Abstract
Here we report the characterization of an Escherichia coli gene (agn43) which encodes the principal phase-variable outer membrane protein termed antigen 43 (Ag43). The agn43 gene encodes a precursor protein of 107 kDa containing a 52-amino-acid signal sequence. Posttranslational processing generates an α43 subunit (predicted Mr of 49,789) and a C-terminal domain (β43) with features typical of a bacterial integral outer membrane protein (predicted Mr of 51,642). Secondary structure analysis predicts that β43 exists as an 18-stranded β barrel and that Ag43 shows structural organization closely resembling that of immunoglobulin A1 protease type of exoprotein produced by pathogenic Neisseria and Haemophilus spp. The correct processing of the polyprotein to α43 and β43 in OmpT, OmpP, and DegP protease-deficient E. coli strains points to an autocatalytic cleavage mechanism, a hypothesis supported by the occurrence of an aspartyl protease active site within α43. Ag43, a species-specific antigen, possesses two RGD motifs of the type implicated in binding to human integrins. The mechanism of reversible phase variation was studied by immunochemical analysis of a panel of well-defined regulatory mutants and by analysis of DNA sequences upstream of agn43. Evidence strongly suggests that phase variation is regulated by both deoxyadenosine methylase (Dam) and by OxyR. Thus, oxyR mutants are locked on for Ag43 expression, whereas dam mutants are locked off for Ag43 expression. We propose a novel mechanism for the regulation of phase switching in which OxyR competes with Dam for unmethylated GATC sites in the regulatory region of the agn43 gene.
Workers in this laboratory (33) have recently identified for Escherichia coli the phase-variable product which determines both colony morphology and the ability of cells to autoaggregate in liquid media (25). The product in question, termed antigen 43 (Ag43), is the major phase-variable protein in the outer membrane and is present in copy numbers exceeding 5 × 104 per cell (55). By multiple criteria, Ag43 has been shown to exist in situ as a hetero-oligomeric complex composed of two chemically and immunologically distinct protein subunits (termed α43 and β43) present in 1:1 stoichiometry. The α43 subunit (apparent Mr of 60,000) is surface expressed, can extend beyond the O side chains of smooth lipopolysaccharide, and is bound to the cell through an interaction of its C-terminal domain with β43, itself an integral outer membrane protein showing pronounced properties of heat modifiability (apparent Mrs of 37,000 [70°C] and 53,000 [100°C]). The α43 and β43 polypeptides contain no detectable carbohydrate, identifiable cofactors, acyl groups, or inter- or intramolecular disulfide bonds. Nearest-neighbor analysis provides evidence that Ag43 is in close proximity to the ferric-enterochelin receptor, FepA (18, 56, 57).
Although detailed electron microscopic studies have failed to reveal any morphologically recognizable structure for Ag43 (58), the antigen displays several properties suggestive of an adhesin. Thus, Ag43 enhances adherence of E. coli to certain tissue culture lines in a manner which can be inhibited by purified α43. The α43 (but not the β43) subunit can be selectively and almost quantitatively released from E. coli outer membranes by brief heating to 60°C. In addition, the N-terminal amino acid sequence of α43 contains a six-residue motif (TVNGGT) which is also present in the N termini of the major subunits of several enterobacterial fimbriae (58). Like expression of many adhesins, expression of Ag43 is subject to reversible phase variation, the rates in liquid minimal medium from positive (Ag43+) to negative (Ag43−) states and vice versa being ≈2.2 × 10−3 and ≈10−3, respectively (18, 56).
We have recently located the gene (agn43) encoding Ag43 to a region of the E. coli K-12 chromosome (min 44.6 to 44.8) between amn and sbcB and established its identity with flu (33), the first metastable gene to be mapped in E. coli (25). In this communication, we report on the sequence of agn43 and flanking regions, show that Ag43 belongs to the class of proteins known as bacterial autotransporters (for a review, see reference 32), and demonstrate that phase switching is regulated by a novel mechanism involving DNA methylation and OxyR, a LysR-type transcriptional activator better known for its ability to control expression of proteins important in oxidative stress (26, 43, 44). Evidence is presented which suggests that OxyR may act as a repressor of Ag43 transcription by binding to unmethylated GATC sites in the regulatory region of the gene.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are described in Table 1. All strains were grown on Luria-Bertani agar or broth, supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), and chloramphenicol (25 μg/ml) as appropriate.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype or description | Reference(s) or source |
|---|---|---|
| Escherichia coli | ||
| 2787 (O126:H27) | AIDA-I+ | 6 |
| BD1302 | metB Δ(ppc-argECBH oxyR) relA spoT (λ+) Hfr P4X | 25 |
| BR83 | topA57 argA supD74 rpsL | 50 |
| C600 | F−thr-1 leuB6 lacY1 fhuA21 supE44 thi-1 mcrA | 4 |
| C600 Δhns | C600 Δhns | 69 |
| CS1291 | F−ompC::Tn5 rpsL gyrA | 68 |
| CSH26 | ara Δ(lac-proP) thi | 76 |
| CSH26 Δcya | CSH26 Δcya | 76 |
| CSH50 | ara Δpro-lac thi | 41 |
| CSH50 fis | CSH50 fis::kan | 41 |
| DL379 | F−araΔ139 Δ(lacIPOZYA-argF)U169 rpsL thi-1 λ246 lysogen (papBAp-lacZYA) | 9 |
| DL850 | DL379 mbf-20::mTn10 | 16 |
| GM2929 | F−ara-14 leuB6 lacY1 tsx-78 supE44 galK2 galT22 hisG4 rfbD rspL136 xyl-5 mtl-1 thi-1 glnV44 fhuA31 dam-13::Tn9(Cmr) dcm-6 hsdR2 recF143 mcrA mcrB | 10, 59 |
| GM3819 | F−thr-1 ara-14 leuB6 Δ(gpt-proA)62 lacY1 tsx-33 supE44 galK2 hisG4(Oc) rfb-1 mgl-51 rpsL31 kdgK51 xyl-5 mtl-1 argE3 thi-1 dam-16::Kanr | 60 |
| GSO5 | SA2692 ΔoxyR::kan (λY2055oxyS-galK) | 43 |
| GSO9 | SA2692 ΔoxyR::kan | G. Storz |
| HB101 | hsdS20 recA13 proA2 leu-6 thi-1 rpsL20 ara-14 galK2 lacY1 xyl-5 mtl-1 supE44 merB endA | 15 |
| IRH10 | ML308-225 dsbA by P1cml from JCB571 | This study |
| IRH11 | ML308-225 degP by P1cml from KS474 | This study |
| IRH12 | ML308-225 dam-16 by P1cml from GM3819 | This study |
| IRH13 | ML308-225 dam-13::Tn9 by P1cml from GM2929 | This study |
| IRH14 | ML308-225 ΔoxyR::kan (λY2055oxyS-galK) by P1cml from GS05 | This study |
| IRH15 | ML308-225 ΔoxyR::kan by P1cml from GS09 | This study |
| IRH16 | IRH14 containing pAQ25 | This study |
| IRH17 | IRH14 containing pGSO68 | This study |
| IRH18 | IRH14 containing pGSO69 | This study |
| IRH19 | BD1302 dam-16 by P1cml from GM3819 | This study |
| JCB571 | araD139 Δ(araABC-leu)7679 galU galK Δ(lac)X74 rpsL thi phoR zih-12::Tn10 dsbA::Kan-1 | 5 |
| KNS4 | MC4100 Φ(hns-lacZ) hyb-2 (Kanr) | 24 |
| KS474 | F− ΔlacX74 galE galK thi rpsL (strA) ΔphoA (PvuII) degP41 (ΔPstI-Kanr) | 72 |
| M182 | Δ(lacIPOZY)X74 galK galU strA | 21 |
| M182 Δcrp | M182 Δcrp | 17 |
| MC4100 | F−araΔ139 Δ(argF-lac)U169 deoC1 flb-5301 relA1 rpsL150 ptsF25 rbsR | 20 |
| ML308-225 (O13:O68:H−) | lacIZ lacY+ lacA+ Ag43+ | 57 |
| OHP250 | C600 hupB::Kanr | 34 |
| OHP251 | C600 hupA::Cmr | 34 |
| RM269 | strR galK2 Δ(srl recA)srl::Tn10 [pGBK1 Φ(gyrB galK)] | 51 |
| SA2692 | HB101 recA+ Δlac Δgal-165 | 43 |
| UT5600 | F−ara-14 leuB6 azi-6 lacYI tsx-67 proC trpE38 rfbD1 rpsL109 xyl-5 mtl-1 thi-1 entA403 Δ(ompT-fecC266) ΔompP | 40 |
| W3110 | F− λ− | 4 |
| ZK126 | W3110 ΔlacU169 tna-2 rpoS+ | 23 |
| ZK1000 | ZK126 rpoS::kan | 11 |
| Edwardsiella tarda ATCC 15947 | Type strain | |
| Enterobacter cloacae ATCC 13047 | Type strain | |
| Erwinia carotovora NCPPB 312 | Type strain | |
| Klebsiella pneumoniae ATCC 13883 | Type strain | |
| Proteus mirabilis ATCC 13315 | Type strain | |
| Salmonella typhimurium ATCC 14028 | Type strain | |
| Serratia marcescens ATCC 13880 | Type strain | |
| Shigella flexneri type 26 ATCC 12022 | Type strain | |
| Plasmids | ||
| pBluescript II SK(+) | ColE1 oriV lacZα Ampr phagemid cloning vector | Stratagene |
| pAQ25 | oxyR wild type in pKK177-3 with altered Shine-Dalgarno sequence to allow overproduction of OxyR; Ampr | 71 |
| pGSO68 | oxyR C199S in pKK177-3; Ampr | 43 |
| pGSO69 | oxyR A233V in pKK177-3; Ampr | 43 |
Protein preparation and analysis.
