Abstract
Background
Many studies have shown that diabetes is often closely related to oral squamous cell carcinoma (OSCC) occurrence and metastasis. Heat shock protein 70 (Hsp70) is a molecular chaperone related to diabetes complications. This study aims to investigate the role of Hsp70 in OSCC in expression of invadopodia-associated proteins.
Methods
The expressions and correlation of HSP70, Hif1α, MMP2, MMP14, and cortactin were examined using bioinformatics analysis and verified by OSCC tissue microarrays. Assay in vitro was performed to analyze cell migration capacity after treatment with or without the HSP70 inhibitor.
Results
The expressions of invadopodia-associated proteins were enhanced in OSCC tissues compared with paracarcinoma tissues and partially correlated with HSP70. Inhibiting HSP70 significantly decreased the cell viability, proliferation, and migration of OSCC cells.
Conclusions
HSP70 may be involved in invadopodia-associated proteins in OSCC cells, which provides a promising method for treatment of OSCC metastasis.
Keywords: invadopodia-associated proteins, Hif1α, heat shock proteins 70, oral squamous cell carcinoma, bioinformatics analysis
Introduction
Oral squamous cell carcinoma (OSCC) is diagnosed with 350,000–400,000 new cases every year around the world (1). Although the advanced multimodal treatment strategy has improved the prognosis and survival rate of OSCC patients, the 5-year survival rate still is about 50% these years (2, 3). The cause of occurrence and metastasis of OSCC is still unclear. Previous studies have proved that the occurrence and development of oral squamous cells may be closely related to diabetes (4).
The role of heat shock proteins 70 (Hsp70) in a variety of cancers and diabetes has been paid more attention in recent years. The expression level of HSP70 was demonstrated to be elevated remarkably in various cancers (5). The high expression level of Hsp70 is associated with the tumor resistance to chemotherapeutic agents (6, 7). HSP70 may play an important role in diabetes mellitus through a mechanism that interferes with the expression of cytokines, matrix metallopeptidase 9 (MMP9), and the antioxidant enzyme superoxide dismutase 2 (8–10). However, as far as we know, whether HSP70 is involved in the metastasis of oral cancer has not been reported.
Invadopodia, a subcellular membrane structure that can degrade the extracellular matrix (ECM), plays an essential role in the invasive migration and metastasis of cancer cells (11, 12). Proteinase like MMP2 and MMP9 are secreted at invadopodia to promote the degradation of ECM (13). The upregulation of some key invadopodia molecules in cancer cells including the matrix metalloproteinase MT1-MMP(MMP14) and the actin assembly protein (cortactin) is associated with patients poor prognosis (14). Tumor microenvironment such as growth factors, hypoxia, and PH was reported to affect the formation and function of invadopodia (15).
In this study, we attempt to explore the potential role of Hsp70 in OSCC expression of invadopodia-associated proteins. The cell viability, proliferation potential, and migratory ability of OSCC cells were examined after inhibiting Hsp70. We discovered that HSP70 may be important for the expression of invadopodia-associated protein.
Materials and Methods
Ethics Statement
The present study was approved by the Medical Ethics Committee of Xiangya Hospital, Central South University (Hunan, China) and was performed according to the Declaration of Helsinki guidelines on experimentation involving human subjects. Written informed consent was obtained from all participants.
Patients
OSCC and matched paracancer tissues in the Department of Pathology, Department of Stomatology, Xiangya Hospital, Central South University were determined by two independent pathologists according to the 2006 WHO Classification System (16). In collaboration with Shanghai Biochip Co., LTD. (China), tumor tissue microarray was constructed, including 117 OSCCs and 56 matched paracancer tissues as previous described (17).
Reagents, Antibodies, Cell Lines, and Culture
Apoptozole (APO, HSP70 inhibitor; catalog no. S8365) was purchased from Selleck China subsidiary (Shanghai, China). Antibodies HSP70 (catalog no. 4873), Hif1α (catalog no. 8690), cortactin (catalog no. 3502), and MMP2 (catalog no. 40994) were acquired from Cell Signaling Technology (Danvers, MA, USA). MT1-MMP (MMP14; catalog no. 14552-1-AP) was purchased from Proteintech (Wuhan, China). Human OSCC cell line (Cal27) was purchased from ATCC. Cal27 cells were cultured in Dulbecco’s minimum essential medium (Hyclone, Logan, UT, USA), containing10% fetal bovine serum (Gibco, Carlsbad, CA, USA) in oxygen concentrations for normoxia (21% O2) at 37°C in Anoxomat chambers (Mart Microbiology, Lichtenvoorde, the Netherlands).
