Abstract
G protein-gated inwardly rectifying K+ (GIRK/Kir3) channels exert a critical inhibitory influence on neurons. Neuronal GIRK channels mediate the G protein-dependent, direct/postsynaptic inhibitory effect of many neurotransmitters including γ-aminobutyric acid (GABA), serotonin, dopamine, adenosine, somatostatin, and enkephalin. In addition to their complex regulation by G proteins, neuronal GIRK channel activity is sensitive to phosphatidylinositol 4,5-bisphosphate (PIP2), phosphorylation, regulator of G protein signaling (RGS) proteins, intracellular Na+ and Ca2+, and cholesterol. The application of genetic and viral manipulations in rodent models, together with recent progress in the development of GIRK channel modulators, has increased our understanding of the physiological and behavioral impact of neuronal GIRK channels. Work in rodent models has also revealed that neuronal GIRK channel activity is modified, transiently or persistently, by various stimuli including exposure drugs of abuse, changes in neuronal activity patterns, and aversive experience. A growing body of preclinical and clinical evidence suggests that dysregulation of GIRK channel activity contributes to neurological diseases and disorders. The primary goals of this review are to highlight fundamental principles of neuronal GIRK channel biology, mechanisms of GIRK channel regulation and plasticity, the nascent landscape of GIRK channel pharmacology, and the potential relevance of GIRK channels to the pathophysiology and treatment of neurological diseases and disorders.
Keywords: GIRK, GPCR, G protein, inhibition, plasticity
INTRODUCTION
G protein-gated inwardly rectifying K+ (GIRK/Kir3) channels are critical mediators of neuronal inhibition in the central nervous system (1–3). GIRK channels are best known as key mediators of the G protein-dependent postsynaptic inhibitory effect of many neurotransmitters, including the most abundant mammalian inhibitory neurotransmitter γ-aminobutyric acid (GABA) (4–6). GIRK activation by inhibitory neurotransmitters hyperpolarizes neurons and limits N-methyl-d-aspartate (NMDA) receptor-dependent activation (7). GIRK channels also contribute to neuron autoinhibition and network inhibition. In dopamine neurons of the substantia nigra (SN), for example, GIRK channels coupled to D2 dopamine receptors (D2R) mediate the autoinhibitory influence of activity-induced dopamine release (8). At the network level, GIRK channel activation shapes the oscillation behavior of thalamic and hippocampal networks (9–11).
GIRK channels belong to the family of K+ channels that exhibit inward rectification (12, 13). Members of this “Kir” family conduct K+ more effectively in the inward direction (into the cell) than in the physiologically relevant outward direction (out of the cell). This property is conferred by blockade of K+ efflux via intracellular Mg2+ and small polyamines. Driven largely by channel crystallization efforts, our understanding of the structural basis of GIRK channel function, including insights related to inward rectification, channel gating, and regulation by G proteins and other interactants has increased dramatically (14–21). These achievements are documented in relevant publications and previous reviews (13, 22). The goal here is to highlight fundamental and emerging principles of neuronal GIRK channel biology, with an emphasis on their regulation and pharmacology, and the relevance and therapeutic potential of GIRK channels in the context of neurological disorders and diseases.
GIRK Channel Subunit Composition
GIRK channels are found in the heart and brain, along with a limited number of other tissues including the pancreas (23–25) and pituitary gland (26). Four mammalian genes encode GIRK channel subunits: KCNJ3/GIRK1/Kir3.1, KCNJ6/GIRK2/Kir3.2, KCNJ9/GIRK3/Kir3.3, and KCNJ5/GIRK4/Kir3.4. Protein biochemistry efforts involving bovine heart tissue (27, 28), studies with linked/concatemeric subunits in expression systems (29, 30), and crystal structures of the purified recombinant GIRK2 homomeric channel (18, 20) support the contention that GIRK channels are homo- and heterotetrameric complexes. GIRK channel subunit composition and stoichiometry are best understood in the heart, where GIRK1 and GIRK4 coassemble in 1:1 stoichiometry to form the muscarinic-gated K+ channel known as IKACh (27, 31). Even in this relatively simple case where only GIRK1 and GIRK4 are present, the possibility that GIRK1:GIRK4 channel subtypes of variable stoichiometries (1:3, 2:2, 3:1) coexist has been neither formally proven nor discounted. Moreover, functional GIRK4 homomers have been identified in atrial myocytes (28, 32, 33).
GIRK channel subunit composition is a more complex issue in the brain (5). GIRK channels containing GIRK1 and GIRK2 are often taken as the prototypical neuronal GIRK channel, a contention based on the broad overlapping distributions of GIRK1 and GIRK2 in the rodent brain (34, 35), the downregulation of GIRK1 in the Girk2–/– mouse brain (36), and the results of electrophysiological investigations in GIRK subunit knockout mice. Indeed, ablation of either GIRK1 or GIRK2 in mice correlates with the complete or near-complete loss of GIRK channel activity in almost all neuron populations evaluated to date (4, 35, 37–40). GIRK3 overlaps with GIRK1 and GIRK2 throughout the rodent brain (34, 35), however, and it can form functional channels with GIRK1 or GIRK2 (41–44). GIRK4 is also found in the rodent brain, though its expression is restricted (34, 45). Alternative splicing of GIRK subunits has also been documented (46–55), and this yields multiple distinct protein variants in the case of GIRK1 and GIRK2. Midbrain dopamine neurons warrant special mention in this conversation, as they do not appear to express GIRK1 (34). The GIRK channel in rat substantia nigra dopamine neurons is formed by two GIRK2 splice variants (GIRK2a and GIRK2c) (51), whereas the GIRK channel in mouse ventral tegmental area (VTA) dopamine neurons is formed by GIRK2c and GIRK3 (43, 44). Thus, although GIRK1 and GIRK2 likely contribute to GIRK channel formation throughout the rodent CNS, there are exceptions to this rule and much remains to be learned about the precise subunit composition of neuronal GIRK channels, even in well-studied rodent models. Importantly, virtually nothing is known regarding GIRK channel subunit expression patterns, alternative splicing, and subtypes in the human brain.
Key Features of Neuronal GIRK Channel Subunits
GIRK subunits contain two transmembrane segments, a hydrophobic pore domain, and cytoplasmic amino- and carboxyl-terminal domains. The cytoplasmic domains are most divergent across the GIRK channel subunits, harboring elements that impact channel trafficking and function, and mediate protein:protein interactions. Brief descriptions of the three primary neuronal GIRK channel subunits (GIRK1, GIRK2, and GIRK3) are detailed in the following sections.
GIRK1.
GIRK1 cannot form functional homomers (31), a deficit linked to the lack of an endoplasmic reticulum (ER) export signal (52). A single-point mutation in the GIRK1 pore (F137S), however, permits surface trafficking of GIRK1 and formation of functional GIRK1 homomultimers (56). This suggests that additional structures and mechanisms supporting the surface trafficking of GIRK channels remain to be identified. Normally, GIRK1 associates with another GIRK subunit (e.g., GIRK2) to achieve surface membrane localization (57, 58). In expression systems, coexpression of GIRK1 yields substantially larger GIRK channel currents than seen with expression of GIRK2 or GIRK4 alone (31, 47, 59, 60). Relatedly, genetic ablation of GIRK1 in mice correlates with small GIRK currents in neurons, despite normal levels of residual GIRK subunit expression (61). Unique residues in the GIRK1 pore region that enhance single-channel conductance and increase mean open time, along with unique elements in the carboxyl-terminal domain, have been implicated in ability of GIRK1 to augment GIRK channel activity (61–64).
GIRK2.
GIRK2 (and GIRK4) can form functional homomeric GIRK channels (47, 52, 59), attributable at least in part to multiple forward trafficking elements, including an ER export signal and post-Golgi surface-promoting motifs (52). Alternative splicing generates multiple distinct GIRK2 variants, two of which (GIRK2a and GIRK2c) are expressed throughout the mouse brain and differ by only 11 amino acids at the carboxyl terminus (50). The longer variant (GIRK2c) possesses a PDZ domain interaction motif (ESKV). When expressed individually in CA1 pyramidal neurons in hippocampal neurons from Girk2–/– mice, the subcellular distributions of GIRK2a and GIRK2c were overlapping but distinct; both subunits were detected in the soma and dendrites, but GIRK2c was found at higher levels that GIRK2a in secondary and tertiary dendrites (65). Although no differences in GPCR-dependent GIRK currents were seen in Girk2–/– neurons expressing either GIRK2a or GIRK2c, the amplitude of synaptic GABABR-GIRK responses evoked by electrical stimulation of proximal (Schaffer collateral) or distal (perforant path) dendritic fields was tracked with the differential dendritic distribution of GIRK2a and GIRK2c.
GIRK3.
Despite its widespread distribution in the rodent brain (34, 35), ablation of GIRK3 in mice yields modest influence on GIRK channel function (35, 38, 39, 66, 67). Nevertheless, several lines of evidence suggest that GIRK3 contributes to neuronal GIRK channel formation. For example, simultaneous ablation of GIRK2 and GIRK3 in mice correlates with a larger reduction in GIRK channel activity in neurons of the locus ceruleus (38) and hippocampus (35) than loss of GIRK2 alone. In addition, the residual GIRK channel (GIRK2c homomer, presumably) in VTA dopamine neurons from Girk3–/– mice is more sensitive to GABABR activation than its wild-type (GIRK2c:GIRK3) counterpart (44). Furthermore, GIRK3 protein levels are decreased in the hippocampus and cerebellum of Girk1–/– and Girk2–/– mice (35, 68), and GIRK3 co-immunoprecipitates with GIRK1 and GIRK2 in the mouse cerebellum (68). Although genetic ablation of GIRK3 is not associated with overt phenotypes (38, 69), Girk3–/– mice show altered sensitivity to drugs of abuse, several classes of analgesic compounds, and sedative hypnotics (40, 70–72), and they are deficient in some forms of GIRK channel plasticity (73, 74).
Like GIRK1, GIRK3 lacks an ER export signal and does not form functional homomers (52). Surprisingly, however, functional GIRK1:GIRK3 heteromers have been reported in expression systems (41, 75, 76), again suggesting that the surface localization of GIRK channels does not depend exclusively on known forward trafficking elements. GIRK3 does harbor a lysosomal targeting sequence (YWSI) that is absent in other subunits, which likely explains why ectopic expression of GIRK3 can suppress GIRK channel activity (52, 59, 77). Although mechanisms that dictate how and when GIRK3 plays channel-forming and/or channel-trafficking roles are unclear, the balance of evidence suggests that GIRK3 titrates GIRK channel activity in neurons.
GIRK CHANNEL REGULATION
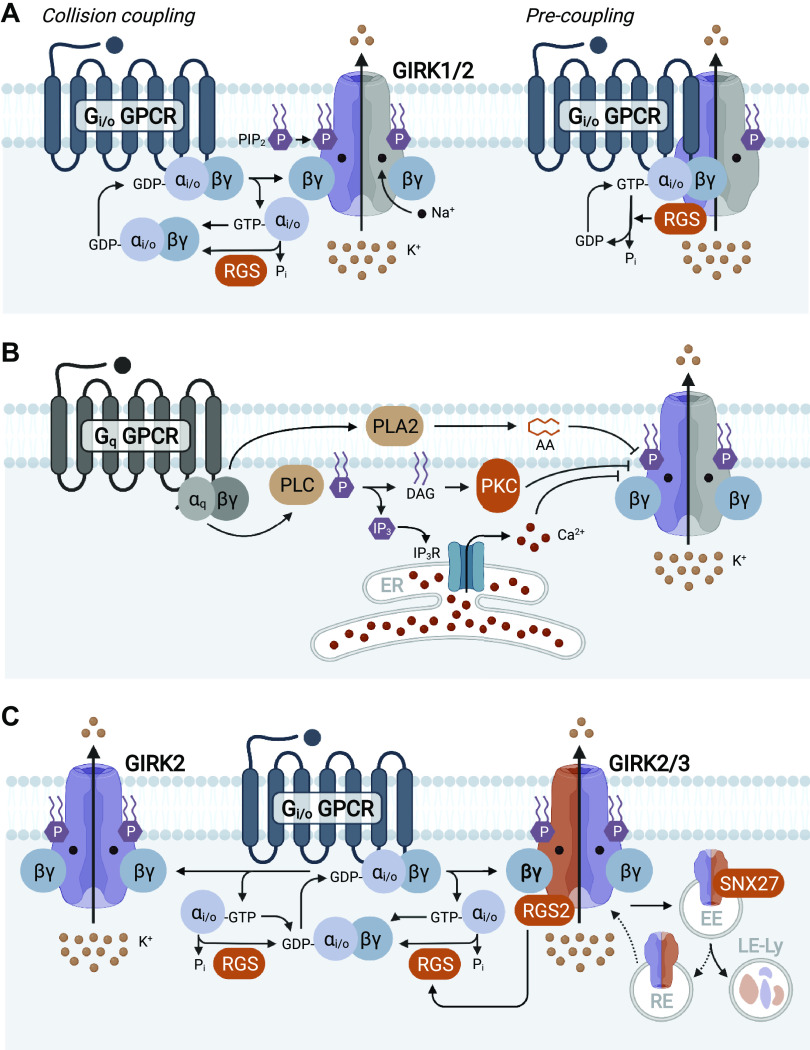
The regulation of GIRK channel activity by G proteins is fascinating and complex (6, 78). GIRK channels are activated by inhibitory (Gi/o) G proteins. The Gi/o G protein family includes three Gαi isoforms, two alternative splice variants of Gαo, and Gαz (79–81). Gαi/o subunits assemble with Gβγ complexes formed by various combinations of four Gβ isoforms and 12 Gγ subunits (82, 83). GIRK channel gating is enhanced by direct binding of Gβγ (84–87) (Fig. 1A). GIRK channels can bind to four Gβγ subunits (20), and GIRK channel activity tracks with Gβγ occupancy of these sites (30, 88). GIRK channels can also bind to Gαi/o, in either GDP- or GTP-bound states (6, 89–94). This interaction likely contributes to the strong functional connection between GIRK channels and Gi/o-linked GPCRs in vivo (94). Efforts in expression systems suggest that Gαi/o isoforms exert unique allosteric influence on GPCR-GIRK signaling and basal activity (6). For example, GPCR-GIRK signaling mediated by Gαo exhibits faster activation rates than seen with Gαi (95). In addition, Gαi isoforms enhance GPCR-GIRK coupling sensitivity (6, 89, 90, 93, 96–98).
Figure 1.
Depictions of G protein-gated inwardly rectifying K+ (GIRK) channel biology, signaling, and regulation. A: the activity of neuronal GIRK channels (e.g., the GIRK1:GIRK2 or GIRK1/2 heterotetramer) is regulated by GPCRs coupled to inhibitory G proteins (Gi/o) G proteins (Gi/o GPCRs). GIRK channel gating is enhanced by direct binding of Gβγ, which stabilizes the interaction between the channel and phosphatidylinositol 4,5-bisphosphate (PIP2). Intracellular Na+ can also facilitate GIRK channel opening. Regulator of G protein signaling (RGS) proteins influence the kinetics and/or sensitivity of GPCR-GIRK signaling. In addition to Gβγ, GIRK channels can also bind to Gαi/o, GPCRs, and RGS proteins, supporting the contention that some or many GPCR-GIRK signaling elements exist in pre-coupled states (right). B: regulation of GIRK channel activity by Gq-coupled GPCRs. Activation of Gq-coupled GPCRs leads to phospholipase C (PLC) activation, which converts PIP2 into diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). DAG activates protein kinase C (PKC), whereas IP3 releases Ca2+ from internal stores (endoplasmic reticulum, ER). PIP2 depletion, PKC activation, and increased intracellular Ca2+ have all been shown to suppress GIRK channel activity. Phospholipase A2 (PLA2) activation and arachidonic acid (AA) production may also mediate the Gq-mediated suppression of GIRK channel activity in some cases. C: schematic depiction of GIRK1-lacking GIRK channels, as found in dopamine neurons of the substantia nigra (GIRK2 homomer, left) and ventral tegmental area (GIRK2c:GIRK3 heteromer, right). GIRK3 interacts with proteins that regulate sensitivity to GPCR activation (RGS2) and channel trafficking (sorting nexin 27, or SNX27). SNX27 interacts with the PDZ interaction domain in GIRK3, targeting GIRK3-containing channels to early endosomes (EE), leading to reduced surface expression of GIRK channels by facilitating their degradation in late endosomes/lysosomes (LE-Ly), rather than recycling to the surface membrane via recycling endosomes (RE). Created with BioRender.com.
Little is known regarding the Gα and Gβγ isoforms that mediate GIRK-dependent signaling in neurons. Although the loss of Gαo in mice prolonged the deactivation kinetics of GPCR-GIRK signaling in CA3 pyramidal neurons (99), GPCR-GIRK response amplitude was preserved. This suggests that multiple Gαi/o isoforms shape neuronal GIRK-dependent signaling. Loss of Gγ3 in mice correlated with a reduction in the number of neurons exhibiting a GABABR-GIRK current, and smaller responses in those exhibiting a response (100). Loss of Gγ3 also correlated with reduced Gαi3 and Gβ2 subunit protein levels (101), suggesting that these G protein isoforms mediate GABABR-GIRK signaling in some neurons. Interestingly, GIRK-dependent responses to adenosine and somatostatin were preserved in Gγ3–/– mice, supporting the contention that different GPCR-GIRK signaling pathways in neurons are composed of distinct signaling elements.
Neuronal GIRK channels exhibit basal/tonic activity (6). Acute inhibition of neuronal GIRK channels, for example, depolarizes neurons and induces epileptiform activity in mouse hippocampal cultures (102). Basal/tonic GIRK channel activity can reflect GPCR-dependent and/or GPCR-independent channel activation. Indeed, the relatively high level of constitutive GIRK channel activity seen in dorsal (but not ventral) rat hippocampal CA1 pyramidal neurons is due to ambient adenosine and tonic activation of GIRK channel by the A1 adenosine receptor (A1R) (103, 104). Basal/tonic GIRK channel activity can also result from intrinsic channel gating and basal G protein turnover (105, 106), or perhaps from direct activation by other regulatory factors.
Phosphatidylinositol 4,5-Bisphosphate
Phosphatidylinositol 4,5-bisphosphate (PIP2) is a requisite cofactor for GIRK channel regulation by Gβγ (78, 107, 108). Gβγ activates GIRK channels by stabilizing the interaction between GIRK channels and PIP2. The structural basis of PIP2 binding to and regulation of GIRK channels has been illuminated with the crystal structure of the GIRK2 homomer (18), along with cryo-EM (109) and molecular dynamics simulations (110, 111).
