Abstract
Extracorporeal membrane oxygenation (ECMO) is increasingly being used for patients with severe respiratory failure and has received particular attention during the coronavirus disease 2019 (COVID-19) pandemic. Evidence from two key randomized controlled trials, a subsequent post hoc Bayesian analysis, and meta-analyses support the interpretation of a benefit of ECMO in combination with ultra-lung-protective ventilation for select patients with very severe forms of acute respiratory distress syndrome (ARDS). During the pandemic, new evidence has emerged helping to better define the role of ECMO for patients with COVID-19. Results from large cohorts suggest outcomes during the first wave of the pandemic were similar to those in non-COVID-19 cohorts. As the pandemic continued, mortality of patients supported with ECMO has increased. However, the precise reasons for this observation are unclear. Known risk factors for mortality in COVID-19 and non-COVID-19 patients are higher patient age, concomitant extra-pulmonary organ failures or malignancies, prolonged mechanical ventilation before ECMO, less experienced treatment teams and lower ECMO caseloads in the treating center. ECMO is a high resource-dependent support option; therefore, it should be used judiciously, and its availability may need to be constrained when resources are scarce. More evidence from high-quality research is required to better define the role and limitations of ECMO in patients with severe COVID-19.
Keywords: Extracorporeal membrane oxygenation, ECMO, Acute respiratory distress syndrome, ARDS, COVID-19, Resource limitations, Extracorporeal circulation, Respiratory failure
Take-home message
| Extracorporeal membrane oxygenation (ECMO) is increasingly being used for patients with severe acute respiratory failure, with the strongest evidence for its use in patients with very severe forms of acute distress respiratory syndrome (ARDS). However, many questions about how best to optimize the care of ECMO patients remain. Future research should focus on better defining the role and limitations of ECMO. |
Introduction
Over the past decade, the use of venovenous extracorporeal membrane oxygenation (VV-ECMO) for adults with respiratory failure has increased markedly worldwide [1]. This trend has continued during the coronavirus disease 2019 (COVID-19) pandemic; however, the optimal approach for providing VV-ECMO for acute respiratory failure, and the most appropriate use of ECMO in resource-constrained settings, remain uncertain [2–4]. In this review, we provide an in-depth overview of the use of VV-ECMO, with a major focus on COVID-19.
Background and evidence
Modern evidence for a potential benefit of ECMO in patients with respiratory failure appeared in 2009, with publication of the CESAR multicenter randomized controlled trial that suggested that transferring adult patients with severe respiratory failure to an ECMO center significantly decreased mortality or severe disability at six months [5]. However, important methodological limitations limited the generalizability of these findings [6].
The 2018 EOLIA trial examined early initiation of ECMO in patients with severe acute respiratory distress syndrome (ARDS) [7]. The EOLIA trial was terminated early for futility; however, there was a substantial, albeit not statistically significant reduction in 60-day mortality in the ECMO-treated group (35 vs. 46%, p = 0.09). A 28% rate of cross-over from the control to the ECMO group contributed to the null finding. However, a subsequent post hoc Bayesian analysis of EOLIA, along with a meta-analysis, an individual patient data meta-analysis, and a network meta-analysis, supported the interpretation of a benefit of ECMO in combination with ultra-lung-protective ventilation for selected patients with very severe ARDS [8–12].
Technical aspects
ECMO is a device used to support or replace pulmonary gas exchange function (venovenous ECMO), or cardiac function (venoarterial ECMO) [1, 12]. During ECMO, blood is withdrawn from the patient and directed through a “membrane lung” consisting of bundles of adjacent hollow fibers that channel fresh gas in close proximity to blood, allowing exchange of oxygen and carbon dioxide by diffusion. Over the past decades, major technological improvements in pumps, tubing and membrane lungs have led to improved gas exchange and reduced complications. Recent polymethylpentene membranes are considered superior to previously used silicone rubber polymer or polypropylene membranes [13]. Centrifugal pumps within current ECMO systems require smaller filling volumes compared to conventional roller pumps, potentially at the expense of increased hemolysis [14]. Overall, the general technical approach when applying ECMO in COVID-19 patients is not different from non-COVID-19 patients [15–17].
Initial experience with venovenous ECMO in COVID-19
At the start of the COVID-19 pandemic, there was a reasonable rationale for using ECMO in patients with very severe COVID-19-related ARDS, based on the EOLIA trial, along with retrospective observational studies, including matching studies (albeit with mixed results), from the 2009 influenza A(H1N1) pandemic [18–20]. However, there were specific concerns that contact of blood with artificial surfaces might induce a greater inflammatory response in COVID-19 compared to non-COVID-19 patients [21, 22]. Furthermore, two papers published early during the pandemic suggested very high mortality rates for ECMO-supported patients, leading to an initially cautious approach to its use, especially when the demand for critical care services was on the verge of exceeding available resources [2, 23–26].
As the pandemic progressed, the central question was whether pathophysiological changes in COVID-19-related ARDS were fundamentally different from other forms of ARDS, and whether these differences could have consequences for clinical management [27]. A retrospective cohort study from France reported an estimated probability of 60 days and 90 days of mortality of 31% and 36% in 83 patients with COVID-19-related ARDS supported with ECMO from March 8 to May 2, 2020 [16]. Potential explanations for substantially lower mortality rates than initially observed include better patient selection, along with optimized mechanical ventilation support and adjunctive therapy provided by very experienced teams in high-volume centers [16].
Mortality rates reported from other cohorts in the United States, Europe, South America and the Middle East were similar, albeit with some heterogeneity. A Chilean population-based study of 85 patients supported from March 3 to August 31, 2020, reported a 90-day mortality of 38.8% [28]. In a study of 307 patients treated in 19 ECMO centers in five countries in the Middle East and India from March 1 to September 30, 2020, survival to home discharge was 45% [29].
A survey conducted of both the Extracorporeal Life Support Organization (ELSO)—and non-ELSO-affiliated intensive care units (ICUs) noted a mortality rate of 43.9% for 1,413 patients supported with ECMO from March to September, 2020 [30]. Data from ELSO complemented these findings. In an initial analysis of 1035 COVID-19 patients who received ECMO between January and May, 2020, at 213 hospitals in 36 countries, estimated 90-day cumulative in-hospital mortality was 37.4% (95% CI 34.4–40.4) [31].