Cell envelopes were isolated and the α43 subunit was purified essentially as described by Caffrey and Owen (18). Cell lysates were analyzed by emulsifying one colony in Laemmli sample buffer, before sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). One dimensional SDS-PAGE was performed as detailed elsewhere (46), using 12.5% (wt/vol) polyacrylamide separating gels and 4.5% (wt/vol) polyacrylamide stacking gels. After electrophoresis, proteins were either stained with Coomassie brilliant blue R250 or transferred to a nitrocellulose filter for Western blotting. Western immunoblotting and colony immunoblotting were performed essentially as described by Caffrey et al. (19), using specific anti-Ag43 or anti-α43 rabbit polyclonal antibodies as appropriate. Slide immunofluorescence microscopy was based on the method of Nowicki et al. (54) and has been described in detail elsewhere (33). The discontinuous SDS-PAGE system of Schägger and von Jagow (67) was used for the separation of protease cleavage products generated following incubation of either undenatured or heat-denatured (100°C, 5 min) α43 with Staphylococcus aureus V8 protease. Peptides for N-terminal amino acid sequencing were separated on 15% (wt/vol) polyacrylamide gels and were electroblotted to polyvinylidene difluoride membranes, using a transfer buffer containing 10 mM 3-[cyclohexylamino]-1-propanesulfonic acid, 10% (vol/vol) aqueous methanol, and sufficient 4 M NaOH to adjust the buffer to pH 11. Transfer was performed at room temperature for 2 h at 200 mA. Amido black-stained bands were excised from the polyvinylidene difluoride membrane, and the N-terminal amino acid sequences were determined by the automated sequential Edman degradation procedure using an Applied Biosystems model 470A protein sequencer. Amino acid sequence alignments were generated by using the FASTA alignment program (62) at Ecole pour les Etudes et la Recherche en Informatique et Electronique, Nimes, France. Electrospray mass spectroscopy was performed by E. C. Barton, Department of Chemistry, University of Cambridge.
Strategy for sequencing the agn43 gene.
To obtain internal amino acid sequence data for cloning purposes, the N-terminal sequences of three internal peptides, αa, αb, and αc, obtained by digestion of heat-denatured α43 with S. aureus V8 protease, were determined and identified as GTANTTVVYAGGDQTV, GAIATGTVINXKGXQVV, and KGSSFTLNAGDTATDTTVN, respectively. Similar proteolysis experiments performed on undenatured α43 revealed two main products. One (Mr of 48,000) had an N-terminal sequence identical to that of native α43 (viz., ADIVVHPGETVNGGTLANH [56]). The other had an apparent Mr and N-terminal sequence identical to those of αc. The N-terminal sequence of β43 was confirmed as PTNVTLASGATWNIPDNA (56).
To localize the agn43 gene, mixtures of oligonucleotides, corresponding to the sequences ADIVVHP and FTLNAGDT from the N-terminal ends of α43 and αc, respectively, were used as primers in PCR performed with chromosomal DNA as the template. An 1,150-bp PCR fragment, amplified from the chromosome, was cloned into pBluescript II (pIH1) and then sequenced. Subcloning from pIH1 of 673-bp NsiI/NcoI, 417-bp NsiI/PstI, and 262-bp PstI/NcoI fragments into complementary sites in pGEM-5 gave rise to pIH2, pIH3, and pIH4, respectively. Additional subclones pIH5 and pIH6 were constructed from the products of further PCRs which used the original α43 and αc primers and oligonucleotides designed from a knowledge of the nucleotide sequence of subclones pIH2 and pIH4. Analysis of the translated sequence of the 1,150-bp PCR product indicated that the cloned fragment represented part of the Ag43 coding sequence.
Previous work had located the agn43 gene to two overlapping λ clones in the Kohara miniset library at 44.6 to 44.8 min on the physical map of the E. coli chromosome (33, 53). However, all attempts to subclone agn43 from these λ clones or from restricted chromosomal DNA into the vectors pBluescriptII SK+, pGEM5, and pUC19 proved unsuccessful. In view of this fact, PCR was used in attempts to isolate and sequence agn43. In this respect, primers were designed from a knowledge of the N-terminal amino acid sequence of β43 and from known sequences within the genetic elements IS2F and sbcB flanking the chromosomal region presumed to contain the agn43 gene. These and other primers were used in PCRs to create clones pIH7 to -10, covering the balance of the agn43 gene together with flanking DNA. DNA was sequenced by primer walking at least twice in both directions using independent clones.
PCR and molecular cloning procedures.
PCR amplifications were performed with 500 ng of chromosomal DNA as the template and 0.2 μM (each) primer in a 100-μl reaction mixture containing 2 U of Taq DNA polymerase, 50 μM (each) deoxynucleoside triphosphate, 1.5 mM MgCl2 and 10 μl of the manufacturer’s buffer. Forty cycles of 1 min of denaturation at 94°C, 1 min of primer annealing at 65°C, and 1-min extension by Taq polymerase at 72°C were carried out. E. coli ML308-225 derivatives were constructed by P1 transduction (52) from E. coli K-12 strains harboring the mutations of interest. DNA analysis and manipulations were performed according to standard methods (3).
DNA sequence determination and analysis.