Immunohistochemistry
The sections of OSCC tissue arrays were deparaffinized in xylene and rehydrated in a graded series of ethanol and double-distilled water before subjected to heat-induced antigen retrieval. After incubated with primary antibodies (HSP70, 1:200; Hif1α, 1:100; MMP2, 1:200; MMP14, 1:50; cortactin, 1:100) overnight at 4°C, the secondary antibody was incubated at room temperature for 1 h. All slices were scanned by an Aperio ScanScope CS scanner (Epistem, Cambridge, MA) and quantified using Aperio Quantification software (Version 9.1, Epistem) for staining quantification. Histoscore of membrane and nuclear staining was calculated as a percentage of different positive cells using the formula (3+) ×3 + (2+) × 2 + (1+) × 1. Histoscore of pixel quantification was calculated as total intensity/total cell number. The threshold for scanning of different positive cells was set according to the standard controls provided by Aperio.
Cell Counting Kit-8 Assay
When plated in 96-well plates at 2 × 103 cells per well, Cal27 cells were cultured with Cell Counting Kit (CCK8; BioTime, Shanghai, China) for 24 and 48 h, respectively. Absorbance at 450nm was measured with the microplate reader (FlexStation 3, Molecular Devices, USA).
EdU (5′-Ethynl-2’-Deoxyuridine) Staining Assay
The effects of Apo on cell proliferation were assessed by the Cell-Light™ 5′-ethynl-2′-deoxyuridine (EdU) Apollo®488 In Vitro Imaging Kit according to the manufacturer’s instructions. The number and proportion of the cells incorporated EdU were visualized and the fluorescence intensity was quantified using Image J1.42.
Wound Healing Assay and Transwell Invasion Assay
Wound healing assay and Transwell invasion assay were performed as previously described (18) and details were described in Supplementary Material and Methods in S1 File.
The Cancer Genome Atlas Analysis
In order to obtain a correlation between the target genes among head and neck cancer (HNSC) patients, gene expression quantification data of HNSC patients were downloaded from The Cancer Genome Atlas (TCGA) database. Then, the expression quantification data of HSPA4, HIF1A, MMP14, MMP2, and CCTN in TCGA HNSC dataset were extracted and processed by R × 64 3.5.1 with the package ggplot2, showing the inner relationship among above genes in HNSC intuitively. Search Tool for the Retrieval of Interacting Genes (STRING; https://string‐db.org/) was used to predict the interactions between proteins (19).
