Abstract
Reductions in brain blood flow are associated with reduced cognitive function and cerebrovascular disease. Acute periods of uninterrupted sitting can lead to endothelial dysfunction, namely due to a reduction in shear stress and subsequent reduction in nitric oxide bioavailability. Little is known of the impact of sitting on brain health. The purpose was to determine the total brain blood flow response following a 60-minute bout of uninterrupted sitting. Using a parallel design, this study evaluated the impact of 60-minutes of sitting on total brain blood flow. Fifteen participants (n=15; age=24 ± 1yr; BMI=25 ± 1 kg/m2) sat, uninterrupted, for 60-minutes during the SIT protocol. To ascertain the contribution of blood pooling effects on total brain blood flow, ten participants (n=10; age=23±2yr; BMI=27±4 kg/m2) sat in a modified sitting (MOD) for 60-minutes. Finally, thirteen participants (n=13; age=23±3yr; BMI=26±4 kg/m2) remained supine for the duration of the 60-minutes as a time-control (TC). Brain blood flow was quantified through Doppler-ultrasound measurements of blood flow through the internal carotid (ICA) and vertebral (VA) arteries: (ICA blood flow + VA blood flow) × 2. Following the 60-minutes of sitting (SIT), there was a significant reduction in brain blood flow with time (p=0.001, ηp2=0.05). Total brain blood flow did not significantly change in MOD (p=0.69, ηp2=0.05) or TC (p=0.06, ηp2=0.58) conditions. These findings indicate 60-minutes of sitting may alter cerebrovascular hemodynamics characterized by a reduction in total brain blood flow.
Keywords: Brain blood flow, sedentary behavior, extracranial arteries, doppler ultrasound, measurement
INTRODUCTION
On average, adults spend two-thirds of their waking day engaged in sedentary behaviors (13), defined as any waking activity of ≤1.5 METs in the sitting, reclining, or lying position. Sedentary behavior, particularly sitting, is a significant risk factor for many chronic diseases, including cardiovascular disease (CVD) and neurodegenerative diseases (5,7,23). While not well-explored, one potential mechanism linking regular sitting exposure to neurodegenerative disease is reduced brain blood flow during the sitting behavior (12). Normal brain function is dependent on the regular supply of blood to deliver adequate delivery of oxygen and glucose (24,25). Prolonged sitting can lead to blood pooling in the lower extremities, resulting in decreased venous return and disrupted brain blood flow (4,22). Indeed, three hours of sitting resulted in a moderate decrease in total brain blood flow (Qbrain) – measured through the vertebral artery (VA) and internal carotid artery (ICA) (1). However, it should be noted that individuals engage in multiple sitting postures, including the traditional sitting posture (i.e., legs at a 90 angle) and a modified sitting posture with legs slightly elevated. The effects of both traditional and modified sitting postures on brain blood flow have not been investigated. Sitting in the modified sitting position with the legs slightly elevated may attenuate the impact of venous pooling in the lower limbs, and, thus, may be a feasible seated position to mitigate the potential reduction in Qbrain in the traditional sitting position.
The purpose of this pilot investigation was to determine the impact of 60-minutes of sitting in different seated position on Qbrain. It was previously reported by Shvartz and colleagues that 60-minutes of uninterrupted sitting was enough to observe any changes in the hemodynamic response (20). To ascertain the ‘normal’ physiological response, healthy young participants were recruited and studied. Using a parallel study design, we tested two hypotheses: 1) uninterrupted sitting would lead to a significant decline in Qbrain, and 2) uninterrupted sitting in the modified sitting position would not lead to a significant decline in Qbrain.
METHODS
Participants
To estimate the sample size required to detect the smallest effect in a parallel study with a Type I Error rate of 0.05 and 80% power, approximately 12 participants per group were required to detect a small effect change (effect size: 0.2) in Qbrain. Participants were recruited from the University of Southern Mississippi campus and surrounding Hattiesburg, Mississippi area. Inclusion criteria consisted of young men and women, free of any diagnosed cardiovascular, neurological, or metabolic diseases, nonsmokers, asymptomatic (e.g., no arrhythmias or resting blood pressure >140/90), and not pregnant. Participants could not be on any vasoactive medications known to affect cardiovascular measures. Premenopausal women were measured in the early follicular phase of their menstrual cycle. Prior to participation in the study, the participants underwent a consenting process and signed an informed consent form. Procedures and protocols conformed to the Declaration of Helsinki (17) and were reviewed and approved by the University of Southern Mississippi Institutional Review Board (Protocol Approval # IRB-20-11). This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (11).