Initial DNA sequencing reactions were performed as instructed by the manufacturer with the FLASH thermal cycle sequencing kit (Genpak Ltd.) and M13 universal forward and reverse primers. Primer-walking sequencing reactions were carried out by using dye terminator chemistry at Kings College School of Medicine and Dentistry, London, England. All samples were electrophoresed with an ABI 373A sequencer. DNA sequence analysis was performed with the Genetics Computer Group software package from the University of Wisconsin. Sequence similarity searches were carried out with the BLAST program at the National Center for Biotechnology Information (2).
Southern hybridization.
Southern blotting was performed by a modification of the method described by Southern (70) in which a vacuum blotter system (VacuGene; LKB) was used for transfer of DNA to nitrocellulose filters. Hybridizations were performed at 65°C with random-labeled probe essentially as described elsewhere (70). The Prime-a-Gene kit (Promega) and 20 μCi of [α-32P]dATP were used to radiolabel purified DNA fragments (25 to 50 ng) by nick translation. After incubation, the labeled probe was passed through a Nick column (Pharmacia) to remove unincorporated radiolabeled nucleotide.
Structural predictions.
Computational analysis was performed with different algorithms suited for secondary-structure predictions developed by Kyte and Doolittle (45), Jähnig (37), or Emini et al. (29) which are available in the HUSAR program package of the Deutsches Krebsforschungszentrum (Heidelberg, Germany). The location of surface sites was also predicted by the method of Parker et al. (61). The presence of coiled-coil segments (8, 48, 49) was assessed by using the MultiCoil program (75a).
Nucleotide sequence accession number.
The DNA sequence described here has been deposited in the GenBank library and has been assigned accession no. U24429.
RESULTS
Nucleotide sequence of the region encompassing agn43.
The total contiguous sequence of 7,738 bp generated by the sequencing strategy detailed in Materials and Methods contained, as expected, an IS2 element 5′ to the putative agn43 gene (positions 38 to 1363), a large open reading frame (ORF) of 3,275 nucleotides (positions 2477 to 5752) and a bacteriophage P2 attachment site (position 6876 to 7168; GenBank entry U24429). That the large ORF encodes agn43 and that the first ATG codon (position 2636) corresponds to the start site of the gene is supported by a putative translation product which shows (i) the presence between residues M1 and A52 of a region bearing all the hallmarks of an extended signal sequence (see below); (ii) the presence, beginning at residues A53, G228, G161, K419, and P552, of sequences of amino acids corresponding precisely to those determined empirically for the N termini of α43, αa, αb, αc, and β43, respectively; and (iii) excellent correlation for both subunits between the predicted and empirically determined amino acid compositions (18). A sequence similar to a rho-independent transcription terminator is present beginning 32 nucleotides beyond the stop codon and contains interrupted inverted repeats with the potential for forming a hairpin structure containing a loop of 11 bases and a stem of 3 bases. The predicted ribosome binding site with a sequence TAAGG was identified 8 bp upstream of the proposed ATG start codon and is followed by a stretch of four adenosine residues which are presumed to confer maximal efficiency of initiation of translation. The overall G+C content of the agn43 gene is 57.6%. Two additional ORFs of 708 and 1,536 bp were identified up- and downstream of agn43, respectively. Neither ORF displayed significant homology to any known genes.
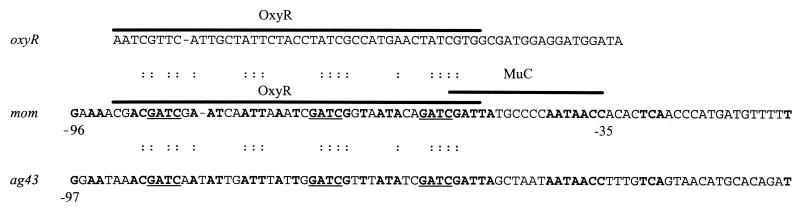
The putative promoter region revealed sequences (TTGTCA and TATGTT) in good agreement with the consensus sequences predicted for the −35 (TTGACA) and −10 (TATAAT) sites of E. coli genes, though they are separated by only 14 nucleotides. An interesting feature of this region was the nucleotide sequence homology (39.5% identity) shared between it and the promoter regions of the mom and oxyR genes, both of which are known to bind OxyR, a LysR-type transcriptional activator (12, 22). Moreover, direct comparison of the OxyR and MuC binding sites of the mom regulatory region with that of agn43 shows a region displaying 65.6% identity at the nucleotide level (Fig. 1). This includes a 6- and a 7-bp stretch of nucleotides identical to those seen at the beginning and the end of the MuC binding domain (13), respectively. The putative promoter region of agn43 also possesses three GATC sites which have the potential for methylation by Dam methylase (Fig. 1). These sites are positioned at points almost identical to those observed for the mom gene. No GATC sites are present in the same region of the oxyR promoter.
FIG. 1.
Locations and alignment of OxyR binding sites. The extents of the OxyR binding sites for mom and oxyR have been determined (12, 44). The MuC binding site has been determined for mom (13). MuC and OxyR binding sites are overlined by thick lines. Dashes represent gaps introduced for better alignment. GATC sites are underlined. Identical nucleotides conserved between the three (putative) promoter regions are indicated by dots; identical nucleotides between agn43 and mom sequences are indicated by boldface letters. Only the autoregulatory site of oxyR is shown. Nucleotides are given in the standard single-letter code.
An interesting feature of the DNA region encoding agn43 is the presence of 14 direct repeats of 10 bp or longer and an additional 31 repeat stretches of 12 bp or longer which contain one nucleotide mismatch, all of which are translated in the same reading frame. These are located predominantly toward the 5′ end of the gene and give rise to repeats in amino acid sequence. The sequence of agn43 of E. coli ML308-225 (U24429) shows 98% identity at the nucleotide level to the corresponding gene subsequently sequenced for E. coli K-12 (AE000291).
Amino acid sequence of the Ag43 precursor.
The Ag43 ORF encodes a primary translation product of 1,039 amino acids with a molecular mass of 107,067 Da. The N-terminal region possesses the characteristics of an enlarged signal sequence with (i) four positively charged amino acids between residues 28 and 33 (R28ARGKR) followed by (ii) a hydrophobic region spanning 12 neutral amino acids and (iii) a sequence (V50LA) compatible with the consensus for a signal peptidase recognition site (36) (Fig. 2).
FIG. 2.
Comparison of the N-terminal segments of Ag43 and other autotransporters. The leader sequence of Ag43 (this work) has been aligned with those of Tsh (GenBank accession no. I54632), AIDA-I (Q03155), EspC (U69128), SepA (Z48219), Pet (AF056581), Hia (U38617), AIDA-I (I64138), Hsf (U41852), EspP (AB011549), ShMu (U35656), UspA1 (U57551), and HMW-1 (A43855). The N-terminal sequence of HMW-2, which is identical to that of HMW-1, is not shown. The cleavage sites are indicated by <>. The positions of the N, H, and C domains which are characteristic of a signal sequence (36) are also shown.
The mature (signal peptidase processed) protein begins at A53, is composed of 987 amino acids, and has a calculated molecular mass of 101,413 Da. This figure approximates the combined molecular masses of both subunits as estimated by SDS-PAGE and gel filtration (18). This, together with the fact that the N terminus of mature β43 starts at P552 (56), suggests that agn43 is initially translated as a polyprotein precursor which undergoes at least two posttranslational processing steps, viz. (i) removal of the 52-residue signal peptide and (ii) internal cleavage between D551 and P552 to yield α43 (predicted Mr of 49,789) and β43 (predicted Mr of 51,642). That release of β43 from the polyprotein precursor involves a single cleavage event occurring between D551 and P552 is supported by the results of electrospray mass spectroscopy, which indicate an Mr for purified α43 of 49,807 ± 24.11, in good agreement with the predicted value.
