Abstract
SARS-CoV-2 (COVID-19) vaccination numbers are globally increasing. Therefore, an increased chance exists that patients undergoing Peptide Receptor Radionuclide Therapy (PRRT) or diagnostic radionuclide imaging for Neuroendocrine Tumours (NETs) may have recently received vaccination. We report the imaging findings of two NETs patients, A—following [177Lu] Lu-DOTATATE PRRT post therapy planar scintigraphy and single photon emission computed tomography with computed tomography (SPECT/CT), and B—following [68 Ga]Ga-DOTA-NOC positron emission tomography with computed tomography (PET/CT) respectively. Both studies were done few days after COVID-19 vaccination. Patient A showed a new focus of uptake in the left deltoid muscle; and Patient B showed uptake in the left deltoid and a left axillary lymph node. Nuclear Physicians need to be aware of pitfalls with somatostatin receptor radionuclide imaging post-vaccination to ensure accurate interpretation, as well as dosimetric considerations with vaccine-related post-therapy uptake.
Keywords: COVID-19 vaccination, [177Lu] Lu-DOTA-TATE, [68 Ga]Ga-DOTA-NOC, PRRT, Neuroendocrine tumour
Spotlight
SARS-CoV-2 (COVID-19) vaccinations have shown high efficacy, with an associated increase in the rates of vaccinations globally. Therefore, an increased chance exists that patients undergoing Peptide Receptor Radionuclide Therapy (PRRT) or diagnostic radionuclide imaging for Neuroendocrine Tumours (NETs) may have been recently vaccinated.
We report two patients- Patient A, with grade 2 gastric NET with liver metastases, who received [177Lu] Lu-DOTA-TATE therapy; and Patient B with grade 2 pancreatic NET with hepatic, and mesenteric nodal metastases who had [68 Ga]Ga-DOTA-NOC positron emission tomography with computed tomography (PET/CT) imaging; both following Pfizer-BioNTech mRNA COVID-19 vaccination.
Cases
Patient A
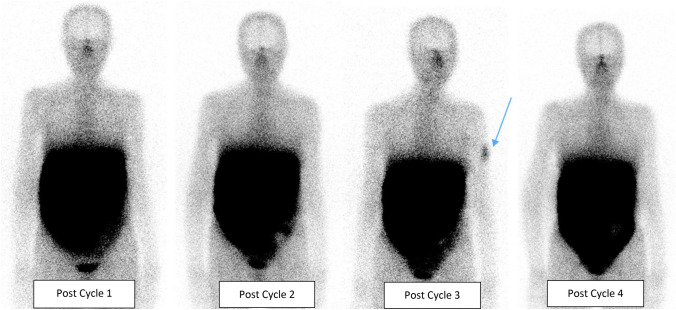
A 73-year-old woman, known with gastric grade 2 neuroendocrine tumour (NET) and extensive inoperable liver metastases was referred to Nuclear Medicine for peptide receptor radionuclide therapy (PRRT) with [177Lu] Lu-DOTA-TATE. The first two cycles of PRRT were uneventful and post-therapy imaging revealed good uptake of [177Lu] Lu-DOTA-TATE in the known gastric mass and liver metastases, concordant to the staging [68 Ga]Ga-DOTA-NOC PET/CT. No new areas of somatostatin receptor avidity were seen. Following Cycle 3 of [177Lu] Lu-DOTA-TATE, the post therapy planar and single photon emission computed tomography with computed tomography (SPECT/CT) images were acquired (Figs. 1, 2, 3). These showed a new focus of mildly increased uptake in the left upper arm, localised to the left deltoid muscle. There were no other sites of new uptake. Ipsilateral and contralateral axillary lymph nodes did not demonstrate increased uptake on SPECT or size-significance on CT. In the absence of any confounders to explain the new uptake, the patient was interviewed, and it was revealed that she had received the first dose of the SARS-CoV-2 (COVID-19) Pfizer/BioNTech mRNA vaccine four days prior to receiving cycle 3 of [177Lu] Lu-DOTA-TATE therapy. The vaccination site corresponded to the site of new uptake. Clinically, mild focal myalgia was noted at this site which progressively decreased within a few days following therapy, without the need for analgesia. Serial follow-up revealed no other adverse outcomes from deltoid muscle uptake. This focus of uptake was no longer seen on subsequent post therapy imaging, following cycle 4 of [177Lu] Lu-DOTA-TATE therapy (Fig. 1).
Fig. 1.
