Abstract
Objective
Camrelizumab is a selective, humanised, high-affinity IgG4 kappa monoclonal antibody against programmed cell death 1 that shows effective antitumour activity with acceptable toxicity in multiple tumour types. The CameL trial demonstrated that camrelizumab plus chemotherapy (CC) significantly prolonged the median progression-free survival and median overall survival versus chemotherapy alone (CA) in patients with advanced non-squamous non-small cell lung cancer (NSCLC). Our study was conducted to investigate the cost-effectiveness of the two strategies in chemotherapy-naive patients with advanced non-squamous NSCLC.
Design, setting and participants
A Markov simulation model was generated based on the CameL trial. The two simulated treatments included CC and CA.
Primary and secondary outcome measures
Utility was derived from published literature, and costs were calculated based on those at our hospital in Chengdu, China. Incremental cost-effectiveness ratios (ICERs) were calculated to compare the cost-effectiveness of the two treatment arms.
Results
In the overall population, the total costs were $27 223.40 and $13 740.10 for CC and CA treatment, respectively. The CC treatment produced 1.37 quality-adjusted life years (QALYs), and the CA treatment produced 1.17 QALYs. Hence, patients who were in the CC group spent an additional $13 483.30 and generated an increase of 0.20 QALYs, resulting in an ICER of $67 416.50 per QALY.
Conclusions
For chemotherapy-naive patients with advanced non-squamous NSCLC, CC is not considered a cost-effective treatment versus CA in China when considering a willingness-to-pay threshold of $31 500 per QALY.
Trial registration number
Keywords: HEALTH ECONOMICS, Health economics, Respiratory tract tumours, CHEMOTHERAPY
Strengths and limitations of this study.
A Markov simulation model was generated based on the published CameL trial.
Survival analysis was applied to the calculation of Markov model parameters for pharmacoeconomic evaluation.
Health outcomes were measured by quality-adjusted life years.
Only direct costs including hospitalisation, costs for drugs, radiology and laboratory tests and treatments for all grades of adverse events were considered.
The reconstructed survival curves cannot be completely fitted with the actual survival curves due to the inevitable bias when capturing the survival probabilities at each time point through the Plot Digitizer.
Introduction
Lung cancer has become one of the leading causes of cancer-related death worldwide and is the most commonly diagnosed cancer in Chinese males.1 2 The most common type of lung cancer is non-small cell lung cancer (NSCLC). More than 30% of patients with NSCLC have locally advanced disease at the time of diagnosis, with a 5-year survival rate of 18%.3 4 The standard of care for patients with advanced NSCLC is mainly platinum-based doublet chemotherapy.3 The treatment paradigm of advanced NSCLC has been changed by immune checkpoint inhibitors (ICIs) in recent years. For example, ipilimumab, a fully human anti-cytotoxic T-lymphocyte antigen 4 antibody, and nivolumab, a fully human anti-programmed cell death 1 (PD-1) antibody, are ICIs that result in improved efficacy in patients with NSCLC with few adverse events (AEs).5 6A significant overall survival (OS) benefit was observed with nivolumab plus ipilimumab compared with chemotherapy as first-line treatment in patients with NSCLC.7 Pembrolizumab, as a first-line monotherapy, improves OS and progression-free survival (PFS) in patients with untreated metastatic NSCLC with programmed death ligand 1 (PD-L1) expression.8
Camrelizumab is a selective, humanised, high-affinity IgG4 kappa monoclonal antibody against PD-1 that shows a great tumour response with acceptable toxicity in multiple tumour types.9 As the outcomes presented in the CameL trial, camrelizumab plus chemotherapy (CC) treatment has shown a clinically significant improvement in PFS versus chemotherapy alone (CA) treatment in all patients with advanced non-squamous NSCLC without sensitive epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations.10 11 Camrelizumab successfully entered the Chinese medical insurance catalogue at the end of 2020, and the price was reduced from $3065.02/200 mg to $453.25/200 mg, a decrease of 85%. Patients can make reimbursement for the drugs included in the medical insurance catalogue in China. Therefore, it is valuable to conduct this study from the perspective of payers in low/middle-income countries with lower willingness-to-pay (WTP) thresholds.
