as type II diabetes, increased adiposity, and muscle mass loss. For the treatment of these complications, bile acids have been standing out, among them, the tauroursodeoxycholic acid (TUDCA). TUDCA is an endogenous bile acid capable of improving insulin secretion and sensitivity, reducing body weight and blood glucose, and inhibiting cell death and endoplasmic reticulum stress in different experimental models. Recent studies have also demonstrated a new role for TUDCA, acting in the reversal of dexamethasone-induced skeletal muscle atrophy. Here, we aimed to investigate the effects of TUDCA on glucose homeostasis and muscle mass and function in aged mice.
Materials and methods: In this study, 3-month-old (Ctl) and 18-month-old (Old) C57BL/6 mice were used. To evaluate the effects of TUDCA, Old mice were treated for 20 days with an intraperitoneal injection of TUDCA at 300 mg/kg (Old+TUDCA) or its vehicle. We evaluated glucose homeostasis through glucose and insulin tolerance tests. In addition, we evaluated the muscle strength of these animals through a maximum voluntary carrying capacity (MVCC) and Kondziela’s inverted screen test. At the time of euthanasia, body and fat depots weights were obtained, as well as the weight of gastrocnemius, soleus, and tibialis anterior (TA) skeletal muscles. Molecular analyses to identify the expression of proteins that activate protein syntheses, such as p-mTOR and p-P70S6K, were obtained by western blotting. Data were analyzed by one-way ANOVA. Data are mean ± SEM and the difference between the groups was considered statistically significant if P ≤ 0,05.
Results: Old mice presented an increase in body weight (BW) (g) (29.20 ± 0.678 Ctl x 33.08 ± 1.025 Old x 29.54 ± 0.841 Old+TUDCA), as well as in adiposity (% BW) (epididimal fat: 0.70 ± 0.28 Ctl x 1.34 ± 0.09 Old x 0.69 ± 0.39 Old+TUDCA; inguinal fat: 0.65 ± 0.07 Ctl x 0.91 ± 0.04 Old x 0.66 ± 0.04 Old+TUDCA). Moreover, Old mice presented reduced skeletal muscle weight (% BW) (gastrocnemius: 0.544 ± 0.015 Ctl x 0.427 ± 0.014 Old x 0.498 ± 0.023 Old+TUDCA; soleus: 0.027 ± 0.0006 Ctl x 0.020 ± 0.001 Old x 0.024 ± 0.001 Old+TUDCA; TA: 0.179 ± 0.009 Ctl x 0.12 ± 0.004 Old x 0.161 ± 0.006 Old+TUDCA) and loss of muscle function, judging by a lower MVCC (% BW) (151.0 ± 7.83 Ctl x 77.4 ± 6.55 Old x 106.1 ± 7.54 Old+TUDCA) and lower time score on Kondiziela’s test (seconds) (121.9 ± 7.08 Ctl x 7.3 ± 1.43 Old x 39.84 ± 7.34 Old+TUDCA). The reduction in muscle weight in these mice was accompanied by lower protein content of phosphorylated mTOR (0.482 ± 0.101 Old x 1.18 ± 0.158 Old+TUDCA, fold change of Ctl) and P70S6K (0.323 ± 0.092 Old x 0.756 ± 0.108 Old+TUDCA, fold change of Ctl), indicating a reduction in protein synthesis signaling. TUDCA treatment also improved glucose tolerance (area under the curve) (17284 ± 446 Ctl x 23179 ± 518 Old x 15224 ± 691 Old+TUDCA), and insulin sensitivity KITT (%/min) (5.16 ± 0.32 Ctl x 1.84 ± 0.12 Old x 4.30 ± 0.27 Old+TUDCA) in Old mice. Once insulin plays a crucial role in activating protein synthesis signaling in skeletal muscle, the improvement in insulin sensitivity may be involved, at least in part, in the higher muscle mass observed in Old mice.
Conclusion: Our results show that the bile acid TUDCA attenuates skeletal muscle loss and improves glucose metabolism in Old mice, pointing TUDCA as a promising compound to treat aging-associated disorders.
Supported by: FAPESP
Disclosure: M. Carvalho: Grants; FAPESP - 2021/13917-6.
504
Empagliflozin effect on renal parameters and metabolism in a prediabetic rat model: urinary proteomic and metabolomic approach
H. Malinska1, I. Markova1, M. Hüttl1, D. Miklankova1, J. Stastny2, P. Kacer2;
1Centre for Experimental Medicine, Institute for Clinical and Experimental Medicine, 2Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences, Prague, Czech Republic.
Background and aims: The treatment of SGLT2 inhibitors favorably affects the progression of renal dysfunction regardless of the presence of diabetes, as shown by a recent meta-analysis of the DAPA-HF and EMPEROR studies. The renoprotective effects of SGLT2 inhibitors are related to metabolic and hemodynamic effects at the systemic as well as directly at the glomerular level. In the kidney, SGLT2 inhibitors reduce intraglomerular pressure, affect renal gluconeogenesis and hypoxia, and may have anti-inflammatory effects, but the exact mechanism is unknown. In our study, we investigated the effect of empagliflozin on renal parameters and the metabolism in the kidney in a non-obese prediabetic rat model without the presence of hyperglycemia and essential hypertension. Metabolic parameters in the kidney were related to the target urinary proteomics and metabolomics analyses.
Materials and methods: Adult male rats of hereditary hypertriglyceridemic (HHTg) strain of rats were fed a standard diet without or with empagliflozin in a dose 10 mg/kg/day for 8 weeks. Urinary target proteomic and metabolomic analyses were evaluated by tandem mass spectrometry methods.
Results: In HHTg rats, empagliflozin administration did not affect body weight, the weight of epididymal as well as perirenal adipose tissue, but markedly decreased non-fasting glucose and insulin (p˂0.01). In addition, empagliflozin reduced serum triglycerides levels (p˂0.001) and led to significantly decreased ectopic triglycerides (p˂0.05) and cholesterol accumulation (p˂0.01) in kidney cortex. According to target urinary proteomic analyses, empagliflozin treatment significantly decreased inflammatory markers (MCP-1, IL-6, IL-8; leukotrienes C4, D4 and E4 class) together with decreased profibrotic marker - epidermal growth factor (p˂0.01). In addition, metabolomic analyses revealed increased urinary secretion of indole and p-cresol related to improved aromatic amino acid metabolism in empagliflozin-treated HHTg rats compared to untreated animals. Empagliflozin also decreased albuminuria and concentration of NGAL in urine (p˂0.05), however plasma creatinine was not affected. In kidney cortex, relative mRNA expression as well as the concentration of MCP-1 (p˂0.05) were decreased in empagliflozin-treated HHTg rats. To renoprotective mechanism of empagliflozin can contribute markedly improved oxidative parameters in kidney cortex: elevated level of glutathione (p˂0.01) and increased activity of antioxidant enzymes glutathione peroxidase and superoxide dismutase (p˂0.05), however, gene expression of transcription factor Nrf2 was not affected. Significantly increased gene expression of β-hydroxybutyrate dehydrogenase 1 in kidney cortex ((p˂0.01) can contribute to higher ketone body utilization and can improved renal fuel efficiency.
Conclusion: Alleviation of inflammation, oxidative stress and the improvement of renal lipid metabolism may be involved in the mechanism of the renoprotective effects of empagliflozin. Metabolic changes in the kidneys correlated well with the results of targeted urinary proteomics, which could be used as a suitable indicator of empagliflozin treatment as well as initial renal disorders in prediabetic conditions.
Supported by: Czech Science Foundation, project 19-06199S and the MH CZ – DRO grant (IKEM, IN 00023001)
Disclosure: H. Malinska: None.
505
SGLT2 inhibition prevents high fat diet-induced glucose intolerance, but not weight gain, in C57BL/6 male mice
T. Li1, W. Zhu1, Z. Sun1, C. Rayner2, R. Young2, T. Wu1,2;
1Southeast University, Medical School, Affiliated Zhongda Hospital, Nanjing, China, 2Adelaide Medical School and Centre of Research Excellence in Translating Nutritional Science to Good Health, The University of Adelaide, Adelaide, Australia.
Background and aims: Excessive intake of dietary fat is linked to increased risk of obesity and type 2 diabetes, but the underlying mechanisms remain incompletely understood. Dietary fat has been shown to upregulate renal glucose transporters and augment renal glucose reabsorption, which may contribute to metabolic derangement. This study determined the effects of a high fat diet (HFD) on body weight, glucose tolerance and expression of renal glucose transporters in C57BL/6 male mice, and whether these were modified by the SGLT-2 inhibitor, empagliflozin.
Materials and methods: Two independent studies were performed in six-week old C57BL/6 mice. In Study 1, mice were fed either a HFD (Research Diet #D12492, USA; 60% fat, 20% carbohydrate and 20% protein; 5.24 kcal/g) or normal chow diet (NCD) for 12 weeks (n = 5 per group), followed by both an intraperitoneal glucose tolerance test (IPGTT) and an intraperitoneal insulin tolerance test (IPITT). Renal expression of glucose transporters, including SGLT1, SGLT2, GLUT1 and GLUT2, at Week 12 was assessed by the real-time quantitative PCR and western blot. In Study 2, mice were fed either a HFD or NCD, with oral gavage of empagliflozin (10mg/kg) or vehicle once daily, for 12 weeks (n = 5 per group). Body weight, fasting blood glucose, and glucose tolerance during an IPGTT were assessed at Weeks 6 and 12.
Results: In Study 1, a 12-week HFD did not affect fasting blood glucose compared to NCD, but increased weight gain by 42.4%, the incremental area under the curve (iAUC) for blood glucose during the IPGTT by 71.1%, and the AUC for blood glucose during an IPITT by 22.2% (P < 0.0001 for each). Relative renal SGLT2 mRNA and protein levels increased by 125% and 36%, while GLUT1 mRNA and protein levels increased by 113% and 23%, respectively (P < 0.05 for each). In Study 2, HFD did not affect fasting blood glucose compared to NCD, but weight gain by 10.3% and 36.4% (P < 0.01 each), and the iAUC for blood glucose during IPGTT by 86.02% and 154.8% (P < 0.0001 for each) at Weeks 6 and 12, respectively. Empaglifozin abolished the increase in iAUC for blood glucose induced by the HFD at both Weeks 6 and 12 (P <0.001), without affecting weight gain.
Conclusion: These observations reveal that the renal SGLT-2 pathway is primarily responsible for HFD-induced glucose intolerance in mic and support the concept of inhibiting SGLT2 to prevent obesogenic diet-induced dysglycaemia.
Disclosure: T. Li: None.
506
Metreleptin robustly increases resting-state brain connectivity in patients with lipodystrophy
H. Schlögl1, A. Villringer2, K. Miehle1, M. Stumvoll1, K. Mueller2;
1Endocrinology, University Hospital Leipzig, 2Neurology, Max-Planck-Institute for Human Cognitive and Brain Sciences, Leipzig, Germany.
Background and aims: In recent years, knowledge about the rare disease lipodystrophy (LD) has improved, and with recombinant leptin (metreleptin) a causal treatment option is now available. Research in LD and substitution with metreleptin not only helped LD patients, but also opened new directions in investigating leptin’s role in insulin signalling, fatty acid metabolism and regulation of eating behavior. Previously, in a study with LD patients undergoing metreleptin substitution using functional magnetic resonance imaging (fMRI), we found significantly increased resting-state connectivity in the three brain areas hypothalamus, insula/superior temporal gyrus, and medial prefrontal cortex. However, as LD is very rare, studies on brain effects of metreleptin have very low case numbers, ranging from one to ten. In addition, fMRI is a scientific method susceptible to false positive results, nevertheless, it is still the best possibility to investigate changes in brain areas like the hypothalamus. Thus, reproductability of findings is seen as one of the key indicators for scientifically meaningful results. In this study, we aimed to reproduce our fMRI findings in an independent sample and compare results to a healthy control group.
Materials and methods: Measurements in four LD patients and three healthy control persons were performed at four different time points (see Figure legend) on a 3-T MAGNETOM Skyra scanner (Siemens Healthineers). To identify treatment-related connectivity changes, eigenvector centrality (EC) mapping was performed using the fastECM software. Within SPM 12, the statistical analysis was performed on the group level using all four EC maps for all patients using the general linear model with a flexible factorial design with factors subject and time. Furthermore, a flexible factorial model was used with the three factors subject, group (patient or control), and time.
Results: In LD patients, we found a significant EC increase over all four measurements in the hypothalamus (cluster-level P=0.006, cluster size kE=160, cluster maximum at [-6 8 -14], T=11.96, Z=4.94). In addition, two further clusters were found in the precuneus (left: P=0.007, kE=151, [-14 -62 28], T=10.84, Z=4.77; right: P=0.007, kE=155, [12 -60 32], T=9.71, Z=4.58). Using the three-factorial model, a significant interaction between group and time was found in the hypothalamus (P=0.020, kE=94, [-4 6 -14], F=27.99, Z=4.30) (Figure).
Conclusion: Here we reproduce in an independent sample of LD patients the increase in connectivity in hedonic and homeostatic central nervous networks through metreleptin treatment. The effects were specific for metreleptin treated LD patients and did not occur in healthy controls. These results are an important contribution to ascertain brain leptin action and build the foundation for further research of central nervous effect of this important metabolic hormone.
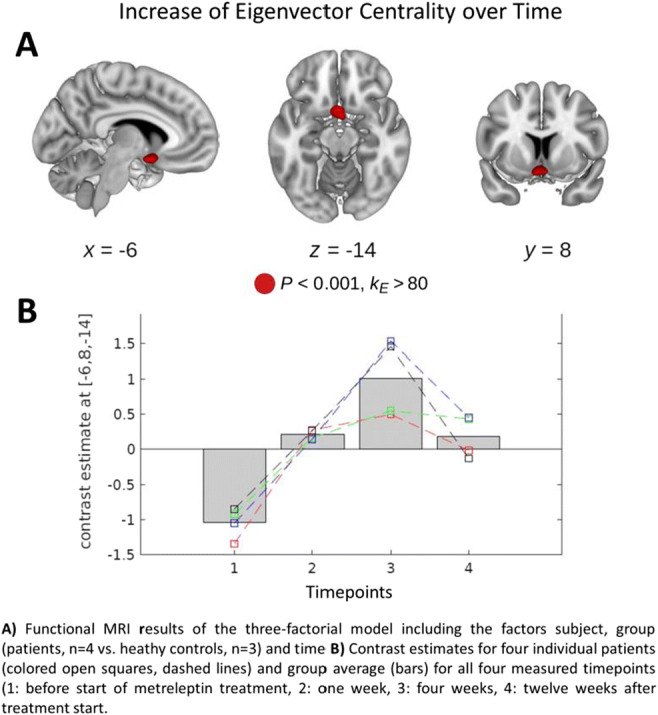
Clinical Trial Registration Number: Registered at the ethics committee of the University of Leipzig, approval no. 147/10-ek
Supported by: DZD, Germany, 82DZD00601; DFG, Germany, SFB 1052/2; BMBF, Germany, 01EO1501
Disclosure: H. Schlögl: Employment/Consultancy; Consultancy for Aegerion Pharmaceuticals in 2018.
SO 33 Insulin in action
507
Different CD36 subcellular localisation in beta cells between diabetes-prone and -resistant mice: possible involvement of CD36 in beta cell dysfunction
M. Nagao1,2, A. Asai1, M. Okazaki-Hada1, H. Sugihara1, L. Eliasson2, S. Oikawa1;
1Department of Endocrinology, Diabetes and Metabolism, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan, 2Islet Cell Exocytosis, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden.
Background and aims: Type 2 diabetes (T2D) is associated with impaired insulin secretion. We have previously shown higher gene and protein expression of fatty acid translocase/CD36 in human islets from obese T2D donors. Moreover, overexpression of CD36 induced impairments of glucose- and K+-stimulated insulin secretion (GSIS/KSIS), associated with suppressed insulin signaling and exocytosis dysfunction (e.g., defects in insulin granule docking), in INS-1 clonal β-cells. To further investigate the importance of CD36 in diabetes development, we aimed to study the implications of CD36 expression for the different insulin secretion capacity between Oikawa-Nagao Diabetes-Prone (ON-DP) and Diabetes-Resistant (ON-DR) mouse islets. These two mouse lines, established by us through selective breeding, have different susceptibilities to diet-induced diabetes. We previously demonstrated that pancreatic islets of pre-diabetic ON-DP mice show higher gene expression of CD36 and lower GSIS/KSIS compared to those of ON-DR mice, which make this a good model for further investigations.
Materials and methods: Islets were isolated from ON-DP and ON-DR mice at 5 weeks of age by collagenase digestion. Protein expression was evaluated by Western blotting. Islet triacylglycerol (TG) content was analyzed by gas-liquid chromatography. Insulin granule distribution in β-cells was assessed in the islet transmission electron micrographs. For subcellular localization analysis of CD36, islet cells were dispersed into single cells and stained immunocytochemically. P<0.05 was considered statistically significant by Student’s t test.
Results: ON-DP mouse islets showed 2.2-fold higher CD36 protein level (n=6, p<0.001 vs ON-DR), 1.6-fold higher TG content (p=0.02), and 50% lower AKT Ser473 phosphorylation level (p<0.01). The number of insulin granules docked to the plasma membrane was smaller by 70% in β-cells of ON-DP mice (n=4, p=0.012). Immunocytochemical analysis revealed that CD36 was expressed in almost all insulin-positive β-cells. Interestingly, while CD36 was widely distributed in the plasma membrane and cytoplasm in β-cells of ON-DP mice, it was localized more centrally in those of ON-DR mice. The ratio of the plasma membrane to cytoplasmic CD36 signal intensity in β-cells of ON-DP mice was 1.8-fold higher than those of ON-DR mice (n=3, p=0.042).
Conclusion: Our data highlight the role of CD36 in lipid accumulation, insulin signaling attenuation, and exocytotic machinery impairments in pancreatic β-cells. The novel finding of different CD36 subcellular localization in β-cells between ON-DP and ON-DR mice provides new insight into the pathophysiological role of CD36 for the development of T2D in relation to obesity.
Supported by: JSPS KAKENHI, ONO Medical Foundation, Swedish Research Council
Disclosure: M. Nagao: None.
508
Preservation of glucose homeostasis in mice with conditional beta cell specific deletion of the dual leucine zipper kinase in a prediabetic condition
J. Duque Escobar1,2, K.A. Köster1,2, M. Dethlefs1,2, S. Schröder1, E. Oetjen1,2;
1Institute of Clinical Pharmacology and Toxicology, University Medical Center Hamburg-Eppendorf, 2DZHK, partner site Hamburg/Kiel/Lübeck, Hamburg, Germany.
Background and aims: (Our) previous studies in a beta-cell line showed that activation of the dual leucine zipper kinase (MAP3K12) by prediabetic signals contributes to the loss of beta-cell function and mass and thereby to the pathogenesis of diabetes mellitus type 2. In line, high glucose-induced apoptosis was reduced in isolated islets from mice with a conditional beta-cell specific Dlk deletion (beta-DLK-/-) compared with islets from DLKfl/fl and RIP-Cre transgenic mice as controls. Hence, it was investigated whether beta-DLK-/- mice maintain physiological glucose homeostasis despite western diet (WD) induced prediabetes.
Materials and methods: Eight-week old mice (strain Bl6J) with beta-DLK-/-, DLKfl/fl and RIP-Cre genotype were fed a WD (21.2% fat, 33.2% sucrose) and sacrificed after 20 weeks of diet. HbA1c, blood glucose, and serum insulin were measured using commercial kits. Ki67, insulin and glucagon staining was assessed by immunohistochemistry or -fluorescence of paraffin-embedded pancreatic slides.
Results: Irrespective of their genotype, all mice gained more weight under WD than under normal diet (ND) (85 ± 3.2% vs 39 ± 2.2%). In histological analysis, the islets of beta-DLK-/- and of RIP-Cre mice showed increased cell proliferation, measured as Ki67 positive cells, and islet area under WD. Consistently, in pancreatic section of beta-DLK-/- and RIP-Cre mice after WD, the number of insulin- and glucagon positive cells was increase to the same extent with 2.75/2.72-fold and 2.1/2.0-fold increase, respectively. At the end of the WD, only in beta-DLK-/- mice no diet-depending increase in HbA1c was detected, however, no difference between the genotypes under ND was observed. Moreover, in RIP-Cre (171 ± 6.2 mg/dl to 213 ± 9.0 mg/dl; p = 0.0003) and in DLKfl/fl (166 ± 6.2 mg/dl to 203 ± 6.7 mg/dl; p = 0.0007) mice, but not in beta-DLK-/- mice (148 ± 5.9 mg/dl to 168 ± 5.6 mg/dl; p = 0.1013) glucose levels were elevated under WD. In line, insulin serum levels after WD were significantly highest in beta-DLK-/- mice (6.3 ± 1.1 μg/ml) than in RIP-Cre (2.2 ± 0.4 μg/ml; p < 0.0001 vs beta-DLK-/-) or DLKfl/fl (3.7 ± 0.8 μg/ml; p = 0.0102 vs beta-DLK-/-) mice. Consequently, the insulin/glucose ratio was significantly increased in beta-DLK-/- (4.6 ± 0.8-fold) compared to RIP-Cre (2.4 ± 0.4-fold; p < 0.0001 vs beta-DLK-/-) and DLKfl/fl (2.7 ± 0.6-fold; p = 0.0009 vs beta-DLK-/-) mice, respectively.
Conclusion: These results show that deletion of Dlk in beta-cells preserves their ability to compensate increased insulin demand in a prediabetic condition and maintain normoglycemia. Thus, the inhibition of DLK will sustain beta-cell function under prediabetic conditions. Inhibition of DLK might therefore be a novel and promising target for the treatment of diabetes.
Supported by: DFG Deutsche Forschungsgemeinschaft
Disclosure: J. Duque Escobar: Grants; Deutsche Forschungsgemeinschaft (DGF): OE181/5-1, DZHK travel grant.
509
Impaired insulin secretion by decreased potassium conductance of beta cells in a prediabetic mouse model
M. Asuaje Pfeifer, K. Grupe, M. Liebmann, I. Rustenbeck, S. Scherneck;
Institute of Pharmacology, Toxicology and Clinical Pharmacy, Technische Universität Braunschweig, Braunschweig, Germany.
Background and aims: Female New Zealand obese (NZO) mice exhibit both impaired glucose-stimulated insulin secretion and impaired glucose tolerance, but do not develop a manifest diabetes. Gene expression data suggest dysfunctional oxidative phosphorylation in the islets of Langerhans, which could lead to disturbances in the function of the ATP-dependent potassium channels (KATP channels). Therefore, the contribution of mitochondrial metabolism and subsequently the KATP channels to this phenotype were investigated.
Materials and methods: Islets of Langerhans from female NZO and NMRI mice (control) were isolated by collagenase digestion. Single cells were harvested by calcium-free incubation and cultured overnight in RPMI 1640 containing 10% FCS and 5 mM glucose. The transmembrane current of single β-cells was measured by the patch-clamp technique in the perforated-patch configuration. Mitochondrial membrane potential was determined in perifused, freshly isolated islets by TMRE fluorescence. Insulin secretion was measured by ELISA and ATP as well as ADP content were determined by luciferase luminescence after static incubation of freshly isolated islets.
Results: In the presence of 1 mM glucose, the outward current was lower in NZO β-cells than in NMRI β-cells (15.47 vs. 34.69 pA, p < 0.001). Blockade of KATP channels by 500 μM tolbutamide reduced it to equal levels, while opening by 250 μM diazoxide enhanced the currents difference (34.77 vs. 73.44 pA, p < 0.01). Insulin secretion was blunted by 250 μM diazoxide in both strains (NMRI: 0.22 vs. 2.41 ng/islet/h, p < 0.01; NZO: 0.37 vs. 1.07 ng/islet/h, p = 0.53). A concentration of 500 μM tolbutamide tended to increased insulin secretion in the NMRI strain but not in NZO mice. Raising the glucose concentration from 1 mM to 20 mM resulted in a comparable elevation of TMRE fluorescence in both NZO and NMRI islets. Compared to 1 mM, 20 mM Glucose increased the ATP content and the ATP/ADP ratio in NMRI (ATP: 12.85 vs. 10.07 pmol/islet, p < 0.05; ATP/ADP: 2.24 vs. 1.65, p < 0.01) and NZO mice (ATP: 7.57 vs. 4.55 pmol/islet, p < 0.01; ATP/ADP: 2.95 vs. 2.15, p < 0.01). The ATP and ADP contents were significantly decreased in NZO islets compared to NMRI islets after incubation with 1 and 20 mM Glucose, while the ATP/ADP ratio was markedly increased in NZO mice.
Conclusion: The altered insulin secretion profile of the NZO mice fits to the decreased outward current of the β-cells at low glucose, which in turn results from a dysfunction of the KATP channels, as evidenced by the use of potassium channel blockers and openers. This could be due to the chronically increased ATP/ADP ratio, which in conjunction with the diminished adenine nucleotide contents suggests a defective regulation of the citrate cycle or the respiratory chain.
Supported by: DDG
Disclosure: M. Asuaje Pfeifer: None.
510
Endogenous glucose-dependent insulinotropic polypeptide (GIP) facilitates postprandial intestinal lipid uptake
L.S. Gasbjerg1, M.M. Helsted2, S. Stensen2, L.S.L. Krogh2, A.H. Sparre-Ulrich3, B. Hartmann1, T. Vilsbøll4, M.B. Christensen5, J.J. Holst1, C. Christoffersen1, F.K. Knop2, M.M. Rosenkilde1;
1University of Copenhagen, Copenhagen N, 2Gentofte Hospital, Hellerup, 3Antag Therapeutics Aps, Copenhagen N, 4Steno Diabetes Center Copenhagen, Herlev, 5Bispebjerg Hospital, Copenhagen N, Denmark.
Background and aims: The gut incretin hormone glucose-dependent insulinotropic polypeptide (GIP) increases triglyceride deposition in subcutaneous adipose tissue when administered to healthy individuals but these effects are attenuated both in patients with type 2 diabetes (T2D) and obesity. Using the selective GIP receptor antagonist GIP(3-30)NH2, we investigated the effect of endogenous GIP on circulating lipoproteins during a meal in healthy men and patients with T2D.
Materials and methods: We measured apolipoprotein B48 (ApoB48) as a marker of chylomicron count and plasma lipoprotein (chylomicrons, VLDL, LDL, HDL) triglyceride and cholesterol content during two four-hour liquid meal tests (1,894 kJ) with double-blind infusions of GIP(3-30)NH2 or placebo (saline) in randomized order in 12 healthy men (19-65 years, BMI 20.3-25.5 kg/m2) and in 10 men with T2D (44-72 years, HbA1c 37-70 mmol/mol (6.2-11%), BMI 27.4-41.2 kg/m2). Increment AUC (iAUC) values were compared with Wilcoxon paired test.
Results: GIP(3-30)NH2 reached ~ 1,000 higher plasma levels than GIP(1-42) (data previously published). Compared to placebo, the GIP(3-30)NH2 infusion lowered postprandial levels of ApoB48 (iAUC0-180 min (median (95% CI)): 215 (156-258) vs 404 (363-541) mg ×l-1 × min, p=0.0049) (Figure A), chylomicron-triglyceride content (iAUC0-270 min: 32.7 (-2.32-72.1) vs 49.5 (1.21-170) mmol × l-1 × min p=0.083) and HDL-triglyceride content (iAUC0-270min: -0.90 (-1.95-0.18) vs 0.95 (-0.72-3.95) mmol × l-1 × min, p=0.016). In patients with T2D, infusion of GIP(3-30)NH2 did not change postprandial levels of ApoB48 (Figure B) or lipoprotein-triglyceride content. In both groups, there were no differences in lipoprotein-cholesterol contents.
Conclusion: We show that GIP receptor antagonism reduces postprandial circulating chylomicrons (ApoB48 levels) and HDL-triglyceride content in healthy individuals suggesting that endogenous GIP facilitates intestinal lipid uptake during a meal; an effect which seems to be reduced in patients with T2D.
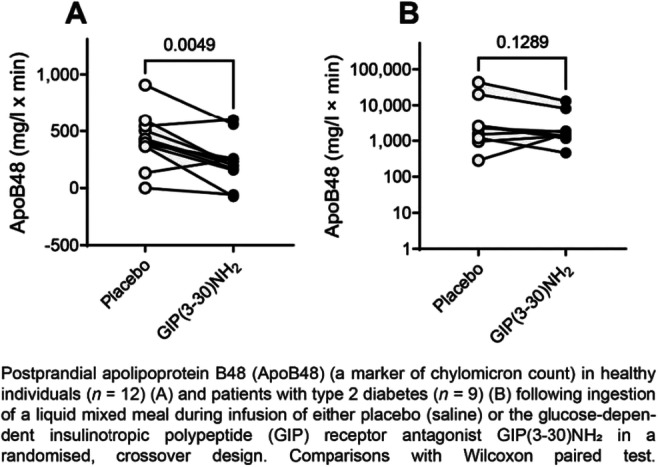
Clinical Trial Registration Number: NCT03702660 // NCT03013296
Supported by: EFSD/Lilly Research Programmes; Gangstedfonden; Herlev-Gentofte Hospital; University of Copenhagen
Disclosure: L.S. Gasbjerg: Stock/Shareholding; Antag Therapeutics Aps.
511
Inhibition of pyruvate dehydrogenase kinase 4 alleviates insulin resistance through the PI3K/AKT signalling pathway
M.-J. Kim1, R. Kim2, S. Kwon1, J.-H. Jeon1,3, I.-K. Lee4,3;
1Internal Medicine, Kyungpook National University Chilgok Hospital, 2Biomedical Science, The Graduate School of Kyungpook National University, 3Kyungpook National University School of Medicine, 4Internal Medicine, Kyungpook National University Hospital, Daegu, Republic of Korea.
Background and aims: Insulin resistance is the main characteristic of type 2 diabetes melitus. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway is considered to be the core of the insulin signaling pathway, the PI3K/AKT signaling pathway is downregulated during hepatic insulin resistance. Previous studies have shown that pyruvate dehydrogenase kinase 4 (PDK4) expression is dramatically increased in the liver and muscle of insulin-resistant patients and rodents.
Materials and methods: We used AML12 hepatocyte used for quantitative real-time PCR, western blot and glucose production assay. Two mouse models of insulin resistance (5 weeks old male KKAy and high fat diet-induced obesity;DIO mice) were used to determine the role of PDK4 in insulin resistance. We performed the indicate tests of insulin resistance and studied its mechanism through pharmacological treatment of PDK4 inhibitors.
Results: We confirmed that the gene expression of PDK4 was significantly increased and AKT pathway was attenuated in the palmitate treated AML12 using publicly available transcriptomics dataset analysis (GSE74059). PDK4 expression was induced obviously after treatment of palmitate in AML12 hepatocytes, shown remarkable decreased AKT phosphorylation. Treatment of two different PDK4 inhibitors ameliorated phosphorylation of ser293 and ser300 in pyruvate dehydrogenase E1α (PDHE1α) and increased AKT activity, respectively. Pharmacological inhibition of PDK4 reduced the levels of fasting blood glucose and the level, increased the insulin levels and significantly improved insulin resistance (glucose tolerance test(GTT), insulin tolerance test (ITT) and homeostasis model assessment of insulin resistance (HOMA-IR), all P<0.05) in the KKAy mice and DIO mice. We compared with commercial anti-diabetic drug;sitagliptin. We found that this novel PDK inhibitors were either more effective or non-inferior for improving insulin resistance.
Conclusion: PDK4 inhibitor regulates PI3K/AKT signaling pathway and alleviates insulin resistance in diabetic KKAy and DIO mice. Targeting PDK4 may be a potential treatment option for diabetes treatment via reducing insulin resistance.
Disclosure: M. Kim: None.
512
The roles of SCD enzyme and appetite hormones in the associations of plasma cysteine with obesity and insulin resistance: The Maastricht Study
E.C. Tore1, B. Adriaans1, A.K. Elshorbagy2, M.C.G. Brouwers1, P.C. Dagnelie1, S.J.P. Eussen3, T.E. Gundersen4, E. Kooi5, Y.H.A. Kusters1, T. Olsen6, H. Refsum6, C.G. Schalkwijk1, C.D.A. Stehouwer1, K.J. Vinknes6, M.M.J. van Greevenbroek1;
1Internal Medicine, CARIM School for Cardiovascular Disease, Maastricht University, Maastricht, Netherlands, 2Pharmacology, University of Oxford, Oxford, UK, 3Epidemiology, CAPHRI Care and Public Health Research Institute, Maastricht University, Maastricht, Netherlands, 4Vitas Analytical Services AS, Oslo, Norway, 5Radiology & Nuclear Medicine, CARIM School for Cardiovascular Disease, Maastricht University Medical Center, Maastricht, Netherlands, 6Nutrition, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway.
Background and aims: Growing evidence links high plasma total cysteine (tCys) concentrations to obesity and type 2 diabetes (T2D). We aimed to examine if potential underlying mechanisms involve the stearoyl-CoA desaturase (SCD) enzyme, a regulator of lipid metabolism, and hormones regulators of food intake.
Materials and methods: Cross-sectional data from a subset of The Maastricht Study enriched with (pre)diabetes individuals (n=1130, 49.4% women, 60±8 yrs) were analyzed. tCys was measured in fasted EDTA plasma with LC-MS/MS. Outcomes included BMI, waist circumference, total and limb fat mass, subcutaneous and visceral adipose tissues (SAT and VAT), and insulin resistance (Matsuda index and HOMA-IR). Two SCD activity indices were estimated as product/precursor ratios of fatty acids in total serum lipids with 16 and 18 carbon atoms, respectively, assayed with GC with flame ionization detection. Serum appetite (ghrelin) and satiety (PYY, GIP, GLP-1, PP) hormones where measured by immunoassays. SCD activity indices and satiety hormones, respectively, were combined in two standardized scores. Two mediation models with z-standardized main exposure (tCys) and outcomes (adiposity measures) were used to examine the mediation effect by SCD activity and appetite/satiety hormones, respectively, with adjustment for cohort structure, demographics and lifestyle. Mediation models by SCD activity were further adjusted for serum triglycerides, since these might affect serum SCD activity index; these analyses might however be overadjusted.
Results: Plasma tCys was associated with all measures of adiposity (e.g., BMI [β=0.27; 95% CI: 0.21, 0.32], limb fat [0.27; 0.22, 0.33] and VAT [0.14; 0.09, 0.19]) and insulin resistance (Matsuda index [-0.19; -0.25, -0.13] and HOMA-IR [0.19; 0.13, 0.25]). SCD activity explained a significant proportion of all associations, with mediated effects (ME) ranging from 5.9% for limb fat [indirect effect: 0.02; 0.01, 0.03] to 19.7% for VAT [0.03; 0.02, 0.04]. Ghrelin mediated all associations, with ME ranging from 2.4% for limb fat [0.01; 0.00, 0.01] to 5.6% for VAT [0.01; 0.01, 0.02]. Similar ME were found in the associations of tCys with Matsuda index (SCD: 14.3% [-0.03; -0.04, -0.01]; ghrelin: 5.4% [-0.01; -0.02, -0.00]) and HOMA-IR (SCD: 18% [0.03; 0.02, 0.05]; ghrelin: 5.4% [0.01; 0.00, 0.02]). No mediation by satiety hormones was found. Adjustment for triglycerides substantially attenuated all MEs by SCD activity index that became non-significant.
Conclusion: Higher SCD enzyme activity and serum ghrelin may represent two pathways by which plasma tCys relates to obesity and insulin resistance. Further research is warranted to confirm these findings and identify effective strategies to limit the effects of tCys on obesity and T2D.
Supported by: JPI and Health~Holland
Disclosure: E.C. Tore: Grants; JPI, Health~Holland.
513
Bone morphogenetic protein 4 (BMP4) and its receptor BMPR1A in human adipose tissue and its regulation by insulin and free fatty acids
M. Dobrzycka1, M. Stefanowicz2, N. Matulewicz2, A. Nikolajuk3, J. Dadan4, P. Mysliwiec4, I. Kowalska1, M. Karczewska-Kupczewska1;
1Department of Internal Medicine and Metabolic Diseases, Medical University of Bialystok, Bialystok, 2Department of Metabolic Diseases, Medical University of Bialystok, Bialystok, 3Department of Prophylaxis of Metabolic Diseases, Polish Academy of Sciences, Olsztyn, 4Department of General and Endocrine Surgery, Medical University of Bialystok, Bialystok, Poland.
Background and aims: Obesity is linked to the development of metabolic syndrome (MS), which increases the risk of type 2 diabetes and cardiovascular disease. Both high body mass index (BMI) and body fat distribution are important causes of metabolic disorders and visceral obesity is a major determinant in the development of MS. BMP4 signaling plays an important role in adipogenesis as well as carbohydrate and lipid metabolism. The aim of the study was to essess the association of BMP4 and BMPR1A expression in SAT and visceral adipose tissue (VAT) with MS in obese subjects, and to examine BMP4/BMPR1A regulation by insulin and free fatty acids (FFA).
Materials and methods: The study included obese 31 subjects (mean age: 38.9±10.95 years) who underwent the bariatric surgery and were divided into subjects without MS (n=15) and with MS (n=16). The mRNA expressions of BMP4, BMPR1A, insulin signaling pathway genes (IRS1, IRS2, PIK3CA, AKT2, SLC2A4) and adipogenesis markers (CEBPA, CEBPB, PPARG, ADIPOQ) were measured in SAT and VAT. In another subgroup of 18 males (mean age: 25.56±3.22 years; mean BMI:27.29±5.0 kg/m2) with normal glucose tolerance the 6-h hyperinsulinemic-euglycemic clamp was performed and after one week another 6-h clamp with the concurrent Intralipid/heparin infusion was conducted. Biopsy of SAT was performed to assess the expression of BMP4 and BMPR1A before and after each 6-h clamp.
Results: In obese subjects with MS the expression of BMP4 (p=0.02) and BMPR1A (p=0.02) in VAT was lower compared to obese subjects without MS. In the entire group, BMP4 expression in VAT was correlated negatively with BMI (r=-0.41, p=0.02), waist circumference (r=-0.55, p=0.005), systolic blood pressure (r=-0.45, p=0.01), triglyceride (r=-0.44, p=0.02), fasting insulin (r=-0.57, p=0.002), HOMA-IR (r=-0.57, p=0.002), and positively with HDL-cholesterol (r=0.52, p=0.003). BMPR1A expression in VAT was correlated negatively with triglyceride (p=-0.40, p=0.03), fasting insulin (r=-0.40, p=0.04), HOMA-IR (r=-0.42, p=0.03). BMP4 and BMPR1A expression in VAT was correlated positively with IRS1 expression (r=0.40, p=0.03 and r=0.40, p=0.03, respectively) and BMPR1A expression was also correlated with SCL2A4 expression (r=0.54, p=0.002). In SAT, BMP4 expression was correlated positively with ADIPOQ (r=0.49, p=0.006) and IRS1 (r-0.48, p=0.007) expression, and BMPR1A expression with PPARG (r=0.43, p=0.02), ADIPOQ (r=0.41, p=0.02), IRS2 (r=0.56, p=0.001), PIK3CA (r=0.50, p=0.004) expression. All these correlations were independent of BMI. In subgroup of 18 males insulin infusion resulted in a decrease expression of both BMP4 (p=0.03) and BMPR1A (p=0.0016) in SAT, but this effect was not observed during the clamp with Intralipid/heparin infusion.
Conclusion: The BMP4/BMPR1A pathway in VAT may play an important role in the induction of metabolic disturbances in obesity. BMP4 may be an important regulator of white adipogenesis in humans. Acute hiperinsulinemia decreases the expression of BMP4/BMPR1A in SAT, but infusion of FFA abolishes this effect.
Supported by: The subsidy of Medical University of Bialystok
Disclosure: M. Dobrzycka: None.
514
Interleukin-33 (IL33) inhibits glucose uptake in human adipocytes and its expression in adipose tissue is elevated in insulin resistance and type 2 diabetes
M.J. Pereira1, A. Azim1, B.N. Jui1, J. Kullberg2,3, M.H. Lundqvist1, J.W. Eriksson1;
1Dept Medical Sciences, Clinical Diabetes and Metabolism, Uppsala University, Uppsala, 2Dept Surgical Sciences, Section of Radiology, Uppsala University, Uppsala, 3Antaros Medical, Mölndal, Sweden.
Background and aims: Interleukin-33 (IL33) is expressed in human adipose tissue and is released upon tissue damage to mediate local immune activation of Th2 cells. IL33 has been associated with obesity-related inflammation and complications. However, to what extent IL33 contributes to metabolic disorders in humans still requires further investigation. We aimed to investigate the expression levels of IL33 in human subcutaneous adipose tissue (SAT) in subjects with and without T2D, its association with genes involved in adipose tissue metabolism and effects of IL33 on human adipocyte glucose uptake.
Materials and methods: Abdominal SAT needle biopsies were collected from 20 control and 20 metformin-treated subjects with T2D matched for sex (F/M 10/10, age (58 ± 11 vs 58 ± 9 yr) and BMI (30.8 ± 4.6 vs 30.7 ± 4.9 kg/m2). IL33 and its receptor (IL1 receptor-like 1, IL1RL1) mRNA levels were measured using transcriptomics and were correlated with markers of insulin resistance and obesity and with the expression of genes involved in lipid storage and differentiation. MRI of body and tissue fat volumes was performed. Furthermore, SAT of 7 healthy control subjects was incubated for 24h without (control) or with human recombinant IL33 (200 and 1000 pg/mL), and adipocytes were isolated to measure effects on glucose uptake.
Results: The T2D group had 1.7-fold higher IL33 mRNA expression in SAT than the control group (P<0.001). IL33 expression in SAT was positively correlated with markers of dysglycemia (fasting glucose, glucose AUC during OGTT, and HbA1c; all P<0.01), insulin resistance HOMA-IR (P<0.001), FFA AUC during OGTT (P=0.01), liver and pancreas fat% (P<0.05), whereas it was negatively correlated with the Matsuda insulin sensitivity index (P<0.001). No associations were found between IL33 with BMI or SAT/VAT volume. Following multivariate analyses, HOMA-IR (st beta coeff= 0.541, P=0.018) remained a predictor of IL33 expression (P<0.001, adjusted R2=0.45, model: glucose, FFA AUC, liver fat%, and T2D status). Expression of the IL33 receptor, IL1RL1, did not correlate with any of the glycemic or insulin resistance parameters but was positively associated with BMI (P=0.009) and the pro-inflammatory cytokine IL1B (p<0.002). Both IL33 and IL1RL1 genes were negatively associated with expression of genes promoting lipid storage and adipogenesis (e.g. FASN, AGPAT1/2/3, DGAT1/2, CEBPA, PPARG, FABP4; P<0.05). In addition, IL33 expression was negatively correlated with adipocyte glucose uptake (basal and insulin-stimulated; P<0.05) and with several genes for glucose transport and insulin signalling (e.g. SLC2A1, SLC2A4, and AKT1; P<0.01). Conversely, following 24h of incubation of SAT with IL33 (1000 pg/ml), both basal and insulin-stimulated glucose uptake in adipocytes were reduced by about 30% (P<0.05) relative to control.
Conclusion: Our findings suggest that T2D patients have higher IL33 expression in SAT compared to healthy controls, and this is associated with insulin resistance and reduced lipid storage and adipogenesis markers. IL33 may reduce adipocyte glucose uptake. These findings open up a potential pharmacological route for reversing insulin resistance associated with obesity.
Supported by: EXODIAB, AstraZeneca, SW DiabFond, Ernfors Found, ALF, Marie Sklodowska Curie TREATMENT, Rudberg AM
Disclosure: M.J. Pereira: None.
SO 34 The hepato-skeletal impact on metabolic control
515
Hepatic OPA1 deficiency impacts mitochondrial plasticity, lipoprotein metabolism and liver steatosis
G. Norata1, A. Moregola1, P. Uboldi1, L. Scorrano2, L. Da Dalt1;
1University of Milan, Milan, 2University of Padua, Padua, Italy.
Background and aims: In liver, mitochondria continuously undergo biogenesis, fusion, fission and mitophagy, and thus participate to the regulation of energy metabolism, calcium homeostasis and lipid metabolism. Aim of this study was to test the role of selective deletion of a key protein involved in inner mitochondrial membrane fusion (OPA1) in hepatocytes on mitochondrial function and lipid metabolism.
Materials and methods: Liver selective OPA-1 deficient mice (Opa1LKO) were generated by crossing OPA-1 flox/flox mice with albumin-CRE+ mice. Opa1LKO and OPA1 flox/flox (controls) mice were fed a high fat diet for 20 weeks and then in vivo metabolic responses, liver and fat characteristics and proteomic signature were profiled.
Results: Opa1LKO mice fed with HFD display reduced weight and weight gain compared to controls. Liver, Visceral Adipose tissue (VAT) and Subcutaneous Adipose Tissue (SCAT) weight to total body weight was also reduced. Opa1LKO presented a significant reduction in fasting glycaemia as well as improved glucose tolerance and insulin tolerance compared to Opa1 WT. Moreover, plasma cholesterol levels and cholesterol distribution in lipoprotein fractions was reduced in Opa1LKO compared to Opa1 WT and the same was true for triglycerides. In vivo metabolic cages experiments revealed a reduced oxygen consumption and increased respiratory exchange rate in Opa1LKO compared to Opa1WT.Liver OPA1 deficiency limited lipid accumulation in the liver compared to Opa1 WT. Moreover, the liver from Opa1LKO mice presented an increased prevalence of smaller LD compared to Opa1 WT suggesting that diminished lipid accumulation and smaller LDs could be associated to changes in liver morphology and structure. In parallel Opa1LKO presented smaller adipocytes both in VAT and in SCAT compared to Opa1 WT. Next we investigated further changes in liver morphology and observed that Opa1LKO develop fibrosis associated to specific signs of chostetasis, potentially suggesting that that Opa1LKO liver could present an impairment in sterol metabolism and bile acid homeostasis. Shotgun liver proteomics was therefore performed to profile proteins involved in bile acids synthesis and maturation as well as proteins involved in lipoprotein metabolism. Interestingly, proteins involved in bile acids Bile Acid-CoA:Amino Acid N-Acyltransferase (BAAT) and 3-Oxosteroid 5β-Reductase (Akr1d1) synthesis and transport such as bile acid CoA ligase (SCL27A5) were reduced in Opa1LKO liver compared to Opa1 WT, in parallel also proteins involved in lipoproteins uptake, lipoprotein synthesis (MTTP) and apolipoproteins (apoA-I, apoA2, apoB) were reduced.
Conclusion: Our data suggest that the impairment of mitochondrial fusion could rewire liver metabolism and leads to cholestasis. This could be the consequence of altered bile acid synthesis, due to impaired mitochondrial activity, which results in reduced lipid absorption and limited liver lipid accumulation under HFD conditions.
Disclosure: G. Norata: None.
516
Adenosine: a new functional marker and therapeutic target for the link insulin resistance-non-alcoholic fatty liver disease
R.E. Figueiredo1, F.O. Martins1, R.O. Oliveira1,2, S.V. Conde1;
1CEDOC- Edifício CEDOC II, 2Unidade de Cirurgia Metabólica e Bariátrica, CTO, Hospital Cruz Vermelha de Lisboa, Lisboa, Portugal.
Background and aims: Nonalcoholic Fatty Liver Disease (NAFLD) is the most common liver disease worldwide, affecting 20-25% of the adult population. It represents a spectrum of diseases ranging from hepatic steatosis (HS) to nonalcoholic steatohepatitis (NASH). NAFLD is the hepatic manifestation of type 2 diabetes mellitus (T2DM), and this association is associated with insulin resistance. Adenosine is known to act on hepatic glucose production and insulin action. In dysmetabolic states, adenosine receptors are known to be altered, having a key function in inflammation and carcinogenesis. The present work aims to investigate the role of adenosine and the adenosinergic system in the pathological paradigm linking insulin resistance and obesity to fibrosis in the settlement and development of NAFLD.
Materials and methods: Human liver biopsies from patients submitted to bariatric surgery were compared with liver biopsies collected from normal weight controls undergoing different types of surgeries. Anthropometric and blood biochemical analysis were performed for the evaluation of body mass index (BMI) and parameters related with dysmetabolism such as glycemia, insulin and triglycerides. The clinical study followed the Helsinki Declaration and was approved by the ethical committee of Hospital da Cruz Vermelha and Nova Medical School. Written informed consent was obtained from all individuals. We evaluated by Western Blot the levels of Adenosine receptors: (A2A and A2B), a protein related to inflammation (interleukin-1 receptor, IL-1R), insulin signaling markers (Insulin receptor, IR) and the levels of Glucokinase. Additionally, hematoxylin-eosin and masson’s trichrome staining were performed to evaluate lipid deposition and fibrosis, and immunohistochemistry to colocalize A2aR within the different liver cell types. Inflammatory profile in plasma samples will be evaluated as well as liver adenosine levels.
Results: Obese individuals present a tendency to decrease in the levels of A2aR in the liver (% from control: CTL= 100.0 ± 8.4; Obese= 89.3 ± 28.1), with obese class III individuals showing the highest A2aR levels decrease (% of decrease from control: Ob Class I= 6%; Ob Class II= 10.2%; Ob Class III:24.4%). A2aR were colocalized within both Kupffer cells and hepatocytes. In contrast, A2bR levels tendentially increased in hepatic tissue from obese individuals (CTL= 100.0 ± 42.6; Obese= 211.9 ± 169.8), without evident dependency on obesity degree. Hepatic levels of IL-1R, IR and GCK were elevated in obese patients, showing that inflammation (IL-1R % from control: CTL= 100.0 ± 0; Obese= 285.5 ± 50.2, p<0.05), insulin resistance (IR % from control: CTL= 100.0 ± 0; Obese= 164.3 ± 13.9, p<0.01) and de novo lipogenesis (GCK % from control: CTL= 100.0 ± 0; Obese= 131.8 ± 55.4) are increased in these subjects.
Conclusion: We have demonstrated that obese patients with hepatic insulin resistance and dysmetabolism have alterations in adenosinergic system, both A2aR and A2bR. Adenosine metabolism should be further assessed to evaluate its possible role as a marker for liver dysfunction and insulin resistance associated with obesity.
Supported by: CEECIND/04266/2017
Disclosure: R.E. Figueiredo: None.
517
Disturbance of hepatic tight junctions by hypercaloric diets precedes intestinal barrier disruption
M.J. Meneses1,2, B. Patrício3, G.D. Belew4, F. Carli3, J.G. Jones4, A. Gastaldelli3, M.P. Macedo1,2;
1Chronic Diseases Research Centre (CEDOC), NOVA Medical School, Faculdade de Ciencias Medicas, Universidade NOVA de Lisboa, Lisbon, Portugal, 2APDP-ERC - Diabetes Portugal Education and Research Center, Lisbon, Portugal, 3National Research Council (CNR), Institute of Clinical Physiology (IFC), Pisa, Italy, 4Center for Neurosciences and Cell Biology, University of Coimbra, Coimbra, Portugal.
Background and aims: Diets high in lipids and carbohydrates are associated with dysbiosis, dysmetabolism and increased intestinal permeability impacting on tight junctions’ integrity. The passage of pro-inflammatory factors into the circulation is one of the risk factors for the development of non-alcoholic fatty liver disease (NAFL). However, hepatic tight junctions (TJ) are also crucial for homeostasis by separating the basolateral domain drained by sinusoidal blood flow and sealing the apical side of adjacent cells to form bile canaliculi. Disruption of hepatic TJ was already described in hepatitis and cancer, but little is known about the effects of diet on hepatic TJ. The hypothesis of this work is that diets rich in lipids and/or carbohydrates alter TJ not only in the intestine, but also in the liver. These changes are associated with alterations in proteins and lipids essential for maintaining the integrity of these TJ.
Materials and methods: C57BL/6J male mice were fed for 18 weeks with standard chow (CTR; n=12), high fat chow (HF; n=12), or with high sucrose (30% in drinking water, i.e., 55% fructose/45% glucose) added to standard diet (HS; n=11) or high fat chow (HFHS; n=11). Intestinal and liver tissues were histologically analyzed, and the levels of proteins involved in occlusion junctions were assessed. Given the importance of lipids for the composition of cell membranes and thus for tight junctions’ integrity, lipidomics of liver tissue was evaluated.
Results: All experimental groups except CTR developed hepatic steatosis. Both HS and HFHS presented decreased hepatic levels of occludin when compared to CTR (p<0.01 and p<0.05, respectively) and HF (p<0.01), indicating sugar-induced damage towards occludin. Besides, HFHS animals also had decreased hepatic expression of tricellulin when compared to CTR (p<0.05). Interestingly, although we found alterations in hepatic TJ, the exposure of the animals to the hypercaloric diets for 18 weeks did not lead to changes in intestinal tissue TJs. HS presented increased hepatic lysophosphatidylcholine C16:0 and C18:0 compared to the CTR (p<0.001) and an overall decrease in hepatic ceramides compared to CTR, especially the long chain (C16-18: p<0.01), which were previously found to negatively impact on membrane permeability and intestinal TJ. On the other hand, HFHS did not show changes in ceramides, rather an overall decrease in hepatic phosphatidylcholines compared to the CTR, namely PC 34:1 and PC 34:2 (p<0.001).
Conclusion: Hypercaloric diets, rich in sucrose, with/without lipids, negatively impact on hepatic TJ proteins occludin and tricellulin before affecting intestinal TJ. These changes are accompanied by changes in membrane lipids, known to be essential for TJ integrity and appropriate permeability.
Supported by: Marie Sklodowska-Curie Grant Agreements No. 722619 & 734719; FCT
Disclosure: M.J. Meneses: Grants; mtFoieGras Marie Sklodowska-Curie RISE Grant Agreement No. 734719, FOIEGRAS Marie Sklodowska-Curie Grant Agreement No. 722619, Portuguese Diabetology Society, iNOVA4Health UIDB/Multi/04462/2020.
518
Fetuin-a promotes lipid induced DPP4 expression in pancreatic beta cells
S. Nag, S. Mandal, R. Kundu;
Department of zoology, Visva-bharati university, Santiniketan, India.
Background and aims: Dipeptidyl peptidase 4 (DPP4) is a ubiquitous proteolytic enzyme that inactivates incretin hormones, GLP-1 and GIP, leading to reduced insulin secretion from beta cells (also called incretin effects). DPP4 is present in endothelial cells and expressed in tissues such as liver, lung, kidney and more recently has been reported in pancreatic beta cells. Palmitate treatment shows upregulation of DPP4 expression in beta cells. The aim of this present study is to investigate the role of fetuin-A, a hepato-adipokine, in DPP4 expression in presence of lipid and its consequence in impaired insulin secretion. Fetuin-A has been extensively studied by our group for quite a few years and has been found to be an endogenous ligand for TLR4. Beta cells incubated with DPP4 inhibitors shows reduced inflammation following TLR4 route and restores insulin secretion from beta cells. Therefore, this present observation focuses on the role of Fetuin-A in DPP4 expression and insulin secretory defects in the beta cells.
Materials and methods: Western blotting was used to study expression of DPP4 and other signal molecules involved in palmitate and palmitate+Fetuin-A treatment in MIN6 cells, a mouse insulinoma cell line. TLR4 and NF-kB inhibitors and gene specific siRNAs (DPP4, fetuin-A, TLR4 etc) were used to see alterations in the gene expressions. Immunofluorescence technique was used to see the expression and localization of DPP4 in palmitate+Fetuin-A treated cells. GSIS (Glucose Stimulated Insulin Secretion) and other ELISA analysis were performed to study the effect of DPP4 inhibitor vildagliptin (20uM), in palmitate treated MIN6. Oil O Red staining was done to see the accumulation of lipid droplets in MIN6 cells. Pancreatic islets collected from standard diet (SD) and 12 weeks HFD fed male mice, C57BL/6J, were subjected to western blot analysis for DPP4, fetuin-A, TLR4 and other relevant proteins. GTT, ITT and HOMA-IR were measured for insulin resistance.
Results: Palmitate treatment for 4h dose dependently (0.25, 0.50 and 0.75mM) increased DPP4 expression (~1.5 fold at 0.75mM palmitate) in beta cells which was abrogated by TLR4 and NF-kB inhibitors. Palmitate also increased phosphoNF-kBp65, IL-6 and IL-1beta levels. When fetuin-A (100ug/ml) was used in combination with palmitate, DPP4 expression was found to be increased by ~3 folds and augments its membrane localization. Inhibition of TLR4 and NF-kB by inhibitors or siRNAs, repressed DPP4 levels by ~1.7 fold. DPP4 inhibitor, vildagliptin (20uM), restored insulin secretion by ~1.5 fold, reduced TLR4, NF-kB, IL-6, IL-1beta and Fetuin-A (~1.4 fold) expression and also upregulates insulin gene transcriptor, Pdx1, in palmitate+fetuin-A treated cells. Vildagliptin significantly reduced lipid accumulation in the beta cells showing its multipronged effects. Islets isolated from 12 weeks HFD mice showed increased DPP4 expression (~1.5 fold) compared to SD mice. Also, there are increased expression of TLR4, pNF-kBp65 and Fetuin-A in the HFD islets. GTT, ITT and HOMA-IR score indicated degree of insulin resistance in HFD.
Conclusion: The results indicated palmitate with Fetuin-A potentiate DPP4 expression in pancreatic beta cells following TLR4-NF-kB pathway. Use of DPP4 inhibitor, Vildagliptin, repress fetuin-A level in beta cells, subside inflammatory cytokines and reduce fat accumulation in palmitate+Fetuin-A treatment indicating restoration of insulin secretion and beta cell function.
Supported by: SERB grant no. ECR/2017/001028
Disclosure: S. Nag: Grants; SERB grant no. ECR/2017/001028 & UGC fellowship awarded to SN.
519
The effects of deferoxamine (DFO) on DEX-induced muscle atrophy in C57BL/6J mice
K.-W. Lee1, S.-E. Choi2, J. Jeong1, S. Park2, M. Song1, Y. Son2, Y. Kang2, T. Kim3, S.-Y. Ahn4, H. Kim1, S. Han1, J. Jeon1, N. Lee1, D. Kim1;
1Department of Endocrinology and Metabolism, Ajou university, Suwon, 2Department of Physiology, Ajou university, Suwon, 3Division of Endocrinology and Metabolism, Seoul Medical Center, Seoul, 4Department of Internal Medicine, Busan Bumin Hospital, Busan, Republic of Korea.
Background and aims: Muscle atrophy is caused by various factors such as aging, insulin resistance, and diabetes. During these events, the balance between protein synthesis and degradation is disrupted and finally muscle loss is induced. This has been reported to occur via the ubiquitin-proteasome dependent pathway. Recently, many studies related to muscle loss have been reported, but the reality is no drug has been approved by the FDA yet. In this study, we investigated the effects of deferoxamine (DFO) on DEX-induced muscle atrophy in C57BL6J mice.
Materials and methods: To investigate the effects of DFO on muscle atrophy, C57BL/6J male mice (8-week-old) were randomly divided into three groups: control group, DEX group, and DEX plus DFO group. The mice were administered DEX (20 mg/kg/day i.p) and followed by injection of DFO (100 mg/kg/day i.p) for 12 days. The body weight of the mice was checked daily. On day 12, the grip strength of the mice was tested to measure the muscle force. After the mice were sacrificed, muscle tissues from the tibialis anterior (TA), gastrocnemius (GA), extensor digitorum longus (EDL), and soleus (SOL) were excised, dissected, and weighed. Each muscle tissue was stained with hematoxylin and eosin. The H&E stained sections were measured for cross-sectional area analyses using ImageJ software. To investigate the molecular mechanisms of DFO several factors related with muscle atrophy were tested using RT-PCR.
Results: Mice treated with DEX for 12 days had a significant decrease in body weight and grip strength. Interestingly, DFO administration alleviated reduction of mice weight and grip strength. After sacrificing the mice, the weight of the muscle tissues were measured. DEX significantly reduced TA muscle only, not GA, EDL, or SOL muscles. However, treatment with DFO prevented weight loss of TA muscle. DFO also prevented DEX-induced reduction of muscle fiber size. In addition, expression of IGF-1, which is a muscle protein synthesis-related factor, was decreased in DEX-administrated TA muscle, but treatment of DFO slightly recovered the expression of IGF-1. DEX dramatically increased expression of myostatin and muscle atrophic factors such as F-box only protein 32 (atrogin) and muscle RING-finger protein-1 (MuFR-1). However, DFO ameliorated the expression of myostatin, atrogin, and MuFR-1 in DEX-administrated mice muscles.
Conclusion: DFO ameliorates muscle atrophy by inhibition of muscle atrophic factors and recovered expression of IGF-1. These results suggest DFO may be a basis for the development of therapeutic drugs as applied to atrophy-related muscular diseases.
Disclosure: K. Lee: None.
520
Meta-inflammation and de novo lipogenesis markers are involved in metabolic associated fatty liver disease progression in BTBR ob/ob mice
S. Mas;
IIS-FJD / CIBERDEM, Madrid, Spain.
Background and aims: Metabolic associated fatty liver disease (MAFLD) is a hepatic manifestation of metabolic syndrome and usually associated with obesity and diabetes. Our aim is to characterize the pathophysiological mechanism involved in MAFLD development in Black Tan and brachyuric (BTBR) insulin-resistant mice in combination with leptin deficiency (ob/ob).
Materials and methods: We studied liver morphology and biochemistry on our diabetic and obese mice model (BTBR ob/ob) as well as a diabetic non-obese control (BTBR + streptozotocin) and non-diabetic control mice (BTBR wild type) from 4-22 weeks. Lipid composition was assessed and lipid related pathways were studied attranscriptional and protein level.
Results: Microvesicular steatosis was evident in BTBR ob/ob from week 6, progressing to macrovesicular in the following weeks. At 12th week, inflammatory clusters, activation of STAT3 and Nrf2 signaling pathways, and hepatocellular ballooning. At 22 weeks, the histopathological features previously observed were maintained and no signs of fibrosis were detected. Lipidomic analysis showed profiles associated with de novo lipogenesis (DNL).
Conclusion: BTBR ob/ob mice develop MAFLD profile that resemble pathological features observed in humans, with overactivation of inflammatory response, oxidative stress and DNL signaling pathways. Therefore, BTBR ob/ob mouse is an excellent model for the study of the steatosis to steatohepatitis transition.
Supported by: ISCIII PI20/00487
Disclosure: S. Mas: None.
521
Skeletal muscle mitochondrial inertia is associated with carnitine acetyltransferase protein activity and physical function in humans
M.K.C. Hesselink1, R. Mancilla1, L. Lindeboom2, L. Grevendonk1, J. Hoeks1, T.R. Koves3, D.M. Muoio3, P. Schrauwen1, V.B. Schrauwen-Hinderling4;
1Dept. of Nutrition and Movement Sciences, Maastricht University, Maastricht, Netherlands, 2Department of Radiology and Nuclear Medicine,, Maastricht University Medical Centre, Maastricht, Netherlands, 3Duke University School of Medicine, Durham, USA, 4Department of Radiology and Nuclear Medicine, Maastricht University Medical Centre, Maastricht, Netherlands.
Background and aims: At the onset of exercise, PCr decreases and restabilizes at a steady-state exercise intensity. The speed of this phenomenon (PCr on-kinetics) reflects the readiness to activate mitochondrial ATP synthesis, which in turn is secondary to acetyl-CoA availability. We hypothesized that PCr on-kinetics is slower in metabolically compromised and older individuals and associates with low carnitine acetyl-transferase (CrAT) activity and markers of physical function.
Materials and methods: In study 1, PCr on-kinetics (quantified as half-time of the mono-exponential change of PCr) and ADP levels were assessed by 31P-Magnetic Resonance Spectroscopy at the onset of exercise in all individuals in this study. In muscle biopsies from individuals with type 2 diabetes (T2D, n=9, 64 ± 7 years), obese sedentary (OB, n=8, 59 ± 7 years), untrained (UT, n=9, 22 ± 4 years and endurance-trained (T, n=12, 25 ± 4 years) CrAT activity and acetylcarnitine levels were measured. In study 2, we examined PCr on kinetics and its relationship with muscle oxidative capacity (measured as post-exercise PCr resynthesis rate) and markers of physical function and exercise efficiency in young (Y, n=10, 23 ± 5 years), older (O, n=15, 70 ± 2 years) and older trained (OT, n=17, 69 ± 2 years) volunteers.
Results: PCr on-kinetics, i.e. half-time of PCr at the onset of exercise, was significantly longer in T2D (49.2 ± 4.3 s) and OB (38.9 ± 2.6 s) compared to T (19.6 ± 2.1 s; P < 0.001). Furthermore, it was significantly longer in T2D as compared to UT (28.3 ± 3.2 s; P < 0.001). PCr on-kinetics strongly associated with skeletal muscle CrAT protein activity (r = -0.56; P = 0.001), in vivo skeletal muscle acetylcarnitine content (r = -0.47; P = 0.005) and ADP levels during exercise (r = 0.70; P < 0.001). This association remained after adjusting for PCr resynthesis rate (r = 0.61; P < 0.001). PCr on kinetics was significantly longer in O (33.1 ± 1.8 s) compared to OT (24.7 ± 1.8 s; P = 0.005) and Y (24.9 ± 2.2 s; P = 0.02). Interestingly, PCr on-kinetics correlated strongly with walking speed (r = -0.48; P = 0.002), chair sit-stand test performance (r = 0.54; P < 0.001) and exercise gross (r = -0.56; P < 0.001) and net efficiency (r = -0.53; P < 0.001) in study 2.
Conclusion: PCr on-kinetics is significantly slower in metabolically compromised and older individuals compared to young, healthy volunteers, regardless of in vivo skeletal muscle mitochondrial PCr recovery rate. Thus, mitochondrial inertia is more pronounced in metabolically compromised older individuals. Lower acetyl-coA availability (due to lower CrAT protein function) or a lower sensitivity to ADP may contribute to deferred mitochondrial activation at the onset of exercise. Functionally, mitochondrial inertia affects performance in daily life physical function parameters and hence is a relevant interventional target in metabolically compromised individuals and in elderly with compromised physical function.
Disclosure: M.K.C. Hesselink: None.
SO 35 Metabolic inflexibility and complications in humans
522
A genetic polymorphism with structural impact, blood levels, expression in adipose tissue, and engineered adipocytes functionally link Olfactomedin 2 to obesity
A. Lluch1,2, J. Latorre1,2, I. Espadas3, J. Moreno-Navarrete1,2, A. Martín-Montalvo3, J. Fernández-Real1,2, F. Ortega1,2;
1Unit of Diabetes, Endocrinology and Nutrition (UDEN), Institut d'Investigació Biomèdica de Girona (IDIBGI), Girona, 2CIBER de la Fisiopatología de la Obesidad y la Nutrición (CIBEROBN), Instituto de Salud Carlos III (ISCIII), Madrid, 3Department of Cell Therapy and Regeneration, Andalusian Center for Molecular Biology and Regenerative Medicine (CABIMER), Junta de Andalucía-University of Pablo de Olavide-University of Seville-CSIC, Sevilla, Spain.
Background and aims: Olfactomedin 2 (OLFM2) is a pleiotropic glycoprotein with a major role in brain development. Recently, we identified central OLFM2 as a new actor in the regulation of whole-body energy homeostasis. Highly expressed in neurons, our pioneering findings also locate OLFM2 in adipose tissue, specifically in fat cells. The current work investigates the role of this molecule in adipocyte biology and adipose tissue function, laying emphasis on the mechanism of action and the relationship with obesity.
Materials and methods: This study primarily stems from observations of highly increased OLFM2 in the adipose tissue of severe obese women following surgery-induced weight loss. These results were validated in cross-sectional samples by real-time PCR (n~200). OLFM2 was also evaluated in ex vivo isolated and in vivo cultured adipocytes. Engineered 3T3-L1, together with changes in the expression patterns (microarrays) of human adipocytes challenged with small interfering (si)RNAs against OLFM2 were used to deepen into the mechanistic consequences of impaired OLFM2 in obese adipocytes. In parallel, circulating measures (ELISA), and the functional consequences of a structural polymorphism previously linked to OLFM2 activity (rs2303100) were assessed in independent cohorts.
Results: In partial agreement with the Olfm2 null mice, significant changes in the polymorphism rs2303100 (c.317G→A), which encodes an arginine to glutamine swift (R106Q), link OLFM2 to obesity (OR=1.38 [1.02-1.86], p=0.028; n=1,808). Also circulating variations in subjects with a wide range of body mass indexes (BMI) associate OLFM2 with the amount of fat. Concurrently, decreased OLFM2 expression in visceral and subcutaneous depots of adipose tissues ran in parallel to an obese phenotype. These variations are likely related to sex (sexual dimorphism) and age, and mainly determined by the predominant expression of OLFM2 in adipocytes, according to our pioneering observations in isolated fat cells. In fact, OLFM2 in human adipocytes is strongly conditioned by the hormonal milieu and compromised by inflammation. Mechanistically, we demonstrate that the loss of OLFM2 in adipose cells displays inflammatory issues and deranged adipogenesis as a result of a rapid and transient deregulation of genes related to cell cycle, also coupled to impaired fatty acid anabolism and energy storage.
Conclusion: Overall, current findings first identify OLFM2 as a new molecular key actor and signalling molecule of relevance in adipocyte biology for proper functioning of fat depots.
Supported by: ISCII (CP19/00109, PI18/00550), PFIS (FI19/00045), FEDER
Disclosure: A. Lluch: None.
523
Development of a predictive model for short and medium-term weight loss in people with type 2 diabetes attending a weight management programme
L. Al-Abdullah1, P. Welsh1, J. Logue2;
1University of Glasgow, Glasgow, 2Lancaster University, Lancaster, UK.
Background and aims: Behavioural weight management programmes are effective in helping some but not all obese patients to lose weight. With emerging novel pharmacological options for weight loss, it may be advantageous to be able to predict successful short and medium-term weight loss within traditional weight management programmes, so patients who are unsuccessful in these programmes can be moved to other therapies at an earlier stage.
Materials and methods: We used routine electronic health records collected in the Greater Glasgow and Clyde health board of the UK National Heath Servic , covering 1.2 million people. We conducted a longitudinal study between 2004-2014 including 1658 adult patients with obesity and type 2 diabetes referred to and attending the Glasgow Weight Management Service. Successful short term outcome was defined as attending 7/9 weight management sessions over 16 weeks and losing >5% body weight. Successful medium-term outcome was defined as losing >5% body weight at 3 years after also attaining successful short term weight loss. As an exposure/predictor, early weight change in programme is defined as weight change in the first 3 session of the programme. Binary logistic regression analysis was used to estimate the strength of association between predictor variables (including demographic and clinical) and successful short and medium term completion. Discrimination of predictors was determined by using area under the receiver operator curve (AUROC).
Results: The mean age of the participants was 57.8 years, 60% were female, T2DM had been diagnosed for a median of 5.31 years and the median BMI was 40.2 kg/m2 (IQR = 35.9 - 44.8). In the short term 333 participants successfully lost weight (20.0%) and of 1152 participants with medium-term outcome data, 139 successfully lost weight over 3 years (12.1%). For the short term outcome, the only clinical or demographic variable associated with short term successful completion was weight change in the first 3 sessions (each 1% weight loss OR 2.86 (95%CI 2.5-3.23) for successful short term completion) and the AUROC curve for the model was 0.839 (95%CI 0.812-0.866). Losing 0.5% weight in the first 3 sessions predicted successful short term completion with sensitivity 90.4% and specificity 53.6% (negative predictive value 95.7%). The only independent predictor of medium term weight loss was also weight change in the first three sessions (each 1% weight loss OR 2.22 (95%CI 1.92-2.56)) and the AUROC curve for the model was 0.816 (95%CI 0.775-0.856). Losing 0.5% weight in the first 3 sessions predicted successful medium-term weight loss success with sensitivity 89.9% and specificity 50.5% (negative predictive value 97.3%).
Conclusion: Demographic and clinical variables do not allow prediction of successful weight loss using a clinical structured weight management programme. However, in programme early weight loss is strongly associated with short term and medium-term success. A threshold of failing to achieve 0.5% body weight loss in the first three sessions is sufficient to identify participants who are unlikely to succeed in programme (>95% accuracy). Such patients may benefit from being switched to other interventions while they are still engaged.
Disclosure: L. Al-Abdullah: None.
524
Metabolic inflexibility in type 2 diabetes is supported by untargeted metabolomics during OGTT
G. Fanni, J.W. Eriksson, M.J. Pereira;
Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Background and aims: Metabolic inflexibility, i.e. the inability to efficiently adapt metabolic processes to physiological and nutritional conditions, is a hallmark of insulin resistance and can be explored extensively with high-throughput metabolomics techniques. These can be integrated with dynamic tests, such as the oral glucose tolerance test (OGTT), to better capture the perturbations of the whole metabolome upon an oral glucose load. We aimed to identify metabolic alterations in response to an OGTT in individuals with type 2 diabetes (T2D) using an untargeted metabolomic and lipidomic approach for the first time on both plasma and subcutaneous adipose tissue (SAT) samples.
Materials and methods: 20 individuals with T2D and 20 healthy controls matched for sex (20F/20M), age (58±9 vs 58±11 years), and BMI (30.7±4.7 vs 30.8±4.6 kg/m2) were metabolically profiled with high-throughput metabolomics and lipidomics analysis both in plasma and in the SAT, during fasting and 90 minutes after an OGTT. We identified 854 known metabolites in plasma samples and 692 metabolites in SAT samples. We compared concentration changes 90 minutes after the glucose load versus fasting levels in all metabolites between patients with T2D and controls. Post-hoc analyses were performed to explore the associations between metabolites that significantly differ in response to OGTT between T2D and controls and markers of obesity, glucose metabolism, sex, and age.
Results: In controls, the glucose load caused a reduction in the plasma concentration of several acylcarnitines (fold-change 0.41 to 0.50), acyl ethanolamines (0.48 to 0.59), xanthine (0.80), mannose (0.79), BCAAs (0.67 to 0.84), and other amino acids (0.78 to 0.94), but this effect was significantly blunted in patients with T2D (all p<0.05; figure 1). Also, patients with T2D had an increased response during OGTT in glucose and fructose levels compared to controls (both p<0.05). Only glucose had significantly different plasma concentrations at fasting between T2D and controls. No differences were seen in the adipose tissue metabolome in response to the glucose challenge between T2D and controls. In multiple regression analyses, the plasma concentration change after the glucose load of most of these metabolites was mainly associated with indices of hyperglycemia (glucose AUCOGTT and HbA1c), rather than insulin resistance (HOMA-IR), insulin secretion (Insulinogenic index), or BMI.
Conclusion: Using a metabolomic approach, we show that T2D patients display attenuated metabolite responses in plasma during an OGTT. This concerns several metabolite families, e.g. amino acids and fatty acid derivatives. This supports inflexibility in several metabolic pathways which can contribute to dysregulated substrate partitioning and turnover in T2D. These findings are not directly associated with changes in adipose tissue metabolism, thus other tissues like muscle and liver may be of importance.
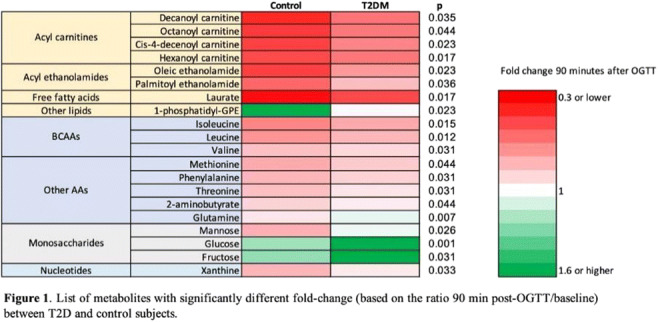
Supported by: EXODIAB, UAS ALF, SSMF, DF, ErforsS
Disclosure: G. Fanni: None.
525
The relationship between obesity and depression is partly dependent on metabolic status: a nationwide inpatient sample database study
Z. Wang, J. Zhao, X. Fan;
Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, Jinan, China.
Background and aims: Studies have found a close association between obesity and depression, whereas others have not. As we know, obese have different metabolic characteristics and exhibit different metabolic obesity phenotypes. These differences might be a possible explanation for these inconsistent results. Some obese individuals do not develop metabolic disorders and are described as having metabolically healthy obesity (MHO). In addition, many nonobese individuals with an abnormal metabolic health status are known to have metabolically unhealthy nonobesity (MUNO). Up to date, evidence about the joint effects of obesity and different metabolic profiles on the risk of depression is limited. In addition, whether this association is modified by sex is also worth investigating. Thus, we combined obesity and metabolism by focusing on metabolic obesity phenotypes and analyzed the Nationwide Inpatient Sample database to comprehensively investigate the relationship of obesity and metabolism with depression.
Materials and methods: This was a cross-sectional study using data from 2016 to 2018 that were obtained from the Nationwide Inpatient Sample (NIS) database. In total, 11,364,644 participants were enrolled. BMI ≥ 25 kg/m2 was used to define obesity. Metabolic risk factors were defined as the following three components of metabolic syndrome based on the diagnostic criteria for metabolic syndrome: (1) hypertension, (2) dyslipidemia (including hypertriglyceridemia and hypercholesterolemia), and (3) hyperglycemia (including prediabetes and diabetes). Participants were classified as one of four obesity phenotypes based on their obesity and metabolic statuses: (1) metabolically healthy nonobesity (MHNO): patients were nonobese and had fewer than two metabolic risk factors; (2) MHO: patients were obese and had fewer than two metabolic risk factors; (3) MUNO: patients were nonobese and had at least two metabolic risk factors; and (4) metabolically unhealthy obesity (MUO): patients were obese and had at least two metabolic risk factors.
Results: Among all participants, a higher risk of depression was observed for MUNO, MHO and MUO than for MHNO. The risk was highest for MUO (OR = 1.709; 95% CI = 1.698, 1.720). However, the association between MHO and depression was different for men and women (OR = 0.937, men; OR = 1.452, women). MHO was related to the risk of depression among women but the opposite associations were observed among men.
Conclusion: The increased risk of obesity-related depression appears to partly depend on metabolic health status. Our findings highlight the importance of both metabolic health status and obesity in depression and suggest a compelling need to improve metabolic health status regardless of the presence of obesity. Furthermore, our results might provide a basis for further studies to investigate individualized MetS-related treatment strategies for depression.
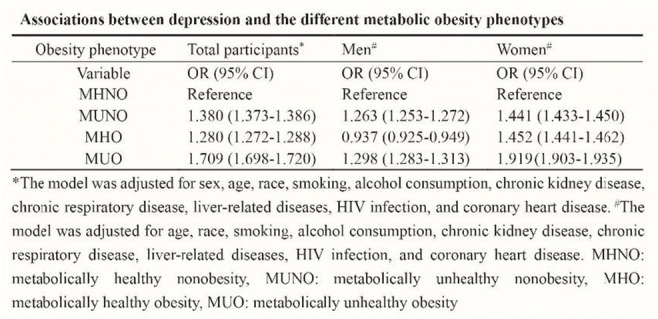
Supported by: National Key Research and Development Program of China 2017YFC1309800
Disclosure: Z. Wang: None.
526
Oxytocin correlates with body dysmorphic concerns and predicts weight loss in patients with obesity after a very low carbohydrate ketogenic diet
R. Tozzi1, R. Rossetti2, S. Basciani2, D. Masi2, R. Risi2, A. Balena2, M. Spoltore2, M. Watanabe2, S. Mariani2, L. Gnessi2, C. Lubrano2;
1Department of Molecular Medicine, Sapienza, University of Rome, 2Department of Experimental Medicine, Sapienza, University of Rome, Rome, Italy.
Background and aims: Oxytocin (OXT) enhances glucose uptake and lipid utilization in adipose tissue and skeletal muscle. Dysfunction of the OXT system could underlie the pathogenesis of weight gain. In addition, its involvement in social-cognitive processes and as a neurotransmitter of the emotional system has recently emerged, also in relation to body image awareness. Men and women with obesity show higher OXT blood levels and are often characterized by eating disorders and altered body perception. In contrast, metabolic syndrome has been associated with reduced fasting serum oxytocin in larger scale mixed gender studies. The purpose of our study was to investigate the concentrations of OXT in a population affected by obesity before and after a very low carbohydrate ketogenic diet (VLCKD) induced weight loss. Moreover, we aimed to investigate the relationship between peripheral OXT levels and the presence of altered perception of physical appearance.
Materials and methods: Subjects with obesity were enrolled at the Center of High Specialization for the treatment of Obesity. At baseline (t0) and after eight weeks of VLCKD (t1), all patients underwent clinical evaluation, biochemical routine assessment, DXA examination for body composition (Hologic Inc., Bedford, MA, USA, QDR 4500W) and venous blood sampling in EDTA plus 500 KIU/mL of aprotinin (Abcam ab146286) for plasma OXT determination (Abcam, Ab133050, ELISA kit). Achievement of ketosis was monitored by measuring urinary seton bodies. For psychometric evaluation, all patients were administered the Body Uneasiness Test-2(BUT2) for dysmorphophobia assessment at baseline.
Results: 40 patients (26 females and 14 males) suffering from obesity were enrolled, (age = 55.5 ±7 years and BMI = 35.7± 4.3 Kg/m2). OXT level at baseline (t0) was 1166 ±403 pg/ml, with no differences between males and females. At t0 OXT positively correlated with BMI, hip circumference and with the BUT2 specific subsection for Positive Symptom Distress Index -PSDI ( r=0.43, p<0.05). After VLCKD, a significant weight reduction was seen (mean BMI = 32.7 kg/m2, mean weight loss = -8.8 kg) and OXT (t1) significantly decreased (728.2± 201.7 pg/ml, p<0.001). Baseline OXT positively correlated with urinary keton bodies, after adjustment for age (r= 0.335, p=0.046). A strong inverse- age adjusted correlation between weight loss and baseline OXT was also reported (r= - 0.458, p<0.005). A regression analysis showed that the reduction in OXT between t0 and t1, the grade of ketosis and baseline % fat mass were all predictors of weight loss (R2 0.422; p=0.025).
Conclusion: Our study demonstrates that higher OXT levels associate with dysmorphophobia, BMI and ketosis induction. A lower OXT reduction during a VLCKD seems to be unfavorable in achieving weight loss. Ketosis, by reducing oxytocin levels, suggested to be a valid diet treatment to reduce body perception disorders. Differences in assay method used for measuring OXT, as well as expression patterns of oxytocin receptors could explain the partial discrepancy of our results with those in literature. Peripheral actions of oxytocin deserve further investigations.
Disclosure: R. Tozzi: None.
527
Type 2 diabetes is associated with decreased activation and increased cytokine production by blood lymphocytes: a human flow cytometry study
D. Gašparini1,2, I. Kavazović1, Ž. Mijolović2, I. Klarić2, A. Prunk Drmić2, V. Peršić2,1, F.M. Wensveen1, T. Turk Wensveen2,1;
1Faculty of Medicine, University of Rijeka, Rijeka, 2Thalassotherapia Opatija, Opatija, Croatia.
Background and aims: Chronic and systemic low-grade inflammation has been recognized as a prominent feature of type 2 diabetes and its complications. Recent studies on the novel coronavirus disease have shown that elevated blood levels of proinflammatory cytokines in patients with diabetes are contributing to an increased risk of morbidity and mortality after viral infection. However, which immune cells are involved in the underlying mechanism is mostly unknown. The aim of this study is to characterize peripheral blood lymphocytes and determine the nature of diabetes-induced changes in the antiviral arm of the immune system, specifically CD8+ T cells, natural killer (NK) cells and gamma delta T cells.
Materials and methods: Study participants underwent initial clinical and laboratory evaluation to exclude subjects whose immune system could be affected by advanced liver or kidney disease, neoplastic or inflammatory disorders and medication use. After signed informed consents were obtained, peripheral blood mononuclear cells were isolated from the blood of patients with diabetes (N=22) and age-matched control subjects (N=11). Multiparametric flow cytometry was used to analyze the phenotype and cytokine production by CD8+ T, NK and gamma delta T cells after in vitro stimulation with Phorbol 12-myristate 13-acetate and Ionomycin. Unpaired t-test was used to determine statistical significance.
Results: The mean age of study participants was 61 (35-72) and 63 (23-79) years old in the control and diabetes groups, respectively. Approximately one-sixth of patients with diabetes were newly-diagnosed, whereas others had a mean disease duration of 10 years (2-40). The mean percentage of glycated hemoglobin A1C in patients with diabetes was 7.6% (5.1%-11.1%). Phenotypic analysis of peripheral blood mononuclear cells showed only minor differences in the percentage of lymphocyte subpopulations between study groups. However, a significantly increased production of tumor necrosis factor by CD8+ T cells (p=0.025, 95%CI 2.30-32.11) and Granzyme B by NK cells and gamma delta T cells after stimulation (p=0.002, 95%CI 5.97-24.68; p=0.032, 95%CI 1.76-37.31, respectively) was observed in patients with diabetes in comparison to control group. Notably, the expression of CD69, the activation marker, on the surface of NK cells and gamma delta T cells was decreased in patients with diabetes compared to control subjects (p=0.063, 95%CI -43.94 to 1.23; p=0.022, 95%CI -39.24 to -3.23, respectively).
Conclusion: Our findings suggest that cytotoxic immune cells permanently change their functional profile in the context of diabetes and may therefore play an important role in diabetes-induced immune dysfunction.
Supported by: UniRi grant 19-41-1551; HRZZ projects: IP-2016-06-8027, IP-CORONA-2020-04-2045, IP-2020-02-7928
Disclosure: D. Gašparini: None.
528
Relationship between soluble receptor for advanced glycation end products (sRAGE) and obesity in individuals with type 1 diabetes during a median follow-up of 12 years
K. Adeshara1,2, E.B. Parente1,2, V. Harjutsalo1,3, M. Lehto1,2, P.-H. Groop1,3, on behalf of FinnDiane Study Group;
1Folkhälsan Institute of Genetics, Folkhälsan Research Center, 2Research Program for Clinical and Molecular Metabolism, University of Helsinki, 3Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: The soluble receptor for advanced glycation end products (sRAGE) has been linked to obesity and hypothesized to neutralize the action of pro-inflammatory RAGE ligands. Although obesity is defined by the excessive accumulation of body fat tissue, the distribution of fat tissue in the body is of utmost importance. Our group has shown that central obesity is associated with diabetes complications in people with type 1 diabetes. In the present study, we aimed to investigate whether sRAGE is associated with body fat distribution as well as with body fat changes during a median follow-up of 12 years of individuals with type 1 diabetes.
Materials and methods: From the Finnish Diabetic Nephropathy (FinnDiane) Study 303 adults with type 1 diabetes were included in this analysis. Central obesity was represented by the waist to height ratio (WHtR) whereas general obesity was represented by BMI. Changes in BMI and WHtR over the follow-up time were calculated by the difference (delta) between the values at the follow-up visit and baseline visit. The serum concentration of sRAGE was determined by ELISA. Wilcoxon rank test was used to compare the changes in BMI and WHtR. We used a linear regression model adjusted for sex, baseline age, HbA1c and the presence of albuminuria to evaluate the associations between sRAGE, central obesity and general obesity at baseline. The same model was used for the associations between sRAGE and the delta of BMI and WHtR. A p-value below 0.05 was considered significant.
Results: The median age at baseline was 34 (IQR 26, 43) years, median duration of diabetes was 14.1 (8.3, 28.3) years, median BMI was 24.5 (22.5, 26.6), median WHtR was 0.49 (0.45, 0.53) and 50% were men. In a median follow-up of 12.23 (IQR 10.26, 13.74) years, BMI increased (p<0.001) in 69% of the individuals [median delta 1.13 (IQR -0.35, 2.91)] whereas WHtR increased (p<0.001) in 75% of them [median delta 0.023 (IQR -0.001, 0.055)]. In the linear regression, sRAGE was inversely associated with BMI (r2=0.09, β=-0.210, p<0.001) and WHtR (r2=0.16, β=-0.247, p<0.001) at baseline. However, baseline sRAGE showed no association with the delta of BMI (p=0.82), not either with the delta of WHtR (p=0.83).
Conclusion: sRAGE is inversely associated with both general and central obesity, suggesting that it is a marker of body fat. However, independent of the fat distribution, it has no association with the changes in body fat over the 12-year follow-up time of adults with type 1 diabetes.
Supported by: Folkhälsan Research Foundation and Academy of Finland
Disclosure: K. Adeshara: None.
529
Probing Zn 2+ -HSA interactions using mutagenesis and isothermal titration calorimetry
A.J. Stewart1, S.J. Hierons1, D. Wu1, S. Arya1, C.A. Blindauer2;
1School of Medicine, University of St Andrews, St Andrews, 2Department of Chemistry, University of Warwick, Coventry, UK.
Background and aims: Zn2+ is an essential element required for many important biological processes including haemostasis. In plasma, Zn2+ is mainly transported by human serum albumin (HSA). Two Zn2+-binding sites on HSA have been identified. The primary site comprises His67, His247 and Asp249 residues. A secondary site which binds Zn2+ more weakly involves His9, Asp13 and Asp255. Interestingly, non-esterified fatty acids (NEFAs), also transported by HSA compromise its Zn2+-binding capacity. This allostery is important as it means that zinc handling by HSA can be disrupted when NEFA levels become elevated. Plasma NEFA levels are chronically raised in several disease states including obesity and type-2 diabetes and our work has recently suggested this mechanism to contribute to the development of Zn2+-dependent thrombotic complications in type 2 diabetes (Sobczak et al. (2021) Chem. Sci. 12:4079). There are 7 NEFA-binding sites on HSA (FA1-FA7) and crystallographic evidence indicates that NEFA binding to the FA2 site may be largely responsible for preventing HSA from binding Zn2+. However, it is unclear the degree to which NEFA binding at FA2 and other sites contributes to the reduction in Zn2+ binding. Here we examined the allosteric mechanism by which NEFAs impact on Zn2+ binding to HSA.
Materials and methods: Oligonucleotide-directed mutagenesis was used to prepare constructs encoding the human albumin proteins (wild-type, H9A, H67A, H247A and a triple mutant Y150F/R257A/S287A involving residues known to be important for NEFA binding at FA2) and resultant clones were transformed into Pichia pastoris competent cells. Recombinant mutant proteins were expressed and purified using a HiTrap Blue HP column. ITC was used to investigate the Zn2+-binding capacity of each protein in the presence and absence of the saturated fatty acid, palmitate (C16:0).
Results: Two Zn2+-binding sites on HSA were captured by ITC, with a high-affinity site (site A with K1ITC = 4.0 x 105 M-1) and a lower-affinity binding site (site B with K2ITC = 8.8 x 103 M-1). As expected, the Zn2+-binding capacity at the primary site exhibited marked reduction (almost no binding was seen) in H67A and H247A mutant forms of HSA, with the binding capacity of site B unaffected. This confirms that His67 and His247 play a significant role in Zn2+ binding under physiological conditions. However, only a small reduction of Zn2+-binding capacity was observed in H9A, consistent with the fact that His9 forms part of the secondary Zn2+-binding site. Zn2+ binding to HSA was greatly compromised by the presence of the long-chain NEFA, palmitate (C16). Upon addition of 5 mol. eq. of palmitate, Zn2+ binding to HSA and H9A was greatly perturbed. However, loading of H67A and H247 with 5 mol. eq. of palmitate had a relatively minor effect on Zn2+ binding. Additionally, the Zn2+ binding ability of the Y150F/R257A/S287A HSA mutant was not adversely affected by the binding of palmitate.
Conclusion: NEFAs influence Zn2+-binding at the primary site but not the secondary site and NEFAs exert this influence solely by binding at the FA2 site which lies close to site A. Previous studies on bovine serum albumin suggested NEFAs influenced Zn2+ binding at both sites, suggesting some differences in this dynamic between species. This work greatly aids understanding of the interplay between circulatory transport of NEFA and Zn2+ in obesity and type-2 diabetes.
Supported by: We thank BBSRC (grant no. BB/V014684/1) and BHF (grant no. FS/20/3/34956) for funding.
Disclosure: A.J. Stewart: None.
SO 36 Modelling obesity and type 2 diabetes
530
Arrest of long-chain acylcarnitine synthesis prevents inflammation and insulin resistance in adipose tissue of high-fat diet-fed TMLHE knockout mice
E. Liepins, B. Svalbe, H. Cirule, M. Videja, M. Dambrova;
Laboratory of Pharmaceutical Pharmacology, Latvian Institute of Organic Synthesis, Riga, Latvia.
Background and aims: Lipid overload contributes to obesity-associated insulin resistance and inflammation in adipose tissue. Fatty acid intermediates long-chain acylcarnitines induce insulin resistance in muscles and heart, however, it is not yet studied whether acylcarnitines participate in immunometabolic crosstalk in adipose tissue. The tissue levels of long-chain acylcarnitines can be regulated through controlling enzymatic activity of enzymes involved in biosynthesis of carnitine. N6-trimethyllysine dioxygenase (TMLH) is the first enzyme in the carnitine/acylcarnitine biosynthesis pathway. Here we tested whether lower levels of long-chain acylcarnitines in TMLHE KO mice can prevent metabolic disturbances and inflammation in adipose tissue.
Materials and methods: Two-month old C57BL/6J and TMLHE KO male and female mice were fed either a control diet (containing 10% calories from fat) or a high-fat diet (HFD) (containing 45% of calories from fat) for 20 weeks.
Results: In wild-type (WT) mice HFD intake induced macrophage shift to inflammatory M1 phenotype and by 70% reduced glucose uptake in adipose tissue Which resulted in hyperinsulinemia, hyperleptinemia, and disturbed glucose and insulin tolerance. In addition, significantly reduced mitochondrial OXPHOS-dependent respiration rate and OXPHOS coupling efficiency were observed in HFD-fed WT mice adipocytes. TMLHE gene inactivation lowered the content of long-chain acylcarnitines in a diverse mixture of specialized immune cells, peripheral blood mononuclear cells, and adipose tissues by 3-fold. In HFD treated TMLH KO mice glucose uptake, insulin sensitivity, and mitochondrial functionality of adipose tissue were fully preserved and were similar to parameters in chow control mice. In addition to significantly lowered adipose tissue macrophage count and a marked shift in macrophage phenotype to M2-like polarization was observed in HFD-fed TMLH KO mice.
Conclusion: Taken together, our results demonstrate that metabolic correction by lowered long-chain acylcarnitine contents can prevent lipid overload-induced macrophage M1-like polarization characteristic for the adipose tissue inflammation and contribute to preserved insulin sensitivity of adipose tissue.
Supported by: FAT4BRAIN No 857394
Disclosure: E. Liepins: None.
531
Haematopoietic cell-derived Oncostatin M regulates metabolic responses to high-fat diet in mice
M. Albiero1,2, S. Ciciliot3, A. Rodella1,2, L. Migliozzi1,2, F.I. Amendolagine1, G. Zuccolotto4, A. Rosato4,5, G.P. Fadini1,2;
1Department of Medicine, University of Padova, Padova, 2Veneto Institute of Molecular Medicine, Padova, 3University of Pavia, Pavia, 4Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, 5Veneto Institute of Oncology - IOV-IRCCS, Padova, Italy.
Background and aims: Innate immune cells infiltrate the growing adipose and propagate inflammatory clues to metabolically distant tissues, thereby promoting unhealthy obesity and insulin resistance. Among such signals, cytokines of the IL-6 family and gp130 ligands have attracted attention. Contrasting findings exist regarding the role played by Oncostatin M (OSM) in the metabolic consequences of overfeeding, largely because the broad distribution of its receptors with tissue-specific sub-unit combinations. Furthermore, the existing models did not allow dissecting OSM sources . Therefore, we aimed to better elucidate the metabolic role of OSM using the high fat diet (HFD)-induced model of obesity in ubiquitous Osm-/- mice. More importantly, using a hematopoietic-restricted Osm-/- mice, we tested the hypothesis that hematopoietic cell-derived OSM contributes to systemic metabolic regulation.
Materials and methods: Wild type and Osm-/- mice, were fed with a control diet or a HFD for 12 weeks before performing a thorough characterisation encompassing metabolic cages phenotyping, glucose and insulin tolerance tests, body composition, tissue glucose uptake, adipose tissue and liver histology and gene expression analysis. Hematopoietic-restricted Osm-/- mice and control mice were generated by transplanting Osm-/- or wild type bone marrow cells, respectively, into lethally irradiated wild type recipients. After bone marrow reconstitution, animals received HFD and were characterised.
Results: OSM deletion did not affect weight gain and adipose tissue remodelling induced by HFD but severely worsened glucose tolerance compared to wild type mice. Furthermore, Osm-/- mice showed worsened liver damage and increased expression of pro-inflammatory cytokines such as Il-1ß and IL-6. The deletion of OSM from hematopoietic cells recapitulated the detrimental metabolic milieu of ubiquitous Osm-/- mice on HFD. Interestingly, Hematopoietic-restricted Osm-/- mice phenocopied the liver inflammatory fingerprint induced by HFD and the the increase in aspartate aminotransferase observed in ubiquitous Osm-/- mice.
Conclusion: Despite being canonically associated with inflammation, our new data support a protective role for OSM in limiting metabolic derangement under western diet. We have also demonstrated that hematopoietic-derived OSM can regulate metabolism, possibly through a crosstalk between immune-inflammatory cells and the liver. Notwithstanding with the evidence that inflammatory cells are recruited during the development of obesity, OSM could represent an exploitable target to treat metabolic diseases once OSM-target cells and the downstream mechanisms regulating metabolism will be fully elucidated.
Disclosure: M. Albiero: None.
532
Irisin protects against obesity-related kidney disease by regulating perirenal adipose tissue function in obese mice
X. Sun, F. Han, C. Kan, N. Hou;
Department of Endocrinology and Metabolism, Affiliated Hospital of Weifang Medical University, Weifang, China.
Background and aims: More studies demonstrated perirenal adipose tissue (PRAT) has a closer association with kidney disease than other visceral fat deposits in obesity. PRAT could predict microalbuminuria and may be an independent predictor of early kidney damage in patients with obesity. Irisin is a novel hormone secreted by myocytes and has been proposed to mediate the beneficial effects of exercise on obesity. We aimed to determine whether irisin reduced urinary albumin excretion and provided renal protective effects by regulating PRAT function in obese mice.
Materials and methods: Six-week male C57BL/6J mice were randomly assigned to control, high-fat diet (HFD) and irisin groups. Mice in the control group were fed a regular diet, while mice in the other groups were fed an HFD. After receiving an HFD for 24 weeks, mice in the irisin group were administered an intraperitoneal injection of irisin (0.5 μg/g)) for another 4 weeks. Blood biochemical and urinary albumin excretion were measured. The morphology of the kidney was analyzed by histopathology. Glomerular VEGF-NO axis and proteins associated with PRAT function were determined by western-blot.
Results: HFD mice showed significant metabolic abnormality and renal injury, including increased body weight, fat mass, urinary albumin excretion, morphologic changes, and lipid accumulation. Irisin treatment significantly decreased the final body weight (44.87 ± 1.42 g vs. 49.67 ± 1.48 g, P<0.05), fat mass (11.75 ± 0.78 g vs. 14.56 ± 0.43 g, P<0.05), fat/weight (26.08 ± 1.09% vs. 29.37 ± 0.75%, P<0.05), but not PRAT mass. Besides, irisin significantly improved glucose hemostasis decreased free fatty acids (0.85±0.04 mmol/L vs.1.33±0.11 mmol/L, P<0.05). Furthermore, irisin decreased UACR (14.47 ± 1.92 μg vs. 45.16 ± 10.21 μg, P<0.05), attenuated renal fibrosis and lipid deposition. Treatment with irisin increased proteins related to browning in PRAT, including SIRT1, uncoupling protein-1, haem-oxygenase-1 and adiponectin. Pre-treated HFD mice-derived PRAT and glomeruli with irisin ex vivo also activated these proteins in PRAT. Additionally, irisin significantly reduced glomerular VEGF levels and increased nitric oxide production, restoring the glomerular VEGF-nitric oxide axis.
Conclusion: Irisin treatment improved metabolic disorders and protected against obesity-related kidney disease by regulating the PRAT-kidney axis. These findings provide a novel mechanism for the protective effects of irisin on kidneys in obesity.
Supported by: NSFC (81870593 and 82170865)
Disclosure: X. Sun: None.
533
The adipocyte-specific deletion of the tumor suppressor gene CDKN2a improves glucose tolerance in high-fat diet-fed mice
Y. Kahoul1, J. Montaigne1, E. Caron2, A. Bongiovanni3, F. Oger1, P. Froguel1, A. Bonnefond1, J.-S. Annicotte1,4, C. Breton1;
1INSERM UMR1283, CNRS UMR8199, EGID, Université de Lille, Institut Pasteur de Lille, Lille, France, 2INSERM U1172, Développement et plasticité du cerveau neuroendocrine Lille Neuroscience et Cognition, Lille, France, 3Plate-forme d'imagerie cellulaire-UMS 2014-US 41, Lille, France, 4Université de Lille, INSERM, Institut Pasteur de Lille, CHU Lille, UMR1167 – RID-AGE, F-59000, Lille, France.
Background and aims: Genetic association studies (GWAS) have established that the tumor suppressor locus CDKN2A (cyclin dependent kinase inhibitor 2a) locus is associated with T2D risk but the underlying mechanisms remain elusive. CDKN2A is expressed in adipose tissue and the expression is increased in obese subjects. Overall, this locus has a dual function in the regulation of the cell cycle and energy metabolism. We previously showed that Cdkn2a-deficient mice (Cdkn2a-/-) exhibit increased energy expenditure via browning of white adipose tissue (WAT) and have improved insulin sensitivity under high-fat diet (DIO). To assess whether adipose browning is cell-autonomously controlled by Cdkn2a and due to the transdifferentiation of mature adipocyte and/or the recruitment of adipocyte precursors, we analyzed the effect of Cdkn2a deficiency restricted to mature adipocytes in mice (Cdkn2aad-/-).
Materials and methods: Metabolic studies were performed under chow diet and DIO. Metabolic, hormonal, histological parameters as well as glucose homeostasis were assessed. Histological studies of adipose tissues were also performed. The adipose tissue transcriptome was analyzed by RNA-sequencing.
Results: Although metabolic parameters were comparable between wild-type and mutant animals in chow diet, metabolic, hormonal, histological parameters as well as glucose homeostasis were significantly different in Cdkn2aad-/- DIO mice compared to control littermates. Despite normal food intake and body fat pad weights, Cdkn2aad-/- DIO mice displayed improved metabolic profiles with increased energy expenditure (p<0.01) and improved glucose tolerance (p<0.05) without any changes in insulin sensitivity. Mice also exhibited reduced circulating levels of leptin and resistin (p<0.05). Histological analysis revealed epididymal WAT hypertrophy and inguinal WAT hypoplasia (p<0.05) without effects on proliferation or browning. Transcriptomic analysis of epididymal WAT showed a global increase in metabolic pathways involved in energy substrate utilization, especially glucose metabolism. In particular, a decrease in p70S6K signaling pathway (-log(p-value) 5.540), which is known to reduce insulin signaling, suggests a better use of glucose in Cdkn2a-deficient adipocytes.
Conclusion: Our results suggest that the deletion of Cdkn2a in fully differentiated adipocytes is not sufficient to induce WAT browning. However, the Cdkn2a locus does play a role in energy metabolism regulation in mature adipocytes, and might contribute to alleviate the deleterious effects of obesity on insulin resistance and glucose homeostasis.
Supported by: SFD, ANR
Disclosure: Y. Kahoul: Grants; Société Francophone du Diabète, Agence Nationale pour la Recherche (ANR-17-CE14-0034; ANR-10-LABX-46).
534
Nbw4 locus on mouse chromosome 4 harbors candidate genes which protect from fat accumulation and obesity
J. Khuong1,2, D. Altenhofen1,2, T. Kuhn1,2, S. Görigk1,2, S. Lebek1,2, B. Knebel1, A. Schürmann3,2, A. Chadt1,2, H. Al-Hasani1,2;
1Institute of clinical biochemistry and pathobiochemistry, German Diabetes Center (DDZ), Düsseldorf, 2German Center for Diabetes Research (DZD), München-Neuherberg, 3Experimental Diabetology, German Institute of Human Nutrition Potsdam-Rehbrücke, Nuthetal, Germany.
Background and aims: Obesity represents a major risk factor for the development of insulin resistance and type 2 diabetes (T2D). The polygenic factors promoting obesity and T2D remain to be identified. Previously, we conducted linkage analyses in a backcross population generated with T2D- prone New Zealand Obese (NZO) and T2D-resistant C3HeB/FeJ mice. We detected a quantitative trait locus (QTL) on chromosome 4 (Nbw4, NZO body weight on chromosome 4, 10-30 cM, LOD: 8) that conferred protection from high-fat diet (HFD)-induced obesity. The aim of the current study is to identify the underlying genetic variants and to assess their molecular functions in energy metabolism.
Materials and methods: Repetitive backcrossing steps were performed to generate a recombinant congenic mouse line (RCS) harboring the locus on Chromosome 4 from C3H on a NZO background. Genotypes were confirmed using a high-density SNP panel. RCS mice were fed a high fat-diet (45% fat/Kcal) and underwent measurements of blood glucose and body composition at different stages of life. At 13 weeks of age, mice were sacrificed and tissues were harvested for gene expression analysis. Blood samples were collected for blood glucose and plasma insulin measurements. Transcriptome analysis conducted in the parental mouse strains combined with in silico sequence analysis were used for the identification of gene variants potentially linked to the phenotype. 3T3-L1 cells were differentiated for 14 days and harvested for gene expression analysis.
Results: Homozygous carriers of the Nbw4-C3H allele (C3H/C3H) demonstrated a significantly lower body weight compared to Nbw4-NZO allele carriers (NZO/NZO), starting at 6 weeks of age (34.7 ± 0.6 g vs. 41.09 ± 2.6 g; p<0.03 n=8-19). The difference in body weight was attributed by a higher fat mass (16.6 ± 0.5 g NZO/NZO vs. 9.2 ± 0.6 g C3H/C3H; p<0.0001 n=8-19, 10 weeks of age). Both random and fasting blood glucose levels were not different among the groups, but NZO/NZO mice presented significantly higher plasma insulin levels (10.6 ± 1.6 ng/mL vs. 6.5 ± 0.6 ng/mL; p<0.01 n=6-13, 13 weeks of age) when compared to C3H/C3H animals. Gene expression profiling in white adipose tissue (WAT) showed differential expression levels of genes from the Orosomucoid (Alpha-1-Acid Glycoprotein) family (Orm1, Orm2, Orm3) between NZO and C3H mice. Haplotype analysis followed by in silico predictions of gene/protein function revealed a potentially deleterious mutation R15Q in the NZO Orm1 gene variant, predicted to impair structure and function (SIFT: 0.01) and studies in 3T3-L1 pre-adipocytes show upregulation of Orm1 during differentiation to mature adipocytes.
Conclusion: Here we identify a novel obesity locus on Chromosome 4 with large effect size on body fat. Recombinant congenic mice carrying the C3H allele of Nbw4 on a NZO background are protected from HFD-induced obesity. Gene expression studies show strain-specific expression of the secreted Orm family genes in WAT, where expression correlated with protection from obesity.
Disclosure: J. Khuong: None.
535
Identification of candidate genes for metabolic traits in an obese mouse model integrating multiomics data
D. Hesse, M. Delpero, M. Sprechert, D. Arends, G.A. Brockmann;
Breeding Biology and Molecular Genetics, Humboldt-Universität zu Berlin, Berlin, Germany.
Background and aims: The Berlin Fat Mouse Inbred line (BFMI) is a polygenic mouse model for obesity and related pathologies such as insulin resistance and fatty liver. It was selected over more than 50 generations. Subsequently, several sub-strains were generated that are genetically close related but differ in several metabolic traits (see figure). A gene on chromosome 3 (chr), Bbs7, was identified to be primarily responsible for obesity. To help unravel the complicated network contributing to the metabolic syndrome, this study aimed to identify the most likely causal genes associated with differences in traits of the metabolic syndrome in two sub-strains of the BFMI (BFMI861-S1 and BFMI861-S2) independent of the obesity locus on chr 3.
Materials and methods: An advanced intercross line (AIL) was generated from the cross BFMI861-S1 x BFMI861-S2. In generation 10, several traits were collected in 397 male mice at 25 weeks. To perform QTL-analysis, mice with the collected traits were genotyped using GigaMUGA SNP chip and KASP assays. For candidate gene identification, a prioritization tree was applied using whole-genome sequencing, transcriptomics, and proteomics data of the parental lines together with literature. In addition, pathway enrichment was performed integrating transcriptomics and proteomics.
Results: QTL mapping identified causal regions on different chromosomes for many traits (chr3:93.5-100.8Mb: adipose tissue weight and blood glucose; chr5:32-37Mb and chr11:114-122Mb: plasma cholesterol; chr15:32-37Mb and chr16:5-21Mb: body weight; chr17:9.4-25.3Mb: adipose tissue weight, liver weight, hepatic triglycerides, pancreatic insulin content, plasma insulin, and glucose). The association of the chr 17 QTL region with many traits reflects the high correlation between the investigated traits. Hierarchical clustering using both transcriptomics and proteomics data indicated gonadal adipose tissue as driver tissue for the metabolic phenotype. In particular, transcriptomics identified 5,477 differentially expressed genes in the adipose tissue between the parental lines, while only 325 were differentially expressed in the liver. The proteomics data analysis showed comparable results: 2,138 differentially expressed proteins in adipose tissue and only 140 differentially expressed in the liver. Pathway analysis in gonadal adipose tissue revealed an overrepresentation of “lysosome” (23 %, p=3.49x10-15), pointing towards an adipose tissue dysfunction in BFMI861-S1 likely caused by elevated lysosomal activity.
Conclusion: Performing QTL mapping together with a detailed prioritization approach using multiomics data, we identified several QTL regions, including strong candidate genes associated with metabolic traits in BFMI mice. Future experiments will clarify the impact of the identified candidates.
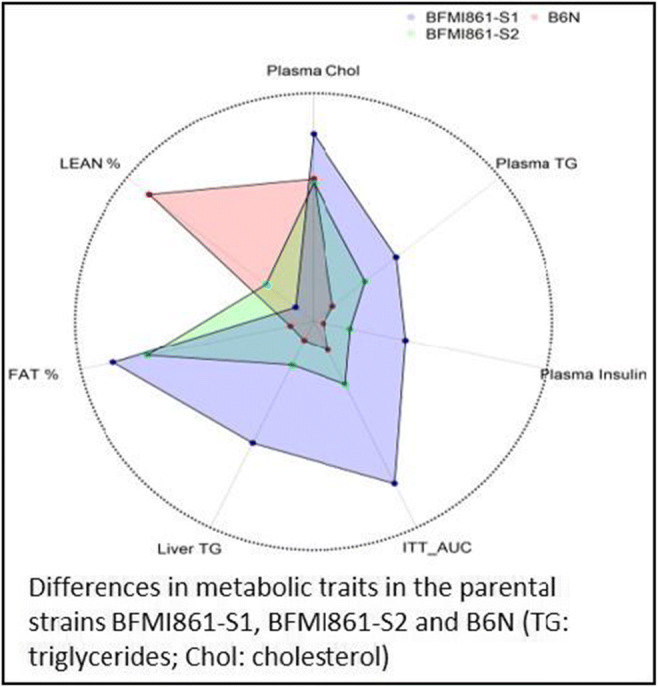
Supported by: DFG: HE8165/1-1
Disclosure: D. Hesse: None.
536
Neurometabolic changes in the hippocampus of type 2 diabetic rats: an imaging perspective towards clinical applications
B. Caramelo1, T. Monteiro-Alfredo1, J. Martins2, J. Sereno2, B. Manadas3, M. Castelo-Branco2, P. Matafome1;
1Institute of Clinical and Biomedical Research (iCBR), Institute of Physiology, Faculty of Medicine, University of Coimbra, 2Institute for Nuclear Sciences Applied to Health (ICNAS), University of Coimbra, 3Centre for Neurosciences and Cell Biology (CNC), University of Coimbra, Coimbra, Portugal.
Background and aims: Type 2 diabetes (T2DM) induces neurologic comorbidities that include neurotoxicity induced by the imbalance between excitatory glutamatergic signalling and gamma-aminobutyric acid (GABA)-induced inhibitory activity. Our objectives were to evaluate the early alterations of such mechanisms in animal models and apply imaging methods that can ensure clinical application.
Materials and methods: Experimental groups: 1) Control group of male Wistar rats only fed standard diet (SD, 10 weeks); 2) high fat diet (HFD)-induced obese rats; 3) Obese rats induced to T2DM by a low dose (35 mg/kg, i.p., 4th week) of streptozotocin (STZ). Rats were subjected to magnetic resonance spectroscopy (MRS) and their hippocampus and visual cortex (VC) were collected for biochemical experiments.
Results: Glutamine, but not glutamate, levels are elevated in the VC of the HFD+STZ group, as well as in the hippocampus of the HFD group (p = 0.0769) and HFD+STZ group. Moreover, HFD potentiates the N-acetylaspartylglutamic acid (NAAG) formation, through the conversion of alanine (r = -0.5430), in the hippocampus, being also correlated to glutathione (GSH) (r = 0.6826). Such suggests that NAAG prevents Glu release, redirecting it into the formation of GSH. However, GABA levels and GABAA receptor were not altered. No changes were observed for catalase, glyoxalase-1, heme-oxygenase and nitrotyrosine, nor the marker for cellular viability N-acetylaspartate (NAA).
Conclusion: In the hippocampus of the HFD model, Glu synthesis is not affected. Through the inhibitory effect of NAAG, Glu release into the synaptic cleft is apparently prevented in order to redirection the excitatory neurotransmitter into the formation of GSH as a compensatory mechanism. However, this effect is lost in the HFD+STZ model, showing the noxious effects of diabetes in the brain even before a compromised cellular viability. The metabolite NAAG may be a valuable early marker of neurologic comorbidities.
Supported by: EFSD/Sanofi Research Grants
Disclosure: B. Caramelo: Grants; European Foundation for the Study of Diabetes.
SO 37 Dietary and nutritional interventions
537
Ketogenic diet administration to mice after a high fat diet regimen promotes histone β-hydroxybutyralion associated to weight loss and improved metabolic fitness
S. Nasser1, T. Solé2, N. Vega1, A. Balcerczyk3, M. Strigini2, L. Pirola1;
1South Lyon Medical Faculty, INSERM Unit 1060, Pierre Bénite, France, 2INSERM U1059 SAINBIOSE, UdL/UJM Saint-Etienne, University Hospital CHU, St-tienne, France, 3Department of Molecular Biophysics, Faculty of Biology and Environmental Protection, University of Lodz, Lodz, Poland.
Background and aims: Ketogenic diets, consisting in the almost complete elimination of dietary carbohydrates, induce the body to rely on ketone bodies as primary energy source. The ketogenic diet (KD), characterized by a very limited carbohydrate content, it used as nutritional treatment for GLUT1-deficiency syndromes and pharmacologically refractory epilepsy. The increase of ketone bodies in the bloodstream at physiological doses confers multiple metabolic, anti-inflammatory and antioxidant benefits. Therefore, the ketogenic diet could be a valuable nutritional option to counter several pathological conditions, including non-alcoholic fatty liver disease, insulin resistance, obesity and osteoarthritis. The objective of this study is to investigate the beneficial impact of the ketogenic diet to promote weight loss and improve metabolic fitness.
Materials and methods: Males C57Bl6/J mice aged 6 weeks were fed a high fat diet for 10 weeks followed by a 8 weeks dietary switch to a chow diet (CD), ketogenic diet (KD) or continuation on a high fat diet (HFD). Body weight, kidney weight, body fat content and serum glucose and β-hydroxybutyrate (BHB) concentrations were evaluated. In liver and kidney, protein expression and histone post-translational modifications were assessed by western blot, and gene expression by quantitative real-time PCR.
Results: After the initial HDF feeding, administration for 8 weeks of a KD or CD induced a comparable weight loss and decrease in fat mass, with better glycemic normalization in the KD group. Histone β -hydroxybutyrylation, but not histone acetylation, was increased in the liver and kidney of mice fed the KD and the rate-limiting ketogenic enzyme HMGCS2 was upregulated - at the gene and protein level - in liver and, to an even greater extent, in kidney. KD-induced HMGCS2 overexpression may be dependent on FGF21, whose expression was dramatically increased by KD in liver. Moreover, we observe a general mild pro-inflammatory effect in the liver of the KD group, which is correlated with increased expression of the NLPR3 inflammasome subunit and an increase of pro-inflammatory IL-1β and Socs3.
Conclusion: Over a period of 8 weeks, KD is equally effective than a chow diet in inducing weight loss, but glycemic normalization was superior. Besides acting as a fuel molecule, BHB may exert its metabolic effects through modulation of the epigenome, via histone β-hydroxybutyrylation and transcriptional modulation in liver and kidney of ketogenic genes. These data show that a transitorily administered ketogenic diet may improve the metabolic status after previous exposure to a high fat diet.
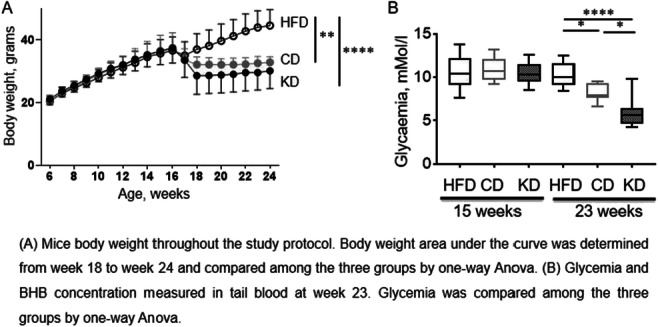
Supported by: EFSD/Boehringer Ingelheim Research Programme
Disclosure: S. Nasser: None.
538
A 36-month high-protein and high-unsaturated fatty-acid dietary intervention improves HbA1c in subjects aged 50-80y: preliminary results of the NutriAct Trial
L. Pletsch-Borba1, C. Wernicke1, N. Meyer1, T. Huong Nguyen1, A. Pohrt2, S. Hornemann3, C. Gerbracht4, A. Pfeiffer1, J. Spranger1, K. Mai1;
1Endocrinology and Metabolism, Charité Universitatsmedizin Berlin, Berlin, 2Institute of Biometry and Clinical Epidemiology, Charité Universitatsmedizin Berlin, Berlin, 3Department of clinical nutrition, German Institute of Human Nutrition Potsdam-Rehbrücke (DIfE), Nuthetal, 4Department of Clinical Nutrition, German Institute of Human Nutrition Postdam-Rehrbrücke (DIfE), Nuthetal, Germany.
Background and aims: It is still unclear whether a long-term dietary intervention not aiming weight loss with high- protein- and unsaturated fatty-acid intake improves glucose parameters and insulin sensitivity in a population aged 50-80y at risk for age-related diseases.
Materials and methods: Within the 36-month dietary intervention trial NutriAct, 502 participants were randomized into either usual care control group including dietary recommendations of the German Nutrition Society (GNS) or an intervention group, which used supplementation of rapeseed oil and specifically designed foods as well as repetitive nutritional counseling to implement a food pattern based on high intake of predominantly plant proteins, UFA and fiber. Fasting glucose, a 3h-oral glucose tolerance test (OGTT) and HbA1c were carried out both at baseline and at month 36. The insulin-sensitivity-index (ISI) based on post-prandial insulin and glucose levels (OGIS), as well as the HOMA-IR were calculated. Independent t-tests and linear regression analyses were carried out to study the effect of the intervention as well as of changes in macronutrients in the mentioned outcomes.
Results: 394 participants (204/190 control/intervention, 37% male, median age 66y, 18% with T2DM, 43% obese) with OGTT/HbA1c/fasting glucose data at baseline and at month 36 were included in the present analyses. HbA1c increased (mean +0.09%) in the control group and decreased (mean -0.02%) in the intervention group (p<0.05 for changes between groups). Changes in the HOMA-Index, OGIS-index and fasting glucose did not differ between intervention and control group in this population (p>0.05). A mild, but similar decline in BMI was seen in control and intervention group (-0.24 kg vs. -0.44 kg, p=0.11). Changes in HbA1c between the intervention groups was attenuated and failed to reach significance (p=0.09) after adjustment for age, sex and BMI changes. Changes in intake of individual macronutrients were not associated with changes in estimates of insulin sensitivity, HbA1c or fasting glucose.
Conclusion: The 36-month NutriAct intervention led to an improvement in the HbA1c in community-dwelling participants aged 50-80y, compared to the usual care group. No changes in insulin sensitivity or fasting glucose were observed. Mechanistic pathways leading to the observed improvements require further research.
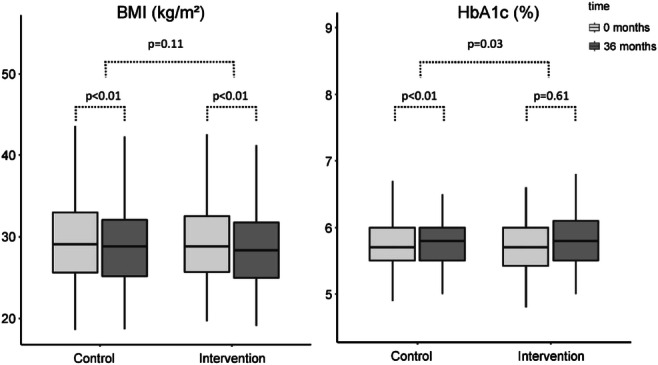
Clinical Trial Registration Number: DRKS00010049
Supported by: Bundesministerium für Bildung und Forschung (BMBF)
Disclosure: L. Pletsch-Borba: None.
539
Intermittent fasting by the 5:2 diet is feasible and improves risk factors for cardio-vascular disease equally in type 2 diabetes and overweight subjects without diabetes
A. Hellberg1, N. Rajamand Ekberg2,3, M.L. Sundqvist4,5, I. Eriksson2, A. Lindén Hirschberg1,6, S.-B. Catrina2,3, K. Brismar2;
1Deparment of Women's and Children's Health, Karolinska Institutet, 2Department of Molecular Medicine and Surgery, Karolinska Institutet, 3Centrum for Diabetes, Academic Specialist Centre, 4Department of Physiology and Pharmacology, Karolinska Institutet, 5Function Area Clinical Nutrition, Karolinska University Hospital, 6Department of Gynecology and Reproductive Medicine, Karolinska University Hospital, Stockholm, Sweden.
Background and aims: Adherence to weight reducing interventions is often poor. One method that recently has gained popularity is intermittent fasting (IF) e.g. the 5:2 diet. The aim of this study was to evaluate the feasibility and effects of the 5:2 diet on weight, metabolic and hormonal risk factors for CVD and glycaemic control in overweight subjects with and without type 2 diabetes (T2D).
Materials and methods: 104 overweight individuals were screened and 97 of them were recruited to the longitudinal study; 35 with T2D not treated with sulfonylurea (SU) or insulin and 62 weight and waist matched subjects without diabetes (controls). The participants followed the 5:2 diet (two days/week on a 500 (women) or 600 (men) kcal menu) during six months. Anthropometric data, blood pressure, metabolic risk factors for CVD including, fasting glucose, HbA1c, insulin, c-peptide, adiponectin, leptin and the IGF system were collected at baseline, 6, 12 and 26 weeks.
Results: Only four (female controls) out of 97 participants discontinued the study before the final visit at 6 months. Mixed model ANOVA was used for exploring changes in biomarkers over time. P < 0.05 was significant. Results are given as mean and CI. A significant weight loss of 4.12 (4.91 to 3.33) kg (4.9%) and 5.25 (6.28 to 4.23) kg (6.4%) was observed in controls and T2D, respectively. HbA1c decreased -3.46 (-5.22 to -1.69) mmol/mol only in the T2D group. In both controls and T2D there was a significant decrease in waist circumference -3.75 (-4.67 to -2.82) cm and -5.46 (-6.66 to -4.26) cm, respectively, total body -1.89 (-2.54 to -1.23) % and -2.52 (-3.37 to -1.67) % and trunk fat -2.18 (-2.98 to -1.39) % and -2.69 (-3.72 to -1.65) %, systolic -4.42 (-8.06 to -0.78) mmHg and -6.79 (-11.57 to -2.01) mmHg and diastolic blood pressure -3.15 (-5.42 to -0.87) mmHg and -3.70 (-6.72 to -0.68) mmHg, respectively. The waist circumference decreased significantly more in T2D compared to controls by -1.72 (-2.98 to -0.45) cm. Metabolic control, insulin sensitivity and serum lipids improved significantly. The age-adjusted IGF-I (IGF-1 SD) and IGF binding protein-1 (IGFBP-1) increased in the T2D group. Adiponectin increased and leptin decreased in both groups. There were no side effects reported.
Conclusion: The 5:2 diet was feasible to follow for 6 months with a low drop out and had an equally good effect on weight reduction and improvement on metabolic and hormonal CVD associated risk factors in overweight subjects with and without T2D. These observations support previous studies that the 5:2 diet can be recommended to patients with T2D not treated with SU or insulin.
Clinical Trial Registration Number: NCT02450097
Supported by: Erling Perssons Stiftelse, ALF
Disclosure: A. Hellberg: None.
540
Short term isocaloric ketogenic diet modulates NLRP3 inflammasome via β-hydroxybutyrate and fibroblast growth factor 21
S. Woo;
Department of Endocrinology, Severance Hospital, Seoul, Republic of Korea.
Background and aims: A ketogenic diet (KD) is known to have beneficial health effects. Various types of KD interventions have been applied to manage metabolic syndrome based on modification of diet parameters such as duration of intervention, macronutrient components, and total calories. Nevertheless, the beneficial health impact of isocaloric KD is largely unknown, especially in healthy subjects.
Materials and methods: The present study investigated the acute effects of a 3-day isocaloric KD. In this non-randomised intervention study, we recruited 15 healthy volunteers aged 24-38 years (7 men and 8 women) and placed them on an isocaloric KD restricting intake of carbohydrates but not energy (75% fat, 20% protein, 5% carbohydrate) for 3 days. Biochemical profiles and laboratory measurements were performed. Peripheral blood monocular cells were cultured, and measured cell stimulated cytokines.
Results: After short-term isocaloric KD, subjects lost body weight and serum free fatty acid levels were increased. These results accompanied elevated serum β-hydroxybutyrate (BHB) concentration and fibroblast growth factor 21 (FGF21) levels and improved insulin sensitivity. Regarding the direct effect of BHB on inflammasome activation, interleukin-1β (IL-1β) and tumor necrosis factor-α secretion in response to adenosine triphosphate or palmitate stimulation in human macrophages decreased significantly after isocaloric KD. In ex-vivo experiments with macrophages, both FGF21 and BHB synergistically reduced IL-1β secretion compared to either BHB or FGF21 alone. The inhibitory effect of FGF21 on IL-1β secretion was blunted with bafilomycin treatment, which blocked autophagy flux.
Conclusion: Isocaloric KD for 3 days is a promising approach to improve metabolic and inflammatory status.
Clinical Trial Registration Number: NCT02964572
Supported by: Severance Hospital Research fund for Clinical excellence (SHRC)
Disclosure: S. Woo: None.
541
Pre-meal low-dose whey protein microgel increases bioavailability of branched chain amino acids in people with type 2 diabetes: a randomised, PBO-controlled crossover study
O. Johansen1, B. Ahrén2, J. Corthesy3, Y. Grzywinski3, M. von Eynatten1, I.J. Neeland4;
1Nestlé Health Science, Vevey, Switzerland, 2University of Lund, Lund, Sweden, 3Analytical Science, Sociéte de Produits Nestlé S.A, Lausanne, Switzerland, 4Center for Integrated and Novel Approaches in Vascular-Metabolic Disease (CINEMA), University Hospitals Cleveland Medical Center, Cleveland, USA.
Background and aims: Whey proteins (WPs) are found in dairy products, and are rich in branched chain amino acids (BCAA) and bioactive peptides. BCAAs are key WP-ingredients with demonstrated biomodulatory effects, e.g., pancreatic beta-cell insulin-stimulatory effects and effects on gastric emptying. We recently reported that a highly-concentrated low-dose (10g, 40 Cal) pre-meal drink (125 mL), generated with novel micelle-technology (WP microgel [WPM]), ingested 15 min ahead of a pizza-meal (622 Cal), vs placebo (PBO), reduced the 2h incremental area under the glucose curve (iAUC) by 22%, increased 2h GLP-1 iAUC by 61% and, delayed 1h gastric emptying with 17%, in 26 people with type 2 diabetes. To better understand the physiologic effects of this novel WPM, we conducted an exploratory analysis of BCAA profiles following a premeal shot of WPM vs PBO.
Materials and methods: This single-intervention crossover study was conducted in 26 individuals with drug-naïve or metformin-treated type 2 diabetes who received 10g WPM (40 kcal) or PBO (0 kcal), provided as a 125 mL shot 15 min ahead of a 250 g pizza meal (622 kcal [29.0 g protein, 22.6 g fat, 72.6 g carbohydrates]). We analyzed 2h free plasma BCAA trajectories using high-performance liquid chromatography/mass spectrometry in 25 persons with available samples (mean age 62 yrs, HbA1c 58 mmol/mol /7.5%, BMI 29 kg/m2) that completed this single dose study.
Results: The pre-meal WPM drink significantly altered the BCAA trajectory vs. PBO (Figure). For leucine, ΔiAUCWPM vs PBO increased by 267%, with a shorter time to maximum (median ΔTmax [min, max]: -60 [-120,0] min, p<0.0001), and peak concentration (mean ΔCmax [SD]: 123 [49] umol/L, p<0.0001). A similar pattern was seen for isoleucine (ΔiAUC +240%, ΔTmax: -60 [-120,0], p<0.0001, ΔCmax: 68 [3], p<0.0001) and valine (ΔiAUC +194%, ΔTmax: -60 [-120, 30], p < 0.0001, ΔCmax: 63 [38], p<0.0001).
Conclusion: We conclude that 10g WPM as a pre-meal drink, induced a rapid plasma increase, and high bioavailibilty, of BCAAs in people with type 2 diabetes. The rapid BCAA availability might be a likely factor for the metabolic modulatory effects observed with WPM.
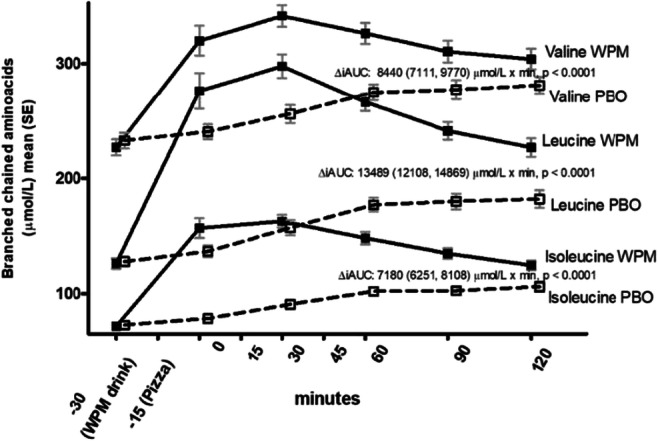
Clinical Trial Registration Number: NCT04639726
Supported by: Nestlé Health Science
Disclosure: O. Johansen: Employment/Consultancy; I am employed by Nestlé Health Science.
542
Effects of 10-h time-restricted eating on body weight and cardiometabolic health in individuals at high risk of type 2 diabetes (RESET): a randomised controlled trial
J.S. Quist1,2, H. Pedersen1,3, M.M. Jensen1,4, K.K.B. Clemmensen1,5, J. Størling1,6, N.J. Wewer Albrechtsen6,7, J.J. Holst6,8, S.S. Torekov6,8, D. Vistisen1,9, M.E. Jørgensen1,10, S. Panda11, C. Brock4,12, G. Finlayson1,2, M.B. Blond1, K. Færch1,6;
1Steno Diabetes Center Copenhagen, Herlev, Denmark, 2School of Psychology, University of Leeds, Leeds, UK, 3iMotions A/S, Frederiksberg, Denmark, 4Department of Clinical Medicine, Aalborg University, Aalborg, Denmark, 5Novo Nordisk A/S, Søborg, Denmark, 6Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark, 7Department of Clinical Biochemistry, University of Copenhagen, Copenhagen, Denmark, 8Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark, 9Department of Public Health, University of Copenhagen, Copenhagen, Denmark, 10National Institute of Public Health, University of Southern Denmark, Copenhagen, Denmark, 11Salk Institute of Biological Studies, La Jolla, USA, 12Mech-Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark.
Background and aims: Time-restricted eating (TRE) has been suggested as a strategy to lose weight. We investigated the effects of a 3-months TRE intervention vs. control on change in body weight and other clinical outcomes in individuals at high risk of type 2 diabetes.
Materials and methods: We randomized 100 men and women aged 30-70 years with (a) overweight (BMI≥25 kg/m2) with concomitant prediabetes (HbA1c 39-47 mmol/mol) or (b) obesity (BMI≥30 kg/m2) to 3 months of TRE or control (habitual living) followed by a follow-up period for 3 months. The TRE group was instructed to consume all foods and beverages (except water) within a self-selected time window of 10 hours/day within 06:00 and 20:00. The primary outcome was change in body weight after 3 months.
Results: After 3 months the baseline corrected differences between the groups were: Body weight: -0.8 kg (95% CI: -1.7; 0.2), p=0.105; body fat: -1.0 kg (95% CI: -1.6,-0.3), p=0.007; body fat percentage -0.6% (95% CI: -1.0,-0.1), p=0.013; fat-free mass: 0.1 kg (95% CI: -0.5; 0.6), p=0.839; HbA1c: -0.7 mmol/mol (95% CI: -1.3,-0.1), p=0.025; fasting plasma glucose: -0.1 mmol/L (95% CI: -0.2,0.1), p=0.205; LDL cholesterol: 0.1 (95% CI: -0.1,0.4), p=0.306. After adjustment for multiple testing, there were no statistically significant changes from baseline to neither 3 nor 6 months. Adherence to the TRE-intervention remains to be evaluated.
Conclusion: We found no clinically relevant effects of a 3-month 10-h TRE intervention on body weight reduction and other clinical outcomes in individuals at high risk of type 2 diabetes.
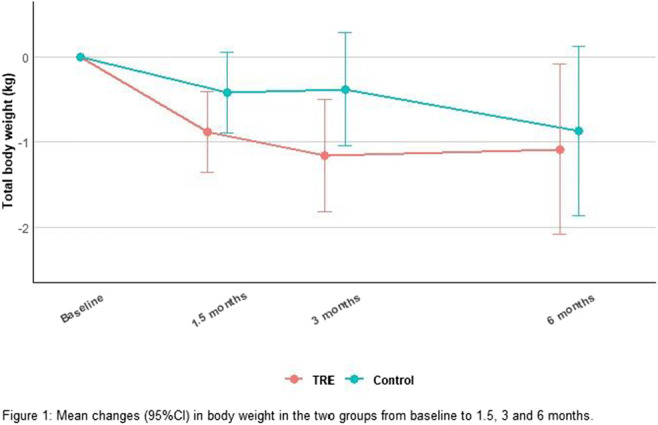
Clinical Trial Registration Number: NCT03854656
Supported by: NNF (NNF17OC0027822); Aalborg University; Innovation Fund Denmark
Disclosure: J.S. Quist: Grants; Novo Nordisk Foundation (NNF17OC0027822), Innovation Fund Denmark. Other; PhD scholarship from Aalborg University, Denmark.
543
Intermittent fasting is safe and improves metabolic parameters in people with insulin treated type 2 diabetes: a randomised controlled trial
A. Obermayer1, N.J. Tripolt1, P.N. Pferschy1,2, H. Kojzar1, F. Aziz1, A. Müller1, A. Jacan2, M. Schauer3, A. Oulhaj4,5, F. Aberer1, C. Sourij6, T.R. Pieber3,2, B. Obermayer-Pietsch7, V. Stadlbauer8,2, H. Sourij1;
1Interdisciplinary Metabolic Medicine Trials Unit, Division of Endocrinology and Diabetology, Medical University of Graz, Graz, Austria, 2CBmed - Center for Biomarker Research in Medicine, Graz, Austria, 3Division of Endocrinology and Diabetology, Medical University of Graz, Graz, Austria, 4Khalifa University, Abu Dhabi, United Arab Emirates, 5United Arab Emirates University, Al Ain, United Arab Emirates, 6Division of Cardiology, Medical University of Graz, Graz, Austria, 7Endocrinology Lab Platform, Division of Endocrinology and Diabetology, Medical University of Graz, Graz, Austria, 8Division of Gastroenterology and Hepatology, Medical University of Graz, Graz, Austria.
Background and aims: Weight reduction and improvement of glycaemic control can be challenging in people with insulin treated type 2 diabetes mellitus (T2DM). The aim of this randomized controlled trial is to determine the impact of a 12 week intermittent fasting regimen (three non-consecutive days of fasting per week) in addition to usual care compared to a control group (usual care) in people with T2DM receiving insulin therapy.
Materials and methods: We enrolled people with T2DM with a glycated haemoglobin A1c (HbA1c) of ≥53 mmol/mol (≥7.0 %) and receiving a minimum insulin dose of 0.3 IU/kg body weight per day. They were randomly assigned to a fasting group or control group (routine healthy diet). All study participants were switched to a basal-bolus insulin regimen (insulin glargine U-300 was used as basal insulin) prior to randomization and received a continuous glucose monitoring device (CGM). For fasting days the fasting group was advised to reduce basal insulin by 20% and to omit prandial insulin. The two co-primary outcomes were i.) the difference in the change of HbA1c from baseline to 12 weeks and ii.) the difference in the proportion of participants achieving a composite endpoint (body weight reduction of at least 2 %, insulin dose reduction of at least 10 % and an absolute HbA1c reduction of at least 3 mmol/mol) between the two groups.
Results: 46 participants (22 women and 24 men) aged 63 ± 7 years, with a mean diabetes duration of 21 ± 9 years, a body mass index (BMI) of 34.3 ± 4.5 kg/m², a mean HbA1c of 67 ± 11 mmol/mol (8,3 %) and a mean total daily insulin dose of 56 ± 27 IU were enrolled. Intermittent fasting significantly reduced HbA1c (-7.3 ± 12.0 mmol/mol) as compared to the control group (0.1 ± 6.1 mmol/mol) after 12 weeks (p=0.012). Body weight decreased in the fasting group (-4.8 % ± 4.6 %) but remained stable in the control group (0.2 % ± 1.2 %) (p<0.001) and total daily insulin dose on non-fasting days was significantly reduced in the fasting group (-9 ± 10 units) as opposed to the control group (4 ± 10 units) after 12 weeks (p<0.001). Eight participants (40%) in the fasting group achieved all 3 components of the composite co-primary endpoint compared to none in the control group (p<0.001). Two severe adverse events (SAE) were recorded in the fasting group and three SAEs in the control group, no severe hypoglycaemic events occurred during the trial.
Conclusion: Intermittent fasting is a safe dietary approach in people with insulin-treated type 2 diabetes using a CGM device over 3 months with the potential to improve HbA1c, accompanied by a reduction in body weight and total daily insulin dose.
Clinical Trial Registration Number: DRKS00018070
Supported by: FWF Programme Clinical Research KLI 851-B
Disclosure: A. Obermayer: Grants; FWF Programme Clinical Research KLI 851-B.
544
Vegetable juice preload ameliorates postprandial glucose concentration in healthy women: a randomised cross-over trial
S. Imai1, S. Kajiyama2, T. Miyawaki1, N. Ozasa3, S. Kajiyama4, Y. Hashimoto5, M. Fukui5;
1Kyoto Women's University, 2Kajiyama Clinic, 3Kyoto University, Kyoto, Japan, 4Japanese Red Cross Kyoto Daini Hospital, 5Kyoto Prefectural University of Medicine, Kyoto, Japan.
Background and aims: Consuming vegetables before carbohydrates was effective to suppress the postprandial glucose concentrations and insulin secretion in people with and without diabetes as we have reported. However, average amounts of vegetable intake in Japan and U.S.A. were under the target of recommendations, due to economical reason, limited cooking time and skills, and low availability of fresh products. In order to explore the benefit of drinking vegetable juice as a substitute of vegetable, we examined the effect of drinking vegetable juice 20 min before consuming carbohydrate on postprandial glucose concentrations in young healthy women.
Materials and methods: This was a randomized controlled three-treatment cross-over open-labeled trial within-participant. The participants consumed 200 g of vegetable juice, 190 g of fresh vegetable (tomato and broccoli), or 200 g of water (control) 20 min before consuming 200 g of boiled white rice for 3 separate days. The test meals of energy, carbohydrate, and dietary fiber in vegetable (energy 380 kcal, carbohydrate 83.7 g, fiber 6.4 g) and vegetable juice (energy 379 kcal, carbohydrate 83.7 g, fiber 4.8 g) were almost equal, but those in water (energy 340 kcal, carbohydrate 74.9 g, fiber 3.4 g) were less than vegetable and vegetable juice. All participants performed the capillary finger pricks and blood glucose concentrations were analyzed by self-monitoring blood glucose device. Pre- and post-meal blood glucose concentrations were measured at -20, 0, 15, 30, 45, 60, 120, and 180 min. The mean blood glucose concentration, standard deviation for blood glucose, large amplitude of blood glucose excursion (LAGE), and other glycaemic parameters were compared among 3 days.
Results: Twenty-four women [age 21.3 ± 0.6 years, HbA1c 35 mmol/mol (5.4 ± 0.2%), mean ± SD] completed the study. The incremental glucose peak at 45 min (vegetable juice 48.3 ± 4.1, vegetable 47.4 ± 3.3 vs. water 66.8 ± 4.3 mmol/L, both p < 0.01, mean ± SEM) and LAGE (vegetable juice 57.1 ± 3.1, vegetable 58.3 ± 3.6 vs. water 78.3 ± 4.3 mmol/L, both p < 0.05) in consuming vegetable juice and vegetable at 20 min before carbohydrate were all significantly lower than those of water. On the other hand, there was no significant difference in glycaemic parameters between vegetable juice and vegetable.
Conclusion: Drinking vegetable juice 20 min before carbohydrate ameliorates the postprandial blood glucose concentrations as well as vegetable preload, despite total amounts of energy and carbohydrate of vegetable juice or vegetable are higher than those of water. Vegetable juice preload is easy, practical, and cost-effective method to lower the postprandial glucose concentrations in women without type 2 diabetes.
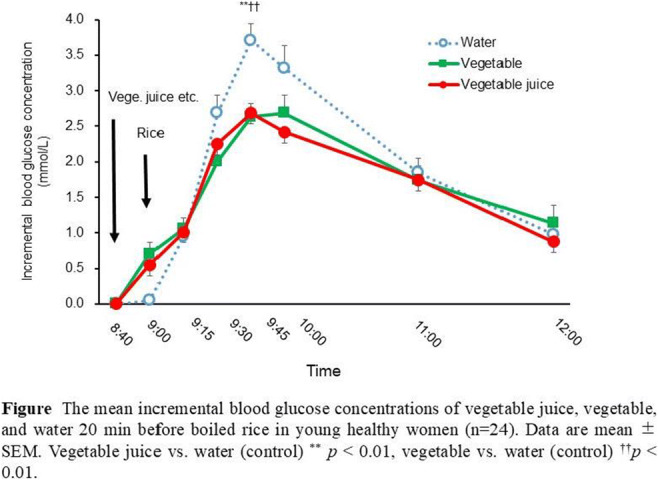
Clinical Trial Registration Number: UMIN 00003494
Supported by: KAKENHIA and Kyoto Women’s University
Disclosure: S. Imai: None.
SO 38 SGLT2 inhibitors and renal outcomes
545
Initiation of the SGLT2 inhibitor canagliflozin to prevent kidney outcome guided by HbA1c and predicted risk of kidney failure
S. Tye1, N. Jong1, S.G. Coca2, J. Sundström3, C. Arnott4,5, B. Neal5, V. Perkovic5, P. Vart1, H.J. Lambers Heerspink1;
1Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, Netherlands, 2Division of Nephrology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, USA, 3Department of Medical Sciences, Uppsala University, Uppsala, Sweden, 4Department of Cardiology, Royal Prince Alfred Hospital, Sydney, Australia, 5The George Institute for Global Health, UNSW Sydney, Sydney, Australia.
Background and aims: The use of sodium glucose co-transporter-2 (SGLT2) inhibitors reduce the risk of kidney and heart failure events independent of glycemic effects. We assessed whether SGLT2 inhibitors initiation guided by multivariable predicted risk based on clinical characteristics and novel biomarkers is more efficient to prevent clinical outcomes compared to a strategy guided by HbA1c alone.
Materials and methods: We performed a post-hoc analysis of the CANVAS trial including 3713 patients with available biomarker measurements. We compared the number of composite kidney outcomes (defined as a sustained 40% decline in eGFR, chronic dialysis, kidney transplantation, or kidney death) prevented per 5 years when canagliflozin was initiated in patients with HbA1c ≥7.5%, versus in patients defined by a multivariable predicted risk model consisting of (1) clinical characteristics or (2) clinical characteristics and novel biomarkers. The difference between models was compared by C statistics.
Results: After a median follow-up of 6.1 years, 137 kidney events were recorded. The clinical risk model included age, previous history of CV disease, systolic blood pressure, urinary albumin creatinine ratio, hemoglobin, body weight, albumin, estimated glomerular filtration rate (eGFR), and treatment variable. The novel biomarkers model included all clinical characteristics, tumor necrosis factor receptor-1, kidney injury molecule-1, matrix metallopeptidase-7, and interleukin-6. Treating all patients with HbA1c ≥7.5% (n=2809) for a median of 6.1 years, 33 kidney outcomes would be prevented. Treating 2809 patients with the clinical and clinical/novel biomarker model resulted in the prevention of 57.0 (95% CI 53.6 to 60.4) and 60.0 (95% CI 56.2 to 63.8) kidney events. The C-statistic for the HbA1c strategy to predict the composite kidney outcome was 0.54 (95% CI 0.50, 0.59). For the clinical and clinical/novel biomarker models, the C statistic was 0.80 (95% CI 0.75, 0.84) and 0.81 (95% CI 0.76, 0.86), respectively.
Conclusion: Initiation of canagliflozin based on an estimated risk-based approach prevented more kidney events than a strategy based on HbA1c alone. The incremental gain of adding novel biomarkers to the clinical risk model was marginal. This finding supports the use of clinical risk-based assessment to guide the initiation of SGLT2 inhibitors in patients with type 2 diabetes.
Clinical Trial Registration Number: NCT01032629
Supported by: EU’s Horizon 2020 under Marie Skłodowska-Curie grant agreement No 754425
Disclosure: S. Tye: None.
546
Effects of dietary sodium and protein intake on early dip of GFR levels in subjects with type 2 diabetes treated with SGLT2 inhibitors
M. Seghieri1, C. Colombi1, C. Gaudio2, C. Merciai2, A. Rosati2, C.M. Baggiore1;
1Diabetes Unit, Nuovo San Giovanni di Dio Hospital, 2Nephrology Unit, Nuovo San Giovanni di Dio Hospital, Firenze, Italy.
Background and aims: SGLT2 inhibitors (SGLT2i) are able to decrease proteinuria and to slow down kidney function decline in patients with type 2 diabetes (T2D). The effect is mainly obtained by increase of natriuresis and glucose-induced osmotic diuresis, resulting in a reduction of intraglomerular pressure. These hemodynamic changes are beneficial in long-term period but up to 28% of patients experience an acute, usually transient, estimated GFR (eGFR) reduction of more than 10%. Moreover, in some case the decrease of eGFR can be more pronounced and compromise the maintenance of therapy. High sodium and protein intake can lead to intraglomerular pressure increase and predispose to a deeper fall of eGFR. We aimed at investigating whether measured creatinine clearance (ClCr) is a more sensitive method to detect the initial dip of GFR and if dietary sodium and protein intake can influence the extent of the early change in GFR.
Materials and methods: Subjects with T2D were consecutively recruited among those referring to the inpatients of combined Nephrology and Diabetology clinic in years 2020-2021. Those eligible for treatment with SGLT2i were asked to collect 24h urinary samples for sodium and urea determination to estimate sodium and protein intake respectively before and 1, 3 and 6 months after drug initiation. Taking account that nephroprotection by these drugs is related a class-effect, a single molecule was not chosen. Creatinine Clearance variation (Δ CrCl), the primary outcome, was calculated as CrCl at 1,3,6 months minus baseline CrCl or as a percentage (Δ CrCl/baseline CrCl*100).
Results: At baseline 28 patients (M 24/ F 4; age 69±7 years; BMI 28.2±3.6 kg/m2; HbA1c 56±16 mmol/mol) had a ClCr of 83.23±25.52 ml/min/1.73 m2 (eGFR 67.32±16.07), which dropped by 19 % at month 1 (eGFR by 6%, although not significantly) and then increased to comparable baseline values at month 6. Exploring the potential correlation between changes in renal function and salt intake, ΔClCr and baseline urinary sodium were inversely related at month 1 (r = -0,61; p <0.01), at month 3 (r = -0.51; p =0.01) and month 6 (r= -0,48; p<0.05). Likewise, an inverse correlation between ΔClCr and baseline urinary urea was demonstrated at month 1 and 3 (r = -0.46; p <0.05 for both), at month 6 a similar, not significant, trend was observed (r = -0.47; p =0.054). A multivariate analysis including age, sex and BMI demonstrated that ΔClCr after one month of treatment was significantly negatively correlated with the interaction of baseline sodium and urea urinary excretion but not with the single terms (beta std=2.09, p<0.04; p=0.04 for the model). Proteinuria showed a significant reduction from baseline (p<0.05), however no significant relationship between change in proteinuria and urinary sodium or urea was observed.
Conclusion: The present study suggests that a higher dietary sodium and protein intake may amplify the extent of early dip in glomerular filtration rate, detected with measured CrCl, in diabetic patients undergoing SGLT2i treatment. We believe that creatinine clearance is a very sensitive method to detect it. Further studies are needed to confirm the results of our pilot study.
Disclosure: M. Seghieri: None.
547
Real-world safety of dapagliflozin in elderly patients with type 2 diabetes in China: post-hoc analysis of the DONATE study
L. Guo1, J. Wang2, L. Li3, L. Yuan4, S. Chen5, H. Wang6, T. Li7,8, L. Qi9, H. Yang10;
1Beijing Hospital, Beijing, 2Weifang Municipal Official Hospital, Weifang, 3Ningbo First Hospital, Ningbo, 4Zhuhai People’s Hospital, Zhuhai, 5The People’s Hospital of Liuyang, Liuyang, 6Yancheng Tinghu People’s Hospital, Yancheng, 7The Second Hospital of Nanjing, Nanjing, 8The 81st Hospital of People’s Liberation Army, Nanjing, 9Beijing Yanhua Hospital, Beijing, 10Zhejiang Rui’an People’s Hospital, Rui'an, China.
Background and aims: Dapagliflozin significantly improves glycaemic control, weight loss and blood pressure (BP) in the patients with type 2 diabetes mellitus (T2DM), but data on dapagliflozin safety in the Chinese patients with T2DM aged ≥65 years are insufficient. We investigated the real-world safety of dapagliflozin in the elderly Chinese patients with T2DM enrolled in the DONATE study.
Materials and methods: The observational DONATE study investigated the safety of dapagliflozin in the patients with T2DM from 88 hospitals in China, who received ≥1 dose of dapagliflozin between August 2017 and July 2020. In this post-hoc analysis, baseline patient characteristics and 24-week safety outcomes, including adverse event (AE)/Serious AE (SAE), and adverse events of special interest (AESI), were analysed by age subgroups (<65 and ≥65 years).
Results: Of the 2,990 patients included in the safety analysis set, 2,482 and 508 patients were aged <65 years and ≥65 years, respectively; mean (SD) age and duration of T2DM were 49.1 (9.6) vs 70.1 (4.8) years, and 7.4 (6.5) vs 13.0 (7.9) years, respectively. There was a higher proportion of male patients in the <65 vs ≥65 years subgroup (68.3% vs 53.3%). Vital signs, including body weight, waist circumference, BMI, BP, and metabolic factors, such as HbA1c and fasting plasma glucose, were similar between age groups at study enrolment. The mean (SD) eGFR was 130.0 (41.1) vs 108.0 (35.5) mL/min/1.73m2 in patients aged <65 and ≥65 years, respectively. Almost half of elderly patients (45.7%) had a history of cardiac disorders, compared with 19.2% of patients aged <65 years. Mean (SD) duration of dapagliflozin exposure was 63.7 (145.8) days and 71.9 (150.0) days in patients aged <65 and ≥65 years, respectively. Overall, 34.5% and 39.8% of patients aged <65 and ≥65 years, respectively, reported ≥1 adverse event (AE) during the 6-month follow-up. Among these, 8.9% vs 9.4% were related to treatment, 5.6% vs 9.4% were serious, and 4.1% vs 7.9% led to treatment discontinuation. The proportion of patients experiencing AESI was similar between age groups (6.0% vs 6.9%), with low rates of hypoglycaemia (1.1% vs 0.8%), urinary tract infections (UTI) (2.3% vs 2.4%), and genital tract infections (GTI) (1.4% vs 1.0%). There were no diabetic ketoacidosis (DKA) events reported in patients aged ≥65 years, while there were two (0.1%) events in patients aged <65 years. Rates of other AESI were also low in patients aged <65 and ≥65 years, respectively: volume depletion (0.2% vs 0.8%), abnormal electrolytes (0% vs 0%), polyuria (0.6% vs 1.2%), renal impairment (0.2% vs 0.6%), hepatic impairment (0.2% vs 0.2%), and haematuria (0.2% vs 0.2%).
Conclusion: Findings from this post-hoc analysis reveal that dapagliflozin safety profile in elderly Chinese patients with T2DM is consistent with that in younger patients. Our data confirm that dapagliflozin is well-tolerated in real-world clinical practice in China, regardless of age.
Clinical Trial Registration Number: NCT03156985
Supported by: This study was funded by AstraZeneca.
Disclosure: L. Guo: None.
548
To protect the kidney by SGLT2 inhibitors, individualised eGFR slope analysis is needed because of the heterogeneity in each person
K. Kashima1, H. Shimizu2, M. Yamada3;
1Kiryu Kosei General Hospital, Kiryu, 2Diabetes Center, Maebashi Hirosegawa Clinic, Maebashi, 3Department of Medicine and Molecular Science, Gunma University, Maebashi, Japan.
Background and aims: Prevention of end stage renal disease (ESRD) is one of the priorities in the diabetes management. But heterogeneity exist in each person on reno-protective effects by SGLT2 inhibitor (SGLT2i) or GLP-1receptor agonist (GLP-1RA). Moreover, eGFR declining rate and timing differ in each person. Individualized eGFR slopes should be analyzed in each person with type 2 diabetes (T2D) with chronic kidney disease (CKD) before and after adding SGLT2i.
Materials and methods: A total of 75 people (70.3±12.2 year-old) with T2D with advanced CKD stage 3-4, treated by SGLT2i (empagliflozin 65% and others) were analyzed. The duration of diabetes at the time of recruitment were as follows: stage 3a (n=29); 11.9±5.4 yrs, stage 3b (n=28); 19.9±12.1 yrs, and stage 4 (n=18); 13.8±8.5 yrs. Rapid baseline slopes were calculated from 45.7±19.9 months before using SGLT2i, and slopes after adding SGLT2i were calculated from 41.1±12.7months. Slopes were analyzed on different CKD stages or on reno-protective responsiveness in each individual. Observation period has extended from the previous study. GLP-1RA (liraglutide 93% and others) was used in 45%, 71% and 44% respectively based on individualized treatment.
Results: 1) In total, SGLT2i decreased eGFR from 46.1±14.1 to 43.6±13.5ml/min/1.73m2 (P<0.001) at first month after initiation. 2) The rate of responders (responder: defined as slope are attenuated vs baseline) in each stage were as follows: stage 3a; (22/29), stage 3b; (21/28), stage 4; (16/18). Slopes in responders (59/75) were improved as follows, -7.6±9.9 to -0.8±3.0 mL/min/1.73m2/year (P<0.001), with GLP-1RA used in 50.8%. Slopes in non-responders (16/75) were worsened as follows, -1.9±2.0 to -3.8±3.1mL/min/1.73m2/year (P<0.001), despite combination with GLP-1RA in 62.5%. Although no clear background differences could be found, responders were more rapid eGFR decliners and highly had macro-albuminuria (52.5% vs 37.5%). In total persons, averaged most rapid eGFR slopes were improved from -6.4±9.1 to -1.4±3.2mL/min/1.73m2/year (P<0.001). 3) Slopes were improved in all stages, stage 3a: -4.1±3.7 to -1.1±3.2 (P<0.001) and stage 3b; -4.9±3.7 to -1.3±3.1 (P<0.001), and stage 4; -12.4±16.3 to -2.0±3.6mL/min/1.73m2/year (P<0.01). 4) Slopes in rapid decliners (defined as most rapid declining slope was greater than -10.0mL/min/1.73m2/year) (13/75) were remarkably improved from -18.9±16.4 to -1.4±3.3mL/min/1.73m2/year (P<0.003), with liraglutide used in 85% (11/13). In rapid decliners, nephrosis highly existed in 54% at the baseline. One female person aged at 30s with nephrosis showed remarkable improvement. Baseline most rapid eGFR slope was at rate of -39.0mL/min/1.73m2/y for 19 months, despite using ARB and keeping HbA1c <7.0%. After adding liraglutide 0.9mg, slope was reduced to -3.8 mL/min/1.73m2/y for next 12 months. Then after adding empagliflozin10mg, slope became to +0.82 mL/min/1.73m2/y for next 50 months, with keeping eGFR~35 mL/min/1.73m2. In these rapid decliners, individualized eGFR slopes show heterogenous eGFR slopes. Adding GLP-1RA and SGLT2i obviously showed stepwise improvement on eGFR declining rate.
Conclusion: To overcome the heterogeneity in effectiveness by SGLT2i and GLP-1RA or different pathophysiology in each person, individualized eGFR slope analysis is needed to improve renal outcomes.
Clinical Trial Registration Number: 3-K018
Disclosure: K. Kashima: None.
549
Combination treatment impacts levels of endotrophin in the drug combinations for rewriting trajectories of renal pathologies in type 2 diabetes (DC-Ren) study
F. Genovese1, A.L. Møller1,2, S. Thöni3, F. Keller3, S. Sharifli3, D.G.K. Rasmussen1, G. Mayer3;
1Nordic Bioscience, Herlev, Denmark, 2Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark, 3Department of Internal Medicine IV (Nephrology and Hypertension), Medical University Innsbruck, Innsbruck, Austria.
Background and aims: DC-Ren focuses on diabetic kidney disease (DKD), a severe complication of type 2 diabetes mellitus (T2DM) often accompanied by cardiovascular disease (CVD). Despite therapeutic advances, new biomarkers for identifying patients at increased risk of disease progression and for predicting response to treatment are of major importance to develop the concept of precision medicine. Increased collagen type VI (COL6) formation has been associated with increased risk of renal and cardiovascular complications and mortality in patients with T2DM. For the first time, we investigate the potential of endotrophin, a pro-fibrotic molecule generated during COL6 formation, as a pharmacodynamic marker in patients with T2DM treated with different treatments.
Materials and methods: Endotrophin was measured by the PRO-C6 ELISA in plasma collected at baseline, and on average after years 1, 2, and 3 from 456 individuals with T2DM enrolled in the DC-Ren study. All individuals were on renin-angiotensin system inhibitor (RASi) treatment at baseline and during follow-up. While some patients remained on RASi treatment only, others received one of the following target drugs on top of RASi: sodium-glucose cotransporter-2 inhibitor (SGLT2i), glucagon-like peptide-1 receptor agonist (GLP-1RA), or mineralocorticoid receptor antagonist (MCRA). 256 patients had at least three follow-ups.
Results: The cohort consisted of 53% males, the age was 67±9.0 and the eGFR was 64±18 ml/min/1.73 m2 (all mean±SD). Higher baseline levels of plasma endotrophin were associated with lower eGFR and higher UACR, respectively, and this relation remained consistent over time. Interestingly, levels of plasma endotrophin were significantly lower in patients treated with SGLT2i in combination with RASi compared to RASi alone, whereas levels increased significantly with MCRA treatment on top of RASi. In patients treated with a SGLT2i on top of RASi, the increase in plasma endotrophin was less pronounced over time which was accompanied by a slower decline in eGFR. In adjusted linear models (for the group on RASi only) a significant association of current plasma endotrophin with future eGFR was found, indicating that plasma endotrophin is a prognostic risk marker for kidney function decline.
Conclusion: Levels of plasma endotrophin decreased with treatment, indicating that RASi in combination with SGLT2i affects COL6 formation and levels of endotrophin. The degree of kidney disease severity increased with increasing plasma endotrophin levels at baseline, indicating that the biomarker identifies patients with high disease activity where the potential of anti-fibrotic effect of treatment becomes more prominent. Further research is needed to understand the mechanism for the treatment’s effect on fibrosis and the association of endotrophin with cardiorenal outcome, mortality, and treatment response in the DC-Ren study.
Supported by: This work was supported by The Innovation Fund Denmark (0172-00270B)
Disclosure: F. Genovese: None.
550
Sodium-glucose link transporter-2 inhibitors in reduced renal function: cross-class comparison from the Association of British Clinical Diabetologists (ABCD) audits
T.S.J. Crabtree1, A. Bickerton2, K. Dhatariya3, A. Gallagher4, J. Elliott5, R. Raghavan6, I.W. Gallen7, R.E.J. Ryder8;
1Diabetes & Endocrinology, Royal Derby Hospital, University Hospitals of Derby and Burton NHS Trust, UK, Derby, 2Diabetes & Endocrinology, Yeovil District Hospital NHS Trust, Yeovil, 3Diabetes & Endocrinology, Norfolk and Norwich University Hospitals NHS Trust, Norwich, 4Diabetes & Endocrinology, University Hospitals of Leicester NHS Trust, Leicester, 5Diabetes & Endocrinology, Sheffield Teaching Hospitals NHS Trust, Sheffield, 6Diabetes & Endocrinology, The Royal Wolverhampton NHS Trust, Wolverhampton, 7Diabetes & Endocrinology, Royal Berkshire Hospitals NHS Trust, Reading, 8Diabetes & Endocrinology, Sandwell & West Birmingham Hospitals NHS Trust, Birmingham, UK.
Background and aims: The ABCD sodium-glucose link transporter-2 inhibitor (SGLT2i) audits launched with the aim of collecting real-world outcome data in UK practice. Previous work from the individual audits suggests that HbA1c reductions persist at lower eGFR levels but are less than in those with preserved renal function. The aim of this analysis is to assess HbA1c outcomes at reduced eGFR levels across the class and to assess for any differences between drugs.
Materials and methods: Data were extracted from the ABCD audits for inclusion; missing data was multiply imputed. Individuals were grouped by baseline renal function using eGFR (mL/min/1.7m2) cut-offs as defined in chronic disease staging as follows: group 1 (eGFR≥90); group 2 (eGFR 60-89); group 3 (eGFR <60). Analysis was performed using a multiple linear regression model to correct for change in other covariates with Bonferroni adjustments for between group comparisons.
Results: Data for 21,338 individuals were included (Empagliflozin=11,234; Dapagliflozin=7,841; Canagliflozin=2,263) with mean±SD age 60.0±10.4years; HbA1c 75.8±2.53mmol/mol; BMI 33.9±7.1kg/m2; weight 97.5±22.1kg and eGFR 81.6±13.3mL/min/1.73m2. Median duration of diabetes 8.1 years (IQR 4.2-12.3). 61.2% were male. The median duration of follow-up across the population was 1.7 years (IQR 0.9-2.8). HbA1c fell by 9.8mmol/mol (95% CI 9.5-10.1, p<0.0001) across the entire population. Including all levels of renal function, empagliflozin was associated with larger reductions in HbA1c than dapagliflozin (10.4mmol/mol [95% CI 10.0-10.7] vs. 9.0mmol/mol [95% CI 8.5-9.5]; p-value between drugs <0.0001) but no other differences between SGLT2i drugs were noted. Group 1 findings mirrored this with HbA1c reduction of 11.4mmol/mol (95% CI 10.9-11.9) with empagliflozin vs. 9.9mmol/mol (95% CI 9.3-10.5) with dapagliflozin (p <0.0001) and no difference was noted between any other drug comparisons. In groups 2 and 3 no difference in HbA1c reductions was noted between drugs. Within each drug group, and across the entire population, there was a trend towards smaller HbA1c reductions in those in group 2 and 3 but failing to reach statistical significance (p>0.3 for all).
Conclusion: In those with preserved renal function (eGFR>90) empagliflozin may have greater glycaemic efficacy than dapagliflozin. No difference in glucose outcomes between the drugs was noted at lower eGFR levels. Interestingly, in this analysis the difference in HbA1c reductions at with lower eGFR failed to reach statistical significance but a trend toward attenuated glucose lowering effects was noted.
Supported by: The ABCD audits is supported by an unrestricted grant from Boehringer Ingelheim, AstraZeneca & NAPP
Disclosure: T.S.J. Crabtree: Honorarium; NovoNordisk, Sanofi, Abbott Diabetes Care. Other; NovoNordisk, Sanofi.
551
Cardiovascular and kidney outcomes with canagliflozin according to type 2 diabetes treatment targets at baseline: data from the CANVAS Program and CREDENCE
J. Seufert1, V. Woo2, M.A. Tsoukas3, S.W. Tobe4, A. Slee5, W. Rapattoni6, F.G. Ang6, D.C. Wheeler7,8;
1University Hospital Freiburg, Freiburg, Germany, 2University of Manitoba, Winnipeg, Canada, 3McGill University Health Centre, Montreal, Canada, 4Sunnybrook Research Institute, Toronto, Canada, 5New Arch Consulting, Seattle, USA, 6Janssen, Inc., Toronto, Canada, 7The George Institute for Global Health, UNSW, Sydney, Australia, 8University College London, London, UK.
Background and aims: In type 2 diabetes (T2D), treatment targets include maintaining HbA1c ≤7.0%, low-density lipoprotein cholesterol (LDL-C) <2 mmol/L (<77 mg/dL), and blood pressure (BP) <130/80 mmHg. It is also recommended to improve urinary albumin:creatinine ratio (UACR), a marker of renal damage and cardiovascular (CV) risk. We examined effects of canagliflozin versus placebo on CV and kidney outcomes in patients with T2D and high CV risk and/or chronic kidney disease according to baseline treatment targets and risk factors.
Materials and methods: Pooled data from the CANVAS Program (N=10,142) and the CREDENCE trial (N=4401) were analyzed according to treatment target achievement and by number of targets achieved at baseline. Hazard ratios and 95% confidence intervals were estimated using Cox regression models.
Results: Of 14,432 participants at baseline, 3683 (26%) had achieved 0, 5851 (41%) had achieved 1, 3680 (25%) had achieved 2, and 1218 (8%) had achieved either 3 or 4 targets. At baseline, 8415 (58%) participants had UACR >2 mg/mmol. Canagliflozin consistently reduced risk of major adverse cardiovascular events (MACE), the composite of hospitalization for heart failure (HHF) or CV death, and the composite of end-stage kidney disease (ESKD) or doubling of serum creatinine (dSCr) versus placebo, regardless of whether baseline targets were met or if UACR was elevated (Figure). The number of uncontrolled targets at baseline did not impact the beneficial effect of canagliflozin on CV and kidney outcomes (all P interaction >0.17).
Conclusion: Overall, canagliflozin demonstrated consistent CV and renal benefits in patients with T2D, regardless of risk factor control.
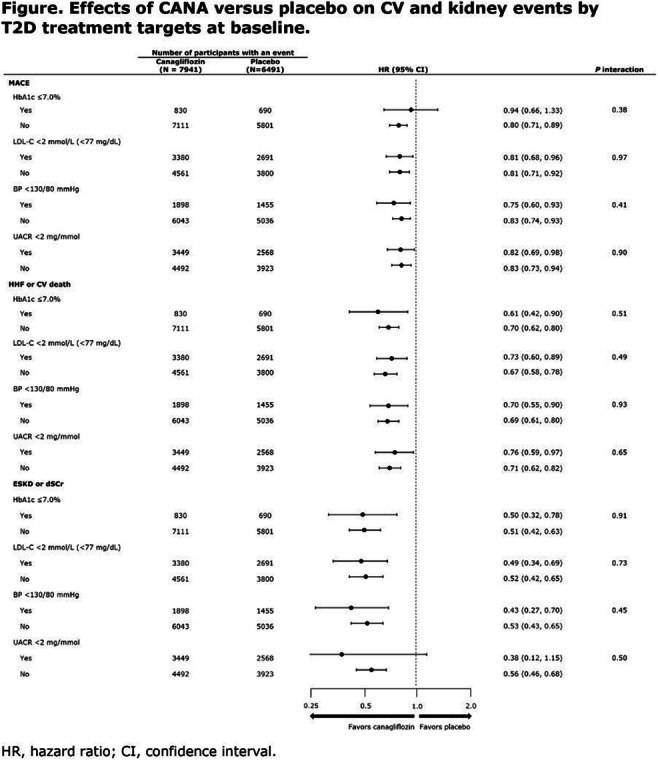
Clinical Trial Registration Number: NCT01032629, NCT01989754, NCT02065791
Supported by: Janssen, Inc.
Disclosure: J. Seufert: None.
552
Albuminuria lowering effect of dapagliflozin, eplerenone and their combination in patients with chronic kidney disease a randomised cross-over clinical trial
M. Provenzano1, H.J.L. Heerspink1, M. Puchades2, C. Garofalo3, N. Jongs1, M. Adreucci4, L. De Nicola3, J. Gorriz2;
1University Medical Centre Groningen, Groningen, Netherlands, 2University of Valencia, Valencia, Spain, 3University of Campania “Luigi Vanvitelli”, Naples, Italy, 4Magna Graecia University of Catanzaro, Catanzaro, Italy.
Background and aims: SGLT2 inhibitors and MRAs reduce urinary albumin-to-creatinine ratio (UACR) and confer kidney and cardiovascular protection in patients with chronic kidney disease (CKD). We assessed the efficacy and safety of the SGLT2 inhibitor dapagliflozin and MRA eplerenone alone and in combination in patients with CKD with and without type 2 diabetes.
Materials and methods: We conducted a randomized open-label cross-over trial in patients with urinary albumin excretion ≥100 mg/24-hour, eGFR 30-90 mL/min/1.73m2 who had been receiving maximum tolerated stable doses of ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB). Patients were assigned to 4-weeks treatment periods with dapagliflozin 10 mg/day, eplerenone 50 mg/day or their combination in random order, separated by 4-week wash-out periods. Primary outcome was the correlation in UACR changes between treatments. Secondary outcome was the percent change in 24-hour UACR from baseline.
Results: Of 57 patients screened, 46 were randomly assigned (mean eGFR 58.1 mL/min/1.73m2, median UACR 401 mg/g) of whom 32 had type 2 diabetes and 14 did not have diabetes. Mean percentage change from baseline in UACR after 4 weeks treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone treatment was -19.6% (95%CI -34.3, -1.5), -33.7% (95%CI -46.1, -18.5), and -53.0% (95%CI -61.7, -42.4; p<0.001 vs dapagliflozin; p=0.0127 vs eplerenone). The change in UACR after 4 weeks treatment with dapagliflozin-eplerenone was consistent in patients with type 2 diabetes (-54.5% [95%CI -64.6, -41.5]) and without diabetes (-49.1[95% -65.1, -28.2]). UACR change during dapagliflozin or eplerenone treatment did not correlate with UACR change during dapagliflozin-eplerenone (r=-0.13; p=0.473; r=-0.08; p=0.658 respectively). Hyperkalemia was more frequently reported with eplerenone (N=8, [17.4%]) compared to dapagliflozin (N=0, [0%]), or dapagliflozin-eplerenone (N=2, [4.3%]; Pbetween-groups=0.0033).
Conclusion: Albuminuria changes in response to dapagliflozin and eplerenone did not correlate supporting systematic rotation of these therapies to optimize treatment. Combining dapagliflozin with eplerenone resulted in a robust additive UACR lowering effect which was similar between patients with and without type 2 diabetes. A larger trial in this population is required to confirm long-term efficacy and safety of combined SGLT2 inhibitor and MRA treatment.
Clinical Trial Registration Number: NTR5602
Disclosure: M. Provenzano: None.
SO 39 Newer agents - cardiovascular outcomes
553
Interleukin-6 and cardiovascular outcome in patients with type 2 diabetes: new insights from CANVAS
A. Koshino1, M. Schechter1, T. Sen1, B.L. Neuen2, C. Arnott2, B. Neal2, V. Perkovic2, P.M. Ridker3, K.R. Tuttle4, M.K. Hansen5, H.J.L. Heerspink1,2;
1University of Groningen, Groningen, Netherlands, 2The George Institute for Global Health, Sydney, Australia, 3Brigham and Women’s Hospital, Harvard Medical School, Boston, USA, 4Providence Medical Research Center, Providence Health Care, Spokane, USA, 5Janssen Research & Development, LLC, Spring House, USA.
Background and aims: Chronic low-grade inflammation is present in both type 2 diabetes (T2D) and chronic kidney disease. Increases in the inflammatory cytokine interleukin-6 (IL-6) were associated with cardiovascular (CV) outcomes in various populations. However, data in patients with T2D are limited. We assessed in a post-hoc analysis of the CANVAS trial the association of circulating IL-6 with CV outcome overall and by baseline kidney function.
Materials and methods: Participants with T2D at high CV risk were randomly assigned to canagliflozin (CANA) or placebo. Plasma IL-6 was measured at baseline and at year 1, 3, and 6 after randomization using electrochemiluminescence. We categorized eGFR at baseline into three strata: ≥90, 60-<90, <60 mL/min/1.73 m2. The CV outcome was defined as a composite of non-fatal myocardial infarction or stroke, or CV death. We used multivariable adjusted Cox proportional hazard regression to estimate the association between baseline IL-6 and the CV outcome adjusted for demographic, clinical, and laboratory characteristics. The change in IL-6 over time and the effect of CANA compared to placebo on IL-6 levels was assessed with a repeated measures mixed effect model.
Results: Of 4,330 CANVAS trial participants, 3,501 (80.9%) had an available IL-6 measurement at baseline. Among them, 1105 (31.6%), 1902 (54.3%), or 494 (14.1%) had eGFR ≥90, 60-<90, or <60 mL/min/1.73 m2, respectively. Higher IL-6 was observed in patients within the lower eGFR subgroups, with respective median (IQR) of 1.55 pg/mL (1.09, 2.22), 1.64 (1.18, 2.39), 1.85 (1.30, 2.77) (p trend<0.01). The CV outcome occurred in 548 (15.6%) individuals over 6.1 years median follow up. Each doubling of baseline IL-6 was associated with 14% (95% CI 4, 24; p<0.01) increased risk for the CV outcome in the overall population. The association between IL-6 and the CV outcome was stronger in the eGFR <60 subgroup and absent among patients with eGFR >90 mL/min/1.73 m2 (p interaction=0.02; Figure). Over 6 years, IL-6 increased by 5.8% (95% CI 3.4, 8.3) in the placebo group. CANA modestly attenuated IL-6 increases by 4.4% (95% CI 1.3, 9.9; p=0.01) compared to placebo, with no evidence that this effect varied by baseline eGFR (p interaction=0.21).
Conclusion: In patients with T2D at high CV risk, higher IL-6 was associated with increased risk for adverse CV outcome. This association was stronger in patients with reduced kidney function. The ongoing ZEUS trial tests whether directed IL-6 lowering therapy improves CV outcomes in patients with CV disease, reduced kidney function, and evidence of inflammation.
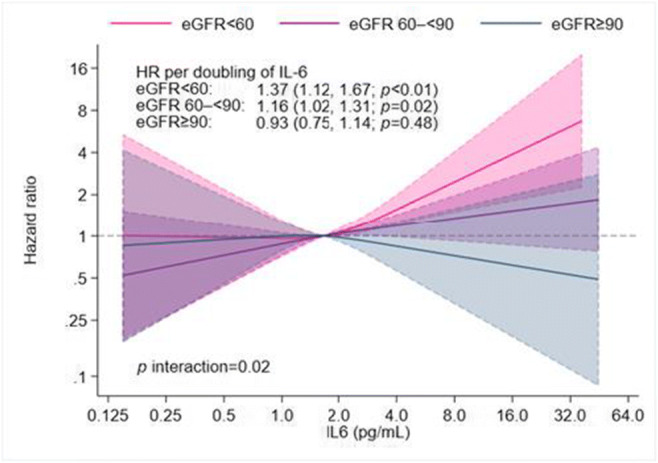
Clinical Trial Registration Number: NCT01032629
Supported by: Janssen Research & Development, LLC
Disclosure: A. Koshino: None.
554
Cardiovascular effectiveness of SGLT-2 inhibitors and GLP-1 receptor agonists in routine care of frail people with type 2 diabetes
A. Kutz, C. Gopalakrishnan, D.H. Kim, E. Patorno;
Department of Medicine, Brigham and Women`s Hospital, Boston, USA.
Background and aims: Frailty is important in diabetes management but its impact on the cardiovascular effectiveness of sodium-glucose cotransporter 2 inhibitors (SGLT-2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RA) as used in routine care is unexplored. We therefore evaluated the cardiovascular effectiveness of SGLT-2i, GLP-1 RA, and dipeptidyl peptidase 4 inhibitors (DPP-4i) in older adults with type 2 diabetes across different strata of frailty.
Materials and methods: In this comparative effectiveness study using Medicare claims data, we identified three pairwise cohorts of older patients with type 2 diabetes who initiated a SGLT-2i, a GLP-1 RA, or a DPP-4i between 04/2013-12/2018, and stratified each cohort by three levels of frailty (non-frail, <0.15; pre-frail, 0.15-0.24; frail, ≥0.25), using a validated claims-based frailty index. Within each frailty stratum, we estimated the propensity score (PS) based on >150 baseline covariates and performed a 1:1 PS matching. The primary outcome was a composite of major adverse cardiovascular events (MACE) including acute myocardial infarction, ischemic stroke, hospitalization for heart failure, and all-cause mortality. For each PS-matched frailty stratum, we estimated hazard ratios (HR), absolute rate differences (RD) per 1`000 person-years, with their 95% confidence intervals (CI), and number needed to treat (NNT). We used the Wald test for homogeneity to assess treatment heterogeneity across frailty strata.
