Abstract
Chicken coccidiosis is an economically significant disease of commercial chicken industry accounting for losses of more than £10.4 billion (according to 2016 prices). Additionally, the costs incurred in prophylaxis and therapeutics against chicken coccidiosis in developing countries (for instance Pakistan according to 2018 prices) reached US $45,000.00 while production losses for various categories of chicken ranges 104.74 to US $2,750,779.00. The infection has been reported from all types of commercial chickens (broiler, layer, breeder) having a range of reported prevalence of 7–90%. The concern of resistance towards major anticoccidials has provided a way forward to vaccine research and development. For prophylaxis of chicken coccidiosis, live virulent, attenuated, ionophore tolerant strains and recombinant vaccines have been extensively trialed and commercialized. Eimeria antigens and novel vaccine adjuvants have elicited the protective efficacy against coccidial challenge. The cost of production and achieving robust immune responses in birds are major challenges for commercial vaccine production. In the future, research should be focused on the development of multivalent anticoccidial vaccines for commercial poultry. Efforts should also be made on the discovery of novel antigens for incorporation into vaccine designs which might be more effective against multiple Eimeria species. This review presents a recap to the overall progress against chicken Eimeria with particular reference to previous decade. The article presents critical analysis of potential areas for future research in chicken Eimeria vaccine development.
Keywords: Eimeria, Vaccine, Poultry, Antigens, Control
Introduction
The chicken industry is a rapidly developing enterprise around the globe. One of the foremost concerns of poultry industry is meeting the ever-rising competitive edge (Bosila et al. 2021). The overall flock health and performance is a critical parameter leading to higher economic returns (Abu et al. 2022). It is important to look up for and counter both clinical and sub-clinical forms of diseases in chicken (Davou et al. 2018; Iraqi et al. 2021; Tahir et al. 2021). Chicken coccidiosis is a disease of commercial poultry holding high economic value (Shahid et al. 2020; Lee et al. 2022). Coccidiosis in chicken causes sub-clinical infections as well as mortalities in persistently infected flocks. The disease can lead to morbidity reflected as increased feed conversion ratios, reduced weight gains, drop in production, lower reproductive performance, continuous Eimeria oocyst shedding, and increased susceptibility towards secondary bacterial infections (Gerhold 2016; Kadykalo et al. 2018; Venkatas and Adeleke 2019). Chicken coccidiosis accounts for more than £10.4 billion (according to 2016 prices) losses annually and has a variable prevalence ranging 7–90%, around the globe (Dalloul and Lillehoj 2006; Guven et al. 2013; Gyorke et al. 2013; Rashid et al. 2019; Blake et al. 2020a). Most recently, the overall economic loss owing to chicken coccidiosis was calculated to be UK £99.2 million (2016 prices) for vaccine, treatment, and other preventive measures’ cost (Blake et al. 2020a). Moreover, it was estimated that there is a range of losses in different categories of chicken ranging US $104.74 to US $2,750,779.00 (Rashid et al. 2019). The economic burden due to production losses indicates the prime value of timely and effective Eimeria vaccines in sparing the economic burden thus created.
It is imperative to recognize at least 7 chicken Eimeria species that have, so far, been recognized as responsible for intestinal disease. These include Eimeria (E.) praecox, E. acervulina, E. mitis, E. brunetti, E. tenella, E. maxima, and E. necatrix (Clark et al. 2017; Idris et al. 2017; Abbas et al. 2019). Among all species, E. maxima, E. tenella, E. acervulina, and E. necatrix have reportedly highest clinical and sub-clinical disease burden, making them economically significant among Eimeria species of poultry (Gerhold 2016; Hinsu et al. 2018). The control options for coccidiosis in chicken have been a mainstay in commercial poultry farming, since almost 100 years (Blake et al. 2017). Prevention and control options like the use of anticoccidials, coccidiostats, anticoccidial vaccines, herbal extracts, plant-derived immunomodulatory agents, essential oils, nano-particles, feed additives, probiotics, and many bioactive compounds, coupled with an extensive list of good farm hygiene and biosecurity practices have been established so far (Dkhil and Al-Quraishy 2016; Awais et al. 2018; Craig et al. 2020; Moryani et al. 2021).
Over many decades, Eimeria vaccines have gained a considerable importance, by virtue of effectively preventing the disease without developing resistance in field strains as in anticoccidial chemotherapeutics (Sander et al. 2019; Liu et al. 2020; Zhao et al. 2020). Rather, the sensitivity towards previously resistant chemical anticoccidials has been enhanced by the use of Eimeria vaccines (Jenkins et al. 2010). Examples of some commercial Eimeria vaccines for chicken include Endrex®, Hipracox®, CoxAbic® (vaccine capable for transmitting maternally derived antibodies from breeders), Hatchpakcocci III® (vaccine available at USA having precocious strains of 3 most important broiler Eimeria), Coccivac ®, Livacox ®, Paracox ®, etc.
Many vaccine candidates for Eimeria have been validated in both lab and animal trials (Ma et al. 2011; Gerhold 2016; Venkatas and Adeleke 2019; Khater et al. 2020). “Paracox® 8” (MSD Animal Health, Madison, NJ, USA) vaccine had been in practice since 1991 to 2015 as the only vaccine in most of the European Union countries. The vaccine which consisted of 8 precocious strains of Eimeria maxima (CP and MFP strain), E. acervulina, E. tenella, E. mitis, E. necatrix, E. praecox, and E. brunetti (MSD Animal Health, 2016) was used both by breeder and by layer producers. Later, two new vaccines were added to prevent disease by Hipra company (Amer, Girona, Spain) and Huvepharma company (Huvepharma, Sophia, Bulgaria). The former company prepared “Hipra Evalon® (E)” containing 5 precocious strains, viz, E. maxima, E. tenella, E. acervulina, E. necatrix, and E. brunetti (Hipra, 2016). The latter company launched “Huveguard® Start” (now named Mmat) containing four precocious strains viz a viz E. acervulina, E. mitis, E. maxima, and E. tenella for its administration today old chick (Huvepharma 2016a). A separate product named “Huveguard® Plus” (now named as NB) was launched with E. necatrix and E. brunetti strains and tended to be given at the 14th day of age (Huvepharma 2016b). These vaccines provided considerable protection to both breeder and layer replacement. The third type of vaccines is non-attenuated vaccines consisting of parasites Eimeria which were not processed with a reduced pathogenicity in the laboratory. Salient examples of such vaccines are Advent™, Inovocox™, Immucox®, and Coccivac® (Chapman et al. 2002). Coccivac® consists of E. acervulina, E. maxima, E. maxima, E. mivati, E. praecox, and E. tenella; Advent™ comprised of E. acervulina, E. maxima, and E. tenella; Inovocox™ was prepared from E. acervulina, E. maxima, E. maxima, and E. tenella; and Immucox® is made of E. acervulina, E. maxima, and E. tenella (Peek and Landman 2011; Price 2012).
The Eimeria vaccines have been developed keeping in view the commercial demands for the control of Eimeria. The potential areas for addressing chicken coccidiosis in commercial flocks need to be focused on terms of cost-effective vaccine production and commercialization on practical basis. The academia and industry linkages should be strengthened to bring forward the commercialization of better vaccinal strains (based on indigenous Eimeria) and integrated strategies against chicken coccidiosis. This paper presents an updated review on development of Eimeria vaccines and future opportunities in the sector.
The antigenic diversity of chicken Eimeria
The Eimeria parasites are obligate, intracellular, apicomplexan parasites belonging to Eimeridae family and genus Eimeria (Brown-Jordan et al. 2018). The epizootiology of chicken Eimeria has shown a wide range of variability of 11–92% (Gyorke et al. 2013; Rashid et al. 2019) and a great antigenic diversity, exhibiting host specificity (Kadykalo et al. 2018). Eimeria have been successfully evading immune systems of hosts including chicken, turkey, pheasant, camel, goat, cattle, sheep, rabbit, mice, fish, and reptiles (Mohsin et al. 2021). Pathogenic species of Eimeria may cast negative trends to overall health and performance of affected species (Conway et al. 1990; Abbas et al. 2011). Infection may elicit symptoms of reduced appetite, lethargy, watery or pasty feces, and appearance of blood in droppings (bloody coccidiosis) (Abbas et al. 2012; Wajiha and Qureshi 2021). Some strains of poultry coccidiosis (e.g., E. maxima) may potentiate the other gut pathogens like Salmonella and Clostridium species (Immerseel et al. 2004). Mixed infection with Eimeria may alter the gut microbiome altogether, damaging the mucosa of intestinal layers (Prescott et al. 2016). The prevalence of Eimeria and the protein meals used in feed have been shown as two risk factors working simultaneously for Clostridial infections in chicken. This protein-rich environment may predispose its host to necrotic enteritis even in the presence of a mild coccidial infection.
Eimeria shows resistance towards varying environmental conditions owing to the hardy nature of oocysts (Jeffers 1974; Zhu et al. 2000). The merozoite stages are the re-infective stages of Eimeria (Rani et al. 2021). Sporozoites invade the intestines (enterocytes) of the host, which leads to appearance of clinical signs usually during the second round of sporozoite replication in chicken.
The parasite may exhibit antigenic variants having lower cross protection among species (Blake et al. 2017). Eimeria, being distinguished from other apicomplexans, possesses exceptionally unique antigenic variation by virtue of retrotransposons that make them a house for several chromoviruses (Morris and Gasser 2006; Reid et al. 2014). These transposons do not have potential of horizontal transfer as the chicken host’s genome lack chromoviruses. This is important as it could help explore Eimeria biology using genomic screens. Moreover, attempts have been made to develop attenuated vaccine wherein issues with being specific in sequence targets were reported (Su et al. 2012).
The natural antigenic diversification and associated disease challenge has been countered by inclusion of more than one strain in the vaccine preparations. Also, E. maxima being the most immunogenic species of Eimeria is a potential vaccine candidate option for antigen isolation and research (Tang et al. 2018). Also, the profilins of Eimeria tenella have been exploited to develop vaccine adjuvants, capable of enhancing immune protection against Toxoplasma gondii (apicomplexan parasite) (Hedhli et al. 2009).