Statistical Analysis
All experiments were performed at least in triplicate and each experiment was repeated for three times. GraphPad Prism Software (GraphPad Software Inc) was used for statistical analysis, and the data are presented as means ± standard error of mean (SEM). One-way analysis of variance (ANOVA) and Student’s t-test were used to analyze the differences as compared with control group and among each group. The relationship among the expression of Hsp70, Hif1α, MMP14, MMP2, and cortactin was examined using two-tailed Pearson’s statistics. The differences were considered statistically significant if the P-value < 0.05.
Results
The Overexpression of Hsp70, Hif1α, MMP2, MMP14, and Cortactin in OSCC
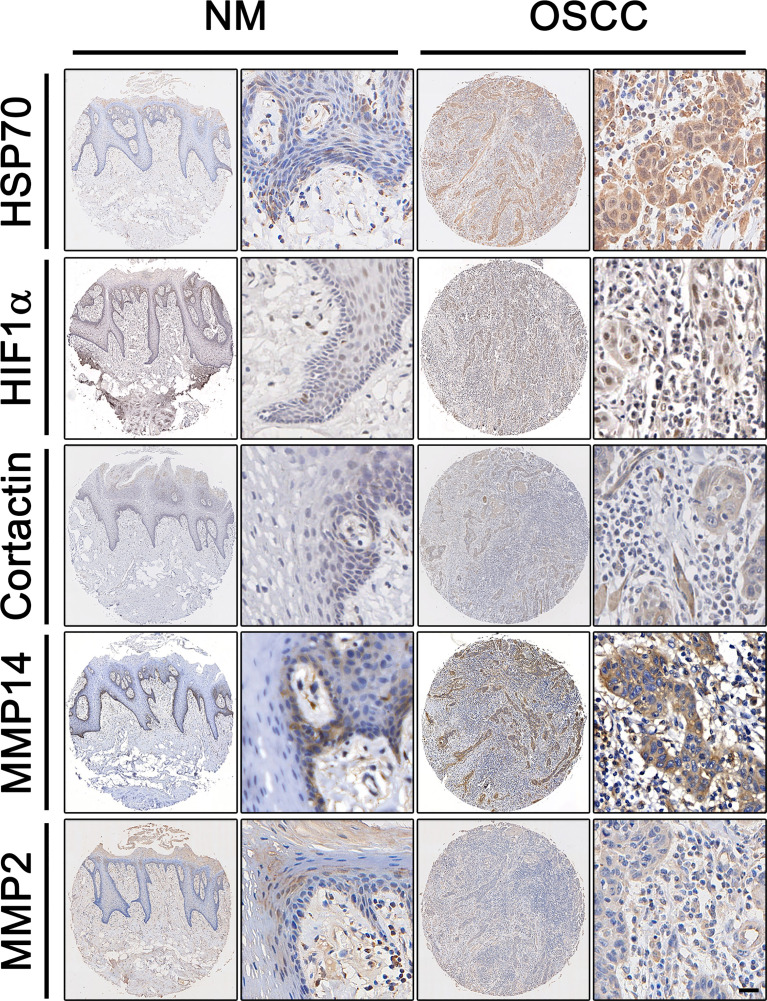
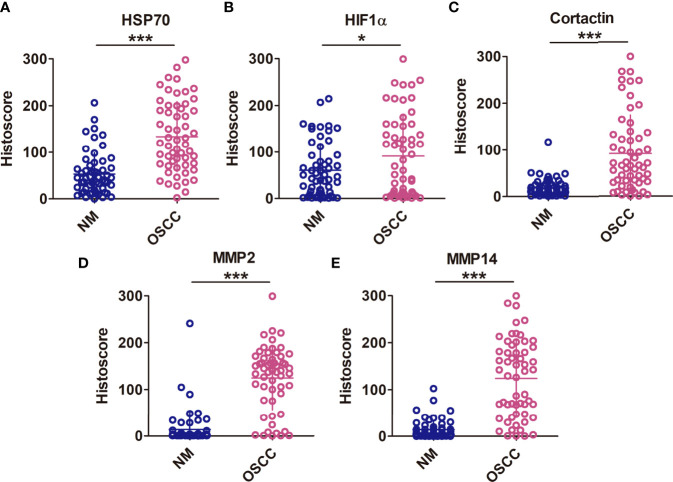
We explored the expression of Hsp70, Hif1α, MMP2, MMP14, and cortactin in OSCC using tissue arrays, in which the normal adjacent tissues were used as control (denoted as normal mucosa). As shown in Figure 1 , a significantly enhanced immunohistochemical staining was found in OSCC tissues compared with normal mucosa. The positive staining of Hsp70, MMP14, MMP2, and cortactin was mostly observed in the cytoplasmic of the carcinoma cells, whereas the nucleic positive signals were detected much more in the Hif1α immunohistochemical staining. The positive staining of MMP14 was mostly observed in the cytoplasmic and cytomembrane of the carcinoma cells. Then, we analyzed the histoscores of Hsp70 ( Figure 2A ), Hif1α ( Figure 2B ), cortactin ( Figure 2C ) MMP14 ( Figure 2D ), and MMP2 ( Figure 2E ) in both tumor and normal tissues and observed significantly higher expression levels of these proteins in OSCC tissues.
Figure 1.
Expression of HSP70, Hif1α, MMP2, MMP14, and cortactin in OSCC. Representative immunohistochemical staining of HSP70, Hif1α, MMP2, MMP14, and cortactin expression in oral squamous cell carcinoma (OSCC) and matched paracarcinoma (NM). Scale bar, 50 μm.
Figure 2.
High expression of HSP70, Hif1α, MMP2, MMP14, and cortactin in OSCC. Histoscores of Hsp70 (A), Hif1α (B), cortactin (C), MMP14 (D), and MMP2 (E) in both tumor and normal tissues, and observed significantly higher expression levels of these proteins in OSCC tissues. Means ± SEM. *P < 0.05; ***P < 0.001 versus the NM group; Student’s t-test analysis.
