Abstract
Purpose of Review
To provide a comprehensive review of drugs and neoplastic, infectious, autoinflammatory, and immunodeficiency diseases causing medium- to large-vessel vasculitis in adults with emphasis on information essential for the initial diagnostic process.
Recent Findings
Entities with medium- to large-vessel vasculitis as clinical manifestations have been described recently (e.g., adenosine deaminase-2 deficiency, VEXAS-Syndrome), and vasculitis in established autoinflammatory or immunodeficiency diseases is increasingly being identified.
Summary
In the diagnostic process of medium- to large-vessel vasculitis in adults, a large variety of rare diseases should be included in the differential diagnosis, especially if diagnosis is made without histologic confirmation and in younger patients. Although these disorders should be considered, they will undoubtedly remain rare in daily practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11926-022-01083-5.
Keywords: Vasculitis, Differential diagnosis, Primary immunodeficiency disease, Autoinflammation, Infectious vasculitis
Introduction
Advanced genetic analyses and novel pharmacotherapeutics or microbes led to the identification of new disease entities with vasculitic manifestations during the last two decades, and we expect more to be identified in the future. Thus, an ever-increasing variety of vasculitides and vasculitis mimics should be considered in the diagnostic process of adults with suspected medium- or large-vessel vasculitis (MVV, LVV, respectively). This is especially important in situations where histological confirmation of a vasculitic process is not possible (i.e., vasculitis of aorta, coronaries, etc.) and where the final diagnosis rests entirely on clinical, laboratory, and imaging findings. Also, histological confirmation is now commonly omitted if imaging is compatible with MVV and/or LVV, but in atypical cases or potential mimics, caution is advised [1, 2].
In this article, autoinflammatory, primary immunodeficiency, neoplastic, or infectious diseases as well as drugs causing MVV and/or LVV are comprehensively reviewed. The primary vasculitides and vasculitis in arthritides, connective tissue, and fibroinflammatory diseases as well as vasculitis mimics are reviewed elsewhere [3]. Single-organ vasculitides have been recently reviewed in this journal [4]. The problem of the inconsistent and variable definition of vessel size has been discussed recently [3]. Because Chapel Hill’s nomenclature remains ambiguous, we defined the vessel sizes according to our daily practice, which is similar to Chapel Hill, but attempts to define large vessels more specifically (see legend of Table 1) [3, 5]. Some of the described diseases exceptionally cause MVV or LVV and are mentioned in the text body only. For diseases that are more regularly associated with MVV and LVV and where a larger body of evidence exists, further details important for recognizing the disease and making the initial diagnosis are presented in the tables: Tables 1 and 2 are abridged versions of the Supplementary Tables S1 and S2, where the complete information (including details on clinical manifestations and important laboratory findings) can be found.
Table 1.
Neoplastic, autoinflammatory, and primary immunodeficiency diseases and drugs causing large- and medium-vessel vasculitis in adults
| Category | Disease | Epidemiology, patient characteristics | Mainly affected large and medium vessels or vessel beds (i) | Diagnostic pearls and pitfalls (ii) | |
|---|---|---|---|---|---|
| Large | Medium | ||||
| Autoinflammatory diseases | Familial Mediterranean fever | FMF: onset childhood to early adulthood, AR inheritance (MEFV gene), prevalence Mediterranean region higher. “PAN-like” MVV: ~ 1% of FMF; m > f = 3.6:1; usually after onset of FMF. BS: associated with FMF in ~ 0.4%, f > m [6–8] | + / − aorta (thoraco-abdominal); CCA, subclavian (“TAK-like”) [9, 10] |
‡ “PAN-like” renal, cerebral, abdominal, cutaneous + / − cardiac [6] |
• Perirenal hematoma in ~ 50% (distinctive feature of “PAN-like” MVV) • “BS-FMF-overlap”: cutaneous, gastrointestinal and CNS involvement more frequent than in isolated BS [8] • “PAN-like” MVV: compared to classical PAN, FMF patients are younger, testicular/cardiac involvement less frequent; CNS involvement more frequent and GN is possible • Hepatitis B Infection is detected in up to ~ 7% of “PAN-like” FMF [6] |
| Inflammatory bowel diseases | IBD onset usually adolescence to early adulthood (onset at any age possible). IBD (esp. CD) and TAK or “TAK-like” at younger age (~ 20 Y/A) than isolated TAK [11, 12] | ‡ “TAK-like” or TAK: aorta, subclavian, vertebral [11, 12] | + / − “TAK-like” or TAK: renal, mesenteric, cerebral, cutaneous, TA (only with associated GCA) [11, 12] |
• IBD preceding vasculitis in most cases in ~ 70% • GCA with IBD rarely relapsing (in contrast to isolated GCA) • IBD usually not active at time of vasculitis onset • Due to limited data, differentiation of concomitant TAK or GCA and IBD vs. “TAK-like” and “GCA-like” disease with IBD not possible |
|
| Chronic recurrent multifocal osteomyelitis | Onset usually in childhood, but possible in adults; f > m; globally. LVV very rare (onset 3 to ~ 50 Y/A) [13, 14] | ‡ “TAK-like”: aorta (ascending, descending), CCA, subclavian [13, 14] | (iii) | • Further associations with pyoderma gangrenosum, synovitis, acne, pustulosis, hyperostosis, osteitis syndrome (SAPHO) and IBD [13] | |
| Primary immunodeficiency diseases | Common variable immunodeficiency | May manifest in childhood or in adulthood at any age; m = f; less common in developing nations; true prevalence of CVID unknown, estimated ~ 0.5–7/106; any form of vasculitis in ~ 2% of CVID [15••, 16, 17•] | ‡ “TAK-like” or TAK: aorta, innominate, CCA, ICA, axillary, subclavian [18–20] | + / − “TAK-like” or TAK: renal, mesenteric, celiac, coronary [18–20] |
• Rule out other causes of hypogammaglobulinemia before diagnosing CVID • Detection of aortic aneurysm in CVID should trigger imaging for LVV • Consider deficiency of adenosin-deaminase-2 in CVID, esp. in patients with MVV • Screen for splenomegaly and lung disease in potential CVID • Limited significance of any serologic test if patient is receiving immunoglobulin replacement therapy |
| Wiskott-Aldrich syndrome | Usually diagnosis in early childhood, exceptionally delayed to early adulthood in milder variants; X-linked recessive disorder; prevalence ~ 4/106; any form of vasculitis in ~ 1 – 29% [21, 22] | ‡ aorta (frequent aortic aneurysms, often panaortic); + / − aortic arch arteries (“TAK-like”) [23, 24] | + / − cerebral, kidney, cardiac, liver, bowel, stomach [25, 26] |
• With longer survival of patients with Wiskott-Aldrich, aneurysms secondary to LVV may increasingly become recognized; screening beginning in childhood might be justified • Isolated presentation with thrombocytopenia is commonly called “X-linked thrombocytopenia”, a mild variant of Wiskott-Aldrich • Missing or reduced expression of the “WAS-protein” can be detected rapidly by lymphocyte flow cytometry in peripheral blood [21] |
|
| Deficiency of adenosin-deaminase-2 | Variable disease onset, can be delayed to adulthood; AR inheritance; m = f; globally (less common in Africa, East Asia); estimated prevalence ~ 4.5/10^6; any vasculitic feature ~ in > 75–90% [27•, 28, 29•] | (iii) |
‡ “PAN-like” (including aneurysms): skin, muscle, mesenteric, celiac, hepatic, renal + / − splenic, testicular, cerebral, coronary, pancreatic, TA [27•, 29•, 30] |
• Screening with ADA2 activity testing (e.g., with dried plasma spots) • Biallelic mutations can be found in asymptomatic individuals (usually through screening of seemingly unaffected family members) • Sneddon syndrome is an important differential diagnosis (livedo racemosa and CNS lesions) • CNS involvement is much more common in DADA2 than in PAN • Skin biopsy can show leukocytoclastic SVV and necrotizing MVV |
|
|
Malignancy—paraneoplasia Myeloid neoplasms |
Myelodysplastic syndromes Myeloproliferative neoplasms |
MDS/CMML: median onset ~ 70–75 Y/A (range 16–90 Y/A), m > f (MDS), m = f (CMML). MVV/LVV less frequent in polycythemia vera or essential thrombocythemia [31–33] | + / − aorta, large veins (“BS-like” manifestation) [31, 34, 35] |
‡ TA (“GCA-like”) + / − “PAN-like” (renal, hepatic, mesenteric, TA (non-GCA), cerebral, cutaneous) [31, 36–35] |
• Consider MDS/MPN in refractory MVV/LVV or with inflammatory dysimmune phenomena [36] • The finding of cytopenia in LVV or MVV should lead to consideration of MDS or MPN as underlying disease process • MDS with GCA has poorer outcome (relapses ↑, steroid dependency) [33] |
| Acute and chronic myeloid leukemia | Any age possible, incidence increases with age. AML: often progression of MDS/MPN. MVV/LVV very rare | + / − “TAK-like”: aorta, CCA/ICA, innominate, subclavian, axillary [37, 38] | + / − “PAN-like”: lower leg, TA (“GCA-like”) [39–38] |
• Basophilia and eosinophilia are common in chronic myeloid leukemia • A differential blood count with visual inspection is advised in the setting of vasculitis with leukocytosis |
|
| Lymphoid neoplasms | Hodgkin and non-Hodgkin lymphomas | Any age possible. Hodgkin and non-Hodgkin lymphoma occasionally with LVV/MVV, multiple myeloma only rarely [39] | + / − aorta, iliac, femoral [37] | ‡ cerebral; + / − “PAN-like”: renal, hepatic, mesenteric, infrabrachial, infrapopliteal, coronary; TA (“GCA-like”) [39, 37, 42–44] |
• Lymphocyte flow cytometry of peripheral blood frequently shows monoclonality • Intravascular lymphoma is a potential mimic of MVV, especially in the CNS or skin [3] |
| Hairy cell leukemia | Mean onset ~ 50 Y/A (any age possible); m > f; MVV very rare [44, 45] | (iii) | ‡ “PAN-like”: cutaneous, hepatic, mesenteric, renal, cerebral, TA (occasionally TA aneurysm) [44–46] |
• MVV is usually diagnosed in patients with known leukemia [44, 45] • “Hairy” cells can typically be identified in the peripheral blood smear |
|
| Other | VEXAS-syndrome | Usually manifests ~ 50–80 Y/A; male > 95%; associated MDS in > 30%; caused by somatic mutation in UBA1-gene; subset with polychondritis [47••, 48] | + / − aorta [47••, 48] |
‡ cutaneous (25%) |
• Consider VEXAS in refractory cases with inflammatory dysimmune phenomena • Elderly patient with autoinflammatory symptoms, cytopenias, and/or polychondritis: look for vacuoles in bone marrow [47••, 48] |
| Drug-induced vasculitis | Minocycline | Young patients (average 30 Y/A) with acne treatment (often long-term); f > m. [49, 50] | (iii) |
‡ “PAN-like”: skin, nerves + / − renal, mesenteric, gall bladder, liver, spleen, cervix [49, 50] |
• Onset of MVV on average ~ 26 months after initiation of minocycline therapy [49, 50] |
| Immune checkpoint inhibitors | Onset ~ 40–70 Y/A; f = m; vasculitis typically 1–3 months after initiation of treatment (ipilimumab, pembrolizumab, nivolumab) [51, 52] | + / − aorta [51] | ‡ TA (“GCA-like”), cerebral (similar to primary angiitis of the CNS), uterine and ovarian vessels; peripheral nerves [51] |
• Vasculitis typically resolves after stopping immunotherapy (and/or a course of oral or intravenous glucocorticoids) • No fatalities related to vasculitis observed [51] • Overlap with other immunotherapy-related adverse events possible (pericarditis, myocarditis, endocrine, gastrointestinal, etc.) [52] |
|
| Granulocyte colony–stimulating factor | Vasculitis occurs in ~ 0.3–0.5% of patients receiving G-CSF; mean ~ 60 Y/A; f > m [53, 54] | ‡ aorta (abdominal < thoracic or panaortic); carotids; + / − iliac, femoral, innominate, subclavian [53, 54] | + / − TA [53] |
• No fatalities related to vasculitis were observed • In about 60%, vasculitis occurs within 10 days after G-CSF initiation (agents: pegfilgrastim; filgrastim; lipefilgrastim; lenograstim) |
|
| Graft versus host disease | Vasculitis is a very rare manifestation | + / − aorta, iliac, femoral, popliteal, subclavian[55] | + / − cerebral [56] | • Cerebral vasculitis can manifest in long-term survivors [56] | |
(i) Main vessels or vessel beds identified by our literature searches (i.e., arteries or vessel beds not listed, could still be affected). If not specified otherwise, the vessel names indicate arteries. The vessel sizes are defined as follows: “Large” (the aorta and distributing vessels of the extremities and neck, originating proximal to the elbow, knee, and dura mater), “small” (arterioles, capillaries, venules, and small intraparenchymal arteries and veins), “medium” (remaining vessels, including visceral arteries). (ii) Pearls mostly reflect the personal experience of the authors and are only partially referenced. (iii) Not identified by our literature search. Special characters: ‡, typically affected vessels or vessel beds; + / − , occasionally to rarely affected vessels or vessel beds; ~ , approximately
AR autosomal recessive; BS Behçet’s syndrome, CCA common carotid artery, CD Crohn’s disease, CNS central nervous system, CMML chronic myelomonocytic leukemia, CVID common variable immunodeficiency, DADA2 deficiency of adenosine-deaminase-2, ESR erythrocyte sedimentation rate, FMF familial Mediterranean fever, GCA giant cell arteritis, G-CSF granulocyte colony–stimulating factor, IBD inflammatory bowel disease, ICA internal carotid artery, LVV large-vessel vasculitis, MDS myelodysplastic syndrome, MPN myeloproliferative neoplasm, MVV medium-vessel vasculitis, PAN polyarteritis nodosa, TA temporal artery, TAK Takayasu arteritis, UC ulcerative colitis, VEXAS vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic, Y/A years of age
Table 2.
