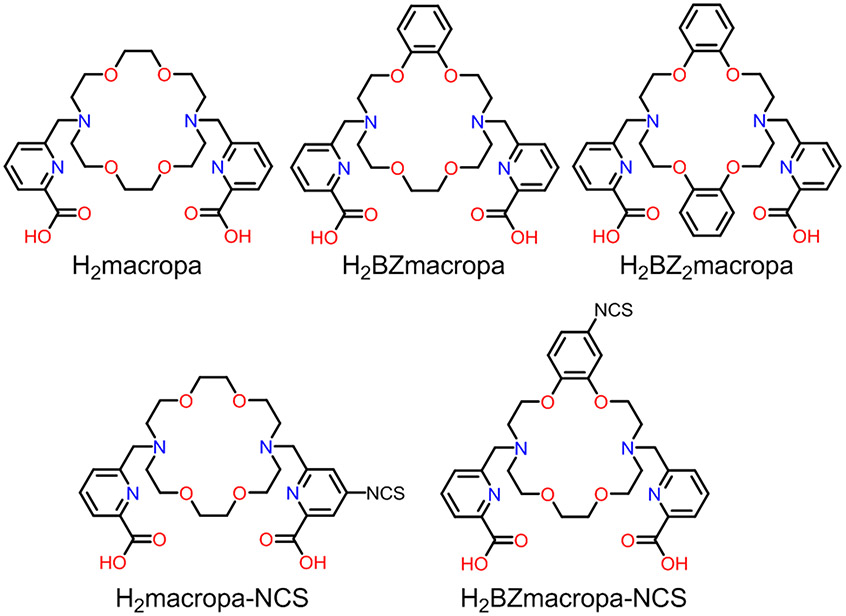
Abstract
Actinium-225 (225Ac) is one of the most promising radionuclides for targeted alpha therapy (TAT). With a half-life of 9.92 days and a decay chain that emits four high energy α particles, 225Ac is well-suited for TAT when conjugated to macromolecular targeting vectors that exhibit extended in vivo circulation times. The implementation of 225Ac in these targeted constructs, however, requires a suitable chelator that can bind and retain this radionuclide in vivo. Previous work has demonstrated the suitability of a diaza-18-crown-6 macrocyclic chelator H2macropa for this application. Building upon these prior efforts, in this study, two rigid variants of H2macropa, which contain either one (H2BZmacropa) or two (H2BZ2macropa) benzene rings within the macrocyclic core, were synthesized and investigated for their potential use for 225Ac TAT. The coordination chemistry of these ligands with La3+, used as a non-radioactive model for Ac3+, was carried out. Both NMR spectroscopic and X-ray crystallographic studies of the La3+ complexes of these ligands revealed similar structural features as those found for the related complex of H2macropa. Thermodynamic stability constants of the La3+ complexes, however, were found to be one and two orders of magnitude lower than those of H2macropa for H2BZmacropa and H2BZ2macropa, respectively. The decrease in thermodynamic stability was rationalized via the use of density functional theory calculations. 225Ac radiolabeling and serum stability studies with H2BZmacropa showed that this chelator compares favorably with H2macropa. Based on these promising results, a bifunctional version of this chelator, H2BZmacropa-NCS, was synthesized and conjugated to the antibody codrituzumab (GC33), which targets the liver cancer biomarker glypican-3 (GPC3). The resulting GC33-BZmacropa conjugate and an analogous GC33-macropa were evaluated for their 225Ac radiolabeling efficiencies, antigen-binding affinity, and in vivo biodistribution in HepG2 liver cancer tumor-bearing mice. Although both conjugates were comparably effective in their radiolabeling efficiencies, [225Ac]Ac-GC33-BZmacropa showed slightly poorer serum stability and biodistribution than [225Ac]Ac-GC33-macropa. Together, these results establish H2BZmacropa-NCS as a new bifunctional chelator for the preparation of 225Ac radiopharmaceuticals.
Graphical Abstract
Introduction
The 2013 FDA approval of 223RaCl2 (Xofigo®) for the management of bone metastases in castration-resistant prostate cancer (mCRPC) patients has heralded a renaissance in targeted alpha therapy (TAT), a treatment modality that uses the high linear energy transfer of alpha (α) particles to annihilate cancer cells.1 Despite the success of this drug, the development of new radiopharmaceutical agents employing 223Ra has been hindered by the difficult chelation chemistry of this ion.2 Fortunately, a number of other α-emitting radionuclides, such as 227Th, 225Ac, 213Bi, 212Bi, 212Pb, and 211At, have suitable properties for use in TAT.3 Among these radionuclides, 225Ac has demonstrated particular promise due to advances in its large-scale production, its ideal 9.92-day physical half-life for conjugation to long-lived biomolecules, and high cytotoxic potency, which arises from the four α particles emitted through its decay chain.4,5 Clinical trials of 225Ac small-molecule6,7 and antibody8 conjugates are underway, and early results have been promising.
A significant limitation in the development of new 225Ac-based TAT radiopharmaceutical agents has arisen from the difficulty in identifying suitable chelating agents for the large Ac3+ ion.5 Although acyclic chelators such as ethylenediamine tetraacetic acid (H4EDTA) and diethylenetriamine pentaacetic acid (H5DTPA) have been shown to possess high affinity for the 225Ac3+ ion, the resulting complexes are labile, leading to release of the free metal ion in biological systems.9 By contrast, many Ac3+ complexes of macrocyclic chelators are substantially more inert. For example, the macrocyclic chelator tetraazacyclododecane-1,4,7,10-tetraacetic acid (H4DOTA) has been used successfully for both small molecule and antibody 225Ac-radioconjugates, albeit with some significant challenges. For example, radiolabeling of H4DOTA with 225Ac either requires high temperature, which are incompatible with macromolecular biomolecules like antibodies, or long incubation times, which yields constructs with relatively low-specific-activities.10-14 Furthermore, serum stability studies have shown that the 225Ac]Ac-DOTA complex dissociates over time,15 a property that is corroborated by animal studies that show deposition of 225Ac in the liver and femur after the administration of DOTA-based conjugates.16 These drawbacks have sparked efforts to develop superior bifunctional chelators for 225Ac.
To accommodate the large ionic radius of Ac3+, researchers have targeted alternative ligands that contain greater than 8 donor atoms to saturate its coordination sphere. For instance, the macrocycles bispa,17 crown,18 macrodipa, and py-macrodipa19 as well as the acyclic chelators octapa,15 py4pa20 and phospha15 have all successfully radiolabeled 225Ac3+ at room temperature in < 60 min. However, not all these chelators form sufficiently stable complexes with 225Ac for biological use.
Among the potential alternative to H4DOTA, the diaza-18-crown-6 macrocyclic chelator H2macropa (Chart 1) has shown significant promise for 225Ac chelation in TAT applications.24,25 This compound is unique by virtue of its high selectivity for large over small lanthanide ions,21 a property that makes it favorable for use with the large Ac3+ ion. In contrast to H4DOTA, this chelator can quantitatively radiolabel 225Ac at room temperature in 5 mins and form complexes that are sufficiently stable for long-term biological applications. The success of this chelator for use with 225Ac has been demonstrated by several studies that have used it in conjunction with small-molecule and antibody-based targeting vectors.26-29
Chart 1.