The Correlations Among Hsp70, Hif1α, MMP2, MMP14, and Cortactin in OSCC
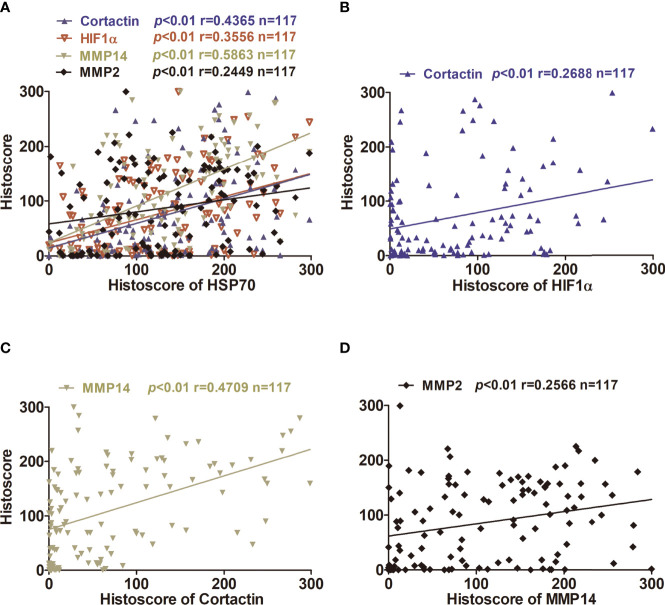
In order to investigate the correlations among the expressions of Hif1α, MMP2, MMP14, cortactin, and Hsp70, we calculated the histoscores of Hsp70, Hif1α, MMP14, MMP2, and cortactin, and then picked every two proteins to analyze their correlations. Our results showed significant positive correlations between the histoscores of Hif1α, cortactin, MMP2, MMP14, and Hsp70. Moreover, the expression of cortactin in OSCC was positively correlated with the expression of Hif1α and MMP14, and the histoscores of MMP14 and MMP2 also showed a positive relationship ( Figures 3A–D ). We also extracted the above genes in OSCC samples from TCGA database and analyzed their correlations. As shown in Figure 4 , the expression of HSPA4 and HIF1A ( Figure 4A ), HIF1A and CTTN ( Figure 4B ), CTTN and MMP4 ( Figure 4C ), as well as MMP14 and MMP2 ( Figure 4D ) also exhibited a significant positive relationship.
Figure 3.
Correlation and regression of HSP70, Hif1α, MMP2, MMP14, and cortactin in OSCC tissues using two-tailed Pearson’s test. (A) A correlation and regression of HSP70, Hif1α, MMP2, MMP14, and cortactin in OSCC tissues using two-tailed Pearson’s test. (B) Correlation between Hif1α and cortactin expression levels in OSCC tissues. (C) Correlation between MMP14 and cortactin expression levels in OSCC tissue. (D) Correlation between MMP2 and MMP14 expression levels in OSCC tissues.
Figure 4.
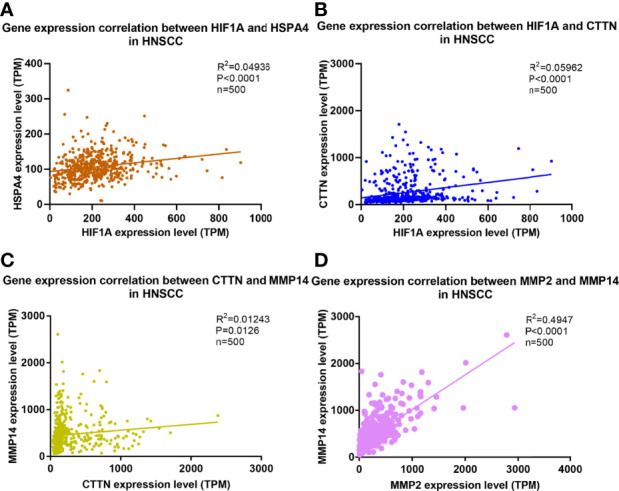
Correlation and regression of HSPA4, HIF-1A, MMP2, MMP14, and CTTN in TCGA database. Correlation and regression of (A) HSPA4, HIF-1A (B) HIF-1A, CTTN (C) CTTN, MMP14 (D) MMP2, and MMP14 in TCGA database.
Inhibiting Hsp70 Suppresses Cell Viability and Proliferation in OSCC Cells
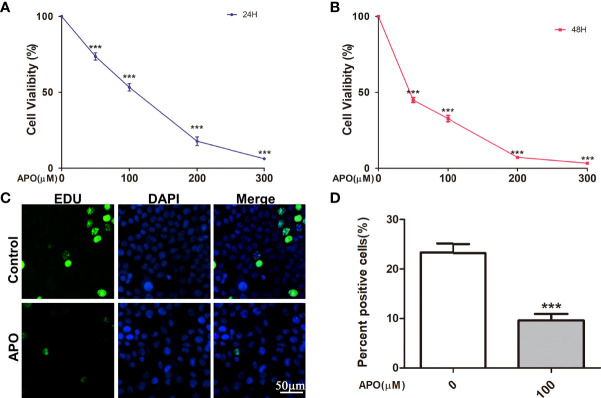
In this study, Cal27 cells were treated with ascending concentrations of Apo for 24 h, and it was displayed in Figure 5A that the OSCC cell viability was reduced dose-dependently as the Apo concentration increased. The suppressive effect of inhibiting Hsp70 on OSCC cells viability was more evident after 48-h treatment ( Figure 5B ). Furthermore, we examined the possible role of inhibiting Hsp70 in cell proliferation using EdU incorporation assays. Compared with control group, Apo treatment group showed decreased EDU positive stained cells significantly ( Figure 5C ). The quantitative data suggested that, at 100 μM, Apo suppressed more than 50% proliferative potentials of OSCC cells ( Figure 5D ). In summary, inhibiting Hsp70 significantly reduced cell viability and inhibited cell proliferation in OSCC cells, exhibiting OSCC cell cytotoxicity.
