Abstract
Ferric iron reductase was purified from magnetotactic bacterium Magnetospirillum (formerly Aquaspirillum) magnetotacticum (ATCC 31632) to an electrophoretically homogeneous state. The enzyme was loosely bound on the cytoplasmic face of the cytoplasmic membrane and was found more frequently in magnetic cells than in nonmagnetic cells. The molecular mass of the purified enzyme was calculated upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be about 36 kDa, almost the same as that calibrated by gel filtration analysis. The enzyme required NADH and flavin mononucleotide (FMN) as optimal electron donor and cofactor, respectively, and the activity was strongly inhibited by Zn2+ acting as a partial mixed-type inhibitor. The Km values for NADH and FMN were 4.3 and 0.035 μM, respectively, and the Ki values for Zn2+ were 19.2 and 23.9 μM for NADH and FMN, respectively. When the bacterium was grown in the presence of ZnSO4, the magnetosome number in the cells and the ferric iron reductase activity declined in parallel with an increase in the ZnSO4 concentration of the medium, suggesting that the ferric iron reductase purified in the present study may participate in magnetite synthesis.
Magnetospirillum magnetotacticum was isolated from the microaerobic zones of freshwater sediments in 1979 by Blakemore et al. (6). The bacterium synthesizes intracellular particles, known as magnetosomes, that are composed of single crystals with ferrimagnetic iron oxide magnetite (Fe3O4) (3, 8) and enclosed by lipid bilayers with some characteristic proteins (9). Under aerobic conditions at neutral pH, iron in the environment is in the oxidized form, resulting in the formation of insoluble polymers of hydroxides, carbonates, and silicates. Microorganisms under these conditions are therefore presented with the problem of obtaining the iron required for growth. M. magnetotacticum uses for iron acquisition a siderophore-mediated system similar to those found in other gram-negative organisms (15). However, the response of this bacterium to the iron concentration of the growth medium appears to differ from those of many other chemoheterotrophs. The bacterium produces significant amounts of a chelator at high iron concentrations and lesser amounts at low iron concentrations. Although Paoletti and Blakemore (15) could offer no explanation for this unusual response of M. magnetotacticum to the iron content of the medium, the bacterium appears to be well adapted for the scavenging of iron necessary to satisfy its very large requirements for magnetite biosynthesis.
On the other hand, Frankel et al. (8) have proposed that M. magnetotacticum synthesizes magnetites in the following sequence: (i) iron uptake with reduction of Fe3+ to Fe2+ in the transport process, (ii) formation of low-density hydrous ferric oxide with reoxidation of Fe2+, (iii) formation of high-density hydrous ferric oxide (ferrihydrite) through the dehydration of low-density hydrous oxide, and (iv) formation of magnetite by the partial reduction of iron and the further dehydration of ferrihydrite. Thus, Fe3+ reduction in the cell is considered to be essential for magnetite formation. Paoletti and Blakemore (16) investigated the localization of iron reductase of M. magnetotacticum and concluded that iron reduction occurs in the periplasm. However, it should be noted that neither the purification nor the function of iron reductase in magnetite synthesis has been reported. In the present study, to elucidate the molecular mechanism of biomineralization for magnetite synthesis in M. magnetotacticum, we purified ferric iron reductase from the bacterium and then characterized its molecular and enzymatic features investigating its involvement in magnetite synthesis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. magnetotacticum MS-1 (ATCC 31632) was cultured microaerobically in chemically defined growth medium (6). The ferric quinate was prepared by combining 2.7 g of FeCl3 and 1.9 g of quinic acid with 1 liter of distilled water, added to a final concentration of 34 μM for magnetite synthesis. Cells were harvested at the stationary phase by centrifugation at 10,000 × g for 15 min and suspended in 10 mM Tris-HCl buffer (pH 8.0) containing 0.2 M NaCl. The magnetic cells were collected with permanent bar magnets, and the nonmagnetic cells remaining in the tube were harvested at 10,000 × g for 15 min. Both types of cells were stored at −80°C until use.
Preparation of membrane, periplasmic, and cytoplasmic fractions from M. magnetotacticum.