The predicted Ag43 polyprotein is notable for the abundance of glycine and threonine residues and the presence of only a single cysteine residue, which features in the leader peptide. Indeed, the four residues A, T, G, and V constitute 45% of the precursor molecule. α43 has a calculated isoelectric point of 4.85, a value which agrees well with experimental values (18). The corresponding value predicted for β43 is 6.15.
Amino acid sequence similarities.
Notable sequence similarities were detected between distinct regions of the Ag43 polyprotein and various secreted or surface-exposed proteins in gram-negative bacteria. For example, similarities were detected in the signal sequence region between the Ag43 precursor and those of several potential virulence determinants (Fig. 2). An interesting common feature of these signal sequences is that, without exception, they begin with M1N(R/K) closely followed at residue 9 of Ag43 by a motif (Y/F/W, X, I/L/V, X, or Y/F/W) containing conserved aromatic and hydrophobic residues. In addition, the sequence V21VASELAR observed for Ag43 features in only slightly modified form in the other sequences. Except in Hsf, aidA-I, and Hia, other regions of the signal sequence, i.e., the N, H, and C domains, show very little sequence similarity.
Analysis of α43 alone revealed strong homology (31.2 to 25% identity; 64.7 to 52.5% similarity) with AIDA-I, Tsh, Yejo, and YpjA of E. coli, SepA and IcsA of Shigella flexneri, Hia, aidA-I, and Hsf of Haemophilus influenzae, and BrkA of Bordetella pertussis. Highest scores were achieved with AIDA-I (where alignment extends for the 499 amino acids of α43, giving 31.2% identity and 62% similarity) and with the E. coli conceptual translation products termed YpjA (30.5% identity and 60.7% similarity) and Yejo (29.6% identity and 58.1% similarity). All alignments began at the first residue of the predicted mature proteins.
In contrast, β43 showed only low homology to the C-terminal domains of the same proteins. However, comparison of the C-terminal extremity of β43 with corresponding domains of known autotransporters revealed commonalities in as much as all terminate with an aromatic residue (F or W), which is preceded by aromatic or aliphatic residues at a periodicity of two residues (32). This is a feature of β-barrel-forming integral membrane proteins (see below).
In view of the sequence similarity between Ag43 and AIDA-I, it was of interest to determine whether the two were immunologically related. Analysis of AIDA-I-producing E. coli 2787 (6) by colony and Western immunoblotting and by immunofluorescence microscopy using anti-α43 antibodies revealed the presence of a 60-kDa phase-variable surface antigen, analogous to α43. However, no cross-reacting proteins in the molecular mass range (∼100 kDa) anticipated for AIDA-I could be detected in either colony lysates or purified cell envelopes (data not shown).
Amino acid motifs.
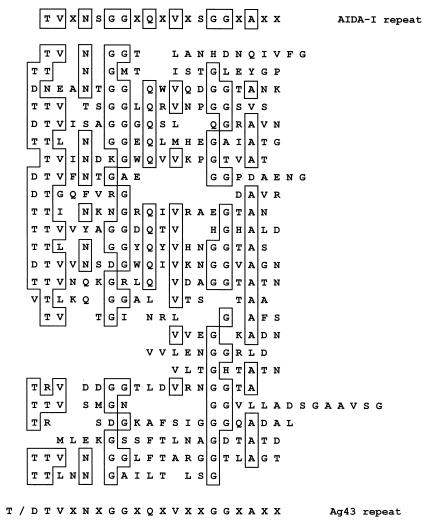
An interesting property of AIDA-I is a region near the N terminus where 21 repeats built up of 19 to 20 amino acid residues are followed, after a short interruption, by an additional 10 repeats (7). In view of the homology displayed between AIDA-I and Ag43, and the repeat structure observed within agn43, the amino acid sequence of Ag43 was aligned with the conserved consensus motif observed in AIDA-I (Fig. 3). The major differences noted between the repetitive regions of Ag43 and AIDA-I are (i) a smaller number of repeats in Ag43 with a more variable length (12 to 20 residues); (ii) a paucity of serine residues in the Ag43 sequence; and (iii) an additional T or D residue at the start of the Ag43 repeat.
FIG. 3.
Comparison of the amino acid sequence of the N-terminal region of Ag43 with the repeat motif of AIDA-I. Alignment begins at T62 of the precursor protein. Amino acids are given in the standard single-letter code. The consensus sequence shown at the bottom represents the amino acids most commonly found in the appropriate position.
With regard to functional motifs, the α43 subunit contains a stretch of amino acids (L386LADSGAAVSGT) whose sequence is compatible with the consensus observed for an aspartyl protease active site (30, 64) and β43 contains a sequence (G631GRATGKT) compatible with that proposed to form a loop (the P loop) involved in binding ATP or GTP (66). In addition, each subunit possesses (i) an RGD motif (at R208 and R970) implicated in the binding of human integrins (35) and (ii) potential glycosaminoglycan attachment sites at S495GNG and S654GKG (14). A recent survey has shown the presence of similar motifs in other autotransporters (32).
Processing of Ag43 in protease-deficient host strains.
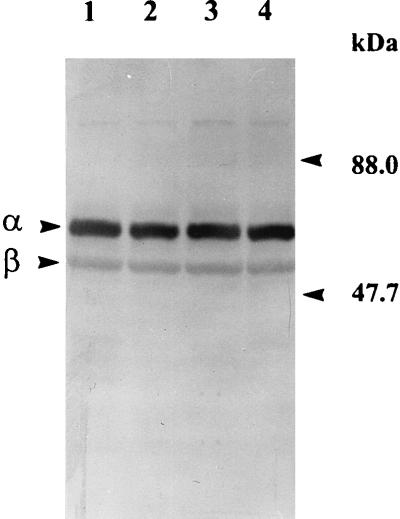
The possible role of several endogenous membrane-associated proteases, viz., DegP, OmpP, and OmpT, in processing the Ag43 precursor to α43 and β43 was investigated in protease-deficient mutants. The degP mutation was transduced from parent strain KS474 (degP) into E. coli ML308-225. The resulting transductant (IRH11) and the ompT ompP strain UT5600 were screened for the correct processing of Ag43 by Western blotting using specific anti-Ag43 antibodies. As shown in Fig. 4, normal processing of the Ag43 precursor to α43 and β43 occurs in the absence of the DegP, OmpP, and OmpT proteases. Additionally, it should be noted that both subunits retain outer membrane localization (57) in the mutants (data not shown) and that all attempts to prevent cleavage of the Ag43 precursor to its α43 and β43 subunits by the addition, at or before lysis, of a cocktail of protease inhibitors (phenylmethylsulfonyl fluoride, Nα-p-tosyl-l-lysine chloromethyl ketone, and benzamidine HCl) have proved unsuccessful. Normal processing of the Ag43 precursor was also observed in dsbA mutants JCB571 and IRH10 lacking a functional periplasmic disulfide oxidoreductase (Fig. 4).
FIG. 4.
Processing of the Ag43 precursor in E. coli strains carrying the ompT, ompP, and degP mutations. Lane 1, E. coli ML308-225 expressing Ag43; lane 2, ompT ompP strain UT5600; lane 3, ML308-225 degP derivative IRH11; and lane 4, dsbA derivative IRH10. After Western blotting, α43 and β43 were detected by using anti-Ag43 antibodies as the primary probe. Note that the differential intensities observed for the α43 and β43 subunits are a function of the polyclonal antiserum used and not a reflection of apparent subunit stoichiometry (which is 1:1). The molecular masses of marker proteins are indicated on the right.
Predicted organization of α43 and β43 in the outer membrane.