Figure 5.
APO inhibits OSCC cell proliferation in vitro. Cell Counting Kit (CCK8) assay shows the suppressive effect of APO after 24-h (A) and 48-h (B) treatment by CCK8 assay and 5′-ethynl-2′-deoxyuridine (EdU) assay (C). Means ± SEM; *** P < 0.001; two-way ANOVA analysis. (D) Quantification of EdU assay. Means ± SEM; *** P < 0.001; Student’s t-test analysis; APO, apoptozole.
Inhibiting Hsp70 Prevents Cell Invasion of OSCC Cells
To further investigate the potential role of inhibiting Hsp70 in tumor invasion and metastasis, we employed wound healing assays and utilized Transwell chamber system to determine the effect of inhibiting Hsp70 on cell invasion. As Figure 6A showed, after treating 100 μM Apo for 12 h, Cal27 cells migrated more slowly in contrast to those treated without Apo. The motility of Cal27 cells was significantly inhibited after Apo treatment ( Figure 6B ). To further explore the correlation between ability of OSCC cells invasion and Apo concentration, Transwell chamber system was utilized in Cal27 cells at 0, 50, 100, and 150 μM Apo, respectively. It is revealed that the number of migrated cells significantly decreased as the Apo concentration improving in OSCC cells ( Figures 6C, D ).
Figure 6.
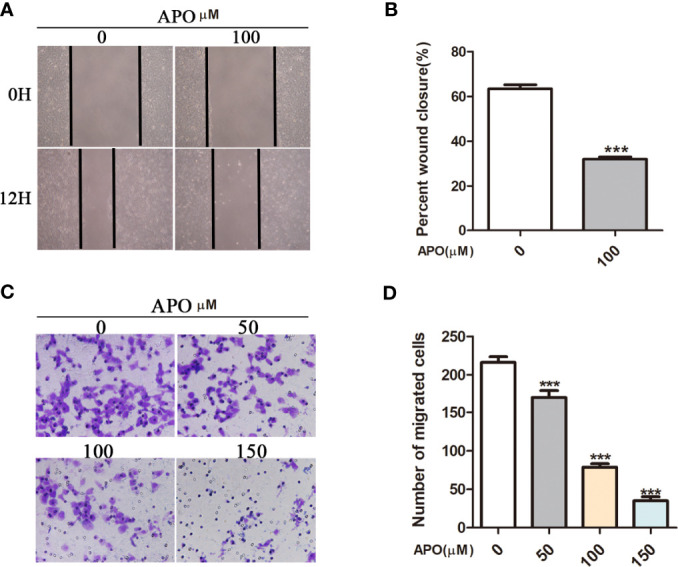
APO inhibits OSCC cell migration and invasion in vitro. Migration assessed by in vitro wound-healing assay (A, B) quantification of EdU assay. Means ± SEM; *** P < 0.001; Student t-test analysis. Invasion assessed and by in vitro Transwell assay (8-μm pore size) (C, D) quantification of EdU assay. Means ± SEM; *** P < 0.001; two-way ANOVA analysis; APO, apoptozole.
Discussion
In previous studies, HSP70, overexpressed in a variety of cancers, can inhibit endogenous and exogenous apoptotic pathways, block oncogene-induced senescence, and lead to treatment resistance (20). HSP70 also mediates the occurrence of tumor-promoting immune microenvironment through TLR4, NF-κB, STAT3, and other signal pathways (20, 21). The HSP70-TLR2 interaction also leads to the activation of neutrophils and the production of pro-inflammatory cytokines in diabetes vascular complications (20). Silencing of Hsp70 enhanced the metastatic properties of the HeLa, A549, and MCF7 cancer cell lines, and Hsp70 (HSP70A1A) inhibited the metastatic ability of cancer cells (22). However, heat shock protein family members such as HSC70 and HSPA2 may play inhibitory roles in cancer cell invasion and metastasis (23, 24). In OSCC, the relationship between HSP70 and tumor metastasis is unclear.