Results: Over a mean follow-up of approximately 9 months, the HR for MACE associated with SGLT-2i vs. DPP-4i (overall, n=91`141 PS-matched pairs; mean age 72.1 years) was 0.82 (95% CI 0.72 to 0.93) in frail, 0.71 (0.67 to 0.75) in pre-frail, and 0.78 (0.71 to 0.86) in non-frail people (p for homogeneity = 0.033). The HR for MACE associated with GLP-1 RA vs. DPP-4i (n=90`988 pairs; age 71.8 years) was 0.84 (0.76 to 0.93) in frail, 0.77 (0.73 to 0.81) in pre-frail, and 0.82 (0.74 to 0.91) in non-frail people (p for homogeneity = 0.150). The HR for MACE associated with SGLT-2i vs. GLP-1 RA (n=67`067 pairs; age 71.7 years) was 0.87 (0.75 to 1.01) in frail, 0.93 (0.87 to 1.01) in pre-frail, and 0.90 (0.79 to 1.03) in non-frail people (p for homogeneity = 0.692). Compared to DPP-4i, the absolute benefit of either SGLT-2i or GLP-1 RA was largest in frail people [RD, -25.0 (-42.1 to -7.9), p for homogeneity <0.001, NNT=40; and RD, -24.9 (-39.0 to -10.7), p for homogeneity <0.001, NNT=41; respectively].
Conclusion: In this population-based study of older adults with type 2 diabetes, while, compared to DPP4i, SGLT-2i and GLP-1 RA were associated with similar relative risk reductions in MACE among people with and without frailty, their absolute benefits were largest in frail people. There was a similar risk of MACE in patients initiating SGLT-2i vs. GLP-1 RA, regardless of frailty level.
Supported by: AK was supported from the Swiss National Science Foundation and Novo Nordisk.
Disclosure: A. Kutz: Grants; Postdoc.Mobility-Grant Nr. P400PM_194479 by the Swiss National Science Foundation, Educational Grant from Novo Nordisk.
555
Precision medicine in type 2 diabetes: integrating trial and real-world evidence can provide accurate estimates of heart failure benefit when initiating SGLT2-inhibitors
K.G. Young1, A.P. McGovern1, R. Hopkins1, D. Raya1, N.A. Sattar2, R.R. Holman3, E.R. Pearson4, A.T. Hattersley1, A.G. Jones1, B.M. Shields1, J.M. Dennis1;
1University of Exeter Medical School, Exeter, 2Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, 3Diabetes Trials Unit, Radcliffe Department of Medicine, University of Oxford, Oxford, 4Division of Population Health & Genomics, University of Dundee, Dundee, UK.
Background and aims: While SGLT2-inhibitors are known to effectively reduce the relative risk of heart failure in people with type 2 diabetes, the impact on absolute risk for the majority of patients, who do not have established cardiovascular disease, is unclear. We aimed to integrate trial and real-world evidence to provide validated individualised estimates of absolute heart failure benefit associated with SGLT2-inhibitor initiation in this population.
Materials and methods: We studied 54,860 patients with type 2 diabetes and without pre-existing heart failure initiating second-line therapy after metformin in UK primary care (Clinical Practice Research Datalink, 2013-2019). The previously validated QDiabetes Heart Failure model was fitted to provide individualised estimates of 5-year heart failure risk. Meta-analysis trial estimates of the relative benefit of SGLT2-inhibitors for heart failure (hazard ratio 0.68 [95%CI 0.61-0.76]) were applied to update the model and derive estimates of the absolute risk benefit of initiating SGLT2-inhibitors for each individual patient. Estimated absolute risk benefits of SGLT2-inhibitor treatment were validated by comparing to observed heart failure outcomes in patients initiating SGLT2-inhibitors as second-line therapy (15,885 [28.9%] of the study population) versus other oral agents.
Results: In the overall study population, model predicted 5-year incidence of heart failure (2.3%) and observed incidence (2.3% [95%CI 2.1-2.4]) were similar, and the model had good predictive performance (C-statistic: 0.74 [95%CI 0.72-0.77]). SGLT2-inhibitor initiation was predicted to reduce overall heart failure incidence by 0.8%. For validation, in the study population quartile with the highest predicted benefit with SGLT2-inhibitors (predicted mean reduction in heart failure incidence 1.9%, range 0.94-12.5%), incidence of heart failure was markedly lower in those initiating SGLT2-inhibitors (3.1% [95%CI 1.9-4.3]) compared to those initiating other therapies (5.8% [95%CI 5.3-6.4]) [p<0.001]. For those in the quartile with the lowest predicted benefit with SGLT2-inhibitors (predicted mean reduction in heart failure incidence 0.18%, range 0.02-0.28%), incidence of heart failure was similar with SGLT2-inhibitors (0.6% [95%CI 0.2-1.1]) and other second-line therapies (0.6% [95%CI 0.4-0.8]) [p=0.81].
Conclusion: Integrating trial and real-world evidence allows identification of patients with a clinically relevant reduction in heart failure incidence when initiating SGLT2-inhibitors versus other second-line therapies. The approach has the potential to support individualised treatment decisions on optimal type 2 diabetes therapy.
Supported by: MRC (MR/W003988/1); BHF-ATI (SP/19/6/34)
Disclosure: K.G. Young: None.
556
GIP infusion acutely decreases blood pressure and increases heart rate in men with type 2 diabetes, concomitantly with increased pro-atrial natriuretic peptide levels
N.M. Sørum1, L.S. Gasbjerg1,2, M.M. Andersen1, S.M.N. Heimbürger1,3, L.S.L. Krogh1, J.J. Holst2,4, T. Vilsbøll1,5, A. Lund1, D. Terzic6, N.C. Bergmann1, F.K. Knop1,5;
1Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, 2Department of Biomedical Sciences, University of Copenhagen, Copenhagen, 3Zealand Pharma A/S, Søborg, 4Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, 5Steno Diabetes Center Copenhagen, Herlev, 6Department of Clinical Biochemistry, Copenhagen University Hospital, Copenhagen, Denmark.
Background and aims: While dual glucagon-like peptide 1 (GLP-1) receptor / glucose-dependent insulinotropic polypeptide (GIP) receptor agonists have shown promising results regarding glycaemic control and weight loss in people with type 2 diabetes (T2D), the independent cardiovascular effects of GIP remain unclear. In this study we examined the effect of acute GIP infusion on blood pressure (BP), heart rate (HR) and circulating pro-atrial natriuretic peptide (proANP) in overweight / obese men with metformin and long-acting GLP-1 receptor agonist-treated T2D. ProANP results were compared with results from a cohort of healthy men also receiving acute GIP infusion.
Materials and methods: Thirteen men with metformin and long-acting GLP-1 receptor agonist-treated T2D received a double-blind continuous infusion of GIP (6 pmol/kg/min) and saline (placebo), respectively, on separate experimental days. After 60 minutes of infusion, the participants ingested a standardised liquid mixed meal (300 kcal). BP, HR, and plasma levels of proANP (measured as mid-regional proANP) were measured regularly throughout the 270 minutes infusion period. Plasma from 10 healthy men undergoing 60 minutes of GIP infusion (1.5 pmol/kg/min) was analysed for proANP levels at baseline and regularly during infusion.
Results: Compared to placebo, GIP infusion decreased mean arterial pressure (MAP) (95±1.8 vs 89±1.5 mmHg p=0.004), mean systolic BP (SBP) (132±2.2 vs 125±2.9 mmHg, p=0.017) and mean diastolic BP (DBP) (77±2.0 vs 72±1.4 mmHg, p=0.003) and increased HR (67±2.2 vs 76±3.4 beats per minute, p<0.001). Plasma proANP increased following GIP infusion (baseline-subtracted AUC 262±79 vs 988±342 minutes×pmol/l, p=0.002). A similar trend was seen in the healthy cohort (316±51 vs 576±159 minutes×pmol/l, p=0.065)
Conclusion: Infusion of GIP in overweight / obese men with metformin and long-acting GLP-1 receptor agonist-treated T2D decreased MAP, SBP, and DBP and increased HR pointing to potentially additive haemodynamic effects of GIP receptor and GLP-1 receptor co-agonism. As plasma levels of proANP increased following GIP infusion, the BP reduction could be mediated by proANP.
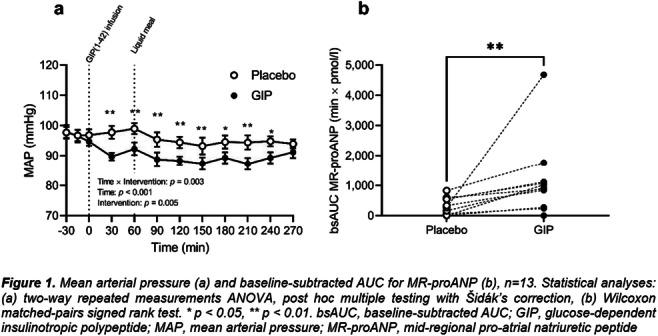
Clinical Trial Registration Number: NCT03526289
Supported by: Innovation Fund Denmark, AP Møller Foundation, Danish Medical Research Grant, Aa. Danielsens Fond
Disclosure: N.M. Sørum: None.
557
Sustainability of HbA1c control of tirzepatide vs insulin glargine in people with type 2 diabetes and increased cardiovascular risk: SURPASS-4
S.E. Kahn1, S. Del Prato2, I. Pavo3, D.R. Franco4, Z. Yang3, R.J. Wiese3, E.R. Pearson5;
1VA Puget Sound Health Care System and University of Washington, Seattle, USA, 2Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands, 3Eli Lilly and Company, Indianapolis, USA, 4CPCLIN, Clinical Research Center, Sao Paulo, Brazil, 5University of Dundee, Scotland, UK.
Background and aims: Tirzepatide (TZP) is a novel dual GIP/GLP-1 receptor agonist in development for glucose-lowering in type 2 diabetes (T2D). The open-label SURPASS-4 trial examined TZP vs insulin glargine (iGlar) in adults with T2D inadequately controlled on oral medications.
Materials and methods: Participants (N=1989) with baseline HbA1c 58-91 mmol/mol (7.5-10.5%) and BMI ≥25 kg/m2 were randomised 1:1:1:3 to once weekly TZP (5, 10, 15 mg) or iGlar (100 U/mL) titrated to <5.6 mmol/L (<100 mg/dL) fasting glucose. This post hoc analysis evaluated the sustainability of glycaemic control after the 52-week (wk) primary endpoint (last on-treatment visit post-52 wks, up to 104 wks), (median=85 wks).
Results: After achieving the 52-wk HbA1c targets of <53 mmol/mol (7%), ≤48 mmol/mol (6.5%), or <39 mmol/mol (5.7%), a participant was considered to have sustained long-term HbA1c control if the final, post-52-wk, on-treatment HbA1c measurement increased ≤2.0 mmol/mol from the 52-wk HbA1c target (Table). More participants in all 3 TZP dose groups maintained each of the 3 HbA1c targets beyond 52 wks vs iGlar (all TZP, p<0.05). Thus, even for the lowest TZP dose (5 mg), 59% of participants achieved an HbA1c ≤48 mmol/mol (6.5%) at 52 wks, with 75% of these having ≤2.0 mmol/mol increase at their last visit. In contrast, for iGlar, 29% achieved an HbA1c ≤48 mmol/mol (6.5%), with 66% of these having ≤2.0 mmol/mol increase.
Conclusion: In conclusion, TZP treatment results in improved long-term HbA1c control for a significantly greater percentage of adults with T2D compared to iGlar.
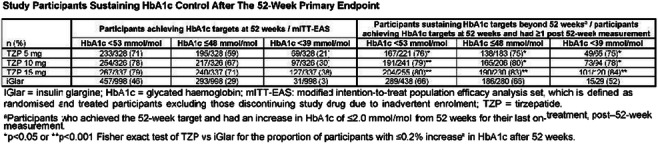
Clinical Trial Registration Number: NCT03730662
Disclosure: S.E. Kahn: Employment/Consultancy; Casma Therapeutics, Eli Lilly and Company, Intarcia, Merck, Novo Nordisk, Pfizer and Third Rock Ventures. Honorarium; Boehringer Ingelheim.
558
Systolic blood pressure reduction with tirzepatide across SURPASS programme: a mediation analysis using weight loss as a factor
O. Mosenzon1, I. Lingvay2, K. Brown3, X. Cui3, L. Fernández Landó3, H. Patel3;
1The Hadassah University Hospital-Ein Kerem, Jerusalem, Israel, 2Southwestern Medical Center, Dallas, USA, 3Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide (TZP) is a novel dual GIP/GLP-1 receptor agonist in development for the treatment of type 2 diabetes. Across the SURPASS 1-5 clinical studies, TZP 5, 10 and 15 mg demonstrated significant improvements in HbA1c (-20.77 to -28.42 mmol/mol) (-1.9 to -2.6%), body weight (-6.6 to -13.9%) and systolic blood pressure (SBP) (-2.8 to -12.6 mmHg) at primary endpoint.
Materials and methods: Patients were randomised 1:1:1:1 to TZP 5 mg, 10 mg, 15 mg or comparator, except in the SURPASS-4 study where patients were randomised 1:1:1:3. Primary endpoint was 40-weeks for (SURPASS-1, 2 and 5) and 52-weeks for (SURPASS-3 and 4). Post-hoc mediation analyses were conducted to evaluate weight loss dependent (WL-D) and independent (WL-IND) effects of TZP on SBP reductions across 5 SURPASS studies. Weight loss was considered an indirect effect (mediator), the effect not mediated by weight loss was considered a direct effect of tirzepatide on SBP. Correlation between SBP change and weight change was also conducted with pooled data of SURPASS 1 to 5.
Results: Concomitant antihypertensive medications were allowed during the SURPASS studies and 47% (SURPASS-1) to 93% (SURPASS-4) of patients were using anti-hypertensive medications at baseline. The difference in mean SBP change from baseline at 40-weeks (Total effect) between TZP and comparator group was -1.3 to -5.1 mmHg (TZP 5 mg), -1.7 to -6.5 mmHg (TZP 10 mg) and -3.1 to -11.5 mmHg (TZP 15 mg). The contribution of WL-D and WL-IND effects on these treatment differences are presented below (Figure). In SURPASS-4 study which enrolled patients with established cardiovascular disease, WL-IND effects explained 33% to 57% of difference in SBP change between TZP and insulin glargine groups, with the remainder of 67% to 43% of the effect being WL-D. In a pooled analysis of SURPASS 1-5 studies, there was a significant (p<0.001) but weak correlation (r = 0.18 to 0.22) between change in body weight and SBP.
Conclusion: In conclusion, TZP induced SBP reduction was largely mediated through weight loss, with different degrees of contributions from weight loss independent effects across the different trials.
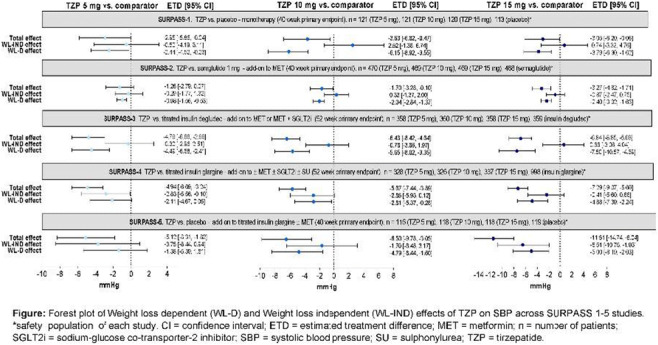
Disclosure: O. Mosenzon: Employment/Consultancy; Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca. Grants; AstraZeneca, Novo Nordisk. Lecture/other fees; AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim.
559
Dapagliflozin improves arterial stiffness, myocardial performance and endothelial glycocalyx in patients with type 1 diabetes after four months treatment
V. Lambadiari1, A. Kountouri1, K. Katogiannis2, G. Pavlidis2, E. Korakas1, L. Pliouta1, G. Kostelli2, K. Balampanis1, J. Thymis2, A. Raptis1, I. Ikonomidis2;
1Second Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Attikon University hospital, 2Second Cardiology Department, Medical School, National and Kapodistrian University of Athens, Attikon University hospital, Athens, Greece.
Background and aims: Patients with type 1 diabetes mellitus (T1DM) present signs of vascular and endothelial dysfunction earlier compared to healthy individuals. According to clinical studies, sodium-glucose-contrasporter-2 inhibitors (SGLT-2i) favorably affect cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM). However, the data regarding the efficacy of SGLT-2i on cardiovascular markers in patients with T1DM are scarce. We investigated the effects of dapagliflozin on endothelial and cardiovascular function in patients with T1DM.
Materials and methods: We recruited in total 30 patients with T1DM and pour glycemic control who were under medication with insulin and received dapagliflozin (n=15) or intensification of insulin treatment (n=15) (control group). We measured at baseline and at four months post-treatment the: a) Carotid-femoral pulse wave velocity (PWV-Complior; ALAM Medical) b) Central systolic blood pressure (cSBP) c) Perfused boundary region (PBR) of the sublingual arterial microvessels (marker of endothelial glycocalyx thickness) and d) Left ventricular global longitudinal strain (GLS) using speckle-tracking echocardiography.
Results: At baseline, patients among the two groups had similar age, sex, HbA1c and markers of endothelial and cardiovascular function (p>0.05). After four months treatment, patients who received dapagliflozin displayed an improvement in PWV (10.72±3.28vs 9.58±3.35m/s, p=0.020), in cSBP (127±17vs 119±12mmHg, p=0.041, in GLS (-19.74±1.59 vs -20.70±1.54, p=0.022) and in PBR (2.02±0.28 vs 1.74±0.2, p=0.022) compared to baseline. However, we did not observe any statistically significant change in PWV (10.19±3.28 vs 10.38±3.72m/s, p=0.606), in cSBP (124±13 vs. 121±13mmHg, p=0.364), in GLS (-19.76±1.28% vs -19.13±1.60%, p>0.113) and in PBR (2.01±0.34 vs. 2.06±0.32μm, p=0.535) in control group. Changes of PBR after four months treatment with dapagliflozin correlated with a concomitant reduction of PWV and cSBP (P<0.05).
Conclusion: Four-months treatment with dapagliflozin improves endothelial glycocalyx and cardiovascular function in patients with T1DM, independently of glycemic control.
Disclosure: V. Lambadiari: None.
560
Impact of empagliflozin on insulin use in patients with heart failure with preserved ejection fraction (HFpEF) and type 2 diabetes: sub-analysis from EMPEROR-Preserved trial
J. Butler1, J.B. Green2, J. Rosenstock3, on behalf of the EMPEROR Trial Committees and Investigators;
1Baylor Scott & White Health, Dallas, 2Duke University School of Medicine, Durham, 3Dallas Diabetes Research Center at Medical City, Dallas, USA.
Background and aims: Type 2 diabetes (T2DM) is highly prevalent among patients with heart failure (HF) and a preserved ejection fraction (HFpEF), often requiring insulin therapy. We assessed the impact of empagliflozin on the need for insulin, and on clinical outcomes according to insulin use in the EMPEROR-Preserved trial.
Materials and methods: Of the 5988 trial patients randomised to empagliflozin 10 mg or placebo, 2938 (49%) had T2DM and 1980 (33%) had preDM at baseline and were included in these analyses. We analysed the effects of empagliflozin vs placebo on time to first sustained insulin initiation (≥2 consecutive study visits) in patients with T2DM or preDM not using insulin at baseline. Further, we analysed the effect on the primary trial endpoint (first hospitalisation for HF [HHF] or cardiovascular [CV] death), first HHF, total HHFs, and CV death in patients with T2DM at baseline according to baseline subgroups of insulin use. All time to event analyses were performed using Cox regression, and total HHFs was analysed by a Joint Frailty Model.
Results: At baseline, 861 patients (434 in empagliflozin, 427 in placebo) were using insulin. Compared with those not using insulin, patients on insulin were slightly younger (69.7 vs 71.5 years), had a longer DM duration (17.6 vs 9.4 years), lower eGFR (54.6 vs 61.9 ml/min/1.73 m2), and had more co-morbidities such as coronary artery disease (47.3 vs 38.0%) and macroalbuminuria (28.2 vs 11.2%). During a median observation time of 26 months, among patients with T2DM or preDM not using insulin at baseline, empagliflozin reduced the risk of sustained initiation of insulin by 31% (HR 0.69 [95% CI 0.49, 0.98], p=0.038). Among patients with T2DM, those using insulin at baseline had higher incidence rates of the primary endpoint, total HHFs and first HHF (p-values for comparison within the placebo group: 0.0001, <0.0001, and 0.0002, respectively) that were reduced by empagliflozin but still remained higher than in the placebo arm of those not using insulin (Figure). The beneficial effects of empagliflozin vs placebo on CV outcomes did not differ by baseline insulin use (Figure).
Conclusion: In EMPEROR-Preserved, empagliflozin reduced the rate of insulin initiation by 31% in patients with HFpEF and T2DM or preDM. Insulin-users at baseline were at significantly higher risk of adverse CV outcomes; still, the effects of empagliflozin on CV outcomes were consistent in patients with T2DM irrespective of insulin treatment.
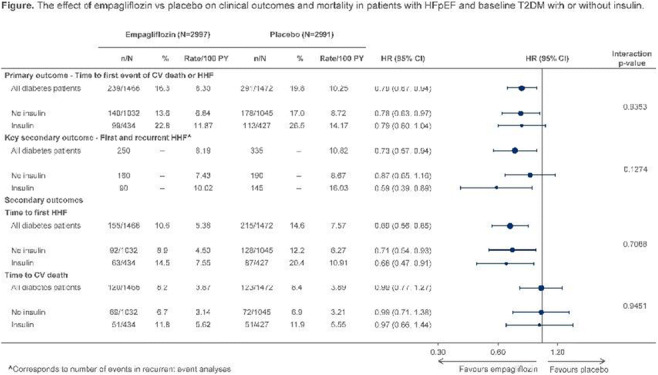
Clinical Trial Registration Number: EMPEROR-Preserved (NCT03057951)
Supported by: Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance
Disclosure: J. Butler: Employment/Consultancy; Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Limited, Vifor.
SO 40 Incretins: impact on BMI
561
Tirzepatide shows beneficial effects on body fat distribution pattern in people with type 2 diabetes
J. Linge1, B. Cariou2, I.J. Neeland3, O. Dahlqvist Leinhard1, M. Petersson1, L. Fernandez4, R. Bray4, A. Rodriguez4;
1AMRA Medical AB, Linköping, Sweden, 2Nantes Université, CHU Nantes, CNRS, Inserm, l’institut du thorax, Nantes, France, 3University Hospitals Cleveland Medical Centre; Case Western University School of Medicine, Cleveland, USA, 4Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide (TZP) is a dual GIP/GLP-1 receptor agonist under development for the treatment of type 2 diabetes (T2D). In the SURPASS-3 MRI substudy, people with T2D (metformin ± SGLT-2i, HbA1c 7-10.5%, BMI ≥25kg/m2, fatty liver index value ≥60) were treated with once-weekly TZP 5 mg (N=71), 10 mg (N=79), 15 mg (N=72) or once-daily insulin degludec (IDeg) (N=74). Overall, TZP showed a mean weight loss of -9.6 kg at week 52 and concurrent reductions of visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (aSAT) and liver fat (LF) assessed by MRI, while people treated with IDeg experienced an overall weight gain (mean +3.2 kg) with increase in VAT and aSAT, and a smaller decrease in LF than TZP. The aim of the current study is to describe overall fat distribution pattern at baseline and week 52 and relate actual changes in fat depots to the changes predicted by the difference in two virtual controls groups (VCGs) matching baseline and 52-week data.
Materials and methods: For each study participant at baseline and week 52, a VCG of ≥150 participants with the same sex, and similar body size (height, weight, BMI) were identified from the UK Biobank imaging study (N=40,174,). Expected levels of VAT, aSAT and LF and deviation from the expected levels (standardized normal z-scores (VATz, aSATz, LFz); units = standard deviation [SD]) were calculated based on individualized matched VCG. The z-score changes from baseline to week 52 were calculated to describe changes in fat distribution pattern, the magnitude of changes in VAT, aSAT and LF, and were compared to that described by the observed weight change.
Results: Baseline fat distribution pattern was similar across pooled TZP and IDeg arms. Compared to matched VCG, study participants had higher baseline VAT (mean (SD) VATz=0.42 (1.23), p<0.001) and LF (LFz=1.24 (0.92), p<0.001), but similar aSAT (aSATz=-0.13 (1.11), p=0.083). People treated with TZP had a significant decrease in VATz (-0.18 (0.58), p<0.001) and LFz (-0.54 (0.84), p<0.001) while aSATz increased (0.11 (0.50), p=0.011), indicating greater loss of VAT and LF, and smaller loss of aSAT than predicted by their observed weight loss. Comparison with the matched VCG showed people treated with TZP on average lost 0.31 L more VAT (p<0.001), 5.64 percent points more LF (p<0.001) and 0.16 L less aSAT (p=0.018) than would be predicted by observed weight loss.
Conclusion: In people with T2D and a skewed fat distribution pattern characterized by higher VAT and LF compared to matched VCG, treatment with TZP resulted in a greater reduction of VAT and LF than would be predicted by observed weight loss alone, indicating a targeted and potentially beneficial shift in fat distribution pattern.
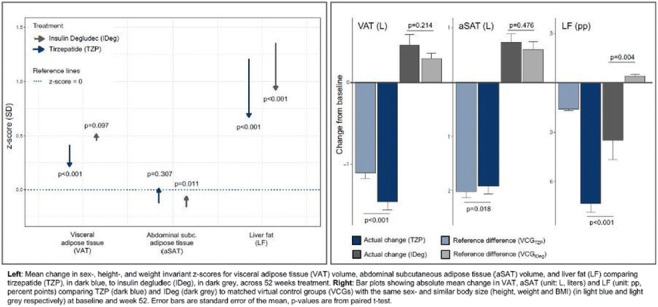
Clinical Trial Registration Number: NCT03882970 substudy
Supported by: Eli Lilly and Company
Disclosure: J. Linge: Employment/Consultancy; Employee - AMRA Medical AB.
562
Semaglutide 2.4 mg reduces the 10-year type 2 diabetes risk in people with overweight or obesity
T.W. Garvey1, T. Holst-Hansen2, P.N. Laursen2, A.R. Rinnov2, L. Wilkinson3;
1Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, USA, 2Novo Nordisk A/S, Søborg, Denmark, 3Novo Nordisk Inc, Plainsboro, USA.
Background and aims: The effect of once-weekly s.c. semaglutide 2.4 mg on the risk of developing type 2 diabetes (T2D) in people with obesity is unknown. Using data from the Semaglutide Treatment Effect in People with obesity (STEP) programme (STEP 1 and STEP 4) we assessed the risk of developing T2D over 10 years.
Materials and methods: Weight management with semaglutide 2.4 mg vs placebo plus diet and exercise was assessed in participants with overweight or obesity in STEP 1 (68 weeks) and STEP 4 (20-week run-in on semaglutide 2.4 mg, 48-week randomised withdrawal). To determine the percentage chance of an individual developing T2D in the next 10 years, the 10-year T2D risk was calculated post-hoc using Cardiometabolic Disease Staging (CMDS), which is a validated staging tool that uses Bayesian logistic regression of T2D risk factors including age, sex, race, BMI, triglycerides, HDL, blood pressure, and blood glucose, to predict an individual's percentage risk of developing T2D in the next 10 years.
Results: In STEP 1 the 10-year risk scores of developing T2D after 68 weeks of treatment decreased from 18.2% to 7.1% with semaglutide 2.4 mg, and 17.8% to 15.6% with placebo (61% vs 13% reduction [p<0.01]; Figure). In STEP 4, most of the risk score reduction with semaglutide 2.4 mg occurred during weeks 0-20, from 20.6% to 11.4%; risk score decreased further to 7.7% with continued semaglutide 2.4 mg during weeks 20-68 but increased to 15.4% with switch to placebo (32% reduction vs 41% increase [p<0.01]; Figure). In STEP 1, week 0 risk scores were higher in participants with prediabetes vs normoglycaemia (Figure), but treatment effects were comparable at week 68 (p=0.45 for interaction in both groups). In STEP 1, risk score changes mirrored weight loss, which was 17% with semaglutide 2.4 mg vs 3% with placebo. In STEP 4, weight loss was 11% for weeks 0-20 with semaglutide 2.4 mg, and a further 9% with continued semaglutide 2.4 mg vs a 6% regain with switch to placebo for weeks 20-68.
Conclusion: In summary, treatment with semaglutide 2.4 mg reduces the 10-year risk of T2D by ~60% regardless of initial glycaemic status, with sustained treatment required to maintain this benefit. These data suggest semaglutide 2.4 mg could help prevent T2D in people with obesity.
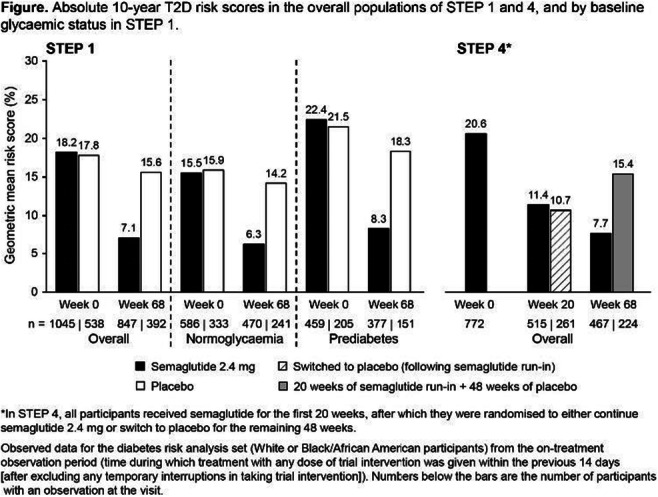
Clinical Trial Registration Number: STEP 1: NCT03548935; STEP 4: NCT03548987
Supported by: Novo Nordisk A/S
Disclosure: T.W. Garvey: Grants; Novo Nordisk. Non-financial support; Boehringer Ingelheim, Jazz Pharmaceuticals, Novo Nordisk, Pfizer.
563
Semaglutide 2.4 mg improved glucose metabolism and reverted prediabetes to normoglycaemia in adults with overweight or obesity vs liraglutide 3.0 mg (STEP 8)
D.M. Rubino1, T.W. Garvey2, B. Goldman3, U. Khalid3, J. Rosenstock4, R. Sørrig3;
1Washington Center for Weight Management and Research, Arlington, USA, 2Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, USA, 3Novo Nordisk A/S, Søborg, Denmark, 4Dallas Diabetes Research Center at Medical City, Dallas, USA.
Background and aims: Body weight change was greater with semaglutide 2.4 mg vs liraglutide 3.0 mg in STEP 8. We evaluated the effects of semaglutide 2.4 mg vs liraglutide 3.0 mg on glucose metabolism, in patients with prediabetes at baseline.
Materials and methods: The STEP 8 trial randomised 338 adults with overweight or obesity to once-weekly, subcutaneous semaglutide 2.4 mg or placebo, or daily subcutaneous liraglutide 3.0 mg or placebo, plus lifestyle intervention for 68 weeks (3:1:3:1). This exploratory analysis assessed effects on glucose metabolism and glycaemic status (normoglycaemia; prediabetes; T2D) at week 68 in participants with prediabetes at baseline. Analyses were not adjusted for multiplicity.
Results: Baseline characteristics for participants with prediabetes at baseline was balanced across treatment groups (n=43 semaglutide 2.4 mg/n=45 liraglutide 3.0 mg; mean HbA1c 5.9%/5.8%; FPG 102 mg/dL/100 mg/dL; HOMA-IR 4.0/3.3 and body weight 104kg/105 kg). Placebo data are not shown. At week 68, 89.5% of participants were normoglycaemic with semaglutide 2.4 mg vs 64.9% with liraglutide 3.0 mg (p=0.01) (Figure); in these participants, 76.5% had weight loss ≥10% with semaglutide 2.4 mg vs 33.3% with liraglutide 3.0 mg. One participant in each group developed T2D (Figure). From baseline to week 68, semaglutide 2.4 mg led to greater reductions vs liraglutide 3.0 mg in HbA1c (Estimated treatment difference [ETD]: -0.22% points; 95% confidence interval [CI]: -0.34 to -0.10; p=0.0002), FPG (ETD: -6.2 mg/dL; 95% CI: -11.5 to -1.0; p=0.02), and HOMA-IR (relative ETD: -28%; 95% CI: -44 to -6; p=0.02).
Conclusion: Both semaglutide 2.4 mg and liraglutide 3.0 mg had clinically beneficial effects on glucose metabolism and glycaemic status in participants with overweight/obesity and prediabetes, though the effect of semaglutide 2.4 mg was greater vs liraglutide 3.0 mg with approximately 25% more participants with prediabetes reverting to normoglycaemia.
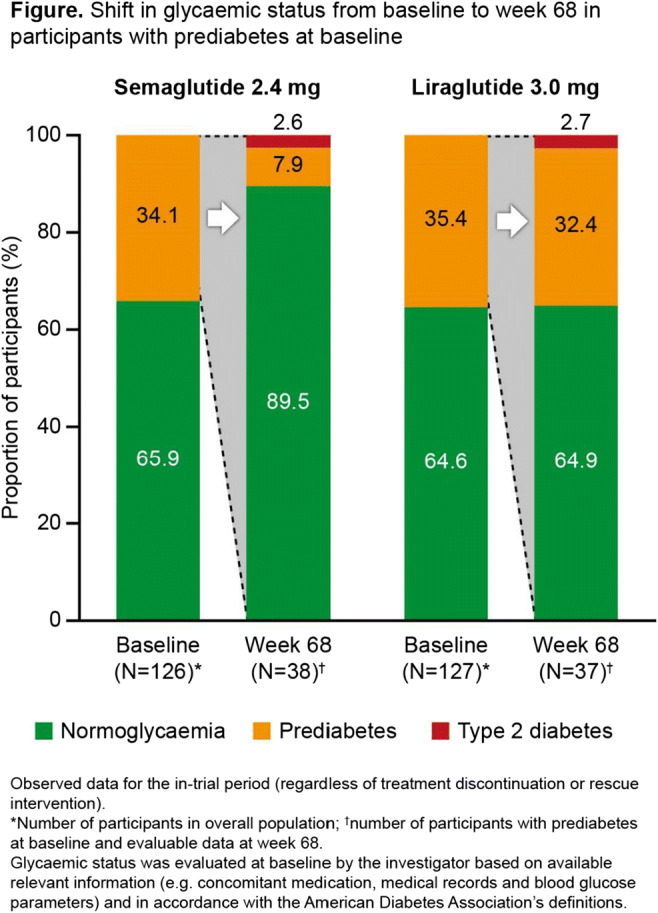
Clinical Trial Registration Number: NCT04074161
Supported by: Novo Nordisk A/S
Disclosure: D.M. Rubino: Employment/Consultancy; Novo Nordisk. Grants; Novo Nordisk, Boehringer Ingelheim, Epitomee. Honorarium; Novo Nordisk, Endocrine Society, Medscape, PeerView Institute. Lecture/other fees; Novo Nordisk. Other; Novo Nordisk, Boehringer Ingelheim, Epitomee.
564
Tirzepatide reduces appetite, energy intake and fat mass in people with type 2 diabetes
T. Coskun1, T. Heise2, J. DeVries3, S. Urva1, J. Li1, E.J. Pratt1, M.K. Thomas1, K.J. Mather1, J. Dunn1, A. Haupt1, Z. Milicevic1;
1Eli Lilly and Company, Indianapolis, USA, 2Profil, Neuss, Germany, 3Faculty of Medicine, Academic Medical Center, Amsterdam, Netherlands.
Background and aims: Tirzepatide (TZP), a dual GIP and GLP-1 receptor agonist (RA) has shown superior improvement in glycaemic control and body weight, than selective GLP-1 RA. Rodent models indicate that GIP RA augments GLP-1 RA induced food intake suppression and causes body weight loss, mainly by reducing fat mass.
Materials and methods: This randomised, double-blind, parallel study compared the effects of TZP 15 mg QW (N=45), semaglutide (SEMA) 1 mg QW (N=44) and placebo (PBO) (N=28) on energy intake (assessed by an ad libitum lunch), appetite (visual analog scale [VAS] ratings of hunger, satiety, prospective food consumption [PFC], and fullness) and body composition (air displacement plethysmography) at baseline and at 28 weeks of treatment.
Results: At 28 weeks, reductions in body weight from baseline were observed with TZP 15 mg (-11.2 kg) and SEMA 1 mg (-6.9 kg), and significantly differed between treatment groups (-4.3 kg [95% confidence interval [CI]: -6.8, -1.9]; p<0.001). Reductions in fat mass from baseline were observed with TZP 15 mg (-9.7 kg) and SEMA 1 mg (-5.9 kg), and significantly differed between treatment groups (-3.8 kg [95% CI: -6.2, -1.4]; p=0.002). Energy intake reductions from baseline observed with TZP 15 mg (-348.4 kcal) and SEMA 1 mg (-284.1 kcal) did not differ between treatment groups (-64.3 kcal [95% CI: -160.3, 31.7]; p=0.187). TZP reduced overall appetite by increasing satiety with decreased PFC (all p<0.05). Appetite ratings did not differ between TZP and SEMA.
Conclusion: TZP achieved greater weight loss than SEMA 1 mg, a selective GLP-1 RA, mostly driven by fat mass loss. Significant and clinically meaningful reductions in fasting overall appetite and energy intake were observed with both TZP and selective GLP-1 RA.. As appetite and energy intake reduction were not significantly different between treatments, additional mechanisms might contribute to the superior weight loss with TZP. Ongoing studies will further elucidate the mechanism of weight loss with TZP.
Clinical Trial Registration Number: NCT03951753
Disclosure: T. Coskun: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
565
Changes in abdominal fat and clinical/analytical parameters in tirzepatide- or insulin degludec-treated patients with type 2 diabetes (SURPASS-3 MRI)
A. Gastaldelli1, K. Cusi2, L. Fernández Landó3, R. Bray3, A. Torcello-Gómez3, B. Brouwers3, Á. Rodríguez3;
1Institute of Clinical Physiology, CNR, Pisa, Italy, 2Division of Endocrinology, Diabetes and Metabolism, The University of Florida, Gainesville, USA, 3Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide (TZP), a novel dual glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonist, significantly reduced liver fat content and volumes of visceral and abdominal subcutaneous adipose tissue (VAT and ASAT) vs insulin degludec (IDeg) in a subpopulation of patients with type 2 diabetes (T2D) in the SURPASS-3 trial. Changes in several biomarkers associated with adipose tissue metabolism and clinical parameters and correlations with changes in abdominal fat were assessed herein.
Materials and methods: This substudy of the phase 3 trial included insulin-naïve patients with T2D inadequately controlled on metformin with/without sodium-glucose co-transporter-2 inhibitors (SGLT-2i) and fatty liver index ≥60 at baseline. Patients had an MRI scan performed prior to randomisation (1:1:1:1) to once-weekly TZP (5, 10, 15 mg) or once-daily IDeg. VAT and ASAT volumes were assessed with MRI techniques. TZP doses were compared vs IDeg at Week 52 for VAT and ASAT volumes, weight, HbA1c, lipids, and biomarkers of adipose tissue dysfunction. Correlation analyses among different parameters used pooled data from all TZP arms.
Results: A total of 296 patients had evaluable MRI data during the study (mean baseline age, 56.2 years; T2D duration, 8.3 years; HbA1c, 67 mmol/mol (8.2%); weight, 94.4 kg; BMI, 33.5 kg/m2; 30% on SGLT-2i). All TZP doses reduced VAT and ASAT volumes from baseline at Week 52, as well as weight, while IDeg increased these (Table). All TZP doses significantly (p<0.001) reduced HbA1c (-22.0 to -25.4 mmol/mol [-2.01% to -2.33%]) vs IDeg (-13.2 mmol/mol [-1.21%]). At Week 52, TZP 10 and 15 mg significantly decreased triglycerides and increased HDL cholesterol vs IDeg (Table). All TZP doses increased adiponectin and decreased leptin levels vs IDeg and TZP 10 and 15 mg significantly (p<0.05) decreased Adipo-IR from baseline (Table). There were significant correlations (p<0.05) between changes in VAT or ASAT, respectively, and changes in weight (ρ=0.76/0.85), HbA1c (ρ=0.29/0.31), HDL cholesterol (ρ=-0.21/-0.25), and adiponectin (ρ=-0.38/-0.28) with TZP. Changes in ASAT were also significantly correlated (p<0.05) with changes in leptin (ρ=0.40), insulin (ρ=0.30), and HOMA-IR (ρ=0.22) with TZP. Changes in VAT were also significantly correlated (p<0.05) with changes in triglycerides (ρ=0.18) with TZP.
Conclusion: In this subpopulation of the SURPASS-3 study, TZP demonstrated significant reductions in VAT and ASAT volumes vs IDeg, which were correlated with improvements in several biomarkers of adipose tissue dysfunction, weight, and HbA1c.
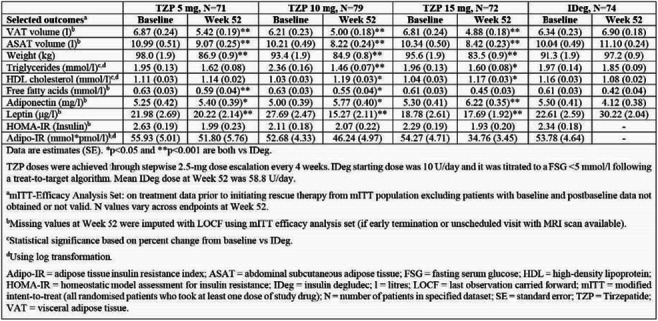
Clinical Trial Registration Number: NCT03882970
Supported by: Eli Lilly and Company
Disclosure: A. Gastaldelli: Employment/Consultancy; Consultant for Inventiva. Honorarium; Eli Lilly and Company, Gilead Sciences, Novo Nordisk and Pfizer. Other; Boehringer Ingelheim and Novo Nordisk.
566
Tirzepatide induces weight loss in patients with type 2 diabetes regardless of baseline BMI: a post hoc analysis of SURPASS-1 through SURPASS-5 studies
J.P.H. Wilding1, A.Y.M. Kwan2, J.M. Maldonado2, H. Wang2, N. Rasouli3;
1University Hospital Aintree, Liverpool, UK, 2Eli Lilly and Company, Indianapolis, USA, 3University of Colorado School of Medicine, Liverpool, USA.
Background and aims: Tirzepatide (TZP) is a novel once-weekly dual GIP and GLP-1 receptor agonist evaluated in patients with type 2 diabetes (T2D). Five Phase 3 SURPASS trials have demonstrated safety and shown robust efficacy of TZP in improvement of glycaemic control and body weight (BW) in adults with T2D. To understand whether the weight-lowering effects of TZP are dependent on baseline BMI, we conducted subgroup analyses of SURPASS-1 through -5.
Materials and methods: BW change in the BMI subgroups (Subgroup 1 [<27 or ≥27 kg/m2] and Subgroup 2 [<30; ≥30 to <35; ≥35 kg/m2]) at primary endpoint was assessed in patients while on treatment without rescue medication (efficacy estimand) in the modified intention-to-treat (mITT) population, defined as all randomised patients who received at least one dose of study drug.
Results: All TZP doses (5, 10, 15 mg) lowered BW in patients with T2D irrespective of baseline BMI in both subgroups (p<0.001). The weight reductions were generally dose-dependent and absolute weight change was generally greater in higher BMI categories. The changes in weight for Subgroup 1 are shown in Figure 1 (SURPASS-1 [S-1] vs placebo; SURPASS-2 [S-2] vs semaglutide 1 mg, add-on to metformin; SURPASS-3 [S-3] vs insulin degludec, add-on to metformin ± sodium-glucose co-transporter 2 inhibitor [SGLT2i]; SURPASS-4 [S-4] vs insulin glargine, add-on to metformin ± SGLT2i ± sulfonylurea; and SURPASS-5 [S-5] vs placebo, add-on to insulin glargine ±metformin). In Subgroup 2, the changes in weight for participants with baseline BMI <30 kg/m2 (S-1 through S-5) ranged from-5.4 kg to -5.9 kg (TZP 5 mg), -7.0 kg to -8.9 kg (TZP 10 mg), -7.5 kg to -11.2kg (TZP 15 mg), and -5.2 kg to +3.0 kg (comparators); with baseline BMI 30 to<35 kg/m2 (S-1 through S-5) ranged from -6.6 kg to -8.0kg (TZP 5 mg), -8.0 to -9.8 kg (TZP 10 mg), -8.2 to -12.8 kg (TZP 15 mg), and-6.1 kg to +2.5 kg (comparators); with baseline BMI ≥35 kg/m2 (S-1 through S-5) ranged from -6.4 kg to -10.2 kg (TZP 5 mg), -7.8 kg to -12.9 kg (TZP 10 mg), -10.8 kg to -15.5 kg (TZP 15 mg), and -6.9 kg to +2.5 kg(comparators).
Conclusion: TZP-treated patients with T2D experienced weight loss across a spectrum of mean baseline BMI values in SURPASS-1 through -5 studies. The most frequent adverse events were gastrointestinal -related events that were mild to moderate in severity and occurred during the dose-escalation period.
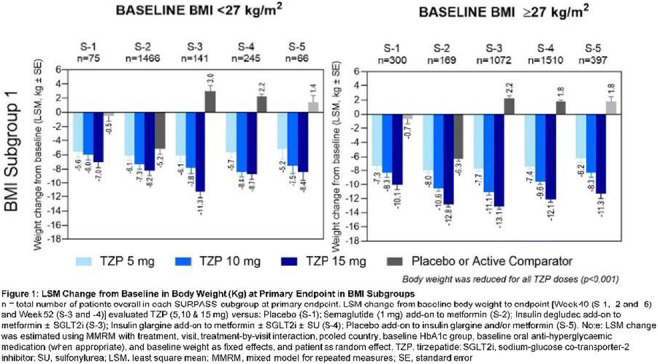
Disclosure: J.P.H. Wilding: Employment/Consultancy; Alnylam, AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Lilly, Napp, Novo Nordisk, Mundipharma, Pfizer, Rhythm Pharmaceuticals, Saniona and Ysopia. Grants; AstraZeneca and Novo Nordisk. Honorarium; AstraZeneca, Boehringer Ingelheim, Napp, Novo Nordisk, Mundipharma, Sanofi and Takeda. Lecture/other fees; AstraZeneca, Boehringer Ingelheim, Napp, Novo Nordisk, Mundipharma, Sanofi and Takeda.
567
Effect of once-weekly semaglutide 2 mg vs 1 mg on HbA1c and body weight by baseline demographic subgroups: SUSTAIN FORTE post hoc analysis
J.P. Frias1, L. Bardtrum2, Y. Hansen2, I. Lingvay3, S. Macura2, N. Tentolouris4, J.B. Buse5;
1Velocity Clinical Research, Los Angeles, USA, 2Novo Nordisk A/S, Søborg, Denmark, 3University of Texas Southwestern Medical Center, Dallas, USA, 4National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece, 5University of North Carolina School of Medicine, Chapel Hill, USA.
Background and aims: Once-weekly (OW) s.c semaglutide 1 mg is an effective treatment option for type 2 diabetes (T2D). However, in the SUSTAIN programme, 20-30% of patients did not reach a target HbA1c <7%. A higher dose of OW s.c. semaglutide was therefore explored in the SUSTAIN FORTE trial, in which superior HbA1c lowering and additional reduction in body weight (BW) were demonstrated with the 2 mg vs the 1 mg dose of OW semaglutide: estimated treatment difference (trial product estimand) [95% CI] for HbA1c -0.23%-points [-0.36;-0.11] and BW -0.93 kg [-1.68;-0.18]. The safety and tolerability profile of the two doses were comparable. Previous analysis showed consistent efficacy across dichotomous subgroups by HbA1c <9% or ≥9% and BMI <35 or ≥35 kg/m2. This post hoc analysis evaluated the effect of semaglutide 2 mg vs 1 mg on glycaemic control and BW across a broader range of baseline demographic subgroups.
Materials and methods: SUSTAIN FORTE was a 40-week, randomised, double-blind, phase 3b trial. Participants with inadequately controlled T2D (mean baseline HbA1c 8.9% and BW 99.3 kg) on a stable dose of metformin alone or in combination with a sulphonylurea were randomised to receive semaglutide 2 mg (n=480) or 1 mg (n=481). HbA1c and BW changes from baseline to end-of-treatment were analysed in the full analysis set in the on-treatment without rescue medication period by ANCOVA (adjusting for either baseline HbA1c or BW) across the following baseline demographic subgroups: age, sex, diabetes duration, HbA1c, BMI, kidney function, background antihyperglycaemic medication and region. No adjustment for multiplicity was made.
Results: This post hoc analysis indicates that greater HbA1c reductions were provided by semaglutide 2 mg vs 1 mg generally across subgroups (Figure). Similar results were observed for BW (data not shown), an exception being the small subgroup of participants with moderately impaired kidney function (n=14), where semaglutide 2 mg did not provide greater BW reduction; thought to be a chance finding driven by a pronounced weight change in the semaglutide 1 mg arm. Except for BW reduction by kidney function subgroup, interaction test p-values indicated no significantly different treatment effect from one level of a subgroup to another.
Conclusion: OW semaglutide 2 mg improved glycaemic control and reduced BW to a greater extent than OW semaglutide 1 mg across baseline demographic subgroups. The results of this post hoc analysis reinforce semaglutide 2 mg as a valuable treatment option for a broad range of patients with T2D, and as a potential treatment intensification option for patients treated with OW semaglutide 1 mg.
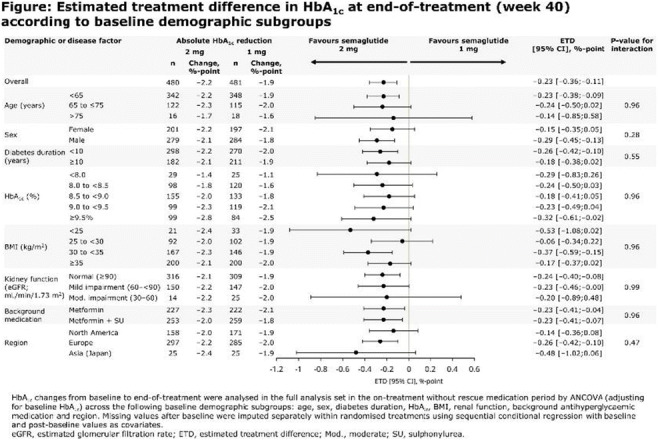
Clinical Trial Registration Number: NCT03989232
Supported by: Novo Nordisk A/S
Disclosure: J.P. Frias: Employment/Consultancy; Akero, Altimmune, Axcella Health, Becton Dickenson, Boehringer Ingelheim, Carmot Therapeutics, Echosens, 89bio, Eli Lilly, Gilead, Intercept, Metacrine, Merck, Novo Nordisk, Pfizer, Sanofi. Grants; Akero, AstraZeneca, Boehringer Ingelheim, BMS, 89bio, Eli Lilly, Intercept, IONIS, Janssen, Madrigal, Metacrine, Merck, NorthSea Therapeutics, Novartis, Novo Nordisk, Oramed, Pfizer, Poxel, Sanofi. Lecture/other fees; Eli Lilly, Merck, Sanofi.
568
Tirzepatide-induced weight loss in type 2 diabetes is independent of nausea, vomiting, or diarrhoea
H. Patel1, K. Khunti2, H.W. Rodbard3, H.S. Bajaj4,5, R. Bray1, Z. Kindracki1, Á. Rodríguez1;
1Eli Lilly and Company, Indianapolis, USA, 2Diabetes Research Centre, University of Leicester, Leicester, UK, 3Endocrine and Metabolic Consultants, Rockville, USA, 4LMC Diabetes and Endocrinology, Brampton, Canada, 5Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, Toronto, Canada.
Background and aims: Tirzepatide (TZP), a novel dual glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonist, demonstrated superior reduction in HbA1c and body weight vs comparators in participants with type 2 diabetes (T2D) across the SURPASS 1-5 randomised clinical trials. The most common adverse events (AEs) in TZP-treated participants were gastrointestinal (GI) in nature. This post hoc analysis evaluated the impact of nausea, vomiting, or diarrhoea AEs on weight loss with TZP across the SURPASS 1-5 trials.
Materials and methods: The phase 3 SURPASS 1-5 trials included participants with T2D (drug-naïve or on background antihyperglycaemic medications). Trial participants were randomised (1:1:1:1, except in SURPASS-4 with 1:1:1:3 randomisation) to once-weekly TZP (5, 10, or 15 mg) or comparator (placebo, semaglutide 1 mg once weekly, or titrated daily basal insulins). Participants within trials were subdivided by self-reporting (yes/no) of any nausea, vomiting, or diarrhoea. Change from baseline in body weight at the primary endpoint was assessed within each trial and subgroup. Mediation analyses were conducted to evaluate the contribution of direct and indirect (mediated by nausea, vomiting, or diarrhoea) effects of TZP on weight change vs comparators.
Results: Across the SURPASS 1-5 trials (SURPASS 1, N=475; SURPASS 2, N=1876; SURPASS 3, N=1435; SURPASS 4, N=1989; SURPASS 5, N=471), 19%-36% of TZP-treated participants reported nausea, vomiting, or diarrhoea vs 5%-26% of those in the comparator arms. Mean weight loss at the primary endpoint with TZP was similar in participants reporting nausea, vomiting, or diarrhoea (-6.16 to -14.86 kg) vs those who did not report these GI AEs (-6.17 to -13.29 kg). Mediation analyses (Figure) suggest that the estimated treatment difference (ETD) of change from baseline in weight (95% confidence interval [CI]) favoured all doses of TZP (total and direct effects). The contribution of nausea, vomiting, or diarrhoea (indirect effects) to TZP-induced weight loss was minimal (Figure), if any, representing ≤5% of the total effect across all trials. In some instances, TZP vs comparators led to slightly greater weight loss in participants without GI AEs compared with those reporting GI AEs, leading to positive ETD values for indirect effects (Figure).
Conclusion: Superior weight loss with TZP appears to be independent of reported nausea, vomiting, or diarrhoea AEs across the SURPASS 1-5 clinical trials.
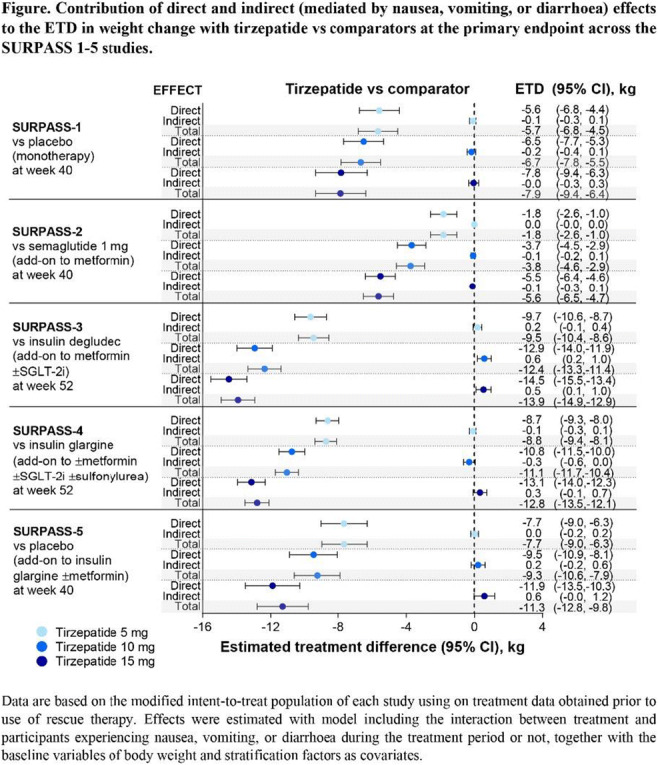
Clinical Trial Registration Number: NCT03954834; NCT03987919; NCT03882970; NCT03730662; NCT04039503
Supported by: Eli Lilly and Company
Disclosure: H. Patel: Employment/Consultancy; Employee of Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
SO 41 Incretins: basic science
569
Comprehensive characterisation of the human gain-of-function GLP-1r variant Ala316Thr in pancreatic beta cells
L. El Eid1, R.-M. Rujan2, G. Deganutti2, B. Jones3, C. Reynolds2, A. Tomas1;
1Section of Cell Biology and Functional Genomics, Imperial College London, 2Centre for Sport, Exercise and Life Sciences, Faculty of Health and Life Sciences, Coventry University, 3Section of Endocrinology and Investigative Medicine, Imperial College London, London, UK.
Background and aims: The glucagon-like peptide-1 (GLP-1) is an important pharmacological target for type 2 diabetes (T2D) due to its insulinotropic effects on pancreatic beta cells and weight lowering actions. GLP-1R action, including signalling and trafficking is modulated by factors such as ligand bias and single nucleotide polymorphisms (SNPs) that alter receptor structure. Here we comprehensively characterize the effect of the GLP-1R missense mutation Ala316Thr (A316T), associated with reduced T2D risk, in pancreatic beta cells by performing atomic level conformational modelling, signalling, and trafficking analyses of the mutant compared to wild type receptor.
Materials and methods: We utilised confocal microscopy and high-content microscopy assays to determine differences in cell surface expression and agonist-induced trafficking responses of wild type versus Thr316 GLP-1Rs in INS-1 832/3 rat pancreatic beta cells, while molecular dynamic (MD) simulations was used to model the conformational space of GLP-1R. We additionally used NanoBiT complementation assays to determine total and endosomal Gαs coupling and β-arrestin2 recruitment propensities for the endogenous agonist GLP-1 as well as the stable analogue exendin-4, and closely related and oppositely biased agonists exendin-phe1 and exendin-asp3.
Results: Consistent with previous studies in HEK and CHO cells, the A316T mutation led to a significant loss of cell surface receptor expression in beta cells (p=0.0114). Moreover, MD simulations revealed that the mutant receptor exhibits altered mobility and flexibility of the extracellular domain (ECD) and important transmembrane regions pre-and post-activation, distinct to each agonist-bound state. Results also revealed a significant decrease in mutant receptor recycling (p=0.0431), associated with increased internalisation (p=0.024) and degradation (p=0.0323) upon GLP-1 binding. We also demonstrate significantly augmented mini-Gs recruitment in response to GLP-1 (p=0.0002), exendin-4 (p=0.0019), exendin-phe1 (p=0.0386), but not for the β-arrestin biased agonist exendin-asp3 by the Thr316 variant versus the wild type. Strikingly, the variant also exhibits significantly increased efficacy (p=0.0083) and potency (p=0.0281) for endosomal over plasma membrane Gαs coupling compared to wild type receptors. Assessment of Gαs coupling vs β-arrestin2 recruitment propensities reveal that mutant receptors exhibit a significant bias towards Gαs coupling versus wild types in response to GLP-1 (p<0.0001), exendin-4 (p<0.0003), and exendin-phe1 (p<0.0001), but not with exendin-asp3 (p=0.5640).
Conclusion: The GLP-1R A316T polymorphism is a determinant of pharmacological responses to GLP-1R-targeting compounds, including biased agonists, with varying responses associated to differentially biased compounds. The mutation causes significant changes in receptor conformational dynamics, signalling, and trafficking signatures highlighting the importance of the precise molecular characterization of GLP-1R variants to predict individual responses to specific GLP-1R therapies.
Supported by: DUK PhD Studentship
Disclosure: L. El Eid: None.
570
Bi 456906, structural properties and pharmacology of the novel glucagon and glucagon-like peptide-1 receptor (GCGR/GLP-1R) dual agonist
R. Augustin1, T. Zimmermann1, L. Thomas1, T. Baader-Pagler1, P. Haebel1, E. Simon1, H. Klein1, R. Santhanam1, W. Reindl1, B. Bajrami1, W. Rist1, I. Uphues1, D. Hamprecht2, H. Neubauer1;
1Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany, 2Pharmaxis Ltd, Frenchs Forest NSW, Australia.
Background and aims: Obesity is a serious global health challenge with a need for safe and effective pharmaceutical interventions. GCG and GLP-1 are peptide hormones that regulate body weight by increasing energy expenditure and reducing energy intake, respectively. We describe the structural properties and pharmacology of the novel GCGR/GLP-1R dual agonist BI 456906.
Materials and methods: In vitro potency of BI 456906 for the GCGR and GLP-1R was determined in cells of recombinant and endogenous receptor expression and in hepatocytes and islets for functional agonism based on glycogen synthesis inhibition and insulin secretion, respectively. In vivo, GLP-1R engagement by BI 456906 was assessed in lean mice for food intake, gastric emptying, and oral glucose tolerance. Subchronic efficacy of BI 456906 on body weight and energy expenditure was investigated in diet-induced obese (DIO) mice. Simultaneous engagement of the GLP-1R and GCGR in vivo was demonstrated in CRE-Luc reporter mice. Liver mRNA sequencing was combined with a computational biology approach to generate mechanistic understanding of GCGR agonism by BI 456906.
Results: BI 456906 is a 29-amino acid peptide designed based on the glucagon sequence. Its in vivo half-life was optimized by integration of an unnatural amino acid and a glycine-serine linker containing a C18 di-acid. BI 456906 is a highly potent agonist for the human GCGR and GLP-1R. It potentiates glucose-stimulated insulin secretion from rodent and perifused human islets and potently inhibits glycogen synthesis. Upon single administration to lean mice, BI 456906 engaged the GLP-1R based on dose-dependent reductions in food intake, gastric emptying, and improvements in oral glucose tolerance. In DIO mice, after daily dosing for 4 weeks, BI 456906 led to superior bodyweight-lowering compared to a maximally effective dose of the GLP-1R agonist semaglutide 32% vs. 27%; p<0.05). The superior efficacy of BI 456906 was attributed to an increase in energy expenditure. Simultaneous, dose-dependent engagement of the GLP-1R and GCGR in vivo was shown for BI 456906 in CRE-Luc reporter mice based on tissue luciferase induction and bioluminescent imaging when compared to semaglutide and a selective GCGR agonist. Bulk mRNA sequencing of livers from DIO mice treated with BI 456906 or the GLP-1R agonist identified a GCG specific signature, e.g. changes in methionine (NNMT, GNMT) and amino acid metabolizing (GOT1, SDS) genes. Engagement of the GCGR by BI 456906 was confirmed based on e.g. liver NNMT mRNA, plasma amino acids, glucagon, and plasma FGF-21. The human relevance of the genes regulated by BI 456906 in livers of mice was supported by a comparative pathway cluster analysis with liver samples from patients with NASH.
Conclusion: BI 456906, by simultaneous activation of the GCGR and GLP-1R, achieved a body weight lowering efficacy that is superior to a GLP-1R agonist and is attributed to an increase in energy expenditure. Transcriptional profiling provided insights into the mechanism of action for BI 456906 in the liver with potential human relevance for patients with NASH. In summary, the pharmacology of BI 456906 suggests clinical benefits for patients with obesity and patients with NASH which is in support of the current clinical programs.
Supported by: BI
Disclosure: R. Augustin: None.
571
Using a BRET based high throughout screening approach to identify previously approved drugs that can be repurposed to ameliorate type 1 diabetes
S. He, G. Lim;
Departement of medicine/Cardiometabolic axis, University of Montreal/The University of Montreal Hospital Research Centre (CRCHUM), Montreal, Canada.
Background and aims: Type I Diabetes (T1D) accounts for approximately 10% of total diagnosed cases, and it is characterized by a progressive loss of functional β-cell mass. A key event that induces β-cell death is the infiltration of autoreactive CD8+ T cells into pancreatic islets, leading to insulitis and the induction of β-cell apoptosis. The underlying event that initiates this process is not known, and exogenous insulin injections still remain the primary therapeutic approach. Considering the role of cytotoxic CD8+ T cells in T1D progression, future approaches may include novel T1D therapies that promote CD8+T cell depletion. Apoptosis is an endogenous mechanism that combats foreign pathogens and clears abnormal cells. Scaffold proteins belonging to the 14-3-3 protein family are critical endogenous regulators of cell survival, and we previously demonstrated that 14-3-3ζ has essential functions in mediating pancreatic β-cell survival through its inhibitory actions on pro-apoptotic BCL-2 proteins, such as BAD. Thus, we hypothesize that identifying compounds with the ability to disrupt 14-3-3ζ:BAD interactions in CD8+T cells may lead to the development of new chemical entities to delay or prevent T1D.
Materials and methods: A BRET (Bioluminescence Resonance Energy Transfer)-based reporter was developed to detect 14-3-3ζ:BAD interactions in NIH-3T3 cells. Stoichiometric expression of Rluc8-14-3-3ζ and mCitrine-BAD (fragment spanning Ser112 and Ser136) is ensured by a bi-directional expression vector. Dose- and time-response studies with established 14-3-3 protein inhibitors, FTY720 and I,2-5, were used to validate the reporter system. High-throughput screening of an FDA-approved drug library (2322 drugs) was used to identify compounds with the ability to cause a 25% of reduction in BRET signal, as assessed by plate reader-based measurements of luciferase. Drugs were tested at concentrations of 200mM, 20mM, 2mM, and 200uM (n=2). Secondary screens will use Hoechst/Propidium Iodide incorporation to confirm the capacity of identified drugs to induce cell apoptosis by using a high-content imaging system.
Results: Co-expression of the mCitrine-BAD(f), and RLuc8-14-3-3ζ in NIH-3T3 cells resulted in a 1.7-fold increase in BRET compared to the absence of the BAD fragment. Treatment of cells with FTY720 and I,2-5 dose- and time-dependently reduced BRET. Scalability studies demonstrated that as little as 20,000 cells per well in a 384-well plate resulted in a measurable BRET signal. To date, screening of 252 out of 2322 drugs has identified 37 drugs with the ability to reduce 25% of BRET signal. Some identified drugs include miglitol, which can maintain normoglycemia, tauroursodeoxycholic acid, which prevents T1D in animal models, DL-α-difluoromethylornithine, which induces apoptosis. By calculating the ratio of dead-to-living-cells, image-based secondary screening data showed that about 65% of these identified compounds can induce cell death but to varying degrees.
Conclusion: These results confirm the ability of our BRET-based reporter to detect 14-3-3ζ:BAD interactions in living cells and identify previously-approved drugs that can disrupt 14-3-3ζ:BAD interactions and induce apoptosis. These hits represent potential chemical backbones that can be modified and translated to new chemical entities to treat T1D.
Supported by: Canada Research Chairs, CIHR; University of Montreal
Disclosure: S. He: None.
572
A novel oral cannabinoid receptor-1 inverse agonist induces additive weight loss and improves metabolic biomarkers in DIO mice in combination with semaglutide or tirzepatide
M. Morningstar, S. Ferreira, A. Kolodziej, T. Blumen, Z. Jin, H. Deng, B. White, R. Brake;
Corbus Pharmaceuticals, Norwood, USA.
Background and aims: The cannabinoid receptor type 1 (CB1) is a clinically validated target for obesity, type 2 diabetes and associated metabolic disorders. First generation CB1 inverse agonists (e.g., rimonabant) were associated with unacceptable psychotropic side effects related to their on-target binding to CB1 in the brain. To address these side effects, Corbus Pharmaceuticals is developing CRB-913, a novel, oral CB1 inverse agonist rationally designed to have markedly reduced brain exposure. Here we explore polypharmacy targeting orthogonal mechanisms (CB1 and GLP-1/GIP) to try to improve the therapeutic index of the existing SOC agents exclusively focused on the incretin receptor family. We show that CRB-913 demonstrates significant single agent activity and additive effects on weight loss in combination with either semaglutide or tirzepatide in a diet-induced obesity (DIO) mouse model. Improvements were realized across a panel of glycemic and metabolic endpoints at doses that could better manage the established adverse event profile of these monotherapy agents.
Materials and methods: In the DIO mouse model, C57BL6/J mice were fed a continuous high fat diet for 22 weeks and during 28 days of treatment. CRB-913 and rimonabant brain exposure were determined in both DIO mice (CRB-913 BID PO for 28-day at 2.5 mg/kg or 5 mg/kg and rimonabant at 10 mg/kg PO QD) and lean mice (single dose CRB-913 and rimonabant at 10 mg/kg PO). Control, single agent CRB-913 (BID PO at 2.5 mg/kg or 5 mg/kg) and combination cohorts with semaglutide (Q3D SC at 0.04 mg/kg or 0.12 mg/kg) or tirzepatide (Q3D SC at 0.024 mg/kg or 0.048 mg/kg) were studied. Body weight and food consumption were recorded daily. Oral glucose tolerance test was performed in week 3 of treatment (1-hour post CRB-913 dose, 16-hr post semaglutide/tirzepatide dose). Blood and liver were collected at end of study.
Results: Compared to rimonabant (10 mg/kg PO lean mice), CRB-913 demonstrated a 24-fold decrease in Cmax and 50-fold decrease in brain/plasma ratio AUC0-24. After 28-day DIO mouse study, CRB-913 (5 mg/kg PO BID) demonstrated similar weight loss (-16.5%) to rimonabant but with 7-fold higher AUC0-24 peripheral distribution and 6-fold lower brain concentration. In the DIO model, CRB-913 (2.5 mg/kg) dosed as a single agent demonstrated weight reduction of -10.2% by day 18, comparable to single agent semaglutide (-7.9% at 0.04 mg/kg) or tirzepatide (-11.9% at 0.024 mg/kg). In combination studies with semaglutide (0.04 mg /kg) or tirzepatide (0.024 mg/kg), CRB-913 (2.5 mg/kg) led to additive weight loss of -19.0% or -24.7% by day 18, respectively. Other combinations at higher doses yielded proportionately additive efficacy. Similarly, improvements were observed in plasma insulin, leptin, and lipid levels, and hepatic steatosis.
Conclusion: CRB-913 demonstrated markedly reduced brain penetration compared to rimonabant and has potential to overcome the clinical safety challenges previously associated with CB1 inverse agonists. The combination of CRB-913 with incretin receptor agonists such as semaglutide and tirzepatide is additive and as such could provide a novel therapeutic strategy that could overcome some constraints observed with these agents as high dose monotherapies. The combination of two such orthogonal mechanisms of action merits exploration in clinical settings.
Disclosure: M. Morningstar: None.
573
Liraglutide treatment attenuates inflammation markers in the cardiac, cerebral and renal microvasculature in streptozotocin-induced diabetic rats
S. Simsek1,2, A. Korn3,4, U. Baylan3,4, R.W. Emmens3,4, C.G. Schalkwijk5,6, H.W.M. Niessen3,4, P.A.J. Krijnen3,4;
1Internal Medicine, Northwest Clinics, Alkmaar, 2Internal Medicine, Amsterdam UMC Location VUmc, Amsterdam, 3Pathology, Amsterdam UMC, Location VUmc, Amsterdam, 4Amsterdam Cardiovascular Sciences, Amsterdam, 5Internal Medicine, Maastricht University Medical Centre, Maastricht, 6Cardiovascular Research Institute Maastricht, Maastricht, Netherlands.
Background and aims: Diabetes mellitus (DM) induces cardiac and cerebral microvascular dysfunction via increased glycation, oxidative stress and endothelial activation. Liraglutide, a glucagon-like peptide-1 receptor analogue, inhibited the reactive oxygen species producing enzyme NOX2 and adhesion molecules in isolated endothelial cells. Here we have studied how Liraglutide affects advanced glycation, NOX expression and inflammatory status of the cardiac and cerebral microvasculature in vivo in diabetic rats.
Materials and methods: DM was induced in Sprague Dawley rats (n=15) via intraperitoneal streptozotocin (STZ) injection (60 mg/kg body weight). 10 control rats remained non-diabetic. From day 9 post-STZ injection, Liraglutide (200 μg/kg bodyweight; n=7) or vehicle (n=8) was injected subcutaneously daily until termination on day 29. The advanced glycation end-product N-ε-(carboxymethyl)lysine (CML), NOX2, NOX4, ICAM-1 and VCAM-1 were subsequently analysed using immunohistochemistry and quantified to compare Liraglutide treatment to placebo.
Results: DM increased CML, NOX2, ICAM-1 and VCAM-1 in the intramyocardial vasculature, although not significant for NOX2. NOX4 was not significantly increased in the ventricles and absent in the atria. In the cerebral vasculature, CML, NOX2 and NOX4 were significantly increased, but not ICAM-1 and VCAM-1. DM significantly increased ICAM-1 and VCAM-1 positive vessels in the kidney, as well as the percentage of ICAM-1 and VCAM-1 positive glomeruli. Liraglutide significantly reduced the DM-associated CML accumulation and expression of NOX2, ICAM-1 and VCAM-1 in the intramyocardial, and CML, NOX2 and NOX4 in the cerebral vasculature. In the renal vasculature, Liraglutide significantly decreased the percentage of VCAM-1 positive glomeruli. Liraglutide had these effects without changing blood glucose levels or body weight.
Conclusion: Our study implies that Liraglutide protects the cardiac, cerebral and renal microvasculature against diabetes-induced dysfunction, independent of a blood glucose lowering effect in a type 1 diabetes rat model.
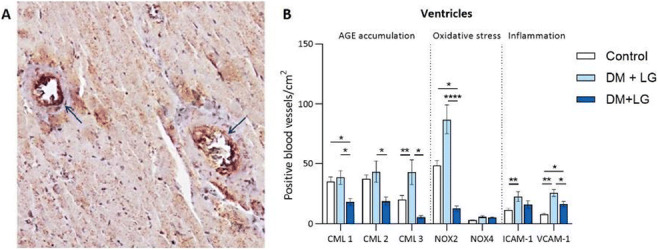
Supported by: This study was financed by Novo Nordisk BV
Disclosure: S. Simsek: None.
574
Dual amylin and calcitonin receptor agonist treatment in type 2 diabetic rats protects against cardiovascular and renal complications
A.T. Larsen1, S.A. Melander1, M.A. Karsdal1,2, K. Henriksen1,2;
1Nordic Bioscience, Herlev, Denmark, 2KeyBioscience AG, Stans, Switzerland.
Background and aims: Dual amylin and calcitonin receptor agonists (DACRAs) are novel candidates for treatment of obesity and type 2 diabetes (T2D) due to their beneficial effects on both body weight, glucose control and insulin action. However, the extent to which they protect the cardio-renal system against damage remains unknown. Here we utilize biomarkers of cardiovascular and kidney damage to characterize the potential of a novel long-acting DACRA as treatment for obesity, T2D and related comorbidities.
Materials and methods: Rat models of both T2D (ZDF) and high fat diet (HFD) induced obesity were treated with the long-acting DACRA KBP to assess the effect on body weight, food intake, fasting glycemia, HbA1c levels as well as glucose tolerance. Furthermore, serum biomarkers of cardiovascular disease (PRO-C6) and kidney complications (Tumstatin) were evaluated to assess the impact of DACRA treatment on these important co-morbidities.
Results: In HFD rats, chronic treatment with KBP resulted in reduced food intake (p<0.01), a significant body weight loss (p<0.001) and improved glucose tolerance (p<0.001). In diabetic ZDF rats, KBP treatment improved glucose control and preserved plasma insulin compared to vehicle (p<0.001), which was reflected in the HbA1c levels at study end (p<0.001). Importantly, the marker of cardiovascular disease, PRO-C6 (endotrophin, a marker of collagen type VI formation) increased during the study in the vehicle treated rats reflecting the disease progression, while KBP treatment attenuated this increase resulting in significantly lower PRO-C6 levels compared to vehicle at study end (p<0.05). Similarly, the marker of kidney damage TUM (tumstatin, a matrikine derived from collagen type IVα3) was also increased over time in the control group, and KBP-treatment led to normalization hereof (p<0.05). Thereby highlighting KBP as a promising treatment of these late complications related to T2D and obesity.
Conclusion: These preclinical studies confirm that long-acting DACRAs have beneficial effects on weight and glucose control, but more importantly also show highly relevant effects on biomarkers associated with cardiovascular and kidney complications. Taken together, these findings highlight KBP as a promising once-weekly agent for treatment of obesity and T2D as well as related late complications.
Supported by: The work was supported by the Danish Research Foundation (SAM) and the Innovation Fund Denmark (ATL)
Disclosure: A.T. Larsen: Employment/Consultancy; All authors are employed by Nordic Bioscience A/S. Grants; The studies were funded by grants from the Danish Research Foundation (Den Danske Forskningsfond) and the Innovation Fund Denmark. Stock/Shareholding; Morten A. Karsdal and Kim Henriksen own stock in Nordic Bioscience A/S.
575
Deorphanisation of black widow spider GLP-1: validation of GLP-1 receptor agonist action relevant to human islet insulin secretion in vitro
G.G. Holz1, A.N. Liles2, R.P. Doyle2, C.A. Leech1, O.G. Chepurny1;
1Medicine, State University of New York (SUNY) Upstate Medical University, Syracuse, 2Chemistry, Syracuse University, Syracuse, USA.
Background and aims: Hybrid peptides incorporating amino acid motifs found within insect venom toxins and mammalian incretin hormones are under preclinical investigation for use in the treatment of type 2 diabetes (T2D). The hybrid peptide Black Widow GLP-1 (BW-GLP-1) consists of amino acid motifs that are predicted to enable its binding to either the GLP-1 receptor (GLP-1R), or the CIRL1 adhesion family GPCR that is a high-affinity receptor for black widow spider (Lactrodectus) alpha-latrotoxin. Interestingly, GLP-1 and alpha-latrotoxin share partial amino acid sequence homology, as is also the case for the GLP-1R and CIRL1. Thus, this study tested for GLP-1- and CIRL1-mediated actions of BW-GLP-1 that might be of relevance to T2D therapeutics.
Materials and methods: Primary amino sequence analysis was used to identify homologous amino acid motifs within GLP-1 and alpha-latrotoxin. The BW-GLP-1 sequence is H-A-E-G-T-F-T-S-D-V-S-S-Y-L-E-G-E-I-V-K-Y-F-V-G-T-L-G-N-GR-amide in which the H-A-E-G-T-F-T-S-D-V-S-S-Y-L-E-G motif at the N-terminus corresponds to residues 1-16 of GLP-1(7-36)amide. It is flanked by E-I-V-K-Y-F-V-G-T-L-G-N that corresponds to residues 970-981 of alpha-latrotoxin, whereas the C-terminus is capped with G-R-amide like that of GLP-1(7-36)amide. For comparison, an alternative hybrid peptide designated as AL07 was tested in which the N-terminus is comprised of residues 1371-1380 of alpha-latrotoxin, a linker comprised of residues 11-16 of GLP-1(7-36)amide, a flanking sequence comprising residues 970-981 of alpha-latrotoxin (as in BW-GLP-1), and a C-terminus capped with G-R-amide like that of GLP-1(7-36)amide (as in BW-GLP-1). This AL07 sequence is H-S-D-G-I-L-T-K-K-L-S-S-Y-L-E-G- E-I-V-K-Y-F-V-G-T-L-G-N-GR-amide.
Results: BW-GLP-1 was tested in FRET assays using the cAMP biosensor H188 expressed in HEK293-GLP-1R cells expressing the recombinant human GLP-1R, or in rat INS-1E cells that expressed H188 and the endogenous GLP-1R. BW-GLP-1 quickly (within 30 sec) raised levels of cAMP in monolayers of HEK293-GLP-1R cells (EC50 4 nM). No such action of BW-GLP-1 was measured in HEK2393 cells expressing GIP, glucagon, or CRF1 receptors. Similarly, BW-GLP-1 raised levels of cAMP in INS-1E cells, and all cAMP-elevating actions of BW-GLP-1 in these two cell types were abolished by the GLP-1R antagonist Exendin(9-39). BW-GLP-1 induced transient oscillations of [Ca2+] in single Fura-2 loaded human beta cells, and it also potentiated 1st and 2nd phase GSIS from perifused human islets when stepping from 2.8 to 16.7 mM glucose. The alternative hybrid peptide AL07 raised levels of cAMP in HEK293-GLP-1R cells and INS-1E cells, thereby demonstrating the surprising finding that GLP-1R agonism was preserved for peptides containing N-terminal sequences corresponding to GLP-1 (as in BW-GLP-1) or alpha-latrotoxin (as in AL07). Finally, for HEK293 cells transfected with CIRL1, BW-GLP-1 was without effect in assays of cAMP and Ca2+.
Conclusion: BW-GLP-1 fails to signal through CIRL1 but is a GLP-1R agonist with insulin secretagogue properties when tested using human islets. The hybrid peptide AL07 is also a GLP-1R agonist, thereby demonstrating that amino acid motifs present within alpha-latrotoxin can be exploited for construction of novel peptides with GPCR activating properties.
Supported by: NIH/NIDDK R01DK128443
Disclosure: G.G. Holz: None.
576
Empagliflozin protects from diet-induced obesity, insulin resistance and hepatic steatosis in mice
B. Radlinger1,2, C. Ress1,2, S. Folie1,2, K. Salzmann1,2, W. Salvenmoser3, M. Graber4, J. Hirsch4, J. Holfeld4, C. Kremser5, P. Moser6, G. Staudacher1,2, T. Jelenik7, M. Roden7,8, H. Tilg1, S. Kaser1,2;
1Internal Medicine I, Medical University Innsbruck, Innsbruck, Austria, 2Christian Doppler Laboratory for Metabolic Crosstalk, Innsbruck, Austria, 3Institute of Zoology and Center of Molecular Biosciences Innsbruck (CBMI), Innsbruck, Austria, 4Department of Cardiac Surgery, Medical University Innsbruck, Innsbruck, Austria, 5Department of Radiology, Medical University Innsbruck, Innsbruck, Austria, 6Innpath GmbH, Innsbruck, Austria, 7Leibniz Center for Diabetes Research at Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany, 8Department of Endocrinology and Diabetology, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany.
Background and aims: Chronic overnutrition, obesity, insulin resistance and ultimately type 2 diabetes are a major global health problem. We hypothesize that SGLT2 inhibition might be beneficial in a preventive setup to ameliorate metabolic dysregulation induced by a western-type diet.
Materials and methods: C57Bl/6 mice were fed a western type diet with (WDE) or without (WD) empagliflozin added to the diet. To control for diet-specific effects a standard diet with (SDE) and without empagliflozin (SD) was used. After 10 weeks metabolic phenotyping including assessment of insulin sensitivity via hyperinsulinemic euglycemic clamps, histological analysis of liver tissue samples, analysis of hepatic insulin signaling and analysis of skeletal muscle mitochondria was performed.
Results: Addition of empagliflozin for 10 weeks prevented significant overweight in WDE mice (mean weight 39.4 g (WD) vs. 32.7 g (WDE), p=<0.0001). WDE mice were hyperglycemic and hyperinsulinemic compared to WD mice (mean blood glucose 215.3 mg/dl (WD) vs. 171.3 mg/dl (WDE), p=0.0147; median insulin 1.02 ng/ml (WD) vs. 0.56 ng/ml (WDE), p=0.0191). Confirmatory, hyperinsulinemic euglycemic clamps revealed increased insulin sensitivity in WDE mice (mean glucose infusion rate 28.01 mg/kg/min (WD) vs. 45.97 mg/kg/min (WDE), p=0.0384). Addition of empagliflozin was associated with less hepatic steatosis in WDE mice compared to WD mice (median steatosis score 1.8 (WD) vs 0.2 (WDE), p=0.0341; median liver triglyceride content 39.4 μg/ml (WD) vs. 14.1 μg/ml (WDE), p=0.0504). S473 AKT phosphorylation was nearly twice as high in WDE mice compared to WD mice indicating increased hepatic insulin sensitivity (median p-AKT / total AKT ratio 0.785 a.u. (WD) vs. 1.675 a.u. (WDE), p=0.0065). Finally, skeletal muscle citrate synthase activity was increased in WDE mice compared to WD mice indicating a larger mitochondrial mass (mean citrate synthase activity 7.126 μmol/ml/min (WD) vs. 9.560 μmol/ml/min (WDE), p=0.0123). Transmission electron microscopy revealed larger mitochondria in WDE and SDE mice compared to WD and SD respectively (median area in type 2 skeletal muscle fiber / individual subsarcolemmal mitochondrion 0.1990 μm² (WD) vs. 0.3170 μm² (WDE), p=<0.0001; 0.1360 μm² (SD) vs. 0.2460 μm² (SDE), p=<0.0001).
Conclusion: SGLT2 inhibition with empagliflozin prevented diet induced overweight, hepatic steatosis and insulin resistance in C57Bl/6 mice after 10 weeks on a western-type diet. Diet independent effects on skeletal muscle mitochondria might contribute to metabolic effects.
Supported by: Austrian BMDW, Austrian FTE, Boehringer Ingelheim
Disclosure: B. Radlinger: None.
SO 42 Clinical epidemiology and pharmacotherapy
577
Patients with type 2 diabetes present similar immunological response to COVID-19 BNT162b2 mRNA vaccine with healthy subjects: a prospective cohort study
S. Paschou1, V. Karalis2, T. Psaltopoulou3, I. Charitaki3, A. Sklirou4, V. Iconomidou4, V. Vasileiou5, G. Kassi5, A. Vryonidou6, A. Kokkinos7, N. Tentolouris7, E. Hatziaggelaki8, I. Trougakos4, E. Terpos3, M. Dimopoulos3;
1Endocrine Unit and Diabetes Center, Department of Clinical Therapeutics, Alexandra Hospital, School of Medicine, National and Kapodistrian University of Athens, 2Faculty of Pharmacy, School of Health Sciences, National and Kapodistrian University of Athens, 3Department of Clinical Therapeutics, Alexandra Hospital, School of Medicine, National and Kapodistrian University of Athens, 4Department of Cell Biology and Biophysics, Faculty of Biology, National and Kapodistrian University of Athens, 5Department of Endocrinology, Alexandra Hospital, 6Department of Endocrinology and Diabetes Center, Hellenic Red Cross Hospital, 7Diabetes Center, First Department of Propaedeutic Internal Medicine, Laiko General Hospital, National and Kapodistrian University of Athens, 8Diabetes Center, Second Department of Internal Medicine, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Background and aims: If a compromised immune response to SARS-CoV-2 is probably a reason for the increased risk for severe COVID-19 in patients with type 2 diabetes mellitus (T2DM), the question whether these patients do also face an impaired immunological response to vaccination is crucial. The aim of this study was to compare the kinetics of neutralizing antibodies (NΑbs) against SARS-CoV-2 after vaccination with BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech) between patients with T2DM and healthy controls.
Materials and methods: This is a single-center prospective cohort study. NAbs levels after BNT162b2 mRNA vaccination were compared between 50 patients with non-insulin treated T2DM (42% men) and 50 age, gender and body mass index (BMI) matched healthy controls (44% men) from the day of the first dose until a period of up to three months after the second dose. NAbs against SARS-CoV-2 were quantified using an FDA approved method (ELISA, cPassTM SARS-CoV-2 NAbs Detection Kit; GenScript, Piscataway, NJ, USA). The median age of both groups was 70 years, and the median BMI value was 29.7 kg/m2 and 27.6 kg/m2 for the T2DM and control group, respectively.
Results: At day 1, the mean NAbs of the control and T2DM groups were 11.13% and 13.82%, respectively (p value=0.926), indicating that participants in both groups started from the same baseline values. Three weeks later, just before the second vaccination dose, the mean NAbs values were 34.39% in the control group and 37.14% in participants with T2DM (p value=0.698). One month after the second vaccination, mean NAbs values increased to 95.12% in the control group and 95.66% in the T2DM group. Three months after the second vaccine dose, the mean inhibitory titers decreased to 90.48% (control group) and 82.56% (T2DM group). It is worth noting that 3 months after the second vaccination, 48 subjects (96%) in the control group and 46 patients with T2DM (92%) were still highly or very highly protected. On all occasions, no significant difference was found between the two groups (all p values>0.05).
Conclusion: This study provided evidence that patients with T2DM present similar immunological response to COVID-19 BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech) with healthy subjects.
Disclosure: S. Paschou: None.
578
Clinically relevant predictors of cardiovascular events in patients with type 2 diabetes: a pooled analysis from clinical studies
A. Malhotra1, G. Bader2;
1Novartis Healthcare Private Limited, Mumbai, India, 2Novartis Pharma AG, Basel, Switzerland.
Background and aims: Patients with type 2 diabetes mellitus (T2DM) are more likely to develop cardiovascular disease (CVD). This study aims to explore the association between several baseline parameters and risk of cardiovascular (CV) events in patients with T2DM and whether evidence from clinical data at T2DM onset could be useful in identifying patients at risk of subsequent CV events.
Materials and methods: Data for baseline visits were extracted from 34 globally conducted clinical studies. The CV risk at baseline was assessed using Framingham risk equation.
Results: At baseline, 2130 (13.6%) patients from the total pooled population (n=18,076) had CVD history. The mean (SD) age of the patients was 56.8 (10.7) years, and the total cholesterol, low-density lipoprotein (LDL) and the systolic blood pressure (SBP) was 196.4 (41.0) mg/dL, 115.4 (36.8) mg/dL and 132.7 (14.6) mmHg, respectively. The disease duration was 5.08 (5.10) years, and the Framingham risk score (FRS) was 20.71 (12.68). Patients with none or multiple risk factors had an incremental risk for subsequent CV events. Patients having CVD history and FRS ≥20% were significantly associated with a higher risk of CV events (HR [95% CI]: 7.8 [5.0, 12.3]; p<0.001; Figure).
Conclusion: Overall, these findings show that clinical data collected at the onset and follow-up of T2DM, such as CVD history, high LDL and SBP, may aid in identifying patients at high risk of subsequent CV events.
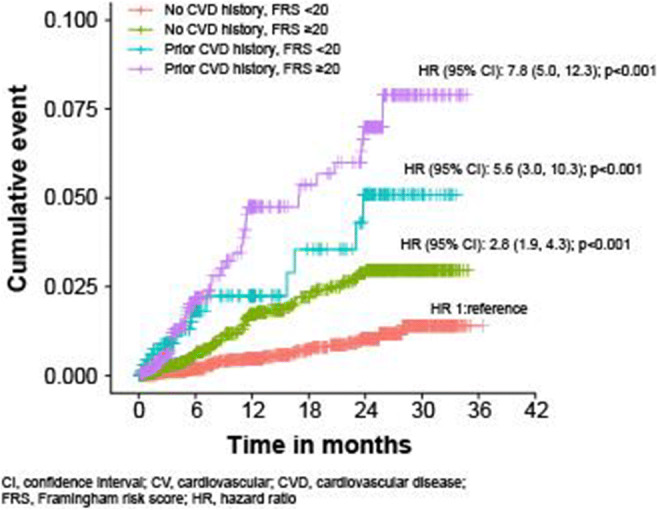
Supported by: Novartis
Disclosure: A. Malhotra: Employment/Consultancy; Novartis.
579
Dramatic changes in use of glucose-lowering drugs in Denmark from 2005 through 2021: a nationwide study
T. Vilsbøll1, J.H. Andersen2, J. Søndergaard3, R.W. Thomsen4, A. Pottegaard2;
1Clinical Research, Steno Diabetes Center Copenhagen, University of Copenhagen, Copenhagen, 2Clinical Pharmacology, Pharmacy and Environmental Medicine, University of Southern Denmark, Odense, 3Research Unit of General Practice, Department of Public Health, University of Southern Denmark, Odense, 4Department of Clinical Medicine, Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark.
Background and aims: The landscape of available type 2 diabetes pharmacotherapy has changed dramatically over the last one-and-a-half decade. We investigated changes in use patterns of glucose-lowering therapy in Denmark during 2005 through 2021.
Materials and methods: Nationwide population-based drug utilization study based on prospective medical databases covering the entire Danish population (~5.8 million). We assessed incident and prevalent use patterns among the 441,205 individuals using at least one non-insulin glucose-lowering drug 2005-2021. Drugs with a primary weight loss indication were excluded.
Results: The rate of new users of glucose-lowering drugs increased from 2005, peaked in 2011, and decreased to a stable level during 2013-2019 and finally increased dramatically in 2020-2021. In parallel, the total prevalence of use increased steadily from 2.1% in 2005 to 5.0% in 2021 of the entire adult population. Metformin comprised 39% of all glucose-lowering drug consumption in 2021 followed by insulin (17%), sodium-glucose cotransporter-2 inhibitors ((SGLT2i) 17%), glucagon-like peptide 1 receptor agonists ((GLP-1RA)16%), and dipeptidyl peptidase 4 inhibitors (7.5%) (Figure, upper panel). Overall, in 2021 57% were on monotherapy, 28% used dual therapy while 12% and 2.4% used three and four drug classes, respectively. Both intensity and diversity of therapies increased substantially over time, with 15 different treatment regimens each covering >1% of users in 2021, an increase from 5 regimens in 2005. General practitioners prescribed 88% of all glucose-lowering drugs in 2021. The distribution of first-line treatments (Figure, bottom panel) initiated by general practitioners versus hospital physicians changed over time, with dramatic changes in initiation of especially GLP-1RAs (general practitioners) and SGLT-2i (hospital physicians) in 2021.
Conclusion: The rate of new users of glucose-lowering drugs is increasing in recent years and the total prevalence of glucose-lowering drug use also increases steadily. Glucose-lowering drugs are mainly prescribed by general practitioners and both the intensity and diversity of glucose-lowering treatment is increasing, with recent dramatic changes in prescribing of GLP-1RAs and SGLT-2i.
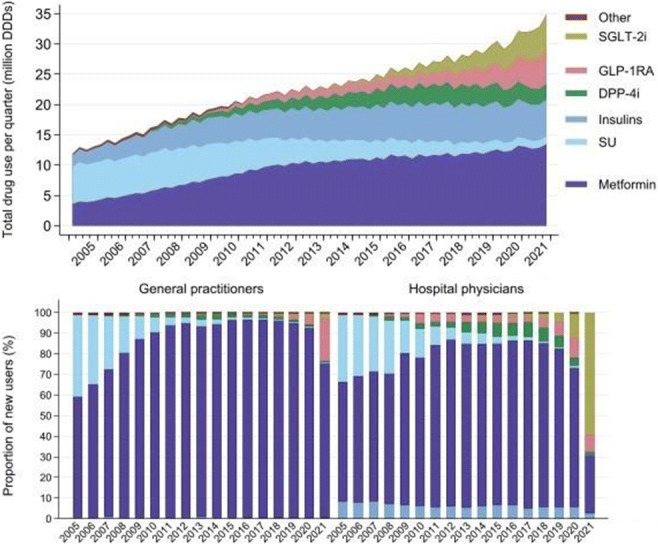
Disclosure: T. Vilsbøll: Grants; Novo Nordisk, Lilly, Boehringer. Honorarium; Amgen, Boehringer, BMS, GSK, Gilead, Mundipharma, Novo Nordisk, Lilly, Sun Pharmaceuticals.
580
Higher adherence and persistence for dulaglutide compared to oral semaglutide at 6- months follow-up with U.S. real-world data
R. Paczkowski1, M.M. Hoog1, J. Peleshok1, M. Yu1, A. Huang2, B. Limone3, J. Manjelievskaia3;
1Eli Lilly and Company, Indianapolis, 2Tigermed-BDM, Somerset, 3IBM Watson Health, Cambridge, USA.
Background and aims: A retrospective, observational analysis of administrative claims data from the IBM MarketScan Databases assessed adherence and persistence among GLP-1 RA naive adult patients with type 2 diabetes (T2D) newly initiating dulaglutide (DU) or oral semaglutide (OS) between Sept 2019 and Nov 2020.
Materials and methods: Patients had continuous enrolment in the 6-month pre-index period and 6-month follow-up periods. The DU patients were propensity-score matched 1:1 to OS patients (6,166 pairs). The median age was 54 years at index date, and 48% were female. At pre-index, mean aDCSI was 0.6 and 10% of patients had ASCVD. Most patients used oral anti-hyperglycaemic medication (87%); a smaller proportion used insulin (15%). The most common prescribing provider was primary care (62%).
Results: More DU patients were adherent (proportion days covered [PDC] ≥80%) (65% vs. 50%, p<0.001) and had higher mean PDC vs. OS (0.78 vs. 0.68, p<0.001). More DU patients were persistent on therapy compared to OS patients (72% vs. 57% p<0.001) and had longer mean duration of persistence (148 vs. 128, p<0.001). Among patients with ≥2 fills of their index drug, 73% of DU and 62% of OS patients were adherent.
Conclusion: During the 6-mo follow-up period, DU patients had significantly higher adherence and persistence compared to OS patients.
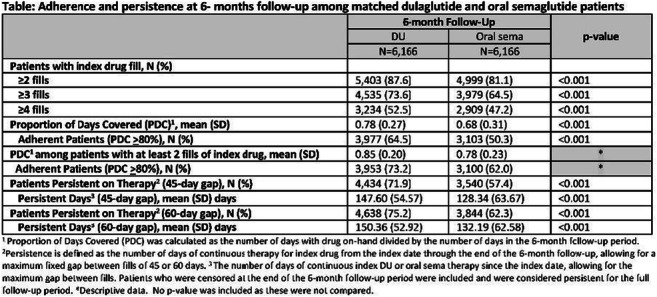
Supported by: Eli Lilly and Company
Disclosure: R. Paczkowski: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
581
Use of metformin in pregnant women with polycystic ovarian syndrome (PCOS): a cohort study in the United Kingdom
K. Brand, C. Foch, E. Boutmy;
Merck Healthcare KGaA, Darmstadt, Germany.
Background and aims: International guidelines support the use of metformin for PCOS-related anovulatory infertility, although other treatment options may have better outcomes (Teede. Fertil. Steril. 2018). Likewise, in the UK, the National Health Services indicate that metformin can be used “off-label” to encourage fertility and control PCOS symptoms, and highlights that taking metformin up to 12 weeks of pregnancy may reduce the risk of spontaneous abortion (NHS 2019). On the other hand, metformin is not licensed for the treatment of PCOS in most countries, including the UK, but it is licensed there for diabetes prevention in high risk patients. This study is describing metformin treatment patterns in pregnant PCOS women.
Materials and methods: A cohort of all pregnant women diagnosed with PCOS and with a last menstrual period (LMP) date between January 2005 and February 2019 was selected in the Clinical Research Practice Datalink (CPRD) and linked to the Pregnancy Registry. The CPRD is a UK database of anonymised medical records from general practitioners. Pregnancies were followed from the LMP date, through the earliest of end of pregnancy (whatever the termination event), or mother’s death.
Results: A total of 2,778 pregnancies were included. Median age was 30 years (q1, q3: 26, 33), and median BMI 30 kg/m2 (25, 36). A total of 528 (19%) pregnancies had already experienced one recorded spontaneous abortion, and 178 (6.4%) at least two. In the 6-month prior the pregnancy, 151 (5.4%) were prescribed clomifene, 222 (8.0%) combined hormonal contraceptives, 114 (4.1%) oral progesterone-only contraceptives, and 329 (11.8%) antidepressants. Overall, 2,519 (90.7%) pregnancies resulted in life birth, 200 (7.2%) in spontaneous abortion, and 11 (0.4%) in still birth. Metformin was prescribed in 525 (18.9%) pregnancies: 405 (77.1%) were already treated in the periconceptional period (90 days prior LMP), 63 (12.0%), 14 (2.7%), and 43 (8.2%) initiated metformin during the 1st , 2nd and 3rd trimester, respectively. Among those treated in the periconceptional period, 276 (68.1%) were still exposed after. Metformin was discontinued prior pregnancy end in most of the cases (447 [85.1%]). The median number of prescriptions per pregnancy was 2 (1, 4), and pregnancies were exposed for a median of 100 days (5, 144). Table 1 displays results per trimester.
Conclusion: In UK clinical practice, most women with PCOS were started on metformin in the periconceptual period and discontinued during the 1st trimester. In February 2022, the European Medicine Agency has approved the use of the originator brand of metformin during pregnancy and in the periconceptional phase, if clinically needed. On the basis of this safety update, the safety of metformin is now understood in the approved indications and if clinically needed. However, further studies are warranted to substantiate the place of metformin in infertility-related indications in PCOS.
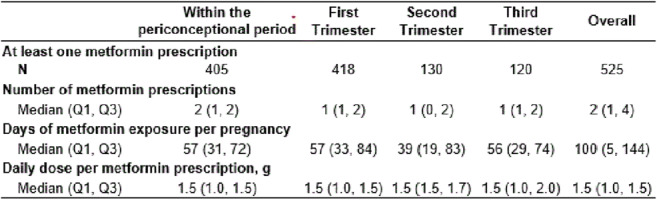
Clinical Trial Registration Number: ISAC protocol number 20_000146
Supported by: Merck (CrossRef Funder ID: 10.13039/100009945)
Disclosure: K. Brand: Employment/Consultancy; Full time employee of Merck Healthcare KGaA, Darmstadt, Germany.
582
Metformin and B12 deficiency: a retrospective study of the prevalence of B12 deficiency in patients on metformin
L. Young, R. Rasheed, H. Bibi;
General Practice, Research & Medical Education, Chapel Street Surgery, Rigg Milner Medical Centre & Corringham Health, Tilbury, UK.
Background and aims: The national prevalence of diabetes is rising. NICE guidelines place metformin as first line medication in all diabetics who can tolerate the side effects. Long term prescribing of metformin is associated with vitamin B12 deficiency. Studies quote prevalence rates of 6% in people below 60 years of age, rising to approximately 20% to those above 60. We undertook a review of the patients’ records on metformin to see the prevalence of B12 levels.
Materials and methods: Diabetic patients were identified from the SystmOne electronic clinical system disease registers. Their records were examined to assess the levels of vitamin B12 in patients prescribed metformin to assess the prevalence of B12 deficiency.
Results: Across a population of circa 10,000 patients there were n=718 diabetic patients with a practice prevalence of 7.18 % across three primary care sites versus a national prevalence of 7.1%. 500 consecutive patient records were examined to review their biochemistry. A total of 96 patients (19%) were B12 deficient and 84 of these patients (16.8%) had been prescribed metformin. Of these, 36 (7.2%) were currently still taking metformin, whereas 41(8.2%) had discontinued metformin due to side effects. The mean interval from starting metformin and developing B12 deficiency was 7.4 years, and this was a dose related effect as 27 patients (75%) were on a dose of 1.5g or higher at the time of their diagnosis. 51(60.7%) patients were male and 33(39.3%) were female. The mean age was 71.2 years with a standard deviation of 12.4 years. Also, we found 12 patients were B12 deficient but had never been on metformin, and 7 developed B12 deficiency prior to being prescribed metformin.
Conclusion: B12 deficiency is a common side effect of metformin therapy. Iatrogenic harm can be prevented by actively screening for B12 deficiency. Studies allude to a prevalence of 6-30%, our prevalence was lower due to our classification of B12 deficiency as <120pg/ml. However due to active screening throughout the therapy time, no patients displayed any overt symptoms of B12 deficiency. Diabetic neuropathy can be mistaken for B12 deficiency induced neuropathy if screening is not undertaken. In addition, patients need to be counselled prior to commencement of therapy of this potential side effect. B12 deficiency can co-exist in patients not on metformin. Awareness to screen for B12 deficiency is necessary to prevent iatrogenic harm from undetected B12 deficiency which could potentially cause neuropathy. This should be part of routine medication reviews and shared decision making in patients commenced upon this therapy.
Disclosure: L. Young: None.
583
HbA1c and weight changes with semaglutide at 6- and 12-months post commencement: updated results from the ABCD semaglutide audit
B.C.T. Field1, T.S.J. Crabtree2, K. Adamson3, D. Barnes4, S. Sivappriyan4, A. Bickerton5, A. Gallagher6, I.W. Gallen7, I. Idris8, R.E.J. Ryder9;
1Faculty of Medical and Health Sciences, University of Surrey, Guildford, 2University of Nottingham, Derby, 3St John's Hospital, Livingston, 4Maidstone & Tunbridge Wells NHS Trust, Kent, 5Yeovil District Hospital NHS Trust, Yeovil, 6University Hospitals of Leicester NHS Trust, Leicester, 7Royal Berkshire Hospitals NHS Trust, Reading, 8Diabetes & Endocrinology, Royal Derby Hospital, University Hospitals of Derby and Burton NHS Trust, UK, Derby, 9Sandwell & West Birmingham Hospitals NHS Trust, Birmingham, UK.
Background and aims: Real-world outcomes derived from the previous UK nationwide ABCD glucagon-like peptide 1 receptor agonist (GLP1RA) Semaglutide audit have demonstrated significant HbA1c and weight improvements at first follow-up. Notably, individuals from this audit have higher HbA1c levels and weights than those recruited from randomised controlled trials, and hence more representative of routine clinical practice. We present updated analysis to report changes observed up to one year after commencing semaglutide.
Materials and methods: Data were extracted from the ABCD semaglutide audit and included relevant baseline and at least one set of follow-up data that had been recorded. Data were analysed at 6 and 12 months. Changes from baseline for HbA1c and weight were assessed using paired t-test and ANOVA between groups in Stata 16 SE. Additional stratified analyses by previous GLP1RA exposure were performed.
Results: Data for 1,652 individuals were extracted of which 797 had sufficient data for inclusion. Those included had mean±SD for: age 59.2±10.6 years; HbA1c 78.4±18.7mmol/mol (9.3±1.7%); weight 107.3±23.2kg; BMI 37.3±7.3kg/m2 and eGFR 87±26mL/min/1.73m2. Median diabetes duration was 10.9 years (IQR 6.4-14.9). 49.8% were male. HbA1c was reduced by 13.3mmol/mol (96% CI 11.0-14.7, p<0.0001) at 6-months and remained 11.2mmol/mol lower than baseline (95% CI 9.1-13.4, p<0.0001) at 12-months. No significant difference was noted in HbA1c between 6-months and 12-months (p=0.47). Among GLP1-RA naïve individuals, HbA1c reductions were 6.1mmol/mol (95% CI 3.0-9.1, p<0.001) greater at 6-months and 4.9mmol/mol (95%CI 0.3-9.4, p=0.036) greater by 12-months compared to those previously treated with an alternative GLP1RA. Compared with baseline, significant weight reduction of 4.0kg (95% CI 3.5-4.5,p<0.0001) and 5.4kg (95% CI 4.1-6.7, p<0.0001) was observed at 6 and 12-months respectively. Where paired results were available, further weight reductions occurred between 6 and 12-months by 2.4kg (95% CI 1.4-3.34, p<0.0001). In the GLP1RA naïve, weight reductions were 2.2kg (95% CI 1.1-3.3, p<0.0001) greater at 6-months compared to those switched from an alternative GLP1RA drug. No statistical significance was noted between these groups at 12-months (p=0.07).
Conclusion: Our observational data suggest that HbA1c reductions associated with semaglutide observed in the first 6-months persist at one year. Weight appears to continue to be lost beyond the initial 6-month period. Although weight and HbA1c changes are smaller in those switched from alternative GLP1RA therapies to semaglutide at 6-months, for weight these differences diminish out to 12-months. Reasons for this are unclear and ongoing work will be needed to clarify this observation.
Supported by: The ABCD audits is supported by an unrestricted grant from NovoNordisk
Disclosure: B.C.T. Field: Honorarium; Abbott Diabetes, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GSK, Janssen, Medtronic, MSD, Napp, Novo Nordisk and Sanofi.
584
Costs associated with healthcare resources utilisation among empagliflozin vs GLP-1RAs initiators in routine clinical care in Denmark
R.W. Thomsen1, L.W.B. Christensen1, J. Kahlert1, J.S. Knudsen1, A. Ustyugova2, S. Sandgaard3, P. Holmgaard3, L.H. Ehlers4, H.T. Sørensen1;
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark, 2Boehringer Ingelheim International GmbH, Ingelheim, Germany, 3Boehringer Ingelheim A/S, Copenhagen, Denmark, 4Nordic Institute of Health Economics, Aarhus, Denmark.
Background and aims: The sodium-glucose cotransporter 2 inhibitor (SGLT2i) empagliflozin has shown reductions in major adverse cardiac events (MACE) similar to glucagon-like peptide-1 receptor agonists (GLP-1RAs) in routine care in Denmark. There is limited evidence how these therapies compare regarding overall costs associated with healthcare resources utilization. We aimed to assess the total average healthcare costs per patient after drug initiation with empagliflozin versus GLP-1RAs in Denmark.
Materials and methods: Nationwide population-based comparative cohort study based on linked prospective healthcare databases for the entire population in Denmark (current population 5.8 million) during Jan 1, 2015, and to Dec 31, 2018. We included 13,249 new users of empagliflozin and 13,747 new users of GLP-1RAs. Propensity scores were applied to balance potential confounders across the two treatment groups through inverse probability treatment weighting (IPTW). We assessed total average costs per year associated with all healthcare resource utilization (inpatient, emergency room, and outpatient clinic hospital care, primary care health services, and prescription medications costs at pharmacies) among drug initiators while on-treatment (i.e., while covered by filled drug prescriptions including a grace period of 60 days).
Results: The two IPTW cohorts were well-balanced at baseline (median age 61 years, 60% men, diabetes duration of 6.7 years, 19% with pre-existing ischemic heart disease, 8% with history of cerebrovascular disease). Healthcare costs in the year prior to drug initiation were also well-balanced. Mean follow-up on-treatment after initiation was 11.3 months (SD 9.8 months) in empagliflozin vs 12.3 (SD 10.9) months in GLP-1 RA initiators, with a total of 12,657 years of drug exposure with empagliflozin and 13,554 years with GLP-1RAs. Average costs per patient per year were very similar in empagliflozin and GLP-1 RA initiators regarding inpatient hospitalizations (13,565 DKK versus 13,275 DKK), hospital outpatient clinic visits (12,007 DKK versus 12,152 DKK), emergency room visits (370 DKK versus 399 DKK), and primary care services (4,108 DKK versus 4,302 DKK). Total costs of any prescription drugs were lower in empagliflozin initiators (8,946 DKK versus 14,029 DKK). Total healthcare costs on-treatment were lower in empagliflozin initiators at 38,995 DKK per person-year than in GLP-1RA initiators at 44,157 DKK per person-year, respectively.
Conclusion: In this nationwide population-based study, average healthcare costs after drug initiation and while on treatment were lower for empagliflozin than GLP-1RAs initiators, driven by lower costs of prescription drugs.
Clinical Trial Registration Number: NCT03993132
Supported by: The study was a NIS study sponsored by Boehringer Ingelheim in collaboration with Aarhus University
Disclosure: R.W. Thomsen: Grants; The study was a NIS study sponsored by Boehringer Ingelheim in collaboration with Aarhus University.
SO 43 Glucose lowering agents
585
Achievement of HbA1c ≤6.5% (47.5 mmol/mol), with ≥10% weight loss, without hypoglycaemia in patients treated with tirzepatide vs comparators in SURPASS program
J.A. Levine1, A. Cheng2, P. Choudhary3, I. Lingvay4, E. Gomez Valderas1, S.E. Allen1, K. Ranta1, V.T. Thieu1;
1Eli Lilly and Company, Indianapolis, USA, 2Department of Medicine, St Michael's Hospital, Toronto, Canada/, Department of Medicine, University of Toronto, Toronto, Canada/, Credit Valley Hospital, Mississauga, Canada, 3Diabetes Research Centre, University of Leicester, Leicester, UK, 4Department of Internal Medicine/Endocrinology and Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, USA.
Background and aims: In the phase 3 SURPASS studies tirzepatide (TZP), a novel dual GIP/GLP-1 receptor agonist in development for type 2 diabetes (T2D) treatment, 66-86% of TZP-treated participants achieved a HbA1c ≤6.5% (47.5 mmol/mol) and 21-69% achieved ≥10% weight loss. Tirzepatide showed a similar safety profile to that of GLP-1 receptor agonists. The purpose of this post-hoc analysis was to compare the percentage of patients who achieved a composite endpoint of HbA1c ≤6.5% (47.5 mmol/mol) with ≥10% weight loss without hypoglycemia in the SURPASS studies.
Materials and methods: We compared the proportion of participants achieving the triple endpoint between the tirzepatide (5, 10, or 15 mg) and respective comparator groups using the efficacy analysis dataset while patients are on treatment and without rescue medication. End of treatment HbA1c and weight were evaluated at week 40 (SURPASS-1, 2, 5) and week 52 (SURPASS-3, 4). Hypoglycemia included blood glucose level <54 mg/dL or severe hypoglycemia. Missing endpoint measures were imputed by predictions using observed data in the efficacy analysis set from the same treatment group.
Results: More participants treated with any dose of TZP achieved the triple endpoint compared to placebo or active comparators in SURPASS 1-5 (FIG). As monotherapy, TZP treatment (5, 10, 15 mg) led to 25%, 36%, 44% of participants achieving the triple endpoint compared to 0% with placebo in SURPASS-1. As add-on to metformin, TZP treatment (5, 10, 15 mg) led to 33%, 50%, 59% achieving the same compared to 22% with semaglutide 1 mg in SURPASS-2. When compared to basal insulin, TZP treatment (5, 10, 15 mg) led to 33%, 50%, 64% achieving the triple endpoint compared to 3% with degludec (SURPASS-3; add-on to metformin) and when added to 1-3 oral antihyperglycemics, TZP treatment (5, 10, 15 mg) led to 28%, 44%, 53% achieving the triple endpoint compared to 1% with glargine U100 (SURPASS-4). Finally, as add-on to basal insulin, TZP treatment (5, 10, 15 mg) led to 16%, 35%, 40% achieving the triple endpoint compared to 1% with placebo in SURPASS-5.
Conclusion: Significantly more participants treated with TZP achieved a HbA1c ≤6.5% (47.5 mmol/mol) with ≥10% weight loss without hypoglycemia than those treated with placebo, semaglutide, or basal insulin in SURPASS-1, 2, 3, 4, and 5 studies.
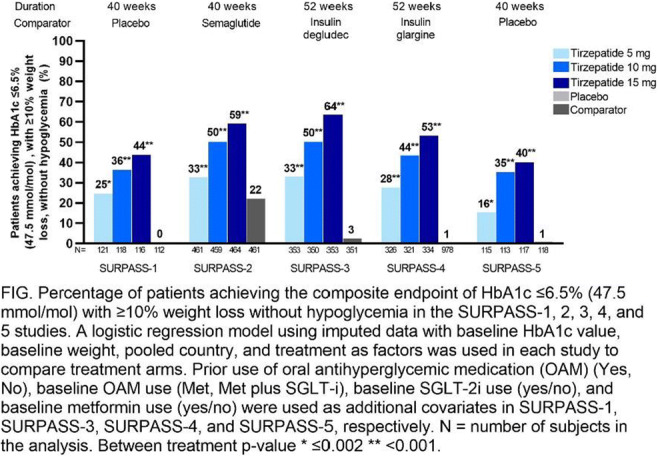
Clinical Trial Registration Number: NCT03954834/NCT03987919/NCT03882970/NCT03730662/NCT04039503
Supported by: Eli Lilly and Company
Disclosure: J.A. Levine: Employment/Consultancy; Joshua A. Levine is an employee and shareholder of Eli Lilly and Company.
586
Tirzepatide leads to significant improvement in postprandial glycaemic control in people with type 2 diabetes (SURPASS 1-5)
C.J. Lee1, E. Spanakis2, R. Bray1, K. Brown1, L.K. Billings3, K. Chivukula1, A. Rodriguez1, F. Giorgino4;
1Eli Lilly and Company, Indianapolis, USA, 2University of Maryland School of Medicine, Baltimore, USA, 3Department of Medicine, NorthShore University HealthSystem/University of Chicago Pritzker School of Medicine, Skokie, USA, 4University of Bari Aldo Moro, Bari, Italy.
Background and aims: In people with type 2 diabetes (T2D), tirzepatide led to greater reductions in HbA1c, fasting blood glucose, and body weight versus active comparator or placebo in Phase 3 trials (SURPASS 1-5) and a higher percentage of Time in Range (3.9-10 mmol/L) (TIR) in a subpopulation of patients in SURPASS-3 with continuous glucose monitoring (CGM) data on tirzepatide vs insulin degludec. Tirzepatide showed a similar safety profile to that of GLP-1 receptor agonists. In this post hoc analysis, we aimed to assess the change in postprandial glucose excursion with tirzepatide vs. comparators.
Materials and methods: Self monitored blood glucose (SMBG) data were collected at baseline and at primary end point for patients randomized across SURPASS 1-5 (baseline characteristics; mean age 54.1-63.6 yrs, duration of T2D 4.7-13.3 yrs, HbA1c 7.9-8.5%/63.3-69.7 mmol/mol, bodyweight 85.9-95.2 kg, BMI 31.9-34.2 kg/m2). For tirzepatide groups vs. comparator, we compared the change from baseline in pre-meal to 2-hour postmeal excursion daily mean and the proportion of patients achieving 2-hour postprandial SMBG target goal of <10 mmol/L and euglycemic goal of <7.8 mmol/L. From the SURPASS-3 CGM substudy of 179 patients, postprandial interstitial glucose (IG) data were identified based on mealtimes recorded during the 7-days of CGM wear at baseline and Week 52. Proportion of patients achieving 2-hour postprandial IG in TIR (3.9-10 mmol/L) and Time in Tight Range (3.9-7.8 mmol/L) (TITR) across all meals were calculated.
Results: Compared to active comparator or placebo, all three doses of tirzepatide led to significant improvement in postprandial glucose excursion (FIG A). All three doses also led to a significantly higher proportion of patients at primary end point achieving 2-hour postprandial SMBG of <10 mmol/L (91.9-99.4% tirzepatide vs 61.7-91.2% comparators, p≤0.019 for all comparisons except tirzepatide 5 mg vs semaglutide 1 mg in SURPASS-2 [96.3% tirzepatide vs 94.9% semaglutide, p=0.276]) and SMBG <7.8 mmol/L (64.6-89.7% tirzepatide vs 12.6-62.1% comparator, p≤0.011) (FIG B). Similarly, for the SURPASS-3 subgroup who completed CGM, all three doses of tirzepatide led to a significantly higher proportion of patients achieving 2-hour postprandial IG in TIR (76.3-91.0% tirzepatide vs 63.3% insulin degludec, p≤0.017) and TITR (48.6-69.4% tirzepatide vs 35.7% insulin degludec, p=0.041) at Week 52.
Conclusion: Tirzepatide treatment led to a significant improvement in postprandial glycemic control with greater proportions of patients achieving target (<10 mmol/L) and euglycemic (<7.8 mmol/L) goals compared to placebo or active comparator in people with T2D.
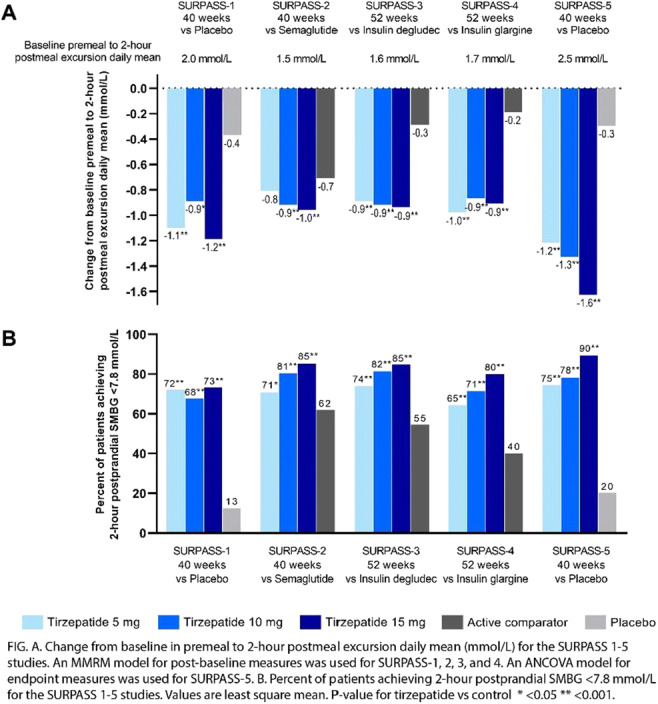
Clinical Trial Registration Number: NCT03954834/NCT03987919/NCT03882970/NCT03730662/NCT04039503
Supported by: Eli Lilly and Company
Disclosure: C.J. Lee: Employment/Consultancy; Clare J. Lee is an employee and shareholder of Eli Lilly and Company.
587
Results from a 12-week proof-of-concept study of a novel oral non-peptide GLP-1 receptor agonist in patients with type 2 diabetes
E. Pratt1, X. Ma2, R. Liu2, D. Robins2, K. Sloop2, A. Haupt2, C. Benson2;
1Lilly Centre for Clinical Pharmacology Pte Ltd, Singapore, Singapore, 2Eli Lilly and Company, Indianapolis, USA.
Background and aims: GLP-1 is an established target for T2D and obesity; however, current treatment options are limited to injectable therapies or oral peptides which require a fasting state to improve bioavailability. LY3502970 (LY) is a novel, highly potent, orally bioavailable non-peptide GLP-1 receptor agonist being developed as treatment for adults with T2D.
Materials and methods: This was a double-blind, placebo-controlled phase 1 study with 5 cohorts. The first cohort established that weekly dose escalation of the daily doses of LY were well tolerated. This enabled a parallel-arm design for the following three cohorts. Patients were randomized 3:1 to multiple daily doses of LY or placebo for 12 weeks. This enabled a parallel design for the following three dose levels. Eligible patients were 18-70 years of age with diagnosis of T2D for ≥6 months and HbA1c ≥7.0% and ≤10.5%.
Results: A total of 51 patients received LY and 17 received placebo. Baseline characteristics were generally balanced between groups. The most commonly reported treatment-emergent adverse events among LY-treated patients were nausea (47.1%), decreased appetite (45.1%), and vomiting (43.1%); these occurred early in the treatment paradigm and decreased over time. One patient reported 2 severe gastrointestinal events (1 each of nausea and vomiting); all other events were mild or moderate. No clinically relevant liver abnormalities or LY-related serious adverse events were reported. Across dose levels on Day 84, Cmax ranged from 60 to 236 ng/mL; median tmax was 4 to 8 hours. On Day 84, mean change from baseline ranged from -1.5% to -1.8% across LY dose levels vs -0.4% with placebo for HbA1c, and -1.6 to -5.0 kg across LY doses vs 0.5 kg with placebo for body weight.
Conclusion: Treatment with the novel oral non-peptide GLP-1 receptor agonist LY showed a safety profile comparable to injectable agents, with robust glucose lowering and substantial reductions in body weight by pharmacokinetics that allow once-daily dosing. These data support further clinical development.
Clinical Trial Registration Number: NCT04426474
Disclosure: E. Pratt: Employment/Consultancy; Lilly Centre for Clinical Pharmacology Pte Ltd.
588
Efficacy, safety and tolerability of danuglipron (PF-06882961) over 12 weeks in adults with type 2 diabetes
D.N. Gorman1, A.R. Saxena2, J. Frias3, R.N. Lopez4, N. Tsamandouras2, M.J. Birnbaum2;
1Pfizer Worldwide Research and Development, Cambridge, UK, 2Pfizer Worldwide Research, Development, and Medical, Cambridge, USA, 3Velocity Clinical Research, Los Angeles, USA, 4Pfizer Inc, Groton, USA.
Background and aims: Danuglipron (PF-06882961) is an oral small molecule glucagon-like peptide-1 receptor (GLP-1R) agonist. This Phase 2a, randomised, double-blind, placebo-controlled, parallel-group study assessed the tolerability, safety and pharmacodynamic effects over 12 weeks of different titration schemes of danuglipron in adults with type 2 diabetes mellitus (T2DM) treated with metformin.
Materials and methods: Participants with T2DM (n=123) were randomised to placebo or 1 of 5 danuglipron titration schemes (to 80, 120 or 200 mg BID, with titration steps of 1 or 2 weeks). Participants with obesity without T2DM (n=28) were enrolled to placebo or danuglipron titration to 200 mg BID for comparison.
Results: In participants with T2DM, robust declines in HbA1c, fasting plasma glucose (FPG) and body weight were observed at multiple dose levels of danuglipron at 12 weeks (Table). The majority of adverse events were mild, with nausea, vomiting and diarrhoea most commonly reported, and a higher incidence of nausea and vomiting at higher doses. In participants with obesity without T2DM, observed weight loss was greater on danuglipron vs placebo; rates of nausea and vomiting were higher vs T2DM. No clinically significant, adverse trends in laboratory measures, electrocardiogram or vital sign abnormalities were apparent.
Conclusion: In adults with T2DM, danuglipron robustly reduced HbA1c, FPG and body weight over 12 weeks. In adults with obesity without T2DM, treatment with danuglipron resulted in greater reductions in body weight vs placebo. In both populations, danuglipron was safe, with a tolerability profile of danuglipron consistent with the mechanism of action.
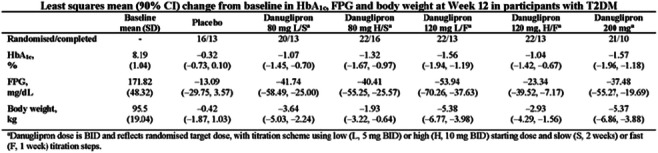
Clinical Trial Registration Number: NCT04617275
Supported by: Sponsored by Pfizer Inc.
Disclosure: D.N. Gorman: Employment/Consultancy; Pfizer Inc. Stock/Shareholding; Pfizer Inc.
589
Oral small molecule GLP-1 receptor agonist danuglipron (PF-06882961) results in glucose lowering and body weight loss over 16 weeks in adults with type 2 diabetes
A.R. Saxena1, J. Frias2, L.S. Brown3, D.N. Gorman4, N. Tsamandouras1, M.J. Birnbaum1;
1Pfizer Worldwide Research, Development, and Medical, Cambridge, USA, 2Velocity Clinical Research, Los Angeles, USA, 3Pfizer Worldwide Research and Development, Collegeville, USA, 4Pfizer Worldwide Research and Development, Cambridge, UK.
Background and aims: Danuglipron (PF-06882961) is an oral small molecule glucagon-like peptide-1 receptor (GLP-1R) agonist with efficacy in nonclinical models comparable to injectable peptidic GLP-1R agonists. This proof of concept, Phase 2b, multicentre, randomised, double-blind, placebo-controlled, parallel-group, dose-ranging study examined the effect of danuglipron on efficacy, safety, tolerability and pharmacokinetics over 16 weeks in adults with type 2 diabetes mellitus (T2DM).
Materials and methods: A total of 411 participants with T2DM (91% on metformin background therapy) were randomised and dosed across 6 arms (placebo or 1 of 5 dose levels of danuglipron); 316 (77%) completed double-blind treatment. HbA1c, fasting plasma glucose (FPG) and body weight were assessed at all clinic visits over 16 weeks.
Results: In adult participants with T2DM, lowering of HbA1c, FPG and body weight at Week 16 was demonstrated at multiple danuglipron dose levels (Table). The majority of adverse events were mild, with nausea, diarrhoea and vomiting most commonly reported. No episodes of severe hypoglycaemia were reported. No clinically significant, adverse trends in laboratory measures, electrocardiogram or vital sign abnormalities were apparent.
Conclusion: In adults with T2DM, danuglipron robustly reduced HbA1c, FPG and body weight over 16 weeks, and was safe, with a tolerability profile consistent with the mechanism of action.
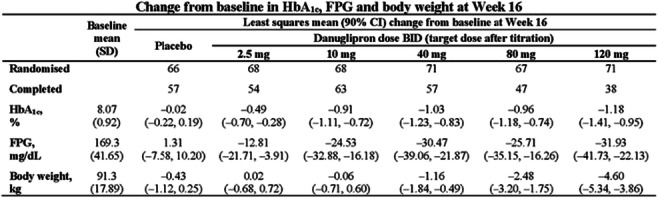
Clinical Trial Registration Number: NCT03985293
Supported by: Sponsored by Pfizer Inc.
Disclosure: A.R. Saxena: Employment/Consultancy; Pfizer Inc. Stock/Shareholding; Pfizer Inc.
590
Oral menin inhibitor, BMF-219, displays a significant and durable reduction in HbA1c in a type 2 diabetes rat model
P. Somanath, S. Mourya, W. Li, T. Archer, B. Law, D. Lu, T. Rughwani, L. Kumar, T. Kinoshita, M. Balakrishnan, T. Butler;
Biomea Fusion, Inc., Redwood City, USA.
Background and aims: Menin is an epigenetic regulatory protein that plays a key role in beta-cell proliferation and function, as previously demonstrated though increased beta-cell mass generation in Men1 knockout mice. The menin-MLL interaction also plays a major role in suppressing islet cell growth through control of cell cycle inhibitor expression. Here, we demonstrate the marked potential of an oral menin inhibitor, BMF-219, in durable glycemic control following a short course treatment in a Type 2 Diabetes Mellitus (T2DM) Zucker Diabetic Fatty (ZDF) Rat model.
Materials and methods: Rats were treated daily with BMF-219, liraglutide or vehicle for 28 days and monitored for an additional 28 days post-treatment for fasting and non-fasting blood glucose levels, HbA1C levels, insulin and c-peptide levels, HOMA-IR and HOMA-B quantitation and oral glucose tolerance test (OGTT).
Results: All animals tolerated BMF-219 well throughout the study. Notably, BMF-219 treatment resulted in a significant reduction in HbA1C at Day 21, which reached 3.5% absolute reduction in HbA1C versus vehicle (p<0.0001), compared to liraglutide (1.7% at Day 29, p<0.05) and remained reduced throughout the entire study, including post-treatment. The high-dose arm of BMF-219 showed a strong reduction in 4-hour fasting blood glucose during the treatment up to Day 29 (p<0.0001). Both BMF-219 dose groups showed improved glycemic control by OGTT on day 25, whereas vehicle and liraglutide-treated animals continued to show high glucose levels. Additionally, insulin levels, HOMA-IR, HOMA-B, OGTT, HbA1C, and C-peptide levels measured at Day 57 across all groups will be reported.
Conclusion: Collectively, our data demonstrate the novel long-acting potential of menin inhibitor, BMF-219, as an oral treatment for T2DM, in maintaining glycemic control after short-term dosing.
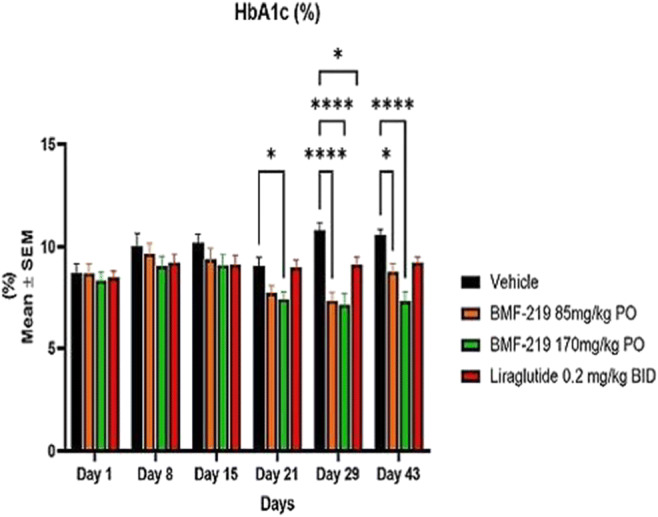
Disclosure: P. Somanath: Employment/Consultancy; Biomea Fusion, Inc.. Stock/Shareholding; Biomea Fusion, Inc..
591
Patients with type 2 diabetes reach glycaemic targets faster with tirzepatide compared to semaglutide and titrated insulin degludec
A. Viljoen1, K.M. Pantalone2, R.J. Galindo3, X. Cui4, R. Huh4, L. Fernández Landó4, H. Patel4;
1Department of Metabolic Medicine/Chemical Pathology, North & East Hertfordshire Hospitals Trust, Stevenage, UK, 2Endocrinology and Metabolism Institute, Cleveland Clinic, Cleveland, USA, 3Division of Endocrinology, Emory University School of Medicine, Atlanta, USA, 4Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide (TZP) is a novel dual GIP/GLP-1 receptor agonist in development for treatment of type 2 diabetes (T2D). TZP 5 mg, 10 mg, and 15 mg demonstrated superiority versus semaglutide 1 mg (SEMA) and titrated insulin degludec (iDeg) in HbA1c change from baseline and proportion of patients reaching HbA1c <53 mmol/mol (7%), ≤48 mmol/mol (6.5%), and <39 mmol/mol (5.7%) at 40-weeks (SURPASS-2) and 52-weeks (SURPASS-3), respectively.
Materials and methods: In SURPASS-2 and 3, TZP was initiated at 2.5 mg once weekly and increased by 2.5 mg every 4 weeks until the assigned dose was reached and maintained for the duration of the study. Semaglutide was initiated at 0.25 mg once weekly and the dose doubled every 4 weeks until 1 mg was reached and maintained for the duration of the study. Insulin degludec was initiated at 10 U/day and titrated weekly to a fasting blood glucose of <5.0 mmol/L following a treat-to-target algorithm. In an exploratory pre-planned analysis, time to achieve glycaemic targets was estimated using Kaplan Meier method and hazard ratio between treatment was calculated using cox proportional-hazards model.
Results: TZP was significantly faster than SEMA and iDeg in time to reach HbA1c <53 mmol/mol (7%) and ≤48 mmol/mol (6.5%) (Table). Median time to achieve HbA1c <53 mmol/mol (7%) was 8.1 weeks for all TZP doses versus 12.0 weeks for SEMA, and to reach ≤48 mmol/mol (6.5%) was 12.1 weeks versus 15.7 weeks, respectively. Additionally, TZP was significantly faster than SEMA in time to lose 5% body weight (Table). Consistently, median time to reach HbA1c <53 mmol/mol (7%) was 8.1 weeks for all TZP doses versus 12.1 weeks for iDeg, and to reach ≤48 mmol/mol (6.5%) was 12.1 weeks versus 24.1 weeks, respectively. Mild to moderate gastrointestinal adverse events were associated with TZP and primarily occurred during the dose escalation period.
Conclusion: In conclusion, patients with T2D reached glycaemic targets faster with TZP compared to SEMA 1 mg and titrated iDeg.
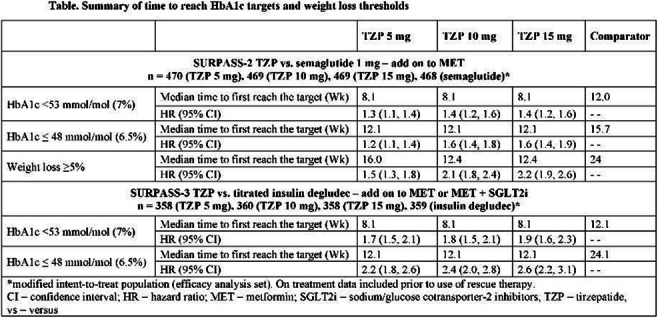
Disclosure: A. Viljoen: Grants; Sanofi. Lecture/other fees; Novartis, Boehringer Ingelheim, Napp, Non-financial support; Lilly, Novo Nordisk, AstraZeneca.
592
Oxyntomodulin analog LY3305677 (LY) improves glycaemic control and weight loss in healthy volunteers and subjects with type 2 diabetes
C. Benson1, L. Tham2, Y. Du1, S. Gurbuz1, K.J. Mather1, C. Tang1, D.A. Robins1, M.K. Thomas1;
1Eli Lilly and Company, Indianapolis, USA, 2Lilly Centre for Clinical Pharmacology, Singapore, Singapore.
Background and aims: Oxyntomodulin Analog LY3305677 (LY) is an acylated peptide analog of oxyntomodulin, a dual glucagon and GLP-1 receptor agonist. We conducted 2 randomised, double-blind, phase 1 multiple ascending subcutaneous dosing studies in healthy subjects (S1) and in type 2 diabetes (T2D) patients (S2) to evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics.
Materials and methods: In S1, 4 cohorts of healthy subjects received placebo or LY once-weekly (QW) for 4 weeks. In S2, T2D subjects received placebo or LY QW in 2 cohorts with doses escalated over 12 or 16 weeks. A total of 54 subjects completed S1 and 24 patients completed S2.
Results: The most common treatment-emergent adverse events in both studies (decreased appetite, nausea, and diarrhoea) were mostly mild in severity, and no deaths or serious adverse events were reported. Pharmacokinetic results were consistent between healthy subjects and T2D patients and supported weekly dosing. In healthy subjects, fasting glucose levels were decreased from baseline with increasing LY doses, and reductions in mean body weight from baseline were generally seen with increasing doses, with the largest change observed on Day 29. In T2D subjects during weeks 12-16, preliminary results of mean HbA1c changes from baseline ranged from -17.1 mmol/mol to -23.6 mmol/mol (-1.56% to -2.16%) in LY-treated vs -4.7 mmol/mol to -7.7 mmol/mol (-0.43% to -0.70%) in placebo subjects, and mean body weight changes from baseline ranged from -2.30 kg to -11.24 kg in LY-treated vs -0.35 kg to -2.03 kg in placebo subjects. Fasting glucose, triglycerides, cholesterol, and glucagon levels were decreased from baseline with LY vs placebo at weeks 12-16.
Conclusion: We conclude that LY was well tolerated in both studies, with a safety profile similar to selective GLP-1 receptor agonists. LY has a promising pharmacodynamic profile with potential for therapeutic benefit in T2D, obesity, or other metabolic diseases.
Clinical Trial Registration Number: NCT03928379
Supported by: Eli Lilly and Company
Disclosure: C. Benson: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
SO 44 Lessons from trials
593
Finerenone in patients across the spectrum of CKD and type 2 diabetes by GLP-1RA use
M.L. Caramori1, P. Rossing2, S. Anker3, G. Filippatos4, B. Pitt5, V. Fonseca6, G.E. Umpierrez7, M. Lambelet8, P. Viswanathan9, R. Lawatscheck10, A. Joseph11, G. Bakris12;
1Department of Medicine and Department of Pediatrics, University of Minnesota, Minneapolis, USA, 2Steno Diabetes Center Copenhagen, Copenhagen, Denmark, 3Department of Cardiology and Berlin Institute of Health Center for Regenerative Therapies, Charité Universitätsmedizin, Berlin, Germany, 4Department of Cardiology, National and Kapodistrian University of Athens, Athens, Greece, 5Department of Medicine, University of Michigan School of Medicine, Ann Arbor, USA, 6Tulane University Health Sciences Center, New Orleans, USA, 7Emory University, Atlanta, USA, 8Chrestos Concept GmbH & Co. KG, Essen, Germany, 9Research & Development, Clinical Development & Operations, Bayer U.S. LLC, Whippany, USA, 10Medical Affairs & Pharmacovigilance, Bayer AG, Berlin, Germany, 11Research and Development, Clinical Development Operations, Bayer AG, Wuppertal, Germany, 12Department of Medicine, University of Chicago Medicine, Chicago, USA.
Background and aims: In FIDELITY, a prespecified pooled analysis of the FIDELIO-DKD and FIGARO-DKD trials, finerenone reduced the risk of cardiorenal outcomes in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D). In FIDELIO-DKD, the effects of finerenone were consistent irrespective of glucagon-like peptide-1 receptor agonist (GLP-1RA) use. This FIDELITY post hoc analysis explores outcomes in a larger CKD population with broader inclusion criteria.
Materials and methods: Patients with T2D and CKD (urine albumin-to-creatinine ratio [UACR] ≥30-<300 mg/g and estimated glomerular filtration rate [eGFR] ≥25-≤90 ml/min/1.73 m2 or UACR ≥300-≤5000 mg/g and eGFR ≥25 ml/min/1.73 m2), treated with optimised renin-angiotensin system blockade were randomised to finerenone or placebo. The efficacy of finerenone on cardiovascular (CV; CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalisation for heart failure) and kidney (kidney failure, sustained ≥57% eGFR decline, or renal death) composite outcomes, and on UACR at month 4, were analysed by GLP-1RA use.
Results: Of 13,026 patients, 944 (7.2%) received a GLP-1RA at baseline. The beneficial effects of finerenone were consistent irrespective of baseline GLP-1RA use on CV (hazard ratio [HR] 0.76; 95% confidence interval [CI] 0.52-1.11 with GLP-1RA; HR 0.87, 95% CI 0.79-0.96 without GLP-1RA; p-interaction=0.63) and kidney (HR 0.82; 95% CI 0.45-1.48 with GLP-1RA; HR 0.77, 95% CI 0.67-0.89 without GLP-1RA; p-interaction=0.79) composite outcomes. The reduction in UACR with finerenone was greater among patients with baseline GLP-1RA use (placebo-corrected change -38% with GLP-1RA; -31% without GLP-1RA use; p-interaction=0.03). Irrespective of baseline GLP-1RA use, finerenone increased the incidence of hyperkalaemia, however the incidence of hyperkalaemia-related permanent discontinuation was low.
Conclusion: The cardiorenal benefits of finerenone on composite CV and kidney outcomes in patients with CKD and T2D were not modified by baseline GLP-1RA use, with an augmented effect observed for UACR reduction with GLP-1RA use at baseline.
Clinical Trial Registration Number: FIDELIO-DKD (NCT02540993) and FIGARO-DKD (NCT02545049)
Supported by: BayerAG supported this analysis, who funded the FIDELIO-DKD & FIGARO-DKD studies & combined analysis
Disclosure: M.L. Caramori: Grants; grants and personal fees from Bayer and grants from Novartis, all these paid to her institution. She has received personal fees from Boheringer Ingelhein and AstraZeneca.
594
Effect of semaglutide versus placebo on cardiovascular outcomes by baseline HbA 1c : SUSTAIN 6 and PIONEER 6 post hoc analysis
L.G. Mellbin1, D.L. Bhatt2, J.-P. David3, M.C. Petrie4, S. Rasmussen3, P.A. Schytz3, T. Vilsbøll5;
1Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden, 2Brigham and Women’s Hospital, Harvard Medical School, USA, 3Novo Nordisk A/S, Søborg, Denmark, 4University of Glasgow, Glasgow, UK, 5Steno Diabetes Center Copenhagen, Herlev, Denmark.
Background and aims: Cardiovascular (CV) outcome trials have demonstrated CV benefits with once-weekly semaglutide vs placebo (SUSTAIN 6), and non-inferiority for reduction in major adverse CV events (MACE) with oral semaglutide vs placebo (PIONEER 6) in people with type 2 diabetes (T2D) at high CV risk. Given the recommended use of glucagon-like peptide-1 receptor agonists in people with T2D and CV risk regardless of HbA1c values, the potential for HbA1c levels to modify treatment effect on CV outcomes is of interest. This post hoc analysis of SUSTAIN 6 and PIONEER 6 evaluated the treatment effect of semaglutide vs placebo on MACE by baseline HbA1c.
Materials and methods: Using pooled data from SUSTAIN 6 and PIONEER 6, MACE (a composite of death from CV causes, nonfatal myocardial infarction [MI] and nonfatal stroke) with semaglutide vs placebo was evaluated across baseline HbA1c. A quadratic spline function of baseline HbA1c by treatment was used to analyse treatment effect on time to first MACE across a continuum of baseline HbA1c values. MACE and its components were also compared between baseline HbA1c subgroups (<8;≥8% [<64;≥64 mmol/mol]; cut-off selected as close to the median). Risk of MACE was analysed using a Cox proportional hazards model, with treatment by baseline HbA1c subgroup as a fixed factor (unadjusted), adding key predictors of CV-renal disease at baseline as covariates (adjusted using inverse probability weighting). The test for heterogeneity in treatment effect across HbA1c subgroups was indicated by interaction p-values.
Results: HRs for risk of MACE favoured semaglutide vs placebo across a continuum of baseline HbA1c values (>6.5-<12.6% [>48-<114 mmol/mol]; Figure). When assessing individual MACE components by baseline HbA1c subgroups, interaction p-values were all >0.05, indicating no statistically significant difference in treatment effect on any CV outcome between baseline HbA1c subgroups (consistent between adjusted and unadjusted analyses). HRs for all CV outcomes were consistently <1.0 with semaglutide vs placebo. HRs in the adjusted analysis [95% CI] for MACE, death from CV causes, nonfatal MI and nonfatal stroke were 0.80 [0.57;1.11], 0.87 [0.49;1.56], 0.98 [0.60;1.59] and 0.52 [0.26;1.05], respectively, with baseline HbA1c <8%, and 0.72 [0.56;0.93], 0.70 [0.46;1.07], 0.83 [0.57;1.20] and 0.74 [0.44;1.22], respectively, with baseline HbA1c ≥8%.
Conclusion: The effect of semaglutide vs placebo on MACE and its components was consistent across baseline HbA1c in the SUSTAIN 6 and PIONEER 6 pooled T2D population. These data indicate that the beneficial CV effect of semaglutide is consistent regardless of HbA1c values.
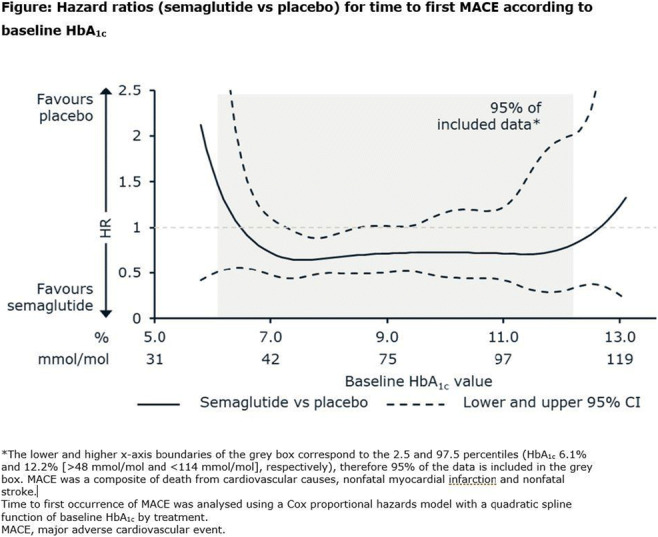
Clinical Trial Registration Number: NCT01720446; NCT02692716
Disclosure: L.G. Mellbin: Employment/Consultancy; No. Grants; No. Honorarium; Yes. Lecture/other fees; Yes. Non-financial support; No. Stock/Shareholding; No. Other; Principal Investigator in Clinical Trial.
595
Tirzepatide reduces serum triglyceride concentrations irrespective of concomitant fibrate use in SURPASS-4 participants with type 2 diabetes at high cardiovascular risk
T. Várkonyi1, S. Del Prato2, I. Pavo3, C. Nicolay3, R.J. Wiese3, S.E. Kahn4;
1University of Szeged, Szeged, Hungary, 2University of Pisa, Pisa, Italy, 3Eli Lilly and Company, Indianapolis, USA, 4University of Washington, Seattle, USA.
Background and aims: In the SURPASS-4 trial, participants with type 2 diabetes (T2D) inadequately controlled with 1-3 oral glucose-lowering medications and high cardiovascular (CV) risk (coronary heart disease, peripheral arterial disease, cerebrovascular disease, chronic kidney disease, or congestive heart failure) received the once weekly dual GIP/GLP-1 receptor agonist tirzepatide (TZP) or titrated insulin glargine (iGlar). In the study, TZP reduced serum triglycerides and low-density lipoproteins cholesterol (LDL-C) levels significantly more than iGlar. In this analysis we evaluated the effect of concomitant use of fibrates or statins on reductions in these lipid parameters.
Materials and methods: Participants (N=2,002) were randomized 1:1:1:3 to once weekly TZP (5, 10, 15 mg) or once daily iGlar. Lipoproteins were measured at baseline, 42, 52, (primary endpoint), 78 weeks and study end (median 85 weeks). This post-hoc analysis assessed percent change from baseline in lipids across subgroup categories in participants while on assigned treatment without rescue medication (efficacy estimand) using mixed-model repeated measures. Participants with baseline and at least 1 post-baseline measurement were included in the analysis.
Results: Overall, 12.4% and 77.8% of the participants were receiving fibrates and statins at baseline, respectively. Baseline triglyceride concentrations of participants receiving fibrates or not receiving fibrates were 2.59 mmol/L and 2.03 mmol/L, respectively, and for those receiving statins or not, they were 2.05 mmol/L and 2.26 mmol/L, respectively. Baseline LDL-C concentrations in participants receiving fibrates or not receiving fibrates were 2.13 mmol/L and 2.15 mmol/L, respectively and for participants receiving statins or not, they were 2.02 mmol/L and 2.59 mmol/L, respectively. At week 52, serum triglycerides and LDL-C were reduced by all 3 TZP doses, whether or not participants were receiving fibrates or statins at baseline (Table). These reductions were maintained until 78 weeks (N = 574 for TZP). TZP dose-dependently reduced serum triglycerides, with the reductions being similar in those on fibrates/statins or not. The marked reduction in triglycerides (14-24%) for TZP in the presence of fibrate therapy and no change for iGlar was clinically meaningful as the participants receiving fibrates still had elevated triglycerides at baseline. In general, TZP also reduced LDL-C more than IGlar. No differential treatment effects (TZP vs iGlar) on the reduction of triglycerides or LDL-C were observed for either fibrate or statin use.
Conclusion: In participants with T2D and high CV risk, TZP lowered triglycerides and LDL-C more effectively than iGlar, regardless of concomitant use of fibrates or statins.
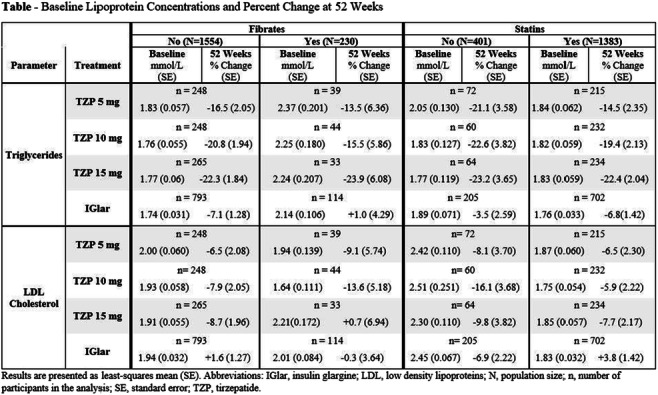
Clinical Trial Registration Number: NCT03730662
Supported by: Eli Lilly and Company
Disclosure: T. Várkonyi: None.
596
Anti-drug antibodies do not impact pharmacokinetics, efficacy, and safety of Tirzepatide: analysis of data from seven phase 3 studies
B. Calderon, G. Mullins, M.E. Hodsdon, Y. Li, G. Anglin, S. Urva, K.B. Schneck, J.N. Bardos, R. Fonseca Martins, K. Brown;
Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide (TZP) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide (GLP)-1 receptor agonist developed for the treatment of type 2 diabetes. Patients receiving TZP may develop an immune response to the 39 amino acid synthetic peptide. This study aimed to evaluate treatment emergent (TE) anti-drug antibodies (ADA) in TZP-treated participants across 7 Phase 3 trials and its potential effect on pharmacokinetics (PK), efficacy, and safety.
Materials and methods: A multi-tiered immunogenicity testing strategy was used to detect and characterize ADA. ADA were characterized for their ability to cross-react to native GIP (nGIP) and native GLP-1 (nGLP-1), neutralize TZP activity on GIP and GLP-1 receptors, and neutralize nGIP and nGLP-1. ADA were assessed at baseline and throughout the course of the study up to week 40 (SURPASS 1, 2, and 5) or week 52 (SURPASS 3, 4, Japan-mono, and Japan-combo).
Results: Among all ADA-evaluable patients across the Phase 3 trials (N=5025), TE ADA developed in 51.1% of patients treated with TZP, and the proportions were similar across the 3 TZP dose groups (5, 10, and 15 mg). At baseline, 7.0% of patients had pre-existing ADA. Maximum ADA titers ranged from 1:20 to 1:81920 (median 1:160) among TE ADA+ patients. Neutralizing antibodies (NAb) against TZP activity on GIP and GLP-1 receptors were observed in 1.9% and 2.1% of the TE ADA+ patients, respectively. Less than 1.0% of TZP-treated TE ADA+ patients had cross-reactive NAb against nGIP or nGLP-1. TE ADA status, ADA titer, and NAb, had no discernible impact on PK profile nor HbA1c effects of TZP. The percentage of TE ADA+ and TE ADA- patients with hypersensitivity reactions was similar. A higher proportion of TE ADA+ patients reported injection site reactions, of which all were nonserious and non-severe, and the vast majority were mild in severity and occurred and/or resolved irrespective of TE ADA status or titer level.
Conclusion: Overall, immunogenicity was not associated with any impact on TZP PK, efficacy, or safety.
Clinical Trial Registration Number: NCT03954834; NCT03987919; NCT03882970; NCT03730662; NCT04039503; NCT03861052; NCT03861039
Supported by: Eli Lilly and Company
Disclosure: B. Calderon: Employment/Consultancy; Employed by Eli Lilly and Company. Stock/Shareholding; Shareholder of Eli Lilly and Company.
597
Greater time spent in glycaemic control with oral semaglutide vs oral comparators
F.K. Knop1,2, B. Cariou3, J. Eliasson4, G. Frappin4, M.S. Kaltoft4, E. Montanya5, J. Rosenstock6;
1Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, 2Steno Diabetes Center Copenhagen, Gentofte, Denmark, 3Nantes Université, CHU Nantes, CNRS, Inserm, l’institut du thorax, Nantes, France, 4Novo Nordisk A/S, Søborg, Denmark, 5Hospital Universitari Bellvitge-IDIBELL, CIBERDEM, and University of Barcelona, Barcelona, Spain, 6Dallas Diabetes Research Center at Medical City, Dallas, USA.
Background and aims: This exploratory analysis aimed to determine how long patients spent with HbA1c <7.0% (53 mmol/mol), and how likely patients were to maintain this glycaemic target in PIONEER efficacy trials of ≥52 weeks’ duration.
Materials and methods: Patients with uncontrolled type 2 diabetes in the PIONEER 2, 3, 4 and 7 trials were randomised to oral semaglutide vs active comparators (empagliflozin 25 mg, sitagliptin 100 mg, liraglutide 1.8 mg once daily). Oral semaglutide dose was escalated, starting at 3 mg once daily and increasing to 7 mg after 4 weeks and then to 14 mg after 8 weeks in all trials except PIONEER 7, which used a flexible dose adjustment approach based on HbA1c and gastrointestinal tolerability. Empagliflozin was initiated at 10 mg and escalated to 25 mg after 8 weeks. Sitagliptin was initiated at 100 mg and not escalated. Liraglutide was initiated at 0.6 mg and escalated to 1.2 mg and then 1.8 mg after 1 and 2 weeks, respectively. Outcomes were evaluated for oral semaglutide vs active comparators using on-treatment without rescue medication data for all randomised patients. A binary endpoint of achieving HbA1c <7.0% (53 mmol/mol) at both week 26 and 52 of each trial (and at week 78 for PIONEER 3) was analysed using a logistic regression model, with treatment, region and strata as categorical fixed effects and baseline value as a covariate.
Results: Mean baseline HbA1c ranged from 8.0 to 8.3% (64-67 mmol/mol). The median duration of time spent with HbA1c <7.0% (53 mmol/mol) was greater with oral semaglutide (26.3-33.7 weeks) vs oral comparators (0-10.9 weeks) (Table). The mean duration of time spent with HbA1c <7.0% (53 mmol/mol) was also greater for oral semaglutide vs oral comparators (Table). The mean and median duration of time spent in glycaemic control with oral semaglutide was similar to that seen with liraglutide. Greater proportions of patients had HbA1c <7.0% (53 mmol/mol) for ≥38 weeks with oral semaglutide than with empagliflozin (46% vs 28%, respectively) and sitagliptin (PIONEER 3: 45% vs 28%, respectively; PIONEER 7: 27% vs 14%, respectively) but not liraglutide (46% vs 48%, respectively). The odds of patients achieving HbA1c <7.0% (53 mmol/mol) at both week 26 and 52 were significantly greater with oral semaglutide vs comparators (Table).
Conclusion: In PIONEER trials of ≥52 weeks, oral semaglutide resulted in greater time spent at glycaemic target and a greater likelihood of maintaining glycaemic control vs oral comparators. Patients receiving oral semaglutide spent a similar time in glycaemic control vs liraglutide, despite a longer dose-escalation with oral semaglutide.
Clinical Trial Registration Number: NCT02863328, NCT02607865, NCT02863419; NCT02849080
Supported by: Funded by Novo Nordisk A/S.
Disclosure: F.K. Knop: Employment/Consultancy; Carmot Therapeutics, Eli Lilly, Novo Nordisk, Zucara (all consultancy only). Grants; AstraZeneca, Boehringer Ingelheim, Gubra, Novo Nordisk, Sanofi, Zealand Pharma. Honorarium; AstraZeneca, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MSD/Merck, Mundipharma, Novo Nordisk, Sanofi, Zealand Pharma, Zucara. Lecture/other fees; AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Mundipharma, Novo Nordisk, Sanofi. Non-financial support; AstraZeneca, Mundipharma, Novo Nordisk, Sanofi. Stock/Shareholding; Antag Therapeutics.
598
Patient characteristics associated with achievement of normoglycaemia following treatment with tirzepatide in the SURPASS 1-4 trials
L. Vázquez1, J. Rosenstock2, S. Del Prato3, D. Reis Franco4, B. Dai5, G. Weerakkody5, L. Fernández-Landó5, B.K. Bergman5, A. Rodríguez5;
1Hospital Marqués de Valdecilla, Santander, Spain, 2Dallas Diabetes Research Center at Medical City, Dallas, USA, 3Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 4CPCLIN/DASA, Clinical Research Center, Sao Paulo, Brazil, 5Eli Lilly and Company, Indianapolis, USA.
Background and aims: Throughout the SURPASS program 23% to 62% of adults with type 2 diabetes (T2D) treated with tirzepatide, a novel dual GIP/GLP-1 receptor agonist, achieved an HbA1c value below the upper limit of the normal range <38.8 mmol/mol (<5.7%) at the primary endpoints. Here, we have performed exploratory analysis to identify baseline characteristics that could be associated with increased odds of achieving a normal HbA1c following treatment with tirzepatide in the SURPASS 1-4 clinical trials.
Materials and methods: Compliant patients (≥75% doses received), on treatment without rescue medication were included in the analyses (N=3229). Background medication at baseline included metformin only (63%), combination of oral anti-diabetic medication (OAM) (26%), and no treatment (9%). Logistic regression models with tirzepatide doses (5 mg, 10 mg, and 15 mg) adjusted as a covariate, were used to obtain odds ratios and assess the impact of individual baseline variables on achieving an HbA1c <38.8 mmol/mol (<5.7%). A multivariate logistic regression model was fitted to further assess the joint impact of these variables.
Results: Higher tirzepatide doses were significantly associated with higher odds of achieving an HbA1c <38.8 mmol/mol (<5.7%). Duration of diabetes, HbA1c, fasting serum glucose (FSG), eGFR and age showed significant associations when assessed by univariate logistic regression models adjusted by tirzepatide doses (Table). Odds of achieving an HbA1c <38.8 mmol/mol (<5.7%) at week 40 was reduced by 13% and 24% with a 5-year increase in age or diabetes duration, respectively. Similarly, a higher baseline HbA1c (10.93 mmol/mol, or 1% increase) and FSG (2.8 mmol/L increase) were associated with decreased odds of achieving an HbA1c <38.8 mmol/mol (42% and 24%, respectively). Additionally, patients receiving metformin-only had a greater chance of achieving this HbA1c target than those on 2 or more antihyperglycaemic medications (Table). A multivariate logistic regression model including age, duration of diabetes, background medication, baseline HbA1c and baseline weight demonstrated similar findings.
Conclusion: Patients with shorter duration of diabetes, receiving only metformin as background therapy were more likely to achieve an HbA1c <38.8 mmol/mol (<5.7%) when treated with tirzepatide in the SURPASS 1-4 clinical trials.
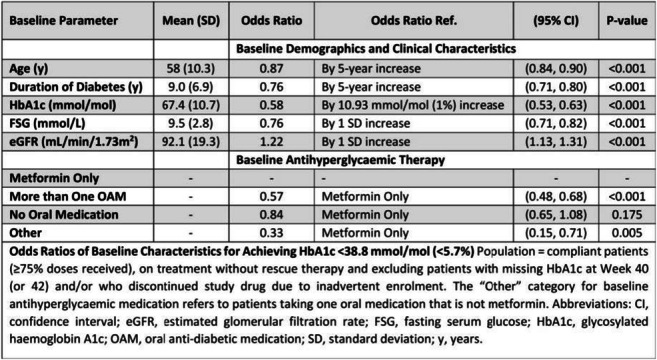
Clinical Trial Registration Number: SURPASS-1: NCT03954834, SURPASS-2: NCT03987919, SURPASS-3: NCT03882970, SURPASS-4: NCT03730662
Disclosure: L. Vázquez: Employment/Consultancy; Former employee: Ely Lilly (2011-2016). Lecture/other fees; Lectures: Abbott, AstraZeneca, Boehringer-Lilly, Fresenius, Lilly, Novo-Nordisk. Stock/Shareholding; Minor shareholder: Ely Lilly. Other; Advisory board: Ely Lilly, Clinical trials: Ely Lilly, Novo-Nordisk, OWL-Liver.
599
Long-term efficacy and safety of evogliptin add-on therapy to dapagliflozin plus metformin combinations in poorly controlled patients with type 2 diabetes
J. Moon1, H. Kim2, C. Chung3, K. Won1, K. Han4, C. Park5, J. Won6, D. Kim7, G. Koh8, E. Kim9, J. Yu10, E. Hong11, C. Lee12, K. Yoon13;
1Department of Internal Medicine, Yeungnam University Hospital, Daegu, 2Department of Internal Medicine, Ajou University Hospital, Suwon, 3Department of Internal Medicine, Wonju Severance Christian Hospital, Wonju, 4Department of Internal Medicine, Nowon Eulgi Medical Center, Seoul, 5Department of Internal Medicine, Kangbuk Samsung Hospital, Seoul, 6Department of Internal Medicine, Inje University Sanggye Paik Hospital, Seoul, 7Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, 8Department of Internal Medicine, Jeju National University Hospital, Jeju, 9Department of Internal Medicine, Ulsan University Hospital, Ulsan, 10Department of Internal Medicine, Hanllym University Kangnam Sacred Heart Hospital, Seoul, 11Department of Internal Medicine, Hanllym University Dongtan Sacred Heart Hospital, Hwaseong, 12Department of Internal Medicine, Hanyang University Guri Hospital, Guri, 13Department of Internal Medicine, Catholic University Seoul St. Mary’s Hospital, Seoul, Republic of Korea.
Background and aims: We aimed to investigate the long-term efficacy and safety of evogliptin add-on therapy in patients with type 2 diabetes who were inadequately controlled by metformin and dapagliflozin combinations.
Materials and methods: In this multi-center, double-blind, randomized, placebo-controlled phase 3 trial, patients with HbA1c 7.0 - 10.5 % (n=283) who had used dapagliflozin 10mg plus metformin (≥ 1,000 mg) (DAPA/MET) for at least 8 weeks were randomly assigned evogliptin 5 mg once daily or placebo (1:1). The primary endpoint was change from baseline in HbA1c at week 24, and the efficacy and safety of evogliptin over 52 weeks were exploratory endpoints in this extension.
Results: Of 283 randomized participants, 234 patients entered the long-term extension and 229 patients completed the 28-week extension period for a total 52 weeks of treatment. At week 52, treatment with evogliptin add-on to DAPA/MET resulted in a greater mean HbA1c reduction than placebo (least square [LS] mean difference -0.55 % [95 % confidence interval [CI] -0.71, -0.39]; p<0.0001). The proportion of patients achieving HbA1c <7% was significantly higher in evogliptin group (32.14 % versus 8.51 % in placebo; odds ratio of 5.62; p<0.0001). Evogliptin treatment also significantly reduced the fasting glucose levels and mean daily glucose levels with the improvement of HOMA-ß (LS mean difference 9.04 [95% CI 1.86, 16.21]; p=0.0138) compared to placebo. Adverse events were similar between groups, and no serious adverse drug reactions were reported in evogliptin group.
Conclusion: Long-term triple combination with evogliptin add-on to dapagliflozin plus metformin showed superior HbA1c reduction and glycemic control compared to placebo at 52 weeks, and is well tolerated to patients with type 2 diabetes who are not adequately controlled with DAPA/MET.
Clinical Trial Registration Number: NCT04170998
Supported by: Dong-A ST
Disclosure: J. Moon: None.
600
Glycaemic effect of tirzepatide by duration of diabetes
J. Kiljanski1, C. De Block2, C. Mathieu3, H. Sapin1, J. Peleshok1;
1Eli Lilly and Company, Indianapolis, USA, 2University Hospital Antwerp and University of Antwerp, Antwerp, Belgium, 3Katholieke Universiteit Leuven, Leuven, Belgium.
Background and aims: Results of SURPASS-1 through -5 trials have shown robust efficacy of novel dual GIP/GLP-1 receptor agonist tirzepatide (TZP) in people with Type 2 Diabetes. To understand whether glucose-lowering effect of tirzepatide depends on the duration of diabetes we conducted a subgroup analysis within each of the 5 Phase 3 trials.
Materials and methods: The primary endpoint of the 5 SURPASS clinical trials was mean change from baseline in HbA1c for 5mg, 10mg, and 15mg to 40 or 52 weeks against various comparators in adults with type 2 diabetes. This subgroup analysis of the 5 trials assessed whether mean change of HbA1c from baseline in the overall population were consistent when assessed by baseline duration of diabetes category (≤5 years, 5 to 10 years, >10 years) in patients while they were on-treatment and without rescue medication (efficacy estimand).
Results: For all 5 studies, analysis of change from baseline in HbA1c at 40 or 52 weeks for all 3 duration of diabetes subgroups were consistent with primary study results, with the treatment differences favoring all 3 doses of TZP compared with placebo or active comparator (Figure). The most frequent adverse events were mild-to-moderate, gastrointestinal-related and occurred during the dose-escalation period.
Conclusion: In conclusion, these results suggest that TZP was effective for mean change from baseline HbA1c for patients regardless of baseline duration of diabetes consistent with the overall patient population across the 5 SURPASS studies.
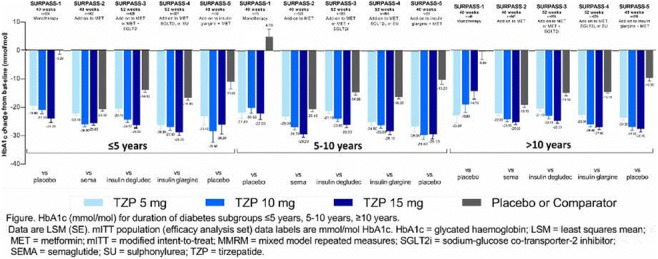
Disclosure: J. Kiljanski: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
SO 45 Beta cell function and glucose control
601
Empagliflozin improves beta cell function independently of relief of glucotoxicity in patients with type 2 diabetes
N.B. Jørgensen1, R. Thirumathyam1, E.A. Richter2, G.V. Hall3, J.P. Gøtze4, M. Fenger5, P.L. Madsen6, J.J. Holst7, S. Madsbad1;
1Department of Endocrinology, Amager Hvidovre Hospital, Hvidovre, 2Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, 3Department of Clinical Biochemistry, Rigshospitalet, Hvidovre, 4D, Rigshospitalet, Hvidovre, 5Department of Clinical, Amager Hvidovre Hospital, Hvidovre, 6Department of Cardiology, Herlev Gentofte Hospital, Herlev, 7Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark.
Background and aims: SGLT2-inhibitors improve beta-cell glucose sensitivity (BGS) and insulin sensitivity in patients with type 2 diabetes (T2D). This effect has been attributed to relief of glucotoxicity, but very few studies have controlled for differences in glycemia. Here, we explore the effects of empagliflozin on BGS first, and peripheral insulin sensitivity (IS) second, compared to similar glycemic control with insulin treatment.
Materials and methods: 17 subjects with T2D (13 males, 58±3 years (mean±sem), BMI 32.9±0.9 kg/m2, HbA1c 52.4±2.4 mmol/L, T2D duration 8.9±1.3 years) were treated with empagliflozin (E) and NPH-insulin (I) for 5 weeks in a randomized cross-over study design with 3 weeks of washout. Insulin was titrated to obtain similar glycemic control as obtained with empagliflozin. An OGTT with stable iv and oral glucose tracers was performed prior to and at the end of each treatment.
Results: Glycemia was similarly reduced on both treatments (Δfasting glucose: E: -1.3±0.2, p<0.01; I: -0.8±0.2, p<0.01; E vs I: p=NS). Fasting insulin concentrations were lower with empagliflozin and higher with insulin (E: 92±12 vs 180±27 pmol/L, p<0.001). Fasting glucagon concentrations were similar during both treatments (E: 19±1, I: 19.5±1 pmol/L) yielding a higher glucagon-to-insulin ratio on empagliflozin than on insulin treatment (E: 0.24±0.03 vs I: 0.14±0.02, p<0.001). Prehepatic insulin secretion (ISR) in response to the OGTT was greater on Empagliflozin compared to insulin treatment (E: AUC ISR 1814±170 pmol/kg, I: 1599±144 pmol/kg, p=0.02), while postprandial glucose excursions did not significantly differ between treatments. As a result, BGS was greater with empagliflozin compared to insulin treatment (E: 0.82±0.1, I: 0.59±0.07 pmol/(kg·min·mM), p=0.001), but postprandial incretin hormone secretion was similar during both treatments (AUC GLP-1: E: 3922±302; I: 3765±262 pmol·min/L; AUC GIP: E:6926±903; I: 6954±565).Fasting hepatic glucose production (HGP) was increased with empagliflozin compared to insulin (E: 19.5±0.8, I: 16.6±0.6 μmol/kg/min, p<0.001), but postprandial HGP did not differ between treatments. IS (AUC Glucose Rd, tissue /AUC Insulin) was numerically lower with empagliflozin, but decreased with insulin compared to washout (0.09±0.01 vs 0.11±0.02 μmol/kg FFM·pM·min), p=0.025). Fatty tissue insulin resistance (Adipo-IR: fasting FFA x fasting insulin) was lower with empagliflozin compared to insulin (E: 77±11, I:110±17 mmol/L x pmol/L, p=0.01). AUC glucagon during the OGTT was higher on empagliflozin treatment compared to insulin (E: 5286±255, I: 4818±345 pmol x min/L, p=0.04).
Conclusion: Improved beta-cell function during empagliflozin treatment is not a result of relieved glucotoxicity or increased incretin secretion. Peripheral hyperinsulinemia may induce insulin resistance.
Clinical Trial Registration Number: EudraCT no. 2017-002101-35
Supported by: Boehringer Ingelheim
Disclosure: N.B. Jørgensen: Grants; Boehringer Ingelheim.
602
Fasting 32-33 split proinsulin and intact proinsulin levels are reduced by liraglutide and metformin in combination. A randomised double-blind trial
C. Anholm1, P. Kumarathurai2, M. Skytte3, O.W. Nielsen2, O.P. Kristiansen2, K. Burling4, P. Barker4, M. Fenger5, S. Madsbad6, A. Sajadieh2, S.B. Haugaard3;
1Department of Endocrinology and Nefrology, University Hospital Nordsjælland Hillerød, Hillerød, Denmark, 2Department of Cardiology, Copenhagen University Hospital Bispebjerg, Copenhagen, Denmark, 3Department of Endocrinology, Copenhagen University Hospital Bispebjerg, Copenhagen, Denmark, 4Core Biochemical Assay Laboratory, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK, 5Department of Clinical Biochemistry, Copenhagen University Hospital, Hvidovre, Copenhagen, Denmark, 6Department of Endocrinology, Copenhagen University Hospital, Hvidovre, Copenhagen, Denmark.
Background and aims: Insulin hypersecretion associates to obesity and type 2 diabetes (T2DM) in its initial stage. Higher circulating concentrations of intact and split proinsulin in absolute amounts and as proportions of insulin and C-peptide suggests defective beta-cell function. The glucagon-like peptide-1 receptor agonist liraglutide increases insulin secretion but reduces the amount of circulating total proinsulin. However, there is no data present of the effects on fasting levels of 32-33 Split Proinsulin (SP) and Intact Proinsulin (IP). We investigated the effects of liraglutide combined with metformin on fasting concentration of IP and SP in patients with newly diagnosed well-controlled T2DM.
Materials and methods: A randomized, double-blind, placebo-controlled, cross-over trial in 12 + 12-weeks periods with ≥2-weeks wash-out before and between intervention periods. Population: Subjects with established coronary artery disease and newly diagnosed T2DM. Intervention: liraglutide/metformin vs. placebo/metformin. Biochemical analysis was done at the beginning and end of each 12-weeks periods. We report data from fasting plasma samples on insulin, C-peptide, IP, SP and glucose.
Results: Of 41 patients randomized data from 28 patients were available for the paired analysis. Data reported as median (IQR). Baseline levels of IP and SP were: 6.3 (3.4:10.7) and 13.4 (8.4:23.9) pmol/L, respectively. Fasting insulin and C-peptide levels were unchanged, but glucose was reduced by liraglutide combined to metformin. Plasma IP was reduced by 29%, i.e. -1.8 (-3.7:-0.3) pmol/L (p = 0.005) and SP by 34%, i.e. -4.6 (-12.1:-0.3) pmol/L (p = 0.005). IP + SP to insulin ratio was reduced by 20% (p = 0.03) and IP + SP to C-peptide ratio by 30% (p = 0.0003), but SP to IP ratio was unchanged.
Conclusion: In patients with newly diagnosed T2DM liraglutide in combination with metformin substantially reduced postabsorptive levels of proinsulin-like molecules and its ratios to insulin and C-peptide suggesting an improvement of beta-cell health.
Clinical Trial Registration Number: ClinicalTrials.gov Identifier: NCT01595789
Supported by: Novo Nordisk
Disclosure: C. Anholm: Grants; Novo Nordisk, The AP Moller Foundation, The Bispebjerg Hospital Research Foundation, The Danish Heart Foundation, Department of Internal Medicine at Amager Hospital, Clinical Research Centre Hvidovre Hospital.
603
Tirzepatide improved markers of islet cell function and insulin sensitivity compared to semaglutide in people with type 2 diabetes
K. Brown, L. Fernández Landó, B. Bergman, M.K. Thomas, B. Liu, C. Lee;
Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide (TZP) achieved significantly greater HbA1c and weight reductions with all doses (5, 10 and 15 mg) vs semaglutide 1 mg (SEMA) in a Phase 3 trial of 1879 people with type 2 diabetes (T2D) on background metformin (mean age 56.6 years; T2D duration 8.6 years; baseline HbA1c 8.3% [67 mmol/mol]; BMI 34.2 kg/m2) (SURPASS-2).
Materials and methods: Changes in fasting markers of islet cell function (fasting glucagon and HOMA2-B) and insulin sensitivity (fasting insulin and HOMA2-IR) were assessed by mixed model repeated measures in the modified intent-to-treat population.
Results: At 40 weeks, all TZP doses improved HOMA2-B, calculated with C-peptide, as indicated by a significant increase by 97-120% on average with TZP, compared to 84% with SEMA. Fasting glucagon levels, adjusted for fasting serum glucose, significantly decreased by 53-55% on average with TZP 10 and 15 mg doses compared with SEMA (48%). All TZP doses improved insulin sensitivity as reflected by a significant decrease by 16-24% on average of HOMA2-IR, calculated with insulin, compared to a decrease by 5% with SEMA. Fasting insulin levels were also significantly reduced by 9-21% on average with all TZP doses compared to an increase of 0.6% with SEMA.
Conclusion: Dual GIP/GLP-1 receptor agonist TZP significantly improved markers of islet cell function and insulin sensitivity compared to selective GLP-1 RA SEMA in people with T2D.
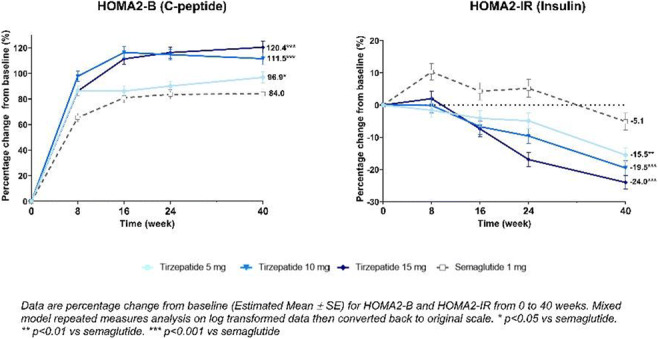
Clinical Trial Registration Number: NCT03987919
Supported by: Study sponsored by Eli Lilly and Company
Disclosure: K. Brown: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
604
Association between variants in TCF7L2, CTRB1/2 and GLP-1R genes and response to therapy with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes
A. Kyriakidou1, A.V. Kyriazou1, T. Koufakis1, Y. Vasilopoulos2, X. Tsekmekidou1, I. Avramidis3, S. Baltagiannis4, D.G. Goulis5, P. Zebekakis1, K. Kotsa1;
1Division of Endocrinology and Metabolism - Diabetes Center, 1st Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, AHEPA University Hospital, Thessaloniki, 2Department of Biology, Section of Genetics, Cell Biology and Development, University of Patras, Patras, 3Diabetes Center, Department of Internal Medicine, G. Papanikolaou General Hospital, Thessaloniki, 4Diabetes Outpatient Clinic, General Hospital of Kastoria, Kastoria, 5Unit of Reproductive Endocrinology, 1st Department of Obstetrics and Gynecology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Background and aims: We aimed to evaluate the role of polymorphisms in TCF7L2, CTRB1/2 and GLP-1R genes in the glycemic and weight loss response to treatment with glucagon-like peptide-1 receptor agonists (GLP-1- RAs) among Greek individuals with type 2 diabetes (T2D).
Materials and methods: 191 adults with T2D (52% female, mean age and BMI 69.3±10.6 years and 35.8±5.7 kg/m2, respectively), who had been treated with liraglutide, exenatide, or lixisenatide for at least 6 months were included. Participants were genotyped for TCF7L2 rs7903146 (C>T), CTRB1/2 rs7202877 (T>G) and GLP-1R rs367543060 (C>T) polymorphisms. Clinical and laboratory parameters were measured at baseline, 3 and 6 months after the initiation of GLP-1 RA. Good glycemic responders were defined as patients fulfilling one of the following criteria: i) HbA1c <7% at 3 or 6 months after the initiation of treatment, ii) reduction of baseline HbA1c by ≥1% after 3 or 6 months of GLP-1 RA administration, and iii) maintenance of HbA1c <7% that a patient had before switching to GLP-1 RA, after 3 or 6 months of treatment. Weight loss responders were defined as subjects who lost ≥3% of their baseline weight after 3 or 6 months of GLP-1 RA use. The dominant genetic model was used in all analyses.