In contrast to these early studies, where mortality was comparable to that in the EOLIA trial, there were outliers. A French study of 302 patients admitted to one of 17 intensive care units (ICUs) in Paris, France, between March and June, 2020, had a higher 90 days of mortality at 54.3% [15]. A study from Germany reported overall in-hospital mortality rate of 68% in 3,397 COVID-19 patients supported with ECMO from March, 2020, through May, 2021 [32]. It is imperative to critically evaluate such high mortality, as seen in a nationwide analysis, for a widely used and established support. These results are particularly disconcerting because resource constraints were not a major issue in Germany during the study period. Importantly, this study represents the only unselected national cohort of COVID-19 patients receiving ECMO, so it is possible that it simply better reflects real-world outcomes.
Differences in mortality in the different cohorts are difficult to interpret, but may include: differences in patient selection, e.g., age limitations; prior experience with ECMO use for ARDS; lack of specific data to guide a consistent approach to managing ARDS; strain on resources, including human resources. One additional explanation for the sobering results may be that ECMO use in Germany is neither regulated nor restricted, such that any hospital may start an ECMO program. Consequently, there are many hospitals with small annual ECMO case volumes and there is a well-known volume-outcome relationship for ECMO [15, 33–35]. Absent randomized data, the degree of confounding in these observational cohorts precludes definitive conclusions [3, 4, 36].
Evolving evidence for the use of ECMO during the COVID-19 pandemic
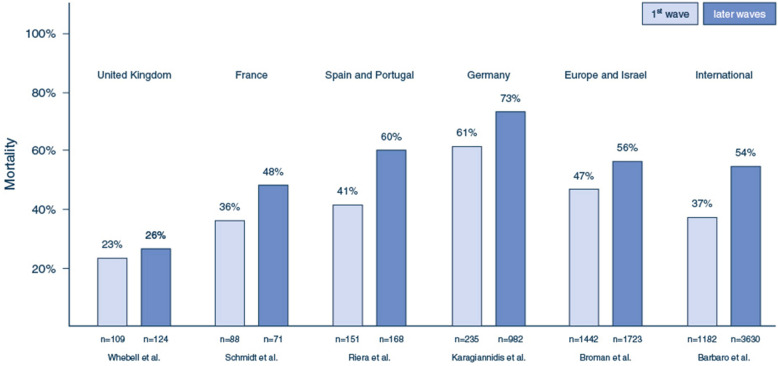
Emerging data suggested that ECMO mortality increased after the first wave (Fig. 1). Comparing 151 patients supported with ECMO from the first wave with 168 from the second wave in 24 ECMO centers in Spain and Portugal, Riera et al. found a significantly higher hospital mortality during the second wave (41.1 vs. 60.1%, p = 0.001) [37]. Schmidt et al. also observed an increased probability of 90-day mortality after the initial wave (36 vs. 48%; HR 2.27, 95% CI 1.02–5.07) [38]. Similar findings were noted in the EuroELSO survey after the first wave [39].
Fig. 1.
Mortality rates from selected ECMO cohorts during the first and later waves of the pandemic. In all reported cohorts, mortality rates were reported for variably defined later waves, compared to the first wave. Differences in outcomes between the cohorts may be, at least in part, explained by differences in inclusion and exclusion criteria (see main text for details). Broman et al. and Barbaro et al. reported data from large international cohorts confirming the observation of increasing mortality with the use of ECMO after the first wave of the pandemic.
ECMO extracorporeal membrane oxygenation, ARDS acute respiratory distress syndrome, COVID-19 coronavirus disease 2019
An updated study from the ELSO was extended to cover all 4,812 COVID-19 patients in the registry who had ECMO initiated in 2020 from 349 hospitals across 41 countries [34]. Patients were grouped into three cohorts: The first cohort consisting of patients initiated on ECMO on or before May 1, 2020, mirroring the initial ELSO registry COVID-19 cohort had an estimated cumulative incidence of 90-day mortality of 36.9% [34]. In the second cohort, consisting of patients initiated on ECMO after May 1, 2020, at those same centers, cumulative 90-day in-hospital mortality was 51.9%; and, in the third cohort, consisting of centers which started providing ECMO for COVID-19 only after May 1, 2020, cumulative 90-day in-hospital mortality higher at 58.9%.
Possible explanations for this increase in mortality include changes in patient selection and treatment standards over the course of the pandemic, the impact of resource limitations in overburdened hospitals, and possible pathophysiological changes due to emerging viral variants [3, 34]. Later during the pandemic, patients were more frequently supported with non-invasive respiratory support before endotracheal intubation, and corticosteroids were given more often [34]. Thus, they may represent a different cohort—with treatment-refractory presentations, an explanation yet to be confirmed.
Efficacy of ECMO in patients with severe COVID-19
A randomized trial similar to EOLIA has not been conducted in patients with severe COVID-19-related ARDS—and such a trial is unlikely, given the lack of clinical equipoise in large ECMO centers, as well as considerable logistical and funding hurdles. However, other analytic approaches are being used. Recently, three target trial emulations have been performed analyzing the efficacy of mechanical ventilation with ECMO compared to mechanical ventilation alone in patients with severe COVID-19-related ARDS [40–42]. This approach may allow causal inferences similar to those from a randomized trial [43, 44].
In an emulated target trial of 190 COVID-19 patients from 35 hospitals in the United States between March and July, 2020, Shaefi et al. reported a lower mortality for patients who received ECMO compared to those who did not (34.6 vs. 47.4%; HR 0.55; 95% CI 0.41–0.74) [41].
A multicenter European COVID-ICU study of 4244 COVID-19 patients with very severe ARDS (PaO2/FiO2 < 80 mmHg or PaCO2 ≥ 60 mmHg) compared patients supported with ECMO within seven days of invasive mechanical ventilation (IMV) vs. those treated without ECMO.[40, 45]. 1,235 patients met eligibility criteria for the emulation trial; 164 patients with ECMO. Probability of day 7 survival was higher for ECMO patients (87 vs. 83%, risk difference: 4%, 95% CI 0%; 9%), but decreased by day 90 (63 vs. 65%, risk difference: − 2%, 95% CI − 10%; 5%). ECMO was associated with higher survival when performed in high-volume ECMO centers (> 30 patients treated with VV-ECMO in the previous year), in regions with a specific ECMO network set-up to handle high demand, when it was initiated within 4 days of IMV and when used in profoundly hypoxemic patients (PaO2/FiO2 < 65 mmHg).
In a larger emulated target trial, the COVID-19 Critical Care Consortium [42] studied 7345 patients with COVID-19-related acute respiratory failure; 844 patients received ECMO. In patients with PaO2/FiO2 < 80 mmHg, ECMO support reduced hospital mortality by 7.1% (95% CI 6.1–8.1%) compared to conventional mechanical ventilation without ECMO (RR 0.78, 95% CI 0.75–0.82). Age (< 65 years), severity of hypoxemia (PaO2/FiO2 < 80 mmHg), duration (≤ 10 days) and intensity of mechanical ventilation (driving pressure > 15 cm H2O) before ECMO were associated with benefit of ECMO.