The periplasmic and cytoplasmic fractions were prepared from the bacterium by the method described by Alefounder and Ferguson (1), with slight modifications. Cells were suspended in 10 mM Tris-HCl buffer (pH 8.0) containing 0.75 M sucrose and incubated with 1.5 mM EDTA plus lysozyme (200 μg per ml) at 30°C for 1 h. The suspension was centrifuged at 104,000 × g for 30 min, and the periplasmic fraction was retained as the supernatant. The precipitates obtained were resuspended in water at 4°C and centrifuged at 104,000 × g for 1 h. The supernatant was retained as the cytoplasmic fraction, and the pellet was resuspended in 10 mM Tris-HCl buffer (pH 8.0) and used as the membrane fraction. The magnetosomes in the membrane fraction were removed with a magnet. The contamination of these fractions was judged by measuring the activities of nitrite reductase (cytochrome cd1) as a periplasmic marker protein and malate dehydrogenase as a cytoplasmic marker protein, respectively.
Ferric iron reductase assay.
Iron reductase activity was measured by the standard method as described by Dailey and Lascelles (7), with trapping of the product as an Fe(II)-3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine (ferrozine) complex. The assay mixture contained 0.1 mM NADH, 0.2 mM ferric citrate, 1 μM flavin mononucleotide (FMN) and 20 mM sodium phosphate buffer (pH 7.0) in a total volume of 1 ml. The reaction was initiated by addition of the enzyme, and the increase in A562 was followed spectrophotometrically at room temperature. Nonenzymatic reduction was scarcely observed under these experimental conditions. The specific activity of ferric iron reductase was expressed as nanomoles of Fe(II)-ferrozine formed per minute per milligram of protein. The effect of pH on activity was tested by using 20 mM sodium phosphate buffer (pH 6.0 to 7.0), 20 mM Tris-HCl buffer (pH 7.0 to 8.5), and 20 mM glycine-NaOH buffer (pH 9.0). All spectrophotometric measurements were performed at room temperature with a Shimadzu MPS-2000 spectrometer, using a cuvette with a 1-cm light path.
Purification of M. magnetotacticum ferric iron reductase.
Unless otherwise noted, all procedures were performed at 4°C under aerobic conditions. The bacterial cells (40 g [wet weight]) obtained from 100 liters of medium were suspended in 80 ml of 10 mM Tris-HCl buffer (pH 8.0) containing 10 μM phenylmethylsulfonyl fluoride and 200 mM NaCl (buffer A). The cells were broken with two passages through a French pressure cell at 1,000 kg/cm2 and centrifuged at 10,000 × g for 15 min. The supernatant was centrifuged at 104,000 × g for 1.5 h, and the resulting supernatant was subjected to ammonium sulfate up to 50% saturation and stirred gently for 1 h. After the precipitate was removed by centrifugation at 10,000 × g for 20 min, ammonium sulfate was added again to the supernatant to 65% saturation. After 1 h, the precipitate was recovered by centrifugation at 10,000 × g for 20 min and suspended in 100 mM Tris-HCl buffer (pH 8.0) containing 10 μM phenylmethylsulfonyl fluoride and 1 μM FMN (buffer B). The fraction was saturated with solid ammonium sulfate to 30% and applied onto a Butyl-Toyopearl column (2.2 by 7 cm) which had been equilibrated with a 30% ammonium sulfate-saturated buffer B. The enzyme was eluted with a linear gradient of 30 to 10% saturation of ammonium sulfate in buffer B. The active fractions were saturated with solid ammonium sulfate to 50% and applied onto a Sepharose CL-6B (2.2 by 5 cm) which had been equilibrated with 50% ammonium sulfate-saturated buffer B and were then eluted with a linear gradient of 50 to 25% saturated ammonium sulfate in buffer B. The fraction containing iron reductase activity was then thoroughly dialyzed against a 50 mM sodium phosphate buffer (pH 7.0) containing 300 mM NaCl and concentrated to approximately 100 μl on Centriflo CF-25 (Amicon) and Centricon-10 (Amicon) concentrators. The concentrated iron reductase was further applied to a COSMOSIL 5 Diol high-performance liquid chromatography (HPLC) column (0.75 by 30 cm) equilibrated in 50 mM sodium phosphate buffer (pH 7.0) containing 0.3 M NaCl and eluted with the same buffer. All HPLC procedures were performed at 25°C. The protein elution was monitored at 220 nm with a Jasco HPLC system. The active fractions were pooled and concentrated to approximately 2 ml on a Centriflo CF-25 concentrator (Amicon).
Molecular mass determination.