Results: The minor allele frequencies for TCF7L2 rs7903146, CTRB1/2 rs7202877 and GLP-1R rs367543060 were 79.1, 15.7, and 0%, respectively. 136 (71.2%) and 125 (65.4%) individuals were classified as glycemic control and weight loss responders, respectively. Carriers of at least one polymorphic rs7903146 T allele presented comparable glucose control and weight loss response to GLP-1 RAs with the homozygous wild-type genotype (OR: 1.08, 95% CI: 0.5, 2.31, P=0.85 and OR:1.35, 95% CI: 0.66, 2.76, P=0.42, respectively). Carriers of at least one polymorphic rs7202877 G allele had similar response to treatment in terms of glycemic control (OR: 1.4, 95% CI: 0.56, 3.47, P=0.47) and weight loss (OR: 1.28, 95% CI: 0.55, 2.98, P=0.57) compared with carriers of two wild type alleles. Regarding GLP-1R rs367543060, all participants were homozygous for the wild-type allele; thus, no comparisons were feasible. Both glycemic responders and non-responders achieved a reduction in weight and BMI from baseline to 6 months (P<0.0001). Fasting glucose and HbA1c decreased in both weight responders (P=0.003 and P<0.0001, respectively) and non-responders (P=0.0004 and P<0.0001, respectively) after 6 months of treatment.
Conclusion: Our findings do not suggest a role for the variants studied in affecting the response to GLP-1 RA treatment in Greek patients with T2D.
Supported by: Novo Nordisk (grant number 133935/2014).
Disclosure: A. Kyriakidou: None.
605
Post-prandial glucose reduction with YG1699 compared to dapagliflozin in subjects with type 1 diabetes
P. Lapuerta1, S. Urbina2, A. Wittle3, J. He1, C. Li1, T. Li1;
1Youngene Therapeutics Co., Ltd., Shanghai, China, 2ProSciento, Inc, Chula Vista, USA, 3Atorus Research, Inc, Chula Vista, USA.
Background and aims: YG1699 is a dual inhibitor of SGLT1 and SGLT2. This phase 2a study compared the effects of YG-1699 to dapagliflozin 10 mg (dapa) in subjects with T1D on insulin pump therapy.
Materials and methods: Subjects with type 1 diabetes in this crossover study received insulin alone for one week (the no treatment period, or NTP), followed by three treatment periods of insulin plus blinded study drug (dapa, YG1699 10 mg, YG1699 25 mg) given in a randomized order, each given once daily and orally for 7 days. There was a washout interval of one week between each treatment period. A mixed meal tolerance test (MMTT) was done at Day 6 of each period. The glucose area under the curve over 120 minutes (AUC120) and GLP-1 AUC180 were measured in the MMTT as measures of SGLT1 activity. The primary endpoint was glucose AUC120 on treatment expressed as a percentage of the NTP value. Insulin withdrawal of up to 6 hours was performed on Day 7 and stopped for capillary ketones ≥ 2.5 mmol/L, plasma glucose ≥350 mg/dL, carbon dioxide <18 mmol/L, or subject symptoms.
Results: 19 subjects enrolled and 18 completed all three treatment periods. Characteristics included mean age 38, BMI 24, 53% female, and baseline hemoglobin A1C 7.4%. The following values are presented (means or %) in the order of YG1699 10 mg, YG1699 25mg, and dapa 10 mg, respectively. Glucose AUC120 values as a percentage of NTP were: 89.5%, 84.8%, and 102.1% (p=0.0003 for YG1699 10 mg vs. dapa 10 mg, and p<0.0001 for YG1699 25 mg vs. dapa 10 mg). Glucose values at 120 minutes were: 128, 115, and 141 mg/dL. Incremental GLP-1 AUC180 values (above the NTP) were: 2135, 3204 and 1424 ng*min/L. Urinary glucose excretion totals during the MMTT were: 6.3, 8.4, and 7.6 g/3 hours (vs. 0.2 in the NTP). Times to stopping insulin withdrawal were: 321, 338 and 328 minutes (vs. 342 in the NTP). Incidences of adverse events were: 94%, 95%, and 95% (mostly mild and moderate hypoglycaemia). Incidences of moderate hypoglycaemia were: 44.4%, 57.9%, and 57.9%. There were no serious adverse events. There was no DKA (diabetic ketoacidosis).
Conclusion: YG1699 reduced post-prandial glucose significantly more than dapagliflozin in subjects with T1D and was well-tolerated in this study. The pattern of lower post-prandial glucose, a greater GLP-1 response, and similar urinary glucose excretion compared to dapagliflozin in the MMTT indicate that YG1699 provided dual inhibition of SGLT1 and SGLT2.
Clinical Trial Registration Number: NCT04956263
Disclosure: P. Lapuerta: Employment/Consultancy; I am employee of Youngene. Stock/Shareholding; And I may own common stock or have been granted stock options or other equity incentive awards..
606
Greater improvement in insulin sensitivity per unit weight loss with tirzepatide compared to selective GLP-1 receptor agonism
M.K. Thomas1, K.J. Mather1, A. Mari2, J. Li1, T. Heise3, J. DeVries4, S. Urva1, T. Coskun1, Z. Milicevic1, A. Haupt1;
1Eli Lilly and Company, Indianapolis, USA, 2Institute of Medicine and National Research Council, Rome, Italy, 3Profil Institute for Clinical Research, Inc, Neuss, Germany, 4Academic Medical Center, Amsterdam, Netherlands.
Background and aims: Tirzepatide (TZP), a novel GIP-GLP-1 dual agonist, improved fasting measures of insulin sensitivity partially attributable to weight loss in Phase 2 studies. Studies in mice demonstrated that GIP receptor agonism with TZP improved insulin sensitivity independent of weight loss.
Materials and methods: To further evaluate insulin-sensitizing actions of TZP, we measured insulin sensitivity with hyperinsulinemic euglycaemic clamps (M-value) in a 28-week double-blind randomised controlled trial comparing effects of TZP 15mg with placebo or semaglutide 1mg (SEMA), a selective GLP-1RA, all subcutaneous QW. Linear regression was used to compare relationships of change in M-value versus change in weight across treatment groups.
Results: Change in M-value was related to absolute change and % change in weight (Figure). Slopes of linear relationships with absolute and % weight change differed between TZP and SEMA (absolute change: p=0.015; % change: p=0.03). Larger negative slopes were observed for TZP compared with SEMA on change in M-value related to absolute change and to % change in weight.
Conclusion: These results suggest that TZP may provide greater improvement in insulin sensitivity than a selective GLP-1RA per unit weight loss, most evident in subjects with greater weight loss.
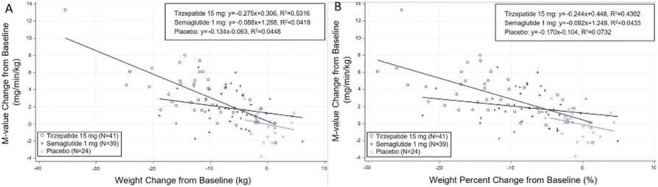
Clinical Trial Registration Number: NCT03951753
Disclosure: M.K. Thomas: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
607
Dipeptidyl peptidase-4 inhibitors and gallbladder or biliary disease in type 2 diabetes: systematic review and meta-analysis of randomised controlled trials
L. He, J. Wang, N. Yang, L. Xu, W. Li, F. Ping, J. Huang, Y. Li, H. Zhang;
Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, Peking U, Beijing, China.
Background and aims: Previous studies showed that both glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) might impact postprandial gallbladder motility, possibly increasing risks of gallbladder or biliary diseases. Since dipeptidyl peptidase-4 (DPP-4) inhibitors enhance the bioavailability of endogenous GLP-1 and GIP-1, we aimed to examine the association between DPP-4 inhibitors and gallbladder or biliary diseases in adults with type 2 diabetes(T2DM).
Materials and methods: We conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) that compared DPP-4 inhibitors with placebo or non-incretin drugs in adults with T2DM. We searched PubMed, EMBASE, Web of Science, and Cochrane Library from inception to 30 June 2021. The primary outcome was the gallbladder or biliary diseases, and the secondary outcomes were cholelithiasis, cholecystitis, and biliary diseases. Overall relative risk (RR) and 95% confidence interval (CI) were calculated.
Results: A total of 82 randomized controlled trials (RCTs) with 104,833 participants were included in the pairwise meta-analysis. Compared with placebo or non-incretin drugs, DPP-4 inhibitors were significantly associated with an increased risk of the composite of gallbladder or biliary diseases (odds ratio 1.22 (95%confidence interval 1.04 to 1.43); risk difference, 11 more (2 to 21 more) events per 10,000 persons in a year) and cholecystitis (odds ratio 1.43 (95% confidence interval 1.14 to 1.79); risk difference, 15 more (5 to 27 more) events per 10,000 persons in a year) but were not with the risk of cholelithiasis and biliary disease. The associations tended to be observed in patients with a longer duration of DPP-4 inhibitors treatment.
Conclusion: DPP-4 inhibitors increased the risk of cholecystitis in RCTs, especially with a longer treatment duration, which requires more attention from physicians in clinical practice.
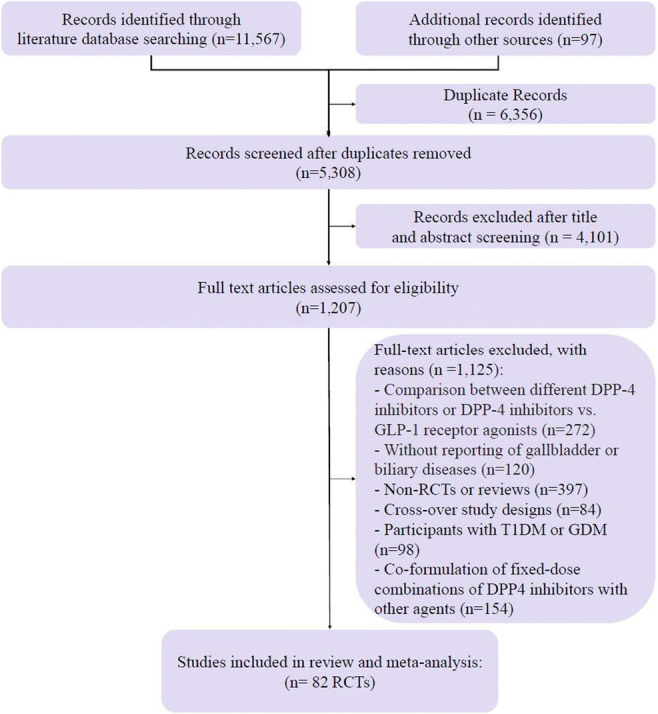
Supported by: Natural Science Foundation of Beijing Municipality Beijing (M22014)
Disclosure: L. He: None.
608
Glycaemic response to meals with a high glycaemic index consumed in the morning and evening among students with early and late chronotype-ChroNu Study
B. Stutz1, B. Krueger1, J. Goletzke1, N. Jankowicz2, C. Herder3, U. Alexy2, A.E. Buyken1;
1Faculty of Natural Sciences, Paderborn University, Paderborn, 2Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Dortmund, 3German Center for Diabetes Research, Düsseldorf, Germany.
Background and aims: Glucose homeostasis is known to deteriorate during the day even among healthy individuals, hence meals with a high glycaemic index (GI) consumed in the evening may be particularly detrimental for glucose homeostasis. On the other hand, recent data suggest that meal consumption against the inner clock could increase the risk for metabolic diseases such as Type 2 Diabetes. Young adults may be particularly vulnerable for dietary misalignment since social schedules commonly impose meals against their biologically later chronotype. Therefore, this study investigates whether the 2-hour post-prandial (2-h pp) glycaemic response to a meal with a high GI is larger in the evening than in the morning both among early and late chronotypes.
Materials and methods: Among 320 students aged 18-25 years those with the earliest (n=22) and latest (n=23) chronotype as determined by the Munich Chronotype Questionnaire were invited to participate in a 4-day randomized controlled cross-over nutrition trial in autumn 2020. On the intervention days (separated by a wash-out day), participants received a high GI meal (GI=72) either at breakfast (7 a.m.) or at dinner (8 p.m.). All other meals had a medium GI (46-59). Glucose responses were recorded by a continuous glucose monitoring device (G6, Dexcom, Inc., San Diego, CA). The outcomes 2-h pp incremental area under the curve (iAUC) and glycaemic variability (mean amplitude of glucose excursion, MAGE) were compared using paired T-Test.
Results: Among earlier chronotypes (median mid-point of sleep 3:43 a.m. (Q1; Q3: 3:10; 4:15)) 2-h pp iAUC responses were higher in the evening than in the morning: iAUC 236 (± 90) vs. 198 (± 87) mmol/L, p= 0.04, yet MAGE was comparable: 3.4 (± 1.3) vs. 3.0 (± 1.8) mmol/L, p= 0.6. In contrast, later chronotypes (median mid-point of sleep 5:37 a.m. (Q1; Q3: 5:10; 6:20)) showed comparable 2-h pp glucose responses in the morning and in evening: iAUC 213 (± 112) vs. 208 (± 96) mmol/L, p= 0.96, MAGE: 3.1 (± 1.6) vs. 3.2 (± 1.2) mmol/L, p= 0.9.
Conclusion: The present study illustrates that diurnal variations in glucose response to a high GI meal differ between earlier and later chronotypes. In line with a deteriorating glucose homeostasis over the day, earlier chronotypes were vulnerable for higher glucose excursions to a high GI meal consumed late in the evening. The fact that this was not seen among later chronotypes suggests that glucose homeostasis was adversely affected also early in the morning following a high GI against the inner clock.
Clinical Trial Registration Number: NCT04302922
Supported by: This research was supported by the German Research Foundation (DFG), BU 1807/3-2
Disclosure: B. Stutz: None.
SO 46 Incretins everywhere
609
Multifactorial risk reduction with oral semaglutide vs comparators in the treatment of type 2 diabetes
V.R. Aroda1, J. Eliasson2, B. Malling2, J.J. Meier3, L.L. Nielsen2, T. Vilsbøll4, K. Khunti5;
1Brigham and Women's Hospital, Harvard Medical School, Boston, USA, 2Novo Nordisk A/S, Søborg, Denmark, 3St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany, 4Clinical Research, Steno Diabetes Center Copenhagen, Gentofte Hospital, University of Copenhagen, Herlev, Denmark, 5Leicester Diabetes Centre, Diabetes Research Centre, Leicester General Hospital, Leicester, UK.
Background and aims: Therapies that address multiple risk factors may help patients with type 2 diabetes (T2D) to improve their cardiometabolic risk profile. This exploratory analysis aimed to evaluate the efficacy of oral semaglutide in improving multiple cardiometabolic risk factors vs comparators.
Materials and methods: In the PIONEER phase 3a clinical trial programme, patients with T2D were randomised to oral semaglutide 14 mg/flex (flexible dose adjustment for PIONEER 7 only) or comparators (empagliflozin 25 mg, sitagliptin 100 mg, liraglutide 1.8 mg or placebo [pbo]). This post hoc analysis evaluated the proportion of patients who achieved specific cardiometabolic endpoints (reductions in HbA1c ≥1%, body weight ≥5%, systolic BP ≥5 mmHg or LDL cholesterol ≥0.5 mmol/L, or an increase in eGFR ≥0 mL/min/1.73 m2) by the end of the PIONEER 1-8 trials, and the proportion achieving 2, 3 and 4 or more of these endpoints. Data were analysed using a logistic regression model with treatment, strata (PIONEER 3-8), interaction strata (PIONEER 5 and 8) and region as categorical fixed effects and baseline value as covariate for the on-treatment without rescue medication data for all randomised patients.
Results: Across trials, greater proportions of patients achieved each endpoint with oral semaglutide vs comparators. Reductions in HbA1c ≥1% occurred in 47.3-77.1% with oral semaglutide vs 32.8-51.4% with active comparators or 8.5-23.7% with pbo. Reductions in body weight ≥5% occurred in 30.8-49.8% with oral semaglutide vs 12.7-41.0% with active comparators or 5.4-16.3% with pbo. Reductions in systolic BP ≥5 mmHg occurred in 47.9-62.5% with oral semaglutide vs 48.8-59.7% with active comparators or 40.1-57.9% with pbo. Reductions in LDL cholesterol ≥0.5 mmol/L occurred in 20.2-27.4% with oral semaglutide vs 13.7-21.1% with active comparators or 12.7-21.7% with pbo. Increases in eGFR ≥0 mL/min/1.73 m2 occurred in 45.1-54.6% with oral semaglutide vs 37.0-50.0% with active comparators or 32.4-44.5% with pbo. Significantly greater proportions of patients achieved improvements in 2, 3 or 4 or more endpoints with oral semaglutide vs comparators across all trials, except for the achievement of 2 endpoints with oral semaglutide 14 mg vs liraglutide 1.8 mg in PIONEER 4 (Table 1).
Conclusion: Multifactorial risk factor management is fundamental to long-term risk reduction in patients with T2D. Oral semaglutide was more effective at improving multiple cardiometabolic risk factors vs comparators, indicating its potential to help address the full cardiometabolic profile for patients with T2D.
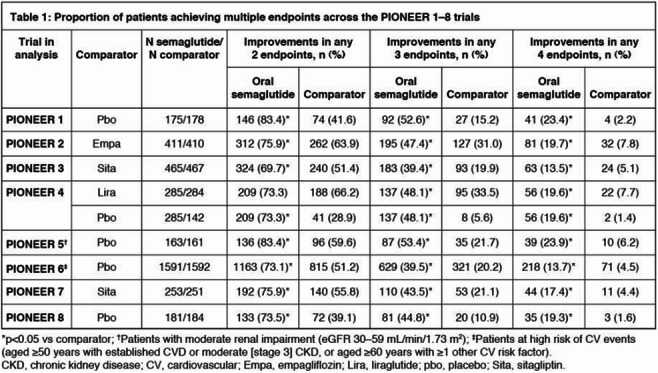
Clinical Trial Registration Number: NCT02906930; NCT02863328; NCT02607865; NCT02863419; NCT02827708; NCT02692716; NCT02849080; NCT03021187.
Supported by: Funded by Novo Nordisk A/S.
Disclosure: V.R. Aroda: Employment/Consultancy; Adocia, AstraZeneca, BD, Novo Nordisk A/S, Sanofi and Zafgen (consultancy fees only). Grants; AstraZeneca/Bristol-Myers Squibb (BMS), Calibra, Eisai, Janssen, Novo Nordisk A/S, Sanofi and Theracos.
610
Reduced risk of kidney function loss in patients with type 2 diabetes treated with GLP-1 receptor agonists versus basal insulin
C. Melzer Cohen1, M. Schechter2,3, I. Yanuv2,3, A. Rozenberg2,3, G. Chodick1,4, T. Abrahamsen5, A. Clark5, J. Lawson5, A. Karasik1,6, O. Mosenzon2,3;
1Maccabi Institute for Research and Innovation, Maccabi Health Services, Tel Aviv, Israel, 2Diabetes Unit, Hadassah Medical Center, Jerusalem, Israel, 3Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel, 4School of Public Health Sackler, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel, 5Novo Nordisk A/S, Copenhagen, Denmark, 6Tel Aviv University, Tel Aviv, Israel.
Background and aims: Several GLP-1 receptor agonists (GLP-1 RAs) have been shown to reduce albuminuria in populations with type 2 diabetes (T2D), with data also suggesting that they may reduce the rate of kidney function decline. This study used data from Maccabi Healthcare Services, a large Israeli state-mandated care provider, to assess changes in kidney outcomes among initiators of GLP-1 RAs compared with matched initiators of basal insulin (BI) in a real-world setting.
Materials and methods: Adults with T2D using ≥2 glucose lowering agents who initiated GLP-1 RAs or BI from 2010 to 2019 were propensity-score matched by 89 baseline characteristics. In the intention to treat (ITT) analysis, subjects were followed until death, the end of data availability or October 2021. In the as-treated (AT) analysis, follow-up was censored also at exposure discontinuation or initiation of the comparator drug. Categorical one-step, single-measurement increase in urine albumin:creatinine (UACR) category (<30; ≥30-<300; ≥300 mg/g, accompanied with >30% increase) was evaluated. Confirmed estimated glomerular filtration rate (eGFR) decline in a composite endpoint (≥40% eGFR decline) including development of end stage kidney disease (ESKD) as well as confirmed declines in eGFR alone (≥30%, 40%, 50%, and 57% respectively) was evaluated. Treatment effects were determined using Cox proportional hazard models.
Results: Before matching, 6797 and 9145 patients were included in GLP-1 RAs and BI groups, respectively. Each exposure group constituted 3424 matched patients (mean age, 59.4 years [SD, 9.4]; 45% women; 49% had T2D for more than 10 years; 21% with history of cardiovascular diseases. The study population was generally healthy from a kidney perspective, with a mean eGFR of 90.6 mL/min/1.73 m2 [SD 19.3] and median UACR of 14.6 mg/g [IQR 0.0-54.9]). The median follow-up times were 82.2 months (ITT) and 22.6 months (AT). Compared with BI, initiation of GLP-1 RA was associated with a lower risk of categorical increase in UACR in both the ITT (hazard ratio (HR) [95% CI] 0.90 [0.83-0.97]) and AT (HR: 0.85 [0.76-0.96]) analyses. The HR of the composite outcome of confirmed ≥40% eGFR decline or new ESKD was 0.96 [0.82-1.11] and 0.71 [0.54-0.95] in the ITT and AT analyses, respectively. The HR for 30%, 40%, 50%, and 57% confirmed eGFR decline in the ITT analyses were 0.98 [0.87-1.11], 0.96 [0.82-1.12], 1.11 [0.91 -1.37] and 1.04 [0.80-1.34]. In the AT analyses, the respective HRs were 0.74 [0.60-0.91], 0.73 [0.54-0.97], 0.74 [0.49-1.12] and 0.62 [0.35-1.10], respectively.
Conclusion: The findings suggest that continuous use of GLP-1 RA may be associated with improved kidney outcomes, including a lower risk for kidney function loss, among patients with type 2 diabetes and preserved baseline kidney function. The ongoing FLOW trial will directly determine whether the GLP-1 RA once-weekly subcutaneous semaglutide reduces the risk of kidney disease progression in people with T2D and CKD.
Supported by: Novo Nordisk A/S
Disclosure: C. Melzer Cohen: None.
611
Chronic administration of the novel hypoglycaemic peptide exhibits robust efficacy in a diet-induced model of obesity and type 2 diabetes
N. Mitkin, V. Pavshintsev, I. Doronin, G. Babkin, A. Malyshev;
Metabico, Inc., Newton, USA.
Background and aims: CHM-273S is a novel peptide, which in recent studies provided pronounced blood glucose lowering effect in the rats with metabolic syndrome during acute administration. This effect was accompanied by improvement of insulin resistance index (HOMA-IR) and induction of insulin signaling pathway in the liver, that indicates the ability of the peptide to enhance insulin sensitivity. In the present work, we aimed to study chronic effects of the peptide in animals affected by diet-induced metabolic disorder.
Materials and methods: The metabolic syndrome was modeled in male C57BL/6 mice, which were kept on a 45% high-fat diet (HFD) for 16 weeks. 40 mice with the maximum weight gain were selected for the subsequent experiment. The control group (n=10) was kept on a standard diet (STD) during this period. Chronic CHM-273S effects were studied in result of its administration for 4 weeks (i.n. or i.p. administration, 5 mg/kg, once-daily) compared with metformin (p.o. administration, 250 mg/kg, once-daily). STD and HFD controls were i.p. injected with saline in the same regime. Bodyweight was monitored three times a week. At the end of the study, blood and visceral adipose tissue samples were collected. Blood serum samples were analyzed for glucose level, insulin concentration, and TNF-α level. Visceral fat mass was weighted, and adipocyte size was estimated applying histological analysis.
Results: HFD for 16 weeks resulted in a 35% and 30% increase in blood glucose level and body weight respectively. Administering CHM-273S (both i.n. and i.p.) for four weeks reduced blood glucose levels by more than 12% demonstrating an effect comparable to that of metformin. In addition, treatment with CHM-273S led to the normalization of HOMA-IR, which was significantly increased after HFD. These data suggest strong hypoglycemic properties of CHM-273S and its ability to attenuate insulin resistance. Animals treated with CHM-273S (i.n.) and metformin exhibited a 30% loss of excess body weight compared to untreated mice, while administration of CHM-273S (i.p.) resulted in a loss of 10%. Chronic CHM-273S (i.n. and i.p.) treatment led to a decreased visceral fat weight, and the linear size of adipocytes compared to the HFD control group, that indicates the association of bodyweight loss with the reduction of fat mass. Serum levels of pro-inflammatory TNF-α cytokine were significantly increased in HFD control mice, indicating systemic inflammation. Chronic i.n. CHM-273S administration reduced TNF-α concentration to the STD control level in the same manner as metformin.
Conclusion: Chronic CHM-273S peptide administration effectively alleviates symptoms of metabolic syndrome induced by the HFD in C57BL/6 mice.
Disclosure: N. Mitkin: None.
612
Effectiveness and tolerability of once-weekly GLP-1 receptor agonists in clinical practice: a focus on switching between once-weekly molecules in type 2 diabetes
G. Formoso1,2, G. Di Dalmazi1,2, S. Coluzzi3, M.P.A. Baldassarre1,2, A. Ghit1,2, G. Graziano4, M.C. Rossi4, B. Ciappini1,2, M. Milo1,2, F. Carrieri1,2, A. Nicolucci4, A. Consoli1,2;
1Department of Medicine and Aging Sciences, “G. d'Annunzio” University of Chieti-Pescara, Chieti, 2Center for Advanced Studies and Technology (CAST), “G. d'Annunzio” University of Chieti-Pescara, Chieti, 3Endocrinology and Metabolic Disease Clinic of Pescara, Pescara, 4CORESEARCH-Center for Outcomes Research and Clinical Epidemiology, Pescara, Italy.
Background and aims: To date, just a few phase III randomized controlled trials (RCTs) directly compared the efficacy of different glucagon-like peptide receptor agonists (GLP-1RAs). In addition, direct comparisons among GLP-1RAs have been relatively limited in Real World Data. Thus, there is the need for a real-world setting comparative analysis of available once-weekly (OW) GLP-1RAs, in order to support data from RCTs and to provide information that may aid physicians in their decision process. Aims of this study were to evaluate the effectiveness and tolerability of OW GLP-1RAs and to assess the clinical benefits of switching from one GLP-1RA to another (switchers) in a routine clinical setting.
Materials and methods: Retrospective, real-world cohort study, based on electronic medical records utilized in one Italian diabetes clinic. Estimated mean changes in HbA1c and body weight after 6 and 12 months from the first prescription of a OW GLP1-RA were evaluated using longitudinal linear mixed models for repeated measures. The effectiveness of the three OW GLP1-RAs was compared separately in the GLP1-RA naïve and switchers cohorts, after propensity score adjustment.
Results: A total of 1,001 patients with type 2 diabetes treated with an OW GLP-1RA were included in this study. Among these, 379 (37.9%) patients received OW semaglutide, 435 (43.4%) dulaglutide or 187 OW exenatide (18.7%). Initiating a long-acting GLP1-RA was associated with statistically significant improvements in HbA1c (-1%) and body weight (-2.6 Kg) after 6 months and benefits were maintained after 12 months. In GLP1-RA naïve cohort, semaglutide showed the largest effect on HbA1c (-1.55%; 95%CI (-1.77; -1.34) and body weight (-3.76 Kg; 95%CI -5.05; -2.47) at 6 months, maintained at 12 months (-1.55%; 95% CI-1.82;-1.28 and -6.29 Kg; 95%CI -7.94;-4.63). In the switchers' cohort, statistically significant reductions at 6 months in HbA1c and body weight were documented with semaglutide and dulaglutide only, with semaglutide associated with the most marked reduction (-0.84%; 95%CI -1.03; -0.65 and -3.43 Kg; 95% -4.67; -2.19). Drop-out rates were 9.2%, 28.5%, and 41.7% in semaglutide, dulaglutide, and exenatide groups, respectively.
Conclusion: The effectiveness and tolerability of the OW GLP-1RAs in the real world were documented. OW semaglutide was associated with the highest response without impact on safety. Clinical improvements were obtained even in switchers, especially in those switching to OW semaglutide.
Disclosure: G. Formoso: None.
613
Multiple dose-ranging study of the novel glucagon/GLP-1 receptor dual agonist BI 456906 vs placebo and open-label weekly semaglutide reference control in type 2 diabetes
J. Rosenstock1, M. Blüher2, B. Schmid3, J. Hoefler3, A. Hennige3;
1Dallas Diabetes Research Center, Dallas, USA, 2University Hospital Leipzig, Leipzig, Germany, 3Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany.
Background and aims: Glucagon-like peptide 1 receptor (GLP-1 R) agonists, developed for treatment of type 2 diabetes (T2D) and obesity, consistently improve glycaemic control and reduce bodyweight with different degrees of magnitude. Glucagon receptor (GCGR) agonism can potentiate the glucose and weight-lowering effects of GLP-1R agonism by increasing lipolysis, lipid oxidation and energy expenditure. The GCGR/GLP-1R dual agonist BI 456906 was tested in this dose-finding Phase II blinded study to assess efficacy and safety vs placebo (PBO) using the weekly GLP-1R agonist semaglutide as an open reference in patients with T2D.
Materials and methods: This PBO-controlled, double-blind study enrolled adults with T2D (≥6 months) and HbA1c 7.0-10.0%, BMI 25-50 kg/m2, on stable metformin background therapy. Participants were randomised to receive masked subcutaneous dose-escalated BI 456906 (up to 0.3, 0.9, 1.8 or 2.7 mg once weekly [qw], or 1.2 or 1.8 mg twice weekly [biw]), or PBO, or open-label semaglutide (up to 1.0 mg qw) for 16 weeks. BI 456906 doses ≥0.9 mg qw were escalated every 1-2 weeks, with at least 10 weeks on a maintenance dose (8 weeks for semaglutide). The primary endpoint was the absolute change from baseline in HbA1c.
Results: Participants (N=411) were randomised to 8 treatment arms (BI 456906 n=50 each per 0.3 mg qw, 0.9 mg qw, 2.7 mg qw; n=52 1.8 mg qw; n=51 1.2 mg biw; n=49 1.8 mg biw; n=50 semaglutide 1.0 mg qw; n=59 PBO) and had generally similar baseline characteristics: 57% male with mean age 57 years, HbA1c 8.1% and BMI 34 kg/m2. Mean absolute HbA1c decreased from baseline, in a dose-dependent manner by -0.93% to -1.88% with BI 456906 treatment at Week 16 (Figure); PBO decreased HbA1c by -0.23%. Low dose BI 456906 treatment reduced HbA1c to approximately the same extent as did semaglutide 1.0 mg qw (−1.47%). The most common AEs in all groups were gastrointestinal (GI) disorders (n=167 [55.3%]), most frequently nausea (20.0-48.1%) and vomiting (11.8-26.0%) in dose-escalated BI 456906 treated participants. Overall, AEs were less frequent in biw dosing groups with slower dose-escalation, compared with qw BI 456906 dosing, particularly for nausea (1.2 mg biw: 27.5%; 1.8 mg biw: 46.9%; 0.3 mg qw: 20.0%; 0.9 mg qw: 28.0%; 1.8 mg qw: 48.1%; 2.7 mg qw: 46.0%) and vomiting (1.2 mg biw: 11.8%; 1.8 mg biw: 22.4%; 0.3 mg qw: 14.0%; 0.9 mg qw: 18.0%; 1.8 mg qw: 23.1%; 2.7 mg qw: 26.0%). The rate of treatment discontinuation due to AEs tended to increase with dose: BI 456906 0.3 mg qw and 0.9 mg qw (10%); 1.8 mg qw (21%); 2.7 mg qw (30%); 1.2 mg biw (8%); 1.8 mg biw (16%); PBO (5%); semaglutide 1.0 mg qw (4%).
Conclusion: The GCGR/GLP-1R dual agonist, BI 456906, effectively lowered HbA1c up to −1.9% (1.8 mg qw) after 16 weeks in patients with T2D. HbA1c reductions were in line with semaglutide 1.0 mg qw (−1.5%). GI adverse events are expected to be mitigated with slower dose escalations.
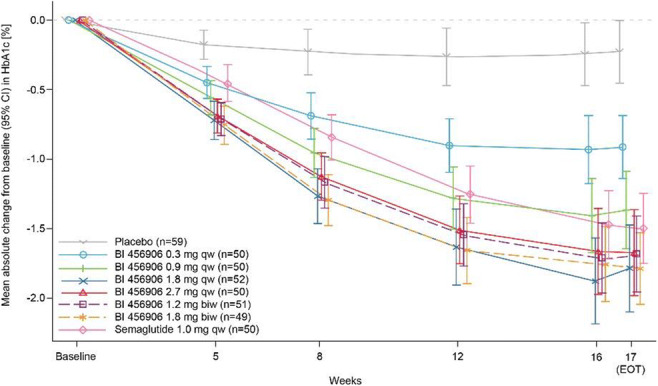
Clinical Trial Registration Number: NCT04153929
Supported by: BI
Disclosure: J. Rosenstock: Non-financial support; Medical writing support.
614
Glycaemic control with tirzepatide in people with type 2 diabetes by baseline HbA1c ≤ 69.4 mmol/mol or >69.4 mmol/mol
C. De Block1, G. Aleppo2, J.A. Levine3, E.G. Valderas3, B.D. Benneyworth3;
1Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Antwerp, Belgium, 2Feinberg School of Medicine, Northwestern University, Chicago, USA, 3Eli Lilly and Company, Indianapolis, USA.
Background and aims: Tirzepatide, a once weekly GIP/GLP-1 receptor agonist in development for type 2 diabetes (T2D), demonstrated superior glycaemic control in the Phase 3 SURPASS clinical trial program. This post-hoc analysis assessed glycaemic control with tirzepatide in participants stratified by baseline (BL) HbA1c (≤69.4 mmol/mol [8.5%], >69.4 mmol/mol [8.5%]).
Materials and methods: Mean change from BL in HbA1c was assessed in tirzepatide-treated participants (5, 10 or 15 mg) from SURPASS-1 (monotherapy), SURPASS-2 (add-on to MET), SURPASS-3 (add on to MET ± SGLT2i), SURPASS-4 (add-on to MET, SGLT2i, or sulfonylurea) and SURPASS-5 (add-on to insulin glargine ± MET) at trial endpoints (40 or 52 weeks). Safety was also presented. Treatment comparisons were estimated using data while participants were on treatment and without rescue medications (efficacy estimand).
Results: Across each SURPASS trial, ranges in mean BL HbA1c were 63.3-69.6 mmol/mol (7.94-8.52%), mean BMI were 31.9-34.2 kg/m2 and mean diabetes duration were 4.7-13.3 years. At trial endpoints, HbA1c reductions from BL ranged from 16.9-23.4 mmol/mol (1.55-2.14%) in the BL HbA1c ≤69.4 mmol/mol (8.5%) subgroup and 29.5-37.8 mmol/mol (2.70-3.46%) in the BL HbA1c >69.4 mmol/mol (8.5%) subgroup (Table). Gastrointestinal side effects were similar to those reported in the incretin class and hypoglycaemic events (blood glucose <3.00 mmol/l [54 mg/dL] or severe) were low.
Conclusion: In conclusion, significant and clinically meaningful HbA1c reductions were observed with tirzepatide, irrespective of BL HbA1c.
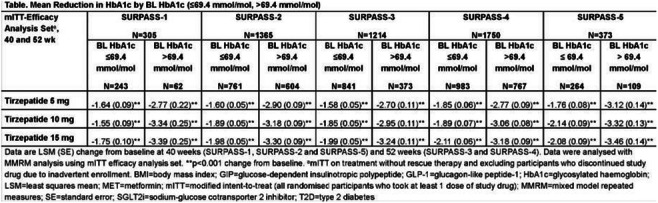
Disclosure: C. De Block: Honorarium; Abbott, AstraZeneca, Boehringer Ingelheim, Menarini Diagnostics, Eli Lilly, Medtronic, Novo Nordisk and Roche. Lecture/other fees; Abbott, AstraZeneca, Boehringer Ingelheim, Menarini Diagnostics, Eli Lilly, Medtronic, Novo Nordisk and Roche.
615
The real-world observational prospective study of health outcomes with dula and liraglutide in type 2 diabetes patients (TROPHIES): 24-m clinical characteristics and treatment patterns
A. Dib1, B. Guerci2, F. Giorgino3, K. Boye4, M. Füchtenbusch5, E. Heitmann6, J. Lebrec7, M. Orsini Federici8, M. Yu9, L.-E. García-Pérez10;
1Lilly France SAS, Neuilly Sur Seine, France, 2University Hospital of Nancy, Vandoeuvre Lès Nancy, France, 3University of Bari Aldo Moro, Bari, Italy, 4Eli Lilly and Company, Indianapolis, USA, 5Marienplatz Diabetes Center, Munich, Germany, 6Lilly Deutschland GmbH, Bad Homburg, Germany, 7HaaPACS GmbH, Schriesheim, Germany, 8Eli Lilly Italia SpA, Sesto Fiorentino, Italy, 9Eli Lilly Canada Inc., Toronto, Canada, 10Lilly, S.A., Alcobendas, Spain.
Background and aims: The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide & Liraglutide in Type 2 Diabetes (T2D) Patients (TROPHIES) was a 24-month, prospective, non-comparative, observational study in adult patients with T2D initiating Dulaglutide (DU) or Liraglutide (LIRA) as their first injectable glucose-lowering treatment. Clinical outcomes and treatment patterns in this real-world setting are presented here.
Materials and methods: Adult patients with T2D started with once-weekly DU (DU; N=1,014) or once-daily LIRA (LIRA; N=991), as per usual practice, in France, Germany and Italy. Secondary objectives included assessments of HbA1c, body weight and treatment patterns.
Results: Clinical characteristics of the DU and LIRA cohorts at 24 months are described in the Table. Most patients receiving DU (856/1,014) initiated treatment at 1.5 mg/week and, among 286 patients with a significant treatment change, 237 were still receiving 1.5 mg/week. In the LIRA cohort, most patients (809/991) initiated at the starting dose 0.6 mg/day and 45% increased dose to 1.2 mg/day by Day 90; however, 17% of patients were already receiving 1.8 mg/day by Day 90. At the first significant treatment change 285/448 patients in the LIRA cohort were receiving 1.2 mg/day. Insulin was initiated by 79 and 97 patients in the DU and LIRA cohorts, respectively.
Conclusion: Both treatments showed clinically meaningful effectiveness on HbA1c and weight, maintained over the 24-month study period, consistent with results of clinical trials.
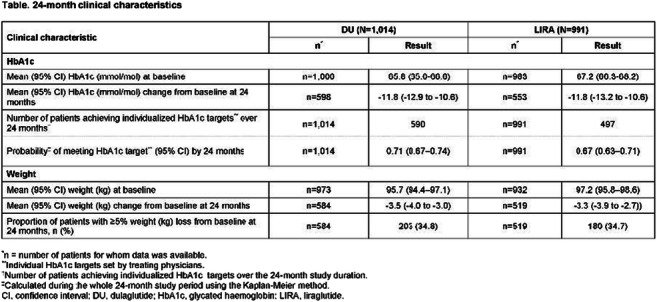
Supported by: Eli Lilly and Company
Disclosure: A. Dib: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
616
Effects of a novel long acting GIP/GLP-1/Glucagon tri-receptor agonist, HISHS-3001, on HbA 1c , body weight and lipid metabolism
R. Thennati1, V. Burade1, A. Garcia-Ocana2, R. Pratley3, G. Rutter4, T. Vilsbøll5, B. Thorens6;
1High Impact Innovations - Sustainable Health Solutions; Sun Pharmaceutical Industries Ltd, Vadodara, India, 2Ichan School of Medicine at Mount Sinai, Diabetes Obesity and Metabolism Institute, New York, USA, 3AdventHealth Translational Research Institute, Orlando, USA, 4Department fo Metabolism, Digestion, Reproduction, CR-CHUM, University of Montreal, Montreal and London, Canada, 5Clinical Research, Steno Diabetes Centre Copenhagen, Harlev, Copenhagen, Denmark, 6Centre for Integrative Genomics, University of Lausanne, Lausanne, Switzerland.
Background and aims: HISHS-3001 is a novel long-acting, Gastric inhibitory peptide (GIP)/Glucagon like peptide-1 (GLP-1)/Glucagon tri-receptor agonist. HISHS-3001 was evaluated in db/db mouse model for its anti-diabetic potentials.
Materials and methods: HISHS-3001, a synthetic peptide, was evaluated for functional assay on GLP-1, GIP and Glucagon receptor expressing cells. Effect on HbA1c, body weight change, triglyceride and Liver FGF-21 was studied in type 2 diabetes model of db/db mouse.
Results: An in-vitro cyclic AMP assay in human receptor- expressing CHO-K1 cells demonstrated that HISHS-3001 had a half-maximal effective concentration 2.9 and 6.5 fold higher than exendin-4 or GIP, respectively, and 355-fold lower than glucagon. Subcutaneous dosing every third day for 4 weeks in diabetic db/db mice of HISHS-3001 at 3 and 20 nmol/kg reduced HbA1c, body weight, triglycerides and glucagon levels robustly and markedly more than tirzepatide (Table). Treatment with HISHS-3001 also led to an increase in serum and liver Fibroblast Growth Factor-21 (FGF-21) levels vs. tirzepatide.
Conclusion: In conclusion, HISHS-3001 is a potent, long acting GIP/GLP-1/glucagon tri-receptor agonist, with higher effects on glucose homeostasis and metabolic biomarkers in diabetic mice than tirzepatide.
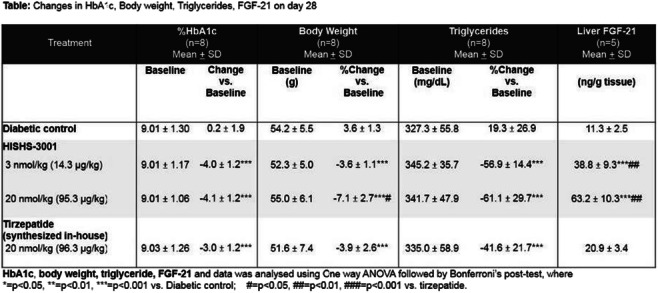
Disclosure: R. Thennati: None.
SO 47 Treatments, molecules and outcomes: a smorgasbord
617
Effects of tirzepatide versus insulin glargine 100 U/mL on kidney outcomes in participants with type 2 diabetes in SURPASS-4
H.J.L. Heerspink1, N. Sattar2, I. Pavo3, A. Haupt3, K.L. Duffin3, Z. Yang3, R.J. Wiese3, K.R. Tuttle4,
D.Z.I. Cherney5;
1Department of Clinical Pharmacy and Pharmacology, University Medical Centre Groningen, Groningen, Netherlands, 2Institute of Cardiovascular and Medical Sciences, Glasgow, UK, 3Eli Lilly and Company, Indianapolis, USA, 4Providence Health Care, University of Washington, Spokane, USA, 5Division of Nephrology, Department of Medicine, Toronto General Hospital, University Health Network, University of Toronto, Toronto, Canada.
Background and aims: Tirzepatide (TZP, 5, 10, 15 mg/week), a dual GIP/GLP-1 receptor agonist, reduced HbA1c levels more than titrated daily insulin glargine (iGlar) in people inadequately controlled on oral diabetes treatments with type 2 diabetes (T2D) and high cardiovascular risk (median study duration 85 weeks). Here, we compared progression to pre-specified kidney endpoints between TZP and iGlar.
Materials and methods: Composite kidney outcomes in SURPASS-4 were analysed: endpoint 1 (eGFR [CKD-EPI] decline ≥40% from baseline, renal death, progression to ESRD, new onset macroalbuminuria) and endpoint 2 (endpoint 1 without new onset macroalbuminuria). Data were examined within the entire study population, and in subgroups defined by baseline SGLT2i use, UACR ≥30 mg/g, eGFR <60 mL/min/1.73m2 and in those at high risk for kidney related outcomes, defined as eGFR <75 mL/min per 1.73 m2 and macroalbuminuria, or eGFR <45 mL/min per 1.73 m2.
Results: At baseline, participants (N=1995, age 63.6 years, HbA1c, 69.7 mmol/mol [8.5%]) had a mean eGFR of 81.3 mL/min per 1.73 m2; 17% had eGFR <60 mL/min per 1.73 m2, 28% microalbuminuria (UACR 30-300 mg/g) and 8% macroalbuminuria (UACR >300 mg/g). During the follow-up to 104 weeks, TZP participants experienced significantly fewer renal outcomes, especially new onset of macroalbuminuria versus iGlar (Table).
Conclusion: In people with T2D and high cardiovascular risk, TZP reduced markers of diabetic kidney disease risk.
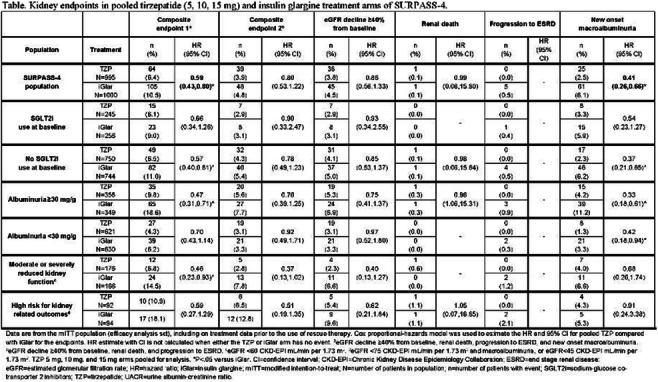
Clinical Trial Registration Number: NCT03730662
Disclosure: H.J.L. Heerspink: Employment/Consultancy; AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe and MundiPharma.
618
Cardiorenal and safety outcomes with finerenone in patients with obesity, CKD and type 2 diabetes
L.K. Billings1, S.D. Anker2, G.L. Bakris3, G. Filippatos4, B. Pitt5, L. Ruilope6, J. Green7, O. Mosenzon8, K.M. Pantalone9, C. Ahlers10, A. Lage11, R. Lawatscheck10, A. Scalise12, P. Rossing13;
1NorthShore University Health System/ UChicago Pritzker School of Medicine, Chicago, USA, 2Charité Universitätsmedizin, Berlin, Germany, 3UChicago Medicine, Chicago, USA, 4Uni of Athens, Attikon University Hospital, Athens, Greece, 5Uni Michigan, Ann Arbor, USA, 6Institute of Research i+12, Madrid, Spain, 7Duke University School of Medicine and Duke Clinical Research Institute, Durham, USA, 8Diabetes Unit, Hadassah Medical Center, Jerusalem, Israel, 9Endocrinology and Metabolism Institute, Endocrinology Metabolism Institute, Cleveland, USA, 10Bayer AG, Berlin, Germany, 11Bayer AG, São Paulo, Brazil, 12Bayer AG, Barcelona, Spain, 13Uni. Copenhagen, Copenhagen, Denmark.
Background and aims: Finerenone, a selective nonsteroidal mineralocorticoid receptor antagonist (MRA), reduced the risk of cardiovascular (CV) and kidney outcomes in patients with type 2 diabetes (T2D) and chronic kidney disease (CKD) in FIDELITY, a prespecified pooled analysis of the FIDELIO-DKD and FIGARO-DKD trials. Patients with visceral adiposity have been shown previously to respond better to MRAs than patients without visceral adiposity; here, we explore the efficacy and safety of finerenone in patients with obesity.
Materials and methods: Patients with T2D and CKD (with urine albumin-to-creatinine ratio [UACR] ≥30-≤5000 mg/g and estimated glomerular filtration rate [eGFR] ≥25 ml/min/1.73 m2), receiving optimised renin-angiotensin system blockade, were randomised 1:1 to finerenone or placebo. The efficacy of finerenone vs placebo on composite CV (CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalisation for heart failure) and composite kidney (kidney failure, a sustained eGFR decline ≥57% from baseline, or renal death) outcomes was assessed in patients with and without obesity. Obesity was defined by a substantially increased waist-hip ratio (WHR) of ≥0.85 in women and ≥0.9 in men.
Results: At baseline, 12,124 (93%) patients had a substantially increased WHR. Of the patients with substantially increased WHR, 56% had a BMI ≥30 kg/m2. There was a numerically higher rate of CV events observed in patients with substantially increased WHR compared to those without (substantially increased, 13.8% [1671/12,124] of patients; not substantially increased, 10.1% [85/844] of patients). The effect of finerenone on the CV composite outcome was not modified by WHR category (substantially increased: HR 0.84, 95% CI 0.77-0.93; not substantially increased: HR 1.15, 95% CI 0.74-1.80; p-interaction 0.11). The effect of finerenone on the kidney composite outcome also was consistent irrespective of WHR category (substantially increased: HR 0.76, 95% CI 0.65-0.87; not substantially increased: HR 0.88, 95% CI 0.54-1.43; p-interaction 0.53). Overall, treatment-emergent adverse events were balanced between finerenone and placebo, and across WHR subgroups. The incidence of hyperkalaemia leading to treatment discontinuation with finerenone vs placebo was 1.7% vs 0.5% and 1.1% vs 1.2% in patients with a substantially increased and not substantially increased WHR, respectively.
Conclusion: In FIDELITY, the efficacy and safety of finerenone in patients with CKD, T2D and obesity (as defined by substantially increased WHR) was broadly consistent with the overall population.
Clinical Trial Registration Number: FIDELIO-DKD (NCT02540993) and FIGARO-DKD (NCT02545049)
Supported by: BayerAG supported this analysis, who funded the FIDELIO-DKD & FIGARO-DKD studies & combined analysis
Disclosure: L.K. Billings: Honorarium; has received honoraria from Eli Lilly, Sanofi, Novo Nordisk, Xeris and Bayer.
619
Betaine: a regulator of mitochondria and endoplasmic reticulum contact points (MAMs) of the hepatocytes
A. Alves1, F. Lamarche2, E. Meugnier1, S. Pesenti1, R. Lefebvre1, A. Bassot3, E. Loizon1, C. Moinard2, B. Morio1;
1CarMeN Laboratory U1060 INSERM INRA 1397, Pierre Benite, France, 2LBFA Laboratory INSERM U1055, Saint-Martin-d'Hère, France, 3Department of Cell Biology, Faculty of Medicine and Dentistry, Edmonton, Canada.
Background and aims: Contact points between mitochondria and the endoplasmic reticulum (ER), called MAMs for Mitochondria-Associated Membranes, have a crucial role in the control of hepatocyte homeostasis. Recent data show that MAMs are altered in the liver of mouse models of insulin resistance and steatosis indicating that MAMs could be a novel therapeutic target for the prevention or treatment of metabolic liver disorders. These dynamic platforms can be regulated by different factors, including nutrients. We hypothesized that betaine, through its biochemical properties, could modulate the structure and function of MAMs of the hepatocytes.
Materials and methods: Primary hepatocytes from 3-month-old male Wistar rats (n=6) were isolated by a collagenase perfusion method and cultured in complete DMEM (3 g/L glucose). Cells were incubated for 18h in the culture medium with 500μmol/L of BSA or palmitate (lipotoxicity model), supplemented or not with betaine (5mmol/L). MAMs structure was explored by transmission electronic microscopy (TEM) and in situ proximity ligation assay (PLA) for the functional unit formed by the IP3R1 (inositol 1,4,5-trisphosphate receptor) receptor of the ER and the VDAC1 (voltage-dependent anion channel) channel of the mitochondria. Cells were also stimulated with insulin (100mmol/L) for 15 min to explore the response to insulin by Western blot (PKB phosphorylation). Lipogenesis was induced with an oleate/palmitate mixture (1mmol/L, 2:1), in order to assess triglyceride storage in hepatocytes by biochemical assay (Triglyceride Method GPO, Biolabo). Mitochondrial respiration was measured by oxygraphy on permeabilized cells with the substrates glutamate (5 mmol/L)/malate (2.5 mmol/L) and succinate (5 mmol/L)/rotenone (5 μmol/L). Gene expression and protein content of key actors at the MAMs (VDAC1, mitofusin 2: Mfn2, and the chaperone Grp75) were also analyzed. Statistical analysis was performed with 2 Way ANOVA and Bonferroni post hoc test, p-value<0.05.
Results: Betaine promoted MAM integrity compared to control. MAM length measured by TEM relative to mitochondrial circumference was increased by 41% under betaine compared to control (betaine 16.2% vs. control 11.5%, p<0.05). The number of VDAC1/IP3R1 interactions per nucleus analyzed by in situ PLA was increased by 22.4% and 39.4% by betaine in BSA and palmitate conditions, respectively (p<0.01). Similarly, insulin response was increased by 80.7% and 138.5% by betaine in BSA and palmitate conditions respectively (p<0.01). Cellular respiration to succinate/rotenone was enhanced by betaine in BSA and palmitate conditions (+62% and +90% respectively, p<0.01). However, betaine did not significantly reduce triglyceride storage. Finally, betaine increased gene expression of Mfn2 and VDAC1 by 18% and 21% respectively in BSA condition (p<0.001), and by 38% and 24% in palmitate condition (p<0.01), but it did not significantly impact the protein expression of the proteins.
Conclusion: Our results support our hypothesis that betaine promotes MAM integrity and insulin response in primary hepatocytes. The increase in cellular respiration evidences a beneficial effect of improved MAM integrity on mitochondrial oxidative function.
Supported by: SFD
Disclosure: A. Alves: None.
620
Effects of finerenone in patients with chronic kidney disease and type 2 diabetes are independent of HbA1c at baseline, HbA1c variability and duration of diabetes
J.B. McGill1, R. Agarwal2, S.D. Anker3, G.L. Bakris4, G. Filippatos5, B. Pitt6, L.M. Ruilope7, A.L. Birkenfeld8, M.L. Caramori9, M. Brinker10, A. Joseph11, A. Lage12, R. Lawatscheck11, C. Scott13, P. Rossing14;
1Washington University School of Medicine, London, USA, 2Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, USA, 3Charité Universitätsmedizin, Berlin, Germany, 4UChicago Medicine, Chicago, USA, 5National and Kapodistrian Uni. Athens, Athens, Greece, 6Uni. Michigan, Ann Arbor, USA, 7Institute of Research i+12, Madrid, Spain, 8Uni. Tübingen, Tübingen, Germany, 9Uni. Minnesota, Minneapolis, USA, 10Bayer AG, Wuppertal, Germany, 11Bayer AG, Berlin, Germany, 12Bayer AG, São Paulo, Brazil, 13Bayer AG, Reading, UK, 14Uni. Copenhagen, Copenhagen, Denmark.
Background and aims: Finerenone reduced the risk of cardiovascular (CV) and kidney outcomes in chronic kidney disease (CKD) and type 2 diabetes (T2D) patients in the FIDELITY prespecified pooled analysis of the FIDELIO-DKD and FIGARO-DKD studies. Here, we evaluate the effect of finerenone by baseline HbA1c, HbA1c variability and diabetes duration.
Materials and methods: Patients with T2D and CKD (urine albumin-creatinine ratio [UACR] ≥30-≤5000 mg/g and eGFR ≥25 ml/min/1.73 m2) were randomised to finerenone or placebo. Effects of finerenone vs placebo on CV (CV death, nonfatal myocardial infarction, nonfatal stroke or hospitalisation for heart failure) and kidney (kidney failure, sustained ≥57% eGFR decline from baseline or renal death) composite outcomes were analysed by baseline HbA1c quartiles, HbA1c variability (first year of treatment) and diabetes duration quartiles.
Results: In 13,026 analysed patients mean baseline HbA1c was 7.7% and diabetes duration was 15.4 years. Higher baseline HbA1c quartiles had longer diabetes duration and more diabetes-related complications. Risk reductions in the CV and kidney composite outcomes with finerenone vs placebo were consistent across HbA1c (p-interaction 0.52 and 0.09, respectively) and diabetes duration quartiles (p-interaction 0.12 and 0.75). HbA1c variability in the first year of treatment was associated with higher cardiorenal risks; each 1 unit increase in mean absolute residual of HbA1c was associated with a 20% increased risk of a CV event (HR 1.20; 95% CI 1.07-1.35; p=0.0016) and a 36% increased risk of a kidney event (HR 1.36; 95% CI 1.21-1.52; p<0.001). The CV and kidney benefits of finerenone were not modified by HbA1c variability (p-interaction 0.48 and 0.09, respectively).
Conclusion: Greater variability in HbA1c was associated with increased risks of cardiorenal outcomes. Cardiorenal risk reductions with finerenone in patients with CKD and T2D were not modified by baseline HbA1c, HbA1c variability or duration of diabetes.
Clinical Trial Registration Number: FIDELIO-DKD (NCT02540993) and FIGARO-DKD (NCT02545049)
Supported by: BayerAG supported this analysis, who funded the FIDELIO-DKD & FIGARO-DKD studies & combined analysis
Disclosure: J.B. McGill: Grants; research funding paid to Washington University from NIH, Beta Bionics, Dexcom and Novo Nordisk. Honorarium; member of steering committees for Bayer and the Jaeb Center, data safety monitoring committees for NIH and the Jaeb Center, and is an associate editor of the Journal of the Endocrine Society & BMJDRC. Non-financial support; personal fees and non-financial support from Bayer, personal fees from Boehringer Ingelheim, Dexcom, Mannkind, Novo Nordisk and Provention Bio.
621
Effects of ertugliflozin on uric acid and gout-related outcomes: post hoc analyses from VERTIS CV
V.S. Sridhar1, F. Cosentino2, S. Dagogo-Jack3, R. Pratley4, R. Frederich5, M. Noyes Essex6, M. Maldonado7, C.-C. Liu8, J.P. Mancuso9, D.Z.I. Cherney1;
1Department of Medicine, Division of Nephrology, University Health Network and University of Toronto, Toronto, Canada, 2Unit of Cardiology, Karolinska Institute & Karolinska University Hospital, Stockholm, Sweden, 3University of Tennessee Health Science Center, Memphis, USA, 4AdventHealth Translational Research Institute, Orlando, USA, 5Pfizer Inc., Collegeville, USA, 6Pfizer Inc., New York, USA, 7MSD Limited, London, UK, 8Merck & Co., Inc., Kenilworth, USA, 9Pfizer Inc., Groton, USA.
Background and aims: The effect of ertugliflozin on serum uric acid and gout-related outcomes in VERTIS CV was investigated.
Materials and methods: Patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease were randomised to ertugliflozin 5 mg (n=2752), 15 mg (n=2747; doses pooled for analyses) or placebo (n=2747). Changes in serum uric acid and composite of gout onset or initiation of anti-gout medication were assessed.
Results: Least squares mean changes from baseline in serum uric acid (mmol/L [95% CI]) at week 260 were −0.0115 (−0.0150, −0.0080) and 0.0041 (−0.0010, 0.0092) in the ertugliflozin and placebo groups, respectively, nominal P<0.001 (Fig. 1a). The hazard ratio (95% CI) for the composite of gout-related outcomes was 0.76 (0.580, 1.002), nominal P=0.052 (Fig. 1b).
Conclusion: Treatment with ertugliflozin was associated with reductions from baseline in serum uric acid, and a non-significant reduction in gout-related outcome events, compared with placebo.
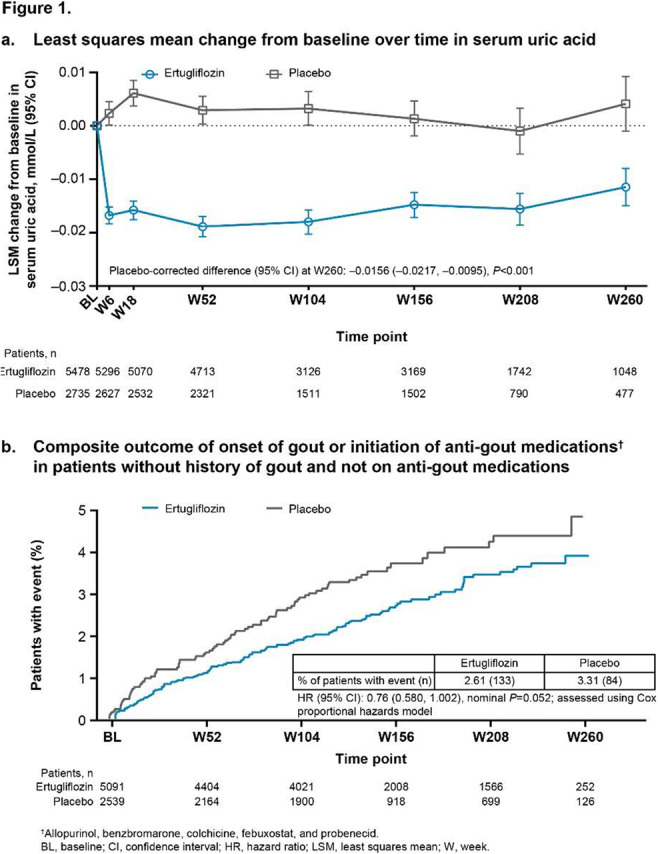
Clinical Trial Registration Number: NCT01986881
Supported by: Merck Sharp & Dohme Corp., Kenilworth, NJ, USA; Pfizer Inc., New York, NY, USA
Disclosure: V.S. Sridhar: None.
622
Interactions between early DPP-4is initiation and HbA1c variability with risk of insulin requirement in type 2 diabetes: real-world evidence from a prospective cohort
A. Yang1, J. Cheung1, H. Wu1, E.S. Lau1, R.C. Ma1, A.P. Kong1, W.Y. So2, A.O. Luk1, J.C. Chan1, E. Chow1;
1The Chinese University of Hong Kong, 2Hong Kong Hospital Authority Head Office, Hong Kong, Hong Kong.
Background and aims: Insufficient insulin secretion and non-suppression of glucagon are key features in type 2 diabetes (T2D). Dipeptidyl peptidase-4 inhibitors (DPP-4is) inhibit degradation of glucagon-like peptide-1 (GLP-1) which suppresses glucagon and augments prandial insulin secretion. Early combination therapy using metformin and DPP-4i reduced treatment escalation and delayed insulin requirement, whilst glycemic variability (GV) might accelerate β-cell loss and worsen glycemic control. Using real-world evidence, we evaluated the interactions between early DPP-4is initiation and HbA1c variability on risk of insulin requirement in T2D.
Materials and methods: This is a territory-wide, population-based prospective cohort including 103,744 patients with T2D initiated on DPP-4is in 2007-2018 followed up till 2019 in Hong Kong. We excluded patients treated with insulin at baseline and those with less than five HbA1c measurements during follow-up. We defined 1-year period before the first date of dispensing of DPP-4is as baseline. Insulin requirement was defined as continuous insulin treatment for at least six months during follow-up. We used Cox models adjusted for baseline and time-dependent variables including medications, risk factors and co-morbidities to compare risk of insulin requirement between patients started early (HbA1c<7.5%) or late (HbA1c≥7.5%) with DPP-4is as well as their interactions with HbA1c variability on outcome. We calculated the HbA1c variability score (HVS) defined as percentage of HbA1c values varying by 0.5% compared with the preceding value.
Results: Of 24,147 patients (53.3% men, age: 63.9±11.6 years, HbA1c: 8.3±1.3%, diabetes duration: 12.3±7.1 years) fulfilling inclusion criteria, 90.1% and 87.4% were treated with metformin and sulphonylureas respectively at baseline. During a mean follow-up of 4.1 years, 20.6% required insulin treatment. Early DPP-4is initiation was associated with lower risk of insulin requirement compared with late initiation with a hazard ratio [HR] of 0.71 (95% CI: 0.66-0.77). Stratified by median HVS value (42.8), the low HVS group had lower risk of insulin requirement than the high HVS group with a HR of 0.59 (0.56-0.63). The lowest risk was observed in patients with early initiation of DPP-4is and low-HVS (P interaction <0.001, Figure 1).
Conclusion: Early initiation of DPP-4is as combination therapy might delay insulin requirement especially in those with low HbA1c variability, possibly through preservation of beta-cell function.
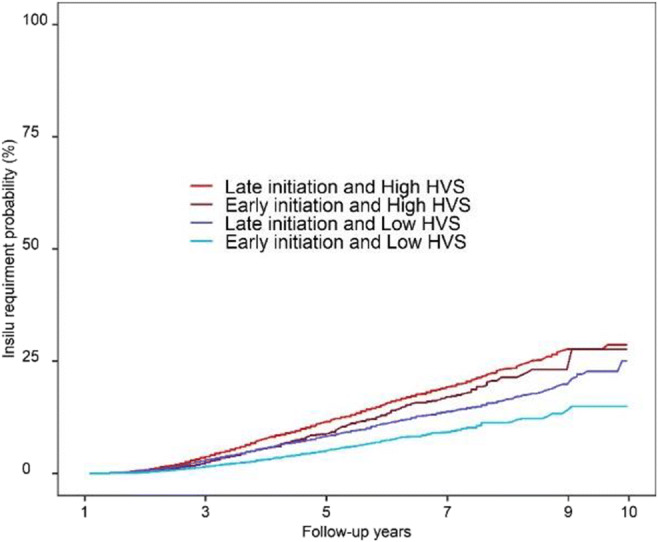
Supported by: We thank the Hong Kong Hospital Authority for providing the data for analysis
Disclosure: A. Yang: None.
623
Effects of acute and sub-chronic treatment with metformin on postprandial blood pressure and heart rate in diet-controlled type 2 diabetes
D.R. Quast1, C. Xie2, M.J. Bound2, J. Grivell2, K.L. Jones2, M. Horowitz2, C.K. Rayner2, M.A. Nauck1, J.J. Meier3, L. Phillips2, T. Wu2;
1Diabetes Division, St. Josef-Hospital Bochum, Bochum, Germany, 2Centre of Research Excellence (CRE) in Translating Nutritional Science to Good Health, The University of Adelaide, Adelaide, Australia, 3Augusta-Hospital, Bochum, Germany.
Background and aims: There is a high prevalence of postprandial hypotension (PPH) (a fall in systolic blood pressure (BP) of ≥ 20 mmHg within two hours of a meal) in type 2 diabetes (T2D), which is associated with syncope, falls and increased mortality. A single dose of metformin (1 g), administered intraduodenally, has been shown to attenuate the fall in blood pressure (BP) induced by an oral glucose drink in T2D patients, suggesting that metformin may reduce the risk of PPH, potentially by slowing of gastric emptying (GE) and stimulation of glucagon-like peptide-1 (GLP-1). We have now investigated the effects of a single dose (acute) and one-week (sub-acute) treatment of oral metformin on postprandial BP and heart rate (HR) in T2D.
Materials and methods: Diet-controlled T2D patients were treated with either metformin (850 mg twice daily) or matching placebo over 7 days in a double-blind, randomised, crossover design; each treatment period was separated by a two-week ‘washout’. At both baseline and the end of each treatment period, a 24-hour ambulatory BP recording was performed to detect PPH (i.e., following standardised breakfast, lunch and dinner). On days 1 and 7 of both treatment periods, participants ingested 850 mg metformin or placebo 30 min before a 75 g glucose drink. BP and HR, GE (scintigraphy), and plasma glucose and total GLP-1 were evaluated at intervals over 4 hours.
Results: 16 T2D patients (5 female, age 69.0 ± 1.5 years, duration of known diabetes 7.8 ± 1.3 years, HbA1C 46.3 ± 1.2 mmol/mol (6.4 ± 2.3 %), BMI 27.2 ± 0.9 kg/m²) were studied. In participants who had PPH at baseline, the probability of PPH was lower during treatment with metformin versus placebo (n = 13 out of 16 (81.3 %); 13 events during 38 post-meal periods recorded (34.2 %) events on metformin vs. 23/38 (60.5 %) events on placebo, P=0.038). After one-week of metformin treatment, HR following 75 g oral glucose was higher compared to placebo (∆HR +2.4 ± 0.9 bpm, P=0.024), although the magnitude of the reduction in systolic BP (~12 mmHg) did not differ between metformin and placebo. Metformin delayed GE (half emptying time (T50) after both acute (82.1 ± 6.8 min vs. 102.3 ± 7.2 min, P<0.001) and sub-acute treatment (83.0 ± 5.3 min vs. 102.4 ± 6.8 min, P<0.001). Following the glucose drink, plasma glucose was reduced by both acute dosing (treatment by time: P<0.001) and sub-acute treatment (treatment effect: P<0.001) of metformin. Plasma GLP-1 was unaffected by acute dosing of metformin but increased after one-week of treatment (area under the curve over 4 hours: 8411 ± 803 pmol/L*min on metformin vs. 6493 ± 610 pmol/L*min on placebo, P<0.001).
Conclusion: In diet-controlled T2D, oral metformin attenuates the hypotensive response to meals to reduce the probability of PPH, in association with augmentation of GLP-1 secretion and slowing of GE.
Clinical Trial Registration Number: ACTRN12619001060134
Supported by: Central Adelaide Local Health Network Inc. and the Royal Adelaide Hospital Research Committee
Disclosure: D.R. Quast: None.
624
Caffeine ameliorates diet-induced insulin resistance by impacting brown adipose tissue function
A.C.F. Soares, I.B. Martins, S.M. Farreca, F.O. Martins, A. Capucho, B.F. Melo, S.V. Conde;
CEDOC, NOVA Medical School, Faculdade de Ciências Médicas, Universidade NOVA de Lisboa, Lisbon, Portugal.
Background and aims: Brown adipose tissue (BAT) has been described as a potential target to control and treat metabolic disorders such as type 2 diabetes, since it is involved in whole-body thermogenesis. When active, BAT can produce bioactive substances like adenosine, a purinergic nucleoside signaling molecule that has been shown to be involved in BAT activation. Caffeine is a non-selective adenosine receptor antagonist that was shown to induce lipolysis, thermogenesis, insulin secretion, and fat oxidation in both non-obese and obese humans, however the impact of caffeine intake on BAT function is lacking. Therefore, the general aim of this study was to investigate the impact of chronic caffeine intake on BAT function in an animal model of insulin resistance induced by hypercaloric diets.
Materials and methods: We used three groups of Male Wistar rats: a control group submitted to 25 weeks of a standard chow, a high fat + high sucrose (HFHSu) group submitted to 25 weeks of 60 % lipid-rich diet + 35% sucrose in drinking water and a HFHSu group submitted to a caffeine intake (1g/kg) in drinking sucrose in the last 11 weeks of diet. Rats were evaluated for body weight gain, insulin sensitivity, by insulin tolerance test (ITT), and glucose tolerance, by oral glucose tolerance test (OGTT), throughout the 25 weeks. BAT samples were collected at the end and used for western blot analysis of the levels of hypoxia-inducible factor2-alpha (Hif-2α), equilibrative nucleoside transporter 1 (ENT1), adenosine receptors (A1, A2A and A2B), inflammation markers as interleukin-1 receptor (IL-1R) and angiogenesis markers as vascular endothelial growth factor VEGF).
Results: HFHSu diet increased weight gain and promoted whole-body insulin resistance and glucose intolerance. Caffeine intake prevented HFHSu-induced increase in weight gain and attenuated insulin resistance, without affecting glucose tolerance in HFHSu animals. In BAT, there was no alteration in A1 receptor levels in HFHSu or HFHSuCAF animals. HFHSu diet decreased the levels of A2B receptor, an effect reverted by caffeine treatment (CTL = 100% ± 29.93; HFHSU = 63.43% ± 19.30, p<0.05 comparing to CTL; HFHSuCAF = 109.77% ± 33.72, p<0.01 comparing to HFHSu). ENT1 levels expression increased with HFHSu diet while caffeine treatment decreased these levels (CTL = 100% ± 25.06; HFHSu = 159.4% ± 59.45, p<0.01 comparing to control; HFHSuCAF = 69.78% ± 24.72, p<0.01 comparing to HFHSu). Hif-2α levels showed a significant increase with the HFHSu diet, effect attenuated by caffeine treatment (CTL = 100% ± 20.15; HFHSu = 162.8% ± 55.48, p<0.05 comparing to CTL; HFHSuCAF = 130.4% ± 21.66). HFHSu diet significantly reduced VEGF levels and caffeine attenuated this reduction (CTL = 100% ± 21.68; HFHSu = 76.98% ± 19.32, p<0.05 comparing to CTL; HFHSuCAF = 90.43% ± 21.45). IL-1R levels was increased by HFHSu diet, being this decreased by caffeine (CTL = 100% ± 21.72; HFHSu = 148% ± 74.20, p<0.05 comparing to CTL; HFHSuCAF = 64.13% ± 21.13, p<0.05 comparing to HFHSu).
Conclusion: Caffeine improvements in weight gain and insulin resistance were accompanied by the reversion of diet-induced dysfunction in BAT. Modulation of BAT adenosinergic system can be a therapeutic target for the treatment of obesity and insulin resistance.
Supported by: FCT, FOM: CEECIND/04266/2017.
Disclosure: A.C.F. Soares: None.
SO 48 Hypoglycaemia: hip hip hooray yet to come
625
Hypo-metrics: associations between hypoglycaemia awareness, blood glucose range and patient-reported hypoglycaemia symptoms in type 1 diabetes
G. Martine-Edith1, P. Divilly1, N. Zaremba1, U. Søholm1,2, Z. Mahmoudi3, M. Gomes3, M. Broadley2, J. Speight2,4, S.A. Amiel1, F. Pouwer2,4, P. Choudhary1,5, Hypo-RESOLVE Consortium;
1Department of Diabetes, King's College London, London, UK, 2Department of Psychology, University of Southern Denmark, Odense, Denmark, 3Department of Digitalization and Modelling, Novo Nordisk A/S, Copenhagen, Denmark, 4Deakin University, Geelong, Australia, 5Diabetes Research Centre, University of Leicester, Leicester, UK.
Background and aims: The experiences of hypoglycaemia vary between- and within-individuals with diabetes. Yet, we lack a tool to measure and document hypoglycaemia symptoms experienced in daily life. Using preliminary data from a novel mobile-phone based app developed for participants to record hypoglycaemia symptom intensity in real time for the Hypo-METRICS study, we evaluated the associations between hypoglycaemia awareness, blood glucose range and patient-reported hypoglycaemia symptoms.
Materials and methods: In the 10-week study, participants with type 1 diabetes used the app to record all episodes of symptomatic or measured hypoglycaemia. At, or near, the time of the hypoglycaemic event, participants reported hypoglycaemia symptoms (e.g., sweating, heart palpitations shaking and hunger) and measured glucose (<2 mmol/L, 2-2.9mmol/L, 3-3.9mmol/L and >3.9mmol/L). A symptom was considered to be absent if participants rated its intensity as ‘Not at all’ and present if rated as ‘A little bit’, ‘Somewhat’, ‘Quite a bit’ or ‘Very much’. Adjusting for capillary or continuous glucose monitoring, we used mixed-effects logistic regression to model the associations between hypoglycaemia awareness (defined by Gold score), glucose range and the presence of autonomic symptoms of hypoglycaemia.
Results: 142 participants (72% using Flash/continuous glucose monitoring and 24% with impaired awareness of hypoglycaemia (IAH)) reported 3,994 hypoglycaemia episodes. Median(IQR) age was 49(37-60) years and diabetes duration 23(11-37) years. Participants with IAH had lower odds of reporting shaking (OR 0.29 (95% CI 0.14-0.61)) than those with normal awareness. As shown in Figure 1, except for hunger, participants had higher odds of reporting autonomic symptoms at lower glucose levels.
Conclusion: Using Hypo-METRICS app data, we identified quantitative differences in the reporting of hypoglycaemia autonomic symptoms according to hypoglycaemia awareness and glucose range. As there is potential to also assess variability in symptom intensity, the app provides a comprehensive tool to evaluate hypoglycaemia symptoms.
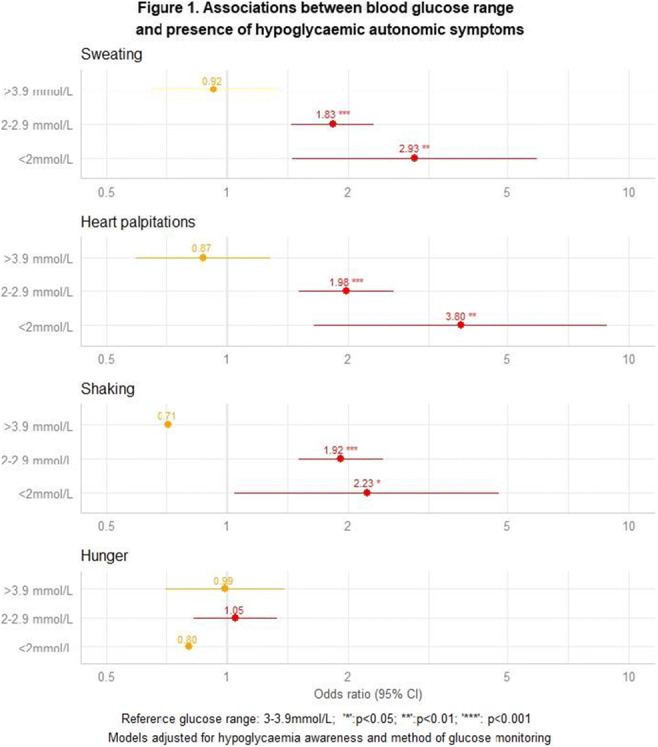
Clinical Trial Registration Number: NCT04304963
Supported by: IMI
Disclosure: G. Martine-Edith: Employment/Consultancy; Novo Nordisk.
626
Persistence of impaired awareness of hypoglycaemia, severe hypoglycaemic events and suboptimal glycaemic control despite advanced diabetes technologies
J. Sherr1, J. Liu2, L. Titievsky3, K. Hagan3, T. Liu3, K. Chandarana3, J. Gaglia3, W. Wolf2, J. Bispham4, K. Chapman2, D. Finan2, J. Pettus5;
1Yale University School of Medicine, New Haven, 2T1D Exchange, Boston, 3Vertex Pharmaceuticals, Boston, 4Evidera PPD, Waltham, 5University of California San Diego, San Diego, USA.
Background and aims: Trials of continuous glucose monitors (CGMs) and hybrid closed-loop systems (HCLS) demonstrate improvements in glycaemia with reductions in hypoglycaemia in T1D, but there is a paucity of real-world data on how these technologies impact prevalence of impaired awareness of hypoglycaemia (IAH), severe hypoglycaemic events (SHEs), and glycaemic control.
Materials and methods: We conducted a one-time online survey of adults with T1D in the T1D Exchange registry and online communities. Self-reported medical histories (insulin delivery method, HbA1c, IAH, SHEs) and CGM data were collected.
Results: 2,044 patients (mean age 43 y, mean T1D duration 26 y, 72% female, 95% White) completed the survey. Most reported using CGM (92%); 953 also used HCLS. Mean HbA1c was 6.89%, 41.5% had a HbA1C of ≥ 7%, 30.7% reported IAH, 19.8% had ≥ 1 SHE in the prior year, 12.0% had ≥ 2 SHEs in the prior year, 9.6% had IAH + ≥ 1 SHE, and 6.6% had IAH + ≥ 2 SHEs (Table). Rates of SHEs and IAH + SHEs were lower in CGM users and in CGM + HCLS users compared to non-CGM users; however, among CGM + HCLS users, 16.6% reported ≥ 1 SHE, 8.7% ≥ 2 SHEs, 7.8% IAH + ≥ 1 SHEs, and 4.7% IAH + ≥ 2 SHEs. Also, 35.6% of CGM + HCLS users had a HbA1C of ≥ 7%.
Conclusion: In a patient cohort with high rates of technology adoption, rates of SHEs and IAH remained high, with a significant proportion of patients not achieving targeted glycaemic control, indicating the need for novel T1D treatments.
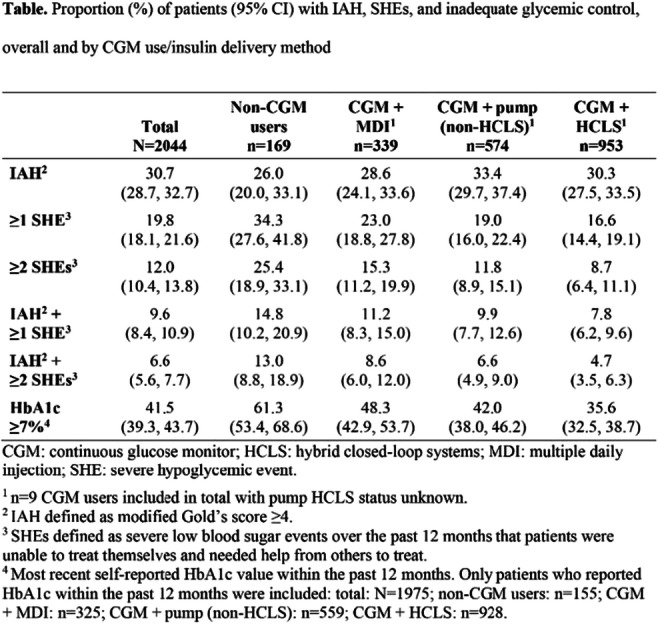
Supported by: Vertex Pharmaceuticals
Disclosure: J. Sherr: Other; J.Sherr has conducted clinical trials for Eli Lilly, Insulet, and Medtronic and has received in-kind support for research studies from Dexcom and Medtronic. She has consulted for Eli Lilly, Lexicon, Medtronic, Sanofi, and Zealand. She has been a member of advisory boards for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, the T1D Fund, and Vertex.
627
The prevalence of hypoglycaemia remains unchanged despite advances in the management of type 1 diabetes
F.A. Baxter1, N. Baillie1, A.R. Dover2, R.H. Stimson1,2, F.W. Gibb2,1, S. Forbes1,2;
1BHF Centre for Cardiovascular Science, University of Edinburgh, 2Department of Diabetes, Royal Infirmary Edinburgh, Edinburgh, UK.
Background and aims: Hypoglycaemia is a well described side-effect of insulin treatment in type 1 diabetes (T1D). Recurrent hypoglycaemia can lead to impaired awareness of hypoglycaemia (IAH) which increases the risk of severe hypoglycaemia (SH), linked to morbidity and mortality. Diabetes technologies have been shown to reduce exposure to hypoglycaemia, however there is little evidence of their impact on IAH. We aimed to survey the prevalence of IAH in the adult T1D clinic of a tertiary teaching hospital and identify factors associated with risk of IAH.
Materials and methods: Adults (age ≥18 years) with a diagnosis of T1D for ≥2 years were approached. They completed a questionnaire which included a Gold score, a Clarke score and questions about history of SH and technology use. Health records were reviewed to collect data on: diabetes history, HbA1c, sex, Scottish Index of Multiple Deprivation (SIMD) quintile and diabetes-related hospital admissions in the previous 12 months. Outcomes were compared between groups with normal awareness of hypoglycaemia and IAH (defined as a Gold score ≥4). Normally distributed data was compared using the independent t-test and non-normally distributed the Mann-Whitney U Test. Categorical data was compared using chi-squared.
Results: 150 people with T1D (58.7% female) completed the survey. Of these 44.7% were insulin pump users and 87.3% used intermittently scanned continuous glucose monitors. 12% of responders were using real-time continuous glucose monitoring and 2.7% hybrid closed-loop therapy. The mean (SD) age of the responders was 40.9 (14.9) years, the time since diagnosis 22 (13.6) years and the age at diagnosis 18.8 (11.2) years. IAH was defined as a Gold or Clarke score ≥4. The proportion of responders with IAH was 18.7%. SH in the previous 12 months was more common in responders with IAH, with 8 (29.6%) vs 13 (10.7%) (p=0.027) reporting an episode. Responders with IAH were more likely to have required treatment with intravenous glucose (p=0.030) and to come from a more deprived SIMD quintile (p=0.007). There was no group difference in the use of diabetes technologies.
Conclusion: This survey has demonstrated that despite advances in the management of T1D the prevalence of IAH has remained unchanged. The fact that there was no group difference in the use of technology highlights the need for further studies exploring the benefit of these systems for people with IAH. The survey also demonstrated that those with IAH are still at increased of SH, although the overall number of episodes was low. The use of the more advanced technologies, such as HCL, in this patient cohort was low but this is reflective of technology use in our patient population, where reimbursement for isCGM and CSII is more common. Worryingly this survey has highlighted ongoing socioeconomic disparities, with those living in the most deprived areas more likely to have IAH.
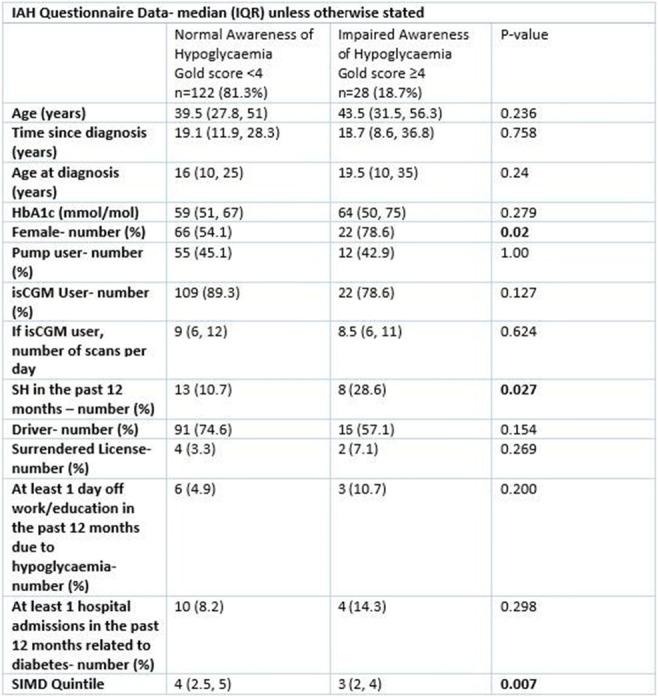
Supported by: FA Baxter and N Baillie are supported by a grant for the Helmsley Charitable Trust
Disclosure: F.A. Baxter: None.
628
Patterns of sensor-detected hypoglycaemia in long-duration CGM data from a diabetes registry
P.M. Baumann, D.A. Hochfellner, M. Cigler, L. Roubik, T. Pöttler, J.K. Mader, on behalf of the Hypo-RESOLVE Consortium;
Division of Endocrinology & Diabetology, Medical University of Graz, Graz, Austria.
Background and aims: Pooled analysis of long-duration CGM data from a diabetes registry allows for large-scale analysis of sensor-detected hypoglycaemic episodes (SDHEs) as well as exploration of daily glucose and hypoglycaemia patterns to supplement conventional analysis of time below range (TBR)
Materials and methods: 66 patients with T1D used CGM over 365.7 (340.6-392.5) days (24.530 days total). In addition to TBR, we explored frequency of SDHE >15 minutes at <70, <54 and 40 mg/dl by time of day and glycaemic control by pooled individual days (12AM-12AM). To determine if (hypo)glycaemic variability is largest within days, between days or between subjects (variance decomposition), we used empty 3-level random intercept models.
Results: TBR was 5.4% (<70), 1.9% (<54) and 0.7% (=40). Proportion of nightly (0-6am) SDHE was 24.7% (<70), 34.4% (<54) and 41.5% (=40). Visual analysis of pooled CGM days (Figure 1) showed a decrease and greater variance in glucose levels during the night, nightly drop of glucose and a surplus of nightly readings below 54mg/dl. Variability in glucose was mainly due to daily fluctuations (64.8% of variance in glucose happens within a day), followed by differences between days (20.2%) and differences between patients (15.0%). For hypoglycaemia, within-day fluctuations (between 40-70mg/dl) are similar to overall glucose (65.1%), but between-day differences are more pronounced (28.3%). Between-patient differences are only minimal (6.6%).
Conclusion: Focus on hypoglycaemic patterns proved to be a valuable addition to TBR. Low SHDEs exhibited a nightly surplus, which is also reflected in the visual analysis. (Low) glucose readings vary mostly within the day, followed by fluctuations between days, and least between patients. Severity of hypoglycaemia hardly varies between patients.
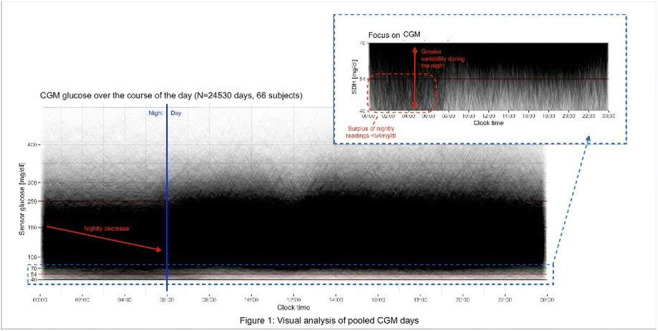
Supported by: IMI2 Grant Agreement number: 777460
Disclosure: P.M. Baumann: None.
629
Real-world estimates of severe hypoglycaemia and associated healthcare utilisation in the US: prospective, longitudinal results of the iNPHORM study
A. Ratzki-Leewing1, J. Black1, A. Kahkoska2, B. Ryan1, S. Harris1;
1Western University, London, Canada, 2UNC School of Medicine, Chapel Hill, USA.
Background and aims: Severe hypoglycemia (SH) surveillance in the United States (US) often uses data from electronic medical or claims records. However, these sources fail to capture hypoglycemia events not requiring healthcare utilization (i.e., paramedical services, emergency department [ED] care, hospitalization). To better understand SH burden in the US, we quantified real-world SH rates and associated healthcare utilization using prospective, longitudinal, self-reported data from the iNPHORM study.
Materials and methods: Adults (18-90 years old) with insulin- and/or secretagogue-treated type 1 or 2 diabetes (T1D, T2D) were recruited from a US-wide probability-based internet panel. Twelve monthly online questionnaires were distributed assessing respondents’ socio-demographic and clinical characteristics, as well as self-reported SH events. Between February 2020 and March 2021, annual crude SH incidence rates and proportions were estimated using negative binomial regression and Wilson’s confidence intervals for binomial proportions, respectively. Frequencies were computed overall, and by diabetes type, medication regimen, and type of healthcare utilization required.
Results: Data from 985 participants were analyzed (T1D: 17%; age: 51 [SD: 14.3] years; male: 49.5%; T1D/T2D duration: 12 [IQR: 14] years; retention rate: 88.6%). Among T2D respondents at baseline, 38% were on insulin alone, 38% secretagogues alone, and 24% insulin plus secretagogues. Overall, the rate of SH was 4.99 (95% CI: 4.14 to 6.01) events per person-year (EPPY), and 33.1% (95% CI: 30.2 to 36.0%) reported ≥1 event. Fewer participants with T2D than T1D reported experiencing ≥1 SH event over follow-up (30.9% [95% CI: 27.9 to 34.1%] vs. 44.2% [95% CI: 36.9 to 51.9%], respectively); however, their SH rates were comparatively higher (T2D: 5.27 [95% CI: 4.25 to 6.53] EPPY; T1D: 3.55 [95% CI: 2.49 to 5.08] EPPY). Among participants with T2D, those using insulin plus secretagogues reported experiencing higher crude rates (14.6 [95% CI: 10.9 to 19.7] EPPY) than those using insulin alone (1.67 [95% CI: 1.23 to 2.26] EPPY) or secretagogues alone (3.78 [95% CI: 2.50 to 5.73] EPPY). Of all SH events (n=4012), over half (62.9%) were treated outside of hospital. ED care was required in 9.1% of events (T1D: 2.7%; T2D: 9.9%), and only 4.6% of events (T1D: 1.6%; T2D: 4.9%) resulted in hospitalization. Participants with T2D, as compared to T1D, reported higher SH frequencies resulting in paramedical services, ED care, or hospitalization (1.34 [95% CI: 0.95 to 1.88] EPPY versus 0.18 [95% CI: 0.07 to 0.41] EPPY, respectively). Rates of SH resulting in healthcare utilization were highest among people with T2D taking insulin plus secretagogues (3.98 [95% CI: 2.85 to 5.55] EPPY) versus insulin alone (0.22 [95% CI: 0.08 to 0.60] EPPY) or secretagogues alone (1.07 [95% CI: 0.52 to 2.21] EPPY).
Conclusion: This analysis offers the first prospective, longitudinal data on self-reported SH rates in the US and associated healthcare utilization. In this study, SH was an alarmingly common issue that affected both T1D and T2D. A minority of events resulted in healthcare utilization, suggesting that surveillance based on healthcare records underestimate the true real-world burden of SH. Future studies should integrate self-reported data with clinical and administrative records to improve epidemiological studies and SH prevention in the real world.
Clinical Trial Registration Number: NCT04219514
Supported by: Sanofi Global
Disclosure: A. Ratzki-Leewing: Grants; Sanofi Global. Honorarium; Eli Lilly, Novo Nordisk, Sanofi Canada.
630
Level of physical activity and risk of severe hypoglycaemia in type 1 diabetes
P.L. Kristensen1, S. Caunt2, R.F. Johansen3, S.E. Sander4, E. Søndergaard3, S. Heller5, S. Mølsted1;
1Nordsjællands Hospital, Hillerød, Denmark, 2Academic Directorate of Diabetes & Endocrinology, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK, 3Steno Diabetes Center Aarhus, Aarhus, Denmark, 4Department of Clinical Research, Nordsjællands Hospital, Hillerød, Denmark, 5University of Sheffield, Sheffield, UK.
Background and aims: Physical activity (PA) has been proposed to improve the counterregulatory response to hypoglycaemia. Therefore, PA may have the potential to improve hypoglycaemia awareness and reduce the risk of severe hypoglycaemia. However, the interplay between awareness status, PA and risk of severe hypoglycaemia is poorly described. The aim of this study was to investigate the association between self-reported PA and risk of severe hypoglycaemia in people with type 1 diabetes (T1D).
Materials and methods: This was a cross-sectional study with data from a survey and patient records. We approached adults with T1D from the outpatient clinics at Northern General Hospital in Sheffield (UK), Nordsjællands Hospital (DK) and Steno Diabetes Center Aarhus (DK). Data on self-reported PA were collected using the Saltin-Grimby scale. Hypoglycaemia awareness was assessed using the Pedersen-Bjergaard method, and clinical variables were collected in patient records. Respondents were stratified into three PA groups: moderate-vigorous PA, light PA, and physically inactive. A log-linear negative binomial regression model was applied with events of self-reported severe hypoglycaemia for the last 12 months as the dependent variable. The independent variables included PA levels and hypoglycaemia unawareness. The analysis was adjusted for age and HbA1c.
Results: In total we included questionnaires from 338 persons with T1D (31% from UK and 69% from DK, 47% females, age 47±18 (mean±SD) years, duration of diabetes 23±16 years, 29% on insulin pumps). 83% had experienced zero episodes of severe hypoglycaemia in the preceding year, 10% had experienced 1-3 episodes, and 7% had experienced 4 or more episodes. In the univariate model, decreasing age (0.022 [0.005; 0.039], p=0.010), normal hypoglycaemia awareness vs unawareness (-0.85 [-1.6; -0.1], p=0.033), and moderate-vigorous PA vs inactivity (-2.1 [-2.8; -1.4], p<0.001) were associated with reduced episodes of severe hypoglycaemia. In the adjusted analysis, moderate-vigorous PA vs inactivity (-2.1 [-2.8; -1.4], p<0.001) was associated with reduced episodes of severe hypoglycaemia, in a dose dependent way. Hypoglycaemia awareness and age were not associated with episodes of severe hypoglycaemia in the adjusted model.
Conclusion: A lifestyle with a low level of physical activity is associated with increased risk of severe hypoglycaemia, even after adjustment for hypoglycaemia awareness status, HbA1c and age. While this suggests a link between physical activity and hypoglycaemia awareness, potential causality and mechanism of action remains to be established.
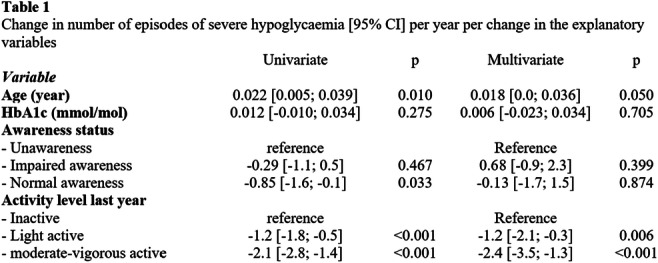
Disclosure: P.L. Kristensen: None.
631
Glycaemic events during exercise can be effectively predicted with machine learning using only start glucose and duration
C.L. Russon1, R.C. Andrews1, R.M. Pulsford2, N. Vaughan1, M. Allen3;
1Exeter Centre of Excellence for Diabetes Research (EXCEED), University of Exeter, 2College of Environmental Sciences, University of Exeter, 3College of Medicine, University of Exeter, Exeter, UK.
Background and aims: When people with Type 1 diabetes (T1D) exercise, we suggest they stay in the range of 7-15mmol/L and take action if glucose moves outside of this range. In a study of 39 people with T1D who took part in a half marathon, 70% had to stop to deal with a low or high glucose event. If people with T1D were warned pre-exercise of the risk of a glycaemic event, it would allow them to take countermeasures to prevent these events occurring. Using standard demographic data, blood tests and information about the exercise bout, we aimed to examine whether machine learning (ML) could predict low or high glucose events during exercise.
Materials and methods: Data came from 2 exercise studies (EXTOD education and EXTOD 101) with each having data on age, sex, length of diabetes, body mass index, HbA1c, C-peptide and time, length, intensity (BORG scale) and type (aerobic, anaerobic and mixed) of exercise sessions. EXTOD education had 2 weeks of Dexcom G6 data from 54 participants and EXTOD 101 8 weeks of Freestyle Libre data from 34 participants. In total there were 976 bouts of exercise. The thresholds for low glucose and high glucose were set to 7 and 15mmol/L. A single reading below or above the threshold was considered as a positive event. 486 bouts contained a glucose reading below 7 and 151 had glucose reading above 15mmol/L. 80% of this data was used to train an ML algorithm (XGBoost) which then determined how important each measure was and what was the best combination. This was then tested on the remaining 20% of data using area under the receiver operator curve (ROC AUC) score as the validation metric.
Results: For predicting a glucose reading below 7mmol/L, the addition of the best 6 measures identified by ML resulted in a ROC AUC score 0.902. These, in order of importance, were glucose at start of exercise (starting glucose), duration of exercise, type of exercise, intensity, time of day and C-peptide. For predicting glucose above 15mmol/L, incorporating the best 7 measures resulted in a ROC score of 0.973. These were start glucose, duration, intensity, sex, years since diagnosis, C-peptide, and time of day. The two most important measures were start glucose and duration of exercise bout in both contexts. Using only these features, a ROC AUC score of 0.890 was achieved for predicting low glucose and 0.968 for high glucose. The model accuracy and sensitivity-specificity intersect was 0.804 and 0.804 for low glucose and 0.949 and 0.900 for high. Using logistic analysis, we have taken these two measures to develop a heat map that can help patients predict their risk of going below 7 or above 15mmol/L during an exercise.
Conclusion: ML has produced a simple heat map to predict risk of glucose going below 7 or above 15mmol/L during exercise. We will go on to look at whether it can predict lower glucose levels during exercise and at times further away from the start of exercise.
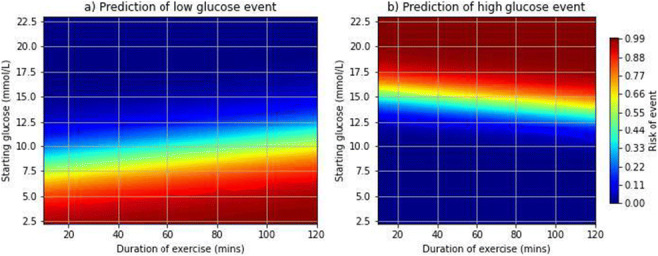
Disclosure: C.L. Russon: None.
632
Sustained pro-inflammatory effects following hypoglycaemia in people with type 1 or type 2 diabetes
J.I.P. van Heck1, C.E.M. Verhulst1, T.W. Fabricius2, R. Stienstra1,3, C.J. Tack1, U. Pedersen-Bjergaard2,4, B.E. de Galan1,5, hypo-RESOLVE consortium;
1Internal Medicine, Radboudumc, Nijmegen, Netherlands, 2Endocrinology and Nephrology, Nordsjællands Hospital, Hillerød, Denmark, 3Nutrition, Metabolism and Genomics Group, Wageningen University, Wageningen, Netherlands, 4Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 5Internal medicine, Maastricht University Medical Centre, Maastricht, Netherlands.
Background and aims: Hypoglycaemia acutely activates the immune system and is associated with an increased risk for atherosclerotic disease. Since atherosclerosis is driven by chronic inflammation, we set out to determine the duration of the pro-inflammatory response to a hypoglycaemic event in people with or without diabetes.
Materials and methods: Adults with type 1 diabetes (n = 47) or insulin-treated type 2 diabetes (n = 15), and age-matched controls without diabetes (n = 32) underwent a hyperinsulinaemic-euglycemic (5.2 ± 0.4 mmol/L)-hypoglycaemic (2.8 ± 0.1 mmol/L) glucose clamp. Participants were 54% male, had an average age of 51 (±17) year, BMI of 26 (±4) kg/m2, HbA1c pf 53 (±16) mmol/mol and a disease duration of 20 (±13) years. Before, during and up to a week thereafter, blood was drawn to determine circulating immune cell composition using flow cytometry combined with measurements of circulating hs-CRP concentrations and a proteomics panel consisting of 92 circulating inflammatory markers.
Results: In response to hypoglycemia, absolute numbers of lymphocytes (1.57 ± 0.53 vs 2.69 ± 0.95·103/μL) and monocytes (0.40 ± 0.14 vs 0.62 ± 0.22·103/μL) increased acutely and remained elevated for one week in all subgroups (all p < 0.001). During hypoglycemia, the proportion of pro-inflammatory CD16+-monocytes increased from 26.9 ± 13.3% to 38.1 ± 14.9% and the proportion of phagocytotic CD14+-monocytes decreased from 65.2 ± 14.4% to 54.5 ± 15.9% in all subgroups (all p < 0.01). Hs-CRP concentrations increased from 2.07 ± 3.20 to 2.71 ± 3.57 μg/mL 1 day after the clamp (p < 0.001) and remained elevated for one week (p < 0.001) to similar extent in all subgroups. Furthermore, 33 inflammatory proteins increased during hypoglycaemia (corrected p-value<0.05), including IL-10 and IL-6. Most inflammatory proteins, increased after 1 day (37 proteins) and remained elevated until 7 days in all subgroups (corrected p-value<0.05). Other proteins, such as IFN-gamma, reached their peak after 7 days (correct p-value<0.05).
Conclusion: Hypoglycaemia induces a prolonged immune response both at the protein and the cellular level in humans, independent of the presence of type 1 or type 2 diabetes.
Clinical Trial Registration Number: NCT03976271
Supported by: Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 777460
Disclosure: J.I.P. van Heck: None.
633
Impact of polymorphism in the beta-2 receptor gene on the metabolic response to epinephrine before and after repeated hypoglycaemia in healthy humans
K.Z. Rokamp1,2, N.V. Olsen3,4, F. Dela5,6, L. Grønlykke2, N.H. Secher7, B. Thorsteinsson1, U. Pedersen-Bjergaard1,8;
1Endocrine Section, Department of Endocrinology, Copenhagen University Hospital, Nordsjællands Hospital, Hillerød, 2Department of Anesthesia, Zealand University Hospital-Køge, Køge, 3Department of Neuroscience and Pharmacology, University of Copenhagen, Copenhagen, 4Department of Neuroanesthesia, Copenhagen University Hospital, Rigshospitalet, Copenhagen, 5Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, 6Department of Geriatrics, Bispebjerg-Frederiksberg Hospital, Copenhagen, Denmark, 7Department of Anesthesiology, Centre for Cancer and Organ Diseases, Copenhagen University Hospital, Rigshospitalet, Hillerød, 8Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Background and aims: The beta-2 adrenergic receptor mediates the metabolic response to epinephrine. The Arg16 variant of the single nucleotide polymorphism Gly16Arg in the beta-2 adrenergic receptor gene (ADRB2) has been associated with an increased risk of severe hypoglycemia in type 1 diabetes. This study investigates the impact of the Arg16 variant on the metabolic response to epinephrine before and after repetitive hypoglycemia.
Materials and methods: Twenty-five healthy male subjects selected according to ADRB2 genotype being homozygous for either Gly16 (GG; n=12) or Arg16 (AA; n=13) participated in 4 trial days (D1-4), D1pre and D4post with epinephrine 0.06 ug kg-1 min-1 infusion, and D2hypo1-2 and D3hypo3 with three periods of hypoglycemia by an insulin-glucose clamp.
Results: At D1pre the insulin (44 ± 8 vs. 93 ± 13 pmol l-1 h; P = 0.0051) and glycerol (79 ± 12 vs. 115 ± 14 μmol l-1 h; P = 0.041) and free fatty acids (FFA) (724 ± 96 vs. 1113 ± 140 μmol l-1 h; P = 0.033) responses (mean ± SEM of area under the curve) to epinephrine were decreased in AA subjects compared to GG subjects but without difference in glucose response. The glycerol and FFA responses were attenuated in GG subjects at D4post compared to D1pre and without difference between genotype groups at D4post.
Conclusion: The initial metabolic substrate response to epinephrine is decreased in AA subjects, but GG subjects are more prone to adrenergic desensitization, with no difference between genotype groups in response to epinephrine after antecedent hypoglycemia.
Supported by: The Novo Nordisk Foundation and the Jascha Foundation
Disclosure: K.Z. Rokamp: None.
SO 49 Is newer (insulin) always better?
634
Ado09, a co-formulation of pramlintide and insulin A21G, improves body weight and treatment satisfaction compared to insulin lispro in people with type 1 diabetes (PwT1D)
R. Eloy1, M. Gaudier1, E. Baumgaertner2, C. Seroussi1, C. Mégret1, B. Kronshage3, Y.-P. Chan1, O. Soula1, T. Heise3, G. Andersen3;
1Adocia, Lyon, France, 2Profil Mainz, Mainz, 3Profil Neuss, Neuss, Germany.
Background and aims: Pramlintide in conjunction with insulin has been shown to improve post-prandial glucose control, but requires additional injections. ADO09 is a co-formulation of pramlintide and insulin (insulin A21G) allowing use of pramlintide-insulin combination therapy without increased injection frequency. This open label, multi-centric, two-arm parallel phase 2 trial investigated the efficacy and safety of ADO09 versus insulin lispro (LIS) in people with type 1 diabetes.
Materials and methods: During a run-in period of 2-4 weeks, basal insulin was switched to insulin degludec or glargine and continuous glucose monitoring (CGM) initiated. Thereafter, baseline assessments including CGM were done over a 3-week period. The treatment period consisted of a 4-week titration period for adjustment of prandial and basal doses followed by a 12-week maintenance period. Dose titration was guided by a Data Monitoring Committee recommending adjustments of insulin doses to PwT1D based on CGM data.
Results: Eighty PwT1D (63 male, 17 female, age 44.2±12.3 years (mean±SD), BMI 28.6±2.6 kg/m2 body weight 90.2±13.5 kg, HbA1c 7.7±0.6%) were randomised 1:1 to ADO09 or LIS. There were no clinically relevant changes in HbA1c with either treatment (LS mean change from baseline 0.13% vs. 0.11% (ADO09 vs. LIS), p=0.81), but ADO09 reduced body weight significantly compared to baseline over the 4 months of treatment while LIS did not (-2.51±3.0 kg vs -0.45±3.1 kg, LSMean difference -2.13 kg, p=0.0045). The incidence of hypoglycaemic events was comparable between both treatments (331 episodes in 31 (77.5%) subjects with ADO09 vs. 326 episodes in 30 (75.0%) subjects with LIS). Severe hypoglycaemia was rare (2 events with ADO09, 1 event with LIS). Both treatments were well tolerated with transient, gastrointestinal adverse events predominantly of mild intensity with ADO09 (15 vs 0 subjects), consistent with the known side effect profile of pramlintide. Other adverse events were similar between treatments. Study participants expressed higher treatment satisfaction with ADO09 versus LIS at the end of the treatment period (standardised questionnaire with 6-point Likert scale). More subjects on ADO09 than on LIS strongly agreed that blood glucose control was more predictable (12.5% vs. 5%) and 45% agreed or strongly agreed that appetite control was easier with ADO09 (LIS 20%). Likewise, 37.5% of PwT1D agreed or strongly agreed that they would like to continue taking ADO09 (LIS 25%) and 30% of study participants agreed or strongly agreed that ADO09 provided benefits that their standard insulin therapy had not provided (LIS 17.5%).
Conclusion: In this 4 months treatment study in PwT1D, ADO09 showed similar overall effects on glycaemic control and hypoglycaemia to LIS, but significantly lowered body weight and improved treatment satisfaction.
Clinical Trial Registration Number: NCT04816890
Disclosure: R. Eloy: Employment/Consultancy; Adocia. Stock/Shareholding; Adocia.
635
Tertiary CGM endpoints comparing second-generation basal insulin analogues glargine 300 U/mL and degludec 100 U/mL in people with type 1 diabetes: inrange randomised controlled trial
R. Bergenstal1, S. Edelman2, P. Choudhary3, T. Danne4, E. Renard5, J. Westerbacka6, B. Mukherjee6, P. Picard7, V. Pilorget6, T. Battelino8;
1International Diabetes Center at Park Nicollet, Minneapolis, USA, 2University of California, San Diego, USA, 3Diabetes Research Centre, University of Leicester, Leicester, UK, 4Diabetes Centre for Children and Adolescents, Children’s and Youth Hospital “Auf Der Bult”, Hannover, Germany, 5Department of Endocrinology, Diabetes and Nutrition, Montpellier University Hospital, University of Montpellier, Montpellier, France, 6Sanofi, Paris, France, 7IVIDATA Life Sciences, Levallois-Perret, France, 8UMC–University Children’s Hospital, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.
Background and aims: The InRange study compared the second-generation basal insulin analogues insulin glargine 300 U/mL (Gla-300) and insulin degludec 100 U/mL (IDeg-100) after 12 weeks of treatment in people with T1D using blinded continuous glucose monitoring (CGM)-derived time spent in glucose range (TIR) 70-180 mg/dL (3.9-10 mmol/L), and total glucose coefficient of variation (CV) as primary and main secondary endpoints. In the primary analyses, the non-inferiority of Gla-300 to IDeg-100 was demonstrated for primary and main secondary endpoints. Here the results for the tertiary CGM endpoints are presented.
Materials and methods: InRange (NCT04075513) was a multicentre, randomised, active-controlled, parallel-group, 12-week, open-label trial comparing Gla-300 vs IDeg-100 in adults with T1D using 20-day CGM profiles (≥10 days evaluable). Here we present descriptive, exploratory data for CGM metrics including mean glucose, Glucose Management Indicator (GMI), and time spent above, within and below glucose ranges, at Week 12.
Results: Overall, 343 participants were randomised; 172 received Gla-300 and 171 IDeg-100. Mean (SD) age was 42.8 (13.3) years, T1D duration was 20.5 (12.8) years and BMI was 27.3 (4.8) kg/m2; a third (33.8%) of participants had at least 1 diabetic complication. HbA1c (%) at Week 12 was 7.5 (0.8) for Gla-300 and 7.4 (0.8) for IDeg-100. Mean number of days of evaluable CGM data at Week 12 was 16 for both treatment groups. Mean (SD) glucose (mg/dL) at Week 12 was 174.9 (30.6) and 167.8 (28.8), for Gla-300 and IDeg-100, respectively (Table). Mean (SD) GMI (%) at Week 12 was 7.5 (0.7) and 7.3 (0.7), respectively. At Week 12, mean (SD) percent TIR 70-180 mg/dL at any time (24 h) for Gla-300 and IDeg-100 was 51.9 (13.8) and 55.4 (13.7), respectively; percent time spent above range (TAR) >180 mg/dL was 42.2 (15.5) and 38.4 (15.4); percent time spent below range (TBR) <70 mg/dL was 5.9 (5.4) and 6.2 (5.9); percent TBR <54 mg/dL was 2.0 (2.8) and 2.1 (3.2). TBR at Week 12 was similar between groups during the night (00:00-05:59 h).
Conclusion: Using clinically relevant CGM-based metrics, the InRange study shows that after 12 weeks of treatment with Gla-300 or IDeg-100, comparable CGM-derived outcomes are observed in people with T1D.
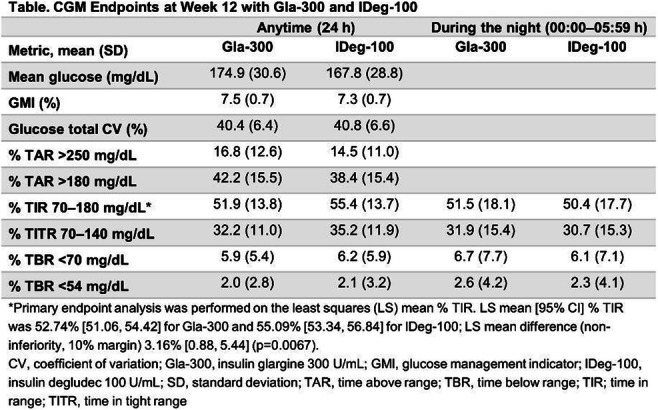
Clinical Trial Registration Number: NCT04075513
Supported by: Sanofi
Disclosure: R. Bergenstal: Other; Dr. Bergenstal’s employer, non-profit HealthPartners Institute, contracts for his services and he receives no personal income for these services.
636
Congenital malformations among offspring of women with type 1 diabetes using insulin pump: a prospective cohort study
I.H. Thorius1, L.L.N. Husemoen1, R.B. Nordsborg1, A.C. Alibegovic2, M.-A. Gall2, J. Petersen3, E.R. Mathiesen4;
1Epidemiology | AA, AI & RWD | Data Science, Novo Nordisk A/S, Søborg, 2Medical & Science, Insulin & Devices, Clinical Drug Development, Novo Nordisk A/S, Søborg, 3Department of Clinical Pharmacology and Center for Clinical research and Prevention, Copenhagen University Phase IV unit, Copenhagen, 4Dept. of Endocrinology ,Rigshospitalet and Dept. of Clinical Medicine, Copenhagen University, Center for Pregnant Women with Diabetes, Copenhagen, Denmark.
Background and aims: Congenital malformations and perinatal mortality are 2-4 fold more frequent in offspring of women with type 1 diabetes (T1D) compared with the background population and associated with poor glycaemic control during early pregnancy. Continuous subcutaneous insulin infusion by insulin pump (pump) has proven superior to improve glycaemic control compared with conventional multiple daily insulin injection (MDI), however, whether pump treatment leads to improved pregnancy outcome regarding congenital malformations and perinatal death remains unknown. The present aim was to evaluate the risk of severe adverse pregnancy outcomes such as malformations, perinatal and neonatal death in pregnant women with T1D treated with insulin using pump or MDI.
Materials and methods: The present study is a secondary analysis of the prospective, multinational cohort of 2088 pregnant women with type 1 diabetes in a real-world setting treated with pump (n= 750) or MDI (n=1338), EVOLVE study. Odds ratios (OR) for offspring with congenital malformations, perinatal or neonatal death were analysed on crude data and using logistic regression on propensity score-matched data.
Results: At enrolment (gestational week 8 (95%CI: 4-14), pump users had higher educational level (university degree: 43.0% vs. 27.4%; P<0.001), better average glycaemic control (HbA1c: 51 (10) mmol/mol (6.8 (0.9) %) vs. 54 (14) mmol/mol (7.1 (1.3) %), P < 0.001). Moreover, a greater proportion of pump users had HbA1c level below 75 mmol/mol (9%) (97.6% vs. 91.9%, P < 0.001) and were more often reporting taking folic acid supplementation compared with MDI users (86.3% vs. 74.9%; P<0.001). All clinically important potential confounders were balanced after propensity score matching and HbA1c remained lower in pump users. The proportion of fetuses with at least one malformation was 13.5% (pump) vs. 11.2% (MDI) (crude OR 1.23 [95%CI 0.94;1.61], P=0.13; propensity score matched (adjusted) OR 1.11 [95%CI 0.81;1.52], P=0.52). The proportion of fetuses with at least one major malformations was 2.8% vs. 3.1% (crude OR 0.89 [95%CI 0.52;1.51], P=0.66; adjusted OR 0.78 [95%CI 0.42;1.45], P=0.43), and fetuses carrying ≥1 minor malformations (but no major) were 10.7% vs. 8.1%; (crude OR 1.36 [95%CI 1.00;1.84], P=0.05; adjusted OR 1.23 [95%CI 0.87;1.75], P=0.25). The proportions of perinatal and neonatal death were 1.6% vs. 1.3% (crude OR 1.23 [95%CI 0.57;2.67], P=0.59; adjusted OR 2.02 [95%CI 0.69;5.93], P=0.20) and 0.3% vs. 0.3% (crude OR 0.90 [95%CI 0.16;4.91], P=0.90; adjusted OR 1.00 [95%CI 0.14;7.12], P=1.00), respectively.
Conclusion: Insulin pump treatment in pregnant women women with T1D was associated with similar risk of congenital malformations, despite better glycaemic control in early pregnancy compared with MDI in this prospective cohort study. Further studies exploring the efficacy and safety of using pump treatment during pregnancy are needed.
Clinical Trial Registration Number: NCT01892319
Supported by: The study was funded by Novo Nordisk A/S
Disclosure: I.H. Thorius: Employment/Consultancy; Novo Nordisk A/S. Stock/Shareholding; Novo Nordisk A/S.
637
Patient and physician experience of hypoglycaemia during basal insulin titration in type 2 diabetes in the US
S. Harris1, K. Mohammedi2, M. Bertolini3, V. Walker4, M. Carlyle4, F. Liz Zhou3, J. Seufert5, J. Anderson6;
1Schulich School of Medicine & Dentistry, The University of Western Ontario, London, Canada, 2Faculty of Medicine, University of Bordeaux, Bordeaux, France, 3Sanofi, Bridgewater, USA, 4Optum, Minnesota, USA, 5Faculty of Medicine, University of Freiburg, Freiburg, Germany, 6The Frist Clinic, Nashville, USA.
Background and aims: Hypoglycaemia after BI initiation can negatively impact adherence to titration and glycaemic target achievement. We report on 2 surveys to better understand patient and physician perspectives/experiences of hypoglycaemia during BI titration.
Materials and methods: Adults with T2D (PWT2D) and ≥2 claims (≥30 days apart in last 12 months) in the Optum Research Database who initiated BI (February-April 2021), and physicians who treated ≥30 PWT2D, ≥1 initiating BI (October 2020-March 2021), completed a mailed survey.
Results: Responders were 416 PWT2D (51% male, 71% white, mean age 70 years, 72% >10 years T2D duration) and 386 physicians (45% general practice). Most physicians reported discussing hypoglycaemia signs/symptoms (93%) and how to titrate BI in response to blood glucose (BG) levels (81%) with PWT2D. Of the 414 responders, 49% experienced hypoglycaemia; 37 (19%) of 194 responders had severe hypoglycaemia. Out of 187 responders experiencing hypoglycaemia, 57% felt very/extremely confident titrating BI during hypoglycaemia (Figure). Only 35% of all patients met fasting BG (FBG) targets. Diabetes Treatment Satisfaction Questionnaire hypoglycaemia score (1.34/6) suggests patients felt hypoglycaemia was infrequent.
Conclusion: While physicians educate PWT2D on hypoglycaemia awareness and BI titration, nearly half of people surveyed experienced hypoglycaemia during titration, and only a third met FBG targets, suggesting new strategies and tools are needed for effective BI titration.
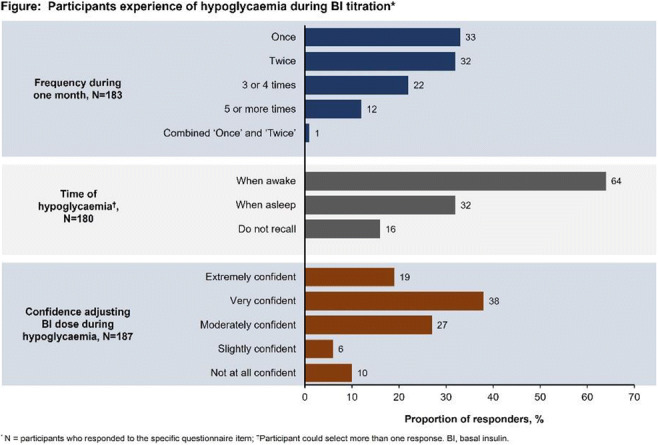
Supported by: Sanofi
Disclosure: S. Harris: Other; Consultant: Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Sanofi, Research support: Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Novo Nordisk, Sanofi.
638
Impact of hypoglycaemia on insulin titration and fasting plasma glucose in basal insulin-treated type 2 diabetes: a subanalysis of the SoliMix trial
R. Ritzel1, R.J. McCrimmon2, J. Rosenstock3, O. Deyneli4, A. Alvarez5, E. Souhami6, L. Melas-Melt7, F. Giorgino8;
1Klinikum Schwabing and Klinikum Bogenhausen, Munich, Germany, 2University of Dundee, Dundee, UK, 3Dallas Diabetes Research Center at Medical City, Dallas, USA, 4Koc University School of Medicine, Istanbul, Turkey, 5Sanofi, Buenos Aires, Argentina, 6Sanofi, Paris, France, 7IviData LIFE SCIENCES, Levallois-Perret, France, 8University of Bari Aldo Moro, Bari, Italy.
Background and aims: The SoliMix trial (EudraCT: 2017-003370-13) found better HbA1c with weight benefit and lower hypoglycaemia risk for iGlarLixi (insulin glargine 100 U/mL + lixisenatide) vs premix BIAsp 30 (biphasic insulin aspart 30) in people with type 2 diabetes advancing from basal insulin plus oral antihyperglycaemic drugs. This exploratory analysis investigated whether hypoglycaemia influenced insulin titration and achievement of fasting plasma glucose (FPG) targets with each therapeutic option.
Materials and methods: Total insulin dose changes from baseline to Week 12 were stratified by hypoglycaemia (yes/no) in each treatment arm and ADA Level 2 hypoglycaemia (<54 mg/dL [<3.0 mmol/L]) event rates during Weeks 0-12 were modeled according to FPG at Week 12 (when most insulin titration occurred).
Results: Lesser insulin dose increases occurred in both treatment groups for those who experienced hypoglycaemia (mean ± SD iGlarLixi: 3.9 ± 12.5 U; BIAsp 30: 11.0 ± 18.8 U) vs those who did not (mean ± SD iGlarLixi: 11.2 ± 9.9 U; BIAsp 30: 19.4 ± 19.3 U). While higher FPG at Week 12 correlated with greater event rates of hypoglycaemia in both treatment arms, event rates were higher for BIAsp 30 than for iGlarLixi irrespective of FPG (Figure).
Conclusion: More frequent hypoglycaemia with premix BIAsp 30 than with iGlarLixi may result in lower insulin doses, thus hampering insulin titration and resulting in higher FPG.
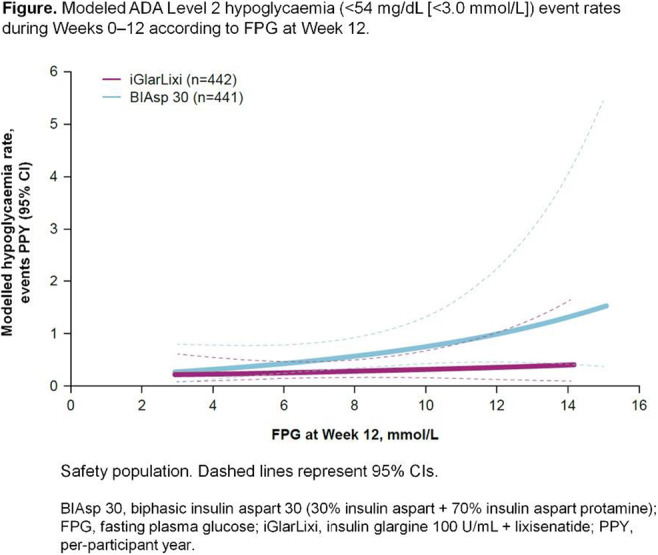
Clinical Trial Registration Number: EudraCT 2017-003370-13
Supported by: Sanofi
Disclosure: R. Ritzel: Honorarium; Novo Nordisk, Sanofi, MSD, AstraZeneca, Lilly, Novartis, and Pfizer. Other; Advisory board/Speaker bureau; Novo Nordisk, Sanofi, MSD, AstraZeneca, Lilly, Novartis, and Pfizer.
639
Once-weekly insulin icodec: comparable total exposure following administration in different subcutaneous injection regions
L. Plum-Mörschel1,2, L.R. Andersen3, S. Hansen4, U. Hövelmann2, P. Krawietz1, N.R. Kristensen4, L.L. Lehrskov4, H. Haahr4;
1Profil, Mainz, Germany, 2Profil, Neuss, Germany, 3Novo Nordisk, Aalborg, Denmark, 4Novo Nordisk, Søborg, Denmark.
Background and aims: Individuals with diabetes may administer subcutaneous insulin in different body regions. In order to address if the choice of injection region affects insulin icodec exposure and glucose-lowering effect, this study compared insulin icodec administration in different subcutaneous injection regions.
Materials and methods: In a randomised, open-label, three-period crossover trial, 25 individuals with type 2 diabetes on basal insulin (22 males; mean±standard deviation age 60±7 years, BMI 30.7±4.6 kg/m2) received single subcutaneous doses of insulin icodec (5.6 U/kg) in the thigh, abdomen and upper arm (separated by 9-13 weeks washout). Blood was sampled for pharmacokinetic analysis until 840 h (35 days) post-dose. Glucose-lowering effect was assessed at 36-60 h post-dose in an automated glucose clamp (plasma glucose target level of 7.5 mmol/L).
Results: Total insulin icodec exposure (AUC0-∞,SD) was similar after single-dose subcutaneous injection in the thigh, abdomen and upper arm (Table). Maximum concentration (Cmax,SD) was higher for abdomen and upper arm versus thigh. Extrapolation of pharmacokinetic profiles to steady state using a pharmacokinetic model showed smaller differences in maximum concentration (Cmax,SS) for abdomen and upper arm versus thigh than after a single dose. Partial glucose-lowering effect at 36-60 h after single dose (AUCGIR,36-60h,SD) was comparable across injection regions (geometric mean [CV%] of 1961 [51], 2130 [52] and 2391 [40] mg/kg for thigh, abdomen and upper arm).
Conclusion: Insulin icodec can be administered subcutaneously in the thigh, abdomen or upper arm with essentially similar exposure and glucose-lowering effect.
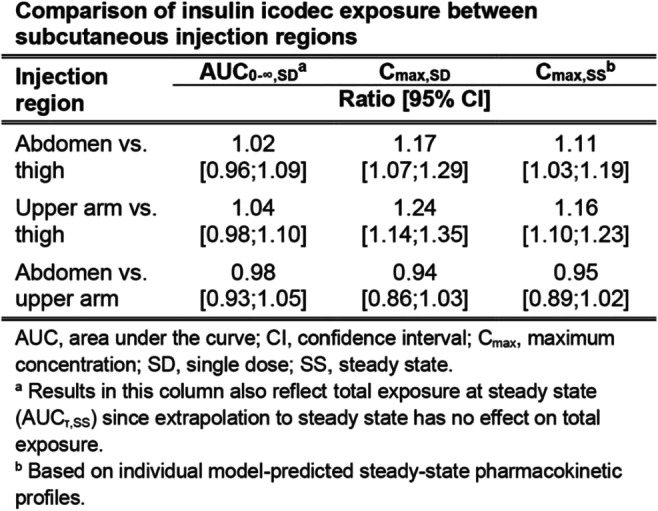
Clinical Trial Registration Number: NCT04582448 (ClinicalTrials.gov)
Supported by: Novo Nordisk
Disclosure: L. Plum-Mörschel: Grants; Novo Nordisk. Lecture/other fees; Novo Nordisk.
640
A novel once weekly basal insulin Fc achieved similar glycaemic control with a comparable safety profile versus insulin degludec in patients with type 1 diabetes
C. Kazda1, J. Bue-Valleskey1, J. Chien1, Q. Zhang1, E. Chigutsa1, W. Landschulz1, P. Wullenweber1, A. Haupt1, D. Dahl2;
1Eli Lilly and Company, Indianapolis, USA, 2Gemeinschaftspraxis für Innere Medizin und Diabetologie, Hamburg, Germany.
Background and aims: Once weekly Basal Insulin Fc (BIF; LY3209590; insulin efsitora alfa) combines a novel single-chain insulin variant with a human IgG2 Fc domain and is designed for once weekly subcutaneous administration. BIF has a 17-day half-life and low peak-to-trough ratio of 1.14. This is the first large Phase 2 trial in T1D for any once-weekly basal insulin. The primary aim of this study was to assess the efficacy and safety of BIF compared with insulin degludec in patients with T1D.
Materials and methods: This randomised, open-label, parallel trial enrolled patients with T1D who were using multiple daily insulin injections for at least 3 months prior to screening. BIF was injected once weekly for the 26-week treatment period, and insulin degludec was injected once daily. Both groups were titrated to fasting blood glucose levels ≤5.6 mmol/L (100 mg/dL). The primary endpoint was HbA1c change from baseline to Week 26 (non-inferiority margin=0.4%).
Results: Patients were randomised to receive BIF (N=139) or insulin degludec (N=126). The baseline characteristics were generally well balanced among treatment groups. The mean±SD age of patients was 46.4±14.5 yrs, the baseline HbA1c was 58.4±9.3 mmol/mol (7.5±0.9%), and the daily basal insulin dose was 28.9±13.3 IU and 27.8±13.7 IU for BIF and insulin degludec, respectively. The HbA1c change from baseline to Week 26 treatment difference for BIF versus insulin degludec was 1.9 mmol/mol (0.17%; [90% CI = 0.01, 0.32]; p = 0.07; Figure 1), meeting the non-inferiority margin. The rates of patient-reported Level 1 hypoglycaemia (≥3.0 and <3.9 mmol/L) were not significantly different between BIF (109.6 events/pt/yr) and insulin degludec (103.3 events/pt/yr; p = 0.59). Similarly, there was no significant difference between the rate of Level 2 hypoglycaemia (<3.0 mmol/L) between BIF (20.1 events/pt/yr) and insulin degludec (18.4 events/pt/yr; p = 0.55). A total of three severe hypoglycemic events occurred during the trial (two after administration of insulin degludec and one after BIF). No difference in the proportion of recurrent or continuous hypoglycemia has been observed between treatment arms. The occurrence of serious adverse events was not significantly different between BIF and insulin degludec. There was no significant difference in the increase in body weight from baseline to study endpoint for BIF (0.1 kg) and insulin degludec (0.5 kg) (treatment difference = -0.4 [-1.0, 0.1]; p=0.198).
Conclusion: Once weekly BIF demonstrated similar glycaemic control compared with once daily insulin degludec and no difference in hypoglycaemia or other safety findings in patients with T1D. These findings support continued development of BIF in Phase 3.
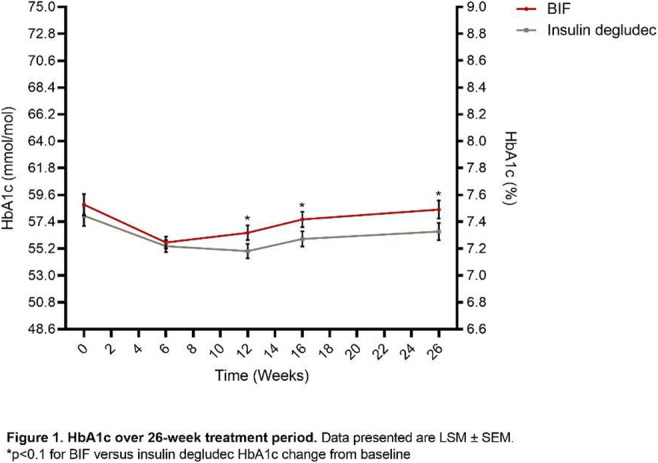
Clinical Trial Registration Number: NCT04450407
Supported by: Eli Lilly and Company
Disclosure: C. Kazda: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
641
Comparison of day and night closed-loop with faster-acting insulin aspart vs insulin aspart mimicking under-estimation of carbohydrates or missed meal bolus
H. Thabit1, W. Mubita1, M.E. Wilinska2, J. Rubio1, M. Karuppan1, J. Schofield1, R. Hovorka2, L. Leelarathna1;
1Manchester Diabetes, Endocrinology and Metabolism Centre, Manchester Royal Infirmary, Manchester, 2Institute of Metabolic Science, University of Cambridge, Cambridge, UK.
Background and aims: The need to estimate carbohydrate content and deliver pre-meal boluses remains a burden for many people with type 1 diabetes. The aim of this study was to determine whether closed-loop using faster-acting insulin aspart will improve glucose control over a 24-hour period compared to insulin aspart under conditions mimicking under-estimation of carbohydrates or missed meal bolus.
Materials and methods: In an open-label, randomised crossover trial, 16 adults with type 1 diabetes on insulin pump therapy (9 females, age 31 ± 9 yrs, diabetes duration 17 ± 8 yrs HbA1c 66 ± 6 mmol/mol) underwent two 23 hour inpatient stays during which glucose levels were controlled by closed-loop with either faster-acting insulin aspart or insulin aspart. During the study periods, participants received standardised meals with half of their usual meal bolus for the evening meals and no meal bolus for lunchtime meals. Primary endpoint was proportion of time in range (3.9 - 10mmol/l) as recorded by sensor glucose during inpatient stay.
Results: Time in range during the study period was comparable in both faster-acting insulin aspart and insulin aspart (61.8 ± 14.3 vs. 61.5 ± 12.8%, p=0.35). Time in hyperglycaemia >10mmol/l (35.5 ±14.9 vs. 35.7 ± 14.8%, p=0.67) and area above the curve >10mmol/l (1.2 ± 0.8 vs. 1.2 ± 0.5 mmol/l min-1, p=0.60) were similar. Time in hypoglycaemia <3.9mmol/l [median (IQR); 1.5 (0.0, 3.1) vs. 1.6 (0.0, 3.1), p=0.91] was not significantly different, while % time and AUC < 3.0 were both low and comparable. Algorithm driven insulin infusion (29.1 ± 9.0 vs. 29.2 ± 11.4 U, p=0.70) and bolus delivered (11.5 ±3.9 vs. 10.9 ± 3.5 U, p=0.76) were not statistically different for both arms.
Conclusion: Glycaemic control during closed-loop with faster-acting insulin aspart was comparable with insulin aspart under conditions mimicking under-estimation of carbohydrates or missed meal bolus. Further improvement in faster-acting insulin action profile is still needed, to enhance the effectiveness and feasibility of fully closed-loop in clinical practice.
Clinical Trial Registration Number: NCT03579615
Supported by: Novo Nordisk A/S
Disclosure: H. Thabit: None.
SO 50 Is longer better? Looking for different basal insulin approaches
642
Effect of insulin degludec vs insulin glargine U100 on continuous glucose monitoring recorded metrics in people with type 1 diabetes and nocturnal severe hypoglycaemia
J.M.B. Brøsen1, R.M. Agesen2, A.C. Alibegovic2, H.U. Andersen3, P. Gustenhoff4, T.K. Hansen5, C. Hedetoft6, T.J. Jensen7, C.B. Juhl8, C.R. Stolberg9, S.S. Lerche10, K. Nørgaard3, H.-H. Parving7, L. Tarnow11, U. Pedersen-Bjergaard1;
1Department of Endocrinology and Nephrology, Copenhagen University Hospital - North Zealand, Hillerød, 2Novo Nordisk A/S, Søborg, 3Steno Diabetes Center Copenhagen, Herlev, 4Steno Diabetes Center Nordjylland, Aalborg, 5Steno Diabetes Center Aarhus, Aarhus, 6Department of internal medicine, Zealand University Hospital, Køge, 7Department of Endocrinology, Copenhagen University Hospital - Rigshospitalet, Copenhagen, 8Department of medicine, Sydvestjysk Sygehus, Esbjerg, 9Department of Endocrinology, Odense University Hospital, Odense, 10Department of Diabetes and Hormonal diseases, Lillebælt Hospital, Kolding, 11Steno Diabetes Center Sjælland, Holbæk, Denmark.
Background and aims: Insulin Degludec (IDeg), in comparison with Insulin Glargine U100 (IGla), reduces the risk of hypoglycaemic events in people with type 1 diabetes (T1D). The impact on CGM assessed metrics such as CV, time in range (TIR), time below range (TBR), and time-above range (TAR) is less known. We present CGM-recorded data from the HypoDeg trial comparing treatment with IDeg and IGla in people with T1D and recurrent nocturnal severe hypoglycaemia.
Materials and methods: This is a pre-defined optional substudy of the HypoDeg trial: a 2-year investigator-initiated, randomized, cross-over trial comparing treatment with IDeg or IGla in 149 participants with T1D and at least one nocturnal severe hypoglycaemic event within the last two years. Participants underwent 2 x 6 days of blinded CGM (Medtronic iPro) during each treatment after 6 and 12 months of treatment. Seventy-four participants completed at least one CGM period in each treatment arm. The endpoints were CV, TIR (3.9 - 10.1 mmol/L), TBR at level 2 (< 3.0mmol/L), and TAR (>10.0, > 13.9 mmol/L). Time spent in ranges is provided as a percentage of readings. In a linear mixed regression model, accounting for repeated measures, we compared the effect of treatments on endpoints.
Results: We collected 261 CGM traces with a mean (SD) observation period of 5.9 (0.7) days. The CV was lower with IDeg than IGla, with a mean (SE) CV of 40.5% (0.9) and 42.5% (0.9), respectively (p=0.009). A difference in CV during the night (23:00h - 07:00h) drove this difference, with a mean CV of 35.7% (1.1) for IDeg versus 39.6% (1.1) for IGla (p=0.001) (Figure 1). There was a significant reduction of all-day TBR at level 2 (< 3.0mmol/L) with IDeg 1.8% (0.4) compared to IGla 3.1% (0.4) (p=0.001). The percentages of TIR and TAR were not different between treatments.
Conclusion: In people with T1D prone to nocturnal severe hypoglycaemia, treatment with IDeg results in a lower mean CV, reaching the definition of stable glucose levels during the night, and a lower all-day TBR at level 2 as compared to IGla.
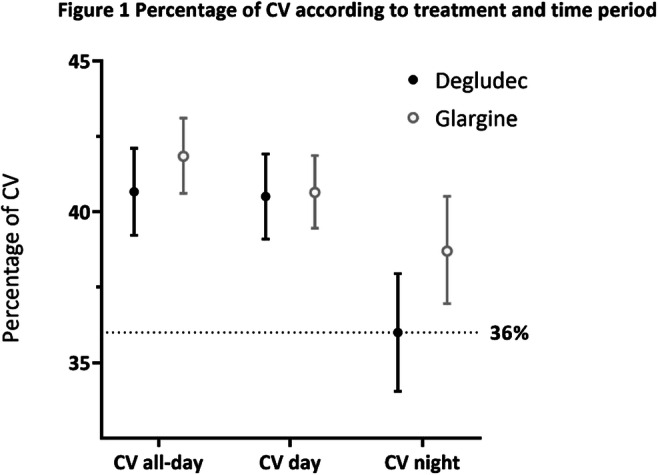
Clinical Trial Registration Number: #NCT02192450
Supported by: NN
Disclosure: J.M.B. Brøsen: None.
643
Efficacy and safety of insulin icodec in patients with type 2 diabetes: a meta-analysis of randomised phase II trials
D. Dimayuga, M. Villa, M. Maningat-Goco;
Center for Diabetes, Thyroid and Endocrine Disorders, St. Luke's Medical Center - Global City, Taguig, Philippines.
Background and aims: Icodec is a novel, long-acting, once-a-week insulin analog. Early evidence on icodec in type 2 diabetes mellitus (T2D) is conflicting. We aimed to conduct a meta-analysis of published randomized controlled trials (RCTs) on the efficacy and safety of icodec in patients with uncontrolled T2D (HbA1c > 7%).
Materials and methods: A systematic search in major online databases was conducted to identify relevant RCTs. Two authors independently performed the study selection and extracted study data. Data on glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), time-in-range of 70-140 mg/dL (TIR), adverse events and hypoglycemia events were noted. Significance level of 95% was used to provide pooled estimates. Random effects model was used for analysis with significant heterogeneity (I²>50%).
Results: Three trials with 606 patients were included. Pooled analysis of available data showed that icodec resulted in a significantly greater mean reduction of HbA1c (mean difference (MD) 0.18 [0.13,0.22], p < 0.00001, I²=84%), mean reduction of FPG (MD=3.87mg/dl [3.18, 4.57], p<0.00001, I²=0%), longer TIR (MD=4.39% [3.94, 4.85], p<0.00001, I²=0%), but had significantly greater propensity to lead to level 1 hypoglycemia (Odds Ratio (OR)=1.59 [1.11, 2.28], p=0.01, I²=29%) when compared to glargine U100. Funnel plot analysis on the propensity for icodec to lead to any hypoglycemia event decreased the I² statistic from 78% to 0% and showed significantly greater hypoglycemia events with icodec (OR=2.59 [1.67,4.01], p<0.001). There was no significant difference in the number of patients with end-of-trial HbA1c <7%, and any adverse event, or hypoglycemia level 2 or 3. Only 1 hypoglycemia level 3 event was noted (patient on icodec).
Conclusion: Once-weekly icodec had significantly better glycemic control compared to daily glargine U100, but had significantly greater hypoglycemia events. Data from ongoing trials on this novel drug can be added to the analysis as they become available.
Disclosure: D. Dimayuga: None.
644
Iglarlixi vs basal plus rapid-acting insulin in adults with type 2 diabetes advancing from basal insulin therapy: the SoliSimplify real-world study
R.J. McCrimmon1, A.Y.Y. Cheng2, G. Galstyan3, K. Djaballah4, X. Li5, M. Coudert4, J. Frias6;
1University of Dundee, Dundee, UK, 2University of Toronto, Toronto, Canada, 3Endocrinology Research Center, Moscow, Russian Federation, 4Sanofi, Paris, France, 5Sanofi, Bridgewater, USA, 6National Research Institute, Los Angeles, USA.
Background and aims: Indirect evidence suggests iGlarLixi (insulin glargine 100 U/mL + lixisenatide) is as efficacious as basal insulin (BI) + rapid-acting insulin (RAI) for management of type 2 diabetes (T2D). However, there are no direct comparisons of iGlarLixi (once-daily [QD]) vs a BI + RAI regimen (multiple daily injections [MDI]). SoliSimplify compared these treatments using real-world data from a US database.
Materials and methods: Electronic medical records were analysed retrospectively using propensity score matching (PSM) to compare therapy advancement with iGlarLixi or BI + RAI in adults ≥18 years with T2D on BI and ≥1 HbA1c available value at baseline and 6-month follow-up. The primary objective was non-inferiority of iGlarLixi to BI + RAI in HbA1c change from baseline to 6 months (margin 0.3%).
Results: PSM generated cohorts with balanced baseline characteristics (N=814 in each group). Participants in both cohorts had a mean age of 61 years and a mean weight of 100 kg, with 27-28% participants having obesity. Almost all participants were prescribed insulin analogues in the baseline period, with insulin glargine (~78%) being the most common, followed by insulin determir (~18%) and insulin degludec (~14-16%). More than 40% of participants received one antihyperglycaemic drug (OAD) and around 39% received ≥2 OADs. HbA1c reduction from baseline to 6 months with iGlarLixi was non-inferior to BI + RAI (p<0.025) (Table). At 6 months, weight gain was significantly lower with iGlarLixi than with BI + RAI (p<0.05 for the difference between treatment groups). Achievement of HbA1c <7% without hypoglycaemia and weight gain was similar between groups at 6 months. Hypoglycaemia was low in both groups, likely due to underreporting.
Conclusion: In this real-world study, QD iGlarLixi was as effective as MDI BI + RAI in HbA1c reduction and had a favourable body weight benefit.
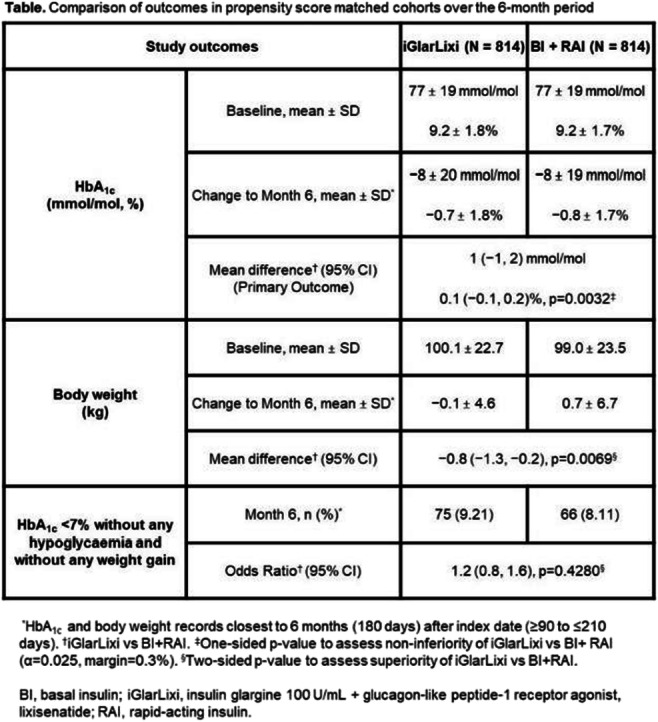
Supported by: Sanofi
Disclosure: R.J. McCrimmon: Other; Advisory board/speaker; Sanofi and Novo Nordisk.
645
Indirect treatment comparison (ITC) of efficacy and safety of insulin glargine 300 U/mL with once-daily-insulin premix in type 2 diabetes
R. Malik1, S. Ghosh2, R. Emral3, R. Malek4, L. Zeng5, Y. Huang6, M.A. Mabunay7, W. Landgraf8, P. Guyot9, E. Schenk10, D. Pushkarna10, R. Ritzel11;
1Weill Cornell Medicine-Qatar, Doha, Qatar, 2Department of Endocrinology, Institute of Post Graduate Medical Education & Research, Kolkata, India, 3Department of Endocrinology and Metabolic Diseases, Ankara University, Faculty of Medicine, Ankara, Turkey, 4Department of internal medicine, Setif university hospital, Setif, Algeria, 5The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, 6Sanofi, Shanghai, China, 7Sanofi, Singapore, Singapore, 8Sanofi, Frankfurt, Germany, 9Sanofi, Chilly Mazarin, France, 10Evidinno Outcomes Research Inc, Vancouver, Canada, 11Division of Endocrinology, Diabetes and Angiology, München Klinik Schwabing, Munich, Germany.
Background and aims: There are no head-to-head randomised clinical trials (RCTs) comparing the efficacy and safety of insulin glargine 300 U/mL (Gla-300), a second-generation basal insulin analogue, with once-daily (OD) insulin premix. An ITC was conducted to fill this gap and compare the efficacy and safety of Gla-300 OD vs Premix OD in people with type 2 diabetes (PWT2D) inadequately controlled on oral antidiabetic drugs (OADs).
Materials and methods: A systematic literature review with predefined criteria was carried out in MEDLINE®, Embase, and CENTRAL (inception to April 23, 2021). RCTs evaluating OD Gla-300 or Premix in insulin naïve PWT2D inadequately controlled on OADs were included. The heterogeneity of the included trials was vetted in a feasibility assessment, which examined the distributions of trial and participant characteristics, and observed treatment effects. Random effects meta-analyses were conducted using the metafor package for R. The Bucher ITC method was used to indirectly compare Gla-300 with Premix by taking the difference between their respective pooled treatment effects vs Gla-100 OD. Changes in HbA1c and body weight, final insulin dose, and hypoglycaemia incidence and rate were assessed at 24-26 weeks.
Results: Six RCTs involving 3,309 insulin naïve PWT2D were eligible for the ITC: two with Gla-300 and four with Premix, all studies were Gla-100 controlled. Baseline BMI (kg/m2) differed between the Asian (25.1-25.6) and intercontinental (29.1-33.0) studies, and mean HbA1c ranged from 8.2-8.9%. Concomitant OADs included metformin (n = 2), pre-study OADs (n = 2), and metformin + glimepiride (n = 1). There was no significant difference for change in HbA1c (mean difference [MD]: 0.13%; 95% CI: −0.04%, 0.31%; p = 0.1), change in weight (MD: −0.42 kg; 95% CI: −1.54, 0.69; p = 0.5), or final insulin dose (MD: 1.31 U; 95% CI: −4.81, 7.44; p = 0.7). The risk of any hypoglycaemia was significantly lower with Gla-300, both with respect to incidence (odds ratio [OR]: 0.64; 95% CI: 0.47, 0.86; p = 0.004) and event rate (rate ratio [RR]: 0.74; 95% CI: 0.57, 0.96; p = 0.02). Additionally, the incidence of confirmed (PG <3.0-3.1 mmol/L) anytime (24 h) hypoglycaemia was lower with Gla-300 (OR: 0.49; 95% CI: 0.33, 0.73; p = 0.0005), as was the rate of confirmed (PG <3.9 mmol/L) anytime hypoglycaemia (RR: 0.37; 95% CI: 0.16, 0.88; p = 0.02). No differences were observed for either severe or nocturnal hypoglycaemia.
Conclusion: These findings suggest that insulin naïve PWT2D inadequately controlled on OADs commencing Gla-300 OD or Premix OD achieve similar glycaemic improvement and differences in insulin dose after 26 weeks, but with a lower risk of clinically significant confirmed (PG <3.0-3.1 mmol/L) or any hypoglycaemia with Gla-300 OD.
Supported by: Sanofi
Disclosure: R. Malik: None.
646
Three types of technology for insulin delivery and their effects on glycaemic related outcomes in patients with type 1 diabetes
L.B.H. Pedersen1, N. Drøjdahl-Ryg1,2, C.B. Juhl1,2, K. Schousboe1;
1Steno Diabetes Center Odense, Odense, 2Medical department, Endocrinology, Hospital South West Jutland, Esbjerg, Denmark.
Background and aims: To ease the burden of type 1 diabetes (T1D), multiple diabetic devices for insulin delivery and measurement of glucose levels have been developed during the last decades. The aim of this study was to compare glycemic related outcomes for three types of insulin delivery devices in patients with T1D, using continuous glucose monitor (CGM).
Materials and methods: In February 2022, an electronic questionnaire was send to all adult patients with T1D treated in an outpatient clinic in the Region of Southern Denmark. Data collection is ongoing and data are prelimenary. The questionnaire included questions about time in range (TIR), time below range (TBR), time above range (TAB), events of hypoglycemia during the last year (hypos), diabetes related distress (DD) and fear of hypoglycemia (FoH). Patients were divided into three groups based on which type of insulin delivery device was used. Users of 1) insulin pen (IPe), 2) insulin pump (excluding hybrid closed loop) (IPu), and 3) hybrid closed loop (HCL). DD and Foh were measured by The Problem Areas in Diabetes 5-item scale (PAID-5) and The 11-item Hypoglycemia Fear Survey-II (HFS-II). PAID-5 and HFS-II both provide sum scores. The total score for PAID-5 ranges from 0-20 and for HFS-II 0-44 points, with higher scores indicating higher DD or FoH. TIR, TAR and TBR were read by the patient from their device, and reported in the questionnaire. To test for differences between groups, ANOVA analyses and an equality-of-median test were performed. ANOVA analyses were adjusted for age, sex, and diabetes duration above or below 3 years. Additionally, analysis for HFS-II and PAID-5 were also adjusted for presence of hypos during the final year.
Results: Of 5840 participants who received the questionnaire, 1484 CGM users (25%) have to date responded (IPe =1094; IPu=250; HCL =134). Mean (SD) age was 55 (15) years, mean diabetes duration was 27 (16) years, and 45% were women. TIR was reported by 998 participants. Mean was lowest for IPe and highest for HCL (IPe: 61% (20), n=752); IPu: 64% (23), n=156; HCLl: 75% (13), n=90). TIR was highest for HCL and lowest for IPe (IPe vs. HCL (p<0.001); IPu vs. HCL (p<0.001); IPe vs. IPu (p<0.01)). TAR was reported by 968 participants. Mean was lowest for HCL and identical for IPe and IPu (IPe: 31% (19), n=723; IPu: 31% (22), n=154; HCL: 22% (14), n=91). TAR was lowest for HCL and highest for IPe (IPe vs HCL (p<0.001); IPu vs Hcl (p=0.001); IPe vs IPu (ns)). TBR was reported by 973 participants. Mean was lowest for HCL and highest for IPe (IPe: 5% (7), n=726; IPU: 3% (4), n= 156; HCLl: 2% (4), n=91). TBR was lowest for HCL and highest for IPe (IPe vs. HCL (p<0.001); IPe vs. IPu (p<0.001); IPu vs. HCL (ns)). During the last year, 87 out of 1484 participants had minimum one incidence of severe hypoglycemia. Mean number of hypos were 0.36 for IPe (n=1094), 0.052 for IPu (n=250) and 0.007 for HCL (n=134) (p=0.002). There were no differences between groups for FoH and DD (HFS-II (n=1478): IPe, 11 (7); IPu, 11(7); HCL, 10 (7) and PAID-5 (n=1478): IPe, 6(5); IPu, 7(4); HCL 6(5).
Conclusion: Patients with T1D using HCL experienced significantly better glycemic control based on TIR, TAR and TBR, compared to pen and pump users. Users of both kinds of pumps had significantly better TIR and TAR compared to pen users. Furthermore, they experienced significantly less hypoglycemic events than pen users. Scores for HFS-II and PAID did not differ between the groups.
Disclosure: L.B.H. Pedersen: None.
647
Hypoglycaemia frequency and physiological response to double or triple doses of once-weekly insulin icodec versus once-daily insulin glargine in type 2 diabetes
T.R. Pieber1, K.N. Arfelt2, R. Cailleteau2, K.M. Due Thomsen3, M. Hart1, I. Mursic1, E. Svehlikova1, M. Urschitz1, H. Haahr2;
1Medical University of Graz, Graz, Austria, 2Novo Nordisk, Søborg, Denmark, 3Novo Nordisk, Aalborg, Denmark.
Background and aims: Insulin icodec is a basal insulin in development for once-weekly administration. The aim of this study was to compare the hypoglycaemia frequency and physiological response after double or triple doses of insulin icodec versus insulin glargine U100.
Materials and methods: In a randomised, open-label, two-period crossover trial, 43 individuals with type 2 diabetes on basal insulin±metformin (mean±SD age 56±9 years, HbA1c 7.2±0.7%) received once-weekly insulin icodec for 6 weeks and once-daily insulin glargine U100 for 12 days. Total weekly doses were equimolar and based on the individual daily run-in insulin glargine U100 dose (mean 30±14 U) titrated to a fasting self-measured plasma glucose target of 4.4-7.2 mmol/L. After reaching steady state, double and triple doses of insulin icodec or insulin glargine U100 were administered. Hypoglycaemia was induced 44 h (insulin icodec) or 7 h (insulin glargine U100) post-dose (the expected time of maximum glucose-lowering effect), as described in the following. First, euglycaemia was maintained at 5.5 mmol/L by variable i.v. glucose. Then, i.v. glucose was terminated and plasma glucose was allowed to decrease to a nadir target of 2.5 mmol/L, whereafter plasma glucose was maintained at nadir for 15 min by re-initiating variable i.v. glucose. Euglycaemia was then restored by constant i.v. glucose infusion. Hypoglycaemic symptoms score (HSS) and concentration of counterregulatory hormones were assessed at plasma glucose 5.5 mmol/L and at predefined plasma glucose levels until nadir plasma glucose.
Results: Following a double dose, clinically significant hypoglycaemia (plasma glucose<3.0 mmol/L) occurred in 40% versus 36% of subjects for insulin icodec versus insulin glargine U100 (odds ratio 1.28; 95% CI [0.46;3.52]; p=0.63), a decline in plasma glucose to ≤2.5 mmol/L occurred in 5% versus 7% of subjects, and mean nadir plasma glucose was 3.2 mmol/L versus 3.3 mmol/L (treatment ratio 0.97; 95% CI [0.94;1.00]; p=0.07). Following a triple dose, clinically significant hypoglycaemia occurred in 53% versus 70% of subjects (odds ratio 0.48; 95% CI [0.18;1.28]; p=0.14), a decline in plasma glucose to ≤2.5 mmol/L occurred in 3% versus 25% of subjects, and mean nadir plasma glucose was 3.1 mmol/L versus 2.9 mmol/L (treatment ratio 1.07; 95% CI [1.04;1.11]; p<0.001) for insulin icodec versus insulin glargine U100. Among the subjects experiencing clinically significant hypoglycaemia after a triple dose, change in HSS at nadir plasma glucose was comparable for insulin icodec versus insulin glargine U100 (treatment difference 0.46; 95% CI [-2.72;3.64]; p=0.77), responses in adrenaline, noradrenaline and cortisol during hypoglycaemia development were greater for insulin icodec versus insulin glargine U100, while glucagon and growth hormone levels increased similarly.
Conclusion: Double or triple doses of once-weekly insulin icodec do not lead to increased risk of hypoglycaemia compared to double or triple doses of once-daily insulin glargine U100. During hypoglycaemia, a comparable symptomatic response and a moderately greater endocrine response were seen for insulin icodec versus insulin glargine U100.
Clinical Trial Registration Number: NCT03945656 (ClinicalTrials.gov)
Supported by: Novo Nordisk
Disclosure: T.R. Pieber: Grants; Novo Nordisk, AstraZeneca, Sanofi. Lecture/other fees; Arecor, Novo Nordisk.
648
Glycaemic control in people with type 2 diabetes (T2D) switching from NPH to insulin glargine 300 U/mL (Gla-300): REALI pooled database
D. Müller-Wieland1, N. Freemantle2, R. Bonadonna3, C. Mauquoi4, G. Bigot5, M. Bonnemaire6, P. Gourdy7, D. Mauricio8;
1Department of Medicine, University Hospital, Aachen, Germany, 2Institute of Clinical Trials and Methodology, University College London, London, UK, 3University of Parma, Parma, Italy, 4Clinical Trial Consulting, International Drug Development Institute, Louvain-la-Neuve, Belgium, 5IVIDATA, Paris, France, 6General Medicines, Sanofi, Paris, France, 7Institute of Metabolic and Cardiovascular Diseases, Toulouse University, Toulouse, France, 8Department of Endocrinology & Nutrition, Hospital of Santa Creu i Sant Pau, Barcelona, Spain.
Background and aims: The effectiveness of Gla-300 in people with T2D switching from NPH is not widely documented.
Materials and methods: The European REALI pooled database included patient-level data from 14 European prospective interventional and non-interventional studies in people with T2D treated with Gla-300. The present analysis evaluated the impact of Gla-300 on HbA1c improvements in people with T2D, previously uncontrolled on basal insulin (BI).
Results: This analysis included data from people with T2D uncontrolled on prior BI: 1,282 switching from NPH and 2,899 from other non-NPH BIs (mainly glargine 100 U/mL, 67%) to Gla-300. In the NPH group, mean±SD age was 63±9.4 years, BMI 32.5±5.8 kg/m², and median diabetes duration 12 years. The majority previously used biguanides (71%), followed by sulfonylureas (20%), and dipeptidyl peptidase 4 inhibitors (18%). HbA1c markedly improved after a 24-week Gla-300 therapy (Figure 1A). Mean±SD fasting plasma glucose (FPG) decreased from 188.8±55.9 mg/dL at Baseline to 143.3±45.5 at Week 24. Gla-300 was started at a mean dose of 29.4 U/day and titrated up to 35.6 at Week 24, with no body weight change. In the non-NPH BI group, baseline characteristics were comparable to those in the NPH group, except for higher baseline HbA1c and FPG in the latter. Figure 1B illustrates HbA1c improvement in the non-NPH BI group, at a mean Gla-300 starting dose of 35.4 U/day (0.37 U/Kg/day) increasing to 41.7 U/day (0.44 U/Kg/day) at Week 24, with no body weight change. Hypoglycaemia was similarly low in both groups.
Conclusion: This analysis shows that people with T2D, previously uncontrolled on BI, benefited from switching to Gla-300 in terms of HbA1c improvement, and this was especially observed in those previously treated with NPH.
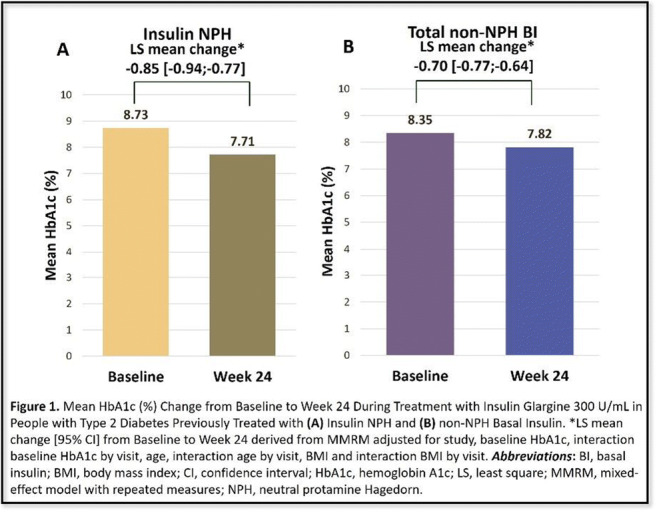
Supported by: Funded by Sanofi
Disclosure: D. Müller-Wieland: Employment/Consultancy; Acted as a consultant and has served on the speaker bureau for Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi.
649
Once weekly basal insulin Fc demonstrated similar glycaemic control to once daily insulin degludec in insulin-naïve patients with type 2 diabetes
J.M. Bue-Valleskey1, C. Kazda1, C. Ma1, J. Chien1, Q. Zhang1, E. Chigutsa1, W. Landschulz1, J. Swan1, A. Haupt1, J. Frias2;
1Eli Lilly and Company, Indianapolis, USA, 2National Research Institute, Los Angeles, USA.
Background and aims: Basal Insulin Fc (BIF; LY3209590), a fusion protein combining a novel single-chain variant of insulin with a human IgG Fc domain, is designed for once weekly (QW) administration. This randomised, parallel, open-label Phase 2 study assessed safety and efficacy of BIF vs DEG in insulin-naïve patients with type 2 diabetes (T2D) previously treated with oral antihyperglycaemic medication.
Materials and methods: BIF was injected QW, and DEG was injected QD. Both groups were titrated to fasting blood glucose (FBG) ≤5.6 mmol/L (100 mg/dL). The primary endpoint was HbA1c change from baseline (CFBL) to Wk 26 (non-inferiority margin=0.4%).
Results: Patients randomised to BIF (N=129) and DEG (N=135) had a mean age of 58.4 years and baseline HbA1c of 63.9 mmol/mol (8.0%). After 26 wks, BIF showed non-inferiority vs DEG for HbA1c CFBL with a treatment difference of 0.06% (90% CI = -0.11, 0.24; p=.56). Flash glucose monitoring performed at baseline and endpoint showed that both groups significantly increased time in range (3.9-10.0 mmol/L [70-180 mg/dL]) from baseline and reported a similar mean % time in range at Wk 26 of 76.0% and 77.4% for BIF and DEG respectively. Both BIF and DEG significantly reduced FBG from baseline with BIF resulting in a small but significantly higher FBG at study endpoint (treatment difference = 0.26 mmol/L [90% CI = 0.01,0.52]; p= 0.09). No severe hypoglycaemia was reported and the rate of Level 2 hypoglycaemia was low and not significantly different (BIF = 0.22; DEG = 0.15 events/pt/yr). Occurrence of treatment-emergent adverse events was similar between BIF and DEG.
Conclusion: BIF injected QW achieved excellent glycaemic control similar to DEG with no concerning hypoglycaemia or other safety findings, supporting continued development of BIF in Phase 3.
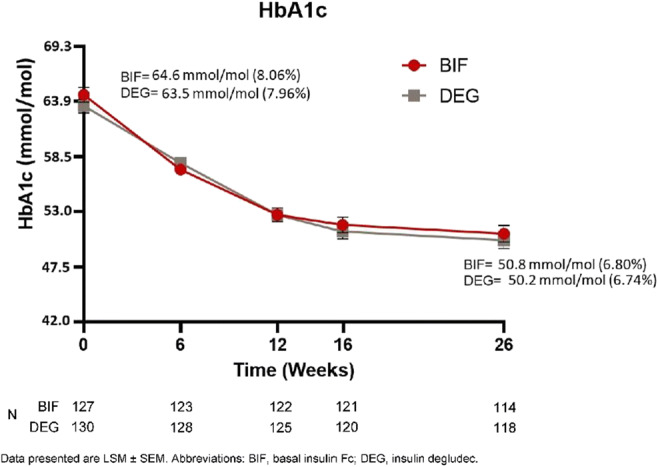
Clinical Trial Registration Number: NCT04450394
Supported by: Eli Lilly and Company
Disclosure: J.M. Bue-Valleskey: Employment/Consultancy; Eli Lilly and Company. Stock/Shareholding; Eli Lilly and Company.
SO 51 Even "old dogs" can learn new tricks
650
Efficacy of therapeutic patient education interventions in metabolic disorders: a systematic review and meta-analysis
J.C. Correia1, A. Waqas2, K. Gariani1, F.R. Jornayvaz1, Z. Pataky1;
1Department of Medicine, Geneva University Hospitals, Geneva, Switzerland, 2Institute of Population Health, Liverpool, UK.
Background and aims: Recent studies have shown the impact of therapeutic patient education (TPE) as a clinically and cost-effective solution to improve biomedical and psychosocial outcomes among people with metabolic disorders. The present systematic review and meta-analysis presents a critical synthesis of development of TPE interventions for obesity and diabetes mellitus (DM) and the efficacy of these interventions across a range of biomedical, psychosocial and psychological outcomes.
Materials and methods: Using a pretested search strategy, the database search for the systematic review was performed in Web of Science, MEDLINE, CINAHL, PsycINFO and COCHRANE database, from inception until August 2019. We considered all those studies which presented the effectiveness of TPE interventions in obesity and diabetes mellitus. We considered randomized and cluster randomized controlled trials conducted among adults > 18 years old. We considered biological parameters, psychological symptomology and quality of life indicators.
Results: A total of 54 RCTs were identified with 46 (85.19%) focused on DM, 6 (11.11%) on obesity and overweight and 2 (3.70%) on hypoglycemia. These interventions were delivered by allied health workers (n= 28), multidisciplinary teams (n=17), research teams (n= 6), peers and peer leaders (n=2) and doctors (n=1). Different delivery formats were used including in groups (n=16), individually (n= 15), electronically (n= 8) and mixed formats (n= 12). Main taught components included prevention of complications (n=49), managing complications (n= 48), implementation of lifestyle changes (n= 45) and self-monitoring (n= 41). There was substantial heterogeneity in reporting of these outcomes (I2= 88.35%, Q= 317.64). We found significant improvement in biomedical outcomes (body weight, glycemia and HbA1c) in the intervention group (SMD=0.36, 95% CI: 0.23 to 0.49) as well as increased adherence to treatment (SMD= 0.310, 95% CI: 0.05 to 0.57). Regarding patient knowledge, although the effect size showed improvement in favor of the intervention group, it was statistically non-significant (SMD= 2.60, 95% CI: -1.44 to 6.64). Similarly, quality of life did not reveal statically significant improvement in favor of the intervention group (SMD= 1.57, 95% CI: -0.54 to 3.68, I2= 98.05%, Q= 51.32). The effect sizes were comparable across interventions delivered by different modes and delivery agents. Risk of bias was low in selective reporting (n= 51), attrition bias (n=38), random sequence generation (n=20), and allocation concealment.
Conclusion: The present systematic review and meta-analysis highlights how TPE interventions are an essential and effective component of patient care. These interventions should be implemented in healthcare and community settings to improve health of patients suffering from chronic metabolic disorders. It also revealed that TPE interventions delivered through different media and delivery formats maybe equally effective. Therefore, these interventions can be tailored to the setting according to availability of human and financial resources.
Disclosure: J.C. Correia: None.
651
Patients find online education programmes helpful, but many feel they learn more from face to face programmes
R. Asir1, N. Burt2, E. Waite2, K.D. Ganapathy3, P. Narendran3,4, R.C. Andrews1,5;
1Diabetes department, Musgrove Park hospital, Taunton, 2Medical School, University of Birmingham, Birmingham, 3Queen Elizabeth hospital diabetes centre, Birmingham, 4Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, 5Exeter centre of excellence for diabetes research, University of Exeter, Exeter, UK.
Background and aims: Type 1 diabetes management including glucose monitoring and insulin administration is predominantly the responsibility of the patient or their care giver, as they may only see a healthcare expert a few times each year. As a result, this places great importance on structured education programmes that provide people with the knowledge and skills to confidently manage their diabetes and make their own decisions effectively. Prior to COVID, these education programmes tend to be delivered face-to-face either as a one-to-one session or in an interactive group, Since COVID, these programmes have moved to being delivered online. To date no studies has looked at how patients access these programmes, when is the best time to offer these programmes and how patients feel these courses compare to face to face programmes.
To understand how patients who have attended an online education programme accessed the course, how they felt it compared to a face-to-face course, and whether they would attend another course.
Materials and methods: 700 patients with Type 1 diabetes were randomly identified from DAFNE databases in a district hospital and a teaching hospital. A questionnaire was posted out to the identified patients with a stamp addressed envelope to return the questionnaire. Data from the returned questionnaire was entered into a database and then analysed. The questionnaire had questions on demographics, technology use, internet accessibility and patient preferences for online courses. For individual who has attended an online course there were additional questions on how they had done the course, what they felt about it and whether they would attend another online course. It is these results that we present here.
Results: 205 (29%) of the 700 questionnaires were returned. Of these 95 (46%) had attended an online course. The demographics of these 95 was as follows; 61 (64%) were female and 34 (36%), mean age + SD was 50 + 14 years and mean length of diabetes + SD was 28 + 14 years. 3 had visual problem (1 visually impaired and 2 colour blind) and 3 used hearing aids. 62% had good internet speed and all but 3 (3%) had time to do the course in the week. 12% of people could only access the course on mobile phone (4) or tablet (7) with the remaining having access to a laptop or desktop. The length of the courses they attended varied from less than a week to more than 5 weeks (59% less than week, 11% 2-4 weeks and 30% >5 weeks). 56% felt that the course was a good as a face-to-face course and 94% stated that they would attend another course. Suggestions to improve the course were, better joining instructions, more technical support, less slides and more discussion, sufficient breaks, and paper copies of what covered.
Conclusion: Most patients who attend an online course can do these during the week and can do it on large screen devices. Almost all would attend another course but nearly half feel that they are not as good as face-to-face courses. Thus, as we come out of COVID we need to ensure that we return to delivering face-to-face courses whilst still offering online courses.
Disclosure: R. Asir: None.
652
What are people's preferences for the 'choice talk' in the diabetes treatment decision-making process?
A. Tichler1,2, D.F.L. Hertroijs1,2, M.C.G. Brouwers3,2, D. Ruwaard1,2, M.J.C. Hiligsmann1,2, J.D. de Jong4,1, A.M.J. Elissen1,2;
1Health Services Research, Maastricht University, Maastricht, 2Care and Public Health Research Institute (CAPHRI), Maastricht University, Maastricht, 3Department of Internal Medicine, Division of Endocrinology and Metabolic Disease, Maastricht University Medical Center+, Maastricht, 4The Netherlands Institute for Health Services Research (NIVEL), Utrecht, Netherlands.
Background and aims: Individuals with type 2 diabetes and their healthcare providers are faced with more difficult treatment decisions due to the increase in choice and complexity of glucose-lowering treatment. Knowledge of individuals’ preferences regarding the treatment decision-making process can support shared decision-making and guide person-centred care. This study aimed to identify what treatment attributes patients find important to discuss in the treatment decision-making process and whether this differs between individuals with different demographic and disease characteristics (e.g. medication use and HbA1c value).
Materials and methods: An object best-worst scaling survey was distributed to all individuals with self-reported diabetes participating in the Dutch Health Care Consumer Panel (N=600) of the Netherlands Institute for Health Services Research. The survey included sixteen choice tasks, each consisting of four attributes and differed in the particular subset of attributes shown. Per choice task, respondents were asked to identify the attribute that they consider most and least important in the treatment decision-making process. Hierarchical Bayes analyses were performed to determine the relative importance score (RIS) of each attribute. A higher RIS indicates a higher level of perceived importance of the attribute. Subgroup analyses were performed to explore whether individuals’ demographic and disease characteristics influence their attribute preferences.
Results: Individuals with type 2 diabetes value ‘quality of life’ (RIS 11.97; 95% CI 11.77-12.16), ‘clinical outcomes’ (RIS 10.40; 95% CI 10.16-10.64), ‘long-term diabetes complications’ (RIS 9.83; 95% CI 9.50-10.16) and ‘short-term adverse events of medication’ (RIS 7.72; 95% CI 7.38-8.07) as most important in the decision-making process for the treatment of type 2 diabetes. Subgroup analyses showed for almost every subgroup the same four attributes valued as most important. The analyses also revealed heterogeneity in the attribute preferences for age, level of education, HbA1c value and medication use. For example, the attribute ‘medication withdrawal’ was considered less important in the decision-making process by individuals aged above 75 years (RIS 5.16; 95% CI 4.48-5.85) compared to individuals aged between 60-75 years (RIS 6.47; 95% CI 5.88-7.06) and individuals aged below 60 years (RIS 8.39; 95% CI 6.58-10.20).
Conclusion: Quality of life is valued as most important by individuals with type 2 diabetes to discuss in the treatment decision-making process, followed by ‘clinical outcomes’ and long-term diabetes complications’. The identified heterogeneity in attribute preference emphasizes the importance of taking people’s values, needs and preferences into account in the treatment decision-making process. It highlights the need for shared decision-making.
Clinical Trial Registration Number: NL8948
Supported by: MSD
Disclosure: A. Tichler: None.
653
Comprehensive diabetes education in care homes: 1 year analysis of impact from the CARES diabetes in care homes national programme
A. Puttanna1, J. Ridgeway2, L. Willcocks2, S. Gregory2, L. Heggs2;
1General Medicines, Sanofi UK, Reading, 2EDEN, Leicester, UK.
Background and aims: People with diabetes living in a care home setting are a clinically vulnerable population with a higher risk of diabetes related complications and hospital admission. In the UK, there is currently no standardised education programme for care home facilities to ensure appropriate knowledge and understanding of diabetes. EDEN (Effective Diabetes Education Now) has previously identified that diabetes education to staff has a positive impact for service users with diabetes. As a result, a targeted diabetes education programme for care home facilities was created (CARES).
The aim of this project was to provide a standardised and structured diabetes education programme across all care homes in the UK to improve on baseline knowledge and confidence in diabetes care.
Materials and methods: A structured education programme focussing on diabetes care and knowledge in the care home setting was created. Care homes were invited to register and participate in the programme on a voluntary basis. All participants undertook a learning journey via an online platform which consisted of a short recorded lesson, 3 eLearning modules and a group mentoring session. To identify if the project aims had been met, each participant completed a pre and post programme questionnaire assessing confidence in competencies on a Likert scale (1 = not confident, 5 = very confident). Data was analysed through Python statistical analysis software.
Results: 458 participants from care homes across the UK registered in the education programme with 105 completing the programme at 1 year. Table 1 identifies the key findings from pre and post attendance questionnaires on basic diabetes competencies. Additionally, competency in checking blood sugars increased from 54% of participants pre-programme to 71% post programme (p = 0.001) whereas competency in insulin initiation went from 45% pre-programme to 50% post programme (p=0.279). There was an increase in the presence of a 'hypo box' on site from 47% to 55% of participants pre and post programme respectively (p<0.0001). Significant reductions in ambulance call outs and hospital admissions for diabetes related problems (self-reported) were noted (p<0.0001).
Conclusion: As one of the first known standardised national diabetes education programme for care home settings, CARES has highlighted that structured diabetes education in care homes increased knowledge and confidence in a broad range of basic diabetes competencies. It also suggests that structured education may result in better care of patients through better treatment of hypoglycaemia (via presence of hypo boxes), reduced ambulance callouts and hospital admissions. Standardised and structured diabetes education should be mandatory for all care homes involved in managing patients with diabetes.
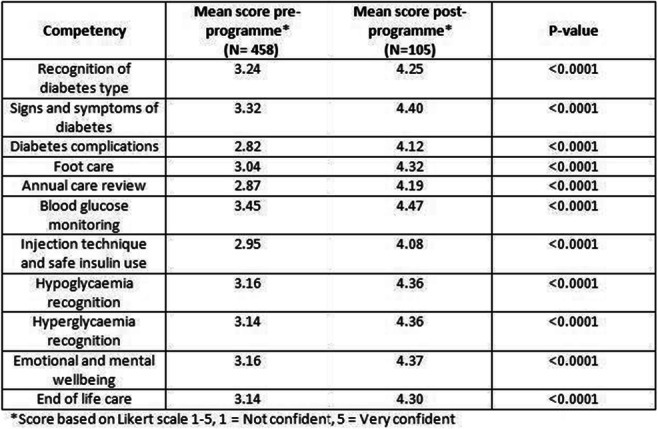
Supported by: S UK supported the costs of programme delivery but had no involvement in content
Disclosure: A. Puttanna: None.
654
Legacy effects of sleep education programme in people with type 2 diabetes and short sleep duration: a prospective follow-up study
J. Cheung1, K. Woo1, R. Cheung1, M. Lee1, E. Lau1, C. Chiu1, E. Chow1, A. Luk1, R. Ma1, J. Chan1, Y. Wing2, A. Kong1;
1Medicine and Therapeutics, The Chinese University of Hong Kong 2Psychiatry, The Chinese University of Hong Kong, Hong Kong, Hong Kong.
Background and aims: Pilot study suggests that sleep education improves sleep quality and glycemia in people with type 2 diabetes (T2D). Here, we aimed to investigate the legacy effects of sleep education on sleep duration and glycemic control in T2D with short sleep duration.
