Abstract
Background:
Myristica fragrans, commonly known as nutmeg, has been shown to have many medicinal properties including reduction of plaque and oral halitosis. In the present study, a mouthwash was prepared from nutmeg oil to compare its effectiveness with 0.2% chlorhexidine (CHX) gluconate mouthwash on halitosis and plaque control.
Materials and Methods:
A total of 60 participants having plaque and plaque-induced oral halitosis were enrolled and randomly divided into two groups as Group A (30) and Group B (30). Nutmeg and 0.2% CHX gluconate mouthwash were administered in Group A and Group B, respectively, for 21 days twice daily. Plaque index and organoleptic score were recorded at baseline, 14th day, and 21st day. The data were collected, tabulated in Microsoft Excel Sheet, and subjected to statistical analysis using appropriate statistical tests.
Results:
The percentage of change in plaque score between baseline and 14th day was 12.3% and 9.44% (P = 0.741), between baseline and 21st day 39.2% and 39.4% (P = 0.889), and between 14th and 21st day 27.7% and 29.9% (P = 0.805) for Group A and Group B, respectively, while the percentage of change in halitosis score between baseline and 14th day was 24.7% and 16.3% (P = 0.201), between baseline and 21st day 54.5% and 56.3% (P = 0.487), and between 14th and 21st day 34.9% and 40.0% (P = 0.393) for Group A and Group B, respectively.
Conclusion:
Nutmeg mouthwash is organic, economical, and equally effective as compared to 0.2% CHX gluconate mouthwash (Clinical Trials Registry-India/2020/10/028540).
Keywords: Antiplaque, halitosis, nutmeg oil, plaque-induced halitosis
INTRODUCTION
Ayurveda is an ancient Indian system of medicine that offers a vast pool of herbal medication effective and is often considered as free of toxicity of the modern allopathic preparations.[1] In recent times, a lot of clinical trials have been conducted, assessing the age-old science of Ayurveda by testing it against the gold standard allopathic preparations used for established traditional treatment modalities.[2] The Ayurvedic concept appeared and formed between 2500 and 500 BC in India. The literal meaning of Ayurveda is “science of life” because the earliest Indian system of health care focused on views of man and his illness.[3] To control the two most challenging problems of the oral cavity, namely gingivitis and halitosis along with their causative agent plaque, an array of chemical agents have been employed in the past. Halitosis is a common problem impacting individuals of all ages and it can affect the persons self-esteem and confidence.[4] Chlorhexidine (CHX) is one such agent, which has stood the test of time and is considered a gold standard in chemical plaque control. CHX is available as a mouthwash, topical gel, and in biodegradable form. It has a broad-spectrum antimicrobial activity. CHX has a substantivity of 12 h and hence is recommended to use twice daily.[5] According to previous studies, long-term use of CHX mouthrinse causes an increase in dental stains, allergy, and burning mouth.[5,6] Hence, the search for herbal mouthwash is continued. Nutmeg is a slightly sweet and delicate spice which is widely used in cooking around the world. It is the only tree which is the source of two distinct spices in the world.[7] The health benefit of nutmeg oil includes medicinal properties such as its character as a sedative, stimulant, relaxing, anti-inflammatory, antiseptic, antifungal, and antibacterial substance. Nutmeg oil is obtained from the seed of nutmeg fruit having the ability to reduce stress, pain, menstrual cramps, heart disorders, indigestion, blood pressure, cough, and bad breath due to the complex structural molecules present in it.[8] Hence, the present clinical trial compares the nutmeg mouthwash with 0.2% CHX gluconate mouthwash for its effectiveness in controlling plaque and halitosis.
MATERIALS AND METHODS
The present study was a double-blinded randomized clinical Ttial, a two-group parallel study conducted at the department of public health dentistry. Before the start of the study, a study protocol was submitted to the Ethical Review Committee and ethical clearance was obtained. The study was registered at Clinical Trial Registry-India (CTRI) and registration number CTRI/2020/10/028540 was obtained. The purpose and procedure of the present study were explained to each and every participant and informed consent was obtained from all. The present study consisted of 60 participants of the age group 20–60 years. Sample size estimation was done by using the student t-test. They were randomly divided into two groups of 30 each by lottery method (Group A and Group B) [Figure 1]. Group A was given nutmeg mouthwash and Group B was given 0.2% CHX gluconate mouthwash and was instructed to use 10 ml of mouthwash twice daily for 21 days. Baseline data were collected using the plaque index[9] and organoleptic diagnosis which is the gold standard for halitosis score.[4,10] Oral prophylaxis was carried out after baseline data collection and participants were instructed to use the given mouthwash and not to use any other oral hygiene aids except toothbrush and toothpaste. Data collection was done on 14th and 21st days. All the examinations and recording of the pro forma were carried out by a single examiner (i.e., primary investigator). Before starting the study, the training and calibration of the examiner were done in the department of public health dentistry.
