Abstract
Background:
Preterm low birth weight (PLBW) is one of the causes of infant mortality and morbidity. Most of the studies have indicated the periodontal-systemic interlink. The association of periodontal pathogen on pregnancy outcome still stands inconclusive. The aim of the study was to detect the prevalence of Porphyromonas gingivalis in umbilical cord blood of new-born infant and correlate the same pathogen in subgingival plaque of pregnant women and to determine the adverse pregnancy outcomes and also to determine the correlation between periodontitis and the association of P. gingivalis in preterm birth/low birth.
Materials and Methods:
The present study included 130 pregnant participants in their full-term and preterm delivery with periodontitis. Periodontal disease was diagnosed clinically using gingival index, pocket depth, plaque index, and clinical attachment level. Umbilical cord blood samples and plaque samples collected using Gracey curette were subjected to culture for the detection of P. gingivalis. The results were subjected to the statistical analysis using the SPSS software.
Results:
The comparison of P. gingivalis in umbilical cord blood and plaque between preterm and full-term group was found to be significantly increased in preterm group. A statistically significant difference was also seen for the clinical parameters between the two groups, with increased values seen in case of preterm labor. Odd's ratio on the comparison of prevalence of periodontitis among full term and preterm group was statistically significant in preterm birth as compared with full-term birth.
Conclusion:
The study results showed statistically significant association of P. gingivalis with PLBW. Periodontal disease significantly affects the adverse pregnancy outcome.
Keywords: Culture method, full term birth, Porphyromonas gingivalis, pregnant women and periodontal diseases, preterm birth, preterm low birth weight
INTRODUCTION
Maternal periodontal disease is regarded as the origin from which oral microorganisms reach the uterine cavity through the circulation. The inflammatory burden that is present in pregnant women is not reflected appropriately by the clinical indicators of periodontitis. A subgingival biofilm with predominantly Gram-negative, lipopolysaccharide producing species plays a causative role in the pathophysiology of preterm low-birth weight (PLBW). The aim of this study was to detect the prevalence of Porphyromonas gingivalis in umbilical cord blood of new born infant and in subgingival plaque of pregnant participants with periodontitis and its relationship with PLBW/preterm birth and to find if any correlation exists.
MATERIALS AND METHODS
Patients who visited the department of obstetrics and gynecology in a rural hospital in Channarayapatna, Hassan, were selected with the following inclusion criteria: (1) healthy pregnant women aged 20–35 years, (2) 9–21 weeks of single gestation, (3) presence of probing pocket depth >3 mm, and (4) ≥20 completely erupted teeth, excluding third molars.
The exclusion criteria were as follows: (1) history of congenital heart disease, diabetes, hyperthyroidism, asthma, or glomerulonephritis, (2) under antibiotic therapy, (3) current use of corticosteroids, (4) pregnant women with Rh factor isoimmunity and twin pregnancy, and (5) tobacco or alcohol consumption.
The study was conducted from August 2017 to May 2018. The study design was approved by the Institutional Ethical Committee, and informed consent was taken from the study participants.
Considering the effect size at 50%, margin of error at 5% and power of study at 80%, total sample size needed was 128, which was rounded off to 130. Hence, the sample size comprised of 65 samples per group (full-term and preterm deliveries).
The bacteriological culture was performed at the Department of Microbiology, Maratha Mandal Nathajirao G. Halgekar College of Dental Sciences and Research Center, Belgaum. For the detection of microorganisms, plaque sample and umbilical blood samples were obtained from each subject in the first or second trimester of pregnancy. Microbial sampling on periodontitis patients was performed with pocket depths of ≥ 5 mm. The site with the deepest pocket depth was chosen for sampling. After the removal of supragingival plaque, the area was isolated with cotton pellets and Gracey curettes were placed into the periodontal pocket to obtain plaque samples [Figure 1]. The plaque samples were transferred into an aliquot with reduced transport fluid medium [Figure 2]. All samples were coded and sent to laboratory for processing within 72 h after sample collection. In order to detect the presence of periodontal pathogens, microbial culture procedures were used to further analyze the samples that were incubated in CO2 for anaerobic culture system and processed at room temperature (25°C). Sheep's blood agar medium had been incubated at 37°C in 10% CO2 for 4 days, and then, colonies were observed on culture plates which were later appreciated as black pigmented colonies after gram staining [Figures 3-6]. The microorganisms found on blood agar media were identified and the percentage of total viable count was calculated for the detection of P. gingivalis [Figure 7]. Umbilical cord blood was collected immediately after each delivery from singleton (primigravida) deliveries [Figure 8] and transferred to a tube with Thyoglycolate medium [Figures 9 and 10] and labeling was done and sent to laboratory where it was processed within 72 hr of sample collection for the detection of P. gingivalis [Figures 11 and 12]. All the clinical parameters: BOP, PD, and CAL. BOP was measured by first examiner using modified sulcus bleeding index[1] and UNC-15 probe was used to record PD and CAL.