Materials and methods: People with T2D and short sleep duration, defined as weekday sleep duration <7 hours/day, exited from a randomized controlled trial in which intervention was sleep education program for 30 minutes through group education followed by an additional 180 minutes individual counseling through telephone (15 minutes per month for 12 months), were evaluated in this follow-up study. Sleep habits were measured by a validated sleep questionnaire through phone interview. Anthropometric index [body mass index (BMI)] and biochemical parameters including fasting plasma glucose (FPG), glycated hemoglobin (HbA1C), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were retrieved from electronic medical record within 3 months of phone interview. The primary outcome was the changes of sleep duration from baseline, whereas secondary outcome was the changes of glycemia as measured by FPG and HbA1C. Generalized estimating equations (GEE) model was used to examine differential changes of sleep duration and HbA1C across the time points at baseline and follow up time.
Results: After a median follow-up of 6.2 years [interquartile range (IQR) 5.0-8.0], 179 out of 267 (n=88 out of 137 in control group; n=91 out of 130 in intervention group) T2D were studied (overall response rate of 67 %). There were no significant differences at baseline, in terms of clinical profile and sleep patterns, between the responders and non-responders. Among the responders studied at follow-up, 48.7% were men with mean age ± standard deviation (SD): 59.0 ± 8.6 years, BMI: 23.4 ± 7.8 kg/m2, weekday sleep duration: 7.0 ± 1.6 hours, weekend sleep duration: 7.7 ± 1.7 hours, FPG: 6.9 ± 1.8 mmol/L and HbA1C 7.2 ± 1.0%. T2D exited from intervention group had improved weekend sleep duration when compared to their counterparts in the control group (β 2021 weekend sleep duration 0.914, 95% Confidence Interval [CI] 0.077 to 1.751, p = 0.032), but there was no difference for weekday sleep duration between the two groups (β 2021 weekday sleep duration 0.371, CI -0.288 to 1.030, p = 0.270), after adjusted for potential confounders, including age, BMI, HbA1C, HDL-C, LDL-C and TG. Moreover, T2D exited from intervention group had a significantly lower FPG than control group [6.9 mmol/L (IQR 6.1-8.3) versus 7.1 mmol/L (IQR 6.0-8.7), p = 0.027]. However, there was no significant difference of HbA1C between the two groups (p = 0.282).
Conclusion: Previous participation in sleep education program was associated with increased weekend sleep duration and decreased fasting glycemia among T2D with short sleep duration.
Supported by: Research Grants Council of the Hong Kong Special Administrative Region, China. Project no. CUHK46671
Disclosure: J. Cheung: None.
655
The importance of education for diabetes control in patients with type 1 diabetes using intermittently scanned continuous glucose monitoring
R. Bem, D. Vávra, Z. Hladikova, J. Mašková, V. Havlová, Z. Lachmanová, K. Sochorová;
IKEM, Prague, Czech Republic.
Background and aims: Intermittently scanned continuous glucose monitoring (isCGM) is a new evolving technology used in the management of Type 1 diabetes leading to improved diabetes control and reduction of hypoglycemia. Education of patients using isCGM should lead to further improvement of glycaemia and other parameters of diabetes control. The aim of the study was to assess the effect of structured educational course on diabetes control parameters in patients with type 1 diabetes using isCGM.
Materials and methods: 125 patients with type 1 diabetes (88 with multiple daily injections of insulin (MDI) and 37 with insulin pump) using isCGM treated in our Diabetes Department were enrolled in the present study between January 2019 and February 2022. All patients completed a 2-hour structured educational course focused on the use of isCGM, including isCGM application, scanning frequency, working with trend arrows, regular downloading and data evaluation. Diabetes control was assessed by the following parameters: HbA1c, mean glycaemia, time in range (TIR) of glucose between 3.9 and 10 mmol/l. The parameters were evaluated before initiating isCGM, at the time of educational course and during regular visits in the diabetes outpatient clinic with a frequency of 3 months for 1 year.
Results: There was no significant improvement of diabetes control during the period between the start of isCGM use and educational course (mean 8.2 months; HbA1C 8.2±1.7 vs 8.0±1.1% ; mean glycemia 9.2±2 vs. 9.0±1.9 mmol/l; TIR 53±19 vs. 54±19), but there was a significantly better diabetes control 12 months following the education course (HbA1C 7.6±1.3%, p<0.01 vs. before isCGM and p<0.05 before course; mean glycemia 8.6±1.5 mmol/l, p<0.05 and p<0.1; TIR 60±17 p<0.05 and p<0.05). There were no significant differences between patient using MDI of insulin pump.
Conclusion: In our study, we demonstrated the essential part of education in the improvement of glycemic parametres in patients with type 1 diabetes using isCGM.
Supported by: MHCR IN 00023001
Disclosure: R. Bem: None.
656
Resources used by HCPs to educate PWD about TIR
A. Sommi1, N. Sainz1, E. Asamoa2, E. Shoger2, R. Wood2, C. Alexander1;
1The diaTribe Foundation, 2dQ&A – The Diabetes Research Company, San Francisco, USA.
Background and aims: Previous studies have shown that the method of communication used by healthcare professionals (HCPs) can influence patient outcomes. The present study aimed to assess the resources used by different types of HCPs when they educate patients about time in range (TIR).
Materials and methods: In an online survey in September 2021, 303 HCPs, each with a minimum of 30 patients with diabetes every month, were asked a series of questions related to their use of TIR and continuous glucose monitoring (CGM), and their challenges, evaluation, and goal-setting when discussing diabetes management with their patients. Respondents were classified as endocrinologists (Endos, n=98), diabetes educators (DEs, n=106), or primary care providers (PCPs, n=99) and by their use of TIR (TIR users, n=234) and CGM (CGM users, n=298) in practice. Respondents received $30-$50 USD for completing the survey. Statistical significance was tested at the 95% confidence level (p<0.05).
Results: There was significant variation in resources used among DEs, Endos, and PCPs. In response to an open-ended question about resources HCPs use to educate patients about TIR, DEs were more likely to use CGM reports than PCPs (57% vs 31%). When discussing CGM data, DEs and Endos were more likely to use a computer printout than PCPs (Endo 78%, DE 70% vs PCP 28%, p<0.0005) while PCPs preferred to provide a verbal summary (PCP 52% vs Endo 27%, DE 29%, p<0.01). DEs were most likely to recommend CGM for all patients (DE 57% vs. Endo 34% vs. PCP 15%, p<0.05) and more likely than Endos to discuss TIR with patients not using CGM (56% vs. 34%, p=0.003).
Conclusion: This data highlights differences in TIR use among HCPs. DEs relied more heavily on TIR and CGM reports while PCPs more often relied on verbal instructions. Interestingly among non-CGM users, a large discrepancy exists between DEs and Endos regarding TIR discussions. This study emphasizes opportunities to bring greater alignment among DEs, Endos, and PCPs to provide standardized quality care to all people with diabetes.
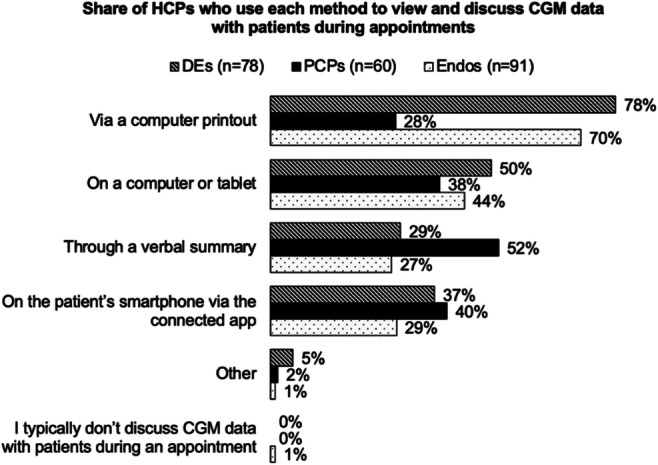
Disclosure: A. Sommi: None.
657
Healthcare professionals’ knowledge, usage, and attitudes towards the use of time in range in diabetes management: an online survey across seven countries
A.Y.Y. Cheng1, A. Ginovker2, T.B. Christensen3, U.R.H. Patted4, Y.M. Kothawade4, C. De Block5;
1Trillium Health Partners, Mississauga & Unity Health, Toronto, Canada, 2The Harris Poll, New York, USA, 3Novo Nordisk A/S, Søborg, Denmark, 4Novo Nordisk GBS, Bangalore, India, 5Department of Endocrinology-Diabetology-Metabolism, Antwerp University Hospital, Edegem, Belgium.
Background and aims: Time in range (TIR) is a metric of glycaemic control derived from continuous glucose monitoring (CGM) data. This multinational study aimed to understand knowledge, usage and attitudes towards TIR among healthcare professionals (HCPs), and gain insights into the benefits and barriers to its use in clinical practice.
Materials and methods: An online survey was completed by 1719 HCPs (specialist physicians [SP; n = 741], general physicians [GP; n = 671] and non-physicians [NP; diabetes nurse specialists, general practice nurses, nurse practitioners, physician assistants, diabetes educators; n = 307]) involved in the direct care of adults with diabetes in Brazil, Canada, South Korea, Spain, Sweden, UK and USA. Participants were sampled from online HCP panels and all were aware of TIR (study definition: amount of time in, below and above target range). HCP subgroups were compared with an exploratory analysis (standard crosstabs) and statistical differences were analysed using Z-test of proportions.
Results: Most HCPs agreed that TIR is changing the course of diabetes management (strongly agreed/somewhat agreed: SP, 90%; GP, 86%; NP, 93%) and is likely to become the standard metric for diabetes management in the future (very likely/somewhat likely: SP, 93%; GP, 87%, NP, 96%). Compared with GPs, SPs and NPs have greater knowledge (know a lot/know a fair amount: SP, 73%; NP, 53% vs GP, 30%; both p<0.05) and comfort using TIR (very comfortable/comfortable: SP, 76%; NP; 63% vs GP, 42%; both p<0.05), and this correlates with experience of using ambulatory glucose profiles (used many times/used a few times: SP, 63%; NP, 49%; vs GP, 28%; both p<0.05). Perceived benefits of TIR include making it easier to manage patients using virtual/telehealth (SP, 60%; GP, 54%; NP, 63%) and saving time for patients and HCPs (SP, 58%; GP, 59%; NP, 72%). Most HCPs believe that TIR is very effective or effective in providing accurate information about hypoglycaemia (SP, 84%; GP, 81 %; NP, 87%), optimising insulin regimens (SP, 87%; GP, 83%; NP, 87%) and optimising non-insulin regimens in type 2 diabetes (SP, 61%; GP, 52%; NP, 66%). The most frequently reported barrier to wider TIR adoption was limited access to CGM (SP, 65%; GP, 74%; NP, 69%), followed by lack of training/education (SP, 45%; GP, 59%; NP, 51%). Factors considered most important in facilitating increased TIR use are shown in the Figure.
Conclusion: Overall, HCPs agreed on the benefits of using TIR for diabetes management. As well as raising awareness among HCPs and patients, healthcare system updates are needed to facilitate increased TIR use including integration into clinical guidelines and recognition by regulators and payors.
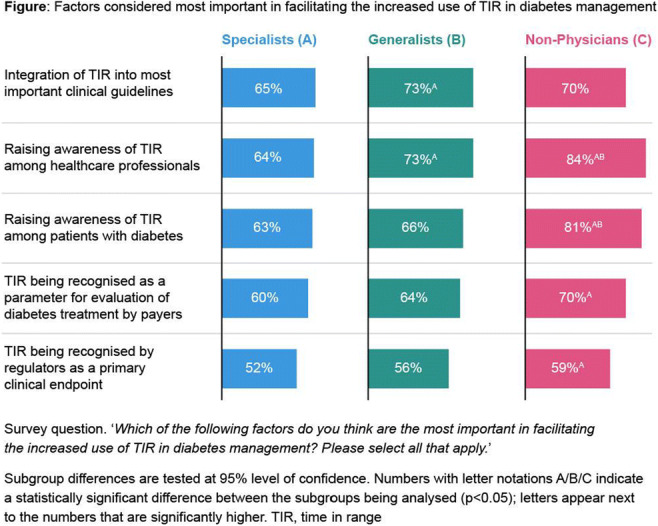
Supported by: Novo Nordisk A/S
Disclosure: A.Y.Y. Cheng: Employment/Consultancy; Novo Nordisk. Honorarium; Novo Nordisk.
SO 52 Money isn't everything?
658
Speed of recovery of testing for management of diabetes in the aftermath of COVID-19 is slower in areas of greater social deprivation: findings from a UK study
D. Holland1, A. Heald2, F. Hanna3, P. Wu4, A. Fryer4;
1The Benchmarking Partnership, Alsager, 2Salford Royal Foundation Trust, Salford, Manchester, 3University Hospital of North Midlands, Staffordshire, 4Keele University, Staffordshire, UK.
Background and aims: We have previously shown that, during the first 6 months of the UK COVID-19-associated lockdown, >6.6M HbA1c tests were missed/delayed, including 1.41M missed/delayed in people with diabetes (0.51M in those with suboptimal control). Studies suggest that COVID-19 has a more significant impact on people with diabetes and those from more deprived backgrounds. We here show data on the variability in speed of recovery of HbA1c testing across 7 UK sites, and its association with demographic characteristics, including deprivation status.
Materials and methods: We examined all HbA1c tests across 570 general practices (GPs; 4.57M population) requested between Jan 2019 and Dec 2021. We compared differences in monthly requests during 4 periods (Apr-Jun 2020 [lockdown 1], Jul-Dec 2020 [inter-lockdown period], Jan-Mar 2021 [lockdown 2] and Apr-Dec 2021 [recovery period]), with their equivalent pre-covid period in 2019. We then examined the effect of practice size, diabetes prevalence, proportion aged over 65 year and deprivation index on these differences for each period.
Results: We showed that, for all 7 centres, monthly requests dropped to 10.6-14.8% of the mean monthly 2019 request numbers in April 2020. During the following 3 periods, degree of recovery to pre-pandemic levels, as well as the drop during lockdown 2, showed greater variability between centres (inter-lockdown period: 74.0-93.2%, lockdown 2: 78.6-94.2%, recovery period: 89.0-105.7%). When examined at a GP practice level, we did not identify any link between age, practice size or diabetes prevalence and post-pandemic recovery. However, we did observe that return to pre-pandemic levels was associated with social deprivation status. Thus, compared with the equivalent pre-pandemic period, levels of HbA1c testing during period 4 (Apr-Dec 2021) were lower in those areas with higher levels of deprivation (91.3-93.5% of 2019 levels for deciles 6-10) than those with lower deprivation (96.2-99.7% of 2019 levels for deciles 1-5) (see figure). Similar findings were also noted during period 2 (inter-lockdown period: Jul-Dec 2020): deprivation deciles 6-10 were 78.2-82.6% of 2019 levels compared with 83.8-88.9% for deciles 1-5. This trend was less evident during lockdown periods 1 and 2.
Conclusion: Our findings reinforce that the COVID-19 pandemic continues to have a major impact on diabetes management, with some centres having yet to return to pre-pandemic levels. This ongoing impact appears most significant in areas of greatest deprivation, thereby adding to the increasing body of evidence showing that those from poorer backgrounds continue to be disproportionately disadvantaged in the context of SARS-CoV-2. There is therefore an onus on healthcare services to implement urgent measures to redress this imbalance.
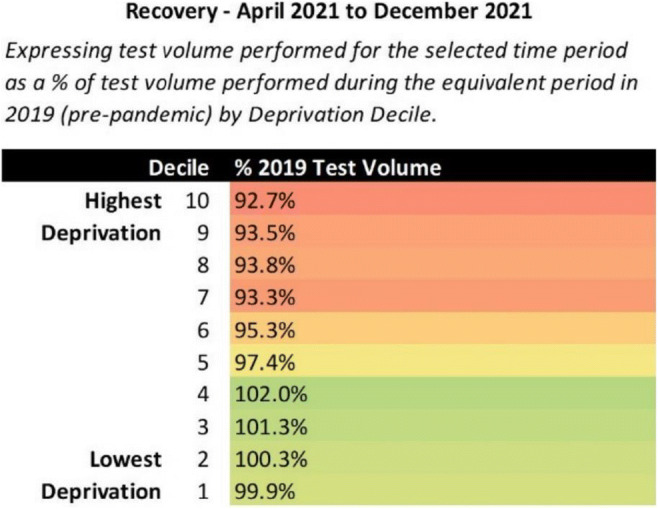
Disclosure: D. Holland: None.
659
Direct and indirect costs of peripheral artery disease in type 2 diabetes: a matched case-control study
K. Nilsson1, K. Carlsson1,2, M. Faurby3, M. Wolden3;
1The Swedish Institute for Health Economics (IHE), Lund, Sweden, 2Lund University, Lund, Sweden, 3Novo Nordisk A/S, Søborg, Denmark.
Background and aims: Peripheral artery disease (PAD) is a risk factor for amputation and other morbidity and mortality, and its prevalence among people with type 2 diabetes (T2D) has been estimated to be 45%. We used national health and social insurance data to quantify direct healthcare costs, indirect costs and other outcomes in people with T2D and PAD, compared with matched controls.
Materials and methods: In this 5-year closed cohort study, individuals ≥16 years old with T2D living in Sweden on 1 January 2012 were identified in an existing database at the Swedish Institute for Health Economics. Individuals with a record of hospital-based care for PAD, including procedure codes related to amputation, in the National Patient Register (1997-2011) were matched pairwise by age, sex and educational status to individuals with T2D without PAD. Study variables included costs of selected hospital-based care and prescription drugs, and mortality from the National Board of Health and Welfare; demographic and socioeconomic information from Statistics Sweden; and work absences from Försäkringskassan, the Swedish Social Insurance Agency. We compared cumulative direct healthcare costs (inpatient, outpatient and drug costs) and indirect costs from lost productivity, and risks of early retirement, cardiovascular events and mortality.
Results: After matching there were 15,397 individuals in each cohort; mean age was 68 years, two-thirds were men, and 41% had completed compulsory education. For each year (2012-2016), cumulative per-person direct costs for people with T2D and PAD were three- to fourfold higher than for people with T2D only (2012 €8240/€2215; 2013 €16,021/€4717; 2014 €23,933/€7528; 2015 €32,173/€10,515; 2016 €40,013/€14,120), and cumulative indirect costs were more than doubled for those with PAD (2012 €7727/€3569; 2013 €14,879/€6581; 2014 €20,758/€8942; 2015 €25,308/€10,969; 2016 €29,064/€12,143). In people with PAD, inpatient costs comprised 70% of total mean direct costs per person over follow-up (€23,889/€33,908). Outpatient costs contributed 20% (€6894) and drug costs contributed 9% (€3125) of total mean direct costs. In controls, these were 68% (€8108), 15% (€1773) and 17% (€2031) respectively of total mean direct costs (€11,912). Individuals with PAD were significantly more likely than controls to experience stroke (HR 2.01) or myocardial infarction (HR 3.30); their all-cause mortality was more than double (HR 2.60) and the risk of early retirement was nearly threefold higher (HR 2.76). During follow-up, 36% (n = 5568) of people in the T2D with PAD cohort died, compared with 13% (n = 1984) of controls (Figure).
Conclusion: This analysis demonstrates that PAD in T2D is associated with increased morbidity, mortality and economic costs, particularly contributed by inpatient admissions and lost productivity.
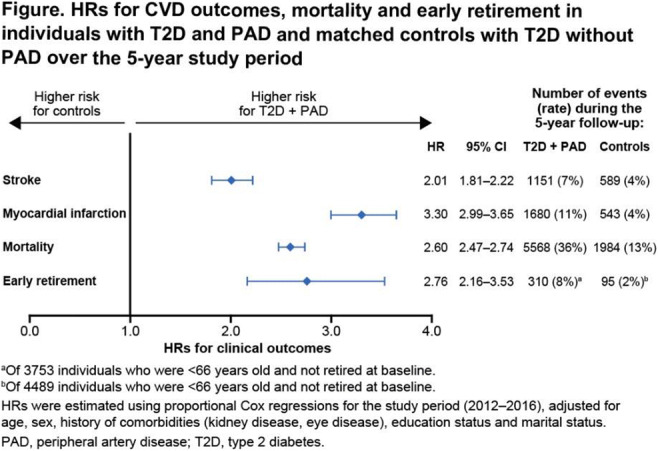
Supported by: Grant from Novo Nordisk A/S to the Swedish Institute for Health Economics
Disclosure: K. Nilsson: Employment/Consultancy; KN is an employee of the Swedish Institute for Health Economics. Grants; Novo Nordisk A/S.
660
Early death and loss of life expectancy years in type 2 diabetes are linked to the effective management of health comorbidities
M. Whyte1, M. Stedman2, A.H. Heald3, I. Laing4;
1Metabolic Medicine, University of Surrey, Salford, UK, 2Res Consortium, Andover, 3Salford Royal Hospital, Salford, 4Department of Clinical Biochemistry, Royal Preston Hospital, Preston, UK.
Background and aims: The age-standardised mortality ratio (SMR) for people with diabetes in England is 1.5-1.7. Intensive, multimodal treatment is believed to have contributed to declining mortality, besides the growth and aging of populations. There are few data quantifying the impact of the management of diabetes co-morbidities on SMR.
Materials and methods: Data from patients with type 2 diabetes in North-West of England were extracted for a 2010 cohort, followed until end 2020, or death. Annual expected deaths were calculated from Office of National Statistics (ONS) mortality rate and life expectancy based upon age, gender and index of multiple deprivation (IMD) which was based on the Lower Layer Super Output Area; LSOA). Linear regression provided a relationship between local IMD and SMR, which was used to calculate a local deprivation factor and adjust the expected national mortality rate for each patient given their LSOA. Comparing the sum of expected patient mortalities with actual deaths provided SMR adjusted for deprivation (SMRd) for the cohort. A life expectancy value multiplied by each actual death added together and then life expectancy values multiplied by fractions of expected deaths can be subtracted to ascertain the total net life expectancy years lost. This value divided by total patient years within that set and multiplied by fifty-two gives an estimate for the life expectancy weeks lost (LEWL) for each patient-year within that group. Average data 2010 to year analysed was termed Historic Average (HA).
Results: The 2020 cohort comprised 9,511 (M 5,228; F 4,283) patients. There were n=3,599 (M=1913, F=1686) deaths to end 2020. The crude SMR was 1.68 (M=1.6, F=1.78), the SMRd was 1.39 (M=1.47, F=1.32), and LEWL was 11.7 weeks (M=11,F=13). Patients with HA HbA1c 49-58 mmol/mol (30% of total cohort) had the lowest SMRd (1.25; M=1.17, W=1.36) and LEWL (M=10, W=11 weeks). Those with HA HbA1c <48 mmol/mol had SMRd = 1.45 and LEWL 15 weeks. If >86 mmol/mol the SMRd = 2.37 & LEWL 26 weeks.Patients with HA-Total Cholesterol (TC) 4-5 mmol/L (37% of total) had SMRd = 1.14, LEWL = 6 weeks. If HA TC was <3 mmol/L, the SMRd = 1.95 (M=1.68, F=3.10) LEWL=37 weeks. If HA TC >6 mmol/L, SMRd=1.39 and LEWL =10 weeks. SMRd falls as HA-Diastolic blood pressure (DBP) increases, HA DBP of 70-80 mmHg had SMRd = 1.21, if DBP 90-100 mmHg SMRd = 1.1 (M=1.0, F=1.2) LEWL =4 weeks. If DBP 50-60 mmHg the SMRd = 1.74 and LEWL 25 weeks.
Conclusion: Optimum control of HBA1c for SMRd is at 48-59mmol/mol, while reduction through higher medication in cholesterol and DBP past the optimum is associated with higher SMR.
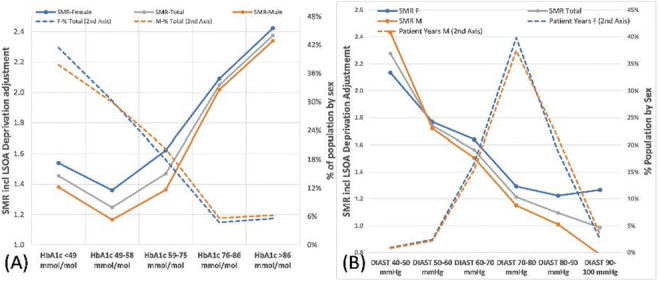
Disclosure: M. Whyte: None.
661
Socioeconomic status and metabolic control in Swedish children with type 1 diabetes
O. Pekkari, J. Ludvigsson, K. Åkesson;
Department of Biomedical and Clinical Sciences, Linköping university, Linköping, Sweden.
Background and aims: The treatment of Type 1 diabetes aiming to a glucose control as close to normal as possible is very demanding, not only for the child but for the entire family. The aim of this study was to investigate how glycaemic control in children with T1D, from families with different socioeconomic status, develops over time and whether paternal or maternal socioeconomic status holds a stronger association to the glycaemic control.
Materials and methods: All children in NDR/Swediabkids, the national Swedish diabetes register, registered with T1D in 2000 - 2020 (n = 19480) were included. One annual HbA1c per patient was randomly chosen. Each child had a mean follow-up of 6.12 years giving 119 276 HbA1c values. Parental information was retrieved from the LISA (The Longitudinal integration database for health insurance and labour market studies)-a national register, and linked to the child through SMR (The Swedish Multi-generation Register). A mixed-effects model for statistical analysis was chosen. Annual long-term trends of HbA1c were stratified for parental sex and then for education, income and marital status.
Results: Parental educational level had the strongest association to the glycaemic control. Paternal and maternal educational level showed similar results. An increase in HbA1c was seen during puberty ( age - years), but not in children to parents with >12 years of education who also showed a larger decrease in HbA1c during the last two decades compared to children where parents had a lower educational level. Children to married parents had a marginally lower HbA1c during puberty compared to children to divorced or single parents. No clear association between glycaemic control and income was shown.
Conclusion: Both the fathers’ and the mothers’ level of education is important for the degree of glycaemic control in their children. Families with less educated parents need extra support from health care.
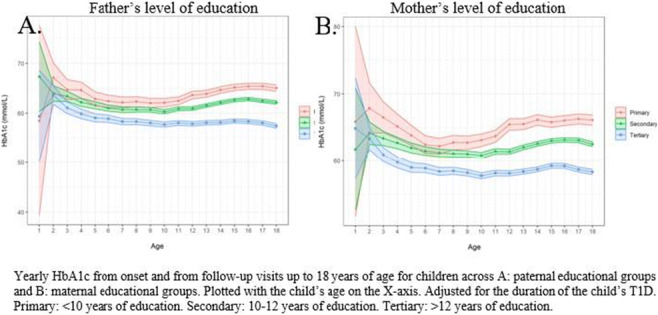
Disclosure: O. Pekkari: None.
662
Early death and loss of life expectancy years in type 2 diabetes are linked to local population demographics and lifestyle
M. Stedman1, A.H. Heald2, M. Whyte3;
1Res Consortium, Andover, 2Salford Royal Hospital, Salford, 3University of Surrey, Basingstoke, UK.
Background and aims: The age-standardised mortality ratio (SMR) for people with diabetes in England is 1.5-1.7. Little work has been carried out on quantifying the impact of the various factors that affect SMR; this can be done by examining sufficient real-world patient records over a reasonable period.
Materials and methods: Data from patients with type 2 diabetes for the Salford region of England was extracted for a 2010 cohort until 2020 or death. Annual expected deaths were calculated from the annual Office of National Statistics (ONS) mortality rate and life expectancy by age and gender plus recorded deaths and population within the 37,400 Lower Layer Super Output Area (LSOA), where the Index of Multiple Deprivation (IMD) are provided. Linear regression provided a relationship between local LSOA IMD and SMR, which was used to calculate a local deprivation factor and adjust the expected national mortality rate for each patient given their LSOA. Comparing the sum of expected patient mortalities with actual deaths provided SMR adjusted for deprivation (SMRd) for the selected cohort. A life expectancy value multiplied by each actual death added together and then life expectancy values multiplied by fractions of expected deaths can be subtracted to ascertain a total net life expectancy years lost. This value divided by total patient years within that set and multiplied by fifty-two gives the life expectancy weeks lost (LEWL) for each patient-year within that group.The impact of demographic and lifestyle factors such as sex, age, BMI, deprivation and smoking on the SMRd was evaluated on a uni-variant basis.
Results: 9,511 (Male 5,228/Female 4,283) patients in 2010 were included with 3,599 (M 1913/F 1686) deaths recorded up to end 2020. A crude SMR was 1.68 (M 1.6 / F 1.78), the SMRd was 1.39 (M 1.47 / F 1.32), and LEWL was 11.7 weeks (M 11/F 13). Expected deaths include for effect of age and sex, the additional effect at Age < 75 SMR 1.77 (M 1.63 / F 2.04), age 75-84 SMR = 1.4 (M 1.27 / F 1.58) and age>=85 SMR = 1.10 (M 1.05 / F 1.15).18,015 (21%) patient-years were smokers (national prevalence is 12%) with SMRd = 2.36 (M 2.07 / F 2.43), 26,527 (31%) non-smokers SMRd was 1.15 (M 1.07 / F 1.20) and 39,705 (47%) ex-smoker SMRd was 1.25 (M 1.19 / F 1.36). SMRd ratio for smokers versus non-smokers is 2.1, in the general population smoking mortality risk ratio is 1.7. The LEWL calculation shows that T2DM smokers might lose 22 weeks (M 20 / F 24) of life expectancy for each year they smoke while non-smokers lose 5 weeks (M 5 / F 6). Average IMD in Salford is 31, 44% above national average 21, with adjustment included, top quartile IMD >50, SMRd Men 1.2 / Women 1.4 and LEWL M 14w / W 18w.
Conclusion: Type 2 diabetes has a considerable variation with large impacts on vulnerable sub-populations. Those at much higher relative risk of earlier mortality from type 2 diabetes are women, of younger age, in deprived areas and smokers. These groups should receive specific interventions that more effectively engage them with their own health care.
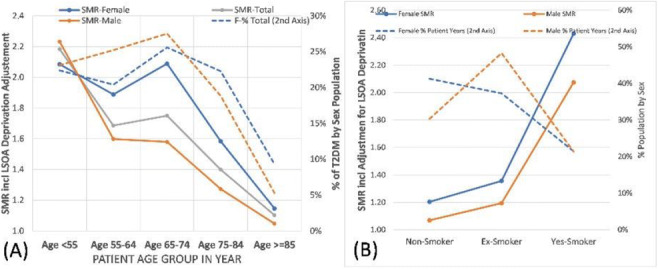
Disclosure: M. Stedman: None.
663
Direct and indirect costs of atherosclerotic CVD in type 2 diabetes: a matched case-control study in 160,000 individuals
K. Steen Carlsson1,2, K. Nilsson2, M. Lyng Wolden3, M. Faurby3;
1Lund University, Lund, Sweden, 2The Swedish Institute for Health Economics (IHE), Lund, Sweden, 3Novo Nordisk A/S, Søborg, Denmark.
Background and aims: Type 2 diabetes (T2D) is associated with risk of atherosclerotic CVD (ASCVD), which is linked to significant morbidity and mortality. We compared direct healthcare costs, indirect costs and other outcomes in people with T2D with and without ASCVD.
Materials and methods: This was a 5-year closed cohort study. Individuals ≥16 years old with T2D alive and resident in Sweden on 1 January 2012 were identified in an existing database at the Swedish Institute for Health Economics. Individuals with ASCVD, defined as ≥1 inpatient admission or outpatient visit with a main or sub-diagnosis relating to stroke, ischaemic heart disease or peripheral artery disease, or procedure codes related to revascularization or amputation, in the National Patient Register (1997-2011), were propensity score matched 1:1 to controls with T2D and without ASCVD by year of birth, sex and educational status. Study variables included costs of selected hospital-based care and prescription drugs, and mortality from the National Board of Health and Welfare; demographic and socioeconomic information from Statistics Sweden; and work absences from Försäkringskassan, the Swedish Social Insurance Agency. We compared annual direct healthcare costs and indirect costs from lost productivity, and risks of early retirement, cardiovascular events and mortality. HRs were estimated using Cox proportional regression adjusted for baseline age, sex, comorbidities, education and marital status.
Results: The cohort with T2D and ASCVD (n = 80,305) had mean age 67 years; 67% were men and 39% had completed only compulsory education. Over the study period, mean annual total direct costs for individuals with T2D and ASCVD were nearly double the costs for controls with T2D and no ASCVD; indirect costs were also 77% higher for those with ASCVD than for those without (Figure). In cases and controls, indirect costs accounted for more than half of total costs. In the group with T2D and ASCVD, inpatient costs constituted 71% of total direct costs, whereas outpatient costs and drug costs contributed 16% and 13%, respectively. In controls with T2D without ASCVD, total direct costs comprised 68% inpatient costs, 15% outpatient costs and 18% drug costs. All-cause mortality was 81% higher in individuals with T2D and ASCVD than in controls (HR 1.81 [95% CI 1.77, 1.86]). Individuals with T2D and ASCVD were also more likely to experience stroke (1.81 [1.73, 1.89]), myocardial infarction (2.61 [2.50, 2.73]) and early retirement (2.23 [2.01, 2.48]).
Conclusion: Individuals with T2D and ASCVD incurred nearly double the total costs of people with T2D and no ASCVD, and had a greater risk of death and other CV events. Efforts to prevent and slow ASCVD in individuals with T2D are needed to reduce mortality, morbidity and the economic impact on healthcare systems and society.
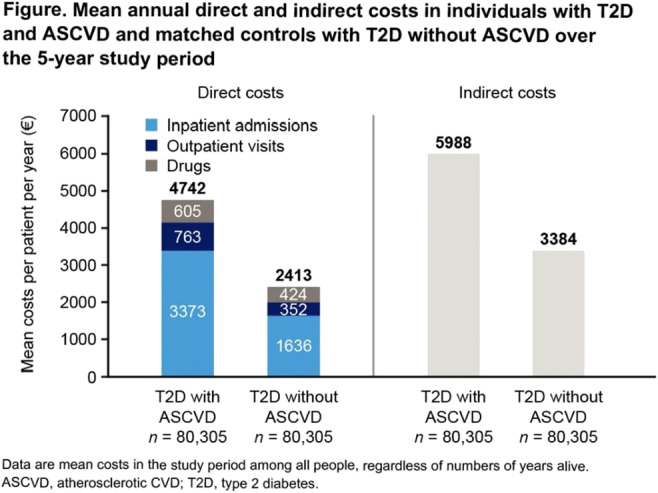
Supported by: Grant from Novo Nordisk A/S to the Swedish Institute for Health Economics.
Disclosure: K. Steen Carlsson: Employment/Consultancy; KSC is an employee of the Swedish Institute for Health Economics.. Grants; Novo Nordisk A/S.
664
Cumulative cardiovascular or renal disease (CVRD) hospital costs for type 2 diabetics free of CVRD at baseline: a 5-year cohort study in the SNDS nationwide claims database
P. Zaoui1, P. Blin2, M. Joubert3, E. Guiard2, D. Sark2, C. Dureau-Pournin2, M.A. Bernard2, R. Lassalle2, F. Thomas-Delecourt4, S. Bineau4, N. Moore2, C. Droz-Perroteau2, P. Jourdain5;
1CHU Grenoble, Grenoble, 2Bordeaux PharmacoEpi INSERM-P CIC1401, University of Bordeaux, Bordeaux, 3CHU Caen, Caen, 4AstraZeneca, Courbevoie, 5Hôpital Kremlin-Bicêtre, Paris, France.
Background and aims: Myocardial infarction (MI), stroke, peripheral arterial disease (PAD), heart failure (HF) and chronic kidney disease (CKD) are common cardiovascular renal disease (CVRD) complications for type 2 diabetes (T2D). The objective was to estimate the 5-year cumulative costs of CVRD hospitalizations for T2D patients free of CVRD at baseline.
Materials and methods: A cohort study of all French T2D patients free of CVRD (5-year history) as of January 1st, 2014, identified and followed-up for 5 years within the French SNDS nationwide claims database. The 5-year cumulative CRVD hospital costs for T2D patients without CVRD at baseline, was estimated for each CVRD over time from the perspective of all payers and taking into account primary and associated diagnoses.
Results: From about 2 million T2D patients without cancer or transplantation, 76.5% were free of CVRD at baseline with a mean age of 65 years, 52% of women, 46% T2D for more than four years and 7% of diabetic complications. For the whole cohort, the 5-year global cost of all CVRD hospitalizations was 3.875 billion € (B€) with an increase weakly exponential over time for specific CVRD manifestations (Figure 1). The 5-year cumulative hospital costs were 1.954 B€ for CKD and 1.169 B€ for HF, respectively 6.6 and 4.0 times more costly than for MI (0.295 B€), 3.2 and 1.9 times more costly than for stroke (0.614 B€), 2.8 and 1.7 time more costly than for PAD (0.690 B€). Hospitalizations with HF or CKD diagnoses together were 1.8 times more costly than for MI, stroke and PAD together (2.742 vs 1.549 B€); sum of hospital costs of each specific CVRD was 21.9% higher than the overall cost because some hospitalizations may involve 2 or more CVRD diagnoses when considering primary and associated diagnoses.
Conclusion: The 5-year global CRVD complication cumulative hospital cost in France was about 2 billion € for those without CVRD at baseline. HF and CKD hospitalizations together were twice as expensive than the classic MI, stroke and PAD complications together. This should encourage the development of specific preventive strategies.
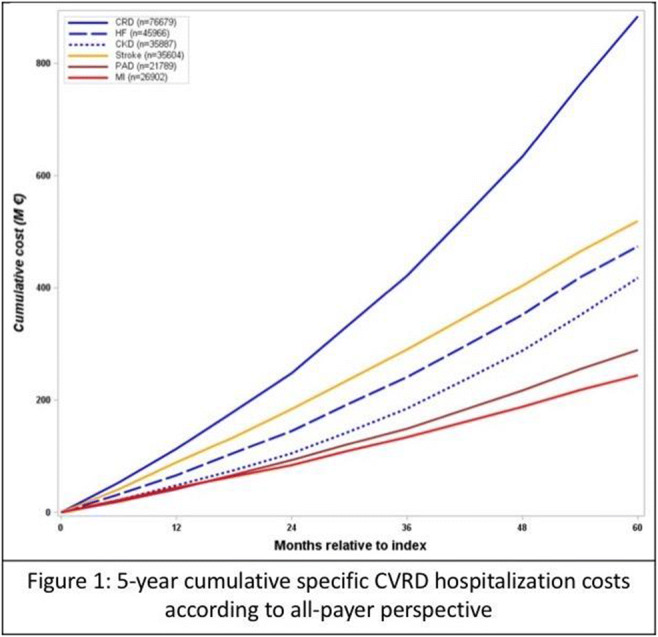
Supported by: Study performed with unconditional funding from AstraZeneca
Disclosure: P. Zaoui: Honorarium; AstraZeneca.
665
Real world healthcare costs among patients using self-monitoring blood glucose compared to continuous glucose monitoring in non-intensively treated type 2 diabetes
F. Giorgino1, E. Repetto2, J. Rötschke3, E. Acmet4, G. Bergman5, R. Maroun6, A. Wu7, I. Duncan8, D. Kerr9;
1Division of Endocrinology, University Hospital Policlinico Consorziale, Bari, Italy, 2Roche Diabetes Care, Inc., Indianapolis, USA, 3Roche Diabetes Care GmbH, Mannheim, Germany, 4Roche Diabetes Care Italy S.p.A., Monza, Italy, 5Roche Diabetes Care B.V., Almere, Netherlands, 6Roche Diagnostics A.G., Bazel, Switzerland, 7Roche Diagnostics, Inc., Santa Clara, USA, 8University of California, Santa Barbara, USA, 9Sansum Diabetes Research Institute, Santa Barbara, USA.
Background and aims: Type 2 diabetes (T2DM) poses a significant economic burden to healthcare systems. The increasing use of continuous glucose monitoring (CGM) in T2DM raises questions about its appropriateness and sustainability in this clinical setting. The aim of this study was to compare all-cause costs and healthcare resource utilization (HCRU) for T2DM individuals treated with oral agents or basal insulin using Self-Monitoring of Blood Glucose (SMBG) compared with CGM.
Materials and methods: A retrospective cost-analysis using the IBM® MarketScan® Databases was performed. Commercially insured T2DM US patients aged ≥18 years who were using SMBG or initiated CGM between Jan 2018 and March 2019 were compared. Inclusion criteria included two consecutive claims for T2DM or one claim for T2DM and a claim for glucose-lowering therapy, at least one pharmacy claim for SMBG strips or CGM sensors, and continuous enrollment for 12 months before and after the index date. Individuals with evidence of CGM in the pre-index period, pregnancy, rapid-acting insulin, glucagon, T1DM, gestational or secondary diabetes were excluded. The analysis examined all-cause costs and HCRU among two subgroups: 1) orally treated T2DM subjects taking oral monotherapy or oral combination therapy, and 2) basal insulin-treated T2DM subjects, including those in combination with oral therapy, but not with other injectables (e.g. GLP-1 and amylin analogues). For each subgroup, SMBG and CGM users were compared using a propensity score matching approach, and all-cause costs during a 12-month follow-up period were evaluated adopting a payer’s perspective.
Results: 1,449 patients were included in each matched cohort in the orally treated subgroup. The average total healthcare costs per person/year were $2,917 less in SMBG users vs CGM users (p<0.001). SMBG users also had lower pharmacy costs (-$1,945, p<0.001, outpatient costs (-$473, p<0.001), and glucose-lowering medication costs (-$1,207, p<0.001). 583 patients were included in each matched cohort in the basal treated subgroup. The total healthcare costs per person/year were $4,426 less in SMBG users vs CGM users (p<0.001). SMBG users had also lower outpatient costs (-$388, p<0.001). In both subgroups, SMBG and CGM cohorts had similar rates for emergency room attendance.
Conclusion: This analysis shows that SMBG is less costly than CGM in T2DM treated by oral or basal insulin regimens. These findings may have implications regarding glucose monitoring technologies resource allocation for non-intensively managed T2D patients.
Disclosure: F. Giorgino: None.
666
Smart insulin pen caps versus standard of care: a Canadian cost-effectiveness analysis
K. Quansah1, K. Hansen1, K. Chan1, S. Muratov2, S. Khoudigian2, M. Lamotte3;
1Novo Nordisk Canada Inc, Mississauga, Canada, 2IQVIA Canada, Mississauga, Canada, 3IQVIA Global, Zaventem, Belgium.
Background and aims: There is growing interest in novel insulin management systems that improve glycemic control without increasing the risk of hypoglycemia. A Bluetooth-enabled smart pen cap for disposable insulin pens allows the user to access data on insulin dose, type and time of injection resulting in safer insulin therapy. This study has evaluated the cost-effectiveness of smart insulin pen caps versus standard of care (SOC) insulin pens in the management of adult patients with type 1 diabetes (T1D) from a Canadian societal perspective.
Materials and methods: The well-validated IQVIA Core Diabetes Model (CDM) version 9.0 was utilized to conduct the analyses. Based on a previously published Swedish non-interventional study, the smart insulin pen cap arm was assumed to bear greater reductions (-0.67%) in glycated hemoglobin from baseline (obtained through a conversion from time in range) and have lower hypoglycemic events compared to the standard arm (-32.87 events per year). Cardiovascular and microvascular risks were predicted using the Epidemiology of Diabetes Interventions and Complications (EDIC) study data. Costs (inflated to 2021 Canadian dollars (CAD)) and utilities were sourced from literature. Core outcomes of the model estimated life years (LYs) and quality-adjusted life-years (QALYs). QALYs were assessed using the additive minimum approach method. The Lauridsen et al diminishing approach was used to estimate disutility for hypoglycemic events. The societal perspective included costs to publicly funded healthcare payer and costs of lost productivity. Both costs and effects were discounted at 1.5% over a 60-year time horizon in the base case. Uncertainty was explored in 18 scenario analyses and a probabilistic sensitivity analysis.
Results: The smart insulin pen cap was associated with lower mean discounted total costs ($221,943 CAD vs. 266,199; $-44,256 CAD), improvements in mean life expectancy (25.78 vs. 24.29; +1.49 years) and gains in QALYs (18.48 vs. 16.74; +1.75 QALYs) over the patient’s lifetime. The scenario analyses either confirmed the dominance of the smart insulin pen cap arm or demonstrated its cost-effectiveness at the willingness to pay threshold of $50,000 CAD. The probabilistic analysis showed dominance in 99.5% of cases and cost-effectiveness in 100% of cases.
Conclusion: For adults with T1D in Canada, a smart insulin pen cap is likely to be a cost-effective treatment option associated with greater clinical benefits and lower costs relative to a standard disposable pen.
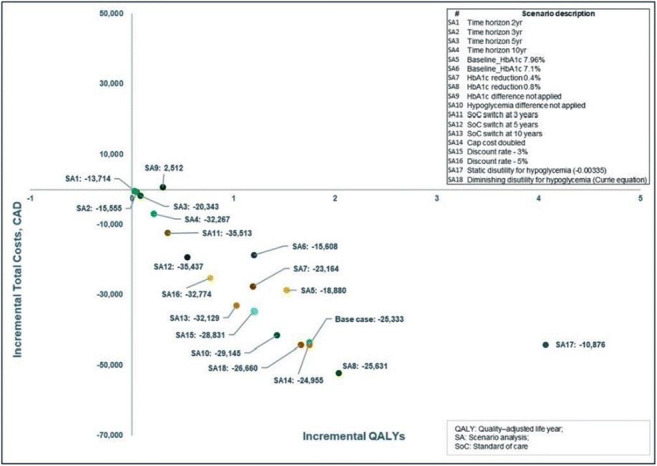
Disclosure: K. Quansah: Employment/Consultancy; Employee of Novo Nordisk Canada Inc.
SO 53 Pumping, looping, freeing
667
Predictors of time in target glucose range in real-world users of the MiniMed™ 780G system
J. Castaneda1, A. Arrieta1, T. van den Heuvel1, O. Cohen2;
1Bakken Research Center, Medtronic, Maastricht, Netherlands, 2Medtronic International Trading Sàrl, Medtronic, Tolochenaz, Switzerland.
Background and aims: Automated insulin delivery use has demonstrated improved glycaemic control in people with type 1 diabetes mellitus. The present analysis investigated predictors of improved sensor glucose (SG) time-in-range (TIR 70-180 mg/dL) based on real-world use of the MiniMed™ 780G advanced hybrid closed-loop (AHCL) system.
Materials and methods: Data uploaded from August 2020-July 2021 by de-identified MiniMed™ 780G system users (in Europe, Middle East and Africa) were analyzed using univariate and multivariable models to identify baseline (pre-AHCL initiation) glycaemic metrics, demographics and system use characteristics associated with TIR post-AHCL initiation. System settings associated with improved TIR post-AHCL were identified and their impact on time spent at <70mg/dL (TBR) post-AHCL was explored. Glycaemic outcomes (e.g., mean±SD of SG, the glucose management indicator (GMI), and mean time spent at SG ranges) post-AHCL were also analyzed.
Results: 12,870 users were included, of whom 2,977 had baseline SG data. Baseline mean TIR and time in AHCL were positively associated with TIR post-AHCL, with higher values predicting greater TIR post-AHCL. Characteristics inversely associated with TIR post-AHCL included percentage of daily basal insulin dose, daily auto correction dose, number of daily AHCL exits triggered by the system and number of daily alarms, wherein higher values predicted lower mean TIR post-AHCL. Settings that predicted the highest TIR post-AHCL were active insulin time (AIT) of 2hrs and glucose target of 100mg/dL. Associations were based on the multivariable model (all p<0.001). Figure 1 shows the glycemic outcomes for subjects with optimized modifiable factors: 5-6 boluses per day, AIT of 2hrs, and 100mg/dL glucose target. Same predictors were found for TBR post-AHCL, except for AIT (p= 0.3104).
Conclusion: Modifiable factors including optimized pump settings can allow users to achieve glycaemic targets with >80% TIR, while maintaining low TBR. The findings from this analysis will potentially guide the optimal use of the MiniMed™ 780G system and facilitate meaningful improvements in safe glycaemic control.
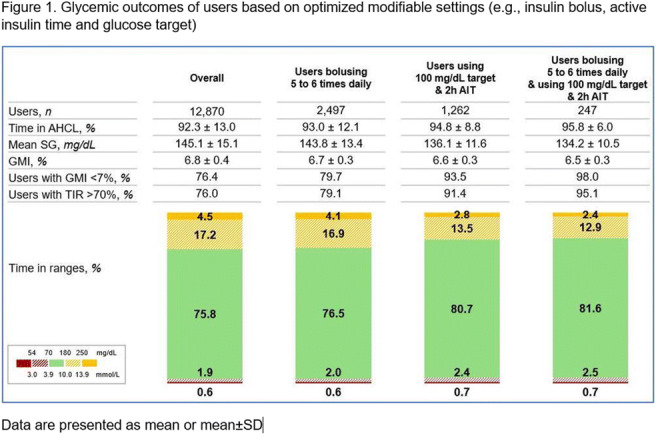
Disclosure: J. Castaneda: Employment/Consultancy; Employer of Medtronic.
668
New ex-vivo method to assess subcutaneous absorption events during basal administration through pump: proof of concept
P. Jacquemier1, Y. Retory2, A. Schmidt2, C. Virbel-Fleischman2, J. Cuenco1, A. Ostertag3, M. Cohen Solal3, J.-B. Julla3, L. Potier4, N. Venteclef1, J.-F. Gautier3, J.-P. Riveline3;
1IMMEDIAB, Institut Necker Enfants Malades, 2Air Liquide Healthcare, Bagneux, France, 3Hôpital Lariboisière APHP, 4Hôpital Bichat APHP, Paris, France.
Background and aims: Glycemic variability and hypoglycemia are still an issue among type 1 diabetic pump users and cannot always be explained by patient co-morbidity or behavior. Erratic subcutaneous (SC) absorption and poor diffusion at the injection site are often among the suspected causes for this phenomenon. However assessing insulin SC propagation is a challenge when it is administered by basal rate through pump and was scarcely studied. We propose a descriptive method linking pump delivery and SC propagation of insulin through continuous follow-up of catheter pressure and high-precision time-spread imaging to screen for events potentially disturbing delivery.
Materials and methods: Administration of insulin (Aspart®) and contrast agent was performed via a pump (t:slim x2, Tandem®) at 1UI/h basal rate in ex vivo human skin explants from bariatric surgery. The sample was placed in a micro-CT scanner (μCT). A 20Hz pressure sensor was inserted between the pump and the sample. 3D images were taken every 5 min via μCT during 3h. Insulin depot was numerically isolated by comparison with image of explant before injection, linear enhancement of histogram and thresholding.
Results: Total opacity of explant images grew linearly with time through all of injection. Insulin impulsions can be detected upon pressure measurements. Pressure build-ups lasting 33.5 min in average (minimum 10 min, maximum 88 min) were detected in 33% of injections. In all other injections, no pressure build-up was observed. First images showed various shapes of insulin depots and interlobular fascia seems to be a preferred path. Different propagation configurations were observed: intradermal injections, propagation along interlobular fascia when the cannula was within hypodermis and close to a fat lobule limits (as displayed in the image, both in 3D and 2D, for the same sample), and bubbles during injection were made clearly visible, as they were once imprisoned in the tissue.
Conclusion: The observation of pressure build-ups in the catheter suggest events in the tissue, but the linearity of opacification indicates a regular delivery, excluding catheter occlusions. The ongoing morphological analysis of insulin depots will enable us to understand the mechanisms of SC propagation and establish if there is a link with pressure events. Analysis of basal rate impact and interpatient variability are to be explored.
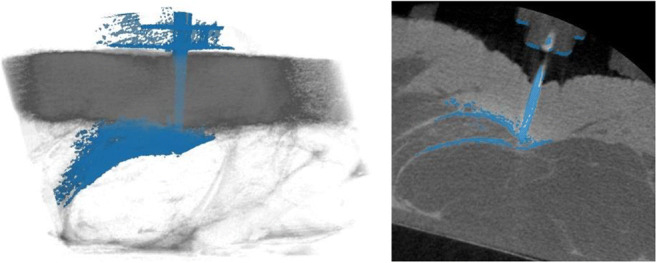
Disclosure: P. Jacquemier: Employment/Consultancy; Air Liquide Healthcare.
669
Recently diagnosed with type 1 diabetes: glycaemic outcomes with the Omnipod® Automated Insulin Delivery System
G.P. Forlenza1, D.J. DeSalvo2, B.W. Bode3, A.B. Criego4, S.A. Brown5, M.J. Schoelwer5, S.N. Mehta6, L.M. Laffel6, S.A. MacLeish7, L.M. Huyett8, J. Boyd8, R.E. Gurlin8, T.T. Ly8, Omnipod 5 Research Group;
1Barbara Davis Center, Aurora, 2Baylor College of Medicine, Houston, 3Atlanta Diabetes Associates, Atlanta, 4International Diabetes Center, Minneapolis, 5University of Virginia, Charlottesville, 6Joslin Diabetes Center, Boston, 7Rainbow Babies and Children's Hospital, Cleveland, 8Insulet Corporation, Acton, USA.
Background and aims: The diagnosis of type 1 diabetes (T1D) is a major life event requiring the rapid acquisition of complex knowledge and skills to safely manage the condition. Automated insulin delivery (AID) systems may improve health and safety while reducing the daily burden of T1D self-management. In the US, an estimated 64,000 people are diagnosed with T1D each year; however, there is little data available evaluating AID use in those recently diagnosed partly due to clinical study design, which typically has required a T1D duration of ≥6mo in children aged <6y, or ≥1y in those aged ≥6y. In this sub-analysis of the Omnipod 5 AID System pivotal trial, we evaluated outcomes of participants who were diagnosed within 1y prior to enrollment.
Materials and methods: Participants were in 3 age groups: 2-5.9y, 6-13.9y, and 14-70y with screening A1C<10% (86 mmol/mol). Inclusion criteria for the pivotal trial were any T1D duration for children <6y, or T1D duration ≥6mo for ages ≥6y. This sub-analysis was restricted to participants with T1D duration <1y prior to study enrollment. In the trial, participants used AID for 3mo at home after 14d of their standard therapy (ST, pump therapy or multiple daily injections [MDI]). Safety endpoints were occurrence of severe hypoglycemia (SH) and diabetic ketoacidosis (DKA). Efficacy endpoints included A1C at end of study compared with baseline and time in range (TIR, % 70-180 mg/dL [3.9-10.0 mmol/L]) during AID compared with the ST phase.
Results: At baseline, participants (N=22, 50% male) had a median age of 5.0y (IQR: 3.2-9.3y, range: 2-43y) with T1D duration (mean±SD) 0.7±0.3y (range: 0.11-0.99y) and a ST total daily insulin dose of 0.56±0.22 U/kg (range: 0.19-0.95 U/kg). Eight participants (36%) were prior MDI users and 3 (14%) were new to continuous glucose monitor (CGM) use. There were no episodes of SH or DKA during the 3-month AID phase in this cohort. With AID, mean A1C improved from baseline to 3 months by 0.8% [8.7 mmol/mol] (p<0.05, Table). TIR improved from 58±18% during ST to 68±11% with AID (p<0.05, Table), equating to an additional 2.4h/d TIR. Median percent time <54 mg/dL (3.0 mmol/L) increased; however, this median time was very low (0.15%), below target, and lower than the full study cohorts (2-5.9y, 6-13.9, and 14-70y) during AID.
Conclusion: The Omnipod 5 AID system was safe and effective in a cohort of recently diagnosed persons with T1D. The absence of SH and DKA are encouraging and suggest that AID could be safe in those without long-term self-management experience. These data support the early adoption of AID.
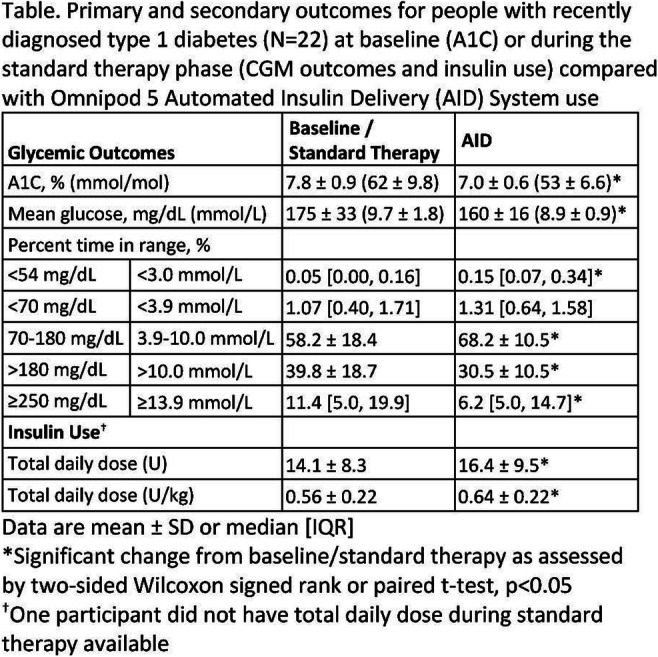
Clinical Trial Registration Number: NCT04196140 & NCT04476472
Supported by: Insulet Corporation
Disclosure: G.P. Forlenza: Grants; Insulet, Medtronic, Dexcom, Abbott, Tandem, Lilly, Beta Bionics. Lecture/other fees; Insulet, Medtronic, Dexcom, Tandem, Lilly, Beta Bionics.
670
Glycaemic outcomes over 15 months in people with type 1 diabetes with the Omnipod® Automated Insulin Delivery System
A. Criego1, A.L. Carlson1, T.T. Ly2, Omnipod 5 Research Group;
1International Diabetes Center, Minneapolis, 2Insulet Corporation, Acton, USA.
Background and aims: Safe and effective use of the Omnipod 5 Automated Insulin Delivery (AID) System was demonstrated in adults and children with type 1 diabetes (T1D) during a 3-month pivotal study. Longer studies evaluating durability of glycemic benefit of AID systems for >1 year are limited. We present results from a 12-month extension study for a total of 15 months of system use.
Materials and methods: In the pivotal study, participants aged 6-70 years with T1D≥6 months and A1C<10% (86 mmol/mol) used the AID system for 3 months, after 14 days of continuous glucose monitor data collection with their standard therapy (pump therapy or multiple daily injections) to assess baseline glycemic outcomes. Participants were invited to continue AID use in a 12-month extension study, with outcomes measured every 3 months. Safety endpoints were occurrence of severe hypoglycemia (SH) and diabetic ketoacidosis (DKA). Efficacy endpoints, including A1C and time in range (TIR, % 70-180 mg/dL [3.9-10.0 mmol/L]), were compared with baseline every 3 months over the 15-month study period (3-month pivotal study and 12-month extension study).
Results: Almost all participants (95%) continued into the extension study. At baseline, children (N=110, ages 6-13.9y) and adolescents and adults (N=114, ages 14-70y) were aged (mean±SD) 10±2y and 37±14y with T1D duration 5±3y and 17±11y, respectively. There were 2 SH and 1 DKA episodes during the 12-month extension. For both age groups, mean A1C improvements first observed at the end of the 3-month pivotal study were sustained through 12 additional months of AID use when compared with baseline (p<0.05, Table). At the end of the 12-month extension, change in A1C from baseline was -0.5% (-5.5 mmol/mol) and -0.3% (-3.3 mmol/mol) for children and adolescents and adults, respectively (p<0.05, Table). For both age groups, TIR improvements were sustained through 15 months of system use compared with standard therapy (p<0.05, Table).
Conclusion: The safety and improved glycemic outcomes from the 3-month pivotal study persisted for an additional 12 months, indicating the potential long-term benefit of the Omnipod 5 AID System.
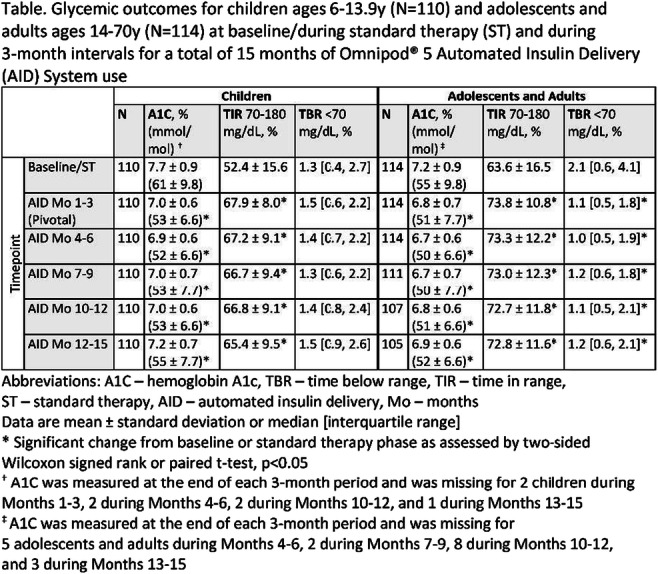
Clinical Trial Registration Number: NCT04196140
Supported by: Insulet Corporation
Disclosure: A. Criego: Grants; Insulet, Dexcom, Medtronic, Abbott Diabetes, Sanofi, Eli Lilly. Lecture/other fees; Medtronic, Sanofi, Eli Lilly, Medscape.
671
Advanced hybrid closed-loop system rapidly achieves and mantains good glycaemic control in type 1 diabetes adults dispite of previous treatment
C. Quiros, A.-C. Nuria, R. Silvia, P. Verónica;
Hospital Universitari Mutua de Terrassa, Terrassa, Spain.
Background and aims: In last years, the improvement in continuous glucose monitoring (CGM) has changed type 1 diabetes (T1D) treatment allowing the automatization of glucose control. The advanced hybrid closed-loop (ACHL) system Minimed 780G adapts basal infusion rates and delivers autocorrection boluses in order to achieve a glucose target decided by the user (100, 110 or 120mg/dL). The aim was to evaluate the effectiveness of Medtronic 780G system in real-life conditions for 6 months.
Materials and methods: Prospective study that includes T1D individuals previously treated with insulin pump without CGM (pump group) or with sensor-augmented pump with predictive low-glucose suspend (SAP-PLGS group) that started Minimed 780G system. Baseline, 1-, 3- and 6-months sensor and pump data were downloaded. Baseline and at 6 months HbA1c were recorded.
Results: Fifty T1D subjects were included. 25 were SAP-PLGS 640G previously users and 25 were using 640G without CGM. Sixty six percent were females, with 48.6 (40-57) years and 20 (12-31.5) years of diabetes duration. Time in range (TIR) improved in total cohort from baseline to 6 months (69 (57.7-76) vs 74 (70-82) %; p=0.01 as well as HbA1c (7.6 (7.1-7.8) vs 7.0 (6.8-7.5) %; p<0.001), with improvement in times <54, >180 and >250 mg/dL. Outcomes at 6 months were not different between groups (TIR pump group 73.5 (68- 78.5) vs TIR SAP-PLGS group 78 (71-85); p>0.05) although SAP-PLGS subjects were prone-to-hypoglycemia individuals and subjects in pump-group had mainly suboptimal metabolic control. All glucose outcomes are depicted in Table 1.
Conclusion: The AHCL system Medtronic Minimed 780G rapidly achieves and maintains good glycemic control during 6 months in real-life conditions in different profiles of type 1 diabetes individuals.
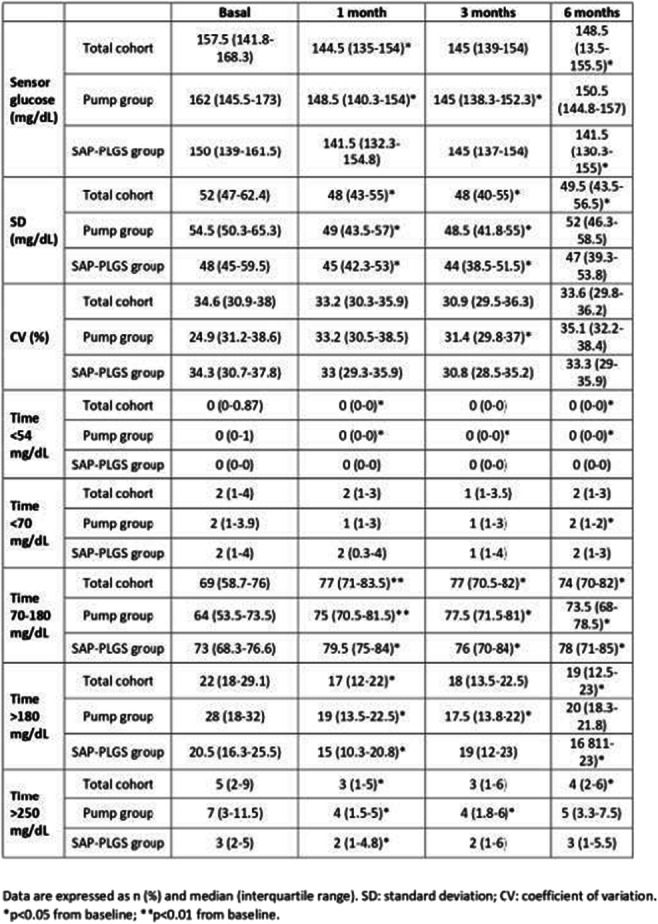
Disclosure: C. Quiros: None.
672
Comparison of hybrid closed-loop systems, AndroidAPS and Control-IQ in patients with type 1 diabetes: the CODIAC study
D.Q. Dat1, A. Hásková1, L. Radovnická2, E. Horová1, G. Grunberger3, C.G. Parkin4, M. Prázný1, J. Šoupal1;
13rd Department of Internal Medicine, 1st Faculty of Medicine Charles University, Prague, Czech Republic, 2Department of Internal Medicine, 1st Faculty of Medicine Charles University, Prague, Czech Republic, 3Grunberger Diabetes Institute, Bloomfield Hills, USA, 4CGParkin Communications, Inc, Henderson, USA.
Background and aims: The number of patients with type 1 diabetes (T1D) using non-certified “Do-It-Yourself” (DIY) automated insulin delivery (AID) systems is rapidly increasing. Non-certified technology has been suggested to bring better treatment outcomes than a certified system. However, there are no studies supporting this statement. Therefore, we compared two AID systems - certified Control-IQ technology (CIQ) and non-certified AndroidAPS (AAPS) in maintaining optimal glycemic control.
Materials and methods: In this prospective open-label single-arm clinical trial, adults with T1D, who had been using the AAPS by their own decision were switched to the CIQ technology for 12 weeks. Results of this treatment were compared with the prior 3-month period on AAPS. The primary endpoint was non-inferiority in time in range (%TIR) at 12 weeks. Time below range (%TBR), time above range (%TAR), mean sensor glucose and glycated hemoglobin (HbA1c) were selected as secondary endpoints.
Results: Twenty-two adults with T1D (mean age 36 ± 10 years; HbA1c 44.8 ± 7.6 mmol/mol [6.2 ± 0.68%]) participated in this study. All participants completed the study. After 12 weeks, there was no difference in %TIR (3.9-10.0 mmol/L [70-180 mg/dL]) between CIQ and AAPS (p = 0.269) but %TBR (<3.9 mmol/L [<70 mg/dL]) was lower with CIQ. (Figure 1) %TAR (>10 mmol/L [180 mg/dL]) was not different between CIQ and AAPS (10.3 ± 6.0 vs. 10.4 ± 8.0, p=0.92). Mean sensor glucose was not different between AAPS and CIQ (6.91 ± 0.87 mmol/L [124.38 ± 15.66 mg/dL] vs. 7.18 ± 0.62 mmol/L [129.24 ± 11.16 mg/dL], respectively, p=0.1). No differences in HbA1c were observed between AAPS and CIQ (44.8 ± 7.6 mmol/mol [6.3 ± 0.68%] vs 45.1 ± 6.1 mmol/mol [6.3 ± 0.55%], respectively, p=0.86) at 12-week. No episodes of severe hypoglycemia or diabetic ketoacidosis were reported.
Conclusion: To our knowledge, this is the first prospective study to compare AAPS and CIQ technology in patients with T1D. The results showed that CIQ and AAPS are non-inferior in achieving time in range. However, patients spent less time in the hypoglycemic range with CIQ use. We believe that these findings will provide further guidance for clinicians and patients when discussing treatment options and contribute to the decision-making process.
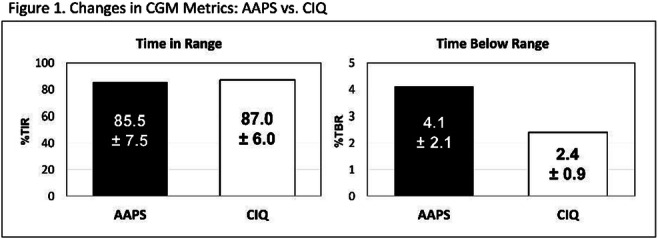
Clinical Trial Registration Number: NCT05165615
Supported by: Ministry of Health, Czech Republic
Disclosure: D.Q. Dat: None.
673
Exploring characteristics and real-world experiences using the software updatable t:slim X2 insulin pump in people with diabetes living with disabilities
H. Singh, G. Alencar, M. Manning, H. Sanchez, S. Habif;
Behavioral Sciences, Tandem Diabetes Care, San Diego, USA.
Background and aims: Diabetes is associated with an increased risk of disabilities including functional, mobility, and work-related impairments. Glycemic and quality of life outcomes may be compromised in people with diabetes living with disabilities as self-care and day-to-day management can become more challenging. Unfortunately, there is very little published data exploring the impact of disabilities on diabetes management and patients’ experiences of different therapy modalities, especially use of advanced diabetes technologies like state-of-the-art, software updatable insulin pumps.
Materials and methods: People with type 1 (T1D) and type 2 diabetes (T2D) using the t:slim X2 insulin pump who were registered with the company’s customer relations management system were sent an email invitation in October 2021 to complete an online survey exploring their experiences using their insulin pump. For purposes of this study, we analyzed demographics, self-reported glycemic data (HbA1c), and patient-reported outcomes (PROs) from US participants reporting one (D1) or more disabilities (D1+) as part of this survey.
Results: In all 28,238 (T1D=25,928; T2D=2310) completed surveys were received. The majority of T1D (85%) and T2D participants (78%) were using the t:slim X2 insulin pump with Control-IQ technology. Of all T1D completers, 26.5% (n=6,882) reported one or more disabilities compared to 39.6% of all PWT2D (n=914). Comparing groups D1 (56.9%) and D1+ (43.1%) from the T1D cohort, D1 participants were younger (mean age=53.9 (18.5) vs. 55.9 (16.7), p<0.001) and reported shorter duration of diabetes (31.8 years (16.7) vs. 33.6 (16.7), p<0.001). The D1+ group was more likely to report HbA1c ≥8% vs. D1 (p<0.001). In contrast there were no significant differences between age and duration of diabetes for T2D participants in the D1 (mean age=63.8 (11.7), duration of diabetes=24.2 (11.1)) and D1+ groups (mean age=62.5 (12.8), duration of diabetes=24.6 (13)). Additionally, no significant difference was observed in number of participants reporting HbA1c ≥8% in both D1 (46.5%) and D1+ (48.6%) groups for the T2D cohort. Vision impairment was the most frequently reported disability in T1D participants (D1=29.5%, D1+=53.1%) while mobility/physical disability was more frequently reported by the T2D group (D1=30%, D1+=65.2%). In terms of PROs, all participants irrespective of type of diabetes and number of disabilities, reported high pump-related satisfaction and trust (scores > 4 out of 5). However, D1 participants from the T1D cohort reported significantly higher pump-related satisfaction (4.44 (0.79) vs. 4.38 (0.84), p=0.002) and trust (4.49 (0.79) vs. 4.44 (0.82), p<0.05) compared to the D1+ group. The T2D cohort did not show significant differences on these variables.
Conclusion: To the best of our knowledge, this is the first study exploring the experience of living with disabilities in people with diabetes using advanced diabetes management technologies. Future, more in-depth, research is encouraged to understand how different types of disabilities impact diabetes management including choice of therapy in people with diabetes.
Disclosure: H. Singh: Employment/Consultancy; Tandem Diabetes Care. Stock/Shareholding; Tandem Diabetes Care.
674
Differences in the efficacy of an advanced hybrid closed-loop system in type 1 diabetes subjects with previous pump therapy experience compared to MDI
P.I. Beato-Vibora, A. Ambrojo-López, M. Fernández-Bueso, E. Gil-Poch, I. Martín-Romo, F.J. Arroyo-Díez;
Badajoz University Hospital, Badajoz, Spain.
Background and aims: The Advanced Hybrid Closed-Loop system (AHCL) MiniMedTM 780G automatically infuses insulin according to interstitial sensor glucose values and it delivers auto-correction boluses. The performance of the system in different subpopulations of people with type 1 diabetes (T1D) needs to be further explored. The aim of the study was to analyse the difference in glycaemic outcomes in previous pump users compared to subjects coming directly from multiple daily injections (MDI), in an adult and paediatric population.
Materials and methods: A prospective longitudinal intervention study was performed. Subjects with T1D consecutively initiated the use of the system. Two-week downloads were collected at baseline and at the last clinical visit. TIR (70-180 mg/dl), time in hypoglycaemia and time in hyperglycaemia were compared, at baseline and end of follow-up for each group (pump group and MDI group) and between groups.
Results: 160 T1D subjects were included (age: 34 ± 15 years, from 7 to 65 years-old, sex: 66% (n = 105) females, children and adolescents: 21% (n = 34), diabetes duration: 21 ± 12 years, baseline HbA1c: 7.27 ± 0.79%, previous treatment: pump therapy 71% (n = 114), MDI 29% (n = 46). Follow-up was 9 [6-12] months.At the end of follow-up, bolus insulin was 57 ± 7%, autocorrection insulin was 31 ± 12% of bolus insulin (27 ± 10 boluses per day). 86% (n = 137) of the participants had a glucose target of 100 mg/dl and 83% (n = 132) had an active insulin time of 2 hours. The percentage of patients with TIR (70-180 mg/dl) > 70% increased from 38% at baseline to 84% at the end of follow-up (p < 0.001). The percentage of patients with TIR (70-180 mg/dl) > 70% and time < 70 mg/dl < 4% increased from 23% at baseline to 55% in the last clinical visit (p < 0.001). The percentage of patients with TIR (70-180 mg/dl) > 70% and time < 54 mg/dl < 1% increased from 20% at baseline to 49% (p = < 0.001). No severe hypoglycaemia or DKA episodes occurred during follow-up.Outcomes at the end of follow-up in subjects coming from pump therapy compared to subjects previously on MDI are summarised in Table 1. In both groups, TIR (70-180 mg/dl) increased, compared to baseline: MDI group (14.11 ± 9.15%, from 63.24 ± 11.91% to 77.35 ± 7.92%, p < 0.001) and pump group (10.30 ± 10.04%, from 67.67 ± 11.91% to 77.97 ± 8.01%, p < 0.001); this increase was higher in MDI patients (p = 0.028). Time in hypoglycaemia was reduced significantly in the MDI group: time < 70 mg/dl: from 3.63 ± 3.86% to 2.22 ± 2.11% (p = 0.009); time < 54 mg/dl: from 0.85 ± 1.46% to 0.43 ± 0.66% (p = 0.031), but not in the pump group: time < 70 mg/dl: from 3.25 ± 3.23% to 3.10 ± 2.97% (p = 0.580); time < 54 mg/dl: from 0.82 ± 0.78% to 0.78 ± 1.17% (p = 0.781), although baseline time in hypoglycaemia < 70 mg/dl and < 54 mg/dl was not significantly different in both groups.
Conclusion: The AHCL MiniMedTM 780G improves glycaemic control independently of the previous experience in pump therapy and the improvement in time in hypoglycaemia could be greater in MDI patients.
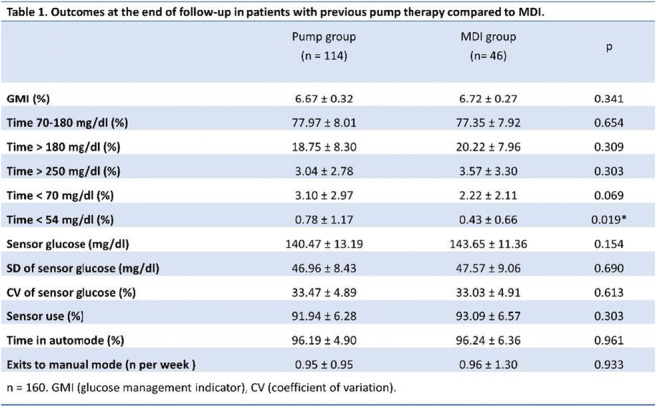
Disclosure: P.I. Beato-Vibora: None.
SO 54 Making sense out of sensors and data
675
Patient-reported outcome measures during the first year at a virtual clinic for type 1 diabetes
O. Tamir1, T. Kolobov1, E. Tarshish2, R. Nissanholtz-Gannot2;
1Pesach Segal Israeli Center for Diabetes Research and Policy, Ramat Gan, 2Ariel University Center, Ariel, Israel.
Background and aims: Lack of access to highly specialized multidisciplinary teams, with the expertise to manage rapidly changing technologies for type 1 diabetes care, remains a major barrier worldwide to achieving disease control. Therapeutic approaches that integrate telemedicine can address this challenge. In studies on telemedicine, type 1 diabetes patients experienced improved satisfaction with care, more efficient follow up, and better HbA1c control compared to standard care. However, patient-reported outcomes (PROMs) were inadequately assessed. In this study, we evaluated PROMs of a newly-established virtual clinic for adult patients with type 1 diabetes at the Sheba Medical Center, Israel, which aims at improving the quality of care provided to the patients. The clinic adopts a patient-centered approach, integrating patient feedback in the design and implementation of its services. It offers a multi-professional healthcare team; a hybrid model of care, with options for virtual and face-to-face appointments; and access to the team beyond regular visits. This study aimed to evaluate the efficacy of the clinic from the standpoint of the patients.
Materials and methods: A total of 65 adult patients, who received care for at least 12 months in the virtual clinic, completed three validated questionnaires at baseline, 6 months, and 12 months: a. Diabetes Treatment Satisfaction Questionnaire (DTSQ), including questions on self-reported hyper and hypo-glycemic events; b. Diabetes Self-Management Questionnaire (DSMQ); and c. Confidence In Diabetes Self-care scale (CIDS). In addition, a questionnaire developed by the research team measured participants’ perspectives on key characteristics of the virtual clinic on a scale of 1-5 (5=very satisfied) at baseline and at 12 months. Data was analysed using descriptive statistics, and participant responses were compared between data collection time points.
Results: Results of the DTSQ show that patient satisfaction with their care increased since joining the virtual service, from an average of 26.68 at baseline to 30.25 at 6 months (MD=-3.57; p=0.002) and to 30.68 at 12 months (MD=-3.88; p=0.001). In addition, participants reported decreased hyperglycemia events from baseline to 6 months (MD=0.55; p=0.005) and 12 months (MD=1.12; p=0.0000) and hypoglycemia events from baseline to 6 months (MD=0.82; p=0.000) and to 12 months (MD=0.78; p=0.0000). There were no significant changes in CIDS and DSMQ scores across times. Patient satisfaction with the virtual service was higher at 12 months in comparison to their previous clinical setting, mainly in relation to: the number of times they were physically required to go to the clinic (MD=-1.42; p=0.003); availability of the physician (MD=-1.17; p=0.005) and the nurse (MD=-1.32; p=0.006) beyond their regular appointments; the diverse ways in which they can communicate with the healthcare team (e.g., telephone, email, text) (MD=-1.27; p=0.002); and costs related to clinic visits (e.g., travel, time off work) (MD=-2.07; p=0.000).
Conclusion: Virtual clinics can help type 1 diabetes patients cope with the challenges of managing their disease. Improved satisfaction with care can facilitate patient involvement and improve outcomes.
Supported by: EFSD Sanofi Research Programme
Disclosure: O. Tamir: None.
676
A pragmatic health informatics systems approach for aiding clinical prioritisation in a hospital based cohort of 4013 people with diabetes
J. Karalliedde1, O. French2, A. Smith2, L. Newcombe2, B. Malhotra1, C. Spellman1, N. Patel1, P. Sen Gupta1, D. Kariyawasam1, D. Rajasingam1, S. Thomas1;
1Guy's and St Thomas NHS Foundation Trust, London, 2Factor 50, Nottingham, UK.
Background and aims: The backlog of diabetes care due to COVID-19 pandemic and subsequent challenges in resource stretched health care systems highlight the need for innovative data driven approaches to facilitate risk stratification and clinical prioritisation. Our aim was to develop a pragmatic health informatics based approach to guide clinicians to prioritise those at highest need.
Materials and methods: We identified modifiable risk factors that could be addressed in 4013 adult people (48% female) with diabetes (type 1 diabetes 20%) attending outpatient clinics in a large university teaching hospital in London. Using data routinely collected from hospital electronic health care records we identified people who had one or more events from six risk (red flag) criteria as agreed by panel of diabetes specialists. These six red flag criteria were new clinical events/results (occurring after their last diabetes clinic attendance): 1. A diabetes related emergency department visit/ hospitalisation 2. HbA1c >96 mmol/mol 3. Concerning HbA1c trend (HbA1c rise >20 mmol/mol) 4. estimated GFR <30 ml/min 5. Concerning eGFR trend (eGFR fall >15 ml/min), or 6. Treatment for advanced diabetic eye disease (e.g. photocoagulation). People with high risk defined as one or more of the six red flag criteria are flagged on a dashboard system. Those who did not have any red flags and who had encouraging HbA1c and eGFR data/trends were categorised as lower risk and ‘green flagged’. We also documented upcoming appointment dates to enable clinicians to decide if given their flagged status whether there was a need for red flagged people to be seen sooner than previously thought and vice versa those with green flags (lower risk) could be seen later than originally intended.
Results: The above criteria were assessed in 4013 people. One third of people were non-Caucasian and this reflects our local population. Of the 4013 people, 656 (16.3%) were identified as having one or more red flags. People with red flags were more likely to be non-Caucasian and had greater deprivation as measured by indices of multiple deprivation. Of the 656 red flagged people, 248 (6.2%) did not have an appointment within 3 months of the review date. These ‘higher risk’ people will be individually assessed and re-prioritised for their next appointment. Similarly a further 364 people (9.1%) had ‘lower risk’ (green flag) and of these 174 (4.3%) were due to be seen within 3 months, and might be considered for rescheduling to enable those at higher risk to be prioritised within currently available clinical resources. The remainder of the cohort (2993, 74.6%) did not have any new risk data for assessment and were therefore not scored as part of this pilot exercise. User feedback from a multidisciplinary group of health care workers including nurses, dieticians, junior doctors and administrative staff was positive.
Conclusion: A pragmatic data-driven method is helpful in identifying people with diabetes at highest need for clinical prioritisation. The related dashboard is a visual aid that can help clinicians assess trends and patterns in risk criteria and guide clinical decision making. Health informatics approaches such as ours are an important tool to enhance care and improve efficiency of clinical service delivery for people with diabetes.
Disclosure: J. Karalliedde: None.
677
Efficacy and safety of an open-source automated insulin delivery system over 48 weeks of use in the CREATE randomised controlled trial
M.J. Burnside1, D.M. Lewis2, H.R. Crocket3, R.A. Meier1, J.A. Williman4, C.A. Jefferies5,6, A.M. Faherty5, R.G. Paul7, C.S. Lever7, S.K.J. Price7, C.M. Frewen8, S.D. Jones8, T.C. Gunn9, B.J. Wheeler8, M.I. de Bock1;
1Paediatric Department, University of Otago Christchurch, Christchurch, New Zealand, 2OpenAPS, Seattle, USA, 3Te Huataki Waiora School of Health, Sport & Human Performance, University of Waikato, Hamilton, New Zealand, 4Department of Population Health, University of Otago, Christchurch, New Zealand, 5Department of Pediatric Endocrinology, Starship Children’s Health, Auckland District Health Board, Auckland, New Zealand, 6Liggins Institute, University of Auckland, Auckland, New Zealand, 7Waikato Regional Diabetes Service, Waikato District Health Board, Hamilton, New Zealand, 8Department of Women’s and Children’s Health, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand, 9Nightscout, Hamilton, New Zealand.
Background and aims: To assess long term efficacy and safety of an open-source automated insulin delivery (AID) system in children, adolescents and adults with type 1 diabetes (T1D) in a 24-week continuation phase following a 24-week multi-site randomised controlled trial.
Materials and methods: Two arms from a 24-week randomised (1:1) controlled trial (RCT) that compared open-source AID (OpenAPS algorithm within a modified version of AndroidAPS in a smartphone, pre-production DANA-i insulin pump and Dexcom G6 continuous glucose monitor), to sensor augmented pump therapy (SAPT), entered into a 24-week continuation phase where the SAPT arm (termed SAPT-AID) crossed over to join the open-source AID arm (termed AID-AID). A hardware switch occurred in the majority of participants in the continuation phase, where the pre-production DANA-i insulin pump was substituted with a pre-production YpsoPump.
Results: In the SAPT-AID arm (n=52), mean percentage of time in range (TIR; 3.9-10mmol/L [70-180mg/dL]) increased from 54.5±16% using SAPT during the RCT to 67.4±10.6% using AID (Δ+12.9%, 95% confidence interval (CI) 9.6 to 16.2; p<0.001), with 44% achieving TIR ≥70% compared to 15% using SAPT (p<0.001). In the AID-AID group (n=42), mean TIR increased from 61.2±12.3% pre-randomisation to 71.2±12.1% during the RCT and remained stable at 69.3±12.5% during the final two weeks of the continuation phase (Δ-1.9% from the RCT, 95% CI -5.6 to 1.8; p=0.310). By the end of the continuation phase mean TIR was almost identical between treatment groups (p=0.92). No episodes of diabetic ketoacidosis or severe hypoglycaemia occurred in either group. Four participants in the SAPT-AID group withdrew; 1 due to infusion site skin irritation, 1 due to a hardware issue, and 2 preferred SAPT.
Conclusion: Further evaluation of the CREATE trial to 48 weeks (24 weeks post RCT) confirms open-source AID using the OpenAPS algorithm within a modified version of AndroidAPS is efficacious and safe with various hardware, and demonstrates sustained glycaemic improvements without additional safety concerns.
Clinical Trial Registration Number: ACTRN12620000034932p
Supported by: Funded by HRC New Zealand, hardware support from SOOIL, Dexcom and Vodafone NZ
Disclosure: M.J. Burnside: None.
678
Perceived benefits of TIR varies between patient CGM users vs HCPs
N. Sainz1, A. Sommi1, E. Asamoa2, E. Shoger2, R. Wood2, C. Alexander1;
1The diaTribe Foundation, 2dQ&A - The Diabetes Research Company, San Francisco, USA.
Background and aims: Previous studies have highlighted the positive patient reported outcomes, both physiological and psychosocial, of using time in range (TIR) among continuous glucose monitoring (CGM) users. The present study aimed to compare the perceived benefits of using TIR among CGM users and healthcare professionals (HCPs).
Materials and methods: 234 HCPs who use TIR to monitor patients’ diabetes (each with 30+ patients with diabetes per month), completed an online survey in September 2021. 253 adults with diabetes who use CGM and TIR and 16 caregivers of children and/or adults with diabetes, completed a separate online survey in October 2021. HCPs and PWD were both asked about their perceived benefits of TIR use. HCPs were classified as either Endocrinologists (Endos, n=88), Diabetes Educators (DEs, n=100), or primary care providers (PCPs, n=46). HCPs received $30-$50 USD and PWD received $10 USD for survey completion. Statistical significance was tested at the 95% confidence level (p<0.05).
Results: When identifying the benefits of TIR, significant differences were found between PWD who use CGM and HCPs. Specifically, 67% of PWD perceived TIR as simple and intuitive for them to understand, whereas 56% of HCPs reported that TIR is simple and intuitive for patients to understand (p=0.0151). 70% of HCPs identified TIR as a metric that informs treatment decisions to manage glucose, compared to 54% of PWD (p=0.0002). Additionally, 68% of HCPs identified TIR as a better indicator of overall glycemic control than A1C, compared to 53% of PWD (p=0.0005). 67% of HCPs reported that TIR provides information needed to individualize care, whereas 46% of PWD reported that TIR provides their healthcare team with the information needed to individualize care (p<0.0001).
Conclusion: This study highlights differences in perceived TIR benefits between HCPs and PWD who use CGM. When considering the benefits of TIR, PWD value the simplicity and clarity of TIR whereas HCPs value the information and management help that TIR provides. An increased and comprehensive understanding of the benefits that TIR can offer may be helpful for patients and providers when assessing the value of TIR.
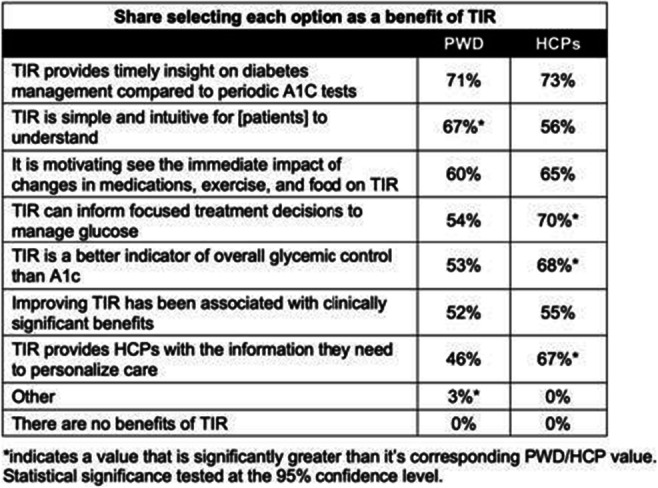
Disclosure: N. Sainz: None.
679
Introduction of isCGM with glucose alarms to regular isCGM users under real-life conditions: What is the benefit?
D.A. Hochfellner, P.M. Baumann, A.K. Reinhard, J.K. Mader;
Division of Endocrinology and Diabetology, Medical University Graz, Graz, Austria.
Background and aims: Intermittently scanned continuous glucose monitoring (isCGM) has proven improvement in glycaemic control, comfort and quality of life in people living with diabetes (PLWD). Alarms for imminent hypo- or hyperglycaemia have been established to emulate continuous glucose monitoring (CGM) technology. Aim of the present analysis was to evaluate the effect of introducing isCGM with alarms to isCGM without alarms in PLWD under real-life conditions.
Materials and methods: A diabetes technology registry at a tertiary centre in Austria was searched for isCGM users (Freestyle Libre, Abbott Laboratories, Chicago, Il, USA) that were introduced to isCGM with alarm functions (Freestyle Libre 2, Abbott Laboratories, Chicago, Il, USA). CSV data of 90 before and 90 days after isCGM switch were analysed to calculate CGM metrics such as Time in Range (TIR), Glucose Monitoring Indicator (GMI), average glucose levels. The local ethics committee approved this investigation (No.: 34-184 ex 21/22).
Results: 18 PLWD were included in the analysis (Age: 41.0, 27.25-48.25 years, female gender: 27.8%, BMI: 24.5, 23.8-26-7 kg/m², C-peptide: 0.02, 0.00-0.05 ng/dL, Creatinine: 0.9, 0.9-1.0 mg/dL). TIR (70-180mg/dL) was 56.6, 46.9-73.5% during isCGM use without alarms and 58.7, 47.1-72.4% with alarms. Time below range (TRB <70mg/dL) was 4.8, 1.9-6.2% vs. 3.6, 2.1-7.2% and time above range (TAR >180mg/dl) was 39.2, 21.9-50.9% vs. 35.7, 22.4-51.7% respectively. Average glucose levels were 167.5, 140.3-186.1mg/dL without alarms and 161.4, 141.0-187.0mg/dL with alarms. GMI was 56.5 49.4-61.3 vs. 54.9, 49.5-61. Insulin doses were not documented thoroughly as data were derived from routine care. Basal insulin doses were obtained in all 18 PLWD before and in 8 PLWD after switch to isCGM with alarms (25.0, 20.0-30.0 Units/24h vs. 24, 19.0-30.3 Units/24h). All data are displayed in median, Q1-Q3.
Conclusion: Introduction of isCGM with alarms to users of isCGM without alerts resulted in a slight increase in TIR and a moderate decrease in TBR. Reductions in TAR were more pronounced, but overall, change in GMI was not clinically relevant. Similar to other CGM trials for the majority of patients glycemic targets were not achieved. Combined use of CGM systems with decision support algorithms might be needed to further improve glycaemic control.
Disclosure: D.A. Hochfellner: None.
680
Continuous glucose monitoring metrics and pregnancy outcomes in insulin treated diabetes: a post-hoc analysis of the GlucoMOMS trial
A. van der Wel1, D. Rademaker1, R. van Eekelen1, GlucoMOMS Study Group, I. Evers2, A. Franx3, S. Siegelaar1, B. van Rijn3, H. DeVries1, R. Painter1;
1Obstetrics & Gynecology, Amsterdam UMC, Amsterdam, 2Obstetrics & Gynecology, MeanderMC, Amersfoort, 3Obstetrics & Gynecology, Erasmus University, Rotterdam, Netherlands.
Background and aims: Worldwide the incidence of diabetes in pregnancy is rapidly increasing. To date, no accurate risk indicator of maternal glycaemia for frequent complications of diabetes in pregnancy, like large for gestational age and neonatal hypoglycaemia, has been identified. Glycaemic metrics derived from continuous glucose monitoring may give more insight in the relationships between maternal glucose patterns and the risk of pregnancy complications. The objective of this analysis was to investigate the association between continuous glucose monitoring metrics and perinatal outcomes in insulin dependent diabetes mellitus in pregnancy.
Materials and methods: We conducted a post-hoc analysis of the GlucoMOMS randomised controlled trial which investigated the value of continuous glucose monitoring in pregnancy with diabetes. Associations between continuous glucose monitoring metrics or HbA1c and perinatal outcomes were analysed per trimester and type of diabetes (type 1 diabetes and a composite group of type 2 or gestational diabetes) using multivariable binary logistic regression analysis. Outcomes of interest were preeclampsia, preterm birth, large for gestational age, neonatal hypoglycaemia, and Neonatal Intensive Care Unit admission.
Results: The current analysis reports the results of 115 participants (n = 42 type 1 diabetes, n = 73 type 2 diabetes or gestational diabetes) who were originally allocated to the continous glucse monitoring study arm of the GlucoMOMS randomised controlled trial. Continuous glucose monitoring metrics and HbA1c values varied between trimesters and type of diabetes. In general, women with type 1 diabetes showed the highest glucose and HbA1c levels, and spent less Time in Range (3.5-7.8 mmol/l) compared to women with type 2 diabetes or gestational diabetes. Mean glucose in type 1 diabetes was 6.3 mmol/L in first trimester and 6.8 in second and third trimester, For the composite group of type 2 diabetes and gestational diabetes mean glucose was 5.8, 6.1 and 6.0 mmol/L in respectively the first, second and third trimester. We found that in pregnancies complicated by type 1 diabetes, greater Time Above Range in second and third trimester was associated with increased large for gestational age (ORs 1.1 (95% CI 1.0-1.1), 1.1 (95% CI 1.0-1.1) respectively). High mean glucose was also related to LGA in second (OR 2.6 (95% CI 1.1-6.2)) and third (OR 2.3 (95% CI 1.0-5.2)) trimester. In contrast, in type 2 diabetes and gestational diabetes, none of the continous glucose monitoring metrics related to large for gestational age (Time Above Range ORs first and second trimester: 1.0 (95% CI 0.9-1.1) and third trimester: 1.1 (95% CI 1.0-1.1)), neonatal hypoglycaemia, preeclampsia, preterm birth and Neonatal Intesive Care admission rates. HbA1c showed no relation with pregnancy outcome in type 1 diabetes nor in type 2 diabetes or gestational diabetes.
Conclusion: Continous glucose monitoring metrics identify pregnancies at risk for large for gestational age in type 1 diabetes. The novelty of this study is that in type 2 diabetes and gestational diabetes, continuous glucose monitoring metrics seem not to identify pregnancies at risk.
Clinical Trial Registration Number: NTR2996
Supported by: ZonMw, project number 80-82310-97-11157
Disclosure: A. van der Wel: None.
681
Comparison between duration and nadir of symptomatic and asymptomatic sensor-detected hypoglycaemia in people with type 1 diabetes
M.M. Gomes1, Z. Mahmoudi1,2, G. Mathine-Edith2, P. Divilly2, N. Zaremba2, U. Søholm2,3, M. Broadley3, F. Pouwer3,4, S.A. Amiel2, P. Choudhary2,5;
1Department of Pharmacometrics, Data Science, Novo Nordisk A/S, Søborg, Denmark, 2Department of Diabetes, King's College London, London, UK, 3Department of Psychology, Syddansk Universitet, Odense, Denmark, 4School of Psychology, Deakin University, Geelong, Australia, 5Diabetes Research Center, University of Leicester, Leicester, UK.
Background and aims: Widespread use of Continuous Glucose Monitoring (CGM) identifies many episodes of sensor detected hypoglycaemia (SDH) that are not reported (either symptomatic or detected by routine glucose monitoring), even in people with normal awareness of hypoglycaemia (NAH) using Gold score. We aim to assess differences in duration and nadir glucose between symptomatic and asymptomatic episodes of SDH in participants with Type 1 diabetes (T1D) in the HypoMETRICS study.
Materials and methods: Participants continued their usual method of glucose monitoring, with additional blinded (CGM) and reported episodes of PRH (episodes with symptoms that resolved with carbohydrate ingestion OR measured <3.9 mmol/l (70mg/dl) on the HypoMETRICS app for 10 weeks. The symptoms included sweating, heart palpitations, shaking, hunger, confusion, headache, difficulties in speaking and difficulties in movement and coordination.
Results: We analyzed data from 81 participants, mean (SD) age 45.2 (14.8) years; diabetes duration of 23 (13.9) years, 61 using Flash/CGM for routine monitoring. Of 6549 SDH episodes below 3.9 mmol/l (70mg/dl), 1707 (26%) were recognised by the participant. SDH that were associated with contemporaneous PRH were mean of 33.8 minutes longer than those without PRH; 89.40 [85.30, 93.50] versus 55.60 [50.80, 60.40] minutes; P< 0.001. These episodes also had a mean 0.18 mmol/l lower nadir glucose values; 3.020 [3.00, 3.04] versus 3.20 [3.18, 3.22], p value <2e-16). Of 1679 SDH episodes below 3.0mmol/l (54mg/dl), 19% were recognised by the participant. SDH associated with PRH were on average 15.70 minutes longer than unreported SDHs (55.40 [49.20, 61.60] versus 39.7 [34.8, 44.4], p value <2e-06). They also had on average 0.06 mmol/L lower nadir than unreported SDHs (2.800 [2.774, 2.810] versus 2.740 [2.705, 2.770], p value <0.001).
Conclusion: Results suggest that episodes of sensor hypoglycaemia that are detected by people with T1D due to symptoms or routine monitoring have longer durations and lower nadirs than undetected episodes. We need to understand the impact of these asymptomatic episodes and consider reviewing the definition of SDH to improve sensitivity of detecting relevant SDH episodes.
Clinical Trial Registration Number: NCT04304963
Supported by: This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU)
Disclosure: M.M. Gomes: Grants; This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 777460.
682
Glycaemic control using intermittently scanned CGM in severe COVID-19 patients with diabetes requiring methylprednisolone therapy: a retrospective observational study
M. Uchihara1, N. Kodani1, R. Bouchi1,2, S. Saito3, Y. Miyazato3, N. Ihana-Sugiyama1,2, M. Ohsugi1,2, A. Tanabe1, K. Ueki1,4, M. Hojo5, H. Kajio1;
1Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine, 2Diabetes and Metabolism Information Center, Research Institute, National Center for Global Health and Medicine, 3Disease Control and Prevention Center, National Center for Global Health and Medicine, 4Department of Molecular Diabetic Medicine, Research Institute, National Center for Global Health and Medicine, 5Department of Respiratory Medicine, National Center for Global Health and Medicine, Shinjuku-ku, Japan.
Background and aims: In patients with severe coronavirus disease 2019 (COVID-19), methylprednisolone therapy is effective in reducing mortality. However, in patients with diabetes, hyperglycemia induced by methylprednisolone is often difficult to control, and there is still no well-established insulin regimen to control hyperglycemia. This study aimed to examine the efficacy of intermittently scanned continuous glucose monitoring (isCGM) in controlling hyperglycemia induced by methylprednisolone therapy in patients with severe COVID-19 with diabetes.
Materials and methods: This retrospective observational study analyzed patients with severe COVID-19 who had diabetes and were hospitalized and treated with methylprednisolone between April 1, 2021, and August 18, 2021, at the National Hospital in Tokyo, Japan. Glycemic control in patients who used isCGM in addition to blood glucose monitoring (BGM) was compared with patients only with BGM. Blood glucose levels were measured before each meal and at bedtime using a glucometer. Total daily insulin requirement was also examined.
Results: During the study period, isCGM was used in five patients, and BGM in 14 patients, who were adjusted for age and severity. The total daily insulin dose from the second to the fourth day after administration was significantly higher in the isCGM group as compared to the BGM group (104.2 ± 61.0 units/day vs 39.5 ± 27.2 units/day, p=0.005), without causing hypoglycemia. Patients with isCGM had a significantly lower average blood glucose level during the 7 days of methylprednisolone therapy than those without isCGM (197.4 ± 29.3 mg/dl vs 239.0 ± 24.9 mg/dl, p = 0.007).
Conclusion: Hyperglycemia induced by methylprednisolone therapy in patients with COVID-19 with diabetes can be effectively and safely managed using isCGM.
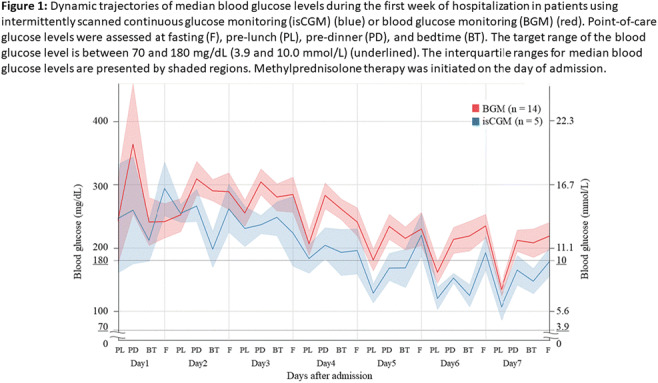
Disclosure: M. Uchihara: None.
SO 55 From low to high and back: the many faces of insulin therapy
683
Comparing the rates and duration of sensor detected hypoglycaemia and patient reported hypoglycaemia based on diabetes type and awareness status: Hypo-METRICS trial
P.M. Divilly1, G. Martine-Edith1, Z. Mahmoudi1, N. Zaremba1, U. Søholm2, F. Pouwer2, S.A. Amiel1, P. Choudhary1;
1Diabetes Research Group, Denmark Hill, Kings College London, London, UK, 2Department of Psychology, University of Southern Denmark, Odense, Denmark.
Background and aims: Continuous glucose monitoring (CGM) is increasingly used to report hypoglycaemia, but many episodes of sensor detected hypoglycaemia (SDH) are asymptomatic. As part of the HypoMETRICS study, we are comparing rates of SDH and patient reported hypoglycaemia (PRH) in real time in people living with Type I (T1D) and Type 2 diabetes (T2D) with impaired (IAH) and normal awareness of hypoglycaemia (NAH).
Materials and methods: Data were collected from 244 participants with T1D (n=158; 72 % using CGM/Flash) or T2D (n=88; 33% using CGM/Flash) who had experienced ≥1 hypoglycaemic episode in the last month. Hypoglycaemic awareness was assessed by Gold score (37 T1D and 20 T2D were IAH). Participants continued their usual method of glucose monitoring, with additional blinded CGM and reported episodes of PRH (episodes with symptoms that resolved with carbohydrate ingestion OR measured ≤3.9 mmol/l (70mg/dl) on the HypoMETRICS app for 10 weeks. Mann-Whitney U tests and Kruskal-Wallis tests were used.
Results: During 400,608 hours of CGM, participants reported 7307 PRH, 15,530 SDH 3.9mmol/l, 4421 SDH 3.0mmol/l, 385 prolonged (>2hours) SDH 3.0mmol/l and 187 SDH 2.2mmol/l, compared to T1D. Median (interquartile range) glucose 8.7mmol/l (3.7), time in range 61.8% (47.25) and time below range (TBR) 3.5% (11.25). T2D reported fewer SDH 3.9mmol/l (7.08 vs 2.54 events/week, p=>0.001), SDH 3.0mmol/l (1.74 vs 0.4 events/week; p=>0.001) and PRH (3.1 vs 1.05 events/week; p=>0.001). There were no differences between T1D IAH and NAH for TBR (5.7% vs 4.5%; p=0.3243), SDH 3.9mmol/l (8.48 vs 6.77 events/week; p=0.229), SDH 3.0mmol/l (2.2 vs 1.72 events/week; p=0.135) or PRH (3.1 vs 3.1 events/week; p=0.56). Similarly, no differences in TBR (1.9% vs 1.6%; p=0.866), SDH 3.9mmol/l (2.84 vs 2.42 events/week; p=0.834), SDH 3.0mmol/l (0.4 vs 0.4 events/week; p=0.723) or PRH (1.1 vs 1.05 events/week; p=0.563) were seen between T2D IAH vs NAH. Duration of SDH at all thresholds were not significantly different between all four groups.
Conclusion: T1D has higher rates of hypoglycaemia than T2D, but we found no difference in the TBR, frequency and duration of hypoglycaemia events between IAH and NAH, within T1D and T2D groups. This suggests CGM data does not differentiate awareness status.
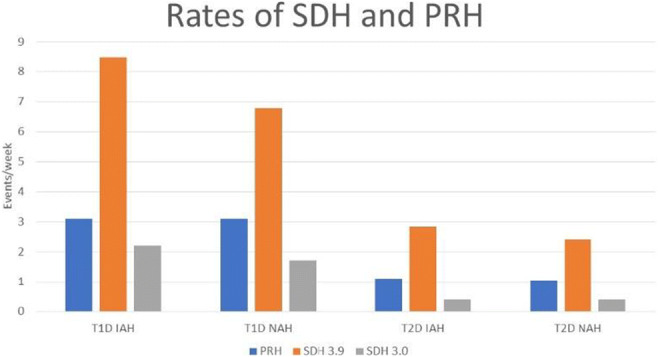
Clinical Trial Registration Number: NCT04304963
Supported by: EU IMI
Disclosure: P.M. Divilly: Grants; IMI.
684
Indirect Treatment Comparison (ITC) of three ready-to-use glucagon treatments for severe hypoglycaemia: nasal glucagon, glucagon injection, and dasiglucagon injection
M. Giménez1, K. Khunti2, K. Syring3, L. Baker3, S. Chenji3, R. Threlkeld3, Y. Yan3, M. Matsuhisa4;
1Hospital Clínic, Barcelona, Spain, 2University of Leicester, Leicester, UK, 3Eli Lilly and Company, Indianapolis, 4Tokushima University, Tokushima, Japan.
Background and aims: To evaluate and compare the efficacy and safety of 3 ready-to-use glucagon treatments for severe hypoglycaemia in adults and children with diabetes: nasal glucagon, glucagon injection, and dasiglucagon injection.
Materials and methods: A systematic literature review was conducted to identify randomised clinical trials assessing the efficacy and safety of ready-to-use glucagon vs reconstituted injectable glucagon. Bayesian fixed-effect network meta-analysis was used to perform the ITC of glucagon injection and dasiglucagon vs nasal glucagon. Endpoints included the proportion of participants achieving treatment success, maximum blood glucose, and treatment-emergent adverse events (TEAEs). Mean time to treatment success and to maximum blood glucose were also analysed for glucagon injection vs nasal glucagon, but not for dasiglucagon due to reporting differences (mean vs median).
Results: Ten clinical trials were included in the ITC, including 4 for nasal glucagon, 3 for glucagon injection, and 3 for dasiglucagon. Nasal glucagon had comparable efficacy with glucagon injection and with dasiglucagon (Table), and all 3 treatments achieved high proportions of treatment success (>98%) in adults and children with diabetes. In adults, results from the combined T1D and T2D analysis were consistent with the T1D analysis, except a statistically significant shorter mean time to achieve treatment success was observed for nasal glucagon vs glucagon injection in the combined analysis. There was a trend towards a lower percentage of participants treated with nasal glucagon experiencing at least one TEAE compared to glucagon injection or dasiglucagon, but no statistical significance was reached.
Conclusion: This study suggests that nasal glucagon, glucagon injection, and dasiglucagon had comparable efficacy in reversing insulin-induced hypoglycaemia, with a shorter mean time to achieve treatment success for nasal glucagon compared to glucagon injection. The findings from this ITC may help discussions between healthcare professionals and patients as they consider available ready-to-use glucagon treatment options.
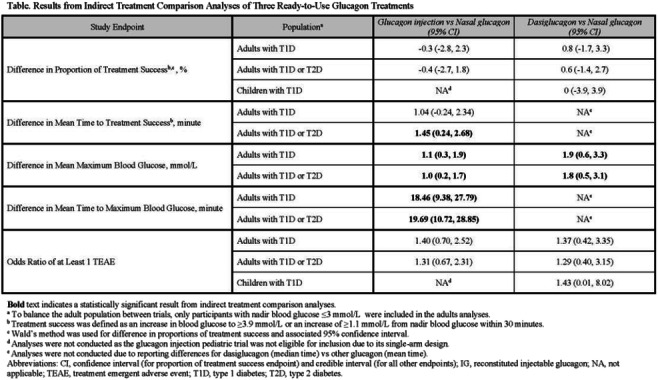
Supported by: Eli Lilly and Company
Disclosure: M. Giménez: Lecture/other fees; Eli Lilly and Company, Medtronic Inc, Novo Nordisk A/S, Sanofi Aventis, AstraZeneca, MSD.
685
Incretin hormone and glucoregulatory responses to nutrients in individuals with and without post-bariatric hypoglycaemia: a systematic review
R.J. Jalleh1, M. Umapathysivam1, J. Louise1, M.P. Plummer1, A. Deane2, K.L. Jones1, M. Horowitz1;
1Adelaide Medical School, The University of Adelaide, Adelaide, 2Intensive Care Unit, Royal Melbourne Hospital, Victoria, Australia.
Background and aims: Bariatric surgery is the most effective treatment to achieve remission of type 2 diabetes. Post-bariatric hypoglycaemia is an increasingly recognised complication for which management remains suboptimal because of a poor understanding of the underlying pathophysiology. The changes in glucoregulatory hormone responses to nutrients in individuals with and without post-bariatric hypoglycaemia have not been systematically examined. We hypothesised that individuals with post-bariatric hypoglycaemia have a greater glucagon-like peptide-1 (GLP-1) and insulin response to nutrients and conducted a systematic review and meta-analysis to evaluate this.
Materials and methods: The study protocol was prospectively registered with PROSPERO. PubMed, EMBASE, Web of Science and the Cochrane databases were searched for publications between January 1990 and November 2021 using MeSH terms related to post-bariatric hypoglycaemia. Studies were included if they evaluated individuals who had bariatric surgery and had measurements of GLP-1, glucose-dependent insulinotropic polypeptide (GIP), insulin, C-peptide or glucagon following a nutrient load and assessed individuals with and without post-bariatric hypoglycaemia. The mean difference in glycated haemoglobin (HbA1c) was also evaluated. A random-effects meta-analysis was performed, and Hedge’s g (standardised mean difference) and 95% confidence interval is reported for all outcomes for which sufficient studies were available. The τ2 estimate and I2 statistic were used as tests for heterogeneity and a funnel plot with the Egger regression-based test was used to evaluate for publication bias.
Results: From 377 identified publications, 13 studies were included in the analysis. In all included studies, the form of bariatric surgery used was Roux-en-Y gastric bypass. Comparing individuals with and without post-bariatric hypoglycaemia, the standardised mean difference in peak GLP-1 following a nutrient load was 0.57 (0.32, 0.82), peak GIP 0.05 (-0.26, 0.36), peak insulin 0.91 (0.52, 1.3), peak C-peptide 0.69 (0.28, 1.1), peak glucagon 0.05 (-0.26, 0.36) and HbA1c -0.46 (-0.78, -0.15). There was no significant heterogeneity in any outcome except for peak insulin: τ2 = 0.23, I2 = 56.6. No publication bias was evident.
Conclusion: Following Roux-en-Y gastric bypass, peak concentrations of GLP-1, insulin and C-peptide were greater after a nutrient load in individuals with post-bariatric hypoglycaemia. The HbA1c was less in those with post-bariatric hypoglycaemia. These observations suggest that antagonism of GLP-1 represents a potential intervention to prevent in hypoglycaemia in patients who suffer from post-bariatric hypoglycaemia after Roux-en-Y gastric bypass.
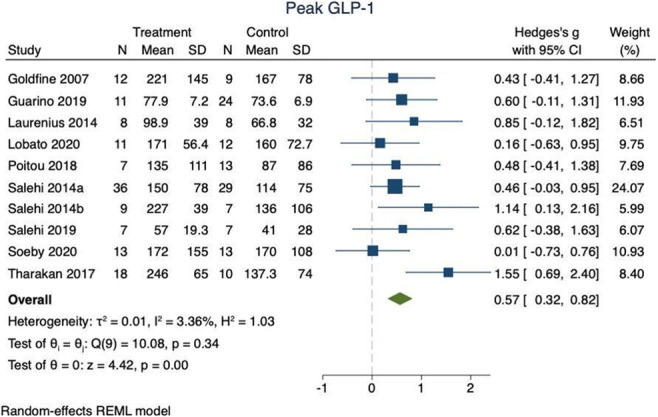
Clinical Trial Registration Number: CRD42021287515
Supported by: NHMRC postgraduate scholarship
Disclosure: R.J. Jalleh: None.
686
Predictive value of fasting C-peptide levels and continuous glucose monitoring-defined coefficient of variation for hypoglycaemia in each stage of chronic kidney disease
S. Kwon1, J. Park2, S. Cho1, S. Park1, Y.-B. Lee1, G. Kim1, K. Hur1, J. Kim1, S.-M. Jin1;
1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, 2Division of Endocrinology and Metabolism, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Republic of Korea.
Background and aims: Although fasting C-peptide levels and continuous glucose monitoring-defined coefficient of variation (CV) are clinically important indicators for the risk of hypoglycemia, their usefulness in advanced stage of CKD has not been determined.
Materials and methods: We analyzed data from 1,186 participants with type 1 and type 2 diabetes who underwent professional continuous glucose monitoring (CGM) between January 2009 and May 2021 at outpatient clinics. Youden indices from receiver operating characteristic (ROC) analyses were used to determine cut-off values of CV and fasting C-peptide levels for the prediction of level 1 and level 2 hypoglycemia, and cut-off values of fasting C-peptide levels for the prediction of CV 36%, in each CKD stage.
Results: For the prediction of level 1 and level 2 hypoglycemia by CV (%), the area under the ROC curve (AUC) was 0.790 (95% CI 0.761-0.818; cut-off value, 30.5) and 0.846 (95% CI 0.818-0.873; cut-off value, 30.5) in stage 1/2 (n = 933), 0.850 (95% CI 0.776-0.925; cut-off value, 31.0) and 0.820 (95% CI 0.721-0.918; cut-off value, 31.0) in stage 3A, 0.845 (95% CI 0.742-0.949; cut-off value, 31.3) and 0.717 (95% CI 0.566-0.868; cut-off value, 31.3) in stage 3B, and 0.782 (95% CI 0.686-0.879; cut-off value, 31.4) and 0.846 (95% CI 0.745-0.947; cut-off value, 37.5) in stage 4/5, respectively. For the prediction of level 1, level 2 hypoglycemia, and CV 36% by C-peptide levels, the AUC was 0.636 (95% CI 0.596-0.677; cut-off value, 0.33 nmol/l), 0.666 (95% CI 0.622-0.711; cut-off value, 0.33 nmol/l), 0.743 (95% CI 0.690-0.778; cut-off value, 0.44 nmol/l) in CKD1/2, respectively. C-peptide levels did not have significant predictive value in other CKD stages.
Conclusion: CV has predictive value for both level 1 and 2 hypoglycemia even in advanced CKD stages, with similar cut-off levels to those of stage 1/2 CKD. Fasting C-peptide levels do not have predictive value for hypoglycemia or CV in those with impaired renal function, indicating that even very high C-peptide levels in advanced CKD stage did not indicate low degree of glycemic variability.
Disclosure: S. Kwon: None.
687
Adjustment of insulin Degludec to Reduce post-Exercise (nocturnal) hypoglycaeMia in people with diabetes: the ADREM study
L.C.A. Drenthen1, E.J. Abbink2, D.H.J. Thijssen3, C.J. Tack1, B.E. de Galan1,4;
1Internal Medicine, section Diabetes, Radboudumc, Nijmegen, 2Internal Medicine, Radboudumc, Nijmegen, 3Physiology, Radboudumc, Nijmegen, 4Internal Medicine, section Diabetes, Maastricht UMC+, Maastricht, Netherlands.
Background and aims: It is common practice for people with type 1 diabetes mellitus (T1DM) to reduce the dose of long-acting insulin at bedtime after exercise to reduce the risk of subsequent nocturnal hypoglycaemia. It is unknown whether this has to be recommended for insulin degludec, a second generation long-acting insulin analogue with a much longer half-life and more stable glucose-lowering profile.
Materials and methods: We performed a randomized controlled cross-over study with 18 adults with T1DM at elevated risk of hypoglycaemia. We investigated three degludec dosing regimens after a 45-minute exercise test on a bicycle ergometer, followed by blinded continuous glucose monitoring for 6 days. The dosing regimens were: 1. No adjustment of the degludec dose (CON); 2. A dose reduction of 40% (D40); and 3. Postponement of degludec for 8 hours at a 20% lower dose (D20-P). The primary outcome was the time (minutes) spent in the hypoglycaemic range (i.e. glucose <3.9 mmol/l) during the night (00:00-05:59h) following the exercise day. Secondary outcomes included time spent in hypoglycaemic and hyperglycaemic range (i.e. glucose >10.0 mmol/l) during the next days.
Results: We recruited 6 women and 12 men (mean±SD age 38±13 years, BMI 24.97±2.72 kg/m2, HbA1c 56±8 mmol/mol and VO2max 40.15±9.55 ml/min/kg). The three exercise tests were performed consistently across all treatment groups regarding heart rate, blood glucose and lactate concentration. No differences between the treatment regimens were found in the time spent in hypoglycaemia the night after the exercise day (table 1). During the subsequent whole day (00:00-23:59h), people spent more time in hypoglycaemia in CON vs. D40 (p=0.043), and more time in hyperglycaemia in D20-P vs. CON (p=0.001) and D40 (p=0.003). During the second night after the exercise day, CON was associated with more time spent in hyperglycaemia vs. D20-P (p=0.022), whereas during the second whole day (00:00-23:59h), D20-P was associated with more time spent in hypoglycaemia vs. D40 (p=0.019).
Conclusion: Adjustment of insulin degludec dosing after exercise has no effect on the time spent in subsequent nocturnal hypoglycaemia in people with T1DM, although a 40% dose reduction may reduce the risk of next-day hypoglycaemia without increasing the risk of hyperglycaemia. These data argue against standard insulin degludec dose adjustment after exercise in people with T1DM.
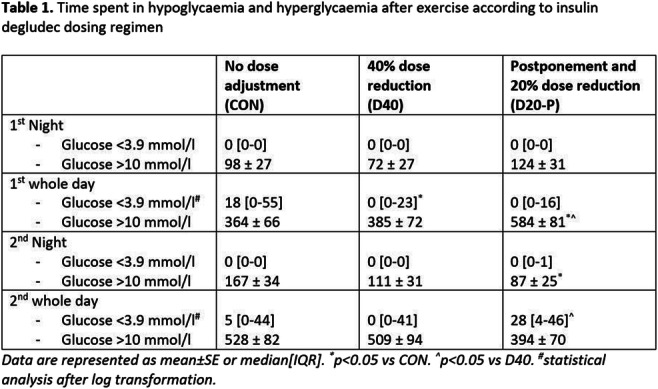
Clinical Trial Registration Number: EudraCT number: 2019-004222-22
Supported by: Novo Nordisk B.V
Disclosure: L.C.A. Drenthen: Other; Novo Nordisk B.V.
688
Glycaemic control with Ultra Rapid Lispro (URLi) vs lispro in children and adolescents with type 1 diabetes: PRONTO-Peds
D.R. Franco1, R. Wadwa2, L.M. Laffel3, M.A. Dellva4, R.K. Pollom4;
1CPCLIN/DASA Clinical Research Center, São Paulo, Brazil, 2Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, USA, 3Joslin Diabetes Center, Harvard Medical School, Boston, USA, 4Eli Lilly and Company, Indianapolis, USA.
Background and aims: This phase 3, treat-to-target study evaluated efficacy and safety of URLi vs lispro in 716 pediatric patients (pts) with type 1 diabetes (T1D).
Materials and methods: After 4-week lead-in to optimise basal insulin, pts were randomised to double-blind URLi (n=280) or lispro (n=298) injected 0-2 min prior to meals, or open-label URLi (n=138) injected up to 20 min after meals (URLi+20). Pts remained on prestudy basal insulin (degludec, detemir, or glargine). Primary endpoint was HbA1c change from baseline after 26 weeks.
Results: Noninferiority was shown in HbA1c change with URLi vs lispro: estimated treatment difference (ETD) -0.02% (95% CI -0.17, 0.13) and with URLi+20 vs lispro: ETD -0.02% (95% CI -0.20, 0.17). Postprandial glucose (PPG) measured by self-monitored blood glucose (SMBG) was lower with URLi vs lispro 1 h after breakfast (p<0.001) and dinner (p=0.006). URLi significantly reduced 1 h postmeal glucose daily mean vs lispro (p=0.001). Total daily insulin dose was similar between treatments. There were no significant differences among treatments in rate or incidence of severe, nocturnal or documented hypoglycaemia (<3.00 mmol/L [<54 mg/dL]). With URLi vs lispro, rate of postdose hypoglycaemia (<3.00 mmol/L [<54 mg/dL]) was higher at ≤2 h (p=0.034). Incidence of treatment-emergent adverse events was similar between groups. More pts reported an injection site reaction related event with URLi (7.9%) and URLi+20 (2.9%) vs lispro (2.7%). All injection site reactions were mild or moderate in severity. Two URLi patients discontinued the study due to injection site reactions.
Conclusion: In children and adolescents with T1D, URLi demonstrated similar overall glycaemic control and greater PPG lowering with an acceptable safety and tolerability profile compared with lispro. URLi dosed at the start of meals or up to 20 min after the start of meals showed noninferiority for HbA1c change from baseline vs lispro. URLi dosed at the beginning of meals showed lower PPG at 1 h after breakfast and dinner and lower 1 h postmeal glucose daily mean vs lispro.
Clinical Trial Registration Number: NCT03740919
Supported by: Eli Lilly and Company
Disclosure: D.R. Franco: Employment/Consultancy; Novo Nordisk, Medtronic, Sanofi, Abbott, and Biomm advisory boards. Lecture/other fees; Novo Nordisk, Lilly, Novartis, AstraZeneca, Medtronic, Biomm, Sanofi, Abbott, and Roche.
689
Titration patterns of insulin glargine 300 U/mL in insulin-naive people with type 2 diabetes and clinical outcomes: a subgroup analysis of ATOS study
A. Tirosh1, A. Singh2, H. Vargas-Uricoechea3, M.A. Mabunay4, G. Bigot5, M. Naqvi6, G. Galstyan7;
1Division of Endocrinology, Diabetes and Metabolism, Chaim Sheba Medical Center, Tel-Aviv University, Tel Hashomer, Israel, 2G.D Hospital and Diabetes Institute, Kolkata, India, 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Universidad del Cauca, Popayan, Colombia, 4Sanofi, Singapore, Singapore, 5IviData LIFE SCIENCES, Levallois-Perret, France, 6Sanofi, Mumbai, India, 7Endocrinology Research Centre of Health Care Ministry of Russian Federation, Dmitriya Ulyanova, Moscow, Russian Federation.
Background and aims: ATOS, a 12-month prospective observational study conducted in Asia, Middle East, North Africa, Latin America, and Eastern Europe, showed that initiation of insulin glargine 300 U/mL (Gla-300) in insulin-naïve people with type 2 diabetes (T2D) resulted in improved glycaemic control with low rates of hypoglycaemia and minimal weight change. This post hoc analysis aimed to explore the baseline factors and outcomes associated with different magnitudes of Gla-300 titration in people initiating basal insulin.
Materials and methods: Participants were categorized based on the magnitude of Gla-300 dose change from baseline to Month 3: no/minimal titration (0-2 U; Group A) and the groups that uptitrated 2-6 U (Group B); 6-10 U (Group C) and >10 U (Group D).
Results: This analysis included 3651 participants; Group A, 1210 (33.1%); Group B, 1076 (29.5%); Group C, 585 (16.0%) and Group D, 780 (21.4%). Age and BMI increased from groups A to D. Baseline HbA1c was 9.31 %, 9.23%, 9.26% and 9.27%, respectively. Group A had lowest usage of sulfonylureas and highest use of dipeptidyl-peptidase 4 inhibitors at baseline compared to the other groups. Baseline dose was highest in Group A. At Month 6, the individualised HbA1c target achievement was 24.0% in Group A and showed an increasing trend from Group B (21.5%) to Group C (23.4%) and to Group D (34.4%). At Month 12, the proportions of participants reaching target were 36.6%, 44.3%, 49.7% and 54.6% in Groups A, B, C and D respectively (Table). Clinically meaningful improvements were seen in HbA1c, fasting plasma glucose and self-monitored blood glucose at 6 and 12 months across the groups. Insulin dose increased over time in the groups at month 6 and 12 with minimal changes in Group A. The hypoglycaemia incidence was low across all groups during the study period (1.3%, 1.6%, 2.1% and 2.7% in Groups A, B, C and D respectively).
Conclusion: This post hoc analysis showed that in a real-world clinical setting, a large proportion of participants have no to minimal titration. Among those who titrate, insulin dose titration occurred gradually and continued beyond 12 weeks. Baseline factors appear to impact the titration patterns. Over the course of time more participants reached HbA1c target with increasing magnitude of titration with low incidence of hypoglycaemia across the groups and no increase in clinically meaningful hypoglycaemia. Basal insulin treatment should be individualised, and titration could be further optimised.
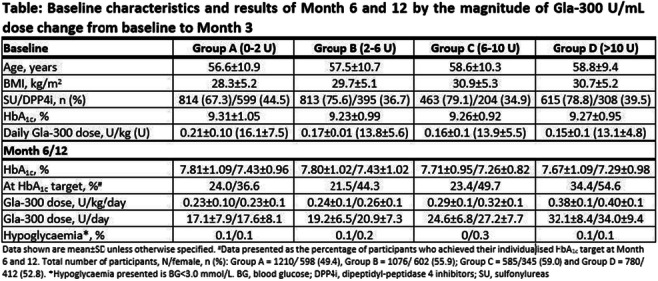
Clinical Trial Registration Number: NCT03703869
Supported by: Sanofi
Disclosure: A. Tirosh: Grants; Medtronic. Honorarium; Member of the ATOS Steering Committee and have received honoraria in relation to ATOS study, Sanofi, NovoNordisk, MSD, AstraZeneca, Boehringer Ingelheim. Lecture/other fees; Sanofi, NovoNordisk, MSD, AstraZeneca, Medtronic.
690
Early deescalation with IDegLira in patients with type 2 diabetes using short-term intensive insulin therapy to correct severe hyperglycaemia
Z. Taybani1, B. Bótyik1, A. Veres1, R. Szerencsi1, K. Fehértemplomi1, M. Zatykó1, G. Csatári1, B. Géczi1, M. Katkó2, T. Varkonyi3;
1Békés County Central Hospital, Dr. Réthy Pál Member Hospital, Bekescsaba, 2Department of Internal Medicine – Division of Endocrinology, University of Debrecen, Debrecen, 3Department of Internal Medicine, University of Szeged, Szeged, Hungary.
Background and aims: In selected patients with type 2 diabetes (T2DM) complex insulin regimens initiated for correcting hyperglycemia and applied at least for 3 months can be safely deescalated with IDegLira. As basal-bolus therapy (BBT) can reverse glucotoxicity in a few days theoretically deescalation can be performed much earlier, even within the first week after the initiation of intensive insulin intervention. We examined prospectively the efficacy and safety of early deescalation with IDegLira in patients with T2DM treated with short-term intensive insulin therapy to correct severe hyperglycemia.
Materials and methods: Human BBT initiated for severe hyperglycemia (HbA1c>11% or HbA1c>9% with clinical symptoms of hyperglycemia and/or fasting glucose>13.9mmol/L or random glucose>16.7mmol/l) was replaced with IDegLira after reaching a blood glucose range below 10 mmol/l usually on the first week of therapy if the previously insulin-naive patient had a c-peptide value >1.1ng/ml, the daily dose of BBT was <60 IU and insulin requirement was <0.6 IU/kg. Patients are followed during the routine ambulatory diabetes care every 4 months.
Results: Between february 2020 and december 2021 early deescalation of short-term intensive insulin therapy with IDegLira was performed in 73 adult patients with T2DM. The first 4-month outpatient control visit has so far been performed in 55 patients (62% newly diagnosed T2DM, mean age 60.4 ±13 years, 49 % women, mean BMI 30.8 ± 6.1 kg/m2). During the follow-up HbA1c decreased from 12.3±1.8% at baseline to 6.3±0.9% at 4-month (p<0.0001) and body weight changed from 87.6 ± 17.9 kg to 86.2 ± 16.9 kg (p=0.299). The mean daily dose of BBT was 42.4 ± 10.3 IU before deescalation, and the dose of IDegLira was 20.6 ± 5.7 units at the 4-month visit. Adverse events (AE) were rare, and severe AEs did not occur. After switching to IDegLira 10 (18%) patients reported at least one episode and 6 (10%) patients had more than one episode of mild hypoglycemia. At the 4-month visit the proportion of individuals reaching an HbA1c<7% without hypoglycemia was 73%.
Conclusion: Early deescalation with IDegLira in selected patients with T2DM using short-term intensive insulin therapy to correct severe hyperglycemia is safe and results in significant glycemic improvement in the short-term.
Disclosure: Z. Taybani: None.
SO 56 Diversity of life with diabetes
691
Impact of the first COVID-19 wave in the Netherlands on glycaemic control in persons with type 2 diabetes
J.M. van den Berg1,2, E.I.H. Holthuis1, B.N. Baak1, J.A. Overbeek1,2, K.M.A. Swart1,2, M.T. Blom2, P.J.M. Elders2, R.M.C. Herings1,3;
1PHARMO Institute for Drug Outcomes Research, Utrecht, 2Department of General Practice and Elderly Care Medicine, Amsterdam Public Health Research Institute, Amsterdam, 3Department of Epidemiology and Data Science, Amsterdam Public Health Research Institute, Amsterdam, Netherlands.
Background and aims: Coronavirus disease 2019 (COVID-19) had a major impact on healthcare use worldwide. Crucial appointments, care and surgical procedures were postponed or canceled. It has been estimated that 34,000 to 50,000 healthy years of life were lost during the first COVID-19 wave in the Netherlands (March 1 to June 30, 2020). General practitioners (GP) saw much less patients concerning chronic care of type 2 diabetes (T2D). To closely monitor glycaemic control, via levels of haemoglobin A1c (HbA1c), and to assess the risk of long-term T2D complications, patients with T2D usually have quarterly check-ups. The aim of this study was to examine whether the first COVID-19 wave in the Netherlands impacted glycaemic control in people with T2D by analysing HbA1c levels and examinations. We hypothesised that HbA1c levels in the first wave were higher compared to HbA1c levels in the same period in 2019.
Materials and methods: A retrospective study was conducted using data from the DIAbetes, MANagement and Treatment (DIAMANT) cohort, derived from electronic medical records from Dutch general practitioners. The first COVID-19 wave was defined as March 1 to June 30, 2020. Descriptive analyses were used to examine patient characteristics and clinical parameters of T2D patients. Categorical variables were described as counts and percentage (%), and continuous variables as means with standard deviation (SD). Paired sample t-test was used to compare HbA1c levels in the first wave to HbA1c levels from March 1 to June 30, 2019. Furthermore, we compared trends in 2019 and 2020 for HbA1c measurements and levels.
Results: Preliminary results show 194,713 patients with T2D (46% women, mean age 68 (± 13) years on March 1, 2020). A decrease in frequency of HbA1c measurements and an increase in HbA1c levels were observed in the first wave (Figure 1). Mean HbA1c in the first wave was 56.1 (± 13.0) mmol/mol, compared to 55.2 (± 12.3) mmol/mol in the same period in 2019. A total of 101,537 (51%) patients had a recorded HbA1c value in the period March 1 to June 30, 2019, of which, 51,433 (51%) also had a recorded HbA1c value in the first wave. In this group, the mean HbA1c differed significantly when comparing levels in 2020 to 2019 (0.39; 95% CI, 0.29-0.48).
Conclusion: The first COVID-19 wave in the Netherlands caused an increase in HbA1c levels in patients with T2D, and a decrease in HbA1c measurements. Further research is needed to examine which patients retained HbA1c measurements and whether the observed increase in HbA1c levels is associated with long-term T2D complications.
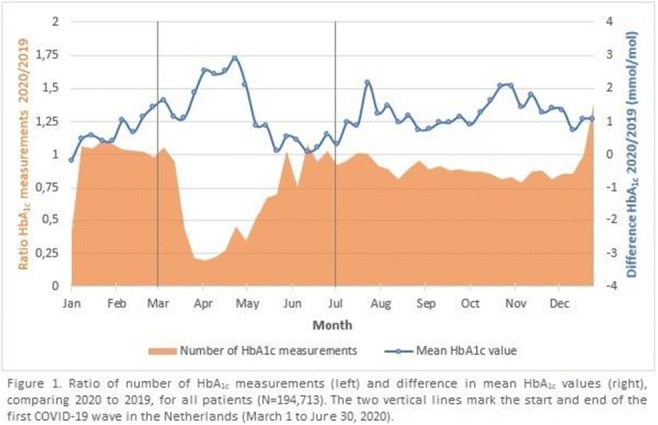
Disclosure: J.M. van den Berg: None.
692
Depression in type 1 diabetes and type 2 diabetes: linking patient experience with clinical records
U. Waheed1, A.H. Heald2, A. Paisley1, K. Grady3, E. Solomon4, M.P. Stedman5;
1Endocrinology & Diabetes, Salford Royal NHS Foundation Trust, Salford, 2Endocrinology & Diabetes, Salford Royal NHS Foundation Trust, Manchester, 3Research for the Future Research and Innovation, Salford Royal NHS Foundation Trust, Salford, 4Department of Clinical Health Psychology, Salford Royal NHS Foundation Trust, Salford, 5Health Research, Res Consortium, Andover, UK.
Background and aims: Diabetes impacts on mental health. The presence of symptoms of depression in people with diabetes is associated with reduced self-care compared to people with diabetes alone. Depressive symptoms are also associated with adverse health outcomes, such as less good blood glucose control. We investigated patient characteristics of those most at risk and consequences for glycaemic control.
Materials and methods: Depression (PHQ-9), Diabetes Distress Screening Scale (DDSS) and Quality of Life (EQ5D) on-line questionnaires were completed by people with diabetes and linked to data extracted data from responders’ clinical records. Sources of distress were also considered through evaluating sub-scales Emotional, Physician, Regimen and Interpersonal DDSS scores.
Results: 130 responses with 34% T1DM, 56% women, median age 59 (IQR47-67) years were recorded. The median scores were EQ5D=0.74 (IQR 0.64 - 0.85) UK median 0.83; DDSS=1.9 (IQR1.3-2.7) (≥2 indicates moderate distress) and PHQ9=5 (IQR2-11) (≥5 indicates depression).Higher DDSS/lower EQ5D5L/higher PHQ-9 within the response group were linked to female sex (DDSS 0.5/25% above median), younger age (<50years DDSS 0.7/35% above median), fewer years after diagnosis (<10years DDSS 0.8/40% above median), and obesity (BMI>35 DDSS 0.6/30% above median).33 people with good (HbA1c≤48mmol/mol) or improving glycaemic control (HbA1c reduced by 2mmol/mol since previous reading) had higher DDSS = 2.4 versus the others = 1.9. The link between individual DDSS and PHQ-9 scores was significant r2=0.4, while the association to EQ5D was less r2=0.11. For 30 people with history of anti-depressant medication use, DDSS was 0.9/45% above median, showing that medical diagnosis of depression confirmed patient self-reported distress.DDSS scores did not significantly link to diabetes type or insulin use.
Conclusion: Physician related DDSS higher scores came from women, younger age, overweight, early years after diagnosis, plus history of antidepressant use. This is potentially remediable.Level of distress is associated/maybe required in achieving and maintaining lower HbA1c. The impact on the mental health of younger women/people with shorter diabetes duration should be noted when considering psychosocial intervention/behaviour change messaging to support diabetes management.
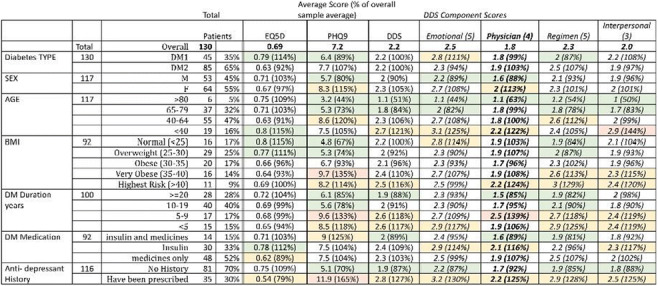
Disclosure: U. Waheed: None.
693
Effects on metabolic control and body composition of insulin therapy optimisation in patients with Cystic Fibrosis Related Diabetes (CFRD)
V. Grancini, L. Porcaro, A. Gaglio, V. Resi, L. Giarratana, E. Orsi;
Fondazione IRCCS Ca' Granda - Policlinico MI, Milan, Italy.
Background and aims: Cystic fibrosis related diabetes mellitus (CFRD) occurs in approximately 19% of adolescents and 40-50% of adults with CF. It’s the result of a partial to complete insulin deficiency, leading not only to hyperglycaemia, but also to loss of muscle mass and induction of a catabolic state. Therefore, insulin is the only recommended therapy for CFRD.Continuous subcutaneous insulin infusion (CSII) has been associated with improved glycaemic control in CFRD in small studies, if compared to multiple daily injection (MDI) therapy, mostly due to better coverage of dietary requirements of these patients (high calories, not carbohydrate restrictions, several snacks during the day). The aim of this study is to evaluate the effects of insulin therapy optimization combined to a structured educational program (training to Sensor Augmented Pump -SAP- therapy and carbohydrates counting) on metabolic control and body composition in patients with CFRD requiring insulin therapy
Materials and methods: 22 patients agreed to participate in the program and, at the end of it, switched to SAP therapy. 29 patients continued the outpatient checks without joining the program. Patients belonging to both groups were re-evaluated 2 years after the baseline evaluation.
Results: Patients in the educational program group demonstrated a substantial improvement in glycometabolic control (HbA1c 7.4±1.4 to 6.5±0.6%; average blood glucose 8.5±1.6 to 7.4±1.1 mmol/L; Glucose management indicator - GMI 7.2±0.9 to 6.5±0.5%; time in range - TIR 64.2±18.0 to 80.38±10.1%; time above range - TAR 31.0±18.8 to 16.2±9.1%; time below range - TBR 4.76±3.5 to 3.0±2.0%, P<0.05) and in body composition, assessed with bioimpedance analysis (fat free mass - FFM 78.9±8.6 to 85.1±7.0%, total body water - TBW 57.9±6.3 to 63.2±6.7 L, P<0.05). The same parameters didn’t differ from basal to final evaluation in patients in the control group.
Conclusion: In conclusion, a structured educational program resulting in therapy optimization leads to a significative improvement in glycometabolic control and, consequently, in body composition, due to the known anabolic effects of insulin therapy.
Disclosure: V. Grancini: None.
694
Retrospective analysis of daily basal bolus insulin requirement in people with type 2 diabetes in non-ICU care
S. Krishnamoorthy1, L. Balakrishnan2, A. Ravi3;
1Department of Internal Medicine, Karunya Sugalaya Diabetes Care & Research Centre Pvt Ltd, 2Department of Diabetology, Karunya Sugalaya Diabetes Care & Research Centre Pvt Ltd, 3Department of Clinical Research, Karunya Sugalaya Diabetes Care & Research Centre Pvt Ltd, Kumbakonam, India.
Background and aims: This study aims to determine the total daily dose of insulin requirement, the proportion of daily basal-bolus insulin requirement in India where people have more carbohydrates in their diet. Incidence of hypoglycemia in non-ICU care and the prescription pattern of insulin ± oral antidiabetic medications needed at the time of discharge in a secondary care centre in south India were also determined.
Materials and methods: A retrospective observational study was conducted at a diabetes care hospital for a period of 6 months. The study included those who have Type 2 Diabetes for at least 6 months, aged 18 years and above, who have been on oral antidiabetic medications and/or insulins, and admitted for various reasons (nonsurgical, non-ICU) who stayed for at least 2 days for glycemic control and managed with analogue basal-bolus insulin therapy. Type 1 diabetes, Gestational diabetes and those who administered steroids for other coexisting illnesses, as well as those who were treated in the hospital exclusively with Premix insulin or oral glucose-lowering agents, were excluded.
Results: A total of 122 patients were enrolled. Males were 31% and females were 69%. The mean ± standard deviation (SD) of age, the duration of diabetes, Body Mass Index were 58.1± 9.8, 13.4 ± 6.8 years, 25.5 ± 4.4 respectively. The mean insulin dose required for optimal control is 1.2 ± 0.6 units per kg of body weight/day. The total daily dose of insulin was 49.6 ± 21.1 units. The average length of hospital stay was 3 days. Of the total cases treated, 11.4% were treated with Oral antidiabetic agents, 2.4% were administered with 0.6mg of liraglutide at bedtime. 69.7% of patients required a higher bolus (>50% of Total daily dose) than basal insulin (30.3%). 7.4% had level 1 hypoglycemia and 2.4% had level 2 hypoglycemia.There is a significant difference (p-value <0.05) in the capillary blood glucose values on discharge (CBG: Baseline-319.9 ± 110.8, Endpoint 168.4 ± 60.29). On discharge, 14.7% were prescribed only oral antidiabetic agents, 50% were prescribed only insulin (basal-bolus:38.5%, Premix:11.5%), 35.3% were prescribed with both insulin and Oral antidiabetic agents (basal-bolus:14%, Premix:21.3%)
Conclusion: Basal insulin requirement was strikingly similar in most, regardless of duration of diabetes with a mean of 22.2 ± 10.1 units/day. Most of the people required more bolus insulin to address prandial glucose excursion. It is observed from this study that, at the time of discharge, people who were preferring fewer pricks were prescribed premix insulin along with oral antidiabetic agents. Incidence of hypoglycemia is low when analogue basal bolus insulin regimen was used in hospital glycemic management
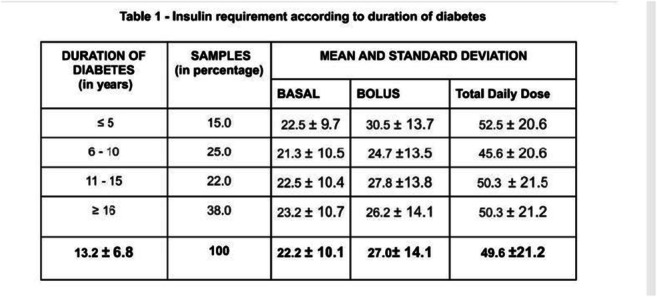
Disclosure: S. Krishnamoorthy: None.
695
Burden of hypoglycaemia and impaired awareness of hypoglycaemia in people with diabetes on peritoneal dialysis
H. Eid1, M. Onyema2, S. Haboosh1, J. Dick2, D. Moutzouris1, P. Vas2,3, N. Paray3, J. Williams3, J. karalliedde1,2;
1Guy’s and St Thomas hospital, London, 2Kings’ College Hospital, London, 3Royal Devon and Exeter NHS Trust, Exeter, UK.
Background and aims: People with diabetes and end stage kidney disease (ESKD) are at high risk of hypoglycaemia. The number of people with diabetes on peritoneal dialysis (PD) is increasing worldwide. The management of glycaemic control in people with diabetes on peritoneal dialysis can be challenging due to use of glucose containing PD solutions, frequent need for insulin treatment, predisposition to glycaemic variability and risks of hypoglycaemia. There is to our knowledge no previously reported data on the burden of hypoglycaemia in people with diabetes on peritoneal dialysis. The aim of this study was to report the prevalence of hypoglycaemia and impaired awareness of hypoglycaemia in this high risk group of people with diabetes on peritoneal dialysis.
Materials and methods: We examined the individual case notes of all people with diabetes on peritoneal dialysis at 3 large university hospital foundation trusts between December 2021-January 2022. Individual people were contacted to obtain detailed history of hypoglycaemic events and assess degree of awareness using Clark and Gold scores.
Results: In total 64 people with diabetes [n=14 (22%) with Type 1 Diabetes] on peritoneal dialysis with median age 63 years (range 29 to 88) were evaluated. Median (interquartile range) for duration of diabetes was 20 years (10 to 25). Similarly, median duration of peritoneal dialysis was 22 months (10 to 36). All participants except one were on glucose containing dialysis regimes. Fourty four people (~70%) were on insulin. Of note 6 people were on sulphonylureas which are contraindicated in ESKD. Of the cohort 58% of people had HbA1c <58mmol/mol, of whom 66% were on a treatment that could be associated with hypoglycaemia (sulphonylurea or insulin). Using Clark score criteria ~13% of cohort (8 people including 4 with type 1 diabetes) had hypoglycaemic unawareness and all of them were on insulin treatment. Similarly, 10 (15%) people had Gold score of 4 or greater indicative of impaired awareness of hypoglycaemia. Self-reported hypoglycaemic events (at least 1 event per month) were reported by 21% of the cohort. In this high risk group of people with type 1 diabetes and ESKD use of continuous subcutaneous insulin infusion (CSII, Insulin pump) therapy was low with only 3 people using this treatment. Further only 7 people were on glucose monitoring systems including intermittent scanned glucose monitoring systems.
Conclusion: We observed that ~15% of people with diabetes on peritoneal dialysis has impaired awareness of hypoglycaemia. In our cohort 58% had an HbA1c <58mmol/mol and of this group two thirds were on treatments that could cause hypoglycaemia. In this group 1 in 5 people reported hypoglycaemic events and would benefit from greater diabetes specialist input to provide related hypoglycaemia education, enhance self-management skills, provide access to newer technologies and advice on insulin regimes and doses. We also noted that in people with diabetes treated with insulin and on peritoneal dialysis the use of technology to help mitigate the burden of hypoglycaemia was low. Our data highlights the need for greater interdisciplinary working and regular input of the diabetes team to help better manage diabetes care in this high risk group of people with diabetes on peritoneal dialysis.
Disclosure: H. Eid: None.
696
The impact of fasting Ramadan on glycaemic indices in people with type 1 diabetes using flash glucose monitoring: an observational study
E.Y. Alyusuf, A. Alrajeh, M.E. Al-Sofiani, A.M. Alguwaihes;
King Saud University, Riyadh, Saudi Arabia.
Background and aims: Fasting in Ramadan remains a challenge for patients with type 1 diabetes (T1D). Our aim was to evaluate the changes in continuous glucose monitoring (CGM) metrics during fasting Ramadan and to identify the potentially contributing factors of changes in glycemic control.
Materials and methods: Flash CGM metrics from 89 individuals with T1D who attempted fasting in Ramadan 2021 were retrospectively compared at three 4-week periods: pre-, during, and post-Ramadan. The association of potential factors with changes of CGM metrics were analyzed.
Results: The sample comprised intermittently-scanned CGM users with ≥ 70% active sensor time (59.6% female, age 28.1±10.3 years, diabetes duration 11.6±8.5 years, 32.6% insulin pump users, and 68.5% received Diabetes-Ramadan-oriented education. During Ramadan, basal insulin was decreased by 2.17 units and nutritional insulin was decreased by 10.73 units. Comparing pre- and during Ramadan, there was a significant increase in average sensor glucose by 9.02 mg/dl (95%CI= 4.25 to 13.79; p <0.001) during Ramadan with a daily average glucose curve typically followed a pattern that was characterized by rapid glucose excursion after sunset meal (Iftar) which was sustained overnight and a second rise after pre-dawn meal (Suhoor) followed by a gradual decline during the fasting hours. Comparing pre- and during Ramadan, glucose management indicator (GMI), time above range (TAR)181-250 mg/dl, and TAR>250 mg/dl have increased significantly by 0.22% (95%CI= 0.1 to 0.34), 2.25% (95%CI= 0.86 to 3.64), and 2.76% (95%CI= 0.81 to 4.71); respectively, all p< 0.05; whereas time in range (TIR) dropped significantly by 3.98% (95%CI= -6.18 to -1.77); p=0.001 without significant change in HbA1c, time below range (TBR) <70 mg/dl, glucose variability, or average scans/day. In contrast, no statistically significant differences were detected in all CGM metrics or HbA1c comparing pre- vs. post-Ramadan. Longer diabetes duration and higher average sensor glucose before Ramadan were associated with increased average sensor glucose during Ramadan β=0.209 (95%CI=0.04 to 1.06); p=0.034 and β=-0.676 (95%CI=-0.71 to -0.17); p=0.002; respectively. Comparing to infrequent scanners (≤ 10 scans/day), frequent scanners during Ramadan (>10 sensor scans/day) had significantly higher TIR (49.3±16.5 vs. 57.9±16.8%, p=0.015), lower TAR >250mg/dl (18.2±13.9 vs. 11.9±14.2%, p=0.036), and lower glucose variability (38.4±8.2 vs. 34.8±8.1%, p=0.035), with comparable TBR <70mg/dl, TAR 181-250 mg/dl, average sensor glucose, and GMI.
Conclusion: Ramadan fasting is associated with worsening glycemic control in people with T1D with no change in time spent in hypoglycemia. While Hypoglycemia is a common concern during fasting Ramadan, hyperglycemia should not be overlooked particularly post-Iftar and Suhoor. Improving pre-Ramadan glycemic control and glucose monitoring with frequent sensor scanning during Ramadan may maintain better glycemic control.
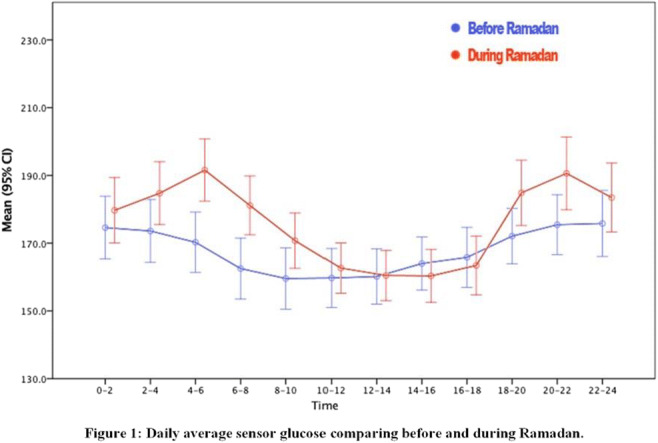
Disclosure: E.Y. Alyusuf: None.
697
Empathy and linguistics in digital health interactions to prevent gestational diabetes in women at risk: a pilot study
E. Rey Velasco1,2, H. Sæderup Pedersen1, T.C. Skinner1;
1Department of Psychology, University of Copenhagen, 2Liva Healthcare, Copenhagen, Denmark.
Background and aims: Lifestyle-related diseases, such as type 2 and gestational diabetes, are among the leading causes of death and disability. Their rapid increase worldwide has called for low-cost, scalable solutions to promote health behavior changes. Digital health coaching has proved to be effective to this aim, with the rising use of text-based interventions. State-of-the-art research has considered wording but lacks meaning and grammar analysis. Furthermore, empathy should be considered as a basis for a good coach-user relationship and positive patient outcomes. We aim to analyze patient cues in a digital health intervention and contribute to future research on coach communication.
Materials and methods: We tested a combination of an empathic and a linguistic approach to code 148 text messages sent by 20 women in the intervention arm of a randomized trial on tele-coaching for gestational diabetes prevention and postnatal weight loss support. The tele-coaching programme was based on the behavior change techniques taxonomy and the motivational interviewing and self-determination theory frameworks.
Results: We identified 143 empathic opportunities present in 42.37% of the corpus. These were mainly negative (82.2%) and implicit (60%). Most of the wording was found in the content (86.2%) with a declarative structure (86.3%). Our transitivity analysis showed that half of the identified processes, or verbal groups, (22.4%) were material (10.7%) and related to food and diet (26.9%), physical activity (26.9%), and lifestyle goals (11.2%).
Conclusion: The combination of empathy and linguistic analysis showed promising results. The identified verbal processes in these patient text messages were in line with the expressed empathic categories. Our findings reveal preliminary insights on the coach-user relationship within a tele-health setting for rpevention of gestational diabetes. The absence of explicit empathic opportunities and direct questions could be attributed to a lack of trust or information on the coach's abilities throughout the intervention. We will be conducting further research to explore additional linguistic features and code coach messages.
Clinical Trial Registration Number: ACTRN12620001240932
Supported by: Industrial PhD Programme
Disclosure: E. Rey Velasco: Employment/Consultancy; The author is employed at Liva Healthcare. Grants; The author received an Industrial PhD funding from Innovationsfonden Danmark to conduct the research.
698
Correlation between the regression of microalbuminuria, blood pressure reduction and remission of type 2 diabetes: results of six month RCCT with whole-body digital twin technology
S. Joshi1, J. Mohammed2, M. Mohammed3, T. Poon2, M. Dharmalingam4, B. Saboo5, S. Damodharan6, A. Vadavi7, M. Thajudeen3, A. Keshavamurthy3, S. Mohammed2, P. Shamanna8;
1Joshi Clinic and Lilavati Hospital, Mumbai, India, 2Twin Health Inc, Mountain View, USA, 3Twin Health, Bangalore, India, 4MS Ramaiah Medical College, Bangalore, India, 5Dia-Care Diabetes Centre, Ahmedabad, India, 6Ramakrishna Hospital and Harvey Speciality clinic, Coimbatore, India, 7Sudha Prevention Center, Bangalore, India, 8Bangalore Diabetes Centre, Bangalore, India.
Background and aims: The prospective study was designed to determine the effect of Twin Precision Treatment Technology (TPT) vs standard care on change in A1C and T2DM remission and regression of microalbuminuria and reduction of blood pressure. The TPT intervention uses the Whole-Body Digital Twin Platform, with AI and Internet of Things, to integrate multi-dimensional data to give precision nutrition and health recommendations via the TPT app and by coaches.
Materials and methods: We analysed the data at six months (intervention n= 206, control n= 71). Intervention arm patients were on TPT: a novel whole-body digital twin enabled precision nutrition that measures weight, physical activity, sleep, and sensor glucose values, for achieving remission. Microalbuminuria (MIC) was defined as urinary albumin excretion of 30-300 mg/day
Results: The mean duration of diabetes was 3.6 years (±2.6, 95% CI 3.3 to 4.04, minimum 0.1, maximum 8). The mean age was 43.3 years (±8.8, 95% CI 42.1 to 44.4, minimum 17, maximum 70). There were 62 patients with microalbuminuria at baseline. Based on the ADA criteria, overall 83.4% (n=172/206) achieved diabetes remission as compared to 71.4% (n=10/14) in the MIC group and 84.3% (n=161/191) in the group without MIC; p=0.257 (NS). One patient had macroalbuminuria. The mean reductions at follow up at six months in MIC -27 mg/day (±92, 95% CI -51 to -4.1, minimum -214, maximum 599), A1c -2.9% (±2.3, 95% CI -3.5 to -2.3, minimum -7.1, maximum 0.9), SBP -9 mmHg (±15, 95% CI -13 to -5.2, minimum -7.1, maximum 0.9) and DBP -6.2 mmHg (±8.5, 95% CI -8.4 to -4.1, minimum -25, maximum 14). There was no significant correlation between the reduction in A1c and the reduction in MIC (Pearson r 0.18, 95% CI -0.06 to 0.41, p=0.14 NS). There was a significant positive correlation between the reduction in MIC and Systolic BP (Pearson r 0.33, 95% CI 0.091 to 0.53, p=0.008). However, there was no significant correlation between the reduction in MIC and Diastolic BP (Pearson r 0.17, 95% CI -0.07 to 0.40, p=0.17). (Refer Image)
Conclusion: There is a significant number of subjects achieving remission of T2DM associated with a significant number of patients achieving regression of microalbuminuria. The reduction in HbA1c and microalbuminuria were independent of each other. The positive correlation between the reduction in Systolic BP and microalbuminuria indicates the additive benefits of TPT yielding synergism for the common pathophysiological pathways in patients with diabetic kidney disease
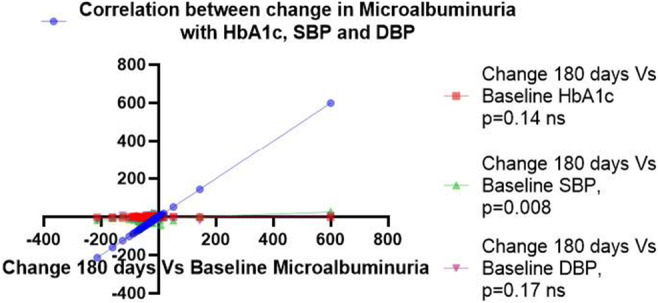
Clinical Trial Registration Number: CTRI/2020/08/027072
Disclosure: S. Joshi: None.
SO 57 Type 1 diabetes: still the challenge number one
699
Effect of 24 months of optimised glucose control on residual C-peptide secretion in youth with new onset type 1 diabetes
J. Ware1,2, C.K. Boughton1,3, J.M. Allen1,2, M.E. Wilinska1,2, A. Thankamony2, T. Randell4, A. Ghatak5, R.E.J. Besser6,7, D. Elleri8, N. Trevelyan9, F.M. Campbell10, R. Bailey11, G. Dunseath12, R. Hovorka1,2, on behalf of the CLOuD Consortium;
1Wellcome-MRC Institute of Metabolic Science-Metabolic Research Laboratories, University of Cambridge, Cambridge, UK, 2Department of Paediatrics, University of Cambridge, Cambridge, UK, 3Wolfson Diabetes and Endocrine Clinic, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK, 4Department of Paediatric Diabetes and Endocrinology, Nottingham Children's Hospital, Nottingham, UK, 5Department of Diabetes, Alder Hey Children's NHS Foundation Trust, Liverpool, UK, 6Department of Paediatrics, University of Oxford, Oxford, UK, 7NIHR Oxford Biomedical Research Centre, Oxford, UK, 8Department of Diabetes, Royal Hospital for Sick Children, Edinburgh, UK, 9Paediatric Diabetes, Southampton Children's Hospital, Southampton, UK, 10Department of Paediatric Diabetes, Leeds Children's Hospital, Leeds, UK, 11Jaeb Center for Health Research, Tampa, USA, 12Diabetes Research Group, Swansea University, Swansea, UK.
Background and aims: We assessed whether improved glucose control with hybrid closed-loop can preserve C-peptide secretion compared to standard insulin therapy in youth with new onset type 1 diabetes.
Materials and methods: In an open-label, multicentre, randomised, parallel trial, youth aged 10 to <17 years were randomised within 21 days of type 1 diabetes diagnosis to hybrid closed-loop using the Cambridge algorithm or standard insulin therapy (control) for 24 months. Primary endpoint was the difference in mixed meal C-peptide AUC 12 months post diagnosis. Analysis was by intention to treat.
Results: We randomised 97 participants (mean±SD age 12±2 yrs), 51 to closed-loop and 46 to control therapy. There was no difference in C-peptide AUC at 12 months (primary endpoint) or 24 months between groups (geometric mean [95% CI] 12 months closed-loop: 0.35 pmol/mL [0.27, 0.43] vs control: 0.46 pmol/mL [0.33, 0.61]; mean adjusted difference -0.06 [-0.14 to 0.03]; p=0.19 and 24 months closed-loop: 0.18 pmol/mL [0.12, 0.24] vs control: 0.24 pmol/mL [0.12, 0.37]; mean adjusted difference -0.04 [-0.14 to 0.06]). Glycated haemoglobin was lower in the closed-loop group by 4 mmol/mol [0.4%] (95% CI 0 to 8 mmol/mol [0.0 to 0.7%]) at 12 months, and 11 mmol/mol [1.0%], (95% CI 7 to 15 mmol/mol [0.5 to 1.5%]; p<0.001) at 24 months. Five severe hypoglycaemic events occurred in closed-loop group (3 participants), and one in control group; one diabetic ketoacidosis occurred in the closed-loop group.
Conclusion: In youth with new onset type 1 diabetes, optimising glucose control for 24 months does not appear to prevent the decline in residual C-peptide secretion.
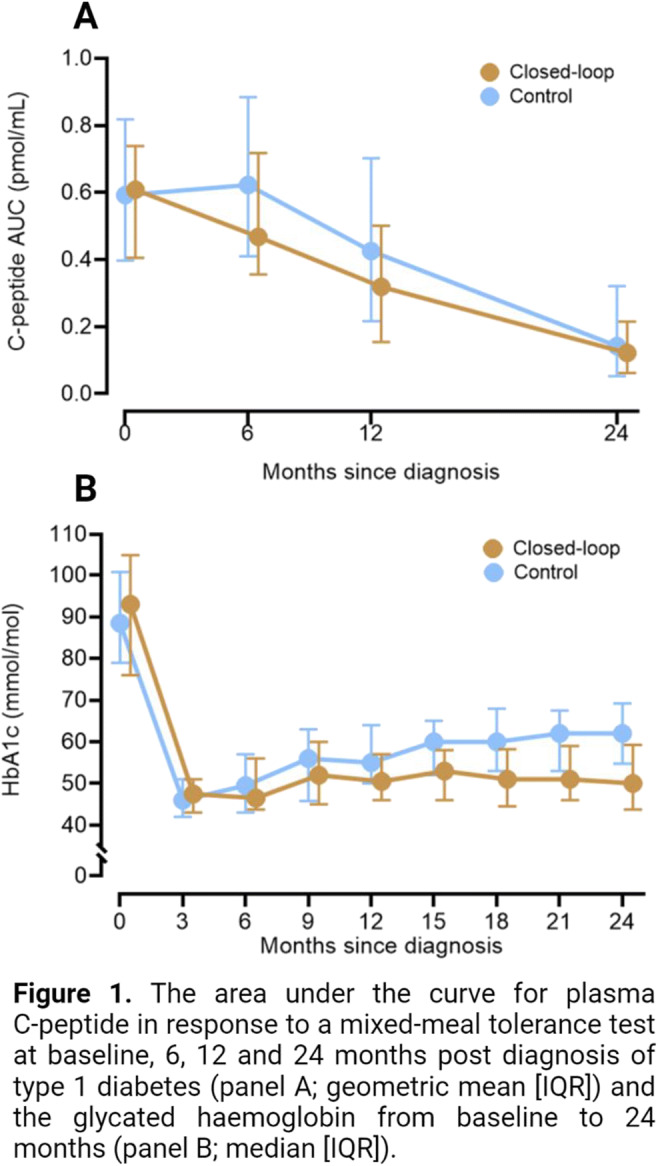
Clinical Trial Registration Number: NCT02871089
Supported by: NIHR EME, Helmsley Trust, JDRF, additional support from NIHR BRC
Disclosure: J. Ware: None.
700
Sociodemographic differences in physical activity in people with type 1 diabetes
S. Elton Sander1, S. Molsted1, S. Caunt2, E. Søndergaard3, R.F. Johansen3, S. Heller4, P.L. Kristensen5;
1Department of Clinical Research, Nordsjællands Hospital, Hillerød, Denmark, 2Academic Directorate of Diabetes and Endocrinology, Sheffield Teaching Hospital NHS, Sheffield, UK, 3Steno Diabetes Center, Aarhus, Denmark, 4Department of Oncology and Metabolism, University of Sheffield, Sheffield, UK, 5Department of Endocrinology and Nephrology, Nordsjællands Hospital, Hillerød, Denmark.
Background and aims: In the general population, the risks of health-related outcomes are unequally distributed between socio-demographic variables including sex, marital status and educational level. This also applies to people with type 1 diabetes (T1D). It is unknown if these inequalities also apply to physical activity (PA) in people with T1D. We investigated the impact of socio-demographic factors on level of PA in T1D, and patient perceived challenges and barriers to PA.
Materials and methods: This was a cross-sectional study. Participants with T1D (>18 years) were recruited from the outpatient clinics at two Hospitals in Denmark and one in the UK. Data on self-reported leisure time PA were collected using the Saltin-Grimby scale and stratified into two categories: moderate-vigorous PA, or physically inactive/light PA. Education level was measured using the International Standard Classification of Education, and highest level was used to stratify into three levels. Participants were asked whether they wanted to be more active, if they had PA related glucose challenges, and if they had received help from health care professionals to manage blood glucose in relation to PA.Multivariate logistic regression analyses identified associations between exposure variables sex and education level on the outcomes. The analyses were adjusted for age and marital status
Results: 334 people with T1D were included in the analyses (69% and 31% from DK and UK, respectively, 47% female, age 50 (30-61) median (IQR) years, Education levels were short in 10%, medium in 34%, and high in 57%. 44% reported moderate-vigorous PA levels, 67% reported that they wanted to be more active, and 60% reported having experienced PA related blood glucose challenges. 47% reported that they had not received help from health care professionals to manage PA related blood glucose challenges. More men than women were moderate-vigorous physically active (51% vs 35 %, p=0.004), and more women wanted to be more active (77% vs 59%, p=0.001). More women reported having PA related blood glucose challenges (53% vs 48%, p=0.008). There were no sex differences in the participants’ reporting of having received help from health care professionals to manage blood glucose in relation to PA. When the participants were stratified by education level, there were no differences in PA level, whether they wanted to become more active, whether they experienced PA related blood glucose challenges, or whether they received help from health care professionals to manage these challenges. In a multivariate logistic regression analysis, being woman was associated with low PA level OR 2.1 [95% CI 1.3; 3.4, p=0.002] and wanting to be more active OR 2.1 [1.3; 3.6, p=0.007]. In the same adjusted model, medium vs high education level was associated with the low PA level OR 1.9 [1.1; 3.02, p=0.018].
Conclusion: Education level was not associated with any of the outcomes. However, there were sex related inequalities, and being woman was associated with a lower PA level and wanting to be more active and experiencing more PA related blood glucose challenges.
Disclosure: S. Elton Sander: None.
701
Which patient-reported outcomes should be measured in routine diabetes care?
K. Hamilton1, R. Forde1, M. Due-Christensen1, K. Eeg-Olofsson2, D. Nathanson3, S. Rossner3, S. Vikstrom-Greve3, A.-K. Porth4, Y. Seidler4, L. Delbecque5, A. Ozdemir Saltik6, Y. Hasler6, T. Stamm4,7, D. Hopkins8, A. Forbes1;
1King's College London, London, UK, 2University of Gothenburg, Gothenburg, Sweden, 3Karolinska University Hospital, Stockholm, Sweden, 4Medical University of Vienna, Vienna, Austria, 5Eli Lilly and Company, Indianapolis, USA, 6Medtronic International Trading Sàrl, Tolochenaz, Switzerland, 7Ludwig Boltzmann Institute for Arthritis and Rehabilitation, Vienna, Austria, 8King’s Health Partners Institute for Diabetes, Endocrinology and Obesity, London, UK.
Background and aims: To improve outcomes people with diabetes must be engaged in their care. This requires more patient-centred models of care. Developing integrated digital data collection systems that include patient-reported outcomes (PROs) may help achieve this. We sought to identify PROs with high relevance to diabetes populations to be considered for integration into international diabetes care systems, to inform care at the individual patient and population level.
Materials and methods: A systematic literature review was undertaken to identify PROs relevant to diabetes populations with potential to inform individual patient care and service development. PubMed, COSMIN and COMET databases were searched. Studies were included if they recommended PROs because they have utility for informing individual patient care (e.g. they provide key insights into diabetes), healthcare decision making (e.g. they are key indicators of care) and/or facilitating patient-centred care (i.e. they are patient-important outcomes). Recommended outcomes were categorised as conceptually distinct PROs. Studies were appraised in terms of relevance for identifying suitable PROs and evidence suggesting the PROs are patient-important, so that the recommended PROs could be considered in light of the evidence endorsing them.
Results: Screening papers (n=2,121) identified 27 studies recommending 53 diabetes-specific PROs, and tools for measuring 72% of these PROs. The PROs were endorsed by evidence representing international stakeholder perspectives, and reflected the experience of living with diabetes, psychosocial effects, symptoms, diabetes self-management performance and potential mediators of this. Sixteen PROs were endorsed by the most relevant studies for identifying suitable PROs (i.e. consensus work on developing an outcome set for clinical practice), and evidence suggesting patient-importance: diabetes-related distress, performance of and self-efficacy for self-management, burdens and restrictions related to diabetes, diabetes-related social support, symptoms, diabetes-related problem solving, fear of hypoglycaemia, diabetes-related eating problems, and treatment side effects.
Conclusion: We identified a comprehensive list of diabetes-specific PROs recommended by international literature that are suitable for informing care at the individual patient and population level. We anticipate these PROs could be applied universally for this purpose. Including these PROs in diabetes care systems will stimulate a more patient-centred approach, supporting the shift toward more precision medicine in diabetes. It could facilitate clinical communication and decision making by highlighting important issues for people with diabetes and/or influences on self-management engagement, and service development by highlighting issues that can be addressed systemically leading to better models of care delivery.
Supported by: H2O has received funding from the IMI 2 Joint Undertaking under grant agreement No 945345-2
Disclosure: K. Hamilton: Grants; H2O has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 945345-2.
702
Continuous monitoring in-vivo with an islet-based microfluidic biosensor
M. Raoux1, E. Puginier1, A. Pirog2, F. Poulletier de Gannes2, J. Gaitan1, A. Hurtier2, M. Monchablon2,1, J. Cieslak2, S. Renaud2, J. Lang1;
1CNRS UMR 5248, CBMN, Univ. Bordeaux, Pessac, 2CNRS UMR 5218, IMS, Bordeaux INP, Univ. Bordeaux, Talence, France.
Background and aims: Continuous monitoring of for glucose levels is currently based on enzyme-linked electrochemical probes. In contrast, monitoring of a few electrogenic islets in a biosensor may harness the computational power of the micro-organ made for nutrient detection and provide a more suitable readout. Extracellular islet electrophysiology detects slow potentials (SPs) reflecting insulin secretion of coupled islet β-cells and is a method of choice for nondestructive long-term monitoring of islet activity in vitro.
Materials and methods: Male Wistar rats (mean 10.4 weeks) were implanted with a microdialysis catheter (Microdialysis AB) in the right interscapular region under anesthesia. Mouse islets from adult male C57BL/6J mice were seeded on microelectrode arrays (MEAs, MCS, Reutlingen, Germany) coated with 2% v/v Matrigel, and cultured at 37°C for 3 days. Microfluidic chips were made of PDMS (channel Ø 0.8 mm) and aligned on plasma-treated MEAs in a clean room. Fluid shear stress was simulated using COMSOL Multiphysics (Burlington, MA, USA), and devices were calibrated by video-microscopy and phenol red. SP recordings were analyzed using MC_Rack software. Glucose was measured after i.p. administration at the tail vein (Abbott, France) and in the dialysate using glucose oxidase (Biolabo, France). Respiration rate was measured by analysis of video files.
Results: We developed a microfluidic microelectrode chip containing a few islets connected via microdialysis to interstitial fluids in living rats. Blood/dialysate glucose levels and electrical activity of the islets were determined simultaneously. Initial off-line experiments showed an excellent correlation between rat or human serum glucose and the frequency or amplitude of SPs (n=25, R2 > 0.96, p < 0.01). Microdialysis at 1 μl/min resulted in 80% recovery and only minimal shear stress in the device. Intraperitoneal glucose testing (2g /kg; n=4-5 animals), with or without subsequent insulin injection (2.5 U/kg), resulted in an excellent correlation (Spearman) between 2h SP recordings and dialysate glucose (mean+SD R2 of 0.81+0.1, p < 0.001, frequency; 0.82+0.1, p < 0.01, amplitude) or blood glucose (0.91+0.04, frequency; 0.92+0.04). Normalized slopes were fairly comparable between experiments, especially for SP frequency (mean+SD; dialysate glucose vs. frequency 1+0.32, amplitude 1+0.68; blood glucose vs. frequency 1+0.17, amplitude 1+0.44).
Conclusion: Our data demonstrate that islet-based biosensors provide continuous glucose monitoring in rodents in vivo for at least 2 hrs. This extracorporeal CGM might control insulin pumps by calculating in real-time the physiological needs.
Supported by: Société Francophone du Diabète; Agence Nationale de la Recherche ANR-DIABLO
Disclosure: M. Raoux: None.
703
Development of non-invasive saliva glucose measuring device (D-SaLife) and clinical evaluation according to ISO 15197:2013 criteria
K. Han1, I.-S. Jang2, M.-S. Kwon2, J.-W. Kye2, E. Shim2, D.-C. Kim2, K. Min1;
1Diabetes Center, Nowon Eulji University Hospital, 2Dongwoon Anatech, Seoul, Republic of Korea.
Background and aims: Glucose is detected not only in blood and interstitial fluid, but also in saliva. If the amount of glucose in saliva could be quantified, noninvasive testing using saliva could be alternative to conventional invasive capillary blood glucose testing. Therefore, we developed a new device D-Salife (Saliva Glucose Monitoring System for self-testing, a prototype of Dongwoon Anatech) that allows consumers to easily measure saliva glucose. The purposes of this study were to investigate the correlation and the precision with between salivary glucose level measured with D-Salife (D-Salife glucose) and the capillary whole blood glucose level (CB glucose), and those also between two different saliva measurements (D-salife vs iron chromatography).
Materials and methods: Fasting saliva and capillary glucose were measured simultaneously in a fasting state from a total of 114 subjects, including nondiabetic, pre-diabetics, and diabetic patients. Oral saliva was collected using a sterile dental sponge. Saliva glucose was measured 6 times with D-Salife and with IC((Model Thermo Scientific DIONEX ICS-6000 IC System), and CB glucose was with a glucometer (Roche Accu-Chek Performa). The Precision was evaluated the repeatability and intermediate measurement precision, according to ISO 15197:2013. consensus Error Grid.
Results: The regression analysis showed that there was a direct correlation between CB glucose and D-Salife glucose (p < 0.001) (adjusted R2 =0.9325, the regression coefficient = 0.95849). Error grid analysis showed that 76 (66.7%) from D-Salife were in zone A, the remaining 38 (33.3%) were in section B, thus meeting the ISO15197 international performance standard for glucose meters.D-Salife glucose showed a high correlation with the IC glucose (Y=23.466x, P<0.001, R2=0.872), and the precision was also excellent according to the ISO_CEG standard (A zone ; 76%, B zone ; 24% ) In the repeatability analysis of 6 measurements of D-Salife glucose, the pooled standard deviation showed 8.7 mg/dL in the sugar concentration range below 100 mg/dL, 5.8 mg/dL in the pooled standard deviation range in the sugar concentration range above 100 mg/dL, and 4.9% in the pooled coefficient of variation.
Conclusion: The D-Salife showed good performance according to the ISO15197 international performance standard for glucose meters, and the acceptable level of repeatability comparable to popular capillary glucometer. So it could be a clinically useful noninvasive self-testing glucometer.
Disclosure: K. Han: None.
704
Cognitive assessment in elderly with type 1 diabetes on insulin pump: a cross-sectional observational study
A. Rizzi1, L. Tartaglione1, F. L'Abbate2, G. Masone Iacobucci2, D. Quaranta2, L. Leo1, G.E. Rizzo1, D. Pitocco1;
1Diabetes Care Unit, Catholic University School of Medicine, 2Institute of Neurology, Catholic University School of Medicine, Rome, Italy.
Background and aims: Aim of the study was to investigate cognitive performance in elderly with type 1 diabetes (T1DM) on insulin pump (CSII) and the correlation between time in range (TIR) and cognitive scores (CS). Differences in CS according to duration of disease were also investigated.
Materials and methods: Subjects with T1DM older than 65 years, on CSII were included. Participants underwent following tests: Rey Auditory Verbal Learning Test (RAVLT), Rey-Osterrieth Figure Test, Verbal Fluency Test, Stroop Color and Word Test and Trail Making Test (TMT) A and B. At visit data from continuous glucose monitoring, according to 2019 Advanced Technology and Treatments in Diabetes Consensus, data on diabetic history and blood tests were collected.
Results: We enrolled 45 subjects, 22 women (49%), mean age 69.5 years. Median duration of disease was 30 years; mean HbA1c was 58 mmol/mol (7.4%). Mean TIR was 66%, mean time below range was 2.62% and time above range (TAR) 29.9%. 5 subjects (11%) presented CS compatible with mild cognitive impairment. No correlation between TIR and CS was detected. Dividing study population on median duration of disease, subjects with longer history presented lower TIR (59.7 %(20.2) vs 71.1% (16.3), p=0.05) and higher TAR (35.7% (17.8) vs 25.2% (15.3), p=0.05). In both groups 26% were using advanced hybrid closed loop systems. Subjects with longer history presented lower scores at RAVLT-Immediate recall (30.1 (12.6) vs 39.3 (13.4), p<0.001) and RAVLT-Accuracy (0.89 (0.09) vs 0.93 (0.05), p=0.08) and longer time at Stroop-Test (28.8 (8.13) vs 20.0 (7.36), p<0.001) and TMT-B (131 (64.2) vs 104 (32.2), p=0.08) test. Groups were comparable for school age (>30 years 12.9 vs <30 years 13.1, p=0.80).
Conclusion: Elderly with T1DM presented good TIR and at a deep evaluation, they presented good CS. Those with longer history presented lower CS in executive and attentive functions, expression of subcortical damage. No association between TIR and CS was detected.
Disclosure: A. Rizzi: None.
705
Reduction of basal insulin to prevent exercise induced hypoglycaemia during two types of exercise in adults and adolescents with type 1 diabetes using insulin pump therapy
J. Molveau1, É. Myette-Côté2, S. Tagougui1, C. Suppère1, E. Heyman3, V. Messier1, L. Legault4, R. Rabasa-Lhoret1;
1Institut de recherches cliniques de Montréal, Montreal, Canada, 2Applied Human Sciences, University of Prince Edward Island, Charlottetown, Canada, 3Université de Lille, Lille, France, 4Montreal's children Hosptial, Montreal, Canada.
