Abstract
Background
Anecdotal reports of menstrual irregularities after receiving COVID-19 vaccines have been observed in post-authorisation and post-licensure monitoring. We aimed to identify and classify reports of menstrual irregularities and vaginal bleeding after COVID-19 vaccination submitted to a voluntary active surveillance system.
Methods
This observational cohort study included recipients of a COVID-19 vaccine who were aged 18 years and older and reported their health experiences to v-safe, a voluntary smartphone-based active surveillance system for monitoring COVID-19 vaccine safety in the USA, from Dec 14, 2020, to Jan 9, 2022. Responses to survey questions on reactions after vaccination were extracted, and a pre-trained natural language inference model was used to identify and classify free-text comments related to menstruation and vaginal bleeding in response to an open-ended prompt about any symptoms at intervals after vaccination. Related responses were further categorised into themes of timing, severity, perimenopausal and postmenopausal bleeding, resumption of menses, and other responses. We examined associations between symptom theme and respondent characteristics, including vaccine type and dose number received, solicited local and systemic reactions reported, and health care sought.
Findings
63 815 respondents reported on menstrual irregularities or vaginal bleeding, which included 62 679 female respondents (1·0% of 5 975 363 female respondents aged ≥18 years). Common themes identified included timing of menstruation (70 981 [83·6%] responses) and severity of menstrual symptoms (56 890 [67·0%] responses). Other themes included menopausal bleeding (3439 [4·0%] responses) and resumption of menses (2378 [2·8%] responses). Respondents submitting reports related to menopausal bleeding were more likely to seek health care than were those submitting reports related to other menstruation and vaginal bleeding themes.
Interpretation
Reports of heterogeneous symptoms related to menstruation or vaginal bleeding after COVID-19 vaccination are being submitted to v-safe, although this study is unable to characterise the relationship of these symptoms to COVID-19 vaccination. Methods that leverage pretrained models to interpret and classify unsolicited signs and symptoms in free-text reports offer promise in the initial evaluation of unexpected adverse events potentially associated with use of newly authorised or licensed vaccines.
Funding
Centers for Disease Control and Prevention.
Introduction
Three COVID-19 vaccines are being used in the USA. Two are mRNA vaccines—BNT162b2 (tozinameran; Pfizer-BioNTech) and mRNA-1273 (elasomeran; Moderna)—each requiring two doses to complete a primary series. The other is a single-dose adenovirus vector-based vaccine, Ad26.COV2.S (Janssen Pharmaceuticals Companies of Johnson & Johnson [Janssen or Johnson & Johnson]). As of June 12, 2022, more than 221 million people have been fully vaccinated in the USA.1 Common side-effects from COVID-19 vaccination include local reactions, such as injection site pain, and systemic reactions, such as fever and fatigue.2 Serious adverse events after COVID-19 vaccination are rare and include anaphylaxis, thrombosis with thrombocytopenia syndrome after receipt of Ad26.COV2.S,3 and myocarditis after receiving mRNA COVID-19 vaccines.4
There have been reports about menstrual irregularities and vaginal bleeding occurring after COVID-19 vaccination in social and traditional media, the Vaccine Adverse Event Reporting System, vaccine monitoring systems in the UK and Norway, and online surveys.5, 6, 7, 8, 9 Symptoms related to menstruation and vaginal bleeding were not solicited adverse events in clinical trials of COVID-19 vaccines, and they were not solicited as prespecified symptoms in v-safe, a voluntary active surveillance system monitoring health status after COVID-19 vaccination in the USA.10 In August, 2021, the National Institutes of Health announced funding of research studies exploring potential links between COVID-19 vaccination and menstruation.11 One study funded through this initiative among users of a fertility tracking application reported that a less than 1-day increase in menstrual cycle length was associated with COVID-19 vaccination;12 however, this study was limited to individuals with consistent normal cycle (24–38 days) lengths at baseline. We aimed to characterise and classify free-text reports of menstrual irregularities and vaginal bleeding to v-safe in response to open-ended prompts about symptoms.
Research in context.
Evidence before this study
We searched PubMed and Google from database inception to June 12, 2022, using the search terms “menstr*”, “COVID-19”, and “vaccine”, without language restrictions. There have been reports of menstrual irregularities and vaginal bleeding after receiving COVID-19 vaccines in social media and traditional media, the United States Vaccine Adverse Event Reporting System, monitoring systems in the UK, Norway, and other countries, and online surveys. A study among users of a fertility tracking application suggests COVID-19 vaccination can increase menstrual cycle length by less than 1 day. The prevalence of these events and their characteristics remain poorly understood.
Added value of this study
In this observational cohort study, we identified free-text reports related to menstruation and vaginal bleeding submitted by 63 815 individuals (of whom 62 679 [98·2%] were female) to an active vaccine safety monitoring system, v-safe. Symptoms related to menstrual timing or severity were most common. People reporting symptoms related to menopausal bleeding were more likely to seek health care. To our knowledge, this study is the first to specifically assess menstrual irregularities and vaginal bleeding after COVID-19 vaccination from a large US vaccine safety monitoring system. Methods that interpret and classify text can help to evaluate symptoms not specifically solicited in vaccine safety surveillance systems.
Implications of all the available evidence
Menstrual irregularities and vaginal bleeding after COVID-19 vaccination are being reported, although this study is unable to assess whether these events are caused by COVID-19 vaccination. Text analytic methods can speed up the identification of potential vaccine reactions that are not prespecified in structured data collection. Further studies are needed to better understand their prevalence and pathophysiology.