Infectious diseases causing large- and medium-vessel vasculitis in adults
| Pathogen | Epidemiology, patient characteristics | Mainly affected large and medium vessels or vessel beds (i) | |
|---|---|---|---|
| Large | Medium | ||
| Bacteria/Mycobacteria | |||
| Gram-positive bacteria | |||
| Gram-positive cocci (staphylococci, streptococci, enterococci) | Any age; predisposing conditions for medium- or large-vessel vasculitis: any infection with bacteremia (typically endocarditis), atherosclerosis, ICH, IVDA | ‡ aorta, femoral, carotid (distal ICA) [101, 102, 104, 105] | ‡ cerebral: mainly anterior circulation, splanchnic. [101, 105, 106•] |
| Listeria spp. | Elderly or young children; ICH; pregnancy; eating of contaminated products, more common spring/summer | ‡ aorta (abdominal > thoracic) [107, 108] | (ii) |
| Mycoplasma spp. | History of atypical pneumonia; exposure to infected children; in context of outbreak | (ii) | ‡ cerebral [106•, 109] |
| Mycobacteria | |||
| M. tuberculosis | Travel (long-term) to or migration from high prevalence countries; close contacts (e.g., family member) with active pulmonary tuberculosis; ICH at risk of reactivation of latent infection | ‡ aorta (abdominal > thoracic); distal ICA [104, 110] | + / − proximal cerebral arteries [106•] |
| Gram-negative bacteria | |||
| Spirochetes | |||
| Treponema pallidum (Syphilis) | Sexual activity; other sexually transmitted infections in the past |
‡ ascending aorta + / − aortic arch or descending aorta (rarely sinus or abdominal aorta) [111, 112] |
+ / − cerebral: anterior circulation (esp. middle cerebral artery) |
| Borrelia spp. | History of tick bite or erythema migrans (< 50%); frequent outdoor activity in endemic regions | (ii) |
‡ cerebral: SVV/MVV (diffuse and mainly leptomeningeal) + / − dural sinus [106•] |
| Leptospira spp. | Travel to warm and tropical regions, outdoor activity and animal contact (farmers, veterinarians, soldiers, canal workers (contact to contaminated soil and water) are at high risk) | ‡ aorta [114] | + / − cerebral (size unclear), dural sinus; coronary [106•, 114] |
| Other Gram-negative bacteria | |||
| Salmonella spp. | Atherosclerosis, diabetes mellitus, ICH, hemoglobinopathies, abnormal intestinal mucosal barrier; eating of contaminated food | ‡ aorta (abdominal > thoracic) [115] | (ii) |
| Coxiella burnetii | Travel to or migration from endemic regions; close contact to animals (e.g., cattle); occupational exposure (veterinarian, farmer); ICH; pregnancy |
‡ aorta, mostly abdominal; |
+ / − TA, “PAN-like” possible (hepatic) [117, 118] |
| Brucella spp. | Occupational exposure (farmer, animal breeder, butcher), travel to or migration from endemic regions, drinking of unpasteurized milk | ‡ aorta (abdominal > thoracic), ICA [119, 120] | + / − cerebral, dural sinus [120] |
| Francisella tularensis | Outdoor activity or outdoor profession (e.g., forester, hunter), exposure to rodents, tick bites, contaminated material | ‡ aorta (abdominal) [121] | (ii) |
| Viruses | |||
| Varicella Zoster virus | Any age, ICH at risk | + / − ICA, vertebral arteries [106•, 122] | + / − cerebral [106•, 122] |
| Herpes Simplex virus | Any age; with or without comorbidities | + / − ICA [123] | ‡ cerebral [123, 124] |
| Cytomegalovirus | Usually in heavily ICH | (ii) | ‡ “PAN-like” (distal extremities, renal, splanchnic) [125, 126] |
| HIV | Migrants from high-burden countries, risk for STD, IVDA, mostly young or middle-aged patients | ‡ aorta, carotid, subclavian, femoral, popliteal [127, 128] |
‡ cerebral + / − “PAN-like” (skin, nerve, muscle, renal splanchnic) [106•, 127] |
| Hepatitis B virus | Migrants from high burden countries (also second-generation: perinatal transmission), IVDA, sex workers | (ii) but highly likely | ‡ “PAN-like” (skin, renal, testicular, splanchnic, peripheral nerves) [101, 105, 129, 130] |
| Fungal infections (yeasts (Cryptococcus, Candida spp.), molds (Aspergillus, Mucor spp.), dimorphic (Histoplasma, Coccidioides spp.)) | ICH, critically ill patients, IVDA; migration from and long-term travel to endemic regions (dimorphic fungi) | + / − aorta, carotids [131–133] | ‡ proximal cerebral arteries (including mycotic aneurysms), esp. Candida and Aspergillus spp. [105, 134, 135] |
| Parasites (iii) | |||
| Taenia solium (neurocysticercosis) | Migration from endemic region (seroprevalence in endemic regions is very high) | + / − ICA [136] | ‡ cerebral (“cysticercal arteritis”) [136] |
| Toxocara spp. | Close contact to dogs/cats (ownership); migration from and travel to endemic regions | (ii) | ‡ cerebral (all vessels can be affected) [137] |
(i) Main vessels or vessel beds identified by our literature search (i.e., arteries or vessel beds not listed, could still be affected). If not specified otherwise, the vessel names indicate arteries. (ii) Not identified by our literature search. (iii) Only two common parasitic infections are mentioned due to the rarity of such cases in Western Europe. Special characters: ‡, typically affected vessels or vessel beds; + / − , occasionally to rarely affected vessels or vessel beds
CNS central nervous system, ICA internal carotid artery, ICH immunocompromised host (e.g., immunosuppressive therapy, solid organ or hematopoietic stem cell transplantation, HIV), IVDA intravenous drug abuse, PAN polyarteritis nodosa, spp species pluralis, STD sexually transmitted diseases
Autoinflammatory Diseases
Autoinflammatory diseases (AIDs) are characterized by systemic inflammation due to dysregulation of the innate immune system. They can be broadly divided into monogenic and polygenic diseases. Some may present with vasculitis in adults [57••, 58•].
Monogenic Autoinflammatory Diseases
Most monogenic AIDs manifest in early childhood, but occasionally onset or delayed diagnosis in adulthood are reported. Vasculitis and vasculopathy of the large- and medium-sized vessels are rare manifestations of these diseases [58•].
Familial Mediterranean fever (FMF) is the most common monogenic AID, typically presenting with recurrent episodes of fever, abdominal pain, rash, and arthralgia, and is also the most strongly associated with systemic vasculitis: IgA vasculitis occurs in 2.7–7%, and a vasculitis similar to polyarteritis nodosa (PAN) can be observed in approximately 1% [6, 59]. Interestingly, perirenal hematomas are a frequent manifestation and some patients present with glomerulonephritis, which is not observed in classical PAN [6]. An independent, FMF-associated form of vasculitis can be postulated, hence the term “PAN-like” vasculitis has been proposed [6]. LVV such as Takayasu arteritis (TAK) or Cogan Syndrome have rarely been described to co-occur with FMF [6, 9, 10]. Particularly noteworthy is the possible co-occurrence of Behçet’s syndrome (BS) and FMF. Both diseases have a higher prevalence in the Mediterranean region. They share certain clinical manifestations (such as fever, arthritis, cutaneous signs, and the self-limited relapsing disease course), both respond to colchicine, and in cohorts of BS, FMF is more common than in the general population in the same region [7, 8]. Pyogenic arthritis, pyoderma gangrenosum, and Acne (PAPA) syndrome is another monogenic AID that can rarely present with vasculitis; a single patient with possible cerebral vasculitis and aneurysm of the posterior cerebral artery was identified [58•, 60]. The tumor necrosis factor receptor–associated periodic syndrome (TRAPS), typically presenting with recurrent episodes of fever, periorbital edema, abdominal pain, arthralgia/myalgia, and rash, is rarely associated with MVV and SVV, but disease onset in adulthood is rare, and so far, vasculitides have primarily been described in children [58•].
