Abstract
Objectives
Patients with acute congestive heart failure (HF) regularly undergo urinary catheterisation (UC) at hospital admission. We hypothesised that UC has no clinical benefits with regard to weight loss during inpatient diuretic therapy for acute congestive HF and increases the risk of urinary tract infection (UTI).
Design
Retrospective, non-inferiority study.
Setting
Geneva University Hospitals’ Department of Medicine, a tertiary centre.
Participants
In a cohort of HF patients, those catheterised within 24 hours of diuretic therapy (n=113) were compared with non-catheterised patients (n=346).
Primary and secondary outcome measures
The primary endpoint was weight loss 48 hours after starting diuretic therapy. Secondary endpoints were time needed to reach target weight, discontinuation of intravenous diuretics and resolution of respiratory failure. Complications included the time to a first UTI, first hospital readmission and death.
Results
A total of 48-hour weight loss was not statistically different between groups and the adjusted difference was below the non-inferiority boundary of 1 kg (0.43 kg (95% CI: −0.03 to 0.88) in favour of UC, p<0.01 for non-inferiority). UC was not associated with time to reaching target weight (adjusted HR 1.0; 95% CI: 0.7 to 1.5), discontinuation of intravenous diuretics (aHR 0.9; 95% CI: 0.7 to 1.2) or resolution of respiratory failure (aHR 1.1; 95% CI: 0.5 to 2.4). UC increased the risk of UTI (aHR 2.5; 95% CI: 1.5 to 4.2) but was not associated with hospital readmission (aHR 1.1; 95% CI: 0.8 to 1.4) or 1-year mortality (aHR 1.4; 95% CI: 1.0 to 2.1).
Conclusion
In this retrospective study, with no obvious hourly diuresis-based diuretic adjustment strategy, weight loss without UC was not inferior to weight loss after UC within 24 hours of initiating diuretic treatment. UC had no impact on clinical improvement and increased the risk of UTI. This evidence, therefore, argues against the systematic use of UC during a diuretic therapy for HF.
Keywords: heart failure, urinary tract infections, health & safety
Strengths and limitations of this study.
The present study is the first to give an insight into the hypothetical clinically relevant benefits of urinary catheterisation (UC) in the context of heart failure (HF).
The study preceded the advent of sacubitril or sodium-glucose cotransporter-2 inhibitors therapy. Nevertheless, in 2021, updated European Society of Cardiology guidelines did not evolve regarding diuretics or the relevance of UC for the management of acute decompensate HF.
The study’s retrospective, observational approach only allowed us to hypothesise that urinary catheters were placed for HF management or to facilitate diuresis.
Since patients are usually not weighed in emergency rooms, we focused on the weight change from days 1 to day 3. Thus, UC’s impact during the first 24 hours of diuretic therapy was not assessed.
A randomised prospective design, with protocols to guide rapid diuretic adaptation, would be better able to explore the UC’s real potential among HF patients. However, considering current evidence and risks, such a study may never occur.
Introduction
Heart failure (HF) is a major public health concern, affecting 2% of the developed world’s population.1 Patients with HF are hospitalised about once a year, on average.2 Due to population ageing and the growing prevalence of comorbidities, the absolute number of hospital admissions for HF is expected to increase by as much as 50% over the next 25 years.1 3 4
Diuretics are the mainstay treatments for volume overload.5 6 Nevertheless, overly aggressive or insufficient treatments can result in acute kidney injury (AKI), electrolytic imbalance, low blood pressure, prolonged hospital length of stay (LOS) or early hospital readmission.7 8 Assessing adequate response to diuretics, for example, measuring diuresis, is therefore important and enables rapid treatment adjustment. This may be as or even more useful than how diuretics are initially administered.7 9
Between one quarter10 and one half of patients11 hospitalised for HF undergoes in-dwelling urinary catheterisation (UC). The rationale for UC in this population sometimes includes managing hypervolaemia11 12 or improving comfort during diuretic treatment.13 By maximising the elimination of liquids while avoiding excessive losses, UC could theoretically have a positive impact on hospital LOS, readmission rates and even death. Although the benefits of UC remain uncertain, the risks of increased urinary tract infections (UTI) and traumatic complications are well known.11 14 15 A recent retrospective study of catheterised HF patients showed no impact on LOS and an increased risk of infection.11 Little evidence exists on UC’s impact on clinically relevant improvements such as weight loss, time to improvement of respiratory failure or time to discontinuation of intravenous therapy. The present study aimed to determine the risks and clinical benefits of UC among patients hospitalised for congestive HF, with the a priori hypothesis that HF management with UC is not better than without it.