Methods
V-safe
V-safe is an active surveillance system for monitoring COVID-19 vaccination safety administered by the US Centers for Disease Control and Prevention (CDC). Participation in this active surveillance system is open to all people in the USA and its territories who receive a COVID-19 vaccination and who can access v-safe using a smartphone. Respondents self-enrol and receive text-message reminders to complete surveys at specified intervals after vaccination.10 For days 0–7 after each dose, the survey asks about local and systemic reactions and allows submission of an unstructured text response to the following prompt: “any other symptoms or health conditions you want to report”. For weeks 2–6 and months 3, 6, and 12 after the dose, the survey allows an unstructured text response to the following prompt: “since your/their last check-in, have you/they experienced any new or worsening symptoms or health conditions? (If yes) Please describe.” When respondents log another dose in their account, survey timing restarts.
Data analysis
We examined reports to v-safe from Dec 14, 2020, to Jan 9, 2022, among adults aged 18 years and older. To identify text responses potentially related to menstrual irregularities or vaginal bleeding, we filtered all 6 168 832 free-text responses to select those containing the word fragments (“menses”, “menst”, “spotting”, “period”, “cycle”, “miscarr”, “menorrh”, or “metrorrh”) or (“bleed” or “blood” and “menop”, “uter”, “vag”, “breakthrough”, “break through”, “endomet”, “gest”, “term”, or “trimester”; appendix p 1). Although the search terms were intended for English text, we did not restrict the responses by language. We evaluated the performance of this search filter by reviewing 2000 random responses manually and identifying those related to menstruation or vaginal bleeding. We applied zero-shot text classification to these filtered responses with the Bidirectional and Auto-Regressive Transformers (known as BART)13 model for natural language inference, pretrained for sentence pair classification on the Multi-Genre Natural Language Inference14 corpus. The Multi-Genre Natural Language Inference contains more than 432 000 labelled sentence pairs and sources a wide range of text not specific to symptom reporting, including transcribed face-to-face and phone conversations, government reports, magazine articles, and contemporary fiction. Zero-shot classification, in which data can be sorted into classes that were not observed in the model's training data, allows for classification of a large amount of text data that would be slow to evaluate and annotate manually.15 This method has advantages over strict keyword searches because it identifies text responses that are closely related semantically, even if different words are used to describe the same concept. We proposed a hypothesis statement, and the BART model assigned a probability score, or entailment probability, that the statement describes the observed text.13, 14, 15 In the analysis, we included responses that had an entailment probability of 0·9 or higher with at least one of the following core hypothesis statements: “this mentions menstruation”, “this mentions spotting”, “this mentions vaginal bleeding”, “this mentions uterine bleeding”, and “this specifically mentions missing or skipping a period” (appendix p 1). We evaluated the performance of this step by manually reviewing 1000 random responses that matched the initial search filter for relevance to menstruation or vaginal bleeding.
We used zero-shot classification to further classify responses into the following themes, which are categories based on entailment probability with the theme's hypothesis statements (appendix p 2): menstrual timing, menstrual severity, and resumption of menses after a period of no menses. We defined an additional theme related to perimenopausal and postmenopausal bleeding, henceforth referred to as menopausal bleeding, comprising responses that contained the string “menop” or were from a respondent aged 59 years or older. This age threshold was chosen to be much higher than existing estimates of the median age of menopause (approximately 51 years)16 so that all formerly menstruating respondents older than the age threshold would be very likely to be postmenopausal. Responses could match multiple themes. Responses that did not match any theme were classified into the category other (appendix p 2). We evaluated performance of zero-shot classification into themes of timing, severity, resumption of menses, and other responses by manually labelling responses from the random sample that matched the initial search filter (n=1000) and that were manually classified as related to menstruation. We calculated sensitivity and specificity with 95% CIs (Wilson score intervals with continuity correction) and F1 scores with bias-corrected and accelerated bootstrapped 95% CIs.
We tested for differences in respondent characteristics by response theme by analysing themes as multiple response categorical variables using the method described by Bilder and colleagues.17 Respondent characteristics included the vaccine received, dose number, ever reporting a solicited injection site or systemic reaction, and seeking health care for the symptoms reported on the survey. Analyses of solicited injection site and systemic reactions were limited to recipients of BNT162b2 and mRNA-1273 vaccines for comparability with other studies2 and because these vaccines are more similar in their dosing regimen and mechanism of action. We calculated a Pearson's χ2 statistic for independence between each respondent characteristic and theme. We reported global bootstrap p values using 4999 pseudosamples with the same number of respondents as the original sample generated with replacement. For analysis of the vaccine received, respondents with other or unknown vaccine type were excluded from statistical testing. To examine timing of v-safe reports relative to national vaccine administration, we compared month of v-safe reports related to menstruation with the number of vaccine doses administered in the USA.18
Python (version 3.7.8) and the transformers package were used for zero-shot classification. R (version 3.6.3) was used for descriptive statistics, multiple response categorical variables analysis,19 data visualisation,20 and bootstrap CIs.21 Activities were reviewed by CDC and were conducted consistent with applicable federal law and CDC policy.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
63 815 respondents reported on menstrual irregularities or vaginal bleeding, which included 62 679 female respondents (1·0% of 5 975 363 female respondents aged ≥18 years). The median age was 37 years (IQR 30–44, range 18–94; table 1 ). The largest number of reports were submitted in April–May, 2021; for comparison, the months with the most vaccine doses administered were March–April, 2021 (appendix p 3). Among the 63 815 respondents who reported menstrual irregularities or vaginal bleeding, most respondents received BNT162b2 (33 149 [51·9%] respondents) or mRNA-1273 (26 741 [41·9%] respondents) vaccines. 57 997 respondents who submitted responses related to menstruation or vaginal bleeding were aged 18–49 years, which included 57 046 female respondents (1·8% of 3 095 361 females aged 18–49 years). 511 respondents who submitted responses related to menstruation or vaginal bleeding were aged 65 years and older, which included 434 female respondents (<1% of 1 181 482 female individuals aged 65 years and older). 49 795 (78·0%) respondents reporting menstrual irregularities or vaginal bleeding were White, 4866 (7·6%) were Black, and 4929 (7·7%) were Asian; 8116 (12·7%) identified as Hispanic or Latino.