Methods
Clinical outcomes
Clinical results were extracted from the CameL trial.11 A total of 412 chemotherapy-naive patients who had histologically confirmed advanced non-squamous NSCLC without sensitive EGFR and ALK alterations were randomly allocated at a 1:1 ratio to the CC group (205) and the CA group (207). Patients in the CC group received intravenous camrelizumab (200 mg) plus carboplatin (area under the curve (AUC), 5 mg/mL per min) and pemetrexed (500 mg/m2) on day 1 every 3 weeks, followed by maintenance therapy with camrelizumab plus pemetrexed. Patients in the CA group received intravenous carboplatin (AUC 5 mg/mL per min) and pemetrexed (500 mg/m2) on day 1 every 3 weeks, followed by maintenance therapy with pemetrexed alone. The median duration of treatment was 34.1 weeks in the CC group and 19.7 weeks in the CA group. For the first 54 weeks, CT scans were conducted every 6 weeks. Laboratory examinations were performed every 3 weeks. In the overall population, both PFS and OS were significantly prolonged in the CC group compared with the CA group (PFS, 11.3 months vs 8.3 months, p=0.0001; OS, 27.9 months vs 20.5 months).
Model structure
A Markov model was conducted in TreeAge Pro software V.2020 (TreeAge Software, Williamstown, Massachusetts, USA) to simulate the disease process, which included three states: PFS, progressive disease (PD) and death. Patients with advanced non-squamous NSCLC were assumed to be in the PFS state until the disease progressed, and then they could either enter the PD state or the death state; however, patients in the PD state could either remain in the same state or enter the death state (figure 1). GetData Graph Digitizer software was used to extract the survival curves from the published CameL trial. Pseudo-individual patient data were generated using the algorithm derived by Hoyle and Henley to minimise the difference between the trial data and our modelled data. The Weibull distributions provided the best fit to the recreated survival data (figure 2).12 Based on the fitted curve, we can estimate the time-dependency transition probability in each cycle using the following formula: P(t→t+1)=1−exp[λ(t)γ−λ(t+1)γ)], where t equals the current cycle number in the Markov model.12 The cycle length was 1 month, and this model defined the time horizon as 10 years. Health outcomes were measured by quality-adjusted life years (QALYs). We assumed that patients in the two groups received docetaxel after PD based on clinical guidelines.13
Figure 1.
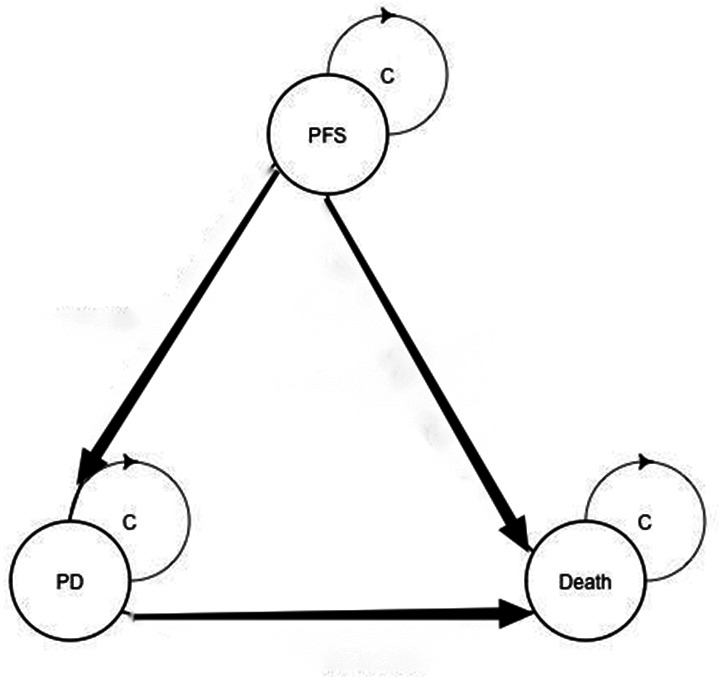
A Markov structure was built to compare two treatment strategies. PD, progressive disease; PFS, progression-free survival.
Figure 2.