Figure 1.
Flow chart of participants in the study. n – number of participants
Inclusion criteria
Participants of age between 20 to 60 years
Participants who were systemically healthy
Participants who suffered halitosis.
Exclusion criteria
Participants having gingivitis or periodontitis
Participants who already using mouthwash for 3 months
Participants who underwent any antibiotic therapy in the past 3 months
Participants using orthodontic or prosthetic appliances
Participants who were pregnant
Participants had adverse oral habits
Participants who used oral hygiene aids apart from toothbrush
Participants who were mentally challenged, physically handicapped, or who were not capable to comply with the study protocol
Dropouts.
Nutmeg mouthwash was formulated and developed in a pharmacy college. Nutmeg oil along with some ingredients such as olive oil, menthol, glycerin, and orange oil was obtained from a store providing Ayurvedic products. Other ingredients such as sucralose, sodium benzoate, Span 80, and Tween 20 were obtained from the laboratory of the same pharmacy college where the mouth wash was formulated.
Method of formulation of nutmeg mouthwash
Nutmeg oil and olive oil were triturated in a uniform direction in mortar and pestle to form a uniform mixture. Later, glycerine, and orange oil were added while triturating. Sucralose, sodium benzoate, and menthol were triturated separately to form a fine powder and added to initially prepared mixture of oils. Water was added to obtain the desired quantity and stirred at 900 rpm with the help of an electronic stirrer. Span 80 and Tween 20 were added for better emulsification as it was an oil-based mouthwash. The prepared mouthwash had white color, acceptable taste, and watery consistency which were easy to gargle. Shelf life of the mouth was 1.5 years on the basis of ingredients used in the preparation.
The concentration of each ingredient used was:
Nutmeg oil – 2%
Olive oil – 5%
Glycerine – 0.25%
Sucralose – 0.1%
Orange oil – 0.1%
Sodium benzoate – 0.05%
Menthol – 1%
Span – 80%–0.1%
Tween – 20%–0.1%
Water – QS – Quantity Sufficient
Participants in both the groups were demonstrated with the modified Bass brushing technique.[11] They were told to brush twice daily and instructed to shake the mouthwash before use. Participants in both the groups were told to use 10 ml of their respective mouthwash twice daily and rinse it for 30 s and were told to spit. Post the end of first, second, and 3rd week, the participants were asked to report to the department to check the compliance and to issue more mouthwash if required.
RESULTS
At baseline, there was no statistical difference between the two treatment options for plaque indices and halitosis scores. PlI: the plaque score difference at different intervals in Group A was 0.069 between baseline and 14th day; between baseline and 21st day, it was 0.001; and between 14th and 21st day, it was 0.001. Likewise in Group B, the difference between baseline and 14th day was 0.349, between baseline and 21st day was 0.001, and between 14th and 21st day was 0.001. Overall statistically significant (P ≤ 0.05) reduction was found in the plaque score of all the pairs in Group A and Group B [Table 1 and Graph 1]. Halitosis Score: the halitosis score difference at different intervals in Group A was 0.001 between baseline and 14th day; between baseline and 21st day, it was 0.001; and between 14th and 21st day, it was 0.001. Likewise in Group B, the difference between baseline and 14th day was 0.010, between baseline and 21st day, it was 0.001; and between 14th and 21st day, it was 0.001. Overall statistically significant (P ≤ 0.05) reduction was found in the halitosis score in all the pairs in Group A and Group B [Table 2 and Graph 2]. The halitosis score difference between Group A and Group B at baseline was 0.03 (P = 0.875), on 14th day was 0.26 (P = 0.222), and on 21st day was 0.10 (P = ‒0.609). Statistically significant (P ≤ 0.05) reduction was found in the halitosis score on the 21st day in both the Groups [Table 3]. The percentage of change in plaque score and halitosis score at each interval showed no statistically significant difference among both the groups [Table 4 and Graphs 3 and 4].