Table 1.
Characteristics of v-safe respondents with reports related to menstruation or vaginal bleeding compared with total female v-safe respondents
| Respondents reporting menstrual irregularities or vaginal bleeding (n=63 815) | Total female v-safe respondents aged ≥18 years*(n=5 975 363) | ||
|---|---|---|---|
| Sex | |||
| Female | 62 679 (98·2%) | 5 975 363 (100·0%) | |
| Male† | 368 (0·6%) | .. | |
| Other | 206 (0·3%) | .. | |
| Unknown | 562 (0·9%) | .. | |
| Age group | |||
| 18–49 years | 57 997 (90·9%) | 3 095 361 (51·8%) | |
| 50–64 years | 5307 (8·3%) | 1 698 520 (28·4%) | |
| 65–74 years | 422 (0·7%) | 887 481 (14·9%) | |
| 75–84 years | 82 (0·1%) | 249 567 (4·2%) | |
| 85+ years | 7 (<0·1%) | 44 434 (0·7%) | |
| Race | |||
| White | 49 795 (78·0%) | 4 078 154 (68·2%) | |
| Black | 4866 (7·6%) | 442 955 (7·4%) | |
| Asian | 4929 (7·7%) | 412 249 (6·9%) | |
| American Indian or Alaska Native | 1230 (1·9%) | 67 631 (1·1%) | |
| Native Hawaiian or other Pacific Islander | 440 (0·7%) | 32 988 (0·6%) | |
| Other | 2948 (4·6%) | 218 213 (3·7%) | |
| Missing | 2685 (4·2%) | 849 895 (14·2%) | |
| Ethnicity | |||
| Hispanic or Latino | 8116 (12·7%) | 619 917 (10·3%) | |
| Not Hispanic or Latino | 52 850 (82·8%) | 4 421 916 (74·0%) | |
| Missing | 2851 (4·5%) | 933 538 (15·6%) | |
| Vaccine received‡ | |||
| BNT162b2 | 33 149 (51·9%) | 2 975 438 (49·8%) | |
| mRNA-1273 | 26 741 (41·9%) | 2 794 014 (46·8%) | |
| Ad26.COV2.S | 4072 (6·4%) | 285 775 (4·8%) | |
| Other/unknown | 3 (<0·1%) | 1662 (<0·1%) | |
| Reported dose§ | |||
| 1 | 54 180 (84·9%) | 4 935 274 (82·6%) | |
| 2 | 55 428 (86·9%) | 4 221 380 (70·6%) | |
| ≥3 | 11 366 (17·8%) | 745 158 (12·5%) | |
| Submitted survey§ | |||
| Dose 1 | |||
| 0–7 days | 51 828 (81·2%) | 4 725 436 (79·1%) | |
| 14 days | 46 934 (73·5%) | 3 766 825 (63·0%) | |
| 21 days | 28 079 (44·0%) | 2 292 256 (38·4%) | |
| 28 days | 8222 (12·9%) | 607 862 (10·2%) | |
| 35 days | 4594 (7·2%) | 216 797 (3·6%) | |
| 42 days | 4435 (6·9%) | 174 458 (2·9%) | |
| 3 months | 3994 (6·3%) | 358 875 (6·0%) | |
| 6 months | 3575 (5·6%) | 468 299 (7·8%) | |
| 12 months | 321 (0·5%) | 57 155 (1·0%) | |
| Dose 2 | |||
| 0–7 days | 52 752 (82·7%) | 3 920 283 (65·6%) | |
| 14 days | 46 192 (72·4%) | 3 262 994 (54·6%) | |
| 21 days | 44 570 (69·8%) | 3 113 097 (52·1%) | |
| 28 days | 42 958 (67·3%) | 2 982 212 (49·9%) | |
| 35 days | 42 625 (66·8%) | 2 913 033 (48·8%) | |
| 42 days | 41 545 (65·1%) | 2 864 136 (47·9%) | |
| 3 months | 41 537 (65·1%) | 2 827 283 (47·3%) | |
| 6 months | 38 616 (60·5%) | 2 783 393 (46·6%) | |
| 12 months | 469 (0·7%) | 50 053 (0·8%) | |
Data are n (%).
Includes respondents who did and did not report menstrual irregularities or vaginal bleeding.
Includes 192 individuals with responses that were unrelated or not definitively related to menstruation (eg, “I suffer from cluster headache and I am in the middle of a cycle”), ten individuals reporting symptoms experienced by someone else, and four individuals who specifically mention being transgender in the response.
150 respondents with responses related to menstruation or vaginal bleeding and 81 465 female v-safe respondents received vaccine doses from more than one manufacturer.
Includes v-safe entries that do not mention menstruation, vaginal bleeding, or other free-text symptoms.
Responses related to menstruation or vaginal bleeding had a median word count of 8 (IQR 4–17). Manual verification of a random sample of responses showed high performance of the search strings filter (100% sensitivity, 99·9% specificity) and subsequent zero-shot classification for identifying menstruation-related or vaginal bleeding-related responses (98·3% sensitivity, 98·6% specificity; appendix p 4). Among menstruation-related or vaginal bleeding-related responses, sensitivity was higher for classification into timing (95·8% sensitivity, 60·1% specificity) and severity (91·2% sensitivity, 76·2% specificity) themes than for the theme of resuming menses (70·8% sensitivity, 99·3% specificity).
