Abstract
Hybrid immunity occurs in those who have been both infected with and vaccinated against SARS-CoV-2. But how well does such hybrid immunity protect against the virus and its emerging variants?
Subject terms: Vaccines, SARS-CoV-2, Viral infection
The COVID-19 pandemic is far from over. SARS-CoV-2 mutates relatively slowly (~1 × 10−6 per base per infection cycle); yet, the world continues to experience a series of viral surges fuelled by the evolving sub-lineages of the Omicron variant. Now, 32 months into the pandemic, more than 66% of the world’s population is estimated to have some form of immunity against SARS-CoV-2, either through infection (‘natural’), vaccination (‘artificial’) or both (‘hybrid’). Hybrid immunity emerged as a form of ‘super immunity’ in 2021, when convalescent people receiving vaccine doses were found to have neutralizing antibody titres on average 50-fold higher than unvaccinated convalescent individuals1. As breakthrough infections emerged in vaccinated people, hybrid immunity became an established arm of the anti-SARS-CoV-2 immune landscape. What do we now know about hybrid immunity and its ‘super immunity’ abilities?
Hybrid immunity against viral variants
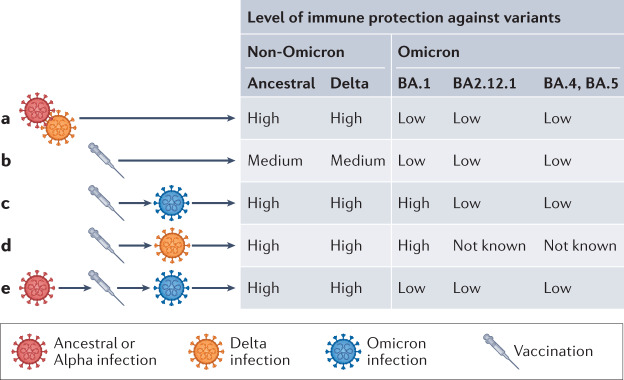
Several studies, including our own, suggest that hybrid immunity confers more effective cross-variant neutralization than a natural infection alone (Fig. 1a,c). Vaccination after infection with the SARS-CoV-2 ancestral Wuhan-Hu-1 strain or with Alpha, Delta and Beta variants increases the number of SARS-CoV-2 memory T cells and B cells by more than an order of magnitude by recruiting new B cell clones into the memory pool and expanding persistent clones1,2 (Fig. 1c). Formation of memory B cells by vaccination and robust enhancement of serologic responses by SARS-CoV-2 infection results in durable2, high levels of cross-variant neutralizing antibodies1 and reduces the risk of reinfection3.
Fig. 1. Protection after different immune-conferring events.
a | Emerging Omicron sub-variants escape immunity conferred by infection with non-Omicron SARS-CoV-2 variants4,5. b | A second dose of mRNA vaccine gives moderate protection for non-Omicron and limited protection against Omicron variants 6–8 months post-vaccination4,5. c | Hybrid immunity elicited by BA.1 breakthrough infection in vaccinated individuals (2 doses) provides cross-variant protection but causes neutralization escape for newly emerging Omicron variants2,8. d | Hybrid immunity generated by Delta infection in vaccinated individuals elicits broader protection against non-micron and Omicron (BA.1) variants6. e | Compared with B.1.617.2, immune imprinting generated by infection with Wuhan/B.1.1.7, vaccination (3 doses) and Omicron reinfection decreases Omicron neutralizing antibodies and T cell recognition, which may increase chances of Omicron reinfection. Consistent with Reynolds et al.9.
These actions hold for Omicron, at least to a certain degree. A BA.1 breakthrough infection elicits a broad cross-variant neutralizing activity against, for example, the ancestral WA1 and Delta variants, whereas BA.1 infection in the absence of prior vaccination only induces neutralization of BA.1 (ref.4). But titres are lower overall: anti-BA.1 neutralizing titres elicited by a BA.1 breakthrough infection average sixfold lower than the anti-Delta neutralizing titres elicited by a Delta breakthrough infection4,5 (Fig. 1d). Consequently, newly emerging Omicron variants, including BA.2.12.1, BA.4 and BA.5, show neutralization escape when tested with sera from BA.1-infected individuals6 (Fig. 1c). The immunity recall after breakthrough infections varies with respect to pathogenicity of variants. For Delta breakthrough infections, the neutralizing antibody titres against Delta and other variants are high and correlate with disease severity5, but Omicron breakthrough infections generate weak immunity against Omicron and emerging sub-lineages. The reasons include the altered antigenic properties of the Omicron subvariants, based on a substantial number of additional spike mutations6, and the lowered pathogenicity of the Omicron subvariants, with replication mostly confined to the upper respiratory tract, mortality reduced in animal models, and ex vivo viral replication weakened.