Chelators Discussed in this Work.
The efficacy of H2macropa suggests that this structural archetype is valuable for Ac3+ chelation. Building upon this scaffold, two new analogues of H2macropa were investigated. These compounds are rigidified versions of the parent chelator, containing either one (H2BZmacropa) or two (H2BZ2macropa) benzene rings within the 18-membered macrocycle (Chart 1). We hypothesized that the decreased conformational flexibility of these new analogues would preorganize them for more effective and stable chelation of the Ac3+ ion.30-35 This study presents a comprehensive comparative investigation of these new chelators with respect to their La3+ and Ac3+-complexing properties. Furthermore, a bifunctional chelator, H2BZmacropa-NCS, and its conjugate to the glypican-3 (GPC3)-targeting antibody codrituzumab36 (GC33) were prepared. The resulting 225Ac-labeled GC33-BZmacropa conjugate was then assessed in an in vivo mouse model of liver cancer. Collectively, the results from this study highlight the importance of ligand design principles in developing effective chelating agents for TAT applications.
RESULTS AND DISCUSSION
Chelate Syntheses.
The previously reported ligands, H2macropa21 and H2BZmacropa,37 and the novel ligand H2BZ2macropa were synthesized via adaptations of procedures that have been used to access related macrocyclic chelators.38 Briefly, the ligands were synthesized by the alkylation of the corresponding diaza-18-crown-6 macrocycle at the secondary amine nitrogen with 6-(bromomethyl)pyridine-2-carboxylic acid methyl ester followed by acid hydrolysis of the ester functional groups (Scheme S1-S2). The ligands were fully characterized by NMR spectroscopy, mass spectrometry, elemental analysis, and analytical HPLC (Figures S1-S29).
Coordination Chemistry with La3+.
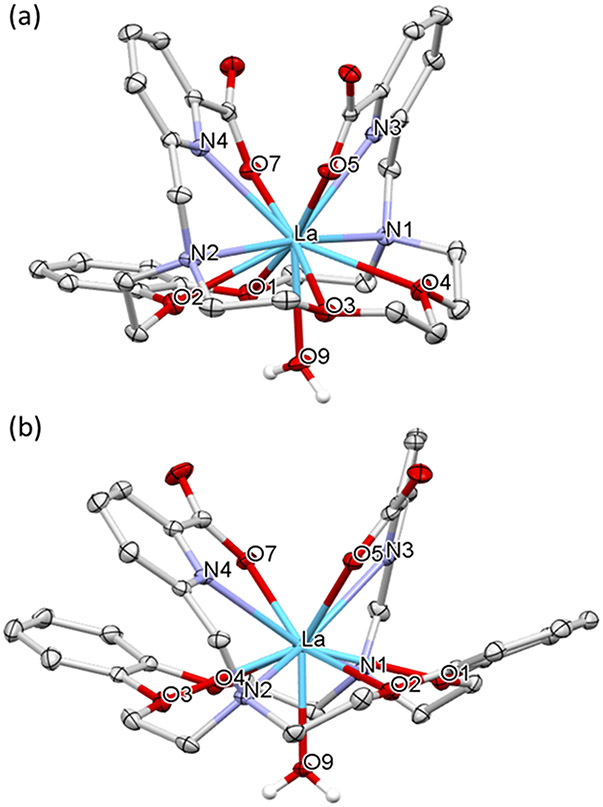
Because no stable isotopes of Ac3+ exist, the coordination chemistries of H2BZmacropa and H2BZ2macropa were investigated using La3+. As the largest lanthanide, this ion has a similar ionic radius, hydrolysis constant, and hard-soft acid-base properties to Ac3+, thus rendering it a suitable model.5,39 Treatment of equimolar ratios of LaCl3 with each compound in water at neutral pH followed by salt metathesis with KPF6 led to the precipitation of the [LaL(H2O)]PF6 complexes, which were crystallized via the slow evaporation of concentrated aqueous solutions at room temperature. The structures of these complexes (Figure 1), as determined by X-ray crystallography, are comparable to that of [La(macropa)(H2O)]+.24 In all three complexes, the La3+ center attains an 11-coordinate geometry with 10 donors provided by the macrocyclic ligands and the 11th arising from an inner-sphere water molecule that interpenetrates the macrocyclic core.
Figure 1.
Crystal structures of (a) [La(BZmacropa)(H2O)](PF6) and (b) [La(BZ2macropa)(H2O)](PF6). Thermal ellipsoids are drawn at the 50% probability level. Outer-sphere solvents, counter-anions, and hydrogen atoms attached to carbon centers are omitted for clarity. Color scheme: La = blue; O = red; N = violet; C = grey.
Within the macropa2− and BZmacropa2− structures, the metal ion sits above the mean plane of the O-atoms in the macrocycle (O1─O4), resulting in near linear N1─La─N2 angles of 178.04° and 172.41°, respectively. By contrast, the structure of [La(BZ2macropa)(H2O)]+ shows that all the donor atoms of the macrocyclic base reside in a plane below the La3+ center, rendering the N1─La─N2 angle to be much more acute (148.77°). This distortion is most likely a consequence of the presence of the two rigid benzene groups, which lead to additional strain in the macrocycle of BZ2macropa2− upon coordination to the La3+ backbone.
In addition to this distortion, there are also significant differences with respect to the interatomic distances between the donor atoms and the La3+ center within these structures. For example, the inner coordination sphere of the La3+ ion in the BZmacropa complex is relatively asymmetric; the La─N distances on the macrocycle are 2.857 and 2.957 Å, representing a significant difference of 0.1 Å. Furthermore, the La─O distances from the aryl ether oxygens of the macrocycle are significantly disparate at 2.772 and 3.058 Å. By contrast, the crystal structure of [La(BZ2macropa)(H2O)]+ is relatively symmetric with chemically equivalent donor atoms interacting with the La3+ center at similar distances. However, the La─O distances within the macrocycle fall between 2.83 and 2.96 Å. By contrast, the crystal structure of [La(macropa)]+ has La─O distances that are much shorter (between 2.70 and 2.79 Å). Thus, the aryl ethers donors conferred by the benzene ring appear to interact more weakly than the aliphatic ethereal donors, as evidenced by the longer interatomic distances in the former.
Solution Thermodynamics with La3+.
To assess the effects of the presence of the rigid benzene groups on the metal-binding properties of H2BZmacropa and H2BZ2macropa, the thermodynamic stabilities of their La3+ complexes were measured via pH potentiometric titrations. The protonation constants of H2BZmacropa and H2BZ2macropa and the stability constants of their La3+ complexes are collected in Table 1. For both the chelators, a total of four protonation constants were observed over a pH range of 2.5–11.3. The first and the second protonation constants most likely correspond to the sequential protonation of the macrocyclic amine nitrogen atoms, whereas the third and the fourth protonation constants are assigned to the protonation at the picolinate pendent arms. Although there are six basic sites on the chelators, the fifth and sixth protonation constants could not be determined using the pH ranges employed in these titrations. The sum of the first and second protonation constants of the rigid compounds are 0.8 and 1.3 log units, respectively, lower than that of macropa, indicating that the addition of phenyl groups increases the acidity of the macrocycles. This result is an expected consequence of the electron-withdrawing nature of the phenyl groups and is consistent with previously reported data on similar macrocyclic chelators.40-42 It should be noted that the protonation constants for H2BZmacropa have been previously reported,37 and these values—in particular the most basic protonation constant—differ somewhat from those measured in this study. The use of different media for ionic strength (0.1 M KCl vs 0.1 M KNO3) could partly explain these discrepancies. In any case, the pH potentiometric titrations in the present study were carried out in triplicate and are reported with a high degree of confidence.