PIP2 depletion has been implicated in the suppression of cardiac GIRK channel activity by Gq-coupled GPCRs (112, 113). Although stimulation of Gq-coupled GPCRs (e.g., muscarinic acetylcholine and group 1 metabotropic glutamate receptors, mGluR) suppressed GABABR-GIRK signaling in rodent CA1 pyramidal neurons (114), the muscarinic receptor-dependent suppression of GABABR-GIRK signaling involved activation of phospholipase C (PLC) and PKC, whereas the group 1 mGluR influence involved activation of phospholipase A2 (PLA2) and production of arachidonic acid. Despite these mechanistic differences, both types of inhibition culminated in the disruption of GIRK channel:PIP2 interactions (115). Interestingly, the group 1 mGluR-mediated suppression of GABABR-GIRK and D2R-GIRK signaling pathways in rat substantia nigra dopamine neurons was unaffected by PLC, PKC, or PLA2 inhibitors. Rather, inhibition of GIRK-dependent signaling in this case involved Ca2+ release from intracellular stores (116). Thus, Gq-coupled GPCRs may negatively regulate neuronal GIRK channels via multiple mechanisms including PIP2 depletion, PKC activation/phosphorylation, PLA2 activation and arachidonic acid production, and/or increased intracellular Ca2+ (Fig. 1B).
Phosphorylation
Phosphorylation has been implicated in GIRK channel biology (117–124), but little is known about kinases and substrates relevant in vivo. Phosphorylation of GIRK1 at an undetermined, protein phosphatase 2A (PP2A)-sensitive site is required for GIRK channel activation by Gβγ (119). The NMDA receptor (NMDAR)- and protein phosphatase 1 (PP1)-dependent dephosphorylation of serine-9 on GIRK2 [GIRK2(S9)] increases the surface trafficking of GIRK channels in rat hippocampal neurons (121). This process has been implicated in the depotentiation of long-term potentiation (LTP) (125). Although kinase(s) targeting GIRK2(S9) or other known or predicted phosphorylation sites on GIRK channels have not been identified, work across multiple models suggests that GIRK channels can be phosphorylated by several serine-threonine protein kinases, including PKA, PKC, and CaMKII (119, 120, 122, 123, 126, 127). Work in Xenopus oocytes suggests that tyrosine phosphorylation of GIRK channels can also regulate GIRK channel trafficking (128) or suppress GIRK channel activity (118, 129).
PKC can phosphorylate recombinant GIRK1 and GIRK2, and PKC phosphorylation suppresses GIRK channel activity (119, 120, 123, 124, 130–132). Some (but not all) of the suppression of GIRK channel activity evoked by Gq-coupled GPCRs can be mimicked by PKC activators (114, 115, 123, 130, 133), or abolished using PKC inhibitors (134). Notably, a phospho-dead variant of GIRK1(S185) diminished the negative influence of PKC on channel function, and precluded the negative regulation of GIRK channel activity by Gq-coupled GPCRs (120). As such, PKC phosphorylation likely contributes to the negative regulation of GIRK channels by Gq-coupled GPCRs (123, 135).
Regulator of G Protein Signaling Proteins
Regulator of G protein signaling (RGS) proteins negatively regulate G protein-dependent signaling (136, 137). RGS proteins enhance the intrinsic GTP hydrolysis rate of Gα, accelerating re-formation of inactive G protein heterotrimers (138). Evidence for neuronal GIRK channel regulation by RGS proteins comes primarily from studies involving two RGS subfamilies:
R7 RGS proteins.
Members of the R7 subfamily of RGS proteins (RGS6, RGS7, RGS9, and RGS11) contain a “Gγ-like” domain that promotes stable dimerization with Gβ5, the atypical/nonsignaling member of the Gβ subunit family (139). R7 RGS/Gβ5 complexes exhibit strong substrate selectivity for Gαo (140, 141), and they are structurally similar to Gβγ (142). The structural similarity with Gβγ likely explains the ability of R7 RGS/Gβ5 complexes to bind GIRK channels (143, 144). Although the RGS7/Gβ5:GIRK channel interaction has been modeled (145), it remains unclear whether R7 RGS/Gβ5 complexes compete with Gβγ for access to the Gβγ binding sites on GIRK channels (20). As occupancy of these sites by Gβγ determines GIRK channel activity and GPCR-GIRK coupling strength (30, 88), resolving this issue is paramount to understanding the regulatory influence of R7 RGS/Gβ5 complexes on GIRK-dependent signaling. What is clear is that R7 RGS/Gβ5 complexes negatively regulate GPCR-GIRK signaling in mouse cardiomyocytes (144, 146) and neurons (143). For example, RGS7 or Gβ5 ablation in mice is correlated with prolonged synaptic GABABR-GIRK currents in CA1 pyramidal neurons (143, 147). GABABR-GIRK currents exhibited slower deactivation rates and increased GABABR-GIRK coupling sensitivity in cultured hippocampal neurons from RGS7–/– or Gβ5–/– mice (143, 148).
Adaptors for R7 RGS/Gβ5 complexes have also been identified, including the small palmitoylated protein R7BP and GPR158 (139, 149). Adaptor binding confers stability to and membrane association of R7 RGS/Gβ5 complexes, while enhancing their catalytic activity (139, 149, 150). Interestingly, RGS7 forms mutually exclusive complexes with R7BP or GPR158 in the mouse hippocampus (147). Whereas loss of R7BP diminished the regulatory influence of RGS7/Gβ5 on GABABR-GIRK signaling in mouse hippocampal pyramidal neurons (148), there was no impact of GPR158 ablation (147). GPR158 overexpression, however, suppressed the negative regulatory influence of RGS7/Gβ5 on GABABR-GIRK signaling. Thus, adaptors may be able to direct the influence of R7 RGS/Gβ5 complexes toward (R7BP) or away (GPR158) from GIRK channels in neurons.
RGS6/Gβ5 is the dominant RGS influence on cardiac GIRK-dependent signaling in the mouse (144, 146, 151), and RGS6/Gβ5 negatively regulates GABABR-GIRK signaling in mouse cerebellar granule cells (152). Since rodent hippocampal pyramidal neurons express RGS6 (153), it is surprising that loss of RGS6 has no impact on GABABR-GIRK signaling in mouse hippocampal pyramidal neurons (147, 148). In light of the finding that RGS6 negatively regulates the 5HT1AR-dependent inhibition of adenylyl cyclase in mouse hippocampal pyramidal neurons (154), it is tempting to speculate that RGS7/Gβ5/R7BP (GIRK channels) and RGS6/Gβ5/GPR158 (adenylyl cyclase) exert negative regulatory influence on discrete branches of inhibitory GPCR signaling pathways.
R4 RGS proteins.
Two members of the R4 RGS protein subfamily—RGS2 and RGS4—have been implicated in GIRK channel regulation. The suppression of GIRK channel activity by Gq-coupled purinergic receptors was diminished by ectopic expression of RGS2 in rat sympathetic neurons (155). RGS2 is a negative regulator of GABABR-GIRK signaling in mouse VTA dopamine neurons (44). Ablation of RGS2 or GIRK3 in mice increased GABABR-GIRK coupling sensitivity in VTA dopamine neurons. There was no additive influence of RGS2 ablation on GABABR-GIRK coupling sensitivity in VTA dopamine neurons from Girk3–/– mice, suggesting that GIRK3 mediates the influence of RGS2 in these neurons (Fig. 1C).
RGS4 exhibits dual specificity for Gαi/o and Gαq (156). RGS4 interacts with GABABR (157, 158), and has been shown to negatively regulate GIRK-dependent signaling in expression systems (157–163). Simultaneous genetic ablation of RGS4 and RGS6 in mice revealed an influence of RGS4 on GPCR-GIRK signaling dynamics in cardiomyocytes (151). In this setting, however, RGS4 opposed the more dominant negative regulatory influence of RGS6/Gβ5 on GPCR-GIRK signaling dynamics. This oppositional influence of RGS4 ablation on GIRK-dependent signaling in cardiomyocytes may reflect enhanced Gαq activity and depletion of PIP2 (107, 108).
Other GIRK Channel Regulators
Several other modes and mechanisms of GIRK channel regulation have been described. For example, GIRK2- and GIRK4-containing GIRK channels are activated by elevated intracellular Na+ (EC50 = 30–40 mM) (47, 164). This regulatory mode may serve as a break on excessive neuronal activity, as intracellular Na+ entry into the cell would ultimately trigger GIRK channel activation. Like Gβγ, Na+ increases the affinity of GIRK channels for PIP2 (108). The binding site for Na+ has been resolved in the GIRK2 homomultimer (18), and Na+-dependent gating of GIRK channels has been modeled by molecular dynamics simulation (111). GIRK channels are also regulated by cholesterol (165), a mode of regulation suggested by early reports of channel association with cholesterol-rich lipid rafts (35, 166). GIRK channel activity is enhanced by increases in membrane cholesterol (167–170). A diet-based increase of cholesterol levels increased GIRK channel activity in rodent hippocampal neurons, whereas cholesterol depletion or statin treatment decreased or normalized GIRK channel currents (168, 171). Recent cryo-EM studies, along with molecular simulation and mutagenesis efforts, have yielded important insights into the cholesterol-binding site in GIRK channels, and mechanisms of GIRK channel gating by cholesterol (168, 172).
Is There a GIRK “Signalosome”?
As indicated earlier, GIRK channels can bind to a number of signaling elements, including Gα, Gβγ, and R7 RGS proteins. These and other data have fueled speculation that macromolecular complexes coordinate selective and efficient GPCR-GIRK signaling in neurons (173, 174). Some evidence suggests that GIRK channels bind to GABABR (157, 175). For example, GABABR is coimmunoprecipitated with GIRK channels in expression systems and in vivo (176, 177). Comprehensive proteomic analysis of GABABR interacting proteins, however, suggests that this interaction is indirect and/or weak (178).
Several plausible signaling elements could facilitate formation of a precoupled GABABR-GIRK signaling pathway, or “signalosome.” For example, given its association with GABABR (157, 179, 180) and scaffolding with GIRK channels (6), Gα could mediate a GABABR:GIRK interaction. Gβ5 is part of the GABABR interactome in the rodent brain (181), and since RGS7/Gβ5 binds to GIRK channels (143), RGS7/Gβ5 may serve as the bridge. Potassium (K+) channel tetramerization domain (KCTD) proteins are core elements of the GABABR interactome and they interact with Gβγ (182). Interestingly, the effect of KCTD elimination in mice on GABABR-GIRK signaling is reminiscent of loss of RGS control (183), suggesting that one or more KCTD family members may participate in GABABR-GIRK signalosome assembly in neurons. Despite evidence supporting the existence of GIRK signalosomes, it is important to note that the functional properties of reconstituted GPCR-GIRK signaling pathways (including the GABABR-GIRK signaling pathway), exhibit functional properties consistent with “collision coupling,” suggesting that at least one untethered or diffusible element is involved (184, 185).
GIRK CHANNEL PLASTICITY
Several examples of GIRK channel plasticity in rodent models have been described (Table 1), including an early demonstration that the same stimulus that evoked LTP in rat hippocampal pyramidal neurons also potentiated the synaptic GABABR-GIRK inhibitory postsynaptic current (186). Subsequently, evidence for GIRK channel plasticity in rodent models has been reported following exposure to drugs of abuse [e.g., opioids (65, 191, 192), psychostimulants (39, 74, 187, 188, 190), alcohol (193,194, 200)], neuronal activity (73, 121), aversive experience (195,196, 201), and amyloid beta (Aβ) (197–199, 202). Studies of these forms of plasticity have shed light on underlying mechanisms, and emerging themes are discussed in the following sections.
Table 1.
Plasticity of neuronal GIRK channel activity
| Stimulus | Brain Region/Neuron | Plasticity | Mechanism | Refs. |
|---|---|---|---|---|
| Neuronal activity | ||||
| NMDAR activation | HPC culture | ↑ A1R-GIRK, GABABR-GIRK | Dephosphorylation/GIRK2 surface trafficking | (121, 125) |
| HFS | HPC slice culture | ↑ GABABR-GIRK | NMDAR, Ca2+/CaMKII | (186) |
| Burst/depolarization | VTA/dopamine | ↑ GABABR-GIRK, D2R-GIRK | NMDAR, Ca2+/CaMKII, GIRK3 | (73) |
| Tonic/LFS | VTA/dopamine | ↓ GABABR-GIRK | NMDAR, GIRK3 | |
| Drugs of abuse | ||||
| Cocaine | ||||
| Single injection | VTA/dopamine | ↓ GABABR-GIRK, D2R-GIRK | Internalization, D2/3 R-dependent | (187) |
| Repeated injection | VTA/dopamine | ↓ GABABR-GIRK | ||
| Single injection | VTA/GABA | ↓ GABABR-GIRK | (188) | |
| VTA/dopamine | - GABABR-GIRK | |||
| Repeated injection | PL/layer 5/6 pyramidal | ↓ GABABR-GIRK | Dephosphorylation/GABABR2 internalization | (39) |
| D1/5R-dependent | ||||
| PL/layer 2/3 pyramidal | - GABABR-GIRK | |||
| IL/layer 5/6 pyramidal | - GABABR-GIRK | |||
| Repeated injection | PL/GABA | - GABABR-GIRK | (189) | |
| Methamphetamine | ||||
| Single injection | VTA/GABA | ↓ GABABR-GIRK | Dephosphorylation/GABABR2 internalization | (188) |
| VTA/dopamine | ↓ GABABR-GIRK | |||
| Single injection | VTA/GABA | ↓ GABABR-GIRK | Dephosphorylation/GABABR2 | (74) |
| Repeated injection | VTA/dopamine | ↓ GABABR-GIRK, - D2R-GIRK | GIRK3, D1R/D2R-dependent | |
| Self-administration | Midbrain/dopamine | ↓ GABABR-GIRK, D2R-GIRK | Ca2+ | (190) |
| Repeated injection | VTA/dopamine | ↓ D2R-GIRK | ||
| Opioids | ||||
| Morphine | HPC culture | ↑ 5HT1AR-GIRK ↓ GABABR-GIRK | ↑ GIRK2, Ca2+/CaMKII, trafficking | (65, 191) |
| Remifentanil | PL/layer 5/6 pyramidal | ↓ GABABR-GIRK | (192) | |
| Ethanol | ||||
| Repeated injection | VTA/dopamine | ↑ D2R-GIRK, - GABABR-GIRK | (193) | |
| CIE/ethanol vapor | lateral OFC/pyramidal | ↓ GPCR-GIRK, ML297-GIRK | (194) | |
| Aversive experience | ||||
| Forced swim | DRN/serotonin | ↓ KOR-GIRK | p38 MAPK, GIRK1(Y12) | (195) |
| Foot-shock | LHb | ↓ GABABR-GIRK | PP2A dephosphorylation internalization | (196) |
| Predator odor | LHb | ↓ GABABR-GIRK | ||
| Restraint | LHb | ↓ GABABR-GIRK | ||
| Foot-shock | LHb | ↓ GABABR-GIRK | ||
| CUS | PL/layer 5/6 pyramidal | ↓ GABABR-GIRK, ML297-GIRK | ||
| Amyloid beta (Aβ) | ||||
| Aβ25–35 | HPC/CA3 pyramidal | ↓ GABABR-GIRK | ↓ GIRK expression | (197, 198) |
| Aβ1–42 | HPC culture | ↑ GABABR-GIRK | NMDAR, trafficking, p75NTR | (199) |
Examples of neuronal GIRK channel plasticity provoked by various stimuli in rodent models. CUS, chronic unpredictable stress; DRN, dorsal raphe nucleus; GABA, γ-aminobutyric acid; GIRK, G protein-gated inwardly rectifying K+; HFS, high-frequency stimulation; HPC, hippocampus; KOR, kappa opioid receptor; LFS, low-frequency stimulation; LHb, lateral habenula; MAPK, mitogen-activated protein kinase; NMDAR, N-methyl-d-aspartate receptor; OFC, orbitofrontal; PL, prelimbic; VTA, ventral tegmental area. Directional arrows, direction of plasticity: up arrows, strengthening; down arrows, weakening.
Subcellular Trafficking
Several forms of GIRK channel plasticity involve increased or decreased trafficking of GIRK channels to the surface membrane. For example, removal of an NMDAR antagonist following long-term incubation in cultured rat hippocampal neurons rapidly increased the surface trafficking of GIRK1 and GIRK2 (121). The increased surface trafficking required activation of protein phosphatase-1 (PP1) and subsequent dephosphorylation of GIRK2(S9), and it led to enhanced A1R-GIRK signaling and basal activity but no change in GABABR-GIRK signaling.
Morphine-dependent plasticity of GIRK-dependent signaling was also reported in cultured rat hippocampal neurons (191). This adaptation is mediated by activation of pertussis toxin-insensitive G proteins and involved activation of CaMKII. Morphine treatment involved an overall increase in GIRK2 protein levels, subcompartment-dependent changes in GIRK2, and an increase in overlap between GIRK2 and the excitatory synapse protein PSD-95. Interestingly, 5HT1AR-GIRK signaling was enhanced by chronic morphine treatment, whereas GABABR-GIRK signaling was suppressed. This adaptation was recapitulated in cultures from Girk2–/– mice expressing either GIRK2a or GIRK2c (65).
Several examples of psychostimulant-induced suppression of GIRK channel activity involving GIRK channel internalization have been described in the mouse VTA and medial prefrontal cortex (39, 187, 188). For example, a single injection of cocaine transiently suppressed GABABR-GIRK and D2R-GIRK signaling in mouse VTA dopamine neurons. This adaptation persisted for 3–5 days and correlated with increased GIRK2 internalization (187). Although mechanisms of plasticity were not resolved, methamphetamine self-administration in mice also triggered a suppression of GABABR-GIRK and D2R-GIRK signaling in midbrain dopamine neurons (190), and a single methamphetamine injection suppressed GABABR-dependent signaling in mouse VTA dopamine neurons (188).
Acute exposure to methamphetamine (or cocaine) suppressed GABABR-GIRK responses in mouse VTA GABA neurons (74, 188). This neuroadaptation persisted for at least 7 days and involved increased internalization of GIRK2 and GABABR. GABABR-GIRK currents were rescued by the PP1/PP2A phosphatase inhibitor okadaic acid, suggesting that the internalization was sustained by phosphatase activity and reversible. Indeed, methamphetamine provoked a decrease in phosphorylation of GABABR2 at serine-783 in the VTA. A subsequent study involving GABABR2(S783A) knock-in mice, which carry a phospho-dead mutation of S783 and are thus resistant to PP2A dephosphorylation (203), did not show this plasticity (74). Notably, the coordinated internalization of GABABR and GIRK2 seen in this neuroadaptation, along with cases of GIRK channel plasticity specific to 1 but not to other inhibitory GPCRs in the same cell (121, 191), supports the signalosome framework for GIRK-dependent signaling.