The purified enzyme was dialyzed against 50 mM sodium phosphate buffer (pH 7.0) containing 300 mM NaCl and concentrated with Centricon-10 concentrators (Amicon). The ferric iron reductase was applied to a COSMOSIL 5 Diol HPLC column (0.75 by 30 cm) equilibrated with 50 mM sodium phosphate buffer (pH 7.0) containing 300 mM NaCl and eluted with the same buffer. All HPLC procedures were performed at 25°C. Protein elution was monitored at 220 nm with a Jasco HPLC system. Molecular mass was calibrated by the HPLC system, and the following protein standards were used: bovine serum albumin (67.5 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and RNase A (13.7 kDa).
Electron microscopy.
The average number of magnetosomes in each cell was determined by counting the electron-dense particles on electron micrographs of about 100 individual cells. For electron microscopy, the washed cells were suspended in sterile distilled water and adsorbed onto copper grids. After the cells were stained with 1% uranyl acetate, the cells were observed in a JEOL JEM-1200EX transmission electron microscope.
Determination of N-terminal amino acid sequence.
The ferric iron reductase was blotted from a sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gel onto a polyvinylidene fluoride membrane (Millipore), and the membrane was then stained with Coomassie brilliant blue R-250. The N-terminal amino acid sequence of the ferric iron reductase was determined by applying the proteins blotted on the membrane to a gas-phase protein sequencer (Shimadzu PPSQ21) equipped with a UV-visible light detector (Shimadzu SPD-10A) and liquid chromatography (Shimadzu LC-10AS).
Determination of zinc concentration in the cell.
Cells (about 0.5 g) were washed with 1 M EDTA and suspended in 10 ml of H2O. The suspension was mixed with 1 ml of HNO3 (for atomic absorption spectrum analysis) and then boiled for 30 min. The resulting ash was suspended with 3.5 ml of 1 N HNO3, and the concentration of zinc in the suspensions was analyzed by an induced coupled plasma atomic spectroscopy system (Seiko Instruments SPS 1500VR).
Physical measurements.
Protein was determined by using the bicinchoninic acid protein assay reagent from Pierce Chemical Co. Tricine-SDS-polyacrylamide gel electrophoresis in the presence of 5% SDS was performed as described by Schägger and von Jagow (17). The protein bands were stained with Coomassie brilliant blue R-250 in isopropanol and acetic acid. The marker proteins were as follows: phosphorylase b (97.4 kDa), bovine serum albumin (67.5 kDa), l-glutamate dehydrogenase (55 kDa), ovalbumin (42.7 kDa), aldolase (40 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (21.5 kDa), and lysozyme (14.4 kDa).
Chemicals.
Ferrozine, NADH, NADPH, glutathione, FMN, and flavin adenine dinucleotide were purchased from Sigma Chemical Co. All other chemicals were the highest grade commercially available.
RESULTS
Localization of M. magnetotacticum ferric iron reductase.
Localization of the ferric iron reductase in M. magnetotacticum was investigated by measuring the enzymatic activity of the periplasmic fraction, the cytoplasmic fraction, and the cytoplasmic membranes prepared as described in Materials and Methods. As summarized in Table 1, ferric iron reductase activity was found in the cytoplasmic but not the periplasmic fraction. On the other hand, the membranes retained about 30% of the total activity detected in the cell extract. However, ferric iron reductase activity was not found in membranes that had been washed with 0.3 M NaCl. Therefore, it seems likely that the ferric iron reductase of M. magnetotacticum is loosely bound to the cytoplasmic face of the cytoplasmic membrane.
TABLE 1.
Localization of ferric reductase in M. magnetotacticuma
| Fraction | Activity
|
||
|---|---|---|---|
| Ferric reductase (nmol min−1) | Nitrite reductase (μmol min−1) | Malate dehydrogenase (μmol min−1) | |
| Periplasm | 0 | 0.43 | 1.9 |
| Cytoplasm | 8.3 | 0 | 16.1 |
| Membrane | 2.4 | NDb | ND |
After cells in 10 mM Tris-HCl buffer (pH 8.0) containing 0.75 M sucrose were incubated with EDTA plus lysozyme at 30°C for 1 h, the membrane, periplasmic fraction, and cytoplasmic fractions were prepared as described in Materials and Methods. N,N,N′,N′-tetramethyl-p-phenylenediamine [Ph(NMe2)2]-nitrite reductase activity was followed by measuring the increase in A606. The reaction mixture contained 10 mM sodium phosphate (pH 6.5), 1 mM Ph(NMe2)2, 0.1 mM sodium nitrite, and the protein sample in a total volume of 1.0 ml. Malate dehydrogenase activity was followed by measuring the decrease in A340. The reaction mixture for the malate dehydrogenase assay contained 100 mM potassium phosphate (pH 7.5), 0.25 mM NADH, 0.2 mM oxaloacetic acid, and protein sample.