A detailed study on the connection of antigenic, genetic, and population diversity of Eimeria tenella with vaccine development revealed the disparity of genetic diversity in other closely related apicomplexans (Blake et al. 2015). Sampling from commercial chicken farms representing 5 different continents suggested high level of Eimeria interbreeding despite great genetic distances between the geographical regions, signposting the lack of Eimeria movement and/or balancing selection. The diversity of nucleotides within immune mapped protein 1 (IMP-1) gene of E. tenella in the USA, UK, China, and India was found to be low (Kundu et al. 2017). The source of nucleotide diversity was owing to repetition of cag triplets and 5 substitutions, 3 of them being non-synonymous. The study is indicative of variable genetic diversity of different Eimeria species from various geographical regions, owing to some contraction/expansion in the nucleotide sequences. Moreover, based on the genetic exploration and likely promise of apical membrane antigen-1 (AMA1) as vaccine candidates against E. tenella infections, the study suggested to employ the combination of 2 or more antigens for targeting each Eimeria specie. Reports of unforeseen differences in the genome have increased research focus to comprehend and design vaccines against Eimeria (Blake et al. 2020b). Similarly, a detailed account regarding the genetic diversity of other Eimeria species and its relevance in field challenge on a global level is required.
The host-parasite immunological relationship
A substance capable of inducing an immunological response in the host is known as antigen. Parasites also have a number of antigens, which are recognizable by the host. Eimeria like other pathogens goes through nonspecific immune response as well as specific. The non-specific consists of physical barriers, and cellular response—leukocytes, phagocytes, and complements. Specific immune response consists of either antibody production (humoral) or cell-mediated response (cellular) against specific antigen of pathogen. The latter involves T lymphocytes, natural killer cells, and phagocytes (Kim et al. 2019). Owing to cross-reactivity, there are several immunoglobulin responses that are not restricted to Eimeria antigens only. Experiments have demonstrated the stimulation of lymphocytes with complex oocyst antigen preparations that were produced after Eimeria infection (Yun et al. 2000). Analysis for species specificity suggests that the adaptive immune response can identify multiple antigens and the species cross-reactive epitopes may be non-protective (Khalafalla et al. 2011). This interaction is not very simple and depends upon factors like genetic makeup of host, parasite involved, and disease history (Mathis et al. 2018).
Eimeria overcomes non-specific defense mechanism of host that comprises of gastric secretions, lysozymes, mucous layer, etc. in the intestine (Figs. 1 and 2). Upon cracking the first line of defence, it faces resistance from mucosa-associated lymphoid tissue (MALT) as the second line of defence. Coupled with MALT, another important component of immunity called gut-associated lymphoid tissue hinders Eimeria by activating both humoral and cellular immune response. Eimeria in its progression invades into host cell with the help of microneme protein (Huang et al. 2015). A formal protocol of immune system starts here at the verge of immune cells like antigen-presenting cells, macrophages, and dendritic cells. These cells process and present antigenic molecules of pathogen to the host for final recognition by lymphocyte particularly T-lymphocytes (Min et al. 2013). Briefly, the process of production of immune response further finds humoral response in the form of Ig M, Ig Y, and Ig A which is in the case of Eimeria, a weak response. Cell-mediated immunity thus becomes a source of control of this disease (Yun et al. 2000). In this situation, cytokines like interferon molecules and interleukins help mediate differentiation of resting T-helper cells (Th0) into Th1 and Th2. Th1 is specific for resolving intracellular pathogens whereas Th2 is specific for extracellular (López-Osorio et al. 2020). Th1 cells produce interferons like IFN-γ that kills parasites and limits its multiplication. Th2 cells produce interleukin like IL2, IL4, and IL10. IL2 is a growth factor for different cells and an increased amount has been found in E. acervulina infection. Similarly, other immune modulators produced by T cells are IL6, IL8, IL12-13, and IL15-18; out of these, IL-17 has been highlighted recently (Kim et al. 2019). Also, the granulocyte–macrophage colony-stimulatory factor, tumor necrosis factor (TNF)-α, T-reg cells, lipopolysaccharide-induced TNF-α factor, TNF-α super family 15, transforming growth factor (TGF)-β, and TLR4 and TLR15 have been found to play roles in immunological responses (Zhou et al. 2013). Recently, a decoy receptor namely the tumor necrosis factor receptor superfamily member-6B has been shown to play a vital role in promoting inflammatory and other immune pathways in chicken against coccidiosis (Guo et al. 2020).
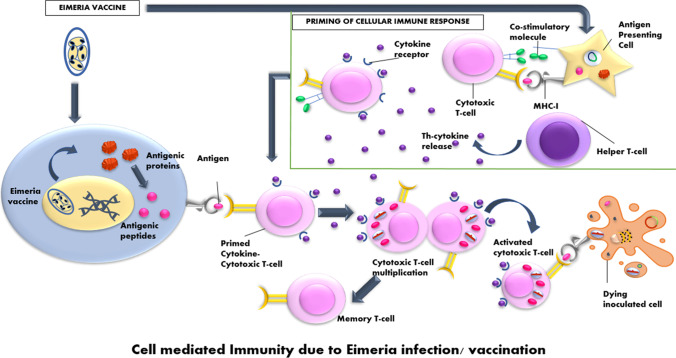
Fig. 1.
Cell-mediated immunity due to Eimeria infection/vaccination. Cellular immune response priming takes place when Eimeria is taken to antigen-presenting cells. Antigenic proteins from Eimeria parasite are converted into peptides that bind with the cytokine primed cytotoxic T cells. These cytotoxic T cells either get activated and start inoculating the cells for death or may lead to formation of memory T cells
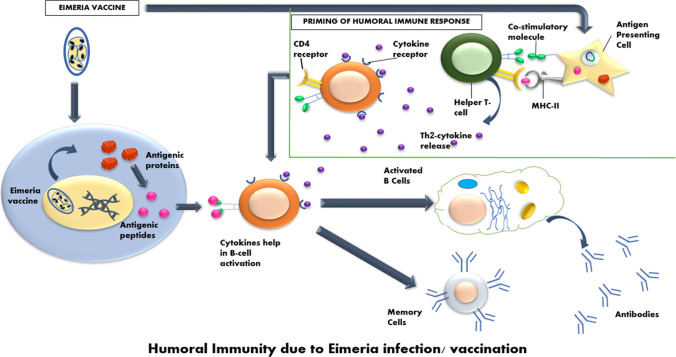
Fig. 2.
CD4/T-helper immunity in Eimeriosis. Humoral immune response priming takes place when Eimeria is taken to antigen-presenting cells. Antigenic proteins from Eimeria parasite are converted into peptides that bind with the cytokine primed helper T cells. The cytokine release help in B cell activation and release of antibodies or they may form memory cells for future immunological response on Eimeria challenge
Typically, the toll-like receptors bind antigen, leading to cellular pathway activation that subsequently activates innate immune players like interleukins and interferons (Mansilla and Capozzo 2017). The Eimeria genome encodes for thousands of proteins at a single time, rendering the entire profile and prediction of complete immunogenic ability difficult (Reid et al. 2014). Phenomenon of host-parasite interaction in Eimeria is therefore highly unpredictive and isolation of the protective antigens remains elusive. It is mainly because the bioassays are limited to extracellular stages of the parasite and partially owing to lack of immunoprotection against some proteins that are considered useful. However, these issues are not restricted to Eimeria species only, and the development of vaccine against certain viruses, bacteria, and protozoan faces a similar problem of antigenic complexity. A study identified four novel, two known, and one unknown gene that encode for immune-protective antigens of E. maxima (Yang et al. 2017). The strategy revolves around genetics, and immunology, it focuses on candidate Eimeria antigens showing immunoprotection in the host and a CDNA library resource intended for screening T cells that influence target antigens in this study.
Eimeria vaccination challenges and other alternative vaccine designs: a recap
Non-judicious use of anticoccidial treatments resulted in resistance development in poultry (Abbas et al. 2015). Also, the sub-clinical infections in poultry can cast a sharp decrease in the production and performance parameters of the birds (Abbas et al. 2019). The pathogenic potential of Eimeria encourages the need for application of vaccine at an early age in chicken. The registration and production of first live vaccine named CocciVac® was achieved in 1952, on a commercial scale in the USA. For immunization of chickens, two types of vaccines are commonly used, i.e., attenuated and virulent vaccines. These vaccines target different Eimeria species and have different routes of administration. Attenuated vaccines have a lower reproducibility and immunogenic potential. Additionally, the production cost of attenuated vaccines is higher from the commercial standpoint. The virulent vaccines on the other hand may be anticoccidial sensitive strains. They may have the ability to decrease level of resistance in diverse coccidial populations owing to lesser genetic selection pressure in contrast with the recombinant vaccines (Blake et al. 2017). On the other hand, the virulent vaccines may lead to the clinical appearance of the disease or may exacerbate other pathogens. One of the major constraints in producing live chicken coccidiosis vaccines is the need to add controlled oocyst doses from all pathogenic species of Eimeria, making vaccine production demanding (labour and technicality wise) and non-economical (Soutter et al. 2020). Attenuated vaccines have been conventionally used to prevent chicken Eimeria in commercial settings. Cost of production, time- and labor-intensive passaging, associated disease challenge, and probability of developing resistance, however, limit their utilization. Moreover, the time and resources required for quality assurance to make vaccine batches of the same standards further limit the practicality of attenuated vaccines.