Following a repeated cocaine injection approach commonly used to evoke locomotor sensitization in rodents, layer 5/6 pyramidal neurons in the prelimbic (PL) region of the mouse medial prefrontal cortex exhibited decreased GABABR-GIRK signaling (39). This form of GIRK channel plasticity was also correlated with elevated internalization of GIRK2 and GABABR and rescued by okadaic acid. Notably, GIRK-dependent signaling in these neurons was also suppressed in male (but not female) mice by chronic unpredictable stress (201). Moreover, remifentanil self-administration correlated with decreased GABABR-GIRK signaling in layer 5/6 PL pyramidal neurons in male but not female mice (192).
Aversive experience (inescapable footshock) suppressed GABABR-GIRK signaling in neurons of the mouse lateral habenula (196). The adaptation emerged after just 1 h and persisted for up to 14 days. This form of experience-dependent plasticity correlated with increased internalization of GABABR1 and GIRK2, and it was rescued by okadaic acid and the more selective and membrane-permeable PP2A inhibitor LB-100.
Phosphoregulation
As alluded to aforementioned, phosphoregulation has been implicated in several examples of GIRK channel plasticity. GIRK2(S9) activity is the best example of phosphoregulation that involves phosphorylation of GIRK channel itself (121). Dephosphorylation of this residue promotes increased surface trafficking of GIRK channels and as such, phosphorylation of GIRK2(S9) should enhance the internalization of GIRK channel.
Several examples of plasticity that involve suppression of GIRK channel activity are rescued by the PP1/PP2A inhibitor okadaic acid (39, 188, 196), or the more selective PP2A inhibitor LB-100 (196). This suggests that dephosphorylation of GIRK channels or a functionally related target(s) underlies the suppression. As treatment with phosphatase inhibitor should enhance the phosphorylation of the relevant target(s), and these forms of GIRK channel plasticity involve suppression of GIRK channel activity, they do not likely involve GIRK2(S9). Indeed, dephosphorylation of GABABR2(S783) was linked to the methamphetamine-induced suppression of GABABR-GIRK signaling in mouse VTA GABA neurons (188) and, strikingly, this adaptation was not observed in phospho-deficient GABABR2(S783A) knock-in mice (74). PP2A-mediated dephosphorylation of GABABR2(S783) has been implicated in the surface expression of GABABR (204), and aversive experience does impact PP2A activity in the central nervous system (CNS) (205). Moreover, a Ca2+-dependent association between PP2A and GABABR1 has been described, and this interaction enhances the dephosphorylation of GABABR2(S783), promoting GABABR internalization (206).
An alternative phosphoregulatory mechanism has been implicated in the stress-induced suppression of KOR-GIRK signaling in serotonergic neurons of the mouse dorsal raphe (195). Stress-induced suppression of KOR-GIRK signaling was lost in mice lacking p38a mitogen-activated protein kinase. A corresponding increase in tyrosine phosphorylation of GIRK1(Y12) suggests that GIRK channel phosphorylation underlies this form of stress-induced plasticity. Phosphorylation of GIRK1(Y12) suppresses GIRK channel activity (118, 129), and increased phosphorylation of GIRK1(Y12) has been reported in several aversive settings in mice including behavioral stress, neuropathic pain, and acute inflammation (195, 207, 208).
GIRK3
GABABR-GIRK signaling in mouse VTA dopamine neurons is subject to bi-directional plasticity triggered by neuronal activity (73). Burst firing of VTA dopamine neurons provoked a rapid increase in GABABR-GIRK and D2R-GIRK signaling in mouse VTA slices. This form of plasticity was mimicked by depolarization of the VTA dopamine neurons and involved NMDAR and CaMKII activation, and was blocked by tetanus toxin, implicating increased receptor/channel insertion in the membrane. This adaptation was not seen in VTA dopamine neurons from Girk3–/– mice. Interestingly, tonic stimulation or low-frequency stimulation of mouse dopamine neurons rapidly suppressed synaptic GABABR-GIRK signaling; this adaptation was also dependent upon GIRK3.
Repeated methamphetamine injections suppressed GABABR-GIRK but not D2R-GIRK signaling in mouse VTA dopamine neurons (74). This form of plasticity was not seen in VTA dopamine neurons from Girk3–/– mice, and indirect evidence suggested that GIRK channel internalization was involved. Interestingly, the methamphetamine-induced suppression of GABABR-GIRK signaling in VTA dopamine neurons was still observed in GABABR2(S783A) knock-in mice, indicating that phosphoregulation of GABABR2(S783A) does not underlie all forms of psychostimulant-induced GIRK channel plasticity.
The involvement of GIRK3 in plasticity driven by neuronal activity or psychostimulants may reflect the unique subunit composition of the GIRK channel in mouse VTA dopamine neurons (GIRK2c:GIRK3) and/or protein:protein interactions mediated by GIRK3 in these neurons. Indeed, GIRK3 can interact with proteins that regulate channel trafficking and/or sensitivity to GPCR activation, including RGS2 (Fig. 1C). RGS2 negatively regulates GABABR-GIRK coupling sensitivity in mouse VTA dopamine neurons, an influence attributable to a physical interaction with GIRK3 (44). Notably, chronic cocaine decreased RGS2 expression in the rat brain (209); a similar effect could explain the enhanced GABABR-GIRK coupling efficiency seen in mouse VTA dopamine neurons following repeated morphine injections (44). GIRK3 coexpression suppresses GIRK channel activity evoked by GIRK2 expression in Xenopus oocytes (59), and ectopic viral expression of GIRK3 suppresses GIRK-dependent signaling in mouse VTA dopamine neurons (210). Thus, stimuli that alter the expression or stability of GIRK3 should lead to predictable changes in GIRK-dependent signaling, at least in VTA dopamine neurons.
GIRK3 has the same carboxyl-terminal PDZ interaction domain as GIRK2c, and this domain promotes an interaction with sorting nexin 27 (SNX27) (211) (Fig. 1C). SNX27 targets GIRK3-containing channels to early endosomes, leading to reduced surface expression of GIRK channels (19, 75, 211). SNX27 mRNA levels in the rat neocortex are elevated following exposure to psychostimulants (212), suggesting a plausible mechanism for the psychostimulant-induced suppression of GIRK-dependent signaling in VTA dopamine neurons. The depolarization-induced enhancement of GIRK channel activity in mouse VTA dopamine neurons was blocked by a dominant negative peptide mimicking the GIRK3 PDZ interaction domain, supporting a role for SNX27-mediated trafficking of GIRK channels in this form of plasticity. Interestingly, the total expression and subcellular trafficking of recombinant GIRK2c homomers is unaltered by SNX27 expression in (213), indicating that other elements of GIRK3, perhaps the lysosomal targeting motif, are required for the influence of SNX27 on GIRK channel trafficking.
NMDA Receptor
NMDAR activation increases surface trafficking of GIRK channels in rat hippocampal neurons by stimulating the PP1-dependent dephosphorylation of GIRK2(S9) (121). Other forms of GIRK channel plasticity also depend upon NMDAR activation, including the high frequency stimulation-induced strengthening of the GABABR-GIRK inhibitory postsynaptic current in rat hippocampal neurons (186), and the bi-directional activity-dependent plasticity of GIRK-dependent signaling in mouse VTA dopamine neurons (73). These forms of plasticity also involved intracellular Ca2+ increases and CaMKII activation.
There is a complex inter-relationship between the strength of GIRK channel and NMDAR activity in neurons. GIRK channels normally serve as an inhibitory break on NMDAR-dependent signaling in neurons (7). Genetic ablation of NMDAR in mouse forebrain pyramidal neurons correlated with decreased GIRK2 levels in cortical synaptic membranes (214). A single injection of the NMDAR antagonist ketamine, which induces a rapid antidepressant response, negatively regulates GABABR-GIRK signaling in the rat spinal cord (215). This intriguing effect is mediated by stabilization of adaptor protein 14-3-3eta, which decouples GABABR from GIRK channels. In a social defeat model of depression in rats, GABABR and 14-3-3eta protein levels were decreased in the hippocampus; NMDAR antagonists increased GABABR and 14-3-3eta protein levels, whereas GIRK2 protein levels were decreased (216).
NEURONAL GIRK CHANNEL PHARMACOLOGY
Although there are relatively few selective pharmacological tools available to study neuronal GIRK channels, progress made over the last decade has offered reason for optimism; this has been the focus of several recent reviews (3, 13, 217–219).
GIRK Channel Activators
ML297 is the prototypical member of a family of small molecule modulators of GIRK1-containing GIRK channels (220). ML297 is a potent (EC50 ∼100 nM) urea-based compound that shows modest selectivity for recombinant neuronal (GIRK1:GIRK2) over cardiac (GIRK1:GIRK4) GIRK channel subtypes. GIRK channel activation by ML297 is PIP2-dependent, but Gβγ-independent (221). Unique residues in the GIRK1 pore helix and second membrane-spanning domain are critical for the ML297-induced activation of GIRK1-containing channels.
ML297 has been used in several studies exploring the physiological relevance and therapeutic potential of GIRK channel activation. Systemic ML297 postponed seizure onset in an electroshock model and prevented convulsions and death in PTZ-induced seizure model in mice (220). Systemic ML297 also decreased multiple measures of anxiety-related behavior in male mice (221–223), without impacting motor activity or depression-related behavior, or inducing a conditioned place preference (CPP) (221). Interestingly, direct infusion of ML297 into the mouse ventral hippocampus recapitulated the apparent anxiolytic effect of systemic ML297, and selective ablation of GIRK channels in CA3 and dentate gyrus subregions of the ventral hippocampus was sufficient to block the anxiolytic effect of systemic ML297 in mice (223). Coadministration of ML297 prevented the synaptic and cognitive deficits provoked by intracerebroventricular (ICV) infusion of Aβ in mice (202, 224, 225). Intrathecal administration of ML297 in rats increased the mechanical nociceptive threshold without impairing motor function (226). ML297 inhibited wake activity, preferentially prolonging nonrapid eye movement sleep without changing sleep intensity in mice (227). Systemic ML297 also rescued the deficits in cognitive flexibility evoked by chronic unpredictable stress in male mice (201).
ML297 displays low solubility and poor blood-brain barrier permeability (220, 228), properties that limit its clinical utility. Two second-generation urea-based compounds stand out following structure activity relationship optimization. VU0466551 is approximately twice as potent as ML297 and displays analgesic effects in mice when dosed alone or together with submaximal morphine (229). GAT1508 is strongly selective for recombinant GIRK1:GIRK2 channels relative to cardiac (GIRK1:GIRK4) channels, and it can serve as a both a direct activator and positive allosteric modulator of neuronal GIRK channels (230). Interestingly, GAT1508 (but not ML297) accelerated the extinction of conditioned fear responses in mice, but was without effect on motor activity and coordination, anxiety-related behavior, or cognition. VU0810464 is a non-urea-based compound with higher GIRK1:GIRK2 channel selectivity and improved brain distribution as compared with ML297 (222), but it has a shorter half-life (228). VU0810464 exhibited no anxiolytic efficacy in mice in the elevated plus maze, but it retained efficacy in the stress-induced hyperthermia test (222).
Structure-based virtual screening against the alcohol binding site in GIRK2 lead to the identification of a compound (NCATS_2) that can activate both homomeric (GIRK2) and heteromeric (GIRK1:GIRK2) GIRK channels (231). An NCATS_2 analog (NCATS_2.3) was identified as selective for GIRK1-containing GIRK channels, but with higher brain distribution than ML297. This analog, also called G protein-independent GIRK activator 1 (GiGA1), activates GIRK1:GIRK2 channels in a G-protein-independent manner. Following systemic administration in mice, GiGA1 suppressed the seizure score and reduces the time in tonic seizure in a PTZ-induced seizure model. As was noted with ML297 (but not GAT1508), GiGA1 induces a dose-dependent decrease in locomotor activity, consistent with a sedative influence.
GIRK Channel Inhibitors
Few compounds selectively block GIRK channels. Recombinant GIRK channels are inhibited by volatile anesthetics (232, 233), as well as several plant-derived compounds including ginsenoside Rf, hesperidin, and diterpenes (234–238). Tertiapin, or the stable synthetic derivative tertiapin-Q (239, 240), is a commonly used GIRK channel inhibitor. Tertiapin is a small peptide isolated from bee venom that blocks GIRK channels, as well as Kir1.1 channels (240, 241) and BK (large-conductance Ca2+-activated K+) channels (242). Tertiapin exerts proconvulsant effects (243) and ICV infusion of tertiapin in mice disrupted the induction and maintenance of LTP in the hippocampus (202, 224). ICV tertiapin in mice also impaired nonassociative and recognition hippocampal-dependent learning, without altering motor function or coordination (244).
Neuronal GIRK Channels as Secondary Drug Targets
Several drugs used in medical practice, whose primary molecular targets are not GIRK channels, nevertheless impact GIRK channel activity. This may contribute to the therapeutic efficacy of these compounds and/or their undesirable side effects. Typical antipsychotic drugs like haloperidol, pimozide, and thioridazine, as well as atypical antipsychotic drugs like clozapine, inhibit recombinant neuronal and cardiac GIRK channels (245). GIRK channel inhibition by these drugs may explain some side effects, including seizures and sinus tachycardia. Some selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, and sertraline), serotonin-norepinephrine reuptake inhibitors (duloxetine), and tetracyclic antidepressants (amoxapine) can inhibit recombinant neuronal and cardiac GIRK channels (246, 247). GIRK channel inhibition may explain some side effects like seizures induced by antidepressant overdose (248). Tipepidine, an antitussive drug with antidepressant effects in adolescent depression and attention deficit hyperactivity disorder (ADHD), has been shown to enhance noradrenergic and dopaminergic transmission in rodent neurons via inhibiting GIRK channels (249–251).
GIRK CHANNELS IN NEUROLOGICAL DISORDERS AND DISEASES
Genetic evidence has implicated GIRK channels in an array of human neurological disorders and diseases (3, 13, 218). For example, a genome-wide association study (GWAS) of schizophrenia identified a single-nucleotide polymorphism (SNP) in KCNJ3/GIRK1 in a Japanese population (252). A subsequent GWAS identified nine SNPs in KCNJ3/GIRK1 in a Chinese population and increased KCNJ3/GIRK1 transcript levels in the dorsolateral prefrontal cortex of postmortem brains from patients with schizophrenia or bipolar disorder (253). One SNP in KCNJ3/GIRK1 is associated with idiopathic generalized epilepsy (254). An epistatic interaction between KCNJ6/GIRK2 and CREB1 [cyclic adenosine 5′-phosphate (adenosine monophosphate)-response element-binding protein] has been linked to rumination, a cognitive abnormality of depression (255). In humans, two SNPs in KCNJ6/GIRK2 are associated with ADHD risk, and functional variants of KCNJ6/GIRK2 influence executive processes (256). The GIRK channel inhibitor tipepidene mildly improves ADHD symptoms in pediatric patients in a pilot study (251). A mutation in the Forkhead-box protein 2 (FOXP2) transcription factor underlies a severe speech and language disorder, and increased GABABR-GIRK signaling in cortical neurons has been implicated (257).
Work in animal models involving gain- or loss-of-function manipulations (1, 3), including recent efforts using brain-region and/or neuron-specific manipulations (Table 2), has complemented these genetic studies in humans, shedding light on potential neuron populations and circuits underlying the impact of GIRK channels on neurophysiology and behavior. What follows is a brief overview of the evidence linking neuronal GIRK channels to addiction and cognitive disorders.
Table 2.
Regional and/or cell-specific manipulation of neuronal GIRK channels
| Subunit | Manipulation Brain Region/Neuron | Approach | Outcome | Refs. |
|---|---|---|---|---|
| GIRK1 ablation | Forebrain/pyramidal | CaMKIICre:Girk1fl/fl | ↑ locomotor activity (cocaine) | (258) |
| GIRK1 ablation | PL/pyramidal | Viral Cre: Girk1fl/fl | ↑ locomotor activity (cocaine) | (189) |
| - Fear conditioning | ||||
| GIRK1 ablation | PL/pyramidal | Viral Cre: Girk1fl/fl | ↑ anxiety-related behavior (EPM) | (201) |
| ↑ depression-related behavior (FST) | ||||
| ↓ cognitive flexibility | ||||
| GIRK1 ablation | IL/pyramidal | Viral Cre: Girk1fl/fl | - Anxiety-related behavior (EPM) | (259) |
| - Depression-related behavior (FST) | ||||
| ↓ cognitive flexibility | ||||
| GIRK1 ablation | Whole-brain/parvalbumin | PVCre: Girk1fl/fl | ↓ anxiety-related behavior (EPM) | (260) |
| ↑ depression-related behavior (FST) | ||||
| - Cognitive flexibility | ||||
| GIRK1 ablation | vHPC/CA3 and DG | Viral Cre: Girk1fl/fl | Abolished anxiolytic effect of ML297 | (223) |
| GIRK2 overexpression | VTA/dopamine | DATCre, viral | - Anxiety-related behavior (EPM) | (77) |
| - Depression-related behavior (FST) | ||||
| ↓ locomotor activity (cocaine) | ||||
| GIRK2 overexpression | VTA/dopamine | SNX27THKO, viral | Rescued cocaine sensitization | (261) |
| GIRK2 overexpression | dHPC/CA1 pyramidal | CaMKIICre: Girk2fl/fl, viral | rescued fear conditioning | (65) |
| GIRK2 ablation | Forebrain/pyramidal | CaMKIICre: Girk2fl/fl | ↑ locomotor activity (cocaine) | (258) |
| GIRK2 ablation | Forebrain/pyramidal | CaMKIICre: Girk2fl/fl | ↓ fear conditioning | (262) |
| - Motor activity, hot-plate | ||||
| - Anxiety-related behavior (EPM) | ||||
| - Spatial learning (Barnes maze) | ||||
| GIRK2 ablation | Whole brain/dopamine | DATCre: Girk2fl/fl | ↑ locomotor activity (morphine) | (40) |
| GIRK2 ablation | Whole brain/dopamine | DATCre: Girk2fl/fl | ↑ locomotor activity (cocaine) | (210) |
| - CPP (cocaine) | ||||
| ↑ self-administration (cocaine) | ||||
| GIRK2 ablation | Whole brain/dopamine | DATCre: Girk2fl/fl | ↓ depression-related behavior (FST) | (263) |
| - Locomotor activity | ||||
| GIRK2 ablation | Whole brain/GABA | GADCre: Girk2fl/fl | - Locomotor activity (morphine) | (40) |
| GIRK2 ablation | Whole brain/GABA | GADCre: Girk2fl/fl | - Motor activity, hot-plate | (262) |
| - Anxiety-related behavior (EPM) | ||||
| - Fear conditioning (Barnes maze) | ||||
| GIRK3 overexpression | VTA/dopamine | DATCre, viral | - Anxiety-related behavior (EPM) | (77) |
| - Depression-related behavior (FST) | ||||
| ↑ locomotor activity (cocaine) | ||||
| GIRK3 overexpression | VTA | Girk3−/−, viral | Rescued morphine sensitivity | (40) |
Studies employing genetic and/or viral approaches to manipulate GIRK channel activity in specific brain regions and/or neuron populations in mouse models. CPP, conditioned place preference; DG, dentate gyrus; dHPC, dorsal hippocampus; EPM, elevated plus maze; FST, forced swim test; GIRK, G protein-gated inwardly rectifying K+; IL, infralimbic cortex; PL, prelimbic cortex; PV, parvalbumin; vHPC, ventral hippocampus; SNX27THKO, dopamine neuron-specific SNX27 knockout; VTA, ventral tegmental area. Directional arrows, impact of manipulation on specified behavior: up arrows, enhancement; down arrows, suppression.