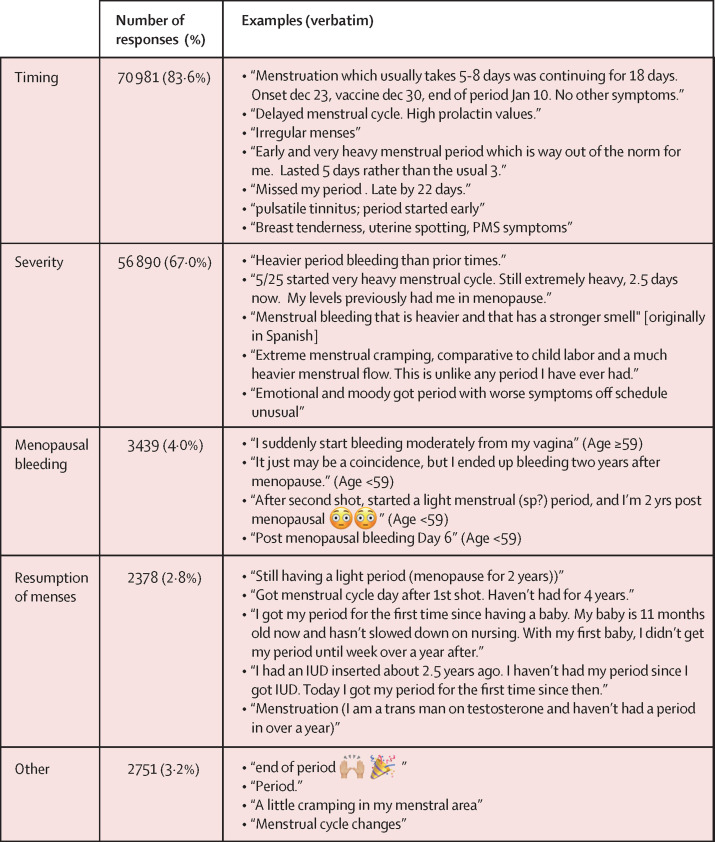
Among 84 943 responses related to menstruation or vaginal bleeding, the most common theme was timing of menses (70 981 [83·6%] responses; figure 1 ). This theme included reports of menstruation starting earlier or later than expected, missed menstrual cycles, and intermenstrual spotting (figure 1). Severity of menstrual symptoms, such as heavy flow, more pain than usual, prolonged bleeding, or subjective descriptors, such as “worse symptoms”, was a theme for 56 890 (67·0%) responses. 45 942 (54·1%) responses met criteria for both timing and severity themes (eg, “early and very heavy menstrual period”). 3439 (4·0%) responses were related to menopausal bleeding and 2378 (2·8%) were related to resumption of menses after a period of no menses; 608 (0·7%) responses overlapped between these themes. Responses related to resumption of menses included those from people whose menstrual cycles were previously suppressed due to medication (eg, hormonal contraception or gender-affirming hormone therapy) or breastfeeding.
Figure 1.
Examples of v-safe survey responses related to menstruation or vaginal bleeding, by theme (N=84 943 responses)
The median age of those reporting symptoms related to menopause (54 years [IQR 50–62]) or resumption of menses (47 years [37–53]) was higher than for timing (37 years [30–43]), severity (37 years [31–44]), and other (37 years [30–44]). Type of vaccine received differed by theme (table 2 ; p=0·016). Among 2912 respondents with menopause-related reports, more than would be expected if vaccine type were independent of them (1301 [44·7%] respondents) received mRNA-1273 and fewer than expected (1425 [48·9%] respondents) received BNT162b2, compared with the number of respondents receiving these vaccines who reported on other themes (ie, timing, severity, resumption of menses, or other) related to menstruation or vaginal bleeding. This distribution is more similar to the vaccine type distribution shown for all female v-safe respondents aged 50–64 years (n=1 698 520): 796 965 (46·9%) mRNA-1273 and 831 395 (48·9%) BNT162b2 (appendix p 5). More respondents reported symptoms related to menstrual irregularities or vaginal bleeding within 21 days after dose 2 (49·0–55·0% of responses across themes) compared with dose 1 (39·2–44·9% of responses across themes) and dose 3 (5·3–8·0% of responses across themes). Among recipients of mRNA-1273 and BNT162b2 vaccines, those reporting menopause-related symptoms were less likely to report local (2374/2726 [87·1%]) and systemic (2390/2726 [87·7%]) reactions than were respondents reporting more common timing-related (local: 46 443/51 259 [90·6%]; systemic: 46 659/51 259 [91·0%]) or severity-related (local: 38 145/41 906 [91·0%]; systemic: 38 444/41 906 [91·7%]) symptoms (p=0·00020; table 2). The proportion of respondents who sought health care was higher among those with reports related to menopausal bleeding (37·4%) than for other themes (13·9–17·7% of responses across remaining themes; p=0·00020).
Table 2.