Immunization with live, attenuated vaccine has been a traditional way of Eimeria prevention compared with other prophylactic or therapeutic options (Panebra and Lillehoj 2019). However, non-attenuated vaccines are more popular in some countries like the USA. It is accompanied by the risk of unwanted infection, lower reproduction index of Eimeria, variable immune response, and limited production capacity (Blake 2015). Substitutive vaccination approach is shown by subunit protein vaccines. Experiments have been conducted to identify and purify immunogenic proteins from different stages of Eimeria life cycle and immunogenic trials have given variable results (Dalloul and Lillehoj 2006). A major hurdle in subunit vaccine preparation is the absence of cross-immunity against other Eimeria species (Ahmad et al. 2016). Proteins isolated from one species are unable to protect against others. Therefore, third-generation vaccines featuring DNA or RNA material are being prioritized over second-generation (sub-unit) and first-generation (live/attenuated/killed) vaccines for Eimeria control (Mathis et al. 2018). Production and use of DNA vaccines is depicted in Fig. 3. The studies on molecular basis of immunological mediators in the face of coccidiosis challenge are warranted (Rothwell et al. 2004). This approach can bring forward the promising innate vaccine adjuvants as future recombinant vaccines against homologous or heterologous Eimeria. As indicated by the pitfalls of attenuated vaccines, recombinant vaccine research and development for chicken Eimeria is required. Various Eimeria antigens have been isolated and cloned from different parts and life-cycle stages of the pathogenic strains including AMA-1, cSZ-JN1, cSZ-JN2, EF-1α, Em6, Em8, Eimeria gametocyte antigens, EMHP-1, EMHP-2, EmCKRS, EmJS-1, Em14-3-3, EMRP, EmSAG, EtMIC1, EtMIC2, Gam 82, GAPDH, IMPI, LDH, MICs, MIF, NA-4, NPmz19, rEtSO7, TA4, etc. for testing as recombinant Eimeria vaccine candidates (Williams 2002; Song et al. 2015a; Lin et al. 2017; Jenkins et al. 2018). A brief overview of chicken Eimeria vaccines, from the past 10 years, is summarized in Table 1. Attenuated vaccines have lower practicality in terms of maintenance of cold storage line throughout rendering the costs of production and administration higher. Live vaccines have multiple risks like development of severe reaction among birds and alteration of resistance in coccidial species (Mathis et al. 2018). Since live vaccines replicate within host cells, they may result in subclinical coccidiosis. The immune response is augmented by re-infection in the case of live vaccine administration. Attenuated vaccines may exhibit lower pathogenicity, but usually high costs are incurred during development. Combination regime of administering attenuated and non-attenuated species has sorted out these problems to some extent (Jenkins et al. 2018). However, the need to chalk out the promising strategy for control of coccidiosis still remains inconclusive.
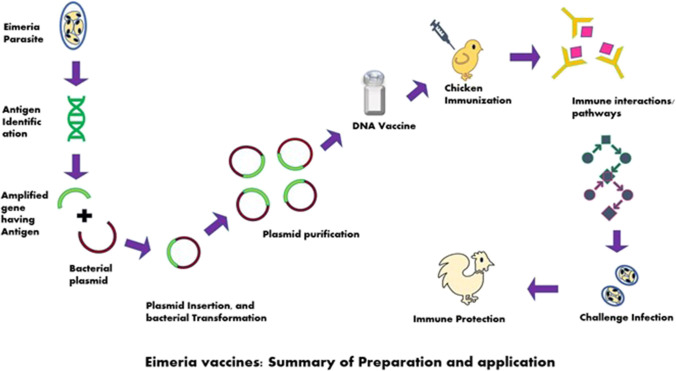
Fig. 3.
Eimeria vaccines: summary of preparation and application. The genes from target antigen are amplified, introduced to the plasmid. Insertion and bacterial transformation take place. The transgenic plasmid is purified to form a vaccine. Chickens are immunized with the dose of vaccine. Immunological interactions take place within host body and provide immune protection in the face of disease
Table 1.
Summary of chicken Eimeria vaccines during previous decade
| Target Eimeria specie (E.) | Antigen/ Antibody/ Protein |
Type of Vaccine | Route of Administration | Vaccinal Response | References |
|---|---|---|---|---|---|
| E. acervulina | LDH, 3-1E & MIF | Multivalent, Subunit | Intramuscular | Partial protection | Song et al. 2015a |
| LDH | DNA | Intramuscular | Protective immunity | Song et al. 2010a, b | |
| Profilin & QCDC | Recombinant | Subcutaneous | Protective immunity | Lee et al. 2010 | |
| Profilin with IMS 1313 or ISA 71 | Recombinant-nano vaccine | Oral | Protective immunity | Jang et al. 2011 | |
| 3-1E and chicken IL-15 | Recombinant | Intramuscular | Partial protection | Ma et al. 2011 | |
| cSZ-JN1, cSZ-JN2 | Recombinant DNA | Intramuscular | Partial protection | Zhu et al. 2012a, b | |
| EaMIC5 | DNA | Subcutaneous | Partial protection | Zhang et al. 2014 | |
| ADF-3-1E | Recombinant DNA | Intramuscular | Protective immunity | Zhao et al. 2014 | |
| EF-1α/ chIL-7 with Montanide Gel 01 adjuvant | DNA | Intramuscular | Protective immunity | Panebra and Lillehoj 2019 | |
| E. maxima | Em6 & Em8 | Multivalent, Subunit | Intramuscular | Partial protection | Song et al. 2015b |
| NP-EMaxIMP1 | Nano-vaccine | Oral | Protective immunity | Jenkins et al. 2018 | |
| Profilin & QCDC | Recombinant | In-ovo | Protective immunity | Lee et al. 2010 | |
| Gam82 | Recombinant | Intramuscular, Oral | Protective immunity | Jang et al. 2010 | |
| Gam56 | DNA | Intramuscular | Protective immunity | Xu et al. 2013 | |
| EmMIC7 | DNA | Intramuscular | Protective immunity | Huang et al. 2015 | |
| EmSAG | Recombinant | Intramuscular | Moderate immunity | Liu et al. 2018b | |
| Et-EmAMA1 and/or Et-EmIMP1 | Vector | Oral | Enhanced immune protection | Tang et al. 2019 | |
| E. necatrix | NA4 & NPmz19 | Multivalent, Subunit | Intramuscular | Partial protection | Song et al. 2015a |
| Gam22 | Recombinant Subunit | Oral | Protective immunity | Liu et al. 2014 | |
| E. tenella | TA4 & SO7 genes | Multivalent, Subunit | Intramuscular | Partial protection | Song et al. 2015a |
| SO7 gene | DNA | Intramuscular | Protective immunity | Yang et al. 2010 | |
| TA4 & Chicken IL-2 | Chimeric DNA | Intramuscular | Protective immunity | Song et al. 2017 | |
| cSZ-2 (E. acervulina antigen) | Recombinant | Intramuscular | Partial protection | Shah et al. 2010 | |
| rBCG co-expressing rhomboid and chIL-2 gene | Recombinant |
Intra-nasal and Subcutaneous |
Protective immunity | Wang et al. 2014 | |
| Profilin | Recombinant nano-vaccine | Subcutaneous | Protective immunity | Zhang et al. 2012a | |
| SO7 & Chicken IL-2 | Chimeric DNA | Oral | Protective immunity | Song et al. 2013 | |
| Rhomboid | Recombinant | Injection followed by oral | Protective immunity | Liu et al. 2013 | |
| DC-derived exosomes (CD80, flotillin & HSP70, MHC-I and MHC- II) | Monovalent | Intramuscular | Protective immunity | del Cacho et al. 2011 | |
| EtHSP70 | Subunit | Subcutaneous | Enhanced protection | Zhang et al. 2012b | |
| EtHSP70+EtMIC2 | |||||
| rEtMIC-1 | Recombinant | Intramuscular | Partial protection | Qi et al. 2013 | |
| IMP1 | Subunit | Subcutaneous | Protective immunity | Yin et al. 2013 | |
| IMP1 with FliC | |||||
| EtMIC-1 (polypeptides-I, II, III) | Recombinant | Oral | Protective immunity | Chen et al. 2015 | |
| 3-1 E (protein) | Recombinant | Oral | Protective immunity | Lin et al. 2015 | |
| 5401(surface antigen) and chicken IFN-γ or IL-2 | Chimeric DNA | Intramuscular | Partial protection | Song et al. 2015a | |
| Serum exosomes | Serum derived | Intramuscular | Protective immunity | del Cacho et al. 2016 | |
| EtCHP559 | Recombinant | Intramuscular | Protective immunity | Zhai et al. 2016 | |
| EtMIC3 | Recombinant | Intramuscular | Protective immunity | Wang et al. 2017 | |
| EtAMA1 | Recombinant | Oral | Protective immunity | Li et al. 2019 | |
| Profilin (E. maxima) | Recombinant Vector | Cloacal Inoculation | Protective immunity | Tang et al. 2018 | |
| EtSO7 | Recombinant | Subcutaneous | Protective immunity | Rafiqi et al. 2018 | |
| MIC-2 | Recombinant | Intramuscular | Partial protection | Yan et al. 2018 | |
| EtAMA1 with L & C binding peptides | Recombinant | Oral | Partial protection | Ma et al. 2019 | |
| EtGam22 | Recombinant | Subcutaneous | Protective immunity | Rafiqi et al. 2019 | |
| EtAN1-ZnFP | Recombinant | Subcutaneous | Partial protection | Zhao et al. 2020 | |
|
Mixed Infections (E. acervulina, E. maxima, E. necatrix& E. tenella) |
TA4-1 and LDH-2- | Multivalent, epitope DNA | Intramuscular | Protective immunity | Song et al. 2015a |
| TA4-1-LDH-2 and IL-2 | Multivalent, epitope DNA | Intramuscular | Protective immunity | Song et al. 2015b | |
|
Mixed infections (E. tenella, E. maxima, & E. acervulina) |
Dendritic Cell derived exosomes | Polyvalent | Intramuscular | Protective immunity | del Cacho et al. 2012 |
| Tachyzoite gene (E. tenella & E. acervulina) and gametocyte gene of E. maxima | Multi-epitope DNA | Intramuscular | Partial protection | Ding et al. 2012 | |
| GAPDH | Multivalent DNA | Intramuscular | Protective immunity | Tian et al. 2017 | |
| 14–3-3 antigen | Multivalent DNA | Intramuscular | Protective immunity | Liu et al. 2018a | |
| E. tenella & E. maxima | EF-1α (E. tenella) | Subunit | Subcutaneous | Protective immunity | Lin et al. 2017 |
Live vaccines harness the ability of host cell–mediated immune response for replication and subsequent protection (Wajiha and Qureshi 2021). Normally, live oocyst vaccine consists of non-attenuated and attenuated parasites. When given orally, these vaccines give coccidial infection of low grade that generates mild immune response (Venkatas and Adeleke 2019). This protection is increased upon re-infection of parasite. Use of non-attenuated live vaccine for the Eimeria control has a major drawback of systemic reaction in poultry that may be associated with decreased bird performance. DNA vaccine has an advantage of stability because of its structural and chemical character and hence attracts scientists to direct their efforts in DNA vaccinology (Blake et al. 2017). The production process is easier with a negligible difference of quality among multiple vaccine batches (Song et al. 2017; Li et al. 2017). DNA vaccines are capable of inducing strong cellular and humoral immune response (Panebra and Lillehoj 2019; Rafiqi et al. 2019). Cytokine administration as adjutants can increase their potential of inducing long-lasting and broader immunity. However, immunity is restricted to homologous strains and there is lack of cross-protective immune response among different strains prevalent over different geographical regions.