ND, not determined.
On the other hand, the level of ferric iron reductase was twofold higher in magnetic cells (10.1 nmol/min/mg) than in nonmagnetic cells (4.7 nmol/min/mg) that had been cultivated under the same growth conditions. This finding suggests that the ferric iron reductase of M. magnetotacticum plays an important role in magnetite synthesis in the cytoplasm.
Purification of M. magnetotacticum ferric iron reductase.
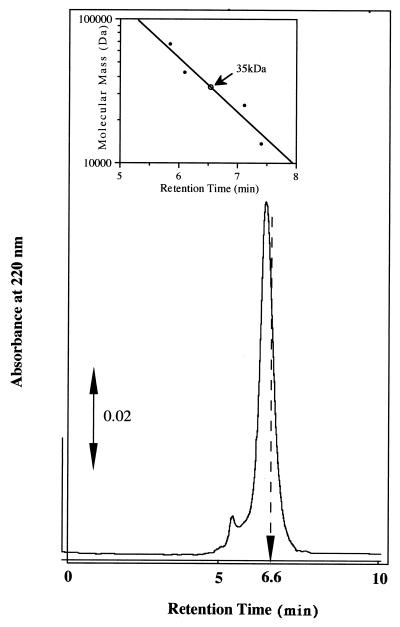
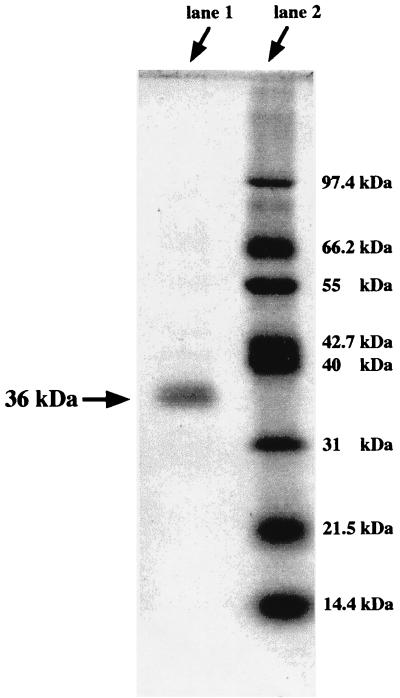
The ferric iron reductase was purified from M. magnetotacticum by ammonium sulfate fractionation, hydrophobic column chromatography, and HPLC; recovery was about 7.3%, as summarized in Table 2. Figure 1 shows the elution profile from the COSMOSIL HPLC gel filtration column. The major peak with high ferric iron reductase activity corresponds to a protein with an apparent molecular mass of 35 kDa. The SDS-polyacrylamide gel electrophoresis profile of the purified enzyme is shown in Fig. 2. M. magnetotacticum iron reductase is composed of a single subunit with molecular mass of 36 kDa. These results indicate that the enzyme is present in a monomeric form in the solution.
TABLE 2.
Purification of ferric iron reductase from M. magnetotacticum
| Fraction | Protein (mg) | Total activity (nmol/min) | Sp act (nmol/min/mg) | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Soluble extract | 1,100 | 11,429 | 10.4 | 1.00 | 100 |
| (NH4)2SO4 fractionation | 385 | 10,000 | 26.0 | 2.50 | 87 |
| Butyl-Toyopearl | 62.7 | 6,605 | 105 | 10.1 | 58 |
| Sepharose CL-6B | 9.7 | 3,142 | 324 | 31.2 | 28 |
| Sephacryl S-200 | 0.27 | 840 | 3,111 | 299 | 7.3 |
FIG. 1.
Elution profile on HPLC column chromatography of M. magnetotacticum ferric iron reductase. The purified ferric iron reductase was applied to a COSMOSIL 5 Diol HPLC column and eluted with buffer as described in Materials and Methods. The active fraction was eluted at 6.6 min (indicated by the arrow).
FIG. 2.
SDS-polyacrylamide gel electrophoresis of M. magnetotacticum ferric iron reductase. The concentration of acrylamide was 5%; the gel was stained with Coomassie brilliant blue. The ferric iron reductase (lane 2) and marker proteins (lane 1) were treated at 100°C for 3 min with 5% SDS in the presence of 1% 2-mercaptoethanol. The marker proteins were phosphorylase b (97.4 kDa), bovine serum albumin (67.5 kDa), l-glutamate dehydrogenase (55 kDa), ovalbumin (42.7 kDa), aldolase (40 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (21.5 kDa), and lysozyme (14.4 kDa).