Patients and methods
We conducted a retrospective, non-inferiority, cohort study using a pre-existing cohort of patients admitted to Geneva University Hospitals’ Department of Medicine for acute HF between 01 January 2006 and 01 January 2010.16 17 Patients were followed for 1 year or until death. Reporting and analyses were performed according to the Strengthening the Reporting of Observational Studies in Epidemiology statement.
Study population
All patients aged 18 years old or more, requiring hospital admission for a primary symptom of dyspnoea and a diagnosis of acute decompensated HF, were eligible.16 17 Acute decompensated HF was diagnosed from patients’ clinical presentation, risk factors and treatment responsiveness and/or was supported by structural or functional echocardiographic anomalies. Patients with a final diagnosis other than HF that explained their dyspnoea, with a low NT-proBNP level (<300 ng/L), who were admitted to the intensive care unit, whose paper medical charts for the index admission were unavailable or who did not receive diuretics during their first 7 days of hospitalisation were excluded. We compared patients who underwent UC within 24 hours of diuretic therapy initiation with those not catheterised.
Outcomes
The primary endpoint was the 48-hour weight loss after starting diuretic therapy. Secondary endpoints were persistent excess weight at 72 hours and at 1 week, the time needed to reach clinical improvement (reaching target weight (±0.5 kg), discontinuation of intravenous diuretics, oxygen supply and continuous positive airway pressure (CPAP)) and hospital LOS.
Complications included the proportion of patients with UTI, initial diuretic treatment failure, worsening kidney function (WKF) and episodes of low blood pressure, and time to a first UTI, first hospital readmission and death. Initial treatment failure was defined as a need for increased doses of diuretics, or a switch from oral to intravenous diuretic therapy or from a bolus to a continuous intravenous diuretic therapy 2 days or more after the initiation of diuretics. Diuretic dose increases before that point were considered to be usual treatment adjustments.
Data collection and variable definition
Data extracted from medical charts included preadmission diuretic use, micturition volumes during diuretic therapy, weight at discharge and, for the first 7 days, daily information on the UC, weight, diuretics administration, clinical parameters, oxygen supply and use of CPAP therapy.
Volume overload (hypervolaemia) was defined as excess weight at diuretic therapy. We calculated excess weight by subtracting target weight from other weights measured during hospitalisation. Target weight was defined as the patient’s weight at discharge or, when unavailable, the lowest weight during hospitalisation that did not result in increased creatinaemia or low blood pressure. Since patients are not always weighed on admission day, 48-hour weight loss was calculated between days 1 and 3 after starting diuretic therapy (day 0). When weight on day 1 or on day 3 was missing, we took double the mean daily weight loss calculated between day 0 and day 4.
Respiratory failure was defined as the need for oxygen supply or CPAP. We noted episodes of low blood pressure (systolic pressure<100 mm Hg) and the need for saline perfusion. Daily doses of torasemide were multiplied by two and doses of oral furosemide were divided by two to convert daily diuretics use into an equivalent intravenous furosemide dosage.18
We obtained patients’ habitual kidney function from their general practitioner.17 Kidney function at hospital admission and during the first week was extracted from the laboratory database. We defined AKI as any kidney function at admission lower than its usual value, and WKF as kidney function that decreased during hospitalisation relative to admission values.17 AKI and WKF were scored according to the Kidney Disease: Improving Global Outcomes (KDIGO) classification. An absolute increase in the creatinine value of 26.4 mmol/L, or a 1.5 to <2 fold increase over the baseline creatinine value, was defined stage 1. A ≥2 to <3 fold increase was defined as stage 2 and a ≥3 fold increase or use of dialysis was determined as stage 3.
Comorbidity burdens were summarised using Charlson index, calculated using the International Classification of Diseases, Tenth Revision codes from electronic medical charts.19 HF types were stratified into intermediate or reduced left ventricular ejection fraction (LVEF<49%), preserved LVEF (LVEF>50%) and unknown LVEF.
We extracted urinary and blood culture information from the microbiology laboratory database for each febrile episode during hospitalisation. A diagnosis of UTI was defined as bacterial growth of 10E3 colony-forming units in a urine sample plus the corresponding symptoms of a UTI.
Information on death was obtained from Switzerland’s national deaths registry and Geneva University Hospitals’ (the only public hospital in the canton) electronic databases. We reviewed charts for hospital LOS, place of discharge (home vs rehabilitation centre or care home) and all-cause and HF-related hospital readmissions within 1 year.
Statistics
For our analyses, timings (day 0) were set from the first day of diuretic use, which could differ from the hospital admission day.
