PURPOSE
Adagrasib (MRTX849) is an oral, highly selective, small-molecule, covalent inhibitor of KRASG12C. We report results from a phase I/IB study of adagrasib in non–small-cell lung cancer, colorectal cancer, and other solid tumors harboring the KRASG12C mutation.
MATERIALS AND METHODS
Patients with advanced KRASG12C-mutant solid tumors were treated with adagrasib 150 mg orally once daily, 300 mg once daily, 600 mg once daily, 1,200 mg once daily, or 600 mg orally twice a day using an accelerated titration design, which transitioned to a modified toxicity probability interval design when a predefined degree of toxicity was observed or target adagrasib exposure was achieved. Safety, pharmacokinetics, and clinical activity were evaluated.
RESULTS
Twenty-five patients were enrolled and received at least one dose of adagrasib. The recommended phase II dose (RP2D) was 600 mg twice a day on the basis of safety, tolerability, and observed pharmacokinetics properties. No maximum tolerated dose was formally defined. After a median follow-up of 19.6 months, eight of 15 patients (53.3%; 95% CI, 26.6 to 78.7) with RECIST-evaluable KRASG12C-mutant non–small-cell lung cancer treated at 600 mg twice a day achieved a confirmed partial response. The median duration of response was 16.4 months (95% CI, 3.1 to not estimable). The median progression-free survival was 11.1 months (95% CI, 2.6 to not estimable). One of two patients with KRASG12C-mutant colorectal cancer treated at 600 mg twice a day achieved a partial response (duration of response, 4.2 months). At the RP2D, the most common treatment-related adverse events (any grade) were nausea (80.0%), diarrhea (70.0%), vomiting (50.0%), and fatigue (45.0%). The most common grade 3-4 treatment-related adverse event was fatigue (15.0%).
CONCLUSION
Adagrasib 600 mg twice a day was well tolerated and exhibited antitumor activity in patients with advanced solid tumors harboring the KRASG12C mutation.
INTRODUCTION
KRAS is the most frequently mutated RAS isoform in cancer, accounting for approximately 85% of RAS family mutations observed in human cancers.1-3 In normal cells, KRAS proteins cycle between guanosine triphosphate (GTP)–bound on and guanosine diphosphate–bound off states and initiate effector binding and intracellular signal transduction when in the GTP-bound on state.1,2 KRAS has a protein resynthesis half-life (t1/2) of approximately 24 hours.1,2 Substitution of Gly12 by cysteine prevents GTP hydrolysis, thereby maintaining KRAS in a constitutively active, GTP-bound state; this results in uncontrolled cellular proliferation and growth, as well as malignant transformation.2 KRASG12C mutations occur in approximately 14% of lung adenocarcinomas, 3%-4% of colorectal cancers (CRCs), and 2% of pancreatic cancers.1,4,5 The discovery of covalent inhibitors targeting the mutated cysteine residue in KRASG12C within the switch II binding pocket has led to the development of clinically active therapies for patients with tumors harboring the KRASG12C mutation.2,6-8
CONTEXT
Key Objective
This KRYSTAL-1 phase I/IB study reports on the safety, tolerability, recommended phase II dose, and preliminary efficacy of adagrasib, a potent covalent KRASG12C inhibitor, in advanced solid tumors harboring KRASG12C mutation.
Knowledge Generated
The recommended phase II dose of adagrasib is 600 mg orally twice daily. At this dose, eight of 15 patients (53.3%; 95% CI, 26.6 to 78.7) with RECIST-evaluable KRASG12C-mutant non–small-cell lung cancer (NSCLC) and one of two patients with KRASG12C-mutant colorectal cancer achieved a confirmed partial response. Median duration of response and median progression-free survival among patients with KRASG12C-mutant NSCLC were 16.4 months and 11.1 months, respectively.
Relevance
This study provides evidence of robust preliminary clinical efficacy to justify developing adagrasib as a novel KRASG12C inhibitor in patients with KRASG12C-mutant NSCLC and colorectal cancer and potentially those with other advanced tumors harboring KRASG12C mutation.
Adagrasib (MRTX849) is an oral, small-molecule, covalent inhibitor of KRASG12C that irreversibly and selectively binds and locks KRASG12C in its inactive, guanosine diphosphate–bound state.9 Adagrasib was optimized for desirable properties of a KRASG12C inhibitor, including high oral bioavailability, long t1/2 (approximately 24 hours), extensive tissue distribution, and central nervous system penetration.9 In preclinical models, adagrasib demonstrated potent inhibition of KRAS-dependent signal transduction (cellular half-maximal inhibitory concentration [IC50]: approximately 5 nM) and cancer cell viability, with > 1,000-fold selectivity for KRASG12C compared with wild-type KRAS.9 At a fixed dose of 100 mg/kg/day, adagrasib demonstrated broad-spectrum antitumor activity across a panel of KRASG12C-positive lung, colon, pancreatic, and other patient- or cell-derived tumor models implanted in mice.9 Additional pharmacokinetics (PK) analysis in these tumor models indicated that maximal and durable antitumor activity was plasma concentration–dependent and dose-dependent and required sustained exposure above a defined threshold to enable inhibition of KRASG12C over the entire dosing interval.9,10
We report results of the phase I/Ib dose-finding component of the first-in-human (FIH) KRYSTAL-1 trial, which evaluated the safety, PK, and clinical activity of adagrasib in patients with KRASG12C-mutant advanced solid tumors (ClinicalTrials.gov identifier: NCT03785249).
MATERIALS AND METHODS
Study Objectives
The objectives of this study were to evaluate the safety and tolerability of adagrasib, characterize its plasma PK parameters, determine biologically relevant dose levels, establish the maximum tolerated dose, identify the recommended phase II dose (RP2D), and assess its clinical activity in patients with advanced KRASG12C-mutant solid tumors.