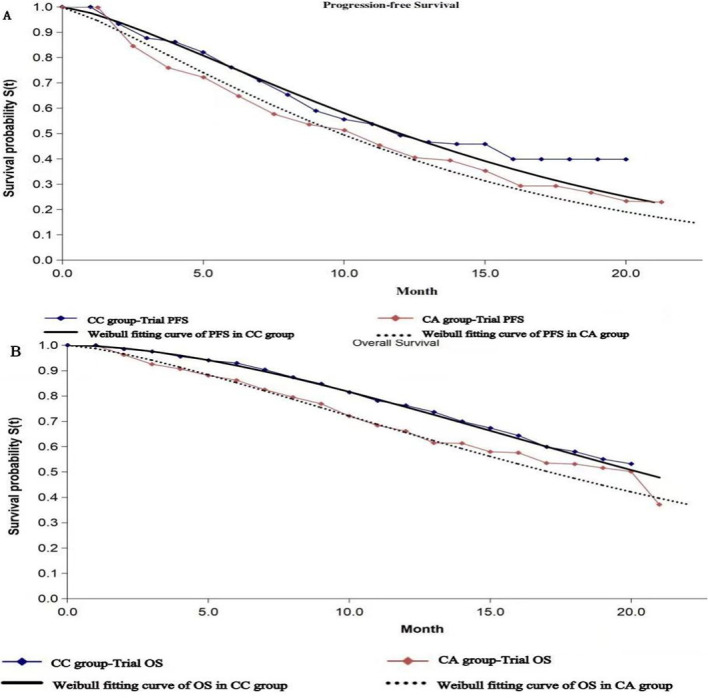
The original Kaplan-Meier PFS (A) and OS (B) curves from the CameL trial. Weibull distributions were fitted to the two groups. CA, chemotherapy alone; CC, camrelizumab plus chemotherapy; OS, overall survival; PFS, progression-free survival.
Costs and utility
In this analysis, we considered only direct costs, including hospitalisation, costs for drugs, radiology and laboratory tests and treatments for all grades of AEs. The prices of all the drugs were based on the price at our hospital in Chengdu, China. We assumed a mean body surface area and a body weight of 1.64 m2 and 65 kg, respectively.14 All costs were measured in US dollars based on the exchange rate on 27 December 2020 (US$1=¥6.46). Health utility scores were 0.81, 0.58 and 0 in the PFS state, PD state and death state, respectively.15 16 The annual discount rate of 3% was calculated (table 1).
Table 1.
Utilities and estimated monthly costs per patient based on the CameL trial
| Parameters | CC group | CA group |
| Costs per month, $ | ||
| Camrelizumab | 3065.17 (3678.20–2452.14) | – |
| Pemetrexed | 1012.95 (1215.54–810.36) | 805.47 (966.56–644.38) |
| Carboplatin | 18.29 (21.95–14.64) | 19.81 (23.77–15.85) |
| Hospitalisation | 25.81 (30.97–20.65) | 15.22 (18.26–12.18) |
| AEs | 58.19 (69.83–46.55) | 63.22 (75.87–50.58) |
| Tests | 201.95 (242.34–161.56) | 166.98 (200.37–133.58) |
| Cost of PD | 183.68 (220.42–146.95) | 249.92 (299.91–199.94) |
| Utility | ||
| PFS | 0.81 | 0.81 |
| PD | 0.58 | 0.58 |
| Discount rate, % | 3 | |
AEs, adverse events; CA, chemotherapy alone; CC, camrelizumab plus chemotherapy; PD, progressive disease; PFS, progression-free survival.
Sensitivity analysis
One-way probabilistic sensitivity analysis was performed to examine the impact of input factors on the model. Key parameters were used within a range of ±20% to explore their impacts on the incremental cost-effectiveness ratios (ICERs). Treatments were considered cost-effective if the ICER was lower than the WTP threshold. According to the WHO recommendations for cost-effective analysis, the threshold of $31 500 per QALY was defined as threefold the gross domestic product per capita of China. In addition, probabilistic sensitivity analysis was performed using Monte Carlo simulation, which included 1000 iterations to further address the uncertainty of all the input parameters.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Baseline analysis
In the overall population, the total costs were $27 223.40 and $13 740.10 for CC and CA treatment, respectively. The CC treatment produced 1.37 QALYs, and the CA treatment produced 1.17 QALYs. Hence, patients who were in the CC group spent an additional $13 483.30 and generated an increase of 0.20 QALYs, resulting in an ICER of $67 416.50 per QALY (table 2).
Table 2.
Results of base case analysis of the CC and CA groups
| Result | CC group | CA group |
| Cost ($) | 27 223.40 | 13 740.10 |
| Incremental costs | 13 483.30 | |
| Effectiveness (QALYs) | 1.37 | 1.17 |
| Incremental effectiveness | 0.2 | |
| ICER ($/QALY) | 67 416.50 |
CA, chemotherapy alone; CC, camrelizumab plus chemotherapy; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years.
