Abstract
The small, stable RNA molecule encoded by ssrA, known as tmRNA or 10Sa RNA, is required for the growth of certain hybrid λimmP22 phages in Escherichia coli. tmRNA has been shown to tag partially synthesized proteins for degradation in vivo by attaching a short peptide sequence, encoded by tmRNA, to the carboxyl termini of these proteins. This tag sequence contains, at its C terminus, an amino acid sequence that is recognized by cellular proteases and leads to degradation of tagged proteins. A model describing this function of tmRNA, the trans-translation model (K. C. Keiler, P. R. Waller, and R. T. Sauer, Science 271:990–993, 1996), proposes that tmRNA acts first as a tRNA and then as a mRNA, resulting in release of the original mRNA template from the ribosome and translocation of the nascent peptide to tmRNA. Previous work from this laboratory suggested that tmRNA may also interact specifically with DNA-binding proteins, modulating their activity. However, more recent results indicate that interactions between tmRNA and DNA-binding proteins are likely nonspecific. In light of this new information, we examine the effects on λimmP22 growth of mutations eliminating activities postulated to be important for two different steps in the trans-translation model, alanine charging of tmRNA and degradation of tagged proteins. This mutational analysis suggests that, while charging of tmRNA with alanine is essential for λimmP22 growth in E. coli, degradation of proteins tagged by tmRNA is required only to achieve optimal levels of phage growth. Based on these results, we propose that trans-translation may have two roles, the primary role being the release of stalled ribosomes from their mRNA template and the secondary role being the tagging of truncated proteins for degradation.
The lambdoid family of bacteriophages has been used extensively as tools in the study of bacterial physiology in Escherichia coli (6). Host functions first identified by mutations that affect the growth of lambdoid phages have often later been found to have important roles in physiological processes of the host cell itself. A small, stable RNA, tmRNA (previously known as 10Sa RNA), encoded by the ssrA locus of E. coli, has been shown to be required for the growth of certain hybrid λimmP22 phages (31, 38). E. coli tmRNA is present at approximately 1,000 copies per haploid genome (23) and is processed at both ends to create a 363-nucleotide mature form from a 457-nucleotide precursor (21, 24, 35, 36, 39). Homologs of tmRNA have been located in every bacterial species whose genome has been searched (4, 12, 29, 42, 43, 45). Analysis of the E. coli tmRNA indicates that this RNA has a secondary structure resembling half of a tRNA molecule (5, 21, 45), including the acceptor stem and TΨC stem-loop of alanyl tRNA in E. coli, and in vitro studies show that it can be charged with alanine by using purified components (21). Phenotypes exhibited by E. coli ssrA mutants, in addition to effects on the growth of certain λimmP22 hybrids, include a slowed growth rate (28), delayed recovery from carbon starvation, reduced motility in soft agar (21), reduced expression of some genes (30), and increased expression of Alp protease (19). Additionally, when an ssrA mutation is present in the same cell as a mutation in the prs gene, encoding phosphoribosyl pyrophosphate synthetase, an enzyme involved in nucleotide synthesis, bacteria are unable to grow at 42°C (1).
In the last few years, evidence has accumulated supporting a model in which tmRNA tags partially synthesized proteins for degradation by cellular proteases (18, 41). This “trans-translation” model, first proposed by Keiler et al. (18), postulates that alanine-charged tmRNA enters ribosomes that have stalled at the 3′ end of a mRNA without having reached a stop codon. The tmRNA acts first as a tRNA, and its alanine is added to the nascent polypeptide. However, after peptide bond formation, the original mRNA is released and tmRNA becomes the template for translation, resulting in the addition of an 11-amino-acid tag sequence to the C terminus of the nascent polypeptide, the last 10 amino acids of which are encoded by tmRNA. The tag sequence contains a protease recognition site at its C terminus, so that tagged proteins become substrates for the proteases Clp, FtsH, and Tsp (10, 13, 18). Recently published work supporting this model has shown that tmRNA is associated with 70S ribosomes but not 30S or 50S subunits or polysomes (20, 40), that charging of tmRNA with alanine is required for association with ribosomes (40), and that translation of polyuridine in vitro, in the presence of tmRNA, produces polyphenylalanine, followed by the tmRNA tag (15).
The effects of ssrA mutation on λimmP22 growth were first described by Strauch et al. for a strain of E. coli with an uncharacterized mutation in ssrA called sip (38). The sip mutation was later shown to result from the excision of a cryptic prophage adjacent to ssrA, resulting in alteration of the 3′ end of tmRNA (19, 31). Certain hybrid λimmP22 phages, formed by crosses between coliphage λ and its relative from Salmonella, phage P22 (8, 33, 49), are unable to grow on E. coli carrying this altered form of ssrA (38). These same studies established that mutation of P22 c1 removes the requirement for tmRNA in λimmP22 phage growth (31, 38). P22 C1 protein activates transcription that is responsible for the establishment of repressor synthesis in phage P22 by binding to the −35 region of the PRE promoter (48). PRE is located downstream of the early promoter, PR, and in the opposite orientation. The PR operon includes genes, 18 and 12, encoding the phage DNA replication functions and located downstream of PRE. It was proposed that tmRNA interacts directly with the P22 C1 protein, reducing its binding at PRE (30). According to this model, in the absence of tmRNA, the increased occupancy of P22 C1 at PRE would lead to decreased expression of phage genes and a defect in phage growth. Consistent with this model, an interaction was observed between tmRNA and DNA-binding proteins in vitro in gel shift experiments (30). However, more recent work indicates that the interaction between tmRNA and P22 C1 protein in vitro is less specific than that observed previously and that any interaction between tmRNA and P22 C1 is probably nonspecific (45a).
In light of these findings, we have explored the possibility that the trans-translation model may provide an explanation for the effects of ssrA mutation on λimmP22 growth in E. coli. To facilitate our analysis, we have divided the trans-translation model into four basic steps: first, charging of tmRNA with alanine; second, transfer of the alanine to the nascent peptide and release of the original mRNA; third, translation of the tmRNA tag and dissociation of the translational ternary complex upon reaching the tmRNA-encoded stop codon; and fourth, degradation of tagged proteins by cellular proteases. We present here the results of experiments showing the effects on λimmP22 growth of ssrA mutations affecting steps 4 and 1 of trans-translation. First, we have made or obtained mutations that change the tag sequence itself, rendering tagged proteins resistant to protease degradation, thus affecting step 4 of trans-translation; and second, we have made mutations that prevent tmRNA from being charged with alanine, affecting step 1. We have also examined the effects that mutation of clpP, the catalytic subunit of the Clp protease (26), has on λimmP22 growth. Our results suggest that charging of tmRNA with alanine is critical for λimmP22 growth in E. coli but that degradation of tagged proteins is required only to achieve an optimal level of phage growth.
MATERIALS AND METHODS
Strains.
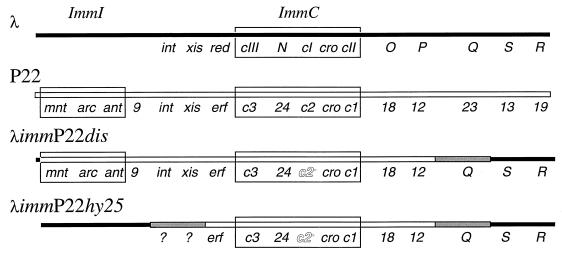
Bacterial strains used in this study are listed in Table 1, and plasmid genotypes are included where relevant. The genetic organization of the relevant regions of the bacteriophages used is shown in Fig. 1.
TABLE 1.