The N-terminal sequence of M. magnetotacticum iron reductase was determined as described in Materials and Methods to be Ser-Ala-Ser-Thr-Pro-Ala-Phe-Arg-Gly-Lys-Ile-Tyr-Asp-Ser-Ile-Ile-?-Thr-Ile-Gly-Ala-Thr-?-Leu-Vla. A search for sequence homology via the BLAST program (2) found no homologous proteins.
Enzymatic properties of M. magnetotacticum ferric iron reductase.
In the standard reaction mixture for ferric iron reductase assay, NADH, FMN, and ferric citrate were used as reductant, electron mediator, and iron source, respectively. Although other bacterial ferric iron reductases have been reported to be able to use NADPH, glutathione, and succinate as reductants and FAD as an electron mediator (4, 12), NADPH and glutathione did not restore enzymatic activity, and interestingly, FAD was not a good electron mediator in the reaction of M. magnetotacticum ferric iron reductase. These results indicate that the ferric iron reductase of M. magnetotacticum requires primarily NADH and FMN as reductant and electron mediator, respectively. The Km values of the enzyme for NADH, FMN, and ferric citrate were 4.3, 0.035, and 14.5 μM, respectively. The Vmax was determined to be 0.87 s−1. The optimal pH was approximately 7.0. These enzymatic properties are summarized in Table 3.
TABLE 3.
Enzymatic assay of ferric iron reductase activity for the purified enzyme of M. magnetotacticum
| Substrate(s) | Activitya (%)
|
Km (μM) | |
|---|---|---|---|
| +substrate | −substrate | ||
| Reductants | |||
| NADH | 1.59b (100) | 0 | 1.3 |
| NADPH | 0.26 (16) | NA | 119.3 |
| Glutathione | 0 | NA | ND |
| Flavin | |||
| FMN | 1.59b (100) | 0.18 (11) | 0.035 |
| FAD | 0.28 (18) | NA | ND |
| Iron source | |||
| Ferric citrate | 1.59b (100) | 0 | 14.5 |
| Ferric quinate | 0.36 (23) | NA | ND |
Determined by measuring the increase in the A562, using a Δɛ562 value of 28 mM−1 cm−1. NA, not assayed. ND, not determined.
Standard conditions: 20 mM sodium phosphate buffer (pH 7.0), 100 μM NADH, 1 μM FMN, 1 mM ferrozine, 200 μM ferric citrate.
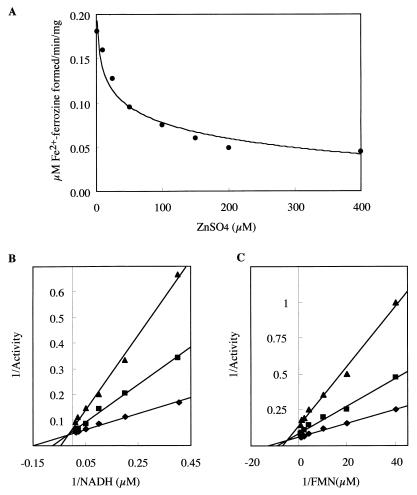
The effects of metal ions on ferric iron reductase activity were examined. Zn2+ strongly inhibited the activity of the ferric iron reductase (Fig. 3A). Kinetic analyses of the enzyme with Zn2+ revealed that Zn2+ affects both Vmax and Km values of the reaction (Fig. 3B and C) and acts as mixed-type inhibitor. The Ki values for Zn2+ were approximately 19.2 and 23.9 μM with respect to NADH and FMN, respectively. Other divalent cations including CaCl2, MgSO4, and MnCl2 had no effects on enzymatic activity.
FIG. 3.
Effect of Zn2+ on the enzymatic activity of M. magnetotacticum ferric iron reductase. (A) Inhibition of ferric iron reductase activity by Zn2+. Activity was followed by measuring the increase in the A562, using a Δɛ562 value of 28 mM−1 cm−1. (B and C) Lineweaver-Burk plots of ferric iron reductase activity in the presence of Zn2+ with respect to NADH (B) and FMN (C) (0 [⧫], 25 [■], and 75 [▴] μM ZnSO4). One unit of the enzymatic activity is defined as 1 μmol of Fe2+-ferrozine formed per min per mg of protein.