Addiction
The mesocorticolimbic dopamine system is an array of interconnected brain regions tasked with reward processing that includes the VTA and medial prefrontal cortex (264). GIRK channels are expressed in key reward-related neuron populations in rodents and they have been implicated in the biological actions of many categories of drugs of abuse (265). Genetic variation in the human KCNJ6/GIRK2 gene is associated with reduced opioid sensitivity and opioid withdrawal symptoms (266–269), nicotine dependence (269, 270), and alcohol dependence and stress-related adolescent drinking (271).
Work in recombinant systems has revealed that GIRK channels are direct targets of some drugs of abuse, activated by ethanol (272, 273) and inhibited by phencyclidine (274). GIRK channels are downstream effectors of GPCRs engaged—directly or indirectly—by drugs of abuse, including D2R (275), GABABR (4), and opioid receptors (MOR, DOR, and KOR) (174). Work in mouse models has shown that GIRK channel activation contributes to many facets of opioid-related physiology and behavior, including respiratory depression (276, 277), withdrawal (278), and systemic and spinal analgesia (70, 279–282). Here, we discuss the inter-relationship between GIRK channel activity and drugs of abuse, as exemplified by studies in mice involving psychostimulants and ethanol.
Psychostimulants.
Exposure to psychostimulants like cocaine weakens inhibitory G protein signaling in the mouse mesocorticolimbic system (283). As noted earlier, cocaine or methamphetamine can suppress GIRK currents in mouse VTA dopamine (187) and GABA (188) neurons, and repeated cocaine injection in mice suppresses GIRK-dependent signaling in layer 5/6 pyramidal neurons of the prelimbic cortex (PL) (39). Drug-naïve Girk1–/– and Girk2–/– mice exhibit enhanced glutamatergic neurotransmission in the nucleus accumbens (67), as well as increased behavioral sensitivity to drugs of abuse (40, 284, 285), similar to observations in wild-type mice following chronic drug exposure.
Genetic and viral genetic approaches in mice have been used to mimic these forms of GIRK channel plasticity, with the goal of understanding their behavioral relevance. Mice lacking GIRK channels in dopamine neurons exhibit increased sensitivity to the motor-stimulatory effect of cocaine, as well as increased responding for and intake of cocaine in a self-administration task (210). Suppression of GIRK channel activity selectively in VTA dopamine neurons, via Cre-dependent virus-driven overexpression of GIRK3 in mice, also increased the motor-stimulatory effect of cocaine, without impacting select measures of anxiety- or depression-related behavior (77). Genetic ablation of GIRK channels in mouse forebrain pyramidal neurons enhanced glutamatergic neurotransmission in D1R-expressing medium spiny neurons in the nucleus accumbens core and increased motor-stimulatory responses to cocaine, comparable with effects seen in wild-type mice given repeated cocaine injections (258). The more selective viral Cre-dependent ablation of GIRK channels in mouse PL pyramidal neurons increased motor-stimulatory effect of cocaine (189), while also impairing working memory and cognitive flexibility in male (but not female) mice (201). Modeling these adaptations in drug-naïve mice supports the contention that psychostimulant-induced plasticity of GIRK channel in reward circuitry contributes to some of the electrophysiological and behavioral hallmarks of addiction.
Alcohol.
GIRK channels play prominent roles in key aspects of alcohol consumption, reward, and withdrawal. Genetic variation in the human KCNJ6/GIRK2 gene has been linked to alcohol dependence and stress-related adolescent drinking (271). In addition, several single-nucleotide polymorphisms in the KCNJ6/GIRK2 promoter were identified in subjects with alcohol use disorder (AUD) and offspring at risk for developing an AUD (286). Variations in Kcnj9/Girk3 expression have been linked to strain differences in key ethanol-related behaviors in mice; lower levels of Kcnj9/Girk3 expression are associated with less severe withdrawal from ethanol, pentobarbital, and zolpidem (71). Withdrawal from ethanol and sedative-hypnotics is less severe in Girk3–/– mice (71). The Kcnj9/Girk3 gene has also been linked to ethanol drinking (287), ethanol-induced conditioned aversion (288), and acute sensitivity to alcohol (289) in mice.
Ethanol binds to and activates recombinant GIRK channels at intoxicating concentrations, independent of Gβγ or cholesterol (17, 21, 169, 272, 273). Many ethanol-induced behaviors are altered in Girk–/– mice. For example, Girk2–/– mice show less severe withdrawal symptoms, including handling-induced convulsions (290), and Girk2–/– mice lack the anxiolytic effect of ethanol, as assessed in the elevated plus maze test (290). Girk2–/– mice also lack ethanol-induced analgesia (291), and exhibit reduced conditioned place preference and conditioned taste aversion (292). Like Girk2–/– mice, Girk3–/– mice exhibit less severe ethanol withdrawal (71). In contrast to Girk2–/– mice, however, Girk3–/– mice exhibit enhanced ethanol reward and CPP (293). The ethanol-induced excitation of VTA dopamine neurons is blunted in Girk3–/– mice (72), a physiological phenotype that correlates with elevated binge-like drinking by Girk3–/– mice. Notably, viral re-expression of GIRK3 in the VTA normalized binge drinking behavior of Girk3–/– mice.
Ethanol also triggers adaptations in GIRK channel activity (294). For example, significant changes in GIRK1 and GIRK3 transcript levels in the mouse nucleus accumbens were observed following chronic intermittent exposure to ethanol vapor (CIE) (200). In addition, withdrawal from repeated in vivo alcohol exposure correlated with enhanced D2R-GIRK (but not GABABR-GIRK) signaling in mouse VTA dopamine neurons (193). Furthermore, following CIE treatment, the GIRK-dependent inhibition of orbitofrontal cortical neurons triggered by D2R, 5HT1AR, and α2 adrenergic receptor activation was blunted in mice (194). GIRK currents evoked by ML297 were also suppressed following CIE, suggesting that this neuroadaptation involved the GIRK channel itself.
Cognitive Disorders
GIRK channels are highly expressed in brain regions related to cognitive function, including the hippocampus, amygdala, and prefrontal cortex (34). Several lines of evidence indicate that dysregulation of GIRK-dependent signaling is associated with cognitive deficits in humans. Perhaps most strikingly, the sequencing results of the exomes from three unrelated human patients with Keppen–Lubinsky syndrome, a rare disease characterized by severe developmental delay and intellectual disability (295), all exhibit de novo heterozygous mutations in KCNJ6/GIRK2 that severely impair GIRK channel function (296).
Work in rodents has shown that an optimal range of GIRK channel activity is required for normal synaptic plasticity. For example, constitutive and forebrain pyramidal neuron-specific Girk2–/– mice, as well as GIRK2 trisomy mice that exhibit elevated GIRK channel activity, are deficient in depotentiation of LTP at CA3-CA1 hippocampal synapses (125, 262). Loss-of-function GIRK1 mutant mice also display impaired depotentiation of LTP, suggesting an essential role of both GIRK1 and GIRK2 in this form of synaptic plasticity (297). Acute ICV injections of either the GIRK channel activator ML297 or inhibitor tertiapin in mice similarly transform LTP into long-term depression (LTD) following high-frequency stimulation (202, 224, 244). The threshold for induction of LTP is higher in dorsal rather than ventral CA1 pyramidal neurons in the rat due to elevated dendritic GIRK channel activity in dorsal CA1 neurons (298). Both gain- and loss-of-function approaches in mice involving GIRK channel activity disrupt associative learning (148, 262, 297, 299, 300). Girk2–/– mice show disrupted contextual fear learning (301), a phenotype recapitulated in forebrain pyramidal neuron-specific Girk2–/– mice (262). Viral restoration of GIRK channel activity in CA1 pyramidal neurons in the dorsal hippocampus rescued contextual fear learning in these mice (65). Mice receiving acute ICV injections of GIRK activator ML297 or tertiapin show learning impairments in open field habituation, novel object recognition, and operant conditioning tasks (244).
Down syndrome.
Down syndrome (DS) is a congenital disorder characterized by intellectual disabilities, mental impairment, and distinct facial features. DS is caused by an extra maternal copy (trisomy) of human chromosome 21 (299, 302), and KCNJ6/GIRK2 is located in the DS critical region (DSCR). A widely-used DS mouse model, Ts65Dn, is a partial trisomy model containing a region of mouse chromosome 16 orthologous to human chromosome 21 and that includes the DSCR (303). In Ts65Dn mice, GIRK2 protein levels are higher and GABABR-GIRK signaling is elevated (304). Ts65Dn mice show various forms of synaptic and cognitive impairments (299, 304, 305), among which several are also seen in mice with trisomy for GIRK2 alone (300). GIRK2 trisomy mice exhibit hampered depotentiation of LTP, accentuated LTD, and impaired hippocampal-dependent contextual fear recall (300). Reducing Kcnj6/Girk2 gene dosage in Ts65Dn mice abolished the impairment of synaptic plasticity and cognitive deficits (305), as well as infantile spasms induced by GABABR agonist (306). Trisomy of Kcnj6/Girk2 alone, however, is not sufficient to confer susceptibility to infantile spasms in this model (307). Thus, although GIRK channel upregulation is not the only mechanism underlying pathological phenotypes in this mouse model of DS, downregulation of GIRK channel activity could be a promising therapeutic approach to ameliorate the symptoms and cognitive deficits in individuals with DS.
Alzheimer's disease.
Although GIRK expression levels are altered in different rodent models of Alzheimer’s disease (AD) (197, 198, 202, 308), divergent outcomes across studies complicates easy synthesis of their role. For example, incubation of rat hippocampal slices with synthetic Aβ reduced GIRK mRNA levels (198), but GIRK1 and GIRK2 protein levels were not impacted (202). Similarly, ICV injection of Aβ in mice decreased GIRK1 protein level in the CA1 subregion of the dorsal hippocampus (202), but had no impact on GIRK1 mRNA or protein levels in the rat hippocampus as assessed after 7 days of incubation (309). Increased GIRK channel internalization was noted in the hippocampus of two transgenic mouse models of AD (308). Effects of Aβ on GIRK channel activity have also been reported and, again, results are divergent. Upregulation of GIRK channel activity was implicated in apoptotic cell death in mouse hippocampus (HPC) cultures (199); this effect was observed with 20 µM Aβ, a concentration higher than typical for Aβ neurotoxicity studies. In contrast, incubation of rat HPC slices with Aβ (1.5 µM) increased CA3 pyramidal neuron excitability, and suppression of GIRK channel activity was implicated (197). Remarkably, coadministration of ML297 rescued synaptic plasticity and memory deficits induced by acute ICV Aβ infusion in mice (202, 224, 225). ICV infusion of either Aβ or tertiapin disrupted novel environment habituation and novel object recognition in mice (202, 244, 310). Thus, GIRK channels may contribute to AD pathogenesis and GIRK channel activators could be useful for rescuing synaptic and behavioral deficits.
CONCLUDING REMARKS
It has been 30 years since the cloning of GIRK1 (311, 312), an achievement that ushered in a new era of research into the molecular mechanisms of inhibitory signaling in the nervous system. The last 15 years have seen a dramatic advance in our understanding of the structural basis of GIRK channel function and regulation. Despite this progress, important questions remain. For example, subunit composition and stoichiometry likely dictate unique aspects of GIRK channel function and regulation, and yet we know little about the precise molecular composition of GIRK channels in the nervous system, particularly in humans. Similarly, our understanding of GIRK channel structure relies heavily on insights from studies of recombinant purified GIRK2 homomers, which are likely a relatively rare channel subtype in the mammalian brain.
The impact on neuronal GIRK-dependent signaling of cholesterol, reversible phosphorylation, RGS proteins, and 14-3-3 proteins warrants rigorous examination given the physiological and/or pathophysiological relevance of these regulatory factors. Elucidating mechanisms underlying distinct forms of GIRK plasticity affords an excellent opportunity to discover additional modulatory influences. These insights may also shed important light on how GIRK-dependent signaling is organized in neurons. Available data indicate that mechanisms are in place to coalesce specific interactions between GIRK channels and discrete sets of signaling elements. As our understanding of GIRK channel regulation and compartmentalization improves, we will be in a better position to consider ways to more selectively intervene when GIRK channel activity is dysregulated.
We now have multiple distinct chemical scaffolds for small molecule GIRK channel modulators. Work with these compounds has already shown their promise in preclinical models of epilepsy, pain, anxiety, and Alzheimer’s disease. These tools afford excellent opportunities to continue building the pharmacological toolkit of GIRK channel activators, focusing on tools with improved pharmacokinetics and channel subtype-specificity. To the latter point, most GIRK channel activators characterized to date target GIRK1-containing GIRK channels. Compounds that selectively modulate GIRK1-lacking channels, like NCATS_2.3 (231), VU0529331 (313), or 3hi2one-G4 (314) are particularly intriguing tools. These compounds could lead to the development of drugs exerting a relatively selective influence on the unique GIRK1-lacking channel subtype in midbrain dopamine neurons; the potential therapeutic applications of these compounds in the context of substance use disorders and movement disorders are exciting to consider.
As we ponder the translational potential of therapeutic approaches targeting GIRK channels, it is worth underscoring the critical point that our current understanding of GIRK channel biology is based almost exclusively on work in rodent models and heterologous expression systems. The extent to which insights gleaned from these studies will align with the contribution of neuronal GIRK channels to human neurophysiology and behavior is unclear. The use of more translational models including human-induced pluripotent stem cells and human postmortem tissues, as well as nonhuman primates, may begin to help fill some of the existing translational gaps in this field. More broadly, translating knowledge gained from rodent models to efficacious and safe therapeutic approaches for human diseases and disorders has proven challenging across an array of neurological contexts (e.g., see Refs. 315 and 316). Despite this cautionary note, the efficacy of manipulations targeting neuronal GIRK channels in preclinical models provides reason for optimism as well as support for advancing efforts toward clinical trials for a variety of neurological disorders and diseases.
GRANTS
This review was completed with support from National Institutes of Health Grants DA034696 and AA027544 (to K.W.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L. prepared figures; H.L., E.M.F.d.V., and K.W. drafted manuscript; H.L., E.M.F.d.V., and K.W. edited and revised manuscript; H.L., E.M.F.d.V., and K.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank members of the Wickman laboratory for providing feedback on this review.
This article is part of the special collection “Inward Rectifying K+ Channels.” Jerod Denton, PhD, and Eric Delpire, PhD, served as Guest Editors of this collection.