Bacterial strains and bacteriophages
| Strain or phage | Parent strain | Plasmid | Relevant genotype | Source |
|---|---|---|---|---|
| K37 | galK Strr | This laboratory | ||
| K8619 | K37 | ssrA::cat | This work | |
| K8664 | K8619 | ssrA+ Ampr (At λatt) | This work | |
| K8637 | K8619 | ssrAO Ampr (At λatt) | This work | |
| K8666 | K8619 | ssrADD Ampr (At λatt) | This work | |
| K8668 | K8619 | Ampr (At λatt) | This work | |
| K8661 | K8619 | pJW28 | ssrA+ Ampr | This work |
| K8810 | K8619 | pJW31 | ssrAGA Ampr | This work |
| K8812 | K8619 | pJW32 | ssrAGC Ampr | This work |
| K8814 | K8619 | pJW34 | ssrAUG Ampr | This work |
| K8895 | K8619 | pJW33 | ssrACG Ampr | This work |
| K8465 | K37 | clpP::kan | This work | |
| K8857 | K8619 | clpP::kan ssrA::cat | This work | |
| K5210 | N953 | lac galE galKam trpam araam T6r Su− | This laboratory | |
| K9281 | K8637 | ssrAO clpP::kan | This work | |
| K9282 | K8666 | ssrADD clpP::kan | This work | |
| ZH1141 | C600 | gal490 bioA mal λcI857 | D. Court | |
| λBDC531 | λimm21 lacZ′::supF::′bla | D. Court | ||
| λimmP22hy25 | λimmP22c25 spi | S. Hilliker | ||
| λimmP22dis | λimmP22c25 dis | This laboratory |
FIG. 1.
Genetic organization of the relevant regions of phages λ and P22 and hybrid phages. Immunity regions are boxed. The solid line indicates λ genetic information, and the open line indicates P22 genetic information. The gray lines indicate areas of the phage chromosomes the origins of which have not been determined. Both hybrids used in this study have a mutation that results in production of an inactive repressor protein, C2, shown by outlined lettering.
Media.
Bacterial cultures were grown in Luria-Bertani broth, described previously (7).
Cloning procedures.
Standard cloning techniques were used (32). Enzymes were purchased from New England Biolabs, Boehringer Mannheim, and Gibco BRL and were used according to the suppliers’ instructions.
Construction of plasmids carrying ssrA mutants.
ssrA mutant alleles were constructed by the PCR splicing by overlap extension method (16) and cloned into plasmid pRS415 (34). Mutant sequences were verified by DNA sequencing with the Thermosequenase kit (Amersham).
Construction of single-copy ssrA mutants.
Single-copy constructs of ssrA tag mutants were constructed by the method of Yu and Court (50). Briefly, phage λBDC531 (imm21) was grown on a strain carrying an allele of ssrA cloned into plasmid pRS415 (34). The resulting phages were used to make lysogens in strain K5210, and the lysogens were selected for ampicillin resistance. Bacteriophage P1 (37) was grown on these lysogens and used to transduce Ampr into strain ZH1141 made resistant to λ. P1 was then grown on this strain, and Ampr was transduced into K8619. The final constructs are sensitive to phages λ and 21 and are resistant to ampicillin and chloramphenicol (ssrA::cat).
EOP.
The efficiency of plating (EOP) of phages was determined as described previously (2).
Bursts of phages.
Phage bursts were measured as described previously (38). Experiments were performed at 37°C for 90 min. Each value given is the average (± the standard deviation) of at least three separate experiments.
Northern blot analysis.
RNA was purified from late-logarithmically growing cultures of the specified strain with the RNeasy total RNA kit (Qiagen). The purified RNA was electrophoresed on a 2% agarose gel containing 20% formaldehyde in 1× formaldehyde-gel running buffer (20 mM MOPS [morpholinepropanesulfonic acid], pH 7.0, 8 mM sodium acetate, 5 mM EDTA, pH 8.0). The RNA was transferred to a GeneScreen Plus hybridization transfer membrane (NEN) by electrotransfer on a SemiPhor apparatus (Hoefer). End-labeled oligonucleotide probes specific for tmRNA or 16S rRNA were hybridized to the immobilized RNA, and the blots were washed as described by Sambrook et al. (32). The hybridized probe was visualized and quantitated with a PhosphorImager system (Molecular Dynamics).
RESULTS
Description of phages and effects of ssrA::cat mutation on λimmP22 growth.
Two different λimmP22 hybrid phages were used to assess the effects of various mutations in ssrA on λimmP22 growth. The two hybrids described here (Fig. 1) were chosen because of the known effects of ssrA mutation upon their growth (31, 38). The first, λimmP22dis (49), has a relatively large region from Salmonella phage P22 replacing a homologous region from phage λ and carries both of the immunity regions, immC and immI, from P22. immC encodes gene products that determine whether the phage assumes the lysogenic pathway or grows lytically, and it is analogous to the immunity region of phage λ. immI regulates the expression of an antirepressor protein, Ant, and an analogous region is not found in λ (14). λimmP22dis also carries the integration and excision genes, recombination function, and DNA replication genes from P22. The remainder of the phage genome is derived from λ. The second hybrid, λimmP22hy25 (14), has a smaller region of the genome from P22 replacing that of λ. This hybrid also carries the DNA replication, recombination, and immC regions from P22 but does not carry the second immunity region, immI. The derivation of the region of the λimmP22hy25 genome between the recombination function locus, erf, and the λ region replacing immI (Fig. 1) has not been determined. The derivation of the transcription factor required for expression of phage late genes, termed Q in phage λ and 23 in phage P22, has not been determined for either hybrid used in this study.
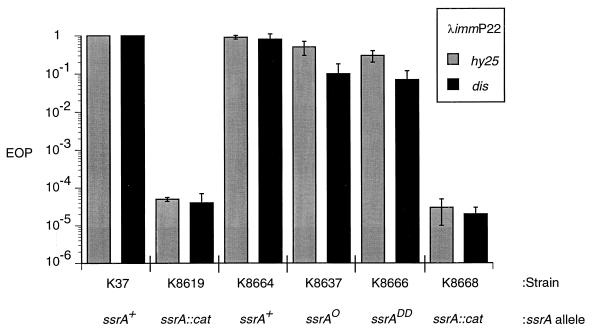
The effects on λimmP22 growth of an ssrA::cat insertional mutation have been previously described by this and another laboratory (19, 31). However, experiments performed with our existing ssrA::cat strain have yielded results that are not entirely consistent, presumably due to acquisition of an additional mutation(s). Therefore, we have constructed a new ssrA::cat strain by crossing an ssrA::cat allele (19) into K37, our standard E. coli strain, creating strain K8619 (Table 1). We tested the ability of the newly constructed ssrA::cat strain to support growth of λimmP22 hybrid phages, (see Fig. 3, 5, and 7). The EOPs of λimmP22hy25 and λimmP22dis in K8619 are decreased by greater than 10,000-fold compared to the EOPs of these hybrid phages seen in the ssrA+ strain. A Northern blot experiment demonstrated, as expected, that the ssrA::cat strain, K8619, failed to produce detectable levels of tmRNA, unlike the ssrA+ strain, K37 (see Table 3, first two lines).
FIG. 3.
EOP of λimmP22 hybrids on E. coli with wild-type or tag mutant ssrA alleles. Strains K8664, K8637, and K8666 have the indicated ssrA allele inserted at the λatt site in addition to the ssrA::cat allele at the normal ssrA locus. K8668 has the cloning vector alone inserted at λatt. The values presented are the averages of three separate experiments, and error bars are included where applicable. The plaques produced by λimmP22dis on K8637 and K8666 were tiny relative to plaques produced by λimmP22dis on other strains.