Yet, the critical limitation of these trials is that, despite their methodological sophistication, they merely simulate randomized controlled trials, while the data still come from retrospective observations. Therefore, we cannot entirely control for selection bias nor can we exclude unmeasured confounding [46].
Data from two other retrospective analyses suggest a survival benefit for patients transferred to an ECMO center [47, 48]. However, here it is difficult to ascertain if the improvements are due to ECMO per se, or to the expertise in managing complex patients at these quaternary referral centers.
Clinical management of patients with severe COVID-19 and ECMO
Indications and timing of ECMO
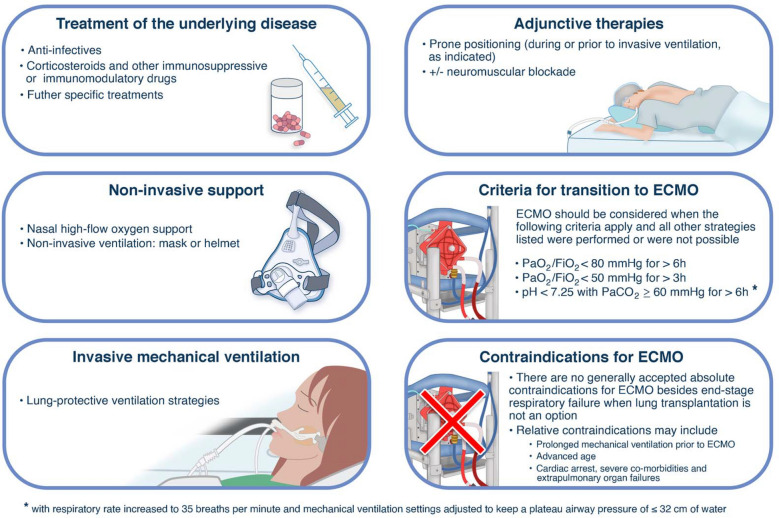
The indications for ECMO in severe COVID-19-related ARDS should follow established criteria from the pre-COVID era until there is evidence to the contrary (Figs. 2 and 3) [27, 49]. Most importantly, optimization of ventilation management and prone positioning (unless contraindicated) should be attempted prior to initiation of ECMO, whenever feasible. However, relative contraindications to ECMO use for COVID-19 patients should be adapted to the local experience, including resource availability [3, 4, 17, 50].
Fig. 2.
Key strategies of care that may be initiated, including consideration of ECMO support in patients with ARDS (non-COVID-19 and COVID-19-related).
ECMO extracorporeal membrane oxygenation, ARDS acute respiratory distress syndrome, COVID-19 coronavirus disease 2019, PEEP positive end-expiratory pressure
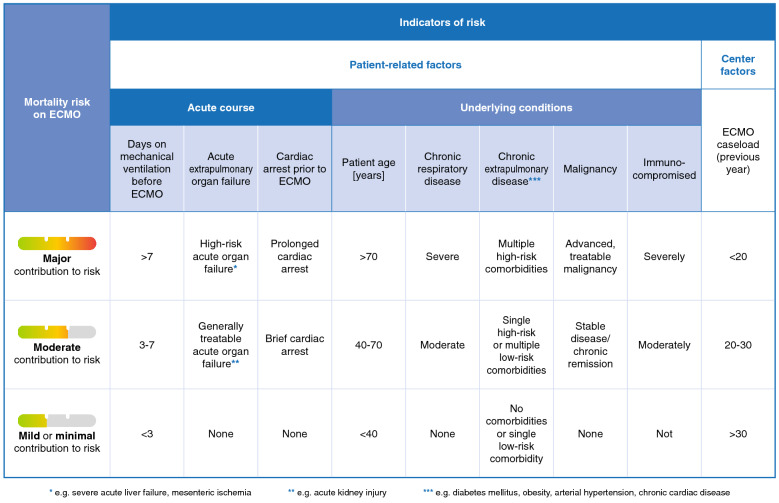
Fig. 3.
Patient-related and center-associated factors contributing to the risk of mortality in patients supported with venovenous ECMO, with a focus on COVID-19-related ARDS.
ECMO extracorporeal membrane oxygenation, ARDS acute respiratory distress syndrome, COVID-19 coronavirus disease 2019
In several retrospective analyses, an association between mortality and duration of invasive mechanical ventilation prior to initiation of ECMO has been described. Lebreton et al. observed significantly lower mortality in patients who received ECMO < 3 days after intubation, compared to longer periods of IMV [15]. Similarly, in a large cohort of ECMO patients in Germany, Karagiannidis et al. observed the lowest in-hospital mortality rate in patients who had been mechanically ventilated ≤ 3 days before ECMO initiation [51]. In an emulated target trial, Urner et al. found that ECMO was most effective if initiated early, and certainly within the first 10 days of IMV [42]. Finally, in another emulated target trial, ECMO was associated with a mortality benefit when initiated within four days of IMV [40]. Yet, observations are mixed, and in other cohorts, duration of IMV prior to ECMO was not that clearly associated with a higher risk of mortality [28, 52].
It is unclear if the duration of non-invasive respiratory support before intubation and ECMO, also relates to outcomes. Data from a small retrospective analysis support this concern—patients who received non-invasive respiratory support for ≥ 3 days had poorer outcomes than patients who received non-invasive respiratory support for < 3 days [53]. Likewise, in the ELSO registry, the later cohort (with higher mortality) was noted to have greater use of non-invasive respiratory support prior to ECMO compared with the early cohort [34].
A scoring system allowing prognostication and risk assessment for individual patients would be desirable for COVID-19 patients. Considering the current lack of such a system, we described known risk factors, and subdivided conditions with respect to the degree to which they contribute to mortality risk (Fig. 3). Current evidence does not allow definite answers as to the right time from intubation or from adjunctive treatment options to start ECMO. Nevertheless, it seems reasonable to follow the approach used in the EOLIA trial for the time being. Caution seems necessary about starting ECMO support in patients with advanced age, serious preexisting conditions, or after prolonged duration of mechanical ventilation.
Prone positioning and invasive mechanical ventilation management
In a recent meta-analysis of studies for non-COVID-19 ARDS patients receiving ECMO, prone positioning was associated with improved survival [54]. Similarly, an individual patient meta-analysis including 889 patients found a lower 60-day mortality in patients treated with prone positioning during ECMO compared to a matched population of ECMO patients managed supine [55]. For COVID-19 patients, a retrospective analysis found that prone positioning during ECMO was associated with reduced mortality [56]. Although these findings have not yet been confirmed in randomized trials, it seems reasonable that the prone position may be beneficial when performed by teams experienced in both prone positioning and ECMO.