Primary analysis and weight evolution
Weight loss analyses during diuretic treatment were restricted to patients with volume overload (online supplemental figure S1). The primary analysis used a linear regression model, where the 48-hour weight loss was the dependent variable and UC was the independent variable. The model was adjusted for age, sex, Charlson index score, preadmission diuretic dose, HF type, admission heart rate and blood pressure, respiratory failure, weight excess at diuretic therapy, first diuretic dose, use of continuous intravenous diuretics, AKI and admission through the emergency room, based on previous studies (online supplemental table S1).9 20 21 To reach non-inferiority, the upper CI of a between-group difference had to be less than 1 kg (in favour of UC). This threshold was determined based on daily minimal clinically significant weight loss of 0.5 kg (1 kg in 2 days; online supplemental statistics S1).22 Unilateral t-test served to test non-inferiority.
bmjopen-2021-053632supp001.pdf (1.4MB, pdf)
We performed five sensitivity analyses on the main outcome (online supplemental statistics S2). First, a multiple imputation method was used to replace missing values. The second matched catheterised patients 1:1 with non-catheterised patients according to sex and the closest value (<10%) of a propensity score. The score included all the variables mentioned above except sex. A paired t-test was used to test mean differences. The third sensitivity analysis excluded patients with urinary retention. The fourth replaced AKI at admission with creatininemia and blood sodium. The last sensitivity analysis split the continuous confounding variables at their median.
We used linear regression, adjusted for confounders, to explore associations between UC and persistent weight excess at 72 hours and 1 week. We also tested the interaction between UC and time in a mixed effects model adjusted for the factors mentioned above. A random intercept for each patient accounted for repeated measures across days. The mean expected excess weights of patients with and without UC was calculated assuming mean values for continuous predictors and a proportion of positive categorical predictors similar to the study sample.
Clinical improvements and complications
The unadjusted impact of UC on time-dependent outcomes was analysed using Kaplan-Meier survival analysis and an unweighted, two-sided, log-rank test to compare groups. Analysis of target weight was restricted to patients with volume overload. Analyses of intravenous diuretics, oxygen supply and CPAP were restricted to patients receiving those therapies. Multivariate Cox models were adjusted for age, sex and Charlson comorbidity index score. For target weight and the time needed to discontinue intravenous diuretics, Cox models were further adjusted for all the confounding factors in the primary analysis. The proportional hazards assumption was verified using Schoenfeld residuals and a visual inspection of the log-minus-log plots (online supplemental statistics S3).
The association between UC and LOS was tested using a linear regression model adjusted for confounding factors and in which LOS was log-transformed to correct for skewed data. Logistic regression was used to adjust binary outcomes for confounders. Comparisons of characteristics between groups were performed using the Χ2 test or Fisher’s exact test, where appropriate, for categorical variables. The Mann-Whitney test was used for continuous variables as these were not normally distributed. Except for the primary outcomes, all analyses were two sided, with a significance level set at 5%. All analyses were performed using STATA, V.12.0, and R statistical software, V.4.0.0.23
Results
Of 640 potential participants in the HF register, 174 had no available paper medical chart and 7 had no diuretic therapy within the first 7 days, leaving a cohort of 459 patients of whom 113 underwent UC within the first 24 hours (24.6%). Only four of these patients had documented urinary retention. Catheterised patients were older, more often women, more frequently experienced respiratory failure or AKI and received higher initial diuretic doses (table 1). Urinary catheters were placed for a median of 4 days (IQR: 2–8). Diuresis was recorded more often among patients with UC (58.0%) than patients without (41.2%, p<0.01).
Table 1.
Characteristics of participants with and without Urinary catheterisation (UC)