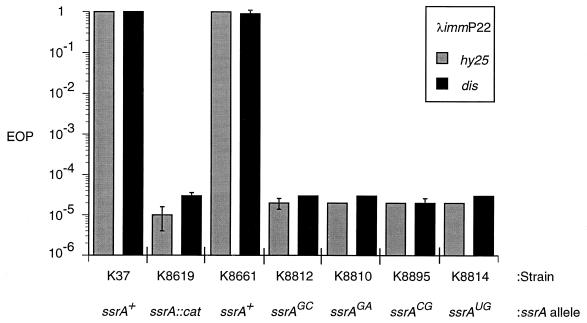
FIG. 5.
EOP of λimmP22 hybrids on E. coli with wild-type or charging mutant ssrA alleles. Strains K8661, K8812, K8810, K8895, and K8814 carry the indicated ssrA allele cloned in plasmid pRS415. The values presented are the averages of three separate experiments, and error bars are included where applicable.
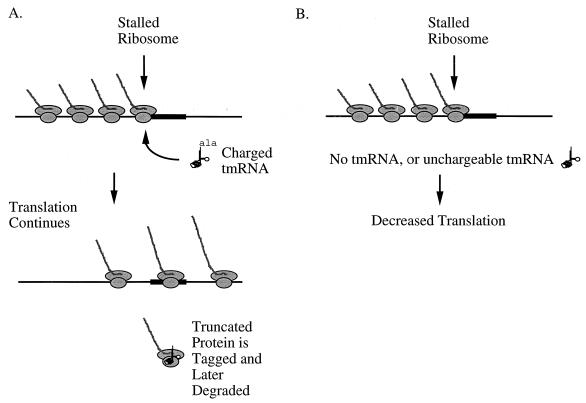
FIG. 7.
Model explaining the effect of tmRNA on translation leading to production of a limiting phage product. mRNA is shown as a black line, ribosomes as gray ovals, and nascent proteins as gray lines. A structure or sequence in the mRNA that causes ribosome stalling is indicated by a heavy black line in the mRNA. (A) Translation in the presence of tmRNA action. An alanine-charged tmRNA molecule enters a ribosome that is stalled at a specific structure or sequence in the phage mRNA. The alanine is attached to the nascent peptide, and the ribosome releases the original mRNA. The ribosomes stacked behind the stalled ribosome are then able to continue translation and will be able to traverse the stall site at a frequency that is sufficient for production of the limiting phage protein at a level above the critical concentration. The released ribosome tags its nascent peptide, but this process is not required for phage growth beyond causing release of the stalled ribosome from the mRNA. (B) Translation in the absence of functional tmRNA. When the tmRNA is incapable of being charged with alanine, it cannot enter the stalled ribosome, so a block in translation occurs. This results in insufficient amounts of the limiting phage protein being produced, and the phages are unable to grow.
TABLE 3.
Relative levels of tmRNA produced in ssrA mutant strains
| Strain | ssrA allele | Location of relevant ssrA allele | Relative tmRNA levela |
|---|---|---|---|
| K37 | ssrA+ | Normal site | 1.0 |
| K8619 | ssrA::cat | Normal site | NDb |
| K8664 | ssrA+ | λatt | 7.5 |
| K8637 | ssrAO | λatt | 1.4 |
| K8666 | ssrADD | λatt | 0.5 |
| K8668 | ssrA::cat | Normal site | ND |
| K8810 | ssrAGA | Plasmid | 12.5 |
| K8812 | ssrAGC | Plasmid | 1.9 |
| K8814 | ssrAUG | Plasmid | 6.4 |
| K8895 | ssrACG | Plasmid | 6.1 |
The ratio of tmRNA to 16S rRNA measured for the wild-type strain, K37, was set equal to 1, and the tmRNA/16S rRNA ratios measured in the other strains were normalized to this value.
ND, not detectable.
Testing the role of the degradation step of trans-translation in λimmP22 growth.
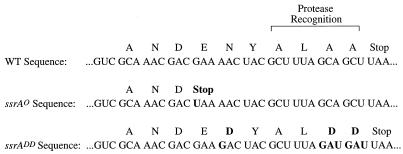
To examine whether the trans-translation model, as proposed, might explain the defect in λimmP22 growth seen in ssrA mutant strains, we first tested whether the final step, degradation of tmRNA-tagged proteins, is required for support of λimmP22 growth. Derivatives of K8619 (ssrA::cat) were constructed that carry a single copy of the wild-type or a mutant allele of ssrA. This second ssrA allele, including its associated wild-type ssrA promoter, was inserted at the λ attachment site. As a control, a derivative of K8619 was constructed in which the cloning vector alone was inserted in single copy at the λatt site. Two ssrA mutants were studied, each having altered sequences in the region of ssrA encoding the tag (Fig. 2). The first, the ssrAO mutant, has a single-base-pair change that creates an ochre stop codon in frame early in the tag coding sequence. Inserting a nonsense mutation in the region of tmRNA encoding the tag was the suggestion of T. Silhavy. A tag produced from this allele would lack the hydrophobic protease recognition sequence found at the C terminus of the tag (Fig. 2). The second mutant, the ssrADD mutant, containing an ssrA allele obtained from R. Sauer, has two aspartic acid residues replacing the two alanines found at the C terminus of the tag in wild-type ssrA as well as an aspartic acid replacing the asparagine earlier in the tag sequence (18). Proteins containing tags from this allele were shown to have an increased half-life in vivo compared to proteins containing a wild-type tmRNA tag, due to disruption of the hydrophobic protease recognition site by the charged aspartic acid residues (18).
FIG. 2.
Sequence of the regions of wild-type and mutant tmRNAs encoding the tag and the presumed amino acid sequence of the resulting tag. Changes from the wild-type sequence are indicated by bold letters. The protease recognition sequence is indicated at the C terminus of the tag.
The EOPs of two λimmP22 phages on these single-copy ssrA constructs were measured, and the results are shown in Fig. 3. As noted above, both λimmP22hy25 and λimmP22dis show a greater-than-10,000-fold reduction in EOP on an ssrA::cat strain compared to the EOP measured on a strain of E. coli with wild-type ssrA. When a wild-type copy of ssrA is present in single copy in addition to the ssrA::cat allele, the EOP returns to wild-type levels. Similarly, when a single copy of either the ssrAO or ssrADD allele is present in an ssrA::cat background, both λimmP22 hybrids show EOPs close to wild-type levels, although the plaques produced by λimmP22dis are much smaller on the ssrAO and ssrADD strains than on ssrA+ E. coli. As expected, both phages failed to grow in a derivative of K8619 with the cloning vector integrated at the λ attachment site, confirming that it was the ssrA alleles that were responsible for growth of the hybrid phages.
Similar experiments were also performed with ssrA alleles carried on multicopy plasmids, and we found that the ssrA+, ssrAO, and ssrADD alleles again supported growth of the hybrid phages while the cloning vector alone did not (data not shown). The small plaque size observed when the EOP of λimmP22dis was measured on the ssrAO and ssrADD strains suggested that the degradation of tagged proteins could play a role in the growth of λimmP22dis. We used burst size as a more quantitative measure of the effectiveness of the ssrAO and ssrADD strains in supporting the growth of the hybrid phages (Table 2, lines 1 to 6). The burst sizes of λimmP22hy25 paralleled the results seen for EOP, i.e., the burst size of λimmP22hy25 on the ssrAO strain was similar to that on the ssrA+ strain while the burst of λimmP22hy25 in the ssrADD strain was slightly lower, ∼40% of that measured on the ssrA+ strain. Significantly, each of these three ssrA alleles supported a burst of λimmP22hy25 that was 400- to 800-fold higher than that produced on the ssrA::cat control strain.