Dose-Escalation Segment Design
The dose-escalation portion of the study used two consecutive phase I designs. The study began with an accelerated titration (AT) design, which transitioned to a modified toxicity probability interval (mTPI) design when a predefined degree of toxicity was observed or target adagrasib exposure was achieved. The AT design was used to limit the number of patients treated at potentially subtherapeutic doses during the dose-escalation segment. For any specific regimen, the maximum tolerated dose was defined as the dose associated with the probability of dose-limiting toxicity (DLT) occurring in 30% of patients during the first treatment cycle. Intrapatient dose escalation was allowed within protocol-defined limits for individual patients during the dose-escalation portion of the trial.
Choice of Starting Dose
The recommended clinical starting dose of adagrasib was chosen on the basis of the highest nonseverely toxic dose (HNSTD), derived from 28-day GLP dog toxicology studies, with dog as the most sensitive species. With the HNSTD of 25 mg/kg/day, taking one sixth of the HNSTD and correcting for the body surface area equate to a recommended human dose of 2.31 mg/kg/day or 162 mg/day for a 70-kg patient. Thus, 150 mg/day was used as a safe starting dose for this FIH trial.
Choice of First Dosing Regimen
On the basis of a projected human oral t1/2 of approximately 15 hours, a once-daily dosing regimen for adagrasib was chosen. The protocol allowed for exploration of alternative dosing regimens during the dose-escalation phase, including twice-a-day dosing or intermittent dosing in 3- or 4-week cycles, depending on the emergent safety/tolerability and PK results.
Patients
Eligible patients were ≥ 18 years old with a histologically confirmed diagnosis of an unresectable or metastatic solid tumor malignancy harboring a KRASG12C mutation in the tumor tissue or circulating tumor DNA on the basis of polymerase chain reaction or next-generation sequencing. Patients were enrolled using the result from a sponsor-approved or local test, and central confirmation of KRASG12C before study entry was not required. Key inclusion criteria were measurable or evaluable disease, adequate bone marrow and organ function, an Eastern Cooperative Oncology Group performance status score of ≤ 1, a life expectancy of at least 3 months, and ability to sign an independent review board–approved informed consent form. Patients' most recent prior systemic therapy and radiation therapy had to be > 2 weeks before the first adagrasib dose. Key exclusion criteria included the presence of active brain metastases or leptomeningeal carcinomatosis. For detailed information on eligibility criteria, see the Data Supplement (online only).
Assessments and End Points
Adverse events (AEs), including clinically significant laboratory abnormalities, were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 reported from the day of the first dose of study treatment until at least 28 days after the last dose.
Patients treated during the dose-escalation phase and selected patients enrolled in dose-expansion cohorts participated in a 96-hour single-dose PK lead-in period. Serial plasma samples for PK evaluation were collected in each patient over 24 hours on specified days after the first dose and during multiple-dose administration.
Baseline disease assessments were performed at screening within 28 days of starting adagrasib using computed tomography or magnetic resonance imaging. On-study disease assessments were performed every 6 weeks after the first dose. Imaging results were evaluated by the investigator to assess disease response as per RECIST 1.1. A best response of complete response/partial response (CR/PR) required a confirmatory assessment at least 4 weeks (≥ 28 days) after the first CR/PR. A best response of stable disease (SD) required a duration of at least 32 days from the date of the first dose; otherwise, it was listed as nonevaluable.
Statistical Analyses
The safety analysis population included all patients who received at least one dose of adagrasib. The PK-evaluable population consisted of all patients who received adagrasib and had adequate and reliable PK data available. Patients who received at least one dose of adagrasib, had an evaluable baseline tumor assessment, and at least one postbaseline tumor assessment were evaluated for clinical response. Descriptive statistics for overall response rate, on the basis of investigator assessment, were analyzed. The time-to-event end points, including duration of response (DOR), progression-free survival (PFS), and overall survival (OS), were reported descriptively and were summarized using the Kaplan-Meier method. Additional details on the statistical analysis can be found in the Data Supplement.
Trial Oversight
This study was approved by an institutional review board at each participating site. The trial was conducted in accordance with Good Clinical Practice guidelines, defined by the International Conference on Harmonization. All patients provided written informed consent before initiation of study procedures.
RESULTS
Patient Demographics and Baseline Characteristics
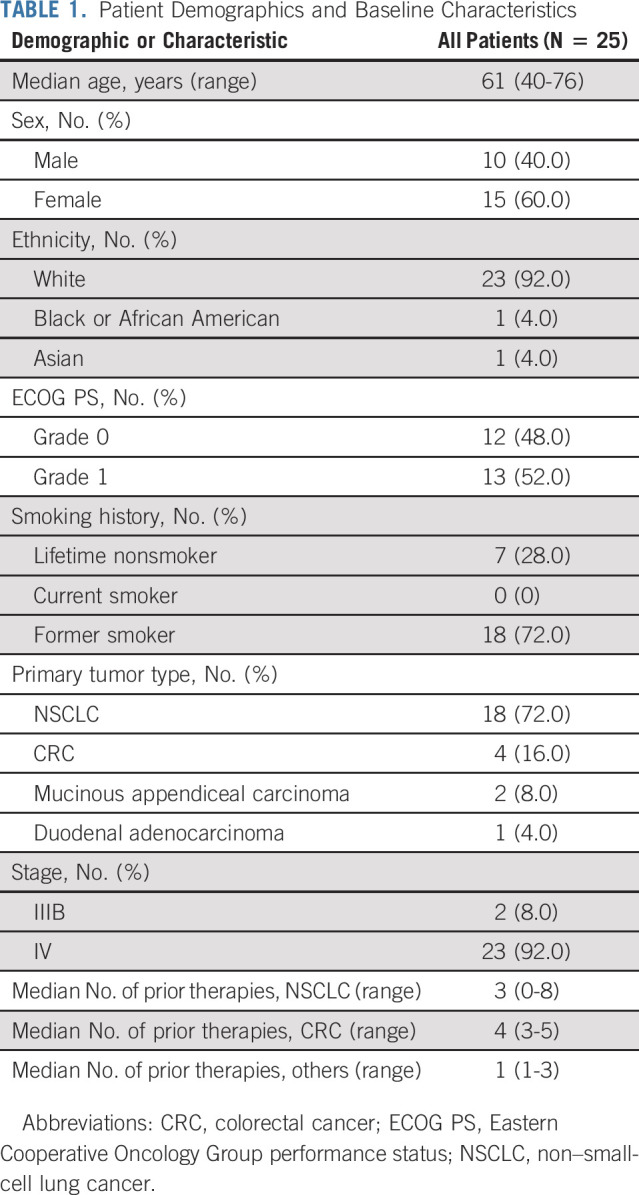
A total of 25 patients were enrolled and received at least one dose of adagrasib. The cutoff date for this analysis was August 15, 2021, with a median follow-up time for the overall population of 22.8 months (95% CI, 18.7 to 23.4). Patient demographics and baseline characteristics are summarized in Table 1.