Table 1.
Protonation Constants of Various Chelators and the Thermodynamic Stability Constants of Corresponding La3+ Complexes Determined by pH Potentiometry (25 °C and I = 0.1 M KCl).
| H2macropa | H2BZmacropa | H2BZ2macropa | H4DOTA | H5DTPA | H4EDTA | |
|---|---|---|---|---|---|---|
| log K1 | 7.41(1)c | 7.06(2) | 6.89(7) | 11.14d | 10.45e | 10.17e |
| log K2 | 6.85(1)c | 6.41(1) | 6.11(3) | 9.69d | 8.53e | 6.11e |
| log K3 | 3.32(1)c | 3.35(2) | 3.36(3) | 4.84d | 4.28e | 2.68e |
| log K4 | 2.36(1)c | 2.41(9) | 2.26(9) | 3.95d | 2.65e | 2.00e |
| log K5 | 1.69(1)c | - | 1.82e | 1.5e | ||
| log KLaL | 14.99(2) c | 13.99(5) | 12.04(6) | 21.7(1) d | 19.48 e | 15.46 e |
| log KLaHL | 2.28(3) c | - | - | 2.5(2) d | - | 2.24 e |
| log K´Laa | 14.63 | 13.81 | 11.92 | 15.67 | 15.27 | 12.67 |
| pLab | 15.58 | 14.77 | 12.87 | 16.62 | 16.22 | 13.62 |
After obtaining the protonation constants, the La3+ stability constants (log KLaL) of the H2BZmacropa and H2BZ2macropa complexes were determined by pH potentiometric titrations (Table 1). The log KLaL values decrease upon the addition of phenyl groups to the macrocyclic backbone, following the sequence log KLamacropa > log KLaBZmacropa > log KLaBZ2macropa. The stability constant for the La3+ complex of H2BZmacropa (log KLaL = 13.99) is approximately 1 log unit lower than that of H2macropa (log KLaL = 14.99). The introduction of a second phenyl group in H2BZ2macropa leads to an even greater destabilization of the complexes, as reflected by log KLaL (log KLaL = 12.04) that is almost 2 log units lower than that of H2BZmacropa. In order to account for the effect of protonation equilibria of the ligands on complex stability, the conditional stability constants (log K′LaL) and pLa (the negative log of the free metal concentration in a solution containing 10−6 M metal ion and 10−5 M chelator) at biologically relevant pH = 7.4 were calculated (Table 1). The trends in these conditional stability constants follow those of the absolute values with log K′Lamacropa = 14.63, log K′LaBZmacropa = 13.81, and log K′LaBZ2macropa = 11.92. Although ligand rigidification should in principle lead to enhanced metal complex stability, this effect may depend on several factors including the type of metal employed. For example, a previous study of ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (H4EGTA) and an analogue of H4EGTA with a phenyl backbone showed that enhanced stability was exhibited for the Ca2+ complex, but stability decreased for the La3+ complex.42 The reduced stability observed in the cases of H2BZmacropa and H2BZ2macropa may thus be a consequence of several different factors.
Although the overall LaL stability constants for the rigid ligands are lower than that of H2macropa, the absolute values exceed 10, suggesting that they form sufficiently strong complexes for use in 225Ac TAT.
DFT Calculations.
In order to better understand the La3+ stability constant trends, density functional theory (DFT) was employed. The geometries of [La(macropa)(OH2)]+, [La(BZmacropa)(OH2)]+, and [La(BZ2macropa)(OH2)]+ were first optimized. The resulting structures are qualitatively similar to the experimentally determined X-ray crystal structures, but exhibit a systematic elongation of the La donor atom distances within the macrocycles of approximately 0.05–0.10 Å. More importantly, however, the relatively asymmetry of [La(BZmacropa)(OH2)]+ is captured by the DFT optimization, thus validating this level of theory. With suitably optimized structures, the ΔG° values for the transchelation reaction between [La(macropa)(H2O)]+ and either BZmacropa2− or BZ2macropa2− were calculated.
For both of these reactions, the ΔG° was found to be > 0, indicating that both BZmacropa2− and BZ2macropa2− form less thermodynamically stable complexes with La3+ than macropa2−. Furthermore, ΔG° for the transchelation with BZ2macropa2− was approximately 10 kJ/mol greater than that for BZmacropa2−. Collectively, these computational results are consistent with our experimental data that show BZ2macropa2− forms the least stable La3+ complex of these three ligands.
Having validated our computational method against the experimental results, we analyzed the different factors that contribute to ΔG° for these reactions. As described in the Supporting Information (Section S1.6), ΔG° can be expressed as the sum ΔΔGS° + ΔΔGB°, where ΔΔGS° is the relative ligand strain energy and ΔΔGB° is the relative metal ion-binding energy (see SI section S1.6 for more details).35,38,43,44 The strain energy (ΔGS°) is defined as the free energy change required to distort the geometrically relaxed free ligand (L) to a conformation that is suitable for metal ion-binding (Lstrain, eq. S1), and the binding energy (ΔGB°) is defined as the free energy change associated with the incorporation of the La3+ ion into the ligand in its preorganized metal-binding conformation (eq. S2). The relative (ΔΔG) values, compared to macropa2−, are given below.
For both BZmacropa2− and BZ2macropa2− ΔΔGS° are negative, indicating that these compounds undergo a significantly smaller free energy penalty for attaining an appropriate metal-binding conformation than macropa2−. This result is consistent with role of the rigid benzene groups in enhancing the metal-binding preorganization of these ligands. By contrast, the ΔΔGB° values for both rigid chelators are positive, a result that suggests that macropa forms stronger binding interactions with the La3+ ion. The weaker binding energies of H2BZmacropa and H2BZ2macropa, compared to H2macropa, is most likely a consequence of their decreased basicity and donor strength. As noted above, the electron-withdrawing phenyl groups embedded in the macrocycle decreases their basicity, as determined experimentally via protonation constant measurements. This decreased basicity also renders them less effective Lewis bases for binding with La3+. To further verify this conclusion computationally, the ΔG° for a simple model ligand substitution reaction on La3+ was calculated. In this substitution reaction, the ΔG° values required to displace dimethyl ether, a model for the aliphatic ether donors in H2macropa, with anisole, an aromatic ether that models the donors on H2BZmacropa and H2BZ2macropa. These calculations revealed that displacement of dimethyl ether by anisole is thermodynamically uphill (ΔG° = 17.22 kJ/mol). Thus, the weaker donor strengths of the aromatic ethers is a plausible explanation for the positive ΔΔGB° determined for H2BZmacropa and H2BZ2macropa. Together, these results reveal a counterbalancing effect of the introduction of the rigid phenyl groups. Although these rigid phenyl groups provide a favorable entropic contribution by preorganizing the ligands for metal binding, their weaker donor strengths provide a poorer enthalpic contribution to La3+ binding. For BZmacropa2− and BZ2macropa2−, the weaker binding strength outweighs the benefits obtained in ligand preorganization.