Hybrid immunity is more durable
The waning of neutralizing titres has become a major obstacle to ending the COVID-19 pandemic. Protection against reinfection decreases as the time increases since the last event of infection or vaccination. Goldberg and colleagues recently reported that at 6–8 months after the last immunity-conferring event, cohorts with hybrid immunity showed the highest level of protection with 10–20 confirmed infections/100,000 persons-days at risk3 (Fig. 1c). For convalescent individuals, this increased to 30 confirmed reinfections, and for individuals with two vaccine doses, reinfections reached 85–90/100,000 persons-days at risk3 (Fig. 1b). Interestingly, hybrid immunity in individuals with just one vaccine dose plus infection conferred the same level of protection as in individuals with three doses of vaccine, and the sequence of immune-conferring events did not matter. Reinfection rates in individuals who recovered from infection and then received one dose of vaccine were similar to reinfection rates in individuals who received one vaccine dose and then got infected. Notably, this study did not include any Omicron variants, but Altarawneh et al. showed ~60% hybrid immunity-mediated protection against BA.1 and BA.2 variants at 8–9 months after infection7.
‘First exposure’ directs hybrid immunity
While neutralizing titres conferred by antibodies wane and are outcompeted by evolving mutations, T cell epitopes remain largely conserved among variants, including Omicron. B cell responses against Omicron are ~10-fold less effective than against pre-Omicron strains, but T cell responses to Omicron are only decreased by 10–30%8. Interestingly, hybrid immunity-conferring events in vaccinated or infected individuals effectively boost humoral responses, but spike-specific T cell immunity appears to be not similarly boosted, compared to what is seen after vaccination of naive individuals8. In addition, hybrid immunity-mediated T cell responses to Omicron depend on previous SARS-CoV-2 exposure9. Unlike Delta, people initially infected by the Wuhan Hu-1 or Alpha variants, then vaccinated and re-infected during the Omicron wave, do not boost T cell immunity against Omicron as measured by levels of live virus neutralizing antibody and T cell recognition (Fig. 1e). This suggests that the first exposure to SARS-CoV-2 confers some form of T cell imprinting that directs future hybrid immune responses. Despite this, T cells are the strongest immune correlate for vaccinated and convalescent individuals avoiding hospitalization8.
Where now for vaccines?
The full extent of how natural, artificial and hybrid immunities will stand against current and future Omicron surges is not yet known. However, the immune trajectories of SARS-CoV-2-infected or vaccinated human populations suggest that hybrid immunity provides the strongest and most durable protection against symptomatic infection, but mostly against variants with similar antigenic phenotypes. So how to safely and effectively widen this protection to include variants with different antigenic phenotypes? Simply including the Omicron spike protein in booster regimes appears insufficient. Gagne et al. showed no advantage in neutralizing titres and protection from Omicron infection in non-human primates boosted with mRNA encoding Omicron spike versus mRNA-1273 encoding the Wuhan-Hu-1 spike10. Similarly, current data from Moderna’s clinical trial with a new bivalent mRNA-1273.214 booster showed only ~twofold higher Omicron BA.1-neutralizing titres than the regular mRNA-1273 booster.
It may be time to change the current ‘one shoe fits all’ approach. In a highly dynamic immune landscape with its various degrees of natural, artificial and hybrid immunity and the regional circulation of Omicron sublineages, vaccination strategies need to be tailored on a regional, perhaps even personalized, basis. Knowing the strength and breadth of a population’s or an individual’s neutralizing titres as well as their T cell responses to existing and new SARS-CoV-2 variants is critical to design and execute effective protective approaches. This requires wide availability of immune testing and a broadened repertoire of vaccine strategies. Pre-existing cross-protective antibody titres mediated by hybrid immunity may be effectively increased by boosting with vaccine candidates spanning conserved regions of SARS-CoV-2 variants other than the spike protein. In addition, vaccination at a mucosal surface induces stronger systemic and mucosal immunity than intramuscular administration, a route that may be particularly beneficial for people with hybrid immunity boosting pre-existing resident immune cells in the mucosa. While hybrid immunity may not be the silver bullet to end the SARS-CoV-2 pandemic, it is a silver lining, from which we can learn to move to the next level of vaccine design and delivery.
Acknowledgements
R.S. and M.O. thank the Ott lab members for their support. M.O. received support from NIH U19AI171110, the James B. Pendleton Charitable Trust, Roddenberry Foundation, P. and E. Taft, and the Gladstone Institutes. M.O. thanks Fast Grants and the Innovative Genomics Institute for support.
Competing interests
The authors declare no competing interests.
References
- 1.Wang Z, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall V, et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg Y, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N. Engl. J. Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suryawanshi RK, et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature. 2022;607:351–355. doi: 10.1038/s41586-022-04865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servellita V, et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. Cell. 2022;185:1539–1548. doi: 10.1016/j.cell.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022 doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altarawneh HN, et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 2022;386:1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent SJ, et al. Disentangling the relative importance of T cell responses in COVID-19: leading actors or supporting cast? Nat. Rev. Immunol. 2022;22:387–397. doi: 10.1038/s41577-022-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds CJ, et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377:eabq1841. doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagne M, et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell. 2022;185:1556–1571. doi: 10.1016/j.cell.2022.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]