TABLE 1.
Patient Demographics and Baseline Characteristics
Patient Disposition and DLTs
The first four patients enrolled were treated using the AT dose-escalation design, with one patient assigned to each of the four dose levels (150 mg, 300 mg, 600 mg, and 1,200 mg once daily). The numbers of patients assigned to each dose and cohorts are shown in Figure 1, and patient characteristics by dose levels are summarized in the Data Supplement. No DLTs were observed at the first three dose levels. At 1,200 mg once daily, one patient was determined to have met the criteria for protocol-defined DLT (< 80% dose intensity of cycle 1 doses) because of postdose emesis likely caused by pill burden (12 pills taken at one time). As a result, once daily dose escalation was discontinued, twice-a-day dosing was initiated, and the phase I segment was transitioned from the AT design to the mTPI design. A total of 23 patients were evaluable for DLTs (two patients missed ≥ 20% of their planned dose because of reasons unrelated to adagrasib). Five patients (21.7%) experienced one or more DLTs. The number and nature of DLTs per dose cohort and detailed descriptions of DLTs are provided in the Data Supplement.
FIG 1.
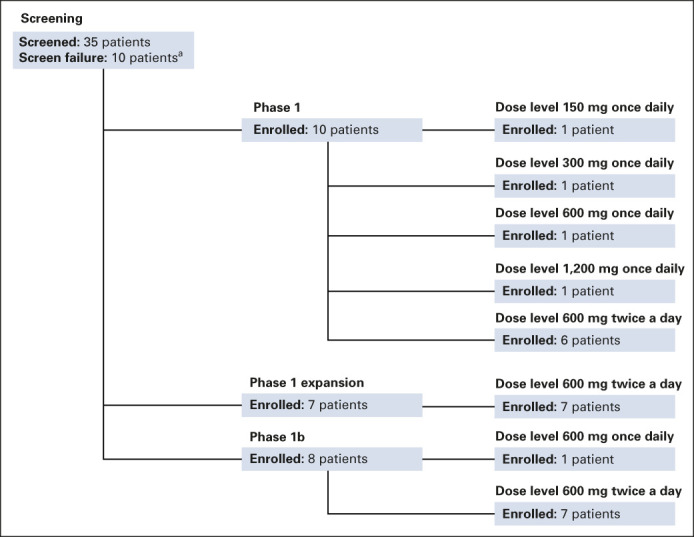
Disposition of study patients. aScreen failures were not distinguished by phase.
PK
Single-dose PK.
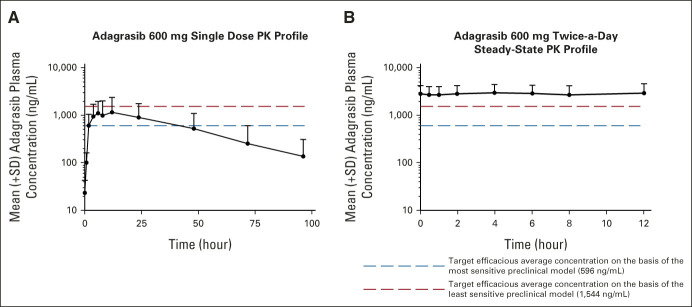
Adagrasib single-dose exposure increased with increasing doses from 150 mg to 600 mg (Data Supplement). However, assessment of dose proportionality in exposure over the 150- to 600-mg dose range was limited by only one patient enrolled at 150-mg and 300-mg doses. As shown in Figure 2A and Table 2, the median time to reach the maximum plasma concentration (tmax) after a single 600-mg oral dose of adagrasib under fasting conditions (n = 5) was 4.17 (range, 2-10.10) hours and the arithmetic mean t1/2 was 23.0 (range, 16.3-27.9) hours.
FIG 2.
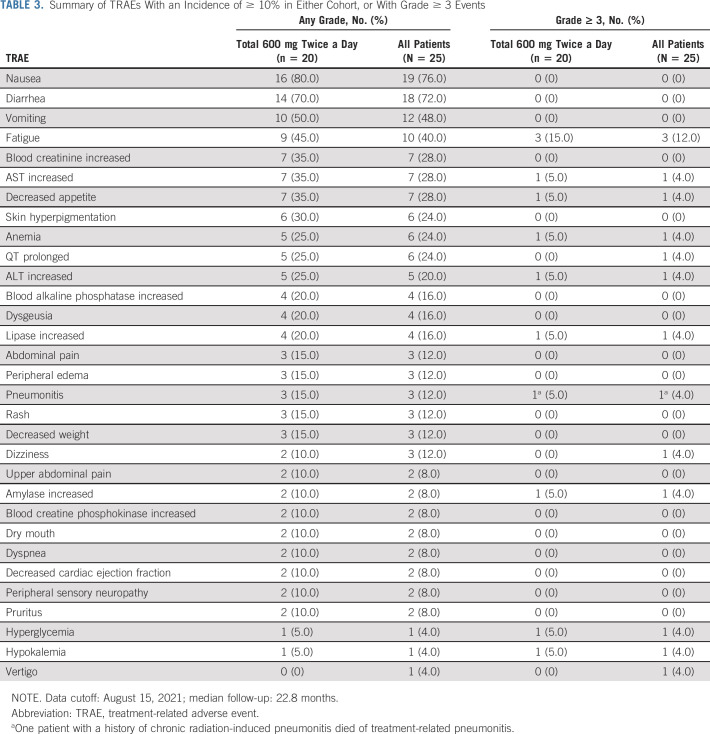
Mean (+SD) plasma adagrasib concentration–time profiles (PK profiles): (A) after a single 600-mg oral dose and (B) after a 600-mg twice-a-day regimen in the steady state under fasting conditions. PK, pharmacokinetics; SD, standard deviation.