The Aicardi-Goutières syndrome (AGS) and STING-associated vasculopathy with onset in infancy (SAVI) are interferonopathies that almost exclusively manifest in childhood. Rare cases of adult-onset or delayed diagnosis in adulthood with vasculitic manifestations are reported. Clinical manifestations of AGS include encephalopathy, dystonia, spasticity and cognitive impairment, characteristic calcifications of the basal ganglia, and various autoimmune features, ranging from low-titer antinuclear antibodies (ANA) to the full clinical spectrum of systemic lupus erythematosus. Cerebral MVV with arterial stenoses and stroke can very rarely occur in adults, especially in patients with mutations in the SAMHD1 gene [57••, 61, 62]. SAVI, an autosomal dominant disease, presents with a broad clinical spectrum, such as interstitial lung disease, skin rashes/plaques, arthritis, and MVV, which may occasionally lead to distal limb ischemia with consecutive need for amputation [61–63].
The spectrum of monogenic AIDs is continuously expanding. Two more recently described diseases, deficiency of adenosine deaminase 2 (DADA-2) and Haploinsufficiency A20 (HA20), can also present with vasculitis in adults [27•, 64••]. HA20 is an autosomal dominant relopathy that usually begins in childhood with a relapsing–remitting course. Rarely, the diagnosis is delayed to adulthood. While the clinical phenotype is very similar to BS, polyarthritis, fever, and fluctuating antibody positivity (e.g., ANA, anti-ds-DNS antibodies or lupus anticoagulant) are more frequently observed in HA20. MVV of the CNS are occasionally observed, and single cases of pulmonary artery and skin vasculitides have been described. The disease should be sought especially in familial early-onset orogenital and gastrointestinal ulcers with articular and ocular symptoms. [3, 64••, 65]. DADA-2 is described below.
Polygenic Diseases with Autoinflammatory Features
Inflammatory bowel disease (IBD)
Crohn’s disease (CD) and ulcerative colitis (UC) have many features of AIDs [66]. Examples of MVV and LVV in patients with IBD are abundant in the literature, but precise epidemiological data are lacking. It has been reported that up to 5% of patients with TAK concomitantly suffer from CD. Gastrointestinal manifestations usually precede vasculitis and LVV is more frequent and often presents with a TAK-pattern [11, 67]. Interestingly, in GCA associated with IBD, male gender seems to be more common [12]. While UC often presents with ANCA (anti-neutrophil cytoplasmic antibodies) and CD with ASCA (anti-Saccharomyces cerevisiae antibodies) positivity, the clinical picture of ANCA-associated vasculitis (AAV) is only rarely observed in IBD [68–70]. Suspected genetic (such as shared associations to HLA-B*52 in patients with TAK and UC) and pathophysiological similarities of LVV and IBD (e.g., granulomatous inflammation in both LVV and CD) have been put forward as arguments for a common genesis of the diseases, but data are limited [11]. Future research may tell whether LVV and MVV are features of IBD or the co-occurrence of two different diseases [11, 69]. The overlapping clinical features (intestinal and extraintestinal; e.g., ocular inflammation, erythema nodosum, or oral aphthae) and similar age range may make it difficult to distinguish IBD from BS, another polygenic disorder with autoinflammatory features [71]. Furthermore, Cogan’s syndrome, a variable vessel vasculitis, can rarely be associated with IBD [72]. BS and Cogan’s syndrome are classified as primary vasculitides [3].
In chronic recurrent multifocal osteomyelitis, an AID in children with rare onset in adulthood, presenting with bone pain and soft tissue swelling, a “TAK-like” LVV has rarely been described [13, 14].
Primary Immunodeficiency Diseases
Primary immunodeficiency (PIDs) are genetically determined diseases which are typically but not always accompanied by susceptibility to infection. The reduced immune tolerance can lead to autoimmune phenomena and/or overt autoimmune diseases. More than 450 PID entities have been identified, and while PIDs are increasingly recognized in adults, they still remain underdiagnosed [73]. Germline mutations of immune genes with only partial alteration of protein function may lead to late-onset manifestations [15••]. In addition, somatic mutations in immune genes may cause phenocopies of PIDs [15••, 73]. In developing countries, access to genetic testing is difficult and undiagnosed PIDs with childhood onset should be considered in migrants, especially from countries with high prevalence of consanguinity. While a presentation with SVV is clearly more common, there are certain PIDs that are associated with MVV and/or LVV (see case study in Fig. 1). The frequent affection of the vasculature of the central nervous system (CNS) in PIDs is noteworthy [25].
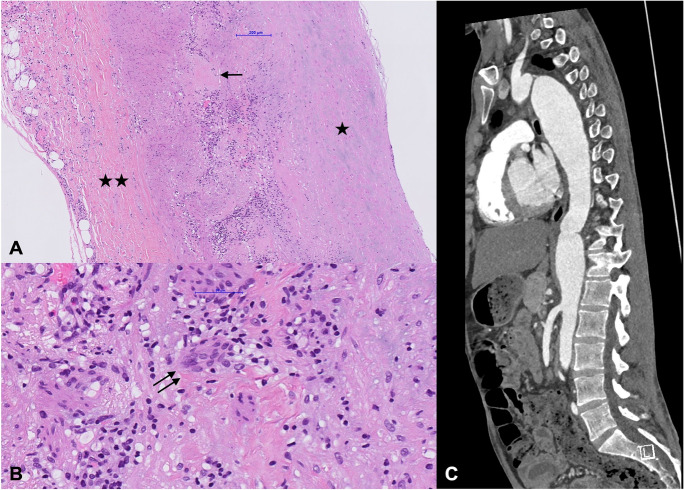
Fig. 1.
Case description: Male patient with a molecularly unclassified primary immunodeficiency (CVID-like) with frequent major infections in early childhood (e.g., meningitis). Laboratory findings consisted of intermittent neutropenia; mild lymphopenia with normal numbers of total T-cells and NK-cells; reduced naïve T cells, total and class-switched B cells; severely reduced IgG/IgM/IgA. Immunoglobulin replacement therapy was started before the age of 10. He developed a panaortic dilatation already in childhood, and a thoracoabdominal aortic replacement was performed at the age of 23. A, B Hematoxylin and eosin staining of the descending aorta showing intimal fibrosis (single star), block-like media necrosis (single arrow), transmural lymphohistiocytic infiltration with presence of giant cells (double arrow), and periadventitial fibrosis (double star); extensive microbial analysis of the specimen remained negative. C CT angiography of aorta (sagittal view) with aneurysm of the suprarenal abdominal and descending aorta
Common variable immunodeficiency (CVID)
CVID is an umbrella diagnosis of patients with suspected PID that present with hypogammaglobulinemia [16, 17•]. CVID in adults is probable, if reduced serum levels of IgG and low IgM or IgA and a poor antibody response to vaccines are discovered in a patient with frequent infections and/or autoimmune features and/or enlarged spleen/lymph nodes. Secondary causes of hypogammaglobulinemia need to be excluded [15••, 16, 17•]. Vasculitis is present in approximately 2% of adults with the diagnosis of CVID [17•]. Because of their rarity, it remains unclear whether the vasculitides in CVID are primary vasculitides in the sense of autoimmune phenomena or whether they are separate entities which may require different therapeutic approaches. All vessel sizes can be involved, including SVV of the CNS and retina, ANCA-positive SVV, or LVV [18–20, 74–76, 77•].
Wiskott-Aldrich syndrome (WAS)
WAS is a rare X-linked PID and is usually diagnosed in childhood, but vasculitis can present in male adults [21]. Thrombocytopenia with small platelets is the signature finding, and susceptibility to bacterial and viral infections, atopy, and autoimmune features are variably present [21, 78]. Vasculitides are the second most common autoimmune feature (in 1–29% of patients) [22]. While SVV, especially IgA vasculitis, is the most frequent vasculitis, necrotizing MVV can occur in various organs and vessels, including the CNS. In adults, lymphohistiocytic and/or granulomatous LVV with propensity to development of aortic aneurysms, is especially noteworthy [22–24, 26].
Deficiency of adenosine deaminase 2
DADA2, first described in 2014, is a rare, autosomal recessive, autoinflammatory, and immunodeficiency disease that manifests with features of a “PAN-like” vasculitis in the majority of cases [27•]. Many aspects of DADA2 remain enigmatic, and it is unclear whether the loss of the enzymatic activity in vitro is directly responsible for the disease manifestations. Many different pathogenic mutations in the ADA2 gene were described, and the phenotype is highly variable with features of vasculitis/vasculopathy, bone marrow disease (e.g., cytopenias), and immune dysregulation (e.g., hypogammaglobulinemia) [28, 29•]. DADA2 is a mimic of various disorders, including early stroke, connective tissue diseases, non-healing leg ulcers, hepatopathy, autoinflammatory diseases, and “PAN-like” MVV [30, 79]. Also, many patients with DADA2 fit under the diagnostic CVID umbrella [77•].
Other PID
A single case of aortitis in an adult with hyper IgM syndrome (typically associated with susceptibility to bacterial infections) was identified [80]. In an adult with TAK and IBD, a gain-of-function germline mutation in STAT1 has been demonstrated [81]. STAT3-dependent hyperimmunoglobulin-E-syndrome (associated with significant IgE elevation, eczema, and eosinophilia) predisposes to aneurysm formation. Multiple cases with dilatations/aneurysms of the aorta, coronaries, carotids, or intracranial medium-sized arteries have been reported in adolescents and adults, some of which had histologically proven vasculitis [82]. DOCK8 deficiency, an autosomal recessive form of hyperimmunoglobulin E syndrome, typically manifests in childhood, but survival into adulthood is common and it can be associated with LVV (aortitis) or cerebral MVV [83].
Malignancy and Paraneoplasia
All forms of vasculitis, but especially SVV (including AAV and IgA vasculitis) and MVV, have been observed in the context of neoplasia [39, 84]. However, a genuine paraneoplastic syndrome with synchronous disease progression of vasculitis and neoplasia is only rarely observed [85, 86]. Due to the relatively high incidence of malignancies and LVV in general, a purely coincidental occurrence of the two diseases is a likely scenario. In addition, patients with suspected vasculitis are often incidentally diagnosed with tumors during detailed imaging studies (such as FDG-PET-CT) [86]. With limited data overall, no specific solid tumor is associated with LVV and MVV [40, 85]. More relevant, however, is the association of vasculitides with hematologic neoplasms.
