PURPOSE
Prospective data on the efficacy of a watch-and-wait strategy to achieve organ preservation in patients with locally advanced rectal cancer treated with total neoadjuvant therapy are limited.
METHODS
In this prospective, randomized phase II trial, we assessed the outcomes of 324 patients with stage II or III rectal adenocarcinoma treated with induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT) and either total mesorectal excision (TME) or watch-and-wait on the basis of tumor response. Patients in both groups received 4 months of infusional fluorouracil-leucovorin-oxaliplatin or capecitabine-oxaliplatin and 5,000 to 5,600 cGy of radiation combined with either continuous infusion fluorouracil or capecitabine during radiotherapy. The trial was designed as two stand-alone studies with disease-free survival (DFS) as the primary end point for both groups, with a comparison to a null hypothesis on the basis of historical data. The secondary end point was TME-free survival.
RESULTS
Median follow-up was 3 years. Three-year DFS was 76% (95% CI, 69 to 84) for the INCT-CRT group and 76% (95% CI, 69 to 83) for the CRT-CNCT group, in line with the 3-year DFS rate (75%) observed historically. Three-year TME-free survival was 41% (95% CI, 33 to 50) in the INCT-CRT group and 53% (95% CI, 45 to 62) in the CRT-CNCT group. No differences were found between groups in local recurrence-free survival, distant metastasis-free survival, or overall survival. Patients who underwent TME after restaging and patients who underwent TME after regrowth had similar DFS rates.
CONCLUSION
Organ preservation is achievable in half of the patients with rectal cancer treated with total neoadjuvant therapy, without an apparent detriment in survival, compared with historical controls treated with chemoradiotherapy, TME, and postoperative chemotherapy.
INTRODUCTION
Neoadjuvant chemoradiotherapy (CRT), total mesorectal excision (TME), and adjuvant chemotherapy is a standard treatment for patients with locally advanced rectal adenocarcinoma.1 This multimodality treatment, although effective in achieving tumor control, is associated with significant morbidity, with bowel, urinary, and sexual dysfunction that can impair patients' quality of life permanently.2 In addition, many patients with distal rectal cancer are left with a permanent colostomy.2 As most of the sequelae from the multimodality therapy are related to surgery,3 there is a strong incentive to explore nonsurgical treatment options.
CONTEXT
Key Objective
Can a potentially rectum-preserving treatment approach for locally advanced rectal cancer achieve outcomes similar to those of standard resection-based treatment?
Knowledge Generated
Oncologic outcomes of patients treated with total neoadjuvant therapy and selective watch-and-wait or total mesorectal excision (on the basis of tumor response) are comparable to the outcomes of patients treated with neoadjuvant therapy and total mesorectal excision. The order of chemoradiation and systemic chemotherapy does not affect disease-free survival, but chemoradiation followed by consolidation chemotherapy appears to be associated with a higher rate of organ preservation.
Relevance
The potentially rectum-preserving treatment approach for locally advanced rectal cancer appears to be safe in patients with a complete or near-complete response to total neoadjuvant therapy who are willing to undergo a strict surveillance protocol. This treatment approach can help patients maintain a higher quality of life compared with standard resection-based treatment.
The excellent survival of patients with a pathologic complete response (pCR) to neoadjuvant therapy4 challenges the added benefit of TME for these patients.5 Retrospective case series and prospective observational studies suggest that organ preservation is feasible for selected patients treated with variable neoadjuvant CRT regimens6,7; however, prospective information on organ preservation for patients with locally advanced rectal cancer treated with total neoadjuvant therapy (TNT) is limited.7
Delivering chemotherapy before surgery, either before or after CRT, is a newer treatment approach referred to as TNT, which aims to improve compliance with systemic chemotherapy and treat micrometastasis earlier.8-11 Although preliminary data suggest that the sequence of chemotherapy and CRT affects the rate of tumor response,12 the impact on organ preservation is unknown.
We conducted a prospective, randomized, multicenter clinical trial (ClinicalTrials.gov identifier: NCT02008656) to test the hypothesis that patients with stage II and III rectal adenocarcinoma treated with TNT followed by either TME or a selective watch-and-wait (WW) approach on the basis of tumor response will have better disease-free survival (DFS) compared with historical DFS in patients treated with a standard therapy of CRT, TME, and intended postoperative adjuvant chemotherapy. We also evaluated whether the organ preservation rates would differ between patients who underwent induction chemotherapy (INCT) followed by CRT (INCT-CRT) and patients who underwent CRT followed by consolidation chemotherapy (CRT-CNCT).
METHODS
Study Design
The Organ Preservation for Rectal Adenocarcinoma (OPRA) Trial was a randomized, nonblinded, phase II trial conducted at 18 US institutions. The trial protocol (Protocol, online only) was approved by the institutional review boards of all participating institutions. All participants provided written informed consent.
Eligible patients were older than 18 years of age and had clinical stage II (T3-4, N0) or stage III (any T, N1-2) biopsy-proven rectal adenocarcinoma staged with magnetic resonance imaging (MRI) according to a specified rectal cancer protocol, a full colonoscopy, and computed tomography (CT) of the chest, abdomen, and pelvis. Patients with recurrent rectal cancer, evidence of distant metastasis at diagnosis, or history of pelvic irradiation were excluded (Protocol).
Random Assignment
Patients were recruited after required assessment and tumor staging and before starting treatment. They were randomly assigned to INCT-CRT or CRT-CNCT (Appendix Fig A1, online only) using the clinical research database CRDBi-Multicenter. Random assignment was stratified per institution and accomplished by the method of random permuted block.
Procedures
Radiotherapy was planned using a multiple-field technique with either intensity-modulated radiotherapy or three-dimensional conformal radiotherapy to deliver 4,500 cGy in 180 cGy over 25 fractions to regional pelvic nodes (including inguinal nodes for primary tumors involving the anus). A total dose of 5,000-5,600 cGy was delivered to the primary tumor and involved nodes with either a simultaneous integrated boost and/or a sequential boost. Patients received either capecitabine (825 mg/m2 twice a day orally) or continuous infusion fluorouracil (FU; 225 mg/m2/d) during radiotherapy per medical oncologist preference. Patients also received eight cycles of infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX; oxaliplatin 85 mg/m2 intravenous [IV], leucovorin 400 mg/m2 IV, FU 400 mg/m2 IV push, and FU 2,400 mg/m2 over 46-48 hours by continuous infusion, repeated on a 14-day cycle) or five cycles of capecitabine and oxaliplatin (CAPEOX; oxaliplatin 130 mg/m2 on day 1 and capecitabine at 1,000 g/m2 twice a day on days 1-14, repeated on a 21-day cycle) before or after CRT.
Tumor restaging was performed by digital rectal examination, endoscopic examination, MRI, and CT of the chest, abdomen, and pelvis, within 8 (±4) weeks after TNT. Response was graded as complete, near-complete, or incomplete according to previously defined criteria. Patients with incomplete clinical response were recommended TME. Patients with clinical complete response (cCR) or near-complete response were offered participation in a standardized WW protocol consisting of digital rectal examination and flexible sigmoidoscopy every 4 months for the first 2 years from the time of assessment of response, and every 6 months for the following 3 years. Rectal MRI was to be performed every 6 months for the first 2 years and yearly for the following 3 years.13 However, patients were evaluated at more frequent intervals if considered necessary by the investigators. Patients with sustained clinical response compared with the previous evaluation were recommended to continue the WW follow-up protocol. Patients with signs of lack of ongoing response compared with their previous evaluation or with signs of tumor regrowth on endoscopy or MRI were recommended to undergo TME. Biopsies of the primary tumor sites for patients on WW were not required by protocol. All patients were to have CT scans of the chest, abdomen, and pelvis at least once a year. Patients who had TME were surveyed according to current guidelines.1 All patients had surveillance colonoscopy according to current guidelines.14
Outcomes
The primary end point was DFS, defined as the interval from random assignment to the first occurrence of locoregional failure, distant metastasis, a new invasive colorectal primary cancer, or death from any cause. Locoregional failure was defined as either an unresectable rectal primary tumor following protocol neoadjuvant treatment, an R2 resection for the rectal primary tumor, or recurrence in the primary tumor bed after an R0-R1 resection. Tumor regrowth in the rectal wall or in regional lymph nodes after a cCR or near-complete response and a period of WW was not considered a locoregional failure if it was followed by an R0-R1 resection. Organ preservation, defined as TME-free survival measured in the intention-to-treat population, was the secondary end point. Other secondary outcomes included adverse events, local recurrence, distant recurrence, and overall survival. Additional end points included bowel, urinary, and sexual function and quality of life. Adverse events during TNT were measured using the Common Terminology Criteria for Adverse Events version 4.0.