Characteristics of v-safe respondents submitting free-text responses related to menstrual irregularities or vaginal bleeding, by response theme*
| Timing | Severity | Menopausal | Resumption of menses | Other | Total | p value† | χ2 statistic | ||
|---|---|---|---|---|---|---|---|---|---|
| Number of unique respondents | 54 671 | 44 785 | 2912 | 2181 | 2625 | 63 815 | .. | .. | |
| Median age, years (IQR) | 37 (30–43) | 37 (31–44) | 54 (50–62) | 47 (37–53) | 37 (31–43) | 37 (30–44) | .. | .. | |
| Number of responses submitted | 70 981 | 56 890 | 3439 | 2378 | 2751 | 84 943 | .. | .. | |
| Vaccine received‡ | .. | .. | .. | .. | .. | .. | 0·016 | 19·11 | |
| BNT162b2 | 28 408 (52·0%) | 23 208 (51·8%) | 1425 (48·9%) | 1106 (50·7%) | 1399 (53·3%) | 33 149 (51·9%) | .. | .. | |
| mRNA-1273 | 22 937 (42·0%) | 18 757 (41·9%) | 1301 (44·7%) | 926 (42·5%) | 1056 (40·2%) | 26 741 (48·9%) | .. | .. | |
| Ad26.COV2.S | 3449 (6·3%) | 2905 (6·5%) | 186 (6·4%) | 151 (6·9%) | 170 (6·5%) | 4072 (6·4%) | .. | .. | |
| Other/unknown | 3 (<0·1%) | 3 (<0·1%) | 1 (<0·1%) | 0 | 0 | 3 (<0·1%) | .. | .. | |
| Respondents submitting responses ≤21 days after a dose | n=32 813 | n=27 363 | n=1324 | n=1234 | n=1397 | n=37 458 | .. | .. | |
| Dose 1 | 13 963 (42·6%) | 11 811 (43·2%) | 519 (39·2%) | 503 (40·8%) | 627 (44·9%) | 16 168 (43·2%) | 0·00020 | 31·41 | |
| Dose 2 | 17 835 (54·4%) | 14 700 (53·7%) | 711 (53·7%) | 679 (55·0%) | 685 (49·0%) | 20 383 (54·4%) | 0·045 | 10·09 | |
| Dose 3 | 2637 (8·0%) | 2184 (8·0%) | 126 (9·5%) | 66 (5·3%) | 93 (6·7%) | 2996 (8·0%) | 0·0024 | 16·96 | |
| Ever reported any solicited injection site reaction§¶ | 46 443/51 259 (90·6%) | 38 145/41 906 (91·0%) | 2374/2726 (87·1%) | 1797/2030 (88·5%) | 2224/2455 (90·6%) | 54 193/59 790 (90·6%) | 0·00020 | 78·69 | |
| Ever reported any solicited systemic reaction§‖ | 46 659/51 259 (91·0%) | 38 444/41 906 (91·7%) | 2390/2726 (87·7%) | 1811/2030 (89·2%) | 2272/2455 (92·5%) | 54 477/59 790 (91·1%) | 0·00020 | 122·10 | |
| Reported seeking health care on the same survey | 9062 (16·6%) | 6920 (15·5%) | 1090 (37·4%) | 386 (17·7%) | 366 (13·9%) | 10 711 (16·8%) | 0·00020 | 1109·42 | |
Data are n, median (IQR), n (%), and n/N (%) unless specified otherwise.
Proportions reported out of the number of respondents.
Bootstrap p values test independence between each respondent characteristic and theme under models for multiple response categorical variables (MRCV).
Respondents reporting multiple doses might have received more than one vaccine type.
Among recipients of BNT162b2 or mRNA-1273 vaccines.
Injection site reactions include pain, redness, swelling, or itching reported ≤7 days after a dose.
Systemic reactions include fatigue, headache, myalgia, chills, fever, joint pain, nausea, vomiting, diarrhoea, abdominal pain, or rash outside of injection site reported ≤7 days after a dose.
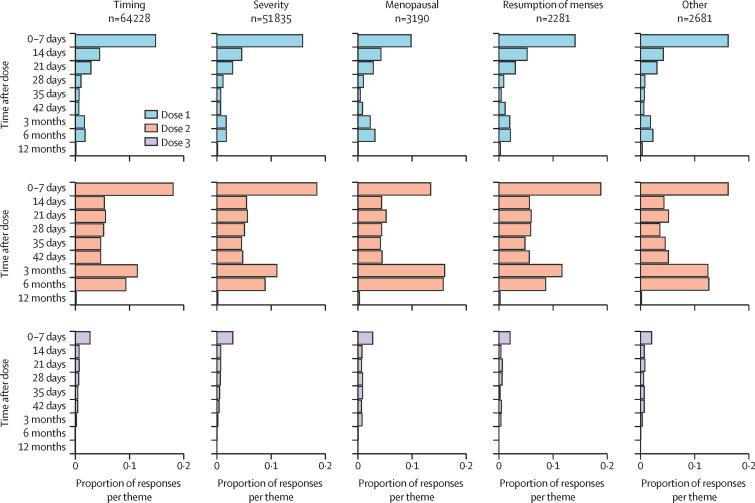
The distribution of responses by survey intervals was similar across all themes, with more responses occurring in the 7 days after each dose and at the 3-month and 6-month surveys after dose 2 (figure 2 ). 18 913 female respondents submitted responses related to menstruation or vaginal bleeding at 3-month surveys or later after any dose number, comprising 0·5% of 3 875 724 female individuals who responded to those surveys. Among these respondents, 15 162 (80·1%) reported symptoms related to timing, 11 760 (62·1%) to severity, 1147 (6·1%) to menopausal bleeding, 555 (2·9%) to resumption of menses, and 785 (4·2%) to other symptoms.
Figure 2.
Timing of v-safe responses related to menstruation irregularities or vaginal bleeding after COVID-19 vaccination, by theme and dose number
Multiple reports of a single event were deduplicated within each time interval.