Figure 1.
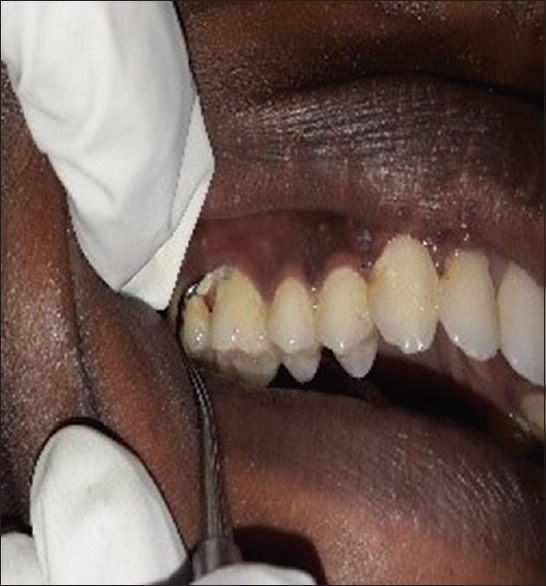
Collecting sub-gingival plaque using Gracey curettes
Figure 2.
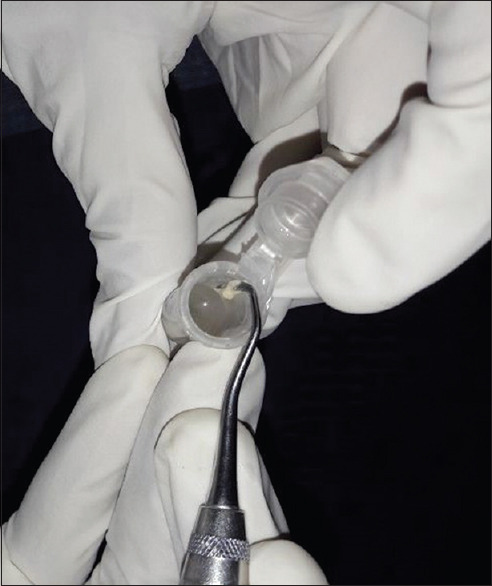
Collected sample is transferred into a transport medium (thioglycolate)
Figure 3.

Anaerobic jar
Figure 6.

Digital colony counter
Figure 7.
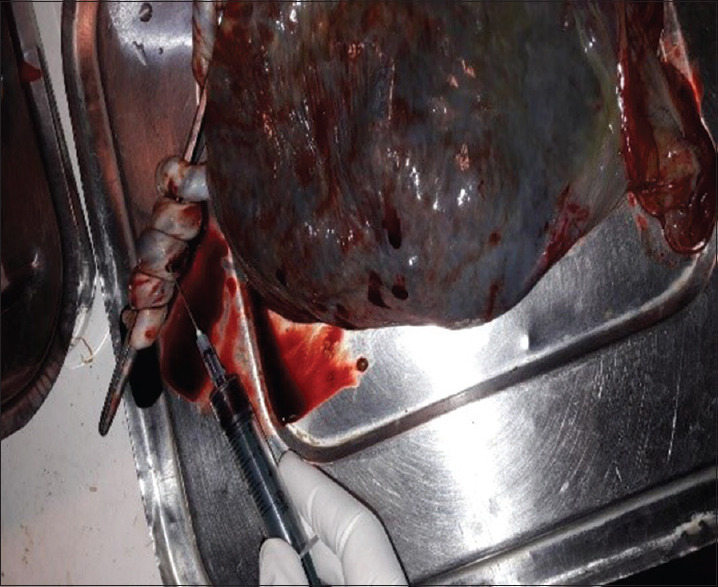
Umbilical cord blood collected
Figure 8.