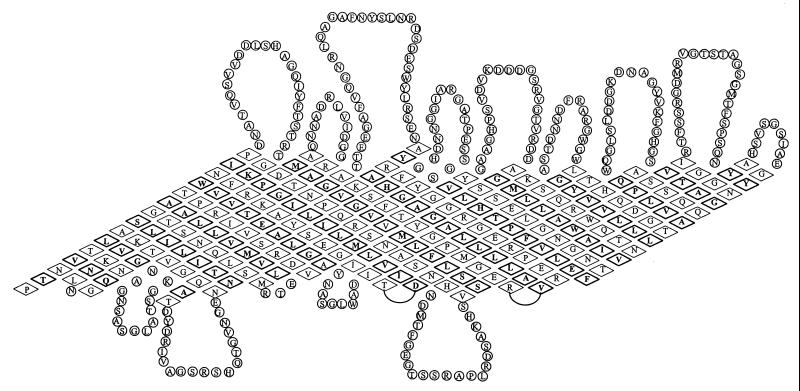
Previous studies have clearly shown that α43 is a peripheral surface-expressed protein anchored to the outer membrane via a noncovalent interaction with β43 (18, 56). To further elucidate the possible organization of the subunits in the outer membrane, secondary-structure analyses were performed. Hydrophobicity plots generated for each subunit according to the algorithm of Kyte and Doolittle (45) showed no linear stretch of 20 to 24 hydrophobic amino acids of the type often associated with the α-helical transmembrane segments of plasma membrane proteins, nor did either subunit show significant evidence of amphipathic α-helical coiled-coil structure (8, 48, 49). In contradistinction, β sheets consisting of alternating hydrophobic and hydrophilic amino acid residues were predicted with a high probability for the β43 subunit. According to Jähnig (37), amphipathic β strands ideally exist with high probability if (i) the calculated values for hydrophobicity [Hβ(i)] vary with a periodicity of two residues between Hβ(i) ≥ 1.6 and Hβ(i + 1) ≤ 0.4; (ii) this occurs over at least 10 residues; and (iii) for the validity of the prediction, the protein must form at least an eight-stranded β-barrel structure. According to these predictions, β43 consists of at least 18 membrane-spanning amphipathic β strands interrupted by external loops and generally short periplasmic loops. Regions of high surface probability, predicted according to the method of Emini et al. (29) or Parker et al. (61), are in good agreement with this topological arrangement since such areas are always located between the β strands (data not shown). Accordingly, we propose that the 18 β sheets are arranged as depicted in Fig. 5 and form a β-barrel structure harboring a pore for the translocation of α43 to the cell surface. α43 itself shows five (i.e., <8) potential amphipathic β strands and numerous regions, including N70 to D72, Y91 to A97, A110 to K112, D171 to G173, T214 to R221, T288 to R294, G336 to A338, A357 to V362, G397 to G402, and T456 to N468, of high surface probability.
FIG. 5.
Proposed topology of β43 in the outer membrane. According to the secondary structure prediction, β43 is composed of 18 amphipathic β strands with 11 to 17 amino acid residues per strand. Amino acids in amphipathic β-sheet structure are indicated by diamonds. Amino acids in boldface diamonds are hydrophobic and face the lipid phase of the outer membrane, while hydrophilic side chains facing the interior pore of the molecule are shown in lightface diamonds. The transmembrane segments are interrupted by large extracellular loops and shorter periplasmic loops. The β-barrel structure is completed by interaction of the first transmembrane strand with the last antiparallel C-terminal strand.
agn43 homologues in other members of the family Enterobacteriaceae.
To assess the presence of agn43 homologues in other members of the Enterobacteriaceae, Southern hybridization experiments were conducted on BamHI- and EcoRI-restricted DNA isolated from the Shigella, Salmonella, Enterobacter, Klebsiella, Edwardsiella, Erwinia, Proteus, and Serratia strains listed in Table 1. However, the only hybridizing signal detected with the α43-specific 1,150-bp gene probe was that arising from control E. coli ML308-225 DNA. Even after prolonged exposure, no signal could be detected from the other enterobacterial DNA preparations, indicating that these strains do not possess genes with strong nucleotide sequence homology to agn43 of E. coli.
Involvement of OxyR and Dam in Ag43 phase variation.
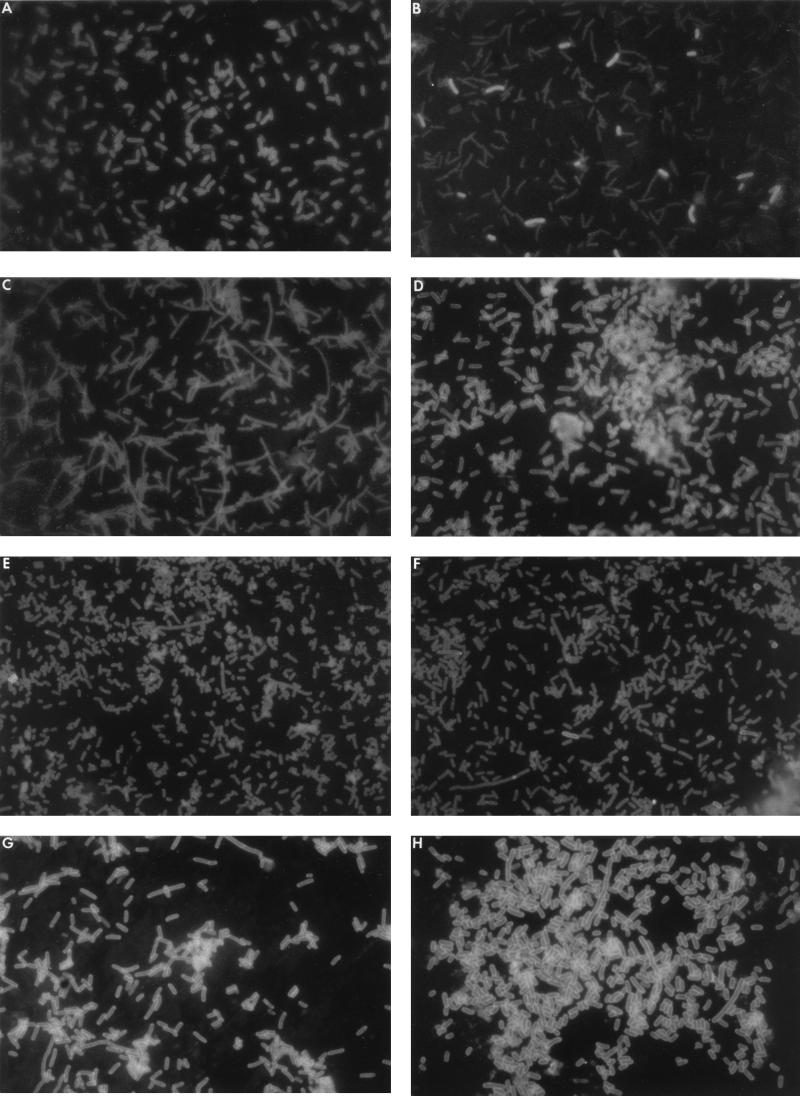
To examine the roles of global regulatory proteins in the expression of Ag43, a variety of E. coli derivatives carrying defined mutations in genes encoding a range of well-documented regulatory proteins were screened (together with parental strains [Table 1]) by immunofluorescence for the presence of Ag43. Subsequently, these same mutations were transduced into control strain ML308-225. Analysis of the resulting transductants containing deletions in the hns, hupA, hupB, crp, cya, mbf, gyrA, gyrB, fis, topA, and rpoS genes by immunofluorescence microscopy and colony immunoblotting revealed that all retained the ability to undergo reversible phase variation (data not shown) in much the same manner as control populations of E. coli ML308-225 (Fig. 6A and B). In contrast, neither Ag43 nor an anti-α43 cross-reactive protein could be detected in either of two Dam methylase mutants (E. coli GM2929 or E. coli GM3819) or corresponding E. coli ML308-225 dam transductants (IRH12 and IRH13) when they were screened by colony immunoblotting, Western blotting (Fig. 6I) or immunofluorescence microscopy (Fig. 6C). These strains all appeared to be locked off.