TABLE 2.
Pharmacokinetics of 600-mg Adagrasib Twice a Day After Single and Multiple Oral Dose Administration Under Fasting Conditions
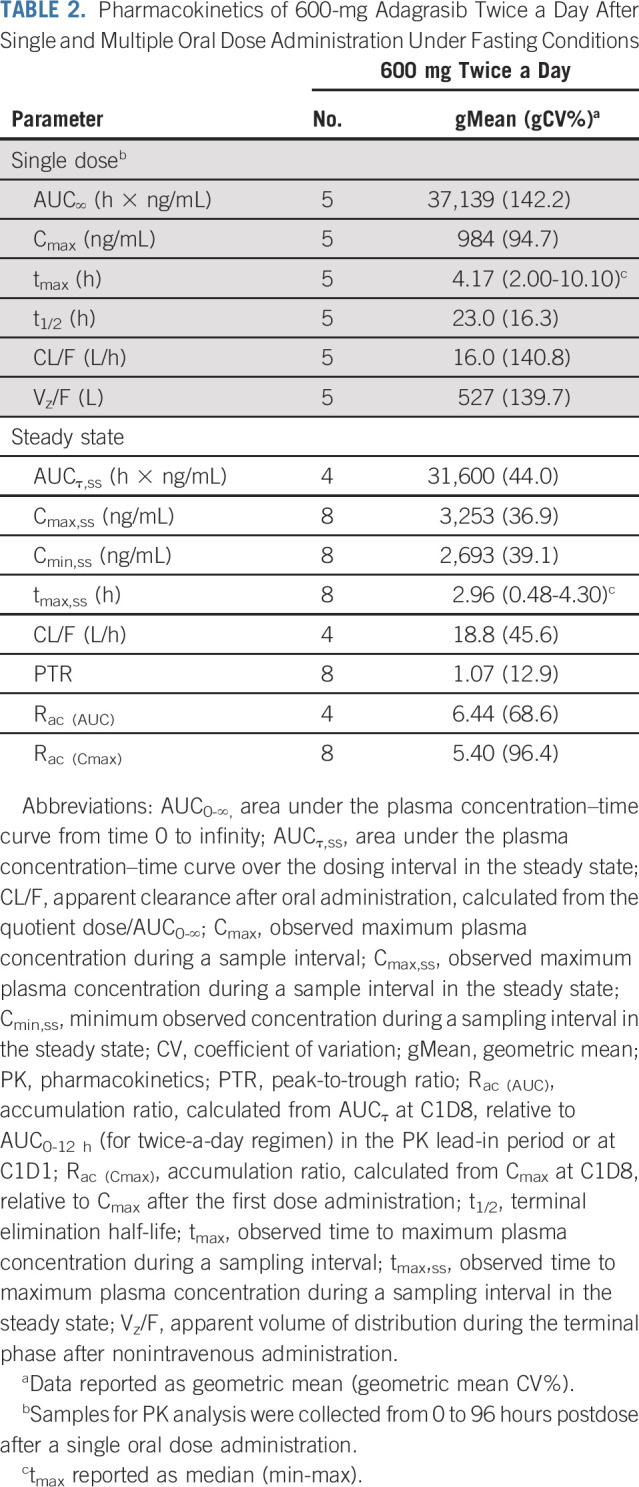
Multiple-dose PK.
After multiple-dose administration of adagrasib 600 mg twice a day under fasting conditions, the steady state was reached by cycle 1, day 8. The median tmax on cycle 1, day 8, was 2.96 (range, 0.48-4.30) hours (Table 2). The geometric mean minimum plasma concentration (Cmin) on cycle 1, day 8 was 2,693 ng/mL, which exceeded the 1,544-ng/mL target efficacious average concentration derived from observed maximal antitumor efficacy in the least sensitive preclinical xenograft model (Fig 2B; Table 2).9 Multiple doses of 600 mg twice a day resulted in an approximately five- to six-fold accumulation of adagrasib and a low peak-to-trough ratio (PTR) of 1.07 in the steady state (Table 2). No additional accumulation of adagrasib was observed after cycle 1, day 8. Compared with the 600-mg twice-a-day regimen, multiple doses of 150 mg and 300 mg once daily resulted in lower drug accumulation (approximately three- to four-fold) and a higher PTR (approximately three) in individual patients (Data Supplement).
Determination of the RP2D
Among the first cohort of six evaluable patients treated at 600 mg twice a day, one patient experienced a DLT. Although the mTPI algorithm recommends dose escalation in this scenario, the sponsor and investigators decided to continue evaluation of 600 mg twice a day, noting that preliminary PK results showed drug concentration levels at or above the predicted efficacious level. Together with two subsequent serial cohorts at the 600-mg twice-a-day dose level, a total of three of the 18 DLT-evaluable patients experienced DLTs. Thus, 600 mg twice a day was chosen as the RP2D.
Safety
Overall, 23 patients (92.0%) experienced treatment-related adverse events (TRAEs), including nine patients (36%) who experienced a grade 3/4 TRAE. The most common TRAEs were nausea (76.0%), diarrhea (72.0%), vomiting (48.0%), and fatigue (40.0%). Among the 20 patients treated at the RP2D of 600 mg twice a day, the most common TRAEs (any grade) were nausea (80.0%), diarrhea (70.0%), vomiting (50.0%), and fatigue (45.0%). The most common grade 3/4 TRAE was fatigue (15.0%). One patient (4.0%) with underlying pneumonitis associated with prior irradiation and systemic therapy experienced treatment-related grade 5 pneumonitis. Thirteen (65.0%) patients required treatment interruption or dose reduction because of TRAEs. The most common TRAEs leading to treatment interruption or dose reduction at 600 mg twice a day were nausea (25.0%), diarrhea, vomiting, and fatigue (each 20.0%). The median dose compliance was 97.1%, and the median relative dose intensity was 90.8%. Table 3 summarizes the most frequently observed TRAEs for all dose cohorts and at the RP2D.