Table 1.
Evaluation of change in plaque index at different intervals among both the groups
| Groups | Interval | SD | F | P | Baseline versus 14 | Baseline versus 21 | 14 versus 21 |
|---|---|---|---|---|---|---|---|
| CHX | Baseline | 0.41 | 31.156 | 0.001* | 0.349 | 0.001* | 0.001* |
| 14 days | 0.44 | ||||||
| 21 days | 0.29 | ||||||
| Nutmeg | Baseline | 0.37 | 38.590 | 0.001* | 0.069 | 0.001* | 0.001* |
| 14 days | 0.41 | ||||||
| 21 days | 0.35 |
*Indicates significant at P≤0.05, Repeated measure ANOVA test; Post hoc Bonferroni test. CHX – Chlorhexidine mouthwash, SD – Standard deviation;
F – Frequency; P – Probability
Graph 1.
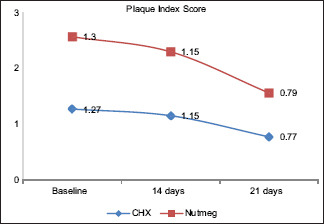
Evaluation of change in plaque index at different intervals among both the groups. CHX – Chlorhexidine mouthwash
Table 2.
Evaluation of change in halitosis at different intervals among both the groups
| Groups | Interval | SD | F | P | Baseline versus 14 | Baseline versus 21 | 14 versus 21 |
|---|---|---|---|---|---|---|---|
| CHX | Baseline | 0.82 | 81.631 | 0.001* | 0.010* | 0.001* | 0.001* |
| 14 days | 0.87 | ||||||
| 21 days | 0.82 | ||||||
| Nutmeg | Baseline | 0.82 | 136.482 | 0.001* | 0.001* | 0.001* | 0.001* |
| 14 days | 0.81 | ||||||
| 21 days | 0.67 |
*Indicates significant at P≤0.05, Repeated measure ANOVA test; Post hoc Bonferroni test. CHX – Chlorhexidine mouthwash, SD – Standard deviation;
F – Frequency; P – Probability
Graph 2.
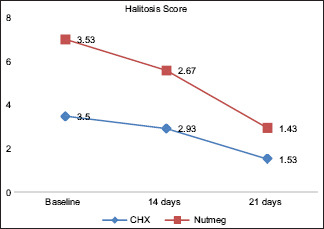
Evaluation of change in halitosis at different intervals among both the groups. CHX – Chlorhexidine mouthwash
Table 3.
Comparison of plaque index and halitosis among both the groups across each interval
| Variable | Interval | Groups | n | Mean | SD | Difference | t | P |
|---|---|---|---|---|---|---|---|---|
| PI | Baseline | CHX | 30 | 1.27 | 0.41 | −0.03 | −0.300 | 0.765 |
| Nutmeg | 30 | 1.30 | 0.37 | |||||
| 14 days | CHX | 30 | 1.15 | 0.44 | 0.00 | 0.030 | 0.976 | |
| Nutmeg | 30 | 1.15 | 0.41 | |||||
| 21 days | CHX | 30 | 0.77 | 0.29 | −0.02 | −0.241 | 0.810 | |
| Nutmeg | 30 | 0.79 | 0.35 | |||||
| Halitosis | Baseline | CHX | 30 | 3.50 | 0.82 | 0.03 | −0.158 | 0.875 |
| Nutmeg | 30 | 3.53 | 0.82 | |||||
| 14 days | CHX | 30 | 2.93 | 0.87 | 0.26 | 1.235 | 0.222 | |
| Nutmeg | 30 | 2.67 | 0.80 | |||||
| 21 days | CHX | 30 | 1.53 | 0.82 | 0.10 | 0.515 | −0.609 | |
| Nutmeg | 30 | 1.43 | 0.67 |
*Indicates significant at P≤0.05, independent t-test; CHX – Chlorhexidine mouthwash, PI – Plaque index, SD – Standard deviation. n – number of participants; t – t value after applying t-test; P – P value
Table 4.