A total of thirty-eight participants were studied and randomized to a specific protocol: SIT (n =15; males =9; age =24 ± 1 years; BMI = 25 ± 1 kg/m2); MOD (n = 10; males = 7; age = 23 ± 2 years; BMI =27 ± 4 kg/m2); TC (n =13; males = 8; age = 23 ± 3 years; BMI = 26 ± 4 kg/m2). All participants were studied in a quiet, dimly lit, temperature (21 – 23 degrees C) and humidity-controlled (50%) room. Participants were asked to refrain from caffeine intake for 12 hours, alcohol and strenuous activity for 24 hours, and be at least 2 hours fasted prior to data collection. The last meal was recorded, and participants were instructed to consume a similar meal for any follow-up study visits. After the consenting process, height and weight data were gathered using a standard stadiometer and digital scale, and participants were positioned supine on an examination table and instrumented for the respective experimental protocol. Three independent protocols were performed, each of which lasted approximately two hours. Participants were monitored throughout each protocol, during which they were asked to refrain from boisterous activities (e.g., listening to heavy music or watching stress-inducing videos), and could move their arms but not their legs, (e.g. no leg fidgeting) (10). The primary outcome was Qbrain, and central hemodynamic measurements were made following each Qbrain assessment.
Protocol
The protocol consisted of three individual conditions—Standard Sitting (SIT), Modified Sitting (MOD), and Time-Control (TC) conditions (See Supplemental Figure). The SIT condition was performed to determine the Qbrain response to 60-minutes of sitting. Participants rested quietly for 10-minutes in the supine position, followed by baseline measurements. Following baseline, participants in the SIT protocol were instructed to step down from the examination table and seat themselves at a desk positioned next to the table. Participants remained in the seated posture for 60-minutes and engaged in non-stimulating tasks. Following 60-minutes, to avoid muscle contractions and limit hemodynamic disruptions, participants were mechanically lifted (Invacare Reliant 450, Invacare, Elyria, Ohio) and returned to the supine position on the examination table. Following five minutes quiet rest - to permit hemodynamic stabilization, measurements were repeated. Measurements were also taken at 10- and 60-minutes of sitting. The MOD condition was performed to limit lower-limb blood pooling determine the impact that that this has on Qbrain during 60-minutes in the MOD position. Participants rested quietly for 10-minutes in the supine position, followed by baseline measurements. Subsequently, participants remained on the examination table but were passively positioned upright with the torso at 90° and their lower limbs slightly elevated (10°) with a positioning pad to reduce lower limb blood pooling (10,15,20). Participants remained in the MOD posture for 60-minutes while performing standard, non-stimulating tasks. Following 60-minutes, participants were passively repositioned to the supine posture. Following five minutes quiet rest the measurements were repeated. Measurements were also taken at 10- and 60-minutes of MOD.
The TC condition was performed to determine how remaining in the supine position for 60-minutes impacted Qbrain. Participants in the TC protocol remained in the supine position for the duration of the 60-minute protocol with measures occurring only at pre- and post-60-minutes (as we expected no significant change in Qbrain to occur during those 60-minutes in the supine position) and engaged in non-stimulating tasks. Similar to the previous protocols, the participants rested quietly for 10-minutes in the supine position, followed by baseline measurements. The participants were then instructed to remain in the supine position for 60-minutes. Following the 60-minutes, post-sitting measurements were taken.
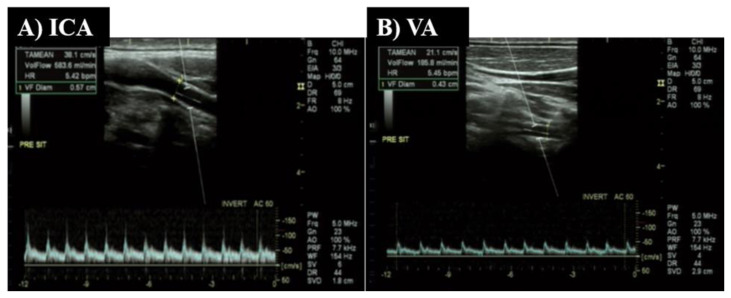
The Qbrain (ml/min) was calculated as (ICA blood flow + VA blood flow) X 2. (1,19). The ICA and VA both perfuse the anastomotic ring in the brain known as the Circle of Willis and are responsible for blood flow directly to both cerebral hemispheres of the brain. One operator (RJ) measured ICA and VA blood flow using duplex-Doppler ultrasound (Logiq P5; GE Medical systems, Milwaukee, WI). Both arteries were scanned on the right side, but the order of scanning was randomized between participants at the beginning of the visit and kept consistent during the protocol. Figure 1 depicts a representative example of the blood velocity pattern and image of the ICA (Panel A) and VA (Panel B).