Statistical Analysis
The trial was designed as two stand-alone phase II studies with similar hypotheses. Power and sample size calculations were based on an estimated 75% 3-year DFS for similar patients treated according to a standard approach of chemoradiation and TME.4 We hypothesized that 85% of patients with locally advanced rectal cancer treated with TNT and selective organ preservation would be alive and disease-free at 3 years. Each group was designed to discriminate between 3-year DFS rates of 75% and 85%. Assuming accrual rates of 2-4 per group and month, and a two-sided type I error of 5%, we required 101 patients evaluable for the primary end point per group to have 85% power.15
For the secondary end point, we used pCR rates from previous data, 20% for the INCT-CRT group and 35% for the CRT-CNCT group (Protocol). If both arms met the end point in primary objective, we planned to use the organ preservation rate to determine the regimen for further study, using a pick-the-winner strategy. We estimated that the 202 patients (plus 10% to account for dropout) required for the primary aim would be sufficient to detect differences in organ preservation rates. Following the accrual of the first 101 evaluable patients in each group, and after finding a higher rate of complete response than anticipated in both arms, the study consortium elected to allow accrual and random assignment to continue to provide additional data for better estimates of survival and organ preservation rates (Protocol, Amendment 18A). All analyses done on the final cohort were also performed on the initial cohort: 101 evaluable patients in the INCT-CRT group and 106 evaluable patients in the CRT-CNCT group.
DFS, organ preservation, local recurrence-free survival, distant metastasis-free survival, and overall survival were analyzed using Cox regression and summarized using Kaplan-Meier curves. Tumor regrowth from restaging for patients with cCR who were offered WW was summarized using similar time-to-event. Treatment compliance and adverse events were summarized by group. Additional analyses directly comparing clinical outcomes using the log-rank test were conducted. Multivariate Cox regression models were fit to the full cohort. All analyses were conducted in R.
RESULTS
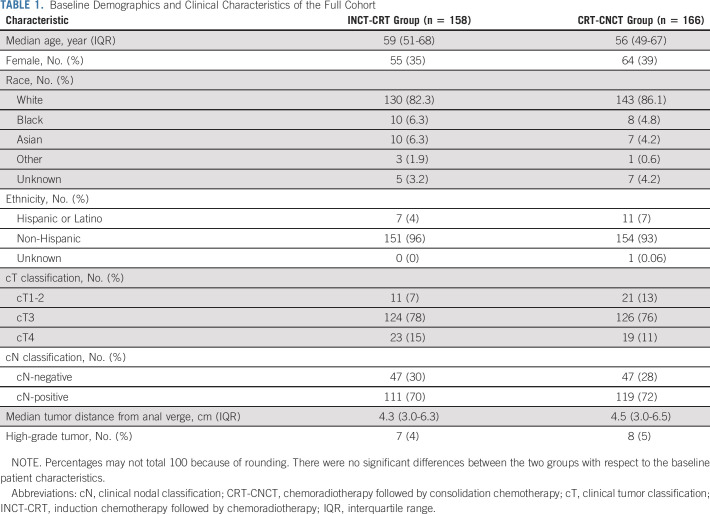
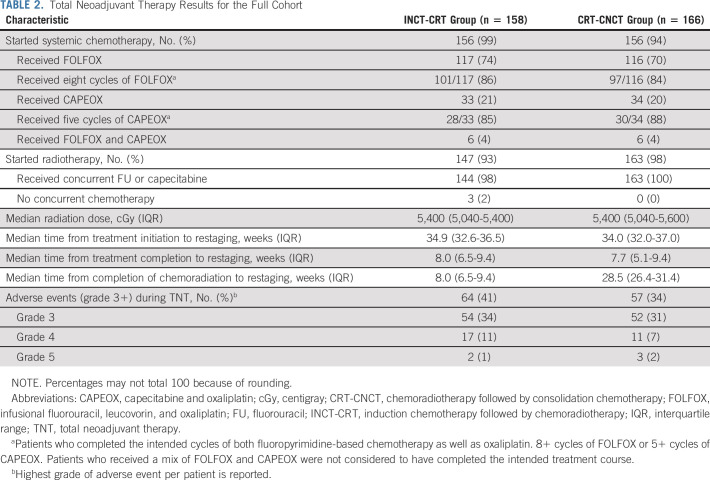
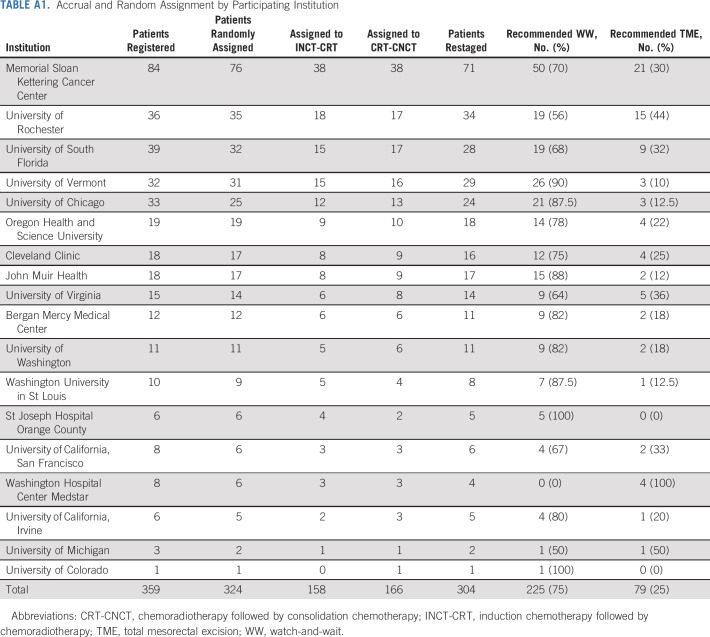
From April 12, 2014, to March 30, 2020, 360 patients were registered to the trial at 18 centers (Appendix Table A1, online only). Of the 324 eligible patients, 158 were randomly assigned to the INCT-CRT group and 166 to the CRT-CNCT group (Fig 1). Most patients were males and had stage III tumors, located at < 5 cm from the anal verge on average. The baseline and clinical characteristics were well balanced between the groups (Table 1, Appendix Table A2, online only). Twelve patients withdrew consent after random assignment, before or after starting neoadjuvant therapy. Most patients in both groups started the assigned treatment (Table 2, Appendix Table A3, online only). The proportion of patients receiving the number of prescribed cycles of systemic chemotherapy was similar in both groups. Overall, more patients received FOLFOX compared with CAPEOX, but the proportion of patients receiving either was similar in both groups. The median dose of radiation was also similar in both groups. The time intervals from the start of treatment to restaging and from the end of treatment to restaging were similar in both groups. The time interval from the end of CRT to tumor restaging was longer in the CRT-CNCT group compared with the INCT-CRT group by study design (Table 2, Appendix Fig A1).
FIG 1.