| Characteristic | Cohort (N=459) | With UC (N=113) | Without UC N=346) | P value |
| Age (years), median (IQR) | 81 (73–86) | 83.5 (76–89) | 80 (71–85) | <0.001 |
| Male | 248 (55.2%) | 52 (47.3%) | 196 (57.8%) | 0.053 |
| Admitted through ER | 398 (86.7%) | 97 (85.8%) | 301 (87.0%) | 0.754 |
| Night-time admission (19:00–07:00) | 169 (36.8%) | 37 (32.7%) | 132 (38.1%) | 0.301 |
| Current smoker | 79 (18.0%) | 18 (17.3%) | 61 (18.1%) | 0.844* |
| High blood pressure | 331 (73.7%) | 83 (73.7%) | 248 (73.2%) | 0.709 |
| Diabetes | 135 (30.1%) | 34 (30.9%) | 101 (29.8%) | 0.812 |
| Myocardial infarct | 27 (6.1%) | 6 (5.5%) | 21 (6.3%) | 0.762* |
| LVEF<50% | 203 (45.2%) | 46 (40.7%) | 157 (45.4%) | 0.686 |
| Stroke | 52 (11.8%) | 18 (16.1%) | 34 (10.2%) | 0.076* |
| Peripheral vascular disease | 76 (17.2%) | 23 (21.1%) | 53 (15.9%) | 0.242* |
| Dementia | 30 (6.8%) | 9 (8.3%) | 21 (6.3%) | 0.482* |
| COPD | 66 (14.7%) | 16 (14.5%) | 50 (14.7%) | 0.958* |
| Oncological disease | 32 (7.2%) | 8 (7.3%) | 24 (7.2%) | 0.963* |
| Liver disease | 24 (5.4%) | 7 (6.4%) | 17 (5.1%) | 0.598* |
| CKD | ||||
| II | 174 (38.7%) | 39 (35.4%) | 135 (39.2%) | 0.380 |
| III | 154 (34.4%) | 40 (36.4%) | 114 (33.6%) | |
| IV | 22 (4.9%) | 6 (5.5%) | 16 (4.7%) | |
| V | 2 (0.5%) | 0 | 2 (0.6%) | |
| Charlson comorbidity index, median (IQR) | 3 (1–4) | 3 (2–4) | 3 (1–4) | 0.115 |
| Preadmission medication | ||||
| ACE/ARB | 290 (64.6%) | 65 (59.1%) | 225 (66.4%) | 0.165 |
| Beta-blocker | 204 (45.4%) | 49 (44.5%) | 155 (45.7%) | 0.829 |
| Aldosterone inhibitor | 62 (13.8%) | 14 (12.7%) | 48 (14.2%) | 0.753* |
| Diuretics | 239 (52.1%) | 66 (58.4%) | 173 (50.0%) | 0.120 |
| Admission characteristics | ||||
| Median sodium level (mmol/L) at admission (IQR) | 137 (134–140) | 137 (133–139) | 138 (135–140) | 0.022 |
| Median NT-proBNP level (ng/L) at admission (IQR) | 6377 (3069–13254) | 7700 (4080–16204) | 6206 (2700–12101) | 0.124 |
| Median haemoglobin level (g/L) at admission (IQR) | 123 (109–137) | 119 (107–133) | 125 (110–138) | 0.115 |
| Median creatinin level (mmol/L) at admission (IQR) | 107 (85–148) | 116 (89–197) | 104 (83–138) | 0.005 |
| Heart rate (beat/min), median (IQR) | 81 (70–94) | 82.5 (70–91) | 80 (69.5–95) | 0.785 |
| Mean blood pressure (mm Hg), median (IQR) | 90 (80–100) | 88.3 (78.3–100) | 90 (80.3–100) | 0.162 |
| AKI | ||||
| I | 143 (31.5%) | 46 (41.8%) | 97 (28.6%) | 0.002* |
| II | 24 (5.3%) | 9 (8.2%) | 15 (4.4%) | |
| III | 5 (1.1%) | 3 (2.7%) | 2 (0.6%) | |
| Respiratory failure | 371 (80.8%) | 105 (92.9%) | 266 (76.9%) | <0.001* |
| Needs oxygen supply | 365 (79.5%) | 104 (92.0%) | 261 (75.4%) | |
| CPAP | 75 (16.3%) | 27 (23.9%) | 48 (13.9%) | |
| Volume overload | 342 (74.5%) | 86 (76.1%) | 256 (74.0%) | 0.654 |
| Target weight (kg), median (IQR) | 69.2 (58.1–80.8) | 70 (58–81.4) | 68.7 (58.6–80.5) | |
| Intravenous therapy | 431 (93.9%) | 108 (95.6%) | 323 (93.4%) | 0.391* |
| Continuous intravenous diuretic therapy | 42 (9.2%) | 15 (13.4%) | 27 (7.8%) | 0.076* |
| Initial diuretic doses (mg), median (IQR) | 40 (30–80) | 60 (40–90) | 40 (20–60) | <0.001 |
Only UC within 24 hours of diuretic therapy initiation was considered. Values are numbers (percentage) unless otherwise stated.
*Fischer test.
ACE/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blockers; AKI, acute kidney injury; CKD, chronic kidney disease by CKD-EPI classification; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure therapy; ER, emergency room; LVEF, left ventricular ejection fraction; UC, urinary catheterisation.
Excess weight under diuretic therapy
At diuretic therapy initiation, 342 patients carried excess weight and were included in the primary analysis (online supplemental figure S1). In adjusted linear regressions, being catheterised was not associated with significantly greater 48-hour weight loss than not being catheterised (0.43 kg in favour of UC (95% CI: −0.03 to 0.88)). The upper CI of between-group difference was below the non-inferiority boundary of 1 kg (p<0.01 for non-inferiority).
In sensitivity analysis, the results were in line with the primary analysis (online supplemental table S2). In the propensity score-matched analysis, 64 patients with UC were matched with 64 patients without a catheter (none had urinary retention). The difference in weight loss was 0.29 kg (95% CI: −0.3 to 0.88; p<0.01 for non-inferiority).