Figure 1.
Example images taken from Doppler-ultrasound. Panel A depicts blood flow through the internal carotid artery (ICA) and Panel B—artery (VA).
The ICA was scanned ~1.0–1.5 cm distal to carotid bifurcation, and the VA between C3 transverse processes and subclavian artery. The arteries were scanned longitudinally using an 11-MHz linear array transducer. The blood velocity was measured simultaneously with the same probe while in pulse-wave mode operating at a frequency of 5-MHz. During all measurements, the probe was held in a stable position (i.e., positioning pad on shoulder supporting the sonographer’s wrist), with an insonation angle of ~60 degrees, and sample volume centered within the vessel and widened to encompass the entire vessel the lumen without extending beyond its borders. For each measurement, the probe position was marked, and ultrasound settings were kept consistent throughout the study.
ICA and VA blood flow (mL/min) using time-averaged mean velocity (Vmean) and vessel diameter, using the equation Vmean X π (diameter/2)2 X 60. For each measurement, a 15-second sweep of simultaneous blood velocity, in pulse wave mode, and B-mode images of the ICA and were captured. The Vmean was estimated using the Doppler-ultrasound internal software. Continuous mean diameters were measured during diastole using calipers positioned perpendicular to the lumen (near and far intima wall) of the vessels. Within-day reliability was determined using a subset of participants (n=8), with the intraclass correlation coefficient calculated as 0.83.
Central cardiovascular hemodynamics were assessed using an automated sphygmomanometer (SphygmoCor XCEL, AtCor Medical, Itasca, Illinois). The XCEL system performs a standard blood pressure assessment, followed by a sub-systolic inflation of the pressure cuff to obtain a brachial waveform, from which a validated transfer function is used to determine central aortic pressures (21). In addition to heart rate (HR), the following variables were recorded: central systolic and diastolic blood pressure (cSBP & cDBP), central pulse pressure (PP), and mean arterial pressure (MAP). Two measures of central hemodynamics were taken at each time point and averaged.
Statistical Analysis
To test the first hypothesis that SIT posture would cause a significant decrease in Qbrain, a one-way repeated measures analysis of variance (ANOVA) was performed to determine the time (baseline, 10-, 60-minutes of sitting, and post-sitting) effect; given a significant time effect, Bonferroni post-hoc analyses were run. The same statistical test was performed for the second hypothesis, that Qbrain reduction would be attenuated with a MOD posture. Effect sizes were calculated and reported using partial eta squared (ηp2), where <0.01 is small, >0.06 is moderate, and >0.14 is large (3). Statistical analyses were performed using IBM SPSS Statistics (Version 25, Armonk, NY). Significance was set a priori at p<0.05.
RESULTS
All cerebrovascular hemodynamic data and effect sizes are reported in Table 1.
Table 1.
Central Cardiovascular and Cerebrovascular Hemodynamic Response to SIT Protocol (n = 15)
| Baseline | Seated (min) | Post-sitting | P-value | Effect Size | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 | 60 | |||||
| Heart Rate (bpm) | 63±10 | 67±10 | 69±11 | 61±11 | 0.06 | 0.18 |
| Central SBP (mmHg) | 118±11 | 116±15 | 117±12 | 120±10 | 0.57 | 0.05 |
| Central DBP (mmHg) | 69±9 | 72±7 | 73±10 | 71±10 | 0.09 | 0.15 |
| Central MAP (mmHg) | 84±9 | 86±9 | 88±10 | 87±9 | 0.45 | 0.06 |
| Central PP (mmHg) | 49±7 | 43±11 | 44±7 | 50±9 | 0.05 | 0.20 |
| VA BF (mL·min−1) | 113±86 | 91±80 | 84±82 | 83±48 | 0.06 | 0.23 |
| ICA BF (mL·min−1) | 368±92 | 279±96* | 277±84* | 294±55* | 0.001 | 0.52 |
| Total Brain BF (mL·min−1) | 962±318 | 740±335* | 722±304* | 753±191* | 0.001 | 0.53 |
Denotes p<0.05 vs. Baseline;
Denotes p<0.05 vs. 10-min;
Denotes p<0.05 vs. 60-min.