In our study, the Hsp70, Hif1α, cortactin, MMP2, and MMP14 expressions were elevated in OSCC tissue compared with normal counterparts. Cortactin is widely recognized as a marker of invadopodia. The main function of cortactin is to facilitate the interaction of Arp2/3 with F-actin to induce the formation of branched actin thereby promoting cell migration and invasion (25). Moreover, cortactin participates in matrix degradation and MMP2, MMP9, and MT1-MMP secretion in OSCC cells (26). Cortactin phosphorylation being regulated through Src-family kinases, Erk1/Erk2, and PAK is essential for invadopodia formation and ECM degradation (27). The recruitment and activation of proteases such as MMP2 and MMP9 at invadopodia sites could promote the degradation of ECM so as to provide traction for cancer invasion and metastasis (28, 29). In addition, MT1-MMP also contributes to the activation of MMP2 in invadopodia and facilitate the digestion of various ECM macromolecules including collagen, fibronectin, and laminins (30).
To evaluate the relationship between the expression of invadopodia-associated proteins and Hsp70, we examined histoscore of those proteins using two-tailed Pearson’s statistics. The results showed that positive relationships between Hsp70 and Hif1α, cortactin, MMP2, and MMP14 were found in OSCC tissue. Thus, we speculated that Hsp70 overexpression may be correlated with the increased invadopodia formation in OSCC. Bioinformatics on HNSC extracted from TCGA database also showed these positive relationships at the gene transcription level.
The life span of Hif1α could be prolonged owing to the formation of persistent complex between Hsp70 and Hif1α (31). HSP70 maintains Hif1α stability dependent on the activation of phosphatidylinositol 3-kinase (PI3K)/Akt and PI3K inhibitors could downregulate HSP70 expression (32). Hif1α not only increases the levels of invadopodia-forming activity but also stimulates its increased degradative activity (15, 33). Diaz et al. found that hypoxia no longer increases invadopodia formation after Hif1α knockdown in HNSC SCC61 cell lines, and it was believed that stabilized Hif1α under hypoxia activated Notch signaling thus increasing invadopodia formation (34). Moreover, Hif1α was reported to elevate the expression of b-PIX which was identified as a fundamental driver of invadopodia formation so that augments the invasive potential in cancer cells (33). Through a literature review, we prospected that inhibiting Hsp70 may affect the formation of invadopodia through downregulating the expression of Hif1α.
In order to further explore the role of Hsp70 in OSCC cells, we cultured Cal27 with distinct concentrations of Hsp70 inhibitor, Apo. We found that Apo could not only impair the cell viability and proliferation ability of OSCC cells in a dose-dependent manner but also attenuate their migratory ability. Overall, the in vitro experiment suggested that inhibiting Hsp70 may cause cytotoxicity to OSCC cells and interrupt their motility. These results further confirmed that HSP70 was involved in regulating the migration ability of OSCC cells.
It is worth mentioning that this study has some limitations. First, experiments on animals actually are needed to further verify the effect of Apo on OSCC. Last, our study discussed a little about the signaling mechanism of APO inhibiting the expression of Hif1α in OSCC cells, which may be meaningful to explore in the future.