Patient A: [177Lu] Lu-DOTA-TATE post-therapy planar images for cycles 1–4. Post Cycle 3 [177Lu] Lu-DOTA-TATE image showing a new focus of increased uptake in the left upper arm (tip of blue arrow). No other sites of new uptake were seen
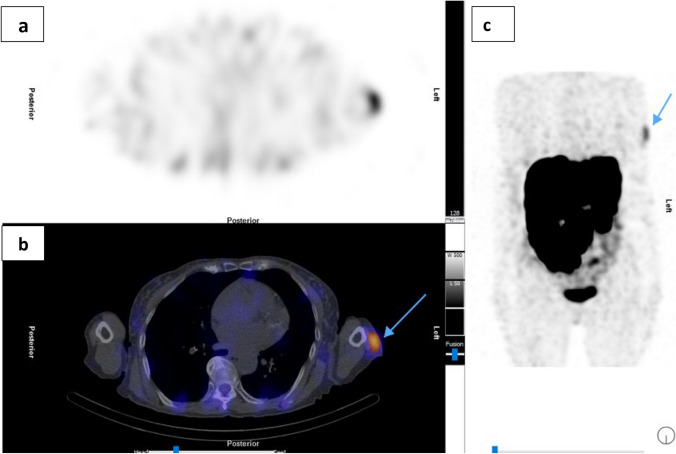
Fig. 2.
Patient A: [177Lu] Lu-DOTA-TATE Cycle 3 post-therapy SPECT/CT. a Transaxial SPECT; b Fused transaxial SPECT/CT; c SPECT Maximum intensity projection (MIP) showing focus of increased uptake localized to the left deltoid (tip of blue arrow)
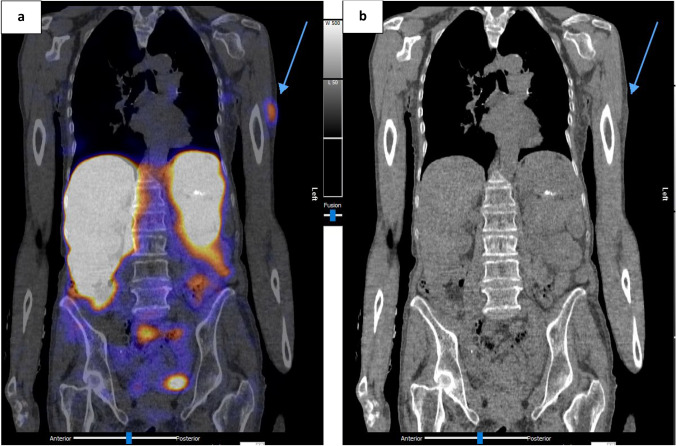
Fig. 3.
Patient A: [177Lu] Lu-DOTA-TATE Cycle 3 post-therapy SPECT/CT showing. a Coronal fused SPECT/CT; b Coronal CT images. Location of the new focus of uptake is denoted with tip of blue arrow
Patient B
A 65-year-old woman, known with metastatic pancreatic grade 2 NET and liver, mediastinal and mesenteric lymph node metastases, had a [68 Ga]Ga-DOTA-NOC PET/CT for response assessment after two cycles of PRRT (Fig. 4). The images revealed new uptake in her left upper arm (Fig. 5) and left axilla (Fig. 6), which localised to the deltoid muscle and a 10 mm axillary lymph node with fatty hilum, respectively. Other areas of uptake were in sites of known disease, demonstrating stable disease. Deltoid muscle uptake corresponded to the COVID-19 vaccination site, for the first dose of Pfizer/BioNTech mRNA received two days prior to imaging.
Fig. 4.