Thioglycolate
Figure 9.
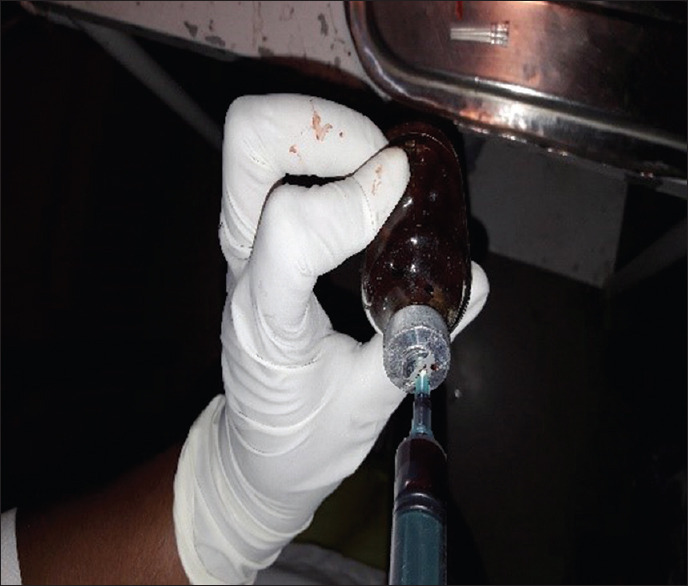
Cord blood transferred to a thioglycolate medium
Figure 10.
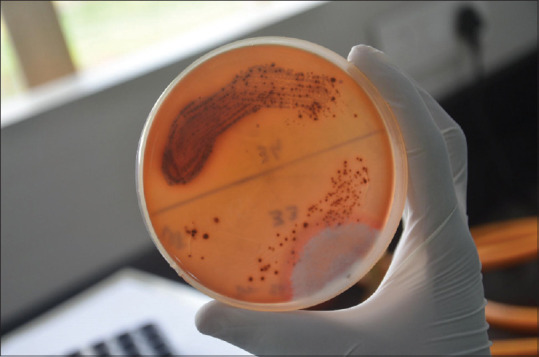
Porphyromonas gingivalis detected on culture plates
Figure 11.

Porphyromonas gingivalis count detected on culture plates-preterm
Figure 12.
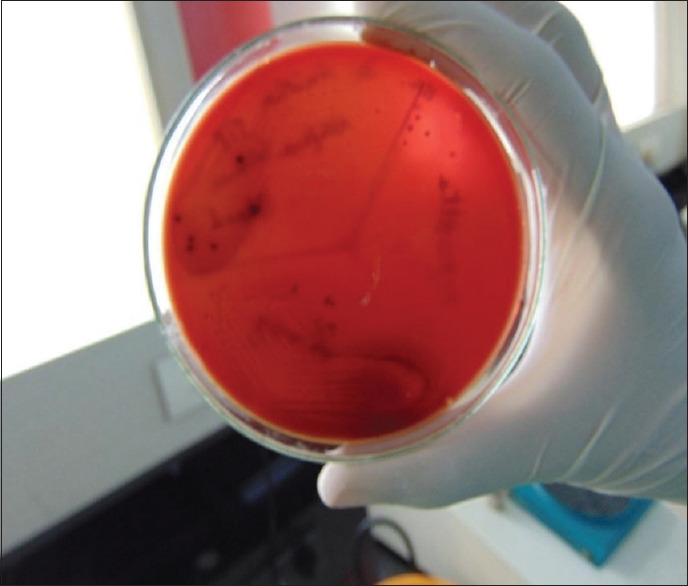
Porphyromonas gingivalis count detected on culture plates-full term
Figure 4.

Incubator
Figure 5.

Streaking culture plates with loops
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS package version 22.0, Inc., an IBM Company, Chicago, Illinois, USA). The Chi-square test was used for the evaluation of significance of differences. The significance of periodontal characteristics between groups was found using Student's t-test. The association between P. gingivalis in subgingival plaque and same pathogen in umbilical cord blood in the two groups was found using the Spearman's correlation coefficient. Significance of the linear relationship between birth outcomes, P. gingivalis load and other clinical variables in the groups was identified using multiple linear regression analysis. P < 0.05 was set as the level of significance (P Value).