Radiolabeling and Stability Studies.
With the comprehensive characterization of the La3+ complexes of BZmacropa2− and BZ2macropa2−, which revealed them to be effective ligands for this ion, their ability to chelate Ac3+ was investigated. The chelators (~300 μM) were incubated at room temperature with 70 μCi (2.6 Mbq) [225Ac]Ac(NO3)3 in water containing NH4OAc (0.1 M) at pH 5.5. Under these conditions, both ligands, as well as macropa, quantitatively radiolabeled 225Ac3+ in only 30 min, resulting in high specific activities of approximately 7.5 Ci/g (260 TBq/g). Instant thin-layer chromatography (ITLC) chromatograms, showing the complex formation for these ligands and macropa, are in the Supporting Information, Figures S63-S66. These results, particularly the room temperature radiolabeling, confirm the high efficacy of expanded macrocyclic ligands like macropa2− for chelation of the large Ac3+ ion.
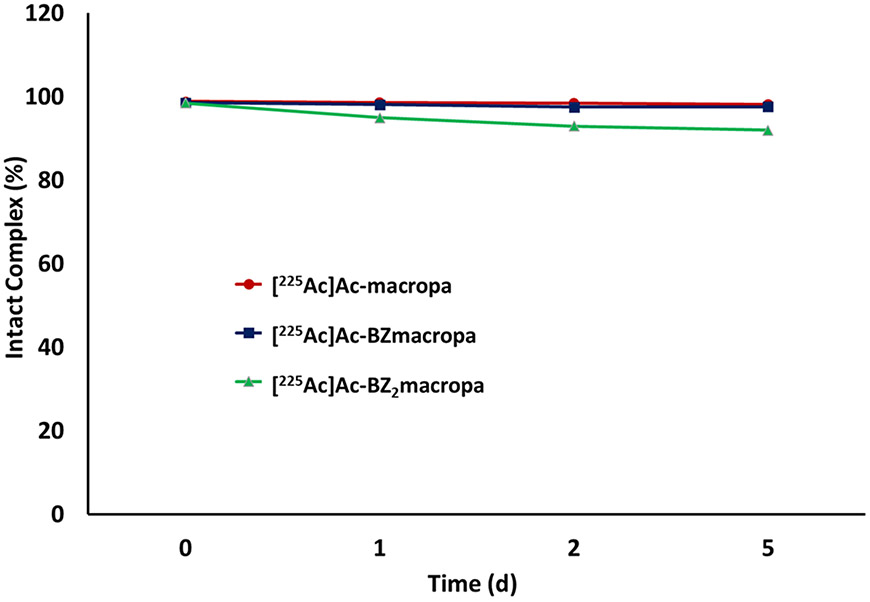
Having demonstrated the rapid and high specific activity radiolabeling with H2BZmacropa and H2BZ2macropa, the stabilities of the resulting Ac3+ complexes in whole human serum at 37°C were evaluated (Figure 2, S70-S72). Under these conditions, all complexes were >90% intact after 5 days. Even with this high uniform stability among the complexes several trends could be discerned. Specifically, increasing stability of the complexes followed the order BZ2macropa2− < BZmacropa2− < macropa2−. This trend matches that observed with the thermodynamic stability constant data of the La3+ complexes that was rationalized with DFT calculations, described above.
Figure 2.
Stability of 225Ac complexes in human serum over time. Stability was measured using ITLC condition 1 as detailed in the Supporting Information.
Bifunctional Chelator, H2BZmacropa-NCS.
The high stability of the 225Ac complex of BZmacropa prompted us to prepare a bifunctional version of this chelator. For this purpose, we targeted the chelator H2BZmacropa-NCS (Chart 1), which contains an amine-reactive isothiocyanate group appended to the phenyl ring of the macrocyclic backbone. The synthesis of this compound (Scheme 1) commenced from the macrocycle 3 which upon treatment with 1 equiv. of KNO3 in trifluoracetic acid afforded the monosubstituted nitro-product 8 in near quantitative yield. The addition of the pendent arms to the nitrated macrocycle was accomplished by alkylation of 8 at the secondary amine nitrogen with 6-(bromomethyl)pyridine-2-carboxylic acid methyl ester. Reduction of the nitrated compound 9 using H2 over Pd/C followed by acid hydrolysis of the ester functional groups yielded H2BZmacrcopa-NH2. Finally, the isothiocyanate functional group was installed onto the macrocycle via the treatment of H2BZmacrcopa-NH2 with excess thiophosgene. The compound H2BZmacrcopa-NCS was fully characterized by NMR spectroscopy, mass spectrometry, and analytical HPLC (Figures S30-S40).
Scheme 1.
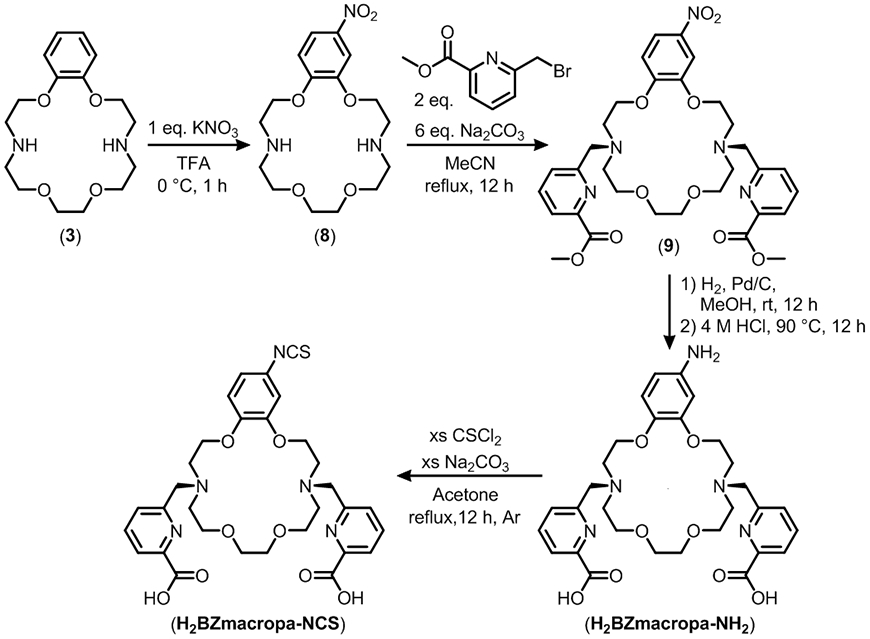
Synthetic scheme for H2BZmacrop-NCS.