TABLE 3.
Summary of TRAEs With an Incidence of ≥ 10% in Either Cohort, or With Grade ≥ 3 Events
Antitumor Activity
The evaluation of antitumor activity reported here was by investigator review and included 20 patients enrolled and treated at the RP2D (Data Supplement and Fig 3). Of these 20 patients, the primary diagnosis was KRASG12C-mutant non–small-cell lung cancer (NSCLC; n = 16), KRASG12C-mutant CRC (n = 2), and KRASG12C-mutant mucinous appendiceal carcinoma (n = 2), respectively.
FIG 3.
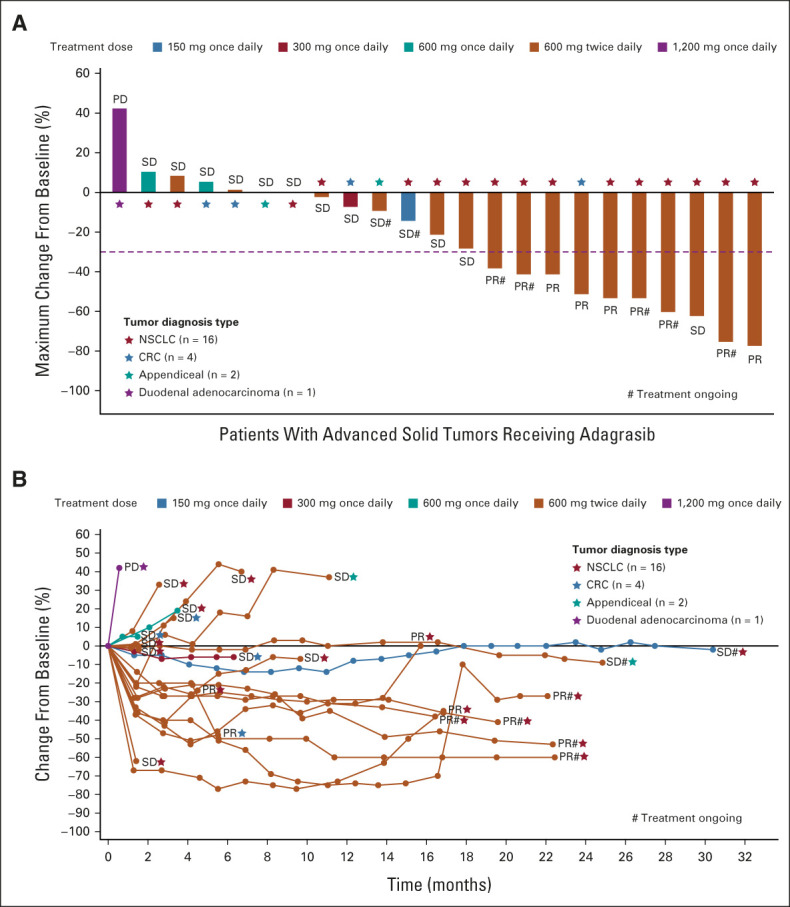
(A) Waterfall plot depicting the best tumor change from baseline. (B) Spider plot depicting the change in tumor measurement over time. Clinical activity evaluable population, n = 24; one patient (NSCLC, treated with 600 mg twice a day) was not included due to having no target lesion; therefore, change from baseline cannot be calculated. Data cutoff date: August 15, 2021. CRC, colorectal cancer; NSCLC, non–small-cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease.
At the time of the data cutoff, 15 of 16 patients with KRASG12C-mutant NSCLC at 600 mg twice a day were evaluable for response. After a median follow-up time of 19.6 months, the confirmed overall response rate was 53.3% (95% CI, 26.6 to 78.7; Fig 3) and the median DOR was 16.4+ months (95% CI, 3.1 to not estimable; range, 2.8-16.9 months). Two responses occurred in later cycles in patients who had been receiving treatment for longer than 10 months. Four patients had ongoing responses with durations of 16.9, 12.6, 5.4, and 2.8 months, respectively, at the time of the data cutoff (Fig 3).
Among the 16 patients with KRASG12C-mutant NSCLC enrolled and treated at the RP2D, the median PFS was 11.1 months (95% CI, 2.6 to not estimable; range, 0-22.4+ months). Kaplan-Meier estimates of PFS at 6 and 12 months were 64.3% (95% CI, 34.3 to 83.3) and 50.0% (95% CI, 22.9 to 72.2), respectively (Data Supplement). The median OS was NR (95% CI, 3.1 to not estimable) and ranged from 2.1 to 23.4+ months (Data Supplement). OS rates for these patients at 6 and 12 months were 73.3% (95% CI, 43.6 to 89.1) and 66.7% (95% CI, 37.5 to 84.6), respectively. Nine of the 16 patients were alive at the time of the data cutoff on August 15, 2021, including five patients who were still receiving treatment.
In addition, a confirmed PR was observed in one of the two patients with KRASG12C-mutant CRC at the 600-mg twice-a-day dose, with a DOR of 4.2 months. SD was reported in the two patients with mucinous appendiceal carcinoma (Fig 3). One patient with metastatic KRASG12C-mutant appendiceal adenocarcinoma and peritoneal carcinomatosis achieved a significant biochemical response, characterized by a decrease in the carcinoembryonic antigen level from a pretreatment baseline of 509 ng/mL to < 5 ng/mL; this patient had a duration of disease control of 24.8 months at the time of the data cutoff.
PFS durations for the two patients with KRASG12C-mutant CRC treated at the 600-mg twice-a-day dose were 3.3 and 5.5 months, respectively. OS durations for these two patients were 9.5 months and 10.5 months, respectively. PFS durations for the two patients with KRASG12C-mutant mucinous appendiceal carcinoma were 8.3 months and 24.8 months, respectively. At the time of the data cutoff, OS durations for these two patients were 23.2 and 26.2 months, respectively, and both patients were still alive. Among the five patients who began treatment at other dose levels, four achieved SD and one had PD as a best response (Fig 3).