Background and aims: Regular exercise is associated with physical and mental health benefits. However, maintaining adequate glucose control around exercise is challenging and the risk of exercise-related hypoglycemia frequently leads people living with type 1 diabetes (PWT1D) to remain sedentary. Strategies such as insulin basal rate reduction (BRR) can limit this risk. However, the impact of physical activity on glycemia varies depending on the type of exercise and the optimal BRR to limit the risk of hypoglycemia during different types of exercises has not been evaluated. We aim to assess the optimal BRR to minimize the risk of hypoglycemia during two types of exercise (High intensity intermittent exercise (INT) and continuous moderate intensity exercise (CONT)) known to have different impacts on glucose profiles.
Materials and methods: Twenty-nine PWT1D treated with insulin pump (20 adults, 9 adolescents; HbA1c = 7.4 ± 1.0%) were included. Participants performed two 60-min exercise sessions matched for energy expenditure (CONT, 60% of VO2peak vs. INT, 2-min, 50%/2-min, 85% of VO2peak). Insulin basal rate was reduced by either 40- or 80%, 90-min before exercise start. The four interventions were carried out in a randomized fashion. Blood glucose was measured every 10-min during exercise.
Results: For CONT, there were no differences in the time spent in hypoglycemia (8.5 ± 15.9, CONT-40%; 9.8 ± 15.9 CONT-80%) or in the number of hypoglycemic episodes (10, CONT-40% vs. 11, CONT-80%). Glucose reduction tended to be higher with 40% BRR (-3.3 ± 2.4, CONT-40% vs. -2.8 ± 2.8, CONT-80%, p=0.9). Time to first hypoglycemic episode was similar in both conditions (36.2 ± 17.5, CONT-40% vs. 40.0 ± 15.9, CONT-80%, p=0.6). For INT, time spent in hypoglycemia was significantly lower with 80% BRR (9.2 ± 16.5, INT-40% vs. 4.9 ± 11.0, INT-80%, p<0.05) as well as the number of hypoglycemic episodes (9, INT-40% vs. 6, INT-80%, p<0.05). Time to first hypoglycemic event was similar in both conditions (44.0 ± 11.3, CONT-40% vs. 46.2 ± 7.5, CONT-80%, p=0.6).
Conclusion: For CONT exercise, reducing insulin basal rate by either 40% or 80% 90 min prior to exercise had no impact on hypoglycemic risk. For INT exercise reducing basal rate by 80% should be prefered. To further reduce hypoglycemic risk, favoring exercise sessions of less than 40min and/or combining basal rate reduction with carbohydrate intake is probably required.
Clinical Trial Registration Number: NCT03845114
Supported by: IRSC/ CMDO
Disclosure: J. Molveau: None.
706
Efficacy and safety of ultra-rapid lispro compared to insulin lispro in adults with type 1 diabetes
Z. Zhou1, J. Ma2, T. Lei3, Z. An4, Y. Xue5, F.P. Manghi6, P. Garcia7, J. Xu8, Y. Yuan8, X. Yan1;
1The Second Xiangya Hospital Of Central South University, Changsha, China, 2Nanjing First Hospital, Nanjing, China, 3Shanghai Putuo District Central Hospital, Shanghai, China, 4West China Hospital, Chengdu, China, 5Changzhou No.2 People's Hospital, Changzhou, China, 6Centro de Investigaciones Metabólicas, Caba, Argentina, 7Hospital Universitario "Dr. Jose Eleuterio Gonzalez", Monterrey, Mexico, 8Eli Lilly and Company, Indianapolis, USA.
Background and aims: Ultra-rapid lispro insulin (URLi), a new formulation of insulin lispro having a faster onset of action and shorter duration of action, may allow more effective control of postprandial glucose (PPG) levels. The aim of this study is to evaluate efficacy and safety of URLi when compared to insulin lispro among patients with Type 1 diabetes (T1D), to demonstrate the noninferiority of URLi to insulin lispro on glycemic control.
Materials and methods: This was a Phase 3, randomized, double-blind, multinational, multicenter, parallel, active-controlled study. Patients with T1D were randomly assigned to URLi or insulin lispro, in combination with basal insulin glargine or insulin degludec. Primary endpoint was efficacy comparison between URLi and insulin lispro at Week 26 from the mixed-effect model repeated measure (MMRM) analysis of change from baseline in HbA1c (noninferiority margin 0.4%). Key secondary endpoints included differences in 1-hour and 2-hour PPG excursion from a mixed-meal tolerance test (MMTT) and change from baseline in 10-point self-monitored blood glucose (SMBG) at Week 26. Safety measures with respect to hypoglycemia or any other adverse events (AEs) were compared between groups.
Results: A total of 354 patients was randomized (URLi: 176; insulin lispro: 178). From baseline to Week 26, the least squares mean (LSMean) of HbA1c decreased by 0.28% (3.0 mmol/mol) in the reference group and by 0.21% (2.3 mmol/mol) in the URLi group with no treatment difference. The LSMean difference (95% CI) between treatment groups in change in HbA1c was 0.07% (-0.11, 0.24) [0.7 mmol/mol (-1.2, 2.7)]. URLi was noninferior to insulin lispro for glycemic control. Treatment with URLi significantly lowered PPG excursions compared to insulin lispro at 1-hour (4.7 mmol/L vs. 5.7 mmol/L, p=0.003) and 2-hour (6.5 mmol/L vs. 7.9 mmol/L, p=0.001), respectively. Significant larger reduction of SMBG values from baseline to Week 26 was observed in the URLi group than in the reference group at morning premeal (-0.57 mmol/L vs. 0.31 mmol/L, p<0.001), morning 1-hour postmeal (-1.13 mmol/L vs. -0.35 mmol/L, p=0.005), and morning 2-hour postmeal (-1.36 mmol/L vs. -0.53 mmol/L, p=0.004) but not at other 10-point SMBG time points. There were no statistical differences with respect to the rate of severe hypoglycemic events, the incidence and rate of documented symptomatic postmeal hypoglycemia, the incidence and rate of documented symptomatic hypoglycemia, or any other AEs during 26-week treatment period.
Conclusion: This study demonstrated that URLi is noninferior to insulin lispro on glycemic control in patients with T1D when administered as prandial insulin, in combination with basal insulin for 26 weeks. URLi was superior to insulin lispro in controlling 1-hour and 2-hour PPG excursions. The overall safety profile was similar between treatment groups.
Clinical Trial Registration Number: NCT03952130
Supported by: Eli Lilly and Company
Disclosure: Z. Zhou: None.
SO 58 Autonomic rhythm
707
Prevalence of peripheral and cardiovascular autonomic neuropathy in Greenlanders with diabetes and prediabetes based on national normative reference data
M.M.B. Christensen1,2, C.S. Hansen3, D. Vistisen1,4, J. Fleischer5,6, D.R. Witte2,5, M.E. Jørgensen7,8;
1Clinical epidemiology research, Steno Diabetes Center Copenhagen, Herlev, Denmark, 2Department of Public Health, Aarhus University, Aarhus, Denmark, 3Complications research, Steno Diabetes Center Copenhagen, Herlev, Denmark, 4Department of Public Health, University of Copenhagen, Copenhagen, Denmark, 5Steno Diabetes Center Aarhus, Aarhus, Denmark, 6Steno Diabetes Center Zealand, Holbæk, Denmark, 7Steno Diabetes Center Greenland, Herlev, Greenland, 8National Institute of Public Health, University of Southern Denmark, Odense, Denmark.
Background and aims: Prevalence of diabetes is increasing in Greenland. However, studies on the prevalence of diabetic peripheral neuropathy (DPN) and cardiovascular autonomic neuropathy (CAN) in Greenland are scarce. Our aim was to determine the prevalence of DPN and CAN in Greenlanders with diabetes and prediabetes by establishment and application of normative reference data from the Greenlandic background population.
Materials and methods: We evaluated the prevalence DPN and CAN among Greenlanders with diabetes and prediabetes who participated in the nationwide Greenlandic Population Survey 2018. DPN was assessed by perception of monofilament and vibration threshold (VPT). A composite measure (DPN composite) for abnormal monofilament and/or VPT was applied. Cardiovascular autonomic reflex tests and power spectral analysis of heart rate variability (HRV) were applied to assess CAN. Normative reference data specific for the Greenlandic population was used to determine the prevalence of DPN and CAN. We estimated normal ranges for VPT and CAN in Greenlanders without diabetes by using piecewise linear quantile regression models at the 5th percentile. The model for normal threshold of VPT was adjusted for age and height. Models for CAN limits were adjusted for age.
Results: A total of 369 participants with either diabetes (37.7%) or prediabetes (62.3%) were examined for DPN and CAN. Women comprised 54.2% and mean age was 60.6 years. DPN composite was diagnosed in 7.6% in the participants with diabetes and in 3.9% of participants with prediabetes. Prevalence of CAN and early CAN in participants with diabetes was 5% and 11.7%, respectively. In participants with prediabetes CAN was diagnosed in 3.2% and early CAN in 11.6%. The prevalence of abnormal VPT, monofilament insensitivity and abnormal HRV measures can be found in table 1.
Conclusion: When compared to prevalence of DPN and CAN in the Western World we have identified a relatively low prevalence of DPN and CAN in Greenlanders with diabetes and prediabetes. However, estimated normative values for VPT and CAN show lower threshold values for peripheral and autonomic function in Greenlanders without diabetes compared to Europeans which may have resulted in an underestimated prevalence of DPN and CAN. This warrants studies investigating the underlying pathophysiology to low nerve function and prevalence of neuropathy in Greenlanders with and without diabetes, respectively.
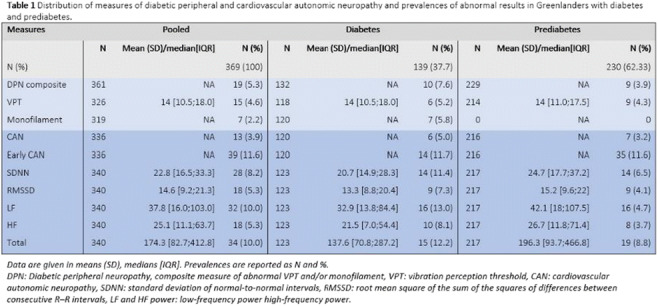
Supported by: Novo Nordisk Foundation, Steno Diabetes Center Aarhus, Institution of Public Health, AU
Disclosure: M.M.B. Christensen: Stock/Shareholding; Novo Nordisk A/S.
708
Diabetic cardiovascular autonomic neuropathy: link between arterial stiffness, insulin resistance, melatonin and some vasoactive peptides
V.A. Serhiyenko1, V.B. Segin2, A.A. Serhiyenko1, L.M. Serhiyenko1;
1Dept of Endocrinology, Danylo Halytsky Lviv National Medical University, 2Lviv Regional Clinical Diagnostical Treatment Endocrinological Center, Lviv, Ukraine.
Background and aims: Diabetes-associated cardiovascular autonomic neuropathy (CAN) is often underdiagnosed complication of type 2 diabetes mellitus (T2DM), that damages autonomic nerve fibers causing abnormalities in heart rate and vascular dynamics. The present study has been designed to determine the relationship between arterial stiffness, insulin resistance (IR) parameters, melatonin, N-terminal pro-brain natriuretic peptidе (NT-proBNP) and endothelin-1 (ET-1) levels among patients with CAN.
Materials and methods: 92 patients with T2DM, among them 46 patients with definite stage of CAN and 46 without CAN were recruited to the study. Patients were aged 50-59 years, BMI 26.8±0.32 kg/m2, the mean duration of diabetes was 7.4±0.5 years, HbA1c level 7.3±0.18%. The diagnosis of CAN was based on the results of 5 standard cardiovascular tests, 24-hour heart rate variability was evaluated using ECG “EC-3H” (“Labtech”), arterial stiffness monitoring - TensioMedTM Arteriograph 24 (Hungary). Homeostasis model assessment IR (HOMA-IR) was calculated: fasting glucose (mmol/L) x fasting immunoreactive insulin (mcIU/ml)/22.5. Melatonin in saline (nocturnal measurement), NT-proBNP and ET-1 contents in blood were determined by enzyme-linked immunosorbent assay (ELISA) using kits by DRG (USA). Statistics: ANOVA.
Results: Development of CAN is associated with decrease in melatonin levels (9.3±0.9 pg/ml vs 19.7±1.2 pg/ml, p<0.001) and increase of HOMA-IR (6.03±0.9 vs 4.12±0.7, p<0.001). We found out that in patients with definite CAN the concentration of NT-proBNP (405.2±14.2 fmol/ml vs 260.3±17.7 fmol/ml, p<0.001) and pulse wave velocity (PWV) (10.95±0.23 m/sec vs 8.7±0.43 m/sec, p<0.001) were statistically significant higher compared to patients without CAN. Arterial stiffness parameters among patients with CAN exceed physiological values and were considered as high. The daily value of PWV was normal in 19.6%, elevated in 47.6% and pathological in 32.6% of cases. Among patients with T2DM without diagnosed CAN value of PWV was normal in 87.0% and elevated in 13.0% of patients. We have not found any statistically significant changes in ET-1 levels among two groups of patients. 60.9% of persons with definite stage of CAN have violations of diurnal blood pressure (BP) profile, namely the daily profile of “non-dipper” was found in 45.7%, and “night-peaker” 19.6%. Multiple regression analysis, after controlling for age, gender, diabetes duration, BP, HbA1c and left ventricular mass index, showed an independent inverse association of melatonin levels with HOMA-IR and PWV (p<0.001).
Conclusion: Development of CAN is associated with increased NT-proBNP levels, IR and arterial stiffness parameters, violation of BP profile and decrease of melatonin levels. Nocturnal melatonin secretion is independently and inversely associated with IR, violation of BP profile and increase of arterial stiffness. Further work is needed to validate this association and perform a prospective analysis to assess whether melatonin supplementation may modify insulin resistance, blood pressure patterns and arterial wall rigidity.
Disclosure: V.A. Serhiyenko: None.
709
Collagen turnover is associated with cardiovascular autonomic and peripheral neuropathy in type 1 diabetes: Novel pathophysiological mechanism?
C. Hansen1, D.G.K. Rasmussen2, T.W. Hansen1, S.H. Nielsen2,3, S. Theilade1,4, M.A. Karsdal2, F. Genovese2, P. Rossing1,4;
1Complications Research, Steno Diabetes Center Copenhagen, Herlev, 2Nordic Bioscience, Herlev, 3Technical University of Denmark, Lyngby, 4Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Background and aims: Diabetic cardiovascular autonomic neuropathy (CAN) and distal symmetric polyneuropathy (DSPN) affect millions of people and increase the risk of morbidity and mortality. Identifying new risk factors for these complications is needed to develop means of intervention. Collagen type VI (COL6) and III (COL3) have been associated with nerve function. We aim to investigate the possible associations between serum markers of COL6 and COL3 and diabetic neuropathy in people with type 1 diabetes (T1DM).
Materials and methods: In a cross-sectional study of 300 people with T1DM, serum PRO-C6 (marker of COL6 formation) and C3M (marker of COL3 degradation) were obtained. CAN was assessed by cardiovascular reflex tests (CARTs): heart rate response to deep breathing (E/I ratio), to standing (30/15 ratio) and to the Valsalva manoeuvre (VM). Two or three pathological CARTs constituted CAN. Peripheral neuropathy was assessed by biothesiometry, where symmetrical vibration sensation threshold above 25 volts constituted DSPN.
Results: Patients were (mean (SD)) 55.7 (9.3) years, 51% where males, diabetes duration was 40.1 (8.9) years, HbA1c was 62.9 (1.9) mmol/mol, estimated glomerular filtration ratio (eGFR) 78.1(24.8) ml/min/1.73m2 with (median(IQR)) serum PRO-C6 7.8 (6.2;11.0) ng/ml and C3M 8.3 (7.1;10.0) ng/ml. Of 259 persons with CARTs measured, 34% was diagnosed with CAN. DSPN was assessed in all participants and was present in 43%. In models adjusted for age, sex (model 1) and additionally adjusted for diabetes duration, HbA1c, BMI, smoking, systolic blood pressure, serum LDL and beta blocker use (latter, only for CAN) (model 2), a doubling of serum PRO-C6, was significantly associated with odds ratio (OD) >2 for CAN and > 1 for DSPN, respectively. Significance was retained after additional adjustment for eGFR (model 3) for CAN (OD 1.7 (95%CI 1.0;2.7 p=0.035), but not for DSPN (OD 1.2 (95%CI 0.8;1.0 p=0.404) (Figure 1, Panel A). A doubling of serum C3M was associated with a higher OD for CAN only in model 1 and 2, (OD 1.7 (95%CI 1.2;2.6 p=0.004) and 1.8 (95%CI 1.2;2.7 p=0.040)). C3M was not associated to DSPN in any model (Figure 1, Panel B).
Conclusion: Our results show previously undescribed associations between collagen remodelling biomarkers and diabetic CAN and DSPN in T1DM, where higher serum levels of PRO-C6 was associated with both CAN and DSPN, and higher levels of C3M was associated with CAN, even though the significance was lost when factoring in eGFR. The pathological mechanisms between collagen remodelling and diabetic neuropathy remain to be explored.
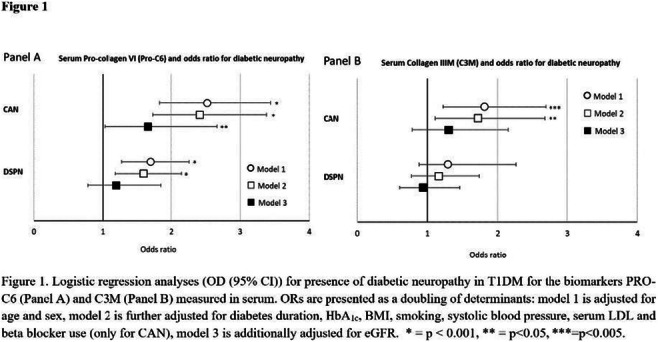
Clinical Trial Registration Number: NCT01171248
Disclosure: C. Hansen: None.
710
Inflammatory markers and cardiovascular autonomic neuropathy in type 1 diabetes
R. Pop-Busui1, L. Ang1, S. Gunaratnam2, Y. Huang2, C. Martin1, A. Burant1, J. Reis1, P. Blakely2, A. Vasbinder2, L. Zhao2, K. Mizokami-Stout1, E.L. Feldman2, S. Hayek2;
1Endocrinology & Diabetes, University of Michigan, 2University of Michigan, Ann Arbor, USA.
Background and aims: Cardiovascular autonomic neuropathy (CAN) is a prevalent serious complication in individuals with type 1 diabetes (T1D). Low-grade inflammation has emerged as a potential mechanism in the development of neuropathy from both experimental and clinical studies. We aimed to assess the role of chronic inflammation in the pathogenesis of CAN in T1D.
Materials and methods: We leveraged an ongoing randomized, placebo-controlled clinical trial targeting inflammation with salsalate in individuals with T1D, to measure a panel of 40 inflammatory markers analyzed on a Luminex xMAP instrument (Luminex Corporation, Austin, TX) (Figure) in baseline samples. Measures of CAN included standardized cardiovascular autonomic reflex tests (CARTs) (E/I, Valsalva, 30:15 ratios) and indices of heart rate variability (HRV). We used Spearman-rank to assess the correlations between inflammatory markers and CAN measures, and linear regression to adjust for age and hemoglobin A1c.
Results: The Figure displays the correlation between biomarkers and CAN measures in 58 T1D participants (mean age 51 ± 13 years, mean diabetes duration 31 ± 15 years, and HbA1c 8.4 ± 1.8%). Amongst all biomarkers, we noted a singular negative correlation between soluble urokinase plasminogen activator receptor (suPAR) and measures of CAN, which remained significant in multivariable analysis (E/I ratio p=0.005, Valsalva p=0.037, RFA p=0.019, LFA p=0.023).
Conclusion: Amongst a large panel of inflammatory markers, we found suPAR to have a notable association with CAN. SuPAR is an immune-mediated signaling glycoprotein which levels are strong predictors of risk in patients with diabetes, which role in CAN warrants further exploration.
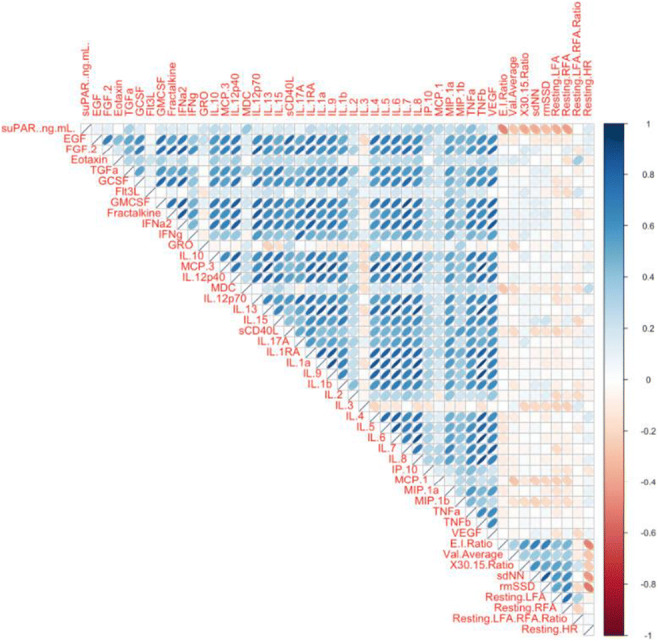
Clinical Trial Registration Number: NCT02936843
Supported by: NIDDK-1-R01-DK-107956-01
Disclosure: R. Pop-Busui: Grants; NIH/NIDDK.
711
Visceral adiposity is associated with autonomic dysfunction in adults with autoimmune diabetes
E. Maddaloni, M. Watanabe, R. Cassano Cassano, D. Masi, R. Amendolara, S. Sterpetti, L. D'Onofrio, C. Moretti, A. Siena, L. Gnessi, R. Buzzetti;
Sapienza University, Rome, Italy.
Background and aims: The prevalence of overweight and obesity is increasing among people with autoimmune diabetes, but little is known about their impact on the risk of chronic complications. We have previously shown that low HDL-cholesterol levels are a major modifiable risk factor of cardiac autonomic neuropathy (CAN), a frequent diabetic complication associated with decreased quality of life and increased risk of death. In this study we aimed to evaluate whether visceral adiposity, another relevant component of metabolic syndrome and a risk factor for dyslipidemia and low HDL-cholesterol levels, is associated with autonomic dysfunction in people with autoimmune diabetes.
Materials and methods: Body mass composition was assessed in 63 adults with autoimmune diabetes (37 females; mean age ± SD: 49.7 ± 12.5 years; disease duration: 16.4 ± 15.7 years; body mass index [BMI]: 25.7 ± 4.9 Kg/m2) using dual energy X-ray absorptiometry (DXA). The Composite Autonomic Symptom Score 31 (COMPASS31) was used to assess the presence of symptoms of autonomic dysfunction in six domains (orthostatic, vasomotor, secretomotor, gastrointestinal, bladder and pupillomotor). Cardiac autonomic reflex tests (CART) were performed to investigate the presence of CAN in 49 participants (14 subjects were not able to perform CART because of contraindications, concomitant therapies, or physical inability). Medical history and biochemical data were retrieved from medical records.
Results: DXA scans showed a mean fat mass of 19.1 ± 8.3 Kg (26.1±7.6% of total body mass), with an estimated mean visceral adipose tissue (VAT) mass of 401.7 ± 226.7 g. Mean weighted COMPASS31 was 15.9±13.2 and CAN was diagnosed in 22 (44.9%) participants. After adjustment for age, sex, HbA1c and HDL cholesterol levels, higher VAT mass was associated with higher COMPASS31 in the gastrointestinal domain (adjusted beta coefficient [95% confidence intervals]: 1.69 [0.14-3.23], p=0.033), but not with total COMPASS31 (adjusted beta coefficient [95% confidence intervals]: -0.30 [-0.76-16.0], p=0.19). Participants with CAN showed higher VAT mass than those without CAN (512.8±217.2 g vs 366.0±221.9 g, p=0.037 after adjustment for age, sex, HbA1c and HDL cholesterol). Conversely, total body fat mass was not associated with autonomic symptoms nor with the presence of CAN.
Conclusion: This study shows that VAT, but not total body fat, is associated with autonomic dysfunction in people with autoimmune diabetes. This observation strengthens the importance of avoiding the accumulation of visceral adiposity in people with diabetes, and also highlights the need for research into the interrelated consequences of obesity among people with autoimmune diabetes.
Supported by: Sapienza University Starting Grant
Disclosure: E. Maddaloni: None.
712
Association of premature ejaculation with diabetic peripheral neuropathy among patients with type 2 diabetes attending at a tertiary level hospital of Bangladesh
M. Ahmed1, F. Yasmin2, A. Morshed3, T. Islam4;
1Medicine, BIRDEM General Hospital, 2Paediatric cardiology, Ibrahim Cardiac hospital and research Institute, 3Psychiatry, Dr. Sirajul Islam Medical College, 4Psychiatry, Sir Salimullah Medical College, Dhaka, Bangladesh.
Background and aims: Diabetes is an important determinant of the risks of premature ejaculation (PE). In patients with diabetes premature ejaculation is three times higher and its onset is 10 to 15 years earlier than non-diabetic person. Diabetes can also affect the blood vessels, leading to sexual dysfunction including premature ejaculation. In addition, a condition called diabetic neuropathy, which involves damage to the nerves, may also causes orgasm without ejaculation. The aim of this study was to find out the association of premature ejaculation with diabetic peripheral neuropathy among patients with Type 2 DM attending in a tertiary care hospital of Bangladesh.
Materials and methods: This descriptive cross sectional study was conducted in BIRDEM general hospital from July 2017 to June 2018. This study was conducted in full accordance with the World Medical Association Declaration of Helsinki, was given a detailed verbal and written description of the proposed study in their own language and signed consent for participation was obtained. Ethical approval for the study was obtained from ethical review committee. All participants’ sexual history had assessed using the Bengali index of Premature Ejaculation Diagnostic Tool. A total of 225 adult patients with type 2 DM were recruited and diagnosis was confirmed as per ADA 2016 criteria. A face to face interview was conducted using premature ejaculation diagnostic tool (PEDT). Diabetic peripheral neuropathy was confirmed by bed side neurological examination including monofilament test and other sensory examination. Data were analyzed by computer with the help of Statistical Package for Social Sciences (SPSS) version 20.0.
Results: Mean age of respondents 39.13 yrs±12.52 and age range was (21-65) years. Most of the respondents were married (73.3%). Among the study population, the prevalence of PE was 55.6%. Mean age of patients having PE was 38.36±8.89 year and age group of 21-30 years had higher incidence rate. Mean age of severe PE (within 15 sec) was found 41.17+_ 9.17 year and age group of 31-40 years had higher incidence rate. Almost half PE patients (48%) had severe PE (within 15 seconds). Among total 225 respondent 135 had neuropathy (60%) and 90 respondent had not develop neuropathy yet (40%). Among those having neuropathy 85 respondent (63%) have premature ejaculation while 10 of them (7.4%) have probable premature ejaculation and 40 respondent (29.6%) are lucky enough to develop premature ejaculation yet. Among those 90 respondent yet to develop neuropathy 40 respondent (44.4%) have developed premature ejaculation already while 15 of them (16.7%) have suffering from probable premature ejaculation and rest 35 respondent (38.9%) have yet to become unlucky.
Conclusion: Erectile dysfunction and PE are very common in DM and may be one of the earliest signs of diabetic neuropathy. PE, which increases in frequency with the age of the patient and the duration of diabetes, may occur in the absence of other signs of diabetic autonomic neuropathy. The health system needs to develop appropriate strategies including early diagnosis, awareness, and health education programs for appropriate treatment.
Disclosure: M. Ahmed: None.
713
Co-existence of peripheral and autonomic neuropathy in type 1 diabetes with and without pain
C.D. Morch1, J. Roeikjer2, S. Santhiapillai Croosu3, T.M. Hansen3, J.B. Frøkjaer4, C. Brock5, N. Ejskjaer6;
1Center for Neuroplasticity and Pain (CNAP), SMI, Department of Health Science and Technology, Aalborg University, 2Department of Health Science and Technology, Aalborg University, Aalborg University Hospital, 3Department of Radiology, Aalborg University Hospital, 4Dept of Radiology, Aalborg University Hospital, 5Department of Gastroenterology, Aalborg University, 6Steno Diabetes Center North Denmark, Depts of Endocrinology and Clinical Medicine, Aalborg University Hospital, Aalborg, Denmark.
Background and aims: Diabetic neuropathy is a complex condition that affects multiple organs. However, the condition is in the literature primarily described as either peripheral (DPN) or autonomic. This study aimed to investigate the association between these two distinct presentations, while also assessing the impact of painful diabetic peripheral neuropathy (PDPN).
Materials and methods: 80 participants (20 type 1 diabetes (T1DM)+PDPN, 20 T1DM+painless DPN, 20 T1DM without DPN or pain and 20 healthy controls (HC)) underwent detailed neurophysiological investigations (including quantitative sensory testing, cardiac autonomic reflex tests (CARTs), and conventional nerve conduction). Cardiac autonomic neuropathy (CAN) was defined as ≥ 2 abnormal CARTs. Differences between groups were analysed with Fischer’s exact tests with Bonferroni correction. After the initial analysis, the participants with diabetes were re-grouped twice based on the presence or absence of small- and large fibre neuropathy.
Results: The prevalence of CAN was highest in T1DM+PDPN (50%), followed by T1DM+DPN (25%) and T1DM-DPN and HC (0%). There was a difference between T1DM+PDPN and T1DM-DPN and HC (p<0.001), while the statistical significance was lost after correction for multiple testing between T1DM+PDPN and T1DM+DPN (p=0.031). When re-grouping based on the presence of small- (SFN) or large fibre neuropathy (LFN), 58% and 55% of the populations had CAN respectively, while no participants without either SFN or LFN had CAN.
Conclusion: This study suggests that CAN only exist when there is concomitant DPN. This appears to be true whether the assessment is based on a clinical assessment or fibre-specific tests. However, larger studies are needed to confirm this relationship.
Disclosure: C.D. Morch: None.
SO 59 Getting a grip on nerves
714
Retinal neurodegeneration as a marker of diabetic peripheral neuropathy and cognitive impairment in type 2 diabetes
F. Picconi1, M. Menduni1, A. Maiorino1, M. Parravano2, B. Russo1, N. Lois3, R. Simo' Canonge4, S. Frontoni1;
1Unit of Endocrinology, Diabetes and Metabolism, S. Giovanni Calibita Fatebenefratelli Hospital, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy, 2IRCCS-G.B. Bietti Foundation Rome, Rome, Italy, 3The Wellcome-Wolfson Institute for Experimental Medicine, Queen's University, Belfast, Ireland, 4Diabetes and Metabolism Research Unit, Vall d'Hebron Research Institute, Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III (ISCIII), Universitat Autònoma de Barcelona, Barcelona, Spain.
Background and aims: The neuropathic process is not only confined to the peripheral sensory nerves but involves the whole nervous system. We suggest that retinal neurodegeneration could parallel brain and peripheral nervous system alterations and therefore retina could be viewed as a new window of the neurodegenerative process. The aim of the study is to evaluate the relationship between the morphological and functional alterations of the neuroretina and both peripheral diabetic neuropathy (DPN) and mild cognitive impairment (MCI) in patients with type 2 diabetes mellitus (DM2).
Materials and methods: 56 DM2 patients (65-90 years), with a disease duration greater than 5 years, with mild diabetic retinopathy were enrolled. DPN was investigated using the Michigan Diabetes Neuropathy Instruments (MNSI), vibration perception threshold (VPT) and warm and cold thermal perception thresholds. Based on the presence of two abnormalities among symptoms and signs, VPT or thermal thresholds we identified two groups: without (DPN-, n:15) or with probable DPN (DPN+, n:41). Participants were also classified in MCI (n:42) or controls (C, n:14), according to Montreal Cognitive Assessment Test (MoCA) (cut-off < 26). All patients underwent microperimetry measuring retinal sensitivity and average threshold macular sensitivity (ATMS), multifocal electroretinogram (mfERG) with implicit time B wave of flash response evaluation and spectral domain optical coherence tomography (SD-OCT), with analysis of retinal central subfield, ganglionar cell layer (GCL) plus inner plexiform layer (IPL) and retinal nerve fiber layer (RNFL) thickness.
Results: In DPN+ group (n=41), ATMS and the GCL+IPL average thickness were significantly reduced in MCI versus C (respectively 24,6 Db Vs. 26,0 Db; p=0.01 and 71,5 μm vs. 79,7 μm; p=0.03). The implicit time B wave of flash response and the implicit time of flicker response were significantly increased in MCI versus C (respectively 28,4 ms vs. 30,9 ms p =0.05 and 31,1 ms vs. 22,8 ms; p= 0.05). No significant differences were observed in the DPN- group between MCI and C. In MCI patients of the DPN+ group, a positive correlation between VPT and implicit time B wave of flash response (r=0.9, p=0.04) and between VPT and the implicit time of flicker response (r=0.9, p=0.03) were observed. We also found a negative correlation between VPT and ATMS (r=-0.4, p=0.05) and between VPT and the GCL+IPL average thickness (r=-0.6, p=0.03).
Conclusion: Morphological and functional alterations of neuroretina are observed in DM2 patients affected by MCI and DPN. A significant relation between neuroretina dysfunction and peripheral neuropathy signs was also evident in MCI with DPN. These results allow to consider the retina as a window for the evolution of peripheral and central neuropathy in the patient with DM2.
Disclosure: F. Picconi: None.
715
Serum 25-hydroxyvitamin D levels in relation to the risk of peripheral neuropathy in Chinese patients with type 2 diabetes
Y. Yang, J. Wang, Y. Ding, L. Zhang;
Qingdao Endocrine&Diabetes Hospital, Qingdao, China.
Background and aims: Vitamin D deficiency is not only related to bone-related diseases but also associated with cardiovascular disease, diabetes, cancer and autoimmune diseases. Previous studies show that vitamin D deficiency increases risk of diabetic peripheral neuropathy (DPN). Data is limited among Chinese patients with type 2 diabetes.
Materials and methods: In this cross-sectional study, we enrolled 5362 patients (2359 men and 3003 women aged 30 to 89 yrs) with type 2 diabetes from Jan 1, 2016 to Oct 31, 2018. Serum 25-hydroxyvitamin D [25(OH)D] level was measured by electrochemiluminescence assay. Vitamin D deficiency was defined as serum 25(OH)D level < 20 ng/mL according to the Endocrine Society Clinical Practice Guideline 2011. Diabetic peripheral neuropathy was diagnosed by motor/sensory nerve conduction velocity.
Results: Patients with diabetic peripheral neuropathy had lower serum 25(OH)D concentration (16.81±3.83 ng/mL v.s 18.05±4.11 ng/mL, P<0.05) compared with those without neuropathy. The prevalence of vitamin D deficiency was higher in patient with neuropathy than those without the complication (83.2% v.s 71.4%, P<0.05). We did not find gender difference on the prevalence of vitamin D deficiency. In the logistical regression model, vitamin D deficiency was associated with increased risk of diabetic neuropathy, after multi-adjustment of age, gender, duration of diabetes, smoking, body mass index, systolic blood pressure, fasting glucose, HbA1c, fasting C-peptide, total cholesterol, and microalbuminuria. The corresponding odds ratio was 1.76 (1.05-3.37) (P<0.05).
Conclusion: Vitamin D deficiency, a common hypovitaminosis in Chinese patients with type 2 diabetes, is independently associated with increased risk for diabetic peripheral neuropathy. Whether vitamin D supplementation improves diabetic peripheral neuropathy deserves further study.
Disclosure: Y. Yang: None.
716
Periodic fasting might delay progression of thermal hypoalgesia in patients with type 2 diabetes and diabetic neuropathy
E. von Rauchhaupt1,2, S. Kopf1,2, Z. Kender1,2, V. Longo3,4, P.P. Nawroth1,2, J. Szendroedi1,2, A. Sulaj1,2;
1Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany, 2German Center of Diabetes Research (DZD), Neuherberg, Germany, 3Department of Biological Sciences, Longevity Institute, School of Gerontology, University of Southern California, USA, 4FIRC Institute of Molecular Oncology, Italian Foundation for Cancer Research Institute of Molecular Oncology, Milan, Italy.
Background and aims: Fasting protocols are rapidly emerging as beneficial interventions inducing ketogenesis and ketone utilization for cell energy. Preclinical studies have shown that ketogenic diets can provide protective effects to peripheral axons and sensory dysfunction. The purpose of this pilot study was to examine the effects of periodic fasting on somatosensory nerve function in patients with type 2 diabetes and diabetic neuropathy.
Materials and methods: Somatosensory nerve function was assessed in thirty-one patients with type 2 diabetes (HbA1c 7.8±1.3% [61.4±14.3 mmol/mol]) before and after a six-month diet intervention, either with a fasting-mimicking diet (FMD) (n=15) or a control Mediterranean diet (n=16). Outcome measures included clinical neuropathy disability score (NDS), neuropathy symptoms score (NSS), nerve conduction velocity and quantitative sensory testing. The pre- and post-intervention comparison was done by a two-sided paired t test with level of significance set at α = 0.05.
Results: Baseline values of clinical neuropathy scores were comparable with values after six-month diet intervention between study groups (NDS in the control group 4.0 [2.5, 5.5] vs. 5.4 [3.4, 7.4]; P=0.31 and NDS in the FMD group 3.7 [2.3, 5.2] vs. 3.4 [1.9, 4.8]; P=0.74, NSS in the control group 5.2 [3.7, 6.7] vs. 5.8 [4.1, 7.5]; P=0.39 and NSS in the FMD group 5.6 [4.0, 7.2] vs. 4.1 [1.9, 6.2]; P=0.18). No significant change was observed between study groups in nerve conduction velocity. In quantitative sensory testing the control group showed a decrease by 47% in heat pain threshold, as a parameter assessing thermal pain (Z-score -0.74 [-1.38, -0.10] vs. -1.09 [-1.72, -0.47]; P=0.03), whereas no such change was observed in the FMD group (Z-score -0.02 [-0.78, 0.73] vs. -0.47 [-1.35, 0.41]; P=0.41). Values of thermal detection, mechanical detection and mechanical pain after six months were comparable with baseline values and did not differ between study groups.
Conclusion: Six-month periodic fasting might delay progression of thermal hypoalgesia in patients with type 2 diabetes and diabetic neuropathy. The role of ketogenesis and utilization of ketone bodies for cell energy in patients with diabetic neuropathy should be further investigated in future studies.
Clinical Trial Registration Number: DRKS-ID: DRKS00014287
Supported by: German Research Foundation DFG (SFB 1118), German Center for Diabetes Research DZD (82DZD07C2G)
Disclosure: E. von Rauchhaupt: None.
717
Sciatic nerve fractional anisotropy and neurofilament light chain protein are related to sensorimotor deficit of the upper and lower limbs in patients with type 2 diabetes
Z. Kender1,2, J.M.E. Jende3, F.T. Kurz3,4, D. Tsilingiris1,2, L. Schimpfle1, A. Sulaj1,2, E. von Rauchhaupt1, P.P. Nawroth1,2, M. Bendszus3, J. Szendroedi1,2, S. Kopf1,2;
1Department of Endocrinology, Diabetology and Clinical Chemistry, Heidelberg University Hospital, Heidelberg, 2German Center of Diabetes Research (DZD), München-Neuherberg, 3Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, 4German Cancer Research Center, Heidelberg, Germany.
Background and aims: Diabetic neuropathy (DPN) is one of the most prevalent and poorly understood microvascular complication of diabetes mellitus. Recent studies have found that fractional anisotropy (FA), a surrogate marker for microstructure of peripheral nerves, is a sensitive parameter for structural and functional nerve damage in patients with DPN. The aim of this study was to investigate the significance of the sciatic nerve’s FA on different nerve fiber deficits of the upper and lower limbs and its correlation with a recently established biomarker of neuronal damage, neurofilament light chain protein (NfL).
Materials and methods: Sixty-four patients with type 2 diabetes and 27 healthy controls underwent detailed clinical and electrophysiological assessments, and complete quantitative sensory testing (QST) as well as diffusion-weighted magnetic resonance neurography (MRN) of the sciatic nerve with subsequent automated calculation of the nerve’s fractional anisotropy (FA), a marker for microstructural nerve integrity. NfL was measured in the serum of all participants.
Results: Patients with DPN showed a 19% decrease in lower sciatic microstructural integrity compared to healthy controls (p<0.001). FA correlated with tibial and peroneal motor nerve conduction velocity (NCV) (r=0.6; p<0.001 and r=0.5; p<0.001) as well as sural sensory NCV (r=0,3; p=0.04). Participants with reduced microstructural nerve integrity of the sciatic nerve showed a loss of function of mechanical and thermal sensation of the upper (r=0.3; p<0.05 and r=0.3; p<0.05) and lower (r=0.5; p<0.001 and r=0.3; p=<0.01) limb as well as reduced functional performance of the upper limb (Purdue Pegboard Test for both hands; r=0.4; p<0.001). Increased levels of NFL were associated with loss of sciatic nerve FA (r=-0.4; p<0.001). Increased HbA1c (r=-0.3; p<0.01) and microvascular surrogate markers (high sensitive Troponin T and urinary albumin-creatinine ratio) were associated with decreased FA (r=-0.4; p<0.001 and -0.4; p<0.001, respectively). There was no correlation between sciatic FA and neuropathic symptoms or pain.
Conclusion: This is the first study showing that non-invasive assessments of microstructural nerve integrity and microvascular markers are associated with nerve fiber damage in diabetic neuropathy. The nerve fiber damage of the lower limb is also associated with functional nerve fiber deficits of the upper and lower limbs, suggesting that diabetic nerve damage involves structural changes of peripheral nerves of upper limbs too. Furthermore, these findings show that proximal nerve damage is related to distal nerve function even before clinical symptoms occur.
Clinical Trial Registration Number: NCT03022721
Supported by: German Research Foundation in the Collaborative Research Center 1158
Disclosure: Z. Kender: None.
718
Dynamic contrast enhanced MRI reveals adverse effects of glucose control on perfusion of the sciatic nerve in type 2 diabetes patients with and without diabetic polyneuropathy
C.M. Mooshage1, L. Schimpfle2, Z. Kender2, J. Szendrödi2, S. Heiland3, M. Bendszus1, S. Kopf2, F.T. Kurz1,4, J.M.E. Jende1;
1Neuroradiology, University Hospital Heidelberg, 2Endocrinology, Diabetology and Clinical Chemistry (Internal Medicine 1), University Hospital Heidelberg, 3Division of Experimental Radiology, Neuroradiology, University Hospital Heidelberg, 4German Cancer Research Center, Heidelberg, Germany.
Background and aims: The pathophysiology of diabetic polyneuropathy (DPN) remains poorly understood, with a lack of causal therapeutic options. Hence, therapy is limited to optimization of serum glucose and modification of risk factors. Clinical studies have come to heterogeneous results with regard to the effectiveness of intensified glucose control as a preventive measure for DPN in patients with Diabetes Type 2 (T2D). In particular, it is still uncertain in how far glycemic control modifies microvascular permeability and perfusion in T2D patients with and without DPN. This study aimed to assess associations of glucose control with parameters of nerve perfusion in patients with T2D using dynamic contrast enhanced (DCE) MRI at 3 Tesla.
Materials and methods: 72 patients with T2D were enrolled in this study. DPN was diagnosed based on electrophysiological criteria (no DPN: 19 women, 19 men; DPN: 18 men, 2 women). Groups were matched for age, BMI, HbA1c, duration of diabetes and eGFR. A complete neurological examination, standard serological and electrophysiological testing and DCE MRI of the sciatic nerve at mid-thigh level were performed. ImageJ and MATLAB were used for post-processing and the constant of the examined nerve’s capillary permeability (Ktrans), the volume fraction of the plasma space (vp), and the volume fraction of the extravascular extracellular space (ve) were determined in accordance with the extended Tofts model.
Results: In patients with DPN Ktrans was lower compared to patients without DPN (DPN 0.034 ± 0.006 min-1; without DPN 0.041 ± 0.014 min-1; p=0.036). In patients with DPN we found positive correlations of Ktrans with HbA1c (r=0.55; p=0.015), Ve (r=0.72; p<0.001) and tibial nerve conduction velocity (NCV; r=0.45; p=0.047). No such correlations were found for age or BMI. In patients without DPN negative correlations were found for Ktrans with HbA1c (r=-0.43; p=0.012), while positive correlations were found for peroneal NCV (r=0.47; p=0.004), Ve (r=0.83; p<0.001), and BMI (r=0.45; p=0.004). In a partial correlation analysis controlled for BMI, the correlation between Ktrans and HbA1c remained significant (r=-0.42; p=0.016).
Conclusion: Ktrans, an indicator of nerve’s capillary permeability, correlated positively with HbA1c in patients with DPN, while the opposite was found in patients without DPN. These findings indicate that lower blood glucose levels in T2D patients without DPN are associated with increased capillary permeability while lower glucose levels in T2D patients with DPN are associated with a reduction of capillary permeability. Hence, our results suggest that the effect of glucose control on the capillary permeability of peripheral nerves may differ between T2D patients with and without DPN. Consequently, the effect of intensified glucose control in T2D patients with and without DPN should be addressed in further longitudinal studies.
Clinical Trial Registration Number: NCT03022721
Supported by: DFG SFB1158
Disclosure: C.M. Mooshage: None.
719
Circulating metabolites associated with diabetic sensorimotor peripheral neuropathy among individuals with type 1 diabetes
B.A. Sørland1,2, C.S. Hansen1, K. Sulek1, C. Legido-Quigley1, P. Rossing1,2, T.S. Ahluwalia1,2;
1Steno Diabetes Center Copenhagen, Herlev, 2University of Copenhagen, Copenhagen, Denmark.
Background and aims: Diabetic sensorimotor polyneuropathy (DSPN) is a common complication of diabetes mellitus associated with risk of amputations, foot ulcers and mortality. DSPN is generally diagnosed in the late stage using present screening methods. Early detection of patients at risk of DSPN development could prevent irreversible nerve damage and support clinical decision making, improving quality of life, and lowering the cost of healthcare. Metabolomics is the study of small biochemicals within the body, in the control of biologic functions such as disease progression. Here, we identified n=71 circulating metabolites and examined those for association with DSPN in subjects with type 1 diabetes (T1D). Knowledge of metabolites associating with DSPN could present new neuropathy risk factors and enable early detection of high risk patients.
Materials and methods: Non targeted serum metabolites (n = 71) and clinical data from 219 individuals with T1D from Steno Diabetes Center Copenhagen were assessed at baseline using gas chromatography coupled to mass spectrometry. Participants had a mean age of 56.4 (IQR 49.6, 64.0) years, 48.0 % (n = 105) were male, 83.6% were smokers (n = 183), 62.6% received statin treatment (n = 137), mean diabetes duration 40.9 (34.3, 47.1) years, BMI 24.7 (22.3, 26.5), HbA1c 63.1 (56.3, 59.4) mmol/mol and total cholesterol 4.8 (4.0, 5.0) mmol/L. DSPN was defined as bilateral vibration sensation threshold >=25V using Biothesiometry test. Association between single metabolites and DPSN were assessed by linear regression models fitted with and without adjustments for clinical factors (age, gender, smoking status, statin use, diabetes duration, BMI, HbA1c and total cholesterol) and corrected for multiple testing. All analysis was performed with R > 4.1.0. Benjamini-Hochberg (BH) was used to correct for multiple testing, PBH < 0.05 were considered significant.
Results: We identified 5 metabolites associated with DSPN in the unadjusted model significant after correction for multiple testing (myo-inositol, 2,4 dihydroxy-butanoic acid, ribitol, 3,4 dihydroxy-butanoic acid, ribonic acid). Increased levels of these 5 metabolites were associated with DSPN. After adjustment for clinical factors 3 metabolites were found to be associated with DSPN, that were earlier not identified by the unadjusted model (p < 0.05; arachidonic acid, heptadecanoic acid, stearic acid). Decreased levels of these 3 metabolites were associated with DSPN. Albeit these were interesting findings, the adjusted results did not go through multiple testing correction.
Conclusion: In this study we identified circulating metabolites associated to DSPN before multiple testing correction. Results suggest that circulating long chain fatty acids might have a role in DSPN pathogenesis. Further studies validating these findings and investigating their potential role using longitudinal data are warranted.
Disclosure: B.A. Sørland: None.
720
Perception threshold tracking: a novel methods for assessing the function of large and small nerve fibers in diabetic peripheral neuropathy
J. Røikjer1,2, S.S. Croosu3, J.B. Frøkjær3,4, T.M. Hansen3,4, N. Ejskjaer1,4, C.D. Mørch2,5;
1Steno Diabetes Center North Denmark, Aalborg University Hospital, 2Department of Health Science and Technology, Aalborg University, 3Department of Radiology, Aalborg University Hospital, 4Department of Clinical Medicine, Aalborg University, 5Center for Neuroplasticity and Pain (CNAP), Aalborg University, Aalborg, Denmark.
Background and aims: Small nerve fibers are important when searching for early signs of diabetic peripheral neuropathy (DPN). The current methods for assessment either have several inherent limitations or are limited to examination of the extent of structural damage. Therefore, the present study aims to assess nerve function.
Materials and methods: We utilized a novel perception threshold tracking technique to selectively assess the function of large and small nerve fibers in four age- and sex-matched groups consisting of 20 participants with type 1 diabetes (T1DM) and: painful DPN (T1DM+PDPN), painless DPN (T1DM+DPN), no DPN or pain (T1DM-DPN), and 20 healthy controls (HC). Nerve fiber function was assessed using weak electrical currents with varying intensity by two different skin electrodes activating large- and small fibers, respectively. Nerve fiber activation was indicated by participants pressing a button. The minimal current needed to activate the nerve fibers were analyzed as the rheobase. Differences between groups were analyzed by Wilcoxon Signed-rank tests.
Results: The rheobase for the large fibers was highest for T1DM+PDPN: 3.94 mA [IQR 1.99-25.0], followed by T1DM+DPN: 2.49 mA [1.74-4.09], T1DM-DPN: 1.68 mA [1.16-1.89], and HC: 1.09 mA [1.02-1.36]. The rheobase for the small fibers was highest for T1DM+PDPN: 1.09 mA [0.52-25.0], followed by T1DM+DPN: 0.78 mA [0.19-1.17], T1DM-DPN: 0.25 mA [0.14-0.45] and HC: 0.14 mA [0.08-0.24]. There was a significant difference in rheobase between all groups and fibre-types (all p<0.05), apart from between large fibres of T1DM+PDPN versus T1DM+DPN and T1DM-DPN versus HC and the rheobase of the small fibres between T1DM-DPN versus HC and T1DM+DPN versus T1DM-DPN.
Conclusion: Perception threshold tracking reveals differences in large- and small nerve fiber function between groups with and without T1DM and pain.
Clinical Trial Registration Number: NCT04078516
Disclosure: J. Røikjer: None.
SO 60 Preventing microvascular complications
721
Safe and efficient 2-year screening intervals allocated by manual and automated grading in nationwide diabetic retinopathy screening
A.D. Fleming1, J. Mellor2, W. Jiang2, A. Storkey3, C.J. Styles4, P.M. McKeigue2, H.M. Colhoun1;
1MRC University Unit for Human Genetics, University of Edinburgh, Edinburgh, 2Usher Institute, University of Edinburgh, Edinburgh, 3School of Informatics, University of Edinburgh, Edinburgh, 4Department of Ophthalmology, NHS Fife, Dunfermline, UK.
Background and aims: In 2021, following evidence from several studies, the Diabetic Eye Service in Scotland introduced a recommendation of 2-year screening interval (2YSI) for patients whose most recent two screens had no diabetic eye disease (ND). Previously the maximum recommended screening interval was 1 year. The study aims were to assess the effectiveness and safety of the 2YSI policy and to compare effectiveness and safety between automatically generated and manual decisions for 2YSI.
Materials and methods: This study used retrospective data containing DR grades (as in table) and fundus images with screening dates from October 2005 to February 2017. Patient inclusion criteria were attendance at the DR screening programme in Scotland during this time. At least yearly screening was offered to people resident in Scotland with age >12 years with diabetes. The null hypotheses were: there is no difference in the distributions of the patient-level DR grades incident during 2YSI whether simulated using manual or automated detection. 50% of subjects were used to train a ResNet101 deep neural network architecture as an automated DR grader. Its outputs were input to a thresholded logistic regression model trained with 12.5% of subjects to detect ND. In the remaining 37.5% of subjects, the test set, we determined which screens would have been omitted if the 2YSI policy had been in place. The screen after two consecutive screens with ND was classed as omitted. Manual and the automated assessments of ND were used for this and so generated two sets of omitted screens. Pearson’s chi2 tests compared the distributions, determined by manual and automated means, of grades in the omitted screens and of grades in the screens following omitted screens. If p<0.05 we planned post-hoc analyses to compare the distributions of each grade versus all others with Holm-Bonferroni correction (8 tests).
Results: There were 135194 subjects with 677871 screens in the test set (5 per subject). The automated ND assessment had 91.0% sensitivity and 82.6% specificity for detection of ND by manual assessment. Manual and automated ND assessment would omit 154818 and 159008 screens of which 1043 and 1006 had more-than-mild disease (MTMD) respectively. The distribution of disease by manual and automated ND assessment in the omitted (p<10−8) and following (p <10−3) screens are shown in the table. There was no difference for individual grades of MTMD.
Conclusion: A DR screening policy with 2YSI would have reduced screens by 23% and missed 2.9% screens with MTMD. Allocation of 2YSI by automated means is safe regarding incident MTMD in the 2YSI and is efficient in that it omitted a further 0.6% of screens. Therefore this automated DR grader could also perform screening interval allocation.
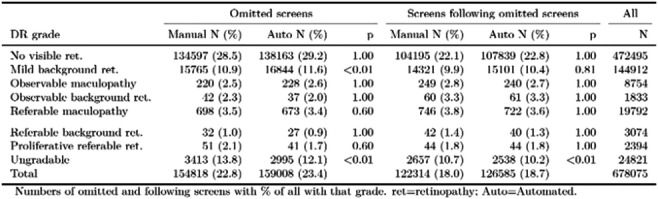
Supported by: JDRF UK
Disclosure: A.D. Fleming: None.
722
The use of the IDx-DR software as a simple screening tool for the detection of diabetic retinopathy in patients at the diabetes outpatient clinic
S. Huber1, V. Parzer1, A. Pollreisz2, B. Ludvik1, J. Brix1;
1Klinik Landstraße, 2Medizinische Universität Wien, Vienna, Austria.
Background and aims: About 200 people in Austria lose their sight every year due to diabetes mellitus. IDx-DR is an algorithm-based software for the screening of diabetic retinopathy (DR). The software provides three different outcomes: negative, moderate DR and visually threatening DR. The aim of this study was to evaluate the benefits of the IDx-DR software as a simple screening tool in diabetes patients at the diabetes outpatient clinic.
Materials and methods: This observational cross-sectional study at our diabetes outpatient clinic started in March 2021. In total, 896 people with diabetes have been included so far (46% women, 54% men, median age 57 years, median BMI 29.7 kg/m², median HbA1c 7.3%, positive smoking history 66.7% with an average of 20 pack years).
Results: A total of 62% of all patients reported an ophthalmologist visit within the last year. The IDx-DR screening showed a DR in 20% of the patients of which 50% had a visually threatening DR. In about 50% of the patients with a positive IDx-DR result no history of eye disease was known. Moreover, a significant difference was found between the baseline HbA1c level (for HbA1c values ≥ 7%) and the presence of DR (p=0.00018). Even no significance could be detected between the regularity of ophthalmological controls (related to an annual control) and the presence of DR, a higher rate was found in patients without annuals checks (15% with DR without annual control vs. 6% with DR with annual control). In 20.5%, the software did not provide a result, due to a significant association to known cataract disease (p =0.003).
Conclusion: Even in a specialized diabetes outpatient clinic, almost 40% (about 2/5) of patients did not have regular check-ups with an ophthalmologist. In 50%, a DR was newly detected, and in the case of a visually threatening DR, an immediate, specialist transfer was made. The detection ability seems to be limited in the case of a pre-existing cataract.The IDx-DR screening might be a promising tool in the early detection of DR, even for internal specialists.
Disclosure: S. Huber: None.
723
Screening of diabetic retinopathy with Aireen medical device: high sensitivity and agreement with ophthalmologists
M. Prázný1, M. Slíva2, T. Humel2, J. Bayer2, M. Šín3, R. Ženíšková3, D. Fillová4, L. Valešová5, J. Hlaváček2;
13rd Dept. of Internal Medicine, Charles University, 1st Faculty of Medicine, 2Aireen, 3Department of Ophthalmology, Military University Hospital and Charles University, 1st Faculty of Medicine, 4Eye center Prague, 5NeoVize Prague, Prague, Czech Republic.
Background and aims: Diabetic retinopathy (DR) is a frequent complication of diabetes, and the screening for DR is recommended to warrant its early diagnosis and treatment. Unfortunately, many patients with diabetes are not seen by ophthalmologists regularly. Here we report the pilot data on effectivity of screening of DR in people with Type 2 diabetes (T2DM) using the automated computer analysis of retinal photography with Aireen artificial intelligence algorithm.
Materials and methods: Aireen (Prague, CZ) is a medical device designed for the screening of DR. Aireen software algorithm was trained on 1.2 million of retinal pictures to learn to identify the signs of DR. The artificial intelligence algorithm was optimized to obtain the highest sensitivity as Aireen is considered a screening medical device. The analysis of the pictures by Aireen gives three outcomes: no signs of DR, signs of DR (check by ophthalmologist necessary), and not suitable for analysis (quality issues). In the pilot study, 1143 pictures were taken with Canon CR-2 AF Digital Non-Mydriatic Retinal Camera (Canon, Japan) from 384 T2DM patients. Pictures were analysed simultaneously by Aireen and two independent ophthalmologists specialized in retinopathies. Chi-Square test with significance level α = 0.01 was used to prove the association between the Aireen and ophthalmologists.
Results: The specificity of Aireen medical device for the screening of DR was 90.9 % and the sensitivity was 99.1 %. From the 107 pictures marked as “DR” by the ophthalmologists was only 1 picture marked as “no DR” with Aireen. The calculated χ2 with one level of freedom gives statistic 544.07 and p < 0.0001 showing significant dependency and a very high level of agreement between the Aireen’s results and ophthalmologist’s evaluation in the screening of DR (table 1).
Conclusion: The screening of DR with Aireen medical device brings a remarkably high level of correlation with ophthalmologist trained in the management of DR. This may identify patients suitable for screening of DR with ophthalmologist and in a substantial proportion limit the burden of patients and ophthalmologist from unnecessary examinations. A clinical study aimed at the screening of DR with Aireen in T2DM patients is planned to start soon and bring more data on the clinical effectivity and safety of this DR screening method.
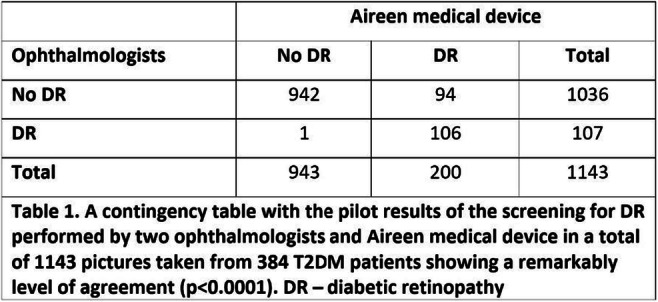
Disclosure: M. Prázný: Employment/Consultancy; Aireen, a.s.
724
Why has the incidence of certifications for sight and severe sight impairment due to diabetic retinopathy increased in Wales: Could the National Diabetes Audit hold the key?
R.L. Thomas1, A. Zeke2, R.V. North3, R. Reynolds4, G. Williams5, D. Flanagan2, C. Bunce6, S.D. Luzio1, D.R. Owens1;
1Diabetes Research Group, Swansea University, Swansea, 2Moorfields Eye Hospital, London, 3Cardiff University, Cardiff, 4Department of Ophthalmology, Aneurin Bevan University Health Board, Newport, 5Department of Ophthalmology, Swansea Bay University Health Board, Swansea, 6The Royal Marsden NHS Foundation Trust, London, UK.
Background and aims: The World Health Organisation highlighted the importance of reporting trends and progress in preventing avoidable blindness in order to evaluate the impact of different strategies. This study aims to understand the trends in the incidence of sight (SI) and severe sight impairment (SSI) certifications over a decade prior to the Covid-19 pandemic
Materials and methods: Certifications for SI and SSI in England and Wales are received by the certification’s office at Moorfields Eye Hospital, London for 2010 - 2020. National Diabetes audit (NDA) data collects data from primary and secondary care across England and Wales about the recommended 8 care processes people with diabetes receive annually (HbA1c, blood pressure, cholesterol, foot surveillance, urine albumin, serum creatinine, weight and smoking cessation).
Results: Since 2010 there has been a gradual reduction in certifications for SI and SSI in England and Wales from 72.8 to 41.3 per100,000 people with diabetes in England and from 82.3 to 55.5 per 100,000 in Wales. However, in Wales since 2016 there has been a gradual increase from 43.5 to 55.5 per 100,000 people with diabetes. Between 2011 and 2020 the number of people completing the recommended 8 care processes has fallen from 43% to 21.4% in those with type 1 diabetes and from 67.7% to 42.5% for those with type 2 diabetes in Wales and from 43.3% to 42.3%, 62.3% to 58.5% respectively in England. In those with type 1 diabetes there has been a gradual increase in those meeting the HbA1c target of ≦59mmol/mol from 22.5% to 26.3% in Wales and from 27.6% to 31.6% in England, however those reaching blood pressures targets of ≦140/80 mm Hg although initially rose from 55.3 to 74.1% in Wales between 2011 and 2013 has fallen to 65.9% in 2020 whereas in England there has been a plateau since the initial rise. For those with type 2 diabetes those reaching the HbA1c target rose to it’s highest level 64.7% in 2016-17 in Wales but has since fallen to 61.1% in 2019-20, whereas in England it has remained around 66%. For blood pressure targets those with type 2 diabetes a similar pattern was observed as for type 1 diabetes.
Conclusion: Over the last decade there has been a gradual decrease in certifications of SI and SSI due to diabetic retinopathy in both England and Wales. However, in contrast to England there has been a slight reversal in this trend in Wales since 2016 which requires further investigation. The NDA indicates the number of people with diabetes completing the 8 care processes and attaining HbA1c and blood pressure targets has been falling in Wales since 2012-13 which may have contributed to the increase in new certifications.
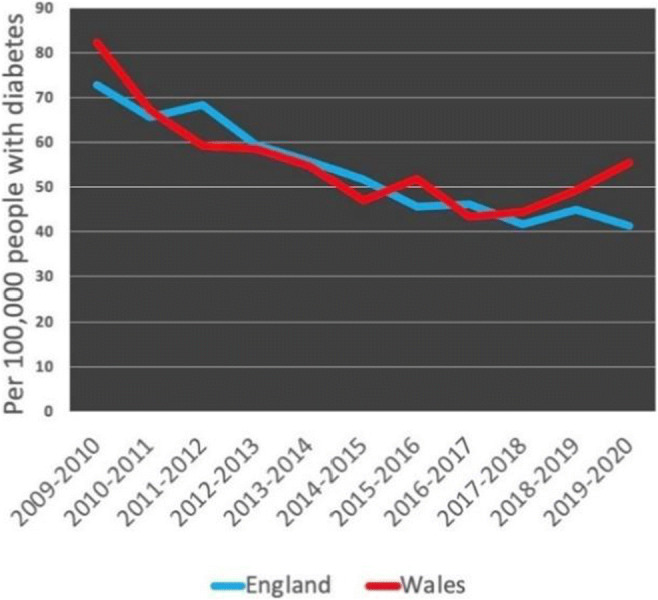
Disclosure: R.L. Thomas: None.
725
How a simple clinical scoring system complements COMPASS 31 questionnaire in predicting cardiovascular autonomic neuropathy in type 1 and type 2 diabetes
I. D'Ippolito, M. Menduni, C. D'Amato, C. Greco, M. Leoni, D. Lauro, V. Spallone;
Endocrinology, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Background and aims: The Composite Autonomic Symptom Score 31 (COMPASS 31) questionnaire has been validated for autonomic symptoms of diabetic neuropathy with a better diagnostic accuracy in type 1 than in type 2 diabetes. A clinical-based scoring system (CAN Risk Score) has been built to predict the presence of cardiovascular autonomic neuropathy (CAN) with sensitivity and specificity for CAN up to 88% and 78% in type 1 and up to 79% and 77% in type 2 diabetes, respectively. This study aimed to evaluate if the use of CAN Risk Score and COMPASS 31 in combination allows a better diagnostic performance for CAN than their single use.
Materials and methods: Seventy-one patients with type 1 (age 42±13 years, duration 25±11 years, 26 men) and 101 with type 2 diabetes (age 62±9 years, duration 10±8 years, 71 men), free from severe comorbidities, conditions affecting autonomic function, and use of beta-blockers, completed the COMPASS 31 questionnaire before undergoing four cardiovascular reflex tests (CARTs). Clinical variables were recorded to measure CAN Risk Score (based on resting heart rate, HbA1c, retinopathy, nephropathy, cardiovascular disease, and in addition on HDL cholesterol, systolic blood pressure, and smoking in participants with type 1 diabetes or insulin treatment and physical activity in those with type 2 diabetes, range 0-10). Early and confirmed CAN were defined by 1 and 2 abnormal CARTs.
Results: In participants with type 1 diabetes, both COMPASS 31 total weighted score (TWS) and CAN Risk Score were associated with CAN (Student's t test: P=0.0011 and P=0.0476, respectively) and related to CARTs results (Spearman rank correlation: P=0.0012 and P<0.0001). In participants with type 2 diabetes, CAN Risk Score was associated with CAN (P<0.0001) and was correlated to CARTs (P<0.0001) while COMPASS 31 TWS showed only borderline association with CAN (P=0.0554). Using ROC analysis to measure the diagnostic accuracy for confirmed CAN, the area under the curve (AUC) of COMPASS 31 TWS and CAN Risk Score was 0.701±0.075 (95% C.I.: 0.553-0.849) and 0.828±0.054 (0.722-0.934) in type 1 diabetes and 0.591±0.100 (0.395-0.787) and 0.867±0.058 (0.752-0.981) in type 2 diabetes, respectively. The Table shows sensitivity, specificity, positive (PPV) and negative predictive value (NPV) for confirmed CAN of COMPASS 31 TWS (at the cut-off of 16.44) and CAN Risk Score (at the cut-off of 4) when used alone or in combination in participants with type 1 and type 2 diabetes.
Conclusion: The use in combination of COMPASS 31 and a clinic-based CAN Risk Score improves their diagnostic performance for CAN in both type 1 and type 2 diabetes and can allow a stepwise screening strategy for CAN, by suggesting CAN absence with combined normality, and increasing the odds of CAN with combined abnormality.
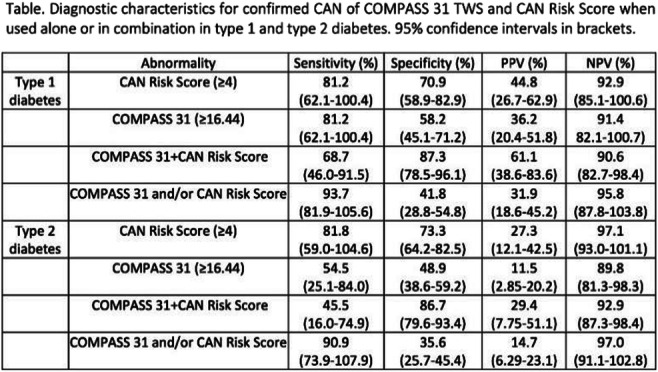
Disclosure: I. D'Ippolito: None.
726
Quantitative sensory testing may identify clinical phenotypes with high risk of progression to sensory loss in diabetic neuropathy
D. Tsilingiris1,2, L. Schimpfle1, A. Sulaj1,2, E. von Rauchhaupt1, P. Nawroth1,2, J. Szendroedi1,2, S. Kopf1,2, Z. Kender1,2;
1Department for Endocrinology, Diabetology, Metabolic diseases and Clinical Chemistry, University Hospital Heidelberg, Heidelberg, 2German Center for Diabetes Research (DZD), Munich-Neuherberg, Germany.
Background and aims: Quantitative sensory testing (QST) allows for sensory profiling in peripheral neuropathy. Sensory loss (SL) is the most common phenotype in diabetic neuropathy (DN), followed by mechanical hyperalgesia (MH) and thermal hyperalgesia (TH), Sensory loss in the lower extremities is related to the manifestation of the diabetic foot syndrome. Our aim was to assess the evolution of lower extremity sensory phenotype among patients with diabetes mellitus (DM) and investigate the course of sensory loss development in the frame of DN.
Materials and methods: We studied a cohort of 287 individuals with DM (Age 59.3±13.9, 38.3% females, 21.3%, T1DM, 78.7% T2DM), of which 225 attended follow-up visits after 1, 2 and 4 years. Allocation into four sensory phenotypes (healthy, TH, MH, and SL) at every timepoint was based on the z-scores of the 13 domains of QST on the right foot, according to published algorithms. Additional measures included the clinical neuropathy assessment (via the neuropathy symptom and disability scores-NSS/NDS, according to clinical practice guidelines) and nerve conduction studies of Suralis, Peroneus and Tibialis nerves.
Results: Among all participants studied at baseline, 76 (26.5%), 84 (29.3%), 69 (24.0%) and 58 (20.2%) were categorized as Healthy, TH, MH, and SL, respectively. The overall prevalence of clinical neuropathy based on NSS/NDS was 33.8%, and across the groups 9.2%, 21.4%, 42.0% and 74.1%, respectively (p for trend <0.001). Accordingly, electrophysiological studies showed an increasing severity of neuropathic findings across heathy, TH, MH, and SL groups. After a mean follow-up of 2.68±1.30 years, 119 of 225 longitudinally followed-up participants showed a cluster change. Those classified as healthy at baseline changed predominantly to TH (37.7%, p< 0.05 vs. MH or SL), those as TH at baseline to MH (32.3%, p< 0.05 vs. Healthy or SL), those with SL on baseline to MH (20.9%, p= 0.01 vs. healthy, p= 0.08 vs. TH), while those with MH changed equally towards TH and SL (28.6% each, p=0.003 vs. healthy). Among those without SL on baseline, there were 35 new cases of SL in the course of follow-up, corresponding to an incidence of 8.13 cases/100*person*years. Compared to the healthy subgroup, the HRs for SL occurrence were 3.40 (95% c.i. 1.10-10.56, p=0.034) for those classified at baseline as TH and 6.06 (2.06-17.83, p=0.001) for those as MH. After adjustment for age and gender, only the MH phenotype remained a significant predictor of SL development (aHR 4.44, 1.48-13.36, p=0.007). Among baseline QST values, the z-score of cold detection threshold (zCDT) showed the best performance for identification of SL occurrence (AUC ROC 0.729, zCDT <-1.00: Sensitivity 70.2%, Specificity 77.1%).
Conclusion: Our findings signify thermal and mechanical hyperalgesia as potential consecutive transitional phenotypes in the course between healthy and sensory loss in DN. This may have profound implications for the use of QST for identification of high-risk patients and primary prevention of diabetic foot, while it may further serve to identify those most likely to benefit from current and future interventions to prevent DN progression.
Clinical Trial Registration Number: NCT03022721
Supported by: SFB 1158
Disclosure: D. Tsilingiris: None.
727
Minimal resource pre-screening tools for chronic kidney disease in people with type 2 diabetes: a global validation study
R. Sisk1, C. Sammut-Powell1, J. Budd1, R. Cameron1, M. Edge1, E. Vazquez-Mendez2, A. Bedenkov3, H. Vasnawala4, S.B. Goncalves5;
1Gendius Ltd, Macclesfield, UK, 2AstraZeneca, Mexico City, Mexico, 3AstraZeneca, Cambridge, UK, 4AstraZeneca, Bangalore, India, 5AstraZeneca, Buenos Aires, Argentina.
Background and aims: Type 2 diabetes (T2DM) is a key risk factor for the development and progression of chronic kidney disease (CKD), and diabetes is now the leading cause of CKD and kidney failure globally. Regular screening is recommended within diabetic populations to identify and manage undiagnosed CKD. Many patients do not undergo screening according to the recommended protocols and frequency.We have, therefore, developed pre-screening models using UK general practice data that identify people with T2DM most at risk of having reduced kidney function (eGFR < 60ml/min/1.73m2) or albuminuria (urine albumin to creatinine ratio (UACR) >= 30mg/g) using readily available patient information. These models can be used to better target CKD screening within T2DM populations, particularly in low- and middle- income countries where resources may be limited. The goal of the present study is to validate our models within global populations, and identify any regional variation in performance.
Materials and methods: We applied our eGFR model to the 3-year observational DISCOVER data study (covering 37 countries in 6 regions globally), and our UACR model to the AstraZeneca TakeCaReofMe programme data (5 countries), and quantified the positive predictive value (PPV) at thresholds that ensured a sensitivity of at least 80%. We compared minimal-resource (MR) models (using only easily obtained patient information) against the screen-all approach using a complete case analysis.
Results: 5123 patients had valid serum creatinine values and were included in the validation of the eGFR model. The screen-all approach for eGFR<60ml/min/1.73m2 resulted in a PPV of 9.5% [95% CI: 8.7% -10.2%]. Using our MR model, this increased to a PPV of 14.5% [95% CI:13.1% - 15.8%] (a relative increase of 53.0%) at a sensitivity of 83.8% [95% CI: 80.3%, 87.2%].9720 patients were included in the UACR validation. The screen-all approach for UACR >= 30mg/g resulted in a PPV of 33.5% [95% CI: 32.6% - 34.4%]. The MR model increased the PPV to 36.2% [95% CI: 35.2% - 37.2%] (a relative increase of 8.0%) at a sensitivity of 81.8% [95% CI: 80.6% - 83.3%]. Regional variation was observed across both renal measures
Conclusion: It is possible to pre-screen people with T2DM for kidney function impairment using models that require only basic patient information, and models developed using UK data perform well in global populations. These models could be used to conduct more targeted screening where resources are limited, and to prioritize screening of high-risk individuals. The models should be adapted and re-calibrated to local populations to ensure adequate performance. Our model to detect albuminuria did not offer significant gains in PPV. This finding highlights the importance of following the recommended guidelines on UACR screening for all people with T2DM.
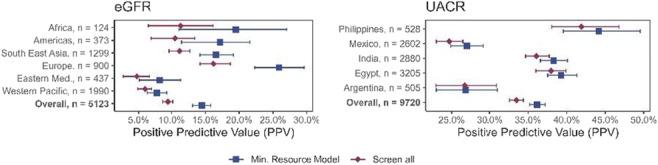
Disclosure: R. Sisk: None.
SO 61 Saving the feet
728
Temporal trends in major, minor and recurrent lower extremity amputations in Belgium from 2009 to 2018
P. Lauwers1, K. Wouters2, H. Avalosse3, J. Vanoverloop4, F. Nobels5, I. Dumont6, G. Matricali7, P. Felix8, J. Hendriks1, E. Dirinck9;
1Department of thoracic and vascular surgery, Antwerp University Hospital, Antwerp, 2Clinical Trial Center, Antwerp University Hospital, Antwerp, 3Landsbond der Christelijke Mutualiteiten/Alliance Nationale des Mutualités Chrétiennes, Brussel, 4Intermutualistisch Agentschap/Agence Intermutualiste, Brussel, 5Department of endocrinology, Onze Lieve Vrouw Ziekenhuis Aalst, Aalst, 6Centre Multidisciplinaire du Pied Diabetique, Ransart, 7Department of orthopedic surgery, Leuven University Hospital, Leuven, 8Department of endocrinology, CHR de la Citadelle, Liège, 9Department of endocrinology, diabetology and metabolism, Antwerp University Hospital, Edegem, Belgium.
Background and aims: In Western countries, diabetes-related complications are the main indication for lower extremity amputations (LEAs). Multidisciplinary diabetic foot clinics (MDFCs) have been implemented to reduce this number. Temporal trends in the incidence of total, major, and minor LEA in people with and without diabetes from 2009 to 2018 in Belgium, and secondary amputation rates were studied.
Materials and methods: Data on all Belgian citizens, both those undergoing LEA and those remaining amputation-free, were collected from the InterMutualistich Agentschap/Agence InterMutualiste, Sex- and age-adjusted annual incidence rates were calculated. Time trends were analysed in negative binomial models. Secondary interventions, defined as an amputation executed on the ipsilateral and/or contralateral limb at any point later in time after the initial amputation, were also studied , with death as competing risk.
Results: In individuals with diabetes, the amputation risk decreased from 143.6/100 000 person-years in 2009 to 109.7 in 2018 (p<0.001). The incidence of major LEAs decreased from 56.2 to 30.7/100 000 person-years (p<0.001); the incidence of minor amputations showed a declining trend in women (54.3 to 45.0/100 000 person- years, p=0.024), but didn’t change significantly for the total population (99.9 to 87.5/100 000 person-years). In individuals without diabetes, the incidence of major amputation didn’t change significantly (6.1 to 5.1/100 000 person-years), whereas minor amputation incidence increased (8.0 to 10.6/100 000 person-years, p<0.001). In individuals with diabetes, one-year secondary intervention rates were high (31.3% after minor LEA, 18.4% after major LEA); the incidence of secondary amputations didn’t change.
Conclusion: In Belgium, with nationwide accreditation of MDFCs since 2005, there was a significant decline in the incidence rate of major LEA in people with diabetes between 2009 and 2018. This decline is particularly successful since it was not accompanied by a significant rise in minor LEA. Nevertheless, secondary interventions were frequent and their incidence remained stable.
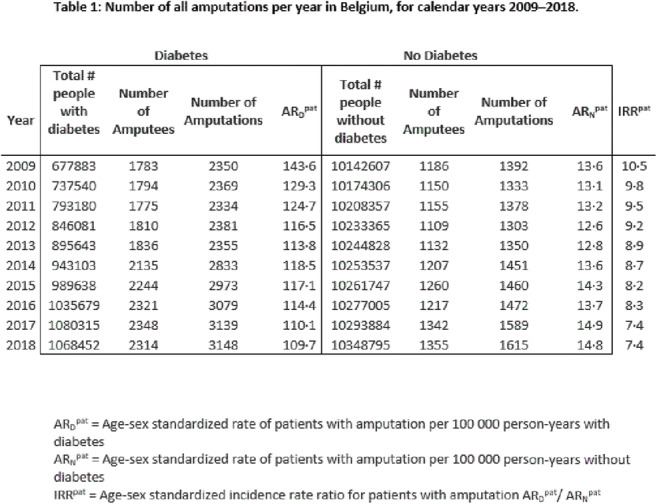
Disclosure: P. Lauwers: None.
729
Mortality rates after lower extremity amputation in Belgium from 2009 to 2018
E. Dirinck1, K. Wouters2, H. Avalosse3, J. Vanoverloop4, F. Nobels5, I. Dumont6, G. Matricali7, P. Felix8, J. Hendriks9, P. Lauwers9;
1Antwerp University Hospital, Antwerp, 2Clinical Trial Center, Antwerp University Hospital, Antwerp, 3Landsbond der Christelijke Mutualiteiten/Alliance Nationale des Mutualités Chrétiennes, Brussel, 4Intermutualistisch Agentschap/Agence Intermutualiste, Brussel, 5Department of endocrinology, Onze Lieve Vrouw Ziekenhuis Aalst, Aalst, 6Centre Multidisciplinaire du Pied Diabetique, Ransart, 7Department of orthopedic surgery, Leuven University Hospital, Leuven, 8Department of endocrinology, CHR de la Citadelle, Antwerp, 9Department of thoracic and vascular surgery, Antwerp University Hospital, Antwerp, Belgium.
Background and aims: Diabetes mellitus and peripheral arterial disease are the main indication for lower extremity amputations (LEA) in Western countries. Reported mortality rates after LEA are high, particulaly with more proximal amputation level and older age. The influence of diabetes is more ambiguous. In this national study, short and long term mortality after minor and major LEA in people with and without diabetes, compared to controls without LEA, was assessed between 2009 to 2018. Temporal trends in one-year mortality rates were studied as well.
Materials and methods: Data on all Belgian citizens undergoing LEA in the study period, and a group of matched controls without LEA, were collected. Age- and sex adjusted survival curves were calculated. A Cox proportional hazard model was used to estimate the relative likelihood of mortality after LEA in individuals with and without diabetes, after correction for age, gender, and district. Matched individuals with and without diabetes but remaining amputation-free were used as controls. Time trends were analysed in negative binomial models.
Results: 41304 amputations (13247 major, 28057 minor) were performed in 26526 individuals. Thirty-day, 1-year and 5-year mortality rates (deaths per 1000 individuals) in people with diabetes after major amputation were 13, 35 and 67, and 4.7, 20 and 51 after minor amputation. In people without diabetes, thirty-day, 1-year and 5-year mortality rates after major amputation were 17, 38 and 65, and 4.9, 21 and 48 after minor amputation. Diabetes was significantly related to reduced survival: hazard ratios for mortality for controls without LEA, minor LEA and major LEA were 1.77 (95% CI 1.56-2.00), 1.40 (95% CI 1.24-1.58) and 1.26 (95% CI 1.12-1.41) respectively. Over the ten-year study period, mortality rates after LEA decreased only for individuals without diabetes.
Conclusion: Early and late mortality rates after LEA were high in Belgium, both in individuals with and without diabetes.
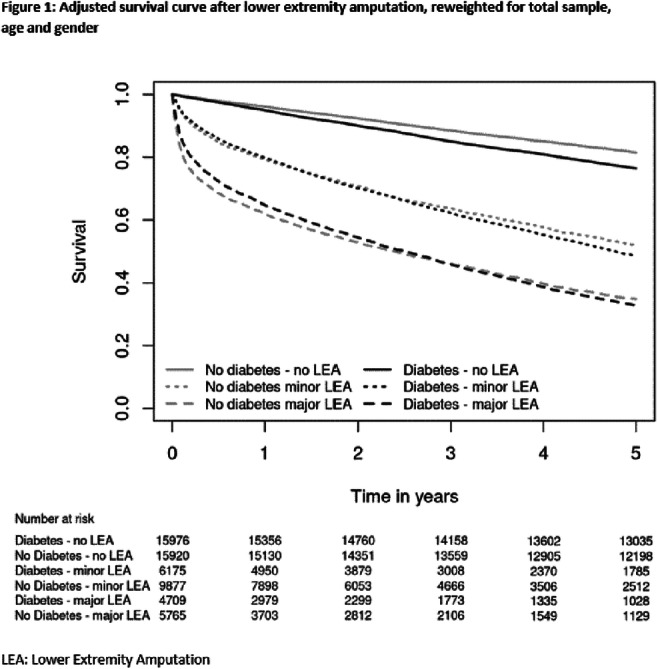
Disclosure: E. Dirinck: None.
730
The association between mortality and use of SGLT-2 inhibitor and GLP-1 receptor agonists younger people with type 2 diabetes and a first PAD diagnosis between 2016 and 2020
E. Uddman1, S. Puvaralingam2, K. Filipsson3, M. Löndahl3;
1Clinical Sciences in Lund, Lund University, 2Clinical Sciences in Lund, Shobitha Puvaralingam, 3Clinical Sciences in Lund, Lund university, Lund, Sweden.
Background and aims: Type 2 diabetes as well as peripheral artery disease (PAD) are significant risk factor for mortality. In people with type 2 diabetes and higher risks of cardiovascular disease, several large randomised controlled trials have identified a reduction in cardiovascular mortality and non-fatal cardiovascular complications after initiating treatment with sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP1-RA). However, the usefulness of these drugs, particular SGLT-2 inhibitors, in people with PAD are less certain. This study aimed to evaluate the effect of prescription of SGLT-2 inhibitors and /or GLP1-RA on all-cause mortality in people younger than 70 years with type 2 diabetes and PAD.
Materials and methods: This study is an observational, population-based cohort study of individuals with type 2 diabetes, aged between 40 and 69 years, and a first diagnosis of PAD between January 2016 and December 2020 in Sweden using data from national registries. People with type 2 diabetes were identified in the National Drug Prescription Registry and defined as people who at least twice had been prescribed an anti-diabetic drug. Prescription of anti-diabetic and antihypertensive drugs and date of the collection were registered. Data on comorbidities and cardiovascular events were collected from the National Patient Registry, which contains nationwide hospital discharge information since 1987. Information on the time of death was retrieved from the Swedish Cause of Death Register. Patients were grouped according to the use of SGLT-2 inhibitors, GLP1-RA, or no prescription of any of these drugs. The primary endpoint, all-cause mortality, is presented as Kaplan-Meier curves, and statistical differences were evaluated using the Log-Rank test.
Results: Of the 2496 identified patients below 70 years of age with a history of type 2 diabetes and a first diagnosis of PAD between 2016 and 2020, 1403 (56.2%) had been prescribed neither an SGLT-2 inhibitor nor a GLP1-RA, while 13.6% was prescribed an SGLT-2 inhibitor, 13.5% a GLP-1 RA and 16.7% drugs from both these classes. During the follow-up period (median 2.9 years), 566 events (22.7%) occurred. The five-year mortality is given in Figure 1.
Conclusion: In this population of younger people with type diabetes and a first diagnosis of PAD between 2016 and 2020, the lowest mortality was seen in the group prescribed both a GLP1-RA and an SGLT-2 inhibitor, followed by single-use of an SGLT-2 inhibitor. These data suggest that early use of SGLT-2 inhibitors in people with type 2 diabetes and PAD seems to be efficient.
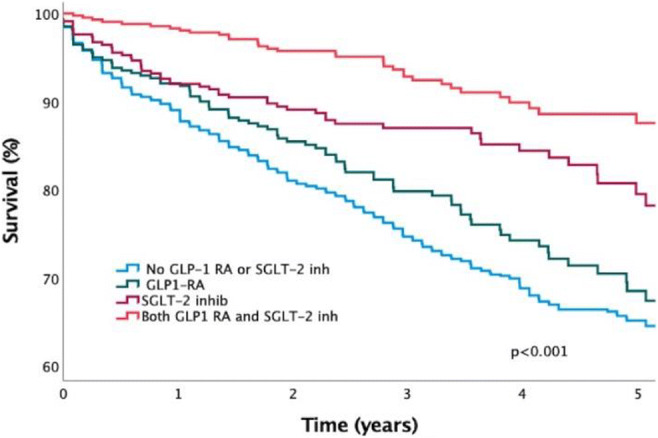
Supported by: M Löndahl unrestricted grant from Boehringer-Ingelheim
Disclosure: E. Uddman: None.
731
The effectiveness of an affordable Toa Uzito (offloading) device for improving diabetic foot ulcer in the developing world
Z.G. Abbas1,2, J.K. Lutale1, L.K. Archibald3;
1MUHAS, Dar es Salaam, United Republic of Tanzania, 2AMC, Dar es Salaam, United Republic of Tanzania, 3University of Florida, Gainesville, USA.
Background and aims: Diabetic foot ulcer (DFU) disease is common in Sub-Saharan Africa (SSA). Callus formation, often seen in persons with peripheral neuropathy, plays a key role in the pathogenesis of DFU by increasing local pressure in susceptible areas on the plantar aspect of the foot. Because callus formation can inhibit DFU healing, offloading remains one of the most useful conservative measures to facilitate DFU healing. Prohibitive costs and paucity of personnel with the required training to implement and manage off-loading devices often preclude this therapeutic option in SSA. For all these reasons, we devised an offloading device that requires no special training to use, is easy to implement, and is very affordable (10 US cents each). We named this device Toa Uzito (Swahili translation for offloading) and carried out this study to ascertain its utility and effectiveness in the management of DFU in Dar es Salaam, Tanzania.
Materials and methods: We carried out a prospective study of persons with plantar DFU who attend a specialist diabetes service in Dar es Salaam, Tanzania. During Feb 2021 - Nov 2021 (study period), and following informed consent, we identified DFU patients (cases) whose management included application of the Toa Uzito off-loading device. Controls were randomly selected patients with plantar DFU who were managed in a similar manner but without the off-loading device. Aggregated clinical and epidemiologic data included demography, initial DFU size, duration of diabetes, and time to complete healing. Data analyses were performed using SAS 9.4 Statistical Software (SAS Institute Inc, Cary, NC, USA). A Cox proportional hazards regression model was used to relate various exposures, considered simultaneously, to time taken for complete healing.
Results: During the study period, 92 patients were managed with the Toa Uzito off-loading device and 1518 control-patients were randomly selected. Cases and controls were similar for sex, type of diabetes, continuous variables (age; BMI; diabetes duration; delay in seeking medical attention), and site of the DFU. Initial mean DFU size was significantly higher in case-patients when compared with controls (3,166 vs. 999 mm2: p <0.0001). The mean number of days to total healing for persons on off-loading was 117 days versus 161 days for persons not treated with offloading (p=0.08). On multivariate analysis, the p-value for offloading was 0.0055 (Hazard Ratio= 0.74), indicating a strong relationship between the use of the Toa Uzito device and decreased time to attain complete ulcer healing. The effectiveness of the Toa Uzito off-loading device is underscored by the fact that the initial size of DFU in cases treated with off-loading was significantly higher than controls to start with.
Conclusion: Toa Uzito is an affordable offloading device and an effective adjunct to DFU management. It renders instant offloading and contributes positively towards DFU healing with potential reduction of amputation rates. This device is simple to implement and is easily adaptable for use at the grass root level anywhere in the developing world.
Disclosure: Z.G. Abbas: None.
732
Exploring MMP9 as a prognostic marker in post debridement wound fluid of diabetes related foot ulcers, and the possibility of a point-of-care test
M.S.G. Longfield1, V. Nube2,3, J.M. White2,3, S.V. McLennan1,4, P. Boughton5, D. Min1,2, S.M. Twigg1,2;
1Greg Brown Diabetes and Endocrinology Research Laboratory, University of Sydney, 2Department of Endocrinology, Royal Prince Alfred Hospital, 3Podiatry Department, Sydney Local Health District, 4NSW Health Pathology, 5Sydney Spine Institute & Sydney Pharmacy School, University of Sydney, Sydney, Australia.
Background and aims: While most diabetes related foot ulcers (DRFU) heal with conservative management, a significant minority do not. Wound grading and wound closure rate (WCR), have some predictive value, helping to identify hard to heal wounds, but a definitive marker of healing has not been integrated into clinical assessment. Evidence in the literature suggests that the pro-inflammatory protein, matrix metalloproteinase-9 (MMP9), is elevated in post debridement wound fluid in DRFU less likely to heal. Therefore, a point-of-care test (POCT) for MMP9 could offer at clinic ulcer healing prediction to guide therapy. This study aimed to determine whether MMP9 concentration in post debridement wound fluid has clinical utility as a predictor of healing outcome and whether a rapid POCT for MMP9 can be adapted and utilised in this setting.
Materials and methods: Post debridement wound fluid was collected by Paperpoint® from 28 DRFU managed at our high risk foot service, as a sub-study of the Diabetes Debridement Study, at three time points, initial visit, week 2, and week 4. Clinical ulcer healing by 12 weeks was then determined. Total MMP9 was quantitated in would-fluid samples by ELISA and the pro and active MMP9 measured by gelatin-zymography. Subsequently, an MMP9 POCT obtained from a commercial source, for tear fluid measures of total MMP9 was adapted to assess its ability to predict DRFU outcome.
Results: Overall, 10 DRFU healed (H) and 18 failed to heal (NH) by 12 weeks. Of those with available samples at week 0 (nH=7, nNH=14), active MMP9 was significantly higher in NH compared to H (10.7(6.02-24.6) vs 2.86(1.91-3.43)ng/mL, P=0.036), with no statistically significant difference in total MMP9 or pro MMP9 These active MMP9 differences were predictive of wound healing with AUROC of 0.786 (P=0.037). At week 4 (nH=5, nNH=16), active MMP9 was no longer differentially expressed between groups however total MMP9 was found to be higher in NH wounds (209 (87.6-335) vs 20.6(9.57-62.9)ng/mL, P=0.015), and was also predictive wound healing with AUROC of 0.867 (P=0.016). Both findings show clinical merit in measuring MMP9 or its derivatives to predict week 12 healing outcome. However, these laboratory-based methods of MMP9 measurement yield little clinical value due to the required equipment and time frame of result turn around. From the ELISA results a 1:2 dilution was determined to test the efficacy of the POCT in wound fluid. Results showed 90.5% accuracy (19/21 correct) reflecting the ELISA results, and a predictive ability for DRFU healing with 71.4% true readings consistent with ROC predictions.
Conclusion: These results indicate that whilst active MMP9 may have an earlier predictive capacity, total MMP9 measured at week 4 shows more clinical utility because of the availability of a relevant POCT. Future large clinical studies should aim to evaluate the POCT in a bedside manner, and thresholds should be examined in fresh collected wound fluid. Additionally, there is potential benefit to designing a POCT, for active MMP9, to predict healing at earlier time-points.
Clinical Trial Registration Number: ACTRN12618000703202
Supported by: TRGS, SMSF-EDRF University of Sydney
Disclosure: M.S.G. Longfield: None.
733
Effect of human amniotic membrane on umbilical vein endothelial cells of gestational diabetic mothers: new insight in wound healing and diabetic foot ulcer
C. Pipino1, I. Cappellacci1, P. Di Tomo1, A. Bernabé-García2, J. Stelling-Férez2, N. Di Pietrantonio1, L. Pelusi1, M.P.A. Baldassarre3, F.J. Nicolás2, A. Pandolfi1;
1Dept. of Medical, Oral & Biotechnological Sciences, University "G. d'Annunzio" Chieti-Pescara, Chieti, Italy, 2Regeneration, Molecular Oncology and TGFß, IMIB-Arrixaca, 30120, El Palmar, Murcia, Spain, 3Department of Medicine and Aging Sciences, University "G. d'Annunzio" Chieti-Pescara, Chieti, Italy.
Background and aims: Diabetic foot ulcer (DFU), a severe diabetes complication affecting 15% of patients with advanced stages of the disease, is the main cause of abnormal wound healing leading to lower limb amputation. This condition, characterized by chronic inflammation and oxidative stress, results in reduced peripheral blood flow and neovascularization. Amniotic membrane (AM) has biological properties that have been found beneficial to the wound healing process in DFU. Here, the potential role of AM on endothelial cells isolated from umbilical cords of gestational diabetes affected women (GD-HUVECs) has been investigated. Indeed, GD-HUVECs have shown a pro-inflammatory phenotype, reduced migration capability and lower vessel formation on Matrigel compared to control HUVECs (C-HUVECs), thus representing a valuable model for studying the role of AM in cell migration and neovascularization of chronic non-healing wounds.
Materials and methods: For GD-HUVECs, stimulated with low doses of the pro-inflammatory Tumour Necrosis Factor-α (TNF-α, 1ng/ml), monocytes adhesion assay was performed to assess the adhesion of monocytes to endothelial cells. In parallel, Vascular and Intercellular adhesion molecules 1 (VCAM-1, ICAM-1), E-selectin (SELE) and chemokine CC ligand-2 (CCL2) gene expression were evaluated by real time PCR. VCAM-1 and ICAM-1 membrane exposure was analysed by flow cytometry. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) nuclear translocation was evaluated by immunofluorescence staining. Nitric oxide/cyclic guanosine monophosphate (NO/cGMP) levels were measured by ELISA. Cell migration was performed by scratch assay and by measuring proteins involved in the focal adhesion turn-over such as Paxillin and Vinculin. Vessel formation was evaluated by Matrigel tube formation assay.
Results: AM significantly reduced TNF-α stimulated monocyte-endothelium interaction and VCAM-1, ICAM-1, SELE and CCL2 expression in C- and GD-HUVECs (p<0.0001 and p<0.01, respectively). That was further confirmed by a reduction on membrane exposure of VCAM-1 and ICAM-1 in both cell types following AM stimulation. Of note, this was associated with a significant decrease of NF-kB cytoplasm-nucleus translocation. Furthermore, AM treatment improved cell migration and vessel formation in GD-HUVECs. Finally, AM-increased NO bioavailability (p<0.01) was affected by endothelial Nitric Oxide Synthase (eNOS) inhibitor L-NAME, suggesting that AM activation of eNOS could be a possible way of regulating this phenomenon. ANOVA and Tukey’s multiple comparison tests were used (n≥3, significance p≤0.05).
Conclusion: Overall these results indicate that, in our in vitro cell model, AM attenuates TNF-α-increased inflammation and improves angiogenesis, possibly through the modulation of NO bioavailability, which plays a key role in the vascular homeostasis balance. This study suggests that AM chronic wound healing improvement may be due to endothelial proper management, therefore explaining its clinical benefit on diabetic foot ulcers.
Disclosure: C. Pipino: None.
734
Proteomic signature reveals potential diagnostic biomarkers for chronic diabetic foot ulcerations
M. Petkovic1, A.E. Sørensen1, E.C. Leal2, R.J. Willemoes1, H. Jenssen1, T.S. Ahluwalia3,4, A. Rasmussen5, E. Carvalho2, L.T. Dalgaard1;
1Roskilde University, Roskilde, Denmark, 2University of Coimbra, Coimbra, Portugal, 3Copenhagen University, Copenhagen, Denmark, 4Department of Biology, University of Copenhagen, Copenhagen, Denmark, 5Steno Diabetes Center Copenhagen, Copenhagen, Denmark.
Background and aims: Chronic foot ulcers (DFU) are a common problem associated with diabetes. Healing outcome is challenging to anticipate in clinical settings, complicating personalized treatment; thus, efficient predictive biomarkers are needed to improve DFU risk stratification and treatment. We aimed to evaluate the proteomic profile of mouse diabetic skin biopsies and human wound swabs from newly detected DFUs and identify prognostic biomarkers for chronic wounds.
Materials and methods: Diabetes was induced in C57BL/6 mice using 5 low dose streptozotocin injections, and skin punch biopsies (n=5) were harvested at days 0, 3 and 10 post-wounding. MicroPredict Study involved 112 diabetic patients with DFU, during a six-month accrual period, admitted to the foot clinic, Steno Diabetes Center Copenhagen. Enrolled patients were categorized according to healing time: rapidly healing (RH, <40 days), slow-healing (SH, >40 days; <6 months) and chronic (CH, >6 months). Wound sampling was performed by brush grafting. Patient wounds (12 RH and 12 CH), matched for sex and age, were preliminarily assessed. Potential proteomic hints of DFU were identified using Liquid Chromatography-Mass Spectrometry (LCMS). MaxQuant analysis was followed by Gene ontology pathway analysis using Perseus.
Results: The animal model revealed the downregulation of adherens and anchoring junctions Elongation factor 1-α2 (EEF1A2), Filamin-C (FLNC), Galectin-1 (LGALS1), Periplakin (PPL) and Filaggrin-2 (FLG2) (3-5-fold) while catalytic Creatine kinase 2 (CK2) and Adenylate kinase 1 (AK1), were 2-fold upregulated in 3 days wounds. The advanced stage of 10 days post wounding was characterized by lowered levels of tubulin chains (TUBA1B) and Myosin-9 (MYH9), while levels of COLL 1A/2A were elevated. Mice skin proteome pathway analysis revealed > 100x upregulated phagocytic activities, at day 3, and cytoskeletal rearrangements 35-fold upregulated 10 days post-wounding. Human swaps analyses identified persistently upregulated NETosis-associated Neutrophil elastase (NE) and lactoferrin (LF), as well as 2-fold higher levels of dermcidin, in CH compared to the RH group. SERPINs D1/ B4 were exclusively detected in the CH wounds, in contrast to tissue repair SERPIN A1/A3, and angiogenic SERPINF1, present in RH wounds only. Furthermore, neutrophil degranulation Annexin A3 was solely detected in the CH. Immunomodulators S100A8/A9 were detected at higher levels in CH over RH wounds. GO: CC and GO: BB pathway analysis revealed over 70-fold upregulation of neutrophil defensin 3 (DEFA3) in RH compared to CH wounds.
Conclusion: The proteomic profile of diabetic mice wounds revealed a decrease in cell adhesion junctions and an increase in phagocytic vesicle processing at the inflammatory wound stage, while predominant proteins in the later healing stages were associated with cytoskeletal rearrangements. The human study showed substantial differences between RH and CH wounds, with pronounced apoptosis, catalytic activity, and host-defence responses increased in CH. Our findings indicate potential biomarkers of chronic wounds for a better, personalized approach, thus merit further investigation.
Clinical Trial Registration Number: H-17018891
Supported by: EFSD/Lilly EXPAND Programme, DDA, SPD
Disclosure: M. Petkovic: None.
735
Nomogram model for predicting the risk of sepsis in diabetic foot patients
P. Huang, X. Zhao, Y. Gu;
Affiliated Hospital of Nantong University, Nantong, China.
Background and aims: To establish an individualized nomogram model for predicting the risk of sepsis in diabetic foot (DF) patients, and provide a reference for clinical prevention and treatment.
Materials and methods: A total of 144 DF patients hospitalized from March 2019 to February 2022 were enrolled in this retrospective study. The general clinical and anthropometric information of participants including height, weight, waist circumference, duration of diabetes and so on were collected. Fasting plasma glucose, glycosylated hemoglobin, serum albumin and other blood biochemical parameters were detected. The pathogenic bacteria in serum and at the site of infection were detected. Firstly, random forest analysis was used to find the primary predictive factors of sepsis in DF patients. Secondly, multiple factor logistic regression analysis was employed to screen the independent influencing factors of sepsis. Thirdly, a nomogram containing these factors was established to predict the sepsis incidence in DF patients. Finally, the performance of the prediction model was evaluated and verified by receiver working characteristic (ROC) curve, corrected calibration curve, and clinical decision curve.
Results: Logistic regression analysis showed that CRP, hypoproteinemia, and wagner ≥ 3 were risk factors for sepsis in DF (P < 0.01). The area under the ROC curve (AUC) of the nomogram model after internal verification was 0.974 (0.953-0.995). The P value of Hosmer-Lemeshow test was 0.98, indicating that the predicted probability of sepsis in the nomogram was consistent with the actual occurrence of sepsis. The standardized net benefit of the nomogram was highest when the predicted probability was between 0.1 and 1.0.
Conclusion: The risk factors for sepsis in DF are CRP, hypoproteinemia, and wagner ≥ 3. The nomogram model drawn on these risk factors has good predictive accuracy and can assist clinicians in formulating targeted sepsis prevention strategies for patients.
Clinical Trial Registration Number: 2018-k016
Supported by: Nantong Municipal Science and Technology Project (MS22019005)
Disclosure: P. Huang: None.
SO 62 Brain, nerve, and heart interaction
736
Functional brain alterations in regions involved in sensory processing in diabetic peripheral neuropathy and neuropathic pain
S.S. Croosu1,2, J. Røikjer2,3, C.D. Mørch3, N. Ejskjaer2,4, J.B. Frøkjær1,4, T.M. Hansen1,4;
1Department of Radiology, Aalborg University Hospital, 2Steno Diabetes Center North Denmark, Aalborg University Hospital, 3Center for Neuroplasticity and Pain (CNAP), SMI, Department of Health Science and Technology, Aalborg University, 4Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Background and aims: The pathogenesis of diabetic peripheral neuropathy and neuropathic pain are poorly understood, but the involvement of the central nervous system has been suggested. This study investigated functional connectivity in brain regions involved in sensory processing, including thalamus, primary somatosensory cortex, and insula in type 1 diabetes mellitus with and without diabetic peripheral neuropathy (DPN) and neuropathic pain. The associations between the functional connectivity to peripheral nerve function and pain scores were further investigated.
Materials and methods: This cross-sectional study included 60 individuals with type 1 diabetes and 20 healthy controls. Resting-state functional MRI was utilized to investigate the functional connectivity of thalamus, primary somatosensory cortex, and insula to other brain areas. Nineteen individuals with type 1 diabetes and neuropathic pain, 19 with type 1 diabetes and DPN, 18 with type 1 diabetes without DPN, and 20 healthy controls were included in the seed-based connectivity analysis. The connectivity parameters were used for correlation analysis to peripheral nerve functions and pain scores.
Results: Overall, the thalamus and primary somatosensory cortex showed higher connectivity to motor areas in diabetes individuals without DPN compared to diabetes individuals with neuropathic pain and healthy controls (all p≤0.029). Poorer peripheral nerve functions and higher pain scores were associated with lower thalamus-to-motor area connectivity and lower primary somatosensory cortex-to-motor area connectivity (all p≤0.043). No connectivity differences were found in insula (all p≥0.071).
Conclusion: The current study demonstrated higher connectivity of thalamus and primary somatosensory cortex to other brain regions in diabetes individuals not having neuropathic complications. The thalamic/primary somatosensory cortex connectivity was associated with peripheral nerve functions and pain intensity. It was possible to distinguish between type 1 diabetes with DPN/neuropathic pain and type 1 diabetes without DPN/neuropathic pain based on thalamic connectivity. These findings may raise the question of whether a higher connectivity pattern of the thalamus and primary somatosensory cortex might maintain normal sensory profiling and whether lower connectivity might contribute to the pathogenesis of DPN and neuropathic pain. The current study contributes to a further understanding of functional alterations of regions involved in sensory processing in diabetes. Longitudinal studies are needed to confirm our findings.
Clinical Trial Registration Number: NCT04078516
Supported by: Augustinus Fonden, Denmark
Disclosure: S.S. Croosu: None.
737
Identification of painful diabetic neuropathy subtypes from functional connectivity resting state MRI patterns: a novel data driven machine learning approach
K. Teh1, J. Mcallister2, A. Anandhanarayanan2, J. Fan2, G. Sloan3, S. Tesfaye3, D. Selvarajah2;
1University of Sheffield, 2Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, 3Diabetes Research Department, Sheffield Teaching Hospitals, Sheffield, UK.
Background and aims: The traditional approach for studying the neurobiology of painful diabetic neuropathy (painful-DN) has followed a diagnostic/clinical framework through case-control studies whereby carefully phenotyped patients are compared with controls. This approach has failed to deliver on hoped-for biomarkers due to the high biological heterogeneity among painful-DN patients and among controls. Our primary aim was to delineate the neurobiological heterogeneity in painful-DN by first defining the functional connectivity subtypes in patients then assessed the clinical significance with respect to prediction of clinical outcome with treatment.
Materials and methods: 82 painful-DN subjects and 36 healthy controls underwent detailed clinical and neurophysiological assessment and resting state functional magnetic resonance imaging (RS-fMRI). Painful-DN subjects were divided into training (n=48) and testing (n=34) datasets. Painful-DN disease subtypes were identified by analysing, via unsupervised and supervised machine learning (k-means algorithm), the RS-fMRI functional connectivity of key somatosensory/nociceptive brain regions. Patients in our testing dataset were divided into responders (VAS<4; n=23) and non-responders (VAS>4; n=11). Data analysis was performed using the NITRC Functional Connectivity Toolbox and SPM8 in MATLAB and Scikit Learn.
Results: RS-fMRI functional connectivity defines two clinically relevant painful-DN subtypes. The two subtypes were characterised by strong functional connectivity differences in the postcentral (p<0.001, 95%CI=0.22:0.65) and precentral (p=0.002, 95%CI=0.15:0.59) parietal cortex functional connectivity. The interhemispheric connectivity between homologous regions was also notable. RS-fMRI functional connectivity differences in the default mode network (p=0.002; 95%CI=-0.35:-0.08) was also identified. The performance of our unsupervised k-means model to predict treatment response was: accuracy=0.77, recall=0.83, precision=0.83 and an AUC=0.73.
Conclusion: We have identified neurobiological markers of clinically relevant subtypes of painful DN from RS-fMRI functional connectivity using an unsupervised data-driven approach. The subtype whose functional connectivity differed most from healthy controls failed to respond to neuropathic pain treatment. Our data-driven approach may constitute a generalisable solution to elucidate the clinical heterogeneity of painful DN.
Supported by: National Institute of Health Research Efficacy and Mechanism Evaluation Programme (NIHR 129921)
Disclosure: K. Teh: None.
738
Mood disorders are related to thalamic H 1 -MRS metabolite parameters in type 1 diabetes
M. Greig1, G. Sloan1, P.R. Shillo2, D. Selvarajah3, I.D. Wilkinson4, S. Tesfaye1;
1Sheffield Teaching Hospitals, Royal Hallamshire Hospital, Sheffield, 2Department of Diabetes, Chesterfield Royal Hospital, Chesterfield, 3Diabetes, University of Sheffield, Sheffield, 4University of Sheffield, Sheffield, UK.
Background and aims: We have previously demonstrated preservation of proton Magnetic Resonance Spectroscopy (1HMRS) neuronal metabolite ratios and increased thalamic blood volume in painful diabetic peripheral neuropathy (pDPN). We hypothesised that perfusion measures and neuronal function measured by metabolite ratios may be related. However, as brain metabolite ratios may also be affected by mood disorders, common in pDPN, this may be a significant confounding factor.
Materials and methods: 52 type 1 diabetes (T1D) subjects (18 pDPN, 23 DPN, 13 without neuropathy-DMNN) and 18 healthy volunteers (HV) were recruited. 1HMRS was performed at 3T (Philips, Netherlands). Single voxel spectra were obtained from a 2.25cm3 (15x10x15mm) volume of interest within the thalamus, TE=135ms, TR=1600ms, NSA=256 using point resolved (PRESS) technique. Metabolite ratios were calculated for choline, Creatine, and N-Acetyl Aspartate (NAA). MR-Dynamic Susceptibility Contrast images were obtained at 3T using a T2*-weighted technique (TR/TE=1250/35ms; 72 dynamics) to assess the passage of bolus intravenous gadolinium-chelate through the thalamus.
Results: There was significant negative correlation between NAA/Cr (a measure of neuronal function) and measures of depression: Hospital Anxiety and Depression Scale R= -0.33 (p=0.01), Becks Depression Inventory R= -0.25 (p=.048); and anxiety: State-Trait Anxiety inventory- State (STAI-S) R= -.37 (p=.002), STAI- Trait (T) R= -.32 (p=0.01) and Behavioural Inhibition R= -0.25 (p=0.04). There were no differences in metabolite ratios between groups and no significant correlations between perfusion measures and metabolite ratios.
Conclusion: This is the first study to show thalamic 1HMRS metabolite ratios are correlated with measures of mood disorders in T1D. The high prevalence of mood disorders in pDPN and DPN have confounded previous 1HMRS studies and may explain conflicting reports in the literature. The link between neuropathy and mood disorders needs further research to understand whether both may arise from a common neurobiological pathway.
Supported by: EFSD/Novo Nordisk Programme
Disclosure: M. Greig: None.
739
HMGB1 inhibition protects Schwann cells from high glucose-induced cytotoxicity
M. Shi, J. Song, X. Zheng, Y. Chen, W. Gu, H. Zhang;
The Affilated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an, China.
Background and aims: Damage to Schwann cells play a crucial role in the pathogenesis of diabetic peripheral neuropathy (DPN). However, it is still unclear whether high mobility group box protein B1 (HMGB1) inhibition can mitigate Schwann cell damage induced by high glucose.
Materials and methods: The effect of different concentrations of HMGB1inhibitor (GA) on Schwann cells cultured in high glucose was investigated. RSC96 cells were divided into 5 groups: NG group (5.6 mmol/L) and HG group (25.0 mmol/L), HG+GA (1μM) group, HG+GA (10μM), HG+GA (100μM). Those cells were cultured for 24h and 48h respectively. HMGB1 synthesis and secretion was detected by western bolt and ELISA. In order to further evaluate the role of HMGB1 in the high glucose induced Schwann cells injury, small interfering RNA (siRNA) was used to knock out HMGB1. CCK8 assay was used to detect the proliferation of RSC96 cells. The levels of inflammatory factors (TNF-α, IL-1β, IL-6, MCP-1 and ICAM-1) were determined by western bolt and ELISA. The mRNA expression of NGF and neuritin-1 was detected by qRT-PCR. The expression levels of NSE, cleaved caspase-3, RAGE, p38MAPK, ERK, JNK and NF-κBp65 in cells were measured by western blot.
Results: Compared with the NG group, HMGB1 was remarkably increased in the HG group and was obviously decreased in HG+GA group (P<0.05). GA at the concentration of 10 μM for 24h had significant inhibitory effect on HMGB1 synthesis and secretion (P<0.05). Schwann cell viability in high glucose was restored due to GA and siHMGB1 by using CCK8 assay (P<0.05). Compared with the NG group, the generation of inflammatory factors (TNF-α, IL-1β, IL-6, MCP-1 and ICAM-1) was remarkably increased in the HG group, and the mRNA expression of NGF and neuritin-1 was decreased, while the cleaved caspase-3 protein and NSE expression were markedly up-regulated (P<0.05). GA intervention and siHMGB1 potently prevented inflammatory substance generation, alleviated the neurotrophic factor reduction, and suppressed cellular apoptosis related activation in Schwann cells exposed to high glucose ambience (P<0.05). Moreover, we found that RAGE expression, phosphorylation of p38MAPK and expression of nuclear NF-κBp65 in high glucose-stimulated Schwann cells was reversed GA intervention or siRNA interference with HMGB1 (P<0.05), while the phosphorylation levels of ERK and JNK wasn’t affected.
Conclusion: 1. HMGB1 was highly expressed in Schwann cells-cultured with high glucose was increased, while cell viability was decreased. 2. GA intervention and siHMGB1 can not only inhibit HMGB1 expression in Schwann cells exposed to high glucose, but also enhance cell viability. 3. GA restored neurotrophic factors level and reduce cleaved caspase-3 expression in Schwann cell due to its anti-inflammatory ability, which may be related to the inhibition of RAGE/p38MAPK/nuclear NF-κBp65 pathway. 4. HMGB1 knockout may antagonize the inflammatory reaction, induce the neurotrophic substance generation and reduce the cleaved caspase-3 production, thereby improving Schwann's survival and DPN. 5. HMGB1 may implicate in Schwann cells lesion in high glucose milieu and HMGB1 inhibition protects Schwann cells from high glucose induced cytotoxicity.
Supported by: National Natural Science Foundation of China Grant Award (81700723), BK20191213
Disclosure: M. Shi: None.
740
Arterial baroreflex vs vascular blood pressure buffering mechanism, potential implication to treat unstable hypertension in diabetes
J. Gmitrov1,2;
1Diabetology Clinic, Krompachy Hospital, Agel SK inc., Slovakia, Krompachy, Slovakia, 2National Institute of Public Health, Tokyo, Japan.
Background and aims: A mounting evidence suggests that enhanced blood pressure (BP) variability including its most dangerous form the catecholamine-induced sudden BP elevation is a major, independent cardiovascular (CV) risk factor especially in the condition of distorted CV autonomic regulation in diabetes. The goal was to compare the effect of the carotid baroreceptor (CB) magnetic stimulation with verapamil, a potent vascular calcium channel blocking agent, effect on sudden, high BP elevation in conjunction with arterial baroreflex sensitivity (BRS) and microvascular dilation, aimed to explain their relationship and potential implementation in diabetic BP regulatory dysfunction.
Materials and methods: Forty-four experiments were performed on conscious rabbits (20 with verapamil, 14 with SMF, and 10 control experiments) sedated using pentobarbital i.v. infusion (5 mg kg-1 h-1). Mean femoral artery BP (MAP), heart rate, BRS and ear lobe skin microcirculatory blood flow, by microphotoelectric plethysmography (MPPG), were simultaneously measured after a 40 min CB exposure to 350 mT SMF, generated by Nd2-Fe14-B alloy magnets, or after 30 min of verapamil i.v. infusion (20 μg kg -1 h-1). BRS was assessed from heart rate and MAP responses to i.v. bolus injection of nitroprusside and phenylephrine.
Results: CB magnetic stimulation significantly increased BRS (74.5%) and microcirculation (23.8%); and decreased resting MAP (-5.8%) and phenylephrine-induced MAP abrupt elevation (MAPAE, -19.1%). Verapamil significantly increased microcirculation (24.87%), decreased MAP (-17.4%), BRS (- 34.84%), and MAPAE (-35.7%) in significantly larger degree than after SMF exposure, Fig. 1. The decrement of the ΔMAPAE inversely correlated with verapamil-induced significant increase in microcirculation ΔMPPG (r = -0.53, p < 0.000) and with SMF-induced increase in ΔBRS (r= -0.47, p=0.016).
Conclusion: Verapamil-potentiated vascular BP buffering mechanism seems to be more effective than SMF baroreflex-mediated, with potential benefit in cardiovascular conditions with sudden, high BP rise. SMF-induced notable increase of microvascular dilation in response to the same dose i.v. nitroprusside, which exercises its vasodilator property as a spontaneous nitric oxide (NO) donor, indicates augmentation of the vessel sensitivity to NO dilatory effect, Fig. 1. We suggest that both, verapamil (by vascular Ca2+ channel blockade) and SMF (throughout decrement of the vascular sympathetic drive), boosters endothelial sheer stress NO-dependent BP buffering mechanism, which by enhancement of the vessel compliance impeded sudden BP rise. The elaboration of pharmacological and non-pharmacological approaches aimed to reinforce intrinsic BP regulatory mechanisms suggested to be the universal solution how to complexly adjust arterial hypertension in its array of diabetic manifestations.
Fig. 1.
Disclosure: J. Gmitrov: None.
741
Impact of reduced eGFR, high HbA1c and cardiovascular disease on mortality in people with Charcot foot
J. Schoug, P. Katzman, M. Löndahl;
Clinical Sciences in Lund, Lund University and Department of Endocrinology, Skåne University Hospital, Lund, Sweden.
Background and aims: Charcot foot (CF) is a potentially devastating foot complication in diabetes mellitus with underlying peripheral neuropathy. The prevalence of risk factors and comorbidities in CF and their impact on mortality have not been fully described. This study aims to review the patient cohort diagnosed with acute CF between 2006-2017 at our University Hospital pertaining to patient characteristics and comorbidities at baseline and whether mortality was affected by cardiovascular disease, renal impairment, or glycemic control.
Materials and methods: Patients with an acute episode of CF admitted between 2006-2017 were retrospectively studied using medical records. Data for diabetes type, sex, age, history of smoking, previous diagnosis of peripheral arterial disease (PAD), ischemic heart disease (IHD), cerebrovascular disease (CVD), systolic left ventricular heart failure (HFrEF; assessed by echocardiography), diabetic foot ulcer (DFU), HbA1c, eGFR (based on creatinine level using revised Lund-Malmö formula), dialysis and kidney transplantation were collected. Death within 5 and 10 years from admission was noted. Medical records were reviewed up to May 28th, 2020. Continuous data are given as median (IQR). Log Rank tests were used for survival analysis (data given as mean and SEM), and Cox regression for estimating hazard ratios. A minimum number of events per variable number of 10 was used for multivariate Cox regression analysis. In case of a limited number of events, a cardiovascular (CV) composite factor was used, combining HFrEF, IHD, CVD, and PAD at the time of referral. A two-tailed p-value < 0.05 was considered statistically significant.
Results: 158 patients, aged 61 (53-68) years, were included and followed for 75 (44-111) months. 66 patients (42%) were women and 60 patients (38%) had type 1 diabetes. Ten patients (6.3%) had a history of kidney transplantation, and eight (5.1%) were on dialysis. The 5-year mortality was 23% (36 deaths) for the entire cohort, and the 10-year mortality was 32% (50 deaths). The prevalence of risk factors, comorbidities, and their association with 10-year mortality risk are given in Table 1. Age, diabetes type, eGFR < 60 ml/min/1.73 m2, and all cardiovascular diseases were associated with higher 10-year mortality. HbA1c > 70 mmol/mol was associated with higher 5-year mortality (p = 0.015), but not 10-year mortality. In a multivariate Cox regression model with age, diabetes type, eGFR, HbA1c, and cardiovascular disease composite, eGFR < 60 ml/min/1.73 m2 and HbA1c > 70 mmol/mol were statistically significant predictors of 10-year mortality. Patients with reduced eGFR had a 10-year mortality risk of 55% compared to 16% in those without reduced eGFR.
Conclusion: In this Swedish cohort of people affected by acute Charcot Foot, reduced eGFR was a more prevalent and stronger predictor of increased 10-year mortality compared to high HbA1c and cardiovascular disease.
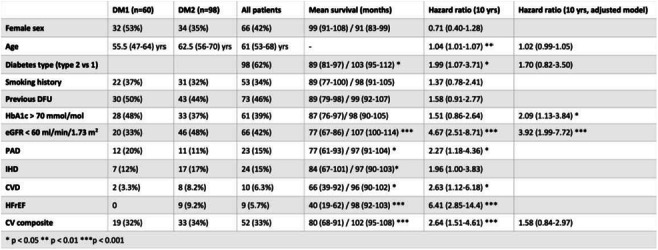
Disclosure: J. Schoug: None.
SO 63 Diversity of the diabetic kidney
742
Chronic kidney disease in a contemporary nationwide register of patients with and without type 2 diabetes
T.G. Jenssen1,2, K. Sveen3, J. Bodegård4, M. Thuresson5, K.I. Birkeland1,2;
1Dept. of Transplantation Medicine, Oslo University Hospital, Oslo, Norway, 2University of Oslo, Oslo, Norway, 3Dept. of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway, 4AstraZeneca Nordic, Oslo, Norway, 5Statisticon AB, Uppsala, Sweden.
Background and aims: Chronic kidney disease (CKD) is an important risk factor for cardiovascular disease and premature mortality regardless of diabetes. Recently, randomized controlled trials have reported beneficial effects of three different sodium glucose transporter-2 inhibitors (SGLT2-is) on progression of CKD in patients with type 2 diabetes (T2D) and those without diabetes, herein referred to as CKD with and without diabetes. We aimed to assess and compare clinical characteristics of diagnosed CKD patients with and without diabetes and the impact of diabetes in a contemporary nationwide cohort.
Materials and methods: Patients with a CKD diagnosis before the index date, 1st January 2020, were identified in the Norwegian Patient Register that covers all in- and out-patient hospital visits, and then linked with the Norwegian Prescription Database and the Cause of death register. Comorbidities were searched in all available data and drug treatment 1-year prior to index. CKD stages were derived from hospital diagnoses, covering 80% of all patients with a CKD diagnosis. Event rates during the year after index were calculated for the first of each outcome and reported as events per 100 patient-years. The following outcomes were reported: All-cause and cardiovascular death as well as inpatient hospitalization with a main diagnosis of CKD, heart failure (HHF) and myocardial infarction, respectively. Standardized differences >10% at baseline were considered to show non negligible difference.
Results: After exclusion of patients with type 1 diabetes, we identified 100 397 patients with CKD, of which 25 589 (25%) had T2D. CKD patients with diabetes were slightly older, 72 vs 70 years, and had more prevalent atherosclerotic cardiovascular disease and heart failure than patients without diabetes (Table). During 1-year follow up, the event rates of hospital stays for CKD (7.1 vs 4.8), HHF (5.7 vs 3.9) and myocardial infarction (3.0 vs 1.7) were higher in diabetes patients, despite more prevalent use of statins (65 vs 40%) and renin angiotensin system inhibitors (62 vs 47%) compared to those without diabetes. All-cause mortality and cardiovascular mortality risks were similar in patients with and without diabetes (10.2 vs 9.8) and (3.1 vs 2.9) respectively. Only 10% of patients with CKD and type 2 diabetes used SGLT2-is.
Conclusion: One out of four patients registered with a CKD diagnosis in the Norwegian National Patient Register has type 2 diabetes. The CKD-patients with type 2 diabetes have more co-morbidities, and higher event rates, particularly for cardiorenal events (HHF or CKD), than non- diabetic CKD patients. Only a minority received treatment with SGLT2-i, and there may be a potential for increased use of therapies that delay progression of renal failure.
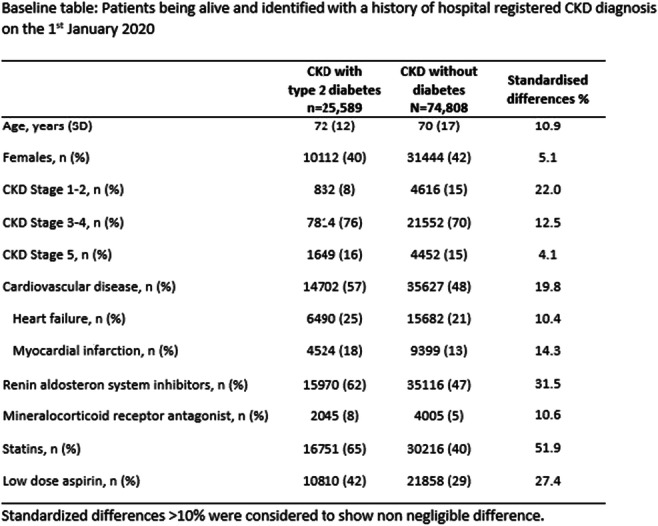
Supported by: KIB Grant from AstraZeneca to University of Oslo
Disclosure: T.G. Jenssen: Honorarium; AstraZeneca, Boehringer Ingelheim.
743
Relationship between genetic risk scores for type 2 diabetes and kidney function and adverse diabetes outcomes
L. Pacal1, D. Galuška1, K. Chalásová1, J.A. Hubáček2, V. Lánská2, K. Kaňková1;
1Department of Pathophysiology, Masaryk University, Brno, 2Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Background and aims: Diabetic kidney disease (DKD) represents a serious diabetic complication with several risk factors contributing to its development or progression (e.g. glucose control, hypertension, dyslipidaemia). Genetic predisposition is also involved, however it is still insufficiently explored. Number of genome-wide association studies (GWAS) identified single nucleotide polymorphisms (SNP) associated type 2 diabetes (T2DM) and also with the kidney function. We selected 21 risk SNPs for T2DM and 9 SNPs associated with kidney function and/or chronic kidney disease and constructed genetic risk scores (GRS). The aim of the work was to find out whether GRS or individual SNPs might predict the progression of DKD or cardiovascular morbidity and mortality in patients with T2DM in the Czech population.
Materials and methods: A total of 400 patients (205 men and 195 women) with T2DM and variable stage of DKD were included with subsequent follow-up for a median of 54.6 (IQR 27.8 - 91) months. Time-to-event analysis considered three end-points: 1. DKD progression (based on GFR), 2. major adverse cardiovascular events (MACE) and 3. all-cause mortality (ACM). DNA was isolated from peripheral blood. 21 T2DM risk SNPs and 9 SNPs associated with kidney function and/or chronic kidney disease were selected for this study. SNPs were determined by quantitative PCR (TaqMan® Genotyping Assay).
Results: At the end of the follow-up period, the cumulative incidence was 49.5 %, 21.5 % and 41.5 % for DKD, MACE and ACM, respectively. We calculated weighted and unweighted GRS for T2DM risk alleles and unweighted GRS for kidney function. Unweighted GRS was calculated by summing-up the number of risk alleles. Weighted GRS considered OR of individual SNPs. First, we analysed the potential correlation between GRS and age, T2DM duration, HbA1c, creatinine and GFR. No statistically significant differences were ascertained (all P > 0.05, Spearman). For further analyses patients were stratified by median and quartiles into two and four groups, respectively. Time-to-event analysis using Kaplan-Mayer curves did not show any statistically significant differences between two or four groups, respectively (P>0.05). Two variants (WSF1 and MTNR1B) were associated with the risk of DKD (P=0.004 and 0.04). WSF1 was associated with MACE (P=0.01) and CDKN2A/B with ACM (P=0.03). Of the variants associated with kidney function CANCAS1 represents a risk for ACM (P=0.04).
Conclusion: The aim of this work was to determine the predictive power of GRS composed of selected SNPs associated with the presence of T2DM and with kidney function in patients with T2DM. The results suggest that GRS does not predict DKD progression, MACE or ACM in our cohort. Only several SNPs seem to have a minor effect.
Supported by: Grant NV18-01-00046 from the Ministry of Health of the Czech Republic
Disclosure: L. Pacal: None.
744
Trajectories of estimated glomerular filtration rate and their association with ethnicity and the risk of progression of chronic kidney disease in people with type1 diabetes
S.A.M. Ayis1, A. Mangelis1, N. Fountoulakis1, L. Gnudi2, S.M. Thomas3, J. Collins3, D. Hopkins2, J. Karalliedde1;
1King's College London, 2King's College Hospital, 3Guys and St. Thomas Hospital, London, UK.
Background and aims: Information on patterns of progression of estimated glomerular filtration rate (eGFR) in ethnically diverse cohorts of people with type 1 diabetes (T1DM) is limited. We aim to evaluate eGFR progression trajectories a multi-ethnic population cohort, with T1DM attending hospital-based clinics in South London with baseline eGFR≥45 mL/min, and 10 years of eGFR follow up measurements with minimum 2 values per year.
Materials and methods: We studied 1,495 people with T1DM diabetes (52% females), median age 44 (range: 18 to 86) years, baseline eGFR median 83 (range: 45 to 168) ml/min, ethnicity distribution of 81% Caucasian, 12% African-Caribbean and 7% others. Group Based Trajectory Models (GBTMs) which analyse the evolution of an outcome over time, were used to identify trajectories of eGFR. The Chronic Kidney Disease (CKD) Epidemiology Collaboration equation was used to estimate eGFR.
Results: Five distinct eGFR trajectories were identified. Group I has rapid eGFR decline with a linear slope of -5.09 (SE:0.48) ml/min/year. Group I (8.5% of people) has baseline eGFR, mean [standard deviation(SD)] 66.2 (17.5) ml/min; Group II (23%), 68.1 (11.9) ml/min; Group III (29.8%), 80.7 (13.6) ml/min, and Groups IV, and V ( 26.3% and 12.4% of people) mean (SD): 96.3(14.4) and 115.87(16.2) at baseline, respectively.There were statistically significant differences between the groups in baseline eGFR , age, gender, and ethnicity. Mean (SD) age for groups (I-V) were 50.9 (15.1); 50.8(13.6); 45.4 (11.8), 39 (10.7), and 31 (8) years respectively, (p<0.05 for trend). Group II, was male dominant (66.28%) while groups IV and V, had greater proportions of females (64.63% and 86%) respectively (p<0.05 for trend). Group I, which met the criteria of fast decline of eGFR (>3 ml/min/ year), had the highest proportion of people from African-Caribbean origin (27.6%), while groups (II-V) had 20.6%, 10.3%, 5.6% and 3.2% respectively (p<0.05 for trend). People in Group I also had higher HbA1c, systolic and diastolic blood pressures, body mass index, (BMI) higher low density lipoprotein and urine ACR, at baseline compared to other groups (P<0.05 for all). At 10 years of follow up 71.8% of Group I, and 5.8% of Group II had progressed to CKD stage 3B or higher (eGFR <45ml/min or lower). One third of people in group I, and 1.2% of Group II being at Stage 4 CKD, and 16.5% of Group I were at stage 5. None from Groups III or IV progressed to CKD but one from group V who had dramatic drop in eGFR at years 8-10.
Conclusion: Distinct clinically relevant patterns of eGFR progression over 10 years, were identified, in an ethnically diverse cohort of people with type 1 diabetes attending specialist clinics in publicly funded hospital. We noted the novel finding that people in Group I who had the fastest eGFR decline and progression towards advanced stages of CKD were characterised by a greater prevalence of people of African-Caribbean ethnicity. This group also had a greater prevalence of traditional risk factors such as older age, raised systolic and diastolic blood pressures, albuminuria, higher HbA1c, BMI and dyslipidaemia. Further studies in similar cohorts are required to validate our findings and for better understanding of the underlying mechanisms that may explain our observations.
Supported by: Grant from Guys and st. Thomas Hospital Charity
Disclosure: S.A.M. Ayis: None.
745
The inflection point of the estimated glomerular filtration rate is higher in people with an increasing to decreasing trend than in people with different trends
S. Katoh1, K. Yokoyama2, M. Zeniya3, Y. Sakamoto4, K. Utsunomiya1, R. Nishimura1;
1Div. of Diabetes, Metabolism & Endocrinology, The Jikei University School of Medicine, 2Harumi Triton Clinic, The Jikei University School of Medicine, 3Sanno Medical Clinic, 4The Jikei University School of Medicine, Tokyo, Japan.
Background and aims: Last year, we showed that baseline hyperfiltration in the estimated glomerular filtration rate calculated from serum creatinine (eGFR) is dominantly associated with a subsequent decline in eGFR. This year, we compared the inflection point of the eGFR among groups of people with different trends.
Materials and methods: This retrospective study comprised 4 periods: from April 2005 to September 2006, from April 2009 to September 2010, from April 2013 to September 2014, and from April 2017 to September 2018. The study involved 4258 people (2843 men and 1415 women; mean age, 49 years) who underwent comprehensive medical check-ups in 3 of the 4 periods for 3-point data analysis. They were assigned to either a Period I-II-III group or a Period II-III-IV group with a midpoint examination in Periods II and III, respectively. We assessed the trends in the ΔeGFR —increasing (Inc) or decreasing (Dec)— from baseline to midpoint and from midpoint to endpoint. Participants were thereby allocated into Inc-Inc, Inc-Dec, Dec-Dec, or Dec-Inc groups in accordance with the ΔeGFR.
Results: There were 34,779 person-years of observation, and the mean observation period was 8.2 years. The baseline eGFR, midpoint eGFR, endpoint eGFR, ΔeGFR from baseline to midpoint, and ΔeGFR from midpoint to endpoint were not significantly different between diabetic (n = 480) and non-diabetic (n = 3778) people. The midpoint eGFR of the Inc-Dec group (79±14 ml/min/1.73 m2, n=767) was significantly higher (Bonferroni correction) than that of the Inc-Inc group (75±14 ml/min/1.73 m2, n=330, p<0.001), Dec-Dec group (73±13 ml/min/1.73 m2, n=1401, p<0.001), and Dec-Inc group (69±11 ml/min/1.73 m2, n=1760, p<0.001). In receiver operating characteristic curve (ROC) analysis of the entire sample, the threshold of the midpoint eGFR between the Inc-Inc and Inc-Dec groups was 75.5 ml/min/1.73 m2 (sensitivity 0.57, specificity 0.54, AUC 0.571, p<0.001). The midpoint eGFR was not significantly different between diabetic and non-diabetic people in the 4 trend groups. Nevertheless, in people with diabetes, the midpoint eGFR was not significantly higher in the Inc-Dec group (81±16 ml/min/1.73 m2, n=83) than in the Inc-Inc group (77±15 ml/min/1.73 m2, n=39, p=0.139). Regression analysis in the Inc-Dec group revealed that the presence of diabetes was significantly associated (standardized β [Sβ] = 0.143, p < 0.001) with the midpoint eGFR as well as midpoint ALT (U/L) (Sβ = 0.180, p = 0.004), smoking index (cigarettes per day × years of smoking) (Sβ = 0.076, p = 0.023), age (Sβ = -0.407, p < 0.001), uric acid (mg/dL) (Sβ = -0.210, p < 0.001), BMI (kg/m2) (Sβ = -0.157, p = 0.028), exercise habit (min/week) (Sβ = -0.069, p = 0.034), while female sex, systolic blood pressure, diastolic blood pressure, LDL cholesterol, triglyceride, AST, Gamma-GTP, ethanol intake, waist circumference, HDL cholesterol, and vegetable intake were not.
Conclusion: In the overall cohort, the inflection midpoint of the eGFR was significantly higher in patients with an increasing to decreasing trend than in other trend groups. Diabetes significantly affected the inflection midpoint of the eGFR in the Inc-Dec group.
Clinical Trial Registration Number: 20-130 5420
Disclosure: S. Katoh: None.
746
Concurrence of diabetic kidney disease and metabolic dysfunction-associated fatty liver disease is associated with heart failure
K.-A. Kim1, J. Yoo2, S. Lee1, H. Choi1, K. Han3;
1Department of Medicine, Dongguk University Ilsan Hospital, Goyang, Gyeonggi-do, 2Department of Biomedicine & Health Science, The Catholic University of Korea, Seoul, 3Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea.
Background and aims: Heart failure (HF) is the leading cause of type 2 diabetes (T2D)-related morbidity. Diabetic kidney disease (DKD) and fatty liver disease are emerging as risk factors for HF. The new classification of fatty liver disease defined as metabolic dysfunction-associated fatty liver disease (MAFLD), has not been deeply studied regarding T2D-related HF. We evaluated the risk of hospitalization for HF (HHF) in DKD according to the presence of MAFLD.
Materials and methods: The study included 1,189,113 patients with T2D from the Korean National Health Insurance Service database, who underwent two cycles of health checkups between 2009-2014. DKD phenotype was classified based on estimated glomerular filtration rate (eGFR(G), Normal(N) vs. Low(L)) and proteinuria(P, - vs. +). Low eGFR was defined by values between 30 and 60 mL/min/1.73 m2. Proteinuric DKD with low eGFR was excluded at baseline because it is a well-known very high-risk category. Three stable DKD phenotypes over 2 years were selected for the study: no-DKD, GNP-(95.2%); nonproteinuric DKD with low eGFR, GLP-(3.4%); proteinuric DKD with normal eGFR, GNP+(1.3%). MAFLD was defined as the presence of hepatic steatosis determined using fatty liver index (FLI)≥60.
Results: The overall baseline HF and MAFLD were 2.1% and 25%, respectively. The prevalence of MAFLD was higher in the presence of proteinuria(GNP+, 42%) compared to GNP- (25%) or GLP- (20%). During an average of 6.5 years of follow-up, 5,781 subjects developed HHF. Without considering MAFLD, when GNP- phenotype was the reference, multivariable-adjusted hazard ratios(aHR) [95% confidence interval, CI] for HHF were 1.90[1.77-2.04] in GLP- and 3.27[2.88-3.71] in GNP+. Regarding combined DKD and MAFLD(F- vs. F+), in the absence of proteinuria, aHR[95% CI] for HHF were 1.37 [1.27-1.50] in the GNP-/F+, 1.93 [1.78-2.09] in the GLP-/F-, and 2.19 [1.88-2.54] in the GLP-/F+ relative to those in GNP-/F-, respectively. In proteinuric DKD(GNP+), aHR for HHF were 3.67[3.16-4.25] in F- and 3.23[2.53-4.13] in F+.
Conclusion: In T2D patients without proteinuria, the presence of MAFLD was associated with a higher risk of HHF. However, in the presence of proteinuria, the association between MAFLD with HHF risk is attenuated. These findings underscore the importance of conducting careful HF risk stratification in T2D with DKD or MAFLD.
Disclosure: K. Kim: None.
747
Association of microvascular complications with death in patients with diabetes and COVID-19: international effort -CORONADO, ABCD-COVID19, AMERICADO studies
S. Hadjadj1, P.-J. Saulnier2, Y. Ruan3,4, X. Zhu5, R. Rea3, P. Gourdy6, B. Cariou7, A. Myers8, K. Khunti9, for the CORONADO, the ABCD COVID-19 diabetes national audit and AMERICADO investigators;
1UMR 1087, L’institut du thorax, Université de Nantes, CHU Nantes, CNRS, Inserm, Nantes, France, 2Centre d’Investigation Clinique CIC 1402, Université de Poitiers, Inserm, CHU de Poitiers, Poitiers, France, 3Oxford Centre for Diabetes, Endocrinology and Metabolism, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, 4Oxford NIHR Biomedical Research Centre, Oxford, UK, 5Institute of Health System Science, Feinstein Institutes for Medical Research, Manhasset, USA, 6CHU de Toulouse & UMR1048/I2MC, Université de Toulouse, Toulouse, France, 7L’institut du thorax, Université de Nantes, CHU Nantes, CNRS, Inserm, Nantes, France, 8Department of Medicine, Division of Endocrinology, Northwell Health, Manhasset, USA, 9University Hospitals of Leicester NHS Trust, Diabetes Research Centre, Leicester General Hospital, Leicester, UK.
Background and aims: The prognostic role of microvascular complications for all-cause death in patients with diabetes hospitalized for coronavirus disease-2019 (COVID-19) has been suggested. However, no detailed analysis of the microvascular burden in these patients is available so far.
Materials and methods: We analyzed data from the French CORONADO initiative and the United Kingdom ABCD Covid-19 audit, two nationwide multicenter studies, and the AMERICADO, a multicenter study in New York area. We assessed the association between the risk of in-hospital death and microvascular complications, in patients with diabetes hospitalized for COVID-19: diabetic retinopathy, presence/absence and/or diabetic kidney disease, and/or history of diabetic foot ulcer.
Results: Among 2951 CORONADO, 3387 ABCD Covid-19 audit and 9327 AMERICADO patients, the status for microvascular diabetic complications was ascertained for 1314 (44.5%), 1809 (53.4%) and 7367 (79.0%) patients: 1010, 1059 and 1800 with ≥1 severe microvascular complication(s) and 304, 750 and 5567 participants free of any complications, respectively. Focusing on patients with DR, after adjustment for age, sex, hypertension and cardiovascular disease, patients with specific microvascular complications had an increased risk of death in the CORONADO, the ABCD COVID-19 diabetes national audit and the AMERICADO participants: adjOR: 2.78 (2.60-2.97), adjOR: 1.25 (1.03-1.52) and adjOR: 1.21 (1.07-1.37), respectively. When meta-analyzing the 3 studies, compared to those patients free of complication, those with specific microvascular complications had an OR for death of 2.03 (1.41-2.92) which decreased to 1.51 (1.08-2.11) after adjustment for age, sex, hypertension and cardiovascular disease.
Conclusion: Microvascular burden is associated with an increased risk of death in patients hospitalized for COVID-19.
Clinical Trial Registration Number: NCT04324736
Supported by: FFRD, SFD, DGOS, CORONADO emergency grant, Novonordisk
Disclosure: S. Hadjadj: Employment/Consultancy; AstraZeneca, Abbott, Boehringer Ingleheim, Eli Lilly, Novartis, Novonordisk, Bayer. Grants; AstraZeneca, LVL, Air Liquide, Novonordisk, Eli Lilly, Bayer, Nantes Metropole, Pierre Fabre Santé, Novartis.
748
Glucose and c-peptide peak times are associated with chronic complications in patients with type 2 diabetes
Y.Q. Jiang1, X.L. Wang1, X.Q. Zhao1, Y. Sun1, P. Huang1, S.H. Ji1, R.Y. Hu1, Y.J. Gu1,2;
1Department of Endocrinology and Metabolism, Affiliated Hospital of Nantong University, 2Department of health medicine, Health and disease management center, Affiliated Hospital of Nantong University, Nantong, China.
Background and aims: To assess the association of chronic complications in patients with type 2 diabetes mellitus (T2DM) with the peak time of glucose and C-peptide (C-p) during the standard steamed bread meal test.
Materials and methods: A total of 1,095 patients with T2DM were divided into two groups: (1) peak time of glucose: Group G1:≤60 minutes (n=84), Group G2: >60 minutes and≤120 minutes (n=648), and Group G3: >120minutes (n=363); (2) peak time of C-p: Group C1:≤60 minutes (n=14), Group C2: >60 minutes and≤120 minutes (n=350), and Group C3: >120 minutes (n=731). Anthropometric and biochemical metabolic parameters, B-cell function and indicators of chronic T2DM complications were compared.
Results: Among patients with delayed glucose peak time, urine albumin-creatinine ratio (UACR) and those with diabetic retinopathy (DR) increased (all P<0.05), while fasting and 120-minuteC-p, the area under the curve for C-p (AUCC-p), insulinogenic index and modified B-cell function index decreased (all P<0.05). Meanwhile, in patients with delayed C-p peak time, UACR and those with DR increased (all P<0.05), whereas C-p index and AUCC-p/AUC for glucose decreased (all P<0.05). On multinominal logistic regression analysis, age and the proportion of patients with DR correlated with delayed glucose and C-p peak time; however, estimated glomerular filtration rate and UACR were correlated with delayed C-p peak time(all P<0.05).
Conclusion: Indicators of diabetic nephropathy were related to delayed C-p peak time, while DR was related to both delayed glucose and C-p peak time. Delayed glucose and C-p peak time in patients with T2DM could be early predictors for diabetic microvascular disease.
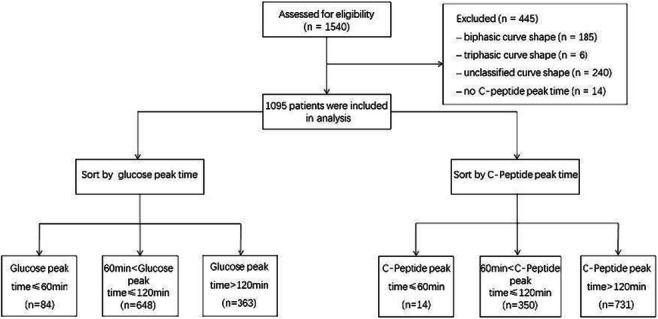
Disclosure: Y.Q. Jiang: None.
749
Kidney biopsy in patients with diabetes and nephrotic range-proteinuria; clinical value and correlation with clinical and laboratory findings
T. Stratigou1, E. Kardalas1, A. Paikopoulou2, D.A. Vassiliadi1, G. Ioannidis1, C. Christodoulidou2, S. Tsagarakis1;
1Endocrinology, Diabetes and Metabolism Department, Evangelismos General Hospital, 2Nephrology Department, Evangelismos General Hospital, Athens, Greece.
Background and aims: Diabetes mellitus (DM) is associated with increased prevalence and evolution of diabetic nephropathy (DN). Interestingly, deteriorated kidney function in diabetic patients can often result from non-diabetic causes and go undiagnosed. Non-diabetic renal disease (non-DRD) could be suspected in case of rapid progression of renal function impairment and/or severe, nephrotic range-proteinuria. While biopsy of the kidneys is the ‘gold standard’ of the diagnostic approach in such cases, no real consensus exists regarding the profile of diabetic patients with renal disease and proteinuria, who should be submitted to kidney biopsy. Thus, this study intends to correlate the clinical and biochemical profile of diabetic patients with deteriorated kidney function to the histopathological data of a kidney biopsy.
Materials and methods: 32 patients, who were treated in our outpatient’s diabetes clinic from a team of endocrinologists and nephrologists at Evangelismos General Hospital, were retrospectively studied. All patients suffered from DM2 and presented with nephrotic range-proteinuria. Biochemical data of glycemic control and renal function, clinical findings related to DN and histological findings of kidney biopsy were documented. All patients were submitted to kidney biopsy and depending on the histopathologic findings were categorized in 3 groups: a) genuine diabetic nephropathy (GDN), b) renal disease due to cause other than diabetes mellitus (ODMRD) and c) mixed renal disease (MRD).
Results: Among the 32 patients, 15 (46.9%) had findings of GDN, while 17 patients (53.1%) suffered from ODMRD (13 patients) or MRD (4 patients). All patients were hypertensive. The patients with GDN were younger (54.1 vs 68.2 vs 70.5 yrs, p=0.016) and had a higher HbA1C value (7.9 vs 6.5 vs 6.8%, p=0.069) at the time of the kidney biopsy in comparison to the ODMRD and MRD patients. On the contrary, patients from the ODMRD group had significantly shorter disease duration compared to the GDN and MRD groups (8.4 vs 11.6 vs 13.3 yrs, p=0.04). Furthermore, the incidence of diabetic retinopathy (DR) was greater among the GDN and MRD patients in comparison to the ODMRD ones (60 vs 75 vs 7.6%, p<0.01). Additionally, higher levels of interstitial fibrosis (>25%) were significantly more prevalent among the GDN patients in comparison to those with ODMRD and MRD respectively (80 vs 38 vs 50%, p=0.018). No statistically significant difference was identified regarding the levels of nephrosclerosis between the 3 groups (27% vs 31% vs 30%, p=0.87). Finally, the presence of diabetic retinopathy (OR 4.88, % 95 CI:1.06-22.38, p=0.04), the identification of higher levels (>25%) of interstitial fibrosis (OR 5.71, % 95 CI:1.16-28.1, p=0.032) and longer DM2 duration (>10 years) (OR 5.04, % 95 CI: 1.1-22.96, p=0.036) were recognized as factors, who were positively associated with GDN.
Conclusion: This study highlights emphatically the clinical usefulness of kidney biopsy in patients with diabetes mellitus and nephrotic range-proteinuria, especially in case diabetic retinopathy is absent and shorter disease duration is observed. Thus, the existing indications for kidney biopsy in diabetic patients with nephrotic-range proteinuria might need to be revised.
Disclosure: T. Stratigou: None.
SO 64 New treatment avenues for the diabetic kidney
750
Albuminuria lowering effect of dapagliflozin, exenatide and their combination in patients with type 2 diabetes: a randomised cross-over clinical trial
A.B. van der Aart-van der Beek1,2, E. Apperloo3, N. Jongs1, D.B. Rouw2, D. Sjöström4, D.H. van Raalte5, K. Hoogenberg2, H.J.L. Heerspink1;
1University of Groningen, Groningen, Netherlands, 2Martini Hospital, Groningen, Netherlands, 3Isala Hospital, Groningen, Netherlands, 4AstraZeneca, Gothenburg, Sweden, 5Amsterdam UMC, Amsterdam, Netherlands.
Background and aims: Sodium-glucose co-transporter 2 (SGLT-2) inhibitors and glucagon-like peptide receptor agonists (GLP1-RA) reduce albuminuria in people with type 2 diabetes (T2D). It is unknown whether the combination of both drug classes confers a more beneficial effect than either treatment alone. We conducted a prospective clinical trial to determine the albuminuria lowering effect of the GLP1-RA exenatide (EXE), SGLT-2 inhibitor dapagliflozin (DAPA) and their combination (COMB) in patients with T2D and albuminuria.
Materials and methods: Patients with T2D, eGFR > 30 ml/min/1.73m2 and urinary albumin:creatinine ratio (UACR) >3.5mg/mmol and ≤100 mg/mmol were randomly assigned to treatment regimen consisting of three 6-week treatment periods in which DAPA 10 mg/day, EXE 2 mg/week and COMB were given in random order. Primary outcome was change in UACR from baseline. Secondary outcomes were correlation between changes in UACR during each treatment period and changes from baseline in Hba1c, body weight (BW), blood pressure (BP) and eGFR. Generalized linear mixed effects models were used to determine change from baseline in primary and secondary outcomes.
Results: Between January 2019 and April 2021 we screened 24 participants of whom 20 were randomized. Median UACR at baseline was 15.4 mg/mmol (IQR 9.4-44.6); mean eGFR 60 ml/min/1.73m2 (SD 18.8) and HbA1c 8.2% (SD 1.3). After 6 weeks of treatment with DAPA, UACR changed by -23.1% (95% CI -34.4,-9.8), versus -9.1% (95% CI -23.1,7.4) with EXE and -27.2% (95% CI -38.3,-14.2) with COMB. There was no correlation in UACR change between DAPA and EXE (r=-0.33, p=0.235), DAPA and COMB (r=-0.099; p=0.716) or EXE and COMB ( r=-0.45; p=0.081). At 6 weeks, reductions in eGFR and SBP were observed with DAPA and COMB, but not EXE (table). Statistical significant reductions in HbA1c and BW were observed with EXE and COMB, but not DAPA (table). With the exception of eGFR, effects were numerically larger with COMB compared to DAPA or EXE alone. No changes in extra- and intracellular volume were observed (table). The study medication was generally well tolerated. Mild hypoglycemia occurred once with DAPA and once with EXE.
Conclusion: This study suggests that COMB has modestly larger effects in various cardiovascular and renal risk factors as compared to DAPA or EXE alone. A larger study is required to assess whether this translates into improved clinical outcomes.
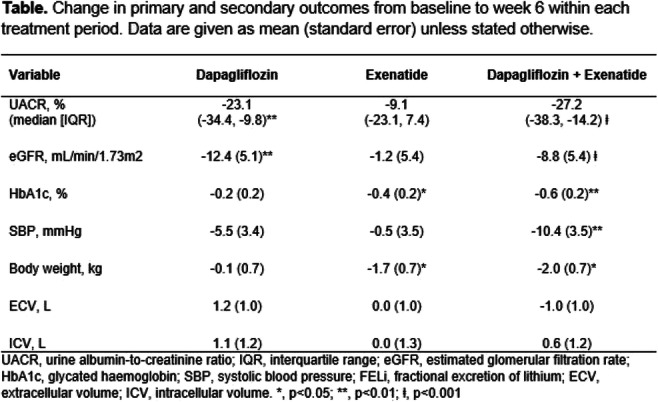
Clinical Trial Registration Number: NL6662 (NTR6839)
Disclosure: A.B. van der Aart-van der Beek: None.
751
Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using an UACR Endpoint study (CONFIDENCE)
J.B. Green1, A.K. Mottl2, G. Bakris3, H.J.L. Heerspink4, J.F.E. Mann5, J.B. McGill6, M. Nangaku7, P. Rossing8,9, C. Scott10, A. Gay11, R. Agarwal12;
1Duke University School of Medicine and Duke Clinical Research Institute, Durham, USA, 2University of North Carolina Kidney Center, UNC School of Medicine, Chapel Hill, USA, 3University of Chicago Medicine, Chicago, USA, 4Department of Clinical Pharmacy and Pharmacology, University of Groningen, Groningen, Netherlands, 5KfH Kidney Center, Munich, Germany, 6Division of Endocrinology, Metabolism and Lipid Research, Washington University in St. Louis, School of Medicine, St. Louis, USA, 7The University of Tokyo Graduate School of Medicine, Tokyo, Japan, 8Steno Diabetes Center Copenhagen, Gentofte, Denmark, 9Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 10Data Science and Analytics, Bayer PLC, Reading, UK, 11Medical Affairs & Pharmacovigilance, Pharmaceuticals, Bayer AG, Berlin, Germany, 12Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, USA.
Background and aims: Despite available interventions, people with type 2 diabetes (T2D) remain at risk of chronic kidney disease (CKD). Finerenone, a potent and selective nonsteroidal mineralocorticoid receptor antagonist, and sodium-glucose co-transporter 2 inhibitors (SGLT2is) may reduce both kidney and cardiovascular risks in people with CKD and T2D. It is unknown whether dual therapy with finerenone and a SGLT2i is superior to either agent alone. We outline the design of a study addressing this clinical question.
Materials and methods: The CONFIDENCE study is a randomised, controlled, double-blind, double-dummy, international, multicentre, three-armed, parallel-group, phase II study of 8 months' duration in approximately 807 adults with CKD and T2D, a urinary albumin-to-creatinine ratio (UACR) of ≥300-<5000 mg/g and an estimated glomerular filtration rate of 30-90 ml/min/1.73 m2 (Figure). The primary objective is to demonstrate that dual therapy comprising finerenone and the SGLT2i empagliflozin is superior for reducing the UACR compared with either monotherapy.
Results: The primary outcome is the relative change from baseline in UACR to 180 days. Secondary outcomes will further characterise the efficacy and safety of dual therapy including reduction in UACR >30% from baseline at 180 days, incidence of hyperkalaemia and acute kidney injury and the assessment of initial and longer-term changes in eGFR. Considering drop-out and screen-failure rates, 269 participants per arm will provide 80% power to reject the null hypothesis of equal means for dual therapy versus either monotherapy.
Conclusion: The CONFIDENCE study will evaluate the additive efficacy, safety and tolerability of finerenone plus a SGLT2i in adults with CKD and T2D. Should an additive effect be shown, early and efficient intervention with dual finerenone and SGLT2i therapy could slow disease progression and provide long-term benefits for people with CKD and T2D.
Clinical Trial Registration Number: NCT05254002
Supported by: The CONFIDENCE study is supported by Bayer AG
Disclosure: J.B. Green: Employment/Consultancy; Duke University School of Medicine and Duke Clinical Research Institute, Boehringer Ingelheim/Lilly, Bayer, AstraZeneca, Sanofi, Hawthorne Effect/Omada, Pfizer, and Novo Nordisk. Grants; Boehringer Ingelheim/Lilly, Merck, Roche, and Sanofi.
752
Optimisation of albuminuria lowering treatment by cross-over rotation to four different drug classes
V. Rotbain Curovic1, M.Y.A. Kroonen2, N. Jongs2, E.H. Zobel1, T.W. Hansen1, G.D. Laverman3, A. Kooy4, F. Persson1, P. Rossing1, H.J.L. Heerspink2;
1Steno Diabetes Center Copenhagen, Herlev, Denmark, 2Department of Clinical Pharmacy and Pharmacology, University of Groningen, Groningen, Netherlands, 3ZiekenhuisGroep Twente, Almelo, Netherlands, 4Bethesda Diabetes Research Center, Hoogeven, Netherlands.
Background and aims: Renin-angiotensin system (RAS) inhibitors decrease urinary albumin:creatinine ratio (UACR) and are guideline recommended drugs for kidney protection but are ineffective in lowering UACR in up to 40% of cases. We hypothesized that rotation to another drug class overcomes resistance to RAS inhibition and tested this hypothesis in a randomized cross-over trial.
Materials and methods: We assigned 26 adults with type 1 diabetes and 37 with type 2 diabetes and UACR ≥30 and ≤500 mg/g to 4-week treatment periods with telmisartan 80 mg, empagliflozin 10 mg, linagliptin 5 mg, and baricitinib 2 mg in random order, separated by 4-week wash-out periods. Participants were then re-exposed for 4-weeks to the individual drug that induced the largest UACR reduction. Primary outcome was the difference in UACR response between the first and second exposure to the best performing drug, versus the difference in UACR response between the best performing drug and the other three drugs.
Results: There was substantial between person variation in the best performing drug: telmisartan was best performing in 33 (52%) participants, followed by empagliflozin and linagliptin in 11 (17%) participants each, and baricitinib in 8 (13%) participants. The individual best performing drug changed UACR during the first exposure by -39.6% (95%CI -44.8, -33.8, p<0.001) and by -22.4% (95%CI -29.7, -12.5, p<0.001) at re-exposure (between exposure difference: 22.1% [95%CI 12.5, 30.8; p<0.001]). The difference in UACR response between the individual best performing drug and the other three drugs was -40.5% (95%CI -45.9, -34.6, p<0.001 vs. between exposure difference). The correlation in UACR response of the best performing drug at exposure and re-exposure was r=0.389, p=0.017.
Conclusion: We demonstrated a large and reproducible variation in UACR lowering responses to different drug classes reinforcing the need for personalized therapy approaches to overcome therapy resistance to guideline recommended treatment.
Clinical Trial Registration Number: NTR5602 and NTR5602
Disclosure: V. Rotbain Curovic: None.
753
Post approval, observational, concurrent study to assess clinical effectiveness and safety of dapagliflozin in diabetic kidney disease: INTENSE - DAPA study
K. Korukonda Dr;
Medical Services, Torrent, Ahmedabad, India.
Background and aims: Type 2 DM continues to evolve with high burden of morbidity and microvascular complications despite the current advances in therapy. Further, TODAY study suggests progressive diabetes and glycemic status with incremental rise in microvascular complications despite background therapy with Metformin. SGLT2is including Dapagliflozin offers ancillary extraglycemic effects including Adenosine modulation for Cardio-renal metabolic benefits. An observational study planned to assess Dapagliflozin in Indian diabetics with Diabetic kidney disease
Materials and methods: A post-approval, concurrent, observational, multicenter single arm study was conducted between Oct ’20 to Aug ’21 at 1550 centres with local institutional ethics committee approval. Descriptive statistics and Chi-square test were employed for categorical and continuous variables including HbA1c, FPG, PPBG and proteinuria were analysed using SPSS Ver. 20.0 software
Results: Intent- to- treat (n=18,600) and PP (n=18166) analysis for treatment completers during the 12 Week observation period for HbA1c, FPG, PPBG and proteinuria variables receiving metformin (1000 mg/d) at background therapy was carried out. Baseline demographics included Age (55.0 ± 9.8 y) , BMI (30.1 ± 20.1 kg/m2 ), Hypertension (n=12048, 66.3%); Dyslipidemia (n=8505,46.8%); Stroke/TIA (n=3505, 19.3%); Chronic Heart Failure (n=1113, 6.1%); Myocardial infarction (n=742 , 4.1%), CKD (n=18166). After 12 week of treatment, the mean change in HbA1c for Dapagliflozin combination therapy was -1.35 ± 1.1 % (p <0.001); -1.32 ± 0.9 % (p <0.001) ; -1.19 ± 1.0 % (p <0.001); -1.36 ± 1.1 % (p <0.001) in overall, Patients with >2 risk factors (RF), microalbuminuria (<300 mg/g) and macroalbuminuria (> 300 mg/g) group respectively. After 12 week of treatment, the mean change proteinuria (mg/g) was -240 ± 32.5 (p<0.001) , -460 ± 36.8 (p<0.001); -80 ± 0.73 (p<0.001); -264± 33.9 (p<0.001) in overall, patients with >2 RF, micro (30 to 300 mg/g) and macroalbuminuria group (>300 mg/g) respectively. five cases with urinary tract infections as TEAE that required no further treatment modification or withdrawal of Dapagliflozin 10 mg. There were no other SAEs
Conclusion: In patients with DKD , Dapagliflozin shows significant improvement in HbA1c, FPG and PPBG. These improvements were consistent in subgroup analysis of high-risk Indian DKD cases with metabolic traits, obesity or albuminuria
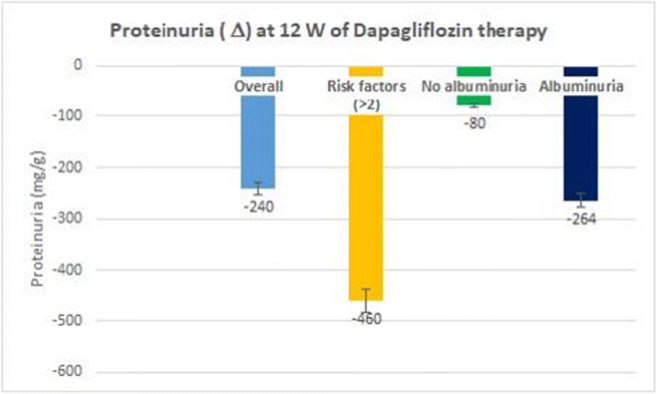
Disclosure: K. Korukonda Dr: None.
754
Anserine is a H 2 S releasing molecule and therefore a therapeutic agent in diabetic nephropathy
C. Wetzel1, T. Pfeffer1, J. Zemva2, R. Bulkescher2, K. Klingbeil1, C.P. Schmitt1, V. Peters1;
1Centre for Paediatric and Adolescent Medicine, University of Heidelberg, 2Department of Medicine I and Clinical Chemistry, University of Heidelberg, Heidelberg, Germany.
Background and aims: Inflammation and oxidative stress are tightly linked and are emerging as key factors in diabetic nephropathy. Hydrogen sulfide (H2S), a gaseous signalling molecule, and anserine, a dipeptide, have several cytoprotective properties. Understanding the interrelation may lead to new therapeutic interventions. We therefore studied a putative interaction between renal anserine and H2S metabolism in mice with increased anserine concentration via Carnosinase 1 knockout (Cndp1-KO) under diabetic conditions.
Materials and methods: Diabetes mellitus type 1 was induced by Streptotozin (STZ) in Wildtype (WT) and Cndp1-KO mice. Diabetic mice were treated with insulin and sacrificed after 12 weeks of diabetes (23-25 weeks old). Maximal H2S synthesis capacity was measured in vitro in human proximal tubular cells (HK2), primary human umbilical artery and vein endothelial cells (HUAEC, HUVEC), in WT and heat shock protein 70 knockout (Hsp70-KO) immortalized murine cardiac endothelial cells (MCEC) and ex vivo in murine renal tissue lysate. For H2S detection a colorimetric assay was used. Anserine concentration was determined via HPLC.
Results: In HK2 cells, addition of anserine dose-dependently increased H2S formation (p≤0.0001). Incubation with 1 mM anserine increased H2S formation by 1.5-fold in HK2, HUAEC and HUVEC (p≤0.0001; p≤0.0001; p≤0.001) and by 1.3-fold in MCEC (p≤0.001) compared to untreated cells. In Hsp70-KO MCEC, H2S formation was 60% lower compared to MCEC WT cells (p≤0.001), incubation with anserine had no effect on H2S formation (all p=n.s.). In WT mice, renal H2S formation was independent on gender (p=n.s.). In Cndp1-KO mice, renal anserine concentration was 5-fold increased in females (3.872 ± 2.464 vs. 0.7839 ± 0.3476 nmol/mg protein, p≤0.01) and 2-fold increased in males (2.0600 ± 0.5851 vs. 0.6927 ± 0.4715 nmol/mg protein, p≤0.01) compared to WT mice. In female Cndp1-KO mice, H2S formation was 2-fold increased (0.0213 ± 0.0045 nmol H2S/mg protein, p≤0.0001) compared to WT mice. In male Cndp1-KO mice, H2S formation was not increased compared to WT mice. In Cndp1-KO mice, H2S formation correlated with renal anserine concentration (r=0.7970). In STZ WT mice, renal anserine concentration and H2S synthesis were similar to non-diabetic WT mice (p=n.s.). In diabetic female Cndp1-KO mice, renal H2S formation was reduced by 65% (0.0138 ± 0.0025 nmol H2S/mg protein, p≤0.0001) but not in respective male mice. In diabetic Cndp1-KO mice, renal anserine concentration was reduced by 50% in female (1.67 ± 0.7719 nmol/mg protein, p≤0.05) and by 30% in male (1.453 ± 0.2907 nmol/mg protein, p=n.s.) compared to non-diabetic Cndp1-Ko mice, but was still 2.5-fold higher in female and 1.9-fold higher in male compared to WT mice (p=n.s.). In diabetic Cndp1-KO mice, reduced H2S formation correlated with decreased anserine concentration (r=0.6519). In renal tissue lysate of female Hsp70-KO mice, H2S formation was halved (p≤0.0001) in comparison to WT.
Conclusion: We provide evidence that anserine is a H2S-releasing molecule, in part Hsp70 dependently, and underline its potential role as a promising therapeutic agent in diabetic nephropathy.
Supported by: DFG, SFB1118
Disclosure: C. Wetzel: None.
755
Therapeutic blocking of NPRC, a podocyte - expressed target, is kidney protective in diabetic nephropathy
D. Dabaghie1, E. Charrin1, M. Lal2, J. Patrakka1;
1Laboratory Medicine, Karolinska Institutet, Stockholm, 2Bioscience Renal, Research and Early Development Cardiovascular, Renal and Metabolism (CVRM), AstraZeneca, Gothenburg, Sweden.
Background and aims: Renal glomerulus is the main target of injury in diabetic nephropathy (DN). Especially, terminally differentiated glomerular podocyte cells play a key role in the progression of DN. Molecular mechanisms of DN-associated glomerulopathy are poorly understood.Natriuretic peptide (NP) system is a key player in the regulation of blood pressure. NP-system has three receptors: NP-receptors A and B (NPRA/B) activate guanyl cyclase to generate key secondary messenger cGMP, whereas NP-receptor C (NPRC) acts as a clearance receptor. Mouse studies have shown that local NP-signalling in the glomerulus is important for the maintenance of renal homeostasis. In patients with DN, transcriptomics studies have shown down-regulation of NPRA and NPRC in the glomerulus. In this study, we aimed to elucidate the role of NPRC in the pathogenesis of glomerulopathies and explore whether glomerular NP-signalling could be a novel therapeutic target for DN.
Materials and methods: Expression of NPRC was studied using qPCR and immunohistochemistry. The functional role of NPRC was analysed using a novel mouse line in which NPRC was inactivated specifically in podocytes (NPRC podKO). NPRC was targeted pharmaceutically using a NPRC blocking peptide in a rat model of DN (uninephrectomized obese ZSF-1 rats (UNx ZSF-1 rats)), followed by studies in a mouse glomerulopathy model.
Results: NPRC was highly expressed by glomerular podocyte cells. NPRC podKO mice showed normal kidney morphology and function, and NPRC-deficiency did not affect the outcome of mouse glomerulopathy model. In diabetic UNx ZSF-1 rats, pharmaceutical targeting with NPRC-antagonist resulted in an increased urinary and plasma cGMP levels, indicating successful target engagement. Histological evaluation of diabetic rats treated only with the antagonist showed an unsignificant trend towards reduced glomerular damage. However, when NPRC blocking was combined with losartan (angiotensin receptor blocker), it potentiated significantly the ameliorative effects on albuminuria and glomerular sclerosis. In line with this, the NPRC-antagonist showed reno-protective effects in a mouse glomerulopathy model as shown by decreased glomerulosclerosis and reduced podocyte loss.
Conclusion: NPRC is highly expressed by renal podocyte cells and its pharmaceutical targeting seems to mediate reno-protective effects in DN. More studies are clearly needed to understand molecular mechanisms of NP-signalling in the glomerulus and to explore whether chronic NPRC inhibition can be a suitable modality to treat DN.
Supported by: AstraZeneca, SSMF, Diabetesfonden
Disclosure: D. Dabaghie: Grants; AstraZeneca, SSMF, Diabetesfonden.
756
Effect of dapagliflozin on arterial stiffness and biomarkers of arterial ageing in people with type 2 diabetes and kidney disease
D. Stathi, N. Fountoulakis, M. Flaquer, A. Corcillo, A. Panagiotou, A. Mangelis, S. Ayis, L. Gnudi, J. Karalliedde;
King's College, London, UK.
Background and aims: Sodium glucose co-transporter 2 (SGLT-2) inhibitors have demonstrated renal benefits in people with type 2 diabetes (T2DM). The exact mechanisms that explain these impressive results remain unclear. Arterial stiffness as measured by aortic pulse wave velocity (Ao-PWV) is an index of arterial ageing and independent predictor of cardio-renal outcomes. The effect of SGLT-2 inhibitors on Ao-PWV and other markers of arterial ageing remains to be defined.
Materials and methods: We performed a 24-week single center randomised controlled trial comparing dapagliflozin and ramipril (D + R) versus ramipril only (R) on markers of arterial ageing in people with T2DM with residual microalbuminuria despite maximum tolerated renin angiotensin system (RAS) inhibition. All participants had estimated glomerular filtration rate >60 ml/min. The study was powered for the primary endpoint of change in urine albumin excretion rate (AER). Secondary endpoints included Ao-PWV (by applanation tonometry), central aortic blood pressure, mediators of the RAS (plasma renin activity, aldosterone, ACE-2 and Angiotensin 1-7/1-9 levels) and bio-markers of arterial ageing [soluble Klotho (sKlotho) and fibroblast growth factor 23 (FGF-23)].
Results: In total, 33 participants (male 72.7%) mean age of 58 years (range 42 to 75 years) were randomized to Dapagliflozin and Ramipril (D+R n=17) or Ramipril (R n=16). After 24 weeks of treatment, AER fell significantly [mean (95% confidence interval)] only in D + R by 43.5% (-57.36% to 29.56%) (p value: <0.01) as compared to 5% (-48.3% to 38.3%) (p value: 0.36) in R. However, Ao-PWV (mean ±SD) did not change significantly from baseline after 24 weeks treatment [D +R 9.06 ±1.91 to 9.13 ±2.03 vs R 9.88 ±2.12 to 10.0 ±1.84]. Similarly, we do not observe any significant changes in central aortic blood pressure, augmentation index, sKlotho or FGF-23. HbA1c fell significantly only in D + R arm, while a modest but significant fall in Angiotensin 1-7 was also noted.
Conclusion: The combination of Dapagliflozin and Ramipril for 24 weeks significantly reduces albuminuria, but did not impact on Ao-PWV or other mediators/bio-markers of arterial ageing. Our data suggest that the early cardio-renal benefits of SGLT-2 inhibitors in people with T2DM are unlikely to be related to improvement in arterial ageing.
Clinical Trial Registration Number: 2013-004042-42
Supported by: AZ
Disclosure: D. Stathi: None.
757
Beneficial effects on metabolic disorders by a long-acting glucagon analogue, HM15136, in pre-clinical models
J. Lee, S. Lee, J. Kim, E. Park, J. Lee, D. Kim, S. Bae, S. Lee, I. Choi;
Hanmi Phaarm. Co., Ltd, Seoul, Republic of Korea.
Background and aims: Obesity is implicated with various metabolic disorders such as hyperlipidemia, cardiovascular disease (CVD) and chronic kidney disease (CKD). In previous studies, robust body weight loss (BWL) in rodent obese models was consistently demonstrated by chronic treatment of HM15136. Also, recent studies unveiled a novel role of GCG on vasodilation and inflammation. Based on these findings, we hypothesized that HM15136 might be able to provide a beneficial effect on not only obesity but also another metabolic disorder such as CKD. Here, potential effects of HM15136 was investigated in animal models of various metabolic disorders.
Materials and methods: HM15136 was administered to diet induced obese (DIO) mice and changes in BW, liver triglyceride (TG) and blood cholesterol were measured. Next, in spontaneously hypertensive rats (SHRs), urinary albumin excretion and albumin/creatinine ratio were determined during 6 weeks treatment of HM15136. To further investigate therapeutic effect on renal disease, unilateral ureteral obstruction mice (UUO mice) was administered with HM15136 for 2 weeks, followed by determining the renal fibrosis markers and histopatological score. Human primary podocyte and HK-2 cells were used for mechanistic study to unveil a MoAs on beneficial effect of HM15136.
Results: In DIO mice, time course changes in BW, liver TG, and blood cholesterol were measured, and all measurements were significantly reduced by HM15136 treatment (-37.5, -72.7, -78.4% vs. vehicle for BW, liver TG, blood CHO at day 17). In SHRs, a known animal model of hypertensive CKD, continuous elevation of urinary albumin excretion and urine albumin/creatinine ratio (ACR) indicates progressive renal function impairment of this model. Interestingly, urinary albumin excretion (-35.2% vs. SHR vehicle at day 46) and ACR (-23.6% vs. SHR vehicle at day 46) were meaningfully improved by HM15136 treatment. Moreover, HM15136 treatment reduced renal fibrosis marker gene expression (-55.6, -63.5, and -67.6% vs. vehicle for TGF-β, collagen-1α1, and collagen-3 α1), and protein level of pro-collagen-1α1 (-48.2% vs. UUO vehicle) in UUO mice, a known animal model of acute kidney injury. Consistently, significant improvement of histopatological score (-2.0 vs. UUO vehicle) was observed, which suggests the potential therapeutic effects of HM 15136 on kidney injury. In mechanistic study, suppression of stress-induced apoptosis in human primary podocyte and reduced level of EMT markers (collagen-1α1 and collagen-3 α1) in HK-2 cells (proximal tubular epithelial cell) well explain how HM15136 could provide a renal protection effects in CKD and acute kidney injury animal models.
Conclusion: These results highlight that HM15136 could have therapeutic potential on not only obesity but also related complications especially renal disorders. Further studies are needed to assess a clinical relevance of these findings.
Disclosure: J. Lee: None.
SO 65 Translating signals in the diabetic kidney
758
Reduced mitochondrial DNA copy number (mtDNA-CN) in type 1 diabetes and kidney damage and correlates thereof and mtDNA-CN changes over time
A.S. Januszewski1,2, L.M. Carroll1, Y. Wen-Loh1, M.L.H. Huang1, D.N. O'Neal2, A.J. Jenkins1,2;
1NHMRC Clinical Trials Center, University of Sydney, Sydney, 2Department of Medicine, University of Melbourne, Melbourne, Australia.
Background and aims: Mitochondrial DNA (mt-DNA) copy number (mtDNA-CN), reflecting the amount of mt vs. nuclear DNA in a cell, is a potential marker / mediator of diabetic kidney disease (DKD). Most studies are in Type 2 diabetes. We determined mtDNA-CN in Type 1 diabetes (T1D) and non-diabetic (CON) adults and associations with T1D complications and risk factors.
Materials and methods: In a cross-sectional study mtDNA-CN was measured in T1D and CON subjects and in a T1D subset was measured at ≥2 time-points. mtDNA-CN was quantified by RT-PCR (ScienCell, Carlsbad, CA) and DNA isolated (Qiagen, Hilden, Germany) from whole venous blood (EDTA) from fasted adults: 178 T1D and 132 CON. C-peptide was measured by ELISA (Mercodia, Uppsala, Sweden), with lower limit of detection 1.25 pM (0.0038 ng/mL). Metabolic syndrome in T1D was defined by ≥2 of: hypertension, increased waist, low HDL-C or high TG and in CON ≥2 of: hypertension, increased waist, low HDL-C, high TG or high glucose. All subjects provided written informed consent.
Results: Subjects: T1D: N=178, (mean(SD) age 38(14)yrs, 21(14)yrs T1D, HbA1c: 8.0(1.5)%, median (LQ,UQ) eGFR 103 (84, 130) mg/ml/kg ; urine ACR 0.60 (0.39, 1.75) mg/mmol , 36% with microvascular complications (CX+) of sight-threatening diabetic retinopathy (STDR) and/or DKD; CON: N=132, age: 37(14)yrs, HbA1c: 5.1(0.4)%), eGFR 104 (92, 119) mg/ml/kg, urine ACR 0.42 (0.29, 0.74) mg/mmol. mtDNA-CN lower in T1D vs. CON: 271 (189, 348) vs. 320 (264, 410); p<0.0001. mtDNA-CN by tertiles in T1D: Tertile 1 (lowest) vs. tertile 3 (highest) mtDNA-CN. Cx were present in 41% of tertiles 1 + 2 vs. 25% in tertile 3, p=0.03 and 31% vs. 14% (p=0.02) had DKD; 14% vs. 29% (p=0.02) used insulin pumps and 61% vs. 79% had detectable C-peptide (p=0.045). mtDNA-CN tertile groups did not differ by sex, smoking, BMI, MetS, self-reported exercise, STDR, HbA1c, statins or ACE/ARB use. mtDNA-CN levels by T1D DKD status were lower in n=43 subjects with vs. without DKD (n=125): 238 (180, 309) vs. 294 (198, 364); p=0.02; and both were lower vs. CON (p=0.002 and p<0.0001 respectively). There were no mtDNA-CN differences by STDR status, coronary artery disease, nor any vs. no complications, all p>0.05. T1D-CX+ (253 (194, 317)) and T1D-CX- (286 (186, 364)) were lower vs. CON (p=0.001 and p=0.005, respectively). Correlates (Spearman) of mtDNA-CN in T1D: FBG (r=-0.19, p=0.02), HDL-C (r=0.17, p=0.03), WCC (r=-0.16, p=0.04), sVCAM-1 (r=-0.17, p=0.03); sICAM-1 (r=-0.20, p=0.02) CON: WHR (r=-0.22, p=0.03); WCC (r=-0.24, p=0.007). Independent determinants of mtDNA-CN in T1D (standardize value±SE; p-value): age (-0.25±0.10; p=0.01), HDL-C (0.24±0.10; p=0.01), sICAM-1 (-0.30±0.10; p=0.004). Area under ROC curve 0.71. CON: lower/medium (0) vs. higher (1) mtDNA-CN level were: pulse pressure (0.25±013; p=0.047), WCC (-0.41±0.15; p=0.005), smoking (never) (0.22±0.13; p=0.09); sex (female) (0.35±0.12; p=0.005). Area under ROC curve 0.74. mtDNA-CN over time: In n=48 T1D mt-DNA levels from 2 - 4 times over 3.4 years tended to decrease 3.4±13.3 p.a., but this was not significant, p=0.80.