A key difference between the first-generation bifunctional chelator H2macropa-NCS (Chart 1) and H2BZmacropa-NCS is the location of the amine-reactive –NCS group. On H2BZmacropa-NCS, this functional group is attached to the macrocycle backbone, whereas for H2macropa-NCS it is on one of the picolinate pendent arms. Consequently, the synthesis of the H2BZmacropa-NCS is more modular in that it proceeds through intermediate 8, which can potentially be functionalized with a diverse range of different pendent donor arms to access a library of different bifunctional chelators. Furthermore, the H2BZmacropa-NCS is relatively easier to synthesize as compared to H2macropa-NCS. For instance, the synthesis of H2macropa-NCS involves a 9-step pathway starting from the macrocycle, while the synthesis of H2BZmacropa-NCS is carried out in 4–5 steps. The location of the –NCS functional group also has important effects on its hydrolytic stability. For instance, a solution of H2macropa-NCS in pH 9.1 NaHCO3 buffer at room temperature completely hydrolyzes to the amine H2macropa-NH2 in approximately 5 h (t1/2 = 1.25 h). Under the same conditions, the hydrolysis of H2BZmacropa is significantly slower (t1/2 = 56 h), requiring over a week for near complete formation of the amine. The location of the –NCS group of H2macropa-NCS directly on the electron-deficient picolinate group increases its electrophilicity, rendering it more reactive and less stable than the –NCS group of H2BZmacropa-NCS that is linked to a more electron-rich phenyl group. The increased stability of H2BZmacropa-NCS marks a potential advantage over H2macropa-NCS, for H2BZmacropa-NCS may be shipped at room temperature and stored for extended periods of time, rendering it more broadly accessible to researchers across the globe.
Antibody Conjugation.
To further evaluate the suitability of H2BZmacropa-NCS as a bifunctional chelator, this compound was conjugated to the antibody GC33, which targets the GPC3 receptor overexpressed in many liver cancers. The H2BZmacropa-NCS conjugate of GC33 (GC33-BZM) was prepared using standard antibody-isothiocyanate coupling conditions, which use a slight molar excess (2.5–3 equiv.) of chelator exposed to the antibody in bicarbonate buffer at 37 °C. These conditions were also applied to prepare the macropa conjugate of GC33 (GC33-M) and the H2BZmacropa-NCS conjugate of Obinutuzumab (OBI-BZM), an antibody that does not bind GPC3. The conjugates were subjected to gel-permeation chromatography to remove any unconjugated bifunctional chelator, and their purities were verified by size-exclusion HPLC (Figures S52-S54).
After purification, the average chelator:antibody ratios were determined. Previous studies have shown that presence of too many covalently attached chelators on antibodies can have negative consequences on their immunoreactivity and pharmacokinetic properties. As such, a chelator:antibody ratio of <5 is generally desirable for radioconjugates.45-47 The chelator:antibody ratios were assessed using a modification of the previously reported colorimetric arsenazo assay (Figures S58-S59).48 All conjugates were found to have chelator:antibody ratios of approximately 1:1 using this method, indicating that minimal alteration of the antibody was achieved. To provide further verification of these results, the chelator:antibody ratios of GC33-M and GC33-BZM were also determined using UPLC-HRMS. The resulting mass spectra, shown in Figures S55-S57, correspond well with the chelate:antibody ratios determined using the arsenazo method.
Having established the degree of functionalization of these conjugates, we next investigated the impact of this modification on the immunoreactivity of GC33 using bio-layer interferometry (BLI). The exposure of various concentrations of antibody-chelator conjugates to immobilized GPC3 antigen revealed concentration-dependent binding of the antibody to the surface and subsequent dissociation. The combined association and dissociation kinetic data across all concentrations were fit using the global fitting model in the Octet Analysis Studio software (Figures S60-S62). These data revealed Kd values of 0.19, 0.14, and 0.042 nM for GC33-BZM, GC33-M, and GC33 respectively. Taken together, the results show that both GC33-BZM and GC33-M retained comparably high binding affinity for GPC3 that was slightly diminished relative to the free GC33 antibody.
Radiolabeling and Stability Studies with Chelator-Antibody Conjugates.
After confirming the identity, purity, and antigen-binding affinity of the antibody conjugates, they were radiolabeled with 225Ac following a previously reported procedure that was used for GC33-M.29 Under these conditions, a solution of antibody conjugates (450 μg) was added to a solution of 225Ac (150 μCi, 5.55 MBq) in aqueous 0.1 M NH4OAc (pH 5.5). The mixtures were incubated at room temperature for 30 min, after which the completion of the radiolabeling was checked with ITLC (Condition 2). Purification of the radioconjugates by gel permeation chromatography afforded them in >95% purity, as verified by ITLC (Condition 2) (Figures S67-S69). The mild conditions and rapid reaction time required for these radiolabelings highlight the suitability of these chelators for antibody radioconjugates of 225Ac. Importantly, the rapid room temperature radiolabeling afforded by these conjugates is critical for minimizing radiolytic damage and thermal aggregation or denaturation of the antibody.
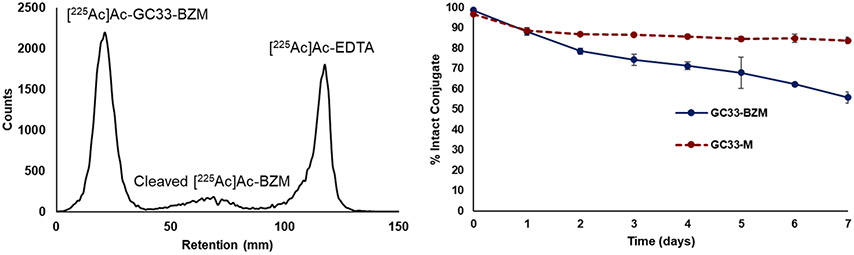
The stabilities of [225Ac]Ac-GC33-BZM and [225Ac]Ac-GC33-M were evaluated in whole human serum at 37°C over several days (Figure 3, S73-S74). Radio-ITLC analysis (Condition 2) revealed that [225Ac]Ac-GC33-M remained > 90% intact over 7 days, highlighting the excellent stability of the 225Ac complex of the bifunctional macropa-NCS2− chelator. By contrast, the radioconjugate [225Ac]Ac-GC33-BZM exhibited marked degradation over the course of the experiment, with approximately 55% intact complex remaining after 7 days. The reduced stability of the [225Ac]Ac-GC33-BZM conjugate relative to the unconjugated ligand-225Ac complex, which showed good stability in human serum, may arise from the electron-withdrawing effect of the thiourea moiety on the ligand backbone, which decreases the donating capacity of the ligand. Notably, for both GC33-M and GC33-BZM radioconjugates, ITLC traces revealed the presence of 3 distinct species in solution: intact conjugate, “free” 225Ac chelated by the mobile phase EDTA, and a third unknown species. This third species reached approximately 10% of the total activity over the course of 1 day, then remained constant throughout the experiment. We hypothesize that this unknown product may be [225Ac]Ac-macropa or [225Ac]Ac-BZmacropa that has been cleaved from the antibody by radiolysis of the thiourea linker, for extensive radiolysis is often observed in solutions containing 225Ac.18,49
Figure 3.
(Left) ITLC chromatogram of [225Ac]Ac-GC33-BZM after 7 day incubation in human serum at 37°C. (Right) Stability over time of [225Ac]Ac-GC33-BZM and [225Ac]Ac-GC33-M in human serum. Data points are the average of 3 independent samples and error bars represent the standard deviation.
Biodistribution Studies.