Abbreviations: SBP-systolic blood pressure, DBP-diastolic blood pressure, MAP-mean arterial pressure, PP-pulse pressure, BF-blood flow, VA-vertebral artery, ICA-internal carotid artery
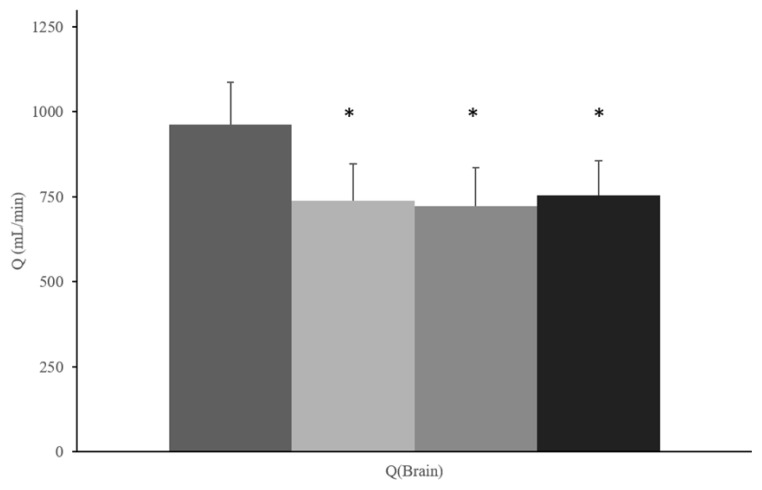
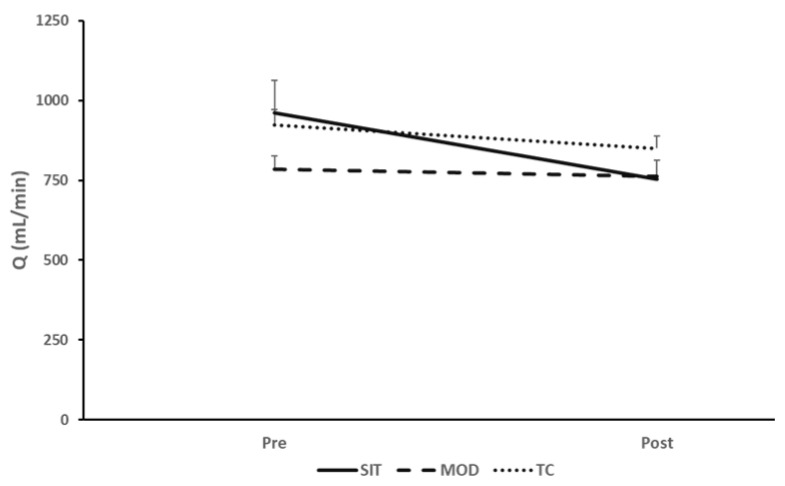
For SIT, there was a significant time effect in estimated total Qbrain (p<0.001, ηp2= 0.53) (Figure 2). ICA blood flow significantly decreased from baseline to 10-minutes of sitting, which persisted into the 60-minute and post-sitting time points (p<0.001, ηp2=0.52). Though not statistically significant (p=0.06), there was a reduction in VA blood flow during all time points of the SIT protocol. There were no statistically significant, or meaningful changes in estimated Qbrain for MOD (799±138 vs. 807±122 mL/min, p=0.69, ηp2=0.05) or TC (923±176 vs. 850±138 mL/min, p=0.06, ηp2=0.58). Though nonsignificant, Qbrain in the TC protocol had a very large effect size, indicating meaningful results. (Table 2). Total brain blood flow interaction data are presented in Figure 3.
Figure 2.
Mean summary data for total estimated head and brain blood flow (Qbrain) response (mL/min) to SIT protocol (n = 15). *Denotes p<0.05 versus baseline.
Table 2.