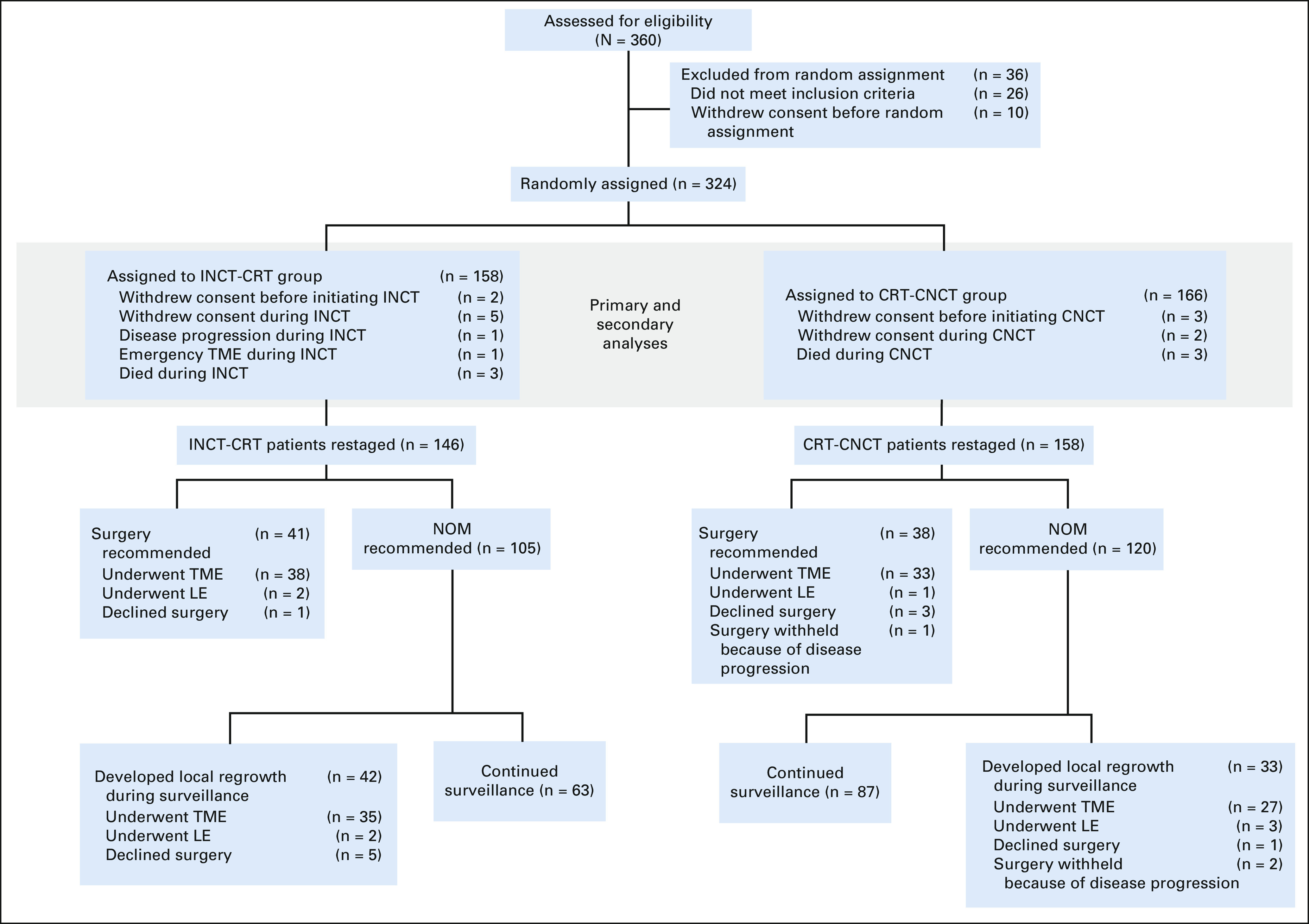
CONSORT diagram illustrating the eligibility, random assignment, outcomes, and follow-up of the trial cohort. Primary and secondary analyses of the 158 INCT-CRT and 166 CRT-CNCT patients followed an intention-to-treat principle. CRT-CNCT, chemoradiotherapy followed by consolidation chemotherapy; INCT-CRT, induction chemotherapy followed by chemoradiotherapy; LE, local excision; NOM, nonoperative management; TME, total mesorectal excision.
TABLE 1.
Baseline Demographics and Clinical Characteristics of the Full Cohort
TABLE 2.
Total Neoadjuvant Therapy Results for the Full Cohort
Six patients (2%), three in each group, died during TNT (Fig 1, Table 2), five from treatment-related toxicity and one from causes unrelated to treatment. Two patients in the INCT-CRT group never had restaging, one because of tumor perforation requiring emergency surgery and one because of disease progression. There was no difference in the rate of adverse events between treatment groups (Table 2).
The data for all outcomes were available up to February 10, 2021. Median follow-up for patients who were alive and event-free at the time of analysis was 3 years (interquartile range [IQR], 1.84-4.06). The event for the primary end point, DFS, occurred in 75 patients, 36 in the INCT-CRT group and 39 in the CRT-CNCT group. The 3-year DFS rates, 76% (95% CI, 69 to 84) for the INCT-CRT group and 76% (95% CI, 69 to 83) in the CRT-CNCT group, did not differ when compared with the historical 3-year DFS rate of 75% (Fig 2, Appendix Fig A2A, online only). Therefore, the primary end point of the study, an improvement in DFS among patients treated with TNT and selective WW compared with historical controls, was not met. Among the 75 observed events for DFS, 41 were distant metastasis, 11 were local recurrence, 10 were persistent disease events, and 10 were death from any cause. The rates of local recurrence-free survival (94% [95% CI, 89 to 99] for INCT and 94% [95% CI, 90 to 98] for CNCT) and distant metastasis-free survival (84% [95% CI, 77 to 91] for INCT and 82% [95% CI, 75 to 89] for CNCT) were similar in both groups (Fig 2). Overall survival is provided, but these data will require additional follow-up. Clinical nodal metastasis (cN+) on baseline MRI was the only variable associated with DFS in univariable and multivariable analyses (hazard ratio [HR] 2.26; 95% CI, 1.23 to 4.17).
FIG 2.
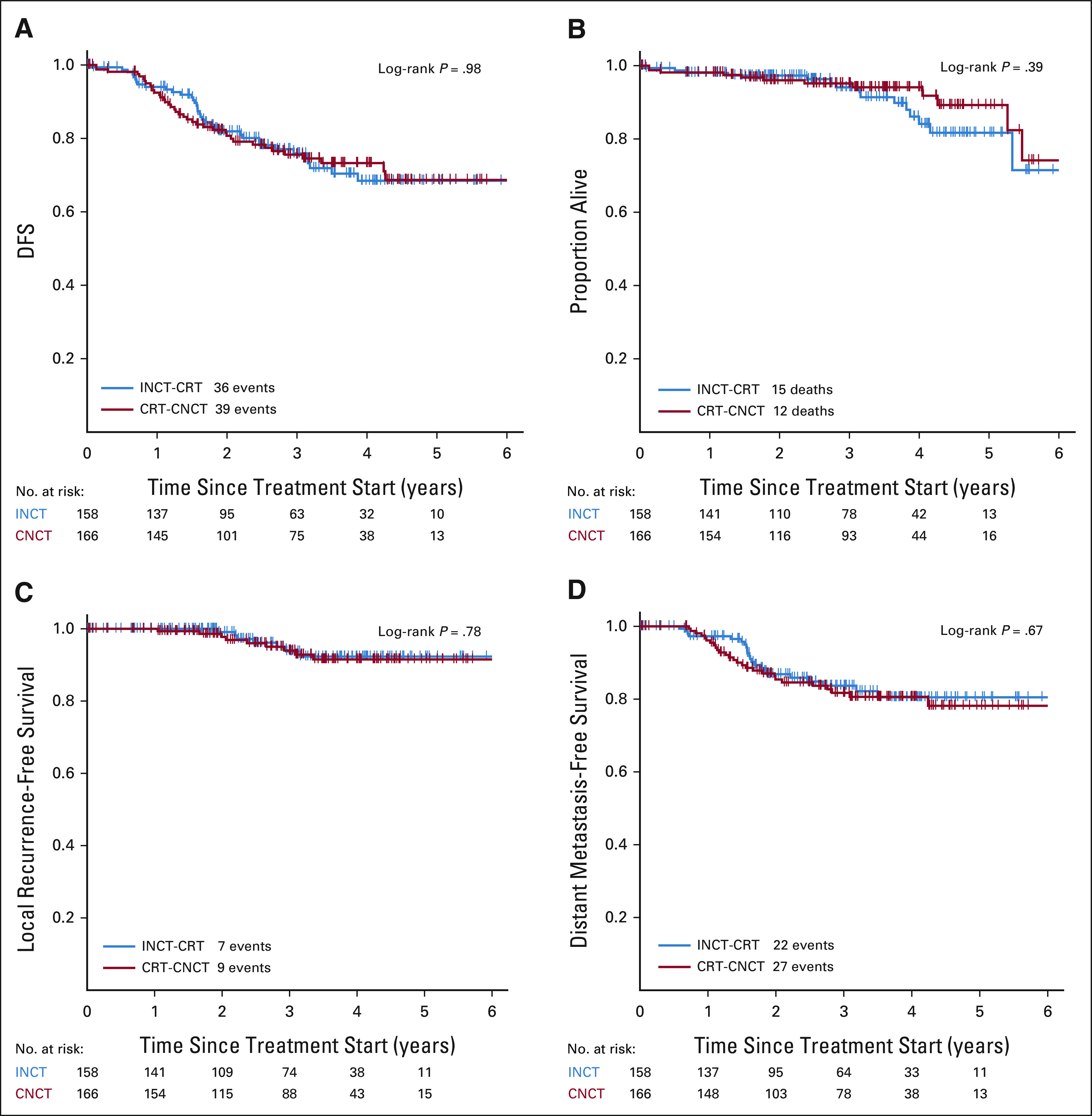
Kaplan-Meier estimates of (A) DFS, (B) overall survival, (C) local recurrence-free survival, and (D) distant metastasis-free survival in the intention-to-treat population by study group. CRT-CNCT, chemoradiotherapy followed by consolidation chemotherapy; DFS, disease-free survival; INCT-CRT, induction chemotherapy followed by chemoradiotherapy.