The fact that viral proteins are expressed in E. tenella and recognized by immune system of birds has shown chances of developing multivalent vaccine vector (Marugan-Hernandez et al. 2016). The recent trends of research on Eimeria focus on transcriptomic, proteomic, and genomic analysis along with genotype diversity and phylogenetic mapping (Blake et al. 2015). These tools can help understand the parasite biology and the genetic diversity and help expand the spectrum of promising vaccine candidates. A newly discovered IMP-1 of E. maxima species is an effective immunogen (Blake et al. 2017). Vaccination against coccidiosis, using immune mapped protein 1 of E. tenella (EtIMP1), reduced the oocyst output up to 60%. Recently, the genome-wide transcriptomic analysis of highly virulent strains of E. tenella has revealed the upregulation of certain rhoptry kinases responsible for overwhelming signaling pathways and immunological responses in these virulent strains (Ribeiro et al. 2021). The transcriptomic data derived from the diverse antigenic profile of Eimeria species would be helpful in designing more efficient vaccine candidates. Additionally, it is imperative to look up for the cost of vaccine production, and vaccine response with a larger number of chicken when commercialization is the target of a certain research trial (Abbas et al. 2017). Vector vaccines also offer an option against coccidiosis. Development of vector vaccine covers three steps: (1) recombinant Eimeria selection system, (2) exogenous antigen-specific immune response, and (3) protection against heterologous pathogen. In first step, transfected sporozoites or merozoites are transferred to cloaca of chick, intravenous route, or meaningfully to the site where sporozoites or merozoites invade the intestine (Duan et al. 2019). It was found that within 5 generations, using single plasmid can give rise to more than 90% of transgenic bacteria. The process of several selection produces stable expression of exogenous gene. This exogenous protein can be integrated into genome of parasites. Although there exist some challenges, success rate is evident (Qin et al. 2014). For the second step, EYFP as model antigen has been applied for lymphocyte proliferation and expression of interferon gamma in CD4 and CD8 T cells, analyzed in transgenic Eimeria-immunized chickens. The findings in terms of EYFP-specific lymphocyte proliferation and stronger IgA production represents that heterologous antigen in recombinant Eimeria influences the immune system. Hence, heterologous recombinant antigen can be considered a significant factor for immune response. In the final step, it is desired from the recombinant vector vaccine that it can elicit higher level immune response against heterologous pathogen infection. In a study, E. tenella expressed Campylobacter jejuni vaccinal candidate (CjaA) was evaluated in chickens. The study concludes 91% protection against C. jejuni. Similarly, recombinant heterologous with Toxoplasma gondii also produced promising results. Hence, Eimeria heterologous vaccine can provide cross-protection (Tang et al. 2016; Clark et al. 2012).
Eimeria vector vaccine faces following major hurdles: (a) parasite hardly finishes its life cycle in in vitro tissue culture; (b) compared to other apicomplexans like Toxoplasma and Plasmodium, the genome of Eimeria is more diverse. Development of vector vaccine gets proof of plasmid-based transfection and successful expression of exogenous lacZ gene by Et mic-1 promotor. However, limitation of sensitivity of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) detection system delayed this process for 10 years after getting proof of successful transfection dynamics. This issue was resolved with development of fluorescent proteins (Hao et al. 2007).
Nano-vaccines are a new generation of modern vaccines which have nano-sized (less than 100nm) adjuvants and/or carriers. In recent times, markedly successful nano-vaccines had been developed and commercialized to counter COVID-19 pandemic, for instance Pfizer and Moderna vaccines (Thi et al. 2021). Similarly, Eimeria nano-vaccines (nanoparticles functionalized Eimeria antigens) have also shown a large promise as they have the ability to elicit both systemic and mucosal immunological responses, following administration via mucosa (Jenkins et al. 2018). Nano-vaccines may supersede the conventional approaches for vaccine development by virtue of efficient immunomodulation, easier engineering, and better immune coverage. It is required to further trial this approach for multivalent Eimeria vaccines.
Adjuvants for coccidia vaccines
Several novel vaccine adjuvants have been trialed for effective immune coverage in chicken coccidiosis (Lin et al. 2020). Studies have shown CD40 ligand (CD40L) as a strong immunological adjuvant. It is expressed on mast cells, activated T cells, and basophils of poultry. The CD40-CD40L interactions activate antigen-presenting cells, upregulate co-stimulatory molecules, and affect T cell–mediated effector function (Yin et al. 2015). The use of plant-mediated adjuvants including saponins, polysaccharides, lectins, and heat shock proteins against coccidia and other apicomplexan parasites may offer safer alternative to conventional adjuvants (Sander et al. 2019). The plant bio-actives have an innate ability to elicit a sustained immune response in the host against target specie. However, there is a knowledge gap in the comparative efficacy of different plant-derived adjuvants against various Eimeria strains. Recently, an oral, yeast-based sub-unit vaccine for E. tenella has been shown to decrease Eimeria replication (Soutter et al. 2022). Nano-vaccine adjuvants in apicomplexan vaccines can offer a sustained immune protection, compared to other adjuvants. Antigen delivery systems utilizing immunostimulatory complexes and virus-like particles, self-assembling polypeptide nanoparticles, have also been shown to enhance immune protective coverage of several recombinant vaccines (Collins et al. 2017). Chicken cytokine genes including IL-1β, IL-2, IL-8, IL-15, IFN-α, IFN-γ, TGF-β4, and lymphotactin have exhibited better immune responses (compared with Eimeria antigens alone) in injected birds (Tian et al. 2017; Rafiqi et al. 2019; Venkatas and Adeleke 2019). The use of ISA 71 (oil based), IMS 1313 (nano-particle based), and adjuvant complex including saponin, cholesterol, Quil A, Carbopol and dimethyldioctadecyl-ammonium bromide, and profilin plus QCDC adjuvant have shown a large promise, and exhibited protective immunity when used with Eimeria antigens (Lee et al. 2010; Jang et al. 2011; Kim et al. 2012). Moreover, chicken genetic/molecular adjuvants like truncated flagellin and interleukin-2 (IL-2) have also been shown to confer protective immunity in the face of coccidiosis challenge (Song et al. 2015a; Yan et al. 2018).
Plant-derived adjuvants for Eimeria vaccine development have also shown promising results (Sander et al. 2019). It could be an eco-sustainable approach if being potentialized and upscaled in collaboration with commercial industry. The innate animal adjuvants like cytokines from chicken have a limitation of rapid degradation and quick clearance from the host, when administered in vivo. To overcome these challenges, the approaches focusing more sustained release are being trialed to efficiently overcome the disease (Wang and Suo 2020). Furthermore, the chimeric and nano-vaccines have opened a gateway to scale-able preparations, possessing excellent physiochemical properties and relatively stable immune responses. Similar vaccines have proven a large promise in other apicomplexan parasites (Collins et al. 2017). The robust immune response shown by virtue of humoral and cell-mediated immunity needs further validation and standardization. It is imperative to uniformly compare and declare a set of “gold-standard” study design and the parameters, evaluating the efficacy of new-generation vaccines at studies on in vitro and in vivo scale (Soutter et al. 2020).
Criteria for efficacy of Eimeria vaccines
The parasite load, reduction of intestinal lesions, or increased weight gain are important metrics to consider for vaccine efficacy. All the other parameters are immunological parameters and do not hold a clear-cut association with the level of host immunity. Performance parameters of birds like reduced feed conversion ratios, decreased oocyst shedding in feces, body weight gains, reduction in severity of intestinal lesions, and higher survivability rates as compared to the control birds can also be taken into account (Chen et al. 2015; Liu et al. 2018a, b; Yan et al. 2018). Serum biochemistry, post-mortem lesion scoring, histopathology, and microscope-assisted visualization can help validate these parameters.
Elevated levels of reactive antibodies (e.g., IgG, IgA) and the increased levels of IFN-γ, IL-2, IL-6, IL-17, and TGF-β; higher levels of CD4+/CD3+ and CD8+/CD3 + T lymphocytes and enhanced IgY antibodies producing cells; and higher antigen-driven proliferation of cells, compared to the control birds, are some of the important immunological indicators (del Cacho et al. 2011; Huang et al. 2018). Patterns of T cells are considered to be more significant criteria for immunogenesis within intestinal mucosa (Min et al. 2013).
A standard set of guidelines based on the modern vaccines in poultry Eimeria is lacking. Most importantly, the animal welfare should be prioritized while experimentation of immunogenicity/pathogenicity trials and guidelines regarding chicken rearing and euthanasia/slaughter must be developed. The line of the chickens and the regimen used for vaccine in research trials may act as confounding factors increasing the statistical variability among different studies (Soutter et al. 2020). The safety and efficacy of vaccines on the basis of bird’s type, age, route of administration, uniform uptake and immunogenesis, cross-protection, and drug sensitivities must be critically evaluated. This is a significant step for distinguishing the type and need of a specific vaccine preparation for particular poultry flocks. Moreover, the safety evaluation standard guidelines could help both manufacturer and farmers in choosing the right preparation for their flocks.