The ROSE trial did not reveal a significant benefit of neuromuscular blockade for mechanically ventilated patients [57]. Nonetheless, neuromuscular blockers may be indicated in select patients to allow for safe prone positioning, to limit respiratory drive, or in patients who despite optimized ventilator settings and sedation, show breathing patterns known to be associated with a risk of ventilator-induced lung injury [58].
Important differences in respiratory mechanics between COVID-19-related and other forms of ARDS have been suggested although the weight of evidence does not support this observation, and thus there is no reason to deviate from established recommendations for IMV [59–62]. COVID-19 patients with ECMO should therefore receive lung-protective ventilation according to recommendations for non-COVID patients [63].
Non-invasive respiratory support and physical rehabilitation during ECMO
The optimal non-invasive ventilatory management during ECMO in COVID-19 patients is not clear. Case reports have described the feasibility of ECMO combined with high-flow nasal oxygen or non-invasive ventilation when IMV was not an option [64, 65]. However, in a retrospective case series, 14/18 patients (78%) were eventually intubated due to complications, after an initial attempt of ECMO without IMV.[66] These patients ultimately had a higher mortality than those not requiring intubation.
Clinical experience and recent observational and pilot randomized data gathered before the COVID-19 pandemic suggest a potential benefit for early mobilization of patients receiving ECMO with or without concomitant IMV [67, 68]. However, data in COVID-19-related ARDS are limited. In a cohort from the United States, 40 patients with COVID-19-related ARDS meeting EOLIA criteria were initiated on ECMO; 88% of these patients were extubated from IMV during ECMO and many participated in active physical rehabilitation [69]. Overall survival was high in this cohort. However, the contribution of rehabilitation to outcomes is unclear given the observational nature of the data.
Infectious complications
Few publications assess the incidence of bacterial and fungal co- and superinfections in COVID-19 patients; with even fewer focusing on ECMO patients. In these cohorts, no clear signal suggesting an increased risk for such infections has been described [45, 70–72]. In the ELSO registry, co-infection later than 2 weeks after initiation of ECMO was associated with an increased risk for mortality in COVID-19 patients [34].
Bleeding complications, thromboembolic events and anticoagulation management
Severe COVID-19 is associated with increased risk of thromboembolic events and bleeding complications, and not surprisingly, these complications have been observed in COVID-19 patients receiving ECMO [15, 41, 73, 74]. However, in the initial ELSO registry study of COVID-19 patients on ECMO, the prevalence of bleeding complications and circuit clotting was similar to rates reported from non-COVID-19 patients, although, observational data may be confounded by changes in practice [31, 75]. Currently, available data suggest that established anticoagulation practices used in non-COVID-19 patients with ECMO should be used in COVID-19 patients [76].
Extracorporeal hemoadsorption
Initial reports suggested that COVID-19 patients with higher interleukin 6 (IL-6) levels had higher mortality rates [77]. Consequently, extracorporeal hemoadsorption of IL-6 and other blood components have been suggested as a therapeutic target in severe courses of the disease [78]. Initial case reports and uncontrolled case series suggested a benefit of extracorporeal hemoadsorption when combined with continuous renal replacement therapy or ECMO [79, 80]. However, in the randomized-controlled CYCOV trial, no benefit for extracorporeal hemoadsorption was found when implemented within the ECMO circuit during the first 72 h of ECMO support [81]. Therefore, the use of hemoadsorption in COVID-19 patients on ECMO cannot be recommended outside of clinical trials at this time, nor is there evidence supporting the general use in non-COVID-19 ECMO patients.
Duration of ECMO support
The duration of ECMO support in COVID-19 patients is prolonged compared to non-COVID-19 patients. Lebreton et al. described a median duration of 14 days; Barbaro et al. found a median duration of 14 days early in the pandemic (patients initiated on ECMO through May 1, 2020),and increased to 20 days later in the pandemic [15, 34]. For patients who survived until day 90, Schmidt et al. described a median ECMO duration of 22 days in patients treated before July 1, 2020, compared to 33 days for those treated after July 1, 2020 [38]. This has clear implications for resource allocation. However, this observation has to be considered with caution as in different cohorts different withdrawal criteria may have been applied, which may have strongly influenced these outcomes.
Lung transplantation
ECMO is a temporary support option that requires ongoing care in an intensive care unit (ICU), ideally as a bridge to recovery when initiated for an acute illness. In prolonged cases, when weaning from ECMO support is no longer likely, lung transplantation may be an option in highly selected patients, with ECMO continued as a bridge to transplantation. Initial experience suggests that patients receiving lung transplantation after severe COVID-19-related ARDS and ECMO may do well and be discharged home, although long-term outcomes, and outcomes relative to those without transplant are unknown [82, 83].
Organization of ECMO systems and implications of resource limitations
ECMO utilizes substantial resources and in many countries, ECMO is not available, or accessible only to a minority of the population. The organization and provision of ECMO services differ from country to country and even between regions within a given country. In some centralized national health systems, ECMO is only provided in dedicated referral centers (e.g., United Kingdom or Singapore) and inter-hospital retrieval systems are available for transferring patients even if they are already on ECMO. In other less centralized jurisdictions, ECMO may be offered in many hospitals, sometimes with lower case volumes in individual centers (e.g., United States or Germany) [35].
Even prior to COVID-19, data from the international ELSO registry suggested that higher annual hospital ECMO volume was associated with lower mortality, a finding confirmed during the COVID-19 pandemic [15, 33, 34]. An analysis from a large hospital network in Paris found that mortality of ECMO patients was significantly lower in centers that had treated ≥ 30 patients with ECMO in the prior year, compared to centers with lower caseloads [15]. Therefore, whenever possible, ECMO should be provided in large and experienced centers, and hospital network coordination and retrieval systems be put in place. However, under special conditions it may be appropriate to offer ECMO in smaller centers, for example in remote regions, if the distance to a large ECMO center may be too long to provide timely care. In these settings, lack of experience may be mitigated through simulation, and telemedicine support to clinicians.
During a pandemic, availability of intensive care resources including ECMO may not meet demand [84]. This may pertain to structural resources (e.g., ICU beds, ventilators, dialysis machines or ECMO consoles), consumables (e.g., specific drugs, ECMO equipment, including membrane lungs, tubing or cannulae), and also human resources (e.g., physicians, nurses and other essential clinical personnel) [85]. Ultimately, rationing and prioritization of scarce resources may be required [17, 86]. In such situations, it may be necessary to care for patients longer in less specialized centers before transfer to a specialized center than under pre-pandemic conditions. However, transport options must be available for transfer to a specialized center, if necessary [87].