DISCUSSION
This FIH, phase I/IB study demonstrated that adagrasib, a highly selective and potent oral small-molecule inhibitor of KRASG12C, was well tolerated and showed evidence of clinical activity at 600 mg twice a day in patients with advanced solid tumors harboring the KRASG12C mutation.
In the current study, adagrasib exhibited favorable PK properties, including oral bioavailability, long t1/2 (approximately 24 hours), extensive tissue distribution (apparent volume of distribution: 527 L), and sustained plasma concentrations, as evidenced by a relatively flat plasma concentration–time profile and low PTR variability.9 The dose-dependent PK and preliminary clinical activity of adagrasib support its ongoing evaluation as both monotherapy and in selected combination therapy strategies.
Studies conducted in nonclinical tumor models have indicated that maintaining plasma concentrations above the target threshold for the full-dose interval is important for maximizing antitumor efficacy, as new protein synthesis can result in uninhibited KRASG12C in the absence of adequate levels of inhibitor.9 The sustained exposure of adagrasib above the target threshold over the entire dosing interval at the 600-mg twice-a-day dose level is predicted to enable inhibition of newly synthesized KRASG12C and prevent a rebound in KRAS-dependent extracellular signal–regulated kinase signaling—a critical factor for durable antitumor activity.9
During the dose escalation (single patient AT design) in this study, a DLT of capsule burden intolerance was observed at 1,200 mg once daily (involving twelve 100 mg pills). Consequently, 600-mg twice-a-day dosing was selected for the phase Ib expansion and ultimately for the phase II expansion because of the desired observed safety and PK and initial signs of efficacy. In addition, on the basis of preclinical modeling, 600-mg twice-a-day PK data have shown that exposure of adagrasib at the steady state dose is 2-fold above the exposure required for a maximal response in the least sensitive animal models, whereas once daily dosing only achieved exposure above that required for maximal responses in the more sensitive preclinical models.
During the study, 65% of patients had treatment interruption or reduction because of TRAEs, which were primarily gastrointestinal in nature and included diarrhea, nausea, or vomiting. These TRAEs were generally low grade, occurred early in treatment, and typically resolved on their own (with occasional prophylaxis—eg, primarily prochlorperazine for nausea/vomiting and loperamide as an option for diarrhea). Although the mechanism of the gastrointestinal AEs is not currently known, the presentation was consistent with local irritation that might have resulted from the capsule formulation. In normal healthy volunteers, data suggest a lower rate of gastrointestinal AEs with a tablet formulation after a single dose [data on file]; the safety of the tablet is being further explored in KRYSTAL-1 (ClinicalTrials.gov identifier: NCT03785249) and in the Expanded Access Program (ClinicalTrials.gov identifier: NCT05162443).
On the basis of these encouraging phase I/Ib results, two pivotal registration-enabling phase III clinical trials are ongoing. KRYSTAL-12 compares adagrasib with docetaxel in previously treated KRASG12C-mutant NSCLC (ClinicalTrials.gov identifier: NCT04685135); KRYSTAL-10 compares adagrasib in combination with cetuximab versus chemotherapy in the second-line treatment of KRASG12C-mutant CRC (ClinicalTrials.gov identifier: NCT04793958). In addition, trials evaluating adagrasib as monotherapy or in combination with other agents in KRASG12C-mutant NSCLC, CRC, and other solid tumors are ongoing (KRYSTAL-2 [ClinicalTrials.gov identifier: NCT04330664], KRYSTAL-7 [ClinicalTrials.gov identifier: NCT04613596], and KRYSTAL-14 [ClinicalTrials.gov identifier: NCT04975256]).
ACKNOWLEDGMENT
We thank the patients, their families, and their caregivers, and the study investigators and their team members at each site for participation in the ongoing KRYSTAL-1 trial. Dr Sai-Hong Ignatius Ou wrote and edited the first version of the manuscript. All the authors reviewed, revised, and approved the final manuscript.
Sai-Hong Ignatius Ou
Stock and Other Ownership Interests: Turning Point Therapeutics, Elevation Oncology
Honoraria: Pfizer, Roche Pharma AG, Genentech/Roche, ARIAD/Takeda, AstraZeneca, BeiGene
Consulting or Advisory Role: Pfizer, Roche/Genentech, AstraZeneca, Takeda, Janssen/JNJ
Speakers' Bureau: AstraZeneca, Genentech/Roche
Research Funding: Pfizer (Inst), Roche Pharma AG (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), ARIAD (Inst), Revolution Medicines (Inst), Mirati Therapeutics (Inst), Janssen/JNJ (Inst)
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals, Loxo Oncology
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, Chugai Pharma, Roche/Genentech, LOXO, Mirati Therapeutics, Araxes Pharma, Ignyta, Lilly, Takeda, Novartis, Biocartis, Voronoi, SFJ Pharmaceuticals Group, Sanofi, Daiichi Sankyo, Silicon Therapeutics, Nuvalent, Inc, Eisai, Bayer, Syndax, AbbVie, Allorion Therapeutics, Accutar Biotech, Transcenta
Research Funding: AstraZeneca, Astellas Pharma, Daiichi Sankyo, Lilly, Boehringer Ingelheim, Puma Biotechnology, Takeda, Revolution Medicines
Patents, Royalties, Other Intellectual Property: I am a coinventor of a DFCI-owned patent on EGFR mutations licensed to Lab Corp. I receive postmarketing royalties from this invention
Ticiana A. Leal
Consulting or Advisory Role: Takeda, Jazz Pharmaceuticals, AstraZeneca, EMD Serono, Merck, Boehringer Ingelheim, Blueprint Medicines, Daiichi Sankyo/Lilly, Bayer, Genentech, Lilly, Janssen, Mirati Therapeutics, Daiichi-Sankyo, Eisai, Daiichi Sankyo/AstraZeneca, Novocure
Igor I. Rybkin
Consulting or Advisory Role: AstraZeneca
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Minal A. Barve
Employment: Texas Oncology
Stock and Other Ownership Interests: Texas Oncology
Research Funding: Mary Crowley Research Center
Lyudmila Bazhenova
Stock and Other Ownership Interests: Epic Sciences
Consulting or Advisory Role: Genentech/Roche, Boehringer Ingelheim, Novartis, Regeneron, Merck, Johnson and Johnson, BMSi, Daichi, NEUVOGEN, Bayer, Sanofi, ORCIC, Turning Point Therapeutics