Conclusion
In conclusion, our work shows that HSP70 inhibition exhibits cytotoxic effect on OSCC cells and prevents invasion and metastasis of OSCC cells through decreasing the expression of invadopodia proteins. These results may provide a new therapeutic prospect in OSCC treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Xiangya Hospital, Central South University (Hunan, China). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
L-XD: concept/design, data analysis. W-MW: concept/design. S-SY, JW, JZ: data analysis. TS, W-MW: critical revision, final approval. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (grant no.81702708,81873717,82170973,82070966), Natural Science Foundation of Hunan (grant no. 2018JJ3862), the Science and Technology Innovation Program of Hunan Province (grant no.2021RC3026), Scientific research Project of Hunan Provincial Health Commission(grant no. 202208024658).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.890218/full#supplementary-material
References
- 1. Csősz É., Lábiscsák P, Kalló G, Márkus B, Emri M, Szabó A, et al. Proteomics Investigation of OSCC-Specific Salivary Biomarkers in a Hungarian Population Highlights the Importance of Identification of Population-Tailored Biomarkers. PloS One (2017) 12:e0177282. doi: 10.1371/journal.pone.0177282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao MX, Zhang WL, Yu XH, Wu JS, Qiao XW, Huang MC, et al. Interplay Between Cancer Cells and M2 Macrophages is Necessary for miR-550a-3-5p Down-Regulation-Mediated HPV-Positive OSCC Progression. J Exp Clin Cancer Res (2020) 39:102. doi: 10.1186/s13046-020-01602-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Jin Y, Qin X. Co-Expression Network-Based Identification of Biomarkers Correlated With the Lymph Node Metastasis of Patients With Head and Neck Squamous Cell Carcinoma. Biosci Rep (2020) 40:BSR20194067. doi: 10.1042/BSR20194067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang WM, Yang SS, Shao SH, Nie HQ, Zhang J, Su T. Metformin Downregulates the Expression of Epidermal Growth Factor Receptor Independent of Lowering Blood Glucose in Oral Squamous Cell Carcinoma. Front Endocrinol (Lausanne) (2022) 13:828608. doi: 10.3389/fendo.2022.828608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciocca DR, Calderwood SK. Heat Shock Proteins in Cancer: Diagnostic, Prognostic, Predictive, and Treatment Implications. Cell Stress chaperones (2005) 10:86. doi: 10.1379/CSC-99r.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabai VL, Budagova KR, Sherman MY. Increased Expression of the Major Heat Shock Protein Hsp72 in Human Prostate Carcinoma Cells is Dispensable for Their Viability But Confers Resistance to a Variety of Anticancer Agents. Oncogene (2005) 24:3328–38. doi: 10.1038/sj.onc.1208495 [DOI] [PubMed] [Google Scholar]
- 7. Ko SK, Kim J, Na DC, Park S, Park SH, Hyun JY, et al. A Small Molecule Inhibitor of ATPase Activity of HSP70 Induces Apoptosis and has Antitumor Activities. Chem Biol (2015) 22:391–403. doi: 10.1016/j.chembiol.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 8. Jheng HF, Tsai PJ, Chuang YL, Shen YT, Tai TA, Chen WC, et al. Albumin Stimulates Renal Tubular Inflammation Through an HSP70-TLR4 Axis in Mice With Early Diabetic Nephropathy. Dis Model Mech (2015) 8:1311–21. doi: 10.1242/dmm.019398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kowluru RA, Mohammad G, dos Santos JM, Zhong Q. Abrogation of MMP-9 Gene Protects Against the Development of Retinopathy in Diabetic Mice by Preventing Mitochondrial Damage. Diabetes (2011) 60:3023–33. doi: 10.2337/db11-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dobrowsky RT. Targeting the Diabetic Chaperome to Improve Peripheral Neuropathy. Curr Diabetes Rep (2016) 16:71. doi: 10.1007/s11892-016-0769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Augoff K, Hryniewicz-Jankowska A, Tabola R. Invadopodia: Clearing the Way for Cancer Cell Invasion. Ann Transl Med (2020) 8:902. doi: 10.21037/atm.2020.02.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor Cell Invadopodia: Invasive Protrusions That Orchestrate Metastasis. Trends Cell Biol (2017) 27:595–607. doi: 10.1016/j.tcb.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacob A, Prekeris R. The Regulation of MMP Targeting to Invadopodia During Cancer Metastasis. Front Cell Dev Biol (2015) 3:4. doi: 10.3389/fcell.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parekh A, Weaver AM. Regulation of Invadopodia by Mechanical Signaling. Exp Cell Res (2016) 343:89–95. doi: 10.1016/j.yexcr.2015.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gould CM, Courtneidge SA. Regulation of Invadopodia by the Tumor Microenvironment. Cell Adh Migr (2014) 8:226–35. doi: 10.4161/cam.28346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer StagingManual: Continuing to Build A Bridge from A Population-based to A More "Personalized" Approach to Cancer Staging. CA Cancer JClin (2017). 67:93–9. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 17. Li JJ, Mao XH, Tian T, Wang WM, Su T, Jiang CH, et al. Role of PFKFB3 and CD163 in Oral Squamous Cell Carcinoma Angiogenesis. Curr Med Sci (2019) 39:410–4. doi: 10.1007/s11596-019-2051-1 [DOI] [PubMed] [Google Scholar]