REFERENCES
- 1.Lujan R, Marron Fernandez de Velasco E, Aguado C, Wickman K. New insights into the therapeutic potential of GIRK channels. Trends Neurosci 37: 20–29, 2014. doi: 10.1016/j.tins.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slesinger PA, Wickman K (Editors). Structure to Function of G Protein-Gated Inwardly Rectifying (GIRK) Channels. New York: Elsevier, 2015. [Google Scholar]
- 3.Jeremic D, Sanchez-Rodriguez I, Jimenez-Diaz L, Navarro-Lopez JD. Therapeutic potential of targeting G protein-gated inwardly rectifying potassium (GIRK) channels in the central nervous system. Pharmacol Ther 223: 107808, 2021. doi: 10.1016/j.pharmthera.2021.107808. [DOI] [PubMed] [Google Scholar]
- 4.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695, 1997. [Erratum inNeuron19: 945, 1997]. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 5.Lujan R, Aguado C. Localization and targeting of GIRK channels in mammalian central neurons. Int Rev Neurobiol 123: 161–200, 2015. doi: 10.1016/bs.irn.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Dascal N, Kahanovitch U. The roles of Gβγ and Gα in gating and regulation of GIRK channels. Int Rev Neurobiol 123: 27–85, 2015. doi: 10.1016/bs.irn.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol 92: 2027–2039, 2004. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- 8.Hikima T, Lee CR, Witkovsky P, Chesler J, Ichtchenko K, Rice ME. Activity-dependent somatodendritic dopamine release in the substantia nigra autoinhibits the releasing neuron. Cell Rep 35: 108951, 2021. doi: 10.1016/j.celrep.2021.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun QQ, Huguenard JR, Prince DA. Somatostatin inhibits thalamic network oscillations in vitro: actions on the GABAergic neurons of the reticular nucleus. J Neurosci 22: 5374–5386, 2002. doi: 10.1523/JNEUROSCI.22-13-05374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietersen AN, Lancaster DM, Patel N, Hamilton JB, Vreugdenhil M. Modulation of γ oscillations by endogenous adenosine through A1 and A2A receptors in the mouse hippocampus. Neuropharmacology 56: 481–492, 2009. doi: 10.1016/j.neuropharm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Johnston A, McBain CJ, Fisahn A. 5-HT1A receptor-activation hyperpolarizes pyramidal cells and suppresses hippocampal γ oscillations via Kir3 channel-activation. J Physiol 592: 4187–4199, 2014. doi: 10.1113/jphysiol.2014.279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 13.Cui M, Cantwell L, Zorn A, Logothetis DE. Kir channel molecular physiology, pharmacology, and therapeutic implications. Handb Exp Pharmacol 267: 277–356, 2021. doi: 10.1007/164_2021_501. [DOI] [PubMed] [Google Scholar]
- 14.Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell 111: 957–965, 2002. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q, Kawano T, Nakata H, Nakajima Y, Nakajima S, Kozasa T. Interaction of G protein β subunit with inward rectifier K(+) channel Kir3. Mol Pharmacol 64: 1085–1091, 2003. doi: 10.1124/mol.64.5.1085. [DOI] [PubMed] [Google Scholar]
- 16.Shin HG, Xu Y, Lu Z. Evidence for sequential ion-binding loci along the inner pore of the IRK1 inward-rectifier K+ channel. J Gen Physiol 126: 123–135, 2005. doi: 10.1085/jgp.200509296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci 12: 988–995, 2009. doi: 10.1038/nn.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147: 199–208, 2011. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balana B, Maslennikov I, Kwiatkowski W, Stern KM, Bahima L, Choe S, Slesinger PA. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc Natl Acad Sci USA 108: 5831–5836, 2011. doi: 10.1073/pnas.1018645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498: 190–197, 2013. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodhinathan K, Slesinger PA. Molecular mechanism underlying ethanol activation of G-protein-gated inwardly rectifying potassium channels. Proc Natl Acad Sci USA 110: 18309–18314, 2013. doi: 10.1073/pnas.1311406110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaaser IW, Slesinger PA. Structural insights into GIRK channel function. Int Rev Neurobiol 123: 117–160, 2015. doi: 10.1016/bs.irn.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer J, Nichols CG, Makhina EN, Salkoff L, Bernstein J, Gerhard D, Wasson J, Ramanadham S, Permutt A. Pancreatic islet cells express a family of inwardly rectifying K+ channel subunits which interact to form G-protein-activated channels. J Biol Chem 270: 26086–26091, 1995. doi: 10.1074/jbc.270.44.26086. [DOI] [PubMed] [Google Scholar]
- 24.Tanizawa Y, Matsubara A, Ueda K, Katagiri H, Kuwano A, Ferrer J, Permutt MA, Oka Y. A human pancreatic islet inwardly rectifying potassium channel: cDNA cloning, determination of the genomic structure and genetic variations in Japanese NIDDM patients. Diabetologia 39: 447–452, 1996. doi: 10.1007/BF00400676. [DOI] [PubMed] [Google Scholar]
- 25.Iwanir S, Reuveny E. Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Arch 456: 1097–1108, 2008. doi: 10.1007/s00424-008-0479-4. [DOI] [PubMed] [Google Scholar]
- 26.Gregerson KA, Flagg TP, O'Neill TJ, Anderson M, Lauring O, Horel JS, Welling PA. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology 142: 2820–2832, 2001. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- 27.Corey S, Krapivinsky G, Krapivinsky L, Clapham DE. Number and stoichiometry of subunits in the native atrial G-protein-gated K+ channel, IKACh. J Biol Chem 273: 5271–5278, 1998. doi: 10.1074/jbc.273.9.5271. [DOI] [PubMed] [Google Scholar]
- 28.Corey S, Clapham DE. Identification of native atrial G-protein-regulated inwardly rectifying K+ (GIRK4) channel homomultimers. J Biol Chem 273: 27499–27504, 1998. doi: 10.1074/jbc.273.42.27499. [DOI] [PubMed] [Google Scholar]
- 29.Hou P, Yan S, Tang W, Nelson DJ. The inwardly rectifying K(+) channel subunit GIRK1 rescues the GIRK2 weaver phenotype. J Neurosci 19: 8327–8336, 1999. doi: 10.1523/JNEUROSCI.19-19-08327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadja R, Alagem N, Reuveny E. Graded contribution of the Gβ γ binding domains to GIRK channel activation. Proc Natl Acad Sci USA 99: 10783–10788, 2002. doi: 10.1073/pnas.162346199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 374: 135–141, 1995. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 32.Bender K, Wellner-Kienitz MC, Inanobe A, Meyer T, Kurachi Y, Pott L. Overexpression of monomeric and multimeric GIRK4 subunits in rat atrial myocytes removes fast desensitization and reduces inward rectification of muscarinic K(+) current (I(K(ACh))). Evidence for functional homomeric GIRK4 channels. J Biol Chem 276: 28873–28880, 2001. doi: 10.1074/jbc.M102328200. [DOI] [PubMed] [Google Scholar]
- 33.Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem 277: 48282–48288, 2002. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- 34.Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16: 3559–3570, 1996. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci 25: 11468–11478, 2005. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA 94: 923–927, 1997. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective γ-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci USA 94: 12210–12217, 1997. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22: 4328–4334, 2002. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, Wickman K. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron 80: 159–170, 2013. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotecki L, Hearing M, McCall NM, Marron Fernandez de Velasco E, Pravetoni M, Arora D, Victoria NC, Munoz MB, Xia Z, Slesinger PA, Weaver CD, Wickman K. GIRK channels modulate opioid-induced motor activity in a cell type- and subunit-dependent manner. J Neurosci 35: 7131–7142, 2015. doi: 10.1523/JNEUROSCI.5051-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jelacic TM, Sims SM, Clapham DE. Functional expression and characterization of G-protein-gated inwardly rectifying K+ channels containing GIRK3. J Membr Biol 169: 123–129, 1999. doi: 10.1007/s002329900524. [DOI] [PubMed] [Google Scholar]
- 42.Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3. J Biol Chem 275: 36211–36216, 2000. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- 43.Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci 7: 153–159, 2004. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- 44.Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci 10: 1559–1568, 2007. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- 45.Perry CA, Pravetoni M, Teske JA, Aguado C, Erickson DJ, Medrano JF, Lujan R, Kotz CM, Wickman K. Predisposition to late-onset obesity in GIRK4 knockout mice. Proc Natl Acad Sci USA 105: 8148–8153, 2008. doi: 10.1073/pnas.0803261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot JP. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett 353: 37–42, 1994. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- 47.Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 270: 28660–28667, 1995. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- 48.Isomoto S, Kondo C, Takahashi N, Matsumoto S, Yamada M, Takumi T, Horio Y, Kurachi Y. A novel ubiquitously distributed isoform of GIRK2 (GIRK2B) enhances GIRK1 expression of the G-protein-gated K+ current in Xenopus oocytes. Biochem Biophys Res Commun 218: 286–291, 1996. doi: 10.1006/bbrc.1996.0050. [DOI] [PubMed] [Google Scholar]
- 49.Nelson CS, Marino JL, Allen CN. Cloning and characterization of Kir3.1 (GIRK1) C-terminal alternative splice variants. Brain Res Mol Brain Res 46: 185–196, 1997. doi: 10.1016/s0169-328x(96)00301-4. [DOI] [PubMed] [Google Scholar]
- 50.Wei J, Hodes ME, Piva R, Feng Y, Wang Y, Ghetti B, Dlouhy SR. Characterization of murine Girk2 transcript isoforms: structure and differential expression. Genomics 51: 379–390, 1998. doi: 10.1006/geno.1998.5369. [DOI] [PubMed] [Google Scholar]
- 51.Inanobe A, Yoshimoto Y, Horio Y, Morishige KI, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci 19: 1006–1017, 1999. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron 33: 715–729, 2002. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 53.Wickman K, Pu WT, Clapham DE. Structural characterization of the mouse Girk genes. Gene 284: 241–250, 2002. doi: 10.1016/s0378-1119(01)00884-8. [DOI] [PubMed] [Google Scholar]
- 54.Wagner V, Stadelmeyer E, Riederer M, Regitnig P, Gorischek A, Devaney T, Schmidt K, Tritthart HA, Hirschberg K, Bauernhofer T, Schreibmayer W. Cloning and characterisation of GIRK1 variants resulting from alternative RNA editing of the KCNJ3 gene transcript in a human breast cancer cell line. J Cell Biochem 110: 598–608, 2010. doi: 10.1002/jcb.22564. [DOI] [PubMed] [Google Scholar]
- 55.Rezania S, Kammerer S, Li C, Steinecker-Frohnwieser B, Gorischek A, DeVaney TT, Verheyen S, Passegger CA, Tabrizi-Wizsy NG, Hackl H, Platzer D, Zarnani AH, Malle E, Jahn SW, Bauernhofer T, Schreibmayer W. Overexpression of KCNJ3 gene splice variants affects vital parameters of the malignant breast cancer cell line MCF-7 in an opposing manner. BMC Cancer 16: 628, 2016. doi: 10.1186/s12885-016-2664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan KW, Sui JL, Vivaudou M, Logothetis DE. Control of channel activity through a unique amino acid residue of a G protein-gated inwardly rectifying K+ channel subunit. Proc Natl Acad Sci USA 93: 14193–14198, 1996. doi: 10.1073/pnas.93.24.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy ME, Nemec J, Clapham DE. Localization and interaction of epitope-tagged GIRK1 and CIR inward rectifier K+ channel subunits. Neuropharmacology 35: 831–839, 1996. doi: 10.1016/0028-3908(96)00132-3. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy ME, Nemec J, Corey S, Wickman K, Clapham DE. GIRK4 confers appropriate processing and cell surface localization to G-protein-gated potassium channels. J Biol Chem 274: 2571–2582, 1999. doi: 10.1074/jbc.274.4.2571. [DOI] [PubMed] [Google Scholar]
- 59.Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G β γ subunits and function as heteromultimers. Proc Natl Acad Sci USA 92: 6542–6546, 1995. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duprat F, Lesage F, Guillemare E, Fink M, Hugnot JP, Bigay J, Lazdunski M, Romey G, Barhanin J. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun 212: 657–663, 1995. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- 61.Wydeven N, Young D, Mirkovic K, Wickman K. Structural elements in the GIRK1 subunit that potentiate G protein-gated potassium channel activity. Proc Natl Acad Sci USA 109: 21492–21497, 2012. doi: 10.1073/pnas.1212019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slesinger PA, Reuveny E, Jan YN, Jan LY. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron 15: 1145–1156, 1995. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 63.Stevens EB, Woodward R, Ho IH, Murrell-Lagnado R. Identification of regions that regulate the expression and activity of G protein-gated inward rectifier K+ channels in Xenopus oocytes. J Physiol 503: 547–562, 1997. doi: 10.1111/j.1469-7793.1997.547bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan KW, Sui JL, Vivaudou M, Logothetis DE. Specific regions of heteromeric subunits involved in enhancement of G protein-gated K+ channel activity. J Biol Chem 272: 6548–6555, 1997. doi: 10.1074/jbc.272.10.6548. [DOI] [PubMed] [Google Scholar]
- 65.Marron Fernandez de Velasco E, Zhang L, B NV, Tipps M, Farris S, Xia Z, Anderson A, Carlblom N, Weaver CD, Dudek SM, Wickman K. GIRK2 splice variants and neuronal G protein-gated K+ channels: implications for channel function and behavior. Sci Rep 7: 1639, 2017. doi: 10.1038/s41598-017-01820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marker CL, Lujan R, Colon J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci 26: 12251–12259, 2006. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arora D, Haluk DM, Kourrich S, Pravetoni M, Fernandez-Alacid L, Nicolau JC, Lujan R, Wickman K. Altered neurotransmission in the mesolimbic reward system of Girk–/– mice. J Neurochem 114: 1487–1497, 2010. doi: 10.1111/j.1471-4159.2010.06864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguado C, Colon J, Ciruela F, Schlaudraff F, Cabanero MJ, Perry C, Watanabe M, Liss B, Wickman K, Lujan R. Cell type-specific subunit composition of G protein-gated potassium channels in the cerebellum. J Neurochem 105: 497–511, 2008. [Erratum inJ Neurochem105: 2053–2054, 2008]. doi: 10.1111/j.1471-4159.2007.05153.x. [DOI] [PubMed] [Google Scholar]
- 69.Pravetoni M, Wickman K. Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav 7: 523–531, 2008. doi: 10.1111/j.1601-183X.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 70.Smith SB, Marker CL, Perry C, Liao GC, Sotocinal SG, Austin JS, Melmed K, Clark JD, Peltz G, Wickman K, Mogil JS. Quantitative trait locus and computational mapping identifies Kcnj9 (GIRK3) as a candidate gene affecting analgesia from multiple drug classes. Pharmacogenet Genomics 18: 231–241, 2008. doi: 10.1097/Fpc.0b013e3282f55ab2. [DOI] [PubMed] [Google Scholar]
- 71.Kozell LB, Walter NA, Milner LC, Wickman K, Buck KJ. Mapping a barbiturate withdrawal locus to a 0.44 Mb interval and analysis of a novel null mutant identify a role for Kcnj9 (GIRK3) in withdrawal from pentobarbital, zolpidem, and ethanol. J Neurosci 29: 11662–11673, 2009. doi: 10.1523/JNEUROSCI.1413-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herman MA, Sidhu H, Stouffer DG, Kreifeldt M, Le D, Cates-Gatto C, Munoz MB, Roberts AJ, Parsons LH, Roberto M, Wickman K, Slesinger PA, Contet C. GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol. Proc Natl Acad Sci USA 112: 7091–7096, 2015. doi: 10.1073/pnas.1416146112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lalive AL, Munoz MB, Bellone C, Slesinger PA, Luscher C, Tan KR. Firing modes of dopamine neurons drive bidirectional GIRK channel plasticity. J Neurosci 34: 5107–5114, 2014. doi: 10.1523/JNEUROSCI.5203-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munoz MB, Padgett CL, Rifkin R, Terunuma M, Wickman K, Contet C, Moss SJ, Slesinger PA. A role for the GIRK3 subunit in methamphetamine-induced attenuation of GABAB receptor-activated GIRK currents in VTA dopamine neurons. J Neurosci 36: 3106–3114, 2016. doi: 10.1523/JNEUROSCI.1327-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balana B, Bahima L, Bodhinathan K, Taura JJ, Taylor NM, Nettleton MY, Ciruela F, Slesinger PA. Ras-association domain of sorting Nexin 27 is critical for regulating expression of GIRK potassium channels. PloS One 8: e59800, 2013. doi: 10.1371/journal.pone.0059800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tabak G, Keren-Raifman T, Kahanovitch U, Dascal N. Mutual action by Gγ and Gβ for optimal activation of GIRK channels in a channel subunit-specific manner. Sci Rep 9: 508, 2019. doi: 10.1038/s41598-018-36833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCall NM, Marron Fernandez de Velasco E, Wickman K. GIRK channel activity in dopamine neurons of the ventral tegmental area bidirectionally regulates behavioral sensitivity to cocaine. J Neurosci 39: 3600–3610, 2019. doi: 10.1523/JNEUROSCI.3101-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logothetis DE, Mahajan R, Adney SK, Ha J, Kawano T, Meng XY, Cui M. Unifying mechanism of controlling Kir3 channel activity by G proteins and phosphoinositides. Int Rev Neurobiol 123: 1–26, 2015. doi: 10.1016/bs.irn.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science 252: 802–808, 1991. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 80.Hildebrandt JD. Role of subunit diversity in signaling by heterotrimeric G proteins. Biochem Pharmacol 54: 325–339, 1997. doi: 10.1016/s0006-2952(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 81.Jiang M, Bajpayee NS. Molecular mechanisms of Go signaling. Neurosignals 17: 23–41, 2009. doi: 10.1159/000186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Senarath K, Kankanamge D, Samaradivakara S, Ratnayake K, Tennakoon M, Karunarathne A. Regulation of G protein βγ signaling. Int Rev Cell Mol Biol 339: 133–191, 2018. doi: 10.1016/bs.ircmb.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Masuho I, Skamangas NK, Muntean BS, Martemyanov KA. Diversity of the Gβγ complexes defines spatial and temporal bias of GPCR signaling. Cell Syst 12: 324–337.e5, 2021. doi: 10.1016/j.cels.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The β γ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325: 321–326, 1987. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 85.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein β γ subunits. Nature 370: 143–146, 1994. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 86.Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein β γ-subunits activate the muscarinic-gated atrial potassium channel. Nature 368: 255–257, 1994. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 87.Krapivinsky G, Kennedy ME, Nemec J, Medina I, Krapivinsky L, Clapham DE. Gβ binding to GIRK4 subunit is critical for G protein-gated K+ channel activation. J Biol Chem 273: 16946–16952, 1998. doi: 10.1074/jbc.273.27.16946. [DOI] [PubMed] [Google Scholar]
- 88.Nemec J, Wickman K, Clapham DE. Gβγ binding increases the open time of IKACh: kinetic evidence for multiple Gβγ binding sites. Biophys J 76: 246–252, 1999. doi: 10.1016/S0006-3495(99)77193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clancy SM, Fowler CE, Finley M, Suen KF, Arrabit C, Berton F, Kosaza T, Casey PJ, Slesinger PA. Pertussis-toxin-sensitive Gα subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol Cell Neurosci 28: 375–389, 2005. doi: 10.1016/j.mcn.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 90.Ivanina T, Varon D, Peleg S, Rishal I, Porozov Y, Dessauer CW, Keren-Raifman T, Dascal N. Gαi1 and Gαi3 differentially interact with, and regulate, the G protein-activated K+ channel. J Biol Chem 279: 17260–17268, 2004. doi: 10.1074/jbc.M313425200. [DOI] [PubMed] [Google Scholar]