Conclusion: T1D is associated with lower mtDNA-CN vs. CON. mtDNA-CN levels are lower in DKD vs. no DKD and with lower C-peptide, age and inflammation. mtDNA-CN levels in T1D remain relatively stable over time. Longitudinal and intervention studies are merited.
Disclosure: A.S. Januszewski: None.
759
Urinary long noncoding RNA to differentiate diabetic kidney disease (DKD) from non-diabetic kidney disease (NDKD)
M. Basu1, S. Neogi1, P. Mukhopadhyay1, N.P. Bhattacharyya1, A. Raychaudhury2, S. Ghosh1;
1Endocrinology, Institute of post graduate Medical Education and Research, 2Nephrology, Institute of post graduate Medical Education and Research, Kolkata, India.
Background and aims: Background: Renal involvement in Type 2 diabetes can be due to diabetes per se (Diabetic Kidney Disease-DKD) or other than diabetes (Non-diabetic Kidney Disease NDKD). Amongst currently available clinical, biochemical and radiological markers none of the covariates were found to be predictors of NDKD. Renal biopsy remains gold standard for diagnosis. lncRNA account for epigenetic changes and are found in the urinary cell, urine supernatant, and as well as in exosomes. The urinary cell has higher nucleic acid content and is relatively unaffected by humoral metabolic factors than supernatant urine. Exosomes are extracellular vesicles that are involved in cell-to-cell communication and act as cargo for the transport of proteins and nucleic acids. In animal models with diabetes and kidney disease, there is dysregulation of several lncRNA. This has not been explored in humans with biopsy-proven kidney disease in T2 DM.Aims: To determine the expression of lncRNA from urine to evaluate if this could be used as non-invasive markers to differentiate DKD from NDKD.
Materials and methods: Methodology: We recruited consecutive patient with renal involvement (eGFR >30ml/min/m2 to <60ml/min/m2 and/or ACR>300mg/mcg who underwent renal biopsy and histopathologically classified (ISN/RPS). 5ml of second-morning urine sample in fasting state was collected and expression of different lncRNAs (MALAT1, NEAT, GAS5, PVT1, HOTAIR) were determined in urinary cell and urinary exosomes. Differences were determined by quantitative real-time PCR with respect to internal control. Mann-Whitney U and Receiver operating characteristic (ROC) curves) were performed
Results: Results: Expression of MALAT1, PVT1, NEAT, and HOTAIR levels were higher in the urinary cells, this was further validated in exosomes. In exosomes, MALAT1 had the best discriminatory ability to differentiate DKD from NDKD (2 fold (p<0.05) with 90% sensitivity and specificity of 82%. We have further validated the data in a second cohort. Using a Δ CT value <8 (as derived from the discovery cohort) MALAT1 had a specificity of 86% and 93% sensitivity to diagnose DKD.
Conclusion: Conclusion: Urinary MALAT1, a lncRNA may help distinguish DKD from NDKD.
Supported by: RSSDI,RSSDI WB,WBDST,ICMR
Disclosure: M. Basu: None.
760
Renal carnosine and anserine concentrations protect against diabetes induced renal interstitial fibrosis and oxidative stress
T. Pfeffer1, P. Kirschner1, C. Wetzel1, M. Bartosova1, T. Poth2, G. Poschet3, J. Zemva4, I. Damgov5, S.F. Garbade1, K. Klingbeil1, C. Schmitt1, V. Peters1;
1Centre for Paediatric and Adolescent Medicine, University Hospital Heidelberg, 2Center for Model System and Comparative Pathology, University Hospital Heidelberg, 3Centre for Organismal Studies, University of Heidelberg, 4Internal Medicine I and Clinical Chemistry, University Hospital Heidelberg, 5Institute of Medical Biometry and Informatics, University of Heidelberg, Heidelberg, Germany.
Background and aims: Exogenous carnosine and anserine mitigate diabetic nephropathy (DN) in rodents. The underlying mechanism, i.e., direct renal protective action versus systemic effects remains unclear. The effect of increased renal carnosine/anserine concentrations in Carnosinase 1 KO (Cndp1-KO) mice under diabetic conditions was analysed in this study.
Materials and methods: Type 1 diabetes mellitus was established in Cndp1-KO and B6 WT mice by Streptotozin (STZ) with high fat (HFD) and normal diet (ND). Mice were treated for 32 weeks with insulin; body mass (BM), energy and liquid intake, blood sugar, urinary albumin-creatinine ratio (ACR) were monthly assessed; at sacrifice kidneys were harvested for histopathology and molecular studies.
Results: Renal carnosine concentration was increased 9-fold and renal anserine concentration was increased 5-fold in Cndp1-KO compared to WT mice. Under diabetic conditions (STZ), carnosine and anserine concentrations were reduced, but still both 2.9-fold higher in Cndp1-KO. In Cndp1-KO mice, HFD increased renal carnosine and anserine concentration by 3.6- and 2.2-fold compared to Cndp1-KO mice under ND (p=0.002/p=0.04). In diabetic Cndp1-KO, HFD led to a less pronounced decrease of carnosine and anserine (21/48%) compared to diabetic Cndp1-KO mice under ND. Carnosine-synthase protein expression was not altered between WT and Cndp1-KO mice, independent of the diet. Oxidative stress (4-HNE) was reduced in diabetic Cndp1-KO mice (STZ and STZ+HFD) compared to the respective WT controls (50/86%, p=0.005/p=0.01). Interstitial fibrosis was reduced in STZ+HFD Cndp1-KO vs. WT (40%, p=0.01), thus not altered under ND and STZ, while Tgf-β mRNA, phospho-SMAD2/3 and TSP-1 protein expression were unchanged. Mesangial expansion was increased in Cndp1-KO vs WT by 25% (p=0.009). Renal vessel/lumen ratio, a marker of vasculopathy, was unaltered in all groups. Body mass, energy/liquid intake, glucose homeostasis, insulin-need/BM and organ weight or ACR were not different between any of the studied groups.
Conclusion: Increased renal anserine/carnosine concentrations reduced renal oxidative stress and interstitial fibrosis, but did not alter glucose metabolism.
Supported by: DFG, SFB1118
Disclosure: T. Pfeffer: None.
761
Association between plasma hypoxanthine and diabetic kidney disease (DKD) in a Japanese population
M. Yamamoto1, S. Harada2, T. Okamura2, K. Fujihara1, S. Kodama1, T. Yamada1, H. Sone1, T. Takebayashi2;
1Department of Hematology, Endocrinology and Metabolism, Niigata University Faculty of Medicine, Niigata, 2Department of Preventive Medicine and Public Health, Keio University School of Medicine, Tokyo, Japan.
Background and aims: Some plasma metabolites in the purine metabolism were reportedly associated with DKD, although results were inconsistent among studies. Also, few studies examined the association between plasma hypoxanthine and DKD in community dwellers. Hypoxanthine is one of the most important derivatives of purine metabolism, and is associated with many diseases including kidney diseases. However, the association between plasma hypoxanthine concentration and the status of DKD including its subtypes in community dwellers is not fully understood.
Materials and methods: To address this, we divided patients with DKD into 3 subtypes: [a] reduced eGFR (eGFR <60 ml/min/1.73m2) and non-proteinuria, [b] normal eGFR (eGFR ≥ 60 ml/min/1.73m2) and proteinuria, and [c] both reduced eGFR (eGFR <60 ml/min/1.73m2) and proteinuria. We used the metabolite database from the Tsuruoka Metabolomics Cohort Study, which had global metabolomics in Japan using capillary electrophoresis-mass spectrometry. Among 10,933 participants, cross-sectionally analyzed were 1,005 who had diabetes mellitus (DM) defined by HbA1c ≥6.5%, and/or fasting blood glucose ≥126 mg/dl and/or antidiabetic drug use. Of these, 231 had DKD, which was defined by eGFR <60 ml/min/1.73m2 and/or test-strip positive proteinuria ([a] 144, [b] 58, and [c] 29 participants). 774 participants did not have DKD.
Results: Baseline characteristics of the total study participants were mean age 64 y, mean HbA1c 6.8±0.9%, mean BMI 25.1±3.8, and mean eGFR 73.4 ± 16.0 ml/min/1.73m2. 87 participants had overt proteinuria (9% of total participants). Plasma hypoxanthine level was positively associated with DKD (Spearman’s rank correlation coefficients 0.129, p <0.001). Univariate and multivariate logistic regression models were used to identify the association between plasma hypoxanthine and DKD including its’ three subtypes in DM patients. Multivariate logistic regression models investigating the association between plasma hypoxanthine and DKD (n = 231) showed that odds ratios per 1 μmol/l increase in hypoxanthine for DKD were 1.42 (95% confidence interval, 1.22-1.65) after adjustment for possible confounders: age, sex, BMI, alcohol intake, smoking habit, energy intake and history of hypertension or dyslipidemia. In addition, we found that plasma hypoxanthine was associated with all 3 subtypes of DKD. Multivariate logistic regression models investigating the association between plasma hypoxanthine and DKD subtypes showed that odds ratios per 1 μmol/l increase in hypoxanthine for DKD subtypes were 1.28 (1.08-1.52) in (a), 1.63 (1.27-2.08) in (b), and 1.69 (1.23-2.34) in (c) as continuous variables.
Conclusion: The results showed that plasma hypoxanthine was elevated in ALL subtypes of DKD regardless of vast differences in pathophysiology. High plasma hypoxanthine levels increase risk of DKD, regardless of its types. These relationships are not affected by covariates. These results implied that plasma hypoxanthine could be a screening marker for DKD regardless of its stage and status, although future prospective studies are required.
Disclosure: M. Yamamoto: None.
762
Advanced glycation endproducts in chronic kidney disease in subjects with and without diabetes: AGEs highly responsive to renal function decline and diabetes
N. Rabbani1, S. Panagiotopoulos2, R.J. MacIsaac2,3, D. Yue4, G.R. Fulcher5, M.A. Roberts6, E. Ekinci2, P.J. Thornalley7;
1College of Medicine, Qatar University, Doha, Qatar, 2Endocrine Centre, University of Melbourne, Melbourne, Australia, 3Department of Endocrinology & Diabetes, St Vincent’s Hospital Melbourne, Melbourne, Australia, 4Diabetes Centre, Royal Prince Alfred Hospital, Sydney, Australia, 5Department of Diabetes, Endocrinology & Metabolism, Royal North Shore Hospital, Sydney, Australia, 6Eastern Health Clinical School, Monash University, Melbourne, Australia, 7Qatar Biomedical Research Institute, Hamad Bin Khalifa University, Doha, Qatar.
Background and aims: Impairment of renal function with and without diabetes has been associated with increased serum levels of advanced glycation endproducts (AGEs). AGEs are formed by degradation of fructosamines, protein modification by glyoxal, methylglyoxal (MG), 3-deoxyglucosone (3DG), and other processes. Serum contains glycation adduct residues in protein and glycation free adducts (glycated amino acids) - the latter formed by proteolysis of damaged proteins with also contributions from absorption of glycated amino acids from digested proteins in food. The aim of this study was to identify which type of AGE is most responsive to stage of chronic kidney disease (CKD) with assay methodology transferrable for assessment by clinical chemistry.
Materials and methods: Study participants were subjects with diabetes (n = 64, 6 type 1 and 58 type 2) or without diabetes (n = 71) in 4 categories of estimated GFR (ml/min/1.73 m2): normal and CKD stages 2, 3 and 4 (n = 7 - 29 per category). Fasting serum samples were obtained prior to a routine clinic visit and stored at -20°C until assay. Analytes were assayed by stable isotopic dilution analysis liquid chromatography tandem-mass spectrometry (LC-MS/MS): AGEs - CML, CEL, G-H1, MG-H1, 3DG-H, CMA, pentosidine, GOLD and MOLD, and oxidative damage markers - N-formylkynurenine, dityrosine and 3-nitrotyrosine. Free adducts were assayed by analysis of 12 kD ultrafiltrate and protein adducts after exhaustive enzymatic hydrolysis of serum protein. Study group medians are compared.
Results: Glycation free adducts were the most responsive to decline in renal function: protein AGE residues increased up to 3-fold in stage 4 CKD whereas AGE free adducts increased up to 8-fold in subjects without diabetes and 30-fold with diabetes. MG-derived MG-H1 free adduct was most responsive to decline in renal function. Glomerular filtration flux of MG-H1 free adduct was increased ca. 5-fold in patients with diabetes. Median, lower - upper quartile (nmol/min): control 11.1 (6.9 - 16.7) versus diabetes 51.7 (32.4 - 67.5); P = 3 x 10-18, Mann-Whitney U. MG-H1 free adduct is readily measured: sample preparation time 60 min, assay time 15 min, with calibration and internal standards available commercially.
Conclusion: AGEs accumulate with decline in renal function with AGE free adducts being the most responsive. MG-H1 free adduct was the AGE free adduct of highest concentration in serum, increased most markedly with decline in renal function and was higher in subjects with diabetes, compared to subjects without diabetes, at all stages of CKD studied. The 5-fold increase in glomerular filtration flux of MG-H1 free adduct in diabetes suggests patients with diabetes have marked increased exposure to this AGE, independent of renal function. Analytical aspects suggest measurement of MG-H1 free adduct by LC-MS/MS is practicable for clinical chemistry profiling.
Supported by: NHMRC and QF
Disclosure: N. Rabbani: None.
763
Use of spot urine urea nitrogen to estimate protein intake in patients with diabetic chronic kidney disease
J.Y. Lee1, Y.H. Kim1, S.J. Kim1, S.S. Kang2, S.B. Kim1;
1Nephrology, internal medicine, Asan medical center, 2Dietetics and nutrition service, Asan medical center, Seoul, Republic of Korea.
Background and aims: Reducing protein intake is important in slowing the progression and in the symptomatic treatment of advanced uremia in patients with diabetic chronic kidney disease (DCKD).
Materials and methods: This cross-sectional study enrolled 305 DCKD patients, none on dialysis, who visited the Nephrology Clinic at the Asan Medical Center. This study evaluated use of spot UUN concentrations in estimating 24 h UUN and daily protein intake in DCKD patients. We calculated a linear regression equation to determine the relationship between spot UUN and 24 h UUN. Correlations between variables were assessed by Pearson’s correlation coefficient (r). Gender differences, assessed according to CKD stage, were compared with the Chi square test.
Results: 24 h UUN and spot UUN were significantly lower in patients with more advanced CKD (p < 0.001 each). Total protein intake assessed by dietary recall, but not protein nutrient density (g protein/1000 kcal), differed significantly among the groups of patients with different stages of CKD (p < 0.001). Protein intake was significantly correlated with 24 h UUN (r = 0.689, p < 0.0001). The correlation coefficient between 24 h UUN and spot UUN was 0.465 (p < 0.001) and that between protein intake and spot UUN was 0.378 (p < 0.001). There was a linear relationship between spot UUN and 24 h UUN as follows: spot UUN (mg/dl)=40.5×24 h UUN(g) +273.8. Therefore, a 24 h UUN of 7.8 g (protein intake 50 g/day) corresponds to a mean spot UUN concentration of 590 mg/ dl.
Conclusion: Measurement of spot urine urea nitrogen is a simple and effective method for monitoring protein intake in DCKD patients. A daily protein intake of 50 g corresponds to a spot urine urea nitrogen concentration of approximately 590 mg/dl in DCKD patients.
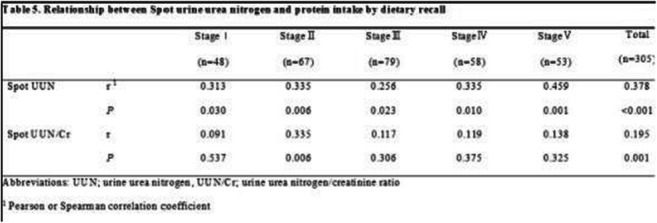
Disclosure: J.Y. Lee: None.
764
Standards of diabetes care in people with diabetes on peritoneal dialysis: an audit at three large university hospital foundation trusts
M. Onyema1, H. Eid2, N.B. Paray3, J. Dick1, D. Moutzouris2, J. Williams3, P. Vas1, J. Karalliedde2;
1King's College Hospital, London, 2Guy's and St Thomas' Hospital, London, 3Royal Devon and Exeter NHS Trust, Exeter, UK.
Background and aims: The number of people with diabetes on peritoneal dialysis as preferred renal replacement therapy is increasing globally. More than 10% of people on dialysis worldwide are on peritoneal dialysis. People with diabetes on all forms of dialysis are at risk of fragmented diabetes care. Moreover the management of their diabetes can be complex and challenging as they have a high predisposition to glycaemic variability and hypoglycaemia. Peritoneal dialysis can further make optimising diabetes control difficult due the use of glucose containing solutions. There is limited information on the standards of diabetes care received by people with diabetes on peritoneal dialysis.
Materials and methods: We audited the care of all people with diabetes on peritoneal dialysis at 3 large university hospital foundation trusts between December 2021-January 2022 against the standards set in the UK Diabetes in Haemodialysis guidance (as no specific guidelines for peritoneal dialysis are currently available) which are : 100% of people should have annual review of glycaemic control by a diabetes specialist, annual eye screening, annual foot risk assessment, target HbA1c 58-68mmol/mol, (>80mmol/mol poor glycaemic control or if <58mmol/mol reduction in treatment should be considered)
Results: In total 65 participant records (type 1 diabetes n=15) were examined, and their demographics were broadly similar across the sites except for ethnicity. All participants except one were on glucose containing dialysis regimes. The percentage of people with diabetes on peritoneal dialysis seen at least annually varied between 63% and 94%. Of those people seen less than annually 58% were on insulin. Of the cohort 92% were in a retinal screening program with at least annual review, 77% had at least an annual foot review, which were broadly similar across the three sites. HbA1c results in a clinically ‘acceptable’ range of 58-80mmol/mol was observed in 32% of people and 9% had poor control (defined as >80mmol/mol). Of the 58% of people who had HbA1c <58mmol/mol, 66% were on a treatment that could be associated with hypoglycaemia (sulphonylurea or insulin). Self-reported hypoglycaemic events (at least 1 event per month) were reported by 21% of the cohort. Of the 65 people 2 had a diabetes related admission in the preceding 12 months.
Conclusion: We observed that frequency with which people with diabetes on peritoneal dialysis were seen by a diabetes specialist team varied both within and between centres. Overall adherence to the retinal screening aims was good but the frequency of foot review was sub-optimal. Indeed, more frequent foot checks should be considered in view of high risk of diabetic foot disease in end stage kidney disease.We observed that 58% of the cohort had an HbA1c <58mmol/mol and of this group two thirds were on treatments that could cause hypoglycaemia. In this group 1 in 5 people reported hypoglycaemic events and would benefit from diabetes care input to review and reduce insulin or sulphonylurea doses. A multidisciplinary team approach is needed to improve the standards of diabetes care for people with diabetes on peritoneal dialysis.
Disclosure: M. Onyema: None.
SO 66 Mechanisms of diabetic kidney disease
765
Systemχ c- overexpression protects renal tubular epithelial cells from oxidative damage by GSH depletion
S. Yoo1,2, M. Kim1,2, J. Bae1, J. Choi3,4, J. Bang2, H. Chin5, G. Koh1,2;
1Department of Internal Medicine, College of Medicine, Jeju National University, 2Department of Internal Medicine, Jeju National University Hospital, 3Department of Rehabilitation Medicine, College of Medicine, Jeju National University, 4Department of Rehabilitation Medicine, Jeju National University Hospital, 5Department of Internal Medicine, Jeju Medical Center, Jeju-si, Republic of Korea.
Background and aims: Oxidative stress in renal tubular cells is known to be one of the etiologies of diabetic nephropathy. 2-Deoxy-D-ribose (dRib) causes oxidative damage by depleting GSH in cells, including beta cells. System χc- is a membrane transporter that moves extracellular cystine into cells independent of sodium, and cystine uptake through system χc- is a rate-limiting step for intracellular GSH synthesis, which is important for protecting cells from oxidative stress. We conducted this study to determine whether dRib causes oxidative damage in renal tubular cells and, specifically, to investigate the mechanism through which dRib increases oxidative stress.
Materials and methods: L-[14C]cystine uptake, GSH content, reactive oxygen species (ROS) levels, and cell viability were measured in NRK-52E cells, a renal tubular cell line, and the mRNA and protein expression of xCT, the functional unit of system χc-, were investigated. The xCT gene was then overexpressed in NRK-52E cells using lentivirus. L-[14C]cystine uptake, GSH, and cell viability were also measured in primary renal tubular epithelial cells isolated from rats.
Results: When NRK-52E cells were stimulated with various concentrations of dRiB, L-[14C]cystine uptake decreased, mRNA and protein expression of xCT increased, and intracellular GSH and ROS levels and cell viability were significantly decreased in a dose-dependent manner. L-[14C]cystine uptake, intracellular GSH and ROS levels, and cell viability reduced by dRib were all significantly recovered by xCT overexpression. In primary renal tubular epithelial cells, dRiB also significantly reduced L-[14C]cystine uptake, GSH content, and cell viability, and these were almost completely recovered by pretreatment with 2-mercaptoethanol, a cystine uptake enhancer.
Conclusion: In renal tubular epithelial cells, dRib depletes GSH by reducing cystine uptake through inhibition of xCT. The resulting oxidative damage can be prevented by overexpressing xCT. Therefore, it is thought that the oxidative damage in renal tubular cells can be prevented through systemχc- regulation.
Disclosure: S. Yoo: None.
766
Tunneling nanotubes-mediated lysosome transfer protects podocytes from diabetes-induced injury
F. Barutta, S. Bellini, I. Gesmundo, G. Togliatto, G. Gruden;
University of Turin, Turin, Italy.
Background and aims: Podocyte injury leading to albuminuria is a characteristic feature of diabetic nephropathy (DN). Dysfunction of the autophagy lysosomal pathway has been implicated in the pathogenesis of the podocyte damage in DN. There is thus increasing interest on novel therapeutic strategies of lysosome replacement/repair. Tunneling nanotubes (TNTs) are membrane channels that interconnect cells and allow transfer of organelles, including lysosomes, from donor to recipient injured cells. The cytosolic protein M-Sec plays a key role in TNT formation and we have previously reported that deletion of M-Sec exacerbates experimental DN. The aim of the present study was to investigate the underlying mechanisms of the detrimental effects of M-Sec deletion focusing specifically on TNT-mediated lysosome transfer and autophagy.
Materials and methods: In vivo study: the study was performed on WT and M-Sec-KO C57Bl6 mice. Animals were made diabetic by streptozotocin injection. Expression of LC3-II (autophagosome marker), p62 (autophagy substrate), and autophagy inducers (beclin, ATG5, ATG7) was assessed by WB, immunohistochemistry, and real-time PCR. In vitro study: M-Sec+/+/M-Sec-/- podocytes were exposed to vehicle, high glucose (HG), or advanced glycation end-products (AGEs). Subset of experiments were carried out on podocytes over/re-expressing M-Sec or treated with latrunculin-B to prevent TNT formation. Autophagy (LC3-II, p62, tandem fluorescent-tagged LC3), lysosomal membrane permeabilisation (acridine orange, cathepsin D distribution), lysosome function (cathepsin D activity), and apoptosis (TUNEL assay/Annexin V staining) were studied by WB, immuno/fluorescence, and functional assays. Formation of TNTs in WGA-stained podocytes was evaluated by both fluorescent and DIC microscopy. TNT-mediated lysosome transfer was assessed by flow cytometry in Cell Tracker Blue-labelled podocytes (recipient cells) exposed to HG/AGEs and co-cultured with donor podocytes carrying GFP-labelled lysosomes and pre-exposed to either vehicle or leupeptin.
Results: Neither diabetes nor M-Sec deletion altered the expression of autophagy inducers. However, M-Sec deletion worsened diabetes-induced glomerular LC3-II/p62 overexpression, indicating blockade of the autophagy flux. Exposure of podocytes to HG/AGEs caused lysosome damage, autophagy blockade, and enhanced apoptosis. Podocyte incubation with HG/AGEs also induced formation of TNTs in a M-Sec-dependent manner and enhanced TNT-mediated transfer of lysosomes from donor cells. Inhibition of TNT formation by either M-Sec deletion or latrunculin B aggravated HG/AGE-induced lysosome/autophagy dysfunction and apoptosis. On the contrary, these alterations were ameliorated/rescued by M-Sec overexpression and by co-culturing with healthy donor podocytes. Pre-treatment with leupeptin that causes lysosome dysfunction abolished the rescuing effect of donor podocytes.
Conclusion: M-Sec deletion aggravated diabetes-induced blockade of the autophagy lysosomal degradation pathway in both cultured podocytes and a model of experimental DN. Collectively, our in vitro results suggest that in the context of diabetes M-Sec is cytoprotective because it allows replacement of diabetes-injured lysosomes via TNTs, thus ameliorating autophagy and reducing apoptosis.
Supported by: JDRF; EFSD/Lilly Research Programme
Disclosure: F. Barutta: None.
767
Accumulation of trimethylamine N-oxide in renal tissue induces mitochondrial dysfunction in insulin-resistant mice
M. Videja1,2, M. Makrecka-Kuka1, S. Korzh1, E. Sevostjanovs1, H. Cirule1, E. Liepinsh1, M. Dambrova1,2;
1Latvian Institute of Organic Synthesis, 2Faculty of Pharmacy, Rīga Stradiņš University, Riga, Latvia.
Background and aims: For more than a decade trimethylamine N-oxide (TMAO) has been linked with the incidence of adverse cardiovascular events, but more recently an association emerged between TMAO levels and impaired renal function in diabetes patients. However, the mechanisms underlying the involvement of TMAO in the pathogenesis of kidney injury are still under debate. Here, our objective was to investigate how increased levels of TMAO affect the development of kidney injury in insulin-resistant mice.
Materials and methods: For this study C57Bl/6NCrL male mice were divided into three groups (n=20) and fed standard R70 chow (R70 control), high-fat diet (HFD control) or HFD with 0.2% TMAO (HFD+TMAO). The animals were weighed weekly, and a glucose tolerance test was performed after 12 weeks. At the endpoint, plasma, urine, and tissue samples were collected and kidney mitochondrial function was assessed using high-resolution fluorespirometry. Biochemical markers of kidney injury were measured in plasma (blood urea nitrogen, creatinine) and urine samples (KIM-1, Cystatin-C, NGAL). Gene expression analysis was carried out in kidney tissue to assess the levels of genes associated with inflammation, kidney damage, production of reactive oxygen species (ROS), and mitochondrial function. TMAO tissue and plasma concentrations were determined by UPLC/MS/MS.
Results: A significant increase in body weight, hyperinsulinemia, and impaired glucose tolerance was observed in HFD control and HFD+TMAO group animals, indicating on advancement of insulin resistance. Both HFD-fed groups showed significantly increased levels of urinary KIM-1 and NGAL, an early markers of kidney injury; however, supplementation of HFD with TMAO did not exacerbate the condition. Data from the analysis of kidney gene expression suggested that some genes responsible for mitochondrial biogenesis and adaptation to oxidative stress (MFN1, TFAM, SIRT3) are upregulated in HFD+TMAO group. This was supported by measurements of mitochondrial respiration, which indicated on lower OXPHOS capacity, leading to energy deficiency in kidney mitochondria after 12 weeks of HFD feeding supplemented with TMAO. An increase in mitochondrial ROS production was characteristic for kidney mitochondria of the HFD+TMAO group. Surprisingly, we observed a 3.3-fold higher accumulation of TMAO in kidney tissue compared to other tissue in all experimental groups. In the HFD+TMAO group, plasma TMAO level was 48.9±9.3 μmol/L and kidney TMAO concentration reached 164±34 nmol/g tissue (a 23-fold increase compared to R70 control).
Conclusion: Taken together, our results demonstrate that TMAO accumulates in kidney tissue at significantly higher concentrations than in other tissue, providing a possible explanation for the observation that kidneys appear to be more affected by high levels of TMAO in patients. However, TMAO does not aggravate the initial stage of kidney injury, but the impact of TMAO on mitochondrial function could potentially result in renal damage and loss of kidney functionality at a later stage.
Supported by: ESF and Latvian state budget within the project no. 8.2.2.0/20/I/004
Disclosure: M. Videja: None.
768
Changes in body composition after kidney-pancreas transplantation and its association with 25-OH vitamin D3 in type 1 diabetes
E.B. Parente1,2, S. Mutter1,2, C. Forsblom1,3, V. Harjutsalo1,3, P.-H. Groop1,3, on behalf of the FinnDiane Study Group;
1Folkhälsan Research Center, 2Research Program for Clinical and Molecular Metabolism, Faculty of Medicine, University of Helsinki, 3Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Diabetes is a progressive chronic disease. Unfortunately, some individuals will develop kidney failure, therefore, requiring kidney transplantation (TX). Considering that after kidney-pancreas TX, individuals with type 1 diabetes are cured of two diseases that impact body composition, in the present study, we evaluated the changes in body fat, muscle and bone tissues after kidney-pancreas TX. Furthermore, since 25-OH vitamin D3 [25(OH)D3] is important for the health of bone and muscle tissues, we analyzed the associations between pre-TX serum 25(OH)D3 concentration and the changes in body composition.
Materials and methods: This observational prospective study included 83 adults with T1D from the Finnish Diabetic Nephropathy Study referred to kidney-pancreas TX. Body composition was assessed by dual-energy X-ray absorptiometry (DXA) and visceral fat was measured by the CoreScan software pre- and post-TX. Changes in body composition were calculated as the difference (delta) between post-TX and pre-TX body compartment measurements and assessed by the Wilcoxon rank test. Linear regression models adjusted for covariates (sex, pre-TX age and BMI) were used to evaluate the associations between pre-TX serum 25(OH)D3 and the changes in body composition. A p-value below 0.05 was considered significant.
Results: The median age pre-TX was 45 (IQR 36,50) years, 66% were men and 35% of the individuals had pre-TX serum 25(OH)D3 below the normal threshold (50 nmol/l). After a median follow-up of 204 (IQR 177, 263) days after kidney-pancreas TX, BMI decreased (median 24.4 to 23.5 kg/m2, p=0.002), but no change was seen in the waist-height ratio. Reductions in total bone mass (TBM) (2.4 to 2.3 kg, p<0.001), bone mineral density (BMD) (1.107 to 1.100, p<0.001), total muscle mass (TMM) (49.2 to 46.5 kg, p<0.001), legs´muscle mass (LegMM) (15.2 to 14.5 kg, p<0.001), and legs´ fat mass (LegFM) (6.2 to 5.1 kg, p=0.019) were observed. No changes occurred in total fat mass or visceral fat mass. Pre-TX 25(OH)D3 was associated with changes in TMM (r2=0.16, β=0.271, p=0.014) and LegMM (r2=0.11, β=0.236, p=0.036). Therefore, the lower pre-TX 25(OH)D3, the lower the delta of TMM and LegMM, which means higher muscle loss. No association was seen between pre-TX 25(OH)D3 and changes in LegFM, TBM or BMD. However, the change in TMM was a predictor of the change in TBM (r2=0.29, β=0.303, p=0.003).
Conclusion: Detrimental findings in body composition, such as a reduction in muscle mass, bone mass and peripheral fat (LegFM) with the maintenance of total fat and visceral fat mass were seen after a short-term follow-up of adults with type 1 diabetes undergoing kidney-pancreas TX. A longer follow-up is necessary to study the impact of these changes on the risk of bone fractures and insulin resistance. Moreover, as pre-TX 25(OH)D3 is associated with muscle loss post-TX, interventional studies are needed to evaluate whether correcting the pre-TX serum 25(OH)D3 concentration will improve the post-TX body composition outcomes.
Supported by: Folkhälsan Research Foundation, Academy of Finland
Disclosure: E.B. Parente: None.
769
Up-regulation of dopamine β-hydroxylase and phenylethanolamine N-methyltransferase by intermittent hypoxia via down-regulation of microRNA-375 in neuronal cells
S. Takasawa, R. Shobatake, Y. Takeda, T. Uchiyama, A. Yamauchi, M. Makino, S. Sakuramoto-Tsuchida, K. Asai, R. Hirota, R. Fujii, H. Ota, A. Itaya-Hironaka;
Biochemistry, Nara Medical University, Kashihara, Japan.
Background and aims: Sleep apnea syndrome (SAS) is characterized by recurrent episodes of oxygen desaturation and reoxygenation (intermittent hypoxia [IH]) and is a risk factor for hypertension and insulin resistance. However, why hypertension is induced by IH has been elusive. Here, we investigated the direct effect of IH on the gene expression(s) of catecholamine synthesizing enzymes in neuronal cells.
Materials and methods: Human and mouse neuronal cells, NB-1 and Neuro-2a cells, were exposed either to normoxia (5% CO2, 21% O2) or 70 cycles/24 h of IH (5 min hypoxia [1% O2]/10 min normoxia), mimicking SAS patients, for 24 h. After the treatment, the mRNA levels of catecholamine synthesizing enzymes, tyrosine hydroxylase, L-3,4-dihydroxyphenylalaninedecarboxylase, dopamine β-hydroxylase (DBH), and phenylethanolamine N-methyltransferase (PNMT) in human NB-1 and mouse Neuro-2a cells were measured by real-time RT-PCR. The protein levels of DBH and PNMT in NB-1 cells were measured by Western blot analysis. Reporter plasmids were prepared by inserting the promoter fragments of human DBH (-1018~+10) and PNMT (-600~+67) upstream of a firefly luciferase reporter gene in pGL4.17 vector. After the reporter plasmids were transfected into NB-1 cells, the cells were exposed either to IH or normoxia for 24 h, and lysed, and the promoter activities were measured. MicroRNA (miR)-375 levels were measured by real-time RT-PCR. To clarify the role of miR-375, miR-375 mimic and non-specific control RNA (miR-375 mimic NC) were introduced into NB-1 cells just before IH/normoxia exposure, and the mRNA levels of DBH and PNMT were measured by real-time RT-PCR.
Results: (1) The mRNA levels of DBH were significantly increased by IH in Neuro-2a (P=0.0135) and NB-1 cells (P=0.0271). (2) The mRNA levels of PNMT were significantly increased by IH in Neuro-2a (P=0.0341) and NB-1 cells (P<0.0001). (3) The protein levels of DBH and PNMT in IH-treated NB-1 cells were significantly increased (P=0.0296 and P=0.0007, respectively). (4) The promoter activities of DBH and PNMT were not increased by IH. (5) Target mRNA search of miR using MicroRNA.org program revealed that both mRNAs have a potential target sequence for miR-375. (6) The miR-375 level of IH-treated cells was significantly decreased than that of normoxia-treated cells (P=0.0222). (7) The IH-induced up-regulation of DBH and PNMT was abolished by introduction of miR-375 mimic but not by miR-375 mimic NC.
Conclusion: These results indicate that IH stress down-regulates the miR-375 in neuronal cells, resulting in the increased levels of DBH and PNMT mRNA via the inhibition of the miR-375-mediated mRNA degradation, leading SAS patients to hypertension as well as diabetes.
Disclosure: S. Takasawa: None.
770
Alternative splicing of the apoptosis gene Bcl-x in diabetic nephropathy
S. Oltean, M. Ayine, M. Stevens;
University of Exeter Medical School, Exeter, UK.
Background and aims: There are several splice events associated with the progression of diabetic nephropathy. Understanding how they are regulated and how they contribute to disease pathogenesis may open new therapeutic avenues. An alternative splicing (AS) event occurring in exon 2 of the Bcl-x gene produces two splice isoforms of antagonistic effect. Use of the proximal 5’ splice site produces the long Bcl-xL isoform, known to be anti-apoptotic while the pro-apoptotic isoform Bcl-xS is formed with the use distal 5’splice site. IL-6 is an inflammatory cytokine known to drive the progression of diabetic nephropathy (DN) that has also been implicated in the alternative splicing of Bcl-x; however, its mechanism is not fully understood yet. The aim of this study is to investigate the regulation of this AS event in kidney cells grown in a diabetic environment as well as the correlation of Bcl-xS/Bcl-xL splicing ratio with various degrees of severity in DN.
Materials and methods: Immortalised Human Embryonic Kidney cells (HEK293) exposed to three different high glucose environment: High Glucose (HG-25mM), Oscillating High Glucose (OHG), Glucose Soup (GS+ -25mM with TNFα 1ng/ml, IL-6 1ng/ml and insulin 100nM), Oscillating Glucose Soup (OGS+), Glucose Soup without IL-6 (GS-), and Oscillating Glucose Soup without IL-6 (OGS--25mM with TNFα 1ng/ml and insulin 100nM). The treatments were compared to Normal Glucose (NG) and Mannitol osmotic control (M). Oscillating treatment involves changing media from NG to a high glucose environment back and forth every 12 hours till 48 hours is reached. The cells were treated for 48 hours and splice isoforms analysed using RT-PCR followed by a bioanalyser quantification. Urine samples were obtained from type I and type II diabetic patients enrolled on the Biomarker Enterprise to Attack DKD (BEAt-DKD) and VIBE (Exeter) studies with varying stages of renal disease. RNA was extracted from the urinary sediment for RT-PCR analysis of Bcl-x splice isoforms. A shift in the Bcl-xS/Bcl-xL splicing ratio was be compared between the glucose environments and controls; for patients’ samples, the Bcl splicing ratio was correlated with various clinical parameters.
Results: A dose-dependent IL-6 treatment caused an increase in the Bcl-xS/Bcl-xL ratio., suggesting an influence of IL-6 on Bcl-x splicing. RT-PCR followed by bioanalyer analysis showed an upregulation of the Bcl-xS/Bcl-xL ratio when HEK293 cells are treated under HG, OGS+. However no significant change was seen in the rest of the treatments showing a combined effect of excessive glucose and IL-6 on the Bcl-x splicing event. Furthermore, pilot data from a small cohort of patients with varying stages of nephropathy indicated that an increase in the pro-apoptotic Bcl-xS in RNA extracted from urinary cells correlated with a decline in GFR, implicating Bcl-x AS as a potential biomarker for DN severity.
Conclusion: High glucose levels combined with IL-6 causes an increase in the pro-apoptotic Bcl-xS splice isoform in HEK 293 cells. Furthermore, detection of the Bcl-x splicing event in the urinary sediment of DN patients indicates that increased Bcl-xS expression correlates with a decline renal function, implicating this AS event as a potential biomarker for DN severity.
Supported by: Diabetes UK
Disclosure: S. Oltean: None.
771
Pegph20 attenuates hyaluronan-CD44 mediated renal injury in obesity related glomerulopathy
B. Qi1,2, V. Musale2, K. Li2;
1Department of Endocrinology, Jilin Province People's Hospital, Changchun, China, 2Division of Systems Medicine, School of Medcine, University of Dundee, Dundee, UK.
Background and aims: Obesity is considered as one of the biggest public health threats worldwide and increases the risk of incident chronic kidney disease. Hyaluronan (HA) is a major component of the extracellular matrix (ECM). We hypothesised that HA content was increased in the ECM of Obesity-Related Glomerulopathy (ORG) and PEGPH20, human recombinant PEGylated hyaluronidase PH-20, reversed high-fat (HF) diet-induced HA accumulation and renal dysfunction. We further investigated whether its renal protective effect was dependent upon the main HA receptor, CD44.
Materials and methods: C57BL/6 mice, global CD44-deficient (cd44-/-) mice, and their wildtype littermate controls (cd44+/+) were fed a chow diet or HF diet for 16 weeks. HF diet-fed mice received injections of either vehicle or PEGPH20 once every 3 days for 24 days. After treatment, mice were euthanized and tissue and blood samples were collected. CD44 expression was detected by Western Blotting. PAS staining was used to observe renal pathology. HA accumulation and α-SMA expression were assessed by histochemistry. Collagen deposition was measured by picrosirius red staining and renal function was assessed by a creatinine assay kit.
Results: HF diet feeding in C57BL/6 mice led to an increase in CD44 expression, HA accumulation, glomerular area and tubular injury score in the renal tissue, as well as an increase in serum creatinine levels. PEGPH20 treatment ameliorated some of these adverse effects including reducing renal CD44 expression (3.02 ± 0.33 (Vehicle HF) vs 1.49 ± 0.16 (PEGPH20 HF) fold increase relative to chow-fed mice, p =0.0092), HA accumulation (31.39 ± 3.48 vs 13.58 ± 1.81 %, p =0.0078), tubular injury score (3.28 ± 0.15 vs 1.39 ± 0.06, p =0.0003) and serum creatinine levels (1.40 ± 0.09 vs 0.79 ± 0.07 mg/dl, p =0.0002),without affecting glomerular area. Furthermore, PEGPH20-treated obese mice had reduced renal fibrosis evidenced by decreased α-SMA expression (5.18 ± 0.17 vs 1.21 ± 0.11 %, p =0.0001) and collagen deposition (9.76 ± 0.76 vs 6.30 ± 0.23 %, p =0.0164) compared with obese mice receiving vehicle. Global deletion of cd44 gene in HF-fed mice significantly reduced renal CD44 expression (1.00 ± 0.05 (cd44+/+ HF) vs 0.13 ± 0.02 (cd44-/- HF) fold change, p =0.0001), HA accumulation (24.44 ± 3.33 vs 6.60 ± 0.58 %, p =0.0001) and tubular injury score (3.28 ± 0.20 vs 1.89 ± 0.06, p =0.0001), as well as reduced α-SMA expression (5.02 ± 0.16 vs 0.43 ± 0.06 %, p =0.0001) and collagen deposition (9.48 ± 0.29 vs 6.08 ± 0.73 %, p =0.0063). PEGPH20 caused further reductions in serum creatinine levels, in HF-fed cd44-/- mice (0.67 ± 0.08 mg/dl) when compared with HF-fed cd44 +/+ mice (1.63 ± 0.05 mg/dl, p =0.0001).
Conclusion: PEGPH20 treatment and global cd44 deletion suppressed HA accumulation during ORG, which prevented obesity-associated renal fibrosis and dysfunction. Our results demonstrate that HA plays an important role in the pathogenesis of ORG, perhaps in part through binding to receptor CD44.
Supported by: BHF Project Grant PG/18/56/33935, BX Qi is supported by a scholarship from CSC
Disclosure: B. Qi: None.
772
Functional and morphological renal changes in a Göttingen Minipig model of obesity-related and diabetic nephropathy
B.O. Christoffersen1, C.A. Kristensen2, R. Lindgaard2, R.K. Kirk1, B.M. Viuff1, P.H. Kvist1, H.D. Pedersen1, T.P. Ludvigsen1, T. Skovgaard1, J.J. Fels1, T. Martinussen3, L.B. Christiansen2, S. Cirera2, L.H. Olsen2;
1Novo Nordisk A/S, Maalov, 2Department of Veterinary and Animal Sciences, University of Copenhagen, Frederiksberg, 3Department of Public Health, Copenhagen University, Frederiksberg, Denmark.
Background and aims: Obesity-related glomerulopathy (ORG) and diabetic nephropathy (DN) are serious complications to metabolic syndrome and diabetes. Here, the effects of a fat, fructose and cholesterol-rich (FFC) diet on kidney biomarkers and morphology were investigated in Göttingen Minipigs with and without streptozotocin-induced diabetes.
Materials and methods: Castrated male Göttingen Minipigs were divided into 4 groups: SD (standard diet, n=8), FFC (FFC diet, n=16), FFC-DIA (FFC diet + diabetes, n=14), FFC-DIA+S (FFC diet with extra salt + diabetes, n=14). Body composition, blood and urine biomarkers, glomerular filtration rate (GFR), blood pressure (BP), heart rate (HR) and resistive index (RI) were evaluated after 6-7 months (T1) and 12-13 months (T2). Histology (glomerular area, fibrosis area and mesangial expansion (ME) scoring), electron microscopy and gene expression profiling (the latter excluding FFC-DIA+S) were performed at T2. T2 data are given below as median and interquartile range [Q1-Q3] in the following order: SD, FFC, FFD-DIA, FFC-DIA+S.
Results: Fat percentage was significantly higher in the three FFC-groups compared to SD (27.6[24.0-30.7] vs. 64.2[61.4-67.6] vs. 54.8[52.7-56.0] vs. 53.1[47.9-61.0] %), and the two diabetic groups had higher plasma glucose than FFC and SD (3.6[3.3-3.9] vs. 3.7[3.6-3.8] vs. 15.4[14.7-19.0] vs. 13.7[13.0-15.6] mM). No differences were observed between groups in BP, whereas HR was higher in the diabetic groups compared to FFC and SD (72[69-80] vs. 72[71-75] vs. 96[83-102] vs. 86[76-105] beats/min). Minipigs fed FFC diet displayed glomerulomegaly compared to SD, and induction of diabetes on top of the FFC diet appeared to further increase the average glomerular area (11383[9202-11602] vs. 14907[13174-21771] vs. 21839[18518-30269] vs. 18262[16066-25095] μm2). The ME score was increased in all FFC groups compared to SD but was not increased further in the presence of diabetes (% glomeruli with a score of 0/1/2/3: 100/0/0/0 vs. 51/22/16/10 vs. 30/31/15/24 vs. 26/33/20/21). GFR was increased in FFC-DIA compared to SD and FFC (40.7[32.9-45.0] vs. 72.6[61.8-76.4] vs. 84.2[78.6-93.5] vs. 67.0[55.9-76.4] ml/min/kg lean mass). RI was increased in all three FFC-fed groups compared to SD (0.37[0.36-0.42] vs. 0.47[0.44-0.50] vs. 0.49[0.44-0.51] vs. 0.52[0.43-0.57]). Urinary NGAL:creatinine ratio was increased in the two diabetic groups compared to FFC and SD (6.6[4.0-9.3] vs. 13.9[10.3-20.0] vs. 51.3[37.1-122.6 vs. 27.0[19.0-42.9] ng/mg), whereas urinary protein:creatinine ratio was only increased in the FFC-DIA group (117[76.2-132.2] vs. 96.1[70.4-115.9] vs. 2181[130.2-461.1] vs. 115.9[106.9-224.7] mg/g). No significant differences were found in urinary albumin:creatinine ratio. Four genes relevant for human DN (CDKN1A, NPHS2, ACE, SLC2A1) were significantly deregulated in FFC and/or FFC-DIA compared to SD. The addition of salt to the diet, did not exacerbate the findings.
Conclusion: Göttingen Minipigs fed FFC diet displayed some of the characteristic features of human ORG. Presence of diabetes on top of FFC diet, lead to changes resembling the early phases of human DN.
Supported by: NGAL kits from BioPorto Diagnostics A/S, Hellerup, Denmark
Disclosure: B.O. Christoffersen: Non-financial support; Kits for NGAL measurements were provided for free from BioPorto Diagnostics A/S, Hellerup, Denmark.
SO 67 Flames and scars in NAFLD: pathogenesis and therapy
773
The diversity of macrophages and their role during non alcoholic fatty liver disease
R. Thibaut1, L. Orliaguet1, T. Ejlalmanesh1, M. Diedisheim1, R. Ballaire1, H. Fohrer-Ting2, C. Klein2, N. Venteclef1, F. Alzaid1;
1Institut Necker Enfants Malades, 2Centre de recherche des Cordeliers, Paris, France.
Background and aims: Non alcoholic fatty liver disease (NAFLD) is an important co-morbidity of type 2 diabetes. It represents a spectrum of conditions ranging from benign steatosis of the liver to non-alcoholic steatohepatitis (NASH), characterised by inflammation and fibrosis. Liver inflammation is characterized by macrophage infiltration. Macrophage are cells of the innate immune system which can adopt different phenotypes, ranging from pro-inflammatory to anti-inflammatory. During NAFLD and NASH, they are pro-inflammatory and drive tissue damages. Recently, a great phenotypic diversity of macrophage phenotypes has been described in multiple organs through the use of single cell omics technologies. Different populations of Kupffer cells (KCs), liver’s resident macrophages, have been described at homeostasis. During NAFLD, it has been proposed that dying KCs are replaced by monocyte-derived KCs which display a distinct phenotype. However, the diversity of macrophage populations in NAFLD and the differential roles of these populations is still unclear. The goal of our study is thus to uncover the diversity and role of liver macrophage sub-populations during progression and resolution of NAFLD.
Materials and methods: We used mice models of NASH (high fat diet, choline-deficient high fat diet) or fibrosis (CCl4), isolated liver macrophages from these mice and performed single-cell RNA sequencing (scRNAseq) analyses. Metabolic tests and histology were carried out to examine the metabolic status of the mice and the extent of liver damage while flow cytometry was used to characterize into more details the different macrophage populations.
Results: Using scRNAseq, we found several sub-populations of liver macrophages. We observed the existence of two populations of KCs, and one population of monocyte-derived macrophages. The relative proportions of these populations changed during NAFLD, with a one sub-population of KCs being less abundant during NAFLD resolution. This sub-population of KCs, that we named oxKCs, was characterized by high expression of metabolism-related genes, which prompted us to study liver macrophage metabolism during NAFLD. We therefore studied macrophage metabolic activity, and more particularly the glycolytic activity and the intensity of mitochondrial respiration. We did not see any change in these metabolic parameters during NAFLD progression. We thus focused on understanding the differential role of the two different KCs populations in the physiopathology of NAFLD. We identified KLF6 as a transcriptional factor particularly expressed by oxKCs in comparison with the other KC population and thus postulated it was pivotal in their function. Using a model of mice expressing the Cre recombinase in macrophages and gRNAs directed KLF6, we plan on identifying KLF6 target genes in macrophages. We also plan on examining the importance of KLF6 in the macrophage response to cellular stressors involved in NAFLD such as lipid overload.
Conclusion: In conclusion, we found that three populations of liver macrophages could be identified. In particular, one particular KC population could be pivotal in NAFLD. This population highly expresses KLF6 which could thus be an interesting therapeutical target. More generally, understanding macrophage diversity and role in NAFLD could lead to the development of therapeutical approaches for management of NAFLD.
Supported by: EFSD/Lilly Young Investigator Research Award
Disclosure: R. Thibaut: Grants; EFSD/Lilly Young Investigator Research Award Application.
774
Sexually dimorphic metabolic response to an obesogenic diet according to the amino acid source in mice
C. Rives1, V. Alquier-Bacquie1, S. Ellero-Simatos1, A. Polizzi1, F. Lasserre1, T. Levade2, F. Sabourdy2, M. Huillet1, N. Loiseau1, H. Guillou1, A. Fougerat1, L. Payrastre1;
1INRAE Toxalim, 2Institut Fédératif de Biologie, CHU, Toulouse, France.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) frequently co-exists with metabolic syndrome and thus is often defined as the liver expression of dyslipidemia, insulin resistance, and obesity. NAFLD is the most common chronic liver disease and affects approximately 25% of the global population. Of note, dietary constituents are key factors in NAFLD pathogenesis. Such factors include a high intake of calories and an excessive consumption of saturated fats and refined carbohydrates. More recently, studies have shown that protein source could also play a role. In this work, we investigated the metabolic effects of different amino acid sources in response to an obesogenic diet in male and female mice.
Materials and methods: The study has been carried out along the “Principles of laboratory animal care” (NIH Publication no. 85-23, revised 1985). Male (n = 12) and female (n = 12) C57BL/6J mice were fed either a Control Diet (CD) or a Western Diet (WD) for 15 weeks. In each diet, amino acid source was either from casein (CD-CAS or WD-CAS) or a free amino acid mixture identical to casein amino acid composition (CD-AA or WD-AA). For each cage, food and water consumption and body weight were evaluated weekly. Plasma, liver, caecum, subcutaneous and epididymal white adipose tissue (WAT) samples were collected.
Results: Male mice fed a WD-AA show reduced WD-induced body weight, glucose intolerance and insulin resistance compared to WD-CAS fed male mice. In addition, hepatic damages (AST and ALT activities), lipid accumulation, expression of genes involved in lipogenesis, β-oxidation and oxidative stress, induced upon WD-CAS feeding were not observed in males fed the WD-AA. By contrast, the metabolic consequences of the WD in female mice were observed regardless of the amino acid source. Besides, males and females fed the WD-CAS showed a decreased caecum weight compared to animal fed a CD-CAS, whereas caecum weight was unchanged in WD-AA fed male and female mice compared to respective controls.
Conclusion: Altogether, these results show that the source of dietary amino acids plays a major role in the metabolic response to an obesogenic diet in mice and is sex-dependent. Microbiota composition and activity, untargeted hepatic genome expression and metabolome are under investigation to identify the mechanisms involved in the distinct metabolic responses to WD according to the amino acid source and sex.
Supported by: FRM
Disclosure: C. Rives: None.
775
Inflammation may prevent hepatocyte damage promoted by steatosis
G. Perrot, C. Ardon-Zitoun, A. Lang, G. Mithieux, F. Rajas;
Laboratoire Nutrition diabète et cerveau, Université Claude Bernard Lyon 1, Lyon, France.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is a common consequence of metabolic stress induced in type 2 diabetes. Usually, NAFLD progresses from simple steatosis to non-alcoholic steatohepatitis, cirrhosis and possibly to hepatocellular carcinomas (HCC). However, some studies reported the development of hepatic tumors, generally hepatocellular adenomas (HCA), on fatty liver associated with low-grade inflammation/fibrosis. Interestingly, HCA/HCC occurs on fatty liver in the absence of inflammation and fibrosis in patients with Glycogen Storage Disease Type Ia (GSDIa), characterized by the loss of glucose-6-phosphatase catalytic subunit (G6PC1). In both GSDIa and type 2 diabetes, the increased flux downstream of glucose-6 phosphate leads to a metabolic reprogramming very similar to that in tumor cells. Here, we used a mouse model of GSDIa to better characterize the inflammatory process in the liver under conditions that favor fibrosis.
Materials and methods: Mice with genetic ablation of G6pc1, specifically in the liver (L.G6pc-/- mice), and wild type (WT) mice were fed either a high fat-methionine and choline deficient diet (HF-MCD), known to promote hepatic fibrosis, or a chow diet. Hepatic tumor development was followed by microscan after injection of contrast agent. Inflammatory and fibrotic parameters was characterized by histology (HPS and Masson’s staining) and marker’s gene expression was analyzed by RT-qPCR and western blot in the mouse livers. Neutrophils (MPO), T lymphocytes (CD3) and Kupffer’s cells (F4/80) were quantified by immunostaining of livers.
Results: After 3 months on HF-MCD diet, no hepatic tumor was detected in WT or L.G6pc-/- mice. As expected, HF-MCD diet promoted inflammatory (Mcp1, Il6, Tnf-α) and fibrotic (Colla1, Tgfβ1, Pai) gene expression in WT mice. Surprisingly, histological analyses showed that inflammation and fibrosis were discrete in livers of L.G6pc-/- mice fed a HF-MCD diet. Moreover, the expression of inflammation/fibrosis markers was lower in L.G6pc-/- mice fed a HF-MCD diet compared to WT mice. Furthermore, in response to MCD diet, Kupffer’s cells and T lymphocytes were highly recruited in the liver of WT mice, while the recruitment of T lymphocytes was drastically decreased in L.G6pc-/- liver. However, Kupffer’s cell population was not affected in L.G6pc-/- liver. In both genotypes, neutrophil recruitment was increased under HF-MCD diet. Finally, it is noteworthy that plasma transaminase activities were strongly increased in L.G6pc-/- mice fed a HF-MCD diet compared to WT mice.
Conclusion: The metabolic perturbations linked to G6P-activated pathways promote steatosis associated or not with inflammation/fibrosis. Our results show that hepatocyte injuries were more important in GSDIa mice, a model of steatosis associated with low-grade inflammation, than in WT mice, in which HF-MCD promoted inflammation/fibrosis. These data strongly support the idea that fine regulation of immune cell activation is essential for the control of hepatic disease and maintenance of liver homeostasis.
Supported by: Fondation pour la Recherche Médicale, Gen et Zic, Association Francophone des Glycogénoses
Disclosure: G. Perrot: None.
776
Hepatocyte-specific CCN1 gene deletion exacerbates the severity of NASH fibrosis in an established mouse model of high fat feeding and diabetes
S.N. Parry1,2, X. Wang1, D. Min1,2, M. Huang3, P.F. Williams1,2, A. Leask4, S.M. Twigg1,2;
1Greg Brown Diabetes and Endocrinology Laboratory, The University of Sydney, Sydney, Australia, 2Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, Australia, 3Department of Anatomical Pathology, St Vincent's Hospital Sydney, Sydney, Australia, 4School of Dentistry, University of Saskatchewan, Saskatoon, SK, Canada.
Background and aims: Type 2 diabetes (T2D) markedly increases the risk of developing non-alcoholic steatohepatitis (NASH) fibrosis. The matricellular protein CCN1, an important regulator of tissue repair, may have a liver protective role via its anti-fibrotic properties. Aim: to examine whether hepatocyte-specific homozygous gene deletion of CCN1 leads to more severe NASH fibrosis compared with control strain mice, in our established NASH fibrosis mouse model induced by high fat feeding and diabetes.
Materials and methods: Male Control mice (4-6 weeks; B6.Cg-Tg(AlbCre)21Mgn/J (AlbCre) and homozygous CCN1fl/fl (CCN1fl/fl)) and hepatocyte-specific CCN1 knockout mice (CCN1flxB6.Cg-Tg(AlbCre)21Mgn/J (CCN1-KO)) were fed either standard chow or high fat diet (HFD; 45% kCal fat). After 15 weeks, HFD mice were rendered diabetic (HFD+DM) with low dose streptozotocin (2-3 x 65 mg/kg ip) and maintained for a further 10 weeks. Liver fibrosis was assessed with Picro Sirius Red (PSR) staining. Gene expression of fibrosis markers were measured by RT-qPCR. Biochemical assays were performed. Data were analysed by two-way ANOVA and shown as mean±SEM.
Results: CCN1-KO HFD+DM mice had ~two-fold increased fibrosis area by PSR staining compared with Control (AlbCre+CCN1fl/fl) HFD+DM mice, including at the Central Vein and Portal Tract zones (Table 1). CCN1-KO HFD+DM mice had increased gene expression of fibrosis markers collagen-I (3.33±1.62 v 5.51±2.32, p<0.001), collagen-III (2.95±0.36 v 4.95±0.71, p<0.001), collagen-IVα1 (2.75±0.19 v 3.90±0.32, p<0.001), collagen-VI (2.09±0.21 v 3.83±0.56, p<0.001) and TGFβ1 (1.76±0.09 v 2.27±0.18, p=0.02), but no change in inflammatory markers TNFα or MCP-1, compared with Control HFD+DM mice. Plasma CCN1 concentration was increased by HFD+DM in the Control mice (0.55±0.04 v 0.78±0.06 ng/mL, p=0.006) but not in the CCN1-KO mice (0.60±0.03 v 0.64±0.05 ng/mL). CCN1-KO HFD+DM mice had greater total body weight (42.19±1.80 v 48.85±1.71 g, p=0.02), fasting insulin (131.25±11.75 v 262.55±48.88 pM, p=0.01) and insulin resistance (HOMA-IR; 17.69±1.26 v 28.44±4.78, p=0.02), but had lesser hyperglycaemia (19.31±1.18 v 15.48±1.13 mmol/L, p=0.03), compared with the Control HFD+DM mice.
Conclusion: Hepatocyte-specific CCN1-KO HFD+DM mice developed more severe NASH fibrosis than Control HFD+DM mice. An atypical metabolic phenotype was identified in the CCN1-KO HFD+DM mice. Studies are now required to determine if the absence of hepatocyte CCN1 has a direct or a systemic effect on the liver. This research indicates hepatocyte CCN1 may have a protective role against NASH fibrosis in the presence of T2D, implicating it as a potential therapy.
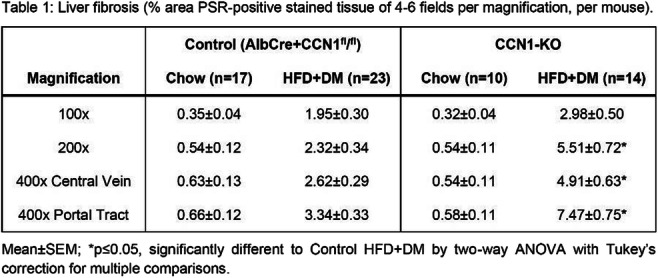
Disclosure: S.N. Parry: None.
777
Elevated fibrosis in non-diabetic obese individuals: comparison of liver fibrosis measured by magnetic resonance imaging
A. Askeland1,2, M. Gjela3,1, R.W. Rasmussen1, K. Højlund4, M. Mellergaard1, J.B. Frøkjær3,2, A. Handberg1,2;
1Department of Clinical Biochemistry, Aalborg University Hospital, Aalborg, 2Department of Clinical Medicine, Aalborg University, Aalborg, 3Department of Radiology, Aalborg University Hospital, Aalborg, 4Steno Diabetes Center Odense, Odense, Denmark.
Background and aims: Obesity is a major risk factor for developing non-alcoholic fatty liver disease (NAFLD). NAFLD represents a spectrum of liver pathologies, including hepatic steatosis and fibrosis. Technological advancements within the field of magnetic resonance imaging (MRI) enable precise and non-invasive quantification of liver steatosis and fibrosis. As such, the aim of this study was to investigate and compare liver steatosis and fibrosis measured by MRI in lean and non-diabetic obese individuals.
Materials and methods: We conducted an observational case-control study comparing hepatic steatosis and fibrosis in age (30-60 years) and gender matched healthy lean (n = 23) and non-diabetic obese individuals (n = 60). Obese individuals were divided into a low-risk (n = 28) and high-risk obesity (n = 32) groups, where participants in the high-risk group had both NAFLD (liver steatosis ≥5%) and metabolic syndrome (IDF criteria). Using a 3T MRI, hepatic proton density fat fraction (PDFF) and T1 relaxation time mapping (T1 mapping) were assessed for evaluation of hepatic steatosis and fibrosis, respectively. Furthermore, the FIB-4 index, a surrogate measure of liver fibrosis, was calculated for participants over the age of 35 years.
Results: Average liver fat fraction (PDFF) was 2.2% (range: 1.2-4.8%) in the lean group, 3.6% (range: 1.6-13.6%) in the low-risk obese group, and 13.7% (range: 5.1-36.1%) in the high-risk obese group. Comparing liver fibrosis (T1 mapping), high- and low-risk obese individuals had a 19.7% (mean±sd: 896±73ms, t-test: p < 0.001) and 8.1% (mean±sd: 810±65au, t-test: p = 0.038) increase compared with lean controls (mean±sd: 749±70ms), respectively. Comparing only the obese groups, liver fibrosis was 10.7% higher in high-risk obese compared with low-risk obese individuals (t-test: p < 0.001). Liver fibrosis showed a positive correlation with liver fat fraction in the combined cohort (Pearson: r = 0.69, p < 0.001). BMI mean was similar in the low- (mean: 35.2 kg/m²) and high risk obese groups (mean: 36 kg/m²). The FIB-4 index was similar between all groups.
Conclusion: In our cohort, both the low-risk and the high-risk obese individuals had increasingly higher MRI-determined liver fibrosis compared with lean controls. These differences were not mirrored in the FIB-4 index. The MRI-measurements revealed a strong association between liver fibrosis and fat fraction, the two major pathologies underlying NAFLD. Further studies are needed to define specific cut-off values for pathological liver fibrosis as determined by T1-mapping, and the clinical relevance of these measures in obesity and related disorders.
Supported by: This work was supported by a research grant from the Danish Diabetes Academy (NNF17SA0031406).
Disclosure: A. Askeland: None.
778
Anti-inflammatory and anti-fibrotic effects of a novel long-acting Glucagon/GIP/GLP-1 triple agonist, HM15211, in TAA induced mouse model of liver injury and fibrosis
J. Kim, J. Lee, Y. Kim, S. Lee, H. Kwon, E. Park, J. Choi, S. Bae, D. Kim, S. Lee, I. Choi;
Hanmi Phaarm. Co., Ltd, Seoul, Republic of Korea.
Background and aims: HM15211 is a novel long-acting triple agonist consisting of Glucagon/GIP/GLP-1 triple agonist conjugated to human IgG FC fragment via short PEG linker. Previously, therapeutic benefits of HM15211 were demonstrated in diet-induced animal models of NASH and fibrosis. Here, the effects of HM15211 on anti-inflammatory and fibrosis (anti-inflammatory and-fibrotic effects of HM15211) were further evaluated in TAA (thioacetamide)-induced liver injury and fibrosis mouse, and underlying mechanism was investigated.
Materials and methods: To induce liver injury and fibrosis, TAA was injected to mouse for 12 weeks with gradual dose increment and HM15211 was administered during last 10 weeks. After the treatment of drug, hepatic hydroxyproline, Sirius red positive area and fibrosis score were determined, and blood level of pro-inflammatory cytokines was measured by using multiplex cytokine analysis. In addition, blood liver enzyme level and hepatic gene expression for fibrosis and inflammation were analysed. To unveil the underlying MoAs, mechanistic study was performed in THP-1 and LX2 cells.
Results: In TAA mice, HM15211 treatment significantly reduced the hepatic hydroxyproline (-51% vs. vehicle, p<0.01), Sirius red positive area (-65% vs. vehicle, p<0.001), and fibrosis score (0.7 for HM15211 vs. 3.0 for vehicle, p<0.001). Considering a baseline fibrosis score of TAA mice at week 2 (1.0), HM15211 treatment could confer both potential reversal effect on pre-existing fibrosis and prevention effect on fibrogenesis. Consistently, expression of hepatic marker genes for fibrosis (i.e. collagen-1α1) and inflammation (i.e. F4/80, TNF-α) were significantly attenuated by HM15211 treatment. Furthermore, multiplex cytokine analysis revealed that HM15211 treatment was associated with robust reduction across blood pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6. Profound reduction in blood level of liver enzymes was also confirmed. Mechanistically, PMA/LPS-induced pro-inflammatory cytokine secretion of THP-1 cells (human monocytes) was significantly attenuated by HM15211, and TGF-β-induced collagen production was also significantly reduced in LX2 cells (human hepatic stellate cells).
Conclusion: HM15211 effectively improved liver inflammation and fibrosis in TAA mice, and related mechanism was elaborated by in vitro studies. Thus, HM15211 could be a novel therapeutic option for fibrosis caused by NASH. Human study is ongoing to assess the clinical relevance of these promising results.
Disclosure: J. Kim: None.
779
Anti-fibrotic potential of a novel long-acting GLP-1/GCG/FGF21/IL-1RA tetra-specific drug (OGB21502) in preclinical mice models
M. Hwang, J. Kim, N. Chang, M. Kim, Y. Kim, D. Im, S. Park;
Onegene Biotechnology Inc., Suwon-si, Republic of Korea.
Background and aims: Non-alcoholic steatohepatitis (NASH) is a complex disease of chronic liver injury and inflammation, causing increased fibrogenesis and ultimately cirrhosis by multiple mechanism. We hypothesized that the simultaneous regulation of multi-target in disease microenvironment is a holistic approach to cure NASH with fibrosis. OGB21502 is a novel tetra-agonist at the GLP-1, GCG, FGF21, and IL-1RA. It efficiently improves hepatic steatosis, inflammation, and fibrosis by regulating lipotoxicity and macrophage/hepatic stellate cell activation. In our previous study, OGB21502 reduced lipid accumulation including triglyceride and cholesterol in DIO mice. OGB21502 also improved cirrhosis progression in 80% of the group in CCl4 model, and the fibrosis score was 2.4 against 4.0 in the vehicle. Here, we evaluated the steatosis resolution and anti-fibrotic effect of OGB21502 in mice models of NASH and fibrosis.
Materials and methods: All animal protocols were completed in agreement through internationally accepted principles of care. To induce NASH, the mice were fed with methionine-choline deficient diet (MCD) for 8 weeks and OGB21502 was subcutaneously administered during the last 4 weeks. Liraglutide and dulaglutide were used as comparative controls. In the second study, CCl4-liver fibrosis model was induced by intraperitoneal injections of CCl4 for 6 weeks. The OGB21502 or comparative control such as Fc-FGF21(RGE) and Ocaliva (OCA) was subcutaneously administered for the last 4 weeks. After treatment, liver tissues and blood samples were prepared and the histopathological feature was evaluated to determine NAS score and the degree of fibrosis. In addition, ELISA and quantitative PCR analysis were performed to determine the steatosis and fibrosis markers. Gene expression profile was analyzed by extracting total RNA from liver tissues in CCl4 model.
Results: In MCD mice, OGB21502 treatment induced decrease of blood ALT (-45.2% vs. vehicle). Histopathological analysis revealed that OGB21502 reduced NAS score (1.1 in OGB21502 vs. 3.4 in vehicle), steatosis score (-57.1% vs. vehicle), and inflammation score (-84.6% vs. vehicle). In CCl4 mice, OGB21502 was associated with the reduced mRNA expression of hepatic fibrosis markers compared to Fc-FGF21(RGE) and OCA in dose-dependent manner. In particular, OGB21502 at a dose of 30 nmol/kg further reduced the expression of TGF-β (-15%), α-SMA (-35%), LOXL-2 (-30%) and Col1A1 (-50%) than Fc-FGF21(RGE). In gene expression profile, OGB21502 more efficiently decreased fibrotic and inflammatory gene expressions compared to Fc-FGF21(RGE). We also confirmed that tetra-specific drug, OGB21502 had more synergistic effects on improving liver fibrosis and inflammation than dual (GLP-1/GCG) or triple (GLP-1/GCG/FGF21) treatment in gene profile. Moreover, LPS-induced pro-inflammatory cytokines were downregulated in Kupffer cells and HSC activation markers by TGF-β were lowered in OGB21502 treated group.
Conclusion: Overall, the composite mechanism of action of OGB21502 significant improved inflammation as well as fibrosis through multiple animal models. It is also expected to be effective in fibrosis in other organs. Therefore, these findings prompt the evaluation of a novel multi-specific therapeutic potential of OGB21502 for NASH with liver fibrosis and other fibrotic diseases.
Disclosure: M. Hwang: None.
780
The association between changes in fatty liver index and BARD score with cardiovascular disease and mortality in patients with new-onset type 2 diabetes
J. Park1, G. Kim2, B.-S. Kim3, K.-D. Han3, S. Kwon2, S. Park2, Y.-B. Lee2, S.-M. Jin2, J.-H. Kim2;
1Division of Endocrinology and Metabolism, Department of Internal Medicine, CHA Bundang Medical Center, CHA university School of Medicine, Gyeonggi-do, 2Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, 3Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) are independently associated with cardiovascular disease (CVD) and mortality. In addition, presence of NAFLD on T2DM have additional increased risk for CVD and mortality. However, NAFLD is a series of continuously changing processes, so there is limited value in predicting its association with CVD at any one point in time. Therefore, we investigated the progression or regression of NAFLD is associated with CVD or mortality in new-onset T2DM.
Materials and methods: Using the Korean National Health Insurance dataset, we included 120,256 patients who had undergone health check-up at least twice for two years and diagnosed with new-onset T2DM without history of any CVD or liver disease except for NAFLD from 2009 to 2012. Hepatic steatosis and advanced hepatic fibrosis were determined using fatty liver index (FLI) and BARD score. According to results of two scores over two years, the individuals were divided into four groups respectively. Outcomes included myocardial infarction (MI), stroke, heart failure (HF), and mortality based on International Classification of Disease 10th revision (ICD-10) codes. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between FLI or BARD score and CV outcome or mortality were analyzed using Cox proportional hazards analysis. Adjusted variables were used age, sex, smoking status, alcohol consumption, physical activity, income, body weight, hypertension, dyslipidemia, fasting glucose, and number of oral hypoglycemic agents used.
Results: Incident hepatic steatosis was associated with increased development of stroke, HF, mortality compared to non-NAFLD (HR [95% CI], stroke 1.47 (1.23 - 1.75), HF 1.36 (1.17 - 1.57), mortality 1.41 (1.20 - 1.65)). Regression of hepatic steatosis was associated with increased risk of MI, stroke, HF and mortality compared to non-NAFLD, and decreased risk of HF and mortality compared to persistent hepatic steatosis (all p<0.05). Persistent hepatic steatosis had increased risk of MI, stroke, HF, and mortality compared to non-NAFLD (HR [95% CI], MI 1.27 (1.10 - 1.47), stroke 1.39 (1.23 - 1.58), HF 1.42 (1.28 - 1.57), mortality 1.66 (1.50 - 1.84)). Persistent advanced fibrosis was associated with increased risk of stroke, HF, and mortality compared to group without advanced fibrosis (all p<0.05). Compared to persistent advanced hepatic fibrosis, regression of hepatic fibrosis had decreased risk of MI, stroke, HF, and mortality (HR [95% CI], MI 0.79 (0.56 - 1.10), stroke 0.58 (0.42 - 0.81), HF 0.77 (0.61 - 0.97), mortality 0.66 (0.51 - 0.86)).
Conclusion: Changes in FLI or BARD score were significantly associated with CVD outcomes and mortality in new-onset T2DM.
Disclosure: J. Park: None.
SO 68 From brain circulation to cognitive dysfunction
781
Inhibition of NLRP3 ameliorates lipid accumulation in diabetic-associated cognitive dysfunction
W. Zhu1,2, T. Niu1,2, T. Li1,2, H. Zhang3, S. Wang1;
1Affiliated Zhongda Hospital of Southeast University, Nanjing, 2Southeast University, Nanjing, 3The First Affiliated Hospital of China University of science and technology, Hefei, China.
Background and aims: Accumulating evidences suggest that type 2 diabetes (T2D) predisposes to neuropathophysiological alterations including inflammatory responses and lipid deposition in brain that eventually culminates into cognitive impairment. Nucleotide leukin-rich polypeptide 3 (NLRP3) is documented as a potent target for treating metabolic diseases and inflammatory disorders. Earlier reports have suggested that NLRP3 deficiency prevents the accumulation of renal cholesterol in diabetic nephropathy. This study was to investigate the effect of NLRP3 on lipid accumulation in the hippocampus and the underlying mechanism in diabetic cognitive impairment.
Materials and methods: In vivo, C57BL/6 mice and NLRP3 knockout mice were fed for 6 months with normal chew diet or high fat diet (60 kJ% from fat) with/without intraperitoneal injections of streptozotocin (STZ) to induce diabetes. After 24 weeks, Morris Water Maze and New Object Recognition Test had be performed to evaluate the learning and memory ability of mice. And the normal and diabetic mice were sacrified to evaluated the protein expression of NLRP3, Interleukin-1β(IL-1β), Tumor necrosis factor α (TNF-α), Interleukin-6 (IL-6) and Hydroxymethyl glutaryl-CoA reductase (HMGCR) by western blot, as well as intracellular lipid droplet deposition in the hippocampal tissues. In vitro, BV2 cells (mouse microglial) were treated with AGEs and palmitic acid over 24 hours. Common microscope was used to observe the activation of BV2 cells. And the expression of NLRP3, inflammatory factors, HMGCR and lipid deposition were also detected.
Results: Diabetic mice with or without NLRP3 knockout showed impaired glucose tolerance. Compared with the normal mice, diabetic mice exhibited reduced learning and memory abilities, and NLRP3, IL-1β, IL-6, TNF-α and HMGCR level were increased, accompanied by lipid accumulation. High-glucose and high-lipid intervention promoted the activation of BV2 cells, the expression of NLRP3, IL-1β, IL-6, TNF-α and HMGCR, as well as lipid accumulation.
Conclusion: In diabetes, activation of NLRP3 may promote lipid accumulation in the hippocampus.
Supported by: 81870568
Disclosure: W. Zhu: None.
782
Cardio-metabolic factors and risk of dementia in people with type 2 diabetes in England: a large retrospective cohort study
E.P. Vamos1, H. Lai1, M. Sharabiani1, E.W. Gregg1, J. Valabhji2, L. Middleton1, C. Millett1, A. Majeed1, A. Bottle1, K. Chang1;
1School of Public Health, Imperial College London, 2Division of Metabolism, Digestion and Reproduction, Imperial College London, London, UK.
Background and aims: Type 2 diabetes (T2D) is a known risk factor for dementia but links between cardio-metabolic factors and T2D-related dementia risk are less well-understood. This study aims to explore and quantify associations of routinely collected cardio-metabolic factors and dementia risk in people with T2D.
Materials and methods: In this retrospective cohort study, we used data from the UK Clinical Practice Research Datalink from 1999 to 2018, enrolling 249,958 adults with T2D aged ≥ 42 years. Continuous cardio-metabolic measures were modelled linearly as well as using tail restricted cubic splines with Cox proportional hazards regression models to estimate hazard ratios (HR) and 95% CI for dementia risk, with age as timescale. Categorised cardio-metabolic measures were also analysed using Cox regression. Models were adjusted for socio-demographic characteristics, duration of diabetes, co-morbid conditions and medications. Analyses were further stratified by follow-up time segmented into three groups (<5, 5-10 and >10 years).
Results: During a median follow-up of 4.0 years, 22,492 people (9%) developed dementia. For patients with ≥10 years follow-up, increased levels of body mass index (BMI) were associated with higher dementia risk (HR 1.03 (95% CI 1.03-1.04 per 1 kg/m2 increment). Cubic splines indicated an increased risk of dementia from 29 kg/m2 onwards compared with BMI<25 kg/m2. Furthermore, BMI in the obesity range was linked to an increased dementia risk (BMI ≥30 kg/m2; HR 1.43 [96% CI 1.31-1.56]). Systolic blood pressure (SBP) level was linked to a 5% lower dementia risk per 10 mm Hg increment, with cubic splines showing an inverse association between SBP and dementia risk throughout the whole range of SBP values. Fasting plasma glucose (FPG) and HbA1c were associated with a 2% and 4% increased dementia risk, respectively (per 1 mmol/L and 5 mmol/mol increments, respectively). No evidence of association was found for total cholesterol and low-density lipoprotein (LDL) while higher levels of high-density lipoprotein (HDL) were linked to a lower dementia risk (0.98 [0.97-0.99] per 0.1 mmol/L increment). When analysing exposures measured ≤ 5 years of follow-up, higher SBP and BMI levels were associated with lower dementia risk (9% and 3% lower risk, per 10 mm Hg and 1 kg/m2 increment, respectively). In these analyses, associations attenuated for glycaemic measures and there was no evidence of associations for lipid measures.
Conclusion: Higher BMI and glycaemic values measured over 10 years before dementia diagnosis were linked to increased dementia risk among people with T2D. The results highlight the importance of long follow-up times to reduce reverse causation. The results may also show the potential importance of modifiable cardio-metabolic risk factors in preventing or delaying dementia, in a patient population at increased risk of dementia.
Supported by: Diabetes UK (Grant No: 18/0005851).
Disclosure: E.P. Vamos: None.
783
Insulin resistance and systemic low-grade inflammation reduce risk of developing dementia in a nationwide cohort study of type 2 diabetes patients
J.S. Nielsen1, N.J. Jensen2, L.W.B. Christensen3, H.T. Sørensen3, K. Højlund1, R.W. Thomsen3, J. Rungby2;
1Odense University Hospital, Odense, 2Bispebjerg University Hospital, Copenhagen, 3Aarhus University Hospital, Aarhus, Denmark.
Background and aims: We examined the hypothesis that increased insulin resistance and systemic low-grade inflammation measured close to diabetes diagnosis associate with future risk of dementia.
Materials and methods: In the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort of patients with recently diagnosed type 2 diabetes, we identified all patients enrolled between 2010-2018 without a previous diagnosis of dementia and with a fasting HOMA2-IR (insulin resistance) and valid hsCRP (low-grade inflammation) measurement. For each of the two exposures the relative risk of receiving a first diagnosis of any type of dementia was computed for each quartile using Cox proportional hazard analysis.
Results: Among 4.579 patients with newly diagnosed type 2 diabetes (median age, 62.4 years; 58.2% males), 52 (1.1%) developed dementia over a median follow-up period of 5.1 (IQR, 4.4-5.9) years. The incidence rate of developing dementia was highest in patients with lowest HOMA2-IR values (1st quartile 3.0 [95% CI, 1.8; 4.8] vs. 2nd quartile 2.2 [1.2; 3.7], 3rd quartile 1.9 [0.9; 0.35], and 4th quartile 1.8 [0.8; 3.2] per 1,000 person-years). This corresponded to an age- and sex-adjusted hazard ratio of 1.47 [0.83; 2.60] when comparing the 1st quartile with 2nd-4th quartiles (Figure 1.A). Similarly, dementia incidence was highest in patients with lowest hsCRP values (1st quartile 3.1 [1.8; 4.9] vs. 2nd quartile 2.1 [1.1; 3.6], 3rd quartile 1.7 [0.8; 3.2], and 4th quartile 2.1 [1.1; 3.6] per 1,000 person-years); which resulted in an age- and sex-adjusted hazard ratio of 1.40 [0.79; 2.49] when comparing the 1st quartile with 2nd-4th quartiles (Figure 1.B).
Conclusion: In contrast to our hypothesis, the risk of developing dementia was about 40% higher in diabetes patients with the lowest degrees of insulin resistance and low-grade inflammation at time of diabetes diagnosis. However, the still low number of dementia cases and short follow-up time in the DD2 cohort limit statistical precision and increase risk of reverse causality.
Disclosure: J.S. Nielsen: None.
784
Retinal microperimetry: a useful tool to predict and monitor the cognitive decline in patients with type 2 diabetes >65 years: a 3 years follow-up study
A. Ciudin1,2, A. Ortiz-Zuñiga1, J. Vasquez-de Sebastian3, C. Tejero4, O. Simó-Servat1, C. Hernandez1, R. Simó1;
1Endocrinology and Nutrition, Vall Hebron University Hospital, Vall Hebron Research Institute-VHIR, Barcelona, 2CIBERDEM, Madrid, 3RE-FiT Barcelona Research Group, Institut de Recerca Vall Hebron-VHIR & Parc Sanitari Pere Virgili, Barcelona, 4Endocrinology and Nutrition, RE-FiT Barcelona Research Group, Institut de Recerca Vall Hebron-VHIR & Parc Sanitari Pere Virgili, Barcelona, Spain.
Background and aims: Current guidelines for management of type 2 diabetes (T2D) recommend screening and annual monitoring for the cognitive function in patients >65 years. T2D acts as accelerator from normal cognition (NC) to mild cognitive impairment (MCI) and from MCI to dementia. However, the accurate assessment of the cognitive status is based on complex neuropsychological tests that cannot be largely incorporated to the current clinical practice. Additionally, at present, there are no reliable biomarkers to identify T2D patients with NC at higher risk of developing MCI. Our group has previously shown that retinal microperimetry (MAIA) is useful for discriminating NC and MCI in patients with T2D, but there are no data on its usefulness as either predictive biomarker of cognitive decline or for its monitoring. On these bases, we performed the present study aimed at exploring the role of retinal microperimetry both as a predictor of cognitive impairment and for the annual monitoring of the cognitive function in patients with T2D>65 years.
Materials and methods: Prospective observational study, including patients with T2D > 65 years without known cognitive impairment, attending the outpatient clinic of our center between March-October 2018, with a 3-year follow-up after the baseline visit. At baseline and after 3 years the following items were assessed: anthropometric data, biochemical analysis, complete neuropsychological battery (RBANS) and retinal microperimetry MAIA 3rd generation-(retinal sensitivity (decibels [dB]), gaze fixation: P1%, P2%, BCEA63, BCEA95). MAIA was also performed at 12 months.
Results: 50 patients were evaluated and identified 36 T2D-NC, 10 T2D-MCI and 4 T2D with dementia. Patients with dementia were excluded. Regarding the MAIA, no significant changes were found in retinal Sb was after 12-month, while all gaze fixation parameters significantly worsened in both groups (NC and MCI). After 3 years’ follow-up, all the patients presented a significant worsening in the RBANS score and gaze fixation MAIA parameters (BCEA95), while retinal sSb significantly changed only in the NC group (24.5±0.6 vs 21.6±4.2, p 0.041). The evolution of the RBANS score significantly correlated with the gaze fixation BCEA95 (r=-0.617, CI [-0.406--0.773], p=0.006). In the NC group, 18 patients converted to MCI after 3 years. These patients had significantly lower baseline retinal Sb (20.6±3.8Db vs 23.8±0.9dB, p<0.001) and worse gaze fixation (BCEA95 23.9±18.1 vs 6.04±5.8°, p 0.017) than subject who remained with normal cognition. The cut-off of 21.15 dB in basal retinal Sb predicted the risk of conversion from NC to MCI at 3 years with a sensitivity and specificity of 100%.
Conclusion: Our data suggest that retinal microperimetry, in particular gaze fixation parameter, is a useful tool for monitoring the cognitive decline both in T2D patients with NC or MCI. Additionally, retinal sensitivity is a useful tool to predict cognitive impairment after 3 years’ in T2D patients with NC.
Disclosure: A. Ciudin: None.
785
Type 2 diabetes and mood, anxiety and stress-related disorders: a genetically informative register-based cohort study
S. Liu1, M. Leone1, J.F. Ludvigsson1, S. Gudbjörnsdottir2, P. Lichtenstein1, S. Bergen1, H. Larsson1, R. Kuja-Halkola1, A. Butwicka1;
1Department of Medical epidemiology and biostatistic, Karolinska Institute, Solna, 2Swedish National Diabetes Register, Gothenburg, Sweden.
Background and aims: Mental health problems, especially mood, anxiety, and stress-related disorders, are common in people with type 2 diabetes (T2D). Yet, limited evidence is available for people with early-onset T2D (diagnosed before age 45), and the underlying factors that contribute to the concomitant occurrence between T2D and these psychiatric disorders remain unclear. To assess the association and familial co-aggregation of early-onset T2D with mood, anxiety, and stress-related disorders and estimate the contribution of genetic and environmental factors to their co-occurrence.
Materials and methods: This nationwide population-based cohort study was conducted using several Swedish national registers. Individuals born in Sweden from 1968 to 1998 were included and followed up to 31st Dec 2013. Within the study cohort, we identified pairs of full-, maternal and paternal half-siblings and cousins. Early-onset T2D and psychiatric disorders were diagnosed before age 45 using the International Classification of Disease, versions 9 and 10. Associations and familial co-aggregations of T2D with mood (including unipolar depression, bipolar disorder), anxiety, and stress-related disorders were estimated by Logistic regression models. Relative contributions of genetic and environmental factors to the association were estimated by quantitative genetic modeling.
Results: Out of a total of 3,061,192 individuals, 7896 (0.3%) were diagnosed with T2D before age 45. Individuals with early-onset T2D showed statistically significantly increased risks of all examined psychiatric disorders (Figure 1). The increased risks were also seen in their full-siblings and half-siblings. These associations seem to be explained by the shared genetic factors to different extents: 51% for unipolar depression, 62% for bipolar disorder, 64% for anxiety, and 58% for stress-related disorders, with the rest being explained by non-shared environments.
Conclusion: Our findings expand the present understanding of the shared etiological liability of the co-occurrence of early-onset type 2 diabetes with mood, anxiety, and stress-related disorders, which could direct future research aiming at identifying the shared biological pathways, environmental risk factors, and intervention targets.
Disclosure: S. Liu: None.
786
Polygenic risk of type 2 diabetes is associated with vascular dementia but not Alzheimer's disease
E. Dybjer1, A. Kumar2,3, K. Nägga2,4, G. Engström5, N. Mattsson-Carlgren1,3, P.M. Nilsson5,6, O. Melander6, O. Hansson3,3;
1Lund University, Malmö, 2Clinical Memory Research Unit, Department of Clinical Sciences Malmö, Lund University, Malmö, 3MultiPark: Multidisciplinary research focused on Parkinson’s disease, Lund University, Malmö, 4Department of Acute Internal Medicine and Geriatrics, Linköping University, Linköping, 5Department of Clinical Sciences Malmö, Lund University, Malmö, 6EpiHealth: Epidemiology for Health Strategic Research Area, Lund University, Malmö, Sweden.
Background and aims: Type 2 diabetes and dementia are associated, but their causal relationship is unclear. Genetic studies, i.e. using polygenic risk scores (PRS) or Mendelian Randomization (MR), and validated dementia endpoints with high diagnostic precision (instead of register-based diagnoses) may clarify causal links between these two entities.
Materials and methods: We tested associations between PRS for type 2 diabetes, fasting glucose, fasting insulin and HbA1c on the one hand and dementia on the other in 29,139 middle-aged participants followed for 23 years. Dementia diagnoses were validated by physicians and included all-cause dementia, mixed dementia, Alzheimer's Disease (AD) and Vascular Dementia. We also tested causal associations between type 2 diabetes and dementia forms through MR analyses.
Results: Seven different PRSs (including SNPs with different significance thresholds between p<5e-02 through -08, named PRS 1-7) for type 2 diabetes were tested. A PRS including N = 4,891 SNPs with p-value<5e-04 showed strongest association with the different outcomes, including all-cause dementia (Hazard Ratio (HR) of 1.11, Bonferroni corrected p = 3.6e-03), mixed dementia (HR 1.18, Bonferroni corrected p = 3.3e-04) and VaD (HR 1.28, Bonferroni corrected p = 9.6e-05), but no significant associations were found with AD. The analyses were adjusted for age, gender and education in Model 1 and the same factors and additionally APOE ε2 and APOE ε4 genotype (0, 1 or 2 alleles) in Model 2. See Figure 1 where bars that rise above the dotted line represent significant associations. Associations were stronger for non-carriers of APOE ε4. MR analyses could, however, not confirm a causal link between genetic risk markers of type 2 diabetes and dementia outcomes.
Conclusion: Polygenic risk of type 2 diabetes is associated with dementia risk, with stronger associations for VaD, in particular in non-carriers of APOE ε4. However, the MR findings did not support a causal relationship between genetic risk markers of type 2 diabetes and VaD.
Supported by: The Swedish Diabetes Fund
Disclosure: E. Dybjer: None.
787
Cerebral microcirculation and cerebrovascular reactivity in type 2 diabetes and obesity, influence of haptoglobin polymorphism
M. Káplár1, B. Cogoi1, R. Esze1, L. Rajnai1, Z. Képes2, S. Somodi1, G. Paragh1, M. Katkó1, M. Emri1, I. Garai2;
1University of Debrecen, 2ScanoMed Ltd, Debrecen, Hungary.
Background and aims: Microcirculation is damaged in diabetic patients, and it has also been observed in obesity. Decreased cerebrovascular reactivity has already been published in type 2 diabetes mellitus (T2DM). Haptoglobin (Hp) modulates atherosclerosis given its antioxidant effect. Hp(1-1) provides a stronger antioxidant effect as compared to Hp(1-2) and Hp(2-2) polymorphisms. A strong association between haptoglobin polymorphism and coronary sclerosis, furthermore carotid artery intima-media thickness has been reported previously, however, no data regarding the impact of Hp polymorphism on cerebral microcirculation and cerebrovascular reactivity are available. Increased platelet activity and advanced glycation end products (AGEs) fundamentally influence vascular function. Our main aim was to investigate the cerebral microcirculation and the cerebrovascular reactivity and to find any association with haptoglobin polymorphism, platelet activity and AGEs levels in obesity and T2DM.
Materials and methods: Participants (diabetic group: 51 patients, mean age: 50.6±7.7 year, BMI: 33.59±5.91 kg/m2; obesity group: 46 patients, mean age: 51.5±9.7 year, BMI: 37.97±6.08 kg/m2) were involved after a written consent was obtained. Tc99m HMPAO dynamic SPECT/CT studies were performed to assess resting cerebral microcirculation. Cerebrovascular reactivity was measured from middle cerebral arteries using transcranial Doppler with the breath-holding method and results were expressed as breath-holding index (BHI). Serum AGEs and P-selectin levels were determined by autofluorescence and ELISA, respectively.
Results: There were no significant differences in hemispheric and regional brain perfusion at rest either between T2DM and obese patients or between groups with different haptoglobin polymorphisms. However, significant differences in BHI between Hp(1-1,1-2) and Hp(2-2) polymorphisms were found both in the whole study group (p=0.005), and in the obese group when evaluating the results separately (p=0.037). The difference in BHI between the two sides correlated negatively and significantly with AGEs levels in patients with Hp(1-1,1-2) polymorphism. There was a significant inverse correlation between BHI and P-selectin levels in those with Hp(2-2) polymorphism.
Conclusion: Hp(2-2) polymorphism significantly reduces the cerebrovascular reactivity of middle cerebral arteries in obese subjects. AGEs and platelet activity have only partial effect on BHI.
Disclosure: M. Káplár: None.
788
Prevalence of cognitive impairment, functional impairment and paroxysmal atrial fibrillation among middle-aged patients with diabetes
C.M. Khoo1,2, K. Htike2, A.M. Reshma1,2, Y.H.K. Eric1, C.S.S. Raymond1,2, Y. Dong1,3, Y.Y. Dan1,2;
1Medicine, National University of Singapore, 2Medicine, National University Hospital, 3Alice Lee Centre for Nursing Studies, Singapore, Singapore.
Background and aims: The standard of care for diabetes mellitus recommends annual diabetes complications screening comprising diabetic eye and foot screening, urine for microalbuminuria and serum creatinine. Here, we examined the prevalence of cognitive impairment, functional impairment, and paroxysmal atrial fibrillation (pAF) that might be important to patient outcomes.
Materials and methods: This was a cross-sectional study of 1,200 middle-aged patients who attended the Diabetes Clinic in an academic hospital. The mean age was 57.7+4.5 years and 55% were males. Ninety-five percent of patients had type 2 diabetes. The mean diabetes duration was 16+9 years, HbA1c 8.4+1.9% and BMI 28.2+5.9 kg/m2. The Montreal Cognitive Assessment (MoCA) test was used for cognitive screening. For functional assessment, the 5 times sit-to-stand test was used for lower body strength, 4-meter walk for mobility and handgrip strength (using Jamar Plus+ Digital Hand Dynamometer) for hand muscle strength. The 72-hour cardiac monitoring device was used to assess for pAF.
Results: There were 41.7% (497/1191) patients with cognitive impairment (defined as MoCA <26). Of these, 59% had a MoCA score between 23 to 25, and 41% a MoCA score ≤22. Those with cognitive impairment were significantly older (59.0 vs 57.5 years), had a longer duration of diabetes (16.7 vs 15.2 years), a higher HBA1c (8.8 vs 8.2%) and BMI (28.8 vs 27.7 kg/m2) levels, a higher pill burden (5.2 vs 4.9) and a greater proportion of insulin users (61% vs 47%) compared to those without cognitive impairment. 922 patients completed all three functional assessments. Of these, 15.2% patients failed Sit-to-Stand-5 test (defined as >12s), 29.8% had reduced handgrip strength (<28kg in males and <18kg in females), and 2.9% had an abnormal 4-m walk (defined as <1 m/s). There were 37.7% of patients with at least one abnormal functional assessment. There was no incident of pAF among the initial 137 patients, thus 72-hour cardiac monitoring was discontinued earlier.
Conclusion: Among middle-aged patients with diabetes, there is a high prevalence of cognitive impairment. Cognitive impairment is associated with older age, longer duration of diabetes and poorer glycaemic control. Up to 40% of middle-aged patients with DM have had possible sarcopenia. Routine screening for pAF is not effective. We recommend the addition of cognitive and functional assessment as part of annual diabetes complications screening among middle-aged patients with diabetes to detect and intervene against early cognitive impairment and possible sarcopenia.
Supported by: NUHS AHS Mission Fund
Disclosure: C.M. Khoo: None.
789
High-mobility group box 1 protein mediates the cross-talk between neuron and microglia results from advanced glycation end products in diabetic mice with neuroinflammation
H. Zhang1, Z. Zhang1, W. Zhu2, T. Niu2;
1Department of Endocrinology, The First Affiliated Hospital of University of Science and Technology of China, Hefei, 2Department of Endocrinology, Affiliated Zhongda Hospital of Southeast University, Nanjing, China.
Background and aims: High-mobility group box 1 protein (HMGB1) is one of the damage-associated molecular patterns (DAMPs) are endogenous molecules released by stressed cells in response to tissue injury. It was demonstrated that neurons were damaged by advanced glycation end products (AGEs) in our previous study. Here, we aim to investigate the effect of HMGB1 released from injured neurons on microglia and associated neuroinflammation in KK-Ay mice with type 2 diabetes mellitus (T2DM).
Materials and methods: In cell research, AGEs were used to treat BV2 cells (Cell line of microglia) and HT22 cells (Hippocampal neuron cell line) as well as primary microglia and neurons. Additionally, medium from neurons with AGEs were used to treat microglia. Furthermore, medium from neurons with AGEs and FPS-ZM1 were also used to treat microglia. Finally, mRNA levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in BV2 cells and primary microglia as well as HMGB1 in cytoplasm, nuclear and medium were tested. In animal experiment, 15 weeks old KK-Ay mice were used as the model of diabetic cognitive impairment and neuroinflammation. Age matched C57/BL6j mice were used as control. Additionally, FPS-ZM1 was utilized to block the function of AGEs receptor. Levels of AGEs peptide in plasma as well as TNF-α, IL-1β and IL-6 mRNA in hippocampus tissue were measured. HMGB1 levels in cytoplasm and nuclear were observed. For the estimating of microglia activation, immunofluorescence was used to detect IBA-1 and CD68 in the brain of mice.
Results: In vitro study, low dose of AGEs cannot stimulate neuroinflammation in BV2 cells or primary microglia of neurons from new born mice. While low dose AGEs increased levels of HMGB1 in cytoplasm and medium, it could not regulate HMGB1 levels in nuclear of HT22 cells and primary neurons from fetal mice. Additionally, medium from AGEs treated HT22 cells and primary neurons increased the inflammatory factors including TNF-α, IL-1β and IL-6 of BV2 cells and primary microglia. Moreover, FPS-ZM1 treatment decreased levels of HMGB1 in cytoplasm and medium of HT22 cells and primary neurons. Compared with medium from AGEs treated HT22 cells and primary neurons, those with AGEs and FPS-ZM1showed reduced levels of TNF-α, IL-1β and IL-6 of BV2 cells and primary microglia. In vivo, microglia associated neuroinflammation was activated. In addition, HMGB1 levels of cytoplasm were elevated in KK-Ay mice with diabetes. Moreover, FPS-ZM1 not only decreased HMGB1 levels of cytoplasm but also reduced activation of microglia and levels of TNF-α, IL-1β and IL-6.
Conclusion: HMGB1, derived from AGEs stressed neurons, mediated the cross-talk between neuron and microglia and stimulated the microglia associated neuroinflammation in diabetic mice with cognitive impairment.
Disclosure: H. Zhang: None.
SO 69 Type 1 diabetes: new findings and complications
790
Exercise-related hypoglycaemia induces hypercoagulable changes in patients with type 1 diabetes
P.G. Hagelqvist1, A. Andersen1, K. Maytham1, S. Engberg2, U. Pedersen-Bjergaard3, P.I. Johansson4, F.K. Knop5, T. Vilsbøll1;
1Clinical Research, Steno Diabetes Center Copenhagen, University of Copenhagen, Herlev, 2Novo Nordisk A/S, Søborg, 3Department of Endocrinology and Nephrology, Nordsjællands Hospital Hillerød, University of Copenhagen, Hillerød, 4Regional Blood Bank, Section for Transfusion Service, Rigshospitalet, University of Copenhagen, Copenhagen, 5Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark.
Background and aims: Conventional plasma coagulation tests have indicated that diabetes constitutes a hypercoagulable state, which may increase susceptibility to thrombotic events. Physical exercise can improve cardiovascular health but may also cause hypoglycaemia in insulin-treated patients. We compared haemostatic profiles of patients with type 1 diabetes (T1D) with healthy controls using new generation whole blood viscoelastic technique and evaluated haemostatic changes during hypoglycaemia, induced with or without exercise, in patients with T1D.
Materials and methods: Thromboelastography (TEG®6s) was used to compare haemostatic profiles of patients with T1D (n=15, [mean±SD] age 29.4±8.1 years, HbA1c 51.0±5.5 mmol/mol, diabetes duration 13.1±6.2 years, BMI 23.7±2.0 kg/m2) with healthy controls individually matched according to age, sex and BMI (n=15). In a randomised, crossover design, patients with T1D underwent two separate hyperinsulinaemic euglycaemic-hypoglycaemic clamp days. Hypoglycaemia was either induced with the participants at rest (Clamp-rest) or under moderate-intensity exercise (Clamp-exercise). TEG was performed at baseline-euglycaemia, after 15 minutes and 60 minutes of hypoglycaemia.
Results: Compared with healthy controls, patients with T1D were more hypercoagulable with shorter R-time (mean difference -1.62 min [95% CI -2.41;-0.84]), greater angle value (3.05° [0.84;5.26]) and shorter K-time (ratio 0.81 [95% CI 0.69;0.96]). Although a numerically greater clot maximum amplitude (MA-CK) was observed in patients with T1D, the result was not significant. Compared with baseline, 15 minutes of exercise-related hypoglycaemia induced hypercoagulable changes with increased angle value, increased MA-CK and decreased fibrinolysis (LY-30), whereas 60 minutes of hypoglycaemia induced at rest increased functional fibrinogen (MA-FF) and increased fibrinolysis (Table 1).
Conclusion: Compared to healthy controls, patients with T1D exhibit hypercoagulability, and exercise-related hypoglycaemia aggravates this without compensatory fibrinolytic activity, which may increase susceptibility to thrombosis.
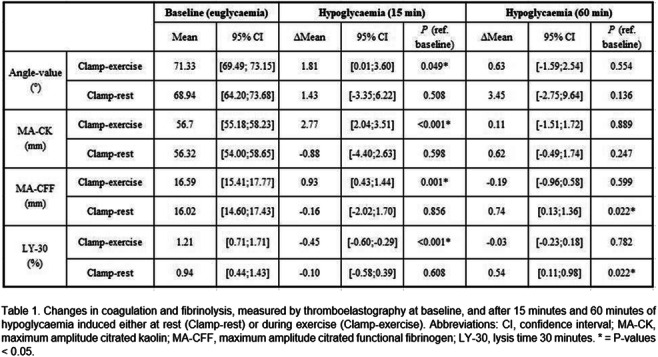
Clinical Trial Registration Number: NCT04650646
Supported by: DFF, Grosserer LF. Foghts foundation.
Disclosure: P.G. Hagelqvist: None.
791
Increased incidence of neurodegenerative diseases in Finnish individuals with type 1 diabetes
S. Satuli-Autere1,2, V. Harjutsalo1,3, M. Eriksson1,2, S. Hägg-Holmberg1,2, H. Öhman4, P.-H. Groop1,5, L. Thorn1,2;
1Folkhälsan Institute of Genetics, Folkhälsan Research Center, 2Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, 3Finnish Institute for Health and Welfare, 4Department of Geriatrics, Helsinki University Hospital, 5Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Diabetes is linked to neurodegenerative diseases (ND), but data in type 1 diabetes is scarce. Our aim is to assess the incidence of different NDs among individuals with type 1 diabetes, compared to the background population, and to evaluate the impact of diabetic vascular complications.
Materials and methods: This substudy includes 4,261 individuals with type 1 diabetes from the Finnish Diabetic Nephropathy Study, initiated in 1997, and 11,653 matched population-based controls without diabetes. The median age at diabetes onset was 13.9 (IQR 9.2-22.1) years, age at baseline study visit 37.4 (28.7-46.6) years, and 47.8% were women. Diabetic vascular complications were assessed at the baseline study visit, and included retinopathy (history of retinal photocoagulation), diabetic kidney disease (presence of albuminuria, <20μg/min), and cardiovascular disease (history of hard cardiovascular events). NDs were identified from registers until end of 2017, and included Alzheimer’s disease, vascular dementia, other dementias, Parkinson’s disease, and multiple sclerosis. Standard incidence ratios (SIR) comparing individuals with type 1 diabetes to controls, were calculated from the diabetes onset for the different NDs and from time of study visit for the different complications.
Results: We identified NDs in 142 (3.3%) individuals with type 1 diabetes and 201 (1.7%) controls, p<0.001, while mortality was higher in type 1 diabetes, 845 (19.8%) vs controls 660 (5.7%), p<0.001. The SIR for any dementia was 2.24 (95%CI 1.79-2.77), for Alzheimer's disease 2.13 (1.55-2.87), for vascular dementia 3.40 (2.08-5.26), and for other dementias 1.70 (1.22-2.31). For multiple sclerosis and other demyelinating diseases SIR was 1.72 (1.22-2.37) and for Parkinson's disease 1.61 (1.04-2.37). Table 1 shows the impact of diabetic complications. Incidence of any dementia was increased already in individuals without albuminuria, SIR 1.99 (1.44-2.68), and increased further with worsening kidney disease: microalbuminuria SIR 2.28 (1.24-3.88), macroalbuminuria 2.92 (1.66-4.78), and kidney replacement therapy 4.93 (2.40-9.04).
Conclusion: ND incidence is increased 1.7-3.4-fold in type 1 diabetes, despite a higher mortality compared to individuals without diabetes. The presence of diabetic complications, especially diabetic kidney disease and cardiovascular disease, further increased the incidence of dementia.
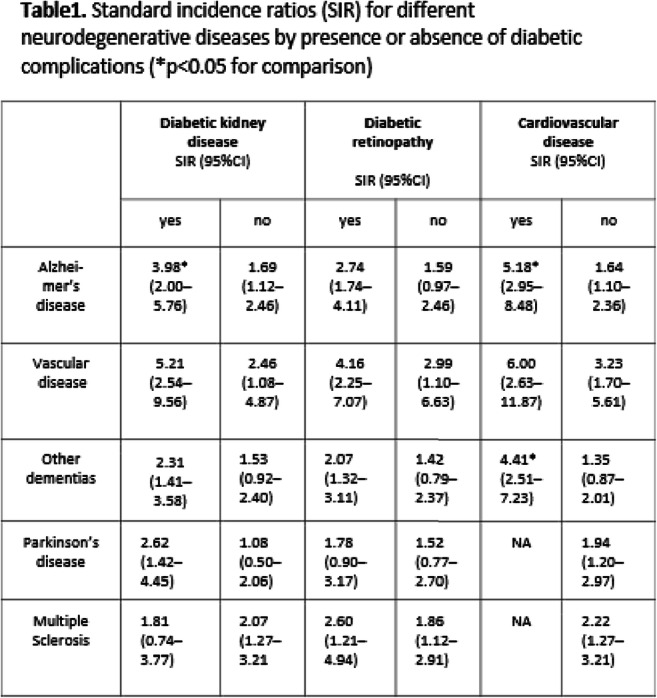
Supported by: Folkhälsan Research Center
Disclosure: S. Satuli-Autere: None.
792
Masp-2 deficiency and the development of diabetic kidney disease in a mouse model of type 1 diabetes
C.B. Holt1,2, L. Halkjær2,3, T. Dudler4, W.J. Schwaeble5, T.K. Hansen1, S. Thiel3, J.A. Østergaard1,6;
1Steno Diabetes Center Aarhus, Aarhus University Hospital, Århus, Denmark, 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 3Department of Biomedicine, Aarhus University, Aarhus, Denmark, 4Department of Research, Omeros Corporation, Seattle, USA, 5Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK, 6Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Background and aims: Diabetic nephropathy affects up to 30% of patients with diabetes and may progress to end-stage renal disease. Mannan-binding lectin (MBL) initiates the lectin pathway of complement and has been linked to albuminuria and mortality in patients with diabetes. We hypothesize that deficiency in MBL-associated serine protease 2 (MASP-2), the effector enzyme of the lectin pathway, will protect against diabetes-induced kidney damage.
Materials and methods: Male C57BL/6J MASP-2 knockout (Masp2-/-) mice and wildtype (WT) mice were divided into a diabetic group and a non-diabetic group. Renal hypertrophy, albumin excretion, glomerular volume, mesangial area, plasma levels of lectin pathway proteins and specific mRNA expressions in the renal cortex were measured after 8 and 12 weeks of diabetes. By two-way ANOVA it was tested if MASP-2 modulated the renal effects of diabetes, i.e., interaction.
Results: Diabetes-induced effects were observed in WT mice and Masp2-/- mice on kidney-to-body weight, albumin-to-creatinine ratio, mesangial area, plasma MBL-C, plasma C3, and renal mRNA expressions of both fibrotic and oxidative stress markers. After 12 weeks of diabetes Masp2-/- diabetic mice had a smaller mesangium at 21.1% of the glomerular area (95% CI 19.7, 22.6) when compared with WT diabetic mice, 25.2% (95% CI 23.2, 27.2), p(interaction)=0.001. After 8 weeks of diabetes, plasma cystatin C was 261.5 ng/ml (95% CI 229.6, 297.8) in the WT diabetic group compared to 459.9 ng/ml (95% CI 385.7, 548.3) in non-diabetic WT mice, p<0.001. By contrast, no difference in plasma cystatin C levels was found between the Masp2-/- diabetic mice, 288.2 ng/ml (95% CI 260.6, 318.6) and Masp2-/- non-diabetic mice, 293.5 ng/ml (95% CI 221.0, 389.7), p=0.86 and p(interaction)=0.001.
Conclusion: We demonstrated a protective effect of MASP-2 deficiency on mesangial hypertrophy after 12 weeks of diabetes as well as an effect on plasma cystatin C level. MASP-2 deficiency did, however, fail to protect against diabetes-induced alterations of kidney weight, albuminuria, and renal mRNA expression of fibrotic- and oxidative stress markers.
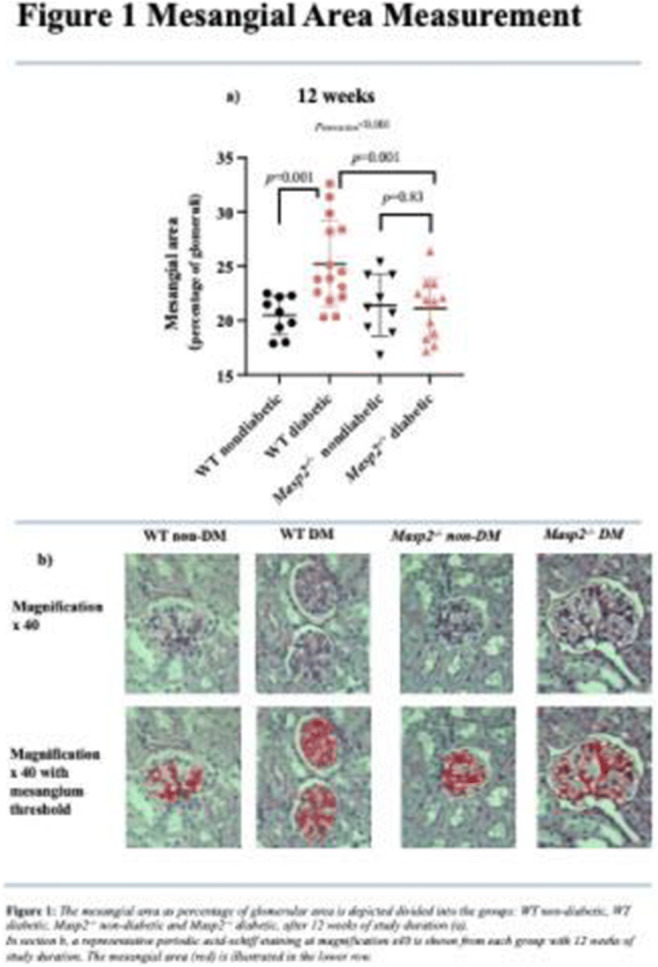
Supported by: AU, A.P Møller, Danish Council for independent research and Health Research Fund, Per Henriksen
Disclosure: C.B. Holt: None.
793
Non-alcoholic fatty liver disease and type 1 diabetes
I. Moreno-Ruiz1, I. Martín-Timón1, J. Modamio-Molina1, S. Bacete-Cebrian1, M. Zubillaga-Gómez1, L. Zeng1, O. Meizoso-Pita1, V. Triviño-Yannuzzi1, I. Huguet-Moreno1, M. Llavero-Valero1, C. Lara-Moreno2, C. Sevillano-Collantes1;
1Endocrinology, Hospital Universitario Infanta Leonor, 2School of Medicine.UCM, Madrid, Spain.
Background and aims: The association between type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD) is well known. Some studies indicate a relevant prevalence also in type 1 diabetes mellitus (T1DM), but so far there is only limited data. The aim of this study is to estimate the prevalence of NAFLD in patients with T1DM using non-invasive scores and compare baseline characteristics of patients with steatosis versus those without it.
Materials and methods: This is a retrospective observational study of 289 T1DM patients of our outpatient clinic of endocrinology. Exclusion criteria were alcohol consumption, pre-existing chronic liver disease (viral or autoimmune hepatitis) and use of chronic steatogenic medication. To predict the prevalence of NAFLD, we calculated the hepatic steatosis index (HSI). NAFLD score, FIB-4 index, GOT-Platelet Related Index (APRI), and GOT/GPT ratio were calculated for every patient and used to estimate the prevalence of advanced fibrosis. The baseline characteristics of patients with steatosis, versus those without it, were compared.
Results: Of the 289 patients included in the analysis 126 were women (43.6%) and 163 (56.4%) men with a mean age of 40.9 ± 12.5 years. The mean duration of DM was 19.7 years (0.5 to 54 years). 254 were on basal-bolus treatment and 35 on continuous subcutaneous insulin infusion (CSII). They had a mean BMI of 26.3 ± 4.4 kg/m2, being 58.1% of the individuals under consideration above normal weight (BMI>25 kg/m2). NAFLD based on HSI > 36 was present in 61.9% of patients. Advanced fibrosis was present in 2.07% based on NFS > 0.676, 0.69% based on FIB-4 > 2.67, 0.69% based on APRI > 1.5, and 11.4% based on AST/ALT > 1.4. Compared to non NAFLD subjects, NAFLD subjects had significantly higher: age (42.9 ± 11.9 years vs 37.6 ± 12.9 years, p<.0001), BMI (28.5 ± 3.8 kg/m2 vs 22.6 ± 2.1 kg/m2, p< .0001), diabetes duration (21.2 ± 11.7 years vs 17.2 ±12.8 years, p=.0081), HbA1c (7.7 ± 1.3% vs 7.3 ± 1.1%, p = .0006), GPT (28 ± 15 U/l vs 19 ± 11 U/l, p<.0001) and triglycerides (108 ± 96 mg/dl vs 76 ± 42 mg/dl, p<.0001) while HDL levels were lower (50 ± 14 mg/dl vs 57 ± 15 mg/dl, p=.0002) in NAFLD patients. NAFLD subjects had higher prevalence of high blood pression (27.9% vs 10,9%, p=.0006), retinopathy (22.35% vs 9.09%, p=.003) and statin use (53.6% vs 28.1%, p<.0001). Doses of insulin/kg was significantly higher in NAFLD subjects (0.64 ± 0,26 vs 0.53 ± 0.2, p=.0001) and the use of CSII is less prevalent in NAFLD patients compared to non NAFLD patients (8.9% vs 17.3%, p=0.03).
Conclusion: In our sample, a remarkable percentage of the individuals showed a BMI higher than normal. Using non-invasive scores, our data reveal a remarkable prevalence of NAFLD, but not of advanced fibrosis, in T1DM. Furthermore, a significant association was found between hepatic steatosis (according to HSI) and age, hypertension, BMI, retinopathy, HbA1c, GOT, HDL and TGs.
Disclosure: I. Moreno-Ruiz: None.
794
Evaluation of deep learning prediction models for cardiovascular risk prediction using fundus images from the Scottish Diabetic Retinopathy Screening programme
J. Mellor1, W. Jiang1, A.D. Fleming2, C.J. Styles3, A. Storkey4, P.M. McKeigue1, H.M. Colhoun2, Scottish Diabetes Research Network Epidemiology Group;
1Usher Institute, University of Edinburgh, Edinburgh, 2Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, 3Department of Ophthalmology, NHS Fife, Dunfermline, 4School of Informatics, University of Edinburgh, Edinburgh, UK.
Background and aims: Cardiovascular disease (CVD) is a leading cause of death worldwide with type 1 and type 2 diabetes as known risk factors. Recent studies have shown deep learning (DL) predictors of CVD risk factors based on fundus images are significantly associated with CVD risk independently of the same risk factors. However, reported increment in CVD risk prediction of the DL models compared to a model using clinical risk factors alone has been small. The objective of this study was to examine whether DL models using retinal images could improve the predictive performance for CVD risk - in people with type 1 diabetes (T1D) and type 2 diabetes (T2D) - when compared with a baseline Poisson model using clinical covariates.
Materials and methods: The cohort was constructed using the Scottish Diabetes Research Network dataset that links all fundus images between 2005-2017 from the Scottish Diabetic Retinopathy Screening (SDRS) programme to eHealth records. We follow 23,103 and 201,957 people with T1D and T2D respectively who were alive any time between 1 January 2008 and 1 January 2018 with no prior CVD event and at least one gradable screening episode prior to follow-up until first incident CVD yielding 166,113.1 and 1,269,142 person-years of follow up respectively. There were 2,003 and 38,569 incident CVD events respectively which included Coronary Heart Disease, stroke, Peripheral Vascular Disease, Cerebrovascular Disease, Atherosclerosis, Coronary Artery Disease, Acute Myocardial Infarction. Using fundus images from the SDRS programme we trained DL networks - for T1D and T2D separately - to yield DL predictors of: CVD, diabetic retinopathy (DR), eGFR, and systolic blood pressure (SBP). Each of the DL predictors is then included in a Poisson regression 10-year CVD risk prediction model containing known CVD risk factors. We compared the predictive performance between Poisson models (a restricted model including age, diabetes duration, and sex as covariates and a full baseline using further known risk factors) and the Poisson models that included the DL predictors. Half of the cohort were used to train all models and half to evaluate performance.
Results: C-statistics and test log-likelihoods are shown in the table. DL contributed no performance improvement to the restricted baseline models, with C-statistics 0.75 and 0.67, nor to the full baseline models, with C-statistics 0.82 and 0.71, for T1D and T2D respectively.
Conclusion: There has been much optimism that DL approaches may significantly improve prediction of CVD risk beyond conventional predictive models. However this study suggests the added value of DL predictors for CVD risk prediction may be small.
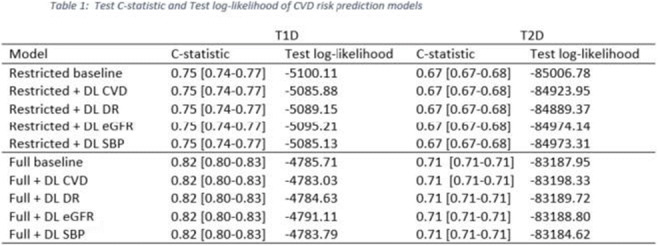
Supported by: JDRF
Disclosure: J. Mellor: Grants; JDRF 2-SRA-2019-857-S-B.
795
The contribution of insulin resistance and glycaemia on fibrin related clot parameters in young adults with type 1 diabetes
S.M. Pearson1, N. kietsiriroje1, R.C. Sagar1, M.D. Campbell2, D.D. Stocken1, S. Plein1, R.A. Ajjan1;
1Leeds institute of cardiovascular and metabolic medicine, University of Leeds, Leeds, 2Faculty of health sciences and wellbeing, University of Sunderland, Sunderland, UK.
Background and aims: CVD is a major problem in individuals with diabetes. Changes in clot density and fibrinolysis (fibrin related clot parameters) can predict adverse outcome following a vascular event in those with type 2 diabetes (T2D) and are also abnormal in type 1 diabetes (T1D). Individuals with T1D can develop insulin resistance which may exacerbate vascular risk. Estimated glucose disposal rate (eGDR) is a validated clinical marker of insulin resistance which predicts mortality in T1D. We hypothesised that insulin resistance in T1D further increases fibrin-related thrombosis risk and we aimed to explore the relative contribution of glycaemia and insulin resistance to changes in fibrin related clot parameters in young adults with T1D.
Materials and methods: Study participants were recruited from an adolescent diabetes clinic in the UK following informed consent. All had established T1D (>3 years) without macrovascular complication. Anthropometric data and a single blood sample were collected and participants were fitted with a blinded continuous glucose monitoring system (Freestyle Libre Pro) for 14 days. Citrated plasma samples were analysed using a validated turbidimetric assay with clot maximum absorbance (Max OD, a measure of clot density) and time to 50% clot lysis (a measure of fibrinolysis potential) measured. Time in range (TIR) and time below range (TBR) was assessed according to international consensus. Two linear regression models were used to identify factors associated with Max OD and 50% lysis time. Model parameters were adjusted by sex and diabetes duration >10 years as potential confounders. Mean values for each variable were calculated and participants were dichotomised accordingly.
Results: A total of 69 participants were recruited with mean±SD age 24.6± 3.5 years (n=43 males), diabetes duration 13.2±6.9 years and HbA1c of 64.5±16.6 mmol/mol. Mean eGDR was 7.96mg/kg/min±2.34. Mean TIR was 49.1%±18.9 and TBR 9.3%±8.3 with 15 patients with sensor wear <7 days excluded from analysis. Max OD was significantly affected by lower eGDR (β=0.063 95%CI: 0.037 to 0.085, p<0.001) and higher WC (β=0.049, 95%CI: 0.021 to 0.079, p<0.001.) 50% lysis time was observed in all patients and was affected by the same variables (β = 184sec, 95%CI: 45-332 and β =183sec, 95% CI: 44-321, all p<0.01.) TIR, TBR and random glucose had no effect on either variable but higher HbA1c was associated with increased Max OD (0.33Au; 0.044-0.62, p=0.028). Hypertension was associated with higher Max OD (0.043Au; 0.01-0.075, p=0.012) but not lysis time. Data is shown in figure 1.
Conclusion: In this study of young adults with T1D markers of insulin resistance (eGDR, hypertension and WC) had the strongest impact on fibrin related clot parameters. Consideration to improving insulin sensitivity should be given when providing care to this group of patients.
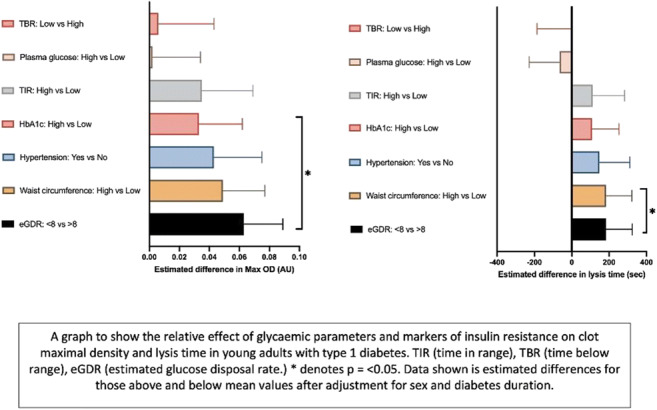
Supported by: The study was generously supported by Abbott diabetes care
Disclosure: S.M. Pearson: None.
796
Sustained QT prolongation 60-min post hypoglycaemic recovery in patients with type 1 diabetes
C.R. Andreasen1, A. Andersen1, P.G. Hagelqvist1, K. Maytham1, J. Lauritsen2, S. Engberg3, B. Hartmann4, J. Faber5, U. Pedersen-Bjergaard6, F.K. Knop2, T. Vilsbøll1;
1Clinical Reseach, Steno Diabetes Center Copenhagen, University of Copenhagen, Herlev, 2Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, 3Novo Nordisk A/S, Søborg, 4Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, 5Department of Endocrinology, Herlev Hospital, University of Copenhagen, Herlev, 6Department of Endocrinology and Nephrology, Nordsjællands Hospital Hillerød, University of Copenhagen, Hillerød, Denmark.
Background and aims: Previous trials suggest that hypoglycaemia may prolong cardiac repolarisation, causing insulin-treated patients with type 1 diabetes to be vulnerable to fatal cardiac arrhythmias. We investigated the effects of recovery from insulin-induced hypoglycaemia to either rebound hyperglycaemia or euglycaemia on cardiac repolarisation in patients with type 1 diabetes.
Materials and methods: We included patients with type 1 diabetes (n=24, [mean±SD] age 53±11 years, HbA1c 57.6±8.9 mmol/mol [7.5±0.8%], diabetes duration 23±14 years, BMI 25.7± 3.1 kg/m2), who underwent two experimental clamps in a crossover design. The two clamps consisted of three steady-state phases: 1) baseline euglycaemia (5-8 mmol/l) for 45 minutes, 2) hypoglycaemia (2.5 mmol/l) for 60 minutes, and 3) recovery hyperglycaemia (clamp A, 20 mmol/l) or euglycaemia (clamp B, 5-8 mmol/l) for 60 minutes.
Results: During hypoglycaemia, heart rate-corrected QT (QTc) (Fridericia’s formula) progressively increased from baseline on both clamp days (Table). In the recovery phase, QTc remained increased during hyperglycaemia and euglycaemia, with no difference between recovery to hyperglycaemia compared to euglycaemia. No significant changes from baseline were observed in QTc dispersion (heterogeneity of myocardial repolarization) during hypoglycaemia or in the recovery phases. Epinephrine levels were increased during hypoglycaemia, with no change from baseline during recovery and no difference between clamps. Potassium decreased during hypoglycaemia with no change from baseline during recovery. However, the potassium levels during recovery were higher during hyperglycaemia than in euglycaemia.
Conclusion: We found clinically significant QT prolongation during hypoglycaemia in patients with type 1 diabetes. The QT interval remained prolonged during a 60-minute recovery from hypoglycaemia with no difference between recovery to hyperglycaemia or euglycaemia. Our findings suggest that vulnerability to serious cardiac arrhythmias and sudden cardiac death may extend beyond a hypoglycaemic event itself.
Clinical Trial Registration Number: NCT03956173
Supported by: NNF, DDA
Disclosure: C.R. Andreasen: None.
797
The presence and extent of microvascular disease is independently associated with subclinical atherosclerosis in patients with type 1 diabetes
A. Barmpagianni1, A. Kountouri2, V. Lambadiari2, S. Liatis1;
1First Department of Propaedeutic Medicine, Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, 22nd Department of Internal Medicine, Propaedeutic and Research Institute,Athens University Medical School, "Attikon" University Hospital, Athens, Greece.
Background and aims: Cardiovascular atherosclerotic disease is a common cause of morbidity and mortality in patients with type 1 diabetes (T1D). Detection of subclinical atherosclerosis can be useful in order to identify individuals at high-risk to develop future cardiovascular events. The exact role of microvascular disease in the progression of atherosclerosis as well as its importance in predicting clinical cardiovascular disease has not been fully established. Aim of the present study is to examine the association between the presence and extent of diabetic microangiopathy and subclinical atherosclerosis in patients with T1D.
Materials and methods: We studied 235 consecutive patients with T1D from two academic diabetes outpatient clinics in Athens, without clinical macrovascular disease, The presence of retinopathy was assessed by an Opthalmologist, nephropathy was defined as an albumin/creatinine ratio (ACR) ≥ 30mg/g and/or eGFR < 60ml/min and cardiac autonomic neuropathy was measured through the battery of three cardiac autonomic tests as previously described. Subclinical atherosclerosis was assessed by measuring pulse wave velocity (PWV) between the common carotid and femoral arteries and its presence was defined according to previously described age-specific PWV limits.
Results: The present analysis was performed in 219 patients with completed data regarding the presence and extent of microvascular disease (76 [34.7%] males with mean age [SD] of 35.2 [12.8] years and median diabetes duration [IQR] of 16 [10-25] years. Seventy-five (34.2%) patients had at least one manifestation of microvascular disease, while 19 (8.7%) and 2 (0.9%) had two and three manifestations respectively. Subclinical atherosclerosis (as expressed by abnormal age-specific PWV value) was detected in 54 (24.7%) individuals, and was significantly related to higher systolic blood pressure (SBP), longer duration of diabetes, higher BMI and the presence of every single microvascular disease manifestation. No association was found between subclinical atherosclerosis and gender, HbA1c, lipid profile and insulin needs. In multivariable analysis, subclinical atherosclerosis was independently related to the presence and extent of microvascular disease (OR [95% CI]: 2.4 [1.1-5.2] for one manifestation: and 9.5 [3.2-28.4] for ≥ 2 manifestations). SBP (OR [95% CI]: 1.03 [1.01-1.05] for each mmHg increase), was the only other factor significantly associated with subclinical atherosclerosis.
Conclusion: The presence and extent of microvascular disease is significantly and independently associated with subclinical atherosclerosis in patients with T1D, free of clinical macrovascular complications. Prospective studies are needed in order to examine a possible predictive value of diabetic microangiopathy on future cardiovascular events.
Disclosure: A. Barmpagianni: None.
SO 70 Circulating markers of cardiovascular risk
798
Long-chain polyphosphates cause a distinct kidney injury phenotype and exacerbate LPS-induced acute kidney injury in mouse
A. Pirttiniemi1,2, H. Salmenkari1,3, J. Lindén4, K. Adeshara1,3, S. Lehtonen2,5, P.-H. Groop1,6, M. Lehto1,3;
1Folkhälsan Institute of Genetics, Folkhälsan Research Center, Helsinki, Finland, 2Research Program for Clinical and Molecular Metabolism, University of Helsinki, Helsinki, Finland, 3Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 4Finnish Centre for Laboratory Animal Pathology, University of Helsinki, Helsinki, Finland, 5Department of Pathology, University of Helsinki, Helsinki, Finland, 6Department of Diabetes, Central Clinical School, Monash University, Melbourne, Australia.
Background and aims: Diabetes and other metabolic disorders are often accompanied by an increased rate of infections and chronic low-grade inflammation, which may participate in the development and progression of kidney disease. Our study in The FinnDiane Study Group aims to assess the renotoxic effects of long-chain polyphosphates, which are commonly found in pathogenic gram-negative bacteria, alongside the more well-known endotoxin, lipopolysaccharide (LPS).
Materials and methods: We used an in vivo experimental acute kidney injury (AKI) mouse model to assess renal damage caused by bacterial components, LPS, and long-chain polyphosphates. AKI was induced by injecting the mice with LPS (5 mg/kg, intraperitoneal injection) and long-chain polyphosphate (700 orthophosphate residues, P700, 10.2 mg/kg intravenous injection). The treatment groups were: [1] healthy control (n=8), [2] LPS treated (n=8), [3] P700 treated (n=4), and [4] LPS + P700 treated (n=8). The mice were sacrificed 20 hours after injection, and the kidneys, sera and urine of the mice were collected for subsequent analysis. The study was conducted using 9-week-old wild-type male BALB/c mice (BALB/cAnNCrl; Scanbur, Karlsunde, Denmark). Kidney injury was assessed based on urine albumin-creatinine ratio (uACR, ELISA/enzymatic assays) as well as assessment of histopathological changes detectable in Hematoxylin-Eosin (H&E) and immunofluorescence (IF) microscopy analysis of the kidneys.
Results: Our study showed all treatment groups to have elevated uACR compared to the healthy control group: LPS (4.5-fold, p<0.001), P700 (8.7-fold, p<0.01), LPS + P700 (25.9-fold, p<0.01). Histopathological analysis (H&E) of the P700-treated mice showed foci of acute tubular necrosis in the outer medulla. The LPS + P700 treated mice exhibited additionally extensive acute tubular necrosis in the cortex, manifested as cytoplasmic eosinophilia, pyknotic nuclei and tubular casts. Further IF-staining revealed P700 and LPS + P700 treated mice to have delocalized and granular deposits of the podocyte marker, nephrin, at the podocyte foot process bifurcations (varying number and degree of affected glomeruli per kidney section). Interestingly, only LPS and LPS + P700 treated mice displayed clinical sepsis-like signs, while P700 treated mice showed no sepsis-like signs despite their observed kidney injury.
Conclusion: Long-chain polyphosphates, P700, worsen LPS-induced AKI and clinical signs of sepsis in mice. P700-treatment alone promotes albuminuria, and a distinct histopathological kidney phenotype with glomerular delocalization of nephrin, without causing clinical signs of sepsis. These findings demonstrate a novel role of polyphosphates for renal damage in mice, implying they could also serve as potential treatment targets for acute and chronic kidney disease associated with bacterial infections in humans.
Supported by: Uni. Helsinki DSHealth, Folkhälsan Research Center
Disclosure: A. Pirttiniemi: Employment/Consultancy; Employment contract by University of Helsinki, The Doctoral School in Health Sciences. Grants; Funding for material costs has been provided by The Folkhälsan Research Center.
799
Increase in specific ceramides associate to heart and kidney outcomes in type 1 diabetes
A. Wretlind1, V. Rotbain Curovic1, T. Suvitaival1, S. Theilade1,2, N. Tofte3, S.A. Winther3, T. Vilsbøll1, H. Vestergaard4,5, P. Rossing1,4, C. Legido-Quigley1,6;
1Steno Diabetes Center Copenhagen, Herlev, Denmark, 2Department of Medicine, Herlev-Gentofte Hospital, Herlev, Denmark, 3Novo Nordisk, Copenhagen, Denmark, 4University of Copenhagen, Copenhagen, Denmark, 5Bornholms Hospital, Rønne, Denmark, 6King's College London, London, UK.
Background and aims: Ceramides are lipid molecules involved in inflammation-related signaling. Recent studies have shown that changes in specific circulating ceramides: Cer(16:0), Cer(18:0) and Cer(24:1) and their ratio to Cer(24:0) are associated with the development of cardiovascular disease up to 5 years before the event. We investigated these ceramides in a well-characterized cohort of persons with type 1 diabetes in relation to cardiovascular disease, end-stage kidney disease and all-cause mortality.
Materials and methods: In this study 662 participants with type 1 diabetes were recruited between 2009 and 2011 for a prospective cohort study. Plasma samples were collected at baseline and mass spectrometry was performed, measuring four ceramides. Cox regression analysis was carried out investigating ceramides, and their ratio to Cer(24:0), against composite cardiovascular events, end-stage kidney disease, as well as all-cause mortality. Hazard ratios (HRs) are reported per doubling in concentration.
Results: The median follow-up time was 6.3 (IQR 0.8) years, during which 94 participants experienced cardiovascular events, 23 progressed to end-stage kidney disease and 58 participants deceased. Cer(16:0) and Cer(18:0) were associated with cardiovascular events with HRs of 2.6 (CI = 1.3-5.4, p = 0.007) and 2.0(1.2-3.4, p = 0.005). The ratios Cer(18:0)/Cer(24:0) and Cer(24:1)/Cer(24:0) were also associated with cardiovascular events with HRs 1.7 (1.1-2.6, p = 0.012) and 2.7 (1.4-5.2, p = 0.002), respectively. Cer(16:0) and the ratio of Cer(24:1)/Cer(24:0) were associated with end-stage kidney disease with HRs 5.4 (1.2-23.5, p = 0.024) and 8.8 (2.4-32.6, p = 0.001), respectively. Finally, Cer(24:0) and the ratios Cer(16:0)/Cer(24:0), Cer(18:0)/Cer(24:0) and Cer(24:1/Cer24:0) were all associated with all-cause mortality with HRs 1.4 (CI = 0.6-3.23), 3.3 (2.0-5.5), 3.3 (1.9-5.7) and 5.3 (2.3-12.1) (all p-values < 0.0001).
Conclusion: Specific ceramides were associated with increase in the 6-year risk of complications in a type 1 diabetes population. This highlights the strength of single ceramide association to vascular complications and presents a new potential tool for early risk assessment.
Clinical Trial Registration Number: NCT01171248
Supported by: SDCC provided internal funding. NNF grant NNF14OC0013659
Disclosure: A. Wretlind: Grants; Novo Nordisk Foundation grant NNF14OC0013659 “PROTON Personalizing treatment of diabetic nephropathy”.
800
Plasma mannose and a first myocardial infarction: associations according to glycaemic state
E. Fortin1, G. Ferrannini1, B. Campi2, L. Mellbin1, A. Norhammar1, P. Näsman3, A. Saba4, E. Ferrannini2, L. Rydén1;
1Department of Medicine K2, Karolinska Institute, Stockholm, Sweden, 2C.N.R. Institute of Clinical Physiology, Pisa, Italy, 3Center for Safety Research, Royal Institute of Technology, Stockholm, Sweden, 4Laboratory of Biochemistry, Department of Surgical, Medical, Molecular and Critical Area Pathology, University of Pisa, Pisa, Italy.
Background and aims: High mannose is associated with diabetes (DM), insulin resistance and coronary atherosclerosis. The relation between mannose levels and a first myocardial infarction (MI) in relation to the glycaemic state has not been explored. We aim at investigating whether mannose is a marker of a first MI in a population with and without dysglycaemia.
Materials and methods: Fasting plasma mannose concentrations were assayed in 777 patients 6-10 weeks after a first MI and in 771 sex- age- and area-matched controls from the Swedish PAROKRANK study, using high-performance liquid chromatography coupled to tandem mass spectrometry. All participants without known DM were categorized as having normal glucose tolerance (NGT), impaired glucose tolerance (IGT) or newly detected DM by means of an oral glucose tolerance test (OGTT). Mannose levels were compared by Mann-Whitney or Kruskal-Wallis test. The relationships between mannose and the glycaemic state, Body Mass Index (BMI), waist circumference and smoking habits were assessed by Spearman’s correlation coefficient. The association between log-transformed mannose and MI was investigated across different glycaemic states (NGT, IGT, newly detected DM and known DM) by logistic regression models, adjusting for significant covariates (age, sex, smoking, family history of cardiovascular disease, education, and civil status).
Results: In the total population mannose gradually increased across different glycaemic states (p<0.0001; Figure 1). Patients with a first MI had higher mannose levels than controls (median 74.5 vs 68.8 μmol/L; p<0.0001). The correlation between mannose and insulin resistance, glycated hemoglobin, fasting plasma glucose and two-hour postload glucose was low as it was for BMI, waist girth, and smoking. Mannose levels were significantly associated with MI in participants with NGT (adjusted Odds Ratio (OR) patients vs controls: 2.27 (95% Confidence Interval (CI) 1.34 - 3.83), however, this was not apparent in dysglycaemic participants i.e. with IGT, newly detected or known DM (adjusted OR: 1.50, 95% CI 0.80 - 2.82).
Conclusion: Mannose increased with increasing levels of dysglycaemia. Overall, the concentrations were significantly higher in patients with a first MI than in controls, but independently associated with MI only in NGT patients. These results reinforce previous findings that mannose is related to coronary atherosclerosis and glucose perturbations, adding that it may be a more sensible marker of risk for a first MI than glucose-related expressions for dysglycaemia. Thus, the prognostic value of mannose in patients at high cardiovascular risk deserves further evaluation.
Supported by: The Swedish Heart-Lung Foundation and Stockholm County Council (ALF project)
Disclosure: E. Fortin: None.
801
Liver function markers predict cardiovascular and renal outcomes in the CANVAS Program
E. Ferrannini1, G. Ferrannini2, N. Rosenthal3, M.K. Hansen4;
1National Research Council, Pisa, Italy, 2Karolinska Institutet, Stockholm, Sweden, 3Janssen Research & Development, LLC, Raritan, USA, 4Janssen Research & Development, LLC, Spring House, USA.
Background and aims: Raised liver function tests (LFTs) have been correlated with multiple metabolic abnormalities and variably associated with cardiorenal outcomes. We sought to systematically test the relationship between LFT levels within the accepted range and major cardiorenal outcomes in a large clinical trial in type 2 diabetes (T2D), and the possible impact of placebo-controlled canagliflozin treatment.
Materials and methods: We measured serum ALT, AST, γGT, ALP, and bilirubin concentrations in 10,142 patients, at baseline and repeatedly over follow-up. The relation of LFTs to first hospitalized heart failure (HHF), cardiovascular (CV) and all-cause mortality, and progression of renal impairment was investigated using multivariate proportional-hazards models.
Results: In univariate association, ALT was reciprocally predictive, and ALP was positively predictive, of all adjudicated outcomes; γGT also was directly associated with CV - but not renal - outcomes. In multivariate models including all 5 LFTs and 19 potential clinical confounders, ALT was independently associated with lower, and γGT with higher, CV outcomes risk. Canagliflozin treatment significantly reduced ALT, AST, and γGT over time. In a fully adjusted model including updated LFT levels and treatment, γGT was independently associated with CV and all-cause mortality, ALP with renal dysfunction progression, and canagliflozin treatment with significant reduction in HHF and renal risk.
Conclusion: Higher γGT levels are top LFT markers of risk of HHF and death in patients with T2D and high CV risk, while ALT are protective. Canagliflozin lowers the risk of HHF and renal damage independently of LFTs and potential confounders.
Clinical Trial Registration Number: NCT01032629
Supported by: Janssen Research & Development, LLC.
Disclosure: E. Ferrannini: Grants; Janssen Research and Development.
802
Therapeutic potential of Crispr/Cas-9 gene edited apolipoprotein B-null cells and insulin signalling in lipoprotein metabolism
Q. Su, H. Wade;
Queen's University Belfast, Belfast, UK.
Background and aims: Hyperlipidaemia, an abnormal high level of very low-density lipoprotein (VLDL) in the blood, is one of the most important risk factors for cardiovascular disease (CVD) in type-2 diabetes patients. A key contributing factor underlying hyperlipidemia is the overproduction of hepatic apolipoprotein B (ApoB), a key structural protein of VLDL. Due to the embryonic lethality upon genetic depletion of apoB, generation of an ApoB-null mouse model had failed, which hinders the development of effective research approaches to investigate the pathophysiological function of ApoB. The advancement of cutting-edge gene-editing tool, Crispr/Cas-9 system, has enabled to deplete ApoB gene in hepatocytes. This greatly broadens the potentials to study the pathological function of VLDL-ApoB and the associated atherosclerosis. Therefore, the objective of this study is to genetically disrupt ApoB in hepatocyte using Crispr/Cas-9 gene editing technology and determine the impact of ApoB on hepatic lipoprotein metabolism and the regulation of insulin on atherogensis.
Materials and methods: The cellular ApoB in both human and mouse hepatocytes were disrupted by Crispr/cas-9 gene editing technology. The ApoB-null cells were further subjected to transcriptome analysis by RNA-sequencing and bioinformatics analysis. A number of molecular biological technology, including q-RT-PCR and immunoblotting analysis, was used to determine regulation of insulin signalling on lipoprotein metabolism.
Results: Transcriptome profile of wild type (WT) and ApoB-null hepatocytes by RNA-sequencing revealed that depletion of ApoB significantly suppressed expression of several apolipoproteins that are key components of VLDL particles, including ApoE and ApoC-1 (P<0.001). Analysing genes involved in vascular endothelial inflammation and atherogenesis we further found that mRNA expression of Toll-like receptor (TLR) -2 (TLR-2), TLR-4, and Icam-1 were markedly reduced in ApoB-null cells compared to WT cells (P<0.001). These indicate the essential role of ApoB in lipoprotein metabolism and the reduced risk of atherogenesis upon ApoB-depletion. Induction of insulin resistance in the WT and ApoB-null cells revealed that expression of TLR-2, TLR-4, Icam-1 and ApoC-1 were significantly upregulated in the WT cells but not in the ApoB-null cells, implicating the lower susceptibility of the ApoB-null cells to metabolic inflammation and atherogenesis at the insulin resistant state. We further reconstituted ApoB function in the ApoB-null cells by transfecting an ApoB expressing plasmid for 48 hours and found that restoration of ApoB upregulated expression of TLR-2, TLR-4, Icam-1, ApoE and ApoC-1 in the transfected ApoB-null cells compared to the un-transfected null cells (P<0.05). These data confirmed the anti-atherogenic effect of ApoB-depletion.
Conclusion: This is the first study that disrupts ApoB function in hepatocytes using Crispr/Cas-9 gene editing system and characterizes the pathophysiological function of ApoB and the regulation of insulin signalling in this context. Novel finding from this study provides mechanistic evidence for developing therapeutic strategy to target ApoB in human subjects with diabetes associated dyslipidaemia and atherosclerosis.
Supported by: BHF
Disclosure: Q. Su: None.
803
Leptin correlates with pulse wave velocity independently of BMI, inflammation, home blood pressure and HbA1c in both men and women in a large population-based cohort
C. Vavruch, C. Östgren, J. Engvall, F.H. Nyström, L. Jonasson;
Department of Health, Medicine and Caring Sciences, Faculty of Medicine and Health Sciences, Linköping, Sweden.
Background and aims: New and clinically useful biomarkers of cardiovascular risk are of essence, since ischemic heart disease is a leading cause of death in the population. Pulse wave velocity (PWV) is one of the most powerful clinical physiological markers of cardiovascular disease and risk, but is currently not regularly used clinically for risk assessment in Sweden. We aimed to find out whether leptin can be used as a marker for PWV.
Materials and methods: We analyzed baseline data from 1 580 men and 1 623 women who participated in “The Swedish CArdioPulmonary bioImage study” (SCAPIS), a population-based multicenter study. This study was performed with data from only the Linköping cohort, recruited during year 2016 to 2018, aged 50-65. Prevalent diabetes, hypertension, dyslipidemia, and smoking status, as well as sleep data, were based on self-reported health in combination with laboratory results. Blood pressure was measured at home, once in the morning and once in the afternoon, for seven consecutive days, for a total of 13 times. Carotid-femoral pulse wave velocity was measured by tonometry to determine arterial (aortic) stiffness.
Results: In a bivariate correlation, leptin, IL18, and hsCRP were all correlated with a p = <0.001 in both men and women. Log transformed Leptin correlated positively with PWV, independent of mean systolic blood pressure, IL18, smoking, LDL/HDL cholesterol ratio, BMI, age, and HbA1c (men: p = <0.001, 95% CI 0.093-0.233; women: p = 0.001, CI 0.056-0.223). In a linear regression with hsCRP instead of IL18 as the marker of inflammation, leptin still associated (men: p = < 0.001, 95% CI 0.095-0.232; women: p = 0.002, 95% CI 0.050-0.213).
Conclusion: Our data support the use of leptin as a marker of PWV and arterial stiffness, that is independent of inflammation, BMI, glucose homeostasis as measured by HbA1c, and traditional risk markers. This has not previously been demonstrated in such a large cohort based on randomized recruitment of participants.
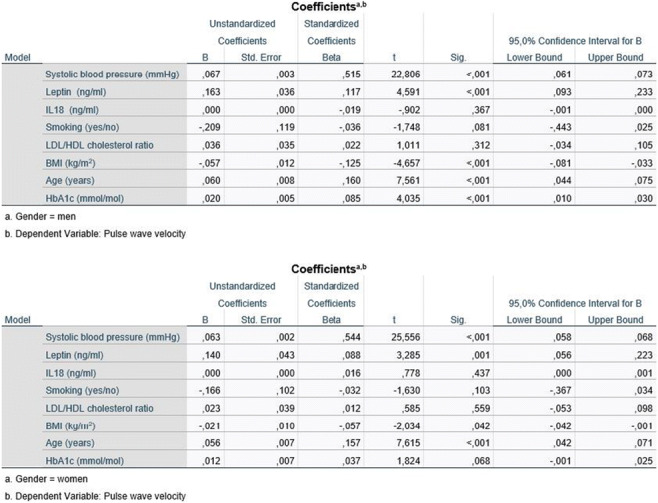
Supported by: The Swedish Heart Lung Foundation
Disclosure: C. Vavruch: None.
804
Low-density lipoprotein-cholesterol levels in migrants and native Danes with type 2 diabetes
A.A. Isaksen1, A. Sandbæk1,2, M.V. Skriver1, G.S. Andersen3, L. Bjerg2;
1Department of Public Health, Aarhus University, Aarhus, 2Steno Diabetes Center Aarhus, Aarhus, 3Steno Diabetes Center Copenhagen, Copenhagen, Denmark.
Background and aims: Elevated low-density lipoprotein-cholesterol (LDL-C) levels is a risk factor for complications and death in type 2 diabetes (T2D), and identification of subgroups with elevated levels is important for appropriate care. We aimed to describe LDL-C levels among migrants with T2D in Denmark, and the risk of exceeding a target level recommended by Danish clinical guidelines.
Materials and methods: In a registry-based nationwide Danish cohort study, we identified individuals with T2D on January 1 2018. Analyses were restricted to individuals with a diabetes duration of at least six months, in order to allow time for clinical measurement of LDL-C, initiation of lipid-lowering treatment and subsequent stabilization of LDL-C levels in newly-diagnosed. The LDL-C level was defined at the most recent measurement, and migrant status was classified based on country of birth and citizenship, and categorized into nine groups. The empirical cumulative distribution (ECD) of LDL-C levels was computed with 95% confidence intervals. The relative risk of having an LDL-C ≥ 2.6 mmol/L was computed in Poisson regression models with robust sandwich variance estimates: model 1 adjusted for sex, age, diabetes duration and prevalent complications (macrovascular complications or diabetic kidney disease), while model 2 additionally adjusted for employment status, equivalised disposable family income, duration of residence and region of residence.
Results: In the origin groups assessed, 254,097 individuals had T2D with a duration of at least six months on January 1 2018 (native Danes: 225,750 (88.8 %), Middle East: 7,865 (3.1 %), Europe: 6,396 (2.5 %), Turkey: 4,472 (1.8 %), Former Yugoslavia: 3,509 (1.4 %), Pakistan: 2,715 (1.1 %), Sri Lanka: 1,653 (0.7 %), Somalia: 957 (0.4 %), Vietnam: 780 (0.3 %)). In this population, 248,813 (97.9 %) had a prior measurement of LDL-C and were included in the analyses. Figure 1 shows the ECD of LDL-C levels, and the RR of exceeding 2.6 mmol/L in each group. In summary, 28.3% of native Danes had an LDL-C ≥ 2.6 mmol/L, and the crude risk of having an LDL-C ≥ 2.6 mmol/L was increased in most migrant groups (RRs from 1.08 [95%CI: 1.03-1.14] in the Former Yugoslavia-group to 1.78 [95%CI: 1.67-1.90] in the Somalia-group). This pattern appeared consistent in the ECD across a range of alternative target values from 2.6 mmol/L to 1.8 mmol/L. In the fully adjusted model, the RR remained elevated in the Middle East, Europe, Pakistan, and Somalia groups (RRs from 1.07 [95%CI: 1.01-1.13] in the Pakistan-group to 1.44 [95%CI: 1.35-1.54] in the Somalia-group).
Conclusion: In the T2D population of Denmark, high levels of LDL-C were common, and most migrant groups had higher levels of LDL-C than native Danes. Migrants from Somalia had particularly high levels of LDL-C, and were almost twice as likely to exceed a guideline target level compared to native Danes.
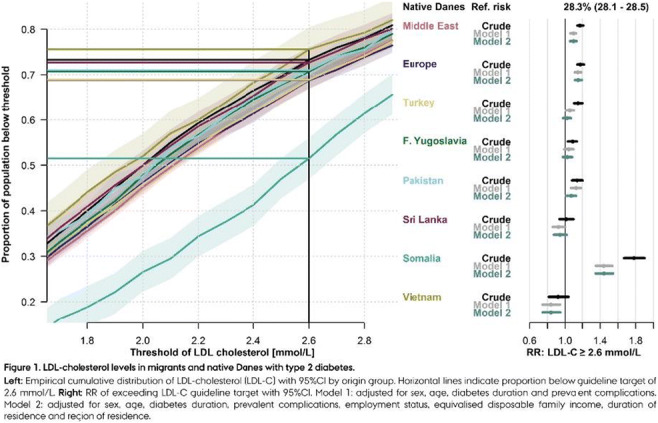
Supported by: Support provided by Steno Diabetes Center Aarhus & the Public Health in Central Denmark Region
Disclosure: A.A. Isaksen: Grants; Support provided by the Steno Diabetes Center Aarhus (SDCA) which is partially funded by an unrestricted donation from the Novo Nordisk Foundation, Support provided by the Public Health in Central Denmark Region-foundation.
805
Serum sphingolipidomic profile and high density lipoprotein subfractions in obese dyslipidaemic type 2 diabetic patients
M. Aslan1, I. Aslan2, D. Aydın3, Y. Koca3, Ç. Yılmaz1, T. Çeker1, A. Öztüzün1;
1Medical Biochemistry, Akdeniz University Faculty of Medicine, 2Endocrinology Clinic, The University of Health Sciences, 3Internal Medicine Clinic, The University of Health Sciences, Antalya, Turkey.
Background and aims: Obese patients with type 2 diabetes mellitus (T2DM) show an altered lipid profile and a certain degree of insulin resistance, which might contribute to changes in both serum sphingolipidomic profile and high-density lipoprotein (HDL) subfractions. We aimed to investigate changes in serum sphingolipid levels and HDL subspecies in relation to low-density lipoprotein cholesterol (LDL-C), non-HDL-C and triglyceride (TG) levels in type 2 diabetic patients.
Materials and methods: Serum was collected from 60 T2DM patients. Levels of C16-C24 sphingomyelins (SMs), C16-C24 ceramides (CERs), sphingosine-1-phosphate (S1P) and C16 CER-1P were determined by an optimized multiple reaction monitoring (MRM) method using ultra fast-liquid chromatography (UFLC) coupled with tandem mass spectrometry (MS/MS). HDL subfraction analysis was done by continuous disk polyacrylamide gel electrophoresis.
Results: C16-C24 SM, C16-C24 CER and C16 CER-1P levels showed a statistically significant increase in T2DM patients with LDL-C above 160 mg/dL compared to those with LDL-C below 100 mg/dL. C16-C24 SM, C22-C24 CER, C16 CER and C16 CER-1P levels showed a statistically significant increase in T2DM patients with non HDL-C above 130 mg/dL compared to those with non HDL-C below 130 mg/dL. C24:C16 SM and C24:C16 CER ratio showed a significant correlation with both LDL-C and non HDL-C levels. Obese T2DM patients (BMI>30) had significantly higher circulating levels of C24 SM, C18-C24 CER compared to those with BMI<30. Type 2 diabetic patients with fasting TG levels above 150 mg/dL showed a significant decrease in HDL-large and a significant increase in HDL-small fractions compared to those with TG levels below 150 mg/dL. Analysis of HDL subfractions independent of TG concentrations showed that HDL large subfractions were significantly higher in female T2DM patients compared to males. Type 2 diabetic patients on steroid therapy had significantly higher levels of HDL large subfractions compared to those who were not treated with steroids.
Conclusion: Plasma sphingomyelins, ceramides and HDL-small fractions are elevated in obese dyslipidemic T2DM patients. Serum long chain CERs, C24:C16 SM, C24:C16 CER ratio and alterations in HDL subfractions may serve as prognostic and diagnostic markers in type 2 diabetic dyslipidemia.
Supported by: Akdeniz University Research Foundation TTU-2021-5605
Disclosure: M. Aslan: None.
SO 71 Prevention and treatment of cardiovascular complications
806
Risk of stroke in patients with type 2 diabetes receiving semaglutide or a dipeptidyl peptidase-4 inhibitor: a real-world US claims database analysis
M. Evans1, M. Husain2, O. Frenkel3, K. Mangla3, A. Srivastava4, I. Lingvay5;
1University Hospital, Llandough, Penarth, Cardiff, UK, 2Ted Rogers Centre for Heart Research, Department of Medicine, University of Toronto, Toronto, Canada, 3Novo Nordisk A/S, Søborg, Denmark, 4Novo Nordisk Global Business Services, Bengaluru, India, 5Department of Internal Medicine and Department of Population and Data Sciences, UT Southwestern Medical Center, Dallas, USA.
Background and aims: People with type 2 diabetes (T2D) have a higher risk of stroke and worse outcomes than those without T2D. A meta-analysis of RCT data has shown that glucagon-like peptide-1 receptor agonists are associated with a significant reduction in the risk of stroke, but there remains a specific evidence gap for the real-world effect of semaglutide. We compared risk of incident stroke in patients with T2D or T2D + atherosclerotic CVD (ASCVD) initiating either semaglutide or a dipeptidyl peptidase-4 inhibitor (DPP-4i).
Materials and methods: Adults (≥18 years) in a US claims database with a claim indicating initiation of semaglutide or a DPP-4i (index date) during the index period (1/1/18-30/9/20), a diagnosis code for T2D on or before index date, and 12 months’ continuous enrolment pre-index were included. Exclusion criteria were a claim for semaglutide, DPP-4i or injectable glucose-lowering medication, or a diagnosis code for type 1 or secondary diabetes in the 12 months pre-index; or a claim associated with pregnancy or gestational diabetes at any time during the study period. Patients were propensity score matched 1:1 on baseline demographic and clinical characteristics (27 variables for T2D; 26 for T2D+ASCVD). Primary outcome was time to first stroke event during follow-up (medical claim with stroke as primary diagnosis during inpatient or emergency room visit).
Results: Post-matching, there were 17,920 pairs with T2D and 4234 pairs with T2D+ASCVD. The groups were well matched on baseline characteristics. Patients with T2D initiating semaglutide had a lower risk of stroke than those initiating a DPP-4i (HR 0.63 [95% CI 0.41, 0.95]; p = 0.029). This was more pronounced for T2D+ASCVD (HR 0.45 [0.24, 0.86]; p = 0.015). Overall, 34 patients with T2D receiving semaglutide (0.2%) experienced a stroke event (incidence rate [IR] per 100 person-years 0.25), compared with 60 patients receiving a DPP-4i (0.3%; IR 0.40; IR ratio [IRR] 0.62 [95% CI 0.40, 0.95]). For T2D+ASCVD, 13 patients receiving semaglutide (0.3%; IR 0.40) and 32 receiving a DPP-4i (0.8%; IR 0.90) experienced a stroke event (IRR 0.44 [0.23-0.85]). The Figure shows cumulative incidence of stroke over the follow-up period (median 237-258 days).
Conclusion: Our results provide preliminary information regarding the potential of semaglutide to reduce stroke in patients with T2D in a real-world setting. Analyses with additional comparison groups and longer follow-up are needed to determine the broader clinical and economic implications.
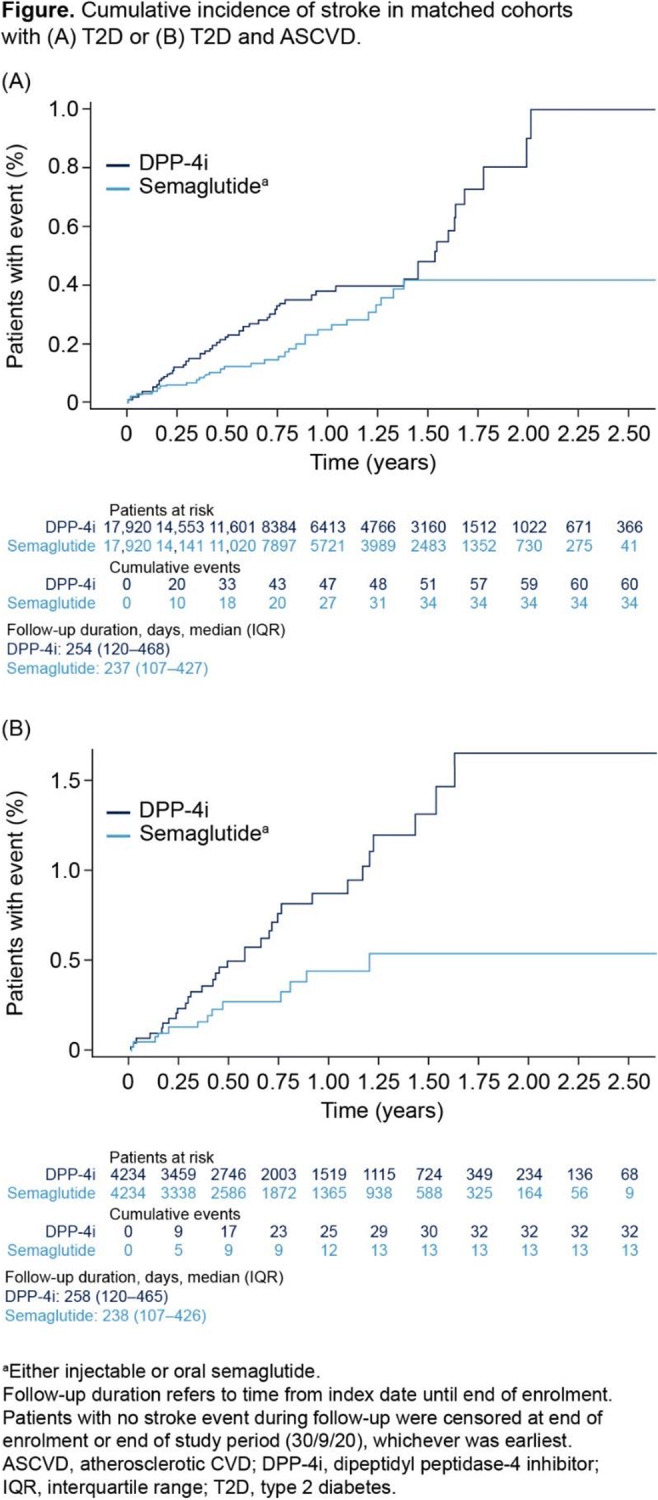
Supported by: Novo Nordisk A/S
Disclosure: M. Evans: Grants; This study was funded by Novo Nordisk A/S. Honorarium; ME has received honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk.
807
Synergistic effects of combination treatment with semaglutide and empagliflozin on 24h and central blood pressure in type 2 diabetes
L. Vernstrøm, S. Gullaksen, K.L. Funck, E. Laugesen, P.L. Poulsen;
Aarhus University Hospital, Aarhus, Denmark.
Background and aims: Glucagon-like peptide-1 receptor agonist (GLP-1ra) and sodium glucose cotransporter-2 inhibitors (SGLT-2i) have each shown cardiovascular and nephroprotective effects. However, the underlying mechanisms are poorly understood, and it is not clear whether combined treatment has synergistic favorable effects on cardiovascular function. In this 32-week randomized, placebo-controlled trial we compared the individual and combined effects of the GLP-1ra semaglutide and the SGLT-2i empagliflozin on measures of cardiovascular function.
Materials and methods: 120 participants with type 2 diabetes were randomized into one of four arms: placebo (n=30), empagliflozin (n=30), semaglutide + placebo (n=30) or the combination of semaglutide + empagliflozin (n=30). Empagliflozin treatment was double blinded. Outcomes comprised: arterial stiffness (carotid-femoral pulse wave velocity [cf-PWV], including 24h PWV measurements), 24h-blood pressure (BP), central BP, and urinary albumin:creatinine ratio (UACR). Outcomes were measured at baseline and after 32 weeks.
Results: Cf-PWV did not change in any of the groups (placebo -0.2 ± 0.22 m/s, p=0.36, empagliflozin 0.07 ± 0.21 m/s, p=0.74, semaglutide 0.4 ± 0.21 m/s, p=0.07 and combination -0.07 ± 0.21 m/s, p=0.75, with no significant intergroup differences). During 24h measurements daytime PWV was reduced in the combination group (-0.23 ± 0.07 m/s, p=0.002, p<0.05 compared to placebo, empagliflozin and semaglutide).At week 32, 24h-systolic blood pressure was reduced with 10 ± 2 mmHg (p<0.001) in the combination group which was significantly different compared to both placebo and either treatment alone (p< 0.05), Figure 1A. Similar changes were seen in central systolic BP (SBP) (-10 ± 2 mmHg, p<0.001 in the combination group, p<0.05 compared to placebo, empagliflozin and semaglutide). Combination treatment resulted in significant reductions in HbA1c and BMI (data not shown), but controlling for these changes did not affect the BP reductions substantially. UACR decreased in all three treatment groups, Figure 1B. The reduction in the combination arm from baseline was -40% [-55;-11], p<0.001 which was significantly higher than the reduction in the placebo arm (difference between placebo and combination; 56 % [4;133], p=0.03).
Conclusion: To our knowledge, this is the first demonstration of combination treatment with semaglutide and empagliflozin leading to substantial and clinically important synergistic reductions in 24h and central blood pressure. Furthermore, UACR was significantly reduced by the combination treatment. We found no significant effect of treatment with empagliflozin, semaglutide or their combination on arterial stiffness, except for a small reduction in daytime PWV in the combination group (probably driven by the reduction in BP).
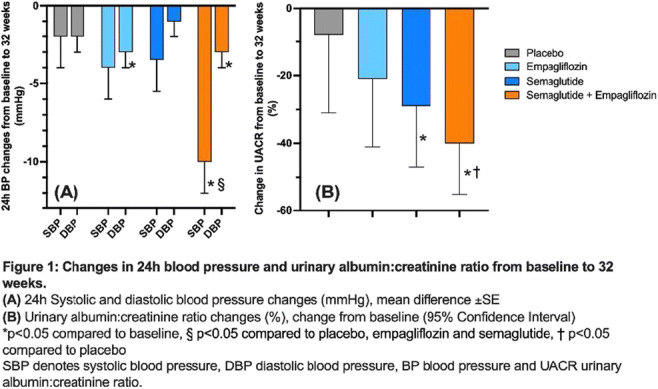
Clinical Trial Registration Number: EudraCT 2019-000781-38
Supported by: NNF, Health Research Foundation of Central Denmark Region, DMA research foundation
Disclosure: L. Vernstrøm: None.
808
Lesser improvement of physical activity after a cadiac rehabilitation program in patients at risk of diabetes or increased artery stiffness
P. Valensi1, M. Nguyen1, M. Duval2, M. Sylva2, G. Amah2, N. Chaib2, S. Gagey2, C. Guiti2, K. Abdennbi2;
1Unit of Endocrinology-Diabetology-Nutrition, Jean Verdier hospital, Paris-Nord University, CRNH-IdF, CINFO, Bondy, 2Centre de Réadaptation cardiovasculaire, hôpital Léopold Bellan, Paris, France.
Background and aims: Artery stiffness, an early marker of atherosclerosis, was reported to be increased in individuals with diabetes, prediabetes or with low physical activity. We hypothesized that prediabetes and higher artery stiffness might be limiting factors for the improvement of aerobic performance after a one-month cardiac rehabilitation program.
Materials and methods: We included 838 patients (men 79%), most of them with coronary artery disease (mostly after an acute coronary syndrome). In the 463 patients without known diabetes, Findrisk score was calculated and an oral glucose tolerance test (OGTT) was performed at admission in the program; artery stiffness was analyzed by measuring carotid-to-femoral pulse wave velocity (PWV) and central blood pressure (Complior®), and a stress test with VO2max measurement was performed at admission and at the end of the program.
Results: Based on OGTT results, 41% of the 463 patients were diagnosed with diabetes or prediabetes. Findrisk score correlated strongly (p<0.0001) with plasma glucose at fasting and 2 hours after glucose load but did not correlate with PWV. VO2max increased significantly after the rehabilitation program (p<0.001) while PWV decreased slightly (p=0.06). VO2max before and after the program correlated negatively with Findrisk score (p<0.0001), more strongly than with glucose levels. Before the program, VO2max correlated negatively with PWV and brachial and central pulsed pressure (p<0.0001). Multivariate analyses showed that before the program lower VO2max was associated with both higher Findrisk score and PWV, independently from age and gender. After the program VO2max and its change (difference after-before) correlated negatively with PWV after the program (p<0.0001 and p=0.003).
Conclusion: In this cohort of patients included in a cardiac rehabilitation program the data show the high prevalence of previously unknown glycemic disorders, and the role of OGTT for their detection. The risk of diabetes (Findrisk) and artery stiffness were associated with a lower physical capacity and might act as limiting factors for the improvement of aerobic performance after rehabilitation.
Disclosure: P. Valensi: None.
809
Calcitriol treatment does not improve left ventricular hypertrophy in patients with type 2 diabetes and stage 3 CKD: results of a randomised controlled trial
L. Gnudi1, N. Fountoulakis1, A. Panagiotou1, A. Corcillo1, G. Maltese1, M. Flaquer1, R. Franks2, A. Chiribiri2, S. Ayis3, J. Karalliedde1;
1School of Cardiovascular and Metabolic Medicine & Science, King's College London, 2School of Biomedical Engineering & Imaging Sciences, King's College London, 3School of Population Health & Environmental Sciences, King's College London, London, UK.
Background and aims: Active vitamin D deficiency is a potential modifiable risk factor for cardiovascular disease (CVD) in chronic kidney disease (CKD). Left ventricular hypertrophy (LVH) independently predicts CVD in patients with CKD and diabetes. There are no randomised controlled trial data on the effects of active vitamin D (calcitriol) treatment on LVH in patients with type 2 diabetes (T2D) and CKD.
Materials and methods: We performed a 48-week duration single center randomised double blind parallel group trial examining the impact of active vitamin D (calcitriol 0.5 mcg once daily) as compared to placebo on a primary endpoint of change from baseline in left ventricular mass index (LVMI) measured by sequential magnetic resonance imaging (MRI). Patients with T2D, LVH and stage 3 CKD, on stable renin angiotensin aldosterone system blockade, with raised intact parathyroid hormone (iPTH) level between 30-300 pg/ml were eligible. Secondary endpoints included urine albumin/creatinine ratio, serum calcium, phosphate levels, iPTH, eGFR, and interstitial myocardial fibrosis (using MRI with gadolinium).
Results: In total, 45 (male 73%) patients with T2D and stage 3 CKD were randomised (calcitriol n=19 or placebo n=26). Baseline, characteristics, mean (standard deviation) or median [interquartile range], respectively in the placebo and calcitriol group were: for age (years) 64.3 (9.76) and 70.3 (8.3), for eGFR (ml/min/1.73 m2) 42.3 (7.75) and 45.1 (9.3), for iPTH (pg/ml) 157 [65-227] and 142 [80-293], for high sensitivity (hs) C-reactive protein (CRP) (mg/L) 6 [1.6-11.4] and 2 [0.7-6.4], and for LVMI (gr/m2) 50.11 (14.74) and 47.28 (12.1). Following 48-week treatment with calcitriol, iPTH was significantly reduced only in the calcitriol group from 142 [80-293] to 76 [41-204] (p=0.04). Changes in LVMI using an analysis of covariance (ANCOVA) with baseline LVMI as covariate was not significantly different between the placebo and calcitriol arms. No significant differences were observed for hsCRP, urine albumin creatinine ratio, interstitial myocardial fibrosis and eGFR between groups. Calcitriol was well tolerated and of the patients enrolled in the calcitriol group, 3 patients (15%) had the calcitriol dose reduced to 0.5 mcg on alternate days as per protocol due to mild hypercalcemia (defined as calcium >2.6 but <2.8 mmol/L).
Conclusion: In patients with T2D, LVH and stage 3 CKD, 48-week treatment with calcitriol as compared to placebo does not improve LVMI. Our data does not support the routine use of active vitamin D for cardiovascular protection in patients with T2D and stage 3 CKD.
Clinical Trial Registration Number: EudraCT Number 2011-003025-10
Supported by: EFSD Clinical Research Grants
Disclosure: L. Gnudi: None.
810
Four months treatment with dapagliflozin or dulaglutide improves cardiovascular function in patients with type 2 diabetes and ischaemic stroke
A. Kountouri1, I. Ikonomidis2, V. Prentza1, K. Katogiannis2, G. Pavlidis2, E. Korakas1, L. Pliouta1, G. Kostelli2, E. Michalopoulou2, J. Thymis2, K. Balampanis1, A. Raptis1, G. Tsivgoulis3, V. Lambadiari1;
1Second Department of Internal Medicine, National and Kapodistrian University of Athens, Attikon hospital, 2Second Cardiology Department, National and Kapodistrian University of Athens, Attikon hospital, 3Second Department of Neurology, National and Kapodistrian University of Athens, Attikon hospital, Athens, Greece.
Background and aims: Patients with type 2 diabetes mellitus (T2DM) and ischemic stroke present impaired markers of vascular and endothelial function. Glucagon-like peptide-1 receptor agonists (GLP-1) and sodium-glucose contrasporter-2 inhibitors (SGLT-2) are novel antidiabetic agents reducing the risk of cardiovascular complications. The aim of the study is to investigate the effect of treatment with GLP-1 or SGLT2 on arterial stiffness, on cardiac perfoemance and on endothelial glycocalyx in patients with T2DM and ischemic stroke.
Materials and methods: We recruited in total 81 patients with T2DM and ischemic stroke who received dulaglutide (n=27), dapagliflozin (n=27) or insulin (n=27). We measured at baseline and at four months post-treatment the: a) Carotid-femoral pulse wave velocity (PWV-Complior; ALAM Medical) b) Augmentation index (Aix) c) Central systolic blood pressure (cSBP) and d) Perfused boundary region (PBR) of the sublingual arterial microvessels (marker of endothelial glycocalyx thickness) e) Left ventricular global longitudinal strain (GLS) using speckle-tracking echocardiography.
Results: At baseline, patients among the three groups had similar age, sex, HbA1c and markers of endothelial and vascular function (p>0.05). After four months treatment, patients on dapagliflozin and on dulaglutide displayed a greater reduction of PWV (12.98±3.23 vs.11.62±1.74m/s, p=0.017,14.77±1.97 vs. 13.59±2.20m/s, p=0.042 respectively), cSBP (132.15±14.03 vs 120.27±10.05mmHg, p=0.035,139±7.25 vs.129±7.75mmHg, p=0.045 respectively), Aix (17.10±17.83 vs 5.97±28.99, p=0.028, 8.59±20 vs 7.46±6.36, p=0.039 respectively) και GLS (-16.87±3.28 vs -18.76±3.35, p=0.001, -16.31±3.42 vs -17.48±3.14, p=0.004 respectively) compared to patients on insulin, despite a similar glycosylated hemoglobin reduction (P<0.05). Patients on dapagliflozin displayed a greater reduction of PWV (-10%), Aix (-65%), cSBP (-9%) and GLS (+11%) compared to patients on dulaglutide or on insulin (PWV, -8% and -1%; Aix, -13% and -3%; cSBP, -8% and +1%, GLS, +7%, and +5%), (P<0.05 for all comparisons). PBR values were improved only in patients who received dulaglutide (2.10±0.16 vs 2.00±0.14, p=0.025 vs dapagliflozin:2.04±0.23 vs 2.00±0.11, p=0.696 vs insulin:2.13±0.3 vs 2.15±0.3, p=0.567).
Conclusion: Four-month treatment with dulaglutide or dapagliflozin improves arterial stiffness and cardiac performance, but only dulaglutide improves endothelial glycocalyx in patients with T2DM and ischemic stroke. Patients treated with dapagliflozin had greater reduction of PWV and central SBP than patients under insulin or dulaglutide.
Disclosure: A. Kountouri: None.
811
Using CVD risk scores to select between GLP-1RA or SGLT2i therapy in type 2 diabetes: modelling the combined effects on both major atherosclerotic and heart failure events
J.W. Sacre1, D.J. Magliano1,2, J.E. Shaw1,2;
1Baker Heart and Diabetes Institute, 2Monash University, Melbourne, Australia.
Background and aims: Current guidelines advise differentiation of major atherosclerotic CVD event (MACE) risk from heart failure hospitalisation (HHF) risk when selecting glucose-lowering therapy. This study investigated: 1) whether this is possible using separate MACE and HHF risk scores; and 2) whether these scores identify groups more likely to benefit from glucagon-like peptide-1 receptor agonist (GLP-1RA) or sodium-glucose cotransporter-2 inhibitor (SGLT2i) treatment.
Materials and methods: Incidence rates of MACE (i.e. CVD death, myocardial infarction, or stroke) and HHF, stratified by the Thrombolysis In Myocardial Infarction (TIMI) Risk Score for MACE (TRS-2°P; range: 1-10 points) or TIMI Risk Score for HHF (TRS-HFDM; range: 0-7 points) were extracted from CVD outcomes trials (DECLARE-TIMI and EMPA-REG OUTCOME; SAVOR-TIMI also provided TRS-HFDM-stratified rates). Placebo-arm event rates were pooled (aggregate data meta-analysis) and compared across MACE and HHF risk groups. Published relative risk reductions with GLP-1RA (HR=0.86 for MACE; 0.89 for HHF) and SGLT2i therapy (HR=0.90 for MACE; 0.68 for HHF) were applied to our pooled event rates to estimate overall (MACE+HHF combined) reductions in CVD events within MACE and HHF risk groups. 95% uncertainty intervals were generated via Monte Carlo simulations (to account for treatment effect and event rate variability).
Results: Rates of HHF increased disproportionately more than MACE with progressively higher CVD risks according to both scores; i.e. HHF was uncommon relative to MACE at low-intermediate HHF risk (18% of the rate of MACE), as well as at low MACE risk (19%). However, incidence of HHF rose to 61% of MACE incidence at highest HHF risk, and 51% of MACE incidence at highest MACE risk. Estimated treatment-mediated reductions in CVD event rates according to MACE risk (panel a) and HHF risk (panel b) are displayed in the Figure (relative proportions of MACE and HHF events prevented are also depicted within each bar). Estimated GLP-1RA- and SGLT2i-mediated reductions in CVD events were similar at low-intermediate MACE or HHF risk, but tended to favour SGLT2is at higher risk levels of both scores.
Conclusion: HHF is relatively uncommon in low-intermediate CVD risk settings, so GLP-1RA and SGLT2i treatment can be expected to offer similar CV protection. However, the greater rise in HHF relative to MACE with progressively higher CVD risk - irrespective of the risk score applied - increasingly favoured SGLT2i treatment. Thus, SGLT2is may offer greater CVD protection in those at highest CVD risk, not just those at highest risk of HHF alone.
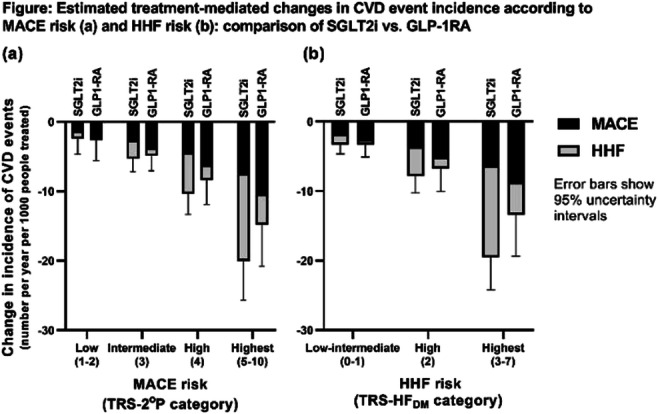
Supported by: the NHMRC and Victorian Government.