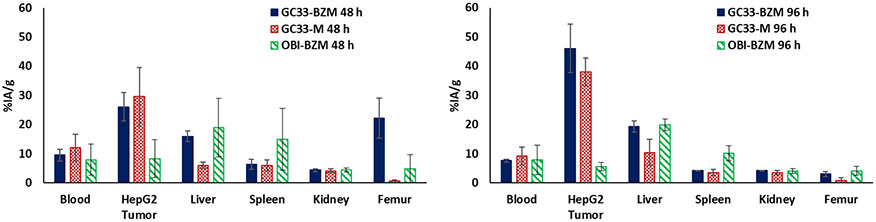
Despite the lower stability of the [225Ac]Ac-GC33-BZM conjugate relative to [225Ac]Ac-GC33-M, we hypothesized that the former may still have sufficient stability for tumor targeting in vivo. To test whether [225Ac]Ac-GC33-BZM could successfully target GPC3+ tumors in vivo, we treated athymic nude mice bearing HepG2 (GPC3+) xenografts with 100 nCi (3.7 kBq) of [225Ac]Ac-GC33-BZM, 225Ac[Ac]-GC33-M (positive control), and [225Ac]Ac-OBI-BZM (isotype control). After 48 and 96 h, mice (n = 3–4) were euthanized, and their organs were weighed and counted using a gamma counter after allowing 24 h for daughter isotope equilibration. The % injected-activity per gram (IA/g) in each organ was then calculated (Figure 4, S75-S77). As expected, the isotype control [225Ac]Ac-OBI-BZM, which does not target the GPC3+ liver cancer xenografts, showed no appreciable tumor uptake at any time point. By contrast, [225Ac]Ac-GC33-BZM demonstrated clear, specific tumor uptake at both 48 and 96 h post-injection, with tumor signal greater than that of all organs at both time points. Importantly, the tumor uptake of [225Ac]Ac-GC33-BZM was effectively indistinguishable from that of 225Ac[Ac]-GC33-M, indicating that these conjugates have comparable targeting capability. “However, the off-target accumulation of 225Ac in both the liver and femur was higher after administration of [225Ac]Ac-GC33-BZM than for 225Ac[Ac]-GC33-M.” A similar degree of liver uptake was also observed in the non-targeted isotype control conjugate [225Ac]Ac-GC33-BZM. Unchelated 225Ac has previously been shown to localize to the liver almost exclusively. Therefore, the higher liver uptake measured for both [225Ac]Ac-GC33-BZM and [225Ac]Ac-OBI-BZM may be a consequence of partial instability of [225Ac]Ac-BZmacropa complex under these conditions.9 Despite these drawbacks, [225Ac]Ac-GC33-BZM still demonstrates significant tumor uptake, suggesting that BZmacropa-NCS may be viable as a bifunctional chelator for in vivo studies of 225Ac-labeled antibody conjugates.
Figure 4.
Selected organ biodistribution of 225Ac-labeled GC33-BZM (solid, blue bar), 225Ac-labeled GC33-M (checkered, red bar), and 225Ac-labeled OBI-BZM (striped, green bar) at 48 h (left panel) and 96 h (right panel) after administration (n = 3–4). Full biodistribution data can be found in the SI, Figures S58-S60.
CONCLUSION
In summary, two rigid variants of the first-generation 225Ac chelator H2macropa were synthesized by incorporating either one or two benzene rings into the macrocyclic base. Our comprehensive coordination chemistry studies of these ligands with La3+, a non-radioactive surrogate for Ac3+, revealed that they form complexes of lower stability than H2macropa. DFT calculations were used to show that the use of phenyl groups in the backbone enhances their preorganization at the cost of their overall enthalpy of metal binding. These results highlight the different factors that need to be taken into consideration in designing and optimizing chelators for nuclear medicine applications. Despite their lower thermodynamic stability with La3+, 225Ac-radiolabeling studies with these ligands proceeded effectively, yielding high specific activity compounds within minutes at room temperature. Thus, these chelators are among the few candidates that can bind 225Ac under such mild conditions, rendering them valuable for use with sensitive macromolecular targeting vectors.
Based on these promising results, the bifunctional variant H2BZmacropa-NCS was synthesized. In contrast to other large macrocyclic bifunctional chelators for 225Ac, H2BZmacropa-NCS places the reactive functional group directly on the macrocycle rather than the pendent arms. This design difference has two important implications. First, the synthetic approach is modular in that it allows for the installation of different pendent donor groups without perturbing the functional group handle on the macrocycle. Second, the stability of the –NCS functional group is substantially enhanced relative to that of the first-generation macropa-NCS. For this first-generation analogue, the –NCS group is installed on the pendent picolinate group and hydrolyzes rapidly both in the solid and solution state. These latter properties have made shipping and storage of this compound challenging. The enhanced hydrolytic stability of H2BZmacropa-NCS, by contrast, should make it more accessible to other researchers. To further evaluate the suitability of this new bifunctional chelator, it was conjugated to the antibody GC33, which can be employed for targeting liver cancers that express GPC3. Consistent with our expectations based on the small-molecule chelators, radiolabeling of the GC33-BZM proceeded rapidly under mild, room temperature conditions. However, our serum stability and biodistribution studies revealed the 225Ac-labeled GC33-BZM to be less stable than the macropa conjugate, yet still capable of delivering this radionuclide to the tumor site. Although the serum and in vivo stability of the 225Ac-labeled GC33-BZM present some concerns, further optimization of this new bifunctional chelator, via modification of either the pendent donor arms or extension of the linker between the reactive –NCS group and the aromatic ring, may afford complexes of enhanced stability suitable for further clinical development. Alternatively, the use of H2BZmacropa-NCS for other promising α-emitting radionuclides also warrants investigation. These efforts are currently underway within our labs.