Sensitivity analyses
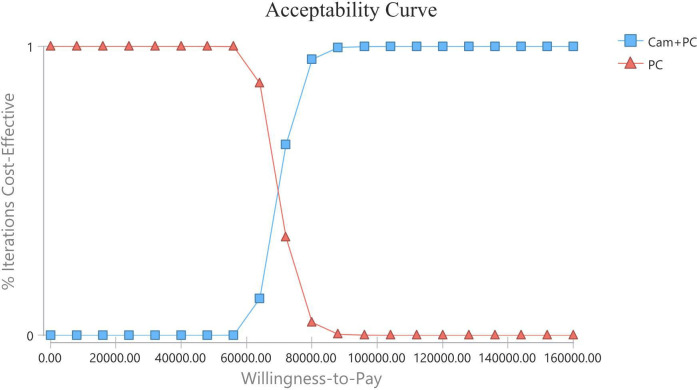
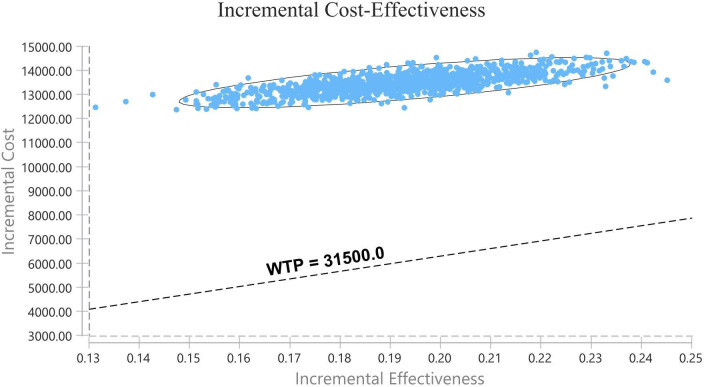
The results of the one-way sensitivity analysis are displayed in tornado diagrams (figure 3). The utility of PFS was the most sensitive parameter influencing the results. The second sensitive parameter was the cost of pemetrexed in the CC group, which ranged from $805.35 to $1 208.02, with ICER ranging from $54 115.08 to $84 422.81 per QALY. Changing other parameters, including the cost of camrelizumab, may result in different results but has little impact on the ICER. Thus, considering the current WTP threshold of $31 500, the acceptable curve shows that CC is not cost-effective for chemotherapy-naive patients with advanced non-squamous NSCLC in China (figure 4). All of the scatter points are located above the WTP threshold, implying the same results (figure 5).
Figure 3.
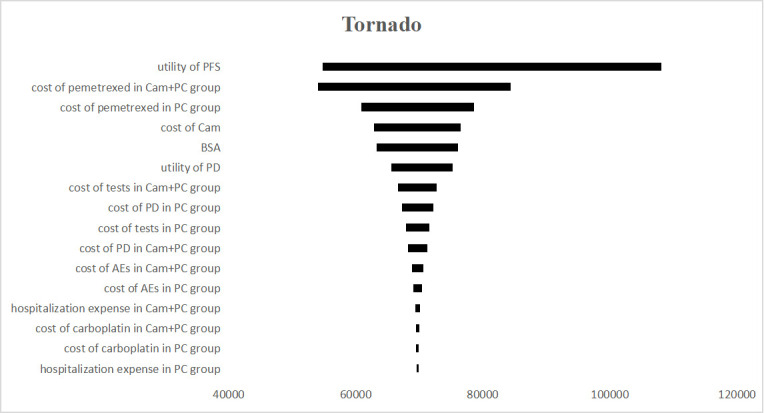
Tornado diagram of one-way sensitivity analyses. The impact of parameters on the ICER was listed. AEs, adverse events; BSA, body surface area; Cam, camrelizumab; ICER, incremental cost-effectiveness ratio; PC, pemetrexed+carboplatin; PD, progressive disease; PFS, progression-free survival.
Figure 4.
The cost-effectiveness acceptability curve showed the probability at different WTP thresholds. Cam, camrelizumab; PC, pemetrexed+carboplatin; WTP, willingness-to-pay.
Figure 5.
The dashed line indicates the WTP threshold. All of the scatter points are located above the WTP threshold, implying that camrelizumab plus chemotherapy is not a cost-effective therapy at the current WTP. WTP, willingness-to-pay.