Disclosure: J.W. Sacre: None.
812
Adherence to antihypertensive and lipid-lowering treatment is associated with better CVD outcomes in individuals with type 1 diabetes and moderate albuminuria
R. Lithovius1,2, S. Mutter1,2, E.B. Parente1,2, V. Harjutsalo1,2, P.-H. Groop1,2;
1Folkhälsan Institute of Genetics, 2Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Individuals with type 1 diabetes are at high lifetime risk of CVD. To gain full benefit of the pharmacological therapies for CVD protection, it is important to ascertain adherence to all prescribed medications. Non-adherence may negatively affect the incidence rates of CVD. While previous studies have estimated adherence to single-drug classes only, data on adherence to concomitant antihypertensive (AHT) and lipid-lowering drugs are limited. Therefore, it is crucial to investigate the adherence to polypharmacy and its impact on CVD outcomes in adults with type 1 diabetes.
Materials and methods: This Finnish Diabetic Nephropathy Study followed 2,708 individuals with type 1 diabetes (mean age 39.0 ± 11.4 years, mean duration of diabetes 22.7 ± 11.7, 54% men), who had purchased AHT or lipid-lowering drugs (based on the Drug Prescription Register) until the first CVD event, death, or the end of 2015 (465 CVD events, median follow-up 14.8 years). Refill adherence was defined as an overall refill adherence to AHT drug classes and lipid-modifying agents, based on the proportion of days covered (PDC) method. The adherence rate was classified into good (> 80%), intermediate (50 - 80%) and poor (< 50%) adherence. Multivariable logistic regression analyses, adjusted for age, sex, duration of diabetes, systolic BP, triglycerides, LDL-cholesterol, HbA1c, waist-to-height ratio, smoking, albuminuria stage and eGFR were performed. Further analyses were done based on urinary AER at baseline: normal AER (AER <20 μg/min or <30 mg/24h; n = 1654), moderate (AER 20 - 200 μg/min or 30 - 300 mg/24h; n = 504) or severe albuminuria (AER >200 μg/min or >300 mg/24h; n = 548).
Results: Overall, the median adherence rate for polypharmacy was 75% (IQR 61%, 85%), and 37% of the individuals had good, 54% intermediate and 9% a poor adherence rate. In the whole cohort, the good (OR 0.56 [95% CI 0.37, 0.84], p = 0.006) and intermediate (0.55 [0.38, 0.81], p = 0.003) adherence rates were associated with lower occurrence of CVD events compared to the poor adherence rate. Similarly, in individuals with moderate albuminuria, both good (0.22 [0.09, 0.51], p = 0.0005) and intermediate (0.31 [0.14, 0.67], p = 0.003) adherence rates were associated with lower occurrence of CVD events than poor adherence rate. However, no differences between the good and poor or between the intermediate and poor adherence rates were observed in those with normal AER or severe albuminuria.
Conclusion: In adults with type 1 diabetes, a refill adherence rate above 50% to prescribed AHT and lipid-lowering treatments is associated with reduced odds of CVD events. These findings are especially important for individuals with moderate albuminuria. This highlights the relevance of ensuring overall adherence to medications in clinical practice, and especially in those individuals at the early stage of kidney disease, who already carry an increased CVD risk.
Supported by: Folkhälsan Research Foundation
Disclosure: R. Lithovius: None.
813
Metformin therapy is associated with higher myocardial perfusion reserve and improved survival in patients with type 2 diabetes: a multicentre study
N. Sharrack1, K.D. Knott2, J.L. Yeo3, T. Kotecha4, S. Thirunavukarasu1, A. Chowdhary1, E. Levelt1, J. Moon2, G.P. McCann3, M. Fontana4, P. Kellman5, C.P. Gale6, J.P. Greenwood1, P.P. Swoboda1, S. Plein1;
1Leeds Institute of Cardiovascular and Metabolic Medicine, Leeds, UK, 2Barts Heart Centre, London, UK, 3Department of Cardiovascular Sciences, University of Leicester, Leicester, UK, 4Royal Free Hospital, London, UK, 5National Heart, Lung, and Blood Institute, Bethseda, USA, 6Division of epidemiology and biostatistics, Leeds, UK.
Background and aims: Metformin is a potent antihyperglycemic agent widely used in the management of type 2 diabetes mellitus (T2DM). Patients with T2DM are at increased risk of cardiovascular disease, including epicardial coronary heart disease, silent myocardial infarction (MI), and coronary microvascular dysfunction (CMD), all of which can be quantified using cardiac magnetic resonance (CMR). We sought to explore the association between metformin use, myocardial perfusion reserve and clinical outcomes in patients with T2DM.
Materials and methods: This was a multicentre, prospective longitudinal cohort study in patients with T2DM. Subjects underwent baseline multiparametric CMR including adenosine stress perfusion imaging with automated inline quantitative myocardial perfusion assessment deriving global myocardial blood flow (MBF) and myocardial perfusion reserve (MPR). Diagnosis of T2DM was based on Hba1c >48mmol/l or a known diagnosis of T2DM. Patients were followed up for death and major adverse cardiovascular events (MACE), including MI, non-fatal stroke, heart failure hospitalisations and death. Kaplan-Meier curves and log rank testing sought associations between baseline metformin use and death and MACE.
Results: A total of 630 patients with T2DM were included (n=412 [67%] on metformin) with a median follow-up of 650 days (interquartile range 422,923) days. There were 27 (4.3%) deaths and 76 MACE events in 62 (12.1%) patients. Baseline characteristics and CMR data, stratified by metformin use, are presented in table 1. Subjects on metformin had a higher prevalence of ischaemic heart disease, higher HbA1c and LVEF. Mean MPR was significantly greater in those on metformin, but stress MBF did not differ between groups. A higher number of deaths occurred in patients not taking metformin, 16/218 (7%), compared to 9/412 (2%) deaths in patients taking metformin. A log rank test showed statistical significance in all-cause mortality between the groups, χ2 (2) =8.74, P=0.003. There was no significant difference in MACE events between patients not taking metformin, 22/218 (10%) and patients on metformin, 36/412 (9%).
Conclusion: In patients with T2DM, metformin use is associated with increased MPR measured automatically using inline artificial intelligence perfusion mapping, as well as statistical improvement in all-cause mortality. We show for the first time that metformin use associates with higher coronary microvascular function and overall survival.
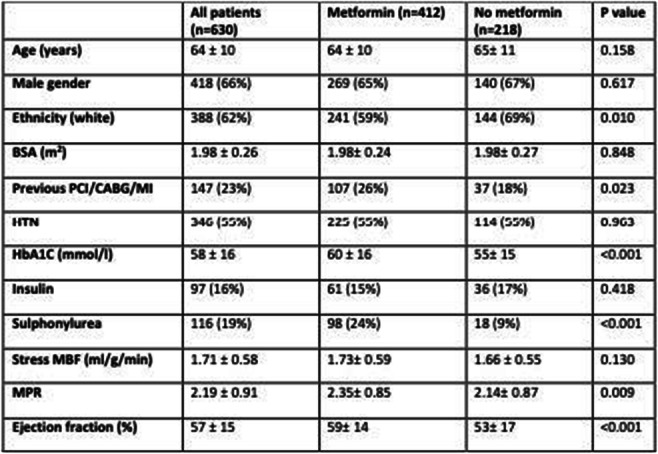
Supported by: REC ID 14/EE/0007, 17/WM/0192, 18/YH/0168, 18/YH/0190, 17/YH/0300
Disclosure: N. Sharrack: None.
SO 72 Diabetes dysmetabolism dialogues with the cardiovascular component
814
Oleate prevents palmitate-induced abnormalities in insulin signalling in human cardiac progenitor cells by inhibiting p38 MAPK and c-Jun phosphorylation
L. Laviola1, R. D'Oria1, I. Calderoni1, C. Caccioppoli1, V.A. Genchi1, G. Santarpino2, A.D. Milano1, A. Leonardini1, A. Natalicchio1, S. Perrini1, A. Cignarelli1, F. Giorgino1;
1University of Bari Aldo Moro, 2General Surgery GVM Bari, Bari, Italy.
Background and aims: Elevated saturated fatty acid deposition in the heart results in insulin resistance, stress kinase activation, and increased cardiovascular risk in humans. The viability of human cardiac progenitor cells (CPC) is essential for myocardium homeostasis. This study investigates the ability of palmitate, a saturated fatty acid, to impair insulin signaling in human CPC, and the potential protective effects of oleate, a mono-unsaturated fatty acid, on palmitate-induced abnormalities.
Materials and methods: Human CPC were obtained from non-diabetic and non-obese subjects undergoing cardiac surgery for coronary artery bypass grafting and/or valve surgery. Human CPC were exposed to 0.25 mM palmitate and/or 0.1 mM oleate for 24 h, and then exposed to 100 nM insulin for the last 15 minutes. Expression of insulin receptor (IR) isoforms, A (IR-A) and B (IR-B), was evaluated by quantitative real-time PCR. IR and Akt protein levels, as well as Akt (S473), p38 MAPK (T180/Y182), and c-Jun (S63) phosphorylation levels were assessed by immunoblotting. p38 MAPK and JNK inhibition was achieved using 15 μM SB202190 and 20 μM SP600125 for 1 h, respectively.
Results: Human CPC were found to express both IR-A and IR-B, with higher IR-A:IR-B ratio. Exposure of human CPC to insulin induced Akt (S473) phosphorylation (p<0.05). Treatment with palmitate, but not with oleate, resulted in impaired insulin-induced Akt phosphorylation (p<0.05), with no effect on total Akt protein levels, and in downregulation of IR protein levels (p<0.05), increased expression of total IR mRNA, IR-A, IR-B, and increased IR-A/IR-B ratio (p<0.05). Palmitate, but not oleate, also induced p38 MAPK (T180/Y182) and c-Jun (S63) phosphorylation (p<0.05). Pretreatment with SB202190 or with SP600125 significantly inhibited the ability of palmitate to impair insulin-induced Akt phosphorylation (p<0.05), but not downregulation of IR protein levels. Interestingly, co-incubation of palmitate with oleate abolished the palmitate-induced p38 MAPK (p<0.05) and c-Jun phosphorylation (p<0.05), and inhibition of insulin-induced Akt phosphorylation (p<0.05). Co-incubation with oleate also prevented the palmitate-induced changes in IR (p<0.05).
Conclusion: Oleate prevents the palmitate-induced abnormalities in insulin signaling in human CPC, largely by counteracting p38 MAPK and JNK activation. Hence, oleate supplementation might limit lipotoxicity in cardiac progenitor cells, thus contributing to cardiac protection.
Disclosure: L. Laviola: None.
815
The effect of methylglyoxal on the pro-angiogenic function of mouse adipose-derived stem cells (mADSCs)
A. Leone, A. Nicolò, I. Prevenzano, D. Conza, F. Fiory, P. Mirra, L. Ulianich, F. Beguinot, C. Miele, C. Nigro;
URT-GDD, National Council of Research & Department of Translational Medicine - Federico II University of Naples, Naples, Italy.
Background and aims: Over the past years, great attention has been paid to the potential role of adipose-derived stem cells (ADSCs) in regenerative medicine. Several studies have demonstrated their crucial role in the repair of damaged tissues, like ischemic wound in diabetic patients. ADSCs play a pivotal role in angiogenesis, through the activation and recruitment of endogenous cells involved in this process and the stabilization of new formed vessels. However, in pathological conditions, including diabetes, ADSCs function is compromised. This represents the main limit for their use in regenerative medicine. Methylglyoxal (MG), a highly reactive dicarbonyl formed as byproduct of glycolysis in chronic hyperglycemia, has harmful effects on vascular function. MG causes cellular dysfunction through several molecular mechanisms including the activation of senescence pathways. This work aims at evaluating MG effect on ADSCs pro-angiogenic function and the molecular mediators involved.
Materials and methods: ADSCs were isolated from the subcutaneous adipose tissue of C57bl6 mice. The presence of CD29, CD44, CD45 and CD31 markers was evaluated by FACS. Adipogenic differentiation was evaluated through the analysis of PPARγ2, GLUT4, AP2 and ADIPOQ mRNA levels by qRT-PCR, and the lipid staining with Oil Red-O. mADSCs viability was evaluated by MTT assay, while the accumulation of MG-adducts and protein levels of p-p38 by western blot. Migration ability was analyzed by transwell assays. p53 and p21 mRNA levels were evaluated by qRT-PCR.
Results: mADSCs were characterized by analyzing the presence of surface markers and their differentiation ability. mADSCs show a 98% and 96% positivity for CD29 and CD44, respectively, while positivity for CD31 and CD45 is less than 1%. mADSCs are able to differentiate into adipocytes as indicated by a 16, 148, 609 and 619-fold increase of PPARγ2 (p=0.03), GLUT4 (p=0.004), AP2 (p<0.001) and ADIPOQ (p<0.001) genes expression, respectively, and by a 5-fold increase of Oil Red-O staining (p=0.001). Treatment with MG 100μM for 16h does not impair mADSCs viability and induces a 50% increase of MG-adducts in mADSCs (p=0.006). mADSCs in co-culture with human retinal endothelial cells (hRECs) induce hRECs migration, which is reduced by 40% (p=0.05) in the presence of MG-treated mADSCs. Conditioned media (CM) from mADSCs induces hREC migration, which is reduced by 30% in response to CM from MG-treated mADSCs (p<0.001). Impaired migration in response to MG treatment associates with reduced p38 activation in hRECs. These data have been also validated in mouse coronary artery endothelial cells. Furthermore, preliminary results indicate that p53 and p21 mRNA levels are increased by 1.7-fold in MG-treated mADSCs compared to control cells.
Conclusion: These results show that MG impairs the pro-angiogenic ability of mADSCs at least in part through the alteration of soluble factors release. This effect associates with the increase of senescence markers in MG-treated mADSCs. The identification of crucial mediators will be useful for optimizing therapeutic strategies involving the use of autologous ADSCs for treatment of diabetic vascular defects.
Disclosure: A. Leone: None.
816
High glucose effects on PCSK9 expression in aortic VSMC: role of PCSK9 inhibitors
C. Barale, E. Melchionda, I. Russo;
University of Turin, Torino, Italy.
Background and aims: Proprotein convertase subtilisin/kexin type 9 (PCSK9) emerged as a valuable pharmacological target in the prevention of the most severe forms of atherosclerotic cardiovascular disease. An increase of the PCSK9 action in vascular smooth muscle cells (VSMC) could play a role in the vascular damage induced by the glycaemic excursions occurring in diabetic patients. Actually, lines of evidence show deleterious effects of PCSK9 overexpression are attributable not only to its influence on cholesterol metabolism but also to its pleiotropic effects. Aim of this study was to evaluate, whether in VSMC: (i) high glucose affects PCSK9 expression; (ii) high glucose influences the LPS-induced increase of PCSK9; (iii) the inhibition of PCSK9 action can interfere with PCSK9 expression; (iv) the high-glucose effects on PCSK9 expression involve the activation of phosphatidylinositol 3-kinase (PI3-K) and mitogen-activated protein kinase (MAPK) pathways.
Materials and methods: In cultured rat aortic VSMC incubated for 30 hours with 25 mmol/L D-Glucose (HG) we measured: (i) the HG effects on PCSK9 expression (by densitometric analysis after Western Blot ) in the absence or in the presence of LPS (50 ng/mL); (ii) the effects on the HG-induced PCSK9 expression exerted by the coincubation with PCSK9 inhibitors, specifically represented by the anti-PCSK9 monoclonal antibodies Alirocumab or Evolocumab (40 mug/mL), whose action is to bind PCSK9 outside the cells- or the synthetic PCSK9-binding peptide PEP 2-8 (10 mumol/L)), whose action may be both within and outside the cells; (iii) the effects of PCSK9 inhibitors on the HG-induced increase of the phosphorylation levels of AKT (pAKT) and ERK-1/2 (pERK-1/2).
Results: In aortic VSMC, HG induced per se an increase of PCSK9 expression (n=6, p<0.0001), and did not significantly modify the LPS-induced PCSK9 expression (n=6, p=ns). The HG-induced increase of PCSK9 expression was reduced by Alirocumab (n=6, p<0.001), Evolocumab (n=6, p<0.005), and PEP 2-8 (n=6, p<0.01). The HG-induced increase of pAKT and pERK-1/2 levels were significantly attenuated by Alirocumab (n=5, p<0.002 and p<0.005 respectively), Evolocumab (n=5, p<0.001 and p<0.0001, respectively), and PEP 2-8 (n=5, p<0.03 and p<0.001, respectively). Similar results were also found for LPS.
Conclusion: In aortic VSMC, HG induces a huge increase of PCSK9 expression and does not modify the increased PCSK9 expression induced by LPS, thus excluding additive effects. Both HG and LPS effects on PCSK9 expression are reduced by monoclonal antibodies or PEP 2-8, indicating that PCSK9 can sustain its expression and secretion in an autocrine manner. Both PI3K and MAPK are involved in this process. Collectively, these findings suggest a new mechanism by which HG may impair vascular function and a positive effect exerted by Alirocumab and Evolocumab by opposing to the autocrine loop of PCSK9.
Supported by: RUSI_RILO_2021
Disclosure: C. Barale: None.
817
Cell-specific role of insulin in a model of restenosis under insulin sensitive and insulin resistant conditions
M. Gonzalez Medina, Z. Liu, A. Giacca;
Physiology, University of Toronto, Toronto, Canada.
Background and aims: Angioplasty and stenting are widely used in the treatment of atherosclerosis. However, these procedures may fail due to post-angioplasty restenosis, which remains a problem for patients with diabetes. Currently, the vascular role of insulin is unclear. Its role on endothelial cells (EC) is vasculoprotective, whereas its effects on vascular smooth muscle cells (SMC) are mainly mitogenic according to in vitro data. We have previously shown a suppressive effect of insulin treatment on neointimal growth in rodent models of restenosis in insulin sensitive conditions that was abolished in insulin resistant conditions. Thus, the objective of this study was to determine the EC-and SMC-specific effects of insulin treatment on neointimal growth in a murine model of restenosis in insulin sensitive and insulin resistant conditions.
Materials and methods: Mice were generated by crossing insulin receptor (IR) floxed mice with mice expressing tamoxifen inducible Cre recombinase under the control of Cdh5 (vascular endothelial cadherin) or SMMHC (smooth muscle heavy chain) promoters. Mice were fed with a low-fat (LFD) or a high-fat-high-sucrose (HFSD) diet staring at four weeks of age and were treated with tamoxifen (50 mg/kg of body weight) at five weeks for ten consecutive days. Mice were then implanted with insulin pellet (0.05 U/day to achieve a 3-fold elevation of plasma insulin) or vehicle (control) three days prior to femoral artery wire injury, which was performed at eight weeks. Following surgery, metabolic parameters were measured until sacrifice. Insulin tolerance test (ITT, 0.5U/kg insulin i.p.) was performed five days prior to sacrifice to examine the effects of diet, insulin treatment and the potential systemic effects of EC- or SMC-specific IR knockdown. Mice were sacrificed 28 days post-surgery for vessel collection following fixation perfusion, and the primary endpoint was neointimal growth.
Results: Compared to controls, tamoxifen-induced IRf/f-Cdh5-Cre+ or IRf/f-SMMHC-Cre+ mice showed a 94% and 80% decrease in IR expression in ECs or SMCs, respectively. Body weight, fasting and fed plasma glucose levels were significantly higher in HFSD-fed mice regardless of genotype or insulin treatment. Additionally, ITT revealed insulin resistance in HFSD fed mice regardless of genotype or insulin treatment. In LFD-fed insulin sensitive conditions, insulin decreased neointimal area (NA) and intima-to-media ratio (I/M) in controls but not in endothelial and SMC IR deficient mice. In HFSD-fed insulin resistant conditions, insulin had no effect in either controls or endothelial and SMC IR deficient mice.
Conclusion: These data demonstrate that insulin action in both ECs and SMCs is required for the anti-restenotic effect of insulin in insulin sensitive conditions. This vasculoprotective effect is abolished but not reversed in insulin resistance even when endothelial IR is deficient. Accordingly, deficiency of IR did not restore the vasculoprotective effect of insulin in insulin resistance Thus, our results suggest that insulin treatment may be beneficial in ECs and VSCMs in insulin sensitive patients with diabetes undergoing revascularization. However, in insulin resistant patients with diabetes undergoing percutaneous intervention, high doses of insulin may be required for its beneficial effects on both ECs and VSMCs. Alternatively, insulin sensitizing agents that could bypass vascular insulin resistance may be preferable to insulin treatment.
Supported by: HS Foundation of Canada
Disclosure: M. Gonzalez Medina: None.
818
Chronic hyperglycaemia inhibits TCA cycle in GLUT4 overexpressing H9C2 cells
B. Stratmann1, B. Eggers2,3, Y. Mattern1, T. Silva de Carvalho1, K. Marcus2,3, D. Tschoepe1,4;
1HDZ NRW, Ruhr-Universität Bochum, Bad Oeynhausen, 2Ruhr-University Bochum, Medizinisches Proteom-Centre, Bochum, 3Medical Proteome Analysis, Centre for Protein diagnostics (PRODI), Ruhr-University Bochum, Bochum, 4Stiftung DHD (Der herzkranke Diabetiker) Stiftung in der Deutschen Diabetes-Stiftung, Bad Oeynhausen, Germany.
Background and aims: Oversupply of nutrients with loss of metabolic flexibility is a hallmark of diabetic cardiomyopathy. Even if excess substrate is offered, the heart suffers energy depletion as metabolic fluxes are diminished. To study effects of high glucose, a stably GLUT4-overexpressing cell line derived from H9C2 (KE2) was established presenting a diabetic cardiomyopathy-like phenotype. Long-term hyperglycaemia effects in KE2 were analysed and compared to H9C2.
Materials and methods: Rat cardiomyoblasts stably overexpressing GLUT4 (KE2) were cultured under cell-specific, normo- (20mM glucose, 20) and hyperglycaemic conditions (30mM glucose, 30) for 9 months. Expression profiles of proteins were compared to non-transfected H9C2 (WT) using RT-qPCR, proteomics-based analysis or Western blotting. GLUT4 surface analysis, glucose uptake, and apoptosis measurements were performed by flow cytometry. BNP levels, ROS formation, glucose consumption and lactate production were quantified.
Results: Hyperglycaemia induced increased GLUT4 presence on the cell surface and exaggerated glucose influx in KE2. The GLUT4 presence was increased in normo- and hyperglycaemic KE2 if compared to WT (2fold, WT30 vs. KE230, p<0.0001/ 1.4fold, WT20 vs. KE220, p<0.0001, t-test). By measuring 2-NBDG-uptake, KE2 proved to increase glucose uptake (3fold; WT30 vs. KE230/ 1.7fold, p<0.0001, WT20 vs. KE220, p<0.0001, t-test). The glucose consumption similarly increased for the same cells (1.6fold, WT30 vs. KE230, p<0.0001/ 1.6fold, WT20 vs. KE220, p<0.01, t-test) and production of L-lactate was doubled (2fold, WT30 vs. KE230L, p<0.0001/ 2fold, WT20L vs. KE220L, p<0.0001, one-way ANOVA). Hyperglycaemia affected the TCA cycle with significantly increased levels of fumarate after chronic hyperglycaemia in KE2 when compared with WT (1.22fold less, WT20 vs KE220, p<0.01/ 1.9fold more, WT30 vs. KE230, p<0.001, t-test), mainly due to decreased fumarase levels in KE2, which, regardless the glucose concentration, had less protein expression of fumarase when compared with WT (1.36fold, WT20L vs KE220L, p<0.05/ 2.10fold, WT30L vs. KE230L, p<0.001, t-test). Hyperglycaemic KE2 showed significant overexpression of BNP when compared with hyperglycaemic WT (8.3fold, WT30 vs. KE230, p<0.0001) or among KE2 (2.5fold, p<0.0001, one-way ANOVA). KE2 showed significantly increased oxidative stress when compared with WT cells (6.9fold, WT20 vs. KE220, p<0.0001; 1.7fold, WT30 vs. KE230, p<0.0001, t-test) or among KE2 (p<0.01) after long-term hyperglycaemia. Increased levels of apoptosis were detected (1.7fold, WT30 vs. KE230, p<0.0001/ 1.6fold, KE220 vs. KE230, p<0.0001, one-way ANOVA).
Conclusion: Chronic glucose overload in cardiomyoblasts induced by GLUT4 overexpression and hyperglycaemia resulted in metabolically stimulated proteome changes and metabolic alterations on TCA level. This indicates that these cells develop DC upon hyperglycaemic stress and reproduce the multi-faceted metabolic effects from chronic hyperglycaemia on cardiomyocyte protein profile and function.
Disclosure: B. Stratmann: None.
819
O-glcnacylation attenuated ages-induced neuroinflammation by inhibiting nf-Κb p65/nlrp3 inflammatory signalling pathway
T. Niu1,2, W. Zhu1,2, H. Zhang3, S. Wang1,2;
1Southeast University, Nanjing,jiangsu Province, 2Affiliated Zhongda Hospital of Southeast University, Nanjing, Jiangsu Province, 3The First Affiliated Hospital of China University of science and technology, Hefei,Anhui Province, China.
Background and aims: The neuroinflammation induced by activation of microglia is one of the major causes of diabetes-related cognitive impairment. The advanced glycation end products (AGEs) induced by hyperglycemia could cause neuroinflammation in microglia. O-GlcNAcylation, which is upregulated by O-GlcNAc tansferase (OGT) and down-regulated by O-GlcNAcase (OGA), exhibite neuroprotective effect in the brain. We aim to investigate the role of O-GlcNAcylation in AGEs-induced neuroinflammation.
Materials and methods: To induce neuroinflammation, primary microglia were isolated from newborn mice, and treated with AGEs. Then, Thiamet G (TMG) was administered to inhibit O-GlcNAcylation. Protein and mRNA levels of OGT, Nuclear factor κB P65 (NF-κB P65), NLRP3(NOD-like receptor protein 3), Tumor necrosis factor α(TNF-α), Interleukin-1β(IL-1β) and Interleukin-6(IL-6) were measured by Western blot and Real-time PCR, respectively. Immunofluorescence of CD68 and Iba1 were detected and observed by confocal microscopy. The interaction between OGT and NF-κB P65 was verified by immunoprecipitation.
Results: AGEs increased CD68 levels in Iba1 positive microglial cells. Additionally, mRNA and protein levels of NF-κB P65, NLRP3, TNF-α, IL-1β and IL-6 were up-regulated, while those of OGT were down-regulated by AGEs. However, the treatment of TMG reversed the effect of AGEs. OGT and NF-κB P65 were observed in the mixture pulled by NF-κB P65 and OGT antibody respectively.
Conclusion: O-GlcNAcylation may alleviate AGEs induced neuroinflammation by inhibiting NF-κB P65 /NLRP3 signaling pathway in primary microglia.
Supported by: 81870568
Disclosure: T. Niu: None.
820
HDL particles' function of cholesterol efflux capacity in newly diagnosed type 1 diabetes: prospective InLipoDiab1 study
A. Uruska1, A. Rohatgi2, A. Grzelka-Wozniak1, S. Saldanha2, J. Flotynska1, A. Cieluch1, A. Kaczmarek1, A. Pypec1, D. Zozulinska-Ziolkiewicz1;
1Poznan University of Medical Sciences, Poznan, Poland, 2University of Texas, Southwestern Medical Center, Dallas, USA.
Background and aims: People with type 1 diabetes are characterized by high serum HDL concentration, which does not translate into a better prognosis in this group. It is probably related to the impaired function of HDL particles. One of the main functions of HDL is the anti-atherosclerotic role related to the promotion of reverse cholesterol transport from macrophages in arterial walls (cholesterol efflux capacity-CEC). The initiation of insulin therapy increases serum HDL-cholesterol levels within the first year of therapy. It is not known whether the quantitative changes are accompanied by an improvement in HDL function. Aim: To evaluate HDL cholesterol efflux capacity in adults with newly diagnosed type 1 diabetes in response to insulin initiation
Materials and methods: The analysis comprised 125 patients (83 men, 66%) with newly diagnosed type 1 diabetes confirmed by the presence of autoantibodies, recruited to the Insulin Therapy and Lipoproteins' Profile in Type 1 Diabetes (InLipoDiab1) study. The median age at onset was 26 (IQR: 22-32) years. Cholesterol efflux capacity was assessed by measuring the efflux of radiolabelled cholesterol from murine J774 macrophages to apolipoprotein B-depleted serum. The study was performed at two points: before the first administration of exogenous insulin and after one year of using intensive insulin therapy.
Results: After one year of observation, a significant increase in serum HDL concentration [46 (36-55) vs 66 (55-82) mg/dl; p<0.0001] and no change in CEC [1.29 (1.11-1.42) vs 1.24 (1.14-1.41); p=0.6] were demonstrated.
Conclusion: In patients with type 1 diabetes, in the first year of the disease, there is no improvement in CEC, a key anti-atherosclerotic HDL function, despite a significant increase in serum HDL concentration. Further prospective studies should be done to explain these discordant findings.
Clinical Trial Registration Number: NCT02306005
Supported by: Kosciuszko Foundation
Disclosure: A. Uruska: None.
821
Serum metabolites and cerebral small-vessel disease in type 1 diabetes
S. Mutter1,2, J. Inkeri1,3, L. Thorn1,2, G.L. King4, H. Shah4, P.-H. Groop1,5, J. Putaala6, J. Martola3, N. Sandholm1,2, D. Gordin5,7, on behalf of the FinnDiane Study Group;
1Folkhälsan Research Center, Helsinki, Finland, 2Research Program for Clinical and Molecular Metabolism, Faculty of Medicine, University of Helsinki, Helsinki, Finland, 3HUS Medical Imaging Center, Radiology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 4Joslin Diabetes Center, Harvard Medical School, Boston, USA, 5Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 6Neurology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 7Minerva Foundation Institute for Medical Research, Helsinki, Finland.
Background and aims: Cerebral small-vessel disease (SVD) is frequent in neurologically asymptomatic individuals with type 1 diabetes (T1D). Previous studies in T1D on BP and albuminuria only moderately explained these findings with no association found with glycaemic control. The severity of diabetic retinopathy was associated with SVD, but the findings might reflect general changes to the microvasculature. Thus, we aim to assess potential mechanisms underlying SVD in T1D by using serum metabolites.
Materials and methods: Our Finnish Diabetic Nephropathy substudy included 189 individuals with T1D (19-51 years of age, diabetes onset < 40 years). Brain MRIs from a 3.0 T scanner were assessed by a neuroradiologist for SVD (n = 66) characterized by white matter hyperintensities (WMH, ≥ 1 on the Fazekas scale) or microbleeds. From non-targeted serum metabolomics, 1,246 out of 1,277 metabolites passed quality control, were normalised and log transformed. Logistic regressions were performed for each metabolite separately (adjusted for sex, age, BP, HDL cholesterol, albuminuria, eGFR, antihypertensive (AHT) and lipid-lowering drugs). Multivariable logistic adaptive LASSO regression was used to define a panel of metabolites that best associated with SVD. A P-value < 2.89 × 10-4 was considered significant as 173 metabolites cover 99% of the variance.
Results: Individuals with SVD were older (41 vs. 37 years), had a higher BP (134 vs. 128 mmHg), were more likely to take AHT drugs (49 vs. 29%) and less likely to have a normal AER (72% vs. 88%). No metabolites were significantly associated with SVD (Figure 1). Oleoylcarnitine (OR 0.53 per 1 SD [95% CI 0.36, 0.76], p = 0.001) and phenylacetate (1.91 [1.29, 2.91], p = 0.002) were suggestively associated with SVD. Phenylacetate was the strongest individual predictor (β = 0.59) in a panel of 29 metabolites that classified individuals according to SVD with a panel AUC of 0.64. Oleoylcarnitine was not selected into the panel. A pathway look-up showed that phenylacetate is linked to hydroxyphenylpyruvate, a suggested marker of WMH.
Conclusion: In T1D serum metabolites show suggestive associations with SVD beyond standard clinical biomarkers. These are linked to known findings and might shed more light on SVD than clinical biomarkers alone.
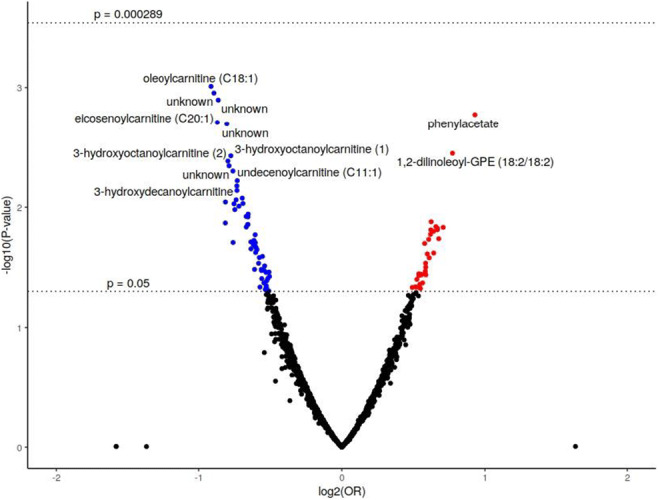
Supported by: Academy of Finland, Folkhälsan, Juselius Foundation, Stockman Foundation, University of Helsinki
Disclosure: S. Mutter: None.
SO 73 Diabetes in the vessels
822
Endotrophin as a risk marker of complications in a type 2 diabetes cohort
N.H. Tougaard1, A.L. Møller2, P.F. Rønn1, T.W. Hansen1, F. Genovese2, M.A. Karsdal2, D.G.K. Rasmussen2, P. Rossing1;
1Steno Diabetes Center Copenhagen, 2Nordic Bioscience A/S, Herlev, Denmark.
Background and aims: Hyperglycemia-mediated tissue injury eventually leads to fibrosis affecting various organ systems. The pro-fibrotic hormone endotrophin reflects collagen type VI formation. Higher level of serum endotrophin has been associated with increased risk of kidney function decline, cardiovascular events and mortality in selected persons with type 2 diabetes and microalbuminuria. We investigate whether endotrophin level in serum and urine is associated with the risk of developing micro- and macrovascular complications in an unselected type 2 diabetes population.
Materials and methods: Endotrophin was measured in serum and urine collected at baseline from individuals enrolled in a cohort study including 774 persons with type 2 diabetes recruited from Steno Diabetes Center Copenhagen, Denmark between 2012 and 2016. Urinary values were normalized to urine creatinine levels. Outcomes were identified through national registries and medical records and included a kidney endpoint (≥40% decline in kidney function or kidney failure), first major adverse cardiovascular event (MACE), all-cause mortality, progression of albuminuria, incident heart failure and sight-threatening eye disease. Cox proportional hazards models with adjustment for conventional risk factors were applied.
Results: The cohort consisted of 254 (33%) females, and the mean age was 65 (±12) years. Depending on event, median follow-up ranged from 3.0 to 6.0 years. A doubling of serum endotrophin was independently associated with the kidney endpoint (n: 49, HR: 1.80, 95% CI: 1.13-2.87, first MACE (n: 66, HR: 1.54, 95% CI: 1.04-2.28), all-cause-mortality (n: 156, HR: 1.69, 95% CI: 1.31-2.19) and incident heart failure (n: 42, HR: 1.63 95% CI: 1.02-2.60), but not with progression of albuminuria and incident sight-threatening eye disease. A doubling of urine endotrophin was independently associated with progression of albuminuria (n: 85, HR: 1.18, 95% CI: 1.01-1.38) and incident heart failure (n: 40, HR: 1.79, 95% CI: 1.09-2.95), but not with the other endpoints.
Conclusion: Serum endotrophin was an independent risk marker of mortality, kidney and cardiovascular complications in type 2 diabetes.
Disclosure: N.H. Tougaard: None.
823
Mitogenic signalling pathways in valvular interstitial cells and their dependency from diabetic conditions
J.I. Selig1, F.A. Kraft1, E. Adler1, D.M. Ouwens2,3, A. Lichtenberg1, P. Akhyari1, M. Barth1;
1Department of Cardiac Surgery, University Hospital of the Heinrich-Heine-University Düsseldorf, Düsseldorf, 2Institute for Clinical Biochemistry and Pathobiochemistry, German Diabetes Center (DDZ), Düsseldorf, 3German Center for Diabetes Research (DZD), München-Neuherberg, Germany.
Background and aims: Type 2 diabetes mellitus is an independent risk factor for the development of calcific aortic valve disease, a progressive disorder that leads to the loss of valve function. Previous analyses showed that valvular interstitial cells (VIC) are sensitive to insulin. Elevated insulin and glucose levels induce insulin resistance with reduced AKT phosphorylation after insulin stimulation. However, knowledge about downstream signalling pathways of insulin and their role in mitogenic processes associated with valve degeneration is still scarce.
Materials and methods: Primary ovine VIC (n=9) were transfected with custom-designed siRNA targeting FOXO1, mTOR, ERK1 and ERK2 one day and four days after seeding. The cells were cultivated either under normoglycaemic conditions (NG; 100 mg/dl glucose) or under diabetic conditions with hyperglycaemia and hyperinsulinemia (HG+HI; 450 mg/dl glucose and 100 nM insulin). VIC transfected with non-targeting siRNA cultivated under NG served as control. After six days, cell differentiation and degeneration as well as extracellular matrix remodelling were examined with qRT-PCR, Western blot analyses and colorimetric assay systems.
Results: All four siRNA targets were significantly downregulated both at mRNA (p<0.001) and protein level (p<0.01). Downregulation of mTOR, ERK1 and ERK2 in combination with HG+HI was associated with an activation of VIC indicated by an increased α-smooth muscle actin expression (mTOR: p<0.01; ERK1: p<0.001; ERK2: p<0.05), while downregulation of FOXO1 led to an elevated expression of the degeneration marker osteopontin (p<0.05). Furthermore, downregulation of mTOR and FOXO1 increased hyaluronan synthase 2 expression (NG: p<0.05; HG+HI: p<0.05) and decreased decorin expression (NG: p<0.0975; HG+HI: p<0.05), respectively. ERK1 and ERK2 in contrast did not influence the extracellular matrix composition. Moreover, a versatile interaction was observed between the different signalling molecules. Downregulation of mTOR caused an upregulation of all three other proteins (FOXO1: p<0.05; ERK1/2: p<0.01). FOXO1 downregulation in contrast caused an upregulation of ERK2 (p<0.01), but did not affect ERK1 or mTOR. In turn, ERK1 and ERK2 downregulation did not influence the expression of mTOR and FOXO1.
Conclusion: AKT downstream signalling pathways as well as MAPK signalling pathways are involved in the homeostasis of VIC and participate putatively in degeneration and remodelling of aortic valves. Additional diabetic conditions can intensify these effects and thus may contribute to a rapid progression of the disorder. Further analysis of signalling pathways involved in mitogenic processes is of particular importance to develop therapeutic treatments for calcified aortic valve disease in future.
Supported by: DFG
Disclosure: J.I. Selig: Grants; German Research Foundation (DFG).
824
Dulaglutide prevents high-glucose induced endothelial senescence and the capture of microvesicles by endothelial cells
F. Moschovaki Filippidou1, N. Sbat1, F. Zobairi1, F. Toti1,2, L. Kessler1,3;
1Inserm 1260 UMR, Regenerative Nanomedicine, University of Strasbourg, 2Faculty of Pharmacy, University of Strasbourg, 3Diabetology, Department of Diabetology, University Hospital of Strasbourg, Strasbourg, France.
Background and aims: In patients with diabetes, higher cardiovascular risk has been associated with release of MV with endothelial origin. The cardiovascular benefit of a treatment by GLP1 receptor agonists (GLP1RA) has been demonstrated in various clinical trials. Because senescence shifts the endothelium to an athero-thrombogenic surface, we investigated the effect of dulaglutide, a GLP1RA, on high-glucose induced senescence in primary coronary artery endothelial cells.
Materials and methods: Porcine coronary artery young endothelial cells (PCAECs) were treated with 1,2 and 3 μM dulaglutide and challenged by high glucose concentration (HG 25mmol/L) for 48 or 96 hours. Senescence was monitored by the SA-β-gal activity and expression of p53 and p21 by western blot. The expression of GLP1R was studied by western blot and confocal microscopy. The effect of dulaglutide on the relative expression of a variety of genes encoding markers and actors in HG-induced endothelial responses was evaluated by RT-qPCR. Endothelial procoagulant MVs were measured by prothrombinase assay in culture medium. In a crosstalk model, effector properties of MVs were studied after 48 hours incubation with naïve young PCAECs at the concentration of 10nM. Kinetics of the MVs capture by naïve target cells was monitored by flow cytometry after MVs were labelled with the fluorescent PKH26 lipid probe.
Results: Dulaglutide (1 μM) significantly attenuates the HG-induced endothelial senescence measured after 96h by SA-β-gal activity to baseline values in normal glucose (113.7±2.9 vs. 103.9±2.1, % of Control, p=0.02, n=8). A dose response effect was observed with higher dulaglutide concentrations, as increasing dulaglutide concentration to 3 μM lead to an early reduction already detectable at 72h by SA-β-gal activity (HG: 337.6, HG+Dula: 289, Mean fluorescence A.U., p=0.0088, n=3). Down-regulation of p21, the p53 down-stream senescence marker (p=0.03, n=5), confirmed HG-induced senescence inhibition after 96h. GLP1R expression remained unchanged under conditions of HG and dulaglutide treatment at 48h and 96h. Analysis of the gene expression levels confirmed the protective effect of dulaglutide on HG-induced senescence. Shc1, encoding protein p66, was downregulated by 34% (p=0.05, n=4) after 48h, while a 26% reduction in Ncf1 gene, encoding p47phox, nearly reached significance after 96h (p=0.087, n=3). HG-induced expression levels were elevated for CdkN1A, encoding p21, after 48h (p=0.042, n=4) and IL6 after 96h (p=0.0255, n=3), thereby confirming the ongoing process of endothelial damage. Dulaglutide did not alter the shedding of MVs in response to HG. Nevertheless, in MV-mediated crosstalk experiments, the percentage of PKH26-labelled target cells was reduced when MVs were isolated from dulaglutide-treated cells (95.98±0.66 vs. 92.04±1.08, p=0.010, n=6).
Conclusion: Dulaglutide may delay HG-induced endothelial senescence, and possibly inhibits the capture of MVs by naïve endothelial cells. Endothelial senescence may be considered as a new target in the prevention of cardiovascular diseases in diabetic patients.
Disclosure: F. Moschovaki Filippidou: Grants; Eli Lilly and Company, France.
825
High glucose concentration-induced dicarbonyl stress in human aortal endothelial cells activates the unfolded protein response and NLRP3 Inflammasome
P. Thornalley1, M. Xue1, N. Rabbani2;
1Diabetes Research Center, Hamad Bin Khalifa University, 2College of Medicine, Qatar University, Doha, Qatar.
Background and aims: Metabolic dysfunction of endothelial cells in hyperglycemia contributes to the development of vascular complications of diabetes. High glucose concentration produces hexokinase-2 linked glycolytic overload, leading to the increased formation and accumulation of the reactive glucose-derived metabolite, methylglyoxal (MG) - called dicarbonyl stress - and other metabolic dysfunction. MG-modified proteins are unfolded and prospective physiological activators of the unfolded protein response (UPR). MG is metabolized by glyoxalase 1 (Glo1) of the glyoxalase system. We investigated the role of dicarbonyl stress in activation of the UPR and NLRP3 inflammasome in endothelial cells during model hyperglycemia in vitro by siRNA silencing of Glo1.
Materials and methods: Human aortal endothelial cells (HAECs) were cultured under an atmosphere of air with 5% CO2, 100% humidity at 37 oC in human large blood vessel endothelial cell growth medium with growth supplements and antibiotics according to the manufacturer’s instructions; used during passages 4 - 6 to maintain the endothelial phenotype. Cultures had low glucose (4.1 mM, LG) and high glucose (20 mM, HG) concentration incubated for 72 h, with and without prior treatment with 25 nM Glo1 siRNA (Glo1KD) and protein abundance of UPR pathway mediators, XBP1, PERK and ATF6, and markers of downstream UPR and NLRP3 inflammasome inflammatory signaling, monocyte chemoattractant-1 (MCP-1), interleukin-8 (IL8) and thioredoxin interacting proteins (TXNIP). Data are mean ± SD (n = 3). The role of the UPR sensor, inositol-requiring enzyme-1α (IRE1α), was explored by use of the specific inhibitor 4μ8C, and role of XBP1 by siRNA silencing,
Results: Glo1 protein was decreased by 92% and 75% in LG and HG cultures, respectively, with siRNA silencing. XBP1 protein was increased 43 ± 16% by Glo1KD in LG (P<0.05), increased 68 ± 3% in HG (P<0.01) and further increased 153 ± 21% by Glo1KD in HG (P<0.001). PERK protein was decreased 58 ± 4% by Glo1KD in LG (P<0.05), decreased 21 ± 2% in HG (P<0.01) and further decreased 63 ± 2% by Glo1KD in HG (P<0.001). PERK is thought to contribute to VEGF-mediated endothelial cell viability. ATF6 protein was unchanged in HG. NLRP3 inflammasome component, TXNIP, was increased 54 ± 21% by Glo1KD in LG (P<0.05), increased 112 ± 12% in HG (P<0.001) and further increased 159 ± 9% by Glo1KD in HG. Inhibition of IRE1α by 1 μM 4μ8C prevented increase of XBP1, TXNIP and MCP-1 mRNA in HG cultures. TXNIP is increased through IRE1α-mediated decrease of miR-17 which de-stabilizes TXNIP mRNA. Surprisingly, silencing of XBP1 potentiated the increase of TNXIP, MCP1 and IL8 in HG. This may suggest MCP1 and IL8 are increased via the NLRP3 inflammasome rather than the XBP-1 pathway of the UPR; knockdown of XBP1 mRNA removing a competing substrate for the cleavage of miR-17 by IRE1α.
Conclusion: We conclude that dicarbonyl stress induced in human aortal endothelial cells in high glucose concentration activates both the UPR and, via IRE1α increase of TXNIP, also the NLRP3 inflammasome. Concurrent decrease of PERK may impair endothelial cell viability and survival. These responses likely contribute to vascular inflammation and endothelial dysfunction in diabetes.
Supported by: QF funded project
Disclosure: P. Thornalley: None.
826
Alpha-tubulin deacetylation impairs angiogenesis and induces endothelial-to-mesenchymal transition
J. Kim, W. Ham, W. Lee;
Chung-Ang University, Seoul, Republic of Korea.
Background and aims: Microtubules are one of the intracellular cytoskeletons which composed of α- and β-tubulin subunits. These proteins form a cellular framework with key roles in cell shaping, division, and intracellular transport. Acetylation of α-tubulin subunits on lysine 40 catalyzed by α-tubulin N-acetyltransferase 1 (αTAT1) has been known to be an essential post-translational modification that regulates microtubule stability. Diabetes is associated with endothelial dysfunction which causes progressive vascular complications. However, functional consequences of tubulin acetylation/deacetylation were mostly remained elusive in endothelium.
Materials and methods: We generated endothelial cell-specific inducible αTAT1 KO mice by crossing αTAT1 floxed mice with Tie2-CreERT2 mice. Eight-week-old male mice were injected i.p. with tamoxifen (40 μg/g) for 4 days and sacrificed after 2 weeks. In vitro, human umbilical vein cells (HUVECs) subjected to αTAT1 knockdown by siRNA.
Results: Endothelial cells isolated from αTAT1 KO mouse lungs showed robust decrease of α-tubulin acetylation levels compared to wild-type. αTAT1 KO mice showed impaired microvessels sprouting based on ex vivo aortic ring assay. αTAT1 siRNA treated HUVECs significantly reduced VEGF-induced tube formation, cell migration, and proliferation compared to the controls. Furthermore, αTAT1 silenced cells showed marked spindle-like morphological changes and upregulation of mesenchymal markers indicating endothelial-to-mesenchymal transition (EndMT).
Conclusion: Our results demonstrate that tubulin deacetylation in endothelial cells has a pathological role by which impaired angiogenesis and induction of EndMT and could contribute to diabetic vascular complications.
Supported by: National Research Foundation of Korea
Disclosure: J. Kim: None.
827
A six-month low-carbohydrate diet high in fat in patients with type 2 diabetes does not affect the vasodilatory reactivity of the brachial artery
E.M. Gram-Kampmann1, T.B. Olesen1, C.D. Hansen2, M.B. Hugger2, J.M. Jensen2, P. Hermann3, M.H. Olsen4, A. Krag2, K. Højlund1;
1STENO Diabetes Center Odense (SDCO), Odense University Hospital, Odense C, 2Department of Gastroenterology and Hepatology, Odense University Hospital, Odense C, 3Department of Endocrinology, Odense University Hospital, Odense C, 4Department of Internal Medicine, Holbæk Hospital, Holbæk, Denmark.
Background and aims: One of the major causes of mortality in type 2 diabetes (T2D) is cardiovascular disease (CVD). Endothelial dysfunction (ED) is an early sign of atherosclerosis which is present before overt clinical CVD. In non-diabetic subjects, low-carbohydrate diets (LCDs) have been demonstrated to improve vasodilatory reactivity in the endothelium, but in T2D patients, the effect of a LCD on measures of ED is mixed. The aim of this study was to examine the effect of a non-calorie-restricted LCD high in fat for six months on measures of ED in T2D.
Materials and methods: Patients with T2D were randomized 2:1 to either a LCD with a max. of 20 E% (percentage of total energy intake) from carbohydrates and min. 50 E% from fat (n = 49) or a control diet with 50-60 E% from carbohydrates (n = 22) for 6 months. Both diets were non-calorie restricted. Flow-mediated vasodilation (FMD) and nitroglycerine-induced vasodilation (NID) were assessed at baseline and after six months using B-mode ultrasound scans of the brachial artery and are reported as dilation in percentage (%). Blood samples were collected and dual-energy x-ray absorptiometry (DXA) and anthropometrics were assessed at baseline and after six months. We report baselines differences and the mean difference in change (MDIC) between groups from baseline to six months.
Results: Participants on the LCD reduced their self-reported carbohydrate intake to ~ 14 E% and increased their fat intake to ~63 E% including a 2.7 fold increase in saturated fatty acids (SFA). The LCD caused a clinically relevant decrease in HbA1c (MDIC: -8.9 ± 1.7 mmol/mol; P < 0.001), a reduction in weight (MDIC: -3.9 ± 1.0 kg; P < 0.01) and improved body composition (P<0.05) after 6 months compared with the control diet. Forty-seven brachial ultrasound scans from the LCD group and 19 from the control group were available for analyses at baseline, and 44 and 18, respectively, at follow-up. At baseline, FMD (5.1 ± 0.3 vs. 4.2 ± 0.4 %; P = 0.046), NID (16.3 ± 0.5 vs. 13.8 ± 1.2 %; P = 0.012) and systolic blood pressure (p<0.05) were higher in the LCD group than in the control group. Baseline FMD was positively associated with NID (P = 0.001), but negatively associated with age and systolic blood pressure (all P < 0.05). Six months of LCD did not significantly change either FMD (MDIC: -0.0 ± 0.0 %; p = 0.82) or NID (MDIC: 1.0 ± 1.0 %; P = 0.29) compared with the control diet.
Conclusion: A non-calorie-restricted LCD high in fat for 6 months does not affect either FMD or NID in patients with T2D. While these results could not confirm a beneficial effect of LCD on ED as observed in non-diabetic individuals, the data also suggest that the higher intake of fat, in particular SFA, associated with a LCD, does not adversely affect endothelial function and hence cardiovascular risk in patients with T2D.
Clinical Trial Registration Number: NCT03068078
Supported by: Novo Nordisk Foundation, Danish Diabetes Academy, Region of Southern Denmark, A.P. Møller
Disclosure: E.M. Gram-Kampmann: None.
828
Perivascular adipose tissue remodelling impairs vasoreactivity in thermoneutral-housed rats
A.C. Keller1, M.M. Henckel1, J. Chun2, L.A. Knaub1, G.B. Pott1, G.G. James3, L.A. Walker4, J.E. Reusch1;
1Department of Medicine/Research, University of Colorado/Rocky Mountain Regional VA Medical Center, Aurora, 2Aquillius Corporation, San Diego, 3Department of Medicine/Research, University of Colorado/Rocky Mountain Regional VA Medical Center, Aurora, 3Cornell College, Mount Vernon, 4Department of Medicine, University of Colorado, Aurora, USA.
Background and aims: Diabetes increases cardiovascular disease (CVD) risk and impacts men and women differently. Vascular pathology, characterized by impaired vasoreactivity and mitochondrial function, also differs between the sexes. We reported that housing rats at thermoneutral (TN) conditions causes vascular dysfunction and perturbed metabolism. We hypothesized that perivascular adipose tissue (PVAT), a vasoregulatory adipose depot with a brown adipose tissue (BAT) phenotype, remodels to a white adipose (WAT) phenotype at TN, driving diminished vasoreactivity and mitochondrial function in a sex-dependent manner.
Materials and methods: Male and female Wistar rats were housed at either room temperature (RT) or TN. Endpoints included vasoreactivity in response to acetylcholine and phenylephrine in vessels with intact PVAT or transferred to PVAT of the oppositely-housed animal; vessel mitochondrial respiration; signaling to mitochondrial biogenesis and OXPHOS proteins; PVAT morphology and adiponectin.
Results: TN animals displayed significantly diminished aorta vasodilation (16.1 ± 4.4% dilation in TN animals vs. 36.9 ± 6.0% dilation in RT animals with 20 μM ACh, p<0.05), lessened by the presence of PVAT in males. Switching PVAT from RT rats onto aorta from TN rats in females corrected vasodilation (42.7 ± 13.8% vs. 11.3 ± 7.2% in control rats with 20 μM ACh) and over-constriction (2.3 ± 0.5 mN/mg vs. 3.4 ± 0.8 mN/mg in control rats, p<0.05 for both). In aorta of all animals housed at TN versus those housed at RT, mitochondrial respiration was significantly diminished in lipid substrate experiments (state 3= 21.5 ± 1.0 pmol/s*mg vs. 26.8 ± 1.6 pmol/s*mg, state 4= 15.3 ± 1.0 pmol/s*mg vs. 21.3 ± 1.5 pmol/s*mg and uncoupled=32.9 ± 1.6 pmol/s*mg vs. 41.8 ± 2.0 pmol/s*mg, p<0.05 for all). In aorta from animals at TN, there was significantly less expression of pAMPK (p<0.05), peNOS (p<0.001), and mitochondrial complexes I (p<0.01) and IV (p<0.05). Male rat aorta had a higher content of mitochondrial biogenesis and OXPHOS proteins; PGC1-α (p<0.0001), MnSOD (p<0.0001), eNOS (p<0.0001), pCaMKII α/β (p<0.0001), and mitochondrial protein complexes II (p<0.05), III (p<0.01), and V (p<0.05). Remodeled PVAT occurred in rats housed at TN: TN rats had 19.7% less BAT phenotype overall as compared with RT rats (p<0.05). UCP-1, a marker of BAT, was less in animals housed at TN (p=0.06). Adiponectin concentrations were significantly lower in those housed at TN (p<0.05), more pronounced in females (0.74 ± 0.08 ng/ml vs. 0.97 ± 0.14 ng/ml).
Conclusion: TN housing leads to impaired vasoreactivity and vascular mitochondrial content; TN housing also leads to decreased adiponectin and UCP-1, more significant in females. Exposure of TN aorta to RT PVAT corrects vasodilation in females. These data support a model wherein diminished adipokine production and signaling from remodeled PVAT to the vasculature causes dysfunctional vasoreactivity via the dampening of nutrient sensor AMPK and/or eNOS activity, in a sex-dependent manner. These results provide insights into sex differences in vascular disease mediated by the adventitia.
Supported by: The authors wish to acknowledge the following funding sources: NIH/NCRR CCTSI UL1 RR025780, VA Merit
Disclosure: A.C. Keller: None.
829
One-year treatment with dulaglutide and dapagliflozin improves endothelial function and albuminuria compared to DPP-4 inhibitors
E. Korakas1, I. Ikonomidis2, J. Thymis2, A. Kountouri1, K. Katogiannis2, G. Pavlidis2, D. Tsilivarakis2, V. Prentza1, K. Balampanis1, L. Pliouta1, F. Kousathana1, G. Kostelli2, G. Dimitriadis1, A. Raptis1, V. Lambadiari1;
1Second Dept of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Attikon University Hospital, 2Second Cardiology Department, Medical School,National and Kapodistrian University of Athens, Attikon University Hospital, Chaidari, Greece.
Background and aims: Dulaglutide and dapagliflozin have favorable effects on albuminuria and arterial stiffness, while Dipeptidyl-peptidase 4 inhibitors (DPP-4is) exert rather neutral actions. In this study, we aimed to compare the early combination of dulaglutide and dapagliflozin with DPP-4is as an add-on to metformin monotherapy in terms of endothelial function and albuminuria in type 2 diabetes mellitus (T2DM).
Materials and methods: Overall, 45 patients with T2DM and albuminuria were included in our study. 23 patients received the combination of dulaglutide and dapagliflozin and were followed immediately prior (baseline), 4 and 12 months afterwards. 22 patients, matched for sex, age and glycemic control, received DPP-4is. In each visit we measured a) Carotid-femoral PWV b) central systolic blood pressure (cSBP) c) perfused boundary region (PBR) of the sublingual arterial microvessels, d) urinary albumin-to-creatinine ratio (UACR), e) glycosylated hemoglobin (HbA1c).
Results: After 4 and 12 months, patients on dulaglutide/dapagliflozin combination significantly improved HbA1c (8±1.5% vs 6.7±0.73% vs 6.6±0.69%, p<0.001), PBR (2.2±0.29 vs 2.1±0.28 vs 1.9±0.28 μm, p<0.05), PWV (11.9±3.2 vs 11±2.2 vs 11.2±2.4 m/s, p<0.05), cSBP (128.8±24.2 vs 121.7±16.3 vs 121.2±12.3 mmHg, p<0.05) and UACR (396.5±341.2 vs 241±195.7 vs 148.8±97.2 mg/g, p<0.001). On the contrary, no statistically significant differences in PBR (2.1±0.32 vs 2.2±0.31 μm vs 2.1±0.27, p>0.05), PWV (11.2±3.5 vs 11.8±3.1 vs 12.9±7.1 m/s, p>0.05), cSBP (125.2±18.3 vs 126.4±18.6 vs 129.0±19.7 mmHg, p>0.05) were noted in the DPP-4is group, despite significant improvements of HbA1c (8.0±1.7 vs 7.2±1.2 vs 6.8±2.2%, p<0.01) and UACR (242.1±96.9 vs 206.2±106.4 vs 201.9±111.6 mg/g, p<0.01).
Conclusion: The combination of dulaglutide and dapagliflozin as an add-on treatment to metformin early in the course of treatment has sustainable favorable effects on endothelial function and albuminuria compared to DPP-4is in patients with T2DM.
Disclosure: E. Korakas: None.
SO 74 Weighing risks of cardiovascular complications
830
Assessment of skin autofluorescence association with glycated haemoglobin, cardiovascular risk markers and concomittant chronic diseases in children with type 1 diabetes
B. Mianowska1, M. Jankowska2, I. Pietrzak1, J. Chrzanowski3, J. Solek3, A. Szadkowska1;
1Dept. of Pediatrics, Diabetology, Endocrinology and Nephrology, Medical University, 2Dept. of Developmental Neurology and Epileptology, Polish Mothers’ Memorial Institute Research Hospital, 3Dept. of Biostatistics and Translational Medicine, Medical University, Lodz, Poland.
Background and aims: Advanced glycation end products (AGEs) are long-lived proteins that play a role in the pathogenesis of chronic diabetes complications. Skin autofluorescence (sAF) is a non-invasive method used to measure tissue AGEs accumulation. It was shown that in adults with type 1 or type 2 diabetes sAF correlates with long term glycemic control and may be regarded as a surrogate marker of chronic diabetes complications, including cardiovascular (CV). This study aim was to assess sAF association with glycated hemoglobin (HbA1c) values, CV risk markers and comorbidity of autoimmune thyroiditis or celiac disease in children with type 1 diabetes (T1D).
Materials and methods: Patients aged 3-18 years with ≥6 months T1D duration participated. sAF (in arbitrary units, AU) was measured with AGE Reader (Diagnoptics BV, Netherlands). Total cholesterol and its fractions (low and high density), triglycerides, HbA1c, C-reactive protein (CRP), ambulatory blood pressure monitoring (ABPM; Spacelabs 90217 Monitor, Spacelabs, USA) records, body mass index (BMI) Z-score and body composition parameters (MC-980MA analyzer, TANITA, Japan) were analysed.
Results: Altogether 348 patients were included in the analysis (182 boys, 166 girls; median age 14.3 years [IQR 11.2-17.1]; T1D duration 5.5 years [3.1-8.8]). Median HbA1c was 7.3% [6.7-8.1%] (56 mmol/mol [50-65 mmol/mol]), 244 patients were treated with insulin pumps. Median sAF was 1.40AU [1.27-1.53] and positively correlated with current HbA1c, mean of previous (since T1D diagnosis) HbA1cs, age, and T1D duration (respectively rho: 0.27, 0.22, 0.15, 0.14, all p<0.01). sAF was significantly higher in girls than in boys (respectively 1.40AU [1.3-1.6] vs 1.37AU [1.27-1.50], p=0.011) and in patients with than without celiac disease (1.53AU [1.43-1.63] vs 1.40AU [1.27-1.53], p=0.001). Moreover, sAF positively correlated with percentage of body fat, body impedance and CRP; and negatively correlated with basic metabolic rate (BMR) in kcal (respectively rho: 0.12, 0.14, 0.17 and -0.16, all p<0.05). No significant correlations were found between sAF and: lipids, BMI z-score, blood pressure load measures (percentages of daytime and nighttime systolic or diastolic readings >95th percentile for sex and hight) or nighttime decrease of systolic or diastolic pressure (dipping status). sAF did not differ between patients with (1.40AU [1.27-1.60AU], p=0.707) or without autoimmune thyroiditis (1.40AU [1.27-1.53AU]). In the final multiple regression model, parameters that remained positively correlated with sAF were: age (β=0.40, p<0.0001), historical HbA1c (β=0.24, p<0.0001), CRP (β=0.15, p=0.014), female sex (β=0.26, p=0.034) and presence of celiac disease (β=0.11, p=0.062); while BMR (β=-0.51, p<0.0001) remained negatively correlated.
Conclusion: In young patients with T1D whose diabetes duration is relatively short sAF reflects well previous glycemic control represented by historical average HbA1c however its associations with classic CV-risk markers are not evident. sAF negative correlation with BMR and positive with celiac disease deserve further research.
Disclosure: B. Mianowska: None.
831
First occurred cardiovascular or renal disease (CVRD) cumulative incidence for type 2 diabetics free of CVRD at baseline: a 5-year SNDS nationwide claims database cohort study
P. Blin1, M. Joubert2, P. Zaoui3, E. Guiard1, D. Sark1, C. Dureau-Pournin1, M.A. Bernard1, R. Lassalle1, F. Thomas-Delecourt4, S. Bineau4, N. Moore1, C. Droz-Perroteau1, P. Jourdain5;
1Bordeaux PharmacoEpi INSERM-P CIC1401, University of Bordeaux, Bordeaux, 2CHU Caen, Caen, 3CHU Grenoble, Grenoble, 4AstraZeneca, Courbevoie, 5Hôpital Kremlin-Bicêtre, Paris, France.
Background and aims: Myocardial infarction (MI), stroke, peripheral arterial disease (PAD), heart failure (HF) and chronic kidney disease (CKD) are common cardiovascular renal disease (CVRD) manifestations for type 2 diabetes (T2D). The main objective was to assess the 5-year incidence of the first occurred CVRD manifestation for T2D patients free of CVRD at baseline, that is currently not well known.
Materials and methods: A cohort study of all French T2D patients free of CVRD (5-year history) as of January 1st, 2014, identified and followed-up for 5 years within the French SNDS nationwide claims database. The cumulative incidence of the first occurred CVRD manifestation (MI, stroke, PAD, HF or CKD) was estimated using the cumulative incidence function, with other CVRD manifestations and death as a competing risk.
Results: From about 2 million T2D patients without cancer or transplantation, 76.5% were free of CVRD at baseline with a mean age of 65 years, 52% of women, 46% T2D for more than four years and 7% of diabetic complications. The most frequent first occurred CVRD manifestation during the 5-year follow-up was CKD followed by HF, then PAD, stroke and MI with a constant linear increase over time (figure 1). After 5 years of follow-up, CKD represented 40.6% of first CVRD manifestation, HF 23.0%, PAD 13.5%, stroke 13.2% and MI 9.7%. HF and CKD together reached about 1 patient out of ten and represented 63.6% of first CVRD manifestations. The cumulative incidence increased with age, and similar proportion of CVRD manifestation was observed whatever the age-class, except for HF which presented a clear increased with age from 14% of the events before 65 years to twice more (30.7%) for those ≥ 75 years.
Conclusion: While MI, stroke and PAD remain classic major risks of complications for CVRD free T2D patients, HF and CKD nowadays represent a higher risk of CVRD manifestation. This should encourage the development of specific preventive strategies.
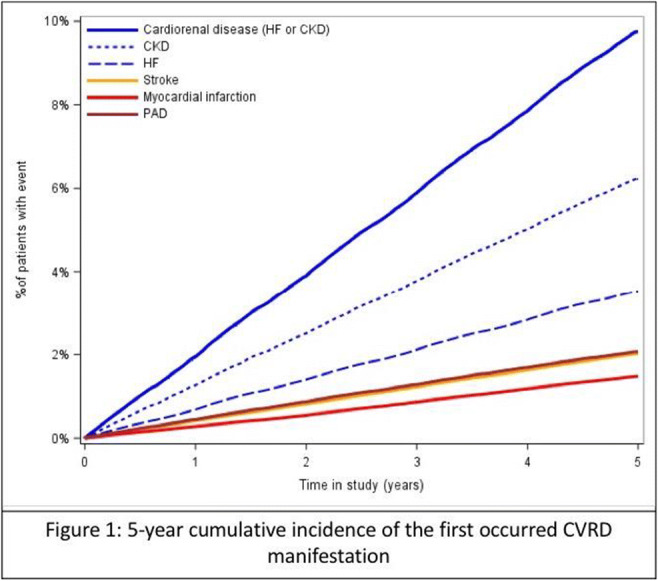
Supported by: Study performed with unconditional funding from AstraZeneca
Disclosure: P. Blin: None.
832
The association between hemoglobin A1c and development of cardiovascular disease in males and females without diabetes at baseline: a population inception cohort study
P. Kaul1, L. Chu2, D.A. Dover1, R.O. Yeung1, D.T. Eurich1, P. Senior1, S. Butalia3;
1University of Alberta, Edmonton, 2Alberta Health Services, Edmonton, 3University of Calgary, Calgary, Canada.
Background and aims: To examine the association between HbA1c and development of CVD in males and females in a large, population-based inception cohort without diabetes at baseline, in a universal health care setting.
Materials and methods: The study population consisted of an inception cohort of individuals between 40 and 80 years without diabetes or CVD on April 1, 2013 in the province of Alberta, Canada (N=1,419,558). Individuals who died, left the province, developed CVD or did not have an HbA1c test during the 3-year enrollment period (April 1, 2013 and March 31, 2016) were excluded. The remaining males and females were divided into the following categories based on their maximum HbA1c during the enrollment period: HbA1c < 5.0%, 5.0 to 5.4%, 5.6 to 5.9%, prediabetes 6.0 to 6.4%, and diabetes ≥6.5%. HbA1c categories for prediabetes and diabetes were based on Canadian diabetes guidelines. The primary outcome of interest was a CVD hospitalization (defined as a hospitalization with a primary diagnosis of heart failure, acute coronary syndromes, cerebrovascular disorders, atrial fibrillation and flutter, ventricular arrhythmia, or sudden cardiac death) over a 5-year follow-up period until March 31, 2021. Cox-proportional hazards models were used to estimate the association (presented as adjusted hazard ratio with 95% CI) between HbA1c categories and outcomes in males and females after adjusting for age, hypertension, material deprivation, urban/rural residence, and comorbidity burden.
Results: A total of 624,096 individuals (55% female) were included in the study. During the enrollment period, 15% and 5.2% of females developed prediabetes and diabetes, respectively, and 15.9% and 8.2% of males developed prediabetes and diabetes, respectively. Compared to the reference category of 5.0% to 5.4%, males with normal HbA1c (5.5% to 5.9%) had an increased risk of CVD hospitalization (aHR 1.16; 95% CI 1.1 to 1.23) whereas females did not (aHR 1.04; 95% CI 0.96 to 1.12) (Figure). Males with prediabetes had a higher risk of CVD hospitalization compared to females (aHR 1.38; 95% CI 1.28 to 1.47 in males; aHR 1.13, 95% CI 1.04 to 1.23 in females). Females and males who developed diabetes during the enrollment period had the highest risk of CVD.
Conclusion: Normal glycemia and prediabetes conferred a higher risk of CVD hospitalization in males than in females. These findings highlight the importance of optimizing cardiovascular risk profiles, particularly in males at all levels of glycemia.
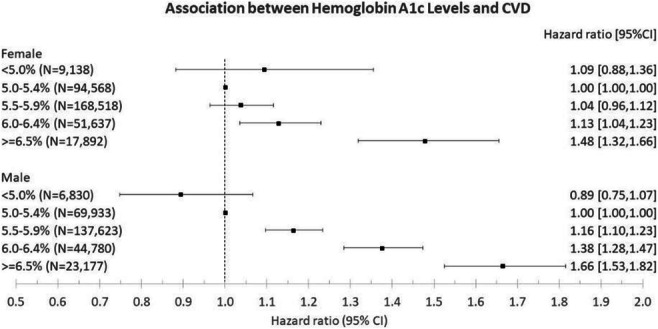
Supported by: Canadian Institutes of Health Research
Disclosure: P. Kaul: None.
833
Association between age at diagnosis of type 2 diabetes and cardiovascular and mortality risks: a nationwide population-based study
D. Seo1, M. Kim2, Y. Cho1, S. Ahn1, S. Hong1, S. Kim1;
1Inha University School of Medicine, Incheon, 2Hanmi Pharm. Co., Ltd., Seoul, Republic of Korea.
Background and aims: The incidence of type 2 diabetes mellitus (T2DM) among young adults is increasing and individuals with young onset T2DM are known to have increased risk of vascular complications. We aimed to investigate the association between mortality and cardiovascular (CVD) outcomes in relation to the age at diagnosis of T2DM.
Materials and methods: In this population cohort study with Korean National Health Insurance Service data (2012-2014), a total of 634,350 patients with newly diagnosed T2DM were included. Controls were randomly selected from the general population and matched for age, sex, and previous history of CVD, using the propensity score matching analysis (1:2 ratio). Participants were followed for CVD outcomes, death or until 2019. Outcomes of interest were total mortality, CVD mortality, coronary heart disease (CHD), acute myocardial infarction (AMI), stroke, hospitalization for heart failure (HHF) and 3-point MACE. We conducted the primary analyses using Cox proportional hazards models in those with no previous CVD and repeated the analysis in the entire cohort.
Results: During a 5.67 years follow-up period, patients with T2DM diagnosed at ≤40 years had the highest excess risk for most outcomes relative to controls with adjusted hazard ratio (HR) (95% CI) of 6.08 (5.50-6.72) for total mortality, 5.53 (4.29-7.14) for CVD-related mortality, 7.19 (6.72-7.70) for HHF, and 5.10 (4.91-5.30) for CHD. All risks attenuated progressively with each increasing decade at diagnostic age but remained significant; by the time T2DM was diagnosed at ≥91 years, the adjusted HR (95% CI) of 1.29 (1.21-1.37) for total mortality, 1.32 (1.15-1.51) for CVD-related mortality, 2.95 (2.54-3.42) for HHF, and 3.66 (3.16-4.24) for CHD.
Conclusion: In this population-based cohort study, younger age at diagnosis of T2DM was associated with a higher risk of mortality and CVD outcomes especially in those without previous CVD. Therefore, awareness should be raised to prevent or delay T2DM in younger individuals.
Supported by: INHA UNIVERSITY Research Grant
Disclosure: D. Seo: None.
834
The added value of ECG abnormalities in predicting incident cardiovascular disease risk for people with type 2 diabetes: the Hoorn Diabetes Care System cohort
P.P. Harms1,2, R.A.R. Herings1,3, S.J.G. Welten1,2, S. Remmelzwaal4,2, F. Rutters4,2, J.W.J. Beulens4,3, G. Nijpels1,2, P.P.J. Elders1,2, M.T. Blom1,2;
1Department of General Practice Medicine, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, 2Health behaviors & chronic diseases, Amsterdam Public Health research institute, Amsterdam, 3Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, 4Department of Epidemiology and Data Science, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, Netherlands.
Background and aims: Cardiovascular disease (CVD) is an important complication and cause of mortality in people with type 2 diabetes. Guidelines recommend the use of prediction models to estimate individualised risk of CVD and help clinicians make informed personalised treatment decisions. However, prediction models currently recommended by various international guidelines perform only moderately in people with type 2 diabetes, with c-statistics for discrimination typically around or below 0.7 (theoretical range: 0.5-1.0). ECG abnormalities are good candidates for improving prediction because they are associated with incident CVD, are common in people with type 2 diabetes without pre-existent CVD, and might be part of the pathophysiology. We aimed to investigate if adding ECG abnormalities as a predictor improves performance of currently recommended incident CVD prediction models for people with type 2 diabetes.
Materials and methods: We evaluated the ASCVD, AD-ON, and ADVANCE Cox-prediction models that are recommended for use by the guidelines of the American College of Cardiology/American Heart Association (ACC/AHA), the European Society of Cardiology (ESC), and the Dutch Society of General Practitioners (NHG), respectively, in 11,277 people with type 2 diabetes without CVD (CHD, heart failure, stroke, thrombosis) from the dynamic Hoorn Diabetes Care System cohort (1998-2018). Baseline measurements, taken at entry into the cohort, included CVD risk factors and ECG abnormalites coded according to the Minnesota Classification as minor or major. Annual follow-up for CVD continued until 2018. After calibration to ensure good model fit, performance of the prediction models was assesed with and without the addition of ECG abnormalities, and compared using c-statistics, net reclassification indeces (NRIs), and the integrated discrimination index (IDI). Prediction horizons and categoric NRI cut-off values were adopted from the guidelines.
Results: For all three models, c-statistics and classification or estimation of people their individual CVD risk improved with ECG abnormalites as an additional predictor (Table1).
Conclusion: The addition of ECG abnormalities to incident CVD risk prediction models consistently improves model performance and the ability of models to correctly classify people with type 2 diabetes in the appropriate CVD risk category.
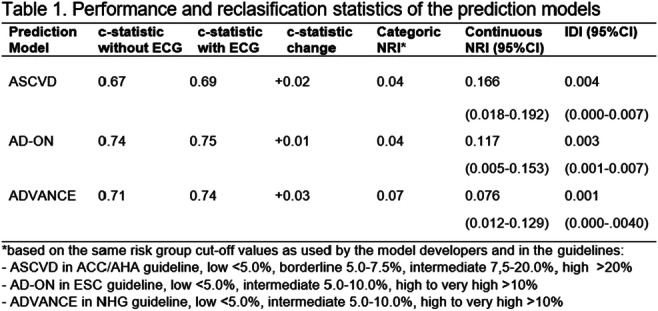
Supported by: EFSD European Pilot Research Grants for Innovative Measurement of Diabetes Outcomes
Disclosure: P.P. Harms: None.
835
Cardiovascular risk factor control in patients with type 2 diabetes and coronary artery disease versus stroke
P. Balasubramanian1, W. Kernan2, K.N. Sheth3, A.P. Ofstad4, M. Mattheus5, N. Marx6, S.E. Inzucchi1;
1Section of Endocrinology, Yale School of Medicine, New Haven, USA, 2Department of Medicine, Yale School of Medicine, New Haven, USA, 3Department of Neurology, Yale School of Medicine, New Haven, USA, 4Boehringer Ingelheim Norway KS, Asker, Norway, 5Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany, 6Department of Internal Medicine, University Hospital RWTH Aachen, Aachen, Germany.
Background and aims: Stroke and coronary artery disease (CAD) share several cardiovascular (CV) risk factors, including type 2 diabetes. Risk factor control is vital for secondary prevention of both conditions, but systems for care of patients after stroke or CAD events differ, and distinctions in care for these patients have not been examined. The aim of this study was to compare CV risk factor control in patients with CAD versus stroke at baseline in three recent large CV outcome trials (CVOTs) involving 11,622 patients with type 2 diabetes at high CV risk.
Materials and methods: We analysed baseline data from the (1) EMPA-REG OUTCOME, (2) CAROLINA, and (3) CARMELINA trials. Risk factors assessed included dyslipidaemia, hypertension, smoking, and use of anti-platelet/anti-coagulant drugs. Risk factor control was defined as (a) low-density lipoprotein cholesterol <100 mg/dL or statin use, (b) systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg, (c) use of anti-platelet/anti-coagulant drugs, and (d) not smoking. The quality of CV risk factor goal attainment was defined as the odds of having 3-4 versus 0-2 risk factors controlled. We calculated the odds ratio (OR) across macrovascular disease groups at baseline and by subgroups of age, sex, and region in the trials, based on logistic regression.
Results: The ORs (95% confidence intervals [CIs]) for CV risk factor control in patients with CAD alone were higher than in patients with stroke alone across all three trials: (1) 2.60 (2.19-3.08), (2) 1.59 (1.18-2.15), and (3) 2.20 (1.81-2.67), respectively. The respective ORs (95% CIs) were lower (and rendered non-significant in CAROLINA) when risk factor control in patients with both CAD and stroke was compared to patients with stroke alone: (1) 2.00 (1.52-2.64), (2) 1.13 (0.72-1.79), and (3) 1.42 (1.08-1.86). Results were consistent across all subgroups.
Conclusion: We found significant disparities in CV risk factor control based on history of CAD and/or stroke in three contemporary CVOTs in patients with type 2 diabetes, with less optimal control in stroke patients, particularly in those with stroke alone. The intermediate results in patients with both CAD and stroke suggest a role for clinician factors. These findings require further study to understand their origins but emphasise the need for improved secondary prevention strategies in patients with stroke.
Clinical Trial Registration Number: EMPA-REG OUTCOME (NCT01131676), CAROLINA (NCT01243424), CARMELINA (NCT01897532)
Supported by: Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance
Disclosure: P. Balasubramanian: None.
836
Deviations from the ADA/EASD treatment algorithm is associated with higher cardiovascular events and death in type 2 diabetes under routine care
M.L. Morieri, E. Longato, B. di Camillo, G. Sparacino, A. Avogaro, G.P. Fadini;
University of Padova, Padova, Italy.
Background and aims: Since current treatment algorithms are only partially evidence-based, we examined whether deviations from therapeutic recommendations for the management of T2D has an impact on long-term outcomes.
Materials and methods: This study was conducted on an outpatient clinical-administrative database in years 2008-2018. We defined 6 domains of inadequate treatment prescription (ITP) based on the 2018 ADA/EASD algorithm: first-line and second-line treatment, intensification, use of insulin, cardioprotective, and weight-affecting drugs. Patients were divided according to presence/absence of ITP at each visit. Study outcomes were major adverse CVD events (MACE) and death. We used Cox proportional hazard models with time-dependent covariates or Cox marginal structural models (Cox MSMs).
Results: We included 5419 patients, on average 66-year old, 41% women, with a diabetes duration of 7.6 years and only 11.7% had pre-existing CVD. At study entry, 57.3% of patients had ITP in at least one domain. By the end of the study (after a median of 7.3 years and 12 visits/patient), 85.8% of subjects had at least one visit with ITP in one or more domains. Overall 1325 MACE occurred (in 1117 subjects [20.6%]), and 917 deaths were observed. As shown in figure 1, in the fully adjusted model, being always on ITP was associated with an 81% relative higher risk of MACE as compared to being always with appropriate treatment. The association was confirmed with Cox MSMs (weighted for the same covariates used in model 3) where ITP was associated with a 57% relative higher risk of MACE (HR 1.57; 95% C.I. 1.39-1.78; p<0.0001). Cumulative ITP was associated with a corresponding 79% higher mortality risk (confirmed also with Cox MSMs - HR: 1.59: 95% C.I. 1.37-1.85; p<0.0001). No individual domain or combination of two explained all the prognostic effect of ITP, which remained significant in all subgroups analyses when population was stratified by key baseline characteristics.
Conclusion: Deviations from internationally recommended standards in the management of T2D is associated with higher risk of MACE and premature mortality. These data provide a validation of the current treatment algorithm. Figure 1 Legend: Estimated impact on MACE (A) and death (B) of cumulative ITP time (i.e., the proportion of time being on ITP up to each visit, ranging from 0 to 1) according to multivariable-adjusted models (Model 1: age, sex, study entry year, diabetes duration, and time-varying covariates: CVD history, kidney disease, macro- and microvascular disease. Model 2: Model 1 with the addition of: line of treatment, latest BMI and HbA1c, other non-diabetic medications, presence of obesity, and presence of HbA1c levels > 10%. Model 3: Model 2 with the addition of: history of cancer, chronic pulmonary disease, systemic inflammatory disease, and hepatic steatosis.
Supported by: Italian Society of Diabetes
Disclosure: M.L. Morieri: None.
837
High levels of physical activity are associated with a reduced risk of CVD regardless of sedentary time among type 2 diabetes patients
Y. Wu1, W. Wang2, J. Zhao1, The MIDiab Study Group;
1Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, 2National Center for Chronic and Noncommunicable Disease Control and Prevention, Beijing, China.
Background and aims: With the rapid development of technology and social economy level, people’s lifestyles are changing. It was estimated that there were 451 million diabetic patients worldwide in 2017, and this number was expected to reach 693 million by 2045. The increasing prevalence of diabetes will inevitably lead to an increase in the proportion of complications of diabetes, especially cardiovascular disease (CVD). Previous studies have illustrated that both a higher level of physical activity and decreased sedentary (SED) behavior were associated with a lower risk of CVD. The aim of this study was to explore the associations of moderate-to-vigorous intensity physical activity (MVPA) time and SED time with multifactorial control status and CVD risk among type 2 diabetes mellitus (T2DM) patients in China.
Materials and methods: A cross-sectional analysis of 9152 diabetic patients from the Multifactorial Intervention on Type 2 Diabetes (MIDiab) Study was performed. These patients covered a total of 223 hospitals and were grouped according to their self-reported MVPA time (low, <150 min/w; moderate, 150-450 min/w; high, ≥450 min/w) and SED time (low, <4 h/d; moderate, 4-8 h/d; high, ≥8 h/d). Multifactorial control status involved blood pressure (BP), body mass index (BMI), low-density lipoprotein cholesterol (LDL-C) and HbA1c. Multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for multifactorial control status and CVD risk.
Results: Participants had a mean ± SD age of 60.87±8.44 years and 25.1% had CVD. The prevalence of CVD decreased with the increase in MVPA time. With the increase in MVPA time, the multifactor control rate showed a positive trend. After adjustment for potential confounding factors, an inverse association between a high physical activity level and CVD risk that was independent of SED time was found, whereas this association was not observed in the moderate-MVPA group. Compared with the referent group (i.e., those with a SED time <4 h/d and MVPA time ≥450 min/week), CVD risk was higher in the low-MVPA group than in the other groups; the OR associated with a SED time <4 h/d was 1.344 (95% CI 1.100-1.624), with a SED time of 4 to <8 h/d was 1.280 (95% CI 1.039-1.578), and with a SED time ≥8 h/d was 1.554 (95% CI 1.193-2.025), full details were shown in the attached table.
Conclusion: In conclusion, high levels of physical activity (i.e., more than 450 min/week MVPA) were associated with a reduced risk of CVD regardless of SED time. Meanwhile, this high physical level seemed to eliminate the increased risk of CVD associated with high SED time in patients with T2DM. These results may provide practical and relatively detailed lifestyle intervention for T2DM patients, thus further improving the multifactor control rate, delaying the occurrence and progress of CVD and relieving the disease burden.
Clinical Trial Registration Number: NCT03430284
Supported by: The National Key Research and Development Program of China (2017YFC1309800)
Disclosure: Y. Wu: None.
SO 75 Emerging comorbidities in diabetes: clinical associations and mechanisms
838
Anti-fibrotic potential of a novel long-acting Glucagon/GIP/GLP-1 triple agonist (HM15211) in preclinical models of idiopathic pulmonary fibrosis
S. Lee, J. Kim, J. Lee, J. Kim, Y.-H. Ban, J. Lee, S. Bae, D. Kim, S. Lee, I. Choi;
Hanmi Phaarm. Co., Ltd, Seoul, Republic of Korea.
Background and aims: Idiopathic pulmonary fibrosis (IPF) is an irreversible and progressive pulmonary disorder of unknown causes in which scarring of lung tissue eventually leads to death. A large number of studies have demonstrated that the core mechanisms of fibrosis across various organs are similar. Previously, excellent preclinical efficacy of HM15211, a novel long-acting Glucagon/GIP/GLP-1 triple agonist, on liver fibrosis was confirmed. Since the major distributed tissues of HM15211 were found to be in liver and lung, such findings prompted us to hypothesize that HM15211 could be also a promising drug candidate for IPF. In the present study, its effect on pulmonary function and mortality was evaluated in bleomycin (BLM)-induced mouse model of IPF.
Materials and methods: To investigate the therapeutic effect of HM15211 on IPF-induced pulmonary function impairment as well as mortality, BLM induced mouse model of lung fibrosis was established. Briefly, at day 0, 1.5 U/kg and 2.5 U/kg of BLM were intratracheally injected to impair pulmonary function and to increase mortality, respectively. HM15211 treatment was initiated from day 7, and continued until day 14 ~ day 21, followed by monitoring the saturation of peripheral oxygen (SpO2) and survival rate. To highlight the therapeutic benefits of HM15211, pirfenidone (PIRF) and nintedanib (NINT) treated groups were included as comparative control.
Results: In 1.5 U/kg BLM mice, markedly impaired lung function was observed at day 7 as indicated by SpO2 (84.0 vs. 95.6% for normal), which was further exacerbated until day 14 (77.1% vs. 96.0% for normal). Interestingly, while PIRF (81.9%) and NINT (81.3%), FDA-approved drugs for IPF management, prevented additional lung function impairment without statistical significance, HM15211 treatment significantly restored the impaired lung function close to normal level at day 14 (91.5%, p<0.001 vs. BLM, vehicle), demonstrating HM15211’s disease modifying potential. In addition, at 21 days after 2.5 U/kg BLM treatment, substantial decline in survival rate (17%) was effectively inhibited by HM15211, NINT, and PIRF. Notably, HM15211 (61%, p<0.05 vs. BLM, vehicle) showed improved survival rate compared to NINT (33%, no significant) and PIRF (28%, no significant), suggesting potential superiority of HM15211 over current IPF drugs.
Conclusion: HM15211 could possess therapeutic potential for IPF with improved treatment effects over current standard of care. On-going mechanistic studies will elucidate the underlying mode of actions, and human study should be followed up (required) to assess the clinical relevance of these findings.
Disclosure: S. Lee: None.
839
Disruption of the intestinal barrier is associated with markers of kidney injury in DSS-induced colitis in mice
H. Salmenkari1,2, K. Adeshara1,2, A. Pirttiniemi1,2, J. Lindén3, S. Lehtonen4,2, P.-H. Groop1,5, M. Lehto1,5;
1Folkhälsan Institute of Genetics, Folkhälsan Research Center, 2Research Program for Clinical and Molecular Metabolism, University of Helsinki, 3Department of Basic Veterinary Sciences, HiLIFE, University of Helsinki, 4Department of Pathology, University of Helsinki, 5Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Background and aims: Intestinal permeability is increased in individuals with diabetes, and this can lead to an increased systemic load of bacterial toxins. On the other hand, increased rate of bacterial infections and use of antibiotics are associated with the progression of microvascular complications, such as diabetic kidney disease. Our aim at the FinnDiane study group was to study how a compromised intestinal barrier may contribute to the development of kidney damage.
Materials and methods: To induce colitis and translocation of bacteria and bacterial products, 7-week-old male BALB/c mice (n=6) were treated with 5% dextran sodium sulfate (DSS) in the drinking water over 7 days followed by four days of fresh water. Healthy mice (n=6) were used as controls. Colitis progression was measured as a change in body weight, colon shortening, and disease scoring by combining stool consistency, colonic bleeding, and colonic edema. Kidney damage was assessed by measuring kidney weight, albuminuria by ELISA, kidney inflammatory infiltration by using immunofluorescence staining for CD45, and RT-qPCR analysis of the proinflammatory cytokines, interleukin 1 beta (Il-1β) and tumor necrosis factor-alpha (Tnfa), tissue damage markers kidney injury molecule -1 (Kim-1) and lipocalin 2/Neutrophil gelatinase-associated lipocalin (Lcn2/Ngal) as well as kininogen 2 (Kng2), encoding a propeptide participating in coagulation and inflammation pathways.
Results: The mice in the colitis group lost an average of 19.8% of their body weight while the control mice gained 2.0%. The colon lengths were shorter in the colitis group than in the healthy mice (5.2 ± 0.8 vs 7.5 ± 0.6 cm, p = 0.0002) and the disease scores were increased (6.5 (3-9) vs 0, p = 0.005). Colitis led to a reduction in kidney weights (0.27 ± 0.02 vs 0.32 ± 0.03 g, p = 0.0048) and an increase in albuminuria (153 ± 72 vs 58 ± 13 mg/l, p = 0.016), when compared with healthy controls. CD45-positive cells, indicative of inflammatory infiltration, were observed around individual distal tubules in the colitis group. Transcriptomic levels of kidney tissue injury markers Lcn2 (8.65-fold, p = 0.006) and Kim-1 (2.02-fold, p = 0.006), as well as the pro-inflammatory cytokine Il-1β (2.05-fold, p = 0.006) were increased in the colitis group, while Tnfa transcription was unchanged. Colitis also caused a small but significant induction of Kng2 mRNA (1.4-fold, p = 0.013) expression.
Conclusion: Intestinal barrier disruption leads to inflammatory responses in the kidneys and an increase in clinically relevant markers of kidney injury, including albuminuria and induction of Kim-1 and Lcn2. This implies that a defective gut barrier function followed by translocation of bacterial products may lead to kidney insults, potentially predisposing individuals to the development and progression of renal damage, such as diabetic kidney disease.
Supported by: Diabetes Research Foundation, Päivikki and Sakari Sohlberg Foundation, Munuaissäätiö
Disclosure: H. Salmenkari: None.
840
Sodium/glucose co-transporter-2 inhibitors and the risk of gout in patients with type 2 diabetes: a propensity score matched, prevalent-new user design study
A. Subramanian, C. Sainsbury, K. Gokhale, K. Nirantharakumar, K. Toulis;
Institute of Applied Health Research,, University of Birmingham, Birmingham, UK.
Background and aims: SGLT2i-induced glycosuria may induce changes in renal handling of urate. The putative effect of SGLT2i on uric acid levels and thus the incidence of gout is of clinical interest in the context of the prevalence of hyperuricaemia and gout in patients with type 2 diabetes mellitus. However, evidence from studies remain inconclusive. To address this discrepancy, we undertook an observational study to explore the incidence of gout in patients with T2DM who are treated with SGLT2i.
Materials and methods: A retrospective open cohort study in IQVIA Medical Research Data - UK was performed estimating the risk of gout in patients with T2DM who were new users of SGLT2i and comparing this to the risk in propensity score (PS)-matched patients who were new users of DPP4i (1st Nov 2012 to 31st Dec 2018). Propensity scores (PS) were estimated from logistic regression model using an extended list of clinical, laboratory and demographic covariates. Proportional hazards were used to estimate the hazard ratios (HRs) and 95% CIs of the outcome (gout), using both intention to treat and at treatment analysis.
Results: A total of 99 incident cases of gout were recorded over 30,577 person-years of observation in 13,617 new users of SGLT2i and 29,426 new users of DPP4i. Crude incidence rates (IR) per 1000 person-years were found to be 3.31 and 3.16 for SGLT2i and DPP4i new users respectively. Unadjusted hazard ratio (HR) was 1.05 [95% Confidence Interval (CI): 0.70-1.55]. After further adjustment for all covariates used in the PS model, the aHR was 1.11 (0.74-1.67). In the at-treatment analysis, crude IRs per 1000 person-years were found to be 2.93 and 3.24 for SGLT2i and DPP4i users respectively. In the fully adjusted model, the adjusted HR was 0.95 (95% CI: 0.58-1.55].
Conclusion: In this nation-wide study, no significant difference in the incidence of gout was documented in patients with diabetes treated with SGLT2i compared to DPP4i users after propensity-score matching. This neutral finding was consistent in both intention-to treat and at-treatment analyses.
Supported by: MN
Disclosure: A. Subramanian: None.
841
Reactivity to angiotensin II and structural changes of diabetic gut in an early model STZ-induced diabetes
M. Esteves-Monteiro1,2, M. Ferreira-Duarte3,2, D. Menezes-Pinto3, P. Dias-Pereira4, M. Morato3,2, M. Duarte-Araújo1,2;
1Department of Immune-Physiology and Pharmacology, Institute of Biomedical Sciences Abel Salazar, University of Porto(ICBAS-UP), 2LAQV@REQUIMTE, 3Laboratory of Pharmacology, Department of Drug Sciences, FFUP, 4Department of Pathology and Molecular Immunology, Institute of Biomedical Sciences Abel Salazar, University of Porto(ICBAS-UP), Porto, Portugal.
Background and aims: Diabetes mellitus (DM) is associated with gastrointestinal complications that negatively impact the health status and quality of life of approximately 75% of patients. These complications are often ignored and the time-course and underlying pathophysiological mechanisms are still poorly characterized. Angiotensin II (AngII) is crucial for cardiovascular function but also has relevant gastrointestinal effects by activating AT1 and AT2 receptors. So, we aimed at evaluating the existence of early structural and functional changes in the streptozotocin (STZ)-induced rat model of DM.
Materials and methods: The project was approved by the local animal welfare body (ORBEA ICBAS-UP) and followed the ARRIVE guidelines for reporting animal experiments. A single intraperitoneal injection of STZ (55 mg/kg) was administered to adult male Wistar rats to induce DM. After 14 days, rats were euthanized and 1cm-long segments of the ileum, proximal (PC), middle (MC) and distal (DC) colon were used for macroscopic and histological analysis, or mounted longitudinally in isolated organ baths. In each segment, we performed: a cumulative concentration-response curve to acetylcholine (ACh); a non-cumulative concentration-response curve to AngII; the response to a single concentration of AngII in the absence and presence of Candesartan (10nM, AT1 antagonist) or PD123319 (100nM, AT2 antagonist). The contractile response to potassium chloride (KCl, 125mM) was recorded after every protocol. Statistical analysis was performed by paired or unpaired Student’s t test or Mann-Whitney U test. Results were expressed as median[IQR]; p<0.05 was considered statistically significant.
Results: STZ-induced rats showed hyperglycemia (>500 mg/dL vs 98 mg/dL in controls). Macroscopically, the colon had an increased length (25.75±0.77 cm vs 19.63±0.47 cm) and circumferential perimeter (15.45±0.58 mm vs 11.00±0.46 mm) compared with controls (p<0.05 for all). The ileum had higher circumferential perimeter (12.55±0.31 mm vs 9.38±0.32 mm) and weight (in g/g, 2.69±0.10 g/g vs 1.80±0.05 g/g) than controls (p<0.001 for both). The intestinal wall was thicker in STZ-induced animals than in controls (Ileum 166%; PC 171.27%; MC 156.63%; DC 132.64%, p<0.05). The contractile responses to ACh and KCl were similar, but the contractile response to AngII was lower in the ileum (305.3[138.6- 620.5] mN/g vs 71.20[12.3 -100.6] mN/g), PC (50.46 [15.32-78.15] mN/g vs 181.5[136.0-297.0] mN/g) and MC (100.6[22.86-163.5] mN/g vs 276.6[246.4-451.1] mN/g) of diabetic rats when compared to controls (p<0.05 for all). The response to AngII was always abolished by Candesartan. PD123319 increased AngII response in the colon of controls but did not alter AngII-induced contraction in the ileum, MC and DC of diabetic rats. PD123319 decreased the contractile response in the control ileum and diabetic PC.
Conclusion: Our study indicates that early STZ-induced DM is already associated with structural remodeling of the gut wall and decreased contractile response to AngII.
Supported by: UIDB-50006/2020 and 2020.06502.BD-FCT
Disclosure: M. Esteves-Monteiro: None.
842
Increased glycaemic variability evaluated by continuous glucose monitoring is associated with osteoporosis in type 2 diabetes patients
R. Huang, H. Wang, Z. Shen, T. Cai, Y. Zhou, Y. Wang, J. Wu, J. Ma;
Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China.
Background and aims: Subjects with type 2 diabetes mellitus (T2DM) are susceptible to osteoporosis. This study was conducted to evaluate the association between glycemic variability evaluated by continuous glucose monitoring (CGM) and osteoporosis in type 2 diabetic patient.
Materials and methods: A total of 362 type 2 diabetic subjects who underwent bone mineral density (BMD) measurement and were monitored by a CGM system from Jan 2019 to May 2020 were enrolled in this cross-sectional study. Glycemic variability was calculated with the Easy GV software, including 24-hour mean blood glucose (24-h MBG), the standard deviation of 24-h MBG (SDBG), coefficient of variation (CV), mean amplitude of glycemic excursions (MAGE), and time in range between 3.9 and 10.0 mmol/L (TIR). Other potential influence factors for osteoporosis were also examined.
Results: Based on the T-scores of BMD measurement, there were 190 patients with normal bone mass, 132 patients with osteopenia and 40 patients with osteoporosis. T2DM patients with osteoporosis showed a higher 24-h MBG, SDBG, CV, and MAGE, but a lower TIR (all p < 0.05). Multivariate logistic regression analysis revealed that age, female, body mass index (BMI), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA) and MAGE independently contribute to osteoporosis, and corresponding odds ratio (95% confidence interval (CI)) was 1.129 (1.072-1.190), 4.215 (1.613-11.012), 0.801 (0.712-0.901), 2.743 (1.385-5.431), 0.993 (0.988-0.999), and 1.380 (1.026-1.857), respectively. Further receiver operating characteristic analysis indicated that the area under the curve and its 95% CI were 0.673 and 0.604-0.742, with the optimal cut-off value of MAGE predicting osteoporosis being 4.31 mmol/L.
Conclusion: In addition to conventional influence factors including age, female, BMI, LDL-C and SUA, increased glycemic variability assessed by MAGE is associated with osteoporosis in type 2 diabetic patients.
Supported by: NKRDP (No. 2018YFC1314100)
Disclosure: R. Huang: None.
843
Increased Fibrosis-4 (Fib-4) score reveals low bone mineralisation in obese individuals with or without type 2 diabetes
I. Barchetta, F.A. Cimini, S. Dule, G. Passarella, A. Dellanno, A. Di Biasio, G. Silecchia, F. Leonetti, M.G. Cavallo;
Sapienza University of Rome, Rome, Italy.
Background and aims: Chronic liver diseases are associated with an increased risk of bone fractures, mostly in presence of end-stage disease. Less consistent evidence is available on the relationship between non-alcoholic fatty liver disease (NAFLD) and increased bone fragility, and data on the association between liver fibrosis, estimated through markers as the Fibrosis-4 (FIB-4) score, and bone mineralization in dysmetabolic individuals are limited. Aim of this study was to investigate the association between parameters of bone mineralization and metabolism, presence of NAFLD and estimated liver fibrosis in individuals with obesity, with or without type 2 diabetes (T2D).
Materials and methods: For this cross-sectional investigation, we recruited 100 severely obese individuals referring the our outpatient clinics of Sapienza University, Rome, Italy. Patients underwent clinical work-up, blood sampling for metabolic profiling, liver US-scan and DXA examination; a subgroup of eighteen patients underwent liver biopsy as for clinical indication. Markers of bone turnover (osteopontin (OPN), osteoprotegerin (OPG), osteocalcin, sclerostin) were measured in plasma by Milliplex, Multiplex.
Results: In our population (female%: 75%, mean±SD age: 46±10.6 years, BMI: 42.6±5.6 kg/m2; 50% with abnormal glucose metabolism (AGM): type 2 diabetes: 20% + impaired fasting glucose: 30%) NAFLD subjects had lower T-score than no-NAFLD (p= 0.04). Reduced T-score at the DXA was associated with increased FIB-4 (r= -0.39, p= 0.01), and presence of AGM (r= -0.39, p= 0.03). Moreover, FIB-4 linearly correlated with circulating OPG levels (r= 0.36, p= 0.004).At the multivariate regression analysis, the FIB-4 predicted lower T-score independently of sex, BMI, presence of AGM, US-assessed fatty liver and smoking status (β standardized= -0.41; p= 0.02).The association between reduced T-score and NAFLD was confirmed in the subgroup of subjects undergoing liver biopsy, where the T-score progressively decreased with NAFLD severity (p< 0.05).
Conclusion: These data indicate the presence of a tight relation between lower bone mineral density and signs of liver fibrosis and NAFLD, suggesting common mechanistic pathways underlying impaired bone homeostasis and the hepatic involvement in insulin-resistance.
Supported by: Fondazione Lilly Italia
Disclosure: I. Barchetta: None.
844
Prognosis in men and women with atrial fibrillation without and with type 1 and type 2 diabetes: a nationwide propensity-matched cohort study
S. Karayiannides1,2, P. Lundman1,3, L. Landstedt-Hallin1, L. Friberg1, A. Norhammar4,5;
1Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet (KI), 2Center for diabetes, Academic Specialist Centrum, 3Department of Cardiology, Danderyd University Hospital, 4Cardiology Unit, Department of Medicine K2, Karolinska Institutet (KI), 5Department of Clinical Physiology, Capio S:t Görans Hospital, Stockholm, Sweden.
Background and aims: There are sex-specific differences in epidemiology, clinical presentation and prognosis in patients with atrial fibrillation (AF). Our aim was to describe the impact of diabetes mellitus type 1 (T1DM) and diabetes mellitus type 2 (T2DM) on the sex-specific prognosis in patients with AF and describe differences in pharmacotherapy between men and women.
Materials and methods: In national registries, we identified all patients with non-valvular AF in Sweden (n= 309 611) during 2013-2014. Using propensity score matching based on age and comorbidities, in total 12 variables including all the components of CHA2DS2-VASc score, we matched 90 222 men and 90 222 women with AF without diabetes, 757 men and 757 women with AF and T1DM and 20 034 men and 20 034 women with AF and T2DM. Cox regression was used in the matched cohorts to calculate the hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality and competing risk regression for myocardial infarction, ischaemic stroke and first-ever diagnosis of heart failure and dementia with all-cause mortality as competing risk in women without diabetes, T1DM and T2DM, using men in the respective group as reference. The follow-up time for mortality was until 27 March 2017 and for the other endpoints until 31 December 2015.
Results: After matching, the median age for patients without diabetes was 77 years (69-84), for patients with T1DM 72 (65-78) and for T2DM 78 (72-84) years. Women were less often treated with warfarin than men in both the cohort without diabetes (35.7 vs. 39.8%, p<0.001), the cohort with T1DM (30.4 vs. 41.6%, p<0.001) and the cohort with T2DM (43.6 vs. 47.1%, p<0.001). Among those without diabetes, women had a better prognosis than men (Figure) regarding all-cause mortality [HR;95% CI: 0.89 (0.88-0.91)], heart failure [0.93 (0.90-0.96)] and myocardial infarction [0.83 (0.79-0.88)] but worse prognosis regarding stroke [1.12 (1.07-1.16)]. Among those with T1DM, women had worse prognosis than men regarding heart failure [1.42 (1.09-1.86)] and myocardial infarction [1.50 (1.09-2.06)]. Among those with T2DM, women had worse prognosis than men for stroke [1.14 (1.06-1.23)] and similar risks for the other events. The risk for dementia was similar regardless of sex, presence or type of diabetes with a trend (non-significant) for lower risk in women with T1DM.
Conclusion: Women with AF without diabetes had generally lower risk than men for death and other events except for stroke risk which was increased. In the presence of diabetes, regardless of type, this apparent advantage of women over men was lost.
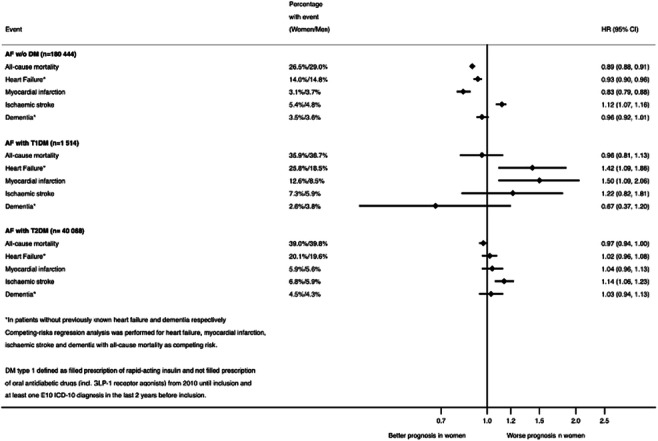
Disclosure: S. Karayiannides: None.
845
Insulin resistence in obstructive sleep apnea: Is there a role for mitochondrial dysfunction in the liver?
J.L. Fernandes1, F.O. Martins1, B.F. Melo1, E. Olea2, J. Prietro-Lloret3, A. Obeso3, A. Rocher3, J.F. Sacramento1, S.V. Conde1;
1Neuronal Control of Metabolic Diseases: Therapeutic Strategies, CEDOC - Centro de Estudos de Doenças Crónicas, Lisboa, Portugal, 2Departamento de Enfermeria, Universidad de Valladolid, Valladolid, Spain, 3Departamento de Bioquimica, Biologia Molecular y Fisiologia, Universidad de Valladolid, Valladolid, Spain.
Background and aims: The relationship between obstructive sleep apnea (OSA) and metabolic diseases is unequivocal, however the mechanisms behind this relationship are not consensual. Mitochondrial dysfunction and oxidative stress in the liver play a major role in the development of metabolic diseases. Chronic intermittent hypoxia (CIH), one of the key features of OSA, induces whole-body metabolic dysfunction with an increase in Glut2 in the liver and lipid peroxidation and ROS production leading to liver inflammation. We hypothesize that mitochondrial dysfunction and oxidative stress in the liver play a role in the development of CIH-induced dysmetabolism.
Materials and methods: Male Wistar Rats were divided into 2 groups: control group (NC) and obese group (HF) fed with 60% lipid-rich diet for 12 weeks. Half of the groups were submitted to a CIH paradigm (40s, 5%O2 /80s air, 8h/day) for 14 days. Liver samples were evaluated by western blot for the levels of oxidative phosphorylation complexes (OXPHOS) and proteins involved in inflammation, hypoxia, glucose homeostasis, insulin signalling and oxidative stress. Hepatic histological and immunohistochemical analyses were performed to evaluate lipid deposition, fibrosis, angiogenesis, mitochondrial density, hypoxia, and macrophages markers. Energy status of the cell was assessed by measuring nucleotide levels by HPLC.
Results: CIH promoted insulin resistance and glucose intolerance and exacerbated metabolic dysfunction and hepatic lipid deposition in HF animals. HF diet increased insulin receptor levels by 55.3% (p<0.01), and CIH and HF diet showed a tendency to decrease the levels of insulin degrading enzyme. Glut 2 levels increased by 29.60% (p<0.05) and 46.10% (p<0.05) in HF and HFIH groups. No significant alterations in HIF-1α were shown, but HIF-2α decreased by 29.47%(p<0.01) in HF group. HF diet promoted a 12.78% reduction in mitochondrial density (p<0.01), which was reversed by hypoxia. CIH decreased OXPHOS complexes I, II, III, IV and V levels by 61.33% (p<0.05), 60.75% (p<0.05), 47.73%, 59.42% (p<0.05), 64.00%(p<0.01) and by 27.17%, 53.77% (p=0.0530), 73.27% (p=0.0521), 34.50%, 64.57% (p<0.05), respectively in NC animals and HF animals. HF diet increased levels of complex I by 105.7% (p<0.01) and showed a tendency to increase complexes II and IV. HF diet increased catalase levels by 86.30% (p<0.01) and decreased Superoxide dismutase 1 (SOD-1) by 30.44% (p<0.05). In contrast, CIH decreased the levels of SOD-1 by 62.52% (p<0.001) but increased the levels of catalase by 35.4% (p<0.01) in NC animals without changes in HF group. TNF-α expression was increased in HF group by 53.00% (p<0.05) without alterations with CIH.
Conclusion: Mitochondrial dysfunction and hepatic oxidative stress may contribute to metabolic dysfunction associated with CIH, justifying the relationship between insulin resistance, hepatic steatosis and OSA. Targeting these mechanisms might be a therapeutic approach to prevent/treat metabolic dysfunction in sleep apnea.
Supported by: CEECIND/04266/2017,FOM;CEECIND/02428/2018,JFS.BFU2015-70616-R (MINECO/FEDER; DGICYfT);VA106G18 (JCyL
Disclosure: J.L. Fernandes: None.
846
The impact of obesity and diabetes chronic complications in COVID 19 patients
A. Andrita1, A.-M. Militaru1, A. Nica2, E. Rusu2, C. Dobjanschi1, G. Radulian2;
1Department of Diabetes, Nutrition and metabolic diseases, ”Nicolae Malaxa” Clinical Hospital, 2Diabetes, Nutrition and metabolic diseases, "Carol Davila" University of Medicine and Pharmacy, Bucharest, Romania.
Background and aims: Obesity, chronic micro and macrovascular complications were observed to be risk factors for COVID 19 poorer outcome in patients with diabetes mellitus. The aim of the study is to identify possible correlations between these factors and the severity of the viral infection.
Materials and methods: We included 237 hospitalized patients with diabetes mellitus and COVID 19, which were divided according to obesity, diabetes complications and hypoglycemic treatment. Inflamatory syndrome and prothrombotic risk parameters, COVID 19 severity, period of hospitalization and the need for oxygen therapy were followed. The data were analyzed using IBM SPSS statistics software for Windows, Version 28.0.
Results: 55,08% (n = 130) of the patients enrolled in the study were women and 44,98 % (n = 106) were men. The average age was 64,89 ±13,75 years and the mean duration of diabetes evolution was 10,14 ±9,29 years. Out of them 44,05% (n = 100) were obese, 55,51 % (n = 127) were Metformin-treated patients and 34,20% (n = 79) were receiving insulin therapy. 21,94 % (n = 52) had microvascular complications and 38,55% (n = 97) had macrovascular complications. Average length of stay in hospital was 15,18 ±11,24 days and 76,5% (n =179) of patients required oxygen therapy. C-reactive protein was statistically significant associated with oxygen therapy (p = 0,049). C-reactive protein value was not statistically significantly higher in obese patients (p = 0.12). We also identified statistically significant correlations between D-dimer (p = 0.004), procalcitonin (p = 0.002), erythrocyte sedimentation rate (p = 0,029) and obesity. Furthermore, correlations between D-dimer (p=0.012), procalcitonin (p = 0.049) and the presence of macrovascular complications were highlighted. Acute myocardial infarction (p = 0.006) and stroke (p <0.001) were correlated with length of stay in hospital. Microvascular complications were correlated with elevated ferritin values (p = 0.011). Ferritin was statistically significantly lower in insulin-treated patients (p = 0.018). On the other hand, COVID 19 severity was not significantly correlated with hypoglycemic treatment involving neither metformin (p = 0.433) or insulin (p = 0.747) nor with the presence of micro (p = 0.331) or macrovascular complications (p = 0.229).
Conclusion: The results suggest that the inflammatory syndrome due to COVID 19 infection is correlated with both weight status, also with the presence of diabetes chronic complications. Although no significant correlation has been identified between diabetes chronic complications and viral infection severity, they may impact the length of stay in hospital of diabetes patients.
Disclosure: A. Andrita: None.
SO 76 Cancer and type 2 diabetes: interconnections and mortality
847
The impact of diabetes associated risk factors on survival among individuals with type 2 diabetes and breast -, lung-, colorectal- or prostate cancer
T. Laurberg1, D.R. Witte1,2, S. Gudbjörnsdottir3, B. Eliasson4,5, L. Bjerg1,2;
1Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus N, Denmark, 2Department of Public Health, Aarhus University, Aarhus, Denmark, 3Department of Molecular and Clinical Medicine, The Wallenberg laboratory, Institution of medicine, Gothenburg University, Gothenburg, Sweden, 4Sahlgrenska University Hospital, Gothenburg, Sweden, 5Swedish National Diabetes Register, Gothenburg, Sweden.
Background and aims: Premature death in diabetes is no longer caused by cardiovascular disease to the same extent as before, but increasingly by cancer. The objectives of this study were to estimate the excess mortality, when individuals with type 2 diabetes (T2DM) were diagnosed with the most common cancer diagnosis (breast-, lung-, prostate- and colorectal cancer), and to examine how mortality risk was associated with modifiable diabetes-related risk factors such as HbA1c, cholesterol, LDL cholesterol, hypertension, BMI, smoking and physical activity.
Materials and methods: This longitudinal nationwide cohort study included individuals with T2DM registered in the Swedish National Diabetes Register any time between 1998 -2019. Each individual was followed and classified according to their time-updated cancer status and the main outcome was all-cause mortality. Poisson models were used to estimate the mortality as a function of the time-updated risk-factor, adjusted for sex, age, diabetes duration, marital status, country of birth, and yearly income.
Results: We included 655,344 individuals with T2DM and during 4,787,324 person-years of follow-up 179,627 individuals died. Overall, the all-cause mortality rate ratio (MRR) was 2.89 [95% confidence interval (CI): 2.85-2.92] for those diagnosed with cancer when compared to those without cancer. The most significant risk factors associated to mortality among individuals with T2DM and a diagnosis of cancer were low physical activity, 1.59 (1.57-1.61) and smoking, 2.15 (2.08-2.22), whereas HbA1c, cholesterol, LDL cholesterol, hypertension, and BMI had weaker associations with survival. In those without a cancer diagnosis, all estimates pointed in the same direction as in those with both T2DM and cancer, and physical activity and smoking were likewise the strongest risk factors.
Conclusion: In a future with more patients with T2DM and a cancer diagnosis, these results suggest that smoking and physical activity may be the two most salient risk factors for mortality in people with type 2 diabetes, both with and without cancer.
Supported by: Danish Diabetes Academy, NNF17SA0031406
Disclosure: T. Laurberg: None.
848
Versican proteoglycan as new link between type 2 diabetes and breast cancer
A. Parascandolo1,2, V. D'Esposito1,2, M.R. Ambrosio1,2, T. Migliaccio1, M.F. Di Tolla1, F. D'Alterio1, L. Zinna1,2, P. Mirra1,2, C. Miele1,2, L. Insabato3, M. Pace3, D. Russo3, S. Pignatiello4, F. Beguinot1,2, P. Formisano1,2;
1Department of Translational Medicine, University of Naples, “Federico II”, 2Institute of Experimental Endocrinology and Oncology, National Council of Research (CNR), 3Department of Advanced Biomedical Sciences, University of Naples, “Federico II”, 4UOC Pathological Anatomy, PO Ospedale del Mare Asl Napoli 1 Centro, Naples, Italy.
Background and aims: Type 2 diabetes increases incidence, progression and death for several cancers including breast cancer (BC). Patients affected by diabetes and early BC, had larger tumors and a worse outcome with respect to patients without diabetes. Hyperglycemia, the hallmark of T2D, acts directly on cancer cells, but also on tumor microenvironment that in mammary gland is highly represented by adipose tissue (AT) leading to imbalanced production proinflammatory factors. However, mechanisms related to mammary AT (MAT) changes induced by T2D and their connection to BC progression need to be clarified. Versican (VCAN) is a chondroitin sulfate proteoglycan within the extracellular matrix involved in inflammation, vascular diseases and different kind of cancers. Interestingly, VCAN has been identified as a hub gene with a key role in diabetic kidney and diabetic bone disease. All together these evidence pointed out VCAN as a target molecule with a strategical role in the connection between diabetes and BC progression. Therefore, the aim of our work is based on the study of VCAN as an AT biomarker involved in BC-MAT bidirectional communication.
Materials and methods: MAT biopsies have been obtained from healthy women undergoing surgical mammary reduction (H-MAT; N=15) and from patients with BC underwent surgical resection (C-MAT; N=20). Total RNA and protein lysates have been collected and analyzed for VCAN expression by qPCR and Western blot analysis respectively. Mesenchymal stem cells (MSC) have been isolated by H-MAT and co-cultured with MCF7 BC cells in transwell systems. VCAN content was investigated by immunohistochemistry (IHC). Data were analyzed using GraphPad Prism 7.
Results: In MSC isolated from H-MAT, VCAN mRNA and protein levels positively correlated with glycemia (p<0.01 **). Moreover, in co-colture with MCF7, MSC expressing high levels of VCAN displayed increased Interleukin 6 (IL6) levels and induced Matrix Metalloprotease-9 (MMP9) expression in BC cells, compared with MSC expressing lower VCAN levels. In C-MAT, VCAN mRNA expression levels were higher compared with H-MAT (p<0.0001 ***). Finally, in C-MAT VCAN was detected by IHC at higher extent in the proximity of the tumor, compared to more distant sites (p<0.0001 ***).
Conclusion: VCAN is expressed in adipose progenitors and positively correlates with glycemia. Its higher expression levels in C-MAT suggest a role in the connection between T2D and breast cancer. Characterization of VCAN in AT surrounding BC may lead to identify a novel biomarker associated to BC progression in metabolically perturbed peri-tumoral AT.
Supported by: This research was funded by Regione CampaniaPORFESR2014–2020 COEPICA Project and by AIRC Association
Disclosure: A. Parascandolo: None.
849
Genetic profiles of whole tumours from individuals with type 3c diabetes indicate immune-mediated mechanisms may not underlie insulin deficiency
J.L.E. Hill1, T.G. Hill2, N.G. Morgan1, S.J. Richardson1;
1College of Medicine and Health, University of Exeter, Exeter, 2Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, Oxford, UK.
Background and aims: Understanding the relationship between pancreatic ductal adenocarcinoma (PDAC) and diabetes is a high research priority, since diabetes can be an early indicator of PDAC, which is associated with poor survival (4-6 months). Previous research has suggested that PDAC with diabetes is a subtype of PDAC with underlying immune-driven mechanisms. We used a cancer registry to investigate the relationships between PDAC and diabetes more fully.
Materials and methods: Patient data were collected from The Cancer Genome Atlas Program. HiSeq illumina RNA expression data were used to compare genetic profiles of whole tumours (84 people with PDAC/no diabetes; 22 people with PDAC/diabetes). CIBERSORT deconvolution methodology was used to compare immune profiles and tissue histology was analysed in islets from subjects with/without diabetes. Presence of immunomodulatory proteins were assessed using PANC-1 and Endo-βH1 cell lines after cytokine challenge.
Results: INS, GCG, and SST expression were significantly (p < 0.05-p < 0.01) reduced in PDAC/diabetes vs PDAC alone. We found no significant upregulation of genes associated with immune mechanisms (PDCD1, CTLA4, PDL1) in PDAC/diabetes. Unexpectedly, the basement membrane of islets was observed to be unusually thickened in subjects with PDAC/diabetes and PDAC alone. We further examined a PDAC-derived cell line (PANC-1) and noted only a modest increase in expression of PD-L1 in response to cytokine challenge (1247 ± 206.6 MFI). By contrast, the beta cell line (Endo-βH1) showed a robust increase in PD-L1 expression when exposed to cytokines (109877 ± 218.1 MFI)
Conclusion: These data imply that immune-mediated changes may not be key drivers underlying the development of diabetes in PDAC.
Disclosure: J.L.E. Hill: None.
850
A pan-cancer analysis of the oncogenic role of regenerating protein1 alpha(REG1A) in human tumors
H. Wang, L. Li;
Department of Endocrinology, Zhongda Hospital, Southeast University, Nanjing, China.
Background and aims: Regenering 1 alpha (REG1A) is a secreted protein containing 166 amino acids that belongs to the calcium-dependent lectin superfamily. In humans, four different REG gene isoforms are known: REG1A, REG1B, REG3A and REG4.Regenerating protein 1 alpha(REG1A) is a member of the regenerating gene (Reg) and first derived from the regenerating islet. REG1A was originally identified by screening a cDNA library of regenerating rat islet cells and was mainly associated with regeneration of pancreatic b-cells and improvement of diabetes.Now it is considered REG1A plays a pivotal role in promoting cell proliferation, differentiation, anti-apoptosis and inflammatory response. REG1A has been implicated in a gamut of maladies including diabetes, various types of cancer. Multiple clinical studies have shown that REG1A is elevated in the serum of patients with a variety of tumors, and the expression of the gene is strongly correlated with the disease progression of patients. Clinical studies suggest that REG1A has the potential to become a new tumor marker.The closely association with REG1A and tumorigenesis and prognosis of has been confirmed, but there is no available pan-cancer analysis of REG1A. Therefore, we explored the potential oncogenic role of REG1A in 33 tumors.
Materials and methods: Raw data on REG1A expression in cancer patients were obtained from TCGA and GTEx databases. Combining the data for normal tissues from databases, we investigated genomic changes, expression patterns and survival analysis of REG1A in pan-cancer,including ‘Gene_DE’ module of TIMER2 and HEPIA2. We used the ‘Survival Map’ module of GEPIA2 to acquire the OS (Overall survival) and DFS (Disease-free survival) significance map data of REG1A across all TCGA tumors. The cBioPortal database is ued to analyse genetic alteration of REG1A.The relationship between REG1A expression and tumor immune invasion was analyzed by TIMER.The proportion of tumor-infiltrating immune cells (TIICs) and the number of stromal and immune components were assessed from the TCGA database using CIBERSORT,QUANTISEQ,XCELL,MCPCOUNTER and EPIC computational methods.STRING website and GEPIA2 were used to obtain REG1A-correlated targeting genes and protein. GO (Gene ontology) enrichment analysis was performed in REG1A-binding and interacted genes.
Results: Overall, expression levels of REG1A were higher in 22 types of tumor tissues than in normal tissues,especially in CHOL (Cholangiocarcinoma),COAD (Colon adenocarcinoma), KICH (Kidney Chromophobe), KIRC(Kidney renal clear cell carcinoma), LIHC (Liver hepatocellular carcinoma), LUAD (Lung adenocarcinoma), LUSC (Lung squamous cell carcinoma), READ (Rectum adenocarcinoma), HNSC (Head and Neck squamous cell carcinoma). Low REG1A expression was associated with poor clinical scenarios of OS and RFS. here were significant negative correlations between REG1A expression and TIICs, including CD8+ T cells, CD4+ T cells, B cells, fifibroblast, neutrophils and macrophage. The phosphorylation of REG1A protein had a decreased level in some tumors, such as clear cell carcinoma. REG1A was related to triglycerides metabolism.The effect of REG1A on tumor pathogenesis may be related to the metabolic pathway.
Conclusion: We observed phosphorylation of REG1A protein had a decreased level in some tumors, such as clear cell carcinoma.Our first pan-cancer study provides a relatively comprehensive understanding of the oncogenic role of REG1A in different tumors.
Disclosure: H. Wang: None.
851
Insulin receptors in pancreatic acinar cells contribute to pancreatic cancer development
A. Zhang1, K.H. Chu1, Y. Xia1, T. Ruiter1, J. Yang1, J. Lin1, N. Chen1, D.F. Schaeffer2, J.L. Kopp1, J.D. Johnson1;
1Department of Cellular and Physiological Sciences, University of British Columbia, 2Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada.
Background and aims: Epidemiological studies showed hyperinsulinemia, a cardinal feature of obesity and type 2 diabetes, is independently associated with an increased risk of pancreatic ductal adenocarcinoma (PDAC). Complementing these epidemiological associations, our previous in vivo studies showed a ~50% reduction in pancreatic intraepithelial neoplasia (PanIN) pre-cancerous lesions in mice with genetically reduced insulin production. Our single-cell transcriptomic analyses suggested that hyperinsulinemia might indirectly promote PDAC development by affecting multiple cell types in the neoplastic microenvironment. However, it remains unclear whether hyperinsulinemia directly accelerates PDAC development through insulin receptor (Insr) and its downstream signaling cascades in pancreatic acinar cells, which are the cellular origins of PDAC.
Materials and methods: We generated Ptf1aCreER;LSL-KrasG12D;nTnG mice (a PDAC mouse model) with an Insrwt/wt (PK-Insrwt/wt), Insrwt/fl (PK-Insrwt/fl), or Insrfl/fl (PK-Insrfl/fl) genotype to reduce insulin receptor gene dosage by 0%, 50%, or 100% in acinar cells. All mice were fed with a high-fat diet to induce hyperinsulinemia. Body weight, fasting glucose, and insulin levels were tracked throughout the study period. The mice were euthanized at 3 months or 10 months, and we performed blinded histopathological analyses and quantification to assess PanIN formation. To investigate the underlying molecular mechanisms, pancreata from each genotype were used for proteomic and phosphoproteomic analyses.
Results: Loss of Insr from acinar cells did not significantly influence body weight, fasting glucose, or insulin levels. Unbiased quantification of PanIN area for mice euthanized at 10 months old showed a ~2.9-fold significant reduction in PanIN and tumor area in PK-Insrf/f (n=6) male mice compared to PK-Insrw/w (n=5) male mice (16.58 ± 5.53% vs 47.92 ± 4.05%, p<0.01), and ~1.8-fold reduction compared to PK-Insrw/f (n=10) male mice (30.54 ± 4.14%). In addition, we found a significant reduction in PanIN and tumor area in PK-Insrf/f (n=9) female mice in comparison to PK-Insrw/f (n=16) and PK-Insrw/w (n=8) female mice (8.96 ± 4.28% vs 24.45 ± 4.48% vs 47.19 ± 5.67%, respectively, p<0.001). By assessing the mice euthanized at 12 weeks of age, we found PK-Insrf/f (n=4-9) had a 5.3-fold and 6.3-fold reduction in PanIN area in males (0.31 ± 0.19%) and females (0.23 ± 0.039%), respectively, compared to PK-Insrw/w (n=3-4) male (1.64 ± 0.16%, p<0.05) and female (1.46 ± 0.77%) control mice. Unbiased proteomic analysis at 12 weeks of age demonstrated that compared to PK-Insrw/w mice, Reg proteins (like Reg3g, Reg3b, and Reg3a) which increase during pancreas injury or inflammation, and digestive enzymes (like Amy1, Cela1, and Prss2), which are secreted by acinar cells were significantly downregulated in PK-Insrf/f mice.
Conclusion: Collectively, our data suggest that Insr in acinar cells promotes both PanIN initiation and development, possibly by decreasing digestive enzyme production in acinar cells and the inflammation associated with those enzymes release during damage. Thus, prophylactic approaches targeting Insr signaling pathways, or hyperinsulinemia itself, may be beneficial in preventing pancreatic cancer.
Supported by: CIHR
Disclosure: A. Zhang: None.
852
Triglyceride-glucose index and all-cause mortality, and incidence of vascular events in subjects with type 2 diabetes: results from a 13-year follow-up observational study
P. Falcetta1, M. Garofolo1, D. Lucchesi1, E. Gualdani2, M. Giambalvo1, P. Francesconi2, S. Del Prato1, G. Penno1;
1Department of Clinical and Experimental Medicine, University of Pisa, Pisa, 2Epidemiology Unit, Regional Health Agency of Tuscany, Firenze, Italy.
Background and aims: Triglyceride-glucose (TyG) index is considered a reliable marker of insulin resistance and an independent predictor of cardiovascular (CV) outcomes; however, the prognostic value of TyG index in type 2 diabetes (T2D) remains scarcely explored.
Materials and methods: This observational prospective cohort study enrolled 961 individuals with T2D between 2002-2004 who have been followed-up for a mean of 13.1±2.8 years. Vital status was retrieved for all individuals on December 31, 2017 by interrogating the Italian Health Card Database; data for major vascular events were available for 947 participants (98.5%), and were obtained, up to the same date, in collaboration with the Regional Health Agency of the Tuscany Region through hospital discharge registers (ICD-9-CM codes). Patients were divided into tertiles (T1-T3) according to their baseline TyG index levels. The TyG index was calculated as ln [fasting triglycerides (mg/dL) x fasting glucose (mg/dL) / 2] and subdivided into tertiles (thresholds 8.97 and 9.51, mean value 9.26±0.63). Following Kaplan-Meier (K-M) analyses, the hazard ratios (HR, 95% confidence intervals, CI) for different outcomes associated with TyG index tertiles were assessed by unadjusted and adjusted Cox regression models.
Results: At the time of census, 229 (23.8%) subjects had died (18.25 x 1000 person-years, PYs; 95% CI 16.03-20.78), major CV events occurred in 273 (28.8%; 25.68 x 1000 PYs; 22.80-28.91), coronary events (CHD) in 181 (19.1%; 16.20 x 1000 PYs, 14.01-18.74), hospitalization for heart failure (hHF) in 82 (8.7%; 6.79 x 1000 PYs, 5.47-8.44), cerebrovascular events (CVE) in 101 (10.7%), peripheral vascular diseases (PVD) in 39 (4.1%), ESKD in 71 subjects (7.5%; 5.87 x 1000 PYs, 4.65-7.41). On K-M analyses, major CVD (24.2%, 25.6% and 36.7%; log-rank 15.098, p=0.001) and coronary events (14.5%, 17.1% and 25.9%; log-rank 14.979, p=0.001) increased across TyG index tertiles. All other outcomes (all-cause death, hHF, CVE, PVD, ESKD) showed a not statistically significant trend for increasing incidence across TyG tertiles. On unadjusted Cox modeling, the cumulative incidence of major CV events and CHD rose significantly with the increase of TyG index (T3 vs T1, HR 1.650, 95% CI 1.236-2.202, p=0.001 for major CVD; HR 1.911, 1.331-2.744, p<0.0001 for CHD) and marginally (T3 vs T1, HR 1.992, 0.926-4.285, p=0.078) for PVD. After adjusting for age, diabetes duration (DD), age at T2D diagnosis, sex, BMI, active smoking, HbA1c, uric acid, fibrinogen, hypertension, dyslipidemia, prior CVD, CKD, retinopathy and peripheral neuropathy, HRs still increased with the rising of the TyG index for major CVD (T3 vs T1, 1.960, 95% CI 1.438-2.672, p<0.0001; with independent effects for age, DD, male sex, BMI, smoking, fibrinogen, prior CVD, CKD, retinopathy and peripheral neuropathy), and for CHD (T3 vs T1, 2.029, 1.404-2.934, p<0.0001; with independent effects for age, DD, male sex, fibrinogen, prior CVD, CKD and retinopathy).
Conclusion: The triglyceride-glucose (TyG) index was significantly and independently associated with major CVD and CHD, suggesting that it may be a valid marker for risk stratification in subjects with T2D.
Disclosure: P. Falcetta: None.
853
The prognostic impact of HbA1c and diabetes on mortality in chronic heart failure
K. Sveen1,2, M. Grundtvig3,4, L. Gullestad5,6, K.I. Birkeland1,7;
1Oslo University Hospital, Oslo, 2Oslo Diabetes Research Center, Oslo, 3Department of Medicine and health care, St. Olavs university hospital, Trondheim, 4dept. Medicine, Innlandet Hospital Trust, Lillehammer, 5Department of Cardiology, Oslo University Hospital, Oslo, 6KG Jebsen Cardiac reasearch Center and Center for Heart Failure Research, Oslo university Hospital, Oslo, 7Institute of clinical medicine, University of Oslo, Oslo, Norway.
Background and aims: Diabetes is associated with an increased cardiovascular morbidity and mortality in chronic heart failure. Previous trials and registers have mostly used definition of diabetes in categories of either present or not from self-reported data or medical records, while less is known about the impact of levels of HbA1c on HF and mortality. Our aim was therefore to examine the prognostic impact of HbA1c and diabetes mellitus (DM) on mortality in a large cohort of outpatients with HF.
Materials and methods: A total of 13768 outpatients with heart failure from the National Norwegian Heart Failure Registry (NNHFR), which is part of the Norwegian Cardiovascular Disease Registry, were registered from 2013 until 1st January 2020. After excluding 1170 with missing HbA1c measurements, 12598 patients were included. We evaluated the impact of glycaemic control (HbA1c) on all-cause mortality according to the presence of DM or not. Cox proportional hazard regression models were used to develop predictors for all-cause mortality and significant variables were entered in a multivariable model to evaluate the association between HbA1c and DM and all-cause mortality during follow-up.
Results: The prevalence of DM in the registry was 31.6 % (3984/12598), 26.6% of the patients were pre-diagnosed with DM and an additional 5 % (n=624) were added due to HbA1c ≥ 48 mmol/mol). During a median (25-75 percentiles) follow up time of 28 (14.6-46.3) months in survivors, 21.8% died. In univariate analyses 26.9% of individuals with DM had died versus 20.2% without DM (p<0.001). Among patients with DM and adjusting for confounders, individuals with HbA1c <48 mmol/mol (n=826) had the worst outcome with HR for death 1.36 (95% CI 1.16 -1.60), p<0001. Individuals with diabetes and HbA1c >64 mmol/mol (n=841) also had a worse prognosis compared to those within HbA1c 48-53 mmol/mol, HR 1.17 (1.05-1.31), p<0.001. HbA1c was not a significant predictor for mortality in patients without diabetes (p=0.12).
Conclusion: This study demonstrates that diabetes is a significant independent predictor for all-cause mortality in outpatients with heart failure. In patients with diabetes, mortality was increased in patients with very low and high levels of HbA1c.
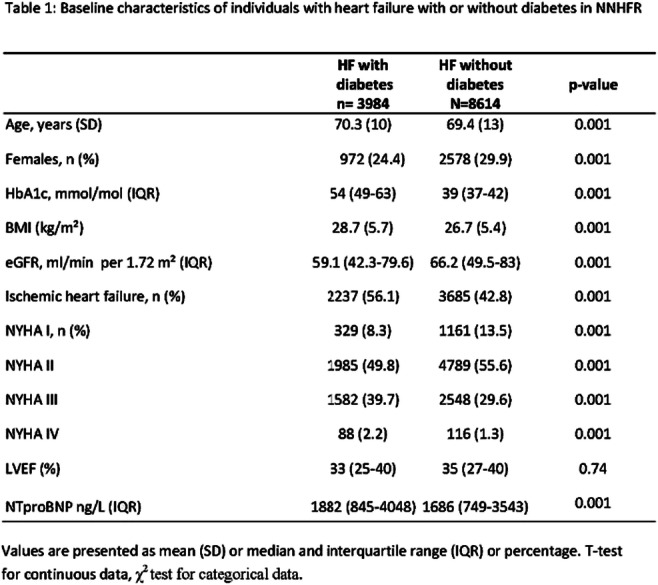
Disclosure: K. Sveen: Lecture/other fees; Lecture fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi.
854
Age-related difference in the impact of diabetes on all-cause mortality after acute myocardial infarction
P.-S. Song, J.-O. Jeong;
Division of Cardiology, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Republic of Korea.
Background and aims: Given the paucity of data on the frequency and prognostic implications of diabetes among patients with acute myocardial infarction (AMI) at a young age, we sought to determine the prevalence of diabetes and associated outcomes in a more contemporary cohort of patients presenting with AMI at age ≤55 years (young group). Also, this study aimed to compare the baseline characteristics and clinical outcomes of diabetic and non-diabetic patients with AMI by age.
Materials and methods: A total of 12,600 AMI patients from the Korea Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-NIH) between November 2011 and December 2015 was classified into young (n=3,590 [28.5%]) and old (n=9,010 [71.5%]). We performed comparisons of baseline characteristics, in-hospital treatments, and long-term clinical outcomes between patients with and without diabetes after stratification according to age group.
Results: The overall prevalence of diabetes mellitus was 34.1%, and 19.7% of individuals were diagnosed newly with diabetes at the index admission. The prevalence of diabetes mellitus was 26.5% in the young AMI group. Diabetic patients were less likely to receive percutaneous coronary intervention with or without coronary stenting in the old group but not in the young group (interaction effect p<0.05). In the entire study cohort, all-cause mortality (539 [12.6%] vs. 566 [6.8%]; adjusted hazard ratio [HR], 1.318; 95% confidence interval [CI], 1.138-1.526; p<0.001), cardiac mortality (284 [6.6%] vs. 283 [3.4%]; adjusted HR, 1.293; 95% CI, 1.050-1.593; p=0.016), recurrent myocardial infarction (219 [5.1%] vs. 247 [3.0%]; adjusted HR, 1.378; 95% CI, 1.125-1.689; p=0.002), and major adverse cardiac events (MACE), a composite of all-cause mortality, recurrent myocardial infarction, and re-hospitalization for heart failure (851 [19.8%] vs. 965 [11.6%]; adjusted HR, 1.173; 95% CI, 1.049-1.311; p=0.005) were significantly higher in diabetic patients than in non-diabetic patients over the three-year clinical follow-up after multiple adjustments. When the entire cohort was subdivided into two age groups, young diabetic patients showed a 107.0% higher mortality rate than those without diabetes (adjusted HR, 2.070; 95% CI, 1.150-3.724; p=0.015). Meanwhile, elderly diabetic patients had a 25.3% higher risk of mortality than non-diabetic patients (adjusted HR, 1.253; 95% CI, 1.076-1.459; p=0.004). The interaction of diabetes with age was statistically significant (adjusted p for interaction = 0.008). Cardiac mortality and MACE of secondary outcomes were similar to the primary endpoint of all-cause mortality over the three-year clinical follow-up.
Conclusion: Diabetes mellitus is not uncommon in younger AMI patients, and the relative risk of long-term mortality is significantly higher in young patients than in older counterparts. More aggressive treatments are needed to prevent future cardiovascular events in younger patients after AMI.
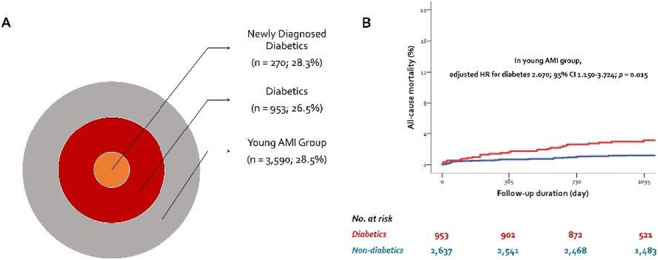
Disclosure: P. Song: None.
SO 77 Disclosing fatty liver disease mechanisms
855
Extracellular vesicle-mediated crosstalk between hepatocytes and endothelial cells in non-alcoholic fatty liver disease condition
S.-H. Lee, J. Lee, M. Rhee, J. Yu, K.-H. Yoon;
The Catholic University of Korea, Seoul, Republic of Korea.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) is an independent risk factor for cardiovascular disease (CVD), although the mechanism of association is still unclear. Extracellular vesicle (EV) is a biological nanoparticle that contains various cargos and plays critical roles in inter-organ crosstalk. We aimed to investigate the role of hepatocyte-derived EVs on endothelial cells in NAFLD condition.
Materials and methods: Primary hepatocytes isolated from C57BL6 mice were exposed to palmitic acid (PA). Hepatocytes-derived EVs were isolated using sequential ultracentrifugation technique and characterized by nanoparticle tracking analysis, western blotting and transmission electron microscopy. Human umbilical vein endothelial cells (HUVEC) and human aortic endothelial cells (HAEC) were treated with EVs released from hepatocytes.
Results: PA treatment increased the expression of genes associated with lipid synthesis, inflammation and oxidative stress in hepatocytes. The amount of EVs derived from PA-treated hepatocytes was greater than the EVs from control hepatocytes. Fluorescence-labeled hepatic EVs uptake were detected in human endothelial cells. Treatment of EVs derived from PA-exposed hepatocytes induced endothelial dysfunction with subsequent upregulation of inflammatory cytokines, adhesion molecules, and oxidative stress markers in HUVEC and HAEC. Small RNA profiling of miRNA isolated from hepatic EVs identified 23 upregulated and 4 downregulated miRNAs (fold change ≥5 and normalized data (log2) ≥4) in PA-treated hepatocytes. miR-23b-3p, miR-30b-5p, and miR-30c-5p were identified as possible candidate cargos confirmed by qPCR.
Conclusion: Our findings suggest a novel role of hepatic EVs that regulate crosstalk between hepatocytes and endothelial cells. This may explain the independent relationship between NAFLD and CVD.
Supported by: National Research Foundation of Korea (2018R1D1A1B07043223, 2021R1F1A1061197)
Disclosure: S. Lee: None.
856
Impact of phenylacetic acid, a microbiota-derived metabolite, on hepatic endoplasmic reticulum-mitochondria interactions and steatosis
R. Lefebvre1, C. Caussy1,2, J. Rieusset1;
1CarMeN Laboratory, UMR INSERM U1060/INRA U1393, 2Hospices Civils de Lyon, Hôpital Lyon Sud, Pierre-Bénite, France.
Background and aims: The gut-liver axis has emerged as an important factor in the development of non-alcoholic fatty liver diseases (NAFLD). Microbiota-derived metabolites such as phenylacetic acid (PAA) have been shown to trigger hepatic steatosis in human primary hepatocytes but the molecular mechanism involved is not elucidated. Moreover, mitochondrial dysfunction is also a key component of NAFLD and oxidative capacities of mitochondria are regulated by the communication of this organelle with endoplasmic reticulum at contact points named mitochondria-associated membranes (MAMs). Hence, MAMs are nutrient sensitive controlling mitochondrial oxidative metabolism and ER-mitochondria miscommunication was associated with hepatic insulin resistance and steatosis. We hypothesized that PAA may induce hepatic steatosis through a disruption of MAMs integrity (Figure 1).
Materials and methods: We investigated the impact of PAA (500 μmol/l) on MAMs integrity and steatosis in Huh7 cell line and primary mouse hepatocytes (PMH) incubated for 16 hours in normal (BSA) and lipotoxic (palmitate 200 μmol/l) conditions. Co-treatments with diazoxide (100 μmol/l) or EGTA (10 mmol/l) allowed to investigate the involvement of electrogenic effects of PAA in Huh7 cells. ER-mitochondria interactions were measured by in situ proximity ligation assay (PLA) targeting VDAC1 (mitochondrial protein) and IP3R1 (reticular protein) proximity and BODIPY labelling was used to evaluate hepatocyte lipid accumulation. Fixed cells were analyzed by fluorescent microscopy and images analyses were performed using either blobfinder (PLA) or ImageJ (BODIPY) to quantify mitochondria-endoplasmic reticulum contacts and lipid droplets size respectively. All experiments were performed in a single time triplicate.
Results: Treatment with PAA reduced MAMs in Huh7 (n = 30 photos, fold change = -72 ± 0.025 %, p = 0.0001) and PMH (n = 30 photos, fold change = -23 ± 0.064 %, p < 0.0001), it also induced lipid accumulation in Huh7 (n = 30 photos, fold change = +20 ± 0.035 %, p = 0.0001) and PMH (n = 20 photos, fold change = +35 ± 0.047 %, p = 0.0001). The effect of PAA on MAM integrity is observed as soon as one hour of treatment (n = 30 photos, fold change = -39% ± 0.029, p < 0.0001) in Huh7. This effect is fully prevented by diazoxide which inhibits membrane depolarization (n = 30 photos, fold change = +9% ± 0.066 % with diazoxide versus -30 ± 0.042 % without it, p < 0.0001) and partially by EGTA treatment, a Ca2+ chelation agent, (n = 30 photos, fold change = -29 ± 0.044 % with EGTA versus -50 ± 0,022 % without it, p < 0.0007). These results suggest an electrogenic mechanism of PAA action on MAMs.
Conclusion: PAA, a gut-microbiome derived metabolite, induces MAM disruption and hepatocyte lipid accumulation. The effect of PAA on MAMs is mediated through an electrogenic mechanism. Whether preventing the electrogenic effect of PAA impacts hepatic steatosis is under investigation.
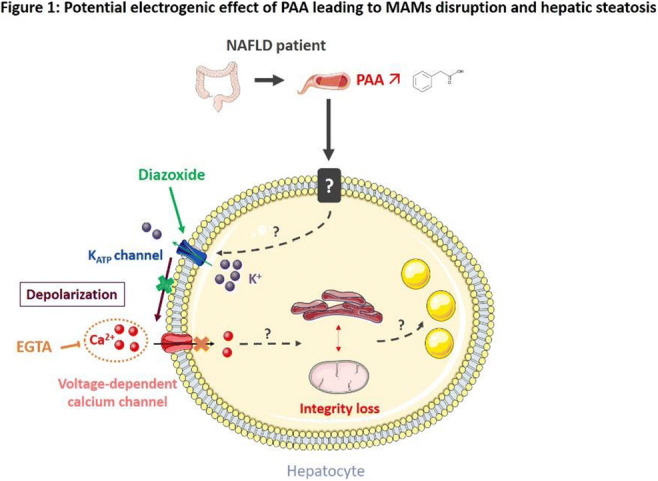
Disclosure: R. Lefebvre: None.
857
Lower serum PRL is associated with the development of non-alcoholic fatty liver disease
P. Xu1,2, P. Zhang2, Y. Zhu2, Y. Bi1,2;
1Department of Endocrinology, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, 2Department of Endocrinology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China.
Background and aims: Non-alcoholic fatty liver disease (NAFLD) has become a worldwide epidemic and associated with a series of metabolic co-morbidities. Prolactin (PRL) is a multifunctional polypeptide with effects on mammary gland development and lactation, and has recently been found to have an important effect on metabolic diseases. Studies have shown that low PRL levels are a risk factor for the prevalent of NAFLD, but a causal relationship is not well-understood. Here we investigated the causative relationship between PRL and NAFLD occurrence.
Materials and methods: In a retrospective cohort study, we studied 355 patients without NAFLD at baseline [215 men and 140 women; mean age 56 (49, 64) years] who had undergone serum PRL testing at 8.00 a.m. and abdominal ultrasonography. NAFLD was diagnosed by abdominal ultrasonography. The patients were divided into the non-NAFLD group and the NAFLD group according to the status of their follow-up endpoint.
Results: This study enrolled 355 persons over a mean 32 (19, 46) months of follow-up, 72 (20.28%) patients who eventually developed NAFLD. Compared with those in the non-NAFLD group, basal serum PRL levels of patients were lower in the NAFLD group in both genders [male: 7.35 (5.48, 10.60)ug/L vs. 9.13 (6.92, 12.50) ug/L, P = 0.002; female: 5.66 (4.67, 9.03) ug/L vs. 9.01 (6.31, 11.60) ug/L, P = 0.009]. A significant decrease in the prevalence of NAFLD was noted in both genders along with the increased quartile of basal serum PRL levels (P <0.05). In multivariate logistic regression, basal serum PRL concentration was independently associated with NAFLD development [male: OR, 0.863 (0.777, 0.960), P = 0.006; female: OR, 0.746 (0.601, 0.925), P = 0.008].
Conclusion: Our study is the first to find that basal serum PRL level can predict the occurrence of NAFLD and it may be a potential biomarker to prevent and treat NAFLD.
Disclosure: P. Xu: None.
858
Association between visceral adipocyte size and non-alcoholic fatty liver disease in subjects with different degrees of adiposity
H. Sun, T. Gu, D. Fang, Y. Yuan, H. Wang, Y. Bi;
Department of Endocrinology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China, Nanjing, China.
Background and aims: With the epidemic of high-caloric diet and obesity, non-alcoholic fatty liver disease (NAFLD) has grown rapidly around the world. Adipose tissue morphology alterations are associated with metabolic diseases, including NAFLD. Previous studies have shown that adipocyte size is associated with liver steatosis. However, few study evaluated the influence of adiposity status on the relationship between adipocyte size and NAFLD. Here, we investigated the association between visceral adipocyte size and the histological severity of non-alcoholic fatty liver disease (NAFLD) in subjects with different degrees of adiposity.
Materials and methods: Omental adipose tissue and liver biopsy were collected from 275 obese subjects. NAFLD was defined according to the NASH Clinical Research Network (NASH-CRN) scoring system. Adipocyte size were measured through pathological section analysis. Adipose tissue insulin resistance (Adipo-IR) was calculated as fasting insulin (pmol/L) × fasting Free fatty acid concentration (mmol/L).
Results: In total, 275 obese patients (206 females and 69 males) were involved in this study. In females, 158 subjects were classified as biopsy-proven NAFLD, and 58 individuals in males were diagnosed with NAFLD. NAFLD patients presented higher levels of fasting glucose, HbA1c, HOMA-IR, ALT and AST (all p<0.05). The adipocyte size was significantly larger in NAFLD participants as compared to the controls (NAFLD vs. control: 99.22±14.08 m vs. 84.14±12.65 m, p<0.001). Among NAFLD patients, subjects with NASH showed a significantly larger adipocyte cell size compared to those with NAFL (NASH vs. NAFL: 101.45±12.77 m vs. 95.79±15.80 m, p=0.015). Further, based on the quartiles of BMI, the subjects were divided into four groups: Q1(BMI<32.5kg/m2), Q2(BMI 32.5-35.5kg/m2), Q3(BMI 35.5-38.8 kg/m2) and Q4(BMI≥38.8kg/m2). Omental adipocyte size was significantly larger in NAFLD subjects in mild to moderate obesity (Q1-Q3: BMI<38.8 kg/m2), but not in severe adiposity (Q4: BMI≥38.8 kg/m2). Mediation analysis showed that adipocyte size impacted NAFLD activity score through Adipo-IR (b=0.0068 [95% bootstrap CI 0.0025,0.0119]). No similar results were observed in males.
Conclusion: We for the first time reported that increased visceral adipocyte size promotes the onset and progression of NAFLD in mild to moderate adiposity but not severe obesity, which may be mediated by adipose tissue insulin resistance.
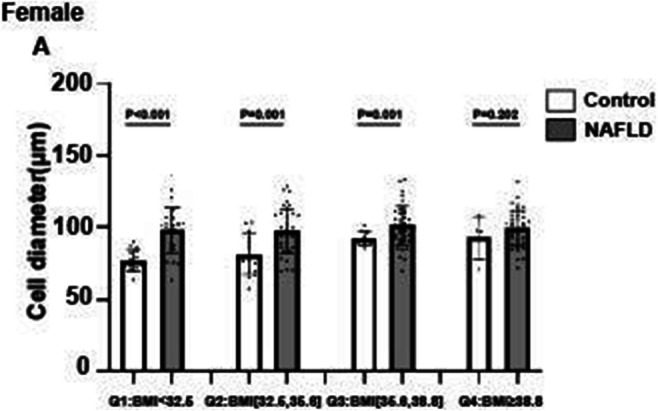
Supported by: NSFC,China
Disclosure: H. Sun: None.
859
Non-alcoholic fatty liver disease (NAFLD) is an independent risk factor for developing new-onset diabetes after acute pancreatitis
Y. Lv, J. Zhang, T. Yang, L. Li;
Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China.
Background and aims: Numerous studies validated frequent glucose dysfunction in patients with acute pancreatitis (AP). However, the prevalence of new-onset diabetes in individuals after a first episode of AP varies widely among previous studies. This study aimed to determine the incidence of post-acute pancreatitis diabetes mellitus (PPDM-A) in Chinese people and further identify potential risk factors that influence diabetes development in patients with AP.
Materials and methods: This was a multi-center retrospective cohort study including 6009 inpatients with a first attack of AP. A total of 1804 patients with AP without known endocrine pancreatic disorders or other pancreatic exocrine diseases were eligible for analysis. Data was collected from medical records by hospital information system and telephone follow-ups after discharge. The multiple logistic regression analysis was established to evaluate the potential influencing factors of PPDM-A.
Results: The prevalence of newly diagnosed diabetes in Chinese patients after a first episode of AP was 6.2%. Data showed that patients who developed PPDM-A were characterized by younger age, longer hospital stays, a higher frequency of overweight or obesity, stress hyperglycemia on admission, accompanied by hyperlipidemia and non-alcoholic fatty liver disease (NAFLD), more likely to be hyperlipidemic AP, and had a higher degree of severity and recurrence rate of AP compared to those with normal glycemia. Regression analysis indicated that stress hyperglycemia, hyperlipidemia, NAFLD and recurrent pancreatitis were the independent influence factors for developing PPDM-A.
Conclusion: Our study first demonstrated the prevalence of secondary diabetes in Chinese patients after AP. The disorder of glucose metabolism in individuals with AP should be regularly evaluated in clinical practice. Further studies are needed to verify the relationship between liver and pancreas in keeping glucose homeostasis under AP condition.
Clinical Trial Registration Number: ChiCTR1800018247
Supported by: This work was supported by National Natural Science Foundation of China (81970717 and 82170845).
Disclosure: Y. Lv: None.
860
A mouse model for metabolic stress-induced nonalcoholic fatty liver disease and subsequent hepatocellular carcinoma
B.-K. Jeong1,2, W. Choi1,3, W.-I. Choi1,2, J. Moon4, P. Kim1,4, J. Park5, H. Kim1,2;
1Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, 2Biomedical Research Center, Korea Advanced Institute of Science and Technology, Daejeon, 3Department of Internal Medicine, Chonnam National University Medical School, Chonnam, 4Graduate School of Nanoscience and Technology, Korea Advanced Institute of Science and Technology, Daejeon, 5Department of Internal Medicine, Yonsei University College of Medicine, Daejeon, Republic of Korea.
Background and aims: Nonalcoholic fatty liver disease (NAFLD) is a rapidly growing metabolic disease that presents with a wide range of clinical manifestations from simple fatty liver to hepatocellular carcinoma (HCC). However, NAFLD researchers are limited by the lack of appropriate animal model that can reflect the full range of the disease in a manageable method with physiological relevance. In this study, we used a controllable method to generate a mouse model of NAFLD and subsequent HCC that closely recapitulates human disease and its physiological relevance.
Materials and methods: To generate NAFLD mouse model, we modified the STAM mouse model (mSTAM). Briefly, male C57BL/6J mice were injected with low dose Streptozotocin (40 mg/kg) for 5 consecutive days from 7 weeks of age. Control mice were fed normal chow diet (NCD) and mSTAM mice were fed high fat diet (HFD) from 8 weeks of age. Hepatic histology, transcriptomes and metabolic phenotypes were evaluated at multiple time points. Mouse transcriptomic data were also compared to human transcriptomic data with similar hepatic histology.
Results: Control mice showed mild hepatic inflammation transiently without hepatic steatosis or fibrosis. In contrast, mSTAM mice developed hepatic steatosis, steatohepatitis, progressive hepatic fibrosis, and HCC at 14, 20, 32, and 38 weeks of age, respectively. HCCs developed in mSTAM mice showed histological features of steatohepatitic variant, characteristically found in human patients with metabolic disease, including ballooning cancer cells, inflammatory cell infiltration and intratumoral fibrosis. They accompanied multiple physiological features of human NAFLD patients including hyperglycemia, obesity, dyslipidemia, adipocyte hypertrophy and adipose tissue inflammation. In terms of overall gene expression alterations at a transcriptome level, there was a substantial association between mSTAM mice and human NAFLD patients with similar liver histology. Furthermore, they shared the key signaling pathways in NAFLD progression, including fatty acid degradation and the p53 signaling pathway.
Conclusion: We have developed a novel and easily manageable mouse model of NAFLD and subsequent NAFLD-related HCC. This model mimics physiological, metabolic, histologic and transcriptomic alterations that occur in human patients as NAFLD progresses.
Supported by: KHIDI MD-Phd/Medical Scientist Training Program
Disclosure: B. Jeong: None.
861
Increased hepatic dicarbonyl stress decreases expression of the drug metabolising enzymes cytochrome (CYP) p450 3A4 and carboxylesterase 1
M. Maandi1, S.C. Maandi2, J.G. Mabley1;
1School of Applied Sciences, University of Brighton, Brighton, 2School of Applied Sciences, University of Brighton, Hove, UK.
Background and aims: Type 2 diabetes mellitus (T2DM) has been linked to hepatic complications including impairment of drug metabolising enzyme activity, which may alter hepatic xenobiotic metabolism capacity. Hyperglycaemia-mediated increase in methylglyoxal levels and the resulting dicarbonyl stress plays a key role in diabetic complications and cellular dysfunction. However, the potential impact of dicarbonyl stress on drug metabolising enzymes has yet to be elucidated. Therefore, the aim of this study was to analyse the effect of dicarbonyl stress induced via glyoxalase I inhibition on the expression of phase I (cytochrome (CYP) P450 3A4) and phase II (carboxylesterase 1 (CES1)) metabolising enzymes.
Materials and methods: The human hepatoma cell line HepG2 was treated with 20 μM S-p-Bromobenzylglutathione cyclopentyl diester (BBGC), a glyoxalase I inhibitor, to induce dicarbonyl stress via increased intracellular methylglyoxal levels. HepG2 cells were treated with BBGC for 24 hours before measuring CYP3A4, CES1 and Interleukin (IL)-8 mRNA levels and protein expression. Nuclear factor kappa B (NF-κB) expression and translocation was analysed, using immunocytochemistry, after treating the cells with BBGC for 4 hours. Data are expressed as mean (or 2(-ΔΔCt) for mRNA expression) ± SEM from n= 5-6 independent experiments. Statistically significant differences were determined using one-way ANOVA with Bonferroni post hoc test orr a Student's t-test.
Results: Increased dicarbonyl stress significantly (p<0.01) decreased mRNA expression of both CYP3A4 (0.67 ± 0. 052) and CES1 (0.6 ± 0. 096), and this was associated with a decrease in protein expression. Dicarbonyl stress also had a proinflammatory effect significantly increasing both IL-8 mRNA expression (2.64 ± 0.23, p<0.05) and secretion (from 2.3 ± 0.18 to 5.33 ± 0.43 pg/μg of protein p<0.01), coupled with an increased expression and translocation of NF-κB (146.7 ± 2.51 %, p<0.05).
Conclusion: In the present study, hepatocytes exposed to increased dicarbonyl stress directly impaired the expression of both Phase I and Phase II drug metabolising enzymes. The increased NF-kB activation and inflammation observed following increased dicarbonyl stress may be mediating the effects on both CES 1 and CYP3A4 expression. Dicarbonyl stress through increased hepatocyte methylglyoxal levels in diabetics may impact drug clearance and therapeutic care.
Disclosure: M. Maandi: None.
862
Lipotoxicity-induced hepatocyte injury is prevented by a novel allosteric modulator of the GABAA receptor
E. Rohbeck1, A. Stein1, C. Niersmann1,2, A. Romero1, B. Knebel3, M. Roden1,4, T. Romacho5, J. Eckel1,6;
1Institute for Clinical Diabetology, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany, 2German Center for Diabetes Research (DZD), Partner Düsseldorf, München-Neuherberg, Germany, 3Institute for Clinical Biochemistry and Pathobiochemistry, German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany, 4Department of Endocrinology and Diabetology, Medical Faculty and University Hospital Düsseldorf, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany, 5ChroCoDiL Group, Department of Nursery Sciences, Physiotherapy and Medicine, Faculty of Health Sciences, University Almería, Almeria, Spain, 6CureDiab Metabolic Research GmbH, Düsseldorf, Germany.
Background and aims: NAFLD is a highly prevalent condition currently lacking an approved pharmacological therapy. Exposure to GABA has been proposed to protect from liver toxicity in both in vivo and in vitro models. Therefore, we aimed to determine if the thioacrylamide-derivative HK4, a novel positive allosteric modulator of the GABAA receptor, protects human hepatocytes against lipotoxicity-induced injury.
Materials and methods: Allosteric modulation of the GABAA receptor was determined by patch clamp in HEK-293 cells, calcium influx measurements in INS-1E cells, and by using the specific GABAA chanel blocker picrotoxin in HepG2 cells. Next generation sequencing was performed of HepG2 cells treated with palmitate in the presence or absence of HK4. Phosphorylated STAT3 and NF-κB, PDI, cleaved caspase 7 and PARP-1 were detected by Western blotting or apoptosis array. Caspase 3/7 activity measurement and TUNEL assay were performed in HepG2 cells and/or human primary hepatocytes.
Results: Patch clamping, calcium influx measurements and assays with the non-competitive GABAA chanel blocker picrotoxin confirmed HK4 as a selective modulator of the GABAA receptor. Hepatocytes were found to display the main pharmacologically-relevant subunits of the GABAA receptor. A preventive effect of HK4 on palmitate-induced apoptosis was demonstrated by reduced caspase 3/7 activity both in HepG2 (47.32 ± 6.92 % vs. 1065 ± 98.68 %, p≤0.0001) and human primary hepatocytes (58.73±16.86 % vs. 147.3 ± 31.3 %, p≤0.05). This effect was further confirmed by TUNEL assay (6.41 ± 1.42 % vs. 11.62 ± 0.99 % TUNEL positive cells, p≤0.01). HK4 reduced cleaved PARP-1 expression and upregulated the ER chaperone PDI (both p≤0.05). The protective effect of HK4 was mediated by prominent inhibition of STAT3 and NF-κB phosphorylation. Transcriptomic analysis showed substantial alterations in gene expression in response to palmitate treatment. For a number of genes, a nearly complete correction of dysregulation was observed in the presence of HK4.
Conclusion: GABAergic signaling reduces lipotoxic-induced apoptosis in hepatocytes by preventing inflammation, DNA damage and ER stress. The effect of HK4 might be mediated by the transcription factors STAT3 and NF-κB. Consequently, HK4 may arise as an innovative pharmacological tool to treat or prevent NAFLD as a first-in-class drug.
Disclosure: E. Rohbeck: None.
SO 78 Screening tools, lipids and novel biomarkers
863
Measures of insulin resistance as a screening tool for dysglycaemia in patients with coronary artery disease: a report from the EUROASPIRE V population
G. Ferrannini1, D. De Bacquer2, I. Erlund3, V. Gyberg1, K. Kotseva4,5, L. Mellbin1,6, A. Norhammar1,7, O. Schnell8, J. Tuomilehto3,9, T. Vihervaara3, D. Wood4, L. Ryden1;
1Medicine, Karolinska Institutet, Stockholm, Sweden, 2Ghent University, Ghent, Belgium, 3Finnish Institute for Health and Welfare, Helsinki, Finland, 4National University of Ireland-Galway, Galway, Ireland, 5Imperial College Healthcare NHS Trust, London, UK, 6Karolinska University Hospital, Stockholm, Sweden, 7Capio St Görans Hospital, Stockholm, Sweden, 8Forschergruppe Diabetes e.V., Munich, Germany, 9King Abdulaziz University, Jeddah, Saudi Arabia.
Background and aims: The optimal screening strategy for dysglycaemia, including type 2 diabetes (T2DM) and impaired glucose tolerance (IGT), in patients with coronary artery disease (CAD) is debated.We tested the hypothesis that measures of insulin resistance by homeostasis model assessment (HOMA) indexes may constitute accurate screening methods in a CAD population.
Materials and methods: Insulin, C-peptide, glycated haemoglobin A1c (HbA1c) and an oral glucose tolerance test (OGTT) were centrally assessed in 3534 CAD patients without known dysglycaemia from the EUROASPIRE V survey. Three different HOMA indexes were calculated: HOMA-IR, HOMA2 based on insulin (HOMA2-ins), and HOMA2 based on C-peptide (HOMA2-Cpep). Dysglycaemia was diagnosed based on the two-hour postload glucose (2hPG) value obtained from the OGTT. Information on study participants was obtained by standardised visits. The optimal thresholds of the three HOMA indexes for dysglycaemia diagnosis were obtained by the maximum value of Youden’s J statistic on receiver operator characteristics curves. The diagnostic performance of such thresholds was tested for both T2DM (i.e. in reference to a 2hPG value ≥11 mmol/L) and dysglycaemia (i.e. in reference to 2hPG value ≥7.8 mmol/L) and their correlation with several clinical parameters was assessed by Spearman’s coefficients.
Results: The mean age of the patients was 63 years and 25% were women. Fifty-four percent of the patients had central obesity, 18% were current smokers, mean blood pressure was 133/80 mmHg and mean LDL-cholesterol 2.4 mmol/L. The OGTT revealed that 41% were dysglycaemic (IGT = 24% and T2DM = 16%). Mean insulin, C-peptide and HOMA indexes were significantly higher in patients with vs. without newly detected dysglycaemia (all p<0.0001). Sensitivity and specificity of the three HOMA indexes for the diagnosis of dysglycaemia were low and the associations between 2hPG and the other parameters in the total sample were weak, with Spearman correlation coefficients of 0.15 for fasting insulin, 0.19 for C-peptide, 0.24 for HOMA-IR, 0.18 for HOMA2-ins and 0.22 for HOMA2-Cpep. HOMA-IR, HOMA2-ins and C-peptide were strongly correlated with body mass index and waist circumference (Spearman correlation coefficients ranging 0.43-0.47).
Conclusion: Screening for dysglycaemia in CAD patients by insulin, C-peptide, HOMA-IR, HOMA2-ins and HOMA2-Cpep had insufficient diagnostic performance to detect dysglycaemia with reference to the yield of an OGTT, which should still be prioritized. Further studies are warranted to assess whether measures of insulin resistance might be better markers of unfavourable metabolic derangement beyond dysglycaemia.
Supported by: European Society of Cardiology, Erling-Perssons Stiftelse (GF, LR) and Irstadska Stiftelsen (LR)
Disclosure: G. Ferrannini: Grants; Erling-Perssons Stiftelse. Honorarium; European Society of Cardiology.
864
Impact of RAGE and IL-6 polymorphisms in the progression for angiopathy in patients with type 2 diabetes
M. Bicho1,2, C. Fonseca3, A.C. Santos1,2, J.F. Raposo4, A. Valente5;
1Genetics Laboratory, Environmental Health Institute, Faculdade de Medicina, Universidade de Lisboa, 2Instituto de Investigação Científica Bento da Rocha Cabral, 3Faculdade de Medicina, Universidade de Lisboa, 4Associação Protetora dos Diabéticos de Portugal, 5Atlântica - Instituto Universitário, Lisbon, Portugal.
Background and aims: Type 2 diabetes mellitus (T2DM) is an inflammatory condition related to an increased expression of pro-inflammatory cytokines, namely interleukin-6 (IL-6), which induces the appearance of microvascular and macro complications, kidney disease and anemia. High levels of circulating IL-6 promote an anti-erythropoietic effect and consequent decrease in the number of circulating erythrocytes. Persistent high blood glucose levels stimulate the formation of the irreversible advanced glycation end products (RAGE), resulting in non-enzymatic glycation and oxidation of proteins and lipids and platelet hyperactivity associated with a high risk of vascular complications. The interaction between RAGE with its ligands results in the activation of multiple signalling pathways, which, in turn, also can increase the secretion of pro-inflammatory cytokines such as IL-6 in T2DM patients. The IL-6 (rs1800795) and RAGE (rs1800624) are functional polymorphisms and have been associated with cardiovascular risk and diabetes comorbidities. This study aimed to evaluate the possible effect of these polymorphisms with anemia and hematological parameters in people with T2DM with the angiopathic disease.
Materials and methods: 148 Portuguese adults (40-75 years) with T2DM. Patients were divided into two groups: GI-n=75 with T2DM and angiopathy and GII-n=73 with T2DM without angiopathy. IL-6 and RAGE genotypes were identified by the endpoint analysis® method. The statistical analysis was performed in SPSS®. Results were considered statistically significant for p<0.05.
Results: Between groups, there were no differences in genotypic and allelic frequencies of the studied polymorphisms. In this cohort, for IL-6 polymorphism, were observed high values of: Mean Corpuscular Volume (MCV) (p=0.026) and Mean Corpuscular Hemoglobin (MCH) (p=0.015) in CC genotype carriers and monocyte/HDL-Cholesterol ratio (MHR) (p=0.038) in C allele carriers (CC+ GC). No differences were observed between hematological parameters and RAGE polymorphism. Selecting the patients by groups and comparing the hematological parameters and the IL-6 polymorphism, it was verified in GI: increased values of MCH (p=0.009) for carriers of the CC genotype. For the RAGE polymorphism, increased values were observed in GI for monocytes (p=0.016) and MHR (p=0.038) in carriers of the AA genotype. Analyzing the relationship of hematological parameters between groups, it was found for IL-6 increased values in GII of: MCV (p=0.033) and monocytes (p=0.007) in carriers of the G allele (GG+GC) and MHR in GC genotype carriers (p=0.019); in the RAGE polymorphism, high values of: Mean Corpuscular Hemoglobin Concentration (p=0.020) were found in carriers of the AA genotype in the GI. In GII, increased monocyte values (p=0.034) and MHR (p=0.005) were observed in carriers of the T (TT+TA).
Conclusion: IL-6 and RAGE polymorphisms appear to influence susceptibility to angiopathy in T2DM patients modulating the disease-associated hematological parameters important to understanding the impact of anemia in these patients.
Supported by: FCT
Disclosure: M. Bicho: None.
865
Erythrocyte membrane fluidity as a novel biomarker for a quantitative evaluation of residual cardiovascular risk in diabetic patients
G. Bianchetti1,2, C. Cefalo3,4, C. Ferreri5, A. Sansone5, C. Serantoni1,2, A. Abeltino1,2, T. Mezza3,4, P. Ferraro6, M. De Spirito1,2, A. Giaccari3,4, G. Maulucci1,2;
1Department of Neuroscience - Biophysics Section, Università Cattolica del Sacro Cuore, Rome, 2Fondazione Policlinico "A. Gemelli" IRCCS, Rome, 3Dipartimento di Scienze Mediche e Chirurgiche - Centro per le Malattie Endocrino-Metaboliche, Fondazione Policlinico "A. Gemelli" IRCCS, Rome, 4Dipartimento di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Rome, 5CNR ISOF, Consiglio Nazionale delle Ricerche ISOF, Bologna, 6U.O.C. di Nefrologia, Fondazione Policlinico "A. Gemelli" IRCCS, Rome, Italy.
Background and aims: Many factors, such as hyperglycemia, atherogenic lipid profile, obesity, and hypertension have been closely associated with the development of cardiovascular diseases (CVD) in type 2 diabetes (T2D). However, despite effective control of the main known variables, a considerable residual cardiovascular risk is still not predictable in diabetic subjects. Here, we propose erythrocyte (RBC) membrane fluidity, reflecting the state of a more complex network of regulatory processes activated in the disease, as a valid biomarker for a quantitative evaluation of residual cardiovascular risk.
Materials and methods: To test whether changes in erythrocyte membrane fluidity may be associated with the development of CVD in diabetic patients, we developed a bioimaging system able to measure with high spatial resolution plasma membrane modifications. Erythrocyte membrane fluidity, quantified by the generalized polarization (GP) index, has been measured on blood samples of 234 diabetic subjects, 86 of whom reported a major cardiovascular event in clinical history, and 32 non-diabetic subjects. Fatty acids (FAs) membrane composition of RBC was obtained for a set of sampled controls and T2D with and without CVD through a gas chromatography-flame ionization detector.
Results: Relying on GP values, ranging from -1 (high fluidity) to +1 (low fluidity), an unsupervised hierarchical clustering method was applied, leading to the determination of a GEL cluster characterized by GP=0.46±0.01 and a High-Risk cluster (HR) characterized by a lower GP value (GP= 0.40±0.03, p<0.05). Though having comparable CVD risk factors according to the UKPDS engine, as well as the same glycaemic and lipid profile, GEL and HR clusters differ in the percentage of patients reporting a major cardiovascular event (GEL: 24%±6%; HR: 41%±4%, p<0.05). The characterization of the RBC FA profile reveals that patients with more fluid membranes (HR) present a clear shift towards a pro-inflammatory condition, reflected in an increased ω6/ω3 inflammatory risk index (HR: 3.6±0.5; GEL: 2.8±0.8, p=0.04), mainly due to higher levels of arachidonic acid (AA-HR=21.6%±1.4%, AA-GEL:19.1%±2.5%, p= 0.02), which is a well-known precursor of inflammatory mediators.
Conclusion: In conclusion, the hereby proposed analysis of RBC membrane fluidity, combining confocal imaging and FA-based lipidomic studies, paves the way for the introduction of RBC fluidity both as a powerful biomarker for CVD and a potential therapeutic target for personalized intervention. Additional studies, following up specific nutritional protocols, will be necessary to further investigate the effect of RBC fluidity normalization in modulating CVD and reducing its associated risk.
Supported by: EFSD and Sanofi European Grants for "Innovative Measurements of Diabetes Outcomes" (2018)
Disclosure: G. Bianchetti: None.
866
Comparison of effects of canagliflozin, dapagliflozin and empagliflozin on cardio-renal biomarkers in 150 patients with type 2 diabetes
V. Teli, V. Gupta;
Tardeo, VG-ADVANTAGE Diabetes Thyroid and Endocrine Center, Mumbai, India.
Background and aims: The aim was to compare the effects of 3 SGLT2i (Canagliflozin {C}, Empagliflozin {E} & dapagliflozin {D} on cardio-renal biomarkers in T2DM patients followed for 1 year.
Materials and methods: Between January to March’20, 150 T2DM patients irrespective of A1c on SGLT2i (dapagliflozin {D} emapgliflozin {E} or canagliflozin {C}) therapy, aged 18-75yrs were included and analysed. They were divided in 3 groups: D (50 on D) E (50 on E) & C (50 on C). Patients were evaluated for cardio-renal {CR} biomarkers [BMI-kg/m2, systolic (S) BP, diastolic (D) BP {mm in Hg}, Lipid profile mg/dl, Hs-CRP {mg/L}, NT-ProBNP pg/mL, creatinine {Cr}, Cystatin-C {Cy-C} eGFR, UACR] & followed 2-3 monthly for 1 yr. Baseline characteristics: analysed using descriptive statistics. Data analysis: SPSS 26, represented as mean ± SE & independant sample t-test used. P value <0.05 considered significant (S).
Results: Baseline (B) characters: well matched D gr: B - 12 mths: S reduction in BMI, SBP, A1c, TC, LDL, TG, Cr, UACR, hs-CRP. E gr: B - 12 mths: S reduction in BMI, SBP, DBP, A1c, TC, LDL, TG, eGFRCy-C, hs-CRP. C gr: B - 12 mths: S reduction in BMI, SBP, DBP, TC, LDL, TG, Apo-B, hs-CRP.
Differences at 12 mths: S reduction in BMI (D 1.6064±0.2935, E 0.698±0.2096, C 1.114±0.2964, p-0.038), DBP (D 1.280±1.571, E 5.840±1.877, C 6.52±1.177, p-0.040) & Apo-B (D 1.1828±3.0408, E 0.6062±0.7705, C 12.087±3.2951, p-0.023). Group D Versus E: S reduction in D gr {Wt (-2.0282±0.84, p-0.017) & BMI (-0.9866±0.36, p-0.007)}. Group E Vs C: None. Group D Vs C: S reduction: SBP (7.480±3.755, p-0.049), DBP 5.240±1.963, p-0.009), Apo-B (10.904±4.913, p-0.029) & hs-CRP (1.574±0.636, p-0.015) favouring C.
Conclusion: Canagliflozin S reduces DBP & Apo-B compared to dapagliflozin & empagliflozin. Canagliflozin S reduces hs-CRP, SBP, DBP & Apo-B compared to dapagliflozin.
Disclosure: V. Teli: None.
867
Prevalence of lipodystrophy and agreement between clinical and radiological imaging methods in diagnosing lipodystrophy in insulin requiring subjects with diabetes
A. Baidya1, S. Dey2, R. Bhadra2;
1Endocrinology, Nil Ratan Sircar Medical College & Hospital, 2Radiodiagnosis, Nil Ratan Sircar Medical College & Hospital, Kolkata, India.
Background and aims: Subjects with diabetes on subcutaneous insulin having lipodystrophy have an almost 6 fold higher incidence of hypoglycaemia compared with subjects without lipodystrophy and 7 fold higher incidence of glycaemic variability which increases the risk of chronic complications of diabetes.1. To study the prevalence of lipodystrophy in insulin requiring subjects with diabetes by clinical and radiological imaging methods. 2. To study the agreement between clinical and imaging methods [Ultrasonography(USG) and Magnetic Resonance Imaging(MRI)] in the diagnosing lipodystrophy.
Materials and methods: Inspection and palpation of insulin injection sites of 362 subjects on subcutaneous insulin for more than 6 months weredone. All subjects were subjected to USG for confirmation of lipodystrophy and proper characterization of the lesions. In 25 subjects, for whom USG was not helpful in proper characterization of lipodystrophy lesions, MRI was performed.
Results: Prevalence of lipodystrophy by clinical and radiological methods is 78.18% and 82.04% respectively. Clinical examination revealed nodular, diffuse hypertrophic, lipoatrophic, lipodystrophy, diffuse plus nodular pattern in 26.5%, 23.7%, 7.4%, 7.4% and 5% patients respectively. Radiological methods revealed diffuse lipohypertrophy, nodular, lipodystrophy, diffuse plus nodular and lipoatrophy pattern in 37.5%, 24.3%, 18.6%, 12.6% and 7.4% patients respectively. In 10 patients MRI helped in the diagnosis of lipodystrophy and in another 10 patients MRI helped in proper subtyping of lipodystrophy. There is a strong agreement between clinical and radiological imaging methods in diagnosing lipodystrophy (Kappa value 0.862; P<0.001)
Conclusion: Prevalence of lipodystrophy is high in insulin requiring subjects with diabetes. There is a strong agreement between clinical and radiological imaging methods in diagnosing lipodystrophy.
Disclosure: A. Baidya: None.
868
Pro-atherosclerotic alterations in lipoprotein particle distribution and size are associated with a high triglyceride to HDL-cholesterol (TG/HDL) ratio
M. Chiriacò1, N. Santoro2, S. Caprio2, D. Tricò3;
1Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 2Department of Pediatrics, Yale School of Medicine, New Haven, USA, 3Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy.