Discussion
We developed a process to identify free-text reports related to menstruation or vaginal bleeding after COVID-19 vaccination and reported such events in 1·0% of female v-safe respondents. These symptoms were not specifically solicited in any of the v-safe surveys. Rather, based on responses to open-ended prompts about symptoms collected through informal surveys, the possibility of menstruation-related symptoms after vaccination was evaluated with natural language processing methods. Several types of menstrual symptoms were reported, with most being related to disruptions in menstrual timing (83·6%) or increased severity of menstrual symptoms (67·0%), such as bleeding or pain. Some respondents also described symptoms related to perimenopausal and postmenopausal bleeding (4·0%), and resumption of menses after a long period of no menses (2·8%).
A link between COVID-19 vaccination and menstruation is biologically plausible. Although one retrospective study (published as a preprint) examining menstrual cycle records did not detect a link between COVID-19 vaccination and menstrual changes,5 a study using prospectively tracked menstrual cycle data showed that COVID-19 vaccination is associated with a less than 1-day increase in menstrual cycle length.12 The process of menstruation, in which endometrial tissue proliferates and breaks down in the absence of egg fertilisation and implantation, is regulated by complex interactions between hormones, inflammation pathways, tissues, and cells.22, 23 Post-vaccine systemic inflammatory reactions2 could disrupt menstruation and trigger breakthrough bleeding. In this study, local and systemic reactions occurred more often among respondents with menstrual timing-related or severity-related symptoms compared with symptoms related to menopause or resumption of menses, but the implications of this finding are unclear. Manual review of v-safe responses suggested some reports of breast tenderness, mood changes, and tinnitus; these symptoms could be related to hormonal changes, which could disrupt normal menstruation, menopause, or pre-existing amenorrhea secondary to medications or other medical conditions.24 Menstrual cycle prolongation has been associated with receiving two COVID-19 vaccine doses within the same cycle,12 supporting the hypothesis that immune response to vaccination can affect the hypothalamic–pituitary–ovarian axis. Temporary menstrual irregularities have been reported after SARS-CoV-2 infection.25, 26 Such irregularities after natural infection could imply suppression of ovarian function in response to the stress of an acute infection or a possible direct effect of the virus. Similar pathophysiology might underlie the occurrence of menstrual irregularities after COVID-19 vaccination. It is possible that menstruation-related reports to v-safe represent multiple overlapping effects, such as those causing immediate symptoms and those that are not recognised or reported until after one to two menstrual cycles. Studies are ongoing that further explore the pathophysiology and epidemiology of menstrual irregularities after COVID-19 vaccination.11
Although a link between COVID-19 vaccination and menstrual irregularities is plausible, menstrual irregularities are also common in the absence of COVID-19 infection or vaccination. In studies conducted in Korea, Japan, and Ethiopia before the COVID-19 pandemic that specifically solicited responses about menstruation among women of reproductive age, the prevalence of menstrual irregularities was estimated to be between 14% and 33%.27, 28, 29 The prevalence of postmenopausal bleeding among postmenopausal Danish women has been estimated to be 11%.30 Stress, anxiety, and uncharacterised factors related to the COVID-19 pandemic might increase incidence of menstrual irregularities.27, 31 A 2020 study in Türkiye of female health-care workers reported a prevalence of menstrual irregularities of 28·7%.31 A survey in the USA conducted via a fertility mobile application between March, 2020, and April, 2021, reported that 36% of participants had menstrual changes, including irregular timing, heavier bleeding, and associated symptoms.32 Unlike these studies, our study was not designed to assess prevalence of menstrual irregularities and did not specifically ask participants about menstruation. However, the proportion of v-safe participants who voluntarily reported menstrual or vaginal bleeding symptoms was similar to findings of a survey among BNT162b2 recipients in Saudi Arabia, for which 0·6% of respondents described menstrual irregularities after COVID-19 vaccination in response to an open-ended question about post-vaccine symptoms.33
The clinical and long-term significance of the heterogeneous symptoms related to menstruation and vaginal bleeding after COVID-19 vaccination described in v-safe responses is unclear. V-safe was designed to collect data for health effects after vaccination, not to determine the duration of reported symptoms; however, reports related to menstruation or vaginal bleeding at more than 3 months after the last dose, at which point any potential effect of vaccination on a single menstrual cycle might have resolved, were rare. Although temporary disruption of menstruation could theoretically affect conception planning, all available evidence suggests that COVID-19 vaccines do not affect fertility,34, 35 and vaccination is recommended for pregnant people and people desiring conception.36 Perimenopausal and postmenopausal bleeding after COVID-19 vaccination is occurring; however, it is unknown whether this bleeding represents a transient benign event, an event that unmasks pre-existing pathology (eg, cancer or polyps), or a purely coincidental event. The American College of Obstetricians and Gynecologists currently recommends that people with postmenopausal bleeding have a diagnostic evaluation for endometrial carcinoma.37 Better understanding of these events is needed to guide clinical follow-up of postmenopausal bleeding when it occurs transiently after COVID-19 vaccination.
In clinical trials and active surveillance, vaccine recipients are asked to report the presence or absence of prespecified symptoms in a routine and structured way. Specifically soliciting menstrual symptoms in clinical trials and surveillance of COVID-19 vaccines would be useful for assessing these outcomes more accurately and completely. For symptoms that are not prespecified, the earliest descriptions are often in the form of free text, such as from social media or traditional media reports, clinical notes, or comment fields of data collection tools. Methods for analysing large amounts of text data can identify important symptom clusters that might represent previously unrecognised adverse events. Zero-shot classification, which leverages pre-trained language models, can interpret and classify free-text descriptions without first requiring humans to manually annotate a large dataset to train a machine learning algorithm.15 Although these methods might not perform as precisely as human reviewers with clinical expertise, and their performance varies based on the specific classification task, they allow large amounts of text data to be analysed consistently and rapidly and, therefore, are useful for hypothesis generation.