Discussion
The domestic ICI camrelizumab has shown promising tumour response in multiple tumour types with manageable toxicities.17–21 In the CameL trial, the incidence of treatment-related AEs of any grade was higher in the CC group than in the CA group. The treatment duration of pemetrexed was longer in the CC group due to longer duration of maintenance therapy, which indicates a better tumour response. Due to the substantial decline in prices of camrelizumab, our research was valuable for assessing a cost-effective strategy for chemotherapy-naive patients with advanced non-squamous NSCLC from a Chinese payer perspective.
The combination therapy in the CC group provided incremental benefits at high incremental costs per QALY in our analysis. The probabilistic sensitivity analyses indicated that CC treatment would be cost-effective at a WTP threshold higher than $67 416.50 per QALY, which is nearly twice the current WTP threshold in China. We conducted the subgroup analysis in the PD-L1-positive population. Patients who were in the CC group spent an additional $20 914.18 and generated an increase of 0.29 QALYs, resulting in an ICER of $72 117.86 per QALY, which was also above the WTP threshold. The higher ICERs in the PD-L1-positive population may be associated with increased healthcare costs due to improved PFS that required more expensive treatment.
Several cost-effectiveness studies of other ICIs demonstrated that pembrolizumab monotherapy was cost-effective compared with chemotherapy both in the USA and France; however, it was not cost-effective in the UK or China as the first-line treatment in patients with advanced NSCLC.22–27 For patients with advanced NSCLC, atezolizumab plus bevacizumab and chemotherapy were not cost-effective;28 on the other hand, nivolumab plus ipilimumab was demonstrated to be cost-effective compared with chemotherapy from the US payer perspective.29
Although we conducted our study in China, the results may provide some enlightenment to other countries. The price of domestic pemetrexed was applied in our study and was demonstrated to be the second most sensitive parameter. However, this parameter was not sufficient to change the economic outcomes according to the sensitivity analysis. Recently, the price of imported pemetrexed (ALIMTA) in China has decreased, which is almost the same as that of domestic pemetrexed. Additionally, the chemotherapy drug price will vary due to different body surface areas; however, the sensitivity analysis shows that it has little impact on the ICER. The ICER in our study was far below the WTP value of $150 000 in the USA.30 Due to the much higher WTP threshold, we assumed that the CC treatment is quite possible to be cost-effective in some developed countries. The healthcare system in China was predominantly government funded, which would make it more likely to negotiate lower drug prices with pharmaceutical companies. If the price of pemetrexed will decrease in the future, it may make CC treatment cost-effective in China. Therefore, our analysis is conducive to the rational allocation of health resources, which is crucial to developing countries with relatively limited health resources.
However, there were limitations to our analysis. First, our model was based on a clinical trial, which may not be completely appropriate for real-world patients. The dose of chemotherapy drugs was calculated based on the average body surface in Chinese individuals, which varies in different individuals. Second, the reconstructed survival curves cannot be completely fitted with the actual survival curves due to the inevitable bias when capturing the survival probabilities at each time point through the Plot Digitizer, which will lead to the loss of the corresponding information of the simulated curves. However, the purpose of adjusting the transition probability is to approach the real results to the greatest extent. Although there are some limitations in applying survival analysis to the calculation of Markov model parameters for pharmacoeconomic evaluation, it is still one of the effective and feasible methods to reasonably solve the problem of time dependence of transfer probability in dynamic Markov models, especially the pharmacoeconomic evaluation of cancer. Third, given the lack of utility data in the CameL trial, the utilities of PFS and PD were derived from published literature. Fourth, the regimen of second-line treatment was not mentioned in the CameL trial, so we assumed that all patients accepted docetaxel after PD as recommended in the National Comprehensive Cancer Network (NCCN), which may differ from actual treatment.13 Additionally, reactive cutaneous capillary endothelial proliferation (RCCEP) is the most common immune-related dermatological toxicity of camrelizumab according to the CameL trial; however, the cost of treating RCCEP was excluded from our study because its effects are mild, reversible and predictable.21
Conclusions
In conclusion, from the Chinese payers’ perspective, CC is not a cost-effective therapy compared with CA in chemotherapy-naive patients with advanced non-squamous NSCLC without sensitive EGFR and ALK alterations at the current WTP of $31 500 per QALY.