The provision of ECMO is particularly resource-intensive—in terms of structural and human resources. Thus, when invoking crisis standards of care, decisions have to be made as to how to allocate resources to ECMO versus other critical demands, such as mechanical ventilation, renal support, or general medical care [50, 88]. These decisions must consider the role of the specific hospital in a particular region, the range of services and capacities of other hospitals in that region, and forecasts of the expected number of patients. The latter requires prediction tools, which are often best implemented regionally. In addition, local outcomes must be taken into account when deciding to expand or even limit availability of ECMO [50].
The way ahead
The COVID-19 pandemic has revealed that many health systems and institutions were not adequately prepared. Admittedly, no healthcare system can be prepared to provide care for dramatic increases in caseloads. Nevertheless, it is necessary to have adequate supplies of key resources, such as personal protective equipment or essential medicines in preparation for a crisis. It is also necessary to strengthen regional coordination and cooperation among hospitals to optimize resources on a larger scale.
In view of the great uncertainties and concerns regarding ECMO support in severe COVID-19-related ARDS at the beginning of the pandemic, it is crucial to emphasize the importance of research. Only with high-quality data and evidence is it possible to offer patients optimal treatment. In some locales, regulations for conducting clinical trials or transferring data to national or international registries are complex and burdensome. There is an urgent need to adapt these requirements so that necessary research can be carried out in everyday clinical practice even during a pandemic, as has been done by the UK RECOVERY trial (www.recovertrial.net) and the international REMAP-Cap trial (www.remapcap.org). Patients should be entered into registries, which should ensure long-term follow-up of patients, including assessment of quality of life.
There are many important questions that remain with respect to the use of ECMO for patients with COVID-19-related ARDS. We suggest that the most important ones relate to identification of which patients are most likely to benefit, optimal timing for ECMO initiation, duration of ECMO support, and long-term, patient-centered outcomes.
Based on observational data, shorter duration of mechanical ventilation prior to ECMO is associated with improved patient survival, suggesting that patients should be transitioned to ECMO relatively early, or perhaps ECMO should be withheld from patients after a longer duration of ventilation. Yet, neither conclusion is fully supported by available evidence, as it is difficult to make firm conclusions from observational data which may be skewed by selection bias, and other factors. Therefore, more research is needed before definitive recommendations can be made.
Finally, every society should make provisions for situations when demand outstrips resources. Ideally, ethical rationing principles should be developed by consensus to be incorporated into triage guidelines for use in crisis situations [50, 88].
Conclusion
The use of ECMO has been increasing for over a decade. However, the role of ECMO has been more prominent than ever in the COVID-19 era. More than two years into the COVID-19 pandemic, we understand that outcomes of patients supported with ECMO for COVID-19-related ARDS appear to have worse outcomes than non-COVID-19-related ARDS. However, there is an important role for ECMO for the temporary support of patients with severe acute respiratory failure, both as a bridge to recovery and, in select cases, as a bridge to transplant. Definitive indications, timing and management with respect to preceding, adjunctive treatment strategies, ventilation management and rehabilitation remain unclear and final recommendations cannot be given at this time. Unexplained high mortality rates observed in some cohorts need to be better understood and measures for improvements to be introduced. Given the resources required to implement ECMO, more evidence is needed for appropriate patient selection and coordination of care to further improve the outcomes of all critically ill patients.
Author contributions
AS wrote the first draft of the manuscript following discussions with DB, AC and ASS. All authors revised the manuscript and added key content. All authors read and approved the final manuscript.
Funding
No funding was provided specific to this work.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
All authors have completed the ICMJE form (available upon request from the corresponding author). AS reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, both outside the submitted work. EF received research support from Abbott and LivaNova and consulting fees from ALung Technologies, Vasomune, Baxter and GE Healthcare. CH is supported by an Australian NHMRC Investigator grant and leads the Australian and New Zealand ECMO Registry. CK received consulting fees from Xenios/ Fresenius and Bayer. He is Chair of the German ICU Registry and President of the German Society of Medical Intensive Care. JR reports lecture and advisory fees from Medtronic and Werfen, outside the submitted work. ASS has been on medical advisory boards for Baxter and Xenios; he is Chair of the Scientific Committee of the Committee of the International ECMO Network (ECMONet). DB receives research support from ALung Technologies. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira and Cellenkos. He is the President-elect of the Extracorporeal Life Support Organization (ELSO) and the Chair of ECMONet. All other authors report no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alain Combes, Arthur S. Slutsky and Daniel Brodie are co-senior authors.
References
- 1.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 2.MacLaren G, Fisher D, Brodie D. Preparing for the most critically Ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 3.MacLaren G, Fisher D, Brodie D. Treating the most critically Ill patients with COVID-19: the evolving role of extracorporeal membrane oxygenation. JAMA. 2022;327:31–32. doi: 10.1001/jama.2021.22580. [DOI] [PubMed] [Google Scholar]