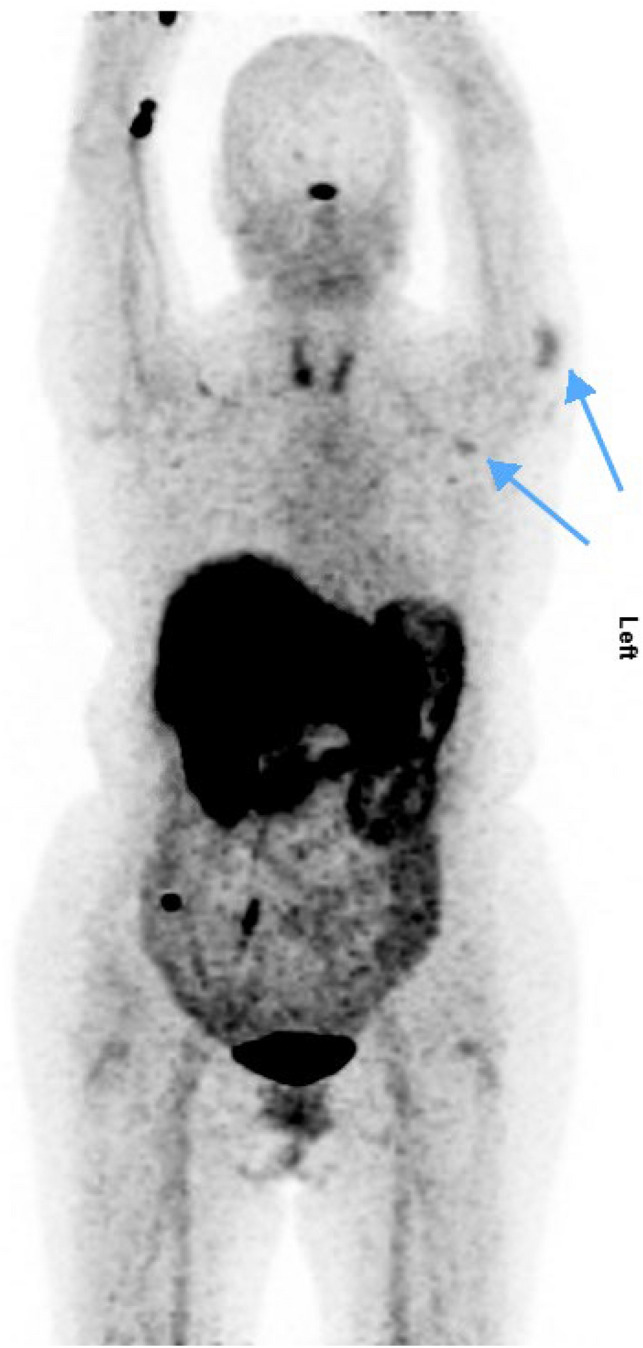
Patient B: [68Ga]Ga-DOTA-NOC PET MIP showing left deltoid and axillary uptake (tip of arrows)
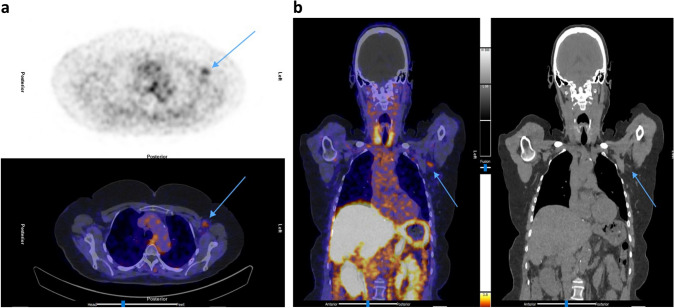
Fig. 5.
Patient B: [68Ga]Ga-DOTA-NOC PET/CT. a Trans axial PET and fused PET/CT showing left deltoid muscle uptake (tip of arrows). b Coronal fused PET/CT, and CT only showing left deltoid uptake (tip of arrows)
Fig. 6.
Patient B: [68Ga]Ga-DOTA-NOC PET/CT. a Trans axial PET and fused PET/CT showing left axillary lymph node (tip of arrows). b Coronal fused PET/CT, and CT only showing left axillary nodal uptake (tip of arrows)
Discussion
Vaccine-induced radiotracer uptake in ipsilateral axillary lymph nodes is well described with [18F]FDG [1, 2], and increasingly so in recent times following COVID-19 vaccination [3–7]. Although the data is limited, [18F]FDG-avid nodal uptake post COVID-19 vaccination has been found to be relatively common, being reportedly demonstrated in over 50% of recently vaccinated patients undergoing [18F]FDG PET/CTs [6–8].
Analogous to [18F]FDG, limited reports exist of similar uptake patterns with [68 Ga]Ga-DOTA-TATE/DOTA-TOC PET/CT studies post COVID-19 vaccination [9–13]. In the case described by Guglielmo et al. [12], the congruence in post-vaccination uptake patterns of both [18F]FDG and [68 Ga]Ga-DOTA-TOC is clearly demonstrated with the reported patient who had sequential studies using both tracers.
In the available case reports and case series describing vaccine-related somatostatin receptor tracer uptake which were reviewed at the time of writing, were imaged using either [68 Ga]Ga-DOTA-TATE or [68 Ga]Ga-DOTA-TOC. This is unlike ours (Patient B) which was imaged with [68 Ga]Ga-DOTA-NOC. These three established somatostatin receptor PET tracers ([68 Ga]Ga-DOTA-TATE, [68 Ga]Ga-DOTA-TOC, and [68 Ga]Ga-DOTA-NOC), though having slightly differing chemistries, are considered comparable in terms of sensitivity and specificity [14, 15]. Patient B’s findings- deltoid and axillary nodal uptake- were congruent with post-COVID-19 vaccination findings on [68 Ga]Ga-DOTA-TATE or [68 Ga]Ga-DOTA-TOC, supporting the posited comparability of these tracers.