Background and aims: A high triglyceride to high-density lipoprotein cholesterol (TG/HDL) ratio has been associated with cardiovascular disease independently of traditional risk factors. We examined whether this association can be explained by pro-atherosclerotic alterations in the lipoprotein subclasses in individuals with high TG/HDL ratio.
Materials and methods: Lipoprotein particle concentration and size were analyzed by proton NMR spectroscopy in a multi-ethnic cohort of 592 adolescents with overweight/obesity (age 13±3 years, 58% females, BMI z-score 2.1±0.8), who were characterized by a 3-hour oral glucose tolerance test (OGTT) and stratified by quartiles of TG/HDL ratio.
Results: Compared with the lowest TG/HDL quartile (<1.3), the highest quartile (>3.4) showed higher BMI z-score (+0.5±0.1), more Caucasians (+12%, p<0.0001) and less African Americans (-32%, p<0.0001), and worse plasma lipid profile (TG +1.6±0.1 mmol/L, HDL cholesterol -0.5±0.03 mmol/L, low-density lipoprotein [LDL] cholesterol +0.4±0.1 mmol/L), glucose tolerance (fasting glucose +0.16±0.06 mmol/L, p=0.007; 2-h glucose +0.84±0.16 mmol/L, p<0.0001) and insulin sensitivity (WBISI -1.38±0.14 mmol/L, p<0.0001), despite similar age and sex distribution. At quantitative lipoprotein analysis, the highest TG/HDL ratio quartile was characterized by a higher concentration of TG-rich very low-density lipoprotein (VLDL) particles (-43±3 nmol/l, p<0.0001) and LDL particles (378±39 nmol/l, p<0.0001), but similar HDL particles concentration compared with the lowest TG/HDL ratio quartile. The average particle size was higher for VLDL (+7.6±1.0 nm, p<0.0001) but lower for LDL (-0.9±0.1 nm, p<0.0001) and HDL (-0.7±0.04 nm, p<0.0001) in the highest TG/HDL ratio quartile, who showed a greater proportion of large VLDL, very small LDL and small HDL within each lipoprotein class (shown in Figure).
Conclusion: In adolescents with obesity, a high TG/HDL ratio is characterized by larger VLDL particles and higher concentrations of smaller LDL and HDL particles, characteristic of a more atherogenic lipid profile. A higher TG/HDL ratio is also associated with insulin resistance and worse glucose metabolism.
Clinical Trial Registration Number: NCT01966627
Supported by: R01-HD-40787, R01-HD-28016, K24-HD-01464 to SC, R01DK114504 to NS, Rising Star Fellowship to DT
Disclosure: M. Chiriacò: None.
869
Triglyceride-glucose index as predictor for re-hospitalisation for heart failure after acute myocardial infarction
J.-O. Jeong, P. Song, S. An;
Division of Cardiology, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Republic of Korea.
Background and aims: Insulin resistance has been extensively demonstrated to be a potential risk factor for cardiovascular diseases, such as acute myocardial infarction (AMI) or heart failure. The triglyceride-glucose (TyG) index has been proposed as a reliable surrogate marker of insulin resistance. The recent studies demonstrated that elevated TyG index level was a strong independent predictor of adverse cardiac events in patients with AMI. However, the prognostic value of the TyG index for re-hospitalisation for heart failure (rHHF) after AMI remains unclear. Heart failure (HF) is a frequent complication of AMI, and insulin resistance was shown to increase the risk of HF hospitalisations following an AMI. Therefore, we sought to evaluate the association between TyG index and the incidence of rHHF in survivors of an AMI.
Materials and methods: We assessed 11,593 patients (8,586 men and 3,007 women) with AMI using data from the Korea Acute Myocardial Infarction Registry-National Institutes of Health (KAMIR-NIH) registry. The KAMIR-NIH is a prospective, multi-centre, nationwide cohort study for patients with AMI. The patients were divided into three groups according to the tertiles of the TyG index, calculated as Ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2]. We assessed hazard ratios (HRs) with 95% confidence intervals (CIs) for rHHF using multivariate Cox proportional-hazards regression models.
Results: The median age of the patients was 64.0 (IQR 54.0 to 74.0) years and the mean TyG index was 8.99 (IQR 8.51 to 9.51). During a 3-years median follow-up period, 452 (3.9%) patients developed an rHHF. Contrary to expectations, the incidence of rHHF after AMI was higher among patients with TyG index levels in the lower tertile (5.1% in lower vs. 3.2% in middle vs. 3.4% in upper tertile). HRs of TyG index for rHHF were 1.313 (95% CI 1.022-1.686) in lower and 0.910 (95% CI 0.695-1.192) in middle tertile, respectively after adjusting for confounding factors (upper tertile of the TyG index as a reference). The area under ROC curve of the TyG index for predicting the occurrence of rHHF was 0.547 [(0.538 to 0.556), p < 0.0007], with the cut-off value of ≤ 8.68. No interaction was found between diabetes and TyG-index for rHHF in multivariate analysis (p value for interaction = 0.749), However, a significant interaction was found between age groups (i.e. ≥ 65 vs < 65 years) and TyG index for rHHF in the multivariate model (p value for interaction < 0.001). The significant trend of TyG-index was observed only among the population aged ≥ 65 for rHHF; HRs of TyG index for rHHF were 1.413 (95% CI 1.067-1.872) in lower and 0.922 (95% CI 0.675-1.258) in middle tertile, respectively, but not among the population aged < 65.
Conclusion: Paradoxically, a lower TyG index appeared to be a worse prognostic factor on 3-year rHHF after AMI, and this issue was more prominent among the older population. Future studies are needed to understand the mechanisms underlying the TyG index paradox and to characterize the TyG index paradox in certain patient subgroups.
Disclosure: J. Jeong: None.
870
Effects of polyphenols on cholesterol efflux and insulin secretion in MIN6 cells
K. Matsuki, Y. Kimura, K. Matsumura, H. Murakami, J. Tanabe, M. Daimon;
Department of Endocrinology and Metabolism, Hirosaki University Graduate School of Medicine, Hirosaki, Japan.
Background and aims: Diabetic dyslipidemia is characterized by elevated levels of triglyceride-rich lipoproteins and reduced levels of HDL-cholesterol (HDL-C). Low levels of HDL-C are associated with an increased risk of atherosclerotic cardiovascular disease, because HDL protects against atherosclerosis through multiple mechanisms include removal of excess cholesterol from macrophages. HDL can also influence cholesterol homeostasis in pancreatic β-cell. It is noteworthy that absence of ATP-binding cassette transporter A1 (ABCA1), which is a cellular cholesterol transporter and regulates islet cholesterol efflux, results in islet cholesterol overload and impaired insulin release in mice. We examined the associations of cholesterol efflux and insulin secretion in MIN6 cells with polyphenols that is reported to increase cholesterol efflux.
Materials and methods: Murine MIN6 cells were labeled with 3H-cholesterol and incubated with ApoA1 (10 μg/ml) or HDL (25 μg/ml) for 24 hours. The percentage cholesterol efflux was calculated by dividing the media-derived radioactivity by the sum of the radioactivity in the media and the cells. The effect of polyphenols on the cholesterol efflux was studied, adding that into the medium before the analysis of the cholesterol efflux. Insulin secretion was assessed by ELISA as the concentration of insulin in the culture medium. The mRNA expression of ABCA1 and ABCG1 were determined by real-time Quantitative RT-PCR.
Results: Under the existence of polyphenols (isorhamnetin), the ApoA1 and HDL-mediated cholesterol efflux had significantly increased by 1.4% or 1.8%, respectively (n=4, p<0.01). Isorhamnetin increased insulin secretion by 882 ng/mg protein/h (n=3, p<0.05) and significantly decreased ABCA1 and ABCG1 expressions.
Conclusion: These data suggest that isorhamnetin increases cholesterol efflux and insulin secretion in MIN6 cells. Isorhamnetin could be the interesting candidate to investigate the new treatment for patients with diabetes mellitus.
Supported by: This work was supported by JSPS KAKENHI Grant Number JP20K11549
Disclosure: K. Matsuki: None.
SO 79 Focus on the heart and beyond
871
Metabolism of valvular interstitial cells is glucose dependent and susceptible to different diabetic conditions
M. Barth1, J.I. Selig1, H.V. Krug1, C. Küppers1, D.M. Ouwens2,3, A. Lichtenberg1, P. Akhyari1;
1Department of Cardiac Surgery, University Hospital of the Heinrich-Heine-University Düsseldorf, Düsseldorf, 2Institute of Clinical Biochemistry and Pathobiochemistry, German Diabetes Center (DDZ), Düsseldorf, 3German Center for Diabetes Research (DZD), München-Neuherberg, Germany.
Background and aims: Diabetes is a risk factor for the development of calcific aortic valve disease. Previous studies showed that the main cell type of aortic heart valves, the valvular interstitial cells (VIC), are susceptible to diabetic conditions such as hyperglycaemia and hyperinsulinemia in terms of impaired glycolysis and mitochondrial respiration. Since knowledge about VIC metabolism especially in the context of diabetes is still scarce, the aim of the present study was to evaluate the utilization and dependency of different fuels of VIC under diabetic conditions.
Materials and methods: Primary VIC of n = 6-7 individual sheep were cultured under normoglycemic and under diabetic conditions (4.5 g/L glucose and 100 nM insulin) for six days. Mitochondrial function and determinants of glycolysis dependent on different mitochondrial fuels like glucose, glutamine and fatty acids were measured using a Seahorse XFe96 extracellular flux analyser. Glycolytic metabolites were analysed by determination of glucose-6-phosphate, pyruvate and lactate with respect to a detailed examination of the impact of hyperinsulinemia (HI), hyperglycaemia (HG) and the combination of both (HI+HG).
Results: Analysis of the glycolytic rate of VIC showed that basal glycolysis is increased by diabetic conditions (p<0.001) independent of the offered fuel. Induced glycolysis was upregulated under diabetic conditions when VIC were restricted to glutamine (p<0.01) or fatty acids (p<0.0001). Analysis of mitochondrial respiration showed that basal respiration is increased by diabetic conditions (p<0.0001) independent of different fuels. Restriction to fatty acids led to a reduction in maximal respiration (p<0.05) and spare respiratory capacity (p<0.01) of VIC under diabetic conditions. ATP production, in contrast, was not altered by the offered fuels but significantly increased by diabetic conditions (p<0.001). Glucose-6-phosphate concentration was significantly increased by HG (p=0.009) but not by HI (p>0.9999) or HI+HG (p=0.059). Pyruvate concentration was significantly decreased by HI (p=0.005), but not by HG (p>0.9999) or HI+HG (p=0.384). Relative quotient of lactate/protein remained unchanged.
Conclusion: Basal mitochondrial respiration and glycolysis is impaired by diabetic conditions, whereas VIC are able to compensate fuel restrictions. Restriction to glutamine or fatty acids, in contrast, impairs the ability of VIC to maintain their metabolic potential under diabetic conditions. These findings are indicative for glucose being the main metabolic fuel of VIC. Moreover, the content of VIC glucose metabolites is affected by different diabetic stimuli, indicating that glucose metabolism of VIC is diversely susceptible to HI and HG.
Supported by: DFG
Disclosure: M. Barth: Grants; German Research Foundation (DFG).
872
Influence of glycaemic status on the risk of cardiovascular events after a myocardial infarction
M. Wijkman1, B. Claggett2, K. Jering2, M. Yilmaz3, M. Claeys4, C. De Pasquale5, P. Kerkar6, T. Moccetti7, M. Ntsekhe8, D. Sim9, M. Studenčan10, M. Tokmakova11, A. Zaman12, Y. Zhou13, E. Lewis14;
1Linköping University, Norrköping, Sweden, 2Brigham and Women´s Hospital, Harvard Medical School, Boston, USA, 3Dokuz Eylul University, Izmir, Turkey, 4University Hospital Antwerp, Edegem, Belgium, 5Flinders Medical Centre, Adelaide, Australia, 6Asian Heart Institute and Research Center, Mumbai, India, 7Cardiocentro Ticino, Lugano, Switzerland, 8University of Cape Town, Cape Town, South Africa, 9National Heart Centre, Singapore, Singapore, 10Teaching Hospital of J.A. Reiman, Prešov, Slovakia, 11Medical University of Plovdiv, Plovdiv, Bulgaria, 12Newcastle University, Newcastle, UK, 13Novartis Pharmaceutical Corporation, East Hanover, USA, 14Stanford University School of Medicine, Palo Alto, USA.
Background and aims: Survivors of myocardial infarction (MI) with a prior diagnosis of diabetes are more likely to develop heart failure (HF) and to experience other cardiovascular (CV) events. Less is known about the CV risk in MI survivors with pre-diabetes or screen-detected diabetes. The aim of this study was to assess the association between glycemic status and CV events in a population of patients with a recent MI and elevated risk of HF.
Materials and methods: In 5661 participants in the PARADISE-MI (Prospective ARNI vs. ACE inhibitor trial to Determine Superiority in reducing heart failure Events after Myocardial Infarction) trial, glycemic status was determined at baseline according to prior medical history and HbA1c values (no diabetes=no prior diagnosis of diabetes and HbA1c <5.7%, pre-diabetes=no prior diagnosis of diabetes and HbA1c 5.7-6.4%, screen-detected diabetes=no prior diagnosis of diabetes and HbA1c ≥6.5%, prior diabetes=prior diagnosis of diabetes regardless of HbA1c). Incidence rates for two adjudicated composite outcomes (CV death/outpatient symptomatic HF/HF leading to hospitalization, and CV death/MI/stroke) were estimated.
Results: During a median follow-up time of 22 months, the incidence rates of HF or CV death was 4.6/100 person-years in those without diabetes (n=1401), 6.5/100 person-years in those with pre-diabetes (n=1576), 7.8/100 person-years in those with screen-detected diabetes (n=283) and 9.0/100 person-years in those with a prior diagnosis of diabetes (n=2401). The corresponding incidence rates for MI/stroke/CV death were 4.4, 5.7, 6.3 and 8.4 per 100 person-years, respectively. After adjustment for additional markers of increased risk, only prior diabetes remained significantly associated with increased risk for both outcomes (adjusted HR for HF/CV death: 1.87, 95% CI 1.52-2.31, P<0.001, and for MI/stroke/CV death: 1.81, 95% CI 1.46-2.23, P<0.001).
Conclusion: In people with a recent MI and additional risk factors for development of HF, incidence rates of CV events were higher in those with a more severe hyperglycemic phenotype. This underscores the importance of early detection of glycemic abnormalities after an MI.
Clinical Trial Registration Number: NCT02924727
Supported by: The PARADISE-MI study was funded by Novartis
Disclosure: M. Wijkman: Lecture/other fees; Advisory boards or lectured for MSD, Lilly, NovoNordisk, and Sanofi.
873
Physical activity and diastolic dysfunction in people with with type 2 diabetes
A.G. Hoek1, I.R. Jankipersad1, E. Dal Canto1,2, N.R. den Braver1, R. Meer1, P.J.M. Elders3, J.W.J. Beulens1,4;
1Epidemiology & Data Science, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, 2Department of Experimental Cardiology, University Medical Center Utrecht, Utrecht, 3Department of General Practice and Elderly Care Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, 4Julius Center for Health Sciences and Primary Care, University Medical Centre Utrecht, Utrecht University, Utrecht, Netherlands.
Background and aims: Heart failure with preserved ejection fraction (HFpEF) is an emerging epidemic, which is especially prevalent in people with type 2 diabetes. In the general population, lower levels of physical activity (PA) have been associated with a higher risk of overall heart failure and left ventricular diastolic dysfunction (LVDD). However, the association between PA, HFpEF and LVDD has never been investigated in a population of people with type 2 diabetes.
Materials and methods: We performed a cross-sectional analysis on 239 participants with type 2 diabetes from the Diabetes Care System cohort, who had no prior diagnosis of heart failure. Percentage of time in moderate-to-vigorous (% in MVPA) of total PA was assessed with accelerometers, wore for 7 days. LVDD was echocardiographically assessed by measuring E/e’ ratio, pulmonary artery pressure, left ventricular mass index and left atrial volume index. Furthermore, the continuous H2FPEF score estimating the probability of HFpEF - using the estimated pulmonary artery pressure, E/e’ ratio, presence of atrial fibrillation, age and body mass index (BMI) - was calculated. Multivariate linear regression models, adjusted for sex, age, BMI, hypertension, hyperlipidemia and diabetes duration were used to analyze the cross-sectional relationship between PA and echocardiographic markers, and effect modification for sex was investigated. For the cross-sectional analysis between PA and the continuous H2FPEF score a similar analysis was used, but BMI was left out of the models since it is embedded in the H2FPEF score.
Results: A 1.0% higher MVPA was significantly associated with a 9.09% decrease in the H2FPEF score (95% CI -14.09;-4.09) for women in the fully adjusted model. For men, no associations were found (-0.67; 95% CI: -3.07;1.73). However, in a separate analysis, a strong association between % in MVPA and BMI (-0.98; 95% CI: -1.54; -0.41) was found, suggesting that the results might be partly driven by BMI. No associations were found between PA and single markers of LVDD; mean E/e’ (-0.003; 95% CI: -0.016;0.010); left atrial volume index (0.013; 95% CI: 0.061;-0.001); left ventricular mass index (0.006; 95% CI: -0.005;0.016) and estimated pulmonary artery pressure (-0.01; 95% CI: -0.048;0.027).
Conclusion: In this study we found that increased PA was associated with a lower H2FPEF score in women with type 2 diabetes. This association was not confirmed when investigating the separate echocardiographic markers of LVDD. Given the strong association between % in MVPA and BMI the found association with the H2FPEF score might be partly driven by BMI.
Supported by: ZonMW NWO-Vidi grant (91 71 8304)
Disclosure: A.G. Hoek: None.
874
Glycaemic variability is associated with diastolic dysfunction in patients with type 2 diabetes
Y.Y. Dzhun1, G.B. Mankovsky1, N.M. Rudenko1,2, Y.Y. Marushko1, Y.A. Saenko1,2, B.M. Mankovsky1,2;
1Government Institution “The Scientific and Practical Medical Center of Pediatric Cardiology and Cardiac Surgery of the Ministry of Health of Ukraine”, 2Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine.
Background and aims: There is convincing body of evidences that diabetes mellitus is associated with an increased risk of heart failure. However, the exact mechanisms of pathogenetic role of diabetes in the development and progression of heart failure remain not fully elucidated. In the last decade, with the widely use of continuous glucose monitoring, indicators of glycemic variability have been increasingly taken into consideration. Glycemic variability could be one of the reason predisposing to heart failure in subjects with diabetes. The aim of the study is evaluation of the relationship between glycemic variability and diastolic dysfunction in patients with type 2 diabetes mellitus without coronary artery disease.
Materials and methods: Seventy-eight patients with heart failure with preserved left ventricular ejection fraction and type 2 diabetes mellitus were examined. Exclusion criteria were: clinically manifesting coronary artery disease, LV ejection fraction <50%, morbid obesity, atrial fibrillation, anemia, severe valvular heart disease (severe mitral, tricuspid, aortic, and/or pulmonary artery insufficiency/or stenosis), hyperthyroidism, hypertrophic or restrictive cardiomyopathy. Diastolic function was assessed by echocardiography; glycemic variability was evaluated by continuous monitoring of glucose. According to the glycemic variability all studied patients were divided into two groups: group I - SD> 2 (high glycemic variability), n = 40; group II - SD ≤ 1.9 (normal glycemic variability), n = 38.
Results: Group I were older (49 (9) vs 46 (5); p<0.05 (the results of the study are presented as mean (SD) or absolute number (%)), with a longer duration of DM (10 (9.5) vs 6 (5.5); p<0.01). In group I compared to group II there were more patients with grade 2 of diastolic dysfunction (25 (62.5%) vs 10 (26.3), p<0.05). Patients in group I had more severe diastolic dysfunction which was characterized by an increased values of E/e', early transmitral flow rate and peak rate of tricuspid regurgitation (E/e': 14.2 (4.2) vs 12.1 (3.5); p<0.01; E, cm / s: 85 (19) vs 75.2 (22); p<0.05 and V max TR, m / s: 2.4 (0.6) vs 2.1 (0.4); p<0.05 in the 2 groups of subjects studied, respectively). In group I patients insulin and sulfonylureas were used more often (11 (27.5%) vs 0 p = 0.0001; 25 (62.5%) vs 10 (26.3%); p<0.01, resepctively); patients of group II were more often treated with iSGLT2 (2 (5%) vs 13 (34.21%); p<0.01).
Conclusion: Increased glycemic variability is associated with diastolic dysfunction and could predispose to development and progression of heart failure with preserved ejection fraction.
Disclosure: Y.Y. Dzhun: None.
875
Prognostic models for heart failure in patients with type 2 diabetes: a systematic review and meta-analysis
G. Kostopoulos1, I. Doundoulakis2, K.A. Toulis1,3, T. Karagiannis4, A. Tsapas4,5, A.-B. Haidich6;
1Department of Endocrinology, 424 General Military Hospital, Thessaloniki, Greece, 2First Department of Cardiology, Hippokration Hospital, Athens, Greece, 3Institute of Applied Health Research, University of Birmingham, Birmingham, UK, 4Clinical Research and Evidence-Based Medicine Unit, Second Medical Department, Aristotle University of Thessaloniki, Thessaloniki, Greece, 5Harris Manchester College, University of Oxford, Oxford, UK, 6Department of Hygiene, Social-Preventive Medicine and Medical Statistics, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Background and aims: Heart failure (HF) is one of the first clinical manifestations of cardiovascular disease in patients with type 2 diabetes (T2D) and can be prevented if addressed early. Recent guidelines advocate risk stratification of patients with T2D to guide treatment decisions. Although several clinical prognostic models (CPMs) for diabetes-related outcomes exist, there is a knowledge gap regarding those predicting HF. We conducted a systematic review and meta-analysis to retrieve, critically appraise, and summarise the performance and generalisability of all available CPMs for HF in patients with T2D.
Materials and methods: A systematic literature search in five electronic databases and grey literature was conducted from inception to October 2021 to identify studies developing and/or validating models predicting HF applicable to patients with T2D. A random-effects model was used for pooling measures of discrimination and 95% prediction intervals (PI) were estimated. A descriptive summary of calibration was also provided. The Prediction Model Risk of Bias Assessment Tool (PROBAST) and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach were used to evaluate risk of bias and certainty in evidence.
Results: Forty-five studies met the eligibility criteria reporting on 53 CPMs: 1) CPMs developed in patients with type 2 diabetes for prediction of heart failure (n =38), 2) CPMs originally developed in non-diabetic cohorts for heart failure prediction and externally validated in patients with type 2 diabetes (n =3) and 3) CPMs originally developed for predicting a different outcome and externally validated for heart failure (n =12). Overall, 90% of the developed CPMs and 40% of the validation efforts were at high risk of bias, mainly due to flaws in the analysis domain. Six CPMs were included in the meta-analysis with the TRS-HFDM (C-statistic = 0.79 95%CI (0.74, 0.83), 95%PI (0.61, 0.90); low certainty) and RECODe (C-statistic = 0.75 95%CI (0.72, 0.78), 95%PI (0.68, 0.81); high certainty) showing the best performance. QDiabetes-HF demonstrated also good discrimination but was externally validated only once and not meta-analysed.
Conclusion: Several prognostic models are available for HF in patients with T2D, but most are at high risk of bias. Three models showed promising performance and thus, they may have a key role in current clinical practice.
Disclosure: G. Kostopoulos: None.
876
Assessment of cardiovascular events in a real-world high-risk cohort with type 2 diabetes or atheroscleortic cardiovascular disease in Germany
M. Verket1, N. Kossack2, J. Brandts1, T. Schönfelder2,3, K. Schütt1, D. Häckl2,4, N. Marx1, D. Müller-Wieland1;
1Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, 2WIG2 – Scientific Institute for Health Economics and Health System Research, Leipzig, 3Chair Health Sciences/Public Health, Medical Faculty, TU Dresden, Dresden, 4Faculty of Economics and Management Science, University Leipzig, Leipzig, Germany.
Background and aims: Heart failure (HF) and peripheral arterial disease (PAD) are common diseases with an impact on healthcare burden. We assessed the incidence of hospitalisation for HF, myocardial infarction (MI), stroke and PAD in a real-world high-risk cohort with type 2 diabetes (T2DM) or atherosclerotic cardiovascular disease (ASCVD) on a standard statin treatment in Germany from 2017-2019.
Materials and methods: The present retrospective longitudinal analyses are based on an anonymized validated research database of WIG2, which contains linkable billing and social data of about 4 million German statutorily health insured persons. We selected three cohorts from 2017-2019 with a follow-up (FU) of 12 months: patients with ASCVD and T2DM, patients with ASCVD and without T2DM, and patients with T2DM and an ASCVD risk factor. Risk factors were defined as older patients (men ≥55 years old; women ≥65 years old), smoking, hypertension, dyslipidemia, microalbuminuria and macroalbuminuria, renal dysfunction and retinopathy within index quarter or the previous 36 months. All patients received a standard statin treatment. Patients with chronic kidney disease stage 4 or 5 within matched time range were excluded.
Results: We identified 73,660 patients with ASCVD and T2DM, 100,959 patients with ASCVD and without T2DM and 29,404 patients with T2DM and a risk factor, all on statin treatment in 2019. Among these patients, around 43.7% (17.7% female), 38.8% (16.9% female) and 26.8% (16.8% female) were over 75 years old in three cohorts: patients with ASCVD and T2DM, patients with ASCVD and without T2DM and patients with T2DM and a risk factor, respectively. The cumulative incidence function (CIF) of HF hospitalization over the FU of 12 months is about 2.9%, 1.5% and 0.56% in patients with ASCVD and T2DM, patients with ASCVD without T2DM and patients with T2DM and a risk factor, respectively (Fig. 1A). There were no differences among ASCVD patients with or without T2DM in CIF of hospitalisation for MI (1.41% and 1.16%, respectively) and stroke (1.64% and 1.27%, respectively). However, CIF of MI and stroke hospitalisation is lower in patients with T2DM and a risk factor (0.08% and 0.1%, respectively) compared to 2 other cohorts. Furthermore, CIF of PAD hospitalization is about 2.3%, 1.4% and 0.03% in patients with ASCVD and T2DM, patients with ASCVD and without T2DM and patients with T2DM and a risk factor, respectively (Fig. 1B).
Conclusion: This German health insured data show that diabetes is a main driver for HF or PAD hospitalization in patients with ASCVD within 12 months. This aspect should be considered in ASCVD risk thresholds.
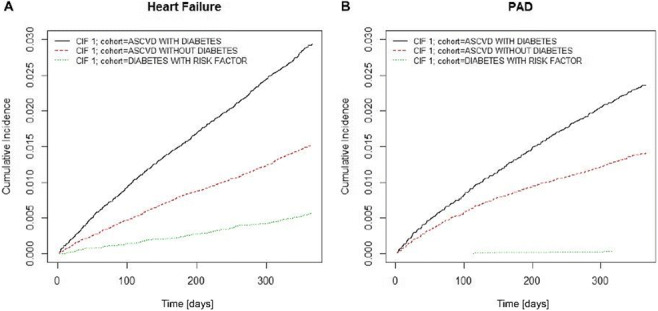
Supported by: Amarin Corporation
Disclosure: M. Verket: None.
877
External validation of SCORE2-OP for predicting 10-year CVD risk in an older population with type 2 diabetes: Edinburgh Type 2 Diabetes study
Z. Huang1, J. Krasauskaite1, L. Klaric2, J.F. Wilson2, J.F. Price1;
1Usher Institute of Population Health Sciences and Informatics, University of Edinburgh, 2MRC Human Genetics Unit, MRC Institute of Genetics and Cancer, University of Edinburgh, Edinburgh, UK.
Background and aims: Cardiovascular disease (CVD) is the leading cause of death in people with type 2 diabetes, and identifying people at higher risk of CVD could facilitate early targeted intervention. Several prediction scores have been developed to stratify CVD risk, however their predictive performance in populations with diabetes was not satisfactory. Recently released by the European Society of Cardiology (ESC), SCORE2-OP is a sex-specific and geography-recalibrated algorithm for predicting 10-year CVD risk in older population including people with diabetes, but its predictive performance in external populations with diabetes is unknown. We aimed to investigate the predictive performance of SCORE2-OP in an older population with type 2 diabetes.
Materials and methods: Performance of SCORE2-OP was assessed in individuals from Edinburgh Type 2 Diabetes Study which is a representative cohort of 1,066 older individuals with type 2 diabetes in Scotland (median follow-up: 10.6 years). Predictors of SCORE2-OP include age, diabetes, smoking, systolic blood pressure, total cholesterol and HDL-cholesterol and interactions between age and each non-age predictor, and scales of low-risk regions were used to recalibrate the final absolute risk. Absolute risk and linear predictors were calculated using the algorithms and scales offered by the ESC. Risk groups were classified by the recalibrated absolute risk <7.5%, 7.5 to <15% and ≥15%, and sex-specific Kaplan-Meier curves of cumulative incidence were drawn. Calibration and discrimination were evaluated for overall and newly incident CVD separately.
Results: Among 1,042 available individuals (mean age: 67.9±4.2 years; male: 51.4%), 258 people developed CVD in 10 years and 110 of them were new cases. When overall incident CVD served as the outcome, the number of people in low, middle and high risk group was 22, 305 and 211 in males and 42, 242 and 220 in females. Kaplan-Meier curves (Figure 1) indicated that generally SCORE2-OP could discriminate the high CVD risk group from the middle and low risk group in males, but it had a poor discriminative performance in females. The slope of calibration was 0.90 (95% CI: 0.52, 1.28) and 0.83 (95% CI: 0.37, 1.28) and c-statistics was 0.60 (95% CI: 0.56, 0.65) and 0.61 (95% CI: 0.55, 0.66) for males and females respectively. Likewise, after prevalent CVD cases at baseline were excluded, the slope of calibration was 1.23 (95% CI: 0.64, 1.83) and 0.44 (95% CI: -0.13, 1.00) and c-statistics was 0.64 (95% CI: 0.58, 0.71) and 0.56 (95% CI: 0.49, 0.64) for males and females.
Conclusion: The predictive performance of SCORE2-OP in an older population with type 2 diabetes was poor, and using this score to predict 10-year CVD risk in this population might not be appropriate. Further independent validations of SCORE2-OP in people with diabetes are warranted.
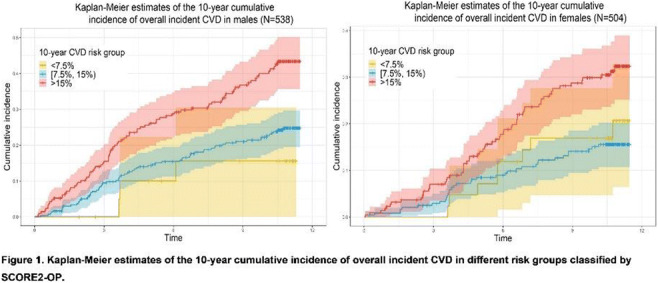
Supported by: The ET2DS was funded by the MRC (UK). Z.H. was supported by a PhD studentship from the Darwin Trust
Disclosure: Z. Huang: None.
878
Weight fluctuation and cardiovascular outcomes: a systematic review and meta-analysis
R.J. Massey, M.K. Siddiqui, E.R. Pearson, A.Y. Dawed;
Population Health & Genomics, University of Dundee, Dundee, UK.
Background and aims: The association between body weight fluctuation and the risk of cardiovascular disease (CVD) has been investigated previously with controversial findings. However, there is no extensive study which systematically evaluates the current evidence. Furthermore, the impact of ethnicity and type 2 diabetes on this phenomena has not yet been investigated. Therefore, the aim of this study is to comprehensively evaluate the effect of weight fluctuation on risk of CVD (any cardiovascular (CV) event, composite CV outcome, CV death, Stroke, Myocardial Infarction) and the influence of ethnicity and type 2 diabetes status on the observed association.
Materials and methods: A systematic review and meta-analysis was performed according to the meta-analyses of observational studies in epidemiology (MOOSE) guidelines. The electronic databases PubMed, Web of Science, and the Cochrane Library were searched for studies that investigated the relationship between body weight or BMI fluctuation and CV diseases using Medical Subject Headings (MeSH) terms and keywords. The relative risks (RRs) for the outcomes were collected from studies, pooled, and analysed using a random-effects model to estimate the overall relative risk.
Results: Of 5645 articles screened, 23 studies with a total population of 15,382,537 fulfilled the prespecified criteria and were included. Individuals in the highest strata of body weight fluctuation were found to have significantly increased risk of any CV events (RR = 1.21; 95% Confidence Interval (CI) 1.13 - 1.28), cardiovascular death (RR = 1.24; 95% CI 1.02 - 1.46), myocardial infarction (RR = 1.14; 95% CI 1.10 - 1.18), stroke (RR = 1.21; 95% CI 1.19 - 1.24), and compound CVD outcomes (RR = 1.40; 95% CI 1.09 - 1.71). Similar RRs were observed regarding BMI fluctuation and per unit standard deviation (SD) increase in body weight fluctuation. This effect was observed regardless of ethnicity or diabetes status.
Conclusion: Appropriate interventions to maintain stable weight could positively influence CVD. Future research is needed to prove a causative link between weight cycling and CVD risk.
Disclosure: R.J. Massey: None.
Author Index
Abad-Santos, F. 214
Abayomi, M. 28
Abbas, F. 430
Abbas, Z. G. 731
Abbink, E. J. 687
Abdelalim, E. M. 298, 304
Abdelmoez, A. M. 81, 161
Abdelsalam, A. 399
Abdennbi, K. 808
Abeltino, A. 865
Abenojar, E. 330
Aberer, F. 427, 430, 543
Abou Azar, F. 28
Abou-Samra, A. 298
Abrahamsen, T. 610
Abrantes, M. 476
Accogli, S. 90
Achenbach, P. 261
Acmet, E. 665
Acosta, F. 141
Acosta-Montalvo, A. 307, 310
Acreman, S. 106, 371
Adamska-Patruno, E. 280
Adamson, K. 583
Adeshara, K. 528, 798, 839
Adler, E. 823
Adreucci, M. 552
Adriaans, B. 512
Afi Leslie, K. 388
Agarwal, R. 620, 751
Agarwal, S. 22
Agesen, R. M. 642
Aghadi, M. 304
Aharon-Hananel, G. 439
Ahlers, C. 618
Ahlholm, N. 146
Ahlqvist, E. 20, 217, 290, 334
Ahlström, H. 183
Ahluwalia, T. S. 20, 719, 734
Ahmad, N. 183
Ahmed, F. 26, 30
Ahmed, M. 712
Ahn, J. 446
Ahn, S. 833
Ahn, S.-Y. 501, 519
Ahrén, B. 541
Ahuja, V. 317
Aiello, E. 311, 327
Aimaretti, E. 495
Ajie, M. 403
Ajjan, R. A. 17, 333, 795
Akagawa, H. 302
Åkesson, K. 315, 661
Akhabir, L. 65
Akhobadze, T. 127
Akhyari, P. 823, 871
Akimoto, Y. 40
Akter, R. 390
Al-Abdullah, L. 523
Albers, D. 375
Albers, J. W. 187
Albiero, M. 531
Albrechtsen, A. 71
Albrechtsen, N. J. W. 167
Aldigeri, R. 364
Alén, R. 485
Alencar, G. 673
Aleotti, F. 418
Aleppo, G. 614
Alexander, C. 656, 678
Alexandre, C. 211
Alexandrou, A. 490
Alexy, U. 608
Alfredsson, L. 290
Alguwaihes, A. M. 696
Alhamar, G. 312
Al-Hasani, H. 260, 296, 534
Ali, M. 148
Alibegovic, A. C. 636, 642
Ali Mondal, S. 116
Allali, S. 28
Allen, J. M. 699
Allen, M. 24
Allen, M. 631
Allen, S. E. 585
Al-Mashhadi, Z. K. 351
Almdal, T. P. 79, 117
Al-Mrabeh, A. 255
Alobaid, A. M. 429
Alquier-Bacquie, V. 91, 774
Alrajeh, A. 696
Al-Sari, N. 136
Al-Selwi, Y. 412
Alshannaq, H. 102
Al-Siddiqi, H. H. 382
Al-Sofiani, M. E. 696
Alssema, M. 454
Altenhofen, D. 296, 534
Altintas, S. 20
Alvarez, A. 638
Álvarez, C. 422
Alvelos, M. 326
Alves, A. 619
Alves, B. L. 452
Alyusuf, E. Y. 696
Alzaid, F. 773
Amadid, H. 190, 367
Amah, G. 808
Amaro, A. 486, 487
Ambrojo-López, A. 674
Ambrosio, M. R. 848
Amendola, L. S. 270
Amendolagine, F. I. 531
Amendolara, R. 411, 711
Amiel, S. A. 625, 681, 683
Amirdelfan, K. 53
Amirruddin, N. 417
Amor, A. J. 220
Ampofo, E. 263, 383
An, S. 869
An, Z. 706
Anandhanarayanan, A. 189, 737
Anastasiou, I. 490
Andersen, A. 224, 790, 796
Andersen, G. S. 804
Andersen, G. 634
Andersen, H. U. 642
Andersen, J. H. 579
Andersen, J. A. 20
Andersen, L. T. 434
Andersen, L. R. 639
Andersen, M. M. 556
Andersen, M. S. 153
Anderson, A. J. 332
Anderson, B. L. 180
Anderson, J. 637
Andersson, T. 217
Andreasen, C. R. 796
Andreozzi, F. 259, 496
Andres-Ejarque, R. 342
Andrews, R. C. 440, 631, 651
Andriessen, C. 8, 115
Andrita, A. 846
Ang, F. G. 551
Ang, L. 710
Angelidi, A. 490
Anglin, G. 596
Anguelova, L. 310
Angwin, C. 174
Anholm, C. 602
Anker, S. D. 593, 618, 620
Annicotte, J.-S. 533
Annuzzi, G. 13, 164, 245
Anstee, Q. M. 18
Antikainen, A. A. 37, 68
Antonini, M. 364
Antonioli, L. 470
Aouadi, M. 145
Apaolaza Gallegos, P. S. 208
Apperloo, E. 750
Aragno, M. 495
Aragona, M. 49
Archer, T. 197, 590
Archibald, L. K. 731
Ardon-Zitoun, C. 775
Arends, D. 535
Arfelt, K. N. 647
Arnoriaga-Rodriguez, M. 159
Arnott, C. 250, 545, 553
Aroda, V. R. 609
Arregui, M. 278
Arrieta, A. 667
Arroyo-Díez, F. J. 674
Arslanian, S. 109
Arya, S. 529
Asai, A. 507
Asai, K. 769
Asamoa, E. 656, 678
Asatiani, N. 127
Åsberg, A. 349
Ashcroft, F. M. 31, 103, 393
Ashcroft, N. 407
Ashcroft, S. P. 231, 232
Ashik, T. 423
Asir, R. 651
ASK Study Group 313
Askeland, A. 777
Askø Andersen, J. 23
Aslan, I. 805
Aslan, M. 805
Assi, E. 262, 399
Astiarraga, B. D. 159
Asuaje Pfeifer, M. 445, 509
Åsvold, B. 290
Atanes, P. 419, 420, 423
Atlan, M. 27
Auclair, M. 27
Auddino, S. 327
Augustin, R. 570
Aukrust, I. 71
Avalosse, H. 728, 729
Avgerinos, I. 147
Avogaro, A. 836
Avramidis, I. 604
Ayachi, C. 331
Aydin, B. K. 472
Aydın, D. 805
Ayers, C. 112
Ayine, M. 770
Ayis, S. A. M. 744, 756, 809
Azim, A. 514
Aziz, F. 427, 430, 543
Azzarello, F. 34
Baader-Pagler, T. 570
Baak, B. N. 691
Baber, R. 321
Babkin, G. 611
Bacete-Cebrian, S. 793
Bacos, K. 265
Bader, G. 578
Badillo Garcia, L. 271
Bae, J. 125
Bae, J. 765
Bae, S. 757, 778, 838
Baechle, C. 1
Baggiore, C. M. 546
Baidya, A. 867
Bailey, R. 699
Baillie, N. 627
Bailly-Maitre, B. 482
Bain, S. 45
Bajaj, H. S. 568
Bajrami, B. 570
Baker, L. 684
Bakker, S. J. L. 6, 358
Bakris, G. 593, 751
Bakris, G. L. 618, 620
Balakrishnan, L. 694
Balakrishnan, M. 590
Balampanis, K. 559, 810, 829
Balasubramanian, P. 835
Balasuriya, C. N. D. 55
Balboa, D. 309
Balcerczyk, A. 537
Baldassarre, M. P. A. 276, 612, 733
Baldi, S. 470
Balena, A. 526
Balestreri, E. 364
Balkau, B. 63, 70
Ballaire, R. 773
Ballesta, S. 222
Ballieux, B. E. P. 409
Bally, L. 52
Baltagiannis, S. 604
Baltrusch, S. 413
Balzano, G. 418
Ban, Y.-H. 838
Banach, M. 329
Banah, A. K. 201
Bang, J. 765
Bangshaab, M. 149
Banke, P. 417
Banks, P. 89
Bany Bakar, R. 460, 466
Baptista, F. I. 486
Baquet, G. 428
Barabash, A. 278
Barahona, I. 94
Barale, C. 816
Barbaresko, J. 1
Barbosa, H. C. L. 213, 503
Barchetta, I. 843
Bardos, J. N. 596
Bardtrum, L. 567
Barg, S. 104, 239
Barhod, E. 439
Barker, P. 602
Barmpagianni, A. 797
Barnes, A. C. 255
Barnes, D. 583
Baron, M. 63
Barra, C. 486, 487
Barral, D. C. 204
Barrès, R. 82
Barreto, C. 444
Barrett, T. 406
Barrientos-Pérez, M. 109
Barsby, T. 179
Bartakova, V. 437
Barth, M. 823, 871
Bartlen, W. 413
Bartosova, M. 760
Bartuskova, H. 497
Barutta, F. 766
Basciani, S. 526
Bassot, A. 619
Basu, M. 759
Bateman, B. T. 273
Bath, L. E. 234
Battelino, T. 54, 247, 635
Batterham, R. L. 498
Battini, L. 49
Baud, G. 459
Baudoux, F. 442
Bauer, W. 280
Baumann, P. M. 628, 679
Baumgaertner, E. 634
Baxter, F. A. 627
Bayazit, M. B. 108
Bayer, J. 723
Baylan, U. 573
Beall, C. 137
Beato-Vibora, P. I. 674
Beaumont, R. 78
Becam, J. 331
Bechtluft, G. 389
Beckerman, M. 196
Beck-Nielsen, H. 43
Bedair, K. 134
Bedenkov, A. 727
Bedou, A. D. 260
Beguinot, F. 269, 815, 848
Bekiari, E. 147
Belew, G. D. 478, 517
Bell-Anderson, K. S. 448
Bellart, J. 441
Bellini, S. 766
Bem, R. 655
Ben, S. 229
Benaiges, D. 222
Benayahu, D. 502
Bencke, J. 23
Bendszus, M. 717, 718
Ben Nasr, M. 262, 399
Benneyworth, B. D. 614
Benninger, R. 330, 375
Bennuri, S. 157
Benomar, Y. 211
Benson, C. T. 111, 587, 592
Berendschot, T. T. M. 121, 155
Berentzen, T. L. 18
Béréziat, V. 27
Bergen, S. 785
Bergenstal, R. 97, 247, 635
Bergman, B. K. 598, 603
Bergman, G. 665
Bergman, M. 83
Bergmann, N. C. 556
Bergsten, P. 472
Berkane, N. 438, 443
Bernabé-García, A. 733
Bernard, M. A. 664, 831
Berney, X. P. 228
Bertolini, M. 637
Bertolotto, A. 49
Bertrand, G. 386
Bertrand, M. 391
Besser, R. E. J. 699
Bettahi, I. 298
Beulens, J. W. J. 339, 345, 346, 834, 873
Bewick, G. A. 342, 423
Bhadra, R. 867
Bhatt, D. L. 594
Bhatta, M. 498
Bhattacharyya, N. P. 759
Bi, Y. 66, 857, 858
Bianchetti, G. 865
Bianchi, C. 49
Bibi, H. 582
Bichisecchi, A. 293
Bicho, M. 864
Bickerton, A. 550, 583
Bielik, V. 493
Bigot, G. 648, 689
Bihan, H. 438, 443
Billings, L. K. 586, 618
Bin Abdul Mu-u-min, R. 382
Bineau, S. 664, 831
Biondi, G. 59
Birkebæk, N. H. 170
Birkeland, K. I. 132, 340, 349, 742, 853
Birkenfeld, A. L. 73, 128, 209, 258, 433, 620
Birnbaum, M. J. 588, 589
Bismuth, E. 109
Bispham, J. 97, 626
Bissenova, S. 405
Bjerg, L. 336, 804, 847
Bjerre, M. 43
Bjørkhaug, L. 71
Björklund, A. 55
Blaak, E. E. 139
Black, J. 629
Blackbourn, L. 234
Blais, C. 359
Blakely, P. 710
Blanc, M. 482
Blanchi, B. 381
Blanco, J. 220
Blasco-Montolio, M. 479
Blin, P. 664, 831
Blindauer, C. A. 465, 529
Blom, M. T. 691, 834
Blom-Høgestøl, I. K. 340
Blond, M. B. 542
Blong, J. 24
Blouin, J.-L. 306
Blüher, M. 25, 321, 613
Blumen, T. 572
Bøås, H. 233
Boavida, J. 253
Boccara, F. 27
Boccia, R. 13
Böckel, S. 427
Bode, B. W. 669
Bodegård, J. 742
Bódis, K. 254
Boettcher, S. 25
Boewe, A. S. 263
Bogdanov, P. 122, 126
Böger, M. 397
Bojsen-Møller, K. N. 110, 477
Boland, A. 194
Bolt, R. 292
Bombrich, M. 254
Bonadonna, R. 364, 648
Bonas-Guarch, S. 266
Bonfanti, R. 293
Bonfini, T. 276
Bongiovanni, A. 533
Bönhof, G. J. 192
Bonnadonna, R. 227
Bonnefond, A. 63, 70, 294, 468, 533
Bonnemaire, M. 648
Bonner, C. 307, 310
Bonnet, F. 151, 152
Bonora, E. 227
Boonpor, J. 319
Borchers, C. H. 257
Borecka, S. 301
Borg, M. 161
Borrelli, A. 59
Borsigova, M. 25
Bos, E. 92
Bosch-Traberg, H. 45
Bosi, E. 293, 369
Boswell, L. 220
Botagarova, A. 60
Botelho, F. 476
Botezelli, J. D. 257
Böttcher, Y. 64
Bottle, A. 782
Bótyik, B. 690
Bouchareb, S. 279
Boucher-Berry, C. 109
Bouchi, R. 682
Boudry, G. 447
Boughton, C. K. 50, 699
Boughton, P. 732
Bouland, G. 20
Bouman, E. J. 345
Bound, M. J. 623
Bourdon, G. 131
Bourry, J. 442
Boutmy, E. 581
Bouzakri, K. 145, 389
Boyd, J. 669
Boye, K. 615
Boyko, E. 181
Bozzetto, L. 13, 164, 245
Brachs, S. 258
Brackley, S. M. 169
Braffett, B. H. 187
Brahma, M. K. 410
Brake, R. 572
Brancato, V. 164
Brand, K. 581
Brandhorst, D. 175, 384
Brandhorst, H. 175, 384
Brandon, A. E. 448
Brandslund, I. 43, 334
Brandt, C. J. 221
Brandts, J. 876
Braun, A. 500
Bray, R. 561, 565, 568, 586
Bressendorff, I. 349
Breton, C. 533
Breyner, N. 119
Briand, F. 119
Briant, L. J. B. 103
Bridge, K. I. 193
Briganti, S. 312
Briggs, J. 375
Brinck, J. 360
Brinker, M. 620
Brinza, C. 65
Brismar, K. 539
Brito-Casillas, Y. 435, 484
Britten, N. 174
Brix, J. 722
Broadley, M. 625, 681
Broca, C. 392
Brock, C. 542, 713
Brockmann, G. A. 535
Broend, J. C. 322
Bron, C. 453
Brøns, C. 148, 184, 185, 334
Brøsen, J. M. B. 642
Brouwers, B. 565
Brouwers, M. C. G. 72, 341, 512, 652
Brown, K. 558, 586, 596, 603
Brown, L. S. 114, 589
Brown, S. A. 669
Brozzi, F. 108, 480
Bruandet, A. 131
Bruinsma, B. 379
Bruls, Y. M. H. 83
Bruni, V. 96
Bruse, J. 91
Brusgaard, K. 458
Bruttini, M. 327
Buckeridge, C. 114
Budd, J. 727
Bue-Valleskey, J. M. 640, 649
Buitinga, M. 405
Bulkescher, R. 120, 754
Bull, S. B. 67
Bunce, C. 724
Bunduc, S. 337
Burade, V. 616
Burant, A. 710
Burchell, G. 339
Burgess, J. 361
Burkart, V. 254
Burkhardt, R. 321
Burling, K. 602
Burnol, A.-F. 447
Burnside, M. J. 677
Bursill, C. A. 47
Burt, N. 651
Bury, Y. 412
Buschard, K. 210
Buse, J. B. 567
Busjahn, A. 162
Butalia, S. 832
Butler, J. 560
Butler, T. 197, 590
Butwicka, A. 785
Buyken, A. E. 608
Buziau, A. M. 72
Buzzetti, R. 411, 711
Caccioppoli, C. 500, 814
Cai, T. 842
Caiazzo, R. 459
Caidahl, K. 82, 229
Cailleteau, R. 647
Cairney-Hill, J. 24
Cajka, T. 11
Calabrese, I. 2
Calderon, B. 596
Calderoni, I. 500, 814
Calders, P. 219
Calero Pérez, S. 94
Callaghan, B. C. 191
Callebaut, A. 405
Calle-Pascual, A. 278
Caluwaerts, S. 328
Camarillo, E. 278
Camell, H. 222
Cameron, R. 727
Campbell, F. M. 699
Campbell, J. A. 361
Campbell, M. D. 429, 795
Campi, B. 800
Campitelli, M. 269
Campos Barros, Á. 303
Cannistraci, R. 95
Cannon, C. P. 252
Canouil, M. 63, 70, 266, 294, 468
Caparrotta, T. M. 234
Capeau, J. 27
Capece, U. 425, 456
Cappellacci, I. 733
Capretti, G. 418
Caprio, S. 868
Capucho, A. 624
Caramelo, B. 536
Caramori, M. L. 593, 620
Caraway, D. 53
Carbillon, L. 438, 443
Carcarino, E. 267
Carceller-López, E. 202
Cardarelli, F. 34
Cardozo, A. K. 391, 410
Carey, D. 78
Caria, E. 81
Cariou, B. 561, 597, 747
Carli, F. 253, 492, 517
Carlier, A. 101
Carlsen, R. 349
Carlson, A. L. 670
Carlson, T. 195
Carlsson, K. 659
Carlsson, S. 217, 284, 290
Carlström, M. 46
Carlyle, M. 637
Carneiro, E. M. 452
Carnovale, D. 293
Caron, E. 533
Carotti, S. 96
Carr, A. 291
Carrasquilla, G. D. 182
Carrero, J.-J. 320
Carrieri, F. 612
Carroll, L. M. 758
Carstensen, B. 87, 367
Carvajal-Gonzalez, S. 114
Carvalho, E. 21, 157, 734
Carvalho, M. 213, 503
Casas, R. 55
Cassano Cassano, R. 711
Castaneda, J. 667
Castaño, L. 278
Castela, Â. 326
Castellani, J. 369
Castelo-Branco, M. 536
Castillo, J. J. 390
Castillo-Armengol, J. 226
Cataldo, L. 35
Catena, C. 408
Cater, N. B. 252
Caton, P. 342
Catrina, S.-B. 19, 539
Caunt, S. 630, 700
Caussy, C. 856
Cauzac, M. 447
Cavaliere, C. 164
Cavallo, M. G. 843
CDPP study group 186
Cefalo, C. M. A. 425, 456, 865
Çeker, T. 805
Celis-Morales, C. 319
Cen, H. H. 36, 257
Cen, J. 387
Cerenius, S. Y. 472
Cersosimo, E. 10
Cesta, C. E. 130, 273
Cetin, B. 380
Cezar, A. 50
Chadt, A. 260, 296, 534
Chae, H. 392
Chaib, N. 808
Chalásová, K. 437, 743
Challa, T. D. 483
Chan, A. Y. L. 130
Chan, J. K. Y. 129, 431
Chan, J. C. 622, 654
Chan, K. 666
Chan, S.-Y. 129, 431
Chan, Y.-P.634
Chandarana, K. 97, 626
Chang, K. 782
Chang, N. 779
Chang, T. 377
Chao, L. 109
Chapman, K. 97, 626
Charitaki, I. 577
Charpentier, G. 63, 70, 294
Charrin, E. 755
Chassaing, B. 91
Chaturvedi, N. 363
Chen, B. 4, 281
Chen, D. 355
Chen, L. 377, 378
Chen, N. 851
Chen, S. 547
Chen, X. 10
Chen, X. 198
Chen, Y. 739
Chen, Y. C. 208
Chen, Y.-C. 32, 401
Chen, Z. 3
Cheng, A. Y. Y. 585, 644, 657
Cheng, Y. 29
Chenji, S. 684
Chepurny, O. G. 575
Cherney, D. Z. I. 252, 617, 621
Cheung, J. 654
Cheung, J. 622
Cheung, R. 654
Chibalin, A. V. 82, 161, 455, 481
Chidsey, K. L. 114
Chien, J. 640, 649
Chigutsa, E. 640, 649
Chillarón, J. J. 222
Chilton, R. 10
Chimienti, R. 305
Chin, H. 765
Chioma, L. 242
Chiral, M. 307, 310
Chiriacò, M. 241, 868
Chiribiri, A. 809
Chiu, C. 654
Chivukula, K. 586
Cho, J. 109
Cho, S. 686
Cho, Y. 833
Chodick, G. 273, 610
Choi, H. 746
Choi, I. 757, 778, 838
Choi, J. 778
Choi, J. 765
Choi, S.-E. 501, 519
Choi, W.-I. 860
Choi, W. 860
Chong, M. 65
Chong, Y. S. 431
Chong, Y. 129
Choudhary, P. 247, 585, 625, 635, 681, 683
Chow, E. 622, 654
Chowdhary, A. 813
Chowdhury, A. I. 472
Christ, A. 165
Christensen, D. H. 191, 334
Christensen, J. R. 221
Christensen, L. W. B. 584, 783
Christensen, M. H. 153
Christensen, M. M. B. 707
Christensen, M. B. 510
Christensen, T. B. 657
Christiansen, L. B. 772
Christodoulidou, C. 749
Christoffersen, B. O. 772
Christoffersen, C. 510
Chrzanowski, J. 830
Chu, J. C. M. 36
Chu, K. H. 851
Chu, L. 832
Chun, J. 828
Chung, C. 599
Chung, H. 467
Chung, S. 446
Cianfarani, S. 242
Ciappini, B. 612
Ciardullo, S. 95, 348
Ciba, I. 472
Ciborowski, M. 280
Ciccaglione, M. 330
Ciccarelli, G. 425, 456
Cichosz, S. L. 98
Ciciliot, S. 531
Cieluch, A. 820
Cieslak, J. 702
Cigler, M. 628
Cignarelli, A. 59, 500, 814
Cilla, M. 369
Cimini, F. A. 843
Cinefra, C. 39
Cinti, F. 425, 456
Cinti, S. 425
Ciregia, F. 58
Cirera, S. 772
Cirule, H. 530, 767
Citro, F. 49
Ciudin, A. 784
Claeys, M. 872
Claggett, B. 872
Clark, A. 610
Clark, L. 255
Clemente-Postigo, M. 479
Clemmensen, K. K. B. 542
Climent, E. 222
CLOuD Consortium 699
Cnop, M. 58, 69, 307, 400
Cobelli, C. 255
Cobo-Vuilleumier, N. 485
Coca, S. G. 545
Coelho, I. 492
Cogoi, B. 787
Cogswell, G. 102
Cohen, O. 667
Cohen, R. 54
Cohen Solal, M. 668
Colbert, K. 195
Colclough, K. 78
Colhoun, H. M. 67, 234, 721, 794
Collado, A. 46
Colli, M. L. 69, 206, 326
Collier, J. 257
Collino, M. 495
Collins, J. 744
Collombat, P. 331
Collotta, D. 495
Colman, P. G. 332
Colombi, C. 546
Colonna, A. 155
Colussi, G. 408
Coluzzi, S. 612
Conde, S. V. 474, 516, 624, 845
Conget, I. 220, 441
Connelly, M. A. 6
Conserva, F. 39
Consoli, A. 612
Conte, C. 369
Conway, B. R. 42
Conza, D. 815
Coppari, R. 194
Corcillo, A. 756, 809
Cordiner, R. L. M. 134
Corrado, A. 164
Corrao, G. 348
Correia, J. C. 650
Correia, M. A. 204
Correig, X. 283
CORONADO, the ABCD COVID-19 diabetes national audit and AMERICADO investigators 747
Cortazar, A. 278
Corthesy, J. 541
Cosentino, C. 108, 480
Cosentino, F. 252, 268, 276, 621
Coskun, T. 111, 462, 564, 606
Cosson, E. 335, 438, 443
Costa, A. 478
Costabile, G. 164
Costa-Silva, B. 492
Coudert, M. 644
Coulter, F. B. 175, 384
Coulthard, R. 412
Couper, J. 332
Cowan, E. 415, 457
Cowley, M. 203
Cox, D. 109
Cox, E. 171
Cózar-Castellano, I. 451
Crabtree, T. S. J. 550, 583
Craig, M. E. 236, 332
Criego, A. B. 669, 670
Crocket, H. R. 677
Croosu, S. S. 720, 736
Csanalosi, M. 165
Csatári, G. 690
Cubbon, R. M. 193
Cuenca-Ardura, M. 309
Cuenco, J. 668
Cugnata, F. 293
Cui, Q. 285
Cui, X. 558, 591
Cui, Y. 281
Cukierman-Yaffe, T. 439
Curcio, F. 408
Curovic, V. 244
Cusi, K. 565
Cypryk, K. 432
Cyranka, G. 31, 103
Czajkowski, P. 280
Dabaghie, D. 755
Dabek, J. 146
Da Costa, L. 453
Da Dalt, L. 515
Dadan, J. 513
D'Addio, F. 262, 399
Dadi, P. K. 376, 414
Dadson, P. 142
Dagnelie, P. C. 72, 121, 282, 324, 512
Dagogo-Jack, S. 252, 621
Dahl, D. 640
Dahl, M. B. 64
Dahlquist, G. 288
Dahlqvist Leinhard, O. 561
Dahlström, E. H. 38, 68
Dahmen, J.-N. 264
Dai, B. 598
Dai, X.-Q. 32
Daimon, M. 338, 870
Dajani, K. Z. 180
Dal Canto, E. 873
Dalgaard, L. T. 20, 157, 734
D'Alterio, F. 848
Daly, A. B. 50
Dam, W. A. 358
Damanti, S. 369
D'Amato, C. 725
Dambeck, U. 163
Dambrova, M. 530, 767
Damgov, I. 760
Damodharan, S. 698
Dan, Y. Y. 788
Dance, A. 294
Dance, B. 236
Daniele, G. 212
Danielsen, E. R. 185
Danielson, K. K. 180
Danne, T. 247, 635
Da Porto, A. 408
Dardano, A. 212, 249
Darwish, T. 216
da Silva, J. 21
Dat, D. Q. 672
Datta, N. 199
Dautzenberg, B. 7
Davani, A. 289
David, J.-P. 594
Davies, M. 499
Davies, M. J. 89
Davila-Batista, V. 274
Davis, E. A. 332
Davis, G. M. 100
Davis, T. M. E. 350, 354
Davis, W. A. 350, 354
Dawed, A. Y. 878
DCCT/EDIC Research Group 187
de Almeida, M. E. 80, 84
Deane, A. 685
De Angelis, R. 245
Deàs-Just, A. 122
De Backer, T. 219
De Bacquer, D. 863
de Barsy, M. 468
De Block, C. 463, 600, 614, 657
de Bock, M. I. 677
de Borst, M. H. 6, 358
De Castro Barbosa, T. 82
Defrance, M. 69
DeFronzo, R. 10
de Galan, B. E. 154, 155, 403, 632, 687
Deganutti, G. 569
de Graaff, B. 361
Dehayem, M. Y. 404
Dehghany, J. 240
Dei Cas, A. 364
de Jong, J. D. 652
de Jonge, C. 54
Dekker, L. H. 320
de Koning, E. J. P. 409
de Kruijk, R. S. 345
Dela, F. 633
Delalleau, N. 307
De la Torre, N. 278
Delbecque, L. 701
Deldicque, L. 468
Delgado, G. E. 73
Del Guerra, S. 34, 58
Delhaey, L. 157
de Ligt, M. 7, 8
Dellanno, A. 843
Della Pepa, G. 13, 164
Delles, C. 123
Dellva, M. A. 688
De Lorenzi, V. 34
De Lorenzo, R. 369
Delpero, M. 535
Del Prato, S. 16, 49, 90, 212, 557, 595, 598, 852
De Luca, C. 58
Del Veliz, S. 28
de Mello Laaksonen, V. 143
Demine, S. 326, 410
den Braver, N. R. 873
Deng, H. 572
Deng, K. 238, 372
De Nicola, L. 552
Dennis, J. M. 235, 555
Denwood, G. 371
de Oliveira, K. M. 452
de Oliveira, R. M. 204, 492
Deotti, S. 175, 384
De Pasquale, C. 872
De Pouvourville, G. 51
Derhourhi, M. 63, 70, 294
Derua, R. 405
DeSalvo, D. J. 669
Descatoire, A. 428
Deshmukh, A. S. 82, 93, 229
Deshmukh, S. 76, 404
Deshpande, S. K. 22
Desiderio, A. 269
de Souza, A. H. 392
De Souza, B. M. 400
De Spirito, M. 865
D'Esposito, V. 848
Deswysen, Y. 468
Dethlefs, M. 508
Detournay, B. 51
De Vas, M. 309
De Vincentis, A. 96
De Vito, F. 259, 496
de Vries, A. P. J. 409
de Vries, S. A. G. 469
DeVries, H. 680
DeVries, J. 57, 564, 606
de Wit-Verheggen, V. H. W. 83
Dey, S. 867
Deyneli, O. 638
Dhami, P. 177
Dharmalingam, M. 698
Dhatariya, K. 550
Dhayal, S. 416
Diane, A. 382
Dias-Pereira, P. 841
Diaz, L. 367
Díaz, M. 435
Diaz Lozano, I. 385
Dib, A. 615
Dib, S. 109
Di Bartolo, B. A. 47
Di Biasio, A. 843
di Camillo, B. 836
Dichman, M.-L. 477
Dick, J. 695, 764
Dickerson, M. T. 376
Di Dalmazi, G. 612
Didisheim, M. 267
Diedisheim, M. 101, 773
Di Giuseppe, G. 425, 456
Dimayuga, D. 643
DiMeglio, P. 342
Dimitriadis, G. 829
Dimopoulos, M. 577
Ding, Y. 715
Ding, Y. 343
Dinh, N. T. T. 361
Di pietrantonio, N. 276, 733
D'Ippolito, I. 725
Dirinck, E. 728, 729
Dirksen, C. 110, 477
Distaso, W. 230
Di Tolla, M. F. 848
Di Tomo, P. 276, 733
Dive, A. 459
Divilly, P. M. 625, 681, 683
Dixen, U. 12
Djaballah, K. 644
Dobiasova, Z. 301
Dobjanschi, C. 846
Dobrzycka, M. 513
Dobson, J. R. 376
Docherty, N. G. 203
Dole, G. 454
Dollet, L. 82, 161, 231
Dom, Z. M. 244
Domazet, S. L. 322
Dombrowski, F. 413
Domingo-Lopez, D. A. 175, 384
Dong, C. X. 180
Dong, F. 313
Dong, X. N. 198
Dong, Y. 788
Donnelly, L. 134
D'Onofrio, L. 411, 711
D'Oria, R. 500, 814
Doria, A. 244
Doronin, I. 611
dos Santos, R. S. 398
Dotta, F. 311, 327, 328
Doucette, C. 300
Doundoulakis, I. 875
Dover, A. R. 99, 627
Dover, D. A. 832
Doyle, R. P. 575
Dravecka, I. 248
Drenthen, L. C. A. 687
Drevon, C. A. 340
Drøjdahl-Ryg, N. 646
Drouin-Chartier, J.-P. 3
Droz-Perroteau, C. 664, 831
Du, Y. 111, 592
Duan, M. F. 320
Duarri, A. 126
Duarte-Araújo, M. 841
Dubois, L. J. 139
Dubois Laforgue, D. 101
Duchampt, A. 453
Duchateau, G. 454
Dudler, T. 792
Due-Christensen, M. 701
Due Thomsen, K. M. 647
Duffin, K. L. 617
Duffy, G. P. 175, 384
Dufour, S. 146
Dule, S. 843
Dullaart, R. P. F. 6, 358
Dumond, A. 145
Dumont, I. 728, 729
Duncan, I. 665
Dunn, J. 564
Dunn, T. C. 333
Dunne, J. L. 286
Dunseath, G. 699
Duque Escobar, J. 508
Dureau-Pournin, C. 664, 831
Durrer, C. 117
Dusaulcy, R. 306
Duval, M. 808
Dwivedi, O. P. 317
Dwulet, J. 375
Dybjer, E. 786
Dzhun, Y. Y. 874
Eason, R. J. 291
Eastwood, S. V. 363
Ebert, T. 15
Eckel, J. 862
Eckstein, M. L. 427, 430
Edelman, S. 635
Edelman, S. 247
Edge, M. 727
Edstorp, J. 290
Eeg-Olofsson, K. 223, 360, 701
Eelen, G. 405
Eggers, B. 818
Egidi, G. 246
Ehlers, L. H. 584
Ehrlich, A. M. 231, 232
Eickelschulte, S. 260
Eickhoff, H. 476
Eid, H. 695, 764
Eizirik, D. L. 58, 69, 206, 326, 400
Ejlalmanesh, T. 773
Ejskjaer, N. 713, 720, 736
Ekelund, J. 223
Ekinci, E. 762
Ekström, S. 462
Elders, P. J. M. 279, 345, 346, 691, 873
Elders, P. P. J. 834
Eldh, M. 385
El Eid, L. 569
El-Ejeh, F. 298
Eletto, E. 364
Elgendy, R. 304
El ghormli, L. 187
Eliasson, B. 223, 360, 847
Eliasson, J. 597, 609
Eliasson, L. 107, 237, 265, 415, 457, 507
Elingaard-Larsen, L. O. 148, 185
Elisabet, B. 157
Elissen, A. M. J. 652
Ellacott, K. L. J. 137
Elleri, D. 699
Ellero-Simatos, S. 91, 144, 774
Ellingsgaard, H. 461
Elliott, J. 550
Ellis, C. 36
Ellis, D. 328, 405
El-Masri, F. 495
Elmquist, J. 194
Eloy, R. 634
Elrakaybi, A. 44
Elsayed, A. 298
Elshorbagy, A. K. 512
Elton Sander, S. 700
Emery, C. 51
Emmens, R. W. 573
EMPEROR Trial Committees and Investigators. 560
Emral, R. 645
Emri, M. 787
ENDIA Study Group 332
Endo, Y. 40
Engberg, S. 23, 790, 796
Engström, G. 786
Engvall, J. 803
EPIC-InterAct consortium 284
Eppel, D. 275
Erazo Tapia, E. 8
Eric, Y. H. K. 788
Eriksson, I. 539
Eriksson, J. W. 26, 30, 214, 475, 514, 524
Eriksson, J. G. 129, 431
Eriksson, M. I. 124, 791
Eriksson, U. 150
Erlmann, M. 427
Erlund, I. 863
Eross, B. 337
Escolano, J.-C. 240
Escrivá, F. 422
Esguerra, J. L. S. 415, 457
Eski, S. E. 410
Esmatjes, E. 220
Espadas, I. 522
Esser, N. 390
Essers, Y. 139
Esterline, R. 7
Esteves, J. V. 270
Esteves-Monteiro, M. 841
Esze, R. 787
Eurich, D. T. 832
Eurola, S. 205
Eussen, S. J. P. 72, 121, 154, 227, 282, 324, 341, 512
Evans, D. M. 340
Evans, E. 342
Evans, M. 806
Evans, M. L. 50
Evers, I. 680
Exner, A. 330
Faber, J. 796
Fabiani, I. 249
Fabricius, T. W. 403, 632
Fabris, M. 408
Facchinetti, A. 52
Fadini, G. P. 531, 836
Færch, K. 542
Færch, L. 499
Færgeman, N. J. K. 84
Fagherazzi, G. 151, 152
Faherty, A. M. 677
Falbo, E. 369
Falcetta, P. 16, 90, 852
Falkevall, A. 150
Fall, T. 19, 183
Fan, J. 189, 737
Fan, L. 285
Fan, X. 525
Fang, D. 858
Fang, M. 181
Fanni, G. 26, 30, 524
Faraj, M. 312
Faraone, A. 212
Fardellas, A. 145
Faros, E. 444
Farreca, S. M. 624
Farrell, C. M. 135
Faurby, M. 659, 663
Fauzi, M. 60
Fayfman, M. 100
Fehértemplomi, K. 690
Feldman, E. L. 187, 710
Felix, P. 728, 729
Fels, J. J. 772
Fenger, M. 601, 602
Fermaut, M. 438
Fernandes, J. L. 845
Fernández-Bueso, M. 674
Fernández Landó, L. 558, 565, 561, 591, 598, 603
Fernández-Marcelo, T. 422
Fernández-Millán, E. 422
Fernández-Pérez, L. 435
Fernández-Real, J. 159, 522
Fernández-Valero, A. 277
Fernández-Veledo, S. 159, 283
Ferrannini, E. 464, 800, 801
Ferrannini, G. 800, 801, 863
Ferrante, M. 369
Ferraro, P. 865
Ferrat, L. 286
Ferreira, I. 492
Ferreira, S. 572
Ferreira, V. 214
Ferreira, V. 26
Ferreira-Duarte, M. 841
Ferrer, J. 266, 309
Ferreri, C. 865
Ferri, G. 34
Feuchtinger, A. 33
Fève, B. 27
Fex, M. 35, 178
Fiedorczuk, J. 280
Field, B. C. T. 583
Fignani, D. 311, 327
Figueiredo, A. 21
Figueiredo, R. E. 516
Filippatos, G. 593, 618, 620
Filippi, V. 275
Filipsson, K. 251, 730
Fillová, D. 723
Finan, D. 626, 97
FinnDiane Study Group 124, 156, 528, 768, 821
Fineman, M. 195
Finlayson, G. 542
Fiorentino, T. V. 259, 496
Fiorentino, V. 262
Fiorina, P. 262, 399
Fiory, F. 815
Firneisz, G. 295
Firnkes, R. 194
Fishman, S. 54
Fiume, M. 39
Flanagan, D. 724
Flanagan, S. E. 308
Flaquer, M. 756, 809
Flatt, P. R. 488
Flaxman, C. S. 416
Fledelius, C. 226
Fleischer, J. 707
Fleming, A. D. 721, 794
Flodström-Tullberg, M. 385
Flores, J. A. 222
Florese, P. 269
Flores-Rodriguez, N. 372
Flotynska, J. 820
Fløyel, T. 210
Foch, C. 581
Fohrer-Ting, H. 773
Fojas, E. G. 471
Fok, K. 32
Folestad, E. 150
Folgueira, C. 214
Folie, S. 576
Folli, F. 262
Folon, L. 63
Fonseca, C. 864
Fonseca, V. 593
Fonseca Martins, R. 596
Font, C. 441
Fontaine, P. 428
Fontana, M. 813
Forbes, A. 701
Forbes, S. 99, 627
Forde, R. 701
Forlenza, G. P. 669
Formichi, C. 311
Formisano, P. 848
Formoso, G. 276, 612
Forotti, G. 241
Forsblom, C. 38, 124, 156, 244, 768
Forslund, A. 472
Fortin, E. 800
Foskett, D. B. 236
Fosse-Edorh, S. 335
Fougerat, A. 91, 144, 774
Fountoulakis, N. 744, 756, 809
Fournier, P. A. 135
Fox, C. 169
Franc, S. 63, 70
Francesconi, P. 16, 852
Franci, G. 312
Franco, D. R. 557, 688
Franco, E. D. 308
Francque, S. 463
Frank, J. 495
Franks, P. W. 297, 321
Franks, R. 809
Franx, A. 680
Franzén, S. 360
Frappin, G. 597
Frayling, T. 78
Frederich, R. 252, 621
Freemantle, N. 648
French, O. 676
Frenkel, O. 45, 806
Frewen, C. M. 677
Frias, J. 588, 589, 644, 649
Frias, J. P. 567
Friberg, L. 844
Frier, B. M. 133
Friis-Hansen, L. 356
Frimodt-Møller, M. 23, 86, 87, 123
Fritsche, A. 73, 128, 433
Fritsche, L. 128, 433
Fritzen, R. 465
Froguel, P. 63, 70, 266, 294, 468, 533
Frohnert, B. I. 313
Frøkjaer, J. B. 713
Frøkjær, J. B. 720, 736, 777
Fromme, T. 143
Frontoni, S. 242, 714
Frørup, C. 210
Frydenberg, H. 54
Fryer, A. 658
Füchtenbusch, M. 615
Fuglsang-Nielsen, R. 351
Fujihara, K. 761
Fujii, R. 769
Fujimoto, H. 60
Fukui, M. 544
Fulcher, G. R. 762
Fulcher, J. M. 32
Funck, K. L. 85, 336, 807
Fung, K. 177
Fung, T. 3
Furu, K. 273
Fusco, C. 312
Gabrielsson, S. 385
Gagey, S. 808
Gaglia, J. 97, 626
Gaglio, A. 693
Gagyi, E. B. 337
Gaitan, J. 702
Gál, V. 295
Galan, V. 53
Gale, C. P. 813
Galecki, A. 244
Galgani, M. 312
Galindo, R. J. 100, 591
Gall, M.-A. 636
Gallagher, A. 550, 583
Gallen, I. W. 550, 583
Galli, A. 480
Gallone, A. 39
Galnobax Study Group 22
Galstyan, G. 644, 689
Galuška, D. 743
Gamet-Payrastre, L. 91
Gan, W. J. 372
Ganapathy, K. D. 651
Gancheva, S. 353
Gansevoort, R. T. 6, 358
Ganss, K. 314
Gantz, I. 252
Gao, L. 130
Gao, Q. 35
Gao, R. 106, 371
Gašparini, D. 527
Garai, I. 787
Garbade, S. F. 760
Garchon, H.-J. 267
Garcia, E. 6
Garcia, P. 706
García-Calzón, S. 334, 352
Garcia-Martinez, I. 485
García-Monzón, C. 202
García Moreno, R. 303
Garcia-Ocana, A. 616
García-Pérez, L.-E. 615
Gariani, K. 650
Garofalo, C. 552
Garofolo, M. 16, 90, 852
Garvey, T. W. 562, 563
Garvey, W. T. 498
Gasbjerg, L. S. 510, 556
Gaspari, S. 225
Gasperikova, D. 301
Gastaldelli, A. 253, 492, 517, 565
Gaudier, M. 634
Gaudio, C. 546
Gauthier, B. R. 485
Gautier, J.-F. 101, 267, 668
Gautier-Stein, A. 453
Gay, A. 751
Gaztambide, S. 278
Géczi, B. 690
Gedebjerg, A. 43
Gee, E. 24
Geisinger-Regeneron DiscovEHR Collaboration 78
Gemmink, A. 7, 160
Genchi, V. A. 500, 814
Geno Rasmussen, C. 313
Genovese, F. 218, 549, 709, 822
Georgitsi, M. 299
Gerbracht, C. 163, 538
Gerring, D. 407
Gerst, F. 209
Gesheva, V. S. 342
Gesmundo, I. 766
Gesualdo, L. 39
Ghatak, A. 699
Gheibi, S. 396
Gheisari, A. 240
Ghit, A. 612
Ghosh, S. 645, 759
Giacca, A. 817
Giaccari, A. 425, 456, 865
Giambalvo, M. 16, 90, 852
Gianfrancesco, S. 13
Giarratana, L. 693
Gibb, F. W. 99, 627
Gil, A. 141
Gill, J. 132
Gilleron, J. 482
Gillespie, K. M. 261
Gilly, A. 71
Gilon, P. 105, 392
Gil-Poch, E. 674
Giménez, M. 220, 441, 684
Ginovker, A. 657
Giorgino, F. 39, 59, 500, 586, 615, 638, 665, 814
Giosuè, A. 2
Girard, D. 267
Girard, M. 89
Giusti, L. 58
Gjela, M. 777
Gloerich, J. 358
Gluckman, P. D. 129, 431
GlucoMOMS Study Group 680
Gmitrov, J. 740
Gmyr, V. 307, 310
Gnessi, L. 526, 711
Gnudi, L. 744, 756, 809
Gobert, M. 459
Göbl, C. S. 275
Godang, K. 436
Gödde, S. 246
Godfrey, K. 431
Godfrey, K. M. 129
Godwin, J. 235
Godzien, J. 280
Goehringer, J. 78
Goerg, A. C. K. 52
Goergen, A. 483
Gojda, J. 370
Gokhale, K. 840
Golden, A. 78
Goldman, B. 499, 563
Goldspink, D. 460
Goletzke, J. 608
Golsäter, M. 315
Gomes, M. M. 625, 681
Gomes, P. 476
Gomes Porras, M. 303
Gomez Valderas, E. 585
Gómez Zumaquero, J. 303
Goncalves, S. B. 727
González-Casimiro, C. M. 451
González-Lleó, A. 274
Gonzalez Medina, M. 817
González-Rodríguez, Á. 202
Good, G. 157
Goossens, G. H. 139
Gopalakrishnan, C. 554
Gordin, D. 821
Görigk, S. 296, 534
Gorman, A. 24
Gorman, D. N. 588, 589
Gorriz, J. 552
Gorska, M. 280
Gorwood, J. 27
Gøtze, J. P. 12, 601
Goulis, D. G. 604
Gourdy, P. 648, 747
Gourley, A. J. 465
Goutchtat, R. 459
Govaere, O. 412
Graber, M. 576
Grace, S. L. 261
Grady, K. 692
Grajales, D. 214
Gram-Kampmann, E. M. 827
Grammatiki, M. 299
Grancini, V. 693
Grasset, E. 119
Gray, S. R. 319
Graziano, G. 612
Greco, C. 725
Green, A. 368
Green, J. B. 560, 618, 751
Greenfield, J. R. 158
Greenfield, S. M. 406
Greenwood, J. P. 813
Gregersen, R. 356
Gregersen, S. 351
Gregg, E. W. 782
Gregory, S. 653
Greig, M. 738
Greve, J.-W. 54
Grevendonk, L. 521
Grewal, K. 208
Gribble, F. M. 216, 306, 460, 466
Grieco, G. E. 311, 327
Grill, V. 55, 290
Grivell, J. 623
Groeneveld, L. 345
Groentved, A. 322
Grønlykke, L. 633
Groop, P.-H. 37, 38, 68, 124, 156, 244, 494, 528, 768, 791, 798, 812, 821, 839
Grove, E. L. 336
Gruden, G. 766
Grunberger, G. 672
Grundtvig, M. 853
Grupe, K. 509
Grzelka-Wozniak, A. 473, 820
Grzywinski, Y. 541
Gu, T. 858
Gu, W. 739
Gu, Y. J. 735, 748
Gual, P. 482
Gualdani, E. 16, 852
Guasch, L. M. 159
Guay, C. 108
Gubitosi-Klug, R. A. 187
Guccio, N. 460, 466
Guck, J. 240
Gudbjörnsdottir, S. 785, 847
Gueddouri, D. 447
Güemes Hidalgo, M. 303
Guensch, D. P. 52
Guerci, B. 51, 615
Guerina, T. 195
Guerra, B. 435
Guiard, E. 664, 831
Guillén, M. 214
Guillou, H. 91, 144, 774
Guilmeau, S. 447
Guion, M. 335
Guirguis, M. N. 53
Guiti, C. 808
Guitton, J. 211, 215
Gullaksen, S. 85, 807
Gullestad, L. 853
Gulseth, H. L. 132, 233, 340, 349
Gunaratnam, S. 710
Gundersen, T. E. 512
Gunn, T. C. 677
Gunnes, N. 233
Guo, L. 547
Guo, W. 198
Gupta, R. 256
Gupta, V. 866
Gupta, Y. 38
Gurbuz, S. 592
Gurlin, R. E. 669
Gurzov, E. A. 391
Gurzov, E. N. 410
Gustenhoff, P. 98, 642
Guttman, A. 295
Guyot, P. 645
Guzmán-Ruíz, R. 479
Gvozdeva, A. 370
Gyberg, V. 863
Gysemans, C. 328, 405, 410
Haahr, H. 639, 647
Habif, S. 673
Haboosh, S. 695
Häckl, D. 876
Hadjadj, S. 747
Haebel, P. 570
Hagan, K. 97, 626
Hagelqvist, P. G. 224, 790, 796
Hagemann, T. 321
Hagerf (Voglova), B. 243
Hägg-Holmberg, S. 68, 124, 156, 791
Hagström, E. 360
Hagström, H. 15
Haidich, A.-B. 875
Hakaste, L. 317
Hakkarainen, A. 494
Hali, M. 395
Halkjær, L. 792
Hall, G. V. 12, 601
Hallahan, N. 238, 372
Hals, I. 55
Haluzik, M. 11
Ham, W. 826
Hameed, A. 280
Hamilton, K. 701
Hamprecht, D. 570
Han, F. 41, 532
Han, K. 599, 703
Han, K.-D. 746, 780
Han, S. 501, 519
Handberg, A. 777
Hanhineva, K. 143
Hanna, F. 658
Hannen, R. 342
Hannon, T. 109
Hansen, A. B. 458
Hansen, A. L. 184
Hansen, B. 230
Hansen, C. D. 827
Hansen, C. S. 20, 86, 190, 707, 719
Hansen, C. 709
Hansen, K. 666
Hansen, M. K. 250, 553, 801
Hansen, S. 639
Hansen, T. M. 713
Hansen, T. M. 720, 736
Hansen, T. W. 87, 123, 218, 709, 752, 822
Hansen, T. 71, 148, 184, 185, 191
Hansen, T. K. 43, 642, 792
Hansen, Y. 567
Hansis-Diarte, A. 10
Hansson, O. 786
Hapca, S. M. 135
Haq, N. 423
Harada, N. 491
Harada, S. 761
Haraldsson, H. 462
Hardikar, A. A. 180
Häring, H.-U. 209
Harjutsalo, V. 68, 156, 528, 768, 791, 812
Härkönen, T. 77
Harms, P. P. 834
Harmsen, M. 271
Harris, M. 332
Harris, S. 629, 637
Harsunen, M. 77
Hart, L. M. T. 20
Hart, M. 647
Hartemann, A. 101
Hartmann, B. 110, 510, 796
Hartnell, S. 50
Hashim, D. 160
Hashimoto, Y. 544
Hásková, A. 672
Hasler, Y. 701
Hassanein, M. 279
Hatoko, T. 491
Hattersley, A. T. 78, 174, 291, 308, 404, 440, 555
Hatziaggelaki, E. 577
Hauck, S. M. 33
Haufe, S. 258
Haugaard, S. B. 356, 602
Haukka, J. K. 244
Haupt, A. 111, 564, 587, 606, 617, 640, 649
Haupt-Jørgensen, M. 210
Havekes, B. 7, 8, 115
Havelund, J. 84
Havlová, V. 655
Hayashi, Y. 491
Haycocks, S. 24
Hayek, S. 710
Haynes, A. 332
Haythorne, E. 31, 103, 393
Haywood, N. J. 193
He, H. H. 64
He, J. 605
He, L. 113, 607
He, S. 571
Heald, A. H. 24, 658, 660, 662, 692
Heckquet, S. K. 86
Hedbäck, N. 477
Hedetoft, C. 642
Heerspink, H. J. L. 45, 358, 552, 553, 617, 750, 751, 752
Heggs, L. 653
Hegyi, J. P. 337
Hegyi, P. 337
Heiland, S. 718
Heimbürger, S. M. N. 556
Heine, R. 57
Heinonen, S. 494
Heise, T. 57, 564, 606, 634
Heitmann, E. 615
Hejlesen, O. 98
Helal, R. 471
Hellberg, A. 539
Helleputte, S. 219
Heller, S. 630, 700
Helsted, M. M. 510
Heltø, A. L. K. 356
Hemi, R. 439
Henckel, M. M. 828
Hendriks, J. 728, 729
Heni, M. 73, 128, 433
Hennayake, C. K. 201
Hennige, A. 613
Henriksen, K. 574
Henriksen, P. 136
Henry, R. M. A. 121, 227, 324
Herbers, E. 494
Herbig, M. 240
Herbsthofer, L. 325
Herder, C. 192, 608
Herings, R. A. R. 834
Herings, R. M. C. 691
Herman, W. 187
Herman, Y. 196
Hermann, P. 827
Hernández, C. 122, 126, 784
Hernandez-Baraza, L. 435
Hernandez Diaz, S. 273
Hertel, J. K. 64
Hertroijs, D. F. L. 652
Herzig, D. 52
Hesse, D. 535
Hesseldal, L. 221
Hesselink, M. K. C. 7, 83, 160, 521
Hetty, S. 26, 30
Heyman, E. 428, 705
Hierons, S. J. 529
Hietala, K. 124
Hiligsmann, M. J. C. 652
Hill, A. V. 76, 169, 291, 292
Hill, J. L. E. 849
Hill, N. 138
Hill, T. G. 103, 849
Hillman, M. 173
Hiltunen, T. 37
Hindle, M. 17
Hindsø, M. 110, 477
Hirota, R. 769
Hirsch, J. 576
Hitos, A. B. 202
Hjelmesæth, J. 64
Hladikova, Z. 243, 655
Hladkou, S. 178
Hlavacek, D. 11
Hlaváček, J. 723
Hlinka, T. 497
Hmeadi, O. M. 104
Ho, F. K. 319
Hochfellner, D. A. 628, 679
Hodsdon, M. E. 596
Hodson, L. 146
Hoebers, N. 139
Hoefler, J. 613
Hoek, A. G. 873
Hoeks, J. 7, 83, 160, 521
Hoeller, V. 407
Hoesli, I. 275
Hoffmann, A. 321
Hoffmann, M. D. 263
Höfler, A. 194
Hogan, M. F. 390
Højlund, K. 80, 84, 184, 191, 322, 334, 368, 458, 777, 783, 827
Hojo, M. 682
Holfeld, J. 576
Holland, D. 658
Hollingsworth, K. G. 255
Holm, L. J. 210
Holman, R. R. 174, 255, 555
Holmberg, S. 173
Holmes-Walker, D. J. 158
Holmgaard, P. 584
Holst, J. J. 12, 110, 456, 477, 510, 542, 556, 601
Holst-Hansen, T. 498, 562
Holt, C. B. 792
Holthuis, E. I. H. 691
Holz, G. G. 575
Hong, E. 599
Hong, O.-K. 446
Hong, S. 833
Hong, S. 467
Hong, Y. 467
Honsek, C. 163
Hoog, M. M. 580
Hoogenberg, K. 750
Hopkins, D. 419, 420, 701, 744
Hopkins, M. 429
Hopkins, R. 235, 555
Horn, K. 321
Hornemann, S. 162, 538
Hornung, I. 383
Horová, E. 672
Horowitz, M. 166, 167, 168, 623, 685
Hosszufalusi, N. 337
Hou, N. 41, 532
Hövelmann, U. 639
Hovorka, R. 50, 641, 699
Howell, S. J. 407
Hribal, M. L. 259, 496
Hric, I. 493
Hsieh, M. H. C. 130
Htike, K. 788
Hu, F. B. 3
Hu, M. 266, 417
Hu, R. Y. 748
Hu, X. 257
Huang, A. 580
Huang, J. 607
Huang, M. 297
Huang, M. L. H. 758
Huang, M. 448, 776
Huang, P. 735, 748
Huang, R. 842
Huang, W. 166, 167, 168
Huang, Y. 710
Huang, Y. 645
Huang, Z. 42, 877
Hubáček, J. A. 743
Huber, J. 169
Huber, S. 722
Hubert, T. 459
Huerta, J. 122
Hug, M. J. 44
Hugger, M. B. 827
Hughes, A. D. 363
Huguet-Moreno, I. 793
Huh, R. 591
Hui, X. 29
Huillet, M. 91, 774
Huising, M. O. 36
Huisman, S. D. 409
Hull, R. L. 390
Hulman, A. 128
Humbert, A. 449
Humel, T. 723
Hummel, J. 433
Huong Nguyen, T. 538
Hur, K. 686
Hurtier, A. 702
Husain, M. 806
Husemoen, L. L. N. 636
Hussain, S. 268
Huttasch, M. 254
Hüttl, M. 497, 504
Huttl, M. 61
Huybrechts, K. F. 273
Huyett, L. M. 669
Hwang, M. 779
Hypo-RESOLVE Consortium 625, 628, 632
Ibfelt, E. H. 170
Ibrahim, H. 205
Iconomidou, V. 577
Idevall-Hagren, O. 237, 373
Idrees, T. 100
Idris, I. 583
Igaz, P. 295
Iglesias, T. 202
Ignácio-Souza, L. M. 215
Ihana-Sugiyama, N. 682
Ihara, K. 244
Iida, H. 464
Ikeguchi-Ogura, E. 491
Ikonomidis, I. 559, 810, 829
Ilonen, J. 77
Im, D. 779
Imai, S. 544
Impronta, F. 425, 456
Inagaki, N. 60, 366, 491
Inashvili, E. 127
Incedal, T. C. 373
Inglada, Y. 222
Inkeri, J. 821
Insabato, L. 848
Inzucchi, S. E. 88, 835
Ioannidis, G. 749
Irvine, K. M. 255
Isaacs, S. R. 236
Isaksen, A. A. 336, 804
Isgrò, C. 312
Ishibashi, R. 9
Isla, H. 126
Islam, T. 712
Isomaa, B. 317
Itaya-Hironaka, A. 769
Ivak, P. 11
Iwasaki, N. 302
Jacan, A. 543
Jacobson, D. A. 376, 414
Jacovetti, C. 108
Jacquemier, P. 668
Jacqueminet, S. 101
Jaffredo, M. 392
Jahn, S. L. 163
Jähnert, M. 353
Jakobsen, P. R. 221
Jalleh, R. J. 166, 685
James, G. G. 828
Jamialahmadi, O. 96
Jan, M. 228
Jang, I.-S.703
Jankipersad, I. R. 873
Jankowicz, N. 608
Jankowska, M. 830
Janku, P. 437
Janona, M. 482
Januszewski, A. S. 473, 758
Jara, M. 18
Jefferies, C. A. 677
Jelenik, T. 576
Jende, J. M. E. 717, 718
Jenkins, A. J. 473, 758
Jensen, A. K. 316
Jensen, D. M. 153, 434
Jensen, J. M. 827
Jensen, M. M. 542
Jensen, M. B. 170
Jensen, M. H. 98
Jensen, N. J. 783
Jensen, T. J. 642
Jensen, T. S. 191
Jenssen, H. 734
Jenssen, T. G. 349, 742
Jenum, A. K. 340
Jeon, J. 501, 519
Jeon, J.-H. 511
Jeong, B.-K. 860
Jeong, J. 501, 519
Jeong, J.-O. 854, 869
Jeong, K. 467
Jering, K. 872
Jesse, K. 87
Jessen, N. 184, 191
Jevon, D. 372
Jeyaparam, S. 272
Jezek, J. 407
Ji, L. 186
Ji, S. H. 748
Jiang, W. 721, 794
Jiang, Y. Q. 748
Jiao, T. 46
Jimenez Sanchez, C. 306
Jin, S.-M. 686, 780
Jin, S. 341
Jin, Y. G. 198
Jin, Z. 572
Jlali, I. 428
Jocken, J. W. E. 139
Joglekar, M. V. 180
Johansen, O. 541
Johansen, R. F. 630, 700
Johansson, K. 173
Johansson, L. 183, 462
Johansson, P. I. 790
Johnson, B. 203
Johnson, J. D. 36, 56, 257, 851
Johnson, P. 175
Johnson, P. R. V. 384
Jonas, J.-C. 392
Jonasson, L. 803
Jones, A. G. 76, 169,174, 261, 291, 292, 404, 440, 555
Jones, B. 569
Jones, J. G. 478, 517
Jones, K. L. 166, 167, 168, 623, 685
Jones, S. D. 677
Jones, T. W. 135
Jong, N. 545
Jongs, N. 250, 552, 750, 752
Jordan, J. 258
Jörgensen, J. 8
Jørgensen, M. E. 71, 542, 707
Jørgensen, N. B. 12,110, 477, 601
Jornayvaz, F. R. 650
Jørsboe, E. 71
Jose, M. D. 361
Joseph, A. 593, 620
Joshi, P. H. 112
Joshi, S. 698
Joubert, M. 664, 831
Jourdain, P. 664, 831
Jozefiak, T. 195
Juhl, C. B. 434, 642, 646
Jui, B. N. 514
Julla, J.-B. 101, 267, 668
Jung, H. 467
Júnior, M. F. 486, 487
Junza, A. 283
Juranek, J. 329
Justesen, L. 148, 185
Juuti, A. 494
Kabisch, S. 163, 165
Kacer, P. 504
Kaci, A. 71
Kaczmarek, A. 820
Kadner, A. 52
Kadolsky, U. 177
Kahkoska, A. 629
Kahl, S. 353
Kahlert, J. 584
Kahn, S. E. 390, 557, 595
Kahoul, Y. 533
Kaiser, G. 209
Kaiser, K. 296
Kajio, H. 682
Kajiyama, S. 544
Kajiyama, S. 544
Kaňková, K. 743
Kalaidzidis, I. 240, 314
Kalaidzidis, Y. 240, 314
Kalamajski, S. 297
Kaltoft, M. S. 597
Kan, C. 41, 532
Kandler, K. 498
Kang, L. 201
Kang, S. S. 763
Kang, Y. 501, 519
Kankova, K. 437
Kannambath, S. 177
Kanzaki, M. 389
Káplár, M. 787
Karagiannis, T. 147, 875
Karagiannopoulos, A. 107, 415, 457
Karaliolios, G. 299
Karalis, V. 577
Karalliedde, J. 676, 695, 744, 756, 764, 809
Karamanakos, G. 365
Karasik, A. 610
Karayiannides, S. 844
Karczewska-Kupczewska, M. 513
Kardalas, E. 749
Kariyawasam, D. 676
Karlsson, H. K. R. 455
Karlstad, Ø. 233
Karnieli, E. 196
Karpe, F. 370
Karsdal, M. A. 218, 574, 709, 822
Karstoft, K. 79, 461
Karuppan, M. 641
Kaser, S. 576
Kashima, K. 548
Kasperova, B. J. 11
Kassi, G. 577
Katayama, M. 81
Katkó, M. 690, 787
Kato, T. 491
Katogiannis, K. 559, 810, 829
Katoh, S. 745
Katte, J. C. 404
Kattner, N. 412
Katzman, P. 741
Kaul, P. 832
Kavazović, I. 527
Kazda, C. 640, 649
K Cardozo, A. 482
Kean, L. S. 379
Kearney, M. T. 193
Keller, A. C. 828
Keller, F. 549
Keller, M. 321
Kellman, P. 813
Kelly, H. 175, 384
Kelly, T. L. 255
Kemper, M. 163
Kender, Z. 716, 717, 718, 726
Kepaptsoglou, O. 444
Képes, Z. 787
Kerkar, P. 872
Kernan, W. 835
Kerr, D. 665
Kerr-Conte, J. 307, 310
Kersten, S. 7
Keshavamurthy, A. 698
Kessler, L. 824
Kesslerova, K. 243
Kettunen, J. L. T. 77
Khalid, U. 563
Khamis, A. A. 266
Khan, D. 488
Khan, I. 65
Khandpur, N. 3
Kharitonenkov, A. 198
Khattab, F. 105
Khoo, C. M. 788
Khoudigian, S. 666
Khunti, K. 18, 568, 609, 684, 747
Khuong, J. 296, 534
Kieffer, T. J. 36
Kietsiriroje, N. 17, 795
Kiflen, M. 65
Kiljanski, J. 600
Kilner, J. 38
Kilpeläinen, T. O. 182
Kim, B.-S. 780
Kim, D. H. 554
Kim, D. 757, 778, 838
Kim, D. 501, 519
Kim, D. 599
Kim, D.-C. 703
Kim, E. 599
Kim, G. 686, 780
Kim, H. 519, 599
Kim, H. 860
Kim, I. 266
Kim, J. 686
Kim, J.-H. 780
Kim, J. 826
Kim, J. 838
Kim, J. 125
Kim, J. 779
Kim, J. 344
Kim, J. 414
Kim, J. 757, 778, 838
Kim, K. W. 236
Kim, K. 125
Kim, K. 125
Kim, K.-A.746
Kim, M. 148, 185
Kim, M.-J.511
Kim, M. 833
Kim, M. 779
Kim, M. 765
Kim, N. 125
Kim, P. 860
Kim, R. 511
Kim, S. 344
Kim, S. J. 763
Kim, S. 125
Kim, S. 833
Kim, S. B. 763
Kim, T. 501, 519
Kim, Y. 778
Kim, Y. 118
Kim, Y. H. 763
Kim, Y. 779
Kimura, Y. 870
Kin, H. 501
Kindracki, Z. 568
King, G. L. 821
Kinoshita, T. 197, 590
Kirk, R. K. 772
Kirketerp-Møller, K. 23
Kirschner, P. 760
Kislev, N. 502
Kitchen, R. R. 256
Kitsos, A. 361
Kivelä, J. 5
Kiyobayashi, S. 60
Kjær, M. S. 18
Kjaergaard, A. D. 43
Kjerpeseth, L. J. 130
Kjölhede, E. 223
Klarić, I. 527
Klaric, L. 877
Klarskov, C. K. 316
Kleber, M. E. 73
Klein, C. 773
Klein, H. 570
Klein Geltink, R. I. 32
Klemp, I. 321
Kliemank, E. 120
Klingbeil, K. 754, 760
Klingenspor, M. 143
Klöppel, G. 412
Klüter, H. 271
Knaub, L. A. 828
Knebel, B. 296, 534, 862
Knight, B. A. 291
Knip, M. 77
Knoch, K.-P. 314
Knop, F. K. 224, 510, 556, 597, 790, 796
Knott, K. D. 813
Knudsen, J. S. 584
Knuppel, A. 363
Koca, Y. 805
Koch, C. 25
Kodama, S. 761
Kodani, N. 682
Koh, G. 599
Koh, G. 765
Kohl, F. 323
Köhler, S. 154
Koistinen, H. A. 199
Kojzar, H. 430, 543
Kokkinos, A. 490, 577
Kolic, J. 56
Kolisek, M. 493
Kolkailah, A. A. 252
Kolobov, T. 675
Kolodziej, A. 572
Komba, M. 32
Konecna, J. 172
Kong, A. P. 622
Kong, A. 654
König, A.-C. 33
Konrad, D. 25, 483
Konrad, N. K. 25
Kontula, K. 37
Koo, D.-J. 14
Kooi, E. 341, 512
Kooman, J. P. 324
Kooy, A. 752
Kopf, S. 120, 716, 717, 718, 726
Kopp, J. L. 851
Korakas, E. 559, 810, 829
Korantzis, A. 444
Kordi-Patime, O. 439
Korn, A. 92, 573
Korukonda Dr, K. 753
Korytko, A. 329
Korzh, S. 767
Koshino, A. 250, 553
Koshizaka, M. 9, 40
Kosinski, M. 432
Kosok, A. 314
Kossack, N. 876
Kostelli, G. 559, 810, 829
Kostenis, E. 209
Koster, A. 154, 324, 341
Köster, K. A. 508
Kostopoulos, G. 875
Kotecha, T. 813
Kothawade, Y. M. 657
Kotnik, P. 54
Kotsa, K. 299, 604
Kotseva, K. 863
Kotzaeridi, G. 275
Koudelková, K. 370
Koufakis, T. 299, 604
Kountouri, A. 559, 797, 810, 829
Kousathana, F. 829
Kovac, L. 353
Kovacs, A. 337
Kovacs, P. 321
Koves, T. R. 7, 521
Kowalska, I. 513
Kowluru, A. 395
Kraenkel, N. 165
Kraft, F. A. 823
Krag, A. 827
Kramer, G. 246
Krasauskaite, J. 42, 877
Krasowska, U. 280
Kravets, V. 375
Krawietz, P. 639
Krbcová, M. 370
Kremers, S. H. M. 346
Kremser, C. 576
Krentz, N. A. J. 36
Kretowski, A. 280
Krijnen, P. A. J. 92, 573
Krishnamoorthy, S. 694
Kristensen, C. A. 772
Kristensen, F. P. 191
Kristensen, N. R. 639
Kristensen, P. L. 316, 630, 700
Kristiansen, K. 143
Kristiansen, O. P. 602
Kristiansen, V. B. 110, 477
Kristinsson, J. A. 64
Krogh, L. S. L. 510, 556
Krohn, K. 321
Krolewski, A. 244
Kronshage, B. 634
Krook, A. 81, 82, 161, 200, 229, 231, 455, 481
Kroon, A. A. 121, 154, 227, 324
Kroonen, M. Y. A. 752
Krueger, B. 608
Krug, H. V. 871
Krus, U. 265
Kruse, M. 162
Kruse, R. 458
Kubanova, L. 493
Kuda, O. 11
Kuefner, M. 200
Kufaishi, H. 86
Kuhn, T. 296, 534
Kuja-Halkola, R. 785
Kukor, Z. 295
Kulkarni, R. N. 456
Kulkarni, S. A. 22
Kullberg, J. 183, 514
Kumar, A. 786
Kumar, L. 590
Kumar, R. 308
Kumarathurai, P. 602
Kun, A. 128
Kundu, R. 518
Kuniß, N. 246
Kunte, P. S. 180
Küppers, C. 871
Kupriyanova, Y. 254
Kurashvili, R. 127
Kurz, F. T. 717, 718
Kuss, O. 323
Kusters, T. 454
Kusters, Y. H. A. 512
Kutz, A. 554
Kuula, J. 494
Kvaskoff, M. 151, 152
Kvist, P. H. 772
Kwan, A. Y. M. 566
Kwon, H. 778
Kwon, M.-S. 703
Kwon, S. 511
Kwon, S. 686, 780
Kye, J.-W. 703
Kyriakidou, A. 604
Kyriazou, A. V. 604
Kzhyshkowska, J. 271
Laakso, M. 199
L'Abbate, F. 704
Labouèbe, G. 225, 228
Lacas-Gervais, S. 482
Lachin, J. M. 187
Lachmanová, Z. 655
Lackner, B. 407
Lacko, D. 172
la Cour Poulsen, L. 64
Laffel, L. M. 97, 669, 688
Lagathu, C. 27
Lage, A. 618, 620
Lahti, K. 317
Lai, E. C. C. 130
Lai, H. 782
Laing, I. 660
Laitinen, I. 462
Lal, M. 755
Lalama, E. 165
Lamarche, F. 619
Lambadiari, V. 559, 797, 810, 829
Lambelet, M. 593
Lambers Heerspink, H. J. 545
Lamotte, M. 666
Lampasona, V. 261
Lampousi, A.-M. 284
Landgraf, W. 645
Landin-Olsson, M. 173
Landschulz, W. 640, 649
Landstedt-Hallin, L. 844
Landstra, C. P. 409
Lang, A. 1, 370
Lang, A. 775
Lang, J. 702
Langin, D. 144
Langlois, A. 389
Lánská, V. 743
Laouali, N. 151, 152
Lapauw, B. 219
Lapière, S. 459
Lapuerta, P. 605
Lara-Moreno, C. 793
Larger, E. 101
Larsen, A. T. 574
Larsen, S. 232
Larsson, H. 785
Larsson, S. C. 183
Laschke, M. W. 263, 383
Lass, A. 144
Lassalle, R. 664, 831
Lasserre, F. 774
Lasserre, F. 91
Latorre, J. 522
Lattuada, G. 95
Latva-Rasku, A. 199
Latz, E. 165
Lau, E. S. 622, 654
Lau, J. 387
Laubner, K. 44, 54
Laugesen, E. G. 85, 807
Laurberg, T. 847
Laurent, V. 442
Lauridsen, J. T. 221
Lauritsen, J. 796
Lauro, D. 725
Laursen, D. H. 221
Laursen, J.-C. 86
Laursen, P. N. 562
Lause, P. 468
Lauwers, P. 728, 729
Laver, T. 78
Laverman, G. D. 752
Laviola, L. 59, 500, 814
Lavoie, G. 28
Law, B. 197, 590
Lawatscheck, R. 593, 618, 620
Lawrence, F. 407
Lawson, J. 610
Le, A. 65
Leal, E. C. 21, 734
Leal, L. G. 256
Leanza, G. 312
Leask, A. 776
Lebek, S. 296, 534
Lebrec, J. 615
Leclerc, J. 359
Lee, C. 599
Lee, C. 603
Lee, C. J. 586
Lee, H. 467
Lee, I.-K. 511
Lee, J. Y. 763
Lee, J. 757, 838
Lee, J. 757, 778, 838
Lee, J. 855
Lee, K.-W. 501, 519
Lee, M. 654
Lee, N. 501, 519
Lee, S. 757, 778, 838
Lee, S. 757, 778, 838
Lee, S. 746
Lee, S.-H. 855
Lee, S. 446
Lee, W. 826
Lee, Y.-B. 686, 780
Lee, Y. S. 431
Lee, Y. 129
Leech, C. A. 575
Leelarathna, L. 641
Lee-Ødegård, S. 132, 340
Leese, G. 134
Lefebvre, R. 619, 856
Legaard, G. 79
Legård, G. E. 117
Legault, L. 705
Legido-Quigley, C. 136, 148, 185, 719, 799
Lehrskov, L. L. 639
Lehrskov, L. 461
Lehtimäki, T. E. 146
Lehto, M. 528, 798, 839
Lehtonen, L. 481
Lehtonen, S. 798, 839
Lehtovirta, M. 317
Lei, T. 706
Leinhard, O. D. 112
Lekka, C. 416
Lekva, T. 436
Lemaitre, M. 131, 442
Lempesis, I. G. 139
Lendeckel, U. 413
Lenne, X. 131
Lenz, J. 430
Leo, L. 704
Leonardini, A. 814
Leone, A. 815
Leone, M. 785
Leonetti, F. 843
Leonetti, S. 241
Leoni, M. 725
Le Pelletier, L. 27
Leppänen, S. 205
Lerche, S. S. 642
le Roux, C. W. 203
Lessan, N. 471
Le Stunff, H. 211, 215
Levade, T. 774
Levelt, E. 813
Levenberg, S. 196
Lever, C. S. 677
Levi, K. 439
Levine, J. A. 585, 614
Levitt, C. H. 374
Levrat-Guillen, F. 51
Lewis, D. M. 677
Lewis, E. 872
Lewis, J. E. 216
L. Heerspink, H. J. 250
Li, A. 410
Li, C. 605
Li, G. 186
Li, J. 285
Li, J. 57, 564, 606
Li, K. 771
Li, L. 547
Li, L. 176, 357, 850, 859
Li, L. J. 431
Li, Q. 186
Li, T. 505
Li, T. 781
Li, T. 605
Li, T. 547
Li, W. 113, 607
Li, W. 197, 590
Li, X. 4, 281
Li, X. 644
Li, Y. 377, 378
Li, Y. 596
Li, Y. 113, 607
Liatis, S. 365, 797
Licata, G. 311, 327, 328
Lichtenberg, A. 823, 871
Lichtenstein, P. 785
Liebetrau, B. 117
Liebmann, M. 445, 509
Liepins, E. 530
Liepinsh, E. 767
Lilao-Garzon, J. 484
Liles, A. N. 575
Lim, G. E. 28, 571
Lima, F. 277
Limone, B. 580
Lin, B. 355
Lin, J. 851
Linares-Pineda, T. M. 277
Lind, L. 183
Lind, T. 288
Lindeboom, L. 115, 521
Lindegaard, B. 316
Lindén, J. 798, 839
Lindén Hirschberg, A. 539
Linder, T. 275
Lindgaard, R. 772
Lindhardt, M. K. 123
Lindholm, E. 20
Lindström, J. 5
Ling, C. 265, 334, 352, 457
Linge, J. 112, 561
Lingvay, I. 499, 558, 567, 585, 806
Liston, A. 307
Lithovius, R. 37, 812
Litorp, H. 462
Liu, B. 603
Liu, C.-C. 621
Liu, E. 313
Liu, J. 97, 626
Liu, L. 239
Liu, R. 587
Liu, S. 785
Liu, T. 97, 626
Liu, Y. 74, 343, 347
Liu, Z. 355
Liu, Z. 817
Liz Zhou, F. 637
Llamas Elvira, J. 141
Llauradó, G. 222
Llaurado, G. 283
Llavero-Valero, M. 793
Lloyd, M. 31, 393
Lluch, A. 522
Löffler, D. 433
Löffler, M. 321
Löfvenborg, J. E. 284
Loghin, C. 111
Logue, J. 523
Loh, M. 111
Lohmann, M. 240
Lois, N. 714
Loiseau, N. 91, 144, 774
Loizon, E. 619
Lombardo, M. T. 305
Löndahl, M. 251, 730, 741
Long, A. E. 261
Longato, E. 836
Longfield, M. S. G. 732
Longo, M. 269
Longo, V. 716
Loos, R. J. F. 182
López, M. 211
Lopez, R. N. 588
López-García, P. 325
López Larrubia, P. 214
Loreggian, L. 399
Loretelli, C. 262, 399
Lortz, S. 397
Lorza-Gil, E. 209
Lotersztajn, S. 91
Lou, Y.-R. 285
Louise, J. 685
Loumaye, A. 468
Loy, S. L. 431
Lu, D. 590
Lu, J. 355
Lu, M. 462
Lu, V. B. 460
Luan, C. 457
Lubrano, C. 526
Lucacchini, A. 58
Lucchesi, D. 16, 90, 852
Lucibello, G. 194
Lucic Fisher, S. G. 448
Ludvigsen, T. P. 772
Ludvigsson, J. 55, 315, 661
Ludvigsson, J. F. 785
Ludvik, B. 722
Luijten, I. H. 140
Luk, A. O. 622, 654
Luk, C. 193
Lund, A. 556
Lund, L. E. 256
Lund, M. A. V. 117
Lund, P.-E. 104
Lund-Blix, N. A. 233
Lundell, L. L. 82
Lundell, L. S. 231
Lundman, P. 844
Lundqvist, M. H. 475, 514
Luongo, D. 164
Lurz, Y. 208
Lutale, J. K. 731
Luukkonen, P. K. 146
Luzio, S. D. 724
Luzza, F. 259, 496
Lv, Y. 357, 859
Ly, T. T. 669, 670
Lynch, J. C. 376
Lynch, P. M. 102
Lyngbaek, M. 79
Lyngbæk, M. 117
Lyng Wolden, M. 663
Lynn, F. C. 36
Lyu, Y. 344
Lyu, Z. 419
Ma, A. 285
Ma, C. 649
Ma, J.-X. 258
Ma, J. 421, 706, 842
Ma, J. 371
Ma, R. C. 622, 654
Ma, X. 587
Maalmi, H. 192
Maandi, M. 861
Maandi, S. C. 394, 861
Maasen, K. 282
Mabley, J. G. 394, 861
Mabunay, M. A. 645, 689
MacDonald, P. E. 32, 33, 180
Macedo, M. P. 204, 253, 492, 517
Macedo, M. 478
Machado, U. F. 270
Machann, J. 162
MacIsaac, R. J. 762
MacLeish, S. A. 669
Macura, S. 567
Maddaloni, E. 411, 711
Mader, J. K. 628, 679
Madsbad, S. 12, 110, 477, 601, 602
Madsen, L. R. 434
Madsen, P. L. 12, 601
Maechler, P. 306
Maeng, M. 43
Maestroni, A. 399
Maezawa, Y. 9, 40
Magliano, D. J. 181, 362, 811
Magnaghi, C. 369
Magnan, C. 215
Magnes, C. 407
M. Aguilera, C. 141
Mahmoudi, Z. 625, 681, 683
Mahrik, J. 11
Mai, K. 538
Mainardi, M. 212
Maiorino, A. 242, 714
Majeed, A. 782
Mäkinen, S. 199
Makino, M. 769
Mašková, J. 655
Makrecka-Kuka, M. 767
Makrilakis, K. 365
Mala, T. 64
Malagon, M. 479
Maldonado, J. M. 566
Maldonado, M. 621
Malek, R. 645
Malhotra, A. 578
Malhotra, B. 676
Malik, R. 645
Malinska, H. 61, 497, 504
Malling, B. 609
Malmberg, F. 183
Maltese, G. 809
Malyshev, A. 611
Mambu-Manbueni, H. 267
Man, K. K. C. 130
Manadas, B. 536
Mancilla, R. 83, 521
Mancini, M. 164
Mancuso, J. P. 252, 621
Mandal, S. 518
Mandatori, D. 276
Mandereau-Bruno, L. 335
Mandrup, S. 267
Manenti, F. 305
Manfrini, S. 96
Mangelis, A. 744, 756
Manghi, F. P. 706
Mangla, K. K. 18, 806
Maningat-Goco, M. 643
Manjelievskaia, J. 580
Mankovsky, B. M. 874
Mankovsky, G. B. 874
Mann, J. F. E. 751
Manning, M. 673
Manolopoulos, K. N. 139
Manson, J. E. 3
Mantzoros, C. 490
Manuel, Y. 129
Mao, S. 65
Marais, G. 428
Marasco, R. 259, 496
Marchetti, P. 34, 58, 311, 326, 400
Marciniak, C. 459
Marcus, K. 818
Mari, A. 57, 456, 464, 606
Mariani, S. 526
Marica, A. 481
Marigowda, G. 379
Marín-Cañas, S. 206, 326
Marinicova, Z. 314
Mariño, E. 410
Markmann, J. 379
Markova, I. 61, 497, 504
Marks, K. P. 170
Marlier, J. 219
Maroun, R. 665
Marrano, N. 59
Marre, M. 294
Marroqui, L. 398
Marselli, L. 58, 311, 326, 400
Marshall, M. 239
Marta, K. 337
Martelli, F. 46
Martin, C. L. 187, 710
Martin, C. 91
Martine-Edith, G. 625, 683
Martinenghi, S. 369
Martinez-Martinez, A. 278
Martinez Mora, A. 183
Martínez-Oca, P. 422
Martinez-Tellez, B. 141
Martinka, E. 248, 289
Martín-Montalvo, A. 522
Martín-Romo, I. 674
Martin-Ruiz, C. 255
Martins, F. O. 474, 516, 624, 845
Martins, I. B. 624
Martins, J. 536
Martín-Timón, I. 793
Martinussen, C. 110
Martinussen, T. 772
Martola, J. 821
Marushko, Y. Y. 874
Marx, N. 835, 876
März, W. 73
Marzetta, F. 226
Mas, S. 520
Masi, D. 526, 711
Masone Iacobucci, G. 704
Massey, R. J. 878
Masszi, T. 295
Mastrocola, R. 495
Matafome, P. 476, 486, 487, 536
Matarese, G. 312
Mather, K. J. 57, 564, 592, 606
Mathiesen, E. R. 636
Mathieu, C. 328, 405, 600
Mathine-Edith, G. 681
Mathioudakis, K. 365
Matran, R. 428
Matricali, G. 728, 729
Matsuhisa, M. 684
Matsuki, K. 870
Matsumura, K. 870
Mattern, Y. 818
Mattheus, M. 88, 835
Matthiessen, K. S. 256
Mattila, I. 136
Mattsson-Carlgren, N. 786
Matulewicz, N. 513
Matuseviciene, L. 104
Maulucci, G. 865
Mauquoi, C. 648
Mauricio, D. 283, 648
Maxwell, A. 38
Maxwell, A. J. 236
Mayer, G. 549
Maytham, K. 224, 790, 796
Mazaki-Tovi, S. 439
Mazzeo, M. 90
Mazzoni, M. R. 58
McAllister, D. A. 234
Mcallister, J. 189, 737
McBride, J. D. 258
McCann, G. P. 813
McCormick, D. 140
McCrimmon, R. J. 135, 638, 644
McCullagh, J. 31
McDaniel, K. 330
McDonald, T. J. 76, 169, 261, 291, 292, 404
McDonough, L. 175, 384
McGill, J. B. 620, 751
McGorm, K. J. 332
McGovern, A. P. 235, 555
McGuire, D. K. 252
McGurnaghan, S. 67, 234
McKay, E. J. 140
McKay, G. J. 38
McKeigue, P. M. 67, 234, 721, 794
McKnight, A. 38
McLennan, S. V. 732
McNeilly, A. D. 135
McQueen, R. B. 286
McVey, Z. 256
Medina-Gali, R. M. 398
Meer, R. 873
Meex, S. J. R. 324
Mégret, C. 634
Mehlem, A. 150
Mehmeti, I. 397, 424
Mehta, S. N. 669
Meier, J. J. 609, 623
Meier, R. A. 677
Meinilä, J. 5
Meizoso-Pita, O. 793
Mekhail, N. A. 53
Melander, O. 786
Melander, S. A. 574
Melas-Melt, L. 638
Melchionda, E. 816
Melenovsky, V. 11
Melidou, E. 299
Melin, E. O. 173
Mellbin, L. 45, 594, 800, 863
Mellergaard, M. 777
Mello-Gomes, R. 486, 487
Mellor, J. 721, 794
Melmer, A. 52
Melo, B. F. 474, 624, 845
Melzer Cohen, C. 610
Melzi, R. 418
Memon, B. 298
Mendez-Gutierrez, A. 141
Mendizabal, L. 278
Menduni, M. 242, 714, 725
Meneses, M. J. 253, 478, 517
Menezes-Pinto, D. 841
Meng, W. 20
Menger, M. D. 263, 383
Menoud, V. 108
Menta, P. L. R. 215
Mercader, J. M. 69
Mercalli, A. 418
Merciai, C. 546
Merom, D. 318
Mertens, J. 463
Mertes, B. 246
Mesa, A. 220
Meschi, T. 364
Messier, V. 705
Meugnier, E. 619
Meulebrouck, S. 70
Mevenkamp, J. 8, 115
Meyer, N. M. T. 163, 538
Meyer-Hermann, M. 240
Mezza, T. 425, 456, 865
Mianowska, B. 830
Michael, N. 129, 431
Michalopoulou, E. 810
Michel, M. 70
Michels, A. 330
Middleton, L. 782
MIDiab Study Group 837
Midtvedt, K. 349
Miedzybrodzka, E. L. 460, 466
Miehle, K. 506
Miele, C. 815, 848
Miettinen, P. J. 77
Migdal, A. L. 100
Migliaccio, T. 848
Migliozzi, L. 531
Mihaly, E. 337
Mijolović, Ž. 527
Miklankova, D. 61, 497, 504
Mikušová, V. 289
Milano, A. D. 500, 814
Milanski, M. 215
Milicevic, Z. 57, 111, 462, 564, 606
Militaru, A.-M. 846
Mill, P. 140
Miller, B. 116
Millett, C. 782
Milo, M. 612
Min, D. 732, 776
Min, K. 703
Minamizuka, T. 40
Miniewska, K. 280
Miras, A. D. 203
Mirra, P. 815, 848
Mirshahi, U. 78
Mischak, H. 123
Mithieux, G. 453, 775
Mitkin, N. 611
Miyamoto, J. E. 215
Miyawaki, T. 544
Miyazato, Y. 682
Mizokami-Stout, K. 710
Mizrak, H. I. 86, 190
Mizushiri, S. 338
Moccetti, T. 872
Modamio-Molina, J. 793
Modi, H. 36
Moen, G.-H. 340
Moffa, S. 425, 456
Moffett, R. C. 488
Moganti, K. 271
Mohammadi-Shemirani, P. 65
Mohammed, J. 698
Mohammed, M. 698
Mohammed, S. 698
Mohammedi, K. 637
Moinard, C. 619
Moissl, A. P. 73
Mokhtar, S. B. A. 121, 155
Molina-Vega, M. 277
Møller, A. L. 218, 549, 822
Møller, N. 149
Møller, P. M. 84
Möllsten, A. 288
Mølsted, S. 630
Molsted, S. 700
Moltke, I. 71
Molveau, J. 705
Monchablon, M. 702
Monfeuga, T. 256
Monfort-Pires, M. 142
Monod, C. 275
Mønsted, M. Ø. 210
Montagner, A. 144
Montaigne, J. 533
Montanya, E. 597
Montaser, H. 179, 205
Monteiro, C. 3
Monteiro, T. 486
Monteiro-Alfredo, T. 476, 536
Moon, J. 813
Moon, J. 860
Moon, J. 599
Moonen-Kornips, E. 8, 160
Moore, N. 664, 831
Mooshage, C. M. 718
Morabito, G. 348
Morato, M. 841
Morch, C. D. 713
Mørch, C. D. 720, 736
Morcillo, S. 277
Moregola, A. 515
Moreno Lopez, M. 307
Moreno-Navarrete, J. 522
Moreno-Ruiz, I. 793
Moretti, C. 711
Morgan, N. G. 137, 388, 416, 849
Mori, A. 327, 328
Moriconi, D. 470
Morieri, M. L. 836
Morio, B. 619
Moritz, T. 35
Morningstar, M. 572
Moroz, M. 280
Morriseau, T. 300
Morrison, K. 65
Morshed, A. 712
Morton, J. I. 362
Morton, R. 65
Moschovaki Filippidou, F. 824
Mosenzon, O. 558, 610, 618
Möser, C. 254
Moser, O. 427, 430
Moser, P. 576
Mossel, M. 271
Mottl, A. K. 751
Mourya, S. 197, 590
Mousovich-Neto, F. 452
Moutzouris, D. 695, 764
Mraz, M. 11
Muammar, T. 471
Mubita, W. 641
Mucci, P. 428
Mueller, A. 430
Mueller, K. 506
Mugabo, Y. 28
Muilwijk, M. 339
Mukherjee, B. 247, 635
Mukhopadhyay, P. 759
Mul, D. 469
Mulangala, J. 47
Mulder, H. 35, 178, 297
Müller, A. 543
Müller, A. 314
Müller, L. 321
Müller-Wieland, D. 648, 876
Mullins, G. 596
Mumu, S. J. 318
Munoz-Descalzo, S. 484
Munro, L. 54
Muoio, D. M. 7, 521
Muraca, E. 95
Murakami, H. 870
Murakami, T. 60, 366
Muralidharan, S. 417
Muratov, S. 666
Murdoch, C. E. 201
Mursic, I. 647
Murzio, C. 495
Musale, V. 201, 771
Mutter, S. 37, 156, 244, 768, 812, 821
Myers, A. 747
Myette-Côté, É. 705
Mysliwiec, P. 513
Mziaut, H. 240
Nachtergaele, C. 438, 443
Nadasdi, A. 295
Nadeem, C. 92
Nag, S. 518
Nagao, M. 507
Nägga, K. 786
Nahirney, N. 56
Nairizi, A. 53
Naji, A. 379
Nakanga, W. P. 440
Nakhe, A. Y. 376, 414
Nalbach, L. 263
Nangaku, M. 751
Nannipieri, M. 470
Nano, R. 418
Napoli, V. 90
Napolitano, T. 331
Naqvi, M. 689
Narendran, P. 406, 651
Narula, S. 65
Naseem, K. 17
Näslund, E. 93, 455
Näsman, P. 800
Nasser, S. 537
Natali, A. 241, 249
Natalicchio, A. 59, 500, 814
Nathanson, D. 360, 701
Nåtman, J. 223, 360
Nauck, M. A. 623
Navez, B. 468
Navis, G. 320
Nawroth, P. P. 120, 716, 717, 726
Neal, B. 250, 545, 553
Neeland, I. J. 112, 541, 561
Nemetova, L. 243
Neogi, S. 759
Neri, E. 90
Nermoen, I. 132
Nesti, L. 241, 249
Netuka, I. 11
Neubauer, H. 570
Neuen, B. L. 553
Neuenschwander, M. 1
Neumark, T. 173
Neusner, R. 286
Neuvonen, M. 494
Newcombe, L. 676
Newman, J. 143
Ng, N. H. J. 417
Ngono Ayissi, K. 27
Nguyen, M. 48, 808
Nguyen, P. M. 237
Nguyen, T. N. 426
Nguyen, Y. H. 199
Ni, Y. 285
Nica, A. 846
Nicholls, S. J. 47
Nicolaisen, S. K. 75, 334
Nicolás, F. J. 733
Nicolay, C. 595
Nicolì, F. 49
Nicolò, A. 815
Nicolucci, A. 612
Niedzwiecki, P. 473
Nielsen, F. E. 356
Nielsen, J. S. 43, 184, 191, 322, 334, 783
Nielsen, J. B. 221
Nielsen, L. L. 609
Nielsen, N. F. 223
Nielsen, N. 79, 461
Nielsen, O. W. 602
Nielsen, R. L. 256
Nielsen, R. 267
Nielsen, S. H. 709
Nielsen, T. S. 232
Niemi, M. 494
Niemi, T. 143
Niersmann, C. 862
Niessen, H. W. M. 92, 573
Nieuwdorp, M. 469
Nigi, L. 311
Nigro, C. 815
Nijhoff, M. F. 409
Nijpels, G. 834
Nikolajuk, A. 513
Nikontovic, A. 98
Nilsson, K. 659, 663
Nilsson, P. M. 786
Nilsson Neumark, A.-S. 173
Nimgaonkar, A. 195
Ning, L. 468
Nirantharakumar, K. 840
Nishimura, R. 745
Nissanholtz-Gannot, R. 675
Nitze, L. M. 18
Niu, T. 781, 789, 819
Nobels, F. 728, 729
Nogueira, G. A. 142
Nohr, E. A. 153
Norata, G. 515
Nordic Pregnancy Drug Safety Studies (NorPreSS) consortium 130
Nordsborg, R. B. 636
Nørgaard, K. 642
Norhammar, A. 800, 844, 863
Norman, G. J. 102
North, R. V. 724
Noursadeghi, N. 36
Nouvenne, A. 364
Novodvorsky, P. 11, 289
Noyes Essex, M. 621
Ntsekhe, M. 872
Nube, V. 732
Nuijts, R. M. A. 155
Nuria, A.-C. 671
Nuutila, P. 143, 199, 464
Nwokolo, M. 50
Nyirenda, M. J. 440
Nyström, F. H. 803
Oakey, H. 332
Obermayer, A. 430, 543
Obermayer-Pietsch, B. 543
Obeso, A. 845
O'Cearbhaill, E. 175, 384
O'Connor, S. 359
Odouard, S. 194
Oetjen, E. 508
Ofori, J. 415
Ofori, J. K. 265, 457
Ofstad, A. P. 88, 835
Ogata, M. 302
Oger, F. 533
Ogura, M. 366
Ogurtsova, K. 181
Öhman, H. 791
Ohsugi, M. 682
Oikawa, S. 507
Oikonen, V. 464
Okamoto, M. M. 270
Okamura, T. 761
Okazaki-Hada, M. 507
Olaniru, O. 177
Oldin, C. 315
Olea, E. 845
Olesen, T. B. 827
Oliveira, L. M. 204
Oliveira, P. 478, 487
Oliveira, R. O. 516
Oliver, N. 138
Olkkonen, V. M. 199
Olleik, H. 381
Olsen, L. H. 772
Olsen, M. H. 184, 221, 827
Olsen, M. T. 316
Olsen, N. V. 633
Olsen, T. 512
Olsesen, T. B. 221
Oltean, S. 770
Omnipod 5 Research Group 669, 670
O'Neal, D. N. 758
Onyema, M. 695, 764
Op de Beeck, A. 326
Op den Kamp, Y. 8
op den Kamp, Y. J. M. 7
Opperhuizen, A. 282
Oram, R. A. 286
Orchard, T. J. 187
Orho-Melander, M. 146
Orioli, L. 468
Orliaguet, L. 773
Orsi, E. 693
Orsini Federici, M. 615
Ortega, F. 522
Ørtenblad, N. 80, 84
Ortiz-Zuñiga, A. 784
Oscarsson, J. 7, 8
Østergaard, J. A. 792
Osterhoff, M. A. 163
Ostertag, A. 668
Östgren, C. 803
Osuna-Prieto, F. 141
Ota, H. 769
Otonkoski, T. 179, 205
Ouřadová, A. 370
Oulhaj, A. 543
Ouni, M. 353
Ouwens, D. M. 823, 871
Overbeek, J. A. 691
Overbergh, L. 405
Overby, P. 56
Owen, B. M. 230
Owens, D. R. 724
Oyaert, K. A. M. 155
Ozasa, N. 544
Ozdemir Saltik, A. 701
Öztüzün, A. 805
Pacal, L. 437, 743
Pace, M. 848
Pack, M. 263
Paczkowski, R. 580
Pagliuca, F. 379
Paikopoulou, A. 749
Painter, R. 680
Paisley, A. 692
Pakseresht, A. 499
Palinkas, D. 337
Pallubinsky, H. 160
Palma, G. 500
Palmer, A. J. 361
Palmer, C. 20
Panagiotopoulos, S. 762
Panagiotou, A. 756, 809
Panagiotou, S. 237
Panczel, P. 337
Panda, S. 542
Pandey, A. 112, 252
Pandolfi, A. 276, 733
Pang, C. 171
Panimolle, F. 411
Pantalone, K. M. 591, 618
Panzhinskiy, E. 36
Papanas, N. 299
Paragh, G. 787
Parascandolo, A. 848
Paray, N. B. 695, 764
Paré, F. 28
Paré, G. 65
Parente, E. B. 528, 768, 812
Park, C. 599
Park, E. 757, 778
Park, J. 686, 780
Park, J. 860
Park, S. 14
Park, S. 501, 519
Park, S. 686, 780
Park, S. 779
Parkin, C. G. 672
Parravano, M. C. 242
Parravano, M. 714
Parrillo, L. 269
Parry, S. N. 776
Partridge, K. M. 137
Parving, H.-H. 642
Parzer, V. 722
Paschou, P. 299
Paschou, S. 577
Pasquel, F. J. 100
Pasquetti, G. 307
Passarella, G. 843
Pataky, Z. 650
Patarrão, R. 253
Patel, H. 558, 568, 591
Patel, K. A. 308
Patel, K. 404, 78
Patel, K. V. 112
Patel, N. 676
Patel, Y. 230
Patera, P. I. 242
Paterson, A. D. 67
Päth, G. 44
Patócs, A. 295
Patorno, E. 273, 554
Patrakka, J. 755
Patrício, B. 517
Patted, U. R. H. 657
Patterson, A. E. 32
Patterson, D. 53
Pattou, F. 307
Pattou, F. 310, 459
Paul, R. G. 677
Pavlidis, G. 559, 810, 829
Pavo, I. 557, 595, 617
Pavshintsev, V. 611
Pawlak-Chaouch, M. 428
Payrastre, L. 144, 774
Pearson, E. R. 134, 174, 261, 555, 557, 878
Pearson, S. M. 17, 795
Pecquet, C. 101
Pedersen, H. 542
Pedersen, H. D. 772
Pedersen, J. 136
Pedersen, K. 210
Pedersen, L. 75
Pedersen, L. B. H. 646
Pedersen, M. G. B. 149
Pedersen, M. L. 71
Pedersen, S. 499
Pedersen-Bjergaard, U. 136, 224, 403, 632, 633, 642, 790, 796
Peghin, M. 408
Pekkari, O. 661
Peleshok, J. 580, 600
Pellegrini, S. 305
Peltoniemi, H. 494
Pelusi, L. 733
Peña-Montero, N. 277
Pendergrast, L. A. 82, 231, 455
Penedo, J. 465
Penesova, A. 493
Peng, L. 100
Penno, G. 16, 90, 852
Penno, M. A. S. 332
Perdomo, G. 451
Perea, V. 220, 441
Pereira, M. J. 26, 30, 214, 475, 514, 524
Pereira, S. 487
Pérez-Serna, A. 398
Perfilyev, A. 265, 352
Peršić, V. 527
Perino, A. 494
Perkins, B. A. 187
Perkovic, V. 250, 545, 553
Pernemalm, M. 385
Pernow, J. 46, 268
Perrini, S. 59, 500, 814
Perrone, G. 96
Perrot, G. 775
Persaud, S. J. 177, 419, 420, 423
Perseghin, G. 95, 348
Persson, F. 87, 89, 123, 752
Pesce, F. 39
Pesce, L. 34
Pesenti, S. 619
Peter, A. 128, 258, 433
Peters, A. 379
Peters, A. 192
Peters, H. P. F. 115
Peters, V. 754, 760
Petersen, E. 53
Petersen, J. 356, 636
Petersen, J. 195
Petersen, K. F. 146
Petersen, M. H. 80, 84
Petersen, T. G. 153
Petersson, M. 561
Petkovic, M. 734
Petrazzuolo, A. 399
Petrelli, A. 293
Petrie, M. C. 594
Pettus, J. 626
Pezzolla, A. 500
Pfeffer, T. 754, 760
Pfeifer, V. 325
Pfeiffer, A. F. H. 162, 163, 165, 538
Pferschy, P. N. 430, 543
Phielix, E. 7, 8
Phillips, L. 623
Picard, A. 225, 228
Picard, P. 247, 635
Picardi, A. 96
Picconi, F. 242, 714
Pichery, E. 331
Picón, M. J. 277
Pieber, T. R. 325, 407, 543, 647
Piemonti, L. 293, 305, 418
Pieralice, S. 312
Pierrou, S. 462
Pietiläinen, K. 494
Pietrowska, K. 280
Pietrzak, I. 830
Pigeyre, M. 65
Pignatiello, S. 848
Pillai, V. 407
Pillon, N. J. 81, 82, 161, 481
Pilmark, N. 461
Pilorget, V. 247, 635
Pina, A. 253
Ping, F. 113, 607
Pinget, M. 145, 389
Pinto, S. 48, 438, 443
Pipino, C. 733
Pires, A. S. 476
Pirinen, E. 494
Pirog, A. 702
Pirola, L. 537
Piron, A. 69, 307
Pirttiniemi, A. 798, 839
Pitha, J. 497
Pitocco, D. 704
Pitt, B. 593, 618, 620
Pivovarova-Ramich, O. 162
Plaisant, M. 331
Plein, S. 795, 813
Pletsch-Borba, L. 538
Pleyerova, I. 11
Pliouta, L. 559, 810, 829
Plummer, M. P. 685
Plum-Mörschel, L. 639
Pociot, F. 210
Pohrt, A. 538
Poignard, P. 48
Poirier, P. 359
Polizzi, A. 91, 144, 774
Pollom, R. K. 688
Pollreisz, A. 722
Polomoscanik, S. 195
Pontoglio, M. 307, 310
Pontrelli, P. 39
Poon, T. 698
Pop-Busui, R. 187, 710
Porcaro, L. 693
Portal, J.-J. 443
Porter-Gill, P. 157
Porth, A.-K. 701
Porthan, K. 146
Poschet, G. 760
Pose-Utrilla, J. 202
Post, A. 358
Postic, C. 144
Poston, L. 128
Poth, T. 760
Potier, J.-B. 145
Potier, L. 101, 267, 668
Potočková, J. 370
Pott, G. B. 828
Pottegaard, A. 579
Pottier, M. 459
Pöttler, T. 628
Poulletier de Gannes, F. 702
Poulsen, C. G. 87
Poulsen, P. L. 85, 807
Pouwer, F. 170, 625, 681, 683
Pozzilli, P. 96, 312
Prasad, R. 178
Prati, B. 364
Pratley, R. E. 252, 616, 621
Pratt, E. J. 57, 564, 587
Prázný, M. 672, 723
Preissl, H. 433
Prentza, V. 810, 829
Prevenzano, I. 815
Price, J. F. 42, 877
Price, S. K. J. 677
Prietl, B. 325
Prietro-Lloret, J. 845
PRIORITY Study Group 123
Provenzano, M. 552
Provenzano, S. 56
Prud’homme, G. 285
Prunk Drmić, A. 527
Prystupa, K. 73, 128
Psaltis, P. J. 47
Psaltopoulou, T. 577
Pucci, L. 470
Puchades, M. 552
Pueyo, I. 220
Puginier, E. 702
Pugliese, N. R. 249
Pulsford, R. M. 631
Pushkarna, D. 645
Putaala, J. 68, 124, 821
Puttanna, A. 653
Puvaneswaralingam, S. 251
Puvaralingam, S. 730
Pypec, A. 820
Qadri, S. 96
Qi, B. 771
Qi, L. 547
Qian, W.-J. 32
Qian, W. 377
Qin, M. 4
Qiu, S. 74, 343, 347
Quansah, K. 666
Quaranta, D. 704
Quast, D. R. 623
Quenon, A. 459
Quinn, L. M. 406
Quiros, C. 671
Quist, J. S. 542
Qvigstad, E. 132, 436
Rabasa-Lhoret, R. 428, 705
Rabassa, M. 441
Rabbani, N. 450, 762, 825
Rabier, T. 459
Raciti, G. A. 269
Rada, P. 202, 214
Rademaker, D. 680
Rádiková, Ž. 493
Radlinger, B. 576
Radovnická, L. 672
Radulian, G. 846
Raghavan, R. 550
Raiko, J. 143
Raja, D. 235
Rajamand Ekberg, N. 19, 539
Rajas, F. 775
Rajasingam, D. 676
Rajnai, L. 787
Rakitzi, P. 299
Rakow, A. 276
Rakza, T. 131
Raleigh, D. P. 390
Ram, Y. 333
Ramadori, G. 194
Ramalheira, T. G. 215
Rambani, V. 301
Ramos, A. 278
Ramos, H. 122, 126
Randell, T. 699
Rangert, A. 315
Ranta, K. 585
Raoux, M. 392, 702
Rapattoni, W. 551
Raposo, J. F. 253, 864
Raptis, A. 559, 810, 829
Rasheed, R. 582
Rasmussen, A. 20, 23, 734
Rasmussen, D. G. K. 218, 549, 709, 822
Rasmussen, R. W. 777
Rasmussen, S. 45, 594
Raso, E. 13
Rasouli, B. 290
Rasouli, N. 566
Rastogi, A. 22
Rathmann, W. 192, 323
Ratzer, M. 407
Ratzki-Leewing, A. 629
Raverdy, V. 459
Ravi, A. 694
Ravier, M. A. 386
Rawlinson, W. D. 236
Raya, D. 555
Raychaudhury, A. 759
Raymond, C. S. S. 788
Rayner, C. K. 166, 167, 168, 505, 623
Rea, F. 348
Rea, R. 747
Rebelos, E. 464
Refsum, H. 512
Regazzi, R. 108, 480
Regeer, H. 409
Reginato, A. 215
Régnier, M. 144
Reimann, F. 216, 306, 460, 466
Reindl, W. 570
Reinhard, A. K. 679
Reinke, J. 258
Reis, F. 476
Reis, J. 710
Reis Franco, D. 598
Rella, M. 59
Remmelzwaal, S. 339, 345, 346, 834
Ren, H. 377, 378
Renard, E. 247, 635
Renaud, S. 702
Repetto, E. 665
Reshma, A. M. 788
Resi, V. 693
Ress, C. 576
Retory, Y. 668
Reusch, J. E. 828
Rewers, M. 286, 313
Rey, E. 202
Reynolds, C. 569
Reynolds, R. 724
Rey Velasco, E. 697
Rhee, M. 855
Ribeiro, R. 253
Ribeiro, R. A. 452
Riccardi, G. 2, 164
Ricci, S. 194
Richard, V. R. 257
Richardson, S. J. 388, 416, 849
Richter, E. A. 12, 601
Rickels, M. R. 379
Ricordi, C. 379
Ridgeway, J. 653
Ridker, P. M. 553
Ried-Larsen, M. 79, 117, 461
Riegel, K. D. 172
Rieusset, J. 449, 856
Rigas, G. 498
Rilstone, S. 138
Rimm, E. B. 3
Rinaldi, E. 227
Rinnov, A. R. 562
Risi, R. 526
Rist, W. 570
Rittig, N. 149
Ritzel, R. 638, 645
Riveline, J.-P. 51, 101, 668
Rivellese, A. A. 13, 164
Rives, C. 91, 774
Rizo-Roca, D. 455
Rizzi, A. 704
Rizzo, G. E. 704
Roberto, L. 59
Roberts, F. 178
Roberts, L. D. 193
Roberts, M. A. 762
Robins, D. A. 587, 592
Robinson, A. 24
Roca, D. 441
Roca Rivada, A. 206
Rocha, M. 487
Rocher, A. 845
Rodbard, H. W. 568
Rodella, A. 531
Rodemer, C. 120
Roden, M. 192, 254, 353, 576, 862
Rodriguez, A. 222
Rodríguez, Á. 565, 561, 568, 586, 598
Rodriguez-Calvo, T. 208
Rodriguez Sanchez-Archidona, A. 225, 226
Roeikjer, J. 713
Rohatgi, A. 820
Rohbeck, E. 862
Rohm, M. 33
Røikjer, J. 720, 736
Rokamp, K. Z. 633
Roland, M.-C. P. 436
Roma, L. P. 263
Romacho, T. 862
Romeo, S. 96
Romeres, D. 255
Romero, A. 862
Rončáková, M. 289
Ronci, M. 58
Rønn, P. F. 218, 367, 822
Rønningen, T. 64
Rooney, M. 181
Rorsman, P. 106, 371
Rosager, E. V. 356
Rosati, A. 546
Rosato, A. 531
Rosenbauer, J. 323
Rosendo-Silva, D. 476
Rosengaard, L. 368
Rosenkilde, M. M. 510
Rosenstock, J. 499, 560, 563, 597, 598, 613, 638
Rosenthal, N. 801
Roshandel, D. 67
Rosicky, I. 275
Rosolova, K. 11
Rosqvist, F. 146
Ross, L. 379
Rossato, S. 3
Rossetti, R. 526
Rossi, M. C. 612
Rossi, P. 90
Rossing, P. 20, 23, 45, 86, 87, 89, 123, 190, 218, 244, 367, 593, 618, 620, 709, 719, 751, 752, 799, 822,
Rossner, S. 701
Rotbain Curovic, V. 123, 752, 799
Rotem, R. 273
Rötschke, J. 665
Rottiers, P. 328
Roubik, L. 628
Roumans, K. H. M. 115
Roumeliotis, A. 299
Roussel, R. 63, 70, 101, 267
Rouw, D. B. 750
Roux, P. P. 28
Rovere-Querini, P. 369
R. Owen, K. 310
Roy, A. 236
Roze, S. 102
Rozenberg, A. 610
Ruan, Y. 747
Rubæk Danielsen, E. 148
Ruban, A. 54
Rubin, K. H. 153
Rubinat, E. 283
Rubino, D. M. 563
Rubio, J. 641
Rudenko, N. M. 874
Ruether, K. 165
Rughwani, T. 590
Ruilope, L. M. 618, 620
Ruissen, M. M. 409
Ruiter, T. 851
Ruiz, P. L. D. 233
Ruiz De Adana, M. 303
Ruiz Ruiz, J. 141
Rujan, R.-M. 569
Rungby, J. 43, 783
Russell, M. A. 388
Russo, B. 242, 714
Russo, D. 848
Russo, I. 816
Russon, C. L. 631
Rustenbeck, I. 509
Rusu, E. 846
Rutter, G. A. 266, 417
Rutter, G. 616
Rutters, F. 345, 346, 834
Ruwaard, D. 652
Ryabov, V. 271
Ryan, B. 629
Rydén, L. 800, 863
Rydén, M. 82, 455
Ryder, R. E. J. 54, 550, 583
Saari, T. 143
Saarimäki-Vire, J. 179, 205
Saarinen, T. 494
Saba, A. 800
Sabater, D. 126
Sabio, G. 202, 214
Saboo, B. 698
Sabourdy, F. 774
Sacchetta, L. 241
Sacramento, J. F. 474, 845
Sacre, J. W. 811
Sadananthan, S. A. 431
Sadananthan, S. 129
Sæderup Pedersen, H. 697
Saenko, Y. A. 874
Sagar, R. C. 17, 795
Sahebekhtiari, N. 458
Saini, V. 180
Sainsbury, C. 840
Saint-Béat, C. 453
Sainz, N. 656, 678
Saito, S. 682
Sajadieh, A. 602
Sakaki, K. 60
Sakamoto, Y. 745
Sakuramoto-Tsuchida, S. 769
Sal, M. 438, 443
Salamone, D. 164
Salas, A. 126
Saldanha, S. 820
Salem, V. 230
Salim, A. 362
Salmenkari, H. 798, 839
Salö, S. 223
Salome, T. 440
Salvatore, M. 164
Salvenmoser, W. 576
Salzmann, K. 576
Samarasinghe, S. 203
Sammut-Powell, C. 727
Sampaio-Pires, D. 474
Sanchez, G. 373
Sanchez, H. 673
Sánchez-Ceinos, J. 268, 276
Sanchez-Delgado, G. 141
Sánchez-Domínguez, R. 422
Sánchez-Roncero, A. 422
Sancho, V. 212
Sandbæk, A. 336, 804
Sandbrink, J. 31
Sander, S. E. 630
Sandforth, L. 258, 433
Sandgaard, S. 584
Sandholm, N. 37, 38, 68, 244, 821
Sánez Tähtisalo, H. 37
Sang, M. 74, 347
Sanlioglu, A. D. 380
Sanna, B. 379
Sansone, A. 865
Santander, C. G. 71
Santarpino, G. 500, 814
Santhanam, R. 570
Santhiapillai Croosu, S. 713
Santo-Domingo, J. 451
Santoro, N. 868
Santos, A. C. 864
Santos, D. 157
Santos, L. B. 503
Santos, R. M. A. 215
Sapin, H. 600
Saponaro, C. 307, 310
Saras, J. 239
Sark, D. 664, 831
Sá-Rocha, M. 486
Sarsenbayeva, A. 26, 214
Sas, T. C. J. 469
Sassi, G. 328
Satake, E. 244
Sathiaseelan, R. 116
Sattar, N. 132, 174, 555, 617
Satuli-Autere, S. 156, 791
Saudek, F. 243
Saulnier, P.-J. 747
Saunder, T. 361
Savaré, L. 348
Savelberg, H. H. C. 121, 324
Savikj, M. 229, 81
Sawadogo, K. 468
Sawatani, T. 400
Saxena, A. R. 114, 588, 589
Sayers, S. 342
Sbat, N. 824
Scalise, A. 618
Scarpa, F. 155
Schaart, G. 8, 160
Schaeffer, D. F. 851
Schalkwijk, C. G. 72, 155, 227, 282, 341, 454, 512, 573
Schaper, N. 121
Schaper, N. C. 72, 324
Scharfmann, R. 240
Schaub, C. M. 376
Schauer, M. 543
Schechter, M. 250, 553, 610
Scheijen, J. L. J. 72, 454
Schenk, E. 645
Scherneck, S. 445, 509
Schimpfle, L. 717, 718, 726
Schirò, S. 364
Schlesinger, S. 1, 370
Schlögl, H. 506
Schmid, B. 613
Schmidt, A. 668
Schmitt, B. M. 263
Schmitt, C. P. 754, 760
Schmitt, H. 44
Schneck, K. B. 596
Schneider, A. 192
Schnell, O. 863
Schoelwer, M. J. 669
Schofield, J. 641
Schoiswohl, G. 144
Scholz, M. 321
Schön, M. 254
Schönfelder, T. 876
Schoonjans, K. 494
Schoonmade, L. 345
Schöppe, T. 264
Schoug, J. 741
Schousboe, K. 646
Schouten, J. 121
Schrader, S. 352
Schram, M. 121
Schram, M. T. 154, 155, 324
Schrauwen, P. 7, 8, 115, 160, 521
Schrauwen-Hinderling, V. B. 7, 8, 83, 115, 254, 521
Schreyer, A. T. H. 256
Schröder, S. 508
Schüler, R. 162
Schulte, A. 240
Schultz, J. 413
Schulze, M. B. 323
Schuppelius, B. 162, 165
Schürmann, A. 296, 353, 534
Schütt, K. 876
Schwaeble, W. J. 792
Schwettmann, L. 192
Schwingshackl, L. 1
Schwitzgebel, V. 306
Schytz, P. A. 594
Sciuto, P. 249
Scorrano, L. 515
Scotney, P. 150
Scott, C. 620, 751
Scott, C. F. 376
Scottish Diabetes Research Network Epidemiology Group 794
Scowcroft, J. A. 53
Sebastian, M. 300
Sebastiani, G. 311, 327, 328
Šebo, V. 370
Secher, N. H. 633
Sechi, L. 408
Seda, O. 61
Sedova, L. 61
Seelam, A. 262, 399
Seely, E. W. 273
Seghieri, M. 546
Segin, V. B. 708
Segovia, J. C. 422
Seguí, N. 441
Seiça, R. 476
Seidel-Jacobs, E. 323
Seidler, Y. 701
Sejling, A.-S. 136
Sekitoleko, I. 440
Self, E. J. 308
Selig, J. I. 823, 871
Sellers, A. J. 160
Selvarajah, D. 188, 189, 737, 738
Selvin, E. 181
Semple, R. K. 140
Sen, I. 81
Sen, T. 553
Sen Gupta, P. 54, 676
Senior, P. 832
Senior, P. A. 180
Sennström, M. 276
Seo, D. 833
Seppänen, W. 146
Serantoni, C. 865
Sereno, J. 536
Serés-Noriega, T. 220
Serhiyenko, A. A. 708
Serhiyenko, L. M. 708
Serhiyenko, V. A. 708
Seroussi, C. 634
Sesti, G. 259, 496
Seufert, J. 44, 54, 551, 637
Severi, I. 425
Sevillano-Collantes, C. 793
Sevostjanovs, E. 767
Shah, H. 821
Shamanna, P. 698
Shannon, R. 157
Shapiro, A. M. J. 180
Sharabiani, M. 782
Sharifi, S. 397, 424
Sharifli, S. 549
Sharma, A. 132
Sharpe, A. 24
Sharrack, N. 813
Shaw, J. A. M. 412
Shaw, J. E. 362, 811
She, R. 136
Shelestova, E. 127
Shen, S. 186
Shen, Z. 842
Shepherd, M. 174
Sherr, J. 626
Sheth, K. N. 835
Shi, J. 41
Shi, M. 739
Shi, R. 387
Shide, K. 366
Shields, B. M. 76, 169, 174, 235, 291, 292, 404, 440, 555
Shih, A. Z. L. 401
Shillo, P. R. 738
Shim, E. 703
Shimazu-Kuwahara, S. 491
Shimizu, H. 548
Shobatake, R. 769
Shoger, E. 656, 678
Shogo, S. 229
Shoyhet, H. 196
Shukla, D. 406
Shulman, G. I. 146, 258
Shumliakivska, M. 276
Siafarikas, C. 365
Sicherre, F. 447
Siddiqui, M. K. 878
Sidi, E. A. L. 359
Siegelaar, S. 680
Sieminska, J. 280
Siena, A. 711
Silecchia, G. 843
Sills, S. M. 53
Silva, G. R. 142
Silva de Carvalho, T. 818
Silva Junior, J. A. 452
Silvano, S. 331
Silverwood, R. J. 363
Silvia, R. 671
Sim, D. 872
Simati, S. 490
Simmons, K. J. 193
Simmons, K. 313
Simó, R. 122, 126, 784
Simo' Canonge, R. 714
Simon, E. 570
Simon, L. 278
Simons, P. I. H. 72
Simó-Servat, O. 784
Simpson, A. M. 180
Simpson, V. 76
Simsek, S. 92, 573
Šín, M. 723
Singh, A. 689
Singh, H. 673
Singh, L. G. 100
Singh, S. P. 410
Singh, T. 178
Sipter, E. 337
Siqueira, B. P. 215
Sisk, R. 727
Sivappriyan, S. 583
Sjöberg, F. 462
Sjöström, D. 750
Skinner, T. C. 697
Sklavounos, I. 444
Sklenar, M. 301
Sklirou, A. 577
Skopkova, M. 301
Skovgaard, T. 772
Skovsø, S. 36, 56, 257
Skriver, M. V. 804
Skytte, M. 602
Slagmolen, L. 370
Slee, A. 551
Sletner, L. 340
Slíva, M. 723
Sloan, G. 188, 189, 737, 738
Sloop, K. 587
Slovak Monogenic Diabetes Study Group 301
Smith, A. 676
Smith, C. A. 460, 466
Smith, G. J. 332
Smith, J. A. B. 81, 161, 481
Smith, U. 269
Smyth, L. J. 38
Snaith, J. R. 158
So, W. Y. 622
Soares, A. C. F. 624
Soares, G. M. 213, 452
Sobngwi, E. 404
Sochorová, K. 655
Søholm, U. 625, 681, 683
Sokooti Oskooei, S. 358
Sola-Gazagnes, A. 101
Soldatos, G. 332
Soldovieri, L. 425, 456
Solé, T. 537
Solek, J. 830
Solheim, M. H. 71
Solimena, M. 240, 314
Solini, A. 241
Solis-Herrera, C. 10
Sollid, S. 132
Solly, E. L. 47
Solomon, E. 692
Solon, C. 213
Solon-Biet, S. M. 448
Solovyova, A. 255
Šoltýs, K. 493
Somanath, P. 197, 590
Sommer, C. 132, 340
Sommi, A. 656, 678
Somodi, S. 787
Somogyi, A. 295
Son, J. 446
Son, Y. 519
Søndergaard, E. 630, 700
Søndergaard, J. 221, 579
Sone, H. 761
Song, D. 467
Song, J. 739
Song, M. 519
Song, P.-S. 854, 869
Song, S. H. 133
Sönmez, A. 240, 314
Sordi, V. 305
Sørensen, A. E. 157, 734
Sørensen, H. T. 43, 75, 184, 584, 783
Sørensen, S. S. 85
Sørgjerd, E. P. 290
Sork, H. 385
Sørland, B. A. 719
Sørrig, R. 563
Sørum, N. M. 556
Sosenko, J. 293
Sosna, T. 243
Souhami, E. 638
Soula, O. 634
Šoupal, J. 672
Sourij, C. 430, 543
Sourij, H. 54, 427, 430, 543
Sousa, D. 486, 487
Sousa de veiga, A. 331
Southekal, S. 462
Sozio, E. 408
Spallone, V. 725
Spanakis, E. 586
Spanakis, E. K. 100
Sparacino, G. 836
Sparre-Ulrich, A. H. 510
Speer, T. 263
Speight, J. 625
Spellman, C. 676
Spigoni, V. 364
Spiliopoulou, A. 67
Spiliotis, I. 310
Spinelli, A. 312
Spinelli, R. 269
Spinhoven, M. 463
Spoltore, M. 526
Spranger, J. 163, 258, 538
Sprechert, M. 535
Sridhar, A. 488
Sridhar, V. S. 621
Srivastava, A. 806
Stabilini, A. 293
Stadlbauer, V. 543
Stæger, F. F. 71
Stahl, M. 313
Stahl, R. 78
Stamm, T. 701
Stanik, J. 301
Stankute, I. 306
Starikova, J. 420
StartRight Study Group 76, 291, 292
Starup-Linde, J. 351
Stastny, J. 504
Stathi, D. 756
Staudacher, G. 576
Stauss, T. G. 53
Steck, A. K. 313
Stedman, M. P. 24, 660, 662, 692
Steen Carlsson, K. 663
Stefana, I. M. 416
Stefanakis, K. 490
Stefanowicz, M. 513
Stehouwer, C. D. A. 72, 121, 154, 155, 227, 282, 324, 341, 454, 512
Stein, A. 862
Stelling-Férez, J. 733
Stemberkova-Hubackova, S. 11
Stene, L. C. 233
Stene, L. 287
Stenlid, R. 472
Stensen, S. 510
Stentebjerg, L. L. 434
Stenvinkel, P. 15
Stermann, T. 260, 296
Sternad, C. 430
Sterpetti, S. 711
Steubl, D. 88
Stevens, M. 770
Stewart, A. J. 465, 529
Sticco-Ivins, M. 330
Stidsen, J. V. 80, 191, 322, 334, 368
Stienstra, R. 403, 632
Stimson, R. H. 99, 627
Stocken, D. D. 795
Stocks, B. 93
Stockton, D. 234
Stolberg, C. R. 642
Stone, V. M. 385
Størdal, K. 233
Storkey, A. 721, 794
Størling, J. 185, 210, 542
Stougaard, E. B. 89
Stout, M. 116
Støving, R. K. 434
Strachan, M. W. J. 99
Strand, R. 183
Strassburger, K. 254
Stratigou, T. 749
Stratmann, B. 818
Strembitska, A. 228
Strigini, M. 537
Strikwerda, M. 346
Strollo, R. 312
Studenčan, M. 872
Stumvoll, M. 506
Stutz, B. 608
Styles, C. J. 721, 794
Su, Q. 802
Suarez, M. 277
Suba, K. 230
Subramanian, A. 840
Subtil, D. 131, 442
Sugihara, H. 507
Sukumar, P. 193
Sulaj, A. 120, 716, 717, 726
Suleiman, M. 58
Suleiman, N. 298
Sulek, K. 719
Sulpice, T. 119
Summanen, P. 124
Sun, D. 454
Sun, G. 56
Sun, H. 66, 858
Sun, J. 176
Sun, N. 186
Sun, Q. 3
Sun, X. 41, 532
Sun, Y. 748
Sun, Y. 166, 168
Sun, Z. 74, 343, 347, 387, 421, 505
Sundberg, C. J. 426
Sundqvist, M. L. 539
Sundström, J. 545
Sung, Y.-A. 467
Suppère, C. 705
Suraci, E. 259, 496
Suvitaival, T. 799
Svalbe, B. 530
Svane, H. M. L. 184
Svane, M. S. 110, 477
Svart, M. V. 149
Sveen, K. 742, 853
Svehlikova, E. 407, 647
Svensson, M. 349
Sverzellati, N. 364
Svoboda, P. 11
Swan, J. 649
Swan, P. 203
Swart, K. M. A. 691
Swensen, A. 32
Swoboda, P. P. 813
Sylva, M. 808
Syreeni, A. 38, 68
Syring, K. 684
Szadkowska, A. 830
Szczerba, E. 1
Szendrödi, J. 718
Szendroedi, J. 120, 716, 717, 726
Szerencsi, R. 690
Szili-Torok, T. 6, 358
Szymczak, F. 326, 400
Tabak, A. 128
Tack, C. 403
Tack, C. J. 632, 687
Taddei, S. 470
Tagougui, S. 428, 705
Taittonen, M. 143
Takasawa, S. 769
Takebayashi, T. 761
Takeda, Y. 769
Takiishi, T. 391
Tamayo, M. 323
Tamir, O. 675
Tan, J. T. M. 47
Tan, K. H. 431
Tan, K. 129
Tan, W. 417
Tanabe, A. 682
Tanabe, J. 870
Tanaka, S. 302
Tang, C. 377, 378
Tang, C. 592
Tanushi, B. 138
Taouis, M. 211
Tapia, G. 233
Tarasov, A. I. 103
Tariq, M. 392
Tarnow, L. 642
Tarp, J. 322
Tarp, J. M. 18
Tarshish, E. 675
Tartaglione, L. 704
Tarussio, D. 228
Tascini, C. 408
Tatsuoka, H. 491
Tatulashvili, S. 438, 443
Tavaglione, F. 96
Tavares, L. 478
Taybani, Z. 690
Taylor, A. J. 32
Taylor, R. S. 53
Taylor, R. 255
Teare, J. 54
Teh, K. 188, 189, 737
Teixeira, P. 194
Tejero, C. 784
Teli, V. 866
Templin, A. T. 390
Tengholm, A. 237
Tentolouris, N. 490, 567, 577
Teo, A. K. K. 417
Teramoto, N. 40
Tercero-Alcazar, C. 479
Ternynck, C. 442
Terpos, E. 577
Terron Exposito, R. 33
Terzic, D. 556
Tesfaye, S. 188, 189, 737, 738
Teshima, A. 204
Tesi, M. 34, 58
Teunissen, M. 454
Thabit, H. 641
Thajudeen, M. 698
Tham, L. 592
Thangam, M. 20
Thankamony, A. 699
Thastum, M. 170
Theilade, S. 709, 799
Thennati, R. 616
Thévenet, J. 307, 310
Thibaut, R. 773
Thiel, S. 792
Thiery, J. 321
Thieu, V. T. 585
Thijssen, D. H. J. 687
Thirumathyam, R. 12, 601
Thirumurugan, K. 129
Thirunavukarasu, S. 813
Thissen, J.-P. 468
Thomakos, P. 444
Thomas, L. 570
Thomas, M. K. 57, 564, 592, 603, 606
Thomas, N. J. 76, 169, 235, 291, 292
Thomas, R. L. 724
Thomas, S. 676
Thomas, S. M. 744
Thomas-Delecourt, F. 664, 831
Thomsen, R. W. 43, 75, 184, 191, 322, 334, 579, 584, 783
Thöni, S. 549
Thorand, B. 192
Thorens, B. 225, 226, 228, 616
Thorius, I. H. 636
Thorn, L. M. 68, 124, 156, 791, 821
Thorn, P. 238, 372
Thornalley, P. J. 450, 762, 825
Thorsteinsson, B. 633
Threlkeld, R. 684
Thuesen, A. C. B. 71, 148, 185
Thulesius, H. 173
Thunander, M. 173
Thuresson, M. 742
Thymis, J. 559, 810, 829
Tiberti, C. 411
Tichler, A. 652
Ticinesi, A. 364
Tiedge, M. 264, 413
Tietge, U. J. F. 6
Tilg, H. 576
Tilling, K. M. 363
Tiniakos, D. 412
Tint, M. 129
Tippett, P. 292
Tirosh, A. 689
Titievsky, L. 97, 626
Tkac, I. 248
Tobe, S. W. 551
Todd, J. A. 416
Tofte, N. 123, 799
Togliatto, G. 766
Tokmakova, M. 872
Tokumoto, S. 491
Tomas, A. 569
Tomic, D. 362
Torcello-Gómez, A. 565
Torchio, S. 305
Tore, E. C. 512
Torekov, S. S. 542
Torsoni, A. S. 215
Torsoni, M. A. 215
Torta, F. 417
Tosi, I. 342
Toti, F. 824
Tougaard, N. H. 218, 822
Toulis, K. 840
Toulis, K. A. 875
Tozzi, R. 526
Tramontana, F. 200
Tramunt, B. 144
Treebak, J. T. 231, 232
Trevelyan, N. 699
Tricò, D. 241, 249, 868
Triggiani, R. 245
Tripathy, D. 10
Tripolt, N. J. 430, 543
Triviño-Yannuzzi, V. 793
Trombetta, M. 227
Trost, K. 35
Trougakos, I. 577
Trusheim, M. R. 286
Tsagarakis, S. 749
Tsamandouras, N. 114, 588, 589
Tsapas, A. 147, 875
Tsatsaris, G. 19
Tschoepe, D. 818
Tsekmekidou, X. 299, 604
Tseng, Y.-H. 21
Tsetsos, F. 299
Tsilingiris, D. 490, 717, 726
Tsilivarakis, D. 829
Tsivgoulis, G. 810
Tsolakidis, A. 365
Tsoukas, M. A. 551
Tsukagoshi, A. 9
Tsukaguchi, R. 366
Tuccinardi, D. 96
Tully, A. 332
Tulmin, H. 416
Tuomi, T. 77, 217, 290, 317
Tuomilehto, J. 5, 863
Tura, A. 275
Turk Wensveen, T. 527
Tuttle, K. R. 553, 617
Twigg, S. M. 732, 776
Tye, S. 545
Uboldi, P. 515
Uchihara, M. 682
Uchiyama, T. 769
Uddman, E. 251, 730
U-Din, M. 142, 143
Uebel, T. 246
Ueki, K. 682
Ulianich, L. 815
Ullrich, S. 209
Ulven, T. 209
Umapathysivam, M. 685
Umpierrez, G. E. 100, 593
Unnikrishnan, A. G. 22
Uphues, I. 570
Urbina, S. 605
Urrutia, I. 278
Urrutia, M. A. 100
Urschitz, M. 407, 647
Ursino, G. 194
Uruska, A. 473, 820
Urva, S. 57, 111, 564, 596, 606
Ustinov, J. 179
Ustyugova, A. 584
Usuelli, V. 262, 399
Utsunomiya, K. 745
Uusitupa, M. 5
Vaag, A. A. 43, 148, 184, 185, 334
Vaccaro, O. 2
Vadavi, A. 698
Vaduva, P. 151, 152
Vähäkangas, E. 179
Vähäsalo, P. 77
Vaikkinen, H. 177
Valabhji, J. 782
Valdecantos, P. 94
Valderas, E. G. 614
Valderhaug, T. G. 64
Valensi, P. 48, 808
Valente, A. 864
Valenti, L. 96
Valešová, L. 723
Valerio, J. 278
Valkovicova, T. 301
Vallejo Mora, R. 303
Valverde, Á. M. 26, 94, 202, 214, 485
Valverde-Tercedor, C. 435
Vambergue, A. 131, 442
Vamos, E. P. 782
van Beek, S. M. M. 160
Van Damme, N. 328
Vandenbempt, V. 410
van den Berg, J. M. 691
van den Heuvel, T. 667
van der Aart-van der Beek, A. B. 750
van der Boog, P. J. M. 409
Vandercappellen, E. J. 324
van der Heide, F. C. T. 121, 155, 227
van der Heijden, A. A. A. 279
van der Kallen, C. J. H. 72, 121,155, 227, 324
Van der Kolk, B. 494
van der Ploeg, I. 426
van der Wel, A. 680
van de Weijer, T. 160
Vandiedonck, C. 267
van Eekelen, R. 680
Vangberg, K. G. 340
Vangelder, V. 459
van Gennip, A. C. E. 154
van Gool, A. J. 358
van Greevenbroek, M. 121, 227
van Greevenbroek, M. M. J. 72, 155, 282, 324, 341, 512
van Hall, G. 148, 185
van Heck, J. I. P. 403, 632
Vanhevel, F. 463
van Krieken, P. P. 25
van Marken Lichtenbelt, W. 160
Vanoverloop, J. 728, 729
van Raalte, D. H. 750
van Rijn, B. 680
van Sloten, T. T. 154
Vargas-Uricoechea, H. 689
Várkonyi, T. 595, 690
Vart, P. 545
Vas, P. 695, 764
Vasbinder, A. 710
Vasileiou, V. 577
Vasilopoulos, Y. 604
Vasnawala, H. 727
Vasquez-de Sebastian, J. 784
Vassiliadi, D. A. 749
Vaughan, N. 631
Vávra, D. 655
Vavruch, C. 803
Vaxillaire, M. 70
Vázquez, L. 598
Vazquez-Mendez, E. 727
Vazzana, A. 364
Veelen, A. 8, 115
Veeraiah, P. 83, 115
Vega, N. 537
Vega Guedes, B. 274
Veijola, R. 77
Vejlstrup, N. 12
Velan, S. 129, 431
Vellanki, P. 100
Velloso, L. A. 142, 213
Veluchamy, A. 20
Vendrell, J. 159, 283
Venteclef, N. 267, 668, 773
Ventriglia, G. 328
Vera-Ramos, J. A. 325
Verchere, B. 208
Verchere, C. B. 32, 401
Veres, A. 690
Veres, D. S. 337
Verheugt, C. L. 469
Verhulst, C. E. M. 403, 632
Verkauskiene, R. 306
Verket, M. 876
Vernstrøm, L. 85, 807
Verónica, P. 671
Vespasiani-Gentilucci, U. 96
Vestergaard, H. 799
Vestergaard, P. 98, 184, 191, 334, 351
Vettoretti, M. 52
Veyrat-Durebex, C. 194
Viana, S. 476
Vicaud, E. 438
Vicaut, E. 51, 443
Vidal-Trecan, T. 101
Videja, M. 530, 767
Viegas, I. 478
Viggers, R. 351
Vihervaara, T. 863
Vikstrom-Greve, S. 701
Viljoen, A. 591
Villa, M. 643
Villringer, A. 506
Villumsen, S. O. 148, 185
Vilsbøll, T. 224, 510, 556, 579, 594, 609, 616, 790, 796, 799
Vinagre, I. 220, 441
Viñals, C. 220
Vinknes, K. J. 512
Vinter, C. A. 153, 434
Viola, V. 312
Virbel-Fleischman, C. 668
Virtanen, K. A. 142, 143
Visentin, F. 194
Vishwanathan, V. 22
Visnovska, M. 64
Vistisen, D. 87, 89, 190, 367, 542, 707
Viswanathan, P. 593
Vitale, M. 2, 164
Vitali, G. 369
Viuff, B. M. 772
Vogl, T. 194
Vogt, A. 52
von Bülow, M. 240
von der Leyen, H. 123
von Eynatten, M. 541
von Rauchhaupt, E. 120, 716, 717, 726
Vranic, M. 26, 30
Vryonidou, A. 577
Vu, H. T. T. 426
Wade, H. 802
Wadwa, R. 688
Waernbaum, I. 288
Wägner, A. M. 274, 435, 484
Wagner, C. 144
Wagner, R. 73, 128, 433
Waheed, U. 692
Waheed-Khan, A. 268
Wahli, W. 144
Waite, E. 651
Wakeling, M. 308
Walker, L. A. 828
Walker, V. 637
Wallace, M. 103
Wallberg-Henriksson, H. 82, 229
Walsby-Tickle, J. 31
Walth, A. A. 33
Wan, J. H. 91
Wanby, P. 173
Wang, C. 379
Wang, H. 66, 858
Wang, H. 547, 566, 850
Wang, H. 421, 842
Wang, J. 113, 607
Wang, J. 343
Wang, J. 547
Wang, J. 715
Wang, J. 64
Wang, Q. 285
Wang, S. 781, 819
Wang, W. 837
Wang, X. 776
Wang, X. L. 748
Wang, X. 186
Wang, X. 347
Wang, Y. 421, 842
Wang, Z. 285
Wang, Z. 525
Wanner, C. 88
Waqas, A. 650
Ware, J. 699
Watanabe, M. 526, 711
Waterstradt, R. 413
Webers, C. A. B. 121, 155
Weedon, M. 78
Weerakkody, G. 598
Wegbrod, C. 314
Wehedy, E. 298
Wei, Y. 217
Weinzierl, A. 263, 383
Weir, C. J. 42
Welsh, N. 387
Welsh, P. 523
Welten, S. J. G. 834
Wen, Q. 472
Wendt, A. 107, 237, 265
Wen-Loh, Y. 758
Wensveen, F. M. 527
Wentorf, E. K. 80
Wentworth, J. M. 332
Wernicke, C. 538
Wesselius, A. 72, 324
West, D. 135
Westerbacka, J. 247, 635
Westermark, G. 387
Westholm, E. 107, 415
Wetzel, C. 754, 760
Wewer Albrechtsen, N. J. 542
Wheatcroft, S. B. 193
Wheeler, B. J. 677
Wheeler, D. C. 551
White, B. 572
White, J. M. 732
White, J. L. 53
White, N. H. 187
White, S. L. 128
Whyte, M. 660, 662
Widman, L. 15
Wiedemann, M. S. F. 25
Wiese, R. J. 557, 595, 617
Wiggins, N. 361
Wijdeveld, M. 489
Wijkman, M. 872
Wild, S. H. 234
Wilding, J. P. H. 566
Wilinska, M. E. 50, 641, 699
Wilkinson, I. 188
Wilkinson, I. D. 738
Wilkinson, L. 562
Willcocks, L. 653
Willemoes, R. J. 734
Willett, W. 3
Williams, A. J. K. 261
Williams, D. M. 363
Williams, G. 724
Williams, J. 695, 764
Williams, P. F. 776
Williman, J. A. 677
Willmes, D. 258
Willnow, T. E. 401
Wilson, J. F. 877
Windum, N. A. 316
Wing, Y. 654
Winther, S. A. 799
Witkowski, P. 379
Witte, D. R. 75, 707, 847
Wittle, A. 605
Wlodek, M. 431
Wojtusciszyn, A. 392
Wolden, M. L. 256
Wolden, M. 659
Wolf, K. 192
Wolf, M. 407
Wolf, W. 97, 626,
Wolke, C. 413
Won, J. 599
Won, K. 599
Wong, I. C. K. 130
Wong, T. 339
Wong, W. K. M. 180
Woo, K. 654
Woo, S. 540
Woo, V. 551
Wood, A. 78
Wood, D. 863
Wood, R. 171, 656, 678
Wood, S. N. 234
Wouters, K. 454
Wouters, K. 728, 729
Wretlind, A. 799
Wright, C. 78
Wright, N. M. 376
Wright, R. J. 99
Wrublewsky, S. 263, 383
Wu, A. 665
Wu, D. 285
Wu, D. 529
Wu, H. 622
Wu, J. 842
Wu, K. 108
Wu, P. 658
Wu, R. 207
Wu, T. 166, 167, 168, 347, 505, 623
Wu, Y. 837
Wueest, S. 25, 483
Wullenweber, P. 640
Wyne, K. 402
Xia, Y. H. 257
Xia, Y. 36, 851
Xiao, P. 391, 410, 482
Xie, C. 166, 167, 168, 347, 623
Xing, J. 319
Xingjing, L. 62
Xu, J. 706
Xu, J. 53
Xu, L. 113, 607
Xu, P. 857
Xu, S. 4, 281
Xu, W. 355
Xu, W. 4, 281
Xu, Y. 333
Xuan, S. 36
Xue, M. 450, 825
Xue, Y. 706
Yabe, D. 491
Yaligar, J. 129, 431
Yamada, M. 548
Yamada, T. 761
Yamamoto, M. 761
Yamane, S. 491
Yamauchi, A. 769
Yan, Q. 281
Yan, X. 706
Yan, Y. 684
Yanes, O. 283
Yang, A. 622
Yang, D. 355
Yang, H. 547
Yang, J. 851
Yang, J. 46
Yang, N. 113, 607
Yang, T. 859
Yang, X. 377, 378
Yang, Y. 715
Yang, Z. 557, 617
Yanuv, I. 610
Yasmin, F. 712
Yasuda, T. 491
Ye, E. 171
Ye, T. 41
Yeo, J. L. 813
Yeung, R. O. 832
Yi, X. 400
Yilmaz, M. 872
Yki-Järvinen, H. 96, 146
Ylinen, A. 124, 156
Ylli, D. 242
Yılmaz, Ç. 805
Yoeli-Ullman, R. 439
Yokote, K. 9, 40
Yokoyama, K. 745
Yoo, J. 746
Yoo, S. 765
Yoon, K.-H. 599, 855
Yoshiji, S. 366
Young, K. G. 235, 555
Young, L. 582
Young, R. 505
Younis, I. 298
Yovos, J. 299
Yu, C. 53
Yu, J. 599
Yu, J. 855
Yu, L. 313
Yu, M. 580, 615
Yu, Y. 377, 378
Yuan, L. 547
Yuan, Y. 706
Yuan, Y. 66, 858
Yue, D. 762
Yuen, S. 28
Yuldasheva, N. Y. 193
Yurchenko, I. 254
Yvan-Charvet, L. 482
Zaborska, K. E. 376, 414
Zaccagnini, G. 46
Zaharia, O. P. 254
Zahedi, R. P. 257
Zahradnicka, M. 243
Zaïmia, N. 386
Zakrzewski, L. 407
Zallocco, L. 58
Zaman, A. 872
Zamarian, V. 305
Zampetti, S. 411
Zangerolamo, L. 213, 503
Zannella, C. 312
Zaoui, P. 664, 831
Zaremba, N. 625, 681, 683
Zatterale, F. 269
Zatykó, M. 690
Zebekakis, P. 604
Zeggini, E. 71
Zeissler, M. L. 416
Zeitelhofer, M. 150
Zeitler, P. 109
Zeke, A. 724
Zemva, J. 120, 754, 760
Zeng, J. 4, 281
Zeng, L. 645
Zeng, L. 793
Zeng, Y. 64
Ženíšková, R. 723
Zeniya, M. 745
Zerbi, A. 418
Zglejc-Waszak, K. 329
Zhai, P. 198
Zhang, A. 851
Zhang, E. 457
Zhang, H. 781, 789, 819
Zhang, H. 739
Zhang, H. 113, 607
Zhang, J. 41
Zhang, J. 165
Zhang, J. 357, 859
Zhang, L. 715
Zhang, L. 285
Zhang, L. 268
Zhang, L. 186
Zhang, P. 857
Zhang, Q. 640, 649
Zhang, Q. 106, 371
Zhang, S. 192
Zhang, X.-M. 146
Zhang, X. 454
Zhang, X. 281
Zhang, Y. Y. 198
Zhang, Y. Y. 198
Zhang, Y. 186
Zhang, Z. 4
Zhang, Z. 789
Zhao, J. 525, 837
Zhao, L. 710
Zhao, M. 419, 420
Zhao, X. Q. 748
Zhao, X. 735
Zhao, X. Y. 198
Zhao, Y. 285
Zhao, Y. B. 36
Zheng, X. 739
Zhou, Q. 44
Zhou, Y. 872
Zhou, Y. 285
Zhou, Y. 343, 421, 842
Zhou, Z. 46
Zhou, Z. 706
Zhu, W. 505, 781, 789, 819
Zhu, X. 747
Zhu, Y. 857
Ziegler, D. 192
Zierath, J. R. 81, 82, 93, 161, 200, 229, 231, 232, 455, 481
Ziko, H. 430
Zimmer, R. T. 427
Zimmermann, T. 570
Zinman, B. 88
Zinna, L. 848
Zobairi, F. 824
Zobel, E. H. 752
Zoega, H. 130
Zou, H. X. 198
Zoupas, C. S. 444
Zozulinska-Ziolkiewicz, D. 473, 820
Zraika, S. 390
Zubiaur, P. 214
Zubillaga-Gómez, M. 793
Zuccolotto, G. 531
Zuccotti, G. 262, 399
Zulueta, M. 278
Zulyniak, M. A. 429
Zurawska-Klis, M. 432
Zusi, C. 227
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.