- 4.Brodie D, Abrams D, MacLaren G, Brown CE, Evans L, Barbaro RP, Calfee CS, Hough CL, Fowles JA, Karagiannidis C, Slutsky AS, Combes A. ECMO During Respiratory Pandemics: Past, Present, and Future. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202111-2661CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, collaboration Ct, Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 6.Abrams D, Brodie D. Extracorporeal membrane oxygenation for adult respiratory failure: 2017 update. Chest. 2017;152:639–649. doi: 10.1016/j.chest.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A, Eolia Trial Group R, Ecmonet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 8.Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M, Dechartres A, Elbourne D. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46:2048–2057. doi: 10.1007/s00134-020-06248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Juni P, Brodie D, Slutsky AS, Combes A. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 10.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 11.Sud S, Friedrich JO, Adhikari NKJ, Fan E, Ferguson ND, Guyatt G, Meade MO. Comparative effectiveness of protective ventilation strategies for moderate and severe acute respiratory distress syndrome. A network meta-analysis. Am J Respir Crit Care Med. 2021;203:1366–1377. doi: 10.1164/rccm.202008-3039OC. [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Schmidt M, Hodgson CL, Fan E, Ferguson ND, Fraser JF, Jaber S, Pesenti A, Ranieri M, Rowan K, Shekar K, Slutsky AS, Brodie D. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020;46:2464–2476. doi: 10.1007/s00134-020-06290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toomasian JM, Schreiner RJ, Meyer DE, Schmidt ME, Hagan SE, Griffith GW, Bartlett RH, Cook KE. A polymethylpentene fiber gas exchanger for long-term extracorporeal life support. ASAIO J. 2005;51:390–397. doi: 10.1097/01.mat.0000169111.66328.a8. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien C, Monteagudo J, Schad C, Cheung E, Middlesworth W. Centrifugal pumps and hemolysis in pediatric extracorporeal membrane oxygenation (ECMO) patients: an analysis of Extracorporeal Life Support Organization (ELSO) registry data. J Pediatr Surg. 2017;52:975–978. doi: 10.1016/j.jpedsurg.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, Lansac E, Sage E, Cholley B, Megarbane B, Cronier P, Zarka J, Da Silva D, Besset S, Morichau-Beauchant T, Lacombat I, Mongardon N, Richard C, Duranteau J, Cerf C, Saiydoun G, Sonneville R, Chiche JD, Nataf P, Longrois D, Combes A, Leprince P, Paris E-C-i Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, Baron E, Beurton A, Chommeloux J, Meng P, Nemlaghi S, Bay P, Leprince P, Demoule A, Guidet B, Constantin JM, Fartoukh M, Dres M, Combes A, Groupe de Recherche Clinique en ReSidPeIRaSU, Paris-Sorbonne E-Ci Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, Agerstrand C, Bartlett RH, Barrett N, Combes A, Lorusso R, Mueller T, Ogino MT, Peek G, Pellegrino V, Rabie AA, Salazar L, Schmidt M, Shekar K, MacLaren G, Brodie D, Members EC-WG. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettila V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M, Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza I Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 19.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 20.Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, Clavel M, Constan A, Marie Richard JC, Brun-Buisson C, Brochard L, Network RR. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 21.Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387. doi: 10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrams D, Lorusso R, Vincent JL, Brodie D. ECMO during the COVID-19 pandemic: when is it unjustified? Crit Care. 2020;24:507. doi: 10.1186/s13054-020-03230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett RH, Ogino MT, Brodie D, McMullan DM, Lorusso R, MacLaren G, Stead CM, Rycus P, Fraser JF, Belohlavek J, Salazar L, Mehta Y, Raman L, Paden ML. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–349. doi: 10.1016/j.hrtlng.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, Salazar PA, Bahamondes JC, Castillo LB, Gajardo AIJ, Kursbaum A, Ferreira LL, Valenzuela J, Castillo RE, Perez-Araos RA, Bravo M, Aquevedo AF, Gonzalez MG, Pereira R, Ortega L, Santis C, Fernandez PA, Cortes V, Cornejo RA. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204:34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabie AA, Azzam MH, Al-Fares AA, Abdelbary A, Mufti HN, Hassan IF, Chakraborty A, Oza P, Elhazmi A, Alfoudri H, Pooboni SK, Alharthy A, Brodie D, Zakhary B, Shekar K, Antonini MV, Barrett NA, Peek G, Combes A, Arabi YM. Implementation of new ECMO centers during the COVID-19 pandemic: experience and results from the Middle East and India. Intensive Care Med. 2021;47:887–895. doi: 10.1007/s00134-021-06451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorusso R, Combes A, Lo Coco V, De Piero ME, Belohlavek J, Euro EC-W, Euro ESC. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47:344–348. doi: 10.1007/s00134-020-06272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, Bartlett RH, Tonna JE, Hyslop R, Fanning JJ, Rycus PT, Hyer SJ, Anders MM, Agerstrand CL, Hryniewicz K, Diaz R, Lorusso R, Combes A, Brodie D, Extracorporeal Life Support O. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karagiannidis C, Slutsky AS, Bein T, Windisch W, Weber-Carstens S, Brodie D. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit Care. 2021;25:413. doi: 10.1186/s13054-021-03831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, Diaz R, Fan E, Hryniewicz K, Lorusso R, Paden ML, Stead CM, Swol J, Iwashyna TJ, Slutsky AS, Brodie D, Extracorporeal Life Support O. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bercker S, Petroff D, Polze N, Karagianidis C, Bein T, Laudi S, Stehr SN, Voelker MT. ECMO use in Germany: an analysis of 29,929 ECMO runs. PLoS ONE. 2021;16:e0260324. doi: 10.1371/journal.pone.0260324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supady A, Biever PM, Staudacher DL, Wengenmayer T. Choosing the right reference cohort for assessing outcome of venovenous ECMO. Crit Care. 2022;26:17. doi: 10.1186/s13054-021-03880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riera J, Roncon-Albuquerque R, Jr, Fuset MP, Alcantara S, Blanco-Schweizer P, Group ES Increased mortality in patients with COVID-19 receiving extracorporeal respiratory support during the second wave of the pandemic. Intensive Care Med. 2021;47:1490–1493. doi: 10.1007/s00134-021-06517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt M, Langouet E, Hajage D, James SA, Chommeloux J, Brechot N, Barhoum P, Lefevre L, Troger A, de Chambrun MP, Hekimian G, Luyt CE, Dres M, Constantin JM, Fartoukh M, Leprince P, Lebreton G, Combes A, Universite GRS. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals. Paris Crit Care. 2021;25:355. doi: 10.1186/s13054-021-03780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broman LM, Eksborg S, Lo Coco V, De Piero ME, Belohlavek J, Lorusso R, Euro EC-WG, Euro ESC. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med. 2021;9:e80–e81. doi: 10.1016/S2213-2600(21)00262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajage D, Combes A, Guervilly C, Lebreton G, Mercat A, Pavot A, Nseir S, Mekontso-Dessap A, Mongardon N, Mira JP, Ricard JD, Beurton A, Tachon G, Kontar L, Le Terrier C, Richard JC, Megarbane B, Keogh RH, Belot A, Maringe C, Leyrat C, Schmidt M, Investigators C-I Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: an emulated target trial analysis. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202111-2495OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaefi S, Brenner SK, Gupta S, O'Gara BP, Krajewski ML, Charytan DM, Chaudhry S, Mirza SH, Peev V, Anderson M, Bansal A, Hayek SS, Srivastava A, Mathews KS, Johns TS, Leonberg-Yoo A, Green A, Arunthamakun J, Wille KM, Shaukat T, Singh H, Admon AJ, Semler MW, Hernan MA, Mueller AL, Wang W, Leaf DE, Investigators S-C. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47:208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urner M, Barnett AG, Bassi GL, Brodie D, Dalton HJ, Ferguson ND, Heinsar S, Hodgson CL, Peek G, Shekar K, Suen JY, Fraser JF, Fan E, Investigators C-CCC. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022;377:e068723. doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ. 2018;360:k182. doi: 10.1136/bmj.k182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Network C-IGobotR, the C-ICUI Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferreyro BL, Fan E. Turning the page on ECMO for ARDS due to severe COVID-19. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202205-0906ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gannon WD, Stokes JW, Francois SA, Patel YJ, Pugh ME, Benson C, Rice TW, Bacchetta M, Semler MW, Casey JD. Association between availability of ECMO and mortality in COVID-19 patients eligible for ECMO: a natural experiment. Am J Respir Crit Care Med. 2022 doi: 10.1164/rccm.202110-2399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whebell S, Zhang J, Lewis R, Berry M, Ledot S, Retter A, Camporota L. Survival benefit of extracorporeal membrane oxygenation in severe COVID-19: a multi-centre-matched cohort study. Intensive Care Med. 2022;48:467–478. doi: 10.1007/s00134-022-06645-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrams D, Ferguson ND, Brochard L, Fan E, Mercat A, Combes A, Pellegrino V, Schmidt M, Slutsky AS, Brodie D. ECMO for ARDS: from salvage to standard of care? Lancet Respir Med. 2019;7:108–110. doi: 10.1016/S2213-2600(18)30506-X. [DOI] [PubMed] [Google Scholar]
- 50.Supady A, Badulak J, Evans L, Curtis JR, Brodie D. Should we ration extracorporeal membrane oxygenation during the COVID-19 pandemic? Lancet Respir Med. 2021;9:326–328. doi: 10.1016/S2213-2600(21)00131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karagiannidis C, Strassmann S, Merten M, Bein T, Windisch W, Meybohm P, Weber-Carstens S. High in-hospital mortality rate in patients with COVID-19 receiving extracorporeal membrane oxygenation in Germany: a critical analysis. Am J Respir Crit Care Med. 2021;204:991–994. doi: 10.1164/rccm.202105-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riera J, Alcantara S, Bonilla C, Fortuna P, Blandino Ortiz A, Vaz A, Albacete C, Millan P, Ricart P, Boado MV, Ruiz de Gopegui P, Santa Teresa P, Sandoval E, Perez-Chomon H, Gonzalez-Perez A, Duerto J, Gimeno R, Colomina J, Gomez V, Renedo G, Naranjo J, Garcia MA, Rodriguez-Ruiz E, Silva PE, Perez D, Veganzones J, Voces R, Martinez S, Blanco-Schweizer P, Garcia M, Villanueva-Fernandez H, Fuset MP, Luna SM, Martinez-Martinez M, Argudo E, Chiscano L, Roncon-Albuquerque R., Jr Risk factors for mortality in patients with COVID-19 needing extracorporeal respiratory support. Eur Respir J. 2022 doi: 10.1183/13993003.02463-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmad Q, Green A, Chandel A, Lantry J, Desai M, Simou J, Osborn E, Singh R, Puri N, Moran P, Dalton H, Speir A, King C. Impact of noninvasive respiratory support in patients with COVID-19 requiring V-V ECMO. ASAIO J. 2022;68:171–177. doi: 10.1097/MAT.0000000000001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papazian L, Schmidt M, Hajage D, Combes A, Petit M, Lebreton G, Rilinger J, Giani M, Le Breton C, Duburcq T, Jozwiak M, Wengenmayer T, Roux D, Parke R, Loundou A, Guervilly C, Boyer L. Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med. 2022;48:270–280. doi: 10.1007/s00134-021-06604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giani M, Rezoagli E, Guervilly C, Rilinger J, Duburcq T, Petit M, Textoris L, Garcia B, Wengenmayer T, Grasselli G, Pesenti A, Combes A, Foti G, Schmidt M, EuroPron EI. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a pooled individual patient data analysis. Crit Care. 2022;26:8. doi: 10.1186/s13054-021-03879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaaqoq AM, Barnett AG, Griffee MJ, MacLaren G, Jacobs JP, Heinsar S, Suen JY, Bassi GL, Fraser JF, Dalton HJ, Peek GJ, Consortium C-CC Beneficial effect of prone positioning during venovenous extracorporeal membrane oxygenation for coronavirus disease 2019. Crit Care Med. 2022;50:275–285. doi: 10.1097/CCM.0000000000005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Heart L, Blood Institute PCTN, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slutsky AS, Villar J. Early paralytic agents for ARDS? Yes, no, and sometimes. N Engl J Med. 2019;380:2061–2063. doi: 10.1056/NEJMe1905627. [DOI] [PubMed] [Google Scholar]
- 59.Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, Laffey J, Carrafiello G, Carsana L, Rizzuto C, Zanella A, Scaravilli V, Pizzilli G, Grieco DL, Di Meglio L, de Pascale G, Lanza E, Monteduro F, Zompatori M, Filippini C, Locatelli F, Cecconi M, Fumagalli R, Nava S, Vincent JL, Antonelli M, Slutsky AS, Pesenti A, Ranieri VM, Collaborators Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E, Aldecoa C, Martinez-Palli G, Martinez-Gonzalez MA, Slutsky AS, Villar J, Network C-SI. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46:2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goligher EC, Ranieri VM, Slutsky AS. Is severe COVID-19 pneumonia a typical or atypical form of ARDS? And does it matter? Intensive Care Med. 2021;47:83–85. doi: 10.1007/s00134-020-06320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiumello D, Busana M, Coppola S, Romitti F, Formenti P, Bonifazi M, Pozzi T, Palumbo MM, Cressoni M, Herrmann P, Meissner K, Quintel M, Camporota L, Marini JJ, Gattinoni L. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46:2187–2196. doi: 10.1007/s00134-020-06281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abrams D, Schmidt M, Pham T, Beitler JR, Fan E, Goligher EC, McNamee JJ, Patroniti N, Wilcox ME, Combes A, Ferguson ND, McAuley DF, Pesenti A, Quintel M, Fraser J, Hodgson CL, Hough CL, Mercat A, Mueller T, Pellegrino V, Ranieri VM, Rowan K, Shekar K, Brochard L, Brodie D. Mechanical ventilation for acute respiratory distress syndrome during extracorporeal life support. Research and practice. Am J Respir Crit Care Med. 2020;201:514–525. doi: 10.1164/rccm.201907-1283CI. [DOI] [PubMed] [Google Scholar]