- 18. Wang WM, Zhao ZL, Ma SR, Yu GT, Liu B, Zhang L, et al. Epidermal Growth Factor Receptor Inhibition Reduces Angiogenesis via Hypoxia-Inducible Factor-1alpha and Notch1 in Head Neck Squamous Cell Carcinoma. PloS One (2015) 10:e0119723. doi: 10.1371/journal.pone.0119723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu J, Zhou L, Song Z, Xiong M, Zhang Y, Yang Y, et al. The Identification of New Biomarkers for Bladder Cancer: A Study Based on TCGA and GEO Datasets. J Cell Physiol (2019) 234:15607–18. doi: 10.1002/jcp.28208 [DOI] [PubMed] [Google Scholar]
- 20. Albakova Z, Armeev GA, Kanevskiy LM, Kovalenko EI, Sapozhnikov AM. HSP70 Multi-Functionality in Cancer. Cells (2020) 9:22. doi: 10.3390/cells9030587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park GB, Chung YH, Kim D. Induction of Galectin-1 by TLR-Dependent PI3K Activation Enhances Epithelial-Mesenchymal Transition of Metastatic Ovarian Cancer Cells. Oncol Rep (2017) 37:3137–45. doi: 10.3892/or.2017.5533 [DOI] [PubMed] [Google Scholar]
- 22. Kasioumi P, Vrazeli P, Vezyraki P, Zerikiotis S, Katsouras C, Damalas A, et al. Hsp70 (HSP70A1A) Downregulation Enhances the Metastatic Ability of Cancer Cells. Int J Oncol (2019) 54:821–32. doi: 10.3892/ijo.2018.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang YL, Zhang Y, Li DD, Zhang FL, Liu HY, Liao XH, et al. RNF144A Functions as a Tumor Suppressor in Breast Cancer Through Ubiquitin Ligase Activity-Dependent Regulation of Stability and Oncogenic Functions of HSPA2. Cell Death Differ (2020) 27:1105–18. doi: 10.1038/s41418-019-0400-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun G, Cao Y, Xu Y, Huai D, Chen P, Guo J, et al. Overexpression of Hsc70 Promotes Proliferation, Migration, and Invasion of Human Glioma Cells. J Cell Biochem (2019) 120:10707–14. doi: 10.1002/jcb.28362 [DOI] [PubMed] [Google Scholar]
- 25. Jeannot P, Besson A. Cortactin Function in Invadopodia. Small GTPases (2020) 11:256–70. doi: 10.1080/21541248.2017.1405773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark ES, Whigham AS, Yarbrough WG, Weaver A. Cortactin is an Essential Regulator of Matrix Metalloproteinase Secretion and Extracellular Matrix Degradation in Invadopodia. Cancer Res (2007) 67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928 [DOI] [PubMed] [Google Scholar]
- 27. Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, et al. Multiple Regulatory Inputs Converge on Cortactin to Control Invadopodia Biogenesis and Extracellular Matrix Degradation. J Cell Sci (2008) 121:369–78. doi: 10.1242/jcs.008037 [DOI] [PubMed] [Google Scholar]
- 28. Clark ES, Weaver AM. A New Role for Cortactin in Invadopodia: Regulation of Protease Secretion. Eur J Cell Biol (2008) 87:581–90. doi: 10.1016/j.ejcb.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weaver AM. Invadopodia: Specialized Cell Structures for Cancer Invasion. Clin Exp Metastasis (2006) 23:97–105. doi: 10.1007/s10585-006-9014-1 [DOI] [PubMed] [Google Scholar]
- 30. Seiki M. The Cell Surface: The Stage for Matrix Metalloproteinase Regulation of Migration. Curr Opin Cell Biol (2002) 14:624–32. doi: 10.1016/S0955-0674(02)00363-0 [DOI] [PubMed] [Google Scholar]
- 31. Tikhonova NS, Moskaleva OS, Margulis BA, Guzhova IV. [Molecular Chaperone Hsp70 Protects Neuroblastoma SK-N-SH Cells From Hypoxic Stress]. Tsitologiia (2008) 50:467–72. doi: 10.1134/S1990519X08030036 [DOI] [PubMed] [Google Scholar]
- 32. Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic Preconditioning Reinforces HIF-Alpha-Dependent HSP70 Signaling to Reduce Ischemic Renal Failure-Induced Renal Tubular Apoptosis and Autophagy. Life Sci (2010) 86:115–23. doi: 10.1016/j.lfs.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 33. Md Hashim NF, Nicholas NS, Dart AE, Kiriakidis S, Paleolog E, Wells CM. Hypoxia-Induced Invadopodia Formation: A Role for β-PIX. Open Biol (2013) 3:120159. doi: 10.1098/rsob.120159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch Increases the Shedding of HB-EGF by ADAM12 to Potentiate Invadopodia Formation in Hypoxia. J Cell Biol (2013) 201:279–92. doi: 10.1083/jcb.201209151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.