Central and Cerebrovascular Hemodynamic Response to MOD and TC Protocols
| Baseline | Seated (min) | Post-Sitting | P-value | Effect Size | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 | 60 | |||||
| MOD Protocol (n = 10) | ||||||
| Heart Rate (bpm) | 64±7 | 67±7 | 65±12 | 62±9 | 0.10 | 0.21 |
| Central SBP (mmHg) | 125±6 | 125±6 | 125±6 | 126±8 | 0.97 | 0.01 |
| Central DBP (mmHg) | 74±6 | 76±7 | 80±9* | 73±8^ | 0.01 | 0.39 |
| Central MAP (mmHg) | 91±5 | 92±6 | 95±7 | 90±7 | 0.09 | 0.26 |
| Central PP (mmHg) | 52±6 | 49±6 | 45±11 | 53±9 | 0.06 | 0.30 |
| VA BF (mL·min−1) | 89±46 | 94±33 | 106±31 | 100±32 | 0.35 | 0.11 |
| ICA BF (mL·min−1) | 303±72 | 306±66 | 298±67 | 281±88 | 0.38 | 0.10 |
| Total Brain BF (mL·min−1) | 784±131 | 799±138 | 807±122 | 763±153 | 0.69 | 0.05 |
|
| ||||||
| TC Protocol (n = 13) | ||||||
|
| ||||||
| Heart Rate (bpm) | 64±11 | 62±11 | 0.35 | 0.33 | ||
| Central SBP (mmHg) | 126±9 | 124±9 | 0.63 | 0.17 | ||
| Central DBP (mmHg) | 73±8 | 74±7 | 0.55 | 0.21 | ||
| Central MAP (mmHg) | 90±7 | 90±7 | 0.88 | 0.05 | ||
| Central PP (mmHg) | 53±9 | 51±8 | 0.54 | 0.21 | ||
| VA BF (mL·min−1) | 111±18 | 109±26 | 0.71 | 0.10 | ||
| ICA BF (mL·min−1) | 350±78 | 316±58 | 0.03 | 0.71 | ||
| Total Brain BF (mL·min−1) | 923±176 | 850±138 | 0.06 | 0.58 | ||
Denotes p<0.05 vs. Baseline;
Denotes p<0.05 vs. 10-min;
Denotes p<0.05 vs. 60-min.
Abbreviations: SBP-systolic blood pressure, DBP-diastolic blood pressure, MAP-mean arterial pressure, PP-pulse pressure, BF-blood flow, VA-vertebral artery, ICA-internal carotid artery
Figure 3.
Pre vs. Post (supine) total brain blood flow response for SIT, MOD, TC Protocols
The central cardiovascular hemodynamic data are summarized in Table 1 and Table 2. No significant time effect for HR (p=0.06, ηp2=0.18), (SBP (p=0.57, ηp2=0.05), DBP (p=0.09, ηp2=0.15), MAP (p=0.45, ηp2=0.06), and PP (p=0.05, ηp2=0.20) was observed. Participants in the MOD protocol only experienced significant increases in DBP (p=0.01, ηp2=0.39), while no significant changes were observed in HR (p=0.10, ηp2=0.21), SBP (p=0.97, ηp2=0.01), MAP (p=0.09, ηp2=0.26), or PP (p=0.06, ηp2=0.30). It is worth noting that PP during the MOD protocol may not have been significant, though the effect size was large. Lastly, during the TC protocol, there were no significant changes in the central cardiovascular hemodynamic measures.
DISCUSSION
The primary purpose of this study was to determine whether uninterrupted sitting could negatively impact brain blood flow. A secondary purpose was to determine whether this brain blood flow response would be impacted when performed in a modified sitting posture with the lower limbs elevated in order to prevent venous pooling. In agreement with our hypotheses, our data support that 60-minutes of sitting can alter cerebrovascular hemodynamics, leading to a decrease in estimated Qbrain. Importantly, Qbrain was did not significantly change for MOD and TC protocol.