Opportunities (the way forward)
Macro-scale epidemiological investigation is needed to understand the diversified antigenic variants of Eimeria globally. Genome-wide analysis of Eimeria species is essential as its screening may bring forward more promising vaccine candidates. High-throughput sequencing tools may be made available at commercially viable scale. Recombinant Eimeria vaccines featuring use of biological agents (BCG, fowl pox virus) can act as vaccine vectors, offering protective immunity (Wang et al. 2014; Tang et al. 2018). The stable transfection by employing E. acervulina expressing the multiple copies of extracellular domain (M2e) of H9N2 influenza virus has been reported (Zhang et al. 2021). These studies encourage more research on further exploration of Eimeria as live vaccine vectors.
Similarly, the genome editing tools (e.g., clustered regularly inter-spaced palindromic repeats (CRISPR)) that have shown a large promise in other species of apicomplexan parasites may also be utilized to probe basic genetics of indigenous coccidial strains (Hu et al. 2020). For instance, epistasis studies on Toxoplasma with the help of CRISPR screens have aided in better understanding of genetic interactions, and the genes that enable the parasite to thrive within a variety of different hosts (Young et al. 2019). Similarly, the CRISPR-aided screening has helped in the determination of functional genes in Plasmodium and related therapeutic and vaccine candidates in this apicomplexan (Thiam et al. 2022). CRISPR has been employed to edit the germ cell lines in chicken, in order to enhance desirable traits related to meat and egg production (Khwatenge and Nahashon 2021). A single gene–based editing by using CRISPR tool for probing systematic analysis of gene functions in E. tenella revealed that the cellular distribution of secreted proteins varies in different life cycle stages (Hu et al. 2020). Another study reported the application of CRISPR for gene function study in E. tenella (Tang et al. 2020). Similarly, a study deploying zinc finger–like proteins revealed partial immune-protective effects of AN-1 like proteins of E. tenella (Zhao et al. 2020).
The multiepitope DNA vaccines could be a game changer to the current scenario of anticoccidial vaccine development. Well-organized cocktail of antigens from different life cycle stages of Eimeria have shown to offer promising immune protection. Recently, a multiepitope vaccine employing cholera toxin adjuvant was designed by immunoinformatic method (Madlala et al. 2021). In this scenario, development of a vaccine comprised of multiple antigens from different species of chicken Eimeria is signposted. One of the potential issues with a multi-valent vaccine against Eimeria would be the confounding factors, which might be eliminated by evaluating the immune responses of each of the component species separately (Soutter et al. 2020).
Conclusion
Coccidiosis is one of the major issues of commercial poultry production worldwide that needs to be prevented in an effective way. Eimeria in chicken reared at open or semi-open houses is still a huge fiscal burden to farm economy. Lessons learnt from other apicomplexan vaccines, employing the most recent approaches, need to be utilized to produce a vaccine that offers wider coverage in terms of both cellular-mediated and humoral immunity. Also, it is recommended to look up for indigenous strains of Eimeria and prepare multivalent vaccines accordingly to manage the drawback of variation with respect to geographical distribution. Most importantly, there’s need to rationalize the use of commercially viable and tailor-made (catering specific bird type and prevalent Eimeria species) new-generation vaccines.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas A, Abbas RZ, Khan MK, Raza MA, Mahmood MS, Saleemi MK, Hussain T, Khan JA, Sindhu ZUD. Anticoccidial effects ofTrachyspermum ammi (Ajwain) in broiler chicken. Pak Vet Journal. 2019;39:301–304. doi: 10.29261/pakvetj/2019.056. [DOI] [Google Scholar]
- Abbas A, Iqbal Z, Abbas RZ, Khan MK, Khan JA. In vitro anticoccidial potential of Saccharum officinarum extract against Eimeria oocysts. Latin American Caribbean Bulletin of Medicinal and Aromatic Plants. 2015;14(6):456–461. [Google Scholar]
- Abbas A, Iqbal Z, Abbas RZ, Khan MK, Khan JA. Immunomodulatory activity of Pinus radaiata against coccidiosis in broiler. Pak Vet J. 2017;37:145–149. [Google Scholar]
- Abbas RZ, Iqbal Z, Blake D, Khan MK, Saleemi MK. Anticoccidial drug resistance in fowl coccidia: the state of play revisited. Worlds Poult Sci J. 2011;67(2):337–35. doi: 10.1017/S004393391100033X. [DOI] [Google Scholar]
- Abbas RZ, Iqbal Z, Blake D, Khan MK, Saleemi MK. Botanicals: an alternative approach for the control of avian coccidiosis. Worlds Poult Sci J. 2012;68(2):203–215. doi: 10.1017/S0043933912000268. [DOI] [Google Scholar]
- Abu SM, Monira N, Asmaul H, Mahbub EATM, Mustafa KAH, Masudur RM. Seroprevalence of Newcastle disease in layer chickens and pathology in clinically affected chickens at Gazipur. Bangladesh. Continental Vet J. 2022;2(1):35–41. [Google Scholar]
- Ahmad TA, El-Sayed BA, El-Sayed LH. Development of immunization trials against Eimeria spp. Trials in Vaccinol. 2016;5:38–47. doi: 10.1016/j.trivac.2016.02.001. [DOI] [Google Scholar]
- Awais MM, Akhtar M, Anwar MI, Khaliq K. Evaluation of Saccharum officinarum L. bagasse-derived polysaccharides as native immunomodulatory and anticoccidial agents in broilers. Vet Parasitol. 2018;249(15):74–81. doi: 10.1016/j.vetpar.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Blake DP. Eimeria genomics: where are we now and where are we going? Vet Parasitol. 2015;212(1-2):68–74. doi: 10.1016/j.vetpar.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Blake DP, Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Jatau ID, Ayoade S, Kawahara F, Moftah A, Reid AJ, Adebambo AO, Álvarez Zapata R, Srinivasa Rao AS, Thangaraj K, Banerjee PS, Dhinakar-Raj G, Raman M, Tomley FM. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci USA. 2015;112(38):E5343–E5350. doi: 10.1073/pnas.1506468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, Ayoade S, Gilbert W, Adebambo AO, Jatau ID, Raman M, Parker D, Rushton J, Tomley FM. Re-calculating the cost of coccidiosis in chickens. J Vet Res. 2020;51(1):1–14. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DP, Pastor-Fernández I, Nolan MJ, Tomley FM. Recombinant anticoccidial vaccines - a cup half full? Infect Genet Evol. 2017;55:358–365. doi: 10.1016/j.meegid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Blake DP, Worthing K, Jenkins MC. Exploring Eimeria genomes to understand population biology: recent progress and future opportunities. Genes. 2020;11(9):1103–16. doi: 10.3390/genes11091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosila MA, Mekky HM, Fedawy HS, Elbayomi KM, Amer MM. Histopathological lesion of arthritis in Mycoplasma synoviae naturally infected breeder chicken in Egypt. Int J Vet Sci. 2021;10(1):72–74. doi: 10.47278/journal.ijvs/2020.006. [DOI] [Google Scholar]
- Brown-Jordan A, Blake D, Beard J, Beharry A, Serrette L, Soleyn A, Oura C. Molecular identification of Eimeria species in broiler chickens in Trinidad. West Indies. Vet Sci. 2018;5(1):12–21. doi: 10.3390/vetsci5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HD, Cherry TE, Danforth HD, Richards G, Shirley MW, Williams RB. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int J Parasitol. 2002;32(5):617–629. doi: 10.1016/S0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Chen P, Lv J, Zhang J, Sun H, Chen Z, Li H, Wang F, Zhao X. Evaluation of immune protective efficacies of Eimeria tenella EtMic1 polypeptides with different domain recombination displayed on yeast surface. Exp Parasitol. 2015;155:1–7. doi: 10.1016/j.exppara.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Clark EL, Tomley FM, Blake DP. Are Eimeria genetically diverse, and does it matter? Trends Parasitol. 2017;33(3):231–241. doi: 10.1016/j.pt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Clark JD, Oakes RD, Redhead K, Crouch CF, Francis MJ, Tomley FM, Blake DP. Eimeria species parasites as novel vaccine delivery vectors: anti-Campylobacter jejuni protective immunity induced by Eimeria tenella-delivered CjaA. Vaccine. 2012;30(16):2683–2688. doi: 10.1016/j.vaccine.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Collins KA, Snaith R, Cottingham MG, Gilbert SC, Hill A. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep. 2017;7(1):1–15. doi: 10.1038/srep46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DP, McKenzie ME, Dayton AD. Relationship of coccidial lesion scores and weight gain in infections of Eimeria acervulina, E. maxima and E tenella in broilers. Avian Pathol. 1990;19(3):489–496. doi: 10.1080/03079459008418702. [DOI] [PubMed] [Google Scholar]
- Craig AD, Khattak F, Hastie P, Bedford MR, Olukosi OA. The similarity of the effect of carbohydrase or prebiotic supplementation in broilers aged 21 days, fed mixed cereal diets and challenged with coccidiosis infection. PloS One. 2020;15(2):e0229281. doi: 10.1371/journal.pone.0229281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006;5(1):143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Davou KP, Lawal I, Joseph A, Lawal S. Some attempted strategies towards the control of avian coccidiosis: a review. SOJ Immunol. 2018;6(2):1–11. doi: 10.15226/2372-0948/6/2/00169. [DOI] [Google Scholar]