Of 304 patients restaged at the end of TNT, 79 (26%) were recommended to undergo TME; 41 of 146 (28%) in the INCT-CRT group and 38 of 158 (24%) in the CRT-CNCT group. The remaining 225 (74%) patients, 105/146 (71%) in the INCT-CRT and 120/158 (76%) in the CRT-CNCT, were offered WW (Fig 1). Of the 225 patients initially entered in the WW protocol, 42/105 (40%) in the INCT-CRT group and 33/120 (27%) in the CRT-CNCT group developed tumor regrowth during follow-up (Figs 1 and 3A). All patients diagnosed with tumor regrowth were recommended for TME. For the intention-to-treat organ preservation analysis, patients who had local excision, patients who refused TME, and patients with TME withheld because of distant progression were all censored as having TME. Patients who refused TME were also censored as having persistent disease for the DFS analysis. The proportion of patients who preserved the rectum at 3 years for the intention-to-treat population was 53% (95% CI, 45 to 62) for the CRT-CNCT group and 41% (95% CI, 33 to 50) for the INCT-CRT group (P = .01; Fig 3B and Appendix Fig A2B). Clinical T3 (HR 2.05; 1.06 to 3.97) or T4 (HR 2.19; 1.02 to 4.70) compared with T1 or T2, clinical nodal metastasis (HR 1.84; 1.22 to 2.78), and INCT-CRT group (HR 1.43; 1.03 to 2.00) were associated with time to TME in multivariable Cox regression analysis. The proportion of patients who actually preserved the rectum (TME-free survival) was 60% (95% CI, 52 to 68) in the CRT-CNCT group and 47% (95% CI, 39 to 56) in the INCT-CRT group (P = .02; Fig 3C).
FIG 3.
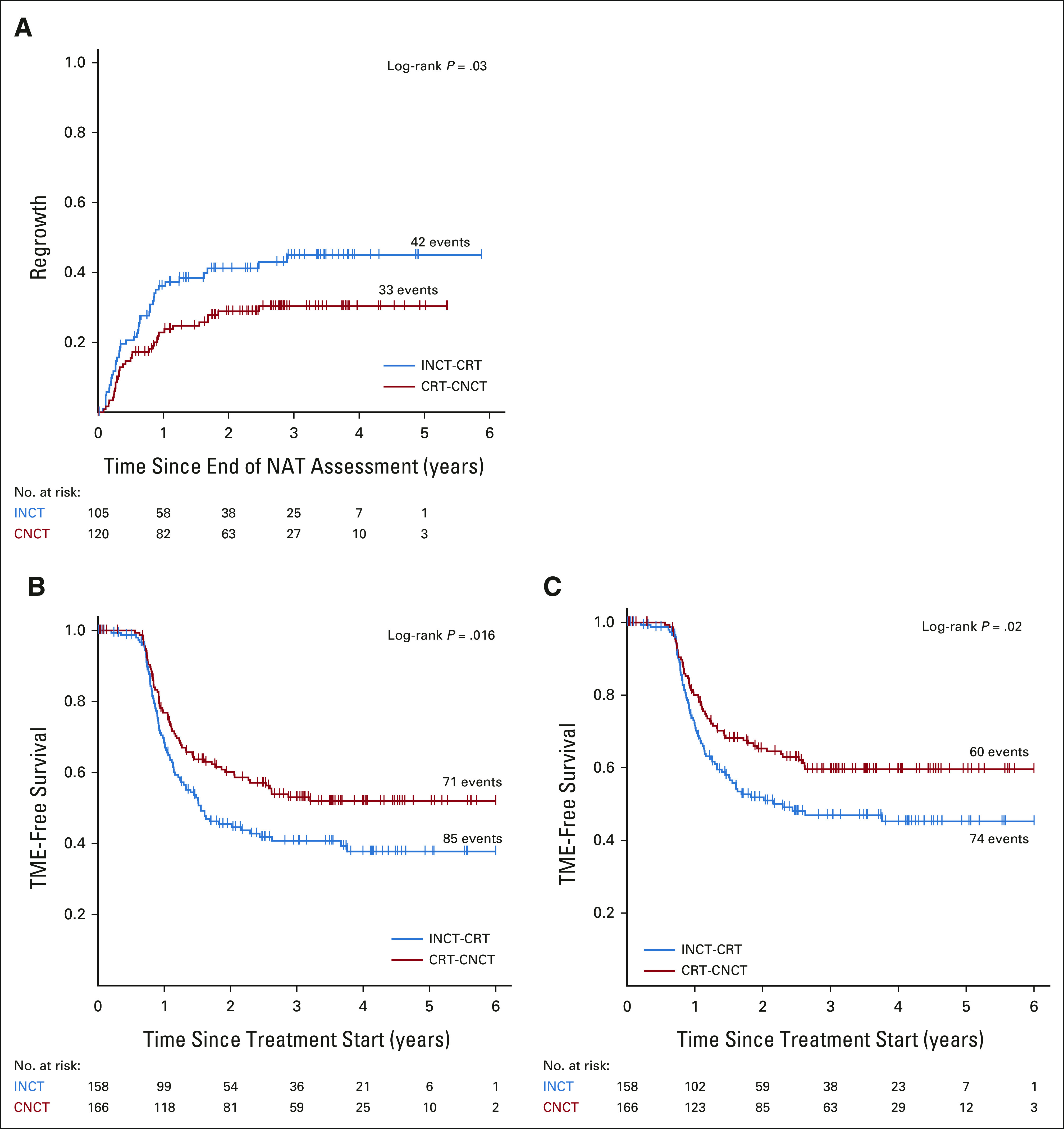
Kaplan-Meier estimates of (A) time to regrowth in watch-and-wait patients, (B) TME-free survival by intention to treat, and (C) for patients who underwent TME. CRT-CNCT, chemoradiotherapy followed by consolidation chemotherapy; INCT-CRT, induction chemotherapy followed by chemoradiotherapy; NAT, neoadjuvant therapy; TME, total mesorectal excision.
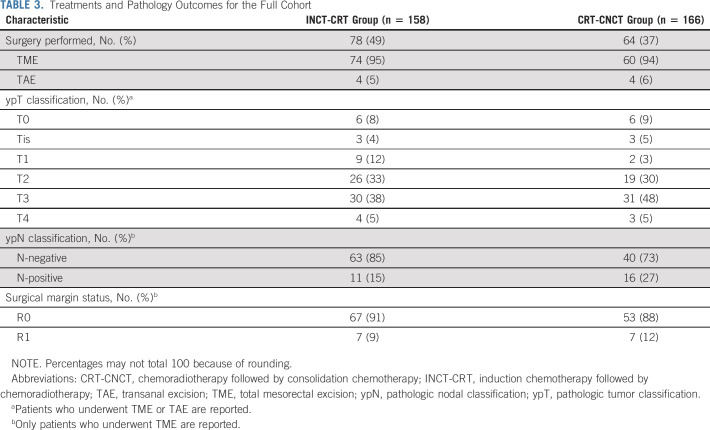
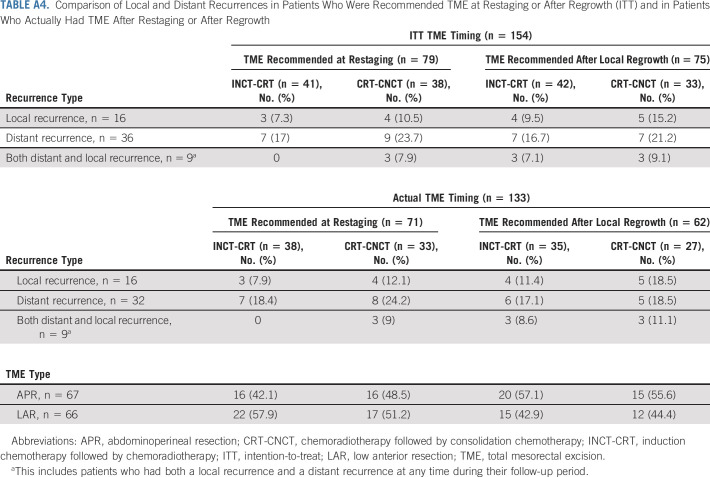
One patient in the INCT-CRT group required emergency TME before finishing neoadjuvant therapy. Of the 304 patients who underwent tumor restaging, 154 patients (79 at restaging for incomplete response and 75 during WW for tumor regrowth) were recommended TME, but only 133 had TME (71 at restaging for incomplete response and 62 during WW for tumor regrowth; Fig 1). The DFS rates were similar for patients having TME after restaging and for patients having TME after regrowth, both for the intention-to-treat population and for the patients who underwent TME (Fig 4). The median time from the day of restaging to the day of TME was 7 (IQR, 3-9.5) weeks in patients recommended TME at restaging and 30 (IQR, 20-103) weeks in patients recommended TME after regrowth. Six patients in each group have not reached the first follow-up clinical assessment after TME. The numbers of patients having TME at restaging or after tumor regrowth who developed local recurrence and distant metastasis after surgery, both for the intention-to-treat and true TME analyses, are presented in Appendix Table A4 (online only). The rate of sphincter-saving surgery was higher in patients having TME at restaging (39/71, 55%) compared with patients having TME after regrowth (27/62, 44%), but the difference was not statistically significant (P = .2). There was no statistically significant difference in the rate of sphincter-saving surgery between treatment groups. The pathologic outcomes for patients having surgery, both local excision and TME, are presented in Table 3.