TABLE 2.
Phage burst sizes on ssrA and clpP mutant strains
| No. | Strain | Relevant alleles | Location of relevant ssrA allele | λimmP22hy25 burst | Fold increase over ssrA::cata | λimmP22dis burst | Fold increase over ssrA::cata |
|---|---|---|---|---|---|---|---|
| 1 | K37 | ssrA+ clpP+ | Normal site | 210 ± 36 | 1,750 | 160 ± 10 | 2,500 |
| 2 | K8619 | ssrA::cat clpP+ | Normal site | 0.12 ± 0.08 | 0.064 ± 0.044 | ||
| 3 | K8664 | ssrA+ clpP+ | λatt | 120 ± 31 | 1,000 | 110 ± 43 | 1,720 |
| 4 | K8637 | ssrAO clpP+ | λatt | 95 ± 30 | 790 | 2.2 ± 1.4 | 34 |
| 5 | K8666 | ssrADD clpP+ | λatt | 50 ± 8.5 | 420 | 2.4 ± 1.4 | 38 |
| 6 | K8668 | ssrA::cat clpP+ | Normal site | 0.078 ± 0.061 | 0.65 | 0.021 ± 0.025 | 0.33 |
| 7 | K8814 | ssrAUG clpP+ | Plasmid | 0.098 ± 0.082 | 0.82 | 0.036 ± 0.041 | 0.56 |
| 8 | K8465 | ssrA+ clpP::kan | Normal site | 90 ± 14 | 750 | 78 ± 23 | 1,220 |
| 9 | K8857 | ssrA::cat clpP::kan | Normal site | 52 ± 33 | 430 | 1.2 ± 0.36 | 19 |
| 10 | K9281 | ssrAO clpP::kan | λatt | NDb | ND | 94 ± 28 | 1,470 |
| 11 | K9282 | ssrADD clpP::kan | λatt | ND | ND | 71 ± 29 | 1,110 |
The indicated burst sizes were divided by the burst size measured for the ssrA::cat strain, K8619.
ND, not determined.
However, the results with λimmP22dis in the ssrAO and ssrADD strains were quite different. λimmP22dis produced bursts that were 34- and 38-fold higher, respectively, than those observed on the ssrA::cat control strain but were ∼50-fold lower than the burst produced by λimmP22dis on an isogenic ssrA+ strain. This intermediate phenotype suggests that tmRNA may function in two ways in supporting λimmP22 growth. First, as clearly indicated by the results with λimmP22hy25, one role of tmRNA is unrelated to the activity that directs peptides toward degradation. Second, as shown by the results with λimmP22dis, the activity of tmRNA that directs proteins toward degradation is also required to achieve optimal growth of this hybrid phage.
Testing the role of tmRNA charging in λimmP22 growth.
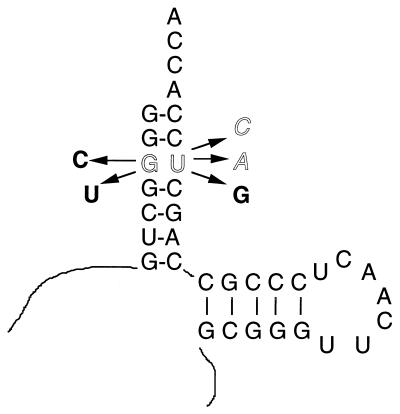
The effects of mutations in the tmRNA acceptor stem that affect its ability to be charged with alanine were next examined to determine whether alanine charging, the first step postulated for trans-translation, is important for λimmP22 growth. Four ssrA alleles with altered nucleotide sequences in the acceptor stem were constructed in plasmid pRS415 (Fig. 4). The G · U base pair at the third position in this stem was targeted for mutagenesis because it is the recognition site for charging by the alanyl aminoacyl-tRNA synthetase, so that tmRNA with a change in either of these nucleotides should not be charged with alanine (17, 27). These “charging mutant” alleles cloned in pRS415 were transformed into the ssrA::cat strain, K8619, and growth of λimmP22 hybrids was measured by EOP (Fig. 5). K8619 carrying an ssrA+ allele in pRS415 supported growth of both hybrid phages tested, as expected. However, derivatives of K8619 carrying ssrA alleles with changes in the G · U base pair to G · A, G · C, C · G, or U · G, respectively, in pRS415 were all unable to support the growth of either hybrid phage tested. It is noteworthy that the final mutation, G · U to U · G, creates an altered sequence, preventing charging of tmRNA with alanine, but does not alter the base-pairing energy and thus should not produce significant differences in structure compared to the wild-type tmRNA, although stacking of bases may be affected by the change. As shown in Fig. 5, this mutant was unable to support λimmP22 growth.
FIG. 4.
Acceptor stem and associated sequence of tmRNA showing changes resulting from ssrA mutation. The resulting tmRNAs should be defective in charging with alanine. The G · U base pair at the third position of the acceptor stem was changed to G · C or G · A, as shown by outlined letters, or C · G or U · G, as shown by bold letters.
To assess more quantitatively the effects of the ssrA alleles defective in alanine charging on phage growth, we measured phage burst size in a strain carrying ssrAUG as the only intact ssrA allele. As shown in line 7 of Table 2, both λimmP22 phages tested on a strain carrying the ssrAUG allele had burst sizes that were essentially identical to that produced in the ssrA::cat control strain.
Northern analysis of ssrA mutants.
To determine whether mutant tmRNAs were being stably expressed, Northern blot analysis was performed, and the results are shown in Table 3. Each of the strains carrying a mutant ssrA allele produced an RNA species of a size equal to that seen in a strain carrying a wild-type ssrA allele when probed with an oligonucleotide specific for ssrA (not shown). A tmRNA signal was not observed in the RNA preparations isolated from strains carrying only the ssrA::cat allele, K8619 and K8668. To control for the amount of RNA loaded into each lane of the gel, the blots were simultaneously probed with an oligonucleotide specific for the 16S rRNA. The ratio of tmRNA to 16S rRNA was calculated by quantitating the signal detected in each lane for the tmRNA and 16S rRNA probes with a PhosphorImager and then dividing the amount of signal detected for tmRNA by the amount detected for 16S rRNA. This number was assigned a value of 1 for the wild-type ssrA strain, K37, while the ratios measured for the other strains were normalized to this value. A representative experiment is shown in Table 3.
The ratio of signals shown in Table 3 suggests that each of the ssrA mutant strains produces stable tmRNA while the two strains lacking a functional ssrA allele do not. While there is some variation among strains, most of the mutant strains produced normalized tmRNA levels at or above the wild-type level of 1. As might be expected, strains K8810, K8812, K8814, and K8895, which carry mutant ssrA alleles on multicopy plasmids, exhibited tmRNA levels severalfold higher than that seen in the wild-type strain, K37, which carries a single copy of ssrA. The two strains carrying single copies of ssrA alleles with altered tag sequences, K8637 and K8666, exhibited tmRNA levels that were quite similar to that seen for the wild-type ssrA strain, K37, although the ssrADD strain, K8666, often produced a tmRNA level slightly lower than that seen in K37. The control strain K8664, carrying what we presume by its construction to be a single copy of wild-type ssrA at λatt, consistently exhibited a tmRNA level higher than that seen in K37, the strain carrying wild-type ssrA at its normal location. However, as seen in the EOP and burst experiments performed with these two strains, there was little or no difference in phage growth due to the difference in tmRNA levels between these two control strains.