RESULTS
A statistically significant difference was seen for probing pocket depth (PPD), clinical attachment level (CAL), P. gingivalis blood, and P. gingivalis plaque on intergroup comparison of mean values of study parameters between the two groups, with increased values seen in case of preterm labor [Table 1].
Table 1.
Inter group comparison of mean values of study parameters between two groups
| Variables | Group | n | Mean | SD | Mean rank | Z-score | P |
|---|---|---|---|---|---|---|---|
| PD | Full term | 63 | 3.46 | 0.59 | 53.88 | −4.213 | <0.001* |
| Preterm | 72 | 4.13 | 0.98 | 80.35 | |||
| CAL | Full term | 63 | 0.06 | 0.35 | 32.63 | −10.424 | <0.001* |
| Preterm | 72 | 3.06 | 1.37 | 98.94 | |||
| P. gingivalis in blood | Full term | 63 | 0.86 | 1.84 | 32.00 | −10.224 | <0.001* |
| Preterm | 72 | 59.82 | 36.95 | 99.50 | |||
| P. gingivalis in plaque | Full term | 63 | 42.40 | 24.80 | 38.42 | −8.227 | <0.001* |
| Preterm | 72 | 124.10 | 63.14 | 93.88 |
*P<0.05-statistically significant. Z-score – Standard score; SD – Standard deviation; PD – Pocket depth; CAL – Clinical attachment level, P. gingivalis – Porphyromonas gingivalis, n – number
Table 2 depicts a statistically significant difference in P. gingivalis blood scores noticed on correlation coefficient relationship between P. gingivalis load in plaque, umbilical cord blood and other clinical parameters in the two groups, with highly significant values seen in preterm group.
Table 2.
Correlation coefficients to establish relationship between Porphyromonas gingivalis load in plaque, umbilical cord blood and other clinical parameters
| Group | Variable | Values | P. gingivalis in plaque | PD | CAL |
|---|---|---|---|---|---|
| Full term | P. gingivalis in blood | Rho | −0.16 | −0.05 | −0.10 |
| P | 0.21 | 0.68 | 0.42 | ||
| n | 63 | 63 | 63 | ||
| Preterm | P. gingivalis in blood | Rho | 0.85 | 0.91 | 0.84 |
| P | <0.001* | <0.001* | <0.001* | ||
| n | 72 | 72 | 72 |
*P<0.05-Statistically significant. Rho – Spearman’s correlation coefficient; PD – Pocket depth; CAL – Clinical attachment level; P. gingivalis – Porphyromonas gingivalis, n – number
Multivariate analysis [Table 3] showed a statistically significant difference in P. gingivalis plaque, PPD, CAL, and umbilical cord blood associated with preterm birth. As a predictive analysis, the multiple linear regression is used to explain the relationship between P. gingivalis blood which was constant and continuous dependent variables between both the groups.
Table 3.
Multiple linear regression analysis to predict porphyromonas gingivalis load in umbilical cord blood with porphyromonas gingivalis in plaque, probing pocket depth and clinical attachment level in two groups
| Group | Model | Variables | Unstandardized coefficients | t | P |
|---|---|---|---|---|---|
|
| |||||
| B | |||||
| Full term | 1 | Constant | 0.71 | 0.47 | 0.64 |
| P. gingivalis plaque | −0.01 | −0.596 | 0.554 | ||
| PPD | 0.12 | 0.288 | 0.775 | ||
| CAL | −0.49 | −0.688 | 0.494 | ||
| Preterm | 1 | Constant | −39.54 | −3.128 | 0.003* |
| P. gingivalis plaque | 0.22 | 2.104 | 0.03* | ||
| PPD | 21.14 | 3.656 | 0.001* | ||
| CAL | 13.09 | 2.616 | 0.01* |
*P<0.05-statistically significant. n – number; t – t value; PD – Pocket depth; PPD – Probing PD; CAL – Clinical attachment level; P. gingivalis – Porphyromonas gingivalis
Table 4 depicts the odd's ratio on the comparison of prevalence of periodontitis among full term and preterm group which was statistically significant in preterm birth as compared with full-term birth.