FIG. 6.
Expression of Ag43 in dam and oxyR mutants assessed by slide immunofluorescence microscopy using anti-α43 antibodies (A to H) and by Western immunoblotting (I). (A) An Ag43+ (phase on) population of E. coli ML308-225 showing ∼65% positive cells; (B) an Ag43− (phase off) population of E. coli ML308-225 showing ∼5% positive cells; (C) dam derivative IRH13; (D) oxyR derivative IRH14; (E) IRH16 (IRH14[pAQ25]); (F) IRH17 (IRH14 [pGSO68]); (G) IRH18 (IRH14[pGSO69]); (H) IRH19. (I) Lanes A to H of the immunoblot contain cell lysates of strains shown in panels A to H, respectively. The position of the α43 subunit is indicated. Previous work has documented that the presence of α43 is indicative of the presence of both α43 and β43 in equal stoichiometry.
The involvement of OxyR in expression of Ag43, suggested from a consideration of promoter sequence homologies (Fig. 1) and other data (33, 75), was confirmed following transduction of the ΔoxyR::kan (λY2055 oxyS-galK) mutation from E. coli GS05 and the ΔoxyR::kan mutation from GS09 into E. coli ML308-225 (IRH14 and IRH15). In contradistinction to the dam mutants, the oxyR mutants are firmly locked on for Ag43 expression, as judged by colony blotting, Western immunoblotting (Fig. 6I), and slide immunofluorescence microscopy (Fig. 6D). Reintroduction into strain IRH14 of the wild-type oxyR gene on plasmid pAQ25 by CaCl2 transformation generated ampicillin-resistant transformants displaying a restored phase-variable phenotype in which both Ag43+ and Ag43− variants could be detected (Fig. 6E), albeit the percentage of positive variants was lower than normal (∼1%). Results similar to those for complementation with pAQ25 were obtained following transcomplementation experiments with pGSO68, which contains an oxyR gene carrying a mutation (C199S) in the redox center (Fig. 6F). This mutation locks the molecule in the reduced form and prevents transcriptional activation of antioxidant genes associated with oxidative response. In contrast, similar experiments conducted with pGSO69, which contains an oxyR gene carrying a mutation (A233V) that causes constitutive expression of antioxidant genes, failed to restore phase variation of Ag43, with the transformants retaining the locked-on state of Ag43 expression observed for recipient strains (Fig. 6G). Analysis of a strain (IRH19) containing mutations in both the dam and oxyR genes indicates that the expression of Ag43 remains in the locked-on state (Fig. 6H).
DISCUSSION
The goals of this study were to identify the gene encoding Ag43, a major bipartite outer membrane protein which mediates autoaggregation and colony form variation in E. coli (33), to elucidate the likely export and assembly mechanisms involved and to establish the factors controlling the phase-variable expression of the antigen.
The strategy used to identify and sequence the agn43 gene (see Results) involved a knowledge of the N-terminal amino acid sequences of α43, β43, and internal peptides αa, αb, and αc, generated by V8 protease digestion of α43. Three separate lines of evidence indicate that the 3,275-bp ORF, identified by this strategy, encodes Ag43: (i) the amino acid sequences determined empirically for the N termini of α43, β43, and the three internal peptides can be found at anticipated locations within the predicted sequence of the primary translation product; (ii) the predicted Mr of α43 (49,789) is consistent with that determined by electrospray mass spectroscopy and gel filtration; and (iii) the predicted amino acid compositions of the two subunits agree with those determined empirically for the purified subunits (18).
The autotransporters, a family of proteins from gram-negative bacteria, utilize the recently designated type IV secretion system to reach the extracellular milieu. The secretion system is intimately linked to the unifying structure possessed by these proteins which, in general, comprises an N-terminal leader sequence, a mature protein (α) which is translocated across the outer membrane, and a C-terminal (β) domain which forms a β-barrel pore to allow translocation of the mature protein (32). On the basis of amino acid sequence similarities and of several structural commonalities, it appears that Ag43 belongs to the autotransporters. Thus, the agn43 gene encodes a precursor of 107 kDa which must be processed to the mature bipartite outer membrane complex by at least two cleavage events. Cleavage by a signal peptidase after A52 is presumed since (i) this residue immediately precedes the sequence corresponding to the N terminus of mature α43 and (ii) the upstream sequence shares many of the primary structural features characteristic of a general secretory pathway (GSP) peptide. Interestingly, the Ag43 signal sequence contains a 27-residue N-terminal extension showing striking sequence similarities with N-terminal extremities of a number of autotransporter proteins and the HMW (high-molecular-weight) proteins of H. influenzae. The presence of a common motif upstream from the classical signal sequences suggests that this region might have a particular function, perhaps in targeting and export of these outer membrane proteins or in induction of correct folding.
Final expression of Ag43 on the cell surface as the mature 1:1 complex (predicted Mr of 101,431) composed of the 495-amino-acid α43 subunit and a 492-amino-acid β43 subunit (Mr of 51,642) also requires posttranslational cleavage of the precursor protein between residues D547 and P548. This could be effected by a membrane-bound protease other than OmpT, OmpP, or DegP (32, 40) or by an autoproteolytic event. The latter explanation is more attractive especially in view of the demonstration of a consensus sequence for an aspartyl protease active site (30) and the observation that retroviral aspartyl protease is encoded as a segment of a polyprotein precursor and is involved in the correct maturation of the polyprotein by autocleavage (64). However, it should be recognized that (i) autoprocessing has not been convincingly demonstrated in vitro for Ag43 or indeed several other autotransporters (see reference 32 for a recent review) and (ii) the aspartyl protease active site of α43 may have an additional or alternative functional role in cleavage of an as yet unidentified host substrate.
One of the most striking features of autotransporters is the ability of the C-terminal domain to form, within the outer membrane, a β-barrel pore composed of amphipathic antiparallel β sheets, through which the N-terminal (α) domain is secreted. Consistent with the proposal that Ag43 is an autotransporter is the secondary structural prediction that the β43 domain forms a pore of 18 amphipathic β sheets. The lengths of these predicted β strands (10 to 16 residues) are in good agreement with the sizes of β strands identified in the OmpF and PhoE porin structure (38). It should be noted that Loveless and Saier have aligned the autotransporter β domains and have predicted that all autotransporters possess 14 conserved nine-residue β strands (47). However, this analysis did not include Ag43, whose unusually long β domain (β43) shows no significant homology with any other autotransporter. Additional evidence that β43 forms a β barrel is indicated by the fact that β43 possesses three terminal residues (VTF) which are observed in similar positions in the β domains of other autotransporters and which are supposedly involved in targeting of the proteins to the outer membrane (32).