Patients with UC did not have a statistically lower persistent excess weight at 72 hours: the difference was 0.27 kg (95% CI: −0.52 to 1.1; p=0.50) in unadjusted and 0.24 kg (95% CI: −0.17 to 0.64; p<0.001 for non-inferiority) in adjusted linear regression. At 1 week, the excess weight difference between patients with and without UC was −0.09 kg (95% CI: −1.0 to 0.8; p=0.84) in unadjusted and −0.14 kg (95% CI: −0.89 to 0.60; p=0.01 for non-inferiority) in adjusted linear regression. Similarly, there was no statistically significant interaction between UC and daily excess weight changes in the mixed effects model (p=0.55; figure 1).
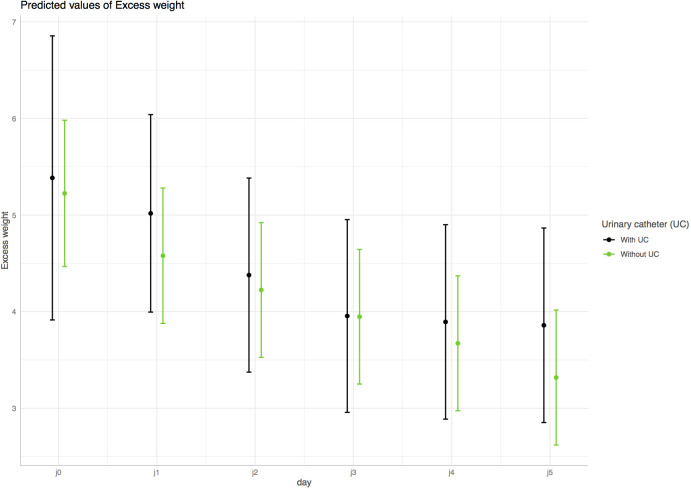
Figure 1.
Predicted excess weight (kg) over time (days) for patients with (black line) and without (green line) urinary catheterisation (UC). Mean expected excess weights and their CIs were calculated using an adjusted mixed effects model assuming mean values for continuous predictors and a proportion of positive categorical predictors similar to the study sample. UC had no statistical effect on excess weight evolution over time (p for interaction=0.55).
Clinical improvement
Time to reach target weight and time needed to discontinue CPAP were not statistically different between patients with and without UC in both unadjusted and adjusted analysis (figure 2, table 2). UC tended to be associated with a longer time to discontinuation of an intravenous diuretic or discontinuation of oxygen supply (figure 2), but the associations disappeared after adjustment for confounders (table 2).
Figure 2.
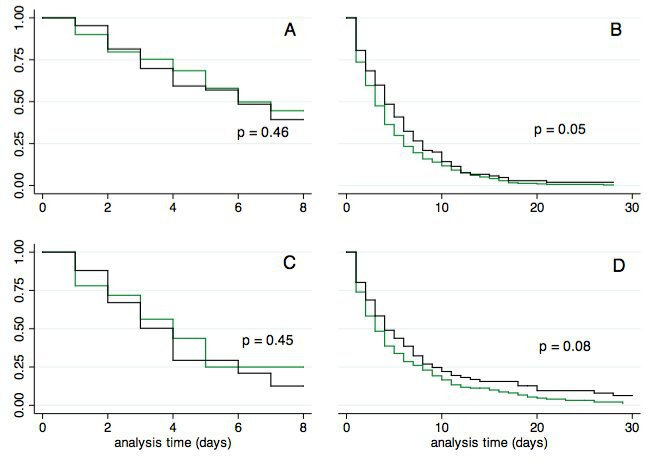
Time to reach clinical improvement for patients with urinary catheterisation (black line) and controls (green line): (A) time to reach target weight; (B) time to discontinuation of intravenous diuretics; (C) time to discontinuation of continuous positive airway pressure therapy; and (D) time to discontinuation of oxygen supply.
Table 2.