- 91.Rusinova R, Mirshahi T, Logothetis DE. Specificity of Gβγ signaling to Kir3 channels depends on the helical domain of pertussis toxin-sensitive Gα subunits. J Biol Chem 282: 34019–34030, 2007. doi: 10.1074/jbc.M704928200. [DOI] [PubMed] [Google Scholar]
- 92.Rubinstein M, Peleg S, Berlin S, Brass D, Keren-Raifman T, Dessauer CW, Ivanina T, Dascal N. Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GαiGDP and Gβγ. J Physiol 587: 3473–3491, 2009. doi: 10.1113/jphysiol.2009.173229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berlin S, Keren-Raifman T, Castel R, Rubinstein M, Dessauer CW, Ivanina T, Dascal N. G α (i) and G βγ jointly regulate the conformations of a G βγ effector, the neuronal G protein-activated K+ channel (GIRK). J Biol Chem 285: 6179–6185, 2010. doi: 10.1074/jbc.M109.085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kano H, Toyama Y, Imai S, Iwahashi Y, Mase Y, Yokogawa M, Osawa M, Shimada I. Structural mechanism underlying G protein family-specific regulation of G protein-gated inwardly rectifying potassium channel. Nat Commun 10: 2008, 2019. doi: 10.1038/s41467-019-10038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Q, Dickson A, Doupnik CA. Gβγ-activated inwardly rectifying K(+) (GIRK) channel activation kinetics via Gαi and Gαo-coupled receptors are determined by Gα-specific interdomain interactions that affect GDP release rates. J Biol Chem 279: 29787–29796, 2004. doi: 10.1074/jbc.M403359200. [DOI] [PubMed] [Google Scholar]
- 96.Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. G(α)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron 33: 87–99, 2002. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 97.Rubinstein M, Peleg S, Berlin S, Brass D, Dascal N. Gαi3 primes the G protein-activated K+ channels for activation by coexpressed Gβγ in intact Xenopus oocytes. J Physiol 581: 17–32, 2007. doi: 10.1113/jphysiol.2006.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mase Y, Yokogawa M, Osawa M, Shimada I. Structural basis for modulation of gating property of G protein-gated inwardly rectifying potassium ion channel (GIRK) by i/o-family G protein α subunit (Gαi/o). J Biol Chem 287: 19537–19549, 2012. doi: 10.1074/jbc.M112.353888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greif GJ, Sodickson DL, Bean BP, Neer EJ, Mende U. Altered regulation of potassium and calcium channels by GABA(B) and adenosine receptors in hippocampal neurons from mice lacking Gα (o). J Neurophysiol 83: 1010–1018, 2000. doi: 10.1152/jn.2000.83.2.1010. [DOI] [PubMed] [Google Scholar]
- 100.Schwindinger WF, Mirshahi UL, Baylor KA, Sheridan KM, Stauffer AM, Usefof S, Stecker MM, Mirshahi T, Robishaw JD. Synergistic roles for G-protein γ3 and γ7 subtypes in seizure susceptibility as revealed in double knock-out mice. J Biol Chem 287: 7121–7133, 2012. doi: 10.1074/jbc.M111.308395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK, Robishaw JD. Mice with deficiency of G protein γ3 are lean and have seizures. Mol Cell Biol 24: 7758–7768, 2004. doi: 10.1128/MCB.24.17.7758-7768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y, Zhang Y, Kong S, Zang K, Jiang S, Wan L, Chen L, Wang G, Jiang M, Wang X, Hu J, Wang Y. GIRK1-mediated inwardly rectifying potassium current suppresses the epileptiform burst activities and the potential antiepileptic effect of ML297. Biomed Pharmacother 101: 362–370, 2018. doi: 10.1016/j.biopha.2018.02.114. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 25: 3787–3792, 2005. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim CS, Johnston D. A1 adenosine receptor-mediated GIRK channels contributes to the resting conductance of CA1 neurons in the dorsal hippocampus. J Neurophysiol 113: 2511–2523. 2015. doi: 10.1152/jn.00951.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rishal I, Porozov Y, Yakubovich D, Varon D, Dascal N. Gβγ-dependent and Gβγ-independent basal activity of G protein-activated K+ channels. J Biol Chem 280: 16685–16694, 2005. doi: 10.1074/jbc.M412196200. [DOI] [PubMed] [Google Scholar]
- 106.Kahanovitch U, Tsemakhovich V, Berlin S, Rubinstein M, Styr B, Castel R, Peleg S, Tabak G, Dessauer CW, Ivanina T, Dascal N. Recruitment of Gβγ controls the basal activity of G-protein coupled inwardly rectifying potassium (GIRK) channels: crucial role of distal C terminus of GIRK1. J Physiol 592: 5373–5390, 2014. doi: 10.1113/jphysiol.2014.283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391: 803–806, 1998. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 108.Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA 95: 1307–1312, 1998. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niu Y, Tao X, Touhara KK, MacKinnon R. Cryo-EM analysis of PIP2 regulation in mammalian GIRK channels. eLife 9: e60552, 2020. doi: 10.7554/eLife.60552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lacin E, Aryal P, Glaaser IW, Bodhinathan K, Tsai E, Marsh N, Tucker SJ, Sansom MSP, Slesinger PA. Dynamic role of the tether helix in PIP2-dependent gating of a G protein-gated potassium channel. J Gen Physiol 149: 799–811, 2017. doi: 10.1085/jgp.201711801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li D, Jin T, Gazgalis D, Cui M, Logothetis DE. On the mechanism of GIRK2 channel gating by phosphatidylinositol bisphosphate, sodium, and the Gβγ dimer. J Biol Chem 294: 18934–18948, 2019. doi: 10.1074/jbc.RA119.010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho H, Youm JB, Ryu SY, Earm YE, Ho WK. Inhibition of acetylcholine-activated K(+) currents by U73122 is mediated by the inhibition of PIP(2)-channel interaction. Br J Pharmacol 134: 1066–1072, 2001. doi: 10.1038/sj.bjp.0704347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cho H, Lee D, Lee SH, Ho WK. Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc Natl Acad Sci USA 102: 4643–4648, 2005. doi: 10.1073/pnas.0408844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sohn JW, Lee D, Cho H, Lim W, Shin HS, Lee SH, Ho WK. Receptor-specific inhibition of GABAB-activated K+ currents by muscarinic and metabotropic glutamate receptors in immature rat hippocampus. J Physiol 580: 411–422, 2007. doi: 10.1113/jphysiol.2006.125914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sohn JW, Lim A, Lee SH, Ho WK. Decrease in PIP(2) channel interactions is the final common mechanism involved in PKC- and arachidonic acid-mediated inhibitions of GABA(B)-activated K+ current. J Physiol 582: 1037–1046, 2007. doi: 10.1113/jphysiol.2007.137265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kramer PF, Williams JT. Calcium release from stores inhibits GIRK. Cell Rep 17: 3246–3255, 2016. doi: 10.1016/j.celrep.2016.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mullner C, Vorobiov D, Bera AK, Uezono Y, Yakubovich D, Frohnwieser-Steinecker B, Dascal N, Schreibmayer W. Heterologous facilitation of G protein-activated K(+) channels by β-adrenergic stimulation via cAMP-dependent protein kinase. J Gen Physiol 115: 547–558, 2000. doi: 10.1085/jgp.115.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rogalski SL, Appleyard SM, Pattillo A, Terman GW, Chavkin C. TrkB activation by brain-derived neurotrophic factor inhibits the G protein-gated inward rectifier Kir3 by tyrosine phosphorylation of the channel. J Biol Chem 275: 25082–25088, 2000. doi: 10.1074/jbc.M000183200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Medina I, Krapivinsky G, Arnold S, Kovoor P, Krapivinsky L, Clapham DE. A switch mechanism for G β γ activation of I(KACh). J Biol Chem 275: 29709–29716, 2000. doi: 10.1074/jbc.M004989200. [DOI] [PubMed] [Google Scholar]
- 120.Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H, Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K(+) channels by protein kinase C. Proc Natl Acad Sci USA 101: 1087–1092, 2004. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA 106: 629–634, 2009. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mullner C, Steinecker B, Gorischek A, Schreibmayer W. Identification of the structural determinant responsible for the phosphorylation of G-protein activated potassium channel 1 by cAMP-dependent protein kinase. FEBS J 276: 6218–6226, 2009. doi: 10.1111/j.1742-4658.2009.07325.x. [DOI] [PubMed] [Google Scholar]
- 123.Adney SK, Ha J, Meng XY, Kawano T, Logothetis DE. A critical gating switch at a modulatory site in neuronal Kir3 channels. J Neurosci 35: 14397–14405, 2015. doi: 10.1523/jneurosci.1415-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niemeyer A, Rinne A, Kienitz MC. Receptor-specific regulation of atrial GIRK channel activity by different Ca(2+)-dependent PKC isoforms. Cell Signal 64: 109418, 2019. doi: 10.1016/j.cellsig.2019.109418. [DOI] [PubMed] [Google Scholar]
- 125.Chung HJ, Ge WP, Qian X, Wiser O, Jan YN, Jan LY. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc Natl Acad Sci USA 106: 635–640, 2009. doi: 10.1073/pnas.0811685106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rusinova R, Shen YM, Dolios G, Padovan J, Yang H, Kirchberger M, Wang R, Logothetis DE. Mass spectrometric analysis reveals a functionally important PKA phosphorylation site in a Kir3 channel subunit. Pflugers Arch 458: 303–314, 2009. doi: 10.1007/s00424-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Treiber F, Rosker C, Keren-Raifman T, Steinecker B, Gorischek A, Dascal N, Schreibmayer W. Molecular basis of the facilitation of the heterooligomeric GIRK1/GIRK4 complex by cAMP dependent protein kinase. Biochim Biophys Acta 1828: 1214–1221, 2013. doi: 10.1016/j.bbamem.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mora SI, Escobar LI. Phosphorylation of a tyrosine at the N-terminus regulates the surface expression of GIRK5 homomultimers. FEBS Lett 579: 3019–3023, 2005. doi: 10.1016/j.febslet.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 129.Ippolito DL, Temkin PA, Rogalski SL, Chavkin C. N-terminal tyrosine residues within the potassium channel Kir3 modulate GTPase activity of Gαi. J Biol Chem 277: 32692–32696, 2002. doi: 10.1074/jbc.M204407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hill JJ, Peralta EG. Inhibition of a Gi-activated potassium channel (GIRK1/4) by the Gq-coupled m1 muscarinic acetylcholine receptor. J Biol Chem 276: 5505–5510, 2001. doi: 10.1074/jbc.M008213200. [DOI] [PubMed] [Google Scholar]
- 131.Brown SG, Thomas A, Dekker LV, Tinker A, Leaney JL. PKC-δ sensitizes Kir3.1/3.2 channels to changes in membrane phospholipid levels after M3 receptor activation in HEK-293 cells. Am J Physiol Cell Physiol 289: C543–C556, 2005. doi: 10.1152/ajpcell.00025.2005. [DOI] [PubMed] [Google Scholar]
- 132.Thomas AM, Brown SG, Leaney JL, Tinker A. Differential phosphoinositide binding to components of the G protein-gated K+ channel. J Membr Biol 211: 43–53, 2006. doi: 10.1007/s00232-006-0014-5. [DOI] [PubMed] [Google Scholar]
- 133.Mark MD, Ruppersberg JP, Herlitze S. Regulation of GIRK channel deactivation by Gα(q) and Gα(i/o) pathways. Neuropharmacology 39: 2360–2373, 2000. doi: 10.1016/s0028-3908(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 134.Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves G(α)q family subunits, phospholipase C, and a readily diffusible messenger. J Biol Chem 276: 16720–16730, 2001. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- 135.Keselman I, Fribourg M, Felsenfeld DP, Logothetis DE. Mechanism of PLC-mediated Kir3 current inhibition. Channels (Austin) 1: 113–123, 2007. doi: 10.4161/chan.4321. [DOI] [PubMed] [Google Scholar]
- 136.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein α subunits. Int J Biol Sci 1: 51–66, 2005. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol 17: 363–376, 2006. doi: 10.1016/j.semcdb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 138.Kimple AJ, Bosch DE, Giguere PM, Siderovski DP. Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev 63: 728–749, 2011. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys 54: 33–46, 2009. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hooks SB, Waldo GL, Corbitt J, Bodor ET, Krumins AM, Harden TK. RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J Biol Chem 278: 10087–10093, 2003. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
- 141.Masuho I, Xie K, Martemyanov KA. Macromolecular composition dictates receptor and G protein selectivity of regulator of G protein signaling (RGS) 7 and 9-2 protein complexes in living cells. J Biol Chem 288: 25129–25142, 2013. doi: 10.1074/jbc.M113.462283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheever ML, Snyder JT, Gershburg S, Siderovski DP, Harden TK, Sondek J. Crystal structure of the multifunctional Gβ5-RGS9 complex. Nat Struct Mol Biol 15: 155–162, 2008. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie K, Allen KL, Kourrich S, Colon-Saez J, Thomas MJ, Wickman K, Martemyanov KA. Gβ5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci 13: 661–663, 2010. doi: 10.1038/nn.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Posokhova E, Wydeven N, Allen KL, Wickman K, Martemyanov KA. RGS6/Gβ5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ Res 107: 1350–1354, 2010. [Erratum inCirc Res108: e3, 2011]. doi: 10.1161/CIRCRESAHA.110.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patil DN, Rangarajan ES, Novick SJ, Pascal BD, Kojetin DJ, Griffin PR, Izard T, Martemyanov KA. Structural organization of a major neuronal G protein regulator, the RGS7-Gβ5-R7BP complex. eLife 7: e42150, 2018. doi: 10.7554/eLife.42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang J, Huang J, Maity B, Gao Z, Lorca RA, Gudmundsson H, Li J, Stewart A, Swaminathan PD, Ibeawuchi SR, Shepherd A, Chen CK, Kutschke W, Mohler PJ, Mohapatra DP, Anderson ME, Fisher RA. RGS6, a modulator of parasympathetic activation in heart. Circ Res 107: 1345–1349, 2010. doi: 10.1161/CIRCRESAHA.110.224220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ostrovskaya OI, Orlandi C, Fajardo-Serrano A, Young SM Jr, Lujan R, Martemyanov KA. Inhibitory signaling to ion channels in hippocampal neurons is differentially regulated by alternative macromolecular complexes of RGS7. J Neurosci 38: 10002–10015, 2018. doi: 10.1523/JNEUROSCI.1378-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ostrovskaya O, Xie K, Masuho I, Fajardo-Serrano A, Lujan R, Wickman K, Martemyanov KA. RGS7/Gβ5/R7BP complex regulates synaptic plasticity and memory by modulating hippocampal GABABR-GIRK signaling. eLife 3: e02053, 2014. doi: 10.7554/eLife.02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, Gregg RG, Martemyanov KA. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol 197: 711–719, 2012. doi: 10.1083/jcb.201202123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orlandi C, Xie K, Masuho I, Fajardo-Serrano A, Lujan R, Martemyanov KA. Orphan receptor GPR158 is an allosteric modulator of rgs7 catalytic activity with an essential role in dictating its expression and localization in the brain. J Biol Chem 290: 13622–13639, 2015. doi: 10.1074/jbc.M115.645374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wydeven N, Posokhova E, Xia Z, Martemyanov KA, Wickman K. RGS6, but not RGS4, is the dominant regulator of G protein signaling (RGS) modulator of the parasympathetic regulation of mouse heart rate. J Biol Chem 289: 2440–2449, 2014. doi: 10.1074/jbc.M113.520742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maity B, Stewart A, Yang J, Loo L, Sheff D, Shepherd AJ, Mohapatra DP, Fisher RA. Regulator of G protein signaling 6 (RGS6) protein ensures coordination of motor movement by modulating GABAB receptor signaling. J Biol Chem 287: 4972–4981, 2012. doi: 10.1074/jbc.M111.297218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci 17: 8024–8037, 1997. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stewart A, Maity B, Wunsch AM, Meng F, Wu Q, Wemmie JA, Fisher RA. Regulator of G-protein signaling 6 (RGS6) promotes anxiety and depression by attenuating serotonin-mediated activation of the 5-HT(1A) receptor-adenylyl cyclase axis. FASEB J 28: 1735–1744, 2014. doi: 10.1096/fj.13-235648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Filippov AK, Fernandez-Fernandez JM, Marsh SJ, Simon J, Barnard EA, Brown DA. Activation and inhibition of neuronal G protein-gated inwardly rectifying K(+) channels by P2Y nucleotide receptors. Mol Pharmacol 66: 468–477, 2004. doi: 10.1124/mol.66.3. [DOI] [PubMed] [Google Scholar]
- 156.Lan KL, Zhong H, Nanamori M, Neubig RR. Rapid kinetics of regulator of G-protein signaling (RGS)-mediated Gαi and Gαo deactivation. Gα specificity of RGS4 AND RGS7. J Biol Chem 275: 33497–33503, 2000. doi: 10.1074/jbc.M005785200. [DOI] [PubMed] [Google Scholar]
- 157.Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABA(B) receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J Physiol 580: 51–65, 2007. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim G, Jung S, Son H, Kim S, Choi J, Lee DH, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. The GABAB receptor associates with regulators of G-protein signaling 4 protein in the mouse prefrontal cortex and hypothalamus. BMB Rep 47: 324–329, 2014. doi: 10.5483/bmbrep.2014.47.6.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA 94: 10461–10466, 1997. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ulens C, Daenens P, Tytgat J. Changes in GIRK1/GIRK2 deactivation kinetics and basal activity in the presence and absence of RGS4. Life Sci 67: 2305–2317, 2000. doi: 10.1016/s0024-3205(00)00820-1. [DOI] [PubMed] [Google Scholar]
- 161.Zhang Q, Pacheco MA, Doupnik CA. Gating properties of GIRK channels activated by Gα(o)- and Gα(i)-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation. J Physiol 545: 355–373, 2002. doi: 10.1113/jphysiol.2002.032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jaen C, Doupnik CA. Neuronal Kir3.1/Kir3.2a channels coupled to serotonin 1A and muscarinic m2 receptors are differentially modulated by the “short” RGS3 isoform. Neuropharmacology 49: 465–476, 2005. doi: 10.1016/j.neuropharm.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 163.Jaen C, Doupnik CA. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J Biol Chem 281: 34549–34560, 2006. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- 164.Sui JL, Chan KW, Logothetis DE. Na+ activation of the muscarinic K+ channel by a G-protein-independent mechanism. J Gen Physiol 108: 381–391, 1996. doi: 10.1085/jgp.108.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosenhouse-Dantsker A. Cholesterol-binding sites in GIRK channels: the devil is in the details. Lipid Insights 11: 1178635317754071, 2018. doi: 10.1177/1178635317754071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Delling M, Wischmeyer E, Dityatev A, Sytnyk V, Veh RW, Karschin A, Schachner M. The neural cell adhesion molecule regulates cell-surface delivery of G-protein-activated inwardly rectifying potassium channels via lipid rafts. J Neurosci 22: 7154–7164, 2002. doi: 10.1523/JNEUROSCI.22-16-07154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bukiya AN, Osborn CV, Kuntamallappanavar G, Toth PT, Baki L, Kowalsky G, Oh MJ, Dopico AM, Levitan I, Rosenhouse-Dantsker A. Cholesterol increases the open probability of cardiac K+ currents. Biochim Biophys Acta 1848: 2406–2413, 2015. doi: 10.1016/j.bbamem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 168.Bukiya AN, Durdagi S, Noskov S, Rosenhouse-Dantsker A. Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus. J Biol Chem 292: 6135–6147, 2017. doi: 10.1074/jbc.M116.753350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Glaaser IW, Slesinger PA. Dual activation of neuronal G protein-gated inwardly rectifying potassium (GIRK) channels by cholesterol and alcohol. Sci Rep 7: 4592, 2017. doi: 10.1038/s41598-017-04681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rosenhouse-Dantsker A. Cholesterol binding sites in inwardly rectifying potassium channels. Adv Exp Med Biol 1135: 119–138, 2019. doi: 10.1007/978-3-030-14265-0_7. [DOI] [PubMed] [Google Scholar]