FIG 4.
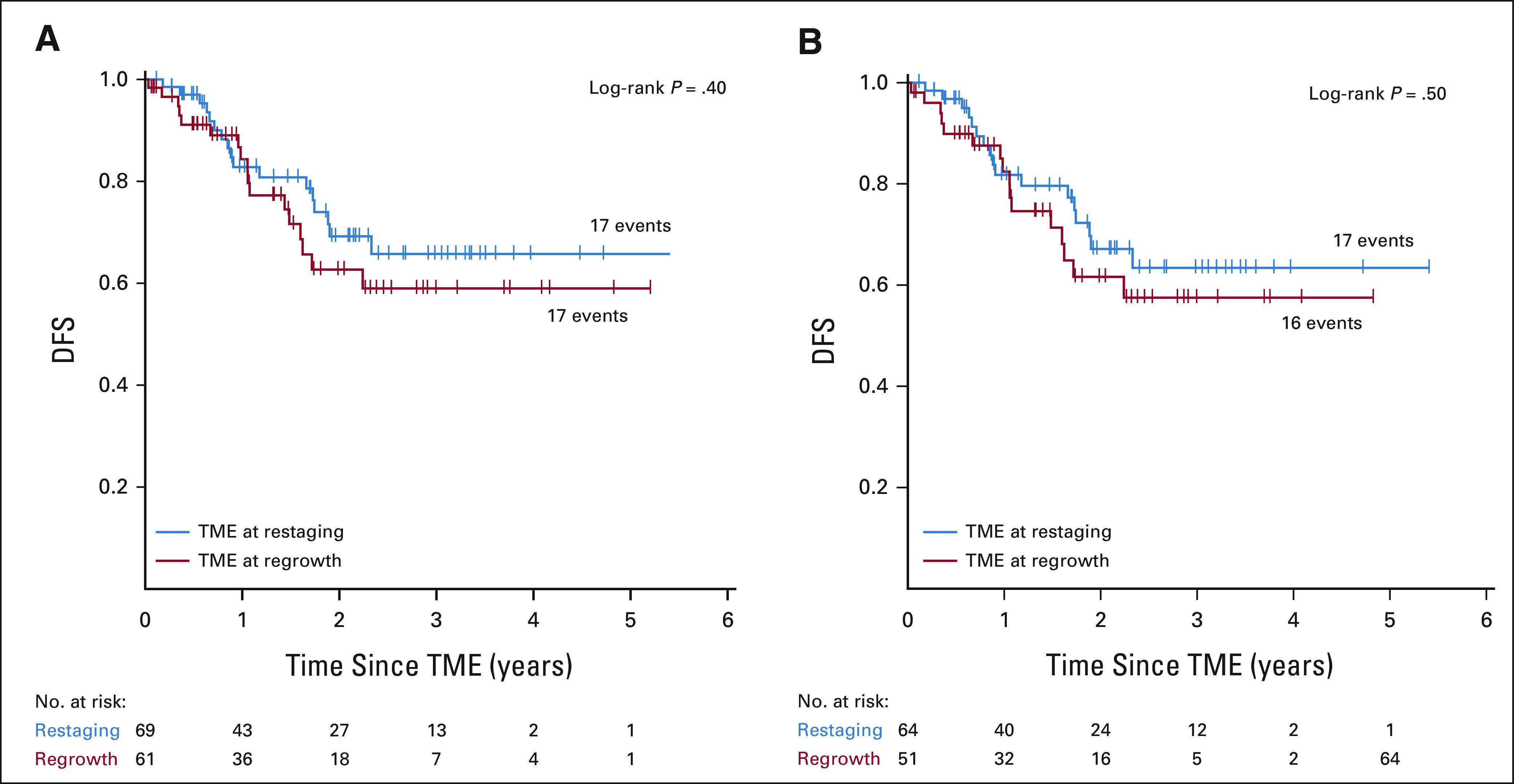
Kaplan-Meier estimates of DFS for (A) patients recommended TME after restaging and after tumor regrowth by intention to treat and (B) patients who actually underwent TME. Patients who developed distant metastasis before TME was recommended (three at restaging and six at regrowth) and patients in whom TME was not performed because of disease progression found at surgery (one at restaging and two at regrowth) are not included in the analysis. Six patients in each group have not reached the first follow-up clinical assessment after TME. DFS, disease-free survival; TME, total mesorectal excision.
TABLE 3.
Treatments and Pathology Outcomes for the Full Cohort
DISCUSSION
For patients with locally advanced rectal cancer, a treatment approach including selective WW for patients with a cCR to TNT provides similar DFS compared with historical control patients treated with neoadjuvant CRT, TME, and intended adjuvant chemotherapy. Although the primary end point of 3-year DFS rates in both arms did not reach the upper critical value specified by the study design, they were in line with historical controls and are within the range reported in recent prospective clinical trials.10,11,16-19 We also found that many patients treated with TNT, approximately half of those receiving CRT-CNCT, achieved a sustained cCR and preserved the rectum at 3 years.
The failure to reject the null hypothesis has several potential explanations. First are potential differences in patient characteristics such as tumor stage and distance from the anal verge between the study population and historical controls. Second, it is possible that TNT may not improve DFS compared with standard CRT, TME, and postoperative chemotherapy. The GCR-3 trial, a small phase II study, failed to demonstrate a difference in pCR or survival in patients with stage II and III rectal adenocarcinoma randomly assigned to chemotherapy before or after CRT and TME.20 By contrast, two recent large studies using different TNT regimens reported lower disease-related treatment failure or DFS in the experimental arms compared with the standard arm of chemoradiation, TME surgery, and postoperative chemotherapy.10,11 However, neither study showed improved overall survival in the experimental compared with the standard arm.10,11 Alternatively, it is possible that the WW strategy had a detrimental effect on patient DFS. A definitive proof of the safety and efficacy of organ preservation in rectal adenocarcinoma would require a large phase III trial with a noninferiority design, where patients achieving a cCR to TNT would be randomly assigned to TME or a WW strategy, although such a trial would be extremely unlikely to accrue.
We found that close to half of the patients treated with TNT achieved a sustained cCR and preserved the rectum. Although the median follow-up is still relatively short, and more patients may develop regrowth and require surgery in the future, the time to TME curve in this study and previously reported retrospective series suggests that the overwhelming majority of cases of regrowth occur during the first 2 years after completion of neoadjuvant therapy.6
The organ preservation rate in the OPRA trial was substantially higher than the pCR rates reported in recent clinical trials for patients with locally advanced rectal adenocarcinoma treated with TNT and TME.8-12 The explanation for this is not clear. These variations in tumor response could be attributed to differences in patient inclusion criteria or treatment plans, but they can also be attributed to the long interval from the end of TNT to the assessment of tumor response and to offering WW to patients with near-complete clinical response. In addition, almost 10% of patients undergoing TME had a pCR (no residual cancer cells in the surgical specimens), further highlighting rectal cancer responsiveness to TNT, as well as the difficulty in identifying patients with a complete response.
The higher organ preservation rate in patients treated with CRT-CNCT compared with INCT-CRT is consistent with the results of the CAO/ARO/AIO-12 trial, which reported a higher rate of pathologic complete response in patients with rectal cancer treated with CRT followed by three cycles of FOLFOX and TME compared with patients treated with three cycles of FOLFOX followed by CRT and TME.12 Similar to the CAO/ARO/AIO-12 trial, the difference in organ preservation in the OPRA trial cannot be attributed to differences in total dose of radiation or chemotherapy received, in total treatment time, or in time intervals from the initiation and from the end of neoadjuvant therapy to assessment of tumor response. The different time interval from the end of chemoradiation to the assessment of response has been considered a potential factor contributing to the difference in organ preservation between groups. However, the results of the trial are not totally consistent with this interpretation, as the proportion of patients recommended for WW at restaging plus the proportion of patients with pCR among those who had surgery immediately after restaging did not differ (78% CRT-CNCT v 75% INCT-CRT). By contrast, the higher rate of tumor regrowth in the INCT-CRT group (40%) compared with the CRT-CNCT group (27%) suggests that the difference in organ preservation may be due, at least in part, to differences in tumor regrowth and not in the proportion of patients initially recommended for WW.