Supplementary Material
ACKNOWLEDGMENT
A.P.K., S.F., K.E.B, and F.E.E acknowledge the support from Intramural Research Program funds ZIA BC 011800 and ZIA BC 010891. K.J.K. and J.J.W. were supported by Department of Energy Basic Energy Sciences (award No. DE-SC0021662) and National Institutes of Biomedical Imaging and Bioengineering of the National Institutes of Health (award numbers R21EB027282 and R01EB02925). J.J.W also acknowledge the support from Research Corporation for Science Advancement through a Cottrell Research Scholar Award. We are grateful to Chugai Pharmaceutical for providing codrituzumab (GC33).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Synthetic procedures, experimental details, and supplementary figures. (PDF)
X-ray crystal data (CIF)
DFT optimized geometries (ZIP)
REFERENCES
- (1).Parker C; Nilsson S; Heinrich D; Helle SI; O’Sullivan JM; Fosså SD; Chodacki A; Wiechno P; Logue J; Seke M; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med 2013, 369 (3), 213–223. [DOI] [PubMed] [Google Scholar]
- (2).Abou DS; Thiele NA; Gutsche NT; Villmer A; Zhang H; Woods JJ; Baidoo KE; Escorcia FE; Wilson JJ; Thorek DLJ Towards the Stable Chelation of Radium for Biomedical Applications with an 18-Membered Macrocyclic Ligand. Chem. Sci 2021, 12 (10), 3733–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Nelson BJB; Andersson JD; Wuest F Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2021, 13 (1), 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).King AP; Lin FI; Escorcia FE Why Bother with Alpha Particles? Eur. J. Nucl. Med. Mol. Imaging 2021, 49 (1), 7–17. [DOI] [PubMed] [Google Scholar]
- (5).Thiele NA; Wilson JJ Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm 2018, 33 (8), 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yadav MP; Ballal S; Sahoo RK; Tripathi M; Seth A; Bal C Efficacy and Safety of 225Ac-PSMA-617 Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Theranostics 2020, 10 (20), 9364–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ballal S; Yadav MP; Bal C; Sahoo RK; Tripathi M Broadening Horizons with 225Ac-DOTATATE Targeted Alpha Therapy for Gastroenteropancreatic Neuroendocrine Tumour Patients Stable or Refractory to 177Lu-DOTATATE PRRT: First Clinical Experience on the Efficacy and Safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47 (4), 934–946. [DOI] [PubMed] [Google Scholar]
- (8).Tagawa ST; Sun M; Sartor AO; Thomas C; Singh S; Bissassar M; Fernandez E; Niaz MJ; Ho B; Vallabhajosula S et al. Phase I Study of 225Ac-J591 for Men with Metastatic Castration-Resistant Prostate Cancer (MCRPC). J. Clin. Oncol 2021, 39 (15_suppl), 5015–5015. [Google Scholar]
- (9).Davis IA; Glowienka KA; Boll RA; Deal KA; Brechbiel MW; Stabin M; Bochsler PN; Mirzadeh S; Kennel SJ Comparison of 225Actinium Chelates: Tissue Distribution and Radiotoxicity. Nucl. Med. Biol 1999, 26 (5), 581–589. [DOI] [PubMed] [Google Scholar]
- (10).Maguire WF; McDevitt MR; Smith-Jones PM; Scheinberg DA Efficient 1-Step Radiolabeling of Monoclonal Antibodies to High Specific Activity with 225Ac for α-Particle Radioimmunotherapy of Cancer. J. Nucl. Med 2014, 55 (9), 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mesude B; Katharina L; Teja K; Phelps ME; Strand S; Morris MJ; Radu CG; Damoiseaux R; Peltola MT; Peekhaus N; et al. Genetic Signature of Prostate Cancer Mouse Models Resistant to Optimized HK2 Targeted α-Particle Therapy. Proc. Natl. Acad. Sci 2020, 117 (26), 15172–15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lakes AL; An DD; Gauny SS; Ansoborlo C; Liang BH; Rees JA; McKnight KD; Karsunky H; Abergel RJ Evaluating 225Ac and 177Lu Radioimmunoconjugates against Antibody–Drug Conjugates for Small-Cell Lung Cancer. Mol. Pharm 2020, 17 (11), 4270–4279. [DOI] [PubMed] [Google Scholar]
- (13).Sudo H; Tsuji AB; Sugyo A; Kaneko MK; Kato Y; Nagatsu K; Suzuki H; Higashi T Preclinical Evaluation of Podoplanin-Targeted Alpha-Radioimmunotherapy with the Novel Antibody NZ-16 for Malignant Mesothelioma. Cells 2021, 10 (10), 2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sudo H; Tsuji AB; Sugyo A; Harada Y; Nagayama S; Katagiri T; Nakamura Y; Higashi T FZD10-Targeted α-Radioimmunotherapy with 225Ac-Labeled OTSA101 Achieves Complete Remission in a Synovial Sarcoma Model. Cancer Sci. 2022, 113 (2), 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ramogida CF; Robertson AKH; Jermilova U; Zhang C; Yang H; Kunz P; Lassen J; Bratanovic I; Brown V; Southcott L; et al. Evaluation of Polydentate Picolinic Acid Chelating Ligands and an α-Melanocyte-Stimulating Hormone Derivative for Targeted Alpha Therapy Using ISOL-Produced 225Ac. EJNMMI Radiopharm. Chem 2019, 4 (1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Deal KA; Davis IA; Mirzadeh S; Kennel SJ; Brechbiel MW Improved in Vivo Stability of Actinium-225 Macrocyclic Complexes. J. Med. Chem 1999, 42 (15), 2988–2992. [DOI] [PubMed] [Google Scholar]
- (17).Comba P; Jermilova U; Orvig C; Patrick BO; Ramogida CF; Rück K; Schneider C; Starke M Octadentate Picolinic Acid-Based Bispidine Ligand for Radiometal Ions. Chem. Eur. J 2017, 23 (63), 15945–15956. [DOI] [PubMed] [Google Scholar]
- (18).Yang H; Zhang C; Yuan Z; Rodriguez-Rodriguez C; Robertson A; Radchenko V; Perron R; Gendron D; Causey P; Gao F; et al. Synthesis and Evaluation of a Macrocyclic Actinium-225 Chelator, Quality Control and In Vivo Evaluation of 225Ac-Crown-AMSH Peptide. Chem. Eur. J 2020, 26 (50), 11435–11440. [DOI] [PubMed] [Google Scholar]
- (19).Hu A; Brown V; MacMillan SN; Radchenko V; Yang H; Wharton L; Ramogida CF; Wilson JJ Chelating the Alpha Therapy Radionuclides 225Ac3+ and 213Bi3+ with 18-Membered Macrocyclic Ligands Macrodipa and Py-Macrodipa. Inorg. Chem 2022, 61 (2), 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Li L; Rousseau J; de G.Jaraquemada-Peláez M; Wang X; Robertson A; Radchenko V; Schaffer P; Lin K-S; Bénard F; Orvig C 225Ac-H4Py4pa for Targeted Alpha Therapy. Bioconjug. Chem 2021, 32 (7), 1348–1363. [DOI] [PubMed] [Google Scholar]
- (21).Roca-Sabio A; Mato-Iglesias M; Esteban-Gómez D; Tóth É; de Blas A; Platas-Iglesias C; Rodríguez-Blas T Macrocyclic Receptor Exhibiting Unprecedented Selectivity for Light Lanthanides. J. Am. Chem. Soc 2009, 131 (9), 3331–3341. [DOI] [PubMed] [Google Scholar]
- (22).Clarke ET; Martell AE Stabilities of Trivalent Metal Ion Complexes of the Tetraacetate Derivatives of 12-, 13- and 14-Membered Tetraazamacrocycles. Inorg. Chim. Acta 1991, 190 (1), 37–46. [Google Scholar]
- (23).Martell AE, Smith Robert M., Critical Stability Constants. Volume 1,; Plenum Press: New York; London, 1974. [Google Scholar]