These results provide compelling evidence that both the derivatives of K8619 carrying copies of ssrA with mutations changing the G · U base pair in the acceptor stem and the derivatives of K8619 carrying single copies of ssrA with altered tag-coding sequences produce stable tmRNA at levels sufficient for a functional tmRNA to support phage growth.
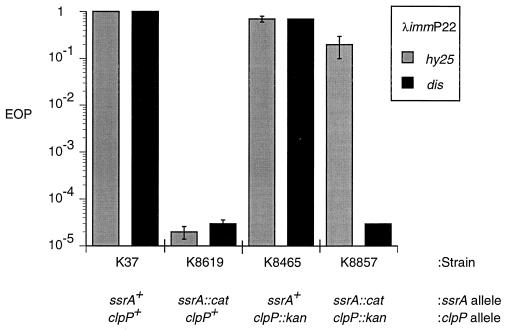
Effect of a clpP mutation on growth of λimmP22.
If degradation of tagged proteins is important for growth of the hybrid phages, then we would expect that the λimmP22 hybrids would also be inhibited if the responsible protease is not active. The Clp protease (11, 44) has been shown to be the major protease responsible for degradation of tagged proteins in vivo; tagged proteins exhibit an increased half-life in a clpP mutant strain of E. coli (10, 13). We introduced a clpP::kan allele, the gift of S. Gottesman, into our strain background and assayed its effects upon the growth of λimmP22 hybrids. As shown in Fig. 6, neither of the λimmP22 hybrids tested showed a significant difference in EOP on lawns formed with the clpP::kan or clpP+ strains.
FIG. 6.
EOP of λimmP22 hybrids on E. coli ssrA and clpP mutant strains. The values presented are the averages of three separate experiments, and error bars are included where applicable. The plaques produced by λimmP22hy25 on K8857 were tiny relative to plaques produced by λimmP22hy25 on other strains.
To obtain a more quantitative assessment, we measured the burst sizes of the λimmP22 phages in the clpP::kan strain. Although EOP measurements failed to detect any significant difference between the wild-type and clpP::kan strains in support of the growth of these phages, measurement of burst sizes revealed a small but reproducible difference in support of the growth of both λimmP22hy25 and λimmP22dis; the burst in the clpP::kan strain is reproducibly twofold lower than that found in the isogenic strain that has a wild-type clpP locus. These twofold differences are relatively insignificant, however, when compared to the 750-fold (λimmP22hy25) or 1,220-fold (λimmP22dis) differences exhibited when bursts produced in clpP::kan and ssrA::cat strains are compared.
The clpP::kan allele was also introduced into K8619, our ssrA::cat strain, to determine whether the absence of the Clp protease could have an effect on phage growth in an ssrA::cat background. Given the results described above, which suggest that the charged form of tmRNA is required for λimmP22 growth, we hypothesized that some aspect of translation could play a role in the action of tmRNA in supporting hybrid-phage growth. One plausible activity could be an influence on the levels of proteins important for the growth of λimmP22. For example, the level of a limiting protein that is protease sensitive could be influenced by tmRNA. If this protein is degraded by the Clp protease, then we would expect that higher levels of this protein would be present in a clpP::kan bacterium and, thus, the effects of the ssrA::cat mutation on the growth of the hybrid phages could be suppressed in a strain that has both ssrA::cat and clpP::kan mutations. When the growth of λimmP22 was assessed in the clpP::kan-ssrA::cat double-mutant strain, K8857, we observed different results with the two λimmP22 hybrids (Fig. 6). Consistent with the outlined hypothesis, λimmP22hy25 has an EOP of close to 1 on a clpP::kan-ssrA::cat double mutant, although the plaques produced on this strain were tiny compared to plaques produced by λimmP22hy25 on an ssrA+ strain. In contrast, λimmP22dis does not shown any difference in EOP when grown on the ssrA::cat strain or the ssrA::cat-clpP::kan strain, suggesting that λimmP22dis may have additional elements involved in the control of its growth that are not affected by Clp protease.
Measurements of phage burst sizes provided significant additional information about the effect of the clpP::kan mutation on hybrid-phage growth. As in the EOP experiments, clpP::kan suppresses the effect of ssrA::cat on the growth of λimmP22hy25 (Table 2, line 9). This hybrid phage produces a burst in the clpP::kan-ssrA::cat strain, K8857, that is 430-fold higher than the burst it produces in the ssrA::cat strain, K8619. However, unlike the results observed in the EOP experiments, the burst experiments revealed that clpP::kan also suppresses the effect of ssrA::cat on λimmP22dis growth, although the suppression is considerably less effective than that observed for λimmP22hy25. λimmP22dis has a burst size in K8857 (clpP::kan ssrA::cat) that is 19-fold higher than the burst size it has in K8619 (ssrA::cat), while this burst size in K8857 is still 130-fold lower than the burst size produced by λimmP22dis in ssrA+ E. coli. These data provide further evidence that λimmP22dis may require more than one activity of tmRNA for optimal growth while λimmP22hy25 requires a single activity of tmRNA, and this activity is unrelated to the degradation of tagged proteins.
Effect of clpP mutation in combination with ssrAO and ssrADD on growth of λimmP22dis.
To examine in another way whether trans-translation has two functions in the bacterium, we constructed strains carrying the clpP::kan allele in the ssrAO and ssrADD strain backgrounds. Our results measuring growth of λimmP22dis on the ssrA::cat-clpP::kan strain suggest that the absence of Clp protease from the bacterium partially suppresses the effect of loss of tmRNA function on λimmP22dis growth. Our results measuring both λimmP22hy25 and λimmP22dis growth on ssrA mutant strains that produce nondegradable tmRNA tags suggest that trans-translation may have dual functions, one being unrelated to degradation of tagged proteins, and that λimmP22dis requires both of these functions for optimal growth. We have suggested that a possible explanation for the observed differences in clpP::kan suppression between λimmP22hy25 and λimmP22dis is that the absence of only one function of tmRNA must be suppressed for λimmP22hy25 growth whereas the absence of both functions of tmRNA must be suppressed for optimal λimmP22dis growth. Based on these considerations, we predicted that when λimmP22dis is grown in a strain supplying one function of tmRNA, absence of Clp protease need only suppress the other function of tmRNA. λimmP22dis should thus grow optimally in such a strain.
This hypothesis was tested by introducing the clpP::kan allele into the ssrAO and ssrADD strain backgrounds and measuring the growth of λimmP22dis on these strains. As shown in Table 2, lines 10 and 11, λimmP22dis burst sizes on ssrAO-clpP::kan and ssrADD-clpP::kan strains, K9281 and K9282, are significantly higher than the burst sizes on the ssrAO and ssrADD strains and only slightly lower than the burst produced by λimmP22dis on ssrA+ E. coli. Significantly, the levels of λimmP22dis growth measured here are equal to the levels of λimmP22dis growth on the clpP::kan strain, K8465, suggesting that the absence of Clp protease in the ssrAO and ssrADD backgrounds allows nearly optimal λimmP22dis growth.
DISCUSSION
The experiments described in this report address the question of whether the trans-translation model for tmRNA function can explain the requirement for tmRNA in the growth of certain λimmP22 hybrid phages. Our results indicate that the action of trans-translation, as originally proposed, is unlikely to explain how this small RNA acts in supporting λimmP22 hybrid-phage growth. By postulating that tmRNA acts to remove partially synthesized proteins from the bacterial cell by tagging them for degradation, the model by necessity has four distinguishable components: charging of tmRNA with alanine, release of the stalled translation complex with the nascent peptide from the mRNA template, addition of the tmRNA-encoded tag to the truncated nascent peptide, and degradation of the tagged peptide by cellular proteases.