A major perceived drawback of the WW strategy is the potential risk of tumor spread among patients with an apparent complete or near-complete clinical response who are initially observed and later develop tumor regrowth. We found no significant difference in outcomes among patients recommended for TME immediately after restaging compared with WW patients recommended for TME after tumor regrowth. The results were similar in the intention-to-treat population and in the patients who actually had TME. Our results are consistent with a report from Dr Habr-Gama's group who described no survival detriment in patients who had delayed surgery for suspected cCR.21
In conclusion, a treatment strategy including TNT and selective WW or TME on the basis of tumor response allows organ preservation in almost half of the patients with locally advanced rectal adenocarcinoma without an apparent adverse impact on oncologic outcomes. Although the sequence of the CRT and systemic chemotherapy did not affect DFS, delivering CRT before systemic chemotherapy appeared to result in a higher rate of organ preservation compared with delivering CRT after systemic chemotherapy.
ACKNOWLEDGMENT
Collaborators who made valuable contributions to this study are listed in Appendix 1 (online only).
APPENDIX
FIG A1.
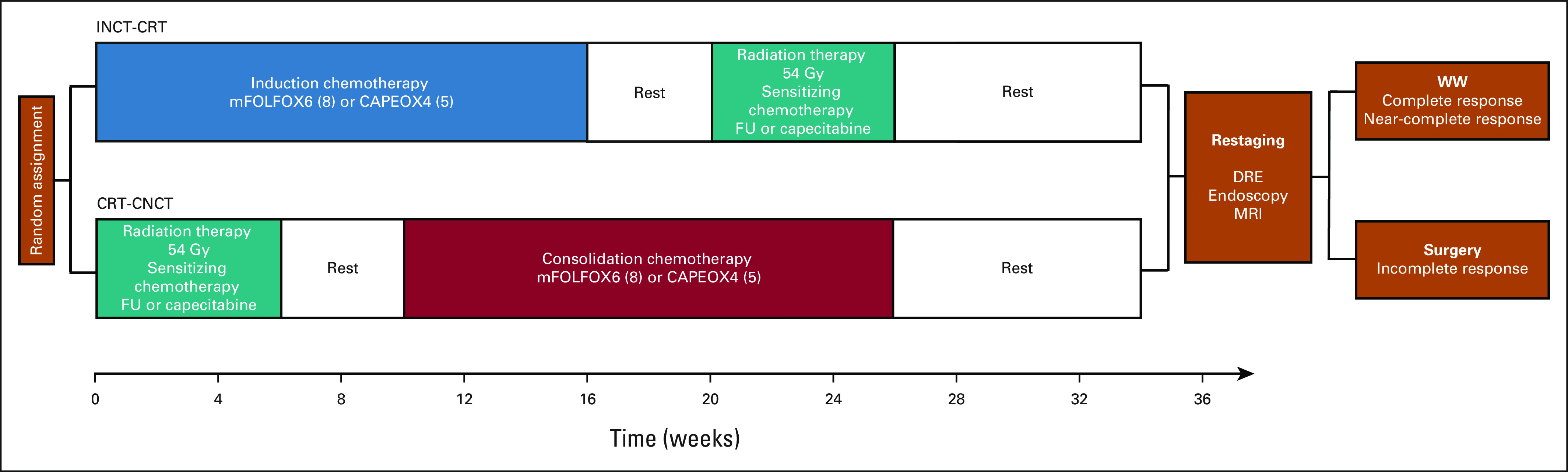
Trial schema. CAPEOX, capecitabine and oxaliplatin; CRT-CNCT, chemoradiotherapy followed by consolidation chemotherapy; DRE, digital rectal exam; FU, fluorouracil; Gy, gray; INCT-CRT, induction chemotherapy followed by chemoradiotherapy; mFOLFOX, modified infusional fluorouracil, leucovorin, and oxaliplatin; MRI, magnetic resonance imaging; WW, watch-and-wait.
FIG A2.
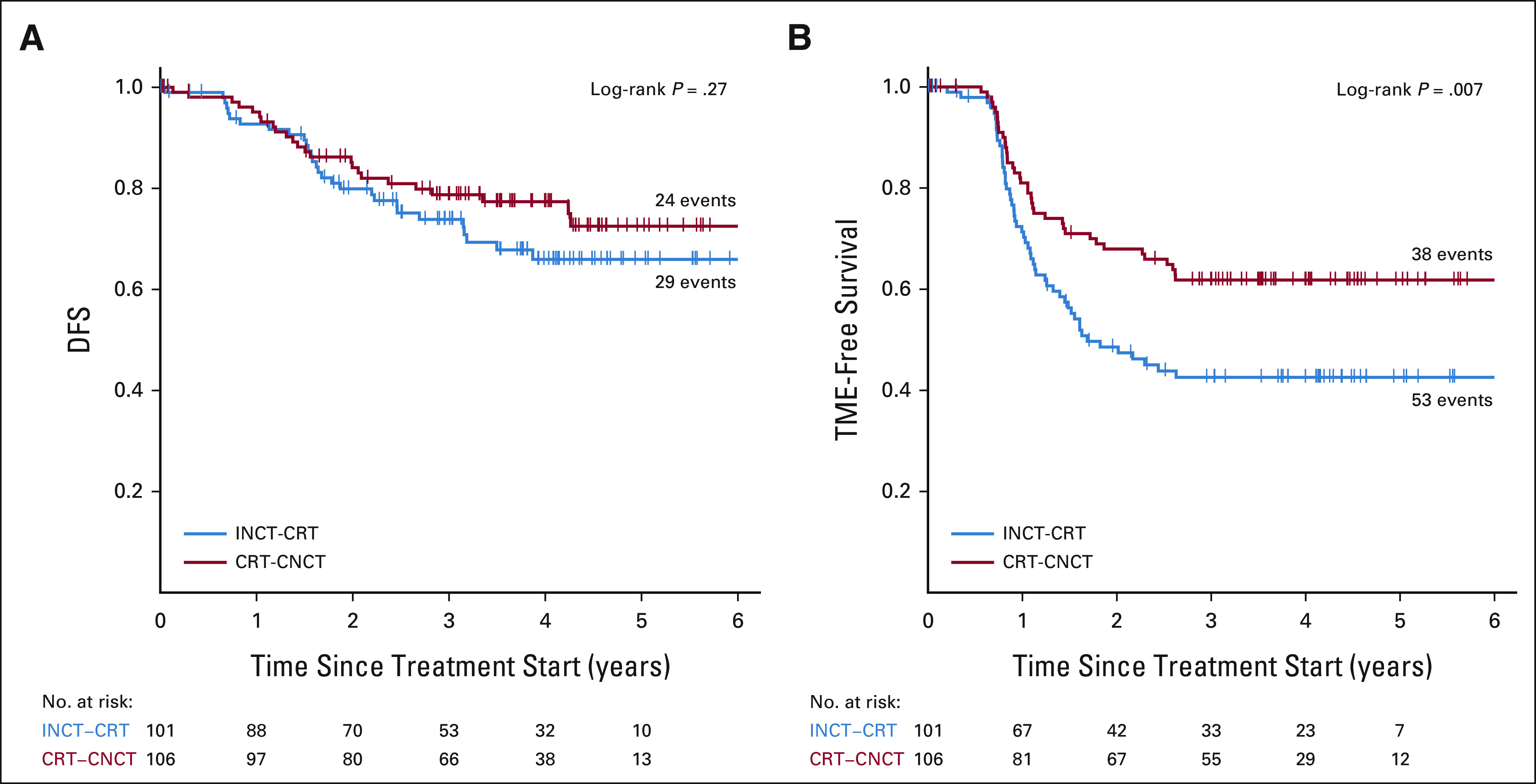
Kaplan-Meier estimates of (A) DFS and (B) TME-free survival in the initial cohort. CRT-CNCT, chemoradiotherapy followed by consolidation chemotherapy; DFS, disease-free survival; INCT-CRT, induction chemotherapy followed by chemoradiotherapy; TME, total mesorectal excision.
TABLE A1.
Accrual and Random Assignment by Participating Institution
TABLE A2.
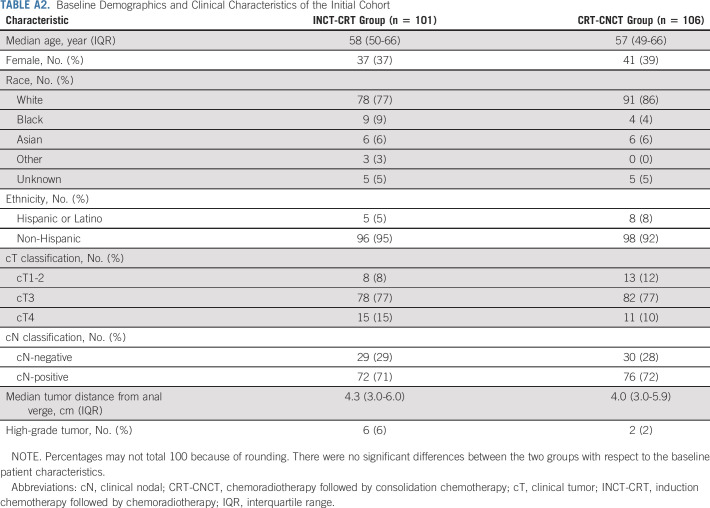
Baseline Demographics and Clinical Characteristics of the Initial Cohort
TABLE A3.