Table 4.
Comparison of prevalence of periodontitis among full-term and preterm group
| Periodontitis | Number of patients | χ2 | P | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Full term, n (%) | Preterm, n (%) | Lower | Upper | ||||
| Present | 3 (4.8) | 21 (29.2) | 13.691 | <0.001* | 1.90 | 1.48 | 2.45 |
| Absent | 60 (95.2) | 51 (70.8) | |||||
*P<0.05-statistically significant. χ2 – Chi-square; n – Number of patients; OR – Odd’s ratio; CI – Confidence interval
The scatterplots [Figures 13-15] represent P. gingivalis count in the umbilical cord blood and plaque sample of the mothers. Clinical parameters evaluated in mothers of preterm versus full-term neonates revealed a significant difference in preterm delivery when compared to full-term delivery.
Figure 13.
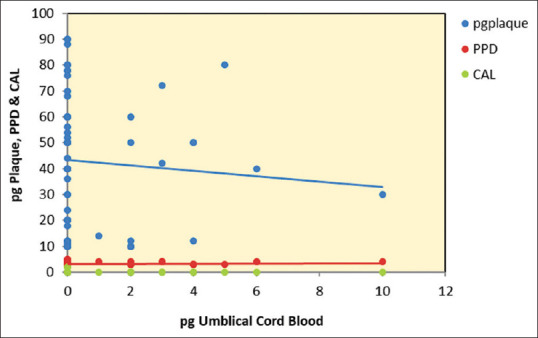
Scatterplot depicting relationship between Porphyromonas gingivalis umbilical cord blood, Porphyromonas gingivalis plaque, probing pocket depth and clinical attachment level in full-term group. pg: Porphyromonas gingivalis; PPD: Probing pocket depth; CAL: Clinical attachment level
Figure 15.
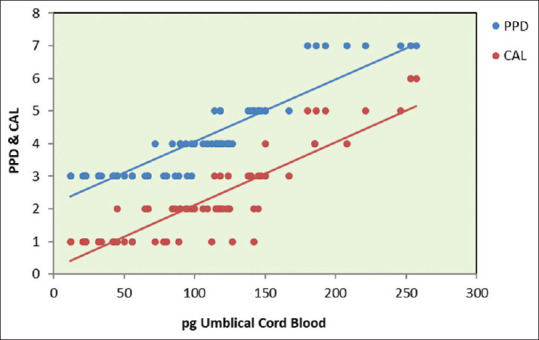
Scatterplot depicting relationship between Porphyromonas gingivalis umbilical cord blood, probing pocket depth, and clinical attachment level in full-term group. pg: Porphyromonas gingivalis; PPD: Probing pocket depth; CAL: Clinical attachment level
Figure 14.
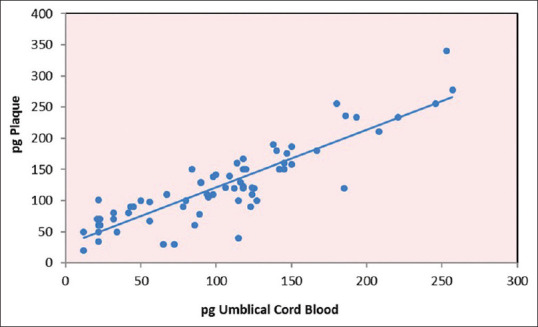
Scatterplot depicting the relationship between Porphyromonas gingivalis umbilical cord blood and Porphyromonas gingivalis plaque in pre-term group. pg: Porphyromonas gingivalis
DISCUSSION
Preterm delivery within 30 weeks of gestation constitutes 1%–2% of all deliveries. This is responsible for about 60% of newborn deaths and 50% of postnatal health-care expenditure.[2] Preterm birth is said to occur if the span of gestation is <37 weeks (259 days). Preterm can be classified as:[3]
Mildly preterm birth <37 weeks
-
Very preterm birth, which includes:
- Moderately preterm birth <32 weeks but >28 weeks
- Extremely preterm birth: <28 weeks.