- 64.Loyalka P, Cheema FH, Rao H, Rame JE, Rajagopal K. Early usage of extracorporeal membrane oxygenation in the absence of invasive mechanical ventilation to treat COVID-19-related hypoxemic respiratory failure. ASAIO J. 2021;67:392–394. doi: 10.1097/MAT.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt M, de Chambrun MP, Lebreton G, Hekimian G, Chommeloux J, Brechot N, Barhoum P, Lefevre L, Juvin C, Molle J, Luyt CE, Combes A. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;203:1571–1573. doi: 10.1164/rccm.202102-0259LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mang S, Reyher C, Mutlak H, Natanov R, Lotz C, Gill-Schuster D, Bals R, Danziger G, Meybohm P, Combes A, Kuhn C, Lepper PM, Muellenbach RM, Group AW-S Awake extracorporeal membrane oxygenation for COVID-19-induced acute respiratory distress syndrome. Am J Respir Crit Care Med. 2022;205:847–851. doi: 10.1164/rccm.202105-1189LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abrams D, Madahar P, Eckhardt CM, Short B, Yip NH, Parekh M, Serra A, Dubois RL, Saleem D, Agerstrand C, Scala P, Benvenuto L, Arcasoy SM, Sonett JR, Takeda K, Meier A, Beck J, Ryan P, Fan E, Hodgson CL, Bacchetta M, Brodie D, Investigators M-P. Early mobilization during extracorporeal membrane oxygenation for cardiopulmonary failure in adults: factors associated with intensity of treatment. Ann Am Thorac Soc. 2022;19:90–98. doi: 10.1513/AnnalsATS.202102-151OC. [DOI] [PubMed] [Google Scholar]
- 68.Investigators E-PS, International EN Early mobilisation during extracorporeal membrane oxygenation was safe and feasible: a pilot randomised controlled trial. Intensive Care Med. 2020;46:1057–1059. doi: 10.1007/s00134-020-05994-8. [DOI] [PubMed] [Google Scholar]
- 69.Mustafa AK, Alexander PJ, Joshi DJ, Tabachnick DR, Cross CA, Pappas PS, Tatooles AJ. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. 2020;155:990–992. doi: 10.1001/jamasurg.2020.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin JM, Marcelin AG, Monsel A, Luyt CE, Blaize M. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med. 2021;203:307–317. doi: 10.1164/rccm.202009-3400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Fernandez-Pittol M, Pitart C, Inciarte A, Bodro M, Morata L, Ambrosioni J, Grafia I, Meira F, Macaya I, Cardozo C, Casals C, Tellez A, Castro P, Marco F, García F, Mensa J, Martínez JA, Soriano A. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pickens CO, Gao CA, Cuttica MJ, Smith SB, Pesce LL, Grant RA, Kang M, Morales-Nebreda L, Bavishi AA, Arnold JM, Pawlowski A, Qi C, Budinger GRS, Singer BD, Wunderink RG. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med. 2021;204:921–932. doi: 10.1164/rccm.202106-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doyle AJ, Hunt BJ, Sanderson B, Zhang J, Mak SM, Benedetti G, Breen KA, Camporota L, Barrett NA, Retter A. A comparison of thrombosis and hemorrhage rates in patients with severe respiratory failure due to coronavirus disease 2019 and influenza requiring extracorporeal membrane oxygenation. Crit Care Med. 2021;49:e663–e672. doi: 10.1097/CCM.0000000000005199. [DOI] [PubMed] [Google Scholar]
- 74.Jimenez D, Garcia-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, Le Mao R, Rodriguez C, Hunt BJ, Monreal M. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nunez JI, Gosling AF, O'Gara B, Kennedy KF, Rycus P, Abrams D, Brodie D, Shaefi S, Garan AR, Grandin EW. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. 2022;48:213–224. doi: 10.1007/s00134-021-06593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMichael ABV, Ryerson LM, Ratano D, Fan E, Faraoni D, Annich GM. 2021 ELSO adult and pediatric anticoagulation guidelines. ASAIO J. 2022;68:303–310. doi: 10.1097/MAT.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 77.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Supady A, Duerschmied D, Bode C, Rieder M, Lother A. Extracorporeal cytokine adsorption as an alternative to pharmacological inhibition of IL-6 in COVID-19. Crit Care. 2020;24:514. doi: 10.1186/s13054-020-03238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rieder M, Zahn T, Benk C, Lother A, Bode C, Staudacher D, Duerschmied D, Supady A. Cytokine adsorption in a patient with severe coronavirus disease 2019 related acute respiratory distress syndrome requiring extracorporeal membrane oxygenation therapy: a case report. Artif Organs. 2021;45:191–194. doi: 10.1111/aor.13805. [DOI] [PubMed] [Google Scholar]
- 80.Song T, Hayanga J, Durham L, Garrison L, McCarthy P, Barksdale A, Smith D, Bartlett R, Jaros M, Nelson P, Molnar Z, Deliargyris E, Moazami N. CytoSorb therapy in COVID-19 (CTC) patients requiring extracorporeal membrane oxygenation: a multicenter, retrospective registry. Front Med (Lausanne) 2021;8:773461. doi: 10.3389/fmed.2021.773461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, Frech F, Muller S, Kuhl M, Benk C, Maier S, Trummer G, Flugler A, Kruger K, Sekandarzad A, Stachon P, Zotzmann V, Bode C, Biever PM, Staudacher D, Wengenmayer T, Graf E, Duerschmied D. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med. 2021;9:755–762. doi: 10.1016/S2213-2600(21)00177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon R, Jr, Kim S, Manerikar A, Pelaez A, Pipkin M, Shahmohammadi A, Rackauskas M, Kg SR, Balakrishnan KR, Jindal A, Schaheen L, Hashimi S, Buddhdev B, Arjuna A, Rosso L, Palleschi A, Lang C, Jaksch P, Budinger GRS, Nosotti M, Hoetzenecker K. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurihara C, Manerikar A, Querrey M, Felicelli C, Yeldandi A, Garza-Castillon R, Jr, Lung K, Kim S, Ho B, Tomic R, Arunachalam A, Budinger GRS, Pesce L, Bharat A. Clinical characteristics and outcomes of patients with COVID-19-associated acute respiratory distress syndrome who underwent lung transplant. JAMA. 2022;327:652–661. doi: 10.1001/jama.2022.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 85.Supady A, Curtis JR, Brown CE, Duerschmied D, von Zepelin LA, Moss M, Brodie D. Ethical obligations for supporting healthcare workers during the COVID-19 pandemic. Eur Respir J. 2021 doi: 10.1183/13993003.00124-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Supady A, Curtis JR, Abrams D, Lorusso R, Bein T, Boldt J, Brown CE, Duerschmied D, Metaxa V, Brodie D. Allocating scarce intensive care resources during the COVID-19 pandemic: practical challenges to theoretical frameworks. Lancet Respir Med. 2021;9:430–434. doi: 10.1016/S2213-2600(20)30580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, MacLaren G, Brodie D, Shekar K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Supady A, Brodie D, Curtis JR. Ten things to consider when implementing rationing guidelines during a pandemic. Intensive Care Med. 2021;47:605–608. doi: 10.1007/s00134-021-06374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.