Research Funding: BeyondSpring Pharmaceuticals
Melissa L. Johnson
Employment: HCA Healthcare
Consulting or Advisory Role: Otsuka, Genentech/Roche (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Loxo (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), Pfizer (Inst), Guardant Health (Inst), Ribon Therapeutics (Inst), Incyte (Inst), AbbVie (Inst), Achilles Therapeutics (Inst), Atreca (Inst), GlaxoSmithKline (Inst), Gritstone Oncology (Inst), Janssen Oncology (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), EMD Serono (Inst), G1 Therapeutics (Inst), WindMIL (Inst), Checkpoint Therapeutics (Inst), Eisai (Inst), Axelia Oncology (Inst), Black Diamond Therapeutics (Inst), CytomX Therapeutics (Inst), EcoR1 Capital (Inst), Editas Medicine (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), ITeos Therapeutics (Inst), Oncorus (Inst), Regeneron (Inst), Turning Point Therapeutics (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stemcentrx (Inst), Novartis (Inst), Checkpoint Therapeutics (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Oncology (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), Jounce Therapeutics (Inst), University of Michigan (Inst), WindMIL (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics, Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca, Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan-Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), RasCal (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics, Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Karen L. Velastegui
Employment: Mirati Therapeutics, Arena Pharma
Stock and Other Ownership Interests: Mirati Therapeutics, Arena Pharma
Cornelius Cilliers
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
James G. Christensen
Employment: Mirati Therapeutics
Leadership: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Consulting or Advisory Role: BridgeBio Pharma
Patents, Royalties, Other Intellectual Property: Multiple patents in the last 2 years while employed at Mirati Therapeutics covering discovery of KRAS, LSD1, and EZH2 inhibitors (Inst)
Xiaohong Yan
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
Richard C. Chao
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics, Pfizer, Merck
Kyriakos P. Papadopoulos
Consulting or Advisory Role: Basilea, Turning Point Therapeutics, Bicycle Therapeutics
Research Funding: AbbVie (Inst), MedImmune (Inst), Daiichi Sankyo (Inst), Regeneron (Inst), Amgen (Inst), Calithera Biosciences (Inst), Incyte (Inst), Merck (Inst), Peloton Therapeutics (Inst), ADC Therapeutics (Inst), 3D Medicines (Inst), EMD Serono (Inst), Syros Pharmaceuticals (Inst), Mersana (Inst), MabSpace Biosciences (Inst), Jounce Therapeutics (Inst), Bayer (Inst), AnHeart Therapeutics (Inst), F-star (Inst), Linnaeus Therapeutics (Inst), Mirati Therapeutics (Inst), Tempest Therapeutics (Inst), Treadwell Therapeutics (Inst), Lilly (Inst), Pfizer (Inst), BioNTech (Inst), Bicycle Therapeutics (Inst), Kezar Life Sciences (Inst)
No other potential conflicts of interest were reported.
See accompanying article on page 2609
PRIOR PRESENTATION
Presented previously at the 2020 EORTC-NCI-AACR (ENA) Virtual Symposium, October 24-25, 2020.
SUPPORT
Support for this study was provided by Mirati Therapeutics, Inc. Medical writing and editorial support were provided by Caleb Rans and Ellen Powers of Axiom Healthcare Strategies (Princeton, NJ).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Mirati will honor legitimate requests for clinical trial data from qualified researchers, upon request, as necessary for conducting methodologically sound research. Mirati will provide access to data and clinical study reports (CSRs) for clinical trials for which results are posted on the clinicaltrials.gov registry for products or indications that have been approved by regulators in the United States and European Union. In general, data will be made available for request approximately 12 months after clinical trial completion. Relevant components of the protocol and statistical analysis plan for the KRYSTAL-1 study will also be made available upon request.
AUTHOR CONTRIBUTIONS
Conception and design: Sai-Hong Ignatius Ou, Pasi A. Jänne, Melissa L. Johnson, Cornelius Cilliers, James G. Christensen, Xiaohong Yan, Richard C. Chao, Kyriakos P. Papadopoulos
Administrative support: Karen L. Velastegui
Provision of study materials or patients: Sai-Hong Ignatius Ou, Pasi A. Jänne, Ticiana A. Leal, Igor I. Rybkin, Joshua K. Sabari, Minal A. Barve, Lyudmila Bazhenova, Melissa L. Johnson, James G. Christensen, Richard C. Chao, Kyriakos P. Papadopoulos
Collection and assembly of data: All authors
Data analysis and interpretation: Sai-Hong Ignatius Ou, Pasi A. Jänne, Ticiana A. Leal, Igor I. Rybkin, Joshua K. Sabari, Minal A. Barve, Lyudmila Bazhenova, Melissa L. Johnson, Cornelius Cilliers, James G. Christensen, Xiaohong Yan, Richard C. Chao, Kyriakos P. Papadopoulos
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRASG12C Solid Tumors (KRYSTAL-1)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sai-Hong Ignatius Ou
Stock and Other Ownership Interests: Turning Point Therapeutics, Elevation Oncology
Honoraria: Pfizer, Roche Pharma AG, Genentech/Roche, ARIAD/Takeda, AstraZeneca, BeiGene
Consulting or Advisory Role: Pfizer, Roche/Genentech, AstraZeneca, Takeda, Janssen/JNJ
Speakers' Bureau: AstraZeneca, Genentech/Roche
Research Funding: Pfizer (Inst), Roche Pharma AG (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), ARIAD (Inst), Revolution Medicines (Inst), Mirati Therapeutics (Inst), Janssen/JNJ (Inst)
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals, Loxo Oncology
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, Chugai Pharma, Roche/Genentech, LOXO, Mirati Therapeutics, Araxes Pharma, Ignyta, Lilly, Takeda, Novartis, Biocartis, Voronoi, SFJ Pharmaceuticals Group, Sanofi, Daiichi Sankyo, Silicon Therapeutics, Nuvalent, Inc, Eisai, Bayer, Syndax, AbbVie, Allorion Therapeutics, Accutar Biotech, Transcenta
Research Funding: AstraZeneca, Astellas Pharma, Daiichi Sankyo, Lilly, Boehringer Ingelheim, Puma Biotechnology, Takeda, Revolution Medicines