Supplementary Material
Footnotes
Contributors: QX wrote the main manuscript text and prepared the two tables and HZ prepared figures 1–5. QL and NS designed the study. QL was in charge of planning, conducting and reporting of the work described in the article and is the guarantor. All authors reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval is not required for the study.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Feng R-M, Zong Y-N, Cao S-M, et al. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun 2019;39:22. 10.1186/s40880-019-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 4.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-Analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90. 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Brahmer J, Hellmann MD, et al. CheckMate 227: a randomized, open-label phase 3 trial of nivolumab, nivolumab plus ipilimumab, or nivolumab plus chemotherapy versus chemotherapy in chemotherapy-naïve patients with advanced non-small cell lung cancer (NSCLC). Annals of Oncology 2017;28:ii50–1. 10.1093/annonc/mdx091.064 [DOI] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 8.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–30. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Wu J, Chen X, et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clin Cancer Res 2019;25:7363–9. 10.1158/1078-0432.CCR-19-1680 [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Huang C, Fan Y, et al. P1.01-61 a phase II umbrella study of Camrelizumab in different PD-L1 expression cohorts in pre-treated advanced/metastatic non-small cell lung cancer. J Thorac Oncol 2019;14:S382–3. 10.1016/j.jtho.2019.08.776 [DOI] [Google Scholar]
- 11.Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (camel): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-314. 10.1016/S2213-2600(20)30365-9 [DOI] [PubMed] [Google Scholar]
- 12.Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11:139. 10.1186/1471-2288-11-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The Tax 320 non-small cell lung cancer Study Group. J Clin Oncol 2000;18:2354–62. 10.1200/JCO.2000.18.12.2354 [DOI] [PubMed] [Google Scholar]
- 14.Wu B, Dong B, Xu Y, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS One 2012;7:e32530. 10.1371/journal.pone.0032530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chouaid C, Agulnik J, Goker E, et al. Health-Related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol 2013;8:997–1003. 10.1097/JTO.0b013e318299243b [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Wu B, Wang X, et al. Health state utilities in patients with advanced non-small-cell lung cancer in China. J Comp Eff Res 2018;7:443–52. 10.2217/cer-2017-0069 [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Qi L, Wang X, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun 2020;40:711–20. 10.1002/cac2.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen T, Zheng S, Geng L, et al. Experience with anti-PD-1 antibody, Camrelizumab, monotherapy for biliary tract cancer patients and literature review. Technol Cancer Res Treat 2020;19:153303382097970. 10.1177/1533033820979703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (escort): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832–42. 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 20.Fan Y, Zhao J, Wang Q, et al. Camrelizumab plus apatinib in extensive-stage SCLC (passion): a multicenter, two-stage, phase 2 trial. J Thorac Oncol 2021;16:299–309. 10.1016/j.jtho.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Lu X, Koral K. The clinical application of camrelizumab on advanced hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2020;14:1017–24. 10.1080/17474124.2020.1807939 [DOI] [PubMed] [Google Scholar]
- 22.Huang M, Lou Y, Pellissier J, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics 2017;35:831–44. 10.1007/s40273-017-0527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartari F, Santoni M, Burattini L, et al. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: recent insights and future challenges. Cancer Treat Rev 2016;48:20–4. 10.1016/j.ctrv.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Georgieva M, da Silveira Nogueira Lima JP, Aguiar P, et al. Cost-Effectiveness of pembrolizumab as first-line therapy for advanced non-small cell lung cancer. Lung Cancer 2018;124:248–54. 10.1016/j.lungcan.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 25.Chouaid C, Bensimon L, Clay E, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer 2019;127:44–52. 10.1016/j.lungcan.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 26.Liao W, Huang J, Hutton D, et al. Cost-Effectiveness analysis of first-line pembrolizumab treatment for PD-L1 positive, non-small cell lung cancer in China. J Med Econ 2019;22:344–9. 10.1080/13696998.2019.1570221 [DOI] [PubMed] [Google Scholar]
- 27.Zhou K, Jiang C, Li Q. Cost-Effectiveness analysis of pembrolizumab monotherapy and chemotherapy in the non-small-cell lung cancer with different PD-L1 tumor proportion scores. Lung Cancer 2019;136:98–101. 10.1016/j.lungcan.2019.08.028 [DOI] [PubMed] [Google Scholar]
- 28.Wan X, Luo X, Tan C, et al. First-Line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer 2019;125:3526–34. 10.1002/cncr.32368 [DOI] [PubMed] [Google Scholar]
- 29.Hu H, She L, Liao M, et al. Cost-Effectiveness analysis of nivolumab plus ipilimumab vs. chemotherapy as first-line therapy in advanced non-small cell lung cancer. Front Oncol 2020;10:1649. 10.3389/fonc.2020.01649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann Intern Med 2021;174:25–32. 10.7326/M20-1392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article.