Participation of ferric iron reductase in magnetite synthesis of M. magnetotacticum.
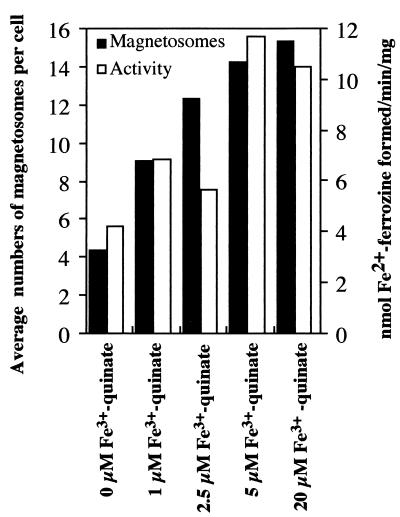
To investigate the effects of extracellular irons on ferric iron reductase activity and magnetite synthesis in the cell, the bacterium was cultivated in media containing different concentrations of ferric quinate. In cells cultivated with medium containing less than 5 μM ferric quinate, the average number of particles per cell decreased in parallel with the ferric iron reductase activity of the soluble fraction (Fig. 4). In these experiments, cell yield was not affected by the ferric quinate concentration. Several researchers have reported that the ferric iron reductases from other bacteria are not regulated by extracellular iron since the enzymatic activity is almost the same as that of iron-enriched cells or iron-deficient cells (13, 14). M. magnetotacticum ferric iron reductase activity, however, decreased in cells cultivated in low concentrations of ferric quinate, but only slight changes in activity were observed in cells cultivated in medium with more than 5 μM ferric quinate.
FIG. 4.
Ferric iron reductase activity and magnetosome numbers of cells cultivated at various concentrations of Fe3+-quinate. To investigate the effects of extracellular irons on ferric iron reductase activity and magnetite synthesis in the cell, the bacterium was cultivated in media containing different concentrations of Fe3+-quinate (0, 1, 2.5, 5 μM, and 20 μM). After 72 h of cultivation, the average numbers of magnetosomes were determined by counting of electron-dense particles in micrographs from a total of about 100 cells in each sample. Iron reductase activity was analyzed as described in Materials and Methods.
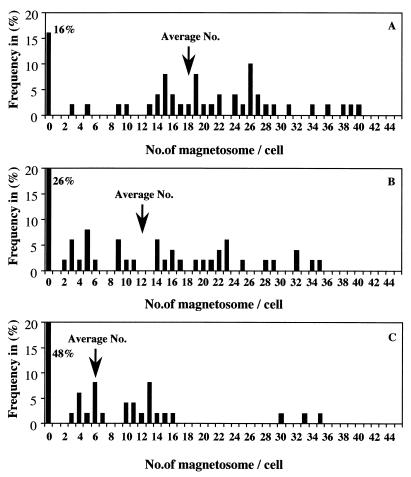
To demonstrate the participation of ferric iron reductase in magnetite synthesis, the effects of Zn2+ in the medium on the magnetite synthesis were investigated by comparing the ferric iron reductase activities of the soluble fractions and the numbers of magnetosomes in cells cultivated in the presence of ZnSO4 at 1 μM (original concentration of ZnSO4 in the medium) and at 20, 75, and 200 μM. Figure 5 shows the distribution of magnetosome numbers per cell. The higher numbers were decreased in parallel with the concentration of ZnSO4 (1 to 75 μM) in the medium, and the yield of cells with no detectable magnetosomes was increased in parallel with the concentration of ZnSO4. The average numbers of magnetosomes were 18.6, 12.1, and 6.4 per cell with 1, 20, and 75 μM ZnSO4, respectively, and about half of the cells grown in the presence of 75 μM ZnSO4 had no magnetic particles. Furthermore, the ferric iron reductase activity of the soluble fraction also decreased in parallel with the concentration of ZnSO4 in the medium (Table 4). However, bacterial growth was affected by 200 μM ZnSO4. These results suggest that although the iron reductase is involved in reduction of intercellular iron for magnetite synthesis, the marked loss of ferric iron reductase activity by high ZnSO4 concentrations affects other aspects of iron metabolism necessary to sustain life.
FIG. 5.
Distribution of magnetosome numbers in cells grown under various extracellular concentrations of Zn2+ in the presence of 20 μM Fe3+-quinate. The average numbers in the samples were 18.6, 12.1, and 6.4 per cell with respect to 1 μM (A), 20 μM (B), and 75 μM (C) ZnSO4, respectively. Values were obtained by counting of electron-dense particles in micrographs from a total of about 100 cells in each sample.