Comparison of change in plaque index and halitosis among both the groups
| Variable | Interval | Groups | Percentage change | t | P |
|---|---|---|---|---|---|
| PI | Baseline -14 days | CHX | 9.44 | −0.332 | 0.741 |
| Nutmeg | 12.3 | ||||
| 14-21 days | CHX | 29.9 | 0.248 | 0.805 | |
| Nutmeg | 27.7 | ||||
| Baseline -21 days | CHX | 39.4 | −0.141 | 0.889 | |
| Nutmeg | 39.2 | ||||
| Halitosis | Baseline -14 days | CHX | 16.3 | −1.293 | 0.201 |
| Nutmeg | 24.7 | ||||
| 14-21 days | CHX | 40.0 | 0.861 | 0.393 | |
| Nutmeg | 34.9 | ||||
| Baseline -21 days | CHX | 56.3 | −0.699 | 0.487 | |
| Nutmeg | 54.5 |
CHX – Chlorhexidine mouthwash, PI – Plaque index, SD – Standard deviation; t – t-test; P – Indicates significant at P≤0.05
Graph 3.
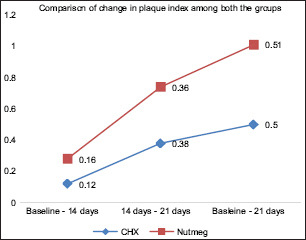
Comparison of change in plaque among both the groups. CHX – Chlorhexidine mouthwash
Graph 4.
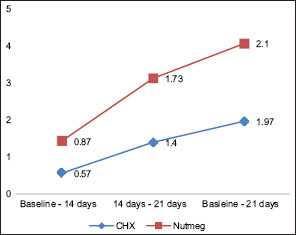
Comparison of change in halitosis among both the groups. CHX – Chlorhexidine mouthwash
DISCUSSION
One cannot be entirely healthy without good oral health and its impact in terms of pain, suffering, impairment of function, and effect on the quality of life cannot be ignored. Oral disease affects all people regardless of their gender, socioeconomic strata, race, age, and it qualifies as major public health problem owing to their high prevalence and incidence around the globe.[12] Poor oral hygiene and the accumulation of bacterial plaque are important predisposing factors of halitosis, gingivitis can further lead to periodontitis.[13] Using a chemical plaque control agent (like a mouthwash) to supplement mechanical plaque removal can produce an antimicrobial effect throughout the oral cavity.[14] CHX is one of the most commonly prescribed chemical plaque control agents in dentistry. It has a long-lasting antibacterial activity with a broad spectrum of action and it has been shown to reduce plaque, gingival inflammation, and bleeding.[15] However, the presence of alcohol in CHX mouthwash is accompanied by side effects, too. Ahmad et al.,[6] in their study, confirmed the effect of long-term use of CHX mouthrinse on increasing the dental stains, allergy, and burning mouth.[6] In recent times, the use of herbal mouthwash is on the rise due to the spread in the awareness of the effect of complementary and alternative medicine. It is due to a stronger belief that the alternative therapy has less side effects.[16] In agreement with the present study, Mitra et al.,[17] in the study conducted on white tea extract mouthwash (herbal) and CHX mouthwash, concluded that the white tea extract mouthwash was as effective as CHX mouthwash in plaque control. Bahadır et al.[18] in their study stated that dental plaque is the primary etiology for halitosis, which typically develops within 10–21 days in the absence of plaque control. Hence, the present study was conducted for 21 days and only primary cause (dental plaque) was taken into consideration.[18] Baseline scores were recorded and follow-up was taken on 14th and 21st days for both the groups as per the study conducted by Mali et al.[19] to assess the anti-plaque efficacy of Arimedadi oil. In the present study, none of the participants have shown any side effect of nutmeg mouthwash after using it for 21 days. In the present study on inter-group comparison for plaque score and halitosis score, it was observed that there was statistically no significant difference (P > 0.05) between both the groups on the 14th day and 21st days. A review of available literature has revealed that no similar studies have been conducted to assess the effectiveness of such nutmeg mouthwash for plaque and halitosis control.
Limitations of our study
Small sample size and the inability to document long-term side effects, if any, of nutmeg mouthwash were the limitations of the study. Further microbiological examinations are needed to assess for the shelf life, substantivity, and long-term efficacy of nutmeg mouthwash.