Myeloid Neoplasms
Myelodysplastic syndrome and myeloproliferative neoplasia (MDS, MPN)
10–25% of patients with MDS or chronic myelomonocytic leukemia (CMML) suffer from concomitant systemic inflammation with autoimmune or autoinflammatory manifestations, of which about one-third correspond to various types of vasculitis [31, 32, 36]. According to a large retrospective study, LVV was the most frequent subset, followed by "Behçet’s-like” syndrome, “PAN-like” vasculitis, and SVV (including AAV and cryoglobulinemic vasculitis). In approximately 44%, vasculitis is diagnosed prior to MDS/CMML and men seem to be affected more frequently. Vasculitis does not seem to have an impact on overall survival, and there is no specific association with MDS/CMML subtypes or their severity [34]. A recent case–control study described less prominent cranial symptoms in GCA in the context of MPN (including essential thrombocythemia, polycythemia vera, and CMML), especially with JAK2 mutations. In this context, corticosteroid dependence and a refractory disease course with shorter overall survival seem more common [33, 35]. Acute myeloid leukemia (AML)/chronic myeloid leukemia (CML): MDS/MPN, including CMML, bear a high risk for progression to AML. Rarely, MVV or LVV can be observed in AML and CML. Apart from constitutional symptoms and symptoms related to cytopenias, leukemia can manifest with bone pain like a polymyalgic syndrome; examining the blood count in detail in is essential [31, 37–39, 41].
Lymphoid Neoplasms
Hodgkin’s and Non-Hodgkin’s lymphoma
Some studies describe an increased incidence of lymphoma (especially non-Hodgkin’s lymphoma) in patients with autoimmune diseases [87]. True paraneoplastic vasculitis is rare but was described in isolated cases, mostly affecting the medium-sized vessels (including the temporal artery in the form of GCA) and rarely the aorta and its major branches [37, 39, 42]. Interestingly, lymphoma (especially Hodgkin lymphoma) can be detected in up to 6% of patients with primary angiitis of the CNS [43, 44]. Hairy cell leukemia (HCL): HCL is a rare lymphoid neoplasm which is occasionally associated with “PAN-like” vasculitis, and potentially affects the temporal arteries. Their simultaneous occurrence is almost certainly not coincidental, and “PAN-like” MVV should be considered a paraneoplastic phenomenon of HCL. Besides MVV, mainly cutaneous SVV can occur. Direct tumor cell infiltration of the vascular wall is also observed (a vasculitis mimic) [44–46].
VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome
The VEXAS-Syndrome is a recently described disease due to somatic mutations in the UBA1 gene on the X-chromosome (which explains why women are only exceptionally affected). It presents with a broad range of refractory inflammatory and hematologic manifestations such as polychondritis, cytopenias and macrocytosis, pulmonary infiltrates, and neutrophilic dermatosis. Various vasculitic manifestations can occur, ranging from cutaneous SVV/MVV (in 26% of patients) to temporal arteritis (in the form of GCA) or aortitis (in 1.7% of patients) [47••, 48]. Characteristic vacuolization in erythroid and myeloid precursor cells on bone marrow analysis is nearly always identified. VEXAS is associated with MDS in > 30% and is typically refractory to conventional immunosuppressive treatment strategies [88].
Drug-Induced and Treatment-Related Vasculitis
Drug-associated vasculitis is common, and the list of triggering agents is long, including a broad range of pharmaceutical and illicit drugs such as cocaine (especially if cut with levamisole). However, SVV is far more common than LVV or MVV and commonly associated with serological abnormalities (elevated ANA, ANCA or antiphospholipid antibodies, low complement levels) [89, 90•].
TNF-alpha inhibitors
Biologics, such as TNF-alpha inhibitors or rituximab, are indispensable therapeutics for immune-mediated diseases, but they can potentially trigger vasculitis, mainly SVV (especially cutaneous leukocytoclastic vasculitis or Henoch-Schonlein purpura) [91]. TNF-alpha inhibitors are reported to rarely induce MVV, including cerebral vasculitis or temporal arteritis, and LVV. The number of reported cases is small, and most of the affected patients suffered from rheumatoid arthritis. The clear differentiation between manifestations of the underlying rheumatoid arthritis with consecutive rheumatoid vasculitis and drug-induced vasculitis is difficult [91–93].
Vaccines
The potential triggering of autoimmune diseases by vaccination is controversial. Various publications describe vasculitides of all forms in association with different vaccines [94]. In adults, GCA following influenza vaccination is the most discussed, and recent pharmacovigilance data revealed a potential safety signal for GCA and PMR with COVID-19 vaccinations [95, 96] There is a lack of studies demonstrating a clear association.
Minocycline
This antibiotic is commonly used for long-term treatment in chronic acne in young patients. Its wide spectrum of side effects includes cutaneous and systemic MVV in the form of “PAN-like” vasculitis [49, 50].
Immune checkpoint inhibitors (ICIs)
Immune therapy revolutionized cancer treatment and has become a well-established treatment modality for various neoplasms. ICIs are monoclonal antibodies that block regulatory immune checkpoints, such as programmed cell death protein 1 (PD-1) and others (PDL1 and CTLA-4), to enforce immunity against tumor-associated antigens [97]. Augmented anti-tumor immunity can be accompanied by the development of autoimmune phenomena, known as immune-related adverse events [97]. It is not surprising that the use of ICIs bears the risk of provoking vasculitis [98••, 99]. Although rare, LVV is the most common vasculitic complication of ICIs (esp. anti-PD1-therapy). It is responsive to the cessation of the ICIs and concomitant corticosteroid therapy. Fatalities attributable to vasculitis have not been reported [51, 52, 100•].
Granulocyte-colony stimulating factor (G-CSF) and chemotherapy
G-CSFs, such as filgrastim, induce proliferation and maturation of neutrophils and are mainly used to treat chemotherapy-associated neutropenia. They bear the potential to induce a rapidly progressive LVV, with disease onset shortly after their first application. G-CSF withdrawal and corticosteroid treatment are effective with short recovery time. Certain chemotherapeutics (especially docetaxel) have been discussed as possible inductors of LVV [53, 54].
Graft versus host disease (GVHD)
GVHD is a multiorgan disease due to donor cells initiating an immune response after engraftment in the host. GVHD rarely causes LVV or cerebral MVV [55, 56].
Infectious Diseases
Infections are a rare but well-recognized etiology of vasculitis. Since inflammation of the vessel wall is observed, we consider infections to be the cause of true vasculitis. The underlying disease mechanisms include direct microbial invasion of the endothelial cells, immune-mediated injury of the vessel wall, stimulation of an immune response to shared epitopes between pathogens, and host or toxin-mediated injury [101, 102, 103•]. However, the available literature mostly consists of case reports or case series. Publications demonstrating a clear and unambiguous infectious etiology of vasculitides are scarce and limited to a few well-studied pathogens (e.g., hepatitis B virus (HBV); hepatitis C virus (HCV), Salmonella spp., and Treponema pallidum or Mycobacterium tuberculosis) [5]. Thus, the true incidence of infectious vasculitis remains unknown.
The enormous spectrum of microbes, the difficulty of pathogen detection from tissue samples, and the frequent necessity to diagnose infections based on serological tests (high risk for false-positive tests) make the unequivocal identification of a pathogen as the cause of vasculitis challenging. Serologic results and molecular detection of a pathogen must be reviewed critically and in the context of the clinical presentation, as testing can detect colonization or residual genetic material after resolved infection. It is often the patient’s medical history (including the social/professional and travel/migration history) that provides diagnostic clues and helps to narrow the spectrum of possible microorganisms and diagnostic workup. Table 2 and supplementary Table S2 summarize the most important microorganisms causing MVV and LVV from a Western European perspective.
Bacterial infections
Staphylococci and streptococci are the most common gram-positive pathogens leading to MVV or LVV, usually of the aorta and the cerebral vessels [101, 102, 104, 105, 106•]. Rare reports describe aortitis or cerebral vasculitis caused by Listeria species (spp.) or Mycoplasma spp. [106•, 107–109]. Similarly, Mycobacterium tuberculosis is a known cause of aortitis as well as cerebral vasculitis [104, 106•, 110]. In the broad spectrum of gram-negative bacteria, Salmonella spp. (representing the Enterobacteriaceae) and Treponema pallidum (representing the Spirochaetaceae) are by far the most likely to cause vasculitis — usually aortitis [106•, 111–113, 115]. For emerging bacteria such as Coxiella burnetii and Brucella spp., Francisella tularensis, and Leptospira spp., cases with aortitis, cerebral or “PAN-like” vasculitis have been described [106•, 114, 116–121]. Borrelia spp. can cause cerebral MVV [106•]. While a single case of aortitis by Tropheryma whipplei was identified, clinically relevant MVV or LVV seem not to be significant manifestations [138].
Viral infections: hepatitis B/C
Many different viruses are recognized as causative agents of vasculitis, but only HBV-associated vasculitis and HCV-associated cryoglobulinemic vasculitis are mentioned specifically in the Chapel Hill 2012 nomenclature [5]. HBV-associated “PAN-like” vasculitis is indeed one of the best studied forms of vasculitis; its prevalence and incidence have gradually declined thanks to extensive vaccination and screening programs [101, 105, 129, 130]. HCV is a frequent cause of cryoglobulinemic vasculitis, typically a SVV, but rarely manifesting in the form of a “PAN-like” vasculitis [3, 105]. HIV: Vasculitis only occurs in approximately 1% of HIV infections, and vessels of any size can be affected. The aorta and its large initial branches are mostly affected, but CNS vasculitis and “PAN-like” disease are also described [106•, 127, 128, 139]. Herpesviridae: Varicella zoster virus is most frequently associated with vasculitis among Herpesviridae; cerebral vasculitis is the typical manifestation [106•, 122]. A possible vasculitis of the temporal arteries has been debated for years, but a causative relationship has been found unlikely [140]. Occasional cases of herpes simplex virus with cerebral vasculitis have been described [123, 124]. Chronic active Epstein-Barr virus infection was linked to MVV or LVV (esp. aorta) in rare cases and more commonly in children; coronary aneurysms seem to be a typical complication [141, 142]. Information on Cytomegalovirus is scarce, but some well-described cases with “PAN-like” MVV were published [105, 125, 126]. Parvovirus B19: Although repeatedly described, it remains uncertain whether Parvovirus B19 truly causes LVV or MVV. “PAN-like” and CNS vasculitis were described mainly in children. A clear etiological link remains to be made. Since cryoglobulinemia and cold agglutinins (vasculitis mimic) can occur, these sequelae and their clinical manifestations (e.g., Raynaud’s phenomenon) need to be considered in patients with Parvovirus B19 [3, 101, 105, 106•, 143]. SARS-CoV-2: Early in the current pandemic, it became apparent that SARS-CoV-2 facilitates the induction of endotheliitis, contributing to thrombus formation in some cases with COVID-19. The multi-systemic inflammatory syndrome in adults can mimic Kawasaki disease, including coronary artery aneurysms, and it is clearly attributed to preceding/ongoing SARS-CoV-2 infection [144]. Further studies are needed to answer the question whether the few cases of vasculitis diagnosed in COVID-19 patients, ranging from SVV to LVV, are coincidental or are due to SARS-CoV-2 infection [103•, 145–148].