TABLE 4.
Effects of extracellular Zn2+ on growth, magnetite synthesis, and ferric reductase activitya
| Zn in medium (μM) | Growth (optical density at 660 nm) | Avg no. of magnetosomes/ cell | Activity (Ub) | Zinc in cell (μg/g of protein) |
|---|---|---|---|---|
| 1 (control) | 0.085 | 18.6 | 11.6 | 50.9 |
| 20 | 0.080 | 12.1 | 3.03 | 559 |
| 75 | 0.080 | 6.4 | 1.68 | 1,726 |
| 200 | 0.065 | 5.3 | 1.42 | NDc |
Cells were grown for 72 h in the presence of various amounts of ZnSO4. The average numbers of magnetosomes in the given samples were obtained by counting of electron-dense particles in electron micrographs from a total of about 100 cells in each sample. Ferric iron reductase activity was determined by measuring the increase in the A562 (7). Zinc concentrations in the cells were determined with an induced coupled plasma atomic spectroscopy system as described in Materials and Methods.
One unit is 1 nmol of Fe2+-ferrozine formed per min per mg of protein.
ND, not determined.
DISCUSSION
This study describes the purification and enzymatic characterization of ferric iron reductase from magnetotactic bacterium M. magnetotacticum. The enzyme is a monomeric protein with a molecular mass of 36 kDa and is localized in the cytoplasm of the bacterial cell. The enzyme requires NADH and FMN as an optimal electron donor and cofactor, respectively.
The Km and Vmax values for NADH are 4.3 μM and 0.87 s−1, respectively, almost the same as the Km (18.2 μM) and Vmax (5.2 s−1) of iron reductase from Rhodopseudomonas sphaeroides (13). However, the Km (0.035 μM) for FMN of M. magnetotacticum iron reductase is much lower than that of R. sphaeroides iron reductase (3.2 μM), indicating that M. magnetotacticum iron reductase has a greater affinity for FMN.
Ferric iron reductases have been found in several other organisms and are considered to be involved in many aspects of intracellular iron metabolism (removal of iron from siderophores, insertion of iron into protoporphyrin, iron supply from ferritin, etc.). In general, the extracellular iron concentration has no effect on the content of ferric iron reductases in the cell (10, 11). However, Rhodobacter sphaeroides ferric iron reductase is not induced equally in aerobically grown cells (4.7 to 5.2 nmol/min/mg) and photosynthetic cells (8.0 nmol/min/mg) (11). The difference in activity may depend on the intracellular iron demand. In the case of M. magnetotacticum, the soluble fraction prepared from the nonmagnetic cells showed about 50% of the ferric iron reductase activity of the soluble fraction prepared from the magnetic cells. These results suggest that the iron reductase of M. magnetotacticum may participate in a biological process requiring large amounts of ferrous iron.
On the other hand, the enzymatic activity is specifically inhibited by Zn2+ as a partial mixed-type inhibitor, and the Ki values for Zn2+ are approximately 19.2 and 23.9 μM for NADH and FMN, respectively. When the bacterium was cultivated in a Zn-containing culture medium, the average number of magnetosomes in the cell decreased in parallel with the concentration of ZnSO4 in the medium. The inhibitor for ferric iron reductase strongly inhibits magnetosome formation in the cell. Furthermore, the ferric iron reductase activity of the soluble fraction decreased markedly from that of the control. Therefore, it seems likely that the ferric iron reductase is related to magnetite synthesis in M. magnetotacticum.
Intracellular iron is essential for heme synthesis and non-heme iron proteins, but most of the iron present is in a poorly defined state. Ferritin or bacterioferritin isolated from bacteria, plant, or mammalian sources is a large 24-subunit protein that can internalize up to 45,000 atoms of iron in an iron oxo-hydroxo mineral lattice (14). As the main intracellular reservoir for iron, ferritin is taken up or released as cellular conditions require. Recently, Bertani et al. reported that M. magnetotacticum has two bacterioferritin genes which show strong similarity to other bacterioferritin subunit proteins (5), although no ferritins have been purified from the bacterium. Furthermore, 200 μM Zn2+ shows inhibitory effects on not only magnetosome formation but also growth of the bacterium. Therefore, ferric iron reductase of M. magnetotacticum may play a role in supplying ferrous iron to these iron crystals as well as to magnetites.