Patients found with bilateral nodal SSTR uptake (axillary and/or supraclavicular) post vaccination, also had at least two doses of the vaccine on alternate deltoid muscles [9, 11]. Our reported patient had only one dose at the time of diagnostic imaging, in keeping with the unilateral findings. We also noted that the gender of all patients with COVID-19 vaccine-related SSTR uptake in the reports available to us (where overtly stated) were female, and all had the Pfizer-BioNTech vaccine—this is, however, unlikely to be of any significance.
COVID-19 vaccine-related uptake is also reported with other tracers asides [18F]FDG and [68 Ga]Ga-DOTA-TOC/TOC, though less frequently. These include [18F]Choline [16], [18F]-fluciclovine [10, 17, 18], [18F]-Fluorthanatrace [10] and [68 Ga]Ga-PSMA/[18F]-PSMA [8, 13].
However, to the best of our knowledge as at the time of writing, there have been no reports of vaccine-induced uptake of [177Lu] Lu-DOTA-TATE in published literature. [177Lu] Lu-DOTA-TATE is a beta-minus-particle emitting therapeutic radiopharmaceutical, utilised in treating neuroendocrine tumours which overexpress somatostatin receptors (SSTR), especially SSTR type 2 [19]. Post-vaccination uptake of [177Lu] Lu-DOTA-TATE, as with [68 Ga]Ga-DOTA-TATE/TOC/NOC, occurs on account of the increased expression of SSTR types 1 and 2 in macrophages following vaccine-induced immune-system activation [13, 20]. Given the potent tumoricidal effect of [177Lu] Lu-DOTA-TATE [19], unintended uptake can potentially cause inadvertent harm. Close monitoring and careful clinical follow-up of such uptake is therefore mandatory, and appropriate intervention must be instituted as deemed necessary. Fortunately, in the case of our reported patient, mild and progressively decreasing myalgia was the only adverse effect. Subsequent [177Lu] Lu-DOTA-TATE post-therapy imaging also revealed complete resolution of uptake at the injection site in keeping with the absence of symptoms. The resolution of the uptake also lends credence to the transient nature of these vaccine-related findings.
It is also noteworthy that available published reports of COVID-19-vaccination-related SSTR uptake were detected on PET imaging. Our report of COVID-19 vaccine related [177Lu] Lu-DOTA-TATE uptake, was performed with planar scintigraphy and single photon emission computed tomography with computed tomography (SPECT/CT). Despite the known decreased sensitivity and image resolution of planar scintigraphy and SPECT compared to PET, it was able to demonstrate vaccine related SSTR uptake despite. To the best of our knowledge, this is the first report of COVID-19 vaccine-related uptake on planar scintigraphy and SPECT-based imaging.
In the clinical practice of Nuclear Medicine, vaccine-related uptake is differentiated from new/progressive disease by the history of recent vaccination and the morphology of the lymph nodes on CT. Additionally, the presence of the typical “double sign”[8]—uptake in the upper arm and ipsilateral axilla—is also useful in delineating vaccine-related uptake from pathology. This sign is demonstrated in our [68 Ga]Ga-DOTA-NOC PET/CT case (Figs. 4, 5, 6). However, the [177Lu] Lu-DOTA-TATE case, only shows the presence of uptake in the deltoid muscle (Figs. 1, 2, 3) without ipsilateral axillary lymph node uptake on SPECT or lymph node enlargement on CT (Figs. 2, 3), potentially making image interpretation more challenging. However, ‘good old’ history taking helped solve the conundrum.
Nuclear Medicine Physicians therefore need to be cognizant of higher SSTR expression in the tissues surrounding the COVID-19 vaccinations injection site, and thereby avoid this pitfall. This will ensure accurate interpretation of SSTR diagnostic and post-therapeutic studies post COVID-19 vaccination. This is especially important in the context of pathologies with a propensity for nodal spread, given the potential of false-positive ‘new disease, to impact management decisions.
Furthermore, in the current era of exponential growth in theranostics, it is critical to consider the potential dosimetric implications of the inadvertent uptake of therapeutic radiopharmaceuticals in vaccination-related sites; the consequences of which may be akin to radiopharmaceutical extravasation [21]. Dosimetric estimation of absorbed doses to such tissue will be useful, and may be a subject for future research.