Clinical improvements and adverse outcomes with and without urinary catheterisation
| Time to clinical improvement | With UC | Without UC | HR | Adjusted HR* |
| Time to target weight (d), median (IQR) | 6 (3–7) | 6 (3–7) | 1.1 (0.8–1.5) | 1.0 (0.7–1.5) |
| Time to switch/discontinuation of intravenous diuretics (d), median (IQR) | 4 (2–8) | 3 (1–6) | 0.8 (0.7–1.0) | 0.9 (0.7–1.2) |
| Time to discontinuation of CPAP (d), median (IQR) | 4 (2–6) | 4 (2–5) | 1.2 (0.7–2.2) | 1.1 (0.5–2.4) |
| Time to discontinuation of oxygen supply (d), median (IQR) | 4 (2–8) | 3 (1–7) | 0.8 (0.6–1.0) | 0.9 (0.7–1.2) |
| Time to adverse events | With UC | Without UC | HR | Adjusted HR† |
| Urinary tract infection, n (%) | 37 (32.7%) | 46 (13.3%) | 2.9 (1.8–4.8)‡ | 2.5 (1.5–4.2)‡ |
| One-year all-cause hospital readmission, n (%) | 56 (50.9%) | 193 (56.9%) | 1.1 (0.8–1.4) | 1.1 (0.8–1.4) |
| One-year heart failure-related hospital readmission, n (%) | 28 (25.5%) | 91 (26.8%) | 1.1 (0.7–1.7) | 1.1 (0.7–1.7) |
| One-year mortality, n (%) | 51 (50.0%) | 101 (33.4%) | 1.7 (1.2–2.4)‡ | 1.4 (1.0–2.1) |
| Other secondary outcomes | With UC | Without UC | OR | Adjusted OR† |
| Initial treatment failure | 23 (26.7%) | 78 (30.5%) | 0.8 (0.5–1.4) | 0.8 (0.4–1.4) |
| Low blood pressure episode Need of saline perfusion |
49 (43.7%) | 154 (44.6%) | 1.0 (0.6–1.5) | 1.1 (0.7–1.7) |
| Need of saline perfusion | 24/49 (49.0) | 67/154 (43.5) | ||
| Worsening of renal function | ||||
| 0 | 82 (74.6%) | 260 (76.7%) | 1.1 (0.7–1.8)§ | 1.1 (0.7–1.8)§ |
| 1 | 27 (24.6%) | 73 (21.5%) | ||
| 2 | 1 (–) | 6 (1.8%) |
Values are numbers unless otherwise stated.
*Models were adjusted for age (continuous), sex (binary), Charlson index score (continuous), preadmission diuretic dose (continuous), heart failure type (categorical), admission heart rate and blood pressure (continuous), respiratory failure (binary), weight excess at diuretic therapy (continuous), first diuretic dose (continuous), use of continuous intravenous diuretics (binary), acute kidney injury (categorical) and admission through the emergency room (binary).
†Models were adjusted for age (continuous), sex (binary), Charlson index score (continuous).
‡P value<0.05.
§OR of changing to a worse category.
CPAP, continuous positive airway pressure therapy; HR, hazard ratio; OR, Odds ratio; UC, urinary catheterisation.
The median hospital LOS was identical for patients with and without UC (12 days, IQR: 9–18). However, fewer UC patients were discharged directly home from hospital (57.3% vs 73.7%; adjusted OR 0.6 (95% CI: 0.3 to 0.9; p<0.01)).
Complications
The proportions of initial treatment failures, low blood pressure episodes and patients with WKF were not statistically different between groups (table 2).
All-cause and HF-related readmissions were not statistically different between patients with and without UC (table 2 and figure 3). Half the patients with UC and one-third without UC were dead at 1 year. The difference was not statistically significant after adjustment (table 2).
Figure 3.
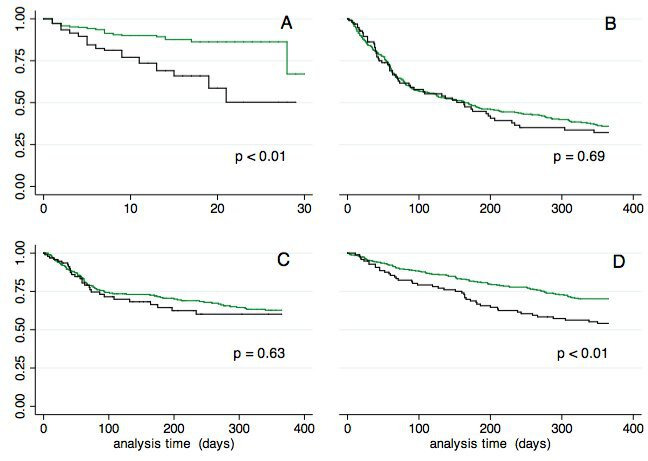
Time to reach unfavourable outcomes for patients with urinary catheterisation (black line) and controls (green line): (A) time to first urinary tract infection; (B) time to first all-cause hospital readmission; (C) time to first heart failure-related readmission; and (D) time to death.
UC patients were at a higher risk of suffering from a UTI, and this association persisted in adjusted analyses (table 2 and figure 3). Multiple UTIs occurred in nine UC patients (8.0%) and in five patients without UC (1.2%, p<0.01). Patients with a UTI had a longer hospital LOS (15 days (IQR: 10–21) vs 11 days (IQR: 8–17), p<0.001) and an increased 1-year mortality rate (52.5% vs 34.0%, adjusted HR 1.5; 95% CI: 1.1 to 2.3; p=0.038).