- 171.Bukiya AN, Blank PS, Rosenhouse-Dantsker A. Cholesterol intake and statin use regulate neuronal G protein-gated inwardly rectifying potassium channels. J Lipid Res 60: 19–29, 2019. doi: 10.1194/jlr.M081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mathiharan YK, Glaaser IW, Zhao Y, Robertson MJ, Skiniotis G, Slesinger PA. Structural insights into GIRK2 channel modulation by cholesterol and PIP2. Cell Rep 36: 109619, 2021. doi: 10.1016/j.celrep.2021.109619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Doupnik CA. GPCR-Kir channel signaling complexes: defining rules of engagement. J Recept Signal Transduct Res 28: 83–91, 2008. doi: 10.1080/10799890801941970. [DOI] [PubMed] [Google Scholar]
- 174.Nagi K, Pineyro G. Kir3 channel signaling complexes: focus on opioid receptor signaling. Front Cell Neurosci 8: 186, 2014. doi: 10.3389/fncel.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.David M, Richer M, Mamarbachi AM, Villeneuve LR, Dupre DJ, Hebert TE. Interactions between GABA-B1 receptors and Kir 3 inwardly rectifying potassium channels. Cell Signal 18: 2172–2181, 2006. doi: 10.1016/j.cellsig.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 176.Ciruela F, Fernandez-Duenas V, Sahlholm K, Fernandez-Alacid L, Nicolau JC, Watanabe M, Lujan R. Evidence for oligomerization between GABAB receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur J Neurosci 32: 1265–1277, 2010. doi: 10.1111/j.1460-9568.2010.07356.x. [DOI] [PubMed] [Google Scholar]
- 177.Fajardo-Serrano A, Wydeven N, Young D, Watanabe M, Shigemoto R, Martemyanov KA, Wickman K, Lujan R. Association of Rgs7/Gβ5 complexes with girk channels and GABAB receptors in hippocampal CA1 pyramidal neurons. Hippocampus 23: 1231–1245, 2013. doi: 10.1002/hipo.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fritzius T, Bettler B. The organizing principle of GABAB receptor complexes: Physiological and pharmacological implications. Basic Clin Pharmacol Toxicol 126, Suppl6: 25–34, 2019. doi: 10.1111/bcpt.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci 119: 2807–2818, 2006. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 180.Laviv T, Vertkin I, Berdichevsky Y, Fogel H, Riven I, Bettler B, Slesinger PA, Slutsky I. Compartmentalization of the GABAB receptor signaling complex is required for presynaptic inhibition at hippocampal synapses. J Neurosci 31: 12523–12532, 2011. doi: 10.1523/JNEUROSCI.1527-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schwenk J, Perez-Garci E, Schneider A, Kollewe A, Gauthier-Kemper A, Fritzius T, Raveh A, Dinamarca MC, Hanuschkin A, Bildl W, Klingauf J, Gassmann M, Schulte U, Bettler B, Fakler B. Modular composition and dynamics of native GABAB receptors identified by high-resolution proteomics. Nat Neurosci 19: 233–242, 2016. doi: 10.1038/nn.419. [DOI] [PubMed] [Google Scholar]
- 182.Zheng S, Abreu N, Levitz J, Kruse AC. Structural basis for KCTD-mediated rapid desensitization of GABAB signalling. Nature 567: 127–131, 2019. doi: 10.1038/s41586-019-0990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fritzius T, Turecek R, Seddik R, Kobayashi H, Tiao J, Rem PD, Metz M, Kralikova M, Bouvier M, Gassmann M, Bettler B. KCTD hetero-oligomers confer unique kinetic properties on hippocampal GABAB receptor-induced K+ currents. J Neurosci 37: 1162–1175, 2017. doi: 10.1523/JNEUROSCI.2181-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kahanovitch U, Berlin S, Dascal N. Collision coupling in the GABAB receptor-G protein-GIRK signaling cascade. FEBS Lett 591: 2816–2825, 2017. doi: 10.1002/1873-3468.12756. [DOI] [PubMed] [Google Scholar]
- 185.Berlin S, Artzy E, Handklo-Jamal R, Kahanovitch U, Parnas H, Dascal N, Yakubovich D. A collision coupling model governs the activation of neuronal GIRK1/2 channels by muscarinic-2 receptors. Front Pharmacol 11: 1216, 2020. doi: 10.3389/fphar.2020.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang CS, Shi SH, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell 123: 105–118, 2005. doi: 10.1016/j.cell.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 187.Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Lujan R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci 31: 12251–12257, 2011. doi: 10.1523/JNEUROSCI.0494-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martinez-Hernandez J, Watanabe M, Moss SJ, Lujan R, Luscher C, Slesinger PA. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron 73: 978–989, 2012. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rose TR, Marron Fernandez de Velasco E, Vo BN, Tipps ME, Wickman K. Impact of acute and persistent excitation of prelimbic pyramidal neurons on motor activity and trace fear learning. J Neurosci 41: 960–971, 2021. doi: 10.1523/JNEUROSCI.2606-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sharpe AL, Varela E, Bettinger L, Beckstead MJ. Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol 18: pyu073–pyu073, 2015. doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nassirpour R, Bahima L, Lalive AL, Luscher C, Lujan R, Slesinger PA. Morphine- and CaMKII-dependent enhancement of GIRK channel signaling in hippocampal neurons. J Neurosci 30: 13419–13430, 2010. doi: 10.1523/JNEUROSCI.2966-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Anderson EM, Engelhardt A, Demis S, Porath E, Hearing MC. Remifentanil self-administration in mice promotes sex-specific prefrontal cortex dysfunction underlying deficits in cognitive flexibility. Neuropsychopharmacology 46: 1734–1745, 2021. doi: 10.1038/s41386-021-01028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology 36: 993–1002, 2011. doi: 10.1038/npp.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Ethanol dependence abolishes monoamine and GIRK (Kir3) channel inhibition of orbitofrontal cortex excitability. Neuropsychopharmacology 42: 1800–1812, 2017. doi: 10.1038/npp.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lemos JC, Roth CA, Messinger DI, Gill HK, Phillips PE, Chavkin C. Repeated stress dysregulates κ-opioid receptor signaling in the dorsal raphe through a p38α MAPK-dependent mechanism. J Neurosci 32: 12325–12336, 2012. doi: 10.1523/JNEUROSCI.2053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D, Mameli M. Rescue of GABA and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med 22: 254–261, 2016. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 197.Nava-Mesa MO, Jiménez-Díaz L, Yajeya J, Navarro-Lopez JD. Amyloid-β induces synaptic dysfunction through G protein-gated inwardly rectifying potassium channels in the fimbria-CA3 hippocampal synapse. Front Cell Neurosci 7: 117, 2013. doi: 10.3389/fncel.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mayordomo-Cava J, Yajeya J, Navarro-Lopez JD, Jimenez-Diaz L. Amyloid-β (25-35) modulates the expression of GirK and KCNQ channel genes in the hippocampus. PLoS One 10: e0134385, 2015. doi: 10.1371/journal.pone.0134385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.May LM, Anggono V, Gooch HM, Jang SE, Matusica D, Kerbler GM, Meunier FA, Sah P, Coulson EJ. G-protein-coupled inwardly rectifying potassium (GIRK) channel activation by the p75 neurotrophin receptor is required for amyloid β toxicity. Front Neurosci 11: 455, 2017. doi: 10.3389/fnins.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rinker JA, Fulmer DB, Trantham-Davidson H, Smith ML, Williams RW, Lopez MF, Randall PK, Chandler LJ, Miles MF, Becker HC, Mulholland PJ. Differential potassium channel gene regulation in BXD mice reveals novel targets for pharmacogenetic therapies to reduce heavy alcohol drinking. Alcohol 58: 33–45, 2017. doi: 10.1016/j.alcohol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Anderson EM, Loke S, Wrucke B, Engelhardt A, Demis S, O'Reilly K, Hess E, Wickman K, Hearing MC. Suppression of pyramidal neuron G protein-gated inwardly rectifying K+ channel signaling impairs prelimbic cortical function and underlies stress-induced deficits in cognitive flexibility in male, but not female, mice . Neuropsychopharmacology 46: 2158–2169, 2021. doi: 10.1038/s41386-021-01063-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sanchez-Rodriguez I, Djebari S, Temprano-Carazo S, Vega-Avelaira D, Jimenez-Herrera R, Iborra-Lazaro G, Yajeya J, Jimenez-Diaz L, Navarro-Lopez JD. Hippocampal long-term synaptic depression and memory deficits induced in early amyloidopathy are prevented by enhancing G-protein-gated inwardly rectifying potassium channel activity. J Neurochem 153: 362–376, 2020. doi: 10.1111/jnc.14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Terunuma M, Revilla-Sanchez R, Quadros IM, Deng Q, Deeb TZ, Lumb M, Sicinski P, Haydon PG, Pangalos MN, Moss SJ. Postsynaptic GABAB receptor activity regulates excitatory neuronal architecture and spatial memory. J Neurosci 34: 804–816, 2014. doi: 10.1523/JNEUROSCI.3320-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Terunuma M, Pangalos MN, Moss SJ. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv Pharmacol 58: 113–122, 2010. doi: 10.1016/S1054-3589(10)58005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mucic G, Sase S, Stork O, Lubec G, Li L. Networks of protein kinases and phosphatases in the individual phases of contextual fear conditioning in the C57BL/6J mouse. Behav Brain Res 280: 45–50, 2015. doi: 10.1016/j.bbr.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 206.Li X, Terunuma M, Deeb TG, Wiseman S, Pangalos MN, Nairn AC, Moss SJ, Slesinger PA. Direct interaction of PP2A phosphatase with GABAB receptors alters functional signaling. J Neurosci 40: 2808–2816, 2020. doi: 10.1523/JNEUROSCI.2654-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ippolito DL, Xu M, Bruchas MR, Wickman K, Chavkin C. Tyrosine phosphorylation of K(ir)3.1 in spinal cord is induced by acute inflammation, chronic neuropathic pain, and behavioral stress. J Biol Chem 280: 41683–41693, 2005. doi: 10.1074/jbc.M507069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Clayton CC, Xu M, Chavkin C. Tyrosine phosphorylation of Kir3 following κ-opioid receptor activation of p38 MAPK causes heterologous desensitization. J Biol Chem 284: 31872–31881, 2009. doi: 10.1074/jbc.M109.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, Gilman AG, Worley PF. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J Neurosci 18: 7178–7188, 1998. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McCall NM, Kotecki L, Dominguez-Lopez S, Marron Fernandez de Velasco E, Carlblom N, Sharpe AL, Beckstead MJ, Wickman K. Selective ablation of GIRK channels in dopamine neurons alters behavioral effects of cocaine in mice. Neuropsychopharmacology 42: 707–715, 2017. doi: 10.1038/npp.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR 3rd, Slesinger PA. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci 10: 1249–1259, 2007. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- 212.Kajii Y, Muraoka S, Hiraoka S, Fujiyama K, Umino A, Nishikawa T. A developmentally regulated and psychostimulant-inducible novel rat gene mrt1 encoding PDZ-PX proteins isolated in the neocortex. Mol Psychiatry 8: 434–444, 2003. doi: 10.1038/sj.mp.4001258. [DOI] [PubMed] [Google Scholar]
- 213.Nassirpour R, Slesinger PA. Subunit-specific regulation of Kir3 channels by sorting nexin 27. Channels (Austin) 1: 331–333, 2007. doi: 10.4161/chan.5191. [DOI] [PubMed] [Google Scholar]
- 214.Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S, White R, Featherstone RE, Ray R, Ortinski PI, Banerjee A, Gandal MJ, Lin R, Alexandrescu A, Liang Y, Gur RE, Borgmann-Winter KE, Carlson GC, Hahn CG, Siegel SJ. Pyramidal cell selective ablation of N-methyl-d-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiatry 77: 556–568, 2015. doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Laffray S, Bouali-Benazzouz R, Papon MA, Favereaux A, Jiang Y, Holm T, Spriet C, Desbarats P, Fossat P, Le Feuvre Y, Decossas M, Heliot L, Langel U, Nagy F, Landry M. Impairment of GABAB receptor dimer by endogenous 14-3-3zeta in chronic pain conditions. EMBO J 31: 3239–3251, 2012. doi: 10.1038/emboj.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Workman ER, Haddick PC, Bush K, Dilly GA, Niere F, Zemelman BV, Raab-Graham KF. Rapid antidepressants stimulate the decoupling of GABAB receptors from GIRK/Kir3 channels through increased protein stability of 14-3-3η. Mol Psychiatry 20: 298–310, 2015. doi: 10.1038/mp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Walsh KB. Screening technologies for inward rectifier potassium channels: discovery of new blockers and activators. SLAS Discov 25: 420–433, 2020. doi: 10.1177/2472555220905558. [DOI] [PubMed] [Google Scholar]
- 218.Zhao Y, Gameiro-Ros I, Glaaser IW, Slesinger PA. Advances in targeting GIRK channels in disease. Trends Pharmacol Sci 42: 203–215, 2021. doi: 10.1016/j.tips.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weaver CD, Denton JS. Next-generation inward rectifier potassium channel modulators: discovery and molecular pharmacology. Am J Physiol Cell Physiol 320: C1125–C1140, 2021. doi: 10.1152/ajpcell.00548.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, Sliwoski G, Morrison RD, Denton J, Niswender CM, Daniels JS, Sulikowski GA, Xie XS, Lindsley CW, Weaver CD. ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci 4: 1278–1286, 2013. doi: 10.1021/cn400062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wydeven N, Marron Fernandez de Velasco E, Du Y, Benneyworth MA, Hearing MC, Fischer RA, Thomas MJ, Weaver CD, Wickman K. Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc Natl Acad Sci USA 111: 10755–10760, 2014. doi: 10.1073/pnas.1405190111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vo BN, Abney KK, Anderson A, Marron Fernandez de Velasco E, Benneyworth MA, Daniels JS, Morrison RD, Hopkins CR, Weaver CD, Wickman K. VU0810464, a non-urea G protein-gated inwardly rectifying K(+) (K(ir) 3/GIRK) channel activator, exhibits enhanced selectivity for neuronal K(ir) 3 channels and reduces stress-induced hyperthermia in mice. Br J Pharmacol 176: 2238–2249, 2019. doi: 10.1111/bph.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vo BN, Marron Fernandez de Velasco E, Rose TR, Oberle H, Luo H, Hopkins CR, Wickman K. Bidirectional influence of limbic GIRK channel activation on innate avoidance behavior. J Neurosci 24: 5809–5821, 2021. doi: 10.1523/JNEUROSCI.2787-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sanchez-Rodriguez I, Gruart A, Delgado-Garcia JM, Jimenez-Diaz L, Navarro-Lopez JD. Role of GirK channels in long-term potentiation of synaptic inhibition in an in vivo mouse model of early amyloid-β pathology. Int J Mol Sci 20: 1168, 2019. doi: 10.3390/ijms20051168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sanchez-Rodriguez I, Temprano-Carazo S, Najera A, Djebari S, Yajeya J, Gruart A, Delgado-Garcia JM, Jimenez-Diaz L, Navarro-Lopez JD. Activation of G-protein-gated inwardly rectifying potassium (Kir3/GirK) channels rescues hippocampal functions in a mouse model of early amyloid-β pathology. Sci Rep 7: 14658, 2017. [Erratum inSci Rep10: 1722, 2020]. doi: 10.1038/s41598-017-15306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kimura M, Shiokawa H, Karashima Y, Sumie M, Hoka S, Yamaura K. Antinociceptive effect of selective G protein-gated inwardly rectifying K+ channel agonist ML297 in the rat spinal cord. PLoS One 15: e0239094, 2020. doi: 10.1371/journal.pone.0239094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zou B, Cao WS, Guan Z, Xiao K, Pascual C, Xie J, Zhang J, Xie J, Kayser F, Lindsley CW, Weaver CD, Fang J, Xie XS. Direct activation of G-protein-gated inward rectifying K+ channels promotes nonrapid eye movement sleep. Sleep 42: zsy244, 2019. doi: 10.1093/sleep/zsy244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wieting JM, Vadukoot AK, Sharma S, Abney KK, Bridges TM, Daniels JS, Morrison RD, Wickman K, Weaver CD, Hopkins CR. Discovery and characterization of 1H-pyrazol-5-yl-2-phenylacetamides as novel, non-urea-containing GIRK1/2 potassium channel activators. ACS Chem Neurosci 8: 1873–1879, 2017. doi: 10.1021/acschemneuro.7b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Abney KK, Bubser M, Du Y, Kozek KA, Bridges TM, Linsdley CW, Daniels JS, Morrison RD, Wickman K, Hopkins CR, Jones CK, Weaver CD. Analgesic effects of the GIRK activator, VU0466551, alone and in combination with morphine in acute and persistent pain models. ACS Chem Neurosci 10: 1294–1299, 2019. [Erratum inACS Chem Neurosci10: 2621, 2019]. doi: 10.1021/acschemneuro.8b00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Xu Y, Cantwell L, Molosh AI, Plant LD, Gazgalis D, Fitz SD, Dustrude ET, Yang Y, Kawano T, Garai S, Noujaim SF, Shekhar A, Logothetis DE, Thakur GA. The small molecule GAT1508 activates brain-specific GIRK1/2 channel heteromers and facilitates conditioned fear extinction in rodents. J Biol Chem 295: 3614–3634, 2020. doi: 10.1074/jbc.RA119.011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhao Y, Ung PM-U, Zahoránszky-Kőhalmi G, Zakharov AV, Martinez NJ, Simeonov A, Glaaser IW, Rai G, Schlessinger A, Marugan JJ, Slesinger PA. Identification of a G-protein-independent activator of GIRK channels. Cell Rep 31: 107770, 2020. doi: 10.1016/j.celrep.2020.107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Weigl LG, Schreibmayer W. G protein-gated inwardly rectifying potassium channels are targets for volatile anesthetics. Mol Pharmacol 60: 282–289, 2001. doi: 10.1124/mol.60.2.282. [DOI] [PubMed] [Google Scholar]
- 233.Yamakura T, Lewohl JM, Harris RA. Differential effects of general anesthetics on G protein-coupled inwardly rectifying and other potassium channels. Anesthesiology 95: 144–153, 2001. doi: 10.1097/00000542-200107000-00025. [DOI] [PubMed] [Google Scholar]
- 234.Choi S, Jung SY, Ko YS, Koh SR, Rhim H, Nah SY. Functional expression of a novel ginsenoside Rf binding protein from rat brain mRNA in Xenopus laevis oocytes. Mol Pharmacol 61: 928–935, 2002. doi: 10.1124/mol.61.4.928. [DOI] [PubMed] [Google Scholar]
- 235.Radad K, Moldzio R, Rausch WD. Ginsenosides and their CNS targets. CNS Neurosci Ther 17: 761–768, 2011. doi: 10.1111/j.1755-5949.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Loscalzo LM, Yow TT, Wasowski C, Chebib M, Marder M. Hesperidin induces antinociceptive effect in mice and its aglycone, hesperetin, binds to μ-opioid receptor and inhibits GIRK1/2 currents. Pharmacol Biochem Behav 99: 333–341, 2011. doi: 10.1016/j.pbb.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 237.Vasas A, Forgo P, Orvos P, Talosi L, Csorba A, Pinke G, Hohmann J. Myrsinane, premyrsinane, and cyclomyrsinane diterpenes from Euphorbia falcata as potassium ion channel inhibitors with selective G protein-activated inwardly rectifying ion channel (GIRK) blocking effects. J Nat Prod 79: 1990–2004, 2016. doi: 10.1021/acs.jnatprod.6b00260. [DOI] [PubMed] [Google Scholar]