Fungal infections
Vasculitides associated with invasive fungal infections are rare and occur almost exclusively in immunocompromised or critically ill patients or in special patient populations, such as intravenous drug users or migrants from endemic regions with dimorphic fungi. Not surprisingly, the related morbidity and mortality can be very high. Candida spp. is the most frequent fungus associated with vasculitis. Cerebral MVV is the most common vasculitis; however, large vessels like the distal carotids or aorta can be affected as well [105, 106•, 131–135].
Parasites and infections in migrants and travelers
Depending on the patient’s migration or travel background and its related exposure risks, less frequently described pathogens like Taenia solium (mainly occurring in the southern hemisphere, causing Neurocysticercosis), Toxocara spp. or West Nile virus (occurring almost worldwide) need to be considered as rare causes of vasculitis, mainly of the cerebral vessels [136, 137, 149].
Conclusion
Including a large variety of rare diseases and vasculitis mimics in the differential diagnosis of medium- to large-vessel vasculitis in adults is essential for correct diagnosis. This seems particularly important, if the diagnosis is made without histologic confirmation and is heavily based on imaging results. This review should be helpful in compiling a list of rarer and novel potential differential diagnoses in the appropriate clinical and epidemiological setting. Although the described disorders should be considered, they will undoubtedly remain rare in daily practice, as the primary vasculitides are much more common [3]. As new entities are constantly being described, we believe it is essential to periodically reevaluate atypical cases that have run for years under diagnoses that do not really fit the clinical phenotype. Also, we suggest performing a detailed diagnostic workup from the very beginning, because the accuracy of many diagnostic tests decreases quickly, once therapy is started.
Although we have attempted to be as comprehensive as possible, including very rare diseases, this was not possible for the section on infectious diseases. Given the enormous number of different microbes that could potentially cause vasculitis, we had to limit ourselves to the most commonly described germs. For tropical regions in particular, the scope of pathogens would have to be expanded considerably.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Yara Banz (Institute of Pathology, University of Bern, Bern, Switzerland) for providing the histopathological images in Fig. 1.
Author Contribution
FL, RP, PS, MR, and LS all wrote parts of the manuscript. RP wrote the infectious disease part. MR co-wrote the section about primary immunodeficiency diseases. LS and FL planned and structured the project, co-wrote and edited all sections, and compiled the tables. All authors approved the final version to be published.
Funding
Open access funding provided by University of Bern
Declarations
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent
The patient described in Fig. 1 gave written consent for publication of the images and clinical information.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Vasculitis
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fabian Lötscher and Roxana Pop contributed equally to this work.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79(1):19–30. doi: 10.1136/annrheumdis-2019-215672. [DOI] [PubMed] [Google Scholar]
- 2.Seitz L, Lötscher F. The intima-media thickness in suspected giant cell arteritis-sometimes it is worth taking a closer look. Rheumatology (Oxford) 2021;60(7):3039–3041. doi: 10.1093/rheumatology/keab316. [DOI] [PubMed] [Google Scholar]
- 3.Seitz L, Seitz P, Pop R, Lötscher F. Spectrum of large and medium vessel vasculitis in adults: primary vasculitides, arthritides, connective tissue and fibroinflammatory diseases. Curr Rheumatol Rep. [DOI] [PMC free article] [PubMed]
- 4.Martins-Martinho J, Dourado E, Khmelinskii N, Espinosa P, Ponte C. Localized forms of vasculitis. Curr Rheumatol Rep. 2021;23(7):49. doi: 10.1007/s11926-021-01012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 6.Abbara S, Grateau G, Ducharme-Bénard S, Saadoun D, Georgin-Lavialle S. Association of vasculitis and familial Mediterranean fever. Front Immunol. 2019;10:763. doi: 10.3389/fimmu.2019.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watad A, Tiosano S, Yahav D, Comaneshter D, Shoenfeld Y, Cohen AD, Amital H. Behçet’s disease and familial Mediterranean fever: Two sides of the same coin or just an association? A cross-sectional study. Eur J Intern Med. 2017;39:75–78. doi: 10.1016/j.ejim.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz T, Langevitz P, Zemer D, Gazit E, Pras M, Livneh A. Behçet’s disease in Familial Mediterranean fever: characterization of the association between the two diseases. Semin Arthritis Rheum. 2000;29(5):286–295. doi: 10.1016/s0049-0172(00)80015-3. [DOI] [PubMed] [Google Scholar]
- 9.Zihni FY, Kalfa M, Ocakçı PT, Tarhan F, Parildar M, Keser G, Aksu K. Coexistence of Takayasu’s arteritis with familial Mediterranean fever. Rheumatol Int. 2012;32(6):1675–1678. doi: 10.1007/s00296-011-1853-7. [DOI] [PubMed] [Google Scholar]
- 10.Zenone T, Puget M. Cogan’s syndrome in a patient with familial Mediterranean fever. Clin Exp Rheumatol. 2012;30(1):141. [PubMed] [Google Scholar]
- 11.Sy A, Khalidi N, Dehghan N, Barra L, Carette S, Cuthbertson D, et al.; Vasculitis Clinical Research Consortium (VCRC); Canadian Vasculitis Network (CanVasc). Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin Arthritis Rheum. 2016;45(4):475–82. 10.1016/j.semarthrit.2015.07.006. [DOI] [PMC free article] [PubMed]
- 12.Bekele DI, Warrington KJ, Koster MJ. Giant cell arteritis associated with inflammatory bowel disease: a case-series and review of the literature. Rheumatol Int. 2021;41(2):487–492. doi: 10.1007/s00296-020-04727-w. [DOI] [PubMed] [Google Scholar]
- 13.Shirai T, Hanaoka R, Goto Y, Kojima I, Ishii Y, Hoshi Y, et al. Takayasu arteritis coexisting with sclerosing osteomyelitis. Intern Med. 2018;57(13):1929–1934. doi: 10.2169/internalmedicine.0329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino A, Sawada T, Matsuda M, Miyagawa S, Nakamura T, Matsubara H. A case of Takayasu’s arteritis and aortic regurgitation, which presented much difficulty in the diagnosing process because of complicated osteomyelitis and non-typical manifestations. J Cardiol. 2009;54(1):148–152. doi: 10.1016/j.jjcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 15.•• Staels F, Collignon T, Betrains A, Gerbaux M, Willemsen M, Humblet-Baron S, et al. Monogenic adult-onset inborn errors of immunity. Front Immunol. 2021;12:753978. 10.3389/fimmu.2021.753978. Very detailed and conceptually strong review about this important topic; includes a practical figure with graphic illustration of the oldest age-of-onset reported for every gene that is reviewed and practical tables. [DOI] [PMC free article] [PubMed]
- 16.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.• Janssen LMA, van der Flier M, de Vries E. Lessons learned from the clinical presentation of common variable immunodeficiency disorders: a systematic review and meta-analysis. Front Immunol. 2021;12:620709. 10.3389/fimmu.2021.620709. Extensive overview about the myriads of associated manifestations of CVID. [DOI] [PMC free article] [PubMed]
- 18.Jerschow E, De Vos GS, Hudes G, Rubinstein A, Lipsitz EC, Rosenstreich D. A case of common variable immunodeficiency syndrome associated with Takayasu arteritis. Ann Allergy Asthma Immunol. 2007;98(2):196–199. doi: 10.1016/S1081-1206(10)60697-7. [DOI] [PubMed] [Google Scholar]
- 19.Tudela P, Bonal J, Romero R, Caralps A. Common variable immunodeficiency and Takayasu’s arteritis. Nephron. 1990;55(3):351–352. doi: 10.1159/000185996. [DOI] [PubMed] [Google Scholar]
- 20.Skeik N, Rumery KK, Udayakumar PD, Crandall BM, Warrington KJ, Sullivan TM. Concurrent Takayasu arteritis with common variable immunodeficiency and moyamoya disease. Ann Vasc Surg. 2013;27(2):240.e13–8. doi: 10.1016/j.avsg.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Candotti F. Clinical manifestations and pathophysiological mechanisms of the Wiskott-Aldrich syndrome. J Clin Immunol. 2018;38(1):13–27. doi: 10.1007/s10875-017-0453-z. [DOI] [PubMed] [Google Scholar]
- 22.Sudhakar M, Rikhi R, Loganathan SK, Suri D, Singh S. Autoimmunity in Wiskott-Aldrich syndrome: updated perspectives. Appl Clin Genet. 2021;14:363–388. doi: 10.2147/TACG.S213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makar RR, Gordins P, Spickett G, Williams R, Lambert D. Endovascular repair of descending thoracic aortic aneurysm in patient with Wiskott-Aldrich syndrome. J Vasc Surg. 2013;58(5):1385–1387. doi: 10.1016/j.jvs.2012.12.081. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SL, Unsworth DJ, Dwight JF, Kennedy CT. Wiskott-Aldrich syndrome, vasculitis and critical aortic dilatation. Acta Paediatr. 2001;90(11):1346–1348. doi: 10.1080/080352501317130452. [DOI] [PubMed] [Google Scholar]
- 25.Byram K, Calabrese LH, Fernandez J. Comorbid vasculitis among patients in a national primary immunodeficiency database [abstract]. Arthritis Rheumatol. 2018; 70(suppl 10). https://acrabstracts.org/abstract/comorbid-vasculitis-among-patients-in-a-national-primary-immunodeficiency-database/. Accessed 8 Jan 2022.