- del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine. 2011;29(21):3818–3825. doi: 10.1016/j.vaccine.2011.03.022. [DOI] [PubMed] [Google Scholar]
- del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C. Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infect Immun. 2012;80(5):1909–1916. doi: 10.1128/IAI.06413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cacho E, Gallego M, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C. Induction of protective immunity against experimental Eimeria tenella infection using serum exosomes. Vet Parasitol. 2016;224:1–6. doi: 10.1016/j.vetpar.2016.04.043. [DOI] [PubMed] [Google Scholar]
- Ding J, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP. Multi-epitope recombinant vaccine induces immunoprotection against mixed infection of Eimeria spp. Parasitol Res. 2012;110(6):2297–2306. doi: 10.1007/s00436-011-2764-y. [DOI] [PubMed] [Google Scholar]
- Dkhil MA, Al-Quraishy S. Nanoparticles against Eimeriosis. In: Nanoparticles in the Fight Against Parasites. Parasitol Res. 2016;8(1):207–210. doi: 10.1007/978-3-319-25292-6_11. [DOI] [Google Scholar]
- Duan CH, Hu DD, Tang XM, Suo JX, Wang S, Zhang SX, Tao GR, Li C, Wang CY, Gu XL, Tang XL, Huang GP, Xiang BQ, Wu SQ, Ben Mamoun C, Suo X, Liu XY. Stable transfection of Eimeria necatrix through nucleofection of second generation merozoites. Mol Biochem Parasitol. 2019;228:1–5. doi: 10.1016/j.molbiopara.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Gerhold RW (2016) Overview of coccidiosis in poultry”. Available from http://www.merckvetmanual.com/poultry/coccidiosis/overview-of-coccidiosis-in-poultry (Accessed: June 11, 2022)
- Guo L, Huang W, Tong F, Chen X, Cao S, Xu H, Luo W, Li Z, Nie Q. Whole transcriptome analysis of chicken bursa reveals candidate gene that enhances the host's immune response to coccidiosis. Front Physiol. 2020;11:573676. doi: 10.3389/fphys.2020.573676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven E, Beckstead RB, Kar S, Vatansever Z, Karaer Z. Molecular identification of Eimeria species of broiler chickens in Turkey. Ank Üni Vet Fak Derg. 2013;60(4):245–250. doi: 10.1501/Vetfak_0000002587. [DOI] [Google Scholar]
- Gyorke A, Pop L, Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. 2013;20(1):50. doi: 10.1051/parasite/2013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LL, Liu XY, Zhou XY, Li JD, Suo X. Transient transfection of Eimeria tenella using yellow or red fluorescent protein as a marker. Mol Biochem Parasitol. 2007;153(2):213–215. doi: 10.1016/j.molbiopara.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Hedhli D, Dimier-Poisson I, Judge JW, Rosenberg B, Mévélec MN. Protective immunity against Toxoplasma challenge in mice by coadministration of T. gondii antigens and Eimeria profilin-like protein as an adjuvant. Vaccine. 2009;27(16):2274–81. doi: 10.1016/j.vaccine.2009.01.100. [DOI] [PubMed] [Google Scholar]
- Hinsu AT, Thakkar JR, Koringa PG, Vrba V, Jakhesara SJ, Psifidi A, Guitian J, et al. Illumina next generation sequencing for the analysis of Eimeria populations in commercial broilers and indigenous chickens. Front Vet Sci. 2018;5:176–184. doi: 10.3389/fvets.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Tang X, Mamoun CB, Wang C, Wang S, Gu X, Duan C, et al. Efficient single-gene and gene family editing in the apicomplexan parasite Eimeria tenella using CRISPR-Cas9. Front Bioeng Biotechnol. 2020;8:28. doi: 10.3389/fbioe.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang Z, Li M, ong X,Yan R, Xu L, Li X (2015) Immune protection of microneme 7 (EmMIC7) against Eimeria maxima challenge in chickens. Avian Pathol 44(5):392-400. 10.1080/03079457.2015.1071780 [DOI] [PubMed]
- Huang X, Liu J, Tian D, Li W, Zhou Z, Huang J, Song X, Xu L, Yan R, Li X. The molecular characterization and protective efficacy of microneme 3 of Eimeria mitis in chickens. Vet Parasitol. 2018;258:114–123. doi: 10.1016/j.vetpar.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Huvepharma NV (2016a) Huveguard Start Product Label. Huvepharma NV Huveguard Start Product Label, Huvepharma NV Antwerp, Belgium
- Huvepharma NV (2016b) Huveguard Plus Product Label, Huveguard Plus Product Label, Huvepharma, NV, Antwerp, Belgium
- Idris M, Abbas RZ, Masood S, Rehman T, Farooq U, Babar W, Hussain R, Raza A, Riaz U. The potential of antioxidant rich essential oils against avian coccidiosis. Worlds Poult Sci J. 2017;73(1):89–104. doi: 10.1017/S0043933916000787. [DOI] [Google Scholar]
- Immerseel FV, Buck JD, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33(6):537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Iraqi M, Nasef SA, El-Enbaawy M. Phenotypic and genotypic characteristics of antimicrobial and disinfectant resistance gram-negative bacteria involved in early broiler chick mortality. Int J Vet Sci. 2021;10(2):129–134. doi: 10.47278/journal.ijvs/2020.033. [DOI] [Google Scholar]
- Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Bertrand F, Dupuis L, Deville S. Montanide TM IMS 1313 N VG PR nanoparticle adjuvant enhances antigen-specific immune responses to profilin following mucosal vaccination against Eimeria acervulina. Vet Parasitol. 2011;182(2-4):163–170. doi: 10.1016/j.vetpar.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Jang SI, Lillehoj SH, Lee HS, Lee KW, Park MS, Chaa S, Lillehoj EP, Subramanian M, Sriraman R, Srinivasan VA. Eimeria maxima recombinant Gam82 gametocyte antigen vaccine protects against coccidiosis and augments humoral and cell-mediated immunity. Vaccine. 2010;28(17):2980–2985. doi: 10.1016/j.vaccine.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Jeffers TK. Eimeria acervulina and E. maxima: incidence and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. 1974;18:331–342. doi: 10.2307/1589101. [DOI] [PubMed] [Google Scholar]
- Jenkins M, Klopp S, Ritter D, Miska K, Fettere R. Comparison of Eimeria species distribution and salinomycin resistance in commercial broiler operations utilizing different coccidiosis control strategies. Avian Dis. 2010;54(3):1002–1006. doi: 10.1637/9137-111109-Reg.1. [DOI] [PubMed] [Google Scholar]
- Jenkins MC, Stevens L, O'Brien C, Parker C, Miska K, Konjufca V. Incorporation of a recombinant Eimeria maxima IMP1 antigen into nanoparticles confers protective immunity against E. Maxima challenge infection. Vaccine. 2018;36(8):1126–1131. doi: 10.1016/j.vaccine.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Kadykalo S, Roberts T, Thompson M, Wilson J, Lang M, Espeisse O. The value of anticoccidials for sustainable global poultry production. Int J Antimicrob Agents. 2018;51(3):304–310. doi: 10.1016/j.ijantimicag.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Khalafalla RE, Daugschies A, Dyachenko V. Cross-reactivity of anti-Eimeria tenella antibody fragments on merozoites and sporozoites of different chicken Eimeria species. Parasitol Res. 2011;108(3):745–9. doi: 10.1007/s00436-010-2171-9. [DOI] [PubMed] [Google Scholar]
- Khater HF, Ziam H, Abbas A, Abbas RZ, Raza MA, Hussain K, Younis EZ, Radwan IT, Selim A. Avian coccidiosis: recent advances in alternative control strategies and vaccine development. Agrobiol Rec. 2020;1:11–25. doi: 10.47278/journal.abr/2020.003. [DOI] [Google Scholar]
- Khwatenge CN, Nahashon SN. Recent advances in the application of CRISPR/Cas9 gene editing system in poultry species. Front. Genet. 2021;12:627714. doi: 10.3389/fgene.2021.627714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Lillehoj HS, Lee SH, Dominowski P, Yancey RJ, Lillehoj EP. Effects of novel vaccine/adjuvant complexes on the protective immunity against Eimeria acervulina and transcriptome profiles. Avian Dis. 2012;56(1):97–109. doi: 10.1637/9720-031711-Reg.1. [DOI] [PubMed] [Google Scholar]
- Kim WH, Chaudhari AA, Lillehoj HS. Involvement of T cell immunity in avian coccidiosis. Front Immunol. 2019;10:2732. doi: 10.3389/fimmu.2019.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu K, Garg R, Kumar S, Mandal M, Tomley FM, Blake DP, Banerjee PS. Humoral and cytokine response elicited during immunisation with recombinant immune mapped protein-1 (EtIMP-1) and oocysts of Eimeria tenella. Vet Parasitol. 2017;244:44–53. doi: 10.1016/j.vetpar.2017.07.025. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lillehoj HS, Jang SI, Lee KW, Yancey RJ, Dominowski P. The effects of a novel adjuvant complex/Eimeria profilin vaccine on the intestinal host immune response against live E. acervulina challenge infection. Vaccine. 2010;28(39):6498–6504. doi: 10.1016/j.vaccine.2010.06.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lu M, Lillehoj HS. Coccidiosis: recent progress in host immunity and alternatives to antibiotic strategies. Vaccines. 2022;10(2):215–239. doi: 10.3390/vaccines10020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang F, Ma C, Huang Y, Wang D, Ma D. Recombinant Lactococcus lactis expressing Eimeria tenella AMA1 protein and its immunological effects against homologous challenge. Exp Parasitol. 2017;191:1–8. doi: 10.1016/j.exppara.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Li C, Yan X, Lillehoj HS, Oh S, Liu L, Sun Z, Gu C, Lee Y, Xianyu Z, Zhao H (2019) Eimeria maxima-induced transcriptional changes in the cecal mucosa of broiler chickens. Parasit Vector 12(1):1–9. 10.1186/s13071-019-3534-4 [DOI] [PMC free article] [PubMed]