Discussion and conclusion
Among patients admitted to medical wards for acute HF, the strategy of abstaining from UC did not lead to inferior initial weight loss when compared with the strategy of UC within the first 24 hours. Besides, UC had no impact on clinical improvement such as the time needed to reach target weight, discontinue intravenous diuretics or improve respiratory failure, and hospital LOS. Furthermore, UC did not prevent excessive diuresis resulting in low blood pressure episodes or WKF, and it was associated with a higher risk of a UTI.
There are few appropriate indications for UC,14 and 7%–50% of UCs are done outside these indications (online supplemental table S3).24 25 Rates of UC subsequent to HF vary greatly and could be very high. In one study, more than half of haemodynamically stable patients underwent UC.11 The rationale for UC in HF is weak yet somehow based on beliefs that it facilitates urine elimination and increases comfort by decreasing toilet visits. However, there is good evidence that UC does not increase the comfort of patients undergoing diuretic therapy, even at high dosages.13 Most guidelines on UC good practice do not list HF as a standard indication.26 Through their Choosing Wisely campaigns, Swiss and American authorities recommend avoiding in-dwelling UC for urine output monitoring in stable patients who can void or for patient or staff convenience.27 28 Indication lists, authorities’ recommendations and financial penalties have reduced the overall inappropriate use of UC.14 An American study showed that the proportion of UC among HF patients decreased by 8% between 2009 and 2014.10
Catheter-associated UTIs (CAUTIs) are the second most common infections associated with patients hospitalised for HF after Clostridium-related infections.29 Previous reports among HF patients found associations between CAUTI and increased risks of discharge to a skilled care facility, longer hospital LOS, higher total hospital costs and in-hospital mortality.11 29 Condom catheters are a better option when diuresis affects older patients with a disability: they lead to fewer complications,30 are more comfortable and are less painful than UC.13
The present study is the first to consider the association between UC and clinically relevant outcomes in the context of HF. Using a register and significant adjustments to potential confounding factors further strengthened our findings. However, the study has limitations. First, the cohort preceded some important advances in HF management (eg, sacubitril treatment or sodium-glucose cotransporter-2 inhibitors) that may have changed readmission risk and mortality. Nevertheless, there were no changes in the 2021 European Society of Cardiology guidelines concerning the management of acute HF using diuretics, or the relevance of UC in this indication.3 We thus believe that our study’s conclusions remain valid today. Second, the study’s retrospective, observational approach only allowed us to hypothesise that urinary catheters were placed for HF management. To minimise these issues, we only selected UCs which occurred in the first 24 hours of diuretic therapy. Third, since patients are usually not weighed in emergency rooms, we focused on the weight change from day 1 to day 3. Thus, UC’s impact during the first 24 hours of diuretic therapy was not assessed. A randomised prospective design, with protocols to guide rapid diuretic adaptation, would be better able to explore the UC’s real potential among HF patients. However, considering current evidence and risks, such a study may never occur. It is of note that records of the amount of urine passed were only available for half of the patients, with or without UC. Thus, checking for adequate diuresis after treatment with diuretics might be a simpler, safer recommendation than UC for improved HF management. Finally, some medical charts could not be retrieved, but their unavailability was random and unrelated to their UC status or outcomes. Thus, there is little risk that unavailable charts biased the results.
In this retrospective study, with no obvious hourly diuresis-based diuretic adjustment strategy, weight loss without UC was not inferior to weight loss after UC within 24 hours of initiating diuretic treatment. UC had no impact on the clinically relevant outcomes of time to reach target weight, time to resolve respiratory failure and hospital LOS. The lack of benefits and the increased risk of a UTI preclude systematic UC for the management of HF.
Supplementary Material
Acknowledgments
We gratefully acknowledge Elise Dupuis for her help with the statistical analysis.