The underlying mechanisms mediating the Qbrain response to sitting are unclear. Bouts of uninterrupted sitting have previously been shown to decrease leg blood flow due to venous pooling (4,15,16). It may be that a similar process occurs in the extracranial vessels that perfuse the brain. A study in 2018 found that engaging in prolonged sitting decreased blood flow through the middle cerebral artery by 5% (2). Given that the middle cerebral artery is a branch of the extracranial, internal carotid artery, we believe that the observed changes may be due to downstream impact of the extracranial vessels. We observed a non-significant reduction in Qbrain response during the MOD and TC protocols, while there was a significant reduction in Qbrain during the SIT protocol. In the MOD protocol, the baseline values were lower than both SIT and TC protocols. Thus, the nonsignificant reduction observed in the MOD protocol may be due to lower baseline Qbrain measures. However, there was not a significant difference in Qbrain at baseline between MOD and the other protocols. Though not significant, the reduction in Qbrain observed in the TC protocol may be trending toward significance and driven by the significant reduction in the ICA BF. The SIT protocol, potentially, resulted in greater venous pooling in the lower limb. Though, we did not directly examine limb pooling (e.g., limb circumference changes, or microvascular perfusion), previous reports from our group and others have shown that uninterrupted sitting does result in an increase in venous pooling (4,15,22). In the MOD protocol, central CBP was significantly higher at the end of the 60-minutes of sitting compared to baseline. This may potentially be due to the postural position of the MOD sitting protocol and the impact of venous return. Venous pooling, particularly related to the SIT protocol could lead to a redistribution of blood volume, resulting in reductions in venous return and subsequent reductions in brain blood flow (9,10,20). Future studies, however, are needed to validate whether this is the underlying mechanism mediating the reduction in estimated Qbrain. Limited evidence exists surrounding the influence of prolonged uninterrupted sitting on brain blood flow (1,2,14). Previous work found that uninterrupted sitting reduced cerebral blood flow, a response mediated by reductions in middle cerebral artery blood flow velocity (MCAv) (2). Another report demonstrated a similar reduction in MCAv were observed during 8 hours of uninterrupted sitting in older overweight and obese adults (14). In agreement with these reports, our results demonstrate a reduction in estimated Qbrain via the reduction in blood flow through extracranial arteries. Additionally, our present study includes a novel perspective on how engaging in a MOD sitting posture may, potentially, impact the Qbrain, which helps to further the knowledge of sitting behaviors impacting brain blood flow.
Previous studies have demonstrated an association between chronic sedentary behavior and cognitive decline and dysfunction (6,8). However, evidence linking the impact of repeated acute exposure to sedentary behavior to cerebrovascular complications is unclear. Because of the association between Qbrain and brain function, reductions in Qbrain can have detrimental consequences for brain health (24). The reduction in blood flow during acute, uninterrupted sitting has the potential to initiate a harmful cerebrovascular health response, leading to cognitive impairments and cerebrovascular disease (18). Long term, habitual exposure to reductions in Qbrain caused by sitting can have considerable implications in the development of the aforementioned impairments and diseases (24). The current area of research should be repeated in an older population, as well as individuals with chronic conditions as to improve generalizability. Future work is needed to better elucidate the mechanisms contributing to the development of cerebrovascular diseases during sedentary behaviors by simultaneously measuring Qbrain through the extracranial arteries and the middle cerebral artery velocity and autoregulation. Additionally, future research is needed to assess cognitive function as it relates to sedentary behavior and the hemodynamic response, as there may be some functional, vascular contributions that plays a vital role in cognitive function.
A discussion of possible limitations of the present study is warranted. One important limitation to note is the design of the study, as different participants were allocated to different groups to compare changes in estimated brain blood flow. This, potentially, could have, introduced individual variation that could not be accounted for. However, we recruited a healthy, young group of participants who were comparable in age and BMI with minimal differences in the distribution of sex between the groups, thus limiting as much variability between groups as possible. Future studies might consider a true crossover study design to observe the impact of sedentary behavior and modified sitting on the hemodynamic response. Additionally, another limitation in our methods was the unavailability of equipment to measure factors that could affect Qbrain, such as end-tidal CO2 (PaCO2), as body positions can influence CO2 levels and consequently impact blood flow to the brain. Finally, this study does not include a measure of cognitive function. Though results of this pilot study do provide evidence to show that 60-minutes of sitting may negatively affect total estimated Qbrain, we cannot determine the impact that this observed reduction has on cognitive function. Future work should include a measure of cognitive function, as there may be some hemodynamic-related impairments during periods of sitting. Finally, some variables in the study were trending toward significance (e.g., MOD Central PP, TC Qbrain, and SIT VA BF) with large effect sizes, thus future work should increase the sample size of the population. As this was a pilot study, future work is needed addressing the aforementioned limitations to confirm the proposed mechanisms and gain insight into the long-term cerebrovascular effects of uninterrupted sitting.
These preliminary findings demonstrate that, in healthy young individuals, 60-minutes of sitting can significantly reduce estimated Qbrain. The underlying mechanism(s) mediating this response is unclear but could be related to venous blood pooling in the lower extremities due to the findings in the MOD sitting group. Future work should determine the long-term cerebrovascular and cognitive health consequences of uninterrupted sitting in healthy, diseased, and aging populations.