We begin by discussing the results of our experiments testing whether the first step in the trans-translation model, charging of tmRNA with alanine, is required to support the growth of the hybrid phages. The ssrA sequence was changed in four ways to alter the nucleotides in the putative acceptor stem of tmRNA that are recognized by the alanyl aminoacyl-tRNA synthetase and are thus required for charging with alanine. One of these mutants, the ssrAUG mutant, has conservative changes from the wild type that result in a change from a G · U to a U · G base pair in tmRNA and thus is unlikely to differ significantly in structure from the wild-type molecule. E. coli expressing any of these altered tmRNAs as the sole form of tmRNA in the bacterium was unable to support λimmP22 growth. The Northern transfer results presented in Table 3 showed that the mutant tmRNAs are found at levels at least as high as the levels of wild-type tmRNA and are therefore sufficient to allow optimal phage growth. These results suggest that it is not the structure of the tmRNA alone that is important for the support of λimmP22 growth; the charging of tmRNA with alanine is critical.
Next we discuss the results of our experiments testing whether the trans-translation model in its entirety can explain why some λimmP22 phages fail to grow in the absence of tmRNA, namely, if tagging and the resulting proteolysis are required for tmRNA to support the growth of these phages. The sequence of tmRNA encoding the peptide tag was changed in two ways to make it ineffective for directing proteolysis. We did this either through the introduction of a nonsense codon, which should terminate tag synthesis upstream of the protease recognition sequence, or by altering the tag sequence in a way previously shown to render it unrecognizable to proteases. Measurement of phage growth by both EOP and burst showed that derivatives of K8619, the ssrA::cat strain, having an additional copy of either of the mutant ssrA alleles, ssrAO or ssrADD, supported growth of λimmP22hy25 similarly to an isogenic strain with wild-type ssrA. The Northern transfer results shown in Table 3 confirm that both of these mutant strains produce stable tmRNAs at or near the level of tmRNA produced in a strain with wild-type ssrA. Since these mutant strains express tmRNAs that are unable to add tags appropriate for signaling proteolysis, it is unlikely that tagging peptides for proteolysis plays a significant role in the action of tmRNA in supporting the growth of this phage. However, similar measurements of phage production showed that these derivatives of K8619 provided an intermediate level of support of the growth of the second hybrid phage, λimmP22dis. Thus, the action of tmRNA in tagging peptides for proteolysis is likely to be important for optimal growth of λimmP22dis. Assuming that the mutant tmRNAs fail to add tags that lead to physiologically significant proteolysis, the fact that there is still partial support of the growth of λimmP22dis means that tmRNA must also contribute to the growth of λimmP22dis independently of its role in fostering proteolysis.
One possible unifying explanation for the action of the mutant tmRNA in support of the growth of the P22 hybrid phages is that the mutant tag sequences, at some low level, signal proteolysis. Accordingly, this level of proteolysis would be sufficient to support efficient growth of λimmP22hy25 but only inefficient growth of λimmP22dis. Although formally possible, we think that this scenario is unlikely. Experiments from the Sauer laboratory (18) show that peptides tagged with ssrADD variants are not targets for proteolysis. Moreover, it is difficult to see how the shortened tag presumably added by the ssrAO mutant tmRNA could be an appropriate signal, especially since it also ends with an aspartate residue. Finally, it is unlikely that a very low level of proteolysis of proteins carrying the mutant tag sequences could act to foster λimmP22 phage growth.
Experiments with a clpP mutant provide further support for the conclusion that tagging peptides for proteolysis cannot explain the full role of tmRNA in supporting the growth of the P22 hybrid phages. If degradation of tagged proteins is critical for λimmP22 growth, then removing the protease primarily responsible for the degradation should result in a failure in λimmP22 growth similar to that seen in an ssrA::cat bacterium. We found that growth of the P22 hybrid phages was only slightly decreased in a strain in which the clpP gene is disrupted by a kanamycin resistance cassette (clpP::kan). Although experiments by Gottesman et al. (10) and Herman et al. (13) provide evidence that Clp is likely to be the protease primarily responsible for degradation of tmRNA-tagged peptides under the conditions of our experiments, it is possible that other proteases can digest tagged proteins in the absence of Clp protease, albeit with a greatly lowered efficiency (10, 13).
If tmRNA has another role in supporting the growth of hybrid phages, what, then, could this role be? Our results with the ssrA::cat-clpP::kan double-mutant strain provide some insight into this question. The finding that tmRNA is not required for growth of the hybrid phages in cells that are deficient in Clp protease activity suggests that a protein(s) sensitive to the Clp protease is central to the tmRNA requirement. Accordingly, in the ssrA::cat strain, the lack of tmRNA may result in a reduced concentration of this protein, with the level further decreased by Clp-mediated proteolysis, resulting in a concentration of the protein that is insufficient for phage growth. When Clp activity is removed from an ssrA::cat cell by introduction of the clpP::kan allele, the level of this Clp-sensitive protein increases to a level that supports growth of the hybrid phages. A precedent for such a protein affecting phage growth is the λ-encoded O protein, a substrate for the Clp protease (9, 46). This protein is thought to be limiting for λ growth (22, 25, 47).
Suppression of the effect of ssrA::cat by the clpP::kan mutation was observed for both hybrid phages tested; however, this suppression was less effective for growth of λimmP22dis than for growth of λimmP22hy25. This observation suggests that λimmP22dis requires an additional function of trans-translation for optimal growth and that the clpP::kan allele cannot compensate for the loss of both functions of trans-translation for λimmP22dis growth. The observation that production of λimmP22dis in E. coli hosts carrying the ssrAO and ssrADD alleles is significantly greater than production of this phage in an ssrA::cat strain also suggests that degradation of tagged proteins cannot be solely responsible for the failure of λimmP22dis to grow in an ssrA::cat strain. Experiments measuring λimmP22dis bursts in strains carrying both the clpP::kan allele and either the ssrAO or ssrADD allele show that λimmP22dis growth in these strains is similar to λimmP22dis growth in a clpP::kan-only strain, suggesting that the absence of Clp protease allows nearly optimal λimmP22dis growth if one function of tmRNA is provided.
Experiments measuring phage growth in strains lacking Clp protease also suggest two separate functions for Clp in phage growth. The first function is the degradation of tmRNA-tagged proteins, as has been described previously (10, 13, 18). This is shown in our experiments by the approximately twofold decrease in bursts of both hybrid phages in a strain lacking Clp protease. The second function of Clp is one that is detrimental to λimmP22 growth in the absence of tmRNA. This is shown by the suppressive effect that clpP mutation has on the growth of both hybrids. A likely candidate for this second function, as argued above, is the degradation by Clp of a protein that is limiting for phage growth.
Our previous observation that P22 c1 mutations permit both λimmP22 hybrids to grow in an ssrA::cat strain is consistent with the idea of a limiting phage protein being responsible for the effect of tmRNA on phage growth. We have previously proposed that the binding of C1 protein in the PR operon reduces expression of downstream functions, including those involved in replication (30, 38). We now suggest that one or more of these functions is a protein that is essential for phage growth, produced in limiting amounts, and sensitive to a protease, most likely Clp. In the presence of C1, while a low level of the limiting protein is expressed, the concentration is sufficient to support phage growth, even with active proteolysis. However, in the absence of tmRNA, lower levels of the protein would be available, and thus it would be reduced to functionally insignificant levels by proteolysis. In the absence of C1, significantly higher levels of the limiting protein would be available and proteolysis would not reduce the protein concentration below the critical level. It should be kept in mind that this proteolysis is unrelated to the proteolysis resulting from tagging by tmRNA.