Footnotes
Contributors: GJ, SC, NG, DC and JS planned the study (protocol). MA and GB collected the data. GJ and JS performed the analyses. GJ wrote the manuscript. All authors reviewed and approved the final manuscript. GJ and JS accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The study received a grant from Geneva University Hospitals (grant: PRD 1-2017-I).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The database, variable explanation and Stata do-file (in Word format) are available at request to gregor.john@h-ne.ch.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
Ethics approval
This study involves human participants and was approved by the Institutional Review Board of Geneva University Hospital, and the Commission cantonale d'éthique de la recherche (CCER number 08-250R). As a retrospective analysis on data of patients hospitalised, the ethics committe waived the need to obtaine an informed consent.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barasa A, Schaufelberger M, Lappas G, et al. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur Heart J 2014;35:25–32. 10.1093/eurheartj/eht278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation 2019;139:659. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 5.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines: developed in collaboration with the International Society for heart and lung transplantation. Circulation 2009;119:1977–2016. 10.1161/CIRCULATIONAHA.109.192064 [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of cardiology. developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 2012;33. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felker GM, O'Connor CM, Braunwald E, et al. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail 2009;2:56–62. 10.1161/CIRCHEARTFAILURE.108.821785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Maaten JM, Dunning AM, Valente MAE, et al. Diuretic response in acute heart failure-an analysis from ASCEND-HF. Am Heart J 2015;170:313–21. 10.1016/j.ahj.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 10.Metersky ML, Eldridge N, Wang Y, et al. National trends in the frequency of bladder catheterization and physician-diagnosed catheter-associated urinary tract infections: results from the medicare patient safety monitoring system. Am J Infect Control 2017;45:901–4. 10.1016/j.ajic.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Jang AY, O'Brien C, Chung W-J, et al. Routine indwelling urethral catheterization in acute heart failure patients is associated with increased urinary tract complications without improved heart failure outcomes. Circ J 2018;82:1632–9. 10.1253/circj.CJ-17-1113 [DOI] [PubMed] [Google Scholar]
- 12.Aoki T. Appropriate use of urinary catheter in acute heart failure patients. Circ J 2018;82:1505–6. 10.1253/circj.CJ-18-0447 [DOI] [PubMed] [Google Scholar]
- 13.Saint S, Kaufman SR, Rogers MAM, et al. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc 2006;54:1055–61. 10.1111/j.1532-5415.2006.00785.x [DOI] [PubMed] [Google Scholar]
- 14.Meddings J, Rogers MAM, Krein SL, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf 2014;23:277–89. 10.1136/bmjqs-2012-001774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollingsworth JM, Rogers MAM, Krein SL, et al. Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Ann Intern Med 2013;159:401–10. 10.7326/0003-4819-159-6-201309170-00006 [DOI] [PubMed] [Google Scholar]
- 16.Garin N, Carballo S, Gerstel E, et al. Inclusion into a heart failure critical pathway reduces the risk of death or readmission after hospital discharge. Eur J Intern Med 2012;23:760–4. 10.1016/j.ejim.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Berra G, Garin N, Stirnemann J, et al. Outcome in acute heart failure: prognostic value of acute kidney injury and worsening renal function. J Card Fail 2015;21:382–90. 10.1016/j.cardfail.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 18.Anisman SD, Erickson SB, Morden NE. How to prescribe loop diuretics in oedema. BMJ 2019;364:l359. 10.1136/bmj.l359 [DOI] [PubMed] [Google Scholar]
- 19.Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–94. 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 20.Shah RV, McNulty S, O'Connor CM, et al. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an analysis from diuretic optimization strategies in acute heart failure. Am Heart J 2012;164:862–8. 10.1016/j.ahj.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voors AA, Davison BA, Teerlink JR, et al. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail 2014;16:1230–40. 10.1002/ejhf.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezekowitz JA, O'Meara E, McDonald MA, et al. 2017 comprehensive update of the Canadian cardiovascular Society guidelines for the management of heart failure. Can J Cardiol 2017;33:1342–433. 10.1016/j.cjca.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 23.R Core Team . R: A language and environment for statistical computing. [program], 2019. R Foundation for Statistical Computing, Vienna, Austria, 2017. Available: https://www.R-project.org/
- 24.Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med 2000;109:476–80. 10.1016/S0002-9343(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 25.Gokula RRM, Hickner JA, Smith MA. Inappropriate use of urinary catheters in elderly patients at a midwestern community teaching hospital. Am J Infect Control 2004;32:196–9. 10.1016/j.ajic.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention . Examples of appropriate indications for indwelling urethral catheter use. Available: https://www.cdc.gov/infectioncontrol/guidelines/cauti/recommendations.html [Accessed 9 Apr 2020].
- 27.Physicians ACoE . Choosing wisely, 2013. Available: https://www.choosingwisely.org/societies/american-college-of-emergency-physicians/ [Accessed 9 Apr 2020].
- 28.Générale SSdmi . Choosing wisely, 2016. Available: https://www.smartermedicine.ch/fr/liste-top-5/infektiologie.html#c15766 [Accessed 9 Apr 2020].
- 29.Miller PE, Guha A, Khera R, et al. National trends in healthcare-associated infections for five common cardiovascular conditions. Am J Cardiol 2019;124:1140–8. 10.1016/j.amjcard.2019.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saint S, Krein SL, Fowler KE, et al. Condom catheters versus indwelling urethral catheters in men: a prospective, observational study. J Hosp Med 2019;14:E1–4. 10.12788/jhm.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-053632supp001.pdf (1.4MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The database, variable explanation and Stata do-file (in Word format) are available at request to gregor.john@h-ne.ch.