Supplementary Data
Showcasing the different positions during the SIT, MOD, and TC Protocols.
Acknowledgments
AKNOWLEDGEMENTS
Part of RJ’s effort for this work is supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) (1T32HD071866). The contents of the study are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health or the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
REFERENCES
- 1.Burnet K, Blackwell J, Kelsch E, Hanson ED, Stone K, Fryer S. Cerebrovascular function response to prolonged sitting combined with a high-glycemic index meal: A double-blind, randomized cross-over trial. Psychophysiol. 2021;58(8):1–13. doi: 10.1111/psyp.13830. [DOI] [PubMed] [Google Scholar]
- 2.Carter SE, Draijer R, Holder SM, Brown L, Thijssen DHJ, Hopkins ND. Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol. 2018;125(3):790–798. doi: 10.1152/japplphysiol.00310.2018. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Statistical Power for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Laurence Erlbaum and Associates; 1988. [Google Scholar]
- 4.Credeur DP, Miller SM, Jones R, Stoner L, Dolbow DR, Fryer SM. Impact of Prolonged Sitting on Peripheral and Central Vascular Health. Am J Cardiol. 2019;123(2):260–266. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Dunstan DW, Barr ELM, Healy GN, Salmon J, Shaw JE, Balkau B. Television viewing time and mortality: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121(3):384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Stamatakis E. Prospective Study of Sedentary Behavior, Risk of Depression, and Cognitive Impairment. Med Sci Sport Exerc. 2014;46(4):718–723. doi: 10.1249/MSS.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 8.Kesse-Guyot E, Charreire H, Andreeva VA, Touvier M, Hercberg S, Galan P. Cross-Sectional and Longitudinal Associations of Different Sedentary Behaviors with Cognitive Performance in Older Adults. PLoS One. 2012;7(10):e47831. doi: 10.1371/journal.pone.0047831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac output and cerebral blood flow: The integrated regulation of brain perfusion in adult humans. Anesthesiol. 2015;123(5):1198–208. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 10.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol - Hear Circ Physiol. 2016;311(1):H177–H182. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navalta JW, Stone WJ, Lyons TS. Ethical Issues Relating to Scientific Discovery in Exercise Science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padilla J, Fadel PJ. Prolonged sitting leg vasculopathy: contributing factors and clinical implications. Am J Physiol Circ Physiol. 2017;313(4):H722–H728. doi: 10.1152/ajpheart.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry S, Straker L. The contribution of office work to sedentary behaviour associated risk. BMC Public Health. 2013;13(1) doi: 10.1186/1471-2458-13-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdomo SJ, Gibbs BB, Kowalsky RJ, Taormina JM, Balzer JR. Effects of Alternating Standing and Sitting Compared to Prolonged Sitting on Cerebral Blood Flow Velocity. Med Sci Sport Exerc. 2017;49(5S):695–696. doi: 10.1007/s11332-019-00526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol. 2015;100(7):829–838. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol. 2016;310(5):H648–H653. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickham PP. Human Experimentation: Code of Ethics of W.M.A. BMJ. 1964;2(5402):177–177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam study. Ann Neurol. 2005;57(6):789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol. 2011;589(11):2847–2856. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shvartz E, Gaume JG, White RT, Reibold RC. Hemodynamic responses during prolonged sitting. J Appl Physiol. 1983;54(6):1673–1680. doi: 10.1152/jappl.1983.54.6.1673. [DOI] [PubMed] [Google Scholar]
- 21.Stoner L, Credeur D, Dolbow DR, Gater DR. Vascular health toolbox for spinal cord injury: Recommendations for clinical practice. Atherosclerosis. 2015;243:373–382. doi: 10.1016/j.atherosclerosis.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Stoner L, Willey Q, Evans WS, Burnet K, Credeur DP, Fryer S. Effects of acute prolonged sitting on cerebral perfusion and executive function in young adults: A randomized cross-over trial. Psychophysiology. 2019;56(12):1–11. doi: 10.1111/psyp.13457. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE. Sedentary Behavior Research Network (SBRN) – Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimer’s Dement Transl Res Clin Interv. 2017;3(3):291–300. doi: 10.1016/j.trci.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willie CK, Tzeng Y-C, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592(5):841–859. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Showcasing the different positions during the SIT, MOD, and TC Protocols.