This classification is analogous with division of birth weight which includes, extremely low birth weight (<1000 g), very low birth weight (<1500 g), and low birth weight (<2500 g).[4] Systemic health, age, low socioeconomic status, and smoking are the various risk factors commonly associated with both periodontitis and PLBW.[5] Poor oral hygiene or periodontal disease can cause systemic infection which leads to release of endotoxins or other microbial products causing inflammation that leads to elevated levels of IL-8 in blood. This enhances the expression of elastases by PMNs and leads to the formation of prostanoids which cause preterm rupture of membranes. The oral bacteria can also be introduced into vaginal by orogenital sexual practice which then enter amniotic fluid through ascending route and may lead to preterm birth.[6]
The periodontal status worsens during pregnancy, irrespective of prepartum periodontal status which may pose a potential risk for fetal exposure and preterm delivery.[7] In support of this, other studies[8] also concluded that mothers with preterm deliveries had more severe periodontal disease than mothers with full-term deliveries. On the other hand, a study[9] found no correlation between low-birth weight and periodontitis but found a correlation between periodontal pockets and prematurity. Although there were numerous studies to find out the association between preterm delivery and specific periodontal microbiota, none of the studies are conclusive. A study[10] has shown that P. gingivalis may affect the pregnancy outcome. On the contrary, other studies[11] showed that there is no relation among P. gingivalis and preterm labor. In fact, a study[12] has shown that P. gingivalis was not at all found in pregnant participants.
The pregnant participants included in the study were all systemically healthy except for periodontitis. This inclusion prevented any bias pertaining to earlier oral infections which could have required antibiotic therapy. Vaginal bacterial counts are found to be associated with overt clinical evidence of gingivitis.[13] For this reason, vaginal infection was taken as excluding criteria. Furthermore, participants with normal ultrasound reports and urine reports were included to reduce the bias.
In the present study, bleeding on probing was considered as a sign of inflammation of gingiva. Both groups showed increased bleeding index scores, relatable with their hormonal changes, and inadequate oral hygiene which was in accordance with a study[14] which reported that bleeding on probing had the highest correlation with preterm birth which may be due to bacteria and their products diffusing more rapidly than usual when vascular permeability of gingival tissues was enhanced because of hormonal influences.
Lopez et al. reported that the prevalence of preterm birth was 11.5% higher in pregnant women with periodontitis as compared to the periodontally healthy group.[15] In the present study, the presence of P. gingivalis in umbilical cord blood of infants suggests a probable passage of transmission from placental tissue through vasculature. P. gingivalis was present in both full term and preterm groups; however, the preterm group was 29.2% higher compared with the normal (full term) group.
P. gingivalis is a Gram-negative microorganism that can occupy and live within the epithelium.[16] P. gingivalis infection disrupts expression of cytokines and enhances cell proliferation while inhibiting apoptosis.[17] We focused on P. gingivalis in our study since this microorganism was predominantly found in severe cases of periodontitis, epidemiologically correlated with preterm birth and expressed significant virulence characteristics in animal models. Zenobia and Hajishengallis have shown that P. gingivalis expresses a microbial community-wide dysbiotic effect which is attributed to it is ability to destabilize host antimicrobial defenses without suppressing inflammation, which in turn enhances the growth of bacterial species that can tolerate and exploit the inflammatory environment.[18]
Our findings showed that P. gingivalis was more frequently found in preterm delivery as compared with full-term delivery which is in accordance with a study[19] which found 49 of 97 (51%) placentas and 40 of 97 (41%) umbilical cord specimens positive for P. gingivalis within the preterm cohort, and more P. gingivalis antigen in preterm specimens within the placenta and umbilical cord compared to term tissues. Further, the unique distribution of P. gingivalis antigen in the villous stroma of preterm placental specimens was linked to shorter gestation length and the need for cesarean section. Another novel finding was the association between preeclampsia and the presence of P. gingivalis antigens in the umbilical cord.[19]
P. gingivalis gains entry into the placental mesenchyme by various ways. One possible explanation could be the crossing over of P. gingivalis from the maternal circulation into the fetal tissues through the syncytiotrophoblast in case of a disruption in the syncytiotrophoblast layer forming a direct port d’entrée. Alternatively, P. gingivalis may invade the placenta through the decidua, at the uterine-trophoblast interface.[20] Here, microbes may spread from the maternal circulation to susceptible extra-villous trophoblast cells by direct invasion or cell-to-cell spread during implantation of the trophoblast into the uterine wall in the first trimester of pregnancy.[20,21] P. gingivalis efficiently invades a variety of host cell types[22] including extravillous trophoblast cells.[23]
While there are several virulence mechanisms associated with P. gingivalis that promote dysbiosis,[18] the ability of P. gingivalis to synthesize different lipopolysaccharide structures that either activate or antagonize TLR4, such as 4′ phosphatases and lipid A1,[24] is particularly significant at the maternal-fetal interface.