This study has important limitations. V-safe text fields were designed to collect information on symptoms that respondents had after COVID-19 vaccination, not to determine whether these symptoms, including menstrual irregularities, were associated causally with vaccine receipt. This study cannot determine whether these groups of outcomes reported after vaccination occurred at a rate that exceeds population baseline incidence. Furthermore, coverage of these symptoms in social media and traditional media during the survey period might have prompted respondents to notice and report their own symptoms in v-safe responses, possibly similar to previous instances of stimulated reporting of symptoms after vaccination.38 In this study, most menstruation-related reports occurred in late spring of 2021, which corresponds to a period of more intense media attention around menstruation-related reports and an increase in vaccine doses administered nationally in the USA. Enrolment in v-safe is voluntary, and findings from the enrolled respondents might not be generalisable to all recipients of the COVID-19 vaccine. V-safe requires participants to respond to surveys using a smartphone; older adults and those with lower incomes or less formal education are less likely to own smartphones and might be under-represented.39 This analysis is limited to adults aged 18 years and older and does not examine menstruation-related symptoms in younger age groups. The prevalence of menstrual symptoms and vaginal bleeding after COVID-19 vaccination might be underestimated, as these symptoms were not solicited reactions. Surveys done 14 days or later after the last dose that ask about new or worsening symptoms might miss stable or waning symptoms reported in an earlier survey. The data in this analysis are self-reported. V-safe respondents might not recognise disruptions to menstrual cycles until months after vaccination, and therefore they might not consider reporting them to v-safe. The search string filters might miss responses for which a key search string is misspelled by the respondent. The language model can misclassify responses, particularly when the language is ambiguous or complex. Responses were grouped into semantic themes, which might not align with clinical categorisation schema. Although some non-English responses were reported in this analysis, the language model is trained primarily on English text, and the search string filters were designed to detect English responses.14
Symptoms related to menstruation or vaginal bleeding after receiving a COVID-19 vaccine are being reported to v-safe in response to open-ended prompts about new symptoms after vaccination. Commonly reported symptoms were related to cycle timing, severity of menstrual symptoms, and postmenopausal bleeding. Vaccine safety monitoring through systems like v-safe and the Vaccine Adverse Event Reporting System can help to identify and characterise symptoms reported after vaccination; however, questions remain about the prevalence of these symptoms after vaccination and how they compare with baseline rates, what physiological changes post-vaccination might underlie potential menstrual cycle disruption, and what the clinical significance is of post-vaccination menstrual irregularities and vaginal bleeding. Ongoing studies,11 including prospective cohort studies that can solicit and describe menstrual symptoms after vaccination, long-term follow-up studies that can characterise the clinical implications of these symptoms, and physiological studies that can explore potential mechanisms underlying menstrual symptoms, can better explore these questions.
Data sharing
Individual participant text responses in v-safe are not available to others because they might contain personally identifying information. Code for data pre-processing, filtering by search strings, and zero-shot classification is available at the code repository: https://github.com/cdcai/zsl-vaccine.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Centers for Disease Control and Prevention. We thank v-safe participants for contributing safety data, Isaac McCullum and Bicheng (Tony) Zhang for their work on data management, and Tom Shimabukuro for critical review of the manuscript. The findings and conclusions in this Article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributors
KKW, CMH, AH, TRM, and DKS contributed to the study concept and design. KKW drafted the manuscript. KKW and CMH contributed to the statistical analysis and data visualisation. DKS, AH, and TRM supervised the conduct of the study. KKW and CMH verified the raw data. All authors contributed to the acquisition, analysis, or interpretation of data; provided administrative, technical, or material support; critically revised the manuscript for important intellectual content; and had access to the data and were responsible for the decision to submit the manuscript.