A number of outer membrane proteins and bacterial surface structures have been shown to be phase variable. In most cases, the precise biological significance of phase variation remains speculative. However, the most likely role is to provide the organism with a strategy to survive and persist in a particular ecological niche (26, 27). An important feature of previous studies was the unequivocal demonstration that Ag43 undergoes reversible phase variation at frequencies similar to those observed for other phase-variable systems (56). It is clear from the present study that Dam plays a critical role in phase variation of agn43 since dam mutants produce no detectable levels of Ag43, as judged by a variety of criteria. Dam binds to GATC sequences and methylates adenosine at the N6 position. Most of the estimated 18,000 GATC sites in E. coli are methylated by Dam in this fashion (31). However, a number of these have been shown to be differentially protected from methylation and that such protection is involved in regulating transcription of certain genes. This is the case with pap, which is controlled by Dam and Lrp (31, 74). However, the mechanisms of regulation of the pap and agn43 genes are quite distinct inasmuch as OxyR and not Lrp appears to act as the methylation blocking factor affecting expression of Ag43. Thus, mutants lacking a functional gene product constitutively express Ag43 and complementation of the oxyR mutation reinitiates the on↔off switch. The fact that the mutagenized oxyR gene (C199S) restores phase variation of Ag43 in complementation experiments, in a manner similar to that of the wild-type gene, suggests that OxyR may have to be in the reduced form to inhibit agn43 transcription. In contradistinction to the above, reintroduction of an oxyR gene carrying an A233V mutation does not lead to restoration of phase variation. The mutated alanine residue (A233) is located in a C-terminal region which shows homology with other LysR-type transcriptional regulators and which may be involved in contact with RNA polymerase and/or in the multimerization of OxyR (44, 73). Thus, a multimerized OxyR may be required to prevent Ag43 transcription.
Based on the extensive nucleotide homology present in the regulatory regions agn43 and other genes (oxyR and mom) known to be negatively regulated by OxyR, one can predict an OxyR binding region upstream of the agn43 gene and extending between positions −95 and −49. Analysis of the OxyR footprint of the mom and oxyR loci indicates that the essential protein-DNA contacts are made with the first 5 bp of the protected region, covering the first GATC site in mom (13). However, analyses of the mom promoter region have indicated that methylation of all GATC sites by Dam methylase is required for successful transcription of the mom gene (39, 63). Of particular note in this respect was the observation of three GATC sites in the putative promoter region of the agn43 gene with a spatial distribution almost identical to that observed in the mom promoter region. This suggests that a similar methylation pattern is required to allow transcription of the agn43 gene and that all sites have to be methylated to prevent binding of OxyR to the agn43 promoter. No GATC sites are present in the oxyR promoter region, an observation consistent with the fact that DNA methylation is not involved in regulating OxyR expression. The presence within the mom and agn43 regulatory regions of 7-bp (AATAACC) and 6-bp (CGATTA) homologous sequences (corresponding to the extremities of the MuC binding domain of mom [13]) suggests that another protein apart from OxyR and perhaps analogous to MuC may be required for agn43 transcription.
In summary, the evidence presented here strongly suggests that Ag43 is an autotransporter whose phase-variable expression is transcriptionally regulated by DNA methylation and by OxyR. Based upon analysis of mutants and upon sequence comparisons, it is proposed that OxyR may act as a repressor of Ag43 transcription by binding to unmethylated GATC sites in the regulatory region of the gene and that methylation of these sites prevents OxyR binding. Although analogous to the system involved in regulating expression of the mom gene of phage Mu, the phase variation mechanism proposed for agn43 is, to the best of our knowledge, a novel one in bacteria and one which is amenable to direct testing, e.g., by mutation of critical GATC sequences and by in vitro and/or in vivo footprint analysis.
ACKNOWLEDGMENTS
This research was supported in part by grant HRB 32-91 from the Health Research Board of Ireland.
We thank James P. Nataro for helpful review of the manuscript and G. Storz for the kind gift of strains. Many thanks go to Mary Meehan for her patience, help, expertise, and scientific discussions.
REFERENCES
- 1.Adley C C, Bukhari A I. Methylation dependent expression of the mom gene of bacteriophage Mu: deletions downstream from the methylation sites affect expression. Nucleic Acids Res. 1984;12:3535–3550. doi: 10.1093/nar/12.8.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1989. [Google Scholar]
- 4.Bachmann B J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972;36:525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulphide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 6.Benz I, Schmidt M A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989;57:1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27) is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 8.Berger B, Wilson D B, Wolf E, Tonchev T, Milla M, Kim P S. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blyn L B, Braaten B A, Low D A. Regulation of pap pilin phase variation by a mechanism involving differential Dam methylation states. EMBO J. 1990;9:4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blyn L B, Braaten B A, White-Ziegler C A, Rolfson D H, Low D A. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 1989;8:613–620. doi: 10.1002/j.1460-2075.1989.tb03416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase-inducible “gear box” promoters: differential effects of katF mutations and role of α70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bölker M, Kahmann R. The Escherichia coli regulatory protein OxyR discriminates between methylated and unmethylated states of the phage Mu mom promoter. EMBO J. 1989;8:2403–2410. doi: 10.1002/j.1460-2075.1989.tb08370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bölker M, Wulczyn F G, Kahmann R. Role of bacteriophage MuC protein in activation of the mom gene promoter. J Bacteriol. 1989;171:2019–2027. doi: 10.1128/jb.171.4.2019-2027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourdon M A, Krusius T, Campbell S, Schwartz N B, Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci USA. 1987;84:3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 16.Braaten B A, Blyn L B, Skinner B S, Low D A. Evidence for a methylation-blocking factor (mbf) locus involved in pap pilus expression and phase variation in Escherichia coli. J Bacteriol. 1991;173:1789–1800. doi: 10.1128/jb.173.5.1789-1800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busby S, Kotlarz D, Buc H. Deletion mutagenesis of the Escherichia coli galactose operon promoter region. J Mol Biol. 1983;167:259–274. doi: 10.1016/s0022-2836(83)80335-0. [DOI] [PubMed] [Google Scholar]
- 18.Caffrey P, Owen P. Purification and N-terminal sequence of the α subunit of antigen 43, a unique protein complex associated with the outer membrane of Escherichia coli. J Bacteriol. 1989;171:3634–3640. doi: 10.1128/jb.171.7.3634-3640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caffrey P, McVeigh T, Owen P. Western immunoblotting. In: Owen P, Foster T J, editors. Immunochemical and molecular genetic analysis of bacterial pathogens. Amsterdam, The Netherlands: Elsevier Science Publishing; 1988. pp. 255–266. [Google Scholar]
- 20.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 21.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 22.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 23.Connell N, Han Z, Moreno F, Kolter R. An E. coli promoter induced by the cessation of growth. Mol Microbiol. 1987;1:195–201. doi: 10.1111/j.1365-2958.1987.tb00512.x. [DOI] [PubMed] [Google Scholar]
- 24.Dersch P, Schmidt K, Bremer E. Synthesis of the Escherichia coli K-12 nucleoid-associated DNA-binding protein H-NS is subjected to growth-phase control and autoregulation. Mol Microbiol. 1993;8:875–889. doi: 10.1111/j.1365-2958.1993.tb01634.x. [DOI] [PubMed] [Google Scholar]
- 25.Diderichsen B. flu, a metastable gene controlling surface properties of Escherichia coli. J Bacteriol. 1980;141:858–867. doi: 10.1128/jb.141.2.858-867.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorman C J. Genetics of bacterial virulence. Oxford, England: Blackwell Scientific Publications; 1994. [Google Scholar]