Declarations
Conflicts of interest
The authors Kolade O; Ayeni A; Brink A; Steyn R; More S, and Prasad V, have no conflicts of interest to declare. This article does not contain any studies with human or animal subjects performed by the any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 2.Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38(5):894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]
- 3.Tu W, Gierada DS, Joe BN. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Soc N Am. 2021 doi: 10.1148/rycan.2021210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed N, Muzaffar S, Binns C, Ilyas MW, Usmani S. COVID-19 vaccination manifesting as incidental lymph nodal uptake on 18F-FDG PET/CT. Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003635. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F] FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48(6):1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter UJ, Maurer A, et al. [18F] FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2022;32(1):508–516. doi: 10.1007/s00330-021-08122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin M, Hyun CY, Choi YH, Choi JY, Lee K-H, Cho YS. COVID-19 vaccination–associated lymphadenopathy on FDG PET/CT: distinctive features in adenovirus-vectored vaccine. Clin Nucl Med. 2021;46(10):814. doi: 10.1097/RLU.0000000000003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orevi M, Chicheportiche A, Ben-Haim S. Lessons learned from post-COVID-19 vaccination PET/CT studies. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y. DOTATATE—Avid bilateral axilla and subpectoral lymphadenopathy induced from COVID-19 mRNA vaccination visualized on PET/CT. Clin Nucl Med. 2021;46(11):931–2. doi: 10.1097/rlu.0000000000003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surasi DSS, Lin L, Ravizzini G, Wong F. Supraclavicular and axillary lymphadenopathy induced by COVID-19 vaccination on 18F-fluorthanatrace, 68Ga-DOTATATE, and 18F-Fluciclovine PET/CT. Clin Nucl Med. 2022;47(2):195–196. doi: 10.1097/RLU.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 11.Pudis M, VercherConejero JL, Martín Marcuartu JJ, Cortés Romera M. 68Ga-DOTATOC-avid lymphadenopathies induced from COVID-19 mRNA vaccination. Jpn J Clin Oncol. 2021;51(12):1765. doi: 10.1093/jjco/hyab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guglielmo P, Muccioli S, Berti S, Sartorello A, Pesella F, Gregianin M. Detection of unilateral axillary nodal uptake both at 68Ga-DOTATOC and 18F-FDG PET/CT after 1 week from COVID-19 vaccine. Clin Nucl Med. 2022;47(2):e123. doi: 10.1097/RLU.0000000000003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eifer M, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Shams J, et al. Covid-19 mRNA vaccination: age and immune status and its association with axillary lymph node PET/CT uptake. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52(12):1864–1870. doi: 10.2967/jnumed.111.091165. [DOI] [PubMed] [Google Scholar]
- 15.Kabasakal L, Demirci E, Ocak M, Decristoforo C, Araman A, Ozsoy Y, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(8):1271–1277. doi: 10.1007/s00259-012-2123-y. [DOI] [PubMed] [Google Scholar]
- 16.Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F] choline PET/CT—not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021;48(8):2657–2658. doi: 10.1007/s00259-021-05279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peacock JG, Banks EA, McWhorter N. 18F-fluciclovine–avid axillary lymph nodes after COVID-19 vaccination on PET/CT for suspected recurrence of prostate cancer. J Nucl Med Technol. 2022;50(1):73–74. doi: 10.2967/jnmt.121.263001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong FC, Martiniova L, Masrani A, Ravizzini GC. 18F-fluciclovine–avid reactive axillary lymph nodes after COVID-19 vaccination. Clin Nucl Med. 2022;47(2):154. doi: 10.1097/RLU.0000000000003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Olmo-García MI, Prado-Wohlwend S, Bello P, Segura A, Merino-Torres JF. Peptide receptor radionuclide therapy with [177Lu] Lu-DOTA-TATE in patients with advanced GEP NENS: present and future directions. Cancers. 2022;14(3):584. doi: 10.3390/cancers14030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armani C, Catalani E, Balbarini A, Bagnoli P, Cervia D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J Leukoc Biol. 2007;81(3):845–855. doi: 10.1189/jlb.0606417. [DOI] [PubMed] [Google Scholar]
- 21.van der Pol J, Vöö S, Bucerius J, Mottaghy FM. Consequences of radiopharmaceutical extravasation and therapeutic interventions: a systematic review. Eur J Nucl Med Mol Imaging. 2017;44(7):1234–1243. doi: 10.1007/s00259-017-3675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]