Periodontal infection by P. gingivalis can induce atopobiosis (translocation) to the placenta and trigger inflammation. There is a pro-inflammatory pattern associated with labor, characterized by Th-1 profile and the activity of cytotoxic cells, which is enhanced by placental infection with urogenital microorganisms. However, placental infection associated with P. gingivalis suggests a switch where the balance of Th-1 profile favors an inflammatory response mediated by monocyte chemoattractant protein-1 and macrophage activity as an explanation of its possible relationship with adverse outcomes during pregnancy.[25] Studies[26] have also shown that an excess of TH17 with a reduced Treg cell profile can lead to adverse pregnancy outcomes.
In our present study, P. gingivalis was statistically significant in umbilical cord blood of infant and plaque sample of pregnant women with preterm delivery compared to full term delivery which was in agreement with a cohort study[7] which observed that out of 546 umbilical cord samples obtained, 317 (58%) were positive for IgM of one or more oral microorganisms, indicating systemic distribution and exposure of the fetus. Another study[27] in rabbits showed placental and fetal exposure to P. gingivalis after the maternal exposure at a site away from reproductive tract. About one-third of placentas, almost one-half of the fetuses and one-third of maternal livers, showed microbial DNA indicating systemic distribution in the mothers and trans-placental passage to their fetus.
Our present study included clinical examination for bleeding on probing, dental plaque, pocket depth, and CAL which were statistically significant in preterm delivery which was in contrast to the results obtained by a cohort study[28] which investigated the correlation between periodontal disease and PLBW and effect of periodontal treatment on pregnancy outcomes which found no differences in the periodontal status between women with normal birth outcome and PLBW cases.
One limitation of the study was that though most of the confounding factors were taken care of during the selection of the subjects, subclinical/asymptomatic inflammation such as chorioamnionitis may precipitate preterm delivery and thus may act as a source of bias in the study. Many potential confounding factors (environmental exposures, psychosocial and behavioral factors, medical conditions, neighborhood characteristics, infertility treatments, conception by assisted reproduction techniques, biological, and genetics factors) and possible effect modifiers for adverse pregnancy outcome must be controlled in future studies.
Another limitation is that the detection of microorganism depends on disease activity. A low count of periodontopathogens may be found in progressive periodontal sites which currently are inactive. Vice versa, a significant number of pathogens may appear in supposedly inactive periodontal sites which are experiencing an increase in disease activity.[29]
Postpartum healthcare was provided in a follow-up period of 6–8 weeks, during which periodontal therapy was also administered.
CONCLUSION
In light of the current observation, following conclusions were drawn:
P. gingivalis was significantly associated with PLBW and periodontitis
The association between P. gingivalis and PLBW can be clearly established. By using different mechanisms, P. gingivalis subverts the host's immune response. Hence, a combination of preexisting periodontal disease and P. gingivalis placental colonization would increase the risk of adverse pregnancy outcomes in part by inducing a TH17/Treg cell imbalance
Therefore, periodontal microorganisms may reach the placenta or the fetus and causes adverse pregnancy outcomes.