- 26.McCluggage WG, Armstrong DJ, Maxwell RJ, Ellis PK, McCluskey DR. Systemic vasculitis and aneurysm formation in the Wiskott-Aldrich syndrome. J Clin Pathol. 1999;52(5):390–392. doi: 10.1136/jcp.52.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.• Pinto B, Deo P, Sharma S, Syal A, Sharma A. Expanding spectrum of DADA2: a review of phenotypes, genetics, pathogenesis and treatment. Clin Rheumatol. 2021;40(10):3883-3896. 10.1007/s10067-021-05711-w. Gives a detailed overview about the clinical manifestations of this novel syndrome. [DOI] [PubMed]
- 28.Jee H, Huang Z, Baxter S, Huang Y, Taylor ML, Henderson LA, et al. Comprehensive analysis of ADA2 genetic variants and estimation of carrier frequency driven by a function-based approach. J Allergy Clin Immunol. 2022;149(1):379–387. doi: 10.1016/j.jaci.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.• Barron KS, Aksentijevich I, Deuitch NT, Stone DL, Hoffmann P, Videgar-Laird R, et al. The spectrum of the deficiency of adenosine deaminase 2: an observational analysis of a 60 patient cohort. Front Immunol. 2022;12:811473. 10.3389/fimmu.2021.811473. Apart from the clinical manifestations, details on laboratory findings in ADA2-deficiency is provided. [DOI] [PMC free article] [PubMed]
- 30.Dimachkie MD, Fraga GR, Moura NS, Springer JM. A rare case of adenosine deaminase 2 deficiency presenting with temporal arteritis. J Clin Rheumatol. 2021;27(7):e251–e252. doi: 10.1097/RHU.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 31.Mekinian A, Grignano E, Braun T, Decaux O, Liozon E, Costedoat-Chalumeau N, et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology (Oxford) 2016;55(2):291–300. doi: 10.1093/rheumatology/kev294. [DOI] [PubMed] [Google Scholar]
- 32.Grignano E, Mekinian A, Braun T, Liozon E, Hamidou M, Decaux O, et al. Autoimmune and inflammatory diseases associated with chronic myelomonocytic leukemia: a series of 26 cases and literature review. Leuk Res. 2016;47:136–141. doi: 10.1016/j.leukres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Papo M, Friedrich C, Delaval L, Boysson H, Viallard JF, Bachmeyer C, et al. Myeloproliferative neoplasms and clonal haematopoiesis in patients with giant cell arteritis: a case-control and exploratory study. Rheumatology (Oxford) 2022;61(2):775–780. doi: 10.1093/rheumatology/keab337. [DOI] [PubMed] [Google Scholar]
- 34.Roupie AL, Guedon A, Terrier B, Lahuna C, Jachiet V, Regent A, et al. Vasculitis associated with myelodysplastic syndrome and chronic myelomonocytic leukemia: French multicenter case-control study. Semin Arthritis Rheum. 2020;50(5):879–884. doi: 10.1016/j.semarthrit.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Roupie AL, de Boysson H, Thietart S, Carrat F, Seguier J, Terriou L, et al. Giant-cell arteritis associated with myelodysplastic syndrome: French multicenter case control study and literature review. Autoimmun Rev. 2020;19(2):102446. doi: 10.1016/j.autrev.2019.102446. [DOI] [PubMed] [Google Scholar]
- 36.Hamidou MA, Boumalassa A, Larroche C, El Kouri D, Blétry O, Grolleau JY. Systemic medium-sized vessel vasculitis associated with chronic myelomonocytic leukemia. Semin Arthritis Rheum. 2001;31(2):119–126. doi: 10.1053/sarh.2001.27717. [DOI] [PubMed] [Google Scholar]
- 37.Nardi-Agmon I, Hamdan A, Eisen A, Orvin K, Porter A, et al. Diffused coronary involvement in Takayasu arteritis with concomitant malignancy. Clin Rheumatol. 2021 doi: 10.1007/s10067-021-06000-2. [DOI] [PubMed] [Google Scholar]
- 38.Yuce Inel T, Gulcu A, Karakas A, Erdogan Yucel E, Onen F. Coexistence of Takayasu arteritis and chronic myeloid leukemia: coincidental or paraneoplastic phenomenon? Int J Rheum Dis. 2021;24(9):1213–1216. doi: 10.1111/1756-185X.14186. [DOI] [PubMed] [Google Scholar]
- 39.Hutson TE, Hoffman GS. Temporal concurrence of vasculitis and cancer: a report of 12 cases. Arthritis Care Res. 2000;13(6):417–423. doi: 10.1002/1529-0131(200012)13:6<417::aid-art13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Deshayes S, Liozon E, Chanson N, Sacré K, Moulinet T, Blanchard-Delaunay C, et al. Concomitant association of giant cell arteritis and malignancy: a multicenter retrospective case-control study. Clin Rheumatol. 2019;38(5):1243–1249. doi: 10.1007/s10067-018-04407-y. [DOI] [PubMed] [Google Scholar]
- 41.Buhl T, Bertsch HP, Raab BW, Kaune KM, Vasko R, Strutz F, et al. Fulminant polyarteritis nodosa associated with acute myeloid leukaemia resulted in bilateral lower leg amputation. Rheumatology (Oxford) 2009;48(9):1170–1172. doi: 10.1093/rheumatology/kep173. [DOI] [PubMed] [Google Scholar]
- 42.Chircop I, Boespflug A, Cini A, Lega JC, Dalle S. Paraneoplastic polyarteritis nodosa in a patient with cutaneous T-cell lymphoma. Lancet Haematol. 2021;8(3):e240. doi: 10.1016/S2352-3026(20)30393-8. [DOI] [PubMed] [Google Scholar]
- 43.Salvarani C, Brown RD, Jr, Christianson TJH, Huston J, 3rd, Ansell SM, Giannini C, et al. Primary central nervous system vasculitis associated with lymphoma. Neurology. 2018;90(10):e847–e855. doi: 10.1212/WNL.0000000000005062. [DOI] [PubMed] [Google Scholar]
- 44.Wooten MD, Jasin HE. Vasculitis and lymphoproliferative diseases. Semin Arthritis Rheum. 1996;26(2):564–574. doi: 10.1016/s0049-0172(96)80044-8. [DOI] [PubMed] [Google Scholar]
- 45.Hasler P, Kistler H, Gerber H. Vasculitides in hairy cell leukemia. Semin Arthritis Rheum. 1995;25(2):134–142. doi: 10.1016/s0049-0172(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 46.Lowe J, Russell NH. Cerebral vasculitis associated with hairy cell leukemia. Cancer. 1987;60(12):3025–3028. doi: 10.1002/1097-0142(19871215)60:12<3025::aid-cncr2820601228>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 47.•• Beck DB, Ferrada MA, Sikora KA, Ombrello AK, Collins JC, et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N Engl J Med. 2020;383(27):2628-2638. 10.1056/NEJMoa2026834. First description of this novel syndrome, which was found using a genotype driven approach. [DOI] [PMC free article] [PubMed]
- 48.Georgin-Lavialle S, Terrier B, Guedon AF, Heiblig M, Comont T, Lazaro E, et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large-scale analysis of a multicentre case series of 116 French patients. Br J Dermatol. 2021 doi: 10.1111/bjd.20805. [DOI] [PubMed] [Google Scholar]
- 49.Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum. 2012;42(2):213–221. doi: 10.1016/j.semarthrit.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Yokota K, Kurihara I, Nakamura T, Nakatsuka S, Miyashita K, Kobayashi S, et al. Remission of angiographically confirmed minocycline-induced renal polyarteritis nodosa: a case report and literature review. Intern Med. 2022;61(1):103–110. doi: 10.2169/internalmedicine.7340-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37(9):2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 52.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taimen K, Heino S, Kohonen I, Relas H, Huovinen R, Hänninen A, et al. Granulocyte colony-stimulating factor- and chemotherapy-induced large-vessel vasculitis: six patient cases and a systematic literature review. Rheumatol Adv Pract. 2020;4(1):rkaa004. 10.1093/rap/rkaa004. [DOI] [PMC free article] [PubMed]
- 54.Hoshina H, Takei H. Granulocyte-colony stimulating factor-associated aortitis in cancer: a systematic literature review. Cancer Treat Res Commun. 2021;29:100454. doi: 10.1016/j.ctarc.2021.100454. [DOI] [PubMed] [Google Scholar]
- 55.Al-Heilfi A, Nataraja C, Cooley H, Batt T. Large-vessel vasculitis in graft-versus-host disease: a case report. J Med Case Rep. 2021;15(1):478. doi: 10.1186/s13256-021-03067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sostak P, Padovan CS, Eigenbrod S, Roeber S, Segerer S, Schankin C, et al. Cerebral angiitis in four patients with chronic GVHD. Bone Marrow Transplant. 2010;45(7):1181–1188. doi: 10.1038/bmt.2009.323. [DOI] [PubMed] [Google Scholar]
- 57.•• Nigrovic PA, Lee PY, Hoffman HM. Monogenic autoinflammatory disorders: conceptual overview, phenotype, and clinical approach. J Allergy Clin Immunol. 2020;146(5):925–937. 10.1016/j.jaci.2020.08.017. Detailed, well-structured and comprehensive overview about the monogenic autoinflammatory disorders. [DOI] [PMC free article] [PubMed]
- 58.• Demir S, Sag E, Dedeoglu F, Ozen S. Vasculitis in systemic autoinflammatory diseases. Front Pediatr. 2018;6:377. 10.3389/fped.2018.00377. Well-structured and comprehensive overview about vasculitis in systemic autoinflammatory diseases. [DOI] [PMC free article] [PubMed]
- 59.Ozen S, Ben-Chetrit E, Bakkaloglu A, Gur H, Tinaztepe K, Calguneri M, et al. Polyarteritis nodosa in patients with Familial Mediterranean Fever (FMF): a concomitant disease or a feature of FMF? Semin Arthritis Rheum. 2001;30(4):281–287. doi: 10.1053/sarh.2001.19958. [DOI] [PubMed] [Google Scholar]
- 60.Khatibi K, Heit JJ, Telischak NA, Elbers JM, Do HM. Cerebral vascular findings in PAPA syndrome: cerebral arterial vasculopathy or vasculitis and a posterior cerebral artery dissecting aneurysm. J Neurointerv Surg. 2016;8(8):e29. doi: 10.1136/neurintsurg-2015-011753.rep. [DOI] [PubMed] [Google Scholar]
- 61.Videira G, Malaquias MJ, Laranjinha I, Martins R, Taipa R, Magalhães M. Diagnosis of Aicardi-Goutières syndrome in adults: a case series. Mov Disord Clin Pract. 2020;7(3):303–307. doi: 10.1002/mdc3.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thiele H, du Moulin M, Barczyk K, George C, Schwindt W, Nürnberg G, et al. Cerebral arterial stenoses and stroke: novel features of Aicardi-Goutières syndrome caused by the Arg164X mutation in SAMHD1 are associated with altered cytokine expression. Hum Mutat. 2010;31(11):E1836–E1850. doi: 10.1002/humu.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staels F, Betrains A, Doubel P, Willemsen M, Cleemput V, Vanderschueren S, et al. Adult-onset ANCA-associated vasculitis in SAVI: extension of the phenotypic spectrum, case report and review of the literature. Front Immunol. 2020;11:575219. doi: 10.3389/fimmu.2020.575219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2016;48(1):67–73. doi: 10.1038/ng.3459.Firstdescriptionofthisnoveldisorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aeschlimann FA, Batu ED, Canna SW, Go E, Gül A, Hoffmann P, et al. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann Rheum Dis. 2018;77(5):728–735. doi: 10.1136/annrheumdis-2017-212403. [DOI] [PubMed] [Google Scholar]