- 238.Kusz N, Orvos P, Bereczki L, Fertey P, Bombicz P, Csorba A, Talosi L, Jakab G, Hohmann J, Redei D. Diterpenoids from Euphorbia dulcis with potassium ion channel inhibitory activity with selective G protein-activated inwardly rectifying ion channel (GIRK) blocking effect. J Nat Prod 81: 2483–2492, 2018. doi: 10.1021/acs.jnatprod.8b00500. [DOI] [PubMed] [Google Scholar]
- 239.Jin W, Lu Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 38: 14286–14293, 1999. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- 240.Jin W, Klem AM, Lewis JH, Lu Z. Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry 38: 14294–14301, 1999. doi: 10.1021/bi991206j. [DOI] [PubMed] [Google Scholar]
- 241.Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 37: 13291–13299, 1998. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- 242.Kanjhan R, Coulson EJ, Adams DJ, Bellingham MC. Tertiapin-Q blocks recombinant and native large conductance K+ channels in a use-dependent manner. J Pharmacol Exp Ther 314: 1353–1361, 2005. doi: 10.1124/jpet.105.085928. [DOI] [PubMed] [Google Scholar]
- 243.Hill E, Hickman C, Diez R, Wall M. Role of A1 receptor-activated GIRK channels in the suppression of hippocampal seizure activity. Neuropharmacology 164: 107904, 2020. doi: 10.1016/j.neuropharm.2019.107904. [DOI] [PubMed] [Google Scholar]
- 244.Djebari S, Iborra-Lazaro G, Temprano-Carazo S, Sanchez-Rodriguez I, Nava-Mesa MO, Munera A, Gruart A, Delgado-Garcia JM, Jimenez-Diaz L, Navarro-Lopez JD. G-protein-gated inwardly rectifying potassium (Kir3/GIRK) channels govern synaptic plasticity that supports hippocampal-dependent cognitive functions in male mice. J Neurosci 41: 7086–7102, 2021. doi: 10.1523/JNEUROSCI.2849-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kobayashi T, Ikeda K, Kumanishi T. Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K(+) (GIRK) channels expressed in Xenopus oocytes. Br J Pharmacol 129: 1716–1722, 2000. doi: 10.1038/sj.bjp.0703224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br J Pharmacol 138: 1119–1128, 2003. doi: 10.1038/sj.bjp.0705172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by different classes of antidepressants. PLoS One 6: e28208, 2011. doi: 10.1371/journal.pone.0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Judge BS, Rentmeester LL. Antidepressant overdose-induced seizures. Psychiatr Clin North Am 36: 245–260, 2013. doi: 10.1016/j.psc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 249.Kawaura K, Honda S, Soeda F, Shirasaki T, Takahama K. [Novel antidepressant-like action of drugs possessing GIRK channel blocking action in rats]. Yakugaku Zasshi 130: 699–705, 2010. doi: 10.1248/yakushi.130.699. [DOI] [PubMed] [Google Scholar]
- 250.Hamasaki R, Shirasaki T, Soeda F, Takahama K. Tipepidine activates VTA dopamine neuron via inhibiting dopamine D2 receptor-mediated inward rectifying K+ current. Neuroscience 252: 24–34, 2013. doi: 10.1016/j.neuroscience.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 251.Sasaki T, Hashimoto K, Tachibana M, Kurata T, Okawada K, Ishikawa M, Kimura H, Komatsu H, Ishikawa M, Hasegawa T, Shiina A, Hashimoto T, Kanahara N, Shiraishi T, Iyo M. Tipepidine in children with attention deficit/hyperactivity disorder: a 4-week, open-label, preliminary study. Neuropsychiatr Dis Treat 10: 147–151, 2014. doi: 10.2147/NDT.S58480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yamada K, Iwayama Y, Hattori E, Iwamoto K, Toyota T, Ohnishi T, Ohba H, Maekawa M, Kato T, Yoshikawa T. Genome-wide association study of schizophrenia in Japanese population. PLoS One 6: e20468, 2011. doi: 10.1371/journal.pone.0020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yamada K, Iwayama Y, Toyota T, Ohnishi T, Ohba H, Maekawa M, Yoshikawa T. Association study of the KCNJ3 gene as a susceptibility candidate for schizophrenia in the Chinese population. Hum Genet 131: 443–451, 2012. doi: 10.1007/s00439-011-1089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chioza B, Osei-Lah A, Wilkie H, Nashef L, McCormick D, Asherson P, Makoff AJ. Suggestive evidence for association of two potassium channel genes with different idiopathic generalised epilepsy syndromes. Epilepsy Res 52: 107–116, 2002. doi: 10.1016/s0920-1211(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 255.Lazary J, Juhasz G, Anderson IM, Jacob CP, Nguyen TT, Lesch KP, Reif A, Deakin JF, Bagdy G. Epistatic interaction of CREB1 and KCNJ6 on rumination and negative emotionality. Eur Neuropsychopharmacol 21: 63–70, 2011. doi: 10.1016/j.euroneuro.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 256.Ziegler GC, Roser C, Renner T, Hahn T, Ehlis AC, Weber H, Dempfle A, Walitza S, Jacob C, Romanos M, Fallgatter AJ, Reif A, Lesch KP. KCNJ6 variants modulate reward-related brain processes and impact executive functions in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 183: 247–257, 2020. doi: 10.1002/ajmg.b.32734. [DOI] [PubMed] [Google Scholar]
- 257.Druart M, Groszer M, Le Magueresse C. An etiological Foxp2 mutation impairs neuronal gain in layer VI cortico-thalamic cells through increased GABAB/GIRK signaling. J Neurosci 40: 8543–8555, 2020. doi: 10.1523/JNEUROSCI.2615-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Marron Fernandez de Velasco E, Carlblom N, Xia Z, Wickman K. Suppression of inhibitory G protein signaling in forebrain pyramidal neurons triggers plasticity of glutamatergic neurotransmission in the nucleus accumbens core. Neuropharmacology 117: 33–40, 2017. doi: 10.1016/j.neuropharm.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Anderson EM, Demis S, Wrucke B, Engelhardt A, Hearing MC. Infralimbic cortex pyramidal neuron GIRK signaling contributes to regulation of cognitive flexibility but not affect-related behavior in male mice. Physiol Behav 242: 113597, 2021. doi: 10.1016/j.physbeh.2021.113597. [DOI] [PubMed] [Google Scholar]
- 260.Anderson EM, Demis S, D'Acquisto H, Engelhardt A, Hearing M. The role of parvalbumin interneuron GIRK signaling in the regulation of affect and cognition in male and female mice. Front Behav Neurosci 15: 621751, 2021. doi: 10.3389/fnbeh.2021.621751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rifkin RA, Huyghe D, Li X, Parakala M, Aisenberg E, Moss SJ, Slesinger PA. GIRK currents in VTA dopamine neurons control the sensitivity of mice to cocaine-induced locomotor sensitization. Proc Natl Acad Sci USA 115: E9479–E9488, 2018. doi: 10.1073/pnas.1807788115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Victoria NC, Marron Fernandez de Velasco E, Ostrovskaya O, Metzger S, Xia Z, Kotecki L, Benneyworth MA, Zink AN, Martemyanov KA, Wickman K. G protein-gated K+ channel ablation in forebrain pyramidal neurons selectively impairs fear learning. Biol Psychiatry 80: 796–806, 2016. doi: 10.1016/j.biopsych.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Honda I, Araki K, Honda S, Soeda F, Shin MC, Misumi S, Yamamura KI, Takahama K. Deletion of GIRK2 subunit containing GIRK channels of neurons expressing dopamine transporter decrease immobility time on forced swimming in mice. Neurosci Lett 665: 140–146, 2018. doi: 10.1016/j.neulet.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 264.Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev 99: 2115–2140, 2019. doi: 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rifkin RA, Moss SJ, Slesinger PA. G protein-gated potassium channels: a link to drug addiction. Trends Pharmacol Sci 38: 378–392, 2017. doi: 10.1016/j.tips.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nishizawa D, Nagashima M, Katoh R, Satoh Y, Tagami M, Kasai S, Ogai Y, Han W, Hasegawa J, Shimoyama N, Sora I, Hayashida M, Ikeda K. Association between KCNJ6 (GIRK2) gene polymorphisms and postoperative analgesic requirements after major abdominal surgery. PloS One 4: e7060, 2009. doi: 10.1371/journal.pone.0007060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lotsch J, Pruss H, Veh RW, Doehring A. A KCNJ6 (Kir3.2, GIRK2) gene polymorphism modulates opioid effects on analgesia and addiction but not on pupil size. Pharmacogenet Genomics 20: 291–297, 2010. doi: 10.1097/FPC.0b013e3283386bda. [DOI] [PubMed] [Google Scholar]
- 268.Bruehl S, Denton JS, Lonergan D, Koran ME, Chont M, Sobey C, Fernando S, Bush WS, Mishra P, Thornton-Wells TA. Associations between KCNJ6 (GIRK2) gene polymorphisms and pain-related phenotypes. Pain 154: 2853–2859, 2013. doi: 10.1016/j.pain.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nishizawa D, Fukuda KI, Kasai S, Ogai Y, Hasegawa J, Sato N, Yamada H, Tanioka F, Sugimura H, Hayashida M, Ikeda K. Association between KCNJ6 (GIRK2) gene polymorphism rs2835859 and post-operative analgesia, pain sensitivity, and nicotine dependence. J Pharmacol Sci 126: 253–263, 2014. doi: 10.1254/jphs.14189fp. [DOI] [PubMed] [Google Scholar]
- 270.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16: 36–49, 2007. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Clarke TK, Laucht M, Ridinger M, Wodarz N, Rietschel M, Maier W, Lathrop M, Lourdusamy A, Zimmermann US, Desrivieres S, Schumann G. KCNJ6 is associated with adult alcohol dependence and involved in gene x early life stress interactions in adolescent alcohol drinking. Neuropsychopharmacology 36: 1142–1148, 2011. doi: 10.1038/npp.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci 2: 1084–1090, 1999. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 273.Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, Kumanishi T. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci 2: 1091–1097, 1999. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- 274.Kobayashi T, Nishizawa D, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by phencyclidine. Curr Neuropharmacol 9: 244–246, 2011. doi: 10.2174/157015911795017407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42: 939–946, 2004. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 276.Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124: 641–650, 2016. doi: 10.1097/aln.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wei AD, Ramirez J-M. Presynaptic mechanisms and KCNQ potassium channels modulate opioid depression of respiratory drive. Front Physiol 10: 1407, 2019. doi: 10.3389/fphys.2019.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, Luscher C. Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J Neurosci 28: 4069–4077, 2008. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Marker CL, Cintora SC, Roman MI, Stoffel M, Wickman K. Hyperalgesia and blunted morphine analgesia in G protein-gated potassium channel subunit knockout mice. Neuroreport 13: 2509–2513, 2002. doi: 10.1097/00001756-200212200-00026. [DOI] [PubMed] [Google Scholar]
- 280.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and α 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci USA 100: 271–276, 2003. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Marker CL, Stoffel M, Wickman K. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci 24: 2806–2812, 2004. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J Neurosci 25: 3551–3559, 2005. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hearing MC, Zink AN, Wickman K. Cocaine-induced adaptations in metabotropic inhibitory signaling in the mesocorticolimbic system. Rev Neurosci 23: 325–351, 2012. doi: 10.1515/revneuro-2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Blednov YA, Stoffel M, Cooper R, Wallace D, Mane N, Harris RA. Hyperactivity and dopamine D1 receptor activation in mice lacking Girk2 channels. Psychopharmacology (Berl) 159: 370–378, 2002. doi: 10.1007/s00213-001-0937-6. [DOI] [PubMed] [Google Scholar]
- 285.Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharmacology 28: 932–938, 2003. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- 286.Kang SJ, Rangaswamy M, Manz N, Wang JC, Wetherill L, Hinrichs T, Almasy L, Brooks A, Chorlian DB, Dick D, Hesselbrock V, Kramer J, Kuperman S, Nurnberger J Jr, Rice J, Schuckit M, Tischfield J, Bierut LJ, Edenberg HJ, Goate A, Foroud T, Porjesz B. Family-based genome-wide association study of frontal theta oscillations identifies potassium channel gene KCNJ6. Genes Brain Behav 11: 712–719, 2012. doi: 10.1111/j.1601-183X.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tarantino LM, McClearn GE, Rodriguez LA, Plomin R. Confirmation of quantitative trait loci for alcohol preference in mice. Alcoholism Clin Exp Res 22: 1099–1105, 1998. doi: 10.1111/j.1530-0277.1998.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 288.Risinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcoholism Clin Exp Res 22: 1234–1244, 1998. doi: 10.1111/j.1530-0277.1998.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 289.Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol 11: 195–269, 2006. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 290.Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther 298: 521–530, 2001. [PubMed] [Google Scholar]
- 291.Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: activation of GIRK2 channels. Proc Natl Acad Sci USA 100: 277–282, 2003. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology (Berl) 169: 108–114, 2003. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- 293.Tipps ME, Raybuck JD, Kozell LB, Lattal KM, Buck KJ. G protein-gated inwardly rectifying potassium channel subunit 3 knock-out mice show enhanced ethanol reward. Alcohol Clin Exp Res 40: 857–864, 2016. [Erratum inAlcohol Clin Exp Res40: 2253, 2016]. doi: 10.1111/acer.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cannady R, Rinker JA, Nimitvilai S, Woodward JJ, Mulholland PJ. Chronic alcohol, intrinsic excitability, and potassium channels: neuroadaptations and drinking behavior. Handb Exp Pharmacol 248: 311–343, 2018. [Erratum inHandb Exp Pharmacol248: 619, 2018]. doi: 10.1007/164_2017_90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Basel-Vanagaite L, Shaffer L, Chitayat D. Keppen–Lubinsky syndrome: expanding the phenotype. Am J Med Genet A 149A: 1827–1829, 2009. doi: 10.1002/ajmg.a.32975. [DOI] [PubMed] [Google Scholar]
- 296.Masotti A, Uva P, Davis-Keppen L, Basel-Vanagaite L, Cohen L, Pisaneschi E, Celluzzi A, Bencivenga P, Fang M, Tian M, Xu X, Cappa M, Dallapiccola B. Keppen–Lubinsky syndrome is caused by mutations in the inwardly rectifying K+ channel encoded by KCNJ6. Am J Hum Genet 96: 295–300, 2015. doi: 10.1016/j.ajhg.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mett A, Karbat I, Tsoory M, Fine S, Iwanir S, Reuveny E. Reduced activity of GIRK1-containing heterotetramers is sufficient to affect neuronal functions, including synaptic plasticity and spatial learning and memory. J Physiol 599: 521–545, 2021. doi: 10.1113/JP280434. [DOI] [PubMed] [Google Scholar]
- 298.Malik R, Johnston D. Dendritic GIRK Channels gate the integration window, plateau potentials, and induction of synaptic plasticity in dorsal but not ventral CA1 neurons. J Neurosci 37: 3940–3955, 2017. doi: 10.1523/JNEUROSCI.2784-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Cramer NP, Best TK, Stoffel M, Siarey RJ, Galdzicki Z. GABAB-GIRK2-mediated signaling in Down syndrome. Adv Pharmacol 58: 397–426, 2010. doi: 10.1016/S1054-3589(10)58015-3. [DOI] [PubMed] [Google Scholar]
- 300.Cooper A, Grigoryan G, Guy-David L, Tsoory MM, Chen A, Reuveny E. Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc Natl Acad Sci USA 109: 2642–2647, 2012. doi: 10.1073/pnas.1109099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19, 2010. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Antonaros F, Pitocco M, Abete D, Vione B, Piovesan A, Vitale L, Strippoli P, Caracausi M, Pelleri MC. Structural characterization of the highly restricted Down syndrome critical region on 21q22.13: new KCNJ6 and DSCR4 transcript isoforms. Front Genet 12: 770359, 2021. doi: 10.3389/fgene.2021.770359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res 360: 263–280, 1990. [PubMed] [Google Scholar]
- 304.Best TK, Siarey RJ, Galdzicki Z. Ts65Dn, a mouse model of Down syndrome, exhibits increased GABAB-induced potassium current. J Neurophysiol 97: 892–900, 2007. doi: 10.1152/jn.00626.2006. [DOI] [PubMed] [Google Scholar]
- 305.Kleschevnikov AM, Yu J, Kim J, Lysenko LV, Zeng Z, Yu YE, Mobley WC. Evidence that increased Kcnj6 gene dose is necessary for deficits in behavior and dentate gyrus synaptic plasticity in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis 103: 1–10, 2017. doi: 10.1016/j.nbd.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Joshi K, Shen L, Michaeli A, Salter M, Thibault-Messier G, Hashmi S, Eubanks JH, Cortez MA, Snead OC. Infantile spasms in down syndrome: rescue by knockdown of the GIRK2 channel. Ann Neurol 80: 511–521, 2016. doi: 10.1002/ana.24749. [DOI] [PubMed] [Google Scholar]
- 307.Joshi K, Shen L, Cao F, Dong S, Jia Z, Cortez MA, Snead OC 3rd.. Kcnj6(GIRK2) trisomy is not sufficient for conferring the susceptibility to infantile spasms seen in the Ts65Dn mouse model of down syndrome. Epilepsy Res 145: 82–88, 2018. doi: 10.1016/j.eplepsyres.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 308.Alfaro-Ruiz R, Martin-Belmonte A, Aguado C, Hernandez F, Moreno-Martinez AE, Avila J, Lujan R. The expression and localisation of G-protein-coupled inwardly rectifying potassium (GIRK) channels is differentially altered in the hippocampus of two mouse models of Alzheimer's disease. Int J Mol Sci 22: 11106, 2021. doi: 10.3390/ijms222011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Akyuz E, Villa C, Beker M, Elibol B. Unraveling the role of inwardly rectifying potassium channels in the hippocampus of an Aβ (1-42)-infused rat model of Alzheimer's disease. Biomedicines 8: 58, 2020. doi: 10.3390/biomedicines8030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mayordomo-Cava J, Iborra-Lazaro G, Djebari S, Temprano-Carazo S, Sanchez-Rodriguez I, Jeremic D, Gruart A, Delgado-Garcia JM, Jimenez-Diaz L, Navarro-Lopez JD. Impairments of synaptic plasticity induction threshold and network oscillatory activity in the hippocampus underlie memory deficits in a non-transgenic mouse model of amyloidosis. Biology (Basel) 9: 175, 2020. doi: 10.3390/biology9070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364: 802–806, 1993. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- 312.Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci USA 90: 10235–10239, 1993. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kozek KA, Du Y, Sharma S, Prael FJ 3rd, Spitznagel BD, Kharade SV, Denton JS, Hopkins CR, Weaver CD. Discovery and characterization of VU0529331, a synthetic small-molecule activator of homomeric G protein-gated, inwardly rectifying, potassium (GIRK) channels. ACS Chem Neurosci 10: 358–370, 2018. doi: 10.1021/acschemneuro.8b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cui M, Xu K, Gada K, Shalomov B, Ban M, Eptaminitaki GC, Kawano T, Plant LD, Dascal N, Logothetis DE. A novel small molecule selective activator of homomeric GIRK4 channels. J Biol Chem 298: 102009, 2022. doi: 10.1016/j.jbc.2022.102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Egan KJ, Vesterinen HM, Beglopoulos V, Sena ES, Macleod MR. From a mouse: systematic analysis reveals limitations of experiments testing interventions in Alzheimer's disease mouse models. Evid Based Preclin Med 3: e00015, 2016. doi: 10.1002/ebm2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sadler KE, Mogil JS, Stucky CL. Innovations and advances in modelling and measuring pain in animals. Nat Rev Neurosci 23: 70–85, 2022. doi: 10.1038/s41583-021-00536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]