- 27.Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol. 1993;10:465–471. doi: 10.1111/j.1365-2958.1993.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 28.Earhart C F, Lundigran M, Pickett C L, Pierce J R. Escherichia coli K-12 mutants that lack major outer membrane protein a. FEMS Microbiol Lett. 1979;6:277–280. [Google Scholar]
- 29.Emini E A, Hughes J V, Perlow D S, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985;55:836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foltmann B. Gastric proteinases—structure, function, evolution and mechanism of action. Essays Biochem. 1981;17:52–84. [PubMed] [Google Scholar]
- 31.Hale W B, van der Woude M W, Low D A. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J Bacteriol. 1994;176:3438–3441. doi: 10.1128/jb.176.11.3438-3441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of autotransporters. Trends Microbiol. 1998;6:370–376. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 33.Henderson I R, Meehan M, Owen P. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol Lett. 1997;149:115–120. doi: 10.1111/j.1574-6968.1997.tb10317.x. [DOI] [PubMed] [Google Scholar]
- 34.Huisman O, Faelen M, Girard D, Jaffé A, Toussaint A, Rouvière-Yaniv J. Multiple defects in Escherichia coli mutants lacking HU protein. J Bacteriol. 1989;171:3704–3712. doi: 10.1128/jb.171.7.3704-3712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isberg R R, Tran Van Nhieu G. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 1994;2:10–14. doi: 10.1016/0966-842x(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 36.Izard J W, Kendall D A. Signal peptides: exquisitely designed transport promoters. Mol Microbiol. 1994;13:765–773. doi: 10.1111/j.1365-2958.1994.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 37.Jähnig F. Structure predictions of membrane proteins are not that bad. Trends Biochem Sci. 1990;15:93–95. doi: 10.1016/0968-0004(90)90188-h. [DOI] [PubMed] [Google Scholar]
- 38.Jeanteur D, Lakey J H, Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991;5:2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 39.Kahmann R. Methylation regulates the expression of a DNA modification function encoded by bacteriophage Mu. Cold Spring Harbor Symp Quant Biol. 1983;47:639–646. doi: 10.1101/sqb.1983.047.01.075. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann A, Stierhof Y-D, Henning U. New outer membrane-associated protease of Escherichia coli K-12. J Bacteriol. 1994;176:359–367. doi: 10.1128/jb.176.2.359-367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch C, Vandekerckhove J, Kahmann R. Escherichia coli host factor for site-specific DNA inversion: cloning and characterization of the fis gene. Proc Natl Acad Sci USA. 1988;85:4237–4241. doi: 10.1073/pnas.85.12.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 43.Kullik I, Stevens J, Toledano M B, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for DNA binding and multimerization. J Bacteriol. 1995;177:1285–1291. doi: 10.1128/jb.177.5.1285-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kullik I, Toledano M B, Tartaglia L A, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J Bacteriol. 1995;177:1275–1284. doi: 10.1128/jb.177.5.1275-1284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 46.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 47.Loveless B J, Saier M H., Jr A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol Membr Biol. 1997;14:113–123. doi: 10.3109/09687689709048171. [DOI] [PubMed] [Google Scholar]
- 48.Lupas A. Prediction and analysis of coiled coil structures. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 49.Lupas A, van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 50.McNairn E, NiBhriain N, Dorman C J. Overexpression of the Shigella flexneri genes coding for DNA topoisomerase IV compensates for loss of DNA topoisomerase I: effect on virulence gene expression. Mol Microbiol. 1995;15:507–517. doi: 10.1111/j.1365-2958.1995.tb02264.x. [DOI] [PubMed] [Google Scholar]
- 51.Menzel R, Gellert M. Fusions of the Escherichia coli gyrA and gyrB control regions to the galactokinase gene are inducible by coumermycin treatment. J Bacteriol. 1987;169:1272–1278. doi: 10.1128/jb.169.3.1272-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 53.Noda A, Courtwright J B, Denor P F, Webb G, Kohara Y, Ishihama A. Rapid identification of specific genes in Escherichia coli by hybridization to membranes containing the ordered set of phage clones. BioTechniques. 1991;10:474–477. [PubMed] [Google Scholar]
- 54.Nowicki B, Rhen M, Väisänen-Rhen V, Pere A, Korhonen T K. Immunofluorescence study of fimbrial phase variation on Escherichia coli KS71. J Bacteriol. 1984;160:691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owen P. The gram-negative outer membrane: structure, biochemistry and vaccine potential. Biochem Soc Trans. 1992;20:1–6. doi: 10.1042/bst0200001. [DOI] [PubMed] [Google Scholar]
- 56.Owen P, Meehan M, de Loughrey-Doherty H, Henderson I. Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol Med Microbiol. 1996;16:63–76. doi: 10.1111/j.1574-695X.1996.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 57.Owen P, Caffrey P, Josefsson L-G. Identification and partial characterization of a novel bipartite protein antigen associated with the outer membrane of Escherichia coli. J Bacteriol. 1987;169:3770–3777. doi: 10.1128/jb.169.8.3770-3777.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owen P, Caffrey P, Josefsson L-G, Meehan M. Outer membrane proteins: old and new. In: Ron E Z, Rottem S, editors. Microbial surface components and toxins in relation to pathogenesis. New York, N.Y: Plenum Press; 1991. pp. 127–139. [Google Scholar]
- 59.Palmer B R, Marinus M G. The dam and dcm strains of Escherichia coli—a review. Gene. 1994;143:1–12. doi: 10.1016/0378-1119(94)90597-5. [DOI] [PubMed] [Google Scholar]
- 60.Parker B, Marinus M G. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene. 1988;73:531–535. doi: 10.1016/0378-1119(88)90517-3. [DOI] [PubMed] [Google Scholar]
- 61.Parker J M R, Gou D, Hodges R S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray derived accessible sites. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- 62.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plasterk R, Vrieling H A, Van de Putte P. Transcription initiation of Mu mom depends on methylation of the promoter region and a phage-coded transactivator. Nature (London) 1983;301:344–347. doi: 10.1038/301344a0. [DOI] [PubMed] [Google Scholar]
- 64.Rao J K M, Erickson J W, Wlodawer A. Structural and evolutionary relationships between retroviral and eucaryotic aspartic proteinases. Biochemistry. 1991;30:4663–4671. doi: 10.1021/bi00233a005. [DOI] [PubMed] [Google Scholar]
- 65.Roberts M, Fairweather N F, Leininger E, Pickard D, Hewlett E L, Robinson A, Hayward C, Dougan G, Charles I G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991;5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 66.Saraste M, Sibbald P R, Wittinghofer A. The P-loop: a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 67.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100-kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 68.Schnaitman C A, McDonald G A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984;159:555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith S G J. Regulation of CS1 fimbrial expression. Ph.D. thesis. Dublin, Ireland: Trinity College; 1995. [Google Scholar]
- 70.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 71.Storz G, Tartaglia L A, Ames B N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 72.Strauch K L, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao K, Zou C, Fujita N, Ishihama A. Mapping of the OxyR protein contact site in the C-terminal region of RNA polymerase alpha subunit. J Bacteriol. 1995;177:6740–6744. doi: 10.1128/jb.177.23.6740-6744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van der Woude M, Braaten B, Low D. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol. 1996;4:5–9. doi: 10.1016/0966-842x(96)81498-3. [DOI] [PubMed] [Google Scholar]
- 75.Warne S R, Varley J M, Boulnois G J, Norton M G. Identification and characterization of a gene that controls colony morphology and auto-aggregation in Escherichia coli K-12. J Gen Microbiol. 1990;136:455–462. doi: 10.1099/00221287-136-3-455. [DOI] [PubMed] [Google Scholar]
- 75a.Wolf E, Kim P S, Berger B. Multicoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 1997;6:1179–1189. doi: 10.1002/pro.5560060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu X-M, Reznikoff W S. Deletion analysis of the CAP-CAMP binding site of the Escherichia coli lactose promoter. Nucleic Acids Res. 1984;12:5449–5464. doi: 10.1093/nar/12.13.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]