- 66.McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3(8):e297. doi: 10.1371/journal.pmed.0030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yavne Y, Tiosano S, Ben-Ami D, Watad A, Guy A, Comaneshter D, et al. Giant cell arteritis and inflammatory bowel disease - is there a connection? Results from a population-based study. Autoimmun Rev. 2018;17(11):1134–1137. doi: 10.1016/j.autrev.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 68.Moiseev S, Cohen Tervaert JW, Arimura Y, Bogdanos DP, Csernok E, et al. 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun Rev. 2020;19(9):102618. doi: 10.1016/j.autrev.2020.102618. [DOI] [PubMed] [Google Scholar]
- 69.Humbert S, Guilpain P, Puéchal X, Terrier B, Rivière S, Mahr A, et al.; French Vasculitis Study Group. Inflammatory bowel diseases in anti-neutrophil cytoplasmic antibody-associated vasculitides: 11 retrospective cases from the French Vasculitis Study Group. Rheumatology (Oxford). 2015;54(11):1970–5. 10.1093/rheumatology/kev199. [DOI] [PubMed]
- 70.Bernstein CN, Eliakim A, Fedail S, Fried M, Gearry R, et al. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: update August 2015. J Clin Gastroenterol. 2016;50(10):803–818. doi: 10.1097/MCG.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 71.Valenti S, Gallizzi R, De Vivo D, Romano C. Intestinal Behçet and Crohn’s disease: two sides of the same coin. Pediatr Rheumatol Online J. 2017;15(1):33. doi: 10.1186/s12969-017-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vavricka SR, Greuter T, Scharl M, Mantzaris G, Shitrit AB, Filip R, et al. Cogan’s syndrome in patients with inflammatory bowel disease -a case series. J Crohns Colitis. 2015;9(10):886–890. doi: 10.1093/ecco-jcc/jjv128. [DOI] [PubMed] [Google Scholar]
- 73.Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. The ever-increasing array of novel inborn errors of immunity: an interim update by the IUIS Committee. J Clin Immunol. 2021;41(3):666–679. doi: 10.1007/s10875-021-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill F, Yonkof J, Chaitanya Arudra SK, Thomas J, Altorok N. Successful treatment of ANCA-associated vasculitis in the setting of common variable immunodeficiency using rituximab. Am J Ther. 2016;23(5):e1239–e1245. doi: 10.1097/MJT.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 75.Najem CE, Springer J, Prayson R, Culver DA, Fernandez J, Tavee J, Hajj-Ali RA. Intra cranial granulomatous disease in common variable immunodeficiency: case series and review of the literature. Semin Arthritis Rheum. 2018;47(6):890–896. doi: 10.1016/j.semarthrit.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Pagnini I, Simonini G, Lippi F, Azzari C, Cimaz R. Cutaneous polyarteritis nodosa and common variable immunodeficiency: a previously unreported association. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S169. [PubMed] [Google Scholar]
- 77.• Schepp J, Proietti M, Frede N, Buchta M, Hübscher K, Rojas Restrepo J, et al. Screening of 181 patients with antibody deficiency for deficiency of adenosine deaminase 2 sheds new light on the disease in adulthood. Arthritis Rheumatol. 2017;69(8):1689-1700. 10.1002/art.40147. A reminder to think of ADA-2-deficiency in patients with antibody deficiencies. [DOI] [PubMed]
- 78.Ochs HD, Filipovich AH, Veys P, Cowan MJ, Kapoor N. Wiskott-Aldrich syndrome: diagnosis, clinical and laboratory manifestations, and treatment. Biol Blood Marrow Transplant. 2009;15(1 Suppl):84–90. doi: 10.1016/j.bbmt.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Fayand A, Chasset F, Boutboul D, Queyrel V, Tieulié N, Guichard I, et al. DADA2 diagnosed in adulthood versus childhood: a comparative study on 306 patients including a systematic literature review and 12 French cases. Semin Arthritis Rheum. 2021;51(6):1170–1179. doi: 10.1016/j.semarthrit.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Staels F, Betrains A, Willemsen M, Corvelyn A, Tousseyn T, Dierickx D, et al. Inflammatory aortitis in a patient with type 2 hyper IgM syndrome. Rheumatology (Oxford) 2021;60(3):e87–e89. doi: 10.1093/rheumatology/keaa573. [DOI] [PubMed] [Google Scholar]
- 81.Maeshima K, Ishii K, Shibata H. An Adult Fatal Case with a STAT1 Gain-of-function mutation associated with multiple autoimmune diseases. J Rheumatol. 2019;46(3):325–327. doi: 10.3899/jrheum.180210. [DOI] [PubMed] [Google Scholar]
- 82.Yavuz H, Chee R. A review on the vascular features of the hyperimmunoglobulin E syndrome. Clin Exp Immunol. 2010;159(3):238–244. doi: 10.1111/j.1365-2249.2009.04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al.; inborn errors working party of EBMT. DOCK8 deficiency: clinical and immunological phenotype and treatment options - a review of 136 patients. J Clin Immunol. 2015;35(2):189–98. 10.1007/s10875-014-0126-0. [DOI] [PubMed]
- 84.Solans-Laqué R, Bosch-Gil JA, Pérez-Bocanegra C, Selva-O’Callaghan A, Simeón-Aznar CP, Vilardell-Tarres M. Paraneoplastic vasculitis in patients with solid tumors: report of 15 cases. J Rheumatol. 2008;35(2):294–304. [PubMed] [Google Scholar]
- 85.Fain O, Hamidou M, Cacoub P, Godeau B, Wechsler B, Pariès J, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473–1480. doi: 10.1002/art.23085. [DOI] [PubMed] [Google Scholar]
- 86.Ungprasert P, Sanguankeo A, Upala S, Knight EL. Risk of malignancy in patients with giant cell arteritis and polymyalgia rheumatica: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(3):366–370. doi: 10.1016/j.semarthrit.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025–2030. doi: 10.1093/annonc/mdu365. [DOI] [PubMed] [Google Scholar]
- 88.Obiorah IE, Patel BA, Groarke EM, Wang W, Trick M, Ombrello AK, et al. Benign and malignant hematologic manifestations in patients with VEXAS syndrome due to somatic mutations in UBA1. Blood Adv. 2021;5(16):3203–3215. doi: 10.1182/bloodadvances.2021004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Misra D, Patro P, Sharma A. Drug-induced vasculitis. Indian J Rheumatol. 2019;14(5):S3–9. [Google Scholar]
- 90.• Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep. 2015;17(12):71. 10.1007/s11926-015-0545-9. Well written overview about the broad spectrum of drug-induced vasculitis. [DOI] [PubMed]
- 91.Gutiérrez-González LA. Biological therapy-induced systemic vasculitis. Curr Rheumatol Rep. 2016;18(7):39. doi: 10.1007/s11926-016-0588-6. [DOI] [PubMed] [Google Scholar]
- 92.Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86(4):242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 93.Kunchok A, Aksamit AJ, Jr, Davis JM, 3rd, Kantarci OH, Keegan BM, Pittock SJ, et al. Association between tumor necrosis factor inhibitor exposure and inflammatory central nervous system events. JAMA Neurol. 2020;77(8):937–946. doi: 10.1001/jamaneurol.2020.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Felicetti P, Trotta F, Bonetto C, Santuccio C, Brauchli Pernus Y, Burgner D, et al. Spontaneous reports of vasculitis as an adverse event following immunization: a descriptive analysis across three international databases. Vaccine. 2016;34(51):6634–6640. doi: 10.1016/j.vaccine.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 95.Liozon E, Parreau S, Filloux M, Dumonteil S, Gondran G, Bezanahary H, et al. Giant cell arteritis or polymyalgia rheumatica after influenza vaccination: a study of 12 patients and a literature review. Autoimmun Rev. 2021;20(2):102732. doi: 10.1016/j.autrev.2020.102732. [DOI] [PubMed] [Google Scholar]
- 96.Mettler C, Jonville-Bera AP, Grandvuillemin A, Treluyer JM, Terrier B, Chouchana L. Risk of giant cell arteritis and polymyalgia rheumatica following COVID-19 vaccination: a global pharmacovigilance study. Rheumatology (Oxford) 2022;61(2):865–867. doi: 10.1093/rheumatology/keab756. [DOI] [PubMed] [Google Scholar]
- 97.Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, et al. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118(3):499–509. doi: 10.1182/blood-2011-01-325266. [DOI] [PubMed] [Google Scholar]
- 98.•• Zhang H, Watanabe R, Berry GJ, Vaglio A, Liao YJ, Warrington KJ, et al. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci U S A. 2017;114(6):E970-E979. 10.1073/pnas.1616848114. Important work linking immunoinhibitory checkpoint deficiency with vasculitis (GCA), providing pathophysiologic insight and opening new therapeutic approaches. [DOI] [PMC free article] [PubMed]
- 99.Watanabe R, Zhang H, Berry G, Goronzy JJ, Weyand CM. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am J Physiol Heart Circ Physiol. 2017;312(5):H1052–H1059. doi: 10.1152/ajpheart.00024.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.• Crout TM, Lennep DS, Kishore S, Majithia V. Systemic vasculitis associated with immune check point inhibition: analysis and review. Curr Rheumatol Rep. 2019;21(6):28. 10.1007/s11926-019-0828-7. Comprehensive overview of immune check point inhibitor–associated vasculitic manifestations that will become increasingly important for rheumatologists and immunologists in the future. [DOI] [PubMed]
- 101.Lidar M, Lipschitz N, Langevitz P, Shoenfeld Y. The infectious etiology of vasculitis. Autoimmunity. 2009;42(5):432–438. doi: 10.1080/08916930802613210. [DOI] [PubMed] [Google Scholar]
- 102.Haq SA, Pagnoux C. Infection-associated vasculitides. Int J Rheum Dis. 2019;22(Suppl 1):109–115. doi: 10.1111/1756-185X.13287. [DOI] [PubMed] [Google Scholar]
- 103.• Theofilis P, Vordoni A, Koukoulaki M, Vlachopanos G, Kalaitzidis RG. Overview of infections as an etiologic factor and complication in patients with vasculitides. Rheumatol Int. 2022;1–12. 10.1007/s00296-022-05100-9. Provides good explanation of mechanisms of infection-associated vasculitis and overview of well-studied infectious etiologies. [DOI] [PMC free article] [PubMed]