Further multicenter, randomized controlled clinical trials, with more meticulous methodology, including precise definitions of exposure, control of confounders and application of periodontal therapy must be carried out so that preventive measurements can be taken to manage each individual case.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mombelli A, van Oosten MA, Schurch E, Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoat MK, Geurs NC, Reddy MS, Goldenberg RL, Hauth JC. Current evidence regarding periodontal disease as a risk factor in preterm birth. Ann Periodontol. 2001;6:183–8. doi: 10.1902/annals.2001.6.1.183. [DOI] [PubMed] [Google Scholar]
- 3.Haram K, Mortensen JH, Wollen AL. Preterm delivery: An overview. Acta Obstet Gynecol Scand. 2003;82:687–704. doi: 10.1034/j.1600-0412.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- 4.NHMRC, National Health and Medical Research Council Clinical Practice Guidelines. Care Around Preterm Birth. 1997 [Google Scholar]
- 5.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 6.Jarjoura K, Devine PC, Perez-Delboy A, Herrera-Abreu M, D’Alton M, Papapanou PN. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol. 2005;192:513–9. doi: 10.1016/j.ajog.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL, Jr, Beck JD, et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001;6:175–82. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- 8.Savitt ED, Socransky SS. Distribution of certain subgingival microbial species in selected periodontal conditions. J Periodontal Res. 1984;19:111–23. doi: 10.1111/j.1600-0765.1984.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Lunardelli AN, Peres MA. Is there an association between periodontal disease, prematurity and low birth weight? A population-based study. J Clin Periodontol. 2005;32:938–46. doi: 10.1111/j.1600-051X.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 10.Collins JG, Smith MA, Arnold RR, Offenbacher S. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect Immun. 1994;62:4652–5. doi: 10.1128/iai.62.10.4652-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souccar NM, Chakhtoura M, Ghafari JG, Abdelnoor AM. Porphyromonas gingivalis in dental plaque and serum C-reactive protein levels in pregnancy. J Infect Dev Ctries. 2010;4:362–6. [PubMed] [Google Scholar]
- 12.Raber-Durlacher JE, Van Steenbergen TJ, van der Velden U, de Graff J, Abraham-Inpijn L. Experimental gingivitis during pregnancy and post-partum: Clincal, endoerinological, and microbiological aspects. J Clin Periodontal. 1994;21:549–58. doi: 10.1111/j.1600-051x.1994.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 13.Persson R, Hitti J, Verhelst R, Vaneechoutte M, Persson R, Hirschi R, et al. The vaginal microflora in relation to gingivitis. BMC Infect Dis. 2009;9:6. doi: 10.1186/1471-2334-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radnai M, Gorzó I, Nagy E, Urbán E, Novák T, Pál A. A possible association between preterm birth and early periodontitis. A pilot study. J Clin Periodontol. 2004;31:736–41. doi: 10.1111/j.1600-051X.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 15.López NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. J Dent Res. 2002;81:58–63. doi: 10.1177/002203450208100113. [DOI] [PubMed] [Google Scholar]
- 16.Lamont RJ, Jenkinson HF. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zenobia C, Hajishengallis G. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence. 2015;6:236–43. doi: 10.1080/21505594.2014.999567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanterpool SF, Been JV, Houben ML, Nikkels PG, De Krijger RR, Zimmermann LJ, et al. Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLoS One. 2016;11:e0146157. doi: 10.1371/journal.pone.0146157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6:e1000732. doi: 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins JR, Bakardjiev AI. Pathogens and the placental fortress. Curr Opin Microbiol. 2012;15:36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett. 2000;187:139–44. doi: 10.1111/j.1574-6968.2000.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 23.Inaba H, Kuboniwa M, Bainbridge B, Yilmaz O, Katz J, Shiverick KT, et al. Porphyromonas gingivalis invades human trophoblasts and inhibits proliferation by inducing G1 arrest and apoptosis. Cell Microbiol. 2009;11:1517–32. doi: 10.1111/j.1462-5822.2009.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zenobia C, Hasturk H, Nguyen D, Van Dyke TE, Kantarci A, Darveau RP. Porphyromonas gingivalis lipid A phosphatase activity is critical for colonization and increasing the commensal load in the rabbit ligature model. Infect Immun. 2014;82:650–9. doi: 10.1128/IAI.01136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez LA, De Avila J, Castillo DM, Montenegro DA, Trujillo TG, Suárez LJ, et al. Porphyromonas gingivalis placental atopobiosis and inflammatory responses in women with adverse pregnancy outcomes. Front Microbiol. 2020;11:591626. doi: 10.3389/fmicb.2020.591626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148:13–21. doi: 10.1111/imm.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192:554–7. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell-Lewis D, Engebretson SP, Chen J, Lamster IB, Papapanou PN. Periodontal infections and pre-term birth: Early findings from a cohort of young minority women in New York. Eur J Oral Sci. 2001;109:34–9. doi: 10.1034/j.1600-0722.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 29.Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetumcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontal. 1986;13:570–7. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]