- (24).Thiele NA; Brown V; Kelly JM; Amor-Coarasa A; Jermilova U; MacMillan SN; Nikolopoulou A; Ponnala S; Ramogida CF; Robertson AKH; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed 2017, 56 (46), 14712–14717. [DOI] [PubMed] [Google Scholar]
- (25).Hu A; Wilson JJ Advancing Chelation Strategies for Large Metal Ions for Nuclear Medicine Applications. Acc. Chem. Res 2022, 55 (6), 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kelly JM; Amor-Coarasa A; Ponnala S; Nikolopoulou A; Williams C; Thiele NA; Schlyer D; Wilson JJ; DiMagno SG; Babich JW A Single Dose of 225Ac-RPS-074 Induces a Complete Tumor Response in an LNCaP Xenograft Model. J. Nucl. Med 2019, 60 (5), 649–655. [DOI] [PubMed] [Google Scholar]
- (27).Aluicio-Sarduy E; Thiele NA; Martin KE; Vaughn BA; Devaraj J; Olson AP; Barnhart TE; Wilson JJ; Boros E; Engle JW Establishing Radiolanthanum Chemistry for Targeted Nuclear Medicine Applications. Chem. Eur. J 2020, 26 (6), 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Reissig F; Bauer D; Zarschler K; Novy Z; Bendova K; Ludik M-C; Kopka K; Pietzsch H-J; Petrik M; Mamat C Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers. 2021, 13 (8), 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bell MM; Gutsche NT; King AP; Baidoo KE; Kelada OJ; Choyke PL; Escorcia FE Glypican-3-Targeted Alpha Particle Therapy for Hepatocellular Carcinoma. Molecules 2021, 26 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).De Sousa AS; Croft GJB; Wagner CA; Michael JP; Hancock RD Effect of Cyclohexylene Bridges on the Metal Ion Size Based Selectivity of Ligands in Aqueous Solution. Inorg. Chem 1991, 30 (18), 3525–3529. [Google Scholar]
- (31).Brechbiel MW; Gansow OA Synthesis of C-Functionalized Trans-Cyclohexyldiethylenetriaminepenta-Acetic Acids for Labelling of Monoclonal Antibodies with the Bismuth-212 α-Particle Emitter. J. Chem. Soc. Perkin Trans. 1 1992, 9, 1173–1178. [Google Scholar]
- (32).Ferreirós-Martínez R; Esteban-Gómez D; Platas-Iglesias C; de Blas A; Rodríguez-Blas T Zn(II), Cd(II) and Pb(II) Complexation with Pyridinecarboxylate Containing Ligands. Dalt. Trans 2008, 42, 5754–5765. [DOI] [PubMed] [Google Scholar]
- (33).Ramogida CF; Cawthray JF; Boros E; Ferreira CL; Patrick BO; Adam MJ; Orvig C H2CHXDedpa and H4CHXOctapa—Chiral Acyclic Chelating Ligands for 67/68Ga and 111In Radiopharmaceuticals. Inorg. Chem 2015, 54 (4), 2017–2031. [DOI] [PubMed] [Google Scholar]
- (34).Wang X; de G. Jaraquemada-Peláez M; Cao Y; Ingham A; Rodríguez-Rodríguez C; Pan J; Wang Y; Saatchi K; Häfeli UO; Lin K-S; et al. H2CHXHox: Rigid Cyclohexane-Reinforced Nonmacrocyclic Chelating Ligand for [Nat/67/68Ga]Ga3+. Inorg. Chem 2020, 59 (7), 4895–4908. [DOI] [PubMed] [Google Scholar]
- (35).Hu A; Aluicio-Sarduy E; Brown V; MacMillan SN; Becker KV; Barnhart TE; Radchenko V; Ramogida CF; Engle JW; Wilson JJ Py-Macrodipa: A Janus Chelator Capable of Binding Medicinally Relevant Rare-Earth Radiometals of Disparate Sizes. J. Am. Chem. Soc 2021, 143 (27), 10429–10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ishiguro T; Sugimoto M; Kinoshita Y; Miyazaki Y; Nakano K; Tsunoda H; Sugo I; Ohizumi I; Aburatani H; Hamakubo T; Kodama T; Tsuchiya M; Yamada-Okabe H Anti–Glypican 3 Antibody as a Potential Antitumor Agent for Human Liver Cancer. Cancer Res. 2008, 68 (23), 9832–9838. [DOI] [PubMed] [Google Scholar]
- (37).Panchenko PA; Zubenko AD; Chernikova EY; Fedorov YV; Pashanova AV; Karnoukhova VA; Fedyanin IV; Fedorova OA Synthesis, Structure and Metal Ion Coordination of Novel Benzodiazamacrocyclic Ligands Bearing Pyridyl and Picolinate Pendant Side-Arms. New J. Chem 2019, 43 (38), 15072–15086. [Google Scholar]
- (38).Thiele NA; Woods JJ; Wilson JJ Implementing F-Block Metal Ions in Medicine: Tuning the Size Selectivity of Expanded Macrocycles. Inorg. Chem 2019, 58 (16), 10483–10500. [DOI] [PubMed] [Google Scholar]
- (39).Deblonde GJ-P; Zavarin M; Kersting AB The Coordination Properties and Ionic Radius of Actinium: A 120-Year-Old Enigma. Coord. Chem. Rev 2021, 446, 214130. [Google Scholar]
- (40).Corbaux P; Spiess B; Arnaud F; Schwing MJ Complexing Properties of an N,N′-Methylated Diaza-Crown-Ether. Polyhedron 1985, 4 (8), 1471–1473. [Google Scholar]
- (41).Fedorova O; Fedorov Y; Oshchepkov M Complexes of Di- and Triazacrown Ethers with Heavy Metal Ions in Water Solution. Electroanalysis 2012, 24 (8), 1739–1744. [Google Scholar]
- (42).Tei L; Baranyai Z; Botta M; Piscopo L; Aime S; Giovenzana GB Synthesis and Solution Thermodynamic Study of Rigidified and Functionalised EGTA Derivatives. Org. Biomol. Chem 2008, 6 (13), 2361–2368. [DOI] [PubMed] [Google Scholar]
- (43).Wilson JJ; Ferrier M; Radchenko V; Maassen JR; Engle JW; Batista ER; Martin RL; Nortier FM; Fassbender ME; John KD; et al. Evaluation of Nitrogen-Rich Macrocyclic Ligands for the Chelation of Therapeutic Bismuth Radioisotopes. Nucl. Med. Biol 2015, 42 (5), 428–438. [DOI] [PubMed] [Google Scholar]
- (44).Hu A; MacMillan SN; Wilson JJ Macrocyclic Ligands with an Unprecedented Size-Selectivity Pattern for the Lanthanide Ions. J. Am. Chem. Soc 2020, 142 (31), 13500–13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Vosjan MJWD; Perk LR; Visser GWM; Budde M; Jurek P; Kiefer GE; van Dongen GAMS Conjugation and Radiolabeling of Monoclonal Antibodies with Zirconium-89 for PET Imaging Using the Bifunctional Chelate p-Isothiocyanatobenzyl-Desferrioxamine. Nat. Protoc 2010, 5 (4), 739–743. [DOI] [PubMed] [Google Scholar]
- (46).Al-Ejeh F; Darby JM; Thierry B; Brown MP A Simplified Suite of Methods to Evaluate Chelator Conjugation of Antibodies: Effects on Hydrodynamic Radius and Biodistribution. Nucl. Med. Biol 2009, 36 (4), 395–402. [DOI] [PubMed] [Google Scholar]
- (47).Sharma SK; Glaser JM; Edwards KJ; Khozeimeh Sarbisheh E; Salih AK; Lewis JS; Price EW A Systematic Evaluation of Antibody Modification and 89Zr-Radiolabeling for Optimized Immuno-PET. Bioconjug. Chem 2021, 32 (7), 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Dadachova E; Chappell LL; Brechbiel MW Spectrophotometric Method for Determination of Bifunctional Macrocyclic Ligands in Macrocyclic Ligand–Protein Conjugates. Nucl. Med. Biol 1999, 26 (8), 977–982. [DOI] [PubMed] [Google Scholar]
- (49).Hooijman EL; Chalashkan Y; Ling SW; Kahyargil FF; Segbers M; Bruchertseifer F; Morgenstern A; Seimbille Y; Koolen SLW; Brabander T; et al. Development of [225Ac]Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of MCRPC. Pharmaceutics 2021, 13 (5), 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.