- 104.Berti A, Moura MC, Sechi E, Squizzato F, Costanzo G, Chen JJ, Warrington KJ. Beyond giant cell arteritis and Takayasu’s arteritis: secondary large vessel vasculitis and vasculitis mimickers. Curr Rheumatol Rep. 2020;22(12):88. doi: 10.1007/s11926-020-00965-w. [DOI] [PubMed] [Google Scholar]
- 105.Pagnoux C, Cohen P, Guillevin L. Vasculitides secondary to infections. Clin Exp Rheumatol. 2006;24(2 Suppl 41):S71–81. [PubMed] [Google Scholar]
- 106.• Younger DS, Coyle PK. Central nervous system vasculitis due to infection. Neurol Clin. 2019;37(2):441-463. 10.1016/j.ncl.2019.01.002. Comprehensive overview of infectious vasculitis of the central nervous system. [DOI] [PubMed]
- 107.Gauto AR, Cone LA, Woodard DR, Mahler RJ, Lynch RD, Stoltzman DH. Arterial infections due to Listeria monocytogenes: report of four cases and review of world literature. Clin Infect Dis. 1992;14(1):23–28. doi: 10.1093/clinids/14.1.23. [DOI] [PubMed] [Google Scholar]
- 108.Clouse WD, DeWitt CC, Hagino RT, DeCaprio J, Kashyap VS. Rapidly enlarging iliac aneurysm secondary to listeria monocytogenes infection: a case report. Vasc Endovascular Surg. 2003;37(2):145–149. doi: 10.1177/153857440303700211. [DOI] [PubMed] [Google Scholar]
- 109.Ahmed AOE, Babikir MMI, Khojali AEM, Arachchige SNM, Abdirahman AM, Mohamed MFH. Central nervous system vasculitis as a rare presentation of mycoplasma pneumoniae: a case report. Case Rep Neurol. 2020;12(3):402–409. doi: 10.1159/000510632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Delaval L, Goulenok T, Achouh P, Saadoun D, Gaudric J, Pellenc Q, et al. New insights on tuberculous aortitis. J Vasc Surg. 2017;66(1):209–215. doi: 10.1016/j.jvs.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 111.Roberts WC, Moore AJ, Roberts CS. Syphilitic aortitis: still a current common cause of aneurysm of the tubular portion of ascending aorta. Cardiovasc Pathol. 2020;46:107175. doi: 10.1016/j.carpath.2019.107175. [DOI] [PubMed] [Google Scholar]
- 112.Paulo N, Cascarejo J, Vouga L. Syphilitic aneurysm of the ascending aorta. Interact Cardiovasc Thorac Surg. 2012;14(2):223–225. doi: 10.1093/icvts/ivr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith JL, Israel CW, Harner RE. Syphilitic temporal arteritis. Arch Ophthalmol. 1967;78(3):284–288. doi: 10.1001/archopht.1967.00980030286005. [DOI] [PubMed] [Google Scholar]
- 114.De Brito T, Silva AMGD, Abreu PAE. Pathology and pathogenesis of human leptospirosis: a commented review. Rev Inst Med Trop Sao Paulo. 2018;60:e23. doi: 10.1590/s1678-9946201860023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gardini G, Zanotti P, Pucci A, Tomasoni L, Caligaris S, Paro B, et al. Non-typhoidal Salmonella aortitis. Infection. 2019;47(6):1059–1063. doi: 10.1007/s15010-019-01344-z. [DOI] [PubMed] [Google Scholar]
- 116.de Worm S, Giot JB, Courtoy C, Gillet E, Amrane S, Huynen P, et al. A case of giant cell arteritis associated with culture-proven Coxiella burnetii aortitis. Int J Infect Dis. 2018;69:50–54. doi: 10.1016/j.ijid.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 117.Odeh M, Oliven A. Temporal arteritis associated with acute Q fever. A case report. Angiology. 1994;45(12):1053–1057. doi: 10.1177/000331979404501209. [DOI] [PubMed] [Google Scholar]
- 118.Lefebvre M, Grossi O, Agard C, Perret C, Le Pape P, Raoult D, Hamidou MA. Systemic immune presentations of Coxiella burnetii infection (Q Fever) Semin Arthritis Rheum. 2010;39(5):405–409. doi: 10.1016/j.semarthrit.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Cascio A, Iaria C. Brucella aortitis: an underdiagnosed and under-reported disease. Int J Rheum Dis. 2014;17(7):825. doi: 10.1111/1756-185X.12449. [DOI] [PubMed] [Google Scholar]
- 120.Turkoglu SA, Halicioglu S, Sirmatel F, Yildiz M, Yildiz N, Yildiz S. Vasculitis and neurobrucellosis: Evaluation of nine cases using radiologic findings. Brain Behav. 2018;8(4):e00947. doi: 10.1002/brb3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Briere M, Kaladji A, Douane F, Breux JP, Touroult-Jupin P, Boisset S, et al. Francisella tularensis aortitis. Infection. 2016;44(2):263–265. doi: 10.1007/s15010-015-0824-4. [DOI] [PubMed] [Google Scholar]
- 122.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70(11):853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hauer L, Pikija S, Schulte EC, Sztriha LK, Nardone R, Sellner J. Cerebrovascular manifestations of herpes simplex virus infection of the central nervous system: a systematic review. J Neuroinflammation. 2019;16(1):19. doi: 10.1186/s12974-019-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fan TH, Khoury J, Cho SM, Bhimraj A, Shoskes A, Uchino K. Cerebrovascular complications and vasculopathy in patients with herpes simplex virus central nervous system infection. J Neurol Sci. 2020;419:117200. doi: 10.1016/j.jns.2020.117200. [DOI] [PubMed] [Google Scholar]
- 125.Kouchi M, Sato S, Kamono M, Taoda A, Iijima K, Mizuma A, et al. A case of polyarteritis nodosa associated with cytomegalovirus infection. Case Rep Rheumatol. 2014;2014:604874. doi: 10.1155/2014/604874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abadeer K, Aslam N, Cortese C, Wadei HM. Cytomegalovirus-induced polyarteritis nodosa in a liver transplant recipient. Am J Transplant. 2017;17(12):3236–3240. doi: 10.1111/ajt.14376. [DOI] [PubMed] [Google Scholar]
- 127.Vega LE, Espinoza LR. Vasculitides in HIV infection. Curr Rheumatol Rep. 2020;22(10):60. doi: 10.1007/s11926-020-00945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brand M, Woodiwiss AJ, Michel F, Nayler S, Veller MG, Norton GR. Large vessel adventitial vasculitis characterizes patients with critical lower limb ischemia with as compared to without human immunodeficiency virus infection. PLoS ONE. 2014;9(8):e106205. doi: 10.1371/journal.pone.0106205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guillevin L. Infections in vasculitis. Best Pract Res Clin Rheumatol. 2013;27(1):19–31. doi: 10.1016/j.berh.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Wang CR, Tsai HW. Human hepatitis viruses-associated cutaneous and systemic vasculitis. World J Gastroenterol. 2021;27(1):19–36. doi: 10.3748/wjg.v27.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeller V, Lortholary O. Vascularites secondaires aux infections fongiques [Vasculitis secondary to fungal infections]. Presse Med. 2004;33(19 Pt 2):1385–8. French. 10.1016/s0755-4982(04)98937-3. [DOI] [PubMed]
- 132.Huang J, Cano EJ, Shweta F, Shah AS, Schuetz AN, Bois M, Gurram PR. Infected aneurysm of the native aorta due to Coccidioides posadasii. Open Forum Infect Dis. 2021;8(6):ofab266. 10.1093/ofid/ofab266. [DOI] [PMC free article] [PubMed]
- 133.Brunner S, Engelmann MG, Näbauer M. Thoracic mycotic pseudoaneurysm from Candida albicans infection. Eur Heart J. 2008;29(12):1515. doi: 10.1093/eurheartj/ehm623. [DOI] [PubMed] [Google Scholar]
- 134.Roberts M, Carmichael A, Martin P. Cerebral vasculitis caused by Aspergillus species in an immunocompetent adult. Infection. 2004;32(6):360–363. doi: 10.1007/s15010-004-3077-1. [DOI] [PubMed] [Google Scholar]
- 135.Varghese B, Ting K, Lopez-Mattei J, Iliescu C, Kim J, Kim P. Aspergillus endocarditis of the mitral valve with ventricular myocardial invasion, cerebral vasculitis, and intracranial mycotic aneurysm formation in a patient with hemophagocytic lymphohistiocytosis. Med Mycol Case Rep. 2018;21:49–51. doi: 10.1016/j.mmcr.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marquez JM, Arauz A. Cerebrovascular complications of neurocysticercosis. Neurologist. 2012;18(1):17–22. doi: 10.1097/NRL.0b013e31823d7a80. [DOI] [PubMed] [Google Scholar]
- 137.Meliou M, Mavridis IN, Pyrgelis ES, Agapiou E. Toxocariasis of the nervous system. Acta Parasitol. 2020;65(2):291–299. doi: 10.2478/s11686-019-00166-1. [DOI] [PubMed] [Google Scholar]
- 138.Depascale R, Pizzi M, Padoan R. Whipple’s aortitis. Rheumatology (Oxford). 2022;keac042. 10.1093/rheumatology/keac042. [DOI] [PubMed]
- 139.Guillevin L. Vasculitides in the context of HIV infection. AIDS. 2008;22(Suppl 3):S27–33. doi: 10.1097/01.aids.0000327513.53255.17. [DOI] [PubMed] [Google Scholar]
- 140.Abendroth A, Slobedman B. Varicella-zoster virus and giant cell arteritis. J Infect Dis. 2021;223(1):4–6. doi: 10.1093/infdis/jiaa567. [DOI] [PubMed] [Google Scholar]
- 141.Kang R, Tanaka TD, Ogasawara Y, Yoshimura M. A rare complication of chronic active Epstein-Barr virus infection. JACC Case Rep. 2020;2(5):756–759. doi: 10.1016/j.jaccas.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jamal O, Sahel N, Saouab R, El Qatni M, Zaizaa M, El Kassimi I, et al. Fatal systemic vasculitis associated with chronic active Epstein-Barr virus infection. Mo Med. 2021;118(3):226–232. [PMC free article] [PubMed] [Google Scholar]
- 143.Douvoyiannis M, Litman N, Goldman DL. Neurologic manifestations associated with parvovirus B19 infection. Clin Infect Dis. 2009;48(12):1713–1723. doi: 10.1086/599042. [DOI] [PubMed] [Google Scholar]
- 144.Patel P, DeCuir J, Abrams J, Campbell AP, Godfred-Cato S, Belay ED. Clinical characteristics of multisystem inflammatory syndrome in adults: a systematic review. JAMA Netw Open. 2021;4(9):e2126456. doi: 10.1001/jamanetworkopen.2021.26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Becker RC. COVID-19-associated vasculitis and vasculopathy. J Thromb Thrombolysis. 2020;50(3):499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oda R, Inagaki T, Ishikane M, Hotta M, Shimomura A, Sato M, et al. Case of adult large vessel vasculitis after SARS-CoV-2 infection. Ann Rheum Dis. 2020;annrheumdis-2020–218440. 10.1136/annrheumdis-2020-218440. [DOI] [PubMed]
- 147.Dhakal P, Khadka S, Clowes JA, Chakinala RC. Aortitis in COVID-19. IDCases. 2021;24:e01063. doi: 10.1016/j.idcr.2021.e01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Manenti A, Farinetti A, Manco G, Mattioli A. Vasculitis and aortitis: COVID-19 challenging complications. J Vasc Surg. 2021;73(1):347–348. doi: 10.1016/j.jvs.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harroud A, Almutlaq A, Pellerin D, Paz D, Linnell GJ, Gendron D. West Nile virus-associated vasculitis and intracranial hemorrhage. Neurol Neuroimmunol Neuroinflamm. 2019;7(1):e641. doi: 10.1212/NXI.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.