- Lin RQ, Lillehoj HS, Lee SK, Oh S, Panebra A, Lillehoj EP. Vaccination with Eimeria tenella elongation factor-1α recombinant protein induces protective immunity against E. tenella and E. maxima infections. Vet Parasitol. 2017;243:79–84. doi: 10.1016/j.vetpar.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Lin X, Mohsin M, Abbas RZ, Li L, Chen H, Huang C, Li Y, Goraya MU, Huang Z, Yin G. Evaluation of immunogenicity and protective efficacy of Eimeria maxima immune mapped protein 1 with EDA adjuvant in chicken. Pak Vet J. 2020;40(2):209–213. doi: 10.29261/pakvetj/2020.043. [DOI] [Google Scholar]
- Lin Z, Shi Y, Deng B, Mao X, Yu D, Li M. Protective immunity against Eimeria tenella infection in chickens following oral immunization with Bacillus subtilis expressing Eimeria tenella 3-1E protein. Parasitol Res. 2015;114:229–3236. doi: 10.1007/s00436-015-4539-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng J, Li J, Gong P, Zhang X (2013) Protective immunity induced by a DNA vaccine encoding Eimeria tenella rhomboid against homologous challenge. Parasitol Res 11(1):251–257. 10.1007/s00436-012-3132-2 [DOI] [PubMed]
- Liu D, Cao L, Zhu Y, Deng C, Su S, Xu J, Jin W, Li J, Wu L, Tao J. Cloning and characterization of an Eimeria necatrix gene encoding a gametocyte protein and associated with oocyst wall formation. Parasit Vector. 2014;7(1):27. doi: 10.1186/1756-3305-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu L, Li L, Tian D, Li W, Xu L, Yan R, Li X, Song X. Protective immunity induced by Eimeria common antigen 14–3-3 against Eimeria tenella, Eimeria acervulina and Eimeria maxima. BMC Vet Res. 2018;14(1):1–11. doi: 10.1186/s12917-018-1665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Huang J, Li Y, Ehsan M, Wang S, Zhou Z, Song X, Yan R, Xu L, Li X. Molecular characterization and the protective immunity evaluation of Eimeria maxima surface antigen gene. Parasit Vector. 2018;11(1):1–12. doi: 10.1186/s13071-018-2906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tuo W, Wu X, Xiong J, Yu E, Yin C, Ma Z, Liu L. Immunoproteomic and mass spectrometric analysis of Eimeria acervulina antigens recognized by antisera from chickens infected with E. acervulina, E. tenella or E. necatrix. Parasit Vector. 2020;13(1):93. doi: 10.1186/s13071-020-3965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM. Overview of poultry Eimeria life cycle and host-parasite interactions. Front Vet Sci. 2020;7:384. doi: 10.3389/fvets.2020.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Huang Y, Ma C, Zhang L, Wang J, Wang D, Li J, Dallou RA. Eimeria tenella: specific EtAMA1-binding peptides inhibit sporozoite entry into host cells. Poult Sci. 2019;9(10):4480–4491. doi: 10.3382/ps/pez298. [DOI] [PubMed] [Google Scholar]
- Ma D, Ma C, Pan L, Li G, Yang J, Hong J, Cai H, Ren X. Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp Parasitol. 2011;127(1):208–214. doi: 10.1016/j.exppara.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Madlala T, Adeleke VT, Fatoba AJ et al (2021) Designing multiepitope-based vaccine against Eimeria from immune mapped protein 1 (IMP-1) antigen using immunoinformatic approach. Sci Rep 11(1):1-17. 10.1038/s41598-021-97880-6 [DOI] [PMC free article] [PubMed]
- Mansilla FC, Capozzo AV. Apicomplexan profilins in vaccine development applied to bovine neosporosis. Exp Parasitol. 2017;183:64–68. doi: 10.1016/j.exppara.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Marugan-Hernandez V, Cockle C, Macdonald S, et al. Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasit Vector. 2016;9(1):1–14. doi: 10.1186/s13071-016-1756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis GF, Newman LJ, Fitz-Coy S, Lumpkins B, Charette R, Fuller L. Comparison of breeder/layer coccidiosis vaccines: part 1-precocity and pathogenicity. J Appl Poult Res. 2018;27(1):33–37. doi: 10.3382/japr/pfx037. [DOI] [Google Scholar]
- Min W, Kim WH, Lillehoj EP, Lillehoj HS. Recent progress in host immunity to avian coccidiosis: IL-17 family cytokines as sentinels of the intestinal mucosa. Dev Comp Immunol. 2013;41:418–28. doi: 10.1016/j.dci.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Mohsin M, Li L, Huang X, Aleem MT, Habib YJ, Shehata AI, Afzal MZ, Abbas RZ, Abbas A, Yin G. Immunogenicity and protective efficacy of probiotics with EtIMP1C against Eimeria tenella challenge. Pak Vet J. 2021;41(2):274–278. doi: 10.29261/pakvetj/2021.009. [DOI] [Google Scholar]
- Morris GM, Gasser RB. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol Advanc. 2006;24(6):590–603. doi: 10.1016/j.biotechadv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Moryani AA, Rajput N, Naeem M, Shah AH, Jahejo AR. Screening of the herbs and evaluation of their combined effects on the health and immunity of coccidiosis challenged broiler chickens. Pak Vet J. 2021;41(2):228–234. doi: 10.29261/pakvetj/2021.005. [DOI] [Google Scholar]
- Panebra A, Lillehoj HS. Eimeria tenella elongation factor-1α (EF-1α) coadministered with chicken IL-7 (chIL-7) DNA vaccine emulsified in Montanide Gel 01 adjuvant enhanced the immune response to E. acervulina infection in broiler chickens. Avian Dis. 2019;6(2):342–350. doi: 10.1637/11976-092418-reg.1. [DOI] [PubMed] [Google Scholar]
- Peek HW, Landman WJM. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Prescott JF, Smyth JA, Shojadoost B, Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45(3):317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Price KR. Use of live vaccines for coccidiosis control in replacement layer pullets. J. Appl. Poult. Res. 2012;21:679–692. doi: 10.3382/japr.2011-00486. [DOI] [Google Scholar]
- Qi NS, Wang YY, Liao SQ. Partial protective of chickens against Eimeria tenella challenge with recombinant EtMIC-1 antigen. Parasitol Res. 2013;112:2281–2287. doi: 10.1007/s00436-013-3389-0. [DOI] [PubMed] [Google Scholar]
- Qin M, Liu XY, Tang XM, Suo JX, Tao GR, Suo X. Transfection of Eimeria mitis with yellow fluorescent protein as reporter and the endogenous development of the transgenic parasite. PLoS One. 2014;9(12):e114188. doi: 10.1371/journal.pone.0114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiqi SI, Garg R, Ram H, Reena KK, Asari M, Kumari P, Kundave VR, Singh M, Banerjee PS. Immunoprophylactic evaluation of recombinant gametocyte 22 antigen of Eimeria tenella in broiler chickens. Parasitol Res. 2019;118(3):945–953. doi: 10.1007/s00436-018-06198-2. [DOI] [PubMed] [Google Scholar]
- Rafiqi SI, Garg R, Reena KK, Ram H, Singh M, Banerjee PS. Immune response and protective efficacy of Eimeria tenella recombinant refractile body protein, EtSO7, in chickens. Vet Parasitol. 2018;258:108–113. doi: 10.1016/j.vetpar.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Rani Z, Abbas RZ, Abbas A, Saeed Z, Rehman T, Hussain MK, Rehman A, Hussain K. In vitro and in vivo anticoccidial effects of butyric acid and its impact on blood and serum chemistry of broiler chickens. Kafkas Univ Vet Fak Derg. 2021;27(5):583–588. doi: 10.9775/kvfd.2021.25907. [DOI] [Google Scholar]
- Rashid M, Akbar H, Bakhsh A, Rashid MI, Hassan MA, Ullah R, Hussain T, Manzoor S, Yin H. Assessing the prevalence and economic significance of coccidiosis individually and in combination with concurrent infections in Pakistani commercial poultry farms. Poult Sci. 2019;98(3):1167–1175. doi: 10.3382/ps/pey522. [DOI] [PubMed] [Google Scholar]
- Reid AJ, Blake DP, Ansari HR, Billington K, Browne HP, Bryant J, Dunn M, et al. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014;24(10):1676–1685. doi: 10.1101/gr.168955.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro E, Silva A, Sausset A, Bussière FI, Laurent F, Lacroix-Lamandé S, Silvestre A. Genome-wide expression patterns of rhoptry kinases during the Eimeria tenella life-cycle. Microorganisms. 2021;9(8):1621–34. doi: 10.3390/microorganisms9081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol. 2004;173(4):2675–2682. doi: 10.3390/microorganisms9081621. [DOI] [PubMed] [Google Scholar]
- Sander VA, Corigliano MG, Clemente M. Promising plant-derived adjuvants in the development of coccidial vaccines. Front Vet Sci. 2019;6:20–35. doi: 10.3389/fvets.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MAA, Yan R, Xu L, Song X, Li X. A recombinant DNA vaccine encoding Eimeria acervulina cSZ-2 induces immunity against experimental E. tenella infection. Vet Parasitol. 2010;16(1-2):185–189. doi: 10.1016/j.vetpar.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Shahid SRA, Shah MA, Riaz A, Malik AM, Hasan MU, Xiangrui L, Babar W, Shahid SKA. Identification and molecular characterization of Eimeria tenella based on EtMic5 gene in Pakistan. Pak Vet J. 2020;40(4):443–448. doi: 10.29261/pakvetj/2020.063. [DOI] [Google Scholar]
- Song H, Xu X, Yan R, Shah MAA, Li X. Changes of cytokines and IgG antibody in chickens vaccinated with DNA vaccines encoding Eimeria acervulina lactate dehydrogenase. Vet Parasitol. 2010;173(3-4):219–227. doi: 10.1016/j.vetpar.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Song H, Yan R, Xu L, Song X, Shah MAA, Zhu H, Li X. Efficacy of DNA vaccines carrying Eimeria acervulina lactate dehydrogenase antigen gene against coccidiosis. Exp Parasitol. 2010;126(2):224–231. doi: 10.1016/j.exppara.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Song H, Qiu B, Yan R, Xu L, Song X, Li X. The protective efficacy of chimeric SO7/IL-2 DNA vaccine against coccidiosis in chickens. Res Vet Sci. 2013;94(3):562–567. doi: 10.1016/j.rvsc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Song X, Gao Y, Xu L, Yan R, Li X (2015a) Partial protection against four species of chicken coccidia induced by multivalent subunit vaccine. Vet Parasitol 212(3–4):80–85. 10.1016/j.vetpar.2015.08.026 [DOI] [PubMed]