Recently, Yamazaki et al. (18) reported that M. magnetotacticum cytochrome cd1, which is located in the periplasmic space, may function as a Fe(II)-oxidizing enzyme under microaerobic conditions using nitrite as an electron acceptor. They proposed that the bacterium synthesizes magnetites in the initial steps: (i) iron uptake by the siderophore-iron transport system, (ii) removal of iron from the siderophore by ferric iron reductase in the cytoplasmic fraction, (iii) transfer of ferrous iron to the periplasmic Fe(II)-oxidizing enzyme by an unknown iron transporter. Magnetosome vesicles, however, are present in the cytoplasm, and interestingly, the magnetosome membrane does not appear to be contiguous with the cytoplasmic membrane (9). Therefore, to elucidate the involvement of ferric iron reductase in magnetite synthesis, the iron transport system in the cytoplasmic membrane should be characterized, and the mechanism of magnetosome vesicle formation should be studied.
ACKNOWLEDGMENTS
This work was supported in part by Grant-in-Aid for Scientific Research (C) 09660076 and Grant-in-Aid for Scientific Research on Priority Areas 10129208 to Y.F. from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Alefounder P R, Ferguson S J. The location of dissimilatory nitrate reductase and the control of dissimilatory nitrate reductase by oxygen in Paracoccus denitrificans. Biochem J. 1980;192:231–240. doi: 10.1042/bj1920231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill D L, Maratea D, Blakemore R P. Ultrastructure of a magnetotactic spirillum. J Bacteriol. 1980;141:1399–1408. doi: 10.1128/jb.141.3.1399-1408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazylinski D A, Blakemore R P. Denitrification and assimilatory nitrate reduction in Aquaspirillum magnetotacticum. Appl Environ Microbiol. 1983;46:1118–1124. doi: 10.1128/aem.46.5.1118-1124.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertani L E, Huang J S, Weir B A, Kischvink J L. Evidence for two types of subunits in the bacterioferritin of Magnetospirillum magnetotacticum. Gene. 1997;201:31–36. doi: 10.1016/s0378-1119(97)00424-1. [DOI] [PubMed] [Google Scholar]
- 6.Blakemore R P, Maratea D, Wolfe R S. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979;140:720–729. doi: 10.1128/jb.140.2.720-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dailey H A J, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977;129:815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel R B, Papaefthymiou G C, Blakemore R P, O’Brien W. Fe3O4 precipitation in magnetotactic bacteria. Biochim Biophys Acta. 1983;763:147–159. [Google Scholar]
- 9.Gorby Y A, Beveridge T J, Blakemore R P. Characterization of the bacterial magnetosome membrane. J Bacteriol. 1988;170:834–841. doi: 10.1128/jb.170.2.834-841.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huyer M, Page W J. Ferric reductase activity in Azotobacter vinelandii and its inhibition by Zn2+ J Bacteriol. 1989;171:4031–4037. doi: 10.1128/jb.171.7.4031-4037.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leong S A, Neilands J B. Relationship of siderophore-mediated iron assimilation to virulence in crown gall disease. J Bacteriol. 1981;147:482–491. doi: 10.1128/jb.147.2.482-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark T, Johnson W. Ferric reductase of Legionella pneumophila. Biometals. 1993;6:107–114. doi: 10.1007/BF00140111. [DOI] [PubMed] [Google Scholar]
- 13.Moody M D, Dailry H A. Ferric iron reductase of Rhodopseudomonas sphaeroides. J Bacteriol. 1985;163:1120–1125. doi: 10.1128/jb.163.3.1120-1125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Halloran T V. Transition metals in control of gene expression. Science. 1993;261:715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- 15.Paoletti L C, Blakemore R P. Hydroxamate production by Aquaspirillum magnetotacticum. J Bacteriol. 1986;167:73–76. doi: 10.1128/jb.167.1.73-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paoletti L C, Blakemore R P. Iron reduction by Aquaspirillum magnetotacticum. Curr Microbiol. 1988;17:339–342. [Google Scholar]
- 17.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki T, Oyanagi H, Fujiwara T, Fukumori Y. Nitrate reductase from the magnetotactic bacterium: a novel cytochrome cd1 with Fe(II):nitrate oxidoreductase activity. Eur J Biochem. 1995;233:665–671. doi: 10.1111/j.1432-1033.1995.665_2.x. [DOI] [PubMed] [Google Scholar]