CONCLUSION
The present study has shown that nutmeg mouthwash has similar efficacy as that of CHX in reducing dental biofilm build-up and halitosis. It is organic and relatively cost-effective compared with CHX. Hence, it can be administered as an alternative to 0.2% CHX gluconate mouthwash. The promotion of nutmeg mouthwash with no side effects accompanied by low cost may motivate the patient, especially the one with low socioeconomic strata for oral hygiene maintenance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mamgain P, Kandwal A, Mamgain RK. Comparative evaluation of triphala and ela decoction with 0.2% chlorhexidine as mouthwash in the treatment of plaque-induced gingivitis and halitosis: A randomized controlled clinical trial. J Evid Based Complementary Altern Med. 2017;22:468–72. doi: 10.1177/2156587216679532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samy RP, Pushparaj PN, Gopalakrishnakone P. A compilation of bioactive compounds from Ayurveda. Bioinformation. 2008;3:100–10. doi: 10.6026/97320630003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramar PS, Peter NP, Ponnampalam G. A compilation of bioactive compounds from Ayurveda. BMJ Innov. 2008;100:10. doi: 10.6026/97320630003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rösing CK, Loesche W. Halitosis: An overview of epidemiology, etiology and clinical management. Braz Oral Res. 2011;25:466–71. doi: 10.1590/s1806-83242011000500015. [DOI] [PubMed] [Google Scholar]
- 5.Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad M, Parichehr B, Naeimeh N, Pedram I, Seyed A, Jaber Y. Comparison of the efficacy and side effects of chlorhexidine mouthrinses with (Hexidine) and without (Epimax) alcohol. Dent Hypotheses. 2016;7:137–41. [Google Scholar]
- 7.Namra N, Rafia R, Ayesha M, Jihene BG. Nutmeg: A review on uses and biological properties. IJCBS. 2016;9:107–10. [Google Scholar]
- 8.Dino R, Mario P, Emma M. Physico-chemical properties of nutmeg (Myristica fragrans houtt) of North Sulawesi Nutmeg. Fullerene J Chem. 2020;5:23–31. [Google Scholar]
- 9.Löe H. The Gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 10.Greenman J, Lenton P, Seemann R, Nachnani S. Organoleptic assessment of halitosis for dental professionals—General recommendations. J Breath Res. 2014;8:1–9. doi: 10.1088/1752-7155/8/1/017102. [DOI] [PubMed] [Google Scholar]
- 11.Poyato-Ferrera M, Segura-Egea JJ, Bullón-Fernández P. Comparison of modified Bass technique with normal toothbrushing practices for efficacy in supragingival plaque removal. Int J Dent Hyg. 2003;1:110–4. doi: 10.1034/j.1601-5037.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 12.Petersen PE. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century--The approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(Suppl 1):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 13.Pawlaczyk-Kamieńska T, Torlińska-Walkowiak N, Borysewicz-Lewicka M. The relationship between oral hygiene level and gingivitis in children. Adv Clin Exp Med. 2018;27:1397–401. doi: 10.17219/acem/70417. [DOI] [PubMed] [Google Scholar]
- 14.Rao SS, Chava VK. Anti-plaque and Anti-gingivitis agents in the control of Supragingival plaque. Ann Ess Den. 2017;9:10–6. [Google Scholar]
- 15.Kumar SB. Chlorhexidine mouthwash - A review. Res J Life Sci Bioinform Pharm Chem Sci. 2017;9:1450–2. [Google Scholar]
- 16.Fischman SL. The history of oral hygiene products: How far have we come in 6000 years? Periodontol 2000. 1997;15:7–14. doi: 10.1111/j.1600-0757.1997.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitra DK, Shah PM, Shah HH, Rodrigues SV, Mehta CJ. The antiplaque efficacy of white tea extract mouthrinse. J Indian Soc Periodontol. 2016;20:514–7. doi: 10.4103/0972-124X.201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aylıkcı BU, Colak H. Halitosis: From diagnosis to management. J Nat Sci Biol Med. 2013;4:14–23. doi: 10.4103/0976-9668.107255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mali GV, Dodamani AS, Karibasappa GN, Vishwakarma P, Jain VM. Comparative evaluation of arimedadi oil with 0.2% chlorhexidine gluconate in prevention of plaque and gingivitis: A randomized clinical trial. J Clin Diagn Res. 2016;10:C31–4. doi: 10.7860/JCDR/2016/19120.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]