Model for tmRNA effect on λimmP22 growth.
The question remains, why would alanine-charged tmRNA be necessary to maintain a critical concentration of a phage product, apart from the role of tmRNA in tagging peptides for proteolysis? Given that tmRNA is known to associate with ribosomes and that alanine charging of tmRNA is required for λimmP22 growth and for association of tmRNA with the ribosome, it is conceivable that the lack of functional tmRNA would result in decreased translation of mRNA encoding the limiting protein(s). A model that may explain this effect is shown in Fig. 7. The mRNA encoding the limiting P22 phage product may have a sequence or structure that causes a ribosome to become stalled at some position upstream of the stop codon. The ribosomes following in the polysome would then be blocked from proceeding with translation and stacking of the ribosomes would result, causing a reduction in translation of the limiting protein. In the presence of alanine-charged tmRNA, as shown in Fig. 7A, the stalled ribosome would be removed from the mRNA by trans-translation and the following ribosomes would be freed to continue translation. The tagged protein resulting from trans-translation may later be digested by proteases, but this degradation step would not significantly affect the expression of the limiting P22 product. In the absence of functional tmRNA, as shown in Fig. 7B, the stalled ribosome would not be removed from the mRNA, translation would be reduced, and levels of the limiting phage product would not rise above the critical concentration. The critical step in trans-translation, based on this model, is thus step 2, release of the stalled translational complex from the original mRNA. This model for a mechanism allowing ribosomes to proceed through a translational arrest is not unlike that proposed for RNA polymerase progressing through an arrest site during transcriptional elongation (3).
While evidence exists in support of pieces of this model, many of the details are speculative at this stage. Most notably, it has not been shown whether tmRNA is capable of acting anywhere other than at the 3′ end of a mRNA. It is possible to determine experimentally whether tmRNA has this capability, and such work is currently in progress. Identification of the putative limiting P22 product will also increase our understanding of this system and allow other aspects of this model to be tested. It must be noted that our results suggesting that charged tmRNA is required for phage growth do not rule out the possibility that this charged tmRNA is involved in some process other than trans-translation that is required for λimmP22 growth.
The model for tmRNA function proposed here, while based on the effects of tmRNA on λimmP22 growth, may also apply universally to tmRNA function in bacteria. Work performed in this laboratory on the tmRNA of Neisseria gonorrhoeae has shown that it may be essential for survival of the bacterial cell (17a). However, the ssrAO allele allows survival when it is present as the only copy of ssrA in N. gonorrhoeae, so degradation of tagged proteins does not appear to be an important element in this system either. The introduction of an ssrA mutation into a nusA1 strain of E. coli yields yet another example of the importance of charging tmRNA with alanine and the relative unimportance of the degradation of tagged proteins. A nusA1 strain, which affects transcription antitermination of phage λ, supports growth of λ at 32°C, whereas nusA1-ssrA double-mutant strains do not support the growth of λ at 32°C (37a). However, the ssrA tag mutant alleles, ssrAO and ssrADD, are able to support the growth of λ at 32°C in the presence of a nusA1 mutation while a charging mutant allele, ssrAUG, is unable to support λ growth at 32°C in a nusA1 cell (29a). These findings further support the major thesis advanced here, namely, that degradation of truncated proteins may not be the only role for tmRNA but instead one of two roles, the second being release of stalled ribosomes from their mRNA template, and that this second function may be the more critical of the two.
ACKNOWLEDGMENTS
We thank Robert Sauer for the ssrADD mutant; Susan Gottesman for the clpP::kan allele, for sharing unpublished results, and for helpful discussion; and Tom Silhavy for suggesting that we make a nonsense mutation in ssrA. We thank Don Court for sharing his method for constructing single-copy chromosomal insertions.
This work was supported by Public Health grant AI11459-10 (to D.F.). J.W. acknowledges support from NIH training grant 2 T32 GM07315.
REFERENCES
- 1.Ando H, Kitabatake M, Inokuchi H. 10Sa RNA complements the temperature-sensitive phenotype caused by a mutation in the phosphoribosyl pyrophosphate synthetase (prs) gene in Escherichia coli. Genes Genet Syst. 1996;71:47–50. doi: 10.1266/ggs.71.47. [DOI] [PubMed] [Google Scholar]
- 2.Bear S E, Court D L, Friedman D I. An accessory role for Escherichia coli integration host factor: characterization of a lambda mutant dependent upon integration host factor for DNA packaging. J Virol. 1984;52:966–972. doi: 10.1128/jvi.52.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borukhov S, Sagitov V, Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993;72:459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- 4.Brown J W, Hunt D A, Pace N R. Nucleotide sequence of the 10Sa RNA gene of the beta-purple eubacterium Alcaligenes eutrophus. Nucleic Acids Res. 1990;18:2820. doi: 10.1093/nar/18.9.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felden B, Himeno H, Muto A, McCutcheon J P, Atkins J F, Gesteland R F. Probing the structure of the Escherichia coli 10Sa RNA (tmRNA) RNA. 1997;3:89–103. [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman D I. Interaction between bacteriophage lambda and its Escherichia coli host. Curr Opin Genet Dev. 1992;2:727–738. doi: 10.1016/s0959-437x(05)80133-9. [DOI] [PubMed] [Google Scholar]
- 7.Friedman D I, Olson E R, Johnson L L, Alessi D, Craven M G. Transcription-dependent competition for a host factor: the function and optimal sequence of the phage lambda boxA transcription antitermination signal. Genes Dev. 1990;4:2210–2222. doi: 10.1101/gad.4.12a.2210. [DOI] [PubMed] [Google Scholar]
- 8.Gemski P, Jr, Baron L S, Yamamoto N. Formation of hybrids between coliphage lambda and Salmonella phage P22 with a Salmonella typhimurium hybrid sensitive to these phages. Proc Natl Acad Sci USA. 1972;69:3110–3114. doi: 10.1073/pnas.69.11.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman S, Clark W P, de Crecy-Legard V, Maurizi M R. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. Sequence and in vivo activities. J Biol Chem. 1993;268:22618–22626. [PubMed] [Google Scholar]
- 10.Gottesman S, Roche E, Zhou Y, Sauer R T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman S, Wickner S, Maurizi M R. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 12.Haring V, Billington S J, Wright C L, Huggins A S, Katz M E, Rood J I. Delineation of the virulence-related locus (vrl) of Dichelobacter nodosus. Microbiology. 1995;141:2081–2089. doi: 10.1099/13500872-141-9-2081. [DOI] [PubMed] [Google Scholar]
- 13.Herman C, Thevenet D, Bouloc P, Walker G C, D’Ari R. Degradation of C-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH) Genes Dev. 1998;12:1348–1355. doi: 10.1101/gad.12.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilliker S, Botstein D. Specificity of genetic elements controlling regulation of early functions in temperate bacteriophages. J Mol Biol. 1976;106:537–566. doi: 10.1016/0022-2836(76)90251-5. [DOI] [PubMed] [Google Scholar]
- 15.Himeno H, Sato M, Tadaki T, Fukushima M, Ushida C, Muto A. In vitro trans translation mediated by alanine-charged 10Sa RNA. J Mol Biol. 1997;268:803–808. doi: 10.1006/jmbi.1997.1011. [DOI] [PubMed] [Google Scholar]
- 16.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 17.Hou Y-M, Schimmel P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature. 1988;333:140–145. doi: 10.1038/333140a0. [DOI] [PubMed] [Google Scholar]
- 17a.Huang, C., et al. Unpublished data.