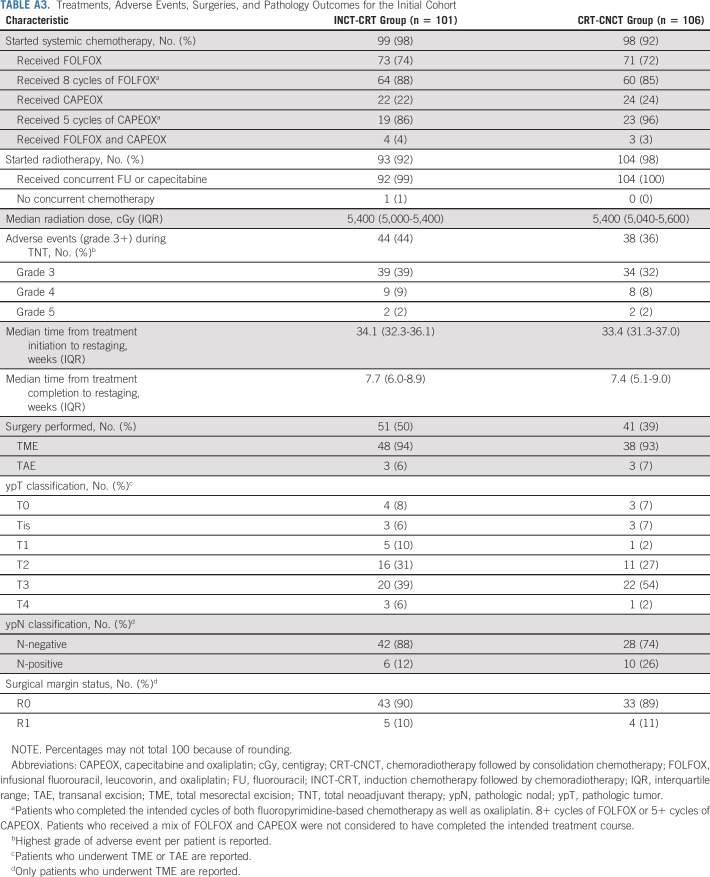
Treatments, Adverse Events, Surgeries, and Pathology Outcomes for the Initial Cohort
TABLE A4.
Comparison of Local and Distant Recurrences in Patients Who Were Recommended TME at Restaging or After Regrowth (ITT) and in Patients Who Actually Had TME After Restaging or After Regrowth
APPENDIX 1.
The authors would like to acknowledge the following for their contributions to the OPRA trial.
Julio Garcia-Aguilar
Stock and Other Ownership Interests: Intuitive Surgical
Consulting or Advisory Role: Medtronic, Intuitive Surgical, Johnson & Johnson
Sujata Patil
Consulting or Advisory Role: ByHeart
Marc J. Gollub
Stock and Other Ownership Interests: Pfizer
Meghan Lee
Employment: ICON Clinical Research
Richard F. Dunne
Consulting or Advisory Role: Exelixis, Helsinn Therapeutics
Jorge Marcet
Consulting or Advisory Role: Baxter, Stryker, Medtronic
Blase Polite
Honoraria: Physicans' Education Resource, Simon-Kucher and Partners, American Journal of Managed Care, HMP, Genzyme
Consulting or Advisory Role: Cancer Expert Now
Speakers' Bureau: Natera
Travel, Accommodations, Expenses: Tapestry Pharmaceuticals, Institute for Clinical and Economic Review
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1196539
David Liska
Consulting or Advisory Role: Olympus Medical Systems
Charles Ternent
Honoraria: Intuitive Surgical
Consulting or Advisory Role: Virtual Incisions Inc
Travel, Accommodations, Expenses: Intuitive Surgical
Andrew L. Coveler
Consulting or Advisory Role: Halozyme, Seattle Genetics, Merrimack, AbbVie
Research Funding: XBiotech (Inst), Newlink Genetics (Inst), Taiho Pharmaceutical (Inst), Immunomedics (Inst), Onconova Therapeutics (Inst), Lilly (Inst), Gilead Sciences (Inst), Genentech (Inst), Seattle Genetics (Inst), AbGenomics International (Inst), Halozyme (Inst), Novocure (Inst), MedImmune (Inst), Amgen (Inst), Actuate Therapeutics (Inst), Surface Oncology (Inst)
Travel, Accommodations, Expenses: Halozyme, AbbVie
Joseph C. Carmichael
Consulting or Advisory Role: Johnson & Johnson
Speakers' Bureau: Johnson & Johnson
John Krauss
Research Funding: Ignyta (Inst), Boston Biomedical (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), ACCRU (Inst), NSABP Foundation (Inst), Amgen (Inst), Isofol Medical (Inst), NSABP Foundation (Inst), Novartis (Inst), Hutchison MediPharma (Inst), Cardiff Oncology, AstraZeneca/MedImmune (Inst), Tempest Therapeutics (Inst), Tempest Therapeutics (Inst), Pfizer (Inst), Alliance for Clinical Trials in Oncology (Inst), Daiichi Sankyo/Arqule (Inst), Bristol Myers Squibb/Medarex (Inst)
Martin R. Weiser
Consulting or Advisory Role: Precisca
Research Funding: Clinical Genomics
Patents, Royalties, Other Intellectual Property: UpToDate Section Editor
Garrett M. Nash
Open Payments Link: https://openpaymentsdata.cms.gov/physician/851428
Emmanouil Pappou
Travel, Accommodations, Expenses: Intuitive Surgical
José G. Guillem
Employment: Intuitive Surgical
Honoraria: Intuitive Surgical, Myriad Genetics
Speakers' Bureau: Intuitive Surgical
Maria Widmar
Employment: BridgeBio (I)
Stock and Other Ownership Interests: BridgeBio
Neil H. Segal
Consulting or Advisory Role: Roche/Genentech, GlaxoSmithKline, ABL Bio, Boehringer Ingelheim, Novartis, Revitope, AstraZeneca
Research Funding: Bristol Myers Squibb, Pfizer, Roche/Genentech, Merck, Immunocore, AstraZeneca, Regeneron (Inst), PureTech (Inst)
Andrea Cercek
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Incyte, Merck, Janssen, Seattle Genetics
Research Funding: Seattle Genetics, Rgenix (Inst), GlaxoSmithKline
Rona Yaeger
Consulting or Advisory Role: Array BioPharma, Natera, Mirati Therapeutics
Research Funding: Array BioPharma (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Mirati Therapeutics (Inst)
J. Joshua Smith
Consulting or Advisory Role: Guardant Health
Karyn A. Goodman
Consulting or Advisory Role: RenovoRx, Roche/Genentech, Novartis, Philips Healthcare
Abraham J. Wu
Stock and Other Ownership Interests: Simphotek
Consulting or Advisory Role: AstraZeneca, MORE Health, NanoVi
Research Funding: CivaTech Oncology
Travel, Accommodations, Expenses: CivaTech Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/368691
Leonard B. Saltz
Consulting or Advisory Role: Genor
Research Funding: Taiho Pharmaceutical
No other potential conflicts of interest were reported.
See accompanying editorial on page 2515
DISCLAIMER
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
PRIOR PRESENTATION
Presented in part at the ASCO Virtual Annual Meeting, May 29-31, 2020.
SUPPORT
Supported by grants from the National Cancer Institute of the United States R01CA182551, P30CA008748, and T32 CA009501.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Upon publication, deidentified participant data will be made available with a signed data access agreement after approval of a proposal e-mailed to the corresponding author.