Patents, Royalties, Other Intellectual Property: I am a coinventor of a DFCI-owned patent on EGFR mutations licensed to Lab Corp. I receive postmarketing royalties from this invention
Ticiana A. Leal
Consulting or Advisory Role: Takeda, Jazz Pharmaceuticals, AstraZeneca, EMD Serono, Merck, Boehringer Ingelheim, Blueprint Medicines, Daiichi Sankyo/Lilly, Bayer, Genentech, Lilly, Janssen, Mirati Therapeutics, Daiichi-Sankyo, Eisai, Daiichi Sankyo/AstraZeneca, Novocure
Igor I. Rybkin
Consulting or Advisory Role: AstraZeneca
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Minal A. Barve
Employment: Texas Oncology
Stock and Other Ownership Interests: Texas Oncology
Research Funding: Mary Crowley Research Center
Lyudmila Bazhenova
Stock and Other Ownership Interests: Epic Sciences
Consulting or Advisory Role: Genentech/Roche, Boehringer Ingelheim, Novartis, Regeneron, Merck, Johnson and Johnson, BMSi, Daichi, NEUVOGEN, Bayer, Sanofi, ORCIC, Turning Point Therapeutics
Research Funding: BeyondSpring Pharmaceuticals
Melissa L. Johnson
Employment: HCA Healthcare
Consulting or Advisory Role: Otsuka, Genentech/Roche (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Loxo (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), Pfizer (Inst), Guardant Health (Inst), Ribon Therapeutics (Inst), Incyte (Inst), AbbVie (Inst), Achilles Therapeutics (Inst), Atreca (Inst), GlaxoSmithKline (Inst), Gritstone Oncology (Inst), Janssen Oncology (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), EMD Serono (Inst), G1 Therapeutics (Inst), WindMIL (Inst), Checkpoint Therapeutics (Inst), Eisai (Inst), Axelia Oncology (Inst), Black Diamond Therapeutics (Inst), CytomX Therapeutics (Inst), EcoR1 Capital (Inst), Editas Medicine (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), ITeos Therapeutics (Inst), Oncorus (Inst), Regeneron (Inst), Turning Point Therapeutics (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stemcentrx (Inst), Novartis (Inst), Checkpoint Therapeutics (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Oncology (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), Jounce Therapeutics (Inst), University of Michigan (Inst), WindMIL (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics, Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca, Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan-Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), RasCal (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics, Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Karen L. Velastegui
Employment: Mirati Therapeutics, Arena Pharma
Stock and Other Ownership Interests: Mirati Therapeutics, Arena Pharma
Cornelius Cilliers
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
James G. Christensen
Employment: Mirati Therapeutics
Leadership: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Consulting or Advisory Role: BridgeBio Pharma
Patents, Royalties, Other Intellectual Property: Multiple patents in the last 2 years while employed at Mirati Therapeutics covering discovery of KRAS, LSD1, and EZH2 inhibitors (Inst)
Xiaohong Yan
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
Richard C. Chao
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics, Pfizer, Merck
Kyriakos P. Papadopoulos
Consulting or Advisory Role: Basilea, Turning Point Therapeutics, Bicycle Therapeutics
Research Funding: AbbVie (Inst), MedImmune (Inst), Daiichi Sankyo (Inst), Regeneron (Inst), Amgen (Inst), Calithera Biosciences (Inst), Incyte (Inst), Merck (Inst), Peloton Therapeutics (Inst), ADC Therapeutics (Inst), 3D Medicines (Inst), EMD Serono (Inst), Syros Pharmaceuticals (Inst), Mersana (Inst), MabSpace Biosciences (Inst), Jounce Therapeutics (Inst), Bayer (Inst), AnHeart Therapeutics (Inst), F-star (Inst), Linnaeus Therapeutics (Inst), Mirati Therapeutics (Inst), Tempest Therapeutics (Inst), Treadwell Therapeutics (Inst), Lilly (Inst), Pfizer (Inst), BioNTech (Inst), Bicycle Therapeutics (Inst), Kezar Life Sciences (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrem JM, Shokat KM: Direct small-molecule inhibitors of KRAS: From structural insights to mechanism-based design. Nat Rev Drug Discov 15:771-785, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Simanshu DK, Nissley DV, McCormick F: RAS proteins and their regulators in human disease. Cell 170:17-33, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network : Comprehensive molecular characterization of human colon and rectal cancer. Nature 487:330-337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network : Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543-550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen JG, Olson P, Briere T, et al. : Targeting Kras(g12c)-mutant cancer with a mutation-specific inhibitor. J Intern Med 288:183-191, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Hu Q, Shokat KM: Disease-causing mutations in the G protein Galphas subvert the roles of GDP and GTP. Cell 173:1254-1264 e11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong DS, Fakih MG, Strickler JH, et al. : KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med 383:1207-1217, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallin J, Engstrom LD, Hargis L, et al. : The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov 10:54-71, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lito P, Solomon M, Li LS, et al. : Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 351:604-608, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Mirati will honor legitimate requests for clinical trial data from qualified researchers, upon request, as necessary for conducting methodologically sound research. Mirati will provide access to data and clinical study reports (CSRs) for clinical trials for which results are posted on the clinicaltrials.gov registry for products or indications that have been approved by regulators in the United States and European Union. In general, data will be made available for request approximately 12 months after clinical trial completion. Relevant components of the protocol and statistical analysis plan for the KRYSTAL-1 study will also be made available upon request.