- 18.Keiler K C, Waller P R, Sauer R T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 19.Kirby J E, Trempy J E, Gottesman S. Excision of a P4-like cryptic prophage leads to Alp protease expression in Escherichia coli. J Bacteriol. 1994;176:2068–2081. doi: 10.1128/jb.176.7.2068-2081.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komine Y, Kitabatake M, Inokuchi H. 10Sa RNA is associated with 70S ribosome particles in Escherichia coli. J Biochem (Tokyo) 1996;119:463–467. doi: 10.1093/oxfordjournals.jbchem.a021264. [DOI] [PubMed] [Google Scholar]
- 21.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S B, Bailey J E. A mathematical model for lambda dv plasmid replication: analysis of wild-type plasmid. Plasmid. 1984;11:151–165. doi: 10.1016/0147-619x(84)90020-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee S Y, Baily S C, Apirion D. Small stable RNAs from Escherichia coli: evidence for the existence of new molecules and for a new ribonucleoprotein particle containing 6S RNA. J Bacteriol. 1978;133:1015–1023. doi: 10.1128/jb.133.2.1015-1023.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarov E M, Apirion D. 10Sa RNA: processing by and inhibition of RNase III. Biochem Int. 1992;26:1115–1124. [PubMed] [Google Scholar]
- 25.Matsubara K. Replication control system in lambda dv. Plasmid. 1981;5:32–52. doi: 10.1016/0147-619x(81)90076-7. [DOI] [PubMed] [Google Scholar]
- 26.Maurizi M R, Clark W P, Katayama Y, Rudikoff S, Pumphrey J. Sequence and structure of ClpP, the proteolytic subunit of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990;265:12536–12545. [PubMed] [Google Scholar]
- 27.McClain W H, Foss K. Changing the identity of a tRNA by introducing a G-U wobble pair near the 3′ acceptor end. Science. 1988;240:793–796. doi: 10.1126/science.2452483. [DOI] [PubMed] [Google Scholar]
- 28.Oh B K, Apirion D. 10Sa RNA, a small stable RNA of Escherichia coli, is functional. Mol Gen Genet. 1991;229:52–56. doi: 10.1007/BF00264212. [DOI] [PubMed] [Google Scholar]
- 29.Oh B K, Chauhan A K, Isono K, Apirion D. Location of a gene (ssrA) for a small, stable RNA (10Sa RNA) in the Escherichia coli chromosome. J Bacteriol. 1990;172:4708–4709. doi: 10.1128/jb.172.8.4708-4709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Resnick, K., J. Withey, and D. Friedman. Unpublished data.
- 30.Retallack D M, Friedman D I. A role for a small stable RNA in modulating the activity of DNA-binding proteins. Cell. 1995;83:227–235. doi: 10.1016/0092-8674(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 31.Retallack D M, Johnson L L, Friedman D I. Role for 10Sa RNA in the growth of lambda-P22 hybrid phage. J Bacteriol. 1994;176:2082–2089. doi: 10.1128/jb.176.7.2082-2089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Semerjian A V, Malloy D C, Poteete A R. Genetic structure of the bacteriophage P22 PL operon. J Mol Biol. 1989;207:1–13. doi: 10.1016/0022-2836(89)90437-3. [DOI] [PubMed] [Google Scholar]
- 34.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava R A, Srivastava N, Apirion D. Characterization of the RNA processing enzyme RNase III from wild type and overexpressing Escherichia coli cells in processing natural RNA substrates. Int J Biochem. 1992;24:737–749. doi: 10.1016/0020-711x(92)90007-n. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava R K, Miczak A, Apirion D. Maturation of precursor 10Sa RNA in Escherichia coli is a two-step process: the first reaction is catalyzed by RNase III in presence of Mn2+ Biochimie. 1990;72:791–802. doi: 10.1016/0300-9084(90)90188-m. [DOI] [PubMed] [Google Scholar]
- 37.Sternberg N L, Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol. 1991;204:18–43. doi: 10.1016/0076-6879(91)04004-8. [DOI] [PubMed] [Google Scholar]
- 37a.Strauch M. Ph.D. thesis. Ann Arbor: University of Michigan; 1983. [Google Scholar]
- 38.Strauch M A, Baumann M, Friedman D I, Baron L S. Identification and characterization of mutations in Escherichia coli that selectively influence the growth of hybrid lambda bacteriophages carrying the immunity region of bacteriophage P22. J Bacteriol. 1986;167:191–200. doi: 10.1128/jb.167.1.191-200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subbarao M N, Apirion D. A precursor for a small stable RNA (10Sa RNA) of Escherichia coli. Mol Gen Genet. 1989;217:499–504. doi: 10.1007/BF02464923. [DOI] [PubMed] [Google Scholar]
- 40.Tadaki T, Fukushima M, Ushida C, Himeno H, Muto A. Interaction of 10Sa RNA with ribosomes in Escherichia coli. FEBS Lett. 1996;399:223–226. doi: 10.1016/s0014-5793(96)01330-0. [DOI] [PubMed] [Google Scholar]
- 41.Tu G F, Reid G E, Zhang J G, Moritz R L, Simpson R J. C-terminal extension of truncated recombinant proteins in Escherichia coli with a 10Sa RNA decapeptide. J Biol Chem. 1995;270:9322–9326. doi: 10.1074/jbc.270.16.9322. [DOI] [PubMed] [Google Scholar]
- 42.Ushida C, Himeno H, Watanabe T, Muto A. tRNA-like structures in 10Sa RNAs of Mycoplasma capricolum and Bacillus subtilis. Nucleic Acids Res. 1994;22:3392–3396. doi: 10.1093/nar/22.16.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ushida C, Muto A. Small stable RNAs in Mycoplasma capricolum. Nucleic Acids Symp Ser. 1993;29:157–158. [PubMed] [Google Scholar]
- 44.Wawrzynow A, Banecki B, Zylicz M. The Clp ATPases define a novel class of molecular chaperones. Mol Microbiol. 1996;21:895–899. doi: 10.1046/j.1365-2958.1996.421404.x. [DOI] [PubMed] [Google Scholar]
- 45.Williams K P, Bartel D P. Phylogenetic analysis of tmRNA secondary structure. RNA. 1996;2:1306–1310. [PMC free article] [PubMed] [Google Scholar]
- 45a.Withey, J., and D. Friedman. Unpublished data.
- 46.Wojtkowiak D, Georgopoulos C, Aylicz M. Isolation and characterization of ClpX, a new ATP-dependent specificity component of the Clp protease of Escherichia coli. J Biol Chem. 1993;268:22609–22617. [PubMed] [Google Scholar]
- 47.Womble D D, Rownd R H. Regulation of lambda dv plasmid DNA replication. A quantitative model for control of plasmid lambda dv replication in the bacterial cell division cycle. J Mol Biol. 1986;191:367–382. doi: 10.1016/0022-2836(86)90133-6. [DOI] [PubMed] [Google Scholar]
- 48.Wulff D L, Rosenberg M. Establishment of repressor synthesis. In: Hendrix R W, Roberts J W, Stahl F W, Weisberg R A, editors. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1983. pp. 53–73. [Google Scholar]
- 49.Yamamoto N, Wohlheiter J A, Gemski P, Baron L S. Lambda immunity-P22 dis: a hybrid of coliphage lambda with both immunity regions of Salmonella phage P22. Mol Gen Genet. 1978;166:233–243. [PubMed] [Google Scholar]
- 50.Yu D, Court D. A new system to place single copies of genes, sites, and lacZ fusions on the Escherichia coli chromosome. Gene. 1998;223:77–81. doi: 10.1016/s0378-1119(98)00163-2. [DOI] [PubMed] [Google Scholar]