- Song X, Ren Z, Yan R, Xu L, Li X (2015b) Induction of protective immunity against Eimeria tenella, Eimeria necatrix, Eimeria maxima and Eimeria acervulina infections using multivalent epitope DNA vaccines. Vaccine 33(24):2764–2770. 10.1016/j.vaccine.2015.04.052 [DOI] [PubMed]
- Song X, Zhao X, Xu L, Yan R, Li X. Immune protection duration and efficacy stability of DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 against coccidiosis. Res Vet Sci. 2017;111:31–35. doi: 10.1016/j.rvsc.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Soutter F, Werling D, Nolan M, Küster T, Attree E, Marugán-Hernández V, Kim S, Tomley FM, Blake DP. A novel whole yeast-based subunit oral vaccine against Eimeria tenella in chickens. Front Immunol. 2022;13:809711–25. doi: 10.3389/fimmu.2022.809711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutter F, Werling D, Tomley FM, Blake DP. Poultry coccidiosis: design and interpretation of vaccine studies. Front Vet Sci. 2020;7:101–12. doi: 10.3389/fvets.2020.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Liu X, Yan W, Shi T, Zhao X, Blake DP, Tomley FM, Suo X. piggyBac transposon-mediated transgenesis in the apicomplexan parasite Eimeria tenella. PLoS ONE. 2012;7:e40075. doi: 10.1371/journal.pone.0040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir A, Khan MA, Bibi K, Bibi S, Rauf F, Ayaz F. Prevalence of colibacillosis in young broiler chicks and antibiogram of Escherichia coli in different areas of Hazara Region. Adv Life Sci. 2021;8(3):238–240. [Google Scholar]
- Tang X, Suo J, Li C, Du M, Wang C, Hu D, Duan C, Lyu Y, Liu X, Suo X. Transgenic Eimeria tenella expressing profilin of Eimeria maxima elicits enhanced protective immunity and alters gut microbiome of chickens. Infect Immun. 2018;86(9):e00888–17. doi: 10.1128/IAI.00888-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Suo J, Liang L, Duan C, Hu D, Gu X, Yu Y, Liu X, Cui S, Suo X. Genetic modification of the protozoan Eimeria tenella using the CRISPR/Cas9 system. Vet Res. 2020;51(1):1–5. doi: 10.1186/s13567-020-00766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wang C, Liang L, Hu D, Zhang S, Duan C, Suo J, Liu X, Suo X, Cui S. Co-immunization with two recombinant Eimeria tenella lines expressing immunoprotective antigens of E. maxima elicits enhanced protection against E. maxima infection. Parasit Vector. 2019;12(1):1–8. doi: 10.1186/s13071-019-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XM, Yin GW, Qin M, Tao GR, Suo JX, Liu XY, Suo X. Transgenic Eimeria tenella as a vaccine vehicle: expressing TgSAG1 elicits protective immunity against Toxoplasma gondii infections in chickens and mice. Sci Rep. 2016;6(1):1–9. doi: 10.1038/srep29379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi TTH, Suys EJ, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021;9(4):359. doi: 10.3390/vaccines9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam LG, Mangou K, Ba A, Mbengue A, Bei AK. Leveraging genome editing to functionally evaluate Plasmodium diversity. Trends Parasitol. 2022;38(7):558–571. doi: 10.1016/j.pt.2022.03.005. [DOI] [PubMed] [Google Scholar]
- Tian L, Li W, Huang X, Tian D, Liu J, Yang X, Liu L, et al. Protective efficacy of coccidial common antigen glyceraldehyde 3-phosphate dehydrogenase (GAPDH) against challenge with three Eimeria species. Front Microbiol. 2017;18(8):1245–57. doi: 10.3389/fmicb.2017.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatas J, Adeleke MA. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res. 2019;118(6):1701–1710. doi: 10.1007/s00436-019-06338-2. [DOI] [PubMed] [Google Scholar]
- Wajiha, Qureshi NA (2021) In vitro anticoccidial, antioxidant activities and biochemical screening of methanolic and aqueous leaves extracts of selected plants. Pak Vet J 41(1):57–63. 10.29261/pakvetj/2020.071
- Wang S, Suo X. Still naïve or primed: Anticoccidial vaccines call for memory. Exp Parasitol. 2020;216:107945–50. doi: 10.1016/j.exppara.2020.107945. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen L, Li J, Zheng J, Cai N, Gong P, Li S, Li S, Zhang X. A novel recombinant BCG vaccine encoding Eimeria tenella rhomboid and chicken IL-2 induces protective immunity against coccidiosis. Korean J Parasitol. 2014;52(3):251–256. doi: 10.3347/kjp.2014.52.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Wu LL, Gao Y, Zhang Y, Weng YB, Lin RQ (2017) Evaluation of the protective effect of pVAX-EtMIC3-recombined plasmid against E. tenella in chicken. Parasitol Res:116 [DOI] [PubMed]
- Williams RB. Fifty years of anticoccidial vaccines for poultry (1952-2002) Avian Dis. 2002;46:775–802. doi: 10.1637/0005-2086(2002)046[0775:FYOAVF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang Y, Tao J. Efficacy of a DNA vaccine carrying Eimeria maxima Gam56 antigen gene against coccidiosis in chickens. Korean J Parasitol. 2013;51(2):147–154. doi: 10.3347/kjp.2013.51.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Cui X, Zhao Q, Zhu S, Huang B, Wang L, Zhao H, et al. Molecular characterization and protective efficacy of the microneme 2 protein from Eimeria tenella. Parasite. 2018;25:60–70. doi: 10.1051/parasite/2018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wang C, Hao F, Zhao D, Zhang Y, Li Y. Studies on construction of a recombinant Eimeria tenella SO7 gene expressing Escherichia coli and its protective efficacy against homologous infection. Parasitol Int. 2010;59(4):517–523. doi: 10.1016/j.parint.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Yang X, Li M, Liu J, Ji Y, Li X, Xu L, Yan R, Song X. Identification of immune protective genes of Eimeria maxima through cDNA expression library screening. Parasit Vector. 2017;10(1):1–10. doi: 10.1186/s13071-017-2029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Lin Q, Qiu J, Qin M, Tang X, Suo X, Huang Z, Liu X. Immunogenicity and protective efficacy of an Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and chicken CD40 ligand. Vet Parasitol. 2015;210(1-2):19–24. doi: 10.1016/j.vetpar.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Yin G, Qin M, Liu X, Suo J, TangX TG, Han Q, Suo X, Wu W. An Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and the TLR-5 agonist Salmonella typhimurium FliC flagellin. Biochem Biophys Res Commun. 2013;440(3):437–442. doi: 10.1016/j.bbrc.2013.09.088. [DOI] [PubMed] [Google Scholar]
- Young J, Dominicus C, Wagener J, et al. A CRISPR platform for targeted in vivo screens identifies Toxoplasma gondii virulence factors in mice. Nat Commun. 2019;10:3963. doi: 10.1038/s41467-019-11855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. 2000;24(2-3):303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Huang B, Dong H, Zhao Q, Zhu S, Liang S, Li S, Yang S, Han H (2016) Molecular characterization and immune protection of a new conserved hypothetical protein of Eimeria tenella. PloS one 11(6):e0157678–e0157698. 10.1371/journal.pone.0157678 [DOI] [PMC free article] [PubMed]
- Zhang DF, Xu H, Sun BB, Li JQ, Zhou QJ, Zhang HL, Du AF. Adjuvant effect of ginsenoside-based nanoparticles (ginsomes) on the recombinant vaccine against Eimeria tenella in chickens. Parasitol Res. 2012;110(6):2445–53. doi: 10.1007/s00436-011-2784-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma L, Liu R, Zhang Y, Zhang S, Hu C, Song M, Cai J, Wang M. Eimeria tenella heat shock protein70 enhances protection of recombinant microneme protein MIC2 subunit antigen vaccination against E. tenella challenge. Vet Parasitol. 2012;188(3-4):239–246. doi: 10.1016/j.vetpar.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huang J, Li M, Sui Y, Wang S, Liu L, Xu L, Yan R, Song X, Li X. Identification and molecular characterization of microneme 5 of Eimeria acervulina. PLoS One. 2014;9(12):e115411–19. doi: 10.1371/journal.pone.0115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Tang X, Wang S, Shi F, Duan C, Bi F, Suo J, Hu D, Liu J, Wang C, Suo X, Liu X. Establishment of recombinant Eimeria acervulina expressing multi-copies M2e derived from avian influenza virus H9N2. Vaccines. 2021;9(7):791–101. doi: 10.3390/vaccines9070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xu R, Zhang Y, Ji X, Zhang J, Liu Y, Bao Y, Qin J (2014) Protective efficacy in chickens of recombinant plasmid pET32a(+)-ADF-3-1E of Eimeria acervulina. Parasitol Res 113(8):3007–3014. 10.1007/s00436-014-3963-0 [DOI] [PubMed]
- Zhao H, Zhao H, Zhao O, Zhu S, Huang B, Lv L, Liu G, et al. Molecular characterization and immune protection of an AN1-like zinc finger protein of Eimeria tenella. Parasitol Res. 2020;119(2):623–635. doi: 10.1007/s00436-019-06545-x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang Z, Cao L, Hu S, Zhang Z, Qin B, Guo Z, Nie K. Upregulation of chicken TLR4, TLR15 and MyD88 in heterophils and monocyte-derived macrophages stimulated with Eimeria tenella in vitro. Exp Parasitol. 2013;133:417–433. doi: 10.1016/j.exppara.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xu L, Yan R, Song X, Tang F, Wang S, Li X. Identification and characterization of a cDNA clone-encoding antigen of Eimeria acervulina. Parasitol. 2012;139(13):1711–1719. doi: 10.1017/S0031182012001163. [DOI] [PubMed] [Google Scholar]
- Zhu H, Yan R, Wang S, Song X, Xu L, Li X. Identification and molecular characterization of a novel antigen of Eimeria acervulina. Mol Biochem Parasitol. 2012;186(1):21–28. doi: 10.1016/j.molbiopara.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Lillehoj HS, Allen PC, Yun CH, Pollock D, Sadjadi M, Emara MG. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult Sci. 2000;79(5):619–625. doi: 10.1093/ps/79.5.619. [DOI] [PubMed] [Google Scholar]