Supplementary Material
References
- 1.Centers for Disease Control and Prevention COVID-19 vaccinations in the United States. 2022. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- 2.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 3.MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Von Woon E, Male V. Effect of COVID-19 vaccination on menstrual periods in a prospectively recruited cohort. medRxiv. 2022 doi: 10.1101/2022.03.30.22273165. published online March 30. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medicines and Healthcare products Regulatory Agency Coronavirus vaccine—weekly summary of yellow card reporting. 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#annex-1-vaccine-analysis-print
- 7.Trogstad L. Increased occurrence of menstrual disturbances in 18- to 30-year-old women after COVID-19 vaccination. SSRN Electron J. 2022 doi: 10.2139/ssrn.3998180. published online January 14. (preprint). [DOI] [Google Scholar]
- 8.Lee KMN, Junkins EJ, Luo C, Fatima UA, Cox ML, Clancy KBH. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination. Sci Adv. 2022 doi: 10.1126/sciadv.abm7201. published online July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Health and Human Services. Public Health Service. Food and Drug Administration. Centers for Disease Control Vaccine Adverse Event Reporting System (VAERS) 2022. https://wonder.cdc.govvaers.html
- 10.Centers for Disease Control and Prevention V-safe active surveillance for COVID-19 vaccine safety: version 3. https://www.cdc.gov/vaccinesafety/pdf/V-safe-Protocol-508.pdf
- 11.National Institutes of Health Item of Interest: NIH funds studies to assess potential effects of COVID-19 vaccination on menstruation. 2021. https://www.nichd.nih.gov/newsroom/news/083021-COVID-19-vaccination-menstruation
- 12.Edelman A, Boniface ER, Benhar E, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a U.S. cohort. Obstet Gynecol. 2022;139:481–489. doi: 10.1097/AOG.0000000000004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis M, Liu Y, Goyal N, et al. BART: denoising sequence-to-sequence pre-training for natural language generation, translation, and comprehension. 2020. https://aclanthology.org/2020.acl-main.703.pdf
- 14.Williams A, Nangia N, Bowman SR. A broad-coverage challenge corpus for sentence understanding through inference. 2018. https://aclanthology.org/N18-1101.pdf
- 15.Yin W, Hay J, Roth D. Benchmarking zero-shot text classification: datasets, evaluation and entailment approach. 2019. https://aclanthology.org/D19-1404/
- 16.McKinlay SM. The normal menopause transition: an overview. Maturitas. 1996;23:137–145. doi: 10.1016/0378-5122(95)00985-x. [DOI] [PubMed] [Google Scholar]
- 17.Bilder CR, Loughin TM, Nettleton D. Multiple marginal independence testing for pick any/c variables. Commun Stat Theory Methods. 2000;29:1285–1316. [Google Scholar]
- 18.Centers for Disease Control and Prevention COVID data tracker: trends in number of COVID-19 vaccinations in the US. 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends
- 19.Koziol N, Bilder C. MRCV: methods for analyzing multiple response categorical variables (MRCVs) 2014. https://rdrr.io/cran/MRCV/f/inst/doc/MRCV-vignette.pdf
- 20.Venables WN, Ripley BD. 4th edn. Springer; New York, NY: 2002. Modern applied statistics with S. [Google Scholar]
- 21.Efron B, Narasimhan B. bcaboot: bias corrected bootstrap confidence intervals. 2021. https://cran.r-project.org/web/packages/bcaboot/bcaboot.pdf
- 22.Critchley HOD, Babayev E, Bulun SE, et al. Menstruation: science and society. Am J Obstet Gynecol. 2020;223:624–664. doi: 10.1016/j.ajog.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berbic M, Fraser IS. Immunology of normal and abnormal menstruation. Womens Health (Lond Engl) 2013;9:387–395. doi: 10.2217/whe.13.32. [DOI] [PubMed] [Google Scholar]
- 24.Yu JN, Nam GE, Han K, et al. Association between menstrual cycle irregularity and tinnitus: a nationwide population-based study. Sci Rep. 2019;9 doi: 10.1038/s41598-019-50559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li K, Chen G, Hou H, et al. Analysis of sex hormone, menstruation and ovarian reserve in COVID-19 women of child-bearing age: a cross-sectional study. Reprod Biomed Online. 2021;42:260–267. doi: 10.1016/j.rbmo.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226:270–273. doi: 10.1016/j.ajog.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung EK, Kim SW, Ock SM, Jung KI, Song CH. Prevalence and related factors of irregular menstrual cycles in Korean women: the 5th Korean National Health and Nutrition Examination Survey (KNHANES-V, 2010-2012) J Psychosom Obstet Gynaecol. 2018;39:196–202. doi: 10.1080/0167482X.2017.1321631. [DOI] [PubMed] [Google Scholar]
- 28.Zeru AB, Gebeyaw ED, Ayele ET. Magnitude and associated factors of menstrual irregularity among undergraduate students of Debre Berhan University, Ethiopia. Reprod Health. 2021;18:101. doi: 10.1186/s12978-021-01156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimamoto K, Hirano M, Wada-Hiraike O, Goto R, Osuga Y. Examining the association between menstrual symptoms and health-related quality of life among working women in Japan using the EQ-5D. BMC Womens Health. 2021;21:325. doi: 10.1186/s12905-021-01462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astrup K, Olivarius NF. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand. 2004;83:203–207. doi: 10.1111/j.0001-6349.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 31.Takmaz T, Gundogmus I, Okten SB, Gunduz A. The impact of COVID-19-related mental health issues on menstrual cycle characteristics of female healthcare providers. J Obstet Gynaecol Res. 2021;47:3241–3249. doi: 10.1111/jog.14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malloy SM, Bradley DE. The relationship between perceived stress during the COVID-19 pandemic and menstrual cycles and symptoms. Fertil Steril. 2021;116:e72. [Google Scholar]
- 33.Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girardi G, Bremer AA. Scientific evidence supporting coronavirus disease 2019 (COVID-19) vaccine efficacy and safety in people planning to conceive or who are pregnant or lactating. Obstet Gynecol. 2022;139:3–8. doi: 10.1097/AOG.0000000000004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesselink AK, Hatch EE, Rothman KJ, et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol. 2022 doi: 10.1093/aje/kwac011. published online Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention COVID-19 vaccines while pregnant or breastfeeding. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 37.ACOG ACOG Committee opinion number 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124–e129. doi: 10.1097/AOG.0000000000002631. [DOI] [PubMed] [Google Scholar]
- 38.McNeil MM, Arana J, Stewart B, et al. A cluster of nonspecific adverse events in a military reserve unit following pandemic influenza A (H1N1) 2009 vaccination—possible stimulated reporting? Vaccine. 2012;30:2421–2426. doi: 10.1016/j.vaccine.2012.01.072. [DOI] [PubMed] [Google Scholar]
- 39.Pew Research Center Mobile Technology and Home Broadband 2021. 2021. https://www.pewresearch.org/internet/2021/06/03/2021-mobile-broadband-methodology/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant text responses in v-safe are not available to others because they might contain personally identifying information. Code for data pre-processing, filtering by search strings, and zero-shot classification is available at the code repository: https://github.com/cdcai/zsl-vaccine.