AUTHOR CONTRIBUTIONS
Conception and design: Julio Garcia-Aguilar, Sujata Patil, Marc J. Gollub, Jorge Marcet, Peter Cataldo, Daniel O. Herzig, Samuel Oommen, Charles M. Friel, Steven Hunt, Brian L. Bello, Philip B. Paty, Martin R. Weiser, Garrett M. Nash, Larissa Temple, Andrea Cercek, J. Joshua Smith, Karyn A. Goodman, Abraham J. Wu, Leonard B. Saltz
Administrative support: Meghan Lee, Philip B. Paty
Provision of study materials or patients: Julio Garcia-Aguilar, Peter Cataldo, David Liska, Charles M. Friel, Charles Ternent, Andrew L. Coveler, Anita Gregory, John Krauss, Philip B. Paty, Martin R. Weiser, Garrett M. Nash, Larissa Temple, Karyn A. Goodman, Leonard B. Saltz
Collection and assembly of data: Marc J. Gollub, Jin K. Kim, Jonathan B. Yuval, Hannah M. Thompson, Floris S. Verheij, Dana M. Omer, Meghan Lee, Richard F. Dunne, Jorge Marcet, Peter Cataldo, Blase Polite, Daniel O. Herzig, David Liska, Samuel Oommen, Charles Ternent, Andrew L. Coveler, Steven Hunt, Anita Gregory, Madhulika G. Varma, Brian L. Bello, Joseph C. Carmichael, John Krauss, Ana Gleisner, Philip B. Paty, Martin R. Weiser, Emmanouil Pappou, Iris H. Wei, Maria Widmar, Andrea Cercek, Rona Yaeger, J. Joshua Smith, Karyn A. Goodman, Abraham J. Wu, Leonard B. Saltz
Data analysis and interpretation: Julio Garcia-Aguilar, Sujata Patil, Marc J. Gollub, Jin K. Kim, Hannah M. Thompson, Floris S. Verheij, Dana M. Omer, Richard F. Dunne, Jorge Marcet, Peter Cataldo, Blase Polite, David Liska, Samuel Oommen, Andrew L. Coveler, Brian L. Bello, Joseph C. Carmichael, Ana Gleisner, Martin R. Weiser, Garrett M. Nash, Emmanouil Pappou, José G. Guillem, Maria Widmar, Sabrina Lin, Neil H. Segal, Andrea Cercek, Rona Yaeger, J. Joshua Smith, Abraham J. Wu, Leonard B. Saltz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Julio Garcia-Aguilar
Stock and Other Ownership Interests: Intuitive Surgical
Consulting or Advisory Role: Medtronic, Intuitive Surgical, Johnson & Johnson
Sujata Patil
Consulting or Advisory Role: ByHeart
Marc J. Gollub
Stock and Other Ownership Interests: Pfizer
Meghan Lee
Employment: ICON Clinical Research
Richard F. Dunne
Consulting or Advisory Role: Exelixis, Helsinn Therapeutics
Jorge Marcet
Consulting or Advisory Role: Baxter, Stryker, Medtronic
Blase Polite
Honoraria: Physicans' Education Resource, Simon-Kucher and Partners, American Journal of Managed Care, HMP, Genzyme
Consulting or Advisory Role: Cancer Expert Now
Speakers' Bureau: Natera
Travel, Accommodations, Expenses: Tapestry Pharmaceuticals, Institute for Clinical and Economic Review
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1196539
David Liska
Consulting or Advisory Role: Olympus Medical Systems
Charles Ternent
Honoraria: Intuitive Surgical
Consulting or Advisory Role: Virtual Incisions Inc
Travel, Accommodations, Expenses: Intuitive Surgical
Andrew L. Coveler
Consulting or Advisory Role: Halozyme, Seattle Genetics, Merrimack, AbbVie
Research Funding: XBiotech (Inst), Newlink Genetics (Inst), Taiho Pharmaceutical (Inst), Immunomedics (Inst), Onconova Therapeutics (Inst), Lilly (Inst), Gilead Sciences (Inst), Genentech (Inst), Seattle Genetics (Inst), AbGenomics International (Inst), Halozyme (Inst), Novocure (Inst), MedImmune (Inst), Amgen (Inst), Actuate Therapeutics (Inst), Surface Oncology (Inst)
Travel, Accommodations, Expenses: Halozyme, AbbVie
Joseph C. Carmichael
Consulting or Advisory Role: Johnson & Johnson
Speakers' Bureau: Johnson & Johnson
John Krauss
Research Funding: Ignyta (Inst), Boston Biomedical (Inst), Boehringer Ingelheim (Inst), AbbVie (Inst), ACCRU (Inst), NSABP Foundation (Inst), Amgen (Inst), Isofol Medical (Inst), NSABP Foundation (Inst), Novartis (Inst), Hutchison MediPharma (Inst), Cardiff Oncology, AstraZeneca/MedImmune (Inst), Tempest Therapeutics (Inst), Tempest Therapeutics (Inst), Pfizer (Inst), Alliance for Clinical Trials in Oncology (Inst), Daiichi Sankyo/Arqule (Inst), Bristol Myers Squibb/Medarex (Inst)
Martin R. Weiser
Consulting or Advisory Role: Precisca
Research Funding: Clinical Genomics
Patents, Royalties, Other Intellectual Property: UpToDate Section Editor
Garrett M. Nash
Open Payments Link: https://openpaymentsdata.cms.gov/physician/851428
Emmanouil Pappou
Travel, Accommodations, Expenses: Intuitive Surgical
José G. Guillem
Employment: Intuitive Surgical
Honoraria: Intuitive Surgical, Myriad Genetics
Speakers' Bureau: Intuitive Surgical
Maria Widmar
Employment: BridgeBio (I)
Stock and Other Ownership Interests: BridgeBio
Neil H. Segal
Consulting or Advisory Role: Roche/Genentech, GlaxoSmithKline, ABL Bio, Boehringer Ingelheim, Novartis, Revitope, AstraZeneca
Research Funding: Bristol Myers Squibb, Pfizer, Roche/Genentech, Merck, Immunocore, AstraZeneca, Regeneron (Inst), PureTech (Inst)
Andrea Cercek
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Incyte, Merck, Janssen, Seattle Genetics
Research Funding: Seattle Genetics, Rgenix (Inst), GlaxoSmithKline
Rona Yaeger
Consulting or Advisory Role: Array BioPharma, Natera, Mirati Therapeutics
Research Funding: Array BioPharma (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Mirati Therapeutics (Inst)
J. Joshua Smith
Consulting or Advisory Role: Guardant Health
Karyn A. Goodman
Consulting or Advisory Role: RenovoRx, Roche/Genentech, Novartis, Philips Healthcare
Abraham J. Wu
Stock and Other Ownership Interests: Simphotek
Consulting or Advisory Role: AstraZeneca, MORE Health, NanoVi
Research Funding: CivaTech Oncology
Travel, Accommodations, Expenses: CivaTech Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/368691
Leonard B. Saltz
Consulting or Advisory Role: Genor
Research Funding: Taiho Pharmaceutical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Benson AB, Venook AP, Al-Hawary MM, et al. : NCCN guidelines insights: Rectal cancer, version 6.2020. J Natl Compr Canc Netw 18:806-815, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Kang SB, Cho JR, Jeong SY, et al. : Quality of life after sphincter preservation surgery or abdominoperineal resection for low rectal cancer (ASPIRE): A long-term prospective, multicentre, cohort study. Lancet Reg Health West Pac 6:100087, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters KC, van de Velde CJ, Leer JW, et al. : Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch colorectal cancer group study. J Clin Oncol 23:6199-6206, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, et al. : Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 11:835-844, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Habr-Gama A, Perez RO, Nadalin W, et al. : Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg 240:711-718, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Valk MJM, Hilling DE, Bastiaannet E, et al. : Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 391:2537-2545, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Appelt AL, Pløen J, Harling H, et al. : High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol 16:919-927, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Cercek A, Roxburgh CSD, Strombom P, et al. : Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 4:e180071, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Aguilar J, Chow OS, Smith DD, et al. : Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol 16:957-966, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahadoer RR, Dijkstra EA, van Etten B, et al. : Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol 22:29-42, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Conroy T, Bosset JF, Etienne PL, et al. : Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22:702-715, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Fokas E, Allgäuer M, Polat B, et al. : Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol 37:3212-3222, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Smith JJ, Chow OS, Gollub MJ, et al. : Organ preservation in rectal adenocarcinoma: A phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 15:767, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Short MW, Layton MC, Teer BN, et al. : Colorectal cancer screening and surveillance. Am Fam Physician 91:93-100, 2015 [PubMed] [Google Scholar]
- 15.Lawless JE: Statistical Models and Methods for Lifetime Data. New York, NY, John Wiley & Sons, 1982 [Google Scholar]
- 16.Gérard JP, Azria D, Gourgou-Bourgade S, et al. : Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 30:4558-4565, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Allegra CJ, Yothers G, O'Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: A phase III randomized clinical trial. J Natl Cancer Inst 107:djv248, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rödel C, Graeven U, Fietkau R, et al. : Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16:979-989, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Fokas E, Schlenska-Lange A, Polat B, et al. : Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: Long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol 8:e215445, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. : Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol 26:1722-1728, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Habr-Gama A, Perez RO, Proscurshim I, et al. : Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: Does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys 71:1181-1188, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon publication, deidentified participant data will be made available with a signed data access agreement after approval of a proposal e-mailed to the corresponding author.